
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 14 May 2018
Sec. Coral Reef Research
Volume 5 - 2018 | https://doi.org/10.3389/fmars.2018.00160
This article is part of the Research TopicCoral Reefs in the AnthropoceneView all 21 articles
Climate change and other anthropogenic disturbances have created an era characterized by the inability of most ecosystems to maintain their original, pristine states, the Anthropocene. Investigating new and innovative strategies that may facilitate ecosystem restoration is thus becoming increasingly important, particularly for coral reefs around the globe which are deteriorating at an alarming rate. The Great Barrier Reef (GBR) lost half its coral cover between 1985 and 2012, and experienced back-to-back heat-induced mass bleaching events and high coral mortality in 2016 and 2017. Here we investigate the efficacy of interspecific hybridization as a tool to develop coral stock with enhanced climate resilience. We crossed two Acropora species pairs from the GBR and examined several phenotypic traits over 28 weeks of exposure to ambient and elevated temperature and pCO2. While elevated temperature and pCO2 conditions negatively affected size and survival of both purebreds and hybrids, higher survival and larger recruit size were observed in some of the hybrid offspring groups under both ambient and elevated conditions. Further, interspecific hybrids had high fertilization rates, normal embryonic development, and similar Symbiodinium uptake and photochemical efficiency as purebred offspring. While the fitness of these hybrids in the field and their reproductive and backcrossing potential remain to be investigated, current findings provide proof-of-concept that interspecific hybridization may produce genotypes with enhanced climate resilience, and has the potential to increase the success of coral reef restoration initiatives.
The rapid increase in atmospheric CO2 to levels not documented for millions of years (Hönisch et al., 2012) and associated ocean warming and acidification have profoundly transformed the marine realm (Pandolfi et al., 2011). Higher-than-usual seawater temperatures can cause coral bleaching, the breakdown of the symbiotic relationship between the coral host and its dinoflagellate endosymbionts (Symbiodinium spp.), and associated coral mortality (Hoegh-Guldberg, 1999). Ocean acidification is reducing carbonate ion availability in seawater and can depress calcification rates of calcifying organisms like corals (Langdon et al., 2000; Doney et al., 2009; Chan and Connolly, 2013). These global changes, coupled with local stressors such as pollution, overfishing, and outbreaks of crown-of-thorns starfish, have drastically altered coral cover and community composition at a global scale. In the last three decades, multiple mass bleaching events have decimated coral reefs worldwide including in 1998, 2010, and 2014–2017 (Eakin et al., 2016; Heron et al., 2016; Hughes et al., 2017, 2018). The Great Barrier Reef (GBR) is no exception with 50–80% coral mortality recorded on many northern reefs following the 2016 mass bleaching event (Great Barrier Reef Marine Park Authority, 2017), followed by another high mortality mass bleaching event in 2017. Climate models predict a <5% chance of reaching the Paris agreement target of limiting the global temperature rise to <2°C compared to pre-industrial times by 2100 (Raftery et al., 2017), and most coral reefs are forecasted to experience annual severe bleaching before the end of the century (van Hooidonk et al., 2016). Several observations of an increase in tolerance of coral bleaching after successive bleaching events suggest that adaptation and/or acclimatization are possible under certain conditions (Maynard et al., 2008; Berkelmans, 2009; Guest et al., 2012; Penin et al., 2013). Nevertheless, over 50% of the world's coral reefs has been lost in the last three decades, with the Caribbean having lost over 80% of its coral cover (50 Reefs, 2017)1, indicating that the rates of natural adaptation and acclimatization are overall insufficient to keep pace with the rate of environmental changes (van Oppen et al., 2017).
Active reef restoration is one way to assist the recovery of coral reefs that are degraded, damaged or destroyed. Reef restoration is still in its infancy and all of the few successful efforts so far occurred on a small spatial scale (e.g., Nakamura et al., 2011; Omori, 2011; Villanueva et al., 2012; Guest et al., 2014; dela Cruz and Harrison, 2017). Traditionally, locally sourced biological material is used for restoration based on the assumption that these populations are locally adapted and therefore most likely to survive (Breed et al., 2013). However, anthropogenic disturbances are rapidly changing the environment and shifting selection pressures (Becker et al., 2013), and locally sourced stock is therefore potentially mismatched with the altered environment. An effective restoration strategy should thus incorporate an understanding of present day ecological characteristics of species, characteristics of future available habitats, and adaptive potential of species (Becker et al., 2013). The use of non-local and climate resilient materials is controversial, but is gaining traction in wildland restoration (Jones and Monaco, 2009), revegetation (Sgrò et al., 2011; Breed et al., 2013), and coral reef restoration (Rau et al., 2012; van Oppen et al., 2017).
One possible way to improve the adaptive potential of species is via hybridization, which can increase genetic variation, break genetic correlations that constrain evolvability of parental lineages, and assist species to acquire adaptive traits (Hoffmann and Sgrò, 2011; Becker et al., 2013; Carlson et al., 2014; van Oppen et al., 2015; Hamilton and Miller, 2016; Meier et al., 2017). Hybridization can be conducted either via targeted crossing of individuals or species carrying desired phenotypic traits (e.g., high thermal tolerance) or via crossing between species with the goal of increasing genetic diversity and new variation for natural selection to act upon, and potentially generating hybrid vigor. The relative fitness of F1 hybrids (Figure 1) depends on whether there are additive (i.e., hybrids are of intermediate fitness between the parental species), dominant (i.e., hybrids are of equal fitness to the dominant parent species), over-dominant (i.e., hybrids are more fit than both parental species), under-dominant (i.e., hybrids are less fit than both parental species) gene effects, and/or maternal effects (i.e., hybrids are of equal fitness to their maternal parent species) (for review, see Lippman and Zamir, 2007; Li et al., 2008; Chen, 2013). Reciprocal hybrids are predicted to have equal fitness, except under maternal inheritance. With maternal effects, the fitness of the hybrids is directly affected by the fitness of the maternal parental species, regardless of the offspring's own genotype (Roach and Wulff, 1987; Bernardo, 1996). In the context of restoration, hybrid vigor which can be driven by dominant or over-dominant mechanisms, is a desirable outcome. The value of hybridization in enhancing fitness has been demonstrated in multiple cases. For instance, hybridization has provided genetic variance in morphology for adapting to changing environments in Darwin's finches (Grant and Grant, 2010), altered chemical defense of hybrid Brassicaceae plants and aided their survival through the Last Glacial Maximum (Becker et al., 2013), and facilitated extensive adaptive radiation in haplochromine cichlid fishes (Meier et al., 2017).
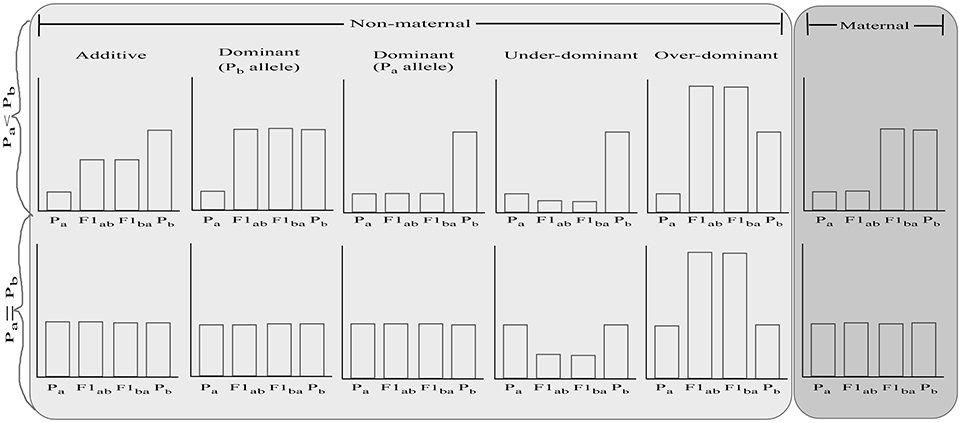
Figure 1. Possible relative fitness of reciprocal F1 hybrids (F1ab and F1ba) based on fitness of the parental species (Pa and Pb) and the driving mechanism. In the top graphs, parental species are assumed to differ in phenotype while the bottom graphs indicate a situation where parental species are similar. For further explanation, see text.
Hybridization is known to occur naturally in some scleractinian corals and has played an important role in the evolution and diversification of the genus Acropora (van Oppen et al., 2001; Willis et al., 2006). In the Caribbean, recent environmental degradation and massive population decline in Acropora cervicornis and Acropora palmata have favored hybridization and expansion of their F1 hybrid, Acropora prolifera (Fogarty, 2012). These hybrids either have equivalent or higher fitness relative to the parent species in most life history stages examined (Fogarty, 2012). In recent years, A. prolifera has been reported in increasingly high abundance in many reef locations (Fogarty, 2012; Japaud et al., 2014; Aguilar-Perera and Hernández-Landa, 2017) and the hybrid has expanded to marginal environments where parent species are absent (Fogarty, 2012).
