- 1Department of Ocean Systems, NIOZ Royal Institute for Sea Research, Utrecht University, Den Burg, Netherlands
- 2Department of Marine Microbiology and Biogeochemistry, NIOZ Royal Institute for Sea Research, Utrecht University, Den Burg, Netherlands
Fe-limited monocultures of the ubiquitous algae Micromonas pusilla and Phaeocystis globosa were infected with their respective viruses (MpV and PgV) to ascertain the effect of Fe-limitation on phytoplankton host-virus dynamics. The effect of the viral shunt on Fe concentrations and bioavailability is starting to gain attention, since not only is Fe released through lysis, but also its solubility is increased by the simultaneous release of Fe-binding dissolved organic ligands. However, the effect of Fe-limitation on the process of viral lysis itself is poorly understood. In this study fine adjustment of a seawater-based culture medium including the use of ultra-clean trace metal conditions and protocols allowed for Fe-limited growth at nanomolar amounts as opposed to micromolar amounts typically employed in culturing. Viral lysates derived from Fe-limited and Fe-replete (for comparison) hosts were cross-inoculated in hosts of both Fe treatments, to judge the quality of the resulting lysate as well as the effect of Fe introduction after initial infection. For both phytoplankton host-virus systems, the virus burst size reduced strongly under Fe stress, i.e., on average 28 ± 1% of replete. Moreover, the MpV virus progeny showed highly reduced infectivity of 30 ± 7%, whereas PgV infectivity was not affected. A small addition of Fe to Fe-limited cultures coming from the Fe-replete lysate counteracted the negative effect of Fe-limitation on phytoplankton virus production to some extent (but still half of replete), implying that the physiological history of the host at the moment of infection was an important underlying factor. These results indicate that Fe-limitation has the strong potential to reduce the loss of phytoplankton due to virus infection, thereby affecting the extent of Fe-cycling through the viral shunt. To what extent this affects the contribution of viral lysis-induced organic ligand release needs further study.
Introduction
Phytoplankton form the base of most marine pelagic food webs and are important in sequestering atmospheric carbon dioxide (CO2) through photosynthesis. The production of phytoplankton is controlled by physicochemical variables (bottom-up) as well as by biological factors (top-down). Main bottom-up controls of phytoplankton are light and nutrient availability (Behrenfeld et al., 2006). The latter can be subdivided into major (nitrate, phosphate, and silicate) and micro-nutrients (e.g., iron; de Baar et al., 1990; Martin et al., 1990). Top-down factors, e.g., grazing and viral infection, influence the organic matter flux differently (Wilhelm and Suttle, 1999; Weitz and Wilhelm, 2012). While grazing transfers photosynthetically fixed carbon and organic nutrients up the food chain (Calbet and Landry, 2004), viral lysis results in the release of the hosts' cellular content into the surrounding water (Gobler et al., 1997; Wilhelm and Suttle, 1999). Thereupon, the flow of nutrients through the microbial food web is stimulated by bacterial recycling of the dissolved and dead particulate matter (Brussaard et al., 2005; Suttle, 2005; Brussaard and Martínez, 2008). Virally-induced mortality of various different natural phytoplankton groups was found to be at least an equally important loss factor as microzooplankton grazing (Baudoux et al., 2007; Mojica et al., 2016).
In order to understand and predict changes in phytoplankton community composition, it is important to elucidate how bottom-up and top-down factors interact and affect phytoplankton population dynamics. Several studies using phytoplankton host-virus culture systems showed that major nutrient availability influences viral production (Maat and Brussaard, 2016 and see review by Mojica and Brussaard, 2014). For example, phosphorus (P) limitation of the virally infected phytoplankton host results in a prolonged latent period, i.e., the time between infection and the initial release of progeny viruses from the host cell, for the infecting viruses (Maat et al., 2014). Moreover, P-stress resulted in reduced viral burst size, i.e., the number of newly formed viruses released per lysed host cell (Bratbak et al., 1998; Maat et al., 2014). These studies proposed shortage of phosphorus as a viral production substrate as well as possible host energy deficiency as reasons for the lower and delayed viral particle yield. There is, however, virtually nothing known on the effect of micronutrient limitation on phytoplankton host-virus interactions.
Furthermore, as iron (Fe) solubility in seawater is low (Millero, 1998; Liu and Millero, 2002), marine phytoplankton depend on Fe-binding ligands to increase solubility and therefore bioavailability (Gledhill and van den Berg, 1994; Rue and Bruland, 1995). The redox state of Fe is an important factor in Fe bioavailability. The oxidized Fe(III) state is the more stable and thus prevalent state in marine conditions, while the reduced Fe(II) state is the more bioavailable (Breitbarth et al., 2010; Shaked and Lis, 2012). Release of reactive oxygen species during phytoplankton growth has been shown to contribute to bioavailability of Fe by facilitating reduction of Fe(III) (Kustka et al., 2005; Garg et al., 2007). Furthermore, organic exudates have been connected to lowered Fe(II) oxidation rates experimentally (González et al., 2014). Part of the Fe-binding ligand pool is thought to be of marine biological origin. Strong Fe-binding organic ligands called siderophores are purposefully produced by bacteria (Butler, 2005; Mawji et al., 2011). Humic acids and polysaccharide excretions are other recognized Fe chelators with a biological origin (Hassler et al., 2011; Laglera et al., 2011). The highest Fe-binding ligand concentrations generally correlate with biological activity (Rue and Bruland, 1995; Gerringa et al., 2006; Ibisanmi et al., 2011). Viral lysis, releasing organic substances in seawater, may well be an important contributor to the ligand pool (Gobler et al., 1997; Poorvin et al., 2004, 2011). In these studies by Poorvin and others, it was found that bacterial and cyanobacterial lysates provided organically bound Fe in a form more bioavailable than supplied inorganic, ethylenediaminetetraacetic acid (EDTA) bound or desferrioxamine B (DFB) bound Fe. In comparison to the studied bacteria's self-produced siderophores, lysates were also found to contain more bioavailable Fe.
Fe-limitation negatively affects phytoplankton physiology and growth (de Baar et al., 1990; Martin et al., 1990; Behrenfeld et al., 1996; Timmermans et al., 2001b). Besides energetic consequences of Fe-limitation in terms of the cell's ability to harvest light energy (Geider and La Roche, 1994), Fe is also found to be an essential micronutrient for DNA replication (Netz et al., 2012; Zhang, 2014). As parasites viruses are dependent on the metabolism of their host for the production of their progeny. We hypothesize that viral production depends on the degree of Fe-stress of the host. Viral lysis in turn affects the production of Fe-binding organic ligands and thus the solubility of the limiting Fe. Thus far, in terms of impact on Fe cycling, studies focussed solely on the release of Fe or Fe-binding organic ligands upon viral lysis and not on the virus growth cycle (Gobler et al., 1997; Poorvin et al., 2004, 2011). Here we examine virus production characteristics under Fe-limitation for two key ecologically relevant phytoplankton hosts: the nanoeukaryotic bloom-forming Prymnesiophyte Phaeocystis globosa and the picoeukaryotic Prasinophyte Micromonas pusilla. Phaeocystis is a globally occurring, bloom-forming genus (Vaulot et al., 1994), with P. globosa ecologically relevant in temperate marine waters (Schoemann et al., 2005). Viruses have been found to drive P. globosa bloom decline (Brussaard, 2004; Brussaard et al., 2005; Baudoux et al., 2006). M. pusilla is a common species that is distributed globally (Not et al., 2004, 2005; Vaulot et al., 2008). It has been speculated that viral control of this species is continuous (Cottrell and Suttle, 1995). As model species for diverse regions and ecological niches, these species were chosen in this study to offer a broad insight in the response to Fe-limitation of phytoplankton host-virus systems in world oceans subject to changing conditions. Limiting concentrations were of ecological relevance to represent a natural context.
