
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 06 December 2017
Sec. Marine Conservation and Sustainability
Volume 4 - 2017 | https://doi.org/10.3389/fmars.2017.00394
This article is part of the Research Topic The Ecological Significance of Marine Top Predators: From Key Stone Species To Environmental Sentinels View all 5 articles
 Audrey Jaeger1,2*
Audrey Jaeger1,2* Chris J. Feare3
Chris J. Feare3 Ron W. Summers4
Ron W. Summers4 Camille Lebarbenchon2,5
Camille Lebarbenchon2,5 Christine S. Larose6
Christine S. Larose6 Matthieu Le Corre1
Matthieu Le Corre1Migration is a fundamental aspect of the ecology and evolutionary history of many animals, driven by seasonal changes in resource availability and habitat structure. Seabird migration has been investigated extensively in highly seasonal temperate and polar environments. By contrast, the relationships between migration and seasonal environmental changes have rarely been studied in tropical marine habitats. The sooty tern Onychoprion fuscatus is the most abundant tropical seabirds, and has been ranked as the most important tropical species in terms of its annual estimated consumption of marine resources. We used global location sensing (GLS loggers) to describe for the first time the year-round at-sea distribution and activity patterns of sooty terns from a large breeding colony in the western Indian Ocean (Bird Island, Seychelles). While breeding, they foraged within 1,074 ± 274 km of the colony. After breeding, birds undertook an extensive post-breeding migration throughout the Indian Ocean; average distances traveled exceeded 50,000 km per individual. Sooty terns used mainly four distinct core oceanic areas during the non-breeding period; in the Bay of Bengal (A), northeast to an area straddling the Chagos-Laccadive plateau (B), southeast to an area on each side of the 90 East Ridge (C) and southwest to an area around Comoros (D). Individuals exhibited a high degree of fidelity to these core areas in successive years. We also established that they performed an unusual behavior for a non-Procellariiformes seabird; most individuals undertook a 1-month pre-laying exodus, during which they foraged in a specific area c. 2,000 km to the south-east of the colony. Year-round at-sea activity of sooty terns revealed that they spent only 3.72% of their time in contact with seawater, so indicating that they must sleep in flight. Activity parameters exhibited seasonal (breeding vs. non-breeding periods) and daily variations; they notably never land on the water at night. In the Seychelles, breeding sooty terns are threatened by commercial egg harvesting. Our discovery of extremely wide non-breeding at-sea distribution highlights the risk of other threats during their non-breeding period, such as over-fishing, marine pollution and climate change.
Animals from groups as diverse as mammals, birds, fish, and insects migrate across different spatial and temporal scales to cope with cyclic changes in resources and habitats (Wassenaar and Hobson, 1998; Egevang et al., 2010; Block et al., 2011). Animal migration represents a fundamental aspect of their ecology and evolutionary history, and contributes in a major way to ecosystem functioning (Bauer and Hoye, 2014; Dingle, 2014). Unraveling patterns of migration is thus essential for our understanding of the ecology, life history, and behavior of most animals (Nathan et al., 2008). Studying animal movements, particularly those of small species, is a difficult logistical and engineering challenge. Researchers have recently made considerable technological progress to track the distribution of individual animals (Bridge et al., 2011), but the movements of some species, even keystone species that have central roles in ecosystems, remain little known.
The sooty tern Onychoprion fuscatus is the most abundant tropical seabirds, with a global population estimated at 60–80 million birds (Schreiber et al., 2002). It nests in dense colonies, sometimes involving more than 1 million pairs (Feare et al., 2007), on islands scattered throughout the tropical Atlantic, Pacific and Indian Oceans. This species has been ranked fourth in terms of its abundance among world's seabirds, and the first of tropical bird species in terms of its annual estimated consumption of marine resources (Brooke, 2004). Their potential impact on marine resources is thereby likely to be significant. Despite the key role of sooty terns play in tropical marine ecosystems, some fundamental aspects of their ecology, especially their at-sea distribution, remain little known (Schreiber et al., 2002).
In terms of some life history traits, sooty terns share common characteristics with highly pelagic birds, such albatrosses, shearwaters, and tropicbirds than most other terns (Dinsmore, 1972). This includes a long lifespan (over 30 years), delayed sexual maturity (5–6 years), low reproductive rate (one egg clutch), and long incubation and fledging periods (Schreiber et al., 2002). It is generally assumed that the extreme life history traits of pelagic seabirds, such as slow growth of chicks, low fecundity, and high adult survival, result from the difficulties in finding food in the pelagic environment (Lack, 1968; Weimerskirch, 2001). According to this idea, sooty tern life history traits could be adaptations that facilitate exploitation of pelagic ecosystems (Dinsmore, 1972). Some at-sea surveys (Ballance et al., 1997; Jaquemet et al., 2005) and stable isotope analyses (Cherel et al., 2008; Young et al., 2010) support this hypothesis but the at-sea distribution and behavior of this species remain poorly known during the breeding and non-breeding periods (Schreiber et al., 2002; Soanes et al., 2015).
Developments of tracking devices have enhanced our understanding of movements of wide-ranging marine predators during both the breeding and non-breeding periods (e.g., Phillips et al., 2006; González-Solís et al., 2007; Weimerskirch et al., 2015). Until recently, device sizes restricted deployments on large seabirds. Geolocation (Global Location Sensing or GLS logging), using archival light-recording tags, offers considerable potential for tracking seabird movements even for small species (Egevang et al., 2010), over long time intervals, such as the non-breeding period (Croxall et al., 2005).
Here, we analyzed data collected using GLS deployed on sooty terns from Bird Island (Seychelles, Indian Ocean) to describe for the first time their year-round at-sea distribution and activity, and address the following questions:
(1) Animal migration is viewed as a biological phenomenon driven by seasonal changes in resource availability and habitat. Accordingly, seabirds perform large migration at polar and temperate latitudes (Croxall et al., 2005; Phillips et al., 2006; Shaffer et al., 2006; González-Solís et al., 2007; Bost et al., 2009; Egevang et al., 2010; Weimerskirch et al., 2015) where environmental conditions show marked seasonality. Seasonality in tropical zones is less marked than at higher latitudes and our study aimed to discover as the extent of migrations undertaken by this tropical species.
(2) Resources in tropical waters are also believed to be less predictable than those in polar or temperate zones, and consequently tropical seabirds should exhibit a lower foraging site fidelity than seabirds of higher latitudes (Weimerskirch, 2007). In the present study, sooty terns were tracked for 2 years in order to investigate the fidelity to their foraging areas from year to year.
(3) Based on the absence of sooty terns at their breeding colonies during the non-breeding season and the aversion of this species to landing on the water, Ashmole (1963) suggested that they do not rest on water and must sleep on the wing. We used the salt water sensor capability of the GLS to investigate the at-sea activity pattern of sooty terns in order to examine Ashmole's hypothesis.
