- 1Seven Seas Marine Consultancy, Amsterdam, Netherlands
- 2Wageningen UR, Wageningen Marine Research, Den Helder, Netherlands
- 3Peruvian Centre for Cetacean Research, Centro Peruano de Estudios Cetológicos, Museo de Delfines, Lima, Peru
Within the Gulf of Guinea high levels of fisheries-related cetacean mortality (bycatch and direct-capture) has been documented. For locally rare species such removals could potentially lead to significant population level effects. However, information on the cetacean abundance and distribution is scarce. Similarly, it remains largely unreported where fishing fleets operate offshore. A cetacean survey took place during geophysical surveys (2013–2014) along the coasts of Ghana and Côte d'Ivoire. This provided a unique opportunity to study both offshore cetacean and fishing communities. Due to large group-sizes, melon-headed whales were the most abundant (0.34 animals km−1) followed by Fraser's dolphins and short-finned pilot whales. Range state records were confirmed for melon-headed whale and Fraser's dolphin in Ivoirian waters and ten further species represented first at-sea sightings. The artisanal fishing canoe was most abundant (92% of all vessels) and recorded up to 99.5 km from the Ghanaian coast. Asian trawlers operated over shelf areas and tuna purse-seine vessels in deep oceanic and slope waters. Fraser's dolphins, melon-headed whales, pantropical spotted dolphins, bottlenose dolphins, and pilot whales were recorded in areas with the highest fishing densities. Melon-headed whales, pilot whales, and rough-toothed dolphins were observed in vicinity of trawlers; bottlenose dolphins, pantropical spotted dolphins, and pilot whales in vicinity of canoes. Some notable differences were found in the species composition between the present surveys and port-based surveys of landed cetaceans (bycatch/direct-captures). These may be explained by (1) feeding strategies (nocturnal vs. diurnal; surface vs. deep water); (2) different attractions to vessels/fishing gear; (3) variable body sizes; and (4) difficulty to positively identify species. Despite these differences, both cetaceans and fishing vessels predominantly occurred in shelf and slope waters (< 1000 m depth contour), making fishery-related mortality likely. The poor knowledge on population trends of cetaceans in this unique upwelling region, together with a high demand for cetacean products for human consumption (as “marine bushmeat”) may lead to a potential decline of some species that may go unnoticed. These new insights can provide a foundation for the urgently required risk assessments of cetacean mortality in fisheries within the northern Gulf of Guinea.
Introduction
Fisheries bycatch (entanglement in fishing gear) is a key threat to cetacean species along the coast of West Africa. The current lack of information regarding the impact on cetacean abundance and population structure hinders assessments of the sustainability of mortality levels (Van Waerebeek and Ofori-Danson, 1999; Van Waerebeek et al., 2000, 2003; Ofori-Danson et al., 2003; Clapham and Van Waerebeek, 2007; Weir and Pierce, 2012). The capture locations and thus the type of habitat (neritic, slope, pelagic) where cetacean mortality occurs remain unreported as fishermen may operate both shoreward and offshore of the continental shelf and at considerable distance from the fishing ports where they land their catches.
Within the Gulf of Guinea [extending from Cape Palmas in Liberia to Cape Lopez in Gabon; International Hydrographic Organization (IHO), 1953], dedicated port-based research on the exploitation of cetaceans in Ghanaian waters has been carried out intermittently since 1995 using specimens and photographic evidence obtained from bycatch in fisheries, directed takes and several strandings (Ofori-Danson and Odei, 1997; Van Waerebeek and Ofori-Danson, 1999; Debrah, 2000; Ofori-Danson et al., 2003; Van Waerebeek et al., 2009, 2014; Debrah et al., 2010). Recent data originating from captured specimens landed at fishing ports, as well as strandings, provided a fully validated list of 18 cetacean species for Ghana (Van Waerebeek et al., 2009). Similarly, using data originating from strandings, captures, bycatch, whaling, and a few at-sea records has provided a list of 16 cetacean species in Côte d'Ivoire (Weir, 2010; Perrin and Van Waerebeek, 2012; Weir et al., 2013a,b).
A longitudinal set of landings data on cetaceans are available from a few fishing villages in southern Ghana (Ofori-Danson et al., 2003; Debrah et al., 2010; Van Waerebeek et al., 2014; Table 3). The Dixcove village holds a large community of drift-gillnet fishermen in the Ahanta district in the Western Region on Ghana's coast, where cetacean landings are highest. During the latest port-based survey in Dixcove, results indicated that daily cetacean landings in a single fishing port may have increased from 0.74 animals per day in 2001–2003 (Debrah et al., 2010) to 2.82 animals per day in 2013–2014 (Van Waerebeek et al., 2014). Generally, cetacean carcasses are often used as bait in shark fisheries but most captured animals seem to be landed, butchered and sold for human consumption, the co-called trade in marine bushmeat (Alfaro-Shigueto and Van Waerebeek, 2001; Ofori-Danson et al., 2003; Clapham and Van Waerebeek, 2007). In Ghana, as well as in the neighboring countries (Togo, Benin, and Côte d'Ivoire), cetaceans are protected species, although at Ghanaian ports cetaceans are landed and sold without impediment (Debrah et al., 2010; Segniagbeto and Van Waerebeek, 2010; Sohou et al., 2013; Segniagbeto et al., 2014). Aquatic mammals are protected by Ghana's 1971 Wildlife Conservation Regulation (Debrah et al., 2010), so direct captures of cetaceans are illegal in Ghana. However, there exists no legislation that we are aware of that outlaws landings of incidentally captured animals (by-catch). In recent years it has become apparent that considerable numbers of dolphins are being directly targeted in the gillnet fisheries or are harpooned or lanced at close quarters (Van Waerebeek et al., 2009). The latter is evident by the deep piercing dorsal wounds in a number of specimens landed at the fishing port of Dixcove and confirmed through fishermen's accounts (Debrah, 2000; Ofori-Danson et al., 2003; Van Waerebeek et al., 2009). Prices paid for dolphins are as high as those for similarly-sized billfishes, such as sailfish, marlin, and swordfish (Debrah et al., 2010). A decline in fish stocks together with a rapidly growing human population has turned formerly less attractive marine resources such as cetaceans and sea turtles into marine bushmeat (Ofori-Danson et al., 2003; Clapham and Van Waerebeek, 2007). This causal relationship was frequently cited by Ghanaian fishermen when interviewed and asked to explain their increasing captures of dolphins and sea turtles (Van Waerebeek et al., 2009). The combination of an increased number of fishermen per boat and overall reduced catch levels per boat, already apparent since 2001, highlights the decline of this sector as a source of gainful employment (Atta-Mills et al., 2004). This explains the increasing pressure upon local communities to exploit all possible marine resources, including cetaceans and turtles.
Along the West African coasts no systematic monitoring of cetacean mortality occurs outside of some ports in Ghana and, more recently, Mauritania (Weir and Pierce, 2012; Mullié et al., 2013). As there are no abundance estimates for cetaceans within the region and no operational national management plans, the levels of exploitations are of great concern, particularly for species such as the Clymene dolphin (Stenella clymene) which accounted for a third of all the captured cetaceans landed between 2013 and 2014 at one Ghanaian fishing village (Van Waerebeek et al., 2014). The International Union for Conservation of Nature (IUCN) recently classified Clymene dolphins as a Data Deficient species (Hammond et al., 2010). However, the documented high mortality of the species in Ghanaian fisheries (Ofori-Danson et al., 2003; Van Waerebeek et al., 2009; Debrah et al., 2010) led to the addition of the eastern tropical Atlantic (ETA) Clymene dolphin to the Conservation of Migratory Species (CMS) of Wild Animals Appendix II in 2008, as a migratory species that needs, or would significantly benefit from, international cooperation (Van Waerebeek and Perrin, 2012).
In the Ivoirian/Ghanaian part of the Gulf of Guinea, the fisheries sector can broadly be categorized into three subsections: Small scale (or artisanal); semi-industrial (or coastal); and industrial fisheries. The artisanal fisheries category, where large dug-out wooden canoes are most commonly used, is the most important with over 11,200 canoes operating actively from over 300 landing sites located along the entire 550 km length of the coastline of Ghana (Aheto et al., 2012). Drift gillnets are used offshore to exploit mainly large pelagic species such as, e.g., blue sharks (Prionace glauca), hammerhead sharks (Sphyrna spp.), yellowfin tuna (Thunnus albacares), skipjack tuna (Katsuwonus pelamis), Atlantic sailfish (Istiophorus albicans), and swordfish (Xiphias gladius) [Food and Agriculture Organization (FAO), 2007]. The semi-industrial fleet operates as purse seiners targeting mainly small pelagic fish during the upwelling periods, and switching to bottom trawling for the rest of the year [Food and Agriculture Organization (FAO), 2007]. The industrial fleet is made up of trawlers and shrimpers exploiting demersal and semi-pelagic species whilst the tuna fishing vessels target mainly yellowfin, skipjack, and bigeyetuna [Thunnus obesus; Food and Agriculture Organization (FAO), 2007].
Here we report on a marine mammal survey that took place from a geophysical seismic survey vessel during two subsequent years along the coasts of Ghana and Côte d'Ivoire. The objective of this study was to gather new information on the poorly monitored local cetacean populations in order to understand the threat posed by interactions with fisheries either due to unintended bycatch (entanglement) or direct capture. The information presented provides (a) a valuable insight into the occurrence, relative abundance, and at-sea distribution of cetaceans; (b) an overview of the distribution of fishing activities; (c) information on those areas where fishing density levels were at their highest; and (d) an indication as to which cetacean species appear to be under the greatest fishing pressure. As such, these findings provide new directions for future assessments of fishing pressure on cetaceans through incidental catches and directed takes.
Methods
Study Area
The Republic of Ghana has borders with the Republic of Côte d'Ivoire to the west and the Republic of Togo to the east. There are two seasonal periods of coastal upwelling per year (major and minor), with differing duration and intensities. During the upwelling season the sea surface temperature (SST) drops whilst the surface salinity levels increase and the dissolved oxygen levels decrease (Koranteng, 2001). The major upwelling of nutrient-rich water (the “long cold season”) occurs between July and September when the SST falls below 25°C. The minor upwelling (the “short cold season”) normally lasts for only about 3 weeks, occurring anytime between December and March. In between the cold seasons are warm seasons during which SST is high (27–29°C) and during which a strong thermocline is formed in continental shelf waters (Koranteng, 2001). The main local surface flow is dominated by the eastward Guinea Current, accompanied by a westward undercurrent (Adamec and O'Brien, 1978).
Survey Design
Effort-corrected cetacean observations (i.e., number of sightings per unit of effort, whereby effort is defined as distance surveyed) were carried out in Ghanaian waters (18 April–31 May 2013) and Ghanaian/Ivoirian waters (13 February–25 May 2014) during a geophysical seismic survey onboard the Geco Eagle (94.8 m) and Geco Triton (82.7 m). The distribution of survey effort was determined by parallel survey transects designed for the geophysical activities and the vessels did not divert from the track-line when sightings were made. The Geco Eagle left Takoradi, Ghana on 18 April 2013 and transited to the study area (04°33′N, 002°53′W) which was located 38–67 km (range) from the Ghanaian coast. The Geco Triton left Takoradi on 12 February 2014 and transited to the study area (04°34′N, 003°09′W) which was located 43–76 km (range) from the Côte d'Ivoire/Ghana border (Figure 1). There were three different survey periods (18 April–31 May 2013; 13 February–25 March 2014; 26 March–25 May 2014) utilizing different teams of observers. The survey area covered in 2013 comprised of water depths between 1200 and 3600 m and the survey area covered in 2014 was located in both Ivoirian and Ghanaian waters situated slightly further West (water depths of 67–3650 m; Figure 1).