Methods
Materials Preparation
Cleaning of equipment and preparation of chemicals took place in a class 100 ultra-clean laboratory environment (Interflow). Culture handling and sampling were carried out in a 15°C climate chamber within the ultra-clean laboratory. When outside the ultra-clean environment, sample handling took place inside class 100 laminar flow hoods (Interflow). All rinsing and chemical dissolution and dilution was done using 18 MΩ deionized Milli-Q water (Merck Millipore), further referred to as MQ.
Prior to use, general labware of high- and low-density polyethylene (HDPE and LDPE, respectively) and fluorinated ethylene propylene (FEP) was cleaned thoroughly by pre-rinsing (5x) with MQ, followed by soaking in 6 M hydrochloric acid (HCl) for a minimum of 24 h, and a final thorough (5x) MQ rinsing. A sub-boiling quartz distillation apparatus (Savillex) was used to purify nitric acid (HNO3) in triplicate, yielding Fe free quartz distilled (3QD) HNO3. A 0.3 M 3QD-HNO3 solution is used to fill stored bottles.
The polycarbonate (PC) culture flasks (50–500 mL, VWR) and bottles (1–2 L, Nalgene) were acid cleaned with 1 M HCl for a minimum of 24 h, after which they were rinsed with MQ (5x). Finally, bottles were sterilized with a 10% volume of boiling MQ, i.e., the bottle with MQ was microwaved at 900 W to boiling point and left boiling for ~20 s. After vigorous shaking the hot MQ was poured out over the inverted lid. Culture vessels were then left to air-dry in a laminar flow bench for at least 2 h.
Culturing
Axenic cultures of the nanoeukaryotic Prymnesiophyte P. globosa G(A) (culture collection of the University of Groningen, the Netherlands) and the picoeukaryotic Prasinophyte M. pusilla LAC38 (Marine Research Center culture collection, Göteborg University, Sweden) were maintained under trace metal clean conditions.
A low Fe-containing medium (Low Trace: LT) based on natural seawater (DFe 0.2 nM, collected west of the Bay of Biscay, Atlantic Ocean after Rijkenberg et al., 2012) was designed without metal chelators such as EDTA. Chelators as EDTA are used as metal buffers in culture media, and are added in 10−6–10−3 M concentrations to ascertain that those transition metals added as micronutrients, e.g., Cu, Co, Fe, Mn, Mo, Ni, V, Zn, are kept in the dissolved phase through complexation by EDTA (Sunda and Huntsman, 1995). However, these high concentrations of artificial ligands completely overrule the natural chemistry of the metals, making the study of natural Fe binding organic ligands impossible (Gerringa et al., 2000). The medium was enriched with the macronutrients NaNO3 (Sigma-Aldrich) and Na2HPO4 (Merck Millipore) to final concentrations of 128 and 8 μM, respectively. The following micronutrients were added: KBr (7.1 μM final concentration), NaF (3.0 μM), CaCl2·2H2O, (17.9 μM), SrCl2·6H2O (0.8 μM), MgCl2·6H2O (2.3 μM) and Na2SeO3 (10.0 nM). Vitamins H, B1, and B12 were added to final concentrations of 2.0, 296.0, and 0.4 nM, respectively. A combined Tris(hydroxymethyl) aminomethane (Tris) and HCl buffer was added to a 4.0 μM final concentration. Nutrient and buffer stock solutions were cleaned of trace metal contaminations by equilibration with MnO2 after van den Berg and Kramer (1979). The MnO2 was removed using a trace metal clean 47 mm diameter 0.2 μm pore size PC filter (Whatman) in a polysulfone (PSU) filter tower (Nalgene) with an electric vacuum pump (Merck Millipore). This cleaning process was performed twice. To maintain constant growth for multiple generations addition of additional trace metals proved essential. These trace metal additions were kept to a minimum in order to avoid influencing Fe speciation, e.g., interactions between Cu and Fe (González et al., 2016). P. globosa received an additional trace solution containing final concentrations of 2.0 nM ZnSO4·7H2O, 1.0 nM CoCl2·6H2O, 4.6 nM MnCl2·4H2O, and 0.6 nM Na2MoO4·2H2O. M. pusilla showed poor physiological condition and growth using this trace solution. Because it was impossible to maintain as steady state under these conditions, a slightly different trace solution was used, containing final concentrations of 4.0 nM ZnSO4·7H2O, 5.0 nM CoCl2·6H2O, 1.0 nM CuSO4·5H2O, 1.0 nM NiSO4·6H2O, 1.0 nM Na3VO4, 1.0 nM K2CrO4, 9.1 nM MnCl2·4H2O, 4.1 nM Na2MoO4·2H2O, and 1.0 nM H2SeO3. Fe was added from an acidified 3 μM FeCl stock solution made using a 1000 mg L−1 ICP stock (Fluka, Sigma-Aldrich). The Fe-limiting medium contained final concentrations of 1.0 and 3.0 nM Fe for P. globosa and M. pusilla, respectively. In comparison, the Fe-replete (control) medium contained 9.0 μM FeCl for both species.
Culture temperature was 15°C and irradiance was supplied at 90 μmol quanta m−2 s−1 under a 16:8 h light:dark cycle. Phytoplankton cultures were maintained semi-continuously to obtain and sustain constant and comparable physiology and growth, i.e., diluting the culture daily with new medium whereby the exact volume was determined by the maximum growth rate possible under Fe-limiting culture conditions without wash-out (Maat et al., 2014). The limiting Fe concentration determined the maximum cell abundance, which was determined before and after dilution using flow cytometry (Marie et al., 1999). At steady state, i.e., after at least 8 volume changes and consistent phytoplankton counts (2.1 ± 0.4 × 106 and 2.1 ± 0.7 × 106 for P. globosa and M. pusilla, respectively), samples were collected for dissolved macronutrients (nitrogen and phosphorus) and Fe, as well as pigment composition. Nutrient samples (5 ml after washing of filter and tube) were 0.2 μm filtered (25 mm diameter Acrodisk, Pall) and frozen at −20°C until analysis. GF/C filtered (1.2 μm nominal pore size, 25 mm diameter, Whatman) algal pigment samples of 50 mL were frozen at −80°C until analysis.
Axenic viral lysate of the double-stranded DNA viruses PgV-07T (Baudoux and Brussaard, 2005) infecting P. globosa G(A) and MpV-08T (Martínez et al., 2014) infecting M. pusilla LAC38 were obtained by 10% v/v inoculation to exponentially growing phytoplankton host and checked for full lysis by flow cytometry (FCM). Fe-limited lysates of both phytoplankton viruses were initiated by 1% v/v inoculations with Fe-replete lysates, after which a minimum of 5 subsequent 10% v/v inoculations followed before use for the experiment. This way the Fe concentration in the Fe-limited lysate was similar to the Fe concentration in the Fe-limited host culture. The number of infective phytoplankton viruses was determined using the most probable number (MPN) endpoint dilution assay according to Suttle (1993). MPN data was analyzed using the University of British Columbia Computer Science department's Assay Analyser software program (Passmore et al., 2000).
Experimental Design
Steady state exponentially growing phytoplankton cultures were subdivided per treatment (Fe-limited and Fe-replete) in 6 replicate 500 mL culture flasks. Two days later the viral infection experiment started 3 h into the light period. For each treatment, 2 replicate cultures received viruses produced on Fe-limited host culture (VL), 2 replicates received viruses produced on Fe-replete host culture (VR), and 2 replicates did not receive viruses and served as non-infected controls (C). Fe-limited lysates were added not only to the respective Fe-limited host cultures, but also to the Fe-replete host in order to test for the reduced infectivity we observed under Fe-limitation (See Results). For this reason and to still guarantee a one-step infection cycle we aimed to add 20–25 viruses per algal cell for P. globosa. Given lower yields for M. pusilla, we endeavored to add at least 5–10 viruses per algal cell, while still maintaining a ~10% v/v addition. Similarly, Fe-replete lysate was also added to Fe-limited host cultures. This caused a Fe-spike of about 0.9 μM (10% v/v of Fe-replete medium containing lysate), which allows testing whether a spike of Fe influences virus proliferation.
