- 1Department of Biological Sciences, Florida International University, North Miami, FL, USA
- 2Coral Restoration Foundation, Key Largo, FL, USA
Coral restoration is gaining traction as a viable strategy to help restore degraded reefs. While the nascent field of coral restoration has rapidly progressed in the past decade, significant knowledge gaps remain regarding the drivers of restoration success that may impede our ability to effectively restore coral reef communities. Here, we conducted a field experiment to investigate the influence of coral density on the growth, habitat production, and survival of corals outplanted for restoration. We used nursery-raised colonies of Acropora cervicornis to experimentally establish populations of corals with either 3, 6, 12, or 24 corals within 4m2 plots, generating a gradient of coral densities ranging from 0.75 corals m−2 to 12 corals m−2. After 13 months we found that density had a significant effect on the growth, habitat production, and survivorship of restored corals. We found that coral survivorship increased as colony density decreased. Importantly, the signal of density dependent effects was context dependent. Our data suggest that positive density dependent effects influenced habitat production at densities of 3 corals m−2, but further increases in density resulted in negative density dependent effects with decreasing growth and survivorship of corals. These findings highlight the importance of density dependence for coral restoration planning and demonstrate the need to evaluate the influence of density for other coral species used for restoration. Further work focused on the mechanisms causing density dependence such as increased herbivory, rapid disease transmission, or altered predation rates are important next steps to advance our ability to effectively restore coral reefs.
Introduction
Coral reefs only cover <0.1% of Earth's surface, yet house more than 30% of total marine biodiversity (Reaka-Kudla, 2005). Reefs are a key source of fisheries production (Moberg and Folke, 1999) and also provide shoreline protection for >100 million people living next to coastlines (Ferrario et al., 2014). However, the invaluable ecosystem services coral reefs provide are increasingly jeopardized as corals decline globally (Bruno and Selig, 2007; Jackson et al., 2014). In the Pacific Ocean, reefs have lost nearly half of their corals over the past four decades (Bruno and Selig, 2007). This alarming trend is even more pronounced in the Western Atlantic Ocean (henceforth, the Caribbean), where coral reefs have lost 50% of their coral cover since the mid 1970's (Gardner et al., 2003; Jackson et al., 2014). On many reefs, coral declines are accompanied by a loss of benthic diversity and increases in algae, weedy coral species, soft corals, and sponges (Burman et al., 2012; Ruzicka et al., 2013; Cardini et al., 2015). Such declines in coral cover and diversity often lead to the loss of structural complexity (Alvarez-Filip et al., 2009), diminished fish populations (Newman et al., 2015), and decreased coral recruitment (Dixson et al., 2014), jeopardizing the ecosystem function and economic value of reefs (Costanza et al., 2014).
To address these declines, coral restoration has gained increasing attention as a viable strategy to help degraded reefs recover, with large-scale restoration efforts now underway across the globe (Young et al., 2012). Current restoration efforts are primarily focused on restoring ecosystem engineers by outplanting nursery-raised corals to degraded reefs (Lirman et al., 2014; Cabaitan et al., 2015; Griffin et al., 2015). These projects have shown that coral size (Garrison and Ward, 2008), genotype (Lirman et al., 2014), and source location (Forrester et al., 2013) all influence the success of restored corals. While coral restoration is a rapidly progressing field, significant knowledge gaps remain regarding the drivers of restoration success.
One such gap is the optimal density and arrangement for outplanting restored corals. Restoration via transplantation of autogenic ecosystem engineers in systems ranging from tropical forests (Zahawi and Augspurger, 2012), to grasslands (Morgan and Scacco, 2006), to mangroves (Elster, 2000) suggests that the density and arrangement of organisms used for restoration can significantly influence their survival, growth (Li and Wilson, 1998) and recruitment (Mulligan et al., 2002). Further, the density of restored ecosystem engineers can mediate important ecological processes that drive community dynamics, such as herbivory and nutrient cycling (Holl et al., 2000).
Similarly, on coral reefs outplant density and arrangement will likely affect important responses such as growth rates, habitat production, disease dynamics, and, ultimately, coral survivorship. Indeed, Griffin et al. (2015) found that short-term growth rates of restored Acropora cervicornis over 3 months in the US Virgin Islands were inversely related to outplant density. In contrast, Shaish et al. (2010) found no differences in mortality or bleaching of restored Montipora digitata in high density, low density, or “patchy” arrangements of nursery-raised corals in the Philippines after 15 months.
Theoretical work suggests that corals outplanted in high densities and arranged with even spacing will maximize the development of topographic complexity on degraded reefs (Sleeman et al., 2005). The creation of habitat may aggregate important fishes such as schooling grunts, which can focus nutrient delivery from excretion and create nutrient hotspots that can increase the growth rates of restored corals as well as important processes such as herbivory (Shantz et al., 2015) and the removal of coral predators (Ladd and Shantz, 2016). However, there is a paucity of long-term studies that investigate the role of outplant density on coral restoration success. Given that coral restoration is an expensive and labor-intensive process, determining the most effective densities in which to outplant restored corals is an important step toward balancing the costs and benefits of coral restoration.
Here, we address this information gap by investigating the influence of coral density on the growth, habitat production, and survivorship of restored corals. Over 13 months we monitored experimentally-established populations of A. cervicornis outplanted in a gradient of densities on a reef in the Florida Keys, USA. We tracked the growth, habitat production, tissue loss, and survivorship of restored corals as proxy for the success of coral restoration. We hypothesized that low-density treatments would demonstrate higher growth rates and per-coral habitat production compared to high-density treatments. Further, we predicted that per capita tissue loss and colony mortality would be greatest in high-density plots.
Methods
Study Species
Acropora cervicornis is a fast-growing, branching coral species with the ability to rapidly expand via asexual fragmentation (Glynn, 1973; Tunnicliffe, 1981). The structural complexity provided by A. cervicornis and its congener, A. palmata, provides essential habitat for a multitude of reef-associated organisms (Reviewed in Bruckner, 2002). Populations of these two species, historically structural dominants on many reefs throughout the Caribbean, have declined 80–90% in the past four decades, with drastic population reductions of >95% in some areas (Hughes, 1994; Aronson and Precht, 2001; Bruckner, 2002; Jackson et al., 2014). Acropora cervicornis populations have failed to recover throughout the majority of their historical range, resulting in their listing as “Threatened” under the US Endangered Species Act (Hogarth, 2006) and contributing to significant declines in structural complexity on many Caribbean reefs (Alvarez-Filip et al., 2009). Currently, coral restoration efforts are primarily focused on A. cervicornis due to life history characteristics amenable to rapid propagation and to the species' critical role on Caribbean coral reefs as habitat.
Experimental Design
Our field site was a low-relief reef in approximately 5–7 m of water located 6.5 km offshore of Plantation Key, Florida, USA (24.924°N, 80.503°W). We established four experimental blocks of six 4 m2 plots, with each plot separated by ≥5 m. Each block of 4 m2 plots contained one replicate of each density treatment: 3-, 6-, 12-, or 24-colonies, as well as control plots in which no corals were outplanted. Each block also contained a 12-colony treatment (hereafter “12-clumped”), in which 12 coral colonies were outplanted within 1 m2 of the plot (Figure 1). Treatments were randomly assigned to plots within a block. Three A. cervicornis genotypes (K-1, K-2, and U24), obtained from the Coral Restoration Foundation's offshore nursery off of Tavernier Key, Florida, were used and present in equal (1-1-1) ratios to create experimental treatments.
