- 1Institute for Marine and Antarctic Studies, University of Tasmania, Hobart, TAS, Australia
- 2Australian Antarctic Division, Kingston, TAS, Australia
- 3Trace and Environmental DNA Laboratory, Department of Environment and Agriculture, Curtin University, Perth, WA, Australia
- 4CSIRO Indian Ocean Marine Research Centre, The University of Western Australia, Perth, WA, Australia
- 5British Antarctic Survey, Natural Environment Research Council, Cambridge, United Kingdom
- 6Marine and Environmental Sciences Centre, ISPA-Instituto Universitário, Lisbon, Portugal
- 7Falklands Conservation, Stanley, Falkland Islands
- 8Centre d'Etudes Biologiques de Chizé, UMR 7372 du Centre National de la Recherche Scientifique-Université de La Rochelle, Villiers-en-Bois, France
- 9Wildlife Conservation Society, Punta Arenas, Chile
- 10Directorate of Natural Resources-Fisheries of the Falkland Islands Government, Stanley, Falkland Islands
- 11Department of Primary Industries, Parks, Water and Environment, Hobart, TAS, Australia
Almost all of the world's fisheries overlap spatially and temporally with foraging seabirds, with impacts that range from food supplementation (through scavenging behind vessels), to resource competition and incidental mortality. The nature and extent of interactions between seabirds and fisheries vary, as does the level and efficacy of management and mitigation. Seabird dietary studies provide information on prey diversity and often identify species that are also caught in fisheries, providing evidence of linkages which can be used to improve ecosystem based management of fisheries. However, species identification of fish can be difficult with conventional dietary techniques. The black-browed albatross (Thalassarche melanophris) has a circumpolar distribution and has suffered major population declines due primarily to incidental mortality in fisheries. We use DNA metabarcoding of black-browed albatross scats to investigate their fish prey during the breeding season at six sites across their range, over two seasons. We identify the spatial and temporal diversity of fish in their diets and overlaps with fisheries operating in adjacent waters. Across all sites, 51 fish species from 33 families were identified, with 23 species contributing >10% of the proportion of samples or sequences at any site. There was extensive geographic variation but little inter-annual variability in fish species consumed. Several fish species that are not easily accessible to albatross, but are commercially harvested or by-caught, were detected in the albatross diet during the breeding season. This was particularly evident at the Falkland Islands and Iles Kerguelen where higher fishery catch amounts (or discard amounts where known) corresponded to higher occurrence of these species in diet samples. This study indicates ongoing interactions with fisheries through consumption of fishery discards, increasing the risk of seabird mortality. Breeding success was higher at sites where fisheries discards were detected in the diet, highlighting the need to minimize discarding to reduce impacts on the ecosystem. DNA metabarcoding provides a valuable non-invasive tool for assessing the fish prey of seabirds across broad geographic ranges. This provides an avenue for fishery resource managers to assess compliance of fisheries with discard policies and the level of interaction with scavenging seabirds.
Introduction
Effective ecosystem-based management of commercial fisheries requires information not just on the sustainability of target stocks, but also on the interactions of other marine organisms with fishing operations. Globally, seabirds frequently interact with commercial fisheries through competition for shared resources (Frederiksen et al., 2004; Okes et al., 2009), incidental mortality in fishing gear (Brothers et al., 1999; Sullivan et al., 2006; Watkins et al., 2008; Tuck et al., 2011) and consumption of fishery discards (Garthe et al., 1996; Gonzalez-Zevallos and Yorio, 2006). Seabird survival and breeding success can be reduced by competition with fisheries (Furness and Tasker, 2000; Frederiksen et al., 2004), and incidental mortality in fishing gear can be a major cause of population declines, particularly of albatrosses and large petrels (Weimerskirch and Jouventin, 1987; Barbraud et al., 2008; Phillips et al., 2016). Physical and operational mitigation measures have been developed to reduce seabird mortality (Løkkeborg, 2008; Phillips et al., 2016), including the reduction of fishery discards, which decreases the attractiveness of vessels (Abraham et al., 2009; Pierre et al., 2012). Scavenging birds are attracted to the supplementary food source provided by discards, which may consist of (i) the head, tail and offal of retained catch (commercial species caught at commercial size); (ii) whole fish of commercial species but caught at a non-commercial size; (iii) non-commercial species and (iv) unused baits (in longline fishing). These discards are often fish or other species that may not be naturally accessible. Some populations benefit from the additional food source, with higher breeding success and survival resulting in population growth (Oro et al., 1995; Bertellotti and Yorio, 2000). However, discards can alter food-web structure by providing nutritionally-poor food (Grémillet et al., 2008), or artificially inflating populations of predatory gulls or skuas, which may not be sustainable in the absence of discards or which also prey on smaller seabirds, with potentially major impacts (Phillips et al., 1999b; Foster et al., 2017). The interactions between seabird populations and fisheries are likely to vary over time, space and species; therefore, understanding the nature and extent of these interactions is imperative for effective ecosystem management.
Seabird dietary studies can inform ecosystem risk assessments for fishery management by identifying interactions between fisheries and seabirds for different populations (Phillips et al., 1999a). Understanding the dietary flexibility of seabirds is also fundamental for predicting the responses of individuals and populations to spatial and temporal changes in natural prey abundance, and availability from fisheries, and hence for the effective management of marine resources (Constable et al., 2000). Stomach content and stable isotope analyses are the two main approaches for assessing seabird diet (Duffy and Jackson, 1986; Barrett et al., 2007). The former primarily relies on the use of otoliths and bones to identify fish prey, enabling prey size and meal mass estimates to be obtained. However, discrimination can be poor or impossible if the prey (including larvae or eggs) is small, has no hard parts, or digests quickly; the hard-parts are eroded; or those from closely-related species cannot be readily distinguished (Duffy and Jackson, 1986; Barrett et al., 2007). These problems apply in particular to items originating as fisheries offal, as viscera float and are therefore easier to ingest than fish heads with otoliths, particularly those from large species (Thompson and Riddy, 1995). More recent studies have used DNA analysis to identify parts that were not taxonomically diagnostic (Alonso et al., 2014). However, studies using stomach samples are usually restricted to the chick-rearing period, thus focusing on chick rather than adult diet across the annual cycle and usually requires handling of birds.
Stable isotope analysis of blood or feathers does not suffer from the biases associated with differential digestion of prey and can be applied to all stages of the breeding season. This method has been used to determine likely fishery overlaps by comparing the estimated proportions of pelagic and demersal prey, on the assumption that the latter were obtained from fisheries (Granadeiro et al., 2013). However, in most systems stable isotope analyses lack the resolution to identify prey beyond broad trophic groups. DNA metabarcoding of predator scats is a useful alternate or complementary method for assessing seabird diet (Deagle et al., 2007; Bowser et al., 2013). It can provide high-level taxonomic resolution and does not require prey remains to be physically identifiable (Pompanon et al., 2012). Although the method cannot be used to identify prey size and meal mass, it does give an indication of species occurrence in the diet. Samples can also be collected during all breeding stages (McInnes et al., 2017a) and the technique is non-invasive and requires minimal field time compared to conventional diet sampling, increasing the options for simultaneous sampling across broad spatial scales (Jarman et al., 2013).
The black-browed albatross (BBA, Thalassarche melanophris) has a circumpolar distribution and is the most abundant albatross species in the southern hemisphere (Phillips et al., 2016). Populations have experienced extensive declines which are strongly linked to incidental mortality in longline and trawl fisheries (Phillips et al., 2016). While the population at South Georgia is still declining (Poncet et al., 2017), numbers in the Falkland Islands and on islands off Chile are currently increasing (Wolfaardt, 2013; Robertson et al., 2014, 2017). The increases in Chile have been attributed to a reduction in incidental seabird mortality due to faster sink rates of baited longline hooks associated with a change in fishing practices, and the use of bird-scaring (streamer or tori) lines, making hooks less accessible to birds (Robertson et al., 2014). However, longline and trawl fisheries are still thought to cause high mortality of this species elsewhere, especially in the wintering grounds (Yeh et al., 2013; Kuepfer, 2015; Tamini et al., 2015). Fishery resource overlaps with the diet of black-browed albatrosses have been shown at all breeding sites where fish have been characterized, including Iles Kerguelen (Cherel et al., 2000), Diego Ramirez (Arata and Xavier, 2003), South Georgia (Reid et al., 1996; Xavier et al., 2003) and the Falkland Islands (Thompson, 1992). However, the most recent samples used in these studies were collected over 15 years ago (1995, 2002, 2000, and 1991, respectively; Data Sheet 1 in Supplementary Material), over which time fishing operations and regulations, including discarding policies and mitigation requirements, have changed substantially in many regions (Phillips et al., 2016).
We used DNA metabarcoding of BBA fecal DNA to investigate the fish prey consumed at six sites across their breeding range to: (1) determine the fish prey diversity and any spatial and temporal variability; (2) identify any fishery target, bycatch and bait species in the diet of BBA to distinguish regions in which rates and risks of vessel interactions may be greater (and hence efforts to improve discard management and monitoring of compliance with seabird bycatch mitigation may be targeted); and (3) evaluate sources of potential resource competition or food supplementation by fisheries. We use this study to show that DNA metabarcoding can quantify fish diversity and the presence of discards in the diet of seabirds, providing a valuable tool for fishery resource and conservation management.
Methods
Study Sites and Sample Collection
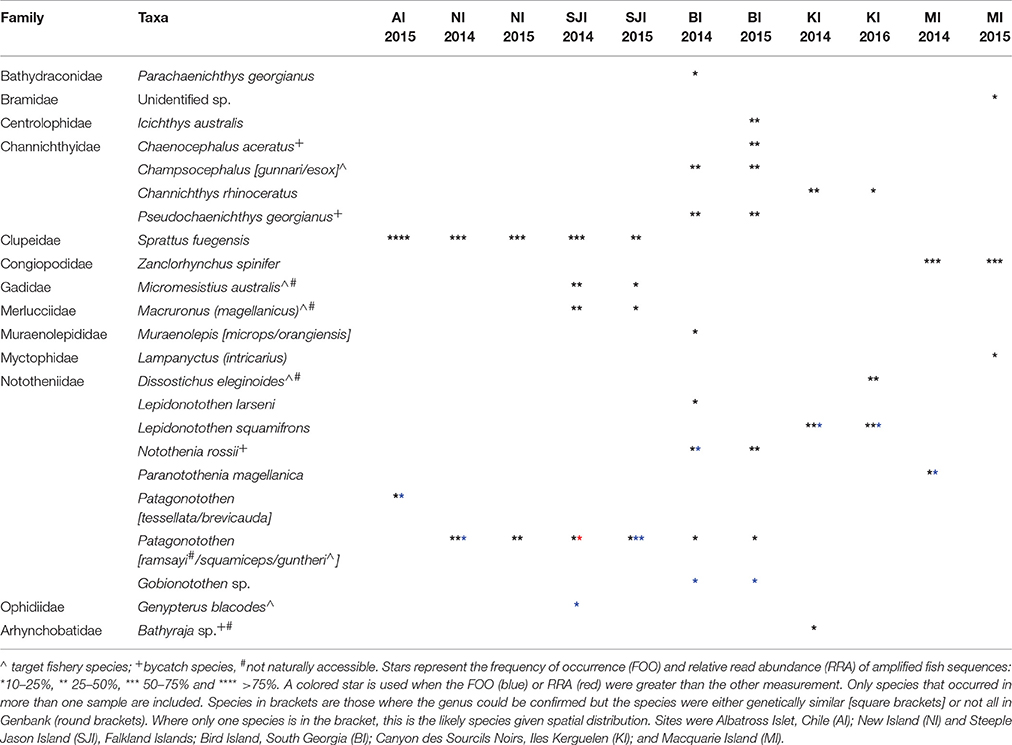
Fresh scat samples were collected from black-browed albatrosses at six breeding colonies over multiple seasons: in austral summers 2013/14 and 2014/15 at New Island and Steeple Jason Island (Falkland Islands), Macquarie Island (Australia) and Bird Island (South Georgia); in 2013/14 and 2015/16 at Canyon des Sourcils Noirs (Iles Kerguelen); and in 2014/15 at Albatross Islet (Chile; Figure 1). The majority of samples were collected during the chick-rearing period (December-March) with additional samples collected during incubation in 2014/15 at Steeple Jason Island and New Island, Kerguelen in 2013/14 and during incubation in both years at Macquarie Island (Table 1). Sampling years are hereafter termed 2014 for samples collected in 2013/14 and 2015 for 2014/15 samples. This project was approved by the University of Tasmania Animal Ethics Committee (Permit A13745).
