- 1International Research Center for Marine Biosciences at Shanghai Ocean University, Ministry of Science and Technology, Shanghai, China
- 2Key Laboratory of Exploration and Utilization of Aquatic Genetic Resources, Ministry of Education, Shanghai Ocean University, Shanghai, China
- 3College of Fisheries and Life Science, Shanghai Ocean University, Shanghai, China
This review provides the updated information and analysis of research development on horseshoe crabs over the past 30 years and raises some suggestions for future studies on horseshoe crabs. Horseshoe crab is a unique marine species and attracts many scholars in various research fields. However, to date, the development of horseshoe crab research has not been analyzed and reported from a bibliometric perspective. Bibliometric analysis is a unique tool for evaluating the development of a specific research area by analyzing relevant publications and researchers from all over the world. In this study, Web of Science, Google Scholar, and PubMed were used to collect relevant data on horseshoe crabs. VOSviewer software was used to visualize cooperation and major keywords in horseshoe crab research. From 1989 to 2019, scholars emphasized the physiological characteristics, medical value, and ecological conservation of horseshoe crabs. Some topics in this field have gradually developed because of some reasons explained in this review. Some important or recent studies have been discussed, and some potential research topics for future research have been suggested.
Introduction
Horseshoe crab belongs to phylum Arthropoda, subphylum Chelicerata, class Merostomata, order Xiphosura, and family Limulidae. It is a crab-like animal with blue hemolymph and a brown body composed of the cephalothorax, abdomen, and sword tail. As ancient creatures (Cartwright-Taylor et al., 2009; Delvaeye and Conway, 2009) that lived earlier than dinosaurs (Rudkin and Young, 2009), they first appeared in the Devonian period of the Paleozoic era, about 450 million years ago (Rudkin and Young, 2009). For 450 million years, they maintained their original traits and lived in the benthic zone and were thus famous living fossils (Sekiguchi and Sugita, 1980; Rudkin and Young, 2009). Horseshoe crab types include only Limulus polyphemus (American horseshoe crab), Tachypleus tridentatus (trispine horseshoe crab), Tachypleus gigas (southern horseshoe crab), and Carcinoscorpinus rotundicauda (mangrove horseshoe crab; Carmichael and Brush, 2012; Mashar et al., 2017). L. polyphemus is mainly distributed in the American Atlantic coast and the Gulf of Mexico. T. tridentatus is spread largely in the southeast and east coastal areas of Asia, including the Beibu Gulf of Guangxi, Hongkong, Taiwan, Japan, and Malaysia. T. gigas and C. rotundicauda are present in the waters of south and southeast Asia (Sekiguchi, 1988; Vestbo et al., 2018).
Horseshoe crabs are widely utilized as biological resources in various fields. L. polyphemus has been mass harvested for use as eel and whelk bait in commercial fishery in the last century (Berkson and Shuster, 1999; Smith et al., 2009). This species is still caught for use in fertilizer and livestock feed. In China and southeast Asia, T. tridentatus and T. gigas are regarded as food and traditional Chinese medicine and thus consumed by local people or travelers (Fu et al., 2019). With the progress in scientific research, specifically, biomedical research (Shuster, 1962), researchers explored and revealed the visual, endocrine, and physiological processes of horseshoe crabs (Berkson and Shuster, 1999; Zaldívar-Rae et al., 2009; Carmichael and Brush, 2012). Horseshoe crabs have medical importance given that they produce reagents for testing bacterial endotoxins (Levin and Bang, 1964; Cooper et al., 1971; Novitsky, 1984; Berkson and Shuster, 1999; Swan, 2002; Kreamer and Michels, 2009) and vaccines (Maloney et al., 2018). In the marine ecological community, horseshoe crabs form prey–predator relationships with many animals and play a vital role in estuarine and coastal communities (Botton, 2009). Horseshoe crabs can be used to detect endotoxin concentration in the environment (Novitsky, 2009) and are regarded as an indicator of coastal ecology (Chen et al., 2004).
However, horseshoe crabs have faced multiple threats (Maloney et al., 2018). The abuse of horseshoe crab biological resources, along with other factors, such as environmental pollution and shoreline retreat, has caused the population of horseshoe crabs to severely decline (Rudloe, 1982; Widener and Barlow, 1999; Cartwright-Taylor et al., 2011; Smith et al., 2017; Vestbo et al., 2018). In the International Union for Conservation of Nature (IUCN) Red List, the status of the American horseshoe crab (Vulnerable) and the trispine horseshoe crab (Endangered) has been recorded; their population is decreasing (Smith et al., 2016; Laurie et al., 2019). Therefore, for conserving horseshoe crabs and promoting further biological research in this field, previous studies on horseshoe crabs should be reviewed.
Bibliometrics, which was established in 1958 (Thelwall, 2008), has been used to analyze various types of information in publications (Borgman, 1989). Norton defined bibliometrics as the measurement of texts and information (Wilson, 2001). Bibliometrics is an important and efficient method to evaluate scientific research. With the external characteristics of literature (including word frequency analysis and simple document counting) as research objects and applying certain mathematical statistical methods for quantitative analysis, bibliometrics can objectively reflect the research status and developmental trends of relevant disciplines (Morris et al., 2002; Daim et al., 2006; Nederhof, 2006). Bibliometrics is performed to generally analyze from two perspectives: the influence of a researcher or a group in a certain field and the internal situation of a research field (including communication, cooperation, and hot topics; Borgman, 1989; Thelwall, 2008). Bibliometrics has been applied in various fields, such as psychology, biodiversity, ecological restoration, and biotechnology (Daim et al., 2006; Godin, 2006; Leydesdorff, 2007; Lv et al., 2015; Cao et al., 2016).
The Web of Science (WOS) is a global authoritative scientific literature retrieval platform (Falagas et al., 2008; Wang et al., 2019). WOS contains approximately 11,000 source journals (Bornmann et al., 2016). Although it is a multidisciplinary database (Bar-Ilan, 2008), WOS is biased toward the natural and life sciences (Bornmann et al., 2016). It is also a common and useful tool for bibliometric analysis (Mongeon and Paul-Hus, 2016). The databases of PubMed and Google Scholar were also applied to obtain additional information on horseshoe crab research. The bibliometric analysis of horseshoe crabs helped researchers understand hot topics and develop potential directions for research on this animal. Thus, this research aimed to provide a framework for understanding the development of studies on horseshoe crab-related topics, including ecology, conservation, and physiology.
Methods and Materials
Data Collection
In the WOS database, the core collection was chosen to extract relevant information. With “horseshoe crab” as the theme of the search, 1,248 results were found on July 26, 2019. The time range was from 1989 to July 26, 2019. The bibliometric plug-in in WOS was used to count the number of the publications of authors, organizations, journals, countries, or regions. All the information of relevant papers (e.g., title, authors, affiliation, address, and keywords) was selected and exported in a tab-delimited format to construct bibliometric maps by using VOSviewer (Version 1.6.11). Bibliometric tools in WOS were also used to extract other relevant information (the number of publications of each author, country, region, organization, field, and journal; the total number of times the publications were cited; and the proportion of all publications).
The bibliometric plug-in in WOS can list the top 20 authors on the basis of the number of publications. Given that WOS may omit relevant information, relevant data were also collected from PubMed and Google Scholar. Two approaches were used to search relevant publications in Google Scholar. The method for collecting publications that included the word “horseshoe crab” only in titles was designated as G1, and the method for collecting publications that included the word “horseshoe crab” in whole articles was designated as G2. The numbers of publications on horseshoe crabs by the top 20 authors were searched in Google Scholar via G1 and G2. In PubMed, the number of publications on horseshoe crab by these 20 authors over the last 30 years was collected.
Important Settings for Bibliometric Map Construction
VOSviewer 1.6.11 was used to generate bibliometric maps. VOSviewer is a free computer program (available in http://www.vosviewer.com/) used primarily for bibliometric visual analysis (van Eck and Waltman, 2010; Vezyridis and Timmons, 2016; Sweileh, 2018). The bibliometric maps generated by VOSviewer, which is supported by the VOS mapping technique, perform well (Van Eck and Waltman, 2007). The VOSviewer can be used to analyze cooperative relationships within a research field and for hot keyword analysis and clustering analysis (van Eck and Waltman, 2017).
The counting method is full counting, which means each link has the same weight. The maximum number of lines is 1,000, and the minimum strength of lines is zero.
The type of analysis was set as coauthored publication and the units of analysis were set as authors, organizations, and countries (regions included) to visualize the research cooperation in this field. Studies that “published by a large number of authors (country or organization)” were not selected. The following thresholds were set: the minimum number of documents of an author (country or organization) was five, and the minimum number of citations of an author (country or organization) was zero. Thus, in this research, the degree of cooperation was defined as the extent of the coauthorship of publications.
“Co-occurrence” and “all keywords” were chosen to identify the hot keywords of papers on horseshoe crabs. The threshold or the minimum number of occurrences of a keyword was five.
Results
Publications Per Year
The annual number of publications on horseshoe crabs in Google Scholar (using method G2) increased from 1989 to 2014 (Figure 1A and Table 1). Although a declining trend has been shown since 2015, the annual number of publications on horseshoe crabs in Google Scholar (using method G2) is higher than that of publications on other topics.
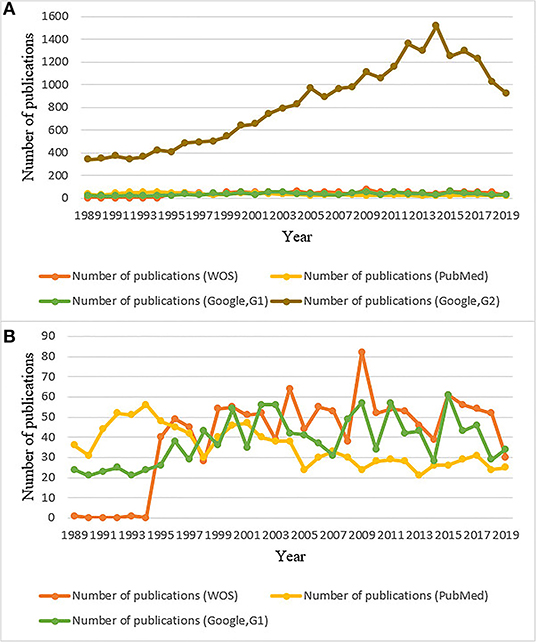
Figure 1. Number of publications of each year from 1989 to 2019 based on WOS, PubMed, and Google Scholar. G1: Publications in Google that include “horseshoe crab” only in titles. G2: Publications in Google that include “horseshoe crab” in whole articles. The number of publications from WOS, PubMed, G1, and G2 are all included in (A), the number of publications excluded G2 are listed in (B).
