
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mar. Sci. , 27 June 2018
Sec. Ocean Observation
Volume 5 - 2018 | https://doi.org/10.3389/fmars.2018.00211
This article is part of the Research Topic Best Practices in Ocean Observing View all 85 articles
 Frank E. Muller-Karger1*
Frank E. Muller-Karger1* Patricia Miloslavich2,3
Patricia Miloslavich2,3 Nicholas J. Bax2,4
Nicholas J. Bax2,4 Samantha Simmons5
Samantha Simmons5 Mark J. Costello6
Mark J. Costello6 Isabel Sousa Pinto7
Isabel Sousa Pinto7 Gabrielle Canonico8
Gabrielle Canonico8 Woody Turner9
Woody Turner9 Michael Gill10
Michael Gill10 Enrique Montes1
Enrique Montes1 Benjamin D. Best11
Benjamin D. Best11 Jay Pearlman12
Jay Pearlman12 Patrick Halpin13
Patrick Halpin13 Daniel Dunn13
Daniel Dunn13 Abigail Benson14
Abigail Benson14 Corinne S. Martin15
Corinne S. Martin15 Lauren V. Weatherdon15
Lauren V. Weatherdon15 Ward Appeltans16
Ward Appeltans16 Pieter Provoost16
Pieter Provoost16 Eduardo Klein3,16
Eduardo Klein3,16 Christopher R. Kelble17
Christopher R. Kelble17 Robert J. Miller18
Robert J. Miller18 Francisco P. Chavez19
Francisco P. Chavez19 Katrin Iken20
Katrin Iken20 Sanae Chiba16,21
Sanae Chiba16,21 David Obura22
David Obura22 Laetitia M. Navarro23,24
Laetitia M. Navarro23,24 Henrique M. Pereira23,24,25
Henrique M. Pereira23,24,25 Valerie Allain26
Valerie Allain26 Sonia Batten27
Sonia Batten27 Lisandro Benedetti-Checchi28
Lisandro Benedetti-Checchi28 J. Emmett Duffy29
J. Emmett Duffy29 Raphael M. Kudela30
Raphael M. Kudela30 Lisa-Maria Rebelo31
Lisa-Maria Rebelo31 Yunne Shin32
Yunne Shin32 Gary Geller33
Gary Geller33Measurements of the status and trends of key indicators for the ocean and marine life are required to inform policy and management in the context of growing human uses of marine resources, coastal development, and climate change. Two synergistic efforts identify specific priority variables for monitoring: Essential Ocean Variables (EOVs) through the Global Ocean Observing System (GOOS), and Essential Biodiversity Variables (EBVs) from the Group on Earth Observations Biodiversity Observation Network (GEO BON) (see Data Sheet 1 in Supplementary Materials for a glossary of acronyms). Both systems support reporting against internationally agreed conventions and treaties. GOOS, established under the auspices of the Intergovernmental Oceanographic Commission (IOC), plays a leading role in coordinating global monitoring of the ocean and in the definition of EOVs. GEO BON is a global biodiversity observation network that coordinates observations to enhance management of the world's biodiversity and promote both the awareness and accounting of ecosystem services. Convergence and agreement between these two efforts are required to streamline existing and new marine observation programs to advance scientific knowledge effectively and to support the sustainable use and management of ocean spaces and resources. In this context, the Marine Biodiversity Observation Network (MBON), a thematic component of GEO BON, is collaborating with GOOS, the Ocean Biogeographic Information System (OBIS), and the Integrated Marine Biosphere Research (IMBeR) project to ensure that EBVs and EOVs are complementary, representing alternative uses of a common set of scientific measurements. This work is informed by the Joint Technical Commission for Oceanography and Marine Meteorology (JCOMM), an intergovernmental body of technical experts that helps international coordination on best practices for observing, data management and services, combined with capacity development expertise. Characterizing biodiversity and understanding its drivers will require incorporation of observations from traditional and molecular taxonomy, animal tagging and tracking efforts, ocean biogeochemistry, and ocean observatory initiatives including the deep ocean and seafloor. The partnership between large-scale ocean observing and product distribution initiatives (MBON, OBIS, JCOMM, and GOOS) is an expedited, effective way to support international policy-level assessments (e.g., the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services or IPBES), along with the implementation of international development goals (e.g., the United Nations Sustainable Development Goals).
“At some level in monitoring and marine ecology more generally, we have to turn the argument around so that we are monitoring recovery and restoration, or even resilience in the face of increasing threats - as well as the many ongoing declines. Monitoring is but a small and necessary cost to sustain or increase the value of the benefits we get from nature”.
Nicholas Bax, Institute for Marine and Antarctic Science, University of Tasmania, Australia.
For millennia, humans have sought to learn about the state of the ocean, driven by the need to know waves, currents, tides, and weather patterns to plan for daily life along the coast and to carry out migrations and commerce. The ocean covers 71% of the Earth's surface and contains 97% of the Earth's water, and thus is a three-dimensional ecosystem holding over 99% of the living space on Earth (Costello et al., 2010). Life in the sea has been of particular interest to humans in the quest to sustain and expand human population and cultures. Yet our ability to characterize and monitor marine life, including its diversity, abundance, productivity, and the interactions among different forms of life and the environment remains primitive. It was not until the late nineteenth century that oceanography was established as a multidisciplinary science by the Challenger Expedition (Rice, 1999). World War II propelled oceanography forward, given the need to understand the physical environment for national security purposes. Afterward, the industrialization of the ocean for increased fishing, offshore oil and gas extraction opportunities, combined with other human uses, continued to drive the need to improve our understanding of the global ocean. Our ability to observe, measure and understand the ocean has again grown dramatically since the 1980s, combining routine observations from satellites and autonomous devices like buoys, drifters and other devices to address critical operational requirements for the protection of human life and property (Costello et al., 2016). Scientific research has been instrumental in improving forecasts of the physical and chemical state of the ocean. It is now possible to assess the state of the global ocean surface from space continuously by using complementary measures of temperature, height (for ocean currents and global sea level estimates), roughness (for estimates of waves and winds), and color (for bulk phytoplankton biomass, colored dissolved matter, and water quality indices) (see summary in Muller-Karger et al., 2013). The ocean interior is monitored by a variety of observing platforms, some of which are organized in observing networks (e.g., Argo1, OceanSITES, the Global Ocean Ship-based Hydrographic Investigations Program or GO-SHIP, the Data Buoy Cooperation Panel or DBCP, OceanGlider, The Global Sea Level Observing System or GLOSS, etc.). Each observing platform is characterized by its specific sampling in time, space, and many have different suites of sensors. The observing networks are the backbone for the Global Ocean Observing System (GOOS) and its regional alliances (GOOS RA). The concerted use of a number of different observing platforms provides the sampling characteristics needed for one specific observing problem (UNESCO, 2015; Liblik et al., 2016). At present, almost all programs record physical data (temperature, salinity, etc.) while biogeochemical sensor and ecosystem parameters sensors are rare. However, there is progress and the networks are engaged in discussions on biological observations. An important international effort is underway to deploy biogeochemical Argo floats (BGC-Argo; Claustre et al., 2010; Johnson et al., 2017). As fishery and mining concerns evolve to extract resources in increasingly deeper waters, discussions are underway to develop a Deep Ocean Observing Strategy (DOOS). Numerical models seek to incorporate all of this information to improve weather and ocean prediction, as well as to understand details of the global heat budget.
In addition to the advances made in understanding the physical environment of the ocean, there is a renewed effort to organize the collection of information on biology and ecosystem characteristics including the status and trends in the abundance, movement, and genetic diversity of species located across large, traditionally undersampled areas of the ocean. For example, two programs under the International Geosphere-Biosphere Programme (IGBP) and the Scientific Committee on Oceanic Research (SCOR), specifically the Global Ocean Ecosystem Dynamics (GLOBEC; 1991–2001) and the Integrated Marine Biosphere Research project (IMBeR; established in 2005 and ongoing as of 2018 with support from SCOR and Future Earth), have sought to understand how global change will affect the abundance, diversity and productivity of marine populations. These programs aim to enable innovation and collaboration in marine ecosystem research to transform the world toward sustainability. This growing interest is sustained by human population growth, the increasing habitation of coastal shores, and accelerating use and industrialization of ocean spaces, both within Exclusive Economic Zones and in areas beyond national jurisdiction (Merrie et al., 2014; McCauley et al., 2015; Golden et al., 2017). Concerns over ecosystem impacts have led to efforts to track and limit harmful change. Examples of such efforts include the Aichi Biodiversity Targets of the UN Strategic Plan for Biodiversity (2010–2020), the negotiation of a new treaty for the conservation and sustainable use of biodiversity beyond national jurisdiction, and the UN Sustainable Development Goals (SDGs; UN Resolution A/RES/70/1 of 25 September 2015; Anderson et al., 2017). Information about life in the sea is needed to sustain a healthy ocean and the ecosystem services it provides, and to promote a vibrant blue economy by managing human activities that affect ocean life in multiple ways (U.S. Commission on Ocean Policy, 2004; Dunn et al., 2016; Golden et al., 2017). Although several of the SDGs touch on the ocean's ecosystem services (e.g., fisheries in relation to food security under SDG 2), SDG 14 specifically has six targets tied to marine life and associated ecosystem services (ICSU, 2017). Many other SDGs are closely linked to SDG 14, such as those concerning food security, health, gender equality, economic growth, climate action, and sustainable cities and responsible consumption (Singh et al., 2017). To address these requirements, the United Nations General Assembly (A/RES/72/73, December 2017) proclaimed the United Nations Decade of Ocean Science for Sustainable Development (2021–2030) and called upon the Intergovernmental Oceanographic Commission to prepare an implementation plan (UNESCO, 2017).
The scientific community has a responsibility to provide the information and knowledge needed to enable wise policy decisions, which can be achieved by assembling measurements of particular variables into indicators (Niemeijer, 2002) useful to governments and users of resources, for instance. The best approach to obtaining this information is through a multidisciplinary and transdisciplinary ocean observing system that promotes regional monitoring efforts and organizes them into a global network. Transdisciplinarity brings the natural, social, and health sciences together, spanning traditional disciplines (Choi and Pak, 2006; Cornell et al., 2013). In extending existing biological observing systems into a global network, we need to identify, obtain consensus on, and measure a core set of variables focused on quantifying biotic and abiotic changes in the ocean. This requires integration of biogeochemical and biological observations with the continually developing physical observing system and expanding even its broad geographic scope. Four main groups are now engaged in this discussion: the Marine Biodiversity Observation Network (MBON), the GOOS, the Ocean Biogeographic Information System (OBIS), and IMBeR. Many other groups are participating in this conversation, including the Marine Global Earth Observatory (MarineGEO) directed by the Smithsonian's Tennenbaum Marine Observatories Network (TMON), animal tracking efforts, deep ocean observing initiatives (e.g., the Deep Ocean Observing Strategy or DOOS, and the International Network for Scientific Investigations of Deep-Sea Ecosystems or INDEEP), the Global Ocean Acidification Observing Network (GOA-ON; Newton et al., 2015), groups focused on land-ocean interactions, and the public and private sector groups interested in ocean development and blue growth (Golden et al., 2017).
