- 1Institute of Oceanography, National Taiwan University, Taipei, Taiwan
- 2Marine Fisheries Division, Fisheries Research Institute, Council of Agriculture, Keelung, Taiwan
- 3Department of Environmental Management, Faculty of Agriculture, Kindai University, Nara, Japan
With 11 species, the genus Chelidoperca is a small group of teleost fishes belonging to the Serranidae. They are bottom-dwelling fishes living on continental shelves/slopes in offshore areas or on remote seamounts/banks at depths ranging from around 40–400 m mostly in the tropical Indo-West Pacific. Over the past few years, efforts have been made to resolve the taxonomy of Chelidoperca, and subsequently four new species were described. However, these recent advances were made with a traditional approach (i.e., morphology) and limited examinable materials, usually preserved specimens, from ichthyological collections. Further investigations are still needed to address the gaps in our knowledge about their diversity, phylogeny, and biogeography. In this study, we collected 65 new samples, mainly during eight biodiversity expeditions carried out between 2007 and 2016 in the West Pacific under the Tropical Deep-Sea Benthos program. Specimens were photographed after collection to record fresh color patterns, which are essential for species diagnosis. Our analytical approach includes state-of-the-art DNA-based methods for species delimitation. The combined evidence from both molecular and morphological examinations, as well as other information such as geography, is used to test species validity. This reveals 15 species, including six new ones. We formally describe herein C. leucostigmata sp. nov., C. microdon sp. nov., and C. barazeri sp. nov. on the basis of specimens collected on Macclesfield Bank in the South China Sea, on the Chesterfield and Island of Pines plateau of New Caledonia, and off the New Ireland Province of Papua New Guinea, respectively. These new species are morphologically distinct from all other known species of Chelidoperca by body color pattern and combinations of a few identified characters. We also redescribe one of the lesser known species, C. lecromi, from fresh specimens collected close to its type locality and a new site in the Coral Sea. The distributional records for this and other known species are updated accordingly. Genetic references of the species as well as an updated identification key to western Pacific species are also provided.
Life Science Identifier (LSID): urn:lsid:zoobank.org:pub:AB996C2C-1669-41E9-923C-ADB086BC6687.
Introduction
The tropical Indo-West Pacific is the largest marine biogeographic ensemble on Earth (Crandall and Riginos, 2014). Its exceptionally high species diversity is well-known; however, our knowledge is largely limited to shallow water reef ecosystems and associated fauna. The mesophotic coral ecosystems and numerous deep-sea habitats such as seamounts or remote ocean banks are also hotspots of biodiversity, but these have received only limited study due to the technical limitations of comprehensive surveys.
Chelidoperca (Boulenger, 1895) is a genus of small-sized perch-like fishes, commonly known as perchlets, belonging to the family Serranidae. The genus is characterized by relatively slender, rounded, and elongate bodies. They are bottom-dwelling fishes usually collected in benthic trawls (Williams and Carpenter, 2015; Matsunuma, 2016). All members except the eastern Atlantic C. africana are known to be distributed in the Indo-West Pacific at depths of 40–400 m (Matsunuma and Motomura, 2016; Matsunuma et al., 2018; this study). Eleven nominal species are currently recognized in this genus (Bineesh et al., 2013; Williams and Carpenter, 2015; Iwamoto and Wirtz, 2018; Matsunuma et al., 2018; Fricke et al., 2019): C. hirundinacea (Valenciennes in Cuvier and Valenciennes, 1831), C. pleurospilus (Günther, 1880), C. investigatoris (Alcock, 1890), C. margaritifera Weber, 1913, C. africana Cadenat, 1960, C. occipitalis Kotthaus, 1973, C. lecromi Fourmanoir, 1982, C. maculicauda Bineesh and Akhilesh in Bineesh et al., 2013, C. santosi Williams and Carpenter, 2015, C. stella Matsunuma and Motomura, 2016, and C. tosaensis Matsunuma et al., 2018. Among these, C. stella is the only species known to be widespread from the Andaman Sea in the eastern Indian Ocean to the South China Sea in the western Pacific Ocean; others are restricted to single ocean basins or maritime areas (Matsunuma et al., 2018). In addition to C. stella, six other species have been reported in the West Pacific (Williams and Carpenter, 2015; Matsunuma et al., 2018). C. lecromi has only been recorded from off the Chesterfield Islands in the Coral Sea and off American Samoa. C. santosi is currently known from the northwestern Pacific from the Philippines to Japan. C. hirundinacea and C. pleurospilus are distributed from Indonesia to Japan and C. margaritifera is known only from its type locality: the Bird's Head Peninsula region, Indonesia. C. tosaensis was the last described species from the genus, based on the specimens found in waters of Japan and the Philippines.
During a series of biodiversity expeditions carried out between 2007 and 2017, mainly in the West Pacific, under the Tropical Deep-Sea Benthos (TDSB) program (Bouchet et al., 2008) and the cooperative project between Taiwan and France entitled “Taiwan-France marine diversity exploration and evolution of deep-sea fauna (TFDeepEvo; 2013–2016), 58 specimens of Chelidoperca species were collected from waters at depths of 77–338 m off Papua New Guinea, EEZ of New Caledonia, the Philippines, and South China Sea, including several specimens that were unable to be identified as existing known species in the West Pacific but recognizable in several morphotypes. After the detailed examinations carried out in this study, we concluded that they belonged to at least four new species. However, during the course of manuscript preparation, one co-author, Mizuki Matsunuma (MM), and his undergraduate student, Akari Ogino (AO), simultaneously examined other specimens of Chelidoperca collected from New Caledonia and Australia, which were deposited in the ichthyological collection of the National Natural History Museum of Paris (MNHN) and the National Museum of Nature and Science of Tsukuba (NSMT), and also concluded that specimens characterized by orange spots on the pectoral and caudal fin bases are a new species. As a paper by MM and AO describing this new species was already in preparation, all four present co-authors agreed to separately describe the other species. The objective of the present paper is thus to use an integrated approach in systematics (see: Dayrat, 2005; Hung et al., 2017; Lo et al., 2017; Puillandre et al., 2017) to explore the phylogeny and species diversity of the Chelidoperca, especially those occurring in the West Pacific. Our results reveal an even greater species diversity of the genus than previously thought and extend the distribution range of two already known species, C. stella and C. tosaensis. We formally describe herein three of the newly discovered species based on morphological and molecular diagnoses. We also redescribe one of the lesser known species, C. lecromi, using fresh specimens newly collected close to its type locality around the Chesterfield Islands in the Coral Sea. Genetic references of the new species as well as an updated identification key to the West Pacific Chelidoperca species are also provided.
Materials and Methods
Sample Collection, Morphological Measurements, and Species Identification
Most of the Chelidoperca specimens (n = 58) examined in the present study were collected during eight exploratory cruises in the West Pacific (campaigns: AURORA 2007, BIOPAPUA 2010, EXBODI 2011, MADEEP 2014, KAVIENG 2014, ZHONGSHA 2015, KANACONO 2016, and KANADEEP 2017) conducted between 2007 and 2017 under the TDSB and the TFDeepEvo programs with the French research vessel ALIS and the Taiwanese research vessel OR1. A few others (n = 7) were collected at fish landing ports in Taiwan (Dashi, Ilan, on 13 March 2014 and Keziliao, Kaohsiung, on 6 May 2017) and Japan (Saga, Tosa bay, on 31 Jan. 2018) (Figure 1; Supplementary Table 1). A small piece of muscle or fin tissue was excised from each specimen, preserved in 95% ethanol, and stored at −20°C in the Marine Biodiversity and Phylogenomics Laboratory at the Institute of Oceanography, National Taiwan University (NTU), Taipei, for subsequent molecular analyses. Specimens were photographed shortly after collection to record fresh color patterns, preserved in formalin, and deposited in the ichthyological collections of the NTU Museums, Taipei (NTUM), Academia Sinica, Taipei (ASIZP), and National Natural History Museum of Paris (MNHN).
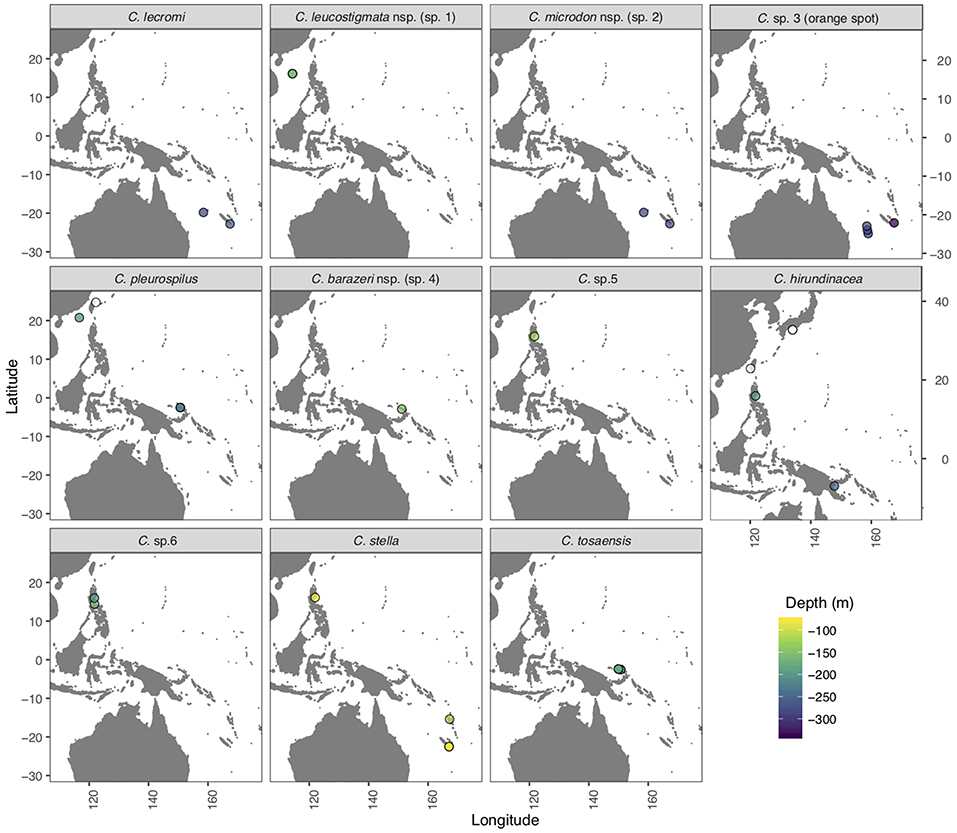
Figure 1. Map of the West Pacific region indicating sampling sites (open and color filled circles) for the specimens of Chelidoperca species examined in the present study. Open circles: specimens collected from fish landing ports with unknown collection depths; color-filled circles: specimens collected during exploratory cruises through the TDSB program and its joint TFDeepEvo project.
Collected specimens were identified to species where possible using morphological features in taxonomic references (Williams and Carpenter, 2015; Matsunuma, 2016; Matsunuma and Motomura, 2016; Matsunuma et al., 2018) and then checked against photographs (if available) in the original papers describing each species. If a specimen could not be identified by morphology or if metric counts overlapped with more than one species, it was first identified to genus level and then checked again after molecular analyses. Methods for morphological examination and specimen measurements generally followed (Matsunuma, 2016) and (Matsunuma and Motomura, 2016). Definitions of some specific morphological features, including the identification of the last anal- and dorsal-fin rays, differentiation of segmented procurrent rays and soft segmented caudal rays, counts of scale rows above the lateral line, and counts of preopercular, opercular, interopercular, and subopercular serrae followed (Matsunuma et al., 2018). Examination of the supraneural dorsal-ray and pattern formula of pterygiophore neural spine interdigitation followed (Anderson and Heemstra, 2012). Specimens examined in this study are listed in Supplementary Table 1. Other type materials examined included: MNHN1981-1436 and MNHN1981-1437 (C. lecormi, holotype, and paratype). All measurements were taken in a straight line, made with a dial caliper, and recorded to the nearest 0.1 mm. Internal osteological features of specimens were recorded based on X-ray photographs.
Collection of Molecular Data and Measurements
Total genomic DNA was extracted from the tissue samples of 47 Chelidoperca individuals and one individual from an outgroup taxon, Centropristis philadelphica, using an automatic extractor: LabTurbo 48 Compact System and LGD 480–500 kits (Taigene Biosciences Corp., Taipei) following the manufacturer's protocol. One mitochondrial protein-coding gene (cytochrome oxidase c subunit I [COI]) and one nuclear protein-coding gene (recombination activating protein 1 [RAG1]) were used as genetic markers in this study for their ability to provide species delimitation (Ward et al., 2005; Hung et al., 2017; Lo et al., 2017) and phylogenetic information (López et al., 2004; Chen et al., 2014; Lo et al., 2015, 2017; Hung et al., 2017), respectively. Protocols for collecting molecular data followed Ward et al. (2005) and the previous study of Lo et al. (2015). Gene amplicons were sequenced with the Sanger sequencing technique at Genomics BioSci &Tech (Taipei) and the Center of Biotechnology (National Taiwan University, Taipei). DNA sequences obtained were edited and aligned with sequence assembly and alignment software CodonCode Aligner v. 7.0.1 (CodonCode Corporation, Dedham, MA, USA) and Se–Al v2.0 (Rambaut, 1996). Sequences (usually at both ends) and base pairs of low quality, i.e., below Q (phred quality value) 20, were verified and trimmed manually. In addition to the sequences newly obtained through our laboratory work, 35 COI sequences and one RAG1 sequence of Chelidoperca plus one outgroup taxon, Paralabrax clathratus, were retrieved from GenBank (NCBI, Nation Center for Biotechnology Information) and the BOLD system (The Barcode of Life Data Systems) (n = 3), and three unpublished COI sequences of Chelidoperca stella samples collected from Palikulo Bay, Vanuatu, through the Santo Marine Biodiversity Survey led by the MNHN in 2006, were provided by Dr. Agnès Dettaï (MNHN) (Supplementary Table 1). Sequences were compiled into two separate gene datasets for the molecular analyses described below. The software PAUP* (Swofford, 2002), MEGA6 (Tamura et al., 2013) and Se–Al were used to obtain the basic statistics of the sequences from the two compiled datasets to compute pairwise genetic distances with the Kimura-2-parameter distance model (K2P) (Kimura, 1980) and to determine and visualize the nucleotides at the COI locus that are apomorphic and unique to the new species.
Phylogenetic Inference
Partitioned maximum likelihood (ML) analysis with the nucleotide substitution model GTR + G (Yang, 1994) was performed on each gene dataset with the software RAxML version 8.0.4 (Stamatakis, 2014) to infer the phylogeny of Chelidoperca species and test the monophyly of recognized species. Partitioning was set by codon position. The final tree with the best likelihood score was selected. Nodal support was assessed by bootstrapping (Felsenstein, 1985) with the ML criterion based on 1,000 pseudo-replicates. Tree topology and bootstrap values were visualized using FigTree v1.4.3 (Rambaut, 2012). According to a previous phylogenetic hypothesis of serranid fishes suggested by Meisler (1987), Chelidoperca appears to be the sister group of all other genera in the serranid subfamily, Serraninae. Here, two taxa belong to the Serraninae, Centropristis philadelphica, and Paralabrax clathratus, were chosen as outgroups and used to root the inferred gene trees.
Species Delimitation Analyses
Two DNA-based species delimitation analyses, the Automatic Barcode Gap Discovery (ABGD) (Puillandre et al., 2012) and Bayesian based Poisson Tree Processes (bPTP) (Zhang et al., 2013), were primarily used to explore the species diversity of the West Pacific Chelidoperca based on the COI dataset. ABGD is a species delimitation method based on pairwise genetic distances. With this tool, sequences are clustered into operational taxonomic units (OTUs) or putative species through the detection of significant differences between intra-specific and inter-specific genetic distances (barcode gap). This analysis was performed at the web interface (http://wwwabi.snv.jussieu.fr/public/abgd/) using a relative gap width (X) value of 1.5 and intraspecific divergence (p) value from 0.001 to 0.1, with 50 steps under K2P pairwise distances.