Capture, handling and deployment of GLS devices on sooty terns were performed under research programs approved by the Center for Research on Bird Population Biology (Program 616; CRBPO; National Museum of Natural History, Paris). Fieldwork in the Seychelles was performed under the approval of the Seychelles Bureau of Standards (A0347) and the Ministry of Environment, Energy and Climate Change.
The study was carried out at a sooty tern colony of c. 500,000 pairs on Bird Island, Seychelles (3°43′S and 55°12′E). Sooty terns nest on the ground and breed annually during a breeding season, which lasts ~5–6 months. Adults arrive at the colony in April-May, females lay in June and incubation takes 28 days (Feare, 1976). Both parents share chick-rearing duties at the colony until fledging when around 8 weeks after hatching; this is followed by a period of post-fledging care (Feare, 1976). We studied the at-sea distribution and activity of individual sooty terns using GLS loggers (Mk13 and Mk18, British Antarctic Survey, Cambridge). These data loggers measured visible light intensity every minute and tested for saltwater immersion every 3 s. The maximum light level, and number of positive tests from 0 (continuously dry) to 200 (continuously wet) were stored at the end of each 10 min block. GLS loggers were attached to plastic leg rings using cable ties and deployed on the tarsus of 86 terns during two successive incubation periods (60 loggers in June 2011 and 26 loggers in June 2012). The GLS loggers were attached to birds that had been incubating for about a week to reduce the risk of desertion. The mass of the logger, ring, and cable tie was 2.6 g, below the limit of 3% of adult body mass (~190 g) recommended for flying birds (Phillips et al., 2003; Ramírez et al., 2013). GLS loggers were recovered during the 2012 and 2013 breeding seasons (incubation stage) by a team of up to five people walking slowly through the colony and catching marked birds using hand nets (Feare and Doherty, 2004). Three searches were undertaken daily in different parts of the colony such that the entire area of the nesting colony was searched every 2 days. During other studies, repeated recapture of ringed sooty terns during incubation has never led to desertion (Feare, pers. obs.), so we consider that the searches for GLS-tagged birds are unlikely to have adversely impacted the marked birds or their near neighbors. Although some established breeders on Bird Island move within the colony or to other colonies between breeding attempts (Feare and Lesperance, 2002), the majority show some degree of site fidelity and so more search effort was devoted to the areas in which birds were originally marked. We recovered 50% (N = 43) of the loggers, but we failed to download data from seven of them. Six GLS loggers were retrieved and successfully downloaded in 2012 (with data recording for 1 year: 2011–2012), 30 in 2013 (17 with data recording for 2 years: 2011–2013, and 13 for 1 year: 2012–2013).
GLS loggers record elapsed time and light level, allowing estimates of geographic position twice per day with an average spatial accuracy of 186 km for birds in flight (Phillips et al., 2004). Latitude was derived from day and night lengths and longitude from the time of local midday and midnight with respect to Greenwich Mean Time (Hill and Braun, 2001). Sooty tern locations were estimated using the “TripEstimation” package in R (Sumner et al., 2009) and following the method of Thiebot and Pinaud (2010). We did not perform the correction with sea-surface temperature because our logger models (Mk13 or Mk18) did not record seawater temperature. The dates of non-breeding, pre-laying and breeding periods were determined by identifying rapid shifts in distance from the colony. Fifty percentage (core area) and 90% (home range) kernel density distributions were used to examine at-sea distribution of sooty terns (Phillips et al., 2006; Weimerskirch et al., 2017). They were calculated with “adehabitat” packages in R (Calenge, 2006).
The GLS loggers also contained a wet/dry sensor that detects immersion in seawater and provides information on foraging/resting activity when birds make contact with the sea (Phalan et al., 2007). We calculated a common activity value derived from the immersion sensors as the percent of time spent in contact with the seawater (Phalan et al., 2007; Mackley et al., 2010a,b; Pinet et al., 2011a). We also calculated the average number of minutes per day that each bird spent in three activity types (1) “floating” (the GLS logger was continuously in contact with seawater for at least one 10 min period, indicating that the bird was sitting on the water; (2) “flying/roosting” (the GLS logger was continuously dry for at least one 10 min period, as would occur when a bird was flying or roosting on land or on floating debris); and (3) “presumed foraging” (the GLS logger recorded intermittent wet and dry states for at least one 10 min period) (McKnight et al., 2011, 2013). We finally calculated the average duration of flying and foraging bouts (Phalan et al., 2007; Mackley et al., 2010b; McKnight et al., 2011). Each activity parameter was calculated in both darkness and daylight (each 10 min block was categorized as daylight or darkness based on the light data) and for both breeding and non-breeding periods (classified with the migration dates derived from geolocation data).
Inter-annual variations were tested on departure dates from the colony, return dates to the colony, durations, distances traveled and geographical ranges during the pre-laying and non-breeding periods, and on the number of individuals that migrated into the four non-breeding core areas. We used student t-tests and χ2 tests because generalized mixed linear models (GLMM), with individuals as random effect, did not produce a better fit compared to generalized linear models (AIC, Akaike Information Criterion, difference of both models <2). The fixed effects of several temporal variations (annual, seasonal and daily) on activity parameters (response variables: percentage of time spent in contact with seawater, flying and foraging bout durations, numbers of foraging bout per night/day) were investigated using GLMM with individuals as random effect. All analyses were performed with R version 3.0.1 (R Core Team, 2016). Values are means ± standard deviations.
All recaptured sooty terns were in good physical condition and no significant difference was detected in their body mass between GLS logger deployment and recovery (N = 40, t = −1.25, p = 0.222, mean ± SD: 191.3 ± 18.3 and 196.9 ± 25.2 g, respectively) or between equipped birds and control birds randomly chosen and weighed at the same time (N = 40, t = −0.376, p = 0.708, mean ± SD: 196.9 ± 25.2 g and 199.4 ± 28.3 g, respectively).
Sooty terns tracked with GLS loggers showed a clear segregation in their core areas during the non-breeding, pre-laying and breeding periods (Figure 1). While breeding, birds were distributed around the colony and their average maximum distance from the island was 1,074 ± 274 km per individual. They departed the colony for their post-breeding migration on 30 September ± 19.0 days and remained at sea during their entire non-breeding period that lasted 6.9 ± 0.7 months (average date of return to the colony: 3 May ± 15.5 days). Total distances covered during the non-breeding period averaged 52,503 ± 6,546 km per individual. Maximum distances from the colony ranged from 2,058 to 8,681 km (4,423 ± 1,190 km). Although the non-breeding home range (90% kernel) covered a wide part of the Indian Ocean (Supplementary Figure 1), four non-breeding core areas (50% kernel) were identified (Figure 1). Eighty percentage of tracked sooty terns visited only one core area during the non-breeding period. Birds that utilized several core areas were categorized in the area in which they spent most of their time. Most birds migrated to the Bay of Bengal (A) or northeast to an area straddling the Chagos-Laccadive plateau (B) (45.2 and 35.9% of birds, respectively). Others traveled southeast to an area on each side of the 90 East Ridge (C) southwest to an area around Comoros (D) (13.2 and 5.7% of birds, respectively).
