- 1Institute of Tropical Aquaculture and Fisheries, Universiti Malaysia Terengganu, Terengganu, Malaysia
- 2Key Laboratory of Marine Biotechnology of Guangdong Province, Institute of Marine Sciences, Shantou University, Shantou, China
- 3STU-UMT Joint Shellfish Research Laboratory, Shantou University, Shantou, China
- 4Marine Biodiversity Group, Department of Biology, University of Bergen, Bergen, Norway
- 5Center for Macroecology and Evolution, University of Copenhagen, Copenhagen, Denmark
Infestation of Sacculina beauforti on an aquaculture species, mud crab Scylla olivacea is alarming due to its high prevalence and the extreme morphological changes in hosts. To further understand its pathological effect on growth and reproduction of S. olivacea, gonadal and hepatopancreatic histological changes of infected individuals were compared with healthy individuals. Also, the histological characteristics of S. beauforti's mature externa was described. Hepatopancreases of infected individuals were loosely packed and rootlets were observed in the intertubular spaces. Although hepatopancreatic tubule count was significantly lower, tubule diameters were unaffected. Gonads, however, were severely affected. No germ cells were found in the infected testes (except for remnants of spermatozoa), indicating the arrest of spermatogenesis. Rootlets were also present in testes of infected individuals. Ovarian tissues of infected females were severely damaged with no rigid cell structures. Gonadosomatic index (GSI) of infected males and females were lower, but the hepatosomatic index (HSI) were higher than their healthy counterparts. No significant differences in GSI and HSI values were observed among infected males and females. Multiple regression analysis revealed that carapace width, GSI and HSI are statistically important for the prediction of infection status. Given the high prevalence of S. beauforti infection and its disruptive effects on the morphology and internal physiology of S. olivacea, this study, albeit fundamental and incomprehensive, highlights to farmers and researchers the emergence of a castrating parasite and the urgency for the development of preventive measures and treatments for this disease in an economically important aquaculture species.
Introduction
Orange mud crab Scylla olivacea is widely distributed around Southeast Asia (SEA) countries, Australia, Japan and Pakistan (Keenan et al., 1998; Alberts-Hubatsch et al., 2016). Together with other Scylla species, i.e., S. paramamosain, S. serrata and S. tranquebarica, mud crabs are economically important, not only as a valuable commodity to support the livelihood of coastal communities but also as a major aquaculture crustacean species that contribute greatly to a country's economic growth. According to FAO (2019), the global aquaculture production of mud crabs (Scylla spp.) was doubled within 3 years, from 41,460 tons in 2013 to 89,390 tons in 2016. The aquaculture production of mud crabs, however, still relies almost entirely on wild-caught seeds and broodstocks (Waiho et al., 2018). In most countries including Malaysia where wild population of mud crabs are still abundant, most aquaculture activities involve fattening and soft-shell crab farming using juveniles. They are often held in earthen ponds that rely on spring tide for water exchange or directly cultured in pens and cages in their natural environment (mangroves, lagoons or estuarine areas) (Ihwan et al., 2013). Due to the increasing domestic and international demand for mud crabs, hatchery and nursery productions are also gaining attention. Private farmers, industrial players and research institutions are working toward optimization of mud crab larval rearing, although broodstocks are still sourced from the wild (Waiho et al., 2018).
Thus far, parasites infecting mud crabs, especially S. olivacea, in Malaysian waters include ciliates, nematodes, copepods and barnacles (Octolasmis spp.). Being the most abundant among other parasites (71.1% prevalence) (Ihwan et al., 2015), Octolasmis barnacles are often found in the respiratory chambers of mud crabs (Jeffries et al., 1989) and pose minimal threat to their hosts as they do not feed on host tissues (Gannon and Wheatly, 1992). Recently, however, the occurrence of rhizocephalan barnacles Sacculina beauforti (Sacculinidae) on mud crab Scylla olivacea was reported, with a high prevalence rate of 42.27% (Waiho et al., 2017b). Unlike barnacles of other superorders, i.e., Thoracica (filter-feeding barnacles) and Acrothoracica (burrowing barnacles), rhizocephalan barnacles are parasitic barnacles that exhibit extremely reduced adult forms (absence of any organ structures, centralized nervous system, feeding appendages and segmentation) (Glenner and Hebsgaard, 2006) and cause severe behavioral changes, and morphological and physiological damages to their hosts (Høeg, 1995; Yang et al., 2014). To date, this rhizocephalan species has only been reported in two Scylla species, i.e., S. olivacea and S. serrata (not found in Malaysian waters) (Fazhan et al., 2017). Although the other two species (S. tranquebarica and S. paramamosain) live sympatrically with S. olivacea, S. beauforti seems to be selective of its host species as no infestation has been found (Waiho et al., 2017b).
All rhizocephalans infect other crustaceans (Høeg, 1995; Høeg and Lützen, 1995). Their unique life cycle begins with the hatching of sexually dimorphic nauplii, which upon several molts, metamorphose into male and female cyprids (Trédez et al., 2017). Female cyprids would develop into kentrogon larvae and infect hosts by penetrating hosts' integument, thereby transferring parasitic material (vermigon) into the hemolymph (Glenner and Høeg, 1994; Glenner, 2001). Eventually, an interna within the host is formed. It possesses numerous rootlets that infiltrate host's organs for nutrient absorption (Nagler et al., 2017). During this period of infection, the external morphology of the host remains normal. After several molts, the host starts to exhibit female-like characters and molting usually stops when an externa emerges on the soft ventral surface of the host's abdomen (Kristensen et al., 2012). Unlike their female counterparts, male cyprids are free-living. Mature externae will start to release nauplii not long after dwarf males have been implemented into a pair of receptacle-like structures formed in the visceral mass of each externa (Høeg, 1995; Glenner et al., 2000).
The adverse effects of S. beauforti infection on the external morphology of S. olivacea are obvious. Among them includes feminization of male hosts (broadened and darkened female-like abdomens), reduction in copulatory appendages (gonopods in males and pleopods in females) and significantly smaller body size (Waiho et al., 2017b). These changes profoundly decrease their market values as they are often regarded as immature females and traded off at a much lower market price (personal communication). However, due to the limited reports on their occurrence (Boschma, 1949; Waiho et al., 2017b; Fazhan et al., 2018), the precise biology and life cycle of S. beauforti, and the effects they have on their hosts remain largely unknown. As the growth and reproduction of crustaceans are primarily regulated by hepatopancreas and gonads (Wang et al., 2014), we investigated the pathological changes of the S. beauforti infection had on these two organs of S. olivacea. With the current reliance of mud crab aquaculture sector on wild stocks, the results of this study, though fundamental, serve as essential baseline data for future prevention, management and treatment of this disease.
