- 1Laboratory of Biochemistry, Scientific Affairs Department, APIVITA S.A., Athens, Greece
- 2Laboratory of Molecular Biology, Department of Biotechnology, School of Food, Biotechnology and Development, Agricultural University of Athens, Athens, Greece
- 3Fitoplanton Marino, Cádiz, Spain
- 4Laboratory of Enzyme Technology, Department of Biotechnology, School of Food, Biotechnology and Development, Agricultural University of Athens, Athens, Greece
Nowadays, there is huge interest in natural products obtained from marine organisms that can promote a state of health and well-being for humans. Microalgae represent a primary source of bioactive compounds that could be used as functional ingredients in cosmetic formulations. The aim of the present study is to evaluate, for the first time, the effects of Nannochloropsis gaditana extract against oxidative stress in human primary fibroblasts so as to investigate the potential applications of it in cosmetics. To gain an insight into the molecular mechanisms of N. gaditana bioactivity, we developed a new RT-qPCR platform for studying transcript accumulation for an array of selected genes (up to 100) involved in many skin-related processes including anti-aging, hydration, oxidative stress response, and DNA damage. For the oxidative stress evaluation, H2O2 was used as a stressor. The study of the transcript accumulation of genes revealed that N. gaditana extract exhibits skin protection properties by mediating oxidative responses and apoptosis (including SOD1, GPX1, BID), positively regulates genes involves in skin texture and hydration (including AQP3, Col6A1, FBN1) and modulates the expression of genes involved in skin irritation, DNA damage and aging (including IL1R, PCNA, FOXO3). These findings indicate that the specific N. gaditana extract possesses significant in vitro skin protection activity against induced oxidative stress, and provide new insights into the beneficial role of microalgae bioactive compounds in cosmetic formulations protecting skin from oxidative stress.
Introduction
Inside the cell, reactive oxygen species (ROS) are apparent in minimal concentration either as aerobic metabolism products or as transduction pathways second messengers. Under normal state, there is a balance between antioxidants and pro-oxidant molecules, necessary to enhance an optimal efficiency of antioxidant defense activity (Calabrese et al., 2008). On the other hand, oxidative stress can damage the cell if free radical generation overtakes antioxidant defenses cell system (Trouba et al., 2002; Bickers and Athar, 2006; Karihtala and Soini, 2007; Mancuso et al., 2007; Valko et al., 2007). In spite of the damaging effect of ROS, cells have developed a defense antioxidant system for prevention of this detrimental activity. This system pertains enzymatic mechanisms such as superoxide dismutase (SOD) (Brawn and Fridovich, 1980; Brenneisen et al., 1997), catalase, glutathione peroxide (GSHPx) (Flohe, 2010) as well as DNA repair genes (Friedberg, 1985) and secondly, antioxidants such as α-tocopherol, carotenoids preventing lipid peroxidation (Diplock, 1994). Skin, the main target for ROS-induced tissue damage (Calabrese et al., 2008), is exposed to various oxidant environmental pollutants, catalyzing directly or indirectly the formation of ROS. (Bickers and Athar, 2006). ROS are able to directly generate damage in DNA, lipid and protein status resulting in an alteration of transcriptional regulation of genes in dermal cells. Additionally, these changes affect the phenotype of the skin causing skin aging (Callaghan and Wilhelm, 2008). Specifically, skin fibroblasts are diminished through apoptosis induction and their regenerative capacity is reducing resulting in ROS - increased skin sagging. Skin wrinkles are related to the amount of collagen, elastin, and hyaluronic acid (Baumann, 2007). On the other hand, fibroblasts are the main players in the production of collagen, elastin, hyaluronic acid in the skin, providing structural support for cell adhesion and migration as well as regulating cellular growth and survival (Beacham et al., 2007).
Recently, there has been huge interest in natural products obtained from marine organisms that can promote a state of health and well-being for humans. Some algae and particular microalgae are a primary source of bioactive compounds and could be used as functional ingredients such as; carotenoids, phycobilins, fatty acids, polysaccharides, peptides, vitamins, and sterols (Raposo et al., 2013). Marine microalgae are easily produced, needing only a simple culture medium with seawater, a source of nitrogen, phosphate, iron, magnesium, and some minor salts. Moreover, microalgae can be described as the largest remaining reservoir of secondary metabolites to evaluate for future therapeutic needs. However, of the approximately 30,000 microalgal species that it are believed to exist, only a few thousand strains are kept in collections around the world, only a few hundred have been chemically explored, and yet fewer have been produced at industrial scale (Gouveia et al., 2006; Macedo et al., 2009). In addition, some extracts of marine microalgae have been shown to have inhibitory effects on the activation of hyaluronidase and suggested to accumulate anti-allergic and anti-inflammatory substances revealing a potential application in cosmetics (Fujitani et al., 2001). It is reported that diatoms and Chlorella stigmatophora extracts have antimicrobial properties (Findlay and Patil, 1984). Rasmussen and Morrissey (2007) presented an overview on the food ingredients produced by marine organisms, highlighting microalgae and/or their bioactive ingredients. In addition, Sansone et al. (2017) recently presented that an extract obtained from the green microalga Tetraselmis suecica had a strong antioxidant and repairing activity in a human cancer cell line. On the other hand, bioactive molecules for cosmetic products are characterized by their capacity to stimulate beneficial properties or to interfere with pathways leading to skin damage (Krutmann, 2001; Yarosh et al., 2006).
Since several lines of research are pointing toward the protective properties of microalgae derived extracts and biomolecules against various environmental biotic and abiotic stresses, including oxidative stress, we evaluated the mass-produced Nannochloropsis gaditana microalgae species as a source for potential applications in cosmetics. N. gaditana belongs to the Eustigmataceae marine microalgae, first described by Hibberd (1981). They are mixotrophic, non-flagellated algae belonging to the class Eustigmatophyceae, which includes unicellular and small (<5 μm) coccoid organisms that lack chlorophyll b (Hoek et al., 1995). Several species belonging to the Nannochloropsis genus are source of eicosapentaenoic acid (EPA), which is of nutritional importance. Nannochloroposis spp. are cultivated for EPA production so as to be used in aquaculture as nutritional ingredient and in pharmaceutical sector as a bioactive ingredient for the prevention of various human diseases (Wang and Chai, 1994; Srinivas and Ochs, 2012). Concerning applications in cosmetology, only Nannochloropsis oculata extracts have been commercially used, since they were proven to have skin-tightening properties (Susan et al., 2012).
NannoG was assessed as a novel cosmeceutical ingredient in this work. A battery of assays on cultured skin cells, analyzing its biological activities of dermatological interests, particularly anti-aging, hydration and antioxidant function was performed. To understand the molecular mechanism of NannoG bioactivity, we employed a newly designed RT-qPCR platform for studying transcript accumulation for an array of selected genes involved in many skin-related processes including anti-aging, hydration and oxidative stress response.
Materials and Methods
N. gaditana Biomass Production and Extract Preparation
Microalgae Cultures
Nannochloropsis gaditana was obtained from the Microalgae Culture Collection of Fitoplancton Marino, S.L. (CCFM). Starter culture for the photobioreactors was grown indoors in 50 mL flask with F/2 culture medium (Guillard and Ryther, 1962) in natural seawater at a salinity of 33 psu and air bubbled with an addition of 2% CO2. All media were prepared fresh from respective dry chemicals. A 24 h light photoperiod was provided by artificial daylight illumination (150 μE m−2 s−1) at 22°C in a thermoregulated growth room. Once the culture reached the stationary phase it was used to inoculate 1 L culture flask. This process of scaling up in volume was repeated until 50 L culture was reached.
Outdoor Culture Systems
N. gaditana was cultured in Fitoplancton Marino's industrial 2,000 L outdoor horizontal tubular photobioreactors routinely used for production. Both temperature and pH were automatically controlled with probes, and kept around 30°C and 7.5°C, respectively. In particular, pH was regulated by addition of CO2. The system was operated as a fed-batch mode in order to prevent nutrient limitation. Growth rate was monitored by cell counting under microscope. Required biomass for further extract preparation was harvested when the culture reached the mid-log phase, with the culture volume to be removed being calculated from cell density data.
Extract Preparation and Chemical Composition
The harvested biomass with 10% dry weight was homogenized, and disrupted cells were macerated for 2 weeks in propylene glycol. Thereafter the macerate was centrifuged at 15,000 g for 1 h at 4°C to eliminate all the cell debris and to obtain a cell free extract, which was then passed through a 0.2 μm filter for further use in in vitro assays. Total proteins were measured by Kjeldahl method, total lipids were measured with Sohxlet method, all vitamins were measured using a HPLC-UV method, total carbohydrates (Tontisirin, 2003), total flavonoids (Chang et al., 2002), total phenols as well as total carotenoids were determined spectrophotometrically.
Human Skin Cell Culture
Primary Normal Human Dermal Fibroblasts (NHDF) isolated from normal human adult skin were purchased from Lonza CloneticsTM (Lonza Walkersville, USA) (Giampieri et al., 2014). Cells were cultured according to Lonza instructions. The cells were grown in a recommended media FGM™-2 BulletKit™ that contained 2% serum. Cells were sub-cultured once theyhad reached 70–80% confluence.
Measurement of Intracellular ATP Levels
ATP determination was based on ViaLight HS BioAssay kit (Lonza). GloMax 20/20 single-tube luminometer (Promega) was used.
H2O2 Treatment
Cells were incubated for 48 h with NannoG. After incubation and prior to H2O2 induction, the cells were washed two times with PBS, to prevent direct extracellular interactions between the extract compounds and H2O2. Subsequently, culture medium containing 0.5 mM H2O2 was added to the cells for 3 h. Then the cells were washed two times with PBS and culture medium was added for 3 h. Finally, after cell lysis, ATP levels were measured.
Transcript Analysis Using Real-Time RT-qPCR
RNA Isolation and cDNA Synthesis
Total RNA was isolated from NHDF using Animal Tissue RNA Isolation kit [Norgen BIOTEK Corporation (ON, Canada)] according to manufacturer's instructions. Eluted RNA (1 μl) was run on a 1% agarose gel to check for degradation, and RNA quantity was determined spectrophotometrically at 260 nm. The RNA concentration and quality has determined by Nannodrop and agarose gel electrophoresis. cDNA was synthesized from 0.8 μg total RNA by reverse transcription according to Super-Script II (Invitrogen, Paisley, United Kingdom) protocol, using Oligo(dT) primers. The reaction mixture was incubated at 42°C for 50 min, followed by heat inactivation at 70°C for 15 min.
