- 1Key Laboratory of Tropical Marine Bio-Resources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China
- 2Guangdong Provincial Key Laboratory of Applied Marine Biology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China
- 3Shanghai Universities Key Laboratory of Marine Animal Taxonomy and Evolution, Shanghai Ocean University, Shanghai, China
- 4Key Laboratory of the Ministry of Education for Coastal and Wetland Ecosystem, The Fujian Provincial Key Laboratory for Coastal Ecology and Environmental Studies, College of the Environment and Ecology, Xiamen University, Xiamen, China
As the typical periphytic ciliate, the genus Euplotes Ehrenberg, 1830 is highly diversified and commonly observed in marine water. In this study, the living morphology, infraciliature and silverline system of two poorly known Euplotes species, E. neapolitanus Wichterman, 1964 and E. antarcticus Fenchel and Lee, 1972, isolated from coastal water of southern China, were investigated. The original description of these two species were brief, and thus we provided detailed redescription based on our Chinese population. Their diagnoses were improved by adding some morphology characteristics and their detailed illustrations and photomicrographs were first supplied here. Based on the sufficient justification for identification of our population by morphology, their small subunit ribosomal RNA gene sequences which have been reported were linked to the accurate species name. Phylogenetic analyses showed that these two species cluster with their congeners which shared high morphological similarities with them. In addition, the geographic distribution of the genus Euplotes in coast of southern China was revealed, and the mangrove was considered as the ideal habitat for them by possessing the higher species richness.
Introduction
The species of the ciliate genus Euplotes Ehrenberg, 1830 are frequently observed in marine samples (Lynn, 2008; Song et al., 2009). This genus is typical periphytic microorganism which can colonize the submerged substrates and are usually the most primary components of aufwuchs in high abundance and richness. They often feed on bacteria and microalgae, and play a crucial role in the flow of energy in microbial food webs (Lynn, 2008).
The genus Euplotes is highly diverse with over 150 species, which were often distinguished using the characteristics including the body size and shape, the dorsal and ventral ridges, the cirral pattern, the adoral zone of membranelles, the macronucleus shape, the dorsal kineties, and the silverline system pattern (Tuffrau, 1960; Borror, 1972; Carter, 1972; Curds, 1975; Lynn, 2008). Recently many reports on this group integrate aspects of their living morphology, infraciliature and molecular sequences, thus offering a more comprehensive understanding of their taxonomy. Despite of many descriptions of new species and redescriptions of insufficiently known species (Lobban et al., 2005; Fan et al., 2010; Jiang et al., 2010a, b; Pan et al., 2012; Chen et al., 2013; Fotedar et al., 2016; Lian et al., 2018, 2019; Yan et al., 2018; Hu et al., 2019; Gao et al., 2020), some species still require a re-investigation due to lacking detailed morphological description in vivo or after protargol preparation, as well as the molecular data (Gao et al., 2017; Liu et al., 2017).
Two poorly known species, namely E. neapolitanus Wichterman, 1964 and E. antarcticus Fenchel and Lee, 1972, were collected in coastal waters of southern China, which gave us an opportunity to re-recognize them. In previous studies, their SSU rRNA gene sequences have been reported, but no detailed morphological data from specimens are linked with them (Yi et al., 2009; Gao et al., 2017; Zhao et al., 2018). In present paper, the morphology of these two species is thus redescribed based on live observation and protargol-impregnated material. For the first time, their detailed illustrations and photomicrographs were supplied here. In addition, the study on the diversity of Euplotes species has been extensively conducted in the coastal waters of southern China in the past decade, and a large number of species have been collected and reported with taxonomy description (Hu et al., 2019; Lian et al., 2020). Based on a compilation of their faunal data, the biogeographical patterns of the genus Euplotes in different sites and habitats of southern China coast were reviewed here.
Materials and Methods
Collection and Identification
Both species were found near a fishing dock of Daya Bay, Huizhou, China (22°42′N; 114°32′E). Euplotes neapolitanus were collected directly using 100 ml bottle from surface water that contained some organic debris on March 31, 2007. The salinity was about 28.6‰, the water temperature 23.1°C and pH 8.2. Euplotes antarcticus was collected using 20 μm mesh plankton nets from the upper 0.5 m water on March 31, 2007. The salinity was about 28.7‰, the water temperature 29.7°C and pH 8.4. After isolation, specimens were maintained in Petri dishes in the laboratory at room temperature. Attempts to culture the two species failed; therefore all studies were carried out on freshly isolated specimens.
Cells were observed in vivo using a light microscope equipped with differential interference contrast (Zhang et al., 2020). Protargol (Wilbert, 1975), silver nitrate (Foissner, 2014) staining methods were used respectively to reveal the infraciliature and silverline systems. Measurements and counts were made at 1,000 × magnification; drawings of stained cells were performed with the help of a camera and photomicrographs. Terminology is mainly according to Curds (1975).
Phylogenetic Analyses
The SSU rRNA gene sequences of E. neapolitanus and E. antarcticus have been published in previous studies with accession number FJ998024 and FJ998023, respectively. The two sequence were aligned with the sequences of 127 other ciliates downloaded from GenBank da ase (see Figure 5 for accession numbers) using the GUIDANCE2 algorithm1. Representative species of Discocephalida, Aspidiscidae, Gastrocirrhidae, Uronychiidae were chosen as outgroup taxa. The final alignment used for phylogenetic analyses included 1,506 sites. Maximum likelihood (ML) analysis with 1,000 bootstrap replicates was performed on the CIPRES Science Gateway applying the GTRGAMMA model (Miller et al., 2010) using RAxML-HPC2 on XSEDE 8.2.9 (Stamatakis, 2014). Bayesian inference (BI) analysis was carried out with MrBayes 3.2.6 on XSEDE (Ronquist et al., 2012) on the CIPRES Science Gateway using the model GTR + I + G selected by AIC in MrModeltest 2.2 (Nylander, 2004). Markov chain Monte Carlo (MCMC) simulations were run for 1,000,000 generations with sampling every 100 generations and a burn-in of 1,000 trees (Dong et al., 2020). MEGA 6 (Tamura et al., 2013) was used to visualize the tree topologies.
Biogeographic Distribution
The regional biogeographic patterns of Euplotes species are inferred based on a compilation of faunal data from different sources include one monographs (Hu et al., 2019) and all papers on the taxonomy and biodiversity of Euplotes in southern China coast. Totally 15 species from six habitats of seven coastal sites were collected as the data set (see Table 2 for a complete list). Considering that the results in these sources come from the extensive and high-frequency samplings in all sites of southern China coast, the compiled faunal data can generally reflect their distribution characters. Therefore, the presences of each species in these sampling sites and habitats were summarized, based on which the distribution variations of these species among different sites were analyzed and the species richness among habitats were compared.
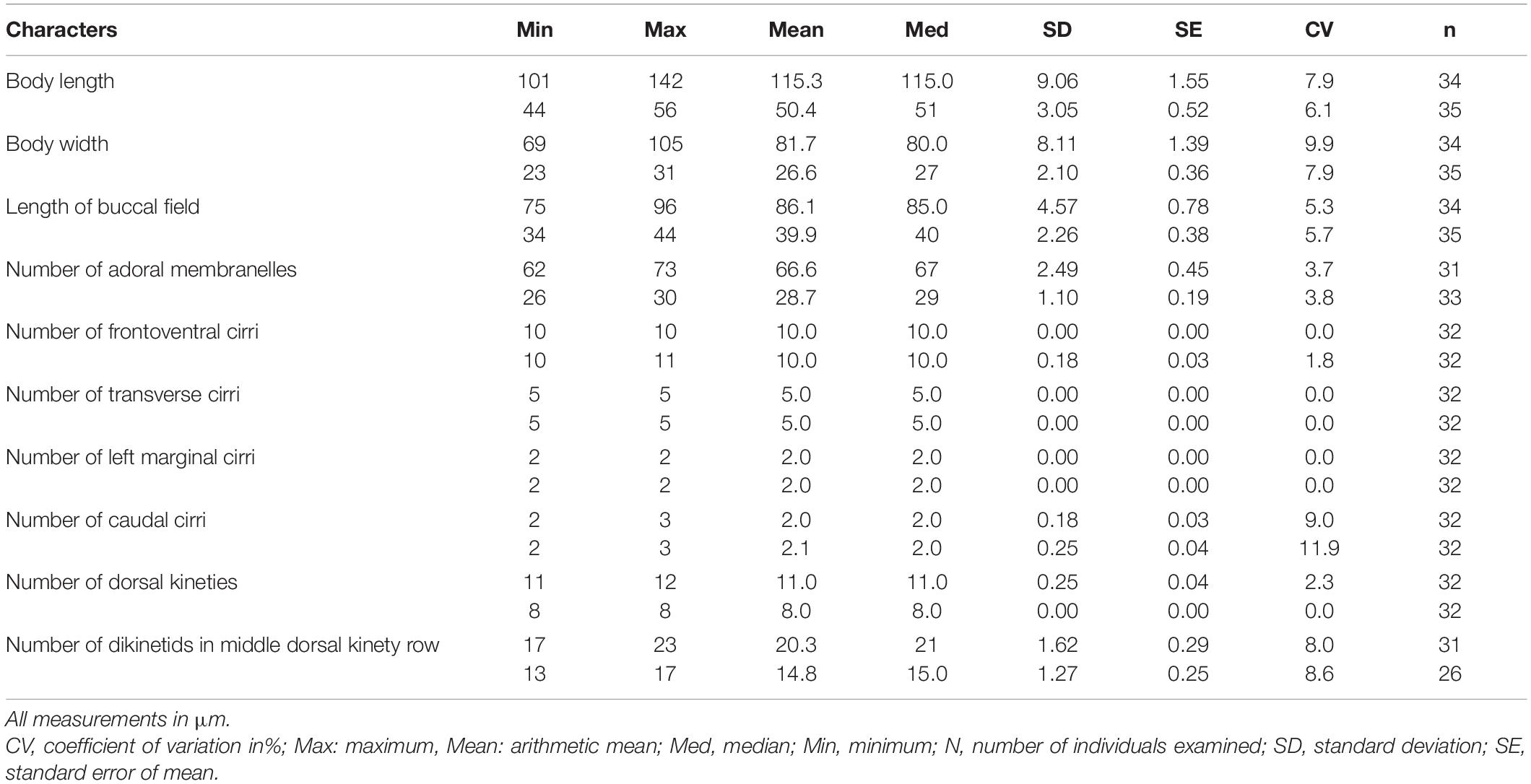
Table 1. Morphometric characteristics of Euplotes neapolitanus (upper rows) and E. antarcticus (lower rows) from protargol impregnated specimens.
