- 1Department of Ocean Systems, NIOZ Royal Netherlands Institute for Sea Research, Den Burg, Netherlands
- 2Fisheries and Oceans Canada (DFO), Bedford Institute of Oceanography, Dartmouth, NS, Canada
- 3Department of Biological Sciences and K.G. Jebsen Centre for Deep-sea Research, University of Bergen, Bergen, Norway
The Scotian Shelf harbors unique aggregations of the glass sponge Vazella pourtalesii that provides an important habitat for benthic and pelagic fauna. Recent studies have shown that these sponge grounds have persisted in the face of strong inter-annual and multi-decadal variability in temperature and salinity. However, little is known of these environmental characteristics on hourly-seasonal time scales. This study presents the first hydrodynamic observations and associated (food) particle supply mechanisms for the Vazella sponge grounds, highlighting the influence of natural variability in environmental conditions on sponge growth and resilience. Near-bottom environmental conditions were characterized by high temporal resolution data collected with a benthic lander, deployed during a period of 10 months in the Sambro Bank Sponge Conservation Area. The lander was equipped with temperature and oxygen sensors, a current meter, a sediment trap and a video camera. In addition, water column profiles of temperature and salinity were collected in an array across the sponge grounds from high to lower sponge presence probability. Over the course of the lander deployment, temperature fluctuated between 8.8–12°C with an average of 10.6 ± 0.4°C. Dissolved oxygen concentration was on average 6.3 mg l–1, and near-bottom current speed was on average 0.12 m s–1, with peaks up to 0.47 m s–1. Semi-diurnal tidal currents promoted constant resuspension of particulate matter in the benthic boundary layer. Surface storm events episodically caused extremely turbid conditions on the seafloor that persisted for several days, with particles being resuspended to more than 13 m above the seabed. The carbon flux in the near-bottom sediment trap peaked during storm events and also after a spring bloom in April, when fresh phytodetritus was observed in the bottom boundary layer. While resuspension events can represent a major stressor for sponges, limiting their filtration capability and remobilizing them, episodes of strong currents and lateral particle transport likely play an important role in food supply and the replenishment of nutrients and oxygen. Our results contextualize human-induced threats such as bottom fishing and climate change by providing more knowledge of the natural environmental conditions under which sponge grounds persist.
Introduction
Sponges are one of the most common megafaunal organisms in the deep sea (Tabachnick et al., 1994). They can have a scattered distribution with low abundances, but under certain environmental conditions may form dense monospecific or mixed sponge aggregations, and even reef-like ecosystems (Maldonado et al., 2017). Sponge aggregations provide ecosystem goods and services by providing shelter from predation, breeding grounds and substrate for many associated species including commercially important fish species (Kutti et al., 2015b), thereby enhancing biodiversity and biomass (Rice et al., 1990; Fuller and Cameron, 1998; Beazley et al., 2013; Beazley et al., 2015; Maldonado et al., 2017; Meyer et al., 2019). Moreover, sponges play a key ecological role in benthic-pelagic coupling by filtering substantial volumes of water (up to 24,000 L per kg–1 of sponge day–1 in temperate and tropical shallow-water sponge grounds; Leys et al., 2007; Maldonado et al., 2012; Pham et al., 2019). Furthermore, they can release particulate detritus to the associated benthic environment (Reiswig, 1971), a pathway referred to as the sponge loop (Rix et al., 2016). Consequently they transfer energy and nutrients from the pelagic to the benthic environment, and play an important role in carbon, nitrogen, phosphate and silicon cycling (Maldonado et al., 2012; Kutti et al., 2013; Cathalot et al., 2015; Maldonado et al., 2020).
In the North Atlantic, deep-sea aggregations of demosponges (especially Astrophorina) and glass sponges (Hexactinellida) have been found along continental shelves, slopes, seamounts, mid-ocean ridges and canyons, especially at depths between 200 to 3000 m (Tabachnick et al., 1994). These sponge ecosystems either occur as mono-specific or multi-species assemblages. At boreal and boreo-arctic latitudes mixed species assemblages have often been found, like for example on the Arctic Mid-Ocean Ridge (Meyer et al., 2019) and on the northwest Atlantic Canadian margin (Murillo et al., 2012; Murillo et al., 2018). At temperate latitudes monospecific aggregations of glass sponges seem to be a dominant feature, such as for example the Pheronema carpenteri grounds in the northeast Atlantic Ocean (Rice et al., 1990), Geodia sp. grounds in the western Barents Sea (Klitgaard and Tendal, 2004) and mono-specific Vazella pourtalesii grounds in the northwest Atlantic along the Canadian continental shelf (Beazley et al., 2013). The dominant presence of particular sponge species has been related to local environmental conditions such as substrate type, topography, as well as the hydrography (Rice et al., 1990; Chu and Leys, 2010; Murillo et al., 2018; Roberts et al., 2018; Davison et al., 2019). Hexactinellids are most abundant and diverse in the deep ocean on steep rocky substrates with low amounts of suspended sediment in the water column and occur less often on flat shelves (Farrow et al., 1983; Maldonado et al., 2017). Other species, such as Pheronema carpenteri, are able to build a basal specular component (comparable to roots) enabling them to anchor in soft substrate (Barthel et al., 1996).
The Scotian Shelf, the continental shelf off Nova Scotia, eastern Canada, harbors unique aggregations of the rosellid glass sponge V. pourtalesii (Schmidt 1870), commonly known as “Russian hats” (Fuller and Cameron, 1998). This species is distributed along the continental margin of eastern North America, from the Florida Keys in the southern US, where it occurs in low densities, to the Scotian Shelf, where it forms extensive monospecific aggregations between ∼150 and 240 m depth in The Emerald Basin, reaching average densities of 3.8 ind. m–2 (Hawkes et al., 2019; Maldonado et al., 2020). Single individuals were found in an even broader depth range between ∼100 to 498 m depth on the Scotian Shelf (Beazley et al., 2018). High species abundance is likely linked to the high primary productivity on the Scotian Shelf (102 g C m–2 year–1; Mills and Fournier, 1979), providing an important food source for the sponges and their associated fauna. Accumulations of V. pourtalesii harbor an enhanced biodiversity and species abundance of other invertebrates such as shrimp, crabs and anemones. Furthermore, analysis of catch data revealed a significantly different fish community associated with the sponge grounds (Fuller, 2011; Hawkes et al., 2019). In order to protect these sponges from bottom fishing gear Fisheries and Oceans Canada (DFO) established two sponge conservation areas in 2013, the Emerald Basin Sponge Conservation Area (197 km2) and the Sambro Bank Sponge Conservation Area (62 km2) (referred to as the ‘closure areas’ thereafter).
Of 35 environmental predictor layers (representing ocean terrain, fishing effort, and physical oceanographic characteristics) minimum bottom temperature and summer primary production layers were most important in a random forest model used to predict the distribution of V. pourtalesii on the Scotian Shelf (Beazley et al., 2018). Variability of environmental factors (e.g., temperature) is defined by the variability of the main water masses entering the Scotian Shelf (McLellan, 1957; Urrego-Blanco and Sheng, 2014), which lies at the confluence of two large ocean gyre currents, the Gulf Stream flowing into a northward direction and the Labrador Current flowing in an equatorward direction (Drinkwater et al., 2003). The contemporary distribution of V. pourtalesii is associated with the deep basins and channels on the Scotian Shelf which are bathed by warm and saline slope waters (Smith, 1978; Loder et al., 1997).
In this study we present the first long-term results of near-bed hydrodynamic observations and (food) particle supply mechanisms to highlight the influence of natural fluctuating environmental conditions on sponge growth and resilience. Observational evidence of the hydrodynamic conditions of the V. pourtalesii sponge grounds on the Scotian Shelf was collected via a long-term deployment (10-month) of a benthic lander in the framework of the EU-SponGES project. Near-bed environmental conditions (e.g., temperature, current speed, turbidity) were measured at high-resolution to determine hourly to seasonal variability. In addition, the lander was equipped with a sediment trap to determine particle and carbon fluxes, which was used as a measure of food availability. Knowledge on the environmental requirements and natural variability experienced by these sponge grounds may help to improve our understanding of their resilience against environmental stressors and ability to recover after natural and anthropogenic-induced disturbances.
Materials and Methods
Topographic and Oceanographic Setting
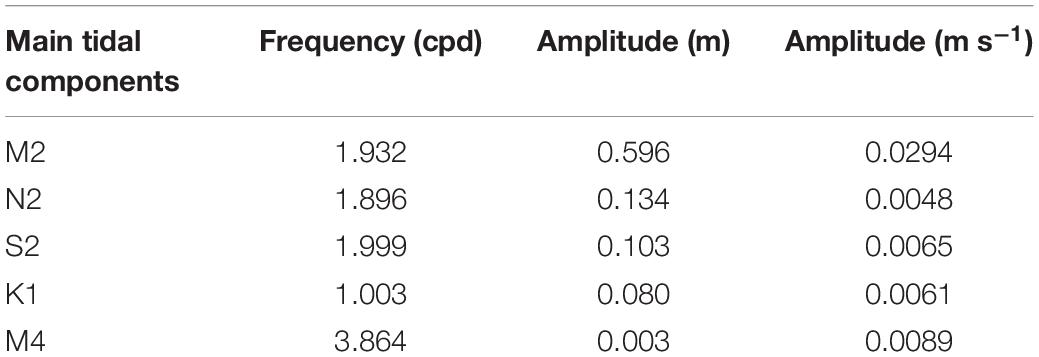
The Scotian Shelf is a 700 km long and 200 km wide section of the continental shelf off Nova Scotia, Canada, bounded by the Laurentian Channel in the northeast, and the Northeast Channel and Gulf of Maine in the southwest. While the average depth of the shelf is 90 m, it is characterized by a heterogonous topography with several banks, channels, canyons and deeper basins (Figure 1; Han et al., 1997). The glass sponge V. pourtalesii is distributed in the Northeast Channel at the mouth of the Gulf of Maine in the west, and on the central Scotian Shelf in the Scotian Gulf, an inlet formed by a deeper cross-shelf channel situated between Emerald and LaHave Banks, which opens up to LaHave and Emerald Basins on the inner shelf (Beazley et al., 2018). The densest known concentration of V. pourtalesii is located above 200 m depth on a saddle between the two deep basins (>200 m water depth) that comprise Emerald Basin (Beazley et al., 2018), with a second notable concentration occurring on the eastern flank of Sambro Bank, Emerald Basin (Figure 1). These two concentrations are mostly encompassed by the Emerald Basin and Sambro Bank closures, respectively.