The technology for synoptic biological observations has lagged behind measurements of physical and biogeochemical properties of the ocean. There are highly selective sampling programs that have accumulated substantial observations of life in the sea. For example, the Sir Alister Hardy Foundation for Ocean Science (SAHFOS) has operated the Continuous Plankton Recorder (CPR), a device towed behind ships of opportunity, since 1946 (Reid et al., 2003; Costello et al., 2016). SAHFOS accumulated remarkable plankton collections along certain ship routes. The CPR surveys are now managed by the Marine Biological Association of the United Kingdom, which continues to expand CPR lines in other locations, although there is still a noticeable lack in the tropical ocean. MarineGEO is focusing on understanding coastal marine life and its role in maintaining resilient ecosystems around the world, where marine biodiversity and people are concentrated and interact most. MarineGEO is making important contributions by deploying simple experiments in different coastal environments around the world. The Bermuda-Atlantic Time-Series (BATS), Hawaii Ocean Time-Series (HOT), and the CARIACO Ocean Time-Series, have integrated a large number of observations of lower trophic level diversity and production with nutrient flux observations at point locations for periods exceeding 20 years (Church et al., 2013; Neuer et al., 2017; among many publications). The California Cooperative Oceanic Fisheries Investigations (CalCOFI), established in 1949 after years of efforts to understand the collapse of the sardine fishery off California, continues a time series of observations that span all trophic levels (Bograd and Lynn, 2003; Wells et al., 2017; and many others).
There is growing interest in networking microbial observatories around the world to monitor EOVs and EBVs at time and space scales relevant to human health (Buttigieg et al., 2018). Matching the broad geographic scope of ship-based observation with the detailed information of point location data is a key requirement for a global system that requires the expansion of existing networks, accompanied with developing new technologies that can gather marine biodiversity observations with increasing detail and efficiency. While progressing quickly, available technology and systems limit the ability of scientists to link observations at these scales to higher trophic levels. Evaluating the density of OBIS records as a function of bottom depth and vertical sampling depth reveals major gaps in the mesopelagic (Sutton et al., 2017; Muller-Karger et al., 2018) and deep ocean zones (Webb et al., 2010; Appeltans et al., 2016). Some of the programs that have provided long time series of observations, such as the CARIACO Time-Series, have subsequently stopped due to lack of funding and conditions that make logistics difficult.
Despite many individual efforts, our overall current set of observations is inadequate to measure, characterize, and monitor the changing life of the sea across trophic levels at most regional scales, and definitely at a global scale. Such links are of direct utility to managing for sustainable use of the ocean. Here we call for a collaboration between research, private, and government sectors to promote observing systems that include ocean biological and biodiversity observations as well as physical and biogeochemical measurements, as a means to address the challenge of sustaining a healthy and vibrant ocean ecosystem. The goal is to detect changes in life in the ocean when and where they occur by field observations being rapidly published into open-access community databases (notably OBIS).
The concept of “Essential Climate Variables” evolved in the late 1990s as a way to focus resources on the collection of minimal sets of “key variables” for which data records were necessary to understand the status and trends in climate variability (Bojinski et al., 2014). The set of Essential Climate Variables (ECVs) was selected by evaluating readiness, feasibility, and impact to address societal needs. The ECVs are now fundamental information used to inform negotiations under the United Nations Framework Convention on Climate Change (UNFCCC)2 and the Intergovernmental Panel on Climate Change (IPCC). The requirements of readiness and feasibility at a global scale have led to a major focus on the physical ocean, with biological ECVs relatively poorly developed (World Meteorological Organization, 2018). Following this example, the Group on Earth Observations Biodiversity Observation Network (GEO BON) proposed a set of Essential Biodiversity Variables (EBVs; Figure 1) for use in monitoring programs to understand patterns and changes in Earth's biodiversity. The EBVs have been grouped into six classes: genetic composition, species populations, species traits, community composition, ecosystem structure, and ecosystem function (Pereira et al., 2013). This concept has taken root within wide segments of the theoretical and applied ecology communities (e.g., Geijzendorffer et al., 2016; Pettorelli et al., 2016; Proença et al., 2016; Turak et al., 2016; Kissling et al., 2018 and others). Within GEO BON, MBON was established in 2016 to frame the EBVs concept in the marine realm (Duffy et al., 2013; Muller-Karger et al., 2014; Costello et al., 2016). The goal of MBON is to coordinate, promote and augment the capabilities of present and future national and international observing systems to characterize and monitor diversity of marine life at the genetic, species, and ecosystem levels using a broad array of in situ and remote sensing observations. MBON is not the observing system, but a network that is contributing to the toolbox and library of standards, methods, and models required by ocean observing systems, such as those operated under the GOOS umbrella. MBON facilitates building an ocean community of practice by participating in the design and development of content of BON in a Box (https://boninabox.geobon.org), which is being developed by GEO BON. MBON also serves as the biodiversity arm of the evolving Blue Planet initiative under GEO, which aims to ensure that these observations are of benefit to society.
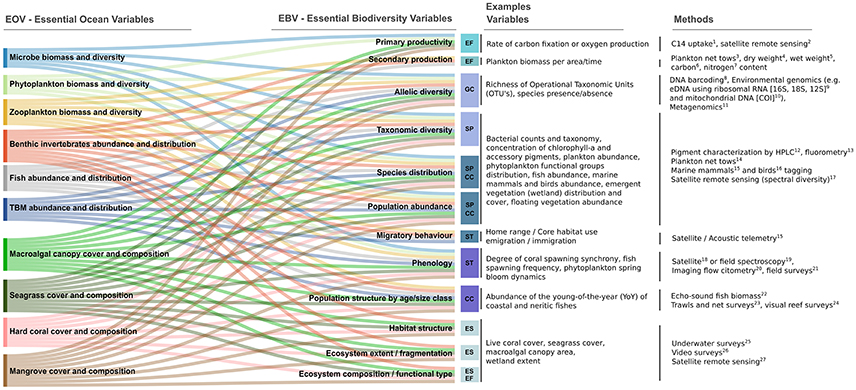
Figure 1. Conceptual relationship between EOVs and EBVs, with simple examples. EOVs (listed on the left hand side) are broadly taxonomically focused around productivity at the base of the food chain (microbes, phytoplankton and zooplankton), higher trophic levels (benthic invertebrates; fish; TBM: marine turtles, birds, and mammals), and habitat forming species (macroalgae, seagrass, mangrove, coral). In contrast, example EBVs (listed on the left hand column) evaluate taxa across scales of spatial and temporal diversity within species (allelic diversity, species distribution, population abundance, population structure by age/size class, phenology), across species (taxonomic diversity), and in terms of ecological context (primary productivity, secondary productivity, habitat structure, ecosystem extent / fragmentation, ecosystem composition / functional type). EOVs are described in Miloslavich et al. (2018). EBV classes from Pereira et al. (2013) are shown as colored boxes which aggregate different example EBVs, specifically: GC, Genetic composition; SP, Species populations; ST, Species traits; CC, Community composition; ES, Ecosystem structure; EF, Ecosystem function. EBVs on primary/secondary production are directly associated with the microbe, phytoplankton, zooplankton, and fish EOVs. Other EBVs can be applied more broadly to the rest of the EOVs. References for the methods listed on the right-hand column of the diagram are as follows: 1Holm-Hansen and Riemann (1978); Knap et al. (1997); 2Behrenfeld and Falkowski (1997); 3 Harris et al. (2000); 4 Madin et al. (2001); 5Omori (1969); 6 Landry et al. (2001); 7 Gordon et al. (1994); 8 Goodwin et al. (2018); 9 Djurhuus et al. (2017); 10 Goodwin et al. (2017); 11Zhao and Bajic (2001); 12Van Heukelem and Thomas (2001); 13Wright (1997); 14 Castellani et al. (2017); 15 Hussey et al. (2015); 16 Robinson et al. (2010); 17Sathyendranath (2014); Bracher et al. (2017); 18 Soto et al. (2009); 19 González-Rivero et al. (2016); 20Sosik and Olson (2007); 21Hill and Wilkinson (2004); 22Simmonds and MacLennan (2005); 23 McIntyre et al. (2015); 24 Caldwell et al. (2016); 25 Short et al. (2001); 26Klemas (2008); 27 Green et al. (2005); Miller et al. (2005); Tiner et al. (2015); Turpie et al. (2015).
The process of developing a multidisciplinary, integrated ocean observing system for operational uses, including sustained scientific research, follows the guidelines of the Framework for Ocean Observing (FOO, 2012). This framework was developed through sponsorship of the Intergovernmental Oceanographic Commission (IOC) of UNESCO. The implementation is coordinated by the GOOS. GOOS was established under the auspices of the IOC in 1991 and has played a lead role in coordinating global monitoring of the physical ocean and in the definition of Essential Ocean Variables (EOVs; Figure 1). EOVs evolved jointly with Essential Climate Variables, and their readiness is evaluated on whether they are at the stage of concept, pilot, or mature. This includes an evaluation of technology maturity, feasibility, and whether the measurements have been or can be implemented in regional or global observing systems. The Framework for Ocean Observing also promotes identifying biological and ecological EOVs in a fit-for-purpose process with user-driven feedback loops (e.g., Kelble et al., 2013; NOC, 2016; Bax et al., submitted; Miloslavich et al., 2018). Today, GOOS is engaged in a continuing process of refining and expanding the EOVs, and has extended the suite of EOVs to include biogeochemical and biological variables. The initial concept for biological EOVs may be traced to the GOOS Coastal Observations Panel (e.g., Malone and Knap, 2004; Malone et al., 2014).
