- 1Center for the Science of Animal Care and Welfare, Chicago Zoological Society, Brookfield, IL, United States
- 2Department of Ocean Sciences, University of California, Santa Cruz, Santa Cruz, CA, United States
- 3Department of Ecology & Evolutionary Biology, University of California, Santa Cruz, Santa Cruz, CA, United States
- 4Department of Process Engineering and Applied Science, Dalhousie University, Halifax, NS, Canada
- 5Marine Mammal Laboratory, Alaska Fisheries Science Center, National Marine Fisheries Service, National Oceanic Atmospheric Administration, Seattle, WA, United States
- 6Field Science Center for Northern Biosphere, Hokkaido University, Hakodate, Japan
- 7Department of Biological Sciences, San Jose State University, San Jose, CA, United States
Mortality from incidental bycatch in longline fishery operations is a global threat to seabird populations, and especially so for the albatross family (Diomedeidae) in which 15 out of 22 species are threatened with extinction. Despite the risks, fisheries remain attractive to many species of seabird by providing access to high-energy foods in the form of discarded fish and offal, target fish, and baited hooks. Current policy regarding fisheries management is increasingly aimed at discard reform, exemplified by a discard ban initiated in the European Union Common Fisheries Policy in 2014. While there is global agreement on the importance of minimizing the waste inherent in bycatch and discards, there is also growing concern that there is a need to understand the extent to which marine animals rely on fisheries-associated resources, especially at the colony and individual levels. We used a novel adaptation of quantitative fatty acid signature analysis (QFASA) to quantify fisheries-associated prey in the diet of two threatened North Pacific albatross species. Diet was estimated with QFASA using multiple lipid classes from stomach oil collected from incubating and chick-brooding Laysan and black-footed albatrosses across three breeding seasons. Prey-specific error was estimated by comparing QFASA estimated diets from known “simulated” diets, which informed the level of precaution appropriate when interpreting model results. Fisheries-associated diet occurred in both albatross species across both the incubation and chick-brood stages; however, neither species relied on fisheries food as the dominant food source (consisting of <10% of the total pooled proportional diet in each species). While total diet proportion was low, the incidence of fisheries-associated resources in albatross diets was highest in the 2009–2010 breeding season when there was a strong central Pacific El Niño. Additionally, the diets of a few individuals consisted almost entirely of fisheries-associated food, indicating that some birds might specialize on this foraging tactic. QFASA proved a tractable method for estimating the importance of fisheries-associated resources in albatross diet, and, as a tool, has the potential to enable long-term monitoring of diet and fisheries reliance of breeding colonies in the northwestern Hawaiian Islands.
Introduction
An ecosystem approach to fisheries (EAF) aims to maintain the health and sustainability of fishery industries and the ecosystems in which they function (Jennings et al., 2014). This recently adopted approach prompted the initiation of large-scale reform to fisheries management and policies, such as the proposed discard ban advocated by the European Commission (Borges, 2015). Wide support of such policy reflects the scientific consensus that modern industrial fishery practices have substantially diminished biodiversity and ecosystem integrity on a global scale (Pauly, 2002; Ormerod, 2003; Worm and Myers, 2003; Pauly et al., 2005; Ainley and Blight, 2009). When taking an ecosystem approach in fisheries management, however, it is important to evaluate both positive and negative impacts of fishing practices across taxa. While many studies and management efforts focus on the negative impacts of fishery bycatch, with obvious conservation benefits, it is also important to consider potentially adverse impacts on species that have learned to exploit fisheries subsidies. Particularly for species that scavenge bait, catch, or fishery discards, policies that reduce access to fisheries-associated resources may simultaneously decrease or eliminate access to what was once a predictable food source. Thus, when projecting demographic consequences of fisheries management for scavenging marine consumers, it is important to consider fishery resource exploitation as well as bycatch mortality rates.
Incidental mortality from fisheries bycatch is a principle threat to seabird biodiversity, particularly for the Procelleriformes (albatrosses and petrels) (Croxall and Gales, 1998; Tuck et al., 2001; Cherel et al., 2017; Pardo et al., 2017). Fifteen out of 22 recognized species of albatrosses are currently threatened or endangered, of which three are critically endangered (IUCN, 2017). Population declines of at least 10 albatross species are attributed, at least in part, to adult mortality from fisheries bycatch, and, specifically, to demersal and pelagic longline operations (Weimerskirch et al., 1997; Gales and Robertson, 1998; Tuck et al., 2001; Lewison and Crowder, 2003; Véran et al., 2007). Despite the risk of injury or death, fisheries remain attractive to many seabird species by providing lipid-rich meals in the form of target fish, baited hooks, and discarded bait and offal (Furness, 2003; Garthe and Scherp, 2003; Grémillet et al., 2008; Anderson et al., 2011; Bicknell et al., 2013). Indeed, diet subsidies from fisheries resources have been linked to breeding success and population growth in some scavenging seabird species (Furness, 2003; McInnes et al., 2017). Furthermore, successful exploitation may be a learned behavior that persists throughout a lifetime, although evidence of specialization on fisheries discards in seabirds appears to vary among species and ecosystem (Granadeiro et al., 2014; Tyson et al., 2015).
While vessel-based studies have significantly advanced our understanding of seabird-fishery interactions and species-level impacts, we know less of the dynamics between fisheries activities and seabirds at the breeding colony and individual level (but see Bearhop et al., 2001; Votier et al., 2008, 2010; Patrick et al., 2015; Collet et al., 2017). For example, we know little about how fisheries exploitation varies across colonies or sex and age-classes or how fisheries exploitation of a breeding colony relates to variability in oceanographic conditions. Additional limitations in vessel-based research are necessary to consider: for one, bycaught birds may be a distinct subset of the populations, introducing a sampling bias, (Edwards et al., 2015). Also, encounters with fishing vessels cannot be used as a proxy for direct interactions with fisheries (Bodey et al., 2014; Sugishita et al., 2015; Collet et al., 2017). Therefore, there is a need to quantify seabird-fishery dynamics independent from vessel-collected data. Quantifying the degree to which populations rely on fisheries subsidies remains an important, yet, in some cases, intractable problem. This is especially true for albatrosses, which rapidly digest prey into a homogenous stomach oil (Warham, 1977; Tickell, 2000), thereby reducing, if not sometimes eliminating, the ability to visually identify fisheries-associated diet in stomach contents.
Biochemical dietary analyses, such as stable isotope, fatty acid, and DNA barcoding, can circumvent some of the biases that occur in traditional stomach content diet estimations and are becoming increasingly important when describing the diet of free-ranging animals that are difficult to observe and/or sample, such as albatross (Inger and Bearhop, 2008; McInnes et al., 2016, 2017; Cherel et al., 2017). Lipids, and specifically fatty acids, have long been used in the characterization of trophic webs both in terrestrial and marine ecosystems (Ackman and Eaton, 1966; Connan et al., 2005). They are effective trophic biomarkers due to their great diversity of structures and because they are often only synthesized de novo in certain organisms (Budge et al., 2006). Quantitative fatty acid signature analysis (QFASA) uses a robust statistical model to quantify predator diet by identifying the combination of prey fatty acid signatures most similar to that of the predator while accounting for predator metabolism. QFASA is widely applied in marine trophic web studies, and diets have been accurately estimated from fatty acids in stomach oil and adipose and blubber tissue in free-ranging fish (Magnone et al., 2015), seabirds (Iverson et al., 2007; Wang et al., 2009), and marine mammals (Beck et al., 2007; Thiemann et al., 2008; Meynier et al., 2010; Bromaghin et al., 2013; Jansen, 2013).
In this study, we evaluated the effectiveness of QFASA to better understand fisheries exploitation in two North Pacific albatross species. We first measured the accuracy of the method and subsequently applied it to estimate the occurrence and significance of fisheries-associated resources in the individual diets of breeding albatross. We developed a novel adaptation of QFASA that incorporates both fatty acids and fatty alcohols derived from multiple lipid classes (triacylglycerol (TAG) and wax esters). Previous studies using QFASA in marine predators universally examine a single lipid class, TAG, because structural tissues (e.g., adipose tissue, blubber) are the common source of predator lipids and are composed almost entirely of TAG. Albatross stomach oil, however, is diet-derived and composed of multiple lipid classes (TAG, wax ester, and glycerol ether) (Lewis, 1969; Cheah and Hansen, 1970; Clarke and Prince, 1976; Imber, 1976; Warham et al., 1976), enabling us to harness trophic information from multiple lipid classes. Furthermore, since lipids in stomach oil are not metabolically transformed into tissues, they do not require the use of calibration coefficients to account for metabolism, eliminating a large source of error in QFASA modeling (Rosen and Tollit, 2012; Bromaghin et al., 2015, 2017). To effectively use QFASA to discriminate fisheries-associated diet from non-anthropogenic diet, lipids that compose fisheries-associated resources must be biochemically distinct from the diet component unrelated to fisheries. North Pacific albatross are predominately surface scavengers and not known to hunt large, strong, and agile epipelagic fish species that are associated with fisheries activities, such as swordfish, tuna, and mackerel (Gould et al., 1997; Walker and Fitzgerald, 2012; Walker et al., 2012). Thus, we approached this study feeling confident that for this particular predator-prey system, the resources we classified as fisheries-associated would be biochemically distinct from the natural diet component.
Our three primary objectives in this study were: (1) to characterize the individual diets of North Pacific albatross across the incubation and chick-brood breeding phases in which diet is relatively unknown, (2) to assess the relative importance of fisheries-associated resources in Laysan and black-footed albatross diet, and (3) to examine the influence of species, breeding phase, and year on fisheries-resource consumption. Furthermore, since QFASA is a modeling exercise and error in any given QFASA exercise will be unique to each prey library (Bromaghin et al., 2015), we emphasize that model uncertainty should be approximated for each unique predator/prey system and results should be interpreted in light of this uncertainty. Therefore, a secondary objective was to estimate the QFASA modeling error associated with each species in our prey library and to use this model uncertainty to inform our subsequent diet analyses and interpretations.
Materials and Methods
Ethics Statement
The animal use protocol for this research was reviewed and approved by the Institutional Animal Care and Use Committee at University of California, Santa Cruz and San Jose State University. All procedures were reviewed and permitted under Papahānaumokuākea Marine National Monument Permit # PMNM-2009-04-A1 and # PMNM-2009-04-M1 and USGS Bird Banding Lab Permit # 23411.
Study Species
Black-footed (Phoebastria nigripes) and Laysan (P. immutabilis) albatrosses are generalist scavengers of the North Pacific and are often considered in policy discussions regarding fisheries management. Population trajectories of both species are vulnerable to incidental mortality from fisheries by-catch (Lewison and Crowder, 2003; Naughton et al., 2007), but particularly so for black-footed albatrosses (Lewison et al., 2004; Véran et al., 2007). Bycatch induced mortality rates for both species are well-monitored from ship-based surveys. Not surprisingly, the highest rates of albatross bycatch in the Hawaiian longline fisheries occur when attendance at breeding colonies is the highest (January-May), presumably due to maximum spatial overlap between birds and these particular fisheries in these months (see Edwards et al., 2015). While bycatch mortality is regularly monitored, detailed information on the diet composition of breeding albatrosses is notably lacking, particularly from periods in the annual cycle outside of chick-rearing (McInnes et al., 2016).
