- 1Deep-Sea Ecology and Biogeochemistry Research Group, The Lyell Centre for Earth and Marine Science and Technology, Heriot-Watt University, Edinburgh, United Kingdom
- 2Monterey Bay Aquarium Research Institute, Moss Landing, CA, United States
- 3Federal Institute for Geosciences and Natural Resources (BGR), Hanover, Germany
In the Clarion-Clipperton Zone (CCZ) in the north-eastern Pacific Ocean ca. 30 billion tonnes of polymetallic nodules, rich in metals critical for frontier technologies, lay on the sediment surface over an area of 4–5 million square kilometres. For this reason, there is accelerating interest in deep-sea mineral mining in the CCZ. Few data exist concerning marine biodiversity in this enormous region and a solid understanding of baseline biodiversity and ecosystem function are necessary in order to inform effective management strategies, conservation and monitoring in the event that mining goes ahead in the CCZ. In this study, 10 in situ baited-camera experiments were carried out in the BGR (The Federal Institute for Geosciences and Natural Resources, Germany) licence area in the eastern CCZ to document the biodiversity of scavengers and their feeding activities. Twelve different bait-attending taxa were identified. The most common of these were macrourid, ophidiid and zoarcid fishes, and dendrobranchiate shrimp. Successional patterns regarding their time of arrival were also observed. The bait consumption rate was measured, and a mean scavenging rate of 878 g d–1 ± 113 (SE, n = 9) was calculated, with the rattail Coryphaenoides spp. responsible for the majority of the bait consumption. Polymetallic nodule size did not significantly affect community structure, diversity, or scavenging rates within the BGR licence area, but significant differences in community structure were found between the BGR area and other areas of the eastern CCZ (the Ocean Minerals of Singapore and United Kingdom 1 areas). This study increases our knowledge of deep-sea scavenging communities in an area targeted for deep-sea mining.
Introduction
Increasing demand for metals and rare earth elements for use in electronics and renewable energy infrastructure is accelerating research into deep-sea minerals and their potential for exploitation (Wedding et al., 2015; Miller et al., 2018). The Clarion-Clipperton Zone (CCZ) in the north-eastern Pacific Ocean is of particular importance due to high abundances of polymetallic nodules – ca. 30 billion tonnes – which cover vast areas of the sedimented abyssal plains (ISA, 2010). Nodules here are rich in manganese, copper, cobalt, nickel, and trace metals such as molybdenum, lithium, and rare earth elements (Wegorzewski and Kuhn, 2014). The International Seabed Authority (ISA) has so far allocated 16 licence areas covering more than 1 million km2 of seabed in the CCZ for polymetallic nodule exploration (ISA, 2019). Mining activity will involve the large-scale removal of nodules and sediments at the seafloor including resident epifauna (Vanreusel et al., 2016) by tethered mining vehicles, and the creation of a bottom water plume of sediment that may spread out over 10 s of kilometres, blanketing adjacent unmined areas (Wedding et al., 2015).
Significant correlations have been found between the coverage of polymetallic nodules and the abundance of megafauna (Radziejewska et al., 2000; Amon et al., 2016; Vanreusel et al., 2016; Leitner et al., 2017; Simon-Lledó et al., 2019), and it has been suggested that this may lead to enhanced prey abundance on and around nodules (Leitner et al., 2017). Since most mobile predators in the deep sea are also scavengers (Drazen et al., 2008), the abundance and size of nodules may play a role in structuring scavenger biodiversity and abundance. Benthic and demersal scavengers play an important role in nutrient cycling and energy flow in deep-sea ecosystems, consuming and redistributing organic material. They depend on the episodic transport of organic material from the pelagic zone in the form of dead fishes, squids, jellyfish, and cetacean carcasses as well as more refractory plant material (Britton and Morton, 1994; Jeffreys et al., 2010, 2011; Fleury and Drazen, 2013; Sweetman et al., 2014; Dunlop et al., 2017, 2018). Fishes, shrimps and amphipods comprise the majority of the abyssal scavenging fauna. Baited-camera studies have revealed that the relative abundance of scavengers increases with depth because of the scarcity of food resources in the abyss; in abyssal ecosystems, up to 46% of fishes are scavengers, compared to just 2% at 100 m off the continental shelf of California (Yeh and Drazen, 2011). Baited-cameras are an effective way to observe these communities due to scavengers’ propensity for constant swimming whilst they look for food, and their relatively scarce abundance over large areas –with hundreds of fish per km2 in the CCZ (Leitner et al., 2017).
Changes in turbidity caused by sediment plumes generated during deep-sea mining activities may have significant impacts on abyssal scavenger diversity and function. Suspended sediments may irritate and damage fish gills, increase pathogen exposure and increase oxygen consumption rates as previously shown for shallow-water, coral reef fishes exposed to sediment plumes (Hess et al., 2015, 2017; Moustaka et al., 2018). The levels of turbidity required to significantly alter scavenging biodiversity and function following mining may be very low given the extremely low sedimentation rates naturally found in the CCZ (Gardner et al., 1984), which likely leads to low resilience to suspended sediments in the scavenging fauna.
Generating a solid understanding of baseline abyssal scavenger biodiversity and function is necessary in exploration licence areas in order to inform effective management strategies, conservation, and monitoring in the event that mining goes ahead in the CCZ. In the eastern CCZ, only a single study has explored abyssal scavenger biodiversity and behaviour, the results of which showed that scavenger biodiversity was significantly correlated to polymetallic nodule abundance (Leitner et al., 2017). In this study we undertook 10 in situ baited camera experiments in the BGR (The Federal Institute for Geosciences and Natural Resources, Germany) licence area in the eastern CCZ, to document the biodiversity of scavengers and their feeding activities, and test the hypothesis that “Polymetallic nodule size affects scavenger biodiversity in the BGR licence area of the CCZ (Hypothesis 1).” We also tested the hypothesis that “Community structure varies significantly between different areas of the eastern CCZ (Hypothesis 2)” by comparing our results with the existing scavenger biodiversity data in Leitner et al. (2017) from the UK Seabed Resources (United Kingdom 1) and Ocean Minerals of Singapore (OMS) licence areas.
Materials and Methods
Study Area
The CCZ refers to an area between the Clarion Fracture Zone to the north and the Clipperton Fracture Zone to the south and spans ∼4–5 million square kilometres from 115°to 155°W and 5°to 20°N. The BGR licence area is situated in the eastern CCZ (Figure 1A), an area between the North Equatorial Current (NEC) and the North Equatorial Counter Current (NECC). Net primary productivity (NPP) is inversely correlated with sea-surface temperature (SST) and reaches a maximum of 379 ± 26.2 mg C m–2 day–1 around March [data derived from the Vertically Generalised Production Model (VGPM); Behrenfeld and Falkowski, 1997a, b; average value retrieved from the Ocean Productivity website1 for the BGR licence area]. Seafloor POC flux is estimated at around 1 g C m–2 yr–1, based on measurements taken at similar depths in regions relatively close to the study site (Lutz et al., 2002). The bottom temperature during the baited-camera deployments was 1.5°C (CTD data) and there is little intra and interannual variability in bottom temperature in this region (Aleynik et al., 2017).
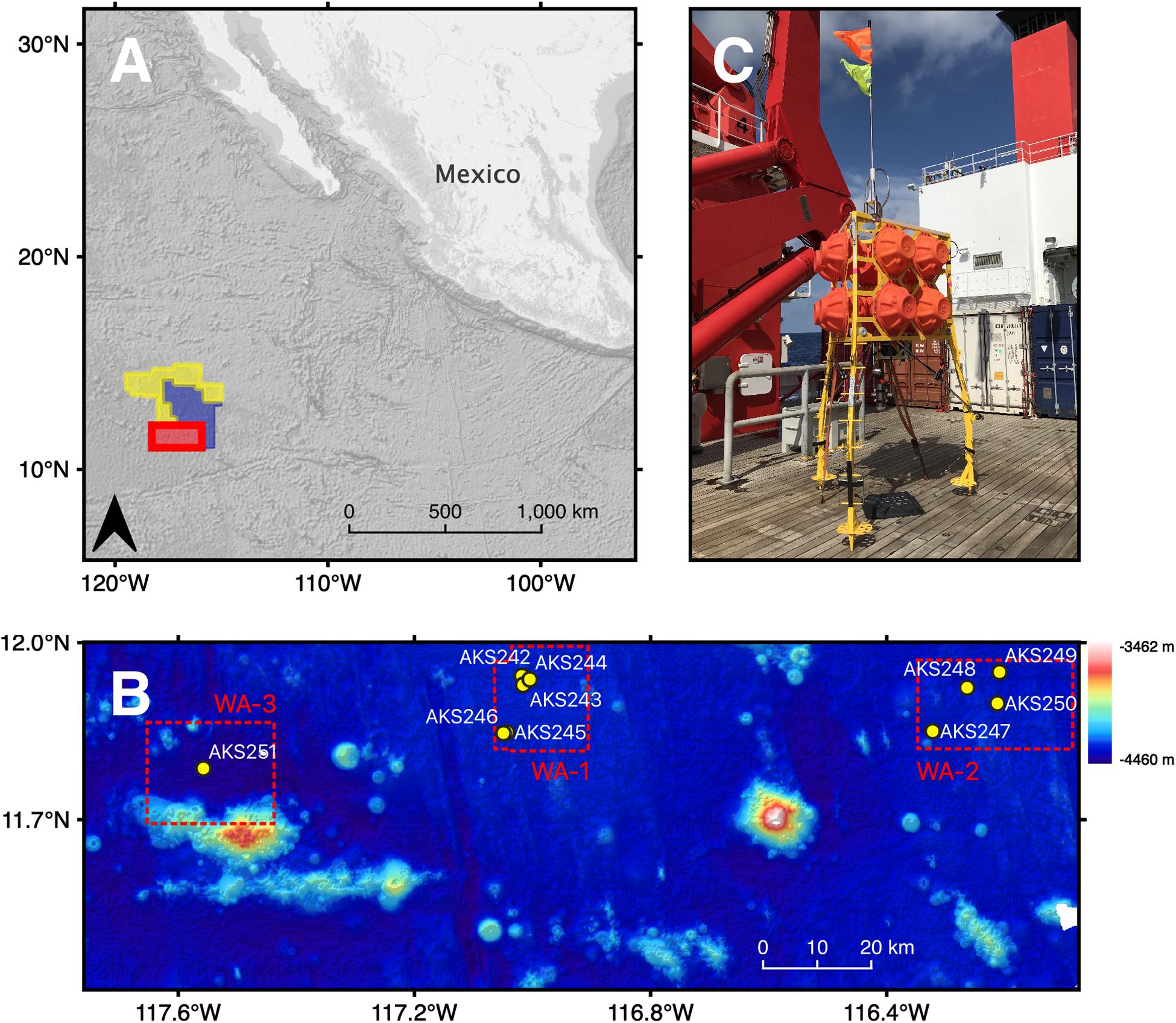
Figure 1. (A) The location of the study area (red polygon), the OMS licence area (yellow polygon) and the UK1 licence area (blue polygon) in the north-eastern tropical Pacific Ocean. (B) Bathymetry map showing the location of each of the 10 baited-camera lander deployments within working areas (WA-1, WA-1, WA-3), colour-coded by depth from dark blue at -4,460 m to white at -3,462 m. (C) The “Anonyx” lander, comprised of a steel tripod frame, standing ∼3 m tall. A cable releasing system connects KUM acoustic releasers, arranged in tandem, to weights (recycled anchor chains weighing 110 kg each). Attached to the upper part of the frame are 12 x Nautilus Marine Service GmbH VITROVEX glass floatation spheres.
