- 1Institute of Tropical Biodiversity and Sustainable Development, University Malaysia Terengganu, Kuala Nerus, Malaysia
- 2Research Division, Association for Biodiversity Conservation and Research (ABC), Balasore, India
- 3School of Marine and Environmental Sciences, Universiti Malaysia Terengganu, Kuala Nerus, Malaysia
- 4Forensic Science Programme, School of Health Sciences, Universiti Sains Malaysia, Kota Bharu, Malaysia
- 5Department of Biotechnology, GIET University, Gunupur, India
- 6Department of Biosciences and Biotechnology, Fakir Mohan University, Balasore, India
Understanding local community attitudes toward wildlife and their environment is critical for making sensitive conservation planning and management decisions particularly for conservation-neglected species like Tachypleus gigas. A questionnaire-based interview was carried out on 388 local households from 12 different villages in Balasore, Odisha, between September 2018 and February 2019, which uses a theoretical mapping on attitudes toward horseshoe crabs. We found that 53% of interviewees accepted the presence of horseshoe crabs in their area, 27% have oppressive attitudes, and the remaining 20% were having mixed feelings. Most respondents (>60%) considered horseshoe crabs to bring tangible benefits such as esthetic, monetary, and cultural significance. However, a handful of respondents expressed oppressive attitudes because horseshoe crabs damage their fishing nets (<20%). Both principal component and stepwise analyses revealed that age, gender, and education were demographic components that closely relate local community perceptions toward the conservation-neglected horseshoe crabs that remain threatened by by-catch. We encourage socioeconomic monitoring particularly during rapid economic and infrastructure development to minimize knowledge erosion and to appreciate local ecological knowledge for better conservation management in India.
Introduction
Expansions of human populations in coastal areas result in exploitation (Brash, 1987; Newmark et al., 1993; Walters, 2004) and the reduction of species (Gregory, 2005; Ndenecho, 2009) as precursors to the sixth extinction era (Ceballos et al., 2015; Corlett, 2015). Humans have overlooked the context of biological diversity. Biotic assemblages are present to serve a certain function in the ecosystem (Manfredo, 2008; Rands et al., 2010; Nelson et al., 2018, 2020; Jaffar et al., 2019). Yet humans portray themselves as stakeholders with commensalism attitudes toward resources and their interaction with wildlife in their area (Nepal, 2002; Braga and Schiavetti, 2013; Fakhrul-Hatta et al., 2018; Khalib et al., 2018; Liao et al., 2019). While planning for development, authorities often proposed actions based on top-down decision that may disregard wildlife and communities who inhabit a certain area of interest (Sutherland et al., 2004; Segan et al., 2011). We propose an “otherwise” approach where the community governs and manage their habitat. In this sense, opinions of Odia community were bottom-up suggestions that call for transparent management decisions, particularly for resource exploitation and human–wildlife co-habitations.
India is a South Asian country with large land plains, and 68% of its boundaries are surrounded by seas that support some 1.38 billion humans that unleash anthropic stress to wildlife and surrounding environments, particularly the water bodies (Schipper et al., 2008; Sodhi et al., 2010). We perceived the local communities are engaging themselves in experiential learning from the environment because their livelihood and well-being relate with resource governance in the area. Hence, the gathering of opinions related to non-keystone species produces genuine opinions from the community particularly, when they are sharing resources with this animal. Therefore, we introduce conservation-neglected horseshoe crabs like Tachypleus gigas and Carcinoscorpius rotundicauda as our tool because they are available in India and also throughout Southeast Asia (John et al., 2018; Nong et al., 2020).
Horseshoe crab species currently under conservation purview are Limulus polyphemus and Tachypleus tridentatus because their respective statuses in the wild were recently changed from “data deficient” to “vulnerable” and “endangered” in International Union for Conservation of Nature (IUCN) Red List (Smith et al., 2018; Laurie et al., 2019). This means that T. gigas and C. rotundicauda statuses in the wild are “data deficient” and yet to receive recommendations for updating. Separately, horseshoe crabs spend their entire life cycle (spawning grounds, embryogenesis, nursery grounds, and feeding grounds) interacting with humans (Smith et al., 2009). Therefore, India has placed horseshoe crabs under Wildlife Protection Act 1972 and Fisheries Act 1897 protection. Further, fishery pressure is relieved during the peak spawning season of horseshoe crabs (between May and September), which falls on no-fishing season (April–July) in India (Pati et al., 2015; Pati, 2017; John et al., 2018). Moreover, the Association for Biodiversity Conservation and Research, a non-governmental organization, has implemented projects related to beach cleaning (Mohanty, 2017b; Kundu, 2018), culture and tradition (Mohanty, 2017a), awareness (Pati and Dash, 2016; Pati et al., 2017; Reuters,, 2020), horseshoe crab rescue (Senapati, 2018; Pati, 2019a, b; ENS,, 2020), celebrations (Mohanty, 2020), and art (Patsani, 2018).
However, all efforts are inadequate if knowledge raised from community engagements is not documented and made available to the public. Hence, with an objective to document community perceptions toward horseshoe crabs after a series of conservation-leadership programs, we design our instrument (questionnaire) using layman’s terms so that community members can freely express their opinions without a scientific stigma. The Odia community opinions and views are respected by maintaining a non-disclosure approach while welcoming rural folks into horseshoe crab conservation-based programs in Balasore (Odisha), northeast India. Overall, this community perception survey enables us to understand community values and their attitudes toward horseshoe crabs in India.
Methodology
Experimental Design
The present study involved the participation of coastal communities in Balasore (Odisha, India) within an 88-km2 (N 21°30′; E 86°56′) intersection of the Subarnarekha, Panchupada-Bardia, and Budhabalanga estuaries (Figure 1). Since both rivers extend into northeast Bay of Bengal (the most productive waters in northeast India), capture fisheries and horseshoe crab (Tachypleus gigas) by-catch are human–wildlife interactions of the Odia community. While coastal communities in Baleswar and Bhograi areas (Balasore, Figure 1) are risking their capture fishery efforts with unpredictable weather and stormy waters of the Indian Ocean, we also learned that horseshoe crabs cause fisher net damage and income loss. This is challenging because T. gigas is placed in Schedule IV of Wildlife Protection Act 1972, and deleterious acts toward the listed wildlife (in either schedules) are punishable with fines (between USD 16 and USD 254) that are unaffordable by these communities. However, out of the six schedules, Schedules I and II provide absolute protection, and offenses under these are prescribed with the highest penalties. The horseshoe crab T. gigas is listed in Schedule IV of Wildlife Protection Act 1972, but penalties for violations are minimal (from USD 16 to USD 50). For effective conservation to stabilize horseshoe crab population structure in the wild, absolute protection is needed, and it is imperative that the current Wildlife Protection Act be updated.
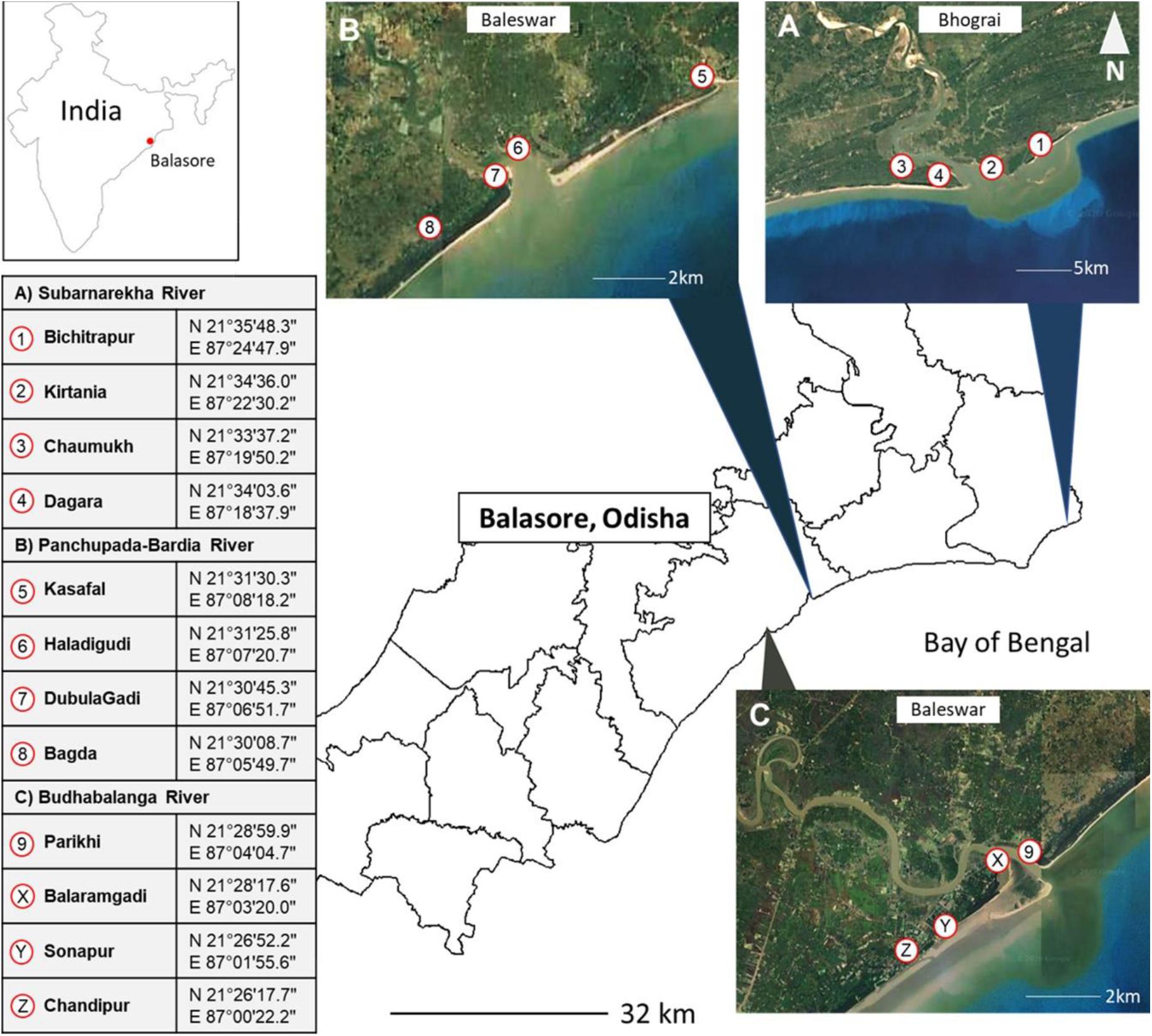
Figure 1. Location of the study site, Balasore in Odisha (East India). This study site indicates the existence of horseshoe crab populations, regardless of Tachypleus gigas or Carcinoscorpius rotundicauda in the area.