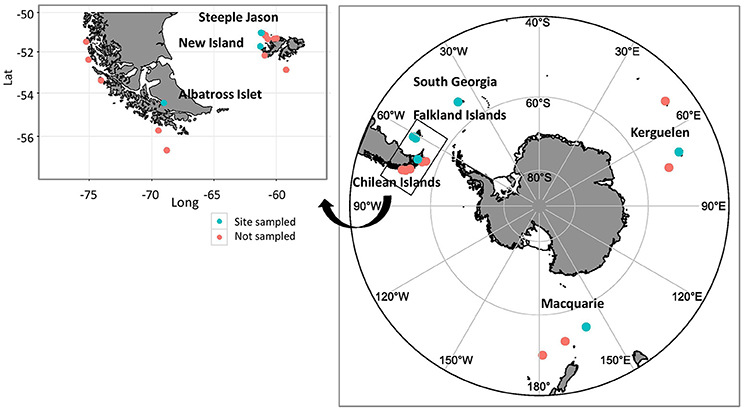
Figure 1. Breeding distribution of black-browed albatrosses and sampling sites. Blue dots represent the six colonies where scat samples were collected, and the red dots the remaining colonies not sampled during the study. The inset shows the individual Chilean and Falkland Island colonies. Samples were collected from Albatross Islet, Chile (40–50 breeding pairs, population increasing); New Island (13,343 breeding pairs, population increasing) and Steeple Jason Island (183,135 pairs, population increasing), Falkland Islands; Bird Island, South Georgia (8,264 breeding pairs, population declining); Canyon des Sourcils Noirs, Iles Kerguelen (~1,200 breeding pairs, population stable); and Macquarie Island (~200 breeding pairs, population stable; ACAP, 2010; Wolfaardt, 2013; Robertson et al., 2014; Phillips et al., 2016; Poncet et al., 2017)
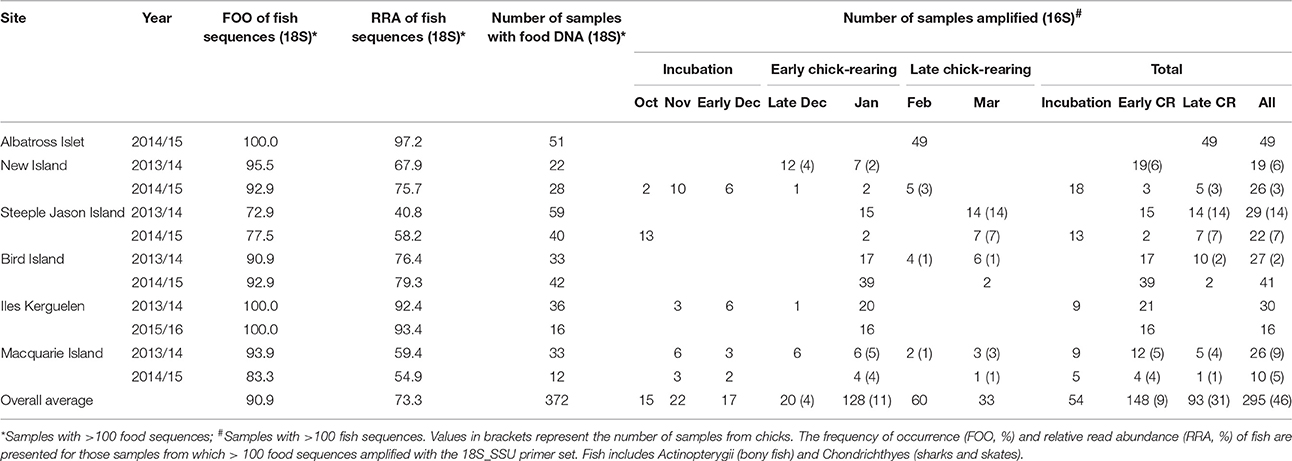
Table 1. The total samples at each site which contained food DNA derived from the 18S_SSU primer set and 16S_Fish primer set.
A small fragment of the non-uric acid portion (dark part) of each scat was collected using tweezers or a spatula and stored in 80% ethanol. Where possible, fresh scats were obtained (where the bird was seen defecating or the sample was on the ground but still wet) and the developmental stage of the bird (chick, juvenile or adult) was recorded. Given the low sample sizes remaining when samples were split by site, age, and month, differences between diet of chicks and adults (self-feeding) could not be explored in this study, and therefore samples from different ages were pooled. Further research with greater sample sizes are required to test partitioning of diet by adults for provisioning compared with self-feeding (Davoren and Burger, 1999; Danhardt et al., 2011), and potential dietary differences between breeders, non-breeders and juveniles (Campioni et al., 2016).
The foraging ranges of black-browed albatross are greater during incubation than chick-rearing, and the magnitudes of these differences depend on the colony (Wakefield et al., 2011). For example, at South Georgia, mean maximum foraging distances of tracked adults were 980–1,690 km (262–327 h) and 275–505 km (45–77 h) during incubation and chick-rearing, respectively (Phillips et al., 2004). The prey detected in scat samples is likely to reflect the most recent meal consumed by albatross, which is similar to stomach contents analysis. The digestion rates of seabirds are influenced by numerous variables, such as predator species, metabolic rate, meal size, food type, and feeding frequency (Hilton et al., 1998). In sooty albatross (Phoebetria fusca), the mean retention rate of prey ranged from 11 to 15 h, however some prey was still detected up to 50 h after eating (Jackson, 1992). In little penguins (Eudyptula minor) prey could be detected for up to 4 days using DNA metabarcoding (Deagle et al., 2010). The retention time is also likely to vary depending on whether the food is consumed for self-feeding or regurgitated to the chick partially digested. During this study, it is assumed that the prey DNA recovered reflects the most recent foraging trip. For extended foraging trips during incubation, some of the food may not be detected.
DNA Metabarcoding
DNA was extracted from albatross scat samples using a Promega “Maxwell 16” instrument and a Maxwell® 16 Tissue DNA Purification Kit. PCR inhibitor concentrations were diluted by mixing a small amount (~30 mg) of the fecal samples in 250 μL of STAR buffer (Roche Diagnostics) prior to extraction. Two different DNA markers were amplified. The first used a metazoan primer set that is highly conserved and amplifies a region of the nuclear small subunit ribosomal DNA (rDNA) 18S gene (McInnes et al., 2017a, Table 2). For this marker the taxonomic resolution is relatively low; however, it recovers DNA from all animal lineages and provides a broad view of the diet. The second primer pair amplifies a region of the 16S rDNA gene specifically from fish and varies enough to allow species-level identification for most of the targeted fish species (Table 2). This primer set was designed based on an alignment of mtDNA 16S sequences from representative Southern Ocean fish that were publicly available on Genbank (a full alignment with sequences in fasta format can be found in Data Sheet 2 of the Supplementary Material). The primer set was designed not to match bird DNA. Primers were tested with fish flesh and scat DNA. All samples were run with the 18S_SSU primer set first, and those that had fish DNA were amplified using the 16S_Fish primer set (See Image 1 of the Supplementary Material for the DNA metabarcoding workflow).
PCR reactions for each primer set were carried out separately as a two stage process. Stage one PCR reactions (10 μL) were performed with 5 μL 2 × Phusion HF (NEB), 1 μL 100 × Bovine Serum Albumin (NEB), 0.1 μL 5 μM of each 18S_SSU or 16S_Fish amplification primer (Table 2), 0.5 μL of Evagreen, 2 μL fecal DNA and 1.3 μL of water. Thermal cycling conditions were 98°C, for 2 min; followed by 35 cycles for 18S_SSU, and 45 cycles for 16S_Fish, of 98°C for 5 s, 67°C for 20 s, 72°C for 20 s, with an extension of 72°C for 1 min. Each sample was run in triplicate on a LightCycler 480 (Roche Diagnostics). A negative control containing no template DNA and positive control containing fish DNA were included in each PCR amplification run. If either the negative amplified or the positive failed to amplify, the PCR was re-run. If ≥2 replicates of each sample had a ct score <30 for the 18S_SSU, or <40 for 16S_Fish, they were combined to reduce biases produced by amplification from samples with low template concentrations (Murray et al., 2015). Pooled samples were diluted 1:10 for the second stage PCR. In the second stage PCR, a unique tag was attached to each sample (Table 2). PCR reactions (10 μL) were performed with 5 μL 2 × Phusion HF (NEB), 1 μL 100 × Bovine Serum Albumin (NEB), 1 μL of 1 μM of each tag primer, and 2 μL of diluted PCR product from stage one. Thermal cycling conditions were 98°C, for 2 min; followed by 10 cycles of 98°C for 5 s, 55°C for 20 s, 72°C for 20 s, with an extension of 72°C for 1 min. Samples were pooled and purified from unincorporated reaction components by washing, utilizing reversible binding to Ampure (Agencourt) magnetic beads following the manufacturer's protocol. Sequencing of PCR products was performed with an Illumina MiSeq high throughput sequencer, using the MiSeq reagent kit V2 (300 cycles).
Bioinformatics
Amplicon pools were de-multiplexed based on unique 10 bp Multiplex IDentifiers (MIDs) incorporated in the Illumina two-step MID protocol. Fastq files were processed using USEARCH v8.0.1623 (Edgar, 2010). Reads R1 and R2 from the paired end sequencing were merged using the fastq_mergepairs function, retaining only merged reads flanked by exact matches to the primers and primer sequences were trimmed. Reads from all samples were pooled and dereplicated, then clustered into broad Operational Taxonomic Units (OTUs) using the cluster_otus command (-otu_radius_pct = 10). Potentially chimeric reads were discarded during this step. Reads for each sample were assigned to these OTUs (usearch_global -id 0.97) and a summary table generated using a custom R script. Each OTU was identified by BLAST and categorized to closest match using MEGAN 5 (Huson et al., 2007) and the Lowest Common Ancestor (LCA) assignment algorithm. LCA parameters were set at a minimum score of 250 and a top-percent of 5% for the 18S_SSU and 340 and 5% for the 16S_Fish. These cut-offs were determined by manually checking a subset of samples against BLAST. Sequences were also manually checked on Genbank to ensure that all species from that genus in the region were represented. Additional flesh samples were obtained at the Falkland Islands (Gras et al., 2016) and through the Australian Antarctic Division and were sequenced and added to Genbank (see Data Availability Section for accession numbers).
OTUs derived from the 18S_SSU primer set were assigned to class, whereas OTUs derived from the 16S_Fish primers were classified to genus or species. OTUs were assigned only to genus if there was any uncertainty in the species match, either due to insufficient difference between species in the 16S region amplified, or if species from that genus were not present on Genbank. The geographic distribution of species in each genus was checked in Gon and Heemstra (1990) and Duhamel et al. (2014), and species was assigned if only one occurred within the foraging range of BBA from a particular site. In such cases, the species name in tables and figures is given in parentheses. Samples amplified with the 18S_SSU primers were included if they contained at least 100 sequences of food DNA, whereas samples amplified for the 16S primers were included if they contained at least 100 sequences of fish DNA (Jarman et al., 2013). Results are presented as the number of samples with a prey item (n), the frequency of occurrence (FOO) and the relative read abundance of sequences (RRA). For FOO calculations, any food item or fish species was deemed present if it comprised >1% of food sequences for 18S_SSU, or fish sequences for 16S_Fish. The RRA for 18S was calculated as the total sequences for that prey group divided by the total food sequences for that sample, whereas the RRA for the 16S was the number of sequences for a fish species divided by the total fish sequences for that sample. The RRA was averaged across island or year groups. These multiple measures of diet composition are presented to reduce potential biases in interpretation that might result from consideration of a single metric. The results from the 18S region are presented to show the fish component of the diet and allow calculations of the overall proportion of the population consuming discards. Further details and discussion on the proportions of each prey group for each site can be found in McInnes et al. (2017b).
Assessing Overlaps between Commercial Fisheries and BBA Prey
Data on fishery catches and target species were provided by the Directorate of Natural Resources of the Falkland Islands Government; the Australian Fisheries Management Authority and the Australian Antarctic Division; the Pecheker database (Martin and Pruvost, 2007) and online Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) Statistical Bulletins (CCAMLR, 2015). These included fishing effort (total hours for trawl and total hooks for longline); the total catch of target species and the main bycatch species (those comprising >1% of the total catch); the fish bait used in long-line fishing operations; and, at Iles Kerguelen, the mass of target and bycatch species that were discarded. No data were available on Illegal, Unreported, and Unregulated (IUU) fishing. During the relevant sampling periods, trawl and longline fisheries were operating during the sampling period within the Falkland Islands Inner and Outer Conservation Zones (FICZ/FOCZ; Table 2), with no trawl fishing during January; longline but no trawl fisheries were operating within the Kerguelen Economic Exclusion Zone (EEZ); trawl but no longline fisheries operated close to South Georgia during the sampling period (CCAMLR Division 48.3; excluding March); no fishery was operating in the Macquarie Island EEZ; nor was there a fishery within the Admiralty Sound or Magellan Strait, which are used by foraging birds from Albatross Islet during chick rearing (Arata et al., 2014). Fishery species were defined as any target fish species, or bycatch fish species that made up >1% of the total catch (Table 3). Bait species used during fishing operations were also identified. For the main fish species (those contributing >10% of amplified sequences), the depth profile for each species during different age stages were compiled from the literature to determine which were likely to be naturally accessible to albatrosses (Table S1 in Supplementary Material). This study focused on the fishing zones adjacent to the breeding sites, as these are likely to be used more intensively than distant waters by foraging birds during chick-rearing (Phillips et al., 2004; Terauds et al., 2006; Catry et al., 2013; Arata et al., 2014), and secondly, the management of these areas is within the same national jurisdiction as the relevant breeding site. However, we acknowledge that birds may have also interacted with fisheries further from colonies, especially during incubation when BBA are foraging farther from the colony than during chick rearing (Phillips et al., 2004; Wakefield et al., 2011)
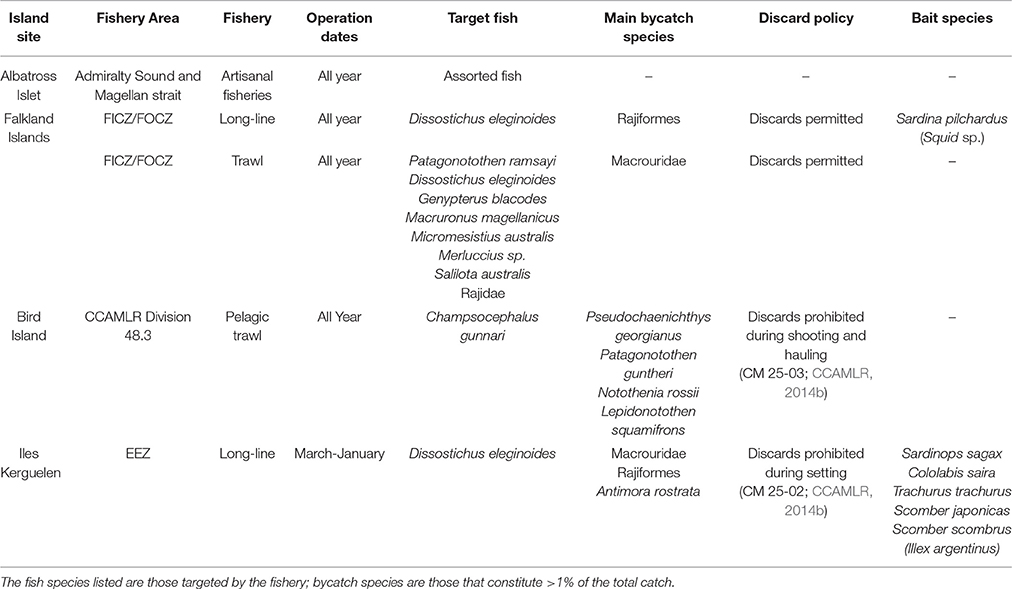
Table 3. Details of commercial fisheries operating in waters adjacent to breeding colonies of black-browed albatrosses during the sampling periods.
