- 1Department of Arctic and Marine Biology, UiT The Arctic University of Norway, Tromsø, Norway
- 2Fram Centre, Norwegian Polar Institute, Tromsø, Norway
- 3Fram Centre, Akvaplan-Niva, Tromsø, Norway
- 4Department of Marine Sciences - Tjärnö, University of Gothenburg, Strömstad, Sweden
- 5Department of Arctic Biology, University Centre on Svalbard, Longyearbyen, Norway
- 6Department of Global Change Research, Mediterranean Institute for Advanced Studies (UIB-CSIC), Esporles, Spain
Population responses to marine climate change are determined by the strength of the selection pressure imposed by changing climate, the genetic variability within the population (i.e., among individuals), and phenotypic plasticity within individuals. Marine climate change research has focused primarily on population-level responses, yet it is at the level of the individual that natural selection operates. We studied individual-level responses of two bivalve species to ocean acidification (OA) at the earliest stage of the life-cycle. We measured sperm activity (swimming speed and percent motility) in the Boreal/Arctic Macoma calcarea and the temperate Mytilus galloprovincialis in response to two pCO2 levels (380 and 1000 ppm) at the ambient temperature at the collection site, i.e., 2 and 16°C, respectively. We also assessed sperm longevity under control conditions. Treatment effects on fertilization success were estimated using fertilization models. At the population level, simulated OA reduced M. galloprovincialis sperm swimming speed by 30%, percent motility by 44%, and fertilization success by 43%, whereas only sperm swimming speed was significantly affected in M. calcarea. Both species showed substantial variability among individuals in response to increased pCO2. This variability was greatest in M. galloprovincialis ranging from non-significant effect to more than 73% reduction in fertilization success in response to OA, whereas M. calcarea responses varied from 8% increase in percent sperm motility to 26% reduction in swimming speed. Further, modeled fertilization success was negatively affected by simulated OA in 10 of 13 studied M. galloprovincialis males and in three of 10 M. calcarea males. We observed sperm longevities (82 h for M. calcarea and 25 h for M. galloprovincialis on average) far longer than the expected time-frame for efficient fertilization accounting for dilution of gametes. Assuming sperm activity is a heritable trait, natural selection might be a possible way for the studied populations to adapt to near-future OA.
Introduction
The rate of anthropogenic climate change may be too rapid for sensitive marine taxa to adapt through new genetic mutations. Hence the response of populations to environmental perturbations will be largely determined by pre-existing genetic variability, the magnitude of the selection pressure caused by changing conditions, and plasticity within individuals (Gienapp et al., 2008; Hoffmann and Sgró, 2011; Reusch, 2014). Therefore, long-term population persistence will not be determined by the average population response, but by the relative responses of different genotypes (Sunday et al., 2011; Schlegel et al., 2012). Genetic variability in traits affected by environmental factors is time-consuming to quantify. Among-individual variability in a measurable trait, however, can give clues as to the initial responses and longer-term adaptability of a population. Average population-level responses to environmental perturbations, on the other hand, may indicate the general sensitivity of a population as well as the magnitude of selection pressure caused by these perturbations. Consequently, population- and individual-level responses provide complementary insights into population vulnerability to future perturbation scenarios.
Most calcifying marine taxa pass through multiple life-stages, each of which may respond differently to selection pressures (Kurihara, 2008; Kroeker et al., 2013). Marine broadcast-spawners shed their gametes directly into the water column, where fertilization takes place (Thorson, 1950), and consequently selection can operate prior to fertilization. Fertilization in broadcast spawners is subject to strong individual selection pressure (Yund, 2000), and depends on complex interactions between gamete traits (Levitan, 2008; Lotterhos and Levitan, 2010; Evans and Sherman, 2013) and the environment (Crimaldi, 2012; Crimaldi and Zimmer, 2013). These interactions determine which gametes—and hence genotypes—meet and fertilize. Hydrodynamic processes lead to a rapid dilution of gametes causing conditions where gamete concentration may become too low for an efficient fertilization (Vogel et al., 1982; Pennington, 1985; Denny and Shibata, 1989; Yund, 2000). The location of an individual within a population of sessile broadcast spawners, spawning synchrony, and the advection of gametes cause high variability in life-time reproductive success among individuals (Denny and Shibata, 1989; Levitan and Young, 1995; Yund, 2000; Himmelman et al., 2008). Nonetheless, gamete traits also affect fertilization success (Vogel et al., 1982; Palumbi, 1999; Styan and Butler, 2000). In particular, percent motility and swimming speed of sperm affect gamete encounter-rate at small scales, favoring males with more active sperm (Figure 1; Levitan et al., 1991; Levitan, 2000; Styan and Butler, 2000; Crimaldi, 2012). Higher swimming speed comes at the cost of reduced longevity, as germ cells have limited energy reserves and few or no homeostatic control mechanisms (Levitan, 2000; Johnson and Yund, 2004). These processes are influenced by pH and temperature (Kupriyanova and Havenhand, 2005; Havenhand et al., 2008; Caldwell et al., 2011), and are presumed to be under genetic control. Consequently, these environmental factors are expected to influence fertilization success and thereby have substantial influence over the contribution of individuals to the next generation (Havenhand et al., 2008; Parker et al., 2009; Schlegel et al., 2012; Van Colen et al., 2012; Albright and Mason, 2013; Barros et al., 2013).
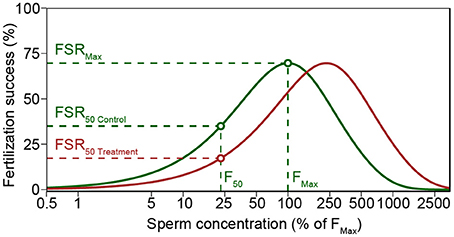
Figure 1. Modeled fertilization success (Fs) for average sperm swimming speed and percent motility of M. galloprovincialis in control (green line) and increased pCO2 treatment (red line). Both control and treatment yield similar maximum Fs (FSRMax), but increased pCO2 treatment lead to a decreased Fs when sperm concentration is low. Treatment and control Fs was compared using the sperm concentration (F50) that yielded 50% of maximum Fs in control (FSR50 Control). Polyspermy block activation time of 10 s was used in the figure to illustrate the decreased fertilization success in high sperm concentrations (see Styan, 1998 and Styan et al., 2008). The figure is based on Schlegel et al. (2012).
In this study, we used sperm swimming speed and percent motility as model traits to compare individual- and population-level responses to CO2-induced experimental ocean acidification (OA). We used two bivalve species in the comparison: the chalky macoma (Macoma calcarea, Gmelin, 1791) is an Arctic to North-Atlantic species (Pedersen, 1994; WoRMS Editorial Board, 2014), whereas the Mediterranean mussel (Mytilus galloprovincialis, Lamarck, 1819) has a cosmopolitan distribution (Gosling, 1984; ISSG, 2014). Both of these species are gonochoristic free-spawning broadcasters. Mytilus galloprovincialis is capable of inhabiting the inter-tidal zone where its gametes might experience a variable pH regime with values that may often reach as low as the predicted open ocean mean values for 2100 (pH drop of ~0.3 units, Hofmann et al., 2011; Ciais et al., 2013; Duarte et al., 2013; but see Vihtakari et al., 2013). M. calcarea, on the other hand, is solely a sub-tidal species, and the pH experienced by its gametes is presumed to be less variable in the short-term. However, M. calcarea is found in the Arctic waters, where OA will be most pronounced during the coming centuries (AMAP, 2013). Sperm activity of M. galloprovincialis has been reported to respond negatively to OA levels projected for 2100 (Vihtakari et al., 2013), but to our knowledge, there are no published results on sperm activity or fertilization success for M. calcarea. Van Colen et al. (2012) found a significant reduction in average fertilization success with a 0.6 pH unit drop in pH for the related species Macoma balthica, but a non-significant effect at pH levels comparable with this study. Both of those studies, however, focused only on population-level responses and did not consider individual variability. The objectives of this study were to (1) identify whether increased pCO2 affects sperm activity at the population-level; (2) quantify variation in these effects among individuals and species, and; (3) model the effects on fertilization success using an established fertilization kinetics model (Model S; Styan and Butler, 2000), a revised version of the popular model by Vogel et al. (1982).
