- 1Department of Earth, Environmental, and Planetary Science, Rice University, Houston, TX, United States
- 2Department of Geological Sciences, The University of Texas at Austin, Austin, TX, United States
- 3Department of Geography and Anthropology, The Coastal Studies Institute, Louisiana State University, Baton Rouge, LA, United States
Shallow water coral reefs and deep sea coral communities are sensitive to current and future environmental stresses, such as changes in sea surface temperatures (SST), salinity, carbonate chemistry, and acidity. Over the last half-century, some reef communities have been disappearing at an alarming pace. This study focuses on the Gulf of Mexico, where the majority of shallow coral reefs are reported to be in poor or fair condition. We analyze the RCP8.5 ensemble of the Community Earth System Model v1.2 to identify monthly-to-decadal trends in Gulf of Mexico SST. Secondly, we examine projected changes in ocean pH, carbonate saturation state, and salinity in the same coupled model simulations. We find that the joint impacts of predicted higher temperatures and changes in ocean acidification will severely degrade Gulf of Mexico reef systems by the end of the twenty-first century. SSTs are likely to warm by 2.5–3°C; while corals do show signs of an ability to adapt toward higher temperatures, current coral species and reef systems are likely to suffer major bleaching events in coming years. We contextualize future changes with ancient reefs from paleoclimate analogs, periods of Earth's past that were also exceptionally warm, specifically rapid “hyperthermal” events. Ancient analog events are often associated with extinctions, reef collapse, and significant ecological changes, yet reef communities managed to survive these events on evolutionary timescales. Finally, we review research which discusses the adaptive potential of the Gulf of Mexico's coral reefs, meccas of biodiversity and oceanic health. We assert that the only guaranteed solution for long-term conservation and recovery is substantial, rapid reduction of anthropogenic greenhouse gas emissions.
1. Introduction
Coral Reefs constitute some of the most biodiverse ecosystems in Earth's oceans. They are critical to the socioeconomic health of 500 million people globally, providing billions of dollars in tourism and food sources for island and coastal communities (Frieler et al., 2013). Coral reefs support 25% of all of Earth's marine species during various stages of their life cycle (NOAA Ocean Service Education, 2017) and throughout geological time reefs have produced high diversity in Earth's oceans (Kiessling et al., 2010). Anthropogenic climate change is threatening reefs globally via multiple stressors including higher water temperatures, changes in water acidity, and fluctuating salinity. Today, there are no coral reefs left on the planet in pristine condition (Jackson et al., 2001; Hughes et al., 2003). Long-term surface temperature observations show a rate of global warming of 0.13°C per decade since 1979 (Trenberth et al., 2007), with an increase to 0.27°C per decade measured from 1985 to 2009 (Chollett et al., 2012).
Anthropogenic climate change affects coral biology via multiple compounding pathways (Rodolfo-Metalpa et al., 2011; Prada et al., 2017); multiple pressures (e.g., warming and acidification) combine to be significantly more damaging than either stressor alone. The majority of shallow-water, reef building corals are a holobiont consisting of an animal host (the coral) and zooxanthellae (photosynthetic endosymbiotic dinoflagellates of the family Symbiodiniaceae; LaJeunesse et al., 2018); this holobiont produces a skeleton made of calcium carbonate (aragonite). Scleractinian corals (or stony corals) are stenohaline and typically prefer a narrow range of water temperatures and carbonate saturation states. While they do have the ability to modify the saturation state (Ωaragonite) of the fluid from which they calcify their skeleton (e.g., Cohen and Holcomb, 2009; Ries et al., 2010; Anthony et al., 2011; Comeau et al., 2017a,b), changes in seawater pH and seawater carbonate chemistry can significantly reduce coral biomineralization, diversity, recruitment, and abundance (Fabricius et al., 2011). During times of extreme stress, in particular elevated sea surface temperatures (SST) or acidification, coral will expel their zooxanthellae, resulting in coral bleaching (Anthony et al., 2008; Baird et al., 2009; Frieler et al., 2013); in some cases on a global, sustained scale (Eakin et al., 2019; Skirving et al., 2019).
While much attention has been cast toward the sharp decline of coral reef systems in the Australia's Great Barrier Reef and across the tropical Pacific since the early 1980s (Frieler et al., 2013), considerably less work has been devoted to examining climate projections focused on corals and reef organisms from the Gulf of Mexico (GoM hereafter). The GoM is home to many coral reefs growing along coastal Texas, Louisiana, Florida, and Mexico in the upper ~1,500 m, and houses a wide array of deep sea coral species (as well as other reef builders, such as sponges) found along the continental shelf and slope (Figure 1, Figures S1, S2). Most of these reefs are within managed areas including Dry Tortugas National Park and Veracruzano Coral Reef System National Park, Flower Garden Banks and Florida Keys National Marine Sanctuaries, and Florida State Park John Pennekamp. Other coral reefs include Campeche Bank, Tuxpan, Tuxtlas, Yucatan Shelf, Florida Middle Grounds, and Pulley Ridge, the deepest stony coral reef in the US (Waddell and Clarke, 2008; Wilkinson and Souter, 2008; Ortiz-Lozano et al., 2013).
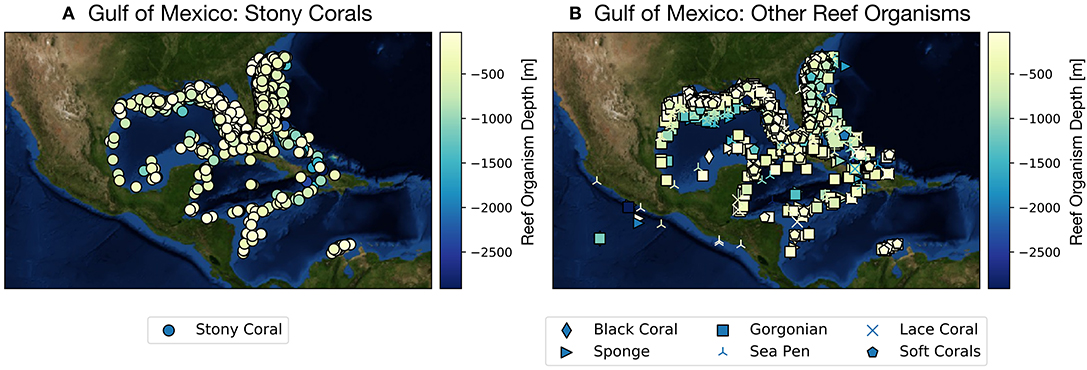
Figure 1. Gulf of Mexico shallow and deep sea coral and sponge sites as reported in the deep sea coral database (NOAA, Figures S1, S2). Color scale along y-axis indicates depth of reef organism and symbols denote organism type (e.g., scleractinian coral vs. sponge). (A) Scleractinian corals (stony corals), (B) all other species reef locations. See also Figures S1, S2. From https://deepseacoraldata.noaa.gov/website/AGSViewers/DeepSeaCorals/mapSites.htm.
GoM reef systems are subject to myriad anthropogenic stressors including rising SSTs, over-fishing, bleaching, chemical pollution and increasing terrestrial runoff, coral mining, and unrestricted tourism (Jordán-Dahlgren and Rodríguez-Martínez, 2003), as well as disease and sedimentation (Tunnell et al., 2007; Carricart-Ganivet et al., 2011; Horta-Puga et al., 2015). The once structurally complex coral reefs in the GoM and Caribbean have declined since the 1970s, and very few reefs still exhibit a mean live coral coverage >10% (Waddell and Clarke, 2008; Wilkinson and Souter, 2008). The majority of GoM coral reefs are reported to be in poor or fair condition with the exception of Flower Garden Banks (a protected National Marine Sanctuary) in the northern Gulf and Dry Tortugas National Park in the westernmost Florida Keys (Waddell and Clarke, 2008; Wilkinson and Souter, 2008; Johnston et al., 2017). The largest changes, documented since the 1970s, indicate that the most prevalent branching corals, the Acroporid corals, have experienced population declines >90% (Acropora Biological Review Team, 2005). Two of these corals, Acropora palmata and Acropora cervicornis, are listed as threatened species under the Endangered Species Act of 2006 (Hogarth, 2006). In 2010, the National Marine Fisheries Service found significant evidence to list 82 coral species as threatened species, including eight Caribbean species (NOAA, 2010).
At present, there is strong evidence that GoM reefs have experienced thermal stress since 1878 (Kuffner et al., 2015) with recent bleaching events in 2016/2017 (Johnston et al., 2019a). In situ SST records show a 0.8°C increase over the last century in the Florida Keys, where corals have declined especially in the later part of the twentieth century. Observed rates of SST warming are spatially and temporally variable throughout the Gulf, but the highest warming rates tend to occur in summer months (June, July, and August); most recently, the highest heating rates have been observed in the central GoM in the Loop Current region (Chollett et al., 2012; del Monte-Luna et al., 2015; Allard et al., 2016). Multiple studies suggest higher probabilities of coral bleaching in mid-latitude reefs (15–20° of latitude) despite similar levels of thermal stress compared to equatorial reefs (Sully et al., 2019). Coral accretion rates must keep up with the current rate of sea level rise for these ecosystems to survive (Toth et al., 2015); today, sea level rise threatens Florida Keys reefs and other GoM reefs, which cannot keep pace (Shinn, 1976).
Many of the climatic changes affecting the future of coral reefs have been examined in climate model projections. Given a business as usual (RCP8.5) greenhouse gas forcing scenario, simulations from the Climate Model Inter-Comparison Project (CMIP5) indicate that by 2090–2100, temperatures will increase, pH will decrease, oxygen content in the oceans will drop, and there will be a decrease in primary productivity (Bopp et al., 2013; Freeman, 2015). Tropical oceans are warming the fastest of any region globally in most of the CMIP5 projections, but with lower acidification rates. GoM SSTs are projected to rise by 0.37°C per decade. This substantial rise in SST would severely stress GoM coral reefs. Indeed, research shows that Gulf corals are stressed when SSTs approach 31°C; today, summertime temperatures frequently reach 30°C in the Florida Keys and Veracruz. These simulations suggest that more than 50% of coral reefs globally will undergo frequent and severe thermal stress by the year 2080 (Donner et al., 2005).
For this special issue on GoM coral reef systems, we zero in on climate change in the GoM and future threats to the region's reef ecosystems. Recent catastrophic environmental events, such as hurricanes Harvey and Irma (Hickerson et al., 2008; Viehman, 2017), have cast justified attention to GoM climate and ocean dynamics, including the well-being of Gulf species and ecosystems (Zavala-Hidalgo et al., 2014). This motivates careful examination of future climate predictions of all relevant variables to accurately capture spatial heterogeneity in reef response. In this work, we address the question: what changes in climate and ocean chemistry will influence the corals and reef systems in the Gulf of Mexico? We hypothesize that new model simulations confirm that the GoM will warm and acidify such that substantial coral bleaching will occur. A general circulation model (GCM) with a fully coupled ocean model is employed to test for changes in multiple environmental stressors that impact coral reefs in the GoM through 2100. The individual impacts of changes in temperature, salinity, and ocean acidification are partitioned to drive a more targeted reef impact mitigation plan. We contextualize future impacts to GoM reefs through the lens of geological time, exploring how present-day corals' predecessor species were able to adapt to analogous climate change events in the past. Finally, we discuss the future of GoM reefs in the Anthropocene, and provide a preview of the threats these ecosystems will soon face in this particular region.
2. Methods
2.1. GCM Simulations
To build a Gulf of Mexico-centric forecast of the various conditions that interfere with coral reef health over the next several decades, we evaluated simulations from the Community Earth System Model version 1.2 (CESM) (Kay et al., 2015). CESM is a state-of-the-art, Intergovernmental Panel on Climate Change (IPCC)-class general circulation model (GCM) developed at the National Center for Atmospheric Research. We compared two periods from a high-CO2 forcing IPCC representative concentration pathway (RCP) scenario (RCP8.5, which corresponds to 8.5 W/m2 of radiative imbalance due to anthropogenic greenhouse gas emissions). RCP8.5 assumes a “business as usual” radiative forcing consistent with minimal mitigation; we chose to employ this high-forcing model ensemble in light of the fact that emissions trends over the past few decades track slightly above RCP8.5 (Peters et al., 2012). CESM 1.2 simulations include a large ensemble (n = 33) of simulations spanning the period 2006–2100, from which we extracted four decades (2006–2026 and 2080–2100) for a modern vs. future comparison. From the early twenty-first century control period and the high-CO2 RCP8.5 scenario, the model ensemble mean was computed for the following variables: SST, salinity (SALT), alkalinity (ALK), dissolved inorganic carbon (DIC), and pH for the upper-most ocean layer of POP2, the ocean model component of CESM. We additionally analyzed the RCP4.5 medium ensemble of CESM1.2 to contextualize the changes in RCP8.5 with those likely under a lower emissions scenario. Note that all of the scripts used in the extraction and analysis of climate model output are documented in section S2, and provided directly in the Supplementary Material.
While the CESM model keeps track of the saturation state of seawater with respect to the carbonate minerals calcite and aragonite, these values are not directly included as part of the standard model output. Thus, we recomputed saturation states (Ω) using the MATLAB implementation of the CO2SYS software (https://www.nodc.noaa.gov/ocads/oceans/CO2SYS/co2rprt.html) (Lewis and Wallace, 1998). The CESM model outputs of Alkalinity, DIC, salinity, water depth (pressure), and temperature were used alongside assumptions of phosphate and silicate concentrations of 0 μM, and the dissociation constants of carbonic acid, bicarbonate, and sulfuric acid from Mehrbach et al. (1973); Dickson and Riley (1979); Dickson and Millero (1987), and Dickson (1990). This “offline” approach to evaluating carbonate mineral saturation states also allows us to apportion the predicted changes between each of the input variables by sequentially holding each variable constant at its 2006–2026 mean values and allowing the remaining variables to change.