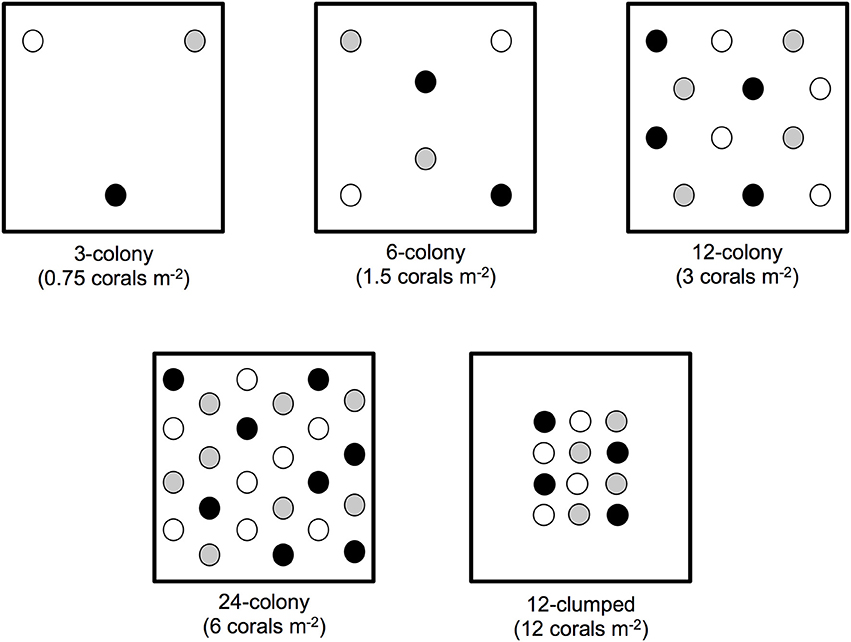
Figure 1. Schematic of experimental design (to scale). Each dot represents an individual coral outplant, different shades indicate the three unique genotypes (K-1, K-2, and U24) used to establish experimental treatments.
To establish experimental treatments we outplanted colonies of A. cervicornis approximately 85 cm in total linear extension (TLE) to each plot in May of 2013. We maximized spacing among coral colonies within a plot such that colonies in low-density plots were farther spaced than those in high-density plots. We also organized colonies to maximize genotype mixing and avoid clumping of the same genotype. Genotype analyses, completed as part of the Coral Restoration Foundation's coral nursery establishment, were done using known microsatellite markers (e.g., Baums, 2008). We outplanted four replicates of each treatment in the randomized block design for a total of 228 corals outplanted into twenty-four 4 m2 plots. Each colony was secured to the substrate using a small amount of marine epoxy where branches contacted the reef substrate and labeled with an individually numbered tag.
Coral Colony Growth, Condition and Predator Surveys
To quantify the effects of density on colony growth, we measured coral colony dimensions (length, width, and height) to the nearest centimeter every 3–6 months. Surveys were conducted in May, August, and December of 2013, and June of 2014. At each sampling event, we also recorded the percent of each coral colony without live tissue and the presence of any disease-like symptoms (e.g., rapid tissue loss, white band disease) via visual assessment. We also counted corallivorous snails (Coralliophila abbreviata) and fireworms (Hermodice carunculata) on each A. cervicornis colony. However, these predators and instances of disease were so rare that we did not explore these data quantitatively.
Statistical Analyses
We estimated total skeletal linear extension (TLE; the sum of the lengths of all branches on a colony) using length, width, and height conversions provided by Kiel et al. (2012). To calculate per capita live TLE for each survey period we used the equation:
Growth rates were calculated for each interval by dividing the TLE accumulated between survey periods by the number of days elapsed to generate a daily growth rate. For all growth rate and TLE calculations, data for corals were not included if: (1) they showed signs of previous breakage, a common natural occurrence in A. cervicornis corals, or (2) displayed 100% tissue loss, which avoided artificially depressing growth rates or TLE measures. We calculated TLE and live TLE at the plot level by summing measures of TLE or live TLE for all corals within a plot. We then used these measures to compare total habitat production (using TLE as a proxy) and live TLE among treatments.
We assessed changes through time in growth rates, per capita TLE, and per capita live TLE via a nested two-way repeated measures ANOVA that considered time and treatment or genotype as predictors and included an interaction between the main factors. For these analyses individual corals were nested within a plot and considered as a random effect to avoid violating assumptions of independence. Among treatment differences within individual survey periods were analyzed via post-hoc tests with Tukey's corrections using the multcomp package in R (Hothorn et al., 2008). Because of the non-normal structure of the tissue loss data, we used a Kruskal-Wallis test with post-hoc analysis to compare median values of the percent of colony without live tissue among treatments at each survey period.
Treatment survivorship was calculated using the percentage of colonies that were alive within a plot at each survey period. Genotype survivorship was calculated as the percentage of coral colonies for each genotype that remained alive at a given survey point. A coral was considered dead when it had no living tissue on the skeleton. Among treatment differences in survivorship, plot level TLE, and plot level live TLE were analyzed via a two-way ANOVA that considered treatment and time as predictors with an interaction between the main factors. Among treatment differences within individual survey periods were analyzed via post-hoc tests with Tukey's corrections using the multcomp package in R (Hothorn et al., 2008). Among genotype differences in survivorship at the end of the experiment were analyzed via a Fisher's Exact Test, followed by pairwise comparisons of the three genotypes using a Bonferonni correction. Colony growth rates and per capita live TLE were square-root transformed, while per capita TLE, plot level TLE, and plot level live TLE were log-transformed to meet ANOVA assumptions.
To investigate the presence of density dependent effects among treatments, we compared treatments that differed 2-fold in coral density by calculating ratios of final plot level TLE (i.e., 6-colony vs. 3-colony treatments). To generate conservative estimates, we first ranked plots within a treatment from highest to lowest plot level TLE. We then paired plots according to ranks and then divided the final plot level TLE of one treatment by the final plot level TLE of the treatment with half the number of corals (e.g., 6-colony/3-colony). If there were no density dependent effects, we expected that a doubling of coral density would result in a doubling of TLE. Thus, if density dependence did not influence habitat production, we expected a ratio of 2:1 for plot level TLE. A ratio >2 would indicate positive density dependence (i.e., a doubling of coral density resulted in more than a doubling in TLE) while a ratio <2 would suggest negative density dependence (i.e., a doubling of colony density resulted in less than a doubling in TLE). We also compared the 12-clumped and 12-colony final plot level TLE. For this comparison, if density did not influence habitat production, we expected a ratio of 1:1, since both treatments contained 12 corals. We used two-tailed t-tests to determine if ratios were significantly different from two, the expected doubling in TLE from a doubling of density (or one in the case of the 12-clumped vs. 12-colony comparison). We conducted these comparisons for both plot level TLE and plot level live TLE. We computed a Pearson product-moment correlation coefficient to assess the relationship between the density of corals and proportion of corals alive within a treatment at the end of the experiment. All analyses were conducted in R version 3.0.2 (R Core Team, 2013). All data reported are means ± SE.
Results
Genotype Effects
Genotype had no effect on restored coral growth rates, total linear extension (TLE), or live TLE at any point during the experiment (Supplementary Table 1). At the conclusion of the experiment, genotype had a marginal effect on the mean percent of a colony with no live tissue [Genotype effect: F(2, 189) = 2.649, p = 0.073] and survivorship of restored corals (Fisher's Exact Test, p = 0.078; Supplementary Figure 1). Genotype U24 was trending toward lower survivorship and having less live tissue per colony. However, genotype appeared to have little influence on coral growth and survivorship as compared to treatment effects described below.
Density Effects
Growth rates of individual corals varied among treatments more than 3-fold, from a low of 0.248 cm day−1 to a high of 0.829 cm day−1 (mean ± SE; 0.49 ± 0.016), and showed a significant time by treatment interaction [F(8, 222) = 4.694, p < 0.001; Figure 2]. Complete statistical results are provided in Supplementary Table 2. Post-hoc tests with Tukey's correction revealed that while growth rates were statistically indistinguishable from May to December 2013, corals in the 12-colony treatments grew nearly two times faster than corals in 24-colony treatments from December 2013 to June 2014 (p = 0.035). All of the other treatments also had ≥50% greater mean growth rates than the 24-colony treatment during this time period, but post-hoc tests did not detect statistical differences likely due to high variability in growth rates.
