
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 11 September 2020
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 7 - 2020 | https://doi.org/10.3389/fmars.2020.00722
This article is part of the Research Topic Marine Aquaculture Impacts on Marine Biota View all 10 articles
 Alvar Carranza1,2*
Alvar Carranza1,2* Philine S. E. zu Ermgassen3
Philine S. E. zu Ermgassen3Farming of marine organisms (mariculture) represented 36% of global aquaculture, with mollusks representing 58.8% in live weight. Mollusk populations in some locations are, however, threatened by degradation of the ecosystems and/or over-fishing. This threat is increasingly being addressed through Restorative Shellfish Mariculture (RSM), as opposed to mariculture alone. There is no general consensus in the literature on what can and cannot be considered RSM. While maximization of benefits other than provisioning services is often considered a prerequisite, in other cases the maximization of fisheries yields is prioritized. Here we define RSM as the farming of marine shellfish, implying some form of intervention during the species life cycle, in order to address negative socio-ecological issues arising from the unsustainable use of marine ecosystems, independent of the final ownership regime of the resource. Strategies for developing RSM were reviewed and classified along a gradient from the most conservation-oriented (e.g., habitat restoration, reintroduction of locally extinct endangered species), to the most fisheries-oriented (including some forms of fisheries enhancement), and classified as Non-hatchery Dependent or Hatchery Dependent strategies. We reviewed the targeted species and strategies implemented across 584 individual projects developed in the last decades in North America, Europe, Asia, Oceania and South America. We found that some 48 species, including 34 bivalves and 15 gastropods were targets of RSM in 34 countries. US projects accounted for ca. three quarters of the total (N = 438), with Philippines, Japan and Australia also being home to a large number. More than 90% of the projects involved five species, namely the eastern oyster (Crassostrea virginica, N = 379), the giant clam (Tridacna gigas, N = 65), the Olympia oyster (Ostrea lurida, N = 25), the bay scallop (Argopecten irradians, N = 25) and the hard clam (Mercenaria mercenaria, N = 15). Of the RSM projects, 51% used Non-hatchery dependent methods, mostly habitat restoration providing substrata for settlement, whereas some 49% involved hatcheries. 3% of the projects combined both methods. This review provides an overview of the breadth, depth and aims of RSM globally, develops a broad definition of the activity, and proposes a structure for classifying RSM.
Capture fisheries have maintained a relatively static production over the last three decades, with the impressive growth in fish supply mostly associated with an expansion of aquaculture (Food and Agriculture Organization [FAO], 2018). In 2016, a peak in global fish production was reached at 171 million tons, with 47% of the total provided by aquaculture. In turn, marine aquaculture (mariculture) rose to 28.664 million tons in 2016, representing ca. 36% of global aquaculture. Of these, mollusks represent 58.8% in live weight, with Asia responsible for more than 85% of mollusk production (Wijsman et al., 2019). Most cultured mollusk species are filter-feeding bivalve shellfish, i.e., clams, mussels, oysters, and scallops (Food and Agriculture Organization [FAO], 2018).
According to the Tacon (2003), aquaculture is distinct from capture fisheries and is defined as “…the farming of aquatic organisms, and implies some form of intervention in the rearing process to enhance production, such as regular stocking, feeding, protection from predators, etc. Farming also implies individual or corporate property of the cultivated stock. For FAO statistical purposes, aquatic organisms which are harvested by an individual or corporate body which has owned them throughout their rearing period contribute to aquaculture, although aquatic organisms which are exploitable as common property resources constitute the harvest of fisheries.”
Although negative environmental impacts of aquaculture have been reported in relation to some commercial mariculture (Fachry et al., 2018; Mau and Jha, 2018), there are a number of categories of mariculture with broad positive socio-ecological impacts. These include subsistence, recreational, restorative, scientific, and remediation mariculture (Phillips, 2009). Subsistence mariculture involves small-scale and artisanal activities carried out primarily to feed family and relatives of the individual or community undertaking the activity. Generally it also implies the use of low tech “artisanal” aquaculture techniques by low-income people, and may include some sale and/or trade of products. Recreational mariculture (e.g., oyster gardens, see Marenghi and Ozbay, 2010), restorative aquaculture (Luckenbach et al., 2005; Beck et al., 2011; La Peyre et al., 2014; Gilby et al., 2018), and remediation using mariculture (Nieves-Soto et al., 2011), are further examples of non-profit mariculture activities targeting either aesthetic or environmental benefits. Finally, scientific mariculture involves the farming of marine shellfish for research, this activity being commonly linked with restorative mariculture or “mariculture-based enhancement.” Here we seek to examine case studies of Restorative Shellfish Mariculture (RSM) to develop a broad definition of RSM, review the potential aims of RSM, and propose a structure for classifying RSM.