To evaluate the accuracy of the model predictions for the modern period, the model output was compared to field data from multiple sites within the GoM. The field data were all taken from the Global Ocean Data Analysis Project Version 2 (GLODAPv2) and include sites off of the coasts of Texas, Louisiana, and Florida that range in depth from the surface to 500 m water depth. Using the same CO2SYS approach, the field measurements of alkalinity, DIC, salinity, and temperature were used to calculate Ωaragonite.
2.2. Defining Coral Reef Stress Factors
Based on CESM's available output history files, we define the following stressors on GoM reefs, and examine changes in these stressors from 2006–2026 to 2080–2100. It should be noted that many of these factors are synergistic (e.g., Rodolfo-Metalpa et al., 2011; Prada et al., 2017).
• Degree Heating Months (DHM), a standard predictor for coral bleaching (Gleeson and Strong, 1995; Liu et al., 2003). DHM = 1 refers to heating in excess of or equal to 1°C above the long-term monthly climatology of the warmest month in a given region (Sully et al., 2019). DHM gives a measure of thermal stress applied to corals, which leads to bleaching. DHM is easier to compute given that coupled GCMs usually archived at monthly time steps; however, degree heating weeks (DHW) is considered the more accurate predictor of bleaching (Liu et al., 2003; Kayanne, 2017; Sully et al., 2019).
• SST Variance: Sully et al. (2019) show in a global survey of coral bleaching from 1998 to 2017 that higher SST variance zones over reefs are less susceptible to bleaching.
• Mean annual SST: an upper temperature limit for coral bleaching in the Pacific has been reported as 28.1°C, but more recent work shows an increasing bleaching threshold of 28.7°C (Sully et al., 2019). In the GoM, reported bleaching thresholds are higher, approaching 30–31°C. Wilkinson and Souter (2008) found corals bleached in the Caribbean when SST reached 31°C and were sustained; Florida Keys and Flower Garden Banks reefs bleach when SST reached 30–31°C (Johnston et al., 2019b) or 29.5°C if temperatures were sustained for 50 days (Johnston et al., 2019a). We consider mean annual SSTs approaching 30°C as high risk for coral bleaching in the GoM.
• Salinity: in laboratory experiments, some species of Acropora corals are sensitive to low salinity values, exhibiting threshold behavior below ~22 g/kg (Berkelmans et al., 2012).
• Carbonate Chemistry: The saturation state of seawater with respect to aragonite (Ωaragonite) is an important control on coral growth as modern scleratinian (stony) corals biomineralize an aragonite skeleton. The saturation state of seawater is also a factor in coral growth and reef stabilization. The modern distribution of coral reefs is largely limited to regions of the ocean where Ωaragonite exceeds 3 (modern distribution threshold; Kleypas et al., 1999; Fine and Tchernov, 2007; Hoegh-Guldberg et al., 2007; Guinotte and Fabry, 2008) and experimental studies suggest that the calcification rate of corals drops to zero when Ωaragonite reaches 2 (experimental calcification threshold; Langdon et al., 2000; Albright et al., 2008). Nevertheless, there are some examples of corals and low diversity reefs growing in more acidified waters in both natural systems (Fabricius et al., 2011; Shamberger et al., 2014) and in controlled experiments (Cohen and Holcomb, 2009; Ries et al., 2010; Anthony et al., 2011). Finally, Ωaragonite = 1 is a strong thermodynamic limit as, below this value, it is more likely for aragonite to dissolve in seawater than to precipitate.
3. The Future of Gulf of Mexico Reefs in 21st Century Projections
3.1. SST Changes
Corals demonstrate species-specific variable responses to increasing surface ocean temperatures (Sully et al., 2019) as well as changing carbonate chemistry (Bahr et al., 2018); this response can also vary regionally and within micro-climates. In the GoM, the CESM RCP8.5 ensemble mean SSTs rise to 28.5°C in the northernmost sector of the Gulf, and 29–30°C in the central and southeastern regions (Figure 2) for the end of the twenty-first century. There is some indication that global mean thermal bleaching thresholds may be shifting toward warmer temperatures as global SSTs rise (Sully et al., 2019). Nevertheless, the projected annual mean warming for the GoM exceeds the most recent thermal threshold estimates of 30°C (Johnston et al., 2019b) in several locations in the southern and central GoM, especially in the Caribbean.
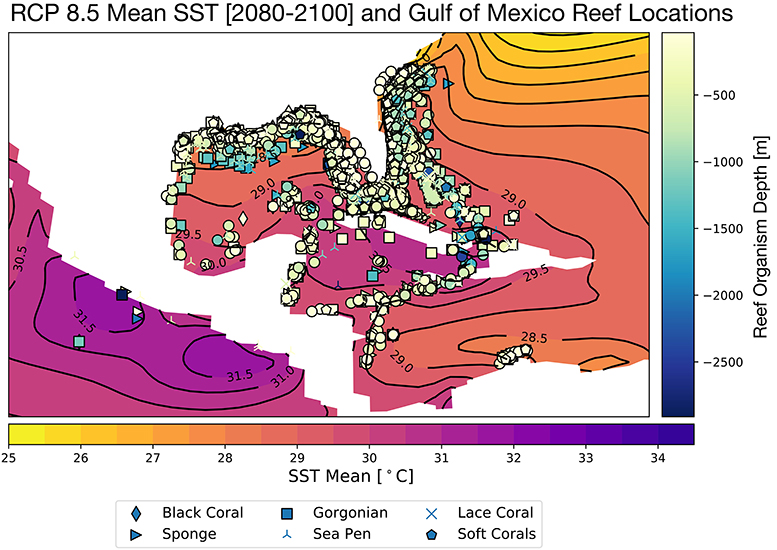
Figure 2. Projected mean Gulf of Mexico SST for 2080–2100, RCP8.5 Large Ensemble, CESM1.2, with all reported Gulf of Mexico reef organism locations overlain. Colors of coral sites indicates reef organism depth [meters] and symbols denote type of reef builder, as in Figure 1. Continental regions are shown in white.
The difference in 2080–2100 and 2006–2026 average SST is given in Figure 3. In most areas of the Gulf of Mexico, temperatures rise by ~3°C; in the central GoM and the Caribbean, the SST changes are closer to ~2.2–2.7°C. At 100 m depth, ocean temperatures increase more modestly by 1–1.5°C (Figure S3). The corals lining the Texas, Louisiana, and Florida coastlines are likely to experience the greatest temperature stress in the coming decades. Previous analyses of coupled climate model simulations (CMIP3) indicate that SST increases of just 1–1.5°C relative to the pre-industrial era places most reefs at a high risk for long-term degradation; an increase of 2°C will increase the risk of degradation or bleaching to 100% (Frieler et al., 2013). In all zones of the GoM, the change in surface ocean temperatures exceeds 2°C. Thus, the RCP8.5 changes in SST suggest wide-spread bleaching is likely by 2100 if a more aggressive mitigation strategy is not adopted in the coming decades.
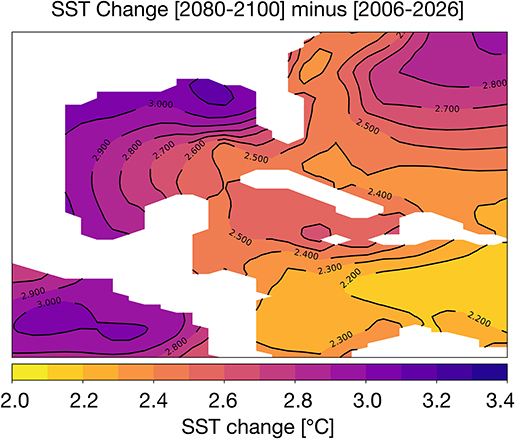
Figure 3. Simulated changes in Gulf of Mexico SST [°C] in the RCP8.5 Large Ensemble, CESM1.2. (2080–2100 mean minus 2006–2026 mean). Continental regions are shown in white.
To explore the potential influence of increased climate mitigation, we performed the same analysis of SST changes in the CESM RCP4.5 medium ensemble, corresponding to a lower greenhouse gas emissions scenario (Figure S4). GoM temperatures rise more modestly by 2060–2080 with lower forcing. While this ensemble only extends to 2080 (precluding a direct comparison of the RCP8.5 2080–2100 conditions), SST changes range from 0.92°C to 1.3°C by 2080. Despite the reduction in warming, this still constitutes changes leading to high risk of long-term degradation as defined by Frieler et al. (2013).
3.1.1. Degree Heating Months
We computed the cumulative number of months above the mean of maximum monthly SST climatology in each GoM grid cell (following our definition of DHM, see section 2.2) (following Liu et al., 2003; Donner et al., 2005; Frieler et al., 2013). Multiple studies (Glynn and D'Croz, 1990; Hoegh-Guldberg, 1999; Sheppard, 2003; Donner et al., 2005) show that a SST exceedance threshold of 1°C in a given month will lead to bleaching; Donner et al. (2005) consider a higher temperature threshold to be anything exceeding 2°C above monthly climatologies, corresponding to a degree heating week (DHW) exceeding 8 weeks of high heating. Figure 4 shows the difference in DHM between the beginning (2006–2026) and end of the twenty-first century. The number of DHM increases for the lower threshold of 1°C throughout the GoM, with the largest increases in DHM in the southern GoM and Caribbean (an increase of 75–90 DHM across the 20 year period). Along the Texas, Louisiana, and Florida coasts, DHM increases by 50–55 months total compared to the 2006–2026 base period. Put another way, GoM corals are likely to experience thermal stress for approximately 2–4 more months of the year by 2080–2100.
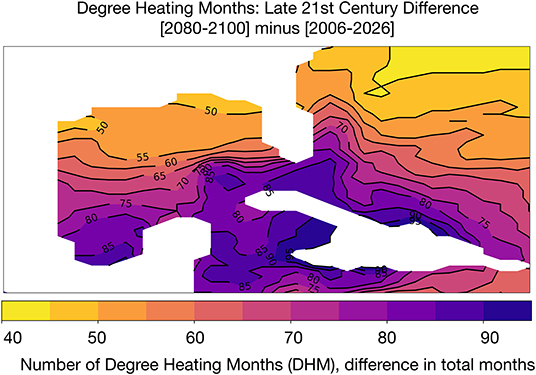
Figure 4. Simulated changes in Degree Heating Months (DHM) in GoM SST [°C] in the RCP8.5 Large Ensemble. DHM climatology is based on the period 2006–2080. DHM are defined as months that exceed 1°C of the hottest month in the grid cell climatology. Continental regions are shown in white.
3.1.2. SST Variance
Coral bleaching is less frequently observed in zones that experience high variance in SST anomalies (see Sully et al., 2019, for a review). To assess the potential for changes in SST variance to either dampen or amplify thermal stressors on GoM reefs, we computed the change in variance in the earlier part of the twenty-first century (Figure 5A) compared to the last 20 years (2080–2100) (Figure 5B). The overall spatial variance of SSTs remains largely unchanged throughout the twenty-first century: higher latitude GoM SSTs are highly variable, with more muted changes in the southern Gulf and Caribbean. The change in variance (Figure 5C) between the two time periods is close to zero degrees across much of the GoM with the exception of a few zones surrounding the Caribbean islands, which show an increase in overall variance. We note that these areas of higher SST variance correspond to main ocean currents driving GoM circulation, the Loop and Caribbean currents.
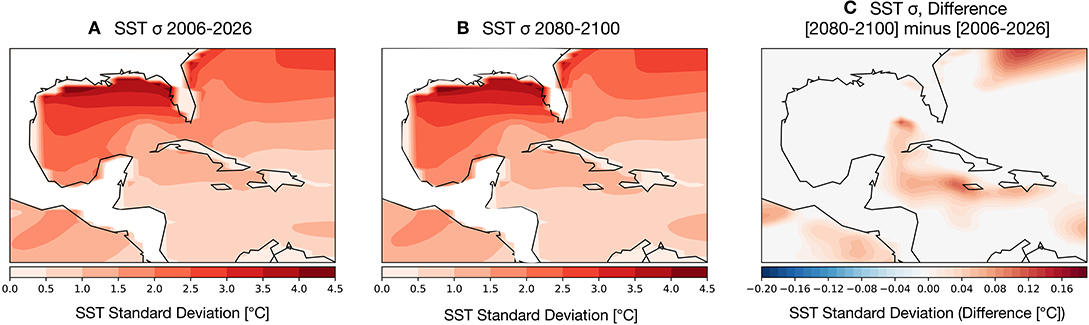
Figure 5. Simulated changes in the standard deviation (1-σ) of GoM SST [°C] in the RCP8.5 Large Ensemble, CESM1.2. (A) 2006–2026 SST σ. (B) 2080–2100 SST σ. (C) (2080–2100 mean σ minus 2006–2026 mean σ). Continental regions are outlined in black contours.
Given that increased variance is likely to help prevent bleaching, there is no modeling evidence that reductions in SST variance in the GoM will contribute to exacerbated coral bleaching.
3.2. Changes in Salinity and Carbonate Chemistry
While warming SSTs are expected to exert a primary influence on coral reefs over the coming decades, other hydrological and chemical changes in the ocean can also impact reef survival. Like SSTs, changes in variables such as salinity and pH exhibit spatial heterogeneity in simulations spanning the twenty-first century.
