
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Mar. Sci. , 18 March 2019
Sec. Deep-Sea Environments and Ecology
Volume 6 - 2019 | https://doi.org/10.3389/fmars.2019.00119
This article is part of the Research Topic Managing Deep-sea Ecosystems at Ocean Basin Scale, Volume 1 View all 29 articles
As “ecosystem engineers,” framework-forming scleractinian cold-water corals (CWC) build reefs that are unique biodiversity hotspots in the deep sea. Studies using common biological techniques such as correlating the spatial occurrence of the most common CWC species with modeled environmental conditions have revealed the ecological requirements and tolerances of these species. However, limited field observations and poorly understood geographical distribution patterns of the CWC restrict the application of existing knowledge toward assessing their fate (e.g., local extinction, newly established populations) under ongoing global change. Hence, the risk to cross ecological tipping points causing the demise (or establishment) of entire CWC reefs remains unclear. A major challenge is to identify the key environmental parameters (or stressors) having the potential to control CWC vitality by providing such tipping points. This is largely hampered by the overall lack of present-day observations of such tipping point crossings. However, evidence for such events is frequently preserved in geological records revealing that entire CWC ecosystems vanished or returned at specific moments in the past. Here, a geological approach is presented that by correlating geological CWC records with paleoceanographic data describing past environmental changes allows to identify a set of key environmental drivers that directly or indirectly control CWC vitality. Thus, by combining such a geological approach with common biological techniques (see above) to describe the ecological tolerance of the most important reef-building CWC has a great potential to better assess their future spatial distribution in times of accelerating global change and to improve the sustainable management of the important deep-sea ecosystems formed by CWC.
Framework-forming scleractinian cold-water corals (CWC) are ecosystem engineers that form the base for biodiversity hotspots being widely distributed in water depths between 200 and 1,000 m in the Atlantic Ocean (Roberts et al., 2009). CWC reefs are considered as vulnerable marine ecosystems (Auster et al., 2011) that potentially provide very important ecosystem services. Consequently, around many CWC sites marine protected areas (MPA) have been established (e.g., off Norway and Ireland, around the Azores) to ban extraction of living and non-living resources invoking activities that are potentially harmful to the CWC (e.g., Armstrong and van den Hove, 2008; Huvenne et al., 2016). However, MPA provide no protection against threats induced by ongoing global change (Jackson et al., 2014) such as ocean warming, acidification, deoxygenation and decreasing particulate organic matter fluxes to the seabed as these cannot be controlled locally (e.g., Sweetman et al., 2017). In addition, this range of environmental parameters might be even more critical for the proliferation or survival of CWC considering a combined effect of multiple stressors (e.g., Büscher et al., 2017). Thus, to assess the vulnerability of CWC to future global change, there is an indispensable need to comprehensively understand their sensitivity to changing environmental conditions.
The ecological requirements and tolerances of the most common framework-forming CWC species have been determined by field observations in the direct vicinity of CWC reefs (Dullo et al., 2008; Freiwald et al., 2009; Brooke et al., 2013; Flögel et al., 2014) or by correlating their spatial occurrence to gridded data bank information describing the ambient environmental setting focusing mainly on physico-chemical properties of the water masses bathing the CWC reefs (Freiwald, 2002; Davies et al., 2008; Davies and Guinotte, 2011). Subsequently, the empirically obtained ranges of individual environmental parameters have been interpreted as generally valid thresholds controlling the occurrence of CWC on a global scale (Davies and Guinotte, 2011). However, recent discoveries of hitherto unknown CWC reefs that exist today under rather “extreme” conditions [e.g., in terms of temperature (Mienis et al., 2014) or oxygen (Ramos et al., 2017)], force us to shift the upper and lower thresholds of environmental parameters beyond formerly described values. In addition, laboratory experiments conducted on several common CWC species (e.g., Lophelia pertusa, Madrepora oculata, and Dendrophyllia) provided additional information on their ecological requirements (e.g., in terms of temperature, carbonate system, food supply, and oxygen) (e.g., Tsounis et al., 2010; Gori et al., 2014; Movilla et al., 2014; Naumann et al., 2014; Maier et al., 2016; Büscher et al., 2017) and also indicate region-specific adaptations of CWC to particular environmental parameters (Dodds et al., 2007; Lunden et al., 2014). Furthermore, exceeding/undercutting such environmental thresholds (“tipping point”) causing a local extinction of CWC so far has never been documented by field observations.
Here, we outline a geological concept and review its applicability as this geological approach has great potential to overcome such lacking observations in order to further delineate the ecological tolerances of CWC. It allows identifying CWC tipping points by taking advantage of the long-term development of CWC reefs. In the geological past, this often is marked by regional extinctions and re-occurrences of CWC concomitant with changes in climate (e.g., Kano et al., 2007; Frank et al., 2011). Correlating such events, i.e., the crossing of tipping points, to the variability of paleoenvironmental parameters allows to identify key parameters that potentially control the (regional) vitality of CWC (e.g., Dorschel et al., 2005; Wienberg et al., 2010; Raddatz et al., 2014b; Van der Land et al., 2014; Victorero et al., 2016). Thus, studying the geological record of CWC reefs enhances our understanding about the response of CWC to past climatic changes. Hence, it provides pivotal information about their likely response to those changes expected for the future. In the end, this information will allow to develop and to optimize management strategies for the sustainable protection of these important deep-sea ecosystems.
Over geological time scales of thousands to millions of years, CWC can form coral mounds (composed of CWC fragments, shells of associated fauna, and hemipelagic sediments), which occur widespread along the continental margins of the Atlantic Ocean (Wienberg and Titschack, 2017) reaching heights of up to >300 m (Mienis et al., 2006). The framework-forming CWC species mostly contributing to the formation of coral mounds are L. pertusa, M. oculata, Solenosmilia variabilis, Bathelia candida, and Enallopsammia profunda (e.g., Mangini et al., 2010; Frank et al., 2011; Muñoz et al., 2012; Hebbeln et al., 2014). Coral mounds are distinct from the surrounding seafloor as they constitute elevated seabed structures causing enhanced hydrodynamics that support the supply of sediment and food particles. Therefore, coral mounds induce re-settlement of CWC (that may develop into reefs) whenever the overall environmental settings turn favorable (Wienberg and Titschack, 2017).
