
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 28 July 2020
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 7 - 2020 | https://doi.org/10.3389/fmars.2020.00593
This article is part of the Research Topic Fish Nutrition, Metabolism and Physiology View all 20 articles
Taste is an important trait of sea urchin quality. Highly contained alanine in gonads of the sea urchin Mesocentrotus nudus results in desirable taste. Past studies hypothesized that high levels of glutamic acid and/or alanine in feed increase the alanine content in the gonads. To identify the amino acids in feed that increase the alanine content in the gonads, the free amino acid content in the gonads of M. nudus of the following five treatment groups were compared at the start and end of the feeding experiment during May–July: sea urchins fed one of four experimental diets, in which glutamic acid (Glu S), alanine (Ala S), aspartic acid (Asp S), or glycine (Gly S) was supplemented, or a control diet without supplementation (Control). The alanine content in the gonads of Ala S increased significantly from the start. The content in the gonads of Glu S increased without significance. There was no significant difference in the alanine content in the gonads between Ala S and Glu S. The content in the gonads of Ala S was significantly higher than that of Asp S, Gly S, and Control. There were no significant differences in the aspartic acid and glutamic acid contents in the gonads among treatments at the end of the experiment. The higher alanine content in the gonads of Ala S than that in Glu S indicates that direct accumulation of alanine from the feed is effective. This study first identified the amino acids in feed that are closely associated with improvement in the taste of sea urchin gonad.
From 1961 to 2016, the average annual increase in global fish and shellfish consumption (3.2%) outcompeted population growth (1.6%) and exceeded the consumption of meat from all terrestrial animals combined (2.8%) (FAO, 2018). In per capita terms, the annual fish and shellfish consumption increased from 9.0 kg in 1961 to 20.2 kg in 2015 (FAO, 2018). Sea urchin gonads are considered a delicious seafood with high commercial value (Walker et al., 2015). The average price increased from 7,363 yen/kg (US$70.5/kg at a 104.46 yen/US$ exchange rate) in 2008 to 14,661 yen/kg (US$131.6/kg at a 111.43 yen/US$ exchange rate) in 2018 at the Tokyo Metropolitan Central Wholesale Market (Metropolitan Central Wholesale Market, 2019), where the largest amount of sea urchins are sold worldwide (Sun and Chiang, 2015). Since 2012, the import of live, fresh or chilled sea urchins to European and Oceanian countries has been underway (FAO, 2019a), indicating an increase in their worldwide popularity. In contrast, global sea urchin production has been decreasing from 109,736 t in 1995 to 70,833 t in 2017 (FAO, 2019b) due to overfishing (Andrew et al., 2002). In response to market demands, many studies on the enhancing growth and gonad production of hatchery-raised larvae, post-settled juveniles and adults have been conducted worldwide (McBride, 2005; Pearce, 2010). Recently, with the decline of the natural population of the sea urchin Paracentrotus lividus, research papers focusing on sea urchin aquaculture in the Mediterranean regions have been published (e.g., Baião et al., 2019; Zupo et al., 2019). In northern Japan, studies focusing on the short-term culture of Mesocentrotus nudus adults, which are densely distributed in crustose coralline red algal communities (barrens) without erect macrophytes (reviewed by Agatsuma, 2013), have been advancing (e.g., Unuma et al., 2015; Inomata et al., 2016). Sea urchins on barrens have small gonads; exhibit undesirable gonad color, texture and taste; and are of no commercial value (e.g., Agatsuma et al., 2005; Takagi et al., 2017). The gonad of M. nudus is the most expensive in the world. The wholesale price per wooden tray with 250–300 g gonads of M. nudus can exceeds 100,000 yen (US$1,000) (Nukui, 2018), whereas the average price of all imported sea urchins is approximately 6,000 yen/kg (US$60/kg) at a 100 yen/US$ exchange rate (Unuma, 2015). There are no quantitatively ranked classes of sea urchin gonad in Japan. Takagi (2020) concluded that M. nudus gonads with 56.0–60.7 of L∗ value, 0.11–0.14 N of hardness, 235–595 mg/100 g of alanine content and 215–334 mg/100 g of arginine content can have commercial value by sensory evaluation and quantitative measurements and analyses of gonad quality of M. nudus.
Studies on development of artificial feeds have been conducted with the sea urchins Evechinus chloroticus (e.g., James et al., 2007; Phillips et al., 2009, 2010), Heliocidaris erythrogramma (e.g., Senaratna et al., 2005), Loxechinus albus (e.g., Lawrence et al., 1997), Lytechinus variegatus (e.g., Watts et al., 1998; Heflin et al., 2012, 2016), Mesocentrotus franciscanus (e.g., McBride et al., 1997, 1999, 2004), P. lividus (e.g., Cook and Kelly, 2007; Cook et al., 2007; Prato et al., 2018), Psammechinus miliaris (e.g., Cook and Kelly, 2007; Suckling et al., 2011), Strongylocentrotus droebachiensis (e.g., Pearce et al., 2002a, b; Siikavuopio et al., 2007), and Tripneustes gratilla (e.g., Cyrus et al., 2014, 2015). Past studies revealed that high protein and β-carotene in feed enhance gonad production (e.g., de Jong-Westman et al., 1995; Hammer et al., 2012) and improve gonad color (e.g., Robinson et al., 2002; Shpigel et al., 2005), respectively, by feeding trials with artificial diets. However, feeds with high protein content lead to undesirable color and taste (e.g., Agatsuma, 1998; Pearce et al., 2002a), low sweet-tasting amino acid (alanine and glycine) contents and high bitter-tasting amino acid (lysine and valine) contents in gonads (Hoshikawa et al., 1998; Liyana-Pathirana et al., 2002; Phillips et al., 2010; Inomata et al., 2016).
Of sea urchin gonad qualities, taste is an important trait. Takagi et al. (2019) first succeeded in improving the gonad taste of sea urchin to a more desirable level than the gonad taste of sea urchin from a fishing ground by feeding fresh Saccharina japonica kelp to M. nudus during May–July. The gonads of M. nudus, which was collected from a barren and fed the basal frond portion of S. japonica or the sporophyll of Undaria pinnatifida kelp during May–July, reached an acceptable level for the sushi restaurants with three or two Michelin stars in Tokyo (Takagi et al., 2020a). Komata (1964) identified umami-tasting glutamic acid, sweet-tasting alanine and glycine, and bitter-tasting methionine and valine as the “taste essential” amino acids in sea urchin gonads by omission test. Our previous studies on M. nudus revealed that the gonad taste can be improved due to the strong sweetness of a high alanine content, a strong umami flavor, and a low arginine content (Takagi et al., 2017, 2019). Phillips et al. (2009) reported that the taste of gonads from the sea urchin E. chloroticus fed feeds containing high amounts of glutamic acid and glycine was sweeter than that of those fed diets containing high amounts of valine and methionine. Takagi et al. (2020c) suggested that a high alanine content in gonads of M. nudus fed the basal frond portion of S. japonica is synthesized from glutamic acid, which is highly contained in the frond portion. Feeding of the sporophyll of U. pinnatifida, which has a markedly high alanine content, increased the alanine content in the gonads of M. nudus, suggesting that the excess alanine in the sporophyll was directly accumulated in the gonads (Takagi et al., 2020b). These studies generated a hypothesis that high levels of glutamic acid and alanine in feed increase the alanine content in the gonads of sea urchins.
