
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 09 May 2017
Sec. Global Change and the Future Ocean
Volume 4 - 2017 | https://doi.org/10.3389/fmars.2017.00123
This article is part of the Research Topic Seasonal to decadal prediction of marine ecosystems: opportunities, approaches and applications View all 11 articles
Since the open-ocean subpolar Atlantic is amongst the most predictable regions in the world, our results hold promise for predicting the general production to seabird populations over a large geographical region adjacent to the northern North Atlantic and the Arctic Mediterranean. Colonies of black-legged kittiwakes Rissa tridactyla in the North Atlantic have declined markedly since the mid-1990s, partly due to repeatedly failing breeding seasons. We show a close link between the breeding success of a kittiwake colony in the Faroe Islands and the subpolar gyre index. Successful breeding follows winters with an expanded subpolar gyre and, by inference, increased zooplankton abundances southwest of Iceland. The environmental conditions in the northwestern Atlantic during the non-breeding and pre-breeding seasons might therefore be important. Furthermore, the subpolar gyre dynamics might influence the local food abundance on the Faroe shelf during the breeding season.
The populations of black-legged kittiwakes Rissa tridactyla (hereafter kittiwake), terns, and auks have declined markedly in the Northeastern (NE) Atlantic and adjacent shelf seas during the last 25 years. This is partly due to large declines in chick production, also referred to as the breeding success (Frederiksen et al., 2007a; Descamps et al., 2017, in press). There is a broad consensus that the decline must be driven by large-scale changes in the climate (Frederiksen, 2010). Climatic changes are often proxied simply as temperature changes (Frederiksen et al., 2007b), while the physical processes responsible have largely remained elusive. The advent of geolocators has revealed that many seabird species from the NE Atlantic occupy northwestern (NW) Atlantic waters during the non-breeding period, likely due to better food availability during winter (Frederiksen et al., 2012). Processes impacting the food abundance in the NW overwintering region (Bogdanova et al., 2011) could therefore have a profound impact on several seabird populations due to “carry-over effects” on the breeding success during the following summer, and also due to the high mortality during the non-breeding season (Harris et al., 2005).
The marked 1990s reduction in the size of kittiwake and Brünnich's guillemots colonies have previously been linked to the dynamics of the subpolar gyre (SPG; Descamps et al., 2013, 2017, in press; Fluhr et al., 2017), although only qualitatively. The SPG is a large body of cold and low-saline subarctic water, which circulates counterclockwise south of Greenland and Iceland (Figure 1). The size and circulation strength of the SPG declined abruptly after the mid-1990s, inducing rapid warming and salinification (Hátún et al., 2005) as well as a major ecosystem shift in the NE Atlantic (Hátún et al., 2009). These changes were initiated by weaker atmospheric forcing and shallower winter convection in the NW Atlantic (Häkkinen and Rhines, 2004). The variable shape and circulation strength of the SPG is intimately related to the morphology of main oceanic fronts (Thierry et al., 2008; Hátún et al., 2016). Such fronts are highly biologically productive and have been identified as feeding hotspots of migratory seabirds while occupying the high seas during the non-breeding period (Edwards et al., 2013; Scales et al., 2014).
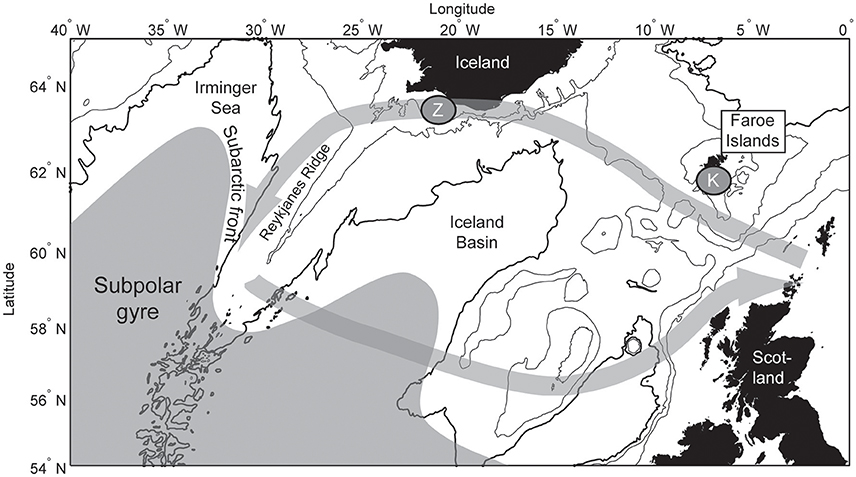
Figure 1. Map of the northeastern Atlantic Ocean. The subpolar gyre is illustrated in gray, and the pre-breeding kittiwake feeding excursion is shown with the gray arrows. The Faroe kittiwake breeding success is sampled at site “K,” and the Icelandic zooplankton record is sampled at site “Z.”