3.2.1. Salinity
GoM salinity is projected to increase; Figure 6 shows the average salinity for 2080–2100 (a) and the change from 2006 to 2026 (b). Salinity in the GoM and Caribbean is quite high, 36–37 g/kg, and the CESM RCP8.5 ensemble exhibits a trend toward saltier water characterizing the twenty-first century. Salinity falling below 22 psu is thus unlikely to stress GoM coral reefs. High salinities can also be a stress on coral reef communities, but the maximum salinities predicted for the GoM in the 2080–2100 simulation are well within the range of naturally observed salinities near reefs, and far lower than some regions (e.g., the Red Sea) (Coles and Jokiel, 1992). That said, changes in community structure among reef dwellers are possible with projected salinity shifts.
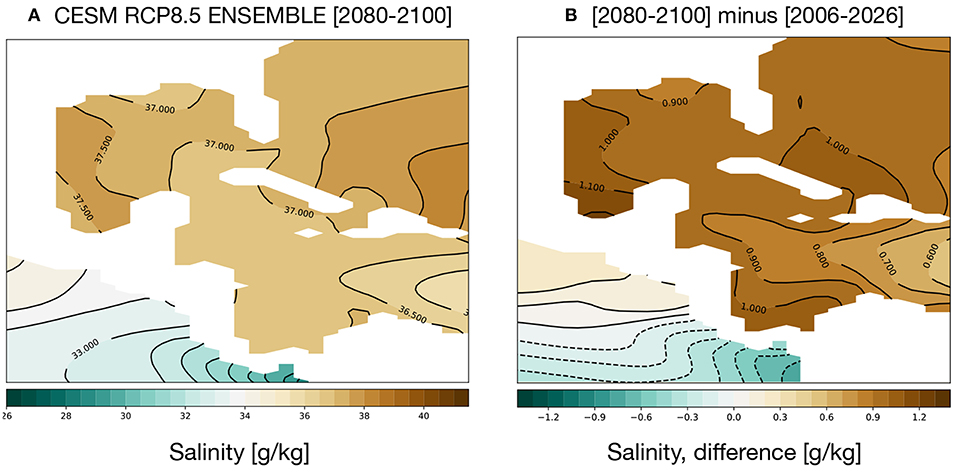
Figure 6. Simulated changes in GoM salinity in the RCP8.5 Large Ensemble, CESM1.2. (A) 2080–2100 mean. (B) (2080–2100) mean minus (2006–2026) mean. Continental regions are shown in white.
3.2.2. Ocean Carbonate Chemistry
To measure the potential for ocean acidification to obstruct aragonite calcification and degrade coral skeleton growth, we examined changes in both pH and Ωaragonite (see section 2). For the modern period, the model predictions under-predict Ωaragonite relative to field observations (Figure 7). For the surface ocean (0–50 m), the offset between the model and data is relatively small with the exception of the highest latitude sites (Figure 7). At 100 m water depth, the model-observation offset is greater (due to higher model-predicted DIC at depth), but a general trend of decreasing Ωaragonite with depth is present in both the field data and the model predictions. We note that the field data were collected in 2007 while the model predictions for the “modern” period span from 2006 to 2026. As a result, it is possible that the model-data discrepancy is due to the impacts of atmospheric CO2 on carbonate chemistry that occurred after the field data were collected. Alternatively, the model predictions for deep-water reefs may be inaccurate in their absolute value.
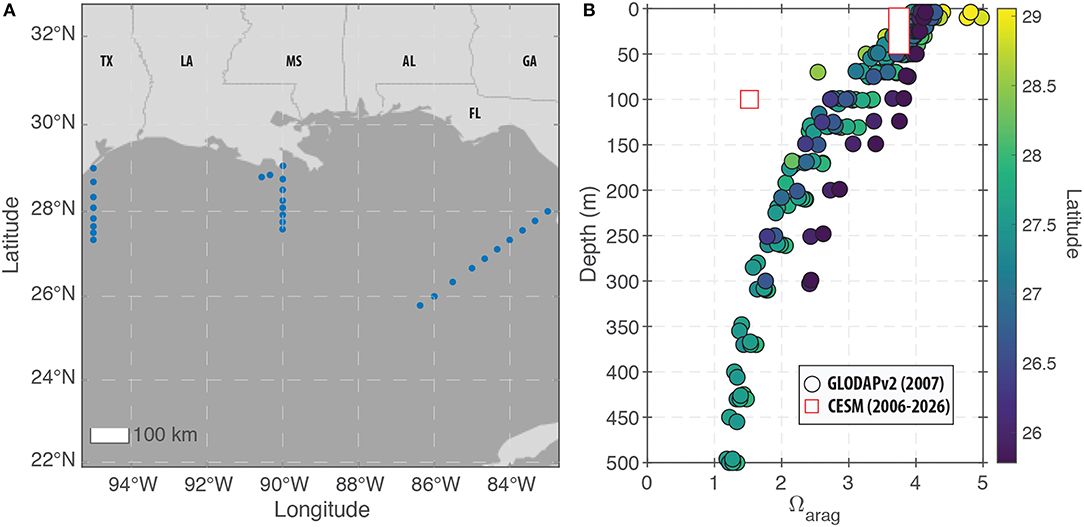
Figure 7. Modern observations of carbonate chemistry in the GoM. (A) Map showing the locations of the GLODAPv2 stations within the GoM that are used for comparison to the CESM predictions. The displayed region (83°W to 95°W and 22°N to 33°N) defines the GoM region used for displaying the model predictions in (B) as well as Figure 10. (B) Field measurements (circles) and model predictions (squares) of changes in Ωaragonite with depth in the GoM. For the surface ocean (0–50 m), the model reproduces most of the data with the exception of the highest latitude sites. However, at 100 m water depth, the model under-predicts Ωaragonite relative to all of the field observations.
Figure 8 shows the CESM results for changes in pH across the GoM for the end of twenty-first century. The surface ocean in the entire GoM regions is predicted to acidify by approximately −0.265 pH points on average, with the largest drop in pH along the northern Gulf coast (Figure 8). To assess the direct impacts of this change in ocean chemistry on coral growth, we computed Ωaragonite in CESM's ensemble mean for the 2080–2100 period (Figure 9). Given potential model biases at depth (see above), we focus on the surface (0–50 m), though model predictions for 100 m depth are shown in Figure S5; additionally, the model prediction for alkalinity changes at the surface is given in Figure S6.
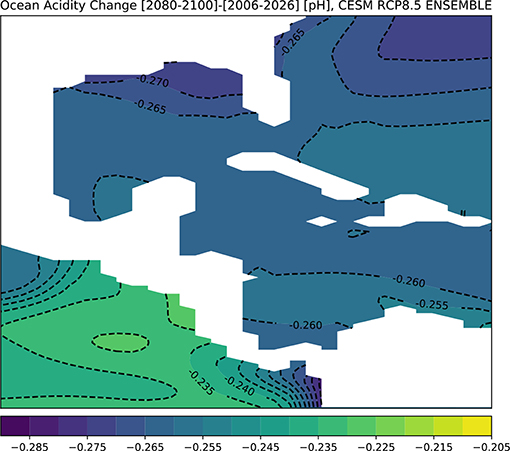
Figure 8. Simulated changes in GoM pH in the RCP8.5 Large Ensemble, CESM1.2. (2080–2100 mean minus 2006–2026 mean). Continental regions are shown in white.
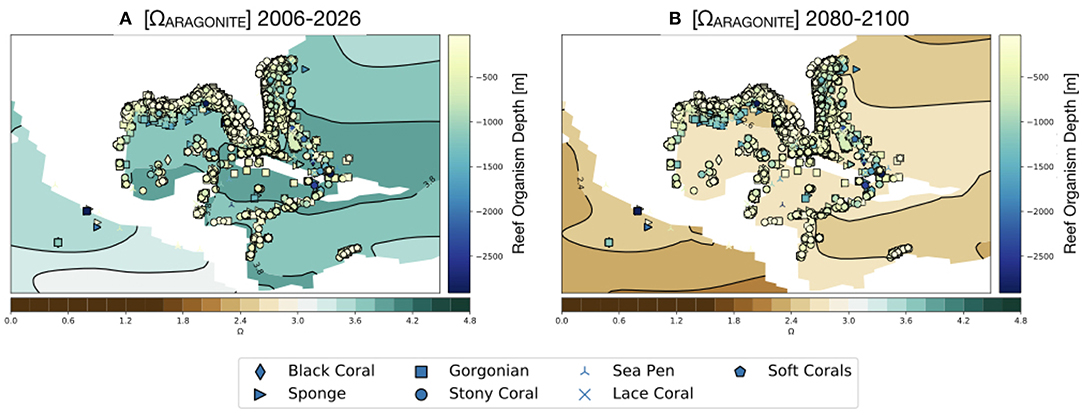
Figure 9. Simulated values of Ωaragonite for the GoMand northern Caribbean region in the RCP8.5 Large Ensemble (CESM1.2) in the surface ocean (0–50 m). Reef organisms and depth at which they occur [m] overlain (see Figure 1). (A) 2006–2026 mean, (B) 2080–2100 mean. Continental regions are shown in white.
As shown in Figure 9, our results suggest that the surface ocean of the entire GoM region will drop below Ωaragonite = 3. This is notable as the modern scleractinian corals are largely restricted to areas of the ocean where Ωaragonite exceeds this value (Kleypas et al., 1999). No surface regions of the Gulf of Mexico are predicted go below the experimental calcification threshold of Ωaragonite = 2 (Langdon et al., 2000; Albright et al., 2008) or to reach the thermodynamic limit for aragonite precipitation (Ωaragonite = 1; Figure 10).
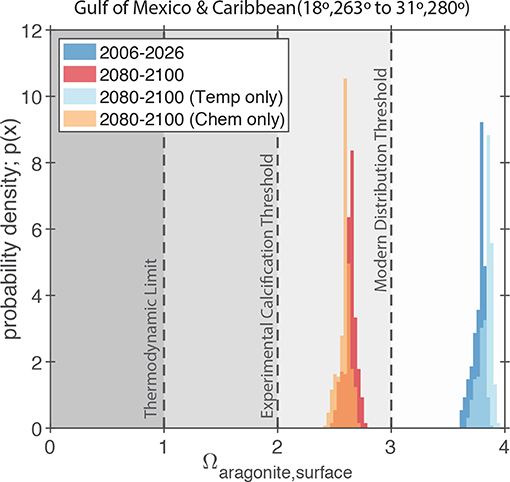
Figure 10. Simulated values of Ωaragonite for the GoM and northern Caribbean Region (i.e., the region shown in Figure 7A) in the RCP8.5 Large Ensemble, CESM1.2. The dark blue and dark red probability density functions (PDFs) show the model predictions for the 2006–2026 and 2080–2100 periods, respectively. The light blue and orange PDFs show the model predictions for the 2080–2100 period where either ocean chemistry or water temperature is held constant at the 2006–2026 values. The gray shading and dashed lines indicate the typical limits for the presence of scleractinian corals in the modern ocean (Ω > 3; Kleypas et al., 1999), the experimentally measured limits for calcification by coral reef communities (Ω > 2; Langdon et al., 2000; Albright et al., 2008), and the thermodynamic limit for aragonite precipitation (Ω = 1).
Compared to reefs in the upper 50 m of the water column, deeper water reefs would experience lower saturation states because aragonite has retrograde solubility (i.e., Ωaragonite decreases with depth due decreased temperature and increased pressure). Modern field observations show that Ωaragonite values at 100 m depth in the GoM are approximately 1 unit lower than surface waters (Figure 7; Wanninkhof et al., 2015; Feely et al., 2018). This means that some of the deeper, mesophotic reefs (even those as shallow as 100 m) may already be experiencing stress due to low Ωaragonite values. Alternatively, deeper-water reef builders (e.g., glass sponges, gorgonian corals, and sea pens) are likely adapted for those conditions.
The model predicted change in Ωaragonite at 100 m depth is much less severe than at the surface; the CESM model estimates only a 0.3 unit change in Ωaragonite at 100 m between the beginning and end of the century, with almost no change at the 500 m horizon (not shown). As mentioned above, the model predicted value for Ωaragonite at 100 m water depth over the modern (2006–2026) period does not match existing field measurements (Figure 7) due to model-predicted DIC concentrations that are too high. While the model is inaccurate in terms of the absolute value for Ωaragonite at depth, this does not necessarily mean that the magnitude of the change predicted by the model for 100 m water depth is also inaccurate. For example, the model prediction that changes in carbonate chemistry are greater in the surface ocean is consistent with the underlying driver being the addition of CO2 to atmosphere, which exchanges more rapidly with the surface ocean relative to below the mixed layer.
The small change in Ωaragonite predicted at depth may stress coral communities. That said, it is also possible that the predicted changes would not be as damaging as the changes predicted for shallow water reefs in that the deeper communities are already adapted/acclimatized to lower Ωaragonite values (Farfan et al., 2018).
Due to the retrograde solubility of aragonite, the predicted increase in temperature acts to slightly increase Ωaragonite (Figure 10); however, the effect of increasing atmospheric CO2 and associated ocean acidification greatly exceeds the effect of temperature and leads to an overall decline in Ωaragonite for the whole region (Figure 10). Similarly, the effect of increasing salinity on Ωaragonite is negligible compared to the predicted pH changes. More importantly, the combination of heat and acidity stresses can often act synergistically (e.g., Rodolfo-Metalpa et al., 2011; Prada et al., 2017), meaning that even moderate heating and Ωaragonite decreases can amplify each other leading to intolerable conditions for coral reefs. Furthermore, with surface oceans getting warmer and more acidic (low Ω) waters at depth, it is possible that surface-adapted reef communities in the GoM will have no suitable refuge by the end of the century (e.g., Pereira et al., 2018; Rocha et al., 2018).