RT-qPCR Platform Design and Analysis
In order to develop a tool for the targeted transcriptomic analysis of target human genes, we designed a new RT-qPCR based platform containing primer sets specific for a carefully selected list of human genes involved in major molecular and physiological skin processes (Supplementary Tables 2, 3). RT-qPCR transcript profiling was selected as the method of choice as it represents a highly sensitive, accurate and reproducible technique, ideal for small to medium scale targeted transcriptomic analysis. Target cDNAs were amplified using gene specific primers, designed from the transcribed region of each gene using Primer Express 1.5 software (Applied Biosystems, Darmstadt, Germany). Quantitative RT-PCR reactions were performed on the Stratagene MX3005P using iTaq™ fast SYBR™ Green supermix with ROX (BioRad Laboratories, Hercules, CA), gene specific primers at a final concentration of 0.2 μM each and 1 μl of the cDNA as template. Detailed information for all the genes in this study, including names, gene symbols, accession numbers, predicted topology and gene specific primers, are presented in Supplementary Material. RT-qPCR cycling started at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The primer specificity and the formation of primer-dimers were monitored by dissociation curve analysis and agarose gel electrophoresis on a 4% (w/v) gel. Six different housekeeping genes were assessed as internal standards. These genes were: actin beta (ACTB), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), glucuronidase beta (GUSB), TATA box binding protein (TBP), succinate dehydrogenase complex (SDHA), and hypoxanthine phosphoribosyltransferase-1 (HPRT1). However, actin beta (ACTB) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were selected as normalization control, as it was confirmed that their regulation was not altered under different biological treatments (Supplementary Figure 1). For the relative quantification of gene expression, a modification of the comparative threshold cycle (Ct) method was used. The ratio of the target gene transcripts (X) in the sample of interest (S), normalized to actin beta (ACTB), and the target gene transcripts in different treatments (R), was calculated as (1+E) − ΔΔCt, where ΔΔCt was calculated as [(CtX.S − CtACTB.S) − (CtX.R − CtACTB.R)]. PCR efficiency (E) for each amplicon was calculated employing the linear regression method on the Log(Fluorescence) per cycle number data, using the LinRegPCR software (Ramakers et al., 2003). All RT-qPCR were performed on three biological repeats.
Enzymatic Assays
Total Antioxidant Capacity Assays
Ferric Reducing Antioxidant Power (FRAP) assays were performed according to Benzie and Strain (1996). FRAP value was expressed as μM ascorbic acid per mg of dry weight of extract. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) assays were adopted from published methods (Pellegrini et al., 2003; Li et al., 2011). ABTS radical scavenging activity was defined as IC50 value (the concentration of extract causing 50% inhibition of absorbance).
Enzyme Inhibition Assays
Elastase inhibitory activity was measured by monitoring the release of p-nitroaniline spectrophotometrically at 410 nm, using succinyl-Ala-Ala-Pro-p-nitroanilide as substrate, at pH 8 at 25°C. (Bieth et al., 1974). The unit of elastase is defined as the amound of enzyme which hydrolyze 1.0 μmole of succinyl-Ala-Ala-Pro-p-nitroanilide per minute at pH 8.0 at 25°C. In addition, tyrosinase inhibitory activity was measured as previously described (Duckworth and Coleman, 1970). Dopachrome concentration was measured at 475 nm. The relative elastase or tyrosinase inhibition (% I) was measured using the following equation:
where Ro is the rate of absorbance increase for the uninhibited reaction and Ri is the rate of increase for the inhibited reaction. Both Ri and Ro correspond to the same substrate concentration. IC50 denotes the concentration of sample (mg/mL) required to inhibit 50% of enzyme activity. IC50 were estimated by non-linear regression analysis employing the program GraFit (Leatherbarrow, 1998). All determinations and assays were carried out in triplicate and at least three replicates of each experiment were performed. The results have been expressed as the mean values ± SD.
Reconstructed Human Skin Model
A cosmetic formulation of NannoG was evaluated concerning skin irritation on the reconstructed human epidermal model, EpiDerm EPI-200 (MatTek Corporation (Ashland, MA, USA). The model consists of normal, human-derived epidermal keratinocytes, which have been cultured to form a multi-layered, highly differentiated model of the human epidermis. The tissue exhibits organized basal, spinous and granular layers, and a multi-layered stratum corneum containing intercellular lamellar lipid layers arranged in patterns analogous to those found in vivo (Cannon et al., 1994). Because it closely mimics the human epidermis, it has proven its scientific relevancy in studies where dermal exposure and toxicity is anticipated: skin corrosion testing of chemicals, skin irritation studies of cosmetic products and raw materials, phototoxicity, and skin penetration studies (Liebsch et al., 1997, 2000; Faller et al., 2002; Schäfer-Korting et al., 2008). Cell viability was measured by the MTT reduction assay and MTT Effective Time 50 (ET50) was determined after topical application of the products for different exposure times (2, 5, and 18 h), according to manufacturer's instructions. As negative and positive control for irritation, Dulbecco's phosphate-buffered saline (DPBS) and 5% Sodium Dodecyl Sulfate (SDS) were used, respectively.
Statistical Analysis
Statistical analysis of the transcriptomic data was based on ANOVA (one-way analysis of variance) at a 95% level of significance. T-test (95% significant level) was used for comparison assessments. Principal components analysis (PCA) was performed using Multibase 2015 software. The above analysis was based on SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Antioxidant Capacity and Enzyme Inhibition Analysis of the NannoG Cell-Free Extract
To address the potential application of the NannoG as a novel cosmeceutical ingredient, we determined its basic chemical composition (Supplementary Table 1), its total antioxidant capacity (TAC), and its inhibitory potential against human elastase and tyrosinase. Both enzymes important in skin physiological processes. The measure of TAC considers the cumulative action of all the antioxidants present in a particular extract (both small molecule antioxidants and proteins), thus providing an integrated parameter rather than the simple sum of measurable antioxidants. In the present work, the TAC of NannoG was assessed using the 2,2-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) and FRAP assays. The activity of crude NannoG against ABTS radical scavenging activity, in terms of the mean inhibition concentration (IC50), was 0.336 mg extract compared to Botryococcus braunii and Nannochloropsis oculata aqueous extracts display ABTS radical scavenging activity with IC50 4.60 ± 0.04 and 4.05 ± 0.18 mg/mL, respectively (Custódio et al., 2015). The FRAP value, expressed as μM ascorbic acid per mg of dry weight was determined as 2.76 μM/mg of dry weight. NannoG also exhibited significant concentration-dependent inhibition activity against human elastase and tyrosinase (Figure 1). Based on these results, the IC50 values were 0.23 ± 0.02 and 2.69 ± 0.13 mg against elastase and tyrosinase, respectively.
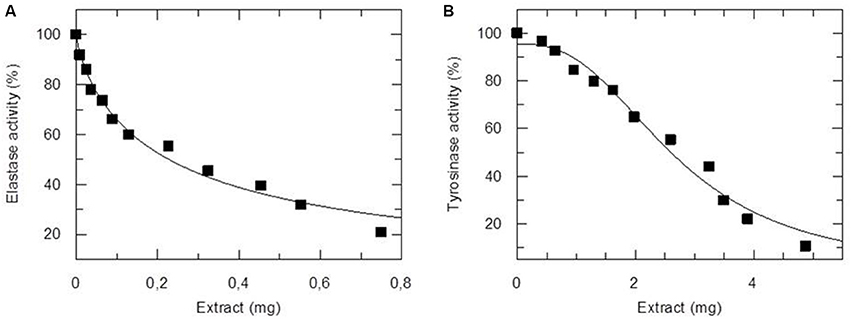
Figure 1. Inhibitory activity of NannoG on elastase (A) and tyrosinase (B) activity. The amount of extract used in enzyme assay was expressed as mg of dry weight. The IC50 values were 0.23 ± 0.02 and 2.69 ± 0.13 mg against elastase and tyrosinase, respectively.
NannoG Increases Fibroblasts Viability In vitro
Primary human dermal fibroblasts (NHDF) were used in vitro to assess the effect of NannoG on cell viability. ATP has been used as a tool for determining the functional integrity of living cells since all cells require ATP to remain alive and carry out their specialized functions. Fibroblasts incubated in culture medium in the presence of two concentrations of NannoG (408, 870 μg/ml) exhibited significantly increased ATP levels compared to control cells and cells treated only with the propylene glycol solvent (Figure 2A). Since oxidative stress is known to promote fibroblast cell death, representing one of the main factors for skin aging, the effect of NannoG on fibroblasts viability was also evaluated under oxidative stress conditions. Thus, cells treated with NannoG (408 μg/ml) for 48 h were subjected for 3 h to oxidative stress induced by the addition of 0.5 mM H2O2 in the culture medium. ATP assay showed that H2O2 induced-oxidative stress reduced cell viability of control fibroblasts up to 30%. Interestingly, the presence of NannoG suppressed the effect of H2O2 resulting in a significantly increased cell viability under oxidative stress (Figure 2B).
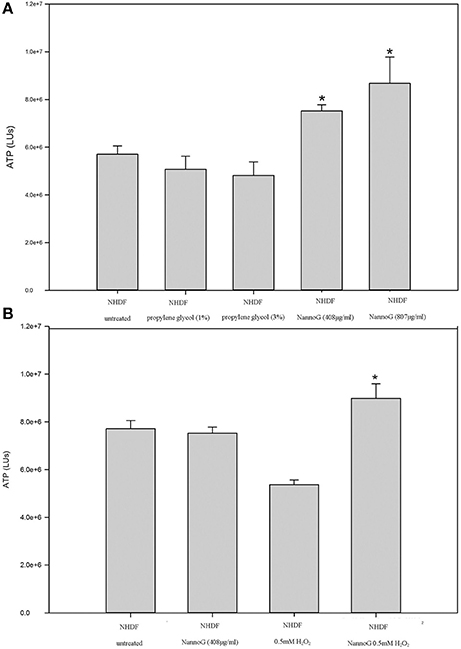
Figure 2. (A) Relative ATP levels (LUs) of cells after 48 h incubation with two concentrations of propylene glycol (1, 3%) and two concentrations of NannoG (408, 870 μg/ml). Data are expressed as mean ± SEM for 8 replicates. *p < 0.05 significantly different from the untreated cells. (B) Relative ATP levels (LUs) for untreated cells, cells treated with H2O2, cells treated with NannoG and cells treated first with NannoG and then with H2O2. Data are expressed as mean ± SEM for 8 replicates. *p < 0.05 significantly different from the control cells and cells treated with 0.5 mM H2O2.