GOOS is developing the implementation plans for the EOVs. An important program that is also engaged in developing the definitions and implementation plans for the biological EOVs is the Ocean Biogeographic Information System (OBIS), which is part of the IOC's International Oceanographic Data and Information Exchange (IODE). OBIS is the data legacy of the Census of Marine Life (CoML; 2000–2010) project and was adopted by IOC in 2009 as its biological information management system (Costello et al., 2015). OBIS has evolved significantly since the days of the CoML (De Pooter et al., 2017), when it was originally designed to document “presence/absence” of species by location based on protocols for natural history museum specimen collections. OBIS works with scientific communities to facilitate free and open access to primary data on species distributions, abundance, and other biodiversity metrics in space and time. It provides a mechanism for data integration and broad access. More than 500 institutions from 56 countries have provided over 50 million observations of nearly 120,000 marine species, which are hosted and shared openly through OBIS for the global community. OBIS has been continuously developing to host new types of global marine biodiversity monitoring data and meet new demands from the global biodiversity monitoring community. Other relevant IODE programs are focused on data organization and management (e.g., World Ocean Database), ocean observation best practices (www.oceanbestpractices.net; Pearlman et al., 2017a), and capacity building programs (e.g., Ocean Data and Information Networks, or ODINs; Large Marine Ecosystems/LME:LEARN; OceanTeacher Global Academy; OBIS training workshops; OceanTeacher Global Academy; OBIS training workshops).
These efforts are closely coordinated with the Joint Technical Commission for Oceanography and Marine Meteorology (JCOMM), which is an intergovernmental body of technical experts that provides a mechanism for international coordination on best practices for observing, data management, and services, and capacity development. These efforts are closely linked together in a complex landscape of ocean observation and data services that the partnership between IOC and GEO BON encompasses (Figure 2). The partnership with JCOMM is implicit.
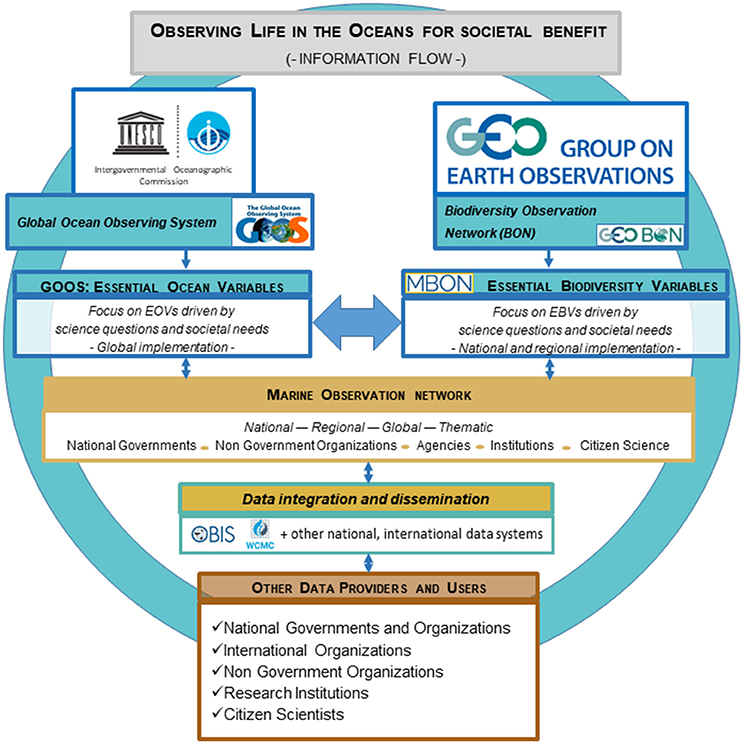
Figure 2. Schematic of the linkages between the GEO BON Marine Biodiversity Observation Network (MBON), the Global Ocean Observing System (GOOS), and the Ocean Biogeographic Information System (OBIS) proposed for biodiversity assessments. The strategies for observing are defined by national government and organizations, non-governmental organizations, and research groups, in an iterative loop that links with the elements that integrate the GOOS, MBON, and OBIS networks. The outer (blue) circle highlights feedbacks, and indicates that there are direct linkages between the users and the top levels in the partnership, including the IOC, GEO BON and JCOMM.
All ocean observing strategies improve understanding of the environment and biodiversity in coastal, neritic, and pelagic areas, including the seafloor from the shelf to the abyss. This helps to conserve and sustainably use life in coastal and pelagic areas. It protects human life and property, improves human health, conserves the environment, and promotes a vibrant economy. An important part of this is to characterize and enable accurate forecasts of environmental and biological change, including trends and impacts of disturbances on ecosystem structure, function and composition, as well as associated services. This requires concurrent measurement of biological EOVs alongside appropriate physical and biogeochemical EOVs. Much work in oceanography considers it sufficient to make bulk measurements about biological properties, such as characterizing stocks and biomass of microbes, plankton, or fish simply in terms of grams of Carbon per unit area in the euphotic zone or in the mixed layer (such as per square meter or gC m−2), or fluxes as changes in stocks over time (gC m−2 per unit time). The concept of the EBVs provides a path to a focused operational monitoring of biodiversity and ecosystem services (Brummitt et al., 2016; Geijzendorffer et al., 2016; Turak et al., 2016; Kissling et al., 2018). EBVs provide a measure of the complexity of life that has direct relevance to community structure and therefore stocks and fluxes, such as productivity.
The integration of marine EBVs into sustained research and operational ocean observing systems should occur following the guidelines of the FOO. In general, physical and biogeochemical ocean variables, already included in EOVs, provide the environmental context for biological EOVs and for EBVs. Yet, to facilitate integration into observing systems and enable broader interpretation, the EBVs need to be co-developed and mapped alongside the biological and ecological EOVs (bio-eco EOVs) in a manner illustrated in Figure 1. Each existing and planned element or node of the ocean observing system will need guidance on which EOVs and EBVs to target, and on the requirements for particular applications.
The bio-eco EOV definitions include “Complementary Variables,” which are other EOVs and/or EBVs that are necessary to fully describe the phenomena or understand impacts on the EOV of natural and anthropogenic pressures (Miloslavich et al., 2018). This establishes a link between the EOVs of GOOS and the EBVs proposed by GEO BON (Pereira et al., 2013). Further, there is consensus in MBON that marine EBVs need to be based on primary species-based data at a level that can be used to estimate biodiversity indices.
Figure 1 is an attempt to illustrate the many-to-many relationship between EBVs and EOVs. These are two systems that identify many (or all) of the same primary measurements, but aggregate them using different approaches. EBVs and EOVs are complementary and the standard operating procedures should be common to both as they develop. However, EBVs are not exclusively complementary to the variables under the GOOS schema. For example, a key difference between the EBVs and EOVs is that the EBVs are cross-domain (land, atmosphere and ocean), while the EOVs focus on the oceans. Other differences between the systems may seem contentious, as one aggregates the species level (or function) data, while the other tends to aggregate across processes or emergent properties. There will be different implications at the biological, ecosystem and domain levels from looking at just one schema or the other. For example, an EBV could include aggregated species level information about a particular group of organisms (e.g., genus, species or higher classification of groups of organisms such as fish, phytoplankton, zooplankton, birds or marine mammals, but without mixing organisms from these different groups such as would be common for evaluating a functional diversity metric). This recognizes that while some taxa may not be easily defined at species level, generally identification would be as close to this as practical. Our recommendation is, therefore, that standard biodiversity indices, such as species richness and α and ß diversity (Tuomisto, 2010a,b) be calculated within a specific taxonomic group (e.g., only within phytoplankton, or only within zooplankton, and so on), or within functional groups (e.g., all animals within a size class that serve as forage for larger predators). Even if there are new or introduced organisms, these should be counted within their taxonomic or functional groups. In this manner, the indices would be valid EBV metrics and they would be Complementary Variables of EOVs. To further illustrate this concept, measures of biomass (e.g., chlorophyll concentration, dry weight, or total weight), productivity (photosynthesis or secondary production), or of abundance (e.g., number of individuals in a population) represent an EBV at ecosystem level and an EOV. These EBVs would also function as Complementary Variables to EOVs. They serve to aggregate EOVs with measures of taxonomic diversity (i.e., richness, abundance, community structure), or functional diversity (i.e., trait data). Increasingly, many efforts focus on the various fields of study in biology that end in '-omics', such as genomics, proteomics, or metabolomics, which hold significant promise to study and categorize biodiversity in terms of species, genetics, physiology, traits and function, etc. We emphasize the importance of proper metadata associated with EBVs since use of different gear, methods (microscopy, 'omics, or pigment detection), or levels of taxonomic resolution can lead to very different outcomes.
EBVs and EOVs remain dynamic and will evolve in response to advances in sampling methodologies and our understanding of the ocean ecosystem. For example, Figure 1 may not adequately address the importance of measuring species interactions in ocean ecosystems. While it includes primary and secondary productivity as EBVs, there are no EBVs for top-down and wasp-waist processes despite our current understanding of marine trophodynamics. These differences in trophic processes are important because they can define the outcome of ecosystem services like fish production or water quality (Hunt and McKinnel, 2006; Litzow and Ciannelli, 2007; Baum and Worm, 2009). This suggests the need to add EOVs and EBVs that measure top-down dynamics, e.g., predation rates, diet compositions, or stable isotope measurements to determine trophic status.
The integration of EOVs and EBVs streamlines the concept of “essential variables,” minimizing duplication across multiple and diverse organizations under the IOC and GEO umbrellas. This addresses the goal of the FOO to integrate biological observations into international elements of a multidisciplinary GOOS. To advance this goal, MBON, GOOS, and OBIS entered into a partnership in December 2016. The partnership, shown in the schematic in Figure 2, seeks to standardize methods wherever possible, document best practices, and build capacity. While complete standardization across the globe is perhaps too high a goal for many EOVs, we recommend verified and calibrated observations and the use of best practices, so that the results from different areas can be compared at some level of aggregation. This is required to detect change across a region or within and between ocean basins, and for interoperability of data collected in different programs and with different gear. This “next step” process (i.e., the discussion, adoption, and implementation of best practices to facilitate comparisons across different regions and over time) is just starting and will evolve as the MBON community becomes organized. Such guidelines are useful to any effort that seeks either to start a new monitoring program or to strengthen existing ones.
There are examples of successes where data have been collected in a way that supports intercomparison. Australia has an initiative to standardize metadata on marine imagery for identifying benthic habitats and organisms. While individual studies collect additional data for their own purposes, key observations can be compared (Althaus et al., 2015). Another example is the World Register of Marine Species (WoRMS), which is expert-controlled, flexible and allows for rapid updates (Horton et al., 2017). Consistent use of WoRMS by the marine community, such as applied within OBIS for taxonomic assessment, has led to important advances in biogeography (Appeltans et al., 2016). This convergence on standards and conventions is now being applied through the partnership to inform monitoring, through consistent use of variables and protocols across ocean observing systems.
