- 1Cawthron Institute, Nelson, New Zealand
- 2Institute of Marine Science, The University of Auckland, Auckland, New Zealand
- 3Sea Education Association, Woods Hole, MA, United States
- 4Environmental and Conservation Sciences, Murdoch University, Murdoch, WA, Australia
- 5Moana New Zealand, Cawthron Aquaculture Park, Nelson, New Zealand
Plankton are central to planetary ecology, generating 50% of Earth’s atmospheric oxygen and forming the largest system of interconnected life at the base of the marine food chain. Yet, current oceanographic models aimed at predicting global climate change lack high-resolution biological data, emphasizing the need for innovative approaches to collect plankton biodiversity and distribution data over larger spatial, temporal, and taxonomic scales. The significant number of boats, ranging from small sailing yachts to large commercial vessels, that ply the world’s oceans every day could help scientists collect thousands of valuable plankton samples. Traditional Plankton Nets (TPN) are not suited to the speed of a recreational craft cruising in the high seas (i.e., at speeds >2 knots). We developed and validated the efficiency of a lightweight, easily deployable Cruising Speed Net (CSN) that enables the collection of ocean surface micro- and mesoplankton at speeds up to 5 knots. Field testing was conducted during two distinct research cruises along coastal and oceanic latitudinal gradients (SSV Robert C. Seamans in New Zealand and RV Investigator in the south-east Indian Ocean). DNA metabarcoding performed on the collected plankton samples showed the TPN and CSN yielded identical sequence-based diversity at low speed, with the CSN also effective at higher speed for characterizing latitudinal distribution of plankton communities. The CSN represents a valuable new tool for expanding the global collection of plankton data.
Introduction
Marine plankton play critical roles in marine ecosystems, being at the basis of oceanic food webs and responsible for generating at least half of the Earth’s oxygen (Sekerci and Petrovskii, 2015). Plankton assemblages in the ocean vary spatially among biogeographic zones in much the same way as terrestrial biomes where different combinations of plant species occupy distinct land regions (Tappan and Loeblich, 1973). Heavily influenced by abiotic and biotic parameters of the water masses, plankton assemblages are also very sensitive to rapid changes in the ecology of the marine ecosystem related to global warming or other anthropogenic influences (Landry et al., 2020). Therefore, comprehensive plankton datasets are critical as indicators of ecological dynamics brought on by stressors, such as pollution or climate change (Hallegraeff, 2010).
Currently, oceanographic models aimed at predicting global ecological changes (Follows et al., 2007; Follows and Dutkiewicz, 2011; Ward et al., 2014) lack good quality, high resolution data on the nature and dynamics of marine plankton biodiversity. However, modeling the complexity of plankton assemblages is challenging due to small- and large-scale spatiotemporal variations that do not necessarily follow classic ecological laws, a paradigm known as “the paradox of the plankton” (Hutchinson, 1961). Fine resolution, large-scale plankton data is therefore required for extrapolation (D’Alelio et al., 2019). Despite the availability of >60 years of extremely valuable and relevant data derived from projects such as the modern Continuous Plankton Recorder series (Reid et al., 2003, 2010; Richardson et al., 2006), there exists no complete global distribution map of plankton assemblages. Furthermore, modern comprehensive sampling of open ocean life is hindered by extremely expensive operational ship-time (typically more than 30,000 USD per day), with more traditional oceanographic research vessels having limited capacity to accommodate a broad range of research projects and/or sampling requirements (Lauro et al., 2014). These factors often influence research cruise logistics and result in minimized collection time with sampling only at specific stations (Sameoto et al., 2000). As a result, some areas of the ocean are almost devoid of plankton composition observations (taxonomic assemblages), including much of the South and tropical Pacific Oceans.
In this context, the significant number of boats, ranging from small sailing yachts to large commercial vessels, that ply the world’s oceans every day represents an extraordinary opportunity for marine, and especially plankton-focused, research. There are approximately 120,000 active commercial ships (e.g., trading cargos, tourism cruises, fishing vessels) in the world fleet (Moser et al., 2015), and an estimated 10,000 yachts sailing the world’s oceans daily (YOREPS, 2020). Most vessels follow long-established routes dictated by predominant weather and trade winds (Cornell, 2014), providing a unique chance for dense spatiotemporal sampling of plankton. However, this concept is not new, and many studies have already exploited its potential (Lauro et al., 2014; Duarte, 2015; Simoniello et al., 2019). Most significant is the Ship of Opportunity Program run by the World Maritime Organization, which has deployed Continuous Plankton Recorders from large commercial vessels for more than 60 years; however, this collection technique is limited to a narrow range of plankton which are susceptible to capture, preservation and visual taxonomic identification in the towed recorders (Reid et al., 2010). The Global Ocean Sampling (GOS) expedition (Rusch et al., 2007; Williamson et al., 2008) paved the way for a new avenue of oceanography based on the use of sailing boats and advanced DNA-based ecology. This concept was expanded with the Tara Oceans expeditions (TO, 2009–2014), during which molecular, taxonomy based on morphological characteristics, and environmental data were collated from plankton assemblages (viruses to animals) across the world’s oceans (Karsenti et al., 2011). These programs resulted in a visionary update to our knowledge of the extraordinary biodiversity and global structure of the marine planktonic ecosystem. However, developing a working understanding of ecological and evolutionary dynamics requires a far greater sampling effort.
One recent initiative, Plankton Planet1, equipped 27 international sailing yachts with a sampling toolkit enabling citizen sailors to collect and isolate sea surface micro- (20–200 μm), meso- (200–2000 μm), and macroplankton (>2000 μm) using a Traditional Plankton Net (TPN, see example in Figure 1) while sailing the high seas. In a single year, this effort revealed a global picture of ocean eukaryotic plankton by using metabarcoding analyses on samples collected from 258 sites (De Vargas et al., 2020). However, a critical limitation to the Plankton Planet initiative was the operational requirement to reduce vessel speed to <2 knots to allow the 20 μm mesh net to tow stably within the water column. Such low speed made it very difficult for sailors to maneuver their vessels in the open sea, even precluding sampling in difficult weather conditions; this was identified as the primary limiting factor for achieving more extensive sampling at a more meaningful scientific scale. Thus, a modified net is needed – a low-cost, small size and effective plankton sampling device capable of collecting undamaged and unbiased surface ocean plankton assemblages at about 5 knots, which would not impact cruising speed and could be easily deployed from any vessel type, small sailing yachts to large commercial vessels.
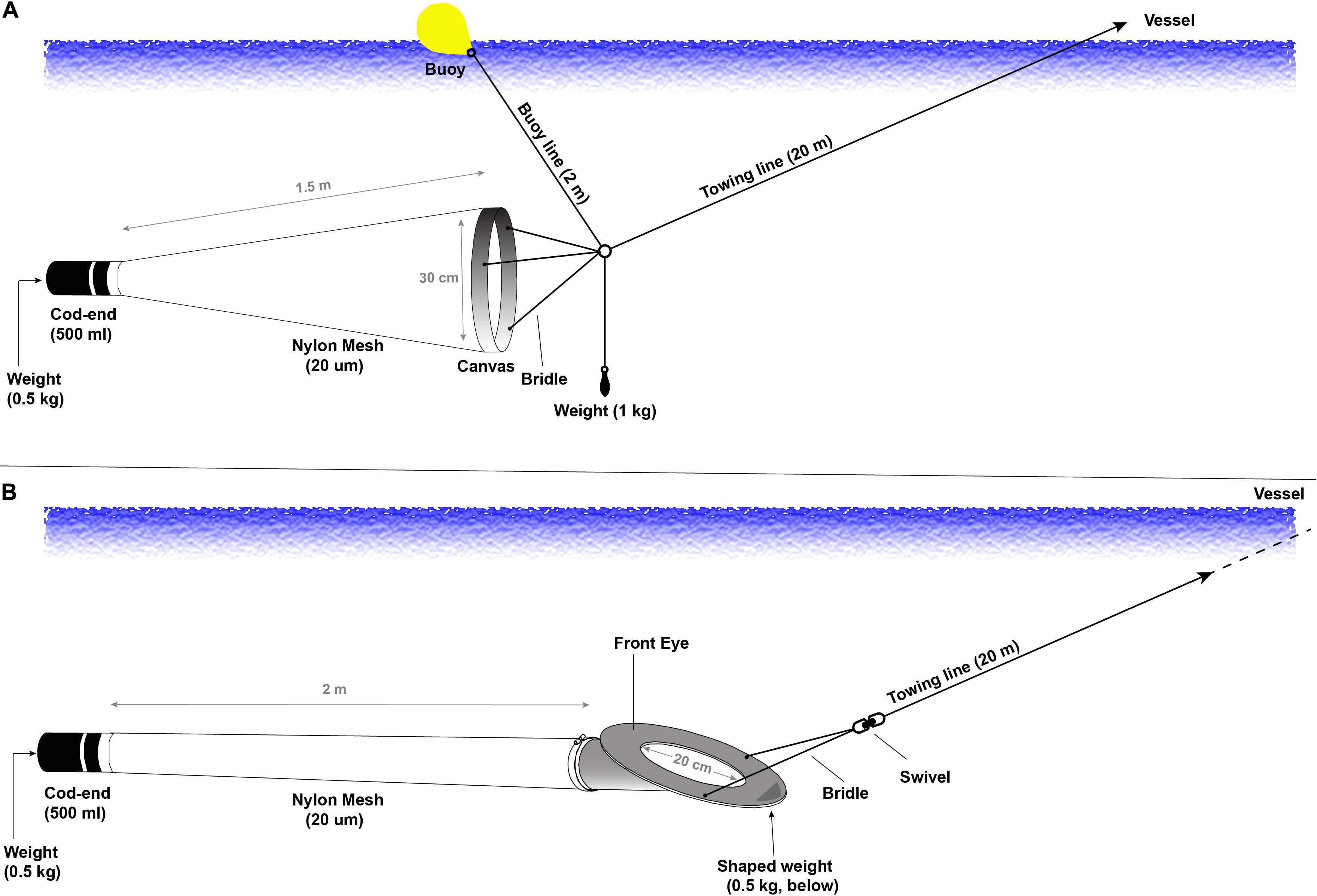
Figure 1. Description of the two plankton sampling devices tested in this study, with (A) the Traditional Plankton Net (TPN) and (B) the Cruising Speed Net (CSN). Complete specifications and general assembly of the CSN are provided in Supplementary Figure 2.
In this study, we designed and built an easily deployable Cruising Speed Net (CSN) enabling the collection of ocean surface marine micro- and mesoplankton by tow from a vessel at a minimum speed of 5 knots. Two case studies were conducted for field-based testing of the plankton sampling devices and comparison of plankton biodiversity derived from metabarcoding analyses of nuclear small-subunit ribosomal DNA sequences. The first case study occurred on-board a brigantine sailing vessel (SSV Robert C. Seamans) dedicated to educational oceanographic research while cruising the eastern coast of New Zealand, and tested two hypotheses: (i) there will be no significant differences in plankton community composition captured by the CSN and TPN devices when used side-by-side at approximately 2 knots, and (ii) when increasing the vessel speed to approximately 5 knots using the CSN, the captured plankton community composition will remain the same. The second case study was conducted on-board a large oceanographic research vessel (RV Investigator) and tested the CSN at 5 knots along a 30° latitudinal transect in the Indian Ocean (off Western Australia), evaluating the hypothesis: (iii) the CSN at 5 knots combined with metabarcoding analysis will be effective in capturing subtle plankton community composition changes associated with the latitudinal gradient. This study also offers complete specifications and general assembly guidelines for the CSN, with the hope that other researchers will use it and contribute to rapidly increasing our knowledge of the diversity, distribution, and evolution of open ocean plankton.