The moment viruses were added, cultures were maintained in-batch. We examined the effect of frequent handling by taking along a control subculture per Fe-limited treatment that was only gently mixed once a day. Samples for phytoplankton and virus abundance as well as photosynthetic capacity (Fv/Fm) were taken every 4 h until full lysis of the cultures (48 h for P. globosa and 96 h for M. pusilla). Phytoplankton abundance and Fv/Fm were determined directly upon sampling, while viral abundance samples were fixed with glutaraldehyde (EM-grade, 0.5% final concentration), flash frozen in liquid nitrogen and stored at −80°C (Brussaard et al., 2010).
Analyses
High Performance Liquid Chromatography (HPLC) pigment analysis was performed on steady state phytoplankton samples after Zapata et al. (2000). Phytoplankton cells in fresh samples were discriminated and counted based on Chlorophyll-a red autofluorescence using a FACSCanto flow cytometer (Becton Dickinson) equipped with a 17 mW 633 nm HeNe red laser. Viral abundances were also determined by flow cytometry using a FACSCalibur flow cytometer (Becton Dickinson) equipped with a 15 mW 488 nm argon-ion blue laser triggered on green fluorescence, following the protocol by Brussaard et al. (2010). In short, samples were diluted 200–1000-fold using a 2 M Tris-HCl buffer at pH 8 and viruses were stained using the nucleic acid-specific fluorescent dye SYBR Green I (Molecular Probes®, Life Technologies, Thermo Fisher). Raw data were analyzed using Cytowin (Vaulot, 1989; Version 4.31 available at http://application.sb-roscoff.fr/Phyto/index.php), whereby PgV and MpV were easily discriminated by plotting green nucleic acid-specific fluorescence vs. side scatter (Baudoux and Brussaard, 2005; Martínez et al., 2015).
The number of infective phytoplankton viruses was determined using the endpoint dilution assay according to Suttle (1993). In short, 10-fold dilution series were set up in 5 replicate tubes using a dilute Fe-replete phytoplankton culture at a density of ~106 cells mL−1. A row of uninfected control tubes was added to each analysis. Cell lysis was regularly scored by eye and the final score after 14 days post-infection was used to calculate the number of infectious viruses. Dividing this number of infectious by the total number of PgV or MpV provided the % infectious viruses.
Photosynthetic capacity (Fv/Fm) measurements were performed using a Chlorophyll Fluorometer with a red emitter-detector unit (Water-PAM, Waltz). Samples were kept in the dark for 30 min at culturing temperature, after which chlorophyll autofluorescence was measured in duplicate in the dark adapted state (F0) and after a saturation pulse of 2.5 s (Fm). Fv is defined as the difference between Fm and F0 (Genty et al., 1989). Concentrations of dissolved inorganic macronutrients nitrate and orthophosphate were verified colorimetrically using a trAAcs 800 auto analyser (Murphy and Riley, 1962; Grasshoff, 1983), and were non-limiting at all times (>128 μM nitrate and >8 μM phosphate). Verification of the dissolved Fe concentration was done using flow injection analysis after Klunder et al. (2011), the detection limit of this method was 0.01 nM.
Results and Discussion
Steady State
Exponential growth rate (μmax) for both Fe-limited and Fe-replete P. globosa and Fe-replete M. pusilla was 0.99 ± 0.11 d−1. M. pusilla showed, however, reduced growth under Fe-limitation (0.63 ± 0.07 d−1). Initial difficulties encountered with consistent semi-continuous culturing of M. pusilla required us to increase the Fe concentration to 3 nM as compared to the 1 nM for P. globosa. Still, the lower steady state μmax found for M. pusilla under Fe-limitation suggests a less efficient Fe-uptake or utilization of M. pusilla as compared to P. globosa. However, the photosynthetic capacity (Fv/Fm) of the Fe-limited M. pusilla remained high around 0.6. Thus, the smaller-sized M. pusilla requires more Fe to grow, albeit at a lower μmax, while it is capable of retaining Fv/Fm at a value similar to Fe-replete conditions. Reduced Fv/Fm and growth rate under Fe-limitation has been reported for a small diatom species (Timmermans et al., 2001a); however, both variables were reduced for the same species and not one or the other as found in present study. Our result do not support the earlier reports that small phytoplankton (diatoms and cyanobacteria) are growing better under Fe-limitation than larger phytoplankton (Price et al., 1994; Timmermans et al., 2001b, 2004). Whether the differences are due to species-specific or phytoplankton group related responses is currently unclear.
The cellular Chlorophyll-a concentration at steady state was lower under Fe-limitation compared to the Fe-replete control cultures for both algal species (Tables 1, 2). For M. pusilla the reduction was larger, i.e., Chlorophyll-a concentration was only 40% of Fe-replete concentration, compared to 63% for P. globosa. In the Fe-limited P. globosa cultures, cellular Chlorophyll-c concentration was reduced (to a similar extent; Table 1), whereas for M. pusilla the Chlorophyll-b concentration also decreased compared to Fe-replete (Table 2). Furthermore, Fe-limitation led to a different distribution of light-harvesting xanthophylls in P. globosa (Table 1). Cellular 19'-hexafucoxanthin concentration was found 5-fold higher in the Fe-limited cultures as compared to Fe-replete P. globosa (4.8 × 10−12 g cell−1), while 19′-butanoyloxyfucoxanthin and fucoxanthin concentrations were 0 and 89% of Fe-replete, respectively. The photoprotective xanthophyll derivatives in Fe-limited P. globosa are increased relative to Chlorophyll-a, i.e., the diadinoxanthin concentration over Chlorophyll-a is 162% of Fe-replete (0.58 and 0.57 × 10−11 g × cell −1, respectively) and the diatoxanthin concentration is 181% of Fe-replete (0.08 and 0.07 × 10−11 g × cell−1, respectively). Fe-limited M. pusilla cultures (Table 2) had lower cellular Chlorophyll-b and light-harvesting xanthophyll concentrations. Relative to the Chlorophyll-a concentration, photoprotective xanthophyll derivatives antheraxanthin, zeaxanthin, and lutein were present in higher amounts. Only violaxanthin remains lower both in absolute and relative terms.
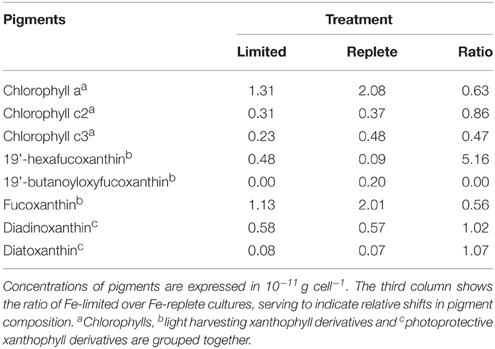
Table 1. Steady state phytoplankton pigment composition in non-infected Fe-limited (first column) and Fe-replete (second column) P. globosa cultures.
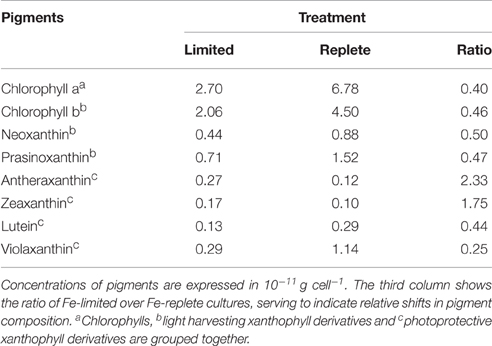
Table 2. Steady state phytoplankton pigment composition in non-infected Fe-limited (first column) and Fe-replete (second column) M. pusilla cultures.
