- 1Department of Marine Sciences, University of the Aegean, Mytilene, Greece
- 2Department of Environment, University of the Aegean, Mytilene, Greece
- 3Department of Biology, University of Miami, Miami, FL, United States
- 4Institute of Marine Biological Resources and Inland Waters, Hellenic Centre for Marine Research, Attica, Greece
Shallow rocky reef fish assemblages were studied in sites of low versus high fishing pressure (FP) across the Aegean Sea, in order to assess community structure at a large scale and investigate spatial variability in relation to FP, depth, and geographic location. A total of 15 pairs of high and low FP sites were selected (18 sites in North Aegean, 12 in South Aegean). The level of FP was defined based on a fishing pressure index specifically developed for coastal small-scale fisheries in the region. In each site, fish communities were investigated at two depth zones (5 and 15 m). Number of species, fish size (Total Length; TL) and abundance were recorded along strip transects through underwater visual surveys. Abundance and TL were used to estimate biomass, and fish species were assigned to distinct trophic and commercial status groups. An 8-fold range in fish density and a 14-fold range in fish biomass were detected, while community structure was affected by all variables considered (FP, depth, geographic location). The N Aegean sites scored higher in number of species and biomass of carnivorous fish, whereas the S Aegean had a higher biomass of several allochthonous and thermophilous species. Abundance and biomass estimates were higher in low FP sites, and primarily at the 15 m depth zone, where low FP sites had the double abundance and 2.8 times higher biomass. Biomass of carnivores was generally very low, except at deep sites of low FP. Given that sites of lower FP represent areas of lower conflicting interests for fisheries whilst providing enhanced biomass levels, they should be included in future marine conservation planning schemes, as they could contribute to the replenishment of fisheries and the boosting of conservation benefits provided by MPAs, once properly managed.
Introduction
Compared to the vast area covered by the open sea, shallow coastal environments are subjected to disproportionately higher pressures due to their dynamic interplay with the terrestrial realm and the direct effects of human activities. Mediterranean shallow rocky reefs are very productive and diverse ecosystems providing important provisioning, regulating and cultural ecosystem services (Salomidi et al., 2012). However, they are exposed to multiple stressors, including overfishing (Sala et al., 2012), pollution (Tornero and Hanke, 2016), invasive species (Katsanevakis et al., 2014), coastal development (Meinesz et al., 1991), destructive fishing practices (Guidetti, 2011), and climate change (Rilov, 2016). Many important Mediterranean rocky reef benthic communities, such as canopy-forming macroalgal forests of the genus Cystoseira and Sargassum are particularly vulnerable to such stressors and have suffered major losses and severe degradation in the recent decades (Thibaut et al., 2015). For this reason, they have been classified as endangered in the European Red List of Habitats (Gubbay et al., 2016). The associated rocky reef fish assemblages are also of high ecological importance, since they play a fundamental role in the functioning of reef ecosystems by regulating food web dynamics and nutrient releases, thus securing ecosystem stability and resilience, and the flow of respective services to humans (Holmlund and Hammer, 1999). For example, predatory fish represent a key component in the structuring of assemblages through trophic cascades (Pinnegar et al., 2000). In Mediterranean rocky reefs, high predatory fish abundance has been observed to control sea urchin populations at low levels, substantially reducing grazing pressure over macroalgal beds (Sala et al., 1998). On the contrary, low abundance of predatory fish can lead to overgrazing by sea urchins, which results to strong declines of algal biomass, and hence to the substantial reduction of the regulating and maintenance services provided by algal communities, with subsequent effects on the entire reef ecosystem (Guidetti, 2006; Ling et al., 2015). Moreover, reef fish have an intrinsic economic value for artisanal fisheries and underwater tourism, greatly supporting coastal economies (Badalamenti et al., 2000).
Despite their ecological and socio-economic importance, shallow rocky reef fish assemblages targeted exclusively by coastal small-scale and recreational fisheries have been much less studied compared to other fish communities targeted by medium- or large-scale fisheries, such as trawlers and purse seiners (Lleonart and Maynou, 2003). Stock assessments for Mediterranean reef fish are generally unavailable in recent published reviews (e.g., Colloca et al., 2013, 2017; Vasilakopoulos et al., 2014; Froese et al., 2018). Most quantitative estimates of fish abundance on rocky reefs have been based on underwater visual surveys, as fisheries-dependent surveys are generally problematic for such estimates due to catch efficiency and selectivity issues (Katsanevakis et al., 2012). However, underwater visual surveys have mainly focused on marine protected areas (MPAs), sometimes including neighboring unprotected areas as reference sites in order to assess MPA benefits on fish communities (e.g,. Rius, 2007; Seytre and Francour, 2009; Claudet et al., 2010; Dimitriadis et al., 2018). Large-scale surveys investigating the status of fish populations in unprotected rocky reefs are generally scarce (but see e.g., Sala et al., 2012; Guidetti et al., 2014), and thus in most Mediterranean countries the status of rocky fish populations and communities remains largely unknown.
The Aegean Sea (NE Mediterranean) has a complex geomorphology, a lengthy coastline of ∼16000 km, and a distinctive insular character with more than 1400 islands or islets. Rocky reefs of the Aegean Sea have traditionally been exploited by small-scale and recreational fisheries, which have played an important role in the shaping of the cultural and socio-economic character of this coastal region (Tzanatos et al., 2005; Giakoumi et al., 2012; Giovos et al., 2018; Keramidas et al., 2018). Yet, little information is available regarding the distribution and conservation status of rocky reefs, as well as of the associated fisheries resources. A recent (under)estimate indicated a rocky cover of at least 164 km2, of which only 3% is included in national parks (Sini et al., 2017). On the other hand, small-scale coastal fishing vessels, which constitute 96% of the total Greek fishing fleet (Petza et al., 2017), exhibit substantial spatial and temporal variability, largely depending on environmental factors and fishers’ behavior (Kavadas et al., 2015). Over and above, the insular character of the Aegean Sea, renders it difficult to monitor the multiple and multifaceted coastal fisheries activities. As a result, data regarding small-scale and recreational fisheries landings are unavailable or non-existent, while no large-scale survey has been conducted in the past to estimate fish population densities and their spatial variability (Tzanatos et al., 2006; Moutopoulos et al., 2013; Kavadas et al., 2015; Giovos et al., 2018).
In the absence of reference conditions, the study of fish community patterns in natural systems across gradients of human or environmental stressors provides some important information to set up current baselines and assess the status of populations and ecosystems (Sala et al., 2012). However, in the Aegean Sea there are no long-term, well-enforced no-take marine reserves (Sini et al., 2017; Fraschetti et al., 2018) that could potentially provide some idea of how unimpacted or unfished areas would look like under the present-day global change scenario. Hence, our study focused in the comparison of areas of low versus high fishing pressure (FP), in order to investigate how small-scale coastal fisheries shape fish community structure. Specifically, we aimed to (a) assess fish communities at a large-scale and investigate spatial variability in relation to FP, depth and geographic location, (b) provide insights regarding the recovery potential of fish populations under reduced fishing pressure, and (c) offer reference data for future management decisions.
Materials and Methods
Study Area and Selection of Sampling Sites
The study area encompasses all shallow Greek territorial waters of the Aegean Sea (NE Mediterranean) (Figure 1), and is divided into two geographic sectors, the North (N) and the South (S) Aegean basin. The two basins are characterized by distinct oceanographic conditions. The N Aegean basin is influenced by the cold, brackish Black Sea waters, and the nutrient-rich freshwater discharge of large rivers, whereas the S Aegean basin is affected by the northward flowing, high-salinity warm waters of the Levantine Sea, has a low riverine input, and is therefore more oligotrophic (Lykousis et al., 2002; Zervakis et al., 2004).
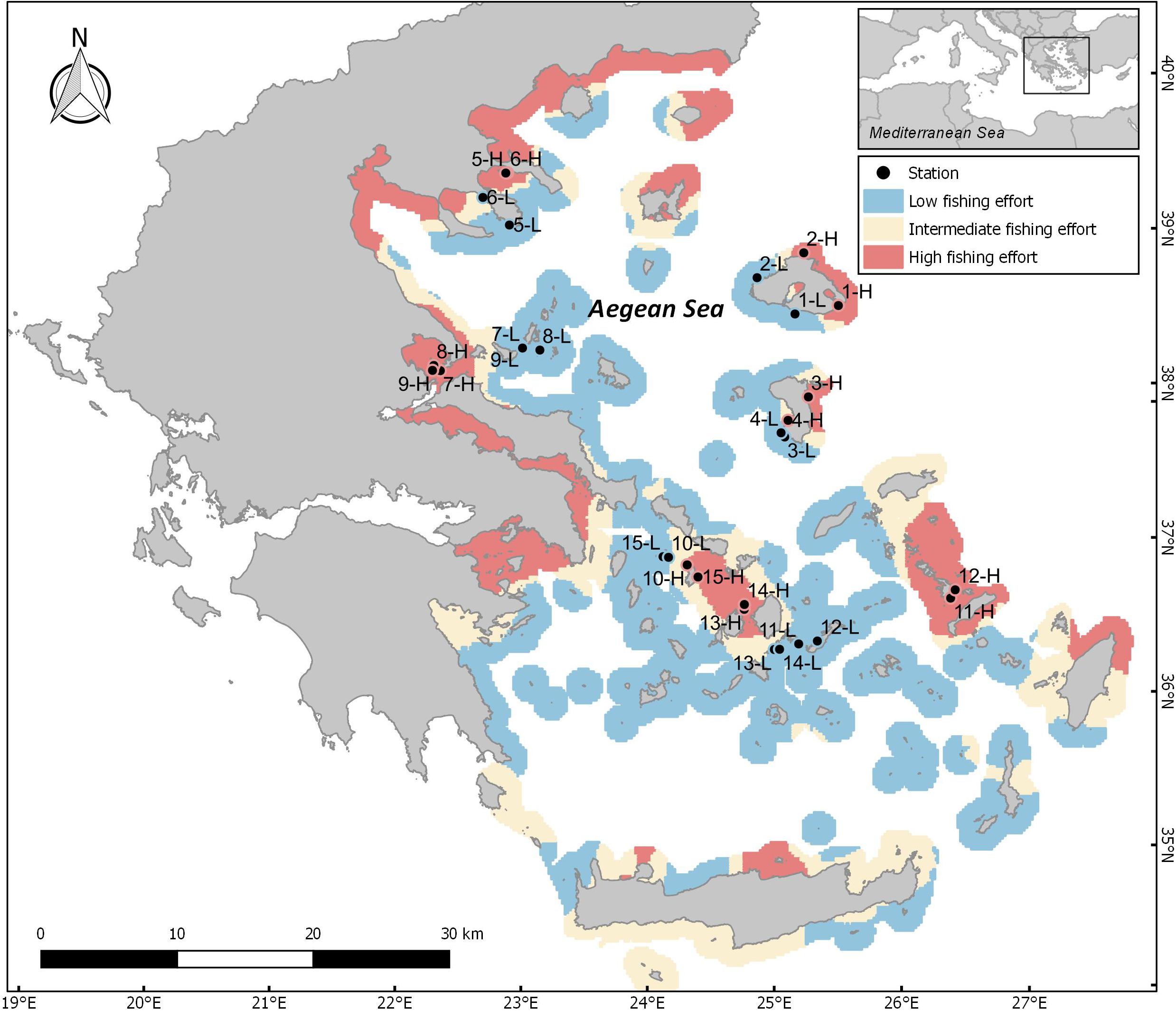
Figure 1. Map of the Aegean Sea depicting areas of different levels of fishing pressure (FP) based on the fishing pressure index developed for small scale fisheries. Blue: areas of low FP, red: areas of high FP, and yellow: areas of intermediate FP. Bullets and numbers indicate pairs of sampling sites; L: low FP sites, H: high FP sites. Station ID numbers 1–9 correspond to N Aegean sites, whereas 10–15 correspond to S Aegean sites.
