- 1Marine Biological Resources Functional Preservation Service, Estación de Ciencias Mariñas de Toralla, Universidade de Vigo, Vigo, Spain
- 2Centro Oceanográfico de Vigo, Instituto Español de Oceanografía, Galicia, Spain
The stated aim of this perspective article is to present new developments and discuss future directions on the applications of cryopreserved organisms to marine water quality assessment. To facilitate this, the authors provide a background of essential knowledge of cryopreservation when applied to ecotoxicology, as well as, practical examples available in literature. An integrated approach with combined monitoring of chemical status plus measurements of biological effects has been recommended extensively by international institutions for the assessment of marine pollution. Among the available techniques, bioassays have been considered as sufficiently robust to be incorporated in marine pollution monitoring programs. However, the routine application of bioassays has also allowed the identification of one of the factors that limits a more extensive use of such biological methods: the availability of biological material throughout the year, regardless of natural spawning periods. A solution to this limitation is the application of cryopreservation techniques. Cryopreservation may, for instance, provide access to stable quality biological material when test species are out of the reproductive season, without the need for maintaining and conditioning organisms in the laboratory. It also guarantees access to a large variety of species that might not be available at the same time of the year and, on top of that, cryopreservation provides opportunities to laboratories that might not have the facilities to keep all these organisms in culture.
Introduction
Water quality assessment is crucial for achieving good chemical and biological status throughout coastal waters and current approaches include the monitoring of responses at different levels of biological organization to indicate effects on the ecosystem. Integrative approaches, intended for the protection of the marine environment, are based mainly on the use of biological tools at different trophic levels in combination with chemical measures, in order to establish environmental damage thresholds (Lyons et al., 2010). In fact, the European Union Marine Strategy Framework Directive (2008/56/EC), which has the objective of achieving and maintaining the Good Environmental Status (GES) in European seas by 2020, emphasizes the need to evaluate and keep within acceptable limits the biological effects of pollutants.
Chemical analyses can identify many contaminants present in the environment, whilst biological methods permit to obtain ecologically relevant information. Among the biological tools that have been considered sufficiently robust for marine pollution assessment, ecotoxicological bioassays present several advantages such as: the detection of new pollutants for which analytical techniques have not yet been developed, provide information about the bioavailability of the pollutants (i.e., the fraction of pollutant that can be incorporated by the organism); they allow to integrate the toxic effects of the different substances present in the environment, and present a good cost/effect ratio (e.g., Stebbing et al., 1980; Calow, 1993).
As useful as they can be, the application of biological techniques using bioassays in routine monitoring has allowed to identify one of the factors that limit a more extensive use of this tools: obtaining biological material of stable high quality throughout the year, regardless of the natural spawning periods (His et al., 1999a).
A great number of response variables can be measured at different levels of biological organization and at different trophic levels in order to determine the GES of the marine environment (e.g., Lyons et al., 2010; Davies and Vethaak, 2012). A wide range of organisms have been considered for marine pollution monitoring, including microorganisms like marine bacteria (Gellert, 2000; Parvez et al., 2006), microalgae (Debelius et al., 2009; Aylagas et al., 2014; Araujo and Moreno-Garrido, 2015), marine invertebrates (Snell and Persoone, 1989; His et al., 1999a; Bellas et al., 2005; Bellas, 2008; Laranjeiro et al., 2015; Perez Fernández et al., 2015) or fish (Hutchinson et al., 1994; EPA, 2002), in all these examples the endpoints are either hatching, growth or normal development along time.
The cryopreservation and cryobanking of test organisms to be used for marine quality assessment, could ensure the accessibility to organisms or their reproductive material all year round as an alternative to either conditioning adults or continuous culture efforts for availability of biological material, which is a very time consuming and expensive process. Biobanking these test organisms in a stable manner (below -135°C) is possible, either using liquid nitrogen or ultrafreezers. At this low temperature, no chemical reactions take place and cellular metabolism is on hold. These stored cells are stopped in time and their viability would only be affected by background radiation, which at normal level will take 2000 years to become a hazard to stored cells (Glenister et al., 1984). There are not many marine cells biobanks apart from culture collections (usually microalgae and/or bacteria), but this is beginning to change (mainly at local level) as cryopreservation becomes a more popular tool and many Marine Biological Research Stations acquire biobanking equipment.
The aim of this perspective paper is to present new developments and discuss future directions on the applications of cryopreserved organisms to marine water quality assessment. To facilitate this, the authors provide a background of essential knowledge of cryopreservation when applied to ecotoxicology, as well as, practical examples available in literature.
