- 1Center for Environment and Water, Research Institute, King Fahd University of Petroleum and Minerals, Dhahran, Saudi Arabia
- 2Geosciences Department, King Fahd University of Petroleum and Minerals, Dhahran, Saudi Arabia
- 3AZTI-Tecnalia, Marine Research Division, Pasaia, Spain
- 4Marine Core Lab, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia
The biota in the Arabian Gulf faces stress both from natural (i.e., hyper salinity and high sea surface temperature), and human (i.e., from oil-related activities) sources. The western Arabian Gulf was also impacted by world's largest oil spill (1991 Oil Spill). However, benthic research in this region is scarce and most of the studies have been conducted only in small areas. Here, we present data on macrobenthos collected during 2002–2003 from the open waters and inner bays in the northwestern Arabian Gulf aimed to assess the ecological status and also to evaluate the long-term impact, if any, of the 1991 Oil Spill. A total of 392 macrobenthic taxa with an average (±SE) species richness (S) of 71 ± 2, Shannon-Wiener species diversity (H′) of 4.9 ± 0.1, and density of 3,181 ± 359 ind. m−2 was recorded from the open water stations. The open waters have “slightly disturbed” (according to AZTI's Marine Biotic Index, AMBI) conditions, with “good-high” (according to multivariate-AMBI, M-AMBI) ecological status indicating the absence of long-term impacts of the oil spill. Overall, 162 taxa were recorded from inner bays with average (±SE) values of S 41 ± 9, H′ 3.48 ± 0.39, and density 4,203 ± 1,042 ind. m−2. The lower TPH (Total Petroleum Hydrocarbons) stations (LTS, TPH concentrations <70 mg kg−2) show relatively higher S, H' and density compared to the higher TPH stations (HTS, TPH concentrations ≥100 mg kg−2). In the inner bays, AMBI values indicate slightly disturbed conditions at all stations except one, which is moderately disturbed. M-AMBI values indicate good status at LTS, while, high, good, moderate, and poor status at HTS. The “moderately disturbed” conditions with “moderate-poor” ecological status in some locations of the inner bays specify a severe long-term impact of the oil spill.
Introduction
The Arabian Gulf (hereafter “the Gulf”), has attracted the attention of marine ecologists due to the following reasons. Firstly, the Gulf is characterized by elevated levels of salinity and sea surface temperature (SST) due to its shallow nature, restricted water exchange, and 1–2 m equivalent of evaporation per year (Sheppard et al., 1992, 2010; Coles, 2003).
Secondly, the widespread activities associated with the enormous oil and gas exploration and processing industry apply immense pressure on the Gulf marine ecosystem. According to a recent estimate, the proved oil reserves in the Arabian Gulf region (including Iran, Iraq, Kuwait, Oman, Qatar, Saudi Arabia, and United Arab Emirates) is 48% of the total world reserves and the total production is 31.8% of the total world daily production (BP, 2013). Similarly, the reserves and production of gas in this region (of the aforementioned countries and Bahrain) are 42.5 and 15.7% of the world reserves and daily world production, respectively (BP, 2013). Gulf countries have a substantial number of offshore and onshore facilities for the exploration, production, distribution, and refining of oil and gas (Literathy et al., 2002), which are being constantly upgraded to meet the future demands worldwide. The fact that oil-related activities cannot be undertaken without any environmental disturbances is well-known (Breuer et al., 2004; Wake, 2005).
Thirdly, the world's largest oil spill (1991 Oil Spill) occurred in the Gulf. The Gulf War in 1991 resulted in the intentional discharge of an estimated 8–11 million barrels of crude oil into the Gulf (Tawfiq and Olsen, 1993). The 1991 Oil Spill affected the coastal areas located between Ras-Al-Khafji in the north and Abu Ali to the south on the Saudi Arabian coast (Figure 1) (Fowler et al., 1993; Michel et al., 1993; Sadiq and McCain, 1993; Tawfiq and Olsen, 1993; Readman et al., 1996; Price, 1998). The concentration of total petroleum hydrocarbons (TPH) in the sediments from the inner bays was about 1,000 mg kg−1 even 1 year after the spill (Michel et al., 1993). The damage to critical habitats in certain areas of the Saudi Arabian coast was irreversible (Fowler et al., 1993; Gerges, 1993; Readman et al., 1996). The progressive recovery of faunal diversity in the shoreline regions of the Gulf has been reported by several researchers (Hoffmann, 1994; Jones et al., 1994; Richmond, 1994; Michel et al., 1993). Price (1998) evaluated the overall status of the Gulf's coastal environment 5 years after the 1991 Oil Spill based on a number of published studies (e.g., McGlade and Price, 1993; Sadiq and McCain, 1993; Price et al., 1994; Jones et al., 1998) and provided evidence for the ecological recovery of most of the spill-affected coastal environments. Further evidence for the recovery from the impacts of the oil spill on a broader context has been provided by Sell et al. (1995), and more recently by Lecklin et al. (2011) by reviewing worldwide literature on the effects of oil on organisms. The time period for recovery suggested by Price (1998) also matches the results of the assessment of the recovery period made by Sell et al. (1995).
Finally, offshore areas claimed for urbanization are a major threat to the marine environment (Bishop, 2002; Khan et al., 2002; Munawar et al., 2002; Jones et al., 2007; Zainal, 2009; Loughland et al., 2012) in the Gulf. Many productive mangroves and other intertidal habitats of the western Arabian Gulf, which were important nursery grounds for fishes and shrimps, have been acquired and used for coastal infrastructure development (Loughland et al., 2012).
Due to their sedentary nature, longevity, which provides long-term exposure to toxic substances, and the presence of diverse taxa which can respond to multiple types of stresses, macrobenthic communities are good indicators of the health of the marine ecosystem (Jewett et al., 1999). The status of the well-being of the marine benthic communities can be used to determine the impact of a range of environmental conditions or effects of man-made perturbations (Pearson and Rosenberg, 1978; Borja et al., 2000; Guidetti et al., 2000; Hampel et al., 2009). Benthic communities are one of the groups that are impacted straightaway, and take a longer time to recover than other organisms, ranging between 2 and 10 years, following an oil spill (Borja et al., 2010). Typically, sensitive taxa such as crustaceans in general, and amphipods in particular, require longer recovery periods. For example, after the Amoco Cadiz Oil Spill, amphipod recolonization took around 15 years in the Bay of Morlaix (Dauvin, 1998; Gómez-Gesteira and Dauvin, 2000). Studies on large-scale oil spills have shown the persistence of hydrocarbons and their long-term effects on aquatic ecosystems (Burns et al., 1994; Reddy et al., 2002; Peterson et al., 2003; Short et al., 2004). Even in the absence of acute toxicity, the persistence of oil in sediments can induce long-term ecological effects through complex biological interactions (Southward et al., 1982; Peterson et al., 2003).
Benthic research from the Gulf is scarce (Basson et al., 1977; McCain, 1984a,b; Coles and McCain, 1990; Jones et al., 1998; Bu-Olayan and Thomas, 2005; Al-Yamani et al., 2009; Joydas et al., 2011, 2012, 2015). A majority of the published studies are based on data from a limited area in each case. In this study, the data on macrobenthic communities collected during 2002 and 2003 from a major part of the open waters and selected sheltered back bays (hereafter “inner bays”) of the Saudi coast of the Gulf, which forms the northwest Gulf waters, are presented. The objectives of the study are: (i) to assess the ecological status of macrobenthic communities in the Saudi waters of the Gulf, and (ii) to assess the long-term impact of the 1991 Oil Spill if any, in the affected area.
This study is based on extensive sampling, conducted in 2002 and 2003 under the Oceanographic Survey in Support of the Marine and Coastal Damage Assessment project intended to assess the long-term impact of the 1991 Oil Spill on the marine resources and environment of the Kingdom of Saudi Arabia.
Materials and Methods
Study Area
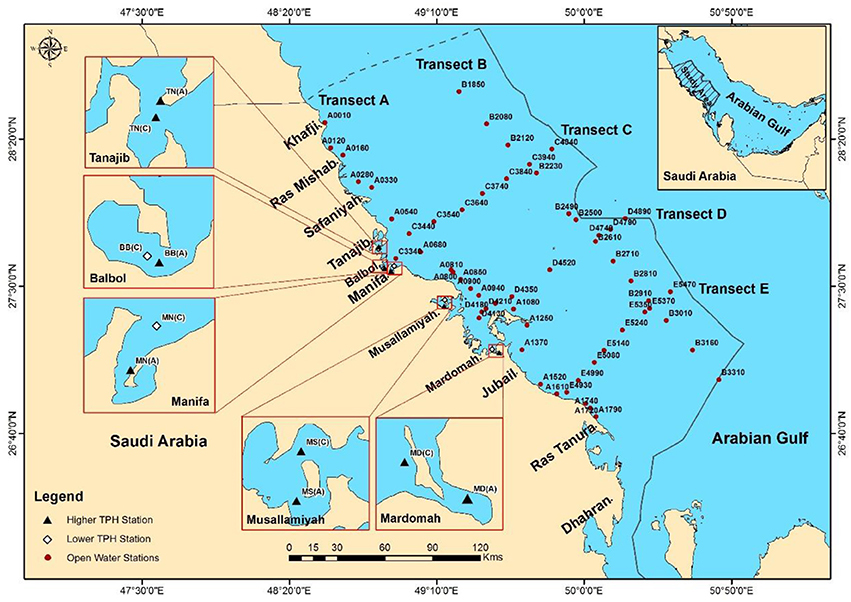
The study area covers the Saudi territorial waters of the Gulf from Khafji to Ras Tanura (latitude: 26°40′–28°30′ N and longitude: 48°10′–51° 00′ E) (Figure 1). The coastline of this region extends in a more or less straight line in the south-easterly direction, and it is highly convoluted with extensive and complex sheltered bay systems (Basson et al., 1977). The most remarkable feature of the western Arabian Gulf is its extremely low relief. The average slope of the seabed is only 35 cm per kilometer. This results in an unusually extensive intertidal zone, width of which in many places can be measured in kilometers. The depth of the Saudi waters of the Gulf is generally below 60 m.
Open Waters
The oceanographic features of the study area do not vary much from the general features of the Gulf. The Gulf receives an average of about 37 km3 year−1 of river runoff from Shatt al Arab, located in the north of the Gulf, and about 23 km3 year−1 of freshwater directly from precipitation (Sheppard et al., 1992). Due to the semi-enclosed nature of the Gulf, the tidal regime is rather complex, with a semi-diurnal pattern in most parts of the Gulf. The tidal range varies between 0.8 and 4 m. The Gulf region is characterized by a pronounced seasonality and the water temperatures at the surface may be as low as 11°C in the winter and may go over 36°C in the summer. The salinity is relatively high (45 in the open waters) in the Gulf as a result of the high atmospheric temperatures and a correspondingly high evaporation rate and a low fresh water input. High sedimentation rates result in a high turbidity and decreased light penetration. Compared to other oceanic regions, even though the Gulf occupies a small area, it has great importance in the Indian Ocean circulation. The counter clockwise currents transport sediments from the Shatt al Arab delta and deposit them along the eastern Saudi Arabian coastline (Zhao and Ghedira, 2014). Primary productivity along the shallow Saudi coastline of the Arabian Gulf is very high due to the recruitment and deposition of sediments and nutrients from the Shatt al Arab delta.