In the present study, we prepared artificial diets in which glutamic acid, alanine and glycine were individually supplemented. We also prepared a diet supplemented with aspartic acid, which is highly abundant in S. japonica fronds and the sporophyll of U. pinnatifida along with the above-mentioned amino acids (Takagi et al., 2020b), and diet without any amino acid supplementation was used as a control. Mesocentrotus nudus adults from a barren were fed each of the diets during May–July, and the free amino acid contents in the gonads were compared at the end of the experiment. This study aimed to identify the amino acids in feed that increase the alanine content in gonads and verify the hypothesis that high levels of glutamic acid and alanine in feed increase the alanine content in the gonads of sea urchins.
The present study does not involve any vertebrate or specified invertebrate animals. Mesocentrotus nudus were collected from a site in the Shizugawa Bay, Miyagi Prefecture, Japan that is not privately-owned or protected in any way. All experimental procedures on animals were in compliance with the guidelines of Miyagi Fisheries Cooperative Association and Miyagi Prefectural Government.
The feeding experiment was designed with the initial group (Initial) and five treatment groups: sea urchins fed one of the experimental diets in which glutamic acid (Glu S), alanine (Ala S), aspartic acid (Asp S), or glycine (Gly S) were supplemented, and sea urchins fed a control diet without these supplements (Control).
On 27 April 2018, a total of 96 M. nudus adults (47–53 mm diameter) were collected from a barren by scuba dive at depths of 2.4–3.9 m off Nojima Island, Shizugawa Bay, Miyagi Prefecture, Japan (38°40′N, 141°30′E). After collection, the sea urchins were placed in a cool box containing moist urethane mats immersed in seawater and transported to Miyagi Prefecture Fisheries Technology Institute in Ishinomaki, Miyagi Prefecture (38°24′N, 141°22′E) within an hour. The rearing equipment was prepared according to Taylor et al. (2017) with modifications. Immediately after transportation, sea urchins were individually held in vertical cylindrical cages (10 cm diameter × 45 cm height) made of polyethylene with a 2 cm mesh, and the cages were randomly placed in 300 L tanks (inside dimension, 116.5 cm length × 78.0 cm width × 38.2 cm high) with a 35 cm water level (Supplementary Figure S1). Each cage was cleaned and randomly relocated every 3–4 days. The sea urchins were reared with running, filtered seawater, which was exchanged two or three times per hour. The seawater was pumped up from offshore waters and aerated. To avoid losing feed through the cage walls, the outside of the bottom and sides (up to a 12 cm height from the bottom) of the cages were enclosed using a polyethylene net with 5 mm mesh. Each enclosed cage was separated by fitting the lower side into PVC pipes (4 cm in length, 11.4 cm outer diameter). The cages were elevated 1 cm above the bottom of tanks using the pipes to defecate sea urchin feces. Furthermore, these were elevated 0.5 cm using polyethylene nets with 2 cm meshes to allow for water circulation beneath the cages. The sea urchins were reared without food for 11 days until the start of the experiment. Of them, 80 sea urchins were used for the feeding experiment from 8 May to 10 July 2018. The seawater temperature in the tanks was measured every 10 min using a wireless data logger (RTR-52A, T&D, Nagano, Japan) and the daily water temperature was calculated as an average of 144 data points over 24 h. The average daily water temperature in the tanks during the rearing was 16.2°C and increased from 11.4°C at the start of the rearing to a maximum of 22.4°C at the end (Supplementary Figure S2).
Five types of artificial diets were fed to the sea urchins ad libitum every 3–4 days during the experiment (16 individuals each). From observation of the amount of uneaten diets, we regulated the amount of diets fed to the sea urchins. The 16 remaining sea urchins at the start of the experiment (Initial) and all sea urchins in the five treatment groups at the end of the experiment were used for measurements and analyses of gonad size, development and free amino acid content.
Five types of artificial diets: four types of experimental diets, in which glutamic acid (≥99.0% glutamic sodium, Kt Msg Co., Ltd., Bangkok, Thailand), alanine (≥98.5% L-alanine, Anhui Huaheng Bioengineering Co., Ltd., Anhui, China), aspartic acid (≥99.0% L-aspartic acid, FUJIFILM Wako Pure Chemical Corporation, Tokyo, Japan) or glycine (≥99.0% glycine, Yuki Gousei Kogyo, Co., Ltd., Tokyo, Japan) was supplemented, and a control diet without amino acid supplementation were prepared at the Fisheries Research Laboratory of Marubeni Nisshin Feed Co., Ltd. in Tahara, Aichi Prefecture, Japan (34°39′N, 139°09′E). Ecklonia maxima, which belongs to the order Laminariales, and alginic acid, which is a major structural polysaccharide of brown algae, were chosen as the main ingredient and the binder, respectively. The flowchart of the production of the artificial diets was shown in Supplementary Figure S3. Dried E. maxima (Andes Trading Co., Ltd., Tokyo, Japan) was pulverized using a mill (160Z, Hosokawa Micron Corporation, Osaka, Japan). A total of 400 g of pulverized E. maxima was mixed with 1,200 ml of tap water and processed using a chopper (Chopper, Hirai Kousakujo Ltd., Kobe, Japan) with a 2 mm diameter die. Then, this solution was mixed with 590 g of alginic acid sodium salt (Maya Trading Co., Ltd., Osaka, Japan) and 1,000 ml of tap water. To prepare the four experimental diets, 10 g of each amino acid was added to the 1,000 ml of tap water before mixing it with the alginic acid sodium salt. The mixture was processed twice using a chopper with a 3 mm die. After processing, the mixture was pressed and cut into moist sheets (approximately 1–8 cm in length, 1.6 cm in width, and 0.4–0.5 cm thick) using a die plate of the chopper. To prepare the control diet, the mixture of Ecklonia, alginic acid sodium salt and tap water was processed twice using the chopper with a 3 mm die, then pressed and cut into moist sheets same as experimental diets. The surface of the diets was coated with a 10% CaCl2 solution to prevent the formation of a ball shape. Then, the diets were immediately frozen at −30°C after blotting excess water. The whole process was conducted ca. 25°C room temperature. This process was repeated 3 times within 60 min. A total of 11 times for each diet was repeated. The percentages of Ecklonia and alginic acid sodium salt in the diets were chosen to coincide with the proximate composition of the sporophyll of U. pinnatifida and the frond of S. japonica (Agatsuma et al., 2002).
The test diameters (TDs) (0.1 mm accuracy) and wet body weights (BWs) (0.1 g accuracy) of the sea urchins in each treatment were measured at the start and end of the experiment using a vernier caliper and an electronic balance, respectively.
