
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mar. Sci., 29 May 2017
Sec. Global Change and the Future Ocean
Volume 4 - 2017 | https://doi.org/10.3389/fmars.2017.00158
This article is part of the Research TopicEffects of climate change across ocean regionsView all 11 articles
Coral reefs are found in a wide range of environments, where they provide food and habitat to a large range of organisms as well as providing many other ecological goods and services. Warm-water coral reefs, for example, occupy shallow sunlit, warm, and alkaline waters in order to grow and calcify at the high rates necessary to build and maintain their calcium carbonate structures. At deeper locations (40–150 m), “mesophotic” (low light) coral reefs accumulate calcium carbonate at much lower rates (if at all in some cases) yet remain important as habitat for a wide range of organisms, including those important for fisheries. Finally, even deeper, down to 2,000 m or more, the so-called “cold-water” coral reefs are found in the dark depths. Despite their importance, coral reefs are facing significant challenges from human activities including pollution, over-harvesting, physical destruction, and climate change. In the latter case, even lower greenhouse gas emission scenarios (such as Representative Concentration Pathway RCP 4.5) are likely drive the elimination of most warm-water coral reefs by 2040–2050. Cold-water corals are also threatened by warming temperatures and ocean acidification although evidence of the direct effect of climate change is less clear. Evidence that coral reefs can adapt at rates which are sufficient for them to keep up with rapid ocean warming and acidification is minimal, especially given that corals are long-lived and hence have slow rates of evolution. Conclusions that coral reefs will migrate to higher latitudes as they warm are equally unfounded, with the observations of tropical species appearing at high latitudes “necessary but not sufficient” evidence that entire coral reef ecosystems are shifting. On the contrary, coral reefs are likely to degrade rapidly over the next 20 years, presenting fundamental challenges for the 500 million people who derive food, income, coastal protection, and a range of other services from coral reefs. Unless rapid advances to the goals of the Paris Climate Change Agreement occur over the next decade, hundreds of millions of people are likely to face increasing amounts of poverty and social disruption, and, in some cases, regional insecurity.
Both warm- and cold-water corals secrete calcium carbonate skeletons that build up over time to create a three-dimensional reef matrix that provides habitat for thousands of fish and other species. The production of limestone-like calcium carbonate is high enough in many warm-water coral reefs to establish carbonate structures. High rates of calcification are sufficient to overcome significant rates of bioerosion and wave driven physical erosion. These structures underpin the framework of barrier reefs and islands, which are critically important to tropical coastlines. Although they occupy less than 0.1% of the ocean floor, tropical coral reef ecosystems provide habitat for at least 25% of known marine species, with many reef species still to be discovered (Fisher et al., 2015). The biological diversity of warm-water coral reefs has been estimated to include ~1–9 million species that live in and around coral reefs (Reaka-Kudla, 1997, Census of Marine Life, http://www.coml.org/census-coral-reef-ecosystems-creefs). In deeper parts of these warm-water reef systems, the tendency toward carbonate dominated reef structures diminishes as light levels decrease (Bongaerts et al., 2010a). At low light levels, erosion and dissolution exceed calcium carbonate production, leading to coral communities that may be abundant yet with little or no three-dimensional calcium carbonate reef framework. Extending from 40 to 150 m, these “mesophotic” (low light) coral reefs also provide extensive habitat, with the rates of discovery of species remaining very high due to these reefs being difficult to visit (Bongaerts et al., 2010a, 2011). Mesophotic reef systems probably cover a comparable area to shallow warm-water coral reefs (Bongaerts et al., 2010a; Slattery et al., 2011).
Both shallow or deeper mesophotic coral reefs are dominated by scleractinian corals that form symbiosis with dinoflagellate protists from the genus, Symbiodinium. On the basis of this symbiosis, their intracellular symbionts (i.e., living within the gastrodermal or digestive tissues of their coral hosts) are able to photosynthesize and provide the host coral with a rich source of sugars, glycerol, lipids, and other organic compounds (Muscatine, 1990). This relationship enables corals to grow and calcify at high rates in the clear, warm, and shallow water conditions along tropical coastlines (Muscatine and Porter, 1977). The abundance of Scleractinian corals hosting Symbiodinium decreases with depth beyond 20–40 m, depending on the clarity of the water column. The deepest Scleractinian corals that are symbiotic with Symbiodinium, are found 100 m or more below the surface of tropical waters (Englebert et al., 2014). The productivity of this symbiosis is complemented by the ability of corals to capture and feed on waterborne particles and plankton (i.e., polytrophy). The combined ability to photosynthesise, as well as feed, underpins the success of the highly productive coral reef ecosystems that line many tropical coastlines. Evidence from isotope signatures within fossils reveal that Scleractinian corals have been symbiotic with Symbiodinium for over 230 million years (Stanley and Fautin, 2001; Muscatine et al., 2005), most probably driving productive and diverse ecosystems that were not too different from those of today.
Cold-water coral reefs extend to depths of 3,000 m although some cold-water corals can be found growing in waters as shallow as 50 m (e.g., Norwegian shelf). Below 200 m depth there is so little light that photosynthesis is no longer possible. As a result, cold-water corals do not form a symbiosis with Symbiodinium and depend instead on particle feeding. Discoveries of the locations and extent of cold-water reefs has primarily been driven by advances in underwater technologies for surveying and mapping (Turley et al., 2007; Ramirez-Llodra et al., 2010). For example, vast extents (~2,000 km2) of cold-water coral reefs, some shown to be thousands of years old (>8,000 years), have been found in Norwegian waters in past decades (Fosså et al., 2005). Cold-water coral reefs have now been discovered in every ocean, forming important assemblages within the deep ocean that provide critical habitat to thousands of other species, including many commercially important species.
Human communities derive many benefits from coral reefs including food, income, recreation, coastal protection, cultural settings, and many other ecological goods and services (Cinner et al., 2009; Costanza et al., 2014). Despite their biological diversity, productivity and importance to humans, both warm and cold-water coral reefs are being heavily impacted by human activities due to both local and global influences (Hall-Spencer et al., 2002; Burke et al., 2011). As a result, many coral reefs are rapidly declining across the world. While local factors can have significant impact on coral reefs (e.g., pollution, overfishing, and the physical destruction of reefs), changes in ocean temperature and chemistry due to anthropogenic activities are dramatically reducing the distribution, abundance, and survival of entire coral reef ecosystems (Gattuso et al., 2014b; Hoegh-Guldberg et al., 2014). Given these risks and the importance of coral reefs to humans and marine biodiversity, the present paper focuses on the challenges that warm and cold-water coral reef ecosystems and their human communities are facing, particularly those posed by rapidly warming and acidifying oceans.
Warm-water coral reefs are prominent ecosystems within coastal areas of the Pacific, Indian, and Atlantic oceans (Figures 1A,B), where they are typically found in a broad band (30°S to 30°N) of warm, sunlit, alkaline, clear, and relatively nutrient deficient ocean waters (Kleypas et al., 1999b). Here, Scleractinian or reef-building corals proliferate, depositing copious amounts of calcium carbonate. As corals die, their dead skeletons build up over time and are “glued” together by the activities of other organisms such as encrusting red coralline algae (Glynn and Manzello, 2015). Other organisms such as calcifying green algae, invertebrates, and phytoplankton also contribute to the overall carbonate budget of warm water coral reefs (Hutchings and Hoegh-Guldberg, 2009), leading to three-dimensional calcium carbonate structures that build up over hundreds and thousands of years. In turn, the three-dimensional structures (Figure 1C) within warm-water reef systems creates habitat for hundreds of thousands of species, many of which support coastal human populations with food, income, and other ecological goods and services such as coastal protection. Coral reefs are also important sources for bio-prospecting and the development of novel pharmaceuticals. The asset value of coral reefs has been estimated as close to $1 trillion (Hoegh-Guldberg, 2015) with the economic value of goods and services from coral reefs exceeding $375 billion annually, with benefits flowing to over 500 million people in at least 90 countries worldwide (Burke et al., 2011; Gattuso et al., 2014b).
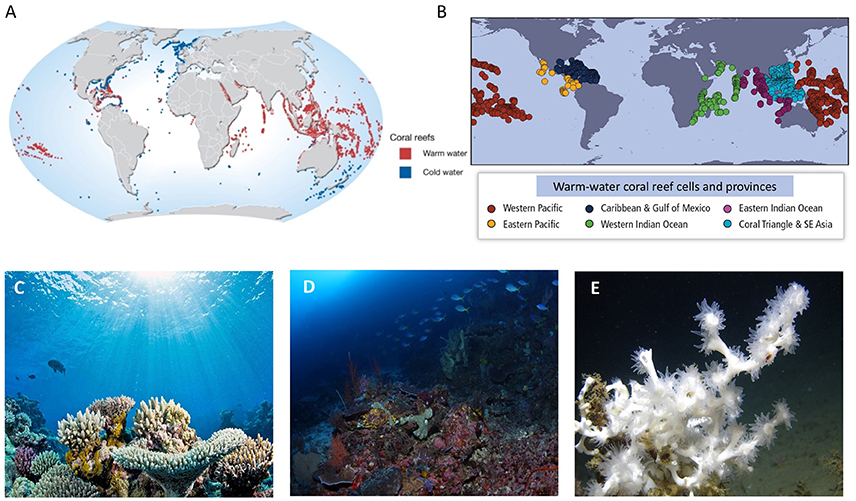
Figure 1. (A) Distribution of warm-water and cold-water coral reefs (credit: Hugo Ahlenius, 2008, UNEP/GRID-Arendal, http://www.grida.no/resources/7197). (B) Location of warm-water coral reef cells and provinces, from Hoegh-Guldberg et al. (2014). (C) Warm-water carbonate coral reef from the Great Barrier Reef, Australia (credit: Ove Hoegh-Guldberg). (D) Mesophotic coral community of North Sulawesi, Indonesia. (Credit: Pim Bongaerts, University of Queensland). (E) Deep-water community of Lophelia pertusa from the Mississippi Canyon at ~450 m depth (Image from NOAA, licensed under the Creative Commons Attribution-Share Alike 2.0 Generic license).
As light levels decrease with depth, decalcification dominates and the overall carbonate balance of reef ecosystems shifts to negative (Barnes and Chalker, 1990; Bongaerts et al., 2010a). Under these conditions, Scleractinian corals and their symbionts persist with reefs being referred to as “mesophotic” (Bongaerts et al., 2010a, 2011; Robinson C. et al., 2010). In these habitats, colonies of Scleractinian corals are often platelike in shape, orientating themselves to maximize light harvesting under these dim light conditions (Figure 1D). Mesophotic reef systems are also primarily restricted to areas where water clarity, carbonate ion concentrations, and temperatures are relatively high. Like their counterparts in shallower regions, mesophotic reef systems play an important role in supporting fisheries and hence human livelihoods. Given the difficulty of working at depths of more than 30 m (beyond SCUBA-diving depth) many species remain to be discovered (Bongaerts et al., 2010a). Mesophotic reefs therefore have an unknown potential to be sources of novel pharmaceuticals and other potentially beneficial compounds (Leal et al., 2012). As a result, their true value has probably been underestimated.
Cold-water corals generally form reefs at much greater depths from 200 to 2,000 m however in some regions they are found at shallower depths (Fosså et al., 2002; Freiwald et al., 2004). Deep-water corals are not dependent on light levels as they are not symbiotic with Symbiodinium. Due to the colder and more CO2 rich (and hence less alkaline) waters, deep-water corals grow slower than warm-water corals, forming aggregations that are variously termed patches, banks, thickets, bioherms, mounds, gardens, and massifs. In the absence of significant wave action, these fragile and slow growing reefs form aggregations that can cover vast tracks of the seabed (e.g., 2,000 km2 in Norwegian waters http://www.lophelia.org/) (Hall-Spencer et al., 2002) and involve near mono-specific stands of Scleractinian corals such as Lophelia pertusa and Oculina varicosa (Figure 1E). In addition to Scleractinian corals, they often exhibit a wide variety of abundant coral-like organisms, including soft corals, gorgonians, and Alcyonaceans.
Coral reefs are facing growing challenges from the local to global effects of human activities. Over the past 200 years, human activities have fundamentally changed coastlines, overexploited resources such as fish stocks, and polluted coastal waters, to a point where many coral reef ecosystems are degrading rapidly (Jackson et al., 2001; Pandolfi et al., 2003; Hoegh-Guldberg, 2014b). Warm-water coral reefs, for example, have declined by at least 50% over the past 30–50 years in large parts of the world's tropical regions (Hughes, 1994; Gardner et al., 2003; Bruno and Selig, 2007; De'ath et al., 2012). Similar conclusions have been reached for cold-water reefs where human activities have put these systems under escalating pressure from the mid-1980s onwards. Key drivers of the destruction of cold-water reefs include commercial bottom trawling, hydrocarbon exploration and production, deep sea mining, cable and pipeline placement, pollution, waste disposal, coral exploitation, and trade, and destructive scientific sampling (Hall-Spencer et al., 2002; Turley et al., 2007; Roberts and Cairns, 2014). The increase in impacts from human activities is a result of rapid advances in technologies for visualizing and exploiting the biological and mineral resources of deep water habitats (Freiwald et al., 2004; Ramirez-Llodra et al., 2010). Many populations of deep-sea corals (Scleractinians, gorgonians) have very slow turn-over rates and may live for centuries, with some species such as black corals (Antipatharians) living for thousands of years. The longevity and slow growth rates of these taxa means that recovery from anthropogenic stressors will be very slow. The areas inhabited by the deep-sea reefs are also a “resource frontier” for hydrocarbon extraction and mining of high value and “high-tech” metals (Roberts and Cairns, 2014). Hence, it is likely that anthropogenic impacts on these reefs will expand. These impacts are also likely to interact with ocean warming and acidification (Figure 2A), which pose growing and serious risks to coral reef ecosystems on their own. The direct impact of these changes to coral reefs have been growing since the early 1980s (Hoegh-Guldberg et al., 2007, 2014; Eakin C. M. et al., 2010; Gattuso et al., 2014b). The latter are the direct result of the burning of fossil fuels and have been driving growing impacts on warm water coral reefs since the early 1980s. Understanding and solving both local and global threats to coral reefs will be critically important if they are to survive some of the greatest rates of environmental change in the recent history of the Earth (Hönisch et al., 2012; Pörtner et al., 2014).