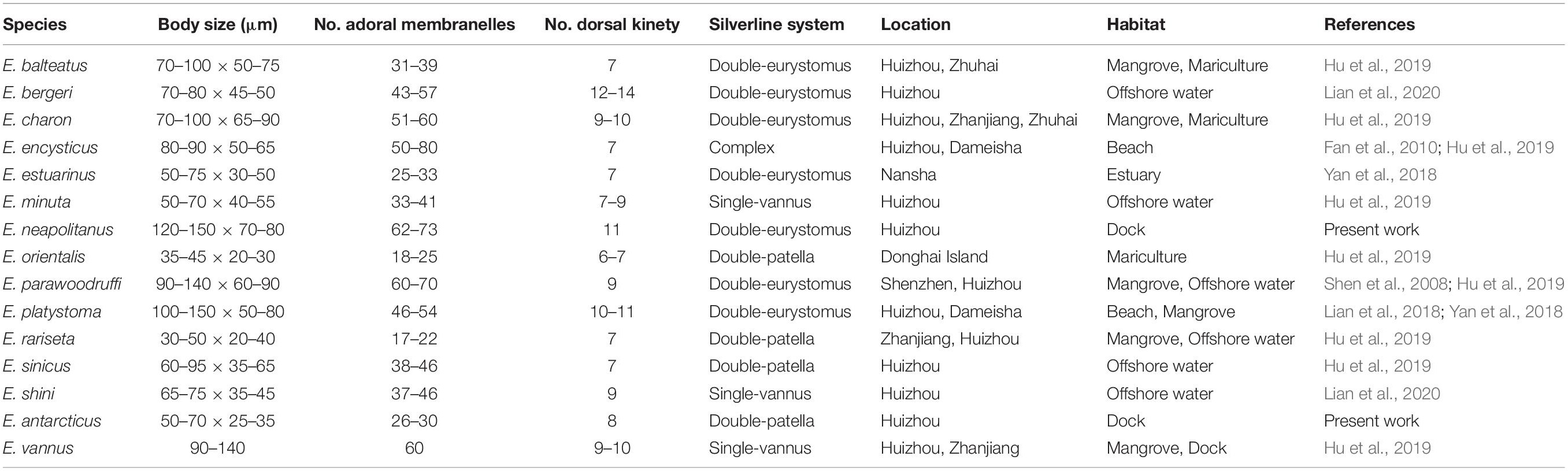
Table 2. Euplotes species collected in the coastal waters of southern China and their morphological comparison.
Results
Euplotes neapolitanus Wichterman, 1964
Improved Diagnosis
Large marine Euplotes with eight or nine low dorsal ridges, 120–150 × 70–80 μm in vivo, ellipsoidal in outline, anterior more truncated than posterior; adoral zone of membranelles about 3/4 of cell length, abruptly curved to anterior end of cell, composed of 62–73 membranelles; always 10 frontoventral, five transversal, two caudal and two left marginal cirri; mostly 11 dorsal kineties with 17–23 dikinetids in mid-kinety rows; Macronucleus 3- or C-shaped; silverline system double-eurystomus type.
Description
Body shape generally ellipsoidal with truncated anterior end and bluntly round posterior end, left margin slightly curved while right margin straight (Figures 1A, 2A,B). Size range 120–150 × 70–80 μm in vivo, dorsoventrally flattened about 3:2 (Figures 1D, 2C), dorsal surface slightly convex with eight or nine low longitudinal ridges extending over entire length of body (Figures 1D, 2D,E); ventral side more or less concave so transverse section slightly arciform (Figure 2D), five or six short ventral ridges among transverse cirri (Figures 1A, 2G). At anterior end of ventral side, a hyaline collar-like plasmatic protrusion formed at base of adoral zone (Figures 1A, 2F). Buccal field prominent, extending about 3/4 of cell length, right border located almost in middle axis of body, obviously indented in middle portion, forming a broad buccal open area (Figures 1A, 2A–C).
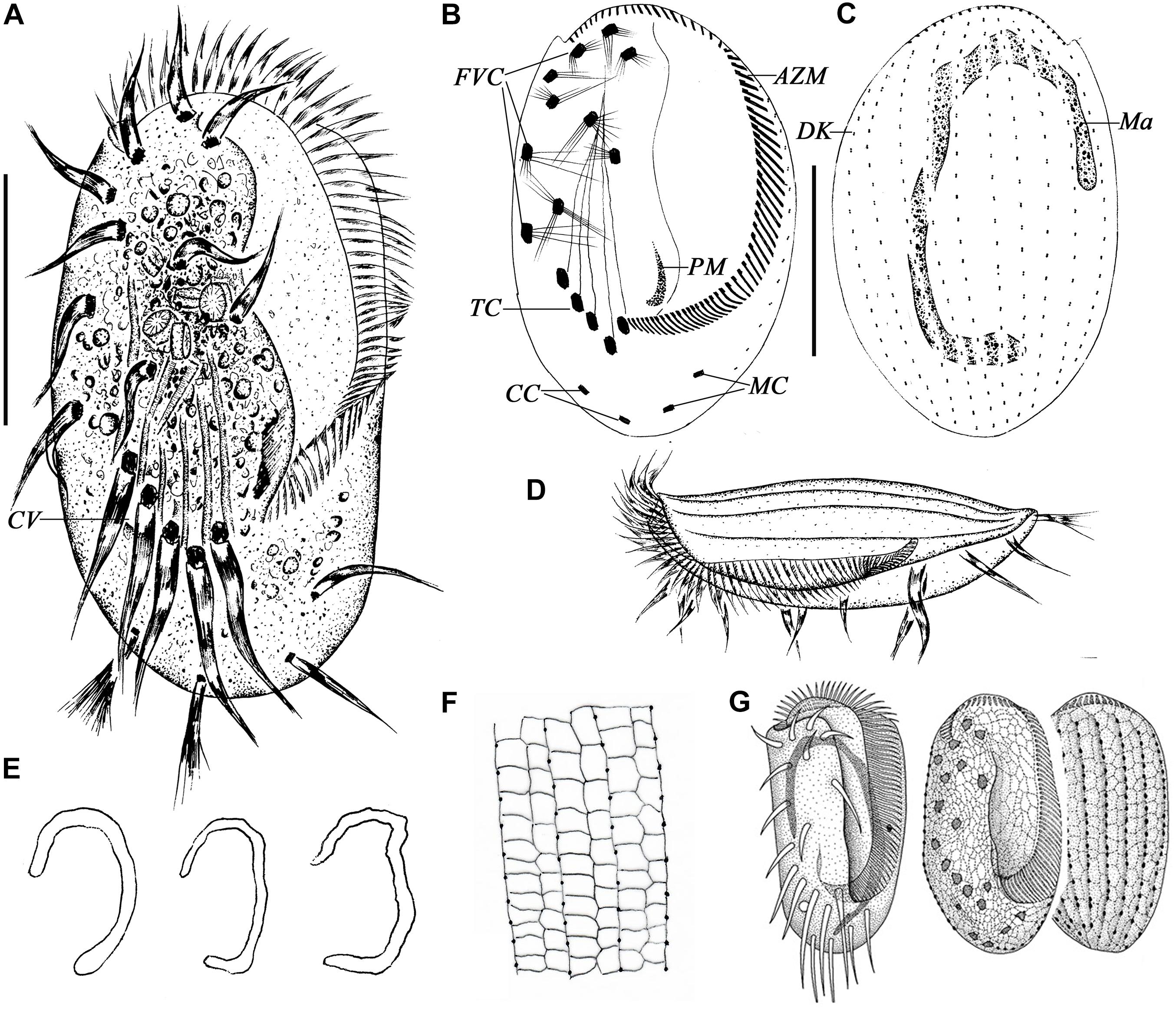
Figure 1. Morphology of Euplotes neapolitanus Wichterman, 1964 in vivo (A,D), after protargol (B,C,E) and silver nitrate impregnation (F,G). (A) Ventral view of a representative specimen. (B,C) Ventral (B) and dorsal (C) view of the same specimen showing the ciliary pattern and nuclear apparatus. (D) Left lateral view showing the AZM and the dorsal ridges. (E) Different shapes of macronucleus. (F) Details of the silverline system of dorsal side. (G) Silverline system on ventral and dorsal sides, from Wichterman (1964). AZM, adoral zone of membranelles; CC, caudal cirri; CV, contractile vacuole; DK, dorsal kinety; FVC, frontoventral cirri; Ma, macronucleus; MC, marginal cirri; PM, paroral membrane; TC, transverse cirri. Scale bars = 50 μm.