The bPTP analysis infers OTUs by analyzing branching events on a rooted dendrogram tree. A rooted COI gene tree of Chelidoperca with identical sequences excluded was inferred using RAxML as our input tree. This analysis was performed at the web server available at https://species.h-its.org/ptp/. The default settings used in the analysis were 100,000 MCMC generations, thinning as 100, burn-in as 0.1, and seed as 123. Congruent results (i.e., common OTUs) from ABGD and bPTP were considered primary evidence for considering putative species. Other criteria applied to evaluate the validity of inferred species included an evaluation of the pairwise genetic distances of the taxa, species monophyly found in gene trees, and the results of morphological comparisons. Finally, as suggested in Kekkonen and Hebert (2014) and applied by Lo et al. (2017) and Hung et al. (2017), resulting allopatric sister OTUs from the COI species delimitation analyses were treated as a single OTU rather than separating them into multiple OTUs when no other evidence suggested the reproductive isolation of sister putative species or OTUs.
According to the distribution data of taxa recorded in previous studies (e.g., Matsunuma et al., 2018), most species within Chelidoperca are restricted to a single ocean basin or marine biogeographic realm (as in Costello et al., 2017). Therefore, we defined three western Pacific geographic regions based on the levels of regional endemicity of marine species (notably in Chelidoperca spp.), ocean currents, and other physical barriers (see: Hung et al., 2017): (1) northwestern Pacific (NWP; including East China Sea, Philippines, Southern Japan, South China Sea, and Taiwan); (2) tropical West Pacific (TWP; including West Papua and the Bismarck Sea off northern Papua New Guinea); and (3) tropical Australia and Coral Sea (TA+CS; including New Caledonia, Vanuatu, and the Solomon Sea). The designation of sympatry or allopatry of inferred sister OTUs was based on these defined regions.
Results and Discussion
Molecular Data and Inferred Phylogeny
The COI dataset comprised 50 newly obtained sequences plus 42 published and unpublished sequences from all (10) nominal Chelidoperca species in the Indo-West Pacific, several undetermined species in the West Pacific, and two outgroups (Supplementary Table 1). The length of the aligned sequences of the dataset is 630 bp. The aligned sequences contained no indels or stop codons and included 235 (37.3%) variable sites, 210 of them being parsimony-informative. Most of the variable sites were found from the third codon positions (203/235; 86.4%).
The RAG1 dataset included 12 newly obtained sequences of five nominal and several undetermined species from the West Pacific, and two outgroups (Supplementary Table 1). The length of the aligned sequences of the dataset is 1,449 bp. The aligned sequences contained no indels or stop codons and included 147 (10.1%) variable sites, 66 of them being parsimony-informative. Most of the variable sites were found from the third codon positions (104/147; 70.7%).
Figures 2, 3 show the phylogenetic trees inferred from the partitioned ML analysis based on COI and RAG1 datasets, respectively. The monophyly of the genus Chelidoperca has only moderate support (BS = 63%) from COI gene analysis, but has maximal support (BS = 100%) from RAG1 analysis. All but one (C. stella) of the included nominal species are confirmed to be monophyletic by COI gene analysis. In addition, those samples that cannot be identified by morphology to any existing Chelidoperca species cluster into six independent lineages in the COI gene tree: C. sp. 1 (samples exclusively from the South China Sea), C. sp. 2 (one sample from the Coral Sea and another from southeastern New Caledonia), C. sp. 3 (samples from the Coral Sea and southeastern New Caledonia), C. sp. 4 (two samples from Papua New Guinea), C. sp. 5 (one sample from the Philippines), and C. sp. 6 (two samples from the Philippines) (Figures 1, 2). Further detailed examination on the specimens (when available) revealed that they represented at least four morpho-species (see below).
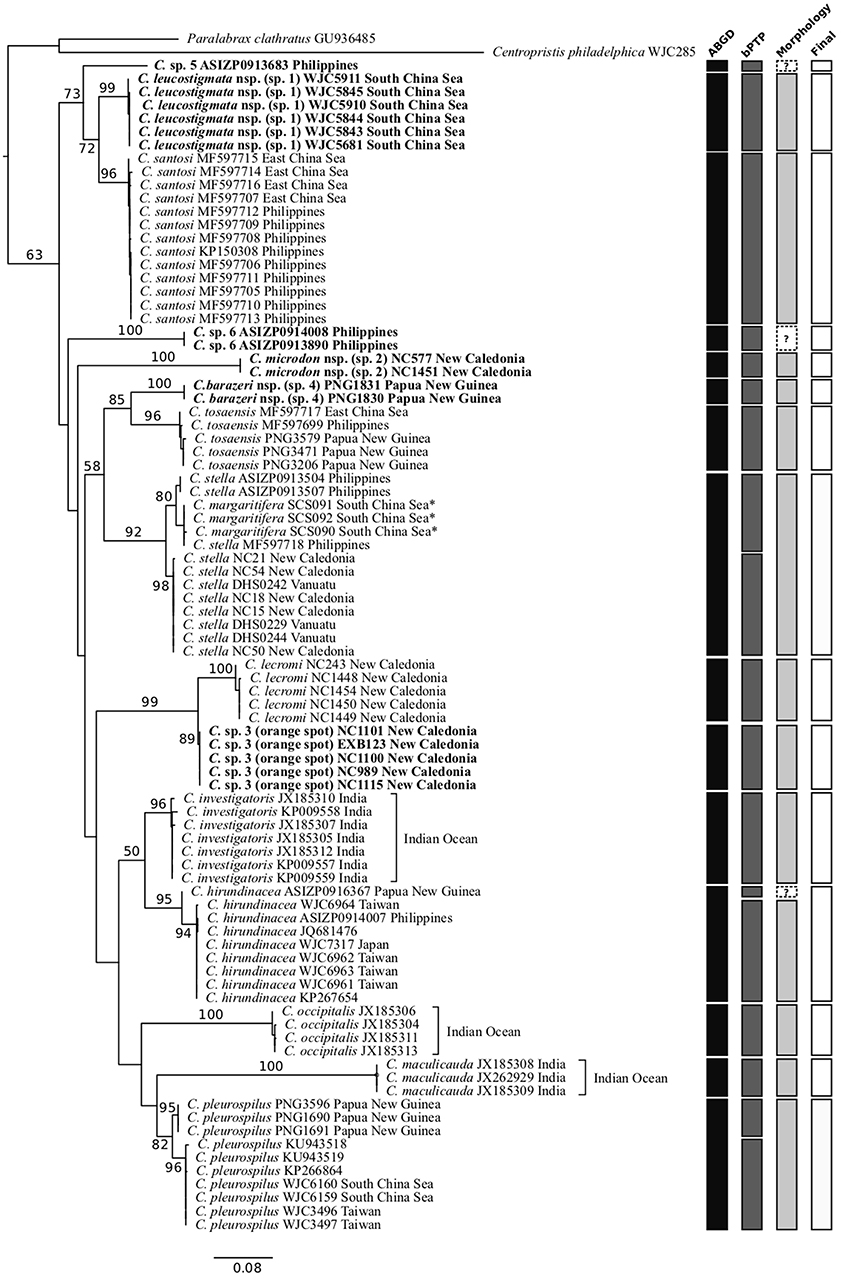
Figure 2. Phylogenetic tree of Chelidoperca species inferred by the partitioned maximum-likelihood method with GTR + G nucleotide substitution model based on the COI gene dataset, species delimitation analyses, and additional criteria (see materials and methods). Branch lengths are proportional to inferred nucleotide substitutions. Numbers at nodes represent bootstrap values in percentages. Values <50% are not shown. Taxa names in bold indicate new or potential new species discovered in this study. *indicates probable misidentified samples. The tree is rooted with two known species from the Serraninae. A summary of determined OTUs (vertical bars) is presented on the right side of the phylogenetic tree.
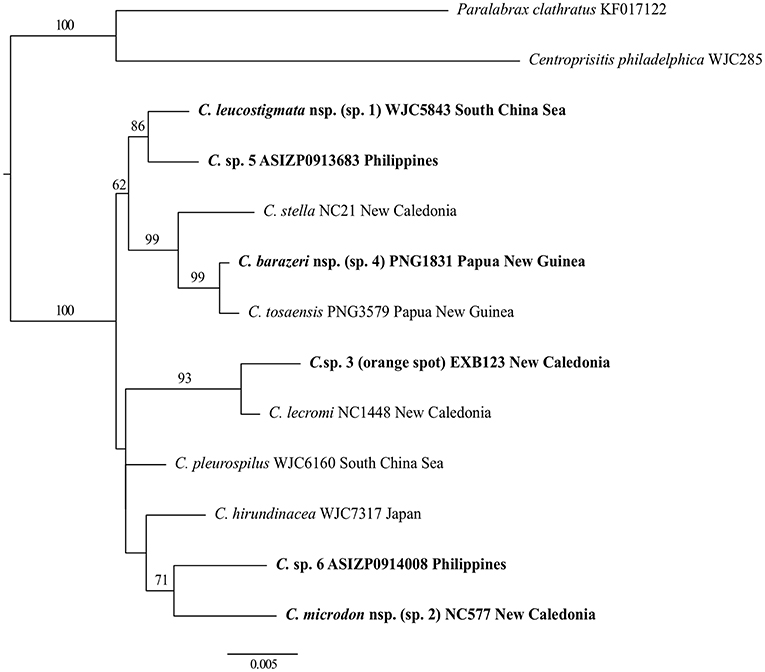
Figure 3. Phylogenetic tree of Chelidoperca species inferred by the partitioned maximum-likelihood method with GTR + G nucleotide substitution model based on the RAG1 gene dataset. Branch lengths are proportional to inferred nucleotide substitutions. Numbers at nodes represent bootstrap values in percentages. Values <50% are not shown. Taxa names in bold indicate new or potential new species discovered in this study. The tree is rooted with two known species from the Serraninae.
For intra-generic relationships, COI gene analysis resolved the following sister-group relationships with strong support (BS > 80%): between C. tosaensis and C. sp. 4, between C. lecromi and C. sp. 3, and between C. pleurospilus and C. maculicauda (Figure 2). The clade comprising the four samples of C. “margaritifera” from the South China Sea is nested within C. stella (Figure 2). RAG1 gene analysis revealed that all the sister-group relationships resolved in the COI tree (except C. pleurospilus/C. maculicauda pair; no sample from C. maculicauda for RAG1 sequencing) were confirmed. In addition, RAG1 gene analysis further resolved C. stella to be the sister group of the clade containing C. tosaensis and C. sp. 4 and the sister-group relationship between C. sp. 1 and C. sp. 5, with strong nodal support (BS = 99 and 86%, respectively) (Figure 3).
Discovery of Hidden Species Diversity of Chelidoperca in the West Pacific
To further explore species diversity, a dataset with COI sequences for 90 Chelidoperca samples including 10 out of 11 nominal species were compiled and analyzed by two species delimitation analyses, ABGD and bPTP. The eastern Atlantic C. africana samples were not included due to a lack of specimens for sequencing. The two analyses presented partially incongruent results, with 15 predicted OTUs (or putative species) from the ABGD and 18 predicted OTUs (or putative species) from bPTP. Regardless of the method used, the number of predicted OTUs (putative species) is much higher than previously recognized. Among them, 12 OTUs are common to both analyses and considered robust support for the inferred species. These are C. investigators, C. lecromi, C. maculicauda, C. occipitalis, C. santosi, C. tosaensis, and the six independent lineages (C. sp. 1–6) discovered during phylogenetic analyses (Figures 2, 3). Incongruent results appeared in two morphologically identified species clades, C. hirundinacea and C. pleurospilus, and in the clade containing the taxa C. “margaritifera” and C. stella (Figure 2). In all of these clades, the ABGD predicted a single OTU (species) whereas the bPTP tended to separate it into two allopatrically distributed entities. By examining the genetic divergence between the sister OTUs within each clade, we found a limited genetic difference of only 1.9% for the C. hirundinacea clade, 2.6% for the C. pleurospilus clade, and 3.1% for the C. stella/C. “margaritifera” clade. When further comparing the morphology from the conspecific and available specimens of each clade, we found only slight coloration differences but no significant difference in morphometrics and meristic counts among conspecific individuals. Here, we followed the criterion of species delimitation suggested by Kekkonen and Hebert (2014); that is, merging allopatric clusters and considering them to be the same species when the results from both species delimitation analyses are in disagreement and when there is no other evidence indicating the presence of reproductive isolation between the compared OTUs. As a result, we validated a total of 15 inferred species in Chelidoperca on the basis of the samples examined in this study (Figure 2).
C. margaritifera is currently known to be distributed only around its type locality, the Bird's Head Peninsula region, West Papua (Matsunuma et al., 2018), and was included in our analyses. However, the C. “margaritifera” samples used for COI gene analysis were from the South China Sea, which is around 1,000 km from the species' type locality. Corresponding sequences were retrieved from the BOLD system, and upon examination we found they were very similar to those from the C. stella samples collected from the Philippines (average K2P distance = 1.1%). We were unable to check the species identification for C. “margaritifera” samples from the South China Sea due to not having access to voucher specimens. However, considering the combined evidence from our species delimitation analyses and the geographic distributions of the two concerned species, we opine that the C. “margaritifera” samples from the South China Sea were misidentified and should be referred to C. stella, which is a widespread species distributed from the Andaman Sea in the eastern Indian Ocean to the South China Sea and to the Coral Sea, including New Caledonia and Vanuatu, according to the new data presented in this study (Figures 1, 2).
Morphological Comparison
Fifty newly collected specimens were used to carry out the morphological examination and comparison. The specimens of C. sp. 5 and C. sp. 6 were not examined because the voucher specimens deposited in the ASIZP were unavailable (specimens were loaned to others). Data from examined specimens are provided in Supplementary Data 1. The comparison of meristic and morphometric data from the specimens of the selected species is summarized in Tables 1–8.
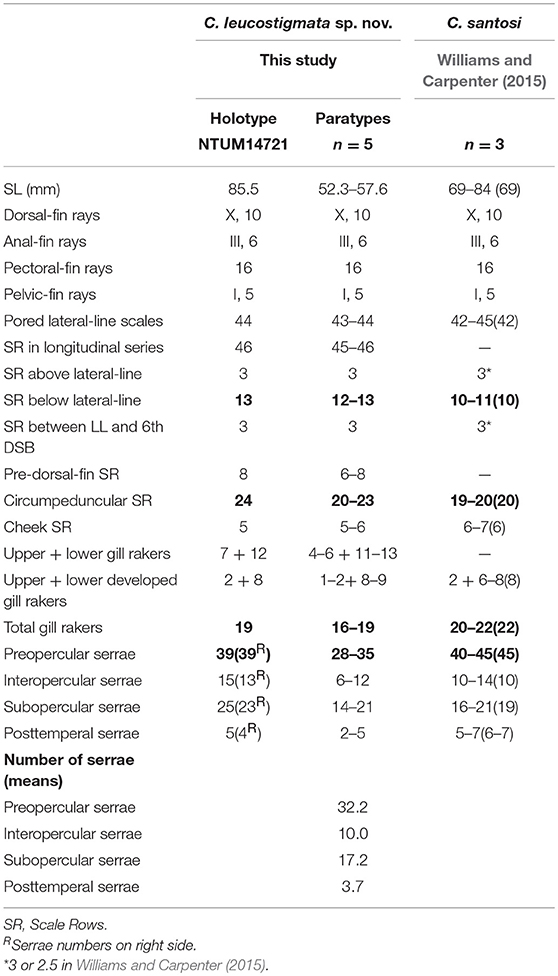
Table 1. Meristic features of Chelidoperca leucostigmata sp. nov. and C. santosi from references; bold font presents distinguishing characteristics; parentheses in Williams and Carpenter (2015) contains holotype data.