The Gulf has diverse habitats, including saline lagoons, sheltered bays, shallow exposed coastal waters, and offshore open waters. In the subtidal region, the largest proportion of the seabed by far, in terms of the area, is composed of soft sandy, or muddy substrata. Sand is normally present from the immediate subtidal levels to a depth of 7–10 m. Below the sandy zone, mud begins to predominate gradually. Seagrass beds occupy large areas of the sandy and silty-sand substrata in the shallow depths. The depths at which seagrass is present are generally limited by the light penetration. In the Gulf, the depth is typically 10–12 m, where the seagrass beds occur as sparse to dense patches.
Inner Bays
The inner bays are characterized by extreme hydrographical conditions. Most of these shallow water areas (<12 m) are occupied by critical marine habitats such as seagrass beds or coral reefs (Basson et al., 1977). These regions are most productive because the shallow seabed lies within the photic region (Sheppard, 1993). However, extreme water temperature variations and hyper salinity are common phenomena in these bays (Coles and McCain, 1990; John et al., 1990; Coles and Fadlallah, 1991; Price et al., 1993). Generally, the restriction caused by the narrow opening of the Strait of Hormuz, high surface evaporation, and minimal freshwater inflow result in the high salinity of the Gulf. Due to the lack of flushing and high evaporation, shallow coastal bays are subjected to a further increase in the salinity and temperature compared to the open sea waters. Annual salinity ranges from 56 to 74 and water temperature from 17 to 40°C in the shallow bays (Coles and McCain, 1990; Coles and Fadlallah, 1991; Jones et al., 1998; Sheppard et al., 2010).
Field Sampling
Sediment samples from the area of the open waters were collected onboard the Cruise 1 of R/V Al-Gosaibi, Saudi Arabia during November–December, 2002. A total of 174 samples from 58 stations (three replicates from each station) along five transects in the Saudi waters of the Gulf (Figure 1) were collected using a Van Veen grab sampler (0.1 m2). Sampling depth ranged from 2.75 to 58 m. Two transects were established along the north-south direction in the coastal (Transects A, 21 stations) and offshore (Transects B, 13 stations) zones, and three transects were established perpendicular to the coast corresponding to the northern (Transects C, 8 stations), central (Transects D, 8 stations), and southern (Transects E, 8 stations) sections of the study area.
Macrobenthic samples were also collected from five locations in two bay systems, viz. Manifa-Tanajib System (MTBS) and Abu Ali Bay System (AABS) during January 2003 (Figure 1). Three stations were located in the MTBS (Tanajib—TN, Balbol—BB, and Manifa—MN) and two in the AABS (Musallamiyah—MS and Mardomah—MD). As oil and gas exploitation in the Gulf is intensive, it is difficult to find control sites (i.e., pristine or undisturbed locations). Hence, the sites were selected based on an initial intensive survey to determine the TPH concentration. Hundreds of core samples were analyzed for the determination of the concentration of TPH in sediments from these locations. Macrobenthic samples and sediment samples were collected from the stations with the lowest (stations including C) and the highest (stations including A) TPH concentrations in these five locations. At each location, three replicate macrobenthic samples were collected from each of two sites.
Altogether 204 macrobenthic samples were used for this study: 174 samples from the open waters and 30 from the bay systems. All the sediment samples collected for the macrobenthic study were sieved through a 0.5 mm mesh sieve and fixed in 5% formalin. Separate grab samples were collected for the sediment characteristics study. As the studies undertaken in the open waters and inner bays were conducted as independent studies, there are differences in the approach of selecting of environmental variables. In the open waters, hydrographical parameters (temperature, salinity, and dissolved oxygen; DO) of bottom waters were measured using a Conductivity Temperature Depth (CTD) profiler and samples were collected for the determination of the grain size and total organic carbon (TOC) in sediments. In the inner bays, the main focus was to test the health of the benthic community, vis-à-vis the concentration of TPH, and hence hydrographical parameters and TOC in sediments were not determined. Sediment samples were collected for the determination of TPH and grain size.
Analyses of Environmental Variables
The grain size of the sediments was determined by a combined sieving and pipette method (Carvar, 1971). Sediment textural types were identified using Sheppard's classification (Sheppard, 1954). The concentration of TOC in the sediments was determined by combustion at 900°C and detection with a non-dispersive infrared sensor (NDIR), using a Shimadzu TOC analyzer with an ASI-V autosampler. Standard carbon solutions of 0.5–20 ppm (mg L−1) prepared with potassium hydrogen phthalate C6H4 (COOK) (COOH) in C-free water were used for the calibration of the analyzer.
TPH in sediment samples was determined according to the Battelle standard operating procedure, SOP 5–203 (NOAA Status and Trends). Briefly, ~2–30 g of the sediments was serially extracted with dichloromethane (DCM) and sodium sulfate (Na2SO4) using an Accelerated Solvent Extraction (ASE) procedure. The sample was weighed into an extraction vessel and spiked with a surrogate internal standard (SIS), solvent was added, and the ASE unit was operated according to the Standard Operating Procedure (SOP). The filtered extract was decanted into an Erlenmeyer flask. The extract was then passed through a 2% deactivated alumina column, and concentrated to 2,000 μl using Kuderna-Danish (KD) and nitrogen evaporation techniques. The TPH concentration in this extract was determined using a gas chromatograph equipped with a flame ionization detector (GC/FID).
According to some guidelines for pollution levels in the sediments of the Gulf (Massoud et al., 1996), they are classified as TPH <15 mg kg−1 is unpolluted, 15–50 mg kg−1 is slightly polluted, 50–200 mg kg−1 is moderately polluted, and >200 mg kg−1 is heavily polluted.
Analysis of Macrobenthos
Initially, the specimens were subjected to group level sorting and then species level identification of the dominant taxa such as polychaetes and major crustaceans such as amphipods, ostracods, mysids, and cumaceans. The taxa were identified down to species level using various identification keys (Day, 1967; Fauchald, 1977; Barnard and Drummond, 1982; Ruffo, 1982, 1989, 1993; Hall, 1985; Jones, 1986; McCain, 1993; LeCroy, 2000). The taxa identified to species level represent about 75% of the total macrobenthos density. The remaining taxa were identified at the genus or family levels.
Statistical Analyses
Macrobenthic species abundance data were analyzed to determine the total density of macrobenthic organisms (N), species richness (S), and Shannon-Wiener (H′) (Log2) for species diversity using PRIMER 6 (Plymouth Routines in Multivariate Ecological Research) (version 6.1.5) (Clarke and Warwick, 2001).
The following biotic indices, which are extensively used by researchers around the world to assess the ecological status of the macrobenthic communities were calculated: (i) the AZTI's Marine Biotic Index (AMBI; Borja et al., 2000), and (ii) the multivariate-AMBI (M-AMBI; Muxika et al., 2007). M-AMBI is a factorial analysis which includes Shannon's diversity, richness, and AMBI. These two indices have been validated as effective ecological status assessment tools for benthic communities in different geographical areas, ranging from tropics to high latitudes, subjected to a range of human pressures, including hydrocarbon impacts (Borja et al., 2003; Muxika et al., 2005; Sigovini et al., 2013; Spagnolo et al., 2014).
AMBI was calculated using AMBI 4.1 software (available in the public domain at http://ambi.azti.es) and the February 2010 species list. Before calculation, the dataset was prepared according to the guidelines in using AMBI (Borja and Muxika, 2005) and M-AMBI (Borja et al., 2008). As some identified species were not included in the species list, the procedure described by Borja and Tunberg (2011) was followed when assigning new species. Additionally, M-AMBI was calculated also using AMBI software. As M-AMBI requires reference conditions to be calculated, and the Gulf is heavily impacted by human activities (see comments and references in the Introduction section), the current structural parameters are not suitable for establishing M-AMBI reference conditions (Borja et al., 2012). Hence, the approach proposed by Borja and Tunberg (2011) and Forchino et al. (2011) was used to establish the reference conditions in the absence of pristine areas. This consisted of increasing the highest diversity and richness values of all replicates by15%, and decreasing AMBI to half of the lowest value. As for the “bad” status, the references were based on the azoic situation (diversity and richness equal to 0 and AMBI equal to 6).
The threshold values for the M-AMBI classification according to Borja et al. (2007) are as follows: “high” ecological status, >0.77; “good,” 0.53–0.77; “moderate,” 0.38–0.53; “poor,” 0.20–0.38; and “bad,” <0.20.
Preliminary analysis indicated that environmental data (depth, bottom water temperature, bottom water salinity, bottom water DO, sand, mud, TOC%, and TPH in the open waters, and sand, mud%, and TPH in the inner bays) and macrobenthic data (S, H′, density, AMBI, M-AMBI, %sensitive species, and %opportunistic species in open waters and inner bays) do not meet the homogeneity of variance assumption. Hence, non-parametric tests were used to analyze the data.
Data from the open waters and inner bays were separately treated due to the different environmental conditions in these two regions, as mentioned in Section Study area. In the case of open waters only two factors were considered—distance to the shore (with two states: nearshore and offshore) and latitude region (with three states: northern, central, and southern); and in the case of inner bays, only one factor was considered—level of TPH (with two states: low and high). In the inner bays, stations MN(C) and BB(C) are lower TPH stations (LTS with TPH concentration <70 mg kg−1) and stations TN(C), TN(A), BB(A), MN(A), MS(C), MS(A), MD(C), and MD(A) are higher TPH stations (HTS with TPH concentration ≥100 mg kg−1). Multivariate analyses were performed on the square-root of the transformed abundance data to detect different macrobenthic assemblages. Bray-Curtis index and group average linkage were used for non-metric multidimensional scaling (MDS) ordination using PRIMER 6.
Significant spatial differences in the environmental and community parameters in the open waters and in the inner bays were established by Permutation Multivariate Analyses of Variance (PERMANOVA) with 999 permutations (Anderson, 2001) in PRIMER 7 (Trial Version 7.0.13). Prior to analysis, the environmental variables were fourth-root transformed and a resemblance matrix was calculated using the Euclidean similarity. Benthic community assemblages and community parameters were square-root transformed and a resemblance matrix was calculated using the Bray Curtis similarity. Significant differences in the nearshore-offshore and northern-southern groups were estimated using the data from the open waters. As weaker dissimilarities were obtained when central was added, it was removed from the tests. In order to test the north-south effect in the nearshore-offshore category, nearshore-offshore was set as the first factor and north-south was set as the second factor. Similarly, in order to test the nearshore-offshore effect in the northern-southern category, northern-southern was set as the first factor and nearshore-offshore was set as the second factor. The interactive effects of both these factors were also tested in the open water data. In the inner bays, significant dissimilarities were established between the LTS and HTS.
Correlations between environmental variables and structural parameters of macrobenthos were calculated using the non-parametric Kendall's tau-b test in SPSS 22 (Release 22.0.0.0). Since multiple analyses were conducted on same variables, to protect from Type I error, Bonferroni correction was applied to keep overall significant level to 0.05.
