
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
TECHNOLOGY REPORT article
Front. Mar. Sci. , 12 October 2017
Sec. Global Change and the Future Ocean
Volume 4 - 2017 | https://doi.org/10.3389/fmars.2017.00321
 Lydia Kapsenberg1*
Lydia Kapsenberg1* Emily E. Bockmon2
Emily E. Bockmon2 Philip J. Bresnahan2
Philip J. Bresnahan2 Kristy J. Kroeker3
Kristy J. Kroeker3 Jean-Pierre Gattuso1,4
Jean-Pierre Gattuso1,4 Todd R. Martz2
Todd R. Martz2Research assessing the biological impacts of global ocean change often requires a burdensome characterization of seawater carbonate chemistry. For laboratory-based ocean acidification research, this impedes the scope of experimental design. Honeywell Durafet® III pH electrodes provide precise and continuous seawater pH measurements. In addition to use in oceanographic sensor packages, Durafets can also be used in the laboratory to track and control seawater treatments via Honeywell Universal Dual Analyzers (UDAs). Here we provide performance data, instructions, and step-by-step recommendations for use of multiple UDA-Durafets. Durafet pH measurements were within ±0.005 units pHT of spectrophotometric measurements and agreement among eight Durafets was better than ±0.005 units pHT. These results indicate equal performance to Durafets in oceanographic sensor packages, but methods for calibration and quality control differ. Use of UDA-Durafets vastly improves time-course documentation of experimental conditions and reduces person-hours dedicated to this activity. Due to the versatility of integrating Durafets in laboratory seawater systems, this technology opens the door to advance the scale of questions that the ocean acidification research community aims to address.
Seawater absorption of carbon dioxide (CO2) emitted by fossil fuel burning and land use change causes a decline in ocean pH, a process known as anthropogenic ocean acidification (Rhein et al., 2013). The expected magnitude of ocean acidification over the coming decades can be estimated from various CO2 emission scenarios and climate mitigation efforts (Ciais et al., 2013). These projections have resulted in a world-wide proliferation of biological studies aiming to quantify the effects of ocean acidification on marine species and ecosystem function (Yang et al., 2016). Critical to these experiments are proper experimental design and documentation of experimental conditions via established practices (Dickson et al., 2007; Riebesell et al., 2010). In ocean acidification biology, documenting seawater carbonate chemistry over the course of an experiment can be extremely time consuming and expensive, reducing resources that could otherwise be invested in quantifying the biological response of interest. In addition, emerging interest in studying the effects of pH variability requires a continuous documentation of experimental conditions. In this paper, we present a method to improve high-frequency documentation of experimental conditions, while at the same time reducing the burden of conducting laboratory-based ocean acidification research.
Our method uses commercially available Honeywell™ Durafet® III pH electrodes. Durafets have been demonstrated to be among the best pH electrodes for continuous measurement of seawater pH and have been incorporated into various autonomous and shipboard underway oceanographic sensor packages (Martz et al., 2010; Bresnahan et al., 2014; Johnson et al., 2016). The value of this electrode lies in its stability and precision over time, achieved by use of Ion Sensitive Field Effect Transistor (ISFET) technology (Martz et al., 2010; Bresnahan et al., 2014). Its near-ideal Nernstian response results in the ability to document pH variation smaller than 0.005 units and performs reliably in the natural range of seawater pH (pHT ~ 7.0–8.5; Takeshita et al., 2014). Calibration of Durafets can be performed at any given pH in this range and remains effective across a wide range of pH conditions and time frames. This also means that, as it does for all sensors, the accuracy of pH data retrieved from Durafets is only as reliable as the calibration itself.
While several research laboratories already use Durafets for biological experiments (Bockmon et al., 2013; O'Donnell et al., 2013; Keppel et al., 2015; Clark and Gobler, 2016), the use of Durafets and processing of the associated data have not yet been described or standardized for seawater applications. In this paper, we test the performance of Durafets using the Universal Dual Analyzer 2182 setup, and recommend a step-by-step protocol for the use in ocean acidification biology experiments. In association with our protocol, we give detailed instructions for setting up sensor communication with LabVIEW™ and provide the LabVIEW VI we developed to log time series data.
The following methods describe communication setup and steps for calibration, sensor performance assessment, and data reporting. These steps, in chronological order, are mentioned throughout the manuscript and used to test Durafets in two separate experiments assessing (i) performance of multiple Durafets and (ii) application in an experimental system.
Honeywell currently provides four options to interface with Durafet electrodes: the Universal Dual Analyzer 2182 (UDA, used in this study), the Analytical Process Analyzer (APT2000/4000), the DirectLine Module (DL421) and the Cap Adapter. The Cap Adapter interface provides only the unconditioned (raw) pH signal and is susceptible to noise pickup (e.g., variable supply voltage and ambient electric fields). Our past work details how to process the raw voltage signal from the Cap Adapter for a Durafet in seawater (Bresnahan et al., 2014), but these methods are not recommended for routine laboratory use due to susceptibility to noise pickup. The other three options perform both temperature compensation using a thermistor embedded in the Durafet and signal conditioning to remove noise. Although, the DL421 is capable of transmitting a conditioned analog pH signal, this device does not transmit the temperature data. The APT series displays temperature data but is less versatile than the UDA. The UDA is the preferred analyzer due to its ability to transmit conditioned pH and temperature signals from multiple Durafets.