In general appearance, the species of Chelidoperca are small-sized fishes with relatively slender and elongated bodies (Figures 4–7). The species are characterized by the following combined characters: dorsal-fin rays usually X, 10; anal-fin rays usually III, 6; two flattened strong opercular spines; and body coloration mostly pale pink (Matsunuma and Motomura, 2016; Matsunuma et al., 2018; this study). In spite of the similarity in overall appearance, the species occurring in the West Pacific (including the new species discovered in this study) can usually be distinguished from each other by the combination of the following characters: body color pattern, number of pored lateral-line scales, number of scale rows between the lateral line and the middle of the spinous dorsal-fin base, presence of scales on the dentary, and presence of enlarged canines on the upper jaw. In color pattern, C. sp. 1 is characterized by having several predominant white spots lying just above the lateral line on the body, which is unique among recognized species (Figure 5). C. margaritifera (no real samples included in this study), C. lecormi (Figure 4), and Chelidoperca sp. 3 (orange spot) (Figure 4) are characterized by having a yellow stripe [along the lateral line in C. lecromi and Chelidoperca sp. 3 (orange spot); and along ventral potion of body in C. margaritifera], which is absent in other congenic species (Williams and Carpenter, 2015; Matsunuma et al., 2018; this study). Among them, C. margaritifera also presents numerous small white spots below the yellow stripe. C. sp. 3 (orange spots) is characterized by having orange spots on pectoral and caudal fin bases (Figure 4).

Figure 4. Chelidoperca lecromi (A–C) from off Chesterfield Island, Coral Sea, and Chelidoperca sp. 3 (orange spot) (D,E) from Kelso Bank, Coral Sea. (A) MNHN1981-1436 (holotype) standard length 125.3 mm (photographed by MM); (B) NTUM13749 (NC1452), collected on 22 Sep 2017; standard length 102.3 mm (photographed by MYL); (C) NTUM13749 (NC1453), collected on 22 Sep 2017; standard length 63.7 mm (photographed by MYL). (D) NTUM13736 (NC1101), collected on 5 Sep 2017; standard length 90.0 mm (photographed by MYL); (E) NTUM13737 (NC1115), collected on 5 Sep 2017; standard length 110.4 mm (photographed by MYL). Bars = 10 mm.
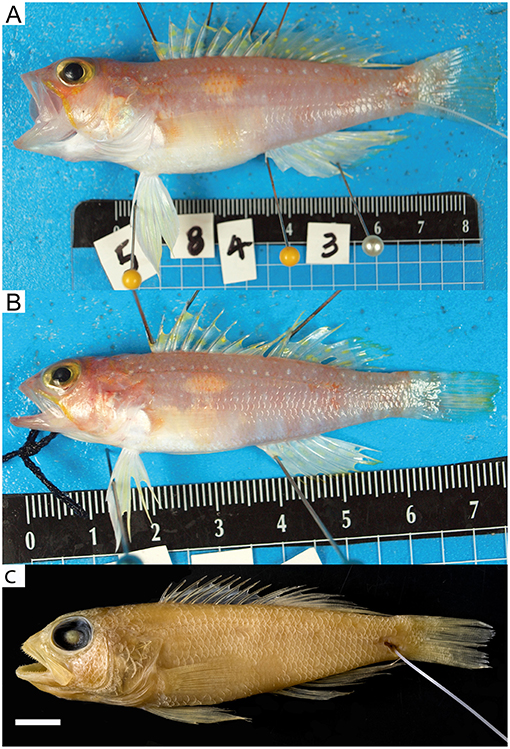
Figure 5. Chelidoperca leucostigmata sp. nov. from north Macclesfield bank. (A) NTUM14721 (WJC5843, holotype), collected on 26 Jul 2015; standard length 85.0 mm (photographed by WJC-Lab); (B) NTUM15634 (WJC5911, paratype), collected on 26 Jul 2015; standard length 57.6 mm (photographed by WJC-Lab); (C) same specimen in (A), showing preserved pigmentation (photographed by MYL). Bars = 10 mm.
The number of pored lateral-line scales is different among Chelidoperca spp., and the West Pacific Chelidoperca can be separated into two groups by this character. The low-number group having 33–37 (usually 34–35) pored lateral-line scales consists of C. stella and Chelidoperca sp. 4; the high-number group having 37–46 (usually ≥ 38) pored lateral-line scales contains nine species: C. hirundinacea, C. lecromi, C. margaritifera, C. pleurospilus C. tosaensis, C. santosi, C. sp. 1, C. sp. 2, and C. sp. 3 (orange spot) (Tables 1–4; Supplementary Data 1) (Williams and Carpenter, 2015; Matsunuma and Motomura, 2016; Matsunuma et al., 2018). In terms of the number of scale rows between the lateral line and the middle of the spinous dorsal fin base (Supplementary Figure 1), the six West Pacific species, C. margaritifera, C. stella, C. tosaensis, Chelidoperca sp. 1, Chelidoperca sp. 2, and Chelidoperca sp. 4, have three scales rows whereas the five other species, C. hirundinacea, C. lecromi, C. pleurospilus, C. santosi, and Chelidoperca sp. 3 (orange spot), have four scales rows [(Williams and Carpenter, 2015; Matsunuma et al., 2018); this study]. While examining the scale rows on the dentary (Supplementary Figures 2B,D,F,H), we observed that they are present (at least one scale row) in the six West Pacific species, C. hirundinacea, C. margaritifera, C. pleurospilus, C. santosi, C. stella, and C. sp. 1, but absent in the other five, C. lecromi, C. tosaensis, C. sp. 2, C. sp. 3 (orange spot), and C. sp. 4 (Williams and Carpenter, 2015; Matsunuma et al., 2018; this study). On their upper jaw, enlarged canines (Supplementary Figure 3) are present in eight West-Pacific species, C. lecromi, C. tosaensis, C. pleurospilus, C. santosi, C. stella, C. sp. 1, C. sp. 3 (orange spot), and C. sp. 4, but absent in C. hirundinacea, C. margaritifera, and C. sp. 2 (Williams and Carpenter, 2015; Matsunuma et al., 2018; this study). In addition to these key morphological features, the fresh pigmentation observed and morphometrics from specimens or photographs are also important in distinguishing them, especially in separating new species from similar and/or closely related congenic species. Detailed comparisons are given in the following section on taxonomy.
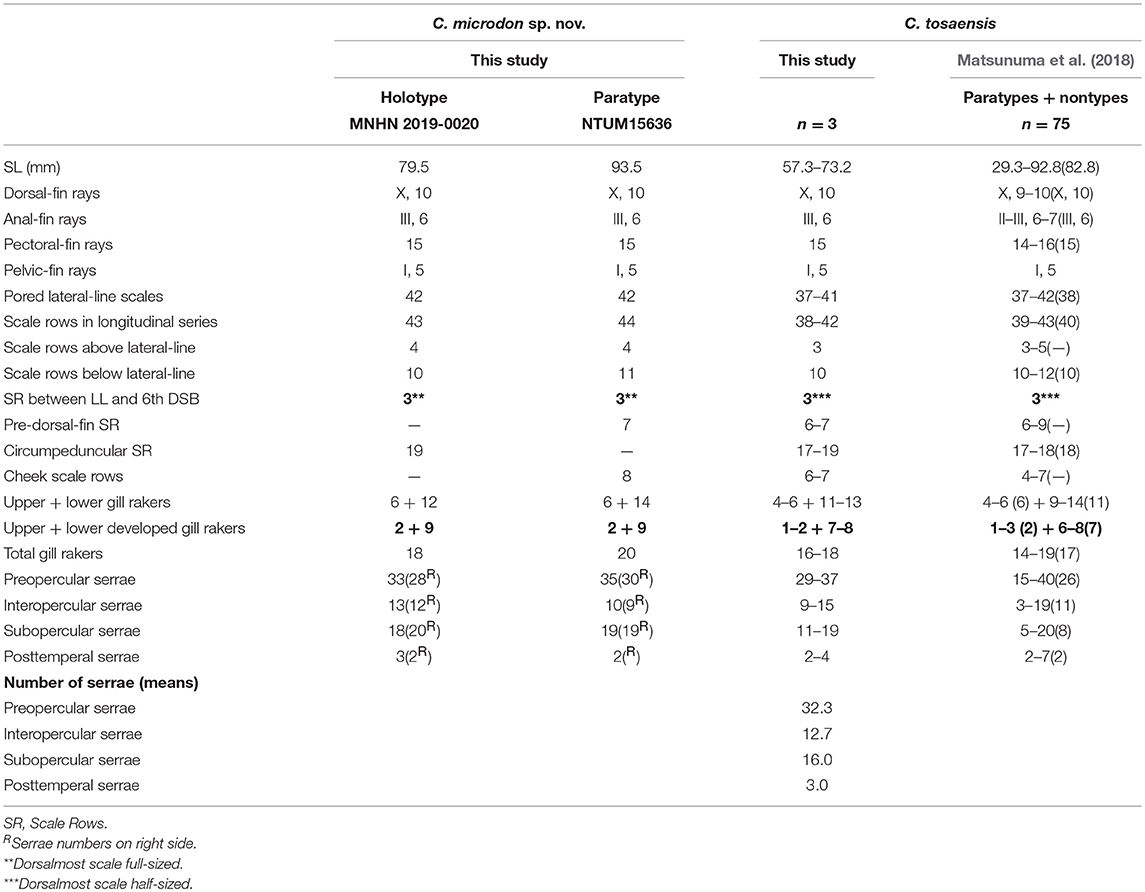
Table 2. Meristic features of Chelidoperca microdon sp. nov. and C. tosaensis (including examined specimens and reference data); bold font presents distinguishing characteristics; parentheses in Matsunuma et al. (2018) contains holotype data.
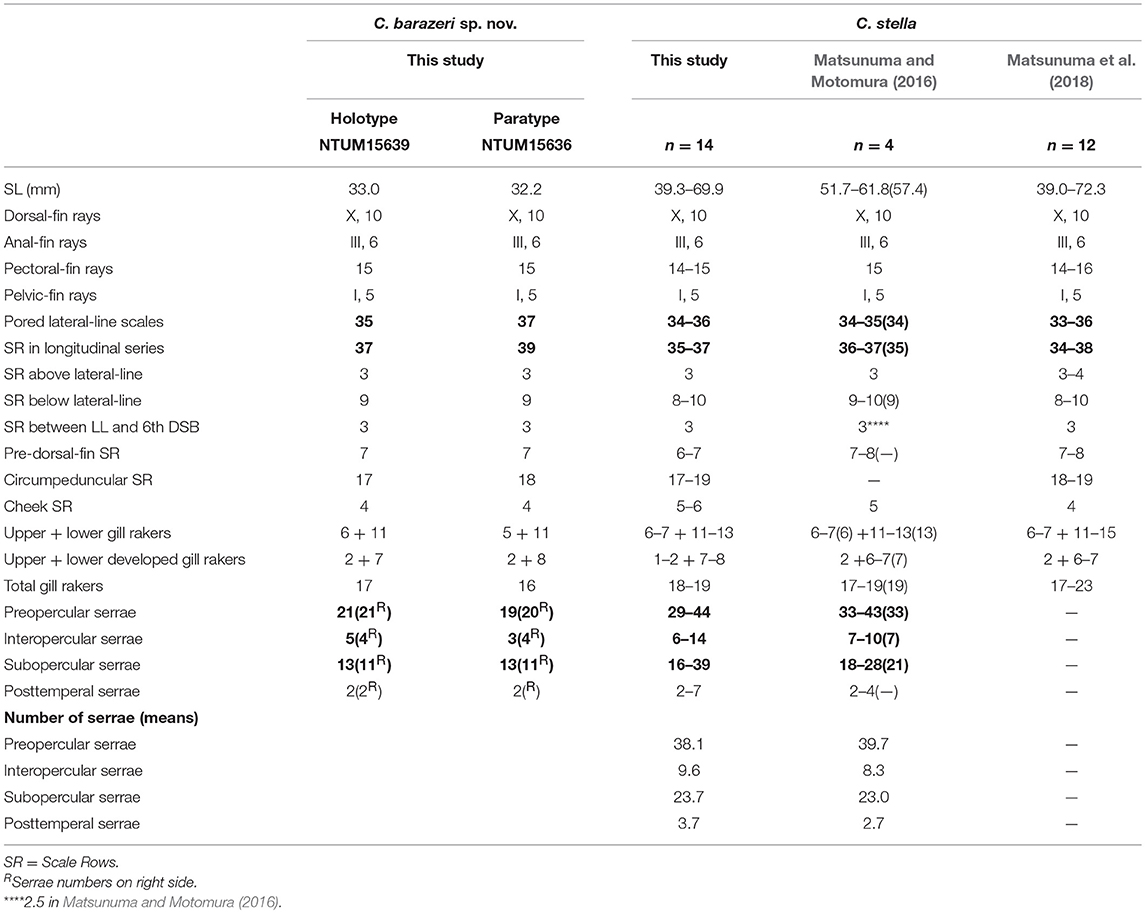
Table 3. Meristic features of Chelidoperca barazeri sp. nov. and C. stella (including examined specimens and reference data); bold font presents distinguishing characteristics; parentheses in Matsunuma and Motomura (2016) contains holotype data.
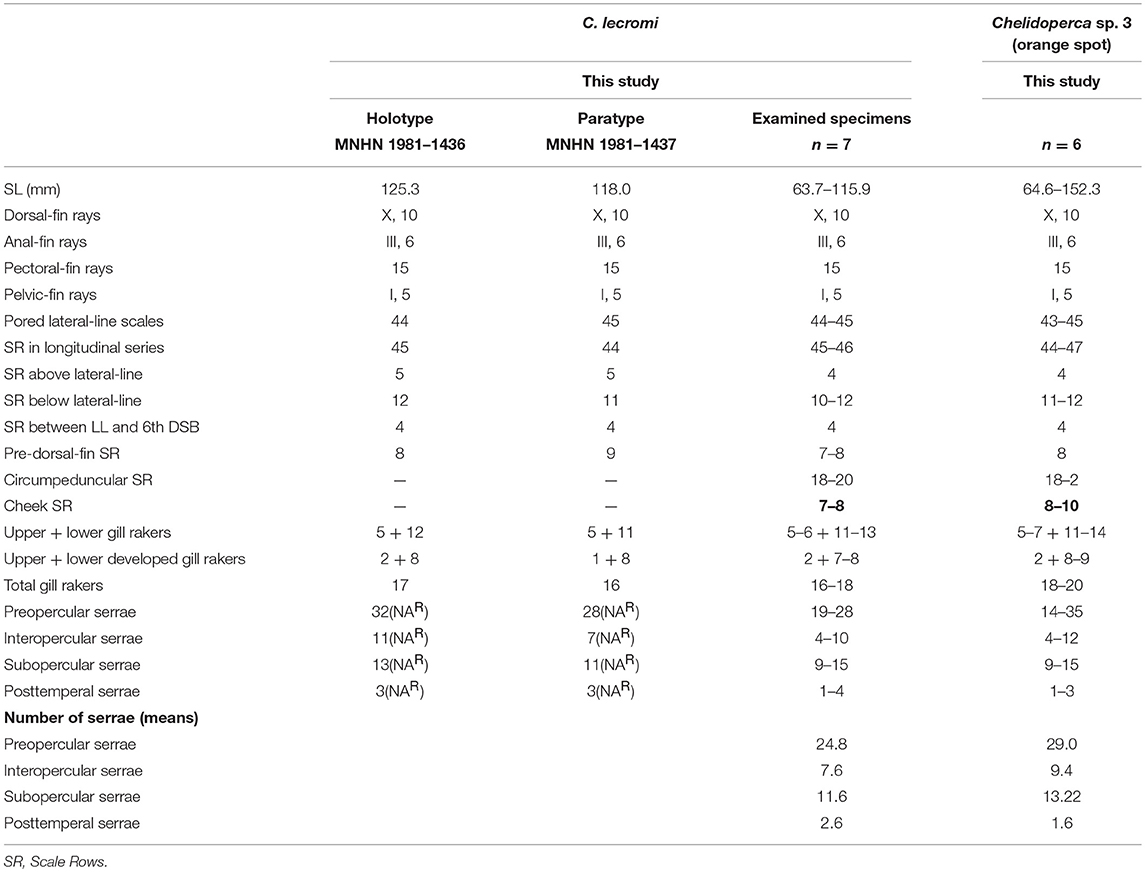
Table 4. Meristic features of Chelidoperca lecromi (including examined specimens and references data) and Chelidoperca sp. 3 (orange spot); bold font presents distinguishing characteristics.
Taxonomy
Below, we formally describe three of the newly discovered species in this study on the basis of available specimens. Subsequent to the original description of C. lecromi, only one holotype (MNHN1981–1436) and one paratype (MNHN1981–1437) have ever been collected. In this study, seven fresh specimens, among which six were collected close to its type locality around the Chesterfield islands in the Coral Sea, were available for examination. Here, and for the first time, we redescribe C. lecromi in detail and provide images of its fresh coloration. Finally, by combining the results of the morphological examination and data from taxonomic references of Chelidoperca, a diagnostic key for the West Pacific Chelidoperca spp. is provided.