Materials and Methods
Description of Traditional Plankton Net (TPN) and Cruising Speed Net (CSN)
The TPN consisted of a conical 1.5 m long (20 μm nylon mesh; NITEXTM, Sefar Ltd., Switzerland) tow net with a 500 ml cod-end (including a 0.5 kg lead weight at the bottom but no mesh window) and a 30 cm diameter metal ring at the mouth, towed by three equally spaced bridle lines (Figure 1A). The bridle lines were connected to a stainless-steel ring (3 cm diameter) to which three additional lines were also attached. The first line was ∼50 cm long with a 1 kg lead weight attached at the bottom, with the purpose of sinking the TPN slightly below the surface whilst being towed. The second line was 2 m long, leading to a buoy at the water surface and ensuring that the TPN remained at <2 m within the water column. The third line was a >20 m long (2 cm diameter) Dynice Dyneema rope used to tow the TPN from the vessel’s rail. The very small nylon mesh size of the TPN combined with the necessary attachments (e.g., buoy and weight) created considerable drag, forcing a maximum tow speed of 2 knots among other problems such as extrusion of biota through the mesh and clogging. Vessel speeds of >3 knots invariably pulled the TPN to the surface, precluding plankton sampling.
CGW Consulting Engineers Ltd. (Nelson, New Zealand) assisted in designing a new and low-cost plankton sampling device able to easily collect plankton assemblages for microscopic and molecular identification, from any vessel type traveling at >3 knots. Design specifications for the sampling device were to: (i) be easily deployable at up to 5 knots, (ii) be relatively light and compact to minimize storage space use on small vessels, (iii) have a small opening of 10–20 cm diameter able to capture 2–5 m3 of seawater in ca. 5 min through a 20 μm mesh size, and (iv) have a solid front head, with or without wings, shaped hydrodynamically to sink and maintain device stability at 2–3 m depth with minimal weights and lines to reduce drag and to facilitate ease of handling during sampling.
Three different candidate models were designed, built and field tested (Supplementary Figure 1). Upon preliminary field testing the first two models (Supplementary Figures 1A,B) were highly unstable in the water column and thus not considered for further improvement. The third model (Supplementary Figure 1C) met most of the requirements and was selected for additional field testing and fine-tuning. This CSN consisted of a specifically shaped PVC head, with a 20 cm wide isometric front eye or mouth of the net that was positioned at a 20° angle, and a slightly conical 2 m long (20 μm nylon mesh; NITEX, Sefar Ltd., Switzerland) tow net with a 500 ml cod-end independently supplied by Whitlock Engineering Ltd., Nelson NZ (Figure 1B and Supplementary Figure 2). Early field testing showed that the CSN performed well at low speed but became unstable at >4 knots, often resulting in the device flipping over and going backward, followed by rapid surfacing. This limitation was resolved by (i) slightly curving the two sides of the front eye in a concave shape (acting as an “underwater kite”), (ii) reducing the CSN attachments to two front bridle lines only, and (iii) adding a swivel to the main towing line (Figure 1B). Further field testing demonstrated that the new modifications allowed the CSN to remain stable within the water column at towed speeds of up to 6.5 knots and it very effectively collected a variety of plankton species, as evidenced by microscopic observations2.
Sampling
New Zealand Case Study
Sampling was conducted on-board a 41 m steel brigantine-rigged sailing vessel (SSV Robert C. Seamans) operated by the Sea Education Association (SEA; Woods Hole, MA, United States) for oceanographic research and education. Field testing occurred between 16 February 2019 and 20 March 2019 along the east coast of New Zealand’s North and South Islands (35.0° S, 174.01° E to 42.8289° S, 173.9039° E) during SEA cruise S284. Samples were collected using the TPN and/or CSN as frequently as possible (i.e., at least once a day – time and weather permitting) following two models: In the first approach, employed in 21 distinct stations, both plankton net devices (TPN and CSN) were towed side-by-side, at 1–2 m below the sea surface from a metal boom extending off the vessel’s port side at approximately 2 knots ship speed for 5 min (Table 1 and Figure 2). The purpose of side-by-side sampling was to compare the TPN and CSN’s plankton capture efficiency, assessed through consistency in the recovered eDNA plankton diversity and community composition. The second sampling scheme involved the deployment of the CSN only, for the same duration, and aimed to assess the CSN’s ability to capture representative plankton assemblages at higher sailing speeds (3–5.1 knots). On eight occasions, this higher-speed sampling was conducted immediately following the side-by-side sampling, hence considered the same stations (Table 1 and Figure 2). Before each new station, sterile gloves were worn and all gear (i.e., sampling nets, cod-ends, collection bottles, and filtration units) were thoroughly cleaned in local seawater mixed with a 2% bleach solution for at least 10 min, then rinsed with seawater from each sampling site to avoid cross contamination from other stations. Directly following each net tow, the cod-end was carefully removed and approximately 500 ml of the collected seawater was carefully poured into a sterile plastic bottle. Geographic location and time were recorded for each station at the start of each tow (Table 1).
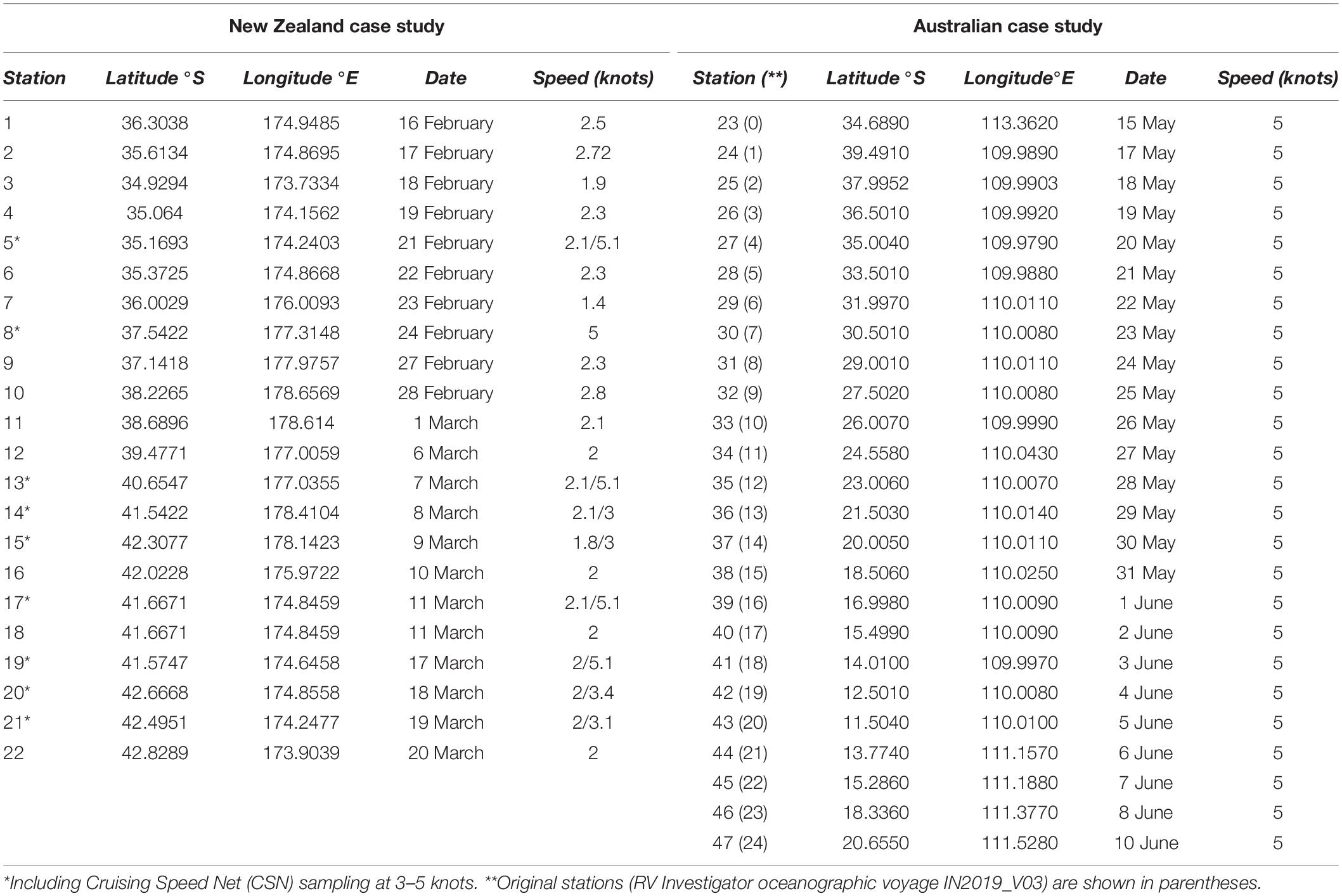
Table 1. Sampling stations for the New Zealand and Australian case studies and corresponding latitude, longitude, date (day and month in 2019), and sampling speed.
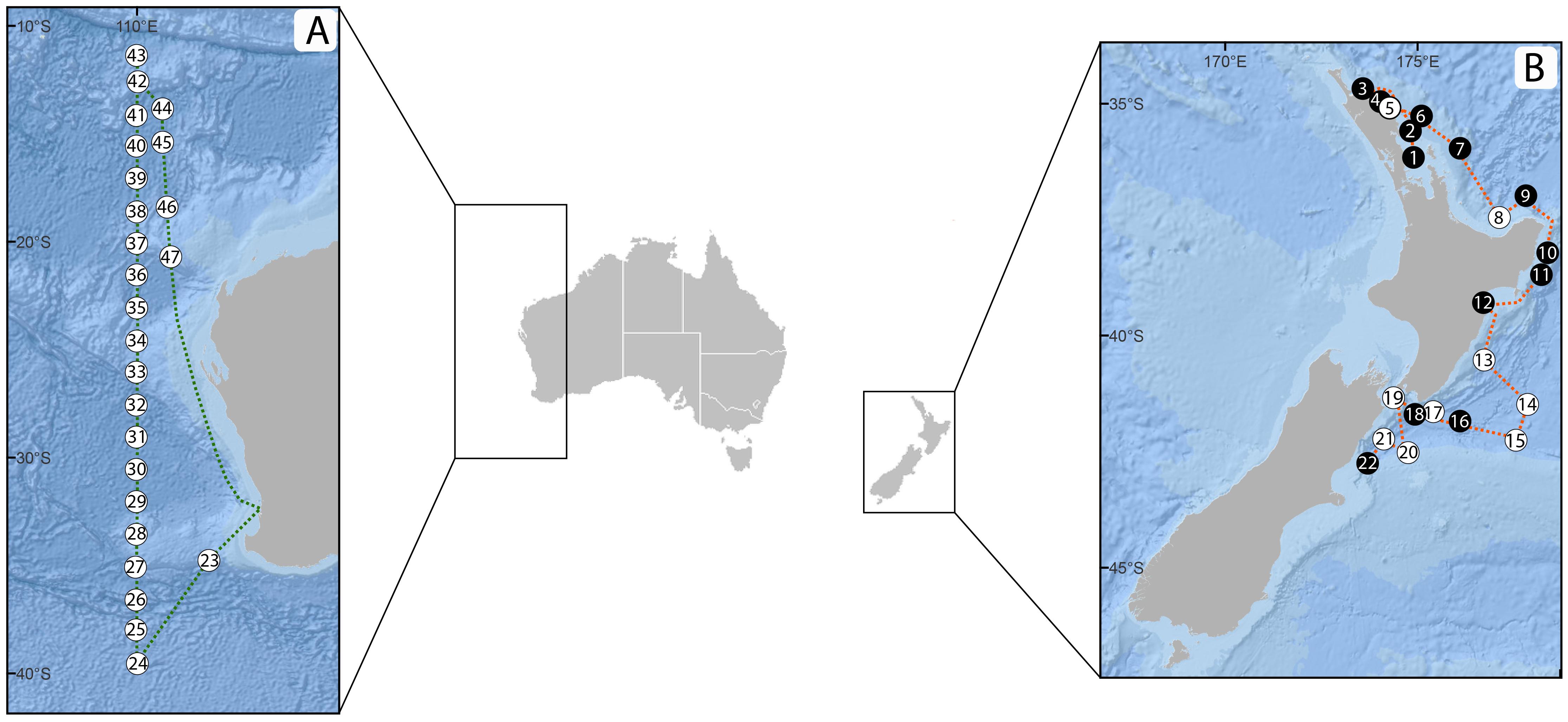
Figure 2. Sampling stations for the (A) Australian case study – RV Investigator voyage IN2019_V03 and (B) New Zealand case study – SSV Robert C. Seamans cruise S284. Numbers correspond to specific sampling stations/dates (Table 1). Black circles, side-by-side sampling using the Traditional Plankton Net (TPN) and Cruising Speed Net (CSN) at 2 knots; white circles, additional and/or unique sampling at 3–5 knots using the CSN.