NannoG Affects the Expression of Skin-Related Genes under Oxidative Stress
To have an insight into the molecular mechanism of the observed NannoG bioactivity under oxidative stress, we measured transcript accumulation for an array of selected genes involved in many skin-related processes. A detailed list of the target genes included in the present study and their respective relative expression levels is presented in Supplementary Table 1. Gene transcripts were measured by quantitative real time RT-PCR (RT-qPCR) using a newly developed platform containing the respective gene-specific primer pairs (Supplementary Table 3). Most genes represented in the qRT-PCR platform code for proteins involved in primary metabolic processes in human skin such as hydration, inflammation, apoptosis, aging, angiogenesis, DNA damage, DNA repair, cell cycle regulation, cell proliferation, and immune response.
Principal component analysis (PCA) revealed a fair differentiation in gene expression among the four experimental conditions used: control cells, cells treated with NannoG, cells treated with H2O2, and NannoG pre-treated cells subjected to H2O2-induced oxidative stress. Specifically, the PCA model based on transcript accumulation of skin related genes explained the 69.7% of the variation of the system allowing a clear separation of the four experimental conditions (Figure 3A). The first component accounted for 42.5% of the variance and allowed distinction of the oxidative stress and no oxidative stress conditions while the second component, which accounted for 27.2% of the variance, allowed distinction of NannoG treated and control cells (Figure 3B). Specific gene transcript levels showing significant changes under our experimental conditions are presented and discussed in detail below.
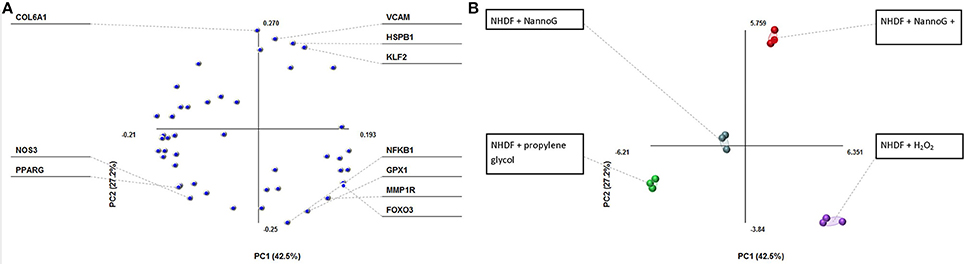
Figure 3. Principal component analysis (PCA) of transcript of genes into four experimental conditions: cells treated with NannoG, cells treated with propylene glycol (control), cells treated with H2O2 and cells treated with NannoG and H2O2. (A) Score plots of PCA, PC1:42.5% and PC2:27.2% based on four experimental conditions: cells treated with NannoG (light blue dots), control cells (green dots), cells treated with H2O2 (purple dots) and cells treated with NannoG first and then with H2O2 (red dots). (B) Loadings of individual identified components showing the highest variability under the studied experimental conditions.
Antioxidant Defense Pathway Related Genes
Under our experimental conditions, H2O2-treatment significantly affected the gene transcripts accumulation of glutathione peroxidase-1 (GPX1) and superoxide dismutase 1 (SOD1) leaving the expression of catalase (CAT) and thioredoxin (TXN) genes unaffected. Interestingly, both the addition of NannoG and the H2O2-induced oxidative stress resulted in a 4-fold up-regulation of the GPX1 transcripts, while in the extract pre-treated stressed cells transcript levels were significantly lower than the respective control cells, indicating a significant modulation of the respective antioxidant defense pathways (Figure 4A). In contrast, SOD1 transcripts were found significantly lower in all treatments in comparison to the control cells (Figure 4B).
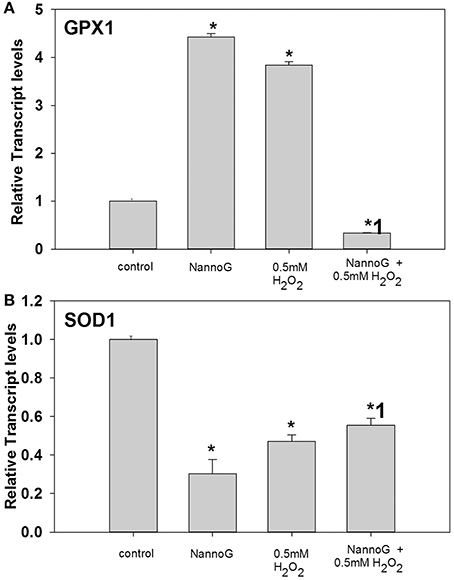
Figure 4. Relative transcript accumulation of glutathione peroxidase-1 (GPX1) (A) and superoxide dismutase-1 (SOD1) (B) genes. The experimental conditions were: control cells, cells treated with H2O2 0.5 mM, cells treated with NannoG (408 μg/ml) and cells treated with NannoG first and then with H2O2 (0.5 mM). *p < 0.05 significantly different from the control, *1 p < 0.05 significantly different from the cell treated with H2O2.
Cell Proliferation-Apoptosis-Angiogenesis Related Genes
The expression of several genes involved in cell proliferation, apoptosis and angiogenesis was studied under our experimental conditions. Transcripts of hepatocytes growth factor (HGF) were found to be more than 2-fold up-regulated in cells treated with NannoG in comparison to the control cells, in contrast to the stressed cells where transcripts were found to be significantly down-regulated (Figure 5A). Interestingly, HGF transcripts were rescored to the control levels in NannoG pretreated stressed cell. Similarly, NannoG could also significantly up-regulate transcripts of heat shock protein B1 (HSPB1) and baculoviral IAP repeat containing 3 (BIRC3), although no significant changes in gene expression were found for HSPB1 upon H2O2-induced stress (Figure 5B). In contrast, BIRC3 was found to be down-regulated upon oxidative stress even after the treatment with the NannoG (Figure 5D). The addition of H2O2 also resulted in a 5-fold reduction in the expression of BH3 interacting domain death agonist (BID), while the pre-treatment with the cell-free extract could at least partially restored its expression (Figure 5C). Finally, endothelial growth factor A (VEGFα) transcripts were found to be 10-fold higher in H2O2 stressed cells, while even higher transcript levels were observed in NannoG pre-treated stressed cells in contrast to unstressed control cells (Figure 5E).
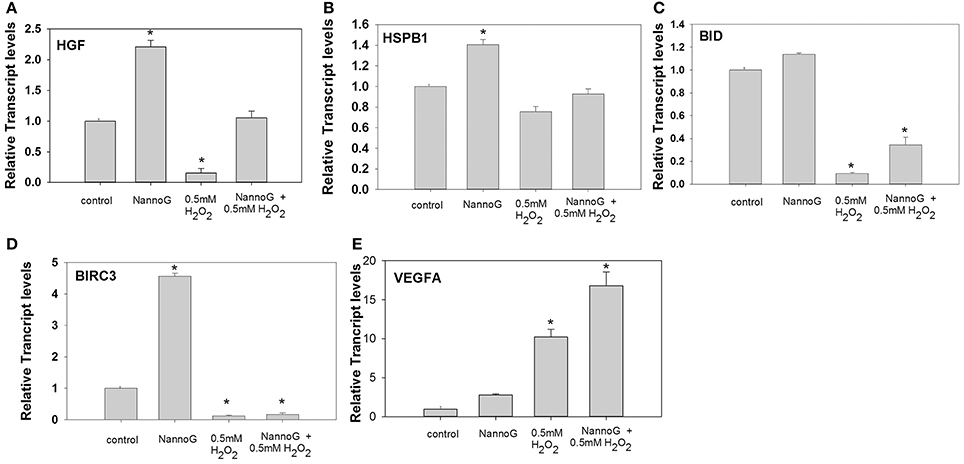
Figure 5. Relative transcript accumulation for: (A) HGF, (B) HSPB1, (C) BID, (D) BIRC3, (E) VEGFα. The experimental conditions are: control cells, cells treated with H2O2 0.5 mM, cells treated with NannoG (408 μg/ml) and cells treated with NannoG first and then with H2O2 (0.5 mM). *p < 0.05 significantly different from the control.
Skin Hydration and Extracellular Matrix Related Genes
To assess whether the NannoG could promote the hydration of skin cells in vitro, we determined the expression of Aquaporine-3 (AQP3) gene. Interestingly, AQP3 transcripts were significantly up-regulated in primary fibroblasts treated with NannoG even after treatment with H2O2 in contrast to control and H2O2-stressed cells (p < 0.05) (Figure 6A). A similar transcript accumulation pattern was observed for Collagen type VI alpha 1 chain (Col6A1), a gene involved in collagen biosynthesis. Transcripts of Col6A1 were found to be up-regulated in all cells pretreated with NannoG in contrast to stressed cells (Figure 6B). Moreover, transcripts for fibrillin-1 (FBN1) were found to be highly up-regulated in cells treated with NannoG in comparison to control cells (p < 0.05) (Figure 6C). Furthermore, we studied the transcript accumulation for collagen breakdown related genes, including metalloprotease-1 receptor (MMP1R) gene transcripts. As expected, MMP1R transcripts were found to be up-regulated in cells stressed with 0.5 mM H2O2 (Figure 6D). Interestingly, although MMP1R transcripts were found to be higher following the NannoG treatment, we observed a drastic down-regulation in H2O2 stressed cells pre-treated with NannoG, suggesting a drastic suppression of collagen breakdown (Figure 6D). Transcripts of cellular retinoic acid binding protein 2 (CRABP2) were found to be more that 4-fold up-regulated in cells treated with NannoG, while oxidative stress completely dismissed the expression of this gene. As observed for other transcripts, the pretreatment of the cells with the extract restored transcripts to their basal control levels (Figure 6E). Another gene showing significant differential regulation under our experimental conditions was prostaglandin E receptor 2 (PTGER) (Figure 6F). These transcripts were found to be highly up-regulated in NannoG treated cells, in contrast to NannoG pretreated stressed cells. Finally, additional extracellular matrix related genes such as serpine mRNA binding protein 1 (SERPINE) and Kruppel-like factor 2 (KLF2) showed significant differential regulation under our experimental conditions (Figures 6G,H). In our in vitro system, SERPINE transcripts were found to be highly induced upon oxidative stress, while the pretreatment with NannoG resulted in the restoration of gene expression to its basal levels. In contrast, H2O2-induced stress even after the NannoG-treatment resulted in strong up-regulation of KLF2 gene expression (Figure 6H).