As an emerging field, consensus on what constitutes RSM is often lacking. While maximization of benefits other than provisioning services is a pre-requisite by some existing definitions (Bersoza Hernández et al., 2018), maximization of fisheries yields predominates in others (e.g., stock enhancement, see Bell et al., 2005). In some cases “restorative aquaculture” is designed primarily to actively deliver ecosystem services, in order to achieve positive impacts on the broad socio-ecological systems, to enhance habitat quality via restoration programs, and simultaneously improve food security and employment opportunities (Theuerkauf et al., 2019). In this case, and in numerous other cases where population and/or species restoration is achieved through “restorative aquaculture,” improved fisheries may be the long-term goal of the restoration activity, but initial stages may be focused on restoring the ecology of the species, biodiversity and other non-harvest related ecosystem services (Fitzsimons et al., 2020). Although the focus of non-commercial strategies is not immediately associated with improving fisheries productivity, the enhanced stocks may often be exploitable by the public as common property resources. Our definition therefore deviates from the FAO definition of mariculture as we consider these activities to be a genuine form of RSM. Thus, we define Restorative Shellfish Mariculture (RSM) as “a multi and/or interdisciplinary approach, involving some form of human intervention during the species life cycle, aiming to address negative socio-ecological impacts derived from the unsustainable use of marine shellfish.” Sustainability is here related to the long-term maintenance (or improvement) of wild stocks and their habitats.
Strategies involved in RSM are classified along a gradient from the most conservation-oriented (e.g., reintroduction of locally extinct or endangered species), to fisheries-oriented (including some forms of fisheries enhancement). As with “traditional” shellfish aquaculture, RSM can also vary with regards to how juvenile mollusks are sourced, i.e., from wild populations or from hatcheries. However, the technology, infrastructure and knowledge needed to develop an operational hatchery may not be readily available in economically less developed countries, and, given the recent increase in the scale of such projects, is frequently also a limiting factor in ecological restoration efforts in developed nations. In this regard, categorizing RSM efforts into Hatchery Dependent (HD) and Non-hatchery Dependent (NHD) techniques will provide insights regarding the feasibility of the mainstreaming of strategies. Our classification of RSM is based on and combines categories defined in Bell et al. (2005); Brumbaugh et al. (2006), Camara and Vadopalas (2009), and Leber (2013).
Non-hatchery Dependent (NHD) strategies involve passive or active approaches to address reduced abundance or local extinctions of shellfish. These include the establishment of no-take areas or sanctuaries to reduce fishing effort and incidental take, analogous to the “Do nothing” strategy (Camara and Vadopalas, 2009). Alternatively, RSM may focus on restoration of the mollusk habitat, where populations have reduced, modified or polluted supporting habitats, or have been overfished. In many cases “do nothing” alone does not result in population recovery. Restoration may require man-made improvements to the environment, such as providing substrate for settlement of larvae where populations are “substrate limited” (Beck et al., 2008; Fitzsimons et al., 2019). Alternatively, mollusk populations may have been reduced below the level where allee effects limit recovery and be “broodstock limited” in which case addition of broodstock or juveniles is necessary to allow for population recovery (Bell et al., 2005; Fitzsimons et al., 2019). Such activities, if they rely on translocations of non-hatchery reared individuals, can be considered NHD Supplementation or Redistribution of natural recruitment. This would also apply to “reintroductions,” where wild juvenile or adult organisms are released in sites where local extirpations/extinctions have occurred. Care must be taken in all NHD translocations and reintroductions, to pay strict attention to biosecurity, so as not to inadvertently cause more harm than good through the accidental introduction of diseases or invasive species (Mineur et al., 2014; Šegvić-Bubić et al., 2020).
In Hatchery Dependent (HD) strategies, juveniles reared in hatcheries are transferred in large numbers into restoration sites, either as a reintroduction or as supplementation of an existing population. HD efforts may rely on wild or genetically improved broodstock. Best practice would also dictate that careful consideration should be given to selecting broodstock so as to maintain genetic diversity (Bromley et al., 2016).
RSM does not include “put and take,” where young are released in order for the same individuals to be captured within their lifetimes. The objective of RSM restocking is to restore a depleted spawning biomass, releasing juveniles into wild, unenclosed population(s). This does not imply that in RSM the stock cannot be sustainably fished. Stock enhancement, which seeks to increase the supply of juveniles and optimize harvests by reducing or eliminating limitations in recruitment may also be considered RSM under sustainable fisheries management if not all individuals which are relayed are later captured (harvesting all individuals would then make it akin to “put and take” or sea ranching) (Leber, 2013). In contrast, sea ranching strategies, in which cultured juveniles are deployed into unenclosed aquatic environments to be harvested at large sizes (Leber, 2013), would not be considered a form of RSM (see e.g., Bell et al., 2005; Lorenzen et al., 2013; Taylor et al., 2017). This is despite the possibility of some positive “spill” from sea ranching to other populations in an open marine environment.