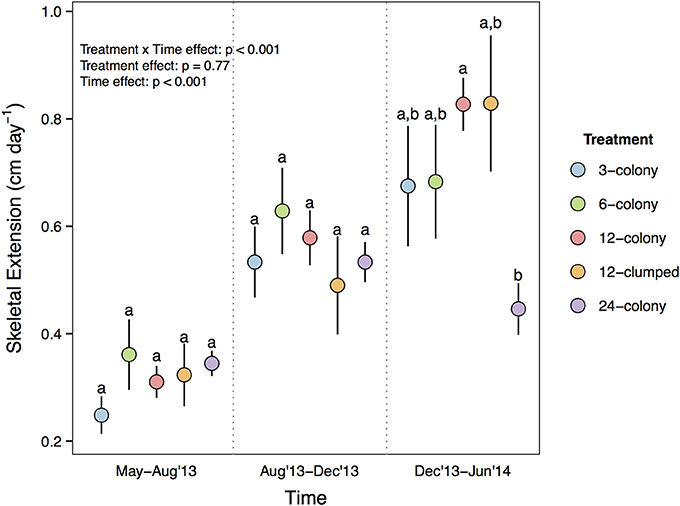
Figure 2. Daily growth rate (cm day−1) of individual corals by treatment through time. Labels on x-axis indicate the time period over which growth rates were calculated. Statistics are from nested two-way repeated measures ANOVA. Letters represent significant differences (p < 0.05) among treatments within a time period from post-hoc tests with Tukey's correction. Data are means ± SE.
Both per capita TLE [Treatment × Time effect: F(12, 476) = 3.96, p < 0.001; Figure 3A] and per capita live TLE [Treatment × Time effect: F(12, 476) = 13.322, p < 0.001; Figure 3B] differed among treatments through time. However, post-hoc tests indicated that the only among treatment differences were for live TLE at the final (June 2014) sampling period where corals from 12-colony treatments had nearly 3x more live TLE than those in the 24-colony treatments (p = 0.03). The other treatments were intermediate in live TLE and did not differ from either the 12- or 24-colony treatments. The patterns for TLE were similar but post-hoc tests did not show statistically significant differences.
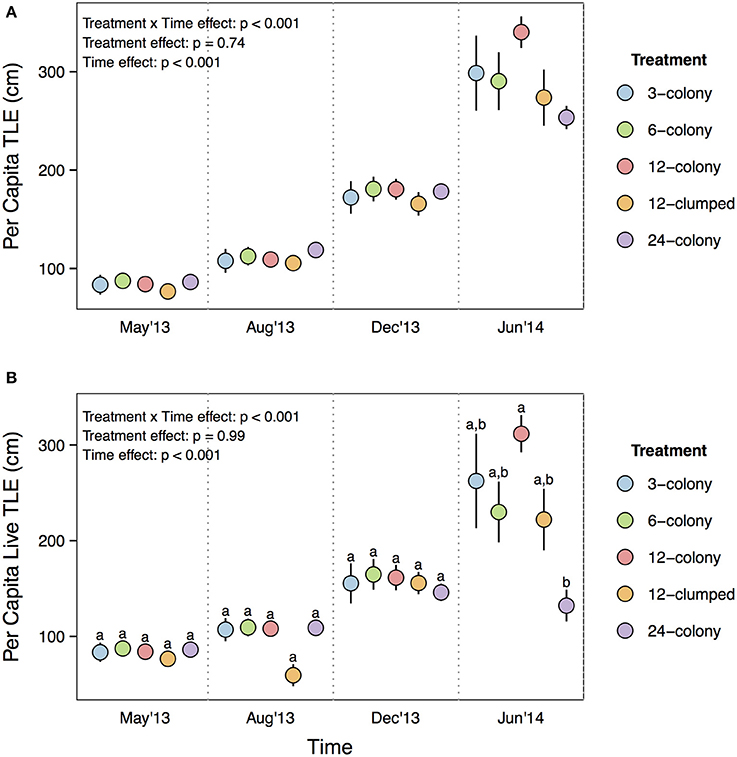
Figure 3. (A) Per capita total linear extension (TLE) and (B) per capita live TLE of individual corals by treatment through time. Labels on x-axis indicate the time at which each survey was conducted. Statistics are from nested two-way repeated measures ANOVA. Letters represent significant differences (p < 0.05) among treatments within a time period from post-hoc tests with Tukey's correction. Data are means ± SE.
Median values for percent of colony without live tissue were significantly higher for corals within the 12-clumped and 24-colony treatments as compared to 3- and 12-colony treatments (χ2 = 43.07, df = 4, p < 0.001; Figure 4). Further, we found clear effects of density treatment on restored coral survivorship [Treatment × Time effect: F(16, 72) = 4.108, p < 0.001; Figure 5], with survivorship significantly decreasing with increasing density. The 12-clumped treatment had the highest initial mortality rates (August 2013) and ended up losing approximately 50% of individual colonies by the end of the experiment, similar to that of the 24-colony treatment. On the other extreme, the 3-colony treatment had 100% survivorship for the duration of the experiment. At the conclusion of the experiment, colony survivorship was negatively correlated the density of corals (Pearson's product-moment correlation coefficient, r = −0.85, df = 3).
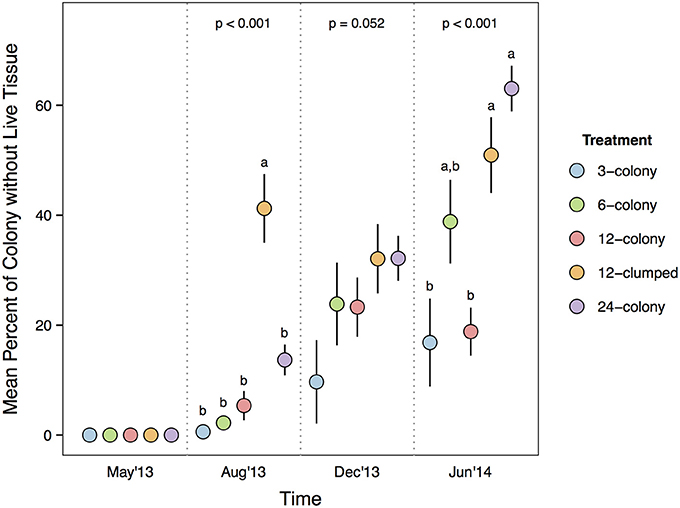
Figure 4. Percent of each coral colony without live tissue compared among treatments within each survey period. P-values for each survey period are from Kruskal-Wallis test. Letters represent significant differences (p < 0.05) among treatments from post-hoc analysis comparing median percent of colony without live tissue values within a survey period. Data are means ± SE.
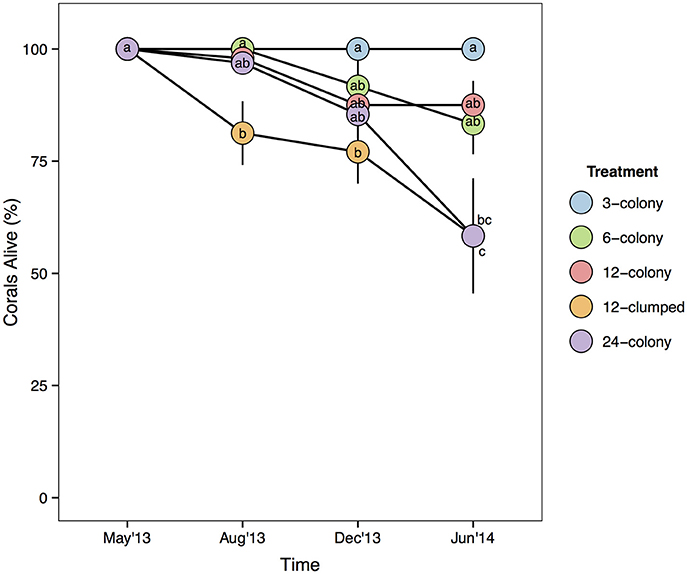
Figure 5. Mean survivorship of coral colonies by treatment over time. Statistics are from nested two-way repeated measures ANOVA. Letters represent significant differences (p < 0.05) among treatments within a time period from post-hoc tests with Tukey's correction. Data are means ± SE.