Materials and Methods
Collection Sites and Animal Handling
Two species of bivalves were used for the experiments: Mediterranean mussel M. galloprovincialis, and Boreal/Arctic M. calcarea, identified as such according to CO1. Studied species were not protected (Artsdatabanken, 2014). Mussels were obtained from a commercial mussel farm (Manuel Cabrera e Hijo S.C.P), Mahón, Menorca, Spain (11 and 14 of April 2011). The mussel farm uses spat collectors to gather juveniles from natural populations. Water temperature (SST—Sea Surface Temperature) in the region ranges between on average 13.8°C in winter (February) and 25.8°C in summer (August; satellite data from NOAA on www.seatemperature.org) At the time of collection, water temperature was at 15.9°C with 37.5 salinity (psu). The range of pH in seagrass meadows around the Balearic islands is between 0.11 and 0.24 within the canopy as the plants are metabolically very active (Hendriks et al., 2014), while the harvested mussels were in an environment with much more limited fluctuations. Mussels were transported dry but kept humid by air from Menorca to Mallorca. Macoma was collected at a depth of 10–25 m, on 29 March and 5 April 2011, at 69°29′ 50″N 18°53′ 35′E, in Balsfjorden, Northern Norway. Sampling for Macoma was conducted outside of protected areas using a Norwegian research vessel (R/V Hyas), and therefore no specific permissions were needed. Water temperature in the fjord ranges between 2.5 and 11.5°C with a salinity range of 14–30 psu (reference sensor in Præbel et al., 2009). M. calcarea were packed in coolers, inserted in incisions in wet (seawater) foam to keep them humid and separated them from the cooler packs on top. They were and transported to Mallorca, Spain, by an airplane the day after collection.
Immediately after transport, upon arrival, the bivalves were transferred to temperature-controlled climate rooms set to 16°C (corresponding to the temperature at the collection site) for M. galloprovincialis and to 4°C for M. calcarea. Water temperature was further lowered to 2°C, the temperature during collection, for tanks holding M. calcarea using chillers. Artificial seawater adjusted to the salinity of the collection site (35 ppt for Mytilus, 28 ppt for Macoma) was made by blending a commercial salt mixture (Instant Ocean Sea Salt) with distilled water. This seawater was thoroughly mixed to ensure a complete dissolution of salt and circulated through a UV lamp for at least 24 h before use to reduce bacterial growth. Adults were kept in tanks with artificial seawater bubbled with ambient air. Measurement of sperm activity was conducted within a week of collection using males that appeared to be in good condition judged by active filtering activity and reaction to tactile stimulus.
Experimental Setup
Spawning
Spawning of M. galloprovincialis was induced by a combination of temperature shock (at 22°C) and fluoxetine (Honkoop et al., 1999). In general the mussels started to spawn 30 min after adding fluoxetine, which was then washed away by changing the water to freshly aerated seawater. The mussels were left to spawn for about 30 min. Sperm suspensions from individual males were pipetted from the highest concentrations close to the bottom of the beakers, and transferred to separate Eppendorf tubes. This concentrated solution was mixed with experimental water just before sperm activity experiments (see Vihtakari et al., 2013). Sperm activity measurements for this species were made within 4.5 h of spawning (Table S2). Sperm of M. calcarea was collected by strip-spawning (Strathmann, 1987), 10–35 μL of sperm was mixed with 1.5 mL of corresponding experimental water leading to an average suspension of 1.58 × 107 ± 4.1 × 105 (SE, n = 23) sperm mL−1 across all studied males in the experiment. Sperm activity of M. calcarea was measured within 1 h of activation (Table S2). Sperm suspensions were held in Eppendorf tubes in incubators set to the relevant treatment temperature (Table 1).
Experimental Water
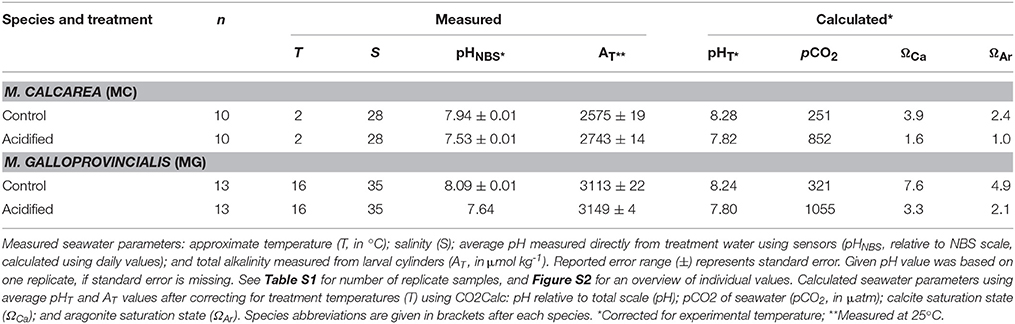
We used pCO2 with two levels (380 ppm as control treatment and 1000 ppm as acidified treatment) as a predictor variable to assess the effects of simulated ocean acidification on sperm swimming speed and percentage motile sperm (See Figure S1 for a schematic overview). Sperm activity was measured at the ambient temperature of the collection site of the bivalves, 2°C for M. calcarea and 16°C for M. galloprovincialis. The pH of the seawater was manipulated by aerating the seawater with air adjusted to the treatment pCO2 to each tank separately. Ambient air was collected via aquarium pumps and passed through soda-lime columns. Precise volumes of CO2-stripped air and CO2 gas were administrated using mass-flow controllers (Aalborg GFC17) and mixed in a container filled with marbles to achieve the desired pCO2 concentrations of 380 ppm (control) and 1000 ppm. These values correspond to the annual average atmospheric pCO2 level of 2005 (Tans and Keeling, 2014) and to the high-end open ocean projected levels for 2100 (Ciais et al., 2013), respectively. pH was measured in the tanks using a spectrophotometer (JASCO 7800) at 25°C following the standard operating procedure (SOP) 6b (Dickson et al., 2007). Total alkalinity (AT) was measured in samples taken for a parallel experiment (see Vihtakari et al., 2013). Sampled seawater was poisoned with 0.2 ml of saturate solution of HgCl2 (Merck, Analar) to avoid biological alteration, and AT was determined by open cell titration as described in SOP3b (Dickson et al., 2007) with a Metrohm Titrando 808, and Tiamo 2.2 titration software. Certified CO2 seawater reference material (CRM Batch 101) from the Andrew Dickson lab at UC San Diego was used to evaluate the accuracy of the results. Detailed overview of carbonate chemistry parameters is presented in Figure S2 and Table S1. Temperature-adjusted carbonate chemistry parameters were calculated using average values in Table 1 and the program CO2Calc (Robbins et al., 2010) (CO2 constant from Mehrbach et al., 1973, refit by Dickson and Millero, 1987, KHSO4 formulation from Dickson, 1990).
Water at the corresponding treatment level was collected from 10 L tanks and transferred to 2 L acid-washed and rinsed glass bottles every morning prior to spawning. Additionally to the spectrophotometric pH measurements, pH in the bottles was measured using NIST buffer (pH 4, 7, and 10) calibrated electrodes (Metrohm, 6.0262.100). Sperm activity was measured once or twice per day until no motile sperm cells were observed. Sperm longevity was accessed only under ambient CO2 conditions, because we were not able to regulate pH in the Eppendorf tubes for the long time period the beyond initial measurements.