Although interspecific hybridization is a potential tool to enhance restoration outcomes, it is often dismissed in restoration initiatives. Concerns raised include the possibility of outbreeding depression in later generations (i.e., F2, F3, backcross), and the loss of diversity through losing part of the parental species' genome (for review, see Hamilton and Miller, 2016). Most examples of outbreeding depression, however, are associated with the admixture of populations or species that are geographically distant, or when life history or phenological differences are large (Hwang et al., 2012; Whiteley et al., 2015). Outbreeding depression can also be transient and can be overcome by natural selection (Jones and Monaco, 2009; Aitken and Whitlock, 2013; Hamilton and Miller, 2016). Instead of reducing genetic diversity, hybridization may conserve diversity by protecting the parental genome from the risk of extinction, and can also increase genetic diversity by combining two divergent genomes within a single organism (Garnett et al., 2011). For example, hybridization has successfully enhanced genetic diversity, improved the population size and rescued the highly inbred, remnant population of Florida panther (Johnson et al., 2010) and the Mt. Buller mountain pygmy-possum (Weeks et al., 2017) from extinction (i.e., genetic rescue).
Here we investigate interspecific hybridization as a novel tool to increase genetic diversity and develop coral stock with increased climate resilience. Parental species were not chosen for their relative climate resilience, but based on our expert knowledge of the probability that they would cross-fertilize as well as their evolutionary relatedness. We examined the performance of hybrids from reciprocal crosses of two Acropora species pairs raised under ambient and elevated seawater temperature and pCO2 conditions, and assessed (1) whether prezygotic barriers exist in interspecific hybrids of Acropora corals from the GBR, and (2) whether hybrids show enhanced fitness and resilience compared to the purebreds. Four phenotypic traits (i.e., survival, recruit size, Symbiodinium uptake, and photochemical efficiency) were measured in hybrid and purebred offspring as proxies for fitness. Surviving hybrids and purebreds at the end of the experiment were transplanted to long-term grow-out tank for rearing with the aim to allow future assessment of their reproductive and backcrossing potential when they reach sexual maturity at ~4 years of age. We continued to monitor these survivors for survival and size during the grow-out period.
A detailed timeline of the experiment, sampling and measurement of each trait is shown in Figure S1. Parental colonies were collected from Trunk Reef, central GBR, prior to full moon on 22nd Nov 2015 and maintained in flow-through aquaria of the National Sea Simulator (SeaSim) at the Australian Institute of Marine Science (AIMS). When signs of imminent spawning were observed (“setting,” i.e., where the sperm-egg bundles begin to protrude through the mouth of the polyps), colonies were isolated in individual tanks to avoid uncontrolled mixing of gametes prior to in vitro crossing. The five most profusely spawning colonies of each parental species were used for crossing to form (1) an Acropora tenuis × Acropora loripes cross, and (2) an Acropora sarmentosa × Acropora florida cross (Figure 2). These two species pairs were chosen to represent a phylogenetically divergent cross and a phylogenetically closely related cross. The phylogeny of Acropora spp. is divided into two distinct groups: the “early spawners” and the “late spawners,” where the “late spawners” spawn about 1.5–3 h before the other group (Fukami et al., 2000; van Oppen et al., 2001; Márquez et al., 2002). A. tenuis (early spawner) and A. loripes (late spawner) are phylogenetically divergent, while A. sarmentosa and A. florida (both are “late spawners”) are closely related and fall within the same phylogenetic clade (Fukami et al., 2000; van Oppen et al., 2001; Márquez et al., 2002). Little information is available from the literature about the relative resilience of these four parental species, but this has limited relevance for this study as our purpose was to increase genetic diversity (and thus adaptive potential) via hybridization, and not to conduct targeted breeding with species of known relative bleaching tolerance. Only two A. florida colonies spawned on the same night as A. sarmentosa, therefore, only two colonies were used for this species.
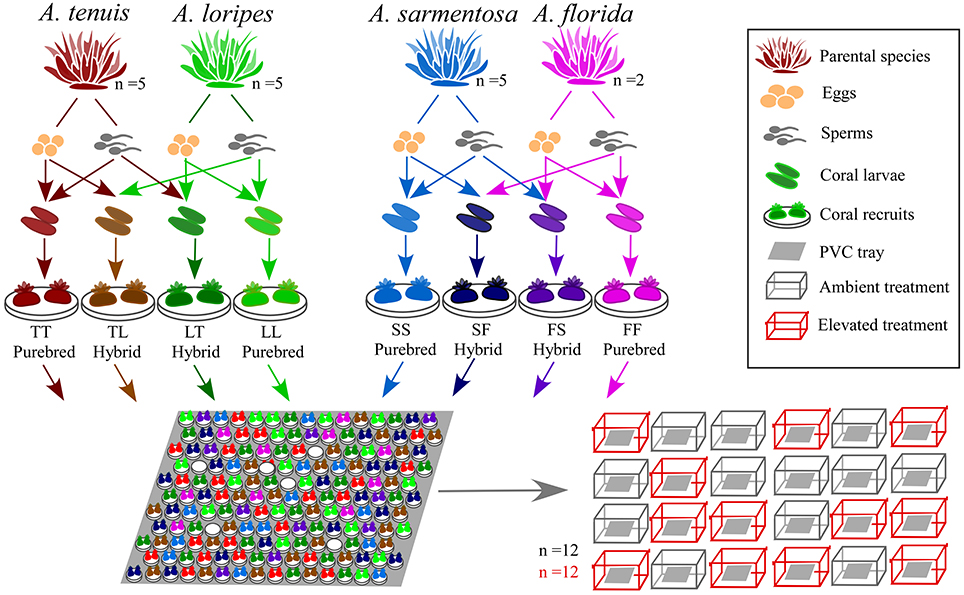
Figure 2. Experimental set up showing the two interspecific crosses [i.e., A. tenuis (T) × A. loripes (L) and A. sarmentosa (S) × A. florida (F)], the four resultant offspring groups from each cross (TT, TL, LT, LL; and SS, SF, FS, FF, respectively), larval settlement, and comparison of hybrid and purebred fitness under ambient and elevated conditions. The abbreviation of the offspring groups throughout this paper is that the first letter represents the origin of the eggs and the second letter the origin of sperm [e.g., SF is a hybrid of A. sarmentosa (S) eggs with A. florida (F) sperm]. The different colors used for the offspring groups in the figure reflect differently colored settlement plugs used for each offspring group.
Egg-sperm bundles of individual colonies were collected and eggs and sperm were separated using a 100 μm filter. Eggs were washed three times with filtered seawater to remove any residual sperm and placed in a 3 L bowl until crosses were set up (within 3 h). Sperm concentration of every colony was measured with a hemocytometer on a compound microscope with 40x magnification. Similar quantities of sperm from each conspecific colony were pooled to create a mixed sperm solution. For the hybrid crosses, the mixed sperm solution of the other parental species was added to the eggs of each interspecific colony. This method prevented intraspecific fertilization by possible remaining sperm that was not washed off the eggs (note that no self-fertilization was observed in any of the crosses performed). Fertilization was conducted under ambient conditions at a sperm concentration of 106 sperm mL−1. Three samples of 100 eggs were collected for each species as a self-fertilization test and a “no sperm” control. Each species pair cross produced four offspring groups, two purebreds and two hybrids (Figure 2). Embryos of each offspring group were then placed in rearing tanks for development under ambient conditions.
Fertilization rates were assessed at 3.5 h, and embryonic development at 9, 15, 21, 33, 45, 57, and 93 h after sperm was added to the eggs. All embryos had reached planula stage and were ready to settle by 93 h. Triplicate samples of 100 embryos of each offspring group were collected and fixed in 4% formaldehyde. Developmental stages were assessed and counted under a dissecting microscope based on the stages described in Randall and Szmant (2009).
Prior to coral spawning, ceramic plugs of eight different colors were preconditioned in the outdoor SeaSim flow-through aquaria under ambient conditions for 6 weeks to develop crustose coralline algae (CCA) and a microbial biofilm to provide a larval settlement cue. Five days after fertilization, planula larvae of the eight offspring groups were each settled onto one assigned color of the pre-conditioned plugs under ambient conditions. Plugs of eight different colors were used so that each offspring groups could easily be identified and randomized in the experimental PVC trays holding the plugs (Figure 2). During settlement, Symbiodinium (i.e., algal symbionts) isolated from the parent colonies were added to achieve a final density of 2 × 106 cells mL−1 in each settlement tank. Larvae of each offspring group only received Symbiodinium from their parental species. To isolate the Symbiodinium, an ~6 cm fragment with three branches was removed from each parental colony with a bone cutter. Soft tissues of the fragment were then removed using an airbrush. The mixed soft tissues/seawater solution was collected and centrifuged at 200 g for 5 min to pellet the Symbiodinium. The Symbiodinium cells were resuspended and washed three times with filtered seawater before being added to the larvae. Symbiodinium uptake was assessed under a dissecting microscope prior to exposure to elevated conditions. Recruits (n = 20 per offspring group) were categorized as either with or without Symbiodinium.