Numerous international policy assessments and processes have highlighted the need to better measure the status and trends of marine biodiversity: the UN Sustainable Development Goals (SDGs) (Lu et al., 2015; Anderson et al., 2017); the Convention on Biological Diversity (CBD); the Convention on Migratory Species (CMS); the Intergovernmental Platform on Biodiversity and Ecosystem Services (IPBES); the UN Regular Process (World Ocean Assessment); and the various efforts coordinated by the Food and Agriculture Organization of the United Nations (FAO). The United Nations General Assembly (UNGA) has initiated a negotiation over a new treaty for the conservation and sustainable use of biodiversity beyond national jurisdiction including, inter alia, mechanisms to develop area-based management tools (e.g., marine protected areas and marine spatial planning), strategic environmental assessments and environmental impact assessments (Gjerde et al., 2016). This requires an understanding of the state of biodiversity in areas beyond national jurisdictions to inform policy development. Requirements are often defined in broad terms, such as those documented in decisions and resolutions taken during Conferences of the Parties, without specifying which variables to measure and the methods required (including data archiving and reporting formats). In cases where data seem abundant, they are often collected using different methods and protocols that make it difficult and time consuming to aggregate the data reliably and at a scale most useful to managers and policy-makers. The need for routine marine biodiversity observations presents important technological and scientific opportunities, but making useful operational measurements needs standards and best practices that are followed internationally. Again, the importance of adequate metadata to accompany the data cannot be overemphasized.
There are several technical and resource challenges to enable the practical, operational use of marine biodiversity information. An important challenge is to design new technologies that can be integrated into existing and planned coastal and ocean observation programs in a practical and cost effective manner. New technologies are required to obtain basic measures of biodiversity to complement, for example, automated estimates of phytoplankton biomass derived from in situ chlorophyll fluorescence observations, or acoustic estimates of fish or zooplankton biomass. At present, there are numerous methods to estimate phytoplankton community composition, or productivity of various functional groups. Many of these are time consuming, such as microscopy, or they may also be costly, such as pigment concentration analyses or genomics assessments. Yet the most common methods used at present to assess phytoplankton abundance are ocean color observations, including from satellite-based sensors, and in situ chlorophyll fluorescence measurements, given the widespread use of chlorophyll fluorometers in profiling, towed, autonomous, and other devices. These in situ devices are undergoing a state change with the introduction of a new generation of compact, low power sensors that can lead to widespread use on autonomous vehicles (Pearlman et al., 2017b).
Today, partnerships such as the one established between MBON, GOOS and OBIS, for instance, provide opportunities for the biological community to make a step change similar to that accomplished by the physical oceanographic community in the early 2000s when the Argo deployments began (Roemmich et al., 2009). This step change would involve developing sensors to observe any and all trophic levels in the ocean, from microbes to marine mammals (e.g., Sigsgaard et al., 2016; Djurhuus et al., 2017). For example, new in situ devices are being designed to collect and process environmental DNA (eDNA) samples and also to identify plankton and microbes (Bowers et al., 2016; Herfort et al., 2016). The Video Plankton Recorder (VPR; Davis et al., 1992), Optical Plankton Recorder, and Imaging FlowCytoBot (Olson and Sosik, 2007; Sosik and Olson, 2007) are examples of submersible microscopes used to collect video or images of small organisms, cells, and particles. Such technologies should incorporate new ways of publishing data directly to regional or international databases such as OBIS. It would be desirable to have compact, inexpensive and practical models of these devices that produce high quality observations for broad and widespread operational use. The automated observations, when combined with ecological models, can lead to the high-throughput biodiversity data that are required for assessments (Bush et al., 2017).
The oceanographic community needs to work in concert and push to integrate existing technologies for biological observations, including biodiversity through imaging and “omics,” into regional and global ocean observing networks such as GO-SHIP, OceanSites, and the Ocean Observatories Initiative (OOI). Observing networks need to develop and share best practices, standards, protocols and observations on bioindicators, and to address significant gaps in geographic coverage (e.g., Buttigieg et al., 2018). Funders, including private investors, have a fundamental role in pushing this agenda forward by requiring research outputs and the underlying data to be made broadly available, and to bring down the costs of instrumentation and deployment.
The EOVs are defined with the goal of propagating standard methods and protocols for specific observations that can be used to address problems of societal relevance. Aligning EOVs with the EBV framework would strengthen understanding of possible causes of changes in biological elements of nature. For example, it is important to add biological diversity information to measures of biomass in order to characterize community structure changes that result in, or that are the result of, cascades of ecosystem disturbance at large and small scales, including anticipated responses to climate change. Similarly, it is important to bring measures of top-down pressures into the EBV framework.
Examples include the coral reef cover or reef fish abundance EOVs. The EBV framework helps design the products required for management. For example, the linkage of multidisciplinary EOVs including EBVs, accounts for the causes of changes in the benthic cover of different corals (i.e., the diversity of corals) as opposed to changes in bulk coral cover alone. Moreover, understanding changes in fish trophic and community structure or in functional diversity, in any location, helps understand whether top-down (e.g., fisheries, predation rates) or bottom-up pressures (e.g., changes in physical, nutrient regime, or climate) control characteristics like the decline in fish length, dominance of a particular species, or possible changes in the geographic distribution of these organisms.
Existing observing systems and plans for new ones should be linking a multidisciplinary set of EOVs with the EBVs to bring biodiversity and the drivers of biodiversity change into the same observing framework.
Animal tagging and animal tracking conducted by programs such as the US Animal Telemetry Network (ATN), Ocean Tracking Network (OTN), Tagging of Pacific Predators (TOPP), and many others, provide information on environmental conditions, animal movement and migration phenology, and animal physiological conditions. These programs offer examples of the complementarity between EOVs and EBVs. The emerging Migratory Connectivity in the Oceans (MiCO) program is synthesizing migratory marine species information to characterize the patterns of connectivity corridors and connected hubs in marine ecosystems. Mapping the concert of migrations of different species over time provides unique insights on the location and timing of biodiversity hotspots and how these may be changing over time. They help explain the trophic and reproductive drivers of migrations. These observations can also detect regime shifts in oceanographic conditions over broad geographic domains driven by climate cycles (e.g., El Niño). This work complements the work of MBON in defining standards for observation of lower, middle, and higher trophic levels. Specifically, quantifying biodiversity requires a time element from the point of view of observing traits of different species which may be expressed at different times to evaluate functional diversity. Phenology itself is also useful to detect species, since some obvious traits may not be apparent at a time when another species may express their traits.
Similarly, the expansion of efforts to coordinate observations of life, such as those planned by the GOA-ON, will help to understand the impacts of changing ocean chemistry on marine life, and vice versa. Linkages are also needed between these programs and those focusing on the deep sea, including the benthos from shelves to the abyss. These have received less attention from large-scale programs due to the difficulty in sampling ability and processing time. However, advances in technologies like new sensors incorporated into tethered and autonomous underwater vehicles (AUVs) are helping to sample the biodiversity of the seafloor. This includes the Monterey Accelerated Research System (MARS), the North-East Pacific Time-Series Underwater Networked Experiment (Neptune), and similar efforts stimulated by the NOAA Ocean Exploration (OE) program and parallel international initiatives. Many of these have started to organize under the Deep Ocean Stewardship Initiative (DOSI) and the International Network for Scientific Investigation of Deep-Sea Ecosystems (INDEEP), with the goal of refining the Deep Ocean Observing Strategy (DOOS) as part of GOOS. These efforts require forethought and planning on which databases should be used to best serve the needs of the International Seabed Authority. Ideally, these should include the same databases used for other parts of the ocean, following standards such as Darwin Core for biological and biodiversity measurements.
There are important linkages that need to be developed or leveraged to identify and share practical and societally relevant measures of biology that can be adopted globally. This partnership aims to achieve this by integrating ocean observations with data from fisheries surveys, Continuous Plankton Recorder data, citizen science observation programs such as the Reef Life Survey (RLS) (Edgar et al., 2017), and networks like the Marine Global Earth Observatory (MarineGEO) of the Smithsonian, the Global Coral Reef Monitoring Network (GCRMN), seagrass.net, and the Kelp Ecosystems Ecology Network (KEEN). By doing so, we will be able to expand our understanding of how different drivers affect marine biodiversity and characterize change to support sustainable management of human activities in the ocean.
Ultimately, understanding life in the sea for broad societal benefit requires a willingness to share observations and observation methodologies. Many countries have agreed to the IOC Oceanographic Data Exchange Policy (Resolution IOC-XXII-6, 2003, http://www.iode.org/policy), the Global Earth Observation System of Systems (GEOSS) “Data Sharing Principles” (GEOSS, 2018), and similar agreements that data should be made open-access (Costello, 2009). Yet considerable biological and biodiversity data falls short of being truly open, including in terms of availability in global databases such as OBIS. Thus, regional comparisons and global assessments remain difficult to make due to the vast disparity of approaches and access to collected data. More openness may occur once individuals who collect and sponsor monitoring programs understand that data sharing (a) opens doors for researchers, including students, to collaborate more broadly and to engage in regional and global scientific and policy developments, and (b) lifts the burden of curating data while minimizing risks of data loss. Indeed, as more data become openly available, the value in collecting comparable data will rapidly increase as existing collections provide greater context for the interpretation of the new observations. Many observations can be lost forever if not deposited in a long-term database. Such loss comes at a great cost to society, since much of these data were collected with public funds. Furthermore, species, ecosystems, and the pressures that affect them are not constrained by political borders, which means that understanding changes requires analyses of uniform data at regional and global scales. However, an analysis of data published in OBIS after 2015 reveals that the majority of effort is still allocated to digitizing historical data to prevent them from being lost, rather than uploading more recent observations (Figure 3).
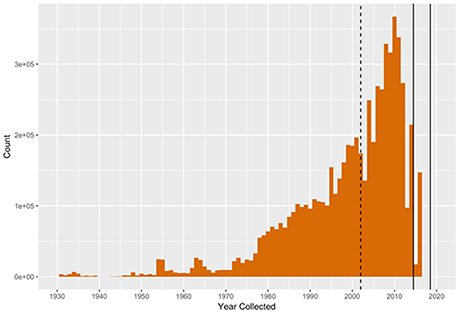
Figure 3. Number of data records by collection year that have been added to the public OBIS database after 2015. The median year is 2000 with a peak between 2008 and 2010. The sharp decline post-2010 illustrates the difficulty of obtaining more recent (< 5 years old) marine biodiversity data for publication in the public domain. There remains an immense, ongoing effort to digitize and publish older data (pre-2000) to OBIS. Updating such global databases is important to establish robust baselines against which change can be detected.