Selection of sites involved a two-step process which included (a) the development of a fishing pressure (FP) index for the identification of areas subjected to different levels of FP, and (b) communication with local experts and stakeholders for validation of the index outputs and the final selection of appropriate sampling sites. The FP index was developed using a Multi-Criteria Decision Analysis (MCDA), and refers to vessels not equipped with an on-board VMS (Vessel Monitoring System) that mainly deploy gillnets, trammel nets and longlines. The MCDA methodology produces a spatial index by taking into account several interactions between anthropogenic and environmental factors (for more details see: Kavadas et al., 2015). This method enabled the evaluation of different areas with regards to their suitability for small-scale coastal fishing in relation to (a) several environmental factors (i.e., bathymetry, distance from the coast, chlorophyll-a concentration), (b) the coexistence of different anthropogenic activities, such as marine traffic1, other types of fishing (Kavadas et al., 2014; Maina et al., 2018), and the presence of fisheries restricted areas (Petza et al., 2017), as well as c) the expected level of small-scale fishing activity based on the spatial distribution of registered coastal fishing vessels in fishing ports (source: European Fleet Register)2 (Kavadas et al., 2013), along with the distance of each location from the closest port. Index values were then used to analyze spatial patterns, and to produce mapping clusters of hot and cold (high and low) FP spots (Figure 1; for a detailed description of the method applied see Supplementary Material). The resulting maps were then cross-checked with the opinions of local experts and stakeholders, in order to find suitable pairs of geographically proximate sites of similar geomorphological characteristics with contrasting FP levels. A total of 30 sites (15 pairs of high and low FP sites) were selected (Figure 1 and Supplementary Table S1), out of which 18 were situated in the N Aegean and 12 in the S Aegean. Although our aim was to cover as many parts of the Aegean Sea as possible in a representative way, the final choice of sampling sites was determined by the objectives of the study, logistic constrains, weather conditions, and the availability of extensive rocky substrates. The majority of sites were sampled along two distinct depth zones (5 and 15 m). However, in one site, sampling was restricted to the 5 m depth zone, due to the lack of extensive rocky substrate at 15 m. As a result, our analysis consists of 30 stations located at the 5 m depth zone, and 28 at the 15 m depth zone.
Field Protocol
Sampling was carried out during September – October 2016. In each site, underwater visual surveys were performed by SCUBA diving at the two distinct depth zones (5 and 15 m). Within each zone, fish surveys were conducted using plot sampling along three successive replicate strip transects (25 m long × 5 m wide, each), positioned several meters apart in a straight line, and covering a total area of 375 m2 per depth zone. Moving one-way along the transects at a constant speed, the fish observer counted and estimated the size (total length - TL in classes of 2 cm) of all fish species encountered within 2.5 m on either side of the transect line. All surveys were carried out on rocky reefs of similar rugosity and selection of the exact positioning of transects was random. To reduce observer-related bias, all fish surveys were carried out by a single experienced fish observer, who was specifically trained for visual surveys of fish communities over rocky bottoms using strip transects, in order to reduce fish length estimation errors (Bell et al., 1985). Also, fish recording and transect deployment were done simultaneously, to minimize disturbance of fish. All species were recorded except from those belonging to the families of Blenniidae, Gobiidae, and Tripterygiidae. Despite their important role in the ecology of shallow rocky reefs (Tiralongo et al., 2016), obtaining reliable diversity, density and biomass estimates for these highly diverse, small, crypto-benthic species would need a different type of sampling approach (e.g., Patzner, 1999; Tiralongo et al., 2016; Prato et al., 2017), which could not be followed due to fieldwork-related logistical constraints.
Data Analyses
Species diversity [i.e., number of species – S; Shannon-Wiener Index – H’; Simpson’s Index of Diversity – (1-D)], population density, and biomass density were the fish population/community state variables considered. Fish biomass was initially estimated for each species using the allometric length-weight relationship: W = aLb, where W: weight in g; L: total fish length in cm; a and b: species-specific parameters obtained from the literature (Moutopoulos and Stergiou, 2002; Giakoumi et al., 2012; Froese and Pauly, 2019). The resulting density and biomass values were converted to individuals and kg per 1000 m2 (i.e., ind. 1000 m–2 and kg 1000 m–2, respectively). Fish species were also aggregated into distinct groups according to their functional trophic status: carnivores, omnivores, grazers (Stergiou and Karpouzi, 2002; Karachle and Stergiou, 2017; Froese and Pauly, 2019), and commercial status depending on the level they are targeted by fisheries and their market value based on scientific literature (Stergiou et al., 2011) and expert judgment: commercial benthic/demersal (C), commercial small pelagic (C-sp), non-commercial or only sold at low price (LC), non-commercial (NC). Bootstrap (with 1000 resamples) was applied on all population/community state variables, in order to estimate the unconditional standard error (Efron and Tibshirani, 1993), as well as the 95% bootstrap-based unconditional confidence intervals per species, trophic group and commercial status group. To test the significance of differences in relation to FP, depth zone, and geographic location, the observed differences were bootstrapped and the 95% bootstrap confidence interval was estimated.
Two-way permutational multivariate analysis of variance (PERMANOVA) was applied using the Bray-Curtis similarity matrix on square root transformed data (Anderson, 2001), in order to assess the effects of geographic location, depth, FP, and their interactions, on fish community structure. “Geographic location” (two levels, North and South Aegean Sea), “Depth” (two levels, 5 and 15 m), and “Fishing pressure” (two levels, High and Low), were all used as fixed factors. For the graphical representation of the multivariate analyses, non-metric Multidimensional Scaling (nMDS) coupled with cluster analysis plots were produced, and similarity percentage analyses (SIMPER) were used to identify the percentage contribution of different species to the observed patterns (no cut-off limit applied). Multivariate analyses were performed with the PRIMER-E v6 (Clarke and Gorley, 2006) and PERMANOVA + software packages (Anderson et al., 2008). The TL and abundance values of the most common commercial fish species were used to construct size structure charts per FP level.
Results
A total of 43 species were recorded (including 2 allochthonous), belonging to 15 families. Overall, the families of Labridae (13 species), Sparidae (10), and Serranidae (5) presented the highest species richness (Table 1). Omnivores were the most diverse group (S: 28 species), followed by carnivores (11) and finally grazers (4). In terms of commercial value, highly-targeted fish included 18 species (C: 14; C-sp: 4), 16 species belonged to the LC group, and 9 species in the NC group. The total number of species per site ranged between 8 (in site 8-H) and 27 (in 8-L) with a mean () value of 18.5 (95% confidence interval - CI: [17, 20]) (Figure 2A). In terms of diversity, of the three biodiversity indices considered (S, H’, and 1-D), statistical differences were only detected in S, where the number of species was significantly higher in the N Aegean sites and at the 15 m depth zone compared to the S Aegean sites and the 5 m depth zone, respectively (Table 2).
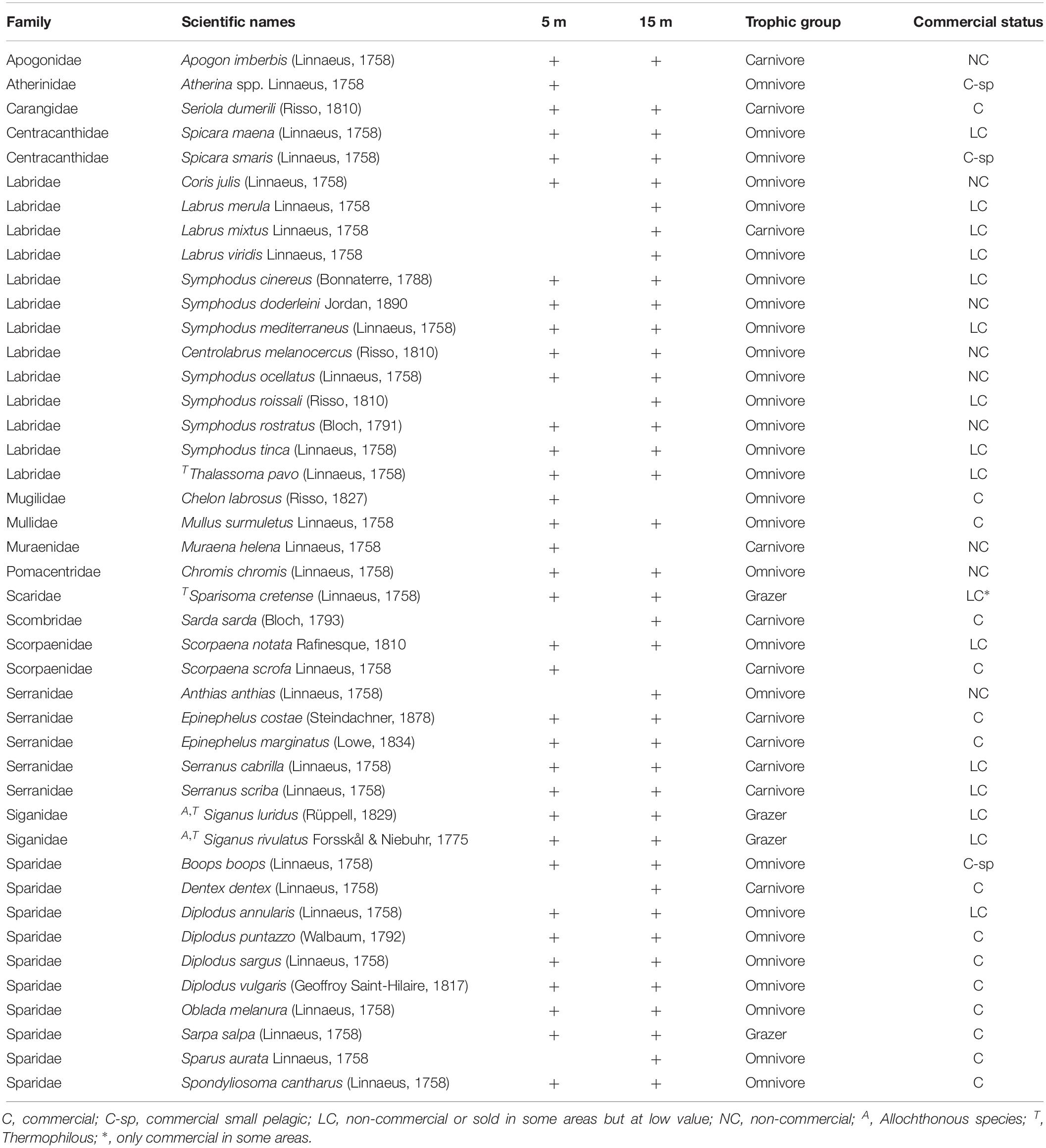
Table 1. Fish species recorded in the Aegean Sea at 5 and 15 m depth zones, their trophic group and commercial status.