Chelidoperca leucostigmata sp. nov. (Figure 5; Supplementary Figures 1B, 2C,D, 3B; Tables 1, 5)
Previously Referred to as Chelidoperca sp. 1
Holotype
NTUM14721 (tissue voucher: WJC5843), 85.5 mm SL, sta. CP4149, 16°07′N, 114°20′E, 162–165 m, north Macclesfield Bank, South China Sea, Ocean Research I, French beam trawl, ZhongSha 2015 expedition, 26 Jul 2015.
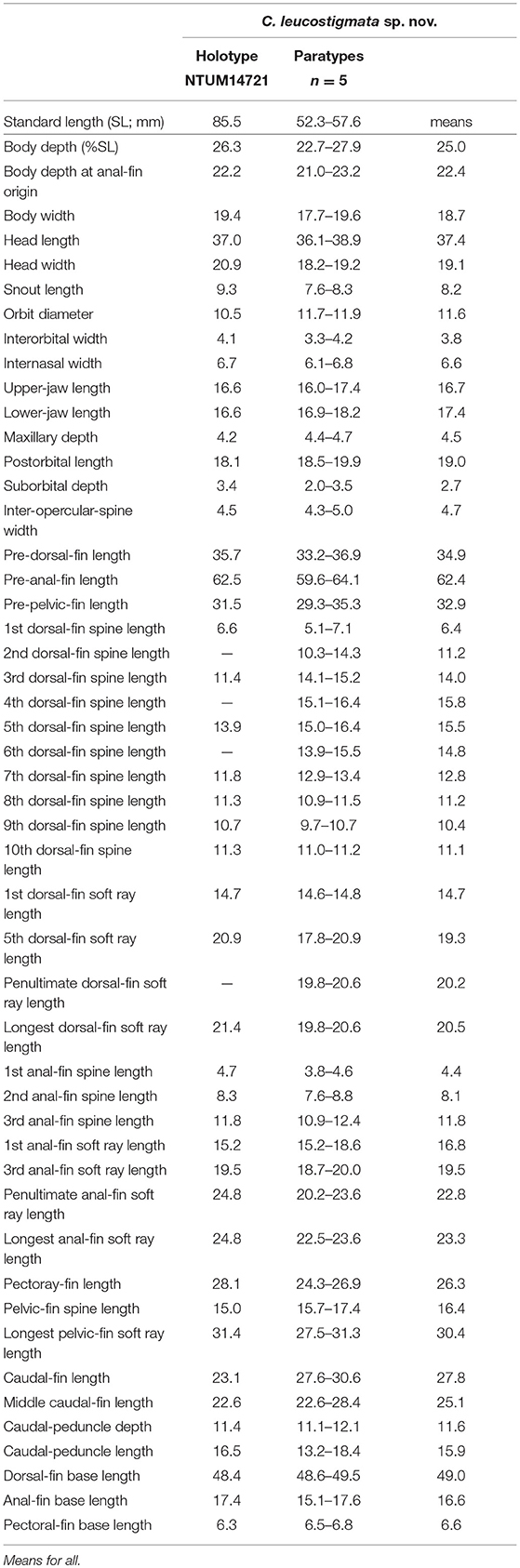
Table 5. Mophometrics expressed as percentages of the standard length for Chelidoperca leucostigmata sp. nov.
Paratypes
Five specimens (52.3–57.6 mm SL): NTUM15632 (tissue voucher: WJC5681), 56.0 mm SL, sta. DW4144, 16°07′S, 114°23′E, 161 m, north Macclesfield Bank, South China Sea, Ocean Research I, Waren Dredge, ZhongSha 2015 expedition, 26 Jul 2015. NTUM15634 (tissue voucher: WJC5910 & 5911), two specimens, 56.1–57.6 mm SL, sta. CP4150, 16°07′N, 114°24′E, 162–163 m, north Macclesfield Bank, South China Sea, Ocean Research I, French beam trawl, ZhongSha 2015 expedition, 26 Jul 2015. NTUM15355 (tissue voucher: WJC5844 & 5845), two specimens, 52.2–55.7 mm SL, sta. CP4149, 16°07′N, 114°20′E, 162–165 m, north Macclesfield Bank, South China Sea, Ocean Research I, French beam trawl, ZhongSha 2015 expedition, 26 Jul 2015.
ZooBank registration
urn:lsid:zoobank.org: act:CF478E5E-3A64-42CA-9CA3-B528287 41EE0.
GenBank registration of the holotype
MK988055 (COI); MK988087 (RAG1).
Diagnosis
Chelidoperca leucostigmata is characterized by the combination of the following morphological characters: 44 pored lateral line scale rows; 3 (dorsal-most scale half-sized) scale rows between the lateral line and middle of spinous dorsal fin base; relatively fewer scale rows 5–6 cheek; scale rows present on ventral side of dentary; scale rows on interorbital area extend beyond the level of the anterior pupil rim, reaching the anterior orbit rim; longitudinal series of clear white spots along lateral line; and elliptical light yellow mark on side of body. Along the COI gene, the following apomorphic sites have unique nucleotides shared by all six specimens of C. leucostigmata examined so far; these nucleotide sites can be used for the diagnosis of the species to other congeners. Nos. 168 (T vs. C), 180 (T vs. A, G or C), 451 (A vs. G), 522 (T vs. A or C), 621 (T vs. C).
Description
Morphometric and selected meristic values are summarized in Tables 1, 5. Dorsal fin rays X, 10; anal-fin rays III, 6; pectoral fin rays 16; pelvic fin rays I, 5. Body fusiform, slightly elongated; snout round; caudal peduncle moderately long. Orbit large, its dorsal margin included in the dorsal contour of the head. Mouth large, slightly oblique; posterior margin of maxilla reaching a vertical through mid-orbit; maxilla expanded posteriorly, with low lateral ridge along dorsal margin; lower jaw slightly protruding beyond upper jaw when mouth closed.
Upper jaw with band of 7 (5–7) rows (in anterior portion) of small, sharp-tipped conical teeth, tooth band becoming narrow posteriorly, outermost row of teeth enlarged, anteriorly projecting antrorse canines (Supplementary Figure 3B); lower jaw with band of about 5 (4–5) rows (in anterior portion) of small, sharp-tipped conical teeth, innermost and outermost rows of teeth enlarged canines, band of small teeth narrowing posteriorly; vomer with V-shaped band of about four rows of small conical teeth; palatine with relatively long band of four rows of small, sharp-tipped conical teeth. Anterior nostrils situated at middle of snout, with small rounded flap rising from posterior rim; posterior nostril with elliptical opening at anterior border of orbit.
Posterior margins of preopercle, interopercle and subopercle finely serrated, serrae on preopercle 39 on both sides (28–39), interopercle 15 (13 on the right side) (6–15); serrae on subopercle about 25 (23 on the right side) (14–25), relatively weaker; number of serrae generally increasing with growth. Opercle with two flat, prominent spines, upper spine slightly longer than lower. Posttemporal with 5 (4) (2–5) serrae tips at beginning of lateral line.
Body covered with ctenoid scales; lateral line slightly arched, gradually descending over pectoral fin, terminating at caudal-fin base. Uppermost row of body scales along dorsal-fin base always about half the size of adjacent lower body scales; tiny and irregularly spaced scales present at bases of spines. Caudal-fin base covered with ctenoid scales, extending onto fin over about half of basal length (depends on specimen condition) of fin. Pectoral fin with ctenoid basal scales, small elongate cycloid scales extending onto fin ventrally. Basal scales absent on either dorsal fin or anal fin. Pelvic-fin base covered with small elongate cycloid scales, but not extending onto fin. Head generally covered with scales, snout and maxilla naked; scales on ventral surface of lower jaw present on angular, and present posteriorly on dentary (Supplementary Figure 2D); opercle, interopercle, and subopercle with both ctenoid and cycloid scales; interorbital region with 1–2 rows of cycloid scales, extending from mid-orbit to around anterior pupil rim (Supplementary Figure 2C). A pair of interorbital canals with numerous small pores along outer margin of interorbital region. Lower jaw with around 4–5 pores anteriorly on each side of dentary symphysis, followed on each side by two pore positions along dentary sensory canal, each position with a single pore, and a fourth slit-like or rounded pore at angular–dentary junction.
Dorsal-fin origin above pectoral-fin base, 5th (or 4th) spine longest, 1st spine shortest; all soft rays branched, subequal in length. Anal-fin origin below base of 1st or 2nd dorsal-fin soft ray, 3rd spine longest; all soft rays branched, 5th ray longest. Posterior tip of dorsal and anal fins reaching a vertical through caudal-fin base when fins adpressed. Pectoral fin with uppermost two rays unbranched, remaining rays branched, 9th longest, its posterior tip being vertical through anal-fin origin. Pelvic-fin origin below pectoral-fin base; spine covered with skin; all soft rays branched, 2nd longest, elongate, slightly expanded distally, its tip reaching anus when adpressed. Caudal fin emarginate; upper lobe with elongate tip, slightly longer than lower lobe; upper lobe with seven unbranched unsegmented procurrent rays, 2–3 unbranched segmented rays, and eight branched segmented rays; lower lobe with 6–7 unbranched unsegmented procurrent rays, 2–3 unbranched segmented rays, and eight branched segmented rays; 19–21 segmented rays in total.
Formula for configuration of supraneural bones, anterior neural spines, and anterior dorsal pterygiophores 0/0/0 + 2/1 + 1/1; vertebrae 10 + 14.
Fresh coloration (based on color photographs of the following specimens when fresh, NTUM14721 [holotype] and NTUM15634) (Figures 5A,B): head pinkish orange, gradually becoming whitish ventrally; tips of upper-jaw and lower-jaw pinkish; snout pinkish; yellow mark extends from suborbital region along dorsal margin of maxilla to ventral corner of cheek, along anteroventral margin of cheek; yellow mark present on suborbital rim; color patterns of cheek and opercle generally the same as that of head. Body pinkish to orange, becoming whitish ventrally; row of clear white spots present dorsally along lateral line (few white spots not in line with the rest sometimes, present ventrally or dorsally), curving upwardly around 7th−8th dorsal-fin ray, descending afterward, terminating at caudal-fin base; row of around 15 poorly defined white spots midlaterally; six irregular, broad orange bands extending from dorsal profile to about ventral profile, distributing from fourth to fifth dorsal-fin spine to caudal fin base; an elliptical light yellow mark below lateral line present at around 5th dorsal-fin spine to 8th dorsal-fin spine. Spinous dorsal-fin membrane translucent white, with two longitudinal rows of yellow spots, one at about a third length of rays, one at about two thirds of length of rays; soft dorsal-fin membrane translucent, with yellow distal margin, and yellow spots scattered over fin in irregularly diagonal rows. Anal fin dusky white, with narrow yellow distal margin and yellow spots scattered over fin in irregularly diagonal rows (sometimes ambiguous). Pectoral fin translucent or with pale yellowish tint, an unclear reddish orange blotch basally on middle rays. Pelvic fin generally pale with yellow tint. Caudal fin generally translucent, with a transverse red to dark brown band on caudal-fin base and irregular diagonal rows of dark spots over fin.
Preserved coloration (based on all examined specimens) (Figure 5C): head and body tan, no melanophore or coloration remain.
Distribution
Chelidoperca leucostigmata is a relatively shallow water perchlet, known only from six specimens collected at depths ranging 161–165 m on Macclesfield Bank (Zhongsha), South China Sea (Figure 1).
Etymology
The name leucostigmata is derived from the Greek, leuco meaning white, and stigmata, meaning spots, in reference to the row of mid-lateral white spots when fresh.
Comparisons
C. leucostigmata can be distinguished from C. hirundinacea, C. lecromi, C. microdon, and C. pleurospilus by having three scale rows between the lateral line and middle of the dorsal-fin base (Matsunuma et al., 2018; this study: Supplementary Figure 1B). Among the species having three scale rows in that region, C. leucostigmata can be distinguished from other congeners except C. santosi by having 43 or more pored lateral-line scales and enlarged canines on the upper jaw. In meristics, C. leucostigmata differs from C. santosi in having more scale rows below the lateral line (12–13 vs. 10–11 in C. santosi), circumpeduncular scale rows (20–24 vs. 19–20 in C. santosi), fewer preopercular serrae (28–39 vs. 40–45 in C. santosi), and fewer total gill rakers (16–19 vs. 20–22 in C. santosi) (Table 1). In pigmentation, C. leucostigmata has a longitudinal series of clear white spots along the lateral line (vs. no white spots along body in C. santosi) and uniformly pigmentation on the snout (vs. two black spots on each side of snout in C. santosi) (this study). C. leucostigmata is phylogenetically close to C. santosi (Figure 2).
Remarks
C. hirundinacea, C. stella, and C. tosaensis sometimes have the row of white spots along the lateral line with different combinations of characters.
Chelidoperca microdon sp. nov. (Figure 6; Supplementary Figures 1C, 2E,F, 3C; Tables 2, 6)
Previously Referred to as Chelidoperca sp. 2
Holotype
MNHN 2019–0020 (tissue voucher: NC577), 79.5 mm SL, sta. CP4730, 22°43′S, 167°16′E, 265–305 m, off Isle of Pines, New Caledonia, Coral Sea, R/V Alis, French beam trawl, KANACONO expedition, 20 Aug 2016.
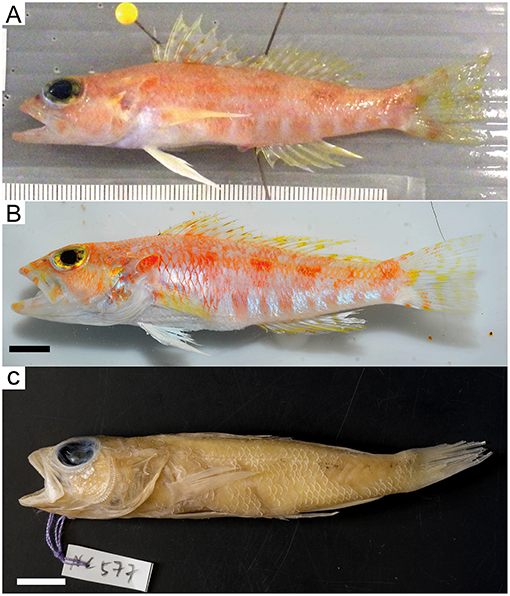
Figure 6. Chelidoperca microdon sp. nov. from Chesterfield Island and Isle of Pines, Coral Sea. (A) MNHN 2019-0020 (NC577, holotype), Collected on 20 Aug 2016; standard length 79.5 mm (photographed by WJC-Lab); (B) NTUM15636 (NC1451, paratype), Collected on 22 Sep 2017; standard length 93.5 mm (photographed by MYL); (C) same specimen in (A), showing preserved pigmentation (photographed by MYL). Bars = 10 mm.
