- 1Department of Animal Science, University of California, Davis, Davis, CA, United States
- 2Dornsife College of Letters, Arts and Sciences, University of Southern California, Los Angeles, CA, United States
- 3Blue Ocean Barns Inc., Redwood City, CA, United States
With increasing interest in feed-based methane mitigation strategies and regional legal directives aimed at methane production from the agricultural sector, identifying local sources of biological feed additives will be critical for rendering these strategies affordable. In a recent study, the red alga Asparagopsis taxiformis harvested offshore Australia was identified as highly effective for reducing methane production from enteric fermentation. Due to potential difference in methane-reduction potential and the financial burden associated with transporting the harvested seaweed over long distances, we examined locally sourced red seaweed A. taxiformis and brown seaweed Zonaria farlowii for their ability to mitigate methane production when added to feed widely used in the Californian dairy industry. At a dose rate of 5% dry matter (DM), California-sourced A. taxiformis and Z. farlowii reduced methane production by up to 74% (p < 0.05) and 11% (p < 0.05) during in vitro rumen fermentation, respectively. No effect on CO2 production was observed for either seaweed. The measured decrease in methane production induced by A. taxiformis and Z. farlowii amendment, suggest that these local macroalgae are indeed promising candidates for biotic methane mitigation strategies in California, the largest milk producing state in the United States. To determine their real potential as methane mitigating feed supplements in the dairy industry, their effect in vivo will need to be investigated.
Introduction
Methane (CH4), produced by human activity, accounts for more than 10% of the total greenhouse gases emitted in the United States (EPA, 2020) and enteric fermentation from ruminant animals accounts for approximately 25% of the total anthropogenic methane produced (NASEM, 2018). Thus, efficient strategies to lower enteric CH4 production would enable a significantly reduced carbon footprint of agriculture and more specifically of animal production.
In vitro studies have demonstrated that some brown and red macroalgae can inhibit microbial methanogenesis (Machado et al., 2014; Roque et al., 2019) and they have been suggested as feed supplements to reduce methanogenesis during enteric fermentation (Wang et al., 2008; Dubois et al., 2013; Machado et al., 2016). Utilization of these macroalgae could also promote higher growth rates and feed conversion efficiencies in ruminants via the potential net energy yield from the redistribution of energy from the microbial methanogenesis pathway into more favorable pathways for the animal (i.e., volatile fatty acids) (Hansen et al., 2003; Marin et al., 2009). Therefore, macroalgae feed supplementation may be an effective strategy to simultaneously improve profitability and sustainability of beef and dairy operations.
In a recent study (Machado et al., 2014), the red macroalgae Asparagopsis taxiformis stood out as the most effective species from a panel of tested seaweed to reduce methane production. In this screening effort, the effect of a variety of macroalgal species including freshwater, green, red, and brown algae on CH4 production during in vitro incubation were compared, and results showed that A. taxiformis amendment yielded the most significant reduction (∼98.9%) of CH4 production.
A major challenge in the implementation of an A. taxiformis-based methane mitigation strategy is the availability of this bioactive seaweed, which has led to the exploration of alternative seaweed species. Given that California, which is the largest milk producing state in the United States, has a shoreline of 1,350 km, it is imperative that local seaweeds are tested for methane mitigation properties in order to reduce the financial burden associated with harvesting the seaweed at locations that require long storage and transportation. Besides the economical aspect, the identification of local seaweed is of particular importance since the key halogenated compound responsible for macroalgae’s methane mitigation abilities, bromoform, differs in concentration across both location and season (Carpenter and Liss, 2000). Here, we tested the potential of two different species of subtidal macroalgae (A. taxiformis and the brown alga, Zonaria farlowii) collected from offshore Southern California for their ability to mitigate methane production during in vitro rumen fermentation.
Materials and Methods
Experimental Design
To determine the effect of two locally sourced macroalgae species on methane production during in vitro rumen fermentation, A. taxiformis and Z. farlowii were supplemented to an in vitro gas production system at a dose rate of 5% DM. This dose rate showed significant methane reduction during in vitro rumen fermentation for Australia-sourced A. taxiformis (Kinley et al., 2016; Roque et al., 2019). Rumen fluid was diluted threefold with artificial saliva buffer (Oeztuerk et al., 2005). After homogenization, 200 mL of the mixture was allocated to 300 mL vessels fitted with Ankom head units (Ankom Technology RF Gas Production System, Macedon, NY, United States). Each vessel received 2 g of rumen solids, and 2 g of a basic ration (Super Basic Ration; SBR; 70% alfalfa, 15% dried distillers’ grain, and 15% rolled corn) commonly used in the dairy industry in California. Rumen solids and SBR were sealed in separate Ankom 5 cm × 5 cm concentrate feed bags and seaweed was included in the respective SBR feed bags (Ankom, Macedon, NY, United States). Control vessels were run in parallel with each seaweed treatment. Vessels were placed and incubated in a temperature controlled shaking water bath (39°C; 40 rpm). Foil gas bags (Restek, United States) were connected to the Ankom head units to collect gas at 24 and 48 h, respectively. The experimental design of this study is summarized in Figure 1.