Settled recruits were randomized and evenly distributed on 24 tailor-made PVC trays to rear under (1) ambient conditions of 27°C, 415 ppm pCO2, or (2) elevated conditions of ambient +1°C, 685 ppm pCO2 (Figure 2). Recruits for the elevated conditions were ramped to the target temperature and pCO2 from ambient at a rate of +0.2°C and + ~50 ppm per day. There were 12 replicate tanks for each of the two treatment conditions and tank positions in the experiment room were randomized (Figure 2). Every tank held one PVC tray with 20 plugs of each offspring group, with the exception of A. florida purebred (FF) and hybrid (FS) which had only 10 plugs due to fewer larvae being available. To avoid sediments from accumulating on top of the recruits, the trays were placed at an approximately 45° angle. Experimental conditions followed Davies Reef (18.83°S, 147.63°E) diurnal and annual temperature variations, a reef in proximity to Trunk Reef were the adult corals used for spawning were collected. A mixed marine microalgae diet of Isochrysis, Pavlova, Tetraselmis, Chaetocerous calcitrans, Thalassiosira weissflogii, and Thalassiosira pseudonana was fed to the recruits twice a day at a final concentration of ~5,000 cells mL−1 in the tank.
Recruits from each tank were imaged using a high-resolution camera (Nikon D810) mounted on a quadpod with a waterproof case. Imaging was conducted fortnightly in the first 8 weeks of the experiment, thereafter every 4 weeks until 28 weeks. The numbers of surviving recruits were visually counted and recorded. Detailed images were taken at 28 weeks for size measurement. Recruit size was estimated as surface area of a circle from the measured recruit diameter since recruits were circular in shape and were not yet forming upright branches. Measurements were made using the software ImageJ and calibrated on the scale chart presented on every image. Recruits were maintained under the treatment conditions for 28 weeks. Surviving juveniles were thereafter relocated to long-term grow-out tanks to accommodate their larger size and maintained under ambient raw water (i.e., unfiltered seawater) to cater for higher feeding demand. Due to the small size of some recruits at 28 weeks and therefore difficulty to make comparisons, size was again measured at 1 year of age (i.e., about 5 months after all surviving recruits were moved to ambient raw water conditions) using the same measurement method. Furthermore, a set of photos of the median sized juvenile were taken at 2 years of age.
Photochemical efficiency (i.e., dark adapted maximum photosystem II quantum yield, Fv/Fm) was measured at week 28 as a proxy for coral health. Measurements were made using Imaging- Pulse Amplitude Modulation (I-PAM) derived by the software ImagingWin (v2.40b). Recruits (n = 15 per offspring group per treatment) were dark adapted overnight, and remained submerged in the treatment seawater during imaging. A recruit would only be measured if: (1) it was not obscured by filamentous algae, and (2) its size was no smaller than the software's area of interest requirement.
Automated controls of seawater chemistry were provided by SeaSim via the SCADA (Supervisory Control and Data Acquisition) system. Experimental conditions are summarized in Table 1. Seawater temperature and pH were recorded every hour using resistance temperature detector (RTD) and a pH probe (Tophit CPS471D). pCO2 was measured bi-weekly using a CO2 equilibrator calibrated to a standard gas of 500 ppm. Total alkalinity (AT) was measured using VINDTA calibrated to Dickson's Certified Reference Material. Salinity was measured weekly with an HACH IntelliCAL™ CDC401 Standard Conductivity Probe calibrated with IAPSO Standard Seawater. Seawater carbonate chemistry parameters, including Ωarag, DIC (dissolved inorganic carbon), , and were calculated using the measured values of seawater AT, pCO2, temperature and salinity, with the program CO2SYS (Lewis and Wallace, 1998 as implemented in Microsoft Excel by Pierrot et al., 2006).
Generalized linear mixed models (GLMM) (McCulloch and Neuhaus, 2013) for binomial data with logistic link functions were used to estimate the effects of treatment and offspring group on recruit survival at week 28. Analyses were conducted separately for the offspring groups of the A. tenuis × A. loripes cross and the offspring groups of the A. sarmentosa × A. florida cross using R Core Team (2016) with packages lme4 (Bates et al., 2014) and multcomp (Hothorn et al., 2008). In order to account for tank differences in the experimental design, a random tank effect was included in the models. Models were checked for overdispersion using a Chi-square test (Bolker et al., 2009) and goodness of fit using Akaike Information Criteria (Akaike, 1974). AIC of the GLMM of the A. tenuis × A. loripes cross was 397, the A. sarmentosa × A. florida cross was 331. Tukey's pairwise comparisons were then conducted and p-values were corrected using the Benjamini–Hochberg method (Benjamini and Hochberg, 1995). To obtain a visual overview of survival over time, longitudinal generalized linear models (GLM) for binomial data were used to estimate the survival for the offspring groups across all time points, and the combined hybrid offspring vs. purebred offspring. A summary table of the mean survival is provided in Table 7. Survival data were also analyzed with Cox proportional hazards regression as a comparison to GLMM. Results of the Cox regression were very similar to those of the GLMM and are shown in the Supplementary Methods and Results section (Table S1).
Statistical analyses of size were conducted separately for the offspring groups of the A. tenuis × A. loripes cross and the offspring groups of the A. sarmentosa × A. florida cross at 28 weeks and at 1 year of age. For the 28-week time point, the absence of growth in a large number of samples under elevated conditions resulted in non-normality of the data, and non-parametric Kruskal–Wallis tests were undertaken followed by Dunn's pairwise comparisons (Dunn, 1964). P-values for the multiple pairwise comparisons were adjusted with the Benjamini–Hochberg method. For the 1 year time point, due to the absence of survivors in some offspring groups, not all size comparisons could be undertaken. Offspring groups with no or less than three survivors were excluded from the analyses. The remaining size data were normally distributed (tested using Shapiro–Wilk tests; Shapiro and Wilk, 1965) and variances were homogeneous (tested by Levene's tests; Levene, 1960) and five pairwise comparisons were undertaken using combined variance t-tests. For the A. tenuis × A. loripes cross, three t-tests were possible for offspring groups that were previously exposed to ambient conditions (note they have been relocated to long-term grow-out tank under raw ambient seawater after 28 weeks). The p-values of these comparisons were adjusted using the Benjamini–Hochberg method. The above analyses were run in R (version 3.3.1). A summary of the mean sizes of the recruits is shown is Table 7.
Generalized linear models (GLM) (McCulloch and Neuhaus, 2013) were used to test the effect of offspring group on rates of Symbiodinium uptake, which was treated as a binomial distributed variable (i.e., Symbiodinium taken up/not taken up). Treatment was not included in this model as Symbiodinium uptake was assessed prior to the start of treatment. For photochemical efficiency (i.e., dark adapted yield, Fv/Fm), generalized linear models were also used to test the effects of offspring group and treatment on the response. Tukey pairwise comparisons were then used and the p-values were adjusted with the Benjamini–Hochberg method. These analyses were run with R packages lme4 (Bates et al., 2014) and multcomp (Hothorn et al., 2008).
The date and time of spawning of the Acropora spp. used in this study are summarized in Table 2. The A. tenuis × A. loripes cross was conducted on the 6th day after the full moon, where A. tenuis spawned at ~19:00–19:30 and A. loripes at ~21:45. The A. sarmentosa × A. florida cross was conducted on the 7th day after the full moon, where A. sarmentosa spawned at ~20:30–20:45 and A. florida at ~21:15–21:30. Fertilization rates of all but one hybrid offspring group were high (averaged 93%) (Figure 3). Hybrid LT had lower fertilization rates (averaged 79%) compared to all other offspring groups. No fertilization was observed in the “no-sperm” control and self-fertilization tests. Purebred and hybrid embryos developed normally and reached the planula stage 93 h after fertilization (Figure S2). The hybrid LT also had a slower initial embryonic development rate with the majority of the LT embryos being at the 2–4 cell stage at 3.5 h after fertilization, while embryos of all other offspring groups were at the 8–16 cell stage (Figure S2). From 9 h onwards, however, all offspring groups developed at similar rates.
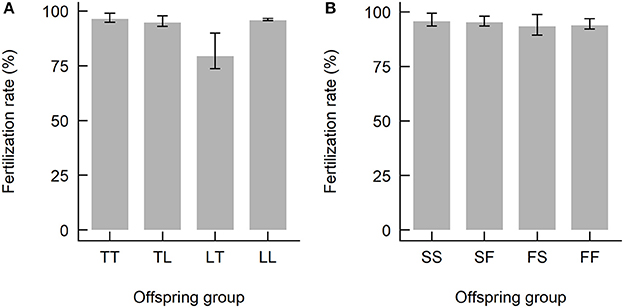
Figure 3. Fertilization rates of the offspring groups from (A) the Acropora tenuis (T) × Acropora loripes (L) cross, and (B) the Acropora sarmentosa (S) × Acropora florida (F) cross. The abbreviation of the offspring groups is that the first letter represents the origin of the eggs and the second letter the origin of sperm, where TL, LT, SF, FS are hybrids and TT, LL, SS, FF are purebreds.Values are mean and error bars represent 95% CI calculated using the angular transformed data back-transformed into percentages.