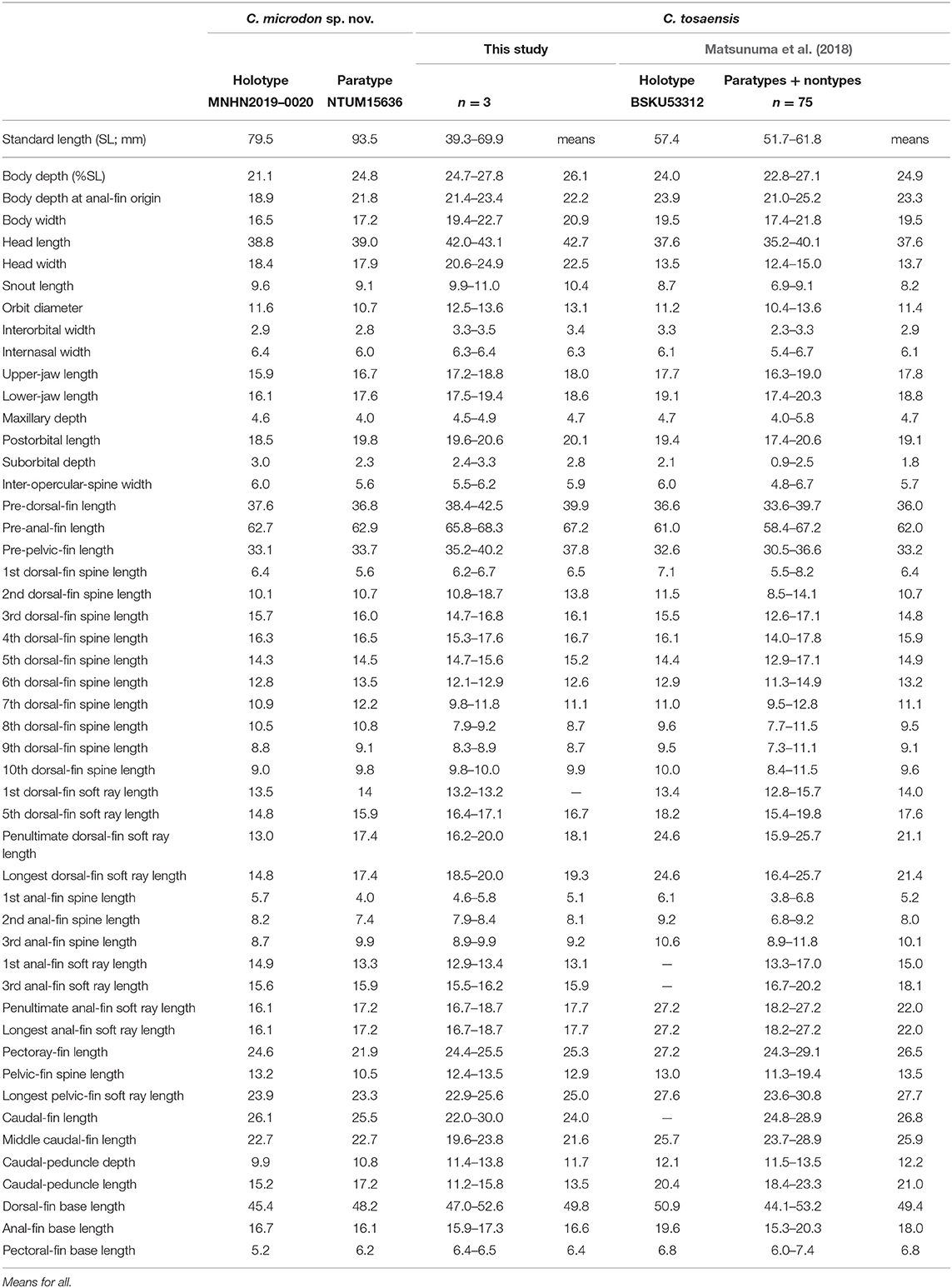
Table 6. Mophometrics expressed as percentages of the standard length for Chelidoperca microdon sp. nov and C. tosaensis (including examined specimens and reference data).
Paratype
NTUM15636 (tissue voucher: NC1451), 93.5 mm SL, sta. CP5032, 19°51′S, 158°29′E, 260–300 m, Chesterfield Plateau, Coral Sea, R/V Alis, French beam trawl, KANADEEP expedition, 22 Sep 2017.
ZooBank registration
urn:lsid:zoobank.org: act:247F62C9-71DF-4746-9CA4-822F9D0 5E223.
GenBank registration of the holotype
MK988060 (COI); MK988088 (RAG1).
Diagnosis
A species of Chelidoperca characterized by the following combination of morphological characters: 42 pored lateral line scale rows; three (dorsal-most scale full-sized) scale rows between lateral line and middle of spinous dorsal fin base; scale rows absent on ventral side of dentary; interorbital scales mostly cycloid, beyond the level of anterior pupil rim, but not reaching anterior orbit rim; five rectangular dark blotches at or slightly above lateral line usually retained; two dark blotches on tip of lower jaw. Along the COI gene, the following apomorphic sites have unique nucleotides shared by the two specimens of C. microdon examined so far; these nucleotide sites can be used for the diagnosis of the species to other congeners. Nos. 36 (C vs. G or T), 42 (C vs. A or G), 108 (T vs. A or C), 141 (G vs. A), 300 (C vs. A or G), 312 (A vs. C or T), 387 (T vs. A or G), 390 (A vs. C or T), 426 (C vs. A or G), 579 (G vs. A).
Description
Morphometric and selected meristic values summarized in Tables 2, 6. Dorsal-fin rays X, 10; anal-fin rays III, 6; pectoral-fin rays 15; pelvic-fin rays I, 5. Body fusiform, slightly elongated; snout pointed; caudal peduncle relatively long. Orbit large, its dorsal margin included in dorsal contour of head. Mouth large, slightly oblique; posterior margin of maxilla extending beyond a vertical through mid-orbit, but not reaching a vertical through posterior margin of orbit; maxilla expanded posteriorly, with low lateral ridge along dorsal margin; lower jaw slightly protruding beyond upper jaw when mouth closed. Upper jaw with band of about 6–8 rows (in anterior portion) of small, sharp-tipped conical teeth, tooth band becoming narrow posteriorly, outermost row of teeth not significantly enlarged; lower jaw with band of about four rows (in anterior portion) of small, sharp-tipped conical teeth, innermost and outermost rows of teeth enlarged slightly, band of small teeth narrowing posteriorly to 1 or 2 rows; vomer with V-shaped band of about four rows of small conical teeth, with several large canines in posterior row directed posteriorly; palatine with relatively long band of four rows of small, sharp-tipped conical teeth. Anterior nostrils situated at middle of snout, with small rounded flap rising from posterior rim; posterior nostril with elliptical opening at anterior border of orbit. Posterior margins of preopercle, interopercle, and subopercle finely serrated, serrae on preopercle 33 (28 on right side) (28–35), interopercle 13 (12 on right side) (9–13), and subopercle 18 (20 on right side) (18–20). Opercle with two flat, prominent spines, upper spine slightly longer than lower. Posttemporal with two (two on right side) (2–3) serrae tips at beginning of lateral line, number of serrae increasing with growth.
Body covered with ctenoid scales; lateral line slightly arched over pectoral fin before gradually descending, terminating at caudal-fin base (rarely with one pored lateral-line scale on caudal fin). Uppermost row of body scales along dorsal-fin base are same size as adjacent lower body scales. Caudal-fin base covered with ctenoid scales (slightly elongate cycloid scales in posterior portion), extending onto fin over about basal two-thirds of fin length. Pectoral fin with ctenoid basal scales, small elongate cycloid scales extending onto fin ventrally. Basal scales absent on dorsal fin. Anal fin without basal scales. Pelvic-fin base and membrane without scales. Head generally covered with ctenoid scales, but snout and maxilla naked; scales on ventral surface of lower jaw present on angular, dentary naked (Supplementary Figure 2F); interopercle, subopercle, and opercle with ctenoid scales; interorbital scales mostly cycloid, beyond the level of anterior pupil rim, but not reaching the anterior orbit rim (Supplementary Figure 2E). A pair of interorbital canals with numerous small pores along outer margin of interorbital region, canals diverging outward anteriorly and reaching a point between anterior and posterior nasal pores; small pores of interorbital canal forming about four rows (about two rows on each side). Lower jaw with one pore anteriorly on each side of dentary symphysis), followed on each side by two pore positions along dentary sensory canal, each position with 2 (sometimes 1) min pore openings, and a fourth slit-like or rounded pore at angular–dentary junction.
Dorsal-fin origin above pectoral-fin base, 4th spine longest, 1st spine shortest; all soft rays branched, subequal in length, 9th (based on paratypes) longest. Anal-fin origin below base of 1st dorsal-fin soft ray, 3rd spine longest; all soft rays branched, 5th ray longest. Posterior tip of dorsal and anal fins not becoming vertical through caudal-fin base when fins adpressed. Pectoral fin with uppermost two rays unbranched, remaining rays branched, 8th longest, its posterior tip not reaching a vertical through anal-fin origin. Pelvic-fin origin below pectoral-fin base; spine entirely covered with skin; all soft rays branched, 2nd longest, elongate, slightly expanded distally, its tip reaching anus when adpressed. Caudal fin truncate, lightly emarginate; upper lobe with pointed tip, slightly longer than lower lobe; upper lobe with seven unbranched unsegmented procurrent rays, three unbranched segmented rays, and eight branched segmented rays; lower lobe with 6–7 unbranched unsegmented procurrent rays, two or three unbranched segmented rays, and seven branched segmented rays; 20–21 segmented rays in total. Formula for configuration of supraneural bones, anterior neural spines, and anterior dorsal pterygiophores 0/0/0 + 2/1 + 1/1; vertebrae 10 + 14.
Fresh coloration (based on color photographs of all specimens when fresh) (Figures 6A,B): body orange to pinkish, gradually whitish ventrally; about five irregular, broad slightly darker orange bands extending from dorsal profile to about midbody, located between the 4th dorsal-fin spine and caudal-fin base, each band with contrasting reddish orange, longitudinally rectangular blotch and melanophores, height of about one scale, at or slightly above lateral midline; about nine irregular reddish orange blotches along ventral portion of body from pectoral-fin axil to caudal-fin base. Background coloration of head similar to body; upper lip orange to pinkish with a dark blurry spot on snout, anterior to anterior orbit rim; maxilla generally dusky white with irregular yellow to reddish orange pattern at anterior tip, middle of anterior tip, posterior edge of maxilla, and premaxilla; lower jaw orange to pinkish with two dark blotches on tip, then an irregular orange spot at mid jaw. Cheek orange to pinkish with a white posteroventral corner; opercle orange to pinkish red above with white ventrally, large ocellated red spot with pinkish white border present between opercular spines. Spinous dorsal-fin membrane translucent whitish with two longitudinal rows of yellow spots, one at about one-third length of rays, another at about two-thirds length of rays, orange to pinkish pattern around fin rays or slightly extended to membrane, and diffuse scattered dark melanophores around fin rays; soft dorsal-fin membrane translucent with numerous small yellow spots scattered over fin in irregularly diagonal row. Anal fin dusky white with longitudinal yellow band distally, narrow translucent margin and about three (paratype) small yellow spots scattered on soft anal-fin membrane. Pectoral-fin with pale yellowish tint, small ambiguous orange to red blotch basally on middle rays. Pelvic fin with bright white or yellow tint. Caudal fin dusky with about three irregular yellow streaks distally, a longitudinal row of three yellow spots (height about 1–2 scales) at middle fin, and about two columns of irregular red streaks of different heights on upper and lower lobes of basal portion of fin membrane.
Preserved coloration (based on all examined specimens) (Figure 6C): head and body tan, scales on nape and along dorsal quarter of body with dark distal margins; about five rectangular dark blotches at or slightly above lateral line usually retained. Small dark blotch on base of 1st dorsal-fin spine.
Distribution
Chelidoperca microdon is a rare and deep-water species, known from two specimens collected at depths ranging 260–305 m on the Chesterfield Plateau, Coral Sea, and on the continental shelf around the Isle of Pines New Caledonia (Figure 1).
Etymology
The name microdon is derived from Latin and means small-toothed in reference to it lacking enlarged canines on both jaws, especially compared with C. tosaensis, a species with similar morphology.
Comparisons
C. microdon can be distinguished from C. hirundinacea, C. lecromi, and C. pleurospilus by having three scale rows between the lateral line and middle of the dorsal-fin base [(Matsunuma et al., 2018); this study: Supplementary Figure 1C]. Among the species having three scale rows in that portion, C. microdon can be distinguished from other congeners except C. tosaensis in lacking scales on the dentary. In meristics, C. microdon differs from C. tosaensis in having three full-sized scales between the lateral line and the middle of the spinous dorsal fin (vs. three half-sized dorsal-most scales in C. tosaensis) (Supplementary Figures 1C,D) and less developed gill rakers on the lower gill arch (9 vs. 6–8 in C. tosaensis) (Table 2). C. microdon pigmentation differs from C. tosaensis in lacking a longitudinal row of white spots on the body (vs. a longitudinal row of about 10–12 white spots along the lateral line in C. tosaensis), 8–9 irregular yellowish to reddish blotches along the ventral portion of body between the posterior part of the pectoral-fin and caudal-fin base (vs. usually 10 irregular yellowish to reddish blotches along the ventral portion of the body between the pectoral-fin axil and caudal-fin base), and presents two dark blotches on the tip of the lower jaw (vs. absent) [(Matsunuma et al., 2018): Figure 3; this study: Figure 6, Supplementary Figure 4]. C. microdon is not closely related to any known Chelidoperca spp. examined in this study; its phylogenetic relationship within the Chelidoperca is still uncertain (Figures 2, 3).
Chelidoperca barazeri sp. nov. (Figure 7; Supplementary Figures 1A, 2A,B, 3A; Tables 3, 7)
Previously Referred to as Chelidoperca sp. 4
Holotype
NTUM15639 (tissue voucher: PNG1830), 33.0 mm SL, sta. CP4262, 2°54′S, 151°07′E, 150–160 m, Gazelle Channel, New Ireland, Bismarck Sea, R/V Alis, French beam trawl, MADEEP expedition, 25 Apr 2014.

Figure 7. Chelidoperca barazeri nsp. from Gazelle Channel, Bismarck Sea (A) NTUM15639 (PNG1830, holotype), collected on 25 Apr 2014; standard length 33.0 mm (photographed by WJC-Lab); (B) NTUM15635 (PNG1831, paratype), collected on 25 Apr 2014; standard length 32.2 mm (photographed by WJC-Lab); (C) same specimen in (A), showing preserved pigmentation (photographed by MYL). Bars = 10 mm.
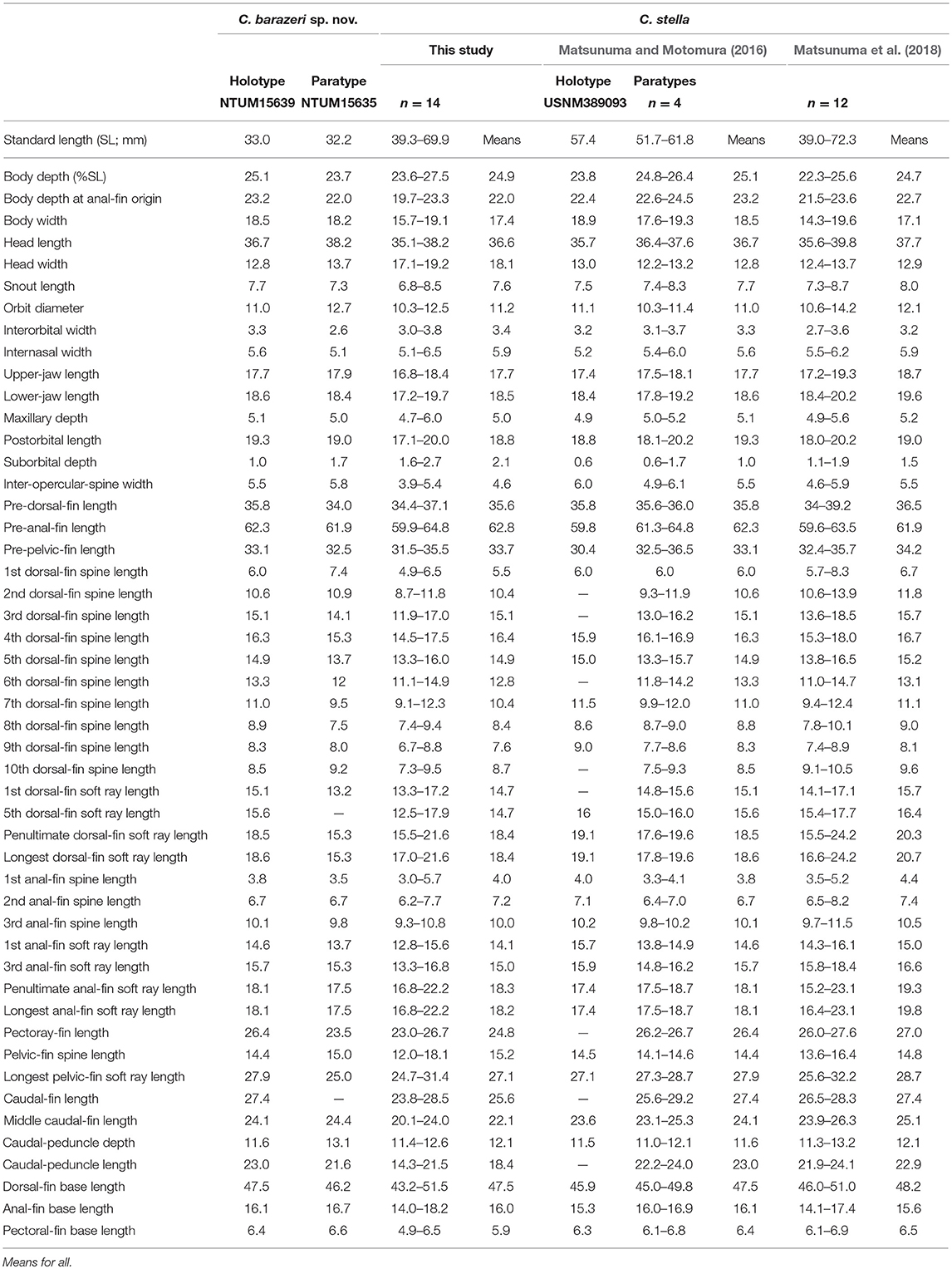
Table 7. Mophometrics expressed as percentages of the standard length from Chelidoperca barazeri sp. nov and C. stella (including examined specimens and reference data).