Statistical Analyses
Analyses were carried out using R software (R Core Team). Poisson generalized linear models (GLM) with a log link function were used to test if there were differences in fish species composition between colonies and years, and between years and breeding stages at each site. The model included the count of samples with fish DNA (n) as the dependant variable and predictor variables included fish species (F), year (Y) and breeding stage (S), or colony (C). The base model included the sample size as a function of the main effects (fish species, year, breeding stage, or colony) as well as the year:stage or year:colony interaction. These terms effectively describe the patterns in the data arising from the experimental sampling process (e.g., total number of samples within a given year). The interaction terms, fish:year, fish:stage, or fish:colony were added to the base model to test the effect of year or stage (or colony for the pooled data) on diet composition. The analysis of deviance (with Chi-squared test) and Akaike's information criterion (AIC) were used to compare fitted models and test the significance of predictor terms (Burnham and Anderson, 2002). A linear regression was used to assess the relationship between the proportion of samples with discards and breeding success, based on monitoring of BBA nesting attempts at each colony in the year that the diet samples were collected. Dissimilarity indices were calculated with the Manhattan method using the command “vegdist” in the package “Vegan” (Oksanen et al., 2016). From these indices, a hierarchical clustering was then constructed using the average agglomeration method, and plots created using the package “ggplot2” (Wickham, 2009). The proportion of samples that amplified with the 16S_Fish primer which contained species that are also caught in fisheries was calculated, and applied to the total number of samples collected that amplified fish with the 18S_SSU primers.
Results
Diversity, and Spatial and Temporal Variability in Fish Prey of BBA
A total of 1,091 scat samples were collected. DNA was amplified in 793 samples using the 18S_SSU primers; 372 samples contained at least 100 sequences of food DNA, and 327 contained fish DNA. These samples were then amplified with the 16S_Fish group specific primers; 295 samples contained at least 100 fish sequences and were included in subsequent analyses (Table 1).
Fish were found to be the most common prey group across all sites and years, based on the 18S_SSU data. In total, 91% of samples contained fish and this made up 72% of sequences (ranging from 73 to 100% of samples, and between 41 and 97% of sequences at different sites; Table 1). Chondrichthyes (sharks and skates) were present in 2.7% of samples and comprised 2% of these sequences (Figure 2).
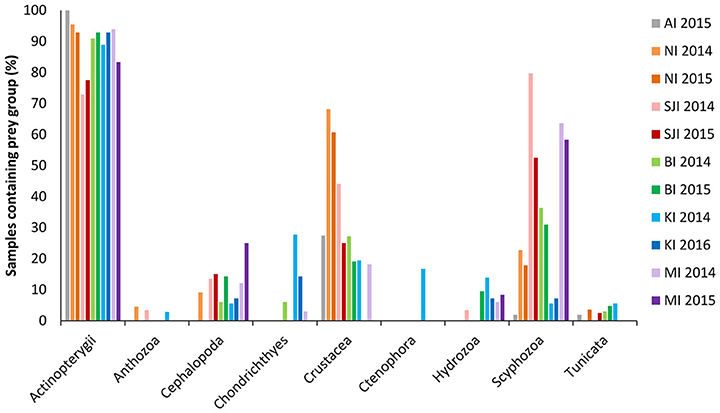
Figure 2. Overall prey groups found in black-browed albatross scats from 2014 to 2016 using 18S_SSU primers. Sites were New Island (NI) and Steeple Jason Island (SJI), Falkland Islands; Bird Island, South Georgia (BI); Canyon des Sourcils Noirs, Iles Kerguelen (KI); and Macquarie Island (MI). Values represent the frequency of occurrence for each site. Lighter colored bars correspond to 2014, darker bars to 2015 (or 2016 for Iles Kerguelen). A prey group was considered to be present if it contributed >1% of the total sequences for that sample. Further details on the non-fish prey groups can be found in McInnes et al. (2017b).
The higher resolution provided by the mtDNA 16S marker identified at least 51 fish species, from 33 families in the diet of BBA across the six breeding sites, with 23 species constituting >10% of the amplified sequences for different colonies and years (Table 4). The most common fish prey belonged to four families: Nototheniidae (notothens), Channichthyidae (crocodile icefishes), Congiopodidae (horsefishes), and Clupeidae (herrings, sardines and allies; Tables 5, 6). Colonies were clustered into four distinct groups according to fish species composition: (1) Falkland Islands and Albatross Islet, (2) Iles Kerguelen, (3) Macquarie Island, and (4) Bird Island (Figure 3). When grouped by family, clusters were similar to fish species, except samples from Steeple Jason in 2015 were more similar to those from Iles Kerguelen due to the high occurrence of Nototheniidae. Fish from the family Nototheniidae were common to all groups. Clupeidae was common in group 1, Channichthyidae in groups 2 and 4, and Congiopodidae in group 3. The differences between years were less marked than those among colonies, as the inclusion of colony provided the best model fit (Base model AIC = 1,618, F:Y AIC = 1,560, F:C AIC = 1,054, F:C+F:Y AIC = 1,175). Two to eight fish species contributed >10% of the fish prey for each colony-year combination (in either FOO or RRA), and were found in more than one sample (Table 4).
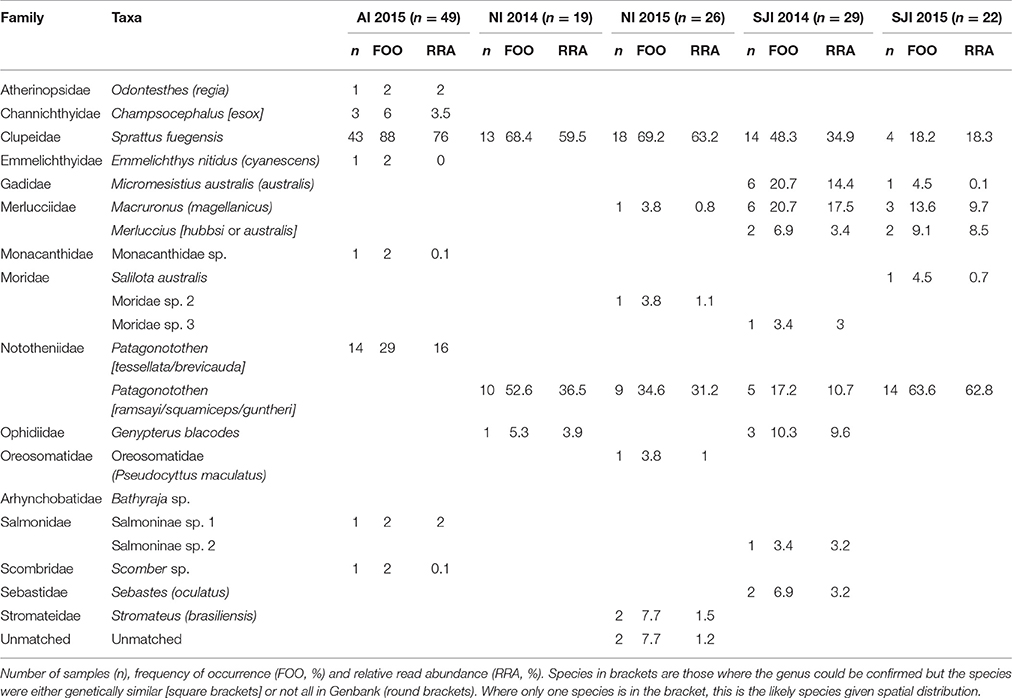
Table 5. Fish prey of black-browed albatrosses at Albatross Islet (AI), Chile; and New Island (NI) and Steeple Jason Island (SJI), Falkland Islands.
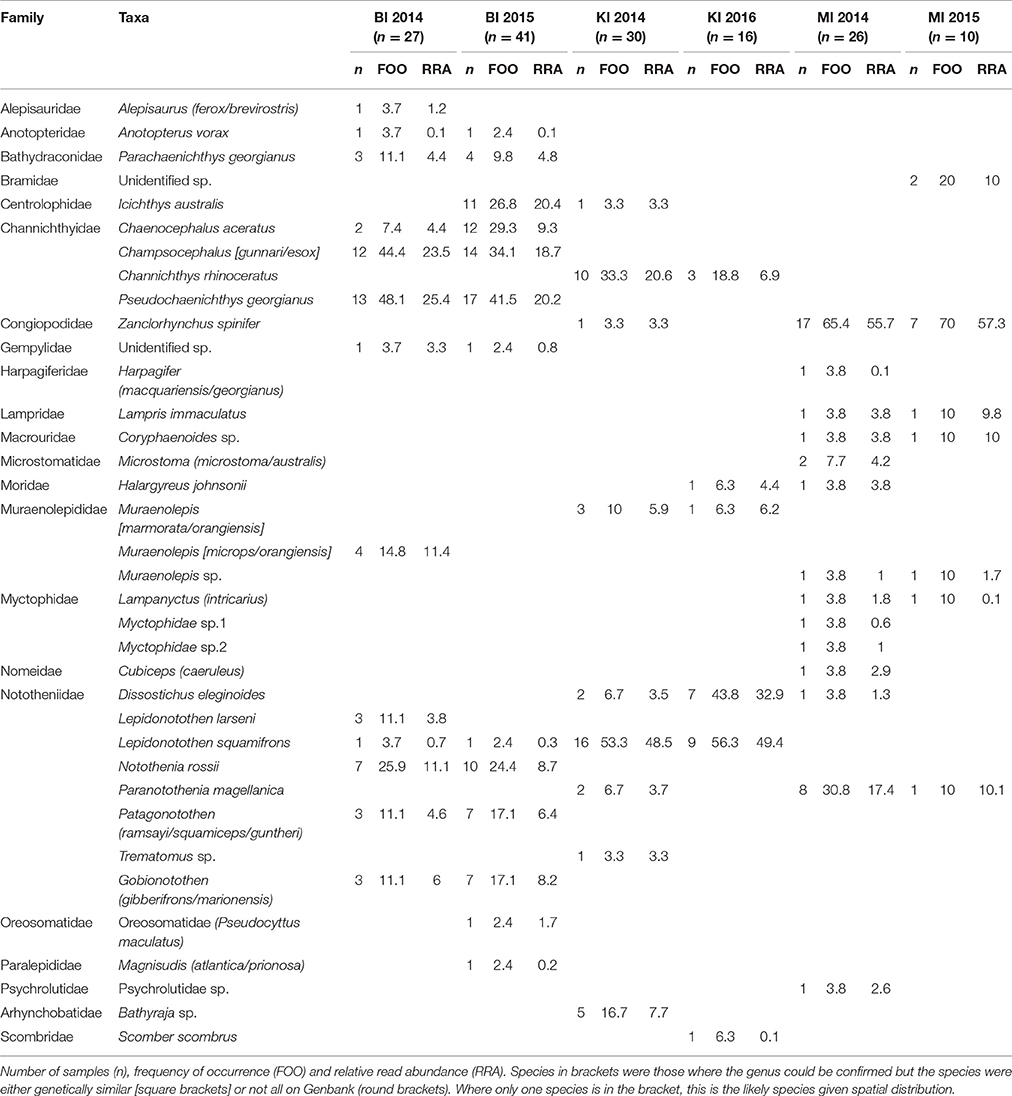
Table 6. Fish prey of black-browed albatrosses at Bird Island, South Georgia UK (BI); Iles Kerguelen, France (KI) and Macquarie Island, Australia (MI).
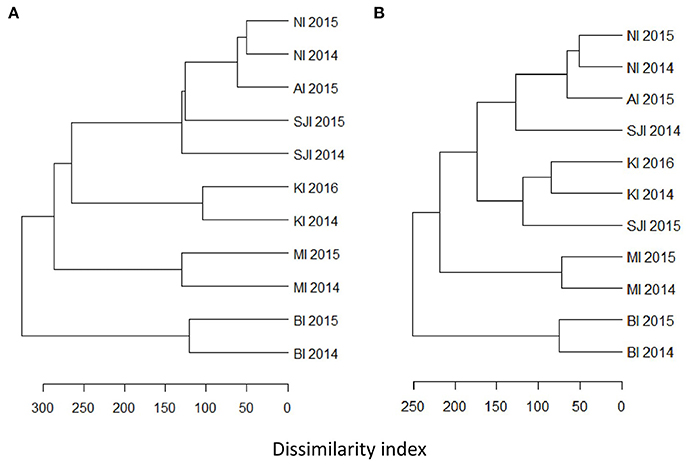
Figure 3. Hierarchical clustering of frequency of occurrence of fish at each site at the level of (A) species, and (B) family. Clusters were based on dissimilarity indices calculated with the Manhattan method, and hierarchical clustering was constructed using the average agglomeration method.
Albatross Islet
Eight fish species were found in the 49 samples from Albatross Islet; the majority contained Fueguian sprat (Sprattus fuegensis; 88% FOO), and black southern cod or Patagonian rockcod (Patagonotothen tessellata or brevicauda) was the second most common item (29% FOO; Table 5, Figure 4).