Measuring Sperm Activity and Fertilization Assays
Sperm swimming speed was determined from replicated digital video clips of sperm suspension (see Videos S1, S2) using four-chamber slides (Leja, Netherlands). The measurement order of replicates (i.e., control first vs. treatment first) was randomized, and the difference between measurements was 14 min at maximum. Sperm swimming speed (VSL—velocity straight line) was measured for each path in 1 s long video clips using CellTrak 1.3 (Motion Analysis Corp., CA, USA). A motility threshold (definition of when a sperm cell was classified as motile) was determined from sperm speed histograms of single video clips from control conditions and set to 10 μm s−1 for M. galloprovincialis and to 9 μm s−1 for M. calcarea. Sperm swimming speed was determined for each video clip by averaging the measured sperm path speeds after excluding non-motile sperm. Percent sperm motility was calculated as a percentage of motile sperm in each video clip. Video clips with fewer than 15 and more than 300 sperm paths were excluded from the dataset as these yielded unreliable measurements. The resulting number of replicates varied between 5 and 11 for start measurements (Table S2). See Vihtakari et al. (2013) for further details about sperm activity measurements. Sperm longevity was estimated as the last observation of continuously swimming sperm for each male under control pCO2 treatment (see Videos S4, S5).
Numerical Methods
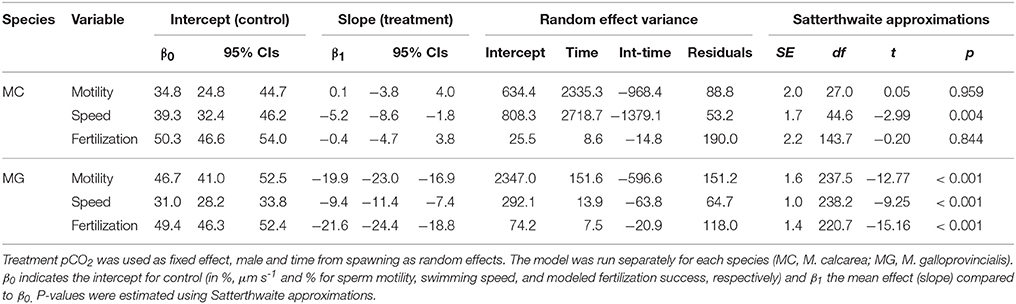
Mean species responses were analyzed by linear mixed-effect models (LMMs) for each species separately using pCO2 treatment levels as a fixed effect, male as random intercept, and time from spawning as random slope using the following R notation (see Bates et al., 2015 for details):
Where y is the response variable (sperm swimming speed, motility or fertilization success) for each species separately, pCO2 a factor specifying the two pCO2 treatment levels, t time from spawning and Male a factor specifying each male. Visual examination of non-transformed response variable (percent sperm motility, swimming speed, and modeled fertilization success) histograms revealed that the distributions on treatment level within each male were approximately normal. Analyses were run with lme4 and lmerTest packages (Bates et al., 2015; Kuznetsova et al., 2015) in the R environment (R Core Team, 2015). The statistical significance (α = 0.05) of model parameters was estimated primarily from 95% confidence intervals (CIs) for fixed effect slopes, but also expressed using P-values using Satterthwaite approximation for denominator degrees of freedom (SAS Technical Report R-101, 1978).
Effects of increased pCO2 and variability among and within males was examined by log-transformed response ratios (LnRR), which were calculated for each male separately using video clips as replicates (Hedges et al., 1999; Nakagawa and Cuthill, 2007). Response ratios (RR) were back-calculated as described the following equation and expressed as percentages (modified from Hedges et al., 1999):
Where X is the average response. The 95% confidence intervals for response ratios were calculated using t-distribution to account for low sample size (modified from Hedges et al., 1999):
where t0.05∕2 is the 95% critical cut point for a t-distribution with degrees of freedom ncontrol + ntreatment − 1, where n is number of replicates, and v combined variance for treatment and control calculated using following equation from Hedges et al. (1999):
Where SD is standard deviation. The equations above are demonstrated as R code in Supplementary Material (Datasheet S1). Mean species response ratios were extracted from the linear mixed effect model results using confidence intervals for the fixed effect slope to calculate response ratio confidence intervals.
Fertilization success (Fs) was calculated using Model S (Styan, 1998; Styan and Butler, 2000; Styan et al., 2008), a revised version of the model by Vogel et al. (1982) (Figure 1). For all comparisons, model parameters were set to be constant with the exception of sperm swimming speed and egg diameter [61 μm for M. galloprovincialis (Sedano et al., 1995), and 95 μm for M. calcarea (Oertzen, 1972)]. The model was run using sperm swimming speed from each replicate video clip after multiplying the initial sperm concentration (S0) by the proportion of motile sperm in each replicate. Afterwards, a mean fertilization model was calculated for each male and treatment. The sperm concentration that yielded 50% of maximum F in control treatments (F50 in Figure 1) was chosen to compare fertilization success among treatments. Response ratios were calculated as described above. The fertilization modeling is demonstrated using one of the males as an example in the R code included in Supplementary Material (Datasheets S1, S2).
Results
Water Chemistry
Difference in pH between low (380 ppm, control hereafter) and high pCO2 (1000 ppm, acidified hereafter) treatments was stable throughout the study, the average difference in pHT being 0.42 for M. calcarea at 2°C and 0.43 for M. galloprovincialis at 16°C (Figure S2, Table 1). Average total alkalinity (AT) was 2659 μmol kg−1 or M. calcarea, and ranged from 2539 to 2770 μmol kg−1 (Figure S2, Table 1). Average AT for M. galloprovincialis was 3130 μmol kg−1 ranging between 3010 and 3164 μmol kg−1. Total alkalinity was significantly higher in acidified treatments compared to control treatments for M. calcarea (168 μmol kg−1 on average; Welch Two Sample t-test: n = 6, t = −7.098, df = 3.761, p = 0.002; Figure S2). There was no significant difference in AT values between acidified and control for M. galloprovincialis (Welch Two Sample t-test: n = 12, t = −1.640, df = 5.289, p = 0.159; Figure S2).
Fertilizing Capacity of Sperm
Fertilization experiments were conducted to examine whether the studied sperm was capable of fertilizing eggs. Sperm from every M. galloprovincialis fertilized eggs successfully and the eggs developed to early trochophore stage, at which point the experiment was terminated. In contrast, M. calcarea did not produce viable embryos. The eggs of this species, which were also stripped, were clearly irregular and immature. Nonetheless, both of the two tested M. calcarea males successfully fertilized eggs, although the eggs did not develop beyond the 8-cell stage.
Acidification Responses
The population-level effects of increased pCO2 on percent sperm motility, and modeled fertilization success of M. calcarea were not significant, while these effects for M. galloprovincialis were significantly negative (Figure 2, Figure S3). There was substantial variability between males in response to increased pCO2 in both species (Figure 2). This variability was largest in M. galloprovincialis, with response ratios varying from a non-significant 100.5% effect to a tenfold reduction (R = 10.1%, CIs 5.4–18.7%) in percent sperm motility, and from a non-significant 98.6% to a two-fold reduction (R = 39.0%, CIs 24.5–61.4%) in sperm swimming speed (Figure 2, Videos S1, S2). Significantly negative pCO2 effects on modeled fertilization success were observed in three of 10 M. calcarea males (Figure 2E). One M. calcarea male showed a positive effect that was marginally non-significant (R = 123.5% CIs 99.3–153.5%). Increased pCO2 had a significant negative effect on modeled fertilization success in 10 of 13 M. galloprovincialis males (Figure 2F). The response ratios varied from 15.3% (CIs 10.3–22.6%) to 83.8% (CIs 67.0–104.7%).