While mollusk mariculture is generally deemed to be among the most sustainable and low-impact forms of food production (see e.g., Shumway et al., 2003), there is also the potential for negative consequences. Poorly managed mariculture can result in negative impacts from invasive species and diseases (Mineur et al., 2014). Furthermore, there is some evidence that mariculture can negatively impact local wild-populations through genetic impacts (Bromley et al., 2016), or through acting as a population sink of wild larvae (Šegvić-Bubić et al., 2020). Furthermore, systems need to account for carrying capacity in order to ensure that local wild-stocks are not energetically impacted. RSM efforts must therefore actively seek to mitigate these potential negative efforts and undertake shellfish growing in an ecologically responsible and holistic way.
To our knowledge, no study has attempted to review or synthesize the breadth and aims of RSM as described above. Here we review, synthesize and compile case studies of RSM from US, Europe, Asia, Oceania, and South America, in order to identify emergent patterns across species and/or ecoregions. We also seek to find the commonalities between at least two somewhat independent epistemic communities: (a) the modern shellfish restoration and (b) the fisheries science/aquaculture “restocking” communities, in order to identify knowledge exchange opportunities that may benefit the mainstreaming of RSM. We hope that this may contribute to a broader view of the efforts so far developed by RSM practitioners worldwide.
In order to identify habitat restoration projects involving RSM, we conducted a review of shellfish restoration networks and databases from across the world. Databases searched included: the NOAA Restoration Atlas1, The Native Oyster Restoration Alliance (NORA)2, a European network aiming at reinforcement and restoration of the native European flat oyster (Ostrea edulis), The Australian Shellfish Reef Restoration Network3 and publications from the Latin American network for Shellfish Conservation and restoration (Carranza et al., 2011). Additional projects were identified from the authors’ experience and review of available literature via web search, either searching by species or selected keywords in English and Spanish. Information relating to all projects meeting previously identified criteria (Table 1) was extracted into a database. Relevant data that were commonly extracted included: the species targeted for restoration, the main restoration strategies employed (as defined above), and the degree of involvement of hatcheries. Each project was geo-referenced, and mapped using Geographic Information Systems. Projects were classified according to the Marine Ecoregions of the World (MEOW) biogeographic classification, a nested system of 12 realms, 62 provinces and 232 ecoregions (Spalding et al., 2007), in order to assess the biogeographic distribution of the projects.
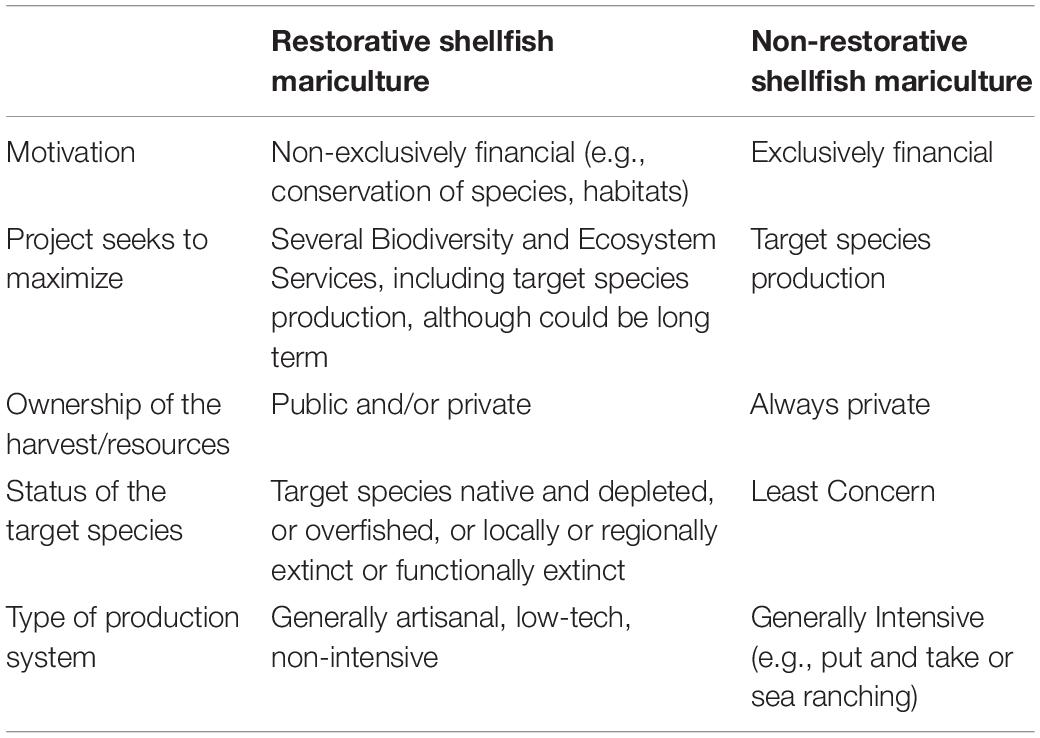
Table 1. List of the criteria involved in the definition of Restorative Shellfish Mariculture, contrasted to “pure” or “commercial” mariculture.