Each UDA contains two sensor inputs that are configured individually by inserting an input card specific to a Honeywell sensor (e.g., pH, dissolved oxygen, conductivity, oxidation-reduction potential; Honeywell, 2009). In this case, we configured both inputs with the Durafet pH card (Table 1).
The UDA displays and transmits data from dual channels to a user-supplied device (e.g., a PC for data storage). Data may be transmitted by either the built-in analog output or digitally (RS485 or Ethernet), after installing a communication card. The analog output can seem like an obvious choice as it can immediately be integrated into existing systems. However, it is important to note that: (i) in transmitting the analog output, additional noise and signal attenuation may be introduced to the analytical signal adding increased uncertainty to the resulting pH, and (ii) the analog output does not include the temperature measurement associated with each Durafet, while the digital output does. The digital output is therefore preferable for use in laboratory settings.
Four UDA-Durafet units were routed through an Ethernet switch, in order to transmit eight pH and temperature data streams to one computer for data logging (Figure 1). A customized National Instruments LabVIEW™ (2013) program was developed in order to control signal averaging and sampling frequency (Supplementary Material. Following methods for Durafets in oceanographic sensor packages, signal averaging was set to 10 measurements per data point, resulting in a maximum sampling frequency of 1 min. For experiments lasting longer than a day, a sampling frequency of 5 min was used. As resolution of the UDA digital output (<0.0001 units pH) is much greater than that of our calibration methods (0.001), data logging was set to a resolution of 0.001.
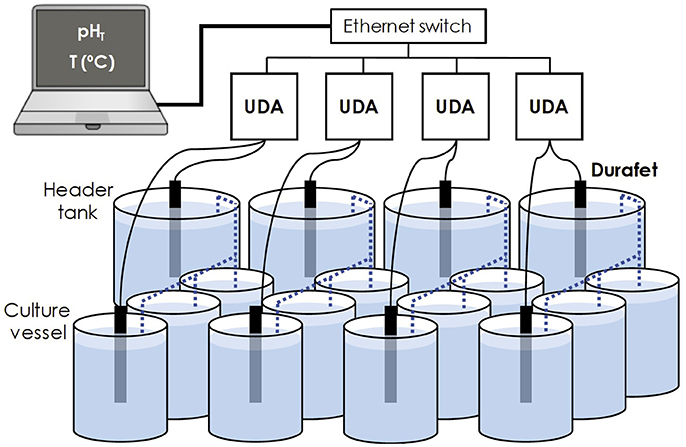
Figure 1. Diagram of four UDA units with a total of eight Durafet pH electrodes in an experimental set up. This design includes four experimental treatments, each of which have three, downstream, (pseudo)replicate larval culture vessels. Dashed lines indicate seawater connections.
UDAs will display a temperature-compensated factory-calibrated value for pH (NBS scale) and temperature for each Durafet. We note that it is necessary to distinguish between the built-in sensor temperature compensation for the Durafet and the optional solution temperature compensation available on the UDA user interface. Solution temperature compensation is an added feature of the UDA that is not appropriate for use in seawater, as it applies only to specific industrial process and/or medical applications. As such, solution temperature compensation should always be turned off when using the UDA for seawater pH measurements.
Durafets are stored in distilled water and require a conditioning period of up to 1 day in seawater prior to use (Step 1, Table 2; Bresnahan et al., 2014). In addition, the black plastic (Ryton) body of the Durafet should be mostly, if not completely, submerged so that the sensor body temperature closely matches that of the calibration vessel and experiment. Note that Durafet temperature sensors can also be calibrated.

Table 2. Step-by-step protocol for use of UDA-Durafets in marine experiments. Durafets should be mostly, if not completely, submerged in seawater.
Although the UDA user guide includes various methods for calibrations, use of the Durafet in seawater requires an additional appreciation for pH scales not found in the factory documentation (Honeywell, 2009). The UDA pH reading needs to be converted to the appropriate pH scale, in this case the total hydrogen ion scale, pHT (Dickson, 2010). There are two modes of calibration: “buffer” and “sample.” Buffer calibration is not appropriate for a Durafet that is to be used in seawater as it would require seawater pH buffers with a ≥2 unit pH difference, and these are not readily available. The “sample” calibration approach is closely aligned with our recommended best practices for in situ pH sensors and so used here (Bresnahan et al., 2014). In this method, a conditioned sensor is calibrated by bringing it into agreement with the pH of a discrete sample taken next to the Durafet and analyzed on a benchtop spectrophotometer. Often, analysis temperature is different from the calibration sample temperature and a temperature correction needs to be performed. This requires additional carbonate chemistry information such as salinity and total alkalinity values (Dickson, 2010).
UDA calibration for seawater pHT is a two-part process: initial calibration via manual entry of a calibration sample value on the UDA (Step 2, Table 2), followed by post-processing of the pH time-series based on the pH of a post-calibration reference sample (Step 3 and 8, Table 2). Manual calibration is recommended to visually track pH conditions during an experiment while the post-processing brings all Durafets into agreement at a higher precision.