Here, we partake discussions with the community before designing our questionnaire. All questions are tested using 50 randomly selected members from the public before every set of questionnaires is validated using Cronbach α test (SPSS v.16, IBM, United States). We adopted the procedures of Creswell and Tashakkori (2007) to amend the questionnaire until Cronbach α test score >0.70 was achieved. A pre-interview (during 16 December 2018) is carried out on another 50 random members from the public to finalize the contents of our questionnaire (Cronbach α = 0.87). With this, our questionnaire covers sociodemographic information, local beliefs on the animal, local knowledge of horseshoe crab, and also their perceptions, attitudes, and opinions toward the horseshoe crab.
Data Collection
We identified a total of 500 households from the Subarnarekha (Bichitrapur, Kirtania, Dagra, and Chaumukha), Panchupada-Bardia (Kasafal, Haladigudi, Dubulagadi, and Bagda), and Budhabalanga (Parikhi, Balaramgadi, Sonapur, and Chandipur) river estuaries using random respondent selection and also “snowball sampling” for their interaction with aquatic species (cf. Newing et al., 2011; Figure 1). We further limited the respondents by their age (between ages 18 and 65), and only one representative is allowed per household (assures responses are independent). Thus, our final sample size is 388 respondents who fulfilled all the desired criteria while also answering most of the questions (>80%). Every respondent needed approximately 1 h both for the face-to-face interview session (verbal consent, identity non-disclosure, and eligibility) in Odia language and to complete the questionnaire with Likert scale (5 = strongly agree, 4 = agree, 3 = neutral, 2 = disagree, and 1 = strongly disagree) and open-ended questions. Our data collection was supported by two volunteers from the Association of Biodiversity Conservation and Research because our intension is to understand coastal community perception on wildlife (marine animal) threats, actions to conserve them, and the willingness to participate in conservation programs (horseshoe crab volunteerism in annual Raksha Bandhan, art contests, participatory horseshoe crab research, beach cleanups, and horseshoe crab tourism) that were held annually in Balasore (Odisha, India).
Data Analysis
We registered all data into both SPSS v.16 (IBM, United States) and also Microsoft Excel as platforms for descriptive and statistical analyses. We develop principal components to analyze the community perceptions using key-word strings. The stepwise analysis that amalgamates Bray–Curtis similarity index and Spearman’s correlation is used to organize data into statistical associations that give the highest value for the best relationship. Both the principal component and stepwise analyses were developed in Primer v.6.1 using protocols of Gorley and Clarke (2006).
Results
Data on Local Knowledge
To explore opinions from 388 respondents, we classify them into age groups of 18–24 (Group 1), 25–34 (Group 2), 35–44 (Group 3), 45–54 (Group 4), and 55–64 (Group 5). The second-line arrangement involved education through the separation of respondents into upper primary (UP), middle elementary (ME), and high school/college (HC) groups (Table 1). However, we only considered opinions of 350 individuals (90.2% of men and women) who possess ME (45.1%) or higher (32.0%) education because they correctly identified the shape and form of horseshoe crab (Tachypleus gigas) from a series of aquatic life pictures (ghost crab, freshwater and marine rays, horseshoe crab in mating pairs, porcelain crab, and hermit crab) presented to them. Only 252 (69.4%, P = 0.921, eigenvalue 5.7, cumulative variation 88.8%) respondents from whom a majority (167 persons) are between 35 and 54 years old (Figure 2A) and possess ME education (Figure 2B) knew about “Nilarakta Kankda” (T. gigas) as mentioned in folk stories from their culture. Comparatively, a majority of 173 individuals aged between 35 and 54 years from the 277 (75.4%, P = 0.927) respondents who knew T. gigas shape and form (particularly women; Figure 2C) associated this arthropod with aphrodisiac tonic, traditional medicine, and alchemy preparations. Only a handful ranging from 12 to 39 female respondents with a majority of them aging 25–54 years were uncertain about T. gigas relevance to their culture (P = 0.898) and tradition (P = 0.726; Table 1), although this arthropod coexist with other fishery resources in their area.
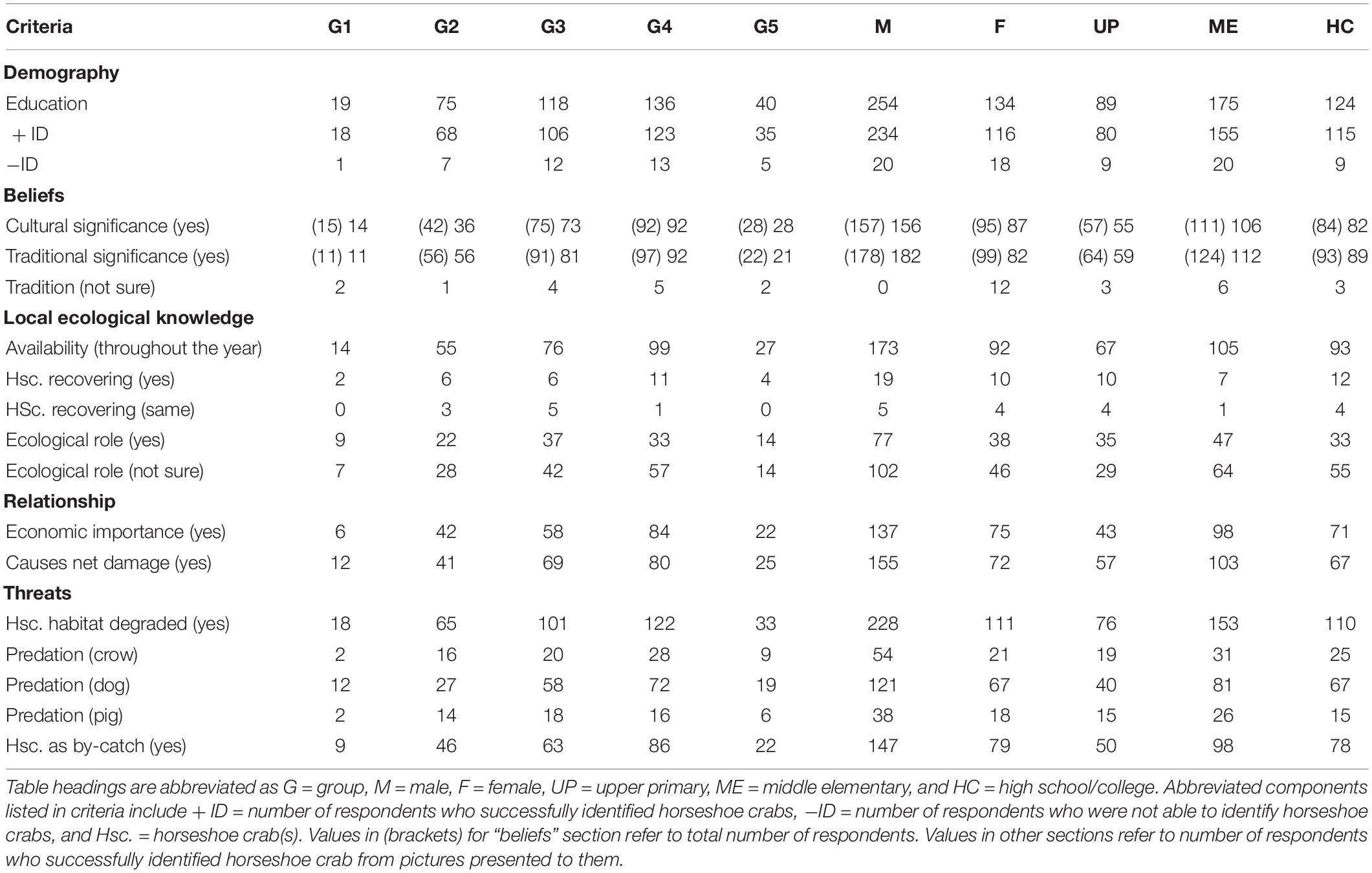
Table 1. Processed data from the instrument (questionnaire) after receiving responses from Odia folks in Balasore.