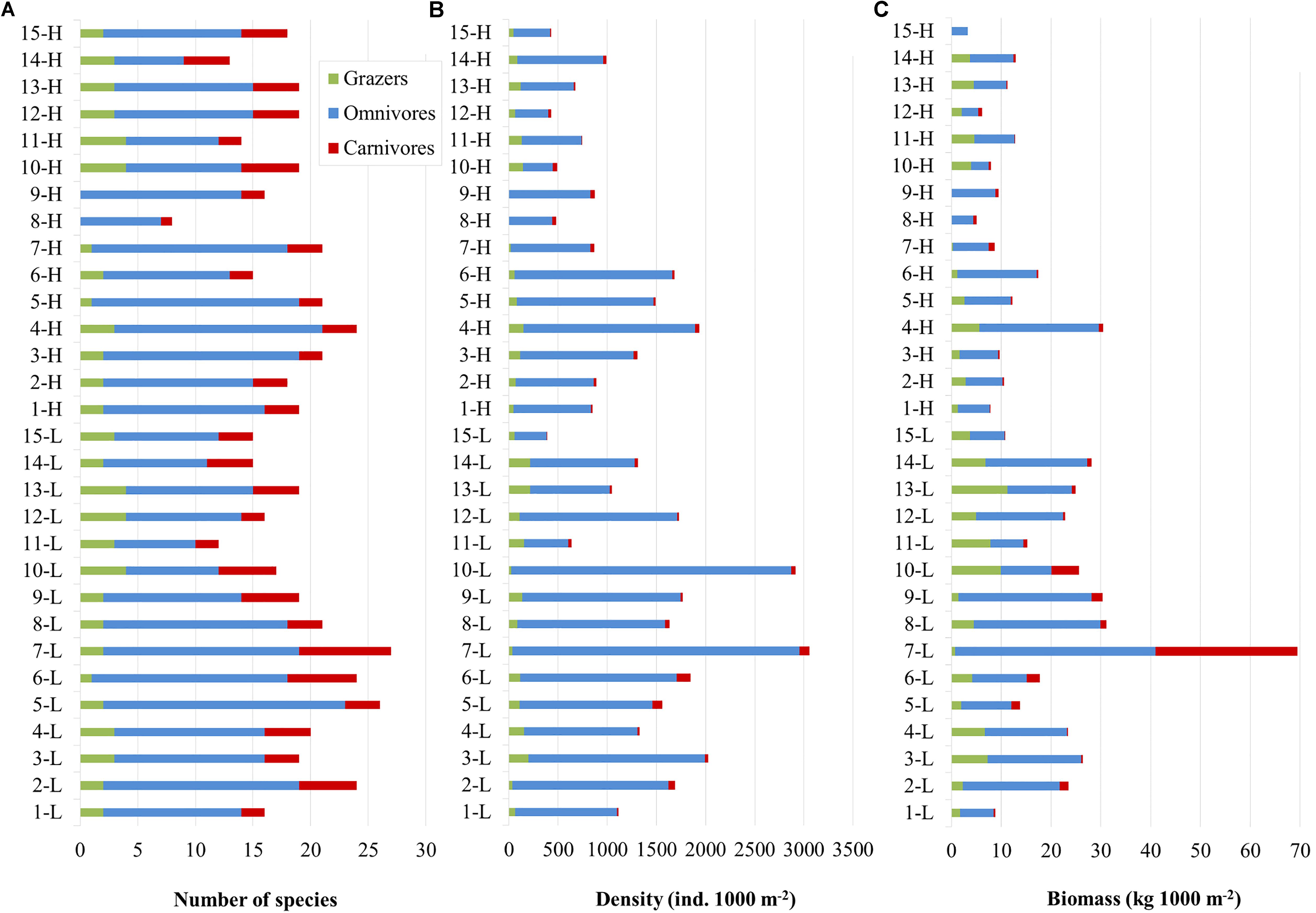
Figure 2. Bar charts displaying fish community parameters per site (5 and 15 m zones pooled); (A) Number of fish species. (B) Total fish abundance. (C) Total fish biomass. L: low fishing pressure site, H: high fishing pressure site, Green: grazers, Blue: omnivores, Red: carnivores. Station ID numbers 1–9 correspond to N Aegean sites, whereas 10–15 correspond to S Aegean sites.
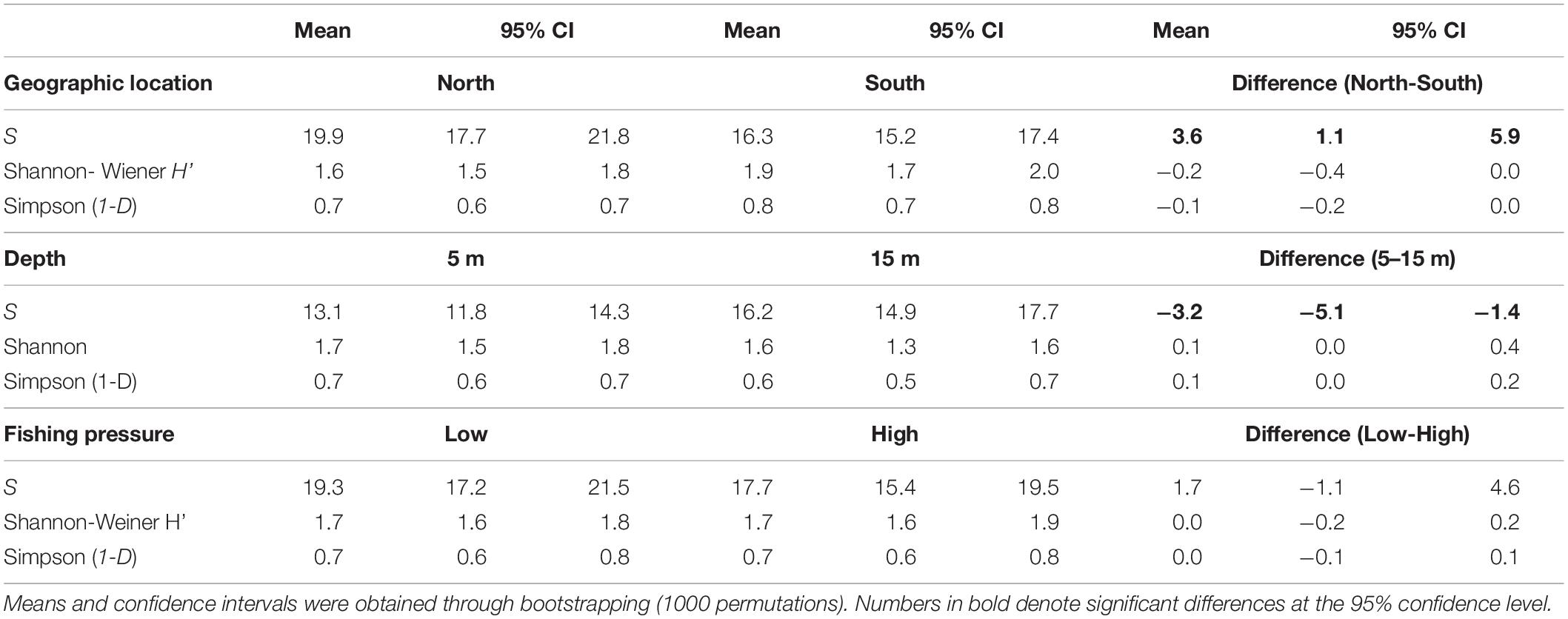
Table 2. Mean values and the 95% confidence intervals (CI) of the three diversity indices (number of species – S, Shannon - Wiener index – H’, and Simpson index – 1-D) that describe diversity of fish species in relation to geographic location, depth, and fishing pressure.
Overall, across our study area, fish density ranged between 386.7 and 3057.3 ind. 1000 m–2 (: 1272.8, CI: [1034.4, 1517.5]) (Figure 2B). All sites and depth zones pooled, the most abundant species were Chromis chromis (: 628.5; CI: [491.9, 817.7]), Boops boops (: 95.9; CI:]37.3, 173.2[), Thalassoma pavo (: 77.6, CI: [55.3, 100.6]), Spicara smaris (: 61.9, CI: [17.9, 113.5]), Coris julis (: 61.7, CI: [48.4, 75.1]), and Diplodus vulgaris (: 55.6, CI: [41.1, 71.0]). Omnivores had the highest density (: 1141.3, CI: [922.1, 1386.6]), followed by grazers (: 93.9; CI: [74.0, 115.5]), and carnivores (: 37.6, CI: [28.5, 49.1]). In terms of commercial status, the NC fish presented the highest density (: 709.6, CI: [544.3, 899.3]), followed by LC (: 205.2, CI: [163.9, 255.0]), C-sp (: 186.7, CI: [100.0, 295.6]), and finally C (: 171.3, CI: [140.7, 200.2]).
The N Aegean sites had a significantly higher fish density (: 1530.0, CI: [1223.1, 1835.3]) than the S Aegean (: 887.0, CI: [687.1, 1115.5]) with a difference: 643.0, CI: [261.8, 1040.7]. Also, density was overall higher in sites of low FP compared to those of high FP (Table 3 and Figures 3A,B), but significant differences were mainly detected at the 15 m depth zone ( difference: 1178.7, CI: [535.1, 1935.0]), where density in low FP sites was double that of high FP sites (Table 3). Similarly, density of the different trophic groups (Table 3 and Figure 3A) and commercial status groups (Table 3 and Figure 3B) was generally higher at the low FP sites, but significant differences were mainly observed at the 15 m depth zone. Results on the mean density of individual fish species per depth zone and FP level are presented in Supplementary Table S2.
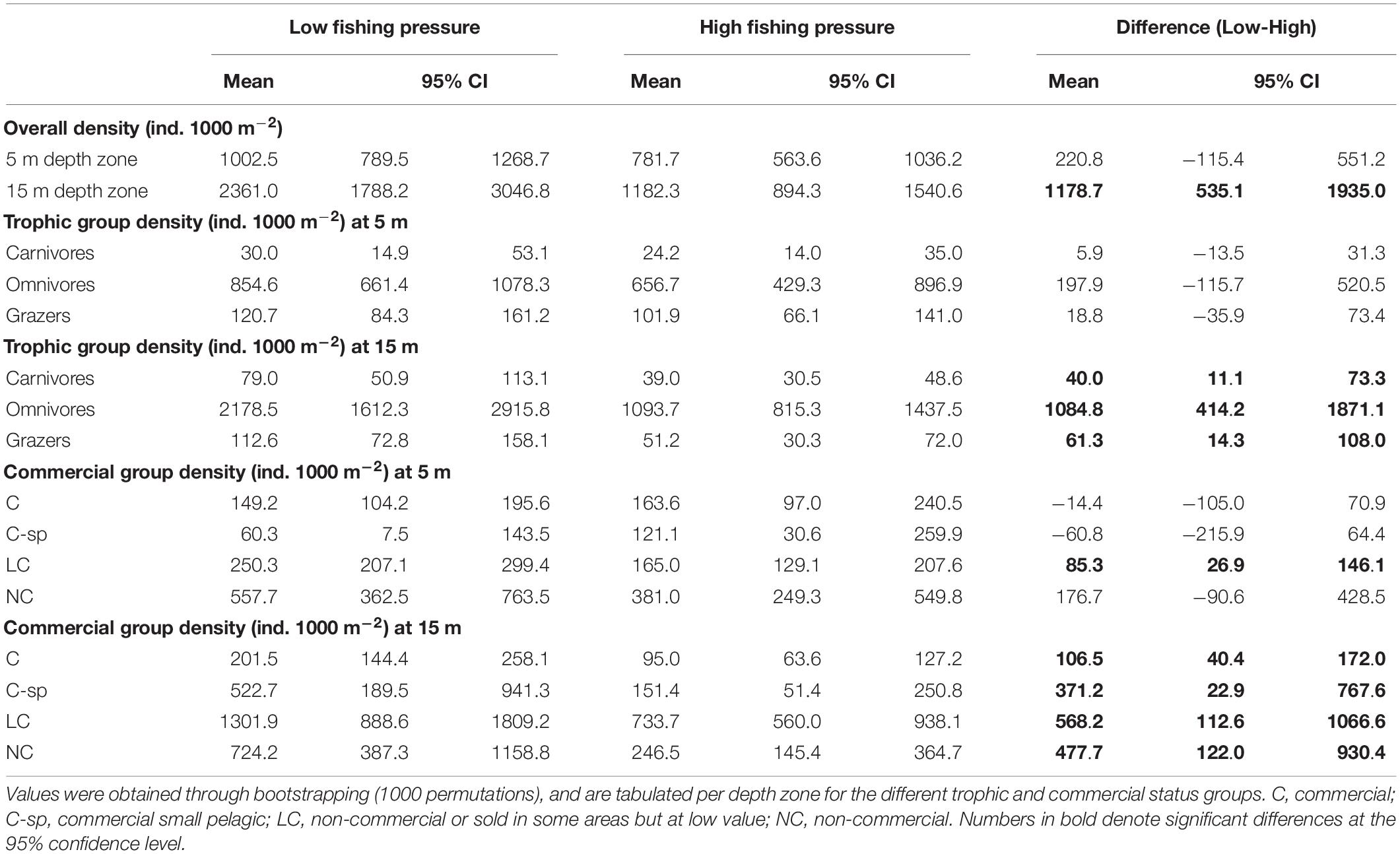
Table 3. Mean density values (ind. 1000 m–2), along with the 95% confidence intervals (CI), of fish species for low and high fishing pressure sites of the Aegean Sea and for the difference between them (Low-High).