Eight conditioned Durafet electrodes were calibrated simultaneously. Durafets were suspended, mostly submerged, in a calibration vessel (8.5 L) with flow-through, pH- and temperature-controlled seawater (pHT 8.0–8.1 at 14°C). The seawater was homogenized with a motorized paddle (6 rpm) to reduce spatio-temporal mismatch sampling errors, which could arise in static water. The pH of discrete seawater samples was determined spectrophotometrically (Ocean Optics, USB2000+ with CUV-UV-10 cuvette holder) via the standard protocol (Dickson et al., 2007). Five replicate seawater calibration samples were drawn sequentially from the calibration vessel into 10-cm path length cuvettes and immediately analyzed for pHT at 22°C using purified m-cresol purple (R. H. Byrne, University of South Florida). Temperature corrections were calculated in R (version 3.2.3) using the seacarb package (Gattuso et al., 2016) and average values of recent total alkalinity and salinity measurements. Total alkalinity (2,559 ± 10 μmol kg−1, N = 92) and salinity (38.3 ± 0.2, N = 92) in our experimental system do not exhibit enough variation to influence the temperature correction. Immediately following in situ pH calculation (<30 min), the pH value was entered manually using the UDA interface for each electrode.
Due to rounding errors and time delays between processing the calibration sample and value entry on the UDA, manual calibration leads to small inter-Durafet differences (~0.01 units pHT, found by comparing pH observations of across multiple Durafets in the same solution). To correct for this error, a post-calibration reference pH sample was collected and analyzed while the LabVIEW program was logging data (Step 3, Table 2). This sample serves two important roles. First, it allows users to check the accuracy of the manual calibration, in order to trust the pH displayed on the UDA during an experiment (or document the offset). Second, it is used as a calibration correction applied to the data at the conclusion of the experiment (a.k.a., post-processing). For this post-processing, the pH value of the post-calibration reference sample collected at the start of the experiment is used to bring all Durafet data into exact agreement at the time-point of sampling (Step 8, Table 2). Post-processing of the pH time series improves pH data agreement among multiple Durafets by roughly one order of magnitude, as it uses the resolution of the UDA digital output and data logging (0.001 units pHT, user-defined in LabVIEW) rather than the resolution of the UDA front panel (0.01). Furthermore, it is straightforward to choose an exact time stamp for calibration in post-processing, while it is quite challenging to simultaneously perform manual calibration at the front panel on multiple UDAs. In summary, post-processing is the only way to bring multiple Durafet electrodes into agreement where uncertainty approaches that of the spectrophotometric pH measurement.
While certified Tris buffer in synthetic seawater could be used for manual calibration and post-calibration correction, there are a few reasons for not doing so. First, a large volume would be necessary to simultaneously calibrate multiple Durafets and this would be expensive in the long-term. Second, the pH of seawater Tris buffer has a strong temperature dependence and therefore requires accurate temperature measurement during analysis (DelValls and Dickson, 1998). Instead, seawater Tris buffer (A. G. Dickson, Scripps Institution of Oceanography) was used to estimate the uncertainty of spectrophotometric pH measurements (section Estimated Uncertainty).
Performance of eight Durafet electrodes was assessed over a range of pH and compared with reference pH samples analyzed via the spectrophotometric pH method. Eight calibrated Durafets were suspended in a calibration vessel, as described in the previous section, set in a sea table controlled at 14°C. Data logging occurred at 1 min frequency and addition of pure CO2 gas was used to decrease pHT to 7.25. Every 30 min, seawater pH was incrementally increased via the addition of high pH seawater (pHT 8.0, bubbled with CO2-free air) or 0.1 M NaOH for pHT > 7.95. Three replicate reference samples were taken at every pH step. The reference samples taken at pHT 8.25 were used for post-processing of the Durafet time series. This post-calibration correction allowed for comparison among Durafets at a resolution greater than that of the UDA front panel.
One goal for experimental applications is high-precision monitoring of different pH treatments without the need for tedious discrete analyses. As such, additional quality control is necessary to verify equal performance by Durafets across time and pH conditions of the experimental design. This was achieved using a combination of “CO2 spikes” and a post-experiment reference sample.
Following calibration of eight Durafets (Step 1–3, Table 2), seawater in the calibration vessel was injected with CO2 gas to reach pHT 6.0 (Step 4, Table 2). Incoming high pH seawater increased pHT to 8.0 overnight. This CO2 spike allowed for comparing Durafet performance across a range of pH that extends beyond the sensitivity of m-cresol purple.
Next, Durafets were placed in the experimental configuration (Step 5, Table 2; Figure 1). The experimental setup was comprised of four header tanks, each of which supply seawater to three downstream, flow-through larval culture vessels fitted with a motorized paddle. All vessels were contained in a temperature-controlled sea table at 14°C. Seawater pH was manipulated via pure CO2 gas addition using a pH control system independent of the Durafets (iks Aquastar, using Tunze pH Electodes). The pH control in header tanks was set for stable pH or variable pH treatments (switching pH condition every 12 h). In this CO2 system, high frequency pH variability in header tanks was generally limited to a range of 0.02–0.08 units pHT. Durafets were used to monitor pH in the two variable pH treatment header tanks and in one or two of the three larval culture vessels per treatment. Once per day, one Durafet was used to check pH in culture vessels without a Durafet to verify stability of pH treatments across all vessels.
