- 1Institute of Oceanography, National Taiwan University, Taipei, Taiwan
- 2Professional Bachelor “Inland Aquaculture & Aquariology”, Institut Universitaire de Technologie Nancy-Brabois, Université de Lorraine, Le Montet, France
- 3Institute of Fisheries Science, National Taiwan University, Taipei, Taiwan
- 4Research Center for Future Earth, National Taiwan University, Taipei, Taiwan
- 5Biology of Marine Organisms and Biomimetics, University of Mons, Mons, Belgium
Scrutinizing the traits of octocorals that could affect their physiological performance becomes increasingly important as several of these species are observed to become dominant on reefs pressured by the Anthropocene. In the present study, we compare the organismal traits of two branching octocorals Litophyton sp. and Stereonephthya sp. commonly populating in sympatry the high-latitude coral communities of northern Taiwan. Using 13 traits, we describe and compare performance traits in these two symbiotic species that we discuss in light of the association they maintain with their algal partners. Litophyton sp. and Stereonephthya sp. hosted Durusdinium and Gerakladium, respectively. Both genera represent singular associations, with the latter further establishing the first solid report of Gerakladium in octocorals. Traits distinguished two groups explained by the two partnerships considered. Litophyton sp. associated with Durusdinium had significantly higher organic matter, chlorophyll (chl) a, total lipid and lower chl c/chl a ratio than Stereonephthya sp. associated with Gerakladium. The δ15N in the host and algae, as well as δ13C in the host were also higher in Litophyton species. Although no significant difference was observed in the δ13C of the algae, Litophyton sp. presented a significantly higher variance for this trait and for chl a content than Stereonephthya species. Altogether, the traits examined suggested contrasting performances among the two octocorals. Both octocoral species clearly deviate from an autotrophic diet. Litophyton sp. appears to complement its heterotrophic diet with photosynthetically acquired energy, while Stereonephthya sp. tends to be more specialized and benefits relatively little from its symbiotic relationship. Our study calls for greater consideration of the individual variation in octocoral physiology and in the definition of their ecological strategies.
Introduction
Octocorals are important contributors to the living three-dimensional structure of the marine animal forests (Sánchez, 2016). These communities, dominated by sessile suspension-feeding organisms, support a diversity of ecological functions. A number of organisms use octocorals for their food (Gerhart, 1990; O’Neal and Pawlik, 2002), their habitat (Reijnen et al., 2011) and/or as a nursery ground (Etnoyer and Warrenchuk, 2007). Octocorals further influence energy flows and nutrient cycling between the pelagic and benthic systems by capturing planktonic organisms and filtering large amounts of dissolved and particulate organic matter (OM) (Coma et al., 1998; Rossi et al., 2017). They play a key role in carbon sequestration (Coppari et al., 2019), and some species contribute substantially to the reef formation in tropical habitats (e.g., Jeng et al., 2011). Therein, octocorals appear to have a relatively high level of tolerance to environmental stressors (Schubert et al., 2017). Accordingly, they have emerged as key potential candidates to prevail in future reefs (Vercelloni et al., 2020). Yet, octocorals spread across more than 40 families and display a wide array of morphologies, sizes, and sclerite architectures (e.g., Aharonovich and Benayahu, 2012). A generalization of these resilience abilities to all reef-associated octocorals seems doubtful, especially considering the variable responses to thermal stress observed among species (Slattery et al., 2019) and the limited knowledge currently available on their physiology.
The combination of features that allows the comparison of individual performance emerged as a decisive approach in defining and comparing species fitness (Violle et al., 2007). In this context, trade-off among traits involved in the flow of energy and matter at the individual level (Kearney et al., 2010) are directly related to their “function” in the ecosystems (sensus Bellwood et al., 2018). This further supplies a basis for the estimation of intraspecific variability and the delineation of species niches (Violle et al., 2012). Nevertheless, mean field theory (the study of the behavior of the mean while ignoring variance, Violle et al., 2012) still remains widely adopted in trait-based community ecology, especially in studies focusing on hyperdiverse ecosystems such as the coral reefs (Hughes et al., 2018; McWilliam et al., 2018). It provides a wide array of opportunities to improve our understanding of organism responses, particularly in species where symbiosis is expected to mediate niche differentiation and expansion (see Gerz et al., 2018). In octocorals, it has relevance in depicting and comparing the variation in individual traits involved in the acquisition and allocation of energy in the holobiont.
A significant proportion of octocorals is mixotrophic in shallow-water tropical reefs, living in association with photosynthetic dinoflagellates of the family Symbiodiniaceae (Schubert et al., 2017). These microalgal symbionts can ensure that most of the daily metabolic needs of the animal host are met (Schlichter et al., 1983), but their contribution has been demonstrated to change in accordance with environmental conditions (Bednarz et al., 2015) or host morphology (Rossi et al., 2018). On the other hand, heterotrophic feeding (Sorokin, 1991; Ribes et al., 1998) may be preponderant in some adult and juvenile individuals of the few known aposymbiotic species (e.g., Eunicella singularis, Gori et al., 2012; Schubert et al., 2017). Heterotrophic diet has also been observed to vary seasonally (Coma et al., 2015) and increase with local pollution (Baker et al., 2010). It may also improve the resilience of species with generalist, facultative associations; which in return, usually appear more sensitive to bleaching than species maintaining specialized, obligate symbioses (Baker et al., 2015). The relative contribution of both heterotrophic and autotrophic feeding is probably a determinant for explaining the performance of many species in a given habitat. Furthermore, it represents a basis upon which natural selection may operate under stressful conditions.
Earlier studies emphasized the variety of responses that can be expected in octocorals and the importance of recognizing species strategies to cope with present-day environmental challenges. In the present study, we targeted octocorals populating high-latitude coral communities from the coastal waters of northern Taiwan (Lin and Denis, 2019). We used 13 organismal traits to describe and compare individual variation in two symbiotic species Litophyton sp. and Stereonephthya sp., chosen for their phylogenetic proximity (McFadden et al., 2006) and morphological similarity (van Ofwegen, 2016). We delineated species performance niches in a constant environment and discussed differences among the two targeted species in light of the association they maintained with their respective algal partners.
Materials and Methods
Study Species and Sampling Site
Two octocoral species (F: Nephtheidae) were targeted in this study: Litophyton sp. and Stereonephthya sp. (Figures 1A,B). Both share similar branching morphology and their populations are observed to thrive in sympatry in the shallow water benthic communities of northern Taiwan. For each taxon, 10 large colonies were sampled in April 2019 at −10 m (water temperature: 24°C) in Bitou (25.1262°N, 121.9131°E). Colonies were tentatively diagnosed as our targeted organisms (general morphology and close-up inspection of the polyps) and were photographed in situ with a tag and scale. Colony fragments, representing a total surface of 10–20 cm2 of the extended colony, were sampled with scissors and placed delicately in Ziploc bags. Samples were collected under Collection Permit No. 1083544868 issued by the Fisheries and Fishing Port Affairs Management Office, New Taipei City Government, Taiwan. Immediately after the dive, octocoral branches were subsampled and fixed in 10% formalin for histological analysis. The remaining samples were fast-frozen for 5 min at −80°C in a cryogenic dry shipper (CX100, Taylor-Wharton, United States), and transferred into an icebox for transportation to the laboratory. Samples were transferred in a −20°C freezer until further processing.