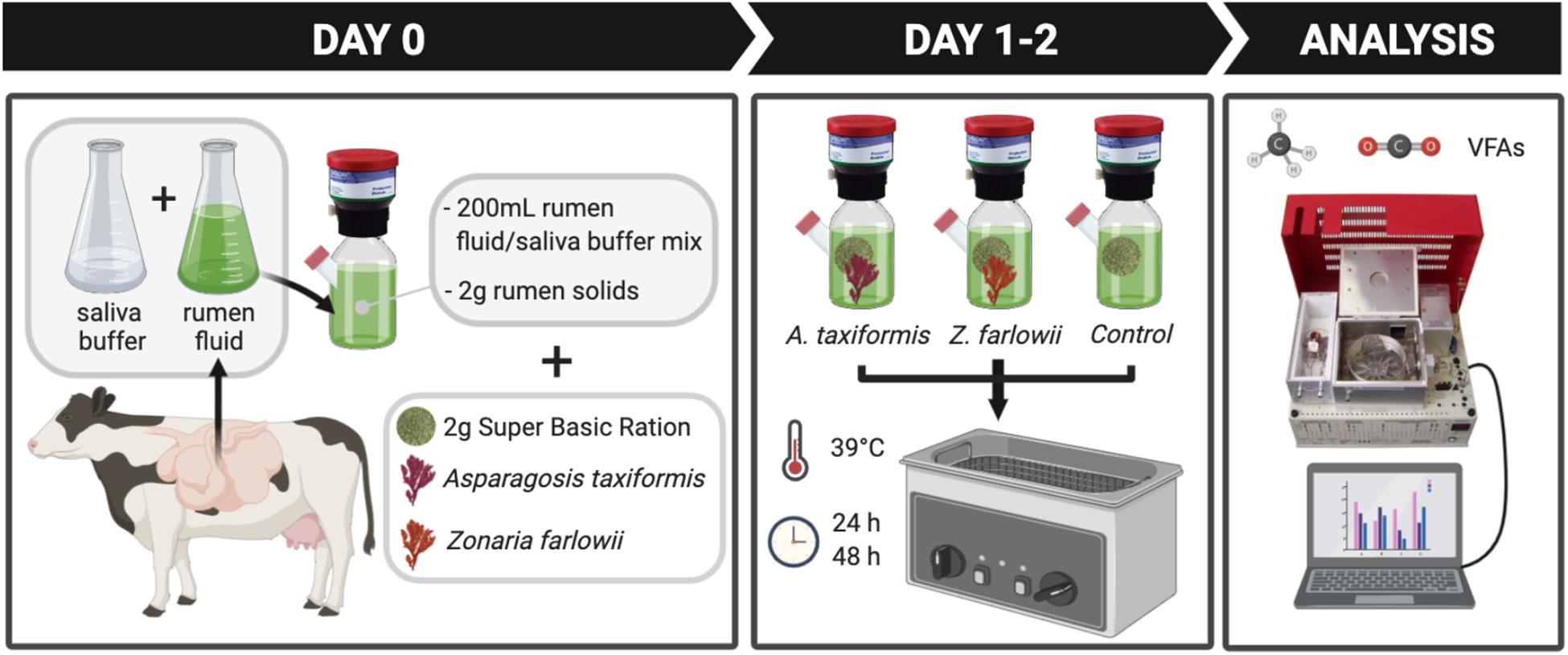
Figure 1. Experimental design. Rumen content was collected from a rumen fistulated Holstein cow and rumen fluid was diluted threefold with saliva buffer after rumen solids and fluid were separated. Fluid/buffer mixture was amended with rumen solids and Super Basic Ration. To test effect of Asparagopsis taxiformis and Zonaria farlowii, fermentation vessels were supplemented with 5% dry matter (DM) of the corresponding seaweed. Carbon dioxide (CO2), methane (CH4) production and volatile acid profiles were determined after 24 and 48 h.
Pacific Coast Seaweed Collection and Preparation, and Biochemical Analysis
Adult (gametophyte) stages of A. taxiformis and Z. farlowii were collected on May 14, 2018 from Little Fisherman’s Cove (33°26′37″ N, 118°29′26″ W) on the leeward side of Santa Catalina Island, ∼35 km off the coast of Southern California, United States (Figure 2). The seaweed was shipped on ice to the University of California, Davis, where it was dried at 55°C for 72 h and ground through a 2 mm Wiley Mill (Thomas Fisher Scientific, Swedesboro, NJ, United States) before being placed into Ankom concentrate feed bags. Biochemical analyses of the seaweeds were performed by Cumberland Valley Analytical Services to determine the biochemical composition of the seaweeds used in this study.