Stomach Oil Collection
The unique gastro-intestinal anatomy of procellariiformes results in the buildup of lipids inside the stomach following slow gastric-emptying that is biased toward proteins, carbohydrates, and aqueous fluid, following mechanical rupture of ingested prey (Place et al., 1989). We collected a total of 94 aliquots of stomach oil (5–10 mL) from 47 adult black-footed albatrosses and 47 adult Laysan albatrosses breeding at Tern Island, French Frigate Shoals (23.870°N, 166.284°W) across the incubation and chick-brood periods during 2010, 2011, and 2012 (Table 1). A small number of chicks were also sampled during the late chick-brood (n = 6 black-footed, n = 6 Laysan, 2010 only). Chick diets were pooled with adult diets to characterize population-level diet metrics from the chick-brood period. Only individual adult diets were used when quantifying individual-level diet metrics (e.g., incidence of fisheries resources). Stomach oil was collected using stomach intubation, with a sterile #5 Kendall catheter tube (18Fr 16” od mm 6.0) attached to a 35 mL plastic Monoject syringe. Stomach intubation is a quick procedure (<3 min.) that is less invasive than other methods of diet sampling, such as forced regurgitations or water off-loading (Wilson, 1984; Karnovsky et al., 2012). Lipid analysis only requires a small amount of oil (1–5 mL) from each bird, substantially less than the volume of total stomach contents. Regardless, stomach oil has an extremely high caloric density and serves as an important source of energy and hydration for birds on the nest, so we supplemented the diet of sampled birds with a 35 mL blended mixture of market squid, Ensure® shake, and Pedialyte® solution. Oil samples were kept frozen in 10 mL conical tubes at −20°C until analysis.
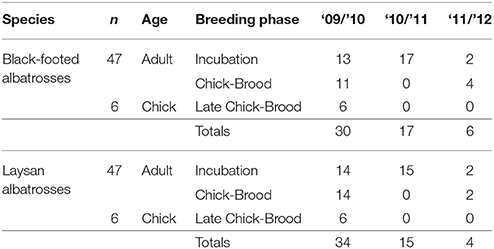
Table 1. Sample sizes of albatross stomach oil samples by species, breeding phase, year, and age class.
Compiling a Lipid Library of Potential Prey Items
QFASA requires a comprehensive library of lipid signatures from all prey species potentially consumed by a predator. North Pacific albatrosses are opportunistic foragers and consume a wide variety of prey using flexible foraging tactics (Harrison et al., 1983; Gould et al., 1997; Tickell, 2000; Walker et al., 2012; Conners et al., 2015); therefore, collecting a library of all potential prey species for lipid analysis would be both impractical and quantitatively unfeasible given limitations of the QFASA model itself. However, since the fatty acid composition of an organism is influenced by its feeding ecology (Budge et al., 2006), collecting species from across the range of functional groups regularly consumed by a predator theoretically should represent the range of lipids consumed by the predator. Therefore, we collected prey species representing 11 functional groups known to be exploited by North Pacific albatrosses (Figure 1, Supplemental Table S1).
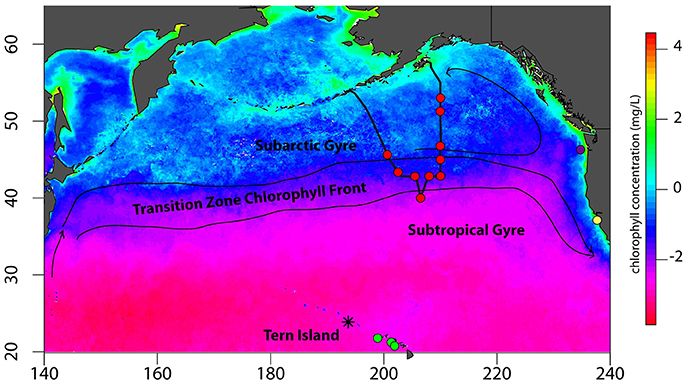
Figure 1. Collection sites across the North Pacific ocean basin for albatross prey specimens. The track of the T/S Oshoromaru is represented by the thick black track-line. Prey collection sites are denoted with colored circles: Red circles represent the locations of midwater trawls and squid jigging in the North Pacific Transition Zone, Subtropical Gyre, and Subarctic Gyre. Green circles identify locations where prey samples were collected in subtropical waters near Tern Island with a cobb trawl. The purple circle identifies the midwater trawl location in the California Current. Lastly, the yellow circle identifies the Año Nuevo breeding colony of northern elephant seals from which blubber samples were collected in a concurrent study (Goetcsh et al. unpublished data). Black lines and arrows are a generalized representation of basin-scale currents that influence frontal zones and foraging habitats of North Pacific albatross. Chlorophyll-a concentrations are the 8 year average (2004:2011) from the month of August. Rasters were downloaded from http://coastwatch.pfeg.noaa.gov/coastwatch/CWBrowserWW360.jsp.
Prey Collection
To represent natural diet items, 22 species of fish, squid, crustaceans, and jellyfish were collected from the Subarctic Gyre and the North Pacific Transition Zone (NPTZ), areas heavily exploited by foraging albatross throughout the year (Supplemental Table S1) (Fernández et al., 2001; Kappes et al., 2015; Thorne et al., 2015). Prey samples were collected using a mid-water trawl net (inner mesh: 10 mm; outer mesh: 70 mm mesh), a frame trawl (3 mm mesh), a neuston tow, and an automated squid jigging machine (MY-10, Towa-denki Seisakusho Co. Ltd) on the T/S Oshoro-maru (Hokkaido University, 72.85 m length, 1779 gross tonnage) 2012 summer cruise from Dutch Harbor to Kodiak, USA (Figure 1), described in detail in Saijo et al. (2016). To represent prey from other regions visited by foraging albatrosses, four additional fish and squid species were collected from a Cobb trawl conducted by National Marine Fisheries Service in 2012 in waters off the main Hawaiian Islands, while one additional species of mesopelagic squid was collected from a demersal fishery off the Oregon-Washington coast in 2014 (Figure 1, Supplemental Table S1). Flying fish eggs are known to be a significant food source provisioned to black-footed albatross chicks (Harrison et al., 1983); however, it is not known if they are consumed by adults outside of chick-provisioning. Fish eggs were collected opportunistically from the albatross breeding colony when found in large, fresh masses. Albatrosses are known scavengers of marine mammal carcasses, so we included elephant seal blubber lipid data from a concurrent study (Goetsch et al., unpublished data). In addition to jellyfishes (unknown species) collected in neuston tows in the NPTZ, we collected the hydrozoan Vellela vellela from the waters off coastal California as V. vellela was previously found in stomach contents of North Pacific albatross chicks in an earlier study (Harrison et al., 1983). Finally, a unique relationship between North Pacific albatrosses and Pacific sunfish was described by Takuzo Abe et al. (2012) who demonstrated that Laysan and black-footed albatrosses consume ecto-parasitic copepods (Pennella pennella) off the skin of sunfish; thus, we opportunistically collected P. pennella from a Pacific pomfret fished off the T/S Oshoromaru in the NPTZ. Fisheries-associated bait and target species were collected from POP Bait Supply in Honolulu, HI, the main supplier to the Hawaiian longline fleet or from markets in coastal California (Supplemental Table S1). When possible, standard lengths of fish, and dorsal mantle lengths of squid were measured to the nearest 0.1 cm; body mass was measured to the nearest 0.1 g. Prey were identified to genus, or species when possible, at the Marine Mammal Laboratory in Seattle, Washington. In total, 34 prey species, spanning all targeted functional groups, were collected for lipid analysis (Supplemental Table S1). All samples were kept frozen at −20°C until analysis.