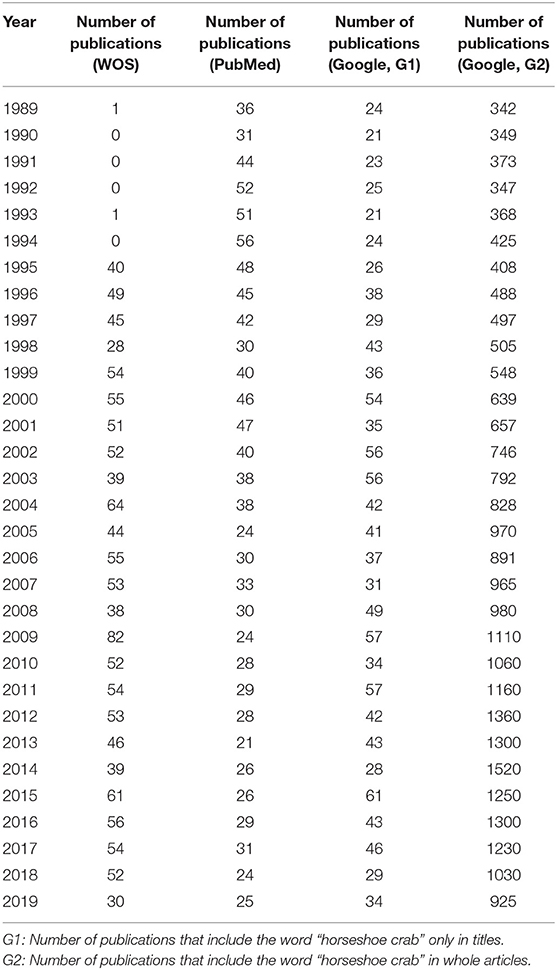
Table 1. Number of publications of each year from 1989 to 2019 based on WOS, PubMed, and Google Scholar.
In the WOS database, the number of total publications about horseshoe crabs fluctuates roughly (Figure 1B and Table 1). Before 1995, few publications on horseshoe crabs were available. In 1995, the proportion of all publications about horseshoe crabs increased sharply. Afterward, the number fluctuated.
The annual number of publications about horseshoe crabs in PubMed decreased. In PubMed, the highest number of publications about horseshoe crabs was recorded in 1994. In Figure 1B, the annual number of publications about horseshoe crabs from 1989 to 1995 in Google Scholar (using method G1) ranged from 20 to 26. It then fluctuated after 1995 and showed a similar trend to WOS.
Research Intensity According to Regions
Countries or Regions
The contribution of the USA is particularly significant with the most publications on horseshoe crabs (Table 2). However, the average number of times each article was cited in the USA did not reach the horizontal dotted line. The average number of times each article was cited in Japan, France, Canada, and Singapore was higher than that of the average level. Japan might be the pioneer in horseshoe crab studies because it has the highest number of publications and number of citations per article.
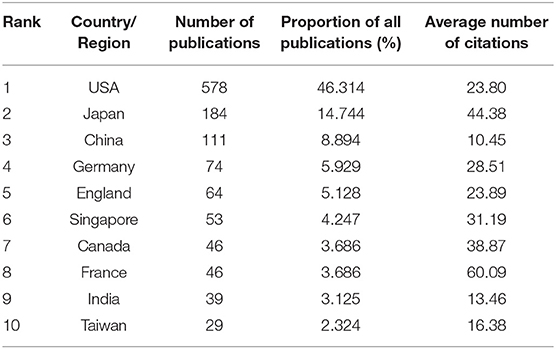
Table 2. Numbers of publications, the proportion of all publications, and the total number of times the top 10 countries or region are cited and ranked by the number of publications based on WOS.
Organizations
Kyushu University had the highest number of publications, and the University of Florida had the second-highest number of publications, followed by the National University of Singapore (Table 3). The number of publications of these three organizations was higher than the average. Kyushu University still had the highest number of average citations per article. In addition, Kyushu University, the National University of Singapore, Marine Biological Laboratory (Woods Hole), and the University of California Davis had a higher number of average citations per article than the average.
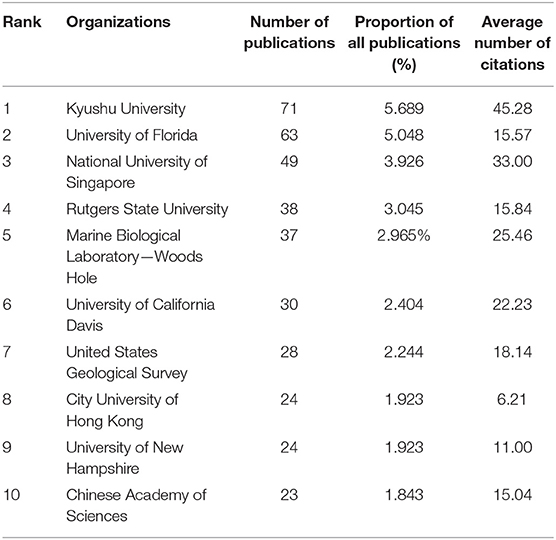
Table 3. Numbers of publications, the proportion of all publications, and the total number of times the top 10 source journals are cited and ranked by the number of publications based on WOS.
Authors
WOS, Google Scholar, and PubMed were applied to analyze the publication records of authors worldwide. As shown in Table 4, the publications on horseshoe crabs by authors have been recorded widely by Google Scholar. PubMed has fewer records of horseshoe crab publications by authors than the two other databases.
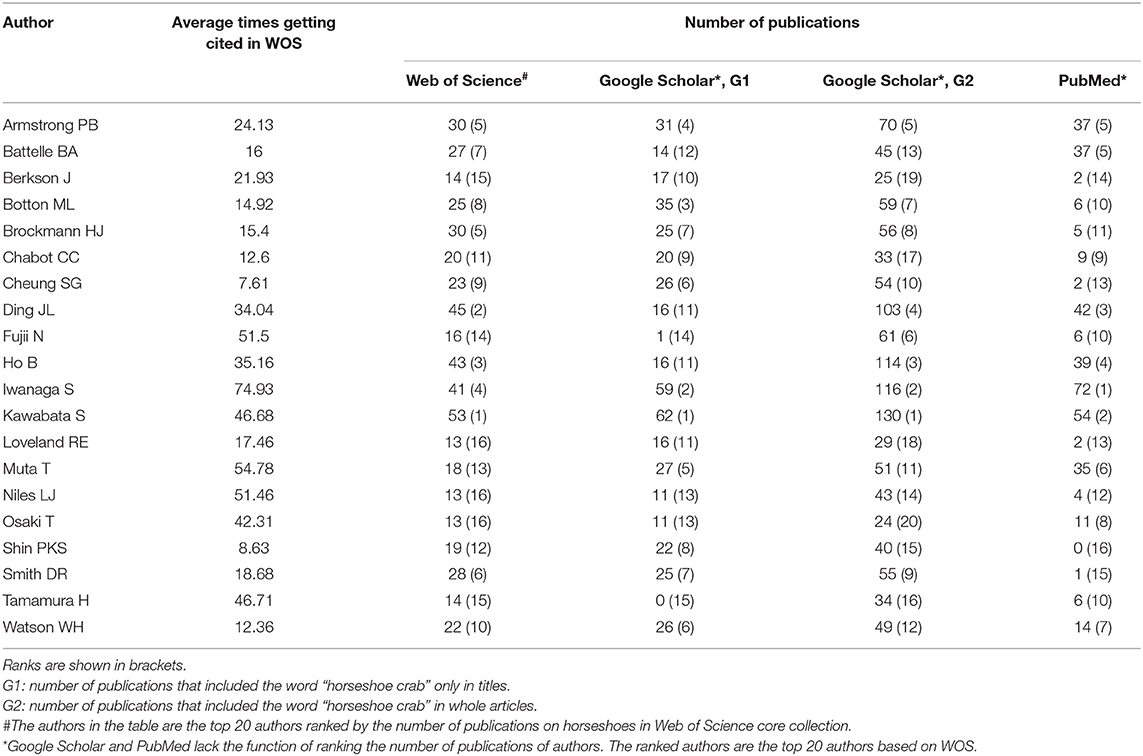
Table 4. The numbers of horseshoe crab publications of top 20 authors based on WOS, PubMed, and Google Scholar.
Journals
The Journal of Biological Chemistry had released 53 papers, which was the highest number among journals. “Biology and Conservation of Horseshoe Crabs” published by Springer in 2017 had the second-highest number of articles. Biological Bulletin had the third-highest number of articles. Only the numbers of publications of these three journals/books were higher than the average. The average number of times each article of the Journal of Biological Chemistry was cited was the highest. The average number of times each article of four journals was cited was higher than the average (Table 5).
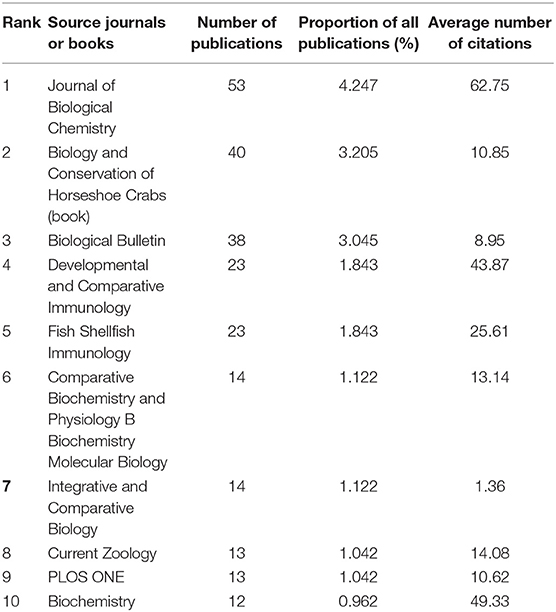
Table 5. Numbers of publications, the proportion of all publications, and the total number of times the top 10 source journals are cited and ranked by the number of publications based on WOS.