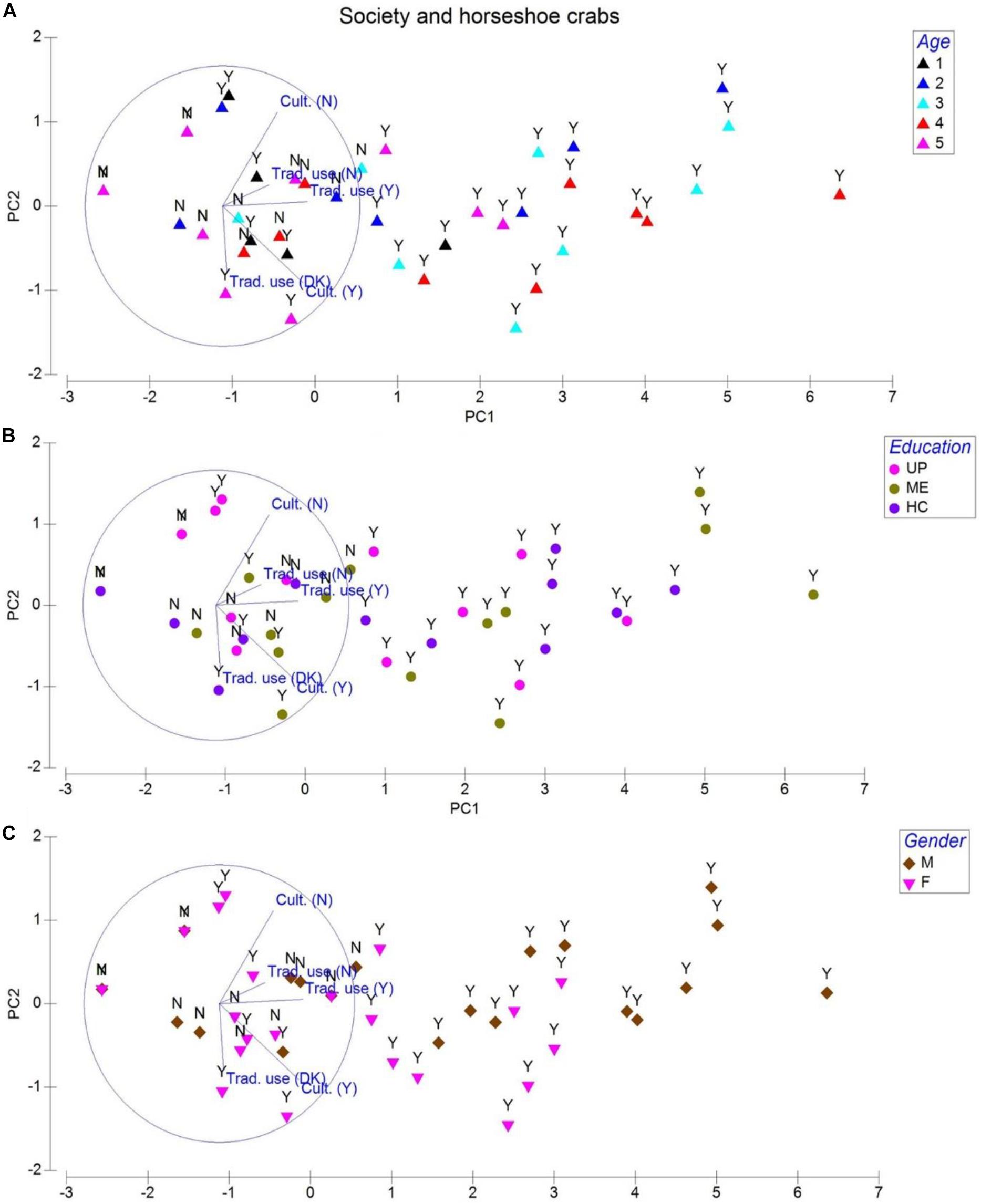
Figure 2. Principal component analysis for society interaction (beliefs) and the horseshoe crab Tachypleus gigas. Abbreviations cult. = culture, Trad. use = traditional use, Y = yes, and N = no are components of the questionnaire that are assigned with values like 1 = yes and 0 = no to develop this analysis. Legends represented by age are 1 = 18–24(Group 1), 2 = 25–34 (Group 2), 3 = 35–44 (Group 3), 4 = 45–54 (Group 4), and 5 = 55–64 (Group 5). Education is denoted with UP = upper primary, ME = middle elementary, and HC = high school. Gender is represented by M = male and F = female respondents.
With 173 male and 92 female respondents acknowledging the year-long availability of T. gigas (P = 0.924), we learned that horseshoe crab emergence into shallow waters was not influenced by season (rain, summer, or winter, P = 0.456, eigenvalue 8.71; cumulative variation 79.9%; Figure 3) in this northeast territory unless the fishermen were fishing exclusively for horseshoe crabs. A total of 29 respondents suggest that environmental conditions vis-à-vis T. gigas populations are recovering (P = 0.454), whereas five male and four female respondents claimed that coastal conditions and horseshoe crab populations remained similar to their first contact with this arthropod (P = 0.457). A vast collection of 321 (91.7%, P = 0.920, Figure 3A) respondents expressed either uncertain or negative views on T. gigas population recovery because their opinions closely relate with wild capture fisheries as resource (Table 1). On the other hand, although 148 individuals (with ME and HC education; Figure 3B) were uncertain about T. gigas role in the environment, 77 (32.9%) male and 38 (32.8%) female respondents expressed views on “biological control” for bivalve and small crab populations after highlighting presence of empty bivalve shells and crab remnants after day-time net casting in the lowest tide (1–1.2 m) waters during the summer months (Figure 3C). These 115 respondents knew about their environment and were able to express their opinions with scientific logic, although these 35 (UP), 47 (ME), and 33 (HC) individuals were deprived of advanced education.
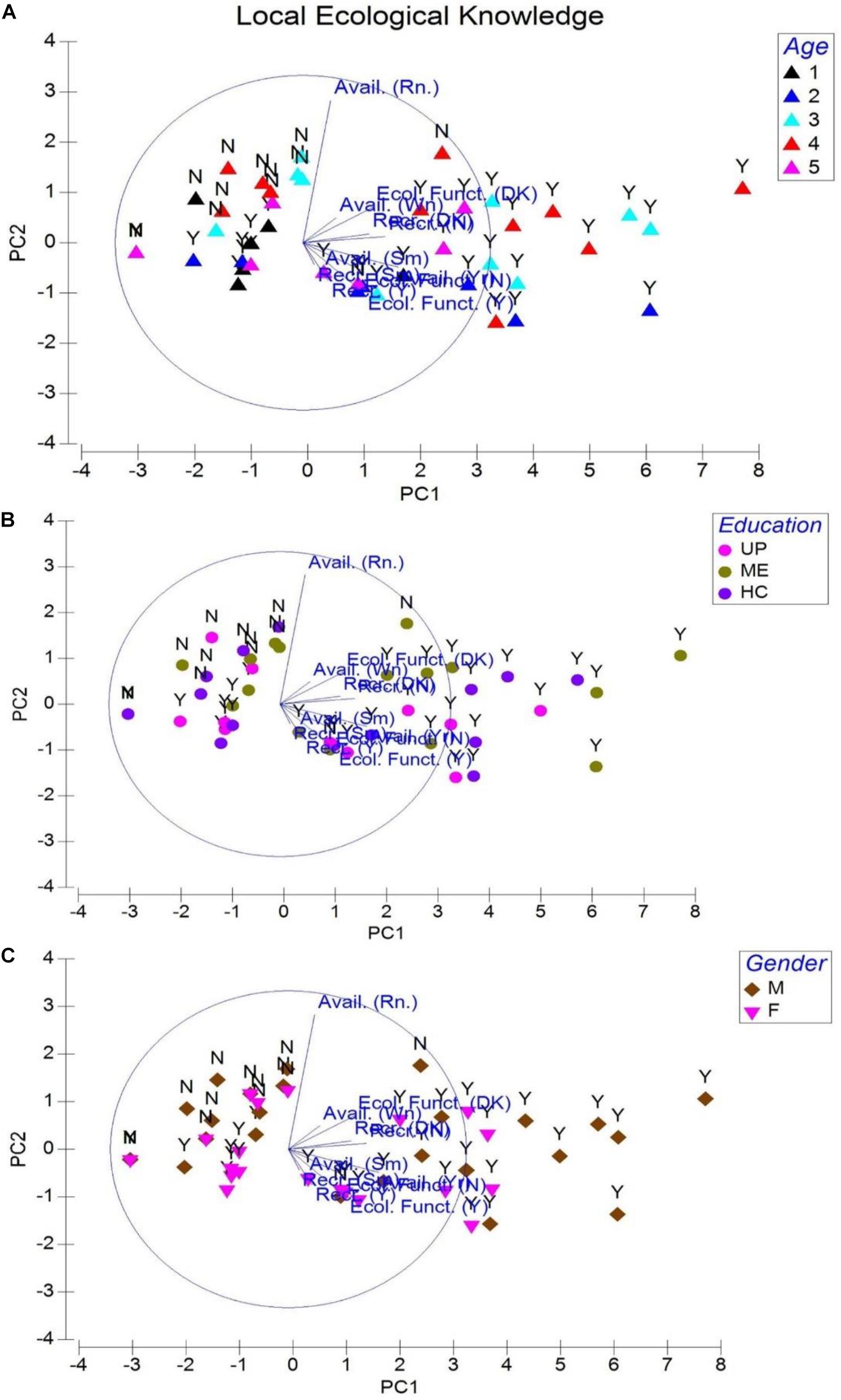
Figure 3. Principal component analysis for local ecological knowledge on Tachypleus gigas. Abbreviations are avail. = availability, Ecol. funct. = ecological function, Recr. = recovery, DK = don’t know, Y = yes and N = no, Rn. = rainy season, Wn. = winter season, and Sm. = summer season are components of the questionnaire that are assigned with values like 1 = yes and 0 = no to develop this analysis. Legends represented by age are 1 = 18–24 (Group 1), 2 = 25–34 (Group 2), 3 = 35–44 (Group 3), 4 = 45–54 (Group 4), and 5 = 55–64 (Group 5). Education is denoted with UP = upper primary, ME = middle elementary, and HC = high school. Gender is represented by M = male and F = female respondents.