During years when the winter mixed layer is anomalously deep in the Labrador-Irminger Sea, the subpolar gyre expands, and the productive subarctic front west of the Reykjanes Ridge (Figure 1) shifts toward Iceland (Hátún et al., 2016). Such years result in increased abundance of the ecologically most important zooplankton species, Calanus finmarchicus, both within the Irminger Sea and on the south Iceland shelf (“Z” in Figure 1; Hátún et al., 2016). The zooplankton concentration southwest of Iceland thus exhibits strong inter-annual variability.
In March, after the kittiwakes return from the NW Atlantic to European waters, many birds make yet another exodus westwards just prior to breeding, along strikingly similar paths (Bogdanova et al., 2011). The westward route follows the Icelandic shelf, from where Hátún et al. (2016) presented long-term on-shelf zooplankton data. In the west the birds gather in a region between 29° and 36°W (Bogdanova et al., 2011), which roughly coincides with the highly productive subarctic front west of the Reykjanes Ridge (Pedchenko, 2005; Hátún et al., 2016).
Environmental conditions both in non-breeding and breeding regions must be significant drivers of the demographic processes of the seabirds. The relative importance of such remote and regional drivers has for example been investigated for the adult kittiwake survival rates in northern Norway (Reiertsen et al., 2014). A large percentage of the variation in adult kittiwake survival rates in northern Norway was explained as a combination of zooplankton variability in the SPG region and the state of a fish stock in the Barents Sea.
The breeding success of kittiwakes on the Faroe shelf (“K” in Figure 1) has, so far, only been compared to the regional food abundances. Significant correlations were identified to the average length of the local O-group sandeel population (r2 = 0.41), and even higher correlations were found when compared to a sandeel biomass index (r2 = 0.56; Eliasen, 2013). Several key ecosystem records from the Faroe shelf are, however, characterized by “high-or-low” variability (Gaard et al., 2002) comparable the zooplankton abundance southwest of Iceland (Hátún et al., 2016). Since the geolocators reveal occupancy of these western waters immediately prior to breeding (Bogdanova et al., 2011), an additional carry-over effect is possible. Acknowledging the importance of local food abundance, we here want to test if food availability in the Icelandic feeding hotspots during the non-breeding/pre-breeding period also might impact the breeding success of the Faroe kittiwakes.
The gyre index is the principal component obtained from an Empirical Orthogonal Function (EOF; Preisendorfer, 1988) analysis of the sea surface height (SSH) field over the entire North Atlantic Ocean (30°–60°N; Häkkinen and Rhines, 2004; Hátún et al., 2005, 2016; Larsen et al., 2012). The high-pass filtered gyre index has previously been used as a metric for the lateral position of the subarctic front (the Frontal position) and the intensity of vertical winter mixing (Hátún et al., 2016). It has previously been demonstrated that the Frontal position (the detrended gyre index) is highly correlated with the zooplankton biomass on the south Iceland shelf (r = 0.80, p <10−8 for 1970–2011), and the abundance of C. finmarchicus within the SPG—abundance increases when the front shifts toward Iceland (Hátún et al., 2016).
A new version of Ssalto/DUACS altimetry products (SSH) was released in April 2014—the so-called DUACS 2014. This included a change of reference period (now 1993–2012 instead of 1993–1999), and a new Cartesian 1/4° resolution is used instead of the 1/3° Mercator grid, which has had a strong impact on the physical information in these data. The spatial resolution improved below 41.5°N, but the grid is now coarser north of this latitude. In order to emphasize the SPG, where the kittiwakes overwinter (Frederiksen et al., 2012), an EOF analysis was applied to the DUACS 2014 data for a more limited domain encompassing the SPG (Figure S1). The obtained new gyre index is more “linear” than the previous one from Larsen et al. (2012), and the important interannual fluctuation associated with deep winter convection (Hátún et al., 2016) have now diminished (Figure 2). Especially, the peak entailing the convection event during winter 1999–2000 is much less evident in the presently used DUACS 2014–based gyre index. The detrended DUACS 2014–based gyre index has been utilized as our updated Frontal position. The model-based Frontal position index from Hátún et al. (2016) is used for the pre-altimetry period (before 1993).
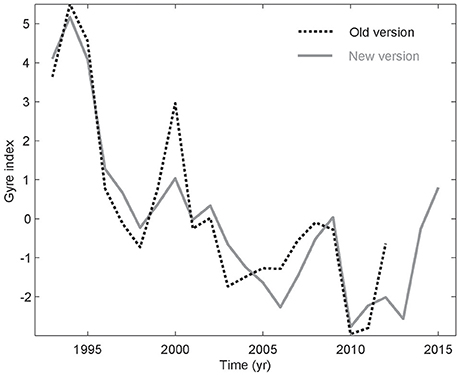
Figure 2. Altimetry-based gyre indices, calculated from the new (gray) and the old AVISO data versions (dashed, Larsen et al., 2012), respectively.