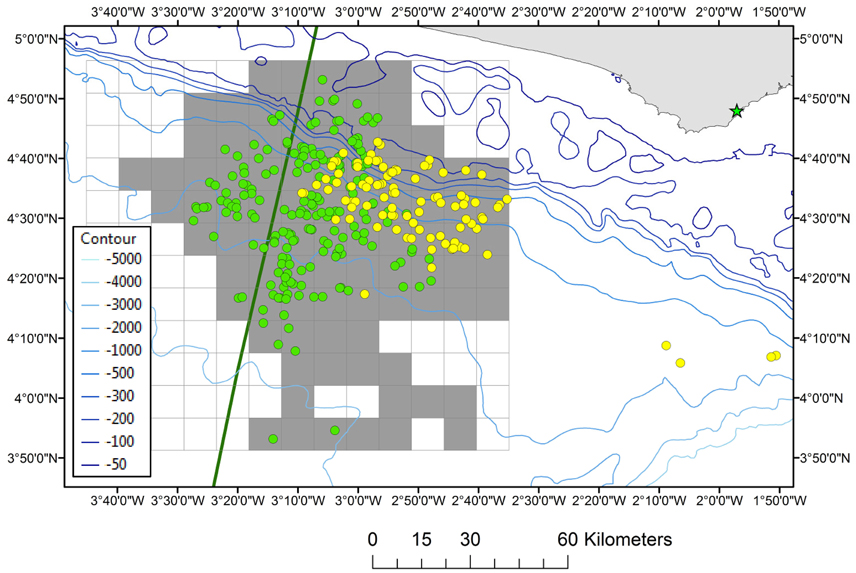
Figure 1. The location of the study area. Grid cells are shaded gray where survey effort (2013 + 2014) took place in the (gridded) survey area. The positions of cetaceans observed in both survey years are depicted as yellow dots (2013) and green dots (2014). The EEZ border between Côte d'Ivoire and Ghana is also shown (green thick line). The position of Dixcove fishing port is presented by a green asterix. Depth contours are displayed from 50 to 5000 m water depth.
Both vessels operated with a speed over ground of ca. 4 knots. Observations were carried out during all weather conditions following guidelines for minimizing the risk of injury and disturbance to marine mammals from seismic surveys (JNCC, 2010) and this took place during all daylight hours (06:00-18:45 UTC). Whilst one observer carried out a 2 h observation watch the other observer was on break. Observational effort was conducted from the bridge wings and foredeck at 18 m height (Geco Eagle) and 14 m height (Geco Triton) with occasional watches carried out from the higher decks (20 m and 18 m respectively). The observers scanned the sea predominately ahead of the vessel with the naked eye and also used binoculars (8 × 43 and 10 × 42) for searching the horizon, aiding species identification, and group-size estimations. When a sighting was made the radial sighting distance was determined using person-specific range-sticks (Heinemann, 1981). The bearing to the sighted animals and their heading were estimated using the ship's mounted compasses which were positioned on both the starboard and portside bridge wings (Geco Eagle) or center console (Geco Triton). Sightings data included the time (UTC), GPS position, water depth, species identification, group size, and the presence of calves and/or sub-adults. DSLR cameras were used to aid species identification, confirm group-sizes and the presence of calves. Zoom lenses (e.g., Canon 7D and Canon EOS 5D Mark II with a 200 mm f2.8 lens and 1.4xconverter; a NIKON D7000 with a 70–300 mm f5.6 lens and a Canon EOS550D with a 100–400 mm f4.5–5.6 lens) were used. Environmental observations were also collected and included wind speed and direction, swell height (low < 2 m, medium 2–4 m, and large > 4 m), and visibility (estimated by eye: Poor < 1 km, moderate 1–5 km, and good > 5 km), glare intensity (strong, weak, variable, or no glare), and Beaufort sea state (BSS). Water depth and SST were routinely measured throughout the survey period (Acoustic Doppler Current Profiler data). A GPSMAP76CSx (Garmin GPS) was used to log the ship's position every minute of the survey. GPS, speed, and course data were not continuously logged by the observers during the second leg; however, this information was provided by the ship's navigators. All the observers had previous experience of conducting cetacean surveys in tropical waters.
Species Categories
Baleen whales located too distant from the vessel to allow definite identification (>1 km) were classified as “balaenopterid” (i.e., large rorqual with vertical blow) or “Bryde's/Sei whale” (i.e., large rorquals with prominent, upright, and falcate dorsal fin). Depending on the sighting distance and glare intensities apparent, dolphins which could not be positively identified were classified as follows: Stenella/Delphinus sp. (definitely one of the five Stenella species and/or Delphinus species); “Stenella sp.” (i.e., definitely a Stenella species with a mid-length beak); “spinner/Delphinus” (i.e., dolphins with a very long thin beak); “spinner/clymene sp.” (i.e., small active dolphins seen “spinning” and likely to be one of these two species); “small blackfish sp.” (i.e., melon-headed whale Peponocephala electra or pygmy killer whale Feresa attenuata); or “large blackfish sp.” (i.e., killer whale Orcinus orca, pilot whale, or false killer whale Pseudorca crassidens). All other unidentified animals were classed as “dolphin sp.”; “large dolphin sp.” or “whale sp.”
Fisheries
The following information was collected on fishing vessels in Ghanaian waters: Date, time of initial observation (UTC), and vessel type: Fishing canoe, trawler, or tuna purse-seiners. During 2013 systematic scans were carried out to record the number of fishing vessels visible to the naked eye. These systematic counts took place early morning (08:00-09:00) and afternoon (14:00-15:00), counting all fishing vessels (≤ 5 km) around the vessel (360°). As such, these systematic scans could be used to estimate fishing vessel density (i.e., the number of vessels per unit of area). In 2014, only the location of fishing vessels was recorded, and the area surveyed was unknown. Hence, fishing vessel density could not be directly estimated. The survey in 2014 was initiated by different observers and these were informed by the client representatives that information regarding fishing vessels was to be collected by two support/chase vessels which were operating ahead of the survey vessel. These support vessels were tasked with guiding the fishing vessels away from the intended track of the seismic survey vessel. Fishing vessels were therefore treated as point observations (i.e., geographic location, date/time, and vessel type). Given the lack of effort-corrected fishing vessel data in 2014, it was assumed that all vessels within half a nautical mile on either side of the seismic survey vessel were recorded.
Data Analysis (Effort-Corrected)
It is extremely unlikely that all animals within a surveyed area are sighted. The ability of the observer to sight a marine mammal is negatively affected by poor weather conditions. Prevailing weather conditions such as sea state, swell height and visibility were therefore considered and only cetacean data collected in “good” conditions, i.e., Beaufort Sea States (BSS 0 to 4), good visibility (≥5 km) and low swells (< 2 m) were used for data analysis. The remaining effort data collected in poor conditions were removed and classified as “off-effort.” Associated sightings were downgraded to off-effort (incidental) status and not included in further analysis. Sightings collected during transits were also downgraded to incidental status as they were made outside of the main survey area.
The bearing and distance to cetacean sightings were used to estimate the position of each sighting taking into account the location of the vessel at the time of the sighting and the observation eye-height. All GPS records were converted to the following coordinate system (from now on referred to as Ghana Projection): Transverse_Mercator; Central_Meridian: –2.9875; Latitude of Origin: +4.5780; Linear Unit: Meter; Geographic Coordinate System: GCS_WGS_1984.
A grid with a resolution of 10 × 10 km was created and the latitude and longitude were assigned to the center of each grid cell when determining the mean water depth. The position of all fishing vessels (2013 and 2014) and cetaceans were imported into GIS (ArcMap 10.2.1). The relative abundance of cetaceans was then measured as the number of animals km−1 (BSS 0–4, Swell < 2 m, visibility ≥ 5 km).
We employed statistical tests using the statistical package PASW for windows (SPSS, Inc., version 18) and the program R (version 3.2.2.) in order to adequately answer the following basic questions: (1) were there significant differences in cetacean data collected between the two surveys and, if not, could these datasets be pooled; (2) did cetacean abundance vary over different depth categories; (3) were fishing vessels heterogeneously distributed; and (4) did the spatial distribution of cetaceans overlap with the distribution of fishing vessels.
Firstly, we studied if there were potentially interannual differences occurring due to changes in survey methods or actual changes in cetacean distribution. We used a pairwise Mann–Whitney's (non-parametric) test to study potential differences between the two surveys by segregation of the relative abundance per grid cell by survey year.
Secondly, water depth is a factor that is known to influence the distribution and abundance of cetaceans (e.g., Cañadas et al., 2002). Cetaceans have shown depth-related trends in their occurrence in the waters off Angola and elsewhere in the wider Gulf of Guinea (Weir, 2011). It is therefore of interest to investigate at which depths the cetacean abundance was peaking within the present study area. We computed the indices of cetacean abundance per grid cell for different water depth categories defined as < 100 m; 100 to 200 m; 200 to 500 m; 500 to 1000 m; 1000 to 2000 m; 2000 to 3000 m and > 3000 m. We then used Kruskal–Wallis to check if the cetacean abundance was uniform distributed over the depth categories. As this was not the case we next carried out pairwise Mann–Whitney's (non-parametric) tests to study in which depth categories the indices of cetacean abundance significantly differed.
Thirdly, in order to investigate whether fishing vessels were heterogeneously distributed in space, and to map their distribution and density within the survey area we fitted a spatial model to the data. Although we could have used other methods (e.g., ordinary kriging or linear interpolation) to map the spatial distribution of fishing vessels, the advantage of fitting a spatial model is that it takes into account the uncertainty in the data, distribution of the response variable (e.g., counts) and only estimates spatial heterogeneity when there is sufficient support in the data to do so.
Because of the difference in (fishing vessel) data collection methods between the 2 years we could not pool the data and therefore modeled the fishing vessels for each year. For 2013, data on fishing vessels consisted of the number of fishing vessels counted during effort-corrected scans. All types of fishing vessels were pooled (fishing canoes, trawlers, Asian trawlers, and tuna vessels). The counts were assumed to follow a negative binomial error distribution, which allows for over-dispersion or clustering in the number of vessels observed. These counts were modeled as a function of a tensor product smooth of latitude and longitude, using a Generalized Additive Model (GAM; Wood 2006). This technique models spatial autocorrelation in the data and a significant spatial smooth implies non-uniform distribution of fishing vessels. This model was subsequently used to make a spatial prediction of the expected number of sighted fishing vessels, which was subsequently divided by the area of the scans (i.e., π r2, where r = 5 km is the maximum sighting distance) to arrive at absolute fishing vessel density.
In 2014, the fishing vessel data consisted only of the registered location of fishing vessels. These data appeared more erroneous, with spurious spatial coordinates and lack of observations in Ivorian waters. Furthermore, the data were not effort-corrected, and only consisted of presences. Therefore, the 2014 data were modeled as a spatial inhomogeneous Poisson point process (IPP), where the distribution of individual vessels was compared with each 5 min point along the entire survey track, with a response value of 1 for the fishing vessels and a response value of 0 for the effort points (e.g., Aarts et al., 2012). Similar to 2013, these response data were modeled as smooth function of latitude and longitude using a GAM (Wood, 2006). The exponent of the linear predictor is proportional to fishing vessel density. This relative fishing vessel density was subsequently multiplied by the total number of vessels observed, divided by the total survey effort (assuming an effective survey strip of the chase vessels of 1 nautical mile) and the average predicted relative density. Under the assumption that all fishing vessels were registered within half a nautical mile of the seismic survey vessel, this leads to an absolute density of fishing vessels.
Finally, because of the poor data quality on the distribution of fishing vessels (particularly 2014), and the likely long-term effects of seismic noise on the distribution of cetaceans no statistical tests were performed to test if cetacean density significantly correlated with the density of fishing vessels. Instead, we visually evaluated the degree of overlap, and thus likely interaction frequency between fisheries and cetaceans, based on the computed fishing vessel density and the index of cetacean abundance.
Results
The total survey effort consisted of 548 h of visual observations in 2013 and 1218 h of visual observations in 2014. Survey effort was concentrated in the eastern half of the study area in 2013, whilst during 2014 survey effort expanded toward deeper waters and into Ivoirian waters as well as along the shelf into both Ghanaian and Ivoirian waters (Figure 1). The start of the survey in 2014 coincided shortly after the minor upwelling season in the Ivoirian/Ghanaian part of the Gulf of Guinea (December–March) although the SSTs never dropped below 25°C (data not shown). The Beaufort sea state (BSS) during the observations in both 2013 and 2014 ranged from 1 to 6. The observation effort was split across BSS 0 (1%), BSS 1 (7.5%), BSS 2 (26.1%), 3 (38.6%), and 4 (24.9%), with only 2% of effort occurring during BSS ≥ 5. Effort-corrected observations (data collected during good conditions) totaled 466 h along 3645 km in 2013 and 1101 h along 8273 km in 2014. A total of 705 h of observations (45%) occurred during seismic operations and 862 h during non-seismic operations (55%).