The gonadal wet weight was measured after blotting excess water, and the gonad index (gonad wet weight × 100/BW) was calculated. A portion of each individual gonad was preserved in 20% formalin. Using standard histological techniques, serial cross-sections (6 μm) were cut and stained with Mayer’s hematoxylin and eosin. Sections were classified based on the stage of development of the germinal cells and the nutritive phagocytes (NPs): stage I, recovering; stage II, growing; stage III, premature; stage IV, mature; stage V, partly spawned; stage VI, spent (Byrne, 1990; King et al., 1994).
Approximately 1.0 g of gonadal tissue from each animal was quickly frozen at −30°C, and the free amino acid (FAA) contents in the tissue were analyzed using the method of Murata et al. (1994) improved by Takagi et al. (2020c). FAAs can be categorized into four groups: umami-tasting (aspartic acid and glutamic acid), sweet-tasting (alanine, glycine, serine, proline, and threonine), bitter-tasting (arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, tyrosine, and valine) amino acids and others according to Kaneko et al. (2009).
Before use in the feeding experiment, a total of 100 g of the control diet was weighed and freeze-dried using a freeze-dryer (FDU-1200, Tokyo Rikakikai Co. Ltd., Tokyo, Japan) for 72 h, and the crude protein, crude lipid, carbohydrate and ash contents in the diet (n = 5) were analyzed according to AOAC methods (AOAC, 1990). The total amino acid (TAA) (total of FAAs and amino acids in proteins and peptides) content of the control diet was analyzed according to Takagi et al. (2020c).
For the determination of residual FAA contents in each artificial diet until the next feeding, on 15 June 2018, the midpoint of the experiment, 8 g of each of the five artificial diets was immersed into seawater in tanks outside of the cages in which sea urchins were reared. Half of the immersed diet sample was collected after 1 h and the remaining half was collected after 3 days (on 18 June). The average daily seawater temperature during 15–18 June was 16.4°C. These samples were frozen at −30°C and had four replicates. The FAA content of each of the artificial diets before the feeding experiment and after immersion for 1 h and 3 days were analyzed using the same method as that for the gonads (n = 4).
All analyses were conducted using JMP 10 (SAS Institute Inc., Cary, NC, United States). The data were tested for homogeneity of variance (Levene’s test). Data that did not show homogeneity of variance were log-transformed. Significant differences in the TD and BW of the sea urchins among the initial values and those of the treatment groups at the start and end of the experiment, in the gonad indices and the FAA contents of gonads among the initial values and those of the treatment groups, and in the supplemented amino acid contents among experimental diets were analyzed using one-way analysis of variance (ANOVA). Tukey’s multiple comparison test was performed as a post hoc test.
The average protein and carbohydrate contents in the control diet were 6.5% and 56.7%, respectively (Table 1). The aspartic acid content was the highest among the amino acids in the control diet, followed by those of glutamic acid, lysine and tyrosine (Table 1). The free glutamic acid, alanine, aspartic acid and glycine contents in the control diet were 1.5 mg/100 g, 5.0 mg/100 g, 1.0 mg/100 g, and 0.2 mg/100 g, respectively. More than 50% of these FAAs were depleted 1 h after immersion. The average content of amino acids supplemented in each diet was 107.1 mg/100 g wet weight. There was no significant difference in the content of supplemented amino acids among the diets (df = 3, MS = 0.016, F = 1.309, p = 0.317). The FAA content decreased to ca. 50.3 mg/100 g 1 h after immersion in seawater (Supplementary Figure S4). Almost all amino acids were depleted 3 days after immersion.
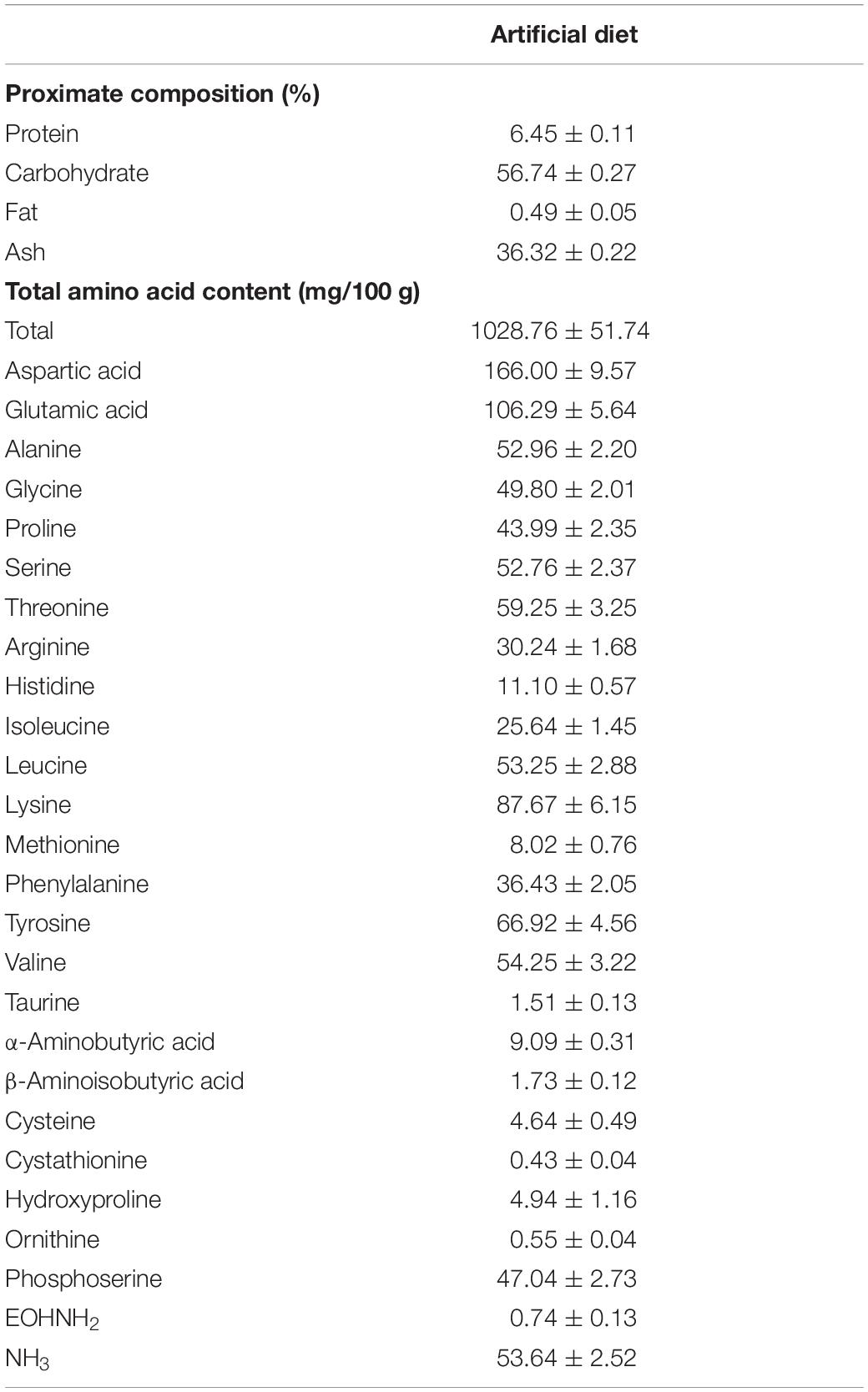
Table 1. Proximate composition (% in dry weight) and total amino acid contents (mg/100 g in wet weight) (mean ± SE) in the artificial diet without amino acid supplementation (control diet) (n = 5).