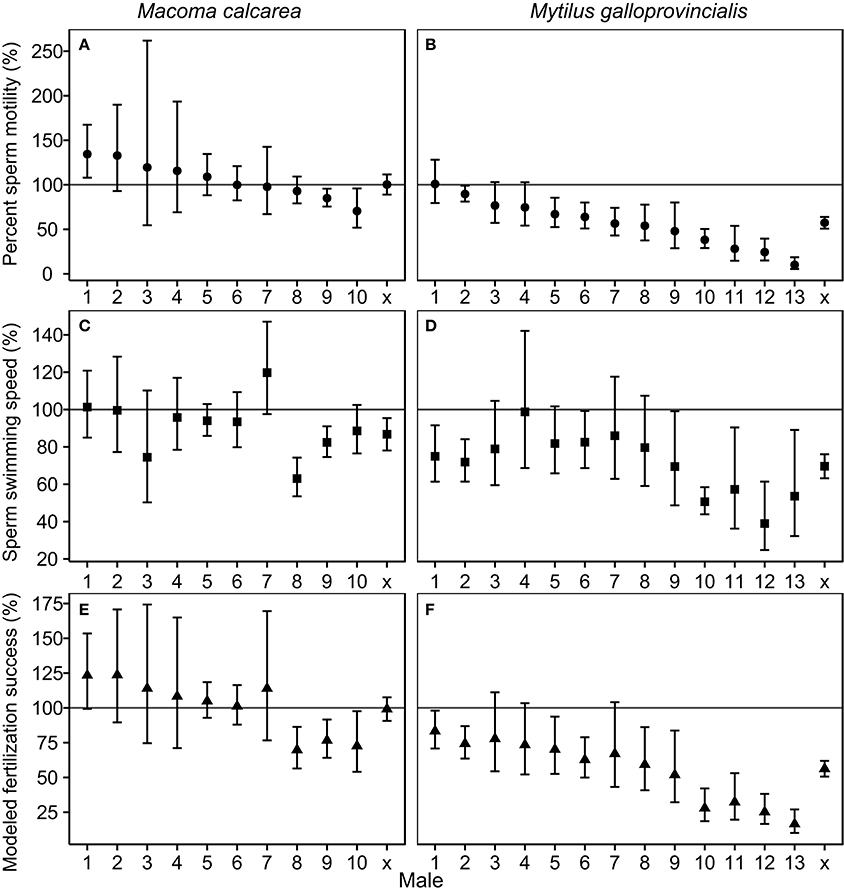
Figure 2. Effect sizes of increased pCO2 for percent sperm motility (A,B), swimming speed (C,D), and modeled fertilization success (E,F). Panels in the first column (A,C,E) represent M. calcarea, and panels in the second column (B,D,F) indicate M. galloprovincialis. Males along x-axis are ordered based on average effect size for percent sperm motility, and x presents the average effect over males for each species estimated using linear mixed models (Table 2). Error bars represent 95% confidence intervals. The line at 100% indicates no significant treatment effect where error bars cross this line. Fertilization success values were calculated using sperm concentrations that give 50 % of maximum fertilization success in control treatment (F50 in Figure 1).
Sperm Longevity
Sperm longevity was estimated only in control pCO2 conditions and at the respective ambient temperature for each species (Table 1, Figure 3, Videos S3, S5). Average sperm longevity for M. calcarea [81.7 ± 11.3 h (SE, n = 9)] was longer than that for M. galloprovincialis [24.7 ± 3.5 h (SE, n = 13)]. Males demonstrated variability in sperm longevity: four M. galloprovincialis males had a markedly shorter sperm longevity (~10 h) than the rest (25.2−46.8 h, Figure 3, Table S3). In M. calcarea, sperm longevity among males ranged between 22.5 and 142.6 h (Table S3).
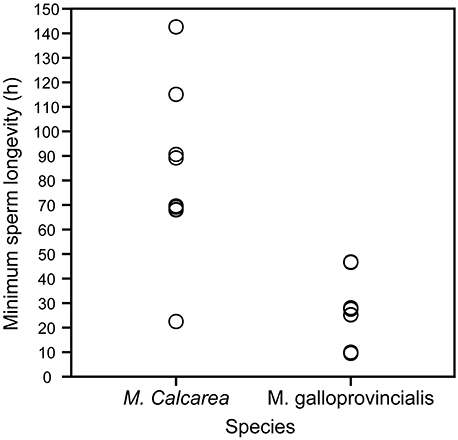
Figure 3. Sperm longevity of M. calcarea and M. galloprovincialis. Each open circle represents a time (h) for the last observation of continuously swimming sperm for an individual male.
Discussion
We observed substantial variability among individuals in response to simulated ocean acidification in both studied species (Figure 2). Fertilization modeling predicted a significant negative effect of acidification on fertilization success for the majority of M. galloprovincialis (10 of 13, Figure 2F), but only for 3 of 10 M. calcarea males (Figure 2E). Among-individual variability in response to OA comparable to that found in M. calcarea has been reported for a sea urchin (Schlegel et al., 2012), and an oyster (Havenhand and Schlegel, 2009), whereas similar strong variability as seen in M. galloprovincialis was previously observed in a polychaete (Schlegel et al., 2014). These results suggest that among-individual variability is likely the norm, rather than an exception, and represents adaptive potential enabling organisms to cope with environmental change. Sperm from males less affected by acidification are likely better adapted to fertilize eggs in a future ocean, and therefore—assuming these beneficial sperm traits are heritable—the offspring of these males will become more abundant in the gene pool (Schlegel et al., 2014).
Simulated ocean acidification (increased pCO2 at 16°C) had substantial and negative population-level effects on M. galloprovincialis sperm activity, as previously described in Vihtakari et al. (2013), and on modeled fertilization success (Figure 2F, Table 2). In contrast, significant population-level reductions were observed only in sperm swimming speed in M. calcarea at 2°C (Figures 2A,C,E). Interestingly, the response was more negative in the intertidal M. galloprovincialis, whose gametes may already occasionally experience pH values as low as projected for 2100 (Ciais et al., 2013; Duarte et al., 2013; Hendriks et al., 2014), than in M. calcarea (Figure 2). This could indicate that variable conditions might maintain a higher level of phenotypic variation in the population as several environmental stressors might act simultaneously (Sunday et al., 2014), although data on more species are needed to draw such conclusions. In many marine invertebrates sperm are stored immotile in the acidic environment of the testis, which inhibits sperm respiration and metabolism (Caldwell et al., 2011; Schlegel et al., 2012; Alavi et al., 2014). The increase in pH upon release is partly responsible for activation of mitochondria in sea-urchin sperm (Christen et al., 1982). Although, sperm activation in marine bivalves appears to be more complex, pH is likely to contribute to sperm activation (Alavi et al., 2014). A weaker pH shock due to lower environmental pH could therefore partly explain the lower percentage of motile sperm in the increased pCO2 treatment.
Van Colen et al. (2012) also found a non-significant decrease in fertilization success of the related species M. balthica at a similar pH reduction (ΔpH ~0.3 units), and a significant 11% reduction in fertilization success at ΔpH of 0.6 units. It is possible that the population-level fertilization success in M. calcarea could also be negatively affected by such pH reductions since our modeling results indicate that some individuals were negatively affected by the studied 0.3 unit drop in pH (Figure 2E), and sperm swimming speed was negatively affected on population level (Figure 2C). Other acidification effects on fertilization success of bivalves include a significant reduction for Saccostrea glomerata starting from a pCO2 level projected for the end of this century (600 ppm; Parker et al., 2009), and a reduction for Crassostrea gigas at a far-future ΔpH of 0.7 units (Barros et al., 2013). Havenhand and Schlegel (2009), on the other hand, found no significant population-level effects on sperm activity or fertilization success of C. gigas for a pH reduction similar to that used in this study (~0.35 units)—a result that is consistent with Barros et al. (2013). Negative population-level effects of OA on fertilization success appear to be common for echinoderms (Havenhand et al., 2008; Morita et al., 2010; Schlegel et al., 2012; Uthicke et al., 2013), corals (Morita et al., 2010; Albright and Mason, 2013), and a polychaete (Schlegel et al., 2014) among free-spawning marine invertebrates, however a positive effect of OA on sperm activity of a sea urchin has also been observed (Caldwell et al., 2011). A recent review concludes that fertilization in benthic marine invertebrates could be relatively robust to OA (Byrne and Przeslawski, 2013). Considering the negative effects that have been reported for a wide range of benthic taxa, and the results of this study, this might not be a good generalization. Although, it is clear that population-level effects of OA on fertilization kinetics of marine invertebrates vary among species, populations, and gamete concentrations, our results together with the literature suggest that OA is likely to decrease the sperm performance in a range of benthic marine invertebrates, potentially resulting to a lower fertilization success in sperm limited low density populations (Levitan and Petersen, 1995; Styan, 1998; Levitan, 2000; Albright and Mason, 2013). The consequences of these reductions are unpredictable considering the complex fertilization kinetics in marine broadcast spawners (Denny and Shibata, 1989; Levitan and Young, 1995; Yund, 2000; Crimaldi and Zimmer, 2013; Evans and Sherman, 2013).