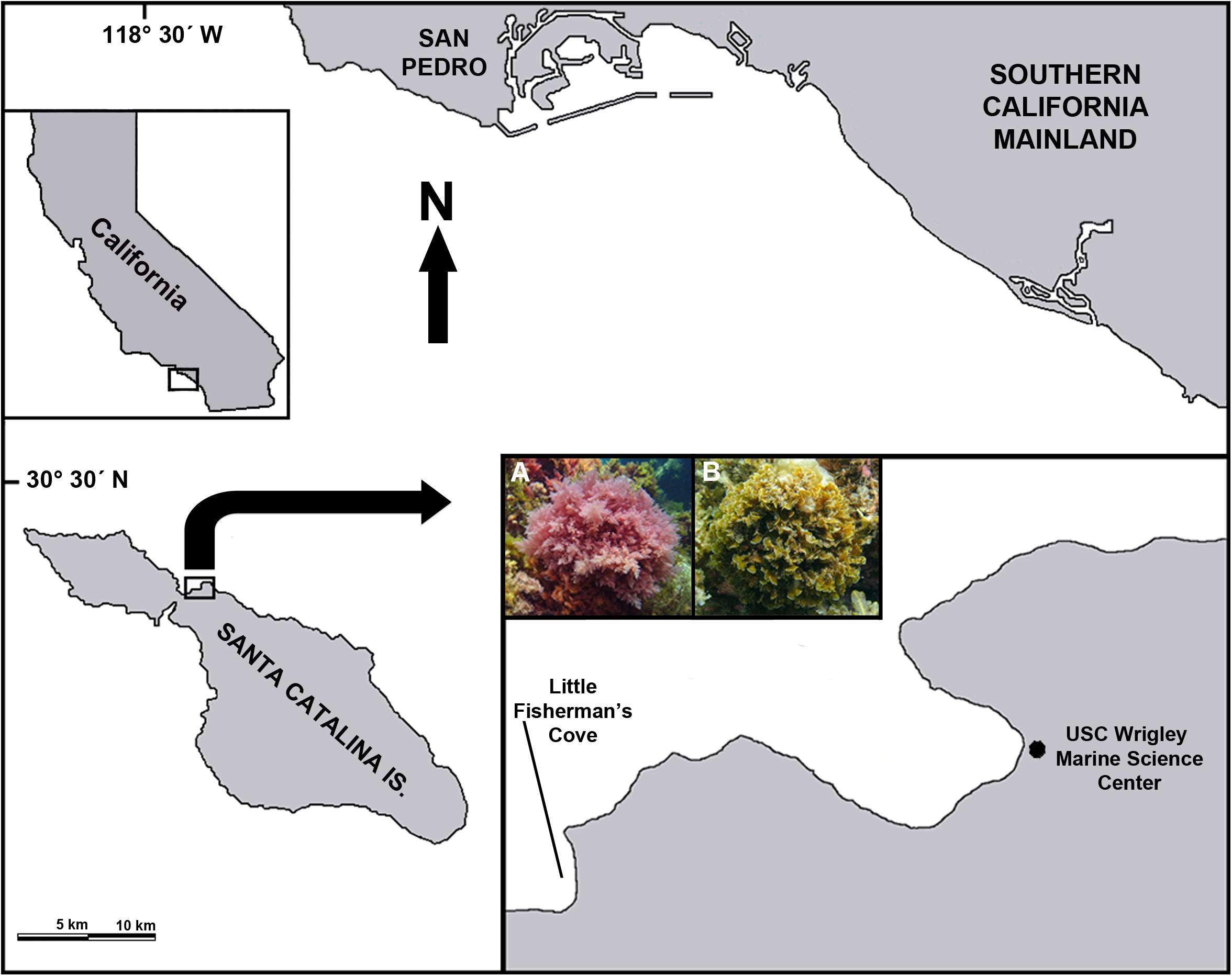
Figure 2. Sampling sites. Map showing the location of Santa Catalina Island relative to the Southern California mainland. Inset on right: the red alga Asparagopsis taxiformis (A) and the brown alga Zonaria farlowii (B). Algae were collected (2–5 m depth) in Little Fisherman’s Cove, located ∼0.6 km from the USC Wrigley Marine Science Center.
Rumen Fluid Collection
All animal procedures were performed in accordance with the Institution of Animal Care and Use Committee (IACUC) at the University of California, Davis, under protocol number 19263. Rumen content was collected from a rumen fistulated Holstein cow, housed at the UC Davis Dairy Research and Teaching Facility Unit. The rumen content donor was fed a dry cow total mixed ration (50% wheat hay, 25% alfalfa hay/manger cleanings, 21.4% almond hulls, and 3.6% mineral pellet). Two liters of rumen fluid and 30 g of rumen solids were collected 90 min after morning feeding. Rumen content was collected via transphonation using a perforated PVC pipe, 500 mL syringe, and Tygon tubing (Saint-Gobain North America, PA, United States). Fluid was strained through a colander and four layers of cheesecloth into a 4 L pre-warmed, vacuum insulated container and transported to the laboratory. Rumen fluid and solids were collected on May 29, 2018 and June 19, 2018 for the in vitro trial to determine methane-mitigation potential of A. taxiformis and Z. Farlowii, respectively. Trials were started on the day of rumen fluid collection.
Greenhouse Gas Analysis
Methane and CO2 were measured from gas bags using an SRI Gas Chromatograph (8610C, SRI, Torrance, CA, United States) fitted with a 3′ × 1/8″ stainless steel Haysep D column and a flame ionization detector (FID) with a methanizer. The oven temperature was held at 90°C for 5 min. Carrier gas was high purity hydrogen at a flow rate of 30 mL/min. The FID was held at 300°C. A 1 mL sample was injected directly onto the column. Calibration curves were developed with Airgas certified CH4 and CO2 standard (Airgas, United States).
Statistical Analysis
Differences in CH4 and CO2 production between sampling time points for each seaweed were determined using unpaired parametric t-tests with Welch’s correction conducted in Graphpad Prism 7 (Graphpad Software Inc., La Jolla, CA, United States). Significant differences among treatments were declared at p < 0.05.