At the start of the experiment, the average TD and BW of the sea urchins in the five treatment groups were 49.8 mm and 54.6 g, respectively. There were no significant differences among the initial group and the treatment groups (TD, df = 5, MS = 1.49, F = 0.499, p = 0.777; BW, df = 5, MS = 86.92, F = 2.049, p = 0.077). No significant differences in TD, BW or gonad indices among the initial group and the treatment groups at the end of the experiment were detected (Table 2). Half of the gonads of sea urchins in the initial group were either in the recovering stage or growing stage (Supplementary Table S1). At the end of the experiment, 44–63% of gonads of the sea urchins in each treatment group were in the premature stage.
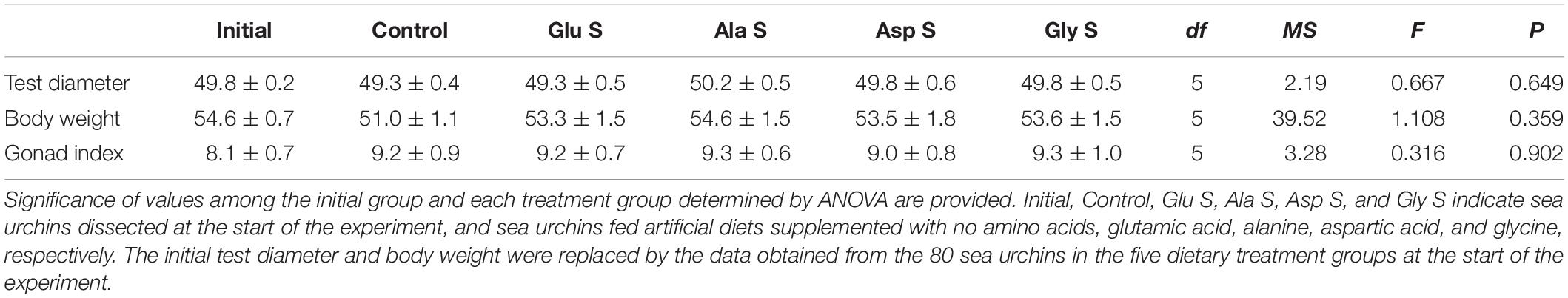
Table 2. Test diameter (mm), body wet weight (g) and gonad indices of Mesocentrotus nudus of the initial group and in each treatment group at the end of the experiment (mean ± SE) (n = 16).
The alanine content in the gonads of Glu S and Ala S increased from the start (Figure 1). There was no significant difference in the alanine content in the gonads between Glu S and Ala S. The alanine content in the gonads of Ala S was significantly higher than that of the initial group and Control, Asp S, and Gly S (p < 0.05). The glutamic acid and aspartic acid contents in the gonads in each treatment increased significantly compared to the initial contents (p < 0.001) (Figure 2). There were no significant differences in these amino acid contents in gonads among the treatments at the end. The glycine content in the gonads of each treatment did not change significantly compared to that at the start except for in Asp S (Figure 2). The contents of bitter-tasting arginine, lysine, methionine and phenylalanine in the gonads of Glu S, Ala S, Asp S, and Gly S decreased significantly compared to those at the start of the experiment (p < 0.05) (Supplementary Table S2). There were no significant differences in the contents of the bitter-tasting amino acids in the gonads among treatments at the end.
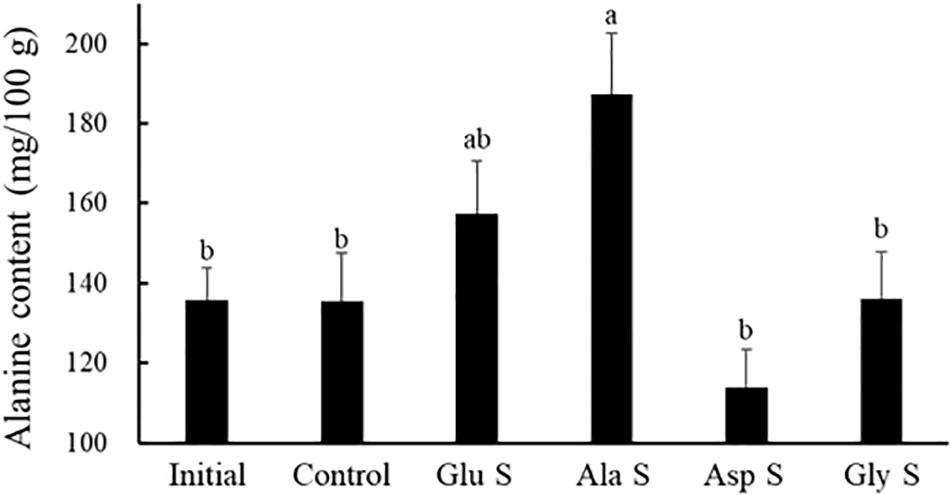
Figure 1. Alanine content (mg/100 g in wet weight) in Mesocentrotus nudus gonads of the initial group and each treatment group at the end of the experiment (mean ± SE) (n = 16). An explanation of Initial, Control, Glu S, Ala S, Asp S, and Gly S is provided in Table 2. Lower-case letters indicate significant differences among groups (p < 0.05 by Tukey’s test).