Research Cooperation Intensity
Countries or Regions
Close cooperative relationships among countries or regions in this field were observed. The United States is the core of academic exchange and cooperation in this field because the dot representing the United States is the largest and is surrounded by numerous lines. The first country to study horseshoe crabs is China, which published articles on horseshoe crabs in 1990. The following countries are Singapore, Japan, France, and Denmark, which mainly published relevant articles between 2000 and 2010. Most countries or regions published articles in 2010. Malaysia, Poland, Wales, and Italy published articles in 2019. In the chart, almost every dot can be connected to another dot with a different color, indicating that each country or region cooperated with other countries at different time spans (Figure 2).
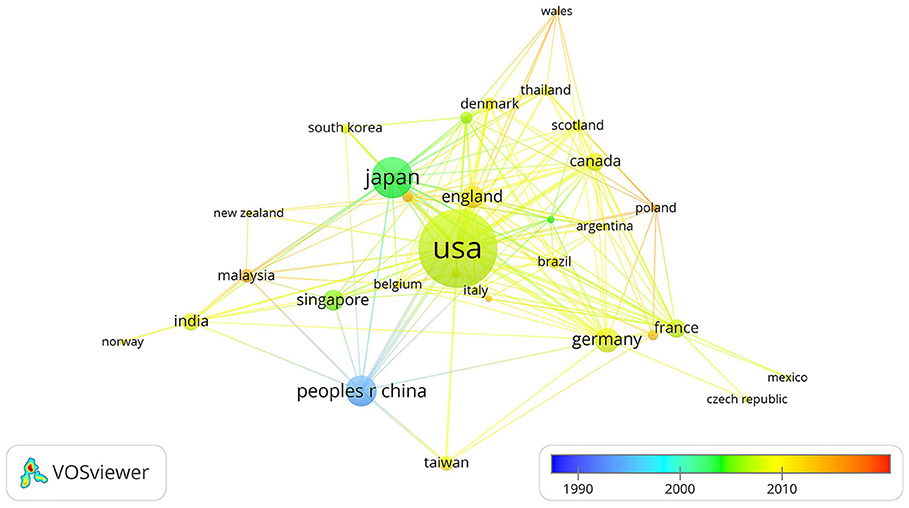
Figure 2. Network showing cooperation among countries. Large dots indicate countries with high numbers of publications. The color of each dot represents when the publication is released. Thick lines between dots indicate cooperation among numerous countries.
Organizations
The relationship among organizations has two situations. In the first situation, some organizations rarely or never cooperated with others in horseshoe crab studies. In the second situation, numerous organizations cooperated with Kyushu University. Among the organizations that seldom or never cooperated with others, the first to study horseshoe crabs may be Florida State University and the Indian Institute of Chemical Biology. They published articles in 2000. The University of Hong Kong, the University of British Columbia, and Boston University published articles from 2000 to 2010. Shenzhen Polytech, Johns Hopkins University, University of Stirling, and University Putra Malaysia published articles after 2010 (Figure 3).
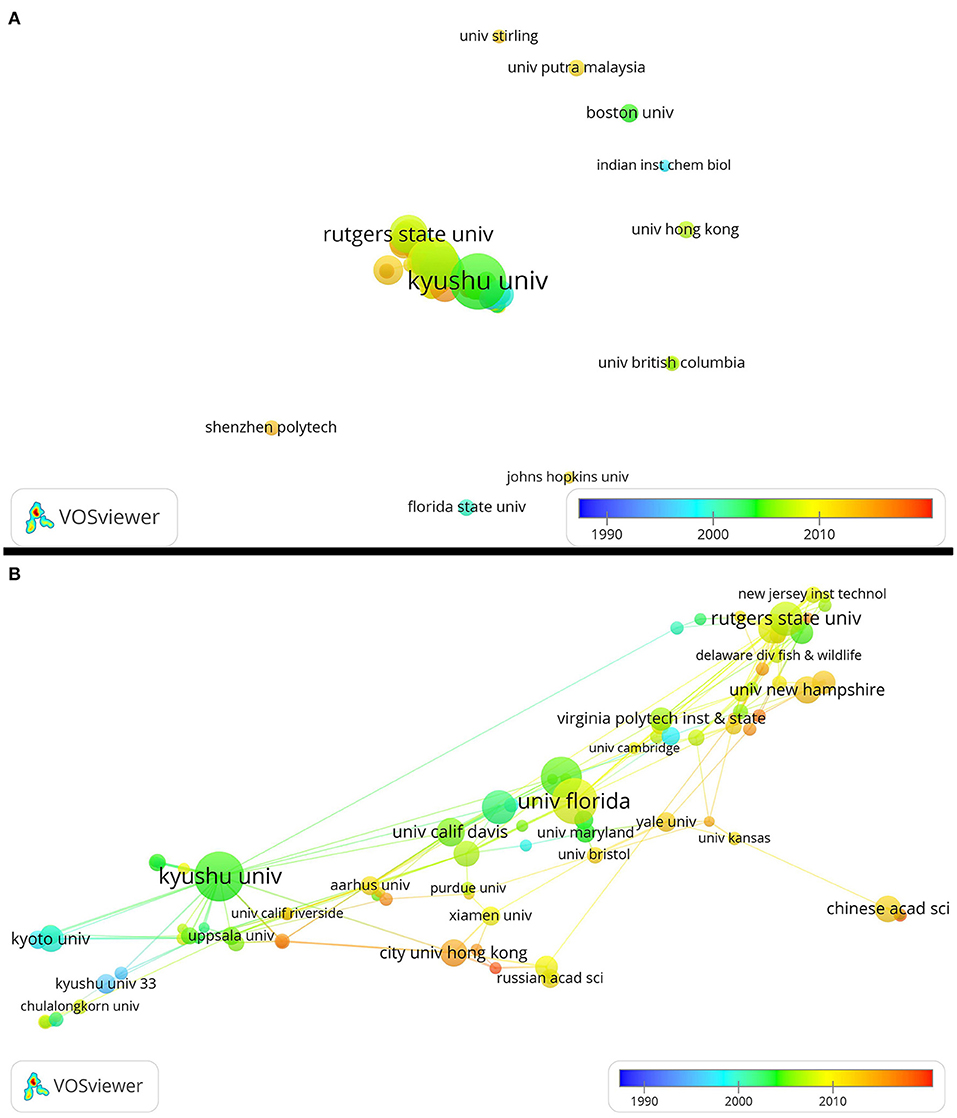
Figure 3. Network showing cooperation among organizations. (B) Is the zoomed-in result from (A). Large dots indicate that organizations have a high number of publications. The color of each dot represents when the publication is released. Thick lines indicate cooperation among numerous organizations.
Three collaboration cores, namely, Kyushu University, University of Florida, and Rutgers State University, were identified. These three organizations published articles mainly from 2000 to 2010. Kyoto University, Kyushu University, and other minor organizations published articles mainly from 1990 to 2000. The City University of Hong Kong, the University of New Hampshire, Yale University, the University of Bristol, the University of Kansas, Aarhus University, the University of California Riverside, and the Chinese Academy of Sciences published articles about horseshoe crabs after 2010. As shown in the figure, each dot is mostly connected to dots of a similar color, which means that organizations cooperated with one another in about 5 years (Figure 3).
Authors
As illustrated in Figure 4, dots and lines are dense in two areas. In the area with Kawabata S as the main core, most authors published articles related to horseshoe crabs in 1999. In the area with Watson and Shin as the main core, most of the papers have been published since 2010. Many authors who have not collaborated with other authors existed outside of these two areas. The dots representing these authors are relatively small, suggesting that they have not published many papers on horseshoe crabs. Some of these authors published articles between 2000 and 2010, whereas others published articles after 2010. To explore the cooperative relationship between authors in detail, we enlarged the image and carefully observed the two dense areas for further discussion. Most authors published horseshoe crab papers in 2000 or earlier (Figure 4). Iwanaga, Kawabata, Ding, Armstrong, Botton, and Battelle were highly prolific authors in 2000. The relationship between Iwanaga and Kawabata was close. Ibuka, Otaka, Yamamoto, Fujii, Tamamura, and Waki had a close relationship. The lines between their dots are dense, and each line is thick (Figure 4B).