Materials and Methods
Orange mud crabs, Scylla olivacea were collected from local fishermen in Marudu Bay, Sabah, Malaysia. Healthy mature adults (carapace width, CW range = 90–105 mm, n = 30 for each sex) (Waiho et al., 2016; Fazhan et al., 2018) and sacculinid-infected crabs with mature externa (CW range = 90–105 mm, n = 21 for females, n = 20 for males) were obtained, with the former healthy individuals serving as controls. Species identity was validated based on the taxonomic key provided by Keenan et al. (1998). The sex of infected crabs was determined based on the presence of gonopods (males) or pleopods (females) (Waiho et al., 2017b; Fazhan et al., 2018). Crabs were classified as “healthy” based on the absence of (1) externa, (2) visible morphological alterations on sexual organs or abdomen, and (3) interna upon dissection (Waiho et al., 2017b). All animal experimental procedures were reviewed approved by the Institute of Tropical Aquaculture and Fisheries, Universiti Malaysia Terengganu.
Measured parameters were CW (to the nearest 0.01 mm), body weight (BW, to the nearest 0.1 g), and the total weight of gonad (whole ovary for females; testis and vas deferens for males) and hepatopancreas (to the nearest 0.001 g). Gonadosomatic index (GSI) and hepatosomatic index (HSI) were calculated based on the following equations:
Gonadal tissues of 20 individuals (healthy males and females, and infected males and females, with five specimens each) and hepatopancreas tissues of 10 individuals (five healthy individuals and five infected individuals) were extracted and fixed in Davidson's solution for 24 h (Waiho et al., 2017c). In addition, a mature externa (slightly cut open using scalpel) and its stalk base were similarly fixed for 48 h to allow full penetration of fixative. Fixed tissues were then transferred to 70% ethanol for 24 h before being dehydrated in ascending (70–100%) ethanol concentrations. The dehydrated tissues were subsequently cleared in xylol, embedded in paraffin wax and sectioned to 5 μm thickness using a rotary microtome (Leica RM2255). Standard Mayer's Hematoxylin-Eosin (HE) staining was applied to all sectioned slides. After staining, sections were mounted on slides using DPX and viewed using Nikon Eclipse 80i Advance Research Microscope. Hepatopancreatic tubules on one transverse cross-section were counted (n = 10 sections) and randomly measured (n = 50). Further, the diameter of S. beauforti's rootlets were also measured (n = 30). All counts and measurements were conducted under 4× magnification.
All data analyses were performed using Microsoft Excel 365 and IBM SPSS Statistic ver. 25. Data were checked for homogeneity of variance using Levene's test and Welch's correction was applied during one-way analysis of variance (ANOVA) if homogeneity of variance is violated (Welch, 1947). Subsequent differences among treatments were detected using Games Howell posthoc test (Kirk, 1995). Multiple regression was conducted, with infection status as dependent variable and sex, CW, BW, GSI and HSI as independent variables to determine if these variables are significant in predicting infection status in S. olivacea. All data were expressed in the form of mean ± standard deviation (s.d.) and a significance level at α = 0.05 was used in all statistical analyses in this study.
Results
The body of the externa is laterally compressed. A thin-walled, muscular mantle surrounds a visceral mass, which contains the connective tissue and the reproductive apparatus. A cuticle clad mantle cavity is located between the mantle cavity and the mantle, and it communicates with the surrounding seawater by a mantle opening (aperture). The aperture is situated at the anterior margin of the externa, opposite the stalk, which through the ventral, abdominal integument of the host connects the external part of the parasite, the externa, with the internal root-like system of the parasite, the interna (Figure 1A). A pair of strait receptacles are located in the muscular basal region of the stalk outside the visceral mass. In lateral cross-section of the basal stalk reveals that it is filled with tubules (Figure 1D). The ovaries in the visceral mass are filled with developing ovules and is traversed by muscle fibers (Figure 1B). The mantle of the externa consists of two layers of collagen fibers enveloping two layers of radial muscles with a longitudinal muscle layer situated in the middle (Figure 1C).
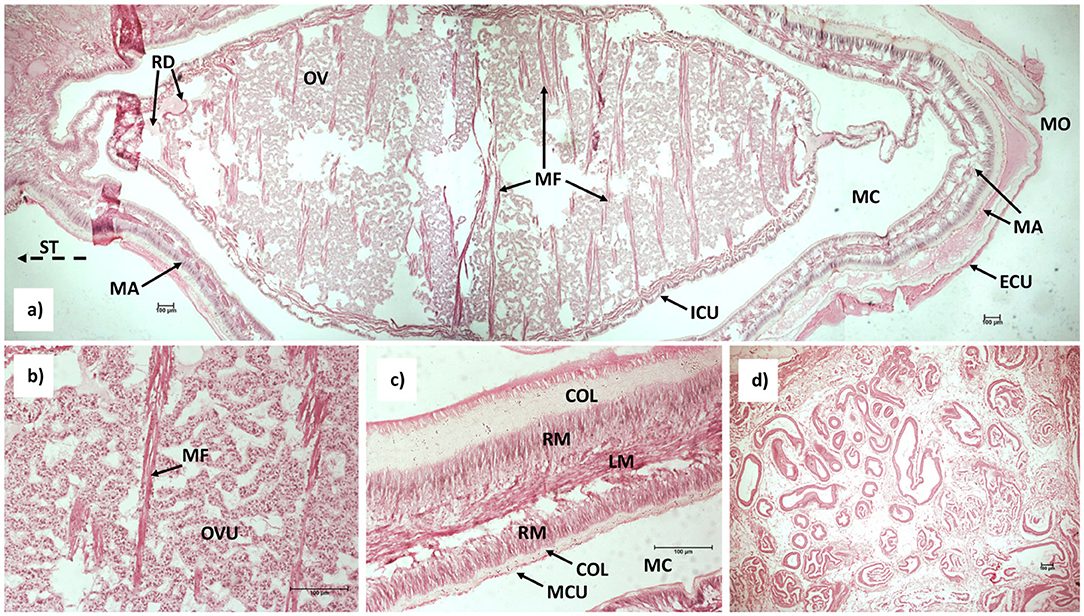
Figure 1. Longitudinal section of the medial portion of (a) mature externa of Sacculina beauforti, magnification 4× (note: three histological sections were combined into a single image, dashed-line arrow point toward stalk); (b) Developing ovules and muscle fibers within the internal cuticle, magnification 20×; (c) Detail of the mantle structure, magnification 20×; (d) Lateral section of the stalk depicting numerous tubules, magnification 4×. Collagen fibers (COL), external cuticle (ECU), internal cuticle (ICU), longitudinal muscle layer (LM), mantle (MA), mantle cavity (MC), mantle cavity cuticle (MCU), muscle fibers (MF), mantle opening (MO), ovary (OV), ovules (OVU), receptacle duct (RD), radial muscle layer (RM), stalk (ST).