4. Contextualizing Anthropogenic Changes With Hot-House Climates of the Past
Scleractinian coral reefs have a long history extending back to the Middle Triassic (242–247 million years ago) (Martindale et al., 2019). Specifically, there are numerous records of reefs in the paleo-GoM, including microbial reefs from the Upper Jurassic (164–153 Ma) (Mancini and Parcell, 2001), coral and rudist bivalve reefs from the Cretaceous (145–90 Ma) (e.g., Enos, 1974; Scott, 1984; Höfling and Scott, 2002; Hattori et al., 2019), sponge and coral reefs from the Paleocene (66 to 59 Ma) (Bryan, 1991), as well as the drowned and living reef banks that initiated during the last deglacial period (~14,500 years ago) (Khanna et al., 2017).
Ancient coral reefs that grew during (or were killed off by) hyperthermal (sudden, extreme heating) events can be seen as analog case studies for changes in reef communities today. When looking at the entire Phanerozoic (the last 541 million years), many of the worst reef collapses are coincident with evidence of thermal stress and ocean acidification (Kiessling and Simpson, 2011). When scleractinian reef systems are considered (the last 250 million years), this trend is even more concerning. Heat stress and acidification occur coincident with the last 3 greatest metazoan reef collapses: the Triassic/Jurassic mass extinction at 201 Ma (a 99.4% loss of reef volume), Pliensbachian/Toarcian extinction and Toarcian Oceanic Anoxic Event at ~183 Ma (a 98.3% loss of reef volume), as well as the early Cenozoic Hyperthermal Events [e.g., Paleocene-Eocene Thermal Maximum at 56 Ma (a 99.6% loss of reef volume) and Early Eocene Climate Optimum (54 Ma)] [reef loss calculated from metazoan reef volumes reported in Kiessling and Simpson, 2011]. It should be noted that since acidification events are so geologically short-lived, it is often difficult to attribute this stress to long-term community change (Hönisch et al., 2012). Ancient reefs are imperfect analogs; modern fast growing coral species, such as A. cervicornis, generally evolved in the last half million years (Hoegh-Guldberg et al., 2007) and thus the reef communities are not identical. Nevertheless, many coral genera (from the Cenozoic in particular) are extant (not extinct), so reasonable comparisons can be made between groups (Weiss and Martindale, 2019). Further, many important reef forming corals were present in the GoM as far back as the early Eocene, including Astrocoenia, Favia, Goniopora, Montastraea, Siderastrea, and Stylophora (Budd, 2000).
The Paleocene-Eocene Thermal Maximum in particular has been noted as one of the better analogs for modern climate change due to the similarities in the cause (i.e., greenhouse gas emissions) and its consequences (e.g., ocean acidification, increases in temperature) (Hönisch et al., 2012). In the Tethys Ocean, Paleocene coral reefs underwent a protracted, three-step collapse, from coral-dominated to foraminiferal or microbial reefs, before the complete demise of reef ecosystems near the Paleocene/Eocene boundary (Scheibner and Speijer, 2008; Zamagni et al., 2012). Nevertheless, these reef ecosystems were able to maintain a relatively high diversity (Zamagni et al., 2012). The main driver of the reef turnover is thought to be elevated temperature, but ocean acidification (Kiessling and Simpson, 2011), excessive sedimentation, and nutrification (Zamagni et al., 2012) are also implicated. In the GoM region, temperature, increased sedimentation, and nutrient input due to tectonism (Galloway et al., 2000) led to the development of sponge and coralline algae dominated reefs. Some zooxanthellate and apozooxanthellate massive and platy corals were also present (Bryan, 1991). Weiss and Martindale (2019) show that corals with particular traits, such as flexible photosymbiosis and feeding strategies and those that lived in siliciclastic environments, were better able to withstand change during the Cenozoic hyperthermal events. Because the GoM is largely siliciclastic, it is possible that GoM corals may prove more resilient than those in carbonate environments. Importantly, the rates of climate change in the modern are faster than during the Paleocene (Zeebe et al., 2016), barring direct comparison between the two time periods.
Finally, the Last Interglacial (LIG, ~129–116 ka) was the last time the Earth was as warm as today with 11% warmer temperatures in the northern hemisphere, a greater loss of Arctic sea ice, and a partial loss of the Greenland ice sheet (Kukla et al., 2002; CAPE-Last Interglacial Project Members, 2006); these conditions are all possible in the near future under realistic carbon emissions scenarios. The Florida Keys in the southeastern GoM had extensive coral reef coverage during the LIG. These reefs contained many of the coral species we find in the Florida Keys today with branching Acropora and Porites, and boulder-shaped Montastraea, Diploria, and Siderastrea corals with ooid banks to the west and east of the reef (Stanley, 1966). Reconstructions from Tropical Atlantic locations find temperature and seasonality variability similar to present (Felis et al., 2015; Brocas et al., 2016, 2018). However, sea level was up to 6 m higher than today during the LIG, and likely exerted a dominant control on coral reef distributions. Evidence from Florida Keys, Bahamas, and Cayman Islands for the LIG found corals grew to about 3 m above current sea level, but not the peak 6 m (Blanchon, 2011). Evidence from the northern Yucatan peninsula suggest that a rapid depth change from 3 to 6 m induced a higher-energy wave environment that remobilized lagoonal sediments and buried or eroded adjacent reef framework resulting in marine sand bodies that prevented the submerged reefs from recovering (Blanchon et al., 2009). While the LIG is not a perfect analog for the current climate change, it provides some insights into coral response to quick pulses in sea level rise due to collapsing ice sheets and shifts in oceanic-atmospheric conditions. Coral reefs did exist and flourish in many locations during the LIG, but rapid changes in sea level given, for example, a partial collapse of the Greenland ice sheet could severely inhibit future growth.
These ancient events provide useful information regarding sensitivities, survival, and recovery during extreme stress events, as well as natural reef ecosystem responses to climate change in the absence of human-induced changes or interventions.
5. Discussion: Gulf Corals in the Anthropocene
This study examines future projections for oceanic conditions in the GoM and the potential impacts of multiple stressors on coral reef ecosystems. Using CESM 1.2, a state-of-the-art coupled GCM, we compared two key 20-year periods: 2006–2026 (a base control) and 2080–2100 (end of twenty-first century). We find that GoM SSTs are likely to warm by 2.5–3°C, elevating mean temperatures to a range of 28–30.5°C in a high-CO2 forcing scenario. While corals do show signs of an ability to evolve toward higher temperatures (e.g., Howells et al., 2012; Palumbi et al., 2014; Dixon et al., 2018), there are annual mean SST thresholds (e.g., 30°C, Wilkinson and Souter, 2008; Johnston et al., 2019b) beyond which corals simply bleach and die. By the end of the twenty-first century, the ensemble mean SST fields are spatially heterogeneous, but a great number of reefs, particularly off the coast of Belize, Florida, and Cuba, may experience mean annual temperatures closer to 30°C by 2100; further, these estimates potentially contain a cold bias (Liu L. et al., 2012), and in reality, GoM temperature observations are already hotter (Johnston et al., 2019b). Taken together, these results suggest that Gulf reef systems will experience frequent and severe thermal stresses by the end of the twenty-first century. Preventing such widespread and severe bleaching to the Earth's coral reef systems likely requires limiting global warming to 1.5°C, which is, at present, a lofty target (Frieler et al., 2013). To avoid widespread degradation of Gulf corals and reef communities, atmospheric CO2 levels would likely need to be stabilized below measured 2005 levels (Donner et al., 2005).
The CESM1.2 RCP8.5 large ensemble provides no evidence that changes in GoM SST variance or salinity will adversely impact coral reef ecosystems. That said, an evaluation of relevant carbonate chemistry variables (i.e., pH and carbonate saturation state), suggest that a threshold may be crossed by the end of the century. Scleractinian coral reefs that are currently growing in supersaturated waters of Ωaragonite greater than 3.4 (Figures 9, 10) will experience significant pH and carbonate saturation state drops (Figures 8, 9). These changes in carbonate chemistry will negatively impact GoM reef biomineralization. Today, scleractinian coral reefs that are found in low pH or Ωaragonite waters have lower biodiversities and abundance of reef builders or dwellers than reefs in higher Ωaragonite waters (Fabricius et al., 2011). Reefs in low Ωaragonite regions are typically poorly cemented, have higher bioerosion, and have fewer structurally complex framework builders, which together result in lower structural integrity of the reef (Fabricius et al., 2011; DeCarlo et al., 2015). If the GoM becomes more acidic (lower Ωaragonite) we should expect the reef ecosystems to become less diverse and structurally weakened with a higher likelihood of significant coral bleaching. These issues are important on their own, but also lead to secondary issues; for example, lessened structural integrity can lead to more significant storm damage during hurricanes (Cheal et al., 2017), which will also increase in intensity and severity with rising temperatures (Molina et al., 2016; Murakami et al., 2018); reef species may also become more susceptible to disease or see a decline in fecundity.
The combination of thermal and chemical stress will make these environmental changes even more damaging (e.g., Donner et al., 2007, 2018; Rodolfo-Metalpa et al., 2011; Prada et al., 2017; Bahr et al., 2018). Deeper water, mesophotic reefs have distinct communities and ecosystems when compared to shallow water reefs (e.g., Bongaerts et al., 2010; Pereira et al., 2018; Rocha et al., 2018) but are, nevertheless, likely to experience severe thermal stress (Schramek et al., 2018). Given the issues of accuracy and resolution in predicting Ωaragonite values at 100 m depth shown in this work, it is hard to make confident conclusions about the fate of mesophotic reefs. On the one hand, the predicted amount of Ωaragonite change at 100 m depth is minimal (especially when compared to the surface), but on the other hand, these values are already below an important calcification threshold for scleractinian corals. If deep Mesophotic reefs are primarily inhabited by organisms that are well adapted to these lower Ωaragonite conditions, the communities may not experience a catastrophic change. Future research should focus on the physiological limits of deep mesophotic reef communities as there is still very little known about these ecosystems (Bongaerts et al., 2010; Kahng et al., 2010).
We acknowledge several important caveats of this work. Coupled atmosphere-ocean GCMs contain significant cold temperature biases in the GoM and Caribbean (Liu L. et al., 2012; Martin and Schumacher, 2012; Ryu and Hayhoe, 2015; Exarchou et al., 2018; McGregor et al., 2018). Analysis of the Coupled Model Intercomparison Project (CMIP3) revealed that SST variability in the Intra-Americas Sea is less than observed in historical simulations (Liu L. et al., 2012); many GCMs produce large cold biases across the Intra-Americas Sea when assessed with gridded SST data products. This is crucially important for coral bleaching thresholds, likely leading to underestimated temperature maxima in the GCM predictions. In Key West, for example, summer water temperatures during 2019 were frequently observed above 32°C (National Data Buoy Center, NOAA). Coral bleaching may thus be underestimated in CESM 1.2 as well, and anthropogenic bleaching events may occur with greater severity than projected in this study.
Recent event-based evidence demonstrates that increases in upwelling from stronger winds, frequent during tropical storms, can lead to cold water events and anoxic conditions, promoting coral death and disease (Lirman et al., 2011). An anomalous cold event in 2010 killed numerous near-shore corals in the Florida Keys (Colella et al., 2012) and in the winter of 1969–1970 (Hudson et al., 1976). Cold coral bleaching events might also be altered by climate change, but the evaluation of changes in frequency in such events requires analysis of daily wind patterns, which is beyond the scope of this work.
Finally, the spatial resolution of CESM is 1 × 1°; reefs typically occupy spatial scales at tens to hundreds of meters (Donner et al., 2005). This discrepancy in scale creates uncertainties related to downscaling and microclimate affects. While GCM simulations afford important future mean state projections, an inability to resolve details at the reef scale and examine local circulation changes may inhibit our ability to make robust predictions about the future of GoM reef bleaching. Advances in regional ocean modeling and downscaling climate model outputs may facilitate the simulation of local upwelling and upper-ocean heating processes; such advances would refine projections of reef impacts (Donner et al., 2005, 2007, 2018). Further, recent work shows 1 × 1° IPCC-class GCMs contain biases in the simulation of the Loop Current, which largely moderates GoM temperatures (Liu Y. et al., 2012). The Loop Current carries warm waters into the central GoM; if this current slows, it could reduce the warming in the central GoM, mitigating the impacts of global warming. Adding embedded, online ecological models that explicitly simulate reef response to temperature and acidity changes would enhance the accuracy of the results presented here. These advances in model development are forthcoming (Bopp et al., 2013; Jones et al., 2019).
6. The Future of the Gulf of Mexico's Coral Reefs
6.1. Climate Change and Biodiversity Loss
Coral reefs are critical ecosystem focal points in marine environments, supporting the world's fisheries, protecting coastlines, and promoting tourism. Through all of these structures, coral reefs generate hundreds of billions of dollars to the global economy each year (Mora et al., 2011; NOAA Ocean Service Education, 2017; Reef Relief, 2019). In the GoM alone, reef-related expenditures generate more than $4.4 billion annually in southeast Florida and reef recreation supports more than 70,000 jobs (Carnes, 2010). The many threats posed by climate change to coral reefs, including bleaching and acidification, motivates a pointed look toward reef systems lining GoM coastlines. The reefs that protect the coastline of the GoM are subject to unique regional ocean changes, warranting this geographically-focused study.