Cryopreservation and Marine Water Quality Assessment
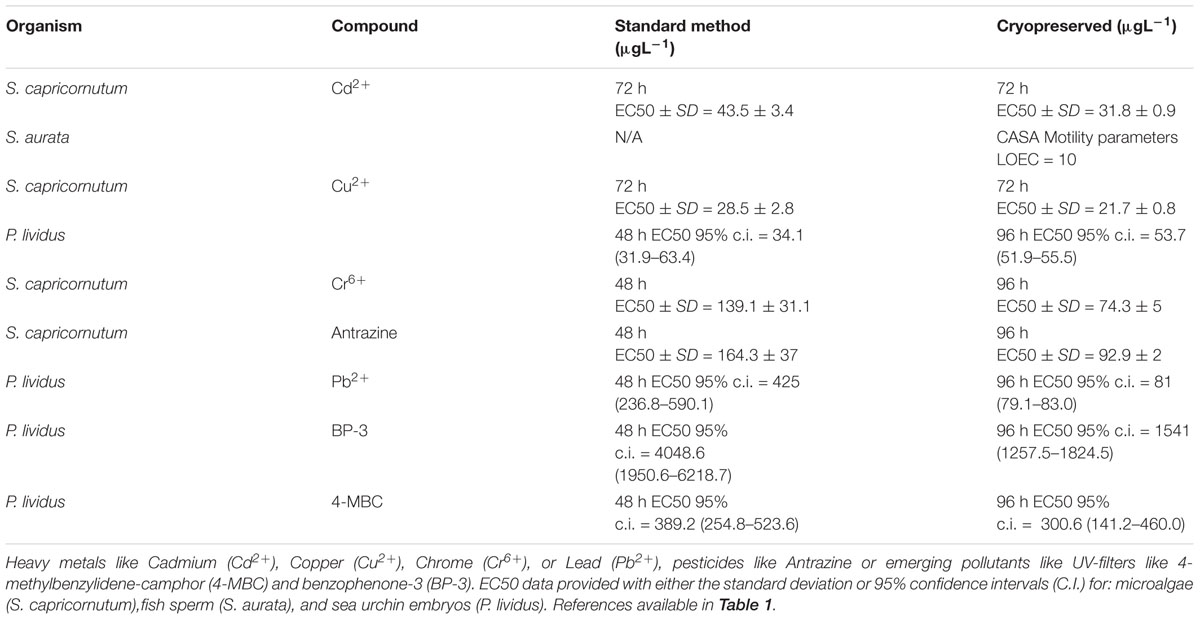
The application of cryopreservation techniques to marine water quality assessment requires the development and standardization of specific cryopreservation protocols for different types of organisms. The main question that needed to be answered was if cryopreserved organisms would be sensitive enough to detect gradual increases of toxic compounds in the water. If so, they could be used to obtain dose-response curves. It was also necessary to compare and establish the differences, or lack thereof, in sensitivity when using fresh and cryopreserved biological material. Regarding the first point, as listed below it has been proved that cryopreserved organisms can be used to detect gradual increases in the concentrations of chemical compounds present in the water, both with single chemicals and with complex natural samples. Cryopreserved organisms can therefore be used to produce dose-response curves and to obtain the No Observed Effect Concentration (NOEC), Lowest Observed Effect Concentration (LOEC), or 10 and 50% Effective Concentrations (EC10 or EC50), as well as their fresh counterparts. In this paper we present a comprehensive list indicating examples of bioassays that specifically reported the use of cryopreserved organisms as an alternative to standard bioassays with fresh organisms (methodological information is indicated in Table 1), each case will be discussed in terms of their comparability with the standard method (toxicological information is indicated in Table 2).
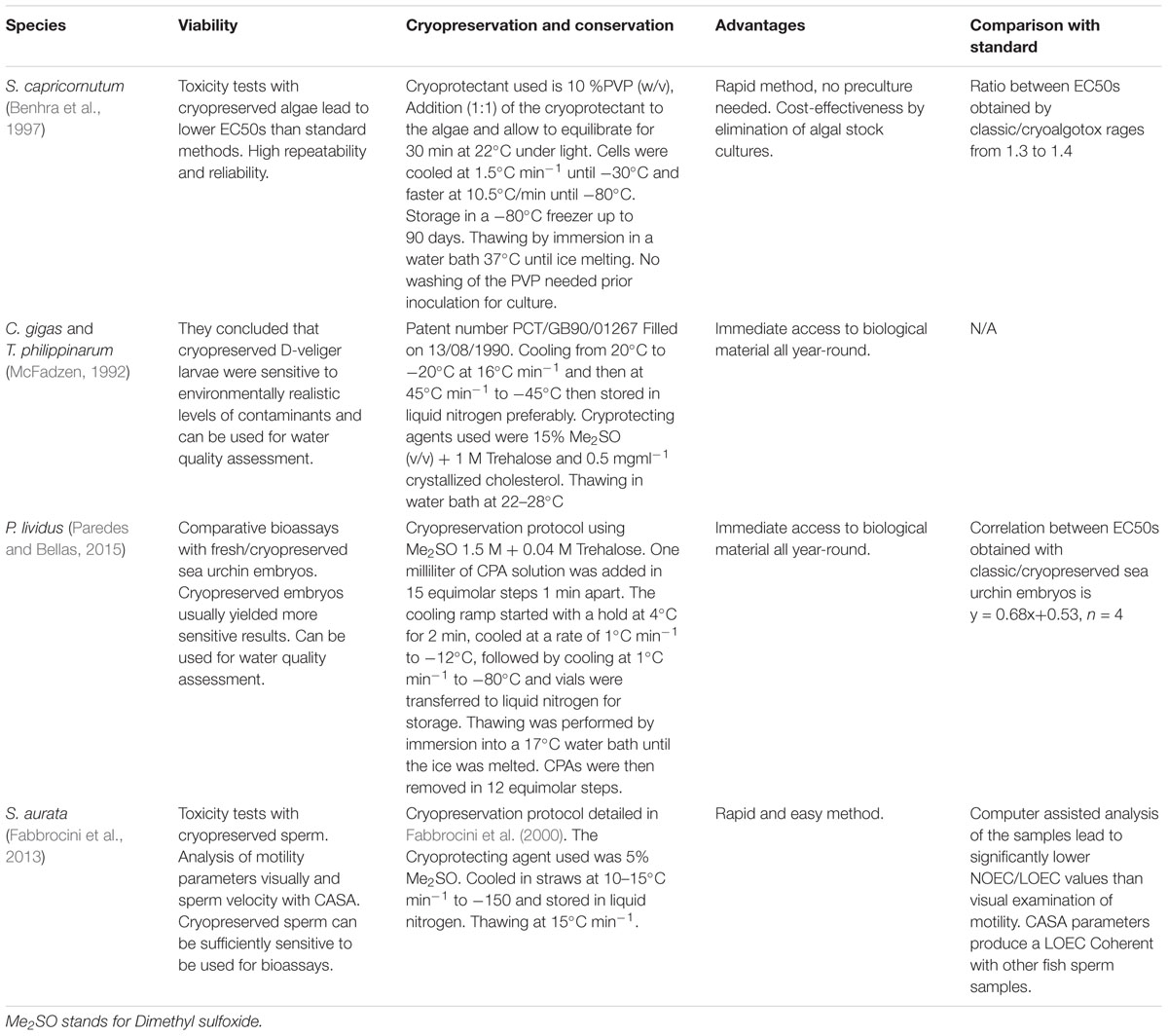
Table 1. Cryopreserved marine organisms that had been used as an alternative to fresh standard methods for evaluating marine water quality.
Cryopreserved Microalgae
Microalgae are an important part of the food chain in the ocean. A disruption of the basis of the food chain would have deep long lasting effects in the ecosystems and therefore they are of high ecotoxicological relevance (Arensberg et al., 1995; Geis et al., 2000). It has been shown that microalgae are more sensitive than other test organisms to some compounds like metals (Wong and Beaver, 1980; Satoh et al., 2005; Araújo et al., 2010) detergents (Lewis, 1990) or herbicides (Pavlic et al., 2006).
Use of cryopreserved freshwater algae Selenastrum capricornutum in ecotoxicity testing has been evaluated by Benhra et al. (1997). Experiments compared the performance of this method, named Cryoalgotox, versus the classic microplate test using fresh algae. S. capricornutum was cryopreserved by slow cooling (Table 1) using 10% (v/w) polyvinylpyrrolidone (PVP) as a cryoprotecting agent (CPA) giving comparable toxicity results. After 72 h incubation, Cryoalgotox produced lower 50% effective concentrations (EC50s) for Cd2+, Cu2+, Cr6+, and atrazine (i.e., higher sensitivity) than the classical microplate tests, which was explained by the periodic renewal of the test medium in the semistatic procedure. This test assay using cryopreserved microalgae produced highly repeatable results (low coefficients of variation).