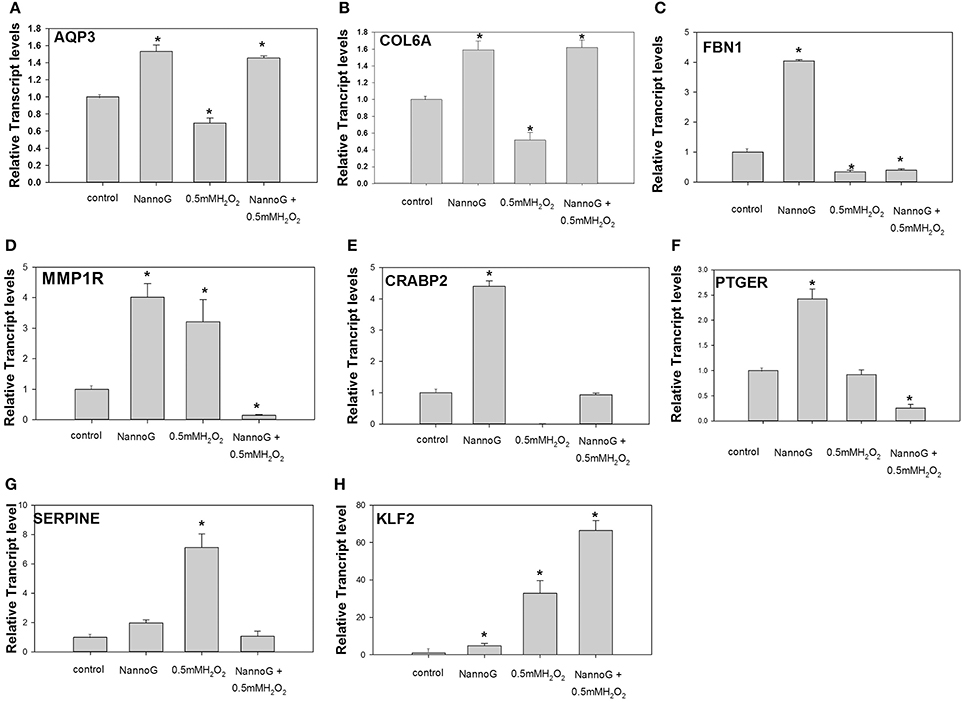
Figure 6. Relative transcript accumulation of: (A) AQP3, (B) COL6A1, (C) FBN1, (D). MMP1R, (E) CRABP2, (F) PTGER2, (G) SERPINE-1, (H) KLF2. The experimental conditions are: control cells, cells treated with H2O2 0.5 mM, cells treated with NannoG (408 μg/ml) and cells treated with NannoG first and then with H2O2 (0.5 mM). *p < 0.05 significantly different from control.
Inflammation and Immune Response Related Genes
Several genes involved in immune response were included in our RT-qPCR platform. Interestingly, among them Interleukin-1 receptor (IL1R) gene showed significant changes in transcript accumulation (Figure 7). NannoG treatment did not alter the expression of IL1R, while the induced oxidative stress resulted in a more than 140-fold induction in transcript levels. Interestingly in cells treated with NannoG prior to the induced H2O2-stress resulted in a significant lower induction of IL1R expression in comparison to the stressed cells (Figure 7).
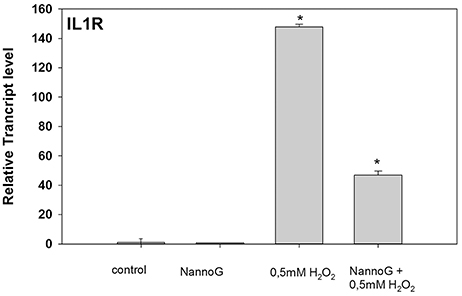
Figure 7. Relative transcript accumulation of IL1R. The experimental conditions are: cells treated with H2O2 0.5 mM, cells treated with NannoG (408 μg/ml), pre-treated cells with NannoG and stressed with H2O2 (0.5 mM). *p < 0.05 significantly different from control.
Cell-Cycle and DNA Repair Related Genes
Among the studied genes involved in cell cycle regulation, the transcript levels of BTG family member-2 (BTG2) were strongly up-regulated in H2O2-stressed cells that were pretreated with NannoG (p < 0.05) (Figure 8A), whereas expression levels in all other treatments were comparable to those found in the control cells. In addition, transcripts of proliferating cell nuclear antigen (PCNA) were found to be more than 8-fold up-regulated upon H2O2-induced oxidative stress and 4-fold higher in NannoG treated control cells, whereas and in this case, gene expression was reverted to the basal control levels when treatment of the cells with the extract preceded the H2O2-induced oxidative stress (Figure 8B).
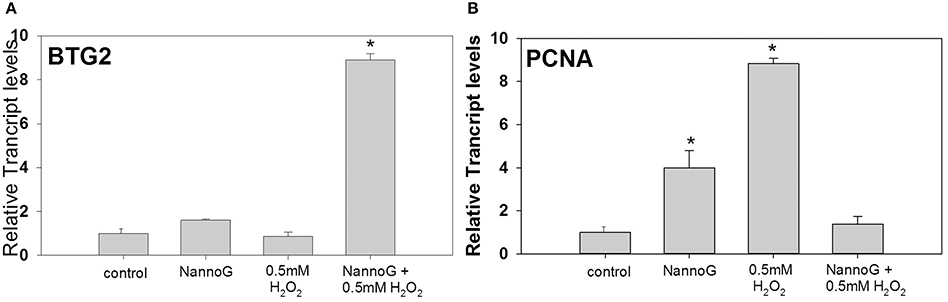
Figure 8. Relative transcript accumulation of BTB2 (A) and PCNA (B) in comparison to control. The experimental conditions are: cells treated with H2O2 0.5 mM, pre-treated cells with NannoG (408 μg/ml), pre-treated cells with NannoG and stressed with H2O2 (0.5 mM). *p < 0.05 significantly different from control.
Aging Related Genes
To evaluate for aging process in NHDF, we assessed the transcripts of the forkhead box O3 (FoxO3) gene (Figure 9). In our study, we observed that transcripts of FoxO3 were highly up-regulated in NannoG treated cells (Figure 9) even after treatment with H2O2, exhibiting 10- and 14-fold higher transcript levels, respectively, in comparison to control cells. Interestingly, H2O2-induced oxidative stress completely abolished the expression of FoxO3.
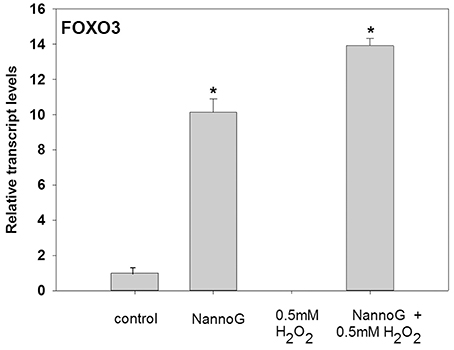
Figure 9. Relative transcript accumulation of FOXO3. The experimental conditions are: cells treated with H2O2 0.5 mM, pre-treated cells with NannoG (408 μg/ml), pre-treated cells with NannoG and stressed with H2O2 (0.5 mM). *p < 0.05 significantly different from all the experimental conditions.
Reconstructed Human Skin Model
Finally, we evaluated skin irritation for both NannoG and cosmetic formulation containing NannoG using an in vitro 3D skin model. Our results clearly demonstrated that NannoG is not irritant for the human skin. In addition, typical cosmetic formulas containing NannoG were proven mild for skin after 16 h of exposure (Supplementary Figures 2, 3).
Discussion
To start with, we evaluated the in vitro effects for N. gaditana extract against oxidative stress in human primary fibroblasts, in an attempt to address its potential applications in cosmetics. According to our results, NannoG exhibited both antioxidant and skin-whitening properties. Natural extracts with the ability to scavenge free radicals are a promising source for novel skin protection, whitening and anti-aging cosmeceuticals (Hamed et al., 2015; Saewan and Jimtaisong, 2015; Wang et al., 2015). In addition, tyrosinase as well as elastase inhibitors are known to be used in various skin related treatments as well as in cosmetics as antiwrinkle and skin-whitening agents (Kim et al., 2012; Kamagaju et al., 2013). On the other hand, elastase enzymatically breaks down elastins fibers, that together with collagen, are responsible for the mechanical properties of connective tissue (Antonicelli et al., 2007). In addition, Imokawa and Ishida (2015) demonstrated that both skin-aging and antiwrinkle effects are significantly correlated with decreased elastase activity. Because of the specific role of elastase in the inflammatory process, its inhibition by NannoG encourages its use in cosmetic products for irritated, reactive and/or senescent epidermis (Henriksen, 2014).
Tyrosinase is the rate-limiting enzyme for controlling the production of melanin, involved in determining the color of mammalian skin and hair (Kim and Uyama, 2005; Kim et al., 2012; Kamagaju et al., 2013). Inhibiting tyrosinase activity has been targeted for the prevention of conditions related to the hyperpigmentation of the skin, such as melasma and age spots (Ando et al., 2007). Our results also demonstrated that NannoG increases the viability of human dermal fibroblasts. Increased ATP intracellular levels in fibroblasts clearly demonstrated that, at least in vitro, NannoG could enhance cell viability, cell proliferation and energy metabolism of dermal fibroblasts even under oxidative stress treatment. According to previous reports, this increase could be associated with higher levels of mitochondrial activity, energy metabolism and cell proliferation (Crouch et al., 1993; Deters et al., 2005) and is indicative of a lack of cytotoxicity (Crouch et al., 1993).
NannoG Exhibits Skin Protection Properties by Mediating Oxidative Responses and Apoptosis
To understand the molecular mechanisms of NannoG bioactivity, we designed a RT-qPCR platform specifically for studying transcript accumulation of key-genes involved in various skin-related mechanisms such as aging, hydration, oxidative stress response, and DNA damage. Under our experimental conditions several skin related processes where found to be transcriptionally modulated by NannoG. Figure 10 summarizes the major results observed under our experimental conditions. Among them, antioxidant defense-related genes including catalase (CAT), superoxide dismutase 1 (SOD1), thioredoxin (TXN), and glutathione peroxidase-1 (GPX1), have been used as molecular markers of oxidative stress. Under our experimental conditions, H2O2-treatment significantly reduced the accumulation of GPX1 and SOD1 gene transcripts leaving the expression of CAT and TXN genes unaffected. Interestingly, the addition of NannoG resulted in a down-regulation of SOD1, while a significant up-regulation of GPX1 was observed. NannoG effect on GPX1 and SOD1 genes expression are in agreement with previous reports, including Malaysian Gelam honey: observed to have oxidative stress-protective properties (Ahmad et al., 2013). GPX1 is one of the most important antioxidant enzymes that lead to the detoxification of H2O2 in the cell (Gurjala et al., 2005). Thus, the strong induction of GPX1 gene expression by NannoG may contribute to the cell-protective effect against H2O2 induced oxidative stress observed in this study.