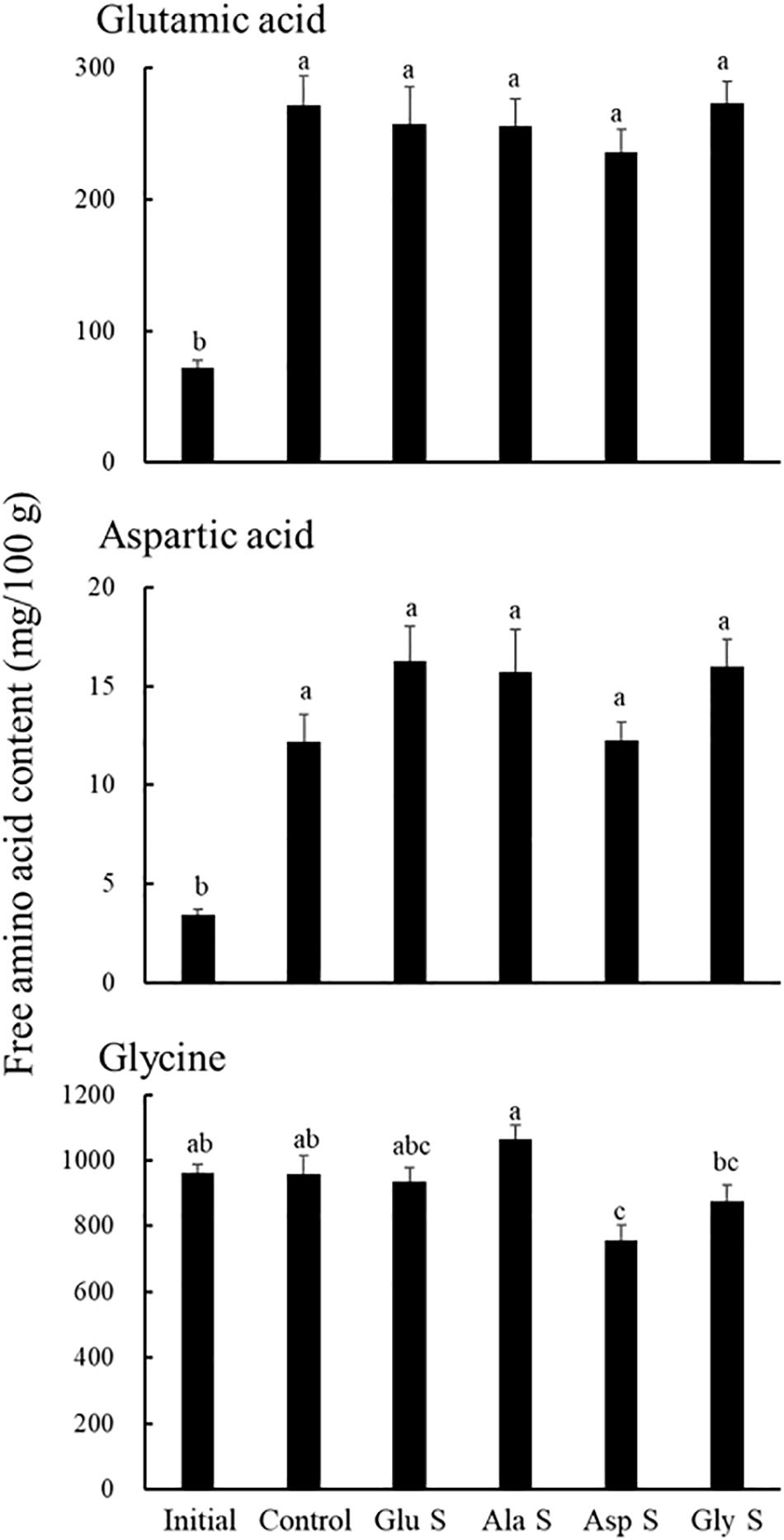
Figure 2. Glutamic acid, aspartic acid and glycine contents in Mesocentrotus nudus gonads of the initial group and each treatment group at the end of the experiment (mean ± SE) (n = 16). An explanation of Initial, Control, Glu S, Ala S, Asp S, and Gly S is provided in Table 2. Lower-case letters indicate significant differences among groups (p < 0.05 by Tukey’s test).
The high protein (e.g., de Jong-Westman et al., 1995; Hammer et al., 2012) and carbohydrate (Heflin et al., 2012, 2016) contents in feeds enhance gonadal production. In contract to no increase in the gonad indices at the end of the experiment in the present study, the approximate composition of each diet was similar to that of S. japonica fronds and U. pinnatifida sporophyll, which are known to enhance gonadal production (Agatsuma et al., 2002). In addition, the gonads of M. nudus in each treatment were shifted from the recovering–growing stages to the growing–premature stages, when M. nudus gonads increase in size (Agatsuma, 1997). The food consumption of sea urchins can be decreased by feed-deterring chemicals (reviewed by Lawrence et al., 2013). Phlorotannin, which is produced as a defensive secondary metabolite (e.g., Taniguchi et al., 1991; Steinberg and van Altena, 1992) in E. maxima (Kannan et al., 2013), might lead to low food intake, resulting in the low gonad production observed in the present study.
Phillips et al. (2009) reported that feeding artificial diets that contained high amounts of glutamic acid and glycine to E. chloroticus enhanced the sweetness of the gonads compared to those of sea urchins fed diets with high contents of valine and methionine. They suggested that the enhanced sweetness of the gonads was attributed to the sweet-tasting glycine in the diet because glycine is known to impart sweetness (Fuke and Konosu, 1991), although the FAA contents in the gonads were not analyzed in the past study. Feeding of S. japonica fronds improves the taste of M. nudus gonads by enhancing the umami taste and sweetness (a high alanine content) and decreasing the arginine content (Takagi et al., 2017, 2019). Takagi et al. (2020c) suggested that a high content of glutamic acid in the basal frond portion of S. japonica could be converted to alanine through digestion. No changes in the glycine and alanine contents in the gonads of Gly S and an increase in the alanine content in the gonads of Glu S indicate that a high glutamic acid content in feed increases the alanine content in gonads. The results of a significant increase in the alanine content in the gonads of Ala S supported the hypothesis that a high alanine content in the gonads of M. nudus fed the sporophyll of U. pinnatifida could be directly accumulated from that in the sporophyll (Takagi et al., 2020b). The present study first identified the amino acids in feed that are closely associated with improvement in the taste of sea urchin gonads. The high alanine content in the gonads of Ala S compared to that in the gonads of Glu S could be due to the direct accumulation of alanine from the feed, which is effective for increasing the alanine content in gonads compared to the process of alanine synthesis from glutamic acid. Past studies reported that feeds containing 19–36% protein notably enhance gonad production (e.g., de Jong-Westman et al., 1995; Pearce et al., 2002b; Inomata et al., 2016). However, these feeds decrease the desirability of taste (e.g., Pearce et al., 2002a; Inomata et al., 2016). Robinson et al. (2002) showed that gonads of sea urchins fed diets containing 250 mg/kg β-carotene (dry weight) are yellower than those fed 50 mg/kg β-carotene (dry weight). Clarification of the correlation between the glutamic acid and alanine contents in feeds, their digestibility and the accumulation rate of alanine in gonads is needed to determine the optimum composition of the amino acids, proteins and β-carotene in feed for efficient improvement in overall gonad quality. No significant difference in the umami and bitter-tasting FAA contents in the gonads among treatments was observed at the end of the experiment, indicating that glutamic acid, alanine, aspartic acid and glycine in the feed did not affect the umami or bitter tastes of the gonads. The cause of the increase in the glutamic acid and aspartic acid contents and the decrease in the bitter-tasting amino acid contents in gonads of all treatments remains unclear.
From the results of the TAA content in the control diet and the FAA content in each experimental diet, the experimental diets supplemented with glutamic acid and alanine contained ca. 195 mg/100 g total glutamic acid and ca. 179 mg/100 g total alanine, respectively. These contents were high when compared with those of the central frond portion of S. japonica (total glutamic acid, 180 mg/100 g; total alanine, 74 mg/100 g), which increased the alanine content in the gonads of M. nudus to > 400 mg/100 g (Takagi et al., 2020c). The leaching of amino acids supplemented with diets after 3 days of immersion would lower the alanine content in the gonads of the Glu S and Ala S (< 200 mg/100 g). Past studies reported the stability of artificial diets by calculating the percentage dry matter lost from diets in seawater or observation on eyes (Pearce et al., 2002a; Senaratna et al., 2005; Cyrus et al., 2015). Pearce et al. (2002a) reported there were no significant differences in feed stability among diets with various binders such as alginate, gelatin, guar gum and starch. It is required to investigate the stability of soluble compounds in diets among various binders or ingredients.