Overall, the pigment analysis shows that for both phytoplankton species the photosystem shifted toward a more photoprotective character under Fe-limited conditions. The lower concentrations of chlorophyll and most light-harvesting xanthophylls furthermore indicated that the Fe-limited cells suffered a lower light-harvesting capacity. Fe is essential for all life and earlier studies showed that Fe-deficiency can lead to anemia in mammals, the dysfunction of Fe-dependent enzymes in yeast, the reduction of the amount of electron-transferring complexes and induction of chlorosis in plants, and a decrease in Fe-intensive light harvesting pigment synthesis and increased photoprotective pigments in phytoplankton (Geider et al., 1993; Mengel, 1994; van Leeuwe and Stefels, 2007; van de Poll et al., 2009; Zhang, 2014). In line with the fact that the Fe concentrations in the Fe-limited cultures were always below the limit of detection, these results confirm that both phytoplankton cultures were indeed Fe-limited, despite that M. pusilla showed healthy Fv/Fm. The more pronounced shift to photoprotective pigment production relative to light-harvesting Chlorophyll-a in M. pusilla compared to P. globosa is in agreement with the strong decline in steady state M. pusilla cell abundance with Fe-limitation while Fv/Fm was unaffected. However, M. pusilla was unable to grow at 1 nM Fe while P. globosa grew well. Our results imply that P. globosa was more affected energetically by Fe-limitation, while M. pusilla suffered instead in overall cellular production.
Viral Infection Characteristics
Infection of both phytoplankton species resulted in one-step infection cycles with full lysis of the cultures whereas the non-infected controls grew or maintained constant cell number (Figures 1A,B, 2A,B). Cell growth in the non-infected Fe-replete cultures reflects the synchronized cell division during the dark period (Brussaard et al., 1999). In the Fe-limited cultures growth of P. globosa halted, which was due to stress from the frequent sampling, since the Fe-limited subcultures that were sampled only once a day did show some growth (data not shown). The Fv/Fm of the uninfected Fe-replete phytoplankton cultures remained constant (Figures 1C,D, 2C,D), whereby the small variations observed in the Fe-replete P. globosa cultures relate to the light:dark cycle (Figure 1D). The Fe-limited non-infected control cultures showed a decline in Fv/Fm over time as a result of the Fe deprivation, but to a lesser extent than the infected cultures.
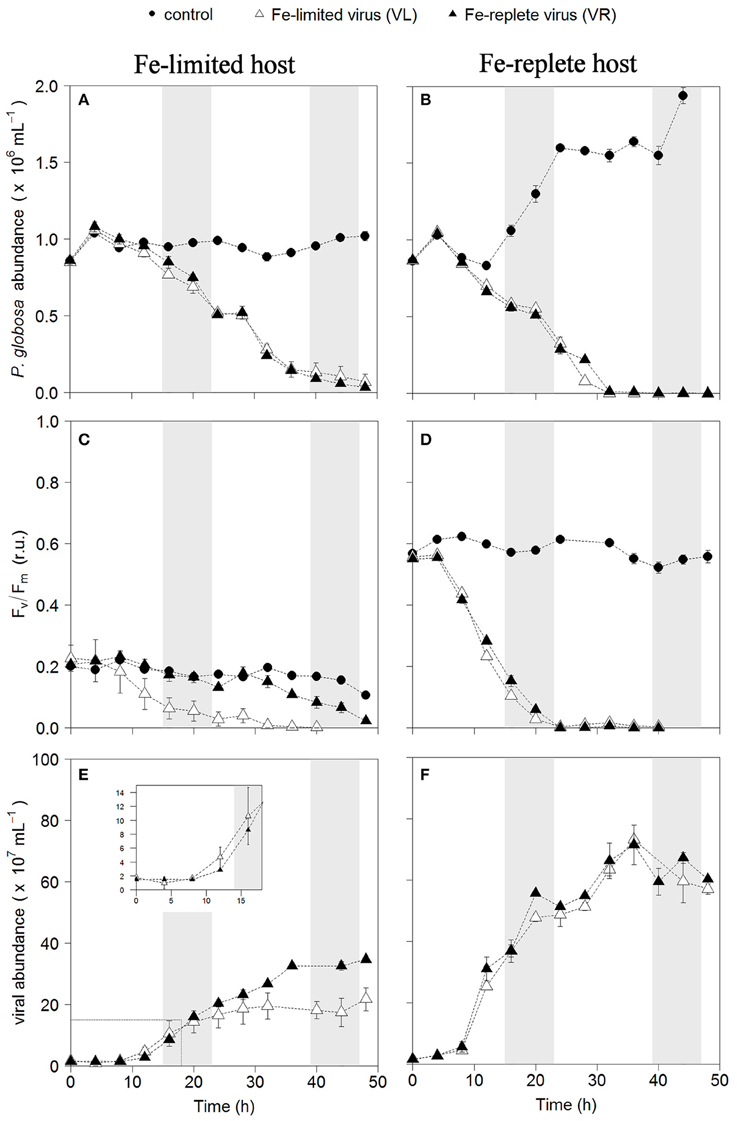
Figure 1. Temporal dynamics of P. globosa viral infections: host cell abundance over time (h) in Fe-limited (A) and Fe-replete cultures (B); photosynthetic capacity (Fv/Fm) over time (h) of Fe-limited (C) and Fe-replete cultures (D); viral abundance in Fe-limited (E) and Fe-replete (F) cultures. Circles represent non-infected controls, black triangles represent infections with Fe-replete host derived viruses (VR) and white triangles represent infections with Fe-limited host derived viruses (VL). Error bars indicate deviation of replicates (n = 2); when not visible they fall within the symbols. Shaded areas indicate dark periods, explaining the slight declines in Fv/Fm for the Fe-replete non-infected host. Furthermore, the dark period illustrates the onset of the synchronized cell division. Inlay in (E) magnifies the first 20 h post-infection.
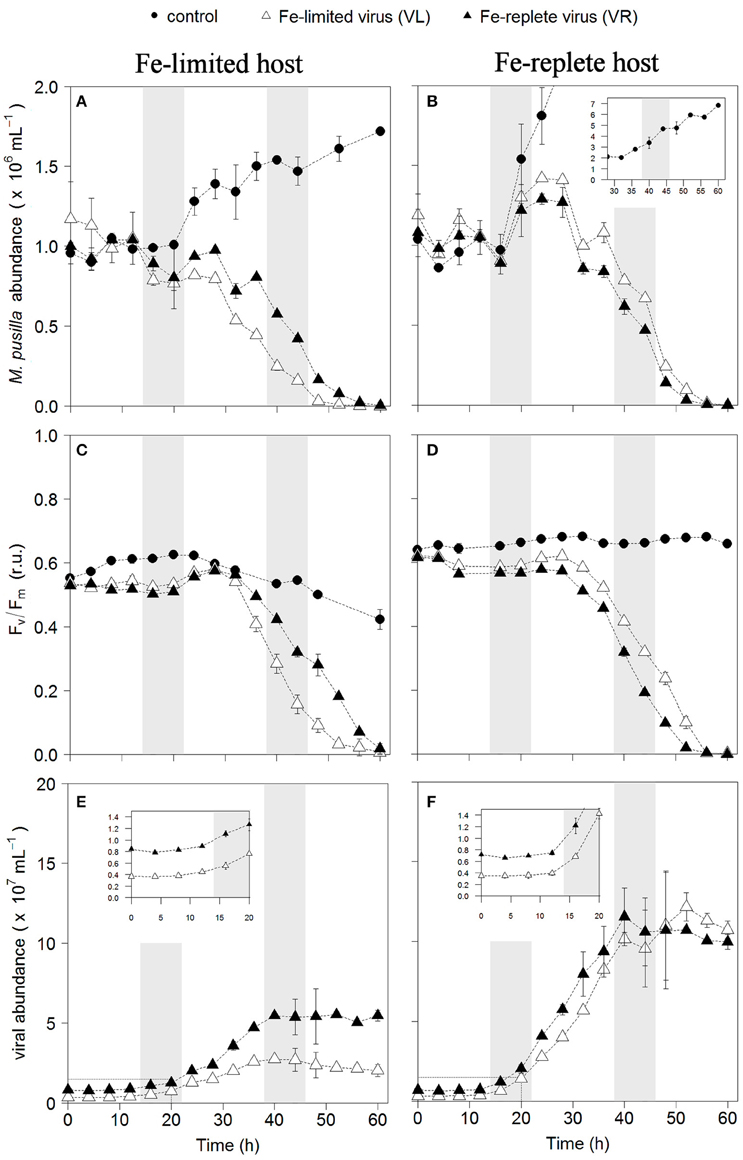
Figure 2. Temporal dynamics of M. pusilla viral infections: host cell abundance over time (h) in Fe-limited (A) and Fe-replete cultures (B); photosynthetic capacity (Fv/Fm) over time (h) of Fe-limited (C) and Fe-replete cultures (D); viral abundance in Fe-limited (E) and Fe-replete (F) cultures. Circles represent non-infected controls, black triangles represent infections with Fe-replete host derived viruses (VR) and white triangles represent infections with Fe-limited host derived viruses (VL). Error bars indicate deviation of replicates (n = 2); when not visible these fall within the symbols. Shaded areas indicate dark periods, explaining the slight declines in Fv/Fm for the Fe-replete non-infected host. Furthermore, the dark period illustrates the onset of the synchronized cell division. Inlay in (B) shows off scale host control growth, inlays in (E,F) magnify the first 20 h post-infection.