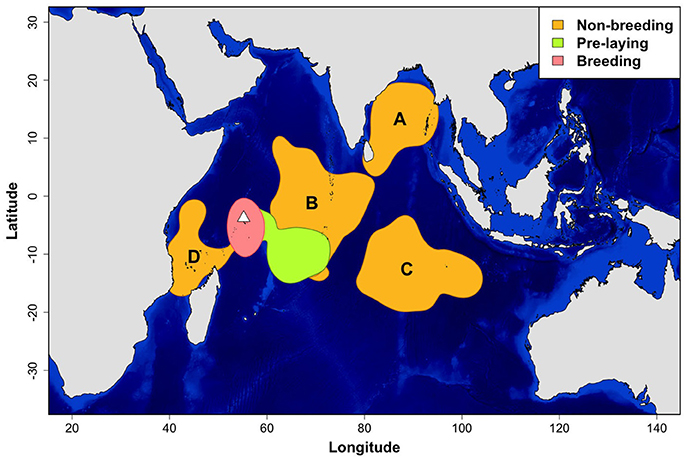
Figure 1. Density distributions of sooty terns (N = 36 birds and 53 annual tracks) from Bird Island (white triangle) during the non-breeding, pre-laying and breeding periods. Density contours encompass core areas (50% kernel). For the non-breeding period, locations were concentrated in four main areas: in the Bay of Bengal (A), on each side of the Chagos-Laccadive plateau (B), on each side of the 90 East Ridge (C), and around Comoros (D). Units are in degrees.
Sooty terns returned to the colony in April/May but only laid eggs in June/July. During the period between first arrival in the Seychelles and egg-laying, 92.5% of individuals traveled east or south-east to forage at an average maximum distance from the colony of 2182 ± 477 km (Figure 1). They performed one or two foraging trips (55.1 and 44.9% of these birds, respectively). The distance traveled was 10,972 ± 3,376 km and took place between 10 May and 6 June, and lasted 31.9 ± 12.1 days.
Seventeen individuals were tracked in 2 years. There was no significant difference in the date, duration, mean distance traveled and maximum distance from the colony during the non-breeding and pre-laying periods between the 2 years of the study (t-tests, all p > 0.367, Table 1). During the first year, birds spent more time in the area on either side of the Chagos-Laccadive plateau (B) (Table 2, χ2 = 5.010, p = 0.025). During the second year, they migrated further east to the 90 East Ridge (C) and to the Bay of Bengal (A), but these differences were not statistically significant (Table 2, χ2 tests: χ2 = 2.355, p = 0.125 and χ2 = 0.915, p = 0.339, respectively). Despite this apparent inter-annual difference in overall distribution, most individuals showed a high degree of site fidelity to their non-breeding grounds between the 2 years (64.7% of birds, see examples on Figure 2).
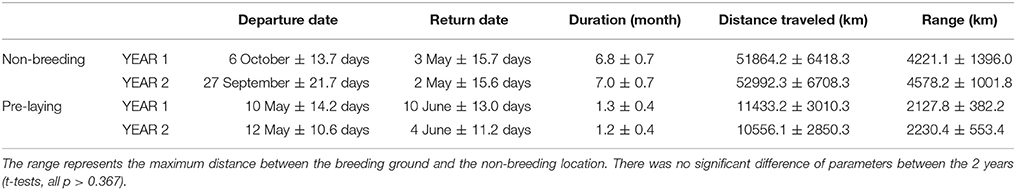
Table 1. Details of the non-breeding and pre-laying at-sea movements performed by sooty terns tracked for 2 years (YEAR1: 2011–2012 and YEAR2: 2012–2013) from Bird Island, Seychelles, Indian Ocean.
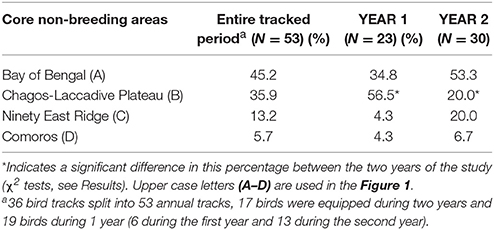
Table 2. Percentage of birds that migrated to the four distinct non-breeding grounds during the entire study period, and split between the two studied years (YEAR1: 2011–2012 and YEAR2: 2012–2013).
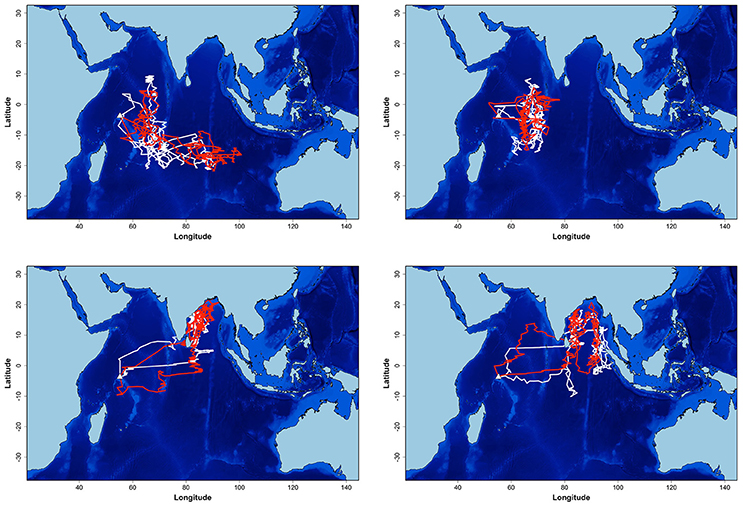
Figure 2. Example of non-breeding at-sea distribution estimation of four sooty terns from Bird Island equipped for 2 years (2011–2012 and 2012–2013). In white: the first year tracks, in red: the second year tracks. Units are in degrees.
Overall, sooty terns spent a low percentage of time in contact with seawater (3.72 ± 1.93%), and consequently a short amount of time foraging (3.59 ± 1.66 h.d−1) or floating (0.38 ± 0.20 h.d−1). Most of their time was devoted to flying (20.72 ± 1.36 h.d−1). The percent of time in contact with seawater exhibited marked daily and seasonal variations (see statistics below) but no inter-annual differences (GLMM, F-value = 0.80, p = 0.372). Firstly, on a daily basis (Figure 3), birds spent a higher percentage of time in contact with the seawater during daylight hours (6.97 ± 3.51%) than during the night (0.03 ± 0.06%; GLMM, F-value = 666.14, p < 0.0001). Accordingly, sooty terns spent 11.51 ± 0.35 h in flight, none floating and 2.23 ± 0.07 h foraging at night. The corresponding times during the day were 9.21 ± 0.96, 0.38 ± 0.17, and 3.36 ± 0.66 h, respectively. Secondly, the percentage of time spent in contact with seawater was lower during the breeding period (1.97 ± 2.15%) compared to the non-breeding period (5.03 ± 5.22%, GLMM, F-value = 129.30, p < 0.0001). This difference was significant only during the daylight period (Table 3, t-test, t = 18.43 and p < 0.0001). During the non-breeding period, birds spent more time floating and foraging during daylight (Table 3, t-tests, both p < 0.0001), and less time flying during both day and night (Table 3, t-tests, both p < 0.0001).