Experimental studies are subject to biases that complicate projecting future population responses (Vihtakari, 2014). Here the lack of adult acclimation and the use of artificial seawater might have influenced results. Adult acclimation in experimental conditions might affect the responses of offspring to ocean acidification through transgenerational plasticity (Miller et al., 2012; Parker et al., 2012; Dupont et al., 2013) as gametes inherit nutrition and other bioactive materials from their parents (Munday et al., 2013; Salinas et al., 2013). Since we did not acclimatize studied males, we cannot assess the possible consequences of such effects. Furthermore, the use of artificial seawater led to 500–700 μmol kg−1 higher total alkalinity (AT) than measured for the Mediterranean for M. galloprovincialis (Millero et al., 1979; Gazeau et al., 2005; Schneider et al., 2007; but see Vihtakari et al., 2013), and 200–400 μmol kg−1 higher than published AT values for the Barents Sea (Chierici et al., 2012) for M. calcarea. Despite this, measured pH and calculated seawater pCO2 values were within the range projected for 2100 in the acidified treatment (Gattuso and Lavigne, 2009; Ciais et al., 2013) and within the range of current values for the control treatment (Millero et al., 1979; Murphy et al., 2001). Temperature could also influence the responses to OA (Byrne et al., 2009, 2010; Parker et al., 2009; Vihtakari et al., 2013), and therefore the observed responses could have been different had we considered the combined effects of OA and global warming in our experimental design. Manipulating temperature would be a logical next step, as OA and temperature will change both in the future. Results of experimental studies should be interpreted with the understanding that no single experiment can accurately assess the consequences of future perturbations to biota under climate change. Instead, these experiments add to the growing body of evidence that ocean acidification might have complex consequences to marine ecosystems.
Sperm longevities estimated for M. calcarea (82 h at 2°C on average, Figure S3) and M. galloprovincialis (25 h at 16°C) in this study are remarkably long considering that effective fertilization is expected to occur within minutes from spawning due to rapid dilution of gametes (Pennington, 1985; Denny and Shibata, 1989; Yund, 2000). Long lasting sperm has been previously reported in bivalves: Powell et al. (2001) found that sperm of the Antarctic bivalve Laternula elliptica were capable of fertilizing eggs more than 90 h after spawning at ~0°C. The highest sperm longevity values in this study (4–6 days for M. calcarea) were of a similar order of magnitude than those found by Alavi et al. (2014) for Pacific oyster C. gigas (4–6 days), Manila clam Ruditapes philippinarum (~7 days), and Japanese scallop Patinopecten yessoensis (2–4 days), all at room temperature. Previous longevities reported for near-shore bivalves are considerably shorter Mytilus edulis: >5 h (Levy and Couturier, 1996); Cerastoderma edule: 4–8 h (André and Lindegarth, 1995). Sperm longevity, i.e., the time that sperm remain motile (Figure 3, Videos S1, S3–S5), however, does not measure the fertilization capability of sperm and consequently might lead overestimating the actual effectiveness of sperm to fertilize eggs. In this study, sperm reached the immotile state after an average of 9 days for M. calcarea and within 38 h for M. galloprovincialis. Filming sperm motility requires relatively high sperm concentrations. The extreme longevities could partly be explained by the high concentration of studied sperm, which could lead to sperm being restricted by space and therefore not swimming as actively as in the field (Johnson and Yund, 2004). Also reduced motility caused by sperm cells sedimenting at the bottom of Eppendorf tubes could decrease the amount of energy consumed compared to the natural environment. Nevertheless, bivalve sperm might stay active far longer than the time-frame of efficient fertilization (Andrè and Lindegarth, 1995; Powell et al., 2001; Alavi et al., 2014) due to hydrodynamic processes leading to a rapid dilution of gametes (Denny and Shibata, 1989; Levitan and Petersen, 1995; Yund, 2000). This is an interesting observation and poses a question whether sperm longevity could be of an advantage in certain conditions. Powell et al. (2001) suggested that long-lived sperm could increase the effectiveness of synchronized mass spawning events in L. elliptica by allowing time for sperm densities to reach the levels needed for high fertilization success. Although this hypothesis could also apply for M. galloprovincialis and M. calcarea, there is currently not enough field-data to identify the potential reasons for sperm remaining active longer than the dilution of gametes.
Concluding Remarks
Ocean acidification negatively affected sperm swimming speed, percent motility and modeled fertilization success in M. galloprovincialis on a population-level. There was a substantial among-male variability in these responses varying from statistically non-significant to a 90% reduction among M. galloprovincialis males. Among-male variability in M. calcarea males ranged from significantly positive in percent sperm motility to a >26% reduction in swimming speed.
Population-level responses may be useful for detecting traits that are vulnerable to climate change, but they tell us little about the adaptive capacity of a population to future conditions. The key determinant for a species' success in the future ocean is the extent of genetic variability in traits that are susceptible to climate change. Inter-individual variability could, thus, mitigate the effects of climate change on future populations (Sunday et al., 2011; Schlegel et al., 2014). Inter-individual variability is likely the norm, rather than an exception, and the responses of populations in the future may therefore differ from the average response of a population today. Nonetheless, the long-term effects of strong selection for acidification on population (and species) viability are difficult to predict. Importantly, there are no data linking robustness of sperm performance under environmental change in a single parameter, such as pH, to robustness of the resulting individual during the remainder of its life-cycle. Reduced individual-variability through selection may have negative consequences for adaptability to ocean acidification during other phases of the life-cycle, or other stressors altogether. The possibility that gamete, larval, and adult robustness are co-evolved is exciting and deserves further attention.
Author Contributions
IH was in charge of the experimental water treatments, and analysis of the carbonate system, MV performed sperm measurements, and analyzed the data, PR, JH, IH, and MV designed the experiment and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We express our gratitude to Martina Jönsson, Johnna Holding, Inés Mazarassa, Clara Gallego, and Regino Martinez for their invaluable help in the laboratory, Carlos M. Duarte for support and guidance, which made this research possible, Haakon Hop for helpful comments on earlier versions of the manuscript, Roar Lorentsen and Peter Leopold for help with bivalve collection, and Marianne Frantzen for loan of camera equipment and guidance with sperm motility assessment. We also want to thank the Stack Exchange community for help in implementing the statistical analyses. This research was financed through the EU 7th Framework Program project Arctic Tipping Points (contract number FP7-ENV-2009-226248; http://www.eu-atp.org; PER IEH), the Fram Centre Ocean Acidification Flagship (PER), and partly by a Linnaeus-grant from the Swedish Research Councils VR and Formas (JNH) of the Linnaeus Centre for Marine Evolutionary Biology (http://www.cemeb.science.gu.se).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2016.00051
Table S1. Water chemistry sample overview over studied males. The first two letters in Male column represent the species (see Table 1 for the species abbreviations) and the number the male number from Figure 1. S, measured salinity in treatment tanks; pHNBS, sensor measurement directly from treatment water; pHT, spectrophotometer measurement from storage tanks; and AT, total alkalinity measurement from larval cylinders. A number in pHNBS, pHT, and AT columns represent the number of replicate measurements. Missing values indicate that measurement was not conducted. Results of the measurements are presented in Table 1.
Table S2. Experimental data averaged over males. The first two letters in Male column represent the species (see Table 1 for the species abbreviations) and the number the male number from Figure 1. pCO2, air pCO2 level; T, temperature (°C); pHNBS, pH in NBS scale; Time, average time from spawning when measurements were conducted (min); C, sperm concentration at the beginning of measurements (sperm μL−1); and n, number of replicate film clips included in the analyses. Average and standard deviation for percentage sperm motility (Motility, %), sperm swimming speed (Speed, μm s−1), and number of sperm paths (Paths) per replicate film clip are given in x and σ columns respectively.
Table S3. Longevity data for Figure 3. Columns from the left: Species, corresponding species (see Table 1 for the species abbreviations); Male, the male number from Figure 1, and x indicates species average; Time, time of the last observation of continuously swimming sperm in hours.