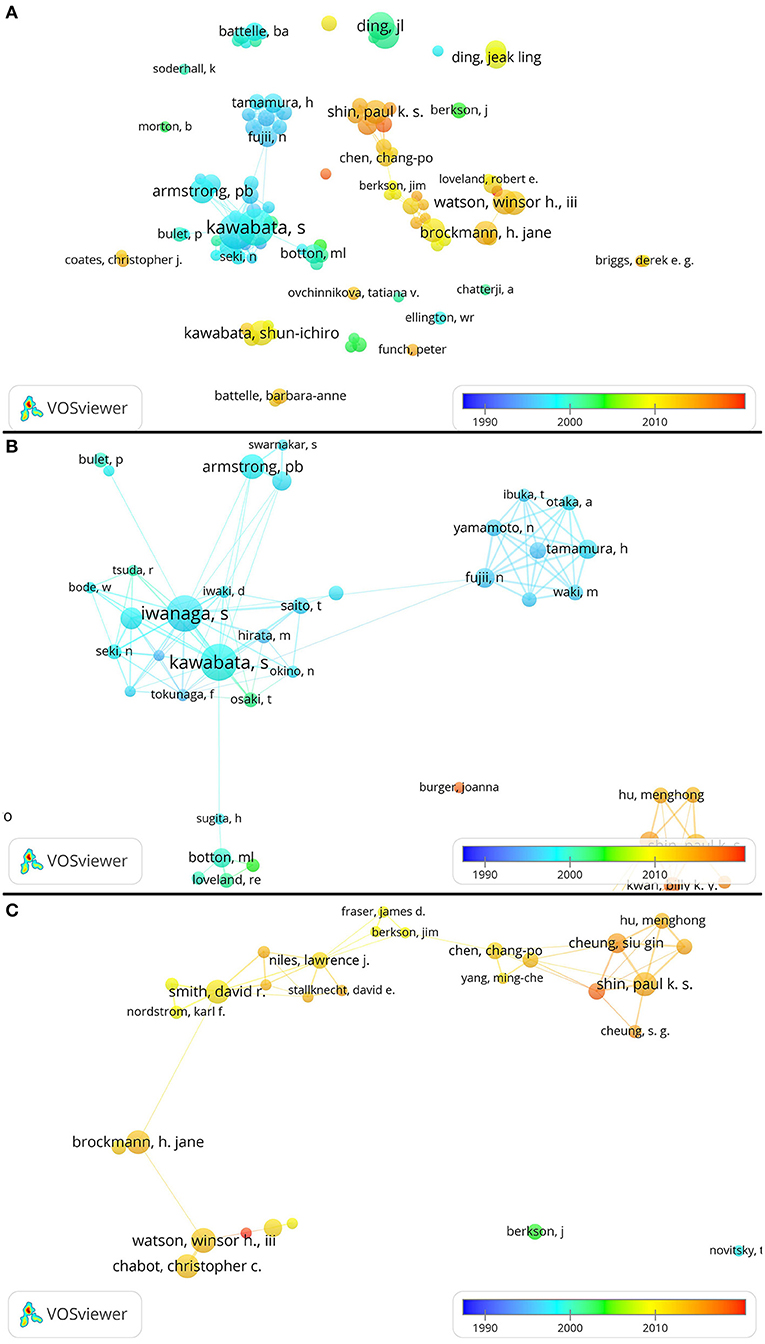
Figure 4. Network showing cooperation among authors. (B,C) Are the zoomed-in results from (A). Large dots indicate that authors have a high number of publications. The color of each dot represents when the publication is released. Thick lines between dots indicate cooperation among numerous authors. The same author may write her or his name in different ways. For example, in this figure, “ding, jl” and “ding, jeak ling” may refer to the same author.
Chabot, Watson, Brockman, Smith, Shin, and Cheung were the authors with a high number of published articles in 2010 (Figure 4C). Chabot, Watson, and Brockman have few cooperative relations with other authors but have a large number of published papers; this result may indirectly indicate that these three authors had a strong scientific research performance. Hu, Shin, and Cheung's studies likely represented the latest studies and frontiers in this field given that most of their papers were published after 2010. These three authors also cooperated with one another often because they were all at the City University of Hong Kong in 2010.
Research Intensity According to Hot Keywords
The phrase “horseshoe crab” had the highest frequency because it was the name of the study object (Figure 5). In 2000, hot terms were population, beach, photosensors, muscle, and immunodeficiency-virus activity. These results indicated that researchers focused on the vision, immune function, and population distribution of horseshoe crabs during this period. In 2005, the main hot key words were hemocytes, Drosophila, vision, hemolymph, cDNA cloning, specificity, signal transduction, Tridentatus, and Limulus. During this period, researchers emphasized the immune ability and vision of horseshoe crabs and began to study the molecular biology of this species. Hot keywords that appeared in 2010 were horseshoe crab, Limulus-polyphemus, antimicrobial peptides, evolution, growth, temperature, eggs, shorebirds, Arthropoda, lectin, bacterial lipopolysaccharides, and behaviors. In 2015, the hot key words were heavy metals, black tiger shrimp, seasonal movements, phylogeny, arthropod trackways, genomic organization, and Xiphosurida. In recent years, scholars focused on the physiology, phylogeny, and behavior of horseshoe crabs.
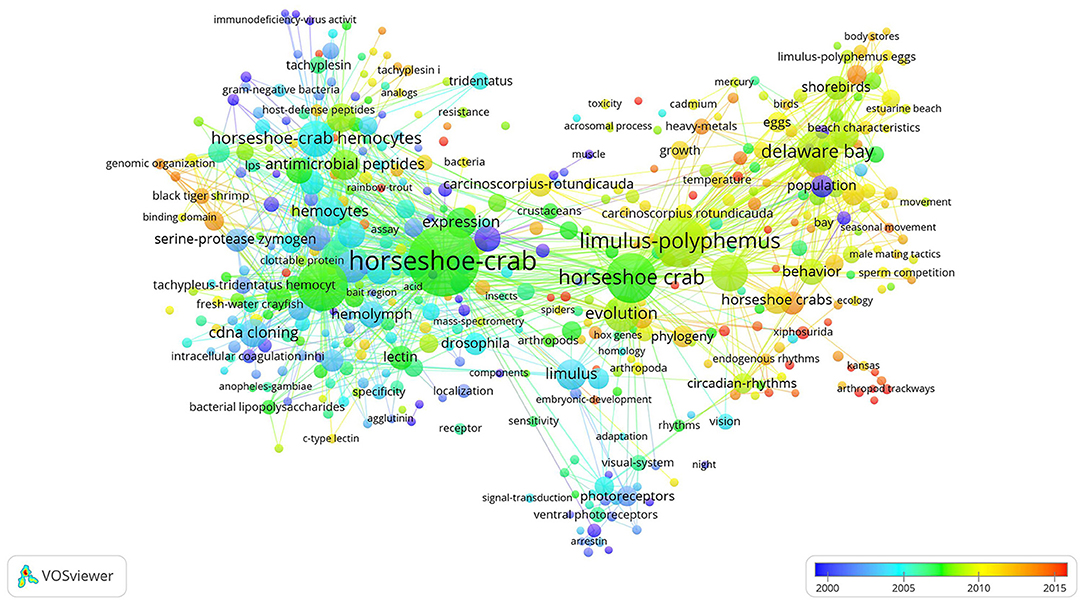
Figure 5. Network showing co-occurrence among keywords. Large dots indicate a high number of publications containing a specific keyword. The color of each dot represents when the publication is released. Thick lines indicate the co-occurrence of a high number of keywords.
Fields
We could infer that studies on horseshoe crab have mainly focused on the biochemical reaction, immune ability, medical application, ecology, and conservation of this animal. Biochemistry molecular biology is the hottest field (Table 6). Marine freshwater biology, zoology, environmental sciences ecology, and immunology have also been highly explored.
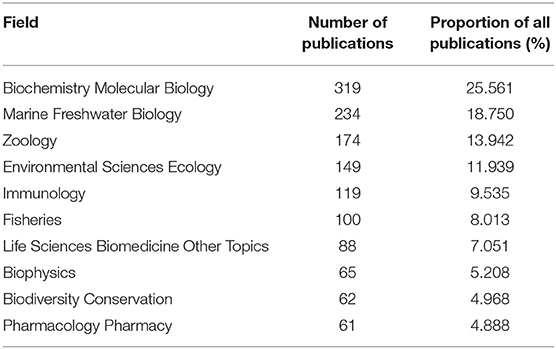
Table 6. Numbers of publications and the proportion of all publications of the top 10 fields ranked by the number of publications based on WOS.
Discussions
Status of Horseshoe Crab Research
Google Scholar (using G2 method) might include articles that mentioned horseshoe crabs but did not focus on them, resulting in much more publications found in Google Scholar G2 than in WOS, PubMed, and Google Scholar G1. For example, through G2 searching, studies about the exoskeleton structure (Raabe et al., 2005) and lectin (Moura et al., 2006) were included, but these studies were not about horseshoe crabs because “horseshoe crabs” in these articles were used as references but did not examine horseshoe crabs. The data showed that horseshoe crabs have been widely explored (Figure 1A), and the trend of the number of publications in PubMed is different from that in WOS and Google Scholar (Figure 1B). The number of horseshoe crab publications from 1989 to 1997 in the PubMed database was higher than that in Google Scholar and WOS databases. Google Scholar and WOS database had a high number of horseshoe crab publications. One possible reason was the selective collection of the PubMed database, which tends to focus on the medical field. From 1989 to 1997, medically relevant studies were published and included; from 2003 to 2019, some medically irrelevant horseshoe crab publications (e.g., conservation and basic physiology researches) were excluded by PubMed. According to WOS, the top 20 pioneers have provided many important studies (Table 4). Thus, new learners who are interested in horseshoe crabs are suggested to read the articles of these pioneers. As shown in Table 4, Google Scholar can provide scholars with a large number of publications.
The large number of publications may indicate efforts in research, whereas a high number of citations per article may indicate that these studies are hot spots and attract considerable attention. According to the results, the USA and Japan finished numerous works in horseshoe crab studies (with the first and second-highest number of publications). Publications from France, Japan, Canada, and Singapore have attracted the attention of researchers (a high number of times each article was cited). Kyushu University and the National University of Singapore have released a large number of publications, which may attract more attention from researchers. Articles in the Journal of Biological Chemistry, Developmental and Comparative Immunology, Fish Shellfish Immunology, and Biochemistry have been widely explored. Publications from these countries, organizations, and journals can be helpful as references for people who want to conduct further research on horseshoe crabs.
Cooperation
Cooperation among countries or regions has improved since 1989. However, many authors and organizations rarely cooperate with others. The USA is the most active in cooperation. Kyushu University, the University of Florida, and Rutgers State University may be cooperation cores. Kawabata, Iwanaga, Watson, and Shin often collaborated with others.