Results
Biochemical Analysis of Asparagopsis taxiformis and Zonaria farlowii
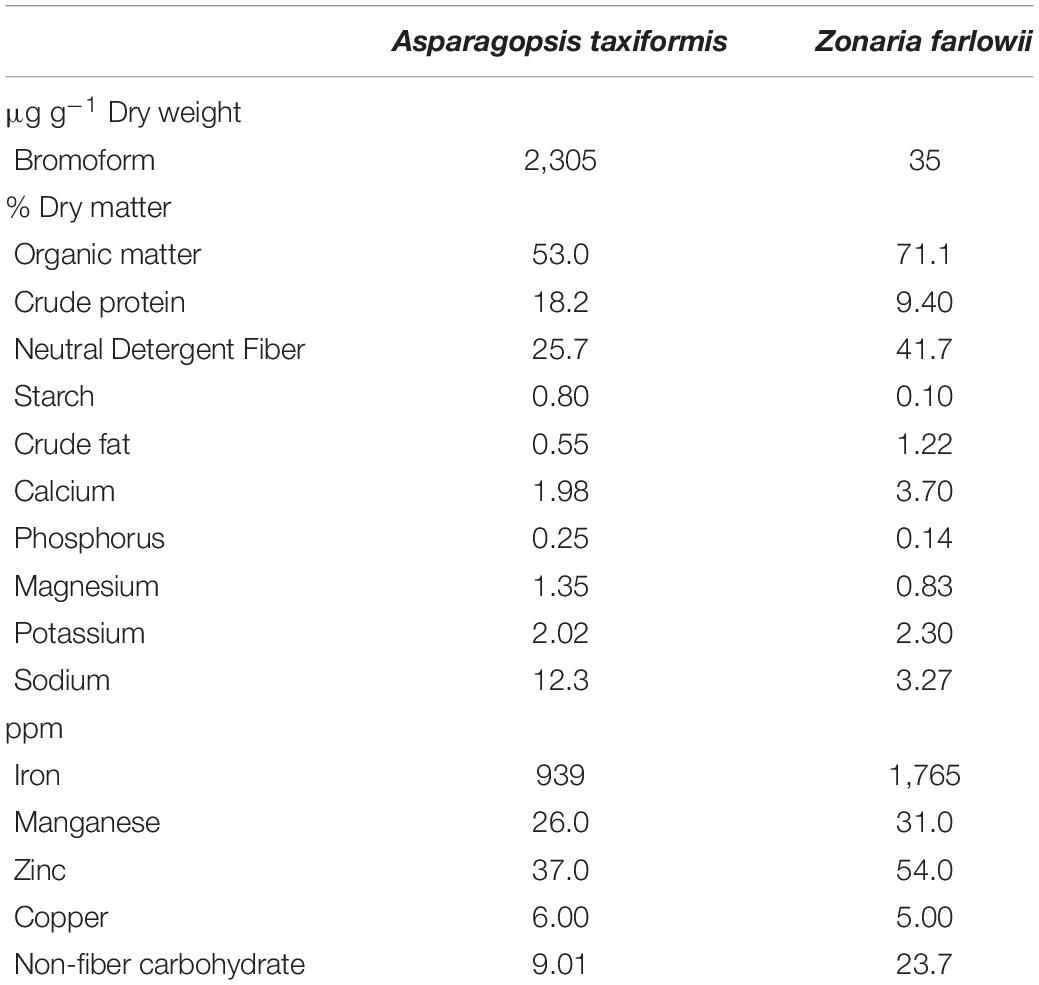
Biochemical analysis of the two Pacific Coast seaweeds revealed a stark difference in their bromoform composition (2,305 μg g–1 dry weight for A. taxiformis and 35 μg g–1 dry weight for Z. farlowii) as well as in their nutritional composition (Table 1). Additionally, crude protein and starch content was significantly (1.9- and 8-fold, respectively) higher, whereas crude fat and non-fiber carbohydrates were (2.2- and 2.6-fold, respectively) lower in A. taxiformis compared to Z. farlowii. Concentrations of iron were almost twice as high in Z. farlowii (1,765 ppm) compared to A. taxiformis (939 ppm).
Gas Production Profile of in vitro Fermentation of Rumen Fluid Amended With 5% A. taxiformis
Amendment of feed with 5% DM of A. taxiformis reduced total gas production by ∼28% (from 142.8 ± 42 mL to 103.4 ± 13.9 mL) and ∼30% (from 214.3 ± 40.3 mL to 151.4 ± 16.4 mL) during 24 and 48 h of rumen incubation. Additionally, the total amount of methane produced remained somewhat similar in A. taxiformis vessels, during both 24 and 48 h of in vitro rumen fermentation [7.1 ± 1.9 mL (g DM)–1 and 6.6 ± 2.5 mL (g DM)–1, respectively), the amount of methane increased over the same time period in the absence of A. taxiformis (control vessels). Methane in the control vessels increased from 20.3 ± 11 mL (g DM)–1 to 35.5 ± 8.5 mL (g DM)–1 during 24 and 48 h of in vitro rumen fermentation, respectively (Figures 3A,C). At a dose rate of 5% DM, A. taxiformis reduced methane production by 65% over 24 h and 74% over 48 h during in vitro rumen fermentation (p < 0.05, Figure 3C). While methane production varied with 5% DM inclusion of A. taxiformis, CO2 production remained similar between treatment [41.9 ± 6.2 mL (g DM)–1 and 65.23 ± 9.1 mL (g DM)–1 at 24 and 48 h, respectively] and control vessels [47.4 ± 13.4 mL (g DM)–1 and 69.0 ± 15.9 mL (g DM) –1 at 24 and 48 h, respectively] (Figures 3B,D).