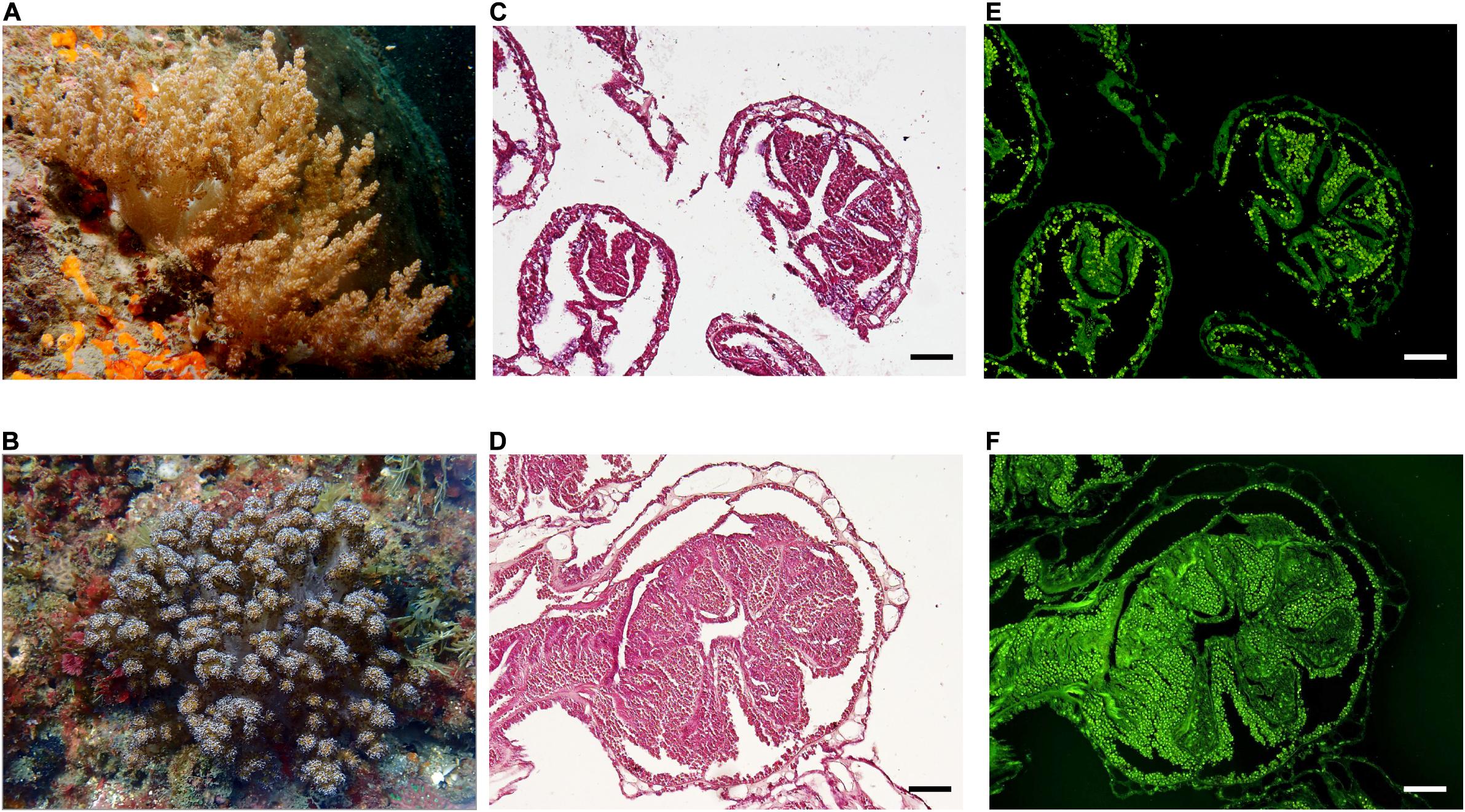
Figure 1. In situ appearance and symbiont distribution in octocoral colonies. Litophyton sp. (A) and Stereonephthya sp. (B) at –10 m depth in Bitou (Taiwan). Histological sections of polyps from Litophyton sp. (C) and Stereonephthya sp. (D) under light microscope with an emphasis on algal endosymbionts using a U-MWB2 filter (E,F). Scale bar 100 μm.
Host Distinction and Algal-Symbiont Identification
Both the confirmation of the host colonies and the identification of their dominant algal symbiont were processed independently. Samples were thawed on ice in the dark. For the confirmation of the host, tissue collected from the top, base, and branches of the coral fragment were bleached to isolate their skeleton elements. Dried sclerites were examined and compared to relevant taxonomic references for the identification of the host species. Other small subsamples were further collected from randomly selected coral colonies of each genus and preserved in 100% EtOH for molecular confirmation of the membership to Litophyton (n = 4) and Stereonephthya (n = 3). DNA was extracted and mitochondrial marker mtMutS was amplified using published primers and protocols (McFadden et al., 2011).
For algal-symbont identification, five colonies of each species were randomly selected to extract DNA from a small subsample preserved in 100% EtOH using a DNeasy PowerSoil Kit (Qiagen, Germany) following the manufacturer’s protocol. The purity of DNA was checked with a NanoDrop 1000 (Thermo Thermo Scientific, United States). For the Symbiodiniaceae identification, the internal transcribed spacer ITS2 was amplified using zITSf (5′-CCGGTGAATTATTCGGACTGACGCAGT-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) as described in Noda et al. (2017). A PCR amplification of 35 cycles was performed (95°C for 30 s, 51°C for 45 s, and 72°C for 2 min). PCR products with around 724 bp were submitted to sanger sequencing, then individually checked for similarity with the Basic Local Alignment Search Tool (BLAST) of the National Center for Biotechnology Information (NCBI) database (Wheeler et al., 2008). Novel sequences have been deposited in GenBank (see accession numbers in Supplementary Table 1).
Algal Symbiont Distribution
Adapted from Rossi et al. (2018), Zinc Formal-Fixx (ShandonTM Thermo Fisher Scientific, CAS no.: 50-00-0) samples were treated in a series of rinsing and decalcification steps using 10% formic acid (Sigma-Aldrich, CAS no.: 64-18-6), after which 8 μM sections of tissue embedded in paraffin wax (Thermo Fisher Scientific, CAS no.: 9003-27-4) were stained with Meyer’s hematoxylin (ShandonTM Thermo Fisher Scientific, CAS no.: 517-28-2) and eosin (ShandonTM Thermo Fisher Scientific, CAS no.: 17372-87-1) procedures, and coverslipped with Organol/Limonene (Sigma-Aldrich, CAS no.: 5989-27-5) mounting medium. Octocoral samples were examined with a fluorescence microscope (Olympus-BX51) under the excitation/emission wavelength of 460–490/520 nm (with U-MWB2 filter) to detect the presence of microalgal pigments in their tissue.
Organismal Trait Measurements
A small fragment (∼1 g of wet weight) was isolated from each thawed sample. The remaining tissue was ground in a mortar, transferred into a beaker, and the slurry was adjusted to an initial volume of 60 mL using artificial seawater (Vi). Vi was homogenized using a T10 basic ULTRA-TURRAX disperser (IKA Works Inc., United States) for 3 min. Dry weight (DW) was determined from a 1 mL aliquot of Vi, vacuum-filtered through a pre-weighed Whatman (United Kingdom) glass fiber filter (Grade: GF/C, ∅ 100 mm, pore size 1.2 μm), and dried overnight at 60°C. The filter was then ashed for 4 h at 450°C to calculate an ash-free dry weight (AFDW) (Pupier et al., 2018), that was used to standardize the following biochemical parameters. It was further used to estimate the % content in OM.
For photosynthetic traits below, a 1 mL aliquot of Vi was centrifuged at 3,000 g for 3 min to pellet algal symbionts. The pellet was re-suspended in 10 mL 90% acetone (J.T. BakerTM Avantor, CAS no.: 67-64-1) and incubated overnight at 4°C. The solution was later centrifuged at 3,000 g for 1 min, and the supernatant was transferred to a cuvette for measuring absorbance at 630, 647, 664, and 750 nm with a spectrophotometer (Optizen Pop, Mecasys, South Korea). Both, chlorophyll a (chl a) and c (chl c) concentrations (μg gAFDW–1) were then estimated following trichromatic equations proposed by Jeffrey and Humphrey (1975). Note that the presence of chl b was checked, but found to be absent in the colonies examined. Algal symbiont content (cells gAFDW–1) was estimated by counting cells from a 10 μL aliquot of Vi in a Neubauer chamber (Bright-Line 3100, Hausser Scientific, United States). Counts were repeated five times and averaged for each sample.
Total protein (μg gAFDW–1) was determined from combining separate measurements of their concentrations in the animal host and algal fractions of a 5 mL aliquot of Vi. Both were isolated by centrifugation (3 min, 3,000 g), and homogenized with a dispenser after adding to the algal pellet a 2 mL 1% sodium dodecyl sulfate (SDS, J.T. BakerTM Avantor, CAS no.: 151-21-3) to ease the release of proteins from the dinoflagellate cytoplasm. Protein concentrations were calculated from triplicate spectrophotometric measurements (SpectraMax i3x, FortéBio, United States) on both fractions using a protein assay kit (PierceTM BCA Thermo Fisher Scientific) with bovine serum albumin as a standard, following manufacturer protocol.
For the stable isotope measurements, the overall methodology was adapted from the protocol recommended for scleractinian corals in Sturaro et al. (2020) to octocorals. Notably, tissue was ground and not air-brushed. A large volume corresponding to a 50 mL of Vi was centrifuged (4°C, 10 min, 2,000 g) to separate both the host and the algal tissue. The purity of the host fraction was checked under an optical microscope, and the process was repeated until no algal cells were observed within the solution. It was then vacuum-filtered through a pre-cleaned (4 h, 450°C) Whatman (United Kingdom) glass fiber filter (Grade: GF/F, ∅ 47 mm, pore size 0.7 μm). The algal pellet was cleaned a minimum of 10 times by adding Milli-Q water to the pellet, resuspending the cells, centrifuging, and discarding the pellet in order to remove host tissue debris from that fraction. Purity was once again checked under a microscope until no significant contamination was visible. To remove carbonates, acidification was performed by adding 1N HCl (FlukaTM Honeywell, CAS no.: 7647-01-0) droplets to the filter and the algal pellet until no bubble was observed. They were both rinsed with Milli-Q water and dried at 50°C overnight. The surface of the filters containing host tissue was then scraped and homogenized into a fine powder as the algal pellet. Both were weighed into tin capsules before stable isotope analysis.
Stable isotope ratios of carbon and nitrogen in both host tissues and algae were measured with an isotope ratio mass spectrometer (DELTA V Advantage, Thermo Fisher Scientific, United States) coupled in continuous flow with an elemental analyzer (Flash 2000, Thermo Fisher Scientific, United States) at the Institute of Oceanography, National Taiwan University. The results were reported using the common δ notation (i.e., δ15N and δ13C) in ‰ relative to Vienna Pee Dee Belemnite and atmospheric N2 for carbon and nitrogen isotopes, respectively. Isotope ratio equation (Coplen, 2011) is as follows:
where X stands for 15N or 13C, and R represents 15N/14N or 13C/12C. USGS40 (L-glutamic acid: δ13C = −26.4 ± 0.1‰; δ15N = −4.5 ± 0.1‰) (IAEA, Vienna, Austria) and protein (δ13C = −27.3 ± 0.1‰; δ15N = 6.0 ± 0.1‰) are used as certified reference material and internal standard, respectively. Carbon and nitrogen contents were expressed as a percentage of the dry mass (%), and the carbon to nitrogen ratio (C:N ratio) was calculated.
