- 1Aix-Marseille Université, CNRS, IRD, INRA, Coll France, CEREGE, Aix-en-Provence, France
- 2Sorbonne Université, CNRS, Laboratoire d’Ecogéochimie des Environnements Benthiques (LECOB), UMR 8222, Observatoire Océanologique de Banyuls, Banyuls-sur-Mer, France
- 3Centre for Tropical Water and Aquatic Ecosystem Research TropWATER, James Cook University, Townsville, QLD, Australia
Groundwater discharge is today recognized as an important pathway for freshwater, nutrients and other dissolved chemical substances to coastal systems. While its effect on supporting primary production in coastal ecosystems is increasingly recognized, its impact on growth of animals at higher trophic level (primary and secondary consumers) is less well documented. Here, we investigate the impact of groundwater discharge on the growth of the Mediterranean mussel (Mytilus galloprovincialis) in a coastal lagoon. Growth rates and condition index (tissue weight/shell weight) of mussels growing at groundwater-exposed sites and at a control site in Salses-Leucate lagoon (France) were measured. The mussels in this lagoon produce circadian (daily rhythm) growth increments in their shell, as opposed to semi-diurnal increments in tidally influenced systems. Mussels from groundwater-influenced sites have higher growth rate and condition index compared to those from a control site. Importantly, growth rates from groundwater-influenced sites are amongst the highest rates reported for the Mediterranean region (41 ± 9 μm d–1). The higher growth rates at groundwater-influenced sites are likely a consequence of both the higher winter temperatures of lagoon water as a result of groundwater discharging with relatively constant temperatures, and the groundwater-driven nutrient supply that increase the food availability to support mussel growth. Overall, this study demonstrates that groundwater discharge to Mediterranean lagoons provides favorable environmental conditions for fast growth of mussels of high commercial-quality.
Introduction
Coastal lagoons are highly productive ecosystems, supporting a wide range of ecosystem services such as aquaculture, fishery, tourism and others (Newton et al., 2014; Riera et al., 2018; Velasco et al., 2018). They are very dynamic environments controlled by physical processes under both marine and terrestrial influence (Kjerfve, 1994). These ecosystems are threatened by climatic and anthropogenic disturbances such as land use change, coastal erosion, sedimentation and excessive nutrient loading (De Wit et al., 2005; Aliaume et al., 2007; Anthony et al., 2009). Whilst the role of surface waters (rivers, streams, runoff, etc.) in supporting biological production in coastal ecosystems has been extensively documented (Middelburg and Nieuwenhuize, 2001; Liu et al., 2010; Bianchi et al., 2014; Cloern et al., 2014), groundwater discharge is only more recently being recognized as an important pathway of nutrients for coastal systems (Slomp and Van Cappellen, 2004; Moore, 2010; Null et al., 2012).
In the Mediterranean Sea, which is an oligotrophic basin characterized by limited surface water inputs, groundwater discharge is a major source of nutrients to coastal systems and may thus affect the primary production in the ecosystems (Herrera-Silveira, 1998; Basterretxea et al., 2010; Tovar-Sánchez et al., 2014; Rodellas et al., 2015). However, the impacts of groundwater discharge are not limited to nutrient loading: a wide range of organisms also respond to changes in water salinity, light penetration into water column, pH and turbulence (Short and Burdick, 1996; Troccoli-Ghinaglia et al., 2010; Lee et al., 2017). For example, groundwater input has been demonstrated to increase meiofauna diversity (Encarnação et al., 2015) and the abundance and body size of Mediterranean mussels (Piló et al., 2018) in Olhos de Agua beach in Portugal, and enhance species richness, abundance and biomass of fishes and invertebrates in Japanese coastal waters, where high groundwater-borne nutrient concentrations have been reported (Hata et al., 2016; Utsunomiya et al., 2017). Conversely, groundwater discharge reduces coral species diversity in coastal systems due to lower salinity (Lirman et al., 2003; Amato et al., 2016). While some studies document the effect of groundwater discharge on primary producers in coastal ecosystems (e.g., Herrera-Silveira, 1998; Charette et al., 2001; Andrisoa et al., 2019), the impact of groundwater discharge on the growth of animals such as mussels and fish species remains largely unstudied (Hata et al., 2016; Piló et al., 2018).
In this study, we assess the effects of groundwater discharge on the growth of the Mediterranean mussel (Mytilus galloprovincialis), which is a commercially important species in the Mediterranean region. Typical for bivalves, mussels sequentially deposit new shell layers during their growth. The shell growth patterns are controlled by both environmental and physiological factors such as temperature, salinity, food availability, tides, day/night cycles and biological clocks (Evans, 1972; Richardson, 1988; Jones et al., 1989; Sato, 1997; Schöne et al., 2004). We here investigate the variations in growth rate and condition index (tissue dry weight/shell dry weight) of mussels growing in the groundwater-fed Salses-Leucate lagoon (France) and examine the role of environmental parameters in mussel growth in this natural environment.
Materials and Methods
Study Sites
Salses-Leucate lagoon is located on the southwestern French Mediterranean coast. The region experiences rainfall during fall and spring (500 mm per year) with little rain during summer, and is characterized by strong northwesterly winds, regularly exceeding 10 m s–1, which play an important role in the hydrodynamics of the lagoon (e.g., Stieglitz et al., 2013; Rodellas et al., 2018).
Groundwater discharges directly into the lagoon from two karstic springs, Font Estramar and Font Dame, with mean water flows of 3.0 × 105 m3 d–1 and 2.0 × 105 m3 d–1, respectively (Fleury et al., 2007; Figure 1). The lagoon is permanently connected with the Mediterranean Sea by three large artificial openings in the eastern part of the lagoon, through which lagoon water efficiently exchanges on a continuous basis. A recent study showed that nutrient inputs driven by the discharge of the two karstic springs are the main source of nitrogen for primary producers in the western basin (Andrisoa et al., 2019). A few small intermittent streams deliver freshwater to the lagoon from the catchment area only during high-rainfall periods.
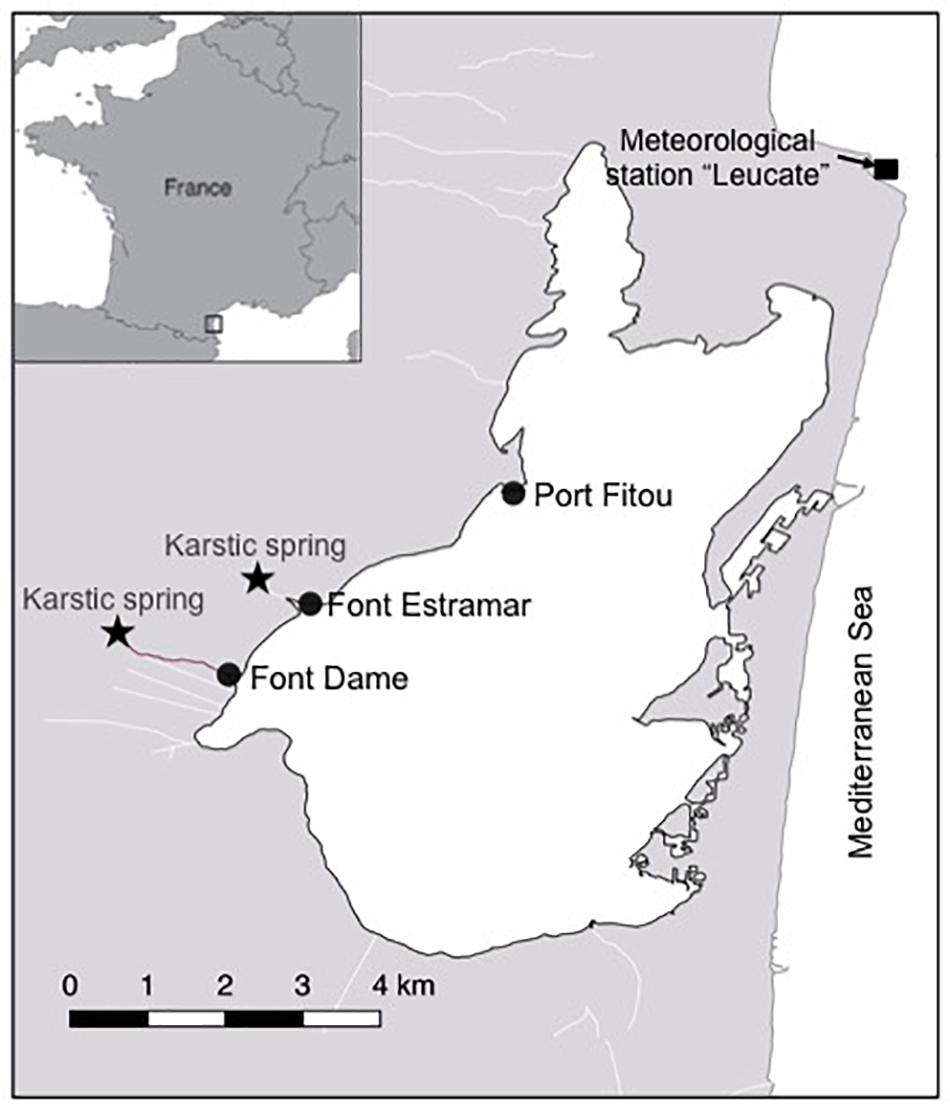
Figure 1. Salses-Leucate lagoon location on the French Mediterranean coastline. The groundwater-influenced sites (Font Dame and Font Estramar) and control site (Port Fitou) are shown, as well as the groundwater springs and the meteorological station.
Installation of Mussel Cages and Monitoring of Environmental Parameters
Three different locations in the western basin of Salses-Leucate lagoon were selected to evaluate the influence of groundwater inputs on mussel growth: two groundwater-influenced sites, located close to the karstic springs of Font Dame and Font Estramar, respectively, and a control site (Port Fitou) far from the groundwater sources and representative for average lagoon conditions (Figure 1). At each location, thirty to sixty (naturally present) specimens of Mediterranean mussels M. galloprovincialis were collected on 10 October 2016 at Font Dame and Font Estramar sites and 17 February 2017 at Port Fitou (Table 1). The initially selected lagoon control sites (i.e., deep cages) were not appropriate due to large salinity variability, requiring a new control site (Port Fitou) installed only 4 month after the beginning of the experiments. New specimens were collected on 27 June 2017 at Font Dame sites but not at Font Estramar sites. Shell length of each individual was measured and specimens were immersed in a calcein solution of 150 mg/L for 1 h. The fluorescent marker calcein is incorporated into growing calcium carbonate structures (Moran, 2000), and used for identification and measurement of growth of various calcifying species (Mahé et al., 2010; Lartaud et al., 2013; Nedoncelle et al., 2013). Sodium bicarbonate (105 g) was added to the solution to adjust the pH to 8.2 and to enhance the solubility of calcein. After shell marking, the calcein-marked mussels were returned to their original location by placing them in cages (25 × 12 cm). At a mean water depth of ca. 70 cm at both Font Dame and Font Estramar sites, cages were installed at 50 cm (surface cage) and 20 cm (bottom cage) above the bottom, experiencing different conditions in the periodically stratified water column due to fresh groundwater inflow. One cage was installed at Port Fitou (vertical homogeneity of the water column). As the mussels were returned to their respective original habitat, it is reasonable to assume that they tolerate the environmental conditions and that growth is not affected by the experimental setup. The calcein-marked mussels were periodically sampled from the cages between November 2016 and January 2018 (Table 1).