At the conclusion of the 12-day experiment, Durafets were placed back into the calibration vessel, a CO2 spike was performed again, and a post-experiment reference sample was taken to identify any potential change in Durafet performance (Step 6 and 7, Table 2; can be performed in reverse). At the conclusion of data collection, a post-calibration correction was applied to the pH time series using the post-calibration reference sample collected on day 0 of the experiment (Step 3 and 8, Table 2; section Calibration).
Using the post-processed time series, sensor performance was evaluated (Step 9, Table 2) by comparing spectrophotometric pH of the post-experiment reference sample to the pH documented by each Durafet at the time of sample collection (Step 6, Table 2). For Durafets that functioned properly, the pH difference should be equal to or smaller than the error associated with the spectrophotometric pH measurement (section Estimated Uncertainty). A pH difference that extends beyond the spectrophotometric measurement error may indicate a performance issue of the Durafet in question, in which case that should be investigated and reported.
In addition to pH, for full documentation of carbonate chemistry conditions during an experiment (e.g., pCO2, saturations states), sampling of other parameters will still be necessary according to standard procedures (Dickson et al., 2007).
In order to evaluate the performance of UDA-Durafets, we estimated the uncertainty (i.e., accuracy) associated with the spectrophotometric pH measurements. For our laboratory, the uncertainty of spectrophotometric pH measurements is 0.008 units pHT, which stems from the accuracy of the purified m-cresol purple based on use in certified synthetic seawater Tris buffer (±0.006 units pHT; Kapsenberg et al., 2017) and precision of replicate measurements pulled sequentially from the calibration vessel (±0.005 units pHT). Precision of replicate measurements (±0.005 units pHT) may also reflect the functional resolution of the spectrophotometer. As such, all Durafet pH data are reported to a precision of 0.001, despite the fact that pH precision of Durafets in seawater extend beyond this (Martz et al., 2010).
In general, at any given time, individual Durafets appeared to exhibit a pH variation on the UDA front panel of pHT ± 0.01 units. When eight calibrated Durafets were placed in the same vessel, this flickering extended to a variation in pHT readings across UDAs of up to 0.02 units at any given time. This occurred because pH input for the sample calibration method is restricted to resolution of the front panel (hundredths of a pH unit). This was not an issue for data logging or quality because (i) UDAs transmit data with greater precision than the front panel, (ii) each logged data point represents an average of 10 measurements as defined in LabVIEW, and (iii) post-processing of the data brings Durafet time-series into agreement at a resolution of 0.001 units pHT (Step 8, Table 2).
Durafets in the UDA configuration exhibited high agreement with one another from pHT 7.25–8.25 (Figure 2). The standard deviation in pHT across eight Durafets was calculated at each time point, with a mean standard deviation of 0.0037 units pHT (Figure 2B). Spikes in the standard deviation among the eight Durafets occurred during step-wise pH increases via the additions of high pH seawater or NaOH, and so are likely a function of the homogenization period of the seawater in the vessel or slight offsets in polling of UDA data by LabVIEW.
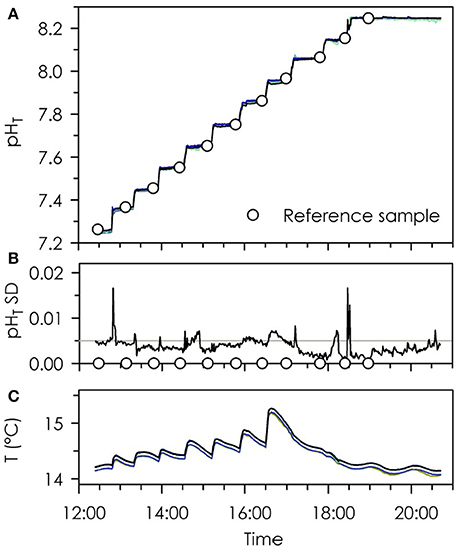
Figure 2. Time-series of pHT (A), pHT standard deviation (B), and temperature (C) of eight Durafet pH electrodes during step-wise increases in seawater pH (lines in A,C are superimposed). Circles represent reference pH samples using the spectrophotometric method with m-cresol purple (A) and sample time points (B). Reference line in (B) indicates a standard deviation of 0.005.
Durafets exhibited high agreement with reference sample pH determined via the spectrophotometric method (Figure 3), so long as measurements were performed within the ideal range of m-cresol purple and the temperature of the seawater was stable. The average standard deviation of three replicate spectrophotometric reference samples was ±0.005 units pHT. For the spectrophotometric pH measurements, the acid dissociation constant for m-cresol purple (pK2) was ~8.04. The increasing offset between the Durafets and reference samples at pHT ≤ 7.55 possibly stem from systematic errors due to the lower limits of m-cresol purple (in this case, pHT22°C ≤ 7.45). At pHT 7.96, Durafet offset was −0.012 units pHT, which occurred when seawater temperature was changing rapidly (−0.016°C min−1, over 7 min). Temperature change at all other sampling points was minimal and ranged from −0.008 to 0.003°C min−1. Temperature fluctuations at pHT < 7.95 occurred as a result of the addition of slightly warmer high pH seawater to raise the pH, followed by cooling of the vessel in a sea table set to 14°C (Figure 2C). At pHT > 7.95, pH was raised via the addition of 0.1 M NaOH, which had no impact on temperature.