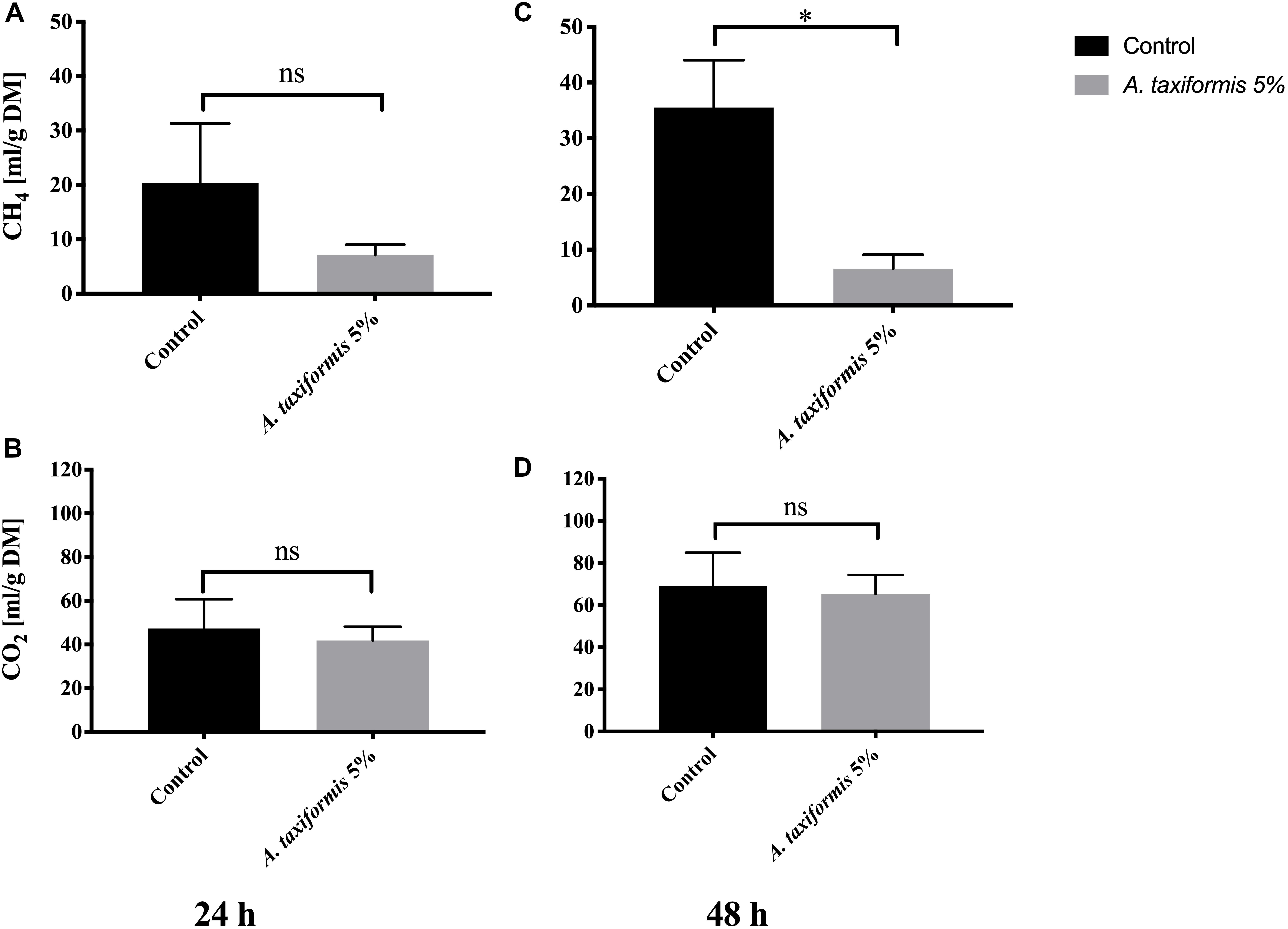
Figure 3. Methane and CO2 production during in vitro fermentation of rumen fluid amended with A. taxiformis. Production of CH4 mL (g DM)–1 and CO2 mL (g DM)–1 from vessels without (n = 4) and with 5% (n = 4) A. taxiformis as additive. Methane and CO2 were measured at 24 h (A,B, respectively) and 48 h (C,D, respectively). “∗” indicates significant difference (p-value < 0.05), “ns” indicates not significant. Error bars represent the standard error from the mean.
Gas Production Profile of in vitro Fermentation of Rumen Fluid Amended With 5% Z. farlowii
Amendment of feed with 5% DM of Z. farlowii did not reduce total gas production beyond a slight reduction of 5% (from 274.2 ± 16.4 mL to 261.8 ± 25.2 mL) during 24 h of rumen incubation. Over 48 h total gas production increased by 2% (from 336.9 ± 23.3 mL to 343.8 ± 23.9 mL). At a dose rate of 5% DM, Z. farlowii reduced methane production by 11.5% after 24 h and 10.7% after 48 h of in vitro rumen fermentation (p < 0.05, Figure 4A). Daily methane production decreased slightly at 48 h compared to 24 h of incubation for both the control and treatment vessels [Control = 62.5 ± 3.3 mL (g DM)–1 and 51.4 ± 2.9 mL (g DM)–1 CH4, at 24 and 48 h, respectively; treatment = 55.3 ± 2.7 and 45.9 ± 3.7 mL (g DM)–1 CH4, at 24 and 48 h, respectively (Figures 4A,C)].