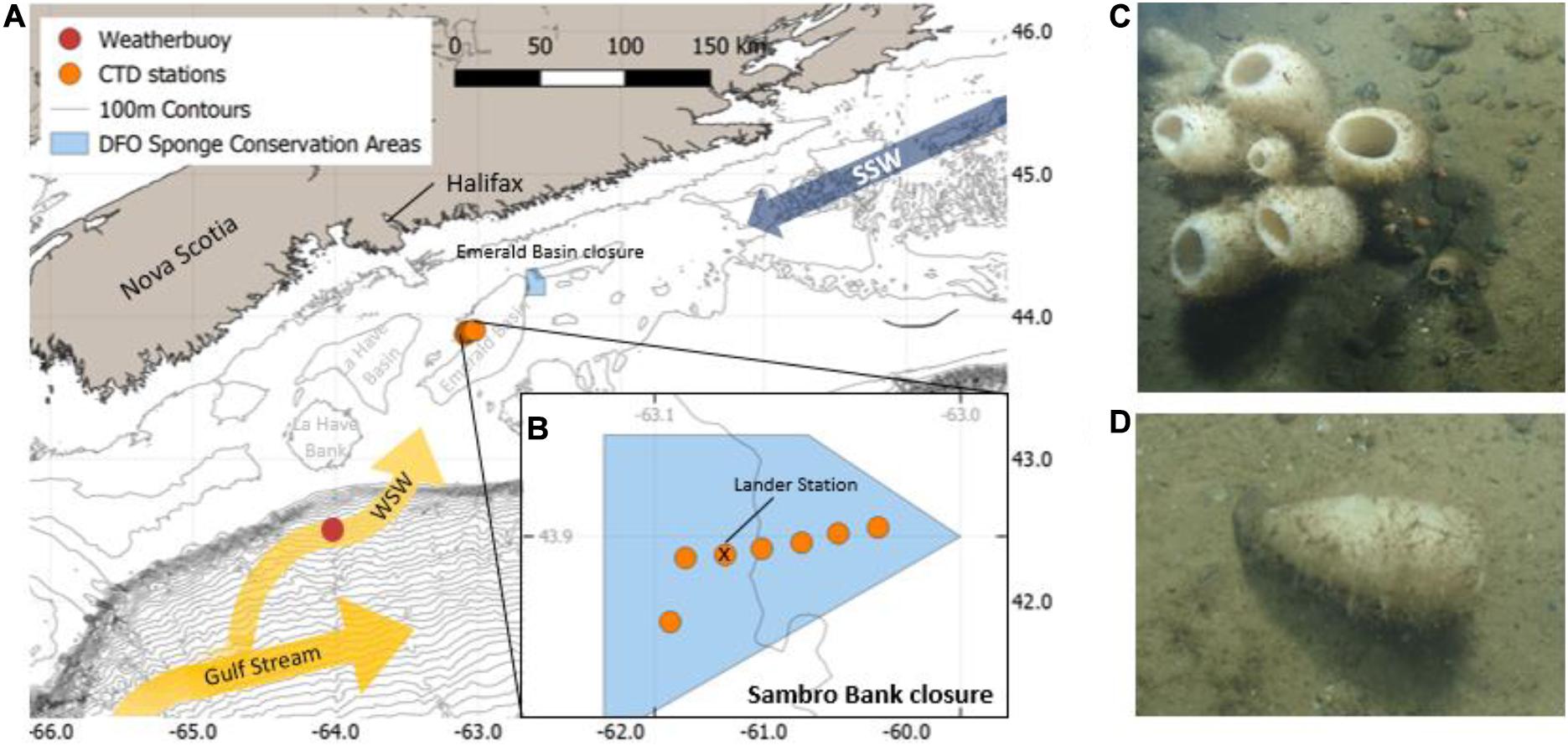
Figure 1. (A) Scotian Shelf with CTD, weather buoy stations and both DFO Sponge conservation areas indicated in light blue. The arrows indicate the flow of the slope waters, WSW (orange arrow) and SSW (dark blue arrow), (B) Sambro Bank closure with CTD and lander stations; (C) V. pourtalesii sponges inside the Sambro Bank closure, (D) some sponges were found in a horizontal position.
Two major ocean currents influence the sponge grounds; the Gulf Stream and the equatorward flowing subpolar Labrador Current (Hannah et al., 2001). The variability of the intensity of the Labrador Current influences all water masses on the shelf and results in the highest inter-annual variability of sea surface temperature (Δ 4.6°C) in the North Atlantic (Petrie and Drinkwater, 1993). When the Labrador Current (seasonally) reaches the seafloor it is known to be strong enough to displace organisms and matter, showing the largest volume transport (up to 0.6 Sv) in winter (Drinkwater et al., 1979; Hannah et al., 2001). The water column on the Scotian Shelf has a two-layered structure consisting of surface and slope waters (Drinkwater et al., 2003). The slope waters consist of two components with different signatures: the Warm Slope Water (WSW) and the Labrador Slope Water (LSW). WSW originates from a mixture of Gulf Stream Water, deep North Atlantic Central Water and shelf waters and flows primarily northeastward adjacent to the Gulf Stream. It is characterized by relatively high temperatures (12°C), high salinities (35.4; Gatien, 1976; Mountain, 2012) and high nutrient concentrations (Townsend and Ellis, 2010). The LSW flows in an equatorward direction and is cold (2°C), less saline (32) and low in nutrients (with an exception of relatively high Si; Petrie and Drinkwater, 1993; Petrie and Yeats, 2000; Mountain, 2012). Onshore movement of the warm slope waters through the major channels and gullies fills the basins at the Scotian Shelf with primarily WSW, resulting in relatively warm waters bathing the sponges year round. The surface water layer (Scotian Shelf Waters, SSW) is mixed by winter winds and contains cold water (2°C) with a relatively fresh signature (32) resulting from terrestrial run-off (McLellan, 1957; Petrie and Drinkwater, 1993). During summer, winter-cooled SSW is sitting in between a warmed up surface layer and the warmer WSW (Drinkwater et al., 2003). The water masses on the Scotian Shelf are influenced by seasonal warming and cooling, whereby the system is usually well stratified in late spring to early autumn, whereas it can be mixed over the shallow banks in winter (Smith et al., 1978). Vertical mixing occurs year round by internal tides, which are generated by the interaction of tidal flow of the stratified water masses over the abrupt bathymetry of the banks and basins (Han and Loder, 2003). This leads to vertical mixing of deep nutrient-rich water into the surface waters (Townsend and Ellis, 2010), but also stirs up sediments and produces intermediate nepheloid layers (Azetsu-Scott et al., 1995). In the Emerald Basin these nepheloid layers have been found at a depth of 140 to 200 m and occur when strong tidal currents flow in a northern direction (Azetsu-Scott et al., 1995). Tidal currents on the Scotian Shelf are dominated by semi-diurnal (M2) tides (Chen et al., 2011).
Water Column Profiling
During a research cruise onboard the Canadian Coast Guard Ship (CCGS) Hudson (HUD2018-021) in June 2018, water column profiles of Conductivity-Temperature-Depth (CTD) were recorded with a Sea-Bird SBE25 V 4.1c system along a transect in the Sambro Bank closure to collect CTD data (each CTD cast was ∼1 km apart) inside and outside of the main sponge grounds (control station in the southwest, Figure 1B), which was based on a presence probability model from Beazley et al. (2018).
Lander
A NIOZ-designed ALBEX lander (Duineveld et al., 2004) was deployed at a depth of 154 m (43.898° N, 63.052° E) near the center of the Sambro Bank closure on September 6, 2017 with the CCGS Martha L. Black, and retrieved on June 22, 2018 during the CCGS Hudson mission (Figure 1B). The lander was equipped with a CT sensor (Sea-Bird SBE37SM-RS232, sampling interval = 15 min, accuracy for temperature of 0.002°C and conductivity of 0.003 mS cm–1), ARO-USB oxygen sensor (JFE-AdvantechTM, sampling interval = 15 min, accuracy of non-linearity ± 2% of full scale, at 1 atm, 25°C). A 2 point calibration was carried out before deployment to compensate for the time drift and ensure reliable and accurate DO data. An Aquadopp 2 MHz (NortekTM, sampling interval = 15 min) acoustic doppler current profiler (ADCP) was attached on the lander at a depth of 3 m above bottom (mab). The ADCP measured acoustic backscatter and velocity in 50 cm bins with an accuracy of ±1% of the measured value (0.05 cm s–1). A NIOZ custom-made HD video camera with white lights recorded every 4 h a short video clip of 20 s of the seabed. The lander was also equipped with a Technicap PPS4/3 sediment trap with 12 bottles with the aperture at 3 mab. Sediment trap vials were filled with a pH-buffered mercury chloride (HgCl2) solution in filtered seawater collected from the deployment site and the carousel was programmed to rotate at 30-day intervals.
Sediment Trap Samples
Upon recovery of the lander, sediment trap samples were stored at 4°C until they were split into five subsamples with a WSD10 McLane manual rotor splitter. Four of the subsamples were split and rinsed with MilliQ water and one subsample was washed with filtered seawater for pigment analysis. After splitting, samples were freeze-dried and weighed to calculate mass fluxes, whereby the average weights of two splits was used. It must be stressed that cylindrical traps tend to under-sample when current speed exceeds 15 cm s–1 (Gardner et al., 1983). Therefore, trap fluxes presented here, especially during high current conditions, are likely underestimated with regards to the near-bed mass flux (Vangriesheim et al., 2001).
One split of each sample was used for isotope analysis. Half of the sample was used for organic carbon analysis and was first decarbonized by hydrochloric acid (2 M HCl supra) and afterward transferred into pressed tin capsules (Elemental Microanalysis). The other half of the sample was used for nitrogen analysis and was directly transferred into pressed tin capsules. Stable isotopes δ15N, δ13C and total weight percent of organic carbon and nitrogen were analyzed by a Delta V Advantage isotope ratio MS coupled to a Flash 2000 Elemental Analyzer (EA-IRMS) by a Conflo IV (Thermo Fisher Scientific Inc.). The reference gas was pure N2. As a standard for δ13C benzoic acid and acetanilide was used, for δ15N acetanilide, urea and casein was used. For δ13C analysis a high signal method was exercised including a 70% dilution. Precision for replicate measurements of δ13C and δ15N was ± 0.15 ‰.
The Chlorophyll-a (Chl-a) concentration of the sediment trap samples was measured by a Fluorescence Spectrometer (F-2500 Hitachi), by comparing the sample absorbance to the absorbance of a known Chl-a standard at two wavelengths (431.0/671.0 nm): Chl-a was extracted in 90% acetone and fluorescence was measured first on the intact extract and a second time after the extract was acidified with HCl (10%) under dark and cold conditions. HCl was added to the sample to convert Chl-a into its degradation products (Phaeopigments). By this the amount of Chl-a can be deducted from its degradation products which otherwise interfere with the measurements since they absorb light at the same wavelength (for details Holm-Hansen et al., 1965).
Data Analysis
Daily moving averages of all environmental variables of the long term data were calculated, as well as monthly averages that were related to the sediment trap sampling intervals. Correlations between the measured environmental variables were calculated using Pearson correlations (n = 27,384) in the program R (R Studio 1.2.1335). The pressure data and near-bed horizontal velocity components at 5 mab obtained with the ADCP were analyzed to determine the most important tidal constituents with the “tidem” package in the program R (R Studio 1.2.1335).
Velocity data of the ADCP at 5 mab were used to make a progressive vector diagram. A single passive particle representing a dissolved or suspended constituent was released and its transport simulated according to the horizontal velocities measured by the current sensor during a period of 30 days; corresponding to the intervals of sediment trap sampling (Duineveld et al., 2007). For this, the velocities per time step were added up in a two-dimensional space simulating the direction of transport. The released particle experiences in any new position the current of the initial position, independently of where the constituent went. Progressive vector diagrams accumulate errors and clearly cannot provide a real estimate of the particle position, but provide an estimate on how far particles or nutrients can disperse after they are released from their original position (Carlson et al., 2010).
Results
Environmental Conditions on the Scotian Shelf
The CTD transect revealed a relatively fresh and solar heated upper layer reaching to a depth of about 20 m below the surface as shown by high temperature (>5°C) and low salinity (<32) (Figure 2). Underneath this layer colder winter-cooled SSW water was found from 20 m until a depth of about 75 m (<5°C, Figure 2B). Underneath the cold layer WSW was observed, which was characterized by a higher temperature (>6°C) and higher salinity (>33) (Figure 2A). Water stratification was very similar at all stations including the control station outside the sponge area, whereas the influence of the cold winter-cooled SSW was decreasing toward the east.
