
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 21 April 2020
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 7 - 2020 | https://doi.org/10.3389/fmars.2020.00242
There is a reluctance to incorporate Fishers’ Ecological Knowledge (FEK) into the evidence base used to underpin marine management decisions. FEK has proved to be useful as an alternative reference of biological changes in data-poor scenarios. Yet, recreational fisher knowledge has rarely been included in scientific studies despite being a source of FEK. Here, the use of recreational FEK to assess the conservation status of marine ecosystems in Galicia (NW Spain) was evaluated. Galicia has a highly complex marine socioecological system that includes both a large global commercial fleet and a powerful recreational sector, alongside other important stakeholders (e.g., tourism, aquaculture). Anglers and spear fishers were asked to provide their perceptions of the conservation status of fish stocks and the impacts on marine ecosystems. Face-to-face interviews were transcribed into text and analyzed using text mining tools. Key concepts were used to quantify fishers’ perceptions of changes in their target fish stocks and quantify the main impacts on marine ecosystems. Overfishing and habitat loss, followed by reduction in biodiversity, pollution, and warming temperatures were considered to be the main drivers of the poor status of cephalopods and finfish stocks. Perceived temporal declines in fish stocks were consistent with available biological data, highlighting the potential for recreational FEK to be used to assess long-term ecological changes. It was important to seek opinions from different users, including fishers from traditional commercial and recreational fisheries, as these groups had good knowledge of the impacts on natural and cultural community heritage. The poor status of ballan wrasse (Labrus bergylta) and kelp beds was identified, which was of concern due to it being a key species in coastal ecosystems. Use of FEK is a good approach to develop knowledge of these systems, but broader monitoring programs are needed to protect the future of these ecosystems.
Responsible management of natural resources and services provided by marine ecosystems needs reliable information on the human-induced changes over time (Lockwood et al., 2012). However, the lack of long-term scientific information on human impacts makes sustainable exploitation of marine populations a challenge as baseline data needed to assess changes do not exist (Halpern et al., 2008). Management decisions based on incorrect baselines can result in the long-term maintenance of marine ecosystems well below natural levels (Pitcher, 2001). This has been described as the “shifting baseline syndrome” where the current conservation status of marine ecosystems is established as a cognitive baseline reference against which to judge future changes (Pauly, 1995). The implications of these shifting baselines is not trivial given the potential for overexploitation of the planet’s ecosystems by humanity (Watson et al., 2018).
Fishers’ ecological knowledge (FEK), alongside other sources of information (Thurstan et al., 2015b), has begun to be used in the last decades to provide alternative long-term references or baselines for management decisions (Hind, 2014). FEK is generated from the long-term use of ecosystems, and integrates practices and perceptions influenced by local culture, differing from traditional science in the way that data are interpreted and organized (Maurstad, 2002). FEK can complement traditional scientific knowledge, increasing spatial and temporal resolution of the derived knowledge framework (Agrawal, 1995; Stephenson et al., 2016; Gourguet et al., 2018). Despite this, its use in the management of natural resources has not become widespread (Huntington, 2000). Fisheries science has been particularly reluctant to incorporate FEK into decision making procedures (Hind, 2014). The lack of recognition by the scientific community of the inseparability of nature and society has also hampered the success of this process (Goldman and Schurman, 2000).
Recreational fisheries are providing an increasing amount of data for researchers about their activity (e.g., Lloret et al., 2008; Giovos et al., 2018), but also about the ecosystems within they operate (e.g., Tiralongo et al., 2019), but the use of their FEK has been limited (see review by Hind, 2014). Given that there are many more recreational than commercial fishers (Hyder et al., 2018; Arlinghaus et al., 2019a), recreational fishers represent a relatively untapped source of long-term information on marine ecosystems (Brewin et al., 2017). Recreational FEK has been used to quantify variations in abundances and distribution of different fish stocks (e.g., Azzurro et al., 2011; Zukowski et al., 2011; Beaudreau and Levin, 2014; Sbragaglia et al., 2020). However, to our knowledge, it has not been used to integrate perceptions of trends of fish stocks with the conservation status of the whole ecosystems.
In this study, the use of recreational FEK to assess the conservation status of marine ecosystems in data-poor situations was evaluated in Galicia (NW Spain). Galicia has one of the largest commercial fleets globally, including large scale high seas vessels operating from the main ports (STECF, 2018), and 4,000 small-scale fisheries vessels in many of the smaller towns and villages (Freire and García-Allut, 2000; Pita et al., 2019). Coastal ecosystems are also exploited by a thriving marine recreational fishery composed by 60,000 fishers and 4,000 boats (Pita et al., 2018b). Furthermore, one of the most important worldwide mussel aquaculture industry is located there (Pérez-Camacho et al., 1991; Villasante et al., 2013), which along with intensive shipping traffic (Suárez de Vivero and Rodríguez Mateos, 2012) and growing tourism demand (Cortés-Jiménez, 2008) shape a highly complex socioecological system. In this context, the lack of long-term scientific ecological data poses serious challenges for setting management baselines from which to develop sustainable exploitation of the coastal ecosystems (Pita and Villasante, 2019; Pita et al., 2019). This is exacerbated because Galicia has the highest social and economic dependence on marine resources in the European Union (Surís-Regueiro and Santiago, 2014).
To address information needs for sustainable management of marine resources, long-term trends in the conservation status of fish stocks and the key changes in marine ecosystems were obtained from recreational FEK. Status of main fish stocks and impacts on marine ecosystems were explored, followed by an analysis to identify topics that could influence fishers’ perceptions of the conservation status of fish stocks and habitats. The level of agreement between fishers’ perceptions and other sources of biological data was assessed.
Very active fishers with a high degree of involvement in the recreational fishery were recruited using a snowball model (Goodman, 1961), starting with a small group of informants that were initially identified by representatives of the main regional recreational fishers’ associations, and expanding through their contacts and social networks [i.e., “peer referencing” (Davis and Wagner, 2003)]. This was designed to recruit avid recreational fishers with a high degree of knowledge of the fishery and ecosystem. This is because they will be most aware of long-term changes, as their catches are dependent on the health of the system and fishing has been a central part of their lifestyle for many years (Arlinghaus et al., 2019b). Top fishers in spearfishing competitions1 (N = 4) and rod and line anglers in the directive board of the main fishers’ clubs in the region2 (N = 10), including old and young people, were selected (mean age was 55.07 ± 11.46 SD years; Table 1).
Face-to-face semi-structured video-recorded interviews were conducted from May 2017 to January 2018 by a single investigator. Semi-structured interviews were used to ensure that participants provided information on key topics, while open-ended questions and probes allowed fishers to expand on the most important items for them (Bryman, 2016).
Filmed interviews have some advantages over other face-to-face methods including sound recordings. For example, video footage can be viewed many times and shared with other investigators, reducing the potential for observer-related biases. Social cues (e.g., voice, intonation, body language) can provide extra information and responses are more spontaneous than with other methods (Opdenakker, 2006). In addition, the accuracy of the interview report remains high without the need to take notes which can distract the interviewer (Wengraf, 2001).
The interviews mostly lasted less than 10 min (7.08 ± 3.49 min). Fishers reported fishing experience (25.07 ± 14.60 years; Table 1) and described their perceptions of: (1) any changes over times in the abundances and sizes of their main target species (i.e., explain how has the abundances and/or sizes changed?); and (2) key changes in the marine ecosystems (i.e., describe what has changed and why?). To identify topics that potentially could influence perceptions, the fishers were also asked to provide information on: (1) main changes in fishing gears and techniques to assess potential hyper-stability in abundance trends (Maunder et al., 2006; Kleiber and Maunder, 2008); and (2) personal motivations for recreational fishing (i.e., why they are recreational fishers?) to assess heterogeneity (Beaudreau and Levin, 2014). Participants were asked to bring pictures of old and recent fishing trips to the interviews to help them to remember and avoid recall bias (Hiett and Worrall, 1977; Pollock et al., 1994). The photographs were requested to show typical fishing scenes, including gears, rather than catches. All fishers who agreed to participate in the study provided verbal informed consent.
The audio of the interviews was transcribed into text by using the googleLanguageR library (Edmondson, 2018) and analyzed by using text mining tools within the tm library (Meyer et al., 2008), both within the statistical software R (R Core Team, 2019). Automatized transcription tools and text mining techniques to process transcribed texts were used because this method can save a considerable amount of time (Bryman, 2016). The texts were subsequently reviewed to avoid transcription errors.
A three-step approach was used to analyze the content of the discourse on each topic. Firstly, the relative frequency of each word included in the transcriptions was obtained using the termdocumentmatrix tool from the tm library. Usual stop words were removed by using the stopwords and removeWords tools. Secondly, the meaning of key concepts represented by the most frequent words was analyzed after translation into English by showing the connections using the Rgraphviz library (Hansen et al., 2017). The maximum correlation threshold was used in each case, allowing at least one association between the words. Finally, hierarchical cluster analysis was done on the dissimilarity matrix of the most recurrent words using Ward’s minimum variance method (Ward, 1963).
The relationships among the key concepts identified were used to quantify fishers’ perceptions of changes in the conservation status of their target fish stocks by using the following scale: −2 (very negative, meaning much less and/or much smaller fishes); −1 (negative, meaning less and/or smaller fishes); 0 (meaning no changes in abundances and/or sizes); 1 (positive, meaning more and/or bigger fishes); and 2 (very positive, meaning much more and/or bigger fishes).
Key concepts were grouped to identify the main impacts on marine ecosystems and access motivations of the recreational fishers. To ensure groups were consistent and coherent (Saldaña, 2015), they were compared with main topics identified in the texts of the transcriptions by using the LDA tool of the topicmodels library in R, that generated a probabilistic framework for the frequency occurrences of the key concepts of the interviews (Hornik and Grün, 2011). Key concepts were also used to quantify changes in fishing gears and techniques separately for anglers and spear fishers by using the following scale: 0 (no changes); 1 (minor improvements); and 2 (great improvements).