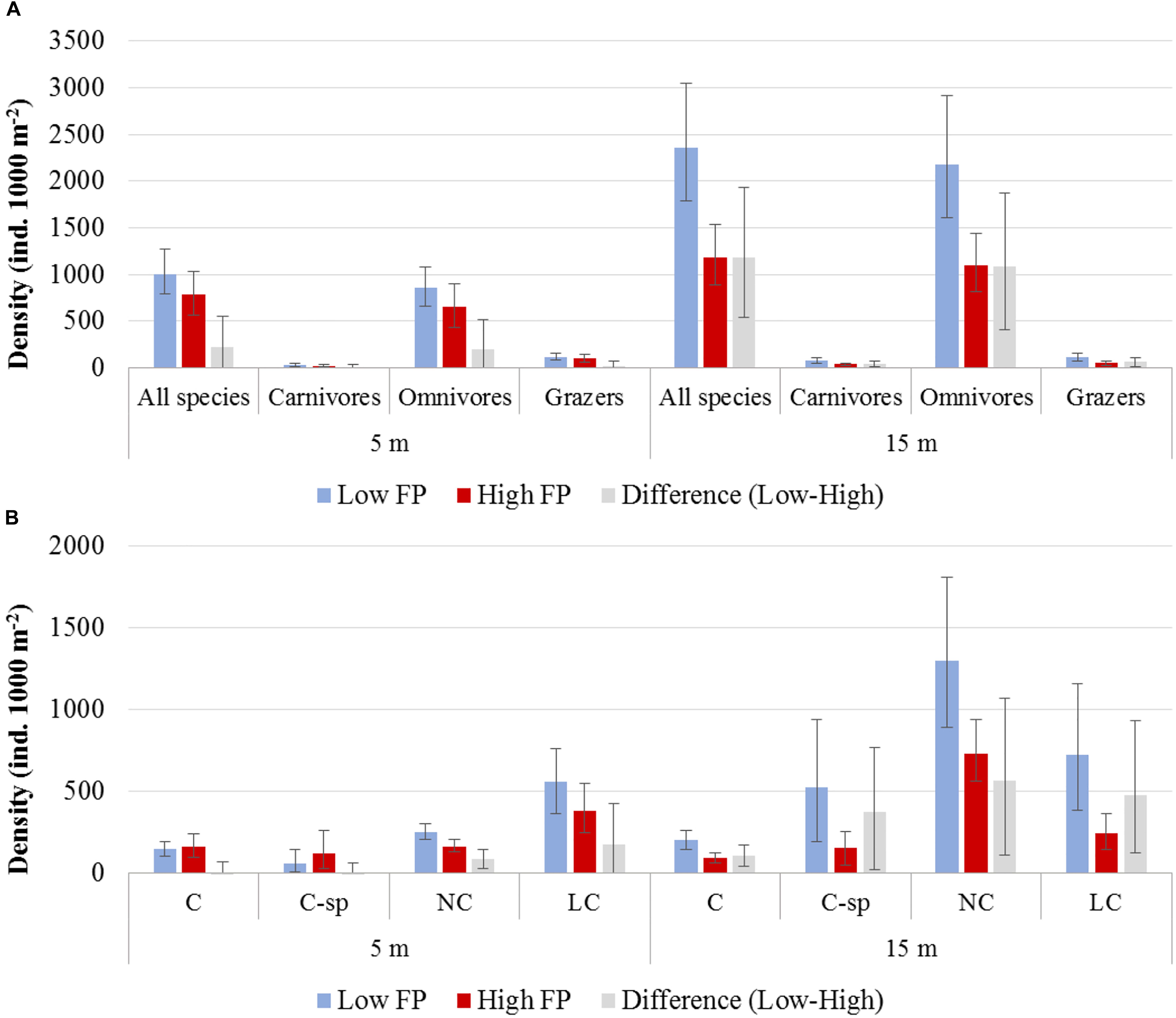
Figure 3. Mean density and the 95% confidence interval (A) of total fish and trophic groups, and (B) of commercial status groups, for low and high fishing pressure (FP) sites, as well as for their difference (Low – High), at the 5 and 15 m depth zones. C, commercial, C-sp, commercial small pelagic, LC, non-commercial or sold in some areas but at low value, NC, non-commercial.
Moreover, across the study area, fish biomass ranged between 5.0 and 69.4 kg 1000 m–2 (: 18.0, CI: [14, 22.9]) (Figure 2C). The species with the highest biomass (all sites and depth zones pooled) were C. chromis (: 3.5, CI: [2.6, 4.6]), B. boops (: 2.2, CI: [0.6, 4.4]), D. vulgaris (: 1.4, CI: [0.9, 1.8]), Sarpa salpa (: 1.2, CI: [0.8, 1.6]), Sparisoma cretense (: 1.2, CI: [0.7, 1.8]), T. pavo (: 1.2, CI: [0.9, 1.5]), and Siganus luridus (: 1.2, CI: [0.6, 1.7]). Omnivores was the trophic group with the highest biomass (: 12.5, CI: [9.6, 15.7]), followed by grazers (: 3.7, CI: [2.8, 4.7]), while carnivores had the lowest biomass (: 1.8, CI: [0.6, 4.3]). In terms of commercial status, the LC group (: 5.6, CI: [4.3, 7.0]) had the highest biomass, followed by C (: 5.0, CI: [3.3, 7.2]), NC (: 4.5, CI: [3.4, 5.7]), and C-sp (: 2.9; CI: [1.2, 5.0]).
Biomass was generally higher in the N Aegean sites (: 19.8, CI: [13.8, 27.7]) than in the S Aegean sites (: 15.3, CI: [12.1, 19.0]), but their difference was non-significant (: 4.4, CI: [−2.6, 13.3]). Biomass was also higher in low FP sites of both depth zones (Table 4 and Figures 4A,B). However, the 15 m depth zone had generally greater biomass values, and differences between low and high FP sites were more pronounced in this depth zone ( difference: 24.3, CI: [11.4, 41.6]), with low FP sites having 2.8 times higher total biomass compared to high FP sites (Table 4). Moreover, at the 5 m depth zone, no significant differences were detected in the biomass of the different trophic groups between high and low FP sites, whereas at the 15 m depth zone, all trophic groups presented significantly higher values in the low FP sites (Table 4 and Figure 4A). With regards to the commercial status groups, at the 5 m depth zone, low FP sites had a higher biomass of LC fish, whereas at the 15 m depth zone most groups (C, C-sp, and LC) had significantly higher biomass in the low FP sites (Table 4 and Figure 4B). Most of the reef-associated commercial fish species also displayed a greater range of size distribution in the low FP sites (Supplementary Figure S3). Tabulations of mean biomass of individual fish species per depth zone and FP level are presented in Supplementary Table S3.
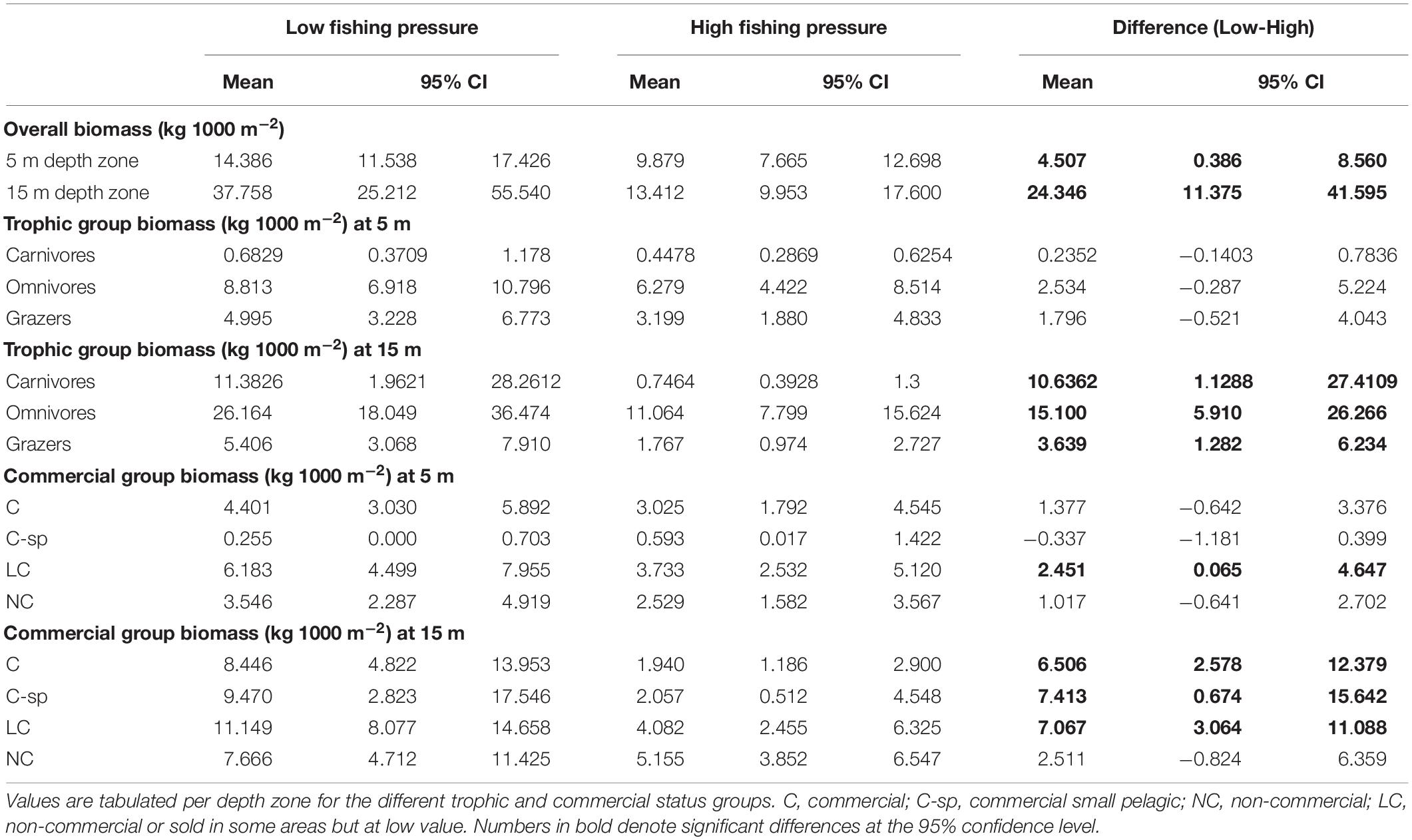
Table 4. Mean biomass values (kg 1000 m–2) and with the 95% confidence intervals (CI), of fish species for low and high fishing pressure sites of the Aegean Sea and for the difference between them (Low-High).