To determine the effect of S. beauforti's infection on the host's gonadal and hepatopancreatic physiology and development, the gonadal and hepatopancreatic histological sections of healthy and infected individuals were compared. Densely packed hepatopancreatic tubules with clear star-shaped luminae were observed in the hepatopancreas of healthy individuals (Figure 2A). A thin layer of connective tissues was found in the intertubular spaces. In the hepatopancreas of infected crabs, hepatopancreatic tubules were loosely packed and S. beauforti's rootlets were found among tubules. The S. beauforti's rootlets had a range of 40.27–125.68 μm in diameter (mean = 80.00 ± 22.74 μm) (Figure 2B). The hepatopancreatic tubule count in healthy individuals [count range (n) = 89–135; mean = 109.00 ± 14.78] was statistically significantly higher than those infected with S. beauforti [count range (n) = 20–44; mean = 33.60 ± 7.46] (Mann-Whitney U; U = 0, P < 0.001). However, the hepatopancreatic tubule diameters were not significantly different between healthy and infected individuals (Student's t test; F1, 98 = 3.099, P = 0.081). Resorptive cells (R-cells) and blister-like cells (B-cells) were still present in the hepatopancreatic tubules of infected individuals.
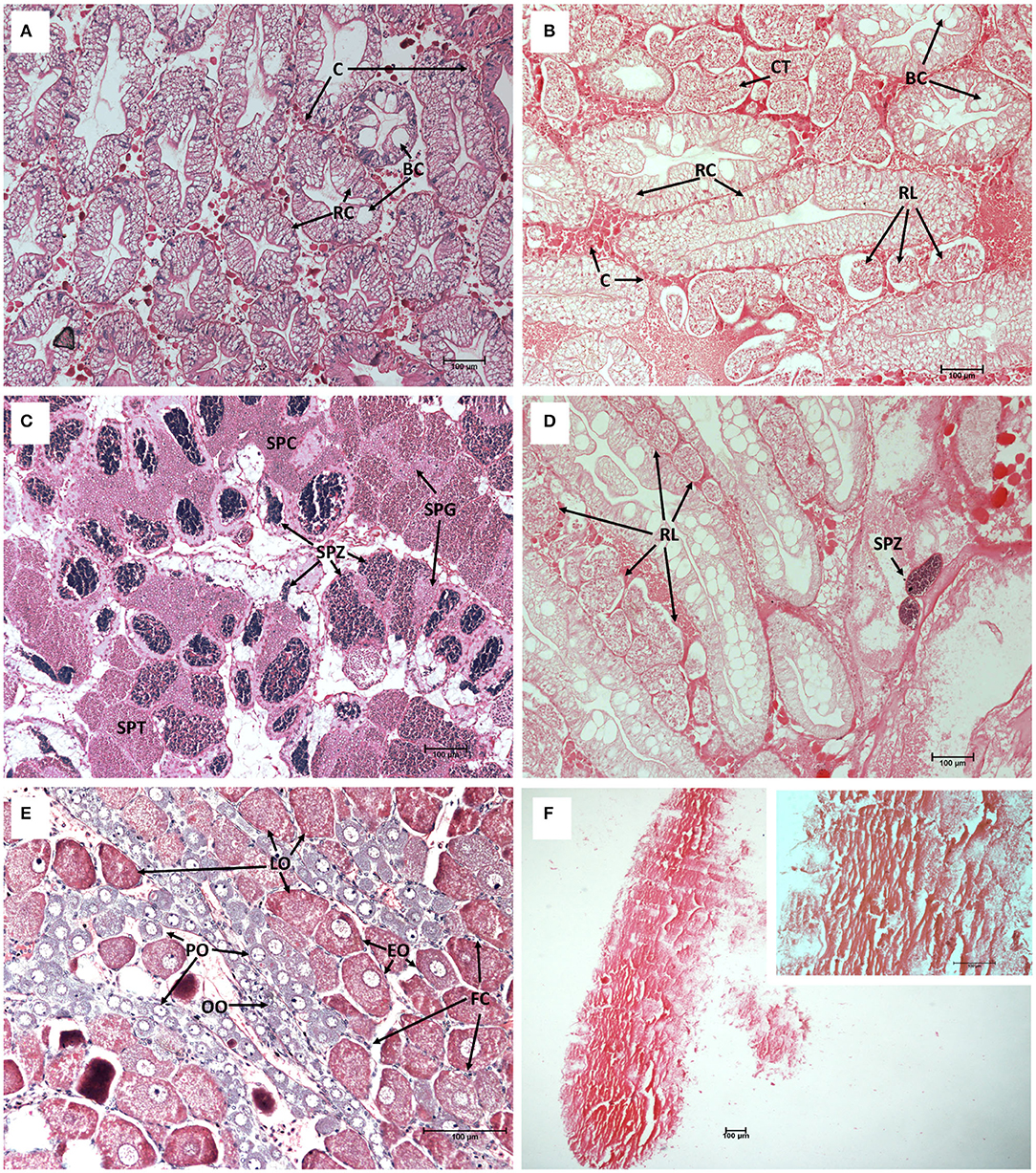
Figure 2. The hepatopancreas and gonad histological cross-sections of healthy (A,C,E) and infected Scylla olivacea (B,D,F) (Mayer's Hematoxylin-Eosin staining). (A) Healthy hepatopancreas with normal tubule shape and arrangement, magnification 10×; (B) Infected hepatopancreas with the presence of rhizocephalan rootlets (RL) and collapsed tubules (CT), magnification 10×; (C) Healthy testis with the presence of spermatogonia (SPG), spermatocytes (SPC), spermatids (SPT) and spermatozoa (SPZ), magnification 10×; (D) Infected testis with the presence of RL, almost no spermatogenesis activity, cell lysis and only remnants of SPZ could be found, magnification 10×; (E) Healthy ovary with the presence of oogonia (OO), primary oocytes (PO), early-maturing oocytes (EO) and late-maturing oocytes (LO), with follicle cells (FC) surrounding oocytes, magnification 20×; (F) Infected ovary appeared degenerated and no rigid cell structures were observed, magnification 4× (large panel) and 20× (upper right panel). Connective tissues (C), R-cells (RC), B-cells (BC).