Feeding diets supplemented with glutamic acid or alanine increased the sweet-tasting alanine content in the gonads of M. nudus, which is attributed to their desirable taste. The higher alanine content in the gonads of sea urchins fed diets supplemented with alanine than in those of sea urchins fed diets supplemented with glutamic acid suggests that direct accumulation of alanine from the feed is effective compared to the process of alanine synthesis from glutamic acid. The present study first identified the amino acids that are closely associated with improvement in the taste of sea urchin gonads. Clarification of the correlation between the glutamic acid and alanine contents in feeds, their digestibility and the accumulation rate of alanine in gonads is needed to determine the optimum composition of amino acids in feed for efficient improvement in gonad taste.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
ST and YA conceived the experiments. ST designed, preformed and managed the experiments, analyzed the data, and wrote the original draft of the manuscript. YM conducted amino acid analyses. TK produced artificial diets. TK, YM, and YA reviewed and edited the manuscript. YA supervised and validated the manuscript. All authors contributed to the article and approved the submitted version.
This study was financially supported by the Grand-in-Aid for JSPS Fellow (Grant No. 17J02308) from the Japan Society for the Promotion of Science to ST.
TK was employed by the company Marubeni Nisshin Feed Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We sincerely thank M. Honjo and other staff members of the Miyagi Prefecture Fisheries Technology Institute and K. Maeda, M. Kuroda, S. Hosoda, Y. Chuma, R. Suzuki, A. Suzuki, and N. Sowanaka of Tohoku University for their cooperation in the feeding experiments. We are grateful to Asst. Prof. T. Nakano of Tohoku University for his advice in making the feeds, and Prof. S. A. Watts of the University of Alabama for his advice in developing the rearing system. We would also like to thank M. Oshima and S. Kodama of the Diving Stage Ariel, the Head of the Youth Division K. Sato and the other staff of Shizugawa Branch, Miyagi Fisheries Cooperative Association for their cooperation in sea urchin collection. We are grateful to Assoc. Prof. K. Takahashi and Asst. Prof. K. Nagasawa for their guidance with the histological techniques.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00593/full#supplementary-material
Agatsuma, Y. (1997). Ecological studies on the population dynamics of the sea urchin Strongylocentrotus nudus. Sci. Rep. Hokkaido Fish. Exp. Stn. 51, 1–66. doi: 10.11501/3122006
Agatsuma, Y. (1998). Aquaculture of the sea urchin (Strongylocentrotus nudus) transplanted from coralline flats in Hokkaido, Japan. J. Shellfish Res. 17, 1541–1547.
Agatsuma, Y. (2013). “Strongylocentrotus nudus,” in Sea Urchins: Biology and Ecology, 3rd Edn, ed. J. M. Lawrence (San Diego: Academic Press), 449–460.
Agatsuma, Y., Sato, M., and Taniguchi, K. (2005). Factors causing brown-colored gonads of the sea urchin Strongylocentrotus nudus in northern Honshu, Japan. Aquaculture 249, 449–458. doi: 10.1016/j.aquaculture.2005.04.054
Agatsuma, Y., Yamada, Y., and Taniguchi, K. (2002). Dietary effect of the boiled stipe of brown alga Undaria pinnatifida on the growth and gonadal enhancement of the sea urchin Strongylocentrotus nudus. Fish. Sci. 68, 1274–1281. doi: 10.1046/j.1444-2906.2002.00565.x
Andrew, N. L., Agatsuma, Y., Ballesteros, E., Bazhin, A. G., Creaser, E. P., Barnes, D. K. A., et al. (2002). Status and management of world sea urchin fisheries. Oceanogr. Mar. Biol. Annu. Rev. 40, 343–425. doi: 10.1201/9780203180594.ch7
AOAC (1990). Official Methods of Analysis of the Association of Official Analytical Chemists, 15th Edn, Vol. 1. Arlington: Association of Official Analytical Chemists Inc.
Baião, L. F., Rocha, F., Costa, M., Sá, T., Oliveira, A., Maia, M. R. G., et al. (2019). Effect of protein and lipid levels in diets for adult sea urchin Paracentrotus lividus (Lamarck, 1816). Aquaculture 506, 127–138. doi: 10.1016/j.aquaculture.2019.03.005
Byrne, M. (1990). Annual reproductive cycles of the commercial sea urchin Paracentrotus lividus from an exposed intertidal and a sheltered subtidal habitat on the west coast of Ireland. Mar. Biol. 104, 275–289. doi: 10.1007/BF01313269
Cook, E. J., Hughes, A. D., Orr, H., Kelly, M. S., and Black, K. D. (2007). Influence of dietary protein on essential fatty acids in the gonadal tissue of the sea urchins Psammechinus miliaris and Paracentrotus lividus (Echinodermata). Aquaculture 273, 586–594. doi: 10.1016/j.aquaculture.2007.10.032
Cook, E. J., and Kelly, M. S. (2007). Effect of variation in the protein value of the red macroalga Palmaria palmata on the feeding, growth and gonad composition of the sea urchins Psammechinus miliaris and Paracentrotus lividus (Echinodermata). Aquaculture 270, 207–217. doi: 10.1016/j.aquaculture.2007.01.026
Cyrus, M. D., Bolton, J. J., De Wet, L., and Macey, B. M. (2014). The development of a formulated feed containing Ulva (Chlorophyta) to promote rapid growth and enhanced production of high quality roe in the sea urchin Tripneustes gratilla (Linnaeus). Aquacult. Res. 45, 159–176. doi: 10.1111/j.1365-2109.2012.03219.x
Cyrus, M. D., Bolton, J. J., Scholtz, R., and Macey, B. M. (2015). The advantages of Ulva (Chlorophyta) as an additive in sea urchin formulated feeds: effects on palatability, consumption and digestibility. Aquacult. Nutr. 21, 578–591. doi: 10.1111/anu.12182
de Jong-Westman, M., March, B. E., and Carefoot, T. H. (1995). The effect of different nutrient formulations in artificial diets on gonad growth in the sea urchin Strongylocentrotus droebachiensis. Can. J. Zool. 73, 1495–1502. doi: 10.1139/z95-177
FAO (2018). The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals. Rome: FAO.
FAO (2019a). Fishery Commodities and Trade. Avaialble at: http://www.fao.org/fishery/statistics/global-commodities-production/en (accessed August 29, 2019).
FAO (2019b). Global Production. Avaialble at: http://www.fao.org/fishery/statistics/global-production/en (accessed April 17, 2019).