Eventually, total lipid (g gAFDW–1) was measured from the small fragment of the colony previously isolated. This subsample was freeze-dried for one day at −80°C and 40 mbar using a lyophilizer (FD6-4P-D-80°C, Fortelice, Taiwan). The subsample was then ground into a fine powder, from which 90% were used to extract lipids following an adapted methodology of Folch et al. (1957) replacing the chloroform by dichloromethane (Riedel-de HaënTM Honeywell, CAS no.: 75-09-2) in the extracting solution (dichloromethane-methanol, 2:1, v/v). The upper organic layer (containing the lipids) was transferred to an aluminum dish and solvents were allowed to evaporate under a chemical hood. The remaining 10% was used for a measurement of the AFDWL which was used to standardize lipid contents. Its measurement follows a similar methodology as described above, but using an aluminum dish instead of a filter.
Trait Analysis
A total of 13 quantitative traits were used to document intra- and inter-specific variations (data available the Dryad Digital Repository: https://doi.org/10.5061/dryad.qz612jmd7): (1) OM; (2) chl a; (3) chl c; (4) ratio chl c/chl a; (5) algal symbiont; (6) total protein; (7) host δ15N; (8) host δ13C; (9) algae δ15N; (10) algae δ13C; (11) host C:N; (12) algae C:N; (13) total lipid. All traits selected are hypothesized to respond to the local environment, and therefore are relevant in delineating realized species performance.
Trait variations were depicted using violin plots and data were compared between species using Welch t-tests, assuming normality but without assumption of the equality of variances among samples. In addition, Levene’s tests was used to assess the equality of variances in traits between species. A Pearson’s r correlation matrix was produced between each pair of trait variables while retaining only individuals with completed observations (see Supplementary Figure 1). Substantial correlation was detected using a cut-off at | r | > 0.8. A principal component analysis (PCA) was generated on scaled trait variables, and variations among individuals visualized on a biplot. Intraspecific variations were assessed by multivariate homogeneity of species dispersions and compared between the two species using a permutation test. A permutational multivariate analysis of variance (PERMANOVA) computed on Euclidean distances was used to test for the difference between the two species. R (v 3.6.1,R Core Team, 2019) and the packages “corrplot,” “factoextra,” “ggplot 2,” “ggpubr,” “tidyr,” and “vegan” were used to analyze the data and produce the figures. The source code for data analysis is available at https://github.com/vianneydenis/stranger-things.git.
Results
Octocorals were confirmed to belong to two distinct genera: Litophyton and Stereonephthya. Histological sections (Figures 1C,D) validated the symbiotic status of both species, with algal cells clearly identified in the endoderm cells (Figures 1E,F). Litophyton sp. was populated by Durusdinium (former clade “D”) while Stereonephthya sp. was populated by Gerakladium algal symbionts (former clade “G”) (Supplementary Table 1).
Litophyton sp. had significantly higher OM, chl a, total lipid, and lower ratio chl c/chl a than Stereonephthya sp. (Figure 2 and Supplementary Table 2). Significantly higher δ15N values were observed in Litophyton sp. animal host and algal fractions compared to Stereonephthya sp. (Figure 3). It was also the case for δ13C values of the animal host fraction, but not for the algal fraction in which there was no significant difference between the two species. In addition, δ13C (Welch’s t-test: t = −11.2, p < 0.001) and δ15N (Welch’s t-test: t = −2.30, p = 0.04) in Stereonephthya sp. were higher in the algal fraction than in the animal host. In contrast, no significant difference was observed in δ13C (Welch’s t-test: t = −2.05, p = 0.06) and δ15N (Welch’s t-test: t = 0.1, p = 0.89) between the algal and host fractions in Litophyton species. Other traits such as chl c, algal symbiont, total protein contents, and C:N ratios did not differ between species. The chl a by cell was also significantly higher in Litophyton sp. than in Stereonephthya sp. (Welch’s t-test: t = 5.2, p < 0.001, inset Figure 2E). Litophyton sp. had a significantly higher variance in chl a (Levene’s test: F = 6.46, p < 0.05) and algae δ13C (Levene’s test: F = 13.28, p < 0.01) values than Stereonephthya species. For host C:N, significantly higher variances were observed in Stereonephthya sp. than in Litophyton sp. (Levene’s test: F = 6.00, p < 0.05). The variances in other selected traits did not significantly differ between the two species.
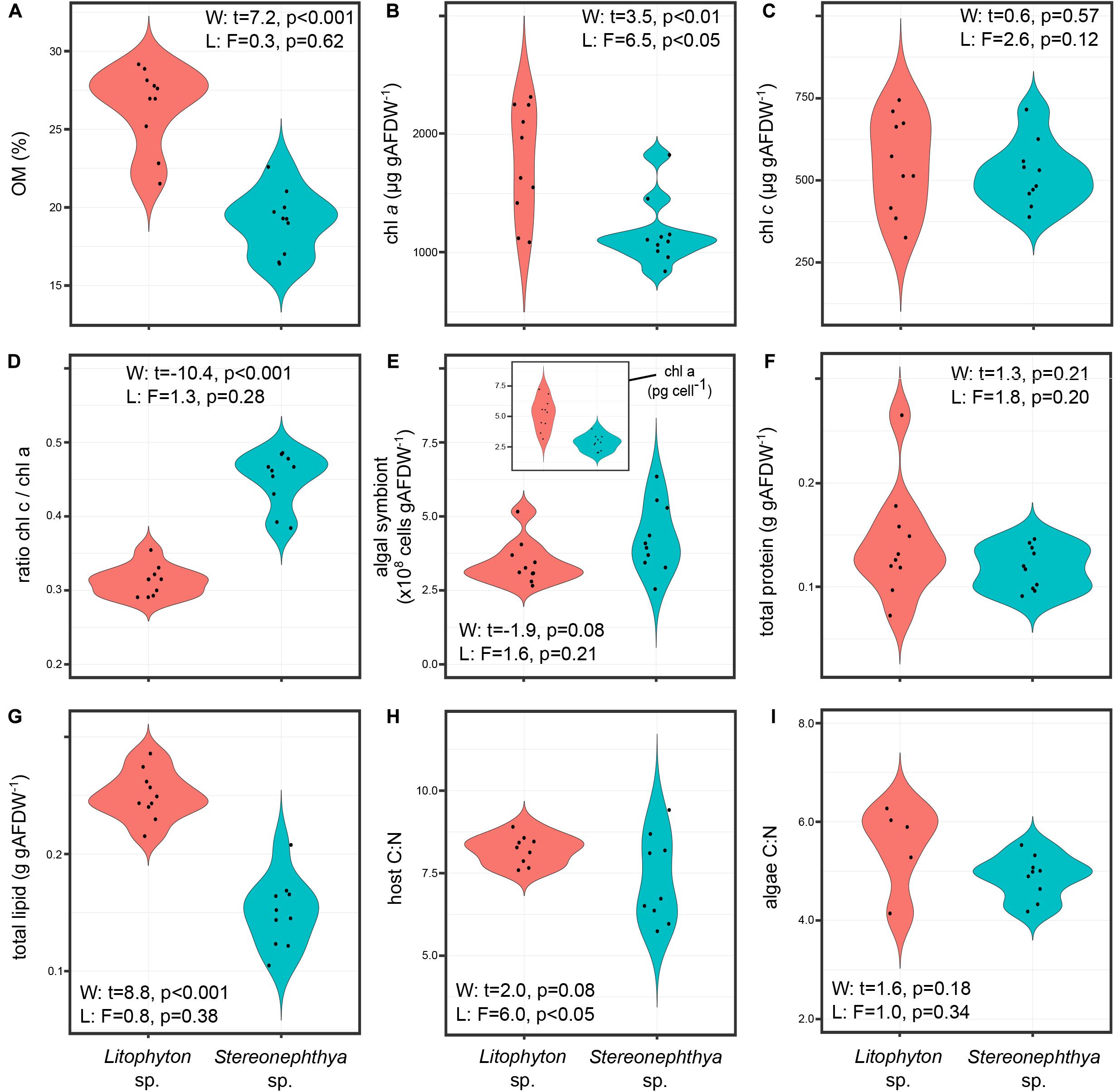
Figure 2. Comparison of the distribution in individual trait values for the two study species: (A) OM, (B) chl a, (C) chl c, (D) ratio chl c/chl a, (E) algal symbiont content – inset content of chl a by algal symbiont cell, (F) total protein, (G) total lipid, (H) host C:N; (I) algae C:N.