Deep-sea scavenger biodiversity and function was quantified in the BGR licence area between March and April 2018 using a baited-camera lander system (Figure 1C). Ten deployments were carried out at depths ranging from 4,000 to 4,400 m (Table 1). The BGR has split the licence area into three working areas (Figure 1B). Five deployments were carried out in WA-1, the northern part of which is the intended location of a collector vehicle test by the Belgian CCZ mining contractor DEME-GSR, scheduled to take place in October 2020. The southern part of WA-1 is designated by the BGR as an “Impact Reference Zone.” Four deployments were carried out in WA-2, an area with a potentially economically viable nodule field spanning 350 km2 that is situated to the east of WA-1. A final deployment was carried out in WA-3, designated as a “Preservation Reference Zone (PRZ).”
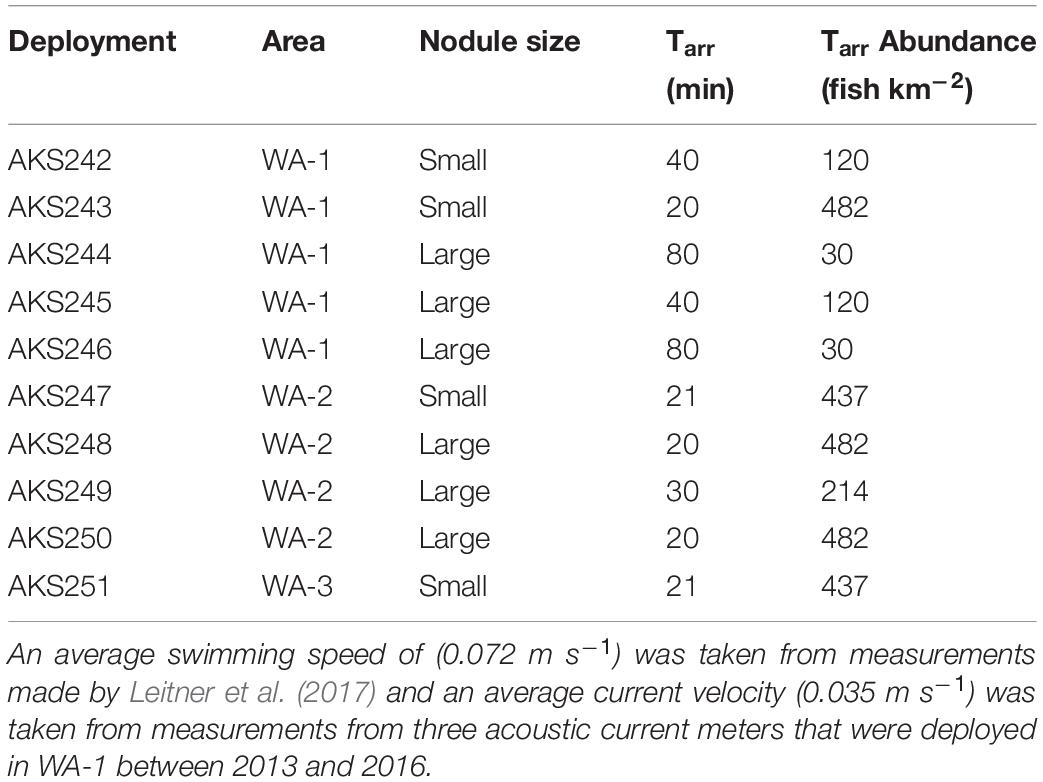
Table 2. Absolute abundance of Coryphaenoides spp. calculated using the time of first arrival model by Priede and Merrett (1996).
Data Collection
This study was undertaken during the BGR exploration cruise MANGAN 2018, aboard the RV “Sonne” from late March to the end of May 2018. It is the first study designed to quantify the diversity of scavengers and their feeding ecology in the BGR licence area.
The “Anonyx” baited-camera lander (Figure 1C) was equipped with a customisable camera bracket positioned above a bait plate (0.25 m2), which held a bespoke BGR HD video camera with a resolution of 1920 × 1080 p and a viewing angle of 70°h × 39°v. The camera was pointed straight down, with an approximate viewing area of 0.75 m2. Video was recorded using the H.264 codec at 5 mb/s. DeepSea Power & Light Sealite Six LED video lights were attached to the crossbeams between the lander legs to provide around 1,500 lm of light at a colour temperature of 6,000 K. The system ran autonomously once activated and was powered by a 670 Wh 24 V Oktopus deep-sea battery, capable of powering the camera and lights for ∼48 h.
A whole albacore tuna was used as bait to attract scavengers. Prior to the lander being deployed, the fish was weighed and photographed, then attached to the bait plate beneath the camera using cable ties. For each deployment, a single fish of a standard wet-weight of 1.5–2 kg was used. Nitrile gloves were worn to attach the bait to the bait plate so as not to transfer any hand oils, sun cream or anything else that could alter the behaviour of bait-attending fauna.
The lander was deployed for a period of 23–40 h; during this time the camera and lights were set to record video for periods of 2 min with an 8 min break in between, in order to conserve battery power. After the first deployments, the video settings were fine-tuned to achieve the best possible image clarity: the maximum gain was lowered to eliminate overexposure of the whiter parts of the image. Once the lander had been recovered, the remaining bait was photographed, then weighed again to calculate the amount of bait that was consumed during the deployment. All video data from the camera were then downloaded to a computer and stored on two hard drives for later analysis. Landers were deployed onto two types of polymetallic nodule fields, identified in previous multibeam backscatter surveys by the BGR, which featured high abundances of either small (<4 cm) or large (>4 cm) nodules (Kuhn et al., 2020). Nodule size was confirmed visually from video captured during each deployment (Table 1).
On one lander deployment, two amphipod traps were deployed attached to the lander legs, at a height of 75 cm above the seafloor. As the traps also contained bait, they were only attached for the final deployment to avoid interference with the baited-camera experiment. Each trap was constructed from PVC tubing with two inward-facing funnels, secured 1.5 cm apart. A piece of albacore tuna was placed inside the trap and the open end was covered with a 35 μm mesh, held in place with a hose clamp. After recovery, the contents of the traps were rinsed with seawater and fixed with buffered 4% formalin for identification on land.
Data Processing
From each 2 min clip, the maximum number of individual scavengers observed in the video frame at one time (MaxN) was recorded for each of the bait-attending megafauna, as well as their time of first arrival (Tarr). Fauna were identified to the lowest taxonomic level possible, based on their morphology, generally to genera or species. The moment the lander touched down on the seabed and the time at which an animal first successfully punctured the flesh of the bait, were also recorded.
The abundance of Coryphaenoides spp. scavengers (Table 3) was calculated using the mathematical “Time of first arrival” model by Priede and Merrett (1996) that uses current velocity (Vw), fish swimming speed (Vf) and the time of first arrival (Tarr) to infer absolute abundances, based on a uniform distribution of fish (Priede and Merrett, 1996). The swimming speed of Coryphaenoides spp. (0.072 m s–1) was taken from measurements made by Leitner et al. (2017) who deployed a stereo video camera system to between 3,600 and 4,300 m in the UK1 licence area of the CCZ. Current velocity measurements were collected from three acoustic current meters that were deployed in WA-1 between 2013 and 2016. These current meters recorded an average velocity of 0.035 m s–1 across the three locations at a height of 11–16 m from the seabed (Aleynik et al., 2017). Average Tarr values were calculated for small and large nodule deployments before inserting these values into the model.
The rate of bait removal (grams of bait removed per day) by benthic and demersal scavengers (Table 4) was calculated by dividing the change in the weight of the bait by the bottom time and multiplying by 24 h.