The differences in per capita live TLE resulted in the 12- and 24-colony treatments ending with similar overall habitat production (plot level TLE) by the end of the experiment, despite the 12-colony treatment starting the experiment with half the number of corals [Treatment × Time effect: F(12, 60) = 1.193, p = 0.309; Figure 6A]. The patterns in plot level live TLE were similar to overall TLE, showing significant Treatment × Time effects [F(12, 60) = 2.240, p = 0.02; Figure 6B]. Similar to the overall plot level TLE the 12- and 24-colony treatments showed similar levels of live TLE. Surprisingly, within 3 months 6-colony treatments had produced as much live TLE at the plot level as 12-clumped treatments.
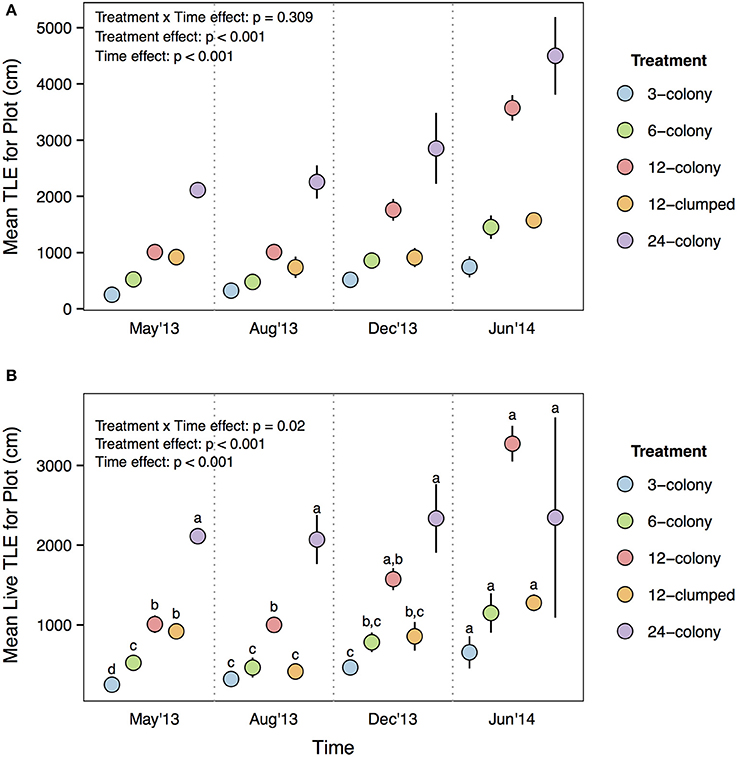
Figure 6. (A) Total linear extension and (B) live TLE at the plot level comparing among treatments across each survey period. Statistics are from nested two-way repeated measures ANOVA. Letters represent significant differences (p < 0.05) among treatments within a time period from post-hoc tests with Tukey's correction. Data are means ± SE.
Analyzing these dynamics over time within treatments showed several interesting patterns. All treatments except the most dense (12-clumped) increased in plot level TLE during the course of the experiment (Figure 7A). On average, 24-colony treatments did not accumulate live TLE at the plot level during the course of the experiment [Time effect: F(3, 12) = 0.10, p = 0.956; Figure 7B] as tissue loss appeared to occur at the same rate as tissue growth. Similarly, 12-clumped treatments actually decreased in live TLE from May to August of 2013, then rebounded to initial levels by December 2013. Conversely, 12-, 6-, and 3-colony treatments significantly increased in plot level live TLE throughout the course of the experiment. The largest increase relative to initial TLE was seen in 12-colony treatments, which more than tripled the amount of live TLE by the end of the experiment.
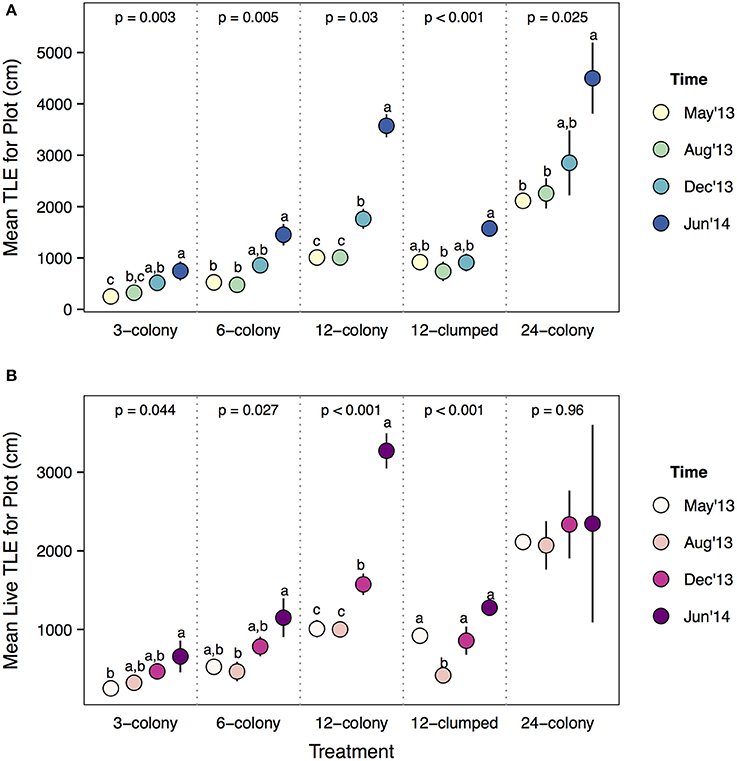
Figure 7. (A) Total linear extension and (B) live TLE at the plot level comparing among survey periods within each treatment. Statistics are from one-way ANOVA. Letters represent significant differences (p < 0.05) among time periods within a treatment from post-hoc tests with Tukey's correction. Data are means ± SE.
We found evidence of density dependent effects when we compared final habitat production (both TLE and live TLE) between treatments. For both TLE and live TLE, the 12-clumped vs. 6-colony and 24- vs. 12-colony comparisons demonstrated negative density dependence, evidenced by the nearly 1:1 ratio of TLE at the end of the experiment compared to the expected 2:1 ratios (Figure 8). Similarly, both TLE and live TLE in 12-clumped (12 corals m−2) treatments demonstrated strong negative density dependence compared to the less dense 12-colony (3 corals m−2) with ratios significantly lower than the expectation of 1:1. There was also evidence for positive density dependence, but it was less strong. The 12-colony treatments had, on average, 2.5 times and 3 times more TLE and live TLE than 6-colony treatments, respectively, although both tests for a difference from the 2:1 ratio were marginally significant (p = 0.07 for TLE and p = 0.09 for live TLE).
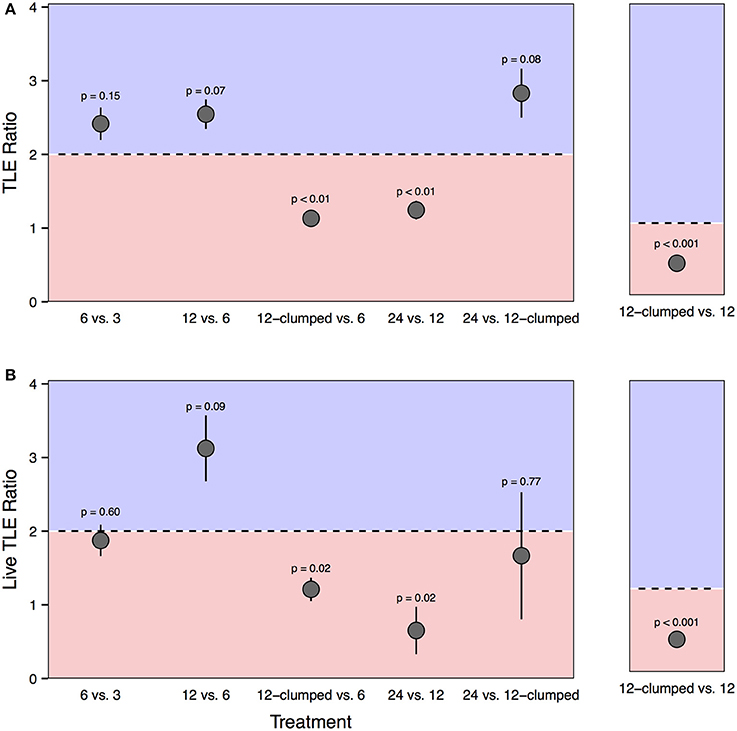
Figure 8. Ratios comparing final plot level (A) TLE and (B) live TLE between treatments differing in coral density. Comparisons are either between treatments that differ two-fold in coral density (e.g., 6-colony vs. 3 colony treatments; left panels) or with equal densities (12-clumped vs. 12-colony treatments; right panels). The black dotted line represents the expected 2:1 (right panels) or 1:1 ratio (left panels). Points that fall within the blue shaded area exceeded expectations of habitat production and suggest positive density dependence. Points that fall within the red shaded area produced less habitat than predicted and suggest negative density dependence.