All four types of germ cells, i.e., spermatogonia, spermatocytes, spermatids and spermatozoa were present in a healthy testis (Figure 2C). However, severe degradation of testis was observed in infected individuals (Figure 2D), with no germ cells of any types but only remnants of spermatozoa could be found. This indicates that spermatogenesis process in the testis of infected individuals was completely arrested. Further, similar to the case of hepatopancreas of infected individuals, infected testis was heavily infiltrated with S. beauforti's rootlets.
Several types of cells could be found in the ovarian tissues of healthy females, i.e., oogonia, primary oocytes, early-maturing oocytes, and late-maturing oocytes, all of which are densely packed and surrounded by follicle cells (Figure 2E). The ovarian tissues of infected individuals, however, were acutely damaged (severe necrosis) and no rigid cell structures could be observed (Figure 2F).
In line with the degradation of gonad and disruption of gametogenesis, significant reduction in gonad overall sizes were observed among healthy and infected males and females (ANOVA; F3, 47.291 = 83.335, P < 0.001). The GSI of infected males and females were significantly smaller than their healthy counterparts (Games Howell; Pmale and Pfemale < 0.001). However, although hepatopancreases of crabs with mature externae were also disrupted, opposite trend was observed in their overall sizes (Figure 3). Differences in HSI values among healthy and infected males and females were obvious (ANOVA; F3, 48.877) = 125.061, P < 0.001), with that of infected males and females significantly larger than their healthy counterparts (Games Howell; Pmale and Pfemale < 0.001) and no significant difference was observed between the HSI values of infected males and females (Games Howell; P = 0.4245). A multiple regression was conducted to predict infection status from sex, CW, BW, GSI, and HSI. These variables significantly predicted infection status, F5, 95 = 119.867, P < 0.001, R2 = 0.856. However, only three variables (CW, P = 0.001; GSI, P = 0.003; HSI, P < 0.001) added statistically significance to the prediction.
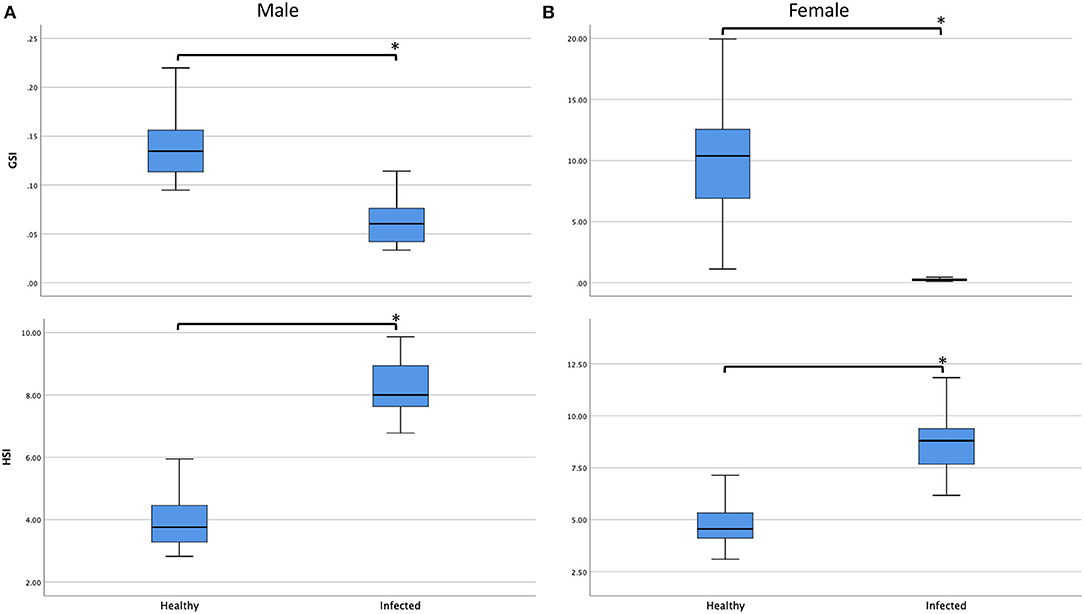
Figure 3. Boxplots showing the gonadosomatic index (GSI, %) and hepatosomatic index (HSI, %) of healthy and infected (A) males and (B) females. All comparisons showed significant differences (P < 0.05, marked with asterisk *). Infection by Sacculina beauforti resulted in significant reduct0069on in GSI but increment in HIS of Scylla olivacea.
Discussion
The high prevalence of S. beauforti in a natural mud crab population has been alarming, as this population is known to supply crabs, both domestically and internationally, to fisheries and aquaculture sectors (Waiho et al., 2017b). Sacculina beauforti belongs to the family Sacculinidae, a family with the most abundant rhizocephalan species (Høeg, 1995).
The internal anatomy of externa provides valuable information on the taxonomy and reproductive biology of the invading parasite (Glenner and Hebsgaard, 2006). Understanding it could provide clues on the future development of prevention or treatment methods, especially in such case where the host (S. olivacea) is an economically important and highly valuable aquaculture species. The externa anatomy of S. beauforti conforms with the classical externa description of most sacculinids (Lützen and Takahashi, 1997; Alvarez et al., 2009; Kobayashi et al., 2018). The double-layered mantle near the mantle opening of S. beauforti provides additional muscle tissues to perform strong contractions during oviposition, fertilization and expulsion of nauplii (Høeg and Lützen, 1995; Alvarez et al., 2009).