Overall, maternal effects were observed in the hybrid offspring groups of the A. tenuis × A. loripes cross and over-dominance in the A. sarmentosa × A. florida cross, with some variations between treatment conditions (Figure 4). Offspring groups differed significantly for survival both in the A. tenuis × A. loripes cross (GLMM, χ2 = 252.2, df = 3, p < 0.001), and the A. sarmentosa × A. florida cross (GLMM, χ2 = 32.2, df = 3, p < 0.001). The values present below are mean survival and the associated 95% confidence intervals are shown in Table 3. For the A. tenuis × A. loripes cross, survival of hybrid LT (49%) and purebred LL (46%) was higher than that of TT (13%) and TL (16%) under ambient conditions (p < 0.001 for all) (Tables 3, 4, 7). Under elevated conditions, survival of hybrid LT (41%) and purebred LL (36%) was also higher than that of TT (7%) and TL (23%) (Tables 3, 4, 7). Survival of hybrids was similar to that of their maternal parental purebred offspring. Under elevated conditions, survival of hybrid TL (23%) was also higher than that of purebred TT (7%) (p < 0.001; Tables 3, 4, 7).
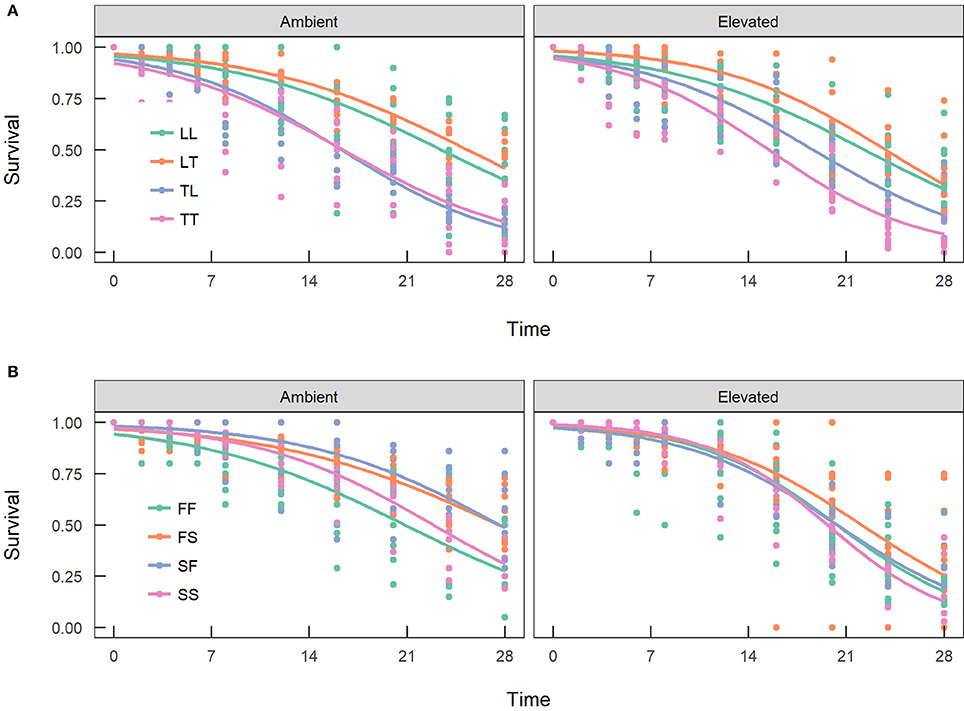
Figure 4. Survival of the offspring groups of (A) the Acropora tenuis (T) × Acropora loripes (L) cross, and (B) the Acropora sarmentosa (S) × Acropora florida (F) cross under ambient and elevated conditions across 28 weeks. The abbreviation of the offspring groups is that the first letter represents the origin of the eggs and the second letter the origin of sperm. Lines represent the estimates of the longitudinal generalized linear models.
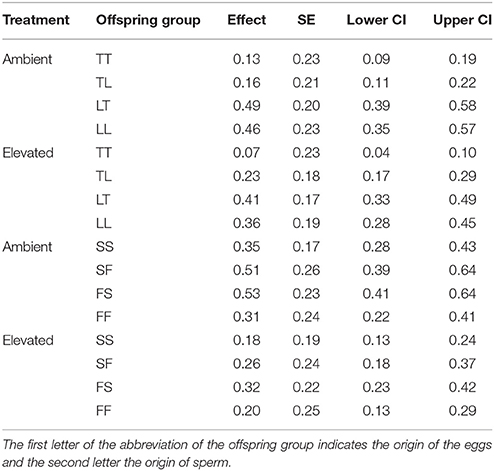
Table 3. Mean survival, SE, as well as lower and upper 95% CI of offspring groups from the Acropora tenuis (T) × Acropora loripes (L) cross and the Acropora sarmentosa (S) × Acropora florida (F) cross under ambient and elevated conditions.
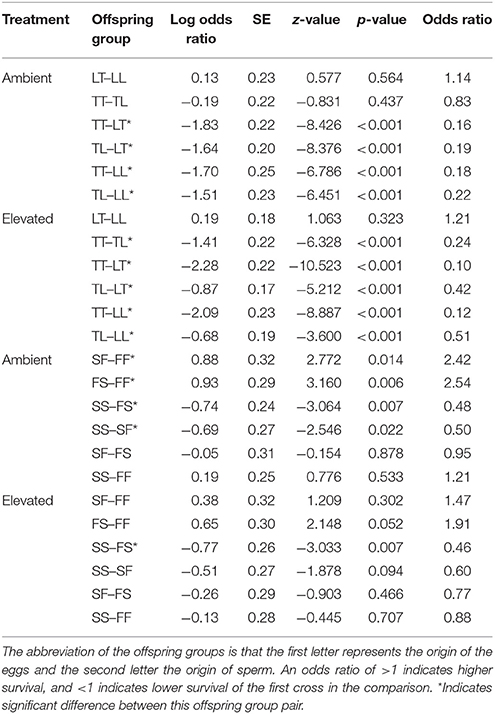
Table 4. Tukey's pairwise comparisons of survival between the offspring groups from the Acropora tenuis (T) × Acropora loripes (L) cross and the Acropora sarmentosa (S) × Acropora florida (F) cross following generalized linear mixed models.
For the A. sarmentosa × A. florida cross, survival of both hybrid SF (51%) and FS (53%) was higher than that of the purebred FF (31%) (p = 0.014, p = 0.006, respectively) and SS (35%) (p = 0.022, p = 0.007, respectively) under ambient conditions (Tables 3, 4, 7). Under elevated conditions, only hybrid FS (32%) had higher survival than purebred SS (18%) (p = 0.007; Tables 3, 4, 7). When combining the data for all hybrid and purebred offspring groups for an overall comparison, hybrid offspring had a consistently higher survival than purebred offspring (Figure S3).
Treatment had a significant effect on survival of both the A. tenuis × A. loripes cross (GLMM, χ2 = 26.9, df = 1, p < 0.001), and the A. sarmentosa × A. florida cross (GLMM, χ2 = 13.6, df = 1, p < 0.001). Tukey pairwise comparisons suggest that TT, SS, SF, and FS had lower survival under elevated conditions compared to ambient conditions at week 28 (p = 0.025, 0.002, 0.007, 0.015, respectively; Table 5).
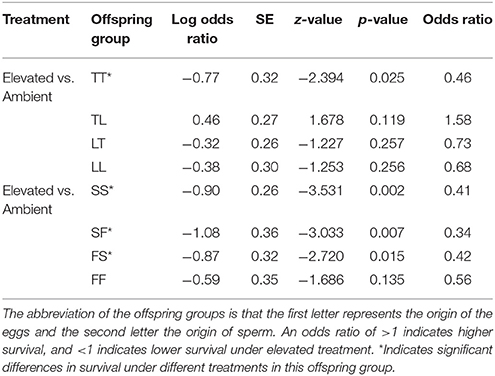
Table 5. Tukey's pairwise comparisons of treatment effect within an offspring group from the Acropora tenuis (T) × Acropora loripes (L) cross and the Acropora sarmentosa (S) × Acropora florida (F) cross following generalized linear mixed models.
For the A. tenuis × A. loripes cross, treatment had a significant effect on recruit size (Kruskal–Wallis, χ2 = 33.6, df = 1, p < 0.001) but offspring group did not (Kruskal–Wallis, χ2 = 6.9, df = 3, p = 0.096; Figure 5). For the A. sarmentosa × A. florida cross, treatment also had a significant effect on recruit size (Kruskal–Wallis, χ2 = 38.2, df = 1, p < 0.001). Offspring group had a significant effect on size under ambient conditions (Kruskal–Wallis, χ2 = 18.2, df = 3, p < 0.001), but not under elevated conditions (Kruskal–Wallis, χ2 = 1.0, df = 3, p = 0.793). Under ambient conditions, the mean size of hybrids FS (41 mm2) and SF (43 mm2) was larger than that of the purebred SS (16 mm2) (z = 3.19, p = 0.003; z = 3.56, p = 0.001, respectively), but not different in size from FF (56 mm2; Table 7).