Discussion
As coral reefs continue to decline globally (Bruno and Selig, 2007; Jackson et al., 2014), the need to develop effective strategies to restore degraded reefs becomes more urgent. Although coral restoration has gained increased attention as a viable strategy to restore reefs, it remains a labor- and cost-intensive strategy (Young et al., 2012; Rinkevich, 2014). Thus, there is a need to maximize ecological benefits while minimizing costs to continue scaling up coral restoration efforts. Here, we show that coral outplant density is a key factor to the success of coral restoration. We found that the survivorship of restored corals decreased with increasing density. Corals outplanted in moderate densities (12-colony treatments; 3 corals m−2) grew faster and lost less live tissue than high-density (24-colony and 12-clumped; 6 and 12 corals m−2) treatments. Further, corals in 12-colony treatments tripled in TLE during the course of the experiment and on average ended up with more live TLE at the plot level as compared to 24-colony treatments, though they started the experiment with half the number of corals. Importantly, our data suggest the presence of both positive and negative density dependent effects. Increasing density to 3 corals m−2 resulted in more coral growth than expected. But, continuing to add more corals (6 or 12 corals m−2) resulted in negative density dependence and less coral growth than expected.
Despite substantial evidence of the impact of coral genotype on coral growth rates, survivorship, and disease prevalence (Vollmer and Kline, 2008; Lirman et al., 2014), we found no effect of genotype on any of our response variables. Instead, we show that density dependence plays a large, yet underappreciated, role in the success or failure of coral restoration efforts. Although we found stronger density effects than genotype effects, we only used three genotypes of A. cervicornis. Promoting genotypic diversity should still remain a restoration priority. Given the uncertain conditions reefs are likely to experience in the future (Pandolfi, 2015; Pendleton et al., 2016), including genotypes with a range of traits and environmental tolerances will likely be essential for successful coral reef restoration.
Density often influences the success of restoring ecosystem engineers in other ecosystems (Li and Wilson, 1998; Holl et al., 2000; Mulligan et al., 2002). Yet, limited research on the role of density exists for coral restoration, particularly in the Caribbean. Our findings suggest that outplanting A. cervicornis for restoration in moderate densities (3 corals m−2 – our 12-colony treatment) maximizes growth rates and habitat production while minimizing tissue loss and coral mortality. To our knowledge, the only other study that manipulated the density of A. cervicornis colonies found a negative relationship between coral density and linear extension (Griffin et al., 2015). However, this study only tracked corals for a period of 3 months, had limited replication (n = 1 per density), and potentially confounded density effects with genotype effects. The longer (13 month) duration of our study allowed us to elucidate the effect of the density of restored corals over timescales more relevant to coral reef community recovery. Similar to Griffin et al. (2015), we found that growth rates in our 24-colony treatment were lower than the others at the end of the experiment. However, we show that density dependence can influence the success of A. cervicornis outplanted for restoration, and that the strength and direction of density dependence changes with coral density. These findings run counter to work done in the Philippines, which found no effect of outplant density or arrangement on M. digitata growth or mortality over a 15-month study, suggesting different species may display variable responses to outplant density (Shaish et al., 2010).
Density dependence has been heavily studied in terrestrial and intertidal systems, often with a particular focus on foundation species (Bertness and Callaway, 1994; Bruno et al., 2003). Work in marine systems, and specifically coral reef ecosystems, has largely focused on the effects of density dependence on the growth and survivorship of coral-associated organisms, such as coral reef fishes, rather than corals themselves (Hixon and Carr, 1997; but see Baird and Hughes, 2000; Hixon and Webster, 2002; Shantz et al., 2011; Marhaver et al., 2013). Here, we found that the densities of our densest treatments resulted in less habitat creation and live coral tissue than would be expected, suggesting negative density dependence.
Although TLE and live TLE increased for the 3-colony, 6-colony, and 12-colony plots, the densest treatments (12-clumped and 24-colony) saw little to no increase in these metrics over the 13-month study. Several mechanisms could be important for driving this negative density dependence. While small-scale alterations in water flow may benefit corals growing in close proximity, at some density threshold, such as in our 24-colony or 12-clumped treatments, coral branches could become so dense as to have a negative effect on water flow. At such high densities, reductions in water flow may reduce mass transfer of nutrients to the coral or efflux of oxygen as a byproduct of photosynthesis, contributing to declines in photosynthesis and the energy available for coral growth (Finelli et al., 2006). Additionally, crowding in high-density treatments could increase shading and intensify competition for light, effectively reducing photosynthesis (Chadwick and Morrow, 2011). Although A. cervicornis relies heavily on photosynthetic endosymbionts for energy, heterotrophic feeding is an important component of growth rates, particularly under stressful conditions (Houlbrèque and Ferrier-Pagès, 2009; Towle et al., 2015). Thus, corals in high-density plots may have experienced increased competition for food particles in the water column, contributing to lower growth rates in these treatments.
Additional mechanisms likely contributed to the negative density-dependent effects at the high-density treatments. High densities of corals may facilitate disease transmission among coral colonies. Some coral diseases can be vectored between A. cervicornis branches that are in direct contact (Williams and Miller, 2005). Thus, disease transmission may have been facilitated in 24-colony and 12-clumped treatments where branches of colonies were more likely to be in direct contact as compared to lower density treatments. The corallivorous snail C. abbreviata, and the fireworm H. carunculata can vector or act as a reservoir for coral diseases (Sussman et al., 2003; Williams and Miller, 2005; Gignoux-Wolfsohn et al., 2012). Consequently, these coral predators, which commonly feed on A. cervicornis (Miller, 1981), may cause initial disease infection or spread diseases within a plot of restored corals. Tightly clustered colonies in high-density treatments may have provided a physical escape from predation for small corallivores, increasing the probability of tissue loss and infection. Further, C. abbreviata prefers A. cervicornis colonies surrounded by conspecifics rather than solitary colonies or those surrounded by heterospecifics (Johnston and Miller, 2014), suggesting that high-density treatments may be preferred by corallivores.
While we did record predator density and disease presence as part of this study, our surveys were not frequent enough to track disease progression or link tissue loss to predator abundance. Although variable among replicates, we observed an average of 30% of corals showing signs of disease (rapid tissue loss) in 12-clumped treatments during our August 2013 survey, which coincided with significant increase in live tissue loss (Figure 4). Thus, the densest treatment appeared to facilitate disease, leading to dramatic loss of live coral. Partial colony mortality, highest in the 12-clumped and 24-coral treatments at the end of the experiment, likely depressed growth rates and contributed to the negative density-dependent effects we observed in the high-density treatments.
Farming damselfish (e.g., Stegastes planifrons) can rapidly colonize A. cervicornis colonies outplanted for restoration, cause significant amounts of partial mortality, and decrease growth rates (Schopmeyer and Lirman, 2015). While we did not specifically quantify damselfish abundance in our experiment, we also did not observe strong colonization by farming damselfish in our study. However, damselfish may selectively recruit to high-density plots that provide more shelter from predators (Almany, 2004) and substrate to create their algal lawns (Ceccarelli et al., 2001). Thus, at other restoration sites, the colonization of high-density coral treatments by territorial damselfish could be an additional mechanism contributing to negative density-dependence of corals used for restoration.