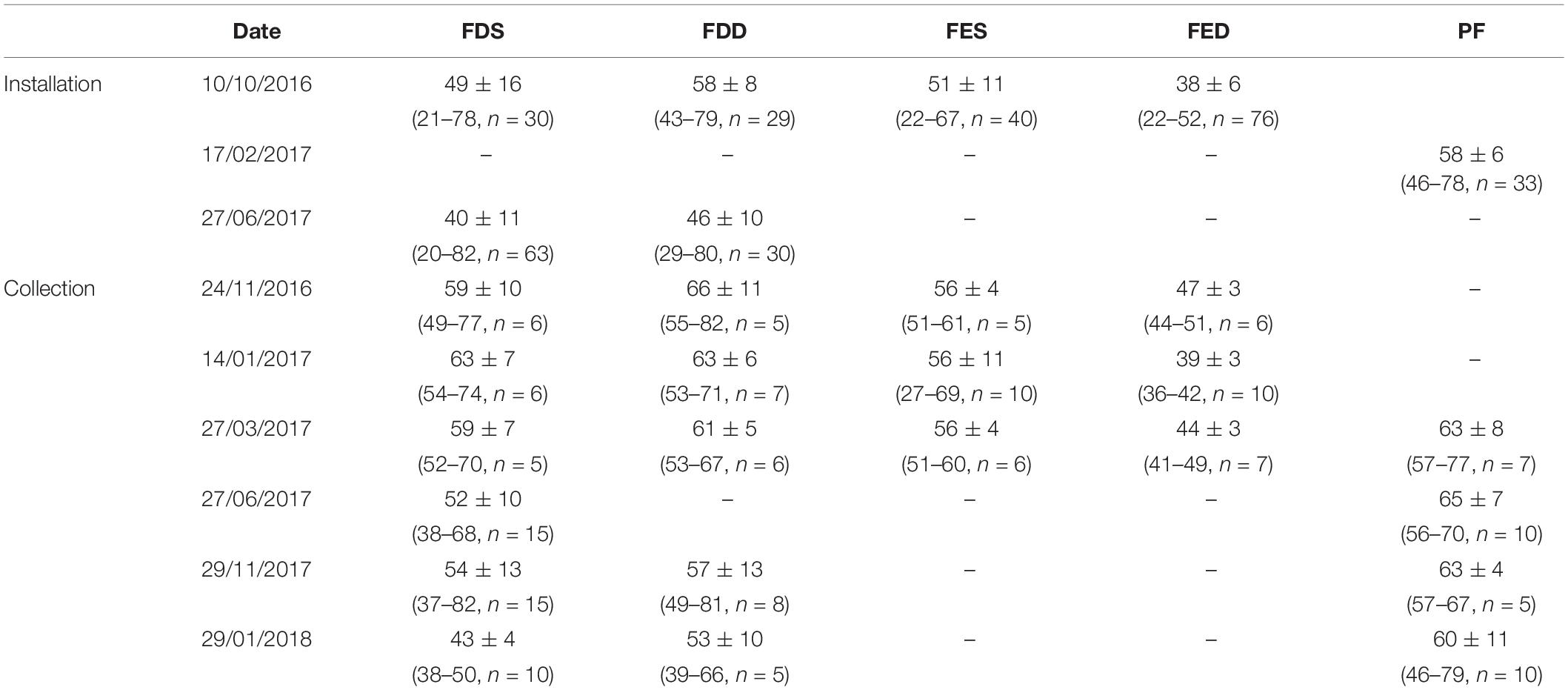
Table 1. The shell length mean (Mean ± SD) and range (Min–Max), and the number (n) of mussels installed/collected from the cages with collection date from the different stations in Salses-Leucate lagoon: FDS: Font Dame Surface, FDD: Font Dame Deep, FES: Font Estramar Surface, FED: Font Estramar Deep, and PF: Port Fitou.
CTD loggers (Solinst LTC Levelogger and NKE S2T600) were installed with all the mussel cages at the three sites to monitor temperature, salinity, and water level variations. Water level was corrected for atmospheric pressure. Loggers were protected with copper mesh to avoid biofouling of the sensors, and were regularly cleaned (once every 1–2 months). Precipitation, wind and atmospheric pressure data at the nearby meteorological station “Leucate” were extracted from the French meteorological service (Météo France).
Sample Preparation
In the laboratory, mussel samples were cleaned to remove all epibionts and other attached organisms. The shell total length was measured along the maximum growth axis using a caliper. The shells were carefully opened and tissues removed. The shell and the flesh of each individual were dried at 60°C overnight and weighted separately.
For the sclerochronological analysis, the right valve of each shell was cut along the maximum growth axis and perpendicular to the growth lines with a Buehler Isomet low-speed saw, using a 0.3 mm thick diamond wafering blade (Figure 2a). The section was mounted on a glass slide using Epoxy Araldite 2020 resin (Figure 2b). A thin section (0.8 mm) of shell was cut along the maximum growth axis, ground with 80, 180, 400, and 800 SiC grit, polished with 3, 1 and 0.3 μm Al2O3 powder and rinsed with deionized water following the protocol in Nedoncelle et al. (2013). In order to resolve growth patterns in the shells (Figure 2c), polished sections were etched in a Mutvei’s solution composed of 500 mL 1% acetic acid, 500 mL 25% glutaraldehyde, and 5 g alcian blue powder for 1 h at 37–40°C (Schöne et al., 2005a) (Figure 2d). Etched samples were immediately rinsed with deionized water and dried.
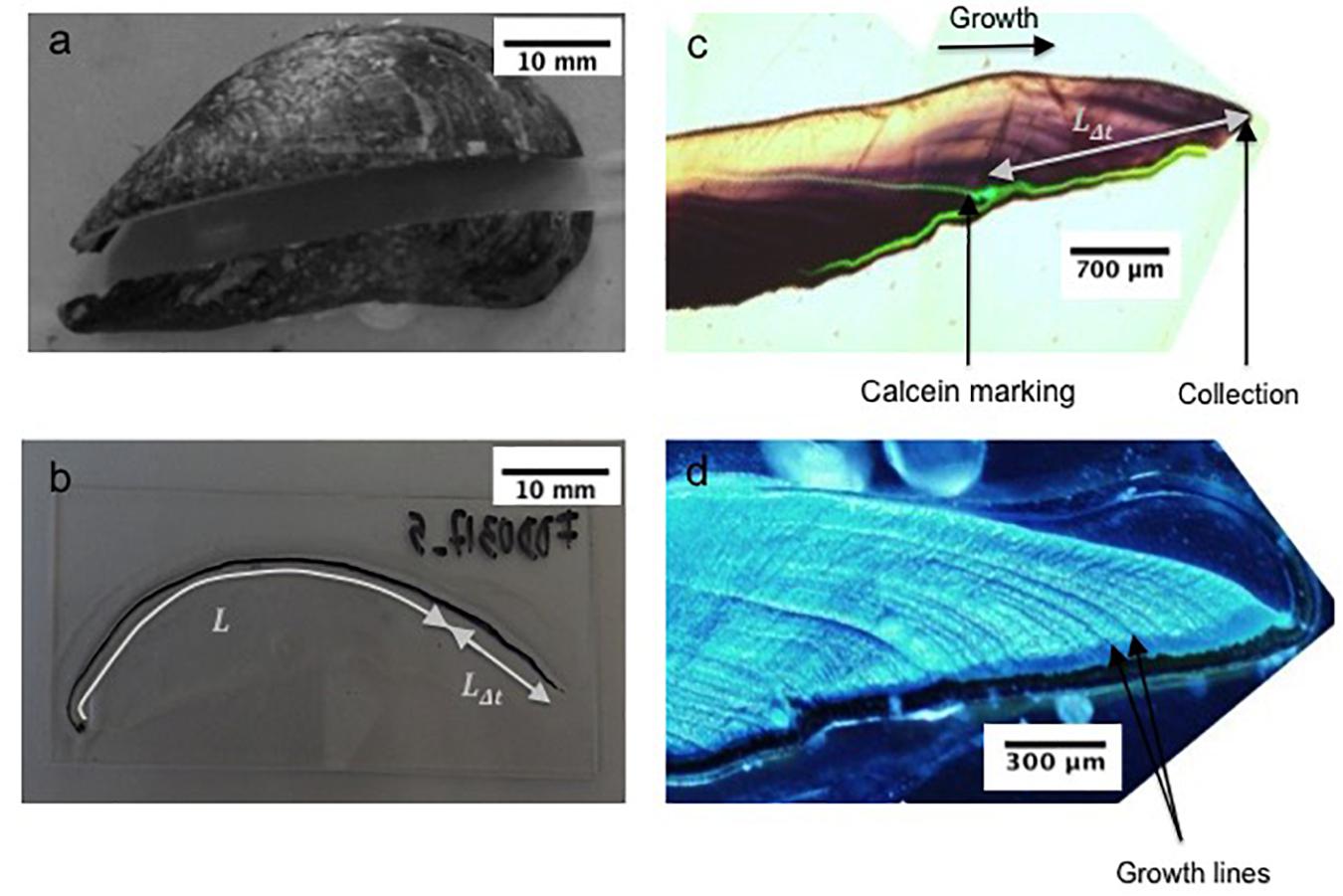
Figure 2. (a) Mussel section along the maximum growth axis, (b) section mounted on glass slide showing the shell length (Lt = L+LΔt), (c) shell under natural light showing the calcein marking, and (d) the shell under fluorescent light showing growth increments.
Condition Index
The condition index (C.I.) is defined as the ratio between the flesh (tissue) dry weight and the shell dry weight (Eq. 1). This index is commonly used to assess the health and the quality of the mussel for scientific and commercial purposes (Lucas and Beninger, 1985; Yildiz et al., 2006; Peharda et al., 2007). It is particularly important in quality assessment and marketing value of bivalves – the higher the proportion of tissue, the better the commercial value (Župan and Šarić, 2014).
Growth Analyses
In order to identify the growth lines marked with calcein, the cross-sections (on the glass slide) were viewed under epifluorescent microscope at magnification 4X (OLYMPUS BX61) and digitized with an OLYMPUS DP 72 camera at the Observatoire Oceanologique de Banyuls-sur-Mer, France (BIOPIC platform). Mutvei-treated sections were analyzed under reflecting light using the same microscope and camera. Growth analyses were carried out on mounted photographs using image processing software Adobe Photoshop and Image J. The distance between the calcein mark and the ventral edge of the shell (LΔt) was measured to allow an estimation of the growth rate during the period held in the cages (Figure 2c). The number of growth increments was counted to estimate the growth periodicity. The width of growth increments was measured to assess the relation between growth and environmental parameters. In some cases, the growth pattern was not well revealed by the Mutvei etching, which may lead to an underestimation of growth increments and an overestimation of the increment width (Nedoncelle et al., 2013). We focused our analysis on the shells with clear growth increments. The periodicities in shell growth were estimated by Fast Fourier Transform (FFT) (Welch, 1967; Walker, 1996).
Growth Curves
The growth rate of individual mussels was modeled using the Von Bertalanffy growth equation as described in Nedoncelle et al. (2013):
where Lt is the shell total length (mm), measured along the maximal growth axis (Figure 2b); L∞is the asymptotic theoretical shell length (mm); K represents the growth coefficient (year–1); and t0 is the time constant obtained from the minimum size at mussel settlement (L0) (L0 and t0 are assumed to be zero in our calculations; Ramón et al., 2007). For each individual, Lt was measured and the shell portion LΔt determined from the distance between the calcein mark and the ventral edge of the shell (Figure 2b).
The linear regression between the total shell length (Lt) and L allows to define the Ford-Walford y-intercept a and regression slope b used to calculate the Von Bertalanffy parameters K and L∞ (Nedoncelle et al., 2013):
The growths of mussels from different sites were compared using commonly used indices of growth performance: the phi-prime index (φ′) and the index P, calculated from the Von Bertalanffy growth parameters K and L∞ (e.g., Brey, 1999; Ragonese et al., 2012):
Statistical Analyses
Data normality and homogeneity of variances were tested with Shapiro–Wilk and Levene’s tests, respectively. All statistical analyses were considered at α = 0.05 level. Analysis of Variance (one-way ANOVA) was used to assess the differences in condition indices between sites Font Dame Surface, Font Dame Deep, Font Estramar Surface, Font Estramar Deep and Port Fitou. We used t-test statistics to determine if there were significant differences in condition indices, growth rates, salinity and water temperature between groundwater-influenced sites (Font Dame Surface, Font Dame Deep, Font Estramar Surface, Font Estramar Deep, which were pooled together for this comparison) and the control site (Port Fitou). The differences between surface cages (Font Dame Surface and Font Estramar Surface) and bottom cages (Font Dame Deep and Font Estramar Deep) were also tested using t-test statistics.
Results
Condition Index
The average condition indices estimated from the sampled mussels during the study period were 8.8 ± 2.3% (n = 50) at Font Dame Surface, 9.9 ± 2.3% (n = 26) at Font Dame Deep, 9.5 ± 3.3% (n = 20) at Font Estramar Surface, 8.8 ± 2.2% (n = 23) at Font Estramar Deep and 5.8 ± 1.4% (n = 30) at Port Fitou. The condition index varied significantly between sites (ANOVA: F = 5.32, p < 0.05) with significantly higher indices at the groundwater-influenced sites over the control site (t-test: t = 5.93, p < 0.05) (Figure 3A).