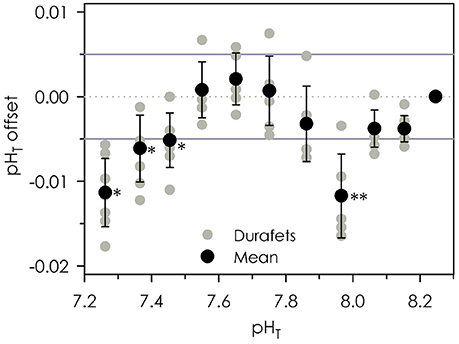
Figure 3. Mean and individual offsets of eight Durafet pH observations compared to reference pH samples analyzed via the spectrophotometric method. Solid reference lines indicate a ±0.005 units pHT error associated with replicate spectrophotometric measurements. *Indicates sampling near the lower pH limit of m-cresol purple. **Indicates sampling that occurred during the fastest observed temperature change.
Eight calibrated Durafets were tested in a seawater pH variability experimental system for culturing marine invertebrate larvae (Figure 4). During the experiment, replicate culture vessels differed no more than 0.01 units pHT on the UDA front panel when checked daily. A post-calibration reference sample (Step 3, Table 2) was used for post-processing pH time series, which resulted in corrections of 0.000 up to 0.008 units pHT (Step 8, Table 2). Based on the corrected time series (Figure 4), a post-experiment reference sample (Step 6, Table 2) revealed a pH offset spanning −0.003 to 0.004 units pHT among Durafets by the end of the 12-day experiment with an average offset of 0.001 ± 0.003, N = 8 (Step 9, Table 2). This variation falls within the uncertainty of the spectrophotometric method for this lab and experimental setup (±0.008 units pHT). In addition, differences among Durafets could stem from spatio-temporal sampling error of the seawater in the calibration vessel as small changes may occur during the sequential sampling of replicates. In summary, offsets spanning −0.003 to 0.004 units pHT indicate no detectable change in performance among the Durafets over the course of the experiment (Step 9, Table 2).
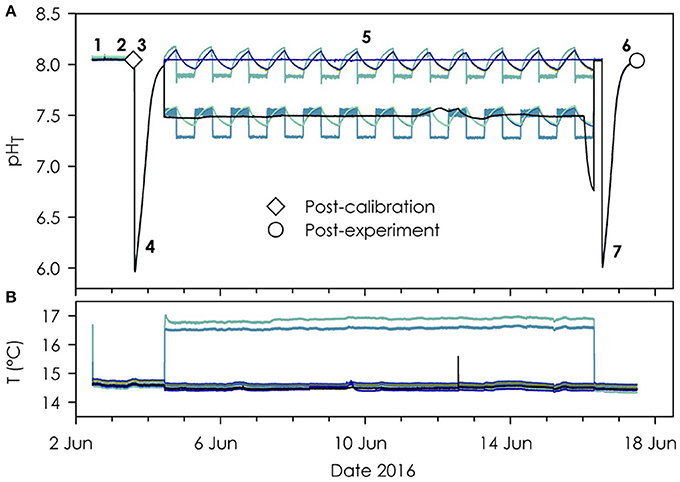
Figure 4. Complete pHT (A) and temperature (B) time-series from eight Durafets during an experiment. Post-calibration (diamond) and post-experiment (circle) reference pH samples and pre- and post-experiment CO2 spikes are included. Numbered steps follow Table 2 (step 6 and 7 are inverted in this example). Header tanks were warmer than culture vessels, and only two header tanks had Durafets (B).
The CO2 spikes revealed high agreement among Durafets across a wide range in pH at the start and end of the experiment, indicating equal performance of all Durafets over the experimental period (Step 9, Table 2; Figure 5). The standard deviation of Durafet pH measurement during CO2 spikes was smaller than ±0.01 units pHT when seawater in the calibration vessel increased from pHT 6.0–8.0 overnight. Once pH change leveled off, agreement among Durafets was again better than ±0.005 units pHT.
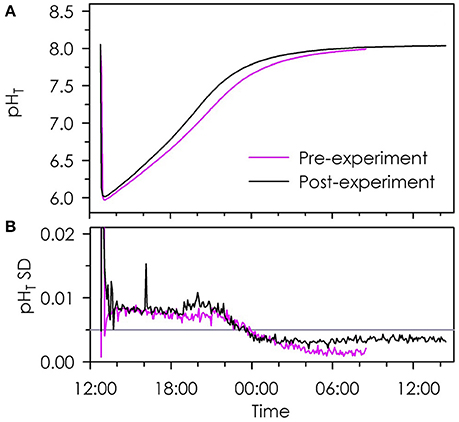
Figure 5. Time-series of mean pHT (A) and standard deviation (B) of eight Durafets during CO2 spikes prior to (pink) and after (black) being used in an experiment (Figure 4). Reference line in (B) indicates a standard deviation of 0.005.