Tachypleus gigas and the Odia Community
We learned that 184 individuals aging between 25 and 54 years (from the total of 350 respondents) who correctly identified horseshoe crabs closely associate their livelihood to T. gigas (P = 0.883, eigenvalue 5.63; cumulative variation 84.0%; Figure 4A; Table 1). In their perception, although horseshoe crabs are not fished as alternative protein or exotic food, occasionally, there are middlemen who purchase both male (small) and female (large) T. gigas. However, these middlemen would only pick healthy-looking crabs (absence of encrusting/fouling organisms on the carapace or absence of morphological abnormalities), as these men inform (some respondents) of lucrative demands in the north and west territories of India. While come respondents routinely fish from the estuary, they would not purposely collect T. gigas and sell (USD 0.50 to USD 0.72) these arthropods to middlemen simply because arrival of middlemen are uncertain. The fishermen do not want to be seen with a large number of horseshoe crabs in their possession and fear heavy fines. Moreover, the crabs are often seen in inundated areas for amplexus, but artisanal fishermen make contact with horseshoe crabs during net casting. These individuals either sell or consume all resources (finfish and shellfish including horseshoe crabs) gathered from their routine activity. With this, a total of 212 respondents (P = 0.794) comprising 137 male and 75 female individuals from all age groups (and having UP or ME education; Figure 4B) referred to horseshoe crabs as by-catch. On the contrary, 155 male and 72 female respondents (P = 0.879) expressed dissatisfaction because entangled horseshoe crabs either damage or make their nets unusable (Figure 4C and Table 1). Although fishermen do not immediately discard their nets into the sea when horseshoe crab entangling is common, there are incidences where horseshoe crabs are forcefully removed, but only when T. gigas’ limbs and tail cease to move (inactive or presume dead).
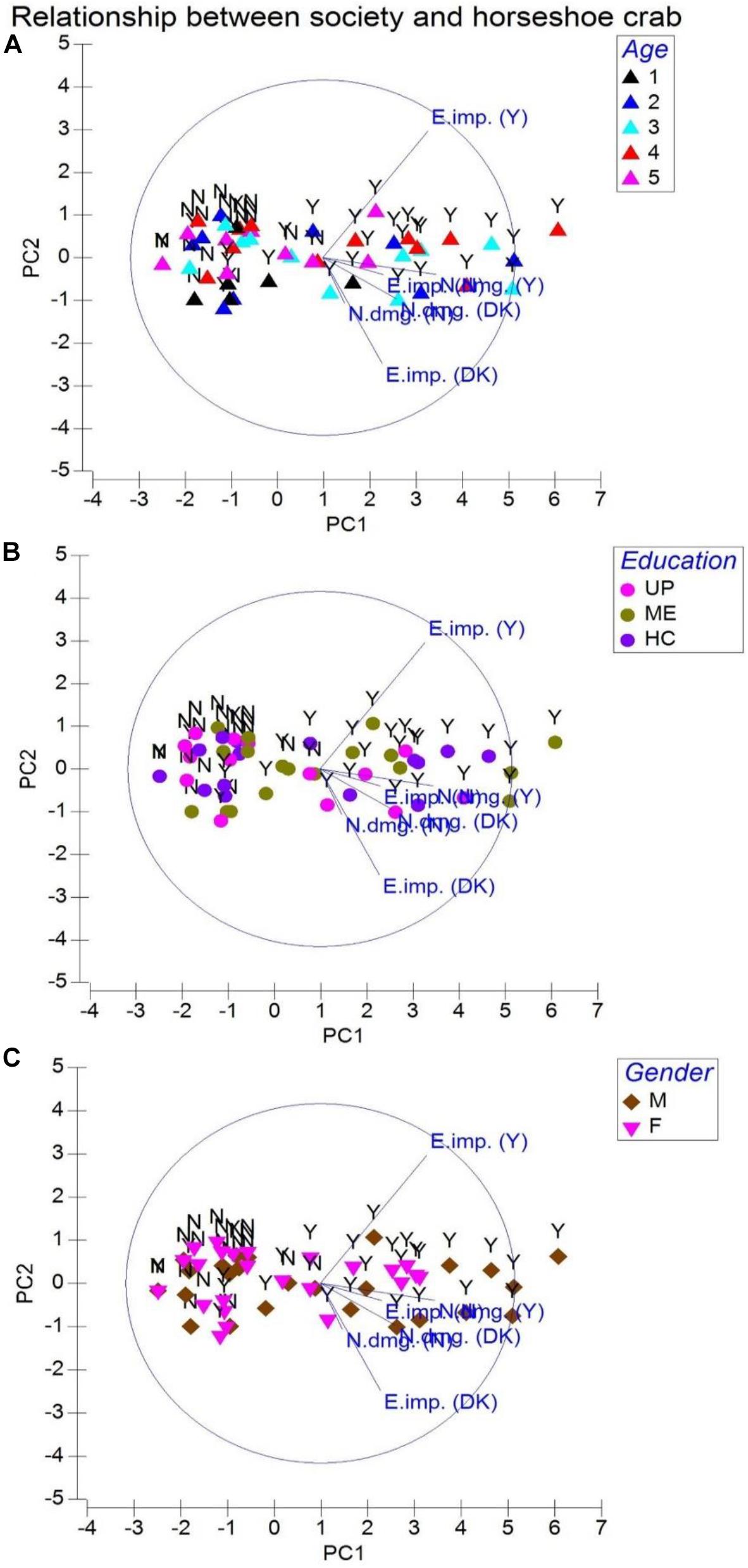
Figure 4. Principal component analysis for society relationships with the horseshoe crab Tachypleus gigas. Abbreviations are E. imp. = ecological importance, N. dmg. = net damage, DK = don’t know, Y = yes, and N = no. Legends represented by age are 1 = 18–24 (Group 1), 2 = 25–34 (Group 2), 3 = 35–44 (Group 3), 4 = 45–54 (Group 4), and 5 = 55–64 (Group 5). Education is denoted with UP = upper primary, ME = middle elementary, and HC = high school. Gender is represented by M = male and F = female respondents.
Capture fisheries are carried out by 226 respondents from age groups 3 and 4 (35–54 years old; 62.9–69.9%, P = 0.941, eigenvalue 8.49; cumulative variation 88.2%; Figure 5A) for their survival, livelihood, and income (Table 1). Therefore, horseshoe crabs are indirectly exploited (by-catch) because all respondents (regardless of their education; Figure 5B) stressed on nets becoming entangled by individual crabs rather than by T. gigas in pairs. The community fears that reduced horseshoe crab capture in pairs relates to reduced finfish and shellfish availability. In their perception, resource depletion may force their community into starvation. On the contrary, 223 respondents (P = 0.958) aging between 35 and 54 were a majority of the 388 respondents who collectively agreed (94.3–100% across all age groups; Figure 5C) that horseshoe crabs and capture fisheries are threatened by degraded habitat. Though not associated, but collectively, 228 men and 111 women made 75 observations of crows, and 188 witnessed accounts of dogs and 56 counts of pig predation on T. gigas (P = 0.735), which suggests that horseshoe crabs are at risk of terrestrial animal predation after becoming stranded or when discarded on the beach (Table 1).