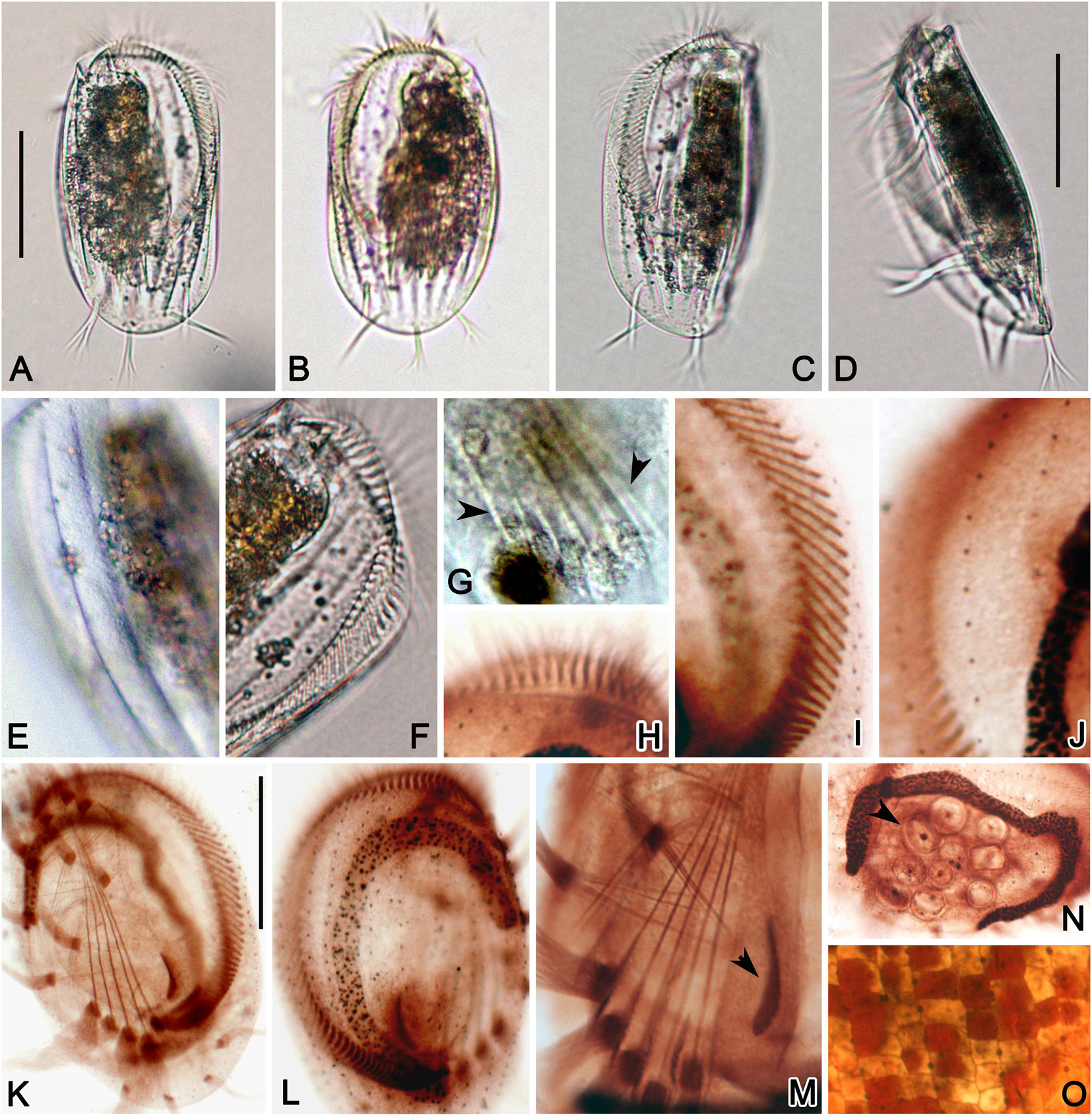
Figure 2. Photomicrographs of E. neapolitanus in vivo (A–G), and after protargol (H–N) and silver nitrate (O) impregnation. (A,B) Ventral (A) and dorsal (B) views of typical specimens. (C,D) Left-dorsal (C) and lateral (D) views. (E) Detail of dorsal side to show the ridges. (F) Anterior part of ventral side showing the abruptly curved AZM. (G) Detail of ventral side, arrowheads show the short ridge between transverse cirri. (H,I) Anterior (H) and middle (I) portions of AZM. (J) Detail of dorsal kinety. (K,L) Ventral (K) and dorsal (L) views of infraciliature. (M) Center portion of ventral side to show the transverse cirri, paroral membrane (arrowhead) and developed fiber system around the cirri. (N) Macronucleus and food (arrowhead) in cell center. (O) Detailed dorsal view of silverline system. Scale bars = 50 μm.
Cytoplasm colorless, highly transparent at marginal area, but dark brownish in right central part due to many lipid globules (3–5 μm) and food vacuoles (10–12 μm) containing yellow alga (Coscinodiscus sp.) (Figures 1A, 2A,B,F). Contractile vacuole posterior to the rightmost transverse cirrus, about 15 μm in diameter (Figure 1A, arrow). Macronucleus usually untypical 3-shaped with inconspicuous concave notch which even disappeared in some individuals (Figures 1E, 2L,N). Locomotion typically by fast crawling on substrate, occasionally remaining stationary for short periods.
Infraciliature as shown in Figures 1B,C. Paroral membrane easily observed in vivo with cilia about 20 μm long, forming an L-shaped kinetosomes area about 20 μm long after staining, with anterior portion thin, and posterior portion thick and slightly curved (Figures 1B, 2M). Adoral zone composed of 62–73 membranelles, which straightly arranged along the left cell margin and abruptly curved to anterior end of cell especially in living cells (Figures 1A,B, 2F,H,I,K). The bases of membranelles up to 10–20 μm long and cilia of membranelles about 15–25 μm long. Consistently 10 frontoventral cirri arranged in normal pattern about 25–30 μm long, and five strong transverse cirri about 40 μm long (Figures 1B, 2K). Usually two, rarely three caudal cirri brush-like, about 30 μm long (Figures 1B, 2K). Two left marginal cirri, positioned below the buccal cavity and close to caudal cirri, the length equal to caudal cirri (Figures 1B, 2K). About 11 or 12 (mostly 11) dorsal kineties almost extending over entire length of cell with the leftmost one usually positioned near body margin on ventral side; middle row with about 17–23 dikinetids (Figures 1C, 2J). Silverline system on dorsal side double-eurystomus type (Figures 1F, 2O).
Euplotes antarcticus Fenchel and Lee, 1972
Improved Diagnosis
Slender marine Euplotes, 50–70 × 25–35 μm in vivo, body elongated ellipsoidal shape with anterior end obliquely truncated and right anterior end protruded; left and right margins bended to ventral side; six longitudinal ridges forming three furrows in neighboring pair on dorsal side; buccal filed about 3/4 of cell length with 26–30 membranelles; ventral cirri fine, including 10 frontoventral, five transversal, two caudal and two left marginal cirri; eight dorsal kineties with 13–17 dikinetids in mid-kinety rows and five or six dikinetids in leftmost one; macronucleus C-shaped or inverted J-shaped; silverline system double-patella type.
Description
Cells measuring 50–70 × 25–35 μm in vivo. Body slender ellipsoidal shaped, anterior end obliquely truncated with right part conspicuously protruded, and posterior end narrowly round (Figures 3A,B, 4A–C,J). Dorsoventrally flattened about 3:2, with left margin strongly and right margin slightly bended to ventral side, which lead to the left margin significantly higher than right one in transverse section of cell (Figures 3E,F, 4D,E). Dorsal surface slightly convex in posterior 2/5 area with about six longitudinal ridges extending over entire length of body (Figures 3E,H, 4D). These ridges separated into three pairs, in each pair two ridges slightly inclined to each other, which thus producing a 2 μm wide groove between the ridge pair on dorsal surface (Figures 3F, 4H). Three conspicuous ridges in ventral center among transverse cirri. The left and right margins of ventral side uplift, forming the ventral bending (Figures 3A, 4G).