This study outlines a straightforward method for high quality data acquisition using the UDA-Durafet configuration for laboratory-based ocean acidification research. Our methods greatly improve time course documentation of pH and temperature and alleviate person-hours devoted to documenting such conditions. To facilitate implementation of this technology in marine research laboratories, we included instructions for setting up UDA-Durafet connections via Ethernet and we have made our LabVIEW program available online (Supplementary Material. This technology enables execution of complex experimental design based on fluctuating pH treatments, multi-drivers, and other long-term manipulations in order to pursue research priorities in global ocean change biology.
Prior to investing in this technology, potential users should evaluate the trade-offs associated with Durafet technology compared to alternatives like discrete analyses and glass-electrode pH characterization. One consideration might be frequency of experiments and person-hours devoted to characterizing seawater chemistry. Durafets are likely to be preferable when high frequency data and sensor stability are required. Examples of this include the ability to measure rapid biological responses in variable pH treatments, the desire to maintain long experiments with minimal effort, or the intent to use data for future meta-analyses where multiple independent studies are being compared.
As expected, Durafets in the laboratory configuration achieved the same level of precision for pHT as compared to Durafets used in autonomous sensor packages. In agreement with Takeshita et al. (2014), our results indicate that a single-point calibration sample can be used, provided that the temperature is stable and the pH falls within the optimal range of the indicator dye. Multiple Durafets can readily be brought into agreement within ±0.005 units pHT in seawater. Due to high-frequency observations, Durafets drastically improve time-course documentation of pH and temperature conditions during ocean acidification biology experiments.
We recommend a step-by-step protocol for use of Durafets in the UDA configuration in laboratory-based seawater systems (see Table 2). This protocol includes, in chronological order, steps for calibration, sensor performance assessment, and data reporting. In short, following sensor conditioning to seawater (Step 1), a manual calibration on all Durafets is performed using the UDA front panel while all Durafets are in the calibration vessel (Step 2). Data logging should be active and a post-calibration reference sample is collected at the same time that Durafets log a data point (Step 3). This pH value is used at the end of the experiment in post-processing of the time series data to gain a more accurate calibration (Step 8). Following calibration, a CO2 spike is performed as a pre-experiment performance assessment that all Durafets exhibit the same response across the range of experimental pH treatments (Step 4). After Step 4, sensors are placed in the experimental configuration to document pH conditions (Step 5). Depending on the length of the experiment, reference samples could be taken to check sensor performance. At the end of an experiment, Durafets are placed back into the calibration vessel and a post-experiment reference sample is taken (Step 6). A CO2 spike is repeated (Step 7) and data logging is stopped. The pH value of the post-calibration reference sample (Step 3) is used to calculate the pH offset with each Durafet pH measurement at the time of sample collection. This offset is then applied as a constant to the full time series (Step 8). Using the post-processed data, sensor performance is assessed and reported (Step 9). This includes two quality control measurements: Durafet pH offsets from the post-experiment reference sample (Step 6), and agreement of Durafets across CO2 spikes.
As reference samples are used to calibrate Durafets (Step 2 and 3) and assess performance (Step 5 and 6), the quality of UDA-Durafet data depends entirely on the quality of the reference samples. It is essential that these samples are collected from seawater at stable temperature and pH. Rapid changes in temperature could interfere with the Durafet's internal reference electrode, resulting in offsets an order of magnitude greater than the precision of spectrophotometric pH measurements. For this same reason, body temperature of the Durafet should match that of the seawater being measured. We also recommend that calibration is performed at a pH condition optimized for the pH indicator (e.g., pHT 8.0 for m-cresol purple).
Likewise, users should attempt to quantify and report the uncertainty associated with their spectrophotometric reference sample pH measurements. We emphasize this activity given the wide range in pH measurement accuracy among laboratories (±0.04 units pHT; Bockmon and Dickson, 2015) and recent improvements for using commercially available unpurified m-cresol (Douglas and Byrne, 2017). For UDA-Durafets, knowing the uncertainty of spectrophotometric pH measurements is necessary in order to interpret mid- and post-experiment reference samples (Step 5 and 6). For example, in a series of three biological experiments (L. Kapsenberg, unpublished), Durafets exhibited an average post-experiment pH offset of −0.001 ± 0.004 units pHT (N = 24) from the spectrophotometric pH measurement (Step 6). For two of these experiments, one Durafet exhibited a post-experiment offset of 0.009 units pHT. This value is slightly larger than normal and extends beyond the uncertainty of the spectrophotometric pH measurement (±0.008 units pHT). If the offset of a Durafet compared to mid- or post-experiment reference samples falls within the uncertainty of the pH measurement, the user cannot conclude sensor failure. However, if offsets extend beyond the magnitude of uncertainty of the pH measurement, the results indicate a potential performance issue with the Durafet, in which case the extent of such an issue should be reported (Step 9).