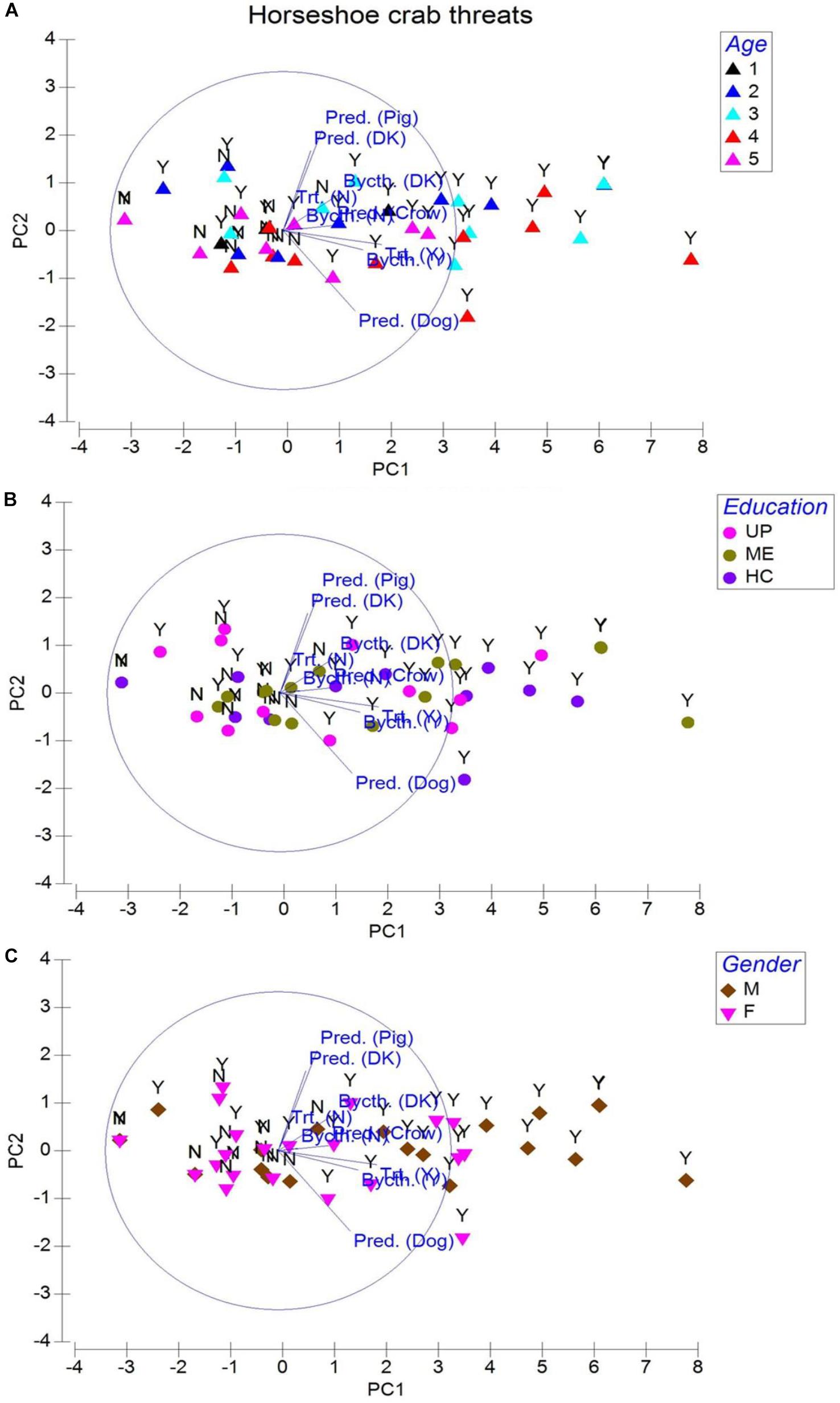
Figure 5. Principal component analysis for society perception of threats to the horseshoe crab, Tachypleus gigas. Abbreviations are pred. = predation, bycth = by-catch, Trt. = threats, DK = don’t know, Y = yes, and N = no. Legends represented by age are 1 = 18–24 (Group 1), 2 = 25–34 (Group 2), 3 = 35–44 (Group 3), 4 = 45–54 (Group 4), and 5 = 55–64 (Group 5). Education is denoted with UP = upper primary, ME = middle elementary, and HC = high school; gender is represented by M = male and F = female respondents.
Discussion
The present study reached out to 117 respondents from Subarnarekha, 119 respondents from Panchupada-Bardia, and 152 respondents from Budhabalanga river estuaries in northeast India where 65% and 35% of the respondents were, respectively, men and women. A vast majority of respondents received ME education (175 persons; 45%) compared with HC (124 persons; 32%) and UP (89 persons; 23%), which means that education was reformed in India to include science, mathematics, and geography (Suchkov, 1959). Education is relevant to their scientific logic when reasoning on biology–ecology opinions (Gafoor and Narayan, 2012). For instance, respondents from Subarnarekha, Panchupada-Bardia, and Budhabalanga estuaries used the term “biological control” for horseshoe crabs against bivalves and small crabs. It shows that these folks understand prey–predator interactions from their basic science education, and also experience working with predators provides them with local ecological knowledge.
We also learned that horseshoe crabs compete with fisher folk for shellfish, but we did not expect that the Odia community ignores the crab’s presence even though this arthropod threatens their livelihood. Instead, the by-catch (horseshoe crab) is removed from nets with caution and returned back to the sea. But when opportunity arises, they are sold to the middlemen for extra income. In addition, most of the respondents were within ages 35–54 (254; 65%), which means that these rural folks may possess traditional and cultural knowledge that was passed from one to the next generation. Perhaps, this passed knowledge allowed them to correctly identify mating horseshoe crab pairs (amplexus), size disparity between male (small) and female (large) crabs, and also their species (“Ram Lakhan,” rounded tail, Carcinoscorpius rotundicauda; and “Nilarakta Kankda,” triangular tail, Tachypleus gigas). Rural communities are known to possess traditional knowledge of forest and aquatic resources (Demunshi and Chugh, 2010), and they pioneer on resource bioprospecting as well as shared-benefit practices (Moran, 2000; Torri, 2011; Pati et al., 2020a). We have learned the benefits of horseshoe crab blood lysate for endotoxin recovery through research, but it was commercialization that lures communities into horseshoe crab exploitation (Bolden et al., 2016). Also, confidence in biomedical bioprospecting concerning blood proteins (tachyplesin-a, b, 1, and 2) gained urban community support for horseshoe crab-derived antibiotics (Dash et al., 2017; Mans, 2017), the use of embryonic peri-vitelline fluid to trigger stem cell differentiation into cardiomyocyte (Ghaskadbi et al., 2008; Alam et al., 2015b) and dendritic cells (Chinnari et al., 2015), and horseshoe crab chitosan use for food preserves and wound healing (Alam et al., 2015a; Pati et al., 2018, 2020b).
We learned about perceived (38 persons) and acquired knowledge (350 persons) through this study because relationships concerning T. gigas and community (culture), its use for traditional applications (tradition), and local ecological knowledge (role, availability, and recovery of T. gigas) were unexplored prior to this study. Tribal traditional knowledge considers arthropods as a dietary and medicinal resource (Jayashankar et al., 2016), whereas coastal communities in India are experts on culture and capture fisheries (Nair et al., 1990; Kumari and Singh, 2020). Unfortunately, past and present research did not focus on T. gigas and C. rotundicauda. Local ecological knowledge that entails sociology and indigenous practices remain top-down and targeted toward community development in India (Davis and Wagner, 2003; Aswani et al., 2018). This means that biodiversity well-being continues to remain neglected. In our approach, community opinions (local economy support and impacts to the community) vis-à-vis expressions on existing threats (resource pool reductions from habitat loss) are relevant with livelihood and ecosystem interactions (cf. Joa et al., 2018) such as social-shared perceptions, values, norms, and also local experiences.
We translate the questionnaire into a conservative mindset for resource pool governance, which also means that there is concern for resource reduction and that the community is attentive toward yield of wildlife. Therefore, our action to replace keystone species like mangroves (Avicennia sp. and Sonneratia sp.) and mud crab (Scylla sp.) with a neglected species like T. gigas allowed us to harness the Odia community’s knowledge about their environment as well as the biology of different types of aquatic species. For instance, we learned from Odia folks that horseshoe crabs are available throughout the year in Subarnarekha, Panchupada-Bardia, and Budhabalanga estuaries. But researchers elsewhere wrongly assumed that horseshoe crabs have seasonal emergence (cf. Moore and Perrin, 2007; John et al., 2012). Compared with researchers, communities reside and constantly utilize their environments. Only after two simultaneous long-term studies were carried out in Malaysia (Nelson et al., 2015, 2016a) and throughout Southeast Asia (John et al., 2018) that it is widely accepted that T. gigas are available throughout the year. Similarly, we learned about the impacts of stranding toward horseshoe crab predation (Fraser et al., 2010; Pati and Dash, 2016), but the Odia community already witnessed this form of opportunistic predation on T. gigas by many other animals like crow, pigs, and dogs; all of these would have remained undocumented if not for this study.
Horseshoe crabs like T. gigas and C. rotundicauda do not receive conservation attention (ca. Smith, 1993; Sekhar, 2003; Tsetan and Ramanibai, 2011; Behera et al., 2014; Gupta et al., 2014) when compared with the Bengal tiger (Panthera tigris tigris), South Asian river dolphin (Platanista gangetica), Indian flapshell turtle (Lissemys punctata), or golden mahseer (Tor putitora), although the horseshoe crabs are listed in Wildlife Protection Act 1972 in India (John et al., 2018). These arthropods are less received for conservation projects in Indonesia. A similar (to the present) type of study was carried out on 277 respondents, but differently, the findings show that T. gigas is impacted by fishing gear (gill nets and bottom trawl), its habitat is impoverished by rubbish dumping, and the crab is consumed by locals in 62 districts of Java, Sumatra, Kalimantan, and Sulawesi Island (Meilana and Fang, 2020). With this, the Indian horseshoe crabs remain excluded from IUCN Red List updating simply because information on their population size and spatial occupancy by country and by region remains incompletely explored. For instance, studies in Thailand, Cambodia, and Vietnam relate horseshoe crabs to tetrodotoxin poisoning, but these studies do not map their availability and population size (John et al., 2018).