Data Analysis
Multivariate community analysis, species accumulation and rarefaction curves, and diversity indices were produced using PRIMER-e 7.0 (Quest Research Ltd.). Analysis of Coryphaenoides spp. absolute abundance, and diversity indices were performed on untransformed data. Resemblance matrices were built using Bray-Curtis similarity, multi-dimensional scaling plots were set to 50 restarts and ANOSIM tests were run to a maximum of 9,999 permutations with the Spearman rank correlation method. The Simpson index of diversity (1-λ) was chosen due to its sensitivity for changes in the most common species, making it less likely that rare species would skew the results.
SPSS Statistics v26 (IBM Corporation) was used for univariate analysis. Normality was checked using a Shapiro-Wilk test and homogeneity of variances were checked with a Levene’s test. Diversity indices and scavenging rates were compared using a One-Way ANOVA test or Student T-test, or where data did not meet parametric assumptions, a Kruskal–Wallis or Mann-Whitney U-test. Tests for correlations between scavenger arrival time, time elapsed until the first bait puncture and absolute abundance were performed using the Spearman Signed Rank test since a normal distribution could not be found following data transformation. An alpha level of 0.05 was used as the criterion for statistical significance.
In order to gather regional insights into scavenger assemblages in the CCZ, our data were compared to data obtained from a similar baited-camera study undertaken in the neighbouring UK Seabed Resources (United Kingdom 1) and Ocean Minerals of Singapore (OMS) licence areas by Leitner et al. (2017). Leitner et al. (2017) deployed their baited camera for similar periods, but used a different bait type, and a lower bait weight (Pacific mackerel, 1 kg). Rarefaction and non-metric Multi-Dimensional Scaling (nMDS) ordinations were carried out after species identifications were standardised between the two datasets. To avoid biasing community differences, three zoarcid species identified in Leitner et al. (2017) were grouped to family level in this study since species level data for this taxon were unavailable in the BGR licence area.
Results
Twelve bait-attending taxa were identified over the course of 10 deployments in the BGR licence area (Figure 2 and Table 4). The most abundant of these were the rattails Coryphaenoides spp., the eelpout Pachycara nazca and the shrimp Hymenopenaeus nereus. Coryphaenoides spp. were the most abundant with an average MaxN of 6 ± 0.55 (standard error or SE, n = 10). The next most abundant were Pachycara nazca with an average MaxN of 4 ± 0.84 (SE, n = 10), however, despite their relatively high abundance in most deployments they were not observed at all during deployment AKS245 in WA-1. Hymenopenaeus nereus had an average MaxN of 4 ± 0.54 (SE, n = 10). The small area of nodule coverage that was visible around the bait plate suggested nodules were similar in their distribution across the sediment surface for each deployment. No significant difference in scavenger assemblage structure was found between the small and large nodule deployment areas within the BGR licence area (ANOSIM, Rho = 0.117, p = 0.23). The mean Simpson Diversity Index (1-λ) for the large nodule sites was 0.80 ± 0.01 (SE, n = 10) and for the small nodule sites it was 0.79 ± 0.01 (SE, n = 10). No significant difference was found between them (independent samples t-test, p = 0.59).
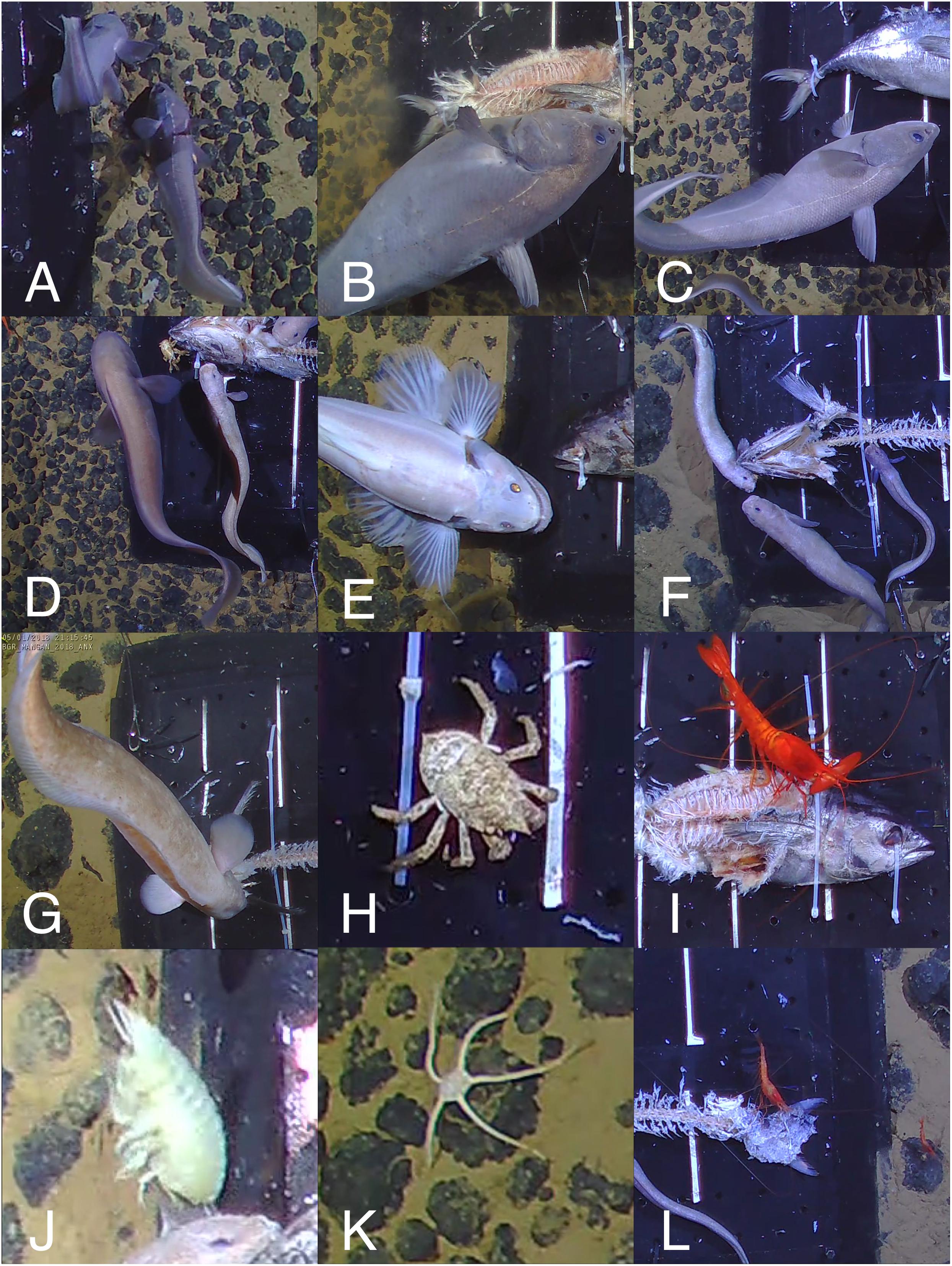
Figure 2. Commonly observed species in the BGR licence area. (A–C) Coryphaenoides spp., (D) Left: Bassozetus sp., Right: P. nazca (E) Bathysaurus mollis, (F) Pachycara nazca (n = 3), (G) Barathrites iris, (H) Munidopsis sp., (I) Cerataspis monstrosus, (J) Eurythenes gryllus, (K) Ophiuroid, (L) Hymenopenaeus nereus (n = 2). For scale estimation, the black bait plate is 50 × 50 cm.
Using the model of Priede and Merrett (1996), the absolute abundance of Coryphaenoides spp. in the BGR licence area ranged between 30 and 482 individuals km–2 (Table 2). When Tarr was averaged for the large nodule deployments, the abundance of Coryphaenoides spp. was 95 individuals km–2, in contrast to an abundance of 296 individuals km–2 calculated for the small nodule region. However, no significant difference in mean Coryphaenoides spp. abundance between small and large nodule sites was detected (independent samples t-test, p = 0.23). No correlation was found between MaxN and the absolute abundance of Coryphaenoides spp. (Pearson Correlation, R = 0.126, p = 0.73) and there were no significant differences in the abundance of Coryphaenoides spp. between the BGR, OMS, and UK1 sites (One-way ANOVA, p = 0.78).
In virtually all of the scavenger deployments, the bait was completely consumed except for the skeleton of the fish and the flesh around the head (Table 3). During the lander’s descent on deployment AKS243 one of the cables securing the bait plate snapped. This deployment was excluded from scavenging rate calculations because, while it attracted the same scavenger assemblage seen in other deployments, the bait remained partially inaccessible for the duration of the deployment. The mean scavenging rate averaged over 9 deployments was 878 g d–1 ± 113 (SE). Higher scavenging rates were recorded in regions with small nodules (1104 g d–1 ± 154 SE, n = 3) compared to regions with large nodules (766 g d–1 ± 136 SE, n = 6), but no significant difference was detected (Mann-Whitney U, p = 0.80). Coryphaenoides sp. were responsible for the majority of the bait consumption. In every deployment, it was the Coryphaenoides spp. that punctured the skin of the bait for the first time, and the time at which this occurred varied considerably between 40 min (AKS249) to 23 h (AKS246) after lander-touchdown. In the latter case, a considerably lower scavenging rate of 94 g d–1 was recorded, however, this was not a consistent trend and while an inverse relationship was seen between the time elapsed before the first puncture and the scavenging rate, the correlation was not significant (Spearman correlation, R = -0.552, p = 0.06). There was a positive relationship seen between the scavenging rate and the absolute abundance of Coryphaenoides spp., but this correlation was also not significant (Spearman correlation, R = 0.398, p = 0.14).