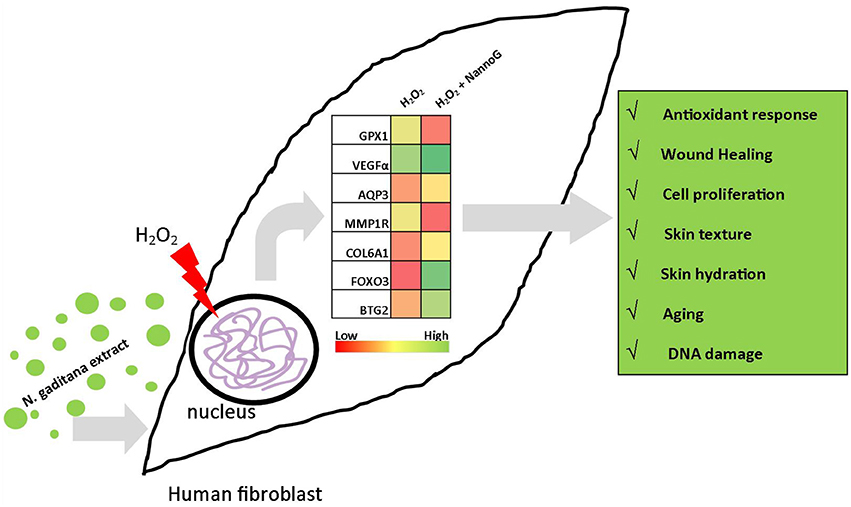
Figure 10. Schematic representation of the major differences observed in gene expression under experimental conditions. Heat charts represents transcript accumulation ratios between H2O2-stressed fibroblasts pretreated with N. gaditana extract to H2O2-stressed control fibroblasts.
We focused on growth factors since they are involved in several cell processes (Zippel et al., 2010), specifically on HGF (Hepatocyte growth factor) which is secreted by mesenchymal cells and acts as a multi-functional cytokine on cells of mainly epithelial origin. Its ability to stimulate mitogenesis, cell motility, and matrix invasion gives it a central role in angiogenesis, tumorogenesis, and tissue regeneration (Nakamura et al., 2011). Hepatocyte growth factor is secreted by mesenchymal cells and acts as a multi-functional cytokine on cells of mainly epithelial origin. Its ability to stimulate mitogenesis, cell motility, and matrix invasion gives it a central role in angiogenesis, tumorogenesis, and tissue regeneration (Nakamura et al., 2011; Tu et al., 2013). In our study, transcripts of HGF where found significantly up-regulated in cells treated with NannoG in comparison to the control cells. Moreover, transcripts of HGF are up-regulated in stressed cells pretreated with NannoG in comparison to stressed control cells. These findings are in line with the increased ATP content of fibroblasts treated with NannoG even under oxidative stress. Interestingly, it has been previously reported that an extract of bitter melon pulp enhanced cell proliferation of human dermal fibroblast through the induction of HGF production mediated by the activation of MAPKs (Ono et al., 2009). In accordance, our results also suggest that the potential beneficial effects of NannoG on dermal fibroblast proliferation under oxidative stress, could at least be partially mediated by a similar mechanism.
As previously reported, ROS induced by oxidative stress reduces the number of skin fibroblasts by inducing apoptosis and decreasing their regenerative capacity (Callaghan and Wilhelm, 2008). Our results on transcript accumulation for several apoptosis-related genes including HSPB1, BID, FAS, and BIRC3 suggest that NannoG could act as a potential anti-apoptosis agent, in agreement with the increased ATP content of NannoG pre-treated fibroblasts both in the absence or presence of oxidative stress. Heat shock proteins (hsp) are known to be produced by fibroblasts in order to protect the surrounding proteins and the cells from necrosis and apoptosis (Takayama et al., 2003). The relevance of HSPB1 to skin regeneration has been thoroughly evaluated. HSPB1 is a constitutive expressed protein, which overexpression protects the cell from apoptosis (Frank et al., 2004; Hirano et al., 2004). In line with these results, transcripts of HSPB1 were found up-regulated in cells treated with NannoG revealing a possible anti-apoptotic response. Among the transcripts under study, BIRC3 encodes a protein that inhibits apoptosis (Smolewski and Robak, 2011). Our results revealed that transcripts of BIRC3 were significantly up-regulated in cells treated with NannoG and down-regulated in H2O2-stressed as well as in H2O2-stressed cells pretreated with NannoG. These results could account for the fact that free radicals upon H2O2 treatment can regulate the expression of the corresponding gene. Although further work is needed in order to fully understand the exact molecular mechanism responsible for the observed gene expression patterns, these are indicative of putative direct anti-apoptotic properties of NannoG that correlate well with the observed enhancement of viability of NannoG pretreated cells that were subsequently stressed with H2O2. Wound-healing is another skin physiological process related to cell proliferation and apoptosis. To evaluate the possible wound-healing properties of NannoG to fibroblasts under oxidative stress we examined the expression of VEGFA. Transcripts of VEGFA were found to be significantly up-regulated in H2O2 stressed cells, while even higher transcript levels were observed in NannoG pre-treated cells in contrast to unstressed control cells. Vascular endothelial growth factor (VEGF) is a angiogenicas well as vasculogenicduring development, indicating that it might also be involved in the regulation of angiogenesis during wound healing (Werner and Grose, 2003; Moens et al., 2011). These results are indicative of a possible positive effect of the NannoG in tissue healing processes, as it is in accordance with previous reports demonstrating that the induction of VEGFA expression correlates with wound healing process (Morgan and Nigam, 2013; Kant et al., 2015).
NannoG Positively Regulated Genes Involves in Skin Texture and Hydration
Our RT-qPCR platform was used also to evaluate the skin texture and hydration effects of NannoG. Aquaporins (AQPs) are integral membrane proteins through which water can flow more rapidly inside the cell than by diffusion through the phospholipid bilayer of the cellular membrane. AQP3 is a transporter of water and glycerol that is mainly expressed in the plasma membrane of keratinocytes of the basal layer of epidermis in normal skin (Fluhr et al., 2008; Hara-Chikuma and Verkman, 2008; Boury-Jamot et al., 2009). Interestingly, transcripts of AQP3 were strongly up-regulated in fibroblasts treated with NannoG even after treatment with H2O2, in contrast to control and H2O2-stressed cells. These results argue for the promotion of skin hydration in NannoG treated cells revealing a highly valuable property of this extract for cosmetology. Collagen production also contributes to skin hydration as well as to skin aging. Collagens are extracellular matrix proteins and have a triple-helical domain as their common structural element, that play a key role in maintaining the integrity of various tissues (Fitzgerald et al., 2013). Transcripts of Col6A1 were found to be up-regulated in all cells pretreated with NannoG in contrast to stressed cells, a response very similar to that observed for AQP3 transcripts. Although there are limited studies focused on the action of Col6A1 in primary fibroblasts (Gruber and Holtz, 2010; Fitzgerald et al., 2013), some evidence supports its wound-healing role as a Col6A1 null mice do not display an overt wound healing process (Lettmann et al., 2014). Transcripts of fibrillin-1 (FBN1) also showed significant changes under our experimental conditions. The encoded protein is a large, extracellular matrix glycoprotein that serves as a structural component of 10–12 nm calcium-binding microfibrils. Fibrillin-containing microfibrils are key architectural structures of the upper dermis and integral components of the dermal elastic fiber network (Haynes et al., 1997). Transcripts for fibrillin-1 (FBN1) were found to be highly up-regulated in cells treated with NannoG in comparison to control cells. This implies a potential role of NannoG as an elasticity improving agent. Evidence for the physiological significance of FBN1 in skin related processes comes from an in vivo study showing that a cosmetic “anti-aging” product is able to induce clinically identifiable improvement in the appearance of facial wrinkles following long-term use. This improvement is associated with deposition of fibrillin-rich microfibrils in the papillary dermis of treated skin. The study further supports the use of fibrillin-1 in a short-term assay as a biomarker for assessing efficacy of potential photoaging repair products (Watson et al., 2009). Furthermore, we studied transcript accumulation for collagen breakdown related genes, including transcripts of gene MMP1R. As expected, MMP1R transcripts were found to be up-regulated in cells stressed with H2O2. Skin aging manifests wrinkles, diminished structural integrity and impaired wound healing due to alterations in the extracellular matrix formed predominantly of Colla1 (Jenkins, 2002; Watson et al., 2009). Furthermore, it has been reported that the expression of MMP1 in dermal fibroblasts is induced under oxidative stress conditions (Buechner et al., 2008; Nazaruk and Galicka, 2014). Interestingly, MMP1R transcripts were found to be drastically down-regulated in H2O2 stressed cells pre-treated NannoG, in contrast to only extract-treated control cells. These results may be indicative of a complex interaction of NannoG with the regulation of MMP1R gene expression under stress conditions. As retinoids display key regulatory function in epidermal growth and differentiation (Roos et al., 1998), we also assessed the expression of retinoid metabolism related genes. Within the cytoplasm, retinol, retinal or retinoic acid are bound to specific cellular binding proteins CRBP-I and II and CRABP I and II. These proteins are involved in the regulation of the intracellular concentration of retinol, retinal and retinoic acid by acting as both storage or shuttle proteins in retinoid metabolism (Roos et al., 1998). In vitro studies have shown that low expression of CRABP2 is associated with loss of elastin g ene expression by retinoic acid in fibroblasts (Plantier et al., 2008; Park et al., 2013). In this study, transcripts of CRABP2 were found to be highly up-regulated in cells treated with NannoG, while oxidative stress completely dismissed the expression of this gene. Following the observed trend, the pre-treatment of the stressed cells with NannoG restored the respective transcripts to control levels, in line with the overall protective effects of the extract under oxidative stress previously discussed. Another gene showing significant differential regulation under our experimental conditions was PTGER. Interestingly, these transcripts were found to be down-regulated in NannoG pre- treated stressed cells. Previous studies have shown that down-regulation of PTGER results in increased collagen synthesis in fibroblasts (Hayashi et al., 2006), a correlation which could at least partially explain our results as the induction of Col6A1 and the down-regulation of MMP1R suggest a net collagen synthesis in NannoG pretreated H2O2-stressed fibroblast. Finally, additional extracellular matrix related genes such as SERPINE and KLF2 showed significant differential regulation under our experimental conditions. Serpine's main targets are proteases, including thrombin, urinary plasminogen activator (uPA), and plasmin. It has also activity toward trypsin and urokinase, and binds heparin. Thus, the physiological target of SERPINE may include several mediators of matrix metabolism. In our study, we observed a significant up-regulation of SERPINE in stressed cells, while pre-treatment with NannoG restored transcripts to the control levels, indicating a possible protective role through the modulation of extracellular matrix metabolism. However, further work is needed to fully understand this interaction. KLFs are a family of broadly expressed zinc finger transcription factors. KLF2 regulates T-cell trafficking by promoting expression of the lipid-binding receptor S1P1 and the selectin CD62L (Weinreich et al., 2009). In our in vitro system, NannoG-treatment as well as H2O2-induced stress resulted in strong up-regulation of KLF2 gene expression in agreement with previous studies (Cullingford et al., 2008).