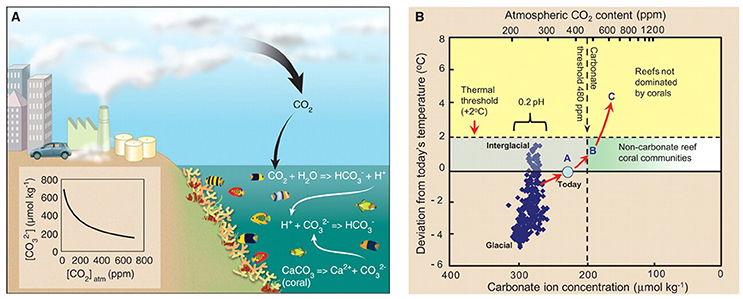
Figure 2. (A) Linkages between the build-up of atmospheric CO2 and the slowing of coral calcification due to ocean acidification. Approximately 30% of the atmospheric CO2 emitted by humans has been taken up by the ocean (IPCC, 2013) where it combined with water to produce carbonic acid, which releases a proton that combines with a carbonate ion. This decreases the concentration of carbonate, making it unavailable to marine calcifiers such as corals. (B) Temperature, [CO2]atm, and carbonate-ion concentrations reconstructed for the past 420,000 years. Carbonate concentrations were calculated (Lewis et al., 1998) from [CO2]atm and temperature deviations from conditions in the decade of the 2000s with the Vostok Ice Core data set (Petit et al., 1999), assuming constant salinity (34 parts per trillion), mean sea temperature (25°C), and total alkalinity (2,300 mmol kg−1). Acidity of the ocean varies by ± 0.1 pH units over the past 420,000 years (individual values not shown). The thresholds for major changes to coral communities are indicated for thermal stress (+2°C) and carbonate-ion concentrations ([carbonate] = 200 μmol kg−1, approximate aragonite saturation ~Ωaragonite = 3.3; [CO2]atm = 480 ppm). Coral Reef Scenarios CRS-A, CRS-B, and CRS-C are indicated as A, B, and C, respectively, with analogs from extant reefs. Red arrows pointing progressively toward the right-hand top square indicate the pathway that is being followed toward [CO2]atm of more than 500 ppm. From Hoegh-Guldberg et al. (2007) with permission of Science Magazine.
Warm-water coral reefs are largely dependent on the physical and chemical changes occurring in the surface of the ocean, whereas cold-water reef systems are tied relatively more to the broad scale conditions of the bulk ocean (Freiwald et al., 2004; Eakin C. M. et al., 2010). In this respect, there are likely to be differences in terms of the rate and characteristics of the changes that are occurring. These differences also translate into different trajectories when it comes to near and long-term projections of planetary warming and ocean acidification.
Warm-water coral reef environments have experienced relatively small amounts of variability in terms of temperature and carbonate ion concentrations, even with the relatively substantial swings in average global temperature and atmospheric CO2 concentration during the glacial cycle (Figure 2B). Warm-water coral reefs contracted toward the equator during glacial periods, and re-expanded along the tropical and subtropical coastlines of the world during the intervening warm periods (Hubbard, 2015). While these changes were rapid relative to geological time frames, they occurred over periods of 10,000 years or more and are slow when compared to climatic changes that have occurred since pre-industrial. While our understanding of how conditions have changed in terms of the habitat of deep-water coral reefs over geological time is limited, it is very likely that conditions varied even less over these long periods than those surrounding the warm-water coral reefs.
It is virtually certain that the upper ocean has warmed between 1971 and 2010 and likely that it has warmed between 1870s and 1971 (IPCC, 2013). These changes are consistent with those expected from the associated rise in greenhouse gas concentrations in the atmosphere (IPCC, 2013). The average sea surface temperatures (SST) of the Indian, Atlantic, and Pacific oceans have increased by 0.65, 0.41, and 0.31°C during 1950–2009 (Table 30-1 in Hoegh-Guldberg et al., 2014). The influence of long-term patterns of climate variability such as the Pacific Decadal Oscillation (PDO) contribute to variability at regional scales and confound efforts to detect and attribute regional changes to anthropogenic greenhouse gas emissions (Hoegh-Guldberg et al., 2014). Nonetheless, examination of the Hadley Centre HadISST1.1 data (Rayner et al., 2003) over 60 years (1950–2009) reveals significant warming trends in SST for many sub-regions of the ocean (Table 30-1 in Hoegh-Guldberg et al., 2014). Significant trends are clearly demonstrated within the six major warm-water coral reef regions, with the exception of the Gulf of Mexico/Caribbean Sea region (Table 1). Rates of increase in SST in the warm-water coral reef regions range from 0.07°C (west Pacific Ocean) to 0.13°C (Coral Triangle and southeast Asia) per decade, resulting in an overall increase in the regions of between 0.44 and 0.79°C during the period from 1950 to 2009.
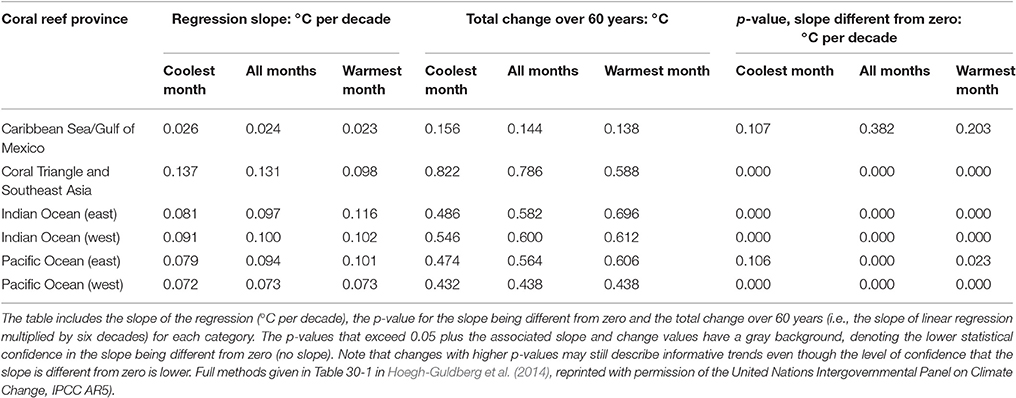
Table 1. Changes in sea surface temperature (SST) in six major warm-water coral reef provinces (Figure 1B) over the period 1950–2009 using 1 × 1 degree monthly SST data extracted from the Hadley Centre HadISST1.1 data set (Rayner et al., 2003).
In addition to the heat content and temperature of the upper layers of the ocean, the research community is virtually certain that ocean chemistry is also changing as a result of the increasing amounts of CO2 entering the Ocean (IPCC, 2013). Observed increases in salinity at tropical latitudes are consistent with the amplification of the global hydrological cycle (Durack and Wijffels, 2010; Durack et al., 2012), including rainfall, which have significant implications for coastal ecosystems such as warm-water coral reefs. At regional levels, changes in storm and rainfall intensity also have the potential to influence coastal water quality, which is important to coral reefs, as a result of the interplay between droughts, coastal and catchment erosion, and sudden inundation (flood) events. The impact of climate change adds to those from other human activities that are already impacting water quality, coastal erosion and biological systems.
Average global sea levels are increasing by an average of 3.2 mm year−1 (over 1993–2010) as a result of warming of the ocean (thus increasing volume) and the melting of land ice (IPCC, 2013). Sea level rise varies between regions as a result of differences in local oceanography and geology and the influence of long-term variation in regional climate. Some areas that have significant warm-water coral reefs, such as Southeast Asia and northern Australia, have reported rates of sea level rise of around 10 mm year−1. While the direct attribution of changes in regional wind strength, storm intensity and frequency to global warming is challenging due to long-term variability, there is considerable evidence that the frequency and intensity of the strongest tropical storms in some regions (e.g., North Atlantic; IPCC, 2013) has increased since the 1970s. The combination of higher sea levels and more intense storm systems is likely to increase the amount of force exerted by wave action on coastal areas, which has implications for coastal infrastructure, as well as the state of ecosystems such as coral reefs, mangroves, and seagrass beds (Hamylton et al., 2013; Saunders et al., 2014).
Changes have also occurred in the pH of ocean surface waters over the past 100 years, a phenomenon which is referred to as ocean acidification (Kleypas et al., 1999a; Caldeira and Wickett, 2003; Gattuso et al., 2014a). As CO2 enters the ocean, it reacts with water increasing hydrogen ion concentration (thus decreasing ocean pH) and decreasing the carbonate ion concentration. While the overall change in ocean pH appears small (0.1 pH units over the past 150 years), this is actually a 26% increase in the concentration of hydrogen ions. Experimental evidence shows a reduction in carbonate ions with ocean acidification is biologically significant, since it can affect the rate at which marine organisms, such as corals build their calcareous structures (Kroeker et al., 2013). However, understanding of the mechanisms driving the sensitivity of coral calcification to ocean chemistry, such as the response of the pH of the internal calcifying fluid in which the coral skeleton forms to the concentration of dissolved organic carbon, are only being untangled (Comeau et al., 2017). These changes in ocean chemistry are temperature dependent, with the CO2 absorption and consequently acidification being highest when waters are cooler. The aragonite (one form of calcium carbonate) saturation state (Ωarag) is essentially the ratio between the concentrations of calcium and carbonate ions (Doney et al., 2009). The aragonite saturation state shows a similar distribution to sea surface temperature with Ωarag being highest in the warmest ocean regions and lowest in polar regions (Jiang et al., 2015). Surface waters of the ocean are generally supersaturated with respect to aragonite (Ωarag > 1). However, in warmer waters where Ωarag is not projected to fall to <1 (thus undersaturated with respect to aragonite, Figure 3), substantial impacts are likely to still occur on calcifying organisms. There is substantial evidence that carbonate accretion on warm-water coral reefs approaches zero or becomes negative when Ωarag falls below 3.3 (Hoegh-Guldberg et al., 2007; Chan and Connolly, 2013), a level likely to be reached in tropical surface waters within the next few decades at current rates of greenhouse gas emission (Hoegh-Guldberg et al., 2007; Ricke et al., 2013).
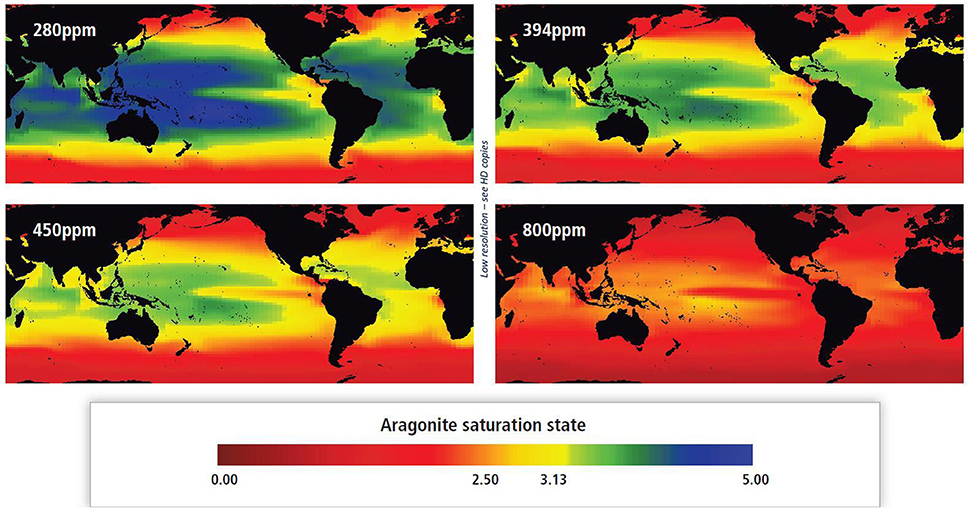
Figure 3. Aragonite saturation state of the surface ocean simulated by the University of Victoria Earth System Model under different atmospheric concentrations of CO2. 280 ppm represents pre-industrial and 394 ppm levels in 2012. Four hundred and fifty ppm is projected to be reached during 2030s under Representative Concentration Pathway (RCP) 4.5, 6.0, and 8.5, and to approach, but not reach 450 ppm, during 2040s under RCP 2.6 (IPCC 2013). Eight hundred ppm is projected to be reached during 2080s under RCP 8.5 only. Fields are calculated from the model output of dissolved inorganic carbon concentration, alkalinity concentration, temperature, and salinity, together with the chemistry routine from the OCMIP-3 project. Modified from Figure SM30-2 in Hoegh-Guldberg et al. (2014; reprinted with permission of IPCC AR5).
The global distribution of cold-water corals is at least partly limited by the depth of the aragonite saturation horizon, Ωarag = 1.0 (Guinotte et al., 2006). Aragonite saturation state diminishes with depth, due partly to hydrostatic pressure and lower temperature, with a distinct aragonite “saturation horizon” below which waters become under-saturated for aragonite (Ωarag <1) (Jiang et al., 2015). The saturation horizon is a complex outcome of ocean circulation, temperature, CO2 concentrations, salinity, metabolic activity, and the concentrations of organic compounds and occurs at depths between 200 and 3,500 m, depending on the latitude and the ocean (Orr et al., 2005; Doney et al., 2009; Rhein et al., 2013; Jiang et al., 2015). Surface waters and waters at 50 m depth are mostly supersaturated throughout the global ocean (Jiang et al., 2015), however in western Arctic waters, the area of under-saturated waters in the upper 250 m north of 70°N has increased from 5 to 31% between 1990s and 2010 (Qi et al., 2017). At 500 m, large areas of undersaturated Ωarag water are found in the northern and equatorial Pacific ocean. At 1,000 m, Ωarag < 1.8 over all ocean basins and at 2.000 m, Ωarag < 1.0 across all the Pacific and Indian Ocean and parts of the Atlantic Ocean. Ocean acidification is proceeding at higher rates at high latitudes than at lower latitudes (Figure 3) resulting in a shoaling of the aragonite saturation horizon. There is now evidence to show that the aragonite saturation horizon has shoaled since the Preindustrial Period (Turley et al., 2007). For example, in the north east Pacific (from 33.5 to 50.0°N) the aragonite saturation horizon has shoaled by 19.6 m in 11 years (2001–2012) and, at this rate, the entire water column in the northern section of this region is projected to become undersaturated within 50–90 years (Chu et al., 2016).
Not surprisingly, the scale and pace of the physical and chemical changes occurring in the ocean are driving a large range of fundamental responses in marine organisms, ecosystems, and regions (Hoegh-Guldberg et al., 2014; Pörtner et al., 2014). Equally significant, is the observation that relatively small amounts of change have resulted in quite substantial biological impacts, with clear evidence of non-linear trends, tipping points, and otherwise complex responses. Coral responses to changes in ocean conditions, in particular mass coral bleaching, provide particularly compelling examples of the consequences of a rapidly changing ocean for organisms, ecosystems, and dependent societies.