Australian Case Study
Sampling was conducted in the Indian Ocean on-board a 94 m oceanographic research vessel (RV Investigator, CSIRO, Hobart, Tasmania) between 15 May 2019 and 10 June 2019, along an approximately 30° latitudinal transect off Western Australia (39.4910° S, 109.9890° E to 11.5040° S, 110.0100° E; Table 1 and Figure 2). For this case study, only the CSN was deployed, once per day in the afternoon and towed for 5 min at 5 knots following the same procedure as described above.
Sample Processing (Both Case Studies)
Filtration
The collected plankton material from TPN and/or CSN tow was carefully resuspended by shaking the plastic sample bottles, of which 200 ml was used for filtration thereby retaining micro- and mesoplankton samples for further analyses. Filtration was done using a sterile Whatman filter (pore size 3 μm; Merck KGaA, Darmstadt, Germany) placed in a Sterifil filtration system (Merck, Darmstadt, Germany) which used a 12V Seaflo 21 Series Water Pressure Pump 3.8LPM (Marine Deals, Auckland, New Zealand). Each filter, and its retained plankton material, was cut in half and stored into two separate 2 ml sterile cryotubes (one of the tubes with the second half of the cut filter was kept as a backup) containing 1.5 ml of LifeGuard Soil Preservation Solution (QIAGEN, Hilden, Germany). Samples were frozen at −20°C immediately after filtration on the research vessels, transported to the Cawthron Institute (Nelson, New Zealand) post cruise and stored at −80°C until further processing.
DNA Extraction
In the laboratory, filters were directly transferred into ZR BashingBead Lysis Tubes (2.0 mm; Zymo Research, CA, United States) containing 1 ml Lysis Buffer from the ZR DuetTM DNA/RNA MiniPrep Kit Plus (Zymo Research). All samples were homogenized via bead beating for 2 min (1600 MiniG Spex SamplePrep, NJ, United States), and centrifuged (10,000 × g, 5 min, 20°C; Eppendorf Centrifuge 5430R, Hamburg, Germany). DNA was extracted from each sample following the manufacturer’s protocol and DNA extraction blanks (i.e., negative controls) were included for each extraction series. The quantity and quality of extracted DNA were measured using a NanoPhotometer (Implen, Munich, Germany).
High-Throughput Sequencing
For the characterization of eukaryotic communities, an approximately 400 base pairs (bp) fragment of the V4 region of the nuclear small subunit ribosomal DNA (18S rRNA gene) was amplified from each sample by a single Polymerase Chain Reaction (PCR). The eukaryotic-specific primers were Uni18SF: 5′AGG GCA AKY CTG GTG CCA GC3′ and Uni18SR: 5′GRC GGT ATC TRA TCG YCT T3′ (Zhan et al., 2013). IlluminaTM overhang adaptors were attached to the primers to allow dual-indexing as described in Kozich et al. (2013). Thermocycling PCR conditions were: 94°C for 3 min followed by 35 cycles of 94°C for 30 s, 52°C for 30 s, 72°C for 1 min, with a final extension step at 72°C for 7 min. Negative controls were included in each PCR analysis.
Amplicons were purified by magnetic separation following the Agencourt AMPure XP protocol (Agencourt Bioscience Corporation, Beverly, MA, United States), quantified (Qubit 2.0 Fluorometer, Invitrogen, Carlsbad, CA, United States) and diluted to 3 ng/μl. Two samples of 20 μl of RNA/DNA free water (UltraPure) were added as negative sequencing controls. Amplicons were sent to Auckland Genomics (The University of Auckland, Auckland, New Zealand) for library preparation. Sequencing adapters and sample specific indices were added to each amplicon using the Nextera Index kit (Illumina). Amplicons were pooled into a single library and paired-end sequences (2 × 250 bp) generated on an Illumina MiSeq using the TruSeq SBS kit (Illumina).
Bioinformatics (Both Case Studies)
Cutadapt version 2.10 (Martin, 2011) was used to remove the primer sequences from the raw reads with a single mismatch being allowed. These trimmed sequences were subsequently processed using the DADA2 package [version 1.12.1; Callahan et al. (2016) within R version 3.6.1; R Core Team (2017)]. The reads were truncated to 250 and 240 bp (forward and reverse reads, respectively) and filtered with a maximum number of “expected errors” (maxEE) threshold of two forward reads and two reverse reads. If reads did not meet this threshold, they were discarded from further analysis. A parametric error matrix was constructed within the program to dereplicate sequence variants for the forward and reverse reads. Paired-end reads were merged with a maximum mismatch of 1 bp and a minimum overlap of 10 bp and singletons were discarded. Chimeric sequences were removed within DADA2 using the consensus option in the removeBimeraDenovo function. The Amplicon Sequence Variants (ASVs) for the 18S rDNA gene were taxonomically classified against the PR2 (Guillou et al., 2013) database. The DADA2 assignTaxonomy function, based on the rdp classifier (Wang et al., 2007), was run with a bootstrap cutoff of 0.9. DNA negative controls were assessed and read numbers for ASVs found in the negative blanks were removed by proportional subtraction. The raw FASTQ sequence data were deposited in the Short Read Archive, BioProject PRJNA657626.
Statistical Analyses
Plankton biodiversity estimates were derived from ASVs, which may include both intra- and inter-organismal genetic variability. Consequently, this approach cannot accurately measure absolute abundances of plankton organisms, but rather estimate plankton composition based on the relative proportions of annotated plankton ASVs recovered from each environmental sample. Therefore, “plankton biodiversity” is hereafter referred to as “ASV-diversity.” The New Zealand and Australian datasets were analyzed separately. Rarefaction curves of sequence reads from all samples were performed using ggrare in the R package ggplot2 (version 3.3.0). For community comparisons, untransformed numbers of reads of both datasets were rarefied down to 23,440 reads per sample using the rarefy_to_even_depth function in the phyloseq R package (version 3.6.1). Samples were fourth root transformed and Bray Curtis dissimilarity matrices were computed for each dataset, as advised in Clarke et al. (2017).
New Zealand Case Study
The New Zealand dataset was statistically analyzed based on two main factors: (i) TPN versus CSN (hereafter referred to as factor “net”) and (ii) Samples collected at <3 knots versus samples collected at >3 knots (hereafter referred to as factor “speed”). Alpha-diversity indices (at ASV level) were calculated for the factors “net” and “speed,” including: Observed ASVs (S), Margalef richness (d) and Shannon diversity (H’), and displayed in boxplots. The effect of “net” and “speed” on these metrics was then examined using a pairwise t-test with 999 permutations.
For beta-diversity analysis, low reads (<10) were removed from the dataset to avoid stochastic effects on the data. Significant differences between the plankton assemblages for the “net” and “speed” factors were tested using distance-based permutational analysis [PERMANOVA; Anderson and Epifanio (2009) implemented in Primer7 PRIMERE Ltd., United Kingdom; Clarke and Gorley (2015)] with “net” and “speed” as fixed factors (“speed” factor nested within “net”).
Exploring differences in ASV-diversity and which taxa accounted for the differences between factors, a two-way crossed species contribution to sample (dis)similarities (SIMPER) analysis was performed based on presence/absence data, as described in San Martín et al. (2005), and splitting the New Zealand dataset into the factors “net” and “speed” with a cutoff percentage set at 70%. As there were no significant taxa associated with each subgroup of the factors (Supplementary Table 2), the 30 most abundant ASVs of the fourth root transformed dataset, assigned at lowest possible taxonomic level, were visualized in a shade plot. A metric Multi Dimensional Scaling (mMDS) plot, based on fourth root transformation and Bray Curtis similarity matrix, was used to visualize similarities of samples for factors “net” and “speed.”
Australian Case Study
The Australian dataset was visualized using Principal Coordinates Analysis (PCO) implemented in Primer7 (Clarke and Gorley, 2015), including the six most abundant plankton species (i.e., ASVs), assigned at lowest possible taxonomic level and overlaid as proportional pie charts.
Results
The 76 plankton samples collected in this study (51 samples from the New Zealand and 25 samples from the Australian case studies) generated a total of 12,088,295 raw reads, which decreased to 5,982,714 reads (2,839 ASVs) post filtering (i.e., denoising, merging and chimera removal). Following the rarefaction analysis (Supplementary Figures 3, 4), each sample was rarefied to 23,440 sequence reads, which resulted in the removal of five low read count samples from the downstream analysis (2 CSN samples at station 21, CSN and TPN samples at stations 22, and CSN at station 34; Table 1). Overall, the taxonomic assignment revealed 74 orders, 133 families, 302 genera, and 379 species (Supplementary Table 1). Residual contamination was detected across all control samples (2,929 sequence reads representing 12 distinct ASVs) and these were removed from the actual dataset following the same procedure as described in Bell et al. (2018).
New Zealand Case Study
The comparison of TPN and CSN data from New Zealand revealed no significant differences between either the sampling devices (factor “net”) or the vessel speeds (factor “speed”) for any of the calculated diversity indices: Observed ASVs, Margalef richness and Shannon diversity (Figure 3 and Table 2A). PERMANOVA analysis resulted in no significant p-values for either “net” or “speed” factors (Table 2B).