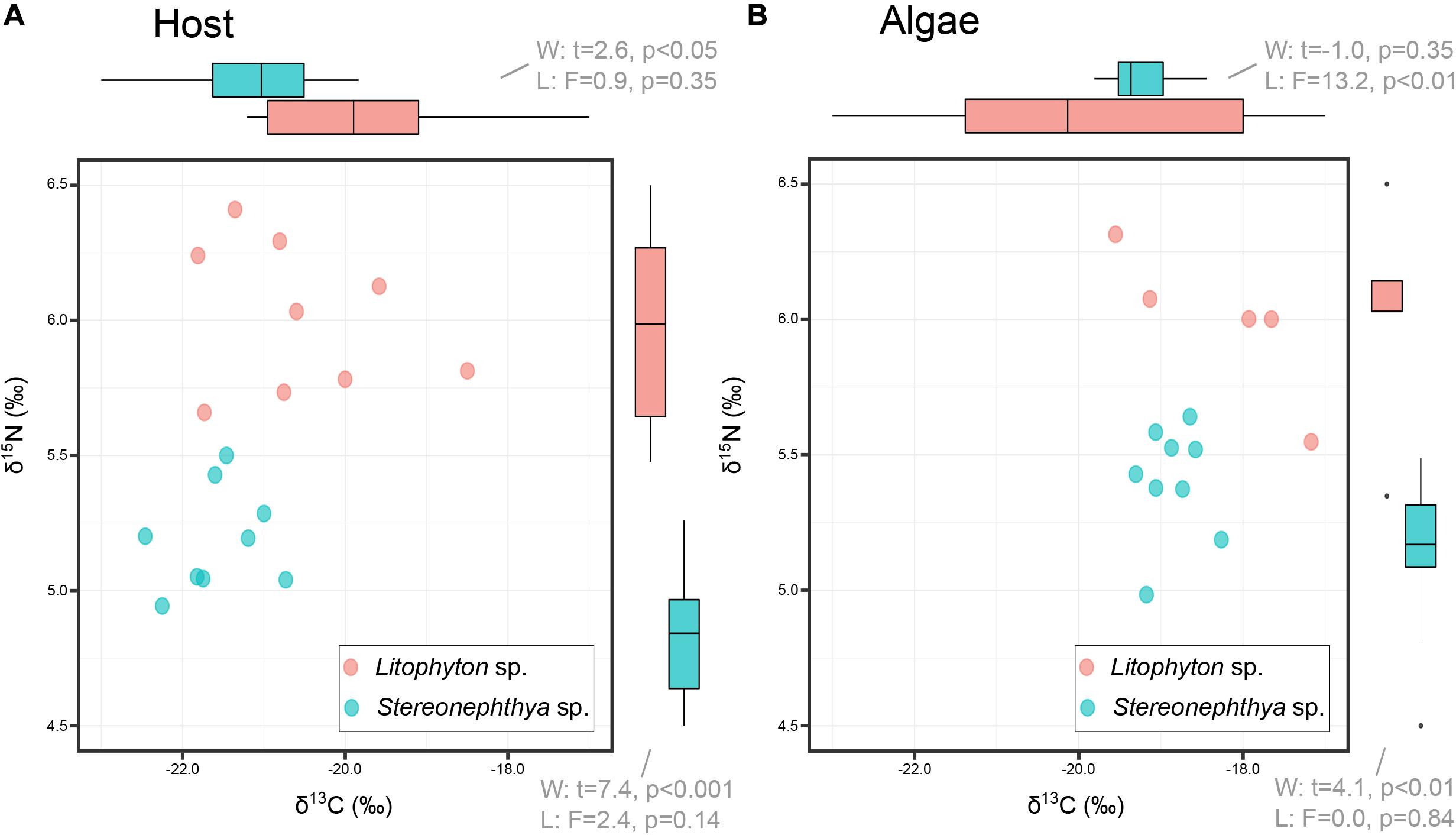
Figure 3. Carbon (δ13C) and nitrogen (δ15N) comparison between octocoral hosts (A) and their associated algae (B). Bi-plots only display full case samples, while boxplot in margins incorporates all measurements available for the variable of interest.
Substantial correlations (Supplementary Figure 1) identified positive relationships between the δ15N in host and algae (Pearson’s r = 0.91, p < 0.001), the chl a and c (Pearson’s r = 0.84, p < 0.001), and the host δ15N and total lipid (Pearson’s r = 0.82, p < 0.01). A negative relationship was detected between the ratio chl c/chl a and host δ15N (Pearson’s r = −0.82, p < 0.001).
The PCA further displayed the variation in individuals for the 13 traits (Figure 4). The first two principal components explained up to 72.2% of the variation observed among individuals, with a clear distinction between the species in the first two dimensions. Intraspecific variations calculated as the dispersion of the species performance niches significantly differed between the two species (Permutation test, F = 9.7, p < 0.05). The multivariate pattern observed in Figure 4 and the significance of the PERMANOVA test (F = 8.3, p < 0.01) further supported a significant difference in how the two species performed, which seemed to be mainly driven by traits differentiating species in their dependence to photosynthetic energy.
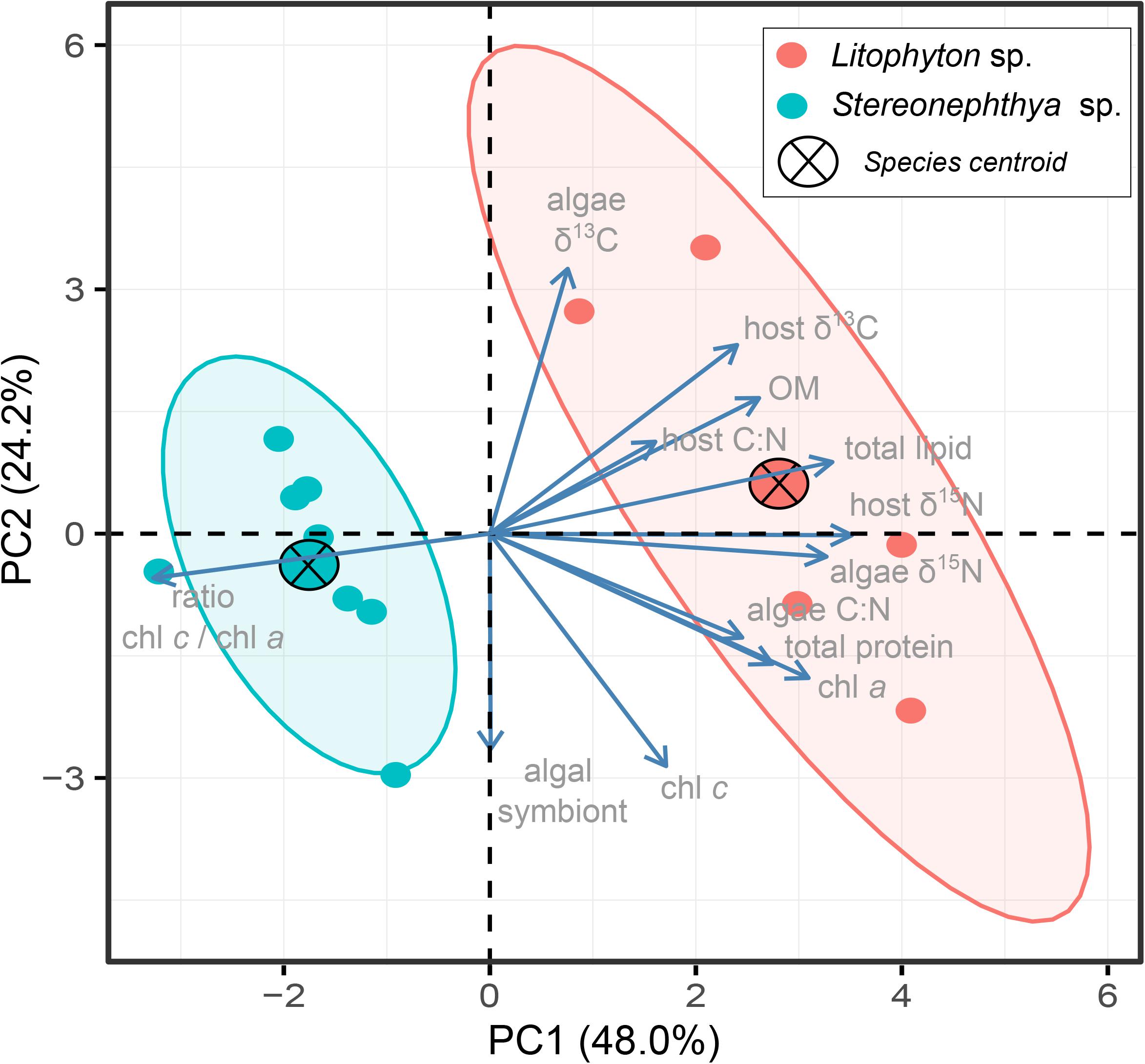
Figure 4. PCA biplot displaying multivariate (trait) variation among individuals. The first two dimensions explained up to 72.2% of the variance observed. Species centroids are displayed, and ellipses are shown with an 80% confidence level for the two species.
Discussion
This study demonstrated that two targeted species are associated with singular Symbiodiniaceae. Traits measured in the partnerships discriminated two distinctive groups supporting contrasting performances. Individual trait variation illustrated differences in species dispersion, which further suggests contrasting niche occupation in these two octocorals living in sympatry in a high-latitude coral community of northern Taiwan.