Multiple secondary impacts are likely to accompany rising temperatures and climate change in the GoM. Warm SSTs drive stronger tropical storms (Molina et al., 2016; Murakami et al., 2018). In recent decades, major hurricanes (e.g., Mitch, which decimated reefs in Belize) have wiped out coral reefs. During the 2005 hurricane season, coral reefs in the GoM (Flower Garden Banks, the Dry Tortugas, and the Florida Keys) experienced extensive damage; however, these reefs were spared from widespread bleaching event that occurred that year because the passing hurricanes reduced water temperatures (Stone et al., 2005; Gierach and Subrahmanyam, 2008; Wilkinson and Souter, 2008). Recovery timescales are on the order of multiple years to decades in a relatively healthy reef. In reefs that are already degraded or experience repeated storm events, recovery from physical disturbance can take even longer (Dollar and Tribble, 1993; Edmunds and Gray, 2014). Given consistent projections showing increases in the frequency and severity of tropical storms and Gulf hurricanes (Balaguru et al., 2018; Klotzbach et al., 2018; Trenberth et al., 2018; Ting et al., 2019), it is likely that high storm surge and wave impacts could further degrade GoM reefs, especially if they are less robust due to weakened cementation. This could initiate a positive feedback loop: coral reefs in the GoM protect local shorelines and infrastructure through wave energy dissipation, prevention of shoreline erosion, import of sediments, and via stabilizing mangrove and seagrass populations (NOAA Ocean Service Education, 2017; Reef Relief, 2019). Storm-driven losses of coral reefs may further reduce the resilience of the built environment along Gulf coastlines. Indeed, the economic damages imparted by hurricane activity in Texas and Florida in 2017 alone surpassed a staggering 125 billion dollars (Klotzbach et al., 2018). Some of these storms also cause unpredictable damages such as low salinity runoff or pollution (Rice University, 2019).
Additional anthropogenic stressors will interact to further degrade coral reefs in the GoM. These include increased sedimentation, fresh-water run-off and pollution due to development (Yeats et al., 1978; Nelsen et al., 1994; Osterman et al., 2008; Liu et al., 2013; Ren et al., 2015), and nutrification, particularly due to agricultural sources such as fertilizers (Nelsen et al., 1994; Osterman et al., 2008) and hydrocarbon extraction (Guzman and Jarvis, 1996). A combination of the above can lead to hypoxia (Justić et al., 2003; Osterman et al., 2008; Rabalais et al., 2010). Many of these effects favor the growth of coral competitors, such as macroalgae, that can further hamper reef development and growth (Gorgula and Connell, 2004; Vermeij et al., 2010). In a healthy ecosystem, herbivorous fishes and invertebrates can help to balance the overgrowth of algae (Williams and Poulnin, 2001; Bellwood et al., 2006; Smith et al., 2010); however, the physiology and reproductive capabilities of these organisms are also compromised by climate change and ocean acidification (Munday et al., 2008; Pankhurst and Munday, 2011). Further, because fish depend on the structural complexity of reefs (Graham and Nash, 2013), losing coral reefs can lead to a feedback where loss of fish leads to algae overgrowth, which then dampens reef development, leading to even fewer fish (Graham et al., 2007; Wilson et al., 2010; Nyström et al., 2012).
6.2. Adaptation and Mitigation
The ability of GoM reefs to adapt to the stressors outlined in section 3 is an open question. Corals may be able to survive in warmer temperatures through adaptation, epigenetic modification, or the utilization of thermal-tolerant symbionts (Howells et al., 2012; Palumbi et al., 2014; Dixon et al., 2018; Sully et al., 2019); however, acclimatization to acidification has not yet been demonstrated (Comeau et al., 2019). The adaptability of coral symbionts will likely play a key role in determining thermal resistance of GoM reefs. Reef structures may acclimate rather than completely dying off if symbiont species more tolerant to high temperatures and bleaching re-occupy coral tissues over time (Hughes et al., 2003) or if corals can acquire thermal tolerant symbionts (Howells et al., 2012). Coral reef generation times are on the order of several years and depend on favorable environmental conditions (Hughes et al., 2003), so if existing corals in a given community are stressed, they will not spawn. Bleaching onset research indicates adaptation via re-population of thermally tolerant symbionts occurs within 0–0.5° warming; given that most GoM SSTs warm by more than 2° in the CESM RCP8.5 ensemble mean, we must not discount the fact that zooxanthellate Gulf corals may disappear completely by 2100. Future reefs may shift toward populations typical of the late Cretaceous and early Cenozoic, when reefs were dominated by non-corals (i.e., rudists, sponges and red algae) and azooxanthellate coral types (Bryan, 1991; Kiessling and Baron-Szabo, 2004).
Coral communities could also migrate toward more favorable environments and regrow. The rate and direction of climatic shifts will likely drive coral species shifts across the GoM, and these changes in climate velocity (Pinsky et al., 2013) are crucial to robust predictions of reef survival (Figure S7). Projected climate velocities for the GoM in terms of SST changes indicate rapid shifts of up to 10 km/yr throughout the forthcoming twenty-first century (Figure S7). While some marine species can migrate rapidly, coral reefs are largely stationary and migrate over generations of new reefs established in new regions. The establishment process requires many factors to encourage reef growth, including the presence of crustose coralline algae (Morse et al., 1994, 1996; Heyward and Negri, 1996), low sediment input (Gilmour, 1999), lithified substrate (Jackson, 1977; Purkis et al., 2011), and precise water quality conditions (Negri and Hoogenboom, 2011). On evolutionary timescales, reefs often shift poleward to avoid thermal stress (e.g., Kiessling, 2001) and this has already been documented in geologically-recent and modern reefs (e.g., Greenstein and Pandolfi, 2008; Yamano et al., 2011; Pandolfi and Kiessling, 2014). Poleward migration may not be a feasible option since GoM corals are limited latitudinally and are already positioned at their northern limit (e.g., Kiessling, 2001; Jones et al., 2019).
It is also hypothesized that reefs may find refuge from thermal stress in the surface waters by migrating to deeper habitats where temperatures are lower (Riegl and Piller, 2003; Bongaerts et al., 2010; Bridge et al., 2013; Padilla-Gamiño et al., 2019); this has occurred to some extent in Pulley Ridge, though recent surveys have found that these deep water hermatypic corals are not surviving (Slattery et al., 2018). Deeper water, mesophotic reefs have distinct communities and ecosystems when compared to shallow water reefs (e.g., Bongaerts et al., 2010; Pereira et al., 2018; Rocha et al., 2018); thus these deeper habitats likely would make poor refuges for shallow-water reef species. Vertical migration in the water column is harder for the reef builders, which require clear water and sunlight for photosynthesis. With sea level rise, many deeper reefs that have narrow depth ranges will not be able to keep pace with increasing water depth. Furthermore, mesophotic reefs inhabit waters with lower Ωaragonite values, leading to the possibility that GoM reef communities would experience an environmental pincer movement: thermal stress from above as well as acidification stress from below (which would also make pole-ward migration problematic). In general, there are myriad conditions that would prevent this from being a feasible adaptation for coral survival on a large scale (Smith et al., 2016; Bongaerts et al., 2017).
Even if corals adapt or acclimatize to some environmental stresses (e.g., temperature or salinity), they may not be able to adapt or acclimate to all of them (e.g., ocean acidification) (Okazaki et al., 2013; Comeau et al., 2019). A controversial solution involves geoengineering via reef-shading, covering large portions of reefs to reduce direct heating via solar radiation (Coelho et al., 2017). This is an expensive and precarious solution which cannot bolster coral resistance to thermal stress. Increasing coral tolerance through assisted evolution, such as selective breeding, assisted gene flow, transplanting of juveniles, epigenetic programming or conditioning, and coral microbiome manipulation may be viable within the next decade (Horoszowski-Fridman et al., 2011; van Oppen et al., 2015; Van Oppen et al., 2017), and would directly bolster reef resiliency to ecosystem collapse.
6.3. Looking Ahead
The marine organisms occupying the GoM evolved in the last 420,000 years; now, atmospheric ρCO2 levels dramatically exceed ice core measurements of greenhouse gas concentrations spanning their entire evolutionary history (Hoegh-Guldberg et al., 2007). In an even broader geologic context, the rate of greenhouse gas emissions, and therefore the rate of climate change, during the Paleocene-Eocene Thermal Maximum was orders of magnitude slower than the modern warming trend, meaning modern climate change is unprecedented in geological history (Zeebe et al., 2016). Given the innumerable benefits that coral reefs in the GoM provide to coastal societies, it is our hope that this work sheds light on future risks specific to this highly vulnerable ecosystem. While reef systems can recover from bleaching events, reefs generally require decades to return to their pre-bleached state (Frieler et al., 2013). It is likely that the accelerating rate of global climate change will exceed the speed at which corals reefs and their symbionts can adapt (multiple decades), a defining feature of abrupt climate change (Hughes et al., 2003; Frieler et al., 2013). Widespread degradation of GoM reefs is especially likely under the RCP8.5 “business-as-usual” scenario considered in this work. To avoid consequential environmental, social, and economic damages (e.g. Chen et al., 2015) and promote long-term conservation and recovery of GoM coral reefs, substantial, rapid reductions of anthropogenic greenhouse gas emissions are past-due.
Data Availability Statement
The datasets analyzed for this study are housed in the Earth System Grid, a publicly available climate model output repository (https://www.earthsystemgrid.org/). Additional data information and our analysis code is available in the Supplementary Material.
Author Contributions
SD, RM, and KD designed the study and formulated scientific questions. SD extracted, post-processed and analyzed climate model data, and formulated coral stressor conditions with assistance from RM and KD. MT modeled the ocean carbon changes and assisted in estimating drivers of acidification as a coral stressor with help from RM. RM, KD, and AW contextualized future GoM change with information surrounding coral communities in past climates. All authors contributed to the writing of the manuscript.
Funding
KD was supported by a Department of the Interior South Central Climate Adaptation Science Center Cooperative Agreement G15AP00136.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Adrienne Correa for support in completing this work. Many thanks to the Gulf of Mexico Reefs: Past, Present, and Future Symposium for instigating this collaboration as well as the Paleontological Society and the Rice University Creative Ventures Fund for conference support. SD thanks the National center for Atmospheric Research Computational & Information Systems Laboratory for their assistance in accessing climate model output.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2019.00691/full#supplementary-material
References
Acropora Biological Review Team (2005). Atlantic Acropora Status Review Document. Report to National Marine Fisheries Service, 152.
Albright, R., Mason, B., and Langdon, C. (2008). Effect of aragonite saturation state on settlement and post-settlement growth of porites astreoides larvae. Coral Reefs 27, 485–490. doi: 10.1007/s00338-008-0392-5
Allard, J., Clarke, J. V. III., and Keim, B. D. (2016). Spatial and temporal patterns of in situ sea surface temperatures within the gulf of mexico from 1901–2010. Am. J. Clim. Change 5:314. doi: 10.4236/ajcc.2016.53025
Anthony, K. R., Kleypas, A. J., and Gattuso, J.-P. (2011). Coral reefs modify their seawater carbon chemistry–implications for impacts of ocean acidification. Glob. Change Biol. 17, 3655–3666. doi: 10.1111/j.1365-2486.2011.02510.x
Anthony, K. R., Kline, D. I., Diaz-Pulido, G., Dove, S., and Hoegh-Guldberg, O. (2008). Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. U.S.A. 105, 17442–17446. doi: 10.1073/pnas.0804478105
Bahr, K. D., Rodgers, K. S., and Jokiel, P. L. (2018). Ocean warming drives decline in coral metabolism while acidification highlights species-specific responses. Mar. Biol. Res. 14, 924–935. doi: 10.1080/17451000.2018.1551616
Baird, A. H., Bhagooli, R., Ralph, P. J., and Takahashi, S. (2009). Coral bleaching: the role of the host. Trends Ecol. Evol. 24, 16–20. doi: 10.1016/j.tree.2008.09.005
Balaguru, K., Foltz, G. R., and Leung, L. R. (2018). Increasing magnitude of hurricane rapid intensification in the central and eastern tropical atlantic. Geophys. Res. Lett. 45, 4238–4247. doi: 10.1029/2018GL077597
Bellwood, D. R., Hughes, T. P., and Hoey, A. S. (2006). Sleeping functional group drives coral-reef recovery. Curr. Biol. 16, 2434–2439. doi: 10.1016/j.cub.2006.10.030
Berkelmans, R., Jones, A. M., and Schaffelke, B. (2012). Salinity thresholds of Acropora spp. on the great barrier reef. Coral Reefs 31, 1103–1110. doi: 10.1007/s00338-012-0930-z
Blanchon, P. (2011). “Last interglacial and reef development,” in Encyclopedia of Modern Coral Reefs: Structure, Form and Process, ed D. Hopley (Dordrecht: Springer), 621–639. doi: 10.1007/978-90-481-2639-2_105
Blanchon, P., Eisenhauer, A., Fietzke, J., and Liebetrau, V. (2009). Rapid sea-level rise and reef back-stepping at the close of the last interglacial highstand. Nature 458, 881–884. doi: 10.1038/nature07933
Bongaerts, P., Ridgway, T., Sampayo, E., and Hoegh-Guldberg, O. (2010). Assessing the ‘deep reef refugia’ hypothesis: focus on Caribbean reefs. Coral Reefs 29, 309–327. doi: 10.1007/s00338-009-0581-x
Bongaerts, P., Riginos, C., Brunner, R., Englebert, N., Smith, S. R., and Hoegh-Guldberg, O. (2017). Deep reefs are not universal refuges: reseeding potential varies among coral species. Sci. Adv. 3:e1602373. doi: 10.1126/sciadv.1602373
Bopp, L., Resplandy, L., Orr, J. C., Doney, S. C., Dunne, J. P., Gehlen, M., et al. (2013). Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosciences 10, 6225–6245. doi: 10.5194/bg-10-6225-2013
Bridge, T. C., Hoey, A. S., Campbell, S. J., Muttaqin, E., Rudi, E., Fadli, N., et al. (2013). Depth-dependent mortality of reef corals following a severe bleaching event: implications for thermal refuges and population recovery. F1000Research 2:187. doi: 10.12688/f1000research.2-187.v2
Brocas, W. M., Felis, T., Gierz, P., Lohmann, G., Werner, M., Obert, J. C., et al. (2018). Last interglacial hydroclimate seasonality reconstructed from tropical atlantic corals. Paleoceanogr. Paleoclimatol. 33, 198–213. doi: 10.1002/2017PA003216
Brocas, W. M., Felis, T., Obert, J. C., Gierz, P., Lohmann, G., Scholz, D., et al. (2016). Last interglacial temperature seasonality reconstructed from tropical atlantic corals. Earth Planet. Sci. Lett. 449, 418–429. doi: 10.1016/j.epsl.2016.06.005
Bryan, J. R. (1991). A paleocene coral 'algal' sponge reef from southwestern alabama and the ecology of early tertiary reefs. Lethaia 24, 423–438. doi: 10.1111/j.1502-3931.1991.tb01497.x
Budd, A. F. (2000). Diversity and extinction in the cenozoic history of caribbean reefs. Coral Reefs 19, 25–35. doi: 10.1007/s003380050222
CAPE-Last Interglacial Project Members (2006). Last Interglacial Arctic warmth confirms polar amplification of climate change. Quat. Sci. Rev. 25, 1383–1400. doi: 10.1016/j.quascirev.2006.01.033
Carnes, K. (2010). Scientific Surveys Show Extent of Cold Impact on Florida Keys Corals. Florida Keys National Marine Sanctuary; National Marine Sanctuary, NOAA.