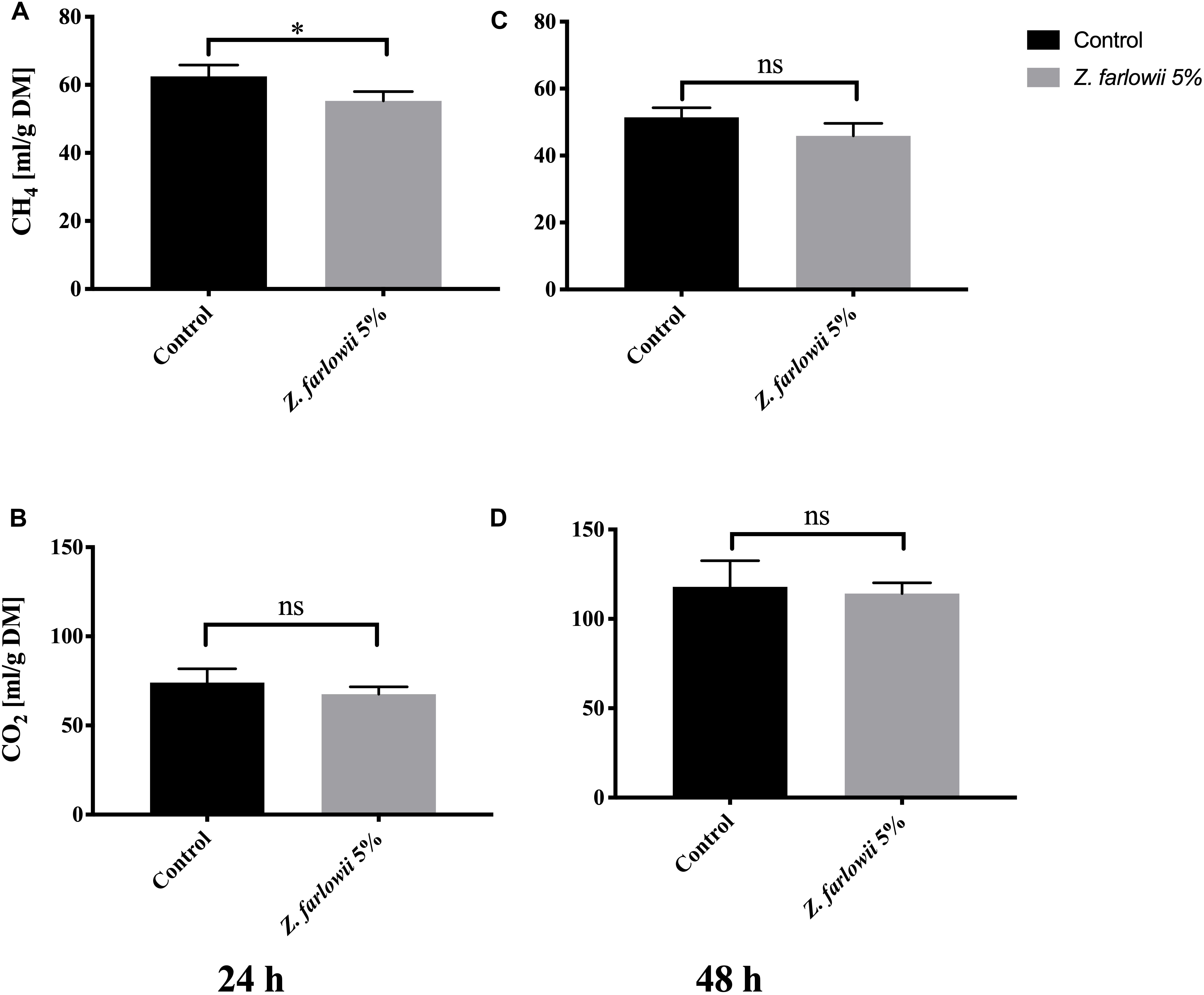
Figure 4. Methane and CO2 production during in vitro fermentation of rumen fluid amended with Z. farlowii. Production of CH4 mL (g DM)–1 and CO2 mL (g DM)–1 from vessels without (n = 3) and with 5% (n = 3) Z. farlowii as additive. Methane and CO2 were measured at 24 h (A,B, respectively) and 48 h (C,D, respectively). “∗” indicates significant difference (p-value < 0.05), “ns” indicates not significant. Error bars represent the standard error from the mean.
While methane production decreased slightly for all vessels at 48 h, CO2 production nearly doubled [Control = 74.1 ± 7.7 mL (g DM)–1 and 117.9 ± 14.6 mL (g DM)–1 CO2, at 24 and 48 h, respectively; treatment = 67.6 ± 4.1 mL (g DM)–1 and 114.2 ± 6.0 mL (g DM)–1 CO2, at 24 and 48 h, respectively]. Carbon dioxide production from vessels amended with 5% DM of Z. farlowii did not differ from the control vessels at 24 or 48 h (p = 0.27 and p = 0.70, respectively; Figures 4B,D).
Discussion
With increasing interest in feed-based methane mitigation strategies, fueled by legal directives aimed at reducing methane production from the agricultural sector, identification of local biotic feed-supplements will be critical to render large-scale methane mitigation strategies economical.
Our data indicate that subtidal macroalgae from Santa Catalina Island, Southern California reduced the in vitro production of CH4 when added to rumen content from California dairy cattle. The addition of A. taxiformis reduced microbial methane production by up to ∼78%, which is slightly lower but similar to the reduction (∼95%) of enteric methane we reported previously when the effect of Australian sourced A. taxiformis on 96 h of in vitro rumen fermentation was determined (Roque et al., 2019). Taking the variations in microbiome composition and function as well as potential experimental variations into consideration a direct comparison of these two different seaweeds would be certainly be of great value to evaluate the real and explicit potential of these promising feed amendments. However, since in vitro assays are only a rough proxy of the in vivo response, such comparison might be better suited for an in vivo follow-up experiment. The effects of adding Pacific Z. farlowii on methane production was significantly less prominent: a reduction of only 11% was measured when Z. farlowii was added at 5% DM. This difference in methane mitigation is not surprising when considering the amount of bromoform concentration associated with the different seaweeds we tested (2,305 μg g–1 dry weight) for A. taxiformis and 35 μg g–1 dry weight for Z. farlowii). In fact, bromoform concentrations for A. taxiformis were maintained throughout our harvesting and sample preparation process at levels similar to the highest values that had been reported for the Australian A. taxiformis (Vucko et al., 2017). Organobromine compounds such as bromomethane (methyl bromide; CH3Br) and bromoform (CHBr3) are abundantly found in marine algae (Gribble, 2000) and have been identified as inhibiting microbial methanogenesis (Tomkins et al., 2009). It is not surprising that California-sourced seaweed, specifically A. taxiformis with its elevated level of bromoform, reduced methanogenesis during in vitro rumen fermentation. Based on these findings, we therefore hypothesize that California-sourced seaweeds might represent a viable option for use in feed-based methane mitigation strategies, especially when considering the significantly reduced carbon footprint associated with utilizing local natural resources.