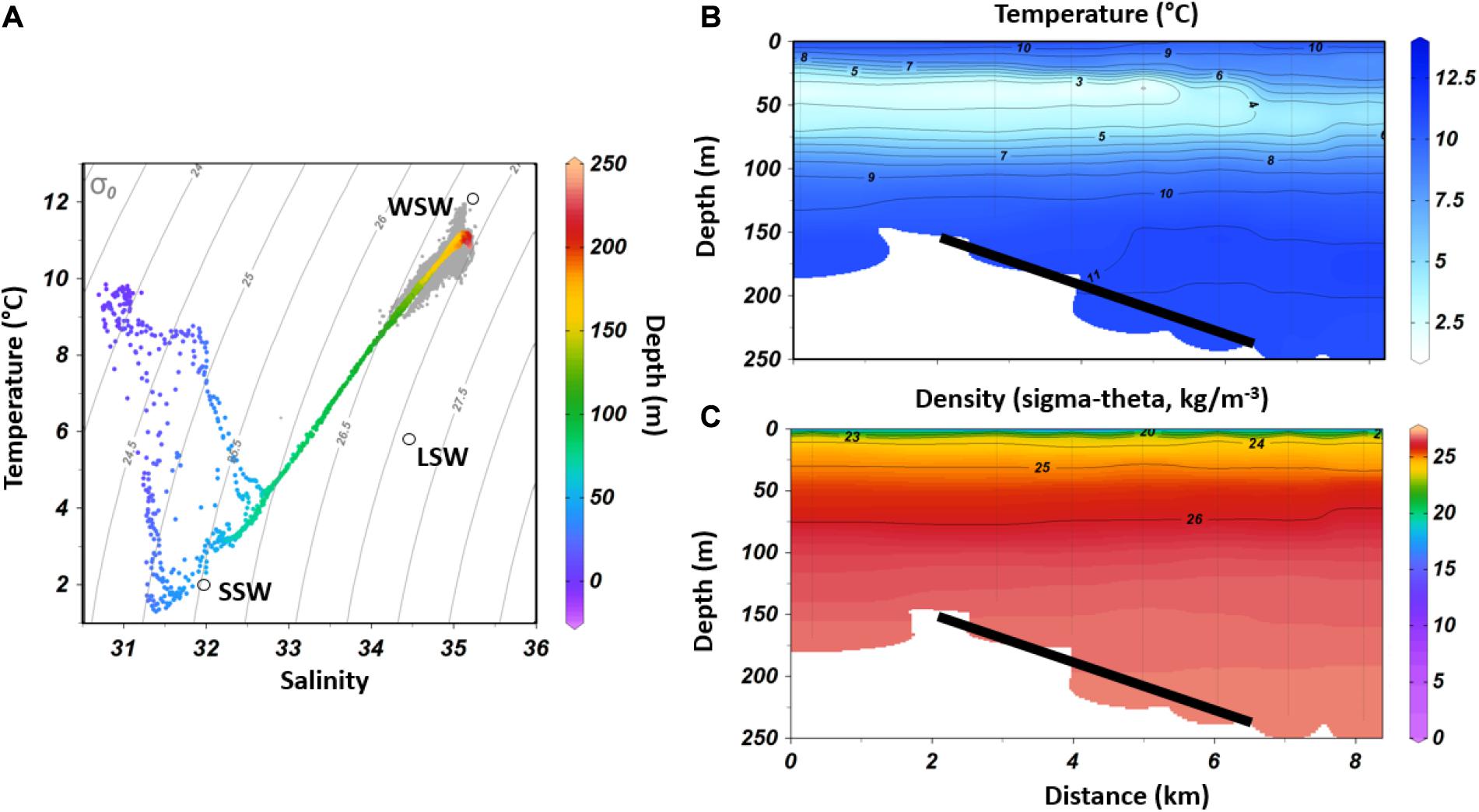
Figure 2. (A) T/S plot of CTD and lander data in gray. The characteristic end members of temperature and salinity for WSW, LSW and SSW are plotted (Mountain, 2012); (B) Temperature transect inside the Sambro Bank closure, with the westernmost station (on the left) located outside of the densest sponge area; (C) Density along the same transect. The black bars indicate the depth distribution of the sponge ground (150–250 m water depth).
Long-term Environmental Conditions in the Sambro Bank Closure
The lander was deployed at a depth of 154 m and data were recorded during the whole deployment period of ten months (Figure 3). Average temperature (T) was 10.6 ± 0.4°C and varied between 8.8 to 12°C (Figure 3A). Average salinity was 35.0 ± 0.1 with a range between 34.1 and 35.3 (Figure 3B). As shown in Figures 3A,B, T and salinity were highly correlated (r2 = 0.84, p < 0.001, Pearsons product-moment correlation). The monthly temperature was on average highest in March and April (10.9°C) and lowest in November (average 10.2°C). Salinity was on average highest in April (35.1) and lowest in November (34.8; Figure 6A). The average dissolved oxygen (DO) concentration during the whole time period was 6.3 ± 0.5 mg l–1 with a range between 4.9 to 8.9 mg l–1 (Figure 3C), corresponding to an oxygen saturation of on average 57%. The dissolved oxygen (DO) concentration was negatively correlated with density (r2 = -0.52, p < 0.001) and was on average highest in spring from March to June (6.7–7.1 mg l–1), while lowest values were found from September to February (6.0 – 6.2 mg l–1, Figure 6A). Currents at 5 mab were on average 0.12 m s–1 with peaks in current speed of up to 0.47 m s–1 (Figure 3E). The direction of the large-scale sub-tidal flow was in a direction of 146°. Tidal flow oscillated between a NW and SE direction with the semi-diurnal tidal cycle (Figure 3D). Current direction was not significantly correlated with temperature, salinity or oxygen concentration (Figure 1 of Supplementary).
Figure 3. Near-bed hydrodynamic data, recorded by the lander from September 2017 to June 2018, with the daily moving average shown by thick lines. (A) Temperature, (B) Salinity, (C) Dissolved Oxygen concentration, (D) Current direction (5 mab), (E) Current speed (5 mab). Weather data from buoy: (F) Wave height; (G) wind direction and (H) wind speed (no continuous record) (DFO). Storm events are highlighted in light blue.
The tidal analysis fitted to the pressure data and revealed that the biggest tidal constituent is the principal semi-diurnal lunar constituent (M2) with an amplitude of 0.596 m, followed by the larger lunar elliptic semidiurnal (N2) and principal solar semidiurnal constituents (S2) with an amplitude of in each case of 0.13 m (Figure 4, Table 1). The tidal analysis fitted to the velocity data revealed that the M2 tide produces the highest velocity with a current speed of 0.03 m s–1. The second important constituent of the velocity data was the M4 constituent producing a velocity of 0.01 m s–1 (Table 1). These tidal signals are evident in the energy spectra in which the largest peaks were found at the above mentioned frequencies (Figure 4).
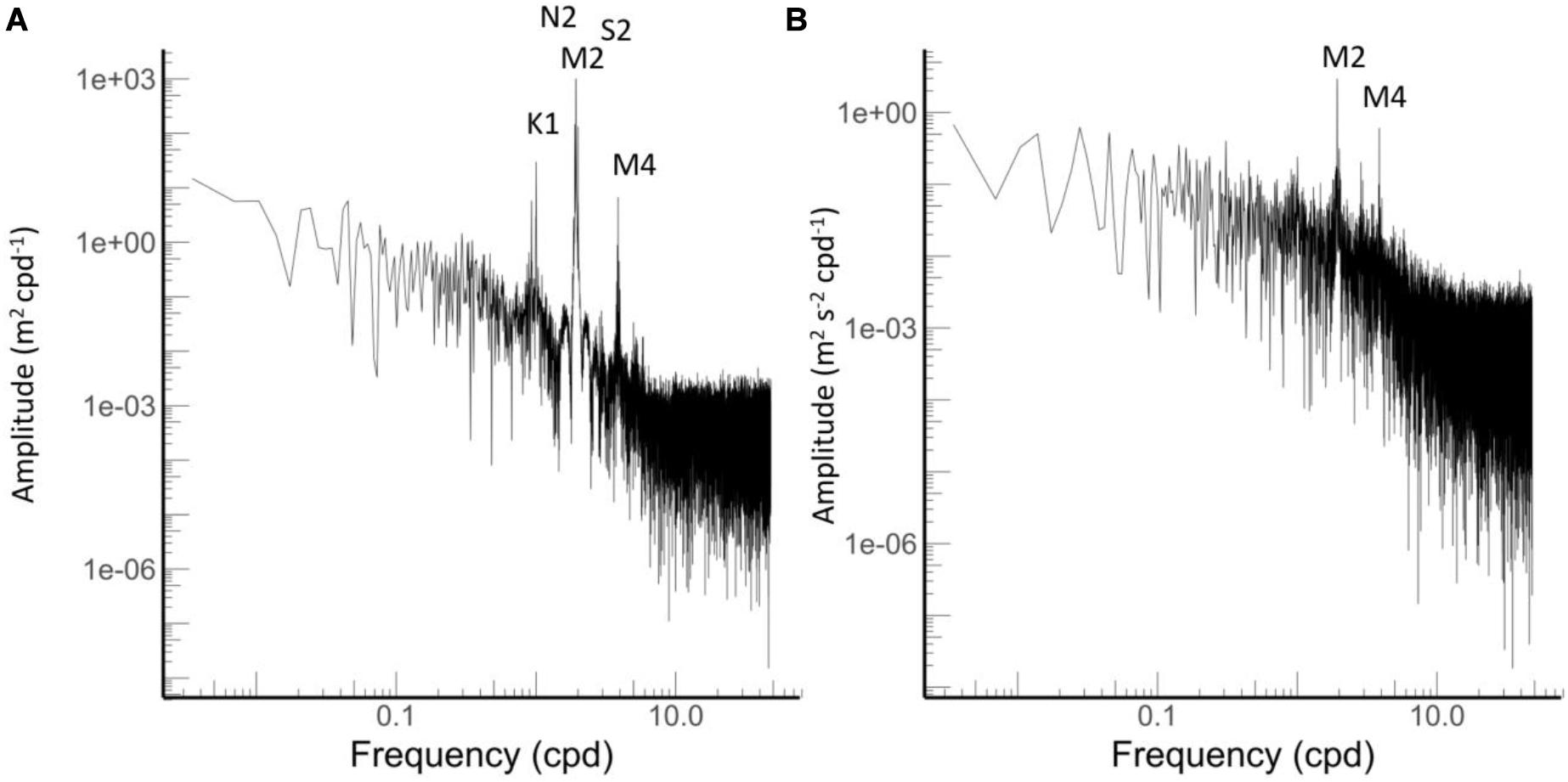
Figure 4. Energy spectra from (A) pressure and (B) velocity data. Main tidal frequencies are indicated.
The acoustic backscatter showed a permanently turbid benthic boundary layer up to a height of about 5 mab throughout the deployment period, whereas periodical peaks reached higher up in the water column, up to 13 mab (Figure 5A). Lowest backscatter was recorded from May to June 2018. The acoustic backscatter at 5 mab showed the strongest correlation with current speed (r2 = 0.25, p < 0.001), temperature and DO concentration (both r2 = 0.2, p < 0.001).