NannoG Modulates the Expression of Genes Involved in Skin Irritation, DNA Damage, and Aging
Several genes involved in immune response were included in our RT-qPCR platform. Among them, IL1R gene showed significant changes in transcript accumulation. The release of cytokines from skin cells plays a crucial role in cellular signaling pathways, linked to cell migration and proliferation, and it is therefore associated to processes necessary for induction of wound healing. Inflammation-related cytokine secretion is a well-founded indicator for irritating and cell-toxicity activity of test compounds. IL1R is an important mediator involved in many cytokine-induced immune and inflammatory responses. Moreover, overexpression of IL1R in both keratinocytes and fibroblasts has been shown to influence the pathophysiology of atopic dermatitis of skin (Arlian et al., 2008). H2O2 treatment strongly enhanced the expression of IL1R, in accordance with previous studies (Higgins et al., 1999). In contrast, NannoG treatment did not alter the expression of IL1R and moreover, cells pretreated with NannoG that were afterwards exposed to H2O2-stress exhibited alleviated induction of IL1R expression. This may suggest that NannoG could protect skin from irritation caused by oxidative stress. DNA damage as a result of ROS generation may have an impact on the expression of genes involved in the cell cycle regulation and DNA repair. The expression of a number genes involved in these processes is known to be enhanced during oxidative stress, thus it was analyzed under our experimental conditions. In our study, the transcript levels of BTG2 were strongly up-regulated in H2O2-stressed cells that were pre-treated with NannoG. BTG2 encodes for a member of the BTG/Tob family, which includes structurally related proteins that appear to have antiproliferative properties. BTG2 is involved in the regulation of the G1/S transition of the cell cycle (Mao et al., 2015) and DNA damage repair (Rouault et al., 1996). This may imply that NannoG treatment provides the cell with the ability, upon stress, to activate cytoprotective processes like cell cycle arrest and DNA repair. In addition, PCNA is an active nuclear protein involved in DNA replication, recombination and repair (Matsukage et al., 1994) and up-regulation of PCNA expression has been observed during induction of cell proliferation (Ducoux et al., 2001; Afaq et al., 2009). In our study, transcripts of PCNA were up-regulated in cells treated with NannoG as well as in H2O2-stressed cells. The first result may be related with the DNA replication properties of PCNA as treatment of cells with NannoG enhances the cellular proliferation, whereas the latter could be related to the DNA repair properties of PCNA. Interestingly, NannoG pre-treated H2O2-stressed cells exhibited basal PCNA transcripts levels, possibly correlating to a less active DNA repair process, as a result of the extract protective effect. As an aging-related process marker in NHDF, we measured the transcripts of FOXO3 gene, a member of the class O of forkhead box transcription factors (FOXO) that have important roles in metabolism, cellular proliferation, stress resistance, apoptosis, autophagy, metabolism, inflammation, cytokine expression, immunity, and differentiation. FoxO proteins have been proposed to have cell protective roles during aging, cell senescence and exercise (Morris, 2005). In cultured human dermal fibroblasts, gene silencing of FoxO3a results in cell morphology consistent with cell senescence, increased cell population doubling times, and the generation of ROS, suggesting that FoxO protein activity may be required to extend cell longevity and to limit oxidative stress (Kyoung Kim et al., 2005). In addition, in vivo experiments suggest that FOXO3 protects against oxidative stress by increasing MnSOD expression and production of catalase and peroxiredoxin III (Lu et al., 2013). In our study, we observed that transcripts of FOXO3 were highly up-regulated in NannoG treated cells even after treatment with H2O2. Similarly, resveratrol and sirtuin-activating compounds have been shown to delay aging, age-related diseases and to increase lifespan via a mechanism that involves enhancement of FOXO3 activity (Stefani et al., 2007; Hubbard and Sinclair, 2014). Although fibroblasts longevity was not directly assayed in our experiments, the strong induction in the expression of FOXO3 gene by the NannoG treatment may have, a positive impact on the lifespan of these cells. In the present study, we combined both biochemical and transcriptomic analysis to gain a deeper insight into the possible bioactivities of NannoG as a source for novel cosmeceuticals. Among the major findings of this work we established that in vitro tested NannoG has potent antioxidant cytoprotective effects to dermal fibroblasts under oxidative stress. In addition, NannoG also induces the dermal fibroblast proliferation under oxidative stress, and transcriptionally regulates several genes involved in skin oxidative stress defense, cell-cycle regulation and DNA repair, moisturization, elasticity, and aging. In order to gain a deeper insight into the molecular mechanisms mediating the observed bioactivities, future work will include high throughput analytical techniques including RNAseq and untargeted metabolomic analysis. Based on present results, we propose that NannoG is a useful ingredient in cosmetic formulations.
Author Contributions
SL design of the work, the aqcuisition, the analysis, interpretate of data and darft the manuscript and final approval of the version to be published. KK substantial contribution to the design of the work and revising it critically for important intellectual content. KG revising it critically for important intellectual content. LM develop the extract. CI develop the extract, and revising it critically for important intellectual content. MC design of the analysis. NL design enzymatic based experiments, and revising it critically for important intellectual content. EF design of the work and darft the manuscript and final approval of the version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The research leading to these results has received funding from the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme FP7/2007–2013/under REA grant agreement no. 286354.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2017.00221/full#supplementary-material
References
Afaq, F., Zaid, M. A., Khan, N., Dreher, M., and Mukhtar, H. (2009). Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 18, 553–561. doi: 10.1111/j.1600-0625.2008.00829.x
Ahmad, T. A., Jubri, Z., Rajab, N. F., Rahim, K. A., Yusof, Y. A., and Makpol, S. (2013). Gelam honey protects against gamma-irradiation damage to antioxidant enzymes in human diploid fibroblasts. Molecules 18, 2200–2211. doi: 10.3390/molecules18022200
Ando, H., Kondoh, H., Ichihashi, M., and Hearing, V. J. (2007). Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Invest. Dermatol. 127, 751–761. doi: 10.1038/sj.jid.5700683
Antonicelli, F., Bellon, G., Debelle, L., and Hornebeck, W. (2007). Elastin-elastases and inflamm-aging. Curr. Top. Dev. Biol. 79, 99–155. doi: 10.1016/S0070-2153(06)79005-6
Arlian, L. G., Morgan, M. S., and Peterson, K. T. (2008). House dust and storage mite extracts influence skin keratinocyte and fibroblast function. Int. Arch. Allergy Immunol. 145, 33–42. doi: 10.1159/000107464
Beacham, D. A., Amatangelo, M. D., and Cukierman, E. (2007). Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr. Protoc. Cell Biol. Chapter 10: Unit 10.9. doi: 10.1002/0471143030.cb1009s33
Benzie, I. F. F., and Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 239, 70–76. doi: 10.1006/abio.1996.0292
Bickers, D. R., and Athar, M. (2006). Oxidative stress in the pathogenesis of skin disease. J. Invest. Dermatol. 126, 2565–2575. doi: 10.1038/sj.jid.5700340
Bieth, J., Spiess, B., and Wermuth, C. G. (1974). The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem. Med. 11, 350–357. doi: 10.1016/0006-2944(74)90134-3
Boury-Jamot, M., Daraspe, J., Bonté, F., Perrier, E., Schnebert, S., Dumas, M., et al. (2009). Skin aquaporins: function in hydration, wound, and skin epidermis homeostasis. Handb. Exp. Pharmacol. 2009, 205–217. doi: 10.1007/978-3-540-79885-9_10
Brawn, K., and Fridovich, I. (1980). Superoxide radical and superoxide dismutases: threat and defense. Acta Physiol. Scand. Suppl. 492, 9–18. doi: 10.1007/978-1-4757-9351-2_24
Brenneisen, P., Briviba, K., Wlaschek, M., Wenk, J., and Scharffetter-Kochanek, K. (1997). Hydrogen peroxide (H2O2). increases the steady-state mRNA levels of collagenase/MMP-1 in human dermal fibroblasts. Free Radic. Biol. Med. 22, 515–524. doi: 10.1016/S0891-5849(96)00404-2
Buechner, N., Schroeder, P., Jakob, S., Kunze, K., Maresch, T., Calles, C., et al. (2008). Changes of MMP-1 and collagen type Ialpha1 by UVA, UVB and IRA are differentially regulated by Trx-1. Exp. Gerontol. 43, 633–637. doi: 10.1016/j.exger.2008.04.009
Calabrese, V. I., Calafato, S., Puleo, E., Cornelius, C., Sapienza, M., Morganti, P., et al. (2008). Redox regulation of cellular stress response by ferulic acid ethyl ester in human dermal fibroblasts: role of vitagenes. Clin. Dermatol. 26, 358–363. doi: 10.1016/j.clindermatol.2008.01.005
Callaghan, T. M., and Wilhelm, K. P. (2008). A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part I: cellular and molecular perspectives of skin ageing. Int. J. Cosmet. Sci. 30, 313–22. doi: 10.1111/j.1468-2494.2008.00454.x
Cannon, C. L., Neal, P. J., Southee, J. A., Kubilus, J., and Klausner, M. (1994). New epidermal model for dermal irritancy testing. Toxicol. In vitro 8, 889–891. doi: 10.1016/0887-2333(94)90095-7
Chang, C.-C., Yang, M.-H., Wen, H.-M., and Chern, J.-C. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food and Drug Anal 10, 178–182.