Hundreds of cryopreservation protocols have been published for both freshwater and marine microalgae that could potentially be used to develop more bioassays with cryopreserved material. Despite most of the microalgae currently held in culture collections are kept cryopreserved and, therefore, most of the microalgae toxicity test are probably carried out with algae that had been cryopreserved at some point, there are no other published comparisons for cultured vs. cryopreserved marine microalgae as far as the authors know.
Cryopreserved Molluscs
Molluscs have been extensively used for several ecotoxicological tests, among which stands out the embryo-larval bioassay (International Council for the Exploration of the Sea (ICES), 1991; His et al., 1999b). The high sensitivity of early-life stages allows the detection of low pollution levels by the identification of effects in the embryonic development (delays or morphological abnormalities) after a short period of exposure/incubation in the presence of a toxicant or a water sample of unknown quality. Oysters, such as Crassostrea gigas (His et al., 1999b; Leverett and Thain, 2013) and mussels, such as Mytilus edulis (Nolan and Duke, 1983) or Mytilus galloprovincialis (His et al., 1997; Beiras and Bellas, 2008), are the star test species for this procedure for being well known and studies species but also for their worldwide distribution.
Cryopreserved bivalve larvae (C. gigas and Tapes philippinarum larvae) have been exposed to different water samples and shown to be sensitive to environmentally realistic levels of contaminants for field monitoring of water quality (McFadzen, 1992). This was the first attempt to use cryopreserved cells of any type for ecotoxicology studies proving that those cells retain the sensitivity to chemicals and could be used for bioassays.
Larvae were cryopreserved at 24 h for C. gigas and 48 h for T. philippinarum at the late trochophore/early D-veliger stages (Table 1) and stored in liquid nitrogen at (-196°C), while using 15% dimethyl sulfoxide (v/v) with 1.0 M Trehalose and 0.5 mg/ml cholesterol as CPAs. Survival was reported as highly variable upon thawing. Despite no comparison between fresh and cryopreserved cells was carried out at the time, cryopreserved cells responded to toxicity and allowed for the calculation of toxicological parameters.
The description of cryopreservation protocols for marine invertebrates is also flourishing and protocols for molluscs like the mussels M. galloprovincialis (Paredes et al., 2013) and Perna canaliculus (Paredes et al., 2012) have been developed. Results with bivalves are promising, since the cryopreservation methods for these organisms have been proven to be reliable, repeatable and sensitive, being on an advanced stage of development. A way forward would be to test the comparison between the procedures with cryopreserved organisms and standard tests, which have not yet been performed.