The Ssalto/Duacs altimeter products were produced and distributed by the Copernicus Marine and Environment Monitoring Service (CMEMS; http://marine.copernicus.eu/).
Zooplankton data were collected during late May and early June 1971–2013 at five stations along a transect extending from the coast and beyond the shelf edge south of Iceland (Figure 1). From 1970 to 1991, the samples were collected with a standard Hensen net (0.42 m2 mouth area, 200 μm mesh size), whereas after that all the samples were collected using a WP2 net (0.25 m2 mouth area, 200 μm mesh size). The volume of water filtered was measured with HydroBios flowmeters fitted in the mouth of the net. For the present analysis, data on zooplankton dry weight biomass standardized per m3 averaged across all stations have been used. Until 2001, the volume of zooplankton samples was measured at sea and the dry weight calculated using a conversion factor from Matthews and Heimdal (1980). From 2002 onwards the samples were deep frozen at sea and dried and weighed on shore (Postel et al., 2000). The copepod C. finmarchicus usually constitutes the dominant component of mesozooplankton biomass in the samples (Astthorsson and Gislason, 1995).
The kittiwake data are from the island Skúvoy (61°47′N and 6°51′W), which is one of the largest seabird colonies in the Faroe Islands. The west facing part of the island is a 5 km long steep seabird cliff, reaching a high of 394 m and holding ~12,000 breeding pairs of kittiwakes in 2008 and 2009. The studied colony, Høvdin, is the northernmost colony on the island. It is about 200 m wide and 130 m high, and kittiwakes have been breeding here for generations. The number of nests fluctuates, but since 2001 the average number of nests has been 2,624 (1,149–4,525).
The counting unit is the number of chicks/nest late in the breeding season when most of the chicks are ready to fly. The start date of counting depends on when the chicks are large enough, and it is determined based on regular visits to the colony before start of the counting. The number is based on a maximum number of fledged chicks as some of the smaller chicks may not survive. Prior to 2000, subsamples at an average of 1,050 (200–1,736) nests were counted, but since 2001 all the nests and chicks have been counted. All the countings but one (in 1990), have been done by the same counter, so inter-counter differences do not impact the results.
The predation in the colony has been rather stable. There are no ground predators on the island like house mouse (Mus musculus), brown rat (Rattus norvegicus), and cat (Felis catus), which do populate many of the other islands. The most common predator of eggs, chicks, and adult kittiwakes is the great skua (Stercorarius skua), however, herring gull (Larus argentatus), great black-backed gull (Larus marinus), raven (Corvus corax varius), and crow (Corvus corone cornix) are also seen preying on the colony although in a lesser extent.
Therefore, the large fluctuation in chick production in the studied colony most likely reflect the adult kittiwake's ability to find food for their chicks. As these fluctuations occur simultaneously in most of the Faroese kittiwake colonies, it is a good proxy for the production in the total Faroese kittiwake population.
The gyre index, and thus the Frontal position metric, increased markedly in 2014 and 2015 (Figure 3A). This reflects the increased convection in the Labrador Sea during winter 2013–2014, and an even deeper mixing during winter 2014–2015, which also involved the Irminger Sea (de Jong and de Steur, 2016; Yashayaev and Loder, 2016). The previously established link between the Frontal position and the zooplankton abundance on the south Iceland shelf still holds (r = 0.70, p < 0.001 for 1993–2015, c.f. Figures 3A,B), and the forecasted zooplankton increase during 2014 (Hátún et al., 2016) indeed did occur.
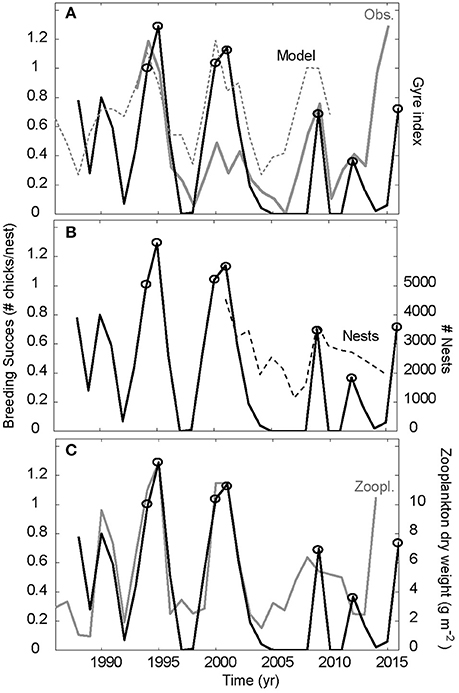
Figure 3. Synchrony between east and west. The Faroe kittiwakes breeding success with full line in all three panels. This is compared to (A) the detrended observed (gray) and simulated (dashed) gyre index, (B) the number of nests in the colony (dashed) and (C) the south Iceland zooplankton abundances (gray). Cold periods with deep convection are associated peaks in the gyre index. The year with peaks in breeding success (1994–1995, 2000–2001, 2009, 2012, and 2016) are highlighted with open circles.