When infected with the corresponding Fe-treatment virus (i.e., Fe-limited host with VL and Fe-replete host with VR), Fe-limitation resulted for the infected P. globosa in slightly delayed (about 4 h) and slower cell lysis than for the Fe-replete cultures (full lysis occurring about a day later; Figures 1A,B). Fv/Fm showed similar differences in temporal dynamics between the Fe-limited and Fe-replete infected cultures upon infection with these viruses (Figures 1C,D). At large, the infected M. pusilla cultures showed similar results (Figure 2). The alterations in host physiological condition in the Fe-limited cultures resulted for both phytoplankton species in slower release of virus progeny and reduced virus yield when infected with VL as compared to Fe-replete cultures (Figures 1E,F, 2E,F). The latent period, e.g., the time period to extracellular release of newly produced viruses, was however unaffected by Fe-limitation of the host. Experiments with the same strain of M. pusilla did show prolonged latent periods when under phosphorus (P) and nitrogen (N) limitation (Maat et al., 2014; Maat and Brussaard, 2016). These were suggested to be due to reduced substrate and host energy availability resulting from impaired photophysiology under major nutrient limitation. In our study Chlorophyll-a concentration was comparably reduced but Fv/Fm of Fe-limited steady state M. pusilla was not impaired. Then again, Fv/Fm of P. globosa cells prior to infection was reduced compared to Fe-replete, still not prolonging the latent period. We cannot be sure of the exact underlying mechanism from the here presented data, but the proliferation of DNA viruses is directly dependent on Fe due to the essential role Fe plays in the catalytic center of ribonucleotide reductase, produced early in infection to support dNTPs production needed for viral DNA synthesis (Romeo et al., 2001). As such, it can be speculated that not the time to produce a virus is affected but instead the number of viruses that can be produced before cell lysis occurs. The burst size of MpV and PgV indeed decreased strongly under Fe-limitation, i.e., to 24 and 29% of the burst size produced under Fe-replete conditions (20 and 196 viruses lysed host cell−1; Table 3). In comparison to P. globosa, the reduced growth rate of Fe-limited steady state M. pusilla did not significantly affect the extent of reduction in virus burst size. Nonetheless, M. pusilla required a higher cellular Fe concentration. This implies that Fe-limited P. globosa, able to grow at the lower Fe concentration, displays a more efficient virus proliferation (despite the low Fv/Fm at the start of infection).

Table 3. Burst sizes (number of virus progeny per lysed host cell) of PgV-07T and MpV-08T infecting Fe-replete and Fe-limited P. globosa (Pg) and M. pusilla (Mp), respectively.
Noteworthy, the higher Fe concentration needed to allow for sustainable growth of M. pusilla under Fe-limitation did not prevent the loss of infectivity of the virus progeny. Infectivity of the Fe-limited MpV lysate was reduced to 30 ± 7%, while in contrast PgV did not show decreased infectivity for Fe-limited hosts. Our results signify that a stronger Fe-stress experienced by the Fe-limited M. pusilla (expressed in reduced growth rate) is more likely responsible for the production of impaired virus progeny than a changed photosynthetic capacity (Fv/Fm 0.2 for P. globosa compared to 0.6 for M. pusilla). The fact that total virus abundance, as measured after staining with a nucleic acid dye, was higher than the infective abundance indicates that (i) virus particles and (ii) viral nucleic acids were produced. Still, impaired capsid proteins or host receptors may explain a loss of infectivity. Alternatively, processes known to be Fe-intensive are DNA replication and repair (Netz et al., 2012; Zhang, 2014). Failure of DNA repair could indeed explain the result of reduced percentage infective viruses. Future research should not only focus on the causal aspects per se but also study what causes the dissimilarities in loss of infectivity between the algal viruses since different responses will directly regulate community composition. Furthermore, future studies should screen if and how other marine taxa and different viruses are affected by virus proliferation under Fe-limitation. For example, non-marine tailed bacteriophages have been found to contain iron ions in the tail proteins that can utilize the siderophore-bound iron receptors on the host cell membrane for attachment of the phage and subsequent infection of the bacterial host (Bartual et al., 2010). It is likely that similar interactions also exists for marine bacteriophages (Bonnain et al., 2016). Still open questions are whether such phages will obtain fewer iron ions in their tail under Fe-deprivation, and if this will negatively affect their infectivity. Furthermore, the recently proposed “Ferrojan Horse Hypothesis” by Bonnain et al. (2016) posits that introduction of phage-attached Fe may aid the host. More study is needed to test this theory and its potential interference with siderophore-specific uptake mechanisms and effect on Fe cycling.
For all Fe-replete cultures (i.e., high Fe concentration and consequently high Fv/Fm of around 0.6) and the Fe-limited M. pusilla the lysis dynamics and decline in Fv/Fm for the VR- and VL-infected cultures were largely comparable (Figures 1D, 2D). However, the Fe-treatment history of the virus (VL or VR) did matter in combination with Fe-limited P. globosa cells, i.e., infection with VR did delay the decline in Fv/Fm with more than a day (Figure 1C). Infection with a VR lysate is analogous to a relief in Fe-limitation at the time of infection. When the Fe-limited P. globosa cultures were infected with a VR lysate, they were effectively spiked with an Fe increase of ~10% relative to Fe-replete conditions (0.9 μM). The 100–300-fold increase of Fe with the addition of an Fe-replete lysate (0.9 μM vs. 1–3 nM) takes the culture Fe concentration well out of limitation ranges which are generally considered to be in nano- to picomolar ranges (de Baar et al., 1990; Martin et al., 1990; Brand, 1991). The concentration increase in our experiment is comparatively drastic to assure lifting of Fe-limitation to levels nearer normal replete cultures. This cross-inoculation provides insight in the potential effects of sudden introduction of Fe, e.g., with (seasonal) dust deposition or terrestrial runoff. The effect of this spike with Fe was apparently enough to prevent instant loss of photosynthetic capacity in the Fe-limited P. globosa cells. For both phytoplankton species, the improved physiological condition upon infection of Fe-limited host with VR virus resulted in enhanced viral production compared to Fe-limited infected with VL virus (by 1.6 and 2.3-fold for PgV and MpV, respectively; Table 3). The effect on burst size is thus stronger for the more Fe-sensitive M. pusilla, indicating that M. pusilla is more capable of mobilizing the Fe added for viral production. Utilization of the limiting macronutrient P when added post infection was also found to stimulate virus production of M. pusilla (Maat et al., 2016). Although, our results indicate that an infected host is capable of mobilizing the limiting Fe for viral production upon addition post infection, it did not lead to a complete recovery of virus production as compared to Fe-replete conditions (around 50% of the replete treatment; Table 2).
