- 1School of Geography, University of Leeds, Leeds, UK
- 2Life Sciences, Natural History Museum, London, UK
- 3School of Marine Science and Technology, Newcastle University, Newcastle, UK
- 4School of Biology, Newcastle University, Newcastle, UK
- 5School of Earth and Environment, University of Leeds, Leeds, UK
Sedimented hydrothermal vents, where hot, mineral-rich water flows through sediment, are poorly understood globally, both in their distribution and the ecology of individual vent fields. We explored macrofaunal community ecology at a sediment-hosted hydrothermal vent in the Southern Ocean. This is the first such study of these ecosystems outside of the Pacific and the furthest south (62°S) of any vent system studied. Sedimentary fauna were sampled in four areas of the Bransfield Strait (Southern Ocean), with the aim of contrasting community structure between vent and non-vent sites. Macrofaunal assemblages were clearly distinct between vent and non-vent sites, and diversity, richness, and density declined toward maximum hydrothermal activity. This variation is in contrast to observations from similar systems in the Pacific and demonstrates the influence of factors other than chemosynthetic primary productivity in structuring infauna at deep-sea vent communities. Vent endemic fauna had limited abundance and were represented by a single siboglinid species at hydrothermally active areas, meaning that that the majority of local biota were those also found in other areas. Several taxa occupied all sampling stations but there were large differences in their relative abundances, suggesting communities were structured by niche variation rather than dispersal ability.
Introduction
Deep-sea hydrothermal vent fields are under imminent threat from mineral extraction (Petersen et al., 2004; Van Dover, 2010), creating an imperative to understand these environments so that their inhabitants may be effectively conserved. Size, distribution, and faunal composition of sediment-hosted hydrothermal vents (SHVs) in particular are poorly understood globally. At SHVs, and in some diffuse venting areas around high-temperature vents, hydrothermal fluid mixes with ambient seawater beneath the seafloor (Bemis et al., 2012) and, in terms of oceanic heat flux, may be more significant than high temperature vents (Larson et al., 2015). The mixing of the hydrothermal fluid with ambient seawater means that it cools too slowly to precipitate mineral structures, creating an environment comprised of hot (generally 10–100°C above ambient), high porosity sediment, typically with high levels of hydrogen sulfide, methane, and reduced metals, such as iron and manganese (Levin et al., 2009; Bernardino et al., 2012; Aquilina et al., 2013; Urich et al., 2014). The vast majority of deep-sea hydrothermal vent research has focused on high-temperature vents, which are typically dominated by vent endemic species (e.g., Rogers et al., 2012). In contrast, there have been only a handful of ecological studies at SHVs (Grassle et al., 1985; Grassle and Petrecca, 1994; Kharlamenko et al., 1995; Levin et al., 2009, 2012; Sweetman et al., 2013) and none from the Southern Ocean. Hydrothermal sites in the Bransfield Strait (Southern Ocean) potentially provide a stepping stone between chemosynthetic communities in the Atlantic, Indian, and Pacific Oceans (Rogers et al., 2012; Roterman et al., 2013) as well as providing an interesting site to explore how ambient deep Southern Ocean benthos respond to reducing environments.
Sediment-hosted vents support communities of chemosynthetic primary producers; free-living, colonial or symbiotic. SHVs thus have direct implications for the wider faunal community, through both in situ organic matter production and the environmental toxicity associated with hydrothermalism (Levin et al., 2009; Bernardino et al., 2012). The relative physical similarity of SHV (compared with high-temperature vents) to non-hydrothermal environments allows more scope for opportunistic interactions between vent endemic fauna and more common deep-sea fauna with profound impacts upon community structure in both macro- and meiofaunal species (Zeppilli et al., 2011, 2015; Bernardino et al., 2012). Bernardino et al. (2012) proposed a conceptual model of how macrofaunal community characteristics (such as abundance and diversity) respond to gradients in environmental toxicity and organic matter (OM) availability at SHVs. These models are consistent with models of non-chemosynthetic macrofaunal response to gradients in pollution and organic enrichment (Pearson and Rosenberg, 1978) and the intermediate disturbance hypothesis (Connell, 1978). Maximum biodiversity is predicted at intermediate levels of toxicity and productivity (Connell, 1978), where higher niche or environmental diversity potentially facilitates higher diversity (Bernardino et al., 2012; Gollner et al., 2015; McClain and Schlacher, 2015). However, high species dominance/low diversity in deep-sea macrofauna is generally rare (McClain and Schlacher, 2015), and often characteristic of reducing or highly disturbed environments (Netto et al., 2009). The models further suggest that the proportion of background fauna generally declines toward a community dominated by vent-endemic species (Bernardino et al., 2012). These changes emulate a distance-decay model (Nekola and White, 1999; McClain et al., 2011, 2012), but one accelerated by the rapid change in environmental conditions (sub-km scale), where dispersal limitations are superseded by habitat selection.