Global cooperation was connected by four rounds of the International Workshop on the Science and Conservation of Horseshoe Crabs, which has been held by the Horseshoe Crab Specialist Group of the International Union for Conservation of Nature (IUCN) every 4 years since 2007. As a result, global cooperation and communication have greatly improved in this field. The first International Symposium on the Science and Conservation of Horseshoe Crabs was held in New York, and proceeding papers were collected in the book “Biology and Conservation of Horseshoe Crabs,” which was edited by Tanacredi, Botton, and Smith (Tanacredi et al., 2009). The second, third, and fourth international workshops on the Science and Conservation of Asian Horseshoe Crabs were held in Hongkong, Nagasaki, and Guangxi, respectively. The change in conference subject indicated that researchers were encouraged to focus on Asian horseshoe crabs. The book “Changing Global Perspectives on Horseshoe Crab Biology, Conservation, and Management” edited by Carmichael, Botton, Shin, and Cheung reported a significant progress on scientific research on horseshoe crabs within the past 10 years and collected proceeding papers from the second and third international conferences (Carmichael et al., 2015). As cochairs of the Horseshoe Crab Specialist Group of the IUCN, Botton and Shin provided considerable contributions to this event and the editing of conference proceedings, which accelerated global collaboration on horseshoe crabs.
Hot Research Orientation
The hot key words and fields indicated that scholars focused on physiology, medical value, and ecological conservation. Before and in 2000, researchers focused on the vision, immune function, and population distribution of horseshoe crabs. In 2010, researchers continued to focus on the immune ability and vision of horseshoe crabs and began to study the molecular biology of this animal. Scholars focused on the physiology and behavior of horseshoe crabs, and the effects of environmental stress on the horseshoe crabs were also investigated. The trend of variations in research focus showed that horseshoe crab physiology, especially the immune system, has been most widely explored, followed by medical application and conservation.
Horseshoe crab is unique with a strong immune system and famous for the utilization of their hemolymph in endotoxin detection (Fennrich et al., 2016). Over the past 30 years, studies have been conducted to identify the mechanism of this strong immunity. Iwanaga and Kawabata discovered the molecular basis for the innate immunity of horseshoe crab and attributed it to the application of horseshoe crabs' inner defense molecules in medicine (Kawabata et al., 1996; Iwanaga et al., 1998; Beisel et al., 1999; Iwanaga, 2002). Ding and Ho explored the hemolymph protein of horseshoe crabs and the mechanism of immune molecules, which greatly contributed to bacterial pyrogen testing (Tan et al., 2000a,b; Ding and Ho, 2001; Frecer et al., 2004; Zhu et al., 2004; Ng et al., 2007; Yu et al., 2009). Armstrong mainly focused on alpha (2)-macroglobulin and found that it is important for horseshoe crab hemolysis and immune system because of its special function (Armstrong et al., 1998; Armstrong and Quigley, 1999). The high citation frequency of these authors' works proved the high degree of concern for the immune system of horseshoe crabs (Table 4).
Given the ability of amebocytes to instantaneously react with endotoxins, horseshoe crabs are hunted for use in safety tests in medical production (Gauvry, 2015; Fennrich et al., 2016; Krisfalusi-Gannon et al., 2018). Tachyplesin in horseshoe crab hemocytes exerts a strong antimicrobial effect on Gram-positive and Gram-negative bacteria (Dorrington and Gomez-Chiarri, 2008) and has an antimicrobial activity against multidrug-resistant pathogens (Liu et al., 2018). Tachyplesin kills bacteria by targeting FabG, which is the conserved β-ketoacyl-acyl carrier protein reductase in unsaturated fatty acid biosynthesis (Liu et al., 2018). However, tachyplesin may target homologous enzymes in mammalian cells, causing cytotoxicity (Liu et al., 2018). In view of the mechanism of tachyplesin, more researchers should focus on the discrimination between bacterial and mammalian FabG proteins to select specific inhibitory ligands and develop safe antibiotics (Liu et al., 2018). Tachyplesin I from horseshoe crabs shows an antitumor activity; however, the action mode of tachyplesin I in tumor cells remains unclear (Li et al., 2017). The new antimicrobial peptide polyphemusin III can induce cell death by destroying the plasma membrane (Marggraf et al., 2018). Purified recombinant Factor C may be applied to inhibit inflammation and septic shock (Li et al., 2007). The 18-mer peptide T22 in horseshoe crab can target cells quickly through a receptor-specific endosomal route, showing a promising capacity for intracellular delivery (Unzueta et al., 2012). CrSPI, a serine protease inhibitor in horseshoe crabs, can modulate immune responses, including complements and PPO-mediated antimicrobial activities (Jiang et al., 2009). The outer shells of horseshoe crabs can be utilized as burn dressings to accelerate wound healing (Kumar et al., 2016).
The population of horseshoe crabs has declined because of marine environment changes and overhunting for feed and medical use (Tanacredi and Portilla, 2015; Krisfalusi-Gannon et al., 2018). Their spawning grounds and habitat have degraded (John et al., 2018). High acid volatile sulfide values contribute to a decline in the horseshoe crab population in Yamaguchi Bay, Japan (Moqsud et al., 2017). In 2015, 583,000 horseshoe crabs were harvested as eel and whelk bait, and 559,903 horseshoe crabs were harvested for biomedical production (Krisfalusi-Gannon et al., 2018). Although horseshoe crabs are released after medical production or studies, they would become vulnerable, and their mortality rate might increase (Walls and Berkson, 2003; Leschen and Correia, 2010). Female horseshoe crabs released back to the sea encounter difficulties in spawning (Anderson and Chabot, 2013). A green alga of the family Ulvaceae might cause harm to horseshoe crabs in their natural habitats (Braverman et al., 2012). Moreover, population decline would affect other organisms in food webs involving horseshoe crabs. Horseshoe crabs and their eggs are the food of many animals, such as shorebirds (Berkson and Shuster, 1999; Smith and Berkson, 2005), American eel, and sand shrimp (Perry, 1931; Price, 1962; Botton and Haskin, 1984; Botton and Ropes, 1989; Botton, 2009). Adult horseshoe crabs feed on bivalves (Botton and Ropes, 1989). Therefore, protecting horseshoe crabs is a matter of concern. Using recombinant Factor C as a synthetic alternative to horseshoe crab blood may be a partial solution for horseshoe crab conservation (Maloney et al., 2018), and placing limitations on the exploitation of this animal and the reasonable management of their habitats are keys to conservation.
Global marine environmental problems, such as ocean acidification and warming, drew increased attention in the twenty-first century. Thus, many studies have focused on the effect of these environmental stresses on marine organisms. As shown in Figure 5, in 2010, researchers began to focus on the effects of the changes in temperature on horseshoe crabs and the advantage of horseshoe crab biological adaptation to environmental stress. Botton et al. contributed valuable research on the distribution and developmental ecology of L. polyphemus (Botton and Loveland, 2003; Botton et al., 2003, 2006, 2010; Botton, 2009) and determined the effect of heavy metals, such as copper and cadmium, on horseshoe crab embryos and larvae (Botton et al., 1998; Itow et al., 1998; Botton, 2000). Shin and Cheung cooperated on many studies on horseshoe crab aquaculture and ecology (Hu et al., 2009, 2013; Shin et al., 2009; Kwan et al., 2015, 2016, 2017). They further explored the effect of stressful habitat conditions, such as starvation and hypoxic exposure, on horseshoe crabs (Hu et al., 2010, 2011; Shin et al., 2014). Studies have provided valuable chromosome-level genome or transcriptome data on horseshoe crab (Gong et al., 2019; Liao et al., 2019b) and may be useful for horseshoe crab research.
A large number of studies in various fields have focused on horseshoe crabs. However, research progress on horseshoe crabs is not so rapid for some reasons. First, more cooperation should be encouraged. Our analysis revealed that authors, countries, regions, and organizations mostly become involved in cooperation (Tables 2–4 (indicating more publications), e.g., the USA and Japan, Kyushu University, and Rutgers State University, Kawabata, and Iwanaga. Cooperation would accelerate and contribute to research on horseshoe crabs by providing more studies. Second, wild horseshoe crabs are difficult to obtain. The habitats of horseshoe crabs are not global and only include a few areas; in addition, the horseshoe crab population is declining (Adibah et al., 2012; Liao et al., 2019a). Captive breeding and artificial rearing should be explored, and some researchers searched for good sperm donors (Sheikh et al., 2019). Lastly, conducting research on horseshoe crabs in labs may be difficult. Time would decelerate our research because of the life span of horseshoe crabs. Horseshoe crabs take 10 years to sexually mature (Smith and Berkson, 2005). Mortality is high during the development of juvenile horseshoe crabs into adults (Gauvry, 2015). Relevant studies have shown that growth and survival decrease over 128 days regardless of diet composition (Carmichael et al., 2009). The survival rate of horseshoe crabs in labs may vary in practice. The hemolymph of horseshoe crabs can clot easily. The addition of LPS-free reagents at low and consistent temperatures may prevent clotting (Armstrong and Conrad, 2008). In the future, attempts to solve these problems are worth making.
Conclusions and Future Directions
We need additional cooperation to discuss and improve horseshoe crab research. The survival of horseshoe crabs is the most important, or we may even have no horseshoe crabs for scientific studies. The conservation of horseshoe crabs is a major cause of concern. The search and widespread application for the substitutes of horseshoe crab blood will benefit the medical industry and the survival of horseshoe crabs. Scientific studies, medical industry, commercial value, and agricultural use may develop rapidly and sustainably.
Author Contributions
ZL collected the data, performed bibliometric analysis, and wrote the manuscript. FM provided important relevant information about horseshoe crabs and wrote the manuscript. MH and YW conceived the idea and structure and wrote the manuscript.