Results
Environmental Parameters
Open Waters
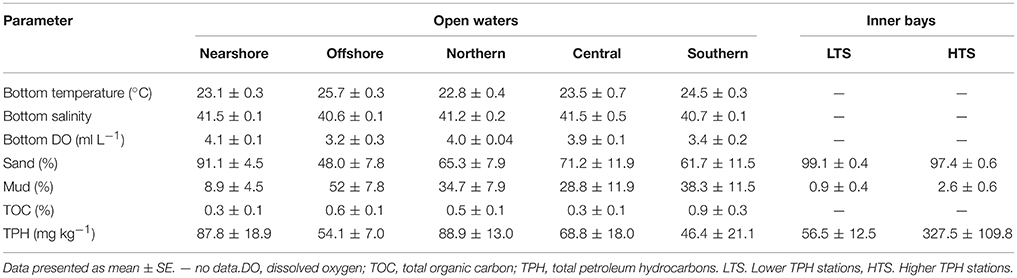
Hydrographical and sediment parameters are summarized in Table 1. There are significant nearshore–offshore variations in the bottom temperature, salinity, and DO, while within these regions, north-south variations are shown only by salinity, and DO (PERMANOVA results in Appendix A). Temperature, DO, and salinity show significant north-south differences with temperature increasing southward and DO and salinity decreasing southward. Within these regions, all three hydrographical parameters show a nearshore-offshore difference (PERMANOVA results in Appendix B). Low oxygen values (0.6 and 1.0 ml L−1) were observed at two offshore stations (B2810 and B2910 respectively), where the depth is >50 m. Interactive effects of nearshore-offshore and north-south factors are observed in temperature and DO (PERMANOVA results in Appendices A,B).
Textural types such as sandy, muddy sand, sandy mud, and mud were observed in the study area. Sand predominated in the nearshore region and mud predominated in the offshore region and there are significant nearshore-offshore differences in sand and mud, while within these regions, north-south differences are absent (PERMANOVA results in Appendix A). A north-south variation in the sediment texture is absent, and similarly there is no nearshore-offshore difference within these two regions (PERMANOVA results in Appendix B). Interactive effects of nearshore-offshore and north-south factors are observed in sand (PERMANOVA results in Appendix A). Seagrass beds, patchy coral flats, and rocky substrata are present in the nearshore region.
Similar to the textural parameters, the percentage of TOC (ranging from nil to 2.8%) also shows nearshore-offshore distinction (PERMANOVA results in Appendix A) with higher values in the nearshore compared to the offshore. There is no north-south difference within the nearshore-offshore regions (PERMANOVA results in Appendix A). Although a significant north-south variation is not evident (PERMANOVA results in Appendix B), the southern region has considerably higher TOC values (ranging from 1.90 to 2.80%) than the northern (0.19–1.10%) and central (0–0.63%) regions. There is no nearshore-offshore difference within the northern-southern regions (PERMANOVA results in Appendix A). There are no interactive effects of nearshore-offshore and north-south factors in TOC (PERMANOVA results in Appendices A,B).
The TPH concentration in the surface sediments ranges from nil to 390 mg kg−1. An unpolluted status was assigned to 10 stations (17% of the stations), which include five stations in the nearshore (two in the central and three in the southern sectors), one in the offshore (southern sector), one in the central and three in the southern regions. A slightly polluted status was assigned to 11 stations (19% of the stations) including stations located in the nearshore, offshore, central, and southern regions, and a moderately polluted status was assigned to 62% of the stations (36 stations). These high TPH concentrations are from samples from the northern and the central sectors of the nearshore and the offshore transects and the northern and the central transects. The heavily polluted status was assigned to the station A0540 located in the nearshore area. Higher average TPH values were recorded for samples from the nearshore area than the offshore area (Table 1). However, the nearshore-offshore difference is not statistically significant and there is no north-south variations within these two regions (PERMANOVA results in Appendix A). On the other hand, average values indicate that there is a progressive decrease in TPH from north to south, and there is a statistically significant difference in TPH between the northern and southern regions without a nearshore-offshore variation within the northern-southern regions (PERMANOVA results in Appendix B). There are no interactive effects of nearshore-offshore and north-south factors in TPH (PERMANOVA results in Appendices A,B).
Inner Bays
In general, the substratum is sandy in nature, composed of either fine sand or medium sand (Table 2, Appendix D). An admixture of gravel and coarse sand was also found to be important at a few stations (e.g., Musallamiyah and Manifa). The percentage of mud is less, ranging from 0.5 to 5.5% in the study area. There is no statistically significant difference in sand or mud between the LTS and HTS (PERMANOVA results in Appendix C).
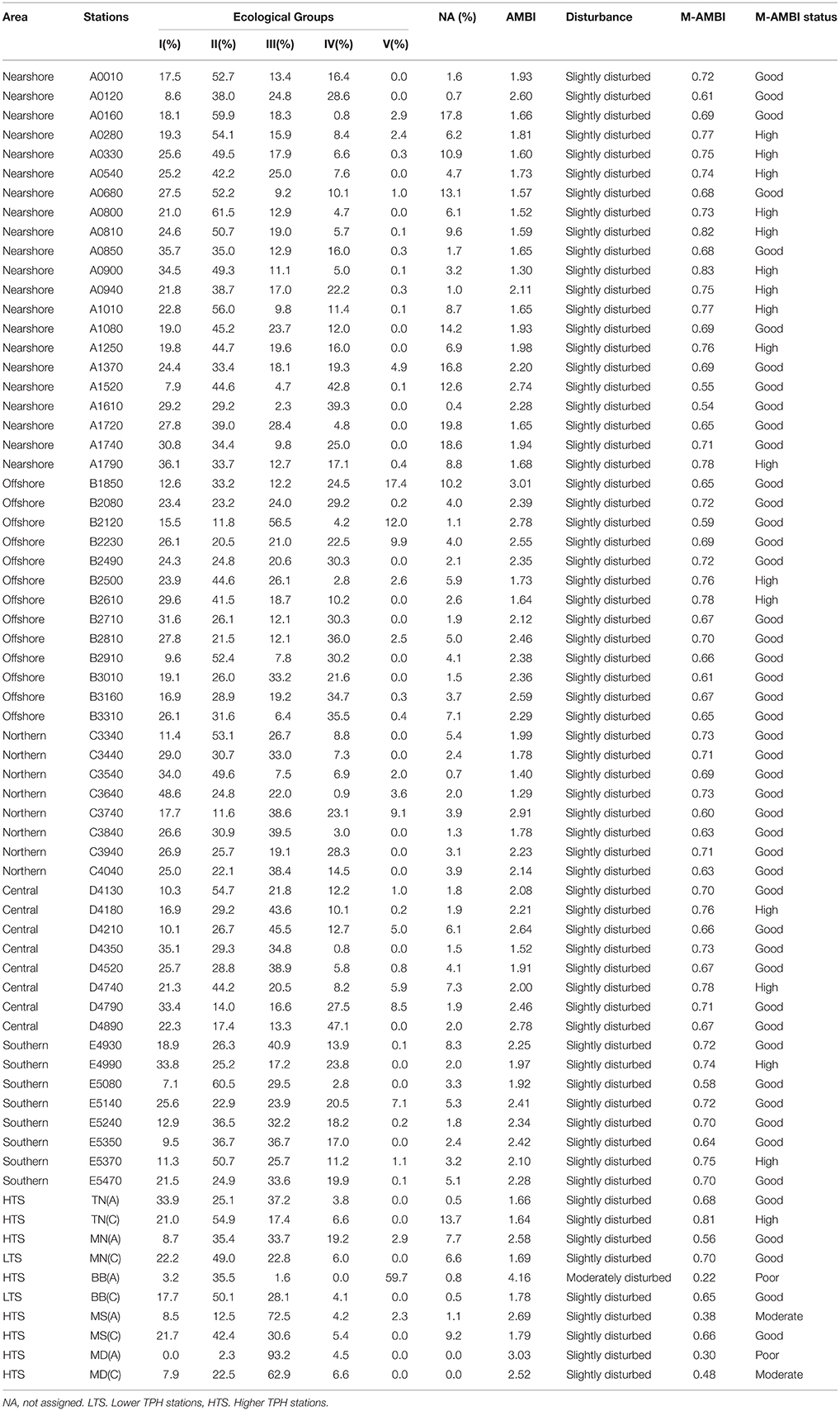
Table 2. The AMBI and M-AMBI values and the status classification for both indices, for each station.
The TPH concentration in the surface sediments of the LTS ranged from 44 mg kg−1 [station MN(C)] to 69 mg kg−1 [station BB(C)], while that in the surface sediments of the HTS ranged from 100 mg kg−1 [station MS(C)] to 1,000 mg kg−1 [station BB(C)] (Table 2, Appendix D). Apart from station BB(C), stations MD(A) (220 mg kg−1), MN(A) (310 mg kg−1), and MS(A) (440 mg kg−1) also show higher TPH concentrations (Appendix D). There are large differences in TPH concentrations between the two stations at Balbol, Manifa, and Musallamiyah (Appendix D). Even though the average values at LTS and HTS show a difference, it is not statistically significant (Table 1 and PERMANOVA results in Appendix C).
Macrobenthic Communities
Open Waters
A total of 392 macrobenthic taxa (216 polychaete species, 114 major crustacean species, and 62 other taxa) were recorded at the open water stations. Of the 114 crustacean species, 60 species belong to amphipods, 14 to ostracods, 37 to cumaceans, and three to mysids. Overall, polychaetes are numerically dominant (50% of the total macrobenthos), followed by crustaceans (25%) and molluscs (7%). Amphipods and ostracods are numerically important among crustaceans, while pelecypodes are the most dominant taxa among molluscs. Remaining taxa constitute 18% of the total macrobenthos, with rhynchocoels, oligochaetes, sipunculids, polyplacophorans, gastropods, copepods, isopods, and ophiuroids contributed >1%.
Appendix E shows the 10 most dominant polychaete and major crustacean species recorded from various regions in the open waters. Of these predominant species, polychaetes, Prionospio sp. 1, and Lumbrineris sp. and crustaceans, Cypridina sp., Euphilomedes sp., and Parasterope sp. dominated in all five regions. In the open water region as a whole, the polychaete assemblage is highly dominated by Prionospio sp.1 (average density 111 ind. m−2), Syllis sp. 1 (110 ind. m−2), and Prionospio bocki (96 ind. m−2), recorded at 54, 47, and 33 stations respectively. The most dominant crustacean species is Gammaropsis atlantica (average density 97 ind. m−2) (Amphipoda) followed by Cypridina sp. (68 ind. m−2) and Euphilomedes sp. (50 ind. m−2) (Ostracoda), recorded at 45, 57, and 55 stations, respectively. There were 88 species (69 polychaete and 19 crustacean species) that could be considered as locally rare, because they were recorded at only one station each. Ampeliscids (amphipods belonging to family, Ampeliscidae), the oil pollution indicators, show significant difference between the nearshore and offshore areas, with a lower density in the nearshore area (average of 16 ind. m−2) compared to the offshore area (average of 107 ind. m−2) with no north-south variations within nearshore-offshore regions (PERMANOVA results in Appendix F). Similarly, northern region shows a lower ampeliscid density (average of 79 ind. m−2) compared to the central (average of 135 ind. m−2) and southern (average of 93 ind. m−2) regions. However, the northern-southern difference is not statistically significant nor the nearshore-offshore variation within the northern-southern regions (PERMANOVA results in Appendix G). Interactive effects of nearshore-offshore and north-south factors are not noticed in ampeliscid density (PERMANOVA results in Appendices F,G).