In addition to demonstrating the potential of the local A. taxiformis for methane mitigation during enteric fermentation, we were also able to demonstrate methane reduction with no obvious impact on total gas and CO2 production (Figures 3A,B, 4A,B) by the brown alga Z. farlowii, a species of seaweed commonly found along the Southern California Bight.
Released CO2 can be used as a highly sensitive proxy capable of detecting changes in the overall metabolic carbon respiration and growth of a microbial community within an ecosystem (Kaschuk et al., 2010; Zheng et al., 2019). Changes in CO2 profiles would therefore indicate significant perturbation and dysbiosis of the healthy rumen microbiome with potentially significant changes in the overall function of the rumen ecosystem as observed for example during acidosis when animals are transition to quickly to feed that contains high level of rapidly digestible carbohydrate such as barley and other cereals and which poses a life-threatening condition to the ruminant animal (Navarre et al., 2012; Eger et al., 2018). The lack of changes of observed CO2 profiles suggest that addition of neither A. taxiformis nor Z. farlowii had an effect on overall microbiome metabolism and healthy function was maintained. To get a more detailed understanding of potential metabolic changes at the sub-microbiome level, which might not be detected by measuring CO2 production and release, more detailed microbiome analyses should be performed before these seaweeds are applied at large scale to ruminant animals.
The effectiveness of macroalgae in reducing methane production during rumen incubation has been linked to the concentration of halogenated bioactives including bromoform and di-bromochloromethane (Machado et al., 2016). In contrast to A. taxiformis, which has been shown to produce several halomethane compounds, Z. farlowii only caused a modest reduction of methanogenesis and consequently higher amounts of emitted enteric methane during in vitro rumen fermentation. These findings suggest that either the bioactives in Z. farlowii are more bioavailable but less effective or concentrated, or methane reduction mediated by Z. farlowii amendment occurs via a different compound or mode of action. Z. farlowii is commonly found along the Southern California Bight, which makes it a potential candidate for non-terrestrial farming operations along the Southern California Coast. In order to understand the molecular mechanism by which Z. farlowii reduces methane production from the rumen microbiota, as well as the nutritional value of Z. farlowii more detailed, follow-up in vitro and in vivo studies involving Z. farlowii as feed additive are required. Only with these data in hand will it be possible to determine Z. farlowii’s value for future methane mitigation strategies and to determine its potential for use in dairy operations.
Conclusion
Asparagopsis taxiformis and Z. farlowii collected off Santa Catalina Island were evaluated for their ability to reduce methane production from dairy cattle fed a mixed ration widely utilized in California operations. The methane reducing effect of A. taxiformis and Z. farlowii described in this study make these macroalgae promising candidates for biotic methane mitigation strategies in the largest milk producing state in the United States, though further follow-up studies will be required to both substantiate methane mitigation ability and elucidate the molecular mechanism by which these macroalgae reduce methane production in livestock. With expected growth in livestock production, it is necessary to investigate and confirm the effect of these macroalgae in vivo in order to ensure that farmers have sufficient incentive to implement such strategies.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by all animal procedures were performed in accordance with the Institution of Animal Care and Use Committee (IACUC) at the University of California, Davis, under protocol number 19263.
Author Contributions
CB, BR, and MH conceived and designed the experiment. DG, MCH, and SN collected seaweed. CB, BR, CS, NN, MG, AP, VD, and MH performed experiments and analyzed data. CB, BR, and MH wrote major part of the manuscript. All authors were involved in writing the final version of the manuscript.
Funding
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s). This work was supported through funds from the College of Agricultural and Environmental Sciences at the University of California, Davis, by Elm Innovations and by the Hellman Foundation. MCH was supported by ARPA-E (Contract# 1726-1513) and SN was supported by a Wyatt Foundation gift.