The breeding success of the Faroe kittiwakes is significantly correlated to the pre-breeding food abundance southwest of Iceland, proxied by the Frontal position (r = 0.71, p < 0.001 for 1993–2013, Figure 3A). The breeding success is highly variable, and the peaks in 1994–1995, 2000–2001, 2009, and 2012 are all associated with deep convection events. The intensified convection during the winters 2013–2015, and the associated increased Frontal position value did not, however, result in the expected increased breeding success. The reaction came in 2016, with a great increase in breeding success. A lag of 1.5 years between deep winter convection and increased chick production, like the event in 2008–2009 (Figure 3A), can be expected (Hátún et al., 2016), but not 2.5 years. When 2014 and 2015 are included, the Frontal position—breeding success correlation drops to 0.58 (which is still significant on a 0.5% level).
The co-variability between the south Iceland zooplankton record and the Faroe kittiwake breeding success was also tight until the years 2005–2008 when no fledlings were produced (Figure 3B). The linkage is much less clear thereafter, but despite the misfit during 2014 the overall correlation is still significant (r = 0.75, p < 0.001 for 1988–2014).
In migratory birds, environmental conditions in both breeding and non-breeding areas may affect breeding success and hence be significant drivers of demographic processes. The kittiwakes occupy the western central SPG during the mid-winter non-breeding season where food is available on the surface (Frederiksen et al., 2012). The gyre index represents the size and circulation strength of the SPG (Hátún et al., 2005), which in turn is inherently associated with the winter convection. When convection is strong, the abundance of C. finmarchicus increases within the SPG (Hátún et al., 2016), and here we show that these years also coincide with increased kittiwake breeding success (Figure 3). This supports previous suggestions that the dynamics of the SPG will impact seabirds in the NE Atlantic, although this inference is still qualitative (Descamps et al., 2013, 2017, in press; Fluhr et al., 2017).
A stronger tie between east and west appears from that fact that the kittiwakes can make a second westward exodus, after having entered the colonies along northwest European shores during spring (Bogdanova et al., 2011). Without a description of the main oceanographic structures, it could not be explained why the birds follow the south Iceland slope on their strikingly parallel westward route. The food production on the south Iceland shelf is much higher than in the adjacent Iceland Basin, which becomes a nutrient depleted “desert” during summer, avoided by e.g., the westward food migrating mackerel stock (Pacariz et al., 2016). Having circumvented the Iceland Basin, the kittiwakes settle in a region between 29° and 36°W (Bogdanova et al., 2011), which is on and just west of the Reykjanes Ridge (Figure 1). This is the location of the subarctic front, which is one of the most productive regions in the North Atlantic (Pedchenko, 2005; Hátún et al., 2016). The metrics presented by Hátún et al. (2016) connect the mixed layer depth and the C. finmarchicus abundance in the Irminger Sea, lateral shifts of the subarctic front, and the zooplankton abundance on the south Iceland shelf. Here, we show that the Faroe kittiwake breeding success co-varies closely with the south Iceland zooplankton abundance, the position of the subarctic front and the C. finmarchicus concentrations within the SPG (not shown, see Hátún et al., 2016). Additionally, since the pre-breeding round-trip takes place as late as March, a carry-over effect to the subsequent summer is likely.
Understanding how the marine climate in the SPG regulates the food production and availability, thus likely holds a common key to understanding the demography of several seabird populations. Furthermore, the potential predictability of the marine climate within the SPG could be utilized to project the general conditions for the seabirds in the near-future. The strongest predictability is likely associated with the highly variable depth of winter convection in the Labrador-Irminger Seas (Hátún et al., 2016). A very strong winter might boost biological production the following season (~0.5 year horizon), and might even precondition the subsequent winter's convection (~1.5 year horizon). The more gradual hydrographic changes in the subpolar Atlantic ascribed to shifts in horizontal gyre circulations (Hátún et al., 2005; Lohmann et al., 2009a) or the strength of the Atlantic Meridional Overturning Circulation (AMOC) (Lohmann et al., 2009b; Robson et al., 2012) has received more attention in the literature, than the more erratic convection-related signal. The subpolar Atlantic is suggested to hold particularly strong decadal-scale prediction potential (Matei et al., 2012a), likely associated with the gyre and/or AMOC circulation dynamics (Matei et al., 2012b). A remaining question is if the strong convection signal in the northwestern Atlantic will disrupt the decadal-scale predictability there—e.g., a strong winter will reset a large part of the “memory” in the horizontally advected water masses.