Although our analyses showed only marginally significant effects for positive density dependence, the patterns were suggestive of positive effects of increasing density to moderate levels. This pattern was most obvious going from the 6-colony to the 12-colony densities where TLE and live TLE increased 2.5–3 times. These data suggest the existence of positive feedback mechanisms for coral growth and habitat creation under moderate increases in density. For corals, more live tissue affords increased opportunity for growth, and therefore increasing structural complexity, particularly for branching corals such as A. cervicornis. Positive density dependence could also be expected for corals through the improvement of microclimatic conditions, as observed for plants in terrestrial systems (Bruno et al., 2003). For example, increased coral density could decrease laminar flow and increase mixing, reducing the boundary layer and enhancing delivery of nutrients and dissolved oxygen to nearby corals (Atkinson and Bilger, 1992; Lesser et al., 1994). For species that rely heavily on asexual fragmentation, such as A. cervicornis, high densities can function to trap and stabilize asexual fragments, contributing to a positive density dependent feedback (Tunnicliffe, 1981). Over time branches can fuse together to form dense thickets that increase resistance to physical disturbance and further promote fragment retention.
Reef fishes, often limited by habitat availability as both juveniles and adults, selectively recruit to live coral, where they grow faster than fishes recruiting to non-living structure (Holbrook et al., 2000; Feary et al., 2009; Kerry and Bellwood, 2015). The topographic complexity provided by corals can aggregate fishes, concentrating fish-derived nutrients that can increase coral growth (Holbrook et al., 2008; Shantz and Burkepile, 2014). These fish-derived nutrient hotspots also increase grazing by herbivorous fishes and decrease algal abundance, both of which likely help facilitate coral growth and survivorship (Shantz et al., 2015). Further, many of the fishes that aggregate around structurally complex corals are invertivores, such as white grunts (Haemulon plumierii), possibly promoting top-down control on coral predators (Lirman, 1999; Ladd and Shantz, 2016).
Our findings have important implications for how we approach coral restoration. Ultimately, the goal of coral restoration is to promote ecological processes and positive feedbacks that foster self-sustaining coral reef communities. While the goal of coral restoration is not focused solely on corals, these ecosystem engineers are the foundation upon which other essential species and ecological processes depend (Mumby and Steneck, 2008; Newman et al., 2015). For example, coral and fish larvae are able to track the smell of corals to use as positive settlement cues (Dixson et al., 2014). Larger corals also have higher reproductive potential (Szmant, 1986) and therefore are more likely to contribute to sexual reproduction, a key component of coral reef recovery. Thus, restoration efforts that maximize coral growth, coral survivorship, and habitat creation will likely promote these important positive feedbacks and more quickly foster the recovery of coral reef communities.
Here, we show that the direction and intensity of density dependence on the success of corals used for restoration is context-dependent. These findings highlight the need for restoration practitioners to consider the density of corals when planning restoration efforts. For A. cervicornis, the primary species used for coral restoration in the Caribbean, our data suggests that outplanting in densities of three corals m−2 can take advantage of positive density dependent processes that maximize habitat production and reduce mortality (Figure 9A). We posit that by capitalizing on positive density-dependent processes, restoration practitioners can maximize the benefits of coral restoration (Figure 9B). Further, this would avoid overloading areas with corals that could be used to restore other areas. Importantly, we found that increasing the density of coral to 6 or 12 corals m−2 can actually induce negative density dependent processes that increase coral mortality and slow coral growth, working against restoration goals. These results demonstrate the need to evaluate the influence of density on the success of other coral species used for restoration, which likely display density-dependent relationships that could be exploited to facilitate coral restoration. Further work is needed to determine the effect density has on important factors such as disease transmission and predator attraction as well as ecosystem processes such as herbivory and nutrient recycling that will likely also influence coral restoration success. Long-term studies investigating how the density of corals influences the development of coral reef communities and the ecological processes that maintain healthy reefs will advance our ability to effectively restore coral reef communities.
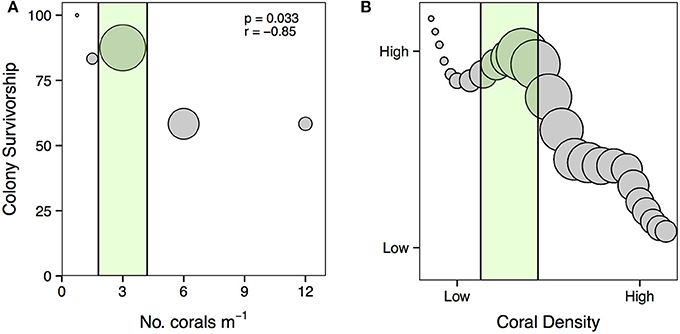
Figure 9. (A) Relationship between the density of A. cervicornis outplanted for restoration and colony survivorship. The size of each point is scaled to the average amount of live TLE (i.e., habitat created) for each treatment at the conclusion of the experiment. The green area represents the densities over which positive density dependence may facilitate coral survivorship and habitat production. Statistics from Pearson's product-moment correlation (df = 3). (B) Proposed relationship between the density of A. cervicornis outplanted for restoration, colony survivorship, and habitat production. Points are scaled to the amount of live habitat created, thus larger circles represent more habitat generation. The green area highlights densities where restoration practitioners can take advantage of positive density-dependence to maximize the benefits of coral restoration.
Author Contributions
ML, DB designed the study. ML, AS conducted fieldwork. ML conducted the analyses and wrote the manuscript with assistance from AS, KN, and DB.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Funding for this work was provided by a Florida Sea Grant Scholar award (PD-012-20) and a Friends of Gumbo Limbo Gordon Gilbert Graduate Research Grant to ML, and was facilitated by a grant from the National Science Foundation, Biological Oceanography program (OCE-1130786) to DB. This work was conducted with permission from the Florida Keys National Marine Sanctuary under permit no. FKNMS-2012-134. Comments from two anonymous reviewers improved the manuscript. We thank the Coral Restoration Foundation for providing the Acropora cervicornis colonies and fieldwork assistance. We thank A. Durán, L. Schrack, B. Pierce, A. Zenone, B. Gunn, C. Lopes, C. Fuchs and many others for assistance in the field.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2016.00261/full#supplementary-material
Supplementary Table 1. Results from nested two-way repeated measures ANOVA for genotype effects on growth rates, TLE, and live TLE.
Supplementary Table 2. Results from nested two-way repeated measures ANOVA for treatment effects on individual colony growth rate, TLE, live TLE, and results from two-way ANOVA for plot level TLE, live TLE, and survivorship.
Supplementary Figure 1. Survivorship of coral colonies by genotype over time.
References
Almany, G. R. (2004). Differential effects of habitat complexity, predators and competitors on abundance of juvenile and adult coral reef fishes. Oecologia 141, 105–113. doi: 10.1007/s00442-004-1617-0
Alvarez-Filip, L., Dulvy, N. K., Gill, J. A., Côté, I. M., and Watkinson, A. R. (2009). Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc. R. 276, 3019–3025. doi: 10.1098/rspb.2009.0339
Aronson, R., and Precht, W. (2001). White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460, 25–38. doi: 10.1023/A:1013103928980
Atkinson, M. J., and Bilger, R. W. (1992). Effects of water velocity on phosphate uptake in coral reef-flat communities. Limnol. Ocean. 37, 273–279. doi: 10.4319/lo.1992.37.2.0273
Baird, A. H., and Hughes, T. P. (2000). Competitive dominance by tabular corals: an experimental analysis of recruitment and survival of understorey assemblages. J. Exp. Mar. Bio. Ecol. 251, 117–132. doi: 10.1016/S0022-0981(00)00209-4
Baums, I. B. (2008). A restoration genetics guide for coral reef conservation. Mol. Ecol. 17, 2796–2811. doi: 10.1111/j.1365-294X.2008.03787.x
Bertness, M. D., and Callaway, R. (1994). Positive interactions in communities. Trends Ecol. Evol. 9, 191–193. doi: 10.1016/0169-5347(94)90088-4
Bruckner, A. W. (2002). Proceedings of the Caribbean Acropora Workshop: Potential Application of the U.S. Endangered Species Act as a Conservation Strategy. Silver Spring, MD: NOAA Technical Memorandum NMFS-OPR-24.