Classification of Functional Groups
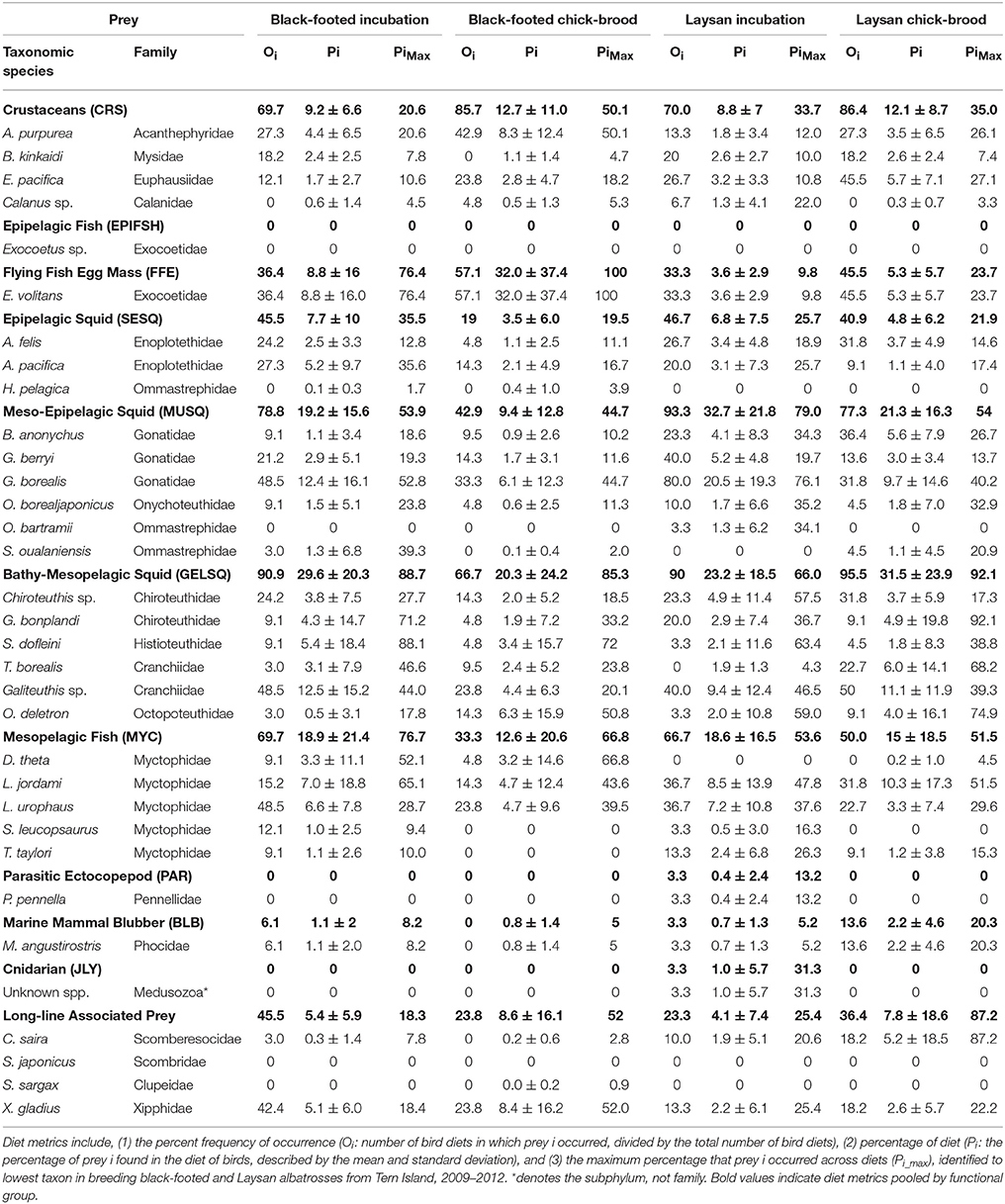
Functional groups were informed by the feeding ecology of prey and by the accessibility of prey to albatross (Supplemental Table S1). Given the large number and diversity of squid species in our prey library, we classified squid into three functional groups: (1) surface-schooling epipelagic squid (SESQ), (2) epi-mesopelagic muscular squid (MUSQ), and (3) bathy-mesopelagic, weakly-muscled, gelatinous squid (GELSQ). The SESQ group consisted of three species of very small oceanic squid (Abraliopsis felis, A. pacifica, and Hyaloteuthis pelagica) that can form dense schools in the upper 100 m at night. The MUSQ group consisted of six species of squid that have thick muscular mantle tissue and that typically occur in mesopelagic depths during the day but travel to the upper water column at night in pursuit of smaller prey. These squid (Gonatopsis borealis, Gonatus berryi, Berryteuthis anonychus, Ommastrephes bartrami, Sthenoteuthis oualaniensis, and Onychoteuthis borealjaponicus) are strong, active predators. Given their size, strength, and habitation of deep mesopelagic waters, they would likely only be consumed by albatrosses if maimed or dead (Pitman et al., 2004; Walker et al., 2012), or as by-catch from (the mostly dismantled) drift-net squid fisheries (Gould et al., 1997). The last functional group of squid (GELSQ) consisted of six species of squid that only occur in meso- to bathy-pelagic waters and never in surface waters while alive. The body composition of these species ranges from a complete lack of musculature (Taonius borealis, Galiteuthis sp., Grimalditeuthis bonplandi, and Chiroteuthis sp. c.f. calyx) to weak musculature in their mantle tissue (Octopoteuthis deletron, Stigmatoteuthis dofleini). All species in this group accumulate buoyant ammonium gas in their bodies upon death, which brings them to surface waters where they can be scavenged by a variety of species, including albatrosses. Species in the Crustacean functional group (CRS) ranged in size from tiny calanoid copepods (Calanus sp.) and euphausiid krill (Euphausiia pacifica) to a larger mysid (Boreomysis kinkaidi) and the very large deep-sea shrimp (Acanthephyra purpurea). Crustaceans may be accessible to albatrosses through a drift foraging strategy or opportunistically (Miller, 1940; Conners et al., 2015). Five species of myctophid fish (Diaphus theta, Lampanyctus jordani, Lampadena urophaos, Stenobrachius leucopsaurus, and Tarletonbeania taylori) were grouped as a mesopelagic fish functional group (MYC). Small, schooling epipelagic fish (EPIFSH) consisted of a single species of flying fish (Exocoetus sp.) obtained from local Hawaiian-waters. It would be unlikely to find epipelagic fish in albatross diets considering albatrosses are primarily scavengers or ambush predators of smaller, minimally agile prey; however, flying fish were included as a quick check on our QFASA model competence. Longline associated bait (LLB) was composed of Asian-sourced chub-mackerel (Scomber japonicas) and Pacific saury (Cololabis saira), and of Pacific sardines (Scomber sargax) from the California Current. Illex argentinus is a moderately sized muscular ommastrephid that is a common bait species in the longline fishery (Bisson, 2008). We were not able to obtain this species for our prey library; however, we did collect O. bartrami from the NPTZ (see above), and since this species is closely-related to I. argentinus and similar in size and trophic niche, it's lipid signature would likely serve as a proxy for I. argentines. As albatross are known to consume not just bait from fishery operations, but also the target fish, particularly swordfish (Bisson, 2008), we included market-purchased Pacific swordfish (Xiphias gladius) sourced from the California Current as long-line associated target species (LLT). The remaining functional groups consisted of only single species within each group: flying fish eggs (FFE, Exocoetus volitans), parasitic ecto-copepod (PAR, Pennella pennella), marine mammal blubber (BLB, Mirounga angustirostris), and jellyfish (JLY, unk. species conglomerate).
Lipid Analysis
Lipid Extraction
Whole, individual prey samples were homogenized using scalpels, a blender, or an industrial meat grinder depending on the size and composition of prey. For large neon flying squid (O. bartrami, ~1.9 kg), we isolated the mantle and tentacles only, because given their large size it is highly unlikely a bird would consume whole individuals of this size. Swordfish and marine mammal samples were also subsampled from the whole specimen (muscle only for swordfish and blubber only for elephant seals). Individual prey items were combined into species-specific homogenates from which three 1.5 g subsamples were collected for lipid analysis. Using averaged prey signatures removes information on the variability of lipids between individuals within a species; however, within-species variability in fatty acid composition is small relative to between-species differences (Budge et al., 2002). For most species, we collected and combined 15 individuals into our prey homogenate; however, for difficult to obtain species, such as some mesopelagic gelatinous squids, we had fewer individuals (prey sample sizes in Supplemental Table S1). Lipids were extracted from prey homogenates using chloroform:methanol:water (8:4:3 v/v/v) following a modified Folch et al. (1957) method (Budge et al., 2006, Appendix 1). Lipids from albatross oil were extracted using a simplified Folch et al. (1957) method for milk (Budge et al., 2006, Appendix 3). V. vellela did not have any recoverable lipids from the wax ester or triacylglycerol lipid classes with the extraction methods used and were excluded from further analysis.
Isolating TAG and Wax Esters
A preliminary assessment of the lipid classes found in albatross stomach oil found high levels of both TAG and wax esters (Budge, pers. comm; also see Warham, 1977; Connan et al., 2007) reflective of the dietary origin of albatross stomach oil and the high amounts of wax esters commonly found in marine organisms (Imber, 1976). We isolated both TAG and wax ester lipid classes from prey and albatross lipid samples with preparative thin layer chromatography (TLC) by streaking each lipid sample at a concentration of 150 mg/mL [CHCl3] onto a silica-coated glass plate before placing the plate into a developing chamber containing hexane:ethyl ether:acetic acid (85:15:1 v/v/v). Plates were developed and dried, then sprayed with 0.2% 2,7-dichlorofluoroscein in ethanol before viewing under UV light. Wax ester and TAG classes were identified in comparison to lipid class standards, scraped off the plate, then eluted with five chloroform rinses. Each lipid class per sample was weighed to the nearest mg, giving a final ratio of TAG:wax ester per sample.
Gas Chromatography and Lipid Signatures
In preparation for gas chromatography, an acid-catalyzed transesterification procedure converted TAG and wax ester samples to fatty acid methyl esters and free fatty alcohols (FAME and FAlc, respectively) using an acid catalyst of H2SO4/MeOH following (Budge et al., 2006). FAME and FAlc were quantified using temperature-programmed gas liquid chromatography on a Perkin Elmer Autosystem II Capillary FID linked to a computerized integration system (Varian Galaxie software). We used a DB-23 30 m Agilent column with a (50%-cyanopropyl)-methylpolysiloxane phase when analyzing FAME from triacyglycerol and a ZB-FFAP 30 m Phenomenex column with a nitroterephthalic acid modified polyethylene glycol phase when analyzing FAME and FAlc in wax ester, both with an internal diameter of 0.25 mm and a film thickness of 0.25 μm. To elute FAs, an initial temperature of 60°C was held for 2 min, then rose 45°C per min to 150°C, held for 2 min, after which it rose 2.5°C per min to a final temperature of 220°C and held for 2 min. For FAlc, an initial oven temperature of 60°C was held for 2 min, then rose 45°C per min to 150°C held for 1 min, after which it rose 4°C per min to a final temperature of 240°C and held for 125 min. Splitless injection with an injector temperature of 250°C was applied for both FAME and FAlc. Helium was used as a carrier gas with flame ionization detection. Resulting chromatograms were individually assessed for correct peak identification and reintegrated when necessary. To create a single lipid signature for each sample, FAME and FAlc constituents from each TAG and wax ester lipid class were weighted relative to the original TAG: wax ester ratio in the oil sample. Weighted FAME and FAlc constituents were then combined into a single lipid signature while keeping FAME and FAlc proportions unique to their lipid class (e.g., the fatty acid methyl ester 16.0 is found in both TAG and wax ester and was merged into the lipid signature as “16.0_wax,” and “16.0_tag”). Any FAME and FAlc constituents that were on average less than 0.1% of the sample were removed unless they were greater than 0.2% in any sample. The remaining FAME and FAlc were renormalized and retained for diet modeling. We report fatty acid and fatty alcohol constituents of each sample as weight percent of total (Table S2). Non-metric multidimensional scaling (NDMS) was used to visualize similarity/dissimilarity in lipid constituents among individual prey species relative to their functional group, taxonomic family, and collection region (Figure 2A). Given the large number of squid in our analysis, we ran a second NMDS ordination on squid species only (Figure 2B).
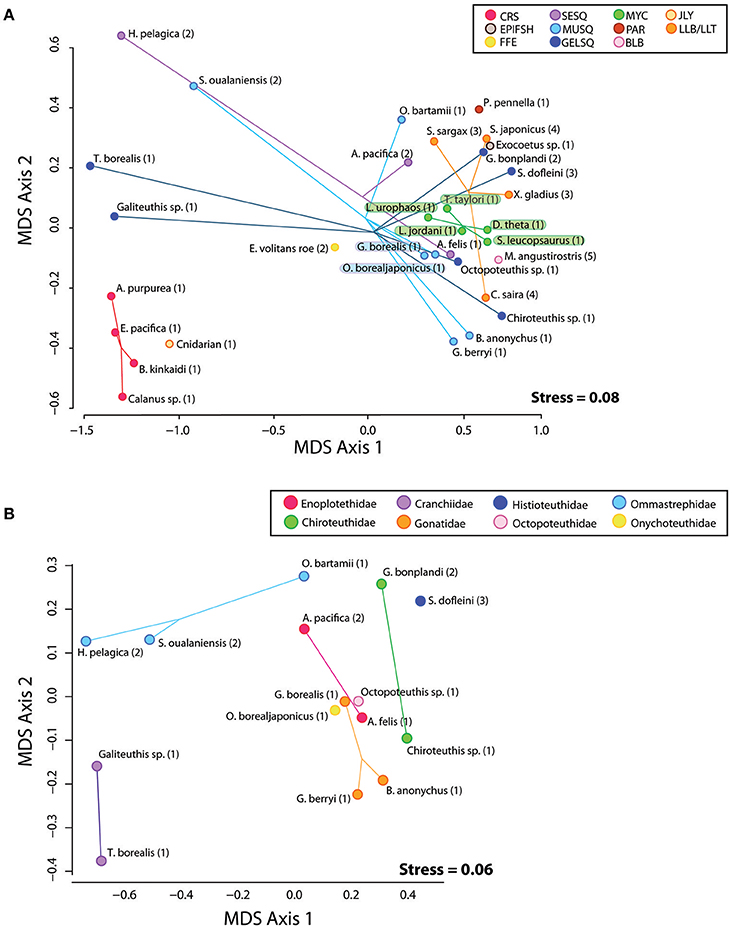
Figure 2. (A) Plot of MDS1 and MDS2 scores derived from a non-metric multi-dimensional scaling analysis of raw FAME and FAlc values from all prey species. Species positions on the plot represent the mean FAME/FAlc composition of the subsamples from each species homogenate. Eleven functional groups are represented by colored spider plots and abbreviations: CRS, Crustacean; SESQ, Small Epipelagic Squid; MUSQ, Epi-Mesopelagic Muscular Squid; GELSQ, Bathy-Mesopelagic Squid; MYC, Mesopelagic Fish; LLA, Longline-Associated Prey; EPIFSH, Epipelagic Fish; FFE, Flying Fish Eggs; PAR, Ectoparasitic Copepod; BLB, Marine Mammal Blubber; JLY, Jellyfish. Collection regions coded by numbers: 1, NPTZ; 2, Hawaii; 3, California Current; 4, Asia; 5, Año. Nuevo breeding colony. (B) Plot of MDS1 and MDS2 scores derived from a non-metric multi-dimensional scaling analysis of raw FAME and FAlc values from squid species only.