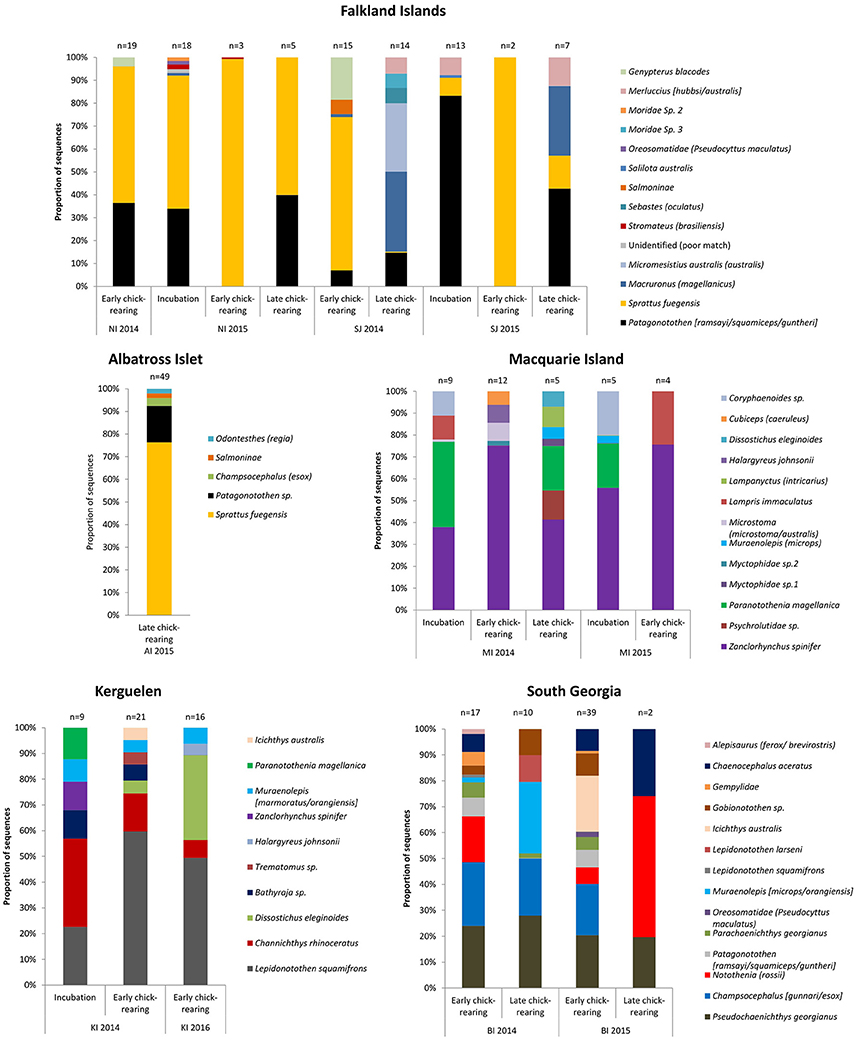
Figure 4. The proportion of fish sequences in the diet of black-browed albatrosses by breeding stage, site and year. Breeding stages were: incubation (October-mid December), early-chick rearing (mid-December-end of January), late-chick-rearing (February onwards). The single sample collected at Macquarie Island during late chick rearing in 2015 was excluded (the DNA sequences were all from the family Bramidae; genus unknown).
Falkland Islands
Eight fish species were identified in the 45 samples from New Island, and contained almost exclusively (>90% of sequences) Fueguian sprat (68% FOO) and rockcod (Patagonotothen sp.; 53% FOO; Table 5). There was no difference in the FOO of fish species consumed between years [Base model AIC = 80.27, F:Y AIC = 84.05; 10.22, p = 0.17] or breeding stages [F:S AIC = 97.45; 10.82, p = 0.70; Figure 4].
Ten fish species were identified in the 51 samples from Steeple Jason Island, of which sprat was the most common species in 2014 (48% FOO), followed by hoki (Macruronus magellanicus; 21% FOO), southern blue whiting (Micromesistius australis australis; 21% FOO), rockcod (17% FOO) and kingclip (Genypterus blacodes; 10% FOO; Table 5). In 2015, rockcod was the main item (64% FOO) followed by sprat (18% FOO) and hoki (14% FOO). There was a difference in the fish species consumed between years [Base model AIC = 157.4, F:Y AIC = 152.51; = 22.90, p < 0.01] and breeding stages [F:S AIC = 129.3; = 64.13, p < 0.001]. When data were adjusted for year, the effect of stage was still significant [F:Y and F:S AIC = 142.5; 46.1, p < 0.001], but not vice versa [F:S and F:Y AIC = 142.5; = 4.83, p = 0.85]. This is likely to be an artifact of the timing of sampling, as no samples were collected in incubation in 2014. During incubation in 2015, samples comprised mostly rockcod, whereas in both years, samples collected during early chick-rearing were mostly of sprat and in 2014 were of kingclip. During late chick-rearing diet was more diverse, including hoki and rockcod in both years, southern blue whiting in 2014, and sprat in 2015 (Table 5, Figure 4).
South Georgia
Sixteen fish species were found in the 68 samples from Bird Island, with two species particularly common in both years: South Georgia icefish (Pseudochaenichthys georgianus; 48 and 42% FOO) and mackerel icefish (Champsocephalus gunnari; 44 and 34% FOO). Marbled rockcod (Notothenia rossii; 26 and 24% FOO), yellow-fin notothen (Patagonotothen guntheri; 11 and 17% FOO) and humped rockcod (Gobionotothen sp.; 11.1 and 17.1% FOO) were also common. In 2014, moray cod (Muraenolepis (microps/orangiensis; 14.8% FOO) was in >10% of samples, whereas in 2015 a large proportion of samples included blackfin icefish (Chaenocephalus aceratus; 29% FOO) and southern driftfish (Icichthys australis; 27% FOO; Table 6, Figure 4). There was an effect of year [Base model AIC = 200.2, F:Y AIC = 196.3; = 33.9, p < 0.01] and breeding stage on fish consumed [F:S AIC = 200.7; = 29.5, p = 0.01]. However, although breeding stage was statistically significant, the base model excluding stage still provided a better fit to the data, even when both year and stage were included [F:Y and F:S AIC = 207.3; = 18.9, p = 0.21; F:S and F:Y AIC = 207.3; = 23.3, p = 0.08].
Iles Kerguelen
Eleven fish species were found in the 46 samples from Iles Kerguelen, with the main fish species gray rockcod (Lepidonotothen squamifrons; 53 and 56% FOO) and unicorn icefish (Channichthys rhinoceratus; 33 and 19% FOO) in both years. In 2014, the other common species were skates (Bathyraja sp.; 17% FOO) and moray cod (Muraenolepis marmoratus/orangiensis; 10% FOO), whereas in 2016, Patagonian toothfish (Dissostichus eleginoides) was the second most common item (44% FOO; Table 6, Figure 4). There were more samples with unicorn icefish during incubation than chick-rearing, whereas all the toothfish was consumed during chick-rearing. There was an effect of year [base model AIC = 113.6, F:Y AIC = 112.8; = 20.8, p = 0.03] and breeding stage on the fish species consumed [F:S AIC = 113.1; = 20.5, p < 0.03].
Macquarie Island
Sixteen species were found in the 36 samples from Macquarie Island (Table 6). In both years, samples mostly contained Antarctic horsefish (Zanclorhynchus spinifer; 65 and 70% FOO) and Magellanic rockcod (Paranotothenia magellanica; 31 and 10% FOO). In 2015, one unidentified species, likely from the family Bramidae, made up 20% of samples, although this may reflect the small sample size (n = 10). Fish species composition did not differ between years [Base AIC = 177.4, F:Y AIC = 190.6; = 16.8, p = 0.33]; the effect of breeding stage was of borderline statistical significance [F:S AIC = 193.5; = 43.9, p = 0.05], but the base model excluding stage still provided a better fit to the data.
Overlaps between Commercial Fishery Species and BBA Prey
Longline Fisheries
Diets of BBA from New Island and Steeple Jason Island did not include any target or bycatch species from longline fisheries operating in the Falkland Islands FICZ/FOCZ. At Iles Kerguelen, diet samples included DNA from the target species, Patagonian toothfish in January of both years (with much higher proportions in 2016; Tables 6, 7) and a bycaught group, skates, in December/January 2014/15 (Table 7, Figure 5). Bait fish, Scomber scombrus also appeared in samples, but occurred infrequently (<2% of sample sequences). This is a northern hemisphere species used as baits in longline fishing, and is therefore only available to BBA from fisheries. In the Kerguelen EEZ, the amount of toothfish discarded was lowest in November and December 2013 (0.19 and 0.18 t, respectively) and highest in January (1.6 t in 2014 and 2.9 t in 2016). More skates were discarded in December and January 2013/14 (0.3 t in November, 5.3 t in December and 9.4 t in January 2013/14; and 2 t in January 2016), which matched with the relative FOO in the diet in the 2 years.
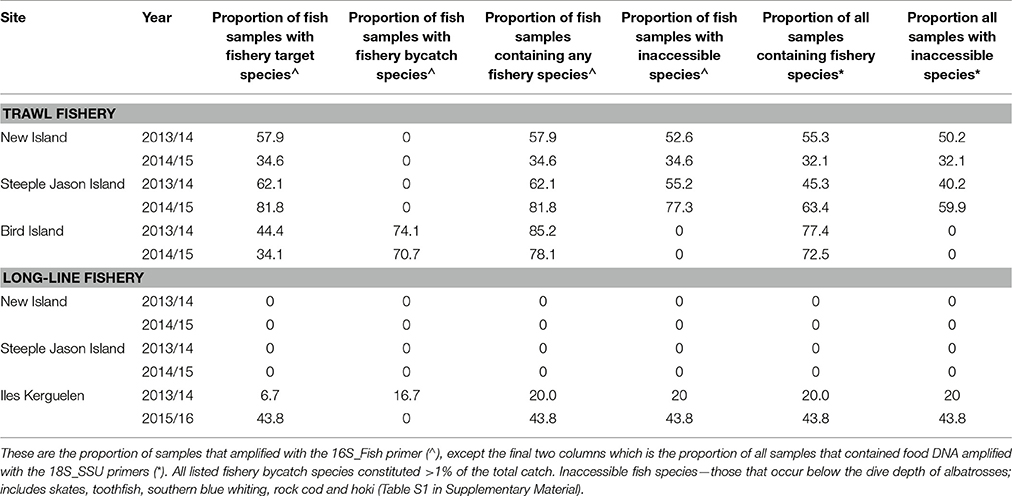
Table 7. The proportion of scat samples that contained DNA from target and bycaught species in commercial fisheries operating in adjacent waters during the study.
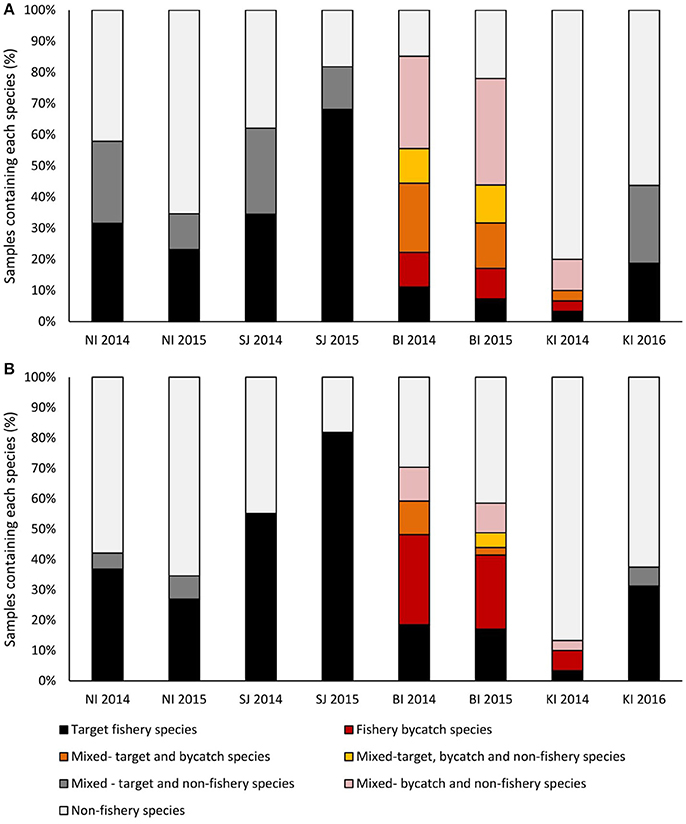
Figure 5. The proportion of samples that contained target, bycatch or non-fishery species as either (A) >1% of sequences in a sample, or (B) >75% of the sequences in a sample. Samples with <75% of sequences in any category were considered to be mixed. Bycatch species each constituted >1% of the total catch. Sites were New Island (NI) and Steeple Jason Island (SJI), Falkland Islands; Bird Island, South Georgia (BI) and Canyon des Sourcils Noirs, Iles Kerguelen (KI).
Trawl Fisheries
The trawl fisheries operating in the Falkland FICZ/FOCZ target eight fish species (Table 3). No bycatch species made up more than 1% of the reported catch. Fishery target species were found in the diet at both sites in each year (Table 7, Figure 5). At New Island, the main fishery target species in the diet was rockcod (91% of those samples with a target species); one sample also contained hoki, and one was of kingclip only. At Steeple Jason Island, BBAs consumed five target species in 2014 (rockcod, hake, hoki, southern blue whiting, and kingclip), whereas samples included three target species in 2015 (rockcod, hoki, and hake; Table 6, Figure 6). The number of samples with fishery target species was lower during early chick rearing (December/January) than either incubation (October-November) or late chick rearing (February-March; Figure 6A). This corresponded to the relative catch in the fishery, particularly during January when it was not operating (Figure 6A). The main catch species in the fishery was rockcod during incubation, and hoki in late chick rearing (Figure 6C). The cluster analysis showed four distinct clusters, with one highlighting the similarity between fishery catch and fish prey of BBA during December 2013 at New Island and October 2014 at both sites, and between fishery catch during March and fish prey of BBA at Steeple Jason (Figure 6D).