Cetacean Occurrence, (Relative) Abundance, and Distribution
A significant difference in the relative abundance of cetaceans was detected between the 2 years (Mann–Whitney's U = 9883,000, p = 0.006). However, when only comparing the abundance of cetaceans within those areas where survey effort from both years overlapped this was no longer significant (Mann–Whitney's U = 143,000; p > 0.05). Therefore, in order to increase sample size it was decided to pool the two data sets for analysis. During effort-corrected observations, approximately 11,181 individual cetaceans were seen in 306 groups during 2013 and 2014 (Table 1); the majority of groups (61.8%) were observed when there were no seismic operations. The highest numbers of cetaceans were seen in Ghanaian waters where the most effort also took place (Figure 1).
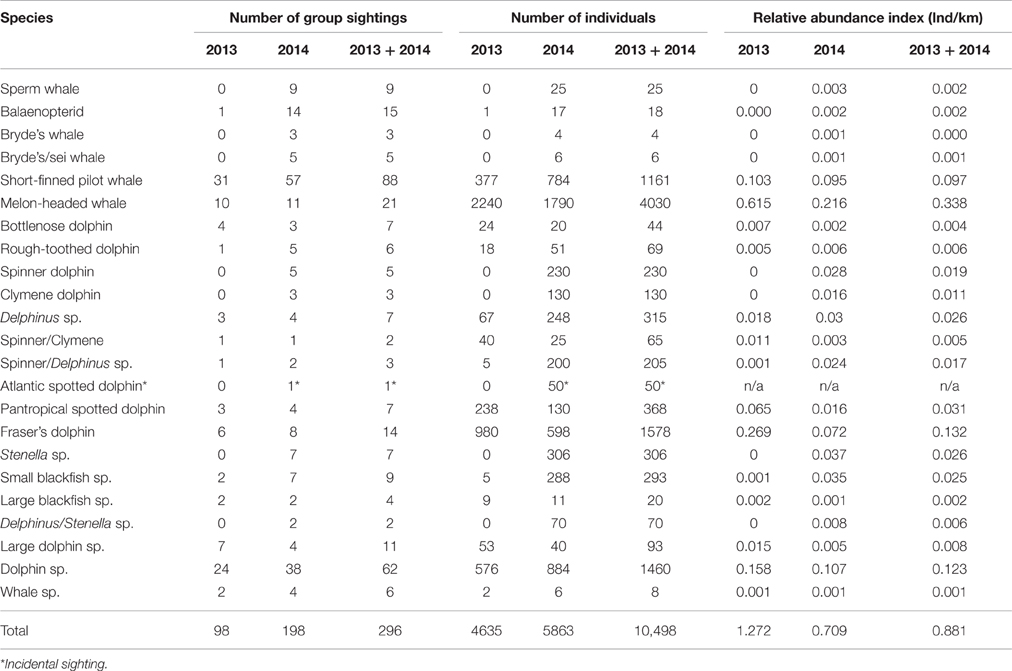
Table 1. Summary of cetacean sightings, individuals (ind), and indices of abundance (the number of individuals per km effort) for the 2 survey years (3,644.9 km effort in 2013 and 8,273.2 km in 2014) and pooled (11,918.1 km: 2013 + 2014).
The relative abundance of cetaceans (all species) were not distributed uniformly through all classes of depth (χ2 = 14.57, df = 6, p = 0.02). The abundance index in 2013 was significantly higher for waters of 200–500 m depth and 500–1000 m depth (p < 0.05; Figure 2A). In 2013, the cetacean abundance was significantly higher for waters of 500–1000 m depth (2.6 animals km−1) compared to the abundance measured in 2014 (0.5 animals km−1; p = 0.009; Figure 2A). The sample sizes for the relative abundance for the first three depth classes in 2014 and class 200–500 m in 2013 were low (≤ 3).
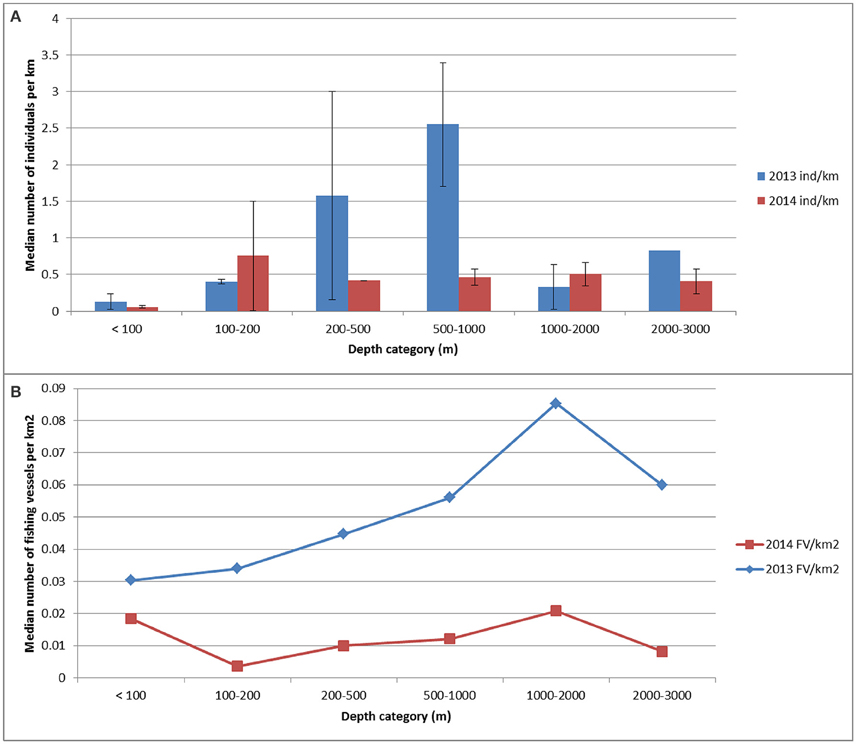
Figure 2. The (median) indices of abundance for cetaceans (number of animals km−1 of search effort) computed for the following water depth categories: < 100 m; 100–200 m; 200–500 m; 1000–2000 m and 2000–3000 m water depth for each survey year (A). The density for fishing vessels (number of fishing vessels km−2) for each depth category is also shown (number of vessels km−2) for each survey year (B).
Species Accounts
Seven cetacean species were documented in 2013 and 12 species in 2014, totaling 12 species during both surveys (Table 1; Figures 3–5). There were 18 confirmed records of mixed-species groups. At least five species were observed in mixed-species groups. Most mixed-species groups (n = 16 records) comprised of two species but there were two groups comprising three species. Mixed-species groups mainly involved melon-headed whales and Fraser's dolphins (Lagenodelphis hosei; n = 6) or short-finned pilot whales and common bottlenose dolphins (Tursiops truncatus; n = 5). Short-finned pilot whales were also observed associating with Fraser's dolphins (n = 3), and rough-toothed dolphins (Steno bredanensis) were observed associating with Fraser's dolphin (n = 2) and melon-headed whales (n = 1). The interspecific associations involving groups of three species comprised of at least 500 melon-headed whales, 8 Fraser's dolphins and 4 rough-toothed dolphins and another record included at least 150 melon-headed whales, 5 rough-toothed dolphins, and 5 unidentified dolphins. Further details on species accounts (e.g., group-sizes, water depths, sea surface temperature, and behaviors) are described in the Supplements (Data Sheet 1 and associated Supplementary Figures 1–6).
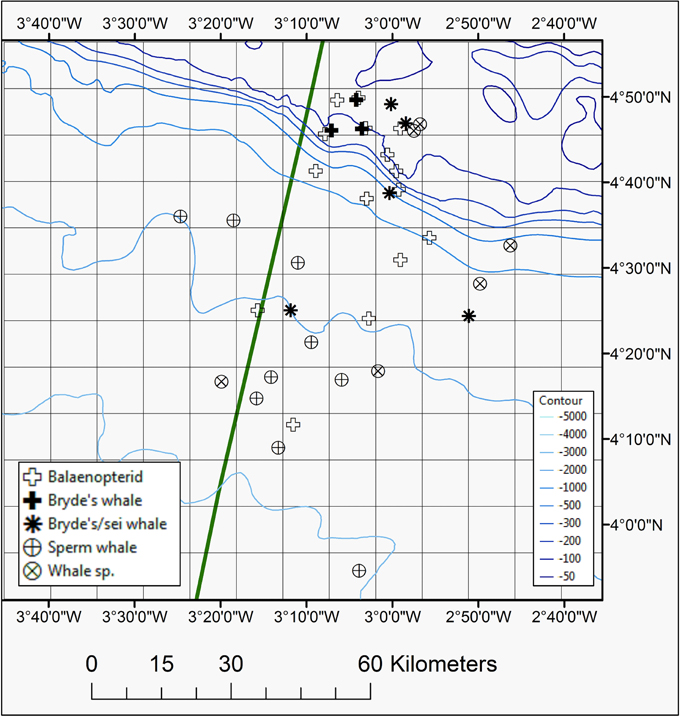
Figure 3. Map showing positions of whales species seen during the 2013 and 2014 surveys, including Bryde's whale, Bryde's/sei whale, sperm whale, unidentified balaenopterid species, or unidentified whale species. The EEZ border between Côte d'Ivoire and Ghana is also shown (green thick line).
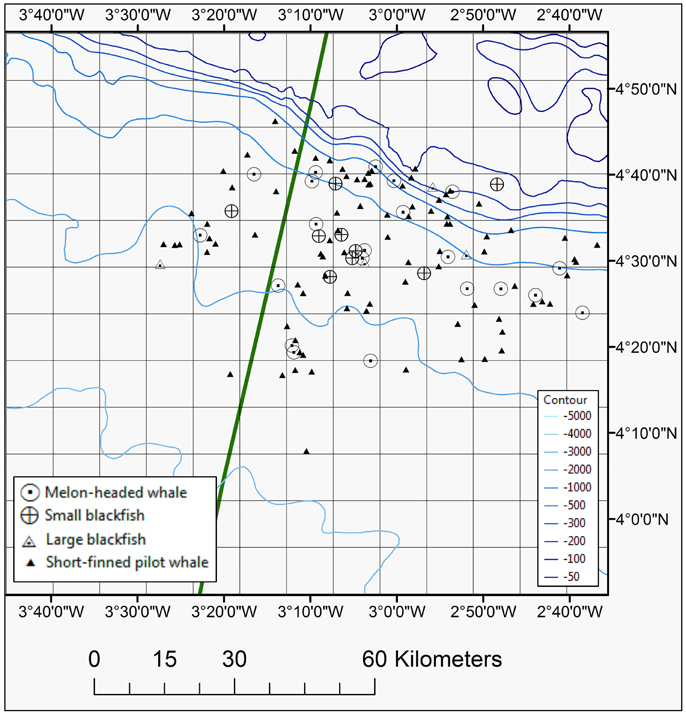
Figure 4. Map showing positions of blackfish species seen during the 2013 and 2014 surveys, including short-finned pilot whale, melon-headed whale, small, and large blackfish species. The EEZ border between Côte d'Ivoire and Ghana is also shown (green thick line).