Sediment cores obtained from coral mounds provide geological records allowing to trace CWC vitality through time (e.g., Dorschel et al., 2005; Kano et al., 2007). For most coral mound sites investigated so far, it has been found that the CWC records show alternating phases of coral vitality and absence (e.g., Eisele et al., 2008; Mienis et al., 2009; Matos et al., 2017). Precise dating by radiometric methods (e.g., uranium-series dating, radiocarbon dating) allows to define the timing of (regional) extinction or re-occurrence of CWC and to relate these “tipping point crossings” with changes in environmental conditions (e.g., Frank et al., 2011).
Interestingly, the timing of crossing CWC tipping points, resulting in either their demise or their re-settlement in a given area, often coincides with major climatic changes. For many sites in the North Atlantic (e.g., off Norway, Ireland, and the United States east coast, in the Mediterranean Sea and Gulf of Mexico), it has been found that after a long-lasting period (tens of thousands of years) of coral absence a re-settlement of CWC occurred during the transition from the last glacial period to the present interglacial along with a significant global warming (e.g., Frank et al., 2009; McCulloch et al., 2010; Taviani et al., 2011; López Correa et al., 2012; Fink et al., 2015; Matos et al., 2015, 2017). However, the exact timing has been quite variable, for instance, starting relatively early at 14,000 years before present (BP) in the Mediterranean Sea (Fink et al., 2015; Stalder et al., 2015) and relatively late at 7,000 years BP off the United States east coast (Matos et al., 2015). In contrast, off NW Morocco and Mauritania, CWC flourished during glacial conditions and went regionally extinct concurrent with the global warming of the recent interglacial (Wienberg et al., 2010, 2018; Frank et al., 2011). This pattern of climate change-driven regional variations in coral proliferation is not just valid for the recent interglacial and last glacial (i.e., the last ∼ 70,000 years), but can also be traced further back in time comprising previous glacial and interglacial stages (Frank et al., 2011; Raddatz et al., 2014b; Van der Land et al., 2014; Matos et al., 2017; Wienberg et al., 2018). Beside major changes in CWC proliferation linked to glacial/interglacial climate variability, CWC development has regionally also been interrupted on shorter time scales, lasting for a few hundreds to thousands of years during interglacial or glacial periods (e.g., Fink et al., 2015; Raddatz et al., 2016; Wienberg et al., 2018).
To identify those environmental changes that are most likely the cause for the demise or re-settlement of CWC, conventional paleoceanographic approaches can be used. At first glance, the aragonitic skeletons of the CWC appear as the prime signal carrier for paleoceanographic changes. They provide an excellent, precisely datable paleoarchive offering a range of innovative proxies to reconstruct the paleoenvironment in which they thrived (Robinson et al., 2014). However, coral-based proxy records provide no environmental information on those times the corals were absent, thus precluding any before-and-after comparisons.
Therefore, the reconstruction of the paleoenvironment controlling the vitality of CWC has to rely on sediment-based approaches. These can be applied either to the hemipelagic sediments deposited among the coral fragments on the coral mounds, so-called on-mound records, or to coral-barren sediments collected nearby ideally from the same water depth to represent the same environmental setting, so-called off-mound records (Figure 1; Dorschel et al., 2005). As the deposition of sediments within the coral framework can be somewhat patchy and is limited to the periods of coral growth (Thierens et al., 2013), the off-mound sites usually provide more continuous paleoenvironmental records (Dorschel et al., 2005; Rüggeberg et al., 2007; Wienberg et al., 2010; Fink et al., 2013).
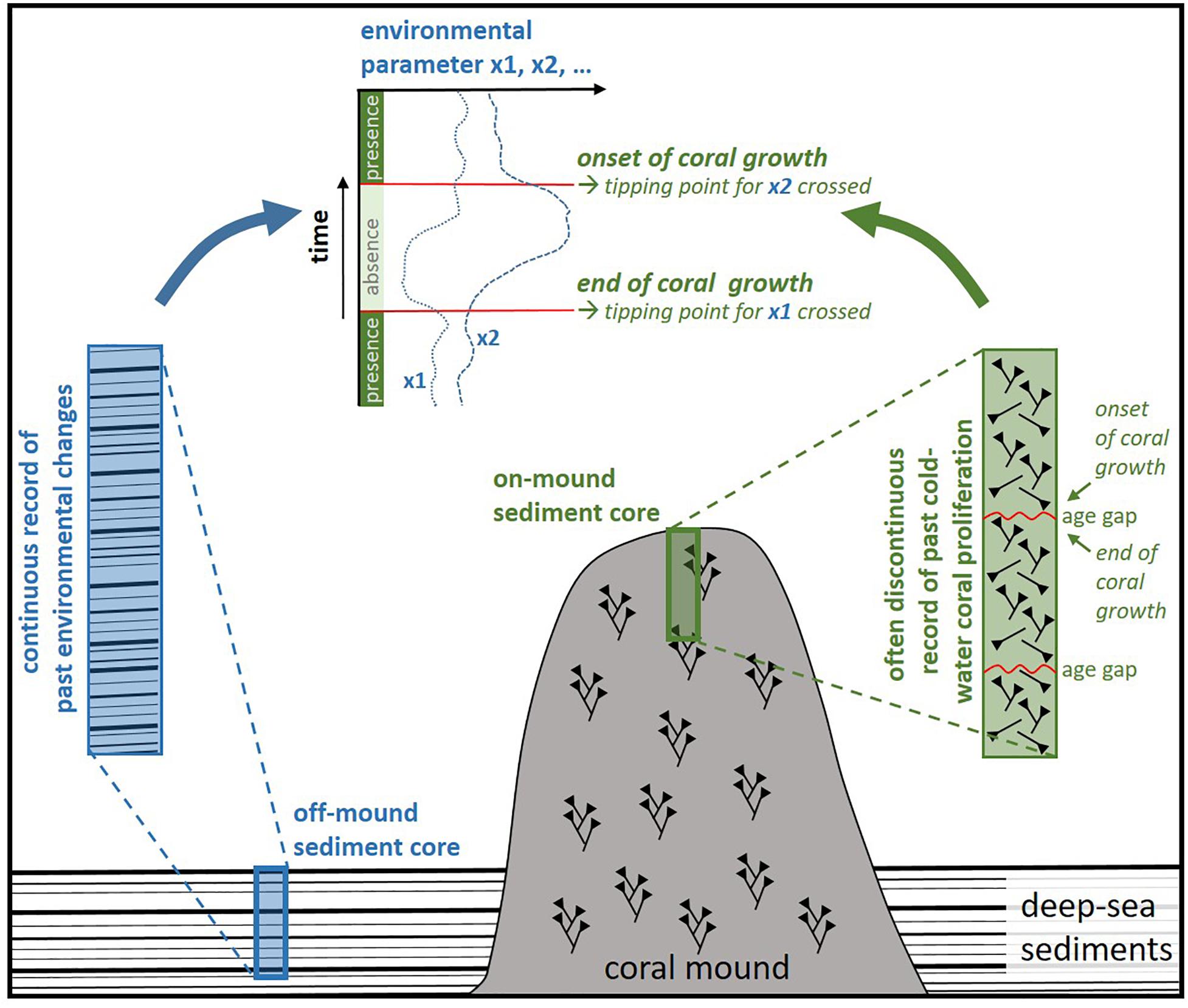