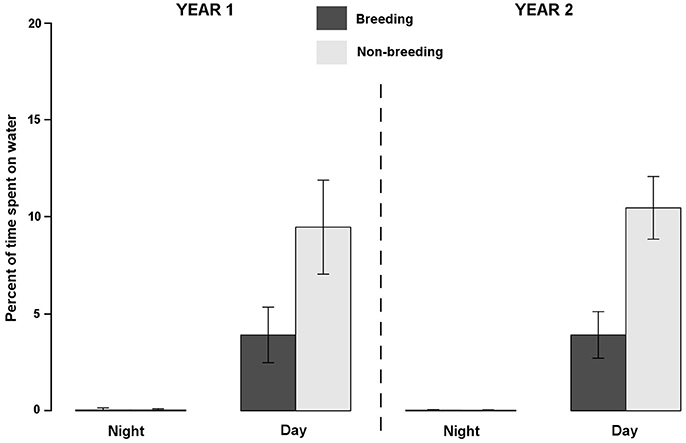
Figure 3. Inter-annual (YEAR1: 2011–2012 and YEAR2: 2012–2013), seasonal (breeding and non-breeding) and diel variations of average ± standard deviation of time spent in contact with the seawater by sooty terns from Bird Island.
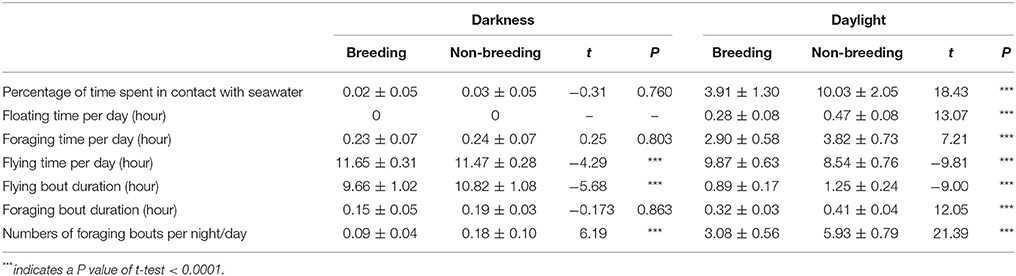
Table 3. Comparisons between darkness and daylight activity parameter values (mean ± SD) during the breeding and non-breeding seasons for sooty terns from Bird Island (N = 36).
The average duration of all foraging bouts was 0.28 ± 0.10 h (16.81 ± 6.12 min). This parameter was significantly higher during the day than at night (0.37 ± 0.06 and 0.19 ± 0.04 h, respectively; GLMM, F-value = 910.89, p < 0.0001). It was also higher during the non-breeding period for day-time values than during the breeding period (Table 3). The average duration of flying bouts was 5.65 ± 4.67 h, it was significantly shorter during day-time than night-time (1.07 ± 0.28 and 10.24 ± 1.20 h, respectively; GLMM, F-value = 10,701.30, p < 0.0001) and significantly longer during the non-breeding period (Table 3, GLMM, F-value = 73.88, p < 0.0001). The number of foraging bouts per day was 2.32 ± 2.46; there were more foraging bouts during the day than at night (4.50 ± 1.59 and 0.14 ± 0.09, respectively; GLMM, F-value = 1403.81, p < 0.0001). Further, there were more foraging bouts during the non-breeding period than during the breeding period (Table 3, GLMM, F-value = 159.19, p < 0.0001).
This study is the first to describe the non-breeding movements and behavior of sooty terns, a seabird species considered to be the world's most abundant with a correspondingly high consumption of marine resources (Brooke, 2004; Danckwerts et al., 2014). Our data revealed that Bird Island sooty terns dispersed widely over the Indian Ocean north of c. 25°S, although individuals appeared to migrate to similar geographical areas in consecutive non-breeding seasons. We also describe for the first time that sooty terns undertake a pre-breeding exodus and are among the most aerial of seabirds.
Our data show that sooty terns undertake extensive post-breeding migrations, the average distances traveled exceeded 50,000 km per individual. In comparison, wandering albatrosses Diomedea exulans perform several circumnavigations around Antarctica, covering more than 120,000 km during a year (Weimerskirch et al., 2015), arctic terns Sterna paradisaea travel more than 80,000 km between Arctic breeding grounds and Antarctic non-breeding areas (Egevang et al., 2010), and sooty shearwaters Ardenna grisea fly 60,000 km (Shaffer et al., 2006). Many seabirds perform latitudinal migrations (Shaffer et al., 2006; González-Solís et al., 2007; Egevang et al., 2010; Kopp et al., 2011) despite some exceptions (Croxall et al., 2005; Bost et al., 2009; Gaston et al., 2015; Weimerskirch et al., 2015). In the present study, most sooty terns migrated eastward, as do several other seabird species breeding in the western Indian Ocean (Catry et al., 2009; Pinet et al., 2011b; Le Corre et al., 2012). These longitudinal migrations are constrained partly by the Eurasian landmass to the north but also by cold water to the south (Kostianoy et al., 2004), acting as physical/environmental barriers for tropical Indian Ocean seabirds.
The area of the Indian Ocean frequented by non-breeding sooty terns from Bird Island is remarkably large. The four main areas identified in this study represent core areas of high density use (50% kernel contours), whilst location estimates that fell outside these core areas covered most of the Indian Ocean north of c. 25°S. Two birds, one ringed when breeding on Bird Island, the other while breeding on Juan de nova, in the Mozambique Channel, were recovered during a die-off of sooty terns on Sri Lanka in February 2011 (Tharaka Prasad, pers. comm.). This suggests that non-breeding areas are shared by birds from different breeding colonies in the western Indian Ocean. Here, sooty terns are extremely abundant with more than 6 million pairs breeding each year in widely spaced colonies, with some exceeding 1 million pairs (Feare et al., 2007). Tropical waters are less productive than in polar or temperate regions (Weimerskirch, 2007). The division of sooty tern non-breeding at-sea distribution into four core areas could be a way for them to reduce competition for scarce resources between numerous conspecifics in the Indian Ocean.