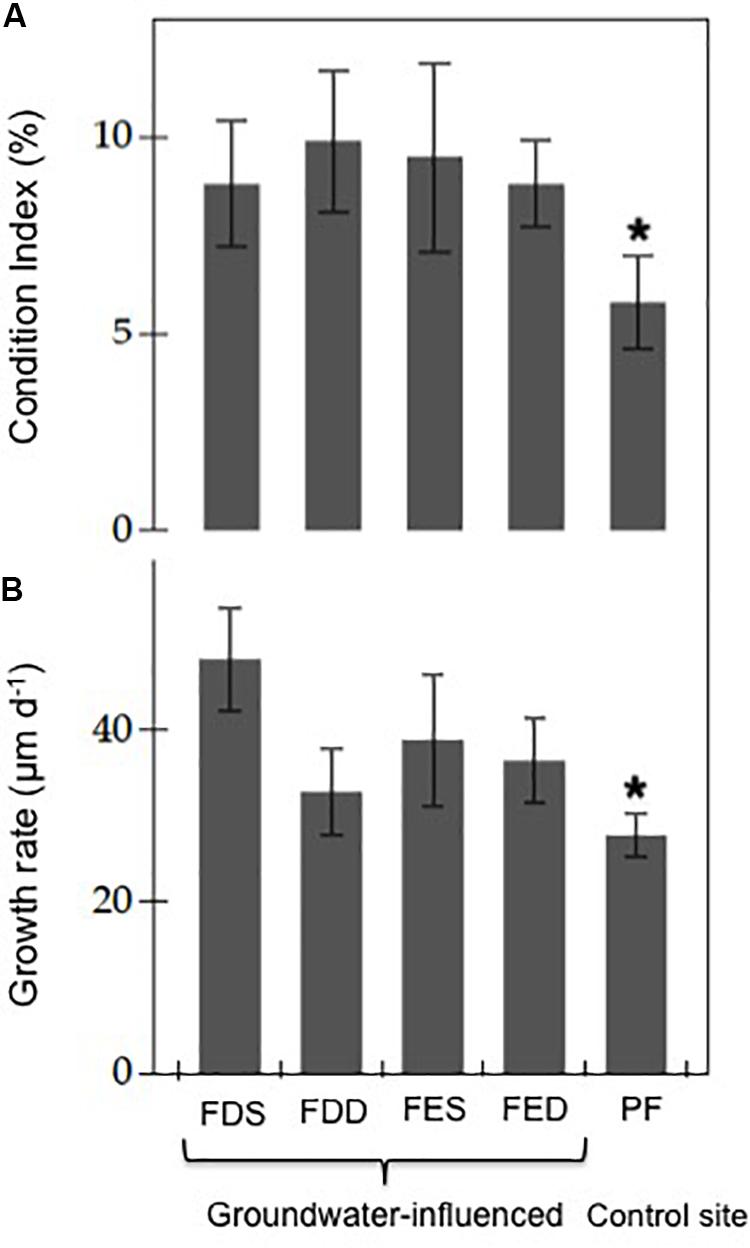
Figure 3. (A) Mean (±SD) condition indices and (B) growth rate in groundwater-influenced sites (FDS: Font Dame Surface, FDD: Font Dame Deep, FES: Font Estramar Surface and FED: Font Estramar Deep) and the control site (PF: Port Fitou) in Salses-Leucate lagoon. The asterisk indicates that p is less than 0.05 for the Student’s t-test between groundwater- influenced sites and control site (condition index: groundwater influenced-sites n = 119, control site n = 30, t-test, t = 5.93, p < 0.05; growth rate: groundwater influenced-sites n = 74, control site n = 8, t-test, t = 5.24, p < 0.05).
Shell Growth Rate
The mean growth rates for sampled mussels were 48.2 ± 6.0 μm d–1 (n = 31) at Font Dame Surface, 32.8 ± 5.0 μm d–1 (n = 17) at Font Dame Deep, 38.8 ± 7.7 μm d–1 (n = 13) at Font Estramar Surface, 36.4 ± 5.0 μm d–1 (n = 13) at Font Estramar Deep and 27.7 ± 2.5 μm d–1 (n = 8) at Port Fitou (Figure 3B). Similar to the condition index, the growth rate of mussels from the groundwater-influenced sites (mean = 40.9 ± 9.2 μm d–1) was significantly higher than that of the control site (27.7 ± 2.5 μm d–1) (t-test: t = 5.24, p < 0.05). At the groundwater-influenced sites (Font Dame and Font Estramar), the growth rates were significantly higher for the mussels from the surface than those from the bottom (t-test: t = 3.97, p < 0.05) with the highest rate observed at Font Dame Surface.
Growth Curves
The Von Bertalanffy growth curves derived from M. galloprovincialis collected in Salses-Leucate lagoon at the groundwater-influenced sites (combined data from Font Dame Surface, Font Dame Deep, Font Estramar Surface and Font Estramar Deep; n = 79, size range = 26.5–81.5 mm) and at the control site (n = 11, size range = 56.0–68.0 mm) are presented in Figure 4, together with curves obtained for the same species growing at other coastal Mediterranean sites. Overall, the growth rates of M. galloprovincialis from Salses-Leucate lagoon (groundwater-influenced sites: K = 0.54, L∞ = 75.0 mm; control site: K = 0.46, L∞ = 63.9 mm) are among the highest reported for this species in the Mediterranean region, particularly for the groundwater-influenced sites (Table 2; Figure 4). As commonly observed, the results indicated a fast growth rate at a younger age and a decrease in growth rates as the shell approaches its maximum size (Figure 4). Individuals collected from the groundwater-influenced sites showed higher total growth rates than individual from the control site. For instance, after 3 years, mussels from the groundwater-influenced sites reached 60 mm while those from the control site reached only 48 mm. Furthermore, the growth performance indices at the groundwater influenced sites (φ′ = 3.48, P = 1.61) and the control site (φ′ = 3.27, P = 1.47) were also among the highest reported to date for this species in the Mediterranean region (Table 2).
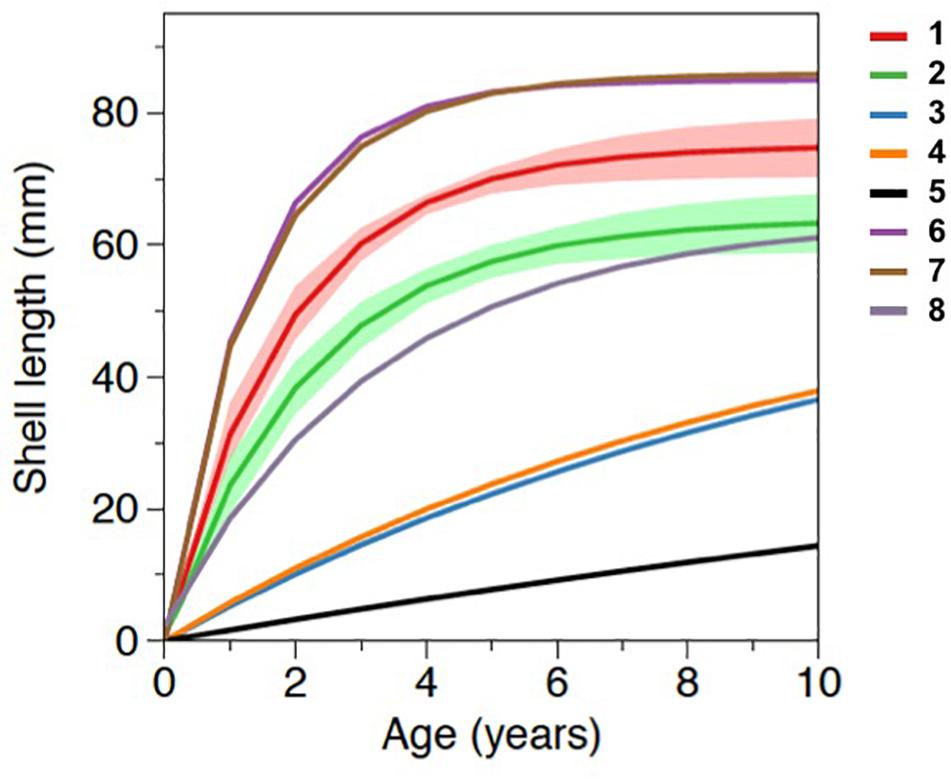
Figure 4. Von Bertalanffy growth curves of Mytilus galloprovincialis from the groundwater-influenced sites and the control site in Salses-Leucate lagoon and from other coastal systems in the Mediterranean region with 1: groundwater-influenced sites in this study (K = 0.54, L∞ = 75.0); 2: control site in this study (K = 0.46, L∞ = 63.9); 3: semi-enclosed basin in Italy (K = 0.09, L∞ = 62.1, Posa and Tursi, 1991); 4: coastal basin in Italy (K = 0.10, L∞ = 58.7, Posa and Tursi, 1991); 5: coastal bay in Italy (K = 0.03, L∞ = 51.3, Sarà et al., 2012); 6: coastal bay in Spain (K = 0.76, L∞ = 85.0, Ramón et al., 2007), 7: coastal lagoon in Italy (K = 0.66, L∞ = 85.9, Ceccherelli and Rossi, 1984), and 8: coastal area in Algeria (K = 0.31, L∞ = 64.0, Abada-Boujemaa, 1996). Shaded areas represent the standard deviations of data obtained in the present study.
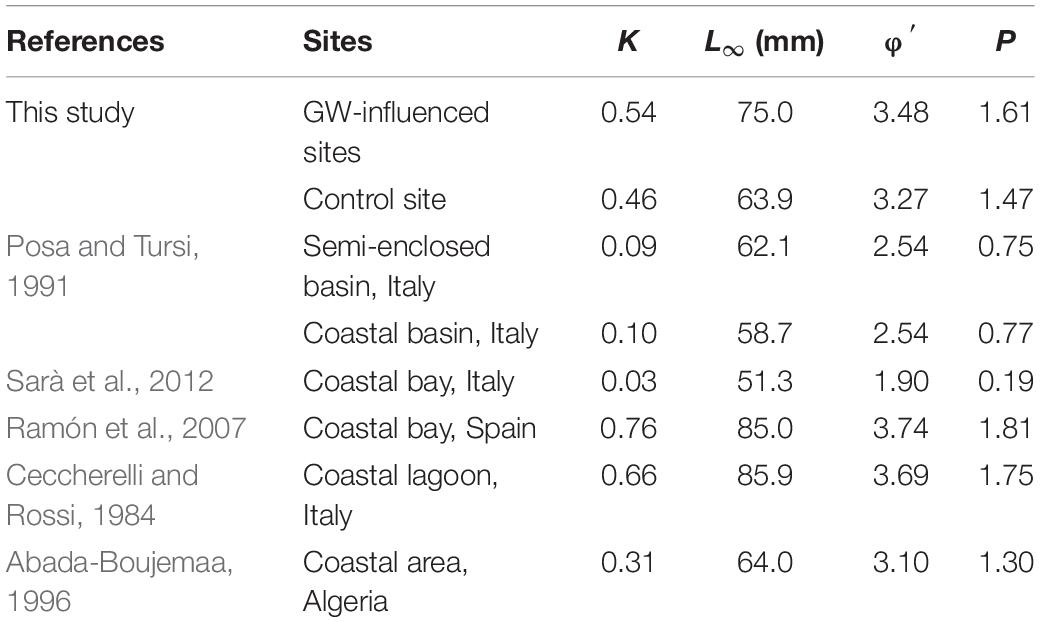
Table 2. The Von Bertalanffy growth parameters and the growth performance indices values of M. galloprovincialis from this study and other coastal systems in the Mediterranean region.
Growth Increments
Mussels growing in Salses-Leucate lagoon formed growth increment with daily (circadian) rhythm. Among the shells showing clear growth pattern, the average number of increments were 0.9 ± 0.2 (n = 17), 0.7 ± 0.1 (n = 15), 0.8 ± 0.2 (n = 9), 0.8 ± 0.1 (n = 9), and 0.7 ± 0.1 (n = 6) per day in Font Dame Surface, Font Dame Deep, Font Estramar Surface, Font Estramar Deep and Port Fitou, respectively. For instance, a mussel collected at Font Estramar Surface formed 93 growth increments during 96 days. The number of increments was consistently lower than the number of days.
Sclerochronological profiles showed a large variability in increment width for a given shell. The periodicities in increments width were estimated by FFT analyses. The analyzed shells exhibited similar patterns and, as an example, three shells (one each site) are presented in Figure 5. The FFT analyses revealed peaks at low frequency corresponding to periodicities of 12.7, 11.2, and 11.3 increments for the shells S1 (FDS0118-3), S2 (FDD0118-4), and S3 (PF0118-3), respectively. Considering the near-daily rhythm of the growth increment, the peaks correspond thus to a period of 11–13 days (near-fortnightly period). The power spectrum for the three shells also showed peaks at high frequencies corresponding to the periods of approximately 3 and 5 days.