Bruno, J. F., and Selig, E. R. (2007). Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS ONE 2:e711. doi: 10.1371/journal.pone.0000711
Bruno, J. F., Stachowicz, J. J., and Bertness, M. D. (2003). Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 18, 119–125. doi: 10.1016/S0169-5347(02)00045-9
Burman, S., Aronson, R., and van Woesik, R. (2012). Biotic homogenization of coral assemblages along the Florida reef tract. Mar. Ecol. Prog. Ser. 467, 89–96. doi: 10.3354/meps09950
Cabaitan, P. C., Yap, H. T., and Gomez, E. D. (2015). Performance of single versus mixed coral species for transplantation to restore degraded reefs. Restor. Ecol. 23, 349–356. doi: 10.1111/rec.12205
Cardini, U., van Hoytema, N., Al-Rshaidat, M. M. D., Schuhmacher, H., Wild, C., and Naumann, M. S. (2015). 37 Years later: revisiting a Red Sea long-term monitoring site. Coral Reefs 34, 1111. doi: 10.1007/s00338-015-1321-z
Ceccarelli, D. M., Jones, G. P., and McCook, L. J. (2001). Territorial damselfishes as determinants of the structure of benthic communities on coral reefs. Oceanogr. Mar. Biol. 39, 355–389.
Chadwick, N. E., and Morrow, K. M. (2011). “Competition among sessile organisms on Coral Reefs,” in Coral Reefs: An Ecosystem in Transition, eds Z. Dubinsky and N. Stambler (Dordrecht: Springer Netherlands), 347–371.
Costanza, R., de Groot, R., Sutton, P., van der Ploeg, S., Anderson, S. J., Kubiszewski, I., et al. (2014). Changes in the global value of ecosystem services. Glob. Environ. Chang. 26, 152–158. doi: 10.1016/j.gloenvcha.2014.04.002
Dixson, D. L., Abrego, D., and Hay, M. E. (2014). Chemically mediated behavior of recruiting corals and fishes: a tipping point that may limit reef recovery. Science 345, 892–897. doi: 10.1126/science.1255057
Elster, C. (2000). Reasons for reforestation success and failure with three mangrove species in Colombia. For. Ecol. Manage. 131, 201–214. doi: 10.1016/S0378-1127(99)00214-5
Feary, D. A., McCormick, M. I., and Jones, G. P. (2009). Growth of reef fishes in response to live coral cover. J. Exp. Mar. Bio. Ecol. 373, 45–49. doi: 10.1016/j.jembe.2009.03.002
Ferrario, F., Beck, M. W., Storlazzi, C. D., Micheli, F., Shepard, C. C., and Airoldi, L. (2014). The effectiveness of coral reefs for coastal hazard risk reduction and adaptation. Nat. Commun. 5, 3794. doi: 10.1038/ncomms4794
Finelli, C. M., Helmuth, B. S. T., Pentcheff, N. D., and Wethey, D. S. (2006). Water flow influences oxygen transport and photosynthetic efficiency in corals. Coral Reefs 25, 47–57. doi: 10.1007/s00338-005-0055-8
Forrester, G. E., Taylor, K., Schofield, S., and Maynard, A. (2013). Colony growth of corals transplanted for restoration depends on their site of origin and environmental factors. Mar. Ecol. 34, 186–192. doi: 10.1111/maec.12000
Gardner, T. A., Côté, I. M., Gill, J. A., Grant, A., and Watkinson, A. R. (2003). Long-term region-wide declines in Caribbean corals. Science. 301, 958–960. doi: 10.1126/science.1086050
Garrison, V., and Ward, G. (2008). Storm-generated coral fragments - A viable source of transplants for reef rehabilitation. Biol. Conserv. 141, 3089–3100. doi: 10.1016/j.biocon.2008.09.020
Gignoux-Wolfsohn, S. A., Marks, C. J., and Vollmer, S. V. (2012). White Band Disease transmission in the threatened coral, Acropora cervicornis. Sci. Rep. 2:804. doi: 10.1038/srep00804
Glynn, P. W. (1973). “Aspects of the ecology of coral reefs in the western Atlantic region,” in Biology and Geology of Coral Reefs, Vol. 2, eds O. A. Jones and R. Endean (New York, NY: Academic Press), 271–324.
Griffin, J. N., Schrack, E. C., Lewis, K.-A., Baums, I. B., Soomdat, N., and Silliman, B. R. (2015). Density-dependent effects on initial growth of a branching coral under restoration. Restor. Ecol. 23, 197–200. doi: 10.1111/rec.12173
Hixon, M. A., and Webster, M. S. (2002). “Density dependence in reef fish populations,” in Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem, ed P. F. Sale (San Deigo, CA: Academic Press), 303–325.
Hixon, M., and Carr, M. (1997). Synergistic predation, density dependence, and population regulation in marine fish. Science. 277, 946–949.
Hogarth, W. (2006). Endangered and Threatened Species: Final Listing Determinations for Elkhorn Coral and Staghorn Coral. Fed. Regist. 71, 26852–26872.
Holbrook, S. J., Brooks, A. J., Schmitt, R. J., and Stewart, H. L. (2008). Effects of sheltering fish on growth of their host corals. Mar. Biol. 155, 521–530. doi: 10.1007/s00227-008-1051-7
Holbrook, S. J., Forrester, G. E., Schmitt, R. J., Holbrook, S. J., Forrester, G. E., and Schmitt, R. J. (2000). Spatial patterns in abundance of a damselfish reflect availability of suitable habitat. Oecologia 122, 109–120. doi: 10.1007/PL00008826
Holl, K., Loik, M., Lin, E., and Samuels, I. (2000). Tropical montane forest restoration in Costa Rica: overcoming barriers to dispersal and establishment. Restor. Ecol. 8, 339–349. doi: 10.1046/j.1526-100x.2000.80049.x
Hothorn, T., Bretz, F., and Westfall, P. (2008). Simultaneous inference in general parametric models. Biometrical J. 50, 346–363. doi: 10.1002/bimj.200810425
Houlbrèque, F., and Ferrier-Pagès, C. (2009). Heterotrophy in tropical scleractinian corals. Biol. Rev. 84, 1–17. doi: 10.1111/j.1469-185X.2008.00058.x
Hughes, T. (1994). Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265, 1547–1551.
Jackson, J. B. C., Donovan, M. K., Cramer, K. L., and Lam, W. (2014). Status and Trends of Caribbean Coral Reefs : 1970-2012. Gland: Global Coral Reeef Monitoring Network, IUCN.
Johnston, L., and Miller, M. W. (2014). Negative indirect effects of neighbors on imperiled scleractinian corals. Coral Reefs 33, 1047–1056. doi: 10.1007/s00338-014-1176-8
Kerry, J. T., and Bellwood, D. R. (2015). Do tabular corals constitute keystone structures for fishes on coral reefs? Coral Reefs 34, 41–50. doi: 10.1007/s00338-014-1232-4
Kiel, C., Huntington, B., and Miller, M. (2012). Tractable field metrics for restoration and recovery monitoring of staghorn coral Acropora cervicornis. Endanger. Species Res. 19, 171–176. doi: 10.3354/esr00474
Ladd, M. C., and Shantz, A. A. (2016). Novel enemies – previously unknown predators of the bearded fireworm. Front. Ecol. Environ. 14, 342–343. doi: 10.1002/fee.1305/suppinfo
Lesser, M. P., Weis, V. M., Patterson, M. R., and Jokiel, P. L. (1994). Effects of morphology and water motion on carbon delivery and productivity in the reef coral, Pocillopora damicornis (Linnaeus): diffusion barriers, inorganic carbon limitation, and biochemical plasticity. J. Exp. Mar. Bio. Ecol. 178, 153–179. doi: 10.1016/0022-0981(94)90034-5
Li, X., and Wilson, S. D. (1998). Facilication among woody plants establishing in an old field. Ecology 79, 2694–2705.