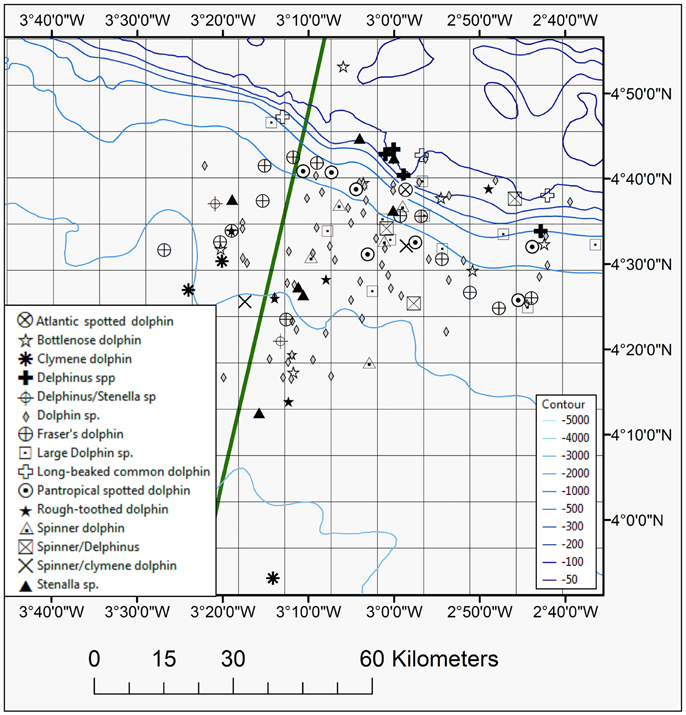
Figure 5. Map showing positions of all dolphin species seen during the 2013 and 2014 surveys, including Atlantic spotted, bottlenose, Clymene, Delphinus spp., long-beaked common, pantropical spotted, rough-toothed, Fraser's, and spinner dolphins. The EEZ border between Côte d'Ivoire and Ghana is also shown (green thick line).
Distribution and Density of Fishing Vessels
The artisanal small-scale fishery was the most frequently recorded with many small wooden canoes present throughout the study area expanding well offshore (Table 2; Figure 6). At times aggregations of up to 25 canoes were recorded. The majority of fishing canoes were engaged with fishing activities (fishing, hauling and setting) mainly recorded between 06:00-08:00 and 15:00-19:00 UTC (Local Time). The most distant fishing canoe was recorded in 2586 m water depth and at a distance of 99.5 km from the Ghanaian coast. Similarly, the farthest canoe in Ivoirian waters was documented 89.8 km from the coast. Asian trawlers were mainly recorded over the shelf area in both Ivorian and Ghanaian waters but commercial trawlers were also recorded in slope waters (Figure 6). The tuna purse seine vessels and Fish Aggregation Devices (FADs) were generally recorded in deeper oceanic waters in the southern part of the study area (Ghanaian waters) (Figure 6).
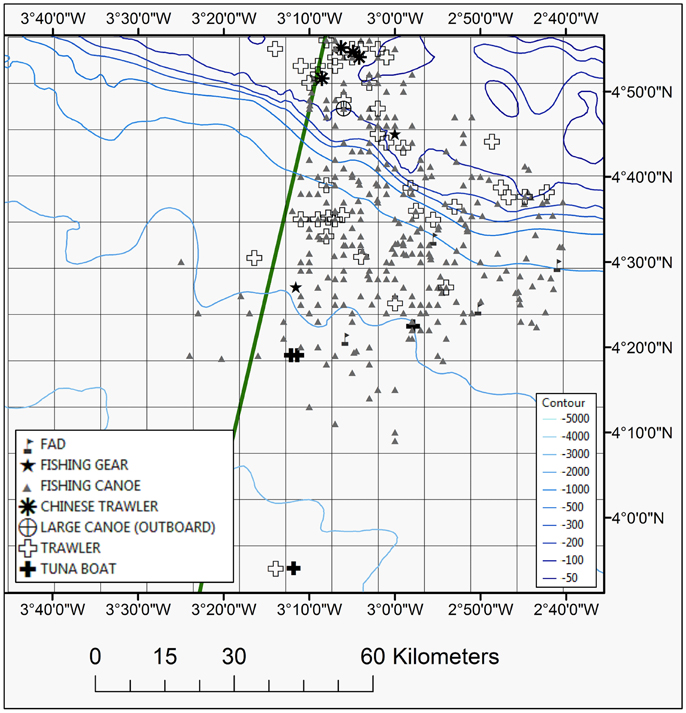
Figure 6. Map showing sighting position of different fishing vessels and fishing gear plotted during the 2013 and 2014 surveys. The EEZ border between Côte d'Ivoire and Ghana is also shown (green thick line).
Although fishing vessels occurred throughout the survey area, at least in 2013, the highest density of fishing vessels was measured for the eastern side of the study area (Figure 7) and specifically in deeper waters (Figure 2B). Since the spatial smooth of the GAM was significant (Chi2 = 32.54, p-value = 0.00031), the distribution of fishing vessels was indeed not uniform in space. In 2013 the cetaceans occurred mainly in areas with a fishing vessel density of ≥ 0.04 vessels km−2 and in 2014 this was between 0.01 and 0.02 vessels km−2 (Figure 7; diagnostic plots are shown in Supplementary Figure 7).
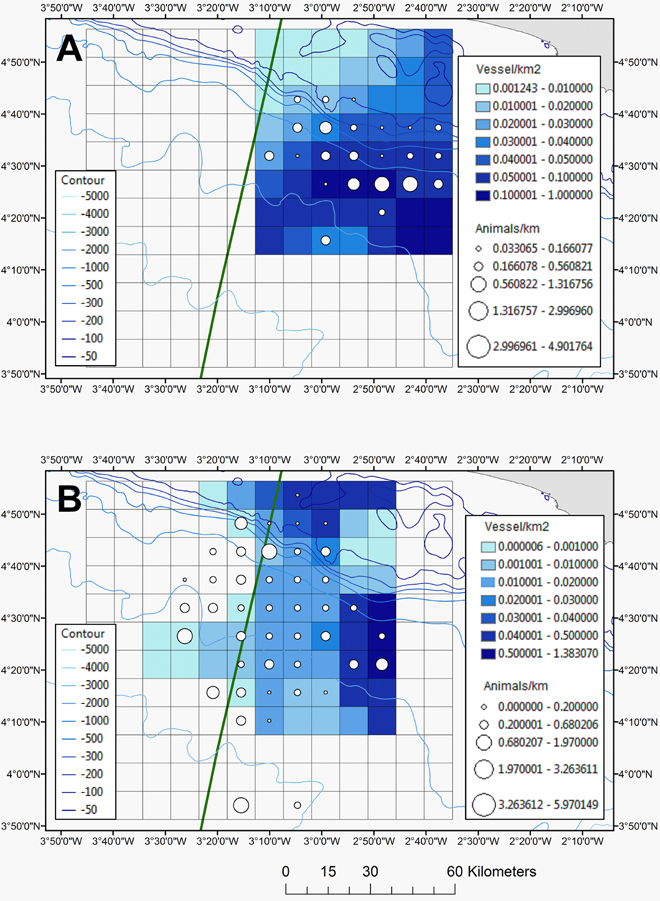
Figure 7. The relative abundance of all cetaceans (animals km−1) together with the density of all fishing vessels (boats km−2) for 2013 (A) and 2014 (B). The EEZ border between Côte d'Ivoire and Ghana is also shown (green thick line).
Interactions between Cetaceans and Fisheries
For safety reasons, the chase vessels would frequently request fishing vessels to move well away from the intended track of the seismic survey vessel, and normally this occurred well before the arrival of the seismic survey vessel. Hence any interactions between cetaceans and fisheries were difficult to observe due to the distances involved. Nevertheless, some interactions were witnessed and these sightings occurred during those times when the fishing vessels were engaged in deploying, soaking or hauling gear (e.g., early morning or late afternoon). On two occasions, short-finned pilot whales were recorded in an area with at least 14 fishing canoes present. On one occasion a group of eight adults and one calf was seen in an area with an operating trawler and two fishing canoes. On 5 May 2013, in an area where one trawler and two fishing canoes (one hauling nets) were operating, a freshly dead melon-headed whale was recorded floating in the water. It seems likely that this animal was bycaught in fishing gear and subsequently lost or discarded at sea. On one other occasion a group of 300 melon-headed whales was seen in the vicinity of an operating trawler. Rough-toothed dolphins were recorded on one occasion in the vicinity of an operating trawler involving a group of 16 adults and two juveniles (water depth: 371 m). On 26 May 2013 (water depth 1280 m), a group of common bottlenose dolphins was observed in close vicinity of a fishing canoe with one dolphin surfacing directly alongside the canoe which was hauling an artisanal drift gillnet. One group of approximately 200 pantropical spotted dolphins, including at least 10 juveniles and three calves, was recorded in the presence of a canoe (water depth 989 m).
In 2013, Fraser's dolphins and melon-headed whales were recorded in the areas with the highest fishing densities (0.09 and 0.08 vessels km−2; Table 3) of which the majority were fishing canoes. Likewise in 2013, Pantropical spotted dolphins were also recorded in areas with a high density of fishing vessels (0.06 vessels km−2; Table 3) closely followed by common bottlenose dolphin and pilot whales (0.05 vessels km−2), common dolphin and rough-toothed dolphin (0.04 vessels km−2; Table 3). In 2014, balaenopterids (including Bryde's whales Balaenoptera brydei) were most abundant in shelf waters where commercial trawlers also operated. Indeed, the areas where Bryde's whales were observed had a relative high fishing density (0.04 vessels km−2; Table 3). In 2014, common bottlenose dolphins and common dolphin also occurred in areas with relatively high fishing vessel densities (0.02 vessels km−2; Table 3) whilst Clymene dolphin, sperm whale and Fraser's dolphin occurred in areas where the fishing density was the lowest (Table 3).
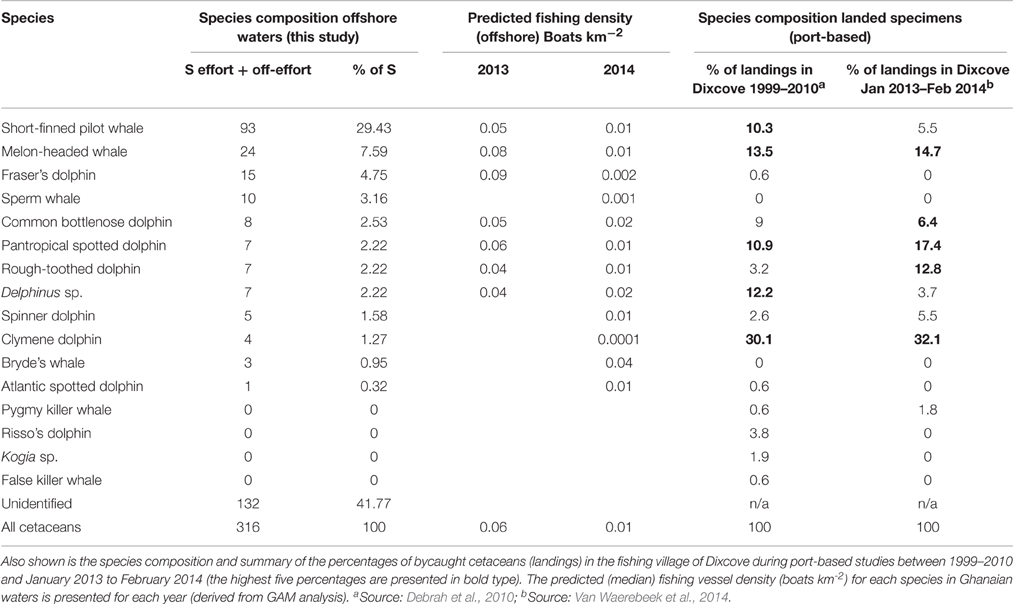
Table 3. Species composition and summary of all cetacean sightings (S; during effort and off-effort search status) pooled from both survey years together with the percentage of all sightings (listed in order of frequency).