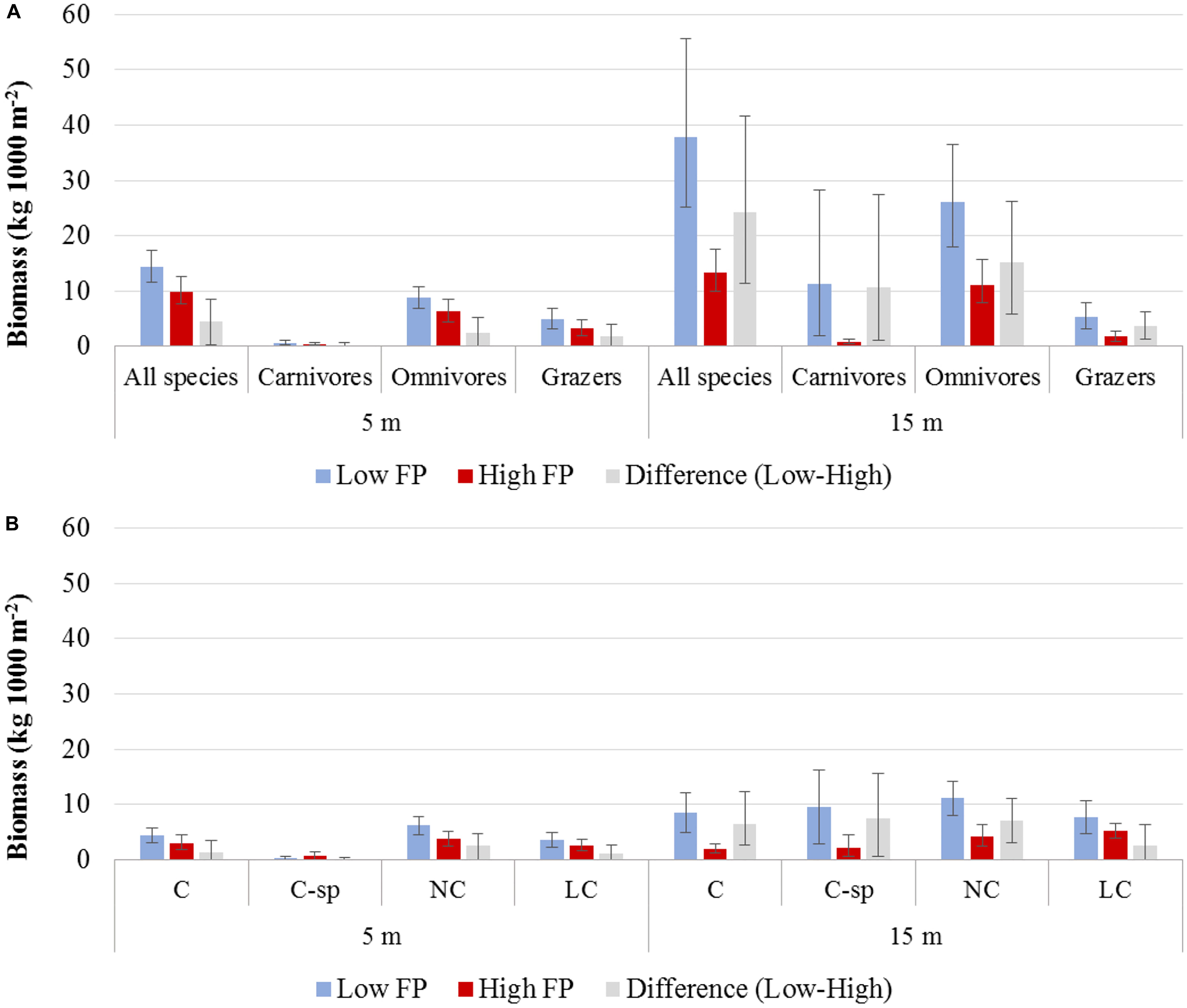
Figure 4. Mean biomass and the 95% confidence interval (A) of total fish and trophic groups, and (B) of commercial status groups, for low and high fishing pressure (FP) sites, as well as their difference (Low – High), at the 5 and 15 m depth zones. C, commercial; C-sp, commercial small pelagic; NC, non-commercial; LC, non-commercial or sold in some areas but at low value.
The PERMANOVA fish community analysis based on density and biomass values, indicated that all three factors, i.e., geographic location of the sites, FP level, as well as depth zone (but not their interactions) had a significant effect on fish community structure (Tables 5, 6). The greatest source of variability was at the level of replicate transects (i.e., residuals), which, despite being located only a few meters apart, displayed an average dissimilarity of 29% in density and 32.2% in biomass, according to the square root of the estimated variance components. Respectively, geographic location provided an additional 22.1 and 23.6% in community dissimilarity, followed by the effect of depth zone (15.1 and 14.7%), and finally the FP level (10.6 and 13.5%).
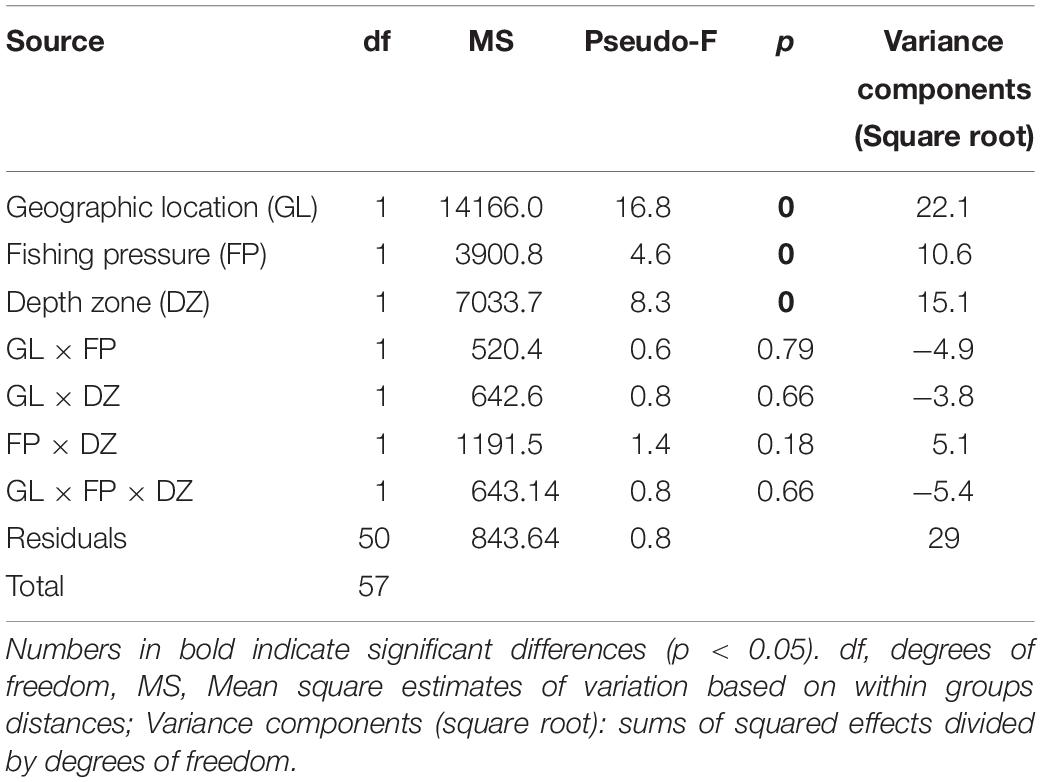
Table 5. PERMANOVA results of rocky reef fish community structure in relation to geographic location, fishing pressure, and depth zone, based on square root transformed density data.
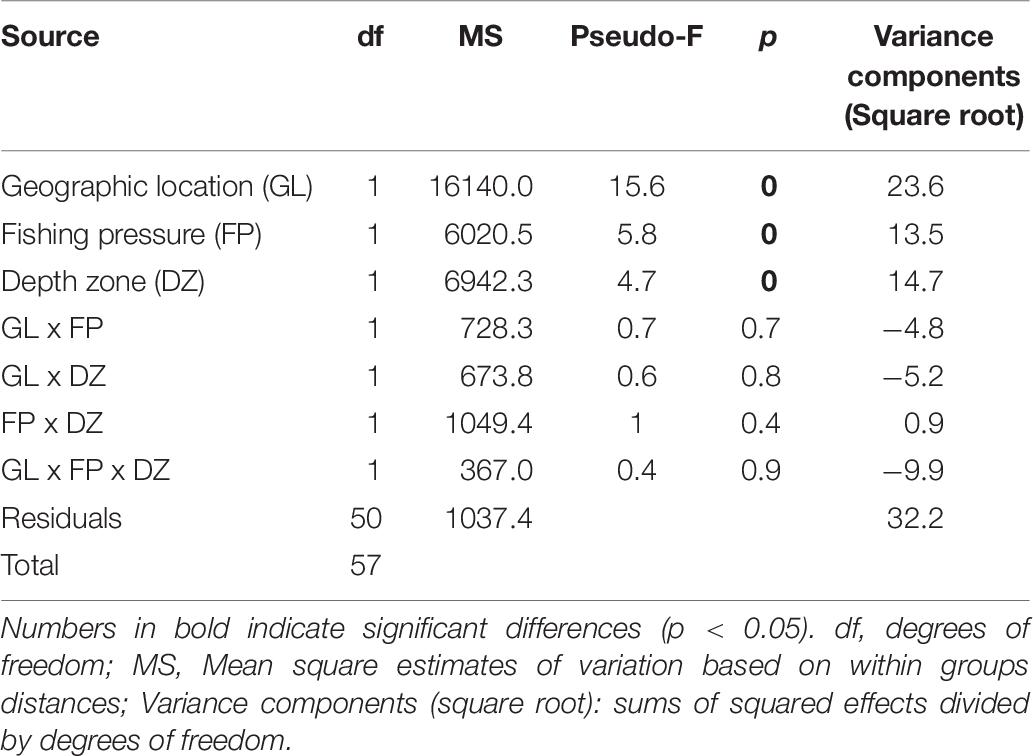
Table 6. PERMANOVA results of rocky reef fish community structure in relation to geographic location, fishing pressure, and depth zone, based on square root transformed biomass data.
The effects of geographic location, depth, and FP depth on fish communities are depicted in the nMDS plots (Figure 5) and the associated cluster analyses (Supplementary Figures S1, S2). Specifically, in Figures 5A,B, the formation of two distinct clusters between North and South at the 50% similarity level, indicates that geographic location had the strongest effect on fish community structure, compared to depth zone (Figures 5C,D) and FP level (Figures 5E,F), where the level of clustering is less distinctive. According to the SIMPER analyses on biomass data, the average dissimilarity between the N and S Aegean sites was 58%, while 16 species made up 80% of the observed differences (Supplementary Table S4). The N Aegean sites had a higher carnivore biomass and a greater number of total species (i.e,. 13 of the species were never recorded in the S Aegean sites). The S Aegean sites had a higher biomass of the allochthonous grazers S. luridus and Siganus rivulatus (the latter being absent from N Aegean areas), as well as of the thermophilous grazer S. cretense, and the omnivore T. pavo. Between low and high FP sites the average dissimilarity was 54.4%, and 17 species (4 grazers, 11 omnivores, and 2 carnivores) made up approximately 80% of the observed differences (Supplementary Table S5). Of these species (including 7 commercial ones), the majority had a higher biomass at the low FP sites. Finally, the average dissimilarity of stations located at different depth zones was 54.9%, and 17 species made up 80% of the observed differences; the majority of the fish species had a higher average biomass value in the 15 m depth zone (Supplementary Table S6).