Quantitative Fatty Acid Signature Analysis
Diet Estimation
Albatross diet was estimated using the QFASA model developed by Iverson et al. (2004) found in the package “QFASApack” written for R v.2.8.1 (R Core Team, www.R-project.org). Since procellariform oil is dietary in origin, and not metabolically reconstituted, we did not subset our suite of FAME/FAlc constituents by those considered “dietary” (vs. “biosynthesized”). Prey signature proportions in diet were weighted by total lipid in prey to give final QFASA diet estimates as proportion of biomass consumed of each prey species in the diet of each individual albatross. To quantify the diet composition of albatrosses and the relative importance of each prey species and prey functional group in albatross diets, we calculated multiple traditional diet metrics for each breeding phase in each albatross species. These include: (1) the percent frequency of occurrence (Oi: number of bird diets in which prey i occurred, divided by the total number of bird diets), (2) percentage of diet (Pi: the percentage of prey i found in the diet of birds, described by the mean and standard deviation), and (3) the maximum percentage that prey i occurred across diets (Pi_max).
Quantifying Prey-Specific Error in the QFASA Model
To quantify the performance of QFASA specific to our suite of 33 species of prey, we modeled QFASA-estimated diets from simulated (and, therefore, known) “pseudo-diet” compositions. Pseudo-diet compositions were created semi-randomly using a modified dirichlet distribution (a multivariate generalization of the beta distribution (0–1)). The dirichlet-derived composition of diets was modified to ensure each prey species was represented equally across the range of compositional possibility (0–1). To do this, we first assigned to each prey (n = 33) a vector of a fixed sequence of proportions, pi(1:11) = (0.01, 0.10, 0.20, 0.30, 0.40, 0.50, 0.60, 0.70, 0.80, 0.90, 0.99). Then, for the ith element of p, a random multivariate composition of n-1 elements that summed to 1-pi was generated from the dirichlet distribution (α = 0.005) to represent the proportion of diet composed of the remaining 32 prey species. In order for each prey species to have a large number of proportions equally represented across the compositional range of 0–1 in the pseudo-diets, we repeated that process 44 times giving 484 (11 * 44) partially supervised pseudo-diets for each of the 33 prey species, resulting in a total of 15,972 (484 * 33) simulated pseudo-diets. The semi-randomly created pseudo-diets were then used to generate pseudo-predator lipid signatures, which were then evaluated by the QFASA model to generate diet estimations. Model error was calculated as the difference between QFASA diet estimates and the known pseudo-diets, giving us a measurement of error specific to each prey species for each of the 15,972 simulations. To assess the performance of QFASA relative to functional groupings of prey, we first summed the diet proportions for all species within each functional group in known diets and in QFASA-estimated diets, and then calculated the error as the difference between those values. Error was summarized across all simulation trials.
Statistical Analyses
Impact of Species, Phase, and Year on Albatross Diet
To visualize differences in diet composition between species and breeding phase, we used a canonical analysis of principal coordinates (CAP) on Bray-Curtis dissimilarity matrices using the capscale function in the vegan package in R (v 3.1.2). CAP analysis is a constrained ordination technique that can reveal ecological patterns masked in unconstrained techniques, such as principal components analysis (Anderson and Willis, 2003). Because regional overlap in foraging habitat shrinks and expands between the two species across the breeding phase (Hyrenbach et al., 2002; Kappes et al., 2015), functional groups of prey that were collected from multiple regions were further classified into subcategories based on region (e.g., Hawaiian small epipelagic squid vs. NPTZ small epipelagic squid). Pearson's correlation coefficients (r) between prey species and correlation axes were then used to explore the relative influence of prey species on multivariate patterns in albatross diet. Correlations were considered weak when r ≥ 0.3 and moderate to strong when ≥ 0.5. The response of multivariate diet composition (by functional group) to the effects of species and breeding phase (and their interaction) was evaluated using a permutational multivariate analysis of variance (per-MANOVA) using the adonis and betadisper functions in the vegan package in R (v 3.1.2). Year was included as a cofactor. Dissimilarity matrices were constructed using Bray-Curtis distances; 4,999 permutations were run.
Fisheries-Associated Prey in Albatross Diet
Given the high number of zeros in our diet composition data, we used zero-inflated negative binomial regression models with the zeroinfl function in the pscl package in R (v 3.1.2) to test for the effect of species, breeding phase, and year on the proportion of fisheries-associated resources in albatross diets. Since fisheries-associated prey was relatively rare in albatross diet (24 out of 106 birds with >10% fisheries-associated prey), sample sizes were not large enough to measure the effect of individual bird characteristics, such as sex, or age class on the proportion of fisheries-associated prey in diets.
Results
The overall lipid content and proportion of TAG and wax ester lipid classes from each prey species (n = 33) and of stomach oil samples from each albatross species are presented in Table S2 along with their FA and FAlc compositions.
Qualitative Assessment of Similarity in Lipid Composition Among Prey
Some functional groups, such as crustaceans (CRU) and myctophids (MYC) displayed distinct clustering in the NMDS ordination plot (Figure 2A), indicating within-group similarity in FA/FAlc constituents. For other functional groups (e.g., all three groups of squid—SESQ, MUSQ, GELSQ) there was a large spread of species within the functional group. We found no clear driver of lipid composition similarity among squid species (Figure 2B). In some cases, squid species were distinctly clustered by taxonomic family (e.g., Cranchiidae, Ommastrephidae), but lipid profiles also were influenced by both oceanographic region and feeding ecology. For example, the two ommastrephid squid from Hawaiian waters (H. pelagica and S. oualaniensis) were clustered closer together in ordination space than an ommastrephid sourced from the NPTZ (O. bartrami). Likewise, both Abraliopsis pacifica (an enoploteuthid) and G. bonplandi (a chiroteuthid) from Hawaiian waters were positioned closer together than their taxonomic family counterparts sourced from the NPTZ. The large muscular gonatid species (G. borealis) was positioned closer to the large muscular onychoteuthid species (O. borealjaponicus) than it was to the smaller gonatids G. berryi and B. anonychus.
Quantifying Error in QFASA Modeling
Model uncertainty varied substantially among species and functional groups (Supplemental Figure S1 and Figure 3). Low error across all crustacean species aligned with the divergent lipid profile in this functional group relative to the others and further indicates distinct lipid profiles among species within this group. Jellyfish, marine mammal blubber, ectoparasitic copepods, and flying fish eggs also had low modeling error. Longline-associated prey also had relatively low error, with sardines and saury baitfish performing better than swordfish. All functional groups of squid and the mesopelagic fish group had high error (Figure 3), mirrored in the proximity and overlap in lipid ordination space. However, while mesopelagic fish were chronically underestimated at higher pseudo-diet proportions, these underestimations were likely compensated by overestimations of other species within this functional group, due to the distinct clustering of myctophids in the NMDS ordination plot (Figure 2A). Large, muscular squid performed poorly overall, driven by large magnitudes of error in two species within this functional group, G. borealis and O. borealjaponicus. These two species are adjacent in NMDS ordination, each likely influencing the error of the other. The other four species of squid in the MUSQ functional group had low error, which is further supported with their distinct positions on the NMDS plot. Mesopelagic gelatinous squid (GELSQ) had the lowest error of squid functional groups, with only two out of seven species showing large magnitudes of error (Galiteuthis sp. and Octopoteuthis sp.). Many, but not all, species had spurious positive diet estimations (of between 0 and 10% on average) when the true diet was zero (Supplemental Figure S1); however, as a species' true pseudo-diet proportion increased > 10%, QFASA estimated diets were rarely overestimated.
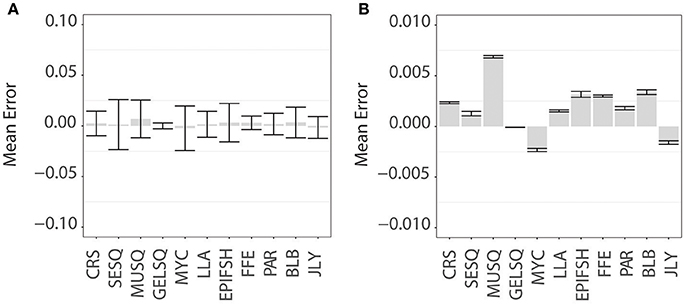
Figure 3. QFASA modeling error for functional groups of prey. Bar plots represent the mean error (calculated as the difference between the QFASA-estimated diet and “true” pseudodiet diet fraction in prey functional groups displayed with (A) standard deviation and (B) 95% confidence intervals. Functional groups denoted by abbreviations: CRS, Crustacean; SESQ, Small Epipelagic Squid; MUSQ, Epi-Mesopelagic Muscular Squid; GELSQ, Bathy-Mesopelagic Squid; MYC, Mesopelagic Fish; LLA, Longline-Associated Prey; EPIFSH, Epipelagic Fish; FFE, Flying Fish Eggs; PAR, Ectoparasitic Copepod; BLB, Marine Mammal Blubber; JLY, Jellyfish.
Albatross Diets Estimated From QFASA
Diet metrics are given in Table 2. Incubating and chick-brooding albatrosses from Tern Island consumed a broad diversity of prey species across most functional groups (Figure 4). Squid dominated the diet in both albatross species across the breeding season, apart from chick-brooding black-footed albatross diets in which fish-eggs were equally as important as the combined squid component (32.0 and 33.3% respectively). Mesopelagic gelatinous squid was the most dominant squid functional group in albatross diets, apart from incubating Laysan albatrosses who consumed a greater proportion of large, muscular squid. Small, epipelagic schooling squid were common, but marginal (by proportion), in the diets of albatrosses across both species and breeding phases (<10% of the overall group diets). Following squid in both species and fish-eggs in brooding black-footed albatrosses, mesopelagic fish (myctophids) were the next most important diet item, ranging between 12.6 and 18.9% of the overall group diets. Crustaceans and longline-associated prey were marginal dietary items in both species and both increased in mean diet proportion from incubation to the chick-brooding phase. Marine mammal blubber, ecto-parasitic copepods, and jellyfish were present, but rare, in the diet of both species, but it is notable that the diet of one chick-brooding albatross contained >30% jellyfish. This indicates that while rare, this functional group may be ephemerally important.