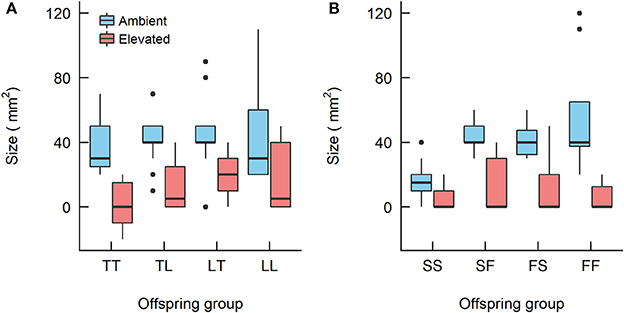
Figure 5. Boxplots showing the size of the Acropora offspring groups at 28 weeks since treatment began from (A) the Acropora tenuis (T) × Acropora loripes (L) cross and (B) the Acropora sarmentosa (S) × Acropora florida (F) cross. The first letter of the offspring groups' abbreviation represents the origin of the eggs and the second letter the origin of sperm. The horizontal bars represent median values, box length represents the interquartile range, and the small circles denote unusual points.
At the 1-year time point, several offspring groups no longer had survivors (Figure 6, Table 7). Note that the treatment condition in this section refers to the treatment conditions that the recruits were exposed to during the 28 week period following settlement, but that they were transferred to long-term grow-out tanks with ambient raw (i.e., unfiltered) seawater afterward. For recruits that were previously under ambient conditions, there were no survivors of purebreds TT and FF, while all hybrid groups had survivors. The mean size of LT (362 mm2) and LL (366 mm2) offspring was larger than that of TL offspring (47 mm2) (t-test, p = 0.008, 0.015, respectively, Tables 6, 7). The size of the LT hybrids was the same as that of the maternal parent species LL (i.e., maternal effect). The mean size of FS hybrids (304 mm2) was larger than that of the pure breds SS (30 mm2) (t-test, p = 0.004, Tables 6, 7). The mean size of hybrid SF (245 mm2, average of 2 recruits) was also relatively larger than SS (30 mm2), however, statistical comparison was not possible due to the low sample size for SF (n = 2; Table 7). For recruits that were previously under elevated conditions, there were no survivors of purebreds TT and SS as well as hybrids TL and SF. The mean size of the hybrid LT recruits (326 mm2) was the same as that of the maternal parent species LL (290 mm2) (Tables 6, 7). Median sized survivors of hybrid and purebred juveniles at 2 years of age and are shown in Figure S4.
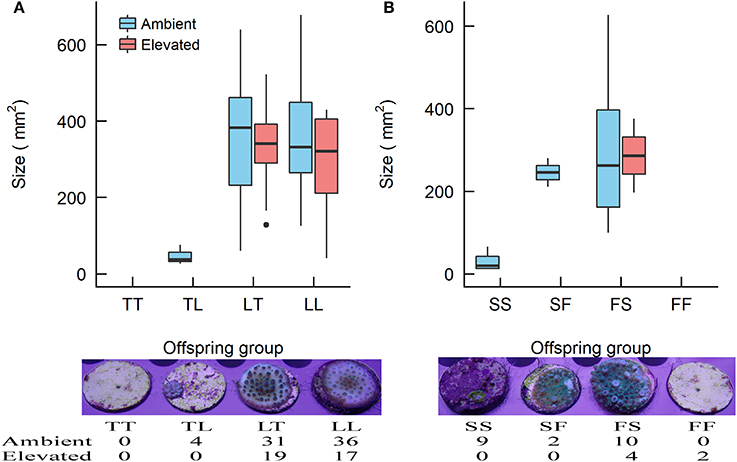
Figure 6. Boxplots showing the size of the Acropora offspring groups at 1 year of age (i.e., ~5 month since relocation to long-term grow-out tank under raw ambient seawater. (A) The Acropora tenuis (T) × Acropora loripes (L) cross and (B) the Acropora sarmentosa (S) × Acropora florida (F) cross. The first letter of the offspring groups' abbreviation represents the origin of the eggs and the second letter the origin of sperm. Where no data are presented there were no survivors in that offspring group. The horizontal bars indicate the medians, box length indicates the interquartile range, and the small circles indicate unusual points. Images below the graphs show examples of median size recruits of the offspring groups reared under ambient conditions in the experiment, and the number of survivors of each offspring group.
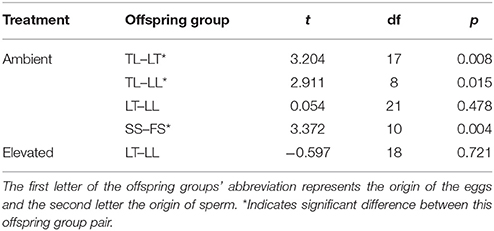
Table 6. Results of t-tests comparing size at the 1-year time point for remaining offspring groups of the Acropora tenuis (T) × Acropora loripes (L) cross, and the Acropora sarmentosa (S) × Acropora florida (F) cross.
There was no significant difference in Symbiodinium uptake between the offspring groups of the A. tenuis × A. loripes cross (GLM, χ2 = 3.25, df = 3, p = 0.354) or the offspring groups of the A. sarmentosa × A. florida cross (GLM, χ2 = 5.35, df = 3, p = 0.148; Figure S5, Table 7). For the A. sarmentosa × A. florida cross, neither treatment nor offspring groups had a significant effect on photochemical efficiency (Treatment: GLM, χ2 = 0.51, df = 1, p = 0.477; offspring group: GLM, χ2 = 4.28, df = 3, p = 0.233). For the A. tenuis × A. loripes cross, treatment had a significant effect on photochemical efficiency (GLM, χ2 = 6.87, df = 1, p = 0.009) but offspring groups did not (GLM, χ2 = 2.43, df = 3, p = 0.488; Figure S6). Tukey pairwise comparisons show that purebreds TT and LL under elevated conditions had lower photochemical efficiency than their counterparts under ambient conditions (p = 0.035, 0.002, respectively).
The results of the various traits measured are summarized in Table 7.
To understand the value of hybridization for coral reef restoration, it is important to establish whether prezygotic barriers exist. Interspecific hybridization among Acropora spp. has been shown to occur in experimental crosses, with varying degrees of prezygotic barriers (Willis et al., 1997; Van Oppen et al., 2002; Fogarty et al., 2012; Isomura et al., 2013). Among multiple pairs of Acropora spp. from the central GBR, crossing resulted in eight pairs with high fertilization (50–80%), seven pairs with moderate fertilization (10–50%) and three pairs with low fertilization (3–10%) (Willis et al., 1997; Van Oppen et al., 2002). The high fertilization rates and normal embryonic development of the interspecific hybrids produced in this study indicate prezygotic barriers are limited in these species pairs. This was unexpected in the case of the A. tenuis × A. loripes cross which involved an “early spawner” and a “late spawner.” These “early spawners” and “late spawners” are believed to have diverged 6.6 Mya (Fukami et al., 2000). We hypothesize that our observations can be explained by the fact that the gametes of these species do not normally encounter one another in the field due to a 2 h difference in spawning times, and selection on prezygotic barriers has therefore been absent. Conversely, A. sarmentosa and A. florida are phylogenetically closely related, occur sympatrically and spawned ~30 min to 1 h apart. Our results indicate a prezygotic barrier has not evolved to maintain reproductive isolation of these two species either, and that hybridization may occasionally occur in nature.
Lower fertilization in one direction in Acropora hybrid crosses, as was observed for hybrid LT, is not uncommon (Fogarty et al., 2012; Isomura et al., 2013). The likelihood of A. palmata eggs being fertilized by A. cervicornis sperm, for instance, is smaller than the likelihood of A. cervicornis eggs being fertilized by A. palmata sperm (Fogarty et al., 2012). The lower fertilization rate of the hybrid LT, however, did not affect recruit size or survival of this offspring group. The slight delay in embryonic development as observed in the hybrid LT was similar to observations for another Acropora cross in Japan (Isomura et al., 2013). This delay, however, was only limited to one early time point and no aberrant development was observed in the hybrids at any time point. The results of this and previous studies suggest that the degree of prezygotic barriers varies between Acropora species, and a range of species with limited prezygotic barriers can be used for hybridization with the aim to enhance climate resilience. Interspecific hybridization may also be applied to several other coral genera. Experimental crosses have successfully hybridized species within the genera Montipora and Platygyra (Willis et al., 1997), but were unsuccessful for species in the genus Ctenactis (Baird et al., 2013). Future studies to test the success of interspecific hybridization in additional coral genera will be valuable to determine the extent to which this approach for coral reef restoration can be applied.