Carricart-Ganivet, J., Beltrán-Torres, A., and Horta-Puga, G. (2011). Distribution and prevalence of coral diseases in the Veracruz reef system, Southern Gulf of Mexico. Dis. Aquat. Organ. 95, 181–187. doi: 10.3354/dao02359
Cheal, A. J., MacNeil, M. A., Emslie, M. J., and Sweatman, H. (2017). The threat to coral reefs from more intense cyclones under climate change. Glob. Change Biol. 23, 1511–1524. doi: 10.1111/gcb.13593
Chen, P.-Y., Chen, C.-C., Chu, L., and McCarl, B. (2015). Evaluating the economic damage of climate change on global coral reefs. Glob. Environ. Change 30, 12–20. doi: 10.1016/j.gloenvcha.2014.10.011
Chollett, I., Müller-Karger, F. E., Heron, S. F., Skirving, W., and Mumby, P. J. (2012). Seasonal and spatial heterogeneity of recent sea surface temperature trends in the Caribbean Sea and Southeast Gulf of Mexico. Mar. Pollut. Bull. 64, 956–965. doi: 10.1016/j.marpolbul.2012.02.016
Coelho, V., Fenner, D., Caruso, C., Bayles, B., Huang, Y., and Birkeland, C. (2017). Shading as a mitigation tool for coral bleaching in three common Indo-Pacific species. J. Exp. Mar. Biol. Ecol. 497, 152–163. doi: 10.1016/j.jembe.2017.09.016
Cohen, A. L., and Holcomb, M. (2009). Why corals care about ocean acidification: uncovering the mechanism. Oceanography 22, 118–127. doi: 10.5670/oceanog.2009.102
Colella, M., Ruzicka, R., Kidney, J., Morrison, J., and Brinkhuis, V. (2012). Cold-water event of january 2010 results in catastrophic benthic mortality on patch reefs in the Florida keys. Coral Reefs 31, 621–632. doi: 10.1007/s00338-012-0880-5
Coles, S. L., and Jokiel, P. L. (1992). Effects of salinity on coral reefs. Pollut. Trop. Aquat. Syst. 147–166. doi: 10.1201/9781351075879-6
Comeau, S., Cornwall, C., DeCarlo, T., Doo, S., Carpenter, R., and McCulloch, M. (2019). Resistance to ocean acidification in coral reef taxa is not gained by acclimatization. Nat. Clim. Change 9:477. doi: 10.1038/s41558-019-0486-9
Comeau, S., Cornwall, C., and McCulloch, M. (2017a). Decoupling between the response of coral calcifying fluid pH and calcification to ocean acidification. Sci. Rep. 7:7573. doi: 10.1038/s41598-017-08003-z
Comeau, S., Tambutté, E., Carpenter, R., Edmunds, P., Evensen, N., Allemand, D., et al. (2017b). Coral calcifying fluid pH is modulated by seawater carbonate chemistry not solely seawater pH. Proc. R. Soc. B Biol. Sci. 284:20161669. doi: 10.1098/rspb.2016.1669
DeCarlo, T. M., Cohen, A. L., Barkley, H. C., Cobban, Q., Young, C., Shamberger, K. E., et al. (2015). Coral macrobioerosion is accelerated by ocean acidification and nutrients. Geology 43, 7–10. doi: 10.1130/G36147.1
del Monte-Luna, P., Villalobos, H., and Arreguín-Sánchez, F. (2015). Variability of sea surface temperature in the Southwestern Gulf of Mexico. Contin. Shelf Res. 102, 73–79. doi: 10.1016/j.csr.2015.04.017
Dickson, A., and Millero, F. J. (1987). A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. Part A Oceanogr. Res. Pap. 34, 1733–1743. doi: 10.1016/0198-0149(87)90021-5
Dickson, A., and Riley, J. (1979). The estimation of acid dissociation constants in seawater media from potentionmetric titrations with strong base. I. the ionic product of water-kw. Mar. Chem. 7, 89–99. doi: 10.1016/0304-4203(79)90001-X
Dickson, A. G. (1990). Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 k. Deep Sea Res. Part A Oceanogr. Res. Pap. 37, 755–766. doi: 10.1016/0198-0149(90)90004-F
Dixon, G., Liao, Y., Bay, L. K., and Matz, M. V. (2018). Role of gene body methylation in acclimatization and adaptation in a basal metazoan. Proc. Natl. Acad. Sci. U.S.A. 115, 13342–13346. doi: 10.1073/pnas.1813749115
Dollar, S. J., and Tribble, G. W. (1993). Recurrent storm disturbance and recovery: a long-term study of coral communities in Hawaii. Coral Reefs 12, 223–233. doi: 10.1007/BF00334481
Donner, S., Heron, S., and Skirving, W. (2018). “Future scenarios: a review of modelling efforts to predict the future of coral reefs in an era of climate change,” in Coral Bleaching (Berlin, Heidelberg: Springer), 325–341.
Donner, S. D., Knutson, T. R., and Oppenheimer, M. (2007). Model-based assessment of the role of human-induced climate change in the 2005 Caribbean coral bleaching event. Proc. Natl. Acad. Sci. U.S.A. 104, 5483–5488. doi: 10.1073/pnas.0610122104
Donner, S. D., Skirving, W. J., Little, C. M., Oppenheimer, M., and Hoegh-Guldberg, O. (2005). Global assessment of coral bleaching and required rates of adaptation under climate change. Glob. Change Biol. 11, 2251–2265. doi: 10.1111/j.1365-2486.2005.01073.x
Eakin, C. M., Sweatman, H. P., and Brainard, R. E. (2019). The 2014–2017 global-scale coral bleaching event: insights and impacts. Coral Reefs 38, 539–545. doi: 10.1007/s00338-019-01844-2
Edmunds, P. J., and Gray, S. C. (2014). Recurrent storm disturbance and recovery: a long-term study of coral communities in Hawaii. Hydrobiologia 734, 143–158. doi: 10.1007/s10750-014-1876-7
Enos, P. (1974). Reefs, platforms, and basins of middle cretaceous in Northeast Mexico. AAPG Bull. 58, 800–809. doi: 10.1306/83D91498-16C7-11D7-8645000102C1865D
Exarchou, E., Prodhomme, C., Brodeau, L., Guemas, V., and Doblas-Reyes, F. (2018). Origin of the warm eastern tropical Atlantic SST bias in a climate model. Clim. Dyn. 51, 1819–1840. doi: 10.1007/s00382-017-3984-3
Fabricius, K. E., Langdon, C., Uthicke, S., Humphrey, C., Noonan, S., De'ath, G., et al. (2011). Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Change 1, 165–169. doi: 10.1038/nclimate1122
Farfan, G. A., Cordes, E. E., Waller, R. G., DeCarlo, T. M., and Hansel, C. M. (2018). Mineralogy of deep-sea coral aragonites as a function of aragonite saturation state. Front. Mar. Sci. 5:473. doi: 10.3389/fmars.2018.00473
Feely, R. A., Okazaki, R. R., Cai, W.-J., Bednaršek, N., Alin, S. R., Byrne, R. H., et al. (2018). The combined effects of acidification and hypoxia on pH and aragonite saturation in the coastal waters of the California current ecosystem and the northern Gulf of Mexico. Contin. Shelf Res. 152, 50–60. doi: 10.1016/j.csr.2017.11.002
Felis, T., Giry, C., Scholz, D., Lohmann, G., Pfeiffer, M., Pätzold, J., et al. (2015). Tropical atlantic temperature seasonality at the end of the last interglacial. Nat. Commun. 6:6159. doi: 10.1038/ncomms7159
Fine, M., and Tchernov, D. (2007). Scleractinian coral species survive and recover from decalcification. Science 315, 1811–1811. doi: 10.1126/science.1137094
Freeman, L. A. (2015). Robust performance of marginal pacific coral reef habitats in future climate scenarios. PLoS ONE 10:e0128875. doi: 10.1371/journal.pone.0128875
Frieler, K., Meinshausen, M., Golly, A., Mengel, M., Lebek, K., Donner, S., et al. (2013). Limiting global warming to 2°c is unlikely to save most coral reefs. Nat. Clim. Change 3, 165–170. doi: 10.1038/nclimate1674
Galloway, W. E., Ganey-Curry, P. E., Li, X., and Buffler, R. T. (2000). Cenozoic depositional history of the Gulf of Mexico basin. AAPG Bull. 84, 1743–1774. doi: 10.1306/8626C37F-173B-11D7-8645000102C1865D
Gierach, M. M., and Subrahmanyam, B. (2008). Biophysical responses of the upper ocean to major Gulf of Mexico hurricanes in 2005. J. Geophys. Res. 113:C04029. doi: 10.1029/2007JC004419
Gilmour, J. (1999). Experimental investigation into the effects of suspended sediment on fertilisation, larval survival and settlement in a scleractinian coral. Mar. Biol. 135, 451–462. doi: 10.1007/s002270050645
Gleeson, M., and Strong, A. (1995). Applying mcsst to coral reef bleaching. Adv. Space Res. 16, 151–154. doi: 10.1016/0273-1177(95)00396-V
Glynn, P., and D'Croz, L. (1990). Experimental evidence for high temperature stress as the cause of el nino-coincident coral mortality. Coral Reefs 8, 181–191. doi: 10.1007/BF00265009
Gorgula, S. K., and Connell, S. D. (2004). Expansive covers of turf-forming algae on human-dominated coast: the relative effects of increasing nutrient and sediment loads. Mar. Biol. 145, 613–619. doi: 10.1007/s00227-004-1335-5
Graham, N. A. J., and Nash, K. L. (2013). The importance of structural complexity in coral reef ecosystems. Coral Reefs 32, 315–326. doi: 10.1007/s00338-012-0984-y
Graham, N. A. J., Wilson, S. K., Jennings, S., Polunin, N. V. C., Robinson, J. A. N., Bijoux, J. P., et al. (2007). Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries, and ecosystems. Conserv. Biol. 21, 1291–1300. doi: 10.1111/j.1523-1739.2007.00754.x
Greenstein, B. J., and Pandolfi, J. M. (2008). Escaping the heat: range shifts of reef coral taxa in coastal Western Australia. Glob. Change Biol. 14, 513–528. doi: 10.1111/j.1365-2486.2007.01506.x
Guinotte, J. M., and Fabry, V. J. (2008). Ocean acidification and its potential effects on marine ecosystems. Ann. N.Y. Acad. Sci. 1134, 320–342. doi: 10.1196/annals.1439.013
Guzman, H. M. and Jarvis, K. E. (1996). Vanadium century record from Caribbean reef corals: a tracer of oil pollution in Panama. Ambio. 25, 523–526. doi: 10.2307/4314533
Hattori, K. E., Loucks, R. G., and Kerans, C. (2019). Stratal architecture of a halokinetically controlled patch reef complex and implications for reservoir quality: a case study from the aptian james limestone in the fairway field, east texas basin. Sediment. Geol. 387, 87–103. doi: 10.1016/j.sedgeo.2019.04.009
Heyward, A. J., and Negri, A. P. (1996). Natural inducers for coral larval metamorphosis. Coral Reefs 18, 273–279. doi: 10.1007/s003380050193
Hickerson, E. L., Schmahl, G., Robbart, M., Precht, W. F., and Caldow, C. (2008). The State of Coral Reef Ecosystems of the Flower Garden Banks, Stetson Bank, and Other Banks in the Northwestern Gulf of Mexico. The state of coral reef ecosystems of the United States and Pacific Freely Associated States, 189–217.
Hoegh-Guldberg, O. (1999). Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshw. Res. 50, 839–866. doi: 10.1071/MF99078
Hoegh-Guldberg, O., Mumby, P. J., Hooten, A. J., Steneck, R. S., Greenfield, P., Gomez, E., et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. doi: 10.1126/science.1152509
Höfling, R., and Scott, R. W. (2002). Early and Mid-Cretaceous Buildups. Society for Sedimentary Geology (SEPM). Phanerozoic Reef Patterns. SEPM Special Publication No. 72, 521–548.