Throughout this process, original video recordings and transcriptions were routinely reviewed especially for cases that raised doubts about the coding and grouping of variables. Furthermore, the coding and grouping were reviewed by a second investigator and discrepancies were discussed until consensus was reached. To assess the degree of completeness of the species lists and key concepts, mean accumulation curves were obtained using bootstrapping with 1,000 permutations (Gotelli and Colwell, 2001). This bootstrapping procedure was used to assess the efficiency in the identification of target species and key concepts (Soberón and Llorente, 1993; Longino and Colwell, 1997) by estimating the potential number of unreported species and key concepts (Palmer, 1990; Colwell and Coddington, 1994). Accumulation curves were calculated using the specaccum tool and the number of unreported species and key concepts was estimated with the specpool tool, both in the vegan package with R (Oksanen et al., 2019).
Generalized linear models (GLMs) were used to assess overall temporal trends in the conservation status of each of the fish stocks by including the fishing experience of recreational fishers as independent variables. GLMs, and Generalized Linear Mixed-Effects Models (GLMMs) to account for correlation in the perceptions provided by each fisher, were used to identify cognitive baselines in the fishers’ perceptions because it was expected that very experienced fishers would perceive larger changes on fish stocks than less experienced fishers (Beaudreau and Levin, 2014). GLMs and GLMMs were also used to assess if perceived changes in fish stocks varied among individuals with different motivations. Different error structures and link functions were assessed, and the best models selected based on an appropriate error structure and Akaike’s information criterion (Akaike, 1973).
The level of agreement between interview-derived and biological trends was assessed where possible for the identified target species. Multidecadal time series of commercial landings (1997–2018) were accessible for some species from the government Pesca de Galicia website3 (Xunta de Galicia, 2019). Temporal trends in the landings of the fish stocks targeted by the interviewed recreational fishers were assessed using GLMs. These were compared against the percentage difference in mean annual landings between the first five and the last 5 years of the available commercial time series to assess population trends.
Long-term data on fish size and abundance of some identified target species were obtained from Pita and Freire (2014) and Alonso-Fernández et al. (2019). In all cases, population trends by species obtained from different sources were normalized to the interview-derived scale (from −2 to 2). The two morphotypes of ballan wrasse Labrus bergylta (Ascanius, 1767; Almada et al., 2016; Quintela et al., 2016), pinto (spotted and reddish) and maragota (plain greenish or brown) (sensu Villegas-Ríos et al., 2013) were treated separately when possible.
Recreational fishers’ perception of the temporal trends in the conservation status of the fish stocks they target was in general negative (mean −1.3 ± 0.8, in a scale between −2 and 2). The greater the experience of the fishers, the worse the perceptions about the status of fish stocks (p = 0.004, R2 = 0.130) (Figure 1). Of the 13 reported species, fishers were mainly concerned about the current conservation status of cephalopods like common octopus Octopus vulgaris (Cuvier, 1797) (mean −2.0 ± 0.0), common squid Loligo vulgaris (Lamarck, 1798) (mean −2.0 ± 0.0) and common cuttlefish Sepia officinalis (Linnaeus, 1758) (mean −1.7 ± 0.8; Table 2). Some fishers explained that catches on cephalopods are highly variable, depending on the season, day and area. This was represented by frequent usage of interconnected words like “cycles,” “sea floor,” “tide,” “variable,” and “year (Supplementary Figures S5–S7). However, there was a common sentiment that abundances of cephalopods had severely declined over time. For example, one boat angler stated “The last cuttlefish season was the worst ever. It was disastrous. Year after year, catches are decreasing” (Participant 4).
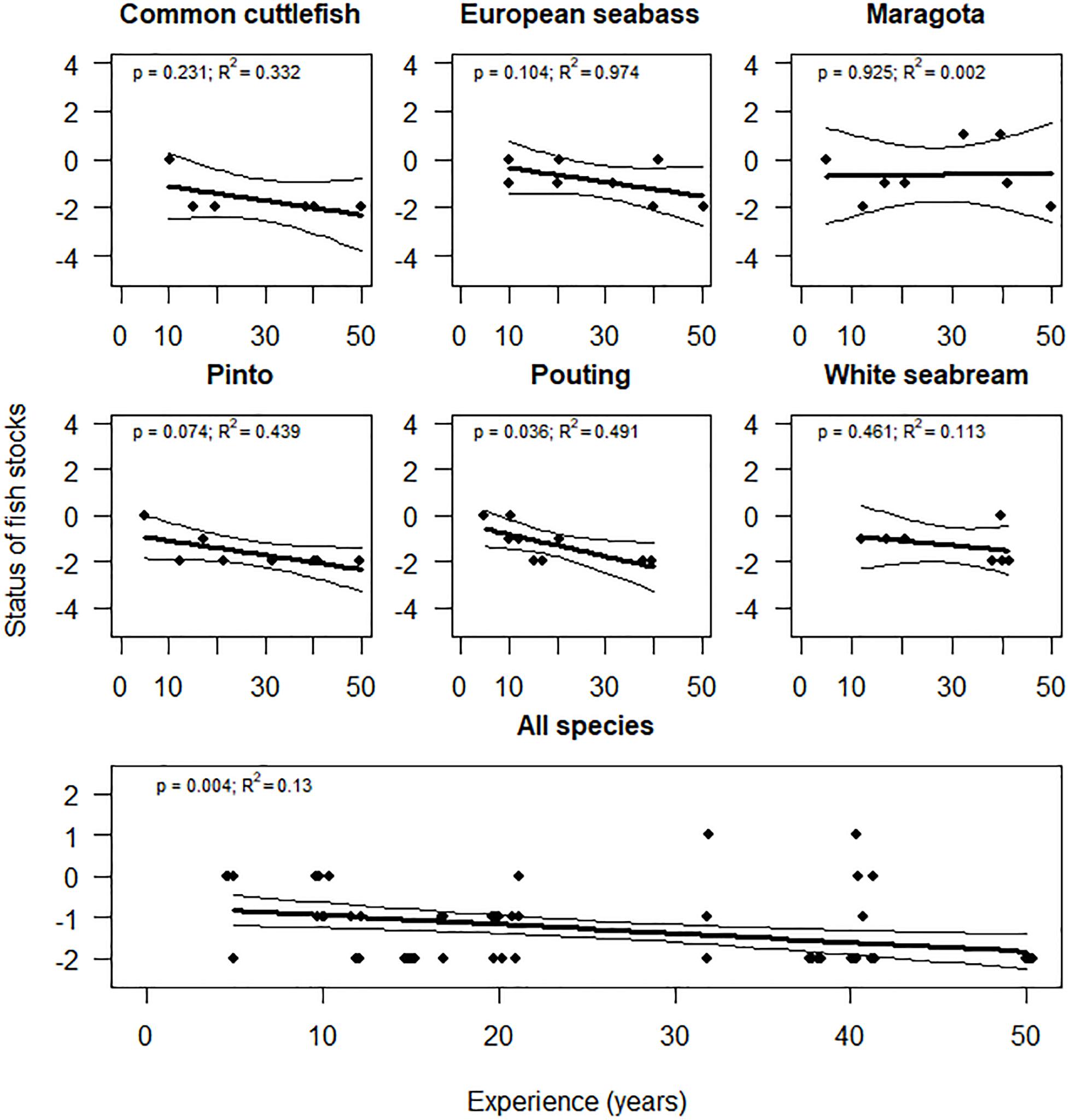
Figure 1. Effect of the experience of recreational fishers on the perceived conservation status of fish stocks (from –2 meaning very negative to 2 meaning very positive). Predictions (thick lines), 95% confidence interval (thin lines) and observations (dots) estimated by GLM are showed. P-values and goodness of fit of the model (R2) are also showed. Only frequently targeted species, identified by more than 20% of the fishers, were included in the results by species.
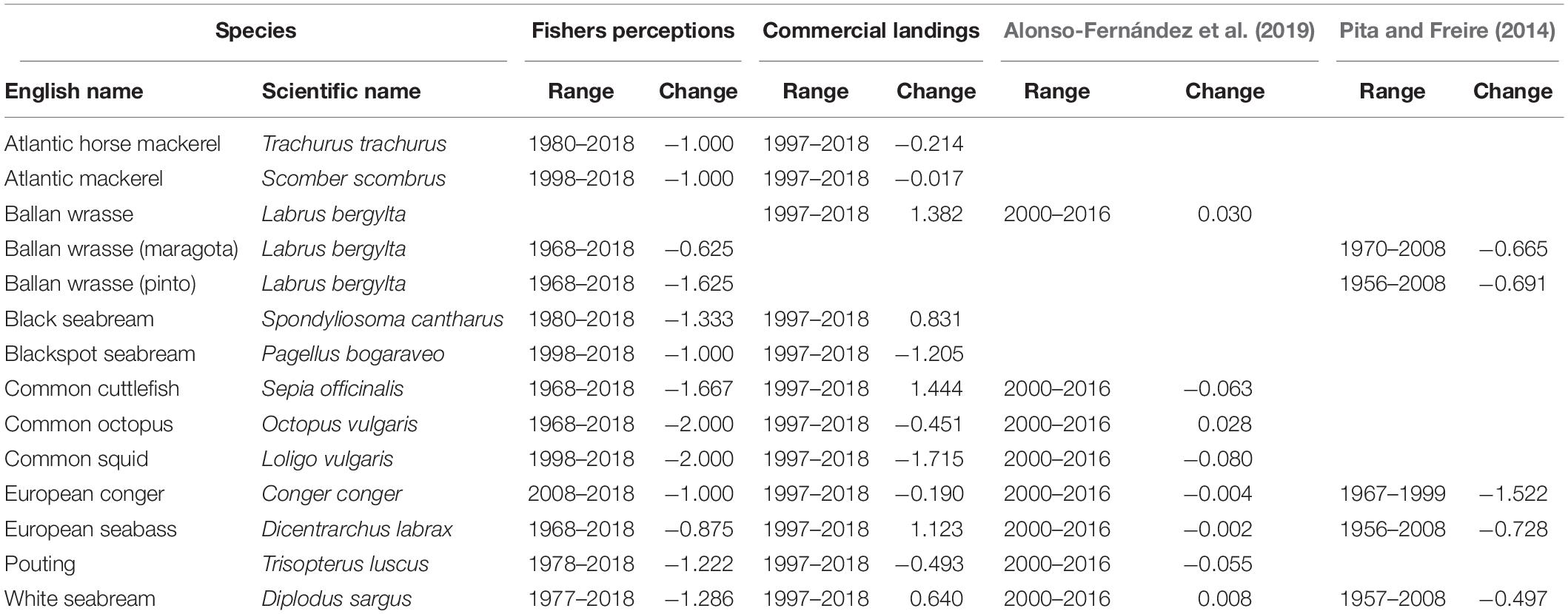
Table 2. Comparison between temporal changes in perceived conservation status of fish stocks targeted by recreational fishers (from −2 meaning very negative to 2 meaning very positive) and temporal trends for the same species in commercial landings [abundance (Alonso-Fernández et al., 2019) and size (Pita and Freire, 2014)].