Toward the region of greatest hydrothermal flux, it was also hypothesized that increased supply of chemosynthetic substrates would support larger populations of vent endemic species and greater OM production rates, in turn enhancing local abundance or biomass (Bernardino et al., 2012). However, there is conflicting evidence as to whether SHV enrich macrofaunal abundance, suggesting that other factors (e.g., supply of other sources of OM or environmental stress) may also be important (Grassle et al., 1985; Grassle and Petrecca, 1994; Levin et al., 2009). The importance of local environmental factors in structuring communities of SHVs remains a key unknown, particularly for non-Pacific SHVs that have not previously been studied quantitatively. The lack of quantitative ecology from SHVs outside the Pacific severely limits our understanding of these ecosystems.
Sedimented hydrothermal systems have been identified in the Bransfield Strait, but ecological investigations have not yet been reported. The only other hydrothermal vent system reported in the Southern Ocean is a high-temperature, hard substratum vent (Rogers et al., 2012), which are not directly comparable to the Bransfield Strait SHVs. The Strait (Figure 1) harbors deep (1050 m) sediment-hosted vents (Klinkhammer et al., 2001; Aquilina et al., 2013) as well as episodic shallow water venting around Deception Island (Somoza et al., 2004). In spite of the wide ranges in temperature in recent geological history (Clarke and Crame, 1992, 2010), some Southern Ocean biota are sensitive to small (ca. 2–3°C) temperature perturbations (Barnes and Peck, 2008; Clarke et al., 2009). Typical seafloor temperature in the Bransfield Strait is around −1.5°C (Bohrmann et al., 1998; Clarke et al., 2009), compared with estimates of 25–50°C at hydrothermally active areas (Dählmann et al., 2001; Klinkhammer et al., 2001; Aquilina et al., 2013). This raises questions as to the ability of Southern Ocean benthos to colonize areas of hydrothermal activity and suggests that it may be crucial to species distribution in the Bransfield Strait.
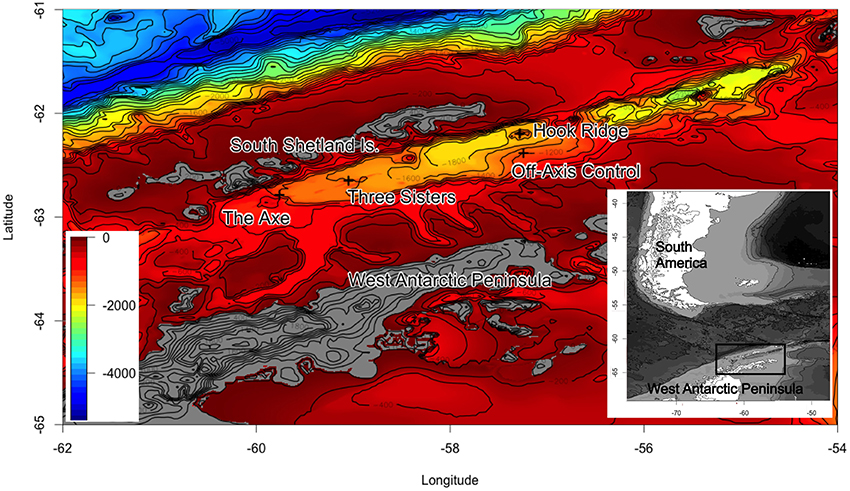
Figure 1. Bathymetric charts of the Bransfield Strait (30 arc-s grids constructed using GEBCO bathymetry) showing sampling locations during JC055. Latitude and longitude given as degrees and decimal minutes.