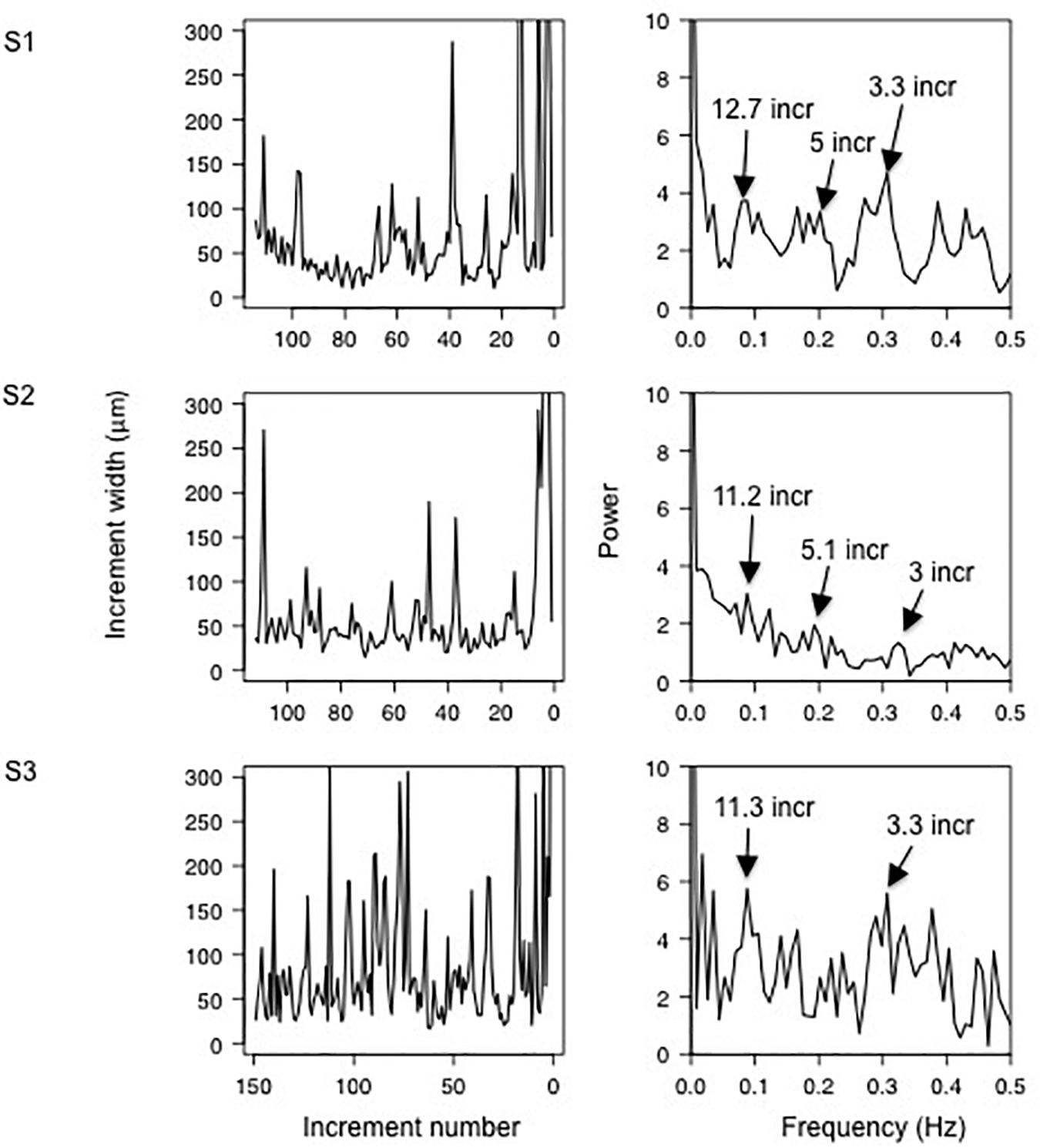
Figure 5. (Left panel) increment number from the calcein mark (right side) to the collection (left side) for three representative Mytilus galloprovincialis shells S1 (FDS0118-3), S2 (FDD0118-4), and S3 (PF0118-3) between June 2017 and January 2018. (Right panel) Fast Fourier Transform showing the periodicities of the increment width.
Environmental Parameters
Time Series Variations
The daily precipitation in Salses-Leucate lagoon ranged between 0.0 and 70.2 mm with a maximum value recorded shortly after the calcein marking (13 October 2016) (Figure 6a). High rainfall events coincided overall with high wind events and occurred chiefly between January and April. The region is characterized by frequent strong winds generally blowing from the northwest. Southeasterly winds also arrive from the Mediterranean Sea but they are less frequent. The wind speed recorded varied between 1.6 and 18.0 m s–1 with an average value of 5.8 ± 2.8 m s–1 (Figure 6b).
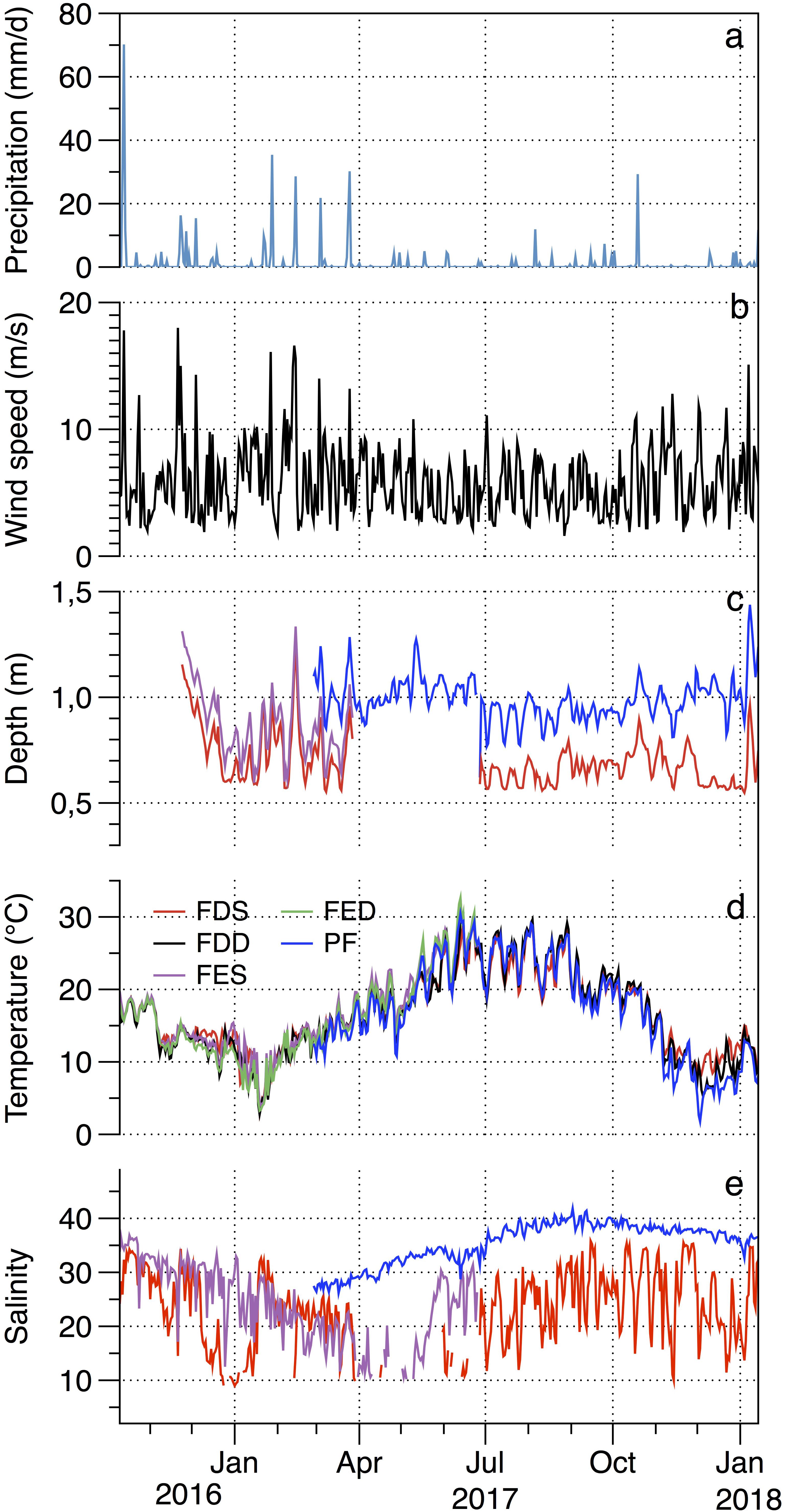
Figure 6. Temporal variations between October 2016 and January 2018 in (a) precipitation, (b) wind speed, (c) water depth, (d) temperature, and (e) salinity in Salses-Leucate lagoon: Font Dame Surface (FDS), Font Dame Deep (FDD), Font Estramar Surface (FES), Font Estramar Deep (FED), and Port Fitou (PF). The salinity data shows large variability and presents similar seasonal pattern for surface and bottom cages. For clarity, we present the surface water salinity only.
Lagoon water levels at Font Dame, Font Estramar and Port Fitou showed similar patterns. They are principally controlled by precipitations as well as winds (Ladagnous and Le Bec, 1997). The water level generally increased with increasing precipitation and wind speed (Figure 6c). The average water levels were 0.7 ± 0.1, 0.8 ± 0.2, and 1.0 ± 0.1 m in Font Dame, Font Estramar and Port Fitou, respectively, indicating that the installed cages (at 0.2 and 0.5 m from the sediment-water interface) were rarely exposed.
The water temperature showed overall similar patterns at the study sites with seasonal minimum values observed in winter and maximum values in summer overlaid by daily variations (Figure 6d). From February 2017 to January 2018 (data available at all sites), the water temperatures were significantly higher at groundwater-influenced sites (mean values in FDS = 18.1 ± 5.1, FDD = 18.1 ± 6.0, FES = 20.5 ± 4.8, and FED = 20.1 ± 5.1°C) than at Port Fitou (mean = 17.3 ± 6.7°C) (t-test: t = 2.34, p < 0.05). Furthermore, for the total period of data collection (October 2016 – January 2018), the water temperatures were significantly higher at the surface (FDS = 16.7 ± 5.1°C; FES = 16.5 ± 5.5°C) than at the bottom (FDD = 16.5 ± 5.5°C; FED = 15.8 ± 5.9°C) (t-test: t = 1.38, p < 0.05).
The salinity was highly variable at the groundwater-influenced sites (Font Dame and Font Estramar) and no clear pattern was observed (Figure 6e), with salinity ranges of 9.0–35.8 (FDS), 7.4–44.4 (FDD), 10.2–36.8 (FES), and 10.0–40.7 (FED). In contrast, salinity in Port Fitou was relatively stable with seasonal values ranging between 26.2 and 41.8, showing small daily variations and a seasonal pattern with maximum values recorded at the end of summer (consistent with an increase of evaporation and a decrease of freshwater inputs). The salinity at Port Fitou was overall considerably higher than that observed at the groundwater-influenced sites, reflecting average lagoon conditions (t-test: t = 37.95, p < 0.05).
Spectral Analyses
At both groundwater-influenced sites, spectral analyses of parameters in bottom and surface waters showed similar patterns and periodicities, and thus here we present surface water data only. The FFT analyses of the temperature data showed overall well-defined peaks centered at 12 h, 1 and 13 days for surface waters at the three study sites (Font Dame, Font Estramar, and Port Fitou) except for Font Estramar at the low frequency (Figures 7A–C). For salinity, peaks were also observed at 12 h, 1 and 13 days for the site Font Dame while no clear peaks were observed at Font Estramar and Port Fitou (Figures 7D–F). Periodicities of 12 h and 11 days were exhibited for the water depth at Font Dame and Port Fitou while at Font Estramar the water depth followed periodicities of 12 and 24 h (Figures 7G–I). The FFT analyses for the wind speed showed clear peaks at 1 and 2.6 days (Figure 7J).