Funding
This research was supported by a grant from the 2017 Beihai City 13th Five-Year Plan Marine Economic Innovation and Development Demonstration Project–Tachypleus Amebocyte Lysate and Chinese Horseshoe Crab Ecological Utilization Industry Chain Collaborative Innovation Project (Grant No. Bhsfs006).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adibah, A. B., Ling, L. P., Tan, S. G., Faridah, Q. Z., and Christianus, A. (2012). Development of single-locus DNA microsatellite markers using 5'anchored ISSR-PCR method for the mangrove horseshoe crab, Carcinoscorpius rotundicauda (Latreille, 1802) in Peninsular Malaysia. Mol. Biol. Rep. 39, 3815–3820. doi: 10.1007/s11033-011-1159-6
Anderson, R. L., and Chabot, C. C. (2013). Sublethal behavioral and physiological effects of the biomedical bleeding process on the American horseshoe crab, Limulus polyphemus. Biol. Bull. 225, 137–151. doi: 10.1086/BBLv225n3p137
Armstrong, P., and Conrad, M. (2008). Blood collection from the American horseshoe crab, Limulus polyphemus. J. Vis. Exp. 20:958. doi: 10.3791/958
Armstrong, P. B., Melchior, R., Swarnakar, S., and Quigley, J. P. (1998). α2-Macroglobulin does not function as a C3 homologue in the plasma hemolytic system of the American horseshoe crab, Limulus. Mol. Immunol. 35, 47–53. doi: 10.1016/S0161-5890(98)80016-3
Armstrong, P. B., and Quigley, J. P. (1999). α2-macroglobulin: an evolutionarily conserved arm of the innate immune system. Dev. Comp. Immunol. 23, 375–390. doi: 10.1016/S0145-305X(99)00018-X
Bar-Ilan, J. (2008). Which h-index? - A comparison of WoS, Scopus and Google Scholar. Scientometrics 74, 257–271. doi: 10.1007/s11192-008-0216-y
Beisel, H. G., Kawabata, S. I., Iwanaga, S., Huber, R., and Bode, W. (1999). Tachylectin-2: crystal structure of a specific glcnac/galnac-binding lectin involved in the innate immunity host defense of the Japanese horseshoe crab Tachypleus tridentatus. EMBO J. 18, 2313–2322. doi: 10.1093/emboj/18.9.2313
Berkson, J., and Shuster, C. N. (1999). The horseshoe crab: The battle for a true multiple-use resource. Fisheries 24, 6–10.
Borgman, C. L. (1989). Bibliometrics and scholarly communication. Commun. Res. 16, 583–599. doi: 10.1177/009365089016005002
Bornmann, L., Thor, A., Marx, W., and Schier, H. (2016). The application of bibliometrics to research evaluation in the humanities and social sciences: an exploratory study using normalized Google Scholar data for the publications of a research institute. J. Assoc. Inform. Sci. Technol. 67, 2778–2789. doi: 10.1002/asi.23627
Botton, M. L. (2000). Toxicity of cadmium and mercury to horseshoe crab (Limulus polyphemus) embryos and larvae. Bull. Environm. Contam. Toxicol. 64, 137–143. doi: 10.1007/s001289910021
Botton, M. L. (2009). “The ecological importance of horseshoe crabs in estuarine and coastal communities: a review and speculative summary,” in Biology and Conservation of Horseshoe Crabs, eds J. Tanacredi, M. Botton, D. Smith (Boston, MA: Springer), doi: 10.1007/978-0-387-89959-6_3
Botton, M. L., and Haskin, H. H. (1984). Distribution and feeding of the horseshoe crab, Limulus polyphemus, on the continental shelf off New Jersey. Fish. Bull. (U.S.) 82, 383–389.
Botton, M. L., Johnson, K., and Helleby, L. (1998). Effects of copper and zinc on embryos and larvae of the horseshoe crab, Limulus polyphemus. Arch. Environ. Contam. Toxicol. 35, 25–32. doi: 10.1007/s002449900344
Botton, M. L., and Loveland, R. E. (2003). Abundance and dispersal potential of horseshoe crab (Limulus polyphemus) larvae in the delaware estuary. Estuaries 26, 1472–1479. doi: 10.1007/BF02803655
Botton, M. L., Loveland, R. E., Tanacredi, J. T., and Itow, T. (2006). Horseshoe crabs (Limulus polyphemus) in an urban estuary (Jamaica Bay, New York) and the potential for ecological restoration. Estuar. Coasts 29, 820–830. doi: 10.1007/BF02786533
Botton, M. L., Loveland, R. E., and Tiwari, A. (2003). Distribution, abundance, and survivorship of young-of-the-year in a commercially exploited population of horseshoe crabs Limulus polyphemus. Mar. Ecol. Progress Series 265, 175–184. doi: 10.3354/meps265175
Botton, M. L., and Ropes, J. W. (1989). Feeding ecology of horseshoe crabs on the continental shelf, New Jersey to North Carolina. Bull. Mar. Sci. Miami 45, 637–647.
Botton, M. L., Tankersley, R. A., and Loveland, R. E. (2010). Developmental ecology of the American horseshoe crab Limulus polyphemus. Curr. Zool. 56, 71–83. doi: 10.1093/czoolo/56.5.550
Braverman, H., Leibovitz, L., and Lewbart, G. A. (2012). Green algal infection of American horseshoe crab (Limulus polyphemus) exoskeletal structures. J. Invertebr. Pathol. 111, 90–93. doi: 10.1016/j.jip.2012.06.002
Cao, Y. Q., Guo, M., Liu, S. R., and Yang, J. (2016). Research on ecological restoration based on bibliometric analysis. Acta Ecol. Sinica 36, 345–353.
Carmichael, R. H., Botton, M. L., Shin, P. K. S., and Cheung, S. G. (2015). Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management. Boston, MA: Springer.
Carmichael, R. H., and Brush, E. (2012). Three decades of horseshoe crab rearing: a review of conditions for captive growth and survival. Rev. Aquacul. 4, 32–43. doi: 10.1111/j.1753-5131.2012.01059.x
Carmichael, R. H., Gaines, E., Sheller, Z., Tong, A., Clapp, A., and Valiela, I. (2009). Diet Composition of Juvenile Horseshoe Crabs: Implications for Growth and Survival of Natural and Cultured Stocks. Boston, MA: Springer. doi: 10.1007/978-0-387-89959-6_33
Cartwright-Taylor, L., Lee, J., and Hsu, C. C. (2009). Population structure and breeding pattern of the mangrove horseshoe crab Carcinoscorpius rotundicauda in Singapore. Aquat. Biol. 8, 61–69. doi: 10.3354/ab00206
Cartwright-Taylor, L., Yap, V. B., Hsu, C. C., and Lou, S. T. (2011). Distribution and abundance of horseshoe crabs Tachypleus gigas and Carcinoscorpius rotundicauda around the main island of Singapore. Aquat. Biol. 13, 127–136. doi: 10.3354/ab00346
Chen, C. P., Yeh, H. Y., and Lin, P. F. (2004). Conservation of the horseshoe crab at Kinmen, Taiwan: strategies and practices. Biodiver. Conservat. 13, 1889–1904. doi: 10.1023/B:BIOC.0000035868.11083.84
Cooper, J. F., Levin, J., and Wagner, H. N. (1971). Quantitative comparison of in vitro and in vivo methods for the detection of endotoxin. J. Lab. Clin. Med. 78:138.
Daim, T. U., Rueda, G., Martin, H., and Gerdsri, P. (2006). Forecasting emerging technologies: use of bibliometrics and patent analysis. Technol. Forecast. Soc. Change 73, 981–1012. doi: 10.1016/j.techfore.2006.04.004
Delvaeye, M., and Conway, E. M. (2009). Coagulation and innate immune responses: can we view them separately? Blood 114, 2367–2374. doi: 10.1182/blood-2009-05-199208
Ding, J. L., and Ho, B. (2001). A new era in pyrogen testing. Trends Biotechnol. 19, 277–281. doi: 10.1016/S0167-7799(01)01694-8
Dorrington, T., and Gomez-Chiarri, M. (2008). Antimicrobial peptides for use in oyster aquaculture: effect on pathogens, commensals, and eukaryotic expression systems. J. Shellfish Res. 27, 365–373. doi: 10.2983/0730-8000(2008)27[365:APFUIO]2.0.CO;2
Falagas, M. E., Pitsouni, E. I., Malietzis, G. A., and Pappas, G. (2008). Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB J. 22, 338–342. doi: 10.1096/fj.07-9492LSF
Fennrich, S., Hennig, U., Toliashvili, L., Schlensak, C., Wendel, H. P., and Stoppelkamp, S. (2016). More than 70 years of pyrogen detection: current state and future perspectives. Atla Alternat. Lab. Anim. 44, 239–253. doi: 10.1177/026119291604400305
Frecer, V., Ho, B., and Ding, J. L. (2004). De novo design of potent antimicrobial peptides. Antimicrob. Agents Chemother. 48, 3349–3357. doi: 10.1128/AAC.48.9.3349-3357.2004
Fu, Y., Huang, S., Wu, Z., Su, M., and Wang, J. (2019). Socio-demographic drivers and public perceptions of consumption and conservation of asian horseshoe crabs in northern beibu gulf, China. Aquatic Conservat. Mar. Freshwater Ecosyst. 29, 1268–1277. doi: 10.1002/aqc.3125
Gauvry, G. (2015). “Current horseshoe crab harvesting practices cannot support global demand for TAL/LAL: the pharmaceutical and medical device industries' role in the sustainability of horseshoe crabs,” in Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management, eds R. Carmichael, M. Botton, P. Shin, S. Cheung (Cham: Springer), 475–482. doi: 10.1007/978-3-319-19542-1_27
Godin, B. (2006). On the origins of bibliometrics. Scientometrics 68, 109–133. doi: 10.1007/s11192-006-0086-0
Gong, L., Fan, G., Ren, Y., Chen, Y., Qiu, Q., Liu, L., et al. (2019). Chromosomal level reference genome of Tachypleus tridentatus provides insights into evolution and adaptation of horseshoe crabs. Mol. Ecol. Res. 19, 744–756. doi: 10.1111/1755-0998.12988