Five hundred and eighty-four completed and ongoing shellfish restoration projects were identified worldwide (Figure 1A). Forty-seven species, including 32 bivalves and 15 gastropods were identified as being targets of RSM. More than 90% of the projects involved only five species, namely the eastern oyster (Crassostrea virginica, N = 379), the giant clam (Tridacna gigas, N = 65), the Olympia oyster (Ostrea lurida, N = 25), the bay scallop (Argopecten iradians, N = 25) and the hard clam (Mercenaria mercenaria, N = 15). The database is strongly biased toward projects developed in the US, partially due to the large number of projects stored in the NOAA database. Nevertheless, even this extensive database under represents US restoration efforts, exemplified by the fact that Bersoza Hernández et al. (2018) lists 1768 projects targeting C. virginicain the US from 1964 to 2017. However, in the case of O. lurida, the Native Olympia Oyster Collaborative4, another known repository, listed no further projects other than those already captured in the NOAA database. Based on the projects we were able to identify, most projects have been developed in the Temperate Northern Atlantic Realm. For example, restoration initiatives targeting mainly C. virginica in the Virginian Ecoregion accounts for 298 cases. This Realm also includes the 10 known O. edulis restoration projects in Europe. In contrast, in the Central Indo-Pacific Realm, at least 95 projects were identified targeting a much larger suite of species, primarily Tridacna spp. in the Philippines and other Pacific islands but also a number of restocking initiatives in Japan regarding some additional species (Table 2 and Figure 1B). Regrettably, we were unable to find further information on these Japanese experiences in the English and Spanish language literature searched. Additional species by country information and supporting references can be found in Supplementary Table 1.
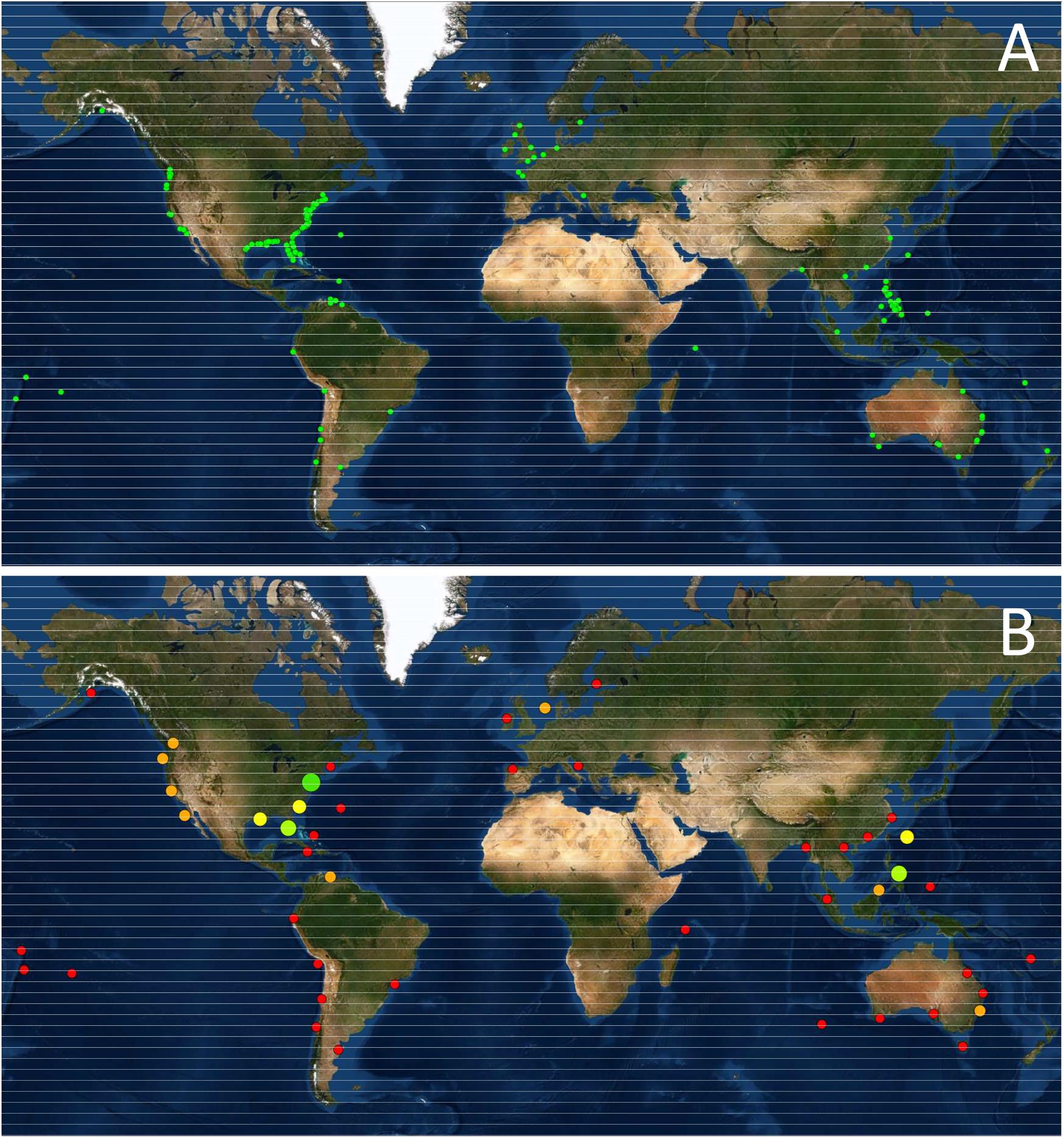
Figure 1. (A) Geographic location of RSM Projects analyzed (N = 575, since some projects could not be geo-referenced); (B) Ecoregion-based RSM effort, showing the relative number of projects.