Cryopreserved Echinoids
Sea urchins are other of the classic models (Bellas et al., 2005; Durán and Beiras, 2010) for water quality testing. Paredes and Bellas (2015) established for the first time a bioassay using cryopreserved sea-urchin embryos (Paracentrotus lividus) (Paredes et al., 2011) and provided a comparison with the already standardized sea urchin embryo larval bioassay for standard chemicals like copper and lead (Figure 1).
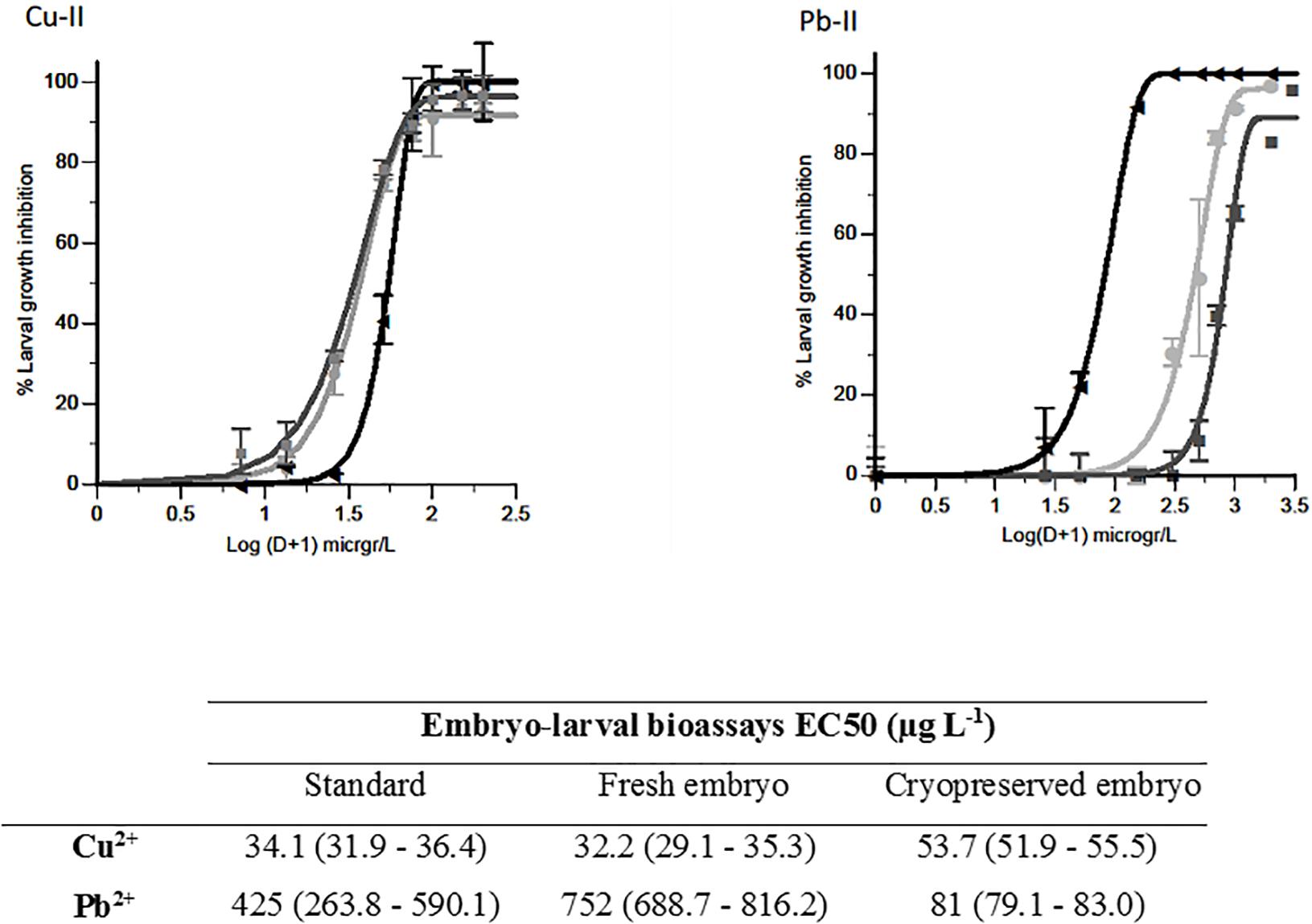
Figure 1. Copper and lead toxicity tests. Larval growth inhibition at each concentration ±SD (n = 35). Light gray, dark gray, and black represent the standard bioassay, the fresh embryo bioassay and cryopreserved embryo bioassay, respectively. Table attached shows EC50s (μg L-1) and 95% confidence intervals (in brackets). Figures modified from Paredes and Bellas (2015).
Sea urchin embryos (early blastula) were cryopreserved using 1.5 M dimethyl sulfoxide plus 0.04 M trehalose and cooled at 1°C min-1 (protocol in Table 1). Samples were then stored in liquid nitrogen. These experiments showed that there was no significant loss in sensitivity when using early blastulas instead of fresh fertilized oocytes. Paredes and Bellas (2015) did find differences in sensitivity when using cryopreserved vs. fresh cells, in some cases the differences were minimal, in other cases the cryopreserved test was clearly more sensitive (Table 2). This increased sensitivity may be because cryopreserved organisms are going through a recovery process after thawing, and might be more sensitive to additional stress, such as toxicant exposure.