Video S1. Video clip of M. galloprovincialis (MG10) sperm under control conditions during initial sperm activity measurements. The video clip shows continuously swimming sperm in good condition.
Video S2. Video clip of M. galloprovincialis (MG10) sperm under increased pCO2 during initial sperm activity measurements. Sperm is clearly affected by the treatment (compare with Video S1).
Video S3. Video clip of M. galloprovincialis (MG3) sperm under control conditions 46 h after induced spawning. The video clip shows several continuously swimming sperm cells.
Video S4. Video clip of M. calcarea (MC5) sperm under control conditions during the initial measurements. The video clip shows several continuously swimming sperm cells in good condition.
Video S5. Video clip of M. calcarea (MC5) sperm under control conditions 115 h after extraction of gametes. One cell adjacent to the lower left corner is swimming continuously. Many cells are still swimming, although not as continuously as in Video S4, and therefore not recorded as “continuously swimming sperm.”
Datasheet S1. Code S1.
Datasheet S2. Code S2.
Figure S1. Experimental setup. On the left gas-mixture, with CO2 bottle, sodalime, Mass Flow Controllers (MFCs) producing gas mixtures with 380 and 1000 ppm CO2. In the middle storage conditions for M. calcarea and Mytilus edulis in separate climate rooms and on the right conditions in which the water used during the video trials was stored. Green for 380 ppm and red for 1000 ppm CO2 gas/water.
Figure S2. Water pH (A,B) and total alkalinity (AT; C) during the study. Circles represent control and triangles acidified treatments. (A) pH sensor values measured directly from treatment water (relative to NBS scale). (B) Water pH values measured from storage tanks using spectrophotometer (relative to total scale). (C) Water AT (mmol kg−1) values measured from larval cylinders. Vertical lines represent days with sperm activity assessments. Individual males from Figure 1 are specified on top of the graph.
Figure S3. Raw data used to calculate the effect sizes (Figure 2). (A,B) percent sperm motility, (C,D) sperm swimming speed, and (E,F) modeled fertilization success. Panels in the first column (A,C,E) represent M. calcarea, and panels in the second column (B,D,F) indicate M. galloprovincialis. Lines between points mark individuals and error bars show standard error of mean. Green indicates control pCO2 treatment, while red increased pCO2 treatment.
References
Alavi, S. M. H., Matsumura, N., Shiba, K., Itoh, N., Takahashi, K. G., Inaba, K., et al. (2014). Roles of extracellular ions and pH in 5-HT-induced sperm motility in marine bivalve. Reproduction 147, 331–345. doi: 10.1530/REP-13-0418
Albright, R., and Mason, B. (2013). Projected near-future levels of temperature and pCO2 reduce coral fertilization success. PLoS ONE 8:e56468. doi: 10.1371/journal.pone.0056468
AMAP (2013). AMAP Assessment 2013: Arctic Ocean Acidification. Technical report, Oslo: Arctic Monitoring and Assessment Programme (AMAP). Available online at: http://www.amap.no/documents/doc/amap-assessment-2013-arctic-ocean-acidification/881
André, C., and Lindegarth, M. (1995). Fertilization efficiency and gamete viability of a sessile, free-spawning bivalve, Cerastoderma edule. Ophelia 43, 215–227. doi: 10.1080/00785326.1995.10429833
Artsdatabanken (2014). The Norwegian Red List for Species. Trondheim: Artsdatabanken. Available online at: http://www.artsdatabanken.no/ (Accessed June 31, 2014).
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1–48. doi: 10.18637/jss.v067.i01
Barros, P., Sobral, P., Range, P., Chícharo, L., and Matias, D. (2013). Effects of sea-water acidification on fertilization and larval development of the oyster Crassostrea gigas. J. Exp. Mar. Biol. Ecol. 440, 200–206. doi: 10.1016/j.jembe.2012.12.014
Byrne, M., Ho, M., Selvakumaraswamy, P., Nguyen, H. D., Dworjanyn, S. A., and Davis, A. R. (2009). Temperature, but not pH, compromises sea urchin fertilization and early development under near-future climate change scenarios. Proc. R. Soc. Lond. B Biol. Sci. 276, 1883–1888. doi: 10.1098/rspb.2008.1935
Byrne, M., and Przeslawski, R. (2013). Multistressor impacts of warming and acidification of the ocean on marine invertebrates' life histories. Integr. Comp. Biol. 53, 582–596. doi: 10.1093/icb/ict049
Byrne, M., Soars, N., Selvakumaraswamy, P., Dworjanyn, S. A., and Davis, A. R. (2010). Sea urchin fertilization in a warm, acidified and high pCO2 ocean across a range of sperm densities. Mar. Environ. Res. 69, 234–239. doi: 10.1016/j.marenvres.2009.10.014
Caldwell, G. S., Fitzer, S., Gillespie, C. S., Pickavance, G., Turnbull, E., and Bentley, M. G. (2011). Ocean acidification takes sperm back in time. Invertebr. Reprod. Dev. 55, 217–221. doi: 10.1080/07924259.2011.574842
Chierici, M., Sørensen, K., Johannessen, T., Børsheim, K. Y., Olsen, A., Yakushev, E., et al. (2012). Tilførselsprogrammet 2011 Overvåking av forsuring av Norske Farvann. Technical report, Oslo: Klima- og forurensingsdirektoriatet. Available online at: http://www.miljodirektoratet.no/old/klif/publikasjoner/2936/ta2936.pdf
Christen, R., Schackmann, R. W., and Shapiro, B. M. (1982). Elevation of the intracellular pH activates respiration and motility of sperm of the sea urchin, Strongylocentrotus purpuratus. J. Biol. Chem. 257, 14881–14890.
Ciais, P., Sabine, C., Bala, G., Bopp, L., Brovkin, V., Canadell, J., et. al. (2013). “Carbon and other biogeochemical cycles,” in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds T. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, and P. M. Midgley (Cambridge, UK; New York, NY: Cambridge University Press), 465–570. Available online at: http://www.ipcc.ch/report/ar5/wg1/
Crimaldi, J. P. (2012). The role of structured stirring and mixing on gamete dispersal and aggregation in broadcast spawning. J. Exp. Biol. 215, 1031–1039. doi: 10.1242/jeb.060145
Crimaldi, J. P., and Zimmer, R. K. (2013). The physics of broadcast spawning in benthic invertebrates. Ann. Rev. Mar. Sci. 6, 7.1–7.25. doi: 10.1146/annurev-marine-010213-135119
Denny, M., and Shibata, M. (1989). Consequences of surf-zone turbulence for settlement and external fertilization. Am. Nat. 134, 859–889. doi: 10.1086/285018
Dickson, A., and Millero, F. (1987). A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. A 34, 1733–1743.
Dickson, A. G. (1990). Standard potential of the reaction: AgCl(s) + 1 H2(g) = Ag(s)+ HCl(aq), and the standard acidity constant of the ion HSO-4 in synthetic sea water from 273.15 to 318.15 K. J. Chem. Thermodyn. 22, 113–127. doi: 10.1016/0021-9614(90)90074-Z
Dickson, A. G., Sabine, C. L., and Christian, J. R. (2007). Guide to Best Practices for Ocean CO2 Measurements. Sidney, BC: PICES; special pu edition.