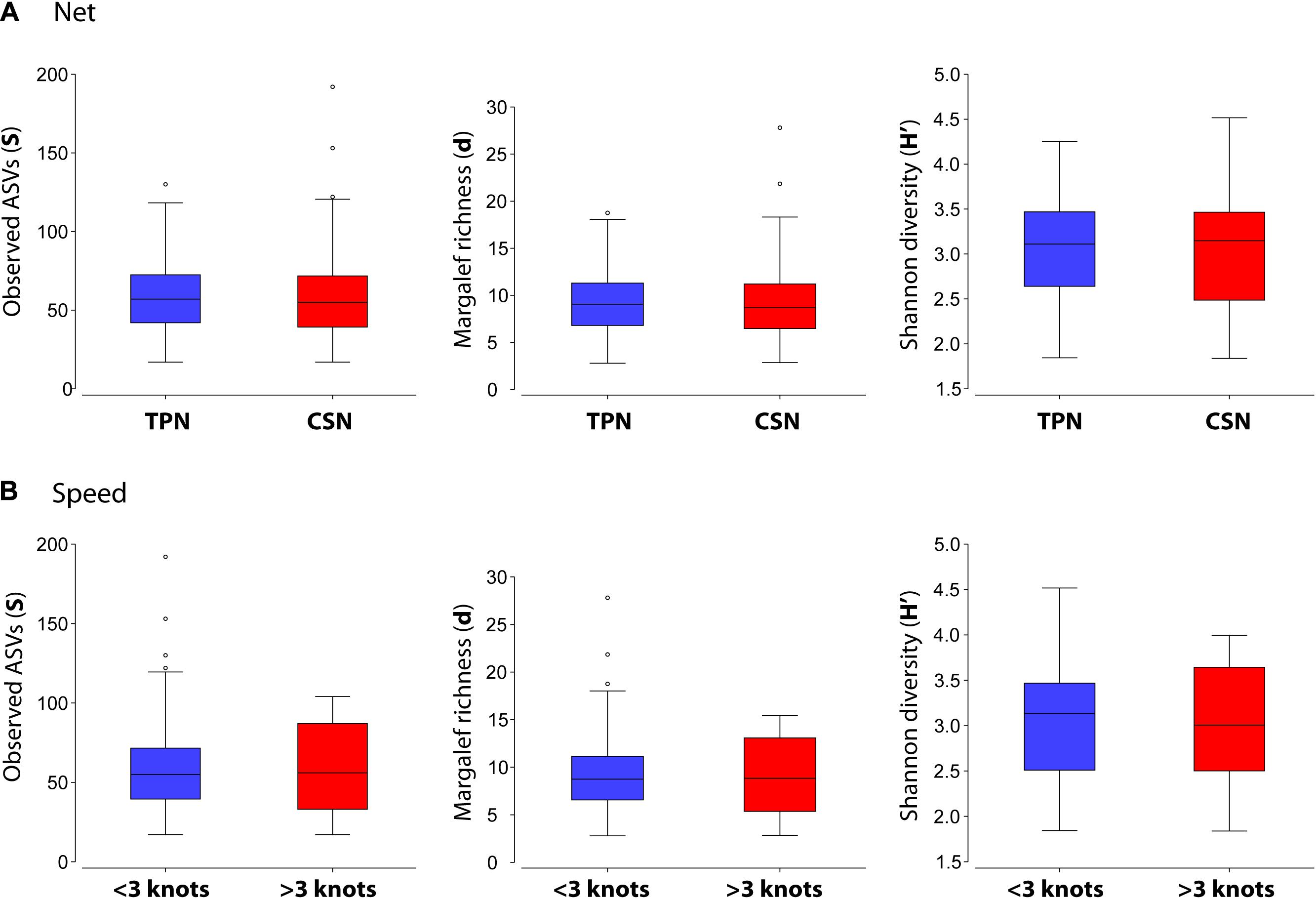
Figure 3. Boxplots comparing diversity indices (Observed ASVs, Margalef richness, and Shannon diversity) obtained with the New Zealand case study dataset for (A) the factors “net” (TPN, Traditional Plankton Net; CSN, Cruising Speed Net), and (B) “speed” (approximately 2/ < 3 knots versus approximately 5/ > 3 knots).
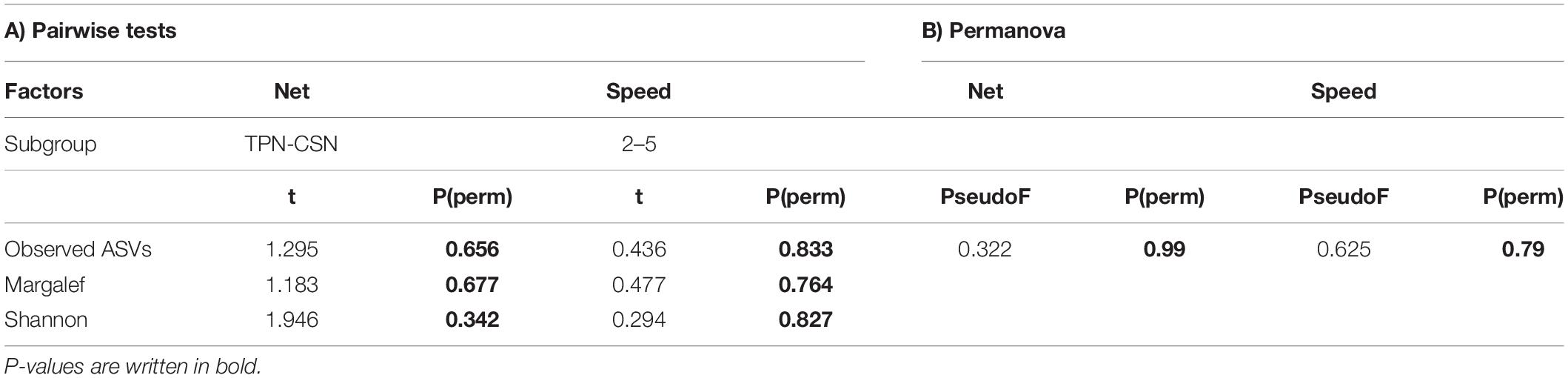
Table 2. (A) Pairwise tests for each calculated diversity index (Observed ASVs, Margalef richness, and Shannon diversity) for factors “net” and “speed.” (B) Permutational multivariate analysis of variance (PERMANOVA) based on Bray Curtis similarities for the “net” and “speed” factors.
Investigating the taxonomic composition between the “net” (TPN versus CSN) and “speed” (<3 versus >3 knots) factor groups, a SIMPER analysis revealed high percentage similarities amongst average taxa abundances between factor groups (Supplementary Table 2). Therefore, average dissimilarity values, even when based on presence/absence transformed data, were too low to be considered for further analysis. For example, the highest listed ASV, ASV_23, contributed with just 0.6% average dissimilarity to differentiation between CSN and TPN. On the other hand, ASV_23 had similar values of 0.45% and 0.59% in the TPN and the CSN, respectively (Supplementary Table 2). Twelve ASVs accounted for a 10% cumulative difference between nets, with high average abundance across both. The same pattern was detected between the speed factor groups (Supplementary Table 2). Overall, no clear indicator taxa were found to be characteristic of just one factor group. Any differences between factors seemed mainly due to random appearances of organisms that sporadically appeared in a given sample within a group. This hypothesis was also supported by the shade (Figure 4) and mMDS (Figure 5) plots, indicating higher affinity of plankton community by sampling station than either the net or speed factors.
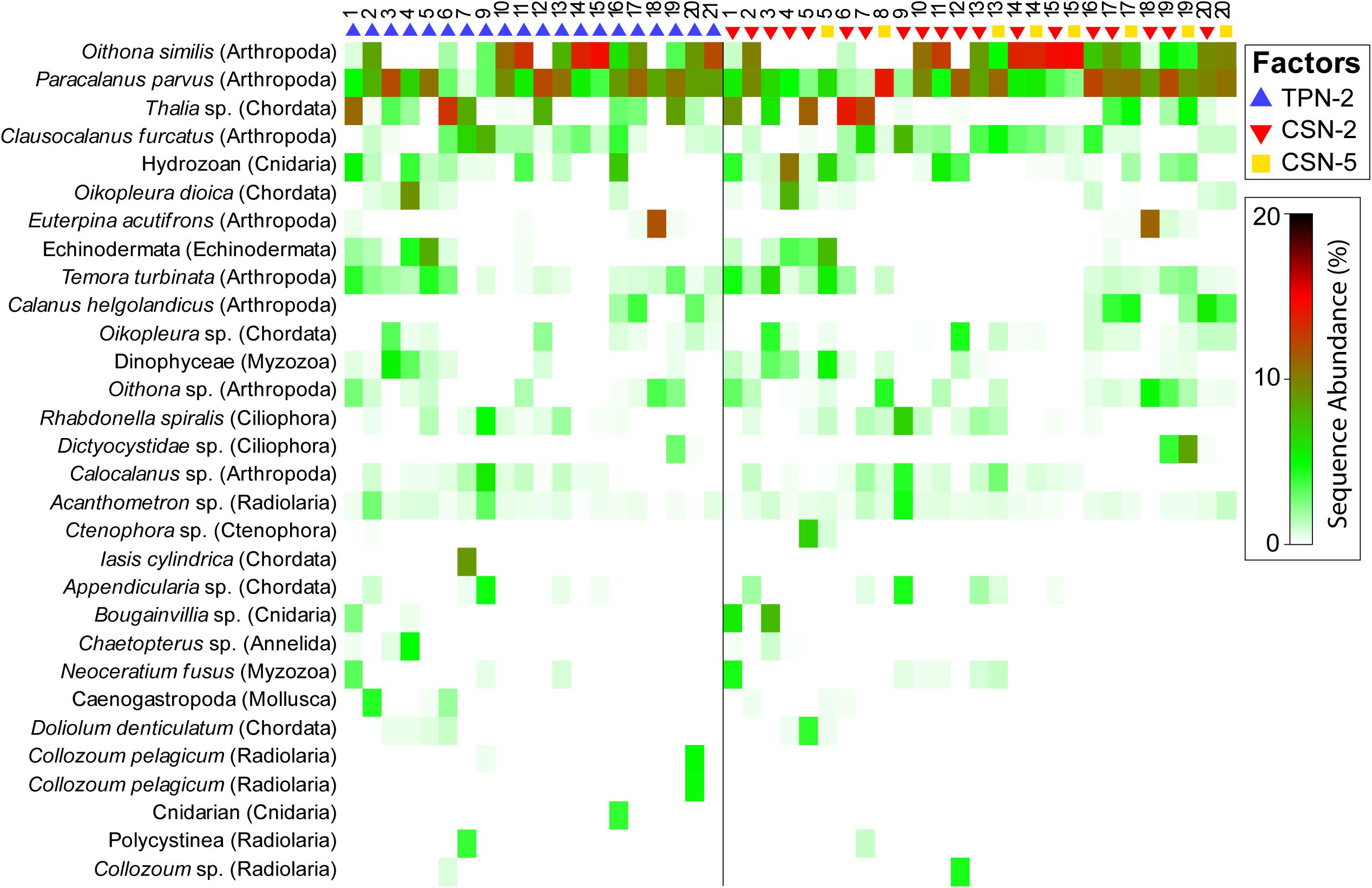
Figure 4. Shade plot displaying the 30 most abundant Amplicon Sequence Variants (ASVs, y-axis, assignment at lowest assignable taxonomic level is indicated to the left, with corresponding phylum in parenthesis) amongst all New Zealand samples. The data are divided into Traditional Plankton Net at <3 knots (TPN2), Cruising Speed Net at <3 knots (CSN2), and at >3 knots (CSN5).