For finfish, the most negative perceptions corresponded to the ballan wrasse pinto variety’s conservation status (mean −1.6 ± 0.7; Table 2). Most agreed that pinto was far less abundant and smaller today than when they started fishing. Words like “kilograms” and “weight” were used regularly and related to this negative perception (Supplementary Figure S3). One spear fisher (Participant 10) commented “You must look for them in deeper waters” and added that “in the past, pintos were often very large, and quite easily you caught pintos weighing three kilos. Nowadays pintos exceeding two kilos are an impressive catch.” Black seabream Spondyliosoma cantharus (Linnaeus, 1758) were also perceived to have shown serious temporal declines (mean −1.3 ± 1.2; Table 2). Catches were quite dependent on local conditions, varying between seasons, so “currents,” “tides,” and “year” were frequently used words (Supplementary Figure S4).
The words “big” and “past” (Supplementary Figure S10) were closely related to negative trends (mean −1.3 ± 0.8; Table 2) in the perceived status of white seabream Diplodus vulgaris (Geoffroy Saint-Hilaire, 1817). One boat angler commented that “in the past you sailed 500 meters and caught some. Nowadays you must travel a lot” (Participant 7). A severe decrease in the average size of white seabream was also explained by one spear fisher (Participant 14), who stated that “my father used to catch huge white seabreams; and when I started fishing, sometimes I caught some good ones, but they were never that big.”
Atlantic horse mackerel Trachurus trachurus (Linnaeus, 1758), Atlantic mackerel Scomber scombrus (Linnaeus, 1758), blackspot seabream Pagellus bogaraveo (Brünnich, 1768), European conger Conger conger (Linnaeus, 1758), and pouting Trisopterus luscus (Linnaeus, 1758) showed moderate negative trends (between −1.2 ± 0.8 and −1.0 ± 1.0; Table 2).
Less concern was shown about the status of the European seabass Dicentrarchus labrax (Linnaeus, 1758) and maragota (mean −0.9 ± 0.8 and −0.6 ± 1.2, respectively; Table 2). Words like “few,” “quantity,” and “year” were used by fishers raising greatest concerns about the state of the European seabass stock (Supplementary Figure S8). However, other fishers explained that this species is still seasonally abundant in the correct habitat, using words like “good,” “more,” “places,” and “same” (Supplementary Figure S8). One spear fisher commented that “they changed the place where I used to find them before. (…) You evolve in your way of fishing; you buy a boat and you can move around, and in the end, you can still find them. Maybe in deeper water, or in more difficult places, but I still catch big seabass” (Participant 1).
Maragota was the only fish that showed some positive perception about the status of the stock (14% of observations; Figure 1). One spear fisher commented that “it has conquered part of the territory of the pintos, or at least I was used to see more pintos and less maragotas” (Participant 1), while another explained “now you see big maragotas quite often. It has increased in size and quantity because it does not have the same commercial value [than pinto] and it is not targeted by any [commercial fishing] gear” (Participant 10). Frequent words like “big,” “more,” and “size” were related with these positive perceptions (Supplementary Figure S2).
Overfishing and habitat loss were reported 35.7% of the interviews (Figure 2A) and were the main perceived impacts on marine ecosystems. “Commercial,” “boats,” “week,” and “weekends” were frequently linked to the fact that commercial boats operate most days, whereas recreational fishers are limited to weekends. The higher fishing effort exerted by commercial boats was one reason used by recreational fishers to suggest that commercial fisheries had severely impacted fish stocks and ecosystems, while the perceived impact of the recreational sector was limited (Supplementary Figure S11).
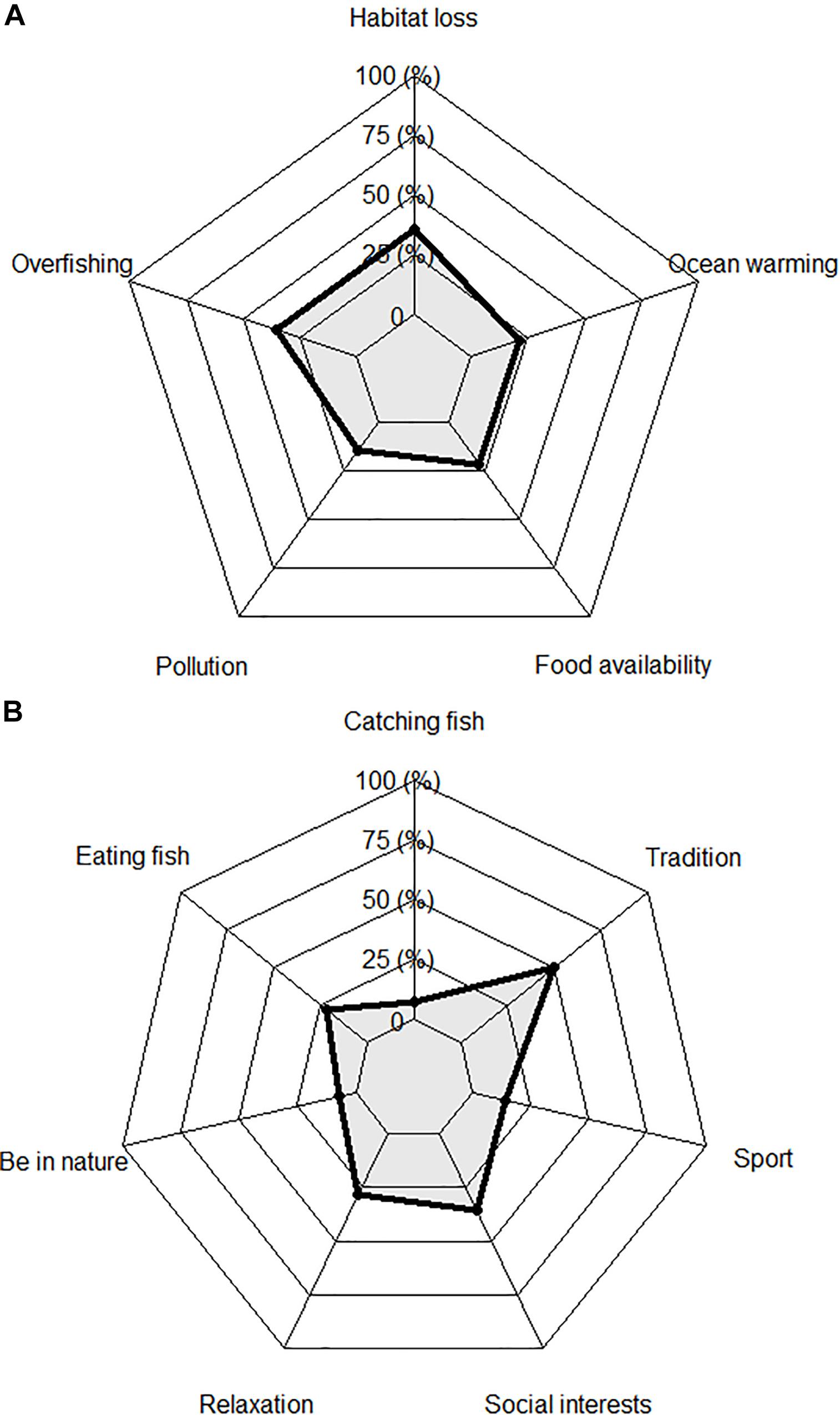
Figure 2. Impacts on ecosystems (A) and individual motivations (B) reported by recreational fishers.
Many recreational fishers complained about the negative impacts of commercial fisheries, especially trawling. For example, “There are some commercial boats trawling in shallow waters, and that kill everything” (Participant 3) and “the commercial boats are trawling close to the shores, some of them even pass the gears under the mussel rafts in the estuaries” (Participant 7). Participant 8 explained that overexploitation occurred because the continental shelf is narrow and many commercial fishing boats operate. He also highlighted technical improvements in commercial fishing had important effects on fish stocks: “before the hydraulic haulers, the nets had to be pushed onboard by hand; now they use several nets in one haul. This have killed a lot.” Illegal practices like unreported landings and lack of compliance with regulations regarding temporary and spatial closures were also identified by some fishers. “If we do not comply with the regulations, how we will know if they are effective or not?” was argued.
Many recreational fishers claimed that their own impact on fish stocks is very low, compared to commercial fishing. “One single hook does not harm the sea at all” (Participant 3), or “you go fishing on the weekends (…), and it is true that we are many, yes, but we are not the reason why the species do not come anymore” (Participant 7). However, some concerns about the impacts of the recreational sector were also shared: “there are many retired commercial [fishers] who sell illegally their catches, and they go fishing absolutely every day of the year. They disguise themselves as recreational fishers when they really are not” (Participant 6).
Severe declines in the presence of algae, especially Laminaria kelp beds, were repeatedly stressed by recreational fishers as the main habitat loss for fish. Frequent words like “aforetime,” “algae,” and “Laminaria,” alongside “less” were related to this impact (Supplementary Figure S11). Spear fishers were especially informed about this issue, with one stating “I believe that what is impacting the volume of fish and their sizes in the ecosystems most is the absence of Laminaria. Some stones were covered in the past by Laminaria and mussels, and nowadays they are clean (…), and the fishes do not stop at those stones anymore. If you go to other stones where Laminaria and mussels still grow, you have the fish there. In many areas of the coast the laminaria has disappeared” (Participant 10). Ocean warming, lack of food availability (reported each in 21.4% of the interviews) and pollution (14.3%) were also perceived as relevant impacts (Figure 2A). One of the fishers commented that the synergistic effects of these impacts could explain the lack of kelp beds, amongst other impacts on biodiversity: “This is the trend; industrial effluent, even when the pipelines discharge far away (…), and the municipalities discharges, and changes in the climate and the currents, all impact on the algae and the sand hoppers at the beaches. There can be no fish because it has neither shelter nor food” (Participant 9).