Table 2. Number of Restorative Shellfish Mariculture (RSM) projects here analyzed, by realm, province, and ecorregion.
We found an even distribution between NHD and HD strategies, with 51% of the projects using Non-hatchery dependent methods, while 49% relied on some form of hatchery production. Within NHD strategies, most projects (74%) involved some form of habitat restoration, while 31% utilized supplementation and/or redistribution of natural recruitment. 3% of the projects combined both methods. HD strategies were the most common in the Central Indo-Pacific Realm, while NHD habitat restoration initiatives were widespread in the US.
Habitat restoration may utilize a variety of materials to add elevation and durability to existing, degraded reef; e.g., deploying fossilized shell material bagged into individual mesh bags; creation of oyster reefs from fresh oyster shell on a relict oyster reef site, or constructing and placing oyster domes and/or bars. Other habitat restoration programs aimed to additionally improve the regulation of salinity in neighboring areas, increasing oysters recovery time following events of natural mortality, but also enhancing oyster reefs resilience concerning the projected scenarios of sea level rise.
In the case of C. virginica, supplementation or redistribution of natural recruitment often takes the form of oyster gardening, although most gardening programs are associated with hatcheries production. For example, the Galveston Bay Foundation (US) worked with local waterfront property owners and other community volunteers to develop an oyster gardening program. Plastic mesh bags full of oyster shells were hung from property owners’ piers in order to collect oyster larvae. Later, all of the oyster gardens are collected and the shells and spat are spread on nearby restoration reefs to enhance the local oyster populations. This approach has also been shown to have wider socio-economic benefits in the form of outreach and education opportunities with the coastal communities in which oyster gardening takes place (De Angelis et al., 2019).
Redistribution of natural recruitment has also had been trialed in Gastropods such as the queen conch (Strombus gigas) in the Florida Keys (Delgado et al., 2004). Results indicate that translocations are more cost-effective than releasing hatchery-reared juveniles, although where the donor source is not local, biosecurity risks should be considered. The redistribution of wild adults provides a rapid increase in reproductive output, and maintains the genetic integrity of the wild stock. Translocations of spat of C. rhizophorae settled on mangrove roots from La Restinga (Isla de Margarita) to Mochima Gulf, are an example of similar approach for bivalves in Venezuela, though regrettably small in scale and not continued due to lack of support (Carranza et al., 2011; zu Ermgassen et al., in press). Some translocations were undertaken in response to environmental impacts as pollution events, such as the transfer of M. mercenaria broodstock from contaminated areas into designated sites within Buzzards Bay following an oil spill. NHD and HD strategies can also be combined. For example, in the Bon Secour Bay oyster Spawner Reef Restoration (Alabama, United States), C. virginica spat raised in the Auburn University Marine Extension and Research Center hatchery and spat-on-shell raised by volunteers from wild settlement in the locality were deployed onto a relict oyster reef.
Oyster gardening is a commonly used approach in HD Supplementation, as hatchery stock are typically very small and prone to high mortality from predation if relayed directly onto the seafloor. For example, in Maryland and Virginia (US), small oyster gardening programs were developed to restore depleted oyster populations and thus improve water quality in Chesapeake Bay. Oysters are grown from spat on shell by volunteer oyster gardeners using floating cages secured to private piers. Juveniles (spats) were provided to volunteers, who monitor and clean the cages and perform some basic monitoring of the oysters. Similarly, to address shellfish injuries from the North Cape oil spill (US), a project using nursery grow-out of and release of quahog M. Mercenaria was conducted to enhance existing populations. Reseeding programs were developed by either purchasing larger sized seed for direct placement in open fishery areas, or smaller sized seed for placement in shellfish nursery growing facilities. Then, floating upwellers were secured and seeded with quahog for restoration of recreational fishing areas.
Typical examples of restocking and stock enhancement are giant clams (Tridacninae) and Trochid gastropods (Trochidae). A network of institutions including the Okinawa Prefectural Fisheries Experimental Station, the University of Papua New Guinea, the Micronesian Mariculture Demonstration Center, the Australian Centre for International Agricultural Research, the Marine Science Institute at the University of Philippines and the WorldFish Center have been restoring stocks of giant clams since the early 1980s, by rearing and propagating juveniles to repopulate coral reef habitats. Juveniles are grown in land-based nurseries until they are large enough for transplantation, usually at (20–25 mm shell length), and then transferred to ocean nurseries (Bell et al., 2005).