Figure 1. Schematic presentation of the “geological approach” to assess the sensitivity of cold-water corals to environmental change and to identify the key ecological drivers being able to push CWC across tipping points causing the demise/return of entire populations. By combining cold-water coral records retrieved from coral mounds with continuous paleoenvironmental records, such key drivers can be identified.
To assess the paleoenvironmental conditions controlling the proliferation of CWC, various proxies can be applied to reconstruct past conditions at the seabed and within the bottom waters bathing CWC reefs. The most common proxy carrier to describe past conditions at the seabed are benthic foraminifera. The chemical composition of their calcitic shells provides information, e.g., on bottom water temperatures (using Mg/Ca ratios, Lear et al., 2002), salinities (by combining Mg/Ca-derived temperatures, Lear et al., 2002, with stable oxygen isotope data, Marchitto et al., 2014), oxygenation (using Mn/Ca ratios, Groeneveld and Filipsson, 2013), pH (using boron isotopes, Rae et al., 2011), and bottom water provenance (using 𝜀Nd and stable carbon isotope data, Mackensen and Bickert, 1999; Tachikawa et al., 2017). In addition, their accumulation rate gives an indication about the food supply to the benthic realm resulting from surface ocean productivity (Herguera and Berger, 1991). Besides the information gained from benthic foraminifera, the grain-size distribution of the terrigenous portion of the sediments (originating from river and wind input) allows to reconstruct the hydrodynamic conditions at the seabed (McCave et al., 2017). These were identified as being one critical parameter for the occurrence of CWC beside productivity and the physical-chemical properties of the bottom waters (e.g., Davies et al., 2008; Hebbeln et al., 2016).
Combining these proxies in a multi-proxy approach allows for a precise description of paleoenvironmental changes (Fischer and Wefer, 1999). However, although most of these proxies provide quantitative results describing former conditions, due to rather large error bars the results usually should be seen as semi-quantitative or sometimes only as qualitative estimates. Nevertheless, these records provide reliable archives of environmental change through time and linking these records with a pattern of coral presence and absence allows to pinpoint the most dramatic paleoenvironmental changes co-occurring with the on-set/off-set of CWC reefs. With such a multi-proxy approach, key environmental drivers pushing the corals across their tipping points can be identified as it is exemplarily demonstrated in the case studies presented below.
To highlight the applicability of the concept outlined above, here we present brief overviews of three case studies. These and other studies (e.g., Wienberg et al., 2010, 2018; López Correa et al., 2012; Fink et al., 2013; Stalder et al., 2015; Raddatz et al., 2016; Matos et al., 2017) already give some clear indications about key environmental drivers, which, can, however, be regionally very different. Nevertheless, a comprehensive approach with a similar set of proxies addressing the most likely critical environmental parameters for various CWC settings is still missing.
The Irish margin is characterized by the widespread occurrence of thriving CWC, which today colonize the top of coral mounds. Coral mounds are arranged in large mound provinces with many of these located within the Porcupine Seabight (White and Dorschel, 2010). There, CWC began to settle ∼2.6. million years ago (Kano et al., 2007; Huvenne et al., 2009), supported by high primary productivity (Raddatz et al., 2014b) and by vigorous hydrodynamics (Thierens et al., 2010) that also play an important role for the food supply to the sessile, suspension-feeding corals (Hebbeln et al., 2016). After a major hiatus in the Irish coral mound records spanning the period from 1.7 to 1 million years ago (Kano et al., 2007), the intensification of the climatic cycles through the Late Quaternary limited CWC proliferation to warm interglacial conditions (at least during the last 300,000 years) with the most recent phase of CWC presence (re-)starting at the onset of the Holocene at 11,500 years BP (Dorschel et al., 2005; Frank et al., 2011).