In the interviews the fishers reported relevant average improvements in fishing gears and techniques (1.21 ± 0.89). Frequent words like “bait,” “expensive,” “line,” “reel,” and “rod” were related to improvements reported by anglers (Supplementary Figure S12). One commented that “people who have more free time, that go fishing more often, and have more disposable income are the ones that try new gear” (Participant 6). In addition to a general trend toward smaller hooks and thinner lines, anglers also identified improvements in artificial baits. One experienced angler explained that “in the past I used to make my own lures with corn leaves; nowadays commercially made lures are available to target surface, medium and deep waters” (Participant 7).
Important improvements in fishing boats and onboard equipment were reported by some anglers. One boat angler commented that “today there are fast boats and you go where you want (.), in the past you had to know where you were; now we have GPS navigation systems, you push the little button and that’s it. Nowadays anyone can catch fish. Fishing is quite easy now” (Participant 7). Some anglers complained about the excessive use of technology by modern recreational anglers “I enjoy traditional fishing. I go bottom fishing with hand line, and I raise the fish with my bare hands. There are people fishing with four electric reels. In my mind you practice sport fishing because you don’t make a living from it; I believe that recreational fishing must be less artificial,” explained one of these anglers (Participant 6).
Major improvements in wetsuits, fins and spearguns were identified by the spear fishers by using words like “carbon,” “fins,” “rubber,” “speargun” or “[wet]suit” (Supplementary Figure S13). One spear fisher commented “the thermal protection offered by today’s wetsuits is well above those of twenty years ago; now you can stay five hours in the water while in the past in the first hour you were freezing” (Participant 10). “When I started fishing my wetsuit had interior lining and right now we use ‘chewing gum’ wetsuits, with no lining, and you can fish for longer at lower temperatures (…), you don’t feel wearing a wetsuit” (Participant 1). In relation to fins, one spear fisher explained that “we have gone from fishing with rubber fins to carbon fiber fins, which require much less physical effort to move in the water” (Participant 10). “In the beginning only two or three people had carbon fins and they were very difficult to obtain,” remembered another spear fisher (Participant 2). Many spearguns are now made of carbon and use bands rather than compressed gas, making them more precise and ergonomic. “When I started fishing, they were all compressed air. They made a lot of noise and tore the fish apart. Nowadays with the rubber ones with thin shafts, you hit them in the head and they die; so they are much more reliable” (Participant 14). Improvements in on-board electronic equipment was also noted (Participant 2), in terms of travel times and finding bait fish. However, one spearfisher highlighted the importance of fishing technique: “How smart you are when waiting underwater looking for the right time to make the shot is what ultimately affects your fishing” (Participant 1).
Family traditions in recreational and/or commercial fisheries was the main motivation for fishing, being reported in half of the interviews (Figure 2B). Fishers following family traditions to access the recreational fishery showed the worst perceptions of the conservation status of fish stocks (Figure 3). Paired comparisons were significatively lower than fishers motivated by eating fish (p = 0.017), relaxing (p = 0.012) and enjoying sport and physical activity (p = 0.041; Figure 3). The frequent use of “father” together with “always” and “sea” was the key concept linked to this motivation (Supplementary Figure S14). Many of the fishers explained that living close to the coastline with family and friends engaged in commercial fishing was an important driver for starting to fish recreationally. “I am from a coastal city and I am a son of a sailor, my father had a little ardora [pilchard] boat, and I sometimes helped him with the fishing gears” (Participant 7). Having a father engaged in recreational fishing was also an important motivation, with one spear fisher stating: “My father was a spear fisher and he used to go with my older brothers. Since I was five years old, if the sea was good, I went with him. (…) If your father has this hobby, and you like it, then you follow it” (Participant 14).
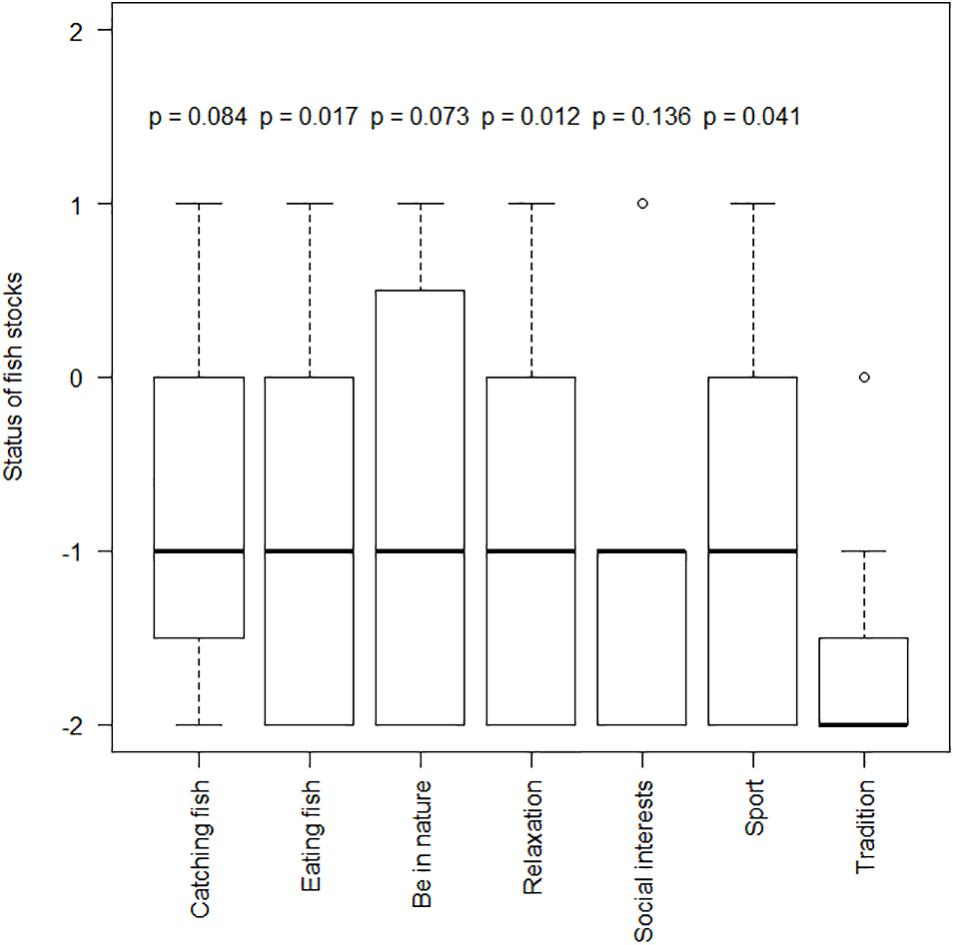
Figure 3. Conservation status of fish stocks (from –2 meaning very negative to 2 meaning very positive) perceived by recreational fishers with different motivations for fishing. The top and bottom of the boxes correspond to the first and third quartiles of the data, the whiskers extend to 1.5 times the interquartile range and the median is indicated with a thick horizontal line. P-values of the paired t-tests estimated by GLM between fishers motived by family traditions and other motivations are also shown.
Building social relationships and enjoying social recognition was another relevant motivation to access the recreational fishery (35.7% of interviews; Figure 2B). Fishers used words like “family” and “people” in the interviews to refer to this motivation (S4_Acceso). “When I arrive home or at meals with friends, this gives me a certain level, a status that I would not have otherwise, and I feel fulfilled” (Participant 1). Fishing was related with relaxation and spiritual peace in 28.6% of the interviews (Figure 2B), using words like “happy” or “hobby.” Spear fishers in particular highlighted this motivation: “Spear fishing creates an emotional and psychological balance” (Participant 2) and “the sea gives me peace out of the ordinary. I believe that it is because it isolates you from everything” (Participant 1). This motivation was also important for some anglers: “Because of my work I am on the road for long; and the sea relaxes me a lot” (Participant 6).
Fish consumption was an important motivation and was linked to frequently used words like “eat” or “fresh” (Supplementary Figure S14) and was reported in 21.4% of the interviews (Figure 2B). “I love fresh fish,” stated one angler (Participant 6). Some fishers valued the sport and the benefits derived from the physical activity (14.3% of the interviews; Figure 2B). Spear fishers were particularly inclined to use words like “capacity,” “fit,” “physical” or “sport,” when asked about their motivations (Supplementary Figure S14). “Being underwater fighting with the faculties that we have as humans is so great. (.). Spear fishing is one of the most physical demanding sports” (Participant 10).
Catching fish and enjoying contact with wild nature were the less relevant motivations for recreational fishers (reported in 7.1% of the interviews; Figure 2B). In this sense, one angler commented that the fishing activity itself gave him deep satisfaction (Participant 6) and one spear fisher explained that “you are in a strange environment, but you have the ability to learn about the habitat of each species. Each species has its own way of fishing that you learn paying attention to their behavior; when do they spawn, why they go deeper at certain periods of the year, when do they get closer to shore…, it is such a wonderful world and we still do not know so much about it” (Participant 10).
Commercial landings of many of the fish stocks targeted by recreational fishers have decreased in the last two decades (Figure 4). Differences in annual landings between the first and the last years of the available time series were negative in the case of Atlantic horse mackerel, Atlantic mackerel, blackspot seabream, common octopus, common squid, European conger and pouting (ranging from −1.7 to −0.02 on a normalized scale between −2 and 2). Commercial landings have been growing in the case of ballan wrasse, black seabream, common cuttlefish, European seabass and white seabream (ranging from 0.6 to 1.4; Table 2).