Assessing the success of RSM efforts is a challenge. However, at least for the US the number of projects can itself be used as a proxy: a total of 5199 ha of C. virginica has been restored in the United States, based in results from 1178 projects from 1987 to 2017 (Bersoza Hernández et al., 2018). Regardless of the restoration strategy applied, all RSM projects will at least temporarily produce positive changes in absolute and/or relative abundances and biomass of the target species. The increases in abundance can, however, be short lived and some exploited species such as Trochus (Trochus niloticus) in the Pacific and M. mercenaria in the Atlantic, were found not to increase significantly after restocking efforts (Heslinga et al., 1984; McCay, 1988), possibly because restoration efforts were focused on marginal habitats where the reproductive contribution of the snails and clams was negligible. When RSM is successful, however, population structure (e.g., size-frequency distribution, sex ratio, age ratio) and population-level processes are also positively affected: For C. virginica, mean oyster recruitment was ∼12 times higher in restored and harvested reefs than in natural, harvested reefs, and potential larval output from restored and protected reefs may be sixfold larger than natural and restored harvested reefs (Theuerkauf et al., 2015; Peters et al., 2017).
Diana (2009) previously highlighted some positive impacts of aquaculture on biodiversity; for example, cultured seafood can reduce pressure on overexploited wild stocks, stocked organisms may enhance depleted stocks, aquaculture often boosts natural production, and employment in mariculture may replace more destructive resource uses. More recently, Alleway et al. (2019) highlighted the role of aquaculture in supporting ecosystem services beyond solely the production of goods, through provisioning services, regulating services, habitat or supporting services, and cultural services. RSM therefore may benefit all hierarchies of biodiversity, considering composition, structural and functional impacts across genetic, species-population, community-ecosystem and landscape levels.
Yet several of these impacts remain to be quantified, and the relative and absolute success of different strategies is yet to be assessed systematically. In particular, the evaluation of impacts on targeted species and other biodiversity benefits due to RSM should receive more attention. Published research points out that, for C. virginica in United States, only half of the projects analyzed by Bersoza Hernández et al. (2018); N = 88 showed positive Returns of Investment (ROI) considering ecosystem services, and that the size of the projects was positively related to ROI. This has also been shown for seagrass restoration projects (van Katwijk et al., 2016), where individual survival and seagrass population growth rate were enhanced with the scale of the restoration trials. Further, although RSM is gaining momentum globally, there is still a lack of documented initiatives in Africa and India and only a few for South America.
In a recent global analysis, Theuerkauf et al. (2019) called for a more integrated, pragmatic, and market-driven approach to ecosystem recovery and management. We believe that RSM has the potential to generate greater positive impacts on the socio-ecological systems should it continue to expand both geographically and taxonomically. More empirical data are needed in order to fully appreciate the positive contributions of RSM to biodiversity and threatened ecosystems, across the functional and taxonomic range of species involved.
The datasets generated for this study are available on request to the corresponding author.
AC designed the manuscript, compiled information, performed the analysis, and wrote the manuscript. PE wrote the manuscript and reviewed and contributed to manuscript structure. Both authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AC wishes to thank Margarida Casadevall for the invitation to contribute to this special issue. Omar Defeo and the TNC Shellfish at Risk team (Mike, Rob, Boze, et al.) are also acknowledged for involving me in shellfish restoration. AC also wish to thank the colleagues from the Latin American Network for Shellfish Conservation and Restoration for sharing experiences and knowledge on South American shellfish conservation. PE thanks the Nature Conservancy for their support.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00722/full#supplementary-material
Alleway, H. K., Gillies, C. L., Bishop, M. J., Gentry, R. R., Theuerkauf, S. J., and Jones, R. (2019). The ecosystem services of marine aquaculture: valuing benefits to people and nature. BioScience 69, 59–68.
Beck, M. W., Brumbaugh, R. D., Carranza, A., Coen, L. D., Defeo, O., Lenihan, H. S., et al. (2008). Shellfish at risk: a global assessment of distribution, condition and threats to habitat-forming bivalves. J. Shellf. Res. 27, 989–990.
Beck, M. W., Brumbaugh, R. D., Airoldi, L., Carranza, A., Coen, L. D., Croefort, C., et al. (2011). Oyster reefs at risk and recommendations for conservation, restoration, and management. BioScience 61, 107–116. doi: 10.1525/bio.2011.61.2.5
Bell, J. D., Munro, J. L., Nash, W. J., Rothlisberg, P. C., Loneragan, N. R., Ward, R. D., et al. (2005). Restocking and stock enhancement of marine invertebrate fisheries. Adv. Mar. Biol. 49, 309–374.