Although the coastal community of Balasore exploits finfish and shellfish through capture fisheries, they are governed by rules and regulations of Fisheries Act 1897. A seasonal fishing ban regulated by Fisheries Act 1897 was imposed for 45 days since 1998 in fishing zones (0–12 nautical miles) of maritime states (east and west coasts). This ban was revised to 60 days (15 April to 14 June onward year 2015) for resource recovery (Narayanakumar et al., 2017). Unfortunately, some respondents assert that horseshoe crab populations are not reducing since the last decade. Perhaps, these folks organize deep sea fishing where chances to entrap T. gigas in their nets are low. Comparatively, vast majority of Odia folks from Subarnarekha, Panchupada-Bardia, and Budhabalanga estuaries (Balasore) are near shore fishers. They witness unusual reductions in mating horseshoe crab pairs becoming entangled in their casted nets particularly when these folks compare the yields during their early fishing experiences. Researchers would rely on sampling time and project money to monitor horseshoe crab populations (Pati et al., 2015, 2017), and the outcome would be similar to ecological knowledge extractions from the Odia community.
While horseshoe crab spawning grounds are situated in shallow surf protected areas (estuaries or shallow seafront), these grounds are easily accessible, attractive for human encroachments (Berkson et al., 2009; Faurby et al., 2010; Lee and Morton, 2016; Nelson et al., 2019; Zauki et al., 2019a), and therefore, would provide substantial evidence on the declining population size of Asian horseshoe crabs (Nelson et al., 2016b; Pati et al., 2017; Fairuz-Fozi et al., 2018; John et al., 2018; Zauki et al., 2019b). Fortunately, the Odia community in Balasore relates “Nilarakta Kankda” with memory, grace, and love, whereas “Ram Lakhan” regards the crab as a goddess (grace and completion). Therefore, our 5-year continuous monitoring through citizen science conservation projects shows that Odia folks do not harm horseshoe crabs, although the crabs become entangled in the nets. This is strong evidence that local ecological knowledge has an outline of conservation awareness, which is constantly practiced by rural communities, although in the presence of local economy threats (net damage and resource competition).
Conclusion and Recommendations
Horseshoe crab entry into the sixth extinction era is possible. Likewise, local ecological knowledge baselines are shifting by each passing human generation. This study shows that we wrongly practice conservation because our efforts are top-down due to community absence in policymaking and land-use decisions. In fact, by acknowledging people’s perceptions, we are reducing expenditure on monitoring programs and, instead, use these funds for awareness projects and compassion instilment among folks in vulnerable areas. This questionnaire communicates science at the community level of understanding, and it appreciates community experiences and their interaction with horseshoe crabs. We highly recommend reliance on artisanal fishers who already bridged gaps between acquired and perceived knowledge in their area through their experiences. This study also creates awareness on fishing gears regardless of their application because these instruments cause horseshoe crab removal and unintentional mortalities in capture fisheries. In short, we recommend conservation projects that educate and empower communities with ecological roles whereby their interaction with communities and leaders enhance monitoring and surveillance. This bottom-up approach allows communities to convey opinions that then are easily translated by researchers into practical applications for policies and resource good-governance, particularly conservation-neglected aquatic species like the Indian horseshoe crabs.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors.
Ethics Statement
Ethical review and approval was not required for the study on human participants, in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author Contributions
SP and BRN conceived and designed and performed the survey, analyzed the data, wrote the manuscript, prepared figures and tables, and reviewed drafts of the manuscript. DA, BRN, BPD, and HAE reviewed drafts of the manuscript and gave their support during fieldwork. SS and HAE analyzed the data and reviewed drafts of the manuscript. SP, SS, HAE, BRN, DA, and BPD approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Conservation Leadership Programme, United Kingdom (CLP ID: 03418018) and Rufford Foundation, United Kingdom (ID: 24630-1).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alam, H., Chinnari, S., Pati, S., Dash, B. P., and Chatterji, A. (2015a). A first record on the role of peri-vitelline fluid of the fertilized eggs of Indian horseshoe crab (Tachypleus gigas; Müller) promoting wound healing process in vitro. Int. J. Adv. Lif. Sci. 8, 411–415.
Alam, H., Chinnari, S., Pati, S., Dash, B. P., and Chatterji, A. (2015b). A horseshoe crab peri-vitelline fluid triggers the human bone marrow stem cell differentiation into cardiomyocyte in vitro. Cell. Dev. Biol. 4, 3–8. doi: 10.4172/2168-9296.1000162
Aswani, S., Lemahieu, A., and Sauer, W. H. (2018). Global trends of local ecological knowledge and future implications. PLoS One 13:e0195440. doi: 10.1371/journal.pone.0195440
Behera, S. K., Singh, H., and Sagar, V. (2014). “Indicator species (Gharial and Dolphin) of riverine ecosystem: an exploratory of River Ganga,” in Our National River Ganga. ed. R. Sanghi, (Cham: Springer), 121–141. doi: 10.1007/978-3-319-00530-0_4
Berkson, J., Chen, C. P., Mishra, J., Shin, P., Spear, B., and Zaldívar-Rae, J. (2009). “A discussion of horseshoe crab management in five countries: Taiwan, India, China, United States, and Mexico,” in Biology and Conservation of Horseshoe Crabs, eds J. Tanacredi, M. Botton, and D. Smith, (Boston, MA: Springer), 465–475. doi: 10.1007/978-0-387-89959-6_29
Bolden, J. S., Warburton, R. E., Phelan, R., Murphy, M., Smith, K. R., De Felippis, M. R., et al. (2016). Endotoxin recovery using limulus amebocyte lysate (LAL) assay. Biologicals 44, 434–440. doi: 10.1016/j.biologicals.2016.04.009
Braga, H., and Schiavetti, A. (2013). Attitudes and local ecological knowledge of experts fishermen in relation to conservation and bycatch of sea turtles (reptilia: testudines). Southern Bahia, Brazil. J. Ethnobiol. Ethnomed. 9:15. doi: 10.1186/1746-4269-9-15
Brash, A. R. (1987). The history of avian extinction and forest conversion on Puerto Rico. Biol. Conserv. 39, 97–111. doi: 10.1016/0006-3207(87)90028-0
Ceballos, G., Ehrlich, P. R., Barnosky, A. D., García, A., Pringle, R. M., and Palmer, T. M. (2015). Accelerated modern human–induced species losses: entering the sixth mass extinction. Sci. Adv. 1:e1400253. doi: 10.1126/sciadv.1400253
Chinnari, S., Pati, S., Dash, B. P., and Chatterji, A. (2015). A new record on inducement of differentiation of dendritic cells by the peri-vitelline fluid of the fertilized eggs of indian horseshoe crab (Tachypleus gigas. Müller). Bio. Forum Int. J. 7, 678–681.
Corlett, R. T. (2015). The anthropocene concept in ecology and conservation. Trends Ecol. Evol. 30, 36–41. doi: 10.1016/j.tree.2014.10.007
Creswell, J., and Tashakkori, A. (2007). Differing perspectives on mixed methods research. J. Mix. Methods Res. 1, 303–308. doi: 10.1177/1558689807306132
Dash, B. P., Pati, S., Mohanty, B. P., and Mahanty, A. (2017). 2-dimensional gel electrophoresis profiles of hemolymph of horseshoe crabs Tachypleus gigas and Carcinoscorprius rotundicauda. J. Inland Fish. Soc. India 49, 70–72.
Davis, A., and Wagner, J. R. (2003). Who knows? on the importance of identifying “experts” when researching local ecological knowledge. Hum. Ecol. 31, 463–489. doi: 10.1023/A:1025075923297
Demunshi, Y., and Chugh, A. (2010). Role of traditional knowledge in marine bioprospecting. Biodiv. Conserv. 19, 3015–3033. doi: 10.1007/s10531-010-9879-9
ENS, (2020). Six Horseshoe Crabs Seized in Odisha, Man Arrested. Available at: https://www.newindianexpress.com/states/odisha/2020/jan/03/six-horseshoe-crabs-seized-in-odisha-man-arrested-2084408.html [Accessed July 23, 2020]
Fairuz-Fozi, N., Satyanarayana, B., Zauki, N. A. M., Muslim, A. M., Husain, M. L., Ibrahim, S., et al. (2018). Carcinoscorpius rotundicauda (Latreille, 1802) population status and spawning behaviour at Pendas coast. Peninsular Malaysia. Glob. Ecol. Conserv. 15:e00422. doi: 10.1016/j.gecco.2018.e00422
Fakhrul-Hatta, S. N. N., Nelson, B. R., Shafie, N. J., Zahidin, M. A., and Abdullah, M. T. (2018). Linkages between chiropteran diversity and ecosystem services for sustainable fragmented forest conservation. Data Brief 21, 2089–2094. doi: 10.1016/j.dib.2018.11.058
Faurby, S., King, T. L., Obst, M., Hallerman, E. M., Pertoldi, C., and Funch, P. (2010). Population dynamics of American horseshoe crabs—historic climatic events and recent anthropogenic pressures. Mol. Ecol. 19, 3088–3100. doi: 10.1111/j.1365-294X.2010.04732.x
Fraser, J. D., Karpanty, S. M., and Cohen, J. B. (2010). Shorebirds forage disproportionately in horseshoe crab nest depressions. Waterbirds 33, 96–100. doi: 10.1675/063.033.0111
Gafoor, K. A., and Narayan, S. (2012). Out-of-school experience categories influencing interest in science of upper primary students by gender and locale: exploration on an Indian sample. Sci. Educ. Int. 23, 191–204.