Structural parameters of macrobenthos (S, H′, and density) recorded from various regions are given in Figure 2. The S at a given station ranges from 46 (northern) to 97 (central region) with an overall average (±SE) of 71 ± 2 species from the open waters. Average values indicate that there is no nearshore-offshore variation in S, while central and southern regions show higher S than the northern region (Figure 2A), but was not statistically significant (PERMANOVA results in Appendixes F, G). The H′ varies between 3.2 (nearshore region) and 5.6 (offshore region) with an average (±SE) of 4.9 ± 0.1. The H′ does not vary between various regions of the open waters (Figure 2B) (PERMANOVA results in Appendixes F,G). The density of macrobenthic organisms ranges from 623 ind. m−2 (northern region) to 19,196 ind. m−2 (central region) with an average (±SE) of 3,181 ± 359 ind. m−2. A higher average density was recorded in the nearshore region compared to offshore and central regions which was higher than in the northern and southern regions (Figure 2C). Of these structural parameters, a statistically significant difference is shown only by the density for the nearshore-offshore variations (PERMANOVA results in Appendices F,G). There are no interactive effects of nearshore-offshore and north-south factors in the structural parameters of macrobenthos (PERMANOVA results in Appendices F,G).
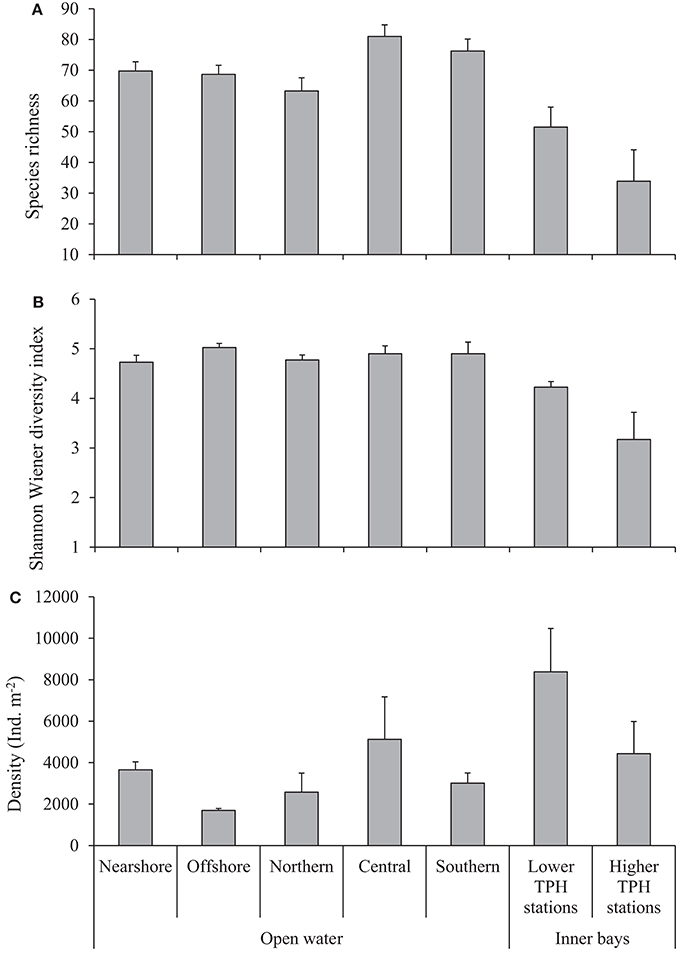
Figure 2. Structural parameters (mean+SE) of macrobenthos in the study area. (A) S, (B) H′, and (C) density.
Classification analyses (using Bray-Curtis similarity) examined through nMDS for fauna show a separation of the nearshore and offshore regions (Figure 3A) and, to some extent, northern from the central and southern regions (Figure 3B). There are significant nearshore-offshore and northern-southern differences between the assemblages and each shows variation within the regions (PERMANOVA results in Appendices F,G). There are also interactive effects of these two factors (PERMANOVA results in Appendices F,G).
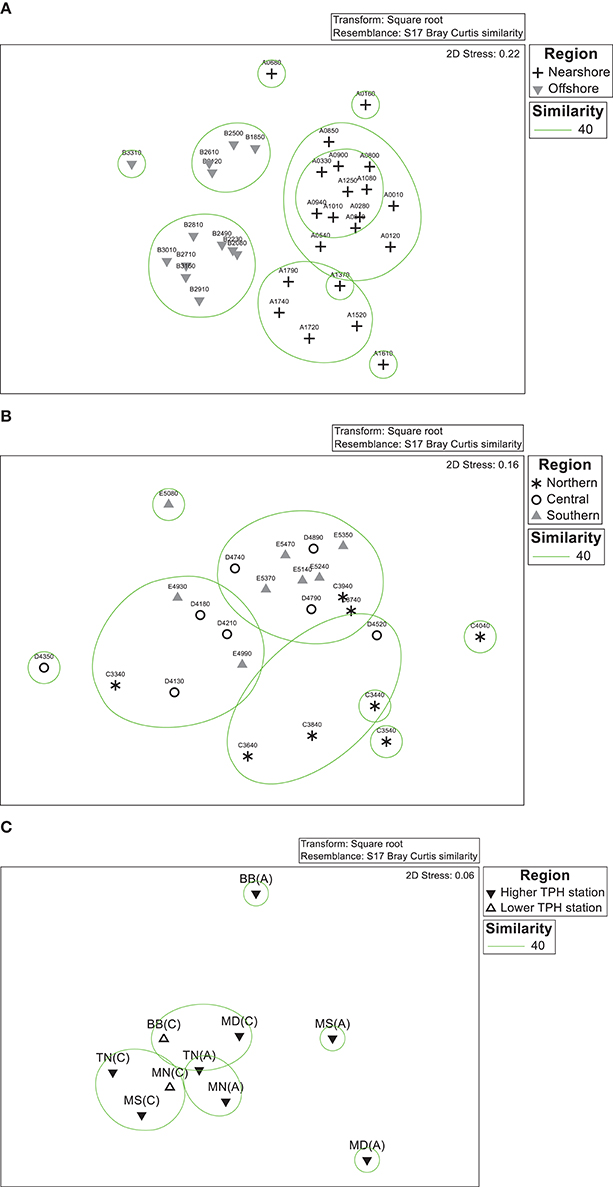
Figure 3. nMDS ordination for stations located in distinct regions. (A) Nearshore and offshore regions of open water, (B) northern, central, and southern regions of open water and (C) control and impacted locations of inner bays.
Inner Bays
In total, 162 taxa were recorded from the inner bay stations. This includes 93 polychaete species, 49 crustacean species, and 20 other taxa. Among the major crustaceans, 20 species belong to amphipods, 10 species to ostracods, 18 species to cumaceans, and one species to mysids. Polychaetes are predominant in the samples (with 60% of the total macrobenthos) followed by molluscs (19%) and crustaceans (16%). Among the crustaceans, amphipods (6.4% of the total macrobenthos), and isopods (5.2% of the total macrobenthos) and among molluscs, bivalves (14% of the total macrobenthos) are dominant. The remaining taxa constitute 5% of the total macrobenthos and in those, only oligochaetes (1.5% of the total macrobenthos) and nemerteans (1.4% of the total macrobenthos) are numerically important.
As the number of samples from the LTS are lower than the number of samples from the HTS, the number of species are also lower in the former as the sampling size affects the number of species. Regarding polychaete and crustacean species, 77 and 43 were recorded from HTS and 56 and 23 from the LTS, respectively.
Only three ampeliscid species (Ampelisca scabripes, Ampelisca tulearensis, and A. insignis) were recorded from the inner bay stations. All three were recorded from the HTS, while only A. scabripes and A. insignis were recorded from the LTS.
Among the 10 dominant polychaete species, Syllis sp., Scoloplos sp., Exogone clavator, and Platynereis sp 1 are abundant and among the dominant crustaceans, Maera quadrimana, Gammaropsis sp., Cypridina sp., and Grandidierella bonnieroides are abundant in the LTS. In the HTS, Platynereis sp 1, Syllis sp 1, Lumbrineris sp, and E. clavator from polychaetes and G. bonnieroides, Maera pacifica, Eusiroides caesaris, and Nannastacus gurneyi from crustaceans are abundant (Appendix E).
Overall average (±SE) values of the structural parameters recorded in the inner bays are, S of 41 ± 9, H′ of 3.48 ± 0.39, and density of 4,203 ± 1,042 ind. m−2. Higher S, H′, and density were recorded from the LTS than that of the HTS (Figure 2). However, none of these parameters show statistically significant variations (PERMANOVA results in Appendix H). The values of S are in the range of 45 [station BB(C)] to 58 [station MC(C)] at the LTS and 6 [station MD(A)] to 86 [station TN(C)] at the HTS. The H′ varies between 4.11 [BB(C)] and 4.3 [MN(C)] at the LTS and 1.34 [station BB(A)] and 4.97 [station TN(C)] at the HTS. The density ranges from 6,284 ind. m−2 [BB(C)] to 10,476 ind. m−2 [station MN(C)] at the LTS and 850 ind. m−2 [station MD(A)] to 13,184 ind. m−2 [station TN(C)] at the HTS. Among the HTS, a lower S (<10) was also recorded from station BB(A) and lower diversity (<2) from stations MS(A) and MD(A).
In the nMDS, most of the stations are close together in the plot, except stations BB(A), MS(A), and MD(A) (Figure 3C). The lower and higher TPH stations do not show a separation and there is no significant difference in the macrobenthic assemblages between these two sets of stations (PERMANOVA results in Appendix H).
AMBI and M-AMBI
A total of 392 taxa were identified in the open waters of the Gulf. After assignment, many new species were incorporated to the new AMBI species list (November 2014), which is available free of charge at the AMBI web site. After this assignment, the percentage of unassigned individuals ranged from 0 to 19.8%, with a mean value of 5.2%. Thus, all samples can be taken into account in the analysis.
In the region of open waters, according to the AMBI results, all stations are slightly disturbed, and according to the M-AMBI results, all stations show a high or good status (Figure 4, Table 2). The sensitive species group (EG I) varies from 7.1% (station E5080, southern region) to 48.6% (station C3640, northern region), and opportunistic species (EGs IV and V) from 0.8% (station D4350, central region) to 47% (station D4890, central region). Despite the AMBI homogeneity distribution, there are statistically significant nearshore-offshore differences (higher AMBI values in offshore, PERMANOVA results in Appendix F). A similar difference is also shown by opportunistic species (PERMANOVA results in Appendix F). However, north-south variations within the nearshore-offshore regions are absent. A statistically significant north-south difference is not shown by any of the parameters. However, AMBI shows nearshore-offshore variations within the northern-southern regions (PERMANOVA results in Appendix G). Interactive effects of nearshore-offshore and north-south factors are not noticed in any of these parameters (PERMANOVA results in Appendices F,G).