The symbiosis between warm-water corals and Symbiodinium (Figures 4A,B) is very sensitive to changes in the physical and chemical environment surrounding corals. Short periods of high or low temperature and/or light, or exposure to toxins like cyanide, can drive the breakdown of the symbiosis, resulting in the loss of the brown symbionts and a subsequent paling (hence “bleaching”) of the coral host (Hoegh-Guldberg, 1999). Coral bleaching involves the breakdown of the symbiosis between Scleractinian corals and Symbiodinium, which may recover if conditions are not too anomalous for too long. While bleaching of coral tissues has been reported on the scale of colonies or groups of colonies for at least 100 years (Yonge and Nichols, 1931), reports of bleaching at large geographic scales (Figures 4C,D, example of affected coral reefs in American Samoa from late 2015) was unknown to the scientific literature until 1979. Since the early 1980s, however, mass coral bleaching has affected entire reefs and regions, often resulting in significant mortality of reef-building corals. The absence of pre 1979 scientific reports in addition to the close relationship between bleaching and elevated sea temperature, plus considerable laboratory, and mesocosm studies, strongly support the conclusion that mass coral bleaching and mortality are novel and are caused by warm water coral reefs being exposed to rising sea temperatures (Hoegh-Guldberg and Smith, 1989; Glynn, 1993, 2012; Hoegh-Guldberg, 1999; Glynn et al., 2001; Hoegh-Guldberg et al., 2007, 2014; Baker et al., 2008; Eakin C. M. et al., 2010; Strong et al., 2011; Gattuso et al., 2014b). The latest cycle of mass coral bleaching in 2016 (Hoegh-Guldberg and Ridgway, 2016) is reputedly the worst on record and accompanies the warmest years on record (King and Hawkins, 2016; https://www.nasa.gov/press-release/nasa-noaa-data-show-2016-warmest-year-on-record-globally).
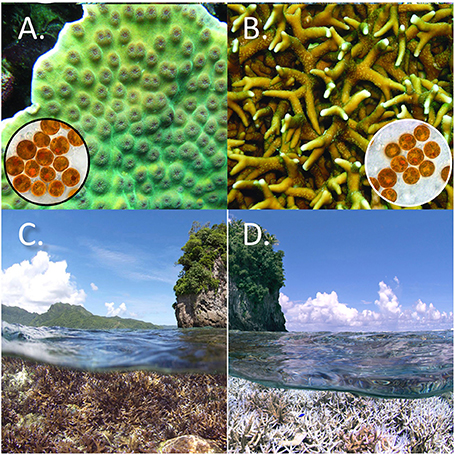
Figure 4. (A) Scleractinian coral (Turbinaria sp) and (B) Hydrozoan coral (Millepora sp) showing respective Symbiodinium symbionts (each brown cell is about 10 μm in diameter) removed from coral tissues; Credit for (A,B): Todd LaJeunesse, from Pennsylvania State University. (https://www.flickr.com/photos/tags/linkflickrset72157631573740050). (C) The photo at left, taken in December 2014, shows coral reef near runway in American Samoa, without obvious bleaching of corals. (D) The photo at right shows the same coral reef, now heavily bleached, in February 2014 (Credit for C,D: Richard Vevers, The Ocean Agency).
Mass coral bleaching and mortality can be triggered by small (1–2°C) SST increases above the long-term summer maxima for a region (Strong et al., 2011). If temperatures are higher for longer, the amount of coral bleaching will increase, driving increased mortality (Hoegh-Guldberg, 1999; Hoegh-Guldberg et al., 2007; Eakin C. M. et al., 2010). There is a strong link between the size and length of temperature extremes and mass coral bleaching and mortality (Hoegh-Guldberg, 1999; Strong et al., 2004, 2011; Eakin C. M. et al., 2010). These relationships are used with satellite data to derive anomalies in SST to monitor the frequency and intensity of mass coral bleaching and mortality (Strong et al., 2004, 2011). For this reason, there is a high level of confidence that the increases in mass coral bleaching and mortality since the early 1980s are due to anthropogenic climate change in particular ocean warming (Hoegh-Guldberg et al., 2014). The loss of symbionts from coral tissues can have immediate effects through the loss of photosynthetic energy, and lead to starvation, disease, reproductive failure, and a loss of competitive ability relative to other organisms on coral reefs (Hoegh-Guldberg and Smith, 1989; Glynn, 1993, 2012; Hoegh-Guldberg, 1999; Baker et al., 2008; Hoegh-Guldberg et al., 2014; Glynn and Manzello, 2015).
Understanding how the positions of ocean isotherms (lines of similar temperatures) are changing and how fast across the ocean surface (“velocity of climate change”, Burrows et al., 2011, 2014) provides insight into whether or not coral populations will be able to move, adapt or acclimatize fast enough to changing sea temperatures (Hoegh-Guldberg, 2012; Pörtner et al., 2014). Some of the highest rates of climate velocity (up to 200 km per decade) were observed in ocean tropical regions (over 1960–2010), driven by shallow spatial gradients in temperature (Burrows et al., 2011, 2014). Observed rates of distribution shifts for individual warm-water coral species linked to increases in sea surface temperatures range from 0 to 150 km per decade, with an average shift rate of 30 km per decade (Yamano et al., 2011; Poloczanska et al., 2013), suggesting that corals and coral ecosystems may be unable to keep up with warming rates (Hoegh-Guldberg, 2012; Burrows et al., 2014; García Molinos et al., 2015).
The possible reduced influence of extremes from climate change with depth has led to the speculation that deeper (>40 m) mesophotic coral reefs may offer a potential refuge against the otherwise rapid changes in temperature, storm intensity, and chemistry that are typical of shallow-water (0–30 m) coral reef environments (Bongaerts et al., 2010a). The “Deep Reef Refugia” hypothesis has been explored by a number of groups who are finding substantial differences in terms of the rate of warming and acidification with depth, as well as examples of species that may span the mesophotic zone to shallow reef areas. Recent work however, has revealed that mesophotic reefs may not be immune to the impacts of storms (Bongaerts et al., 2013). Also, populations of what appear to be the same coral species appear to have considerable genetic structure as a function of depth. This is important given that it implies a high degree of specialization, local adaptation, and even speciation, by corals living at different depths, with the implication that mesophotic corals may not be able to survive in shallow-water environments and vice versa, reducing the potential for mesophotic environments to provide refugia for shallow water Scleractinian corals. This reduces the significance of deeper water populations as a source of recruits for regenerating damaged areas on shallow water coral reefs (Bongaerts et al., 2010b, 2015). In addition to warming oceans, corals are also sensitive to changes to the pH and the carbonate chemistry of seawater as a result of ocean acidification (Kleypas et al., 1999a; Gattuso et al., 2014a). These changes affect organisms in a variety of ways, including reducing calcification rates in a wide array of corals and other organisms in laboratory, mesocosm, and field studies (Gattuso et al., 1998; Reynaud et al., 2003; Kleypas et al., 2006; Dove et al., 2013; Kroeker et al., 2013; Gattuso et al., 2014a).
Long-lived corals from the field have provided an opportunity for retrospective analysis of how growth has varied over long periods of time (De'ath et al., 2009; Lough, 2010, 2011). Calcification measurements from coral cores from 328 colonies of the massive coral Porites growing on the Great Barrier Reef in Australia, for example, have revealed that calcification by these corals has declined by 14.2% since 1990. This appears to unprecedented on the Great Barrier Reef for at least the last 400 years (De'ath et al., 2009) (but see D'Olivo et al., 2013; Figure 5). Given the complexity of the environmental changes occurring in places like the Great Barrier Reef, it is difficult to assign specific drivers of this decline. However, the combined effects of elevated warming and acidification from climate change, along with declining water quality, appear to be significant drivers of the changes observed (D'Olivo et al., 2013). Declining growth and calcification rates have also been detected for Porites colonies in the Red Sea (Cantin et al., 2010) and at several locations in Southeast Asia (Tanzil et al., 2009).
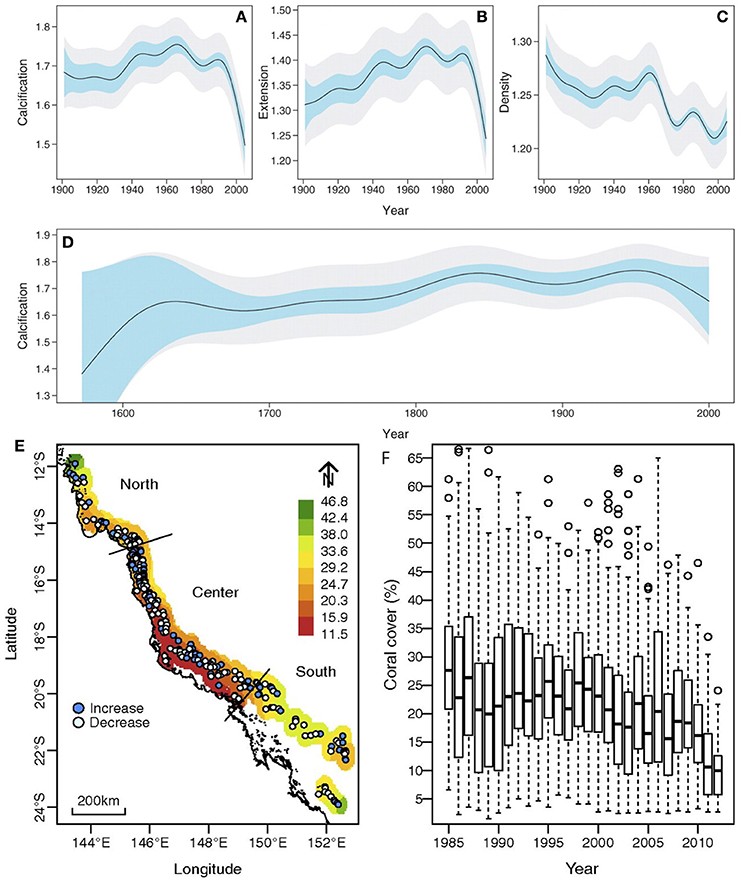
Figure 5. (A–D) Partial-effects plots showing the variation of calcification (grams per square centimeter per year), linear extension (centimeters per year), and density (grams per cubic centimeter) in Porites from the Great Barrier Reef (GBR), Australia, over time. From De'ath et al. (2009). Plots (A–C) are based on 1900–2005 data from 328 Porites colonies, and plot (D) on data for ten long cores. Light blue bands indicate 95% confidence intervals for comparison between years, and gray bands indicate 95% confidence intervals for the predicted value for any given year. Calcification declines by 14.2% from 1990 to 2005 (A), primarily due to declining extension (B). Density declines from 1900 onward (C). The 1572–2001 data show that calcification increased weakly from ~1.62 before 1,700 to ~1.76 in ~1850, after which it remained relatively constant (D) before a weak decline since ~1960. (D–F) Decline coral cover of the GBR over 1985–2012. (E) Map of GBR with color shading indicating mean coral cover averaged over 1985–2014. Points show the location of 214 survey reefs in the northern, central, and southern regions, and their color indicates the direction of change in cover over time. (F) Box plots indicate the percentiles (25, 50, and 70%) of the coral cover distributions within each year and suggest a substantial decline in coral cover over the 27 years. Adapted from De'ath et al. (2012) and with the permission according to PNAS policy.
Studies of the influence of rapidly warming and acidifying conditions on mesophotic coral reefs are absent. Given that these reef systems cover roughly the equivalent area of shallow water coral reefs, understanding how environmental changes are likely to influence these important areas in terms of habitat the fisheries and biodiversity is important and should be a priority of future research (Bongaerts et al., 2010a). Linking the physiological and ecological response of mesophotic reefs to changes in pH and carbonate ion concentration will also be important in the context of understanding how mesophotic coral reef ecosystems will be affected by the shoaling of the saturation horizon in regions such as off Hawaii.
Our understanding of how deep ocean environments are likely to respond to changes in ocean temperature and chemistry are at an early stage. Like mesophotic coral reefs, little is known about the sensitivity of cold-water coral reefs to changes in temperature. As cold-water corals tend not to have a mutualistic symbiosis with Symbiodinium, their response is naturally different to that of symbiotic Scleractinian corals. As with mesophotic coral reefs, there is much more to be discovered with respect to how these critically important cold-water coral reefs are likely to respond to steadily warming and acidifying ocean. Coral reefs in the deep-sea have been identified as particularly vulnerable to the effects of ocean acidification; in part because of the numerical predominance of calcifying taxa, and in part because the pre-industrial carbonate levels at the depths and temperatures they inhabit were already low (Freiwald et al., 2004). Experimental studies reveal that short-term exposures of important deep-water corals such as L. pertusa to a reduction in pH of around 0.15–0.3 units resulted in a decrease in calcification rates of between 30 and 56% (Maier et al., 2009). However, subsequent work has shown that L. pertusa can acclimatize (i.e., maintain considerable calcification) to declining aragonite levels modifying skeletal structure and skeletal strength (Form and Riebesell, 2012; Hennige et al., 2015). Observations of deep-sea corals in under-saturated waters from the SW Pacific also suggest some species-specific tolerance, however growth rates are extremely low and in under-saturated conditions dead coral skeletons dissolve rapidly (Bostock et al., 2015; Thresher et al., 2015). Whether cold-water corals will be able to adjust to rapid warming and ocean acidification projected for the coming century is unknown. However, analyses of cold-water coral fossils suggest that a combination of declining aragonite and oxygen saturations will reduce the distribution of cold-water corals (Thiagarajan et al., 2013).
While coral species and their symbionts have received a major amount of focus in terms of the effect of ocean warming and acidification on warm-water coral reef ecosystems, there is a growing number of studies that have revealed impacts on a broader range of reef organisms. Among the most affected are calcifying algae, calcareous phytoplankton, molluscs, and echinoderms, with the larval stages of some organisms being more sensitive than the adult phase (Kroeker et al., 2013). Bioeroding organisms also respond to both warmer and more acidic conditions (Dove et al., 2013; Fang et al., 2013; Reyes-Nivia et al., 2013). The sponge, Cliona orientalis, increased biomass and bioerosion capability when exposed to warmer and more acidic conditions, implicating a role of this sponge in helping tip the carbonate balance of reefs toward net erosion (Dove et al., 2013; Fang et al., 2013). Similar observations have been made for bio-eroding endolithic algal communities, where small shifts in ocean temperature and acidity (i.e., CO2 levels) enhanced skeletal dissolution and was associated with increased endolithic biomass and respiration under elevated temperatures and CO2 levels (Reyes-Nivia et al., 2013).