Successional patterns were evident in four out of the five most abundant scavengers (Figure 3). Coryphaenoides spp. were always the first to reach the bait, arriving 37 ± 24 mins (standard deviation or SD, n = 10) after lander touch-down. Coryphaenoides spp. were present around the bait plate throughout the duration of the deployments but were seen in fewer numbers after the bulk of the flesh had been removed, which had usually occurred after 16 h. As Coryphaenoides spp. numbers declined, Hymenopenaeus nereus appeared in greater numbers and were seen feeding on the bait. This smaller shrimp arrived at the bait on average 264 ± 196 mins (SD, n = 10) after touch-down. Pachycara nazca was the slowest to arrive at the bait appearing in video footage 571 ± 192 mins (SD, n = 10) after lander touchdown. The initial individuals that arrived at the bait plate remained there and were joined by other P. nazca as the deployment progressed. P. nazca were very rarely observed consuming the bait itself; instead they seemed to be eating bait-attending amphipods. Successional patterns were not seen in any of the other dominant bait-attending fauna, but this may have been because there were so few individuals recorded.
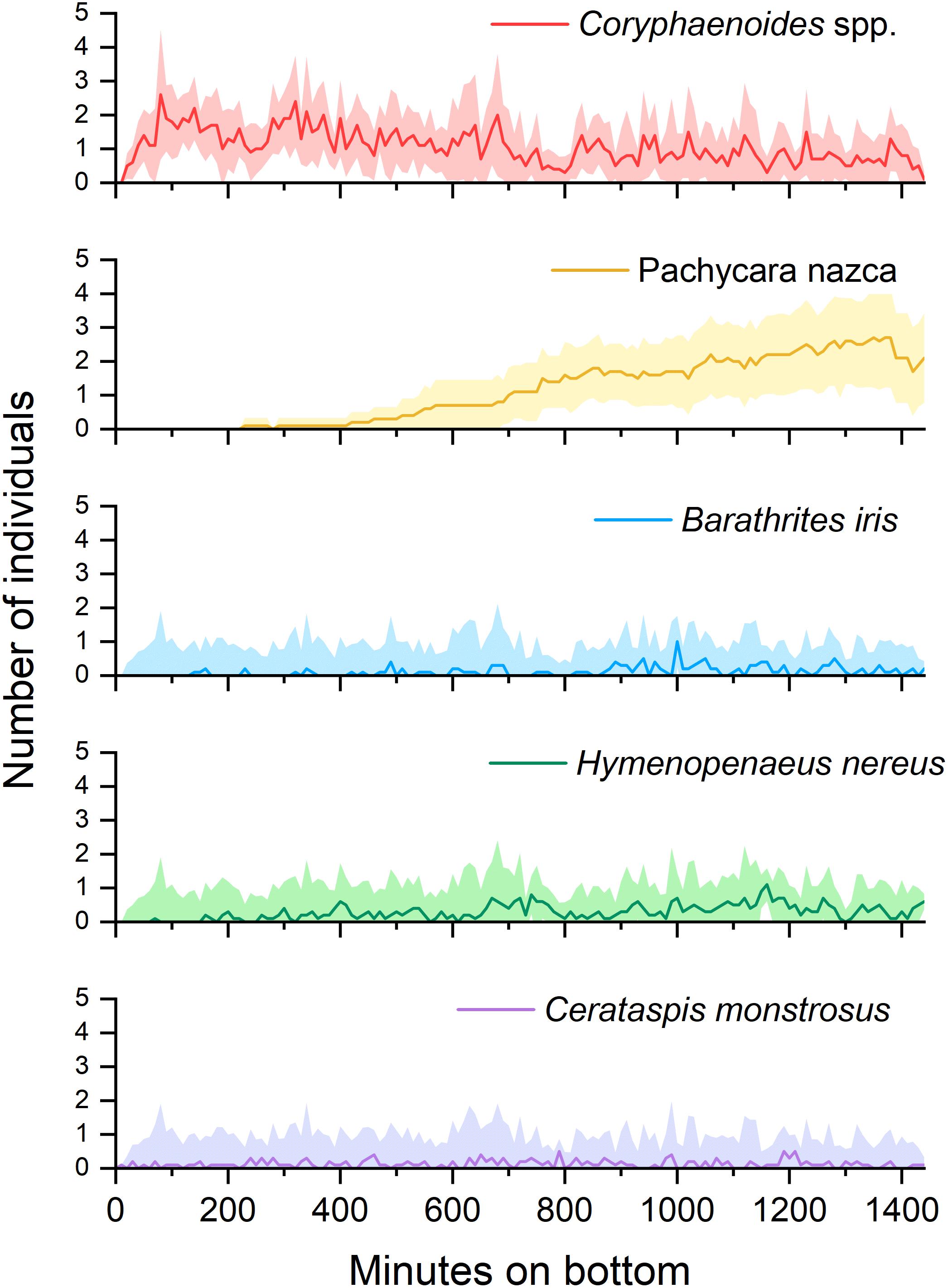
Figure 3. The appearance of the 5 most commonly observed species produced from mean MaxN figures for each 2-min clip across the 10 deployments. Shaded areas represent ± 95% confidence interval.
Species-accumulation curves revealed that the majority of the scavengers had arrived after ∼100 video clips, which equated to 990 min (16.5 h) after the lander reached the seabed (Figure 4A). Clear asymptotes after 8–10 deployments were also visible in the species accumulation curves where species observed were plotted against the number of deployments. This indicated that 10 deployments were sufficient to provide an accurate overview of the full scavenger assemblage in the BGR region.
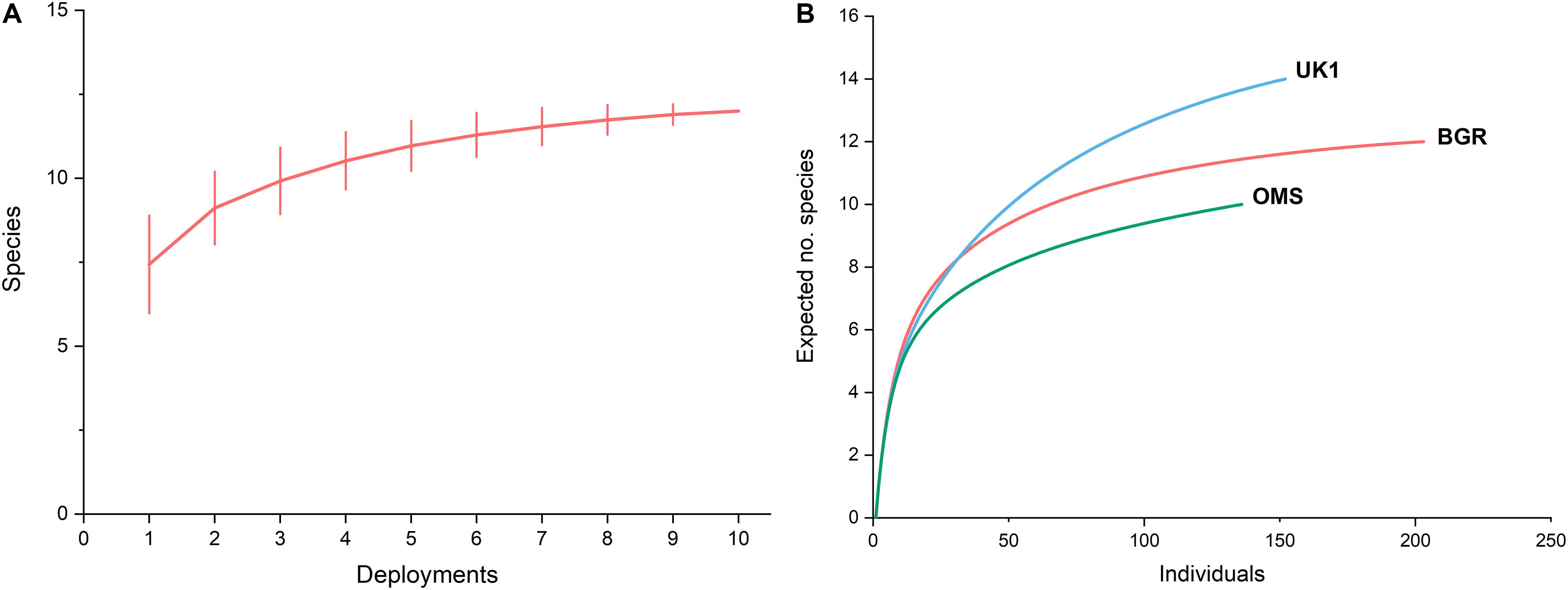
Figure 4. (A) Mean species accumulation curve for the BGR licence area (n = 10) generated from 9,999 permutations of the data, (B) Rarefaction curves for the BGR licence area (n = 10) compared with data collected by Leitner et al. (2017) from the UK1 (n = 6), and OMS (n = 6) licence areas.
Rarefaction curves were generated using pooled MaxN data to estimate species richness in the BGR licence area (Figure 4B). These were compared to the rarefaction curves of the OMS and UK1 areas in Leitner et al. (2017). Rarefied to ES(n)100, species richness in the BGR licence area was ∼11, compared to 9 in the OMS area and 13 in the UK1 area.