Paratype
NTUM15635 (tissue voucher: PNG1831), 32.2 mm SL, sta. CP4262, 2°54′S, 151°07′E, 150–160 m, Gazelle Channel, New Ireland, Bismarck Sea, R/V Alis, French beam trawl, MADEEP expedition, 25 Apr 2014.
ZooBank registration
urn:lsid:zoobank.org act:8CA3E1B1-C4A9-4097-ACFC-535BC5 E80D45.
GenBank registration of the holotype
MK988040 (COI).
Diagnosis
Chelidoperca barazeri is characterized by a combination of the following morphological characters: 35–37 pored lateral line scale rows; 3 (dorsal-most scales half-sized) scale rows between lateral line and middle of spinous dorsal fin base; eight scale rows below lateral line; preopercular serrae 19–21; interopercular serrae 3–5; subopercular serrae 11–13; dentary naked, without scales; scale rows on interorbital area reach or slightly extend beyond midorbit level; opercular spine present; large red ocellus spot; four red blotches with dark melanophores midlaterally; clear red stripe from lower eye to ventral corner of cheek. Along the COI gene, the following apomorphic sites have unique nucleotides shared by the two specimens of C. barazeri examined so far; these nucleotide sites can be used for separating the species from other congeners. Nos. 279 (T vs. A or G), 291 (T vs. A or G), 579 (G vs. A or C), 606 (T vs. A or G), 624 (G vs. A).
Description
Morphometric and selected meristic values are summarized in Tables 3, 7. Dorsal-fin rays X, 10; anal-fin rays III, 6; pectoral-fin rays 15; pelvic-fin rays I, 5. Body fusiform, slightly elongated; snout round; caudal peduncle moderately long. Orbit large, its dorsal margin included in dorsal contour of head. Mouth large, slightly oblique; posterior margin of maxilla reaching a vertical through posterior margin of pupil; maxilla expanded posteriorly, with low lateral ridge along dorsal margin; lower jaw slightly protruding beyond upper jaw when mouth closed.
Upper jaw with band of 7 (5–7) rows (in anterior portion) of small, sharp-tipped conical teeth, tooth band becoming narrow posteriorly, outermost row of teeth enlarged, anteriorly projecting antrorse canines (Supplementary Figure 3A); lower jaw with band of about 6 (5–6) rows (in anterior portion) of small, sharp-tipped conical teeth, innermost and outermost rows of teeth have enlarged canines, band of small teeth narrowing posteriorly; vomer with V-shaped band of about five rows of small conical teeth; palatine with relatively long band of four rows of small, sharp-tipped conical teeth. Anterior nostrils situated at middle of snout, with small rounded flap rising from posterior rim; posterior nostril with elliptical opening at anterior border of orbit.
Posterior margins of preopercle, interopercle, and subopercle finely serrated, serrae on preopercle 21 on both sides (19–21), interopercle five (four on the right side) (3–5); serrae on subopercle about 13 (11 on the right side) (11–13), relatively weaker. Opercle with two flat, prominent spines, upper spine slightly longer than lower. Posttemporal with two serrae on both sides, tips at beginning of lateral line.
Body covered with ctenoid scales; lateral line slightly arched, gradually descending over pectoral fin, terminating at caudal-fin base. Uppermost row of body scales along dorsal-fin base about half of adjacent lower body scale size. Caudal-fin base covered with ctenoid scales, extending onto fin over about half of basal length (based on paratype) of fin. Pectoral fin with ctenoid basal scales, small elongate cycloid scales extending onto fin ventrally. Basal scales absent on either dorsal fin or anal fin. Pelvic-fin base covered with small elongate cycloid scales, but not extending onto fin. Head generally covered with scales, snout and maxilla naked; scales on ventral surface of lower jaw present on angular, dentary naked (Supplementary Figure 2B); interopercle and subopercle with both ctenoid and cycloid scales; interorbital region with single row of cycloid scales, reaching or slightly extending beyond midorbit level, but not extending to anterior margin of orbit (Supplementary Figure 2A). A pair of interorbital canals with numerous small pores along outer margin of interorbital region. Lower jaw with around four pores anteriorly on each side of dentary symphysis, followed on each side by two pore positions along dentary sensory canal, each position with single pore, and a fourth slit-like or rounded pore at angular–dentary junction.
Dorsal-fin origin above pectoral-fin base, 4th spine longest, 1st spine shortest; all soft rays branched, subequal in length. Anal-fin origin below base of 1st dorsal-fin soft ray, 3rd spine longest; all soft rays branched, 5th ray longest. Posterior tip of dorsal and anal fins only reaching a vertical through caudal-peduncle base when fins adpressed. Pectoral fin with uppermost two rays unbranched, remaining rays branched, 8th (9th in paratype) longest, its posterior tip reaching a vertical through anal-fin origin. Pelvic-fin origin below pectoral-fin base; spine covered with skin; all soft rays branched, 2nd longest, elongate, slightly expanded distally, its tip reaching anus when adpressed. Caudal fin emarginate; tip broken on both upper and lower lobe; upper lobe with seven unbranched unsegmented procurrent rays, three unbranched segmented rays, and eight branched segmented rays; lower lobe with 6–7 unbranched unsegmented procurrent rays, 2–3 unbranched segmented rays, and eight branched segmented rays; 19–20 segmented rays in total.
Formula for configuration of supraneural bones, anterior neural spines, and anterior dorsal pterygiophores 0/0/0 + 2/1 + 1/1; vertebrae 10 + 14.
Fresh coloration (based on color photographs of all specimens when fresh) (Figures 7A,B): body reddish pink, becoming whitish ventrally; four irregular, broad, slightly darker pinkish red bands extend from dorsal profile to about midbody, each band with contrasting dark red, longitudinally rectangular blotches (height of about 4–5 scale) with melanophores above lateral line; about 7–8 irregular yellowish orange to orange blotches along ventral portion of body from anus to base of caudal fin. Background coloration of head similar to body; lips of upper-jaw and lower-jaw yellowish-pink; snout orange; one red stripe extends from suborbital region along the dorsal margin of maxilla to ventral corner of cheek along anteroventral margin of cheek; yellow mark present on suborbital rim; color patterns of cheek and opercle generally same as head. Opercle reddish pink with large red ocellus located between opercular spines.
Spinous dorsal-fin membrane translucent white, with several rows of yellow spots; soft dorsal-fin membrane translucent, with yellow distal margin and yellow spots scattered over fin in irregularly diagonal rows. Anal fin uniformly dusky yellow. Pectoral fin translucent yellow, an unclear yellowish-orange blotch basally on middle rays. Pelvic fin generally light yellow. Caudal fin generally translucent, with a transverse yellow to brown band on caudal-fin base and irregular diagonal rows of dark spots over fin.
Preserved coloration (based on all examined specimens) (Figure 7C): head and body tan, melanophores of red blotches still observable.
Distribution
Chelidoperca barazeri is a rare and relatively shallow water species, known only from two specimens collected at depths ranging 150–160 m on seamounts and ocean banks off NW New Ireland, Bismarck Sea (Figure 1).
Etymology
This species is named barazeri for honor of Mr. Jean-François Barazer, the captain of R/V Alis. He is an expert in organizing trawling operations, deep-sea biodiversity surveys, and cruise arrangements. Without his support and great efforts, the discovery of new species in many studies including ours carried out through the TDSB program would not be possible.
Comparisons
C. barazeri can be distinguished from C. hirundinacea, C. lecromi, C. microdon, and C. pleurospilus by having three scale rows between the lateral line and middle of the dorsal-fin base (Matsunuma et al., 2018; this study: Supplementary Figure 1A). Among the species having three scale rows in that region, C. barazeri can be distinguished from other congeners except C. stella by having much fewer pored lateral-line scales (35–37) (Table 3). In meristics, C. barazeri differs with C. stella by having fewer preopercular serrae (19–21 vs. 29–44 in C. stella), interopercular serrae (3–5 vs. 6–14 in C. stella), and subopercular serrae (11–13 vs. 16–39 in C. stella). In pigmentation, C. barazeri differs from C. stella in having four midlateral red blotches with dark melanophores above the lateral line (vs. three narrow red stripes midlaterally with two longitudinal rows of white spots in C. stella), a red stripe from the lower eye to the ventral corner of the cheek (vs. a uniformly red background from eye to cheek in C. stella), and a clear red ocellus located between the opercular spines (vs. lacking a clear ocellus or blotch in C. stella). Except for those distinguishing characters, the dentary of C. barazeri is naked without scales (vs. at least two rows of cycloid scales covered in C. stella). C. barazeri is sister to C. tosaensis (Figures 2, 3); the two species can be separated from each other by meristic characters and body pattern. Compared to C. tosaensis, C. barazeri has fewer pored lateral-line scales (35–37 vs. 37–42), fewer scale rows above the lateral line (9 vs. 10–12), and fewer cheek scale rows (4 vs. 4–7). C. barazeri also differs from C. tosaensis by lacking white spots on the body (a series of longitudinal white spots along the lateral line are present in C. tosaensis) and by having a red stripe from the lower eye to the ventral corner of the cheek (Figure 7).
Remarks
Morphometric characters usually display an ontogenetic variation among size and life stages in species of Chelidoperca (Matsunuma and Motomura, 2016; Matsunuma et al., 2018). C. barazeri is defined from only two specimens at the sub-adult stage, so morphometric data might present a slightly different range than the adult. A redescription of this species based on adult specimens is required, and can be resolved with more samples from additional surveys in the region. Matsunuma et al. (2018) pointed out that the number of serrae increases with growth and usually overlap at similar sizes. However, C. barazeri (32.2–33.0 mm SL) is still different from C. stella (39.0–72.3 mm SL) in having fewer, non-overlapping numbers of serrae. The numbers of serrae present a distinguishable difference between C. barazeri and C. stella.
Chelidoperca lecromi (Fourmanoir, 1982) (Figure 4; Supplementary Figures 2G,H; Tables 4, 8)
Chelidoperca lecromi (Fourmanoir, 1982): 63, Figure 4 (Chesterfield Island, Coral Sea, 19°40′S, 158°31′E, 300 m depth. Holotype: MNHN 1981–1436). Fricke et al. (2011): 384 (record type specimens in regional checklist). Bineesh et al. (2014): 117 (listed in introduction). Williams and Carpenter (2015): 287 (listed and compared with C. santosi). Matsunuma and Motomura (2016): 388 (listed and compared with C. stella). Matsunuma et al. (2018): 210 (listed and compared with C. tosaensis).
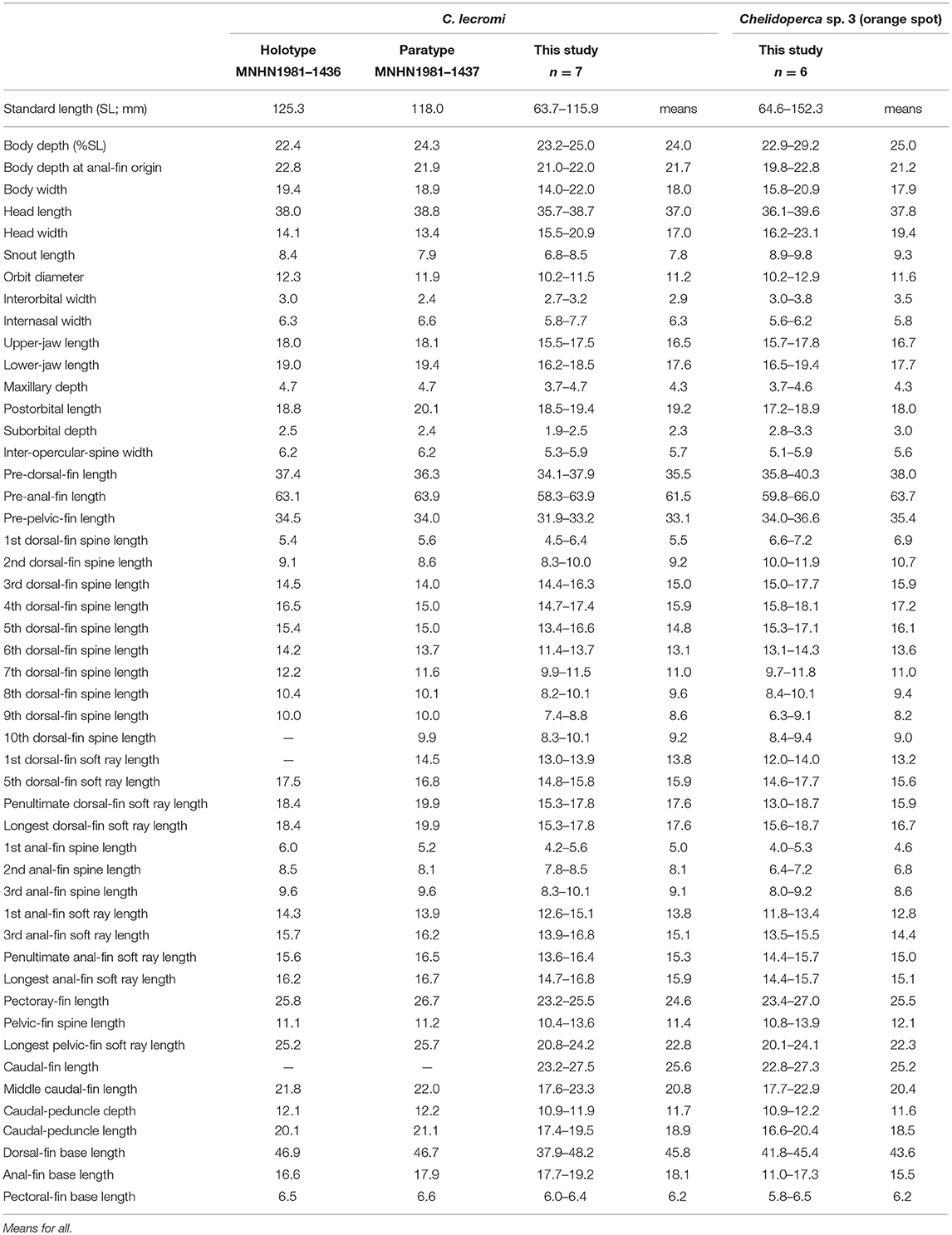
Table 8. Mophometrics expressed as percentages of the standard length from Chelidoperca lecromi (including examined specimens and references data) and Chelidoperca sp. 3 (orange spot).
Holotype
MNHN 1981–1436, 125.3 mm SL, Chesterfield Island, Coral Sea, New Caledonia (19°40′N, 158°31′E), 300 m.
Paratype
MNHN 1981–1437, 118.0 mm SL, collected with holotype.
Other Examined Specimens (All From New Caledonia)
Seven specimens (64.3–114.1 mm SL): NTUM13739 (tissue vouchers: NC1448–1450, NC1452–1454), six specimens, 63.7–115.9 mm SL, sta. CP5032, 19°51′S, 158°29′E, 260–300 m, Chesterfield Plateau, Coral Sea, R/V Alis, French beam trawl, KANADEEP expedition, 22 Sep 2017. NTUM15631 (tissue voucher: NC243), 96.0 mm SL, sta. CP4673, 22°47′S, 167°27′E, 244–285 m, off Isle of Pines, New Caledonia, Coral Sea, R/V Alis, French beam trawl, KANACONO expedition, 13 Aug 2016.