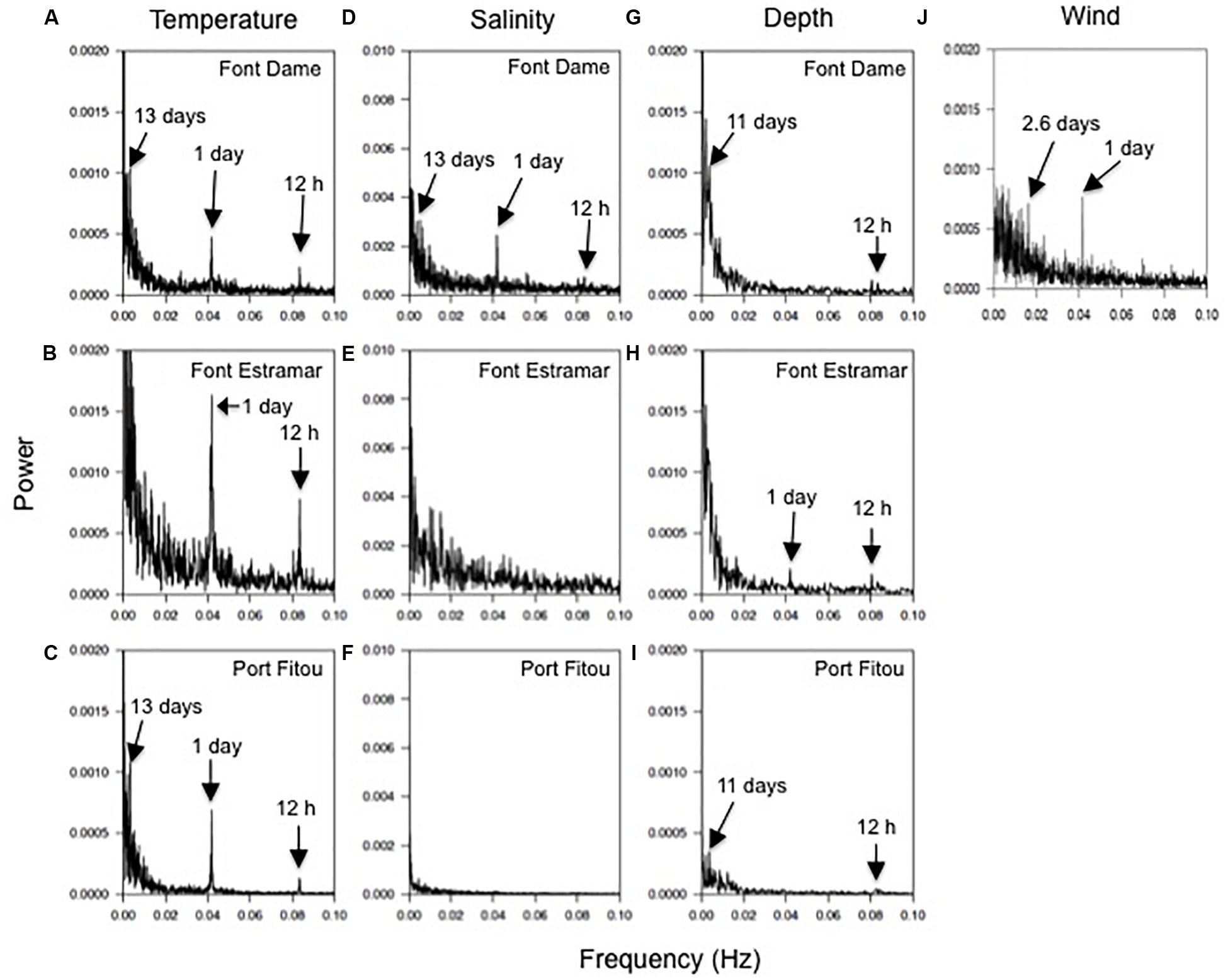
Figure 7. (A–C) Fast Fourier Transformations of the temperature, (D–F) salinity and (G–I) water depth at Font Dame (FD), Font Estramar (FE) and Port Fitou (PF), and (J) the wind speed at the study. Arrows indicate peaks of the power spectrum.
Discussion
Periodicity in Shell Growth and Environmental Influences
The growth increment count demonstrates that M. galloprovincialis in Salses-Leucate lagoon forms growth increment on a near-daily basis (circadian rhythm). Generally, growth patterns reported in mytilid species refer rather to tidal cycles (Langton, 1977; Richardson, 1989; Buschbaum and Saier, 2001; Zaldibar et al., 2004) and circa-tidal periodicity has been observed in several bivalves (Pannella and Macclintock, 1968; Evans, 1972; Richardson, 1988; Schöne et al., 2003; Miyaji et al., 2007; Connor and Gracey, 2011). For instance, Richardson (1989) showed that M. edulis growing under tidally submerged conditions exhibits a clearly defined growth pattern coinciding with the number of emersions. In Salses-Leucate lagoon, tidal variations are relatively small (<0.05 m) as a consequence of the small tidal cycles in the Mediterranean Sea and the restricted exchange between the lagoon and sea, and thus tidal cycles have a negligible influence on mussel growth patterns. The circadian rhythm observed in mussels from Salses-Leucate lagoon has been reported in other bivalve mollusks (Pannella and Macclintock, 1968; Richardson, 1988; Parsons et al., 1993; Chauvaud et al., 2005), and is often related to cycles governed by biological clocks (Pittendrigh and Daan, 1976; Schöne, 2008; Connor and Gracey, 2011). The biology of most organisms is closely related to the changes in their environmental conditions, which often present clear daily patterns of, mainly, temperature and light. As a consequence, bivalves and other organisms have developed behavioral and physiological daily patterns (Connor and Gracey, 2011). As commonly observed in coastal environments, temperature, salinity, water depth, and wind speed variations in Salses-Leucate lagoon exhibit daily periodicity, suggesting that these environmental factors contribute to the daily pattern of mussel growth in the lagoon.
Although the growth pattern is oriented toward a daily pattern, the number of growth increments counted in the shell was generally slightly lower than the number of days, suggesting that either growth halts during some part of the study period or that the growth pattern was not well revealed by the Mutvei etching, resulting in a potential underestimation of the number of growth increments (Nedoncelle et al., 2013). Similar observations have been reported previously for Arctica islandica (Witbaard et al., 1994; Schöne et al., 2005b), Pecten maximus (Chauvaud et al., 2005), and Phacosoma japonicum (Tanabe, 2007). They observed that the number of growth increment formed during a limited interval of time is (sometimes significantly) lower than the number of days (or tides) at the study sites. Winter growth cessation is indeed common in bivalves (Jones and Quitmyer, 1996; Tanabe, 2007; Okaniwa et al., 2010), because the production of shell carbonate ceases below species-specific growth temperature thresholds. For instance, Margaritifera margaritifera in northern Sweden stops producing shell carbonate below 5°C (Schöne, 2008) while M. galloprovincialis from Tokyo Bay, Japan, stops growing or barely grows for water temperature between 8 and 14°C (Okaniwa et al., 2010). Growth cessation may also occur all year round as a result of an abrupt change in the environmental conditions due to strong wind events, drop in salinity and/or phytoplankton bloom (due to toxicity or clogging of the gill system) (Page and Hubbard, 1987; Chauvaud et al., 1998; Schöne, 2008; Okaniwa et al., 2010). The observed discrepancy in days and growth increments in this study is small in comparison to those observed at many other sites, likely due to the comparatively stable environmental conditions in the lagoon, but nevertheless suggests that the conditions temporarily cause either a complete cessation of growth during specific days or a desynchronization in the circadian rhythm of growth increment deposition (Chauvaud et al., 2005).
Aside from the (near-) daily cycles, the spectral analysis of the increment width shows spectral peaks at frequencies corresponding to periods of 11–13 days (Figure 5) The temperature, salinity and water depth patterns also exhibit periodicity of approximately 11–13 days, suggesting a spring-neap cycle influence on the growth of M. galloprovincialis in Salses-Leucate lagoon. Tidal patterns in (non-lagoonal) bivalves are widespread and are expressed by thin increments altering with groups of relatively thick ones, forming periodic pattern of 13–14 days, corresponding to the fortnightly spring-neap tide cycles (Evans, 1972; Richardson, 1989; Miyaji et al., 2007), and suggested to be related to valve activity and/or current velocity changes modifying the food availability (Clark, 2005; Lartaud et al., 2010; Tran et al., 2011). Whilst spring-neap cycles can affect groundwater discharge rate in tidal systems (e.g., Kim and Hwang, 2002; Taniguchi et al., 2002; Sieyes et al., 2008), this is unlikely the case in Salses-Leucate lagoon due to the quasi-negligible tidal amplitude. However, spring-neap cycles are likely affecting the exchange between the lagoon and the Mediterranean Sea (Sylaios et al., 2006), and may therefore play a role in controlling the temperature, salinity, water depth and eventually the nutrient supply in Salses-Leucate lagoon.
The origin of the periodicity of approximately 3 and 5 days in the increment growth remains uncertain (Figure 5). It may be related to frequent wind events, which show a periodicity of 2.6 days (close to the 3–5 days periodicities of the growth increments), and thus drive the circulation within the lagoon and exchange with the sea, thereby controlling environmental factors in the lagoon (Figure 6). For instance, southeasterly winds favor the input of seawater in the lagoon while northwesterly winds reduce its input. Furthermore, wind-driven circulation of lagoon water through sediments is recognized as an important source of nutrient in coastal lagoons (Rodellas et al., 2018). This “wind-driven” nutrient supply may increase phytoplankton abundance, which in turn may control mussel growth, albeit with a small temporal lag governed by primary production timescales.
Growth of M. galloprovincialis in the Mediterranean Region
The Von Bertalanffy curves show that the growth rates of M. galloprovincialis from Salses-Leucate lagoon are among the highest rates recorded for this species in the Mediterranean region (Figure 4). This clearly indicates that Salses-Leucate lagoon is well suitable for the growth of M. galloprovincialis. The time required to grow to a length of 30 mm (ca. 1 year) is significantly shorter than that reported for the same species from the coastal bay of Mare Grande and the semi-enclosed basin of Mare Piccolo (Italy) of approximately 7 years (Posa and Tursi, 1991) or longer in the Gulf of Castellammare (Sarà et al., 2012). The growth rates of M. galloprovincialis observed in this study (particularly from the groundwater-influenced sites) are only a little below those reported from the coastal lagoon of Sacca di Scardovari on the Adriatic coast (Ceccherelli and Rossi, 1984) and Fangar Bay in Spain (Ramón et al., 2007). The Sacca di Scardovari lagoon and Fangar Bay are both located at the mouth of big rivers (Po River and Ebro River, respectively), and thus these areas are likely receiving nutrient inputs from rivers. Moreover, these areas are well known for agricultural activities, which may also be a relevant source of nutrients (Busch, 2013; Di Giuseppe et al., 2014). Despite the seasonal variations in water temperature in Salses-Leucate lagoon, the water temperature ranges (Median = 16.0ºC, Q1 = 12.3ºC and Q3 = 20.4°C) includes the optimal temperature range for growth of M. galloprovincialis (17–20°C) (Blanchette et al., 2007).
Role of Groundwater Discharge
Mytilus galloprovincialis at the groundwater influenced sites shows higher growth rate and condition index compared to that of the control site, suggesting that groundwater influenced sites are favorable for their growth (Figures 3, 4). Three compounding effects of groundwater inputs to coastal areas can explain the differences between mussel growth at groundwater-influenced and non-influenced sites, i.e., groundwater-driven variations in (i) temperature, (ii) nutrient availability, and (iii) salinity.
(i) Despite the seasonal variations of water temperature in the lagoon (Figure 6d), the average water temperature at the groundwater-influenced sites is significantly higher than the temperature at the control site. Since temperatures in groundwater sources are relatively constant throughout the year (17–19°C), groundwater inputs in winter (when lagoon waters temperatures “elsewhere” are below 10°C) produce an increase of lagoon water temperatures at the groundwater-influenced sites. The higher growth rate and condition index observed at the groundwater-influenced sites may thus (at least in part) be related to this groundwater-driven increase in temperature (Schöne et al., 2002, 2005b).
(ii) In addition to water temperature, food availability is a major factor controlling shell growth and condition indices. Bivalve growth increases with increasing phytoplankton abundance (Page and Hubbard, 1987; Sato, 1997), with food availability controlling 64–70% of the variation in growth of M. galloprovincialis (His et al., 1989). Sato (1997) demonstrated that growth of bivalve Phacosoma japonica in Ariake Bay (Japan) is also primarily influenced by food availability. High phytoplankton abundance has been directly linked to groundwater input in several costal systems worldwide (Valiela et al., 1990; McClelland et al., 1997; Herrera-Silveira, 1998). A recent study demonstrates that groundwater discharge from Font Dame and Font Estramar sustains primary production of the lagoon investigated here (Andrisoa et al., 2019). Indeed, the concentrations of particulate organic nitrogen in Salses-Leucate lagoon [which is dominated by phytoplankton in this lagoon (Carlier et al., 2009)], are higher in groundwater-influenced sites (62 ± 40 μg N L–1) than in the control site (52.8 ± 21.9 μg N L–1) (A. Andrisoa, unpublished data). The high nutrient concentrations driven by groundwater inputs likely favor phytoplankton growth at the groundwater-influenced sites, which is readily available for mussel growth at these sites.