In contrast, oceanographers, navy personnel, and climate scientists worked together in the 1980s to implement international data archeology efforts to recover basic hydrographic observations of variables such as salinity, temperature, oxygen and nutrient concentrations (Teague et al., 1987; Parker, 1992; Levitus et al., 1994). Further, they worked to ensure that newly collected data were shared openly as soon as practical, which was often within 24 h of collection. In this instance, the first 1,000 profiling ARGO floats used to measure temperature and salinity profiles at different depths were in the water by the end of 2003, and were supported and analyzed by a multinational community (Gould et al., 2004). This step-change in observing capacity for the physical oceanographic community required defining common measures, harmonizing protocols for sampling, and agreeing on the use of common frameworks for data and metadata storage and sharing. It also required a willingness to share data. The physical oceanography community, like the weather community, understood the opportunities for collaboration and became engaged in big regional and global science and policy developments, as well as in implementing strategies to support industry sectors related to maritime activities and marine science (i.e., “blue growth”; Golden et al., 2017).
The biological community has initiated this process, but we are only in the early stages of taking it forward. Some data, like commercial fisheries data, have routinely been collected and aggregated for international management, but they are often not publicly available due to protection for specific commercial, personal identification, or political reasons. For those biological data that are shared, they are frequently made available at an aggregated level (geographic, time, etc.). Yet fishery research trawl data comprises some of the largest well-georeferenced standardized time-series data in OBIS. Thus, even with existing data, inefficient data sharing habits and a lack of public availability inhibit our ability to detect change over time across coastal and open ocean habitats. The molecular biology community, including journal editors, referees, authors and readers, expect publication of data in open-access database before publication (Costello and Wieczorek, 2014). Unfortunately such best practice is not mandated in the biodiversity and conservation journals (Costello et al., 2015). Our ability to conduct regional, national, and international time series-based assessments for international policy processes is at present compromised due to poor data availability. The information and advice provided to policy makers regarding trends in biodiversity, such as the state of threatened species populations, spread of invasive species, and change in ecosystems, needs to be current and not delayed by years as is presently the case.
The GEO BON and GOOS communities need to foster better and more efficient means for digitally cataloging data with appropriate metadata, and need to promote and build capacity in the use of data storage formats and data exchange formats that have been accepted and understood by a wide community. The uncalibrated data from the Argo floats are released for public use within 24 h. The quality controlled data are available within 6 months. There is a clear and present need to build the capacity in the biological oceanography community to deliver observations within the timeframes needed to detect changes and to make relevant decisions. This requires a new paradigm to ensure that quality of the data is not compromised.
OBIS provides a mechanism for integrating and making accessible standardized marine biodiversity data, including abundance and associated environmental data. The “OBIS 2.0” currently in development will include a workbench for scientists to process data and for the data to be kept “private” (e.g., data can be kept by the eventual provider but still be checked using OBIS quality control services such as outlier detection). The data holder can then push the data to OBIS at their command. The database conforms to international standards (e.g., OBIS-ENV-DATA format of Darwin Core). This system is being adopted by the European Marine Observation and Data Network (EMODnet), Fisheries and Oceans Canada (DFO), the US Integrated Ocean Observing System (IOOS), and the Integrated Marine Observing System (IMOS) in Australia. More national and regional systems may ideally follow this model.
The data should be available in defined formats and be supported by complete metadata. The provenance of the data, including methods of collecting observations, data processing, and quality control should be well described and available to the ocean research community. Such methodologies, sometimes called best practices or standard operating procedures, can vary significantly across teams or communities, often with limited or out-of-date documentation, or no documentation. To address repeatability of observations and to retain historical access to measurement and data processing techniques, a long-term sustained repository of best practices is needed. This has been defined as an extended operational capability of the IODE (Pearlman et al., 2017a) that will permit storage, advanced discovery, and access to provide widespread use of best practices. Development of this resource needs to be coordinated with the incipient GEO BON's BON in a Box efforts.
A separate but related challenge is the reconciliation between operational taxonomic units (OTUs) derived from molecular biology observations and other measures of taxonomic diversity. One issue is that the databases against which sequences of aminoacids or genes extracted from organisms or the environment are compared to identify species or taxonomic groups are still not well populated. The algorithm for these comparisons is referred to as BLAST (Basic Local Alignment Search Tool). As more genetic samples are collected and available databases grow, the computational cost to conduct BLAST comparisons increases. Molecular methods are being refined and will become more widespread. This includes environmental DNA (eDNA) techniques, which seek to capitalize on the genetic material that is derived from whole microbial cells or shed from multicellular organisms via metabolic waste, damaged tissue, or sloughed skin cells, all of which are ubiquitous in the environment (Thomsen et al., 2012; Andruszkiewicz et al., 2017; Djurhuus et al., 2017). The “omics” community delivers genetic observations to nucleotide databases, including several databases that are linked via the International Nucleotide Sequence Database Collaboration (e.g., DNA Data Bank of Japan, GenBank in the USA, and the European Nucleotide Archive). These records, however, typically have minimal environmental or biological data other than the nucleotide data. The opportunity exists to link these with biogeographic and environmental databases, including the Global Biodiversity Information Facility (GBIF) and OBIS. Marine species nomenclature, classification and associated information are managed in the World Register of Marine Species (WoRMS) which is expert-controlled and dynamic, allowing for rapid updates (Costello et al., 2013). WoRMS is used for “cleaning” species synonyms and misspellings by OBIS, GBIF and many other databases.
Implementing these standards is becoming especially important as species move with the changing oceans or are transported by human intervention, changing the makeup of tomorrow's communities. Individual researchers and groups that constitute the marine biological research and monitoring communities need to better collaborate, adopt advanced data and metadata schema like Darwin Core, and deliver data to national and international databases such as OBIS and GBIF.
In general, another challenge is the increased informatics. This includes high capacity computing and networking requirements, expected for current processing, analysis, and visualization of eDNA, imagery, or other geographically-distributed data. It also includes further adoption of data format and control standards, to allow easier assimilation into numerical models. These require significant machine-learning and human resources to develop reliable products and applications. Making such resources available, as well as addressing the quality assurance and quality control (QA/QC) of the processes, needs to be part of EOV/EBV implementation strategies.
An important aim of the partnership is to create, advertise, and generate capacity to implement a set of standard methods or documented best practices (Costello et al., 2016). This would facilitate the start of observation programs and activities in areas where they do not exist, and facilitate the interoperability of data collected through different programs and by different people and equipment. MBON, BON in a Box, OBIS, and some of the GOOS Regional Alliances are initiating specific activities to layer biological observations onto present and planned observing systems by allocating efforts to strengthen capacity internationally, with the aim to ensure global participation and global ocean coverage. As part of this process, OBIS partners are collaborating with the regional training centers of IODE's OceanTeacher Global Academy to organize OBIS training sessions around the world (seven of these took place in 2017 involving 152 people). These experiences are being used to develop online training modules that explain the steps required for publication of and access to biodiversity-relevant data, including short chapters that contain focused online tutorials. Additional activities will include training in the observation of essential variables, building the systems and capacity to organize and share observations using international data standards (e.g., Darwin Core), and best practices in data quality control (e.g., obistools R package, github.com/iobis/obistools).
How do we implement a sustained system of biological observations based on EOVs? Overcoming the various challenges outlined above for operational collection of biological data requires substantial scientific work, a commitment to integrate biological EOVs into the GOOS Regional Alliances, and collaboration among nations. A sustained system of biological observations also requires clearly defined and shared measures, documented protocols for sampling, and agreement on the use of common frameworks for data and metadata storage and publication. To initiate this process, it is necessary to set specific and feasible targets, and to focus on short-term goals. Table 1 provides a roadmap for these goals.

Table 1. Roadmap to implementation of biological and biodiversity variables into ocean observing systems with outputs relevant to societal need.
It is incumbent on the scientific community to harmonize the EOV and EBV concepts and work with operational agencies around the world to implement a multidisciplinary ocean observing system that delivers information that is relevant and timely for the SDGs and the United Nations Decade of Ocean Science for Sustainable Development. We invite these communities to enter the dialogue occurring between MBON, GOOS, OBIS, and JCOMM to refine the concepts and the roadmap laid out in this paper further. Together, we will build an integrated system that includes proper observations of life in the sea and has the power to influence regional and global decisions. The partnership will provide guidelines for IPBES and for planning the United Nations Decade of Ocean Science for Sustainable Development 2021-2030 (IOC XXIX-1, 2017). As this information improves, it will serve the intended purpose of supporting the sustained development of the ocean, or what is now called the “Blue Economy” (Golden et al., 2017).
FM-K conceived and wrote the bulk of the article. PM, NB, SS, MC, IS, GC, WT, MG, EM, BB, JP, PH, DD, AB, CM, LW, WA, PP, EK, CK, RM, FC, KI, SC, DO, LN, HP, VA, SB, LB-C, JD, RK, L-MR, YS, GG all contributed significant ideas and text to the article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This manuscript is a contribution to the Marine Biodiversity Observation Network (MBON) of the Group on Earth Observations Biodiversity Observation Network. It is also a contribution to the Integrated Marine Biosphere Research (IMBeR) project, which is supported by the Scientific Committee on Oceanic Research (SCOR) and Future Earth. The work leading up to the manuscript was funded under the US National Ocean Partnership Program (NOPP RFP NOAA-NOS-IOOS-2014-2003803 in partnership between NOAA, BOEM, NASA, the US Integrated Ocean Observing System (IOOS) Program Office, and NSF. Specifically, the MBON work was funded through NASA grant NNX14AP62A [National Marine Sanctuaries as Sentinel Sites for a Demonstration Marine Biodiversity Observation Network (MBON)], and NASA Grant NNX14AR62A and BOEM award MC15AC00006 (The Santa Barbara Channel Biodiversity Observation Network). MBON work in the Arctic [Initiating an Arctic Marine Biodiversity Observing Network (AMBON)] was funded by NOPP grant NA14NOs0120158 with support from BOEM, NOAA, and Shell Oil Company. Funds for AMBON were also provided by NSF through Supplement grant 1723374 to NSF 1204082 (Collaborative Research: The Distributed Biological Observatory (DBO)-A Change Detection Array in the Pacific Arctic Region). Additional support was provided by NSF grant number 1728913 (Research Coordination Networks (RCN): Sustained Multidisciplinary Ocean Observations—the OceanObs RCN). Mention of trade names or commercial products does not constitute endorsement or recommendation for use by the US Government. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of US government agencies. Corinne Martin and Lauren Weatherdon are supported by ODYSSEA (http://odysseaplatform.eu/), a project funded by the European Union's Horizon 2020 research and innovation programme, under grant agreement No 727277. OBIS staff has received funding from EU's H2020 ECOPOTENTIAL project (grant agreement No 641762). The manuscript benefitted substantially from the comments and recommendations received from two reviewers (Emmanuel Devred and Maciej Telszewski) and from the editors, including Johannes Karstensen.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2018.00211/full#supplementary-material
1. ^ Argo 2018. http://www.argo.ucsd.edu/About_Argo.html, visited January 16, 2018.