Non-metric Multi-Dimensional Scaling analyses showed distinct clustering of deployment sites by region (Figure 5). Scavenger community composition was found to be significantly different between the BGR and OMS sites (ANOSIM, R = 0.7, p < 0.001) and also between the BGR and UK1 sites, albeit with a lower Rho coefficient (ANOSIM, R = 0.6, p < 0.001). SIMPER analysis showed that the BGR and OMS sites were 48% dissimilar, with Zoarcidae contributing 16.4%, H. nereus contributing 15.6% and Cerataspis monstrosus contributing 15.5% to the dissimilarity matrix. The BGR and UK1 sites were 44.2% dissimilar with C. monstrosus contributing 18.5%, Zoarcidae contributing 16.1%, and H. nereus contributing 15.9% of the dissimilarity. In terms of biodiversity average species richness for the BGR licence area was 7 ± 0.31 (SE, n = 10) but no significant difference was detected between the BGR, OMS, and UK1 areas (One-way ANOVA, p = 0.25). The average Simpson Diversity Index (1-λ) for the BGR licence area was 0.79 ± 0.01 (SE, n = 10) but again, no significant differences were found between the BGR, OMS, and UK1 licence areas (One-way ANOVA, p = 0.23).
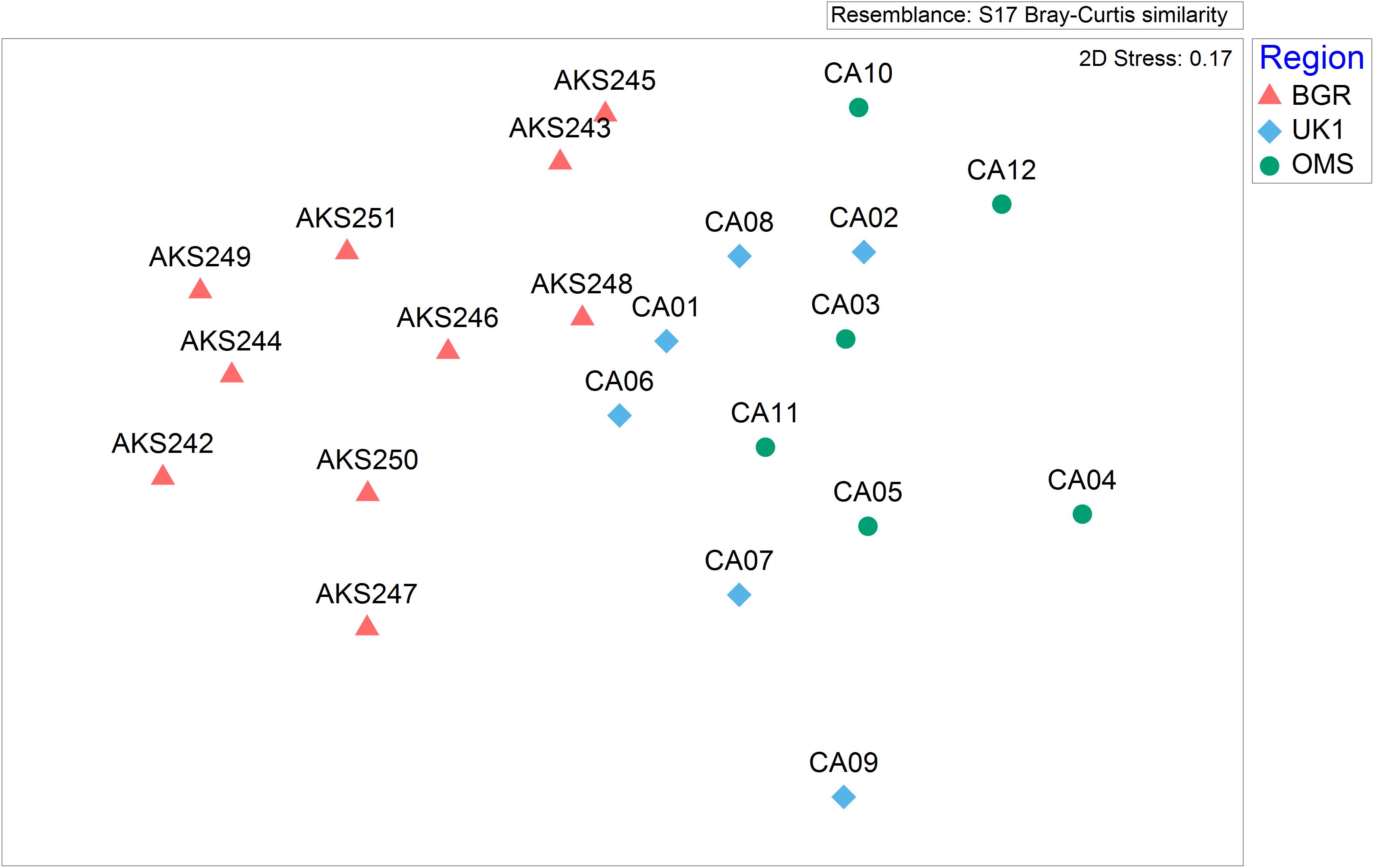
Figure 5. A non-metric Multidimensional Scaling Plot representing the community composition and abundance observed in the BGR licence area (n = 10) and data collected by Leitner et al. (2017) from the UK1 (n = 6), and OMS (n = 6) licence areas.
Discussion
Once mining in the CCZ begins it will significantly disturb 300–800 km2 of seafloor per mining operation per year (Smith et al., 2008; Levin et al., 2016). This activity is likely to severely change abyssal habitats both directly by sediment and nodule removal, and as a result of the sediment plume, which may disperse for 10 s of kilometres smothering adjacent, unmined areas (Aleynik et al., 2017). Given that benthic and demersal scavenger communities play key ecosystem function roles in abyssal ecosystems and may be significantly impacted by sediment and nodule removal as well as sediment plumes, assessing baseline scavenger diversity and behaviour before mining begins is essential in order to inform future management decisions and conservation.
We did not find any significant variation in scavenger biodiversity or abundance in areas with different sized nodules, thus our first hypothesis can be rejected. However, our results show that community structure varies significantly between different regions of the eastern CCZ, which supports our second hypothesis. These differences were most pronounced between the BGR and OMS licence areas and were predominantly caused by an increase in the number of zoarcids in the BGR area (Figure 6).
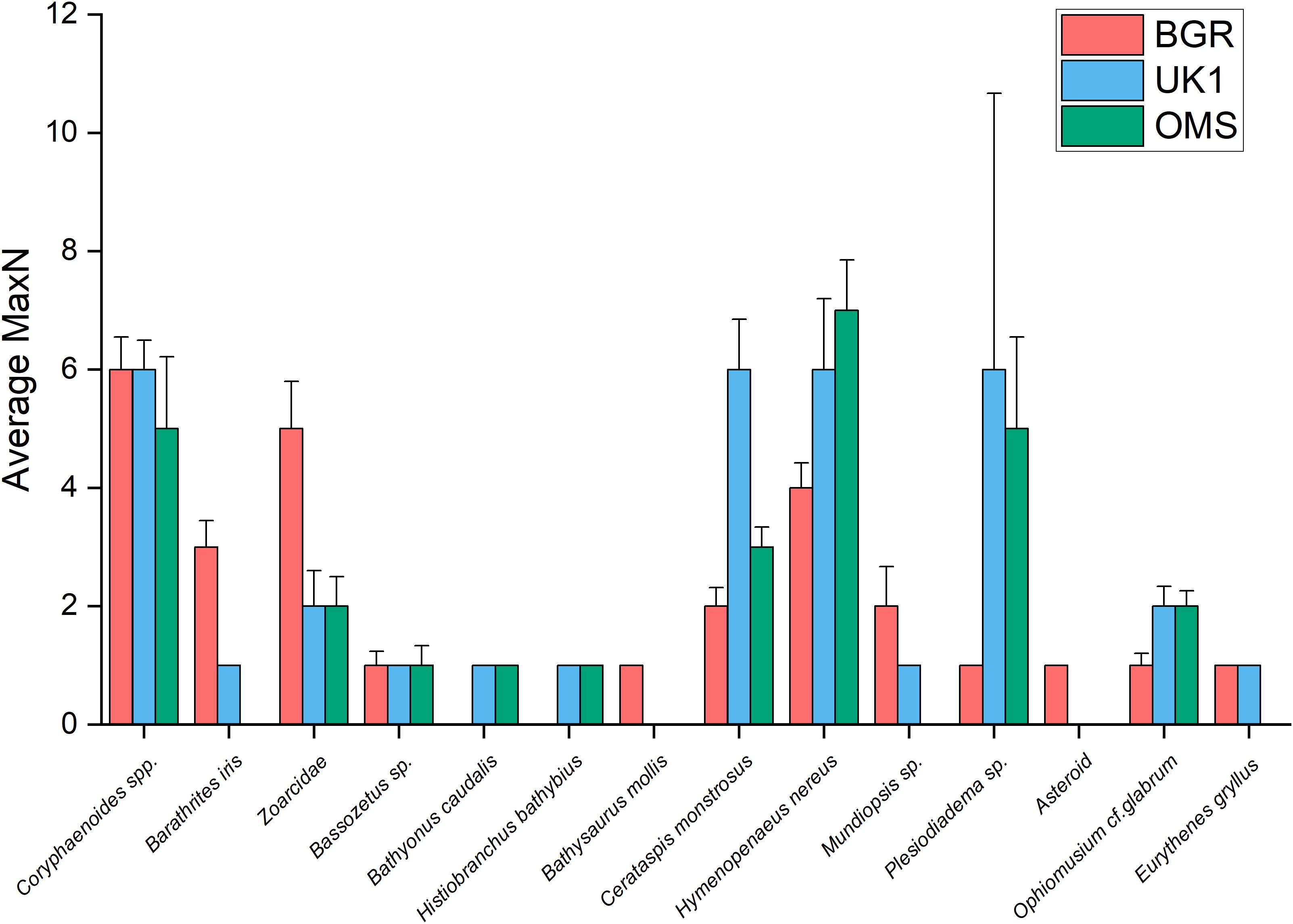
Figure 6. A comparison of the average MaxN for each species between the BGR licence area (n = 10) and data collected by Leitner et al. (2017) from the UK1 (n = 6) and OMS (n = 6) licence areas, with + 1 SEM as error bars.