Hepatopancreas plays an essential role in nutrient absorption and storage in crustaceans (Wu et al., 2020). In addition, hepatopancreas stores lipid, an essential nutrient that could provide huge amount of energy during critical moments, such as during starvation, molting, maturation or reproduction (Wang et al., 2014). It is also involved in the ovarian development by regulating the synthesis of vitellogenin and vital sex steroid hormones, including that of mud crab genus Scylla (Warrier et al., 2001; Jia et al., 2013). Due to these critical roles, the whole organ is rich in nutrient and the rhizocephalan S. beauforti take advantage of this – penetrating intertubular spaces within the hepatopancreas of its host with numerous rootlets, potentially absorbing nutrient directly from it. Similar finding of sacculinid rootlets in the hepatopancreas of its host was also reported in portunid blue crab Callinectes sapidus infected by Loxothylacus texanus (Bortolini and Alvarez, 2008) and shore crab Carcinus maenas infected by Sacculina carcini (Powell and Rowley, 2008). The adverse metabolic effect of S. beauforti on S. olivacea is postulated to be at the maximum when externa matures (Robles et al., 2002). Both healthy and infected individuals exhibited similar tubule diameters and with the presence of tubular R- and B-cells. R-cells are involved in nutrient absorption and metabolization, lipid and glycogen storage, and is crucial in the detoxification process and uric acid excretion (Vogt and Quinitio, 1994; Johnston et al., 1998; Vogt, 2019); B-cells play important role in intracellular digestion and assimilation (Al-Mohanna and Nott, 1986) as they might be involved in the production and recycling of fat emulsifiers (Vogt, 2019). This implies that although S. beauforti's infection and the infiltration of numerous rootlets might occupy most intertubular spaces and resulted in an increase in mass (as evident by the significant increase of infected individuals' HSI and decrease in tubular counts), the tubular structure integrity of some hepatopancreatic tubules remains intact and might still be functional. The preservation of the function of host's hepatopancreas is vital for the survival of rhizocephalans as it is their main nutrient absorption site and its destruction will result in the death of the host.
The gonads of hosts, however, were even more affected. Unlike healthy gonads with various types of germ cells (Quinitio et al., 2007; Azmie et al., 2017; Waiho et al., 2017c), the testes of infected individuals were significantly reduced in size, degenerated and non-functional (no active germ cells were found), indicating that spermatogenesis was completely inhibited. In addition, ovaries of infected S. olivacea were so severely damaged that no rigid cell structures could be observed. Similar gonadal atrophy and disruption of vitellogenesis and spermatogenesis are commonly reported in sacculinid-infected hosts (Rubiliani, 1983; Høeg, 1995; Isaeva et al., 2001). The extent of damage, however, varies among species. For example, Polyascus polygenea disrupted spermiogenesis but not spermatogenesis in the testis of its host Hemigrapsus sanguineus (Isaeva et al., 2001; Glenner et al., 2003); and in C. maena parasitized by S. carcini, virtually no weight difference could be found in the testes of infected and healthy crabs, - and nothing indicated that spermatophores of infected crabs would not be functional (Zetlmeisl et al., 2011). The severe damage observed in the ovaries of infected S. olivacea in this study is similar to that reported in spider crab Macropodia longirostris infected by sacculinid Drepanorchis neglecta (Hartnoll, 1962), where disintegration of ovarian cells and total ovary degeneration (in some cases) were observed. The size of testes and ovaries of infected hosts (S. olivacea) with mature externa were extremely reduced, to the extent that the mean of their GSI values (meanmale = 0.06 ± 0.02%; meanfemale = 0.26 ± 0.14%) were even smaller than the mean GSI values of immature males (mean = 0.10 ± 0.04%) (Waiho et al., 2017c) and females (mean = 2.46 ± 1.31%) (Azmie et al., 2017) of the same species reported in previous studies. Based on the size (CW) of the hosts collected in this study, they should be sexually mature with fully developed gonads (Waiho et al., 2016; Fazhan et al., 2017). However, S. beauforti infestation resulted in gonadal atrophy of both sexes, to such degree that the hosts are physiologically infertile.
Gonadal development and maturation are essential processes in the life history of crustaceans (Waiho et al., 2017a). Aside from being directly involve in reproduction, gonads are responsible for the morphological differentiation of most crustacean species which leads to sexual dimorphism (Parvizi et al., 2017; Waiho et al., 2019). Therefore, it is possible that rhizocephalans induce morphological changes (feminization) by negatively regulating the development of gonads in hosts as gonads play vital role in sex differentiation of an organisms, including crustaceans (Waiho et al., 2019). For example, doublesex gene (Dsx)—a male sex-determining gene—is found in the testes of crustaceans and involves directly in the regulation of sexual dimorphism and the silencing of Dsx1 gene in male Daphnia magna embryos is known to induce the development of female secondary characteristics (Kato et al., 2011). Another important organ in crustaceans that is involved in sexual dimorphism is the androgenic gland (AG). As demonstrated in intersex individuals of red-claw crayfish Cherax quadricarinatus (Rosen et al., 2010) and juveniles of freshwater prawn Macrobrachium rosenbergii (Ventura et al., 2009), the silencing of an AG specific insulin-like gene (Cq-IAG in C. quadricarinatus; Mr-IAG in M. rosenbergii) resulted in feminization (impede the regeneration of male secondary sexual characteristics) and severe testicular degeneration – two characteristics similar to the effects induced by rhizocephalans on their respective hosts. By injecting healthy male crabs Rhithropanopeus harrisii with crude extracts of roots from sacculinid Loxothylacus panopei, Rubiliani (1983) showed that proteinaceous substances from rootlets are responsible, not only for the inhibition of spermatogenesis, but cytolysis of androgenic gland and sinus gland as well. Thus, future research on the potential effect of S. beauforti infestation on S. olivacea's AG and in-depth study on the molecular changes in gonads and AG after infected are recommended to uncover the regulatory mechanism of rhizocephalans that would result in feminization and gonad degeneration in infected hosts.
The mud crab S. olivacea serves as important fisheries commodity and aquaculture candidate in Southeast Asia where this species is abundantly found. Infestation of rhizocephalan barnacles (S. beauforti) on S. olivacea did not only affect external morphologies, but also severely damaged their internal organs, rendering them infertile. This could adversely affect their market price as mature females with ripe ovaries are known to fetch significantly higher price compared to males of the same size (Waiho et al., 2020). Further, since aquaculture activities such as crab fattening, soft-shell crab production and hatchery rearing of mud crab still rely heavily on sourcing juveniles and broodstocks from the wild, they could be render fruitless if infected individuals are accidentally sourced as infected juveniles are more susceptible to other infections and have a higher chance of mortality (Keogh et al., 2017) while broodstocks are not able to produce any larvae due to the degenerated gonads. Thus, farmers would endure great losses—financial, effort and time—if infected crabs were unknowingly cultured. Also, as most mud crab aquaculture activities are conducted in large earthen ponds with direct water intake from nearby brackish mangrove estuary ecosystems, and infected hosts might be physically healthy until the emergence of externa [which could take months from infection to externa emergence, i.e., 33 days in crab Rhithropanopeus harrissi infected with Loxothylacus panopaei (Alvarez et al., 1995) and 9 months in littoral crab Carcinus maenas infected with Sacculina carcini (Foxon, 1940)], farmers might not be aware of the presence of infected individuals being co-cultured with healthy individuals. Eventually, this could lead to an outbreak of S. beauforti infestation within the culture ponds if mature externae were fertilized by free-living male cyprids (Glenner et al., 2003; Kobayashi et al., 2018) potentially introduced via the water intake system. Therefore, if infected individuals were detected during culture (i.e., with the presence of externa), especially in facilities where water intake is unfiltered and untreated, it is important to remove and discard (kill) them to prevent unwanted outbreaks (Waiho et al., 2021).