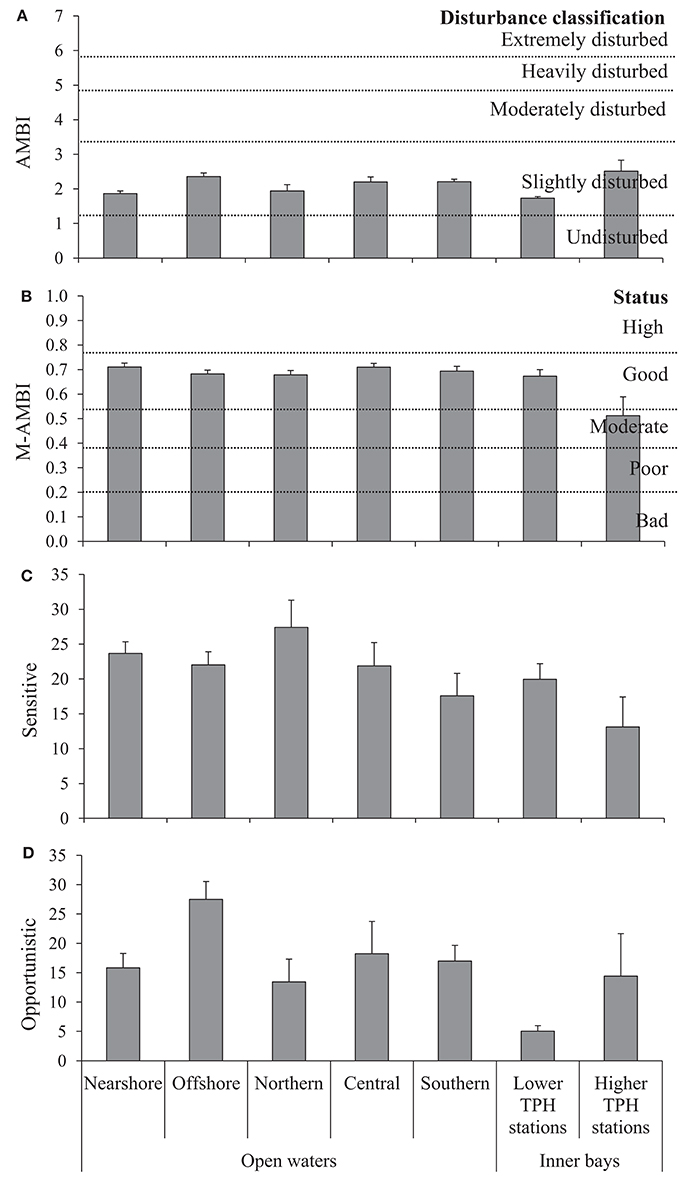
Figure 4. The AMBI (A) and M-AMBI (B) values, and the percentage of sensitive (C), and opportunistic (D) species (addition of the ecological groups IV and V) (mean + SE) calculated for the study area.
In the inner bays, AMBI values indicate slightly disturbed conditions at all stations except one [LTS, BB(A)], which is moderately disturbed (Figure 4, Table 2). M-AMBI values show good status for LTS, while, high, good, moderate, and poor status for HTS (Table 2). The sensitive species group varies from 0% [HTS, MD(A)] to 33.9% [HTS, TN(A)], and opportunistic species from 3.8% [HTS, TN(A)] to 59.7% [HTS, BB(A)]. The average values of AMBI, M-AMBI, sensitive species, and opportunistic species show worst status for the HTS compared to the LTS (Figure 4). However, none shows statistically significant differences between the two sites (PERMANOVA results in Appendix H).
Environmental Correlations of Macrobenthos
The results of the Mann-Kendall correlation analyses between environmental variables and faunal parameters are given in Appendix I. In the open waters, after Bonferroni correction, AMBI is positively correlated to depth (p < 0.05) and mud% (p < 0.05) and negatively correlated to sand% (p < 0.05). Ampeliscid density is positively influenced by depth (p < 0.05), and negatively influenced by salinity (p < 0.05) and DO (p < 0.05).
In the inner bays, significant correlations were not recorded after Bonferroni correction (Appendix I).
Discussion
Open Waters
Parameters of the bottom waters exhibit nearshore-offshore and north-south variations. A general increase in the temperature and decrease in the salinity and DO toward the south are attributed to the inflow of Indian Ocean water through the Strait of Hormuz (Reynolds, 1993; Abdelrahman and Ahmad, 1995). The high evaporation and turbulence in the coastal areas cause higher salinity and DO in the nearshore waters compared to those of the offshore waters. During late fall, winter, and spring, when the coastal waters are comparatively cooler, the temperature increases toward the offshore areas.
In the Gulf, sediments and their characteristics generally follow distributions that align with the northwest-southeast axis of the Gulf (Wagner and van der Togt, 1973). The predominance of sand in the coastal regions, which are closer to the shore, and shallow in nature, can be attributed to the water circulation prevalent in this area. The gradual replacement of sand by mud fractions from the nearshore to offshore regions is likely to be due to the increasing protection from wave action in the center of the basin, resulting in transport of only fine materials to the center of the Gulf basin (Wagner and van der Togt, 1973). In an ideal situation, higher TOC values are associated with a lower sediment particle size (Khalaf et al., 1986). With the increase of the mud fraction with the depth, TOC also increases, as found in the present study.
Based on the published reports on the TPH concentrations of sediments, Massoud et al. (1996) suggested a TPH value of 10–15 mg/kg (in dry, silt/clay sediment fractions), as the unpolluted or natural background level in the Gulf. In the present study, 17% of the stations, which are mainly in the central and southern regions, has the natural background level of TPH. About 64% of the stations has TPH concentrations >50 mg kg−1.
In general, the benthic density (3,181 ± 359 ind. m−2) and H′ (4.9 ± 0.1) in the region of the open waters are comparable to the values recorded in earlier studies in the Saudi waters of the Gulf. For example, a density of 4,710 ind. m−2 and 2,022 ind. m−2 have been reported by Coles and McCain (1990) and McCain (1984b), respectively. Also, values in the range of 0.5–5.5 have been reported for H′ by McCain (1984b). A comparatively higher density was observed in the nearshore areas compared to that in the offshore areas, which can be due to the rich primary production in nearshore waters. The supply of food to the subtidal benthic environment depends on the proximity to the shore and the water depth (Levinton, 1982). In general, diversity indices recorded are high at many stations. Diversity values >3 were recorded at all stations.
Spatially related structures in the macrobenthic assemblages along the nearshore-offshore and north-south axes are evident from the MDS and PERMANOVA analyses. The hydrographical and sediment parameters which show variations as a function of the depth, also influence the fauna. However, prominent spatial variations in the structural parameters of macrobenthos are not observed. Fauna at the two stations where low DO values were recorded do not show any sign of impoverishment, as indicated by the total density (2,433–3,090 ind.m−2), S (57–66 species), and relatively high H′ values (4.0–4.3).
Despite such high TPH concentrations at a large number of stations, “slightly disturbed” conditions, with either “high” or “good” ecological status indicate that the benthic communities have experienced very low stress in the open waters, 12 years after the 1991 Oil Spill. During the spill, the oil has moved from the coast of Kuwait down to Abu Ali Island in Saudi Arabia along the nearshore area (Fowler et al., 1993; Michel et al., 1993; Sadiq and McCain, 1993; Tawfiq and Olsen, 1993; Readman et al., 1996; Price, 1998). Hence, the residual impact, if any, can be expected mostly at the stations located close to the shore (transect A) from the north down to Abu Ali and nearshore stations of the northern (transect C) and central (transect D) regions. The oil spill has not affected the nearshore area south of Abu Ali (Tawfiq and Olsen, 1993; Readman et al., 1996). Our results indicate that the health of the benthic communities in the nearshore area north of Abu Ali (shown by the average values of AMBI, M-AMBI, S, and H′ are 1.8, 0.72, 64, and 4.63, respectively) is better than that of the nearshore area south of Abu Ali (shown by the values of the same parameters of 2.09, 0.68, 59, and 4.38, respectively) even though the concentration of TPH north of Abu Ali (average of 104 mg kg−1) is higher than that south of Abu Ali (average of 35 mg kg−1). Coles and McCain (1990) have reported the status of benthic community based on samples collected during the 1985–1986 period from three stations in the nearshore area north of Abu Ali. They have reported an average S of 45 species per station from the sand/silt habitat, which is lower than that recorded from the same area in the present study (64 species per station). As the benthos samples collected by Coles and McCain (1990) are not from polluted stations, the comparison confirms the absence of a long-term impact from the 1991 Oil Spill.
Recovery of a marine ecosystem from an oil spill can be defined as the re-establishment of a biological community in which the plants and animal characteristics of the community are present and functioning normally (Kingston, 2002). However, in the recovery assessment it is impossible to say whether the benthic communities that have reestablished after the oil spill are the same or are different from those which would have existed prior to the oil spill. The western Gulf has a large number of oil-related facilities in the form of production wells, platforms, underwater pipelines, refineries etc. Periodically, new installations are built and the existing facilities are upgraded. These oil-related activities have the potential to cause disturbances to the benthic communities due to the physico-chemical alterations of the benthic habitat. As such activities existed prior to the 1991 oil spill, it can be assumed that an “undisturbed condition” with “high′' ecological status for the benthic communities may not have existed in the impacted open waters prior to the spill. In that case, the recovery of the benthic communities to the level of a pristine condition as per the definition of Kingston (2002) may not be possible in the western Gulf as these oil-related activities still continue.
The spatial trends in the structural parameters of the macrobenthos observed in the present study are assumed to be due to the oil-related activities in this region. A great majority of the offshore oil production facilities are situated in the northern region and they decrease toward the south. Accordingly, there is a north-south decrease in the concentration of TPH with a corresponding increase in the oil sensitive organisms such as ampeliscids. Although, relatively lower than the nearshore region, the offshore region also have high concentrations of TPH (Table 1), which have the potential to pose a threat to the marine environment. Despite the slightly higher H′, in the offshore region compared to that of the nearshore region, more disturbed conditions (according to AMBI) and a slightly inferior ecological status (according to M-AMBI) are observed in the offshore region compared to the nearshore region. As the Oil Spill has moved toward south through the nearshore region, the high concentration of TPH in the offshore region cannot be attributed to the 1991 Oil Spill. The high concentration of TPH and the relatively poorer ecological status of the benthic communities in the offshore region may be due to the effects of the offshore platforms and the increased vessel traffic.
TOC values directly proportional to the concentration of TPH have been reported in some parts of the world after oil spill incidents (for example, Wattayakorn and Rungsupa, 2011). However, in most cases, such relationships have not been observed in the Gulf (Abdali et al., 1993; Al-Ghadban et al., 1994; Al-Lihaibi and Al-Omran, 1996; Massoud et al., 1996; Al-Lihaibi and Ghazi, 1997; de Mora et al., 2010) and the conclusions of the present study is in accord with the conclusions of the previous studies. The concentration of TOC can be considered as an indicator of hydrocarbon pollution only when the concentration of TPH is very high (Al-Ghadban et al., 1994).