During the early Holocene, the food supply to the seabed off Ireland increased, triggered by enhanced surface ocean productivity after the polar front has moved to the north of the region (Rüggeberg et al., 2007). At the same time, the bottom water dynamics also strengthened significantly due to the return of the Mediterranean Outflow Water to those water depths (600–800 m) inhabited by the CWC (Dorschel et al., 2005; Rüggeberg et al., 2007). From a record obtained slightly further south, the intermediate water conditions for the last glacial period are described as well-oxygenated and marked by relatively warm temperatures (Mojtahid et al., 2017). Thus, the major environmental changes associated with the return of the CWC to the Irish margin are an increase in surface water productivity as well as a re-organization of the regional water column structure affecting the bottom water hydrodynamics. Combined, both changes clearly increased the food supply to the corals, which probably triggered the re-establishment of CWC reefs on the Irish margin at around 11,500 years BP (Frank et al., 2011).
Off the United States east coast, CWC reefs exist off Cape Lookout where they are occasionally reached by meanders of the Gulf Stream exposing them to highly variable environmental conditions especially in terms of bottom-water temperature and dynamics (Mienis et al., 2014). Sediment cores revealed that this vivid CWC site was re-established at ∼7,000 years BP, i.e., well after full interglacial conditions have been reached globally. Also at this site, bottom-water temperatures and surface ocean productivity reached interglacial levels already at ∼12,000 years BP, however, without enabling the CWC to return. The major environmental change affecting this site at ∼7,000 years BP was a dramatic increase of the hydrodynamic energy at the seabed (Matos et al., 2015). This change, most likely caused by the impingement of the Gulf Stream on the upper continental slope, is assumed to have triggered the re-establishment of the CWC by creating a setting that could provide sufficient food probably by delivering food particles at a higher rate (Matos et al., 2015).
Off Santa Maria di Leuca on the Apulian margin in the Ionian Sea, CWC reefs occur under relatively high temperatures and salinities (Taviani et al., 2005; Freiwald et al., 2009). At this site, CWC reef development started at around 13,000 years BP (McCulloch et al., 2010; Fink et al., 2012). Most interesting here is an interruption of CWC proliferation between 11,000 and 6,000 years BP (Fink et al., 2012) that coincided with the Sapropel S1 event during which the entire eastern Mediterranean Sea below 1,800 m turned anoxic (De Lange et al., 2008). Decreasing oxygen contents also affected the CWC site at intermediate depths (∼600 m) causing a drastic reduction of the dissolved oxygen concentrations from relatively high “Holocene” values of ∼4 mL L-1 (Freiwald et al., 2009; Fink et al., 2012) to concentrations of <2 mL L-1 prevailing during the Sapropel S1 event (Fink et al., 2012). In contrast, cooler water temperatures and lower salinities combined with enhanced eutrophication during the Sapropel S1 event probably would have improved the conditions for the CWC. Thus, their temporal demise most likely has to be attributed to the poor oxygenation of the bottom waters at that time acting as a stressor to the CWC (Fink et al., 2012).
Analyzing the long-term development of CWC in the past reveals that CWC are apparently quite tolerant to environmental change as crossings of tipping points are mostly limited to major environmental overturns, although also short-term extinction events have been described on a regional scale (Fink et al., 2012, 2015; López Correa et al., 2012; Raddatz et al., 2016). While major environmental overturns associated with transitions from glacial to interglacial conditions and vice versa might cause the demise of CWC at one site, they might support coral re-settlement at another site (e.g., Frank et al., 2011) highlighting the complex response of CWC to environmental changes. Still, open questions regarding the sensitivity of CWC to environmental change relate to (i) the rate of their adaptation capability in relation to the rate of environmental change and (ii) their strategies to cope with the effects of multiple stressors.
With respect to ongoing/future global warming, increasing temperature is often mentioned as a serious threat for CWC in the future (e.g., Lunden et al., 2014; Sweetman et al., 2017). However, the still few available studies indicate that CWC react very sensitive to changes in the food supply, either driven by changing surface ocean productivity (e.g., Rüggeberg et al., 2007; Wienberg et al., 2010) or by changing hydrodynamics (e.g., Dorschel et al., 2005; Fink et al., 2013; Matos et al., 2015, 2017), and in bottom-water oxygenation (Fink et al., 2012). Nevertheless, in a recent study, the combined impact of high temperatures and low oxygenation in limiting CWC proliferation has been identified (Wienberg et al., 2018), highlighting the importance of considering the effects of multiple stressors when investigating the sensitivity of CWC to environmental change.
Although proxies are available (e.g., Raddatz et al., 2014a; McCulloch et al., 2017), the effects of ocean acidification are difficult to cover by this geological approach, as the younger geological history provides no equivalent to pH variability expected until the end of this century. However, although ocean acidification has been identified as another major future threat for CWC (Guinotte et al., 2006), the discovery of their capacity to cope with more acidic conditions by pH upregulation in the coral calcifying fluid (Anagnostou et al., 2012) makes it presently difficult to assess the sensitivity of CWC to increasing pH in the future.
Combining observational data and multivariate statistical analyses has provided a wealth of information on ranges of individual environmental parameters tolerated by CWC (e.g., Davies et al., 2008; Davies and Guinotte, 2011). In addition, laboratory experiments provided some insight into maximum and/or minimum tolerated levels of some environmental parameters (e.g., Tsounis et al., 2010; Gori et al., 2014; Movilla et al., 2014; Naumann et al., 2014; Maier et al., 2016; Büscher et al., 2017), however, with partly contrasting results from different studies and areas (e.g., Dodds et al., 2007; Lunden et al., 2014) probably hinting to regional adaptations.
However, to what extent any reported environmental ranges define tipping points that upon crossing might cause the local/regional extinction of a population remains largely unanswered, in particular because during the observational/instrumental period no crossing of such an environmental tipping point for CWC has been documented. By adding a geological perspective to this problem, the approach outlined here offers significant additional information on environmental changes that indeed ultimately resulted in the crossing of a tipping point for the CWC. Understanding the response of CWC to past environmental changes allows to identify key drivers able to push a population beyond such a tipping point – in terms of demise and of re-establishment. Interestingly, the available data indicate food supply as the most prominent key driver, which appears to be controlled by a complex interplay of surface-ocean productivity and bottom-water hydrodynamics. However, this observation might point to a hidden impact of multiple stressors that often are energetically challenging for the metabolism of marine species, which potentially can be compensated by the availability of large quantities of high quality organic matter (Diaz and Rosenberg, 1995).