Hogarth, W. (2006). Endangered and threatened species: final listing determinations for elkhorn coral and staghorn coral. Feder. Regis. 71, 26852–26861.
Hönisch, B., Ridgwell, A., Schmidt, D. N., Thomas, E., Gibbs, S. J., Sluijs, A., et al. (2012). The geological record of ocean acidification. Science 335, 1038–1063. doi: 10.1126/science.1208277
Horoszowski-Fridman, Y., Izhaki, I., and Rinkevich, B. (2011). Engineering of coral reef larval supply through transplantation of nursery-farmed gravid colonies. J. Exp. Mar. Biol. Ecol. 399, 162–166. doi: 10.1016/j.jembe.2011.01.005
Horta-Puga, G., Tello-Musi, J. L., Beltrán-Torres, A., Carricart-Ganivet, J. P., Carriquiry, J. D., and Villaescusa-Celaya, J. (2015). “Veracruz reef system: a hermatypic coral community thriving in a sedimentary terrigenous environment,” in Aportes al Conocimiento del Sistema Arrecifal Veracruzano: Hacia el Corredor Arrecifal del Suroeste del Golfo de México, eds A. Granados-Barba, L. Ortiz-Lozano, D. Salas-Monreal, and C. González-Gándara (Universidad Autonoma de Campeche), 181–208.
Howells, E., Beltran, V., Larsen, N., Bay, L., Willis, B., and Van Oppen, M. (2012). Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat. Clim. Change 2, 116–120. doi: 10.1038/nclimate1330
Hudson, J. H., Shinn, E. A., Halley, R. B., and Lidz, B. (1976). Sclerochronology: a tool for interpreting past environments. Geology 4, 361–364. doi: 10.1130/0091-7613(1976)4<361:SATFIP>2.0.CO;2
Hughes, T. P., Baird, A. H., Bellwood, D. R., Card, M., Connolly, S. R., Folke, C., et al. (2003). Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933. doi: 10.1126/science.1085046
Jackson, J. B., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., et al. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637. doi: 10.1126/science.1059199
Jackson, J. B. C. (1977). Competition on marine hard substrata: the adaptive significance of solitary and colonial strategies. Am. Nat. 111, 743–767. doi: 10.1086/283203
Johnston, M. A., Hickerson, E. L., Nuttall, M. F., Blakeway, R. D., Sterne, T. K., Eckert, R. J., et al. (2019a). Coral bleaching and recovery from 2016 to 2017 at east and west flower garden banks, Gulf of Mexico. Coral Reefs 38, 787–799. doi: 10.1007/s00338-019-01788-7
Johnston, M. A., Nuttall, M. F., Eckert, R. J., Blakeway, R. D., Sterne, T. K., Hickerson, E. L., et al. (2019b). Localized coral reef mortality event at East Flower Garden Bank, Gulf of Mexico. Bull. Mar. Sci. 95, 239–250. doi: 10.5343/bms.2018.0057
Johnston, M. A., Sterne, T. K., Eckert, R. J., Nuttall, M. F., Embesi, J. A., Walker, R. D., et al. (2017). Long-Term Monitoring at East and West Flower Garden Banks: 2016 Annual Report. National Oceanic and Atmospheric Administration Institutional Repository, United States.
Jones, L. A., Mannion, P. D., Farnsworth, A., Valdes, P. J., Kelland, S.-J., and Allison, P. A. (2019). Coupling of palaeontological and neontological reef coral data improves forecasts of biodiversity responses under global climatic change. R. Soc. Open Sci. 6:182111. doi: 10.1098/rsos.182111
Jordán-Dahlgren, E., and Rodríguez-Martínez, R. E. (2003). “The Atlantic coral reefs of Mexico,” in Latin American Coral Reefs, ed J. Cortés (Amsterdam: Elsevier), 131–158.
Justić, D., Rabalais, N. N., and Turner, R. E. (2003). Simulated responses of the Gulf of Mexico hypoxia to variations in climate and anthropogenic nutrient loading. J. Mar. Syst. 42, 115–126. doi: 10.1016/S0924-7963(03)00070-8
Kahng, S., Garcia-Sais, J., Spalding, H., Brokovich, E., Wagner, D., Weil, E., et al. (2010). Community ecology of mesophotic coral reef ecosystems. Coral Reefs 29, 255–275. doi: 10.1007/s00338-010-0593-6
Kay, J., Deser, C., Phillips, A., Mai, A., Hannay, C., Strand, G., et al. (2015). The Community Earth System Model (CESM) large ensemble project: a community resource for studying climate change in the presence of internal climate variability. Bull. Am. Meteorol. Soc. 96, 1333–1349. doi: 10.1175/BAMS-D-13-00255.1
Kayanne, H. (2017). Validation of degree heating weeks as a coral bleaching index in the Northwestern Pacific. Coral Reefs 36, 63–70. doi: 10.1007/s00338-016-1524-y
Khanna, P., Droxler, A. W., Nittrouer, J. A., Tunnell, J. W. Jr., and Shirley, T. C. (2017). Coralgal reef morphology records punctuated sea-level rise during the last deglaciation. Nat. Commun. 8:1046. doi: 10.1038/s41467-017-00966-x
Kiessling, W. (2001). Paleoclimatic significance of phanerozoic reefs. Geology 29, 751–754. doi: 10.1130/0091-7613(2001)029<0751:PSOPR>2.0.CO;2
Kiessling, W., and Baron-Szabo, R. C. (2004). Extinction and recovery patterns of scleractinian corals at the cretaceous-tertiary boundary. Palaeogeogr. Palaeoclimatol. Palaeoecol. 214, 195–223. doi: 10.1016/S0031-0182(04)00421-3
Kiessling, W., and Simpson, C. (2011). On the potential for ocean acidification to be a general cause of ancient reef crises. Glob. Change Biol. 17, 56–67. doi: 10.1111/j.1365-2486.2010.02204.x
Kiessling, W., Simpson, C., and Foote, M. (2010). Reefs as cradles of evolution and sources of biodiversity in the phanerozoic. Science 327, 196–198. doi: 10.1126/science.1182241
Kleypas, J. A., McManus, J. W., and Menez, L. A. (1999). Environmental limits to coral reef development: where do we draw the line? Am. Zool. 39, 146–159. doi: 10.1093/icb/39.1.146
Klotzbach, P. J., Bowen, S. G., Pielke Jr, R., and Bell, M. (2018). Continental us hurricane landfall frequency and associated damage: Observations and future risks. Bull. Am. Meteorol. Soc. 99, 1359–1376. doi: 10.1175/BAMS-D-17-0184.1
Kuffner, I. B., Lidz, B. H., Hudson, J. H., and Anderson, J. S. (2015). A century of ocean warming on florida keys coral reefs: historic in situ observations. Estuar. Coasts 38, 1085–1096. doi: 10.1007/s12237-014-9875-5
Kukla, G. J., Bender, M. L., de Beaulieu, J.-L., Bond, G., Broecker, W. S., Cleveringa, P., et al. (2002). Last interglacial climates. Quater. Res. 58, 2–13. doi: 10.1006/qres.2001.2316
LaJeunesse, T. C., Parkinson, J. E., Gabrielson, P. W., Jeong, H. J., Reimer, J. D., Voolstra, C. R., et al. (2018). Systematic revision of symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 28, 2570–2580. doi: 10.1016/j.cub.2018.07.008
Langdon, C., Takahashi, T., Sweeney, C., Chipman, D., Goddard, J., Marubini, F., et al. (2000). Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Glob. Biogeochem. Cycles 14, 639–654. doi: 10.1029/1999GB001195
Lewis, E., and Wallace, D. (1998). CO2SYS-Program Developed for the CO2 System Calculations. Carbon Dioxide Inf Anal Center Report ORNL/CDIAC-105.
Lirman, D., Schopmeyer, S., Manzello, D., Gramer, L. J., Precht, W. F., Muller-Karger, F., et al. (2011). Severe 2010 cold-water event caused unprecedented mortality to corals of the florida reef tract and reversed previous survivorship patterns. PLoS ONE 6:e23047. doi: 10.1371/journal.pone.0023047
Liu, G., Strong, A. E., and Skirving, W. (2003). Remote sensing of sea surface temperatures during 2002 barrier reef coral bleaching. Eos Trans. Am. Geophys. Union 84, 137–141. doi: 10.1029/2003EO150001
Liu, L., Hong, Y., Hocker, J. E., Shafer, M. A., Carter, L. M., Gourley, J. J., et al. (2012). Analyzing projected changes and trends of temperature and precipitation in the southern USA from 16 downscaled global climate models. Theor. Appl. Climatol. 109, 345–360. doi: 10.1007/s00704-011-0567-9
Liu, M., Tian, H., Yang, Q., Yang, J., Song, X., Lohrenz, S. E., et al. (2013). Long-term trends in evapotranspiration and runoff over the drainage basins of the Gulf of Mexico during 1901–2008. Water Resour. Res. 49, 1988–2012. doi: 10.1002/wrcr.20180
Liu, Y., Lee, S.-K., Muhling, B. A., Lamkin, J. T., and Enfield, D. B. (2012). Significant reduction of the Loop Current in the 21st century and its impact on the Gulf of Mexico. J. Geophy. Res. 117:C05039. doi: 10.1029/2011JC007555
Mancini, E. A., and Parcell, W. C. (2001). Outcrop analogs for reservoir characterization and modeling of Smackover microbial reefs in the northeastern Gulf of Mexico area. Gulf Coast Assoc. Geol. Soc. Trans. 51, 207–218.
Martin, E. R., and Schumacher, C. (2012). The relationship between tropical warm pool precipitation, sea surface temperature, and large-scale vertical motion in IPCC AR4 models. J. Atmos. Sci. 69, 185–194. doi: 10.1175/JAS-D-11-0104.1
Martindale, R. C., Foster, W. J., and Velledits, F. (2019). The survival, recovery, and diversification of metazoan reef ecosystems following the end-permian mass extinction event. Palaeogeogr. Palaeoclimatol. Palaeoecol. 513, 100–115. doi: 10.1016/j.palaeo.2017.08.014
McGregor, S., Stuecker, M. F., Kajtar, J. B., England, M. H., and Collins, M. (2018). Model tropical Atlantic biases underpin diminished pacific decadal variability. Nat. Clim. Change 8, 493–498. doi: 10.1038/s41558-018-0163-4
Mehrbach, C., Culberson, C. H., Hawley, J. E., and Pytkowicx, R. M. (1973). Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907. doi: 10.4319/lo.1973.18.6.0897
Molina, M., Timmer, R., and Allen, J. (2016). Importance of the Gulf of Mexico as a climate driver for US severe thunderstorm activity. Geophys. Res. Lett. 43, 12295–12304. doi: 10.1002/2016GL071603
Mora, C., Aburto-Oropeza, O., Bocos, A. A., Ayotte, P. M., Banks, S., Bauman, A. G., et al. (2011). Global human footprint on the linkage between biodiversity and ecosystem functioning in reef fishes. PLoS Biol. 9:e1000606. doi: 10.1371/journal.pbio.1000606
Morse, A. N. C., Iwao, K., Baba, M., Shimoike, K., Hayashibara, T., and Omor, M. (1996). An ancient chemosensory mechanism brings new life to coral reefs. Biol. Bull. 191, 149–154. doi: 10.2307/1542917
Morse, D. E., Morse, A. N. C., Raimondi, P. T., and Hooker, N. (1994). Morphogen-based chemical flypaper for agaricia humilis coral larvae. Biol. Bull. 186, 172–181. doi: 10.2307/1542051
Munday, P. L., Jones, G. P., Pratchett, M. S., and Williams, A. J. (2008). Climate change and the future for coral reef fishes. Fish Fish. 9, 261–285. doi: 10.1111/j.1467-2979.2008.00281.x
Murakami, H., Levin, E., Delworth, T., Gudgel, R., and Hsu, P.-C. (2018). Dominant effect of relative tropical Atlantic warming on major hurricane occurrence. Science 362, 794–799. doi: 10.1126/science.aat6711
Negri, A. P., and Hoogenboom, M. O. (2011). Water contamination reduces the tolerance of coral larvae to thermal stress. PLoS ONE 6:e19703. doi: 10.1371/journal.pone.0019703
Nelsen, T. A., Blackwelder, P., Hood, T., McKee, B., Romer, N., Alvarez-Zarikian, C., et al. (1994). Time-based correlation of biogenic, lithogenic and authigenic sediment components with anthropogenic inputs in the Gulf of Mexico necop study area. Estuaries 17, 873–885. doi: 10.2307/1352755
NOAA (2010). Endangered and Threatened Wildlife; Notice of 90-Day Finding on a Petition to List 83 Species of Corals as Threatened or Endangered Under the Endangered Species Act (ESA). National Oceanic and Atmospheric Administration (NOAA), National Marine Fisheries Service (NMFS), Department of Commerce. Washington, DC: Federal Register. 75, 6616–6621.