Ghaskadbi, S., Patwardhan, V., Banerjee, M., Agarwal, S., Lenka, N., Verma, M. K., et al. (2008). Enhancement of vertebrate cardiogenesis by a lectin from perivitelline fluid of horseshoe crab embryo. Cell. Mol. Life Sci. 65, 3312–3324. doi: 10.1007/s00018-008-8246-4
Gorley, C. K., and Clarke, K. R. (2006). PRIMER v6: User Manual/Tutorial. Plymouth: PRIMER-E, 65–132.
Gregory, T. (2005). Conflict between global and local land use values in Larvia’s Gauja National Park. Landsc. Res. 30, 415–430. doi: 10.1080/01426390500171227
Gupta, N., Sivakumar, K., Mathur, V. B., and Chadwick, M. A. (2014). The ‘tiger of Indian rivers’: stakeholders’ perspectives on the golden mahseer as a flagship fish species. Area 46, 389–397. doi: 10.1111/area.12124
Jaffar, M., Yunus, N. M., and Nelson, B. R. (2019). Regional tinfoil barb imports can alter its native species genetic makeup. J. Sustain. Sci. Manag. 14, 51–65.
Jayashankar, M., Charles, M., Arya, V. V., and Hegde, J. (2016). “utility of arthropods by indigenous communities: sustaining natural resources. in economic and ecological significance of arthropods,” in Diversified Ecosystems, eds A. Chakravarthy and S. Sridhara, (Singapore: Springer), 117–131. doi: 10.1007/978-981-10-1524-3_6
Joa, B., Winkel, G., and Primmer, E. (2018). The unknown known–A review of local ecological knowledge in relation to forest biodiversity conservation. Land use policy 79, 520–530. doi: 10.1016/j.landusepol.2018.09.001
John, B. A., Kamaruzzaman, B., Jalal, K., and Zaleha, K. (2012). Feeding ecology and food preferences of Carcinoscorpius rotundicauda collected from the Pahang nesting grounds. Sains Malays. 41, 855–861.
John, B. A., Nelson, B. R., Sheikh, H. I., Cheung, S. G., Wardiatno, Y., Dash, B. P., et al. (2018). A review on fisheries and conservation status of Asian horseshoe crabs. Biodiv. Conserv. 27, 3573–3598. doi: 10.1007/s10531-018-1650-7
Khalib, N. K. A., Shafie, N. J., Basri, H. H., Nelson, B. R., and Abdullah, M. T. (2018). Non-volant small mammal data from fragmented forests in Terengganu State. Data Brief 21, 1514–1520. doi: 10.1016/j.dib.2018.10.061
Kumari, A., and Singh, S. K. (2020). Crab culture practices in sundarbans. Stud. Indian Place Names 40, 2212–2221.
Kundu, S. (2018). Beach Cleaning Drive by ABC. Available at: https://www.thestatesman.com/cities/beach-cleaning-drive-abc-1502617498.html [Accessed July 23, 2020]
Laurie, K., Chen, C. P., Cheung, S. G., Do, V., Hsieh, H., John, A., et al. (2019). Tachypleus Tridentatus (errata version published in 2019)- the IUCN Red List of Threatened Species. e. T21309A149768986. Available at: http://dx.doi.org/10.2305/IUCN. UK. 2019-1.RLTS.T21309A149768986.en [Accessed Dec 13, 2019]
Lee, C. N. W., and Morton, B. (2016). Changes in the distributions of juvenile horseshoe crabs (Arthropoda: Chelicerata)(2002–2014) related to environmental perturbations at Pak Nai and Ha Pak Nai, Deep Bay, Hong Kong SAR, China. Mar. Pollut. Bull. 108, 134–146. doi: 10.1016/j.marpolbul.2016.04.037
Liao, Y., Hsieh, H. L., Xu, S., Zhong, Q., Lei, J., Liang, M., et al. (2019). Wisdom of Crowds reveals decline of Asian horseshoe crabs in Beibu Gulf. China. Oryx 53, 222–229. doi: 10.1017/S003060531700117X
Manfredo, M. J. (2008). “Attitudes and the study of human dimensions of wildlife,” in Who Cares About Wildlife?, ed. M. J. Manfredo (New York, NY: Springer), 75–109. doi: 10.1007/978-0-387-77040-6_4
Mans, D. R. (2017). Exploring the global animal biodiversity in the search for new drugs–spiders, scorpions, horseshoe crabs, sea spiders, centipedes, and millipedes. J. Transl. Sci. 3, 1–18. doi: 10.15761/JTS.1000197
Meilana, L., and Fang, Q. (2020). Local knowledge-based study on the status of horseshoe crabs along the Indonesian coast. Reg. Stud. Mar. Sci. 36:101252. doi: 10.1016/j.rsma.2020.101252
Mohanty, K. K. (2020). International Horseshoe Crab Day Celebrated. http://odiabarta.in/32495/?fbclid=IwAR0GeRPmL3ydSwFTSPkK4O7A7O mnZlu432K6FkAYXGSNU8us1I8vQ73k6rc [Accessed July 23, 2020]
Mohanty, U. (2017a). B’swar Conservationists tie Rakhi to Crabs. https://www.dailypioneer.com/2017/state-editions/bswar-conservationists-tie-rakhi-to-crabs.html [Accessed July 23, 2020]
Mohanty, U. (2017b). Chandipur Beach Cleaned of Litter for Crab Breeding. https://www.dailypioneer.com/2017/sunday-edition/chandipur-beach-cleaned-of-litter-for-crab-breeding.html[Accessed July 23, 2020]
Moore, S., and Perrin, S. (2007). Seasonal movement and resource-use patterns of resident horseshoe crab (Limulus polyphemus) populations in a Maine, USA estuary. Estuaries Coasts 30, 1016–1026. doi: 10.1007/BF02841392
Moran, K. (2000). Bioprospecting: lessons from benefit-sharing experiences. Int. J. Biotechnol. 2, 132–144. doi: 10.1504/IJBT.2000.000133
Nair, S. R., Parulekar, A. H., and Desai, B. N. (1990). Research in the assessment of capture and culture fisheries along the Indian coast. Bull. Natl. Symp. Res. Dev. Mar. Fish. Ses. III & IV 1987, 297–305.
Narayanakumar, R., Shyam, S. S., Geetha, R., Swathi Lekshmi, P. S., Jayasankar, J., Ganga, U., et al. (2017). Transaction cost of implementation of seasonal fishing ban in selected maritime states of India. Mar. Fish. Infor. Sew. T & E Ser. 232, 7–10.
Ndenecho, E. N. (2009). Ecological planning and ecotourism development in Kimbi Game Reserve. Cameroon. J. Hum. Ecol. 27, 105–113. doi: 10.1080/09709274.2009.11906198
Nelson, B. R., David, G., Mokhtar, A. F., Mamat, M. A., and Rahman, A. J. A. (2018). Avian data from Kenyir rainforest trail. Data Brief 21, 2633–2637. doi: 10.1016/j.dib.2018.11.119
Nelson, B. R., Mamat, M. A., Cheeho, W., and Shahimi, S. (2020). Forest birds as diversity indicator in suburban and residential areas. Ecofem. Climate Change 1, 1–6.
Nelson, B. R., Satyanarayana, B., Moh, J. H., Ikhwanuddin, M., Chatterji, A., and Shaharom, F. (2016a). The final spawning ground of Tachypleus gigas (Müller, 1785) on the east Peninsular Malaysia is at risk: a call for action. PeerJ 4:e2232. doi: 10.7717/peerj.2232
Nelson, B. R., Satyanarayana, B., Moh, J. H., and Shaharom, F. (2016b). Does human infringement at the spawning grounds challenge horseshoe crab eggs and their embryogenesis? J. Sustain. Sci. Manag. Special Issue 1: Int. Sem. Straits Malacca South China Sea 1–10.
Nelson, B. R., Satyanarayana, B., Zhong, J. M. H., Shaharom, F., Sukumaran, M., and Chatterji, A. (2015). Episodic human activities and seasonal impacts on the Tachypleus gigas (Müller, 1795) population at Tanjung Selangor in Peninsular Malaysia. Estuar. Coast. Shelf. Sci. 164, 313–323. doi: 10.1016/j.ecss.2015.08.003
Nelson, B. R., Zhong, J. M. H., Zauki, N. A. M., Satyanarayana, B., and Chowdhury, A. J. K. (2019). Effects of shore sedimentation to Tachypleus gigas(Müller, 1785) spawning activity from Malaysian waters. J. Sustain. Sci. Manag. 14, 41–60.