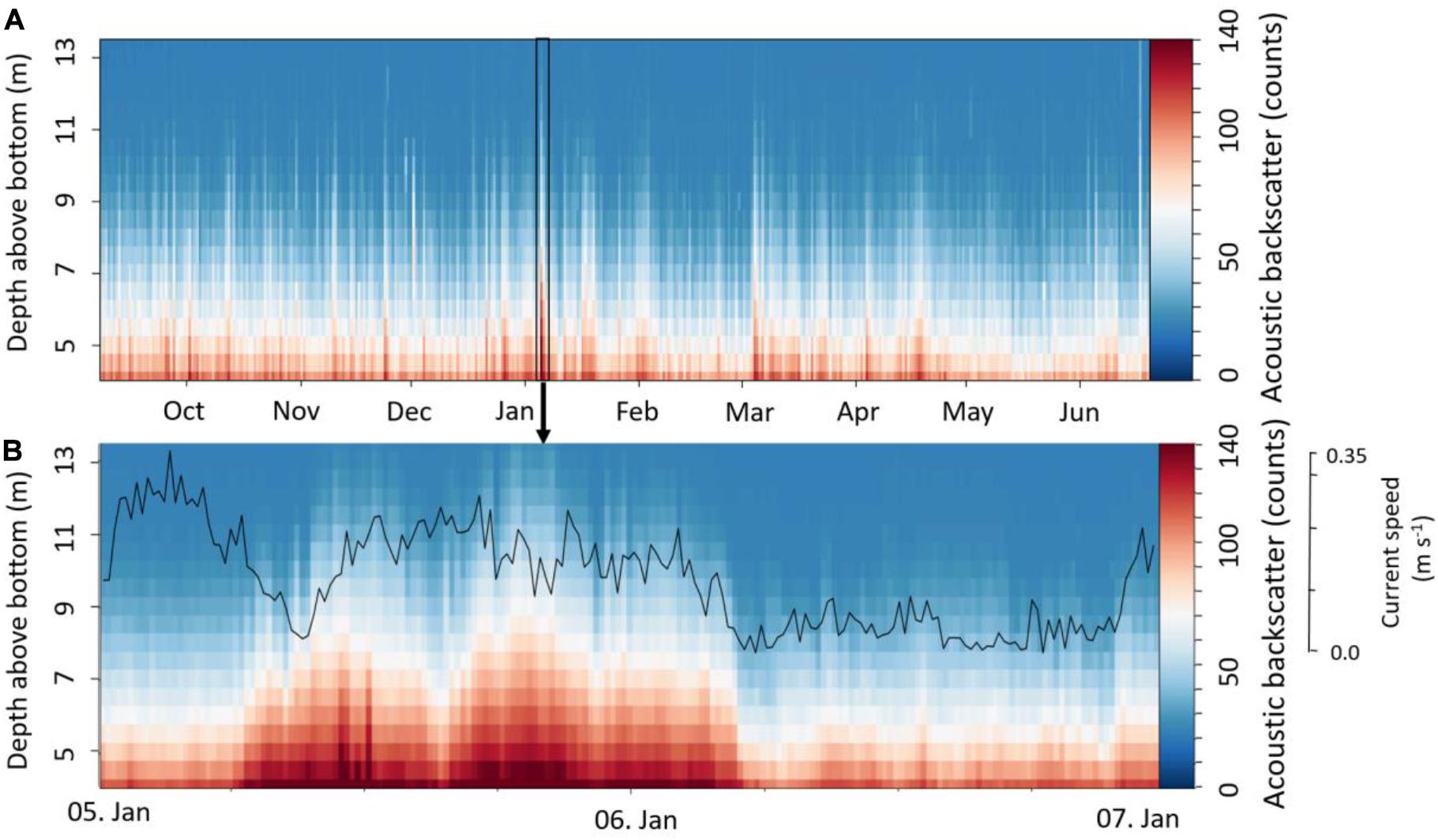
Figure 5. (A) Acoustic backscatter (counts) in 3.5 to 13 m above the sea bottom; (B) Resuspension event in January with current speed at 5 mab (bin2).
Although several high turbidity events were observed throughout the year, two events are highlighted here in more detail, showing an altered daily amplitude of T, salinity, DO concentration (Figure 3), and corresponding peak in turbidity (Figure 5). Both events were correlated to storm events as observed by weather buoy data, being characterized by peaks in wave height at the 5th of January (max. 15.5 m at 4:20) and the 3th of March (max. 10.6 m at 21:20, Figure 3). Wind speed also peaked on these two days, reaching 24.2 m s–1 and 21.1 m s–1, respectively, blowing into an eastern direction (DFO, 2020). Based on the acoustic backscatter data, the first extreme event in January resulted in a high load of particles in up to 13 mab for about 24 h (Figure 5B). During this event three smaller turbidity peaks were found between the 05th of January and the 06th of January (Figure 5B), which were related to a change in current direction due to the semi-diurnal tides. The second storm event in March showed a slightly lower surface wave height, surface wind speed and turbidity than during the winter peak (Figure 5A). Additionally, a temporal drop in temperature and salinity was observed during this event, where both parameters fell to a minimum of 8.8°C and 34.1, respectively (Figure 3). On the contrary DO increased during the event. Several smaller events with similar characteristics were observed from March to May, showing the same alterations in T, salinity, DO and peaks in acoustic backscatter.
Sediment Trap
The vertical mass flux during the deployment period was on average 3166 ± 3421 mg m–2 day–1 (Figure 6F), while the average carbon flux was 100 ± 72 mg C m–2 day–1 (Figure 6G). Highest vertical mass flux, i.e., 12,390 mg m–2 day–1, was observed in the period from December 2017 to January 2018. Highest carbon flux was likewise found in December/January coinciding with the winter storm event (281 mg C m–2 day–1, Figure 6). The C:N ratio was on average 8.3 ± 0.2, ranging from 7.8 to 8.6 (Figure 6B) and the δ15N was 6.7 ± 0.4 ‰, ranging from 6.2 to 7.3‰ (Figure 6C). The δ13C ratio ranged from −23.3 to −22.8‰ with an average of −23 ± 0.1 ‰ (Figure 6D). The Chl-a concentration was on average 2.1 ± 1.1 μg l–1, with an average Chl-a: Phaeo ratio of 0.00069 ± 0.00077 (Figure 6E). The largest peak in Chl-a (4.05 μg l–1), being two times higher than the average Chl-a concentration, was observed in March/April, indicating the arrival of fresh phytodetritus at the seafloor, which occurred after the second storm event in March.
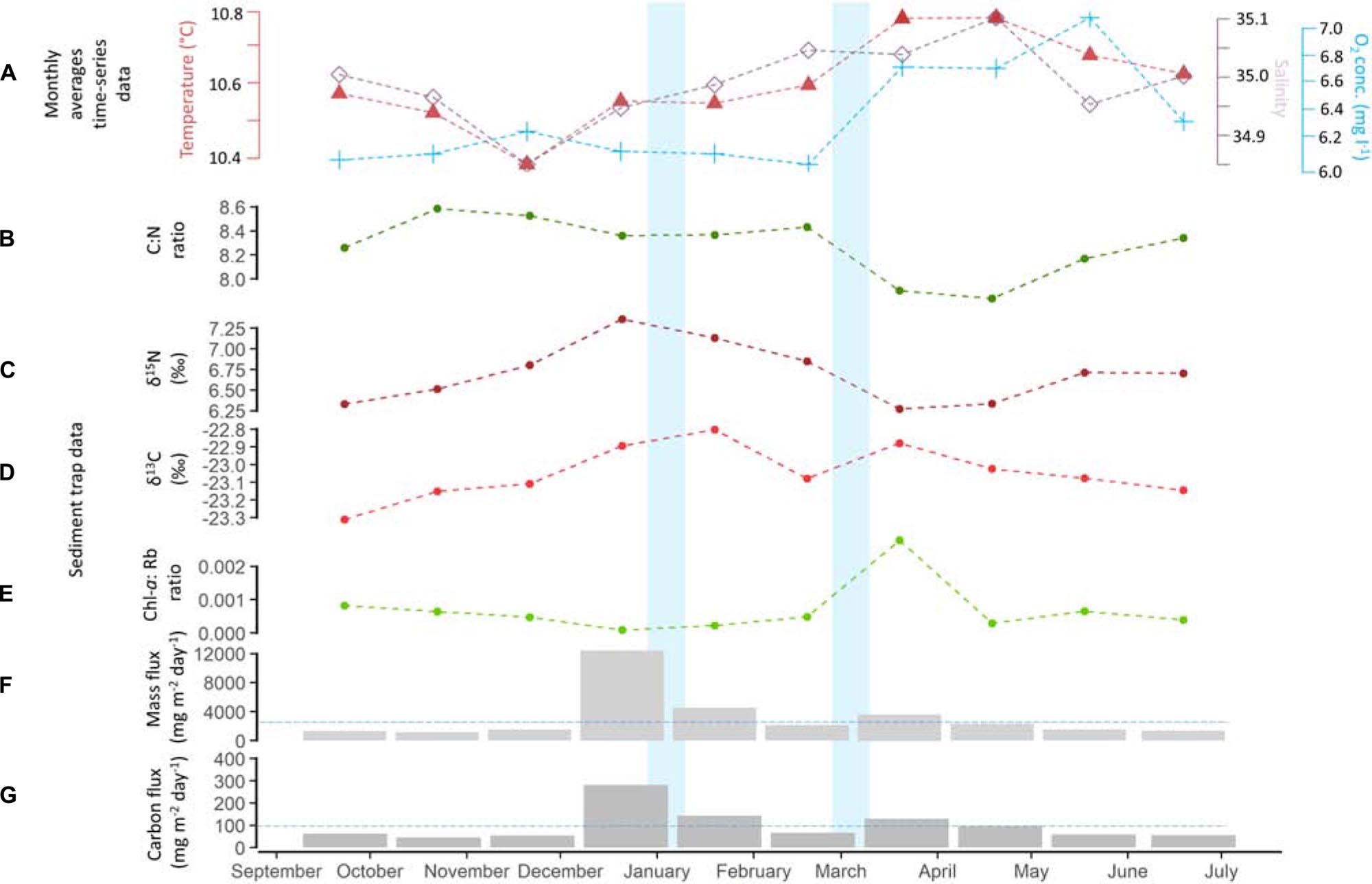
Figure 6. (A) Monthly averages of temperature, salinity and oxygen concentration recorded by the lander, Sediment trap data: (B) C:N ratio; (C) δ15N; (D) δ13C; (E) Chlorophyll-a: Phaeopigments ratio of the sediment trap material; (F) Mass and (G) Carbon flux recorded by the sediment trap. Blue horizontal lines are indicating the average mass and carbon flux. Line charts and histograms represent the average value during the 30 day sediment trap interval. Storm events are highlighted in light blue.
The winter peak (December/January) in mass flux corresponded to a peak in acoustic backscatter (Figure 5B), which is likely the result of resuspension of bottom material as indicated by the presence of a large quantity of sponge spicules in the sediment trap sample. The quality of the organic matter flux in this period was low as indicated by the C:N ratio (8.4), the δ15N (7.3 ‰) and the Chl-a: Phaeopigment ratio (0.000009), indicating that high amounts of more degraded organic matter are trapped. This is in contrast to the fresh organic matter observed in March/April with a C:N ratio of 7.9, the δ15N of 6.3 ‰ and the Chl-a: Phaeopigment ratio of 0.003 (Figure 6).
Particle Displacement
The progressive vector plot showed that particulate suspended matter released at the deployment site was estimated to travel in a mainly southern direction (min. 71 km in June/July to a max. 233 km in December/January) during each 30-day sampling interval (Figure 7). SE-NW movements of particles are related to the semi diurnal tidal flow. In autumn, the particles generally traveled in a SSE direction, while after the storm event in January, the residual current direction shifted into a southern direction and showed the longest distance. After the storm event in spring (March/April) particles were transported relatively fast to the southwest. During spring residence time on the shelf was the longest as indicated by the shortest distances. With the shelf edge around 100 km to the south from the lander site, particles would be washed off the shelf in a period between 14 to >30 days.