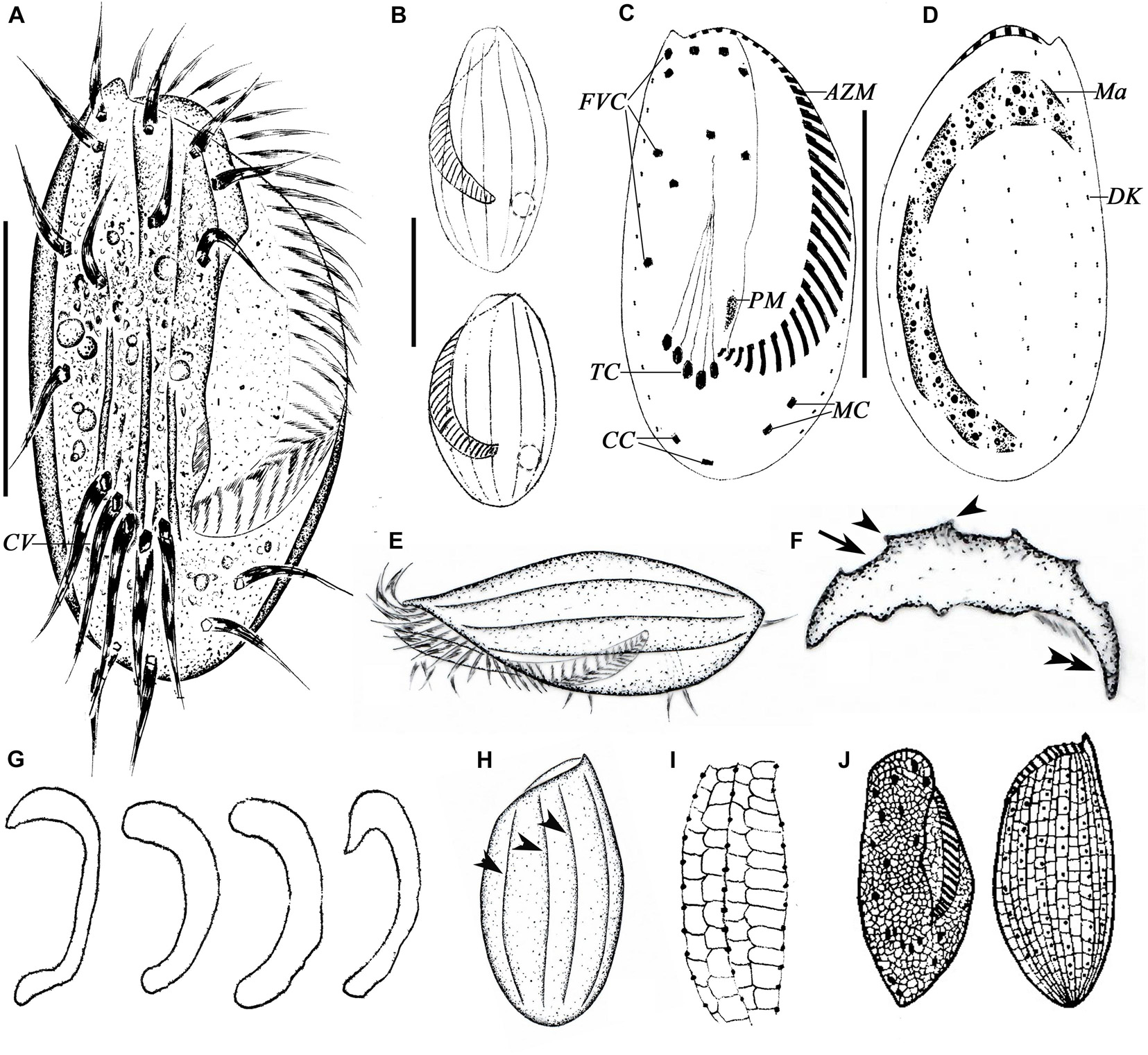
Figure 3. Morphology of E. antarcticus Fenchel and Lee, 1972 in vivo (A,B,E,F,H), after protargol (C,D,G) and silver nitrate (I,J) impregnation. (A) Ventral view of a representative specimen. (B) Dorsal views of different body shapes. (C,D) Ventral (C) and dorsal (D) views of the same specimen showing the ciliary pattern and nuclear apparatus. (E) Left lateral view showing the AZM and the dorsal ridges. (F) Cross section view to show bended left and right margins (double-arrowhead), note the furrows (arrow) and ridges (arrowheads) on dorsal side. (G) Different shapes of macronucleus. (H) Dorsal view to show the ridges (arrowheads). (I) Details of the silverline system of dorsal side. (J) Ventral and dorsal views, from Curds (1975). AZM, adoral zone of membranelles; CC, caudal cirri; CV, contractile vacuole; DK, dorsal kinety; FVC, frontoventral cirri; Ma, macronucleus; MC, marginal cirri; PM, paroral membrane; TC, transverse cirri. Scale bars = 30μm.
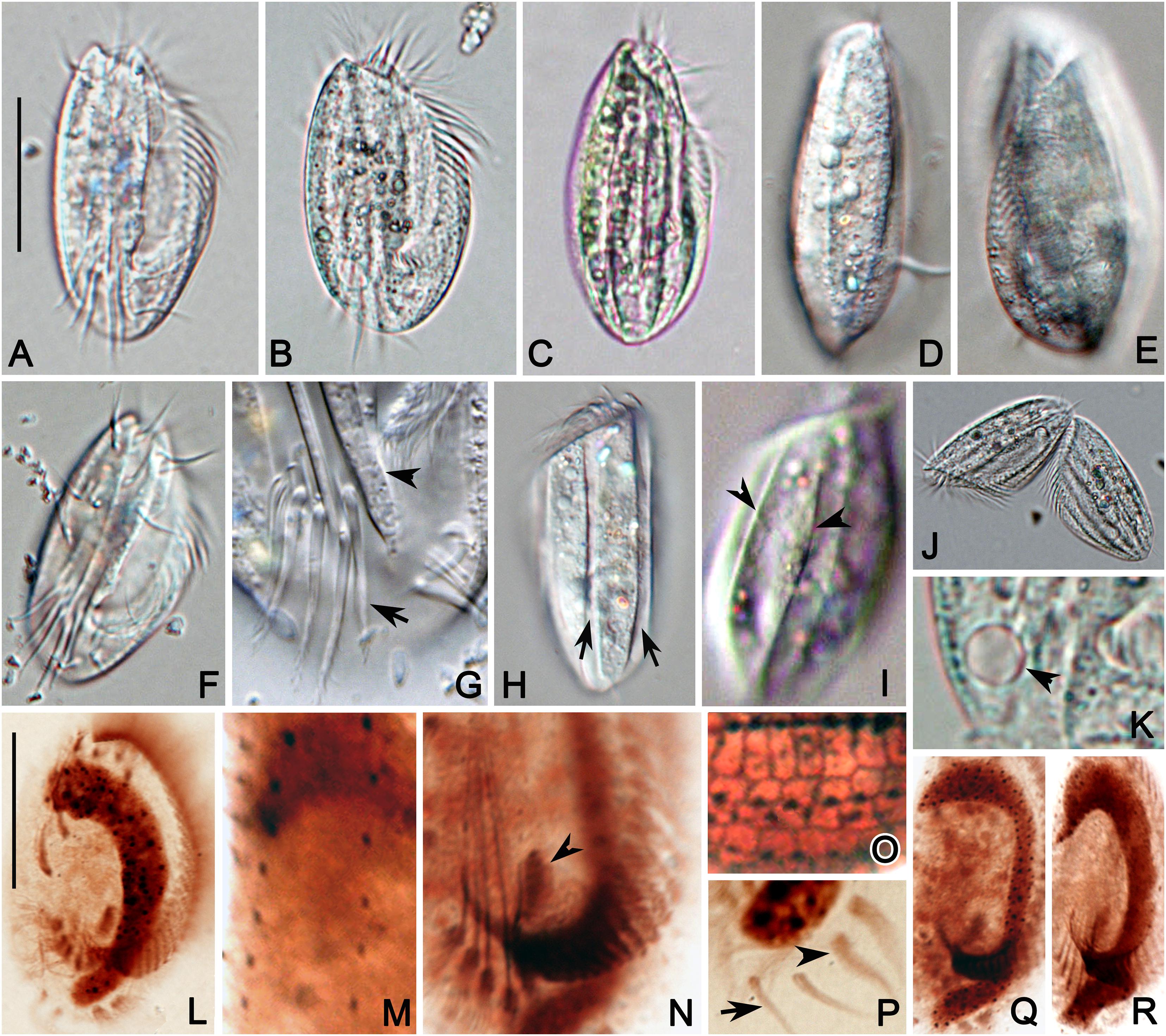
Figure 4. Photomicrographs of E. antarcticus in vivo (A–K), and after protargol (L–N,P–R) and silver nitrate (O) impregnation. (A) A representative specimen. (B,C) Cells of different shapes. (D,E) Right (D) and left (E) views showing the slightly convex dorsal side. (F) Ventral view showing the cirri. (G) Detail of posterior portion of ventral side to show the transverse cirri (arrow) and ridges (arrowhead). (H,I) Dorsal views to show the grooves (arrow) formed by inclined ridges (arrowhead). (J) A late divider. (K) Detail of contractile vacuole (arrowhead). (L) Ventral view of infraciliature. (M) Detail of dorsal kineties. (N) Center portion of ventral side to show the transverse cirri, paroral membrane (arrowhead) and posterior end of AZM. (O) Detail of dorsal view of silverline system. (P) Posterior portion of ventral side showing the marginal (arrowhead) and caudal (arrow) cirri. (Q,R) Macronucleus of different shapes. Scale bars = 30 μm.
Cytoplasm colorless, several lipid droplets (2–4 μm across) and food vacuoles (3–6 μm across) scattered in cell, rendering cells brown in color at low magnification (Figures 3A, 4B,C). Contractile vacuole located right posterior 1/4 and below the last two ventral cirri, about 8 μm in diameter (Figures 3A, 4B,K). Macronucleus usually open C-shaped and the posterior curve not conspicuous in some individuals which presents an inverted J-shape of macronucleus (Figures 3G, 4L,Q,R). Locomotion untypical, cells usually keep swimming slowly by rotation around the main axis, and occasionally crawls on the substrate.
Buccal field extending to 3/4 of body length, right border slightly indented in lower half, forming a buccal open area accounting for left 40% of body width (Figures 3A, 4A,F). Adoral zone composed of 26–30 membranelles, proximal portion slightly curved and anterior portion obliquely extend along the left cell shoulder to the dorsal anterior end of cell (Figures 3A,C, 4A,L). The bases of membranelles up to 5–9 μm long and cilia of membranelles about 8–12 μm long. Paroral membrane composed of many irregularly arranged kinetosomes which forming a clavate shape about 7 μm long (Figures 3C, 4N).
The ventral cirri generally fine. Invariably 10 frontoventral cirri, about 12 μm long; five transverse cirri about 21 μm long, and two caudal cirri about 15 μm long (Figures 3A,C, 4F,G,L). Two marginal cirri obliquely arranged below the proximal end of adoral membranelles, bearing thicker cilia and larger basal plaque than caudal cirri but equal to the latter in length (Figures 3C, 4P). Eight dorsal kineties with the leftmost and rightmost one usually positioned on ventral side. Most dorsal kineties extending over the entire length of the cell, except for the leftmost one which is shortest and positioned below the proximal end of adoral membranelles. The mid-kinety kinety with 13–17 dikinetids, while the rightmost one with only five or six dikinetids (Figures 3D, 4M). The dorsal silverline system double-patella type while in groove area the polygons slightly shrunk due to the inclination of ridges and seem to be double-eurystoma type (Figures 3I, 4O).