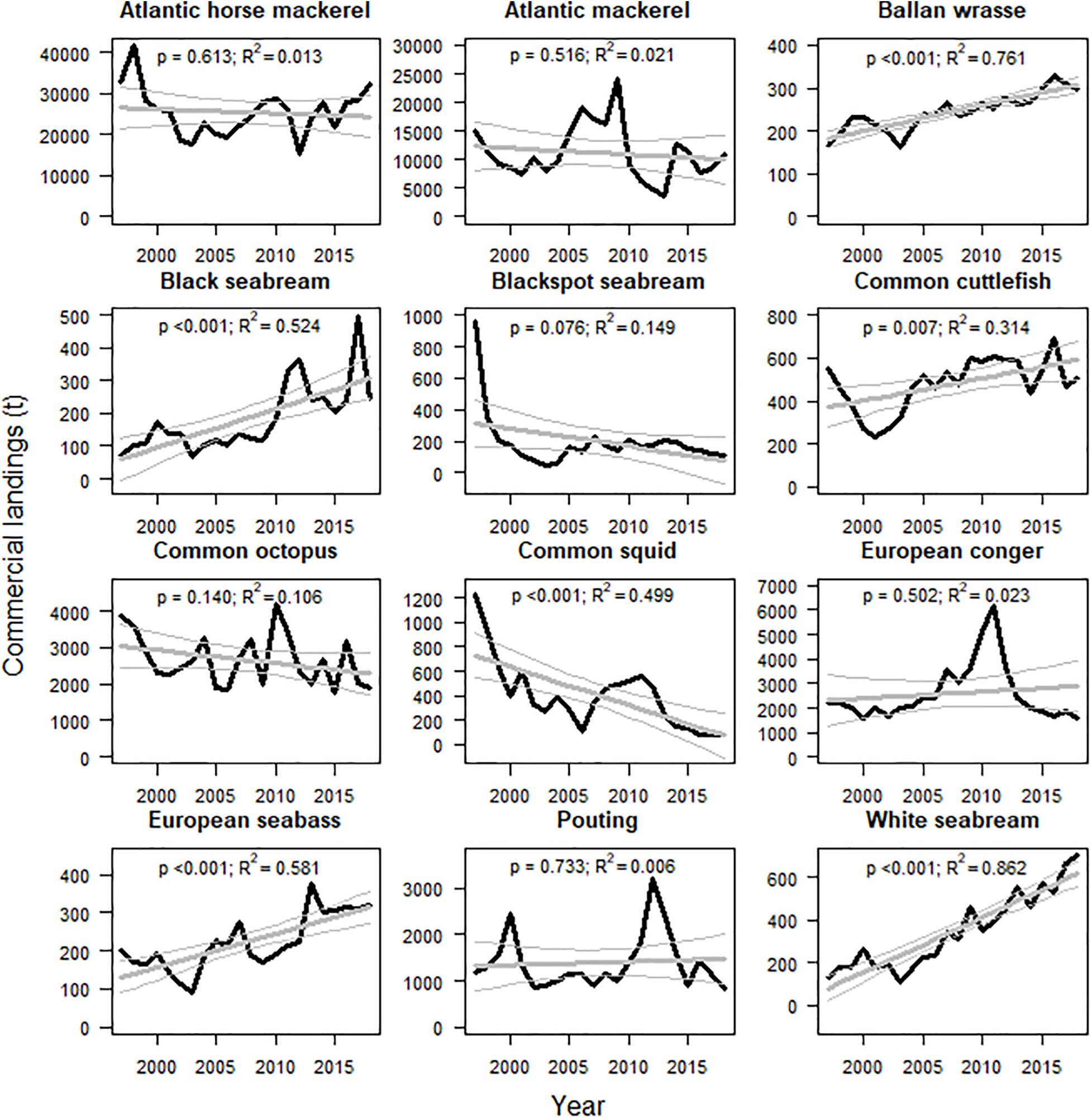
Figure 4. Temporal trends in annual commercial landings in Galician ports of main species targeted by recreational fishers (1997–2018). Predictions (thick lines) and 95% confidence interval (thin lines) of the partial effect of the year on the landings estimated by GLM are showed in gray color. P-values and goodness of fit of the models (R2) are also shown.
Pita and Freire (2014) compiled recreational fishing competitions data between 1967 and 2008. This was used to estimate relevant decreases in the sizes of European conger, European seabass, maragota, pinto and white seabream, that ranged from −1.5 to −0.5 (Table 2). Alonso-Fernández et al. (2019) used data from independent observers on board commercial vessels between 2000 and 2016 and reported little decreases in relative abundances of common cuttlefish, common squid, European conger, European seabass and pouting, ranging from −0.08 to −0.002. Abundances of ballan wrasse, common octopus and white seabream showed minor increases between 0.01 and 0.07 (Table 2).
Perceived conservation status of fish communities showed an inversely proportional relationship with the experience of the interviewed fishers in this study. This finding is similar to a growing body of literature (Sáenz-Arroyo et al., 2005; Ainsworth et al., 2008; Beaudreau and Levin, 2014) that supports empirically the “shifting baseline syndrome” (Pauly, 1995). However, this interpretation should be treated with caution as suggested by Beaudreau and Levin (2014) because fishers tend to overestimate their effort and catches with long recall periods (Hiett and Worrall, 1977; Pollock et al., 1994; Daw, 2010). Fishers were asked to look at pictures of old and recent fishing trips before the interviews to minimize the potential influence of recall bias.
Other cognitive processes can modulate people’s perceptions of natural environments, especially when facing challenging issues (Balcetis and Dunning, 2007). For example, the relatively stable perception on the conservation status of the maragota stock could have been affected by the comparisons that many fishers made with pinto’s status. Participant 1 explained that he had seen more pinto than maragota in the past and fishers perceived a marked decrease in pinto, so this could have masked the negative trend for maragota. The changes in the relative abundances of the two morphotypes found in the study is of concern and should be investigated further to ensure conservation of ballan wrasse.
There were limited time series of biological data being available to make a rigorous comparisons with trends in fish conservation status derived from interviews. However, concordance with the available temporal trends in abundances, fish sizes and commercial landings corroborates the use of FEK as an alternative source of long-term information for data-poor situations (see e.g., Zukowski et al., 2011; Beaudreau and Levin, 2014; Thurstan et al., 2015a). In this sense, progressive reduction in the conservation status of fish stocks perceived by the recreational fishers was supported by decreases up to 76% in body size of European conger, European seabass, maragota, pinto and white seabream estimated by Pita and Freire (2014) in the last 50 years (Table 2). Changes in conservation status were also similar for several fish stocks to abundance trends estimated by Alonso-Fernández et al. (2019). The smaller time series analyzed by Alonso-Fernández et al. (2019) (16 years) than that in this study (up to 50 years) could explain the lower decreases that they found, and even the small increases shown for ballan wrasse, common octopus and white seabream (Table 2). Furthermore, fishery-dependent data derived from onboard programs could be hyper-stable, because many factors influence to levels of landing beyond simply the status of the stock (Maunder et al., 2006; Kleiber and Maunder, 2008). For instance, skippers acquire better materials and new skills as their fishing experience increases, changing their behavior (e.g., opening new fishing grounds and abandoning unproductive or unprofitable ones) (Hilborn et al., 1995; Walters, 2003; Ahrens and Walters, 2005).
In a similar way, some of the decreases in the conservation status of fish stocks showed in this study could also be hyper-stable, masking negative trends. As explained by the fishers in the interviews (e.g., participants 1, 7, and 10) and in other studies (e.g., Olson and Cunningham, 1989), fishers are continuously searching for new fishing places, further from the coast and/or deeper water, evolving to adapt to eventual lower abundances to keep their catches stable. Furthermore, improvements in fishing equipment in recent years (see e.g., Young et al., 2015) highlighted in the interviews could also have increased the fishery catchability, masking perceived trends in conservation status. However, as suggested by Pita and Freire (2014), although the changes in conservation status shown in this study might not be fully accurate, these estimates are valuable conservative proxies of long-term alterations in the fish stocks.
The improvements in the monitoring, reporting, enforcement and control of commercial fisheries in the last decade could have also hyper-stabilize some trends in commercial landings (Villasante et al., 2016). In spite of a reduction in the fisheries effort (Alonso-Fernández et al., 2019; Pita et al., 2019), technical operational improvements (e.g., hydraulic haulers) could have also have an impact on commercial landings. Nevertheless, trends in commercial catches are in general consistent with recreational fishers’ perceptions about declines in stocks. Commercial fisheries should be showing signs of depletion for some of the stocks observed here. With the exception of Atlantic horse mackerel (ICES, 2018c), this view is supported by the need for conservation measures in the relevant areas for species under European catch management [i.e., Atlantic mackerel (ICES, 2019) and blackspot seabream (ICES, 2018a)]. This assessment highlights the need for assessment and management of a broader set of stocks in Galicia at European level. European seabass has been assessed in Biscay and biomass has been declining (ICES, 2018b). The growing market demand for species like ballan wrasse, common cuttlefish, European seabass and white seabream could also increase targeting by commercial fishers despite declines in their populations. As a result, it is vital to obtain detailed and reliable monitoring of the abundance and catches of all the species that are targeted by recreational or commercial fishing to ensure sustainable exploitation of the stocks.
One of the challenges of using FEK to assess long-term changes in the conservation status of ecosystems has been an inability to integrate fishers’ perceptions on trends of fish stocks with environmental impacts. Perceptions of the impacts driving changes in ecosystems differ both within and between user groups (Eden and Bear, 2011a). For example, recreational anglers are the main resource users in European freshwater ecosystems (Arlinghaus et al., 2019a), where inland commercial fisheries are virtually absent (Boisneau et al., 2016). Freshwater anglers perceive that healthy ecosystems include human interventions like stocking as acceptable or even desirable practices (Eden and Bear, 2012). Conversely in marine ecosystems, recreational fishers can be less powerful than the commercial sector and the potential interventions are very limited. Therefore, their perceptions of impacts on ecosystems tend to be similar to scientists and fisheries managers (Beaudreau and Levin, 2014). It is therefore not surprising that fishers interviewed in this study identified overfishing and habitat loss as the main threats to marine ecosystems, in concordance with mainstream science (Jackson et al., 2001). Although most fishers stressed that commercial fisheries are primarily responsible for overfishing, other (e.g., Participant 6) also recognized their role and responsibility (Hyder et al., 2018; Pita et al., 2018b; Radford et al., 2018).
Fishers born in coastal populations, following family traditions of commercial and/or recreational fisheries evidenced higher sensitivity toward changes in ecosystems than other groups of fishers. These “traditional fishers” are more aware of the impacts that can alter their traditional way of life because they play a role in the socioecological legacy of the community. Therefore, it is recommended that future studies of fish abundance using FEK also analyze impacts on ecosystems using inputs from different resource users, including those more prone to protect natural and cultural community heritage. Identifying these “traditional fishers” is important because social and emotional components of access motivations evidenced by Galician fishers seem more relevant than that shown in other regions, where reconnection with natural environments (Walsh et al., 1989; Snyder, 2007; Eden and Bear, 2011b) and fishing for food are stronger motivations for recreational fisheries (Cooke et al., 2017).