Inner Bays
This study clearly shows the presence of residual effects of the 1991 Oil Spill in the sheltered inner bays of the Gulf, where the grain size of the sediment is more or less the same as that in other areas. Of the five locations in the inner bays evaluated in this study, the TPH concentration is very high in the MTBS, specifically at the station located in Balbol. In a study conducted in 2003, a TPH concentration of 1,000 mg kg−1 was recorded in the surface sediments of an impacted station, indicating that the situation was at an alarming level even 12 years after the oil spill. Sauer et al. (1993) has reported the occurrence of sunken oil from the 1991 Oil Spill at a depth of 0–5 cm from the sediment surface inside the sheltered inner bays. A core sample from Balbol Bay analyzed along with the present study shows a concentration of 38,000 mg kg−1 at a depth of about 15 cm from the seabed. This indicates that the rate of accumulation of oil in the sediments was rapid in the sheltered inner bays due to the absence of strong current or wave action. The post-1991 Oil Spill studies have reported higher TPH values in the sediments of the MTBS and AABS compared to those in the sediments in the open water areas (Fowler et al., 1993; Michel et al., 1993; Readman et al., 1996). About seven months after the Spill, Fowler et al. (1993) recorded sediment hydrocarbon concentrations up to 1,140 mg kg−1 from the open water regions and up to 1,400 mg kg−1 from the sheltered inner bays. Michel et al. (1993) also reported sediment hydrocarbon concentrations up to 900 mg kg−1 from the inner bays of the AABS 1 year after the spill. Even though a consistent reduction in the contamination of the bays has been reported in the subsequent years, the recovery has been slow. Readman et al. (1996) reported an increase of the contamination at certain sites on the impacted coasts in the sheltered bays when the same sites were surveyed during the months of August in 1991, 1992, and 1993. They attributed this to the patchiness in the distribution and the re-deposition of a remobilized oil pockets from the original contamination event.
According to the results of the present study, the four stations from the inner bays can be categorized as having a “moderate-poor” ecological status. Even though, the highest concentration of TPH was observed at the station in Balbol, the other two locations in the MTBS, viz., Tanajib and Manifa, can be categorized as having a “good-higH′' ecological status. On the other hand, both sites studied in the AABS, Mussallamiya and Mardomma, can be categorize as having a “moderate-poor” status, indicating a higher stress on the benthic community compared to the MTBS. However, an accurate picture will be revealed only if a large number of samples are collected from both MTBS and AABS and evaluated.
In this study samples were not collected from the intertidal region. However, high levels of polyaromatic hydrocarbons (PAHs) along the shoreline of the sheltered bays of Saudi Arabia, originating from the 1991 Oil Spill, have been reported (Bejarano and Michel, 2010). These authors note that the concentration of the sum of PAHs in these samples are similar to the levels found in the sheltered areas 1year after the oil spill (100,000–300,000 ng g−1). Persisting high concentration of PAHs indicates that the physical removal or microbial degradation of the oil in these heavily oiled intertidal sediments is very low. As alarming levels of oil are present in the intertidal (Bejarano and Michel, 2010) and subtidal (present study) zones more than even a decade after the spill, the complete recovery may take decades more. Chances of the re-distribution of the sunken oil due to dredging/trenching activities are also present. This area, being in close proximity to the oil fields, may host future facilities. Any activities that require dredging/trenching can cause the re-introduction of the oil into the water column, and thereby the re-distribution to the surface of the seabed. This can impact the resident benthic community and cause further delays in the recovery of the inner bay habitats from the 1991 Oil Spill.
AMBI and M-AMBI and the Baseline Conditions
The indices AMBI and M-AMBI have not been commonly used in studies of the Gulf waters. These two indices were used in this study because of their extensive use in different habitats worldwide for assessing the ecological status of benthic communities (Borja et al., 2012). The worldwide acceptance of these indices is due to the fact that the benthic status of a given area determined by AMBI and M-AMBI is highly correlated with the benthic status assigned by professional judgment (Borja et al., 2014). However, one difficulty of using such indices in the Gulf is the selection of reference conditions. Commonly, reference conditions are derived from: (i) comparison with an existing “pristine”site; (ii) past data and information; (iii) numerical models; and (iv) best professional judgment (Borja et al., 2004, 2012; Muxika et al., 2007; Forchino et al., 2011). Due to the widespread oil-related activities in the Gulf, and the likelihood of the resulting stress on the marine environment (Bishop, 2002; Khan et al., 2002; Munawar et al., 2002; Jones et al., 2007; Khan, 2007), finding pristine sites is a great challenge. The Gulf is a naturally stressed water body with high salinity and high seasonal temperature variations. In such environments, generally, the biota will be living at their upper tolerance levels and the impacts of any human pressures can be increased many fold compared to their effects in a normal environment. Considering all these facts, Sheppard et al. (2010) recently categorized the Gulf as a young sea in decline. In this region, the majority of the historical data have been generated from a multitude of Environmental Impact Assessment studies. One drawback of this type of data in serving as a good baseline is that, in most cases, the baseline chosen is the already severely degraded marine ecosystem (Sheppard et al., 2010). Hence, a comparison with the previous data is generally of no use. In such circumstances and in the absence of numerical models, expert judgment may be the best option to determine the reference conditions (Borja et al., 2014). In the present study, the approach used for establishing the reference condition is by selecting the highest richness and diversity values determined and increasing them by 15%. This has been considered to be a reasonable approach (Borja and Tunberg, 2011; Forchino et al., 2011; Paganelli et al., 2011). Based on this reference condition, about 25% of the stations were categorized as having a “higH′' ecological status. These stations have an average S value of 82 species and H′ of 5.1. These values are expected to be used as reference data in the future impact assessments on benthic communities in the Gulf.
Conclusions
This study evaluated a major part of the northwestern Gulf and compared the macrobenthic communities in the nearshore, offshore, northern, central, and southern regions of the open waters and locations in the inner bays affected by the 1991 Oil Spill. More than a decade after the spill incident, while the surficial sediments in the open waters have only low levels of hydrocarbon concentrations, the sediments in the sheltered bays have alarming levels. The macrobenthic communities in the open waters can be categorized as having either a good or high ecological status, while those in the sheltered bays have a moderate-poor status. The results indicate a severe long-term impact of the oil spill in the inner bays. In this study only a limited number of benthos samples were collected from the oil-spill-impacted sheltered bays. To fully comprehend the spread and the quantum of the residual impact of the 1991 Oil Spill on the benthic communities in the sheltered bays it is necessary to conduct an extensive study in future. The slightly inferior ecological status of the benthic communities in the offshore areas compared to that of the nearshore areas, reported in this study, maybe due to the effects of the oil-related activities in the open waters of the Saudi waters of the Gulf.
Author Contributions
TJ and AA designed the study, conducted sampling, and laboratory analyses. TJ, MQ, AB, and PK structured the manuscript, conducted data analyses, and interpretations. TJ prepared a first draft of the manuscript and all coauthors contributed to the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Center for Environment & Water (CEW) at the Research Institute of King Fahd University of Petroleum & Minerals, Dhahran, Saudi Arabia, for providing research facilities and the Marine Studies Section team of CEW for performing the fieldwork and laboratory analyses. We express our sincere thanks to the General Authority of Meteorology and Environmental Protection, Saudi Arabia and Saudi Company for Environmental Works Ltd. for their constant support. We also thank M/S Al-Gosaibi for providing services of their Oceanographic Vessel. We are grateful to Dr. Ajmal Khan, Dr. Murugesan, Dr. Linoy Libini, and Dr. Thomas Jacob of Annamalai University, India and Dr. Sajan Sebastian of the Cochin University of Science and Technology, India for their valuable support. We are also thankful to Dr. Chandana Senaratne, KFUPM/RI, for editing the manuscript. The authors highly appreciate the two reviewers for their suggestions to improve the quality of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2017.00248/full#supplementary-material
References
Abdali, F., A1-Najjar, H. A., Bahloul, M., Jacob, P. G., and Ghadban, A. N. (1993). “Pore diffusion transport of petroleum hydrocarbons within sediment column in ROPME Sea Area,” in Presented at the Scientific Workshop on Results of the R/V Mt Mitchell Open Sea Cruise (Kuwait), 24–28.
Abdelrahman, A. S. M., and Ahmad, F. (1995). A note on the residual currents in the Arabian Gulf. Cont. Shelf Res. 15, 1015–1022. doi: 10.1016/0278-4343(95)80006-Y
Al-Ghadban, A. N., Jacob, P. G., and Abdali, F. (1994). Total organic carbon in the sediments of the Arabian Gulf and need for biological productivity investigations. Mar. Poll. Bull. 28, 356–362. doi: 10.1016/0025-326X(94)90272-0
Al-Lihaibi, S. S., and Al-Omran, L. (1996). Petroleum hydrocarbons in offshore sediments from the Gulf. Mar. Poll. Bull. 32, 65–69. doi: 10.1016/0025-326X(95)00161-F
Al-Lihaibi, S. S., and Ghazi, S. J. (1997). Hydrocarbon distributions in sediments of the open area of the Arabian Gulf following the 1991 Gulf war oil spill. Mar. Poll. Bull. 34, 941–948. doi: 10.1016/S0025-326X(97)00069-6
Al-Yamani, F., Boltachova, N., Revkov, N., Makarov, M., Grintsov, V., Kolesnikova, E., et al. (2009). Winter species composition, diversity and abundance of macrobenthos in Kuwait's waters, Arabian Gulf. Zookeys 31, 17–38. doi: 10.3897/zookeys.31.74
Anderson, M. J. (2001). A new method for non parametric multivariate analysis of variance. Austral Ecol. 26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x
Barnard, J. L., and Drummond, M. M. (1982). Gammaridean Amphipoda of Australia, Part V: Superfamily Haustorididea. Smithsonian contribution to zoology, Smithsonian Institution Press. 1–148. doi: 10.5479/si.00810282.360
Basson, P., Burchard, J. H., and Price, A. (1977). Biotopes of the Western Arabian Gulf: Marine life and environments of Saudi Arabia. Saudi Arabia: Aramco Department of Loss Prevention and Environmental Affairs, Dhahran.
Bejarano, A. C., and Michel, J. (2010). Large-scale risk assessment of polycyclic aromatic hydrocarbons in shoreline sediments from Saudi Arabia: environmental legacy after twelve years of the Gulf war oil spill. Environ. Pollut. 158, 1561–1569. doi: 10.1016/j.envpol.2009.12.019
Bishop, J. M. (2002). “Fishing and mariculture,” in The Gulf Ecosystem, Health and Sustainability, eds N. Y. Khan, M. Munawar, and A. R. G. Price (Leiden: Backhuys Pub), 253–278.