Phylogenetic Position of E. neapolitanus and E. antarcticus Based on SSU rRNA Gene Sequence Data
In phylogeny trees, E. neapolitanus clusters with another population from Korea, which, however, was weakly supported (53ML/0.76BI), and then forms a clade with E. harpa and E. platystoma (Figure 5). E. antarcticus groups together with E. trisulcatus with high support value (1.00BI/100ML), which then form a sister branch with the clade consisting of E. euryhalinus, E. sp. 6 and E. magnicirratus (Figure 5).
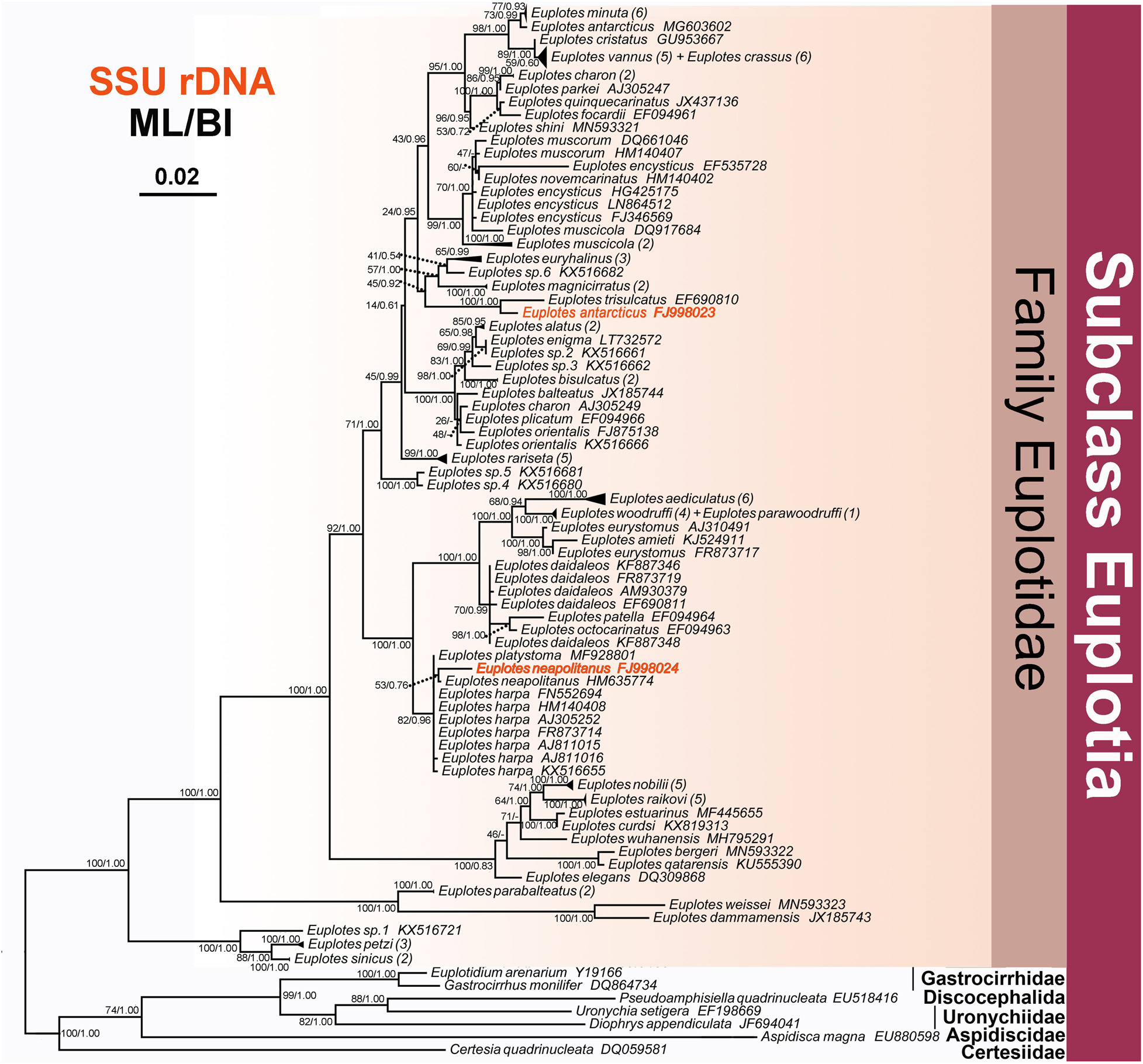
Figure 5. The Maximum likelihood (ML) tree inferred from 18S rRNA gene sequences, showing the position of E. neapolitanus and E. antarcticus. Numbers in the brackets mark the number of populations for each species. Numbers at nodes represent the bootstrap values of the ML analysis and the posterior probability of the Bayesian inference (BI) analysis. “–” indicates topologies that differ between the ML and BI phylogenies. Scale bar corresponds to five substitutions per 100 nucleotide positions.
Geographic Distribution of the Euplotes Species in Coast of Southern China
Totally 15 Euplotes species have been found and reported with taxonomy description in seven sites of coast of southern China (Figure 6 and Table 2). Among these species, E. charon has a most extensive distribution and was detected in three sites, followed by E. balteatus, E. encysticus, E. parawoodruffi, E. platystoma, E. rariseta, and E. vannus which were detected in two sites (Figure 7). The rest eight species were only found in one site. The species richness of Euplotes is variable among these sites. The highest species richness (13 species) was occurred in Huizhou and the lowest one was occurred in Donghai Island, Zhuhai and Shenzhen with only one species collected there (Figure 7). In addition, regarding the distributions among the six habitat types, the Euplotes has highest species number in both mangrove and offshore waters and lowest one in estuary habitat (Figure 7).
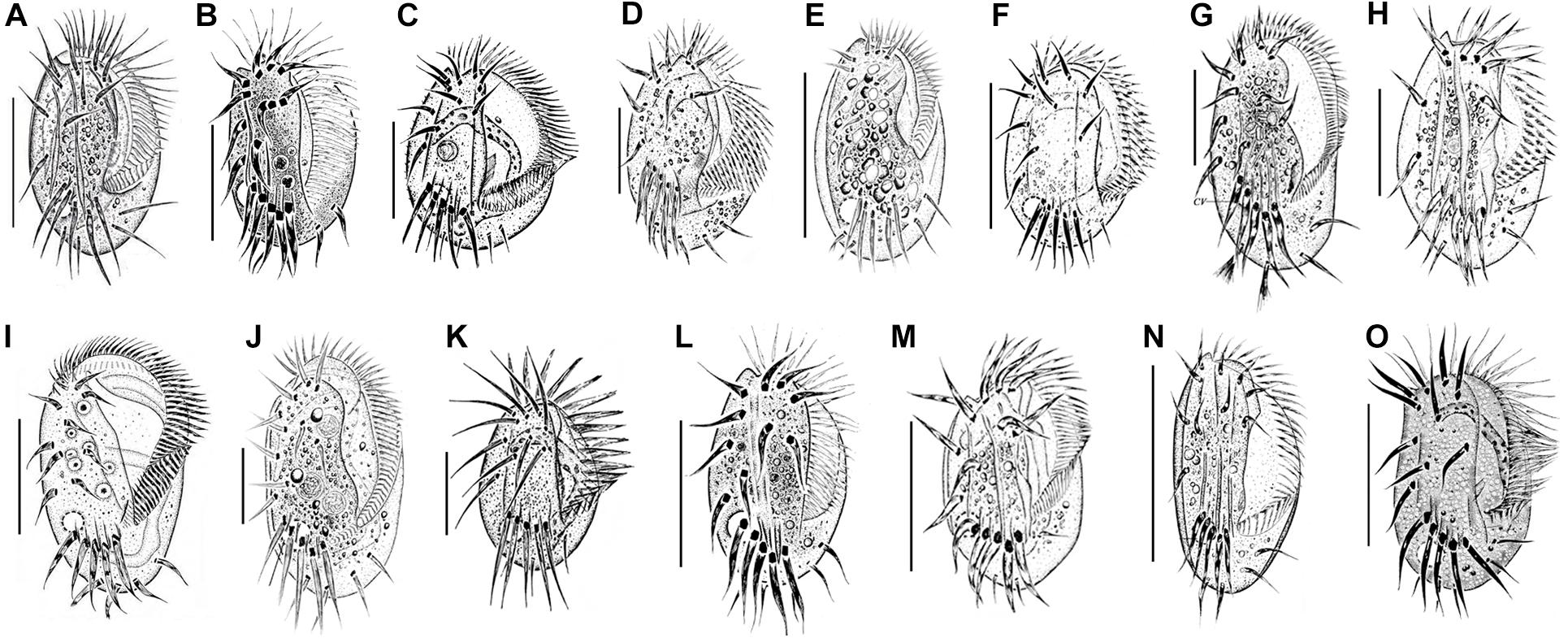
Figure 6. Euplotes species collected in the coastal waters of southern China. (A) E. balteatus (Dujardin, 1841) Kahl, 1932 (from Chen et al., 2013); (B) E. bergeri Lian et al., 2020 (from Lian et al., 2020); (C) E. charon (Müller, 1773) Ehrenberg, 1830 (from Song and Packroff, 1997); (D) E. encysticus Yonezawa, 1985 (from Fan et al., 2010); (E) E. estuarinus Yan et al., 2018 (from Yan et al., 2018); (F) E. minuta (Yocum, 1930) Borror and Hill, 1995 (from Song and Wilbert, 1997); (G) E. neapolitanus Wichterman, 1964 (present work); (H) E. orientalis Jiang et al., 2010 (from Jiang et al., 2010b); (I) E. parawoodruffi Song and Bradbury, 1997 (from Shen et al., 2008); (J) E. platystoma Dragesco and Dragesco-Kernéis, 1986 (from Yan et al., 2018); (K) E. rariseta Curds et al., 1974 (from Song and Packroff, 1997); (L) E. shini Lian et al., 2020 (from Lian et al., 2020); (M) E. sinicus Jiang et al., 2010 (from Jiang et al., 2010a); (N) E. antarcticus (present work); (O) E. vannus (Müller, 1786) Borror and Hill, 1995 (from Song and Packroff, 1997). Scale bars: 50 μm (A–G,I,J,L–O); 20 μm (H,K).