Ocean warming, biodiversity loss and pollution are global threats to ocean conservation that fishers also identified (Jackson, 2008). However, it is important to highlight that fishers identified the decrease in kelp beds as one of the major impacts on marine ecosystems. Kelp beds are structural and ecological complex habitats (Teagle et al., 2017) distributed in worldwide cold and temperate waters (Dayton, 1985). These ecosystems provide shelter and food for many marine animals, increase primary production and genetic diversity, regulate climate by capturing and storing carbon, recycle essential nutrients, keep water quality and protect against storms (Steneck et al., 2002; Smale et al., 2013). Kelp beds are therefore important sources of commercial, recreational and cultural resources (Harvey et al., 2001; Bennett et al., 2016). However, they are highly vulnerable to direct human impacts (Pérez-Matus et al., 2017) and global warming (Wernberg et al., 2010; Harley et al., 2012; Provost et al., 2017). Kelps create key habitats in temperate rocky reefs around the world, including Galicia (Pita et al., 2018a). Kelps are especially vulnerable to impacts in this region because it is located at their southern distribution limit (Flores-Moya, 2012; Tuya et al., 2012; Voerman et al., 2013). Participant 9 explained that ocean warming and land-based pollution are the main drivers of kelp reduction. This was consistent with the progressive weakening of the coastal upwelling in Galicia (Bode et al., 2009) that introduces the cold water and nutrients needed for critical life stages of kelps (Alvarez et al., 2012), and with studies that link wastewater treatment plants with impacts on kelp beds (Connell et al., 2008). Fishers’ perceptions on the negative trends of kelps are a first wake-up call about the important consequences of a reduction of kelp beds on the sustainability of coastal ecosystems and on the services provided by them (Krumhansl et al., 2016). Ballan wrasse is a key species in kelp beds because it controls sea urchin populations (Pita and Freire, 2017) preventing regime shifts due to overgrazing (Ling et al., 2015). Thus, decreases in the abundances of ballan wrasse showed in this study, together with the increase in the direct exploitation of kelps by humans (Pita et al., 2019), could cause abrupt changes in coastal ecosystems. Careful assessment of the conservation state of ballan wrasse and kelp exploitation is recommended to develop adaptive, responsible management of coastal ecosystems.
The results of this study are limited by the sample size and range of demographics, in this case middle-aged fishers. Furthermore, experienced fishers that are highly engaged in the recreational fishery have been recruited. Thus, this study would not capture potentially lower levels of awareness of young and/or less avid fishers of the state of fish stocks and ecosystems. However, this does not detract from the outcomes of this study, since it is not intended to generalize these results to the entire population of recreational fishers. Moreover, the number of new concepts and/or results associated with each additional interview tends to diminish after a relatively small number of interviews (Morgan, 2002; Tashakkori and Teddlie, 2010; Green and Thorogood, 2013; Ritchie et al., 2013). In this study, theoretical saturation was reached in the identification of main target species and in the groupings of key changes in marine ecosystems and access motivations (Supplementary Figure S15). In addition, the findings of this study are consistent with available biological data and with similar studies using FEK in other regions. Therefore, despite direct regulatory guidance derived from this study being limited, the validity of recreational FEK to assess changes on the conservation status of fish stocks and ecosystems in data-poor situations has been demonstrated, offering a potential methodology to be further developed in the future.
The perceptions of experienced fishers generated key information on long-term ecological impacts that will support conservation of ecosystems and reduce the impact of the “shifting baseline syndrome” (Pauly, 1995). Fishers following family traditions in commercial and/or recreational fisheries were identified as key informants to detect impacts on natural and cultural community heritage, so must be included in FEK studies. Most fish stocks in this study were perceived to be declining that was driven by overfishing, habitat and biodiversity losses, pollution and ocean warming. The poor status of ballan wrasse and kelp beds was identified, which was of concern due to representing key species in coastal ecosystems. Use of FEK is a good approach to develop knowledge of these systems, but broader monitoring programs are needed to protect the future of these ecosystems.
The datasets generated for this study will not be made publicly available. They include personal information.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
PP performed the interviews and analyzed the data. PP, MA, KH, and SV reviewed the data and wrote the manuscript. JV participated in the design of the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was funded by the Xunta de Galicia (RECREGES I and II projects under Grants ED481B2014/034-0 and ED481B2018/017), and Fundación Biodiversidad, Ministerio para la Transición Ecológica, Gobierno de España (SICORE project). SV acknowledges the financial support of the European COST Action “Ocean Governance for Sustainability—challenges, options and the role of science,” the ICES Science Fund Project “Social Transformations of Marine Social-Ecological Systems,” and the CYTED program for the ECOMAR Network.
We appreciate the involvement of the recreational fishers who voluntarily shared their knowledge during this study. We dedicate this article to the memory of JV who recently passed away.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00242/full#supplementary-material
Agrawal, A. (1995). Dismantling the divide between indigenous and scientific knowledge. Dev. Change 26, 413–439. doi: 10.1111/j.1467-7660.1995.tb00560.x
Ahrens, R., and Walters, C. (2005). “Why are there still large pelagic predators in the oceans? Evidence of severe hyper-depletion in longline catch-per-effort,” in Proceedings of the 1st Meeting of the Scientific Committee of the Western and Central Pacific Fisheries Commission, (Noumea: Western and Central Pacific Fisheries Commission).
Ainsworth, C. H., Pitcher, T. J., and Rotinsulu, C. (2008). Evidence of fishery depletions and shifting cognitive baselines in Eastern Indonesia. Biol. Conserv. 141, 848–859. doi: 10.1016/j.biocon.2008.01.006
Akaike, H. (1973). “Information theory and an extension of the maximum likelihood principle,” in Second International Symposium of Information Theory, eds B. N. Petrov and F. Csaki (Tsahkadsor: Akademiai Kiado), 267–281.
Almada, F., Casas, L., Francisco, S. M., Villegas-Ríos, D., Saborido-Rey, F., Irigoien, X., et al. (2016). On the absence of genetic differentiation between morphotypes of the ballan wrasse Labrus bergylta (Labridae). Mar. Biol. 163, 1–6. doi: 10.1007/s00227-016-2860-8
Alonso-Fernández, A., Otero, J., Bañón, R., Campelos, J. M., Quintero, F., Ribó, J., et al. (2019). Inferring abundance trends of key species from a highly developed small-scale fishery off NE Atlantic. Fish. Res. 209, 101–116. doi: 10.1016/j.fishres.2018.09.011
Alvarez, I., Prego, R., Castro, M., and De Varela, M. (2012). Galicia upwelling revisited: out-of-season events in the rias (1967–2009). Ciencias Mar. 38, 143–159.
Arlinghaus, R., Abbott, J. K., Fenichel, E. P., Carpenter, S. R., Hunt, L. M., Alós, J., et al. (2019a). Opinion: governing the recreational dimension of global fisheries. Proc. Natl. Acad. Sci. U.S.A. 116, 5209–5213. doi: 10.1073/pnas.1902796116
Arlinghaus, R., Beardmore, B., Riepe, C., and Pagel, T. (2019b). Species-specific preference heterogeneity in German freshwater anglers, with implications for management. J. Outdoor Recreat. Tour. (in press).
Azzurro, E., Moschella, P., and Maynou, F. (2011). Tracking signals of change in mediterranean fish diversity based on local ecological knowledge. PLoS One 6:e24885. doi: 10.1371/journal.pone.0024885
Balcetis, E., and Dunning, D. (2007). Cognitive dissonance and the perception of natural environments. Psychol. Sci. 18, 917–921. doi: 10.1111/j.1467-9280.2007.02000.x
Beaudreau, A. H., and Levin, P. S. (2014). Advancing the use of local ecological knowledge for assessing data-poor species in coastal ecosystems. Ecol. Appl. 24, 244–256. doi: 10.1890/13-0817.1
Bennett, S., Wernberg, T., Connell, S. D., Hobday, A. J., Johnson, C. R., and Poloczanska, E. S. (2016). The’Great Southern Reef’: social, ecological and economic value of Australia’s neglected kelp forests. Mar. Freshw. Res. 67, 47–56.
Bode, A., Alvarez-Ossorio, M. T., Cabanas, J. M., Miranda, A., and Varela, M. (2009). Recent trends in plankton and upwelling intensity off Galicia (NW Spain). Prog. Oceanogr. 83, 342–350. doi: 10.1016/j.pocean.2009.07.025
Boisneau, P., Stolzenberg, N., Prouzet, P., and Moreau, D. (2016). “How to Transmit Information and Maintain Knowledge in the Context of Global Change for French Inland Commercial Fishers,” in Freshwater, Fish and the Future: Proceedings of the global cross-sectoral conference, eds W. W. Taylor, D. M. Bartley, C. I. Goddard, N. J. Leonard, and R. Welcomme (Bethesda: Food and Agriculture Organization of the United Nations), 289–300.
Brewin, R. J. W., Hyder, K., Andersson, A. J., Billson, O., Bresnahan, P. J., Brewin, T. G., et al. (2017). Expanding aquatic observations through recreation. Front. Mar. Sci. 4:351. doi: 10.3389/fmars.2017.00351
Colwell, R. K., and Coddington, J. A. (1994). Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 345, 101–118. doi: 10.1098/rstb.1994.0091
Connell, S. D., Russell, B. D., Turner, D. J., Shepherd, S. A., Kildea, T., Miller, D., et al. (2008). Recovering a lost baseline: missing kelp forests from a metropolitan coast. Mar. Ecol. Prog. Ser. 360, 63–72. doi: 10.3354/meps07526
Cooke, S. J., Twardek, W. M., Lennox, R. J., Zolderdo, A. J., Bower, S. D., Gutowsky, L. F. G., et al. (2017). The nexus of fun and nutrition: recreational fishing is also about food. Fish Fish. 19, 201–224. doi: 10.1111/faf.12246
Cortés-Jiménez, I. (2008). Which type of tourism matters to the regional economic growth? The cases of Spain and Italy. Int. J. Tour. Res. 10, 127–139. doi: 10.1002/jtr.646
Davis, A., and Wagner, J. R. (2003). Who knows? On the importance of identifying “experts” when researching local ecological knowledge. Hum. Ecol. 31, 463–489.