Hu, M., Wang, Y., Chen, Y., Cheung, S., Shin, P., and Li, Q. (2009). Summer distribution and abundance of juvenile Chinese horseshoe crabs Tachypleus tridentatus along an intertidal zone in southern China. Aquatic Biol. 7, 107–112. doi: 10.3354/ab00194
Hu, M., Wang, Y., Cheung, S. G., and Shin, P. K. S. (2013). Comparison of different frozen natural foods on survival and growth of juvenile Chinese horseshoe crab Tachypleus tridentatus (leach, 1819): implications on laboratory culture. Aquacul. Res. 44, 567–573. doi: 10.1111/j.1365-2109.2011.03059.x
Hu, M., Wang, Y., Tsang, S. T., Cheung, S. G., and Shin, P. K. S. (2010). Effect of prolonged starvation on body weight and blood-chemistry in two horseshoe crab species: Tachypleus tridentatus and Carcinoscorpius rotundicauda (Chelicerata: Xiphosura). J. Exp. Mar. Biol. Ecol. 395, 112–119. doi: 10.1016/j.jembe.2010.08.023
Hu, M., Wang, Y., Tsang, S. T., Cheung, S. G., and Shin, P. K. S. (2011). Effect of starvation on the energy budget of two asian horseshoe crab species: Tachypleus tridentatus and Carcinoscorpius rotundicauda (Chelicerata: Xiphosura). Mar. Biol. 158, 1591–1600. doi: 10.1007/s00227-011-1672-0
Itow, T., Loveland, R. E., and Botton, M. L. (1998). Developmental abnormalities in horseshoe crab embryos caused by exposure to heavy metals. Arch. Environ. Contamin. Toxicol. 35, 33–40. doi: 10.1007/s002449900345
Iwanaga, S. (2002). The molecular basis of innate immunity in the horseshoe crab. Curr. Opin. Immunol. 14, 87–95. doi: 10.1016/S0952-7915(01)00302-8
Iwanaga, S., Kawabata, S. I., and Muta, T. (1998). New types of clotting factors and defense molecules found in horseshoe crab hemolymph: their structures and functions. J. Biochem. 123, 1–15. doi: 10.1093/oxfordjournals.jbchem.a021894
Jiang, N., Thangamani, S., Chor, C. F., Wang, S. Y., Winarsih, I., Du, R. J., et al. (2009). A novel serine protease inhibitor acts as an immunomodulatory switch while maintaining homeostasis. J. Innate Immun. 1, 465–479. doi: 10.1159/000209224
John, B. A., Nelson, B. R., Sheikh, H. I., Cheung, S. G., Wardiatno, Y., Dash, B. P., et al. (2018). A review on fisheries and conservation status of Asian horseshoe crabs. Biodiver. Conservat. 27, 3573–3598. doi: 10.1007/s10531-018-1633-8
Kawabata, S. I., Nagayama, R., Hirata, M., Shigenaga, T., Agarwala, K. L., Saito, T., et al. (1996). Tachycitin, a small granular component in horseshoe crab hemocytes, is an antimicrobial protein with chitin-binding activity. J. Biochem. 120, 1253–1260. doi: 10.1093/oxfordjournals.jbchem.a021549
Kreamer, G., and Michels, S. (2009). “History of horseshoe crab harvest on delaware bay,” in Biology and Conservation of Horseshoe Crabs, eds. J. T. Tanacredi, M. L. Botton, and D. R. Smith (Boston, MA: Springer), 299–313. doi: 10.1007/978-0-387-89959-6_19
Krisfalusi-Gannon, J., Ali, W., Dellinger, K., Robertson, L., Brady, T. E., Goddard, M. K. M., et al. (2018). The role of horseshoe crabs in the biomedical industry and recent trends impacting species sustainability. Front. Mar. Sci. 5:13. doi: 10.3389/fmars.2018.00185
Kumar, V., Roy, S., Sahoo, A. K., and Kumar, V. (2016). Horseshoe crabs: biomedical importance and its potential use in developing health-care products. Indian J. Geo Mar. Sci. 45, 1234–1244. Available online at: http://nopr.niscair.res.in/handle/123456789/35707
Kwan, B. K. Y., Chan, A. K., Cheung, S. G., and Shin, P. K. S. (2017). Marine microalgae as dietary supplements in the culture of juvenile Chinese horseshoe crabs, Tachypleus tridentatus (Xiphosura). Aquacul. Res. 48, 3910–3924. doi: 10.1111/are.13218
Kwan, B. K. Y., Cheung, S. G., and Shin, P. K. S. (2015). A dual stable isotope study for diet composition of juvenile Chinese horseshoe crab Tachypleus tridentatus (Xiphosura) on a seagrass-covered intertidal mudflat. Mar. Biol. 162, 1137–1143. doi: 10.1007/s00227-015-2647-3
Kwan, B. K. Y., Hsieh, H. L., Cheung, S. G., and Shin, P. K. S. (2016). Present population and habitat status of potentially threatened asian horseshoe crabs Tachypleus tridentatus and Carcinoscorpius rotundicaudain hong kong: a proposal for marine protected areas. Biodivers. Conservat. 25, 673–692. doi: 10.1007/s10531-016-1084-z
Laurie, K., Chen, C.-P., Cheung, S. G., Do, V., Hsieh, H., John, A., et al. (2019). Tachypleus tridentatus (errata version published in 2019). The IUCN Red List of Threatened Species 2019: e.T21309A149768986.
Leschen, A. S., and Correia, S. J. (2010). Mortality in female horseshoe crabs (Limulus polyphemus) from biomedical bleeding and handling: implications for fisheries management. Mar. Freshwater Behav. Physiol. 43, 135–147. doi: 10.1080/10236241003786873
Levin, J., and Bang, F. B. (1964). A description of cellular coagulation in the Limulus. Bull. Johns Hopkins Hospital 115:337.
Leydesdorff, L. (2007). Betweenness centrality as an indicator of the interdisciplinarity of scientific journals. J. Am. Soc. Inform. Sci. Technol. 58, 1303–1319. doi: 10.1002/asi.20614
Li, P., Ho, B., and Ding, J. L. (2007). Recombinant Factor C competes against LBP to bind lipopolysaccharide and neutralizes the endotoxicity. J. Endot. Res. 13, 150–157. doi: 10.1177/0968051907079573
Li, X., Dai, J., Tang, Y., Li, L., and Jin, G. (2017). Quantitative proteomic profiling of tachyplesin I targets in U251 gliomaspheres. Mar. Drugs 15:25. doi: 10.3390/md15010020
Liao, Y., Hsieh, H.-L., Xu, S., Zhong, Q., Lei, J., Liang, M., et al. (2019a). Wisdom of crowds reveals decline of Asian horseshoe crabs in Beibu Gulf, China. Oryx 53, 222–229. doi: 10.1017/S003060531700117X
Liao, Y. Y., Xu, P. W., Kwan, K. Y., Ma, Z. Y., Fang, H. Y., Xu, J. Y., et al. (2019b). Draft genomic and transcriptome resources for marine chelicerate Tachypleus tridentatus. Sci. Data 6:190029. doi: 10.1038/sdata.2019.29
Liu, C. B., Qi, J. L., Shan, B., and Ma, Y. B. (2018). Tachyplesin causes membrane instability that kills multidrug-resistant bacteria by inhibiting the 3-Ketoacyl carrier protein reductase FabG. Front. Microbiol. 9:825. doi: 10.3389/fmicb.2018.00825
Lv, M. Q., Wu, S. J., Chen, C. D., Jiang, Y., Wen, Z. F., Chen, J. L., et al. (2015). A review of studies on water level fluctuating zone (WLFZ) of the Three Gorges Reservoir(TGR) based on bibliometric perspective. Acta Ecol. Sinica 35, 3504–3518. doi: 10.5846/stxb201309252366
Maloney, T., Phelan, R., and Simmons, N. (2018). Saving the horseshoe crab: a synthetic alternative to horseshoe crab blood for endotoxin detection. PLoS Biol. 16:e2006607. doi: 10.1371/journal.pbio.2006607
Marggraf, M. B., Panteleev, P. V., Emelianova, A. A., Sorokin, M. I., Bolosov, I. A., Buzdin, A. A., et al. (2018). Cytotoxic potential of the novel horseshoe crab peptide Polyphemusin III. Marine Drugs 16:19. doi: 10.3390/md16120466
Mashar, A., Butet, N. A., Juliandi, B., Qonita, Y., Hakim, A. A., and Wardiatno, Y. (2017). “Biodiversity and distribution of horseshoe crabs in northern coast of java and southern coast of Madura,” in 3rd International Symposium on Lapan-Ipb Satellite for Food Security and Environmental Monitoring 2016, ed Y. Setiawan (Bogor). doi: 10.1088/1755-1315/54/1/012076
Mongeon, P., and Paul-Hus, A. (2016). The journal coverage of Web of Science and Scopus: a comparative analysis. Scientometrics 106, 213–228. doi: 10.1007/s11192-015-1765-5
Moqsud, M. A., Sato, K., Hasan, M., Yukio, N., and Omine, K. (2017). Seasonal variation of contaminated geo-environmental condition of Yamaguchi bay tidal flat, Japan. Reg. Stud. Mar. Sci. 10, 27–31. doi: 10.1016/j.rsma.2017.01.002
Morris, S., Deyong, C., Wu, Z., Salman, S., and Yemenu, D. (2002). Diva : a visualization system for exploring document databases for technology forecasting. Comput. Indust. Eng. 43, 841–862. doi: 10.1016/S0360-8352(02)00143-2
Moura, R. M., Queiroz, A. F. S., Fook, J. M. S. L. L., Dias, A. S. F., Monteiro, N. K. V., Ribeiro, J. K. C., et al. (2006). CvL, a lectin from the marine sponge Cliona varians: Isolation, characterization and its effects on pathogenic bacteria and Leishmania promastigotes. Comparat. Biochem. Physiol. Part A Mol. Integrat. Physiol. 145, 517–523, doi: 10.1016/j.cbpa.2006.08.028
Nederhof, A. J. (2006). Bibliometric monitoring of research performance in the social sciences and the humanities: a review. Scientometrics 66, 81–100. doi: 10.1007/s11192-006-0007-2
Ng, P. M. L., Le Saux, A., Lee, C. M., Tan, N. S., Lu, J., Thiel, S., et al. (2007). C-reactive protein collaborates with plasma lectins to boost immune response against bacteria. EMBO J. 26, 3431–3440. doi: 10.1038/sj.emboj.7601762
Novitsky, T. J. (1984). Discovery to commercialization: the blood of the horseshoe crab. Oceanus 27, 13–18.