Diagnosis
Chelidoperca lecromi is characterized by a combination of the following morphological characters: 44–45 pored lateral line scale rows; four (dorsal-most scale full-sized) scale rows between lateral line and middle of spinous dorsal fin base; relatively fewer scale rows 8–9 on cheek; scale rows absent on ventral side of dentary; scale rows on interorbital area extend beyond level of mid-orbit but not reaching posterior nasal pores; no dark stripe or blotch on body; numerous small yellow spots scattered on dorsal fin and middle caudal fin. Along the COI gene, the following apomorphic sites have unique nucleotides shared by the seven specimens of C. lecromi examined so far; these nucleotide sites can be used for the diagnosis of the species to other congeners. Nos. 186 (A vs. C), 543 (A vs. G, C or T), 558 (T vs. A or G).
Description
Following description based on seven NTUM specimens from New Caledonia. Morphometric and selected meristic values summarized in Tables 4, 8. Dorsal-fin rays X, 10; anal-fin rays III, 6; pectoral-fin rays 15; pelvic-fin rays I, 5. Body fusiform, slightly elongated; snout round; caudal peduncle moderately long. Orbit large, its dorsal margin included in dorsal contour of head. Mouth large, slightly oblique; posterior margin of maxilla slightly extending a vertical through mid-orbit; maxilla expanded posteriorly, with low lateral ridge along dorsal margin; lower jaw slightly protruding beyond upper jaw when mouth closed.
Upper jaw with band of 6–7 rows (in anterior portion) of small, sharp-tipped conical teeth, tooth band becoming narrow posteriorly, outermost row of teeth enlarged, anteriorly projecting antrorse canines; lower jaw with band of about 4–5 rows (in anterior portion) of small, sharp-tipped conical teeth, innermost and outermost rows of teeth with enlarged canines, band of small teeth narrowing posteriorly; vomer with V-shaped band of about four rows of small conical teeth, posterior teeth canine-like; palatine with relatively long, narrow band of two rows of small, sharp-tipped conical teeth. Anterior nostrils situated at middle of snout, with small rounded flap rising from posterior rim; posterior nostril with elliptical opening at anterior border of orbit.
Posterior margins of preopercle, interopercle, and subopercle finely serrated, serrae on preopercle 32 (unavailable on right side) (19–32), interopercle 11 (NA) (4–11), and subopercle 13 (NA) (9–15). Opercle with two flat, prominent spines, upper spine slightly longer than lower. Posttemporal with 3 (NA) (1–4) serrae tips at beginning of lateral line, number of serrae increasing with growth.
Body covered with ctenoid scales; lateral line slightly arched, gradually descending over pectoral fin, terminating at caudal-fin base. Uppermost row of body scales along dorsal-fin base always similar in size to adjacent lower body scales; tiny and irregularly spaced small scales sometimes present at bases of spines. Caudal-fin base covered with ctenoid scales, extending onto fin over about half of basal length of fin. Pectoral fin with ctenoid basal scales, small elongate cycloid scales extending onto fin. Basal scales absent on either dorsal fin or anal fin. Head generally covered with scales, snout and maxilla naked; scales on ventral surface of lower jaw present on angular, but not extending onto dentary (Supplementary Figure 2H); opercle, interopercle, and subopercle with both ctenoid and cycloid scales; interorbital scales extend from the level of mid-orbit to around the level of anterior pupil rim, but not reaching the level of posterior nasal pores (Supplementary Figure 2G). A pair of interorbital canals with numerous small pores along outer margin of interorbital region.
Dorsal-fin origin above pectoral-fin base, 4th spine longest, 1st spine shortest; all soft rays branched, 9th ray longest. Anal-fin origin below base of 1st dorsal-fin soft ray, 3rd spine longest; all soft rays branched, 5th ray longest. Posterior tip of dorsal fin reaching a vertical through caudal-fin base when fins adpressed; that of anal fin not reaching a vertical through caudal-fin base. Pectoral fin with uppermost two rays unbranched, remaining rays branched, 9th longest, its posterior tip reaching a vertical through anus. Pelvic-fin origin below pectoral-fin base; spine covered with skin; all soft rays branched, 2nd longest, elongate, slightly expanded distally, its tip not reaching anus when adpressed. Caudal fin emarginate; upper lobe with elongate tip, slightly longer than lower lobe.
Fresh coloration (based on color photographs of all NTUM specimens when fresh) (Figures 4B,C): body orange to pinkish, gradually whitish ventrally; about six irregular, broad orange bands extending from dorsal profile to about midbody, the last two bands close to each other; about seven irregular yellowish to reddish blotches along ventral portion of body; a longitudinal yellow stripe (covering about 1–2 scales) under and through lateral line from posterior orbit rim to caudal fin. Ground coloration of head similar to body; tips of upper-jaw and lower-jaw pinkish; snout yellowish to pinkish; a yellow mark at ventral orbit rim and a yellow mark at posterodorsal margin of maxilla; cheek yellow to pinkish; posterior margin of preopercle white; opercle yellow to pinkish above with white ventrally. Spinous dorsal-fin membrane translucent yellowish scattered entirely with numerous yellow to red spots; soft dorsal-fin membranes translucent yellowish scattered with numerous yellow spots. Anal fin whitish, with a longitudinal yellow stripe present mid-distally, with translucent white margin. Pectoral fin translucent yellow. Pelvic fin generally white with pale yellow anterior margin. Caudal fin translucent yellow with white distal margin and tips of both lobes; numerous small yellow spots scattered centrally.
Preserved coloration (based on all examined specimens) (Figure 4A): head and body tan or cream white, most markings disappear; small dark spots scattered on dorsal fin and middle caudal fin sometimes retained in large specimens.
Distribution
Chelidoperca lecromi is a deep-water species known from nine examined specimens taken in depths ranging 244–300 m by bottom trawl on seamounts at Chesterfield Plateau, Coral Sea, and on the continental shelf off Isle of Pines, New Caledonia (Figure 1).
Comparisons
Chelidoperca lecromi possesses four scale rows between the lateral line and the base of middle dorsal-fin spinous portion, and distinguished from the following congeners with three scale rows in that area: C. africana, C. barazeri, C. investigatoris, C. leucostigmata, C. maculicauda, C. margaritifera, C. microdon, C. occipitalis, C. santosi, C. stella, and C. tosaensis (Bineesh et al., 2013; Williams and Carpenter, 2015; Matsunuma, 2016; Matsunuma and Motomura, 2016; Matsunuma et al., 2018; this study). Among the species having four scale rows in that area, C. lecromi can be readily distinguished from remaining congeners by coloration (reddish body with a large dark blotch in C. hirundinacea, and a longitudinal row of dark blotches on the mid-body in C. pleurospilus) (this study). Furthermore, C. lecromi can be also distinguished from C. hirundinacea by having interorbital scales not reaching the level of posterior nasal pores (vs. reaching in the latter) and scales on the ventral side of lower jaw restricted to the angular (vs. extending onto the dentary) (this study). Chelidoperca lecromi is sister to Chelidoperca sp. three (orange spots) (undescribed species confirmed herein; see below) (Figures 2, 3); they are similar in overall body appearance, including morphometrics, meristics, and coloration. However, the latter is characterized by having diagnostic orange spots on the pectoral- and caudal-fin bases and the apomorphic and unique nucleotides at the COI locus shared by the five specimens of the species examined so far: no. 126 (C vs. T). Otherwise, the two species display an average genetic divergence of 4.43% at the COI locus.
Remarks
Chelidoperca lecromi was originally described by Fourmanoir (1982) based on the holotype (Figure 4A) and single paratype from off Chesterfield Island, Coral Sea. Although the species has not been recorded since the original description, the present six specimens newly collected from the type locality and a single specimen from Isle of Pines were identical to that species. Photographs of fresh specimens revealed the fresh coloration of the species for the first time (Figure 4).
Diagnostic Key to Species of Chelidoperca in the West Pacific
1a. Pored lateral line scale rows 33–37 (usually 34–35) . . . . . . . . 2
1b. Pored lateral line scale rows 37–46 (usually ≥38) . . . . . . . . . . 3
2a. Two rows of cycloid scales on dentary, preopercular serrae 29–44; interopercular serrae 6–14, subopercular serrae 16–39, two rows of white blotches along ventral body midlaterally . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Chelidoperca stella
2b. Dentary without scales, preopercular serrae 19–21; interopercular serrae 3–5, subopercular serrae 11–13; no white blotches along body . . . . Chelidoperca barazeri (sp. 4)
3a. Three scale rows (dorsal-most scale in full- or half-size) between the lateral line and the middle of the spinous dorsal-fin base . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
3b. Four scale rows between the lateral line and the middle of the spinous dorsal-fin base . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
4a. Dentary without scales, a large red ocellus at opercular spines area . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
4b. At least one scale row on dentary, lacking red ocellus at opercular spines area . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
5a. Pored lateral line scale rows 37–42 (usually 38–40), three (dorsal-most scale half-sized) scale rows between the lateral line and the middle of the spinous dorsal-fin base, 10–12 white spots along lateral line, usually 10 irregular yellowish to reddish blotches start at pectoral-fin axil along ventral portion of body . . . . . . . . . . . . . . . . . . . . . . . . . Chelidoperca tosaensis
5b. Pored lateral line scale rows 42, three (dorsal-most scale full-sized) scale rows between the lateral line and middle of the spinous dorsal-fin base, no white spots along the lateral line, usually 8–9 irregular yellowish to reddish blotches start at posterior part of pectoral-fin, along ventral portion of body . . . . . . . . . . . . . . . . . . . . . . . . . . . . Chelidoperca microdon (sp. 2)
6a. Outermost row of canines not significantly enlarged on upper jaw, scale rows below lateral line eight, a yellow stripe along the ventrolateral body from the posterior opercular tip to caudal peduncle . . . . . . . . . . . Chelidoperca margaritifera
6b. Outer row has enlarged canines on upper jaw, scale rows below lateral line 10–13, no yellow stripe along body . . . . . . 7
7a. A series of longitudinal white spots along lateral line, 5–6 cheek scale rows, no black spots on snout . . . . . . . . . . . . . . . . . . . . . . . Chelidoperca leucostigmata (sp. 1)
7b. No white spots along lateral line, 6–7 cheek scale rows, two black spots present on each side of snout . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Chelidoperca santosi
8a. Several black blotches along the body midlaterally, anteriormost interorbital scale not reach the middle point of orbit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Chelidoperca pleurospilus
8b. No black blotches along the body midlaterally, anteriormost interorbital scale extend over the middle point to anterior margin of orbit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
9a. Multiple interorbital scale rows, anteriormost scale extend over anterior margin of orbit, usually reach the anterior nostril, dentary covered by at least one row of scales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Chelidoperca hirundinacea
9b. Single interorbital scale row, anteriormost scale only reaches the anterior margin of orbit, dentary naked without scales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
10a. Clear orange spot located at middle area of pectoral-fin and caudal-fin base, dorsal-fin yellow with a white stripe, caudal-fin yellow with white margin . . . . . . . . . . . . . . . . . . Chelodoperca sp. three (orange spot)
10b. No orange spot at pectoral- and caudal-fin base, dorsal-fin translucent white with numerous small yellow spots scattered through fin, caudal-fin translucent white with several rows of yellow spots in middle area . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Chelodoperca lecromi
Concluding Remarks
Records of misidentification could lead to erroneous information on species distribution data and therefore mislead our understanding of the biodiversity pattern and evolution of the species (Hung et al., 2017). In Chelidoperca, for instance, the name C. “margaritifera” has repeatedly appeared in the literature along with regional species records or checklists (e.g., in Katayama, 1984; Yamada et al., 2007; Fricke et al., 2011; Senou, 2013). With detailed morphological examinations of types and other voucher specimens of available Chelidoperca species deposited in museums, Matsunuma et al. (2018) resolved this long-standing taxonomic issue and reported that all extralimital specimens previously identified as C. margaritifera should refer to C. santosi, C. stella, or C. tosaensis. Although recent advances in the systematics of Chelidoperca have been made (Williams and Carpenter, 2015; Matsunuma and Motomura, 2016; Iwamoto and Wirtz, 2018; Matsunuma et al., 2018), a more efficient workflow to further investigate the diversity and resolve taxonomic issues is still required, especially when confronted with limited examinable materials and insufficient information on species identification keys. In this study, we adopted an integrated approach including the recently developed DNA-based analyses to increase the pace and rigor of our biodiversity assessments and hypothesis testing on species validity. Our sampling included 65 specimens freshly caught mainly during eight expeditions organized under the TDSB program and its joint TFDeepEvo project, and is the most comprehensive biodiversity survey of this group of fishes. Samples were collected from several limited access areas, for instance the Macclesfield Bank in the South China Sea and Chesterfield Islands and seamount chain on the Lord Howe Rise in the Coral Sea. Our results reveal six previously unknown species, among which three, C. microdon, C. leucostigmata, and C. barazeri, are formally described here. The number of nominal species in Chelidoperca now totals 14, all of which are regarded as valid. The results of our investigation also allow us to depict more precisely the biogeographic pattern of several previously recognized species.
Chelidoperca stella is possibly the shallowest occurring species of Chelidoperca. It was originally described on the basis of specimens collected from the Andaman Sea, northeastern Indian Ocean, at depths of 58–66 m (Matsunuma and Motomura, 2016) and the North-West Pacific and South China Sea (Matsunuma et al., 2018). With the new data obtained in this study, we confirm its existing records of species distribution and further indicate its range extends to the waters around New Caledonia and Vanuatu (depths: 77–120 m), which are located in the tropical Australia and Coral Sea region (TA+CS) (Figure 1; Supplementary Table 1). However, an obvious phylogeographic break within the West Pacific for the species is observed from our molecular analyses (Figure 2), which implies a restriction of continued gene flow between TA+CS and NWP populations. This is similarly observed for two other species, C. hirundinacea and C. pleurospilus (Figure 2).
Chelidoperca tosaensis is another species of Chelidoperca that may occur in relatively shallower waters (60–302 m) (Matsunuma et al., 2018). The species was first described on the basis of specimens from the NWP region. Here, five samples identified as C. tosaensis were newly caught on seamounts and ocean banks at depths of 133–197 m off NW New Ireland in the Bismarck Sea. Another poorly preserved specimen (EXB607) found in the New Caledonian water might belong to the species (no tissue sample preserved for the molecular validation) (Supplementary Table 1). This indicates a wider species distribution ranging from NWP to tropical West Pacific (TWP) (Figure 1). However, no clear phylogeographic pattern is observed in this species; the average genetic distance within the species is only 0.5%.
Chelodoperca lecromi is a rare species of Chelidoperca and was thought to be restricted to the waters off the Chesterfield Islands in the Coral Sea (Fourmanoir, 1982; Fricke et al., 2011). Actually, no additional specimens have been collected since the species was described. However, a photograph of an adult-sized C. lecromi specimen from American Samoa was provided by Randall (unpublished) from the author's unpublished sources. The photograph is available at http://www.fishbase.org/photos/PicturesSummary.php?ID=56308&what=species. By examining its coloration, it is unlike C. lecromi or any other known Chelidoperca species. In this study, we collected six specimens from the Chesterfield Plateau, Lord Howe Rise, close to the type locality of the species, and one additional specimen from off the Isle of Pines, SE New Caledonia. Based on these updated data, the species distribution range is wider than previously thought but still restricted to the EEZ of New Caledonia. Its range (geographically and bathymetrically) overlaps with one of the new species, C. microdon, that we discovered in this study. It is also worthy to note that another new species, which is the sister species of C. lecromi, Chelidoperca sp. three (orange spot), is also recorded in the EEZ of New Caledonia (Figure 1). However, these two sibling species do not share the same habitats, as the latter specimens were collected on seamounts Argo, Kelso, and Capel, which are located on the southern half of the Lord Howe Rise, and from Gifford Guyot at Norfolk Ridge. Oceanic topography may impact the distribution and speciation of these two sibling species.
Data Availability
The datasets generated for this study can be found in NCBI GenBank, under accession numbers: MK988034-MK988093.