Discussion
Melon-headed whales were found to be the most abundant cetacean and it is therefore not surprising that the species is also regularly landed in the fishing ports in Ghana (Debrah et al., 2010; Van Waerebeek et al., 2014). The Dixcove village in the Western Region is located approximately 70 km from the present study area (Figure 1). Because it can be expected that the Dixcove fishermen set their driftnets in, or in close vicinity of the present study area, it is of interest to compare the cetacean species composition of landings to that observed at sea in the present study during the same survey years. The cetacean species mainly landed at Dixcove between January 2013 and February 2014 included, in order of frequency, Clymene dolphin, pantropical spotted dolphin, melon-headed whale, rough-toothed dolphin, common bottlenose dolphin, spinner dolphin, pilot whale, and occasionally long-beaked common dolphin and pygmy killer whale (Van Waerebeek et al., 2014). There are some interesting differences when comparing the species composition of landings to that of the present survey (Table 3). Most strikingly, Clymene dolphins were rarely identified at sea (1.3% of all sightings) yet they were the most frequently landed cetacean at Dixcove between 2013 and 2014 (32.1%; Van Waerebeek et al., 2014; Table 3). On the other hand Fraser's dolphins were encountered regularly offshore (4.8% of all sightings) and often in large groups (13.6% of all individuals counted offshore) yet were rarely landed at Dixcove (Debrah et al., 2010; Van Waerebeek et al., 2014; Table 3).
Species Accounts and Vulnerability to Fishing Mortality
We next discuss our findings for each species and confirm new species records for Ghana and Côte d'Ivoire. In addition, and based on our findings, we evaluate the species' vulnerability to fishing mortality through entanglement or direct capture in fishing gear in these waters.
Bryde's Whales
Bryde's whales occurred mainly in shelf waters. It seems likely that the start of the 2014 survey may have been influenced by the tail of the upwelling season which created feeding opportunities for the Bryde's whales in these shelf waters consisting of small pelagic fish (e.g., sardines, Sardinella spp. and European anchovy, Engraulis crassicolus). Indeed, balaenopterids have been reported to show a close relationship with SST in areas with recently upwelled water (e.g., Gill et al., 2011). Bryde's whales have only previously been documented during the 1970s whaling activities (Best, 1996) and a stranded individual has been confirmed from Togo (Segniagbeto et al., 2014). Our records therefore present the first confirmed at-sea sightings for Ghanaian waters. Bryde's whales occurred in areas where trawlers and fishing canoes were operating (0.04 vessels km−2; Table 3). Large balaenopterids, such as Bryde's whales, are known to occasionally become entangled in fishing gear, but due to their large size they do not appear to be especially susceptible (Reilly et al., 2008). We opine that overall there is a low risk of entanglement/capture in fisheries in Ghana with the higher chance of entanglement in drift gillnets in shelf waters, particularly involving smaller Bryde's whales.
Sperm Whales
Sperm whales with juveniles were recorded in the present study and these nursing groups probably occur year-round. The historical “Coast of Africa” sperm whaling ground between latitudes 03–23°S (Townsend, 1935) combined with authenticated specimens (n = 5) from Benin, Ghana, and Togo suggests that this stock is also present in the northern Gulf of Guinea (Sohou et al., 2013). While confirmed at-sea sightings in offshore Ivoirian waters have previously been reported by Best (1974), our records present the first confirmed at-sea sightings in Ghanaian waters. Of the large cetaceans, the sperm whale is the most affected by entanglement in drift net fishing gear in the Mediterranean Sea (Reeves and Notarbartolo di Sciara, 2006). The deliberate capture of a small sperm whale by the crew of a large fishing canoe was previously reported in Ghanaian waters (Debrah et al., 2010). The only authenticated case of a sperm whale entangled in artisanal fishing gear within the region is an animal flensed at Bakingili, Cameroon (Ayissi et al., 2011). Other well documented interactions between sperm whales and fisheries include long-line fishing gear. In the equatorial waters of the Gulf of Guinea, and further south, long-line fishermen targeting tuna and sharks report regular predation of hooked fish by sperm whales (Van Waerebeek et al., 2009). We opine that there is a low risk of entanglement/capture in fisheries of sperm whales in Ghana although smaller whales may form an occasional target for deliberate capture.
Short-Finned Pilot Whales
Short-finned pilot whales have been sighted off Côte d'Ivoire (Cadenat, 1959) and landed in Ghana (Ofori-Danson et al., 2003; Van Waerebeek et al., 2009, 2014; Debrah et al., 2010), Togo (Segniagbeto et al., 2014), and Benin (Sohou et al., 2013). Our records present the first confirmed at-sea sightings in Ghanaian waters. Despite their large size and deep-water foraging habits they do infrequently become entangled in artisanal fishing gear (5.5–10.3% since 1999; Van Waerebeek et al., 2014 Table 3). Interactions with other fisheries within tropical and sub-tropical zones involve pelagic long-liners that target tuna (Thunnus spp.) and swordfish (Xiphiius gladius) which, in other regions, are often depredated not only by killer whales (O. orca) and false killer whales (P. crassidens), but also short-finned pilot whales (Dalla Rosa and Secchi, 2007; Hernandez-Milian et al., 2008). However, during the present survey, commercial boats targeting tuna were only recorded on four occasions all of which occurred in deep waters. We opine that there is a moderate risk of entanglement or capture of pilot whales in Ghana. Pilot whales were encountered in areas with a comparably high fishing vessel density (in 2013: 0.05 vessels km−2; Table 3). The species is common in these waters and particularly immature pilot whales may become entangled in artisanal gillnets.
Melon-Headed Whales
Melon-headed whales have not previously been recorded off Côte d'Ivoire (Perrin and Van Waerebeek, 2012). Two of our records occurred in Ivoirian waters (April) and present a new range state record for Côte d'Ivoire with the remainder presenting first confirmed at-sea sightings for Ghanaian waters. Melon-headed whales were observed associating with Fraser's dolphins on multiple occasions probably during multi-species feeding frenzies. Indeed, the two species are known to occur in mixed-species groups elsewhere in the tropical Atlantic [e.g., International Fund for Animal Welfare (IFAW), 1996; de Boer, 2015]. In comparison, melon-headed whales were observed to be less abundant further south and only occasionally encountered during surveys off Angola and Gabon (de Boer, 2010a; Weir, 2011). The melon-headed whale is the third most frequently captured cetacean in artisanal fishing gear (13.5–14.7% since 1999 in Dixcove landings; Van Waerebeek et al., 2014) and these figures match our observations where the species was found to be the most abundant of all cetaceans (Table 3). We opine that there is a high risk of melon-headed whales to become entangled or captured in fisheries in Ghana. They commonly occurred in areas with a high fishing vessel density (in 2013: 0.08 vessels km-2; Table 3). In addition, due to their large group formations, melon-headed whales are at risk of becoming entangled as a group (“group entanglement”) rather than single individuals. Such simultaneous or group entanglement of genetically related dolphins (mother-offspring or related/reproductive pairs) in fishing gear has been reported for the Franciscana (Pontoporia blainvillei) in Argentina, and this might exacerbate the demographic consequences of bycatch, and the loss of groups of relatives means that significant components of genetic diversity could be lost together (Mendez et al., 2010).
Rough-Toothed Dolphins
Rough-toothed dolphins were recorded on six occasions during the present survey (2.2% of all sightings; Table 3) which is slightly higher compared to offshore surveys in Angola (0.6%; Weir, 2011) and Gabon (1.2%; de Boer, 2010a). Relatively little is known regarding rough-toothed dolphins along the West African coast but a few records are known for Mauritania, Senegal, Cape Verde, Gabon, Angola, and St Helena (Van Waerebeek et al., 2000; Findlay et al., 2006; MacLeod and Bennett, 2007; de Boer, 2010a; Weir, 2010). The species has been previously described for both Gabon and Côte d'Ivoire: (1) two sightings in 1972 at sea off Ghana; (2) reported landed in Ghana (e.g., Van Waerebeek et al., 2009, 2014); and (3) three specimens were captured in 1958 off Abidjan, Côte d'Ivoire (Cadenat, 1959). An increase in rough-toothed dolphins caught in artisanal fishing gear (from 3.2 to 12.8%) was reported from Dixcove (Debrah et al., 2010; Van Waerebeek et al., 2014; Table 3). Rough-toothed dolphins are known to interact with trawl fishing gear off West Africa (Addink and Smeenk, 2001) and FADs (de Boer, 2010b). We opine that the risk of entanglement or direct capture of rough-toothed dolphins in Ghana is quite high. Particularly, the tendency to interact with fisheries together with the relatively high mortality levels confirms that rough-toothed dolphins are particularly susceptible to bycatch. They also regularly form large groups of (multi-species) feeding associations which enhance the risk of group-entanglement. Finally, because of their attraction to boats they also are an easy target for direct capture.
Common Bottlenose Dolphins
Common bottlenose dolphins were observed associating with pilot whales on five occasions. This association has also been previously described in Angolan waters (Weir, 2008a) and Northwest African waters (Djiba et al., 2015). The species is known to occur in both the inshore and offshore waters of Angola (Weir, 2011) however in Ghana most landed bottlenose dolphins are thought to belong to an offshore stock (Van Waerebeek et al., 2009). While the inshore stock of bottlenose dolphins are thought to be largely depleted in Ghanaian waters, they still occur off Benin (Van Waerebeek et al., 2009; Sohou et al., 2013). The offshore numbers of bottlenose dolphins (2.5%; Table 3) are comparable to other offshore regions (Gabon: 3.6% and Angola: 2.1%; de Boer, 2010a; Weir, 2011). Three bottlenose dolphins were deliberately caught in Ivoirian waters in 1957–1958 (Cadenat and Lassarat, 1959). Our records present the first confirmed at-sea sightings in Ghanaian waters. At Dixcove, between 6.4 and 9.0% of small cetaceans landed consisted of common bottlenose dolphin during the 1999–2014 port monitoring period (Debrah et al., 2010; Van Waerebeek et al., 2014; Table 3). During this study, an observation was made involving a small group of bottlenose dolphins foraging in the direct vicinity of artisanal fishing gear and a similar interaction was recently reported from near-shore waters in Benin (Van Waerebeek et al., 2009; Sohou et al., 2013). We opine that there is a moderate to high risk of bottlenose dolphins to become entangled or captured in fisheries in Ghana. Although, their coastal tendency makes this species particularly susceptible to entanglement in fishing gear they only infrequently become entangled. However, their readiness to approach boats makes them an easy target for capture. Furthermore, there is a risk of group-entanglement.
Pantropical Spotted Dolphins
Pantropical spotted dolphins accounted for 2.2% of all offshore sightings (Table 3) which is higher than recorded during offshore surveys off Angola (0.24%) and Gabon (1.2%; de Boer, 2010a; Weir, 2011). They were recorded in waters over the shelf edge and indeed further offshore which is consistent with records elsewhere in deep tropical waters specifically off Ghana, Gabon, and Angola (Picanço et al., 2009; Weir, 2010; de Boer, 2010a; Perrin and Van Waerebeek, 2012). Published group sizes of pantropical spotted dolphins within the region are mostly in the range of 50–150 animals which matched our observations (MacLeod and Bennett, 2007; Weir, 2007; de Boer, 2010a). The species has previously been reported in Ghanaian offshore waters, albeit non-authenticated (Jefferson et al., 1997) but its occurrence in Côte d'Ivoire remains unconfirmed (Weir, 2011). Pantropical spotted dolphins occurred in areas where there was a high fishing vessel density (in 2013: 0.06 vessels km−2; Table 3). An increase in the landings of pantropical spotted dolphins from 10.9% in 2010 to 17.4% in 2014, was reported at Dixcove (Van Waerebeek et al., 2014; Table 3), making this the second most commonly landed cetacean. It is unclear whether the species is also captured in tuna purse seine fisheries in the tropical Atlantic. In the eastern tropical Pacific (ETP) seiners target pantropical spotted dolphin and spinner dolphin in order to locate and catch yellowfin and skipjack tuna (Culik, 2010a). We opine that the risk of entanglement or capture in fisheries of pantropical spotted dolphins in Ghana is high. The species frequently interacts with vessels and together with a tendency of interacting with fishing gear makes it particularly susceptible to bycatch in fishing gear within the region. These dolphins also form an easy target for direct capture and because of their formation into large groups there is a risk of group-entanglement.