2. ^ GCOS Implementation Plan for the Global Observing System for Climate in Support of the UNFCCC (2010 Update) (World Meteorological Organization, Geneva, 2010), p. 180.
Althaus, F., Hill, N., Ferrari, R., Edwards, L., Przeslawski, R., Schönberg, C. H., et al. (2015). A standardised vocabulary for identifying benthic biota and substrata from underwater imagery: the CATAMI classification scheme. PLoS ONE 10:e0141039. doi: 10.1371/journal.pone.0141039
Anderson, K., Ryan, B., Sonntag, W., Kavvada, A., and Friedl, L. (2017). Earth observation in service of the 2030 agenda for sustainable development. Geo-spatial Inform Sci. 20, 77–96. doi: 10.1080/10095020.2017.1333230
Andruszkiewicz, E. A., Starks, H. A., Chavez, F. P., Sassoubre, L. M., Block, B. A., and Boehm, A. B. (2017). Biomonitoring of marine vertebrates in Monterey Bay using eDNA metabarcoding. PLOS ONE 12:e0176343. doi: 10.1371/journal.pone.0176343
Appeltans, W., Dujardin, F., Flavell, M., Miloslavich, P., and Webb, T. J. (2016). “Biodiversity baselines in the global ocean,” in The Open Ocean: Status and Trends, ed UNESCO-IOC UNEP, xxxiii, 220–238.
Baum, J. K., and Worm, B. (2009). Cascading top-down effects of changing oceanic predator abundance. J. Anim. Ecol. 78, 699–714. doi: 10.1111/j.1365-2656.2009.01531.x
Behrenfeld, M. J., and Falkowski, P. G. (1997). Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol. Oceanograp. 42, 1–20. doi: 10.4319/lo.1997.42.1.0001
Bograd, S. J., and Lynn, R. J. (2003). Long-term variability in the southern California Current System. Deep Sea Res. II 50, 2355–2370. doi: 10.1016/S0967-0645(03)00131-0
Bojinski, S., Verstraete, M., Peterson, T. C., Richter, C., Simmons, A., and Zemp, M. (2014). The concept of essential climate variables in support of climate research, applications, and policy. Bull. Amer. Meteor. Soc. 95, 1431–1443, doi: 10.1175/BAMS-D-13-00047.1
Bowers, H. A., Marin, R. III., Birch, J. A., Scholin, C. A., and Doucette, G. J. (2016). Recovery and identification of Pseudo-nitzschia frustlules from natural samples acquired using the Environmental Sample Processor (ESP). J. Phycol. 52, 135–140. doi: 10.1111/jpy.12369
Bracher, A., Bouman, H. A., Brewin, R. J. W., Bricaud, A., Brotas, V., Ciotti, A. M., et al. (2017). Obtaining phytoplankton diversity from ocean color: a scientific roadmap for future development. Front. Mar. Sci. 4:55. doi: 10.3389/fmars.2017.00055
Brummitt, N., Regan, E. C., Weatherdon, L. V., Martin, C. S., Geijzendorffer, I. R., Rocchini, D., et al. (2016). Taking stock of nature: Essential biodiversity variables explained. Biol. Conser. 213, 252–255. doi: 10.1016/j.biocon.2016.09.006
Bush, A., Sollmann, R., Wilting, A., Bohmann, K., Cole, B., Balzter, H., et al. (2017). Connecting Earth observation to high-throughput biodiversity data. Nat. Ecol. Evol. 1:176. doi: 10.1038/s41559-017-0176
Buttigieg, P. L., Fadeev, E., Bienhold, C., Hehemann, L., Offre, P., and Boetius, A. (2018). Marine microbes in 4D – using time series observation to assess the dynamics of the ocean microbiome and its links to ocean health. Curr. Opin. Microbiol. 43, 169–185. doi: 10.1016/j.mib.2018.01.015
Caldwell, Z. R., Zgliczynski, B. J., Williams, G. J., and Sandin, S. A. (2016). Reef Fish survey techniques: assessing the potential for standardizing methodologies. PLoS ONE. 11:e0153066. doi: 10.1371/journal.pone.0153066
Castellani, C., and Edwards, M. (2017). Marine Plankton: A Practical Guide to Ecology, Methodology, and Taxonomy. Oxford: Oxford University Press.
Choi, B. C., and Pak, A. W. (2006). Multidisciplinarity, interdisciplinarity, and transdisciplinarity in health research, services, education and policy. 1. Definitions, objectives, and evidence of effectiveness. Clin. Invest. Med. 29, 351–364.
Church, M. J., Lomas, M. W., and Muller-Karger, F. E. (2013). Sea change: charting the course for biogeochemical ocean time series research in a new millennium. Deep Sea Res. II 93, 2–15. doi: 10.1016/j.dsr2.2013.01.035
Claustre, H., Bishop, J., Boss, E., Bernard, S., Berthon, J. FCoatanon, C., et al. (2010). “Bio-optical profiling floats as new observational tools for biogeochemical and ecosystem studies,” in Proceedings of the “OceanObs'09: Sustained Ocean Observations and Information for Society” Conference, Vol. 2, (Venice), 21–25.
Cornell, S., Berkhout, F., Tuinstra, W., Tàbara, J. D., Jäger, J., Chabay, I., et al. (2013). Opening up knowledge systems for better responses to global environmental change. Environ. Sci. Policy. 28, 60–70. doi: 10.1016/j.envsci.2012.11.008
Costello, M. J., and Wieczorek, J. (2014). Best practice for biodiversity data management and publication. Biol. Conserv. 173, 68–73. doi: 10.1016/j.biocon.2013.10.018
Costello, M. J., Basher, Z., McLeod, L., Asaad, I., Claus, S., Vandepitte, L., et al. (2016). “Chapter 7. Methods for the study of marine biodiversity,” in The GEO Handbook on Biodiversity Observation Networks, eds M. Walters and R. J. Scholes (Springer), 129–163.
Costello, M. J., Bouchet, P., Boxshall, G., Fauchald, K., Gordon, D., Hoeksema, B. W., et al. (2013). Global coordination and standardisation in marine biodiversity through the World Register of Marine Species (WoRMS) and related databases. PLoS ONE 8:e51629. doi: 10.1371/journal.pone.0051629
Costello, M. J., Cheung, A., and De Hauwere, N. (2010). Topography statistics for the surface and seabed area, volume, depth and slope, of the world's seas, oceans and countries. Environ. Sci. Technol. 44, 8821–8828. doi: 10.1021/es1012752
Costello, M. J., Vanhoorne, B., and Appeltans, W. (2015). Conservation of biodiversity through taxonomy, data publication and collaborative infrastructures. Conserv. Biol. 29, 1094–1099. doi: 10.1111/cobi.12496
Costello, M. J. (2009). Motivation of online data publication. BioScience 59, 418–427. doi: 10.1525/bio.2009.59.5.9
Davis, C. S., Gallager, S. M., Berman, M. S., Haury, L. R., and Strickler, J. R. (1992). The Video Plankton Recorder (VPR): design and initial results. Arch. Hydrobiol. Beiheft Ergeb. Limnol. 36, 67–81.
De Pooter (2017). Toward a new data standard for combined marine biological and environmental datasets - expanding OBIS beyond species occurrences. Biodiv. Data J. 2017:e10989. doi: 10.3897/BDJ.5.e10989
Djurhuus, A., Closek, C. J., Port, J., Starks, H., Yamahara, K., Romero, O., et al. (2017). Standardizing filter type and extraction method for marine biodiversity monitoring using environmental DNA. Front. Mar. Sci. 4:314. doi: 10.3389/fmars.2017.00314
Duffy, J. E., Amaral-Zettler, L. A., Fautin, D. G., Paulay, G., Rynearson, T. A., Sosik, H. M., et al. (2013). Envisioning a national marine biodiversity observation network. BioScience 63, 350–361. doi: 10.1525/bio.2013.63.5.8
Dunn, D. C., Maxwell, S. M., Boustany, A. M., and Halpin, P. N. (2016). Dynamic ocean management increases the efficiency and efficacy of fisheries management. Proc. Natl. Acad. Sci. U.S.A. 113, 668–673. doi: 10.1073/pnas.1513626113
Edgar, G. J., Alexander, T. J., Lefcheck, J. S., Bates, A. E., Kininmonth, S. J., Thomson, R. J., et al. (2017). Abundance and local-scale processes contribute to multi-phyla gradients in global marine diversity. Sci. Adv. 3:e1700419, doi: 10.1126/sciadv.1700419
FOO (2012). A Framework for Ocean Observing. By the Task Team for an Integrated Framework for Sustained Ocean Observing, UNESCO 2012, IOC/INF-1284. Paris.
GEOSS (2018). Available online at: https://www.earthobservations.org/geoss_dsp.shtml.
Geijzendorffer, I. R., Regan, E., Pereira, H. M., Brotons, L., Brummitt, N., Gavish, Y., et al. (2016). Bridging the gap between biodiversity data and policy reporting needs: an Essential Biodiversity Variables perspective. J. Appl. Ecol. 53, 1341–1350. doi: 10.1111/1365-2664.12417
Gjerde, K. M., Nordtvedt reeve, L. L., Harden-Davies, H., Ardron, J., Dolan, R., Durussel, C., et al. (2016). Protecting Earth's last conservation frontier: scientific, management and legal priorities for MPAs beyond national boundaries. Aquat. Conserv. Mar. Freshw. Ecosyst. 26(Suppl. 2), 45–60. doi: 10.1002/aqc.2646
Golden, J. S., Virdin, J., Nowacek, D., Halpin, P., Bennear, L., and Patil, P. G. (2017). Making sure the blue economy is green. Nat. Ecol. Evol. 1:0017 doi: 10.1038/s41559-016-0017
González-Rivero, M., Beijbom, O., Rodriguez-Ramirez, A., Holtrop, T., González-Marrero, Y., Ganase, A., et al. (2016). Scaling up ecological measurements of coral reefs using semi-automated field image collection and analysis. Remote Sens. 8:30. doi: 10.3390/rs8010030
Goodwin, K. D., Muller-Karger, F. E., Djurhuus, A., Zeigler Allen, L., Allen, A. E., McCrow, J. P., et al. (2018). “Molecular Approaches for an Operational Marine Biodiversity Observation Network. Chapter 35,” in World Seas: An Environmental Evaluation, Vol. III: Ecological Issues and Environmental Impacts. 2nd Edn. ed C. Sheppard (Elsevier).