The aim of the present study is to show how hydrothermalism influences infaunal communities in the Bransfield Strait. We present macrofaunal assemblage data and critically evaluate conceptual models of SHV ecology, (Bernardino et al., 2012) adding perspectives from high-latitude SHVs. The following hypotheses were explored: (1) Hydrothermally active sites will support a greater macrofaunal density than background sites due to increased OM availability (2) Macrofaunal diversity will be different between areas with or without active hydrothermalism; (3) Abundance of vent endemic species (Sclerolinum contortum Georgieva et al., 2015) will be highest in areas of moderate hydrothermal activity, as suggested by Sahling et al. (2005). We also explore the influence of environmental gradients upon community composition and results are quantitatively compared to Bernardino et al. (2012)'s conceptual models of abundance, diversity and proportions of vent endemic fauna, using a measure of environmental distance.
Materials and Methods
Ethics Statement
In accordance with the Antarctic Act (1994) and the Antarctic Regulations (1995), necessary permits (S5-4/2010) were acquired from the South Georgia and South Sandwich Islands Government.
Sample Collection and Processing
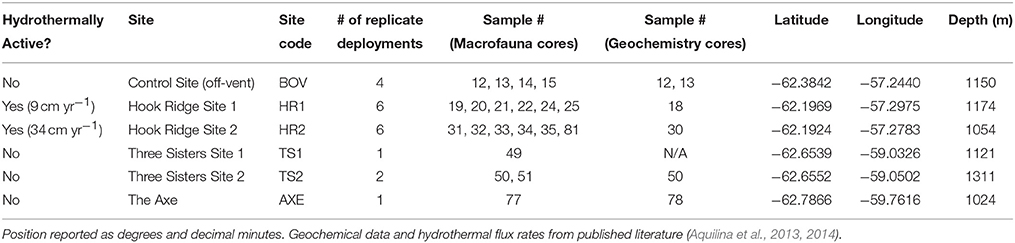
The Bransfield Strait is located between the West Antarctic Peninsula and the South Shetland Islands. Two subaerial volcanoes (Deception and Bridgeman Islands) divide the Strait into three sub-basins, the central of which hosts several volcanic edifices: Hook Ridge; the Three Sisters, and the Axe (Whiticar and Suess, 1990; Bohrmann et al., 1998; Dählmann et al., 2001; Klinkhammer et al., 2001; Aquilina et al., 2013; Figure 1). At Hook Ridge, remnants of a high temperature mineral chimney venting shimmering water were seen during a video survey (Aquilina et al., 2013) and hot, sulphidic sediments have been recovered (Dählmann et al., 2001; Klinkhammer et al., 2001). Of the four study areas visited in the Bransfield Strait during RRS James Cook cruise 55 (Figure 1) only Hook Ridge was found to be hydrothermally active and cores from different areas of the ridge were subject to fluid advection rates of 9–34 cm yr−1 (Aquilina et al., 2013). Sediments from the off-vent site, the Three Sisters and the Axe showed no signatures consistent with active hydrothermalism.
Samples were collected in January 2011, aboard RRS James Cook cruise 55 (Tyler et al., 2011). Following bathymetric and water column surveys to identify the source of venting (see Aquilina et al., 2013), replicate corer deployments were made (3–6 per site except at the Axe). All samples were collected using a Bowers and Connelly dampened megacorer (Gage and Bett, 2005), from each of the three raised edifices in the central basin, and one off-axis/ off-vent control site (Figure 1; Table 1). A single deployment was made at the Axe, owing to shallow sediment coverage and the absence of water column signals indicative of hydrothermal activity (Aquilina et al., 2013). From each deployment, multiple 10 cm diameter sediment cores were collected. Samples from individual cores from the same deployment were pooled into single samples, as cores from the same deployment are pseudoreplicates.