In addition to impacts on growth, calcification, and reproduction, there is growing evidence of impacts on a range of physiological systems of coral reef organisms. Ocean acidification, for example, impairs the homing ability and olfactory discrimination of some coral reef fish, with potential consequences for the ability of fish to detect and avoid predators (Munday et al., 2009; Dixson et al., 2010). At present, there are few reports on the influence or not of ocean acidification on the metabolic performance of tropical fish species. In this regard, it will be important to explore whether or not tropical fish have the same challenges that temperate fish have when it comes to respiratory gas transport and acid–base balance (Esbaugh et al., 2012; Pörtner et al., 2014). Physiological impacts combined with ecological impacts and habitat degradation, are likely to generate “surprises” for complex ecosystems such as those associated with both cold and warm water coral reefs.
The impact of climate change on coral reef organisms has ramifications for ecosystems, some of which may be transformative in terms of their effects on primary productivity, food web dynamics, habitat forming species, disease ecology, and many other aspects (Hoegh-Guldberg and Bruno, 2010). The recent decline in the abundance of warm-water coral reefs (Hughes, 1994; Gardner et al., 2003; Bruno and Selig, 2007; De'ath et al., 2012), however, illustrates the complex yet fundamental ways that marine ecosystems are changing in response to rapid rates of ocean warming and acidification. The ecological ramifications of rapid global change for mesophotic coral reefs are less well-known or understood than those of warm-water shallow reef systems. Similarly, threats to cold-water coral reefs less well-understood and undoubtedly involve a different mix of local and global drivers (Turley et al., 2007; Roberts and Cairns, 2014).
The major ecological responses of warm-water coral reefs to climate change have their origins in the response of reef-building corals to warming and acidification, and their role as framework builders within typical carbon reef systems (Gattuso et al., 1998; Kleypas et al., 1999a; Reynaud et al., 2003; Maier et al., 2009; Kroeker et al., 2013). As described above, corals are sensitive to small changes in temperature, light, and a number of other environmental variables, responding by disassociating from the dinoflagellate symbionts that populate their tissues (i.e., bleaching). Small changes in temperature are driving decreased growth and reproduction and increased mortality of corals in many parts of the world (Hoegh-Guldberg and Smith, 1989; Hoegh-Guldberg, 1999; Hoegh-Guldberg et al., 2014). As corals lose their symbionts, they become vulnerable to death and disease, as well as being less able to compete with other benthic organisms. These changes have driven episodes of coral mortality associated with thermal stress, with the catastrophic loss of corals in particular regions over the past 30 years (Hoegh-Guldberg, 1999; Baker et al., 2008; Eakin C. M. et al., 2010; Glynn, 2012). While some coral reefs have recovered over subsequent decades many others have not. Regional differences in the ability to recover are linked to the presence and absence of other factors affecting the resilience of reef building corals and other reef related organisms such as levels of herbivory, macroalgal cover, and coral recruitment rates (Baker et al., 2008). The reduced resilience of reef building corals as a result of thermal stress is likely to be exacerbated by increasing ocean acidification, which has the potential to reduce the ability of corals to grow, calcify, and recover from disturbances. While teasing apart the effects of rising temperatures and increasing amounts of ocean acidification is difficult, both thermal stress, and acidification have the potential to reduce the ability of corals to recover from stresses (Hughes et al., 2007). This may help explain why stressors such as cyclones, which do not appear to have increased in frequency over the past 30 years (Callaghan and Power, 2011; IPCC, 2013), appear to be having longer-lasting impacts on coral communities on the Great Barrier Reef (De'ath et al., 2012).
Mass coral bleaching reduces the energy available to corals, leading to physiological compromise. Warm-water corals, for example, exude mucus which is rich with the excess carbohydrates which provides food for a large number of molluscs, crustaceans, worms, ciliates, fish, and many other organisms (Baker et al., 2008; Wild et al., 2011). It also appears to play an important role in preventing the settlement of fouling and disease organisms. Mucus secretion, however, is reduced in bleached corals, potentially leading to increased disease (Harvell et al., 2007). Bleaching can also directly influence growth and reproduction of corals, as well is their tendency to succumb to a range of diseases (Harvell et al., 1999, 2007; Bruno and Selig, 2007; Baker et al., 2008). A reduction in reef-building corals raises the threat that a considerable proportion of the mega-diversity associated with coral reefs will face extirpation or, for some species, global extinction (Glynn, 2012). A meta-analysis of 17 independent studies, undertaken by Wilson et al. (2006), revealed that fish species reliant on live coral cover for food and shelter (some 62% of reef fish species) decreased in abundance within 3 years of disturbance events such bleaching, storms, and outbreaks of crown-of-thorns starfish that reduced coral cover by 10% or more.
The loss of calcifiers such as corals and calcareous algae due to warming and other stressors contributes to a reduced rate of community calcification, which is exacerbated by increases in dissolution and bioerosion as the water column becomes more acidified. Coral bleaching events driven by elevated temperatures has also been shown to shift carbonate budgets of coral reefs from net accretion to net erosion (DeCarlo et al., 2017; Januchowski-Hartley et al., 2017). Sixteen years later, a third of reefs that were considered ecologically recovering (Graham et al., 2015) did not show positive carbon budgets (Januchowski-Hartley et al., 2017). Reefs remaining in negative carbonate budgets were those where massive coral loss was high and recovery of branched corals was low. The composition of reef benthic communities, which are sensitive to thermal stress, have an influence on the sensitivity of coral reef ecosystems to ocean acidification (DeCarlo et al., 2017). In long-term studies done in mesocosms, carbonate balance of reefs tips toward overall dissolution under concentrations of CO2 of more than 450 ppm (Dove et al., 2013), which matches similar conclusions from previous experimental work (Anthony et al., 2008; Wild et al., 2011; Andersson and Gledhill, 2013) and from the geographical distribution of coral reefs in relation to the aragonite saturation state of seawater (Kleypas et al., 1999b; Hoegh-Guldberg et al., 2007).
The strong relationship between short periods of elevated sea temperature in mass coral bleaching and mortality within warm-water coral reefs has been used to project how communities of reef building corals might change as ocean temperatures increase as a result of anthropogenic climate change (Hoegh-Guldberg, 1999; Done et al., 2003; Donner et al., 2005; Frieler et al., 2012). Inherent to the conclusions of these studies, however, is the requirement that the thermal threshold of corals remain relatively constant over time. Evidence from the past 25 years, over which time satellite measurement programmes have used a simple algorithm based on sea surface temperature anomalies (relative to the average summer-time maxima 1985–1993) to predict when and where mass coral bleaching and mortality is likely to occur. This strongly suggests that little change has occurred in the sensitivity of reef building corals to thermal stress (Eakin C. et al., 2010; Strong et al., 2011; Hoegh-Guldberg, 2012). Nonetheless, it is important to consider potential evolutionary responses of reef-building corals over the next 100 years as well as the potential for coral reef ecosystems to relocate, as conditions change. Due to the dearth of information available about mesophotic and cold-water corals, this discussion will be restricted to the evidence for warm-water coral reefs.
Other than dying, corals have the option of acclimatizing, evolving or relocating as conditions within a region become sub-optimal (Hoegh-Guldberg, 2014a). Reef -building corals, like all organisms, can adjust their phenotype or acclimate to match local conditions to some extent (Gates and Edmunds, 1999; Middlebrook et al., 2010, 2012). However, there is little or no evidence that acclimatization has resulted in an upward shift in the thermal tolerance of reef-building corals (Eakin C. et al., 2010; Hoegh-Guldberg, 2012; Hoegh-Guldberg et al., 2014). Corals appear able to shift the relative ratio of different genetic clades or varieties of Symbiodinium within the one coral colony, which is correlated with tolerance to extreme temperatures (Rowan et al., 1997; Berkelmans and van Oppen, 2006; Jones et al., 2008). Further investigation of these putatively more tolerant varieties reveals a physiological trade-off in terms of reduced growth and competitiveness (Jones and Berkelmans, 2011).
A few studies (Glynn et al., 2001; Maynard et al., 2008a,b) have proposed that the thermal tolerance of reef building corals has increased over time, with less corals bleaching for similar amounts of thermal stress. The problem with these studies is several-fold (Hoegh-Guldberg, 2009). For example, the assessment of stress levels was restricted to temperature alone despite the fact that variation in parameters such as the light intensity (Hoegh-Guldberg, 1999; Mumby et al., 2001) and water flow rates over a reef (Nakamura and Van Woesik, 2001) can significantly modify the overall stress levels arising from elevated temperature at small scales (Hoegh-Guldberg, 2014a). As well, studies like that of Maynard et al. (2008a) investigated community level responses, and hence are unable to distinguish the loss of fragile species as opposed to the specific acclimatization and/or adaptation of individual species. Evidence for acclimatization in other reef organisms has exposed some intriguing possibilities, such as transgenerational acclimatization, where organisms inherit improved tolerances from parents that have been previously exposed to high levels of stress. For example, some coral reef fishes being exposed to higher CO2 levels prior to producing the next generation (Donelson et al., 2012; Miller et al., 2012). Whether or not this mechanism operates within corals is unknown, although the very observation that the same satellite temperature threshold still works after more than 25 years is evidence that thresholds are not changing very rapidly. As observed by Donner et al. (2005), the required rate of adaptation needs to match the rate of increase in sea temperatures or ~0.1–0.2°C per decade.
Genetic adaptation has also been suggested as a mechanism by which coral populations might be able to keep up with rapid changes in ocean temperature. Like all organisms, corals and their symbionts have adapted to local temperature conditions, a fact embodied by the fact that thresholds used by satellites for predicting mass coral bleaching and mortality are tied closely to local temperature conditions (Strong et al., 2011). Adapting to local conditions, however, has probably taken hundreds if not thousands of years and is slowed by the fact that reef building corals have generation times from 5 to over 100 years (Babcock, 1991). As a result, reef building corals do not have the population characteristics that would favor rates of evolution that would enable them to keep up with an environment that is changing faster than any time in the past 65 million years if not 300 million years (Hönisch et al., 2012). Several researchers have suggested that corals might “evolve” by swapping their symbionts for more thermally adapted varieties (Buddemeier and Fautin, 1993). Evidence of this, however, has not eventuated. These propositions also suffer from the problem that both the coral and the symbiont need to adapt to temperature change (Hoegh-Guldberg et al., 2002; Stat et al., 2006, 2009; Hoegh-Guldberg, 2012, 2014a). There are several observations of the shuffling of strains of Symbiodinium within the one host in response to warming (Rowan et al., 1997). These changes, however, are examples of acclimatization as opposed to genetic adaptation (Hoegh-Guldberg et al., 2002). In this regard, the advent of completely new symbiotic association between coral and a novel strain of Symbiodinium, hence a “new symbiotic genotype” has never been observed.
A third and final response by organisms facing rapidly changing conditions might be to relocate to new areas, which has been documented for a large number of marine plants and animals (Poloczanska et al., 2013, 2014). New records of several coral reef species have been reported at high latitude locations (Precht and Aronson, 2004; Yamano et al., 2011) which is consistent with the proposition that corals might shift to higher latitudes. There is also ample evidence that small increases in ocean temperature in the past have resulted in the appearance of coral reefs at slightly higher latitudes than where they are found today (Precht and Aronson, 2004; Greenstein and Pandolfi, 2008). While these reports are interesting, they are not sufficient to support the notion that whole coral reef ecosystems will shift successfully to higher latitudes as anthropogenic warming of the ocean continues which raises some important considerations. Firstly, how will ecosystem structure and function of coral reefs be affected if only a portion of species in the ecosystem shift, and which of these are the critical components for ecosystem services. Secondly, reduced light levels along with decreasing aragonite saturation are also critical factors in determining whether or not carbonate coral reef ecosystems will form successfully at higher latitudes. As recognized by Greenstein and Pandolfi (2008), other factors (e.g., available shallow water shelf habitats) are crucially important for determining whether or not coral reef ecosystems will be able to move to higher latitudes. Thirdly, analogies to past shifts are limited given that current changes on coral reefs today include a multitude of other pressures in addition to temperature (e.g., pollution, ocean acidification). And finally, shifts in the past occurred over long periods of time during which conditions were relatively stable as compared to the extremely rapid changes typical of today. Current changes in ocean temperature and acidity will continue for centuries, if not millennia, under the current greenhouse gas emission pathway, thereby severely limiting the ability of populations and adaptive processes to keep up with a rapidly changing climate (Hoegh-Guldberg, 2012).
The close relationship between mass coral bleaching and mortality, and short periods of elevated sea temperature, provides an opportunity to explore how warm-water coral reefs are likely to be affected under different climate change scenarios (Hoegh-Guldberg, 1999). Using projections of sea surface temperature (SST), future temperatures could be compared to established thermal thresholds for corals, and the frequency and intensity of future mass coral bleaching and estimated mortality. This led to the conclusion, which was somewhat controversial at the time, that coral reefs would experience mass coral bleaching and mortality on a yearly basis as early as 2030–2040. With field observations concluding that recovery from disturbances such as mass coral bleaching and mortality takes at least 10–20 years, the predictions of yearly mass coral bleaching and mortality events suggest strongly that coral dominated ecosystems would be unable to cope, and would start to disappear around this time. Subsequent studies revealed that these conclusions are not far-fetched and matched the expectations of a thermal threshold of corals was relatively fixed, as it appears to be (Hoegh-Guldberg, 1999; Done et al., 2003; Donner et al., 2005; Eakin C. et al., 2010; Eakin C. M. et al., 2010; Frieler et al., 2012).
Hoegh-Guldberg et al. (2014) repeated the analysis of Hoegh-Guldberg (1999) using Coupled Model Intercomparison Project Phase 5 (CMIP5) data from an ensemble of 10–16 independent models. Historical and unforced temperature trends were compared with those from Representative Concentration Pathway (RCP) 2.6 (global temperature “about as likely as not” to exceed 1.5°C by 2100 relative to 1986–2005) and RCP 8.5 (global temperature “very likely” to exceed 3.0°C by 2100 relative to 1986–2005) in terms of model projections of the future for coral reef provinces. Model outputs were constrained to geographic areas (coral regions) known to contain warm-water coral reefs. The range in each case represents differences between models and model assumptions. Three things become apparent. First, the amount of SST warming that we have seen so far is very significant in each coral region given that average global temperature has warmed by 0.85°C over the period 1880–2012 (Table 1, Figure 6). Second, differences between the two RCP scenarios do not become evident until mid to late century (Table 2, Figure 6). Third, only conditions associated with the RCP 2.6 scenario stabilize, which is important if evolutionary processes are to be able to operate and re-establish coral reef ecosystems in these regions. In the context of the preceding discussion, this is the only scenario in which coral reefs have any chance of replenishing tropical coastal regions.