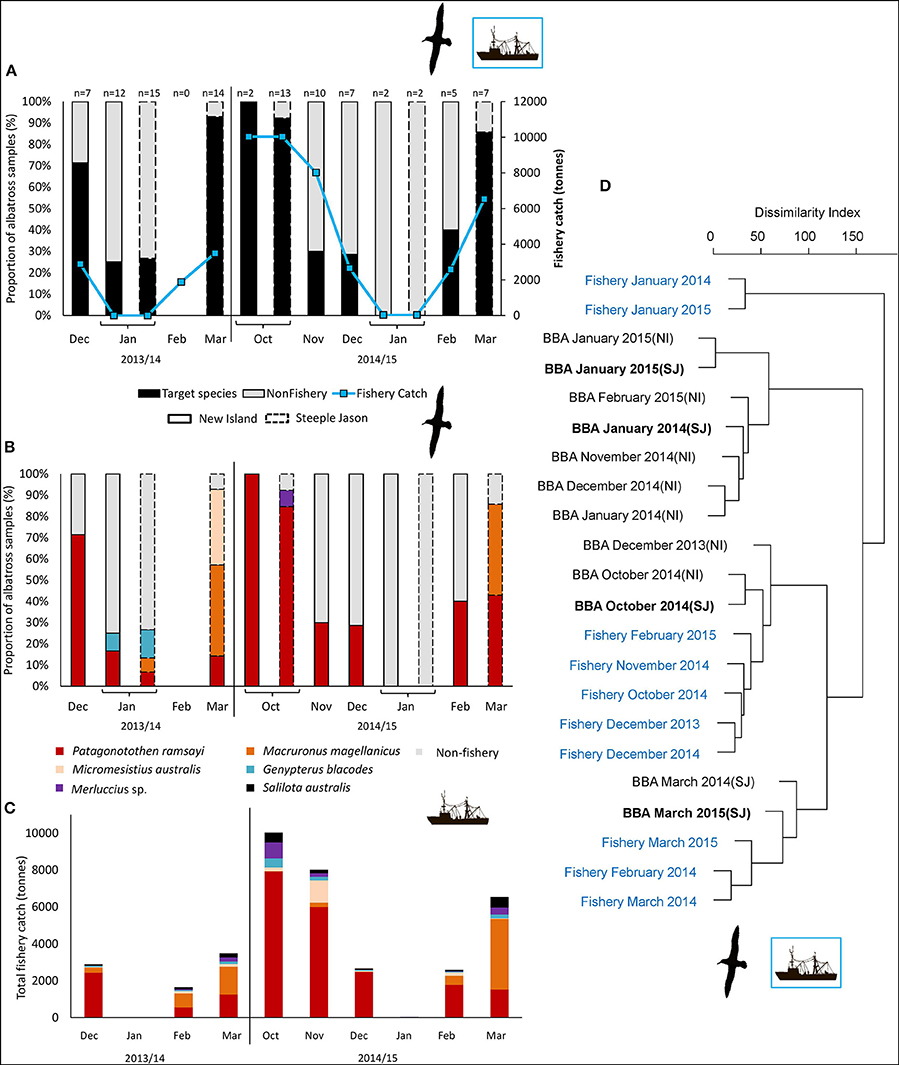
Figure 6. Comparison between back-browed albatross fish prey and fishery catch amounts at the Falkland Islands by month from December 2013-March 2014 (excluding February) and October 2014 to March 2015. Solid borders represent New Island; dashed borders represent Steeple Jason. (A) Scats with or without fishery target species (black and gray bars, respectively), compared to the total catch in the fishery (blue line). (B) The proportion of scat samples containing each of the target species. (C) Total catches in the fishery by species. (D) The hierarchical clustering of albatross diet and fishery catch data by month, based on the proportion of sequences (RRA, black text) and proportion of catch (blue text). Clusters were based on dissimilarity indices calculated with the Manhattan method, and hierarchical clustering was constructed using the average agglomeration method (note low sample sizes during January 2014 and 2015). As food DNA may persist in scats for several days (Deagle et al., 2010), there may be some carry-over of prey caught in the previous month if samples were collected early in the month, which was the case in January of both years.
At South Georgia, the fishery target species (mackerel icefish) and four bycatch species (South Georgian icefish, yellow-fin notothen, gray rockcod, and marbled rockcod) were all recorded in the diets of BBA (Table 6), and in a substantial proportion of the samples in both years (Table 7, Figure 5). The amount of target and bycatch fish species in the diet of BBA did not correspond to the relative catch rates in the fishery. During the sampling period the fishery caught very little mackerel icefish during January 2014 (65 kg), and only 3 tons during February 2014, with 1 ton of South Georgia icefish as bycatch. Over the same period in 2015, the fishery caught 133 tons of mackerel icefish in January, and 144 tons in February, with 70 and 51 tons of yellow-fin notothen bycaught in each month, respectively. Other bycatch included 1 ton of South Georgia icefish, 2 tons of gray rockcod and 4 tons of marbled rockcod, all of which were caught during the 2015 South Georgia groundfish survey.
Breeding Success and Use of Discards
The proportion of sampled birds that had consumed discards was estimated to range from zero at Bird Island to 60% at Steeple Jason. This is based on the conservative assumption that any species which was also available naturally to albatrosses was not considered to have been obtained as a discard (Table 7). Breeding success (chicks fledged/eggs laid) during the years that diet samples were collected ranged from zero at Albatross Islet to 84% at New Island. There was a positive correlation between the proportion of samples that contained discarded fish, and breeding success (r = 0.81, p < 0.001; Figure 7).
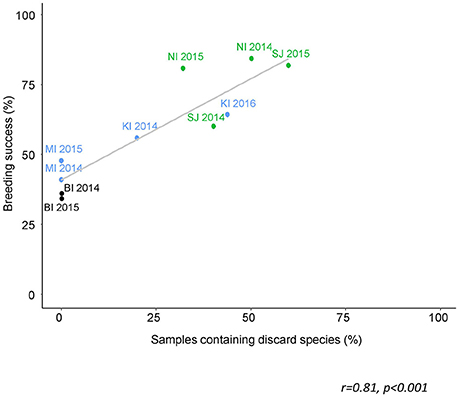
Figure 7. The proportion of scat samples from black-browed albatrosses that contained discard species in relation to breeding success for that site and year. The colors corresponds to the current population trend: green—increasing; blue—stable; black—declining (ACAP, 2010; Wolfaardt, 2013; Poncet et al., 2017). These are minimum estimates of discard occurrence, including only the species that are not known to be naturally accessible to albatrosses (Table S1 in Supplementary Material). Albatross Islet suffered complete breeding success failure due to mammalian predation and was therefore excluded from the analysis.
Discussion
This is the first study to use DNA metabarcoding to identify the fish prey diversity of seabird and use this to evaluate the occurrence of fishery discards in the diet across a broad geographic scale. This technique enabled us to identify an extensive diversity of fish in the diet of BBA, including a similar number of species and families as that recorded in all previous published studies for this species combined (31 families and 52 species). We also detected more fish species on average at each sampling site than in the conventional studies based on otoliths and similar numbers to studies using multiple body parts (Data Sheet 1 in Supplementary Material). There was a clear overlap between the species targeted by fisheries operating in adjacent waters, and the diet of BBA at the local colony. This was most evident at the Falkland Islands and Iles Kerguelen where the higher catch rates of target and bycatch species (or the amount discarded, where known) in a particular year corresponded with the relative occurrence in the diet. Our data also highlighted regions, such as South Georgia, where BBA diet overlapped with fishery target species, but the birds likely obtained the fish naturally. In this situation, there is the potential for resource competition but no reason to assume direct interaction with vessels or incidental mortality of foraging adults.
Amplification Success
The number of samples that contained food DNA in this study varied between sites and in some cases were quite low. There are numerous factors that can affect the amplification of food DNA including the primers/markers chosen, whether blocking primers were used, sample selection, timing during the breeding season and experience of the field personnel. We chose the combination of the universal metazoan marker (18S) and group specific markers (16S) to get a broad picture of the diet at the population level and specific information on the fish species consumed. Universal metazoan markers are useful dietary markers as they amplify DNA from all eukaryotes, which enables all possible prey groups to be identified. However, they also amplify non-food DNA such as plant, parasite, and consumer DNA (McInnes et al., 2017a). A consumer blocking primer can increase the detection of food DNA (Vestheim and Jarman, 2008), but was not used in this study as they may inadvertently block similar groups such as other vertebrates like fish (Piñol et al., 2015). This likely reduced the sample size, but provided more reliable results from higher quality samples containing more food DNA. During our study, fresh samples were targeted and the inadvertent collection of dirt and vegetation was minimized where possible. However, with such a large study across a range of environmental conditions this was not always possible. In addition, many samples collected during incubation had little food DNA due to birds fasting. Subsequent to the data collection for this study, optimized scat collection protocols for DNA dietary metabarcoding have been developed that will hopefully improve the amount of DNA detected in future studies allowing diet data to be collected during all breeding stages (McInnes et al., 2017a).
Fish Prey Diversity
The fish prey consumed by BBAs varied considerably across their breeding range. Species in the family Nototheniidae were common in scat samples from the sub-Antarctic sites, as were icefish (Channichthyidae) and horsefish (Congiopodidae). However, aside from the genus Patagonotothen, there were no nototheniids found in the samples from the Falkland Islands and Chile, whereas sprat (Clupeidae) was common. Of those species that contributed >10% of the sequences/samples at any site, 80% of these fish species were likely to be obtained naturally, as they are known to occur at depths accessible to albatrosses (maximum 4.5 m; Prince and Huin, 1994). The remaining species are not known to occur in waters shallower than 4.5 m and are hence likely to be obtained as discards.
This study detected several species that were not identified, or were very uncommon, in the diet of BBA in previous studies, particularly the Fueguian sprat, Antarctic horsefish, and southern driftfish. This was the first study of fish in the diet of BBA at Albatross Islet, and the first published study of BBA diet at Macquarie Island, which may explain some of these discoveries. Sprat was not recorded previously in the diet of BBA at any site (Data Sheet 1 in Supplementary Material), despite being the most common item in our study at the Falkland Islands and Chile. There was an unidentified clupeid in the diet at Diego Ramirez in 2001 and 2002 (Arata and Xavier, 2003), and a small unidentified fish at New Island in 1987 that made up 80% of the fish prey (Thompson, 1992), which may have been sprat. This species has a high biomass across the southern Patagonian shelf as far as the Magellan Strait (Sánchez et al., 1995), Chilean channel waters (Diez et al., 2012) and around the Falkland Islands (Agnew, 2002), and is common in the diet of other seabirds and marine mammals in the region (Thompson, 1993; Baylis et al., 2014; Handley et al., 2016). There is also a sprat hotspot to the west of the Falkland Islands, close to both Steeple Jason and New Island, which may explain the prevalence in the diet at these sites (Gras et al., 2017).
Antarctic horsefish was the main fish species consumed at Macquarie Island and is endemic to the Macquarie and Kerguelen plateaus (Duhamel et al., 2014). Antarctic horsefish has been detected previously, but only in low frequency in BBA diets at Iles Kerguelen (Cherel et al., 2000, 2002). Very little is known about the abundance of horsefish or other fish around Macquarie Island. Horsefish have been detected in the diet of gentoo penguins (Pygoscelis papua; 39% FOO) and itinerant New Zealand sea lions at Macquarie Island (Phocarctos hookeri; 63% FOO; Robinson and Hindell, 1996; McMahon et al., 1999); however the majority of fish consumed by other seabirds and seals are myctophids, and to a lesser extent nototheniids (Goldsworthy et al., 2001).
The southern driftfish was detected in a quarter of samples at Bird Island in 2015 and one sample at Iles Kerguelen. It has only been recorded once in the diet of BBA at South Georgia, in 1986 (Reid et al., 1996; Croxall et al., 1997), and rarely in the diet of other seabirds (Croxall et al., 1995; Catard et al., 2000), though has been detected more commonly in seal diets (Guinet et al., 2001; Lea et al., 2008). Southern driftfish have a circumpolar distribution (Gon and Heemstra, 1990), although are rarely caught during trawls in the Scotia Sea (Collins et al., 2012) and none were recorded during a groundfish survey in January 2015, at the time when the scat samples were obtained (Belchier et al., 2015). It is surprising given our results that only one sample was detected in 20 years of conventional diet studies at South Georgia (British Antarctic Survey unpublished data). There are a few possible explanations: most of the previous studies were later in the season (February onwards) and represent chick diet, whereas our samples were from adults; alternatively, driftfish may be consumed as larvae and therefore the hardparts may be undetectable in stomach contents.
The other main fish prey at Bird Island and Iles Kerguelen were similar to the previous studies at each site (Data sheet 1 in Supplementary Material). At South Georgia, mackerel icefish are common prey (Prince, 1980; Reid et al., 1996; Croxall et al., 1997, 1999; Xavier et al., 2003). However, the diversity of fish in our study was much higher than in previous studies at Bird Island using only otoliths, which identified ten fish species overall, and less than five in any year (Data Sheet 1 in Supplementary Material). In comparison, we detected 16 species using DNA metabarcoding, with 13 in each year. Some of this diversity could relate to secondary ingestion; however, all of the species that contributed >10% of the diets (n = 8 and n = 7) were the sole prey item in at least one sample, suggesting they were the primary prey. At Iles Kerguelen, gray rockcod and unicorn icefish are two of the most abundant fish species in the Kerguelen EEZ (Duhamel and Hautecoeur, 2009) and were common in the diet of BBA at Canyon des Sourcils Noirs in a previous study (Cherel et al., 2000). When sample size differences were taken into account, the fish diversity was similar to previous studies at Iles Kerguelen where otoliths, bones and vertebrae were used (Cherel et al., 2000). In our study, there were some fish species identified in just one sample that are not usually found at those sites, such as Trematomus sp. at Iles Kerguelen. These could have originated from scats produced by juvenile or non-breeding birds, or as residual DNA from previous foraging trips far from the islands. For these reasons, we focused on fish species present in at least 10% of samples.