Cores were sliced into upper (0–5 cm) and lower (5–10 cm) partitions and were passed through a 300 μm sieve to extract macrofauna (e.g., Levin et al., 2009). Differences between upper and lower core partitions were only analyzed where there were measureable hydrothermal differences between each partition. Fauna were preserved in either 80% ethanol or 10% buffered formalin in seawater. In the laboratory, macrofauna were sorted to highest possible taxonomic level using a dissecting microscope. All annelid and bivalve specimens were sorted to species or morphospecies level, with other taxa sorted to family or higher. Incomplete specimens (those without heads) were not counted, to prevent over-estimating their density. Feeding modes were determined by morphology, and validated against taxonomic keys (Fauchald, 1977; Fauchald and Jumars, 1979; Barnard and Karaman, 1991; Beesley et al., 2000; Smirnov, 2000; Poore, 2001; Kilgallen, 2007; Keuning et al., 2011; Reed et al., 2013; Jumars et al., 2015), the Ocean Biogeographic Information System (OBIS) and studies from similar areas/ environments that have conducted stable isotope analyses (Fauchald and Jumars, 1979; Mincks et al., 2008; Levin et al., 2009).
Samples of freeze-dried sediment were acidified (6 M HCl) to remove inorganic carbon and then analyzed for carbon content. Samples were analyzed at EK using a continuous flow isotope ratio mass spectrometer using a Vario-Pyro Cube elemental analyser (Elementar), coupled with a Delta plusXP mass spectrometer (Thermo Electron).
Statistical Analyses
The lack of replicates taken from the Axe and TS1 meant that it was excluded from any permutational analyses aimed at discriminating differences by site. Abundance per deployment was standardized to give abundance per m2. Unless otherwise specified, all subsequent analyses were conducted in the R environment (R Core Team, 2013).
Species Diversity and Richness
Calculations of diversity and richness were made from species-level discriminated fauna only (annelids and bivalves only). Shannon-Wiener diversity (H′) was calculated in VEGAN (v2.0-8) (Oksanen et al., 2013) for whole deployments and individual vertical core partitions. Species-accumulation curves and shared species were calculated using EstimateS (v9.1.0; Gotelli and Colwell, 2001; Colwell et al., 2012; Colwell, 2013). Individual-based species-accumulation curves (999 permutations) were constructed from species level data for each of the sites (n = 100). Pairwise shared species were calculated from abundance data summed by site using the abundance-based Chao-Sørenson index (Chao et al., 2005) to reduce under-sampling bias given the inconsistent number of replicate deployments per site.
Community Structure
Untransformed community composition data were used to create a Bray-Curtis similarity matrix and visualized using the metaMDS method in VEGAN (Oksanen et al., 2013). Significant structure within the data was tested with a similarity profile routine (SIMPROF, 10 000 permutations, average linkage) using the clustsig package (v1.0) (Clarke et al., 2006, 2008; Whitaker and Christmann, 2013). Similarity between sites was compared using permutational ANOVA (PERMANOVA, 999 permutations; Anderson, 2001), using site as a factor. The validity of PERMANOVA was checked using the PERMDISP test for homogeneity of variance (betadisper and anova in Vegan), since PERMANOVA is sensitive to multivariate dispersion (Anderson, 2001). No differences between sites were observed (ANOVA: F-value; 0.535, df = 16; P > 0.05).
Environmental Variables
To compare the relative influence of hydrothermal fluids at different sites, and to test the theoretical model suggested by Bernardino et al. (2012), a proxy index for hydrothermal activity (the Hydrothermal Index – HI) was developed. Sites with comparable levels of relevant environmental parameters were considered to be likely to be more similar in faunal characteristics than those with greater differences (Bernardino et al., 2012; McClain et al., 2012; Gollner et al., 2015). This approach is analogous to the “Glaciality index” (Brown et al., 2010) and “benthic index” (Robertson et al., 2015) that have shown how various biological trends in separate systems can be explained by collating environmental parameters.
A single core profile from each site (except TS1) of geochemical data were available and initial selection criteria were based upon results of an envfit visualization (Oksanen et al., 2013) that identified influential (e.g., H2S) and co-linear species (e.g., Cl− and SO). Environmental data were not available from every site used for ecological analyses, hence more conventional ordination techniques (e.g., CCA) were not possible, which would also not have permitted comparisons with conceptual models as presented here. Temperature profiles were not available for inclusion. Biologically and hydrothermally relevant parameters were selected for the index, comprised of two chemosynthetic substrates (H2S and CH4), and an indicator of temperature changes and hydrothermal flux (Cl−). Low chlorinity (relative to seawater) is characteristic of hydrothermal fluid and so the reciprocal for Cl− concentration was given as a proxy for hydrothermal fluid input (Ginsburg et al., 1999; Aquilina et al., 2013; Larson et al., 2015).