Borja, A., Dauer, D., Elliott, M., and Simenstad, C. (2010). Medium- and long-term recovery of estuarine and coastal ecosystems: patterns, rates and restoration effectiveness. Estuar. Coast. 33, 1249–1260. doi: 10.1007/s12237-010-9347-5
Borja, A., Dauer, D. M., and Grémare, A. (2012). The importance of setting targets and reference conditions in assessing marine ecosystem quality. Ecol. Indic. 12, 1–7. doi: 10.1016/j.ecolind.2011.06.018
Borja, A., Franco, J., and Pérez, V. (2000). A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar. Poll. Bull. 40, 1100–1114. doi: 10.1016/S0025-326X(00)00061-8
Borja, A., Franco, J., Valencia, V., Bald, J., Muxika, I., Belzunce, M. J., et al. (2004). Implementation of the European water framework directive form the Basque country (north Spain): a methodological approach. Mar. Poll. Bull. 48, 209–218. doi: 10.1016/j.marpolbul.2003.12.001
Borja, A., Josefson, A. B., Miles, A., Muxika, I., Olsgard, F., Phillips, G., et al. (2007). An approach to the intercalibration of benthic ecological status assessment in the North Atlantic ecoregion, according to the European Water Framework Directive. Mar. Poll. Bull. 55, 42–52. doi: 10.1016/j.marpolbul.2006.08.018
Borja, A., Mader, J., Muxika, I., Rodríguez, J. G., and Bald, J. (2008). Using M-AMBI in assessing benthic quality within the Water Framework Directive: some remarks and recommendations. Mar. Poll. Bull. 56, 1377–1379. doi: 10.1016/j.marpolbul.2007.12.003
Borja, A., and Muxika, I. (2005). Guidelines for the use of AMBI (AZTI's Marine Biotic Index) in the assessment of the benthic ecological quality. Mar. Poll. Bull. 50, 787–789. doi: 10.1016/j.marpolbul.2005.04.040
Borja, A., Muxika, I., and Franco, J. (2003). The application of a Marine Biotic Index to different impact sources affecting soft-bottom benthic communities along European coasts. Mar. Poll. Bull. 46, 835–845. doi: 10.1016/S0025-326X(03)00090-0
Borja, A., Prins, T. C., Simboura, N., Andersen, J., Berg, T., Marques, J.-C., et al. (2014). Tales from a thousand and one ways to integrate marine ecosystem components when assessing the environmental status. Front. Mar. Sci. 1:72. doi: 10.3389/fmars.2014.00072
Borja, A., and Tunberg, B. G. (2011). Assessing benthic health in stressed subtropical estuaries, eastern Florida, USA using AMBI and M-AMBI. Ecol. Indic. 11, 295–303. doi: 10.1016/j.ecolind.2010.05.007
BP (2013). BP Statistical Review of World Energy June 2013. Available online at: http://www.bp.com
Breuer, E., Stevenson, A. G., Howe, J. A., Carroll, J., and Shimmield, G. B. (2004). Drill cutting accumulations in the Northern and Central North Sea: a review of environmental interactions and chemical fate. Mar. Poll. Bull. 48, 12–25. doi: 10.1016/j.marpolbul.2003.08.009
Bu-Olayan, A. H., and Thomas, B. V. (2005). Validating species diversity of benthic organisms to trace metal pollution in Kuwait Bay, off the Arabian Gulf. Appl. Ecol. Environ. Res. 3, 93–100. doi: 10.15666/aeer/0302_093100
Burns, K. A., Garrity, S. D., Jorissen, D., MacPherson, J., Stoelting, M., Tierney, J., et al. (1994). The Galeta oil spill. II. Unexpected persistence of oil trapped in mangrove sediments. Estuar. Coast. Shelf Sci. 38, 349–364. doi: 10.1006/ecss.1994.1025
Clarke, K. R., and Warwick, R. M. (2001). Change in Marine Communities: an Approach to Statistical Analysis and Interpretation, 2nd Edn. Plymouth: PRIMER-E.
Coles, S. L. (2003). Coral species diversity and environmental factors in the Arabian Gulf and the Gulf of Oman: a comparison to the Indo-Pacific region. Atoll Res. Bull. 507, 1–19. doi: 10.5479/si.00775630.507.1
Coles, S. L., and Fadlallah, Y. H. (1991). Reef coral survival and mortality at low temperatures in the Arabian Gulf: new species-specific lower temperature limits. Coral Reefs 23, 1–237. doi: 10.1007/BF00290427
Coles, S. L., and McCain, J. C. (1990). Environmental factors affecting benthic infaunal communities of the western Arabian Gulf. Mar. Environ. Res. 29, 289–315. doi: 10.1016/0141-1136(90)90024-I
Dauvin, J. C. (1998). The fine sand Abra alba community of the Bay of Morlaix twenty years after the Amoco Cadiz oil spill. Mar. Poll. Bull. 36, 669–676. doi: 10.1016/S0025-326X(98)00058-7
Day, J. H. (1967). A Monograph on the Polychaete of Southern Africa, Part I (Errantia) and Part II (Sedentaria). London: Trustees of the British Museum. 878.
de Mora, S., Tolosa, I., Fowler, S. W., Villeneuve, J.-P., Cassi, R., and Cattini, C. (2010). Distribution of petroleum hydrocarbons and organochlorinated contaminants in marine biota and coastal sediments from the ROPME Sea Area during 2005. Mar. Poll. Bull. 60, 2323–2349. doi: 10.1016/j.marpolbul.2010.09.021
Fauchald, K. (1977). The Polychaetes Worms, Definitions and keys to the Orders, Families and Genera. California: Natural History Museum of Los Angeles County, Science Series 28.
Forchino, A., Borja, A., Brambilla, F., Rodríguez, J. G., Muxika, I., Terova, G., et al. (2011). Evaluating the influence of off-shore cage aquaculture on the benthic ecosystem in Alghero Bay (Sardinia, Italy) using AMBI and M-AMBI. Ecol. Indic. 11, 1112–1122. doi: 10.1016/j.ecolind.2010.12.011
Fowler, S. W., Readman, J. W., Oregioni, B., Villeneuve, J.–P., and Mckay, K. (1993). Petroleum hydrocarbons and trace metals in nearshore Gulf sediments and biota before and after the 1991 War: an assessment of temporal and spatial trends. Mar. Poll. Bull. 27, 171–182. doi: 10.1016/0025-326X(93)90022-C
Gerges, M. (1993). On the impacts of the 1991 Gulf War on the environment of the region: general observations. Mar. Poll. Bull. 27, 305–314. doi: 10.1016/0025-326X(93)90038-L
Gómez-Gesteira, J. L., and Dauvin, J. C. (2000). Amphipods are good bioindicators of the impact of oil spills on soft bottom macrobenthic communities. Mar. Poll. Bull. 40, 1017–1027. doi: 10.1016/S0025-326X(00)00046-1
Guidetti, P., Modena, M., Mesa, G. L., and Vacchi, M. (2000). Composition, abundance and stratification of macrobenthos in the marine area impacted by tar aggregates derived from the Haven oil spill (Ligurian Sea, Italy). Mar. Poll. Bull. 40, 1161–1166. doi: 10.1016/S0025-326X(00)00079-5
Hall, S. J. (1985). Four new species of Myodocopine ostracodes (Sarsiellidae) from Lizard island, North Queensland. Crustacean Biol. 7, 738–763. doi: 10.2307/1548657
Hampel, H., Elliot, M., and Cattrijsse, A. (2009). Macrofaunal communities in the habitats of intertidal marshes along the salinity gradient of the Shelde estuary. Estuar. Coast. Shelf Sci. 84, 45–53. doi: 10.1016/j.ecss.2009.05.029
Hoffmann, L. (1994). Distribution, species composition and status of the intertidal blue-green algal mats. Cour. Forsch. Inst. Senc. Ken. 116, 16–17.
Jewett, S. C., Dean, T. A., Smith, R. O., and Blanchard, A. (1999). Exxon Valdez oil spill: impacts and recovery in the soft-bottom benthic community in and adjacent to eelgrass beds. Mar. Ecol. Prog. Ser. 185, 59–83. doi: 10.3354/meps185059
John, V. C., Coles, S. L., and Abozed, A. I. (1990). Seasonal cycles of temperature, salinity and water masses of the Western Arabian Gulf. Oceanol. Acta 13, 273–281.
Jones, D. A. (1986). A Field Guide to the Sea Shores of Kuwait and the Arabian Gulf. London: Blanford press.
Jones, D. A., Ealey, T., Baca, B., Livesey, S., and Al-Jamali, F. (2007). Gulf desert developments encompassing a marine environment, a compensatory solution to the loss of coastal habitats by infill and reclamation: the case of the Pearl City Al-Khiran, Kuwait. Aquat. Ecosyst. Health Manag. 10, 268–276. doi: 10.1080/14634980701512814
Jones, D. A., Plaza, J., Watt, I., and Al Sanei, M. (1998). Long-term (1991-1995) monitoring of the intertidal biota of Saudi Arabia after the 1991 Gulf War oil spill. Mar. Poll. Bull. 36, 472–489. doi: 10.1016/S0025-326X(98)00009-5
Jones, D. A., Watt, I., Woodhouse, T. D., and Richmond, M. D. (1994). Inter- tidal recovery in the Dawhat at Dafi and Dawhat al Musallamiya Waters (Saudi Arabia) after the Gulf War oil spill. Cour. Forsch. Inst. Senc. Ken. 166, 27–33.
Joydas, T. V., Krishnakumar, P. K., Qurban, M. A., Ali, S. A., Al-Suwailem, A., and Al-Abdulkader, K. (2011). Status of macrobenthic community of Manifa–Tanajib Bay System of Saudi Arabia based on a once-off sampling event. Mar. Poll. Bull. 62, 1249–1260. doi: 10.1016/j.marpolbul.2011.03.012
Joydas, T. V., Qurban, M. A., Al–Suwailem, A., Krishnakumar, P. K., Nazeer, Z., and Cali, N. A. (2012). Macrobenthic community structure in the northern Saudi waters of the Gulf, fourteen years after the 1991 oil spill. Mar. Poll. Bull. 64, 325–335. doi: 10.1016/j.marpolbul.2011.11.007
Joydas, T. V., Qurban, M. A., Manikandan, K. P., Ashraf, T. T. M., Ali, S. M., Al-Abdulkader, K., et al. (2015). Status of macrobenthic communities in the hypersaline waters of the Gulf of Salwa, Arabian Gulf. J. Sea Res. 99, 34–46. doi: 10.1016/j.seares.2015.01.006
Khalaf, F., Literathy, P., Al-Bakri, D., and Al-Ghadban, A. M. (1986). “Total organic carbon distribution in the Kuwait marine bottom sediments,” in Marine Environment and Pollution, Proceedings of the First Arabian Gulf Conference on Environment and Pollution, eds R. Halwagy, D. Clayton, and M. Behbehani (Kuwait: faculty of Sciences, Kuwait University), 117–126.
Khan, N. Y. (2007). Multiple stressors and ecosystem-based management in the Gulf. Aquat. Ecosyst. Health Manag. 103, 259–267. doi: 10.1080/14634980701551168
Khan, N. Y., Munawar, M., and Price, A. R. G. (2002). “Environmental trends and integrated management of the Gulf,” in The Gulf Ecosystem, Health and Sustainability, eds N. Y. Khan, M. Munawar, and A. R. G. Price (Leiden), 483–494.
Kingston, P. F. (2002). Long-term environmental impact of oil spills. Spill Sci. Technol. 7, 53–61. doi: 10.1016/S1353-2561(02)00051-8
Lecklin, T., Ryoma, R., and Kuikka, S. (2011). A Bayesian network for analyzing biological acute and long–term impacts of an oil spill in the Gulf of Finland. Mar. Poll. Bull. 62, 2822–2835. doi: 10.1016/j.marpolbul.2011.08.045
LeCroy, S. E. (2000). An Illustrated Identification Guide to the Nearshore Marine and Estuarine Gammaridean Amphipoda of Florida. Florida Department of Environmental Protection, Tallahassee, Annual Report.
Literathy, P., Khan, N., and Linden, O. (2002). “Oil and petroleum industry,” in The Gulf Ecosystem: Health and Sustainability, eds N. Khan, M Munawar, and A. Price (Leiden: Backhuys Publishers), 127–156.