(iii) Groundwater from Font Dame and Font Estramar discharges substantial amounts of freshwater into Salses-Leucate lagoon, considerably affecting the salinity at the groundwater-influenced sites. Salinity is one of the dominant environmental factors controlling growth. Generally, M. galloprovincialis exhibits highest growth at salinity 35 (His et al., 1989). Typical responses of mussels to lower salinity include reduced feeding activity, slower growth and valve closure (Navarro, 1988; Riisgård et al., 2012). For example, due to low salinities in the Baltic Sea (salinity between 6 and 8 in the northern part), mussels are dwarfed in this area (Kautsky, 1982; Vuorinen et al., 2002). Similarly, Riisgård et al. (2012) showed that mussels growing at salinity 10 have slower growth rates than those growing at salinity 30. However, acclimation to reduced salinities may take place, and mussels are able to adjust growth at changing salinities (Davenport, 1979; Qiu et al., 2002). The higher mussel growth rates measured at the groundwater-influenced sites despite lower salinity suggest that mussels growing there are either acclimated to low salinity environments or that salinity has a less important effect on mussel growth compared to temperature and food availability in this lagoonal environment. Also note that salinity at the groundwater-influenced sites is highly variable (Figure 6e), which may cause stress to the animals. Many bivalves can tolerate small changes in salinity, but salinity outside their acceptable range may negatively affect their growth (Peteiro et al., 2018).
It should be noted that due to sampling constraints (see section Materials and Methods), specimens from different sites were not sampled for exactly the same periods. This could have implications for our results since both growth rates and condition indices highly reflect species reproductive dynamics. Growth rates and condition indices are generally lower during the resting phase (usually from November to February) and higher during gonad maturation and ripening (from April to October) (e.g., Karayücel and Ye, 2010; Vural et al., 2015). Mussels from Font Dame and Port Fitou were studied throughout almost a year, thus covering the different phases of the reproductive cycle, therefore the comparison between groundwater-influenced and control sites must be considered robust. However, specimens from Font Estramar site were monitored from October 2016 to late March 2017 only, and therefore biased toward winter months. Considering the expected lower growth rates in winter periods, the results obtained from the groundwater-influenced site Font Estramar are likely an underestimation of mussel growth rate and condition index in the annual cycle. Further, mussels at the control site Port Fitou were naturally present in a narrower range size than at the groundwater sites, potentially biasing the results. A transplantation of specimen from other sites to cover the same size distribution would likely have introduced an unknown, potentially large bias. Despite these experimental limitations, mussel growth rates and condition indices at groundwater influenced sites are significantly higher than those estimated at control site.
The growth rates of mussels in the upper (shallower) cages are slightly higher than those of bottom cages (Figure 3B). Despite the shallow water depth (≈0.8 m), the water column at the groundwater-influenced sites is generally stratified (except during wind events). The upper cages are relatively more influenced by lower-density groundwater inputs (mean salinity FDS = 21.8 ± 7.1; FES = 24.3 ± 7.0) in comparison to the bottom cages (mean salinity FDD = 27.6 ± 6.5; FED = 27.2 ± 6.1). Thus, temperatures and nutrient concentrations are expected to be higher in surface waters than in bottom waters as a consequence of groundwater inputs, favoring mussel growth rates in the upper cages. The high light availability and temperatures driven by direct solar radiation in surface waters may also favor phytoplankton and thus mussel growth. Higher growth rate of mussels in surface waters than in deeper layers has previously been reported and attributed either to differences in temperature (Fuentes et al., 2000) or the high availability of phytoplankton (Page and Hubbard, 1987). In addition, the lower growth rates observed in the bottom cages may partially result from siltation. Sediment resuspension occurs often in the study area due to frequent wind events and may explain in part the difference in growth observed between upper and lower cages. Silts and clay can clog the gills of mussels, interfere with filter feeding and affect indirectly by reducing light availability for phytoplankton, inhibiting the growth of bivalves (Bricelj et al., 1984; Box and Mossa, 1999).
Economic Implications
The Mediterranean mussel (M. galloprovincialis) is a highly valuable commercial species. The world production of mussels from aquaculture reach an annual value of 1.2 million tons corresponding to an economic value of over 500 million United States dollars (Okumuş et al., 2014). About 80.000 tons are produced annually in France (Župan and Šarić, 2014), and in Thau lagoon (a neighboring site on the French Mediterranean coast), annual mussel production is estimated at 5.400 tons (Gangnery et al., 2004). In particular the competitive price compared to other bivalves makes mussels a sought after seafood (Orban et al., 2002). However, in recent years, the production of mussel is leveling off due to reduced number of suitable coastal sites for high productivity mussel farming, as consequences of human activities (Cataudella et al., 2015). The results of this study clearly show that coastal sites influenced by groundwater inputs represent ideal environments for mussel growth (and thus potential mussel farming), resulting in higher growth rates (1.5 cm yr–1) and condition index. Higher condition index indicates high quality of a marketed product, i.e., better health status and fatness, especially during the periods of gonad maturation and ripening. Mussel aquaculture is traditionally placed in coastal waters with large primary productivity (e.g., Peharda et al., 2007). Results from this study suggest that groundwater-influenced sites can offer environmental conditions well suited for mussel aquaculture, and therefore, it may be economically profitable to farm mussels in groundwater-influenced areas.
Conclusion
M. galloprovincialis in Salses-Leucate lagoon produce circadian (daily rhythm) shell growth increments and have amongst the highest growth rates to date reported for the Mediterranean region. In Salses-Leucate lagoon, mussels from groundwater-influenced sites have higher growth rate and condition index compared to those from a control site (chiefly influenced by seawater), demonstrating that groundwater inflows are favorable for mussel growth. Groundwater discharging to coastal areas is characterized by relatively constant temperatures and is an important source of nutrients, providing thus significant food resources to filter feeders like mussels. This study indicates that higher temperature and food availability associated with groundwater inputs may explain the fast growth rate of M. galloprovincialis at groundwater-influenced sites in Salses-Leucate lagoon, and thus provides direct evidence for the “downstream” ecological impacts of groundwater discharge on this commercially important species.
Identifying suitable sites for profitable production is a considerable challenge in mussel aquaculture. Groundwater-influenced sites are suitable sites for mussel farming, particularly in oligotrophic waters like the Mediterranean Sea. In addition to its increasingly recognized ecological role, this study suggests that groundwater inputs to coastal areas can have non-negligible economic effects on fisheries products in coastal socio-ecosystems.
Ethics Statement
In this work invertebrates (mussels) are investigated. No ethics approval is required or available for this work in France.
Author Contributions
TS conceived and led the study. FL, AA, and TS designed the experiments. IN and VR contributed to laboratory and field work, respectively. All authors contributed to the manuscript writing.
Funding
This research was supported by the French National Research Agency (ANR) through ANR @RAction chair medLOC (ANR-14-ACHN-0007-01 – project leader TS). AA was partially supported by Labex OT-Med (ANR-11-LABEX-0061), and VR acknowledges support from the European Union’s Horizon 2020 Research and Innovation Program under the Marie Skłodowska-Curie Grant Agreement No 748896.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Michel and Henry Comte (Salses-le-Château), L. Fonbonne (Rivage Leucate), R de Wit (MARBEC Montpellier), A. Fiandrino, M. David, and D. Munaron (IFREMER Sete), V. Bailly Comte (BRGM Montpellier), and P. Cook (Flinders University) for sharing their expert knowledge on regional lagoon and groundwater processes. The community from “Le domaine de Pedros” facilitated access to Fitou. We acknowledge the facilities of Biology platform of imaging and cytometry (BioPIC) from Banyuls Oceanographical Observatory, and thank the reviewers for their thorough and constructive comments on the manuscript.
References
Abada-Boujemaa, Y. M. (1996). Cinétique, Croissance, Production et Composition Biochimique des deux bivalves Mytilidés: Perna perna (.) et Mytilus galloprovincialis (Lmk) du Littoral Algérois. France: Muséum National d’Histoire Naturelle.
Aliaume, C., Do Chi, T., Viaroli, P., and Zaldívar, J. M. (2007). Coastal lagoons of Southern Europe: recent changes and future scenarios. Trans. Waters Monogr. 1, 1–12.
Amato, D. W., Bishop, J. M., Glenn, C. R., Dulai, H., and Smith, C. M. (2016). Impact of submarine groundwater discharge on marine water quality and Reef Biota of Maui. PLoS One 11:e0165825. doi: 10.1371/journal.pone.0165825
Andrisoa, A., Stieglitz, T. C., Rodellas, V., and Raimbault, P. (2019). Primary production in coastal lagoons supported by groundwater discharge and porewater fluxes inferred from nitrogen and carbon isotope signatures. Mar. Chem. 210, 48–60. doi: 10.1016/j.marchem.2019.03.003
Anthony, A., Atwood, J., August, P., Byron, C., Cobb, S., Foster, C., et al. (2009). Coastal lagoons and climate change: ecological and social ramifications in US Atlantic and Gulf coast ecosystems. Ecol. Soc. 14:8.
Basterretxea, G., Tovar-Sanchez, A., Beck, A. J., Masqué, P., Bokuniewicz, H. J., Coffey, R., et al. (2010). Submarine groundwater discharge to the coastal environment of a Mediterranean island (Majorca, Spain): ecosystem and biogeochemical significance. Ecosystems 13, 629–643. doi: 10.1007/s10021-010-9334-5
Bianchi, T., Allison, M., and Cai, W.-J. (2014). Biogeochemical Dynamics at Major River-Coastal Interfaces: Linkages with Global Change. Cambridge: Cambridge University Press.
Blanchette, C. A., Helmuth, B., and Gaines, S. D. (2007). Spatial patterns of growth in the mussel, Mytilus californianus, across a major oceanographic and biogeographic boundary at Point Conception, California, USA. J. Exp. Mar. Biol. Ecol. 340, 126–148. doi: 10.1016/j.jembe.2006.09.022
Box, J. B., and Mossa, J. (1999). Sediment, land use, and freshwater mussels: prospects and problems. J. North Am. Benthol. Soc. 18, 99–117. doi: 10.2307/1468011
Brey, T. (1999). Growth performance and mortality in aquatic macrobenthic invertebrates. Adv. Mar. Biol. 35, 153–243.
Bricelj, V. M., Malouf, R. E., and de Quillfeldt, C. (1984). Growth of juvenile Mercenaria mercenaria and the effect of resuspended bottom sediments. Mar. Biol. 84, 167–173. doi: 10.1007/BF00393001
Busch, J. A. (2013). Phytoplankton Dynamics and Bio-Optical Variables Associated with Harmful Algal Blooms in Aquaculture Zones. PhD thesis, Bremen University.
Buschbaum, C., and Saier, B. (2001). Growth of the mussel Mytilus edulis L. in the Wadden Sea affected by tidal emergence and barnacle epibionts. J. Sea Res. 45, 27–36. doi: 10.1016/S1385-1101(00)00061-7
Carlier, A., Riera, P., Amouroux, J., Bodiou, J., Desmalades, M., and Grémare, A. (2009). Spatial heterogeneity in the food web of a heavily modified Mediterranean coastal lagoon: stable isotope evidence. Aquat. Biol. 5, 167–179. doi: 10.3354/ab00147
Cataudella, S., Crosetti, D., and Massa, F., General Fisheries Commission for the Mediterranean Food and Agriculture Organization of the United Nations, and Food and Agriculture Organization of the United Nations, (2015). Mediterranean Coastal Lagoons: Sustainable Management and Interactions Among Aquaculture, Capture Fisheries and the Environment. Rome: Food abd Agriculture Organization of the United Nations.