Nepal, S. K. (2002). Mountain ecotourism and sustainable development. Mt. Res. Dev. 22, 104–109. doi: 10.1659/0276-4741(2002)022[0104:measd]2.0.co;2
Newing, H., Eagle, C., Puri, R., and Watson, C. (2011). Conducting Research in Conservation: A Social Science Perspective. Abingdon. Routledge, doi: 10.4324/9780203846452
Newmark, W. D., Leonard, N. L., Sraiko, H. I., and Gamassa, D. M. (1993). Conservation attitudes of local people living adjacent to five protected areas in Tanzania. Biol. Conserv. 63, 177–183. doi: 10.1016/0006-3207(93)90507-w
Nong, W., Qu, Z., Li, Y., Owen, T. B., Wong, A. Y., Yip, H. Y., et al. (2020). Horseshoe crab genomes reveal the evolutionary fates of genes and microRNAs after three rounds (3R) of whole genome duplication. bioRxiv [preprint] doi: 10.1101/2020.04.16.045815
Pati, S. (2017). Cleaning the breeding beaches and awareness event for horseshoe crab conservation along Chandipur coast and nearby estuaries in Odisha. Zoo’s Print 32, 46–48. doi: 10.13140/RG.2.2.15191.37289
Pati, S. (2019a). Three Hundred Horseshoe Crabs Rescued in Bay of Bengal, India. Available at: http://www.conservationleadershipprogramme.org/news/three-hundred-horseshoe-crabs-rescued-in-bay-of-bengal-india/ [Accessed July 23, 2020]
Pati, S. (2019b). Trade and by-catch Assessment of Indian Horseshoe Crab Along with its Conservation by Integrating Education and Awareness Among Community Along Balasore Coast. Final Report Produced for Rufford Foundation. Available at: https://www.rufford.org/files/24630-1%20Final%20Report.pdf, [Accessed 31August, 31 2019]
Pati, S., and Dash, B. P. (2016). Horseshoe crab (Tachypleus gigas) as prey of domestic pig (Susdomesticus) in Khandia estuary, Balasore, Odisha, India. Zoo’s Print 31, 14–15. doi: 10.11609/zp.v31i5.677
Pati, S., Biswal, G. C., and Dash, B. P. (2015). Availability of Tachypleus gigas (Müller) along the river estuaries of Balasore district, Odisha, India. Int. J. Fish. Aquat. Stud. 2, 334–336.
Pati, S., Chatterji, A., and Dash, B. P. (2018). Chitosan from the carapace of Indian horseshoe crab (Tachypleus gigas, Müller): isolation and its characterization. Adv. Biores. 9, 52–64. doi: 10.15515/abr.0976-4585.9.4.5264
Pati, S., Jena, P., Shahimi, S., Raveen Nelson, B., Acharya, D., Dash, B. P., et al. (2020a). Characterization dataset for pre- and post-irradiated shrimp waste chitosan. Data Brief. 32:106081 doi: 10.1016/j.dib.2020.106081
Pati, S., Chatterji, A., Dash, B. P., Raveen Nelson, B., Sarkar, T., Shahimi, S., et al. (2020b). Structural Characterization and Antioxidant Potential of Chitosan by γ-Irradiation from the Carapace of Horseshoe Crab. Polymers 12:2361. doi: 10.3390/polym12102361
Pati, S., Tudu, S., Rajesh, A., Biswal, G. C., Chatterji, A., Dash, B. P., et al. (2017). Manmade activities affected the breeding ground of horseshoe crab (Tachypleus gigas) along Balasore coast: call for immediate conservation. E-Planet 15, 145–154.
Patsani, M. (2018). Artist from Odisha Displays Paintings Using Lacquer in New Delhi. Available at: https://www.mycitylinks.in/artist-from-odisha-displays-paintings-using-lacquer-in-new-delhi [Accessed July 23, 2020]
Rands, M. R., Adams, W. M., Bennun, L., Butchart, S. H., Clements, A., Coomes, D., et al. (2010). Biodiversity conservation: challenges beyond 2010. Science 329, 1298–1303. doi: 10.1126/science.1189138
Reuters, (2020). Wildlife Groups Pressure Pharmas to Curb Crab Blood Addiction. Available at: https://economictimes.indiatimes.com/news/science/wildlife-groups-pressure-pharmas-to-curb-crab-blood-addiction/crab-blood-used-for-covid-vaccine-tests/slideshow/76171434.cms [Accessed July 23, 2020].
Schipper, J., Chanson, J. S., Chiozza, F., Cox, N. A., Hoffmann, M., Katariya, V., et al. (2008). The status of the world’s land and marine mammals: diversity, threat, and knowledge. Science 322, 225–230. doi: 10.1126/science.1165115
Segan, D. B., Bottrill, M. C., Baxter, P. W., and Possingham, H. P. (2011). Using conservation evidence to guide management. Conserv. Biol. 25, 200–202. doi: 10.1111/j.1523-1739.2010.01582.x
Sekhar, N. U. (2003). Local people’s attitudes towards conservation and wildlife tourism around Sariska Tiger Reserve. India. J. Environ. Manage. 69, 339–347. doi: 10.1016/j.jenvman.2003.09.002
Senapati, A. (2018). In 2 Years, 1 Lakh Endangered Horseshoe Crabs Saved in Odisha. Down to Earth. Available online at: https://www.downtoearth.org.in/news/wildlife-biodiversity/in-2-years-1-lakh-endangered-horseshoe-crabs-saved-in-odisha-62101 (accessed July 23, 2020).
Smith, B. D. (1993). 1990 Status and conservation of the Ganges River dolphin Platanista gangetica in the Karnali River. Nepal. Biol. Conserv. 66, 159–169. doi: 10.1016/0006-3207(93)90002-I
Smith, D. R., Beekey, M. A., Brockmann, H. J., King, T. L., Millard, M. J., and Zaldívar-Rae, J. A. (2018). Limulus Polyphemus- the IUCN Red List of Threatened Species 2016: e. T11987A80159830. Gland: IUCN. doi: 10.2305/IUCN.UK.2016-1
Smith, D. R., Millard, M. J., and Carmichael, R. H. (2009). “Comparative status and assessment of Limulus polyphemus with emphasis on the New England and Delaware Bay populations,” in Biology and Conservation of Horseshoe Crabs, eds J. Tanacredi, M. Botton, and D. Smith, (Boston, MA: Springer), 361–386. doi: 10.1007/978-0-387-89959-6_23
Sodhi, N. S., Posa, M. R. C., Lee, T. M., Bickford, D., Koh, L. P., and Brook, B. W. (2010). The state and conservation of Southeast Asian biodiversity. Biodiv. Conserv. 19, 317–328. doi: 10.1007/s10531-009-9607-5
Suchkov, N. V. (1959). The reform of elementary education in India. Sov. Educ. 1, 54–58. doi: 10.2753/RES1060-9393011254
Sutherland, W. J., Pullin, A. S., Dolman, P. M., and Knight, T. M. (2004). The need for evidence-based conservation. Trends Ecol. Evol. 19, 305–308. doi: 10.1016/j.tree.2004.03.018
Torri, M. C. (2011). Bioprospecting and commercialisation of biological resources by indigenous communities in India: moving towards a new paradigm? Sci. Technol. Soc. 16, 123–146. doi: 10.1177/097172181001600201
Tsetan, C., and Ramanibai, R. (2011). Reptilian fauna of agricultural landscapes of Chembarambakkam Lake, Chennai, Tamil Nadu. Reptile Rap 13, 2–8.
Walters, B. B. (2004). Local management of mangrove forests in the Phillipines: successful conservation or efficient resource exploitation? Hum. Ecol. 32, 177–195. doi: 10.1023/b:huec.0000019762.36361.48
Zauki, N. A. M., Satyanarayana, B., Fairuz-Fozi, N., Nelson, B. R., Martin, M. B., Akbar-John, B., et al. (2019a). Citizen science frontiers horseshoe crab population regain at their spawning beach in East Peninsular Malaysia. J. Environ. Manage 232, 1012–1020. doi: 10.1016/j.jenvman.2018.12.002
Keywords: community, threats, estuary, relationship, fisheries, management
Citation: Pati S, Shahimi S, Edinur HA, Nelson BR, Acharya D and Dash BP (2020) Extraction of People’s Perception Toward Horseshoe Crab Existence in Northeast Coast of India. Front. Mar. Sci. 7:587335. doi: 10.3389/fmars.2020.587335
Received: 25 July 2020; Accepted: 12 October 2020;
Published: 12 November 2020.
Edited by:
Siu Gin Cheung, City University of Hong Kong, Hong KongReviewed by:
Brett W. Molony, Oceans and Atmosphere (CSIRO), AustraliaMenghong Hu, Shanghai Ocean University, China
Copyright © 2020 Pati, Shahimi, Edinur, Nelson, Acharya and Dash. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bryan Raveen Nelson, YnJ5YW4ubmVsc29uQHVtdC5lZHUubXk=; Hisham Atan Edinur, ZWRpbnVyQHVzbS5teQ==; Siddhartha Pati, cGF0aXNpZGRoYXJ0aGFAZ21haWwuY29t
 Siddhartha Pati
Siddhartha Pati Salwa Shahimi1,3
Salwa Shahimi1,3 Hisham Atan Edinur
Hisham Atan Edinur Bryan Raveen Nelson
Bryan Raveen Nelson Diptikanta Acharya
Diptikanta Acharya