Bersoza Hernández, A., Brumbaugh, R., Frederick, P., Grizzle, R., Luckenbach, M., Peterson, C. H., et al. (2018). Restoring the eastern oyster: how much progress has been made in 53 years? Front. Ecol. Environ. 16:463–471. doi: 10.1002/fee.1935
Bromley, C., McGonigle, C., Ashton, E. C., and Roberts, D. (2016). Badmoves: Pros and consofmovingoysters - a case studyof global translocations of Ostrea edulis Linnaeus, 1758 (Mollusca: Bivalvia). Ocean Coast Manag. 122, 103–115. doi: 10.1016/j.ocecoaman.2015.12.012
Brumbaugh, R. D., Beck, M. W., Coen, L. D., Craig, L., and Hicks, P. (2006). A Practitioners Guide to the Design & Monitoring of Shellfish Restoration Projects: An Ecosystem Services Approach. Arlington, VA: The Nature Conservancy.
Camara, M., and Vadopalas, B. (2009). Genetic aspects of restoring Olympia oysters and other native bivalves: balancing the need for action, good intentions, and the risks of making things worse. J. Shellf. Res. 1, 121–146. doi: 10.2983/035.028.0104
Carranza, A., Defeo, O., Gracia, A., Gamarra, A., Pascual, M., Henriques, M., et al. (2011). Towards a South American network for shellfish conservation and restoration Tentacle. Newslett. IUCN 19, 3–10.
De Angelis, B., Birch, A., Malinowski, P., Abel, S., DeQuattro, J., Peabody, B., et al. (2019). “A variety of approaches for incorporating community out reach and education in Oyster Reef Restoration Projects: Examples from the United States,” in Goods and Services of Marine Bivalves, eds Smaal, A. C., Ferreira, J. G., Grant, J., and Petersenand, J. K. ø. Strand. (Cham, Springer International Publishing), 335–354.
Delgado, G. A., Bartels, C. T., Glazer, R. A., Brown-Peterson, N. J., and McCarthy, K. J. (2004). Translocation as astrategy to rehabilitate the queen conch (Strombus gigas) population in the Florida Keys. Fishery Bulletin 102, 278–288.
Diana, J. S. (2009). Aquaculture production and biodiversity conservation. BioScience 59, 27–38. doi: 10.1525/bio.2009.59.1.7
Fachry, M. E., Sugama, K., and Rimmer, M. A. (2018). The role of small-holder seed supply in commercial mariculture in South-east Asia. Aquaculture 495, 912–918. doi: 10.1016/j.aquaculture.2018.06.076
Fitzsimons, J., Branigan, S., Brumbaugh, R. D., McDonald, T., and zu Ermgassen, P. S. E. (eds) (2019). Restoration Guidelines for Shellfish Reefs. Arlington VA: The Nature Conservancy.
Fitzsimons, J., Branigan, S., Gillies, C., Brumbaugh, R., Cheng, J., and DeAngelis, B. (2020). Restoring shellfish reefs: global guidelines for practitioners and scientists. Conserv. Sci. Pract. 2:e198. doi: 10.1111/csp2.198
Food and Agriculture Organization [FAO] (2018). The State of World Fisheries and Aquaculture 2018 - Meeting the Sustainabledevelopment Goals. Rome: Food and Agriculture Organization of the United Nations.
Gilby, B. L., Olds, A. D., Peterson, C. H., Connolly, R. M., Voss, C. M., Bishop, M. J., et al. (2018). Maximizing the benefits of oyster reef restoration for finfish and their fisheries. Fish Fish. 19, 931–947. doi: 10.1111/faf.12301
Heslinga, G. A., Orak, O., and Ngiramengior, M. (1984). Coral reef sanctuaries for trochus shells. Mar. Fisher. Rev. 46, 73–80.
La Peyre, M., Furlong, J., Brown, L. A., Piazza, B. P., and Brown, K. (2014). Oyster reef restoration in the northern Gulf of Mexico: extent, methods and outcomes. Ocean Coast. Manag. 89, 20–28. doi: 10.1016/j.ocecoaman.2013.12.002
Leber, K. M. (2013). “Marine fisheries enhancement: coming of age in the new millennium,” in Sustainable Food Production, ed. P. Christou (Cham: Springer), 1139–1157. doi: 10.1007/978-1-4614-5797-8_188
Lorenzen, K., Agnalt, A. L., Blankenship, H. L., Hines, A. H., Leber, K. M., Loneragan, N. R., et al. (2013). Evolving context and maturing science: aquaculture-based enhancement and restoration enter the marine fisheries management toolbox. Rev. Fish. Sci. 21, 213–221. doi: 10.1080/10641262.2013.837358
Luckenbach, M. W., Coen, L. D., Ross, P. G. Jr., and Stephen, J. A. (2005). Oyster reef habitat restoration: relationships between oyster abundance and community development based on two studies in Virginia and South Carolina. J. Coast. Res. 21, 64–78.