Daw, T. (2010). Shifting baselines and memory illusions: what should we worry about when inferring trends from resource user interviews? Anim. Conserv. 13, 534–535. doi: 10.1111/j.1469-1795.2010.00418.x
Dayton, P. K. (1985). Ecology of kelp communities. Annu. Rev. Ecol. Syst. 16, 215–245. doi: 10.1146/annurev.es.16.110185.001243
Eden, S., and Bear, C. (2011a). Models of equilibrium, natural agency and environmental change: lay ecologies in UK recreational angling. Trans. Inst. Br. Geogr. 36, 393–407. doi: 10.1111/j.1475-5661.2011.00438.x
Eden, S., and Bear, C. (2011b). Reading the river through ‘watercraft’: environmental engagement through knowledge and practice in freshwater angling. Cult. Geogr. 18, 297–314. doi: 10.1177/1474474010384913
Eden, S., and Bear, C. (2012). The good, the bad, and the hands-on: constructs of public participation, anglers, and lay management of water environments. Environ. Plan. A 44, 1200–1218. doi: 10.1068/a4495
Edmondson, M. (2018). googleLanguageR: Call Google’s ‘Natural Language’ API, ‘Cloud Translation’ API, ‘Cloud Speech’ API and ‘Cloud Text-to-Speech’ API. R Package Version 0.2.0. Available online at: https://CRAN.R-project.org/package=googleLanguageR (accessed June 21, 2018).
Flores-Moya, A. (2012). “Warm temperate seaweed communities: a case study of deep water kelp forests from the alboran sea (SW Mediterranean Sea) and the Strait of Gibraltar,” in Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization, eds C. Wiencke and K. Bischof (Cham: Springer-Verlag), 315–327. doi: 10.1007/978-3-642-28451-9_15
Freire, J., and García-Allut, A. (2000). Socioeconomic and biological causes of management failures in European artisanal fisheries: the case of Galicia (NW Spain). Mar. Policy 24, 375–384. doi: 10.1016/s0308-597x(00)00013-0
Giovos, I., Keramidas, I., Antoniou, C., Deidun, A., Font, T., Kleitou, P., et al. (2018). Identifying recreational fisheries in the Mediterranean Sea through social media. Fish. Manag. Ecol. 25, 287–295. doi: 10.1111/fme.12293
Goldman, M., and Schurman, R. A. (2000). Closing the “great divide”: new social theory on society and nature. Annu. Rev. Sociol. 26, 563–584. doi: 10.1146/annurev.soc.26.1.563
Gotelli, N. J., and Colwell, R. K. (2001). Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 4, 379–391. doi: 10.1046/j.1461-0248.2001.00230.x
Gourguet, S., Briand, F., Marçalo, A., Ünal, V., Liu, Y., Kaiser, B., et al. (2018). “Engaging marine scientists and fishers to share knowledge and perceptions – An overview,” in CIESM Monograph 50. Engaging marine scientists and fishers to share knowledge and perceptions-Early lessons, ed. F. Briand (Monaco: CIESM Publisher), 5–27.
Green, J., and Thorogood, N. (2013). Qualitative Methods for Health Research. 2nd Edn. Thousand Oaks, CA: Sage.
Halpern, B. S., Walbridge, S., Selkoe, K. A., Kappel, C. V., Micheli, F., D’Agrosa, C., et al. (2008). A global map of human impact on marine ecosystems. Science 319, 948–952.
Hansen, K. D., Gentry, J., Long, L., Gentleman, R., Falcon, S., Hahne, F., et al. (2019). Rgraphviz: Provides Plotting Capabilities for R Graph Objects. R Package Version 2.30.0. Available online at: https://bioconductor.org/biocLite.R
Harley, C. D. G., Anderson, K. M., Demes, K. W., Jorve, J. P., Kordas, R. L., Coyle, T. A., et al. (2012). Effects of climate change on global seaweed communities. J. Phycol. 48, 1064–1078.
Harvey, E., Fletcher, D., and Shortis, M. (2001). A comparison of the precision and accuracy of estimates of reef-fish lengths determined visually by divers with estimates produced by a stereo-video system. Fish. Bull. 99, 63–71.
Hiett, R. L., and Worrall, J. W. (1977). Marine Recreational Fishermen’s Ability to Estimate Catch and to Recall Catch and Effort Over Time. Pretoria: Human Sciences Research.
Hilborn, R., Walters, C. J., and Ludwig, D. (1995). Sustainable exploitation of renewable resources. Annu. Rev. Ecol. Syst. 26, 45–67.
Hind, E. J. (2014). A review of the past, the present, and the future of fishers’ knowledge research: a challenge to established fisheries science. ICES J. Mar. Sci. 72, 341–358. doi: 10.1093/icesjms/fsu169
Hornik, K., and Grün, B. (2011). topicmodels: an R package for fitting topic models. J. Stat. Softw. 40, 1–30.
Huntington, H. P. (2000). Using traditional ecological knowledge in science: methods and applications. Ecol. Appl. 10, 1270–1274. doi: 10.1890/1051-0761(2000)010
Hyder, K., Weltersbach, M. S., Armstrong, M., Ferter, K., Townhill, B., Ahvonen, A., et al. (2018). Recreational sea fishing in Europe – Participation rates, fishing effort and expenditure in a global context. Fish Fish. 19, 225–243.
ICES (2018a). ICES Advice on Fishing Opportunities, Catch, and Effort Bay of Biscay and the Iberian Coast Ecoregion: Blackspot Sea Bream (Pagellus bogaraveo) in Subarea 9 (Atlantic Iberian Waters). New York, NY: ICES.
ICES (2018b). ICES Advice on Fishing Opportunities, Catch, and Effort Bay of Biscay and the Iberian Coast Ecoregion: Seabass (Dicentrarchus labrax) in Divisions 8.a–b (Northern and Central Bay of Biscay). New York, NY: ICES.
ICES (2018c). Report of the Working Group on Southern Horse Mackerel, Anchovy and Sardine (WGHANSA). Lisbon. New York, NY: ICES.
ICES (2019). Norway Special Request for Revised 2019 Advice on Mackerel (Scomber scombrus) in Subareas 1–8 and 14, and in Division 9.a (the Northeast Atlantic and Adjacent Waters). New York, NY: ICES.
Jackson, J. B. C. (2008). Ecological extinction and evolution in the brave new ocean. Proc. Natl. Acad. Sci. U.S.A. 105, 11458–11465. doi: 10.1073/pnas.0802812105
Jackson, J. B. C., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., et al. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637. doi: 10.1126/science.1059199
Kleiber, P., and Maunder, M. N. (2008). Inherent bias in using aggregate CPUE to characterize abundance of fish species assemblages. Fish. Res. 93, 140–145. doi: 10.1016/j.fishres.2008.03.013
Krumhansl, K. A., Okamoto, D. K., Rassweiler, A., Novak, M., Bolton, J. J., Cavanaugh, K. C., et al. (2016). Global patterns of kelp forest change over the past half-century. Proc. Natl. Acad. Sci. U.S.A. 113, 13785–13790.
Ling, S. D., Scheibling, R. E., Rassweiler, A., Johnson, C. R., Shears, N., Connell, S. D., et al. (2015). Global regime shift dynamics of catastrophic sea urchin overgrazing. Phil. Trans. R. Soc. B 370:20130269. doi: 10.1098/rstb.2013.0269
Lloret, J., Zaragoza, N., Caballero, D., and Riera, V. (2008). Biological and socioeconomic implications of recreational boat fishing for the management of fishery resources in the marine reserve of Cap de Creus (NW Mediterranean). Fish. Res. 91, 252–259. doi: 10.1016/j.fishres.2007.12.002
Lockwood, M., Davidson, J., Hockings, M., Haward, M., and Kriwoken, L. (2012). Marine biodiversity conservation governance and management: regime requirements for global environmental change. Ocean Coast. Manag. 69, 160–172. doi: 10.1016/j.ocecoaman.2012.07.015
Longino, J. T., and Colwell, R. K. (1997). Biodiversity assesment using structured inventory: capturing the ant fauna of a tropical rain forest. Ecol. Appl. 7, 1263–1277. doi: 10.1890/1051-0761(1997)007
Maunder, M. N., Sibert, J. R., Fonteneau, A., Hampton, J., Kleiber, P., and Harley, S. J. (2006). Interpreting catch per unit effort data to assess the status of individual stocks and communities. ICES J. Mar. Sci. 63, 1373–1385. doi: 10.1016/j.icesjms.2006.05.008
Maurstad, A. (2002). Fishing in murky waters—ethics and politics of research on fisher knowledge. Mar. Policy 26, 159–166. doi: 10.1016/S0308-597X(01)00045-8
Meyer, D., Hornik, K., and Feinerer, I. (2008). Text mining infrastructure in R. J. Stat. Softw. 25, 1–54.
Morgan, M. G. (2002). Risk Communication: A Mental Models Approach. Cambridge, MA: Cambridge University Press.
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2019). vegan: Community Ecology Package. R Package Version 2.5-6. Available online at: https://CRAN.R-project.org/package=vegan (accessed September 1, 2019).
Olson, D. E., and Cunningham, P. K. (1989). Sport-fisheries trends shown by an annual Minnesota fishing contest over a 58-year period. North Am. J. Fish. Manag. 9, 287–297. doi: 10.1577/1548-8675(1989)009<0287:sftsba>2.3.co;2
Opdenakker, R. (2006). Advantages and disadvantages of four interview techniques in qualitative research. Forum Qualitative Sozialforschung 4:11.