Crouch, S. P., Kozlowski, R., Slater, K. J., and Fletcher, J. (1993). The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 160, 81–88. doi: 10.1016/0022-1759(93)90011-U
Cullingford, T. E., Butler, M. J., Marshall, A. K., Tham el, L., Sugden, P. H., and Clerk, A. (2008). Differential regulation of Krüppel-like factor family transcription factor expression in neonatal rat cardiac myocytes: effects of endothelin-1, oxidative stress and cytokines. Biochim. Biophys. Acta 1783, 1229–1236. doi: 10.1016/j.bbamcr.2008.03.007
Custódio, L., Pereira, F. H., Rodrigues, M. J., Barreira, L., Rauter, A. P., Alberício, F., et al. (2015). Botryococcus braunii and Nannochloropsis oculata extracts inhibit cholinesterases and protect human dopaminergic SH-SY5Y cells from H2O2-induced cytotoxicity. J. Appl. Phycol. 25, 839–848. doi: 10.1007/s10811-014-0369-4
Deters, A. M., Schröder, K. R., and Hense, A. (2005). Kiwi fruit (Actinidia chinensis L.). polysaccharides exert stimulating effects on cell proliferation via enhanced growth factor receptors, energy production, and collagen synthesis of human keratinocytes, fibroblasts, and skin equivalents. J. Cell Physiol. 202, 717–722. doi: 10.1002/jcp.20161
Duckworth, H. W., and Coleman, J. E. (1970). Physicochemical and kinetic properties of mushroom tyrosinase. J. Biol. Chem. 245, 1613–1625.
Ducoux, M., Urbach, S., Baldacci, G., Hübscher, U., Koundrioukoff, S., Christensen, J., et al. (2001). Mediation of proliferating cell nuclear antigen (PCNA). -dependent DNA replication through a conserved p21Cip1-like PCNA-binding motif present in the Third Subunit of Human DNA Polymerase. J. Biol. Chem. 276, 49258–49266. doi: 10.1074/jbc.M106990200
Faller, C., Bracher, M., Dami, N., and Roguet, R. (2002). Predictive ability of reconstructed human epidermis equivalents for the assessment of skin irritation of cosmetics. Toxicol. In vitro 16, 557–572. doi: 10.1016/S0887-2333(02)00053-X
Findlay, J. A., and Patil, S. D. (1984). Antibacterial constituents of the diatom Navicula delognei. J. Nat. Prod. 47, 815–818. doi: 10.1021/np50035a010
Fitzgerald, J., Holden, P., and Hansen, U. (2013). The expanded collagen VI family: new chains and new questions. Connect. Tissue Res. 54, 345–350. doi: 10.3109/03008207.2013.822865
Flohe, L. (2010). Changing paradigms in thiology from antioxidant defense toward redox regulation. Meth. Enzymol. 473, 1–39. doi: 10.1016/S0076-6879(10)73001-9
Fluhr, J. W., Darlenski, R., and Surber, C. (2008). Glycerol and the skin: holistic approach to its origin and functions. Br. J. Dermatol. 159, 23–34. doi: 10.1111/j.1365-2133.2008.08643.x
Frank, S., Oliver, L., Lebreton-De Coster, C., Moreau, C., Lecabellec, M. T., Michel, L., et al. (2004). Infrared radiation affects the mitochondrial pathway of apoptosis in human fibroblasts. J. Invest. Dermatol. 123, 823–831. doi: 10.1111/j.0022-202X.2004.23472.x
Fujitani, N., Sakari, S., Yamagushi, Y., and Takenaka, H. (2001). Inhibitory effects of microalgae on activation of hyaluronidase. J. Appl. Phycol. 13, 489–492. doi: 10.1023/A:1012592620347
Giampieri, F., Alvarez-Suarez, J. M., Mazzoni, L., Forbes-Hernandez, T. Y., Gasparrini, M., Gonzàlez-Paramàs, A. M., et al. (2014). Polyphenol-rich strawberry extract protects human dermal fibroblasts against hydrogen peroxide oxidative damage and improves mitochondrial functionality. Molecules 19, 7798–7816. doi: 10.3390/molecules19067798
Gouveia, L., Raymundo, A., Batista, A. P., Sousa, I., and Empis, J. (2006). Chlorella vulgaris and Haematococcus pluvialis biomass as colouring and antioxidant in food emulsions. Eur. Food Res. Technol. 222:362. doi: 10.1007/s00217-005-0105-z
Gruber, J. V., and Holtz, R. (2010). Examining the genomic influence of skin antioxidants in vitro. Mediat. Inflamm. 2010:230450. doi: 10.1155/2010/230450
Guillard, R. R., and Ryther, J. H. (1962). Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 8, 229–239. doi: 10.1139/m62-029
Gurjala, A. N., Liu, W. R., Mogford, J. E., Procaccini, P. S., and Mustoe, T. A. (2005). Age-dependent response of primary human dermal fibroblasts to oxidative stress: cell survival, pro-survival kinases, and entrance into cellular senescence. Wound Repair Regen. 13, 565–575. doi: 10.1111/j.1524-475X.2005.00079.x
Hamed, I., Özogul, F., Özogul, Y., and Regenstein, J. M. (2015). Marine bioactive compounds and their health benefits: a review. Compr. Rev. Food Sci. 14, 446–465. doi: 10.1111/1541-4337.12136
Hara-Chikuma, M., and Verkman, A. S. (2008). Roles of aquaporin-3 in the epidermis. J. Invest. Dermatol. 128, 2145–2151. doi: 10.1038/jid.2008.70
Hayashi, T., Nishihira, J., Koyama, Y., Sasaki, S., and Yamamoto, Y. (2006). Decreased prostaglandin E2 production by inflammatory cytokine and lower expression of EP2 receptor result in increased collagen synthesis in keloid fibroblasts. J. Invest. Dermatol. 126, 990–997. doi: 10.1038/sj.jid.5700227
Haynes, S. L., Shuttleworth, C. A., and Kielty, C. M. (1997). Keratinocytes express fibrillin and assemble microfibrils: implications for dermal matrix organization. Br. J. Dermatol. 137, 17–23. doi: 10.1111/j.1365-2133.1997.tb03695.x
Henriksen, P. A. (2014). The potential of neutrophil elastase inhibitors as anti-inflammatory therapies. Curr. Opin. Hematol. 21, 23–28. doi: 10.1097/MOH.0000000000000001
Hibberd, D. J. (1981). Notes on the taxonomy and nomenclatureof the algal classes Eustigmatophyceae and Tribophyceae (synonymXanthophyceae). Bot. J. Linn. Soc. 82, 93–99. doi: 10.1111/j.1095-8339.1981.tb00954.x
Higgins, G. C., Wu, Y., and Postlethwaite, A. E. (1999). Intracellular IL-1 receptor antagonist is elevated in human dermal fibroblasts that overexpress intracellular precursor IL-1 alpha. J. Immunol. 163, 3969–3975.
Hirano, S., Shelden, E. A., and Gilmont, R. R. (2004). HSP27 regulates fibroblast adhesion, motility, and matrix contraction. Cell Stress Chaperones 9, 29–37. doi: 10.1379/471.1
Hoek, C. V. D., Mann, D. G., and Jahns, H. M. (1995). “Heterokontophyta: class Eustigmatophyceae,” in Algae: An Introduction to Phycology, ed G. Thieme (New York, NY: Cambridge University Press), 130–131.
Hubbard, B. P., and Sinclair, D. A. (2014). Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci. 35, 146–154. doi: 10.1016/j.tips.2013.12.004
Imokawa, G., and Ishida, K. (2015). Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 16, 7753–7775. doi: 10.3390/ijms16047753
Jenkins, G. (2002). Molecular mechanisms of skin ageing. Mech. Ageing Dev. 123, 801–810. doi: 10.1016/S0047-6374(01)00425-0
Kamagaju, L., Morandini, R., Bizuru, E., Nyetera, P., Nduwayezu, J. B., Stévigny, C., et al. (2013). Tyrosinase modulation by five Rwandese herbal medicines traditionally used for skin treatment. J. Ethnopharmacol. 146, 824–834. doi: 10.1016/j.jep.2013.02.010
Kant, V., Gopal, A., Kumar, D., Pathak, N. N., Ram, M., Jangir, B. L., et al. (2015). Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J. Surg. Res. 193, 978–988. doi: 10.1016/j.jss.2014.10.019
Karihtala, P., and Soini, Y. (2007). Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. APMIS 115, 81–103. doi: 10.1111/j.1600-0463.2007.apm_514.x
Kim, M., Park, J., Song, K., Kim, H. G., Koh, J. S., and Boo, Y. C. (2012). Screening of plant extracts for human tyrosinase inhibiting effects. Int. J. Cosmet. Sci. 34, 202–208. doi: 10.1111/j.1468-2494.2012.00704.x
Kim, Y. J., and Uyama, H. (2005). Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 62, 1707–1723. doi: 10.1007/s00018-005-5054-y
Krutmann, J. (2001). New developments in photoprotection of human skin. Skin Pharmacol. Appl. Skin Physiol. 14, 401–407. doi: 10.1159/000056374
Kyoung Kim, H., Kyoung Kim, Y., Song, I. H., Baek, S. H., Lee, S. R., Hye Kim, J., et al. (2005). Downregulation of a forkhead transcription factor, FOXO3a, accelerates cellular senescence in human dermal fibroblasts. J. Gerontol. A Biol. Sci. Med. Sci. 60, 4–9. doi: 10.1093/gerona/60.1.4
Lettmann, S., Bloch, W., Maaß, T., Niehoff, A., Schulz, J. N., Eckes, B., et al. (2014). Col6a1 null mice as a model to study skin phenotypes in patients with collagen VI related myopathies: expression of classical and novel collagen VI variants during wound healing. PLoS ONE 9:e105686 doi: 10.1371/journal.pone.0105686
Li, X., Wang, X., Chen, D., and Chen, S. (2011). Antioxidant activity and mechanism of protocatechuic acid in vitro. FFHD 1, 232–244.
Liebsch, M., Barrabas, C., Traue, D., and Spielmann, H. (1997). Entwicklung eines neuen in vitro Tests auf dermale Phototoxizitat mit einem Modell menschlicher Epidermis (EpiDerm™). ALTEX 14, 165–174.
Liebsch, M., Traue, D., Barrabas, C., Spielmann, H., Upholl, P., Wilkins, S., et al. (2000). The ECVAM prevalidation study on the use of EpiDerm for skin corrosivity testing. ATLA 28, 371–401.