Marenghi, F., and Ozbay, G. (2010). Preliminary habitat assessment of floating oyster (Crassostrea virginica) gardens (Delaware). Ecol. Restorat. 28, 254–256. doi: 10.3368/er.28.3.254
Mau, A., and Jha, R. (2018). Aquaculture of two commercially important molluscs (abalone and limpet): existing knowledge and future prospects. Rev. Aquac. 10, 611–625. doi: 10.1111/raq.12190
McCay, B. J. (1988). Muddling through the clambeds: cooperative management of New Jersey’s hard clam spawner sanctuaries. J. Shellfish Res. 7, 327–340.
Mineur, F., Le Roux, A., Maggs, C. A., and Verlaque, M. (2014). Positive feedback loop between introductions of non-native marine species and cultivation of oysters in Europe. Conserv. Biol. 28, 1667–1676. doi: 10.1111/cobi.12363
Nieves-Soto, M., Enriquez-Ocaña, F., Piña-Valdez, P., Maeda-Martínez, A. N., Almodóvar-Cebreros, J. R., and Acosta-Salmón, H. (2011). Is the mangrove cockle Anadara tuberculosa a candidate for effluent bioremediation? Energy budgets under combined conditions of temperature and salinity. Aquaculture 318, 434–438. doi: 10.1016/j.aquaculture.2011.05.041
Peters, J. W., Eggleston, D. B., Puckett, B. J., and Theuerkauf, S. J. (2017). Oyster demographics in harvested reefs vs. No-Take reserves: implications for larval spillover and restoration success. Front. Mar. Sci. 4:326. doi: 10.3389/fmars.2017.00326
Phillips, M. (2009). “Mariculture overview,” in Encyclopedia of Ocean Sciences, eds K. Turekian, J. Steele, and S. A. Thorpe (Amsterdam: Elsevier), 537–544. doi: 10.1016/b978-012374473-9.00752-9
Šegvić-Bubić, T., Žužul, I., Talijančić, I., Ugrin, N., LepenPleić, I., Žuvić, L., et al. (2020). Translocation and aquaculture impact on genetic diversity and composition of wild self-sustainable ostrea edulis populations in the Adriatic Sea. Front. Mar. Sci. 7:84. doi: 10.3389/fmars.2020.00084
Shumway, S. E., Davis, C., Downey, R., Karney, R., Kraeuter, J., Parsons, J., et al. (2003). Shellfish aquaculture–in praise of sustainable economies and environments. World Aquac. 34, 8–10.
Spalding, M. D., Fox, H. E., Allen, G. R., Davidson, N., Ferdaña, Z. A., Finlayson, M., et al. (2007). Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience 57, 573–583. doi: 10.1641/b570707
Tacon, A. J. (2003). “Aquaculture production trends analysis,” in Review of the State of World Aquaculture. FAO Fisheries Circular (Rome: FAO), 5–29.
Taylor, M. D., Chick, R. C., Lorenzen, K., Agnalt, A. L., Leber, K. M., Blankenship, H. L., et al. (2017). Fisheries enhancement and restoration in a changing world. Fish. Res. 186, 407–412. doi: 10.1016/j.fishres.2016.10.004
Theuerkauf, S. J., Burke, R. P., and Lipcius, R. N. (2015). Settlement, growth, and survival of eastern oysters on alternative reef substrates. J. Shellf. Res. 34, 241–250. doi: 10.2983/035.034.0205
Theuerkauf, S. J., Morris, J. A. Jr., Waters, T. J., Wickliffe, L. C., Alleway, H. K., and Jones, R. C. (2019). A global spatial analysis reveals where marine aquaculture can benefit nature and people. PLoS One 14:e0222282. doi: 10.1371/journal.pone.0222282
van Katwijk, M. M., Thorhaug, A., Marbà, N., Orth, R. J., Duarte, C. M., Kendrick, G. A., et al. (2016). Global analysis of seagrass restoration: the importance of large-scale planting. J. Appl. Ecol. 53, 567–578. doi: 10.1111/1365-2664.12562
Wijsman, J. W. M., Troost, K., Fang, J., Roncarati, A. (2019). “Global production of marine bivalves,” in Trends and Challenges, eds Smaal, A., Ferreira, J., Grant, J., and Petersen, J. ø. Strand (Cham: Springer). doi: 10.1007/978-3-319-96776-9_2
Keywords: aquaculture, shellfish, oyster reefs, mussel beds, marine habitats, coastal habitats
Citation: Carranza A and zu Ermgassen PSE (2020) A Global Overview of Restorative Shellfish Mariculture. Front. Mar. Sci. 7:722. doi: 10.3389/fmars.2020.00722
Received: 04 November 2019; Accepted: 10 August 2020;
Published: 11 September 2020.
Edited by:
Cornelia E. Nauen, Mundus Maris, BelgiumReviewed by:
Muhammed Alolade Oyinlola, The University of British Columbia, CanadaCopyright © 2020 Carranza and zu Ermgassen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alvar Carranza, YWx2YXIuY2FycmFuemFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.