Ceccherelli, V. U., and Rossi, R. (1984). Settlement, growth and production of the mussel Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 16, 173–184. doi: 10.3354/Meps016173
Charette, M. A., Buesseler, K. O., and Andrews, J. E. (2001). Utility of radium isotopes for evaluating the input and transport of groundwater-derived nitrogen to a Cape Cod estuary. Limnol. Oceanogr. 46, 465–470. doi: 10.4319/lo.2001.46.2.0465
Chauvaud, L., Lorrain, A., Dunbar, R. B., Paulet, Y.-M., Thouzeau, G., Jean, F., et al. (2005). Shell of the Great Scallop Pecten maximus as a high-frequency archive of paleoenvironmental changes: GREAT SCALLOP Pecten maximus. Geochem. Geophys. Geosystems 6, 1–15. doi: 10.1029/2004GC000890
Chauvaud, L., Thouzeau, G., and Paulet, Y.-M. (1998). Effects of environmental factors on the daily growth rate of Pecten maximus juveniles in the Bay of Brest (France). J. Exp. Mar. Biol. Ecol. 227, 83–111. doi: 10.1016/S0022-0981(97)00263-3
Clark, G. R. (2005). Daily growth lines in some living Pectens (Mollusca: Bivalvia), and some applications in a fossil relative: time and tide will tell. Palaeogeogr. Palaeoclimatol. Palaeoecol. 228, 26–42. doi: 10.1016/j.palaeo.2005.03.044
Cloern, J. E., Foster, S. Q., and Kleckner, A. E. (2014). Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences 11:25. doi: 10.5194/bg-11-2477-2014
Connor, K. M., and Gracey, A. Y. (2011). Circadian cycles are the dominant transcriptional rhythm in the intertidal mussel Mytilus californianus. Proc. Natl. Acad. Sci. U.S.A. 108, 16110–16115. doi: 10.1073/pnas.1111076108
Davenport, J. (1979). The isolation response of mussels (Mytilus edulis L.) exposed to falling sea-water concentrations. J. Mar. Biol. Assoc. U. K. 59, 123–132. doi: 10.1017/S0025315400043423
De Wit, R., Leibreich, J., Vernier, F., Delmas, F., Beuffe, H., Maison, Ph, et al. (2005). Relationship between land-use in the agro-forestry system of les Landes, nitrogen loading to and risk of macro-algal blooming in the Bassin d’Arcachon coastal lagoon (SW France). Estuar. Coast. Shelf Sci. 62, 453–465. doi: 10.1016/j.ecss.2004.09.007
Di Giuseppe, D., Bianchini, G., Vittori Antisari, L., Martucci, A., Natali, C., and Beccaluva, L. (2014). Geochemical characterization and biomonitoring of reclaimed soils in the Po River Delta (Northern Italy): implications for the agricultural activities. Environ. Monit. Assess. 186, 2925–2940. doi: 10.1007/s10661-013-3590-8
Encarnação, J., Leitão, F., Range, P., Piló, D., Chícharo, M. A., and Chícharo, L. (2015). Local and temporal variations in near-shore macrobenthic communities associated with submarine groundwater discharges. Mar. Ecol. 36, 926–941. doi: 10.1111/maec.12186
Evans, J. W. (1972). Tidal Growth Increments in the Cockle Clinocardium nuttalli. Science 176, 416–417. doi: 10.1126/science.176.4033.416
Fleury, P., Bakalowicz, M., and de Marsily, G. (2007). Submarine springs and coastal karst aquifers: a review. J. Hydrol. 339, 79–92. doi: 10.1016/j.jhydrol.2007.03.009
Fuentes, J., Gregorio, V., Giráldez, R., and Molares, J. (2000). Within-raft variability of the growth rate of mussels, Mytilus galloprovincialis, cultivated in the Rıa de Arousa (NW Spain). Aquaculture 189, 39–52. doi: 10.1016/s0044-8486(00)00357-4
Gangnery, A., Bacher, C., and Buestel, D. (2004). Application of a population dynamics model to the Mediterranean mussel, Mytilus galloprovincialis, reared in Thau Lagoon (France). Aquaculture 229, 289–313. doi: 10.1016/S0044-8486(03)00360-0
Hata, M., Sugimoto, R., Hori, M., Tomiyama, T., and Shoji, J. (2016). Occurrence, distribution and prey items of juvenile marbled sole Pseudopleuronectes yokohamae around a submarine groundwater seepage on a tidal flat in southwestern Japan. J. Sea Res. 111, 47–53. doi: 10.1016/j.seares.2016.01.009
Herrera-Silveira, J. A. (1998). Nutrient-phytoplankton production relationships in a groundwater-influenced tropical coastal lagoon. Aquat. Ecosyst. Health Manag. 1, 373–385. doi: 10.1016/s1463-4988(98)00015-3
His, E., Robert, R., and Dinet, A. (1989). Combined effects of temperature and salinity on fed and starved larvae of the mediterranean mussel Mytilus galloprovincialis and the Japanese oyster Crassostrea gigas. Mar. Biol. 100, 455–463. doi: 10.1007/BF00394822
Jones, D. S., Arthur, M. A., and Allard, D. J. (1989). Sclerochronological records of temperature and growth from shells of Mercenaria mercenaria from Narragansett Bay. Rhode Island. Mar. Biol. 102, 225–234. doi: 10.1007/bf00428284
Jones, D. S., and Quitmyer, I. R. (1996). Marking time with bivalve shells: oxygen isotopes and season of annual increment formation. PALAIOS 11, 340–346. doi: 10.2307/3515244
Karayücel, S., and Ye, M. (2010). Growth and production of raft cultivated mediterranean mussel (Mytilus galloprovincialis Lamarck, 1819) in Sinop, Black Sea. Turk. J. Fish. Aqua. Sci. 10, 09–17.
Kautsky, N. (1982). Growth and size structure in a baltic Mytilus edulis population. Mar. Biol. 68, 117–133. doi: 10.1007/BF00397599
Kim, G., and Hwang, D.-W. (2002). Tidal pumping of groundwater into the coastal ocean revealed from submarine 222Rn and CH4 monitoring. Geophys. Res. Lett. 29, 23–21. doi: 10.1029/2002GL015093
Ladagnous, H., and Le Bec, C. (1997). Lagune de Salses-Leucate. I-Analyse bibliographique. Available at: https://archimer.ifremer.fr/doc/00073/18422/.
Langton, R. W. (1977). Digestive rhythms in the mussel Mytilus edulis. Mar. Biol. 41, 53–58. doi: 10.1007/BF00390581
Lartaud, F., Chauvaud, L., Richard, J., Toulot, A., Bollinger, C., Testut, L., et al. (2010). Experimental growth pattern calibration of Antarctic scallop shells (Adamussium colbecki, Smith 1902) to provide a biogenic archive of high-resolution records of environmental and climatic changes. J. Exp. Mar. Biol. Ecol. 393, 158–167. doi: 10.1016/j.jembe.2010.07.016
Lartaud, F., Pareige, S., de Rafelis, M., Feuillassier, L., Bideau, M., Peru, E., et al. (2013). A new approach for assessing cold-water coral growth in situ using fluorescent calcein staining. Aquat. Living Resour. 26, 187–196. doi: 10.1051/alr/2012029
Lee, E., Shin, D., Hyun, S. P., Ko, K.-S., Moon, H. S., Koh, D.-C., et al. (2017). Periodic change in coastal microbial community structure associated with submarine groundwater discharge and tidal fluctuation. Limnol. Oceanogr. 62, 437–451. doi: 10.1002/lno.10433
Lirman, D., Orlando, B., Maciá, S., Manzello, D., Kaufman, L., Biber, P., et al. (2003). Coral communities of Biscayne Bay, Florida and adjacent offshore areas: diversity, abundance, distribution, and environmental correlates. Aquat. Conserv. Mar. Freshw. Ecosyst. 13, 121–135. doi: 10.1002/aqc.552
Liu, K.-K., Atkinson, L., Quiñones, R., and Talaue-McManus, L. (eds) (2010). Carbon and Nutrient Fluxes in Continental Margins. Berlin: Springer.
Lucas, A., and Beninger, P. G. (1985). The use of physiological condition indices in marine bivalve aquaculture. Aquaculture 44, 187–200. doi: 10.1016/0044-8486(85)90243-1
Mahé, K., Bellamy, E., Lartaud, F., and de Rafélis, M. (2010). Calcein and manganese experiments for marking the shell of the common cockle (Cerastoderma edule): tidal rhythm validation of increments formation. Aquat. Living Resour. 23, 239–245. doi: 10.1051/alr/2010025
McClelland, J. W., Valiela, I., and Michener, R. H. (1997). Nitrogen-stable isotope signatures in estuarine food webs: a record of increasing urbanization in coastal watersheds. Limnol. Oceanogr. 42, 930–937. doi: 10.4319/lo.1997.42.5.0930
Middelburg, J., and Nieuwenhuize, J. (2001). Nitrogen isotope tracing of dissolved inorganic nitrogen behaviour in tidal estuaries. Estuar. Coast. Shelf Sci. 53, 385–391. doi: 10.1006/ecss.2001.0805
Miyaji, T., Tanabe, K., and Schöne, B. R. (2007). Environmental controls on daily shell growth of Phacosoma japonicum (Bivalvia: Veneridae) from Japan. Mar. Ecol. Prog. Ser. 336, 141–150. doi: 10.3354/meps336141
Moore, W. S. (2010). The effect of submarine groundwater discharge on the ocean. Annu. Rev. Mar. Sci. 2, 59–88. doi: 10.1146/annurev-marine-120308-081019
Moran, A. L. (2000). Calcein as a marker in experimental studies newly-hatched gastropods. Mar. Biol. 137, 893–898. doi: 10.1007/s002270000390
Navarro, J. M. (1988). The effects of salinity on the physiological ecology of Choromytilus chorus (Molina, 1782)(Bivalvia: Mytilidae). J. Exp. Mar. Biol. Ecol. 122, 19–33. doi: 10.1016/0022-0981(88)90209-2
Nedoncelle, K., Lartaud, F., de Rafelis, M., Boulila, S., and Le Bris, N. (2013). A new method for high-resolution bivalve growth rate studies in hydrothermal environments. Mar. Biol. 160, 1427–1439. doi: 10.1007/s00227-013-2195-7
Newton, A., Icely, J., Cristina, S., Ana, B., Ana Cristina, C., Franciscus, C., et al. (2014). An overview of ecological status, vulnerability and future perspectives of European large shallow, semi-enclosed coastal systems, lagoons and transitional waters. Estuar. Coast. Shelf Sci. 140, 95–122. doi: 10.1016/j.ecss.2013.05.023
Null, K. A., Dimova, N. T., Knee, K. L., Esser, B. K., Swarzenski, P. W., Singleton, M. J., et al. (2012). Submarine groundwater discharge-derived nutrient loads to san francisco bay: implications to future ecosystem changes. Estuar. Coast. 35, 1299–1315. doi: 10.1007/s12237-012-9526-7
Okaniwa, N., Miyaji, T., Sasaki, T., and Tanabe, K. (2010). Shell growth and reproductive cycle of the Mediterranean mussel Mytilus galloprovincialis in Tokyo Bay, Japan: relationship with environmental conditions. Plankton Benthos Res. 5, 214–220. doi: 10.3800/pbr.5.214
Okumuş, Ý, Başçinar, N., and Özkan, M. (2014). The effects of phytoplankton concentration, size of mussel and water temperature on feed consumption and filtration rate of the mediterranean mussel (Mytilus galloprovincialis Lmk). Turk. J. Zool. 26, 167–172.
Orban, E., Di Lena, G., Nevigato, T., Casini, I., Marzetti, A., and Caproni, R. (2002). Seasonal changes in meat content, condition index and chemical composition of mussels (Mytilus galloprovincialis) cultured in two different Italian sites. Food Chem. 77, 57–65. doi: 10.1016/s0308-8146(01)00322-3
Page, H. M., and Hubbard, D. M. (1987). Temporal and spatial patterns of growth in mussels Mytilus edulis on an offshore platform: relationships to water temperature and food availability. J. Exp. Mar. Biol. Ecol. 111, 159–179. doi: 10.1016/0022-0981(87)90053-0
Pannella, G., and Macclintock, C. (1968). Biological and environmental rhythms reflected in molluscan shell growth. J. Paleontol. 42, 64–80. doi: 10.1017/S0022336000061655
Parsons, G. J., Robinson, S. M. C., Roff, J. C., and Dadswell, M. J. (1993). Daily growth rates as indicated by valve ridges in postlarval giant scallop (Placopecten magellanicus) (Bivalvia: Pectinidae). Can. J. Fish. Aquat. Sci. 50, 456–464. doi: 10.1139/f93-053
Peharda, M., Župan, I., Bavčević, L., Frankić, A., and Klanjšček, T. (2007). Growth and condition index of mussel Mytilus galloprovincialis in experimental integrated aquaculture. Aquac. Res. 38, 1714–1720. doi: 10.1111/j.1365-2109.2007.01840.x
Peteiro, L. G., Woodin, S. A., Wethey, D. S., Costas-Costas, D., Martínez-Casal, A., Olabarria, C., et al. (2018). Responses to salinity stress in bivalves: evidence of ontogenetic changes in energetic physiology on Cerastoderma edule. Sci. Rep. 8:8329. doi: 10.1038/s41598-018-26706-9
Piló, D., Barbosa, A. B., Teodósio, M. A., Encarnação, J., Leitão, F., Range, P., et al. (2018). Are submarine groundwater discharges affecting the structure and physiological status of rocky intertidal communities? Mar. Environ. Res. 136, 158–173. doi: 10.1016/j.marenvres.2018.02.013
Pittendrigh, C. S., and Daan, S. (1976). A functional analysis of circadian pacemakers in nocturnal rodents. J. Comp. Physiol. 106, 333–355. doi: 10.1007/BF01417860
Posa, D., and Tursi, A. (1991). Growth Models of Mytilus galloprovincialis lamarck on the mar grande and on the mar Piccolo of Taranto (Southern Italy). Stat. Appl. 3, 135–143.