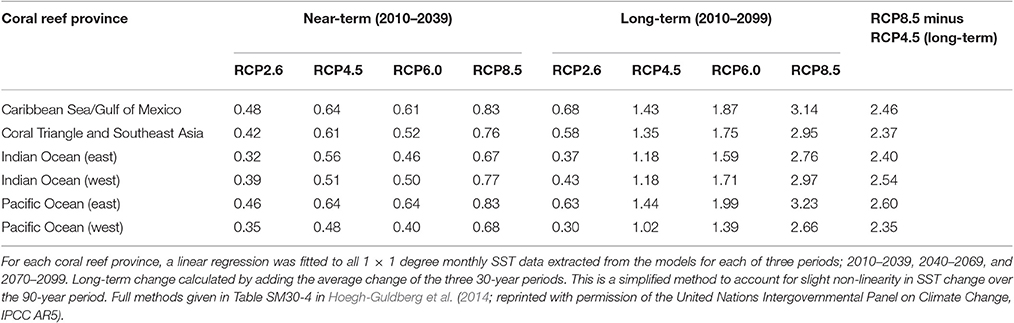
Table 2. Projected changes in sea surface temperature (SST °C) over the next 90 years for coral reef provinces (Figure 1B) from AOGCM model simulations from the Coupled Model Intercomparison Project Phase 5 (CMIP5, http://cmip-pcmdi.llnl.gov/cmip5/).
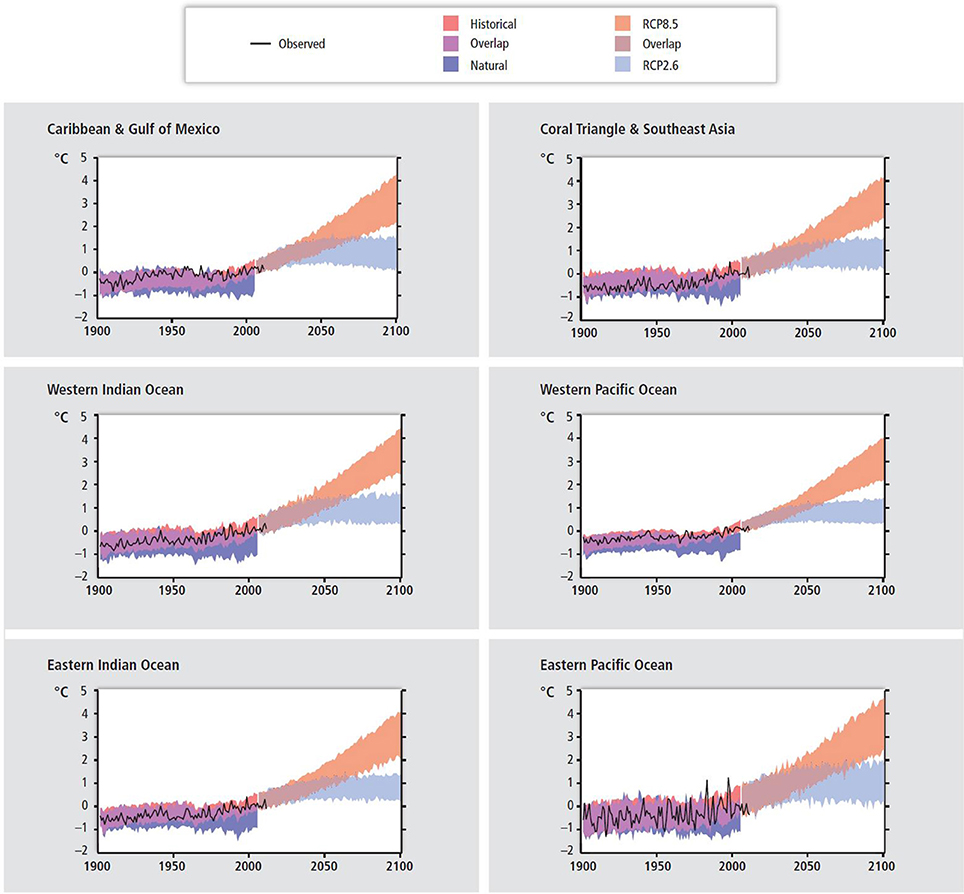
Figure 6. Past and future sea surface temperatures (SST) in six major coral reef provinces and locations (Figure 1B) under historic, unforced (natural), and Representative Concentration Pathways (RCP)4.5 and 8.5 scenarios from Coupled Model Intercomparison Project Phase 5 (CMIP5) ensembles (see Table SM30-3 in Hoegh-Guldberg et al., 2014). Observed and simulated variation in past and projected annual SST over various sites where coral reefs are prominent ecosystems. The black line shows estimates from the Hadley Centre Interpolated sea surface temperature 1.1 (HADISST1.1) data set (Rayner et al., 2003) reconstructed historical SST dataset. Shading denotes the 5–95 percentile range of climate model simulations driven with “historical”changes in anthropogenic and natural drivers (62 simulations), historical changes in “natural” drivers only (25), the RCP4.5 emissions scenario (62), and the RCP8.5 (62). Data are anomalies from the 1986 to 2006 average of the HADISST1-1 data (for the HadISST1.q time series) or of the corresponding historical all-forcing simulations. Figure SM30-3 with the permission of IPCC AR5 (Hoegh-Guldberg et al., 2014).
Hoegh-Guldberg et al. (2014) also looked at the annual incidence of bleaching and mortality events. The proportion of a coral grid cells with a reef province (Figures 1B, 7A) that would have a particular stress level in any 1 year was calculated and the maximum for each decade then plotted. Two levels of stress were examined. The first being the amount of warming required to trigger mass bleaching, which is around one Degree Heating Months (DHM) (Strong et al., 2011) and is shown in Figure 7B. The second was the amount of heat stress required to trigger mass mortality events like those that occurred in the Maldives, Okinawa, North-West Australia and Palau in 1998, and is calculated as five Degree Heating Months Hoegh-Guldberg, 1999; Figure 7C). The conclusions from this analysis are very clear. Firstly, the risk of mass coral bleaching (DHM ≥ 1) increases steadily over the next few decades, affecting all regions of the Caribbean, Gulf of Mexico and eastern Pacific. By contrast, the Western Pacific Ocean, Coral Triangle, and Indian Ocean are likely to experience less stress and will still have large areas unaffected by annual mass coral bleaching by the end of the century. Secondly, conditions that drive mass mortality events today (DHM > 5) will spread across most regions by the end of the century under RCP 8.5. This risk decreases from RCP 8.5 to zero under RCP 2.6 with no regions experiencing annual conditions that would cause mass mortality event. Given the time that it takes for coral reefs to recover from mass mortality events (10–20 years), there is significant risk associated with high greenhouse gas emission scenarios given that the damage from these events, even in managed reef systems. Even 10% of grid cells being at risk of experiencing a mass mortality event would eventually add up to a very low number of unaffected areas by the end of century.
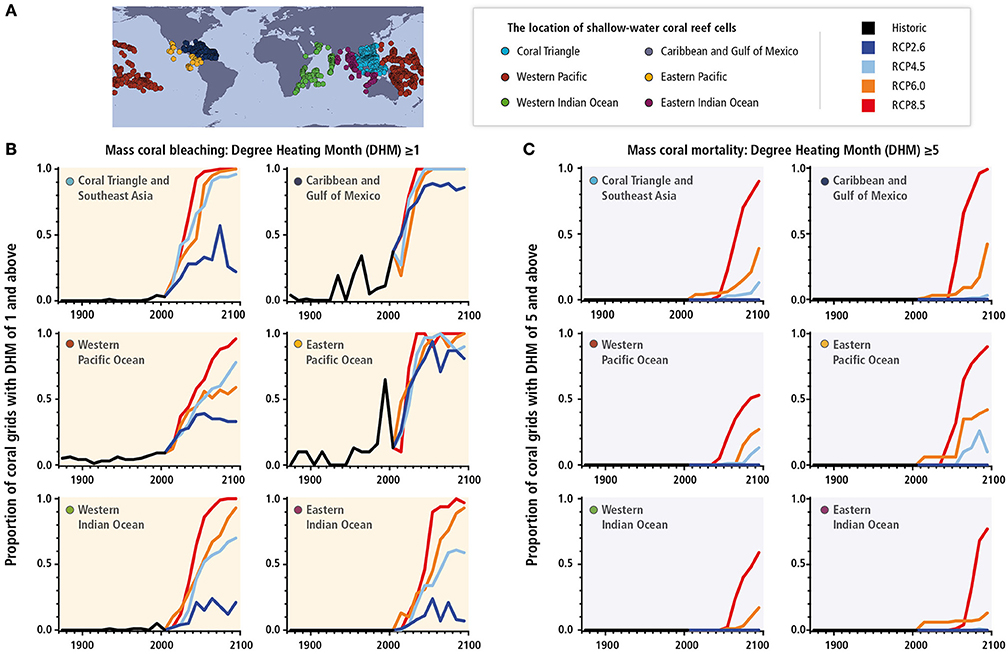
Figure 7. Annual maximum proportions of reef pixels with Degree Heating Months (DHM, Donner et al., 2007) for each of the six coral regions (A). (B) DHM ≥1 (used for projecting the incidence of coral bleaching; Strong et al., 1997, 2011) and (C) DHM ≥5 (associated with bleaching followed by significant mortality; Eakin C. M. et al., 2010) for the period 1870–2009 using the Hadley Centre Interpolated sea surface temperature 1.1 (HadISST1.1) data set. The black line on each graph is the maximum annual area value for each decade over the period 1870–2009. This value is continued through 2010–2099 using Coupled Model Intercomparison Project Phase 5 (CMIP5) data and splits into the four Representative Concentration Pathways (RCP2.6, 4.5, 6.0, and 8.5). DHM were produced for each of the four RCPs using the ensembles of CMIP models. From these global maps of DHM, the annual percentage of grid cells with DHM ≥1 and DHM ≥5 were calculated for each coral region. These data were then grouped into decades from which the maximum annual proportions were derived. The plotted lines for 2010–2099 are the average of these maximum proportion values for each RCP. Monthly sea surface temperature anomalies were derived using a 1985–2000 maximum monthly mean climatology derived in the calculations for Figure 30-4 in Hoegh-Guldberg et al. (2014). This was done separately for HadISST1.1, the CMIP5 models, and each of the four RCPs, at each grid cell for every region. DHMs were then derived by adding up the monthly anomalies using a 4-month rolling sum. Figure SM30-3 presents past and future sea temperatures for the six major coral reef provinces under historic, un-forced (no anthropogenic forcing), RCP4.5 and RCP8.5 scenarios. Reprinted with permission of the PCC AR5, Figure 30-10 (Hoegh-Guldberg et al., 2014).
Considering cold-water coral ecosystems, at pre-industrial atmospheric CO2 level, 9% of known cold-water coral ecosystems were in under-saturated water (Cao et al., 2014). Under emission scenario IS92a (atmospheric CO2 concentration 713 ppm and temperature increase of about 2.4°C by 2100), an estimated 70% of cold-water corals could be in under-saturated water by the end of the century with some ecosystems experiencing under-saturation by 2020s (Guinotte et al., 2006; Turley et al., 2007). Even if mitigation efforts (e.g., through geoengineering) could reduce atmospheric CO2 levels to pre-industrial by the end of the century, the lag in the recovery of deep ocean chemistry would result in longer-lasting threats to cold-water coral ecosystems (Cao et al., 2014).
Overall, the evidence presented above confirms earlier work (Hoegh-Guldberg, 1999; Done et al., 2003; Donner et al., 2005; Frieler et al., 2012) and substantiates the serious concern regarding the vulnerability of carbonate coral reef systems to a rapidly changing world. Given the importance of coastal ecosystems such as warm-water coral reefs for hundreds of millions of humans (Burke et al., 2011; Hoegh-Guldberg, 2015), these changes are likely to have implications for people and livelihoods, as well as regional security in some instances. It is also clear that we must increase our understanding of the effects of warming and acidifying oceans on mesophotic and cold-water coral reefs. These coral reefs represent important stores of biodiversity as well as habitat for fish, many of which are commercially important. As the deep ocean warms, the aragonite saturation horizon shoals and dissolved oxygen declines, it will be important to understand how these ecosystems will be affected. It will also be important to get a better understanding of how environmental conditions differ in the case of mesophotic reefs and whether or not they have the potential to act as refugia for coral reef species from the greater environmental extremes of shallow regions (Bongaerts et al., 2010a, 2013, 2015).
With regard to cold-water corals, management interventions are likely to be limited to regulating or banning fishing and mineral extraction in the locality of reefs (Thresher et al., 2011). The highest priority for these sensitive ecosystems is to locate and protect sites that are likely to be refugia areas (Thresher et al., 2015).
The recent consensus of the Intergovernmental Panel on Climate Change (IPCC, 2014) identified a number of risks and vulnerabilities for coral reefs under rapid ocean warming and acidification, as well as exploring the ramifications and adaptation options (see Table 30.4 in Hoegh-Guldberg et al., 2014). Changes to the structure of ecosystems such as a loss of coral reefs, underpin a series of risks and vulnerabilities to fisheries production and consequently food and income security in tropical regions thus rates of unemployment and poverty. As coral reef ecosystems degrade or disappear, there is the risk that coastal fisheries production is reduced, decreasing food security and increasing unemployment. There is also a risk that the tourist appeal of tropical coastal assets may decrease as ecosystems take on less desirable states (i.e., from coral to seaweed domination), affecting the potential to attract tourist dollars. Reduced availability to food and income is likely to exacerbate coastal poverty in many equatorial countries. Strategies to reduce risks in both these cases involve strengthening integrated coastal zone management to reduce contributing stresses such as coastal pollution, overexploitation and physical damage to coastal resources.