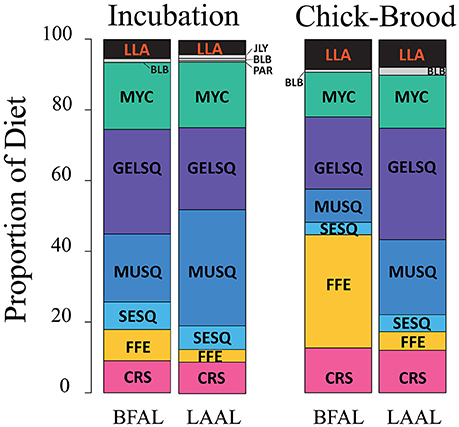
Figure 4. Diet composition of black-footed (BFAL) and Laysan (LAAL) albatrosses across the incubation and chick-brood breeding phases. While the two species show high similarity in the functional groups exploited during incubation, the diet of black-footed albatrosses diverges during the chick-brood with a large increase in flying fish egg consumption. Outside of chick-brooding BFAL, squid (“SESQ,” “GELSQ,” “MUSQ”) composed, by far, the largest proportion of breeding Hawaiian albatross diet. Longline-associated prey (“LLA,” in red) is a marginal diet across both breeding phases and species, but increases slightly in proportional diet in the chick-brood breeding phase. Functional groups denoted by abbreviations: CRS, Crustacean; SESQ, Small Epipelagic Squid; MUSQ, Epi-Mesopelagic Muscular Squid; GELSQ, Bathy-Mesopelagic Squid; MYC, Mesopelagic Fish; LLA, Longline-Associated Prey; EPIFSH, Epipelagic Fish; FFE, Flying Fish Eggs; PAR, Ectoparasitic Copepod; BLB, Marine Mammal Blubber; JLY, Jellyfish.
Impact of Species, Phase, and Year on Albatross Diet
Fish eggs, muscular squid, crustaceans, and small epipelagic squid had the largest influence on differences in diet composition between species and breeding phases (Figure 5). Flying fish eggs correlated strongly (r = 0.81) with black-footed albatross in both the incubation and chick-brood breeding phases. Moderate correlations with crustaceans and black-footed albatross diet (r = 0.47) also occurred predominately during the chick-brood phase (r = 0.35). Large, mesopelagic squid from the NPTZ correlated strongly (r = 0.64) with Laysan albatross diet and weakly with incubation (r = 0.30). We also saw a moderate correlation with mesopelagic fish and Laysan albatross diet (r = 0.41) relative to black-footed diet, across breeding phases. Small, epipelagic squid from the NPTZ correlated moderately with the brooding phase (r = 0.53) and weakly with Laysan albatrosses (r = 0.33), whereas small epipelagic squid from Hawaii did not correlate more strongly with a particular species but did show a moderate correlation with diet from the incubation period (r = 0.50) vs. the chick-brood. Both mesopelagic squid from the NPTZ and longline bait showed a weak correlation (r = 0.39 each) with diets from the chick-brood phase relative to incubation. Results from per-MANOVA analyses indicated that both species and breeding phase (but not their interaction) significantly influenced albatross diet composition [F(1, 105) = 4.45, p < 0.001; F(1, 105) = 4.36, p = 0.001, respectively]. Year had no significant effect [F(1, 105) = 0.013, p = 0.189].
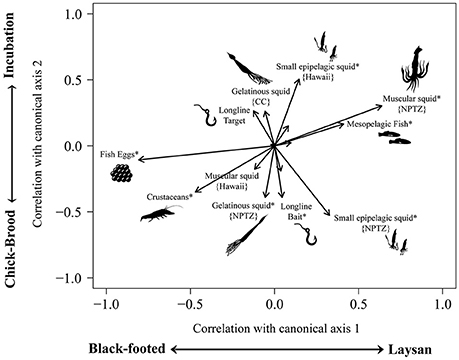
Figure 5. A canonical analysis of principal components displays the functional groups of prey that have the strongest influence on dietary differences between Laysan and black-footed albatrosses during the incubation and chick-brood breeding phases. Because of differences in foraging grounds between the species, particularly during incubation, we further classified functional groups by sub-region, wherever applicable. Lengths and directions of arrows represent the strength and sign of the correlation of functional groups with the two canonical axes and asterisks(*) represent moderate significant correlations (>0.03).
Fisheries-Associated Prey in Albatross Diet
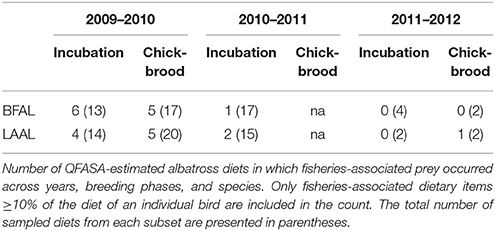
We took a conservative approach when interpreting long-line associated diet to avoid any spurious overestimation, given the management implications inherent with this functional group. When evaluating the relationship between fisheries-associated resources and influential factors (e.g., species and year), we only used data from birds in which fisheries-associated diet composed 10% or more of their proportional diet. This resulted in 12 out of 53 black-footed albatross diets and 12 out of 53 Laysan albatrosses across all years (Table 3). While the overall contribution of fisheries-associated resources to diet was low (ranging between 4AND8% of pooled proportional diet), the incidence of fisheries-associated resources was notable particularly in the incubation months of 2009–2010. Here, 46 and 29% of incubating black-footed and Laysan diets contained fisheries-associated resources. Incidence then decreased in the chick-brooding months to 29 and 25%. During the incubation season of the following year, 2010–2011, incidence of fisheries-associated resources in the diets of both species decreased to 3 and 16%, respectively. While black-footed albatross diets contained only longline target species, Laysan albatross diets contained both longline bait and longline target-fish species. Interestingly, all 24 albatross diets with evidence of fisheries exploitation, had either bait or target species, but never both.
The distribution of fisheries resources in diets was highly skewed with most individuals having low percentages of fisheries-associated prey items (between 10 and 20%), while a few birds had very high percentages (e.g., >80% bait fish in one Laysan albatross diet estimation and between 35 and 55% target fish in three black-footed albatross diet estimations). There was no significant effect of species, phase, or year on fisheries exploitation in the zero-inflated binomial models; however, sample sizes were low due to our conservative approach and larger sample sizes may reveal hidden influences. As with the incidence results described above, there was a tendency for black-footed albatrosses to have diets that included a greater proportion of longline target-fish and for Laysan albatross diets to have a larger proportion of longline bait-fish (Figure 5).
Discussion
Our study provides the first characterization of diet for adult North Pacific albatross during the incubation season, a period when foraging success and efficiency plays an important role in reproductive success since most nest abandonments occur during this breeding phase. Our evaluation of the robustness of QFASA using simulated diets and prey-specific model uncertainty made it possible to interpret diet estimates with an appropriate degree of caution and confidence. These data allowed a robust estimation of the individual and colony level exploitation of fisheries-associated resources for North Pacific albatross.
Incidence of Fisheries-Associated Resources in Breeding Albatross Diet
Bisson (2008) observed that black-footed albatrosses predominately scavenged swordfish carcasses at vessels, while Laysan albatrosses tended to target bait from hooks. Our estimated diets agree. Interestingly, these observed behavioral and dietary differences in fisheries exploitation may incur health consequences as black-footed albatrosses have 400% higher mercury body burdens than Laysan albatrosses (Finkelstein et al., 2006). Although Finkelstein et al. (2006) found a robust relationship between mercury loads and habitat differences, our results (and those observations of Bisson, 2008) suggest that the greater consumption of swordfish by black-footed albatrosses may also contribute to these higher mercury loads, as swordfish meat is extremely high in mercury compared to other fish (USFDA, 2017).
Fisheries-associated prey occurred in the diets of Laysan and black-footed albatrosses across both breeding stages, but neither species appeared to rely on fisheries-associated resources as a primary food source in either breeding phase. While the total proportion of fisheries resources in diet was low, the incidence of fisheries exploitation was not insignificant (e.g., 46% for incubating black-footed albatrosses in incubation 2009–2010) and fisheries resources should be considered by managers, conservation planners, and policy makers as a relevant anthropogenic food source. There is a clear trend of increased seabird interactions with Hawaii longline fisheries since 2004 despite inter-annual variability (Figure 6). However, it is unclear whether this increase is due to an increased reliance on fisheries resources, a shift in the overlap of foraging grounds with fisheries, or the reflection of a proportional increase in albatross population size. Monitoring fisheries exploitation from colony-based studies over multiple years would help clarify whether inter-annual variability in fisheries exploitation correlates with fluctuating bycatch rates.
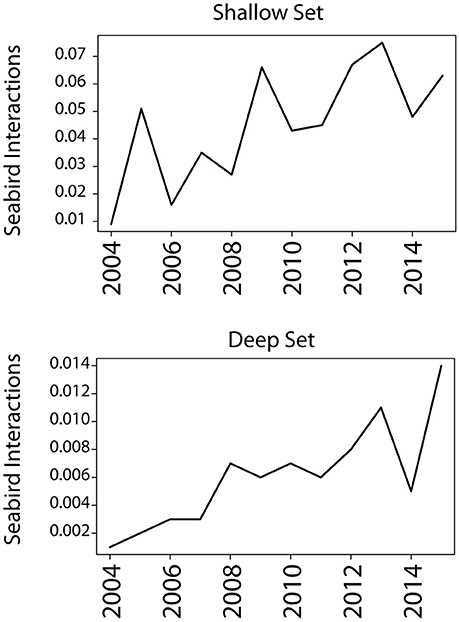
Figure 6. Seabird interactions with Hawaii longline fisheries in the central Pacific are increasing. Seabird interaction rate was measured as the number of interactions per 1,000 hooks; interactions are defined as a bird caught on a hook, whether dead or alive. Interactions across 2004–2015 increased in both deep and shallow set longline fisheries. Species included in the count are black-footed albatrosses, Laysan albatrosses, and sooty shearwaters; however, the two albatross species represent >90% of all interactions across the years. Data was taken from the 2015 Annual Report “Seabird Interactions and Mitigation Efforts in Hawaii Longline Fisheries” from NOAA Fisheries Pacific Islands Regional Office (January 2017).