Conclusion
Although it is still early to demonstrate, unequivocally, the total adverse effect of rhizocephalan barnacle S. beauforti have on its host S. olivacea, the high prevalence of this parasite on an economically important aquaculture crustacean species, and the severe damage it causes on two essential organs—hepatopancreas and gonads—warrant an urgent report to highlight to researchers, farmers and other industry players on its emergence and manifestation. It is hoped that through this report, occurrence of S. beauforti on mud crab genus Scylla in other geographically distinct populations would be reported to aid in understanding S. beauforti's distribution, seasonal abundance, life cycle and pathology, all of which are important for future research into its prevention and cure.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
HF and KW conceived and designed the research, performed the field work, and wrote the manuscript. MH and JM processed the data. HG contributed to the further analysis of data. HG, MH, and MI revised the manuscript and contributed to the writing. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by the Ministry of Higher Education, Malaysia, under the Fundamental Research Grant Scheme (59617) (FRGS/1/2020/STG03/UMT/03/2).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Suhairi Mazelan for his help in editing and merging of the externa's histological sections. We thank Institute of Tropical Aquaculture and Fisheries, Universiti Malaysia Terengganu for providing the facilities needed for this study.
References
Alberts-Hubatsch, H., Lee, S.Y., Meynecke, J.-O., Diele, K., Nordhaus, I., and Wolff, M. (2016). Life-history, movement, and habitat use of Scylla serrata (Decapoda, Portunidae): current knowledge and future challenges. Hydrobiologia 763, 5–21. doi: 10.1007/s10750-015-2393-z
Al-Mohanna, S.Y., and Nott, J.A. (1986). B-cells and digestion in the hepatopancreas of Penaeus semisulcatus (Crustacea: Decapoda). J. Mar. Biol. Assoc. U. K. 66, 403–414. doi: 10.1017/S0025315400043034
Alvarez, F., Bortolini, J.L., and Høeg, J.T. (2009). Anatomy of virgin and mature externae of Loxothylacus texanus, parasitic on the dark blue crab Callinectes rathbunae (Crustacea: Cirripedia: Rhizocephala: Sacculinidae). J. Morphol. 271, 190–199. doi: 10.1002/jmor.10790
Alvarez, F., Hines, A.H., and Reaka-Kudla, M.L. (1995). The effects of parasitism by the barnacle Loxothylacus panopaei (Gissler) (Cirripedia: Rhizocephala) on growth and survival of the host crab Rhithropanopeus harrisii (Gould) (Brachyura: Xanthidae). J. Exp. Mar. Biol. Ecol. 192, 221–232. doi: 10.1016/0022-0981(95)00068-3
Azmie, G., Noordin, N.M., Abol-Munafi, A.B., Azra, M.N., and Ikhwanuddin, M. (2017). Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malays. 46, 2273–2280. doi: 10.17576/jsm-2017-4612-03
Bortolini, J.L., and Alvarez, F. (2008). Hepatopancreas alteration of the blue crab Callinectes sapidus by the rhizocephalan barnacle Loxothylacus texanus. J. Invertebr. Pathol. 99, 354–356. doi: 10.1016/j.jip.2008.08.004
Boschma, H. (1949). Sacculina beauforti and Loxolhyiacils ihlei, two Rhizocephala of the crab Scylla serrata (Forsk.). Bijdragen Tot De Dierkunde, 28, 41–46.
FAO (2019). Cultured Aquatic Species Information Programme: Scylla serrata (Forsskål, 1755). Food and Agriculture Organization of the United Nations. Available online at: http://www.fao.org/fishery/culturedspecies/Scylla_serrata/en (accessed October 20, 2019).
Fazhan, H., Waiho, K., Darin Azri, M.F., Al-Hafiz, I., Wan Norfaizza, W.I., Megat, F.H., et al. (2017). Sympatric occurrence and population dynamics of Scylla spp. in equatorial climate: Effects of rainfall, temperature and lunar phase. Estuar. Coast. Shelf Sci. 198, 299–310. doi: 10.1016/j.ecss.2017.09.022
Fazhan, H., Waiho, K., Wee, H.B., Surzanne, M.A., Ma, H., and Ikhwanuddin, M. (2018). Predicting the sacculinid Sacculina beauforti infection status of the orange mud crab Scylla olivacea by discriminant analysis. Aquaculture 491, 128–134. doi: 10.1016/j.aquaculture.2018.03.009
Foxon, G.E.H. (1940). Notes on the life history of Sacculina carcini Thompson. J. Mar. Biol. Assoc. U. K. 24, 253–264. doi: 10.1017/S0025315400054552
Gannon, A.T., and Wheatly, M.G. (1992). Physiological effects of an ectocommensal gill barnacle, Octolasmis muelleri, on gas exchange in the blue crab Callinectes sapidus. J. Crust. Biol. 12, 11–18. doi: 10.2307/1548714
Glenner, H. (2001). Cypris metamorphosis, injection and earliest internal development of the rhizocephalan Loxothylacus panopaei (Gissler). Crustacea: Cirripedia: Rhizocephala: Sacculinidae. J. Morphol. 249, 43–75. doi: 10.1002/jmor.1040
Glenner, H., and Hebsgaard, M.B. (2006). Phylogeny and evolution of life history strategies of the parasitic barnacles (Crustacea, Cirripedia, Rhizocephala). Mol. Phylogen. Evol. 41, 528–538. doi: 10.1016/j.ympev.2006.06.004
Glenner, H., and Høeg, J.T. (1994). Metamorphosis in the Cirripedia Rhizocephala and the homology of the kentrogon and trichogon. Zool. Scr. 23, 161–173. doi: 10.1111/j.1463-6409.1994.tb00382.x
Glenner, H., Høeg, J.T., O'Brien, J.J., and Sherman, T.D. (2000). Invasive vermigon stage in the parasitic barnacles Loxothylacus texanus and L. panopaei (Sacculinidae): closing of the rhizocephalan life-cycle. Mar. Biol. 136, 249–257. doi: 10.1007/s002270050683
Glenner, H., Lützen, J., and Takahashi, T. (2003). Molecular and morphological evidence for a monophyletic clade of asexually reproducing rhizocephalan: Polyascus, new genus (Cirripedia). J. Crust. Biol. 23, 548–557. doi: 10.1651/C-2361
Hartnoll, R. G. (1962). Parasitic castration of Macropodia longirostris (Fabricius) by a sacculinid. Crustaceana, 4, 295–300.