Overlaps between Commercial Fisheries and Albatross Diet
There were five fish species detected in the scats that were unlikely to be naturally accessible to BBAs during the sampling period due to the known depth profile of fish. These included skates and Patagonian toothfish at Iles Kerguelen, and rockcod, hoki and southern blue whiting at the Falkland Islands. These species were present in fishery catches from the same time period, suggesting vessels were the likely source. At Iles Kerguelen, Patagonian toothfish and skates have no developmental stage where they have been observed at an accessible depth to albatross (Table S1 in Supplementary Material). Skates are demersal and the closest to the surface that toothfish have been recorded is during their larval stage (~50 m depth), during winter and spring at Iles Kerguelen (Loeb et al., 1993; Mori et al., 2016). Patagonian toothfish were the most common fish in previous BBA dietary studies at Iles Nuageuses in 1994, and second most common at Canyon des Sourcils Noirs in 1994 (Cherel et al., 2000, 2002). These studies and others on wandering albatross (Diomedea exulans) suggest that albatross can consume Patagonian toothfish naturally (Weimerskirch et al., 1997), but how they obtain demersal prey is largely unknown (Cherel et al., 2000). It is possible that albatrosses scavenge prey brought up by deep-diving predators such as seals or whales (Sakamoto et al., 2009). In our study, the occurrence of Patagonian toothfish in the diet of birds from Iles Kerguelen did increase with an increase in discards, however, the amount of discarded toothfish from the fishery was low relative to the large albatross population. This result suggests that albatross may also be consuming Patagonian toothfish as natural prey as well as fishery discards during this study in unknown respective proportions. Although seabird bycatch rates in this fishery were very high in the 1990s and early 2000s (Delord et al., 2005), no albatross mortalities have been reported in recent years (CCAMLR, 2014a). This reflects the adoption of mitigation measures which include night setting, streamer lines, retention of offal during setting and fast hook sink rates (CCAMLR, 2014b). Discarding is still permitted in the Kerguelen toothfish fishery, which is still of concern. Discards increase vessel attractiveness and it is difficult to ensure mitigation is 100% effective for the smaller, more maneuvrable, deeper-diving species, particularly those such as white-chinned petrels (Procellaria aequinoctialis) which, unlike albatrosses, scavenge behind vessels in large numbers during darkness (Phillips et al., 2016). Moreover, individual birds will associate vessels with food, which is problematic if they overlap with fisheries under a different jurisdiction where there is poor compliance with seabird bycatch mitigation. Indeed, wandering albatrosses, which habitually follow vessels, may alter their flight path from 30 km away to approach a fishing vessel (Collet et al., 2015).
At the Falkland Islands, the frequency of rockcod and hoki in the diet corresponded to the relative fishery catches of these species, suggesting they were likely obtained as discards. Although this correlation could also reflect availability of fish stocks, these species are not known to be naturally accessible to albatross. Occurrence varied between sites and breeding stages, and was lowest during early chick rearing, which is consistent with the previous stable isotope study which found that pelagic fish were more common than demersal species (Granadeiro et al., 2013). During early chick-rearing, the fishery catch was zero to low, and therefore there was limited opportunity to exploit discards. However, during incubation and late chick rearing, the frequency of target fishery species in the albatrosses' diet was much higher. The occurrence of fishery species in BBA samples was greater at Steeple Jason than New Island, which is 70 km further south. This may be an artifact of the sampling month, given differences in timing; however, this does not explain all of the variation. The samples collected in the same month (e.g., January) were comparable, but no trawl fishery was operating. The few samples with fishery target species at New Island in November, when catch rates were relatively high, does not seem to match the trend at Steeple Jason for the preceding month. Previous studies at the Falklands also found more offal and discards in the diet of BBA at Steeple Jason than New Island, however, as there were few heads and therefore otoliths, the fish species could not be identified (Thompson, 1992). In the western part of the FICZ, there are two types of fishing grounds: one is to the northwest of Steeple Jason where trawlers target rockcod and one in deeper waters (>200 m) to the west-southwest which targets primarily hoki and southern blue whiting. Previous tracking studies found that Steeple Jason birds were more likely to attend vessels even when the distance to the fishing ground was similar for each colony (Granadeiro et al., 2011). Further research is needed to understand this observation.
The consumption of fishery discards by black-browed albatrosses at both sites in the Falklands puts birds at risk of incidental mortality. An estimated 800 BBAs are killed annually in Falkland Island trawl fisheries (Kuepfer, 2015). Although use of paired streamer lines is compulsory on all vessels, continuous discarding is still permitted (Quintin and Pompert, 2014). At the time of this study, the fishing fleet had limited capacity to retain offal on-board or process this into fishmeal; however, it has been recommended that any new vessels entering the fishery should have capabilities for more effective waste management (Sancho, 2009). Strict discard policies employed by trawl vessels operating in waters within the jurisdiction of CCAMLR have minimized exposure of birds to warp cables by retaining discards on-board until after shooting or hauling of fishing gear; consequently, the occurrence of incidental mortality is close to zero (CCAMLR, 2014b). Implementation of improved discard management measures around the Falkland Islands will be essential to reduce incidental mortality in trawl fisheries in the future (Abraham et al., 2009; Pierre et al., 2012).
Competition with Fisheries and Reliance on Fishery Discards
South Georgian and mackerel icefishes were the two most common fish consumed by BBA at South Georgia in both years. Although, mackerel icefish is targeted by the fishery, and South Georgia icefish is bycaught, the BBA at Bird Island were likely to have obtained these species naturally. Very few fish were caught by the fishery during the diet sampling period of 2014 and they are known to occur at an accessible depth to albatross. Five other common prey species of BBAs at South Georgia are also caught in the icefish fishery, with bycatch limits set by CCAMLR (South Georgia icefish, marbled rockcod, yellow-fin notothen, humped rockcod, blackfin icefish). Mackerel icefish was the most common fish in the diet of BBA at South Georgia from 1996 to 2000 (Xavier et al., 2003) and in more recent years (British Antarctic Survey unpublished data). Icefish and BBA are both krill predators, and in years of low krill availability, icefish are likely to provide a valuable alternate food source for albatrosses (Reid et al., 1996). The BBA population at South Georgia is declining, and although this appears to be due mainly to incidental mortality during the non-breeding period (Poncet et al., 2017), their breeding success is also lower than conspecifics in the Indian Ocean (Nevoux et al., 2010). During our study, the proportion of krill in the diet was low (Figure 2), and over the last 20 years of conventional sampling (in mid-late chick rearing), krill has contributed <20% of the diet in only 4 years, two of which were 2014 and 2015 (18.5 and 5.6%, respectively; British Antarctic Survey unpublished data). Given the decline in krill, and high consumption by BBAs of species that are also targeted or bycaught in the icefish fishery, continued monitoring and evaluation of potential competition for resources is particularly important at this breeding site.
Another area of potential resource competition is off Chile, where there is currently a sprat fishery between 41 and 45°S, with annual catch limits of 26,000 tons (Leal et al., 2013). There was a proposal to expand this fishery into Chilean channel waters, where it would be likely to overlap with the foraging areas of BBAs from Chilean colonies. Given the importance of sprat in diets, any expansion of the fishery should consider the resource requirements of other marine species, including BBA, especially at the Albatross Islet colony where the foraging range is restricted (Arata et al., 2014). Globally, a third of fish stocks are fished at unsustainable levels (FAO, 2016), and fisheries are fishing down the food web (Pauly et al., 1998), including smaller fish species like sprat (Leal et al., 2013).
Although, competition with commercial fisheries could have a negative impact on albatrosses by reducing available prey, discards from fisheries can provide a supplementary food source (Bugoni et al., 2010; this study). In our study, breeding success was higher at colonies which had a greater occurrence of fishery discards in the diet samples. At the Falkland Islands where the occurrence of discards was high, the BBA population is increasing (Wolfaardt, 2013). Population increases at Chilean colonies have been attributed to a reduction in bird bycatch in longline fisheries (Robertson et al., 2017). However, high breeding success and a population increase could also reflect greater discard availability. Conversely, at Macquarie Island, where there is no local fishery operating during the breeding season, breeding success of BBAs was lower and the population is stable (ACAP, 2010), and at South Georgia, where the icefish fishery is small and provides few discards, BBAs have the lowest breeding success and the population is declining (Poncet et al., 2017). Many factors can impact breeding success, and a snapshot of diet over 2 years is not definitive. For example, the total failure at Albatross Islet was likely due to predation of eggs and chicks by American mink (Neovison vison; WCS unpublished data). However, availability of discards can influence seabird population trends (Foster et al., 2017), and DNA metabarcoding provides a means of further investigation.
Discards create an unnatural food-web structure, and if they are of low nutritional quality, there may be impacts on growth, breeding success and survival (Rosen and Trites, 2000; Grémillet et al., 2008). For BBA, the increasing population trend and high breeding success at sites where discards were common suggests that these were not nutritionally poor. However, discards could be sustaining an artificially high population and their removal might increase inter and intra-specific competition for available resources. Indeed, the European Union is phasing out the practice of discarding bycatch species and offal from 2015 to 2019, and there are concerns about the negative consequences for scavenging seabirds (Bicknell et al., 2013). Southern blue whiting was the main prey targeted by trawl fisheries around the Falklands Islands up until 2006, at which point the stock collapsed, and rockcod (Patagonotothen ramsayi) increased rapidly (Laptikhovsky et al., 2013). Rockcod is now the main target of the trawl fishery and one of the most common fish in the diet of BBAs at the Falklands during this study. Recent rockcod stock assessments indicate that this species is also beginning to decline (Gras et al., 2017). Monitoring the impact on BBA breeding success and their ability to switch to other resources will be important for assessing the degree to which they have been relying on discards. Similarly, improved discard management in the local trawl fisheries may have implications for the BBA population, particularly in the short-term, and any negative effects might be exacerbated by other threats such as climate change, habitat degradation, introduced pests, or disease, which affect many albatrosses globally (Phillips et al., 2016).
Conclusions
This circumpolar study has revealed extensive fish diversity in the diet of BBA using DNA metabarcoding. Many of the fish species in the diet are not known to be naturally available to albatrosses, and were likely obtained by scavenging on discards (non-target fish, processing waste or used longline bait) from fisheries operating adjacent to the colony. Consumption of discards by black-browed albatrosses was detected from the Falkland Islands trawl fishery during incubation and late chick-rearing and from the Iles Kerguelen longline fishery during brood-guard. Our study indicates that improvements in discard management to reduce the attractiveness of vessels and hence incidental mortality of seabirds is likely to have major implications for some albatross populations. DNA metabarcoding of scat samples provides a non-invasive mechanism for quantifying and evaluating the level of interaction between seabirds and fisheries through identification of target and non-target fish, as well as the presence of baits. This provides an avenue for assessing compliance of fisheries with discard policies, and the effects on the level of interaction with scavenging seabirds.
Author Contributions
JM, RA, SJ, ML, and BR conceived and designed the project; RA, RP, AS, PC, HW, AK, contributed samples; JM and SJ designed primers; JM performed laboratory work; JM and BD performed bioinformatics; JM and BR performed statistical analyses; MG, DM, and YC provided fishery data or information on fish biology; JM wrote the first draft of the manuscript, and all authors contributed substantially to revisions.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was approved by the University of Tasmania Animal Ethics Committee (Permit A13745). Funding was provided by Australian Antarctic Science Grant (4014 and 4122) and the Winifred Violet Scott Charitable Trust; further funding was received from the Falkland Islands Government and from FCT—Portugal through the strategic project UID/MAR/04292/2013 granted to MARE. Falkland Islands fishery catch data were provided by the Directorate of Natural Resources—Fisheries Department of the Falkland Islands Government. Iles Kerguelen fishery data were provided through the Pecheker database with thanks to Guy Duhamel, Nicolas Gasco, Alexis Martin, Patrice Pruvost and Charlotte Chazeau. Macquarie Island data were provided by the Australian Fisheries Management Authority with assistance from Dirk Welsford at the Australian Antarctic Division. South Georgian fishery data was obtained through CCAMLR statistical bulletins. Thanks to the large number of field personnel for scat collections including Javier Arata; fishery observers for obtaining catch data; Mark Belchier and Phillipe Koubbi for advice regarding fish diversity data; the Wildlife Conservation Society for access to Steeple Jason Island and permission to collect samples; and James Marthick and the Menzies Institute (UTAS) for the use of the Miseq Genome Sequencer.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2017.00277/full#supplementary-material
Data Availability
Fish sequences not currently of Genbank were added including: Sprattus fuegensis, Genypterus blacodes, Iluocoetes fimbriatus, Salilota australis, Icichthys australis, Anotopterus vorax, Halargyreus johnsonii (GenBank accession numbers MF346066-074).
References
Abraham, E. R., Pierre, J. P., Middleton, D. A. J., Cleal, J., Walker, N. A., and Waugh, S. M. (2009). Effectiveness of fish waste management strategies in reducing seabird attendance at a trawl vessel. Fish. Res. 95, 210–219. doi: 10.1016/j.fishres.2008.08.014
ACAP (2010). Agreement on the Conservation of Albatrosses and Petrels: Species assessment: Black-browed Albatross Thalassarche melanophris. Available online at: http://www.acap.aq on (Accessed Nov 7, 2016).
Agnew, D. J. (2002). Critical aspects of the Falkland Islands pelagic ecosystem: distribution, spawning and migration of pelagic animals in relation to oil exploration. Aquat. Conserv. Mar. Freshw. Ecosyst. 12, 36–50. doi: 10.1002/aqc.474
Alonso, H., Granadeiro, J. P., Waap, S., Xavier, J., Symondson, W. O. C., Ramos, J. A., et al. (2014). An holistic ecological analysis of the diet of Cory's shearwaters using prey morphological characters and DNA barcoding. Mol. Ecol. 23, 3719–3733. doi: 10.1111/mec.12785
Arata, J. A., Vila, A. R., Matus, R., Droguett, D., Silva-Quintas, C., Falabella, V., et al. (2014). Use and exploitation of channel waters by the black-browed albatross. Polar Biol. 37, 565–571. doi: 10.1007/s00300-014-1458-1
Arata, J., and Xavier, J. C. (2003). The diet of black-browed albatrosses at the Diego Ramirez Islands, Chile. Polar Biol. 26, 638–647. doi: 10.1007/s00300-003-0530-z
Barbraud, C., Marteau, C., Ridoux, V., Delord, K., and Weimerskirch, H. (2008). Demographic response of a population of white-chinned petrels Procellaria aequinoctialis to climate and longline fishery bycatch. J. Appl. Ecol. 45, 1460–1467. doi: 10.1111/j.1365-2664.2008.01537.x
Barrett, R. T., Camphuysen, K., Anker-Nilssen, T., Chardine, J. W., Furness, R. W., Garthe, S., et al. (2007). Diet studies of seabirds: a review and recommendations. ICES J. Mar. Sci. 64, 1675–1691. doi: 10.1093/icesjms/fsm152
Baylis, A. M. M., Arnould, J. P. Y., and Staniland, I. J. (2014). Diet of South American fur seals at the Falkland Islands. Mar. Mamm. Sci. 30, 1210–1219. doi: 10.1111/mms.12090
Belchier, M., Gregory, S., Fallon, N., McKenna, J., Hill, S., Soffker, M., et al. (2015). WG-FSA-15/30: Report of the UK groundfish survey at South Georgia (CCAMLR Subarea 48.3) in January 2015. C.W.G.O.F.S. Assessment. Hobart, TAS: Commission for the Conservation of Antarctic Marine Living Resources.