Consequently, in order to estimate the likely response of individual CWC species to those environmental changes expected until the end of this century (and beyond), future geological as well as biological studies investigating their respective sensitivity need to consider the role of multiple stressors and possibly compensating factors. Of similar importance will be a better understanding of regionally varying tolerances of CWC, a topic that has just begun to emerge.
In the future, a close cooperation of biologists and geologists (and oceanographers) to define the sensitivity of CWC to environmental change bears a great potential to assess the fate of CWC in the course of ongoing global change. Knowledge gained from such a cooperation will provide pivotal information to optimize strategies for the management of the unique deep-sea ecosystems formed by CWC.
DH and RP-R developed the concept for this overview. JT and CW compiled information for the case studies. DH wrote the manuscript. All authors contributed substantially to the discussion of the approach outlined here.
This work is a contribution to the ATLAS project and has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 678760. The paper reflects the authors’ views and the European Union is not responsible for any use that may be made of the information it contains.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The support through the European Union is greatly acknowledged. Special thanks go to all colleagues who contributed over the last years to the development of the “geological approach” outlined here.
Anagnostou, E., Huang, K. F., You, E. F., Sikes, E. L., and Sherrell, R. M. (2012). Evaluation of boron isotope ratio as a pH proxy in the deep-sea coral Desmophyllum dianthus: evidence of physiological pH adjustment. Earth Planet. Sci. Lett. 349–350, 251–260. doi: 10.1016/j.epsl.2012.07.006
Armstrong, C. W., and van den Hove, S. (2008). The formation of policy for protection of cold-water coral off the coast of Norway. Mar. Policy 32, 66–73. doi: 10.1016/j.marpol.2007.04.007
Auster, P. J., Gjerde, K., Heupel, E., Watling, L., Grehan, A., and Rogers, A. D. (2011). Definition and detection of vulnerable marine ecosystems on the high seas: problems with the “move-on” rule. ICES J. Mar. Sci. 68, 254–264. doi: 10.1093/icesjms/fsq074
Brooke, S., Ross, S. W., Bane, J. M., Seim, H. E., and Young, C. M. (2013). Temperature tolerance of the deep-sea coral Lophelia pertusa from the southeastern United States. Deep Sea Res. Part 2 Top. Stud. Oceanogr. 92, 240–248. doi: 10.1016/j.dsr2.2012.12.001
Büscher, J. V., Form, A. U., and Riebesell, U. (2017). Interactive effects of ocean acidification and warming on growth, fitness and survival of the cold-water coral Lophelia pertusa under different food availabilities. Front. Mar. Sci. 4:101. doi: 10.3389/fmars.2017.00101
Davies, A. J., and Guinotte, J. M. (2011). Global habitat suitability for framework-forming cold-water corals. PLoS One 6:e18483. doi: 10.1371/journal.pone.0018483
Davies, A. J., Wisshak, M., Orr, J. C., and Roberts, J. M. (2008). Predicting suitable habitat for the cold-water coral Lophelia pertusa (Scleractinia). Deep Sea Res. Part I Oceanogr. Res. Pap. 55, 1048–1062. doi: 10.1016/j.dsr.2008.04.010
De Lange, G. J., Thomson, J., Reitz, A., Slomp, C. P., Speranza Principato, M., Erba, E., et al. (2008). Synchronous basin-wide formation and redox-controlled preservation of a Mediterranean sapropel. Nat. Geosci. 1, 606–610. doi: 10.1038/ngeo283
Diaz, R. J., and Rosenberg, R. (1995). Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. Ann. Rev. 33, 245–303.
Dodds, L. A., Roberts, J. M., Taylor, A. C., and Marubini, F. (2007). Metabolic tolerance of the cold-water coral Lophelia pertusa (Scleractinia) to temperature and dissolved oxgen change. J. Exp. Mar. Biol. Ecol. 349, 205–214. doi: 10.1016/j.jembe.2007.05.013
Dorschel, B., Hebbeln, D., Rüggeberg, A., Dullo, W. C., and Freiwald, A. (2005). Growth and erosion of a cold-water coral covered carbonate mound in the Northeast Atlantic during the Late Pleistocene and Holocene. Earth Planet. Sci. Lett. 233, 33–44. doi: 10.1016/j.epsl.2005.01.035
Dullo, W. C., Flögel, S., and Rüggeberg, A. (2008). Cold-water coral growth in relation to the hydrography of the celtic and nordic european continental margin. Mar. Ecol. Prog. Ser. 371, 165–176. doi: 10.3354/meps07623
Eisele, M., Hebbeln, D., and Wienberg, C. (2008). Growth history of a cold-water coral covered carbonate mound-galway mound, porcupine seabight, NE-Atlantic. Mar. Geol. 253, 160–169. doi: 10.1016/j.margeo.2008.05.006
Fink, H. G., Wienberg, C., De Pol-Holz, R., and Hebbeln, D. (2015). Spatio-temporal distribution patterns of Mediterranean cold-water corals (Lophelia pertusa and Madrepora oculata) during the past 14,000 years. Deep Sea Res. Part I Oceanogr. Res. Pap. 103, 37–48. doi: 10.1016/j.dsr.2015.05.006
Fink, H. G., Wienberg, C., DePol-Holz, R., Wintersteller, P., and Hebbeln, D. (2013). Cold- water coral growth in the alboran sea related to high productivity during the late pleistocene and holocene. Mar. Geol. 339, 71–82. doi: 10.1016/j.margeo.2013.04.009