Lirman, D. (1999). Reef fish communities associated with Acropora palmata: relationships to benthic attributes. Bull. Mar. Sci. 65, 235–252.
Lirman, D., Schopmeyer, S., Galvan, V., Drury, C., Baker, A. C., and Baums, I. B. (2014). Growth dynamics of the threatened Caribbean Staghorn Coral Acropora cervicornis: influence of host genotype, symbiont identity, colony size, and environmental setting. PLoS ONE 9:e107253. doi: 10.1371/journal.pone.0107253
Marhaver, K. L., Vermeij, M. J., Rohwer, F., and Sandin, S. A. (2013). Janzen-Connell effects in a broadcast-spawning Caribbean coral : distance-dependent survival of larvae and settlers. Ecology 94, 146–160. doi: 10.1890/12-0985.1
Miller, A. C. (1981). Cnidarian prey of the snails Coralliophila abbreviata and C. caribaeae (Gastropoda- Muricidae) in Discovery Bay, Jamaica. Bull. Mar. Sci. 31, 932–934.
Moberg, F., and Folke, C. (1999). Ecological goods and services of coral reef ecosystems. Ecol. Econ. 29, 215–233. doi: 10.1016/S0921-8009(99)00009-9
Morgan, J. W., and Scacco, P. J. (2006). Planting designs in ecological restoration: insights from the Button Wrinklewort. Ecol. Manag. Restor. 7, 51–54. doi: 10.1111/j.1442-8903.2006.00248.x
Mulligan, M. K., Kirkman, L. K., and Mitchell, R. J. (2002). Aristida beyrichiana (Wiregrass) establishment and recruitment: implications for restoration. Restor. Ecol. 10, 68–76. doi: 10.1046/j.1526-100X.2002.10107.x
Mumby, P. J., and Steneck, R. S. (2008). Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol. Evol. 23, 555–563. doi: 10.1016/j.tree.2008.06.011
Newman, S. P., Meesters, E. H., Dryden, C. S., Williams, S. M., Sanchez, C., Mumby, P. J., et al. (2015). Reef flattening effects on total richness and species responses in the Caribbean. J. Anim. Ecol. 84, 1678–1689. doi: 10.1111/1365-2656.12429
Pandolfi, J. M. (2015). Incorporating uncertainty in predicting the future response of coral reefs to climate change. Annu. Rev. Ecol. Evol. Syst. 46, 281–303. doi: 10.1146/annurev-ecolsys-120213-091811
Pendleton, L. H., Hoegh-Guldberg, O., Langdon, C., and Comte, A. (2016). Multiple stressors and ecological complexity require a new approach to coral reef research. Front. Mar. Sci. 3:36. doi: 10.3389/fmars0.2016.00036
R Core Team (2013). R: A Language and Environment for Statistical Computing. Available online at: http://www.r-project.org/
Reaka-Kudla, M. L. (2005). “Biodiversity of Caribbean coral reefs,” in Caribbean Marine Biodiversity, eds P. Miloslavich and E. Klein (Lancaster, PA: DesTech Publishers), 259–276.
Rinkevich, B. (2014). Rebuilding coral reefs: does active reef restoration lead to sustainable reefs? Curr. Opin. Environ. Sustain. 7, 28–36. doi: 10.1016/j.cosust.2013.11.018
Ruzicka, R., Colella, M., Porter, J., Morrison, J., Kidney, J., Brinkhuis, V., et al. (2013). Temporal changes in benthic assemblages on Florida Keys reefs 11 years after the 1997/1998 El Niño. Mar. Ecol. Prog. Ser. 489, 125–141. doi: 10.3354/meps10427
Schopmeyer, S. A., and Lirman, D. (2015). Occupation dynamics and impacts of damselfish territoriality on recovering populations of the threatened staghorn coral, Acropora cervicornis. PLoS ONE 10:e141302. doi: 10.1371/journal.pone.0141302
Shaish, L., Levy, G., Katzir, G., and Rinkevich, B. (2010). Employing a highly fragmented, weedy coral species in reef restoration. Ecol. Eng. 36, 1424–1432. doi: 10.1016/j.ecoleng.2010.06.022
Shantz, A. A., Ladd, M. C., Shrack, E., and Burkepile, D. E. (2015). Fish-derived nutrient hotspots shape coral reef benthic communities. Ecol. Appl. 25, 2142–2152. doi: 10.1890/14-2209.1
Shantz, A. A., and Burkepile, D. E. (2014). Context-dependent effects of nutrient loading on the coral-algal mutualism. Ecology 95, 1995–2005. doi: 10.1890/13-1407.1
Shantz, A., Stier, A., and Idjadi, J. A. (2011). Coral density and predation affect growth of a reef-building coral. Coral Reefs 30, 363–367. doi: 10.1007/s00338-010-0694-2
Sleeman, J. C., Boggs, G. S., Radford, B. C., and Kendrick, G. A. (2005). Using agent-based models to aid reef restoration: enhancing coral cover and topographic complexity through the spatial arrangement of coral transplants. Restor. Ecol. 13, 685–694. doi: 10.1111/j.1526-100X.2005.00087.x
Sussman, M., Loya, Y., Fine, M., and Rosenberg, E. (2003). The marine fireworm Hermodice carunculata is a winter reservoir and spring-summer vector for the coral-bleaching pathogen Vibrio shiloi. Environ. Microbiol. 5, 250–255. doi: 10.1046/j.1462-2920.2003.00424.x
Towle, E. K., Enochs, I. C., and Langdon, C. (2015). Threatened Caribbean coral is able to mitigate the adverse effects of ocean acidification on calcification by increasing feeding rate. PLoS ONE 10:e0123394. doi: 10.1371/journal.pone.0123394
Tunnicliffe, V. (1981). Breakage and propagation of the stony coral Acropora cervicornis. Proc. Natl. Acad. Sci. U.S.A. 78, 2427–2431. doi: 10.1073/pnas.78.4.2427
Vollmer, S. V., and Kline, D. I. (2008). Natural disease resistance in threatened staghorn corals. PLoS ONE 3:e3718. doi: 10.1371/journal.pone.0003718
Williams, D. E., and Miller, M. W. (2005). Coral disease outbreak : pattern, prevalence and transmission in Acropora cervicornis. Mar. Ecol. Prog. Ser. 301, 119–128. doi: 10.3354/meps301119
Young, C., Schopmeyer, S., and Lirman, D. (2012). A Review of reef restoration and coral propagation using the threatened genus Acropora in the Caribbean and Western Atlantic. Bull. Mar. Sci. 88, 1075–1098. doi: 10.5343/bms.2011.1143
Keywords: coral restoration, Acropora cervicornis, density dependence, ecological restoration, coral reef, endangered species, restoration design
Citation: Ladd MC, Shantz AA, Nedimyer K and Burkepile DE (2016) Density Dependence Drives Habitat Production and Survivorship of Acropora cervicornis Used for Restoration on a Caribbean Coral Reef. Front. Mar. Sci. 3:261. doi: 10.3389/fmars.2016.00261
Received: 01 October 2016; Accepted: 25 November 2016;
Published: 12 December 2016.
Edited by:
Ines Stuhldreier, University of Bremen, GermanyReviewed by:
Rob Ruzicka, Florida Fish and Wildlife Research Institute, USADouglas Fenner, NMFS-NOAA, USA
Copyright © 2016 Ladd, Shantz, Nedimyer and Burkepile. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark C. Ladd, bWFyay5sYWRkQGxpZmVzY2kudWNzYi5lZHU=
†Present Address: Mark C. Ladd, Andrew A. Shantz, and Deron E. Burkepile, Department of Ecology, Evolution and Marine Biology, University of California Santa Barbara, Santa Barbara, CA, USA
 Mark C. Ladd
Mark C. Ladd Andrew A. Shantz
Andrew A. Shantz Ken Nedimyer
Ken Nedimyer Deron E. Burkepile
Deron E. Burkepile