Novitsky, T. J. (2009). “Biomedical applications of limulus amebocyte lysate,” in Biology and Conservation of Horseshoe Crabs, eds J. Tanacredi, M. Botton, and D. Smith (Boston, MA: Springer), 315–329. doi: 10.1007/978-0-387-89959-6_20
Perry, L. M. (1931). Catfish feeding on the eggs of the horseshoe crab, Limulus polyphemus. Science 74, 312–312. doi: 10.1126/science.74.1917.312
Price, K. S. (1962). Biology of the sand shrimp, Crangon septemspinosa, in the shore zone of the Delaware Bay region. Chesapeake Sci. 3, 244–255. doi: 10.2307/1350631
Raabe, D., Al-Sawalmih, A., Romano, P., Sachs, C., Brokmeier, H. G., Yi, S. B., et al. (2005). Structure and crystallographic texture of arthropod bio-composites. Mater. Sci. Forum 495–497, 1665–1674. doi: 10.4028/www.scientific.net/MSF.495-497.1665
Rudkin, D. M., and Young, G. A. (2009). “Horseshoe crabs - an ancient ancestry revealed,” in Biology and Conservation of Horseshoe Crabs, eds. J. T. Tanacredi, M. L. Botton, and D. R. Smith (Boston, MA: Springer), 25–44. doi: 10.1007/978-0-387-89959-6_2
Rudloe, A. (1982). Man's influence as an environmental threat to Limulus. Prog. Clin. Biol. Res. 81, 297–300.
Sekiguchi, K., and Sugita, H. (1980). Systematics and hybridization in the four living species of horseshoe crabs. Evolution 34:712. doi: 10.1111/j.1558-5646.1980.tb04010.x
Sheikh, H. I., Kamaruzzaman, B. Y., John, A. B., Zaleha, K., and Ichwan, S. J. A. (2019). Spermiogram of wild and captive malaysian horseshoe crab (Tachypleus gigas) from Pantai Balok, Kuantan, Pahang, Malaysia. Sains Malaysiana 48, 325–328. doi: 10.17576/jsm-2019-4802-08
Shin, P. K. S., Chan, C. S. K., and Cheung, S. G. (2014). Physiological energetics of the fourth instar of Chinese horseshoe crabs (Tachypleus tridentatus) in response to hypoxic stress and re-oxygenation. Mar. Pollut. Bull. 85, 522–525. doi: 10.1016/j.marpolbul.2013.10.023
Shin, P. K. S., Li, H., and Cheung, S. G. (2009). “Horseshoe crabs in Hong Kong: current population status and human exploitation,” in Biology and Conservation of Horseshoe Crabs, eds J. Tanacredi, M. Botton, and D. Smith (Boston, MA: Springer) 347–360. doi: 10.1007/978-0-387-89959-6_22
Shuster, C. N. (1962). Serological Correspondence Among Horseshoe “Crabs” (Limulidae). New York, NY: Zoologica scientific contributions of the New York Zoological Society.
Smith, D. R., Beekey, M. A., Brockmann, H. J., King, T. L., Millard, M. J., and Zaldívar-Rae, J. A. (2016). Limulus polyphemus. The IUCN Red List of Threatened Species 2016: e.T11987A80159830.
Smith, D. R., Brockmann, H. J., Beekey, M. A., King, T. L., Millard, M. J., and Zaldívar-Rae, J. (2017). Conservation status of the American horseshoe crab, (Limulus f): a regional assessment. Rev. Fish Biol. Fisheries 27, 1–41. doi: 10.1007/s11160-016-9461-y
Smith, D. R., Millard, M. J., and Carmichael, R. H. (2009). “Comparative status and assessment of Limulus polyphemus with emphasis on the new england and delaware bay populations,” in Biology and Conservation of Horseshoe Crabs, eds J. Tanacredi, M. Botton, and D. Smith (Boston, MA: Springer), 361–386. doi: 10.1007/978-0-387-89959-6_23
Smith, S. A., and Berkson, J. (2005). Laboratory culture and maintenance of the horseshoe crab (Limulus polyphemus). Lab. Anim. 34, 27–34. doi: 10.1038/laban0705-27
Swan, B. L. (2002). “A Unique Medical Product (LAL) from the horseshoe crab and monitoring the delaware bay horseshoe crab population,” in Limulus in the Limelight, ed. J. T. Tanacredi (Boston, MA: Springer), 53–62. doi: 10.1007/0-306-47590-1_5
Sweileh, W. M. (2018). Bibliometric analysis of peer-reviewed literature in transgender health (1900-2017). BMC Int. Health Hum. Rights 18:16. doi: 10.1186/s12914-018-0155-5
Tan, N. S., Ho, B., and Ding, J. L. (2000b). High-affinity lps binding domain(s) in recombinant Factor C of a horseshoe crab neutralizes LPS-induced lethality. Faseb J. 14, 859–870. doi: 10.1096/fasebj.14.7.859
Tan, N. S., Ng, M. L. P., Yau, Y. H., Chong, P. K. W., Ho, B., and Ding, J. L. (2000a). Definition of endotoxin binding sites in horseshoe crab Factor C recombinant sushi proteins and neutralization of endotoxin by sushi peptides. Faseb J. 14:1801. doi: 10.1096/fj.99-0866com
Tanacredi, J. H., Botton, M. L., and Smith, D. (2009). Biology and Conservation of Horseshoe Crabs. Boston, MA: Springer. doi: 10.1007/978-0-387-89959-6
Tanacredi, J. T., and Portilla, S. (2015). “Habitat inventory trend analysis of Limulus polyphemus populations on long Island, U.S.A.: from the tip of brooklyn to the tip of Montauk, 2003–2014,” in Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management, eds R. Carmichael, M. Botton, P. Shin, S. Cheung (Cham: Springer), 229–236. doi: 10.1007/978-3-319-19542-1_12
Thelwall, M. (2008). Bibliometrics to webometrics. J. Inform. Sci. 34, 605–621. doi: 10.1177/0165551507087238
Unzueta, U., Cespedes, M. V., Ferrer-Miralles, N., Casanova, I., Cedano, J., Corchero, J. L., et al. (2012). Intracellular CXCR4(+) cell targeting with T22-empowered protein-only nanoparticles. Int. J. Nanomed. 7, 4533–4544. doi: 10.2147/IJN.S34450
van Eck, N. J., and Waltman, L. (2007). “VOS: a new method for visualizing similarities between objects,” in Advances in Data Analysis. Studies in Classification, Data Analysis, and Knowledge Organization, eds R. Decker and H. J. Lenz (Berlin; Heidelberg: Springer), 299–306. doi: 10.1007/978-3-540-70981-7_34
van Eck, N. J., and Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538. doi: 10.1007/s11192-009-0146-3
van Eck, N. J., and Waltman, L. (2017). Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics 111, 1053–1070. doi: 10.1007/s11192-017-2300-7
Vestbo, S., Obst, M., Fernandez, F. J. Q., Intanai, I., and Funch, P. (2018). Present and potential future distributions of Asian horseshoe crabs determine areas for conservation. Front. Mar. Sci. 5:164. doi: 10.3389/fmars.2018.00164
Vezyridis, P., and Timmons, S. (2016). Evolution of primary care databases in UK: a scientometric analysis of research output. Bmj Open 6:e012785. doi: 10.1136/bmjopen-2016-012785
Walls, E. A., and Berkson, J. (2003). Effects of blood extraction on horseshoe crabs (Limulus polyphemus). Fishery Bull. Natl. Oceanic atmosphere. Administr. 101, 457–459. Available online at: https://vtechworks.lib.vt.edu/bitstream/handle/10919/48009/22wallsf.pdf;jsessionid=CE5A3B480A64885D7CA9F73E7D78B825?sequence=1
Wang, X., Guo, J., Gu, D., Yang, Y., Yang, X., and Zhu, K. (2019). Tracking knowledge evolution, hotspots and future directions of emerging technologies in cancers research: a bibliometrics review. J. Cancer 10, 2643–2653. doi: 10.7150/jca.32739
Widener, J. W., and Barlow, R. B. (1999). Decline of a horseshoe crab population on Cape Cod. Biol. Bull. 197, 300–302. doi: 10.2307/1542664
Wilson, T. D. (2001). Introductory concepts in information science. Inf. Process. Manag. 37, 764–766. doi: 10.1016/s0306-4573(00)00044-3
Yu, L., Guo, L., Ding, J. L., Ho, B., Feng, S., Popplewell, J., et al. (2009). Interaction of an artificial antimicrobial peptide with lipid membranes. Biochim. Biophys. Acta Biomembr. 1788, 333–344. doi: 10.1016/j.bbamem.2008.10.005
Zaldívar-Rae, J., Sapién-Silva, R. E., Rosales-Raya, M., and Brockmann, H. J. (2009). “American horseshoe crabs, Limulus polyphemus, in Mexico: Open Possibilities,” in Biology and Conservation of Horseshoe Crabs, eds J. Tanacredi, M. Botton, D. Smith (Boston, MA: Springer), 97–113. doi: 10.1007/978-0-387-89959-6_6
Keywords: bibliometric analysis, marine biology, horseshoe crab, VOSviewer, physiology, conservation, Limulus polyphemus, Tachypleus tridentatus
Citation: Luo Z, Miao F, Hu M and Wang Y (2020) Research Development on Horseshoe Crab: A 30-Year Bibliometric Analysis. Front. Mar. Sci. 7:41. doi: 10.3389/fmars.2020.00041
Received: 26 September 2019; Accepted: 22 January 2020;
Published: 11 February 2020.
Edited by:
Zhijun Dong, Yantai Institute of Coastal Zone Research (CAS), ChinaReviewed by:
Mark Botton, Fordham University, United StatesZhaohong Weng, Fisheries College, Jimei University, China
Copyright © 2020 Luo, Miao, Hu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youji Wang, eW91aml3YW5nMiYjeDAwMDQwO2dtYWlsLmNvbQ==; Menghong Hu, bWhodSYjeDAwMDQwO3Nob3UuZWR1LmNu
†These authors have contributed equally to this work
 Zhen Luo
Zhen Luo Fengze Miao
Fengze Miao Menghong Hu
Menghong Hu Youji Wang
Youji Wang