In the present study, the fidelity of individuals to a core area in successive years could imply that individual migration patterns are fixed. Several other studies have already reported this behavior (Bradshaw et al., 2004; Broderick et al., 2007; Phillips et al., 2007; Yamamoto et al., 2014; Ramírez et al., 2016), suggesting that the individual fidelity to a specific non-breeding site is a relatively common characteristic among migratory marine animals. Further data from other breeding colonies are needed to understand how birds select their non-breeding foraging areas, whether they are selected when young through individual long-term selection processes (Péron and Grémillet, 2013), and how the movements to multiple non-breeding feeding areas by birds from a single colony evolved. A study of the distributions and habitats of young sooty terns during the 5–6 years prior to their first breeding attempt will be needed to ascertain at what age distant foraging sites are selected. Longer-term data with tracking in years with different oceanic conditions will be also necessary to confirm site fidelity to non-breeding grounds.
In addition to the wide at-sea distribution of sooty terns revealed in this investigation, the study is the first to demonstrate that sooty terns undertake a pre-laying exodus between the arrival at the colony in May and egg laying in June. This behavior is common in petrels and shearwaters (Warham, 1964) but has not previously been observed in terns, and has been recorded in few other non-procellariid seabirds (Phillips et al., 2007). The pre-laying exodus is considered as a strategy available to Procellariiformes because they are able to carry out long-distance foraging trips (Warham, 1964). Having re-established their nest sites and pair bonds at the start of the breeding season, the females, and in some species also the males, desert the breeding area for a period of days or weeks immediately before egg laying (Phillips et al., 2006, 2007). This behavior provides a period of uninterrupted foraging, during which birds build the reserves required to produce eggs (for females) and to prepare for their food deprivation periods during long shifts of incubation (Warham, 1964).
The core area used during the pre-laying exodus of Bird Island sooty terns included the Saya de Malha Bank, a submarine plateau c. 850–1,100 km south-east of Bird Island, and the Chagos Bank, a large plateau at the south of the Chagos-Laccadive Ridge (Figure 1). These are areas of high primary productivity (Burnett et al., 2001; Sheppard et al., 2012) and presumably offers good feeding opportunities. Nonetheless, birds that undertook a pre-laying exodus traveled on average around 10,000 km. This additional energetic cost immediately following the long-distant return migration suggests that the area used is predictably productive. The relative proximity of high productivity areas may facilitate the establishment of large sooty colonies on nearby islands, as in the western Indian Ocean. Some birds were recorded making two trips to the pre-laying core area, possibly arising from birds losing their first egg and preparing to lay a replacement.
Overall, sooty terns spent 3.72% of their time in contact with the seawater. This is very low compared to other seabirds (Mackley et al., 2010a,b; Pinet et al., 2011a), including tern species (Nisbet et al., 2011). Arctic terns S. paradisaea also exhibited low values of time spent floating; the authors suggested that this behavior was virtually absent because they rest on logs, basking turtles or floating ice (McKnight et al., 2013). In fact, a low percent of time spent on water (i.e., a dry sensor) should imply that birds, (1) rest on floating debris or on land, (2) withdraw one leg and/or foot into the plumage while resting at sea (Linnebjerg et al., 2014), or (3) fly with few resting periods on water. In the present study, the estimated locations and the core areas of distribution were mainly over ocean instead of land, indicating that sooty terns do not rest on land. Moreover, the average movement speed measures, from estimated locations during non-breeding nights and days, were 10.03 ± 6.45 and 10.02 ± 6.36 km.h−1, respectively. These results indicate that birds were moving and thus did not rest on floating debris or on water.
Sooty terns experience difficulty in taking off from the surface of the water (Mahoney, 1984) and become waterlogged after resting on water (Watson and Lashley, 1915). Their uropygial gland produces preen oil with less lipid content than in terns that frequently land on the water (Johnston, 1979). These results have led to speculation that sooty terns have poor feather waterproofing and do not rest on water (Ashmole, 1963; Dinsmore, 1972). In this study, we recorded extended non-stop flights at night (more than 10 h per night) and almost no resting time on the water during the day (<30 min per day). This seems to confirm the long-held belief that sooty terns are among the most aerial of birds, and sleep in flight (Ashmole, 1963; Schreiber et al., 2002). Some other seabird species, such as frigatebirds, are also suspected of sleeping in flight (Rattenborg, 2006).
Sooty tern diet during the breeding season comprises small epipelagic fish, fish larvae and flying squid. The proportions of these main dietary constituents vary between colonies (Jaquemet et al., 2008), within breeding seasons (Feare, 1976) and between years (Feare, unpublished data), suggesting opportunistic foraging. Prey are obtained at or above the sea surface through association with schools of predatory fish, especially tuna. These drive their prey to the surface, making them available to sooty terns. The diet of sooty terns during migration is unknown.
In this study, we showed that most of the time spent in contact with seawater is foraging behavior (89.9%) with almost no floating on the sea surface (10.1%). Foraging bouts occurred mainly during daytime, suggesting sooty terns mainly fed during the day. At-sea observations revealed that sooty terns normally feed on the wing by “air dipping” or “contact dipping” and occasionally by briefly plunging into the surface without complete submersion (Ashmole and Ashmole, 1967). During ocean voyages in the Seychelles, c. 30 flocks of <500 feeding sooty terns were observed, always in association with predatory fish, such as tuna and mackerel (Feare, unpublished data). Most commonly, the birds chased prey at the water surface or above it by means of highly aerobatic maneuvers and did not settle on the water. This likely accounted for the low values of contact with seawater recorded by the GLS loggers.
This is the first study describing the at-sea movements of the sooty tern, the most abundant tropical seabird. The results support the long-held belief that the sooty tern is one of the most pelagic of all terns. Those that breed on Bird Island (Seychelles), have extensive longitudinal migrations and four large pelagic non-breeding core areas. They practically never enter the water and probably sleep in flight. The findings of this study have also important consequences for sooty tern conservation. Our finding that different individuals of the Bird Island population utilize different core areas during the non-breeding period, and yet another area for the pre-laying exodus, shows that the birds can be vulnerable to adverse conditions far from the breeding colonies. These can include excessive commercial exploitation of pelagic predatory fish, especially tuna (Pillai and Satheeshkumar, 2013; Danckwerts et al., 2014). The most used core area, the Bay of Bengal, is highly contaminated by industrial pollutants and by plastic microparticles (Eriksen et al., 2014). The latter have a capacity to adsorb and concentrate toxicants and pharmaceuticals (Gaw et al., 2014). Some of these could have implications for the birds' breeding performance when in Seychelles. The northern Indian Ocean, and especially the Bay of Bengal, is prone to cyclones, whose frequency and intensity are increasing (Singh et al., 2001). This has been associated with increasing atmospheric pollution (Evan et al., 2011). The 2011 die-off of sooty terns in Sri Lanka, mentioned above, was contemporaneous with the development of a tropical depression off the east coast of Sri Lanka (IMD, 2011). This suggests that while the use of large core foraging areas might buffer sooty terns against some vagaries in food availability, there are risks that environmental changes in some core areas could threaten birds that utilize these.