In conclusion, viral infection of both phytoplankton species is distinctly influenced by Fe-limitation. The differences in sensitivity of the host to Fe-limitation subsequently affected the progeny virus growth properties. Phaeocystis and Micromonas occur in Fe-limited and Fe-replete conditions alike (Not et al., 2004, 2005; Schoemann et al., 2005). Viral lysis has been shown to be an important mortality term for P. globosa under natural conditions, and also M. pusilla is readily infected (Cottrell and Suttle, 1995; Brussaard, 2004; Brussaard et al., 2005; Baudoux et al., 2006; Martínez et al., 2015). Virus burst sizes became strongly reduced (on average by 70%) under Fe-limiting relative to Fe-replete conditions. Although, addition of Fe at the time of infection was utilized by the infected Fe-limited host to increase virus production, it was not to the level found under Fe-replete conditions. Thus, Fe-limitation irrevocably interfered with viral productivity. Additionally, Fe-limited M. pusilla demonstrated an evident effect on the quality of the viruses produced as only 30% were infective. The lowered virus infectivity and/or virus yield impair viral control of the specific host species under Fe-limitation. However, at the same time, reduced viral lysis may affect the productivity of remaining non-infected cells and of other phytoplankton species, because viral lysis is considered a driver of Fe cycling by releasing ligand-bound dissolved Fe-species back into the dissolved organic matter pool (Gobler et al., 1997; Poorvin et al., 2004, 2011). What relative contributions different ligands have, and in how far their release is facilitated or impaired by viral lysis requires further study. Further experimental and in-situ study of phytoplankton host-virus dynamics under Fe-limitation is essential to elucidate the level of response specificity, but also the effects on the viral shunt in terms of nutrient cycling in general as well as Fe speciation.
Author Contributions
LG, CB, and HS were responsible for the design of this research. HS, CB analyzed and interpreted data. HS, LG, and CB wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank captain and crew of R.V. Pelagia and Micha Rijkenberg for their assistance in obtaining trace metal clean seawater stocks. We furthermore thank Josje Snoek for her assistance, Patrick Laan for dissolved Fe analysis, Swier Oosterhuis for pigment composition analysis, and Karel Bakker and Sharyn Ossebaar for nutrient analyses. We appreciate discussions with Douwe Maat, Klaas Timmermans and Willem van de Poll. This work was funded through a grant by the Netherlands Organization for Scientific Research (NWO) under contract number 822.01.018 to LG, and from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement n 311975 (MaCuMBA) to CB.
References
Bartual, S. G., Otero, J. M., Garcia-Doval, C., Llamas-Saiz, A. L., Kahn, R., Fox, G. C., et al. (2010). Structure of the bacteriophage T4 long tail fiber receptor-binding tip. Proc. Natl. Acad. Sci. U.S.A. 107, 20287–20292. doi: 10.1073/pnas.1011218107
Baudoux, A. C., and Brussaard, C. P. D. (2005). Characterization of different viruses infecting the marine harmful algal bloom species. Phaeocystis globosa. Virology 341, 80–90. doi: 10.1016/j.virol.2005.07.002
Baudoux, A. C., Noordeloos, A. A. M., Veldhuis, M. J. W., and Brussaard, C. P. D. (2006). Virally induced mortality of Phaeocystis globosa during two spring blooms in temperate coastal waters. Aquat. Microb. Ecol. 44, 207–217. doi: 10.3354/ame044207
Baudoux, A. C., Veldhuis, M. J. W., Witte, H. J., and Brussaard, C. P. D. (2007). Viruses as mortality agents of picophytoplankton in the deep chlorophyll maximum layer during IRONAGES III. Limnol. Oceanogr. 52, 2519–2529. doi: 10.4319/lo.2007.52.6.2519
Behrenfeld, M. J., Bale, A. J., Kolber, Z. S., Aiken, J., and Falkowski, P. G. (1996). Confirmation of iron limitation of phytoplankton photosynthesis in the Equatorial Pacific Ocean. Nature 383, 508–511. doi: 10.1038/383508a0
Behrenfeld, M. J., Worthington, K., Sherrell, R. M., Chavez, F. P., Strutton, P., McPhaden, M., et al. (2006). Controls on tropical Pacific Ocean productivity revealed through nutrient stress diagnostics. Nature 442, 1025–1028. doi: 10.1038/nature05083
Bonnain, C., Breitbart, M., and Buck, K. N. (2016). The Ferrojan Horse Hypothesis: iron-virus interactions in the ocean. Front. Mar. Sci. 3:82. doi: 10.3389/fmars.2016.00082
Brand, L. E. (1991). Minimum iron requirements of marine phytoplankton and the implications for the biogeochemical control of new production. Limnol. Oceanogr. 36, 1756–1771. doi: 10.4319/lo.1991.36.8.1756
Bratbak, G., Jacobsen, A., Heldal, M., Nagasaki, K., and Thingstad, F. (1998). Virus production in Phaeocystis pouchetii and its relation to host cell growth and nutrition. Aquat. Microb. Ecol. 16, 1–9. doi: 10.3354/ame016001
Breitbarth, E., Achterberg, E. P., Ardelan, M. V., Baker, A. R., Bucciarelli, E., Chever, F., et al. (2010). Iron biogeochemistry across marine systems–progress from the past decade. Biogeosciences 7, 1075–1097. doi: 10.5194/bg-7-1075-2010
Brussaard, C. P. D. (2004). Viral control of phytoplankton populations - a review. J. Eukaryot. Microbiol. 51, 125–138. doi: 10.1111/j.1550-7408.2004.tb00537.x
Brussaard, C. P. D., Mari, X., Van Bleijswijk, J. D. L., and Veldhuis, M. J. W. (2005). A mesocosm study of Phaeocystis globosa (Prymnesiophyceae) population dynamics: II. Significance for the microbial community. Harmful Algae 4, 875–893. doi: 10.1016/j.hal.2004.12.012
Brussaard, C. P. D., and Martínez, J. M. (2008). Algal bloom viruses. Plant Virus. 2, 1–10. Available online at: http://www.globalsciencebooks.info/Journals/PV.html
Brussaard, C. P. D., Payet, J. P., Winter, C., and Weinbauer, M. G. (2010). Quantification of aquatic viruses by flow cytometry. Man. Aquat. Viral Ecol. 11, 102–109. doi: 10.4319/mave.2010.978-0-9845591-0-7.102
Brussaard, C. P. D., Thyrhaug, R., Marie, D., and Bratbak, G. (1999). Flow cytometric analyses of viral infection in two marine phytoplankton species, Micromonas pusilla (Prasinophyceae) and Phaeocystis pouchetii (Prymnesiophyceae). J. Phycol. 35, 941–948. doi: 10.1046/j.1529-8817.1999.3550941.x
Butler, A. (2005). Marine siderophores and microbial iron mobilization. Biometals 18, 369–374. doi: 10.1007/s10534-005-3711-0
Calbet, A., and Landry, M. R. (2004). Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine systems. Limnol. Oceanogr. 49, 51–57. doi: 10.4319/lo.2004.49.1.0051
Cottrell, M. T., and Suttle, C. A. (1995). Dynamics of lytic virus infecting the photosynthetic marine picoflagellate Micromonas pusilla. Limnol. Oceanogr. 40, 730–739. doi: 10.4319/lo.1995.40.4.0730
de Baar, H. J. W., Buma, A. G. J., Nolting, R. F., Cadee, G. C., Jacques, G., and Treguer, P. J. (1990). On iron limitation of the southern ocean?: experimental observations in the Weddell and Scotia Seas. Mar. Ecol. Prog. Ser. 65, 105–122. doi: 10.3354/meps065105
Garg, S., Rose, A. L., and Waite, T. D. (2007). Superoxide-mediated reduction of organically complexed iron(III): impact of pH and competing cations (Ca2+). Geochim. Cosmochim. Acta 71, 5620–5634. doi: 10.1016/j.gca.2007.08.002
Geider, R. J., and La Roche, J. (1994). The role of iron in phytoplankton photosynthesis, and the potential for iron-limitation of primary productivity in the sea. Photosyn. Res. 39, 275–301.