Down core geochemical profiles from pore fluid data (Aquilina et al., 2013) were split into upper and lower partitions, as per the faunal samples (Data Sheet S3). Pore fluid concentrations of H2S, CH4 and Cl− were averaged into 0–5 and 5–10 cm below seafloor (b.s.f.) sections. Concentrations of each were then normalized to the mean value (i.e., mean = zero) for each component (thus each was equally weighted) and the sum for each site was given as the HI (two values per site, one per partition). Initially, faunal variation between upper and lower core partitions at non-hydrothermally active sites contributed the majority of the residual error, although relationships between HI and abundance and diversity were still significant (GLM, df = 1, p < 0.05). Since the hydrothermal index focuses upon the relationship between faunal characteristics and hydrothermalism, variation between upper and lower partitions of non-hydrothermally active sites was excluded from subsequent analyses.
Three statistical models were developed to determine the extent that HI could explain patterns in diversity, density, and species richness. Data (mean values for each site) were fitted to generalized linear models using the glm and Anova functions in the CAR package (v2.0-18) (Fox et al., 2013). Normality tests (Shapiro-wilk) were run on each of the responses and used to inform selection of distribution for the models. Diversity data were fitted to a Gaussian model. Count data (abundance and species richness) were fitted to Poisson models but residual deviance exceeded degrees of freedom, indicating over-dispersion, so the models were corrected using a quasi-Poisson dispersion factor (Dobson, 1990; Dobson and Barnett, 2008).
Results
Abundance and Community Composition
A total of 2320 individuals (73–780 ind. per site), of 81 macrofaunal taxa (25–50 per site) were collected. Fifty-one polychaete species, from 32 families represented the most abundant taxon (981 individuals). Peracarid crustaceans were the second most abundant taxon (755 ind. from 18 families), followed by oligochaetes (402 ind. from two families). Sites were dominated numerically by polychaetes (41–56% by abundance), except at Hook Ridge and the Axe where peracarids, mostly isopods, dominated (41–55%; Figure 2; Data Sheet S1). Oligochaetes were very abundant at the control site (37% of abundance) and relatively rare elsewhere (6–18%). The dominant feeding group (Figure 2) was generally comprised of carnivores/ scavengers at Hook Ridge (44–56%) and motile deposit feeders dominated at non-Hook Ridge sites (42–78%). Functional composition of taxa varied significantly (Pearson's χ2 = 178.37, p < 0.01) and was dissimilar between all pairwise comparisons (p < 0.05).
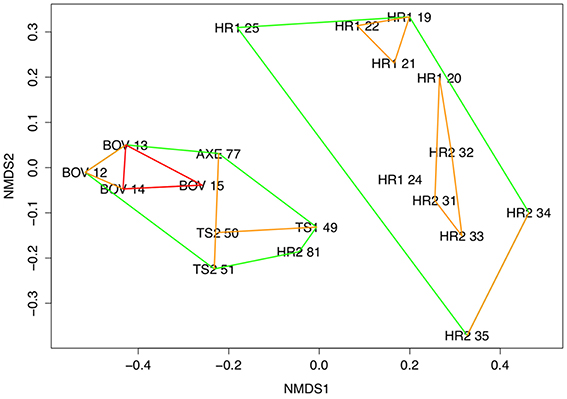
Figure 2. Multidimensional scaling ordination with ordinal hulls. Ordinal hulls based on Manhattan distance computed during cluster analysis. Hulls indicate areas of < 0.7 dissimilarity (green), < 0.5 (blue), and < 0.3 (red). 2-D stress = 0.13. Site numbers refer to deployments (Table 1), using whole core data.
The most abundant morphospecies groups present in the Bransfield strait were idoteid isopods at Hook Ridge 1 (2022 ind. m2 S.E. ± 752) and Limnodriloides sp. B (Oligochaeta: Naididae) at the control site (1958 ind. m2 S.E. ± 510) although the dominance of the Isopoda may have reflected the coarser taxonomic resolution used (Data Sheet S1). The most abundant polychaete species in the Bransfield strait was Aricidea antarctica (Paraonidae, Hartmann-Schröder and Rosenfeldt, 1988), comprising 23% of total macrofaunal density at the control site (1313 ind. m2 S.E. ± 269) but generally occurring in low relative abundances of 1–5% elsewhere.