AJ helped design the study, collected field data, performed data analysis and drafted the manuscript. CF conceived and designed the study, collected field data and drafted the manuscript. RS helped design the study, collected field data and helped draft the manuscript. CL and CSL collected field data. ML helped design the study and helped draft the manuscript.
Funding for this study was provided by the Percy Sladen Memorial Fund, James Cadbury, Robert Gaines-Cooper, Kang Nee, Brian and Margaret Jasper, Amanda O'Keefe, Colin and Fiona Short and WildWings Bird Management. ML provided GLS with funding from the Pew Environment Group (Pew Fellowship Award in Marine Conservation). AJ and CL post-doctoral fellowships were funded by the European Union's Seventh Framework Program (FP7/2007-2013; under grant agreement n°263958).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are very grateful to Marie France and Guy Savy for their warm hospitality on Bird Island and their continuing support of island's seabird studies. Muriel Dietrich and Bozena Kalejta-Summers are thanked for invaluable assistance in the field.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2017.00394/full#supplementary-material
Supplementary Figure 1. Density distributions of sooty terns (N = 36 birds and 53 annual tracks) from Bird Island (white triangle) during the non-breeding, pre-laying and breeding periods Density contours encompass home ranges (90% kernel). For the non-breeding period, locations were concentrated in four main areas: in the Bay of Bengal (A), on each side of the Chagos-Laccadive plateau (B), on each side of the 90 East Ridge (C), and around Comoros (D). Units are in degrees.
Ashmole, N. P. (1963). The biology of the wide-awake or sooty tern Sterna fuscata on ascension Island. Ibis 103, 297–364. doi: 10.1111/j.1474-919X.1963.tb06757.x
Ashmole, N. P., and Ashmole, M. J. (1967). Comparative Feeding Ecology of Sea Birds of A Tropical Oceanic Island. New Haven: Peabody Museum of Natural History, Yale University.
Ballance, L. T., Pitman, R. L., and Reilly, S. B. (1997). Seabird community structure along a productivity gradient: importance of competition and energetic constraint. Ecology 78, 1502–1518. doi: 10.1890/0012-9658(1997)078[1502:SCSAAP]2.0.CO;2
Bauer, S., and Hoye, B. J. (2014). Migratory animals couple biodiversity and ecosystem functioning worldwide. Science 344:1242552. doi: 10.1126/science.1242552
Block, B. A., Jonsen, I. D., Jorgensen, S. J., Winship, A. J., Shaffer, S. A., Bograd, S. J., et al. (2011). Tracking apex marine predator movements in a dynamic ocean. Nature 475, 86–90. doi: 10.1038/nature10082
Bost, C. A., Thiebot, J. B., Pinaud, D., Cherel, Y., and Trathan, P. N. (2009). Where do penguins go during the inter-breeding period? Using geolocation to track the winter dispersion of the macaroni penguin. Biol. Lett. 5, 473–476. doi: 10.1098/rsbl.2009.0265
Bradshaw, C. J. A., Hindell, M. A., Sumner, M. D., and Michael, K. J. (2004). Loyalty pays: potential life history consequences of fidelity to marine foraging regions by southern elephant seals. Anim. Behav. 68, 1349–1360. doi: 10.1016/j.anbehav.2003.12.013
Bridge, E. S., Thorup, K., Bowlin, M. S., Chilson, P. B., Diehl, R. H., Fléron, R. W., et al. (2011). Technology on the move: recent and forthcoming innovations for tracking migratory birds. Bioscience 61, 689–698. doi: 10.1525/bio.2011.61.9.7
Broderick, A. C., Coyne, M. S., Fuller, W. J., Glen, F., and Godley, B. J. (2007). Fidelity and over-wintering of sea turtles. Proc. R. Soc. B 274, 1533–1539. doi: 10.1098/rspb.2007.0211
Brooke, M. (2004). The food consumption of the world's seabirds. Proc. R. Soc. B 271, S246–S248. doi: 10.1098/rsbl.2003.0153
Burnett, J. C., Kavanagh, J. S., and Spencer, T. (2001). Marine Science, Training and Education in the Western Indian Ocean: Shoals of Capricorn Programme Field Report 1998–2001. London: Royal Geographical Society (with the Institute of British Geographers).
Calenge, C. (2006). The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol. Model. 197, 516–519. doi: 10.1016/j.ecolmodel.2006.03.017
Catry, T., Catry, T., Ramos, J. A., Ramos, J. A., Le Corre, M., Le Corre, M., et al. (2009). Movements, at-sea distribution and behaviour of a tropical pelagic seabird: the wedge-tailed shearwater in the western Indian Ocean. Mar. Ecol. Prog. Ser. 391, 231–242. doi: 10.3354/meps07717
Cherel, Y., Corre, M. L., Jaquemet, S., Ménard, F., Richard, P., and Weimerskirch, H. (2008). Resource partitioning within a tropical seabird community: new information from stable isotopes. Mar. Ecol. Prog. Ser. 366, 281–291. doi: 10.3354/meps07587
Croxall, J. P., Silk, J. R., Phillips, R. A., Afanasyev, V., and Briggs, D. R. (2005). Global circumnavigations: tracking year-round ranges of nonbreeding albatrosses. Science 307, 249–250. doi: 10.1126/science.1106042
Danckwerts, D. K., McQuaid, C. D., Jaeger, A., McGregor, G. K., Dwight, R., and Le Corre, M. (2014). Biomass consumption by breeding seabirds in the western Indian Ocean: indirect associations with fisheries and implications for management. ICES J. Mar. Sci. 71, 2589–2598. doi: 10.1093/icesjms/fsu093
Egevang, C., Stenhouse, I. J., Phillips, R. A., Petersen, A., Fox, J. W., and Silk, J. R. D. (2010). Tracking of Arctic terns Sterna paradisaea reveals longest animal migration. Proc. Natl. Acad. Sci. U.S.A. 107, 2078–2081. doi: 10.1073/pnas.0909493107
Eriksen, M., Lebreton, L. C. M., Carson, H. S., Thiel, M., Moore, C. J., Borerro, J. C., et al. (2014). Plastic pollution in the world's oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 9:e111913. doi: 10.1371/journal.pone.0111913
Evan, A. T., Kossin, J. P., Chung, C., and Ramathanan, V. (2011). Arabian Sea tropical cyclones intensified by emissions of black carbon and other aerosols. Nature 479, 94–97. doi: 10.1038/nature10552
Feare, C. J. (1976). The breeding of the sooty tern Sterna fuscata in the Seychelles and the effects of experimental removal of its eggs. J. Zool. 179, 317–360. doi: 10.1111/j.1469-7998.1976.tb02299.x
Feare, C. J., and Doherty, P. F. (2004). Survival estimates of adult sooty terns Sterna fuscata from Bird Island, Seychelles. Ibis 146, 475–480. doi: 10.1111/j.1474-919x.2004.00288.x
Feare, C. J., and Lesperance, C. (2002). Intra-and inter-colony movements of breeding adult sooty terns in Seychelles. Waterbirds 25, 52–55. doi: 10.1675/1524-4695(2002)025[0052:IAIMOB]2.0.CO;2
Feare, C. J., Jaquemet, S., and Le Corre, M. (2007). An inventory of sooty terns (Sterna fuscata) in the western Indian Ocean with special reference to threats and trends. Ostrich 78, 423–434. doi: 10.2989/OSTRICH.2007.78.2.49.129
Gaston, A. J., Hashimoto, Y., and Wilson, L. (2015). First evidence of east–west migration across the North Pacific in a marine bird. Ibis 157, 877–882. doi: 10.1111/ibi.12300
Gaw, S., Thomas, K. V., and Hutchinson, T. H. (2014). Sources, impacts and trends of pharmaceuticals in the marine and coastal environment. Philos. Trans. R. Soc. B 369, 20130572–20130572. doi: 10.1098/rstb.2013.0572
González-Solís, J., Croxall, J. P., Oro, D., and Ruiz, X. (2007). Trans-equatorial migration and mixing in the wintering areas of a pelagic seabird. Front. Ecol. Environ. 5:2. doi: 10.1890/1540-9295(2007)5[297:TMAMIT]2.0.CO;2
Hill, R. D., and Braun, M. J. (2001). Geolocation by Light Level. In Electronic Tagging and Tracking in Marine Fisheries. Dordrecht: Springer Netherlands.