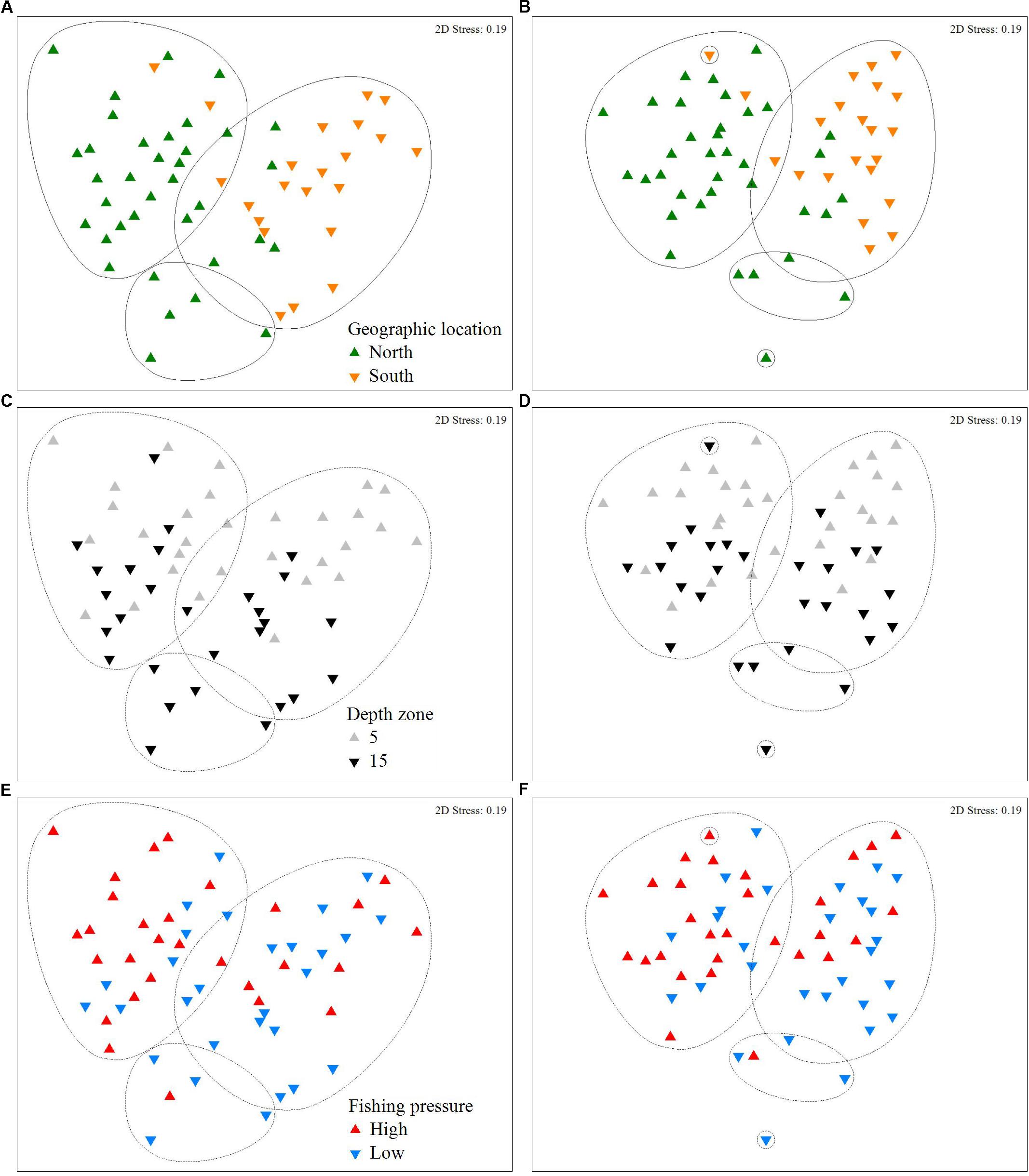
Figure 5. Non-metric Multidimensional Scaling (nMDS) plot of fish density (A,C,E) and biomass (B,D,F), in relation to sites located in different geographic locations (north or south Aegean Sea), depth zones (5 and 15 m), and which are subjected to different levels of fishing pressure (high or low). Points represent different depth stations (i.e., 28 stations, as in two stations only data from the 5 m depth zone were considered; for details see description in section “Materials and Methods”). Clusters represent 50% similarity; continuous line indicates the most discrete clustering.
Discussion
Across the 30 sites sampled at the shallow rocky reefs of the Aegean Sea, we found an 8-fold range in fish density and a 14-fold range in fish biomass. Although community structure and composition was affected by all the spatial variables considered (i.e., geographic location, depth zone, and FP), our results indicate that FP by small-scale coastal fisheries plays an important role in the shaping of fish communities, and that the effects of a reduction in FP can readily be evidenced even among areas that are not under some strict protection status. Species diversity was generally homogenous between low and high FP sites, but low FP sites had significantly higher density and biomass values compared to high FP sites, and this trend was consistent for all trophic and commercial status groups. Furthermore, biomass results are comparable to those reported from other unprotected areas of the Aegean Sea (Sala et al., 2012; Guidetti et al., 2014; Salomidi et al., 2016). Overall, community composition in the majority of sites reflects a largely exploited ecosystem, as the main contributors to the increased density and biomass values were fish of low or no commercial value. On the contrary, biomass of large carnivorous and commercially targeted fish species was considerably lower than what has been reported from Mediterranean MPAs (Sala et al., 2012; Guidetti et al., 2014). Nevertheless, it is interesting to note that some exceptional sites, mainly of low FP, displayed biomass values of ≥30 kg 1000 m–2, and fall within the range of sites with intermediate or high protection status in the Mediterranean basin (Sala et al., 2012; Guidetti et al., 2014; Dimitriadis et al., 2018). These results indicate that regardless the oceanographic differences between the eastern and western Mediterranean (e.g., trophic status, temperature levels, and topographic complexity), shallow rocky reef fish in the Aegean Sea have the potential to reach biomass levels equivalent to those observed in sites of intermediate or even high protection status of the western Mediterranean (which is generally conceived as more productive; Coll et al., 2010).
With regards to depth, although the Shannon-Wiener and Simpson’s diversity indices were not statistically different between the 5 and the 15 m depth zones, the number of species was significantly higher at 15 m. Moreover, community structure displayed significant differences. Fish density and biomass were generally greater at 15 m depth, while differences between low and high FP were more pronounced at this deeper zone. Also, fish biomass values were higher than those reported by Giakoumi et al. (2012) from the 3 m depth zone in the Cyclades Islands, suggesting the existence of some depth-related trend in fish abundance and biomass. In several cases, the protection provided by deeper waters (>10 m) has been shown to enhance diversity and abundance of fish species in coral reef ecosystems (e.g., Tyler et al., 2009; Pereira et al., 2018) and Mediterranean MPAs (Claudet et al., 2010). The main reasoning in support of the “deep reef refugia” hypothesis is that as depth increases, deeper parts of the reefs are less exposed to existing pressures (e.g., overfishing, pollution, temperature rise) than shallow reefs (Bongaerts et al., 2010). For example, certain recreational fishing activities such as spear-fishing, which also form a common practice in coastal Mediterranean rocky reefs, are subjected to diver physiology-related depth limitations; hence their effects are reduced with increasing depth. Besides, the bathymetric distribution of Mediterranean rocky reef fish is known to be affected by several factors, such as feeding habits and seasonal variations in water temperature (Bell, 1983; Vergés et al., 2012). Several commercially important fish, such as groupers and sea breams, undertake ontogenetic migrations during their life cycle, and larger individuals are more common in deeper waters (Sala et al., 2012). As our underwater visual survey can only provide a snapshot in time, it is possible that the present depth-related observations reflect a natural seasonal variation in the distribution of fish communities. Nevertheless, similar to Pereira et al. (2018), our results also suggest that fishing pressure, along with other natural or anthropogenic disturbances, may be attenuated in deeper waters, hence providing some protection that allows the persistence of higher biomass levels.
Geographic location was found to have the most pronounced effect on fish community structure. Overall, the N Aegean sites scored significantly higher values in fish species number and density, and presented a higher carnivore biomass. On the other hand, the S Aegean sites had a higher biomass of thermophilous and allochthonous species. A N Aegean to S Aegean gradient, in terms of species richness and abundance, is commonly reported in the literature, especially regarding benthic sessile species, such as sponges, gorgonians and corals (e.g., Voultsiadou, 2005; Sini et al., 2017), but also for commercial fish species (e.g., Katsanevakis et al., 2010a, b). This distinctive pattern is usually linked to the more productive waters of the N Aegean Sea, which are greatly affected by the inflowing waters of the Black Sea, and the freshwater input of large rivers (e.g., Coll et al., 2010; Würtz, 2010). As the distribution pattern of allochthonous and/or northward expanding thermophilous species has been rather well documented in the Aegean Sea, it is interesting to note that S. luridus was one of the fish species with the highest overall biomass. The species was recorded in all S Aegean sites, where it presented particularly high values compared to other common native species, whereas in the N Aegean it was only observed at the southern coasts of Chios Island (sites 3-L, 4-L, 4-H in the NE Aegean). Although occasional records of few S. luridus individuals have been reported from several sites of the NE Aegean since 2013 (Evagelopoulos et al., 2015; Ismen et al., 2015; Tsirintanis and Katsanevakis, 2017), the large numbers recorded in Chios Island may be a forerunner of the further expansion and successful establishment of the species in the N Aegean Sea. It is also worth mentioning that, of the four grazing fish species existing in the Aegean rocky reefs, the biomass of the native S. sarpa in the S Aegean sites was considerably lower than that of S. luridus and S. cretense, while it had the highest biomass in the N Aegean sites. This is in contrast to the report of Giakoumi (2014), when S. salpa represented 79% of the grazers’ abundance in the S Aegean. These observations suggest a potential northward displacement of the species due to competition pressure for space and food resources following the expansion of the siganids, and the subsequent lowering of algal biomass (Azzurro et al., 2007; Giakoumi, 2014).
Our work provides the most comprehensive assessment of shallow rocky fish community structure in the Aegean Sea to date, and offers important reference data to be used in future monitoring and management decisions. Fish communities present a rather degraded status, with particularly low density and biomass values of commercially important fish species belonging to higher trophic levels. Nevertheless, most species (including the commercially targeted ones) were at a better state in areas of reduced fishing pressure, as fish density, biomass and size structure were notably enhanced, sometimes reaching biomass values equivalent to those reported from strictly enforced MPAs. By changing the population structure and size composition of fish communities, fishing can alter marine food webs with cascading effects on the structure of habitats and the surrounding ecosystems (Turner et al., 1999; Pinnegar et al., 2000; Ling et al., 2015). Recovery requires the elimination or reduction of fishing activities to sustainable levels for sufficient time to allow different species or ecosystem components to recuperate to a more resilient state that will ensure the long term provision of ecosystem goods and services, and hence, the profitability of local fisheries (McClanahan et al., 2007; Libralato et al., 2010; Garciá-Rubies et al., 2013). Provided that sites of lower FP represent areas of lower conflicting interests for fisheries, whilst enhancing fish biomass, it is suggested that such areas should be taken into account in future marine conservation planning efforts aiming at the sustainable management of living marine resources, either as MPAs or as other important sites for conservation (Petza et al., 2019). As the establishment of well-designed and effectively enforced MPAs involves important trade-offs between socio-economic interests and ecological benefits, it is essential that other fisheries management schemes are also effectively implemented in areas outside MPAs, in order to enhance conservation benefits, and to provide supplementary protection to fish species with a greater level of mobility or larger home-ranges (Giakoumi et al., 2017; Petza et al., 2019). In the same respect, the potential of deeper parts of rocky reefs in the provision of additional protection, and the reinforcement of biomass levels, should be further highlighted in respective management schemes (Claudet et al., 2010; Lindfield et al., 2014; Pereira et al., 2018).
Along with the need to increase the coverage of well-established MPAs, an overall reduction of fishing effort at the low FP levels would contribute to a significant improvement of the status of the rocky reef communities in the Aegean Sea, as on average a double increase of density and an almost triple in biomass were observed in areas of reduced pressure by small-scale fisheries. The main bottleneck is that, as data on fishing effort by coastal small-scale and recreational fisheries is currently not available, it is not possible to estimate the exact difference in fishing effort between low and high FP sites, or even better, the relation between fish abundance or biomass and fishing effort, in order to provide policy makers with guidelines on the desirable level of FP reduction. Despite that, policies to reduce the fishing effort over rocky reefs are urgently needed to assist the recovery of these ecosystems and secure the sustainability of their ecosystem services.