Strong winter cooling over the Labrador-Irminger Seas in winters 2013–2015 resulted in exceptional deep convection (de Jong and de Steur, 2016; Yashayaev and Loder, 2016) and induced a large “cold blob,” which was widely discussed in the media during 2015. As suggested by Hátún et al. (2016), this should have led to increased production in several ecosystem components during 2014, 2015, and maybe even 2016. There was a marked increase in the south Iceland zooplankton abundance (Figure 3B), the breeding success of the Atlantic puffins (Fratercula arctica) and the Arctic terns (Sterna paradisaea) in Faroese colonies (Unpublished data, Bergur Olsen), as well as the kittiwake breeding success in 16 Shetland colonies during 2014 (Pers. Comm. Martin Heubeck). The poor breeding performance of the Faroe kittiwakes in 2014 and 2015 therefore at first appears as a conundrum.
One issue which could disturb predictability is that the general intensity of winter convection has weakened over the last 25 years (Hátún et al., 2016), and the nutrient concentrations over the entire subpolar North Atlantic have declined markedly during the same period (Hátún et al., Submitted). This trend is likely to impact the phytoplankton community composition and thus higher trophic levels and food quality over large spatial scales. No fledged chicks were observed in 2005–2008 at the studied Faroese colony (Figure 3) and a similar breeding failure was also observed in Britain between 2001 and 2008 (Coulson, 2011). One could thus speculate if the general food productivity in both the non-breeding and breeding waters has reached critically low levels for sustaining the kittiwake population. If this is correct, it may disturb the demographic processes and thus the identified predictability of the breeding succes.
Kittiwake chicks are fledged late in July and early August and crave food during this period. After just a few days of food shortage, chicks will starve, and die (Coulson, 2011). The local food availability is therefore also very important. The O-group from the local stock of sandeel (Ammodytes marinus) is a principal food item for the kittiwake chicks (Eliasen, 2013). Sandeel lay demersal eggs between November and January, and their planktonic larvae are present in the water column from late January to May (Eliasen, 2013). Sandeel prey on younger stages and eggs of zooplankton and obtain as much energy as fast as possible (refs in Eliasen, 2013), thereby becoming a favorable prey to numerous higher trophic level species.
It has previously been suggested, that variable influx of C. finmarchicus from core gyre regions to adjacent shelves regions around the subpolar Atlantic might regulate the on-shelf biomass of this species (Gislason and Astthorsson, 2000; Harms et al., 2000; Sundby, 2000) and intermittent northward expansions of the SPG do result in higher zooplankton abundances on the south Iceland shelf (Hátún et al., 2016). We here tentatively suggest that SPG-related processes might also regulate the influx of ecologically important zooplankton products onto the Faroe shelf. Additional work is required to investigate this suggestion.
The on-shelf primary production must, however, also be important, which is supported by the positive correlation between the Faroe shelf primary production and the abundance of sandeel (Eliasen et al., 2011). The early phase of the on-shelf spring bloom, which mainly consists of large fast-growing diatoms, becomes silicate limited during late May-early June (Eliasen et al., in press). Furthermore, winters with deep convection increase the pre-bloom silicate content in the North Atlantic Ocean (Hátún et al., submitted), and all years with a strong gyre (high Frontal position index, 1994–1995, 2000–2001, and 2008–2009, Figure 3A) coincide with elevated Faroe shelf primary production (see Eliasen et al., 2011). The strong-gyre years 2014 and 2015 also resulted in very high chlorophyll concentrations on the shelf, but the blooms were much delayed compared to the previous years (Eliasen et al., 2017). Productive years have typically been characterized by high chlorophyll concentrations in May, but the blooms in 2014 and 2015 were initiated during mid-June and early July, respectively, and such late blooms have not been observed before.
The Atlantic puffins and the Arctic terns were apparently able to adapt to this marked shift in bloom timing (phenology), and were able to produce fledlings in 2014 and 2015 (Pers. Comm. Bergur Olsen). The kittiwakes are less phenologically flexible. They build their nests and decide whether or not to start breeding during May, and collectively lay their eggs during late May (Coulson, 2011). An investigation of daily pictures of the kittiwake colony reveals increased nest building and numbers of chicks during both 2014 and 2015 (not shown), but most of the chicks suddenly died during their most food craving period these years. We interpret this as sign of good conditions during the pre-breeding phase, but that local food shortage, possibly caused by the anomalously late blooms, acted as a severe “bottle neck.” The causes underlying the recent delay of the spring bloom are not well-understood and require further attention.