Geider, R. J., La Roche, J., Greene, R. M., and Olaizola, M. (1993). Response of the photosynthetic apparatus of Phaeodactylum tricornutum (Bacillariophyceae) to nitrate, phosphate, or iron starvation. J. Phycol. 29, 755–766. doi: 10.1111/j.0022-3646.1993.00755.x
Genty, B., Briantais, J.–M., and Baker, N. R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990, 87–92. doi: 10.1016/S0304-4165(89)80016-9
Gerringa, L. J. A., de Baar, H. J. W., and Timmermans, K. R. (2000). A comparison of iron limitation of phytoplankton in natural oceanic waters and laboratory media conditioned with EDTA. Mar. Chem. 68, 335–346. doi: 10.1016/S0304-4203(99)00092
Gerringa, L. J. A., Veldhuis, M. J. W., Timmermans, K. R., Sarthou, G., and de Baar, H. J. W. (2006). Co-variance of dissolved Fe-binding ligands with phytoplankton characteristics in the canary basin. Mar. Chem. 102, 276–290. doi: 10.1016/j.marchem.2006.05.004
Gledhill, M., and van den Berg, C. M. G. (1994). Determination of complexation of iron(III) with natural organic complexing ligands in seawater using cathodic stripping voltammetry. Mar. Chem. 47, 41–54. doi: 10.1016/0304-4203(94)90012-4
Gobler, C. J., Hutchins, D. A., and Fisher, N. S. (1997). Release and bioavailability of C, N, P, Se, and Fe following viral lysis of a marine chrysophyte. Limnol. Oceanogr. 42, 1492–1504. doi: 10.4319/lo.1997.42.7.1492
González, A. G., Pérez-Almeida, N., Santana-Casiano, J. M., Millero, F. J., and González-Dávila, M. (2016). Redox interactions of Fe and Cu in seawater. Mar. Chem. 179, 12–22. doi: 10.1016/j.marchem.2016.01.004
González, A. G., Santana-Casiano, J. M., González-Dávila, M., Pérez-Almeida, N., and Suárez De Tangil, M. (2014). Effect of Dunaliella tertiolecta organic exudates on the Fe(II) oxidation kinetics in seawater. Environ. Sci. Technol. 48, 7933–7941. doi: 10.1021/es5013092
Grasshoff, K. (1983). “Determination of nitrate.” in Methods of Seawater Analysis, eds K. Grasshoff, M. Erhardt, and K. Kremling (Weinheim: Verlag Chemie), 143–87.
Hassler, C. S., Schoemann, V., Nichols, C. M., Butler, E. C. V., and Boyd, P. W. (2011). Saccharides enhance iron bioavailability to southern ocean phytoplankton. Proc. Natl. Acad. Sci. U.S.A. 108, 1076–1081. doi: 10.1073/pnas.1010963108
Ibisanmi, E., Sander, S. G., Boyd, P. W., Bowie, A. R., and Hunter, K. A. (2011). Vertical distributions of iron-(III) complexing ligands in the southern ocean. Deep Sea Res. II 58, 2113–2125. doi: 10.1016/j.dsr2.2011.05.028
Klunder, M. B., Laan, P., Middag, R., De Baar, H. J. W., and van Ooijen, J. C. (2011). Dissolved iron in the southern ocean (Atlantic Sector). Deep Sea Res. II 58, 2678–2694. doi: 10.1016/j.dsr2.2010.10.042
Kustka, A. B., Shaked, Y., Milligan, A. J., King, D. W., and Morel, F. M. M. (2005). Extracellular production of superoxide by marine diatoms: contrasting effects on iron redox chemistry and bioavailability. Limnol. Oceanogr. 50, 1172–1180. doi: 10.4319/lo.2005.50.4.1172
Laglera, L. M., Battaglia, G., and van den Berg, C. M. G. (2011). Effect of humic substances on the iron speciation in natural waters by CLE/CSV. Mar. Chem. 127, 134–143. doi: 10.1016/j.marchem.2011.09.003
Liu, X., and Millero, F. J. (2002). The Solubility of iron in seawater. Mar. Chem. 77, 43–54. doi: 10.1016/S0304-4203(01)00074-3
Maat, D. S., and Brussaard, C. P. D. (2016). Both phosphorus and nitrogen limitation constrain viral proliferation in marine phytoplankton. Aquat. Microb. Ecol. 77, 87–97. doi: 10.3354/ame01791
Maat, D. S., Crawfurd, K. J., Timmermans, K. R., and Brussaard, C. P. D. (2014). Elevated CO2 and phosphate limitation favor Micromonas pusilla through stimulated growth and reduced viral impact. Appl. Environ. Microbiol. 80, 3119–3127. doi: 10.1128/AEM.03639-13
Maat, D. S., Van Bleijswijk, J. D. L., Witte, H. J., and Brussaard, C. P. D. (2016). Virus production in phosphorus limited Micromonas pusilla stimulated by a supply of naturally low concentrations of different phosphorus sources, far into the lytic cycle. FEMS Microbiol. Ecol. 92:fiw136. doi: 10.1093/femsec/fiw136
Marie, D., Partensky, F., Vaulot, D., and Brussaard, C. P. D. (1999). Enumeration of phytoplankton, bacteria, and viruses in marine samples. Curr. Protoc. Cytom. Chapter 11, Unit 11.11. doi: 10.1002/0471142956
Martin, J. H., Fitzwater, S. E., and Gordon, R. M. (1990). Iron deficiency limits phytoplankton growth in antarctic waters. Global Biogeochem. Cycles 4, 5–12.
Martínez, J. M., Boere, A., Gilg, I., van Lent, J. W. M., Witte, H. J., van Bleijswijk, J. D. L., et al. (2015). New lipid envelope-containing dsDNA virus isolates infecting Micromonas pusilla reveal a separate phylogenetic group. Aquat. Microb. Ecol. 74, 17–28. doi: 10.3354/ame01723
Martínez, J. M., Swan, B. K., and Wilson, W. H. (2014). Marine viruses, a genetic reservoir revealed by targeted viromics. ISME J. 8, 1079–1088. doi: 10.1038/ismej.2013.214
Mawji, E., Gledhill, M., Milton, J. A., Zubkov, M. V., Thompson, A., Wolff, G. A., et al. (2011). Production of siderophore type chelates in Atlantic Ocean Waters enriched with different carbon and nitrogen sources. Mar. Chem. 124, 90–99. doi: 10.1016/j.marchem.2010.12.005
Mengel, K. (1994). Iron availability in plant tissues - iron chlorosis on calcareous soils. Plant Soil 165, 275–283. doi: 10.1007/BF00008070
Mojica, K. D. A., and Brussaard, C. P. D. (2014). Factors affecting virus dynamics and microbial host-virus interactions in marine environments. FEMS Microbiol. Ecol. 89, 495–515. doi: 10.1111/1574-6941.12343
Mojica, K. D. A., Huisman, J., Wilhelm, S. W., and Brussaard, C. P. D. (2016). Latitudinal variation in virus-induced mortality of phytoplankton across the North Atlantic Ocean. ISME J. 10, 1–14. doi: 10.1038/ismej.2015.130
Murphy, J., and Riley, J. P. (1962). A modified single solution method for the determination of phosphorous in natural waters. Anal. Chim. Acta 27, 31–36.