Bertellotti, M., and Yorio, P. (2000). Utilisation of fishery waste by Kelp Gulls attending coastal trawl and longline vessels in northern Patagonia, Argentina. Ornis Fennica 77, 105–115.
Bicknell, A. W. J., Oro, D., Camphuysen, K. C. J., and Votier, S. C. (2013). Potential consequences of discard reform for seabird communities. J. Appl. Ecol. 50, 649–658. doi: 10.1111/1365-2664.12072
Bowser, A. K., Diamond, A. W., and Addison, J. A. (2013). From puffins to plankton: a DNA-based analysis of a seabird food chain in the northern Gulf of Maine. PLoS ONE 8, e83152. doi: 10.1371/journal.pone.0083152
Brothers, N., Cooper, J., and Løkkeborg, S. (1999). “The incidental catch of seabirds by longline fisheries: worldwide review and technical guidelines for mitigation,” in Fisheries Circular No.937 (Rome: FAO).
Bugoni, L., McGill, R. A. R., and Furness, R. W. (2010). The importance of pelagic longline fishery discards for a seabird community determined through stable isotope analysis. J. Exp. Mar. Biol. Ecol. 391, 190–200. doi: 10.1016/j.jembe.2010.06.027
Burnham, K. P., and Anderson, D. R. (2002). Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach. New York, NY: Springer.
Campioni, L., Granadeiro, J. P., and Catry, P. (2016). Niche segregation between immature and adult seabirds: does progressive maturation play a role? Behav. Ecol. 27, 426–433. doi: 10.1093/beheco/arv167
Catard, A., Weimerskirch, H., and Cherel, Y. (2000). Exploitation of distant Antarctic waters and close shelf-break waters by white-chinned petrels rearing chicks. Mar. Ecol. Prog. Ser. 194, 249–261. doi: 10.3354/meps194249
Catry, P., Lemos, R. T., Brickle, P., Phillips, R. A., Matias, R., and Granadeiro, J. P. (2013). Predicting the distribution of a threatened albatross: the importance of competition, fisheries and annual variability. Prog. Oceanogr. 110, 1–10. doi: 10.1016/j.pocean.2013.01.005
CCAMLR (2014a). Fishery Report 2014: Dissostichus eleginoides Kerguelen Islands French EEZ (Division 58.5.1). Hobart, TAS: Commission for the Conservation of Antarctic Marine Living Resources.
CCAMLR (2015). CCAMLR Statistical Bulletin, Vol 28. Hobart, TAS: Commission for the Conservation of Antarctic Marine Living Resources.
Cherel, Y., Weimerskirch, H., and Trouvé, C. (2000). Food and feeding ecology of the neritic-slope forager black-browed albatross and its relationships with commercial fisheries in Kerguelen waters. Mar. Ecol. Prog. Ser. 207, 183–199. doi: 10.3354/meps207183
Cherel, Y., Weimerskirch, H., and Trouvé, C. (2002). Dietary evidence for spatial foraging segregation in sympatric albatrosses (Diomedea spp.) rearing chicks at Iles Nuageuses, Kerguelen. Mar. Biol. 141, 1117–1129. doi: 10.1007/s00227-002-0907-5
Collet, J., Patrick, S. C., and Weimerskirch, H. (2015). Albatrosses redirect flight towards vessels at the limit of their visual range. Mar. Ecol. Prog. Ser. 526, 199–205. doi: 10.3354/meps11233
Collins, M. A., Stowasser, G., Fielding, S., Shreeve, R., Xavier, J. C., Venables, H. J., et al. (2012). Latitudinal and bathymetric patterns in the distribution and abundance of mesopelagic fish in the Scotia Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 59–60, 189–198. doi: 10.1016/j.dsr2.2011.07.003
Constable, A. J., De La Mare, W. K., Agnew, D. J., Everson, I., and Miller, D. (2000). Managing fisheries to conserve the Antarctic marine ecosystem: practical implementation of the Convention on the Conservation of Antarctic Marine Living Resources (CCAMLR). ICES J. Mar. Sci. 57, 778–791. doi: 10.1006/jmsc.2000.0725
Croxall, J. P., Hall, A. J., Hill, H. J., North, A. W., and Rodhouse, P. G. (1995). The food and feeding ecology of the white-chinned petrel Procellaria aequinoctialis at South Georgia. J. Zool. 237, 133–150. doi: 10.1111/j.1469-7998.1995.tb02752.x
Croxall, J. P., Prince, P. A., and Reid, K. (1997). Dietary segregation of krill-eating South Georgia seabirds. J. Zool. 242, 531–556. doi: 10.1111/j.1469-7998.1997.tb03854.x
Croxall, J. P., Reid, K., and Prince, P. A. (1999). Diet, provisioning and productivity responses of marine predators to differences in availability of Antarctic krill. Mar. Ecol. Prog. Ser. 177, 115–131. doi: 10.3354/meps177115
Danhardt, A., Fresemann, T., and Becker, P. H. (2011). To eat or to feed? Prey utilization of Common Terns Sterna hirundo in the Wadden Sea. J. Ornithol. 152, 347–357. doi: 10.1007/s10336-010-0590-0
Davoren, G. K., and Burger, A. E. (1999). Differences in prey selection and behaviour during self-feeding and chick provisioning in rhinoceros auklets. Anim. Behav. 58, 853–863. doi: 10.1006/anbe.1999.1209
Deagle, B. E., Chiaradia, A., McInnes, J., and Jarman, S. N. (2010). Pyrosequencing faecal DNA to determine diet of little penguins: is what goes in what comes out? Conserv. Genet. 11, 2039–2048. doi: 10.1007/s10592-010-0096-6
Deagle, B. E., Gales, N. J., Evans, K., Jarman, S. N., Robinson, S., Trebilco, R., et al. (2007). Studying seabird diet through genetic analysis of faeces: a case study on macaroni penguins (Eudyptes chrysolophus). PLoS ONE 2:e831. doi: 10.1371/journal.pone.0000831
Delord, K., Gasco, N., Weimerskirch, H., Barbraud, C., and Micol, T. (2005). Seabird mortality in the Patagonian toothfish longline fishery around Crozet and Kerguelen Islands, 2001-2003. CCAMLR Sci. 12, 53–80.
Diez, M. J., Pe'Rez-Barros, P., Romero, M. C., Scioscia, G., Tapella, F., Cabreira, A. G., et al. (2012). Pelagic swarms and beach strandings of the squat lobster Munida gregaria (Anomura: Munididae) in the Beagle Channel, Tierra del Fuego. Polar Biol. 35, 973–983. doi: 10.1007/s00300-011-1144-5
Duffy, D. C., and Jackson, S. (1986). Diet studies of seabirds: a review of methods. Colonial Waterbirds 9, 1–17. doi: 10.2307/1521138
Duhamel, G., and Hautecoeur, M. (2009). Biomass, abundance and distribution of fish in the Kerguelen Islands EEZ (CCAMLR Statistical Division 58.5.1). CCAMLR Sci. 16, 1–32.
Duhamel, G., Hulley, P.-A., Causse, R., Koubbi, P., Vacchi, M., Pruvost, P., et al. (2014). “Chapter 7: biogeographic patterns of fish,” in Biogeographic Atlas of the Southern Ocean, eds C. De Broyer, P. Koubbi, H. J. Griffiths, B. Raymond, C. D'udekem D'acoz, A. Van De Putte, B. Danis, B. David, S. Grant, J. Gutt, C. Held, G. Hosie, F. Huettmann, A. Post, and Y. Ropert-Coudert (Cambridge: Scientific Committee on Antarctic Research), 328–362.
Edgar, R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. doi: 10.1093/bioinformatics/btq461
FAO (2016). The State of World Fisheries and Aquaculture 2016 - Contributing to Food Security and Nutrition for All. Rome.
Foster, S., Swann, R. L., and Furness, R. W. (2017). Can changes in fishery landings explain long-term population trends in gulls? Bird Study 64, 90–97. doi: 10.1080/00063657.2016.1274287
Frederiksen, M., Wanless, S., Harris, M. P., Rothery, P., and Wilson, L. J. (2004). The role of industrial fisheries and oceanographic change in the decline of North Sea black-legged kittiwakes. J. Appl. Ecol. 41, 1129–1139. doi: 10.1111/j.0021-8901.2004.00966.x
Furness, R. W., and Tasker, M. L. (2000). Seabird-fishery interactions: quantifying the sensitivity of seabirds to reductions in sandeel abundance, and identification of key areas for sensitive seabirds in the North Sea. Mar. Ecol. Prog. Ser. 202, 253–264. doi: 10.3354/meps202253
Garthe, S., Camphuysen, K., and Furness, R. W. (1996). Amounts of discards by commercial fisheries and their significance as food for seabirds in the North Sea. Mar. Ecol. Prog. Ser. 136, 1–11. doi: 10.3354/meps136001
Goldsworthy, S. D., He, X., Tuck, G. N., Lewis, M., and Williams, R. (2001). Trophic interactions between the Patagonian toothfish, its fishery, and seals and seabirds around Macquarie Island. Mar. Ecol. Prog. Ser. 218, 283–302. doi: 10.3354/meps218283
Gon, O., and Heemstra, P. (1990). Fishes of the Southern Ocean. Grahamstown: JLB Smith Institute of Ichthyology.
Gonzalez-Zevallos, D., and Yorio, P. (2006). Seabird use of discards and incidental captures at the Argentine hake trawl fishery in the Golfo San Jorge, Argentina. Mar. Ecol. Prog. Ser. 316, 175–183. doi: 10.3354/meps316175
Granadeiro, J. P., Brickle, P., and Catry, P. (2013). Do individual seabirds specialize in fisheries' waste? The case of black-browed albatrosses foraging over the Patagonian Shelf. Anim. Conserv. 17, 19–26. doi: 10.1111/acv.12050
Granadeiro, J. P., Phillips, R. A., Brickle, P., and Catry, P. (2011). Albatrosses following fishing vessels: how badly hooked are they on an easy meal? PLoS ONE 6:e17467. doi: 10.1371/journal.pone.0017467
Gras, M., Pompert, J., Blake, A., Boag, T., Grimmer, A., Iriarte, V., et al. (2016). Report of the 2016 Finfish and Rock Cod Biomass Survey ZDLT1–02–2016. Stanley: Fisheries Department, Directorate of Natural Resources, Falkland Islands Government.
Gras, M., Pompert, J., Blake, A., Busbridge, T., Derbyshire, C., Keningale, B., et al. (2017). Report of the 2017 Ground Fish Survey ZDLT1–02–2017. Stanley: Directorate of Natural Resources - Fisheries, Falkland Islands Government.
Grémillet, D., Pichegru, L., Kuntz, G., Woakes, A. G., Wilkinson, S., Crawford, R. J. M., et al. (2008). A junk-food hypothesis for gannets feeding on fishery waste. Proc. R. Soc. B Biol. Sci. 275, 1149–1156. doi: 10.1098/rspb.2007.1763
Guinet, C., Dubroca, L., Lea, M.-A., Goldsworthy, S., Cherel, Y., Duhamel, G., et al. (2001). Spatial distribution of foraging in female Antarctic fur seals Arctocephalus gazella in relation to oceanographic variables: a scale-dependent approach using geographic information systems. Mar. Ecol. Prog. Ser. 219, 251–264. doi: 10.3354/meps219251
Handley, J. M., Baylis, A. M. M., Brickle, P., and Pistorius, P. (2016). Temporal variation in the diet of gentoo penguins at the Falkland Islands. Polar Biol. 39, 283–296. doi: 10.1007/s00300-015-1781-1
Hilton, G. M., Houston, D. C., and Furness, R. W. (1998). Which components of diet quality affect retention time of digesta in seabirds?. Funct. Ecol. 12, 929–939.
Huson, D. H., Auch, A. F., Qi, J., and Schuster, S. C. (2007). MEGAN analysis of metagenomic data. Genome Res. 17, 377–386. doi: 10.1101/gr.5969107
Jackson, S. (1992). Do seabird gut sizes and mean retention times reflect adaptation to diet and foraging method. Physiol. Zool. 65, 674–697. doi: 10.1086/physzool.65.3.30157976
Jarman, S. N., McInnes, J. C., Faux, C., Polanowski, A. M., Marthick, J., Deagle, B. E., et al. (2013). Adélie penguin population diet monitoring by analysis of food DNA in scats. PLoS ONE 8:e82227. doi: 10.1371/journal.pone.0082227
Kuepfer (2015). An Assessment of Seabird By-Catch in Falkland Islands Trawl Fisheries July 2014-June 2015. Stanley: Falkland Islands Fisheries Department.
Laptikhovsky, V., Arkhipkin, A., and Brickle, P. (2013). From small bycatch to main commercial species: explosion of stocks of rock cod Patagonotothen ramsayi (Regan) in the Southwest Atlantic. Fish. Res. 147, 399–403. doi: 10.1016/j.fishres.2013.05.006
Lea, M. A., Guinet, C., Cherel, Y., Hindell, M., Dubroca, L., and Thalmann, S. (2008). Colony-based foraging segregation by Antarctic fur seals at the Kerguelen Archipelago. Mar. Ecol. Prog. Ser. 358, 273–287. doi: 10.3354/meps07305
Leal, E., Canales, C., Aranis, A., Gomez, A., Ramirez, M., Saavedra, J. C., et al. (2013). Estatus y Posibilidades de Explotación Biológicamente Sustentable de los Principales Recursos Pesqueros Nacionales, a-o 2013. Sardina austral. Informe Final, Instituto de Fomento Pesquero.