The genus Stereonephthya is described as an azooxanthellate genus (Fabricius and Alderslade, 2001). However, in the present study, all Stereonephthya sp. colonies were associated with Gerakladium algal symbionts (former clade “G”). A similar partnership was reported with a single unidentified Stereonephthya colony from the Great Barrier Reef (Van Oppen et al., 2005). This association could be commonly occurring in colonies from northern Taiwan, yet its seasonality cannot be excluded and the possibility that this association could be facultative should be further examined. In any case, although intrageneric variation in photosymbiosis is observed in octocorals (e.g., Williams et al., 2010; Aurelle et al., 2017), this proves to be the first confirmed case of an Alcyoniina genus that either exists symbiotically or asymbiotically with Symbiodiniaceae. The association with Gerakladium, a rare taxon for which there is still limited ecological information (LaJeunesse et al., 2018), is also noteworthy. These Symbiodiniaceae were previously found to be associated with some foraminifera (genus Marginopora) (Pochon et al., 2001), excavating sponges (genus Cliona) (Hill et al., 2011), and a black coral (genus Cirrhipathes) (Bo et al., 2011). Our study confirmed its prevalence in one octocoral species, Stereonephthya. Also of interest is the association of Litophyton sp. with Durusdinium algal symbionts (former clade “D”), whereas previous studies documented this genus in association with Symbiodinium (former clade “A”) (Barneah et al., 2004; Pupier et al., 2019). Durusdinium is not unusual in octocorals (Van Oppen et al., 2005), but its occurrence here associated with Litophyton sp. could be related to the environmental conditions of northern Taiwan. For instance, some microalgae symbiotic with scleractinians are known to be extremophile presenting adaptations to survive in regions with large temperature and turbidity fluctuations (LaJeunesse et al., 2018). When applied to octocorals, elucidating the ecological significance of both Symbiodiniaceae requires additional in situ observations and specific laboratory experiments which are beyond the aim of our study.
Litophyton sp. had higher OM, total lipid, chl a, and lower chl c/chl a ratio than Stereonephthya species. Those differences were accompanied by higher host tissue δ13C and δ15N in Litophyton sp. than in Stereonephthya sp., while only algae δ15N was significantly higher in Litophyton sp. (“Durusdinium” associated) than in Stereonephthya sp. (“Gerakladium” associated). In a shallow Caribbean reef lagoon (<3 m, Puerto Morelos, Mexico), Gorgoniidae such as the sea fan, Gorgonia ventalina (58 ± 4%), or the sea rods, Antillogorgia bipinnata (44 ± 2%) and Antillogorgia americana (57 ± 1%), presented higher OM contents (Rossi et al., 2018) than those reported here in the two targeted Alcyoniidae (27 ± 3% and 19 ± 2% in Litophyton sp. and Stereonephthya sp., respectively). In contrast, our values were higher than those reported on other Gorgoniidae sea whip (Pterogorgia citrina, 17 ± 0.2%) or Plexauridae sea rods such as Eunicea sp. (13 ± 1%), Eunicea tourneforti (12 ± 0.4%), and Plexaurella nutans (15 ± 0.3%). However, records of higher values in the Mediterranean Plexauridae sea whip, Paramuricea clavata (40%, Rossi et al., 2006), and similarities between OM content in our bushy Litophyton sp. with the sea rod, Eunicea mammosa (27 ± 1%, Rossi et al., 2018), suggest that neither the morphology nor the phylogenetic information represent a reliable proxy of the OM content in octocorals. Therefore, in contrast with previous hypotheses (Rossi et al., 2018), we suggest that OM content is holobiont-specific while varying to some extent with the changes in environmental conditions. Lipids (0.25 ± 0.02 g gAFDW–1 and 0.15 ± 0.03 g gAFDW–1 in the Litophyton and Stereonephthya, respectively) were within the same range of content reported in Mediterranean P. clavata (0.12–0.32 g gAFDW–1, Rossi et al., 2006). Converted to contribution to the whole-body tissue, lipid concentrations were 7 ± 1% and 3 ± 1% of the DW in our two species, respectively. These concentrations are below values reported in sexually mature colonies of Heteroxenia fuscescens in the northern Red Sea (11 ± 4%, Ben-David-Zaslow and Benayahu, 1999). Protein contents did not differ between species (0.14 ± 0.05 g gAFDW–1 and 0.12 ± 0.02 g gAFDW–1 in Litophyton and Stereonephthya, respectively), yet values reported here were lower than those in horny colonies of P. clavata in which stem and branches are stiffened by gorgonin. Reported to whole-body tissue contribution, proteins represent 37 ± 10% and 22 ± 3% of the DW in Litophyton and Stereonephthya, respectively. This was higher compared to the aforementioned fleshy H. fuscescens, for which proteins averaged 19 ± 6% (Ben-David-Zaslow and Benayahu, 1999). Both lipid and protein contents may vary seasonally with reproductive cycles and abiotic features of the water (Ben-David-Zaslow and Benayahu, 1999). However, to date, no information exists about the reproduction of the two Alcyoniidae in northern Taiwan. Algal symbiont contents (3.4 ± 0.7 × 108 cells gAFDW–1 and 4.3 ± 1.2 × 108 cells gAFDW–1 in Litophyton and Stereonephthya, respectively) were high compared to Gorgoniidae and Plexauridae species previously examined in the Caribbean (Rossi et al., 2018). Of nine species, only one unidentified Eunicea sp. presented comparable contents in algal symbionts (3.9 ± 0.6 × 108 cells gAFDW–1). Values observed were in the range of the ones reported in the other Alcyoniidae (Rhytisma fulvum fulvum) at shallow (−8 m) and mesophotic (−40 m) depths from the northern Red Sea (Pupier et al., 2019). However, Pupier et al. (2019) also documented contents 2.7 times (9.4 × 108 cells gAFDW–1) and 1.8 times (6.4 × 108 cells gAFDW–1) for an unidentified Litophyton populating both depths, respectively. In the latter species, total chlorophyll (a + c2) exhibited lower concentrations in shallow waters (1,591 μg gAFDW–1) than in deeper waters (2,240 μg gAFDW–1, Pupier et al., 2019). Here, total chlorophyll concentrations differed in Litophyton sp. (2,321 ± 613 μg gAFDW–1) and Stereonephthya sp. (1,684 ± 373 μg gAFDW–1), but both ranged within values previously reported among depths [although it is worthwhile to mention that that the concentration of the extracting solvent differed: 100% acetone in Pupier et al. (2019) vs. 90% in the present study]. These values are also within the ranges of values previously documented in species of Gorgoniidae and Plexauridae.
The chl a content in the Red Sea (−4 m, northern Gulf of Eilat) Litophyton arboretum presented intracolonial difference and increased under low light (Berner et al., 1987). Both light-acclimated (0.9 ± 0.1 pg cell–1) and shade-acclimated (2.2 ± 0.5 pg cell–1) areas showed lower concentration of chl a per cell than the two species in the present study (5.2 ± 1.3 pg cell–1 and 2.8 ± 0.6 pg cell–1 in Litophyton and Stereonephthya, respectively), which may infer weaker light intensity at −10 m at Bitou – while the influence of other factors such as the nutrient availability (e.g., Courtial et al., 2018) and/or differential algal symbiont content (e.g., Scheufen et al., 2017) cannot be entirely ruled out. Similarly, Sinularia flexibilis showed an increase of chl a with the attenuation of light from 1,000 to 100 μmol quanta m–2 s–1 (Khalesi et al., 2009). In the scleractinian coral Stylophora pistillata, the increase of chl a content in algal symbiont cells with depth represents another well documented example of photoacclimation to low light habitat (Falkowski and Dubinsky, 1981; Dubinsky et al., 1984; Mass et al., 2010), and this could also be the case here in northern Taiwan (Denis et al., 2019).
Ratio chl c/chl a was significantly higher in Stereonephthya sp. (“Gerakladium” associated) (0.45 ± 0.04) than in Litophyton sp. (“Durusdinium” associated) (0.31 ± 0.02). These values were in the higher range of values reported in Caribbean octocorals (Rossi et al., 2018), suggesting a possible chromatic adaption (quality of light) of algal symbionts to acclimatize to predominantly short-wavelength, blue-light habitat. For instance, this hypothesis was suggested for the scleractinian coral Leptoseris fragilis (Kaiser et al., 1993) but it, generally, remains overlooked in the interpretation of coral photo-acclimatization (Nir et al., 2011). However, this could be a dynamic pattern, as two of the species examined by Rossi et al. (2018) also presented a lower chl c/chl a ratio when sampled at the same location near Puerto Morelos reef lagoon (Ramsby et al., 2014). Algal symbiont cells in hospite being less sensitive to the quality of the light spectra than in isolation, an alternative explanation for this increase would be the total amount of light received by the algal symbionts (quantity of light) that enlarges the size of the photosynthetic antenna to enhance light harvest in low light habitats. Both hypotheses are likely to be holobiont specific [see Reynolds et al. (2008) for the differences in photoprotection among Symbiodiniaceae genera], and the photo-physiology of both partnerships should further be scrutinized in detail in future studies.