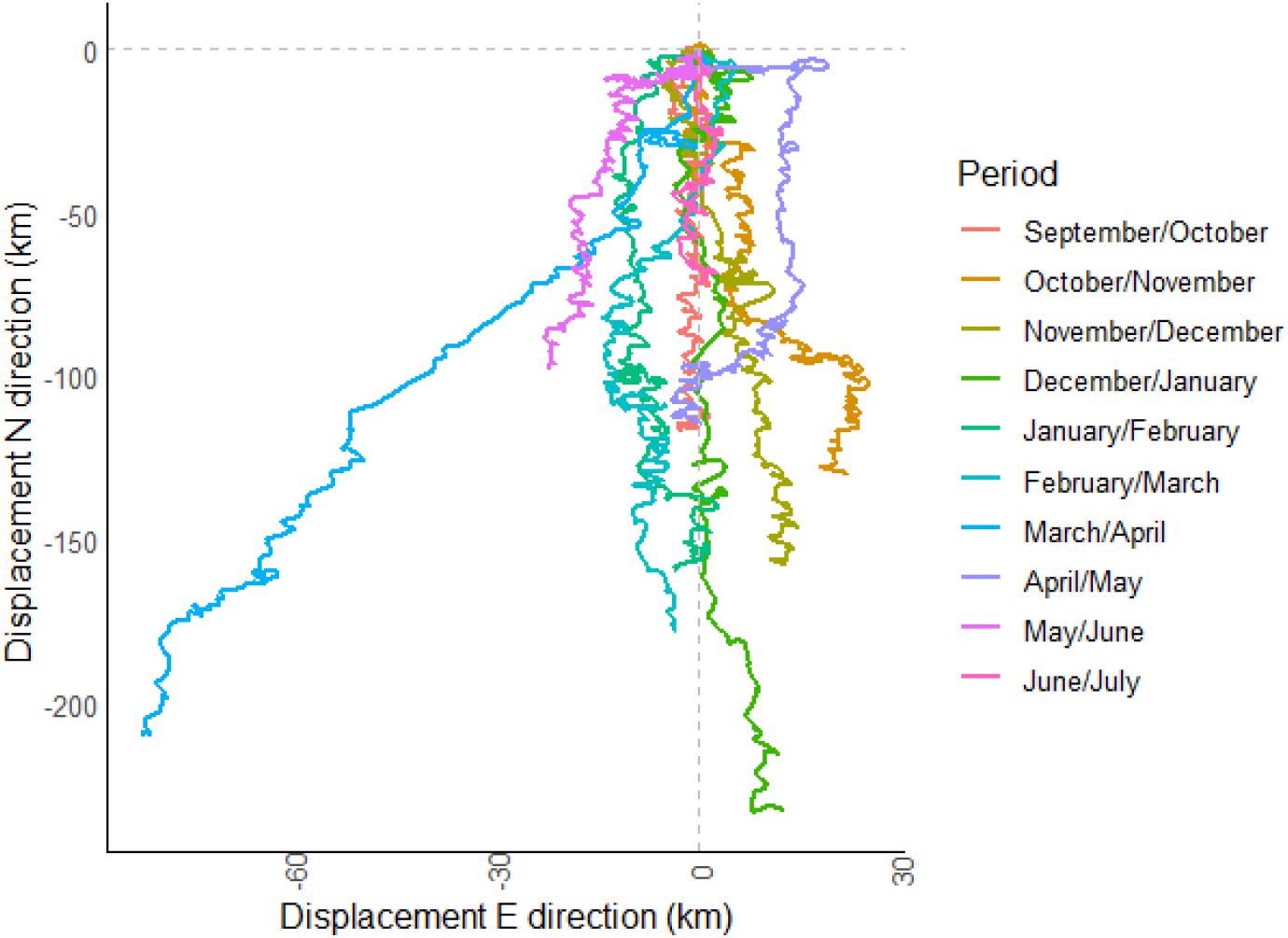
Figure 7. Progressive vector plot of 30-day intervals, corresponding to the sampling interval of the sediment trap. The site where the passive particle was released is the coordinate 0/0, the lander site.
The view of the camera showed several upright V. pourtalesii individuals as well as many anemones (Figure 8, 4th Jan.). Mobile species, such as pollock (Pollachius virens) and especially redfish (Sebastes spp.) also appeared in the recordings. Pollock were observed to swim relatively quickly through the video frame, mostly in groups of several individuals. Redfish were rather stationary or moving with the currents in a resting stage on the seafloor as single individuals or in small groups. The storm events were clearly captured by the video data. The video observations revealed that during the January storm event an extreme high load of particles was in resuspension for one day, whereas conditions gradually normalized within 2 days (Figure 8A), corresponding to the peaks in turbidity. After the water cleared up, sponges and anemones were observed to be dislocated, whereas the lander frame also moved during the storm and another view of the camera was visible. Sponges in the camera view did not appear to be negatively influenced by this dislodgement, since the sponges were white and unaffected in appearance. Nevertheless, some sponges transported in the view of the camera after a high current event, were observed to be dead (Figure 8B). Likely they have been ripped off from their initial position and substrate by the high currents. One of the bigger sponge individuals was observed to decompose in a time period of about three weeks (Figure 8B).

Figure 8. Photographs showing the camera view (A) before, during and after the storm event (4. January). Large amounts of suspended matter were observed in the benthic boundary layer from the 4. January to the 7. January; (B) Dead sponge in February indicated by a white arrow and its subsequent decomposition until its barely detectable at the 9th of March.
Discussion
To date the focus of most studies of the V. pourtalesii grounds has been on the spatial distribution of the sponge grounds, their associated fauna and biodiversity (Fuller and Cameron, 1998; Beazley et al., 2018; Hawkes et al., 2019). Little is known of the natural environmental characteristics and food supply mechanisms on short to seasonal time scales that influence these habitats. For the first time, we measured the environmental characteristics at high temporal resolution over a 10-month period in the V. pourtalesii sponge ground located in the Sambro Bank Sponge Conservation Area.
Environmental Conditions on the Scotian Shelf
Based on the modeling results of Beazley et al. (2018), minimum bottom water temperature is the most important determinant for the distribution of V. pourtalesii on the Scotian Shelf, with the highest presence probabilities associated with minimum bottom temperatures of 5°C. In our study, bottom water temperature varied between 8.8 to 12.0°C during the deployment period. The sponge grounds were observed below the boundary of two water masses, mainly bathed in WSW. During most of the year seasonal water column stratification on the Scotian Shelf prevents mixing of the WSW and SSW (McLellan, 1957), and in this study high and relatively stable bottom water temperatures were measured. Short-term daily fluctuations in temperature and salinity were linked to internal tides (M2) moving stratified waters up and down the sloping topography of the Emerald Basin (Wunsch, 1975; van Haren et al., 1994). Extreme temperature fluctuations during the lander deployment were only observed during some of the storm events in spring, when colder, less saline and oxygen-rich water, typical of winter-cooled surface waters was observed near the bottom (Figure 3). These events resulted in abrupt fluctuations in bottom water temperature of up to 3.2°C, which might be related to wind-driven downwelling on the shelf (Greenberg et al., 1997). These fluctuations did not exceed multi-decadal variability (4 to 12°C), which is even higher with bottom temperatures varying by up to 8°C and being as low as ∼4°C (Beazley et al., 2018). That sponges can thrive in a large temperature range was also observed in other studies: glass sponge accumulations in Norway were correlated with temperatures below 12°C (Strand et al., 2017) and even as low as -0.5°C along the arctic mid-Atlantic Ridge (Roberts et al., 2018), whereas P. carpenteri were modeled to appear over a temperature range of 2.7 to 20.9°C (Howell et al., 2016). Even though lower and upper temperature ranges of most sponge species are still largely unknown, it seems that they can thrive at larger temperature ranges than other deep-sea species forming hotspots like cold-water coral reefs (4 to 12°C) (Roberts et al., 2006).
The DO concentration of 6.3 ± 0.53 mg l–1 measured in this study was about three times higher than the DO concentration (2.05 mg l–1) measured in a sponge reef on the western Canadian margin (Whitney et al., 2005). So far it is not evident which DO concentrations are suitable for sponges. They seem very resilient even at low DO concentrations, as observed at the Namibian margin where DO concentrations were far below 2 mg l–1 (Hanz et al., 2019), which seems to be the threshold for growth and survival of many other marine organisms (Gray et al., 2002).
Another important environmental factor for the occurrence and sustenance of sponge grounds is the interaction between hydrography and seafloor topography (van Haren et al., 2017; Davison et al., 2019). Sponge grounds are often associated with areas where the slope angle of the bottom matches the slope of internal tides, producing internal waves. Internal waves, generated by wind or flow over topography are the main causes of strong bottom currents, enhanced turbulence and mixing and resuspension of particulate matter near the seafloor (Garrett and Laurent, 2002; Hosegood and van Haren, 2004), thereby influencing food supply by increasing the vertical and lateral flux (Davison et al., 2019) as has also been observed in cold-water coral ecosystems (Cyr et al., 2016). An example is the P. carpenteri belt along the slope of the Porcupine Seabight in the NE Atlantic (Rice et al., 1990) or the Faroe Shetland channel where sponges were observed in regions with intensified internal wave activity and associated with pronounced changes in temperature (Davison et al., 2019). Such conditions have also been found on seamounts and canyons, where sponge aggregations have been observed in areas of critical slope in the Arctic (Schulz Bank seamount; Busch et al., 2020) and the Azores (Rainbow Basin; van Haren et al., 2017). Based on our data we can only link the environmental fluctuations to the presence of tidal currents. However, other studies have described the presence of internal wave activity in the Emerald Basin (van Haren et al., 1994). Intermediate nepheloid layers were found to coincide with the critical depth for possible generation and amplification of internal waves with semi-diurnal (M2) internal tidal frequency (Azetsu-Scott et al., 1995). The nepheloid layers observed by Azetsu-Scott et al. (1995) in their vertical profiles had an intermittent character that was explained by recurrent resuspension and variable currents. This intermittency was also clearly visible in the near-bed data (current data and video) from this study (Figures 5, 8) showing pulse-like increases of particulate matter concentrations in the benthic boundary layer pointing to local resuspension. These frequent resuspension and subsequent lateral transport of high near-bed particle concentrations might be an important driver of food supply for V. pourtalesii sponge grounds on the Scotian Shelf.
Food Supply
Sponges are very efficient filter feeders and feed on particulate as well as dissolved resources (Reiswig, 1971; Pile and Young, 2006). While the average daily carbon flux in the near-bed sediment trap was 100 ± 72 mg C m–2 day–1 during the deployment period, carbon flux was highly variable and peaked in December/January (281 mg C m–2 day–1) and March/April (130 mg C m–2 day–1). During the December peak no major fluctuations in temperature, salinity or DO concentration were observed, likely indicating that the organic matter is not delivered from the surface but rather due to resuspension events inside the bottom boundary layer. Even though this winter event was characterized by a high carbon flux, mainly degraded organic matter was resuspended as shown by the low concentration of Chl-a and a high C:N ratio (Figure 6), likely related to resuspension and lateral transport of more degraded material from the seafloor. Hill and Bowen (1983) calculated that a current speed of 0.15 m s–1 is required to resuspend the coarser sand, whereas fine sand and mud is transported at a speed of 0.11 m s–1. Current speed as measured in this study would be sufficient to resuspend coarse sediment during 31% of the time and 56% for the finer fraction. This was also apparent in the ADCP turbidity data (Figure 5) which showed an almost permanent turbid layer close to the bottom. The video recordings showed that during storm-induced resuspension events, particularly the winter event, particles stayed in resuspension for several days, which was however not detected in the acoustic backscatter data of the ADCP, implying that turbidity close to the bottom might be even more persistent. This could possibly be due to the particle size of the particles in resuspension, whereby the finer particles are not resolved by measurements of the ADCP (Bunt et al., 1999).
In March/April the highest amounts of fresh (high concentration of Chl-a and lower C:N ratio) organic matter were observed, likely related to a surface algal bloom. The algal bloom period seems indirectly related to the mixing of the water column during the storm event in March (Figure 6), when nutrients are stirred up to the surface. Our data on the importance of a mixed water column for the surface productivity was confirmed by an ecosystem model, which predicted highest primary production to appear in the beginning of April (Song et al., 2011).