Data Availability Statement
Data are available upon request to PK (cGthcmFjaGxlQGhjbXIuZ3I= – coordinator of the PROTOMEDEA project), and to the authors responsible for the generation of the different datasets: SlK (katsanevakis@marine.aegean.gr) and MS (mariasini@marine.aegean.gr) for data on fish community structure, SfK (stefanos@hcmr.gr) and IM (imaina@hcmr.gr) for the map on small-scale fishing pressure.
Ethics Statement
Ethical review and approval was not required for this study as it was restricted to the use of non destructive visual methods in order to minimize disturbance to the organisms and the marine environment. No animal material was extracted.
Author Contributions
SlK and MS designed and organized the fieldwork. IM and SfK developed the small-scale fisheries index. MS, KV, ZT, and CK contributed to underwater sampling. MS carried out the data analysis, interpreted the results, and prepared the first draft of the manuscript. SlK and IM wrote the sections of the manuscript. All authors have contributed to manuscript revision, and have read and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work is a contribution to the Research Project “PROTOMEDEA – Toward the Establishment of Marine Protected Area Networks in the Eastern Mediterranean” (https://www.protomedea.eu), funded by DG for Marine Affairs and Fisheries of the European Commission, under Grant Agreement SI2.721917. Any opinions, findings, and conclusion or recommendations expressed in this material are those of the authors and do not necessarily reflect those of the European Commission or the authors’ affiliated institutions.
Acknowledgments
The authors would like to thank all diving centers that provided technical support during fieldwork, namely Aquacore Divers, Athos Scuba Diving Center, Azure Diving Center, Lesvos Scuba Oceanic Center, Tortuga Diving Center Mesta Chios, and Mystic Blue Eco-sailing and Diving.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2019.00599/full#supplementary-material
Footnotes
References
Anderson, M. J. (2001). Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish Aquat. Sci. 58, 626–639. doi: 10.1139/f01-004
Anderson, M. J., Gorley, R. N., and Clarke, K. R. (2008). PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. Plymouth: PRIMER-E.
Azzurro, E., Fanelli, E., Mostarda, E., Catra, M., and Andaloro, F. (2007). Resource partitioning among early colonizing Siganus luridus and native herbivorous fish in the Mediterranean: an integrated study based on gut-content analysis and stable isotope signatures. J. Mar. Biol. Ass. U.K. 87, 991–998. doi: 10.1017/S0025315407056342
Badalamenti, F., Ramos, A. A., Voultsiadou, E., Lizaso, L. J. S., D’Anna, G., Pipitone, C., et al. (2000). Cultural and socio-economic impacts of Mediterranean marine protected areas. Environ. Conserv. 27, 110–125. doi: 10.1017/S0376892900000163
Bell, J. D. (1983). Effects of depth and marine reserve fishing restrictions on the structure of a rocky reef fish assemblage in the north-western Mediterranean Sea. J. Appl. Ecol. 20, 357–369. doi: 10.2307/2403513
Bell, J. D., Craik, G. J. S., Pollard, D. A., and Russell, B. C. (1985). Estimating length frequency distributions of large reef fish underwater. Coral Reef 4, 41–44. doi: 10.1007/BF00302203
Bongaerts, P., Ridgway, T., Sampayo, E. M., and Goegh-Guldberg, O. (2010). Assessing the ‘deep reef refugia’ hypothesis: focus on caribbean reefs. Coral Reefs 29, 309–327. doi: 10.1007/s00338-009-0581-x
Claudet, J., García Charton, J. A., and Lenfant, P. (2010). Combined effects of levels of protection and environmental variables at different spatial resolutions on fish assemblages in a marine protected area. Conserv. Biol. 25, 105–114. doi: 10.1111/j.1523-1739.2010.01586.x
Coll, M., Piroddi, C., Steenbeek, J., Kaschner, K., Ben Rais Lasram, F., Aguzzi, J., et al. (2010). The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS One 5:e11842. doi: 10.1371/journal.pone.0011842
Colloca, F., Cardinale, M., Maynou, F., Giannoulaki, M., Scarcella, G., Jenko, K., et al. (2013). Rebuilding Mediterranean fisheries: a new paradigm for ecological sustainability. Fish. Fish. 14, 89–109. doi: 10.1111/j.1467-2979.2011.00453.x
Colloca, F., Scarcella, G., and Libralato, S. (2017). Recent trends and impacts of fisheries exploitation on Mediterranean stocks and ecosystems. Front. Mar. Sci. 4:244. doi: 10.3389/fmars.2017.00244
Dimitriadis, C., Sini, M., Trygonis, V., Gerovasileiou, V., Sourbès, L., and Koutsoubas, D. (2018). Assessment of fish communities in a Mediterranean MPA: can a seasonal no-take zone provide effective protection? Estuar. Coast. Shelf. Sci. 207, 223–231. doi: 10.1016/j.ecss.2018.04.012
Efron, B., and Tibshirani, R. J. (1993). An Introduction to the Bootstrap. New-York, NY: Chapman and Hall.
Evagelopoulos, A., Poursanidis, D., Papazisi, E., Gerovasileiou, V., Katsiaras, N., and Koutsoubas, D. (2015). Records of alien marine species of Indo-Pacific origin at Sigri Bay (Lesvos Island, north-eastern Aegean Sea). Mar. Biodivers. Rec. 8, e35. doi: 10.1017/S1755267215000123
Fraschetti, S., Pipitone, C., Mazaris, A. D., Rilov, G., Badalamenti, F., Bevilacqua, S., et al. (2018). Light and shade in marine conservation across European and contiguous seas. Front. Mar. Sci. 5:420. doi: 10.3389/fmars.2018.00420
Froese, R., and Pauly, D. (eds) (2019). Fishbase. World Wide Web Electronic Publication. Available at: www.fishbase.org (accessed May, 2019).
Froese, R., Winker, H., Coro, G., Demirel, N., Tsikliras, A. C., Dimarchopoulou, D., et al. (2018). Status and rebuilding of european fisheries. Mar. Policy 93, 159–170. doi: 10.1016/j.marpol.2018.04.018
Garciá-Rubies, A., Hereu, B., and Zabala, M. (2013). Long-term recovery patterns and limited spillover of large predatory fish in a Mediterranean MPA. PLoS One 8:e73922. doi: 10.1371/journal.pone.0073922
Giakoumi, S. (2014). Distribution patterns of the invasive herbivore Siganus luridus (Rüppell, 1829) and its relation to native benthic communities in the central Aegean Sea, northeastern Mediterranean. Mari. Ecol. 35, 96–105. doi: 10.1111/maec.12059
Giakoumi, S., Cebrian, E., Kokkoris, G. D., Ballesteros, E., and Sala, E. (2012). Relationships between fish, sea urchins and macroalgae: the structure of shallow rocky sublittoral communities in the Cyclades, eastern Mediterranean. Estuar. Coast. Shelf. Sci. 109, 1–10. doi: 10.1016/j.ecss.2011.06.004
Giakoumi, S., Scianna, C., Plass-Johnson, J., Micheli, F., Grorud-Colvert, K., Thiriet, P., et al. (2017). Ecological effects of full and partial protection in the crowded Mediterranean Sea: a regional meta-analysis. Sci. Rep. U.K. 7:8940. doi: 10.1038/s41598-017-08850-w
Giovos, I., Keramidas, I., Antoniou, C., Deidun, A., Font, T., Kleitou, P., et al. (2018). Identifying recreational fisheries in the Mediterranean Sea through social media. Fish. Manag. Ecol. 25, 287–295. doi: 10.1111/fme.12293
Gubbay, S., Sanders, N., Haynes, T., Janssen, J. A. M., Rodwell, J. R., Nieto, A., et al. (2016). European Red List of Habitats. In: Part 1. Marine Habitats. Gland: IUCN.
Guidetti, P. (2006). Marine reserves reestablish lost predatory interactions and cause community changes in rocky reefs. Ecol. Appl. 16, 963–976. doi: 10.1890/1051-0761(2006)016
Guidetti, P. (2011). The destructive date mussel fishery and the persistence of barrens in Mediterranean rocky reefs. Mar. Pollut. Bull. 62, 691–695. doi: 10.1016/j.marpolbul.2011.01.029
Guidetti, P., Baiata, P., Ballesteros, E., Di Franco, A., Hereu, B., Macpherson, E., et al. (2014). Large-scale assessment of Mediterranean marine protected areas effects on fish assemblages. PLoS One 9:e91841. doi: 10.1371/journal.pone.0091841
Holmlund, C. M., and Hammer, M. (1999). Ecosystem services generated by fish populations. Ecol. Econ. 29, 253–268. doi: 10.1016/S0921-8009(99)00015-4
Ismen, A., Ayaz, A., and Yildirim, Z. D. (2015). Northernmost record of the dusky spinefoot Siganus luridus in the Aegean Sea (Turkey coast). Mar. Biodivers. Rec. 8, e42. doi: 10.1017/S175526721500017
Karachle, P. K., and Stergiou, K. I. (2017). An update on the feeding habits of fish in the Mediterranean. Medit. Mar. Sci. 18, 43–52. doi: 10.12681/mms.1968
Katsanevakis, S., Maravelias, C. D., and Kell, L. T. (2010a). Landings profiles and potential métiers in Greek set longliners. ICES J. Mar. Sci. 67, 646–656. doi: 10.1093/icesjms/fsp279
Katsanevakis, S., Maravelias, C. D., and Vassilopoulou, C. (2010b). Otter trawls in Greece: landing profiles and potential métiers. Medit. Mar. Sci. 11, 43–59. doi: 10.12681/mms.90
Katsanevakis, S., Wallentinus, I., Zenetos, A., Leppäkoski, E., Çinar, M. E., Oztürk, B., et al. (2014). Impacts of marine invasive alien species on ecosystem services and biodiversity: a pan-European review. Aquat. Invasions 9, 391–423. doi: 10.3389/fmars.2014.00032
Katsanevakis, S., Weber, A., Pipitone, C., Leopold, M., Cronin, M., Scheidat, M., et al. (2012). Monitoring marine populations and communities: review of methods and tools dealing with imperfect detectability. Aquat. Biol. 16, 31–52. doi: 10.3354/ab00426
Kavadas, S., Barberá, C., Belardinelli, A., Carpi, P., Cataudella, S., Croci, C., et al. (2014). Common Methodological Procedures for Analysis of VMS Data, Including Web-Based GIS Applications Related to the Spatial Extent and Intensity of Fishing Effort. Available at: http://www.perseus-net.eu/assets/media/PDF/deliverables/5138.4.pdf (accessed May, 2019).