Palmer, M. W. (1990). The estimation of species richness by extrapolation. Ecology 71, 1195–1198. doi: 10.2307/1937387
Pauly, D. (1995). Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 10:430. doi: 10.1016/s0169-5347(00)89171-5
Pérez-Camacho, A., González, R., and Fuentes, J. (1991). Mussel culture in Galicia (NW Spain). Aquaculture 94, 263–278. doi: 10.1016/0044-8486(91)90122-n
Pérez-Matus, A., Ospina-Alvarez, A., Camus, P. A., Carrasco, S. A., Fernandez, M., Gelcich, S., et al. (2017). Temperate rocky subtidal reef community reveals human impacts across the entire food web. Mar. Ecol. Prog. Ser. 567, 1–16. doi: 10.3354/meps12057
Pita, P., Fernández-Márquez, D., Antelo, M., Macho, G., and Villasante, S. (2019). Socioecological changes in data-poor S-fisheries: a hidden shellfisheries crisis in Galicia (NW Spain). Mar. Policy 101, 208–224. doi: 10.1016/j.marpol.2018.09.018
Pita, P., Fernández-Márquez, D., and Freire, J. (2018a). Spatiotemporal variation in the structure of reef fish and macroalgal assemblages in a north-east Atlantic kelp forest ecosystem: implications for the management of temperate rocky reefs. Mar. Freshw. Res. 69, 525–541. doi: 10.1071/MF17193
Pita, P., and Freire, J. (2014). The use of spearfishing competition data in fisheries management: evidence for a hidden near collapse of a coastal fish community of Galicia (NE Atlantic Ocean). Fish. Manag. Ecol. 21, 454–469. doi: 10.1111/fme.12095
Pita, P., and Freire, J. (2016). Assessing the impact of spear fishing by using competitions records and underwater visual census. Sci. Mar. 80, 27–38. doi: 10.3989/scimar.04352.15A
Pita, P., and Freire, J. (2017). Trophic ecology of an Atlantic kelp forest fish assemblage targeted by recreational fishers: implications for coastal management. J. Mar. Biol. Assoc. United Kingdom 99, 19–29. doi: 10.1017/S0025315417001862
Pita, P., Hyder, K., Gomes, P., Pita, C., Rangel, M., Veiga, P., et al. (2018b). Economic, social and ecological attributes of marine recreational fisheries in Galicia, Spain. Fish. Res. 208, 58–69. doi: 10.1016/j.fishres.2018.07.014
Pita, P., and Villasante, S. (2019). The building of a management system for marine recreational fisheries in Galicia (NW Spain). Ocean Coast. Manag. 169, 191–200. doi: 10.1016/j.ocecoaman.2018.12.027
Pitcher, T. J. (2001). Fisheries Managed to Rebuild Ecosystems? Reconstructing the Past to Salvage the Future. Ecol. Appl. 11, 601–617. doi: 10.1890/1051-0761(2001)011
Pollock, K. H., Jones, C. M., and Brown, T. L. (1994). Angler survey methods and their application in fisheries management. Rev. Fish Biol. Fish. 5, 378–380.
Provost, E. J., Kelaher, B. P., Dworjanyn, S. A., Russell, B. D., Connell, S. D., Ghedini, G., et al. (2017). Climate-driven disparities among ecological interactions threaten kelp forest persistence. Glob. Chang. Biol. 23, 353–361. doi: 10.1111/gcb.13414
Quintela, M., Danielsen, E. A., López, L., Barreiro, R., Svåsand, T., Knutsen, H., et al. (2016). Is the ballan wrasse, Labrus bergylta, two species? Genetic analysis reveals within-species divergence associated with plain and spotted morphotype frequencies. Integr. Zool. 11, 85–173.
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Radford, Z., Hyder, K., Zarauz, L., Mugerza, E., Ferter, K., Prellezo, R., et al. (2018). The impact of marine recreational fishing on key fish stocks in European waters. PLoS One 13:e0201666. doi: 10.1371/journal.pone.0201666
Ritchie, J., Lewis, J., and Elam, G. (2013). “Designing and selecting samples,” in Qualitative Research Practice: A Guide for Social Science Students and Researchers, eds J. Ritchie and J. Lewis (Thousand Oaks, CA: Sage), 77–108.
Sáenz-Arroyo, A., Roberts, C. M., Torre, J., and Cariño-Olvera, M. (2005). Using fishers’ anecdotes, naturalists’ observations and grey literature to reassess marine species at risk: the case of the Gulf grouper in the Gulf of California, Mexico. Fish Fish. 6, 121–133. doi: 10.1111/j.1467-2979.2005.00185.x
Sbragaglia, V., Cerri, J., Bolognini, L., Dragiæeviæ, B., Dulæiæ, J., Grati, F., et al. (2020). Local ecological knowledge of recreational fishers reveals different meridionalization dynamics of two Mediterranean subregions. Mar. Ecol. Prog. Ser. 634, 147–157. doi: 10.3354/meps13193
Smale, D. A., Burrows, M. T., Moore, P., O’Connor, N., and Hawkins, S. J. (2013). Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol. Evol. 3, 4016–4038. doi: 10.1002/ece3.774
Snyder, S. (2007). New streams of religion: fly fishing as a lived, religion of nature. J. Am. Acad. Relig. 75, 896–922. doi: 10.1093/jaarel/lfm063
Soberón, J. M., and Llorente, J. B. (1993). The use of species accumulation functions for the prediction of species richness. Conserv. Biol. 7, 480–488. doi: 10.1046/j.1523-1739.1993.07030480.x
STECF (2018). The 2018 Annual Economic Report on the EU Fishing Fleet (STECF 18-07). Luxembourg: STECF.
Steneck, R. S., Graham, M. H., Bourque, B. J., Corbett, D., Erlandson, J. M., Estes, J. A., et al. (2002). Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ. Conserv. 29, 436–459.
Stephenson, R. L., Paul, S., Pastoors, M. A., Kraan, M., Holm, P., Wiber, M., et al. (2016). Integrating fishers’ knowledge research in science and management. ICES J. Mar. Sci. 73, 1459–1465. doi: 10.1093/icesjms/fsw025
Suárez de Vivero, J. L., and Rodríguez Mateos, J. C. (2012). The Spanish approach to marine spatial planning. Marine strategy framework directive vs. EU integrated maritime policy. Mar. Policy 36, 18–27. doi: 10.1016/j.marpol.2011.03.002
Surís-Regueiro, J. C., and Santiago, J. L. (2014). Characterization of fisheries dependence in Galicia (Spain). Mar. Policy 47, 99–109. doi: 10.1016/j.marpol.2014.02.006
Tashakkori, A., and Teddlie, C. (2010). Sage Handbook of Mixed Methods in Social & Behavioral RESEARCH. London: Sage.
Teagle, H., Hawkins, S. J., Moore, P. J., and Smale, D. A. (2017). The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J. Exp. Mar. Bio. Ecol. 492, 81–98. doi: 10.1016/j.jembe.2017.01.017
Thurstan, R. H., Buckley, S. M., Ortiz, J. C., and Pandolfi, J. M. (2015a). Setting the record straight: assessing the reliability of retrospective accounts of change. Conserv. Lett. 9, 98–105.
Thurstan, R. H., McClenachan, L., Crowder, L. B., Drew, J. A., Kittinger, J. N., Levin, P. S., et al. (2015b). Filling historical data gaps to foster solutions in marine conservation. Ocean Coast. Manag. 115, 31–40.
Tiralongo, F., Lillo, A. O., Tibullo, D., Tondo, E., Martire, C., Lo, D., et al. (2019). Monitoring uncommon and non-indigenous fishes in Italian waters: one year of results for the AlienFish project. Reg. Stud. Mar. Sci. 28:100606.
Tuya, F., Cacabelos, E., Duarte, P., Jacinto, D., Castro, J. J., Silva, T., et al. (2012). Patterns of landscape and assemblage structure along a latitudinal gradient in ocean climate. Mar. Ecol. Prog. Ser. 466, 9–19.
Villasante, S., Macho, G., Antelo, M., Isusi de Rivero, J., Rivero Rodriguez, S., Zeller, D., et al. (2016). Estimates of Total Removals of Marine Fisheries Catches in the Northwest of Spain (1950-2010). Washington, DC: University of British.
Villasante, S., Rodríguez-González, D., Antelo, M., Rivero-Rodríguez, S., and Lebrancón-Nieto, J. (2013). Why are prices in wild catch and aquaculture industries so different? Ambio 42, 937–950.
Villegas-Ríos, D., Alonso-Fernández, A., Fabeiro, M., Bañón, R., and Saborido-Rey, F. (2013). Demographic variation between colour patterns in a temperate protogynous hermaphrodite, the ballan wrasse labrus bergylta. PLoS One 8:e71591. doi: 10.1371/journal.pone.0071591
Voerman, S. E., Llera, E., and Rico, J. M. (2013). Climate driven changes in subtidal kelp forest communities in NW Spain. Mar. Environ. Res. 90, 119–127. doi: 10.1016/j.marenvres.2013.06.006
Walsh, R. G., John, K. H., McKean, J. R., and Hof, J. G. (1989). Comparing long-run forecasts of demand for fish and wildlife recreation. Leis. Sci. 11, 337–351.
Walters, C. (2003). Folly and fantasy in the analysis of spatial catch rate data. Can. J. Fish. Aquat. Sci. 60, 1433–1436.
Ward, J. J. H. (1963). Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 58, 236–244.
Watson, J. E. M., Venter, O., Lee, J., Jones, K. R., Robinson, J. G., Possingham, H. P., et al. (2018). Protect the last of the wild. Nature 563, 27–30.
Wengraf, T. (2001). Qualitative research interviewing: Biographic narrative and semi-structured methods. London: Sage.
Wernberg, T., Thomsen, M. S., Tuya, F., Kendrick, G. A., Staehr, P. A., and Toohey, B. D. (2010). Decreasing resilience of kelp beds along a latitudinal temperature gradient: potential implications for a warmer future. Ecol. Lett. 13, 685–694.
Xunta de Galicia (2019). Pesca de Galicia. Estadísticas oficiales de pesca de la. Spain: Xunta de Galicia.
Young, M. A. L., Foale, S., and Bellwood, D. R. (2015). Dynamic catch trends in the history of recreational spearfishing in Australia. Conserv. Biol. 29, 784–794. doi: 10.1111/cobi.12456
Keywords: perceptions, long-term trends, shifting baselines, ecological knowledge, recreational fisheries, stock assessment, fisheries science
Citation: Pita P, Antelo M, Hyder K, Vingada J and Villasante S (2020) The Use of Recreational Fishers’ Ecological Knowledge to Assess the Conservation Status of Marine Ecosystems. Front. Mar. Sci. 7:242. doi: 10.3389/fmars.2020.00242
Received: 15 November 2019; Accepted: 27 March 2020;
Published: 21 April 2020.
Edited by:
Tomaso Fortibuoni, Higher Institute for Environmental Protection and Research (ISPRA), ItalyReviewed by:
Ernesto Azzurro, Higher Institute for Environmental Protection and Research (ISPRA), ItalyCopyright © 2020 Pita, Antelo, Hyder, Vingada and Villasante. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pablo Pita, cGFibG8ucGl0YUB1c2MuZXM=; cHBpdGFvcmR1bmFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.