We found that QFASA-estimated diets from albatrosses sampled in 2009-2010 had a much higher incidence of fisheries resources than the following year. This is particularly interesting given that 2009–2010 was a year with record-breaking warm sea surface temperatures in the central Pacific due to a strong central Pacific El Niño that rapidly decayed into a strong La Niña (Kim et al., 2011). Thorne et al. (2015, 2016) evaluated the impact of oceanographic variability from EÑSO conditions on Hawaiian albatross foraging behavior, energy expenditure, and reproductive success and found that habitat accessibility is poorer in La Niña conditions, driving breeding albatrosses to fly longer distances in unfavorable winds to find suitable foraging habitat. If years of poorer environmental quality or reduced prey accessibility impose increased constraints on breeding albatross, birds may switch strategies to one that more heavily utilizes fisheries resources, as seen in Cape gannets in years of low pelagic fish biomass (Tew Kai et al., 2013). Therefore, long-term monitoring of albatross diets from breeding colonies in the Hawaiian Islands would provide important information on the dynamics of inter-annual oceanographic variability and albatross feeding strategies, including their reliance on anthropogenic food sources.
QFASA Diet Estimation—A Critical Evaluation
Overall, QFASA-estimated diets showed high accordance with direct observations of albatross diet from previous studies (Harrison et al., 1983; Gould et al., 1997; Pitman et al., 2004; Bisson, 2008; Henry, 2011; Walker and Fitzgerald, 2012; Walker et al., 2012), particularly when interpreting diet by functional group. The present study and Walker et al. (2012) both highlight Cranchiidae and Histioteuthidae as the most important squid families in North Pacific albatross diet (Table 2). Our results agree with the differences in squid composition observed previously, with higher Gonatidae, lower Histioteuthidae, and similar abundances of Chranchiidae in Laysan relative to black-footed albatrosses (Table 2, Walker et al., 2012). Species differences in squid consumed is consistent with the distinct at-sea segregation of the two albatross species, where Laysan albatrosses forage in colder, higher-latitude waters than black-footed albatrosses for most of the breeding season (Hyrenbach et al., 2002; Kappes et al., 2010, 2015). These patterns are coincident with the general worldwide distribution of histioteuthids in warm-temperate and tropical regions and gonatids in cool-temperate and Arctic regions (Kubodera and Jefferts, 1984; Nesis, 1987; Voss et al., 1998). Small epipelagic squid were slightly more prevalent in Laysan than black-footed albatross diets, as they consumed a larger proportion of the small gonatid B. anonychus (consistent with Walker et al., 2012). Similarly, the two small Enoploteuthid species were consumed in similar proportions between the two species; however, Laysan albatrosses consumed more of the Enoploteuthid that is more widely distributed in cool-temperate waters (A. felis), while black-footed albatrosses consumed more A. pacifica, which occurs in warmer, southern waters.
Lower prey-specific error was seen in groups that were trophically distinct from the majority of prey, such as crustaceans and flying fish eggs, while the highest errors characterized squid and myctophid species that co-occur in the mesopelagic zone of the North Pacific Transition Zone. This is likely due to close trophic relationships that result in high overlap in lipid profiles. Squid, as a group, introduced an additional complication since the majority of their lipids are contained in their large digestive organs and dietary in origin (Phillips et al., 2002), potentially obscuring the distinctness of the squid lipid signature from that of their prey. This is akin to the complication that arises from stomach content analyses when undigested parts may actually be sourced from the stomach contents of the prey itself. It is illuminating that O. bartrami, a large predatory squid, had low model error. For this species (given its large size) we only analyzed lipids from mantle tissue, excluding the digestive organs. Similarly, many bathy-mesopelagic squid species had low model uncertainty and were collected at sea with little to no digestive organs, further supporting the idea that diet-derived lipids in squid digestive glands may increase error in QFASA diet modeling.
The overall high concordance between direct observations from previous diet studies with our estimated diets supports QFASA modeling as an effective method for quantifying diet in procellariform seabirds. The accuracy of the QFASA model will be dependent on each specific study system and was likely augmented in our study by (1) incorporating two lipid classes in the QFASA model (wax ester and TAG), (2) not requiring calibration coefficients to account for predator metabolism, and (3) collecting a large and comprehensive library of prey lipid signatures across the foraging ranges of Hawaiian albatrosses. Additionally, modeling error in the QFASA model at the species and functional group levels provided essential information on the interpretation of diet estimates. However, we re-emphasize that to estimate fisheries exploitation using QFASA, it is critical that the fisheries-resources available to the predator of interest are biochemically distinct from other prey types, otherwise they cannot be discriminated.
QFASA as a Species Management Tool
We recommend QFASA from albatross oil as an effective tool to monitor procellariform diet from both natural and anthropogenic food sources. A combination of diet sampling methods would provide the most comprehensive characterization of diet, with QFASA providing information on food sources not measurable with traditional methods (e.g., jellyfish, fisheries resources) and traditional methods providing more refined details on the squid component in particular. While collecting stomach oil from individual adult albatrosses is not as logistically simple as collecting regurgitated boluses from the colony floor, the information gained from this method is valuable and worthy of consideration in management plans for monitoring these species. Collecting stomach oil is a minimally invasive procedure that is easy to train and with no known adverse consequences on the individual, especially compared to other available methods of collecting stomach contents from adult albatrosses (e.g., water offloading). Furthermore, the most resource intensive component of this method (in time and cost) is developing the prey lipid library, since albatross are wide-ranging generalist consumers that consume a great diversity of prey. This work provides a large and comprehensive prey lipid library for North Pacific albatross (and potentially other predators) as a resource for managers to use and also to build upon and refine.
Conclusion
A novel variation of QFASA was developed, evaluated, and subsequently applied to quantify diet from individual adult North Pacific albatrosses during the incubation and chick-brood breeding phases for the first time. We found QFASA to be a tractable method for estimating the importance of fisheries-associated resources in the diet of albatrosses and potentially other procellariiformes. Future work should expand the prey lipid library to include more nuanced lipid profiles of potential prey (such as different tissue types of marine mammal carcasses (e.g., eyes and organs) and more prey items used in longline fisheries (e.g., Illex argentinus). We recommend QFASA as a method to develop colony-specific monitoring programs to quantify the exploitation of fisheries-associated resources across years. Having refined diet information from specific colonies across years would help target populations most at risk to fisheries-associated activities. Populations that rely on fishery-associated resources during critical phases of the breeding cycle may be vulnerable to changes in fishery policies (Oro et al., 1995). The overall exploitation of fisheries-associated resources in both species of Hawaiian albatrosses was low in the current study; however, we do stress our estimates were constructed very conservatively. Additionally, the high inter-annual variability in the incidence of fisheries resources in our study indicates that longitudinal information is needed, particularly in light of the increasing numbers of by-caught North Pacific albatross in longline fisheries.
Author Contributions
MC, SB, and SS conceived and designed the study. MC collected albatross stomach oil in the PMNM. MC, CG, and YM designed prey collection methods and conducted fieldwork on the T/S Oshoromaru. WW identified prey species. MC and CG conducted laboratory work for lipid analyses. SB provided all equipment, supplies, and methods for the lipid analyses, and contributed to the interpretation of lipid results. MC conducted QFASA modeling and statistical analyses. MC, CG, and WW interpreted diet results. MC drafted the manuscript and all authors contributed to revisions, approved the final version of the manuscript, and agree to be accountable for all aspects of the work. The research presented in the manuscript was conducted as part of MC's doctoral work at UCSC.
Funding
This research was predominantly funded by the Tagging of Pacific Predators program (TOPP), the Pacific Islands Climate Change Cooperative (PICCC), United States Fish & Wildlife Service, and San Jose State University. This study was partly supported by JSPS KAKENHI Grant Number 23255001 and 15H05709, the Office of Naval Research grants N00014-10-1-0356 and N00014-13-1-0134, Friends of Long Marine Lab, and the Dr. Earl H. Myers and Ethel M. Myers Oceanographic and Marine Biology Trust. MC was supported by a UCSC Regents fellowship and a NOAA's Dr. Nancy Foster graduate fellowship.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank our field team: Sarah Youngren, Ruth Brown, Dan Rapp, Caitlin Kroeger, Morgan Gilmour, and Abram Fleishman, and to PMNM/USFWS support for logistics and funding: Pete Leary, Ty Benally, Paula Hartzell and especially Elizabeth Flint and Maura Naughton. Thank you M/V Kahana & crew for numerous journeys to and from the NWHI. We are grateful to Robert Henry III for the original encouragement and advice to explore quantitative fatty acid analysis in albatross. We are indebted to the student, professors, and crew on the T/S Oshoromaru for help collecting prey and to Daisuke Mizuguchi and Adam Fox for help sorting, weighing, and homogenizing prey. Carrie Greene and Christopher Barry provided technical support in the marine lipid lab at Dalhousie University. Thanks to Robert Humphreys at NOAA's PIFSC for access to prey from Hawaiian waters and to Gregor Calliet for help identifying species of pelagic crustaceans in our prey library. Lisa Schwarz provided statistical advice on constructing balanced pseudo-diets for estimating model error.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2018.00113/full#supplementary-material
References
Abe, T., Sekiguchi, K., Onishi, H., Muramatsu, K., and Kamito, T. (2012). Observations on a school of ocean sunfish and evidence for a symbiotic cleaning association with albatrosses. Mar. Biol. 159, 1173–1176. doi: 10.1007/s00227-011-1873-6
Ackman, R. G., and Eaton, C. A. (1966). Some commercial Atlantic herring oils; fatty acid composition. J. Fish. Res. Board Can. 23, 991–1006. doi: 10.1139/f66-092
Ainley, D. G., and Blight, L. K. (2009). Ecological repercussions of historical fish extraction from the Southern Ocean. Fish Fish. 10, 13–38. doi: 10.1111/j.1467-2979.2008.00293.x
Anderson O, Small C, Croxall J, Dunn E, Sullivan B, Yates O, et al. (2011). Global seabird bycatch in longline fisheries. Endanger. Species Res. 14, 91–106.
Anderson, M., and Willis, T. (2003). Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84, 511–525. doi: 10.1890/0012-9658(2003)084[0511:CAOPCA]2.0.CO;2
Bearhop, S., Thompson, D. R., Phillips, R. A., Waldron, S., Hamer, K. C., Gray, C. M., et al. (2001). Annual variation in great skua diets: the importance of commercial fisheries and predation on seabirds revealed by combining dietary analyses. Condor 103, 802–809. doi: 10.1650/0010-5422(2001)103[0802:AVIGSD]2.0.CO;2
Beck, C. A., Iverson, S. J., Bowen, W., and Blanchard, W. (2007). Sex differences in grey seal diet reflect seasonal variation in foraging behaviour and reproductive expenditure: evidence from quantitative fatty acid signature analysis. J. Anim. Ecol. 76, 490–502. doi: 10.1111/j.1365-2656.2007.01215.x
Bicknell, A. W. J., Oro, D., Camphuysen, K. C. J., and Votier, S. C. (2013). Potential consequences of discard reform for seabird communities. J. Appl. Ecol. 50, 649–658. doi: 10.1111/1365-2664.12072
Bisson, J. R. (2008). Diet Dynamics and Trophic Relations of Laysan and Black-Footed Albatrosses Associated With Pelagic Longline Fishing. Available online at: http://scholarspace.manoa.hawaii.edu/handle/10125/20932.