Taking the estimated carbon demand of a deep-water glass sponge reef (ca. 160 m depth, ∼10°C) at the Canadian coast (1800–4100 mg C m–2 day–1, Kahn et al., 2015), the carbon derived from the vertical flux would provide on average approximately <8% of the required carbon demand. Ex situ experiments have shown that in the Canadian sponge reefs carbon consumption was much higher (360 mg m–2 day–1), compared to the average carbon flux (100 mg m–2 day–1), showing that the carbon demand of the Vazella grounds will not be satisfied (Bart et al., 2020b). A potential carbon deficit may be alleviated from the resuspended organic-rich sediment itself (Grant et al., 1987), but also from the filtration of bacteria and uptake of DOC from the water column (Bart et al., 2020b). Bacteria are expected to be an even more important food source than suspended particulate matter for some glass sponges and will be assimilated efficiently (Pile and Young, 2006; Kahn et al., 2015; Bart et al., 2020a). On the Scotian Shelf an important source of bacteria can be the resuspended sediments, which are 100–1000 times enriched in heterotrophic bacteria compared to the overlaying water (Kuwae and Hosokawa, 1999). These benthic bacteria are attached to sediment particles with extracellular polymeric substances and can be taken up by the ingestion of sediment particles (Kuwae and Hosokawa, 1999). It was also shown that particles remaining in suspension become more densely packed with bacteria (Wainright, 1987). This was suggested to occur on the Scotian Shelf by Grant et al. (1987), who found a high N content in sand particles, which they hypothesized was due to attachment by bacteria. Considering the fact that several large resuspension episodes were observed during the deployment period, the resuspended sediment with microbes attached and subsequent lateral transport might constitute a major food source for the sponges. The fine carbon- and nitrogen-rich suspended material and even the coarse sandy fraction is constantly resuspended and transported over the shelf (Grant et al., 1987) in a mainly southern direction as was also shown by the progressive vector analysis (Figure 7). Even though this type of analysis only provides an estimate, we showed that suspended matter stays on the shelf for 14–>30 days. During the event in March the wind direction was toward the east and the residual current direction changed to the SW in an along slope direction that likely has resulted in downwelling (Greenberg et al., 1997; Li et al., 2014), which was indicated by a decrease in temperature related to the cooler SSW. Due to the occurrence of natural resuspension events throughout the 10-month lander deployment, the question arises if these events and resulting lateral transport of (food) particles are essential for the occurrence of V. pourtalesii on the Scotian Shelf.
Events
The extreme events at the seafloor at the 154 m deep lander site were mainly driven by storm events and occurred predominantly in the winter and spring months, influencing the water column structure and particle distribution on the shelf. Only the storm events in spring were observed to introduce colder and less saline water to the sponge ground, which was during the event in March coinciding with a peak in surface wave height as well as an on average more easterly wind direction (Figure 3). Water column mixing will result in an increased availability of nutrients from the deeper water masses toward the surface (Townsend et al., 2006), triggering primary productivity and increased benthic-pelagic coupling (Ji et al., 2006). Topography-hydrography interactions can also influence biogeochemistry and microbiology either by mixing or by resuspension as described above, which can result in different sponge associated microbial communities defined by the surrounding near-bed environment (Busch et al., 2020). This mixing will indirectly promote an increased vertical flux and therefore food supply to the sponge grounds and their associated fauna, as was also observed in the Gulf of Mexico and on the Namibian margin (Mienis et al., 2012; Hanz et al., 2019). Wind-induced mixing also can play a major role in the replenishment of for instance oxygen as was observed during the spring storm events, where colder SSW with a lower salinity and higher DO concentration was observed amidst the sponge ground. Replenishment of nutrients and DO have shown to be vital for the occurrence of sponges as well as other deep-sea ecosystems, like cold-water coral reefs (van Haren et al., 2014; Hanz et al., 2019). However, these events can also act as stressors for the sponge ground. During periods of high current speed sponges and anemones were observed to be dislocated and tipped over into a horizontal position and a dead individual was observed in front of the camera in late winter (February-March). Accumulations of dead individuals have been observed in the Sambro Bank Sponge Conservation Area (Hawkes et al., 2019), caused either by the displacement by high currents or by fishing activities and subsequent transport by the current (trawling and dumping). Our video data showed that large dead individuals were disintegrating in a period of several weeks, which might be important for the silicon cycling on the Scotian Shelf (Maldonado et al., 2020).
Besides the observed events, the Labrador Current which varies seasonally in extent, is in general known for the displacement of biological organisms and materials to the southwest (Hannah et al., 2001). The association of the V. pourtalesii with movable hard substrate like stones and pebbles might hence have a benefit for the sponges at the Scotian Shelf. The strong currents during winter and spring storm events might be destructive if sponges would be attached to non-movable hard substrate since they can be ripped off their attachment. Additionally, small stones and pebbles might play a role in helping the sponges to stay upright or regain an upright position after being moved by transferring their mass center to their bottom. That would also explain why only in deeper areas in the Fundian Channel, which are likely less affected by storm activity and high current speeds, sponges are also found on bigger boulders (Fuller and Cameron, 1998; Lacharité and Metaxas, 2017).
High amounts of resuspended material can also be a stressor for V. pourtalesii since high amounts of suspended sediment or larger particles can clog the aquiferous system of sponges and could negatively impact their filtering ability and even their survival (Maldonado et al., 2008; Tjensvoll et al., 2013; Kutti et al., 2015a). To avoid complete clogging, sponges exhibit adaptations to high particle loads and can arrest pumping and ‘cough’ (a clearing response) when exposed to high concentrations of suspended sediment. The exact concentration of suspended particulate matter in the water column during the winter storm events was not measured in this study. However, video observations showed that the extreme conditions in the benthic boundary layer lasted up to several days (Figure 8). If V. pourtalesii arrest their pumping activity during these periods they would need to do so for a much longer time than has been shown so far in other studies (Tjensvoll et al., 2013; Kutti et al., 2015a; Grant et al., 2019). The Scotian Shelf is subject to severe storms as well as hurricanes on a regular basis, caused by extratropical cyclonic storms that develop along the U.S. east coast and travel northward (Anderson et al., 1989). Modeling studies have indicated that with predicted climate change storm intensity will increase, which likely will have an effect on the sponge grounds situated on the Scotian Shelf (Knutson et al., 2010).
In addition to naturally driven suspension events, sponges on the Scotian Shelf are also influenced by anthropogenic impacts like fishing activities that can also resuspend large amounts of particulate matter. The Vazella sponge grounds are situated in traditional fishing grounds for groundfish (Beazley et al., 2018). Bottom-tending fishing gears can directly impact sponge grounds through removal and incidental damage, or through indirect effects such as resuspension of sediment and smothering (Tjensvoll et al., 2013), whereby impacts of plumes were observed kilometers away from the source of the plume (Grant et al., 2019). As mentioned above, resuspension events and lateral transport of particles can positively and negatively influence sponges. Particles suspended by fishing activity are known to decrease the filtering capacity and efficiency in many species which can result in adverse effects on overall metabolism and health of sponges (Bell et al., 2015; Grant et al., 2019). How the concentration of suspended sediment observed during storm events compares to resuspension induced from bottom trawling remains unknown. Nevertheless, V. pourtalesii overall does not appear to suffer from the natural resuspension events and showed healthy appearances after the events. Results from this study contextualize the response of sponges to human-induced threats such as bottom fishing and climate change by providing detailed site-specific knowledge of the natural conditions under which these sponges persist and their resilience during changing environmental conditions.
Conclusion
Resiliency against environmental and anthropogenic stressors requires that the surrounding environmental conditions, such as temperature, DO concentration and food supply, be within a species’ optimal physiological tolerance limits (Odum, 1971; Sokolova et al., 2012). Overall the near-bed environmental conditions in the Vazella sponge grounds on the Scotian Shelf fit within the range as described for other glass sponge species. V. pourtalesii experienced temperature fluctuations of up to 3.2°C on a seasonal timescale, with maximum temperatures of 12°C in spring and on average lowest temperatures in Autumn (November). Major storm events in winter and spring were additionally observed to cause extreme resuspension events, which were expected to exhibit a stressor but also an important mechanism for food supply. V. pourtalesii appeared to withstand these events even for several days, suggesting that sponges are resilient to natural as well as anthropogenic (e.g., fishing) resuspension events of short duration. The vertical flux of organic matter was estimated to not cover the food demand of the sponges as also observed in other sponge grounds, whereas semi-diurnal tidal currents play an important role in the resuspension of sediments, which likely constitute a constant source of particulate food for the sponges. The only directly observed stressor was high currents, which relocated benthic fauna and sometimes caused sponges to dislodge from their upright position. Our findings show how resilient these sponges are to environmental changes over short time scales, which might be vital for their survival under human-induced threats such as bottom fishing and climate change.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
UH wrote the main manuscript text, collected the data and is responsible for the analysis and interpretation of the data. FM developed the study concept, sample collection, and helped with interpretation of the data. HR initiated the study. LB and EK organized the fieldwork. All authors, except HR, reviewed the manuscript and contributed to the critical discussion.
Funding
This research has been performed in the scope of the SponGES project, which received funding from the European Union’s Horizon 2020 Research and Innovation Program under grant agreement no. 679849. Canadian cruises and contributions were funded by Fisheries and Ocean’s Canada’s International Governance Strategy Science Program through project “Marine Biological Diversity Beyond Areas of National Jurisdiction (BBNJ): 3-Tiers of Diversity (Genes-Species-Communities)” led by EK (2017–2019) and Strategic Program for Ecosystem-Based Research and Advice (SPERA) project “Evaluation of the Effectiveness of Two Sponge Conservation Areas in the Maritimes Region: Identifying Patterns of Dispersal, Connectivity, and Recovery Potential of the Russian Hat Sponge Vazella pourtalesii” led by LB and EK. FM was supported financially by the Innovational Research Incentives Scheme of the Netherlands Organisation for Scientific Research (NWO-VIDI grant 016.161.360).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the captain and crew of the CCGS Martha L. Black and CCGS Hudson for their help with the deployments and recoveries of the research gear. We also thank Barry MacDonald, Francisco Javier Murillo, and the scientific party of both cruises for their help in collecting the samples. Furthermore we thank Ronald van Bommel (NIOZ) for his help with the stable isotope analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.597682/full#supplementary-material
Supplementary Figure 1 | Pearsons correlations between long-term record data.
Supplementary Figure 2 | u and v components of the current and wind data with the daily moving average shown by thick lines. Main storm events are highlighted in light blue.
Abbreviations
ADCP, Acoustic Doppler current profiler; CTD, Conductivity Temperature Depth; Chl-a, Chlorophyll-a; DO, dissolved oxygen; mab, meters above bottom; WSW, Warm Slope Water; LSW, Labrador Slope Water; SSW, Shelf Slope Water; V. pourtalesii, Vazella pourtalesii.
References
Anderson, C., Dobson, F., Perrie, W., Smith, P., Toulany, B., and Schwing, F. (1989). Storm response in the coastal ocean: the oceanographic component of the Canadian Atlantic Storms Program (CASP). Eos, Trans. Am. Geophys. Union 70, 562–572. doi: 10.1029/89eo00142
Azetsu-Scott, K., Johnson, B. D., and Petrie, B. (1995). An intermittent, intermediate nepheloid layer in Emerald Basin, Scotian Shelf. Continental Shelf Res. 15, 281–293. doi: 10.1016/0278-4343(93)e0003-q
Bart, M. C., De Kluijver, A., Hoetjes, S., Absalah, S., Mueller, B., Kenchington, E., et al. (2020a). Differential processing of dissolved and particulate organic matter by deep-sea sponges and their microbial symbionts. Sci. Rep. 10:17515.