Fink, H. G., Wienberg, C., Hebbeln, D., McGregor, H. V., Schmiedl, G., Taviani, M., et al. (2012). Oxygen control on holocene cold-water coral development in the eastern mediterranean sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 62, 89–96. doi: 10.1016/j.dsr.2011.12.013
Fischer, G., and Wefer, G. (eds.) (1999). Use of Proxies in Paleoceanography. Heidelberg: Springer. doi: 10.1007/978-3-642-58646-0
Flögel, S., Dullo, W. C., Pfannkuche, O., Kiriakoulakis, K., and Rüggeberg, A. (2014). Geochemical and physical constraints for the occurrence of living cold-water corals. Deep Sea Res. Part II Top. Stud. Oceanogr. 99, 19–26. doi: 10.1016/j.dsr2.2013.06.006
Frank, N., Freiwald, A., López Correa, M., Wienberg, C., Eisele, M., Hebbeln, D., et al. (2011). Northeastern atlantic cold-water coral reefs and climate. Geology 39, 743–746. doi: 10.1130/G31825.1
Frank, N., Ricard, E., Lutringer-Paquet, A., Van der Land, C., Colin, C., Blamart, D., et al. (2009). The holocene occurrence of cold-water corals in the NE atlantic: implications for coral carbonate mound evolution. Mar. Geol. 266, 129–142. doi: 10.1016/j.margeo.2009.08.007
Freiwald, A. (2002). “Reef-forming cold-water corals,” in Ocean Margin Systems, eds G. Wefer, D. Billett, D. Hebbeln, B. B. Jorgensen, M. Schlüter, and T. C. E. van Weering (Heidelberg: Springer), 365–385. doi: 10.1007/978-3-662-05127-6_23
Freiwald, A., Beuck, L., Rüggeberg, A., Taviani, M., and Hebbeln, D. (2009). The white coral community in the central mediterranean sea revealed by ROV surveys. Oceanography 22, 58–74. doi: 10.5670/oceanog.2009.06
Gori, A., Grover, R., Orejas, C., Sikorski, S., and Ferrier-Pagès, C. (2014). Uptake of dissolved free amino acids by four cold-water coral species from the mediterranean sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 99, 42–50. doi: 10.1016/j.dsr2.2013.06.007
Groeneveld, J., and Filipsson, H. L. (2013). Mg/Ca and Mn/Ca ratios in benthic foraminifera: the potential to reconstruct past variations in temperature and hypoxia in shelf regions. Biogeoscience 10, 5125–5138. doi: 10.5194/bg-10-5125-2013
Guinotte, J. M., Orr, J. C., Cairns, S. D., Freiwald, A., Morgan, L., Georgeet, R., et al. (2006). Will human-induced changes in seawater chemistry alter the distribution of deep-sea scleractinian corals? Front. Ecol. Environ. 4:141–146. doi: 10.1890/1540-929520060040141
Hebbeln, D., Van Rooij, D., and Wienberg, C. (2016). Good neighbours shaped by vigorous currents: cold-water coral mounds and contourites in the north atlantic. Mar. Geol. 378, 114–126. doi: 10.1016/j.margeo.2016.01.014
Hebbeln, D., Wienberg, C., Wintersteller, P., Freiwald, A., Becker, M., Beuck, L., et al. (2014). Environmental forcing of the campeche cold-water coral province, southern gulf of mexico. Biogeoscience 11, 1799–1815. doi: 10.5194/bg-11-1799-2014
Herguera, J. C., and Berger, W. H. (1991). Paleoproductivity from benthic foraminifera abundance: glacial to postglacial change in the west-equatorial pacific. Geology 19, 1173–1176. doi: 10.1130/0091-7613(1991)019<1173:PFBFAG>2.3.CO;2
Huvenne, V. A. I., Bett, B. J., Masson, D. G., Le Bas, T. P., and Wheeler, A. J. (2016). Effectiveness of a deep-sea cold-water coral marine protected area, following eight years of fisheries closure. Biol. Conserv. 200, 60–69. doi: 10.1016/j.biocon.2016.05.030
Huvenne, V. A. I., Van Rooij, D., De Mol, B., Thierens, M., O’Donnell, R., and Foubert, A. (2009). Sediment dynamics and palaeo-environmental context at key stages in the challenger cold-water coral mound formation: clues from sediment deposits at the mound base. Deep Sea Res. Part I Oceanogr. Res. Pap. 56, 2263–2280. doi: 10.1016/j.dsr.2009.08.003
Jackson, E. L., Davies, A. J., Howell, K. L., Kershaw, P. J., and Hall-Spencer, J. M. (2014). Future-proofing marine protected area networks for cold-water coral reefs. ICES J. Mar. Sci. 71, 2621–2629. doi: 10.1093/icesjms/fsu099
Kano, A., Ferdelman, T. G., Williams, T., Henriet, J. P., Ishikawa, T., Kawagoe, N., et al. (2007). Age constraints on the origin and growth history of a deep-water coral mound in northeast Atlantic drilled during integrated ocean drilling program expedition 307. Geology 35, 1051–1054. doi: 10.1130/G23917A.1
Lear, C. H., Rosenthal, Y., and Slowey, N. (2002). Benthic foraminiferal Mg/Ca-paleothermometry: a revised core-top calibration. Geochim. Cosmochim. Acta 66, 3375–3387. doi: 10.1016/S0016-7037(02)00941-9
López Correa, M., Montagna, P., Joseph, N., Rüggeberg, A., Fietzke, J., Flögel, S., et al. (2012). Preboreal onset of cold-water coral growth beyond the arctic circle revealed by coupled radiocarbon and U-series dating and neodymium isotopes. Quat. Sci. Rev. 34, 24–34. doi: 10.1016/j.quascirev.2011.12.005
Lunden, J. J., McNicholl, C. G., Sears, C. R., Morrison, C. L., and Cordes, E. E. (2014). Acute survivorship of the deep-sea coral Lophelia pertusa from the gulf of mexico under acidification, warming, and deoxygenation. Front. Mar. Sci. 1:78. doi: 10.3389/fmars.2014.00078
Mackensen, A., and Bickert, T. (1999). “Stable carbon isotopes in benthic foraminifera: proxies for deep and bottom water circulation and new production,” in Use of Proxies in Paleoceanography - Examples from the South Atlantic, eds G. Fischer and G. Wefer (Heidelberg: Springer),229–254.