Over the course of this study, 12 different bait-attending taxa were identified in the BGR licence area. The most abundant of the bait-attending fauna were Coryphaenoides spp. fishes (Figures 2A–C). Because traps were not used in this experiment, it was not possible to be sure of the species of rattail. It has been suggested that the identification of C. yaquinae and C. armatus can be achieved using photography alone, based on the differences in light reflection from their scales (Jamieson et al., 2012). However, this method was deemed unreliable in our study due to the differences observed in the reflection based on the subject’s distance from the downward-facing camera and lighting. Twenty-one of the 22 fish trapped by Leitner et al. (2017) in the adjacent OMS and UK1 areas were C. armatus. Therefore, we hypothesise that the Coryphaenoides spp. fishes in our study are predominantly C. armatus. C. armatus is the most usual bait-attending fish found between 2,200 and 5,400 m in the Pacific ocean, and is known to swim against the current tracking odour plumes in search of carrion (Priede and Merrett, 1996; Bailey et al., 2007). These fishes usually arrived less than an hour after lander touch-down and remained at the bait until the bulk of the flesh was consumed (after around 16 h), at which point their numbers diminished and a variety of other bait-attending fauna became more common at the bait (Figure 3). This observation was consistent with C. armatus behaviour seen at cetacean-fall experiments at abyssal depths in the north-east Atlantic (Jones et al., 1998), as well as at fish carcasses deployed by baited camera landers in the OMS and UK1 licence areas of the CCZ (Leitner et al., 2017).
Estimates of the absolute abundance of Coryphaenoides spp. fishes using the Priede and Merrett (1996) model were similar to those of Leitner et al., 2017 and subject to large variations across different deployments (Table 2). There is considerable uncertainty associated with calculating abundances of even the most common scavengers (Farnsworth et al., 2007). For example, the time of first arrival (Tarr) metric, on which the Priede and Merrett (1996) method relies can be influenced by factors other than population density, such as fish that may arrive and swim close to the bait but do not enter the camera frame. In addition, the model is sensitive to changes in current speed, which we were unable to measure directly – our calculations were dependent on average current speed data from the local area. Furthermore, both speed and direction can change regularly (over hours) by tidal movement, “surface winds” and passing eddies (Aleynik et al., 2017). For these reasons, our modelled absolute abundance estimates should be taken with caution; they are included here for the purpose of illustrating differences between the BGR licence area and the OMS and UK1 areas where the same model was used with similar caveats (Leitner et al., 2017). Estimating animal population densities is a key attribute to baseline studies, as well as for planning conservation and monitoring programmes (Wedding et al., 2015), and while MaxN data is an effective proxy, robust estimates will most likely also require ground-truthing over large-scale areas using AUV and ROV vehicles (Leitner et al., 2017).
Coryphaenoides spp. fishes were responsible for the majority of the bait consumption; we observed them tearing the bait into smaller pieces on which other smaller rattails and other animals were able to feed, with the exception of the late-arriving P. nazca. These fish took up residence on the bait plate and behaved like sit-and-wait predators, eating the scavenging amphipods. During dissection of trapped P. nazca fish from the OMS and UK1 licence areas, Leitner et al. (2017) found that their stomachs were full of amphipods, which confirmed our observations. P. nazca were the second most abundant bait-attending species during our experiments although in deployment AKS245 they did not attend the bait at all. This was in contrast to the findings of Leitner et al. (2017) who found that Hymenopenaeus nereus was the second most abundant species in the OMS and UK1 areas (Figure 6). There was no obvious reason for the reduced abundance of P. nazca during deployment AKS245, which was not one of the shorter deployments (26 h); we can only speculate that either this slow-moving fish was not abundant in the area (despite finding similar numbers of zoarcids to the other sites less than a kilometre away, during AKS246), or that there was a drop in current velocity that reduced the odour plume (Sainte-Marie and Hargrave, 1987). In our study, H. nereus was observed feeding on the bait in greater abundances toward the end of deployments when the majority of the flesh had been stripped away by the larger scavenging fauna, and there was a drop in the number of Coryphaenoides spp. fishes around the bait. It is likely that this was due to predator-avoidance, as Coryphaenoides spp. are known to feed on live crustaceans as well as carrion (Drazen et al., 2008); Leitner et al. (2017) observed these fish attacking crustaceans, including the large shrimp Cerataspis monstrosus (unpublished data).
Our scavenging rate calculations revealed a mean bait consumption of 878 g d–1 ± 113 (SE), which is slightly lower than reported in other abyssal studies, though within the same order of magnitude. In almost all of the scavenger deployments, the bait was completely consumed except for the skeleton of the fish and the flesh around the head, this suggests that using a larger bait may have resulted in increased bait consumption. Previous studies have shown that scavenging rates tend to increase with water depth (Hessler et al., 1978; Jones et al., 1998; Collins et al., 1999). For example, Collins et al. (1999) observed rapid scavenging on the Patagonian slope between 900 and 1,300 m, where high abundances of specialised hagfish consumed bait at rates up to 200 g h–1. Experiments conducted at 2,500 m in the highly productive Arctic Ocean that used a variety of different teleost fishes as bait showed voracious scavenging activity by amphipods, and recorded scavenging rates of up to 5,600 g d–1 (Premke et al., 2006). When tuna was used as bait in the Arabian Sea at 3,000–4,000 m, scavenger rates of 2,690 and 4,320 g d–1 were documented (Janßen et al., 2000). These rates were considerably higher than our results, however, the Arabian Sea is subject to intense upwelling, seasonal productivity and Oxygen Minimum Zones (OMZ), meaning scavenger numbers and activities are likely to be higher than the oligotrophic eastern Pacific Ocean (Witte, 1999; Lutz et al., 2002). In addition, the tuna bait used in Janßen et al. (2000) was 1–3 kg heavier and the deployments ran for up to 104 h.
The scavenging rates presented here must be viewed as estimations of actual scavenging activity in the BGR licence area, and it is possible that methodology and differences in bait size and type also play a role in driving the differences between our results and the previously mentioned studies, in addition to their geographic locations. Differences in the duration of the deployments and the way the scavenging rate was calculated (Tables 2, 4) may considerably affect the measured rates. Limited ship-time for baited-camera work meant that it was not possible to standardise each deployment to the same time. The scavenging rate calculation assumes that bait consumption is more or less evenly distributed over the duration, so that it can be standardised to a 24-h period, but this was not always the case. In deployment AKS246, which was a 26-hr deployment, the scavenging rate was 94 g d–1. However, we observed no visible consumption of the bait until after 23 h (despite the regular attendance of scavenging fishes and other animals from 2 h onward), at which point a large Coryphaenoides spp. punctured the flesh and other animals started to feed. In contrast, the scavenging rate for deployment AKS249 was 868 g d–1 despite a similar 21 h delay before the bait consumption began. The duration of the latter deployment was 38 h, which meant other animals had a further 17 h to feed after the first bite, compared to 3 h in AKS246. In our experiment, Coryphaenoides spp. seemed to be the only fishes capable of puncturing the relatively tough skin of the albacore tuna used as bait. During almost all of the deployments there was video footage to corroborate this assertion, and where the first bite happened during a gap in the footage, large Coryphaenoides spp. were present in clips immediately preceding it. No other animal was observed puncturing the bait. A weak inverse relationship was found between the first time the bait was punctured by Coryphaenoides spp. fishes and the scavenging rate, but this correlation was not significant. Had the deployment length been the same for each deployment, we hypothesise that a stronger relationship between the time at which bait consumption commenced and the scavenging rate would have been seen.
The scavenging community in the BGR licence area appeared to be relatively species rich. Rarefaction curves generated for the different study sites (Figure 4B) surveyed showed that species richness was 11 when rarefied to 100 animals, and similar at sites characterised by small and large nodules (data not shown). Species accumulation curves showed that most of the bait-attending community was photographed after 10 deployments, so our species richness estimates appear to be reliable (Figure 4A). Our species richness estimates were greater than those of the Hawaiian Islands at depths > 4,000 m (Yeh and Drazen, 2009) but similar to species richness estimates by Leitner et al. (2017) who found species richness at their OMS sites were slightly below 10 and > 14 for UK1 sites (Figure 4B). A power analysis based on our data showed that only 11 lander deployments would be required in the same area to detect a 25% drop in species richness due to mining (power = 0.80, p < 0.05), which should be considered in future baseline and monitoring studies.
In our study, all of the deployments were placed in areas known to feature dense nodule coverage in one of two size classes, small and large (Table 1). No relationship was found between the diversity or abundance of scavengers and the size of the polymetallic nodules in the regions. This is despite Simon-Lledó et al. (2019) finding that the presence of metazoan fauna and xenophyophores doubled in areas where only small increases in nodule coverage were observed, though they did not observe significant changes in diversity (Simon-Lledó et al., 2019). Amon et al. (2016) found a positive correlation between the abundance of megafauna and the presence of nodules and similarly, Leitner et al. (2017) found a significant effect of nodule coverage on scavenger diversity as well as the size of Coryphaenoides spp. fishes. It has been hypothesised that the reason for increased abundances of highly mobile megafauna in areas of nodule coverage is an increase in epifauna, which may be prey for predatory fish (Amon et al., 2016). We were unable to test the hypothesis that the presence of nodules increases scavenger diversity and abundance compared to regions without nodules, or the effects of changes in percentage cover of nodules. In our observations, albeit of a small border (∼10–20 cm) surrounding the bait plate, the small nodule sites appeared to have a greater coverage of nodules, potentially making them similar to the large nodule sites in terms of available surface area for epifauna. In addition, the surface texture of nodules, which may vary between small and large nodules, has been shown to significantly affect epifaunal assemblages (Mullineaux, 1989).