Although C:N ratio can represent a reliable proxy of lipid content in marine organisms (e.g., in crabs, Bodin et al., 2007), this was not true of the two species examined here, which further presented similar ratios in both the host and the algal fractions. The δ13C values of hosts suggested slight differences in carbon resource use. Coral host δ15N values were more positive for Litophyton sp. than for Stereonephthya sp. (∼ 1‰), indicating a difference in their trophic positions. For both species, since the δ15N values of the host and algal tissues are similar, the differential utilization of nitrogen by the symbionts that would have been translocated to the host seems limited. The most likely hypothesis is the feeding of the larger size-fractions of phytoplankton and zooplankton by Litophyton sp. and a probable greater contribution of detritus or small size phytoplankton for Stereonephthya species. Several studies have already demonstrated a depletion in δ15N values with decreasing plankton size (Bănaru et al., 2013). Further, stable isotope values observed are representative of scleractinian coral hosts that have acquired carbon and nitrogen by heterotrophic feeding of suspended particulate OM (generally around −22 to −24‰ for carbon; e.g., Muscatine et al., 1989; Ferrier-Pagès et al., 2011). The same range of δ13C values were reported in tissues from non-symbiotic Mediterranean octocorals (−19 to −24‰; Cocito et al., 2013). The clear differences between host and symbiont δ13C values (Δ13C = ∼−3‰) indicate clear deviations from an autotrophic diet (Fox et al., 2018). Although heterotrophy was the main energy source of certain gorgonian species (Baker et al., 2015), findings show that autotrophic nutrition had great importance in octocorals (Rossi et al., 2020). The δ13C range of Litophyton sp. suggests higher trophic plasticity than for Stereonephthya sp., indicating diversification of basal resources with varying δ13C values. Litophyton sp. seems to vary its use of heterotrophic nutrient sources, but may also use the autotrophic pathway. In scleractinians corals, facultative associations are commonly associated with parasitic behavior (Lesser et al., 2013). Therefore, it could here explain why Gerakladium does not appear to contribute to the diversification of Stereonephthya diet. However, the paucity of information available on this Symbiodiniaceae lineage prevents further discussion. A more detailed view of the trophic ecology of these two partnerships will require additional samples from various habitats and conditions (e.g., reproduction period; Rossi et al., 2020), as well as from all their possible food sources. Those data, together with insight from 13C-labeling experiment (e.g., Ezzat et al., 2017), might further support evidence of niche partitioning that may explain the coexistence of these two phylogenetically and morphologically similar species.
Altogether, the examined traits elucidated two distinct groups which could be further explained by both partnerships considered. They illustrated contrasting behaviors where Litophyton sp., in association with Durusdinium algal symbionts, appears to complement its heterotrophic diet with slight autotrophically acquired energy. This diversification was reflected among individuals of Litophyton with chl a and δ13C measured in the algal fraction presenting higher variance than Stereonephthya colonies. This variation may have further relevance at a biogeographical level and/or within the Litophyton genus. Pupier et al. (2019) typified a Red Sea Litophyton characterized by its high ratio between photosynthetically acquired carbon and respiration. In contrast, Stereonephthya sp. seems to be much more specialized and may only receive limited benefit from its symbiotic relationship with Gerakladium. Partnership performance niches are clearly distinctive in terms of their positions and sizes, which could be of major importance in individuals naturally selected through exposure to stressors. Interestingly, recent bleaching survey (August 2020) revealed that Litophyton sp. was virtually the only species affected −10 m at the study site. All colonies were severely bleached while no sign of mortality was recorded (V. Denis, personal observation). Quantification of performance traits of both species in variable (extreme) conditions would lead to a better understanding of their individual variation and physiological flexibility. Although species niche delineation will eventually require a detailed examination of the temporal and spatial variation in traits and partnerships, our study already paves the way for the integration of the intraspecific variance in the definition of octocoral strategies.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication. VD conceived and designed the experiments. T-HH, LC, YH, T-YL, C-WW, C-YH, S-HY, and P-LW performed the experiments. T-HH and LC analyzed the data. T-HH, LC, NS, and VD wrote the manuscript.
Funding
This work was funded by the Ministry of Science and Technology (MOST) of Taiwan through grants to VD (nos. 108-2611-002-013 and 109-2611-M-002-017) and P-LW (108-2116-M-002-004). The Ministry of Education of Taiwan also provided funds to P-LW for this study (108L901002). LC was the recipient of a grant from the Foundation Pierre Ledoux (2018/19).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Authors would like to express their gratitude to Yehuda Benayahu and Catherine S. McFadden’s help with the identification of the octocorals and their comments on this manuscript. In addition, the authors thank Sen-Lin Tang for his support for molecular experiments and Jing-Yi Tseng for the stable isotope analysis. Authors are further grateful to Vicky Yuting Lin for contributing to the collection of the wrong species in the field which led to this interesting discovery of Gerakladium associated with Stereonephthya. Authors also acknowledge all of the FRE lab members for their advice on the preparation of this manuscript. Finally, authors thank reviewers for their constructive comments and suggestions on this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.606601/full#supplementary-material
Supplementary Figure 1 | Pearson correlation matrix of trait values. Correlation coefficients (r) are provided with a color scale indicating either a highly positive (r = 1) or negative (r = −1) correlation. Only significant and substantial correlations with a | r | > 0.8 were mentioned in the text. Non-significant coefficient (p < 0.05) is shown crossed.
Supplementary Table 1 | Samples examined for the identity of dominant algal symbiont and accession numbers for the corresponding sequences deposited in GenBank.
Supplementary Table 2 | Mean, standard deviation (SD), coefficient of variation (CV), maximum (Max), and minimum (Min) values of the 13 traits and chl a content by symbiont algal cell in the two octocoral species. Significant differences detected by Welch t-tests are indicated by level of significance ∗p < 0.05, ∗∗p < 0.005, ∗∗∗p < 0.001; details on statistics in Figures 2, 3 (English editing).
References
Aharonovich, D., and Benayahu, Y. (2012). Microstructure of octocoral sclerites for diagnosis of taxonomic features. Mar. Biodivers. 42, 173–177. doi: 10.1007/s12526-011-0102-103
Aurelle, D., Pivotto, I. D., Malfant, M., Topçu, N. E., Masmoudi, M. B., Chaoui, L., et al. (2017). Fuzzy species limits in Mediterranean gorgonians (Cnidaria, Octocorallia): inferences on speciation processes. Zool. Scripta 46, 767–778. doi: 10.1111/zsc.12245
Baker, D. M., Freeman, C. J., Knowlton, N., Thacker, R. W., Kim, K., and Fogel, M. L. (2015). Productivity links morphology, symbiont specificity and bleaching in the evolution of Caribbean octocoral symbioses. ISME J. 9, 2620–2629. doi: 10.1038/ismej.2015.71
Baker, D. M., Webster, K. L., and Kim, K. (2010). Caribbean octocorals record changing carbon and nitrogen sources from 1862 to 2005. Global Change Biol. 16, 2701–2710. doi: 10.1111/j.1365-2486.2010.02167.x
Bănaru, D., Carlotti, F., Barani, A., Grégori, G., Neffati, N., and Harmelin-Vivien, M. (2013). Seasonal variation of stable isotope ratios of size-fractionated zooplankton in the Bay of Marseille (NW Mediterranean Sea). J. Plankton Res. 36, 145–156. doi: 10.1093/plankt/fbt083
Barneah, O., Weis, V. M., Perez, S., and Benayahu, Y. (2004). Diversity of dinoflagellate symbionts in red sea soft corals: mode of symbiont acquisition matters. Mar. Ecol. Prog. Ser. 275, 89–95. doi: 10.3354/meps275089
Bednarz, V. N., Cardini, U., van Hoytema, N., Al-Rshaidat, M. M. D., and Wild, C. (2015). Seasonal variation in dinitrogen fixation and oxygen fluxes associated with two dominant zooxanthellate soft corals from the northern Red Sea. Mar. Ecol. Prog. Ser. 519, 141–152. doi: 10.3354/meps11091
Bellwood, D. R., Streit, R. P., Brandl, S. J., and Tebbett, S. B. (2018). The meaning of the term ‘function’ in ecology: a coral reef perspective. Funct. Ecol. 33, 948–961. doi: 10.1111/1365-2435.13265
Ben-David-Zaslow, R., and Benayahu, Y. (1999). Temporal variation in lipid, protein and carbohydrate content in the Red Sea soft coral Heteroxenia fuscescens. J. Mar. Biol. Assoc. U K. 79, 1001–1006. doi: 10.1017/s002531549900123x
Berner, T., Achituv, Y., Dubinsky, Z., and Benayahu, Y. (1987). Pattern of distribution and adaptation to different irradiance levels of zooxanthellae in the soft coral Litophyton arboreum (Octocorallia, Alcyonacea). Symbiosis 3, 23–39.