As outlined by Poloczanska et al. (2013, 2014, 2016), marine species are already redistributing toward higher latitudes. This has the potential to reorganize ecosystems including commercial fish stocks, drive changes to the distribution, and abundance of predators and prey, as well as increasing the risks of invasive species taking hold in new locations and ecosystems. Changes to the distribution of fish species in coral reef regions are expected as oceans warm (Cheung et al., 2010; Pörtner et al., 2014; García Molinos et al., 2015). This has the potential to change national income in either direction, depending on location, as important fisheries stocks redistribute, increasing the likelihood of disputes over national ownership of fisheries resources (Robinson J. et al., 2010). In this regard, key management actions to reduce risks include increased international cooperation over key fisheries as well as developing a better understanding of the linkages between ocean productivity, recruitment and fisheries stock levels (Bell et al., 2011). International cooperation on measures which enable sustainable fishing of these valuable stocks that take into account the influence of climate change across international boundaries, are important adaptation measures that need to be implemented as soon as possible (Robinson J. et al., 2010). Changing ecosystem structures in a warming and acidifying ocean is also likely to increase the risk of disease such as ciguatera and harmful algal blooms, with ramifications for human health and well-being. Strategies in this case involve increased monitoring and education surrounding key risks, plus the development of alternative fisheries and income for periods in which disease incident increases (Bell et al., 2013).
A recurrent theme within this review is the fact that we are already seeing major and fundamental change occurring in the world's ocean in response to climate change and that the rate of change is largely outstripping the ability for coral reefs to adapt genetically or relocate. If greenhouse gas emissions are not mitigated, it is very clear that the ocean will be a vastly different place by the mid to late century (Gattuso et al., 2015). It is also clear that there are few or no adaptation strategies for humans to counter the risks of ocean warming and acidification at global scales. If they did exist, they would almost certainly be prohibitively expensive relative to the costs of developing solutions to the unprecedented rise of CO2 in the earth's atmosphere.
This leaves us with two clear options with respect to preserving invaluable ecosystems such as coral reefs. The first is to stabilize planetary temperature and CO2 concentrations as quickly as possible. Only then will biological responses such as acclimation and genetic adaptation have any chance of operating. The second is to dramatically reduce local stresses which are currently acting on coral reefs and which are reducing their resilience to climate change. By reducing these non-climate stresses, coral reefs will have the opportunity to develop greater robustness or resilience to the challenges of a changing planet. However, if this is not combined with stabilization of temperatures and acidification then it is likely to only temporarily put off the inevitable. If we do these two things, there is a chance that conditions will stabilize on planet earth by mid-to-late century, ensuring that some of the spectacular coral reef ecosystems will be able to flourish across the world's tropical regions.
OH led the project and wrote 50% of this manuscript. EP contributed to core concepts in the manuscript and contributed 30% of text. SD and WS contributed 15 and 5% respectively to the writing.
Funded primarily by the Australian Research Council (Canberra; FL120100066, LP110200874, CE140100020), The University of Queensland, and the National Oceanic and Atmospheric Administration (Washington, DC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are grateful for support from the Australian Government, Australian Research Council, National Oceanic and Atmospheric Administration, and Queensland Government. OH was supported by an ARC Laureate Fellowship during the development and publications of this study, and was a member of the ARC Centre for Excellence in Coral Reef Studies through the University of Queensland. SD and OH would like to recognize the generous support of the Centre Scientifique de Monaco for the final analysis and writing stages of this study.
Andersson, A. J., and Gledhill, D. (2013). Ocean acidification and coral reefs: effects on breakdown, dissolution, and net ecosystem calcification. Annu. Rev. Mar. Sci. 5, 321–348. doi: 10.1146/annurev-marine-121211-172241
Anthony, K., Kline, D., Diaz-Pulido, G., Dove, S., and Hoegh-Guldberg, O. (2008). Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. U.S.A. 105, 17442–17446. doi: 10.1073/pnas.0804478105
Babcock, R. C. (1991). Comparative demography of three species of scleractinian corals using age-and size-dependent classifications. Ecol. Monogr. 61, 225–244. doi: 10.2307/2937107
Baker, A., Glynn, P. W., and Riegl, B. (2008). Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast. Shelf Sci. 80, 435–471. doi: 10.1016/j.ecss.2008.09.003
Barnes, D. D., and Chalker, B. B. (1990). “Calcification and photosynthesis in reef-building corals and algae,” in Coral Reefs. Ecosystems of the World, Vol. 25, ed Z. Dubinsky (Amsterdam: Elsevier Science Publishing), 109–131.
Bell, J. D., Ganachaud, A., Gehrke, P. C., Griffiths, S. P., Hobday, A. J., Hoegh-Guldberg, O., et al. (2013). Mixed responses of tropical Pacific fisheries and aquaculture to climate change. Nat. Clim. Change 3, 591–599. doi: 10.1038/nclimate1838
Bell, J., Reid, C., Batty, M., Allison, E., Lehodey, P., Rodwell, L., et al. (2011). “Implications of climate change for contributions by fisheries and aquaculture to Pacific Island economies and communities,” in Vulnerability of Tropical Pacific Fisheries and Aquaculture to Climate Change, eds J. D. Bell, J. E. Johnson, and A. J. Hobday (Noumea: Secretariat of the Pacific Community), 733–801.
Berkelmans, R., and van Oppen, M. J. (2006). The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc. Biol. Sci. 273, 2305–2312. doi: 10.1098/rspb.2006.3567
Bongaerts, P., Bridge, T. C. L., Kline, D., Muir, P., Wallace, C., Beaman, R., et al. (2011). Mesophotic coral ecosystems on the walls of Coral Sea atolls. Coral Reefs 30, 335–335. doi: 10.1007/s00338-011-0725-7
Bongaerts, P., Frade, P. R., Hay, K. B., Englebert, N., Latijnhouwers, K. R. W., Bak, R. P. M., et al. (2015). Deep down on a Caribbean reef: lower mesophotic depths harbor a specialized coral-endosymbiont community. Sci. Rep. 5:7652. doi: 10.1038/srep07652
Bongaerts, P., Muir, P., Englebert, N., Bridge, T. C. L., and Hoegh-Guldberg, O. (2013). Cyclone damage at mesophotic depths on Myrmidon Reef (GBR). Coral Reefs 32, 935–935. doi: 10.1007/s00338-013-1052-y
Bongaerts, P., Ridgway, T., Sampayo, E., and Hoegh-Guldberg, O. (2010a). Assessing the ‘deep reef refugia’ hypothesis: focus on Caribbean reefs. Coral Reefs 29, 309–327. doi: 10.1007/s00338-009-0581-x
Bongaerts, P., Riginos, C., Ridgway, T., Sampayo, E. M., van Oppen, M. J. H., Englebert, N., et al. (2010b). Genetic divergence across habitats in the widespread coral Seriatopora hystrix and its associated Symbiodinium. PLoS ONE 5:e10871. doi: 10.1371/journal.pone.0010871
Bostock, H. C., Tracey, D. M., Currie, K. I., Dunbar, G. B., Handler, M. R., Mikaloff Fletcher, S. E., et al. (2015). The carbonate mineralogy and distribution of habitat-forming deep-sea corals in the southwest pacific region. Deep Sea Res. I 100, 88–104. doi: 10.1016/j.dsr.2015.02.008
Bruno, J. F., and Selig, E. R (2007). Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS ONE 2:e711. doi: 10.1371/journal.pone.0000711
Buddemeier, R. W., and Fautin, D. G. (1993). Coral bleaching as an adaptive mechanism - a testable hypothesis. Bioscience 43, 320–326. doi: 10.2307/1312064
Burke, L., Reytar, K., Spalding, M., and Perry, A. (2011). Reefs at Risk Revisited. Washington, DC: World Resources Institute.
Burrows, M. T., Schoeman, D. S., Buckley, L. B., Moore, P., Poloczanska, E. S., Brander, K. M., et al. (2011). The pace of shifting climate in marine and terrestrial ecosystems. Science 334, 652–655. doi: 10.1126/science.1210288
Burrows, M. T., Schoeman, D., Richardson, A., García Molinos, J. G., Hoffmann, A., Buckley, L., et al. (2014). Geographical limits to species-range shifts are suggested by climate velocity. Nature 507, 492–495. doi: 10.1038/nature12976
Caldeira, K., and Wickett, M. E. (2003). Oceanography: anthropogenic carbon and ocean pH. Nature 425, 365–365. doi: 10.1038/425365a
Callaghan, J., and Power, S. B. (2011). Variability and decline in the number of severe tropical cyclones making land-fall over eastern Australia since the late nineteenth century. Clim. Dynam. 37, 647–662. doi: 10.1007/s00382-010-0883-2
Cantin, N. E., Cohen, A. L., Karnauskas, K. B., Tarrant, A. M., and McCorkle, D. C. (2010). Ocean warming slows coral growth in the central Red Sea. Science 329, 322–325. doi: 10.1126/science.1190182
Cao, L., Zhang, H., Zheng, M., and Wang, S. (2014). Response of ocean acidification to a gradual increase and decrease of atmospheric CO2. Environ. Res. Lett. 9:024012. doi: 10.1088/1748-9326/9/2/024012
Chan, N. C. S., and Connolly, S. R. (2013). Sensitivity of coral calcification to ocean acidification: a meta-analysis. Glob. Change Biol, 19, 282–290. doi: 10.1111/gcb.12011
Cheung, W. W. L., Lam, V. W. Y., Sarmiento, J. L., Kearney, K., Watson, R., Zeller, D., et al. (2010). Large scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Glob. Change Biol. 16, 24–35. doi: 10.1111/j.1365-2486.2009.01995.x
Chu, S. N., Wamg, Z. A., Doney, S. C., Lawson, G. L., and Hoering, K. A. (2016). Changes in anthropogenic carbon storage in the Northeast Pacific in the last decade. J. Geophys. Res. Oceans 121, 4618–4632. doi: 10.1002/2016JC011775
Cinner, J. E., McClanahan, T. R., Daw, T. M., Graham, N. A., Maina, J., Wilson, S. K., et al. (2009). Linking social and ecological systems to sustain coral reef fisheries. Curr. Biol. 19, 206–212. doi: 10.1016/j.cub.2008.11.055
Comeau, S., Tambutte, E., Carpenter, R. C., Edmunds, P. J., Evensen, N. R., Allemand, D., et al. (2017). Coral calcifying fluid pH is modulated by seawater carbonate chemistry not soley seawater pH. Proc. Natl. Acad. Sci. U.S.A. 284:20161669. doi: 10.1098/rspb.2016.1669
Costanza, R., de Groot, R., Sutton, P., van der Ploeg, S., Anderson, S. J., Kubiszewski, I., et al. (2014). Changes in the global value of ecosystem services. Glob. Environ. Change 26, 152–158. doi: 10.1016/j.gloenvcha.2014.04.002
D'Olivo, J., McCulloch, M., and Judd, K. (2013). Long-term records of coral calcification across the central Great Barrier Reef: assessing the impacts of river runoff and climate change. Coral Reefs 32, 999–1012. doi: 10.1007/s00338-013-1071-8
De'ath, G., Fabricius, K. E., Sweatman, H., and Puotinen, M. (2012). The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. U.S.A. 109, 17995–17999. doi: 10.1073/pnas.1208909109
De'ath, G., Lough, J. M., and Fabricius, K. E. (2009). Declining coral calcification on the Great Barrier Reef. Science 323, 116–119. doi: 10.1126/science.1165283
DeCarlo, T. M., Cohen, A. L., Wong, G. T. F., Shiah, F.-K., Lentz, S. J., Davis, K. A., et al. (2017). Community production modulates coral reef pH and the sensitivity of ecosystem calcification to ocean acidification. J. Geophys. Res. 122, 745–761. doi: 10.1002/2016JC012326
Dixson, D. L., Munday, P. L., and Jones, G. P. (2010). Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 13, 68–75. doi: 10.1111/j.1461-0248.2009.01400.x
Done, T., Whetton, P., Jones, R., Berkelmans, R., Lough, J., Skirving, W., et al. (2003). Global Climate Change and Coral Bleaching on the Great Barrier Reef. Final Report to the State of Queensland Greenhouse Taskforce through the Department of Natural Resources and Minings, Brisbane.
Donelson, J., Munday, P., McCormick, M., and Pitcher, C. (2012). Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat. Clim. Change 2, 30–32. doi: 10.1038/nclimate1323
Doney, S., Fabry, V., Feely, R., and Kleypas, J. (2009). Ocean acidification: the other CO2 problem. Ann. Rev. Mar. Sci. 1, 169–192. doi: 10.1146/annurev.marine.010908.163834
Donner, S. D., Knutson, T. R., and Oppenheimer, M. (2007). Model-based assessment of the role of human-induced climate change in the 2005 Caribbean coral bleaching event. Proc. Natl. Acad. Sci. U.S.A. 104, 5483–5488. doi: 10.1073/pnas.0610122104
Donner, S. D., Skirving, W. J., Little, C. M., Oppenheimer, M., and Hoegh-Guldberg, O. (2005). Global assessment of coral bleaching and required rates of adaptation under climate change. Glob. Change Biol. 11, 2251–2265. doi: 10.1111/j.1365-2486.2005.01073.x
Dove, S. G., Kline, D. I., Pantos, O., Angly, F. E., Tyson, G. W., and Hoegh-Guldberg, O. (2013). Future reef decalcification under a business-as-usual CO2 emission scenario. Proc. Natl. Acad. Sci. U.S.A. 110, 15342–15347. doi: 10.1073/pnas.1302701110
Durack, P. J., and Wijffels, S. E. (2010). Fifty-year trends in global ocean salinities and their relationship to broad-scale warming. J. Clim. 23, 4342–4362. doi: 10.1175/2010JCLI3377.1
Durack, P. J., Wijffels, S. E., and Matear, R. J. (2012). Ocean salinities reveal strong global water cycle intensification during 1950 to 2000. Science 336, 455–458. doi: 10.1126/science.1212222
Eakin, C. M., Morgan, J. A., Heron, S. F., Smith, T. B., Liu, G., Alvarez-Filip, L., et al. (2010). Caribbean corals in crisis: record thermal stress, bleaching, and mortality in 2005. PLoS ONE 5:e13969. doi: 10.1371/journal.pone.0013969
Eakin, C., Donner, S., Logan, C., Gledhill, D., Liu, G., Heron, S., et al. (2010). “Thresholds for coral bleaching: Are synergistic factors and shifting thresholds changing the landscape for management?” in Fall Meeting 2010 (San Francisco, CA: American Geophysical Union).