Although no significant difference was observed in scavenger species richness or Simpson Diversity Index (1-λ) between our study and that of Leitner et al. (2017), we did find significant differences in scavenger community composition between the BGR, OMS, and UK1 areas, with the most dissimilarity found between the BGR and OMS areas. This indicates that scavenger community composition in the eastern CCZ may change significantly over relatively short distances (<20 km), and as such, this variability should be taken into account when conducting baseline studies and future monitoring. The differences we observed were predominantly caused by higher numbers of zoarcids and both species of shrimp, H. nereus and C. monstrosus, in the BGR licence area (Figure 6). There were, however, similarities in terms of the ophidiid fishes with MaxN values for Barathrites iris and Bassozetus spp. in the BGR licence area ranging between 1 and 2, which was similar to those recorded in the OMS and UK1 areas (Figure 6). In the CCZ, the numbers of ophidiid fishes tend to be greater in the western, compared to the eastern end (Leitner et al., 2017) and this is consistent with observations of only small numbers of these species in our experiments. In contrast, bait-attending communities documented between 3,000 and 4,700 m off the coast of Hawaii were found to be dominated by ophidiids, eels, and aristeid shrimp, with Coryphaenoides spp. and zoarcids rarely seen (Yeh and Drazen, 2009). Baited-camera studies conducted on the eutrophic Californian slope at shallower depths (100–3,000 m), however, found the bait-attending community to be dominated by macrourids and zoarcids (Yeh and Drazen, 2011), which is similar to the findings of this study. Geographically, the BGR and OMS deployments are closest to one another, so the fact that the communities here showed more dissimilarity compared to UK1 area was unexpected. The BGR and OMS lander deployments were also placed at similar depths and have similarly flat bathymetry (Table 1), so it may be that abrupt bathymetrical features (e.g., abyssal depressions and hills) lay between the two locations and affected benthic and demersal community structure, analogous to what has been shown for abyssal benthic invertebrate megafauna (Durden et al., 2017; Leitner et al., 2017). An additional reason could relate to variations in overlying primary production and food-supply regimes in the different licence areas (Lutz et al., 2007; Vanreusel et al., 2016). It is also possible that the differences observed could simply be an artefact produced by the use of different camera systems; differences in viewpoint have been shown to affect faunal abundance counts in baited camera studies (Whitmarsh et al., 2018). Leitner et al. (2017) used a stereo video system which viewed a wider area of the seafloor (and hence more of the scavenging fauna), while this study used a downward-facing camera system with a much narrower view of the seafloor and the scavenger community around the bait. Finally, Leitner et al. (2017) used Pacific mackerel as bait (rather than the albacore tuna used in this study) so it is possible that the different bait types also have influenced the behaviour of deep-sea scavenging communities, as shown in upper bathyal-depth studies (e.g., Sweetman et al., 2014). However, both Pacific mackerel and albacore tuna are oily fish from the family Scombridae, that should both promote a strong odour plume, and in such a food deprived region we hypothesise that the influence of each of these bait types on the community of scavengers attracted, would be similar.
Conclusion
Our results show a relatively high diversity of benthic and demersal scavengers in the BGR licence area of the CCZ, in line with other similar experiments performed in adjacent licence areas. Numerically, the scavenger assemblages observed were dominated by Coryphaenoides spp., followed by zoarcid eelpouts and these taxa were responsible for the majority of the dissimilarity in community structure found between the BGR, OMS, and UK1 areas. Species richness in the BGR area was much greater than species richness off the coast of Hawaii at similar depths (>4,000 m) but was very similar in terms of the dominant fauna to the bait-attending community at similar depths off the coast of California. Bait consumption rates in the BGR licence area were in the same order of magnitude as scavenging rates documented for other bathyal and abyssal studies. No relationship was found between the diversity of scavengers and the size of the polymetallic nodules, but we hypothesise that in terms of overall area of hard substrate, the small and large nodule sites are similar, which could dampen a scavenger diversity-nodule-size signal. We have also shown that the full scavenger community can be observed with as few as 10 lander deployments in the CCZ, and future monitoring studies will need to undertake at least 11 baited camera experiments in this region to detect a change in the scavenger community resulting from mining. This study provides important information to ongoing ecological baseline studies in the CCZ and will help inform effective management strategies in the event that mining eventually goes ahead in this region.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the animal study because animals were photographed in situ and not disturbed.
Author Contributions
RH: fieldwork, analysis, and wrote the manuscript. AL: provided the data, helped with analysis, changes, and corrections to the manuscript. CR: chief scientist on ship, fieldwork scheduling and advice, changes, and corrections to the manuscript. AV: fieldwork scheduling and advice on ship, changes, and corrections to the manuscript. AS: principle investigator, Ph.D. supervisor to RH, changes and corrections to the manuscript, and analysis help. All authors contributed to the article and approved the submitted version.
Funding
AS was contracted by the BGR (The Federal Institute for Geosciences and Natural Resources, Germany) to investigate scavenger diversity in the German licence area of the CCZ. RH and AS were funded by EC MERCES, Grant Agreement No. 689518. AS was also funded by the GCRF One Ocean hub. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No. 818123 (iAtlantic). This output reflects only the author’s view and the European Union cannot be held responsible for any use that may be made of the information contained therein.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank Milena Wales for her help in carrying out the experiment and her hours spent watching “Fish TV,” Henning Wedemeyer for his invaluable technical expertise and advice throughout the cruise, and to the fantastic crew of the RV “Sonne” for their assistance in making this experiment successful.
Footnotes
References
Aleynik, D., Inall, M. E., Dale, A., and Vink, A. (2017). Impact of remotely generated eddies on plume dispersion at abyssal mining sites in the Pacific. Sci. Rep. 7:16959. doi: 10.1038/s41598-017-16912-2
Amon, D. J., Ziegler, A. F., Dahlgren, T. G., Glover, A. G., Goineau, A., Gooday, A. J., et al. (2016). Insights into the abundance and diversity of abyssal megafauna in a polymetallic-nodule region in the eastern Clarion-Clipperton Zone. Sci. Rep. 6:30492. doi: 10.1038/srep30492
Bailey, D. M., Wagner, H.-J., Jamieson, A. J., Ross, M. F., and Priede, I. G. (2007). A taste of the deep-sea: the roles of gustatory and tactile searching behaviour in the grenadier fish Coryphaenoides armatus. Deep Sea Res. Part I Oceanogr. Res. Pap. 54, 99–108. doi: 10.1016/j.dsr.2006.10.005
Behrenfeld, M. J., and Falkowski, P. G. (1997a). A consumer’s guide to phytoplankton primary productivity models. Limnol. Oceanogr. 42, 1479–1491. doi: 10.4319/lo.1997.42.7.1479
Behrenfeld, M. J., and Falkowski, P. G. (1997b). Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol. Oceanogr. 42, 1–20. doi: 10.4319/lo.1997.42.1.0001
Britton, J. C., and Morton, B. (1994). Marine carrion and scavengers. Oceanogr. Mar. Biol. Annu. Rev. 32, 369–434.
Collins, M. A., Yau, C., Nolan, C. P., Bagley, P. M., and Priede, I. G. (1999). Behavioural observations on the scavenging fauna of the Patagonian slope. J. Mar. Biol. Assoc.U. K. 79, 963–970. doi: 10.1017/S0025315499001198
Drazen, J. C., Popp, B. N., Choy, C. A., Clemente, T., De Forest, L., and Smith, K. L. (2008). Bypassing the abyssal benthic food web: macrourid diet in the eastern North Pacific inferred from stomach content and stable isotopes analyses. Limnol. Oceanogr. 53, 2644–2654. doi: 10.4319/lo.2008.53.6.2644
Dunlop, K. M., Jones, D. O. B., and Sweetman, A. K. (2017). Direct evidence of an efficient energy transfer pathway from jellyfish carcasses to a commercially important deep-water species. Sci. Rep. 7:17455. doi: 10.1038/s41598-017-17557-x
Dunlop, K. M., Jones, D. O. B., and Sweetman, A. K. (2018). Scavenging processes on jellyfish carcasses across a fjord depth gradient. Limnol. Oceanogr. 63, 1146–1155. doi: 10.1002/lno.10760
Durden, J. M., Ruhl, H. A., Pebody, C., Blackbird, S. J., and van Oevelen, D. (2017). Differences in the carbon flows in the benthic food webs of abyssal hill and plain habitats. Limnol. Oceanogr. 62, 1771–1782. doi: 10.1002/lno.10532
Farnsworth, K. D., Thygesen, U. H., Ditlevsen, S., and King, N. J. (2007). How to estimate scavenger fish abundance using baited camera data. Mar. Ecol. Prog. Ser. 350, 223–234. doi: 10.3354/meps07190
Fleury, A. G., and Drazen, J. C. (2013). Abyssal scavenging communities attracted to sargassum and fish in the sargasso Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 72, 141–147. doi: 10.1016/j.dsr.2012.11.004
Gardner, W. D., Sullivan, L. G., and Thorndike, E. M. (1984). Long-term photographic, current, and nephelometer observations of manganese nodule environments in the Pacific. Earth Planet. Sci. Lett. 70, 95–109. doi: 10.1016/0012-821X(84)90212-7
Hess, S., Prescott, L. J., Hoey, A., McMahon, S., Wenger, A., and Rummer, J. (2017). Species-specific impacts of suspended sediments on gill structure and function in coral reef fishes. Proc. R. Soc. BBiol. Sci. 284:20171279. doi: 10.1098/rspb.2017.1279
Hess, S., Wenger, A. S., Ainsworth, T. D., and Rummer, J. L. (2015). Exposure of clownfish larvae to suspended sediment levels found on the great barrier reef: impacts on gill structure and microbiome. Sci. Rep. 5:10561. doi: 10.1038/srep10561
Hessler, R. R., Ingram, C. L., Aristides Yayanos, A., and Burnett, B. R. (1978). Scavenging amphipods from the floor of the Philippine trench. Deep Sea Res. 25, 1029–1047. doi: 10.1016/0146-6291(78)90585-4
ISA (2010). Development of Geological Models for the Clarion Clipperton Zone Polymetallic Nodule Deposits. ISA technical studies, 6. Fountain Valley, CA: Kingston.