Bart, M. C., Mueller, B., Rombouts, T., Van De Ven, C., Tompkins, G. J., Osinga, R., et al. (2020b). Dissolved organic carbon (DOC) is essential to balance the metabolic demands of North-Atlantic deep-sea sponges. bioRxiv [preprint] doi: 10.1101/2020.09.21.305086
Barthel, D., Tendal, O., and Thiel, H. (1996). A wandering population of the hexactineliid sponge pheronema carpenteri on the continental slope off morocco, Northwest Africa. Mar. Ecol. 17, 603–616. doi: 10.1111/j.1439-0485.1996.tb00420.x
Beazley, L., Kenchington, E., Yashayaev, I., and Murillo, F. J. (2015). Drivers of epibenthic megafaunal composition in the sponge grounds of the Sackville Spur, northwest Atlantic. Deep Sea Res. Part I: Oceanographic Res. Papers 98, 102–114. doi: 10.1016/j.dsr.2014.11.016
Beazley, L., Wang, Z., Kenchington, E., Yashayaev, I., Rapp, H. T., Xavier, J. R., et al. (2018). Predicted distribution of the glass sponge Vazella pourtalesi on the Scotian Shelf and its persistence in the face of climatic variability. PLoS One 13:e0205505. doi: 10.1371/journal.pone.0205505
Beazley, L. I., Kenchington, E. L., Murillo, F. J., and Sacau, M. D. M. (2013). Deep-sea sponge grounds enhance diversity and abundance of epibenthic megafauna in the Northwest Atlantic. ICES J. Mar. Sci. 70, 1471–1490. doi: 10.1093/icesjms/fst124
Bell, J. J., Mcgrath, E., Biggerstaff, A., Bates, T., Bennett, H., Marlow, J., et al. (2015). Sediment impacts on marine sponges. Mar. Pollut. Bull. 94, 5–13. doi: 10.1016/j.marpolbul.2015.03.030
Bunt, J. A., Larcombe, P., and Jago, C. F. (1999). Quantifying the response of optical backscatter devices and transmissometers to variations in suspended particulate matter. Continental Shelf Res. 19, 1199–1220. doi: 10.1016/s0278-4343(99)00018-7
Busch, K., Hanz, U., Mienis, F., Mueller, B., Franke, A., Roberts, E. M., et al. (2020). On giant shoulders: how a seamount affects the microbial community composition of seawater and sponges. Biogeosciences 17, 3471–3486. doi: 10.5194/bg-17-3471-2020
Carlson, D. F., Muscarella, P. A., Gildor, H., Lipphardt, B. L. Jr., and Fredj, E. (2010). How useful are progressive vector diagrams for studying coastal ocean transport? Limnol. Oceanography: Methods 8, 98–106. doi: 10.4319/lom.2010.8.0098
Cathalot, C., Van Oevelen, D., Cox, T. J., Kutti, T., Lavaleye, M., Duineveld, G., et al. (2015). Cold-water coral reefs and adjacent sponge grounds: hotspots of benthic respiration and organic carbon cycling in the deep sea. Front. Mar. Sci. 2:37. doi: 10.3389/fmars.2015.00037
Chen, C., Huang, H., Beardsley, R. C., Xu, Q., Limeburner, R., Cowles, G. W., et al. (2011). Tidal dynamics in the Gulf of Maine and New England Shelf: an application of FVCOM. J. Geophys. Res.: Oceans 116:12010.
Chu, J. W., and Leys, S. P. (2010). High resolution mapping of community structure in three glass sponge reefs (Porifera, Hexactinellida). Mar. Ecol. Prog. Ser. 417, 97–113. doi: 10.3354/meps08794
Cyr, F., Van Haren, H., Mienis, F., Duineveld, G., and Bourgault, D. (2016). On the influence of cold-water coral mound size on flow hydrodynamics, and vice versa. Geophys. Res. Lett. 43, 775–783. doi: 10.1002/2015gl067038
Davison, J. J., Van Haren, H., Hosegood, P., Piechaud, N., and Howell, K. L. (2019). The distribution of deep-sea sponge aggregations (Porifera) in relation to oceanographic processes in the Faroe-Shetland Channel. Deep Sea Res. Part I: Oceanographic Res. Papers 146, 55–61. doi: 10.1016/j.dsr.2019.03.005
DFO (2020). Canadian Wave Data ID44150 - La Have Bank”, in: 2006/03/01 to 2020/01/14. Canada: Department of Fisheries and Oceans Canada.
Drinkwater, K., Petrie, B., and Smith, P. (2003). Climate variability on the Scotian Shelf during the 1990s. ICES Mar. Sci. Symp. 219, 40–49.
Drinkwater, K., Petrie, B., and Sutcliffe, W. Jr. (1979). Seasonal geostrophic volume transports along the Scotian Shelf. Estuarine Coastal Mar. Sci. 9, 17–27. doi: 10.1016/0302-3524(79)90003-3
Duineveld, G., Lavaleye, M., and Berghuis, E. (2004). Particle flux and food supply to a seamount cold-water coral community (Galicia Bank, NW Spain). Mar. Ecol. Prog. Ser. 277, 13–23. doi: 10.3354/meps277013
Duineveld, G. C., Lavaleye, M. S., Bergman, M. J., De Stigter, H., and Mienis, F. (2007). Trophic structure of a cold-water coral mound community (Rockall Bank, NE Atlantic) in relation to the near-bottom particle supply and current regime. Bull. Mar. Sci. 81, 449–467.
Farrow, G. E., Syvitski, J. P., and Tunnicliffe, V. (1983). Suspended particulate loading on the macrobenthos in a highly turbid fjord: knight Inlet, British Columbia. Can. J. Fish. Aquatic Sci. 40, s273–s288.
Fuller, S., and Cameron, P. (1998). Marine Benthic Seascapes: Fishermen’s Perspectives. Halifax, NS: Ecology Action Centre.
Fuller, S. D. (2011). Diversity of Marine Sponges in the Northwest Atlantic. PhD thesis, Dalhousie University: Halifax, NS.
Gardner, W., Richardson, M., Hinga, K., and Biscaye, P. (1983). Resuspension measured with sediment traps in a high-energy environment. Earth Planetary Sci. Lett. 66, 262–278. doi: 10.1016/0012-821x(83)90140-1
Gatien, M. G. (1976). A study in the slope water region south of Halifax. J. Fish. Board Canada 33, 2213–2217. doi: 10.1139/f76-270
Grant, J., Volckaert, F., and Roberts-Regan, D. L. (1987). Resuspendable organic matter in Nova Scotian shelf and slope sediments. Continental Shelf Res. 7, 1123–1138. doi: 10.1016/0278-4343(87)90102-6
Grant, N., Matveev, E., Kahn, A., Archer, S., Dunham, A., Bannister, R., et al. (2019). Effect of suspended sediments on the pumping rates of three species of glass sponge in situ. Mar. Ecol. Prog. Ser. 615, 79–100. doi: 10.3354/meps12939
Gray, J. S., Wu, R. S.-S., and Or, Y. Y. (2002). Effects of hypoxia and organic enrichment on the coastal marine environment. Mar. Ecol. Prog. Ser. 238, 249–279. doi: 10.3354/meps238249
Greenberg, D. A., Loder, J. W., Shen, Y. S., Lynch, D. R., and Naimie, C. E. (1997). Spatial and temporal structure of the barotropic response of the Scotian Shelf and Gulf of Maine to surface wind stress: a model-based study. J. Geophys. Res.-Oceans 102, 20897–20915. doi: 10.1029/97jc00442
Han, G., Hannah, C. G., Loder, J. W., and Smith, P. C. (1997). Seasonal variation of the three-dimensional mean circulation over the Scotian Shelf. J. Geophys. Res.: Oceans 102, 1011–1025. doi: 10.1029/96jc03285
Han, G., and Loder, J. W. (2003). Three-dimensional seasonal-mean circulation and hydrography on the eastern Scotian Shelf. J. Geophys. Res.: Oceans 108. doi: 10.1029/2002JC001463
Hannah, C. G., Shore, J. A., Loder, J. W., and Naimie, C. E. (2001). Seasonal circulation on the western and central Scotian Shelf. J. Phys. Oceanography 31, 591–615. doi: 10.1175/1520-0485(2001)031<0591:scotwa>2.0.co;2
Hanz, U., Wienberg, C., Hebbeln, D., Duineveld, G., Lavaleye, M., Juva, K., et al. (2019). Environmental factors influencing benthic communities in the oxygen minimum zones on the Angolan and Namibian margins. Biogeosciences (BG) 16, 4337–4356. doi: 10.5194/bg-16-4337-2019
Hawkes, N., Korabik, M., Beazley, L., Rapp, H. T., Xavier, J. R., and Kenchington, E. (2019). Glass sponge grounds on the Scotian Shelf and their associated biodiversity. Mar. Ecol. Prog. Ser. 614, 91–109. doi: 10.3354/meps12903
Hill, P. R., and Bowen, A. J. (1983). “Modern Sediment Dynamics at the shelf-slope Boundary off Nova Scotia,” in The Shelfbreak: Critical Interface on Continental Margins: eds D. J. Stanley and G. T. Moore (Tulsa: Society Economic Paleontologists Mineralogists Special Publication 33), 265–276. doi: 10.2110/pec.83.06.0265
Holm-Hansen, O., Lorenzen, C. J., Holmes, R. W., and Strickland, J. D. (1965). Fluorometric determination of chlorophyll. ICES J. Mar. Sci. 30, 3–15. doi: 10.1093/icesjms/30.1.3
Hosegood, P., and van Haren, H. (2004). Near-bed solibores over the continental slope in the Faeroe-Shetland Channel. Deep Sea Res. Part II: Top. Stud. Oceanography 51, 2943–2971. doi: 10.1016/j.dsr2.2004.09.016
Howell, K.-L., Piechaud, N., Downie, A.-L., and Kenny, A. (2016). The distribution of deep-sea sponge aggregations in the North Atlantic and implications for their effective spatial management. Deep Sea Res. Part I: Oceanographic Res. Papers 115, 309–320. doi: 10.1016/j.dsr.2016.07.005
Ji, R., Chen, C., Franks, P. J., Townsend, D. W., Durbin, E. G., Beardsley, R. C., et al. (2006). The impact of Scotian Shelf Water “cross-over” on the plankton dynamics on georges bank: a 3-D experiment for the 1999 spring bloom. Deep Sea Res. Part II: Top. Stud. Oceanography 53, 2684–2707. doi: 10.1016/j.dsr2.2006.08.007
Kahn, A. S., Yahel, G., Chu, J. W., Tunnicliffe, V., and Leys, S. P. (2015). Benthic grazing and carbon sequestration by deep-water glass sponge reefs. Limnol. Oceanography 60, 78–88. doi: 10.1002/lno.10002
Klitgaard, A. B., and Tendal, O. S. (2004). Distribution and species composition of mass occurrences of large-sized sponges in the northeast Atlantic. Prog. Oceanography 61, 57–98. doi: 10.1016/j.pocean.2004.06.002
Knutson, T. R., Mcbride, J. L., Chan, J., Emanuel, K., Holland, G., Landsea, C., et al. (2010). Tropical cyclones and climate change. Nat. Geosci. 3, 157–163.