Høeg, J.T. (1995). The biology and life cycle of the rhizocephalan (Cirripedia). J. Mar. Biol. Assoc. U. K. 75, 517–550. doi: 10.1017/S0025315400038996
Høeg, J.T., and Lützen, J. (1995). Life cycle and reproduction in the Cirripedia Rhizocephala. Oceanogr. Mar. Biol. Ann. Rev. 33, 427–485.
Ihwan, Z., Azmie, G., Abol-Munafi, A.B., and Ikhwanuddin, M. (2013). Mud Crab Culture System and Practice in Malaysia. Terengganu: UMT Publisher.
Ihwan, Z., Wahidah, W., Ambak, M.A., Ikhwanuddin, M., and Marina, H. (2015). Investigation of parasites and ecto-symbiont in wild mud crab, genus Scylla from Terengganu coastal water, Malaysia, prevalence and mean intensity. Int. J. Zool. Res. 11, 151–159. doi: 10.3923/ijzr.2015.151.159
Isaeva, V.V., Shukalyuk, A.I., Trofimova, A.V., Korn, O.M., and Rybakov, A.V. (2001). The structure of colonial interna in Sacculina polygenea (Crustacea: Cirripedia: Rhizocephala). Crust. Res. 30, 133–146. doi: 10.18353/crustacea.30.0_133
Jeffries, W.B., Voris, H.K., and yang, C.M. (1989). A new mechanism of host colonization: pedunculate barnacles of the genus Octolasmis on the mangrove crab Scylla serrata. Ophelia 31, 51–58. doi: 10.1080/00785326.1989.10430850
Jia, X., Chen, Y., Zou, Z., Lin, P., Wang, Y., and Zhang, Z. (2013). Characterization and expression profile of Vitellogenin gene from Scylla paramamosain. Gene 520, 119–130. doi: 10.1016/j.gene.2013.02.035
Johnston, D.J., Alexander, C.G., and Yellowlees, D. (1998). Epithelial cytology and function in the digestive gland of Thenus orientalis (Decapoda, Scyllaridae). J. Crust. Biol. 18, 271–278. doi: 10.2307/1549320
Kato, Y., Kobayashi, K., Watanabe, H., and Iguchi, T. (2011). Environmental sex determination in the branchiopod crustacean Daphnia magna: deep conservation of a double sex gene in the sex-determining pathway. PLoS Genet. 7:e1001345. doi: 10.1371/journal.pgen.1001345
Keenan, C.P., Davie, P., and Mann, D. (1998). A revision of the genus Scylla De Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bull. Zool. 46, 217–245.
Keogh, C.L., Miura, O., Nishimura, T., and Byers, J.E. (2017). The double edge to parasite escape: invasive host is less infected but more infectable. Ecology 98, 2241–2247. doi: 10.1002/ecy.1953
Kobayashi, M., Wong, Y.H., Oguro-Okano, M., Dreyer, N., Høeg, J.T., Yoshida, R., et al. (2018). Identification, characterization, and larval biology of a rhizocephalan barnacle, Sacculina yatsui Boschma, 1936, from northwestern Japan (Cirripedia: Sacculinidae). J. Crust. Biol. 38, 329–340. doi: 10.1093/jcbiol/ruy020
Kristensen, T., Nielsen, A.I., Jørgensen, A.I., Mouritsen, K.N., Glenner, H., Christensen, J.T., et al. (2012). The selective advantage of host feminization: a case study of the green crab Carcinus maenas and the parasitic barnacle Sacculina carcini. Mar. Biol. 159. 2015–2023. doi: 10.1007/s00227-012-1988-4
Lützen, J., and Takahashi, T. (1997). Sacculina polygenea, a new species of rhizocephalan (Cirripedia: Rhizocephala) from Japan, parasitic on the intertidal crab Hemigrapsus sanguineus (De Haan, 1835) (Decapoda: Brachyura: Grapsidae). Crust. Res. 26, 103–108. doi: 10.18353/crustacea.26.0_103
Nagler, C., Hörnig, M.K., Haug, J.T., Noever, C., Høeg, J.T., and Glenner, H. (2017). The bigger, the better? Volume measurements of parasites and hosts: parasitic barnacles (Cirripedia, rhizocephalan) and their decapod hosts. PLoS ONE 12:e0179958. doi: 10.1371/journal.pone.0179958
Parvizi, E., Naderloo, R., Keikhosravi, A., and Schubart, C.D. (2017). Life history traits and patterns of sexual dimorphism in the freshwater crab Potamon ibericum (Bieberstein, 1809) (Decapoda: Brachyura: Potamidae) from the western Alborz Mountains, Iran. J. Crust. Biol. 37, 323–331. doi: 10.1093/jcbiol/rux029
Powell, A., and Rowley, A.F. (2008). Tissue changes in the shore crab Carcinus maenas as a result of infection by the parasitic barnacle Sacculina carcini. Dis. Aquat. Organ. 80, 75–79. doi: 10.3354/dao01930
Quinitio, E.T., Pedro, J.D., and Parado-Estepa, F.D. (2007). Ovarian maturation stages of the mud crab Scylla serrata. Aquac. Res. 38, 1434–1441. doi: 10.1111/j.1365-2109.2007.01650.x
Robles, R., Alvarez, F., and Alcaraz, G. (2002). Oxygen consumption of the crab Callinectes rathbunae parasitized by the rhizocephalan barnacle Loxothylacus texanus as a function of salinity. Mar. Ecol. Prog. Ser. 235, 189–194. doi: 10.3354/meps235189
Rosen, D., Manor, R., Weil, S., Gafni, O., Linial, A., Aflalo, E.D., et al. (2010). A sexual shift induced by silencing of a single insulin-like gene in crayfish: ovarian upregulation and testicular degeneration. PLoS ONE 5:e15281. doi: 10.1371/journal.pone.0015281
Rubiliani, C. (1983). Action of a rhizocephalan on the genital activity of host male crabs: characterization of a parasitic secretion inhibiting spermatogenesis. Int. J. Invertebr. Reprod. 6, 137–147. doi: 10.1080/01651269.1983.10510036