Ribeiro et al. (2018) developed a cryopreservation protocol for Echinometra lucunter sperm and they are studying the cryopreservation of embryos for water quality assessment. There is a cryopreservation protocol described for P. lividus sperm (Fabbrocini et al., 2014) that yields good motility. Cryopreservation protocols already exist or are under development for different developmental stages for 10–14 different sea urchin species (embryos and sperm), and since sea urchins are a highly demanded model, more applications will probably be further developed soon using cryopreserved cells, including toxicology (Paredes, 2015a).
Cryopreserved Fish Sperm
The case of fish cryopreservation (but also crustaceans) is more complicated, since these organisms are very sensitive to low temperatures and have proven exceptionally difficult to cryopreserve, being fish sperm the only exception. There has been exhaustive research on marine fish sperm cryopreservation and protocols have been described for most farmed species (Sparus aurata by Fabbrocini et al., 2012, 2016; Dicentrarchus labrax by Fauvel et al., 1998; or Mugil cephalus by Balamurugan and Munuswamy, 2017), any of which could be used as a biomonitoring test organism.
The study by Fabbrocini et al. (2013) evaluated the feasibility of using cryopreserved S. aurata spermatozoa to be used in toxicity tests (Table 1). Sperm motility parameters were evaluated after thawing by a computer-assisted analysis. The sensitivity of the sperm (motility percentages and velocities) to a reference toxicant (cadmium) was comparable to what has been recorded for the fresh sperm of other aquatic species (Table 2). The test was found to be sensitive, rapid, easy to perform and showed good reliability.
Discussion
Bioassays have been widely reported to provide a lot of information and be very useful for water quality assessment but in many cases there is either a need for maintaining breeding animals in the lab for out of season use (if possible) or some tests have a very marked seasonality (matching the spawning season of the test species). Using cryopreserved biological material is a good option to overcome this constraint, but it is crucial to be able to compare the results of the procedure with cryopreserved material to the standard tests.
According to Cairns and Pratt (1989) extrapolations from bioassays on one species to another species are not straightforward and results are only comparable in some cases. From the studies reviewed here, the same principle can be applied to the comparison between bioassays with cryopreserved cells and standard bioassays. Cryopreservation is a very useful add-on to an already developed testing methodology that could help increasing the use of bioassays. On the other hand, until an exhaustive comparison and compilation of data takes place and a robust correspondence between bioassays with cultured vs. cryopreserved organisms can be obtained with a reliable level of certainty, these results should be taken with precaution.
The advantages of using cryopreserved biological material for bioassays are many: from providing a reliable source of cells and organisms that can be stored for out of season need, to provide flexibility to the analyser. Making possible the simultaneous testing with a battery of organisms that do not reproduce at the same time of the year, without having to hold the animals in the lab for out of season production, which is costly and labor intensive. Last but not least, it also aligns with the 3R’s of animal welfare principle of reduction, by allowing the storage of unused material for other experiments therefore reducing the number of animals used per trial. As more marine organisms have been successfully cryopreserved, including different cells or development stages, there is great potential for this to continue to develop (Suquet et al., 2000; Paredes, 2015b).
Many of the microalgae currently held in culture collections are kept cryopreserved, there are also available protocols for different molluscs (Wang et al., 2011; Paredes et al., 2013) and being mussels the most widely used organism for biomonitoring, this is another potential candidate for the development of a cryopreserved toxicity test in the near future. Regarding sea urchins, right now there are cryopreservation protocols developed or under development for different cells for 10–14 different sea urchin species, and being sea urchins a highly demanded model soon more applications will be developed, including toxicology (Paredes, 2015b).
Crustaceans are, as of today, not on the table as they have no reliable cryopreservation protocol. Fish are very sensitive to low temperatures and had proven exceptionally difficult to cryopreserve, being the only exception fish sperm. There has been exhaustive research on marine fish sperm cryopreservation and protocols have been described for the most farmed species (S. aurata by Fabbrocini et al., 2012; D. labrax by Fauvel et al., 1998; or M. cephalus by Balamurugan and Munuswamy, 2017), any of these could be used as a biomonitoring test organism and cryopreservation could enhance the possibilities of development of this test. There is potential for the further use of this biotechnology applied to marine water quality assessment.
The parameters used as endpoints in the classic bioassays were characterized by good reliability and sensitivity but, when using cryopreserved cells those parameters might need a little adjusting in order to obtain the best results, For instance, cryopreserved cells develop slower in the first hours post-thaw, therefore experimental protocols need to be adjusted in terms of exposure duration; cryopreserved microalgae can show sensitivity to high light intensities immediately post-thaw so that light intensity needs to be lowered during the first hours of exposure. Cryopreserved samples can be easily stored and transferred, making it possible to perform bioassays in different sites or at different times and can even be part of long-term monitoring programs. Finally, the application of certain bioassays with cryopreserved material in environmental monitoring and risk assessment schemes, may allow the detection of lower concentrations of toxic substances that classical bioassays, which would offer a higher level of protection to marine ecosystems.