Qiu, J.-W., Tremblay, R., and Bourget, E. (2002). Ontogenetic changes in hyposaline tolerance in the mussels Mytilus edulis and M. trossulus: implications for distribution. Mar. Ecol. Prog. Ser. 228, 143–152. doi: 10.3354/meps228143
Ragonese, S., Vitale, S., Mazzola, S., Pagliarino, E., and Bianchini, M. L. (2012). Behavior of some growth performance indexes for exploited Mediterranean hake. Acta Adriat. Int. J. Mar. Sci. 53, 105–123.
Ramón, M., Fernández, M., and Galimany, E. (2007). Development of mussel (Mytilus galloprovincialis) seed from two different origins in a semi-enclosed Mediterranean Bay (N.E. Spain). Aquaculture 264, 148–159. doi: 10.1016/j.aquaculture.2006.11.014
Richardson, C. A. (1988). Tidally produced growth bands in the subtidal bivalve Spisula Subtruncata (Da Costa). J. Molluscan Stud. 54, 71–82. doi: 10.1093/mollus/54.1.71
Richardson, C. A. (1989). An analysis of the microgrowth bands in the shell of the common mussel Mytilus Edulis. J. Mar. Biol. Assoc. U. K. 69, 477–491. doi: 10.1017/S0025315400029544
Riera, R., Tuset, V. M., Betancur, R.-R., Lombarte, A., Marcos, C., and Pérez-Ruzafa, A. (2018). Modelling alpha-diversities of coastal lagoon fish assemblages from the Mediterranean Sea. Prog. Oceanogr. 165, 100–109. doi: 10.1016/j.pocean.2018.05.003
Riisgård, H. U., Bøttiger, L., and Pleissner, D. (2012). Effect of salinity on growth of mussels, Mytilus edulis, with special reference to great belt (Denmark). Open J. Mar. Sci. 02:167. doi: 10.4236/ojms.2012.24020
Rodellas, V., Garcia-Orellana, J., Masqué, P., Feldman, M., and Weinstein, Y. (2015). Submarine groundwater discharge as a major source of nutrients to the Mediterranean Sea. Proc. Natl. Acad. Sci. 112, 3926–3930. doi: 10.1073/pnas.1419049112
Rodellas, V., Stieglitz, T. C., Andrisoa, A., Cook, P. G., Raimbault, P., Tamborski, J. J., et al. (2018). Groundwater-driven nutrient inputs to coastal lagoons: the relevance of lagoon water recirculation as a conveyor of dissolved nutrients. Sci. Total Environ. 642, 764–780. doi: 10.1016/j.scitotenv.2018.06.095
Sarà, G., Reid, G. K., Rinaldi, A., Palmeri, V., Troell, M. A. L. M., and Kooijman, S. (2012). Growth and reproductive simulation of candidate shellfish species at fish cages in the Southern Mediterranean: dynamic Energy Budget (DEB) modelling for integrated multi-trophic aquaculture. Aquaculture 32, 259–266. doi: 10.1016/j.aquaculture.2011.10.042
Sato, S. (1997). Shell microgrowth patterns of bivalves reflecting seasonal change in phytoplankton abundance. Paleontol. Res. 1, 260–266.
Schöne, B., Tanabe, K., Dettman, D., and Sato, S. (2003). Environmental controls on shell growth rates and δ18O of the shallow-marine bivalve mollusk Phacosoma japonicum in Japan. Mar. Biol. 142, 473–485. doi: 10.1007/s00227-002-0970-y
Schöne, B. R. (2008). The curse of physiology—challenges and opportunities in the interpretation of geochemical data from mollusk shells. Geo-Mar. Lett. 28, 269–285. doi: 10.1007/s00367-008-0114-6
Schöne, B. R., Dunca, E., Fiebig, J., and Pfeiffer, M. (2005a). Mutvei’s solution: an ideal agent for resolving microgrowth structures of biogenic carbonates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 228, 149–166. doi: 10.1016/j.palaeo.2005.03.054
Schöne, B. R., Houk, S. D., FreyreCastro, A. D., Fiebig, J., Oschmann, W., Kroncke, I., et al. (2005b). Daily Growth Rates in Shells of Arctica islandica: assessing sub-seasonal environmental controls on a long-lived Bivalve Mollusk. PALAIOS 20, 78–92. doi: 10.2110/palo.2003.p03-101
Schöne, B. R., Freyre Castro, A. D., Fiebig, J., Houk, S. D., Oschmann, W., and Kröncke, I. (2004). Sea surface water temperatures over the period 1884–1983 reconstructed from oxygen isotope ratios of a bivalve mollusk shell (Arctica islandica, southern North Sea). Palaeogeogr. Palaeoclimatol. Palaeoecol. 212, 215–232. doi: 10.1016/j.palaeo.2004.05.024
Schöne, B. R., Goodwin, D. H., Flessa, K. W., Dettman, D. L., and Roopnarine, P. D. (2002). Sclerochronology and growth of the bivalve mollusks Chione (Chionista) fluctifraga and C. (Chionista) cortezi in the northern Gulf of California. Mexico. Veliger 45, 45–54.
Short, F. T., and Burdick, D. M. (1996). Quantifying eelgrass habitat loss in relation to housing development and nitrogen loading in Waquoit Bay. Massachusetts. Estuar. 19, 730–739. doi: 10.2307/1352532
Sieyes, N. R., de Yamahara, K. M., Layton, B. A., Joyce, E. H., and Boehm, A. B. (2008). Submarine discharge of nutrient-enriched fresh groundwater at Stinson Beach, California is enhanced during neap tides. Limnol. Oceanogr. 53, 1434–1445. doi: 10.4319/lo.2008.53.4.1434
Slomp, C. P., and Van Cappellen, P. (2004). Nutrient inputs to the coastal ocean through submarine groundwater discharge: controls and potential impact. J. Hydrol. 295, 64–86. doi: 10.1016/j.jhydrol.2004.02.018
Stieglitz, T. C., van Beek, P., Souhaut, M., and Cook, P. G. (2013). Karstic groundwater discharge and seawater recirculation through sediments in shallow coastal Mediterranean lagoons, determined from water, salt and radon budgets. Mar Chem. 156, 73–84. doi: 10.1016/j.marchem.2013.05.005
Sylaios, G. K., Tsihrintzis, V. A., Akratos, C., and Haralambidou, K. (2006). Quantification of water, salt and nutrient exchange processes at the mouth of a Mediterranean coastal lagoon. Environ. Monit. Assess. 119, 275–301. doi: 10.1007/s10661-005-9026-3
Tanabe, K. (2007). Age and growth rate determinations of an intertidal bivalve, Phacosoma japonicum, using internal shell increments. Lethaia 21, 231–241. doi: 10.1111/j.1502-3931.1988.tb02075.x
Taniguchi, M., Burnett, W. C., Cable, J. E., and Turner, J. V. (2002). Investigation of submarine groundwater discharge. Hydrol. Process. 16, 2115–2129. doi: 10.1002/hyp.1145
Tovar-Sánchez, A., Basterretxea, G., Rodellas, V., Sánchez-Quiles, D., García-Orellana, J., Masqué, P., et al. (2014). Contribution of groundwater discharge to the coastal dissolved nutrients and trace metal concentrations in majorca island: karstic vs detrital systems. Environ. Sci. Technol. 48, 11819–11827. doi: 10.1021/es502958t
Tran, D., Nadau, A., Durrieu, G., Ciret, P., Parisot, J.-P., and Massabuau, J.-C. (2011). Field chronobiology of a molluscan bivalve: how the moon and sun cycles interact to drive oyster activity rhythms. Chronobiol. Int. 28, 307–317. doi: 10.3109/07420528.2011.565897
Troccoli-Ghinaglia, L., Herrera-Silveira, J. A., Comín, F. A., and Díaz-Ramos, J. R. (2010). Phytoplankton community variations in tropical coastal area affected where submarine groundwater occurs. Cont. Shelf Res. 30, 2082–2091. doi: 10.1016/j.csr.2010.10.009
Utsunomiya, T., Hata, M., Sugimoto, R., Hinda, H., Kobayashi, S., Miyata, J., et al. (2017). Higher species richness and abundance of fish and benthic invertebrates around submarine groundwater discharge in Obama Bay. Japan. J. Hydrol. Reg. Stud. 11, 139–146. doi: 10.1016/j.ejrh.2015.11.012
Valiela, I., Costa, J., Foreman, K., Teal, J. M., Howes, B., and Aubrey, D. (1990). Transport of groundwater-borne nutrients from watersheds and their effects on coastal waters. Biogeochemistry 10, 177–197. doi: 10.1007/bf00003143
Velasco, A. M., Pérez-Ruzafa, A., Martínez-Paz, J. M., and Marcos, C. (2018). Ecosystem services and main environmental risks in a coastal lagoon (Mar Menor, Murcia, SE Spain): the public perception. J. Nat. Conserv. 43, 180–189. doi: 10.1016/j.jnc.2017.11.002
Vuorinen, I., Antsulevich, A. E., and Maximovich, N. V. (2002). Spatial distribution and growth of the common mussel Mytilus edulis L. in the archipelago of SW-Finland, northern Baltic Sea. Boreal Environ. Res. 7, 41–52.
Vural, P., Yildiz, H., and Acarli, S. (2015). Growth and survival performances of Mediterranean mussel (Mytilus galloprovincialis, Lamarck, 1819) on different depths in Cardak lagoon, Dardanelles. Mar. Sci. Tech. Bull. 4, 7–12.
Welch, P. (1967). The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoustics 15, 70–73. doi: 10.1109/TAU.1967.1161901
Witbaard, R., Jenness, M. I., Van Der Borg, K., and Ganssen, G. (1994). Verification of annual growth increments in Arctica islandica L. from the North Sea by means of oxygen and carbon isotopes. Neth. J. Sea Res. 33, 91–101. doi: 10.1016/0077-7579(94)90054-X
Yildiz, H., Mustafa, P., and Musa, B. (2006). Condition indices of Mediterranean Mussels (Mytilus galloprovincialis L. 1819) growing on suspended ropes in Dardanelles. J. Food Technol. 4, 221–224.
Zaldibar, B., Cancio, I., and Marigómez, I. (2004). Circatidal variation in epithelial cell proliferation in the mussel digestive gland and stomach. Cell Tissue Res. 318, 395–402. doi: 10.1007/s00441-004-0960-0
Keywords: groundwater discharge, coastal lagoon, Mytilus galloprovincialis, Mediterranean mussel, growth rate, condition index
Citation: Andrisoa A, Lartaud F, Rodellas V, Neveu I and Stieglitz TC (2019) Enhanced Growth Rates of the Mediterranean Mussel in a Coastal Lagoon Driven by Groundwater Inflow. Front. Mar. Sci. 6:753. doi: 10.3389/fmars.2019.00753
Received: 22 January 2019; Accepted: 19 November 2019;
Published: 03 December 2019.
Edited by:
Alberto Basset, University of Salento, ItalyReviewed by:
Ryo Sugimoto, Fukui Prefectural University, JapanPaulo Vasconcelos, Portuguese Institute of Ocean and Atmosphere (IPMA), Portugal
Copyright © 2019 Andrisoa, Lartaud, Rodellas, Neveu and Stieglitz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aladin Andrisoa, YW5kcmlzb2FAY2VyZWdlLmZy
 Aladin Andrisoa
Aladin Andrisoa Franck Lartaud
Franck Lartaud Valentí Rodellas
Valentí Rodellas Ingrid Neveu
Ingrid Neveu Thomas C. Stieglitz
Thomas C. Stieglitz