In conclusion, the dynamics of the North Atlantic subpolar gyre likely impacts the feeding conditions for the black-legged kittiwakes (Rissa tridactyla) in the northwest Atlantic overwintering regions during the non-breeding and pre-breeding seasons. A winter with deep convection and an intensifying gyre increases the zooplankton food availability and improves the physical condition of the birds, which in turn could have a positive carry-over effect on the breeding success the subsequent summer. Gyre-related oceanic dynamics could, furthermore, influence the Faroe shelf food availability during early summer through onto-shelf influxes of oceanic zooplankton species Calanus finmarchicus and their eggs nauplii, and/or nutrients which can boost the on-shelf primary production. The promising predictability of the marine climate adjacent to the subpolar gyre could thus carry over into the general production of seabird populations surrounding the northern North Atlantic Ocean. The less predictable conditions around the nesting sites of individual populations might, however, act as a “bottle-neck” for making realistic forecasts of the kittiwake breeding success. Over the next century, climate models predict global declines in nutrients and thus in primary production, due to shallower winter mixing, with a particularly strong imprint on the North Atlantic (Groeger et al., 2013). If correct, this would change seabird life in the subpolar regions from what is known today.
Authors declare that for this study no ethics approval was required as per institutional and national guidelines. Data for this study are based on visual observations of birds in their natural environment. No animal experiments were performed.
HH has composed the story told, worked up the altimetry data, and led the writing process. BO produced the kittiwake data. He has initiated and maintained the counting unit at Høvdin all years. BO has also helped in writing and approving of the document. SP has participated in developing the underlying idea, and in the writing process.
The research leading to these results has received funding from the European Union 7th Framework Program (FP7 2007–2013), under grant agreement no. 308299 NACLIM www.naclim.eu. HH was partly funded through the Danish project NAACOS 10-093903/DSF. SP was funded by the Danish government through the program “Marine climate in the North Atlantic and its effects on plankton and fish.”
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Astthor Gislason for supplying the Icelandic zooplankton data, and our colleague Jan Arge Jacobsen for discussing the paper.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2017.00123/full#supplementary-material
Astthorsson, O. S., and Gislason, A. (1995). Long-term changes in zooplankton biomass in Icelandic waters in spring. ICES J. Mar. Sci. 52, 657–668. doi: 10.1016/1054-3139(95)80079-4
Bogdanova, M. I., Daunt, F., Newell, M., Phillips, R. A., Harris, M. P., and Wanless, S. (2011). Seasonal interactions in the black-legged kittiwake, Rissa tridactyla: links between breeding performance and winter distribution. Proc. R. Soc. B Biol. Sci. 278, 2412–2418. doi: 10.1098/rspb.2010.2601
de Jong, M. F., and de Steur, L. (2016). Strong winter cooling over the Irminger Sea in winter 2014–2015, exceptional deep convection, and the emergence of anomalously low SST. Geophys. Res. Lett. 43, 7106–7113. doi: 10.1002/2016GL069596
Descamps, S., Anker-Nilssen, T., Barrett, R. T., Irons, D. B., Merkel, F., Robertson, G. J., et al. (2017). Circumpolar dynamics of a marine top-predator track ocean warming rates. Glob. Change Biol. doi: 10.1111/gcb.13715. [Epub ahead of print].
Descamps, S., Strom, H., and Steen, H. (2013). Decline of an arctic top predator: synchrony in colony size fluctuations, risk of extinction and the subpolar gyre. Oecologia 173, 1271–1282. doi: 10.1007/s00442-013-2701-0
Edwards, E. W. J., Quinn, L. R., Wakefield, E. D., Miller, P. I., and Thompson, P. M. (2013). Tracking a northern fulmar from a Scottish nesting site to the Charlie-Gibbs Fracture Zone: evidence of linkage between coastal breeding seabirds and Mid-Atlantic Ridge feeding sites. Deep Sea Res. Part II Top. Stud. Oceanogr. 98, 438–444. doi: 10.1016/j.dsr2.2013.04.011
Eliasen, K. (2013). Sandeel, Ammodytes spp., as a Link between Climate and Higher Trophic Levels on the Faroes Shelf. PhD Thesis, Faroe Marine Research Institute.
Eliasen, K., Reinert, J., Gaard, E., Hansen, B., Jacobsen, J. A., Grønkjær, P., et al. (2011). Sandeel as a link between primary production and higher trophic levels on the Faroe shelf. Mar. Ecol. Prog. Ser. 438, 185–194. doi: 10.3354/meps09301
Eliasen, S. K., Hátún, H., Larsen, K. M. H., Hansen, B., and Rasmussen, T. A. S. (2017). Phenologically distinct phytoplankton regions on the Faroe Shelf-identified by satellite data, in-situ observations and model. J. Mar. Syst. 169, 99–110. doi: 10.1016/j.jmarsys.2017.01.015
Eliasen, S. K., Hátún, H., Larsen, K. M. H., and Jacobsen, S. (in press). Faroe shelf bloom phenology - regulated by ocean-to-shelf nutrient fluxes. Cont. Shelf Res.