Atlantic Spotted Dolphins
Atlantic spotted dolphins (Stenella frontalis) were recorded once during the offshore surveys and this is reflected in their very occasional presence among landings at Dixcove (Van Waerebeek et al., 2014; Table 3) or other Ghanaian fishing ports (Debrah et al., 2010). The species is more abundant further south in Gabon (3.6% of all sightings) and Angola (3.2%; de Boer, 2010a; Weir, 2011). Two Atlantic spotted dolphins were captured for research in Côte d'Ivoire (Cadenat, 1959) and our record presents a first confirmed at-sea sighting for Ghanaian waters. It seems that there is a low risk of entanglement or capture in fisheries in Ghana. This is mainly based on the rare occurrence of this species in these waters. Because of the species' readiness to approach vessels they do form an easy target for direct capture but considering small group sizes (typically < 20 off NW Africa; Djiba et al., 2015) the risk of group-entanglement seems limited.
Spinner Dolphins
Spinner dolphins have previously been recorded on three occasions in the offshore waters of Ghana, all occurring in deep waters (> 3500 m) and in groups of 20–200 animals (Weir, 2011). Skulls of specimens originating from Côte d'Ivoire have been described by van Bree (1971) but at-sea sightings remain unconfirmed. Spinner dolphins are infrequently captured at Dixcove, i.e., 2.6–5.5% of landings in, respectively, 1999–2010 (Debrah et al., 2010) and 2013–2014 (Van Waerebeek et al., 2014; Table 3) similar to the percentage of pilot whale landings. This is not reflected in the offshore survey where spinner dolphins comprised 1.6% of all sightings whereas pilot whales comprised 29.4% (Table 3). Elsewhere, they are known to rest during the day and feed at night on mesopelagic fish, squids, and shrimps (Dolar et al., 2003). Spinner dolphins are also well-documented as bycatch in the tuna fishery in the ETP [Inter-American Tropical Tuna Commission (IATTC), 2009] however there is a lack of information on the bycatch of dolphins in the industrial tuna purse-seine fisheries within the Gulf of Guinea (Maigret, 1981; Van Waerebeek and Perrin, 2012). Because of their infrequent occurrence in Ghanaian waters we opine that the risk of entanglement or capture in fisheries of spinner dolphins in Ghana is moderate. Their presumed nocturnal foraging activities would make them particularly vulnerable to entanglement in night-time operated artisanal fishing gear. These dolphins readily approach vessels to bow-ride and they are an easy target for direct capture. Because of their formation into large groups (hundreds) the risk of group-entanglement is enhanced.
Clymene Dolphins
Clymene dolphins have been recorded in Ghanaian offshore waters in 1972 (Perrin et al., 1981). The first documented record of a Clymene dolphin in Ghana was a bycaught specimen from Keta in 1956 (Van Waerebeek et al., 2009). Recent Clymene dolphin records from Côte d'Ivoire and Ghana all occurred in waters > 1999 m depth but off Angola and Gabon the species was also recorded in continental slope waters of 466 and 684 m depth (Weir et al., 2014). The Clymene dolphin is the most common cetacean landed at Ghanaian fishing ports (1998–2000, 34.5%; Ofori-Danson et al., 2003). This is consistent with the larger and more recent samples at Dixcove alone, where Clymene dolphins represented 30.1 and 32.1% of landed cetaceans occurring year-round (Debrah et al., 2010; Van Waerebeek et al., 2014; Table 3). This is however not reflected in the offshore survey where Clymene dolphins comprised of only 1.3% of all sightings (Table 3). There was a large amount of sightings that were classified as unidentified during the present survey or were classified only to species group level (42% of all sightings; Table 3). Naturally, some of these “unidentified” dolphins may have involved species such as the Clymene dolphin which can be very difficult to identify (Weir et al., 2014). In the western Atlantic, Clymene dolphins are known to be nocturnal foragers for mesopelagic fish and squid (Fertl et al., 1997). There is a lack of information regarding the bycatch of dolphins in the industrial tuna purse-seine fisheries in the Gulf of Guinea (Maigret, 1981; Van Waerebeek and Perrin, 2012). The occurrence of Clymene dolphin bycatch is undocumented in those countries neighboring Ghana (Sohou et al., 2013; Segniagbeto et al., 2014) yet when taking into account the multiple sighting records recently confirmed for the area (Weir et al., 2014) then bycatch is likely to occur throughout the region. We opine that the risk of entanglement or capture in fisheries of Clymene dolphins in Ghana is high. Their nocturnal foraging activities particularly make them vulnerable to entanglement in night-time operated artisanal fishing gear. They are generally weary of boats (they do not readily bow-ride) and therefore the risk of direct capture is probably low. If our observed sighting rate of Clymene dolphins is unbiased and representative of Ghana waters, the landing rate (34.5%; Ofori-Danson et al., 2003) is very high compared to the population abundance. Hence, bycatch may have a negative influence on their population status.
Long-Beaked Form of Common Dolphins
Long-beaked form of common dolphins (Delphinus sp.) comprised 2.2% of all sightings (Table 3). Specimens of “short-beaked” and “long-beaked” common dolphins have previously been described in Côte d'Ivoire (Cadenat, 1959; Van Bree and Purves, 1972; Van Waerebeek et al., 2009). Our records present the first confirmed at-sea sightings in both Ivoirian and Ghanaian waters. There has been a notable drop in the percentage of their landings at Dixcove (12.2–3.7%; Debrah et al., 2010; Van Waerebeek et al., 2014; Table 3). The continuation of at least occasional directed captures of this species has also been documented recently (Van Waerebeek et al., 2014). Bycatches of common dolphins have been reported in tuna purse-seine nets at the border between Liberia and Côte d'Ivoire (Simmons, 1968) and also off southern Africa (Best and Ross, 1977). Short-beaked common dolphins frequently become bycaught in trawl fisheries in the NE Atlantic (Morizur et al., 1999) and studies have shown that their relative abundance and group size were significantly higher in the presence of trawlers and that bycatch mostly occurred at night (de Boer et al., 2012). We opine that there is a high risk of entanglement or capture in fisheries of common dolphins in Ghana. Their tendencies to readily approach vessels to bow-ride and to interact with fisheries make them susceptible to bycatch and an easy target for direct capture. Furthermore, there is risk of group-entanglement.
Fraser's Dolphins
Fraser's dolphins were common in the present study and with a high index of abundance (partly due to its formations into large group sizes). They are known to associate with other species (Dolar, 2002; Dolar et al., 2006) but, to our knowledge, this is the first documented case of an association between Fraser's dolphin and rough-toothed dolphin. There have been ten previous confirmed records of Fraser's dolphin in the ETA (from Senegal, Cabo Verde, Ghana, Angola, Gabon) and one further probable record off Nigeria (Debrah, 2000; Van Waerebeek et al., 2000, 2009; Ofori-Danson et al., 2003; Weir et al., 2008, 2013a; Torda et al., 2010). The previous records for Ghana involved specimens landed in Axim and Dixcove in 2000 (Debrah, 2000; Ofori-Danson et al., 2003). Our records contribute to the understanding of the geographical distribution range of this species by more than doubling the number of published records in West Africa and present the first verified at-sea sighting for Ghanaian waters and a new range state record for Côte d'Ivoire. Fraser's dolphins were recorded in deep waters of 942–2317 m depth and their affinity for foraging at night in deep waters can be explained by the type of prey they habitually target (mesopelagic fish, crustaceans, and cephalopods; Dolar et al., 2003). However, the feeding ecology of this species in the Gulf of Guinea is unknown. Fraser's dolphins were occurring in areas with the highest fishing vessel densities in 2013. Despite their common occurrence during the present survey, the species is rarely landed in Dixcove (0.6% of all landings between 1999–2010 and 0% between 2013–2014; Debrah et al., 2010; Van Waerebeek et al., 2014; Table 3). Fraser's dolphins are reported as bycatch in the tuna purse seine fishery in the ETP (Gerrodette and Wade, 1991) but the potential of bycatch in purse seine fisheries within the Gulf of Guinea remains to be assessed. Due to the fact that Fraser's dolphins were rarely landed in the Ghanaian fishing ports, but present in relative high abundance in our survey, we opine that there is a low risk of entanglement or capture in fisheries of Fraser's dolphins in Ghana. Fraser's dolphins do not readily approach vessels to bow-ride (Culik, 2010b) and therefore its shy nature together with its habitually deep diving foraging behavior (in spite of the fact that foraging takes place mainly at night) may help avoid entanglement in drift gillnets. Because of their formation into very large groups (often multi-species associations) there is however a risk of group-entanglement.
Potential Sources of Bias
There was a marked difference in the fishing vessel density between both survey years which was probably caused partly by the difference in data-collection methods used. However, the differences in the two areas surveyed may also explain some of these differences as the area in 2014 covered a much wider region and expanded overall further westward. Furthermore, the fishing vessels in 2014 appeared more widely dispersed (and less aggregated) compared to those recorded in 2013. Nevertheless, caution is needed when comparing the fishing vessel densities between both years. In 2013, the data-collection method for fishing vessels was effort-corrected (unlike the data collected in 2014) and data were collected by the marine mammal observers onboard the seismic survey vessel. The 2013 fishing vessel data are therefore believed to be more accurate and the least biased, and therefore best represent the fishing vessel density for Ghanaian offshore waters.
Just under half of the survey effort was conducted during times when the seismic source was active (45%) and this is a lower percentage when compared to other seismic surveys (de Boer, 2010c, 2013, 2015). We highlight that caution is required when interpreting the results because overt responses to the seismic sound source by some cetaceans may have occurred. For example, responses to seismic by short-finned pilot whales, Atlantic spotted dolphins and pantropical spotted dolphins have been documented off West Africa (Weir, 2008a,b; Gray and Van Waerebeek, 2011). Avoidance of the area by some species in response to seismic sound levels may also have occurred as research on other marine mammals has shown (temporal) avoidance or a reduction in overall cetacean detection rates in response to loud noises (e.g., pile driving; Southall et al., 2007; Paiva et al., 2015). In addition, further disturbance to marine fauna was likely caused in 2014 by other seismic vessels that were operating nearby. With no information available regarding the detection rates of cetaceans within the region prior to/or after these seismic surveys it is not possible to assess if and how the distribution of cetaceans was affected or if there were significant overlaps between cetaceans and fisheries. Future surveys would benefit from a dedicated (line-transect) survey taking place prior to, and after, the geophysical seismic surveys in order to detect changes in detection rates and the distribution of cetaceans.
Conclusions
The present survey provided a unique opportunity to study both the cetacean community and fishing activities in the poorly studied Ivoirian/Ghanaian part of the Gulf of Guinea. New insights into the occurrence of cetaceans were made with ten cetacean species representing first at-sea sightings and two species new range state records. Our findings confirmed that fishing occurred well offshore, including the small-scale artisanal fishery (Figure 6). However, it must be noted that near-shore areas were not surveyed. Both cetaceans and fishing vessels predominantly occurred in shelf and slope waters, specifically up to the 1000 m depth contour, and it is here where fishing activities are likely to be causing anthropogenic mortality through bycatch and direct captures. The gillnets of artisanal fishermen are most commonly soaked throughout the night and hauled in the early morning hours (E.A. Johnson, Dixcove fisheries officer, pers. comm. to K. Van Waerebeek) and at Dixcove, the landing of catches, including cetaceans, typically occurs during the morning (K. Van Waerebeek, pers. observations). This leads us to believe that most interactions between cetaceans and fisheries probably occurred during the hours of darkness. There are no indications that some species are more sought after than others (i.e., for consumption). The majority of cetaceans are landed freshly dead following entanglement, but occasionally if animals are alive when retrieved they are killed, with piercing lance-like metals, cutlasses, hand harpoons, or sticks (Debrah, 2000). No changes in the handling of landed cetaceans and commercial practices in Dixcove, in comparison with former years, were recently reported (Van Waerebeek et al., 2014). However, it is unknown whether the landings data based on one fishing village alone can be considered representative of broader fishing patterns for the entire region.