Bo, M., Baker, A. C., Gaino, E., Wirshing, H. H., Scoccia, F., and Bavestrello, G. (2011). First description of algal mutualistic endosymbiosis in a black coral (Anthozoa: Antipatharia). Mar. Ecol. Prog. Ser. 435, 1–11. doi: 10.3354/meps09228
Bodin, N., Le Loc’h, F., and Hily, C. (2007). Effect of lipid removal on carbon and nitrogen stable isotope ratios in crustacean tissues. J. Exp. Mar. Biol. Ecol. 341, 168–175. doi: 10.1016/j.jembe.2006.09.008
Cocito, S., Ferrier-Pagès, C., Cupido, R., Rottier, C., Meier-Augenstein, W., Kemp, H., et al. (2013). Nutrient acquisition in four Mediterranean gorgonian species. Mar. Ecol. Prog. Ser. 473, 179–188. doi: 10.3354/meps10037
Coma, R., Llorente-Llurba, E., Serrano, E., Gili, J.-M., and Ribes, M. (2015). Natural heterotrophic feeding by a temperate octocoral with symbiotic zooxanthellae: a contribution to understanding the mechanisms of die-off events. Coral Reefs 34, 549–560. doi: 10.1007/s00338-015-1281-1283
Coma, R., Ribes, M., Gili, J.-M., and Zabala, M. (1998). An energetic approach to the study of life-history traits of two modular colonial benthic invertebrates. Mar. Ecol. Prog. Ser. 162, 89–103. doi: 10.3354/meps162089
Coplen, T. B. (2011). Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass. Spectrom 25, 2538–2560. doi: 10.1002/rcm.5129
Coppari, M., Zanella, C., and Rossi, S. (2019). The importance of coastal gorgonians in the blue carbon budget. Sci. Rep. 9:13550. doi: 10.1038/s41598-019-49797-49794
Courtial, L., Planas Bielsa, V., Houlbreque, F., and Ferrier-Pages, C. (2018). Effects of ultraviolet radiation and nutrient level on the physiological response and organic matter release of the scleractinian coral Pocillopora damicornis following thermal stress. PLoS One 13:e0205261. doi: 10.1371/journal.pone.0205261
Denis, V., Soto, D., De Palmas, S., Lin, Y. T. V., Benayahu, Y., Huang, Y. M., et al. (2019). “Taiwan,” in Mesophotic Coral Ecosystems, eds Y. Loya, K. A. Puglise, and T. C. L. Bridge (Cham: Springer International Publishing), 249–264. doi: 10.1007/978-3-319-92735-0_14
Dubinsky, Z., Falkowski, P. G., Porter, J. W., Muscatine, L., and Smith, D. C. (1984). Absorption and utilization of radiant energy by light- and shade-adapted colonies of the hermatypic coral Stylophora pistillata. Proc. Royal Soc. Lond. Ser. B. Biol. Sci. 222, 203–214. doi: 10.1098/rspb.1984.0059
Etnoyer, P., and Warrenchuk, J. (2007). A catshark nursery in a deep gorgonian field in the Mississippi Canyon, Gulf of Mexico. Bull. Mar. Sci. 81, 553–559.
Ezzat, L., Fine, M., Maguer, J.-F., Grover, R., and Ferrier-Pagès, C. (2017). Carbon and nitrogen acquisition in shallow and deep holobionts of the scleractinian coral S. pistillata. Front. Mar. Sci. 4:102. doi: 10.3389/fmars.2017.00102
Fabricius, K., and Alderslade, P. (2001). Soft Corals and Sea Fans: A Comprehensive Guide to the Tropical Shallow Water Genera of the Central-west Pacific, the Indian Ocean and the Red Sea. Townsville, TSV: AIMS.
Falkowski, P. G., and Dubinsky, Z. (1981). Light-shade adaptation of Stylophora pistillata, a hermatypic coral from the Gulf of Eilat. Nature 289, 172–174. doi: 10.1038/289172a0
Ferrier-Pagès, C., Peirano, A., Abbate, M., Cocito, S., Negri, A., Rottier, C., et al. (2011). Summer autotrophy and winter heterotrophy in the temperate symbiotic coral Cladocora caespitosa. Limnol. Oceanog. 56, 1429–1438. doi: 10.4319/lo.2011.56.4.1429
Folch, J., Lees, M., and Sloane Stanley, G. H. (1957). A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509.
Fox, M. D., Williams, G. J., Johnson, M. D., Radice, V. Z., Zgliczynski, B. J., Kelly, E. L. A., et al. (2018). Gradients in primary production predict trophic strategies of Mixotrophic corals across spatial scales. Curr. Biol. 28, 3355–3363.e4. doi: 10.1016/j.cub.2018.08.057
Gerhart, D. J. (1990). Fouling and gastropod predation: consequences of grazing for a tropical octocoral. Mar. Ecol. Prog. Ser. 62, 103–108. doi: 10.3354/meps062103
Gerz, M., Guillermo Bueno, C., Ozinga, W. A., Zobel, M., Moora, M., and van der Heijden, M. (2018). Niche differentiation and expansion of plant species are associated with mycorrhizal symbiosis. J. Ecol. 106, 254–264. doi: 10.1111/1365-2745.12873
Gori, A., Viladrich, N., Gili, J. M., Kotta, M., Cucio, C., Magni, L., et al. (2012). Reproductive cycle and trophic ecology in deep versus shallow populations of the Mediterranean gorgonian Eunicella singularis (Cap de Creus, northwestern Mediterranean Sea). Coral Reefs 31, 823–837. doi: 10.1007/s00338-012-0904-901
Hill, M., Allenby, A., Ramsby, B., Schonberg, C., and Hill, A. (2011). Symbiodinium diversity among host clionaid sponges from Caribbean and Pacific reefs: evidence of heteroplasmy and putative host-specific symbiont lineages. Mol. Phylogenet. Evol. 59, 81–88. doi: 10.1016/j.ympev.2011.01.006
Hughes, T. P., Kerry, J. T., Baird, A. H., Connolly, S. R., Dietzel, A., Eakin, C. M., et al. (2018). Global warming transforms coral reef assemblages. Nature 556, 492–496. doi: 10.1038/s41586-018-0041-42
Jeffrey, S. W., and Humphrey, G. F. (1975). New spectrophotometric equations for determining chlorophyll a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochemie und Physiol. der Pflanzen 167, 191–194. doi: 10.1016/s0015-3796(17)30778-3
Jeng, M. S., Huang, H. D., Dai, C. F., Hsiao, Y. C., and Benayahu, Y. (2011). Sclerite calcification and reef-building in the fleshy octocoral genus Sinularia (Octocorallia: Alcyonacea). Coral Reefs 30, 925–933. doi: 10.1007/s00338-011-0765-z
Kaiser, P., Schlichter, D., and Fricke, H. W. (1993). Influence of light on algal symbionts of the deep water coral Leptoseris fragilis. Mar. Biol. 117, 45–52. doi: 10.1007/BF00346424
Kearney, M., Simpson, S. J., Raubenheimer, D., and Helmuth, B. (2010). Modelling the ecological niche from functional traits. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 3469–3483. doi: 10.1098/rstb.2010.0034
Khalesi, M. K., Beeftink, H. H., and Wijffels, R. H. (2009). Light-dependency of growth and secondary metabolite production in the captive zooxanthellate soft coral Sinularia flexibilis. Mar. Biotechnol. (NY) 11, 488–494. doi: 10.1007/s10126-008-9164-z
LaJeunesse, T. C., Parkinson, J. E., Gabrielson, P. W., Jeong, H. J., Reimer, J. D., Voolstra, C. R., et al. (2018). Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 28, 2570–2580. doi: 10.1016/j.cub.2018.07.008
Lesser, M. P., Stat, M., and Gates, R. D. (2013). The endosymbiotic dinoflagellates (Symbiodinium sp.) of corals are parasites and mutualists. Coral Reefs 32, 603–611. doi: 10.1007/s00338-013-1051-z
Lin, Y. T. V., and Denis, V. (2019). Acknowledging differences: number, characteristics, and distribution of marine benthic communities along Taiwan coast. Ecosphere 10:e02803. doi: 10.1002/ecs2.2803
Mass, T., Kline, D. I., Roopin, M., Veal, C. J., Cohen, S., Iluz, D., et al. (2010). The spectral quality of light is a key driver of photosynthesis and photoadaptation in Stylophora pistillata colonies from different depths in the Red Sea. J. Exp. Biol. 213, 4084–4091. doi: 10.1242/jeb.039891
McFadden, C. S., Benayahu, Y., Pante, E., Thoma, J. N., Nevarez, P. A., and France, S. C. (2011). Limitations of mitochondrial gene barcoding in Octocorallia. Mol. Ecol. Resour. 11, 19–31. doi: 10.1111/j.1755-0998.2010.02875.x
McFadden, C. S., France, S. C., Sanchez, J. A., and Alderslade, P. (2006). A molecular phylogenetic analysis of the Octocorallia (Cnidaria: Anthozoa) based on mitochondrial protein-coding sequences. Mol. Phylogenet. Evol. 41, 513–527. doi: 10.1016/j.ympev.2006.06.010
McWilliam, M., Hoogenboom, M. O., Baird, A. H., Kuo, C. Y., Madin, J. S., and Hughes, T. P. (2018). Biogeographical disparity in the functional diversity and redundancy of corals. Proc. Natl. Acad. Sci. U S A. 115, 3084–3089. doi: 10.1073/pnas.1716643115