Fluhr, J., Strom, H., Pradel, R., Duriez, O., Beaugrand, G., and Descamps, S. (2017). Weakening of the subpolar gyre as a key driver of North Atlantic seabird demography: a case study with Brunnich's guillemots in Svalbard. Mar. Ecol. Prog. Ser. 563, 1–11. doi: 10.3354/meps11982
Frederiksen, M. (2010). Appendix I: Seabirds in the North East Atlantic. A review of status, trends and anthropogenic impact. TemaNord 587, 27–122.
Frederiksen, M., Edwards, M., Mavor, R., and Wanless, S. (2007b). Regional and annual variation in black-legged kittiwake breeding productivity is related to sea surface temperature. Mar. Ecol. Prog. Ser. 350, 137–143. doi: 10.3354/meps07126
Frederiksen, M., Mavor, R., and Wanless, S. (2007a). Seabirds as environmental indicators: the advantages of combining data sets. Mar. Ecol. Prog. Ser. 352, 205–211. doi: 10.3354/meps07071
Frederiksen, M., Moe, B., Daunt, F., Phillips, R. A., Barrett, R. T., Bogdanova, M. I., et al. (2012). Multicolony tracking reveals the winter distribution of a pelagic seabird on an ocean basin scale. Divers. Distrib. 18, 530–542. doi: 10.1111/j.1472-4642.2011.00864.x
Gaard, E., Hansen, B., Olsen, B., and Reinert, J. (2002). “Ecological features and recent trends in the physical environment, plankton, fish stocks, and seabirds in the Faroe Shelf ecosystem,” in Large Marine Ecosystems of the North Atlantic, eds K. Sherman and H. R. Skjoldal (Amsterdam: Elsevier Science), 245–265. doi: 10.1016/s1570-0461(02)80060-x
Gislason, A., and Astthorsson, O. S. (2000). Winter distribution, ontogenetic migration, and rates of egg production of Calanus finmarchicus southwest of Iceland. ICES J. Mar. Sci. 57, 1727–1739. doi: 10.1006/jmsc.2000.0951
Groeger, M., Maier-Reimer, E., Mikolajewicz, U., Moll, A., and Sein, D. (2013). NW European shelf under climate warming: implications for open ocean - shelf exchange, primary production, and carbon absorption. Biogeosciences 10, 3767–3792. doi: 10.5194/bg-10-3767-2013
Häkkinen, S., and Rhines, P. B. (2004). Decline of subpolar North Atlantic circulation during the 1990s. Science 304, 555–559. doi: 10.1126/science.1094917
Harms, J. H., Heath, M. R., Bryant, A. D., Backhaus, J. O., and Hainbucher, D. A. (2000). Modelling the Northeast Atlantic circulation: implications for the spring invasion of shelf regions by Calanus finmarchicus. ICES J. Mar. Sci. 57, 1694–1707. doi: 10.1006/jmsc.2000.0981
Harris, M. P., Anker-Nilssen, T., McCleery, R. H., Erikstad, K. E., Shaw, D. N., and Grosbois, V. (2005). Effect of wintering area and climate on the survival of adult Atlantic puffins Fratercula arctica in the eastern Atlantic. Mar. Ecol. Prog. Ser. 297, 283–296. doi: 10.3354/meps297283
Hátún, H., Lohmann, K., Matei, D., Jungclaus, J., Pacariz, S., Bersch, M., et al. (2016). An inflated subpolar gyre blows life towards the northeastern Atlantic. Prog. Oceanogr. 147, 49–66. doi: 10.1016/j.pocean.2016.07.009
Hátún, H., Payne, M., Beaugrand, G., Reid, P. C., Sandø, A. B., Drange, H., et al. (2009). Large bio-geographical shifts in the north-eastern Atlantic Ocean: from the subpolar gyre, via plankton, to blue whiting and pilot whales. Prog. Oceanogr. 80, 149–162. doi: 10.1016/j.pocean.2009.03.001
Hátún, H., Sandø, A. B., Drange, H., Hansen, B., and Valdimarsson, H. (2005). Influence of the Atlantic subpolar gyre on the thermohaline circulation. Science 309, 1841–1844. doi: 10.1126/science.1114777
Larsen, K. M. H., Hátún, H., Hansen, B., and Kristiansen, R. (2012). Atlantic water in the Faroe area: sources and variability. ICES J. Mar. Sci. 69, 802–808. doi: 10.1093/icesjms/fss028
Lohmann, K., Drange, H., and Bentsen, M. (2009a). A possible mechanism for the strong weakening of the North Atlantic subpolar gyre in the mid-1990s. Geophys. Res. Lett. 36:L15602. doi: 10.1029/2009GL039166