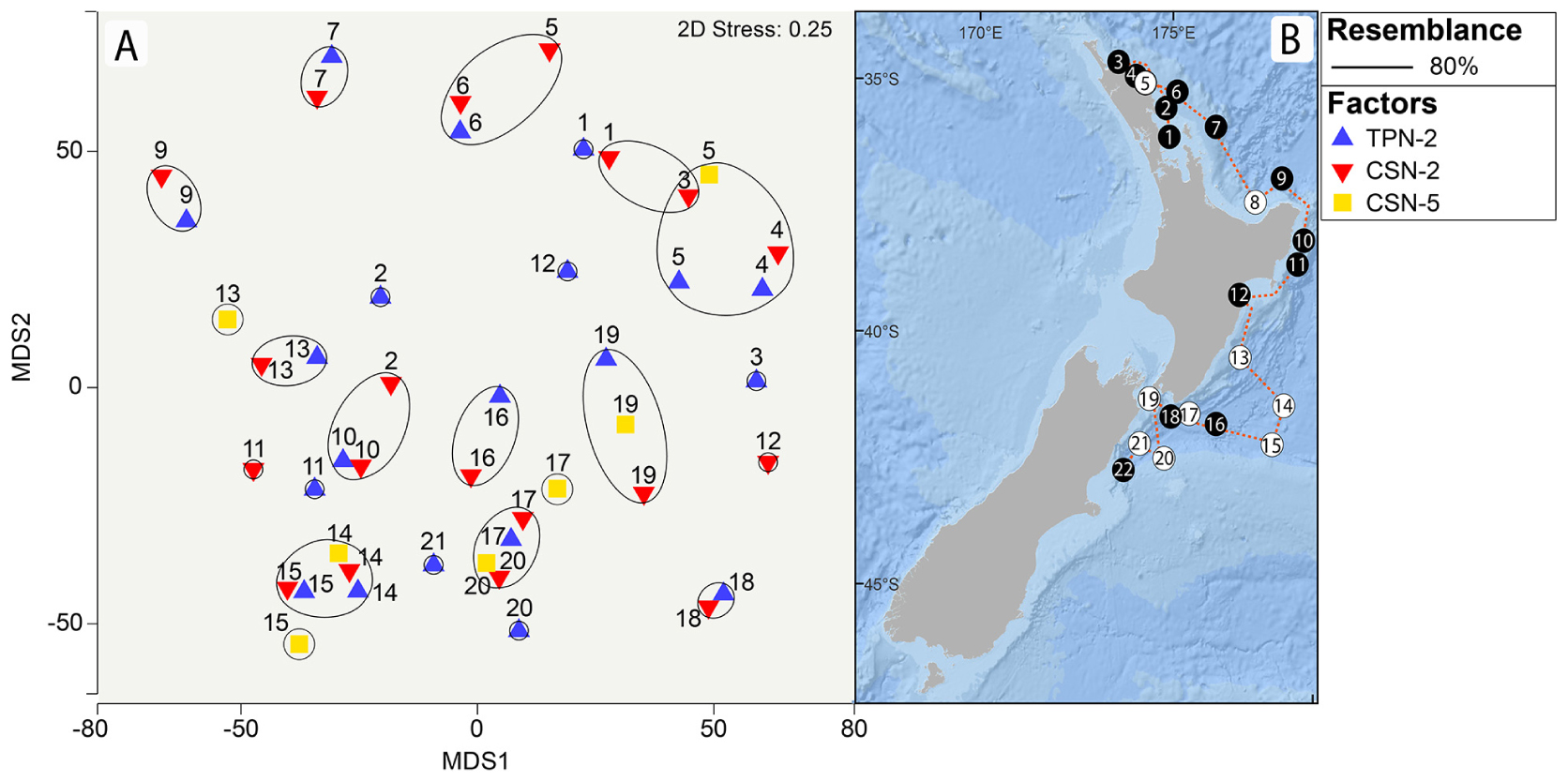
Figure 5. Metric Multi Dimensional Scaling (mMDS) ordination plots based on fourth root transformed data and Bray Curtis similarities obtained from the New Zealand dataset (A), with the corresponding sampling station numbers (1–22) as described in Table 1 and Figure 2. (B) Colored symbols correspond to different factors: Traditional Plankton Net at <3 knots (TPN2), Cruising Speed Net at <3 knots (CSN2) and at >3 knots (CSN5). Circles connect samples with at least 80% community resemblance.
Plotting the 30 most abundant planktonic ASVs amongst all New Zealand samples revealed zooplankton taxa with highest abundances (top of shade plot) were largely mirrored between factor groups (Figure 4). Gelatinous taxa such as the salp Thalia sp. and the tunicate Oikopleura dioica, but also copepods such as Temora turbinata, as well as two unidentified Hydrozoan and Echinodermata ASVs, were observed with higher abundances in early stations (19) for both TPN and CSN devices. In later stations (10–22), a community shift was apparent in both nets as evidenced by the highly abundant copepod species, Oithona similis and Paracalanus parvus. While a few taxa were restricted to a single sample (e.g., Euterpina acutifrons at station 18 and the ciliophoran Dictyocystidae at station 19), the majority of dominant taxa were recovered in multiple samples, regardless of nets or speed. Nevertheless, single stations revealed rare taxa, including a radiolarian species (Collozoum pelagicum), a salp species (Iasis cylindrica) and an unidentified cnidarian taxon, exclusively associated with the TPN device. Similarly, a ctenophoran taxon as well as another Collozoum sp. were only captured by the CSN device.
The mMDS analysis (Figure 5) yielded a relatively strong clustering of planktonic assemblages from each sampling station, regardless of the “net” and “speed” factors (e.g., Stations 13–15, 17, 19, and 20), which was consistent with the PERMANOVA results (Table 2). There was also some evidence of regional separation along the latitudes and longitudes of the cruise track. Both geographic location and proximity to shore were influential; for example, stations 1, 3, 4, 5, and 6 reflect nearshore sampling in the Northland region of New Zealand while 7 and 9 cluster further away, potentially because they were collected at the continental shelf break. Stations around or south of the Coromandel Peninsula clustered on the left side of the mMDS plot (samples 9, 10, and 11). However, the 0.25 stress values represented a complicated fit of the samples’ similarities in the two-dimensional space. Station 2 appeared as an outlier from its “Northland neighbors” and contained high abundances of the copepods Oithona similis and Paracalanus parvus that characterized later sampling sites 10–22 (Figure 4). Station 3, 5, and 12 exhibited high variability between sample subgroups, attributable to the isolated presence of rare taxa. The cnidarian Bougainvillia sp. appeared only in station 3’s CSN sample while Thalia sp. was detected in only one tow at station 5 (CSN2) as well as with only the TPN at station 12 (Figure 4).
Australian Case Study
A PCO (Figure 6) demonstrated that the metabarcoding based plankton assemblages captured by the CSN were heavily influenced by the latitudinal gradient, which broadly corresponded with increasing sea surface temperature from 12 to 28°C. Plankton composition exhibited successive assemblage changes from the southernmost station 24 (latitude 39.491° S) to station 33 (latitude 29.001° S), followed by a clustering of assemblages at the northernmost sampling stations 35–43 (latitudes 24.558°–11.504° S). Interestingly, samples from multiple stations at similar latitudes captured almost identical plankton assemblages despite being collected several days and 100’s of kilometers apart (e.g., station 23/27/28 and stations 35 – 46/46/47 and stations 41/43/44).
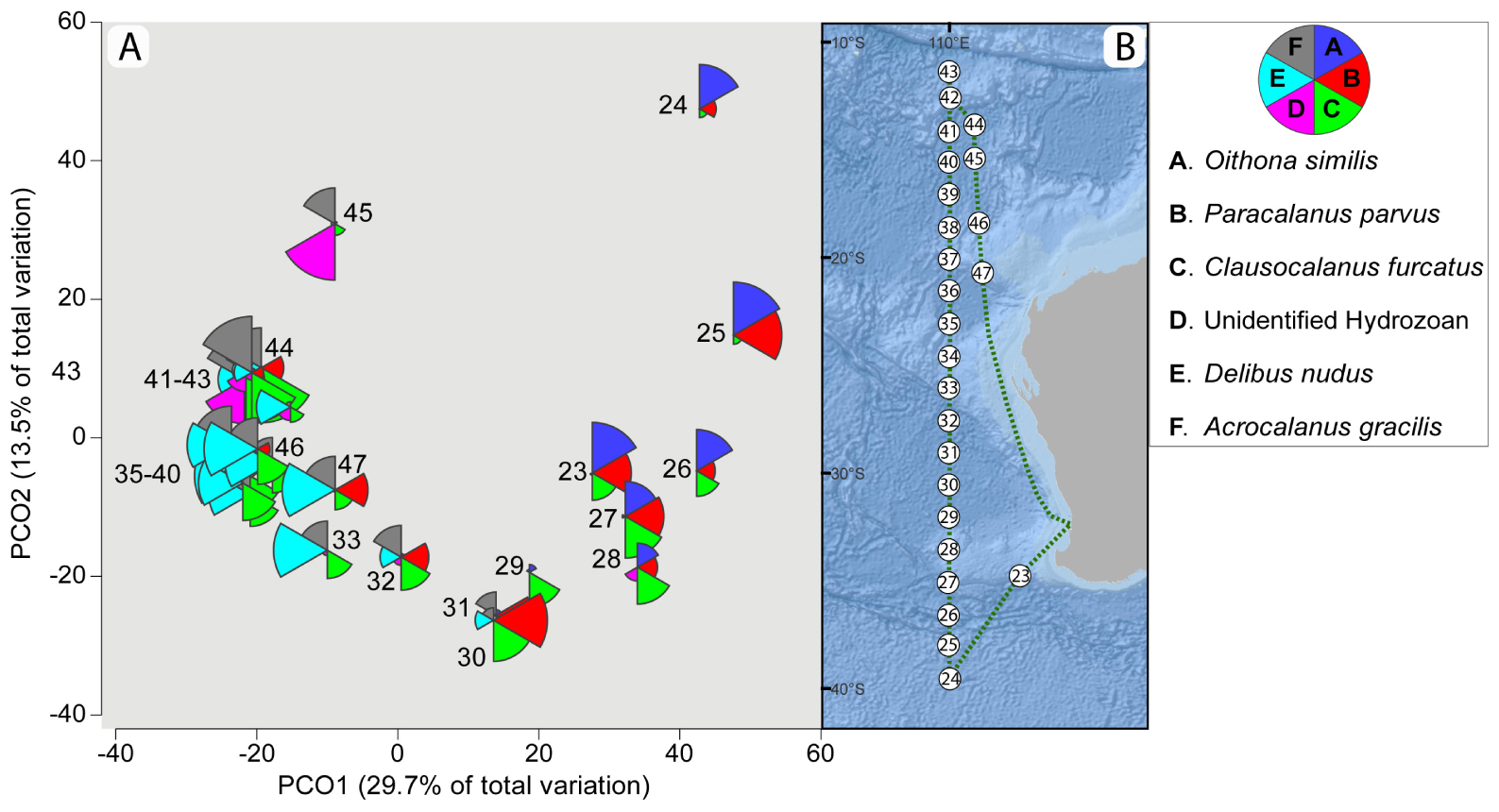
Figure 6. Principal Coordinate Ordination (PCO) based on Bray Curtis similarities of square root transformed plankton (18S rRNA gene) metabarcoding data along a latitudinal transect in the Indian Ocean investigated during the Australian case study (A) with the corresponding sampling station numbers (23–47) as described in Table 1 and Figure 2. (B) The color pie charts represent the relative prevalence [i.e., Amplicon Sequence Variants (ASVs) proportions] of the six most abundant taxa (at the species level; see legend).