Englebert, N., Bongaerts, P., Muir, P., Hay, K., and Hoegh-Guldberg, O. (2014). Deepest zooxanthellate corals of the Great Barrier Reef and Coral Sea. Mar. Biodivers. 45, 1–2, doi: 10.1007/s12526-014-0221-8
Esbaugh, A. J., Heuer, R., and Grosell, M. (2012). Impacts of ocean acidification on respiratory gas exchange and acid–base balance in a marine teleost, Opsanus beta. J. Comp. Physiol. B 182, 921–934. doi: 10.1007/s00360-012-0668-5
Fang, J. K. H., Mello-Athayde, M. A., Schoenberg, C. H. L., Kline, D. I., Hoegh-Guldberg, O., and Dove, S. (2013). Sponge biomass and bioerosion rates increase under ocean warming and acidification. Glob. Change Biol. 19, 3581–3591. doi: 10.1111/gcb.12334
Fisher, R., O'Leary, R. A., Low-Choy, S., Mengersen, K., Knowlton, N., Brainard, R. E., et al. (2015). Species richness on coral reefs and the pursuit of convergent global estimates. Curr. Biol. 25, 500–505. doi: 10.1016/j.cub.2014.12.022
Form, A. U., and Riebesell, U. (2012). Acclimation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa. Glob. Change Biol. 18, 843–853. doi: 10.1111/j.1365-2486.2011.02583.x
Fosså, J. H., Lindberg, B., Christensen, O., Lundälv, T., Svellingen, I., Mortensen, P. B., et al. (2005). “Mapping of Lopehlia reefs in Norway: experiences and survey methods,” in Cold-Water Corals and Ecosystems, eds A. Freiwald and J. M. Roberts (Berlin; Heidelberg: Springer), 359–391.
Fosså, J., Mortensen, P., and Furevik, D. (2002). The deep-water coral Lophelia pertusa in Norwegian waters: distribution and fishery impacts. Hydrobiologia 471, 1–12. doi: 10.1023/A:1016504430684
Freiwald, A., Fosså, J. H., Grehan, A., Koslow, T., and Roberts, J. M. (2004). Cold-Water Coral Reefs. Cambridge: UNEP-WCMC.
Frieler, K., Meinshausen, M., Golly, A., Mengel, M., Lebek, K., Donner, S. D., et al. (2012). Limiting global warming to 2°C is unlikely to save most coral reefs. Nat. Clim. Change 3, 165–170. doi: 10.1038/nclimate1674
García Molinos, J., Halpern, B. S., Schoeman, D. S., Brown, C. J., Kiessling, W., Moore, P. J., et al. (2015). Climate velocity and the future global redistribution of marine biodiversity. Nat. Clim. Change 6, 83–88. doi: 10.1038/nclimate2769
Gardner, T., Côté, I., Gill, J., Grant, A., and Watkinson, A. (2003). Long-term region-wide declines in Caribbean corals. Science 30, 958–960. doi: 10.1126/science.1086050
Gates, R. D., and Edmunds, P. J. (1999). The physiological mechanisms of acclimatization in tropical reef corals. Am. Zool. 39, 30–43. doi: 10.1093/icb/39.1.30
Gattuso, J.-P., Brewer, P. G., Hoegh-Guldberg, O., Kleypas, J. A., Pörtner, H. O., and Schmidt, D. N. (2014a). “Cross-chapter box on ocean acidification,” in Climate Change 2014: Impacts, Adaptation, and Vulnerability Part A: Global and Sectoral Aspects Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel of Climate Change, eds. C. B. Field, V. R. Barros, D. J. Dokken, K. J. Mach, M. D. Mastrandrea, T. E. Bilir, et al. (Cambridge; New York, NY: Cambridge University Press), 129–131.
Gattuso, J.-P., Hoegh-Guldberg, O., and Pörtner, H. O. (2014b). “Cross-chapter box on coral reefs,” in Climate Change 2014: Impacts, Adaptation, and Vulnerability Part A: Global and Sectoral Aspects Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel of Climate Change, eds C. B. Field, V. R. Barros, D. J. Dokken, K. J. Mach, M. D. Mastrandrea, T. E. Bilir, et al. (Cambridge; New York, NY: Cambridge University Press), 97–100.
Gattuso, J.-P., Magnan, A., Billé, R., Cheung, W., Howes, E., Joos, F., et al. (2015). Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 349:aac4722. doi: 10.1126/science.aac4722
Gattuso, J.-P., Frankignoulle, M., Bourge, I., Romaine, S., and Buddemeier, R. W. (1998). Effect of calcium carbonate saturation of seawater on coral calcification. Glob. Planet. Change 18, 37–46.
Glynn, P. W. (1993). Coral reef bleaching: ecological perspectives. Coral Reefs 12, 1–17. doi: 10.1007/BF00303779
Glynn, P. W. (2012). “Global warming and widespread coral mortality: evidence of first coral reef extinctions,” in Saving a Million Species: Extinction Risk from Climate Change, ed L. Hannah (Washington, DC: Island Press), 103–119.
Glynn, P. W., and Manzello, D. P. (2015). “Bioerosion and coral reef growth: a dynamic balance,” in Coral Reefs in the Anthropocene, ed C. Birkeland (Amsterdam: Springer Netherlands), 67–97.
Glynn, P. W., Maté, L. J., Baker, A. C., and Calderón, M. O. (2001). Coral bleaching and mortality in Panama and Ecuador during the 1997-1998 El Niño-Southern Oscillation Event: spatial/temporal patterns and comparisons with the 1982-1983 event. Bull. Mar. Sci. 69, 79–109. Available online at: http://www.ingentaconnect.com/search/article?option2=author&value2=Glynn&operator4=AND&option4=volume&value4=69&freetype=unlimited&sortDescending=true&sortField=default&pageSize=10&index=2#expand/collapse
Graham, N. A. J., Jennings, S., MacNeil, M. A., Mouillot, D., and Wilson, S. K. (2015). Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518, 94–97. doi: 10.1038/nature14140
Greenstein, B. J., and Pandolfi, J. M. (2008). Escaping the heat: range shifts of reef coral taxa in coastal Western Australia. Glob. Change Biol. 14, 513–528. doi: 10.1111/j.1365-2486.2007.01506.x
Guinotte, J. M., Orr, J., Cairns, S., Freiwald, A., Morgan, L., and George, R. (2006). Will human induced changes in seatwater chemistry alter the distribution of deep-sea scleractinian corals? Front. Ecol. Environ. 4, 141–146. doi: 10.1890/1540-9295(2006)004[0141:WHCISC]2.0.CO;2
Hall-Spencer, J., Allain, V., and Fosså, J. H. (2002). Trawling damage to Northeast Atlantic ancient coral reefs. Proc. R. Soc. Lond. B Biol. Sci. 269, 507–511. doi: 10.1098/rspb.2001.1910
Hamylton, S. M., Pescud, A., Leon, J. X., and Callaghan, D. P. (2013). A geospatial assessment of the relationship between reef flat community calcium carbonate production and wave energy. Coral Reefs 32, 1025–1039. doi: 10.1007/s00338-013-1074-5
Harvell, C. D., Kim, K., Burkholder, J. M., Colwell, R. R., Epstein, P. R., Grimes, D. J., et al. (1999). Emerging marine diseases - climate links and anthropogenic factors. Science 285, 1505–1510. doi: 10.1126/science.285.5433.1505
Harvell, D., Jordán-Dahlgren, E., Merkel, S., Rosenberg, E., Raymundo, L., Smith, G., et al. (2007). Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography 20, 172–195. doi: 10.5670/oceanog.2007.91
Hennige, S., Wicks, L., Kamenos, N., Perna, G., Findlay, H., and Roberts, J. (2015). Hidden impacts of ocean acidification to live and dead coral framework, Proc. R. Soc. Lond. B Biol. Sci. 282:20150990. doi: 10.1098/rspb.2015.0990
Hoegh-Guldberg, O. (1999). Coral bleaching, climate change and the future of the world's coral reefs. Mar. Freshw. Res. 50, 839–866. doi: 10.1071/MF99078
Hoegh-Guldberg, O. (2009). Climate change and coral reefs: Trojan horse or false prophecy? Coral Reefs 28, 569–575. doi: 10.1007/s00338-009-0508-6
Hoegh-Guldberg, O. (2012). The adaptation of coral reefs to climate change: is the Red Queen being outpaced? Sci. Mar. 76, 403–408. doi: 10.3989/scimar.03660.29A
Hoegh-Guldberg, O. (2014a). Coral reef sustainability through adaptation: glimmer of hope or persistent mirage? Curr. Opin. Environ. Sustain. 7, 127–133. doi: 10.1016/j.cosust.2014.01.005
Hoegh-Guldberg, O. (2014b). Coral reefs in the Anthropocene: persistence or the end of the line? Geol. Soc. Spec. Publ. 395, 167–183. doi: 10.1144/SP395.17
Hoegh-Guldberg, O. (2015). Reviving the Ocean Economy: the Case for Action - 2015. Gland: WWF International.
Hoegh-Guldberg, O., and Bruno, J. (2010). The impact of climate change on the world's marine ecosystems. Science 328, 1523–1528. doi: 10.1126/science.1189930
Hoegh-Guldberg, O., and Ridgway, T. (2016). Coral Bleaching Comes to the Great Barrier Reef as Record-Breaking Global Temperatures Continue. The Conversation, March 21, 2016 theconversation.com.
Hoegh-Guldberg, O., and Smith, G. J. (1989). The effect of sudden changes in temperature, light and salinity on the population density and export of zooxanthellae from the reef corals Stylophora pistillata and Seriatopra hystrix. J. Exp. Mar. Biol. Ecol. 129, 279–303. doi: 10.1016/0022-0981(89)90109-3
Hoegh-Guldberg, O., Cai, R., Poloczanska, E. S., Brewer, P. G., Sundby, S., Hilmi, K., et al. (2014). “The Ocean,” in Climate Change 2014: Impacts, Adaptation, and Vulnerability Part A: Global and Sectoral Aspects Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel of Climate Change, eds. C. B. Field, V. R. Barros, D. J. Dokken, K. J. Mach, M. D. Mastrandrea, T. E. Bilir, et al. (Cambridge; New York, NY: Cambridge University Press), 1655–1731.
Hoegh-Guldberg, O., Jones, R. J., Ward, S., and Loh, W. L. (2002). Is coral bleaching really adaptive? Nature 415, 601–602. doi: 10.1038/415601a
Hoegh-Guldberg, O., Mumby, P. J., Hooten, A. J., Steneck, R. S., Greenfield, P., Gomez, E., et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. doi: 10.1126/science.1152509
Hönisch, B., Ridgwell, A., Schmidt, D. N., Thomas, E., Gibbs, S. J., Sluijs, A., et al. (2012). The geological record of ocean acidification. Science 335, 1058–1063. doi: 10.1126/science.1208277
Hubbard, D. K. (2015). “Reef biology and geology – not just a matter of scale,” in Coral Reefs in the Anthropocene, ed C. Birkeland (Dordrecht: Springer Netherlands), 43–66.
Hughes, T. P. (1994). Catastrophes, phase shifts, and large-Scale degradation of a Caribbean coral reef. Science 265, 1547–1551. doi: 10.1126/science.265.5178.1547
Hughes, T. P., Rodrigues, M. J., Bellwood, D. R., Ceccarelli, D., Hoegh-Guldberg, O., McCook, L., et al. (2007). Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 17, 360–365. doi: 10.1016/j.cub.2006.12.049
Hutchings, P. A., and Hoegh-Guldberg, O. (2009). “Calcification, erosion and the establishment of the framework of coral reefs,” in Great Barrier Reef: Biology, Environment and Management, eds P. Hutchings, M. Kingsford, and O. Hoegh-Guldberg (Collingwood, VIC: CSIRO Publishing), 74–84.
IPCC (2013). “Climate change 2013: the physical science basis,” in Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, et al. (Cambridge; New York, NY: Cambridge University Press).
IPCC (2014). “Climate change 2014: impacts, adaptation, and vulnerability,” in Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds. V. R. Barros, C. B. Field, D. J. Dokken, M. D. Mastrandrea, K. J. Mach, T. E. Bilir, et al. (Cambridge; New York, NY: Cambridge University Press).
Jackson, J. B., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., et al. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637. doi: 10.1126/science.1059199
Januchowski-Hartley, F. A., Graham, N. A. J., Wilson, S. K., Jennings, S., and Perry, C. T. (2017). Drivers and predictions of coral reef carbonate budget trajectories. Proc. R. Soc. Lond. B Biol. Sci. 284:20162533. doi: 10.1098/rspb.2016.2533
Jiang, L.-Q., Feely, R. A., Carter, B. R., Greeley, D. J., Gledhill, D. K., and Arzayus, K. M. (2015). Climatological distribution of aragonite saturation state in the global oceans. Global Biogeochem. Cycles 29, 1656–1673. doi: 10.1002/2015GB005198
Jones, A. M., and Berkelmans, R. (2011). Tradeoffs to thermal acclimation: energetics and reproduction of a reef coral with heat tolerant Symbiodinium type-D. J. Mar. Biol. 2011:185890. doi: 10.1155/2011/185890
Jones, A., Berkelmans, R., Van Oppen, M., Mieog, J., and Sinclair, W. (2008). A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc. R. Soc. Lond. B Biol. Sci. 275, 1359–1365. doi: 10.1098/rspb.2008.0069
King, A., and Hawkins, E. (2016). 2016 is Likely to be the World's Hottest Year: Here's Why. The Conversation, May 18, 2016.
Kleypas, J. A., Buddemeier, R. W., Archer, D., Gattuso, J.-P., Langdon, C., and Opdyke, B. N. (1999a). Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284, 118–120. doi: 10.1126/science.284.5411.118
Kleypas, J. A., Feely, R. A., Fabry, V. J., Langdon, C., Sabine, C. L., and Robbins, R. R. (2006). Impacts of Ocean Acidification on Coral Reefs and Other Marine Calcifiers: A Guide for Future Research, Report of a Workshop Held 18-20 April 2005, St Petersburg, FL, sponsored by NSF, NOAA and the US Geological Survey.
Kleypas, J. A., McManus, J. W., and Menez, L. A. B. (1999b). Environmental limits to coral reef development: where do we draw the line? Am. Zool. 39, 146–159. doi: 10.1093/icb/39.1.146
Kroeker, K. J., Kordas, R. L., Crim, R., Hendriks, I. E., Ramajo, L., Singh, G. S., et al. (2013). Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Change Biol. 19, 1884–1896. doi: 10.1111/gcb.12179
Leal, M. C., Puga, J., Serôdio, J., Gomes, N. C. M., and Calado, R. (2012). Trends in the Discovery of New Marine Natural Products from Invertebrates over the Last Two Decades Ű Where and What Are We Bioprospecting? PLoS ONE 7:e30580. doi: 10.1371/journal.pone.0030580
Lewis, E., Wallace, D., and Allison, L. J. (1998). Program Developed for CO2 System Calculations. Environmental Sciences Division Publication No. 4735; Carbon Dioxide Information Analysis Center; Oak Ridge National Laboratory, Oak Ridge, TN.