Lohmann, K., Drange, H., and Bentsen, M. (2009b). Response of the North Atlantic subpolar gyre to persistent North Atlantic oscillation like forcing. Clim. Dyn. 32, 273–285. doi: 10.1007/s00382-008-0467-6
Matei, D., Baehr, J., Jungclaus, J. H., Haak, H., Muller, W. A., and Marotzke, J. (2012a). Multiyear prediction of monthly mean atlantic meridional overturning circulation at 26.5 degrees N. Science 335, 76–79. doi: 10.1126/science.1210299
Matei, D., Pohlmann, H., Jungclaus, J., Muller, W., Haak, H., and Marotzke, J. (2012b). Two tales of initializing decadal climate prediction experiments with the ECHAM5/MPI-OM Model. J. Clim. 25, 8502–8523. doi: 10.1175/JCLI-D-11-00633.1
Matthews, J. B. L., and Heimdal, B. R. (1980). “Pelagic productivity and food chains in fjord systems,” in Fjord Oceanography, eds H. J. Freeland and C. D. Levings (New York, NY: Plenum Press), 377–398. doi: 10.1007/978-1-4613-3105-6_34
Pacariz, S., Hátún, H., Jacobsen, J. A., Johnson, C., Eliasen, S. K., and Rey, F. (2016). Nutrient-driven poleward expansion of the Northeast Atlantic mackerel (Scomber scombrus) stock: a new hypothesis. Elem. Sci. Anth. 4:105. doi: 10.12952/journal.elementa.000105
Pedchenko, A. P. (2005). The role of interannual environmental variations in the geographic range of spawning and feeding concentrations of redfish Sebastes mentella in the Irminger Sea. ICES J. Mar. Sci. 62, 1501–1510. doi: 10.1016/j.icesjms.2005.08.004
Postel, L., Fock, K., and Hagen, W. (2000). “Biomass and abundance,” in ICES Zooplankton Methodology Manual, eds R. Harris, P. Wiebe, J. Lenz, H. R. Skjoldal, and M. Huntley (New York, NY: Academic Press), 83–192. doi: 10.1016/B978-012327645-2/50005-0
Preisendorfer, R. (1988). Principal Component Analysis in Meteorology and Oceanography. Amsterdam: Elsevier Science Publishing Company Inc., 425.
Reiertsen, T. K., Erikstad, K. E., nker-Nilssen, T., Barrett, R. T., Boulinier, T., Frederiksen, M., et al. (2014). Prey density in non-breeding areas affects adult survival of black-legged kittiwakes Rissa tridactyla. Mar. Ecol. Prog. Ser. 509, 289–302, doi: 10.3354/meps10825
Robson, J., Sutton, R., Lohmann, K., Smith, D., and Palmer, M. D. (2012). Causes of the rapid warming of the North Atlantic Ocean in the mid-1990s. J. Clim. 25, 4116–4134. doi: 10.1175/JCLI-D-11-00443.1
Scales, K. L., Miller, P. I., Hawkes, L. A., Ingram, S. N., Sims, D. W., and Votier, S. C. (2014). On the front line: frontal zones as priority at-sea conservation areas for mobile marine vertebrates. J. Appl. Ecol. 51, 1575–1583. doi: 10.1111/1365-2664.12330
Sundby, S. (2000). Recruitment of Atlantic cod stocks in relation to temperature and advection of copepod populations. Sarsia 85, 277–298. doi: 10.1080/00364827.2000.10414580
Thierry, V., de Boisseson, E., and Mercier, H. (2008). Interannual variability of the subpolar mode water properties over the Reykjanes ridge during 1990–2006. J. Geophys. Res. Oceans 113:C04016. doi: 10.1029/2007JC004443
Keywords: North Atlantic subpolar gyre, predictability, seabirds, breeding success, oceanic front, Calanus finmarchicus, sub-decadal variability
Citation: Hátún H, Olsen B and Pacariz S (2017) The Dynamics of the North Atlantic Subpolar Gyre Introduces Predictability to the Breeding Success of Kittiwakes. Front. Mar. Sci. 4:123. doi: 10.3389/fmars.2017.00123
Received: 03 February 2017; Accepted: 18 April 2017;
Published: 09 May 2017.
Edited by:
Desiree Tommasi, Princeton University, USAReviewed by:
Donald F. Boesch, University of Maryland Center for Environmental Sciences, USACopyright © 2017 Hátún, Olsen and Pacariz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hjálmar Hátún, aGphbG1hcmhAaGF2LmZv
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.