Five of the six most dominant plankton species in the dataset were copepods, and a clear shift in species distribution was noted along the latitudinal gradient (Figure 6). For example, two copepod species, Oithona similis and Paracalanus parvus were prevalent at the southernmost sampled latitudes in the Indian Ocean; these were also the two most dominant species at similar latitudes in the New Zealand dataset (Figure 1 and Figure 4). Oithona similis was abundant between stations 24 and 28, Paracalanus parvus between stations 25 and 32, Clausocalanus furcatus between stations 26 and 43. Lower latitudes (from 26.007° S) were dominated by two other copepod species (Delibus nudus and Acrocalanus gracilis) and an unidentified Hydrozoa.
Discussion
In the present study, we designed a novel plankton sampling device that operates effectively at the average cruising speed (∼ 5 knots) of a standard sailing vessel. This innovative CSN exploits hydrodynamics rather than weight to remain stabilized below the sea surface, eliminating the unnecessary drag encountered with traditional nets.
Many distinct plankton sampling techniques have been developed over time (Tranter and Fraser, 1968). Some, such as Niskin bottles (Pennington et al., 2016) or pumps (Bishop et al., 1992), can effectively capture plankton assemblages, but these methods require the vessel to stop thereby limiting the sampling area that can be covered. Plankton nets are one of the oldest, simplest, effective and least expensive methods of sampling plankton (Gutkowska et al., 2012) and can be used for both morphological and molecular taxonomic analyses (Harvey et al., 2017; Schabacker et al., 2020). Thus, the more effective the net is in performance (e.g., sample capture and towing), as well as ease of handling, the greater the sampling opportunity and capacity.
The main advantage of towing a plankton net, horizontally or vertically, is that it considerably increases sampling volume and spatiotemporal coverage, e.g., towing a net at about 5 knots represents about 10 km per hour (Sameoto et al., 2000). A major limitation for open ocean towing is the size of the net mesh, usually between 5 and 80 μm for phytoplankton, 125 and 300 μm for mesozooplankton, and 1000 μm for micronekton (John et al., 2001; Castellani and Edwards, 2017). These mesh sizes can create considerable drag and typically require the vessel to not exceed a speed of approximately 2 knots, a situation that is often impossible to meet in marine areas with large waves and swell. Moreover, nets designed for higher towing speeds have reduced sampling capacity, as mesh sizes are increased (ca. > 300 μm) to reduce drag and handling becomes more cumbersome as weight and size of the net tends to increase, too. These constraints mean that existing high-speed nets can only be operated from large vessels able to tow such heavy equipment because they generally have sufficient storage capacity and more crew members.
The first high-speed plankton samplers were developed in the early 1900s, including the Hardy Continuous Plankton Recorder (Wiebe and Benfield, 2003). Off-the-shelf devices, such as the high-speed plankton collector “nackthai” (Schnack, 1974) perform well at up to 8 knots of vessel speed3. Another high-speed net example is the AVANI trawl; developed for collecting sea surface microplastics, it performs well in conditions of moderate sea state under 8 knots of boat speed over long transects (Eriksen et al., 2018). More recently, the Tara Ocean expedition developed a high-speed net able to collect macrozooplankton and neuston at 5–9 knots from the ocean atmosphere interface (Gorsky et al., 2019). All of these “high-speed” devices, however, present two major limitations. First, they are all equipped with nylon mesh sizes from >300 to 1000 μm, missing the vast majority of micro- and mesoplankton (<200 μm) taxa that are critical for holistic community assessments. In fact, the predicted 0.5–2.2 million plankton species do not even take into account the enormous diversity of small (20–200 μm) protist groups mainly associated with unknown taxa (De Vargas et al., 2015). Second, these devices are heavy and bulky. For example, the “nackthai” has a 150 cm long metallic frame with a 22 kg V-fin depressor while the Tara Ocean high-speed net consists of an 80 kg stainless steel head mounted with a 200 cm long nytrel net and cod-end. Furthermore, the high cost of these devices tends to preclude citizen science engagement in plankton collection.
The CSN represents a lightweight, affordable (estimated at ∼500 USD), compact and easily deployable alternative for the collection of ocean surface plankton (micro- and mesoplankton) data from a wider range of vessels and over much broader spatiotemporal scales than currently possible. We conducted two case studies to systematically test the performance and ability of the CSN to capture ocean surface plankton assemblages at two distinct cruising speeds and across two geographical gradients. The New Zealand case study showed that the CSN and TPN performed equally well at low (∼2 knots) speed, and that the same results were obtained when increasing the CSN towing speed to approximately 5 knots. Derived plankton composition was consistent between the two nets and speeds, with some variability likely explained by sporadic detection of rare and sparsely distributed taxa. No consistent bias toward over or underrepresentation of certain groups of organisms was noted. Some taxonomic outliers, such as different Collozoum species (Radiolaria), were detected in individual TPN or CSN samples which shows that single organisms randomly caught in the net can lead to some variability between devices due to sampling stochasticity but not device performances. Enhanced replication could have helped confirm our assumption that these taxa were incidental and do not indicate systematic taxonomic biases of one specific sampling approach. Indeed, other radiolarian taxa previously considered as rare in that area (Taylor, 1978), such as Acanthometron sp., appeared across all stations in equal abundance from both nets. Despite their important contribution to marine ecosystems (Countway et al., 2007; Edgcomb et al., 2011), radiolarians (including Collozoum sp.) are known to be difficult to capture under conventional plankton sampling due to their small size and fragile silica skeleton (Biard et al., 2017). In general, the sampling approach using both nets revealed geographic patterns between the northern and southern New Zealand stations, with the majority of TPN and CSN samples displaying similar community clustering. An overall shift was visible for gelatinous taxa (e.g., Thalia sp. and Oikopleura sp.) known to prefer the warmer, nutrient rich, coastal areas sampled along the Northland areas of New Zealand (Wiebe et al., 1979; Franco et al., 2016). Nearshore nutrient rich sampling stations also captured a high abundance of meroplanktonic forms, such as Chordata, Hydrozoa, and Echinodermata, as well as neritic copepods, including Temora turbinata, all consistent with previous observations (Bradford, 2010). Holoplanktonic copepod species, such as Oithona similis and Paracalanus parvus, dominated at sampling stations further offshore, confirming prior studies that reported higher abundance of these pelagic copepods in global oceanic waters (Cornils et al., 2010; Jose et al., 2017).
The Australian case study demonstrated that the CSN can be successfully operated from a large oceanographic research vessel traveling at a constant cruising speed of 5 knots. The device was very effective in capturing subtle biogeographic changes in plankton species distribution along a 30° latitudinal gradient in the Indian Ocean, confirming data collected in the historic voyage of the International Indian Ocean Expedition (IIOE) in the 1960s by Tranter (1977). The most remarkable result was the very strong and linear successional shift in community distribution from the highest latitude in the South (station 24) to the lowest latitude in the North (station 43) of the transect (Figure 6), consistent with previous studies in the region (Eriksen et al., 2019). Also supporting published observations was the predominance and differential distribution of copepods species across latitudinal zones (Tranter, 1977; Schnack-Schiel et al., 2010). Interestingly, two oceanic copepod species (Oithona similis and Paracalanus parvus) were prevalent at similar (30°–40° S) latitudes in both case studies, substantiating the CSN’s ability to reveal consistent geographically driven plankton diversity patterns and to reach a reliable comparability between different field trips. Plankton assemblages at lower latitudes were most significantly correlated with higher sea surface temperatures that favored gelatinous Hydrozoa (Wiebe et al., 1979) and tropical copepod taxa (Clausocalanus furcatus and Delibus nudus) (Schnack-Schiel et al., 2010; Peralba et al., 2017). Finally, from local to global geographies, plankton are not homogenous but highly scattered and variable throughout the water column (Lima-Mendez et al., 2015). Many environmental variables such as salinity, oxygen and nutrients play a significant role in plankton distribution (Lynch et al., 2014; Landry et al., 2020), and should be incorporated in future CSN studies. We intentionally do not further discuss the fine-scale ecological patterns of plankton diversity reported in the present study, as they are beyond the scope of this paper. The aim of this second case study was to prove that the CSN device can be effectively used, from any vessel type able to reduce its cruising speed to 5 knots, to detect subtle shifts in sea surface planktonic assemblages and will boost upcoming studies seeking to identify driving environmental factors associated with current and future biodiversity patterns in open ocean environments.
Combining the CSN device with DNA metabarcoding to analyze plankton assemblages offers greater efficiency for taxa identification compared with conventional methods (e.g., microscopy), which can be time-consuming and require considerable taxonomic expertise and in spite of that, miss cryptic diversity and species devoid of conspicuous morphology. However, metabarcoding also presents some well acknowledged pitfalls, including possible biases due to primer or marker choice and the incompleteness of sequence reference databases (Harvey et al., 2017). The present study targeted the universal V4 region of 18S rRNA gene followed by sequence assignment using the PR2 database, enabling analysis of a broad range of taxonomic assemblages and including zoo- and phytoplanktonic taxa (De Vargas et al., 2015; Zamora-Terol et al., 2020). However, our data showed zooplankton (especially copepods) to be highly abundant compared with phytoplankton (e.g., Dinophyceae and Spirotrichea, see Supplementary Table 1) which were relatively scarce and mostly restricted to coastal sampling stations. Future studies aiming to capture a wider representation of phytoplankton taxa should consider using serial filtration methods before DNA extraction (Not et al., 2009) and/or include copepods-blocking oligos during PCR (Zamora-Terol et al., 2020). Additionally, simple DNA isolation methods should be considered while sampling plankton from sailing vessels with limited freezer (−20°C) capacity. These may include the desiccation of filters or the use of DNA/RNA isolation buffers that can be kept cold (4°C) for an extended period of time (Thomas et al., 2019; De Vargas et al., 2020). Finally, comparative morphological observations will also be critical for relating the sequence data to the plankton cell sizes and absolute abundances. Beyond purely scientific endeavors, it is also important to provide the general public with the widest range of accessible analytical toolkits to better understand the different “windows of the planktonic world” and improve the future management of our marine ecosystems. The recent development of a low-cost yet high-throughput PlanktoScope for citizen science applications (Pollina et al., 2020) represents an exciting opportunity for future surveys.