Kavadas, S., Damalas, D., Georgakarakos, S., Maravelias, C., Tserpes, G., Papaconstantinou, C., et al. (2013). IMAS-Fish: integrated management system to support the sustainability of Greek Fisheries resources. A multidisciplinary web-based database management system: implementation, capabilities, utilization & future prospects for fisheries stakeholder. Medit. Mar. Sci. 14, 109–118. doi: 10.12681/mms.324
Kavadas, S., Maina, I., Damalas, D., Dokos, I., Pantazi, M., and Vassilopoulou, V. (2015). Multi-criteria decision analysis as a tool to extract fishing footprints: application to small scale fisheries and implications for management in the context of the maritime spatial planning directive. Medit. Mar. Sci. 16, 294–304. doi: 10.12681/mms.1087
Keramidas, I., Dimarchopoulou, D., Pardalou, A., and Tsikliras, A. C. (2018). Estimating recreational fishing fleet using satellite data in the aegean and Ionian Seas (Mediterranean Sea). Fish. Res. 208, 1–6. doi: 10.1016/j.fishres.2018.07.001
Libralato, S., Coll, M., Tempesta, M., Santojanni, A., Spoto, M., Palomera, I., et al. (2010). Food-web traits of protected and exploited areas of the Adriatic Sea. Biol. Conserv. 143, 2182–2194. doi: 10.1016/j.biocon.2010.06.002
Lindfield, S. J., McIlwain, J. L., and Harvey, E. S. (2014). Depth refuge and the impacts of SCUBA spearfishing on coral reef fishes. PLoS One 9:e92628. doi: 10.1371/journal.pone.0092628
Ling, S. D., Scheibling, R. E., Rassweiler, A., Johnson, C. R., Shears, N., Connell, S. D., et al. (2015). Global regime shift dynamics of catastrophic sea urchin overgrazing. Phil. Trans. R. Soc. B 370:20130269. doi: 10.1098/rstb.2013.0269
Lleonart, J., and Maynou, F. (2003). Fish stock assessments in the Mediterranean: state of the art. Sci. Mar 67, 37–49. doi: 10.3989/scimar.2003.67s137
Lykousis, V., Chronis, G., Tselepidis, A., Price, N. B., Theocharis, A., Sikou-Frangou, I., et al. (2002). Major outputs of the recent multidisciplinary biogeochemical researches undertaken in the Aegean Sea. J. Mar. Syst. 3, 313–334. doi: 10.1016/S0924-7963(02)00064-7
Maina, I., Kavadas, S., Damalas, D., Pantazi, M., and Katsanevakis, S. (2018). Dynamics of trawling effort in the Aegean Sea: investigating the potential of Vessel Monitoring System (VMS) data. ICES J. Mar. Sci. 75, 2265–2275. doi: 10.1093/icesjms/fsy083
McClanahan, T. R., Graham, N. A. J., Calnan, J. M., and MacNeil, M. A. (2007). Toward pristine biomass: reef fish recovery in coral reef marine protected areas in Kenya. Ecol. Appl. 17, 1055–1067. doi: 10.1890/06-1450
Meinesz, A., Lefevre, J. R., and Astier, J. M. (1991). Impact of coastal development on the infralittoral zone along the southeastern Mediterranean shore of continental France. Mar. Pollut. Bull. 23, 343–347. doi: 10.1016/0025-326X(91)90698-R
Moutopoulos, D. K., Katselis, G., Kios, K., Tsotskou, A., Tsikliras, A. C., and Stergiou, K. I. (2013). Estimation and reconstruction of shore-based recreational angling fisheries catches in the Greek Seas (1950-2010). J. Biol. Res. Thess. 20, 376–381.
Moutopoulos, D. K., and Stergiou, K. I. (2002). Length-weight and length-length relationships of fish species from the Aegean Sea (Greece). J. Appl. Icthyol. 18, 200–203. doi: 10.1046/j.1439-0426.2002.00281.x
Patzner, R. A. (1999). Habitat utilization and depth distribution of small cryptobenthic fishes (Bleniidae, Gobiesocidae, Gobiidae, Tripterygiidae) in Ibiza (western Mediterranean Sea). Environ. Biol. Fish. 55, 207–214. doi: 10.1023/A:1007535808710
Pereira, P. H. C., Macedo, C. H., Nunes, J. D. A. C. C., Marangoni, L. F. D. B., and Bianchini, A. (2018). Effects of depth on reef fish communities: insights of a “deep refuge hypothesis” from Southwestern Atlantic reefs. PLoS One 13:e0203072. doi: 10.1371/journal.pone.0203072
Petza, D., Chalkias, C., Koukourouvli, N., Coll, M., Vassilopoulou, V., Karachle, K. K., et al. (2019). An operational framework to assess the value of fisheries restricted areas for marine conservation. Mar. Policy 102, 28–39. doi: 10.1016/j.marpol.2019.01.005
Petza, D., Maina, I., Koukourouvli, N., Dimarchopoulou, D., Akrivos, D., Kavadas, S., et al. (2017). Where not to fish – reviewing and mapping fisheries restricted areas in the Aegean Sea. Medit. Mar. Sci. 18, 310–323. doi: 10.12681/mms.2081
Pinnegar, J. K., Polunin, N. V. C., Francour, P., Badalamenti, F., Chemello, R., Harmelin-Vivien, M. L., et al. (2000). Trophic cascades in benthic marine ecosystems: lessons for fisheries and protected-area management. Environ. Conserv. 27, 179–200. doi: 10.1017/S0376892900000205
Prato, G., Thiriet, P., Di Franco, A., and Francour, P. (2017). Enhancing fish underwater visual census to move forward assessment of fish assemblages: an application in three Mediterranean Marine protected areas. PLoS One 12:e0178511. doi: 10.1371/journal.pone.0178511
Rilov, G. (2016). Multi-species collapses at the warm edge of a warming sea. Sci. Rep. U.K. 6:36897. doi: 10.1038/srep36897
Rius, M. (2007). The effect of protection on fish populations in the Ses Negres Marine Reserve (NW Mediterranean. Spain). Sci. Mar. 71, 499–504. doi: 10.3989/scimar.2007.71n3499
Sala, E., Ballesteros, E., Dendrinos, P., Di Franco, A., Ferretti, F., Foley, D., et al. (2012). The structure of Mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PLoS One 7:e32742. doi: 10.1371/journal.pone.0032742
Sala, E., Boudouresque, C. F., and Harmelin-Vivien, M. (1998). Fishing, trophic cascades, and the structure of algal assemblages: evaluation of an old but untested paradigm. Oikos 82, 425–439. doi: 10.2307/3546364
Salomidi, M., Giakoumi, S., Gerakaris, V., Issaris, Y., Sini, M., and Tsiamis, K. (2016). Setting an ecological baseline prior to the bottom-up establishment of a marine protected area in Santorini Island, Aegean Sea. Medit. Mar. Sci. 17, 720–737. doi: 10.12681/mms.1802
Salomidi, M., Katsanevakis, S., Borja, Á, Braeckman, U., Damalas, D., Galparsoro, I., et al. (2012). Assessment of goods and services, vulnerability, and conservation status of European seabed biotopes: a stepping stone towards ecosystem-based marine spatial management. Medit. Mar. Sci. 13, 49–88. doi: 10.12681/mms.23
Seytre, C., and Francour, P. (2009). The cap roux MPA (Saint-Raphaël, French Mediterranean): changes in fish assemblages within four years of protection. ICES J. Mar. Sci. 66, 180–187. doi: 10.1093/icesjms/fsn196
Sini, M., Katsanevakis, S., Koukourouvli, N., Gerovasileiou, V., Dailianis, T., Buhl-Mortensen, L., et al. (2017). Assembling ecological pieces to reconstruct the conservation puz zle of the Aegean Sea. Front. Mar. Sci. 4:347. doi: 10.3389/fmars.2017.00347
Stergiou, K. I., Karachle, P. K., Tsikliras, A. C., and Mamalakis, E. (2011). Shouting fishes: Fishes from the Greek Seas – Biology, Fisheries and Management (in Greek). Athens: Patakis Publishers.
Stergiou, K. I., and Karpouzi, V. S. (2002). Feeding habits and trophic levels of Mediterranean fish. Rev. Fish. Biol. Fisher. 11, 217–254. doi: 10.1023/A:1020556722822
Thibaut, T., Blafuné, A., Boudouresque, C. F., and Verlaque, M. (2015). Decline and local extinction of fucales in the french riviera: the harbinger of future extinctions? Medit. Mar. Sci. 16, 206–224. doi: 10.12681/mms.1032
Tiralongo, F., Tibullo, D., Brundo, M. V., Paladini De Mendoza, F., Melchiorri, C., and Marcelli, M. (2016). Habitat preference of combtooth blennies (Actinopterygii: Perciformes: Blenniidae) in very shallow waters of the Ionian Sea, South-Eastern Sicily, Italy. Acta Ichthyol. Piscat. 46, 65–75. doi: 10.3750/AIP2016.46.2.02
Tornero, V., and Hanke, G. (2016). Chemical contaminants entering the marine environment from sea-based sources: a review with a focus on European seas. Mar. Pollut. Bull. 112, 17–38. doi: 10.1016/j.marpolbul.2016.06.091
Tsirintanis, K., and Katsanevakis, S. (2017). “Additional records of Siganus luridus from Lesvos, the northern limit of its range in the Aegean Sea,” in New Mediterranean Biodiversity Records eds L. Lipej, I. Acevedo, E. H. K. Akel, A. Anastasopoulou, A. Angelidis, E. Azzurro, et al. (Attiki: HCMR), 179–201.
Turner, S. J., Thrush, S. F., Hewitt, J. E., Cummings, V. J., and Funnell, G. (1999). Fishing impacts and the degradation or loss of habitat structure. Fish. Manag. Ecol. 6, 401–420. doi: 10.1046/j.1365-2400.1999.00167.x
Tyler, E. H. M., Speight, M. R., Henderson, P., and Monica, A. (2009). Evidence for a depth refuge effect in artisanal coral reef fisheries. Biol. Conserv. 142, 652–667. doi: 10.1016/j.biocon.2008.11.017
Tzanatos, E., Dimitriou, E., Katselis, G., Georgiadis, M., and Koutsikopoulos, C. (2005). Composition, temporal dynamics and regional characteristics of small-scale fisheries in Greece. Fish. Res. 73, 147–158. doi: 10.1016/j.fishres.2004.12.006
Tzanatos, E., Somarakis, S., Tserpes, G., and Koutsikopoulos, C. (2006). Identifying and classifying small-scale fisheeries métiers in the Mediterranean: a case study in the Patraikos Gulf. Greece. Fish. Res. 81, 158–168. doi: 10.1016/j.fishres.2006.07.00
Vasilakopoulos, P., Maravelias, C. D., and Tserpes, G. (2014). The alarming decline of Mediterranean fish stocks. Curr. Biol. 24, 1643–1648. doi: 10.1016/j.cub.2014.05.070
Vergés, A., Tomas, F., and Ballesteros, E. (2012). Interactive effects of depth and marine protection on predation and herbivory patterns. Mar. Ecol. Prog. Ser. 450, 55–65. doi: 10.3354/meps09599
Voultsiadou, E. (2005). Demosponge distribution in the eastern Mediterranean: a NW–SE gradient. Helgol. Mar. Res. 59, 237–251. doi: 10.1007/s10152-005-0224-8
Würtz, M. (2010). Mediterranean Pelagic Habitat: Oceanographic and Biological Processes, An overview. Gland, Switzerland and Malaga. Spain: IUCN.
Keywords: fish communities, small-scale fisheries, underwater visual surveys, marine conservation, Mediterranean Sea
Citation: Sini M, Vatikiotis K, Thanopoulou Z, Katsoupis C, Maina I, Kavadas S, Karachle PK and Katsanevakis S (2019) Small-Scale Coastal Fishing Shapes the Structure of Shallow Rocky Reef Fish in the Aegean Sea. Front. Mar. Sci. 6:599. doi: 10.3389/fmars.2019.00599
Received: 28 May 2019; Accepted: 05 September 2019;
Published: 15 October 2019.
Edited by:
Alberto Basset, University of Salento, ItalyReviewed by:
Francesco Tiralongo, Ente Fauna Marina Mediterranea (EFMM), ItalyErika M. D. Porporato, Ca’ Foscari University of Venice, Italy
Copyright © 2019 Sini, Vatikiotis, Thanopoulou, Katsoupis, Maina, Kavadas, Karachle and Katsanevakis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Sini, bWFyaWFzaW5pQG1hcmluZS5hZWdlYW4uZ3I=
 Maria Sini
Maria Sini Konstantinos Vatikiotis1
Konstantinos Vatikiotis1 Irida Maina
Irida Maina Stefanos Kavadas
Stefanos Kavadas Paraskevi K. Karachle
Paraskevi K. Karachle Stelios Katsanevakis
Stelios Katsanevakis