Conclusion
This is a perspective on the state of the art and critical analysis of the application of cryopreservation as a tool to improve toxicity testing. As of today, cryopreservation holds great potential as a tool to improve toxicity testing by solving, for instance, the seasonal shortage of biological material. On the other side, there is a need for extensive comparative testing in order to select those cryopreserved cells/protocols that can be more useful, either by developing new protocols for key cell types or making sure the cryopreservation outcome of the existing protocols is specifically designed to be used in a bioassay. There is also a need to obtain good and reliable correlations between methods with both fresh and cryopreserved biological material for a wide variety of chemical compounds. An extensive battery of comparisons using both methods will establish a frame of comparison that would enable researchers to use one or the other according with their practical needs and keep increasing the historical databases. Currently, the cryopreservation of P. lividus embryos and S. aurata sperm are in an advanced stage of development and present promising perspectives for their use in water quality assessment. As cryopreservation of aquatic marine resources continues to develop, the application of those preserved cells to toxicity testing will continue to expand.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by the ASSEMBLE plus, grant from the European Union’s Horizon 2020 Research and Innovation Programme (No. 730984).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Araújo, C. V. M., Diz, F. R., Lubián, L. M., Blasco, J., and Moreno-Garrido, I. (2010). Sensitivity of cylindrotheca closterium to copper: influence of three test endpoints and two test methods. Sci. Total Environ. 408, 3696–3703. doi: 10.1016/j.scitotenv.2010.05.012
Araujo, C. V. M., and Moreno-Garrido, J. (2015). Toxicity Bioassays on Benthic Diatoms. Amsterdam: Elsevier, 539–545.
Arensberg, P., Hemmingsen, V. H., and Nyholm, N. (1995). A miniscale algal toxicity test. Chemosphere 30, 2103–2115. doi: 10.1016/0045-6535(95)00090-u
Aylagas, E., Menchaca, I., Laza-Martínez, A., Seoane, S., and Franco, J. (2014). Evaluation of marine phytoplankton toxicity by application of marine invertebrate bioassays. Sci. Mar. 78, 173–183. doi: 10.3989/scimar.03957.26c
Balamurugan, R., and Munuswamy, N. (2017). Cryopreservation of sperm in grey mullet mugil cephalus (Linnaeus, 1758). Anim. Reprod. Sci. 185, 205–213. doi: 10.1016/j.anireprosci.2017.08.022
Beiras, R., and Bellas, J. (2008). Inhibition of embryo development of the mytilus galloprovincialis marine mussel by organic pollutants; assessment of risk for its extensive culture in the galician rias. Aquaculture 277, 208–212. doi: 10.1016/j.aquaculture.2008.03.002
Bellas, J. (2008). Prediction and assessment of mixture toxicity of compounds in antifouling paints using the sea urchin embryo-larval bioassay. Aquat. Toxicol. 88, 309–315. doi: 10.1016/j.aquatox.2008.05.011
Bellas, J., Beiras, R., Mariño-Balsa, J. C., and Fernandez, N. (2005). Comparison between the Sea urchin embryogenesis bioassay and alternative test species. Ecotoxicology 14, 337–353. doi: 10.1007/s10646-004-6370-y
Benhra, A., Radetski, C. M., and Ferard, J. F. (1997). Cryoalgotox: use of cryopreserved alga in a semistatic microplate test. Environ. Toxicol. Chem. 16, 505–508. doi: 10.1002/etc.5620160316
Cairns, J. Jr., and Pratt, J. R. (1989). The scientific basis of bioassays. Hydrobiologia 18, 5–20. doi: 10.1007/bf00027769
Calow, P. (1993). General Principles and Overview. En: Handbook of Ecotoxicology I. Hoboken, NJ: Blackwell Scientific Publications.
Davies, I. M., and Vethaak, A. D. (2012). Integrated marine environmental monitoring of chemicals and their effects. ICES Cooperative Res. Rep. 315:277.
Debelius, B., Forja, J. M., DelValls, A., and Lubian, L. M. (2009). Toxicity and bioaccumulation of copper and lead in five marine microalgae. Ecotoxicol. Environ. Saf. 72, 1503–1513. doi: 10.1016/j.ecoenv.2009.04.006
Durán, I., and Beiras, R. (2010). Assessment criteria for using the sea-urchin embryo test with sediment elutriates as a tool to classify the ecotoxicological status of marine water bodies. Environ. Toxicol. Chem. 29, 1192–1198. doi: 10.1002/etc.136
EPA (2002). Method 1004.0. Sheepshead Minnow, Cyprinodon variegatus, Larval Survival and Growth Test; Chronic Toxicity. Excerpt from: Short-term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Marine and Estuarine Organisms. Washington, DC: EPA.