Muscatine, L., Porter, J. W., and Kaplan, I. R. (1989). Resource partitioning by reef corals as determined from stable isotope composition. I. δ13C of zooxanthellae and animal tissue vs depth. Mar. Biol. 100, 185–193. doi: 10.1007/Bf00391957
Nir, O., Gruber, D. F., Einbinder, S., Kark, S., and Tchernov, D. (2011). Changes in scleractinian coral Seriatopora hystrix morphology and its endocellular Symbiodinium characteristics along a bathymetric gradient from shallow to mesophotic reef. Coral Reefs 30, 1089–1100. doi: 10.1007/s00338-011-0801-z
Noda, H., Parkinson, J. E., Yang, S. Y., and Reimer, J. D. (2017). A preliminary survey of zoantharian endosymbionts shows high genetic variation over small geographic scales on Okinawa-jima Island, Japan. PeerJ 5:e3740. doi: 10.7717/peerj.3740
O’Neal, W., and Pawlik, J. R. (2002). A reappraisal of the chemical and physical defenses of Caribbean gorgonian corals against predatory fishes. Mar. Ecol. Prog. Ser. 240, 117–126. doi: 10.3354/meps240117
Pochon, X., Pawlowski, J., Zaninetti, L., and Rowan, R. (2001). High genetic diversity and relative specificity among symbiodinium-like endosymbiotic dinoflagellates in soritid foraminiferans. Mar. Biol. 139, 1069–1078. doi: 10.1007/s002270100674
Pupier, C. A., Bednarz, V. N., and Ferrier-Pagès, C. (2018). Studies with soft corals – recommendations on sample processing and normalization metrics. Front. Mar. Sci. 5:348. doi: 10.3389/fmars.2018.00348
Pupier, C. A., Fine, M., Bednarz, V. N., Rottier, C., Grover, R., and Ferrier-Pages, C. (2019). Productivity and carbon fluxes depend on species and symbiont density in soft coral symbioses. Sci. Rep. 9:17819. doi: 10.1038/s41598-019-54209-54208
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Ramsby, B. D., Shirur, K. P., Iglesias-Prieto, R., and Goulet, T. L. (2014). Symbiodinium photosynthesis in Caribbean octocorals. PLoS One 9:e106419. doi: 10.1371/journal.pone.0106419
Reijnen, B. T., van der Meij, S. E., and van Ofwegen, L. P. (2011). Fish, fans and hydroids: host species of pygmy seahorses. Zookeys 103, 1–26. doi: 10.3897/zookeys.103.953
Reynolds, J. M., Bruns, B. U., Fitt, W. K., and Schmidt, G. W. (2008). Enhanced photoprotection pathways in symbiotic dinoflagellates of shallow-water corals and other cnidarians. Proc. Natl. Acad. Sci. U S A. 105, 13674–13678. doi: 10.1073/pnas.0805187105
Ribes, M., Coma, R., and Gili, J.-M. (1998). Heterotrophic feeding by gorgonian corals with symbiotic zooxanthella. Limnol. Oceanog. 43, 1170–1179. doi: 10.4319/lo.1998.43.6.1170
Rossi, S., Coppari, M., and Viladrich, N. (2017). “Benthic-Pelagic coupling: new perspectives in the animal forests,” in Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots, eds S. Rossi, L. Bramanti, A. Gori, and C. Orejas (Cham: Springer International Publishing), 855–885. doi: 10.1007/978-3-319-21012-4_23
Rossi, S., Gili, J.-M., Coma, R., Linares, C., Gori, A., and Vert, N. (2006). Temporal variation in protein, carbohydrate, and lipid concentrations in Paramuricea clavata (Anthozoa, Octocorallia): evidence for summer–autumn feeding constraints. Mar. Biol. 149, 643–651. doi: 10.1007/s00227-005-0229-225
Rossi, S., Schubert, N., Brown, D., Gonzalez-Posada, A., and Soares, M. O. (2020). Trophic ecology of Caribbean octocorals: autotrophic and heterotrophic seasonal trends. Coral Reefs 39, 433–449. doi: 10.1007/s00338-020-01906-w
Rossi, S., Schubert, N., Brown, D., Soares, M. O., Grosso, V., Rangel-Huerta, E., et al. (2018). Linking host morphology and symbiont performance in octocorals. Sci. Rep. 8:12823. doi: 10.1038/s41598-018-31262-31263
Sánchez, J. A. (2016). “Diversity and evolution of octocoral animal forests at both sides of tropical America,” in Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots, eds S. Rossi, L. Bramanti, A. Gori, and C. Orejas (Cham: Springer International Publishing), 1–33. doi: 10.1007/978-3-319-17001-5_39-1
Scheufen, T., Kramer, W. E., Iglesias-Prieto, R., and Enriquez, S. (2017). Seasonal variation modulates coral sensibility to heat-stress and explains annual changes in coral productivity. Sci. Rep. 7:4937. doi: 10.1038/s41598-017-04927-4928
Schlichter, D., Svoboda, A., and Kremer, B. P. (1983). Functional autotrophy of Heteroxenia fuscescens (Anthozoa: Alcyonaria): carbon assimilation and translocation of photosynthates from symbionts to host. Mar. Biol. 78, 29–38. doi: 10.1007/BF00392968
Schubert, N., Brown, D., and Rossi, S. (2017). “Symbiotic versus non-symbiotic octocorals: physiological and ecological implications,” in Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots, eds S. Rossi, L. Bramanti, A. Gori, and C. Orejas (Cham: Springer International Publishing), 887–918. doi: 10.1007/978-3-319-21012-4_54
Slattery, M., Pankey, M. S., and Lesser, M. P. (2019). Annual thermal stress increases a soft coral’s susceptibility to bleaching. Sci. Rep. 9:8064. doi: 10.1038/s41598-019-44566-44569
Sorokin, Y. (1991). Biomass, metabolic rates and feeding of some common reef zoantharians and octocorals. Aus. J. Mar. Freshwater Res. 42, 729–741. doi: 10.1071/MF9910729
Sturaro, N., Hsieh, Y. E., Chen, Q., Wang, P. L., and Denis, V. (2020). Toward a standardised protocol for the stable isotope analysis of scleractinian corals. Rapid Commun. Mass Spectrom. 34:e8663. doi: 10.1002/rcm.8663
van Ofwegen, L. (2016). The genus litophyton forskål, 1775 (Octocorallia, Alcyonacea, Nephtheidae) in the Red Sea and the western Indian Ocean. ZooKeys 567, 1–128. doi: 10.3897/zookeys.567.7212
Van Oppen, M. J., Mieog, J. C., Sanchez, C. A., and Fabricius, K. E. (2005). Diversity of algal endosymbionts (zooxanthellae) in octocorals: the roles of geography and host relationships. Mol. Ecol. 14, 2403–2417. doi: 10.1111/j.1365-294X.2005.02545.x
Vercelloni, J., Liquet, B., Kennedy, E. V., Gonzalez-Rivero, M., Caley, M. J., Peterson, E. E., et al. (2020). Forecasting intensifying disturbance effects on coral reefs. Glob. Chang Biol. 26, 2785–2797. doi: 10.1111/gcb.15059
Violle, C., Enquist, B. J., McGill, B. J., Jiang, L., Albert, C. H., Hulshof, C., et al. (2012). The return of the variance: intraspecific variability in community ecology. Trends Ecol. Evol. 27, 244–252. doi: 10.1016/j.tree.2011.11.014
Violle, C., Navas, M.-L., Vile, D., Kazakou, E., Fortunel, C., Hummel, I., et al. (2007). Let the concept of trait be functional! Oikos. Oikos 116, 882–892. doi: 10.1111/j.0030-1299.2007.15559.x
Wheeler, D. A., Srinivasan, M., Egholm, M., Shen, Y., Chen, L., McGuire, A., et al. (2008). The complete genome of an individual by massively parallel DNA sequencing. Nature 452, 872–876. doi: 10.1038/nature06884
Keywords: stable isotope, intraspecific variation, trade-off, plasticity, functionality, algal symbiont, trait-based approach, soft corals
Citation: Hsu T-HT, Carlu L, Hsieh YE, Lai T-YA, Wang C-W, Huang C-Y, Yang S-H, Wang P-L, Sturaro N and Denis V (2020) Stranger Things: Organismal Traits of Two Octocorals Associated With Singular Symbiodiniaceae in a High-Latitude Coral Community From Northern Taiwan. Front. Mar. Sci. 7:606601. doi: 10.3389/fmars.2020.606601
Received: 15 September 2020; Accepted: 07 December 2020;
Published: 29 December 2020.
Edited by:
Oren Levy, Bar-Ilan University, IsraelReviewed by:
Susana Enríquez, National Autonomous University of Mexico, MexicoClaudia Pogoreutz, University of Konstanz, Germany
Copyright © 2020 Hsu, Carlu, Hsieh, Lai, Wang, Huang, Yang, Wang, Sturaro and Denis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vianney Denis, dmlhbm5leWRlbmlzQGcubnR1LmVkdS50dw==; dmlhbm5leS5kZW5pc0BnbWFpbC5jb20=
 Tsai-Hsuan Tony Hsu
Tsai-Hsuan Tony Hsu Lilian Carlu
Lilian Carlu Yunli Eric Hsieh
Yunli Eric Hsieh Tzu-Yu Angel Lai
Tzu-Yu Angel Lai Ching-Wei Wang
Ching-Wei Wang Ching-Yun Huang
Ching-Yun Huang Shan-Hua Yang
Shan-Hua Yang Pei-Ling Wang
Pei-Ling Wang Nicolas Sturaro
Nicolas Sturaro Vianney Denis
Vianney Denis