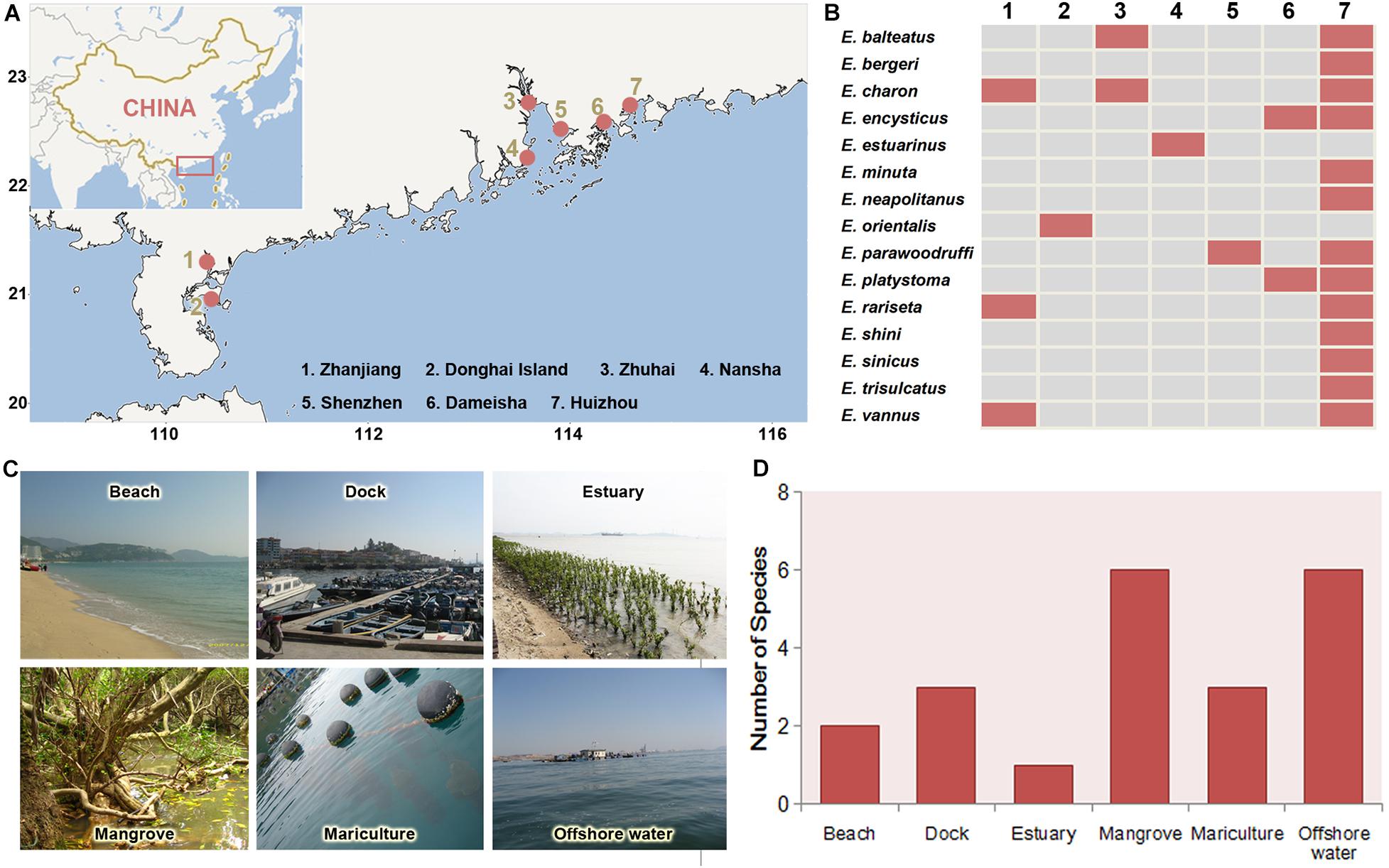
Figure 7. Geographic distribution of the Euplotes species in coastal waters of southern China. (A) Locations (red spots) where the species were collected. (B) Distributions of each species in the seven sites. (C) Main habitat types. (D) Species richness in each habitat types.
Discussion
Comparison of Euplotes neapolitanus With Related Congeners
Euplotes neapolitanus was first found in Bay of Naples in Mediterranean and described briefly by Wichterman (1964). There was no other record or redescription of this species until present work. The organism we collected corresponds well with the original description referring to the large cell size, ellipsoidal body shape with wider and truncated anterior end, conspicuous buccal field with adoral zone of membranelles abruptly curved in anterior end, as well as the basic infraciliature.
In terms of the large size (more than 100 μm), the double-eurystomus type silverline system, 10 frontoventral, two caudal and two marginal cirri, E. neapolitanus is similar with five congerens: E. platystoma Dragesco and Dragesco-Kernéis, 1986, E. harpa Stein, 1859, E. shanghaiensis Song et al., 1998, E. focardii Valbonesi and Luporini, 1990.
Besides with above aspects, E. harpa is similar to E. neapolitanus in having dorsal ridges. However, E. harpa differs from E. neapolitanus by the larger body size (150–160 vs. 130–150 μm), more dorsal kineties (13 vs. 11), and more dikinetids in mid-kinety rows (40–45 vs. 17–23) (Kahl, 1932).
Although most of the morphometric data of E. platystoma overlap with E. neapolitanus, E. neapolitanus can be separated from former by the truncated anterior end of cell (vs. rounded), the anterior adoral zone abruptly curved to dorsal side (vs. evenly curved), and the present of dorsal ridges (vs. absent) (Yan et al., 2018).
Euplotes shanghaiensis can be distinguished from E. neapolitanus by the smaller body size (80–120 × 30–35 vs. 130–150 × 70–75μm in vivo), fewer adoral membranelles (53–58 vs. 62–73), more dorsal kineties (12–13 vs. 11), and freshwater habitat (vs. marine) (Song et al., 1998).
Euplotes focardii differs from E. neapolitanus by the smaller body size (38–110 × 30–92 vs. 130–150 × 70–75 μm in vivo), fewer dorsal kineties (10 vs. 11), and the absent of dorsal ridges (vs. present) (Valbonesi and Luporini, 1990).
Beside, E. damammensis Chen et al., 2013 is also a large Euplotes with 10 frontoventral, two caudal and two marginal cirri as well as 11 dorsal kineties, although its silverline system was not revealed yet. It can be separated from E. neapolitanus by fewer adoral membranelles (44–51 vs. 62–73), and the shape of the macronucleus (sigmoidal vs. C-shaped) (Chen et al., 2013).
In phylogeny trees, E. neapolitanus clusters with E. harpa and E. platystoma, which agrees with their high morphologic similarities. There is another available SSU rRNA gene sequence under the species name E. neapolitanus [Korean isolation; GenBank accession number: HM635774; submitted by Khan et al., unpublished] in the GenBank, which, however, display a significant difference with our population in 55 nucleotides and 5% dissimilarity but a high similarity to E. platystoma with only a difference of four nucleotides. Considering the sufficient justification for identification of our population by morphology, the SSU rRNA gene sequence under the name E. neapolitanus should be undoubtedly linked to our population. Any conclusion about the taxonomy identification of the Korean isolations shouldn’t be made until its morphological data are available.
Comparison of Euplotes antarcticus With Related Congeners
Euplotes antarcticus was initially reported by Fenchel and Lee (1972) with some brief description by emphasizing its oblong body outline. The specimens studied here corresponds well with the original reports regarding its body shape, six dorsal ridges and basic infraciliature such as the numbers of adoral membranelles, ventral cirri, and especially the eight dorsal kineties of which six were on the dorsal surface. Some slight differences could be observed between these two populations, in terms of the cell size in vivo (50–70 × 25–35 μm in our specimens vs. 85–90 × 30–35 μm), and the sampling location (subtropic water in our specimens vs. Antarctica). In addition, the dorsal furrows observed in our specimens were not mentioned in previous descriptions, which was probably because that the dorsal ridges didn’t incline to forming the obvious groove in original population and suggest that the furrows are population-dependent characteristics for this species. Curds (1975) reviewed the original study and supplied some detailed characteristics such as rectangular outline with pointed posterior end, and a cleft presented in the right peristomial margin, which were not prominent or lacked in our specimens. Considering that these supplementary characters were obtained based on the observation on the original figures, those may be over-interpreted and cannot represent the general characters for this species. Moreover, the dargyrome also displayed dissimilarity between them (double-patella type in our specimens vs. double-eurystomus type in Curds’s description). Given that the inclined ridges were observed in vivo in our specimens, the size of each argyrome grid close to the ridge would be shrunk in the appearance in this case and thus double-patella might visually shift to double-eurystomus. Similar result was proposed in the study of Foissner et al. (1991).
Petz et al. (1995) described a Euplotes species under the name of E. antarcticus. However, it is clearly different from the original description E. antarcticus in terms of the cell size in vivo (90–145 × 30–80 vs. 85–90 × 30–35 μm), the numbers of adoral membranelles (38–70 vs. about 30 in original description), dorsal kineties (9–14 vs. 8 in original description), dorsal ridges (7–10 vs. 6 in original description), and caudal cirri (three or two vs. two in original description). Therefore, it was probably a misidentification.
Several congeners with elongated ellipsoidal body shape and double-type silverline system should be compared with E. antarcticus, namely E. affinis (Dujardin, 1841) Kahl, 1932, E. trisulcatus Kahl, 1932, E. dogieli Agamaliev, 1967, E. poljanskyi Agamaliev, 1966 and E. zenkewitchi Burkovsky, 1970.