Community composition was significantly different between all sites (PERMANOVA, F = 3.359, df = 4, p < 0.01; Figure 3) and clearly distinguished the Hook Ridge sites from the other, non-hydrothermally-active areas (SIMPROF, p = 0.05). Relative abundances of annelid and peracarid taxa were the main discriminators in assemblage structure between vent and non-vent conditions. Deployment 81 at Hook Ridge 2 was significantly different to other Hook Ridge sites (Figure 2; SIMPROF, p = 0.05) and reflected the relative lack of terebellids (Polychaeta) and isopods and increased abundance of naidid oligochaetes at HR2 81, giving it an assemblage more characteristic non Hook-Ridge sites, particularly the control site were naidids were highly abundant.
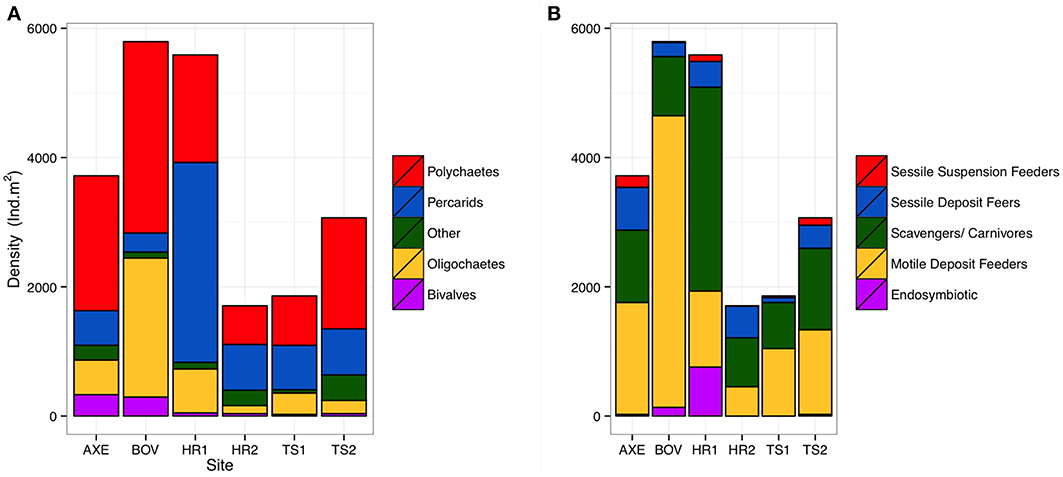
Figure 3. Comparative density (ind. m−2) of (A) major taxa and (B) functional groups by site for whole core data.
Hook Ridge 1 and the control site had the greatest mean macrofaunal density (Table 2). At Hook Ridge, macrofaunal density ranged widely, including the most (deployment 25: 14324 ind. m2) and least (deployment 35: 1061 ind. m2) densely populated sites across the whole Strait. Whilst elevated faunal density was observed in the immediate vicinity of a patch of siboglinids (deployment 25), mean density was similar between non-hydrothermally active sites (5180 ind. m2, S.E. ± 580, n = 12) and Hook Ridge (4150 ind. m2, S.E. ± 1077, n = 9) (Wilcoxon rank sum test, W = 25, p = 0.08) (Figure 3).
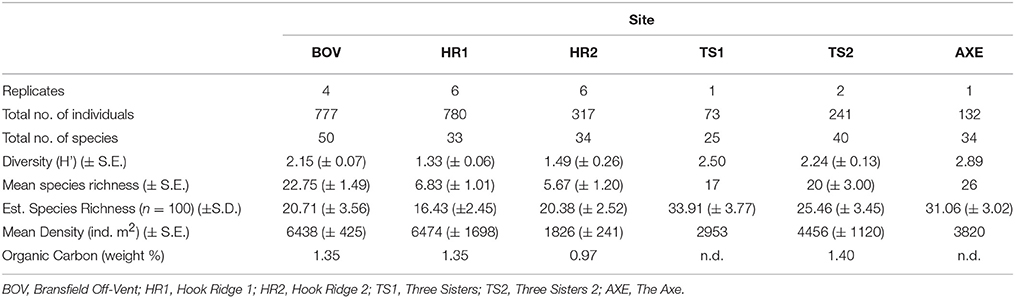
Table 2. Species diversity and mean and estimated richness (for species-level discriminated fauna only), total density and organic carbon levels in the Bransfield Strait.