IMD (2011). IMD Cyclonic Bulletin 04 for Depression BOB 01. India Meteorological Department. Available online at: https://www.webcitation.org/5wCHwmPFv?url=http://www.imd.gov.in/section/nhac/dynamic/cwind.htm. (Accessed October 5, 2011).
Jaquemet, S., Le Corre, M., Marsac, F., Potier, M., and Weimerskirch, H. (2005). Foraging habitats of the seabird community of Europa Island (Mozambique Channel). Mar. Biol. 147, 573–582. doi: 10.1007/s00227-005-1610-0
Jaquemet, S., Potier, M., Cherel, Y., Kojadinovic, J., Bustamente, P., and Le Corre, M. (2008). Comparative foraging ecology and ecological niche of a superabundant tropical seabird: the sooty tern Sterna fuscata in the southwest Indian Ocean. Mar. Biol. 155, 505–520. doi: 10.1007/s00227-008-1049-1
Johnston, D. W. (1979). The uropygial gland of the sooty tern. Condor 81, 430–432. doi: 10.2307/1366977
Kopp, M., Peter, H. U., Mustafa, O., Lisovski, S., Ritz, M. S., Phillips, R. A., et al. (2011). South polar skuas from a single breeding population overwinter in different oceans though show similar migration patterns. Mar. Ecol. Prog. Ser. 435, 263–267. doi: 10.3354/meps09229
Kostianoy, A. G., Ginzburg, A. I., Frankignoulle, M., and Delille, B. (2004). Fronts in the Southern Indian Ocean as inferred from satellite sea surface temperature data. J. Mar. Syst. 45, 55–73. doi: 10.1016/j.jmarsys.2003.09.004
Le Corre, M., Jaeger, A., Pinet, P., Kappes, M. A., Weimerskirch, H., Catry, T., et al. (2012). Tracking seabirds to identify potential marine protected areas in the tropical western Indian Ocean. Biol. Conserv. 156, 83–93. doi: 10.1016/j.biocon.2011.11.015
Linnebjerg, J. F., Huffeldt, N. P., Falk, K., Merkel, F. R., Mosbech, A., and Frederiksen, M. (2014). Inferring seabird activity budgets from leg-mounted time–depth recorders. J. Ornithol. 155, 301–306. doi: 10.1007/s10336-013-1015-7
Mackley, E. K., Phillips, R. A., Silk, J. R., Wakefield, E. D., Afanasyev, V., Fox, J. W., et al. (2010a). Free as a bird? Activity patterns of albatrosses during the nonbreeding period. Mar. Ecol. Prog. Ser. 406, 291–303. doi: 10.3354/meps08532
Mackley, E. K., Phillips, R. A., Silk, J. R., Wakefield, E. D., Afanasyev, V., and Furness, R. W. (2010b). At-sea activity patterns of breeding and nonbreeding white-chinned petrels Procellaria aequinoctialis from South Georgia. Mar. Biol. 158, 429–438. doi: 10.1007/s00227-010-1570-x
McKnight, A., Allyn, A. J., Duffy, D. C., and Irons, D. B. (2013). Stepping stone pattern in Pacific Arctic tern migration reveals the importance of upwelling areas. Mar. Ecol. Prog. Ser. 491, 253–264. doi: 10.3354/meps10469
McKnight, A., Irons, D. B., Allyn, A. J., Sullivan, K. M., and Suryan, R. M. (2011). Winter dispersal and activity patterns of post-breeding black-legged kittiwakes Rissa tridactyla from Prince William Sound, Alaska. Mar. Ecol. Prog. Ser. 442, 241–253. doi: 10.3354/meps09373
Nathan, R., Getz, W. M., Revilla, E., Holyoak, M., Kadmon, R., Saltz, D., et al. (2008). A movement ecology paradigm for unifying organismal movement research. Proc. Natl. Acad. Sci. U.S.A. 105, 19052–19059. doi: 10.1073/pnas.0800375105
Nisbet, I. C. T., Mostello, C. S., Veit, R. R., Fox, J. W., and Afanasyev, V. (2011). Migrations and winter quarters of five common terns tracked using geolocators. Waterbirds 34, 32–39. doi: 10.1675/063.034.0104
Péron, C., and Grémillet, D. (2013). Tracking through life stages: adult, immature and juvenile autumn migration in a long-lived seabird. PLoS ONE 8:e72713. doi: 10.1371/journal.pone.0072713
Phalan, B., Phillips, R. A., Silk, J., and Afanasyev, V. (2007). Foraging behaviour of four albatross species by night and day. Mar. Ecol. Prog. Ser. 340, 271–286. doi: 10.3354/meps340271
Phillips, R. A., Catry, P., Silk, J., Bearhop, S., McGill, R., Afanasyev, V., et al. (2007). Movements, winter distribution and activity patterns of Falkland and brown skuas: insights from loggers and isotopes. Mar. Ecol. Prog. Ser. 345, 281–291. doi: 10.3354/meps06991
Phillips, R. A., Silk, J. R. D., Croxall, J. P., Afanasyev, V., and Briggs, D. R. (2004). Accuracy of geolocation estimates for flying seabirds. Mar. Ecol. Prog. Ser. 266, 265–272. doi: 10.3354/meps266265
Phillips, R. A., Silk, J. R. D., Croxall, J. P., and Afanasyev, V. (2006). Year-round distribution of white-chinned petrels from South Georgia: relationships with oceanography and fisheries. Biol. Conserv. 129, 336–347. doi: 10.1016/j.biocon.2005.10.046
Phillips, R. A., Xavier, J. C., Croxall, J. P., and Burger, A. E. (2003). Effects of satellite transmitters on albatrosses and petrels. Auk 120, 1082–1090. doi: 10.1642/0004-8038(2003)120[1082:EOSTOA]2.0.CO;2
Pillai, N., and Satheeshkumar, P. (2013). Conservation and management of tuna fisheries in the Indian Ocean and Indian, E. E. Z. Int. J. Mar. Sci. 3, 187–192. doi: 10.5376/ijms.2013.03.0024