Euplotes trisulcatus is most similar to E. antarcticus in the body shape and having dorsal ridges and furrows. However, E. antarcticus has a larger body size (50–90 × 25–35 vs. 35–50 × 25–40 μm in vivo), more dorsal kineties (eight vs. seven), and more dikinetids in the middle row (13–17 vs. 11) (Tuffrau, 1960; Carter, 1972). Therefore, it can be clearly separated from E. trisulcatus.
Both E. dogieli and E. poljanskyi can be easily distinguished from E. antarcticus by the absent of dorsal ridges (vs. present), the number of adoral membranelles (35–38 in E. dogieli and 36–40 in E. poljanskyi vs. 26–30 in E. antarcticus), dorsal kineties (seven in E. dogieli and E. poljanskyi vs. eight in E. antarcticus) frontoventral (nine in E. dogieli and eight in E. poljanskyi vs. 10 in E. antarcticus) and marginal cirri (one in E. dogieli and E. poljanskyi vs. two in E. antarcticus) (Agamaliev, 1966, 1967).
Euplotes zenkewitchi differs from E. antarcticus in having more adoral membranelles (50–55 vs. 26–30), more dorsal kineties (ten vs. eight), fewer frontoventral cirri (nine vs. ten), and distinctively curved macronucleus (vs. slightly curved with angular posterior end) (Burkovsky, 1970).
Euplotes affinis is a small organism and resembles E. antarcticus with prominent dorsal ridge. It differs from the latter, however, by the feature of freshwater habitat (vs. marine), 3-shaped macronucleus (vs. C-shaped), having fewer adoral membranelles (18–20 vs. 26–30), and fewer frontoventral cirri (nine vs. ten) (Kahl, 1932).
Besides, E. aberrans Dragesco, 1960 is also similar to E. antarcticus in the body shape although its silverline system was not revealed yet. It can be separated from E. antarcticus by the feature of macronucleus shape (horseshoe shaped with ends almost meet one another vs. obviously opened C-shape), having more adoral membranelles (about 50 vs. 26–30), fewer dorsal ridges (four vs. six) and fewer frontoventral cirri (eight vs. ten) (Dragesco, 1960).
In previous phylogeny studies, the gene sequences of our population of E. antarcticus have been published under the name E. cf. antarcticus. There is another available SSU rRNA gene sequence under the species name E. antarcticus [Korean isolation; accession number: MG603602; submitted by Park et al. (2019)] in the GenBank, which, however, display a significant difference with our population in 94 nucleotides and 5.2% dissimilarity. Considering the sufficient justification for identification of our population by morphology, we suggest to link the SSU rRNA gene sequence of E. antarcticus with our population. Detailed morphological description of the Korean specimen (EF690810) is necessary to reconsider the identification. In phylogeny trees, our population of E. antarcticus clusters with E. trisulcatus, which is consistent with their high morphologic similarities.
Remarks on the Distribution of Genus Euplotes
According to the compilation of faunal data, 15 Euplotes species have been found in southern China coast, which is significantly more than eight species reported in northern China coast such as Bohai and Yellow seas (Song et al., 2009). Moreover, six species, i.e., E. vannus, E. charon, E. minuta, E. rariseta, E. sinicus, E. parawoodruffi were found in both southern and northern China coasts (Song et al., 2009), suggesting their widely distibution in China.
Our results revealed that the highest species richness was occurred in Huizhou, which probably attributes to the diverse habitats there being suitable for different species (Table 2). In addition, mangrove wetlands possess the highest species richness of Euplotes species among the habitats in coast of southern China. The high diversity in mangrove has also been reported for other ciliate groups such as oligotrichs and stichotrichs (Zhang et al., 2018; Liang et al., 2019; Liu et al., 2019; Song et al., 2019), which suggest mangrove is an ideal habitat for ciliates. There are several reasons supporting the large ciliate species diversity in mangrove (Langenheder et al., 2010). First, the water in mangrove is normally eutrophic with high productivity due to the litter decomposition of plants, which supplies sufficient food source for ciliates. Second, the intricate root network of mangrove plant can dissipate the waves and conserve water and soil, which produces a stable habitat for ciliates especially for periphytons. Moreover, the dominant plants and terrain features of the mangroves are spatially varied, which leads to extensive environmental difference among the mangroves and thus supplies a wide range of niches for ciliates.
Data Availability Statement
All data generated or used during the study appear in the article.
Author Contributions
WL and XL conceived the research. WL wrote the manuscript. JJ and YT critically reviewed the findings and improved the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Guangdong MEPP Fund [No. GDOE (2019) A23], the Natureal Science Foundation of China (Nos. 31761133001 and 32070517), Science and Technology Planning Project of Guangdong Province, China (No. 2017B030314052), Science and Technology Basic Resources Investigation Program of China (No. 2017FY201404), and the Science and Technology Planning Project of Guangzhou (No. 202002030489).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Agamaliev, F. G. (1966). New species of psammobiotic ciliates of the western part of the Caspian Sea. Acta Protozool. 4, 169–183.
Agamaliev, F. G. (1967). Faune des ciliés mésopsammiques de la côte ouest de la. Mer Caspienne. Cahiers de Biol. Mar. 8, 359–402.
Borror, A. C. (1972). Revision of the order Hypotrichida (Ciliophora. Protozoa). J. Protozool. 19, 1–23. doi: 10.1111/j.1550-7408.1972.tb03407.x
Burkovsky, I. V. (1970). The ciliates of the mesopsammon of the Kandalaksha Gulf (White Sea). Acta Protozool. 7, 475–489.
Carter, H. P. (1972). Infraciliature of eleven species of the genus Euplotes. Trans. Am. Microsc. Soc. 91, 466–492. doi: 10.2307/3225477
Chen, X. R., Zhao, Y., Al-Farraj, S. A., Al-Quraishy, S. A., El-Serehy, H. A., Shao, C., et al. (2013). Taxonomic descriptions of two marine ciliates, Euplotes dammamensis n. sp. and Euplotes balteatus (Dujardin, 1841) Kahl, 1932 (Ciliophora, Spirotrichea, Euplotida), collected from the Arabian Gulf, Saudi Arabia. Acta Protozool. 52, 73–89.
Curds, C. R. (1975). A guide to the species of the genus Euplotes (Hypotrichida, Ciliatea). Bull. Br. Mus. Nat. Hist. 28, 3–61.
Dong, J. Y., Li, L. F., Fan, X. P., Ma, H. G., and Warren, A. (2020). Two Urosoma species (Ciliophora, Hypotrichia): A multidisciplinary approach provides new insights into their ultrastructure and systematics. Eur. J. Protistol. 72:125991.
Dragesco, J. (1960). Ciliés mésopsammiques littoraux. Systématiquemorphologie, écologie. Trav. Stn. Biol. Roscoff 12, 1–356.
Fan, X. P., Huang, J., Lin, X. F., Li, J. Q., Al-Rasheid, K. A. S., and Hu, X. Z. (2010). Morphological and molecular characterization of Euplotes encysticus (Protozoa: Ciliophora: Euplotida). J. Mar.Biol. Assoc. UK 90, 1411–1416. doi: 10.1017/s002531541000038x
Fenchel, T., and Lee, C. C. (1972). Studies on ciliates associated with the sea ice from Antarctica. I. The nature of the fauna. Arch. Protistenk. 114, 231–236.
Foissner, W. (2014). An update of basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa. Int. J. Syst. Evol. Microbiol. 64, 271–292. doi: 10.1099/ijs.0.057893-0
Foissner, W., Berger, H., Blatterer, H., and Kohmann, F. (1991). Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft 1991, 1–471.
Fotedar, R., Stoeck, T., Filker, S., Fell, J. W., Agatha, S., Marri, M. A., et al. (2016). Description of the halophile Euplotes qatarensis nov. spec. (Ciliophora Spirotrichea, Euplotida) isolated fromthe hypersaline Khor Al-Adaid lagoon in Qatar. J. Eukaryot. Microbiol. 63, 578–590. doi: 10.1111/jeu.12305
Gao, F., Huang, J., Zhao, Y., Li, L. F., Liu, W. W., Miao, M., et al. (2017). Systematic studies on ciliates (Alveolata, Ciliophora) in China: progress and achievements based on molecular information. Eur. J. Protistol. 61, 409–423. doi: 10.1016/j.ejop.2017.04.009
Gao, Y. Y., Gong, R. T., Jiang, Y. H., Pan, B., Li, Y., Warren, A., et al. (2020). Morphogenetic characters of the model ciliate Euplotes vannus (Ciliophora, Spirotrichea): Notes on cortical pattern formation during conjugational and postconjugational reorganization. Eur. J. Protistol. 73:125675. doi: 10.1016/j.ejop.2020.125675
Hu, X. Z., Lin, X. F., and Song, W. B. (2019). Ciliate atlas: species found in the South China Sea. Beijing: Science Press.
Jiang, J. M., Zhang, Q. Q., Hu, X. Z., Shao, C., Al-Rasheid, K. A. S., and Song, W. B. (2010a). Two new marine ciliates, Euplotes sinicus sp. nov. and Euplotes parabalteatus sp. nov., and a new small subunit rRNA gene sequence of Euplotes rariseta (Ciliophora, Spirotrichea, Euplotida). Int. J. Syst. Evol. Microbiol. 60, 1241–1251. doi: 10.1099/ijs.0.012120-0
Jiang, J. M., Zhang, Q. Q., Warren, A., Al-Rasheid, K. A. S., and Song, W. B. (2010b). Morphology and SSU rRNA gene-based phylogeny of two marine Euplotes species, E. orientalis spec. nov. and E. raikovi Agamaliev, 1966 (Ciliophora, Euplotida). Eur. J. Protistol. 46, 121–132. doi: 10.1016/j.ejop.2009.11.003
Kahl, A. (1932). Urtiere oder Protozoa. I: Wimpertiere oder Ciliata (Infusoria) 3. Spirotricha. Tierwelt Dtl. 25, 399–650.