Kutti, T., Bannister, R. J., and Fosså, J. H. (2013). Community structure and ecological function of deep-water sponge grounds in the Traenadypet MPA—Northern Norwegian continental shelf. Continental Shelf Res. 69, 21–30. doi: 10.1016/j.csr.2013.09.011
Kutti, T., Bannister, R. J., Fosså, J. H., Krogness, C. M., Tjensvoll, I., and Søvik, G. (2015a). Metabolic responses of the deep-water sponge Geodia barretti to suspended bottom sediment, simulated mine tailings and drill cuttings. J. Exp. Mar. Biol. Ecol. 473, 64–72. doi: 10.1016/j.jembe.2015.07.017
Kutti, T., Fosså, J. H., and Bergstad, O. A. (2015b). Influence of structurally complex benthic habitats on fish distribution. Mar. Ecol. Prog. Ser. 520, 175–190. doi: 10.3354/meps11047
Kuwae, T., and Hosokawa, Y. (1999). Determination of abundance and biovolume of bacteria in sediments by dual staining with 4’, 6-diamidino-2-phenylindole and acridine orange: relationship to dispersion treatment and sediment characteristics. Appl. Environ. Microbiol. 65, 3407–3412. doi: 10.1128/aem.65.8.3407-3412.1999
Lacharité, M., and Metaxas, A. (2017). Hard substrate in the deep ocean: how sediment features influence epibenthic megafauna on the eastern Canadian margin. Deep Sea Res. Part I: Oceanographic Res. Papers 126, 50–61. doi: 10.1016/j.dsr.2017.05.013
Leys, S. P., Mackie, G. O., and Reiswig, H. M. (2007). The biology of glass sponges. Adv. Mar. Biol. 52, 1–145. doi: 10.1016/s0065-2881(06)52001-2
Li, Y. Z., He, R. Y., and Mcgillicuddy, D. J. (2014). Seasonal and interannual variability in Gulf of Maine hydrodynamics: 2002-2011. Deep-Sea Res. Part Ii-Top. Stud. Oceanography 103, 210–222. doi: 10.1016/j.dsr2.2013.03.001
Loder, J. W., Han, G., Hannah, C. G., Greenberg, D. A., and Smith, P. C. (1997). Hydrography and baroclinic circulation in the Scotian Shelf region: winter versus summer. Can. J. Fish. Aquatic Sci. 54, 40–56. doi: 10.1139/f96-153
Maldonado, M., Aguilar, R., Bannister, R. J., Bell, J. J., Conway, K. W., Dayton, P. K., et al. (2017). “Sponge grounds as key marine habitats: a synthetic review of types, structure, functional roles, and conservation concerns,” in Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots, eds S. Rossi, L. Bramanti, A. Gori, V. Saco del, and O. Covadonga (Cham: Springer International Publishing), 145–183. doi: 10.1007/978-3-319-21012-4_24
Maldonado, M., Beazley, L., Lopez-Acosta, M., Kenchington, E., Casault, B., Hanz, U., et al. (2020). Massive Silicate Utilization Facilitated by a Benthic-pelagic Coupled Feedback Sustains Deep-Sea Sponge Aggregations. Hoboken, NJ: John Wiley & Sons, Inc.
Maldonado, M., Giraud, K., and Carmona, C. (2008). Effects of sediment on the survival of asexually produced sponge recruits. Mar. Biol. 154, 631–641. doi: 10.1007/s00227-008-0956-5
Maldonado, M., Ribes, M., and Van Duyl, F. C. (2012). Nutrient fluxes through sponges: biology, budgets, and ecological implications. Adv. Mar. Biol. 62, 113–182. doi: 10.1016/b978-0-12-394283-8.00003-5
McLellan, H. (1957). On the distinctness and origin of the slope water off the Scotian Shelf and its easterly flow south of the Grand Banks. J. Fish. Board Canada 14, 213–239. doi: 10.1139/f57-011
Meyer, H., Roberts, E., Rapp, H., and Davies, A. (2019). Spatial patterns of arctic sponge ground fauna and demersal fish are detectable in autonomous underwater vehicle (AUV) imagery. Deep Sea Res. Part I: Oceanographic Res. Papers 153:103137. doi: 10.1016/j.dsr.2019.103137
Mienis, F., Duineveld, G., Davies, A., Ross, S., Seim, H., Bane, J., et al. (2012). The influence of near-bed hydrodynamic conditions on cold-water corals in the Viosca Knoll area, Gulf of Mexico. Deep Sea Res. Part I: Oceanographic Res. Papers 60, 32–45. doi: 10.1016/j.dsr.2011.10.007
Mills, E., and Fournier, R. (1979). Fish production and the marine ecosystems of the Scotian Shelf, eastern Canada. Mar. Biol. 54, 101–108. doi: 10.1007/bf00386589
Mountain, D. G. (2012). Labrador slope water entering the Gulf of Maine—response to the North Atlantic Oscillation. Continental Shelf Res. 47, 150–155. doi: 10.1016/j.csr.2012.07.008
Murillo, F. J., Kenchington, E., Tompkins, G., Beazley, L., Baker, E., Knudby, A., et al. (2018). Sponge assemblages and predicted archetypes in the eastern Canadian Arctic. Mar. Ecol. Prog. Ser. 597, 115–135. doi: 10.3354/meps12589
Murillo, F. J., Muñoz, P. D., Cristobo, J., Ríos, P., González, C., Kenchington, E., et al. (2012). Deep-sea sponge grounds of the Flemish Cap, Flemish Pass and the Grand Banks of Newfoundland (Northwest Atlantic Ocean): distribution and species composition. Mar. Biol. Res. 8, 842–854. doi: 10.1080/17451000.2012.682583
Petrie, B., and Drinkwater, K. (1993). Temperature and salinity variability on the Scotian Shelf and in the Gulf of Maine 1945–1990. J. Geophys. Res.: Oceans 98, 20079–20089. doi: 10.1029/93jc02191
Petrie, B., and Yeats, P. (2000). Annual and interannual variability of nutrients and their estimated fluxes in the Scotian Shelf-Gulf of Maine region. Can. J. Fish. Aquatic Sci. 57, 2536–2546. doi: 10.1139/f00-235
Pham, C. K., Murillo, F. J., Lirette, C., Maldonado, M., Colaço, A., Ottaviani, D., et al. (2019). Removal of deep-sea sponges by bottom trawling in the Flemish Cap area: conservation, ecology and economic assessment. Sci. Rep. 9:15843.
Pile, A. J., and Young, C. M. (2006). The natural diet of a hexactinellid sponge: benthic–pelagic coupling in a deep-sea microbial food web. Deep Sea Res. Part I: Oceanographic Res. Papers 53, 1148–1156. doi: 10.1016/j.dsr.2006.03.008
Reiswig, H. M. (1971). Particle feeding in natural populations of three marine demosponges. Biol. Bull. 141, 568–591. doi: 10.2307/1540270
Rice, A., Thurston, M., and New, A. (1990). Dense aggregations of a hexactinellid sponge, Pheronema carpenteri, in the Porcupine Seabight (northeast Atlantic Ocean), and possible causes. Prog. Oceanography 24, 179–196. doi: 10.1016/0079-6611(90)90029-2
Rix, L., De Goeij, J. M., Mueller, C. E., Struck, U., Middelburg, J. J., Van Duyl, F. C., et al. (2016). Coral mucus fuels the sponge loop in warm-and cold-water coral reef ecosystems. Sci. Rep. 6:18715.
Roberts, E., Mienis, F., Rapp, H., Hanz, U., Meyer, H., and Davies, A. (2018). Oceanographic setting and short-timescale environmental variability at an Arctic seamount sponge ground. Deep Sea Res. Part I: Oceanographic Res. Papers 138, 98–113. doi: 10.1016/j.dsr.2018.06.007
Roberts, J. M., Wheeler, A. J., and Freiwald, A. (2006). Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312, 543–547. doi: 10.1126/science.1119861
Smith, P. C. (1978). Low-frequency fluxes of momentum, heat, salt, and nutrients at the edge of the Scotian Shelf. J. Geophys. Res.: Oceans 83, 4079–4096. doi: 10.1029/jc083ic08p04079
Smith, P. C., Petrie, B., and Mann, C. (1978). Circulation, variability, and dynamics of the Scotian Shelf and slope. J. Fish. Board Canada 35, 1067–1083. doi: 10.1139/f78-170
Sokolova, I. M., Frederich, M., Bagwe, R., Lannig, G., and Sukhotin, A. A. (2012). Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 79, 1–15. doi: 10.1016/j.marenvres.2012.04.003
Song, H., Ji, R., Stock, C., Kearney, K., and Wang, Z. (2011). Interannual variability in phytoplankton blooms and plankton productivity over the Nova Scotian Shelf and in the Gulf of Maine. Mar. Ecol. Prog. Ser. 426, 105–118. doi: 10.3354/meps09002
Strand, R., Whalan, S., Webster, N. S., Kutti, T., Fang, J. K.-H., Luter, H. M., et al. (2017). The response of a boreal deep-sea sponge holobiont to acute thermal stress. Sci. Rep. 7:1660.
Tabachnick, K., Van Soest, R., Van Kempen, T. M., and Braekamn, J. (1994). Distribution of Recent Hexactinellida. Sponges in Time and Space. Rotterdam: Balkema, 225–232.
Tjensvoll, I., Kutti, T., Fosså, J. H., and Bannister, R. (2013). Rapid respiratory responses of the deep-water sponge Geodia barretti exposed to suspended sediments. Aquatic Biol. 19, 65–73. doi: 10.3354/ab00522
Townsend, D., and Ellis, W. (2010). “Primary production and nutrient cycling on the Northwest Atlantic continental shelf,” in Carbon and Nutrient Fluxes in Continental Margins: A global synthesis, eds K.-K. Liu, L. Atkinson, R. Quinones, and L. Talaue-McManus (Berlin: IGBP Book Series. Springer).
Townsend, D. W., Thomas, A. C., Mayer, L. M., Thomas, M. A., and Quinlan, J. A. (2006). Oceanography of the northwest Atlantic continental shelf (1, W). Sea: Global Coastal Ocean: Interdisciplinary Reg. Stud. Syntheses 14, 119–168.
Urrego-Blanco, J., and Sheng, J. (2014). Study on subtidal circulation and variability in the Gulf of St. Lawrence, Scotian Shelf, and Gulf of Maine using a nested-grid shelf circulation model. Ocean Dynamics 64, 385–412. doi: 10.1007/s10236-013-0688-z
van Haren, H., Hanz, U., De Stigter, H., Mienis, F., and Duineveld, G. (2017). Internal wave turbulence at a biologically rich Mid-Atlantic seamount. PLoS One 12:e0189720. doi: 10.1371/journal.pone.0189720
van Haren, H., Mienis, F., Duineveld, G. C., and Lavaleye, M. S. (2014). High-resolution temperature observations of a trapped nonlinear diurnal tide influencing cold-water corals on the Logachev mounds. Prog. Oceanography 125, 16–25. doi: 10.1016/j.pocean.2014.04.021
van Haren, H., Oakey, N., and Garrett, C. (1994). Measurements of internal wave band eddy fluxes above a sloping bottom. J. Mar. Res. 52, 909–946. doi: 10.1357/0022240943076876
Vangriesheim, A., Springer, B., and Crassous, P. (2001). Temporal variability of near-bottom particle resuspension and dynamics at the Porcupine Abyssal Plain, Northeast Atlantic. Prog. Oceanography 50, 123–145. doi: 10.1016/s0079-6611(01)00051-9
Wainright, S. C. (1987). Stimulation of heterotrophic microplankton production by resuspended marine sediments. Science 238, 1710–1712. doi: 10.1126/science.238.4834.1710
Whitney, F., Conway, K., Thomson, R., Barrie, V., Krautter, M., and Mungov, G. (2005). Oceanographic habitat of sponge reefs on the Western Canadian Continental Shelf. Continental Shelf Res. 25, 211–226. doi: 10.1016/j.csr.2004.09.003
Keywords: Vazella pourtalesii, sponge ground, environmental conditions, storm events, particle flux, Scotian Shelf
Citation: Hanz U, Beazley L, Kenchington E, Duineveld G, Rapp HT and Mienis F (2021) Seasonal Variability in Near-bed Environmental Conditions in the Vazella pourtalesii Glass Sponge Grounds of the Scotian Shelf. Front. Mar. Sci. 7:597682. doi: 10.3389/fmars.2020.597682
Received: 21 August 2020; Accepted: 10 December 2020;
Published: 08 January 2021.
Edited by:
Chiara Romano, Center for Advanced Studies of Blanes (CEAB), Spanish National Research Council, SpainReviewed by:
Mar Flexas, California Institute of Technology, United StatesFrine Cardone, Stazione Zoologica Anton Dohrn, Italy
Copyright © 2021 Hanz, Beazley, Kenchington, Duineveld, Rapp and Mienis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ulrike Hanz, VWxyaWtlLmhhbnpAbmlvei5ubA==
†Deceased
 Ulrike Hanz
Ulrike Hanz Lindsay Beazley
Lindsay Beazley Ellen Kenchington
Ellen Kenchington Gerard Duineveld
Gerard Duineveld Hans Tore Rapp
Hans Tore Rapp Furu Mienis
Furu Mienis