Lu, Q., Zhai, Y., Cheng, Q., Liu, Y., Gao, X., Zhang, T., et al. (2013). The Akt-FoxO3a-manganese superoxide dismutase pathway is involved in the regulation of oxidative stress in diabetic nephropathy. Exp. Physiol. 98, 934–945. doi: 10.1113/expphysiol.2012.068361
Macedo, M. F., Miller, A. Z., Dionísio, A., and Saiz-Jimenez, C. (2009). Biodiversity of cyanobacteria and green algae on monuments in the Mediterranean Basin: an overview. Microbiology 155, 3476–3490. doi: 10.1099/mic.0.032508-0
Mancuso, C., Scapagini, G., Currò, D., Giuffrida, S. A. M., De Marco, C., Butterfield, D. A., et al. (2007). Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front. Biosci. 12, 1107–1123. doi: 10.2741/2130
Mao, B., Zhang, Z., and Wang, G. (2015). BTG2: a rising star of tumor suppressors. Int. J. Oncol. 46, 459–464. doi: 10.3892/ijo.2014.2765
Matsukage, A., Hirose, F., and Yamaguchi, M. (1994). Transcriptional regulation of DNA replication-related genes in cell growth, differentiation and oncogenesis. Jpn. J. Cancer Res. 85, 1–8. doi: 10.1111/j.1349-7006.1994.tb02878.x
Moens, S., Goveia, J., Stapor, P. C., Cantelmo, A. R., and Carmeliet, P. (2011). The multifaceted activity of VEGF in angiogenesis - implications for therapy responses. Cytokine Growth Factor Rev. 25, 473–482. doi: 10.1016/j.cytogfr.2014.07.009
Morgan, C., and Nigam, Y. (2013). Naturally derived factors and their role in the promotion of angiogenesis for the healing of chronic wounds. Angiogenesis 16, 493–502. doi: 10.1007/s10456-013-9341-1
Morris, B. J. (2005). A forkhead in the road to longevity: the molecular basis of lifespan becomes clearer. J. Hypertens. 23, 1285–1309. doi: 10.1097/01.hjh.0000173509.45363.dd
Nakamura, T., Sakai, K., Nakamura, T., and Matsumoto, K. (2011). Hepatocyte growth factor twenty years on: much more than a growth factor. Gastroenterol. Hepatol. 26, 188–202. doi: 10.1111/j.1440-1746.2010.06549.x
Nazaruk, J., and Galicka, A. (2014). The influence of selected flavonoids from the leaves of Cirsium palustre (L.) Scop. on collagen expression in human skin fibroblasts. Phytother Res. 28, 1399–1405. doi: 10.1002/ptr.5143
Ono, T., Tsuji, T., Sakai, M., Yukizaki, C., Ino, H., Akagi, I., et al. (2009). Induction of hepatocyte growth factor production in human dermal fibroblasts and their proliferation by the extract of bitter melon pulp. Cytokine 46, 119–126. doi: 10.1016/j.cyto.2008.12.016
Park, N. H., Park, J. S., Kang, Y. G., Bae, J. H., Lee, H. K., Yeom, M. H., et al. (2013). Soybean extract showed modulation of retinoic acid-related gene expression of skin and photo-protective effects in keratinocytes. Int. J. Cosmet. Sci. 35, 136–142. doi: 10.1111/ics.12014
Pellegrini, N., Serafini, M., Colombi, B., Del Rio, D., Salvatore, S., Bianchi, M., et al. (2003). Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutrition. 133, 2812–2819.
Plantier, L., Rochette-Egly, C., Goven, D., Boutten, A., Bonay, M., Lesèche, G., et al. (2008). Dysregulation of elastin expression by fibroblasts in pulmonary emphysema: role of cellular retinoic acid binding protein 2. Thorax 63, 1012–1017. doi: 10.1136/thx.2007.093302
Ramakers, C., Ruijter, J. M., Deprez, R. H., and Moorman, A. F. (2003). Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339, 62–66. doi: 10.1016/S0304-3940(02)01423-4
Raposo, M. F., de Morais, R. M., and Bernardo de Morais, A. M. (2013). Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs. 11, 233–252. doi: 10.3390/md11010233
Rasmussen, R. S., and Morrissey, M. T. (2007). Marine biotechnology for production of food ingredients. Adv. Food Nutr. Res. 52, 237–292. doi: 10.1016/S1043-4526(06)52005-4
Roos, T. C., Jugert, F. K., Merk, H. F., and Bickers, D. R. (1998). Retinoid metabolism in the skin. Pharmacol. Rev. 50, 315–333.
Rouault, J. P., Falette, N., Guéhenneux, F., Guillot, C., Rimokh, R., Wang, Q., et al. (1996). Identification of BTG2, an antiproliferative p53-dependent component of the DNA damage cellular response pathway. Nat. Genet. 14, 482–486. doi: 10.1038/ng1296-482
Saewan, N., and Jimtaisong, A. (2015). Natural products as photoprotection. J. Cosmet. Dermatol. 14, 47–63. doi: 10.1111/jocd.12123
Sansone, C., Galasso, C., Orefice, I., Nuzzo, G., Luongo, E., Cutignano, A., et al. (2017). The green microalga Tetraselmis suecica reduces oxidative stress and induces repairing mechanisms in human cells. Sci. Rep. 7:41215. doi: 10.1038/srep41215
Schäfer-Korting, M., Bock, U., Diembeck, W., Düsing, H. J., Gamer, A., Haltner-Ukomadu, E., et al. (2008). The use of reconstructed human epidermis for skin absorption testing: results of the validation study. ATLA 36, 161–187.
Smolewski, P., and Robak, T. (2011). Inhibitors of apoptosis proteins (IAPs) as potential molecular targets for therapy of hematological malignancies. Curr. Mol. Med. 11, 633–649. doi: 10.2174/156652411797536723
Srinivas, R., and Ochs, C. (2012). Effect of UV-A irradiance on lipid accumulation in Nannochloropsis oculata. Photochem. Photobiol. 88, 684–689. doi: 10.1111/j.1751-1097.2012.01091.x
Stefani, M., Markus, M. A., Lin, R. C., Pinese, M., Dawes, I. W., and Morris, B. J. (2007). The effect of resveratrol on a cell model of human aging. Ann. N.Y. Acad. Sci. 1114, 407–418. doi: 10.1196/annals.1396.001
Susan, I., Blackburn, J., and Volkman, K. (2012). “Microalgae: a renewable source of bioproducts,” in Food and Industrial Bioproducts and Bioprocessing, ed N. T. Dunford (Iowa: Wiley-Blackwell), 221–241.
Takayama, S., Reed, J. C., and Homma, S. (2003). Heat-shock proteins as regulators of apoptosis. Oncogene 22, 9041–9047. doi: 10.1038/sj.onc.1207114
Tontisirin, K. (2003). Food Energy-Methods of Analysis and Conversion Factors. Rome: Food and Agriculture Organization of the United Nations.
Trouba, K. J., Hamadeh, H. K., Amin, R. P., and Germolec, D. R. (2002). Oxidative stress and its role in skin disease. Antioxid. Redox Signal. 4, 665–673. doi: 10.1089/15230860260220175
Tu, Y. J., Ye, A. F., Pan, Z. M., Zheng, C., Wu, T. L., Cheng, X. G., et al. (2013). Regulation of expression of HGF in BM-MSCs by baculovirus-mediated transduction. Cell Biol. Int. 37, 659–668. doi: 10.1002/cbin.10071
Valko, M., Leibfritz, D., Moncol, J., Cronin, M. T., Mazur, M., and Telser, J. (2007). Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39, 44–84. doi: 10.1016/j.biocel.2006.07.001
Wang, H. M. D., Chen, C. C., Huynh, P., and Chang, J. S. (2015). Exploring the potential of using algae in cosmetics. Bioresour. Technol. 184, 355–362. doi: 10.1016/j.biortech.2014.12.001
Wang, K. S., and Chai, T. (1994). Reduction in omega-3 fatty acids by UV-B irradiation in microalgae. J. Appl. Phycol. 6:415. doi: 10.1007/BF02182158
Watson, R. E. B., Ogden, S., Cotterell, L. F., Bowden, J. J., Bastrilles, J. Y., Long, S. P., et al. (2009). A cosmetic ‘anti-ageing’ product improves photoaged skin: a double-blind, randomized controlled trial. Br. J. Dermatol. 161, 419–426. doi: 10.1111/j.1365-2133.2009.09216.x
Weinreich, M. A., Takada, K., Skon, C., Reiner, S. L., Jameson, S. C., and Hogquist, K. A. (2009). KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity 31, 122–130. doi: 10.1016/j.immuni.2009.05.011
Werner, S., and Grose, R. (2003). Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 83, 835–870. doi: 10.1152/physrev.00031.2002
Yarosh, D. B., Galvin, J. W., Nay, S. L., Peña, A. V., Canning, M. T., and Brown, D. A. (2006). Anti-inflammatory activity in skin by biomimetic of Evodia rutaecarpa extract from traditional Chinese medicine. J. Dermatol. Sci. 42, 13–21. doi: 10.1016/j.jdermsci.2005.12.009
Keywords: Nannochloropsis gaditana, RT-qPCR, primary human dermal fibroblasts
Citation: Letsiou S, Kalliampakou K, Gardikis K, Mantecon L, Infante C, Chatzikonstantinou M, Labrou NE and Flemetakis E (2017) Skin Protective Effects of Nannochloropsis gaditana Extract on H2O2-Stressed Human Dermal Fibroblasts. Front. Mar. Sci. 4:221. doi: 10.3389/fmars.2017.00221
Received: 06 April 2017; Accepted: 27 June 2017;
Published: 11 July 2017.
Edited by:
Weiqi Fu, New York University Abu Dhabi, United Arab Emirates and University of Iceland, IcelandReviewed by:
Tania Pozzo, University of California, Davis, United StatesWon-Kyo Jung, Pukyong National University, South Korea
Copyright © 2017 Letsiou, Kalliampakou, Gardikis, Mantecon, Infante, Chatzikonstantinou, Labrou and Flemetakis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emmanouil Flemetakis, bWZsZW1AZ21haWwuY29t
 Sophia Letsiou
Sophia Letsiou Katerina Kalliampakou2
Katerina Kalliampakou2 Marianna Chatzikonstantinou
Marianna Chatzikonstantinou Nikolaos E. Labrou
Nikolaos E. Labrou Emmanouil Flemetakis
Emmanouil Flemetakis