Fuke, S., and Konosu, S. (1991). Taste-active components in some foods: a review of Japanese research. Physiol. Behav. 49, 863–868. doi: 10.1016/0031-9384(91)90195-T
Hammer, H. S., Powell, M. L., Jones, W. T., Gibbs, V. K., Lawrence, A. L., Lawrence, J. M., et al. (2012). Effect of feed protein and carbohydrate levels on feed intake, growth, and gonad production of the sea urchin, Lytechinus variegatus. J. World Aquacult. Soc. 43, 145–158. doi: 10.1111/j.1749-7345.2012.00562.x
Heflin, L. E., Gibbs, V. K., Powell, M. L., Makowsky, R., Lawrence, J. M., Lawrence, A. L., et al. (2012). Effect of dietary protein and carbohydrate levels on weight gain and gonad production in the sea urchin Lytechinus variegatus. Aquaculture 358–359, 253–261. doi: 10.1016/j.aquaculture.2012.06.009
Heflin, L. E., Raubenheimer, D., Simpson, S. J., and Watts, S. A. (2016). Balancing macronutrient intake in cultured Lytechinus variegatus. Aquaculture 450, 295–300. doi: 10.1016/j.aquaculture.2015.08.001
Hoshikawa, H., Takahashi, K., Sugimoto, T., Tuji, K., and Nobuta, S. (1998). The effects of fish meal feeding on the gonad quality of cultivated sea urchins, Strongylocentrotus nudus (A. AGASSIZ). Sci. Rep. Hokkaido Fish. Exp. Stn. 52, 17–24.
Inomata, E., Murata, Y., Matsui, T., and Agatsuma, Y. (2016). Gonadal production and quality in the sea urchin Mesocentrotus nudus fed a high-protein concentrated red alga Pyropia yezoensis. Aquaculture 454, 184–191. doi: 10.1016/j.aquaculture.2015.12.003
James, P. J., Heath, P., and Unwin, M. J. (2007). The effects of season, temperature and initial gonad condition on roe enhancement of the sea urchin Evechinus chloroticus. Aquaculture 270, 115–131. doi: 10.1016/j.aquaculture.2007.03.011
Kaneko, K., Shirai, T., Tanaka, M., Kamei, M., Matsumoto, H., and Osako, K. (2009). Taste characteristics of the gonad of longspine black urchin Diadema setosum. Nippon Suisan Gakkaishi 75, 689–694. doi: 10.2331/suisan.75.689
Kannan, R. R. R., Aderogba, M. A., Ndhlala, A. R., Strik, W. A., and Van Staden, J. (2013). Acetylcholinesterase inhibitory activity of phlorotannins isolated from the brown alga, Ecklonia maxima (Osbeck) Papenfuss. Food Res. Int. 54, 1250–1254. doi: 10.1016/j.foodres.2012.11.017
King, C. K., Hoegh-Guldberg, O., and Byrne, M. (1994). Reproductive cycle of Centrostephanus rodgersii (Echinoidea), with recommendations for the establishment of a sea urchin fishery in New South Wales. Mar. Biol. 120, 95–106. doi: 10.1007/BF00381945
Komata, Y. (1964). Studies on the extractives of “Uni” —IV. Taste of each component in the extractives. Bull. Jpn. Soc. Sci. Fish. 30, 749–756. doi: 10.2331/suisan.30.749
Lawrence, J. M., Lawrence, A. L., and Watts, S. A. (2013). “Feeding, digestion and digestibility of sea urchins,” in Sea Urchins: Biology and ecology, 3rd Edn, ed. J. M. Lawrence (San Diego: Academic Press), 135–154. doi: 10.1016/b978-0-12-396491-5.00009-5
Lawrence, J. M., Olave, S., Otaiza, R., Lawrence, A. L., and Bustos, E. (1997). Enhancement of gonad production in the sea urchin Loxechinus albus in Chile fed extruded feeds. J. World Aquacult. Soc. 28, 91–96. doi: 10.1111/j.1749-7345.1997.tb00966.x
Liyana-Pathirana, C., Shahidi, F., Whittick, A., and Hooper, R. (2002). Effect of season and artificial diet on amino acids and nucleic acids in gonads of green sea urchin Strongylocentrotus droebachiensis. Comp. Biochem. Physiol. A 133, 389–398. doi: 10.1016/S1095-6433(02)00178-2
McBride, S. C., Lawrence, J. M., Lawrence, A. L., and Mulligan, T. J. (1999). Ingestion, absorption, and gonad production of adult Strongylocentrotus franciscanus fed different rations of a prepared diet. J. World Aquacult. Soc. 30, 364–370. doi: 10.1111/j.1749-7345.1999.tb00687.x
McBride, S. C., Pinnix, W. D., Lawrence, J. M., Lawrence, A. L., and Mulligan, T. M. (1997). The effect of temperature on production of gonads by the sea urchin Strongylocentrotus franciscanus fed natural and prepared diets. J. World Aquacult. Soc. 28, 357–365. doi: 10.1111/j.1749-7345.1997.tb00282.x
McBride, S. C., Price, R. J., Tom, P. D., Lawrence, J. M., and Lawrence, A. L. (2004). Comparison of gonad quality factors: color, hardness and resilience, of Strongylocentrotus franciscanus between sea urchins fed prepared feed or algal diets and sea urchins harvested from the Northern California fishery. Aquaculture 233, 405–422. doi: 10.1016/j.aquaculture.2003.10.014
Metropolitan Central Wholesale Market (2019). Market Statistical Information. Avaialble at: http://www.shijou-tokei.metro.tokyo.jp/ (accessed August 29, 2019).
Murata, Y., Henmi, H., and Nishioka, F. (1994). Extractive components in the skeletal muscle from ten different species of scombroid fishes. Fish. Sci. 60, 473–478. doi: 10.2331/fishsci.60.473
Nukui, N. (2018). Interview with a Middle Trader of the Tsukiji FishMarket in Tokyo. Tokyo: Asahi Shinbun News Paper. Available online at: https://digital.asahi.com/articles/ASL9W6GKLL9WUTIL03W.html (accessed October 24, 2019) (in Japanese).