ISA (2019). Current Status of the Reserved Areas With the International Seabed Authority [WWW Document]. Avaliable at: https://www.isa.org.jm/contractors/reserved-areas (accessed 9.2.19).
Jamieson, A. J., Priede, I. G., and Craig, J. (2012). Distinguishing between the abyssal macrourids Coryphaenoides yaquinae and C. armatus from in situ photography. Deep Sea Res. Part I Oceanogr. Res. Pap. 64, 78–85. doi: 10.1016/j.dsr.2012.02.001
Janßen, F., Treude, T., and Witte, U. (2000). Scavenger assemblages under differing trophic conditions: a case study in the deep Arabian Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 47, 2999–3026. doi: 10.1016/S0967-0645(00)00056-4
Jeffreys, R. M., Lavaleye, M. S. S., Bergman, M. J. N., Duineveld, G. C. A., and Witbaard, R. (2011). Do abyssal scavengers use phytodetritus as a food resource? Video and biochemical evidence from the Atlantic and Mediterranean. Deep Sea Res. Part I Oceanogr. Res. Pap. 58, 415–428.
Jeffreys, R. M., Lavaleye, M. S. S., Bergman, M. J. N., Duineveld, G. C. A., Witbaard, R., and Linley, T. (2010). Deep-sea macrourid fishes scavenge on plant material: evidence from in situ observations. Deep Sea Res. Part I Oceanogr. Res. Pap. 57, 621–627. doi: 10.1016/j.dsr.2010.01.007
Jones, E. G., Collins, M. A., Bagley, P. M., Addison, S., and Priede, I. G. (1998). The fate of cetacean carcasses in the deep sea: observations on consumption rates and succession of scavenging species in the abyssal north-east Atlantic Ocean. Proc. Biol. Sci. 265, 1119–1127. doi: 10.1098/rspb.1998.0407
Kuhn, T., Uhlenkott, K., Vink, A., Rühlemann, C., and Martinez Arbizu, P. (2020). “Chapter 58 - manganese nodule fields from the Northeast Pacific as benthic habitats,” in Seafloor Geomorphology as Benthic Habitat, 2nd Edn, eds P. T. Harris and E. Baker (Amsterdam: Elsevier), 933–947.
Leitner, A. B., Neuheimer, A. B., Donlon, E., Smith, C. R., and Drazen, J. C. (2017). Environmental and bathymetric influences on abyssal bait-attending communities of the Clarion Clipperton Zone. Deep Sea Res. Part I Oceanogr. Res. Pap. 125, 65–80. doi: 10.1016/j.dsr.2017.04.017
Levin, L. A., Mengerink, K., Gjerde, K. M., Rowden, A. A., Dover, C. L. V., Clark, M. R., et al. (2016). Defining “serious harm” to the marine environment in the context of deep-seabed mining. Mar. Policy 74, 245–259. doi: 10.1016/j.marpol.2016.09.032
Lutz, M., Dunbar, R., and Caldeira, K. (2002). Regional variability in the vertical flux of particulate organic carbon in the ocean interior. Global Biogeochem. Cycles 16:1037. doi: 10.1029/2000GB001383
Lutz, M. J., Caldeira, K., Dunbar, R. B., and Behrenfeld, M. J. (2007). Seasonal rhythms of net primary production and particulate organic carbon flux to depth describe the efficiency of biological pump in the global ocean. J. Geophys. Res. 112:C10011. doi: 10.1029/2006JC003706
Miller, K. A., Thompson, K. F., Johnston, P., and Santillo, D. (2018). An Overview of seabed mining including the current state of development, environmental impacts, and knowledge gaps. Front. Mar. Sci. 4:418. doi: 10.3389/fmars.2017.00418
Moustaka, M., Langlois, T. J., McLean, D., Bond, T., Fisher, R., Fearns, P., et al. (2018). The effects of suspended sediment on coral reef fish assemblages and feeding guilds of north-west Australia. Coral Reefs 37, 659–673. doi: 10.1007/s00338-018-1690-1
Mullineaux, L. S. (1989). Vertical distributions of the epifauna on manganese nodules: implications for settlement and feeding. Limnol. Oceanogr. 34, 1247–1262. doi: 10.4319/lo.1989.34.7.1247
Premke, K., Klages, M., and Arntz, W. E. (2006). Aggregations of arctic deep-sea scavengers at large food falls:: temporal distribution, consumption rates and population structure. Mar. Ecol. Prog. Ser. 325, 121–135. doi: 10.2307/24870670
Priede, I. G., and Merrett, N. R. (1996). Estimation of abundance of abyssal demersal fishes; a comparison of data from trawls and baited cameras. J. Fish Biol. 49, 207–216. doi: 10.1111/j.1095-8649.1996.tb06077.x
Radziejewska, T., Stoyanova, S. V., and Stoyanova, V. (2000). Abyssal epibenthic megafauna of the clarion-clipperton area (NE Pacific): changes in time and space versus anthropogenic environmental disturbance. Oceanol. Stud. 29, 83–101.
Sainte-Marie, B., and Hargrave, B. T. (1987). Estimation of scavenger abundance and distance of attraction to bait. Mar. Biol. 94, 431–443. doi: 10.1007/BF00428250
Simon-Lledó, E., Bett, B. J., Huvenne, V. A. I., Schoening, T., Benoist, N. M. A., Jeffreys, R. M., et al. (2019). Megafaunal variation in the abyssal landscape of the clarion clipperton zone. Progr. Oceanogr. 170, 119–133. doi: 10.1016/j.pocean.2018.11.003
Smith, C. R., De Leo, F. C., Bernardino, A. F., Sweetman, A. K., and Arbizu, P. M. (2008). Abyssal food limitation, ecosystem structure and climate change. Trends Ecol. Evol. 23, 518–528. doi: 10.1016/j.tree.2008.05.002
Sweetman, A. K., Smith, C. R., Dale, T., and Jones, D. O. B. (2014). Rapid scavenging of jellyfish carcasses reveals the importance of gelatinous material to deep-sea food webs. Proc. R. Soc. Lon. BBiol. Sci. 281:20142210. doi: 10.1098/rspb.2014.2210
Vanreusel, A., Hilario, A., Ribeiro, P. A., Menot, L., and Arbizu, P. M. (2016). Threatened by mining, polymetallic nodules are required to preserve abyssal epifauna. Sci. Rep. 6, 1–6. doi: 10.1038/srep26808
Wedding, L. M., Reiter, S. M., Smith, C. R., Gjerde, K. M., Kittinger, J. N., Friedlander, A. M., et al. (2015). Managing mining of the deep seabed. Science 349, 144–145. doi: 10.1126/science.aac6647
Wegorzewski, A. V., and Kuhn, T. (2014). The influence of suboxic diagenesis on the formation of manganese nodules in the clarion clipperton nodule belt of the pacific ocean. Mar. Geol. 357, 123–138. doi: 10.1016/j.margeo.2014.07.004
Whitmarsh, S. K., Huveneers, C., and Fairweather, P. G. (2018). What are we missing? Advantages of more than one viewpoint to estimate fish assemblages using baited video. R. Soc. Open Sci. 5:171993. doi: 10.1098/rsos.171993
Witte, U. (1999). Consumption of large carcasses by scavenger assemblages in the deep Arabian Sea:observations by baited camera. Mar. Ecol. Prog. Ser. 183, 139–147. doi: 10.3354/meps183139
Yeh, J., and Drazen, J. C. (2009). Depth zonation and bathymetric trends of deep-sea megafaunal scavengers of the Hawaiian Islands. Deep Sea Res. Part I Oceanogr. Res. Pap. 56, 251–266. doi: 10.1016/j.dsr.2008.08.005
Keywords: Clarion Clipperton Zone CCZ, polymetallic nodules, scavenger, baited-camera, deep-sea mining, scavenging rate
Citation: Harbour RP, Leitner AB, Ruehlemann C, Vink A and Sweetman AK (2020) Benthic and Demersal Scavenger Biodiversity in the Eastern End of the Clarion-Clipperton Zone – An Area Marked for Polymetallic Nodule Mining. Front. Mar. Sci. 7:458. doi: 10.3389/fmars.2020.00458
Received: 29 January 2020; Accepted: 22 May 2020;
Published: 30 June 2020.
Edited by:
Stefán Áki Ragnarsson, Marine and Freshwater Research Institute, IcelandReviewed by:
Pedro A. Ribeiro, University of Bergen, NorwayMichael Vecchione, National Oceanic and Atmospheric Administration (NOAA), United States
Copyright © 2020 Harbour, Leitner, Ruehlemann, Vink and Sweetman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rob P. Harbour, cnBoMkBody5hYy51aw==
 Rob P. Harbour
Rob P. Harbour Astrid B. Leitner
Astrid B. Leitner Carsten Ruehlemann3
Carsten Ruehlemann3 Andrew K. Sweetman
Andrew K. Sweetman