Goodwin, K. D., Thompson, L. R., Duarte, B., Kahlke, T., Thompson, A. R., Marques, J. C., et al. (2017). DNA sequencing as a tool to monitor marine ecological status. Front. Mar. Sci. 4:107. doi: 10.3389/fmars.2017.00107
Gordon, N., Angel, D. L., Neori, A., Kress, N., and Kimor, B. (1994). Heterotrophic dinoflagellates with symbiotic cyanobacteria and nitrogen limitation in the Gulf of Aqaba. Mar. Ecol. Progr. Ser. 107, 83–88. doi: 10.3354/meps107083
Gould, J., Roemmich, D., Wijffels, S., Freeland, H., Ignaszewsky, M., Jianping, X., et al. (2004). Argo profiling floats bring new era of in situ ocean observations. Eos Trans Am Geophys Union 85, 185–91. doi: 10.1029/2004EO190002
Green, E. P., Mumby, P. J., Edwards, A. J., and Clark, C. D. (2005). Remote Sensing Handbook for Tropical Coastal Management. Coastal Management Sourcebooks 3. Paris: UNESCO.
Harris, R. P., Wiebe, P. H., Lenz, J., Skjoldal, H. R., and Huntley, M. (2000). Zooplankton Methodology Manual. London; San Diego, CA: Academic Press.
Herfort, L., Seaton, C., Wilkin, M., Roman, B., Preston, C., Marin, R., et al. (2016). Use of continuous, real-time observations and model simulations to achieve autonomous, adaptive sampling of microbial processes with a robotic sampler. Limnol. Oceanogr. Methods 14, 50–67. doi: 10.1002/lom3.10069
Hill, J., and Wilkinson, C. (2004). Methods for Ecological Monitoring of Coral Reefs. Townsville, QLD: Australian Institute of Marine Science. Available online at: https://www.cbd.int/doc/case-studies/tttc/tttc-00197-en.pdf
Holm-Hansen, O., and Riemann, B. (1978). Chlorophyll a Determination: Improvements in Methodology. Copenhagen: Oikos. Available Online at: https://www.cbd.int/doc/case-studies/tttc/tttc-00197-en.pdf
Horton, T., Gofas, S., Kroh, A., Poore, G. C. B., Read, G., Rosenberg, G., et al. (2017). Improving nomenclatural consistency: a decade of experience in the World Register of Marine Species. Eur. J. Taxon. 2017:389. doi: 10.5852/ejt.2017.389
Hunt, G. L., and McKinnel, S. (2006). Interplay between top-down, bottom-up, and wasp-waist control in marine ecosystems. Prog. Oceanograp. 68, 115–124. doi: 10.1016/j.pocean.2006.02.008
Hussey, N. E., Kessel, K., Aarestrup, Cooke, S. J., Cowley, P. D., Fisk, A. T., et al. (2015). Aquatic animal telemetry: A panoramic window into the underwater world. Science. 348:1255642. doi: 10.1126/science.1255642
ICSU (2017). A Guide to SDG Interactions: From Science to Implementation, eds D. J.Griggs, M. Nilsson, A. Stevance, and D. McCollum. Paris: International Council for Science.
Johnson, K. S., Plant, J. N., Coletti, L. J., Jannasch, H. W., Sakamoto, C. M., Riser, S. C., et al. (2017). Biogeochemical sensor performance in the SOCCOM profiling float array. J. Geophys. Res. Oceans 122, 6416–6436, doi: 10.1002/2017JC012838
Kelble, C. R., Loomis, D. K., Lovelace, S., Nuttle, W. K., Ortner, P. B., Fletcher, P., et al. (2013). The EBM-DPSER conceptual model: integrating ecosystem services into the DPSIR framework. PLoS ONE 8:e70766. doi: 10.1371/journal.pone.0070766
Kissling, W. D., Ahumada, J. A., Bowser, A., Fernandez, M., Fernández, N., García, E. A., et al. (2018). Building essential biodiversity variables (EBVs) of species distribution and abundance at a global scale. Biol. Rev. 93, 600–625. doi: 10.1111/brv.12359
Klemas, V. (2008). “Sensors and techniques for observing coastal ecosystems,” in Remote Sensing and Geospatial Technologies for Coastal Ecosystem Assessment and Management, 1st Edn. Chapter 2, ed X. Yang (Berlin; Heidelberg: Springer).
Knap, A. H., Michaels, A. F., Steinberg, D., Bahr, F., Bates, N., Bell, S., et al. (1997). BATS Methods Manual. Version 4. U.S. JGOFS Planning Office, 136.
Landry, M. R., Al-Mutairi, H., Selph, K. E., Christensen, S., and Nunnery, S. (2001). Seasonal patterns of mesozooplankton abundance and biomass at Station ALOHA. Deep-Sea Res. II. 48, 2037–2061. doi: 10.1016/S0967-0645(00)00172-7
Levitus, S., Gelfeld, R. D., Boyer, T., and Johnson, D. (1994). Results of the NODC and IOC Oceanographic Data Archaeology and Rescue Projects: Report 1. Key to Oceanographic Records Documentation No. 19. National Oceanographic Data Center. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Environmental Satellite, Data, and Information Service, 73.
Liblik, T., Karstensen, J., Testor, P., Alenius, P., Hayes, D., Ruiz, S., et al. (2016). Potential for an underwater glider component as part of the Global Ocean Observing System. Methods Oceanogr. 17, 50–82. doi: 10.1016/j.mio.2016.05.001
Litzow, M. A., and Ciannelli, L. (2007). Oscillating trophic control induces community reorganization in a marine ecosystem. Ecol. Lett. 10, 1124–1134. doi: 10.1111/j.1461-0248.2007.01111.x
Lu, Y., Nakicenovic, N., Visbeck, M., and Stevance, A. (2015). Five priorities for the UN sustainable development goals. Nature 520, 432–433. doi: 10.1038/520432a
Madin, L. P., Horgan, E. F., and Steinberg, D. K. (2001). Zooplankton at the Bermuda Atlantic Time-series Study (BATS) station: diel, seasonal and interannual variation in biomass, 19941998. Deep-Sea Res. II 48, 2063–2208. doi: 10.1016/S0967-0645(00)00171-5
Malone, T. C., and Knap, A. H. (2004). The Integrated, Strategic Design Plan for the Coastal Observations Module of the Global Ocean Observing System and early warning diagnostics. Havana: MarCuba.
Malone, T. C., DiGiacomo, P. M., Gonçalves, E., Knap, A. H., Talaue-McManus, L., de Mora, S., et al. (2014). Enhancing the Global Ocean Observing System to meet evidence based needs for the ecosystem-based management of coastal ecosystem services. Nat. Resour. Forum 38, 168–181. doi: 10.1111/1477-8947.12045
McCauley, D. J., Pinsky, M. L., Palumbi, S. R., Estes, J. A., Joyce, F. H., and Warner, R. R. (2015). Marine defaunation: animal loss in the global ocean. Science 347:1255641. doi: 10.1126/science.1255641
McIntyre, F., Neat, F., Collie, N., Stewart, M., and Fernandes, P. (2015). Visual surveys can reveal rather different ‘pictures' of fish densities: comparison of trawl and video camera surveys in the Rockall, NE Atlantic Ocean. Deep Sea Res. Part I 95, 67–74. doi: 10.1016/j.dsr.2014.09.005
Merrie, A., Dunn, D. C., Metian, M., Boustany, A. M., Takei, Y., Oude, A., et al. (2014). An ocean of surprises – trends in human use, unexpected dynamics and governance challenges in areas beyond national jurisdiction. Glob. Environ. Chang. 27, 19–31. doi: 10.1016/j.gloenvcha.2014.04.012
Miller, R. L., Del Castillo, C. E., and McKee, B. A. (2005). Remote Sensing of Coastal Aquatic Environments. Springer Netherlands, 347.
Miloslavich, P., Bax, N., Simmons, S. E., Klein, E., Appeltans, W., Aburto-Oropeza, O., et al. (2018). Essential Ocean Variables for sustained observations of marine biodiversity and ecosystems. Global Change Biol. 24, 2416–2433. doi: 10.1111/gcb.14108
Muller-Karger, F. E., Hestir, E., Ade, C., Turpie, K., Roberts, D. A., Siegel, D., et al. (2018). Satellite sensor requirements for monitoring essential biodiversity variables of coastal ecosystems. Ecol. Appl.. 28, 749–760. doi: 10.1002/eap.1682
Muller-Karger, F. E., Kavanaugh, M. T., Montes, E., Balch, W. M., Breitbart, M., Chavez, F. P., et al. (2014). A framework for a marine biodiversity observing network within changing continental shelf seascapes. Oceanography 27, 18–23, doi: 10.5670/oceanog.2014.56
Muller-Karger, F., Roffer, M., Walker, N., Oliver, M., Schofield, O., Abbott, M., et al. (2013). Satellite remote sensing in support of an integrated ocean observing system. Geosci. Remote Sens. Mag. IEEE 1:8. doi: 10.1109/MGRS.2013.2289656
NOC (National Ocean Council) (2016). Biological And Ecosystem Observations Within United States Waters II: A Workshop Report To Inform Priorities For The United States Integrated Ocean Observing System. US National Ocean Council, December 2016. Avialable online at: https://obamawhitehouse.archives.gov/sites/default/files/microsites/ostp/NSTC/biological_and_ecosystem_observations_within_united_states_waters2.pdf (Accessed 3 February, 2018).