Bodey, T. W., Jessopp, M. J., Votier, S. C., Gerritsen, H. D., Cleasby, I. R., Hamer, K. C., et al. (2014). Seabird movement reveals the ecological footprint of fishing vessels. Curr. Biol. 24, R514–R515. doi: 10.1016/j.cub.2014.04.041
Borges, L. (2015). The evolution of a discard policy in Europe. Fish Fish. 16, 534–540. doi: 10.1111/faf.12062
Bromaghin, J. F., Budge, S. M., Thiemann, G. W., and Rode, K. D. (2015). Assessing the robustness of quantitative fatty acid signature analysis to assumption violations. Methods Ecol. Evol. 7, 51–59. doi: 10.1111/2041-210x.12456
Bromaghin, J. F., Budge, S. M., Thiemann, G. W., and Rode, K. D. (2017). Simultaneous estimation of diet composition and calibration coefficients with fatty acid signature data. 7, 6103–6113. doi: 10.1002/ece3.3179
Bromaghin, J. F., Lance, M. M., Elliott, E. W., Jeffries, S. J., Acevedo-Gutiérrez, A., and Kennish, J. M. (2013). New insights into the diets of harbor seals (Phoca vitulina) in the Salish Sea revealed by analysis of fatty acid signatures. Fish. Bull. 111, 13. doi: 10.7755/FB.111.1.2
Budge, S. M., Iverson, S. J., and Koopman, H. N. (2006). Studying trohic ecology in marine ecosystems using fatty acids: a primer on analysis and interpretation. Mar. Mamm. Sci. 22, 759–801. doi: 10.1111/j.1748-7692.2006.00079.x
Budge, S. M., Iverson, S. J., Bowen, W. D., and Ackman, R. G. (2002). Among- and within-species variability in fatty acid signatures of marine fish and invertebrates on the Scotian Shelf, Georges Bank, and southern Gulf of St. Lawrence. Can. J. Fish. Aquat. Sci. 59, 886–898. doi: 10.1139/f02-062
Cheah, C. C., and Hansen, I. A. (1970). Wax esters in the stomach oil of petrels. Int. J. Biochem. 1, 198–202. doi: 10.1016/0020-711X(70)90094-7
Cherel, Y., Xavier, J. C., Grissac, S., De Trouvé, C., and Weimerskirch, H. (2017). Feeding ecology, isotopic niche, and ingestion of fishery-related items of the wandering albatross Diomedea exulans at Kerguelen and Crozet Islands. Mar. Ecol. Prog. Ser. 565, 197–215. doi: 10.3354/meps11994
Clarke, A., and Prince, P. A. (1976). The origin of stomach oil in marine birds: analyses of the stomach oil from six species of subantarctic procellariiform birds. J. Exp. Mar. Bio. Ecol. 23, 15–30. doi: 10.1016/0022-0981(76)90082-4
Collet, J., Patrick, S. C., and Weimerskirch, H. (2017). Behavioral responses to encounter of fishing boats in wandering albatrosses. Ecol. Evol. 7, 3335–3347. doi: 10.1002/ece3.2677
Connan, M., Cherel, Y., and Mayzaud, P. (2007). Lipids from stomach oil of procellariiform seabirds document the importance of myctophid fish in the Southern Ocean. Limnol. Oceanogr. 52, 2445–2455. doi: 10.4319/lo.2007.52.6.2445
Connan, M., Mayzaud, P., Boutoute, M., Weimerskirch, H., and Cherel, Y. (2005). Lipid composition of stomach oil in a procellariiform seabird Puffinus tenuirostris: implications for food web studies - Google Scholar. Mar. Ecol. Ser. 290, 277–290. doi: 10.3354/meps290277
Conners, M. G., Hazen, E. L., Costa, D. P., and Shaffer, S. A. (2015). Shadowed by scale: subtle behavioral niche partitioning in two sympatric, tropical breeding albatross species. Mov. Ecol. 3, 28. doi: 10.1186/s40462-015-0060-7
Croxall, J. P., and Gales, R. (1998). “An assessment of the conservation status of albatrosses,” in Albatross Biology and Conservation, eds G. Robertson and R. Gales (Chipping Norton, NSW: Surrey Beatty), 46–65.
Edwards, A. E., Fitzgerald, S. M., Parrish, J. K., Klavitter, J. L., Romano, M. D., Fischer, K. N., et al. (2015). Foraging strategies of laysan albatross inferred from stable isotopes: implications for association with fisheries. PLoS ONE 10:e0133471. doi: 10.1371/journal.pone.0133471
Fernández, P., Anderson, D. J., Sievert, P. R., and Huyvaert, K. P. (2001). Foraging destinations of three low-latitude albatross (Phoebastria) species. J. Zool. 254, 391–404. doi: 10.1017/S0952836901000899
Finkelstein, M., Keitt, B. S., Croll, D. A., Tershy, B., Jarman, W. M., Rodriguez-Pastor, S., et al. (2006). Albatross species demonstrate regional differences in North Pacific marine contamination. Ecol. Appl. 16, 678–686. doi: 10.1890/1051-0761(2006)016[0678:ASDRDI]2.0.CO;2
Folch, J., Lees, M., and Sloane-Stanley, G. H. (1957). A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509.
Furness, R. W. (2003). Impacts of fisheries on seabird communities. Sci. Mar. 67, 33–45. doi: 10.3989/scimar.2003.67s233
Gales, R., and Robertson, G. (1998). Albatross Biology and Conservation. (Chipping Norton: Surrey Beatty & Sons).
Garthe, S., and Scherp, B. (2003). Utilization of discards and offal from commercial fisheries by seabirds in the Baltic Sea. ICES J. Mar. Sci. 60, 980–989. doi: 10.1016/S1054-3139(03)00099-7
Gould, P. J., Ostrom, P., and Walker, W. A. (1997). Trophic relationships of albatrosses associated with squid and large-mesh drift-net fisheries in the North Pacific Ocean. Can. J. Zool. 75, 549–562. doi: 10.1139/z97-068
Granadeiro, J. P., Brickle, P., and Catry, P. (2014). Do individual seabirds specialize in fisheries' waste? the case of black-browed albatrosses foraging over the patagonian shelf. Anim. Conserv. 17, 19–26. doi: 10.1111/acv.12050
Grémillet, D., Pichegru, L., Kuntz, G., Woakes, A. G., Wilkinson, S., Crawford, R. J. M., et al. (2008). A junk-food hypothesis for gannets feeding on fishery waste. Proc. R. Soc. Lond. B. Biol. Sci. 275, 1149–1156. doi: 10.1098/rspb.2007.1763
Harrison, C. S., Hida, T. S., and Seki, M. P. (1983). Hawaiian seabird feeding ecology. Wildl. Monogr. 85, 3–71.
Henry, R. W. III. (2011). Consequences of Range Expansion in Laysan Albatrosses. Santa Cruz, CA: University of California.
Hyrenbach, K., Fernández, P., and Anderson, D. (2002). Oceanographic habitats of two sympatric North Pacific albatrosses during the breeding season. Mar. Ecol. Prog. Ser. 233, 283–301. doi: 10.3354/meps233283
Imber, M. J. (1976). The origin of petrel stomach oils: a review. Condor 78, 366–369. doi: 10.2307/1367697
Inger, R., and Bearhop, S. (2008). Applications of stable isotope analyses to avian ecology. Ibis 150, 447–461. doi: 10.1111/j.1474-919X.2008.00839.x
IUCN (2017). The IUCN Red List of Threatened Species. Version 2017-3. Available online at: http://www.iucnredlist.org (Accessed 01 January 2017).
Iverson, S. J., Field, C., Don Bowen, W., and Blanchard, W. (2004). Quantitative fatty acid signature analysis: a new method of estimating predator diets. Ecol. Monogr. 74, 211–235. doi: 10.1890/02-4105
Iverson, S. J., Springer, A. M., and Kitaysky, A. S. (2007). Seabirds as indicators of food web structure and ecosystem variability: qualitative and quantitative diet analyses using fatty acids. Mar. Ecol. 352, 235–244. doi: 10.3354/meps07073
Jansen, O. E. (2013). Fishing for Food: Feeding Ecology of Harbour Porpoises Phocoena Phocoena and White-Beaked Dolphins Lagenorhynchus albirostris in Dutch waters. Ph.D. dissertation, Wageningen University.
Jennings, S., Smith, A. D. M., Fulton, E. A., and Smith, D. C. (2014). The ecosystem approach to fisheries: management at the dynamic interface between biodiversity conservation and sustainable use. Ann. N. Y. Acad. Sci. 1322, 48–60. doi: 10.1111/nyas.12489
Kappes, M. A., Shaffer, S. A., Tremblay, Y., Foley, D. G., Palacios, D. M., Bograd, S. J., et al. (2015). Reproductive constraints influence habitat accessibility, segregation, and preference of sympatric albatross species. Mov. Ecol. 3, 34. doi: 10.1186/s40462-015-0063-4
Kappes, M. A., Shaffer, S. A., Tremblay, Y., Foley, D. G., Palacios, D. M., Robinson, P. W., et al. (2010). Hawaiian albatrosses track interannual variability of marine habitats in the North Pacific. Prog. Oceanogr. 86, 246–260. doi: 10.1016/j.pocean.2010.04.012
Karnovsky, N. J., Hobson, K. A., and Iverson, S. J. (2012). From lavage to lipids: estimating diets of seabirds. Mar. Ecol. Ser. 451, 263–284. doi: 10.3354/meps09713
Kim, W., Yeh, S.-W., Kim, J.-H., Kug, J.-S., and Kwon, M. (2011). The unique 2009–2010 El Niño event: a fast phase transition of warm pool El Niño to La Niña. Geophys. Res. Lett. 38. doi: 10.1029/2011gl048521
Kubodera, T., and Jefferts, K. (1984). Distribution and abundance of the early life stages of squid, primarily Gonatidae (Cephalopoda, Oegopsida), in the northern North Pacific (part 1 and part 2). Bull. Natl. Sci. Museum 10, 91-106-193.