Citizen Science (i.e., the involvement of volunteers who collect and/or process data as part of a scientific inquiry) has expanded considerably over the last 20 years across societies and biomes (Fore et al., 2001; Cohn, 2008; Newman et al., 2012). Citizen scientists now participate in various projects targeting various areas such as climate change, invasive species, conservation biology, ecological restoration, water quality monitoring, and population ecology (Silvertown, 2009). In the marine realm, the democratization of ocean observation has the potential to add a vast amount of observations every day. The rapidly decreasing costs of environmental DNA sequencing, imaging, sensing, and other analytical diagnostic tools (Cybulski et al., 2014; McLeod et al., 2015; Contreras-Naranjo et al., 2016; Srivathsan et al., 2019) creates new opportunities for researchers and citizen scientists to collect and analyze oceanographic data at unprecedented scales. This revolution has already started, with existing initiatives aiming to capitalize on the tens of thousands of sailing boats plying the world’s oceans every day (Rusch et al., 2007; Williamson et al., 2008; Lauro et al., 2014; Simoniello et al., 2019; De Vargas et al., 2020). It is our ambition that the prototype CSN developed in this study will serve as an incentive for larger scale monitoring plankton surveys, empowering citizen science programs such as Plankton Planet and ultimately improving our knowledge on the diversity, distribution and evolution of open ocean plankton assemblages and the associated ecological processes. Future testing and improvements of the CSN are already underway and include (i) trialing the behavior and flow of the device when confronted with different particle sizes using Particle Imaging Velocimetry (PIV; Keane et al., 1994), (ii) visualizing the diversity and integrity of plankton cells captured by the CSN using the PlanktonScope (Pollina et al., 2020), and (iii) working toward an even smaller and lighter version of the CSN similar to the Plankton Indicator device produced in the 1950s (Glover, 1953; Hardy, 1956; Planque and Reid, 2002).
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
XP, AZ, UA, and EM conceived and designed the experiments. DG and LB facilitated sampling cruises. UA, AJ, and AR performed the experiments. UA analyzed the data. UA, AJ, AZ, AR, DG, LB, EM, and XP contributed reagents, material, and tools and reviewed the manuscript drafts. UA, AZ, and XP wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors XP.
Acknowledgments
We authors kindly acknowledge the opportunities through Dieter Paulmann at Okeanos Foundation and the New Zealand Royal Society Dumont D’Urville (DDUCAW1501) and Strategic Seeding (16CAW008CSG) Funds Pacific Ocean Initiative. In kind support from Cawthron Institute, The University of Auckland, and SEA Semester. Sea Education Association provided sampling opportunities on the SSV Robert C. Seamans; thanks to the students, crew and faculty of SEA voyage S284 for at sea assistance. The authors wish to thank the CSIRO Marine National Facility (MNF) for its support in the form of sea time on RV Investigator (voyage IN2019_V03), support personnel, scientific equipment, and data management. Zooplankton net tows at Australian voyage stations were taken under Australian Fisheries Management Authority scientific permit 1004152. This research included sampling in the Abrolhos Marine Park under permit number PA201800651, issued by the Director of National Parks, Australia, and permit AUCOM2019446, issued by the Department of the Environment and Energy, Australia. The views expressed in this publication do not necessarily represent the views of the Director of National Parks or the Australian Government. This research is a contribution to the Worldwide Universities Network project; Ocean Eddies in a Changing Climate: Understanding the Impact of Coastal Climates and Fisheries Production. All data and samples acquired on the voyage are made publicly available in accordance with MNF Policy. We thank Heide Friedrich (The University of Auckland) for valuable discussion on nets and Andy Reid (CGW Consulting Engineers Ltd.). This is Plankton Planet contribution #3.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.615458/full#supplementary-material
Footnotes
- ^ www.planktonplanet.org
- ^ https://www.youtube.com/watch?v=hRiRFvaz-W4&feature=youtu.be
- ^ https://www.hydrobios.de/highspeedplanktoncollectornackthai/
References
Anderson, J. A., and Epifanio, C. E. (2009). Induction of metamorphosis in the Asian shore crab Hemigrapsus sanguineus: characterization of the cue associated with biofilm from adult habitat. J. Exp Mar. Biol. Ecol. 382, 34–39. doi: 10.1016/j.jembe.2009.10.006
Bell, K. L., Burgess, K. S., Botsch, J. C., Dobbs, E. K., Read, T. D., and Brosi, B. J. (2018). Quantitative and qualitative assessment of pollen DNA metabarcoding using constructed species mixtures. Mol. Ecol. 28, 431–455. doi: 10.1111/mec.14840
Biard, T., Bigeard, E., Audic, S., Poulain, J., Gutierrez-Rodriguez, A., Pesant, A., et al. (2017). Biogeography and diversity of Collodaria (Radiolaria) in the global ocean. ISME J. 11, 1331–1344.
Bishop, J. K. B., Smith, R. C., and Baker, K. S. (1992). Springtime distributions and variability of biogenic particulate matter in Gulf Stream warm-core ring 82B and surrounding N.W. Atlantic waters. Deep Sea Res. Part A Oceanogr. Res. Pap. 39, 295–S325.
Bradford, J. M. (2010). Distribution of the pelagic copepod Temora turbinata in New Zealand coastal waters, and possible trans-tasman population continuity. N. Z. J. Mar. Freshw. Res. 11, 131–144. doi: 10.1080/00288330.1977.9515666
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J., and Holmes, S. P. (2016). DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Castellani, C., and Edwards, M. (2017). Marine Plankton: A Practical Guide to Ecology, Methodology, and Taxonomy. Oxford: Oxford University Press.
Clarke, L. J., Beard, J. M., Swadling, K. M., and Deagle, B. E. (2017). Effect of marker choice and thermal cycling protocol on zooplankton DNA metabarcoding studies. Ecol. Evol. 7, 873–883. doi: 10.1002/ece3.2667
Contreras-Naranjo, J. C., Wei, Q., and Ozcan, A. (2016). Mobile phone-based microscopy, sensing, and diagnostics. IEEE J. Selected Top. Quantum Electron. 22, 1–14. doi: 10.1109/jstqe.2015.2478657
Cornell, J. (2014). World Cruising Routes: 1000 Sailing Routes in all Oceans of the World. London: A&C Black.
Cornils, A., Schulz, J., Schmitt, P., Lanuru, M., Richter, C., and Schnack-Schiel, S. B. (2010). Mesozooplankton distribution in the Spermonde Archipelago (Indonesia, Sulawesi) with special reference to the Calanoida (Copepoda). Deep Sea Res. II Top. Stud. Oceanogr. 57, 2076–2088. doi: 10.1016/j.dsr2.2010.09.011
Countway, P. D., Gast, R. J., Dennett, M. R., Savai, P., Rose, J. M., and Caron, D. A. (2007). Distinct protistan assemblages characterize the euphotic zone and deep sea (2500 m) of the western North Atlantic (Sargasso Sea and Gulf Stream). Environ. Microbiol. 9, 1219–1232. doi: 10.1111/j.1462-2920.2007.01243.x
Cybulski, J. S., Clements, J., and Prakash, M. (2014). Foldscope: origami-based paper microscope. PLoS One 9:e98781. doi: 10.1371/journal.pone.0098781
D’Alelio, D., Eveillard, D., Coles, V. J., Caputi, L., Ribera d’Alcalà, M., and Iudicone, D. (2019). Modelling the complexity of plankton communities exploiting omics potential: from present challenges to an integrative pipeline. Curr. Opin. Syst. Biol. 13, 68–74. doi: 10.1016/j.coisb.2018.10.003
De Vargas, C., Audic, S., Henry, N., Decelle, J., Mahe, F., Logares, R., et al. (2015). Ocean plankton. Eukaryotic plankton diversity in the sunlit ocean. Science 348:1261605. doi: 10.1126/science.1261605
De Vargas, C., Pollina, T., Romac, S., Le Bescot, N., Henry, N., Berger, C., et al. (2020). Plankton Planet: ‘seatizen’ oceanography to assess open ocean life at the planetary scale. bioRxiv [Preprint]. doi: 10.1101/2020.08.31.263442
Duarte, C. M. (2015). Seafaring in the 21st century: the Malaspina 2010 circumnavigation expedition. Limnol. Oceanogr. Bull. 24, 11–14.
Edgcomb, V., Orsi, W., Bunge, J., Jeon, S., Christen, R., Leslin, C., et al. (2011). Protistan microbial observatory in the Cariaco Basin, Caribbean. I. Pyrosequencing vs Sanger insights into species richness. Multidisciplinary J. Microb. Ecol. 5, 1344–1356. doi: 10.1038/ismej.2011.6
Eriksen, M., Liboiron, M., Kiessling, T., Charron, L., Alling, A., Lebreton, L., et al. (2018). Microplastic sampling with the AVANI trawl compared to two neuston trawls in the Bay of Bengal and South Pacific. Environ. Pollut. 232, 430–439. doi: 10.1016/j.envpol.2017.09.058
Eriksen, R. S., Davies, C. H., Bonham, P., Coman, F. E., Edgar, S., McEnnulty, F. R., et al. (2019). Australia’s long-term plankton observations: the integrated marine observing system national reference station network. Front. Mar. Sci. 6:161. doi: 10.3389/fmars.2019.00161
Follows, M. J., and Dutkiewicz, S. (2011). Modeling diverse communities of marine microbes. Annu. Rev. Mar. Sci. 3, 427–451. doi: 10.1146/annurev-marine-120709-142848
Follows, M. J., Dutkiewicz, S., Grant, S., and Chisholm, S. W. (2007). Emergent biogeography of microbial communities in a model ocean. Science 315, 1843–1846.
Fore, L. S., Paulsen, K., and O’Laughlin, K. (2001). Assessing the performance of volunteers in monitoring streams. Freshw. Biol. 46, 109–123.
Franco, P., Chen, H., and Hwang, J. S. (2016). Taxonomic composition and seasonal distribution changes of pelagic tunicates in the waters off nuclear power plants in northern taiwan in relation to environmental conditions. Zool. Stud. 55:e28. doi: 10.6620/ZS.2016.55-28
Glover, R. S. (1953). The Hardy plankton indicator and sampler: a description of the various models in use. Bull. Mar. Ecol. 4, 7–20.
Gorsky, G., Bourdin, G., Lombard, F., Pedrotti, M. L., Audrain, S., Bin, N., et al. (2019). Expanding tara oceans protocols for underway, ecosystemic sampling of the ocean-atmosphere interface during tara pacific expedition (2016–2018). Front. Mar. Sci. 6:750. doi: 10.3389/fmars.2019.00750
Guillou, L., Bachar, D., Audic, S., Bass, D., Berney, C., Bittner, L., et al. (2013). The protist ribosomal reference database (PR2): a catalog of unicellular eukaryote small sub-unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 41, D597–D604. doi: 10.1093/nar/gks1160
Gutkowska, A., Paturej, E., and Kowalska, E. (2012). Qualitative and quantitative methods for sampling zooplankton in shallow coastal estuaries. Ecohydrol. Hydrobiol. 12, 253–263. doi: 10.2478/v10104-012-0022-2
Hallegraeff, G. M. (2010). Ocean climate change, phytoplankton community responses, and harmful algal blooms: a formidable predictive challenge. J. Phycol. 46, 220–235. doi: 10.1111/j.1529-8817.2010.00815.x
Hardy, A. C. (1956). The Open Sea: Its Natural History. Part 1: The world of plankton. London: Collins.
Harvey, J. B. J., Johnson, S. B., Fisher, J. L., Peterson, W. T., and Vrijenhoek, R. C. (2017). Comparison of morphological and next generation DNA sequencing methods for assessing zooplankton assemblages. J. Exp. Mar. Biol. Ecol. 487, 113–126. doi: 10.1016/j.jembe.2016.12.002
John, E. H., Batten, S. D., Harris, R. P., and Hays, G. C. (2001). Comparison between zooplankton data collected by the continuous plankton recorder survey in the english channel and by WP-2 nets at station L4. Plymouth (UK). J. Sea Res. 46, 223–232.