Fabbrocini, A., D’Adamo, R., Del Prete, F., Langellotti, A. L., Barone, C. M. A., Rinna, F., et al. (2013). Motility of cryopreserved spermatozoa for the ecotoxicological evaluation of aquatic environments. chemistry and ecology: motility of cryopreserved spermatozoa for the ecotoxicological evaluation of aquatic environments. Chem. Ecol. 29, 660–667. doi: 10.1080/02757540.2013.810723
Fabbrocini, A., D’Adamo, R., Del Prete, F., Langellotti, A. L., Rinna, F., Silvestri, F., et al. (2012). Cryopreserved semen in ecotoxicological bioassays: sensitivity and reliability of cryopreserved Sparus aurata spermatozoa. Ecotoxicol. Environ. Saf. 84, 293–298. doi: 10.1016/j.ecoenv.2012.07.024
Fabbrocini, A., D’Adamo, R., Del Prete, F., Maurizio, D., Specchiuli, A., Oliveira, F. L., et al. (2016). The sperm motility pattern in ecotoxicological tests. The CRYO-Ecotest as a case study. Ecotoxicol. Environ. Saf. 123, 53–59. doi: 10.1016/j.ecoenv.2015.08.018
Fabbrocini, A., D’Adamo, R., Pelosi, S., Oliveira, L. F. J., Silvestri, F., and Sansone, G. (2014). Gamete cryobanks for laboratory research: developing a rapid and easy to perform protocol for the cryopreservation of the sea urchin Paracentrotus lividus (Lmk, 1816) Spermatozoa. Cryobiology 69, 149–156. doi: 10.1016/j.cryobiol.2014.06.009
Fabbrocini, A., Lavadera, S. L., Rispoli, S., and Sansone, G. (2000). Cryopreservation of Seabream (Sparus aurata) spermatozoa. Cryobiology 40, 46–53. doi: 10.1006/cryo.1999.2220
Fauvel, C., Suquet, M., Dreanno, C., Zonno, V., and Menu, B. (1998). Cryopreservation of sea bass (Dicentrarchus labrax) spermatozoa in experimental and production simulating conditions. Aquat. Living Resour. 11, 387–394. doi: 10.1016/s0990-7440(99)80004-7
Geis, S. W., Fleming, K. L., Korthals, E. T., Searle, G., Reynolds, L., and Karner, D. A. (2000). Modifications to the algal growth inhibition test for use as a regulatory assay. Environ. Toxicol. Chem. 19, 36–41. doi: 10.1002/etc.5620190105
Gellert, G. (2000). Sensitivity and significance of luminescent bacteria in chronic toxicity testing based on growth and bioluminescence. Ecotoxicol. Environ. Saf. 45, 87–91. doi: 10.1006/eesa.1999.1849
Glenister, P. H., Whittinham, D. G., and Lyon, M. F. (1984). Further studies on the effect of radiation during the storage of frozen 8-cell mouse embryos at -196°C. J. Reprod. Fert. 70, 229–234. doi: 10.1530/jrf.0.0700229
His, E., Beiras, R., and Seaman, M. N. L. (1999a). “The assessment of marine pollution-bioassays with bivalve embryos and larvae,” in Advances in Marine Biology, eds A. I. Southeward, P. A. Tyler, and C. M. Young (Cambridge, MA: Academic Press), 1–178. doi: 10.1016/s0065-2881(08)60428-9
His, E., Heyvang, I., Geffard, O., and Montaudouin, X. (1999b). A comparison between oyster (Crassostrea gigas) and sea urchin (Paracentrotus lividus) larval bioassays for toxicological studies. Water Res. 33, 1706–1718. doi: 10.1016/s0043-1354(98)00381-9
His, E., Seaman, M. N. L., and Beiras, R. (1997). A simplification the bivalve embryogenesis and larval development bioassay method for water quality assessment. Water Res. 31, 351–355. doi: 10.1016/s0043-1354(96)00244-8
Hutchinson, T. H., Williams, T. D., and Eales, G. J. (1994). Toxicity of cadmium, hexavalent chromium and copper to marine fish larvae (Cyprinodon variegatus) and copepods (Tisbe battagliai). Mar. Environ. Res. 38, 275–290. doi: 10.1016/0141-1136(94)90028-0
International Council for the Exploration of the Sea (ICES) (1991). Techniques in Marine Environmental Sciences, No 11.
Laranjeiro, F., Pérez, S., Navarro, P., Carrero, J. A., and Beiras, R. (2015). The usefulness of a sediment bioassay with the gastropod Nassarius reticulatus in tributyltin monitoring programs. Chemosphere 139, 550–557. doi: 10.1016/j.chemosphere.2015.07.076
Leverett, D., and Thain, J. (2013). Oyster embryo-larval bioassay revised. Tech. Mar. Environ. Sci. 54:34.