Lewis, R. W. (1969). Studies on the stomach oils of marine animals—II. oils of some procellariiform birds. Comp. Biochem. Physiol. 31, 725–731. doi: 10.1016/0010-406X(69)92072-6
Lewison, R. L., and Crowder, L. B. (2003). Estimating fishery bycatch and effects on a vulnerable seabird population. Ecol. Appl. 13, 743–753. doi: 10.1890/1051-0761(2003)013[0743:EFBAEO]2.0.CO;2
Lewison, R. L., Freeman, S. A., and Crowder, L. B. (2004). Quantifying the effects of fisheries on threatened species: the impact of pelagic longlines on loggerhead and leatherback sea turtles. Ecol. Lett. 7, 221–231. doi: 10.1111/j.1461-0248.2004.00573.x
Magnone, L., Bessonart, M., Rocamora, M., Gadea, J., and Salhi, M. (2015). Diet estimation of Paralichthys orbignyanus in a coastal lagoon via quantitative fatty acid signature analysis. J. Exp. Mar. Bio. Ecol. 462, 36–49. doi: 10.1016/j.jembe.2014.10.008
McInnes, J. C., Jarman, S. N., Lea, M.-A., Raymond, B., Deagle, B. E., Phillips, R. A., et al. (2017). DNA metabarcoding as a marine conservation and management tool: a circumpolar examination of fishery discards in the diet of threatened albatrosses. Front. Mar. Sci. 4:277. doi: 10.3389/fmars.2017.00277
McInnes, J. C., Raymond, B., Phillips, R. A., Jarman, S. N., Lea, M.-A., and Alderman, R. (2016). A review of methods used to analyse albatross diets—assessing priorities across their range. ICES J. Mar. Sci. 73, 2125–2137. doi: 10.1093/icesjms/fsw105
Meynier, L., Morel, P. C. H., Chilvers, B. L., Mackenzie, D. D. S., and Duignan, P. J. (2010). Quantitative fatty acid signature analysis on New Zealand sea lions: model sensitivity and diet estimates. J. Mamm. 91, 1484–1495. doi: 10.1644/09-MAMM-A-299.1
Miller, L. (1940). Observations on the Black-footed Albatross. Condor 42, 229–238. doi: 10.2307/1364482
Naughton, M., Romano, M. D., and Zimmermann, B. (2007). A Conservation Action Plan for Black-footed Albatross (Phoebastria nigripes) and Laysan Albatross (P. immutabilis), version 1.0. Available online at: https://alaskafisheries.noaa.gov/protectedresources/seabirds/albatross_action_plan1007.pdf (Accessed 14 January 2017)
Nesis, K. N. (1987). Cephalopods of the World: Squids, Cuttlefishes, Octopuses, and Allies. (New Jersey: TFH Publications).
Ormerod, S. J. (2003). Current issues with fish and fisheries: editor's overview and introduction. J. Appl. Ecol. 40, 204–213. doi: 10.1046/j.1365-2664.2003.00824.x
Oro, D., Bosch, M., and Ruiz, X. (1995). Effects of a trawling moratorium on the breeding success of the yellow-legged gull Larus cachinnans. Ibis 137, 547–549. doi: 10.1111/j.1474-919X.1995.tb03265.x
Pardo, D., Forcada, J., Wood, A. G., Tuck, G. N., Ireland, L., Pradel, R., et al. (2017). Additive effects of climate and fisheries drive catastrophic declines in an albatross community. Proc. Natl. Acad. Sci. U.S.A.114, E10829–E10837 doi: 10.1073/pnas.1618819114
Patrick, S. C., Bearhop, S., Bodey, T. W., Grecian, W. J., Hamer, K. C., Lee, J., et al. (2015). Individual seabirds show consistent foraging strategies in response to predictable fisheries discards. J. Avian Biol. 46, 431–440. doi: 10.1111/jav.00660
Pauly, D. (2002). Towards sustainability in world fisheries. Nature 418, 689–695. doi: 10.1038/nature01017
Pauly, D., Watson, R., and Alder, J. (2005). Global trends in world fisheries : impacts on marine ecosystems and food security rapid response email alerting service global trends in world fisheries : impacts on marine ecosystems and food security. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 5–12. doi: 10.1098/rstb.2004.1574
Phillips, K. L., Nichols, P. D., and Jackson, G. D. (2002). Lipid and fatty acid composition of the mantle and digestive gland of four Southern Ocean squid species: implications for food-web studies. Antarct. Sci. 14, 212–220. doi: 10.1017/S0954102002000044
Pitman, R. L., Walker, W. A., Everett, W. T., and Gallo-Reynoso, J. P. (2004). Population status, foods and foraging of Laysan albatrosses Phoebastria immutabilis nesting on Guadalupe Island, Mexico. Mar. Ornithol. 32, 159–165. Available online at: http://www.marineornithology.org/content/get.cgi?rn=617
Place, A. R., Stoyan, N. C., Ricklefs, R. E., and Butler, R. G. (1989). Physiological basis of stomach oil formation in Leach's storm-petrel (Oceanodroma leucorhoa). Auk 106, 687–699.
Rosen, D., and Tollit, D. J. (2012). Effects of phylogeny and prey type on fatty acid calibration coefficients in three pinniped species: implications for the QFASA dietary quantification technique. Mar. Ecol. Prog. Ser. 467, 263–276. doi: 10.3354/meps09934
Saijo, D., Mitani, Y., Abe, T., Sasaki, H., Goetsch, C., Costa, D. P., et al. (2016). Linking mesopelagic prey abundance and distribution to the foraging behavior of a deep-diving predator, the northern elephant seal. Deep Sea Res. II Top. Stud. Oceanogr. 19, 433–449. doi: 10.1016/j.dsr2.2016.11.007
Sugishita, J., Torres, L. G., and Seddon, P. J. (2015). A new approach to study of seabird-fishery overlap: connecting chick feeding with parental foraging and overlap with fishing vessels. Glob. Ecol. Conserv. 4, 632–644. doi: 10.1016/j.gecco.2015.11.001
Tew Kai, E., Benhamou, S., van Der Lingen, C. D., Coetzee, J. C., Pichegru, L., Ryan, P. G., et al. (2013). Are Cape gannets dependent upon fishery waste? a multi-scale analysis using seabird gps-tracking, hydro-acoustic surveys of pelagic fish and vessel monitoring systems. J. Appl. Ecol. 50, 659–670. doi: 10.1111/1365-2664.12086
Thiemann, G., Iverson, S., and Stirling, I. (2008). Polar bear diets and arctic marine food webs: insights from fatty acid analysis. Ecol. Monogr. 78, 591–613. doi: 10.1890/07-1050.1
Thorne, L. H., Conners, M. G, Hazen, E. L., Bograd, S. J., Antolos, M., Costa, D. P., et al. (2016). Effects of El Nino-driven changes in wind patterns on North Pacific albatrosses. J. R. Soc. Interface 13:20160196. doi: 10.1098/rsif.2016.0196
Thorne, L. H., Hazen, E. L., Bograd, S. J., Foley, D. G., Conners, M. G., Kappes, M. A., et al. (2015). Foraging behavior links climate variability and reproduction in North Pacific albatrosses. Mov. Ecol. 3, 27. doi: 10.1186/s40462-015-0050-9
Tuck, G. N., Polacheck, T., Croxall, J. P., and Weimerskirch, H. (2001). Modelling the impact of fishery by-catches on albatross populations. J. Appl. Ecol. 38, 1182–1196. doi: 10.1046/j.0021-8901.2001.00661.x
Tyson, C., Shamoun-Baranes, J., Van Loon, E. E., Camphuysen, K., and Hintzen, N. T. (2015). Individual specialization on fishery discards by lesser black-backed gulls (Larus fuscus). ICES J. Mar. Sci. 72, 1882–1891. doi: 10.1093/icesjms/fsv021
USFDA (2017). Mercury Levels in Commercial Fish and Shellfish (1990-2012). Available online at: https://www.fda.gov/food/foodborneillnesscontaminants/metals/ucm115644.htm (Accessed April 7, 2017).
Véran, S., Gimenez, O., Flint, E., Kendall, W. L., Doherty, J. R. P. F., and Lebreton, J.-D. (2007). Quantifying the impact of longline fisheries on adult survival in the black-footed albatross. J. Appl. Ecol. 44, 942–952. doi: 10.1111/j.1365-2664.2007.01346.x
Voss, N. A., Nesis, K. N., and Rodhouse, P. G. (1998). “The cephalopod family histioteuthidae (Oegopsida): systematics, biology, and biogeography (Volume II),” in Smithsonian Contributions to Zoology, 586, ed M. Sweeney (Washington, DC: Smithsonian Institution Press).
Votier, S. C., Bearhop, S., Fyfe, R., and Furness, R. W. (2008). Temporal and spatial variation in the diet of a marine top predator- links with commercial fisheries. Mar. Ecol. Prog. Ser. 367, 223–232. doi: 10.3354/meps07621
Votier, S. C., Bearhop, S., Witt, M. J., Inger, R., Thompson, D., and Newton, J. (2010). Individual responses of seabirds to commercial fisheries revealed using GPS tracking, stable isotopes and vessel monitoring systems. J. Appl. Ecol. 47, 487–497. doi: 10.1111/j.1365-2664.2010.01790.x
Walker, W. A., and Fitzgerald, S. (2012). “Preliminary results on the diet of laysan albatross and the use of fisheries by-caught marine birds in investigations of natural feeding strategy,” in Poster Presented at the 38th Annual Pacific Seabird Group Meeting. 2012 (Haleiwa).
Walker, W. A., Pitman, R. L., and Ballance, L. T. (2012). “Wanted: dead or alive? Hawaiian albarosses feed mainly by scavenging on mesopelagic cephalopods,” in Poster Presented at the 38th Annual Pacific Seabird Group Meeting. 2012. (Haleiwa).
Wang, S. W., Hollmén, T. E., and Iverson, S. J. (2009). Validating quantitative fatty acid signature analysis to estimate diets of spectacled and Steller's eiders (Somateria fischeri and Polysticta stelleri). J. Comp. Physiol. B 180, 125–139. doi: 10.1007/s00360-009-0393-x
Warham, J. (1977). The incidence, functions and ecological significance of petrel stomach oils. Proc. New Zeal. Ecol. Soc. 24, 84–93.
Warham, J., Watts, R., and Dainty, R. J. (1976). The composition, energy content and function of the stomach oils of petrels (order, procellariiformes). J. Exp. Mar. Bio. Ecol. 23, 1–13. doi: 10.1016/0022-0981(76)90081-2
Weimerskirch, H., Brothers, N., and Jouventin, P. (1997). Population dynamics of wandering albatross Diomedea exulans and Amsterdam albatross D. amsterdamensis in the Indian Ocean and their relationships with long-line fisheries: conservation implications. Biol. Conserv. 79, 257–270. doi: 10.1016/S0006-3207(96)00084-5
Wilson, R. P. (1984). An improved stomach pump for penquins and other seabirds. J. F. Ornithol. 55, 109–112.
Keywords: biochemical diet analysis, QFASA, fisheries resource management, Phoebastria, Procelleriformes, seabird-fishery interaction, seabirds, bycatch
Citation: Conners MG, Goetsch C, Budge SM, Walker WA, Mitani Y, Costa DP and Shaffer SA (2018) Fisheries Exploitation by Albatross Quantified With Lipid Analysis. Front. Mar. Sci. 5:113. doi: 10.3389/fmars.2018.00113
Received: 16 November 2017; Accepted: 15 March 2018;
Published: 04 April 2018.
Edited by:
Romuald Lipcius, Virginia Institute of Marine Science, United StatesReviewed by:
Andrew M. Fischer, University of Tasmania, AustraliaLeslie Cornick, Eastern Washington University, United States
Copyright © 2018 Conners, Goetsch, Budge, Walker, Mitani, Costa and Shaffer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melinda G. Conners, Y29ubmVyc21AZ21haWwuY29t
 Melinda G. Conners
Melinda G. Conners Chandra Goetsch
Chandra Goetsch Suzanne M. Budge
Suzanne M. Budge William A. Walker
William A. Walker Yoko Mitani
Yoko Mitani Daniel P. Costa3
Daniel P. Costa3 Scott A. Shaffer
Scott A. Shaffer