Given only limited prezygotic barriers exist, we explored whether hybrid offspring had increased resilience and may be used to enhance coral reef restoration efforts. If hybrids are comparatively resilient, interspecific hybridization may be combined with methods being developed for deploying coral larvae or recruits onto reefs requiring restoration (e.g., Nakamura et al., 2011; Omori, 2011; Villanueva et al., 2012; Guest et al., 2014; dela Cruz and Harrison, 2017). Overall, maternal effects were observed in hybrids of the A. tenuis × A. loripes cross and over-dominance in hybrids of the A. sarmentosa × A. florida cross, with some variations between traits and treatment conditions. Possible benefits of hybridization in enhancing reef restoration can be observed in both crosses (Table 7). In the A. tenuis × A. loripes cross, hybrids of both directions exhibited ~16–34% higher survival than purebred A. tenuis under conditions with elevated temperature and pCO2 (Table 7), suggesting hybrids have higher climate resilience than the purebred species. Both purebred species also showed reduced photochemical efficiency under elevated compared to ambient conditions while both hybrid species did not. Furthermore, purebred A. tenuis had no survivors at the 1-year time point, yet hybrids from both directions survived. For the A. sarmentosa × A. florida cross, hybrids of one or both directions showed ~14–22% higher survival and were larger in size than both or one parental purebred species (Table 7). One hybrid offspring group (FS) had survivors in both ambient and elevated conditions at the age of 1 year, while both purebred species had no survivors in one of these conditions (Table 7). The FS hybrid was also 10 times larger in size than the only surviving purebred species (A. sarmentosa) at 1 year of age under ambient conditions (Table 7).
Across all traits measured, hybrids were either equivalent to or more fit than at least one parent, and none of the hybrids performed worse than both parents. These patterns are similar to those seen in some other comparisons. The natural hybrid A. prolifera in the Caribbean (Fogarty, 2012) and experimentally produced hybrids of A. millepora × A. pulchra (Willis et al., 2006) had equivalent or higher fitness compared to their parental species. Experimentally produced hybrids of A. millepora × A. pulchra grow larger in size than purebreds in the reef-flat environment (Willis et al., 2006). In this study, photochemical efficiency was the same between offspring groups in the same treatment, suggesting that (1) the observed differences in recruit size of the offspring groups were unlikely caused by carbon translocation from Symbiodinium, and (2) there was no coral host effect on photochemical efficiency of the Symbiodinium.
A comprehensive assessment of the value of interspecific hybridization to coral reef restoration requires multi-generation fitness examinations of hybrids and backcrosses. Such an assessment will require years given the long generations time of corals (3–7 years to reach reproductive maturity). The present study is one of the few studies that examines the long-term fitness of F1 hybrids and provides detailed assessments from fertilization to embryonic development, Symbiodinium uptake, photosynthesis efficiency, survival and size. The results provide evidence that hybridization may have value to reef restoration. From the restoration point of view, hybridization increases genetic variation which can potentially enhance adaptive capacity and release a population from adaptive limits (Hoffmann and Sgrò, 2011; Becker et al., 2013; Carlson et al., 2014; van Oppen et al., 2015; Hamilton and Miller, 2016; Meier et al., 2017). In this study, genetic diversity would have increased in the F1 hybrids and positive effects on survival and recruits size were observed in some cases. Furthermore, none of the hybrid offspring groups performed worse than the purebreds across all traits, suggesting that there was no negative effect of hybridization in the F1. Higher survival and larger recruit size as observed in some hybrids can enhance reef restoration initiatives by reducing post-settlement mortality. Moreover, reproductive maturity of a coral is related to its size (Soong and Lang, 1992; Smith et al., 2005). Corals that achieve a large size earlier can begin to reproduce sooner, which may further assist the recovery of degraded reef systems.
In the A. tenuis × A. loripes cross, maternal effects were observed in fitness. Hybrid survival and size were similar to that of the maternal purebred species, although it exceeded purebred values in some occasions/conditions. Maternal effects have previously been shown for survival of interspecific hybrid larvae from an A. florida × A. intermedia cross (Isomura et al., 2013), and for thermal tolerance and gene expression levels of intraspecific A. millepora hybrid larvae from a higher and lower latitude cross (Dixon et al., 2015). It is unclear whether the observed fitness for the F1 hybrids from our study is due to nuclear or cytoplasmic maternal effects. If survival and size are governed by nuclear maternal effects, F2 hybrids of both directions will have similar fitness to each other, which will be different from their maternal parent. If survival and size are controlled by cytoplasmic maternal effects, fitness of the F2 hybrids will follow maternal fitness (Roach and Wulff, 1987; Bernardo, 1996). In this case, species that are known to carry desirable traits under climate change may therefore be ideal candidates as a source of eggs for creating coral stock for restoration via interspecific hybridization.
When selecting species pairs for hybridization to facilitate reef restoration, both targeted crossing with species or individuals that carry phenotypic traits of value under climate change (e.g., high thermal tolerance) and non-targeted crossing between species could be considered. Species with high climate resilience (e.g., A. loripes in the present study) may be useful for targeted hybridization efforts. Alternatively, non-targeted crossing among related species could be used to generate hybrid vigor and increase genetic diversity for future adaptation.
This study provides the first steps toward the assessment of interspecific hybridization as an approach to create coral stock with augmented climate resilience. While our findings are supportive of this novel strategy, additional research is required. Three important outstanding questions are: (1) The fitness of hybrids vs. purebreds in the field. While laboratory studies are ideal to investigate the responses of corals to one or two specific stressor(s), corals in the wild are subjected to other selection pressures difficult to simulate in the laboratory. An important next stage of this research would involve outplanting the hybrids and purebreds to the field and monitoring their relative fitness. (2) The reproductive and backcrossing potential of F1 hybrids. Isomura et al. (2016) have shown that experimentally produced A. intermedia × A. florida F1 hybrids were fertile and able to produce F2 offspring with high fertilization rates. Transgressive segregation can happen in F2 hybrids, where segregating variation of parental species recombines in hybrids at multiple loci to produce extreme phenotypes and may result in some F2 hybrids with extremely high fitness (for review, see Hamilton and Miller, 2016). Conversely, outbreeding depression may become apparent in the F2 generations and result in hybrids with low fitness. F1 hybrids of the A. intermedia × A. florida cross were able to backcross with either both parent species or the maternal parent species only, depending on the direction of the hybrid cross (Isomura et al., 2016). In the Caribbean, molecular evidence has shown unidirectional gene flow from A. palmata into A. cervicornis, suggesting that their hybrid A. prolifera is fertile and able to backcross with at least one parental species (Vollmer and Palumbi, 2002, 2007). Current knowledge on fertility of F1 coral hybrids remains limited and future studies in the area will be invaluable. (3) The fitness of advanced generation hybrids and backcrosses. If F1 hybrids are fertile and sexual reproduction is successful, high fitness will have to be maintained in the F2, backcrosses and advanced generation hybrids for interspecific hybridization to be beneficial to reef recovery and resilience in the long-term.
F1 hybrids can theoretically propagate via asexual reproduction, and via sexual reproduction with other hybrids or the parental species. The likelihood of the latter depends on the spawning time of the F1 hybrids and the parental species. The A. tenuis × A. loripes cross in the present study had 2 h difference in spawning time and the A. sarmentosa × A. florida cross had 30 min to 1 h difference. While the spawning time of the hybrids remains unknown until they reach reproductive maturity, Isomura et al. (2016) showed that F1 Acropora hybrid spawned at the same time as their maternal parental species. This suggests that the F1 in the present study will likely be able to at least backcross with the maternal parent species for the A. tenuis × A. loripes cross, and potentially with both parental species for the A. sarmentosa × A. florida cross due to closer spawning time. Asexual reproduction (i.e., fragmentation, polyp bail-out) is a common reproductive strategy of broadcast spawning scleractinian corals (Highsmith, 1982; Sammarco, 1982) and F1 hybrids may also persist in the wild via this method. A. prolifera, the natural F1 hybrid in the Caribbean for example, is known to persist and colonize large reef areas through asexual reproduction (Irwin et al., 2017).
In sum, it is likely that interspecific Acropora hybrids are able to propagate over extended periods of time, either sexually, asexually, or via both reproductive methods. Before hybrids can safely be used as stock for restoration, however, it must be demonstrated that the risk of this strategy is low by showing that the fitness of later generations remains equal or superior to that of the parental species in the wild.
WC, MvO, LP, and AH: designed the experiment; MvO: developed the concept for this study; WC and LP: conducted the experiment and collected the data; PM, WC, and AH: undertook statistical analyses; WC and MvO: wrote the manuscript and all authors contributed to the final edited version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was funded by the Paul G. Allen Family Foundation and the Australian Institute of Marine Science (AIMS). We thank staffs of the National Sea Simulator at AIMS for technical support. Thanks are also extended to M. Nordborg for her contribution to analysing the coral embryos, K. Damjanovic, R. Alfred, and L. Blackall for help with coral spawning work, and M. Shanahan for seawater alkalinity measurements. WC acknowledges receipt of the University of Melbourne International Research Scholarship and Fee Remission Scholarship.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2018.00160/full#supplementary-material
1. ^ https://50reefs.org/ (Accessed April 3, 2018).