Nyström, M., Norström, A. V., Blenckner, T., de la Torre-Castro, M., Eklöf, J. S., Folke, C., et al. (2012). Confronting feedbacks of degraded marine ecosystems. Ecosystems 15, 695–710. doi: 10.1007/s10021-012-9530-6
Okazaki, R., Swart, P. K., and Langdon, C. (2013). Stress-tolerant corals of florida bay are vulnerable to ocean acidification. Coral Reefs 32, 671–683. doi: 10.1007/s00338-013-1015-3
Ortiz-Lozano, L., Pérez-España, H., Granados-Barba, A., González-Gándara, C., Gutiérrez-Velázquez, A., and Martos, J. (2013). The reef corridor of the southwest Gulf of Mexico: Challenges for its management and conservation. Ocean Coast. Manage. 86, 22–32. doi: 10.1016/j.ocecoaman.2013.10.006
Osterman, L. E., Poore, R. Z., and Swarzenski, P. W. (2008). The last 1000 years of natural and anthropogenic low-oxygen bottom-water on the louisiana shelf, Gulf of Mexico. Mar. Micropaleontol. 606, 291–303. doi: 10.1016/j.marmicro.2007.10.005
Padilla-Gamiño, J. L., Roth, M. S., Rodrigues, L. J., Bradley, C. J., Bidigare, R. R., Gates, R. D., et al. (2019). Ecophysiology of mesophotic reef-building corals in Hawaii is influenced by symbiont–host associations, photoacclimatization, trophic plasticity, and adaptation. Limnol. Oceanogr. 64, 1980–1995. doi: 10.1002/lno.11164
Palumbi, S. R., Barshis, D. J., Traylor-Knowles, N., and Bay, R. A. (2014). Mechanisms of reef coral resistance to future climate change. Science 344, 895–898. doi: 10.1126/science.1251336
Pandolfi, J. M., and Kiessling, W. (2014). Gaining insights from past reefs to inform understanding of coral reef response to global climate change. Curr. Opin. Environ. Sustain. 7, 52–58. doi: 10.1016/j.cosust.2013.11.020
Pankhurst, N. W., and Munday, P. L. (2011). Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 62, 1015–1026. doi: 10.1071/MF10269
Pereira, P. H. C., Macedo, C. H., José de Anchieta, C., de Barros Marangoni, L. F., and Bianchini, A. (2018). Effects of depth on reef fish communities: insights of a ‘deep refuge hypothesis’ from Southwestern Atlantic reefs. PLoS ONE 13:e0203072. doi: 10.1371/journal.pone.0203072
Peters, G. P., Andrew, R. M., Boden, T., Canadell, J. G., Ciais, P., Le Quéré, C., et al. (2012). The challenge to keep global warming below 2°c. Nat. Clim. Change 3:4. doi: 10.1038/nclimate1783
Pinsky, M. L., Worm, B., Fogarty, M. J., Sarmiento, J. L., and Levin, S. A. (2013). Marine taxa track local climate velocities. Science 341, 1239–1242. doi: 10.1126/science.1239352
Prada, F., Caroselli, E., Mengoli, S., Brizi, L., Fantazzini, P., Capaccioni, B., et al. (2017). Ocean warming and acidification synergistically increase coral mortality. Sci. Rep. 7:40842. doi: 10.1038/srep40842
Purkis, S. J., Renegar, D. A., and Riegl, B. M. (2011). The most temperature-adapted corals have an achilles' heel. Mar. Pollut. Bull. 62, 246–250. doi: 10.1016/j.marpolbul.2010.11.005
Rabalais, N. N., Diaz, R. J., Levin, L. A., Turner, R. E., Gilbert, D., and Zhang, J. (2010). Dynamics and distribution of natural and human caused hypoxia. Biogeosciences 7, 585–619. doi: 10.5194/bg-7-585-2010
Reef Relief (2019). The Importance of Coral Reefs. Available online at: ReefRelief.org.
Ren, W., Tian, H., Tao, B., Yang, J., Pan, S., Cai, W., et al. (2015). Large increase in dissolved inorganic carbon flux from the mississippi river to Gulf of Mexico due to climatic and anthropogenic changes over the 21st century. J. Geophys. Res. 120, 724–736. doi: 10.1002/2014JG002761
Riegl, B., and Piller, W. E. (2003). Possible refugia for reefs in times of environmental stress. Int. J. Earth Sci. 92, 520–531. doi: 10.1007/s00531-003-0328-9
Ries, J., Cohen, A., and McCorkle, D. (2010). A nonlinear calcification response to co 2-induced ocean acidification by the coral oculina arbuscula. Coral Reefs 29, 661–674. doi: 10.1007/s00338-010-0632-3
Rocha, L. A., Pinheiro, H. T., Shepherd, B., Papastamatiou, Y. P., Luiz, O. J., Pyle, R. L., et al. (2018). Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science 361, 281–284. doi: 10.1126/science.aaq1614
Rodolfo-Metalpa, R., Houlbrèque, F., Tambutté, É., Boisson, F., Baggini, C., Patti, F. P., et al. (2011). Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat. Clim. Change 1, 308–312. doi: 10.1038/nclimate1200
Ryu, J.-H., and Hayhoe, K. (2015). Regional and large-scale influences on seasonal to interdecadal variability in Caribbean surface air temperature in CMIP5 simulations. Clim. Dyn. 45, 455–475. doi: 10.1007/s00382-014-2351-x
Scheibner, C., and Speijer, R. P. (2008). Decline of coral reefs during late paleocene to early eocene global warming. Electron. Earth 3, 19–26. doi: 10.5194/ee-3-19-2008
Schramek, T., Colin, P., Merrifield, M., and Terrill, E. (2018). Depth-dependent thermal stress around corals in the tropical pacific ocean. Geophys. Res. Lett. 45, 9739–9747. doi: 10.1029/2018GL078782
Scott, R. (1984). Evolution of early Cretaceous reefs in the Gulf of Mexico. Palaeontogr. Am. 54, 406–412.
Shamberger, K. E., Cohen, A. L., Golbuu, Y., McCorkle, D. C., Lentz, S. J., and Barkley, H. C. (2014). Diverse coral communities in naturally acidified waters of a Western Pacific reef. Geophys. Res. Lett. 41, 499–504. doi: 10.1002/2013GL058489
Sheppard, C. R. (2003). Predicted recurrences of mass coral mortality in the indian ocean. Nature 425, 294–297. doi: 10.1038/nature01987
Shinn, E. A. (1976). Coral reef recovery in Florida and the Persian Gulf. Environ. Geol. 1:241. doi: 10.1007/BF02407510
Skirving, W., Heron, S., Marsh, B., Liu, G., De La Cour, J., Geiger, E., et al. (2019). The relentless march of mass coral bleaching: a global perspective of changing heat stress. Coral Reefs. 38, 547–557. doi: 10.1007/s00338-019-01799-4
Slattery, M., Moore, S., Boye, L., Whitney, S., Woolsey, A., and Woolsey, M. (2018). The Pulley Ridge deep reef is not a stable refugia through time. Coral Reefs 37, 391–396. doi: 10.1007/s00338-018-1664-3
Smith, J. E., Hunter, C. L., and Smith, C. M. (2010). The effects of top-down versus bottom-up control on benthic coral reef community structure. Oecologia 163, 497–507. doi: 10.1007/s00442-009-1546-z
Smith, T. B., Gyory, J., Brandt, M. E., Miller, W. J., Jossart, J., and Nemeth, R. S. (2016). Caribbean mesophotic coral ecosystems are unlikely climate change refugia. Glob. Change Biol. 22, 2756–2765. doi: 10.1111/gcb.13175
Stanley, S. M. (1966). Paleoecology and diagenesis of key largo limestone, Florida. Am. Assoc. Petrol. Geol. Bull. 50, 1927–1947. doi: 10.1306/5D25B6A9-16C1-11D7-8645000102C1865D
Stone, G. W., Walker, N. D., Hsu, A., Babin, A., Liu, B., Keim, B. D., et al. (2005). Hurricane ivan's impact along the northern Gulf of Mexico. EOS Trans. Am. Geophys. Union 86, 497–501. doi: 10.1029/2005EO480001
Sully, S., Burkepile, D., Donovan, M., Hodgson, G., and van Woesik, R. (2019). A global analysis of coral bleaching over the past two decades. Nat. Commun. 10:1264. doi: 10.1038/s41467-019-09238-2
Ting, M., Kossin, J. P., Camargo, S. J., and Li, C. (2019). Past and future hurricane intensity change along the US east coast. Sci. Rep. 9:7795. doi: 10.1038/s41598-019-44252-w
Toth, L. T., Aronson, R. B., Cobb, K. M., Cheng, H., Edwards, R. L., Grothe, P. R., et al. (2015). Climatic and biotic thresholds of coral-reef shutdown. Nat. Clim. Change 5:369. doi: 10.1038/nclimate2541
Trenberth, K., Jones, P., Ambenje, P., Bojariu, R., Easterling, D., Klein Tank, A., et al. (2007). Chapter 3: Observations: surface and atmospheric climate change. Climate Change, 235–336.
Trenberth, K. E., Cheng, L., Jacobs, P., Zhang, Y., and Fasullo, J. (2018). Hurricane harvey links to ocean heat content and climate change adaptation. Earth's Future 6, 730–744. doi: 10.1029/2018EF000825
Tunnell, J. W., Withers, K., and Chavez, E. (2007). Coral Reefs of the Southern Gulf of Mexico. College Station, TX: Texas A&M University Press.
Van Oppen, M. J., Gates, R. D., Blackall, L. L., Cantin, N., Chakravarti, L. J., Chan, W. Y., et al. (2017). Shifting paradigms in restoration of the world's coral reefs. Glob. Change Biol. 23, 3437–3448. doi: 10.1111/gcb.13647
van Oppen, M. J., Oliver, J. K., Putnam, H. M., and Gates, R. D. (2015). Building coral reef resilience through assisted evolution. Proc. Natl. Acad. Sci. U.S.A. 112, 2307–2313. doi: 10.1073/pnas.1422301112
Vermeij, M. J. A., Van Moorselaar, I., Engelhard, S., Hörnlein, C., Vonk, S. M., and Visser, P. M. (2010). The effects of nutrient enrichment and herbivore abundance on the ability of turf algae to overgrow coral in the caribbean. PLoS ONE 5:14312. doi: 10.1371/journal.pone.0014312
Viehman, S. (2017). Assessment of Hurricane Impacts to Coral Reefs in Florida and Puerto Rico. National Centers for Coastal Ocean Science.
Waddell, J. E., and Clarke, A. M., (eds.). (2008). The State of Coral Reef Ecosystems of the United States and Pacific Freely Associated States: 2008. NOAA Technical Memorandum NOS NCCOS 73. Silver Spring, MD: NOAA/NCCOS Center for Coastal Monitoring and Assessment's Biogeography Team.
Wanninkhof, R., Barbero, L., Byrne, R., Cai, W.-J., Huang, W.-J., Zhang, J.-Z., et al. (2015). Ocean acidification along the gulf coast and east coast of the usa. Contin. Shelf Res. 98, 54–71. doi: 10.1016/j.csr.2015.02.008
Weiss, A. M., and Martindale, R. C. (2019). Paleobiological traits that determined scleractinian coral survival and proliferation during the late paleocene and early eocene hyperthermals. Paleoceanogr. Paleoclimatol. 34, 252–274. doi: 10.1029/2018PA003398
Wilkinson, C. R., and Souter, D. (2008). Status of Caribbean Coral Reefs After Bleaching and Hurricanes in 2005. National Oceanic and Atmospheric Administration.
Williams, I., and Poulnin, N. V. C. (2001). Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs 19, 358–366. doi: 10.1007/s003380000121
Wilson, S. K., Fisher, R., Pratchett, M. S., Graham, N. A., Dulvy, N. K., Turner, R. A., et al. (2010). Habitat degradation and fishing effects on the size structure of coral reef fish communities. Ecol. Appl. 20, 442–451. doi: 10.1890/08-2205.1
Yamano, H., Sugihara, K., and Nomura, K. (2011). Rapid poleward range expansion of tropical reef corals in response to rising sea surface temperatures. Geophys. Res. Lett. 38:L04601. doi: 10.1029/2010GL046474
Yeats, P. A., Brewers, J. M., and Walton, A. (1978). Sensitivity of coastal waters to anthropogenic trace metal emissions. Mar. Pollut. Bull. 9, 264–268. doi: 10.1016/0025-326X(78)90608-2
Zamagni, J., Mutti, M., and Košir, A. (2012). The evolution of mid paleocene-early eocene coral communities: how to survive during rapid global warming. Palaeogeogr. Palaeoclimatol. Palaeoecol. 317, 48–65. doi: 10.1016/j.palaeo.2011.12.010
Zavala-Hidalgo, J., Romero-Centeno, R., Mateos-Jasso, A., Morey, S. L., and Martinez-Lopez, B. (2014). The response of the Gulf of Mexico to wind and heat flux forcing: what has been learned in recent years? Atmósfera 27, 317–334. doi: 10.1016/S0187-6236(14)71119-1
Keywords: climate change, coral reefs, coral bleaching, hot-house paleoclimates, adaptation, ocean acidification
Citation: Dee SG, Torres MA, Martindale RC, Weiss A and DeLong KL (2019) The Future of Reef Ecosystems in the Gulf of Mexico: Insights From Coupled Climate Model Simulations and Ancient Hot-House Reefs. Front. Mar. Sci. 6:691. doi: 10.3389/fmars.2019.00691
Received: 24 July 2019; Accepted: 28 October 2019;
Published: 20 November 2019.
Edited by:
Carla Zilberberg, Federal University of Rio de Janeiro, BrazilReviewed by:
Kai Zhu, University of California, Santa Cruz, United StatesJuan Pablo Carricart-Ganivet, National Autonomous University of Mexico, Mexico
Guilherme Ortigara Longo, Federal University of Rio Grande Do Norte, Brazil
Copyright © 2019 Dee, Torres, Martindale, Weiss and DeLong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sylvia G. Dee, c3lsdmlhLmRlZUByaWNlLmVkdQ==
 Sylvia G. Dee
Sylvia G. Dee Mark A. Torres1
Mark A. Torres1 Rowan C. Martindale
Rowan C. Martindale Anna Weiss
Anna Weiss Kristine L. DeLong
Kristine L. DeLong