Pinet, P., Jaeger, A., Cordier, E., Potin, G., and Le Corre, M. (2011a). Celestial moderation of tropical seabird behavior. PLoS ONE 6:e27663. doi: 10.1371/journal.pone.0027663
Pinet, P., Jaquemet, S., Pinaud, D., Weimerskirch, H., Phillips, R. A., and Le Corre, M. (2011b). Migration, wintering distribution and habitat use of an endangered tropical seabird, Barau's petrel Pterodroma baraui. Mar. Ecol. Prog. Ser. 423, 291–302. doi: 10.3354/meps08971
Ramírez, I., Paiva, V. H., Fagundes, I., Menezes, D., Silva, I., Ceia, F. R., et al. (2016). Conservation implications of consistent foraging and trophic ecology in a rare petrel species. Anim. Conserv. 19, 139–152 doi: 10.1111/acv.12227
Ramírez, I., Paiva, V. H., Menezes, D., Silva, I., Phillips, R. A., Ramos, J. A., et al. (2013). Year-round distribution and habitat preferences of the Bugio petrel. Mar. Ecol. Prog. Ser. 476, 269–284 doi: 10.3354/meps10083
R Core Team (2016). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org/.
Rattenborg, N. C. (2006). Do birds sleep in flight? Naturwissenschaften 93, 413–425. doi: 10.1007/s00114-006-0120-3
Schreiber, E. A., Feare, C. J., Harrington, B. A., Murray, B. G., Robertson, W., Robertson, M. J., et al. (2002). Sooty tern (Sterna Fuscata). Birds North Am. 665, 1–31. doi: 10.2173/bna.665
Shaffer, S. A., Tremblay, Y., Weimerskirch, H., Scott, D., Thompson, D. R., Sagar, P. M., et al. (2006). Migratory shearwaters integrate oceanic resources across the Pacific Ocean in an endless summer. Proc. Natl. Acad. Sci. U.S.A. 103, 12799–12802. doi: 10.1073/pnas.0603715103
Sheppard, C. R. C., Ateweberhan, M., Bowen, B. W., Carr, P., Chen, C. A., Clubbe, C., et al. (2012). Reefs and islands of the Chagos Archipelago, Indian Ocean: why it is the world's largest no-take marine protected area. Aqu. Conserv. 22, 232–261. doi: 10.1002/aqc.1248
Singh, O. P., Kahn, A., and Rahman, S. (2001). Has the frequency of intense tropical cyclones increased in the North Indian Ocean? Curr. Sci. 80, 575–580. Available online at: http://www.jstor.org/stable/24104250
Soanes, L. M., Bright, J. A., Brodin, G., Mukhida, F., and Green, J. A. (2015). Tracking a small seabird: first records of foraging movements in the sooty tern Onychoprion fuscatus. Mar. Ornithol. 43, 235–239.
Sumner, M. D., Sumner, M. D., Wotherspoon, S. J., Wotherspoon, S. J., Hindell, M. A., and Hindell, M. A. (2009). Bayesian estimation of animal movement from archival and satellite tags. PLoS ONE 4:e7324. doi: 10.1371/journal.pone.0007324
Thiebot, J.-B., and Pinaud, D. (2010). Quantitative method to estimate species habitat use from light-based geolocation data. Endang. Species. Res. 10, 341–353. doi: 10.3354/esr00261
Wassenaar, L. I., and Hobson, A. (1998). Natal origins of migratory monarch butterflies at wintering colonies in Mexico: new isotopic evidence. Proc. Natl. Acad. Sci. U.S.A. 95, 15436–15439. doi: 10.1073/pnas.95.26.15436
Watson, J. B., and Lashley, K. S. (1915). Homing and Related Activities of Birds. Washington, DC: Carnegie institution of Washington.
Weimerskirch, H. (2001). “Seabird demography and its relationship with the marine environment,” in Biology of Marine Birds, eds E. A. Schreiber and J. Burger (Boca Raton, FL: CRC Press), 115–136.
Weimerskirch, H. (2007). Are seabirds foraging for unpredictable resources? Deep Sea Res. 54, 211–223. doi: 10.1016/j.dsr2.2006.11.013
Weimerskirch, H., Borsa, P., Cruz, S., Grissac, S., Gardes, L., Lallemand, J., et al. (2017). Diversity of migration strategies among great frigatebirds populations. J. Avian Biol. 48, 103–113. doi: 10.1111/jav.01330
Weimerskirch, H., Delord, K., Guitteaud, A., Phillips, R. A., and Pinet, P. (2015). Extreme variation in migration strategies between and within wandering albatross populations during their sabbatical year, and their fitness consequences. Sci. Rep. 5:8853. doi: 10.1038/srep08853
Yamamoto, T., Takahashi, A., and Sato, K. (2014). Individual consistency in migratory behaviour of a pelagic seabird. Behaviour 151, 683–701. doi: 10.1163/1568539X-00003163
Keywords: at-sea distribution, at-sea activity, GLS, non-breeding, pre-laying, sooty tern, Indian Ocean, seabird
Citation: Jaeger A, Feare CJ, Summers RW, Lebarbenchon C, Larose CS and Le Corre M (2017) Geolocation Reveals Year-Round at-Sea Distribution and Activity of a Superabundant Tropical Seabird, the Sooty Tern Onychoprion fuscatus. Front. Mar. Sci. 4:394. doi: 10.3389/fmars.2017.00394
Received: 30 July 2017; Accepted: 22 November 2017;
Published: 06 December 2017.
Edited by:
Filipe Rafael Ceia, University of Coimbra, PortugalReviewed by:
Lucas Krüger, Centro de Ciências do Mar e do Ambiente (MARE), PortugalCopyright © 2017 Jaeger, Feare, Summers, Lebarbenchon, Larose and Le Corre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Audrey Jaeger, YXVkcmV5LmphZWdlckB1bml2LXJldW5pb24uZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.