Aguilar-Perera, A., and Hernández-Landa, R. C. (2017). Occurrence of large thickets of Acropora prolifera (Scleractinia: Acroporidae) in the southern Gulf of Mexico. Mar. Biodiv. 1–3. doi: 10.1007/s12526-017-0685-4
Aitken, S. N., and Whitlock, M. C. (2013). Assisted gene flow to facilitate local adaptation to climate change. Annu. Rev. Ecol. Evol. Syst. 44, 367–388. doi: 10.1146/annurev-ecolsys-110512-135747
Akaike, H. (1974). A new look at the statistical model identification. IEEE Trans. Automat. Contr. 19, 716–723. doi: 10.1109/TAC.1974.1100705
Baird, A. H., Cumbo, V. R., Figueiredo, J., and Harii, S. (2013). A pre-zygotic barrier to hybridization in two con-generic species of scleractinian corals. F1000Res 2:193. doi: 10.12688/f1000research.2-193.v2
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2014). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Becker, M., Gruenheit, N., Steel, M., Voelckel, C., Deusch, O., Heenan, P. B., et al. (2013). Hybridization may facilitate in situ survival of endemic species through periods of climate change. Nat. Clim. Change 3, 1039–1043. doi: 10.1038/nclimate2027
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300.
Berkelmans, R. (2009). “Bleaching and mortality thresholds: how much is too much?,” in Coral Bleaching: Patterns, Processes, Causes and Consequences, eds M. J. H. van Oppen and J. M. Lough (Berlin; Heidelberg: Springer), 103–119.
Bernardo, J. (1996). Maternal effects in animal ecology. Integr. Comp. Biol. 36, 83–105. doi: 10.1093/icb/36.2.83
Bolker, B. M., Brooks, M. E., Clark, C. J., Geange, S. W., Poulsen, J. R., Stevens, M. H. H., et al. (2009). Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. doi: 10.1016/j.tree.2008.10.008
Breed, M. F., Stead, M. G., Ottewell, K. M., Gardner, M. G., and Lowe, A. J. (2013). Which provenance and where? Seed sourcing strategies for revegetation in a changing environment. Conserv. Genet. 14, 1–10. doi: 10.1007/s10592-012-0425-z
Carlson, S. M., Cunningham, C. J., and Westley, P. A. H. (2014). Evolutionary rescue in a changing world. Trends Ecol. Evol. 29, 521–530. doi: 10.1016/j.tree.2014.06.005
Chan, N. C., and Connolly, S. R. (2013). Sensitivity of coral calcification to ocean acidification: a meta-analysis. Glob. Chang. Biol. 19, 282–290. doi: 10.1111/gcb.12011
Chen, Z. J. (2013). Genomic and epigenetic insights into the molecular bases of heterosis. Nat. Rev. Genet. 14, 471–482. doi: 10.1038/nrg3503
dela Cruz, D. W., and Harrison, P. L. (2017). Enhanced larval supply and recruitment can replenish reef corals on degraded reefs. Sci. Rep. 7:13985. doi: 10.1038/s41598-017-14546-y
Dixon, G. B., Davies, S. W., Aglyamova, G. V., Meyer, E., Bay, L. K., and Matz, M. V. (2015). Genomic determinants of coral heat tolerance across latitudes. Science 348, 1460–1462. doi: 10.1126/science.1261224
Doney, S. C., Fabry, V. J., Feely, R. A., and Kleypas, J. A. (2009). Ocean acidification: the other CO2 problem. Ann. Rev. Mar. Sci. 1, 169–192. doi: 10.1146/annurev.marine.010908.163834
Dunn, O. J. (1964). Multiple comparisons using rank sums. Technometrics 6, 241–252. doi: 10.1080/00401706.1964.10490181
Eakin, C. M., Liu, G., Gomez, A. M., De La Cour, J. L., Heron, S. F., Skirving, W. J., et al. (2016). Global coral bleaching 2014-2017? Status and an appeal for observations. Reef Encounter 31, 20–26.
Fogarty, N. D. (2012). Caribbean acroporid coral hybrids are viable across life history stages. Mar. Ecol. Prog. Ser. 446, 145–159. doi: 10.3354/meps09469
Fogarty, N. D., Vollmer, S. V., and Levitan, D. R. (2012). Weak prezygotic isolating mechanisms in threatened Caribbean Acropora corals. PLoS ONE 7:e30486. doi: 10.1371/journal.pone.0030486
Fukami, H., Omori, M., and Hatta, M. (2000). Phylogenetic relationships in the coral family Acroporidae, reassessed by inference from mitochondrial genes. Zool. Sci. 17, 689–696. doi: 10.2108/zsj.17.689
Garnett, S. T., Olsen, P., Butchart, S. H. M., and Hoffmann, A. A. (2011). Did hybridization save the Norfolk Island boobook owl Ninox novaeseelandiae undulata? Oryx 45, 500–504. doi: 10.1017/S0030605311000871
Grant, P. R., and Grant, B. R. (2010). Conspecific versus heterospecific gene exchange between populations of Darwin's finches. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 1065–1076. doi: 10.1098/rstb.2009.0283
Great Barrier Reef Marine Park Authority (2017). Final Report: 2016 Coral Bleaching Event on the Great Barrier Reef. Great Barrier Reef Marine Park Authority.
Guest, J. R., Baird, A. H., Maynard, J. A., Muttaqin, E., Edwards, A. J., Campbell, S. J., et al. (2012). Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS ONE 7:e33353. doi: 10.1371/journal.pone.0033353
Guest, J. R., Baria, M. V., Gomez, E. D., Heyward, A. J., and Edwards, A. J. (2014). Closing the circle: is it feasible to rehabilitate reefs with sexually propagated corals? Coral Reefs 33, 45–55. doi: 10.1007/s00338-013-1114-1
Hamilton, J. A., and Miller, J. M. (2016). Adaptive introgression as a resource for management and genetic conservation in a changing climate. Conserv. Biol. 30, 33–41. doi: 10.1111/cobi.12574
Heron, S. F., Maynard, J. A., van Hooidonk, R., and Eakin, C. M. (2016). Warming trends and bleaching stress of the world's coral reefs 1985–2012. Sci. Rep. 6:38402. doi: 10.1038/srep38402
Highsmith, R. C. (1982). Reproduction by fragmentation in corals. Mar. Ecol. Prog. Ser. 7, 207–226. doi: 10.3354/meps007207
Hoegh-Guldberg, O. (1999). Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshw. Res. 50, 839–866.
Hoffmann, A. A., and Sgrò, C. M. (2011). Climate change and evolutionary adaptation. Nature 470, 479–485. doi: 10.1038/nature09670
Hönisch, B., Ridgwell, A., Schmidt, D. N., Thomas, E., Gibbs, S. J., Sluijs, A., et al. (2012). The geological record of ocean acidification. Science 335, 1058–1063. doi: 10.1126/science.1208277
Hothorn, T., Bretz, F., and Westfall, P. (2008). Simultaneous inference in general parametric models. Biom. J. 50, 346–363. doi: 10.1002/bimj.200810425
Hughes, T. P., Anderson, K. D., Connolly, S. R., Heron, S. F., Kerry, J. T., Lough, J. M., et al. (2018). Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83. doi: 10.1126/science.aan8048
Hughes, T. P., Kerry, J. T., Álvarez-Noriega, M., Álvarez-Romero, J. G., Anderson, K. D., Baird, A. H., et al. (2017). Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. doi: 10.1038/nature21707
Hwang, A. S., Northrup, S. L., Peterson, D. L., Kim, Y., and Edmands, S. (2012). Long-term experimental hybrid swarms between nearly incompatible Tigriopus californicus populations: persistent fitness problems and assimilation by the superior population. Conserv. Genet. 13, 567–579. doi: 10.1007/s10592-011-0308-8
Irwin, A., Greer, L., Humston, R., Devlin-Durante, M., Cabe, P., Lescinsky, H., et al. (2017). Age and intraspecific diversity of resilient Acropora communities in Belize. Coral Reefs 36, 1–10. doi: 10.1007/s00338-017-1602-9
Isomura, N., Iwao, K., and Fukami, H. (2013). Possible natural hybridization of two morphologically distinct species of Acropora (Cnidaria, Scleractinia) in the Pacific: fertilization and larval survival rates. PLoS ONE 8:e56701. doi: 10.1371/journal.pone.0056701
Isomura, N., Iwao, K., Morita, M., and Fukami, H. (2016). Spawning and fertility of F1 hybrids of the coral genus Acropora in the Indo-Pacific. Coral Reefs 35, 851–855. doi: 10.1007/s00338-016-1461-9
Japaud, A., Fauvelot, C., and Bouchon, C. (2014). Unexpected high densities of the hybrid coral Acropora prolifera (Lamarck 1816) in Guadeloupe Island, Lesser Antilles. Coral Reefs 33, 593–593. doi: 10.1007/s00338-014-1169-7
Johnson, W. E., Onorato, D. P., Roelke, M. E., Land, E. D., Cunningham, M., Belden, R. C., et al. (2010). Genetic restoration of the Florida Panther. Science 329, 1641–1645. doi: 10.1126/science.1192891
Jones, T. A., and Monaco, T. A. (2009). A role for assisted evolution in designing native plant materials for domesticated landscapes. Front. Ecol. Environ. 7:28. doi: 10.1890/080028
Langdon, C., Takahashi, T., Sweeney, C., Chipman, D., Goddard, J., Marubini, F., et al. (2000). Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Glob. Biogeochem. Cycles 14, 639–654. doi: 10.1029/1999GB001195
Lewis, E., and Wallace, D. W. R. (1998). CO2SYS Program Developed for the CO2 System Calculations. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN.