Duarte, C. M., Hendriks, I. E., Moore, T. S., Olsen, Y. S., Steckbauer, A., Ramajo, L., et al. (2013). Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries Coast. 36, 221–236. doi: 10.1007/s12237-013-9594-3
Dupont, S., Dorey, N., Stumpp, M., Melzner, F., and Thorndyke, M. (2013). Long-term and trans-life-cycle effects of exposure to ocean acidification in the green sea urchin Strongylocentrotus droebachiensis. Mar. Biol. 160, 1835–1843. doi: 10.1007/s00227-012-1921-x
Evans, J. P., and Sherman, C. D. H. (2013). Sexual selection and the evolution of egg-sperm interactions in broadcast-spawning invertebrates. Biol. Bull. 224, 166–183. Available online at: http://www.biolbull.org/content/224/3/166.long
Gattuso, J., and Lavigne, H. (2009). Technical note: approaches and software tools to investigate the impact of ocean acidification. Biogeosciences 6, 2121–2133. doi: 10.5194/bg-6-2121-2009
Gazeau, F., Duarte, C. M., Gattuso, J. P., Barrón, C., Navarro, N., Rúız, S., et. al. (2005). Whole-system metabolism and CO2 fluxes in a Mediterranean Bay dominated by seagrass beds (Palma Bay, NW Mediterranean). Biogeosciences 2, 43–60. doi: 10.5194/bg-2-43-2005
Gienapp, P., Teplitsky, C., Alho, J. S., Mills, J. A., and Merilä, J. (2008). Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178. doi: 10.1111/j.1365-294X.2007.03413.x
Gosling, E. M. (1984). The systematic status of Mytilus galloprovincialis in Western Europe. Malacologia 25, 551–568.
Havenhand, J. N., Buttler, F. R., Thorndyke, M. C., and Williamson, J. E. (2008). Near-future levels of ocean acidification reduce fertilization success in a sea urchin. Curr. Biol. 18, 651–652. doi: 10.1016/j.cub.2008.06.015
Havenhand, J. N., and Schlegel, P. (2009). Near-future levels of ocean acidification do not affect sperm motility and fertilization kinetics in the oyster Crassostrea gigas. Biogeosciences 6, 3009–3015. doi: 10.5194/bg-6-3009-2009
Hedges, L. V., Gurevitch, J., and Curtis, P. S. (1999). The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156. doi: 10.1890/0012-9658(1999)080[1150:TMAORR]2.0.CO;2
Hendriks, I. E., Duarte, C. M., Olsen, Y. S., Steckbauer, A., Ramajo, L., Moore, T. S., et al. (2014). Biological mechanisms supporting adaptation to ocean acidification in coastal ecosystems. Estuar. Coast. Shelf Sci. 152, A1–A8. doi: 10.1016/j.ecss.2014.07.019
Himmelman, J., Dumont, C., Gaymer, C., Valliéres, C., and Drolet, D. (2008). Spawning synchrony and aggregative behaviour of cold-water echinoderms during multi-species mass spawnings. Mar. Ecol. Prog. Ser. 361, 161–168. doi: 10.3354/meps07415
Hoffmann, A. A., and Sgró, C. M. (2011). Climate change and evolutionary adaptation. Nature 470, 479–485. doi: 10.1038/nature09670
Hofmann, G. E., Smith, J. E., Johnson, K. S., Send, U., Levin, L. A., Micheli, F., et al. (2011). High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6:e28983. doi: 10.1371/journal.pone.0028983
Honkoop, P. J. C., Luttikhuizen, P. C., and Piersma, T. (1999). Experimentally extending the spawning season of a marine bivalve using temperature change and fluoxetine as synergistic triggers. Mar. Ecol. Prog. Ser. 180, 297–300. doi: 10.3354/meps180297
ISSG (2014). Global Invasive Species Database. Available online at: http://www.issg.org/ (Accessed January 31, 2014).
Johnson, S. L., and Yund, P. O. (2004). Remarkable longevity of dilute sperm in a free-spawning colonial ascidian. Biol. Bull. 206, 144–151. doi: 10.2307/1543638
Kroeker, K. J., Kordas, R. L., Crim, R., Hendriks, I. E., Ramajo, L., Singh, G. S., et al. (2013). Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 19, 1884–1896. doi: 10.1111/gcb.12179
Kupriyanova, E. K., and Havenhand, J. N. (2005). Effects of temperature on sperm swimming behaviour, respiration and fertilization success in the serpulid polychaete, Galeolaria caespitosa (Annelida: Serpulidae). Invertebr. Reprod. Dev. 48, 7–17. doi: 10.1080/07924259.2005.9652166
Kurihara, H. (2008). Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar. Ecol. Ser. 373, 275–284. doi: 10.3354/meps07802
Kuznetsova, A., Bruun Brockhoff, P., and Haubo Bojesen Christensen, R. (2015). lmerTest: Tests in Linear Mixed Effects Models. R package version 2.0-29. Available online at: https://CRAN.R-project.org/package=lmerTest
Levitan, D., and Young, C. (1995). Reproductive success in large populations: empirical measures and theoretical predictions of fertilization in the sea biscuit Clypeaster rosaceus. J. Exp. Mar. Biol. Ecol. 190, 221–241. doi: 10.1016/0022-0981(95)00039-T
Levitan, D. R. (2000). Sperm velocity and longevity trade off each other and influence fertilization in the sea urchin Lytechinus variegatus. Proc. R. Soc. Lond. B Biol. Sci. 267, 531–534. doi: 10.1098/rspb.2000.1032
Levitan, D. R. (2008). Gamete traits influence the variance in reproductive success, the intensity of sexual selection, and the outcome of sexual conflict among congeneric sea urchins. Evolution 62, 1305–1316. doi: 10.1111/j.1558-5646.2008.00378.x
Levitan, D. R., and Petersen, C. (1995). Sperm limitation in the sea. Trends Ecol. Evol. 10, 228–231. doi: 10.1016/S0169-5347(00)89071-0
Levitan, D. R., Sewell, M. A., and Chia, F.-S. (1991). Kinetics of fertilization in the sea urchin Strongylocentrotus franciscanus: interaction of gamete dilution, age, and contact time. Biol. Bull. 181, 371–378. doi: 10.2307/1542357
Levy, L. A., and Couturier, C. A. (1996). Effects of sperm longevity and gamete concentrations on fertilization success in the blue mussel. Bull. Aquacult. Ass. Can. 96, 71–73.
Lotterhos, K., and Levitan, D. (2010). “Gamete release and spawning behavior in broadcast spawning marine invertebrates,” in The Evolution of Primary Sexual Characters in Animals, eds J. Leonard and A. Cordoba-Aguilar (New York, NY: Oxford University Press), 99–120.
Mehrbach, C., Culberson, C. H., Hawley, J. E., and Pytkowicz, R. M. (1973). Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 8, 897–907. doi: 10.4319/lo.1973.18.6.0897
Miller, G. M., Watson, S.-A., Donelson, J. M., McCormick, M. I., and Munday, P. L. (2012). Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat. Clim. Chang. 2, 858–861. doi: 10.1038/nclimate1599
Millero, F., Morse, J., and Chen, C. (1979). The carbonate system in the western Mediterranean Sea. Deep Res 26A, 1395–1404. doi: 10.1016/0198-0149(79)90007-4
Morita, M., Suwa, R., Iguchi, A., Nakamura, M., Shimada, K., Sakai, K., et al. (2010). Ocean acidification reduces sperm flagellar motility in broadcast spawning reef invertebrates. Zygote 18, 103–107. doi: 10.1017/S0967199409990177
Munday, P. L., Warner, R. R., Monro, K., Pandolfi, J. M., and Marshall, D. J. (2013). Predicting evolutionary responses to climate change in the sea. Ecol. Lett. 16, 1488–1500. doi: 10.1111/ele.12185
Murphy, P. P., Nojiri, Y., Harrison, D. E., and Larkin, N. K. (2001). Scales of spatial variability for surface ocean pCO2 in the Gulf of Alaska and Bering Sea: toward a sampling strategy. Geophys. Res. Lett. 28, 1047–1050. doi: 10.1029/2000GL012375
Nakagawa, S., and Cuthill, I. C. (2007). Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. Camb. Philos. Soc. 82, 591–605. doi: 10.1111/j.1469-185X.2007.00027.x
Oertzen, J. A. (1972). Cycles and rates of reproduction of six Baltic Sea bivalves of different zoogeographical origin. Mar. Biol. 14, 143–149. doi: 10.1007/BF00373213
Palumbi, S. R. (1999). All males are not created equal: fertility differences depend on gamete recognition polymorphisms in sea urchins. Proc. Natl. Acad. Sci. U.S.A. 96, 12632–12637. doi: 10.1073/pnas.96.22.12632
Parker, L. M., Ross, P. M., and O'Connor, W. A. (2009). The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould 1850). Glob. Chang. Biol. 15, 2123–2136. doi: 10.1111/j.1365-2486.2009.01895.x
Parker, L. M., Ross, P. M., O'Connor, W. A., Borysko, L., Raftos, D. A., and Pörtner, H.-O. (2012). Adult exposure influences offspring response to ocean acidification in oysters. Glob. Chang. Biol. 18, 82–92. doi: 10.1111/j.1365-2486.2011.02520.x
Pedersen, S. A. (1994). Population parameters of the Iceland scallop (Chlamys islandica (Müller)) from West Greenland. J. Northw. Atl. Fish. Sci. 16, 75–87. doi: 10.2960/J.v16.a7
Pennington, J. T. (1985). The ecology of fertilization of echinoid eggs: the consequences of sperm dilution, adult aggregation, and synchronous spawning. Biol. Bull. 169, 417–430. Available online at: http://www.biolbull.org/content/169/2/417.short (Accessed February 28, 2013).