Langenheder, S., Bulling, M. T., Solan, M., and Prosser, J. I. (2010). Bacterial biodiversity-ecosystem functioning relations are modified by environmental complexity. Plos One. 5:e10834. doi: 10.1371/journal.pone.0010834
Lian, C. Y., Luo, X. T., Fan, X. P., Huang, J., Yu, Y. H., Bourland, W., et al. (2018). Morphological and molecular redefinition of Euplotes platystoma Dragesco & Dragesco Kernéis, 1986 and Aspidisca lynceus (Müller,1773) Ehrenberg, 1859, with reconsideration of a “well-known”Euplotes ciliate, Euplotes harpa Stein, 1859 (Ciliophora, Euplotida). J. Eukaryot. Microbiol. 65, 531–543. doi: 10.1111/jeu.12499
Lian, C. Y., Wang, Y. R., Li, L. F., Al-Rasheid, K. A. S., Jiang, J. M., and Song, W. B. (2020). Taxonomy and SSU rDNA-based phylogeny of three new Euplotes species (Protozoa, Ciliophora) from China seas. J. King. Saud. Univ. Sci. 32, 1286–1292. doi: 10.1016/j.jksus.2019.11.013
Lian, C. Y., Zhang, T. T., Al-Rasheid, K. A. S., Yu, Y. H., Jiang, J. M., and Huang, J. (2019). Morphology and SSU rDNA-based phylogeny of two Euplotes species from China: E. wuhanensis sp. n. and E. muscicola Kahl, 1932 (Ciliophora, Euplotida). Eur. J. Protistol. 67, 1–14. doi: 10.1016/j.ejop.2018.10.001
Liang, Z., Shen, Z., Zhang, Y., Ji, D. D., Li, J. Q., Warren, A., et al. (2019). Morphology and phylogeny of four new Vorticella species (Ciliophora: Peritrichia) from coastal waters of southern China. J. Eukaryot. Microbiol. 66, 267–280. doi: 10.1111/jeu.12668
Liu, W. W., Jiang, J. M., Xu, Y., Pan, X. M., Qu, Z. S., Luo, X. T., et al. (2017). Diversity of free-living marine ciliates (Alveolata, Ciliophora): faunal studies in coastal waters of China during the years 2011-2016. Eur. J. Protistol. 61, 424–438. doi: 10.1016/j.ejop.2017.04.007
Liu, W. W., Zhang, K. X., Chen, C. Z., Li, J. Q., Tan, Y. H., Warren, A., et al. (2019). Overview of the biodiversity and geographic distribution of aloricate oligotrich ciliates (Protozoa, Ciliophora, Spirotrichea) in coastal waters of southern China. Syst. Biodivers. 17, 787–800. doi: 10.1080/14772000.2019.1691081
Lobban, C., Modeo, L., Verni, F., and Rosati, G. (2005). Euplotes uncinatus (Ciliophora, Hypotrichia), a new species with zooxanthelae. Mar. Biol. 147, 1055–1061. doi: 10.1007/s00227-005-0024-3
Lynn, D. H. (2008). The ciliated protozoa: characterization, classification, and guide to the literature, 3rd Edn. Dordrecht: Springer.
Miller, M., Pfeiffer, W., and Schwartz, T. (2010). “Creating the CIPRES Science Gateway for inference of large phylogenetic trees,” in Proceedings of the Gateway Computing Environments Workshop (GCE), (New Orleans, LA), 1–8.
Pan, Y., Li, L. Q., Shao, C., Hu, X. Z., Ma, H. G., Al-Rasheid, K. A., et al. (2012). Morphology and ontogenesis of a marine ciliate, Euplotes balteatus (Dujardin, 1841) Kahl, 1932 (Ciliophora, Euplotida) and definition of Euplotes wilberti nov. spec. Acta Protozool. 51, 29–38.
Park, M., Jung, J., Jo, E., Park, K., Baek, Y., Kim, S., et al. (2019). Utility of mitochondrial CO1 sequences for species discrimination of Spirotrichea ciliates (Protozoa, Ciliophora). Mitochondrial DNA A. 30, 148–155. doi: 10.1080/24701394.2018.1464563
Petz, W., Song, W. B., and Wilbert, N. (1995). Taxonomy and ecology of the ciliate fauna (Protozoa, Ciliophora) in the Endopagial and Pelagial of the Weddell Sea, Antarcitica. Stapfia. 40, 1–223.
Ronquist, F., Teslenko, M., Van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Shen, Z., Lin, X. F., and Li, J. Q. (2008). Morphological redescription of a rare marine Euplotids, Euplotes parawoodruffi (Protozoa, Ciliophora, Euplotida). Acta. Zootaxon. Sin. 33, 335–339.
Song, W. B., and Packroff, G. (1997). Taxonomische Untersuchungen an marinen Ciliaten aus China mit Beschreibungen von zwei neuen Arten, Strombidium globosaneum nov. spec. und S. platum nov. spec. (Protozoa, Ciliophora). Arch. Protistenkd. 147, 331–360. doi: 10.1016/S0003-9365(97)80059-0
Song, W. B., and Wilbert, N. (1997). Morphological investigations on some free living ciliates (Protozoa, Ciliophora) from China Sea with description of a new hypotrichous genus, Hemigastrostyla nov.gen. Arch. Protistenkd. 148, 413–444. doi: 10.1016/S0003-9365(97)80020-6
Song, W. B., Warren, A., and Hill, B. F. (1998). Description of a new fresh-water ciliate, Euplotes shanghaiensis nov. spec. from China(Ciliophora, Euplotidae). Eur. J. Protistol. 34, 104–110. doi: 10.1016/s0932-4739(98)80019-9
Song, W. B., Warren, A., and Hu, X. Z. (2009). Free-living ciliates in the Bohai and Yellow Seas, China (Eds.). Beijing: Science Press.
Song, W., Xu, D. P., Zhang, Q. Q., Liu, W. W., Warren, A., and Song, W. B. (2019). Taxonomy and phylogeny of two poorly studied genera of marine oligotrich ciliates including descriptions of two new species: Cyrtostrombidium paraboreale sp. n. and Apostrombidium orientale sp. n. (Ciliophora: Spirotrichea). Eur. J. Protistol. 70, 1–16. doi: 10.1016/j.ejop.2019.05.001
Stamatakis, A. (2014). RaxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Tuffrau, M. (1960). Révision du genre Euplotes, fondée sur la comparaison des structures superficielles. Hydrobiologia. 15, 1–77. doi: 10.1007/bf00048080
Valbonesi, A., and Luporini, P. (1990). Description of two new species of Euplotes and Euplotes rariseta from Antarctica. Polar Biol. 11, 47–53.
Wichterman, R. (1964). Description and life cycle of Euplotes neapolitanus sp. nov. (Protozoa, Ciliophora, Hypotrichida)from the Gulf of Naples. Trans. Am. Microsc. Soc. 83, 362–370. doi: 10.2307/3224748
Wilbert, N. (1975). Eine verbesserte Technik der Protargolimprägnation für Ciliaten. Mikrokosmos. 64, 171–179.
Yan, Y., Fan, Y. B., Luo, X. T., El-Serehy, H. A., Bourland, W., and Chen, X. R. (2018). New contribution to the species-rich genus Euplotes: morphology, ontogeny and systematic position of two species (Ciliophora; Euplotia). Eur. J. Protistol. 64, 20–39. doi: 10.1016/j.ejop.2018.03.003
Yi, Z. Z., Song, W. B., Clamp, J., Chen, Z. G., Gao, S., and Zhang, Q. Q. (2009). Reconsideration of systematic relationships within the order Euplotida (Protista, Ciliophora) using new sequences of the gene coding for small-subunit rRNA and testing the use of combined data sets to construct phylogenies of the Diophrys-like species. Mol. Phylogen. Evol. 50, 599–607. doi: 10.1016/j.ympev.2008.12.006
Zhang, T. Y., Dong, J. Y., Cheng, T., Duan, L. L., and Shao, C. (2020). Reconsideration on taxonomy of the marine ciliate Neobakuella aenigmatica Moon et al., 2019 (Protozoa, Ciliophora, Hypotrichia). Mar. Life. Sci. Technol. 2, 97–108. doi: 10.1007/s42995-020-00032-4
Zhang, T. Y., Qi, H. L., Zhang, T. T., Sheng, Y. L., Warren, A., and Shao, C. (2018). Morphology, morphogenesis and molecular phylogeny of a new brackish water subspecies, Neourostylopsis flava paraflava nov. subsp. (Ciliophora, Hypotrichia, Urostylidae), with redefinition of the genus Neourostylopsis. Eur. J. Protistol. 66, 48–62. doi: 10.1016/j.ejop.2018.07.004
Keywords: Euplotes neapolitanus Wichterman, 1964, Euplotes antarcticus Fenchel and Lee, 1972, biogeography, morphology, phylogeny
Citation: Liu W, Jiang J, Tan Y and Lin X (2020) Novel Contributions to the Taxonomy of the Ciliates Genus Euplotes (Ciliophora, Euplotida): Redescription of Two Poorly Known Species, With a Brief Note on the Distributions of This Genus in Coastal Waters of Southern China. Front. Mar. Sci. 7:615413. doi: 10.3389/fmars.2020.615413
Received: 09 October 2020; Accepted: 16 November 2020;
Published: 08 December 2020.
Edited by:
Xiaoshou Liu, Ocean University of China, ChinaReviewed by:
Yuan Xu, East China Normal University, ChinaYong Jiang, Ocean University of China, China
Copyright © 2020 Liu, Jiang, Tan and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofeng Lin, bGlueGZAeG11LmVkdS5jbg==
 Weiwei Liu
Weiwei Liu Jiamei Jiang3
Jiamei Jiang3 Yehui Tan
Yehui Tan