Pearce, C. M., Daggett, T. L., and Robinson, S. M. C. (2002a). Effect of binder type and concentration on prepared feed stability and gonad yield and quality of the green sea urchin, Strongylocentrotus droebachiensis. Aquaculture 205, 301–323. doi: 10.1016/S0044-8486(01)00685-8
Pearce, C. M., Daggett, T. L., and Robinson, S. M. C. (2002b). Effect of protein source ratio and protein concentration in prepared diets on gonad yield and quality of the green sea urchin, Strongylocentrotus droebachiensis. Aquaculture 214, 307–332. doi: 10.1016/S0044-8486(02)00041-8
Phillips, K., Bremer, P., Silcock, P., Hamid, N., Delahunty, C., Barker, M., et al. (2009). Effect of gender, diet and storage time on the physical properties and sensory quality of sea urchin (Evechinus chloroticus) gonads. Aquaculture 288, 205–215. doi: 10.1016/j.aquaculture.2008.11.026
Phillips, K., Hamid, N., Silcock, P., Sewell, M. A., Barker, M., Weaver, A., et al. (2010). Effect of manufactured diets on the yield, biochemical composition and sensory quality of Evechinus chloroticus sea urchin gonads. Aquaculture 308, 49–59. doi: 10.1016/j.aquaculture.2010.07.030
Prato, E., Chiantore, M., Kelly, M. S., Hughes, A. D., James, P., Ferranti, M. P., et al. (2018). Effect of formulated diets on the proximate composition and fatty acid profiles of sea urchin Paracentrotus lividus gonad. Aquacult. Int. 26, 185–202. doi: 10.1007/s10499-017-0203-5
Robinson, S. M. C., Castell, J. D., and Kennedy, E. J. (2002). Developing suitable colour in the gonads of cultured green sea urchins (Strongylocentrotus droebachiensis). Aquaculture 206, 289–303. doi: 10.1016/S0044-8486(01)00723-2
Senaratna, M., Evans, L. H., Southam, L., and Tsvetnenko, E. (2005). Effect of different feed formulations on feed efficiency, gonad yield and gonad quality in the purple sea urchin Heliocidaris erythrogramma. Aquacult. Nutr. 11, 199–207. doi: 10.1111/j.1365-2095.2005.00340.x
Shpigel, M., McBride, S. C., Marciano, S., Ron, S., and Ben-Amotz, A. (2005). Improving gonad colour and somatic index in the European sea urchin Paracentrotus lividus. Aquaculture 245, 101–109. doi: 10.1016/j.aquaculture.2004.11.043
Siikavuopio, S. I., Dale, T., and Carlehög, M. (2007). Sensory quality of gonads from the green sea urchin, Strongylocentrotus droebachiensis, fed different diets. J. Shellfish Res. 26, 637–643. doi: 10.2983/0730-8000(2007)26[637:sqogft]2.0.co;2
Steinberg, P. D., and van Altena, I. (1992). Tolerance of marine invertebrate herbivores to brown algal phlorotannins in temperature Australasia. Ecol. Monogr. 62, 189–222. doi: 10.2307/2937093
Suckling, C. C., Symonds, R. C., Kelly, M. S., and Young, A. J. (2011). The effect of artificial diets on gonad colour and biomass in the edible sea urchin Psammechinus miliaris. Aquaculture 318, 335–342. doi: 10.1016/j.aquaculture.2011.05.042
Sun, J., and Chiang, F. S. (2015). “Use and exploitation of sea urchins,” in Echinoderm Aquaculture, eds N. P. Brown and S. D. Eddy (Hoboken: Wiley Blackwell), 25–45. doi: 10.1002/9781119005810.ch2
Takagi, S. (2020). Study for Production of High-Quality Gonads in the Sea Urchin Mesocentrotus nudus from Barrens. Ph.D. thesis, Tohoku University, Sendai.
Takagi, S., Murata, Y., and Agatsuma, Y. (2020a). Feeding the sporophyll of Undaria pinnatifida kelp shortens the culture duration for the production of high-quality gonads of Mesocentrotus nudus sea urchins from a barren. Aquaculture 528:735503. doi: 10.1016/j.aquaculture.2020.735503
Takagi, S., Murata, Y., Inomata, E., and Agatsuma, Y. (2020b). Sporophyll of Undaria pinnatifida: a potential feed for the production of high-quality gonads in the sea urchin Mesocentrotus nudus (A. Agassiz, 1864). J. Appl. Phycol. 32, 1467–1475. doi: 10.1007/s10811-020-02041-3
Takagi, S., Murata, Y., Inomata, E., Aoki, M. N., and Agatsuma, Y. (2020c). Pronounced effects of the basal frond portion of the kelp Saccharina japonica on gonad qualities of the sea urchin Mesocentrotus nudus from a barren. Aquaculture 516:734623. doi: 10.1016/j.aquaculture.2019.734623
Takagi, S., Murata, Y., Inomata, E., Aoki, M. N., and Agatsuma, Y. (2019). Production of high quality gonads in the sea urchin Mesocentrotus nudus (A. Agassiz, 1864) from a barren by feeding on the kelp Saccharina japonica at the late sporophyte stage. J. Appl. Phycol. 31, 4037–4048. doi: 10.1007/s10811-019-01895-6
Takagi, S., Murata, Y., Inomata, E., Endo, H., Aoki, M. N., and Agatsuma, Y. (2017). Improvement of gonad quality of the sea urchin Mesocentrotus nudus fed the kelp Saccharina japonica during offshore cage culture. Aquaculture 477, 50–61. doi: 10.1016/j.aquaculture.2017.04.033
Taniguchi, K., Kurata, K., and Suzuki, M. (1991). Feeding-deterrent effect of phlorotannins from the brown alga Ecklonia stolonifera against the abalone Haliotis discus hannai. Nippon Suisan Gakkaishi 57, 2065–2071. doi: 10.2331/suisan.57.2065
Taylor, A. M., Heflin, L. E., Powell, M. L., Lawrence, A. L., and Watts, S. A. (2017). Effects of dietary carbohydrate on weight gain and gonad production in small sea urchins, Lytechinus variegatus. Aquacult. Nutr. 23, 375–386. doi: 10.1111/anu.12403
Unuma, T. (2015). “Introduction: sea urchin fisheries in Japan,” in Echinoderm Aquaculture, eds N. P. Brown and S. D. Eddy (Hoboken: Wiley Blackwell), 77–85.
Unuma, T., Murata, Y., Hasegawa, N., Sawaguchi, S., and Takahashi, K. (2015). Improving the food quality of sea urchins collected from barren grounds by short-term aquaculture under controlled temperature. Bull. Fish. Res. Agen. 40, 145–153.
Walker, C. W., Böttger, S. A., Unuma, T., Watts, S. A., Harris, L. G., Lawrence, A. L., et al. (2015). “Enhancing the commercial quality of edible sea urchin gonads—Technologies emphasizing nutritive phagocytes,” in Echinoderm Aquaculture, eds N. P. Brown and S. D. Eddy (Hoboken: Wiley Blackwell), 263–286. doi: 10.1002/9781119005810.ch12
Watts, S. A., Boettger, S. A., McClintock, J. B., and Lawrence, J. M. (1998). Gonad production in the sea urchin Lytechinus variegatus (Lamarck) fed prepared diets. J. Shellfish Res. 17, 1591–1595.
Keywords: sea urchin, gonad taste, alanine, glutamic acid, Mesocentrotus nudus
Citation: Takagi S, Murata Y, Koshiishi T and Agatsuma Y (2020) The Amino Acids Glutamic Acid and Alanine in Feed Increase the Alanine Content in Gonads of the Sea Urchin Mesocentrotus nudus. Front. Mar. Sci. 7:593. doi: 10.3389/fmars.2020.00593
Received: 22 March 2020; Accepted: 29 June 2020;
Published: 28 July 2020.
Edited by:
Samad Rahimnejad, University of South Bohemia in České Budějovice, CzechiaReviewed by:
Adele Fabbrocini, Institute of Marine Science, National Research Council (CNR), ItalyCopyright © 2020 Takagi, Murata, Koshiishi and Agatsuma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yukio Agatsuma, eXVraW8uYWdhdHN1bWEuYzdAdG9ob2t1LmFjLmpw; eWFnYXRzdW1hMEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.