As Durafets exhibit stability over several months (Bresnahan et al., 2014; Kapsenberg and Hofmann, 2016), we do not recommend mid-experiment re-calibration. For long experiments, biofouling presents a greater risk to data quality than drift stemming from electrolyte gel or liquid junction issues (repairable with replacement; Bresnahan et al., 2014). If desired, users could track data quality of Durafets by collecting mid-experiment reference samples (Step 9). However, users should consider the impact of this on the experiment (e.g., removing all Durafets from experimental vessels to put in a common vessel). Mid-experiment re-calibration could introduce artificial corrections to the pH data resulting from variation in spectrophotometric pH measurements. If Durafets are left in the experimental vessels, a large number of in situ reference samples would be required and quality of the samples may be compromised depending on the stability of the pH and temperature treatments.
Finally, as most research groups replicate experimental units, there is the additional need for monitoring each replicate. As done for the system described in Figure 1, variation among replicate culture vessels was established prior to conducting biological experiments (Figure 4). Negligible differences across (pseudo)replicates allowed us to use one Durafet to check pH values in the other two culture vessels once per day, in order to monitor pH in experimental units without Durafets. The exact configuration in which Durafets can be used depends on the experimental design, an important aspect of ocean acidification biology (Cornwall and Hurd, 2016). In the case of Figure 1, where three culture vessels per header tank are not independent and could be considered pseudo-replicates, biological experiments could be repeated (randomized treatment × header tank combinations) in order to verify a consistent biological response to each pH treatment.
Beyond monitoring the pH and temperature of long-term manipulative experiments, UDA-Durafets can also be used in feedback systems to directly control pH in experimental containers. Each UDA has four built-in relays. In the most basic configuration, a desired pH set point for an experimental container can be programmed directly into the UDA. For an experimental container receiving a constant flow of ambient seawater, a relay switch can be activated to release pre-equilibrated low pH seawater into the vessel containing the Durafet via a solenoid valve when the pH increases above that of the set point. When mixing a continuous flow of ambient seawater with episodic releases of acidified seawater (pHT ~ 5.6), variation in experimental vessels was ± 0.01 and ± 0.03 units pHT in treatments of pHT ~ 7.8 and ~7.4, respectively (±SD over 7 days at 2 s observation frequency; K. J. Kroeker, unpublished). More complex systems using mass flow controllers to mix N2 and CO2 gas at constant flow rates yielded pH stability around 0.005 units pHT (Bockmon et al., 2013), while systems using active feedback between the mass flow controllers and Durafets have achieved stability of 0.002 units pHT (T. R. Martz, unpublished).
One of the benefits of using Durafets in feedback systems is that it allows for automated pH control in flow-through systems where the incoming water characteristics may be changing through time. For advanced applications addressing environmental variability, the relay set point can be programmed to vary through time using a computer program such as LabVIEW. In addition to Durafets, UDAs can also be outfitted with other electrodes (salinity, dissolved oxygen, etc.). Such versatility in experimental parameters and automated control opens the door to pursue new and complex experimental design necessary to advance knowledge of global ocean change impacts on marine biology.
LK and TM conceived and designed the study and LK conducted experiments. PB and EB developed sensor-LabVIEW communication methods with adaptations and associated LabVIEW program by LK. JPG provided laboratory support and KK contributed data on feedback systems. LK prepared the manuscript with contributions from all authors.
This research was funded by the United States National Science Foundation Ocean Sciences Postdoctoral Research Fellowship awarded to LK (OCE-1521597). EB was supported by the National Science Foundation (OCE-1233648). The performance of the experimental system with feedback from Durafets was funded by the David and Lucile Packard Foundation, The Alfred P. Sloan Foundation, and the National Science Foundation (OCE-1524377) to KK. Work at Scripps was funded in part through the Benedek Chair in Ocean Sensor Science.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Samir Alliouane for lab assistance and maintenance of the spectrophotometer and total alkalinity titration unit and Yui Takeshita for contributory discussions on the topic of Durafet sensors. Additionally, we acknowledge Emily Donham and Dr. Takeshita for data contributions of feedback systems.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2017.00321/full#supplementary-material
Bockmon, E. E., and Dickson, A. G. (2015). An inter-laboratory comparison assessing the quality of seawater carbon dioxide measurements. Mar. Chem. 171, 36–43. doi: 10.1016/j.marchem.2015.02.002
Bockmon, E. E., Frieder, C. A., Navarro, M. O., White-Kershek, L. A., and Dickson, A. G. (2013). Technical note: controlled experimental aquarium system for multi-stressor investigation of carbonate chemistry, oxygen saturation, and temperature. Biogeosciences 10, 5967–5975. doi: 10.5194/bg-10-5967-2013
Bresnahan, P. J. J., Martz, T. R., Takeshita, Y., Johnson, K. S., and LaShomb, M. (2014). Best practices for autonomous measurement of seawater pH with the Honeywell Durafet. Methods Oceanogr. 9, 44–60. doi: 10.1016/j.mio.2014.08.003
Ciais, P., Sabine, C., Bala, G., Bopp, L., Brovkin, V., Canadell, J., et al. (2013). “Carbon and other biogeochemical cycles,” in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds. T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, and P.M. Midgley (Cambridge; New York, NY: Cambridge University Press), 465–570.