Loughland, R. A., Al-Abdulkader, K. A., Wyllie, A., and Burwell, B. O. (2012). “Anthropogenic induced geomorphological change along the western Arabian Gulf coast,” in Studies on Environmental and Applied Geomorphology, ed T. Piacentini (Croatia: Tech Europe), 191–218.
Massoud, M. S., Al-Abdali, F., Al-Ghadban, A. N., and Al-Sarawi, M. (1996). Bottom sediments of the Arabian Gulf—II. TPH and TOC contents as indicators of oil pollution and implications for the effect and fate of the Kuwait oil slick. Environ. Pollut. 93, 271–284. doi: 10.1016/S0269-7491(96)00042-5
McCain, J. C. (1984a). Marine ecology of Saudi Arabia: The intertidal infauna of the sand beaches in the northern area, Arabian Gulf, Saudi Arabia. Fauna Saudi Arabia 6, 53–78.
McCain, J. C. (1984b). Marine Ecology of Saudi Arabia: The near shore soft-bottom benthic communities of the Northern Area, Arabian Gulf, Saudi Arabia. Fauna Saudi Arabia 6, 79–101.
McCain, J. C. (1993). Illustrated Keys to the Flora and Fauna of the Arabian Gulf, Prepared for Arabian American Oil Company. Dhahran.
McGlade, J. M., and Price, A. R. G. (1993). Multidisciplinary modelling: an overview and practical implications for the governance of the Gulf region. Mar. Poll. Bull. 27, 361–375. doi: 10.1016/0025-326X(93)90044-K
Michel, J., Hayes, M. O., Keenan, R. S., Sauer, T. C., Jensen, J. R., and Narumalani, S. (1993). Contamination of nearshore subtidal sediments of Saudi Arabia from the Gulf war oil spill. Mar. Poll. Bull. 27, 109–116. doi: 10.1016/0025-326X(93)90015-C
Munawar, M., Price, A. R. G., Munawar, I. F., Carou, S., Niblock, H., and Lorimer, J. (2002). “Aquatic ecosystem health of the Arabian Gulf: status and research needs,” in The Gulf Ecosystem, Health and Sustainability, eds N. Y. Khan, M. Munawar, and A. R. G. Price(Leiden: Backhuys Pub), 303–326.
Muxika, I., Borja, Á., and Bald, J. (2007). Using historical data, expert judgement and multivariate analysis in assessing reference conditions and benthic ecological status, according to the European Water Framework Directive. Mar. Poll. Bull. 55, 16–29. doi: 10.1016/j.marpolbul.2006.05.025
Muxika, I., Borja, A., and Bonne, W. (2005). The suitability of the marine biotic index (AMBI) to new impact sources along European coasts. Ecol. Indic. 5, 19–31. doi: 10.1016/j.ecolind.2004.08.004
Paganelli, D., Forni, G., Marchini, A., Mazziotti, C., and Occhipinti-Ambrogi, A. (2011). Critical appraisal on the identification of reference conditions for the evaluation of ecological quality status along the Emilia-Romagna coast (Italy) using MAMBI. Mar. Poll. Bull. 62, 1725–1735. doi: 10.1016/j.marpolbul.2011.05.027
Pearson, T. H., and Rosenberg, R. (1978). Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. Annu. Rev. 16, 229–311.
Peterson, C. H., Rice, S. D., Short, J. W., Esler, D., Bodkin, J. L., Ballachey, B. E., et al. (2003). Long-term ecosystem response to the Exxon Valdez oil spill. Science 302, 2082–2086. doi: 10.1126/science.1084282
Price, A. R. G. (1998). Impact of the 1991 Gulf war on the coastal environment and ecosystems: current status and future prospects. Environ. Int. 24, 91–96. doi: 10.1016/S0160-4120(97)00124-4
Price, A. R. G., Downing, N., Flower, S. W., Hardy, J. T., Le Tissier, M., Mathews, C. P., et al. (1994). The 1991 Gulf War: Environmental Assessments of IUCN and Collaborators. IUCN Marine Conservation and Development Report. Gland: International Union for Conservation of Nature and Natural Resources.
Price, A. R. G., Wrathall, T. J., Medley, P. A. H., and Al–Moamen, A. H. (1993). Broadscale changes in coastal ecosystems of the western Arabian Gulf following the 1990–91 Gulf War. Mar. Poll. Bull. 27, 143–147. doi: 10.1016/0025-326X(93)90018-F
Readman, J. W., Bartocci, J., Tolosa, I., Fowler, S. W., Oregioni, B., and Abdulraheem, M. Y. (1996). Recovery of the coastal marine environment in the Gulf following the 1991 war-related oil spills. Mar. Poll. Bull. 32, 493–498. doi: 10.1016/0025-326X(95)00227-E
Reddy, C. M., Eglinton, T. I., Hounsel, A., White, H. K., Xu, L., Gaines, R. B., et al. (2002). The West Falmouth Oil Spill after thirty years: The persistence of petroleum hydrocarbons in marsh sediments. Environ. Sci. Technol. 36, 4754–4760. doi: 10.1021/es020656n
Reynolds, R. M. (1993). Physical oceanography of the Gulf, Strait of Hormuz, and the Gulf of Oman—Results from the Mt Mitchell Expedition. Mar. Poll. Bull. 27, 35–59. doi: 10.1016/0025-326X(93)90007-7
Richmond, M. D. (1994). Ecological status of the marine subtidal habitats and the effects of the 1991 Gulf War oil spill, with special reference to soft-substrata communities. Cour. Forsch. Inst. Senc. Ken. 116, 50–60.
Ruffo, S. (1982). Amphipoda of the Mediterranean, Part 1: Gammaridea (Acanthonotozomatidae to Gammaridae). Memories de institute Oceanographique.
Ruffo, S. (1989). Amphipoda of the Mediterranean, Part 2: Gammaridea (Haustoriidae to Lysianassidae). Memories de institute Oceanographique.
Ruffo, S. (1993). Amphipoda of the Mediterranean, Part 3: Gammaridea (Melphidippidae to Talitridae) Ingolfiellidae, Caprellidae). Memories de institute Oceanographique.
Sadiq, M., and McCain, J. C. (1993). The Gulf War Aftermath, an Environmental Tragedy. Dordrecht: Kluwer Academic Publishers.
Sauer, T. C., Brown, J. S., Bjoem, P. D., Aurand, D. V., Michel, J., and Hayes, O. M. (1993). Hydrocarbon source identification and weathering characterization of intertidal and subtidal sediments along Saudi Arabian Coast after the Gulf war Oil Spill. Mar. Poll. Bull. 27, 117–134. doi: 10.1016/0025-326X(93)90016-D
Sell, D., Conway, L., Clark, T., Picken, G. B., Baker, J. M., Dunnet, G. M., et al. and Clark, R.B. (1995). “Scientific criteria to optimise oil spill clean-up,” in Proceedings of the 1995 Oil Spill Conference (Washington, DC: American Petroleum Institute), 95–611.
Sheppard, C., Al-Husiani, M., Al-Jamali, F., Al-Yamani, F., Baldwin, R., Bishop, J., et al. (2010). The Gulf: A young sea in decline. Mar. Poll. Bull. 60, 13–38. doi: 10.1016/j.marpolbul.2009.10.017
Sheppard, C., Price, A. R. G., and Roberts, C. (1992). Marine Ecology of the Arabian Region: Patterns and Processes in Extreme Tropical Environments. London: Academic Press.
Sheppard, C. R. C. (1993). Physical environment of the Gulf relevant to marine pollution: an overview. Mar. Poll. Bull. 27, 3–8. doi: 10.1016/0025-326X(93)90003-3
Sheppard, F. P. (1954). Nomenclature based on sand-silt-clay ratios. J. Sediment. Petrol. 24, 151–158.
Short, J. W., Lindeberg, M. R., Harris, P. M., Maselko, J. M., Pela, J. J., and Rice, S. D. (2004). Estimate of oil persisting on the beaches of Prince William Sound 12 years after the Exxon Valdez oil spill. Environ. Sci. Technol. 38, 19–25. doi: 10.1021/es0348694
Sigovini, M., Keppel, E., and Tagliapietra, D. (2013). M-AMBI revisited: looking inside a widely-used benthic index. Hydrobiologia 717, 41–50. doi: 10.1007/s10750-013-1565-y
Southward, A. J., White, B., Vader, S. L., Gray, J. S., and Crisp, D. J. (1982). An ecologist's view of the implications of the observed physiological and biochemical effects of petroleum compounds on marine organisms and ecosystems. Philos. Transact. R. Soc. Lond. Ser. B Biol. Sci. 297, 241–255. doi: 10.1098/rstb.1982.0040
Spagnolo, A., Punzo, E., Santelli, A., Scarcella, G., Strafella, P., Grati, F., et al. (2014). Offshore platforms: comparison of five benthic indicators for assessing the macrozoobenthic stress levels. Mar. Poll. Bull. 82, 55–65. doi: 10.1016/j.marpolbul.2014.03.023
Tawfiq, N. I., and Olsen, D. A. (1993). Saudi Arabia's Response to the 1991 Gulf Oil Spill. Mar. Poll. Bull. 27, 333–345. doi: 10.1016/0025-326X(93)90041-H
Wagner, C. W., and van der Togt, C. (1973). “Holocene sediment types and their distribution on the southern Persian Gulf,” in The Persian Gulf, ed B. H. Purser (New York, NY: Springer-Verlag), 123–156.
Wake, H. (2005). Oil refineries: a review of their ecological impacts on the aquatic environment. Estuar. Coast. Shelf Sci. 62, 131–140. doi: 10.1016/j.ecss.2004.08.013
Wattayakorn, G., and Rungsupa, S. (2011). Petroleum hydrocarbon residues in the marine environment of Koh Sichang-Sriracha, Thailand. Coast. Mar. Sci. 35, 1–7.
Zainal, K. (2009). The Cumulative Impacts of Reclamation and Dredging Activities. Report for ROPME, Kuwait.
Keywords: oil spills, environmental assessment, sediment analysis, benthos, AMBI, M-AMBI, Saudi Arabia
Citation: Joydas TV, Qurban MA, Borja A, Krishnakumar PK and Al-Suwailem A (2017) Macrobenthic Community Structure in the Northwestern Arabian Gulf, Twelve Years after the 1991 Oil Spill. Front. Mar. Sci. 4:248. doi: 10.3389/fmars.2017.00248
Received: 31 March 2017; Accepted: 19 July 2017;
Published: 03 August 2017.
Edited by:
Alberto Basset, University of Salento, ItalyReviewed by:
José Lino Vieira De Oliveira Costa, Universidade de Lisboa, PortugalAkkur Vasudevan Raman, Andhra University, India
Copyright © 2017 Joydas, Qurban, Borja, Krishnakumar and Al-Suwailem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thadickal V. Joydas, dHZqb3lkYXNAa2Z1cG0uZWR1LnNh
 Thadickal V. Joydas
Thadickal V. Joydas Mohammad A. Qurban1,2
Mohammad A. Qurban1,2 Angel Borja
Angel Borja Periyadan K. Krishnakumar
Periyadan K. Krishnakumar Abdulaziz Al-Suwailem
Abdulaziz Al-Suwailem