Loeb, V. J., Kellermann, A. K., Koubbi, P., North, A. W., and White, M. G. (1993). Antarctic larval fish assemblages: a review. Bull. Mar. Sci. 53, 416–449.
Løkkeborg, S. (2008). Review and Assessment of Mitigation Measures to Reduce Incidental Catch of Seabirds in Longline, Trawl and Gillnet Fisheries. FAO Fisheries and Aquaculture Circular No. 1040.
Martin, A., and Pruvost, P. (2007). Pecheker, Relational Database for Analysis and Management of Fisheries and Related Biological Data from the French Southern Ocean Fisheries Monitoring Scientific Programs. Muséum National d'Histoire Naturelle.
McInnes, J. C., Alderman, R., Deagle, B. E., Lea, M. A., Raymond, B., and Jarman, S. N. (2017a). Optimised scat collection protocols for DNA metabarcoding in vertebrates. Methods Ecol. Evol. 8, 192–202. doi: 10.1111/2041-210X.12677
McInnes, J. C., Alderman, R., Raymond, B., Lea, M.-A., Deagle, B., Phillips, R. A., et al. (2017b). High occurrence of jellyfish predation by black-browed and Campbell albatross identified by DNA metabarcoding. Mol. Ecol. doi: 10.1111/mec.14245. [Epub ahead of print].
McMahon, C. R., Holley, D., and Robinson, S. (1999). The diet of itinerant male Hooker's sea lions, Phocarctos hookeri, at sub-Antarctic Macquarie Island. Wildlife Research 26, 839–846. doi: 10.1071/WR98079
Mori, M., Corney, S. P., Melbourne-Thomas, J., Welsford, D. C., Klocker, A., and Ziegler, P. E. (2016). Using satellite altimetry to inform hypotheses of transport of early life stage of Patagonian toothfish on the Kerguelen Plateau. Ecol. Modell. 340, 45–56. doi: 10.1016/j.ecolmodel.2016.08.013
Murray, D. C., Coghlan, M. L., and Bunce, M. (2015). From benchtop to desktop: important considerations when designing amplicon sequencing workflows. PLoS ONE 10:e0124671. doi: 10.1371/journal.pone.0124671
Nevoux, M., Forcada, J., Barbraud, C., Croxall, J., and Weimerskirch, H. (2010). Bet-hedging response to environmental variability, an intraspecific comparison. Ecology 91, 2416–2427. doi: 10.1890/09-0143.1
Okes, N. C., Hockey, P. A. R., Pichegru, L., Lingen, C. D. V. D., Crawford, R. J. M., and Grémillet, D. (2009). Competition for shifting resources in the southern Benguela upwelling: seabirds versus purse-seine fisheries. Biol. Conserv. 142, 2361–2368. doi: 10.1016/j.biocon.2009.05.031
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2016). Vegan: Community Ecology Package. R Package Version 2.4-1.
Oro, D., Bosch, M., and Ruiz, X. (1995). Effects of a trawling moratorium on the breeding success of the Yellow-legged Gull Larus cachinnans IBIS 137, 547–549. doi: 10.1111/j.1474-919X.1995.tb03265.x
Pauly, D., Christensen, V., Dalsgaard, J., Froese, R., and Torres, F. Jr. (1998). Fishing down marine food webs. Science 279, 860–863. doi: 10.1126/science.279.5352.860
Phillips, R. A., Gales, R., Baker, G. B., Double, M. C., Favero, M., Quintana, F., et al. (2016). The conservation status and priorities for albatrosses and large petrels. Biol. Conserv. 201, 169–183. doi: 10.1016/j.biocon.2016.06.017
Phillips, R. A., Petersen, M. K., Lilliendahl, K., Solmundsson, J., Hamer, K. C., Camphuysen, C. J., et al. (1999a). Diets of northern fulmars Fulmarus glacialis: reliance on commercial fisheries? Mar. Biol. 135, 159–170. doi: 10.1007/s002270050613
Phillips, R. A., Silk, J. R., Phalan, B., Catry, P., and Croxall, J. P. (2004). Seasonal sexual segregation in two Thalassarche albatross species: competitive exclusion, reproductive role specialization or foraging niche divergence? Proc. Biol. Sci. 271, 1283–1291. doi: 10.1098/rspb.2004.2718
Phillips, R. A., Thompson, D. R., and Hamer, K. C. (1999b). The impact of great skua predation on seabird populations at St Kilda: a bioenergetics model. J. Appl. Ecol. 36, 218–232. doi: 10.1046/j.1365-2664.1999.00391.x
Pierre, J. P., Abraham, E. R., Richard, Y., Cleal, J., and Middleton, D. A. J. (2012). Controlling trawler waste discharge to reduce seabird mortality. Fish. Res. 131–133, 30–38. doi: 10.1016/j.fishres.2012.07.005
Piñol, J., Mir, G., Gomez-Polo, P., and Agustí, N. (2015). Universal and blocking primer mismatches limit the use of high-throughput DNA sequencing for the quantitative metabarcoding of arthropods. Mol. Ecol. Resour. 15, 819–830. doi: 10.1111/1755-0998.12355
Pompanon, F., Deagle, B. E., Symondson, W. O., Brown, D. S., Jarman, S. N., and Taberlet, P. (2012). Who is eating what: diet assessment using next generation sequencing. Mol. Ecol. 21, 1931–1950. doi: 10.1111/j.1365-294X.2011.05403.x
Poncet, S., Wolfaardt, A. C., Black, A., Browning, S., Lawton, K., Lee, J., et al. (2017). Recent trends in numbers of wandering (Diomedea exulans), black-browed (Thalassarche melanophris) and grey-headed (T. chrysostoma) albatrosses breeding at South Georgia. Polar Biol. 40, 1347–1358. doi: 10.1007/s00300-016-2057-0
Prince, P. A. (1980). The food and feeding ecology of grey-headed albatross Diomedea chrysostoma and black-browed albatross Diomedea melanophris. Ibis 122, 476–488. doi: 10.1111/j.1474-919X.1980.tb00902.x
Prince, P. A., and Huin, N. (1994). Diving Depths of Albatrosses. Antarctic Science 6, 353–354. doi: 10.1017/S0954102094000532
Quintin, M., and Pompert, J. (2014). Falkland Islands National Plan of Action for Reducing Incidental Catch of Seabirds in Trawl Fisheries. Stanley: Department of Fisheries, Falkland Islands Government.
Reid, K., Croxall, J. P., and Prince, P. A. (1996). The fish diet of black-browed albatross Diomedea melanophris and grey-headed albatross D. chrysostoma at South Georgia. Polar Biol. 16, 469–477. doi: 10.1007/BF02329065
Robertson, G., Moreno, C., Arata, J. A., Candy, S. G., Lawton, K., Valencia, J., et al. (2014). Black-browed albatross numbers in Chile increase in response to reduced mortality in fisheries. Biol. Conserv. 169, 319–333. doi: 10.1016/j.biocon.2013.12.002
Robertson, G., Wienecke, B., Suazo, C. G., Lawton, K., Arata, J. A., and Moreno, C. (2017). Continued increase in the number of black-browed albatrosses (Thalassarche melanophris) at Diego Ramírez, Chile. Polar Biol. 40, 1035–1042. doi: 10.1007/s00300-016-2028-5
Robinson, S. A., and Hindell, M. A. (1996). Foraging ecology of gentoo penguins Pygoscelis papua at Macquarie Island during the period of chick care. IBIS 138, 722–731. doi: 10.1111/j.1474-919X.1996.tb08829.x
Rosen, D. A. S., and Trites, A. W. (2000). Pollock and the decline of Steller sea lions: testing the junk-food hypothesis. Can. J. Zool. 78, 1243–1250. doi: 10.1139/z00-060
Sakamoto, K. Q., Takahashi, A., Iwata, T., and Trathan, P. N. (2009). From the eye of the albatrosses: A bird-borne camera shows an association between albatrosses and a killer whale in the Southern Ocean. PLoS ONE 4:e7322. doi: 10.1371/journal.pone.0007322
Sánchez, R. P., Remeslo, A., Madirolas, A., and De Ciechomski, J. D. (1995). Distribution and abundance of post-larvae and juveniles of the Patagonian sprat, Sprattus fuegensis, and related hydrographic conditions. Fish. Res. 23, 47–81. doi: 10.1016/0165-7836(94)00339-X
Sancho, E. (2009). Falkland Islands National Plan of Action for Reducing Incidental Catch of Seabirds in Trawl Fisheries. Stanley: Falklands Conservation.
Sullivan, B. J., Reid, T. A., and Bugoni, L. (2006). Seabird mortality on factory trawlers in the Falkland Islands and beyond. Biol. Conserv. 131, 495–504. doi: 10.1016/j.biocon.2006.02.007
Tamini, L. L., Chavez, L. N., Góngora, M. E., Yates, O., Rabuffetti, F. L., and Sullivan, B. (2015). Estimating mortality of black-browed albatross (Thalassarche melanophris, Temminck, 1828) and other seabirds in the Argentinean factory trawl fleet and the use of bird-scaring lines as a mitigation measure. Polar Biol. 38, 1867–1879. doi: 10.1007/s00300-015-1747-3
Terauds, A., Gales, R., Baker, G. B., and Alderman, R. (2006). Foraging areas of black-browed and grey-headed albatrosses breeding on Macquarie Island in relation to marine protected areas. Aquat. Conserv. Mar. Freshw. Ecosyst. 16, 133–146. doi: 10.1002/aqc.709
Thompson, K. R. (1992). Quantitative analysis of the use of discards from squid trawlers by black-browed albatrosses Diomedea melanophris in the vicinity of the Falkland Islands. IBIS 134, 11–21. doi: 10.1111/j.1474-919X.1992.tb07223.x
Thompson, K. R. (1993). Variation in magellanic penguin Spheniscus magellanicus diet in the Falkland Islands. Mar. Ornithol. 21, 57–67.
Thompson, K. R., and Riddy, M. D. (1995). Utilization of offal and discards from Finfish trawlers around the Falkland Islands by black-browed albatross Diomedea melanophris IBIS 137, 198–206. doi: 10.1111/j.1474-919X.1995.tb03240.x
Tuck, G. N., Phillips, R. A., Small, C., Thomson, R. B., Klaer, N. L., Taylor, F., et al. (2011). An assessment of seabird-fishery interactions in the Atlantic Ocean. ICES J. Mar. Sci. 68, 1628–1637. doi: 10.1093/icesjms/fsr118
Vestheim, H., and Jarman, S. N. (2008). Blocking primers to enhance PCR amplification of rare sequences in mixed samples - a case study on prey DNA in Antarctic krill stomachs. Front. Zool. 5:12. doi: 10.1186/1742-9994-5-12
Wakefield, E. D., Phillips, R. A., Trathan, P. N., Arata, J., Gales, R., Huin, N., et al. (2011). Habitat preference, accessibility, and competition limit the global distribution of breeding Black-browed Albatrosses. Ecol. Monogr. 81, 141–167. doi: 10.1890/09-0763.1
Watkins, B. P., Petersen, S. L., and Ryan, P. G. (2008). Interactions between seabirds and deep-water hake trawl gear: an assessment of impacts in South African waters. Anim. Conserv. 11, 247–254. doi: 10.1111/j.1469-1795.2008.00192.x
Weimerskirch, H., and Jouventin, P. (1987). Population dynamics of the wandering albatross, Diomedea exulans, of the Crozet Islands: causes and consequences of the population decline. OIKOS 49, 315–322. doi: 10.2307/3565767
Weimerskirch, H., Cherel, Y., Cuenotchaillet, F., and Ridoux, V. (1997). Alternative foraging strategies and resource allocation by male and female Wandering Albatrosses. Ecology 78, 2051–2063. doi: 10.1890/0012-9658(1997)078[2051:AFSARA]2.0.CO;2
Wolfaardt, A. (2013). “An assessment of the population trends and conservation status of black-browed albatrosses in the Falkland Islands,” in First Meeting of the Population and Conservation Status Working Group of the Agreement on the Conservation of Albatrosses and Petrels (La Rochelle).
Xavier, J. C., Croxall, J. P., and Reid, K. (2003). Interannual variation in the diets of two albatross species breeding at South Georgia: implications for breeding performance. IBIS 145, 593–610. doi: 10.1046/j.1474-919X.2003.00196.x
Keywords: scat, trawl fishery, fisheries resource management, Southern Ocean, Thalassarche melanophris, seabird-fishery interaction, fish diversity, seabirds
Citation: McInnes JC, Jarman SN, Lea M-A, Raymond B, Deagle BE, Phillips RA, Catry P, Stanworth A, Weimerskirch H, Kusch A, Gras M, Cherel Y, Maschette D and Alderman R (2017) DNA Metabarcoding as a Marine Conservation and Management Tool: A Circumpolar Examination of Fishery Discards in the Diet of Threatened Albatrosses. Front. Mar. Sci. 4:277. doi: 10.3389/fmars.2017.00277
Received: 31 May 2017; Accepted: 14 August 2017;
Published: 31 August 2017.
Edited by:
Mark Meekan, Australian Institute of Marine Science, AustraliaReviewed by:
Maelle Connan, Nelson Mandela Metropolitan University, South AfricaNuno Queiroz, University of Porto, Portugal
Copyright © 2017 McInnes, Jarman, Lea, Raymond, Deagle, Phillips, Catry, Stanworth, Weimerskirch, Kusch, Gras, Cherel, Maschette and Alderman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julie C. McInnes, anVsaWUubWNpbm5lc0B1dGFzLmVkdS5hdQ==
 Julie C. McInnes
Julie C. McInnes Simon N. Jarman
Simon N. Jarman Mary-Anne Lea
Mary-Anne Lea Ben Raymond
Ben Raymond Bruce E. Deagle
Bruce E. Deagle Richard A. Phillips
Richard A. Phillips Paulo Catry
Paulo Catry Andrew Stanworth7
Andrew Stanworth7 Alejandro Kusch
Alejandro Kusch Michaël Gras
Michaël Gras Yves Cherel
Yves Cherel Dale Maschette
Dale Maschette