Clark, H. R., and Gobler, C. J. (2016). Diurnal fluctuations in CO2 and dissolved oxygen concentrations do not provide a refuge from hypoxia and acidification for early-life-stage bivalves. Mar. Ecol. Prog. Ser. 558, 1–14. doi: 10.3354/meps11852
Cornwall, C. E., and Hurd, C. L. (2016). Experimental design in ocean acidification research: problems and solutions. ICES J. Mar. Sci. 73, 572–581. doi: 10.1093/icesjms/fsv118
DelValls, T. A., and Dickson, A. G. (1998). The pH of buffers based on 2-amino-2-hydroxymethyl-1,3-propanediol (‘tris’) in synthetic sea water. Deep Sea Res. A 45, 1541–1554. doi: 10.1016/S0967-0637(98)00019-3
Dickson, A. (2010). “The carbon dioxide system in seawater: equilibrium chemistry and measurements,” in Guide to Best Practices for Ocean Acidification Research and Data Reporting, eds U. Riebesell, V. J. Fabry, L. Hansson, and J.-P. Gattuso (Luxembourg: Publications Office of the European Union), 17–40.
Dickson, A. G., Sabine, C. L., and Christian, J. R. (eds.). (2007). Guide to Best Practices for Ocean CO2 Measurements. PICES Special Publication 3, p. 191. Available online at: http://cdiac.ess-dive.lbl.gov/ftp/oceans/Handbook_2007/Guide_all_in_one.pdf
Douglas, N. K., and Byrne, R. H. (2017). Achieving accurate spectrophotometric pH measurements using unpurified meta-cresol purple. Mar. Chem. 190, 66–72. doi: 10.1016/j.marchem.2017.02.004
Gattuso, J.-P., Epitalon, J.-M., and Lavigne, H. (2016). Seacarb: Seawater Carbonate Chemistry. R Package Version 3.1.1. Available online at: https://cran.r-project.org/package=seacarb
Honeywell (2009). UDA2182 Universal Dual Analyzer Product Manual. Phoenix, AZ: Honeywell International Inc.
Johnson, K. S., Jannasch, H. W., Coletti, L. J., Elrod, V. A., Martz, T. R., Takeshita, Y., et al. (2016). Deep-Sea DuraFET: a pressure tolerant pH sensor designed for global sensor networks. Anal. Chem. 88, 3249–3256. doi: 10.1021/acs.analchem.5b04653
Kapsenberg, L., Alliouane, S., Gazeau, F., Mousseau, L., and Gattuso, J. P. (2017). Coastal ocean acidification and increasing total alkalinity in the northwestern Mediterranean Sea. Ocean Sci. 3, 411–426. doi: 10.5194/os-13-411-2017
Kapsenberg, L., and Hofmann, G. E. (2016). Ocean pH time-series and drivers of variability along the northern Channel Islands, California, USA. Limn. Oceanogr. 61, 953–968. doi: 10.1002/lno.10264
Keppel, A. G., Breitburg, D. L., Wikfors, G. H., Burrell, R. B., and Clark, V. M. (2015). Effects of co-varying diel-cycling hypoxia and pH on disease susceptibility in the eastern oyster Crassostrea virginica. Mar. Ecol. Prog. Ser. 538, 169–183. doi: 10.3354/meps11479
Martz, T. R., Connery, J. G., and Johnson, K. S. (2010). Testing the Honeywell Durafet® for seawater pH applications. Limn. Oceanogr. Methods 8, 172–184. doi: 10.4319/lom.2010.8.172
O'Donnell, M. J., George, M. N., and Carrington, E. (2013). Mussel byssus attachment weakened by ocean acidification. Nat. Clim. Chang. 3, 587–590. doi: 10.1038/nclimate1846
Rhein, M., Rintoul, S. R., Aoki, S., Campos, E., Chambers, D., Feely, R. A., et al. (2013). “Observations: Ocean,” in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds T.F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, and P. M. Midgley (Cambridge; New York, NY: Cambridge University Press), 255–315.
Riebesell, U., Fabry, V. J., Hansson, L., and Gattuso, J.-P. (2010). Guide to Best Practices for Ocean Acidification Research and Data Reporting. Luxembourg: Publications Office of the European Union.
Takeshita, Y., Martz, T. R., Johnson, K. S., and Dickson, A. G. (2014). Characterization of an ion sensitive field effect Transistor and chloride ion selective electrodes for pH measurements in seawater. Anal. Chem. 86, 11189–11195. doi: 10.1021/ac502631z
Keywords: ocean acidification, Durafet, seawater pH, pH manipulation experiments, pH sensor
Citation: Kapsenberg L, Bockmon EE, Bresnahan PJ, Kroeker KJ, Gattuso J-P and Martz TR (2017) Advancing Ocean Acidification Biology Using Durafet® pH Electrodes. Front. Mar. Sci. 4:321. doi: 10.3389/fmars.2017.00321
Received: 10 June 2017; Accepted: 25 September 2017;
Published: 12 October 2017.
Edited by:
Silvana Noemi Raquel Birchenough, Centre for Environment, Fisheries and Aquaculture Science, United KingdomReviewed by:
Laura Ramajo, Adolfo Ibáñez University, ChileCopyright © 2017 Kapsenberg, Bockmon, Bresnahan, Kroeker, Gattuso and Martz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lydia Kapsenberg, bHlkaWEua2Fwc2VuYmVyZ0BvYnMtdmxmci5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.