Li, L., Lu, K., Chen, Z., Mu, T., Hu, Z., and Li, X. (2008). Dominance, overdominance and epistasis condition the heterosis in two heterotic rice hybrids. Genetics 180, 1725–1742. doi: 10.1534/genetics.108.091942
Lippman, Z. B., and Zamir, D. (2007). Heterosis: revisiting the magic. Trends Genet. 23, 60–66. doi: 10.1016/j.tig.2006.12.006
Márquez, L. M., Van Oppen, M. J. H., Willis, B. L., Reyes, A., and Miller, D. J. (2002). The highly cross-fertile coral species, Acropora hyacinthus and Acropora cytherea, constitute statistically distinguishable lineages. Mol. Ecol. 11, 1339–1349. doi: 10.1046/j.1365-294X.2002.01526.x
Maynard, J. A., Anthony, K. R. N., Marshall, P. A., and Masiri, I. (2008). Major bleaching events can lead to increased thermal tolerance in corals. Mar. Biol. 155, 173–182. doi: 10.1007/s00227-008-1015-y
McCulloch, C. E., and Neuhaus, J. M. (2013). “Generalized linear mixed models,” in Encyclopedia of Environmetrics (JohnWiley & Sons, Ltd), 28–33. doi: 10.1002/9780470057339.vag009.pub2
Meier, J. I., Marques, D. A., Mwaiko, S., Wagner, C. E., Excoffier, L., and Seehausen, O. (2017). Ancient hybridization fuels rapid cichlid fish adaptive radiations. Nat. Commun. 8:14363. doi: 10.1038/ncomms14363
Nakamura, R., Ando, W., Yamamoto, H., Kitano, M., Sato, A., Nakamura, M., et al. (2011). Corals mass-cultured from eggs and transplanted as juveniles to their native, remote coral reef. Mar. Ecol. Prog. Ser. 436, 161–168. doi: 10.3354/meps09257
Omori, M. (2011). Degradation and restoration of coral reefs: experience in Okinawa, Japan. Mar. Biol. Res. 7, 3–12. doi: 10.1080/17451001003642317
Pandolfi, J. M., Connolly, S. R., Marshall, D. J., and Cohen, A. L. (2011). Projecting coral reef futures under global warming and ocean acidification. Science 333, 418–422. doi: 10.1126/science.1204794
Penin, L., Vidal-Dupiol, J., and Adjeroud, M. (2013). Response of coral assemblages to thermal stress: are bleaching intensity and spatial patterns consistent between events? Environ. Monit. Assess. 185, 5031–5042. doi: 10.1007/s10661-012-2923-3
Pierrot, D., Lewis, E., and Wallace, D. W. R. (2006). CO2SYS DOS Program Developed for CO2 System Calculations. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN.
Raftery, A. E., Zimmer, A., Frierson, D. M. W., Startz, R., and Liu, P. (2017). Less than 2 °C warming by 2100 unlikely. Nat. Clim. Chang. 7:637. doi: 10.1038/nclimate3352
Randall, C. J., and Szmant, A. M. (2009). Elevated temperature affects development, survivorship, and settlement of the elkhorn coral, Acropora palmata (Lamarck 1816). Biol. Bull. 217, 269–282. doi: 10.1086/BBLv217n3p269
Rau, G. H., McLeod, E. L., and Hoegh-Guldberg, O. (2012). The need for new ocean conservation strategies in a high-carbon dioxide world. Nat. Clim. Change 2, 720–724. doi: 10.1038/nclimate1555
Roach, D. A., and Wulff, R. D. (1987). Maternal effects in plants. Annu. Rev. Ecol. Syst. 18, 209–235. doi: 10.1146/annurev.es.18.110187.001233
R Core Team (2016). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Sammarco, P. W. (1982). Polyp bail-out: an escape response to environmental stress and a new means of reproduction in corals. Mar. Ecol. Prog. Ser. 10, 57–65. doi: 10.3354/meps010057
Sgrò, C. M., Lowe, A. J., and Hoffmann, A. A. (2011). Building evolutionary resilience for conserving biodiversity under climate change. Evol. Appl. 4, 326–337. doi: 10.1111/j.1752-4571.2010.00157.x
Shapiro, S. S., and Wilk, M. B. (1965). An analysis of variance test for normality (complete samples). Biometrika 52, 591–611. doi: 10.1093/biomet/52.3-4.591
Smith, L. D., Devlin, M., Haynes, D., and Gilmour, J. P. (2005). A demographic approach to monitoring the health of coral reefs. Mar. Pollut. Bull. 51, 399–407. doi: 10.1016/j.marpolbul.2004.11.021
Soong, K., and Lang, J. C. (1992). Reproductive integration in reef corals. Biol. Bull. 183, 418–431. doi: 10.2307/1542018
van Hooidonk, R., Maynard, J., Tamelander, J., Gove, J., Ahmadia, G., Raymundo, L., et al. (2016). Local-scale projections of coral reef futures and implications of the Paris Agreement. Sci. Rep. 6:srep39666. doi: 10.1038/srep39666
van Oppen, M. J. H., Gates, R. D., Blackall, L. L., Cantin, N., Chakravarti, L. J., Chan, W. Y., et al. (2017). Shifting paradigms in restoration of the world's coral reefs. Glob. Change Biol. 23, 3437–3448. doi: 10.1111/gcb.13647
van Oppen, M. J. H., McDonald, B. J., Willis, B., and Miller, D. J. (2001). The evolutionary history of the coral genus Acropora (Scleractinia, Cnidaria) based on a mitochondrial and a nuclear marker: reticulation, incomplete lineage sorting, or morphological convergence? Mol. Biol. Evol. 18, 1315–1329. doi: 10.1093/oxfordjournals.molbev.a003916
van Oppen, M. J. H., Oliver, J. K., Putnam, H. M., and Gates, R. D. (2015). Building coral reef resilience through assisted evolution. Proc. Natl. Acad. Sci. U.S.A. 112, 2307–2313. doi: 10.1073/pnas.1422301112
van Oppen, M. J., Willis, B. L., Van Rheede, T., and Miller, D. J. (2002). Spawning times, reproductive compatibilities and genetic structuring in the Acropora aspera group: evidence for natural hybridization and semi-permeable species boundaries in corals. Mol. Ecol. 11, 1363–1376. doi: 10.1046/j.1365-294X.2002.01527.x
Villanueva, R. D., Baria, M. V. B., and Cruz, D. W. (2012). Growth and survivorship of juvenile corals outplanted to degraded reef areas in Bolinao-Anda Reef Complex, Philippines. Mar. Biol. Res. 8, 877–884. doi: 10.1080/17451000.2012.682582
Vollmer, S. V., and Palumbi, S. R. (2002). Hybridization and the evolution of reef coral diversity. Science 296, 2023–025. doi: 10.1126/science.1069524
Vollmer, S. V., and Palumbi, S. R. (2007). Restricted gene flow in the caribbean staghorn coral Acropora cervicornis: implications for the recovery of endangered reefs. J. Hered. 98, 40–50. doi: 10.1093/jhered/esl057
Weeks, A. R., Heinze, D., Perrin, L., Stoklosa, J., Hoffmann, A. A., Rooyen, A., et al. (2017). Genetic rescue increases fitness and aids rapid recovery of an endangered marsupial population. Nat. Commun. 8:1071. doi: 10.1038/s41467-017-01182-3
Whiteley, A. R., Fitzpatrick, S. W., Funk, W. C., and Tallmon, D. A. (2015). Genetic rescue to the rescue. Trends Ecol. Evol. 30, 42–49. doi: 10.1016/j.tree.2014.10.009
Willis, B. L., Babcock, R. C., Harrison, P. L., and Wallace, C. C. (1997). Experimental hybridization and breeding incompatibilities within the mating systems of mass spawning reef corals. Coral Reefs 16, S53–S65. doi: 10.1007/s003380050242
Keywords: hybridization, restoration, coral reefs, climate change, Acropora, assisted evolution, genetic rescue, hybrid vigor
Citation: Chan WY, Peplow LM, Menéndez P, Hoffmann AA and van Oppen MJH (2018) Interspecific Hybridization May Provide Novel Opportunities for Coral Reef Restoration. Front. Mar. Sci. 5:160. doi: 10.3389/fmars.2018.00160
Received: 19 January 2018; Accepted: 23 April 2018;
Published: 14 May 2018.
Edited by:
Anastazia T. Banaszak, Universidad Nacional Autónoma de México, MexicoReviewed by:
James R. Guest, Newcastle University, United KingdomCopyright © 2018 Chan, Peplow, Menéndez, Hoffmann and van Oppen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wing Yan Chan, dy5jaGFuQGFpbXMuZ292LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.