Maier, C., Popp, P., Sollfrank, N., Weinbauer, M. G., Wild, C., and Gattuso, J.-P. (2016). Effects of elevated pCO2 and feeding on net calcification and energy budget of the mediterranean cold-water coral Madrepora oculata. J. Exp. Biol. 219, 3208–3217.
Mangini, A., Godoy, J. M., Godoy, M. L., Kowsmann, R., Santos, G. M., Ruckelshausen, M., et al. (2010). Deep sea corals off brazil verify a poorly ventilated southern pacific ocean during H2, H1 and the younger dryas. Earth Planet. Sci. Lett. 293, 269–276.
Marchitto, T. M., Curry, W. B., Lynch-Stieglitz, J., Bryan, S. P., Cobb, K. M., and Lund, D. C. (2014). Improved oxygen isotope temperature calibrations for cosmopolitan benthic foraminifera. Geochim. Cosmochim. Acta 130, 1–11.
Matos, L., Mienis, F., Wienberg, C., Frank, N., Kwiatkowski, C., Groeneveld, J., et al. (2015). Interglacial occurrence of cold-water corals off cape lookout (NW Atlantic): first evidence of the gulf stream influence. Deep Sea Res. Part I Oceanogr. Res. Pap. 105, 158–170.
Matos, L., Wienberg, C., Titschack, J., Schmiedl, G., Frank, N., Abrantes, F., et al. (2017). Coral mound development at the campeche cold-water coral province, southern gulf of mexico: implications of antarctic intermediate water increased influence during interglacials. Mar. Geol. 392, 53–65.
McCave, I. N., Thornalley, D. J. R., and Hall, I. R. (2017). Relation of sortable silt grain-size to deep-sea current speeds: calibration of the ‘Mud Current Meter’. Deep Sea Res. Part I Oceanogr. Res. Pap. 127, 1–12.
McCulloch, M., Taviani, M., Montagna, P., López Correa, M., Remia, A., and Mortimer, G. (2010). Proliferation and demise of deep-sea corals in the mediterranean during the younger dryas. Earth Planet. Sci. Lett. 298, 143–152.
McCulloch, M. T., D’Olivo, J. P., Falter, J., Holcomb, M., and Trotter, J. A. (2017). Coral calcification in a changing world and the interactive dynamics of pH and DIC upregulation. Nat. Commun. 8:15686. doi: 10.1038/ncomms15686
Mienis, F., Duineveld, G. C. A., Davies, A. J., Lavaleye, M. M. S., Ross, S. W., Seim, H., et al. (2014). Cold-water coral growth under extreme environmental conditions, the cape lookout area, NW Atlantic. Biogeoscience 11, 2543–2560.
Mienis, F., Van der Land, C., De Stigter, H. C., Van de Vorstenbosch, M., De Haas, H., Richter, T., et al. (2009). Sediment accumulation on a cold-water carbonate mound at the southwest rockall trough margin. Mar. Geol. 265, 40–50.
Mienis, F., Van Weering, T., De Haas, H., De Stigter, H., Huvenne, V., and Wheeler, A. (2006). Carbonate mound development at the SW rockall trough margin based on high resolution TOBI and seismic recording. Mar. Geol. 233, 1–19. doi: 10.1038/ncomms15686
Mojtahid, M., Toucanne, S., Fentimen, R., Barras, C., Le Houedec, S., Soulet, G., et al. (2017). Changes in northeast atlantic hydrology during termination 1: insights from celtic margin’s benthic foraminifera. Quat. Sci. Rev. 175, 45–59.
Movilla, J., Gori, A., Calvo, E., Orejas, C., Lopez-Sanz, A., Dominguez-Carrio, D., et al. (2014). Resistance of two mediterranean cold-water coral species to low-pH conditions. Water 6, 59–67. doi: 10.1371/journal.pone.0062655
Muñoz, A., Cristobo, J., Rios, P., Druet, M., Polonio, V., Uchupi, E., et al. (2012). Sediment drifts and cold-water coral reefs in the patagonian upper and middle continental slope. Mar. Petrol. Geol. 36, 70–82.
Naumann, M. S., Orejas, C., Wild, C., and Ferrier-Pagès, C. (2014). First evidence for zooplankton feeding sustaining key physiological processes in a scleractinian cold-water coral. J. Exp. Biol. 214, 3570–3576. doi: 10.1242/jeb.061390
Raddatz, J., Liebetrau, V., Trotter, J., Rüggeberg, A., Flögel, S., Eisenhauer, A., et al. (2016). Environmental constraints on holocene cold-water coral reef growth off norway: insights from a multiproxy approach. Paleoceanography 31, 1350–1367.
Raddatz, J., Rüggeberg, A., Floegel, S., Hathorne, E., Liebetrau, V., Eisenhauer, A., et al. (2014a). The influence of seawater pH on U/Ca ratios in the scleractinian cold-water coral Lophelia pertusa. Biogeoscience 11, 1863–1871.
Raddatz, J., Rüggeberg, A., Liebetrau, V., Foubert, A., Hathorne, E. C., Fietzke, J., et al. (2014b). Environmental boundary conditions of cold-water coral mound growth over the last 3 million years in the porcupine seabight, northeast atlantic. Deep Sea Res. Part II Top. Stud. Oceanogr. 99, 227–236.
Rae, J. W. B., Foster, G. L., Schmidt, D. N., and Elliott, T. (2011). Boron isotopes and B/Ca in benthic foraminifera: proxies for the deep ocean carbonate system. Earth Planet. Sci. Lett. 302, 403–413.