Netz, D. J. A., Stith, C. M., Stümpfig, M., Köpf, G., Vogel, D., Genau, H. M., et al. (2012). Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat. Chem. Biol. 8, 125–132. doi: 10.1038/nchembio.721
Not, F., Latasa, M., Marie, D., Cariou, T., Vaulot, D., and Simon, N. (2004). A Single Species, Micromonas pusilla (Prasinophyceae), dominates the eukaryotic picoplankton in the Western English Channel. Appl. Environ. Microbiol. 70, 4064–4072. doi: 10.1128/AEM.70.7.4064-4072.2004
Not, F., Massana, R., Latasa, M., Marie, D., Colson, C., Eikrem, W., et al. (2005). Late summer community composition and abundance of photosynthetic picoeukaryotes in Norwegian and Barents Seas. Limnol. Oceanogr. 50, 1677–1686. doi: 10.4319/lo.2005.50.5.1677
Passmore, R., Hsu, J., Liu, R. X., Tam, E., Cai, Y. W., Su Frasca, J., et al. (2000). MPN Assay Analyzer. Available online at: http://www.ocgy.ubc.ca/~suttle/
Poorvin, L., Rinta-Kanto, J. M., Hutchins, D. A., and Wilhelm, S. W. (2004). Viral release of iron and its bioavailability to marine plankton. Limnol. Oceanogr. 49, 1734–1741. doi: 10.4319/lo.2004.49.5.1734
Poorvin, L., Sander, S. G., Velasquez, I., Ibisanmi, E., LeCleir, G. R., and Wilhelm, S. W. (2011). A comparison of Fe bioavailability and binding of a catecholate siderophore with virus-mediated lysates from the marine bacterium Vibrio alginolyticus PWH3a. J. Exp. Mar. Biol. Ecol. 399, 43–47. doi: 10.1016/j.jembe.2011.01.016
Price, N. M., Ahner, B. A., and Morel, F. M. M. (1994). The Equatorial Pacific Ocean: grazer controlled phytoplankton populations in an iron-limited ecosystem. Limnol. Oceanogr. 39, 520–534.
Rijkenberg, M. J. A., Steigenberger, S., Powell, C. F., van Haren, H., Patey, M. D., Baker, A. R., et al. (2012). Fluxes and distribution of dissolved iron in the Eastern (Sub-) Tropical North Atlantic Ocean. Global Biogeochem. Cycles 26, GB3004. doi: 10.1029/2011gb004264
Romeo, A. M., Christen, L., Niles, E. G., and Kosman, D. J. (2001). Intracellular chelation of iron by bipyridyl inhibits DNA virus replication: ribonucleotide reductase maturation as a probe of intracellular iron pools. J. Biol. Chem. 276, 24301–24308. doi: 10.1074/jbc.M010806200
Rue, E. L., and Bruland, K. W. (1995). Complexation of iron(III) by natural organic ligands in the central North Pacific as determined by a new competitive ligand equilibration/adsorptive cathodic stripping voltammetric method. Mar. Chem. 50, 117–138. doi: 10.1016/0304-4203(95)00031-L
Schoemann, V., Becquevort, S., Stefels, J., Rousseau, V., and Lancelot, C. (2005). Phaeocystis blooms in the global ocean and their controlling mechanisms: a review. J. Sea Res. 53, 43–66. doi: 10.1016/j.seares.2004.01.008
Shaked, Y., and Lis, H. (2012). Disassembling iron availability to phytoplankton. Front. Microbiol. 3:123. doi: 10.3389/fmicb.2012.00123
Sunda, W. G., and Huntsman, S. A. (1995). Iron uptake and growth limitation in oceanic and coastal phytoplankton. Mar. Chem. 50, 189–206. doi: 10.1016/0304-4203(95)00035-P
Suttle, C. A. (1993). “Enumeration and isolation of viruses,” in Handbook of Methods in Aquatic Microbial Ecology, 1st Edn., eds P. F. Kemp, B. F. Sherr, E. B. Sherr, and J. J. Cole (Boca Raton, FL: CRC Press), 121–134.
Timmermans, K. R., Davey, M. S., van der Wagt, B., Snoek, J., Geider, R. J., Veldhuis, M. J. W., et al. (2001a). Co-Limitation by iron and light of Chaetoceros brevis, C. dichaeta and C. calcitrans (Bacillariophyceae). Mar. Ecol. Prog. Ser. 217, 287–297. doi: 10.3354/meps217287
Timmermans, K. R., Gerringa, L. J. A., de Baar, H. J. W., van der Wagt, B., Veldhuis, M. J. W., de Jong, J. T. M., et al. (2001b). Growth rates of large and small southern ocean diatoms in relation to availability of iron in natural seawater. Limnol. Oceanogr. 46, 260–266. doi: 10.4319/lo.2001.46.2.0260
Timmermans, K. R., van der Wagt, B., and de Baar, H. J. W. (2004). Growth rates, half saturation constants, and silicate, nitrate, and phosphate depletion in relation to iron availability of four large open-ocean diatoms from the southern ocean. Limnol. Oceanogr. 49, 2141–2151. doi: 10.4319/lo.2004.49.6.2141
van den Berg, C. M. G., and Kramer, J. R. (1979). Determination of complexing capacities of ligands in natural waters and conditional stability constants of the copper complexes by means of manganese dioxide. Anal. Chim. Acta 106, 113–120.
van de Poll, W. H., Janknegt, P. J., van Leeuwe, M. A., Visser, R. J. W., and Buma, A. G. J. (2009). Excessive irradiance and antioxidant responses of an antarctic marine diatom exposed to iron limitation and to dynamic irradiance. J. Photochem. Photobiol. B 94, 32–37. doi: 10.1016/j.jphotobiol.2008.09.003
van Leeuwe, M. A., and Stefels, J. (2007). “Photosynthetic responses in Phaeocystis antarctica towards varying light and iron conditions,” in Phaeocystis, Major Link in the Biogeochemical Cycling of Climate-Relevant Elements, eds M. A. van Leeuwe, J. Stefels, S. Belviso, C. Lancelot, P. G. Verity, and W. W. C. Gieskes (Dordrecht: Springer), 61–70.
Vaulot, D. (1989). CYTOPC: Processing Software for Flow Cytometric Data. signal and noise 2:8. Available online at: http://application.sb-roscoff.fr/Phyto/index.php
Vaulot, D., Birrien, J.-L., Marie, D., Casotti, R., Veldhuis, M. J. W., Kraay, G. W., et al. (1994). Morphology, ploidy, pigment composition, and genome size of cultured strains of Phaeocystis (Prymnesiophyceae). J. Phycol. 30, 1022–1035.
Vaulot, D., Eikrem, W., Viprey, M., and Moreau, H. (2008). The diversity of small eukaryotic phytoplankton (≤ 3 Mm) in marine ecosystems. FEMS Microbiol. Rev. 32, 795–820. doi: 10.1111/j.1574-6976.2008.00121.x
Weitz, J. S., and Wilhelm, S. W. (2012). Ocean viruses and their effects on microbial communities and biogeochemical cycles. F1000 Biol. Rep. 8, 2–9. doi: 10.3410/B4-17
Wilhelm, S. W., and Suttle, C. A. (1999). Viruses and nutrient cycles in the sea. Bioscience 49, 781. doi: 10.2307/1313569
Zapata, M., Rodríguez, F., and Garrido, J. L. (2000). Separation of chlorophylls and carotenoids from marine phytoplankton: a new hplc method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 195, 29–45. doi: 10.3354/meps195029
Keywords: algae, marine phytoplankton, marine viruses, iron, Fe speciation, Fe limitation
Citation: Slagter HA, Gerringa LJA and Brussaard CPD (2016) Phytoplankton Virus Production Negatively Affected by Iron Limitation. Front. Mar. Sci. 3:156. doi: 10.3389/fmars.2016.00156
Received: 29 March 2016; Accepted: 16 August 2016;
Published: 30 August 2016.
Edited by:
Sylvia Gertrud Sander, University of Otago, New ZealandReviewed by:
Jessica Nicole Fitzsimmons, Texas A&M University, USAAridane G. Gonzalez, University of Western Brittany, France
Copyright © 2016 Slagter, Gerringa and Brussaard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hans A. Slagter, aGFucy5zbGFndGVyQG5pb3oubmw=
 Hans A. Slagter
Hans A. Slagter Loes J. A. Gerringa
Loes J. A. Gerringa Corina P. D. Brussaard
Corina P. D. Brussaard