Ethics Statement
This research was performed at National Taiwan University (NTU) in accordance with NTU's guidelines regarding animal research. As this project did not involve experiments on live fish, no ethics statement was required. Most of the specimens examined in the present study were from museum specimens collected during exploratory cruises. A few samples were from local fish landing sites. See details in materials and methods, and acknowledgments.
Author Contributions
W-JC led the project including the search of funding support for completing this study. W-JC and M-YL contributed to the conception, the design of the work, and collection of the samples. S-HL generated most of the data and did the analyses. S-HL, M-YL, MM, and W-JC interpreted the results and wrote the paper.
Funding
This research was supported by research funding from the Ministry of Science and Technology, Taiwan (MOST 102-2923-B-002-001-MY3 and MOST 107-2611-M-002-007- to W-JC; MOST 107-2119-M-001-048 [SCSMART]) and the French National Research Agency (ANR 12-ISV7-0005-01 to Sarah Samadi).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the participants of the oceanography expeditions (AURORA 2007, BIOPAPUA, EXBODI, MADEEP, KAVIENG 2014, ZhongSha 2015, KANACONO, and KANADEEP 2017), crews of the R/V Alis and Ocean Researcher 1 under the Tropical Deep- Sea Benthos program and its joint research project entitled Taiwan France marine diversity exploration and evolution of deep-sea fauna (TFDeepEvo) supported by French ANR and Taiwanese MOST (Principal Investigators: S. Samadi and W-JC). The Papua New Guinea expeditions were also supported from Papua New Guinea's National Fisheries Authority. They were conducted under a Memorandum of Understanding with the University of Papua New Guinea (UPNG) and a permit from the Papua New Guinea Department of Environment and Conservation (DEC). We also thank all the members in the marine biodiversity and phylogenomics lab, IONTU and NTUM, especially H.-C. Lin and J.-N. Chen, for specimen management and assistance in laboratory work/analysis, A. Ogino (Kindai University) for providing morphological data on her examined specimens of Chelidoperca, Dettaï (MNHN) for providing her unpublished sequences, Patrice Pruvost (MNHN) for providing catalog number of type specimens.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2019.00465/full#supplementary-material
Supplementary Figure 1. Scale rows between the lateral line and the middle of the spinous dorsal-fin base in Chelidoperca barazeri sp. nov. (A); C. leucostigmata sp. nov. (B); C. microdon sp. nov. (C); and C. tosaensis (D). Red dotted scales included in scale rows between lateral line and sinous dorsal-fin base; blue dotted scales excluded. F and H indicate full and half-sized scales, respectively. (A) NTUM15639 (PNG1830, holotype), collected on 25 Apr 2014; standard length 33.0 mm (photographed by MYL); (B) NTUM14721 (WJC5843, holotype), collected on 26 Jul 2015; standard length 85.0 mm (photographed by MM); (C) MNHN 2019-0020 (NC577, holotype), collected on 20 Aug 2016; standard length 79.5 mm (photographed by MM); (D) NTUM12346 (PNG3471), collected on 6 Sep 2014; standard length 73.2 mm SL (photographed by MM).
Supplementary Figure 2. Interorbital regions (A,C,E,G) and ventral sides on dentary (B,D,F,H) in Chelidoperca barazeri sp. nov. (A,B); C. leucostigmata sp. nov. (C,D); C. microdon sp. nov. (E,F); and Chelidoperca lecromi (G,H). Red bar, mean of the extended position of the anterior-most interorbital scale row. (A,B) NTUM15639 (PNG1830, holotype), collected on 25 Apr 2014; standard length 33.0 mm (photographed by MYL); (C,D) NTUM14721 (WJC5843, holotype), collected on 26 Jul 2015; standard length 85.0 mm (photographed by MM); (E) MNHN 2019-0020 (NC577, holotype), collected on 20 Aug 2016; standard length 79.5 mm (photographed by MM); (F). NTUM15636 (NC1451, paratype), collected on 20 Aug 2016; standard length 93.5 mm (photographed by MM); (G) NTUM13749 (NC1449), collected on 22 Sep 2017; standard length 90.6 mm (photographed by MM); (H) NTUM13749 (NC1452), collected on 22 Sep 2017; standard length 102.3 mm (photographed by MM).
Supplementary Figure 3. Teeth on both jaws in Chelidoperca barazeri sp. nov. (A); C. leucostigmata sp. nov. (B); C. microdon sp. nov. (C); and C. tosaensis (D). (A) NTUM15639 (PNG1830, holotype), collected on 25 Apr 2014; standard length 33.0 mm (photographed by MYL); (B) NTUM14721 (WJC5843, holotype), collected on 26 Jul 2015; standard length 85.0 mm (photographed by MM); (C) MNHN 2019-0020 (NC577, holotype), collected on 20 Aug 2016; standard length 79.5 mm (photographed by MM); (D) NTUM12346 (PNG3471), collected on 6 Sep 2014; standard length 73.2 mm SL (photographed by MM).
Supplementary Figure 4. Pigmentation on the tips of the lower jaw of C. microdon sp. nov. (A) and C. tosaensis (B). (A) MNHN 2019-0020 (NC577, holotype), collected on 20 Aug 2016; standard length 79.5 mm (photographed by MM); (B) NTUM12346 (PNG3471), collected on 6 Sep 2014; standard length 73.2 mm SL (photographed by MM).
Supplementary Table 1. Taxa examined in this study, sample localities and depths (if known), corresponding accession numbers to tissues/vouchers (if any), and corresponding GenBank accession numbers for gene sequences (new sequences highlighted in bold).
Supplementary Data 1. Raw data from morphological examination of specimens of Chelidoperca spp. used in this study.
References
Alcock, A. W. (1890). Natural history notes from H. M. Indian marine survey steamer ‘Investigator', Commander R. F. Hoskyn, R. N., commanding. No. 16. On the bathybial fishes collected in the Bay of Bengal during the season 1889–1890. Ann. Mag. Nat. Hist. 33, 197–222. doi: 10.1080/00222939008694027
Anderson, W. D. Jr., and Heemstra, P. C. (2012). Review of Atlantic and eastern Pacific anthiine fishes (Teleostei: Perciformes: Serranidae), with descriptions of two new genera. Trans. Am. Phil. Soc. 102, 1–73.
Bineesh, K. K., Akhilesh, K. V., Abdussamad, E. M., and Pillai, N. G. K. (2013). Chelidoperca maculicauda, a new species of perchlet (Teleostei: Serranidae) from the Arabian Sea. Aqua. 19, 71–78.
Bineesh, K. K., Akhilesh, K. V., Abdussamad, E. M., Pillai, N. G. K., Thiel, R., Jena, J. K., et al. (2014). Redescriptions of Chelidoperca investigatoris (Alcock, 1890) and Chelidoperca occipitalis Kotthaus, 1973 (Perciformes: Serranidae) from the south-west coast of India. Indian J. Fish. 61, 117–122.
Bouchet, P., Héros, V., Lozouet, P., and Maestrati, P. (2008). A quarter-century of deep-sea malacological exploration in the South and West Pacific: where do we stand? How far to go? Mém. Mus. Natl. Hist. Nat. 196, 9–40.
Boulenger, G. A. (1895). Catalogue of the Perciform Fishes in the British Museum. 2nd Edn. Vol. 1. Containing the Centrarchidae, Percidae, and Serranidae (Part). London: British Museum.
Cadenat, J. (1960). Notes d'Ichtyologie oust-africaine XXX.–poissons de mer ouest-africains observés du Sénégal au Cameroun et plus spécialement au large des côtes de Sierra-Leone et du Ghana. Bull. de l'Inst. Fran. Afr. Noire. Sér A. Sci. Nat. 22, 1358–1423.
Chen, W.-J., Santini, F., Carnevale, G., Chen, J.-N., Liu, S.-H., Lavoue, S., et al. (2014). New insights on early evolution of spiny-rayed fishes (Teleostei: Acanthomorpha). Front. Mar. Sci. 1:53. doi: 10.3389/fmars.2014.00053
Costello, M. J., Tsai, P., Wong, P. S., Cheung, A. K. L., Basher, B., and Chaudhary, C. (2017). Marine biogeographic realms and species endemicity. Nat. Commun. 8:1057. doi: 10.1038/s41467-017-01121-2
Crandall, E. D., and Riginos, C. (2014). Magnificent dimensions, varied forms, and brilliant colors: the molecular ecology and evolution of the Indian and Pacific oceans. Bull. Mar. Sci. 90, 1–11. doi: 10.5343/bms.2013.1086
Cuvier, G., and Valenciennes, A. (1831). Histoire Naturelle Des Poissons, Vol. 7. Paris: F. G. Levrault Press
Dayrat, B. (2005). Towards integrative taxonomy. Biol. J. Linn. Soc. 85, 783–791. doi: 10.1111/j.1095-8312.2005.00503.x
Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using bootstrap. Evolution. 39, 783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x
Fourmanoir, P. (1982). Trois nouvelles espe‘ces de serranidae des philippines et de la mer du corail Plectranthias maculatus, Plectranthias barroi, Chelidoperca lecromi. Cybium 6, 57–64.
Fricke, R., Kulbicki, M., and Wantiez, L. (2011). Checklist of the fishes of New Caledonia, and their distribution in the Southwest Pacific Ocean (Pisces). Stuttg. Beitr. Naturk. A Neue Serie 4, 341–463.
Fricke, R., Eschmeyer, W. N., and van der Laan, R. (eds) (2019). Catalog of Fishes: Genera, Species, References. Available online at: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (Electronic version accessed January 2, 2019).
Günther, A. (1880). Report on the shore fishes procured during the Voyage of H.M.S. Challenger in the Years 1873–1876. Zoology 1, 1–82. doi: 10.5962/bhl.title.51598
Hung, K.-W., Russell, B. C., and Chen, W.-J. (2017). Molecular systematics of threadfin breams and relatives (Teleostei, Nemipteridae). Zool. Scr. 46, 536–551. doi: 10.1111/zsc.12237
Iwamoto, T., and Wirtz, P. (2018). A synopsis of the Eastern and Central Atlantic combers of the genus Serranus (Teleostei: Scorpaeniformes: Serranidae). Proc. Calif. Acad. Sci. 65, 1–39.
Katayama, M. (1984). “Chelidoperca margaritifera,” in The Fishes of the Japanese Archipelago, eds H. Masuda, K. Amaoka, C. Araga, T. Uyeno, T. Yoshino (Tokyo: Tokai University), 130.
Kekkonen, M., and Hebert, P. D. (2014). DNA barcode-based delineation of putative species: efficient start for taxonomic workflows. Mol. Ecol. Resour. 14, 706–715. doi: 10.1111/1755-0998.12233
Kimura, M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16, 111–120. doi: 10.1007/BF01731581
Kotthaus, A. (1973). Fische des Indischen Ozeans. des forschungsschiffes ‘Meteor' in der Indischen Ozean, Oktober 1964 bis Mai 1965. A. Systematischer teil, X. Percomorphi (3). Meteor Forschungsergebnisse. Reihe D Biol. 16, 17–32.
Lo, P.-C., Liu, S.-H., Chao, N. L., Nunoo, F. K., Mok, H.-K., and Chen, W.-J. (2015). A multi-gene dataset reveals a tropical New World origin and Early Miocene diversification of croakers (Perciformes: Sciaenidae). Mol. Phylogenet. Evol. 88, 132–143. doi: 10.1016/j.ympev.2015.03.025
Lo, P. C., Liu, S. H., Nor, S. A. M., and Chen, W.-J. (2017). Molecular exploration of hidden diversity in the Indo-West Pacific sciaenid clade. PLoS ONE 12:e0176623. doi: 10.1371/journal.pone.0176623
López, J. A., Chen, W.-J., and Ortí, G. (2004). Esociform phylogeny. Copeia 2004, 449–464. doi: 10.1643/CG-03-087R1
Matsunuma, M. (2016). Records of Chelidoperca maculicauda and C. occipitalis (Serranidae) from the Arabian Sea, with comments on diagnostic characters. Spec. Divers. 21, 161–170. doi: 10.12782/sd.21.2.161
Matsunuma, M., and Motomura, H. (2016). Chelidoperca stella, a new species of perchlet (Perciformes: Serranidae) from the Andaman Sea, eastern Indian Ocean. Zootaxa 4092, 388–400. doi: 10.11646/zootaxa.4092.3.4
Matsunuma, M., Yamakawa, T., and Williams, J. T. (2018). Chelidoperca tosaensis, a new species of perchlet (Serranidae) from Japan and the Philippines, with geographic range extension of C. stella to the northwestern Pacific Ocean. Ichthyol. Res. 65, 210–230. doi: 10.1007/s10228-017-0604-5
Meisler, M. R. (1987). Limits and Relationships Ofserranine Seabasses, With Revisions of Serranus and Mentiperca (Pisces: Serranidae) (dissertation/Ph.D thesis). Los Angeles, CA: University of Southern California
Puillandre, N., Fedosov, A. E., Zaharias, P., Aznar-Cormano, L., and Kantor, Y. (2017). A quest for the lost types of Lophiotoma (Gastropoda: Conoidea: Turridae): integrative taxonomy in a nomenclatural mess. Zool. J. Linn. Soc. 181, 243–271. doi: 10.1093/zoolinnean/zlx012
Puillandre, N., Lambert, A., Brouillet, S., and Achaz, G. (2012). ABGD, automatic barcode gap discovery for primary species delimitation. Mol. Ecol. 21, 1864–1877. doi: 10.1111/j.1365-294X.2011.05239.x
Rambaut, A. (1996). Sequence Alignment Editor Version 1.0 a1. Available online at: http://tree.bio.ed.ac.uk/software/seal/ (Seal-v2.0a11-8 Aug 2002)
Rambaut, A. (2012). Figtree v 1.4.0. Available online at: http://tree.bio.ed.ac.uk/software/figtree/ (figtree-v1.4.4-25 Nov 2018)
Senou, H. (2013). “Serranidae. Groupers, basslets and soapfishes” in Fishes of Japan With Pictorial Keys to the Species, 3rd Edn, ed T. Nakabo (Hadano: Tokai University), 757–802, 1960–1971.
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Swofford, D. L. (2002). PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sunderland, MA: Sinauer Associates.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Ward, R. D., Zemlak, T. S., Innes, B. H., Last, P. R., and Hebert, P. D. (2005). DNA barcoding Australia's fish species. Philos. T. R. Soc. B 360, 1847–1857. doi: 10.1098/rstb.2005.1716
Williams, J. T., and Carpenter, K. E. (2015). A new fish species of the subfamily Serraninae (Perciformes, Serranidae) from the Philippines. Zootaxa 3911, 287–293. doi: 10.11646/zootaxa.3911.2.10
Yamada, U., Tokimura, M., Horikawa, H., and Nakabo, T. (eds.). (2007). Fishes and Fisheries of the East China and Yellow seas. Hadano: Tokai University.
Yang, Z. (1994). Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: approximate methods. J. Mol. Evol. 39, 306–314. doi: 10.1007/BF00160154
Keywords: fishes, new species, distribution, integrated taxonomy, diversity, West-Pacific, Tropical Deep-Sea Benthos
Citation: Lee S-H, Lee M-Y, Matsunuma M and Chen W-J (2019) Exploring the Phylogeny and Species Diversity of Chelidoperca (Teleostei: Serranidae) From the Western Pacific Ocean by an Integrated Approach in Systematics, With Descriptions of Three New Species and a Redescription of C. lecromi Fourmanoir, 1982. Front. Mar. Sci. 6:465. doi: 10.3389/fmars.2019.00465
Received: 12 March 2019; Accepted: 10 July 2019;
Published: 06 August 2019.
Edited by:
Tito Monteiro da Cruz Lotufo, University of São Paulo, BrazilReviewed by:
Matthew Thomas Craig, National Marine Fisheries Service (NOAA), United StatesRichard L. Pyle, Bernice P. Bishop Museum, United States
Copyright © 2019 Lee, Lee, Matsunuma and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Jen Chen, d2pjaGVuLmFjdGlub3BzQGdtYWlsLmNvbQ==
 Szu-Hsuan Lee
Szu-Hsuan Lee Mao-Ying Lee
Mao-Ying Lee Mizuki Matsunuma
Mizuki Matsunuma Wei-Jen Chen
Wei-Jen Chen