Lewis, M. A. (1990). Chronic toxicities of surfactants and detergent builders to algae: a review and risk assessment. Ecotoxicol. Environ. Saf. 20, 123–140. doi: 10.1016/0147-6513(90)90052-7
Lyons, B. P., Thain, J. E., Stentiford, G. D., Hylland, K., Davies, I. M., and Vethaak, A. D. (2010). Using biological effects tools to define good environmental status under the european union marine strategy framework directive. Mar. Pollut. Bull. 60, 1647–1651. doi: 10.1016/j.marpolbul.2010.06.005
McFadzen, I. R. B. (1992). Growth and survival of cryopreserved oyster and clam larvae along a pollution gradient in the German Bight. Mar. Ecol. Prog. Ser. 91, 215–220. doi: 10.3354/meps091215
Nolan, C. V., and Duke, E. J. (1983). Cadmium accumulation and toxicity in mytilus edulis: involvement of metallothioneins and heavy-molecular weight protein. Aquat. Toxicol. 4, 153–163. doi: 10.1016/0166-445x(83)90052-8
Paredes, E. (2015b). Exploring the evolution of marine invertebrate cryopreservation – landmarks, state of the art and future lines of research. Rev. Artic. Cryobiol. 71, 198–209. doi: 10.1016/j.cryobiol.2015.08.011
Paredes, E. (2015a). Biobanking of a marine invertebrate model organism: the sea urchin. J. Mar. Sci. Eng. 3:7. doi: 10.3390/jmse4010007
Paredes, E., Adams, S. L., Tervit, H. R., Smith, J. F., McGowan, L. T., Gale, S. L., et al. (2011). Cryopreservation of GreenshellTM mussel (Perna canaliculus) trochophore larvae. Cryobiology 65, 256–262. doi: 10.1016/j.cryobiol.2012.07.078
Paredes, E., and Bellas, J. (2015). The use of cryopreserved sea urchin embryos (Paracentrotus lividus) in marine quality assessment. Chemosphere 128, 278–283. doi: 10.1016/j.chemosphere.2015.02.007
Paredes, E., Bellas, J., and Adams, S. L. (2012). Comparative cryopreservation study of trochophore larvae from two species of bivalves: pacific oyster (Crassostrea gigas) and Blue mussel (Mytilus galloprovincialis). Cryobiology 67, 274–279. doi: 10.1016/j.cryobiol.2013.08.007
Paredes, E., Bellas, J., and Adams, S. L. (2013). Comparative cryopreservation study of trochophore larvae from two species of bivalves: pacific oyster (Crassostrea gigas) and blue mussel (Mytilus galloprovincialis). Cryobiology 67, 274–279. doi: 10.1016/j.cryobiol.2013.08.007
Parvez, S., Venkataraman, C., and Mukherji, S. (2006). A review on advantages of implementing luminescence inhibition test (Vibrio fischeri) for acute toxicity prediction of chemicals. Environ. Int. 32, 265–268. doi: 10.1016/j.envint.2005.08.022
Pavlic, Z., Stjepanovic, B., Horvatic, J., Persic, V., Puntaric, D., and Culig, J. (2006). Comparative sensitivity of green algae to herbicides using erlenmeyer flask and microplate growth-inhibition assays. Bull. Environ. Contam. Toxicol. 76, 883–890. doi: 10.1007/s00128-006-1001-3
Perez Fernández, S., Rial, D., and Beiras, R. (2015). Acute toxicity of selected organic pollutants to saltwater (mysid Siriella armata) and freshwater (cladoceran Daphnia magna) ecotoxicological models. Ecotoxicology 24, 1229–1238. doi: 10.1007/s10646-015-1489-6
Ribeiro, R. C., Caroline da Silva Veronez, A., Tristão Tovar, T., Adams, S. L., Bartolomeu, D. A., Peronico, C., et al. (2018). Cryopreservation: extending the viability of biological material from sea urchin (Echinometra lucunter) in ecotoxicity tests. Cryobiology 80, 139–143. doi: 10.1016/j.cryobiol.2017.10.002
Satoh, A., Vudikaria, L. Q., Kurano, N., and Miyachi, S. (2005). Evaluation of the sensitivity of marine microalgal strains to the heavy metals. Cu, As, Sb, Pb and Cd. Environ. Int. 31, 713–722. doi: 10.1016/j.envint.2005.01.001
Snell, T. W., and Persoone, G. (1989). Acute toxicity bioassays using rotifers. I. A test for brackish and marine environments with Brachionus plicatiliss. Aquat. Toxicol. 14, 65–80. doi: 10.1016/0166-445x(89)90055-6
Stebbing, A. R. D., Akesson, B., Calabrese, A., Gentile, J. H., Jensen, A., and Lloyd, R. (1980). The role of bioassays in marine pollution monitoring. bioassay panel report, rapp. P.-v. Réuni. Cons. Int. Explor. Mer. 179, 322–332.
Suquet, M., Dreanno, C., Fauvel, C., Cosson, J., and Billard, R. (2000). Cryopreservation of sperm in marine fish. Aquacult. Res. 31, 231–243. doi: 10.1046/j.1365-2109.2000.00445.x
Wang, H., Li, X., Wang, M., Clarke, S., Gluis, M., and Zhang, Z. (2011). Effects of larval cryopreservation on subsequent development of the blue mussels, mytilus galloprovincialis Lamarck. Aqua. Res. 42, 1816–1823. doi: 10.1111/j.1365-2109.2010.02782.x
Keywords: cryopreservation, water quality assessment, cryobiology, bioassay, model organisms
Citation: Paredes E and Bellas J (2019) The Use of Cryopreserved Biological Material for Water Quality Assessment. Front. Mar. Sci. 6:454. doi: 10.3389/fmars.2019.00454
Received: 12 December 2018; Accepted: 08 July 2019;
Published: 24 July 2019.
Edited by:
Naser A. Anjum, Aligarh Muslim University, IndiaReviewed by:
Gabriela Verónica Aguirre-Martínez, Universidad Arturo Prat, ChileBenoit Xuereb, University of Le Havre, France
Copyright © 2019 Paredes and Bellas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Estefania Paredes, ZXBhcmVkZXNAdXZpZ28uZXM=
†These authors have contributed equally to this work
 Estefania Paredes
Estefania Paredes Juan Bellas
Juan Bellas