Conflict of Interest
JS was employed by Blue Ocean Barns Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript has been released as a Pre-Print on BioRxiv at https://doi.org/10.1101/434480.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00561/full#supplementary-material
References
Carpenter, L. J., and Liss, P. S. (2000). On temperate sources of bromoform and other reactive organic bromine gases. J. Geophys. Res. 105, 20539–20547. doi: 10.1029/2000JD900242
Dubois, B., Tomkins, N., Kinley, R. D., Bai, M., Seymour, S., Paul, N. A., et al. (2013). Effect of tropical algae as additives on rumen in vitro gas production and fermentation characteristics. Am. J. Plant Sci. 4, 34–44. doi: 10.4236/ajps.2013.412A2005
Eger, M., Riede, S., and Breves, G. (2018). Induction of a transient acidosis in the rumen simulation technique. J. Anim. Physiol. Anim. Nutr. (Berl) 102, 94–102. doi: 10.1111/jpn.12662
Gribble, G. W. (2000). The natural production of organobromine compounds. Environ. Sci. Pollut. Res. Int. 7, 37–47. doi: 10.1065/espr199910.002
Hansen, H. R., Hector, B. L., and Feldmann, J. (2003). A qualitative and quantitative evaluation of the seaweed diet of North Ronaldsay sheep. Anim. Feed Sci. Technol. 105, 21–28. doi: 10.1016/S0377-8401(03)00053-1
Kaschuk, G., Alberton, O., and Hungria, M. (2010). Three decades of soil microbial biomass studies in Brazilian ecosystems: Lessons learned about soil quality and indications for improving sustainability. Soil Biol. Biochem. 42, 1–13. doi: 10.1016/j.soilbio.2009.08.020
Kinley, R. D., De Nys, R., Vucko, M. J., Machado, L., and Tomkins, N. W. (2016). The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in-vitro fermentation with rumen fluid. Anim. Product. Sci. 56, 282–289.
Machado, L., Magnusson, M., Paul, N. A., de Nys, R., and Tomkins, N. (2014). Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLoS One 9:e85289. doi: 10.1371/journal.pone.0085289
Machado, L., Magnusson, M., Paul, N. A., Kinley, R., de Nys, R., and Tomkins, N. (2016). Dose-response effects of Asparagopsis taxiformis and Oedogonium sp. on in vitro fermentation and methane production. J. Appl. Phycol. 28, 1443–1452. doi: 10.1007/s10811-015-0639-9
Marin, A., Casas-Valdez, M., Carrillo, S., Hernandez, H., Monroy, A., Sangines, L., et al. (2009). The marine algae Sargassum spp. (Sargassaceae) as feed for sheep in tropical and subtropical regions. Rev. Biol. Trop. 57, 1271–1281. doi: 10.15517/rbt.v57i4.5464
NASEM (2018). Improving Characterization of Anthropogenic Methane Emissions in the United States. Washington, DC: The National Academies Press.
Navarre, C. B., Baird, A. N., and Pugh, D. G. (2012). “Chapter 5 - diseases of the gastrointestinal system,” in Sheep and Goat Medicine (Second Edition), eds D. G. Pugh and A. N. Baird (Saint Louis, MO: W.B. Saunders), 71–105.
Oeztuerk, H., Schroeder, B., Beyerbach, M., and Breves, G. (2005). Influence of living and autoclaved yeasts of Saccharomyces boulardii on in vitro ruminal microbial metabolism. J. Dairy Sci. 88, 2594–2600. doi: 10.3168/jds.S0022-0302(05)72935-0
Roque, B., Brooke, C., Ladau, J., Polley, T., Marsh, L., Najafi, N., et al. (2019). Effect of the macroalgae Asparagopsis taxiformis on methane production and the rumen microbiome assemblage. Anim. Microb. 1:3. doi: 10.1186/s42523-019-0004-4
Tomkins, N. W., Colegate, S. M., and Hunter, R. A. (2009). A bromochloromethane formulation reduces enteric methanogenesis in cattle fed grain-based diets. Anim. Product. Sci. 49, 1053–1058.
Vucko, M. J., Magnusson, M., Kinley, R. D., Villart, C., and de Nys, R. (2017). The effects of processing on the in vitro antimethanogenic capacity and concentration of secondary metabolites of Asparagopsis taxiformis. J. Appl. Phycol. 29, 1577–1586. doi: 10.1007/s10811-016-1004-3
Wang, Y., Xu, Z., Bach, S. J., and McAllister, T. A. (2008). Effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on in vitro ruminal digestion of mixed forage or barley grain. Anim. Feed Sci. Technol. 145, 375–395. doi: 10.1016/j.anifeedsci.2007.03.013
Keywords: Asparagopsis taxiformis, feed supplementation, macroalgae, methane mitigation, in vitro rumen fermentation, Zonaria farlowii
Citation: Brooke CG, Roque BM, Shaw C, Najafi N, Gonzalez M, Pfefferlen A, De Anda V, Ginsburg DW, Harden MC, Nuzhdin SV, Salwen JK, Kebreab E and Hess M (2020) Methane Reduction Potential of Two Pacific Coast Macroalgae During in vitro Ruminant Fermentation. Front. Mar. Sci. 7:561. doi: 10.3389/fmars.2020.00561
Received: 23 December 2019; Accepted: 18 June 2020;
Published: 17 July 2020.
Edited by:
Maria Angeles Esteban, University of Murcia, SpainReviewed by:
Francisco Javier Alarcón, University of Almería, SpainPengcheng Fu, Hainan University, China
Copyright © 2020 Brooke, Roque, Shaw, Najafi, Gonzalez, Pfefferlen, De Anda, Ginsburg, Harden, Nuzhdin, Salwen, Kebreab and Hess. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthias Hess, bWhlc3NAdWNkYXZpcy5lZHU=
†ORCID: Charles G. Brooke, 0000-0002-2612-6141; Claire Shaw, 0000-0002-2495-0612; Matthias Hess, 0000-0003-0321-0380
 Charles G. Brooke
Charles G. Brooke Breanna M. Roque1
Breanna M. Roque1 Claire Shaw
Claire Shaw David W. Ginsburg
David W. Ginsburg Maddelyn C. Harden
Maddelyn C. Harden Sergey V. Nuzhdin
Sergey V. Nuzhdin Ermias Kebreab
Ermias Kebreab Matthias Hess
Matthias Hess