Ramos, A., Sanz, J. L., Ramil, F., Agudo, L. M., and Presas-Navarro, C. (2017). “The giant cold-water coral mounds barrier off Mauritania,” in Deep-Sea Ecosystems Off Mauritania: Research of Marine Biodiversity and Habitats in the Northwest African Margin, eds A. Ramos, F. Ramil, and J. L. Sanz (Dordrecht: Springer), 481–525.
Roberts, J. M., Wheeler, A. J., and Freiwald, A. (2009). Cold-Water Corals — The Biology and Geology of Deep-Sea Coral Habitats. Cambridge: Cambridge University Press.
Robinson, L. F., Adkins, J. F., Frank, N., Gagnon, A. C., Prouty, N. G., Brendan Roark, E., et al. (2014). The geochemistry of deep-sea coral skeletons: a review of vital effects and applications for palaeoceanography. Deep Sea Res. Part II Top. Stud. Oceanogr. 99, 184–198.
Rüggeberg, A., Dullo, C., Dorschel, B., and Hebbeln, D. (2007). Environmental changes and growth history of a cold-water coral mound (Propeller Mound, Porcupine Seabight). Int. J. Earth Sci. 96, 57–72.
Stalder, C., Vertino, A., Rüggeberg, A., Pirkenseer, C., Camozzi, O., Rappo, S., et al. (2015). Microfossils, a key to unravel cold-water carbonate mound evolution through time: evidence from the eastern alboran sea. PLoS One 10:e0140223. doi: 10.1371/journal.pone.0140223
Sweetman, A. K., Thurber, A. R., Smith, C. R., Levin, L. A., Mora, C., Wei, C. L., et al. (2017). Major impacts of climate change on deep-sea benthic ecosystems. Elem. Sci. Anth. 5:4. doi: 10.1525/elementa.203
Tachikawa, K., Arsouze, T., Bayon, G., Bory, A., Colin, C., Dutay, J.-C., et al. (2017). The large-scale evolution of neodymium isotopic composition in the global modern and holocene ocean revealed from seawater and archive data. Chem. Geol. 457, 131–148. doi: 10.1016/j.chemgeo.2017.03.018
Taviani, M., Remia, A., Corselli, C., Freiwald, A., Malinverno, E., Mastrototaro, F., et al. (2005). First geo-marine survey of living cold-water lophelia reefs in the ionian sea (Mediterranean basin). Facies 50, 409–417.
Taviani, M., Vertino, A., López Correa, M., Savini, A., De Mol, B., Remia, A., et al. (2011). Pleistocene to recent scleractinian deep-water corals and coral facies in the eastern mediterranean. Facies 57, 579–603.
Thierens, M., Browning, E., Pirlet, H., Loutre, M. F., Dorschel, B., Huvenne, V. A. I., et al. (2013). Cold-water coral carbonate mounds as unique palaeo-archives: the plio-pleistocene challenger mound record (NE Atlantic). Quat. Sci. Rev. 73, 14–30.
Thierens, M., Titschack, J., Dorschel, B., Huvenne, V. A. I., Wheeler, A. J., Stuut, J.-B., et al. (2010). The 2.6 Ma depositional sequence from the challenger cold-water coral carbonate mound (IODP Exp. 307): sediment contributors and hydrodynamic paleo-environments. Mar. Geol. 271, 260–277.
Tsounis, G., Orejas, C., Reynaud, S., Gili, J. M., Allemand, D., and Ferrier-Pagès, C. (2010). Prey-capture rates in four mediterranean cold water corals. Mar. Ecol. Prog. Ser. 398, 149–155.
Van der Land, C., Eisele, M., Mienis, F., De Haas, H., Hebbeln, D., Reijmer, J. J. G., et al. (2014). Carbonate mound development in contrasting settings on the irish margin. Deep Sea Res. Part II Top. Stud. Oceanogr. 99, 297–306.
Victorero, L., Blamart, D., Pons-Branchu, E., Mavrogordato, M. N., and Huvenne, V. A. I. (2016). Reconstruction of the formation history of the darwin mounds, N rockall trough: how the dynamics of a sandy contourite affected cold-water coral growth. Mar. Geol. 378, 186–195.
White, M., and Dorschel, B. (2010). The importance of the permanent thermocline to the cold-water coral carbonate mound distribution in the NE Atlantic. Earth Planet. Sci. Lett. 296, 395–402.
Wienberg, C., Frank, N., Mertens, K., Stuut, J.-B., Marchant, M., Fietzke, J., et al. (2010). Glacial cold-water corals growth in the gulf of Cádiz: implications of increased palaeo-productivity. Earth Planet. Sci. Lett. 298, 405–416. doi: 10.1016/j.epsl.2010.08.017
Wienberg, C., and Titschack, J. (2017). “Framework-forming scleractinian cold-water corals through space and time: A late Quaternary North Atlantic perspective,” in Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots, eds S. Rossi, L. Bramanti, A. Gori, and C. Orejas Saco del Valle (Cham: Springer), 699–732.
Keywords: cold-water corals, tipping points, key environmental drivers, geological record, global change
Citation: Hebbeln D, Portilho-Ramos RdC, Wienberg C and Titschack J (2019) The Fate of Cold-Water Corals in a Changing World: A Geological Perspective. Front. Mar. Sci. 6:119. doi: 10.3389/fmars.2019.00119
Received: 23 November 2018; Accepted: 27 February 2019;
Published: 18 March 2019.
Edited by:
Telmo Morato, Universidade dos Açores, PortugalReviewed by:
Lorenzo Angeletti, Istituto di Scienze Marine (ISMAR), ItalyCopyright © 2019 Hebbeln, Portilho-Ramos, Wienberg and Titschack. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dierk Hebbeln, ZGhlYmJlbG5AbWFydW0uZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.