Trédez, F., Rabet, N., Bellec, L., and Audebert, F. (2017). Synchronism of naupliar development of Sacculina carcini Thompson, 1836 (Pancrustacea, Rhizocephala) revealed by precise monitoring. Helgol. Mar. Res. 70:26. doi: 10.1186/s10152-016-0479-2
Ventura, T., Manor, R., Aflalo, E.D., Weil, S., Raviv, S., Glazer, L., et al. (2009). Temporal silencing of an androgenic gland-specific insulin-like gene affecting phenotypical gender differences and spermatogenesis. Endocrinology 150, 1278–1286. doi: 10.1210/en.2008-0906
Vogt, G. (2019). Functional cytology of the hepatopancreas of decapod crustaceans. J. Morphol. 280, 1405–1444. doi: 10.1002/jmor.21040
Vogt, G., and Quinitio, E.T. (1994). Accumulation and excretion of metal granules in the prawn, Penaeus monodon, exposed to water-borne copper, lead, iron and calcium. Aquat. Toxicol. 28, 223–241. doi: 10.1016/0166-445X(94)90035-3
Waiho Fazhan, H., Baylon, J.C., Madihah, H., Noorbaiduri, S., Ma, H., and Ikhwanuddin, M. (2017a). On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. J. Shellfish Res. 36, 807–839. doi: 10.2983/035.036.0330
Waiho, K., Fazhan, H., Glenner, H., and Ikhwanuddin, M. (2017b). Infestation of parasitic rhizocephalan barnacles Sacculina beauforti (Cirripedia, Rhizocephala) in edible mud crab, Scylla olivacea. PeerJ 5:e3419. doi: 10.7717/peerj.3419
Waiho, K., Fazhan, H., and Ikhwanuddin, M. (2016). Size distribution, length-weight relationship and size at the onset of sexual maturity of the orange mud crab, Scylla olivacea, in Malaysian waters. Mar. Biol. Res. 12, 726–738. doi: 10.1080/17451000.2016.1200726
Waiho, K., Fazhan, H., Jasmani, S., and Ikhwanuddin, M. (2017c). Gonadal development in males of the orange mud crab, Scylla olivacea (Herbst, 1796) (Decapoda, Brachyura Portunidae). Crustaceana 90, 1–19. doi: 10.1163/15685403-00003622
Waiho, K., Fazhan, H., Quinitio, E.T., Baylon, J.C., Fujaya, Y., Azmie, G., et al. (2018). Larval rearing of mud crab (Scylla): what lies ahead. Aquaculture 493, 37–50. doi: 10.1016/j.aquaculture.2018.04.047
Waiho, K., Fazhan, H., Zhang, Y., Li, S., Zhang, Y., Zheng, H., et al. (2020). Comparative profiling of ovarian and testicular piRNAs in the mud crab Scylla paramamosain. Genomics 112, 323–331. doi: 10.1016/j.ygeno.2019.02.012
Waiho, K., Fazhan, H., Zhang, Y., Zhang, Y., Li, S., Zheng, H., et al. (2019). Gonadal microRNA expression profiles and their potential role in sex differentiation and gonadal maturation of mud crab Scylla paramamosain. Mar. Biotechnol. 21, 320–334. doi: 10.1007/s10126-019-09882-1
Waiho, K., Glenner, H., Miroliubov, A., Noever, M., Hassan, M., Ikhwanuddin, M., et al. (2021). Rhizocephalans and their potential impact on crustacean aquaculture. Aquaculture 531:735876. doi: 10.1016/j.aquaculture.2020.735876
Wang, W., Wu, X., Liu, Z., Zheng, H., and Cheng, Y. (2014). Insights into hepatopancreatic functions for nutition metabolism and ovarian development in the crab Portunus trituberculatus: gene discovery in the comparative transcriptome of different hepatopancreas stages. PLoS ONE 9:e84921. doi: 10.1371/journal.pone.0084921
Warrier, S.R., Tirumalai, R., and Subramoniam, T. (2001). Occurrence of vertebrate steroids, estradiol 17beta and progesterone in the reproducing females of the mud crab Scylla serrata. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 130, 283–294. doi: 10.1016/S1095-6433(01)00385-3
Welch, B. L. (1947). The generalization of “Student's” problem when several different population variances are involved. Biometrika 34, 28–35.
Wu, Q., Waiho, K., Huang, Z., Li, S., Zheng, H., Zhang, Y., et al. (2020). Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture 515:734560. doi: 10.1016/j.aquaculture.2019.734560
Yang, C.-P., Li, H.-X., Li, L., and Yan, Y. (2014). Occurrence and effects of the rhizocephalan parasite Diplothylacus sinensis (Cirripedia: Rhizocephala: Thomsoniidae) in the swimming crab Portunus sanguinolentus (Decapoda: Portunidae) in Honghai Bay, South China Sea. J. Crust. Biol. 34, 573–580. doi: 10.1163/1937240X-00002270
Keywords: sacculinid, rhizocephalan, mud crab, Scylla olivacea, histology, externa
Citation: Fazhan H, Waiho K, Glenner H, Moh JHZ, Hassan M and Ikhwanuddin M (2020) Gonadal Degeneration and Hepatopancreas Alteration in Orange Mud Crab Scylla olivacea Infected With Sacculina beauforti (Crustacea; Rhizocephala; Sacculinidae). Front. Mar. Sci. 7:534443. doi: 10.3389/fmars.2020.534443
Received: 12 February 2020; Accepted: 27 November 2020;
Published: 15 December 2020.
Edited by:
Yngvar Olsen, Norwegian University of Science and Technology, NorwayReviewed by:
Alexey Miroliubov, Zoological Institute (RAS), RussiaChi-Ying Lee, National Changhua University of Education, Taiwan
Copyright © 2020 Fazhan, Waiho, Glenner, Moh, Hassan and Ikhwanuddin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khor Waiho, d2FpaG9AdW10LmVkdS5teQ==; Mhd Ikhwanuddin, aWtod2FudWRkaW5AdW10LmVkdS5teQ==
 Hanafiah Fazhan
Hanafiah Fazhan Khor Waiho
Khor Waiho Henrik Glenner4,5
Henrik Glenner4,5 Julia Hwei Zhong Moh
Julia Hwei Zhong Moh Mhd Ikhwanuddin
Mhd Ikhwanuddin