Powell, D. K., Tyler, P. A., and Peck, L. S. (2001). Effect of sperm concentration and sperm ageing on fertilisation success in the Antarctic soft-shelled clam Laternula elliptica and the Antarctic limpet Nacella concinna. Mar. Ecol. Prog. Ser. 215, 191–200. doi: 10.3354/meps215191
Præbel, K., Christiansen, J. S., and Fevolden, S. E. (2009). Temperature and salinity conditions in a sub-Arctic intertidal spawning habitat for capelin. Mar. Biol. Res. 5, 511–514. doi: 10.1080/17451000902729670
R Core Team (2015). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Reusch, T. B. H. (2014). Climate change in the oceans: evolutionary versus phenotypically plastic responses of marine animals and plants. Evol. Appl. 7, 104–122. doi: 10.1111/eva.12109
Robbins, L., Hansen, M., Kleypas, J., and Meylan, S. (2010). CO2calc: A User-Friendly Seawater Carbon Calculator for WINDOWS, Max OS X, and iOS (iPhone). U.S. Geological Survey Open-File Report 20101280. Technical Report, St. Petersburg, FL. Available online at: http://pubs.usgs.gov/of/2010/1280/
Salinas, S., Brown, S. C., Mangel, M., and Munch, S. B. (2013). Non-genetic inheritance and changing environments. Non Genet. Inheritance 1, 38–50. doi: 10.2478/ngi-2013-0005
SAS Technical Report R-101 (1978). Tests of Hypotheses in Fixed-Effects Linear Models. Cary, NC: SAS Institute Inc.
Schlegel, P., Havenhand, J. N., Gillings, M. R., and Williamson, J. E. (2012). Individual variability in reproductive success determines winners and losers under ocean acidification: a case study with sea urchins. PLoS ONE 7:e53118. doi: 10.1371/journal.pone.0053118
Schlegel, P., Havenhand, J. N., Obadia, N., and Williamson, J. E. (2014). Sperm swimming in the polychaete Galeolaria caespitosa shows substantial inter-individual variability in response to future ocean acidification. Mar. Pollut. Bull. 78, 213–217. doi: 10.1016/j.marpolbul.2013.10.040
Schneider, A., Wallace, D. W. R., and Körtzinger, A. (2007). Alkalinity of the Mediterranean Sea. Geophys. Res. Lett. 34, L15608. doi: 10.1029/2006GL028842
Sedano, F. J., Rodriguez, J. L., Ruiz, C., Garciamartin, L. O., and Sanchez, J. L. (1995). Biochemical composition and fertilization in the eggs of Mytilus galloprovincialis (Lamarck). J. Exp. Mar. Biol. Ecol. 192, 75–85. doi: 10.1016/0022-0981(95)00062-V
Strathmann, M. F. (1987). Reproduction and Development of Marine Invertebrates of the Northern Pacific Coast: Data and Methods for the Study of Eggs, Embryos, and Larvae. Seattle, WA: University of Washington Press.
Styan, C. A. (1998). Polyspermy, egg size, and the fertilization kinetics of free-spawning marine invertebrates. Am. Nat. 152, 290–297. doi: 10.1086/286168
Styan, C. A., and Butler, A. J. (2000). Fitting fertilisation kinetics models for free-spawning marine invertebrates. Mar. Biol. 137, 943–951. doi: 10.1007/s002270000401
Styan, C. A., Kupriyanova, E., and Havenhand, J. N. (2008). Barriers to cross-fertilization between populations of a widely dispersed polychaete species are unlikely to have arisen through gametic compatibility arms-races. Evolution 62, 3041–3055. doi: 10.1111/j.1558-5646.2008.00521.x
Sunday, J. M., Calosi, P., Dupont, S., Munday, P. L., Stillman, J. H., and Reusch, T. B. H. (2014). Evolution in an acidifying ocean. Trends Ecol. Evol. (Amst). 29, 117–125. doi: 10.1016/j.tree.2013.11.001
Sunday, J. M., Crim, R. N., Harley, C. D. G., and Hart, M. W. (2011). Quantifying rates of evolutionary adaptation in response to ocean acidification. PLoS ONE 6:e22881. doi: 10.1371/journal.pone.0022881
Tans, P., and Keeling, R. (2014). Trends in Atmospheric Carbon Dioxide. National Oceanic and Atmospheric Administration. Available online at: http://www.esrl.noaa.gov/gmd/ccgg/trends/ (Accessed January 31, 2014).
Thorson, G. (1950). Reproductive and larval ecology of marine bottom invertebrates. Biol. Rev. 25, 1–45. doi: 10.1111/j.1469-185X.1950.tb00585.x
Uthicke, S., Pecorino, D., Albright, R., Negri, A. P., Cantin, N., Liddy, M., et al. (2013). Impacts of ocean acidification on early life-history stages and settlement of the coral-eating sea star Acanthaster planci. PLoS ONE 8:e82938. doi: 10.1371/journal.pone.0082938
Van Colen, C., Debusschere, E., Braeckman, U., Van Gansbeke, D., and Vincx, M. (2012). The early life history of the clam Macoma balthica in a high CO2 world. PLoS ONE 7:e44655. doi: 10.1371/journal.pone.0044655
Vihtakari, M. (2014). Bivalves as Indicators of Environmental Perturbations Related to Climate and Ocean Acidification. Ph.D. thesis, UiT The Arctic University of Norway, Tromsø.
Vihtakari, M., Hendriks, I., Holding, J., Renaud, P., Duarte, C., and Havenhand, J. (2013). Effects of ocean acidification and warming on sperm activity and early life stages of the Mediterranean mussel (Mytilus galloprovincialis). Water 5, 1890–1915. doi: 10.3390/w5041890
Vogel, H., Czihak, G., Chang, P., and Wolf, W. (1982). Fertilization kinetics of sea urchin eggs. Math. Biosci. 58, 189–216. doi: 10.1016/0025-5564(82)90073-6
WoRMS Editorial Board (2014). World Register of Marine Species. Available online at: http://www.marinespecies.org (Accessed January 31, 2014).
Keywords: individual variability, sperm motility, bivalves, natural selection, Mytilus galloprovincialis, Macoma calcarea
Citation: Vihtakari M, Havenhand J, Renaud PE and Hendriks IE (2016) Variable Individual- and Population- Level Responses to Ocean Acidification. Front. Mar. Sci. 3:51. doi: 10.3389/fmars.2016.00051
Received: 16 November 2015; Accepted: 01 April 2016;
Published: 29 April 2016.
Edited by:
Jacob Carstensen, Aarhus University, DenmarkReviewed by:
Folco Giomi, University of Padua, ItalyMichael Yu Roleda, Norwegian Institute for Bioeconomy Research, Norway
Copyright © 2016 Vihtakari, Havenhand, Renaud and Hendriks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iris E. Hendriks, aXJpc0BpbWVkZWEudWliLWNzaWMuZXM=
 Mikko Vihtakari
Mikko Vihtakari Jon Havenhand
Jon Havenhand Paul E. Renaud
Paul E. Renaud Iris E. Hendriks
Iris E. Hendriks