Some notable differences were found in the species composition between the present at-sea surveys and the port-based landings data (Ofori-Danson et al., 2003; Debrah et al., 2010; Van Waerebeek et al., 2014). The wide discrepancy between the comparatively large number of landed Clymene dolphins in Ghana and the fact that the species was rarely confirmed at sea, is at least partly explained by the difficulty to positively identify this smallish stenellid from a distance. Clymene dolphins were only observed in the absence of seismic operations and it is possible that these dolphins were either avoiding or keeping a greater distance to the vessel during operations. On the other hand, the wide discrepancy between the low number of landed Fraser's dolphins and the relatively high numbers encountered offshore cannot be explained by identification challenges as there are none. The differences in feeding strategies (nocturnal vs. diurnal; surface vs. deep water), different degrees of attraction to vessels and their gear as well as variable body sizes may influence the vulnerability of a species to become entangled or captured. Adult short-finned pilot whales and false killer whales may rarely be landed because they are more likely to break through nets, and escape, following entanglement but large species may also be difficult to retrieve from the nets and may subsequently be discarded at sea.
Based on our data on fishing density, cetacean (relative) abundance together with the previously reported information on cetacean landings we opine that in particular melon-headed whales, common dolphins, pantropical spotted dolphin, rough-toothed dolphin, common bottlenose dolphin, and Clymene dolphins are at high risk of entanglement or direct capture within these waters. This, together with the increase in the sale of cetacean products for human consumption as marine bushmeat in the Gulf of Guinea (e.g., Ofori-Danson et al., 2003; Van Waerebeek et al., 2015) may well contribute to a rapid and potentially localized decline of these species within this unique upwelling region. It is necessary to rapidly improve and implement feasible conservation measures directed to address this effectively unmanaged exploitation of small cetaceans in the Gulf of Guinea and the wider problem of an uncontrolled trade in marine bushmeat in western Africa.
Although our findings have given a new insight into the distribution of cetaceans and the problem of bycatch and direct takes in Ghanaian waters, it is clear that future risk assessments of fishing pressure on cetaceans through directed takes or incidental bycatch are urgently needed. Firstly, onboard observations are essential to study the dynamics of the catch process; while systematic studies of the ecology and natural history of all exploited species should also be undertaken. The lack of information on population status of cetacean species in this area hampers the understanding of which species-specific vulnerability characteristics drive the probability of a species to become entangled or captured in fishing gear, and this complicates future assessments of fishing pressure on cetaceans. There are also likely to be strong spatial and temporal (seasonal and inter-annual) variations in the distribution and abundance of both cetaceans and fisheries. Introducing biological factors into the analysis would lead to a clearer picture of how cetaceans use their habitat. This would not only improve our understanding of the ecology of the different species involved, but should also lead to more effective management and conservation measures.
Author Contributions
Conceived and designed the survey: Md, JS. Collected and analyzed the data: Md, JS, with contributions from KV, GA. Wrote the manuscript: Md, with textual contributions and re-drafting, critical review, and editorial input from JS, KV, GA.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MS and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
Special thanks to observers Sue Travers, Andy Williams for their support and for providing contributing information. Of equal importance has been the support and goodwill of the crews, clients and the fisheries liaison officers of the Geco Eagle and Geco Trident. Special thanks to Tullow Ghana Limited and Dave Bolger and John Doherty from Tullow Oil for their support. Tullow Oil funded this project and GA has been partly funded by NWO-ZKO grant “Effects of underwater noise on marine mammals in the North Sea.” Finally, we are grateful to Steve Geelhoed for reviewing an earlier draft of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2016.00178
References
Aarts, G., Fieberg, J., and Matthiopoulos, J. (2012). Comparative interpretation of count, presence-absence and point methods for species distribution models. Methods Ecol. Evol. 3, 177–187. doi: 10.1111/j.2041-210X.2011.00141.x
Adamec, D., and O'Brien, J. J. (1978). The seasonal upwelling in the Gulf of Guinea due to remote forcing. J. Phys. Oceanogr. 8, 1050–1060.
Addink, M. J., and Smeenk, C. (2001). Opportunistic feeding behaviour of rough-toothed dolphins Steno bredanensis off Mauritania. Zool. Verh. Leiden 334, 38–48.
Aheto, D. W., Asare, N. K., Quaynor, B., Tenkorang, E. Y., Asare, C., and Okyere, I. (2012). Profitability of small-scale fisheries in Elmina, Ghana. Sustainability 4, 2785–2794. doi: 10.3390/su4112785
Alfaro-Shigueto, J., and Van Waerebeek, K. (2001). “Drowning in a sea of silence: the bushmeat concept applied to marine wildlife,” in Zoos and Aquariums: Committing to Conservation, Symposium hosted by Brevard Zoo (Orlando, FL), 16.
Atta-Mills, J., Alder, J., and Sumaila, U. R. (2004). The decline of a regional fishing nation: the case of Ghana and West Africa. Nat. Resour. Forum 28, 13–21. doi: 10.1111/j.0165-0203.2004.00068.x
Ayissi, I., Van Waerebeek, K., and Segniagbeto, G. (2011). “Report on the exploratory survey of cetaceans and their status in Cameroon,” in CMS Scientific Council UNEP/CMS/ScC17/Inf.10 (Bergen), 31.
Best, P. B. (1974). “The biology of the sperm whale as it relates to stock management,” in The Whale Problem: A Status Report, ed W. E. Schevill (Cambridge: Harvard University Press), 257–293.
Best, P. B. (1996). Evidence of migration by Bryde's whales from the offshore population in the southeast Atlantic. Rep. Int. Whal. Commn. 46, 315–322.
Best, P. B., and Ross, G. J. B. (1977). Exploitation of small cetaceans off Southern Africa. Rep. Int. Whal. Commn. 34, 494–497.
Cadenat, J. (1959). Rapport sur les petits cétacés ouest-africaines. Résultats des recherches enterprises sur ces animaux jusqu'au mois de mars 1959. Bull. IFAN 21A, 1367–1440.
Cadenat, J., and Lassarat, A. (1959). Notes sur les Delphinidés ouest-africains. III. Notes complémentaires sur Tursiops truncatus. Bull. IFAN 21A, 416–419.
Cañadas, A., Sagarminaga, R., and Garcia-Tiscar, S. (2002). Cetacean distribution related with depth and slope in the Mediterranean waters off southern Spain. Deep Sea Res. 1 Oceanogr. Res. Pap. 49, 2053–2073. doi: 10.1016/S0967-0637(02)00123-1
Clapham, P., and Van Waerebeek, K. (2007). Bushmeat and bycatch: the sum of the parts. Mol. Ecol. 16, 2607–2609. doi: 10.1111/j.1365-294X.2007.03378.x
Culik, B. (2010a). Odontocetes - The Toothed Whales: Stenella attenuata. Bonn: UNEP/CMS Secretariat.
Culik, B. (2010b). Odontocetes - The Toothed Whales: Lagenodelphis hosei. Bonn: UNEP/CMS Secretariat.
Dalla Rosa, L., and Secchi, E. R. (2007). Killer whale (Orcinus orca) interactions with the tuna and swordfish longline fishery off southern and south-eastern Brazil: a comparison with shark interactions. J. Mar. Biol. Assoc. U. K. 87, 135–140. doi: 10.1017/S0025315407054306
de Boer, M. N. (2010a). Cetacean distribution and relative abundance in offshore Gabonese waters. J. Mar. Biol. Assoc. U. K. 90, 1613–1621. doi: 10.1017/S0025315410001165
de Boer, M. N. (2010b). First record of a white rough-toothed dolphin (Steno bredanensis) off West Africa including notes on rough-toothed dolphin surface behaviour. Mar. Biodivers. Rec. 3, e66. doi: 10.1017/S1755267210000539
de Boer, M. N. (2010c). Spring distribution and density of minke whale Balaenoptera acutorostrata along an offshore bank in the central North Sea. Mar. Ecol. Prog. Ser. 408, 265–274. doi: 10.3354/meps08598
de Boer, M. N. (2013). Elusive Marine Mammals Explored - Charting Under-Recorded Areas to Study the Abundance and Distribution of Cetaceans using Multi-Method Approaches and Platforms of Opportunity. Ph.D. thesis, Wageningen University, Wageningen.
de Boer, M. N. (2015). Cetaceans observed off Suriname and adjacent waters. LAJAM 10, 2–19. doi: 10.5597/lajam00189
de Boer, M. N., Saulino, J. T., Leopold, M. F., Reijnders, P. J. H., and Simmonds, M. P. (2012). Interactions between short-beaked common dolphin (Delphinus delphis) and the winter pelagic pair-trawl fishery off Southwest England (UK). Int. J. Biodivers. Conserv. 4, 481–499. doi: 10.5897/ijbc12.016
Debrah, J. A., Ofori-Danson, P. K., and Van Waerebeek, K. (2010). “An update on the catch composition and other aspects of cetacean exploitation in Ghana,” in International Whaling Commission SC/62/SM10 (Agadir), 8.
Debrah, J. S. (2000). Taxonomy, Exploitation and Conservation of Dolphins in the Marine Waters of Ghana. M.Phil. thesis (GH), University of Ghana.
Djiba, A., Bamy, I. L., Samba Ould Bilal, A., and Van Waerebeek, K. (2015). “Biodiversity of cetaceans in coastal waters of Northwest Africa: new insights through platform-of-opportunity visual surveying in 2011-2013,” in Oceanographic and Biological Features in the Canary Current Large Marine Ecosystem, IOC Technical Series, No. 115, eds L. Valdés and I. Déniz-González (Paris: IOC-UNESCO), 283–297.
Dolar, M. L. L. (2002). “Fraser's dolphin, Lagenodelphis hosei,” in Encyclopedia of Marine Mammals, eds W. F. Perrin, B. Würsig and J. G. M. Thewissen (San Diego, CA: Academic Press), 485–487.
Dolar, M. L. L., Perrin, W. F., Taylor, B. L., and Kooyman, G. L. (2006). Abundance and distributional ecology of cetaceans in the central Philippines. J. Cet. Res. Manage. 8, 93–111.
Dolar, M. L. L., Walker, W. A., Kooyman, G. L., and Perrin, W. F. (2003). Comparative feeding ecology of spinner dolphins (Stenella longirostris) and Fraser's dolphins (Lagenodelphis hosei) in the Sulu Sea. Mar. Mammal Sci. 19, 1–19. doi: 10.1111/j.1748-7692.2003.tb01089.x
Fertl, D., Schiro, A. J., and Peake, D. (1997). Coordinated feeding by clymene dolphins (Stenella clymene) in the Gulf of Mexico. Aquat. Mamm. 23, 111–112.
Findlay, K. P., Collins, T., and Rosenbaum, H. C. (2006). Environmental Impact Assessment and Mitigation of Marine Hydrocarbon Exploration and Production in the Republic of Gabon. New York, NY: Report of the Wildlife Conservation Society.
Food Agriculture Organization (FAO) (2007). Ghana Fishery Country Profile. Rome: Food and Agriculture Organization of the United Nations.
Gerrodette, T., and Wade, P. R. (1991). Monitoring trends in dolphin abundance in the eastern Tropical Pacific: analysis of 1989 data. Rep. Int. Whal. Commn. 41, 511–515.
Gill, P. C., Morrice, M. G., Page, B., Pirzi, R., Levings, A. H., and Coyne, M. (2011). Blue whale habitat selection and within-season distribution in a regional upwelling system off southern Australia. Mar. Ecol. Prog. Ser. 421, 243–263. doi: 10.3354/meps08914
Gray, H., and Van Waerebeek, K. (2011). Postural instability and akinesia in a pantropical spotted dolphin, Stenella attenuata, in proximity to operating airguns of a geophysical seismic vessel. J. Nat. Conserv. 19, 363–367. doi: 10.1016/j.jnc.2011.06
Hammond, P. S., Bearzi, G., Bjørge, A., Forney, K., Karczmarski, L., Kasuya, T., et al. (2010). Stenella clymene. IUCN Red List Threatened Species 4, 1–5. doi: 10.2305/IUCN.UK.2012.RTLS.T20730A17840531.en
Hernandez-Milian, G., Goetz, S., Varela-Dopico, C., Rodriguez-Gutierrez, J., Romon-Olea, J., Fuertes-Gamundi, J. R., et al. (2008). Results of a short study of interactions of cetaceans and longline fisheries in Atlantic waters: environmental correlates of catches and depredation events. Hydrobiologia 612, 251–268. doi: 10.1007/s10750-008-9501-2
Inter-American Tropical Tuna Commission (IATTC) (2009). Report of the Inter-American Tropical Tuna Commission for the year 2007. Annual Report. La Jolla, CA: IATTC.