Neuer, S., Benway, H. M., Bates, N., Carlson, C. A., Church, M., DeGrandpre, M., et al. (2017). Monitoring ocean change in the 21st century. Eos 98. doi: 10.1029/2017EO080045
Newton, J. A., Feely, R. A., Jewett, E. B., Williamson, P., and Mathis, J. (2015). Global Ocean Acidification Observing Network: Requirements and Governance Plan, 2nd Edn. GOA-ON. Available online at: http://www.goa-on.org/documents/resources/GOA-ON_2nd_edition_final.pdf
Niemeijer, D. (2002). Developing indicators for environmental policy: data-driven and theory-driven approaches examined by example. Environ. Sci. Policy. 5, 91–103. doi: 10.1016/S.1462-9011(02)00026-6
Olson, R. J., and Sosik, H. M. (2007). A submersible imaging-in-flow instrument to analyze nano and microplankton: Imaging FlowCytobot. Limnol. Oceanogr. Methods 5, 2007, 195–203. doi: 10.4319/lom.2007.5.195
Omori, M. (1969). Weight and chemical composition of some important oceanic zooplankton in the North Pacific Ocean. Mar. Biol. 3, 4–10. doi: 10.1007/BF00355587
Palacz, A. P., Pearlman, J., Simmons, S., Hill, K., Miloslavich, P., Telszewski, M., et al. (2017). Report of the Workshop on the Implementation of Multi-Disciplinary Sustained Ocean Observations (IMSOO). Global Ocean Observing System (GOOS) Report No. 223. Avialable online at: http://www.goosocean.org/imsoo-report.
Parker, B. (1992). Oceanographic data archaeology: finding historical data for climate and global change research. Oceanography. 5, 124–125.
Pearlman, J., Buttigieg, P. L., Simpson, P., Mu-oz Mas, C., Heslop, E., and Hermes, J. (2017a). “Accessing Existing and Emerging Best Practices for Ocean Observation, a new approach for end-to-end management of Best Practices,”in OCEANS 2017 (Anchorage, AK: IEEE Xplore), 1–7.
Pearlman, J., Pearlman, F., Ferdinand, O., Delory, E., Meme, S., et al. (2017b). “NeXOS, developing and evaluating a new generation of in situ ocean observation systems,” in OCEANS 2017 (Aberdeen: IEEE Xplore).
Pereira, H. M., Ferrier, S. Walters, M., Geller, G. N., Jongman, R. H., Scholes, R. J., et al. (2013). Essential biodiversity variables. Science 339, 277–278. doi: 10.1126/science.1229931
Pettorelli, N., Wegmann, M., Skidmore, A., Mücher, S., Dawson, T. P., Fernandez, M., et al. (2016). Framing the concept of satellite remote sensing essential biodiversity variables: challenges and future directions. Remote Sens. Ecol. Conserv. 2, 122–131. doi: 10.1002/rse2.15
Proença, V., Martin, L. J., Pereira, H. M., Fernandez, M., McRae, L., Belnap, J., et al. (2016). Global biodiversity monitoring: from data sources to Essential Biodiversity Variables. Biol. Conserv. 213, 256–263. doi: 10.1016/j.biocon.2016.07.014
Reid, P. C., Colebrook, J. M., Matthews, J. B. L., and Aiken, J. (2003). The Continuous Plankton Recorder: concepts and history, from Plankton Indicator to undulating recorders. Prog. Oceanogr. 58, 117–173. doi: 10.1016/j.pocean.2003.08.002
Rice, A. L. (1999). The Challenger Expedition. Understanding the Oceans: Marine Science in the Wake of HMS Challenger. Routledge.
Robinson, R. A., Lawson, B., Toms, M. P., Peck, K. M., Kirkwood, J. K., Chantrey, J., et al. (2010). Emerging infectious disease leads to rapid population declines of common british birds. PLoS ONE 5:e12215. doi: 10.1371/journal.pone.0012215
Roemmich, D., Boebel, O., Freeland, H., King, B., LeTraon, P. Y., Molinari, R., et al. (2009). On The Design and Implementation of Argo: A Global Array of Profiling Floats. UCSD. Available online at: http://www.argo.ucsd.edu/argo-design.pdf (Accessed January 16, 2018).
Sathyendranath, S. (2014). Phytoplankton Functional Types From Space. Dartmouth, NS: IOCCG report. No. 15.
Short, F. T., Coles, R. G., and Pergent-Mantini, P. (2001). “Global seagrass distribution,” in Global Seagrass Research Methods, eds F. T. Short and R. G. Coles (Elsevier Science B.V.), 5–30.
Sigsgaard, E. E., Nielsen, I. B., Bach, S. S., Lorenzen, E. D., Robinson, D. P., Knudsen, S. W., et al. (2016). Population characteristics of a large whale shark aggregation inferred from seawater environmental DNA. Nat. Ecol. Evol. 1:0004. doi: 10.1038/s41559-016-0004
Simmonds, E. J., and MacLennan, D. N. (2005). Fisheries Acoustics: Theory and Practice. Oxford: Blackwell Science
Singh, G. G., Cisneros-Montemayor, A. M., Swartz, W., Cheung, W., Guy, J. A., Tiff-Annie, K., et al. (2017). A rapid assessment of co-benefits and trade-offs among Sustainable Development Goals. Mar. Policy. 93, 223–231. doi: 10.1016/j.marpol.2017.05.030
Sosik, H. M., and Olson, R. J. (2007). Automated taxonomic classification of phytoplankton sampled with imaging-in-flow cytometry. Limnol. Oceanogr. Methods. 5, 204–216. doi: 10.4319/lom.2007.5.204
Soto, I., Andrefouet, S., Hu, C., Muller-Karger, F. E., Wall, C. C., Sheng, J., et al. (2009). Physical connectivity in the Mesoamerican Barrier Reef System inferred from 9 years of ocean color observations. Coral Reefs. 28, 415–425. doi: 10.1007/s00338-009-0465-0
Sutton, T. T., Clark, M. R., Dunn, D., Halpin, P. N., Rogers, A. D., Guinotte, J., et al. (2017). A global biogeographic classification of the mesopelagic zone. Deep Sea Res. Part I Oceanogr. Res. Papers, 126, 85–102. doi: 10.1016/j.dsr.2017.05.006
Teague, W. J., Molinelli, E. J., and Carron, M. J. (1987). A new system for management of the “Master Oceanographic Observation Data Set” (MOODS). Eos, Trans. Am. Geophys. Union 68, 553–559. doi: 10.1029/EO068i022p00553-02
Thomsen, P. F., Kielgast, J., Iversen, L. L., Møller, P. R., Rasmussen, M., and Willerslev, E. (2012). Detection of a diverse marine fish fauna using environmental DNA from seawater samples. PLoS ONE 7:e41732. doi: 10.1371/journal.pone.0041732
Tiner, R. W., Lang, M. W., and Klemas, V. V. (2015) Remote Sensing of Wetlands: Applications Advances. Boca Raton, FL: CRC Press.
Tuomisto, H. (2010a). A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography 33, 2–22 doi: 10.1111/j.1600-0587.2009.05880.x
Tuomisto, H. (2010b). A diversity of beta diversities: straightening up a concept gone awry. Part 2. Quantifying beta diversity and related phenomena. Ecography 33, 23–45. doi: 10.1111/j.1600-0587.2009.06148.x
Turak, E., Brazill-Boast, J., Cooney, T., Drielsma, M., et al. (2016). Using the essential biodiversity variables framework to measure biodiversity change at national scale. Biol. Conserv. 213, 264–271. doi: 10.1016/j.biocon.2016.08.019
Turpie, K. R., Klemas, V. V., Byrd, K., Kelly, M., and Jo, Y.-H. (2015). Prospective HyspIRI global observations of tidal wetlands. Remote Sens. Environ. 167, 206–217. doi: 10.1016/j.rse.2015.05.008
U.S. Commission on Ocean Policy (2004). An Ocean Blueprint for the 21st Century. Final Report of the U.S. Commission on Ocean Policy. Washington, DC.
UNESCO (2017). Available online at: https://en.unesco.org/news/united-nations-announces-decade-ocean-science-2021-2030 (Accessed 30 December, 2017).
Van Heukelem, L., and Thomas, C. S. (2001). Computerassisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Oceanograp. A 910, 31–49. doi: 10.1016/S0378-4347(00)00603-4
Webb, T. J., Vanden Berghe, E., and O'Dor, R. (2010). Biodiversity's big wet secret: the global distribution of marine biological records reveals chronic under-exploration of the deep pelagic ocean. PLoS ONE 5:e10223. doi: 10.1371/journal.pone.0010223
Wells, B. K., Schroeder, I. D., Bograd, S. J., Hazen, E. L., Jacox, M. G., Leising, A., et al. (2017). State of the California Current 2016-2017: Still Anything but “Normal” in the North. CalCOFI Reports, 58, 55.
World Meteorological Organization (2018). Available online at: https://public.wmo.int/en/programmes/global-climate-observing-system/essential-climate-variables
Wright, S. W., Jeffrey, S. W., and Mantoura, R. F. C. (1997). Evaluation of methods and solvents for pigment extraction. En: phytoplankton pigments in oceanography. Monogr. Oceanogr. Methodol. 10, 261–228.
Keywords: essential ocean variables (EOV), essential biodiversity variables (EBV), marine biodiversity observation network (MBON), global ocean observing system (GOOS), ocean biogeographic information system (OBIS), marine global earth observatory (MarineGEO), integrated marine biosphere research (IMBeR)
Citation: Muller-Karger FE, Miloslavich P, Bax NJ, Simmons S, Costello MJ, Sousa Pinto I, Canonico G, Turner W, Gill M, Montes E, Best BD, Pearlman J, Halpin P, Dunn D, Benson A, Martin CS, Weatherdon LV, Appeltans W, Provoost P, Klein E, Kelble CR, Miller RJ, Chavez FP, Iken K, Chiba S, Obura D, Navarro LM, Pereira HM, Allain V, Batten S, Benedetti-Checchi L, Duffy JE, Kudela RM, Rebelo L-M, Shin Y and Geller G (2018) Advancing Marine Biological Observations and Data Requirements of the Complementary Essential Ocean Variables (EOVs) and Essential Biodiversity Variables (EBVs) Frameworks. Front. Mar. Sci. 5:211. doi: 10.3389/fmars.2018.00211
Received: 11 March 2018; Accepted: 29 May 2018;
Published: 27 June 2018.
Edited by:
Johannes Karstensen, GEOMAR Helmholtz-Zentrum für Ozeanforschung Kiel, GermanyReviewed by:
Emmanuel Devred, Fisheries and Oceans Canada, CanadaCopyright © 2018 Muller-Karger, Miloslavich, Bax, Simmons, Costello, Sousa Pinto, Canonico, Turner, Gill, Montes, Best, Pearlman, Halpin, Dunn, Benson, Martin, Weatherdon, Appeltans, Provoost, Klein, Kelble, Miller, Chavez, Iken, Chiba, Obura, Navarro, Pereira, Allain, Batten, Benedetti-Checchi, Duffy, Kudela, Rebelo, Shin and Geller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frank E. Muller-Karger, Y2FyaWJAdXNmLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.