Lough, J. M. (2010). Climate records from corals. Wires Clim. Change. 1, 318–331. doi: 10.1002/wcc.39
Lough, J. M. (2011). Great Barrier Reef coral luminescence reveals rainfall variability over northeastern Australia since the 17th century. Paleoceanography 26:PA2201. doi: 10.1029/2010pa002050
Maier, C., Hegeman, J., Weinbauer, M. G., and Gattuso, J. P. (2009). Calcification of the cold-water coral Lophelia pertusa under ambient and reduced pH. Biogeosciences 6, 1671–1680. doi: 10.5194/bg-6-1671-2009
Maynard, J., Anthony, K., Marshall, P., and Masiri, I. (2008a). Major bleaching events can lead to increased thermal tolerance in corals. Mar. Biol. 155, 173–182. doi: 10.1007/s00227-008-1015-y
Maynard, J., Baird, A., and Pratchett, M. (2008b). Revisiting the Cassandra syndrome; the changing climate of coral reef research. Coral Reefs 27, 745–749. doi: 10.1007/s00338-008-0432-1
Middlebrook, R., Anthony, K. R., Hoegh-Guldberg, O., and Dove, S. (2010). Heating rate and symbiont productivity are key factors determining thermal stress in the reef-building coral Acropora formosa. J. Exp. Biol 213, 1026–1034. doi: 10.1242/jeb.031633
Middlebrook, R., Anthony, K., Hoegh-Guldberg, O., and Dove, S. (2012). Thermal priming affects symbiont photosynthesis but does not alter bleaching susceptibility in Acropora millepora. J. Exp. Mar. Biol. Ecol. 432, 64–72. doi: 10.1016/j.jembe.2012.07.005
Miller, G. M., Watson, S.-A., Donelson, J. M., McCormick, M. I., and Munday, P. L. (2012). Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat. Clim. Change 2, 858–861. doi: 10.1038/nclimate1599
Mumby, P. J., Chisholm, J. R., Edwards, A. J., Andrefouet, S., and Jaubert, J. (2001). Cloudy weather may have saved Society Island reef corals during the 1998 ENSO event. Mar. Ecol. Prog. Ser. 222, 209–216. doi: 10.3354/meps222209
Munday, P. L., Dixson, D. L., Donelson, J. M., Jones, G. P., Pratchett, M. S., Devitsina, G. V., et al. (2009). Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl. Acad. Sci. U.S.A. 106, 1848–1852. doi: 10.1073/pnas.0809996106
Muscatine, L. (1990). “The role of symbiotic algae in carbon and energy flux in reef corals,” in Ecosystems of the World 25 Coral Reefs, ed Z. Dubinsky (Amsterdam: Elsevier Science Publishing Company), 75–87.
Muscatine, L., and Porter, J. W. (1977). Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27, 454–460. doi: 10.2307/1297526
Muscatine, L., Goiran, C., Land, L., Jaubert, J., Cuif, J. P., and Allemand, D. (2005). Stable isotopes (delta13C and delta15N) of organic matrix from coral skeleton. Proc. Natl. Acad. Sci. U.S.A. 102, 1525–1530. doi: 10.1073/pnas.0408921102
Nakamura, T., and Van Woesik, R. (2001). Water-flow rates and passive diffusion partially explain differential survival of corals during the 1998 bleaching event. Mar. Ecol. Prog. Ser. 212, 301–304. doi: 10.3354/meps212301
Orr, J. C., Fabry, V. J., Aumont, O., Bopp, L., Doney, S. C., Feely, R. A., Gnanadesikan, A., et al. (2005). Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686. doi: 10.1038/nature04095
Pandolfi, J. M., Bradbury, R. H., Sala, E., Hughes, T. P., Bjorndal, K. A., Cooke, R. G., et al. (2003). Global trajectories of the long-term decline of coral reef ecosystems. Science 301, 955–958. doi: 10.1126/science.1085706
Petit, J. R., Jouzel, J., Raynaud, D., Barkov, N., Barnola, J., Basile, I., et al. (1999). Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature 399, 429–436. doi: 10.1038/20859
Poloczanska, E. S., Brown, C. J., Sydeman, W. J., Kiessling, W., Schoeman, D. S., Moore, P. J., et al. (2013). Global imprint of climate change on marine life. Nat. Clim. Change 3, 919–925. doi: 10.1038/nclimate1958
Poloczanska, E. S., Burrows, M. T., Brown, C. J., Garcia Molinos, J., Halpern, B. S., Hoegh-Guldberg, O., et al. (2016). Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 3:62. doi: 10.3389/fmars.2016.00062
Poloczanska, E., Hoegh-Guldberg, O., Cheung, W., Pörtner, H.-O., and Burrows, M. T. (2014). “Cross chapter box on observed global responses of marine biogeography, abundance, and phenology to climate change,” in: Climate Change 2014: Impacts, Adaptation, and Vulnerability Part A: Global and Sectoral Aspects Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel of Climate Change, eds C. B. Field, V. R. Barros, D. J. Dokken, K. J. Mach, M. D. Mastrandrea, T. E. Bilir, et al. (Cambridge; New York, NY: Cambridge University Press), 123–127.
Pörtner, H. O., Karl, D., Boyd, P. W., Cheung, W., Lluch-Cota, S. E., Nojiri, Y., et al. (2014). “Ocean systems,” in Climate Change 2014: Impacts, Adaptation, and Vulnerability Part A: Global and Sectoral Aspects Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel of Climate Change, eds C. B. Field, V. R. Barros, D. J. Dokken, K. J. Mach, M. D. Mastrandrea, T. E. Bilir, et al. (Cambridge; New York, NY: Cambridge University Press), 411–484.
Precht, W. F., and Aronson, R. B. (2004). Climate flickers and range shifts of reef corals. Front. Ecol. Environ. 2, 307–314. doi: 10.1890/1540-9295(2004)002[0307:CFARSO]2.0.CO;2
Qi, D., Chen, L., Chen, B., Gao, Z., Zhong, W., Feely, R. A., et al. (2017). Increase in acidifying water in the western Arctic Ocean. Nat. Clim. Change 7, 195–199. doi: 10.1038/nclimate3228
Ramirez-Llodra, E., Brandt, A., Danovaro, R., De Mol, B., Escobar, E., German, C. R., et al. (2010). Deep, diverse and definitely different: unique attributes of the world's largest ecosystem. Biogeosciences 7, 2851–2899. doi: 10.5194/bg-7-2851-2010
Rayner, N., Parker, D., Horton, E., Folland, C., Alexander, L., Rowell, D., et al. (2003). Global analyses of sea surface temperature, sea ice, and night marine air temperature since the late nineteenth century. J. Geophys. Res. 108:4407. doi: 10.1029/2002JD002670
Reaka-Kudla, M. L. (1997). “Global biodiversity of coral reefs: a comparison with rainforests,” in Biodiversity II: Understanding and Protecting Our Biological Resources eds M. L. Reaka-Kudla and D. E. Wilson (Washington, DC: Joseph Henry Press), 83–108.
Reyes-Nivia, C., Diaz-Pulido, G., Kline, D., Hoegh-Guldberg, O., and Dove, S. (2013). Ocean acidification and warming scenarios increase microbioerosion of coral skeletons. Glob. Change Biol. 19, 1919–1929. doi: 10.1111/gcb.12158
Reynaud, S., Leclercq, N., Romaine-Lioud, S., Ferrier-Pages, C., Jaubert, J., and Gattuso, J. (2003). Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification in a scleractinian coral. Glob. Change Biol. 9, 1660–1668. doi: 10.1046/j.1365-2486.2003.00678.x
Rhein, M., Rintoul, S. R., Aoki, S., Campos, E., Chambers, D., Feely, R. A., et al. (2013). “Observations: Ocean,” in Climate Change 2013: The Physical Science Basis Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. eds T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, et al. (Cambridge; New York, NY: Cambridge University Press), 255–316.
Ricke, K. L., Orr, J. C., Schneider, K., and Caldeira, K. (2013). Risks to coral reefs from ocean carbonate chemistry changes in recent earth system model projections. Environ. Res. Lett. 8:034003. doi: 10.1088/1748-9326/8/3/034003
Roberts, J. M., and Cairns, S. D. (2014). Cold-water corals in a changing ocean. Curr. Opin. Environ. Sustain. 7, 118–126. doi: 10.1016/j.cosust.2014.01.004
Robinson, C., Steinberg, D. K., Anderson, T. R., Aristegui, J., Carlson, C. A., Frost, J. R., et al. (2010). Mesopelagic zone ecology and biogeochemistry - a synthesis. Deep Sea Res. I 57, 1504–1518. doi: 10.1016/j.dsr2.2010.02.018
Robinson, J., Guillotreau, P., Jimenez-Toribio, R., Lantz, F., Nadzon, L., Dorizo, J., et al. (2010). Impacts of climate variability on the tuna economy of Seychelles. Clim. Res. 43, 149–162. doi: 10.3354/cr00890
Rowan, R., Knowlton, N., Baker, A., and Jara, J. (1997). Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388, 265–269. doi: 10.1038/40843
Saunders, M. I., Leon, J. X., Callaghan, D. P., Roelfsema, C. M., Hamylton, S., Brown, C. J., et al. (2014). Interdependency of tropical marine ecosystems in response to climate change. Nat. Clim. Change 4, 724–729. doi: 10.1038/nclimate2274
Slattery, M., Lesser, M. P., Brazeau, D., Stokes, M. D., and Leichter, J. J. (2011). Connectivity and stability of mesophotic coral reefs. J. Exp. Mar. Biol. Ecol. 408, 32–41. doi: 10.1016/j.jembe.2011.07.024
Stanley, G. D., and Fautin, D. G. (2001). The origins of modern corals. Science 291, 1913–1914. doi: 10.1126/science.1056632
Stat, M., Carter, D., and Hoegh-Guldberg, O. (2006). The evolutionary history of Symbiodinium and scleractinian hosts—symbiosis, diversity, and the effect of climate change. Perspect. Plant Ecol. Evol. Syst. 8, 23–43. doi: 10.1016/j.ppees.2006.04.001
Stat, M., Loh, W., LaJeunesse, T., Hoegh-Guldberg, O., and Carter, D. (2009). Stability of coral–endosymbiont associations during and after a thermal stress event in the southern Great Barrier Reef. Coral Reefs 28, 709–713. doi: 10.1007/s00338-009-0509-5
Strong, A. E., Barrientos, C. S., Duda, C., and Sapper, J. (1997). “Improved satellite techniques for monitoring coral reef bleaching,” in Proceedings of the 8th International Coral Reef Symposium, Vol.2 (Panama: Smithsonian Tropical Research Institute), 1495–1498.
Strong, A. E., Liu, G., Meyer, J., Hendee, J. C., and Sasko, D. (2004). Coral Reef Watch 2002. Bull. Mar. Sci. 75, 259–268. Available online at: http://www.ingentaconnect.com/search/article?option1=tka&value1=Coral+Reef+Watch&operator2=AND&option2=author&value2=Strong&operator4=AND&option4=volume&value4=75&freetype=unlimited&sortDescending=true&sortField=default&pageSize=10&index=1
Strong, A. E., Liu, G., Skirving, W., and Eakin, C. M. (2011). NOAA's Coral Reef Watch program from satellite observations. Ann. GIS 17, 83–92. doi: 10.1080/19475683.2011.576266
Tanzil, J. T. I., Brown, B. E., Tudhope, A. W., and Dunne, R. P. (2009). Decline in skeletal growth of the coral Porites lutea from the Andaman Sea, South Thailand between 1984 and 2005. Coral Reefs 28, 519–528. doi: 10.1007/s00338-008-0457-5
Thiagarajan, N., Gerlach, D., Roberts, M. L., Burke, A., McNichol, A., Jenkins, W. J., et al. (2013). Movement of deep-sea coral populations on climatic timescales. Paleoceanography 28, 227–236. doi: 10.1002/palo.20023
Thresher, R. E., Guinotte, J. M., Matear, R. J., and Hobday, A. J. (2015). Options for managing impacts of climate change on a deep-sea community. Nat. Clim. Change 5, 635–639. doi: 10.1038/nclimate2611
Thresher, R. E., Tilbrook, B., Fallon, S., Wilson, N. C., and Adkins, J. (2011). Effects of chronic low carbonate saturation levels on the distribution, growth and skeletal chemistry of deep-sea corals and other seamount megabenthos. Mar. Ecol. Prog. Ser. 442, 87–99. doi: 10.3354/meps09400
Turley, C., Roberts, J., and Guinotte, J. (2007). Corals in deep-water: will the unseen hand of ocean acidification destroy cold-water ecosystems? Coral Reefs 26, 445–448. doi: 10.1007/s00338-007-0247-5
Wild, C., Hoegh-Guldberg, O., Naumann, M. S., Colombo-Pallotta, M. F., Ateweberhan, M., Fitt, W. K., et al. (2011). Climate change impedes scleractinian corals as primary reef ecosystem engineers. Mar. Freshw. Res. 62, 205–215. doi: 10.1071/MF10254
Wilson, S. K., Graham, N. A. J., Pratchett, M. S., Jones, G. P., and Polunin, N. V. C. (2006). Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Glob. Change Biol. 12, 2220–2234. doi: 10.1111/j.1365-2486.2006.01252.x
Keywords: corals, climate change, ecosystems goods and services, decline, warming ocean, ocean acidification
Citation: Hoegh-Guldberg O, Poloczanska ES, Skirving W and Dove S (2017) Coral Reef Ecosystems under Climate Change and Ocean Acidification. Front. Mar. Sci. 4:158. doi: 10.3389/fmars.2017.00158
Received: 07 January 2017; Accepted: 10 May 2017;
Published: 29 May 2017.
Edited by:
Iris Eline Hendriks, University of the Balearic Islands, SpainReviewed by:
Steeve Comeau, University of Western Australia, AustraliaCopyright © 2017 Hoegh-Guldberg, Poloczanska, Skirving and Dove. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ove Hoegh-Guldberg, b3ZlaEB1cS5lZHUuYXU=
†Present Address: Elvira Poloczanska, Alfred Wegener Institute for Polar and Marine Research, Integrative Ecophysiology, Bremerhaven, Germany
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.