International Fund for Animal Welfare (IFAW) (1996). Cetacean field research conducted from Song of the Whale off Dominica and Grenada: Spring 1996 (Unpublished Report). London: IFAW.
International Hydrographic Organization (IHO) (1953). Limits of Oceans and Seas. Special Publication 23, 3rd Edn. Monaco: International Hydrographic Organization.
Jefferson, T. A., Curry, B. E., Leatherwood, S., and Powell, J. A. (1997). Dolphins and porpoises of West Africa: a review of records (Cetacea: Delphinidae, Phocoenidae). Mammalia 61, 87–108.
Joint Nature Conservation Committee (JNCC) (2010). Guidelines for Minimising Acoustic Disturbance to Marine Mammals from Seismic Surveys. Peterborough, ON: Joint Nature Conservation Committee.
Koranteng, K. A. (2001). Structure and dynamics of demersal assemblages on the continental shelf and upper slope off Ghana, West Africa. Mar. Ecol. Progr. Ser. 220, 1–12. doi: 10.3354/meps220001
MacLeod, C. D., and Bennett, E. (2007). Pan-tropical spotted dolphins (Stenella attenuata) and other cetaceans around St Helena. J. Mar. Biol. Assoc. U. K. 87, 339–344. doi: 10.1017/S0025315407052502
Maigret, J. (1981). Rapports entre les cétacés et la pêche thonière dans l'Atlantique tropical oriental. Notes Afr. 171, 75–84.
Mendez, M., Rosenbaum, H. C., Wells, R. S., Stamper, A., and Bordino, P. (2010). Genetic evidence highlights potential impacts of bycatch to cetaceans. PLoS ONE 5:e15550. doi: 10.1371/journal.pone.0015550
Morizur, Y., Berrow, S. D., Tregenza, N., Couperus, A. S., and Pouvreau, S. (1999). Incidental catches of marine mammals in pelagic trawl fisheries of the north-east Atlantic. Fish. Res. 41, 297–307.
Mullié, W. C., Wagne, M. M., Elmamy, C. A., Mint Yahya, F., Veen, J., and Van Waerebeek, K. (2013). “Large number of stranded harbour porpoises Phocoena phocoena as by-catch victims in Mauritania,” in International Whaling Commission SC/65a/HIM03 (Jeju), 5.
Ofori-Danson, P. K., and Odei, M. A. (1997). “Preliminary observations of the common dolphin, Delphinus delphis, (Order: Cetacea; fam: Delphinidae) in the Ghanaian coastal waters,” in International Whaling Commission SC/49/SM3 (Monaco), 8.
Ofori-Danson, P. K., Van Waerebeek, K., and Debrah, S. (2003). A survey for the conservation of dolphins in Ghanaian coastal waters. J. Ghana Sci. Assoc. 5, 45–54.
Paiva, E. G., Salgado Kent, C. P., Gagnon, M. M., McCauley, R., and Finn, H. (2015). Reduced detection of Indo-Pacific bottlenose dolphins (Tursiops aduncus) in an inner harbour channel during pile driving activities. Aquat. Mamm. 41, 455–468. doi: 10.1578/AM.41.4.2015.455
Perrin, W. F., Mitchell, E. D., Mead, J. G., Caldwell, D. K., and van Bree, P. J. H. (1981). Stenella clymene, a rediscovered tropical dolphin of the Atlantic. J. Mamm. 62, 583–598.
Perrin, W. F., and Van Waerebeek, K. (2012). The small-cetacean fauna of the west coast of Africa and Macaronesia: diversity and distribution. CMS Tech. Ser. 26, 7–17.
Picanço, C., Carvalho, I., and Brito, C. (2009). Occurrence and distribution of cetaceans in São Tomé and Príncipe tropical archipelago and their relation to environmental variables. J. Mar. Biol. Assoc. U. K. 89, 1071–1076. doi: 10.1017/S0025315409002379
Reeves, R., and Notarbartolo di Sciara, G. (2006). The status and distribution of cetaceans in the Black Sea and Mediterranean Sea. Malaga: IUCN Centre for Mediterranean Cooperation.
Reilly, S. B., Bannister, J. L., Best, P. B., Brown, M., Brownell, R. L. Jr., Butterworth, D. S., et al. (2008). Balaenoptera edeni. IUCN Red List Threatened Species 2014.3, 1–5. doi: 10.2305/IUCN.UK.2008.RLTS.T2476A9445502.en
Segniagbeto, G. H., Van Waerebeek, K., Bowessidjaou, J. E., Ketoh, K., Kpatcha, T. K., Okoumassou, K., et al. (2014). Annotated checklist and fisheries interactions of cetaceans in Togo, with evidence of Antarctic minke whale in the Gulf of Guinea. Integr. Zool. 9, 1–13. doi: 10.1111/1749-4877.12011
Segniagbeto, G., and Van Waerebeek, K. (2010). “A note on the occurrence and status of cetaceans in Togo,” in International Whaling Commission SC/62/SM11 (Agadir), 8.
Sohou, Z., Dossou-Bodjrenou, J., Tchibozo, S., Chabi-Yaouré, F., Sinsin, B., and Van Waerebeek, K. (2013). Biodiversity and status of cetaceans in Benin, West Africa: an initial assessment. WAJAE 21, 121–134.
Southall, B. L., Bowles, A. E., Ellison, W. T., Finneran, J. J., Gentry, R. L., Greene, C. R. Jr., et al. (2007). Marine mammal noise exposure criteria: initial scientific recommendations. Aquat. Mamm. 33, 411–521. doi: 10.1080/09524622.2008.9753846
Torda, G., López Suárez, P., and López Jurado, L. F. (2010). First records of Fraser's dolphin Lagenodelphis hosei for the Cape Verde Islands. Zool. Caboverdiana 1, 71–73.
Townsend, C. H. (1935). The distribution of certain whales as shown by logbook records of American whale ships. Zoologica 19, 1–50.
van Bree, P. J. H. (1971). On skulls of Stenella longirostris (Gray, 1828) from the eastern Atlantic (Notes on Cetacea, Delphinoidea IV). Beaufortia 19, 21–25.
Van Bree, P. J. H., and Purves, P. E. (1972). Remarks on the validity of Delphius bairdii (Cetacea Delphininae). J. Mamm. 53, 372–374.
Van Waerebeek, K., Barnett, L., Camara, A., Cham, A., Diallo, M., Djiba, A., et al. (2003). Conservation of Cetaceans in the Gambia and Senegal 1999-2001 and Status of the Atlantic Humpback Dolphin. Bonn: WAFCET-2 Rep. CMS/UNEP. doi: 10.13140/RG.2.1.3917.9602
Van Waerebeek, K., Debrah, J., and Ofori-Danson, P. K. (2014). “Cetacean landings at the fisheries port of Dixcove, Ghana in 2013-14: a preliminary appraisal,” in International Whaling Commission SC/65b/SM17 (Bled), 4.
Van Waerebeek, K., Ndiaye, E., Djiba, A., Diallo, M., Murphy, P., Jallow, A., et al. (2000). A Survey of the Conservation Status of Cetaceans in Senegal, The Gambia and Guinea-Bissau. Bonn: WAFCET-1 Rep, CMS/UNEP. doi: 10.13140/RG.2.1.2538.6003
Van Waerebeek, K., and Ofori-Danson, P. K. (1999). “A first checklist of cetaceans of Ghana, Gulf of Guinea, and a shore-based survey of interactions with coastal fisheries,” in International Whaling Commission SC/51/SM35 (Grenada), 9.
Van Waerebeek, K., Ofori-Danson, P. K., and Debrah, J. (2009). The cetaceans of Ghana: a validated faunal checklist. WAJAE 15, 61–90. doi: 10.4314/wajae.v15i1.49428
Van Waerebeek, K., and Perrin, W. F. (2012). “Conservation status of the Clymene dolphin in West Africa,” in CMS Scientific Council CMS/ScC14/Doc.5 (Bonn), 9.
Van Waerebeek, K., Uwagbae, M., Segniagbeto, G., Bamy, I. L., and Ayissi, I. (2015). New records of Atlantic humpback dolphin in Guinea, Nigeria, Cameroon and Togo underscore fisheries pressure and generalised marine bushmeat demand. BioRxiv/2015/035337. doi: 10.1101/035337
Weir, C. R. (2007). Occurrence and distribution of cetaceans off northern Angola, 2004/05. J. Cet. Res. Manage. 9, 225–239.
Weir, C. R. (2008a). Overt responses of humpback whales (Megaptera novaeangliae), sperm whales (Physeter macrocephalus), and Atlantic spotted dolphins (Stenella frontalis) to seismic exploration off Angola. Aquat. Mamm. 34, 71–83. doi: 10.1578/AM.34.1.2008.71
Weir, C. R. (2008b). Short-finned pilot whales (Globicephala macrorhynchus) respond to an airgun ramp-up procedure off Gabon. Aquat Mamm. 34, 349–354. doi: 10.1578/AM.34.3.2008.349
Weir, C. R. (2010). A review of cetacean occurrence in West African waters from the Gulf of Guinea to Angola. Mamm. Rev. 40, 2–39. doi: 10.1111/j.1365-2907.2009.00153.x
Weir, C. R. (2011). Ecology and Conservation of Cetaceans in the Waters between Angola and the Gulf of Guinea, with Focus on the Atlantic Humpback Dolphin (Sousa teuszii). Ph.D. thesis (UK), University of Aberdeen.
Weir, C. R., Coles, P., Ferguson, A., May, D., Baines, M., Figueirdo, I., et al. (2014). Clymene dolphins (Stenella clymene) in the eastern tropical Atlantic: distribution, group size, and pigmentation pattern. J. Mamm. 95, 1289–1298. doi: 10.1644/14-MAMM-A-115
Weir, C. R., Collins, T., Cross, T., Gill, A., Elwen, S., Unwin, M., et al. (2013b). False killer whale (Pseudorca crassidens) sightings in continental shelf habitat off Gabon and Côte d'Ivoire (Africa). Mar. Biodivers. Rec. 6, e65. doi: 10.1017/S1755267213000389
Weir, C. R., Debrah, J., Ofori-Danson, P. K., Pierpont, C., and Van Waerebeek, K. (2008). Records of Fraser's dolphin Lagenodelphis hosei Fraser 1056 from the Gulf of Guinea and Angola. Afr. J. Mar. Sci. 30, 241–245. doi: 10.2989/AJMS.2008.30.2.4.554
Weir, C. R., Goncalves, L., and May, D. (2013a). New Gulf of Guinea (Africa) range state records for pygmy killer whale (Feresa attenuata) and Fraser's dolphin (Lagenodelphis hosei). Mar. Biodivers. Rec. 6, e35. doi: 10.1017/S1755267212001303
Weir, C. R., and Pierce, G. J. (2012). A review of the human activities impacting cetaceans in the eastern tropical Atlantic. Mamm. Rev. 43, 258–274. doi: 10.1111/j.1365-2907.2012.00222.x
Keywords: cetacean mortality, fisheries, drift gillnet, anthropogenic impact, cetacean distribution, population decline, Gulf of Guinea, seismic survey
Citation: de Boer MN, Saulino JT, Van Waerebeek K and Aarts G (2016) Under Pressure: Cetaceans and Fisheries Co-occurrence off the Coasts of Ghana and Côte d'Ivoire (Gulf of Guinea). Front. Mar. Sci. 3:178. doi: 10.3389/fmars.2016.00178
Received: 12 March 2016; Accepted: 05 September 2016;
Published: 21 September 2016.
Edited by:
Andrew Butterworth, University of Bristol, UKReviewed by:
Mark Peter Simmonds, University of Bristol, UKCatherine Sarah Longo, Marine Stewardship Council, UK
Copyright © 2016 de Boer, Saulino, Van Waerebeek and Aarts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marijke N. de Boer, bWFyaWprZWRlYm9lckBzZXZlbnNlYXNtYXJpbmUubmw=
 Marijke N. de Boer
Marijke N. de Boer James T. Saulino1
James T. Saulino1