Jose, J., Thanagaraj, M., Lincy, A., and Lipton, A. P. (2017). Population genetics of Oithona similis Claus, 1866 (Crustacea: Cyclopoida) in Arabian Sea: preliminary evidence of haplotype sharing in two populations. Indian J. Geomarine Sci. 46, 147–154.
Karsenti, E., Acinas, S. G., Bork, P., Bowler, C., De Vargas, C., Raes, J., et al. (2011). Tara oceans consortium. A holistic approach to marine eco-systems biology. PLoS Biol. 10:e1001177. doi: 10.1371/journal.pbio.1001177
Keane, R.D., Adrian, R., and Zhang, Y. (1995). Super-resolution particle imaging velocimetry. Meas. Sci. Technol. 6:754. doi: 10.1088/0957-0233/6/6/013
Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K., and Schloss, P. D. (2013). Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120. doi: 10.1128/AEM.01043-13
Landry, M. R., Hood, R. R., and Davies, C. H. (2020). Mesozooplankton biomass and temperature-enhanced grazing along a 110°E transect in the eastern Indian Ocean. Mar. Ecol. Prog. Ser. 649, 1–19. doi: 10.3354/meps13444
Lauro, F. M., Senstius, S. J., Cullen, J., Neches, R., Jensen, R. M., Brown, M. V., et al. (2014). The common oceanographer: crowdsourcing the collection of oceanographic data. PLoS Biol. 12:e1001947. doi: 10.1371/journal.pbio.1001947
Lima-Mendez, G., Faust, K., Henry, N., Decelle, J., Colin, S., Carcillo, F., et al. (2015). Determinants of community structure in the global plankton interactome. Science 348:1262073.
Lynch, T. P., Morello, E. B., Evans, K., Richardson, A. J., Rochester, W., Steinberg, C. R., et al. (2014). IMOS National Reference Stations: a continental-wide physical, chemical and biological coastal observing system. PLoS One 9:e113652. doi: 10.1371/journal.pone.0113652
Martin, M. (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12.
McLeod, E., Wei, Q., and Ozcan, A. (2015). Democratization of nanoscale imaging and sensing tools using photonics. Anal. Chem. 87, 6434–6445. doi: 10.1021/acs.analchem.5b01381
Moser, C. S., Wier, T. P., Grant, J. F., First, M. R., Tamburri, M. N., Ruiz, G. M., et al. (2015). Quantifying the total wetted surface area of the world fleet: a first step in determining the potential extent of ships’ biofouling. Biol. Invasions 18, 265–277. doi: 10.1007/s10530-015-1007-z
Newman, G., Wiggins, A., Crall, A., Graham, E., Newman, S., and Crowston, K. (2012). The future of Citizen science: emerging technologies and shifting paradigms. Front. Ecol. Environ. 10:298–304. doi: 10.1890/110294
Not, F., del Campo, J., Balague, V., de Vargas, C., and Massana, R. (2009). New insights into the diversity of marine picoeukaryotes. PLoS One 4:e7143. doi: 10.1371/journal.pone.0007143
Pennington, J. T., Blum, M., and Chavez, F. P. (2016). Seawater sampling by an autonomous underwater vehicle: “Gulper” sample validation for nitrate, chlorophyll, phytoplankton, and primary production. Limnol. Oceanogr. 14, 14–23. doi: 10.1002/lom3.10065
Peralba, À, Mazzocchi, M. G., and Harris, R. P. (2017). Niche separation and reproduction of Clausocalanus species (Copepoda, Calanoida) in the Atlantic Ocean. Prog. Oceanog. 158, 185–202. doi: 10.1016/j.pocean.2016.08.002
Planque, B., and Reid, P. C. (2002). What we have learned about plankton variability and its physical controls from 70 years of CPR records. ICES Mar. Sci. Symposia 215, 237–246.
Pollina, T., Larson, A. G., Lombard, F., Li, H., Colin, S., de Vargas, C., et al. (2020). PlanktonScope: affordable modular imaging platform for citizen oceanography. bioRxiv [Preprint]. doi: 10.1101/2020.04.23.056978
Reid, P. C., Bathmann, U., Batten, S. D., Brainard, R. E., Burkill, P. H., Carlotti, F., et al. (2010). “A global continuous plankton recorder programme. in proceedings of OceanObs’09,” in Sustained Ocean Observations and Information for Society, eds J. Hall, D. E. Harrison, and D. Stammer (Oxford: ESA Publication WPP-306), 12.
Reid, P. C., Colebrook, J. M., Matthews, J. B. L., and Aiken, J. (2003). the continuous plankton recorder: concepts and history, from plankton indicator to undulating recorders. Prog. Oceanogr. 58, 117–173. doi: 10.1016/j.pocean.2003.08.002
Richardson, A. J., Walne, A. W., John, A. W. G., Jonas, T. D., Lindley, J. A., Sims, D. W., et al. (2006). Using continuous plankton recorder data. Prog. Oceanogr. 68, 27–74. doi: 10.1016/j.pocean.2005.09.011
Rusch, D. B., Halpern, A. L., Sutton, G., Heidelberg, K. B., Williamson, S., Yooseph, S., et al. (2007). The sorcerer II global ocean sampling expedition: northwest atlantic through eastern tropical pacific. PLoS Biol. 5:e77. doi: 10.1371/journal.pbio.0050077
Sameoto, D., Wiebe, P., Runge, J., Postel, L., Dunn, J., Miller, C., et al. (2000). “Collecting zooplankton,” in ICES Zooplankton Methodology Manual, eds R. Harris, P. Wiebe, J. Lenz, H.-R. Skjoldal, and M. Huntley (Amsterdam: Elsevier), 55–81.
San Martín, A. M., Gerdes, D., and Arntz, W. E. (2005). Distributional patterns of shallow-water polychaetes in the Magellan region: a zoogeographical and ecological synopsis. Sci. Mar. 69, 123–133.
Schabacker, J. C., Amish, S. J., Ellis, B. K., Gardner, B., Miller, D. L., Rutledge, E. A., et al. (2020). Increased eDNA detection sensitivity using a novel high-volume water sampling method. Environ. DNA 2, 244–251. doi: 10.1002/edn3.63
Schnack, D. (1974). “On the reliability of methods for quantitative surveys of fish larvae,” in The Early Life History of Fish, ed. J. H. S. Blaxter (Berlin: Springer), 201–212.
Schnack-Schiel, S. B., Mizdalski, E., and Cornils, A. (2010). Copepod abundance and species composition in the Eastern subtropical/tropical Atlantic. Deep Sea Res. Part II Top. Stud. Oceanogr. 57, 2064–2075. doi: 10.1016/j.dsr2.2010.09.010
Sekerci, Y., and Petrovskii, S. (2015). Mathematical modelling of spatiotemporal dynamics of oxygen in a plankton system. Math. Model. Nat. Phenom. 10, 96–114. doi: 10.1051/mmnp/201510207
Silvertown, J. (2009). A new dawn for citizen science. Trends Ecol. Evol. 24, 467–471. doi: 10.1016/j.tree.2009.03.017
Simoniello, C., Jencks, J., Lauro, F. M., Loftis, J. D., Weslawski, J. M., Deja, K., et al. (2019). Citizen-science for the future: advisory case studies from around the globe. Front. Mar. Sci. 6:225. doi: 10.3389/fmars.2019.00225
Srivathsan, A., Hartop, E., Puniamoorthy, J., Lee, W. T., Kutty, S. N., Kurina, O., et al. (2019). Rapid, large-scale species discovery in hyperdiverse taxa using 1D MinION sequencing. BMC Biol. 17:96. doi: 10.1186/s12915-019-0706-9
Tappan, H., and Loeblich, A. R. Jr. (1973). Evolution of the Oceanic Plankton. Earth Sci. Rev. 9, 207–240.
Taylor, F. J. (1978). Records of marine algae from the Leigh area. Part II. Records of phytoplankton from Goat Island Bay. Tane. 213–218.
Thomas, A. C., Nguyen, P. L., Howard, J., Goldberg, C. S., and Jentoft, S. (2019). A self-preserving, partially biodegradable eDNA filter. Methods Ecol. Evol. 10, 1136–1141. doi: 10.1111/2041-210x.13212
Tranter, D. J. (1977). Further studies of plankton ecosystems in the eastern Indian Ocean. V. Ecology of the Copepoda. Austr. J. Mar. Freshw. Res. 28, 593–625.
Tranter, D. J., and Fraser, J. H. E. (1968). Zooplankton Sampling. UNESCO Monographs on Oceanographic Methodology 2. Paris: UNESCO.
Wang, Q., Garrity, G. M., Tiedje, J. M., and Cole, J. R. (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267. doi: 10.1128/AEM.00062-07
Ward, B. A., Dutkiewicz, S., and Follows, M. J. (2014). Modelling spatial and temporal patterns in size-structured marine plankton communities: top–down and bottom–up controls. J. Plankton Res. 36, 31–47. doi: 10.1093/plankt/fbt097
Wiebe, P. H., and Benfield, M. C. (2003). From the Hensen net toward four-dimensional biological oceanography. Prog. Oceanogr. 56, 7–136. doi: 10.1016/s0079-6611(02)00140-4
Wiebe, P. H., Madin, L. P., Haury, L. R., Harbison, G. R., and Philibin, L. M. (1979). Diel vertical migration by Salpa aspera and its potential for large-scale particulate organic matter transport to the deep-sea. Mar. Biol. 53, 249–255. doi: 10.1007/BF00952433
Williamson, S. J., Rusch, D. B., Yooseph, S., Halpern, A. L., Heidelberg, K. B., Glass, J. I., et al. (2008). The Sorcerer II Global Ocean sampling expedition: metagenomic characterization of viruses within aquatic microbial samples. PLoS One 3:e1456. doi: 10.1371/journal.pone.0001456
YOREPS (2020). YOREPS. Avaliable at: https://sailwx.info/shiptrack/yotreps.phtml (accessed 23.09.2020).
Zamora-Terol, S., Novotny, A., and Winder, M. (2020). Reconstructing marine plankton food web interactions using DNA metabarcoding. Mol. Ecol. 29, 3380–3395. doi: 10.1111/mec.15555
Keywords: plankton net, eDNA, metabarcoding, plankton, 18S (SSU) rRNA gene
Citation: von Ammon U, Jeffs A, Zaiko A, van der Reis A, Goodwin D, Beckley LE, Malpot E and Pochon X (2020) A Portable Cruising Speed Net: Expanding Global Collection of Sea Surface Plankton Data. Front. Mar. Sci. 7:615458. doi: 10.3389/fmars.2020.615458
Received: 09 October 2020; Accepted: 24 November 2020;
Published: 23 December 2020.
Edited by:
Stelios Katsanevakis, University of the Aegean, GreeceReviewed by:
Wiebe Hendrik Cornelis Frederik Kooistra, University of Naples Federico II, ItalyPaul Tett, Scottish Association For Marine Science, United Kingdom
Copyright © 2020 von Ammon, Jeffs, Zaiko, van der Reis, Goodwin, Beckley, Malpot and Pochon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xavier Pochon, eGF2aWVyLnBvY2hvbkBjYXd0aHJvbi5vcmcubno=
 Ulla von Ammon
Ulla von Ammon Andrew Jeffs
Andrew Jeffs Anastasija Zaiko
Anastasija Zaiko Aimee van der Reis2
Aimee van der Reis2 Deb Goodwin
Deb Goodwin Lynnath E. Beckley
Lynnath E. Beckley Xavier Pochon
Xavier Pochon