Species Richness and Diversity
Individual-based estimates of species richness, grouped by site, showed that the Three Sisters and the Axe were the most species rich sites and that Hook Ridge 1 was the least species rich (Table 2). The Hook Ridge sites had the lowest mean diversity, consistent with a higher proportion of endemic species and a more reducing environment. Mean shared species between sites was 53% with the highest being between the two Hook Ridge sites (82%). The non-Hook Ridge sites shared between 18–77% of their species with the lowest shared species between the control site BOV and Three Sisters 1 (18%).
Vent Endemic Species
Siboglinid polychaetes were found at Hook Ridge 1 (S. contortum) and the control site, Three Sisters and the Axe (Siboglinum sp.). Complete specimens of both siboglinid species never co-occurred (Data Sheet S1) but fragments (sections of tube, with or without body tissue fragments) of S. contortum were found at all sites. Siboglinids occurred in low abundances (0–71 individuals with heads per deployment) and were never the dominant functional group at any site (0–14% of total abundance per site; Figure 2). Complete specimens of S. contortum were counted in two deployments from Hook Ridge 1 only (HR1: 21 and 25, 1 and 70 ind., respectively) and ranged in abundance between 32 and 4520 ind. m2, comprising 14% of total faunal abundance at Hook Ridge 1. Owing to the high proportion of S. contortum at Hook Ridge 1 deployment 25 (31% of abundance compared with 0–1% elsewhere), this deployment was distinct from all others (SIMPROF, p = 0.05) (Figure 3). The patchy or limited distribution of S. contortum meant that replicates within the same site showed considerable variation in proportions of vent endemic species. Siboglinum sp. occurred in its highest abundance at the control site (2% of fauna, 64–159 ind. m2).
Environmental Drivers of Community Composition
The HI was elevated at both Hook Ridge sites (particularly site 2), relative to other areas of the Bransfield Strait (Table 3). HI was highest in downcore sections (5–10 cm below seafloor (b.s.f)) from Hook Ridge (0.44–4.02) and was always higher than in upper cores (0–5 cm b.s.f.; Table 3). HI was greater at Hook Ridge 2 than Hook Ridge 1 and Hook Ridge 1 upper partition had comparable levels of HI to off-vent sites. Mean diversity, species richness and density was fitted to values of HI for each site (1 or 2 partitions per site, Table 4), using generalized linear models. Mean diversity, species richness and density all significantly declined with increasing HI (GLM: p < 0.05, R2 0.38– 0.52; Figure 2, 4, Table 4). Ecological distance was also consistent with environmental distance (PERMANOVA: p < 0.001).
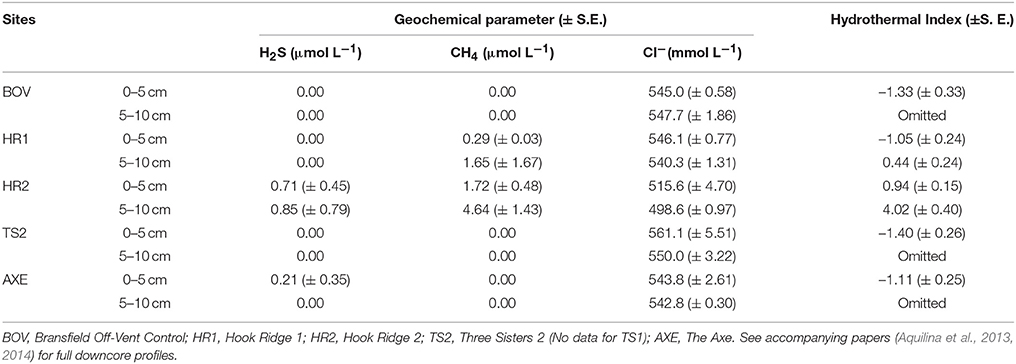
Table 3. Mean concentrations of four geochemical parameters (binned into upper and lower partitions) used to calculate the hydrothermal index (Data from Aquilina et al., 2013, 2014).

Table 4. Results of generalized linear models for various biotic measures with hydrothermal index (HI).
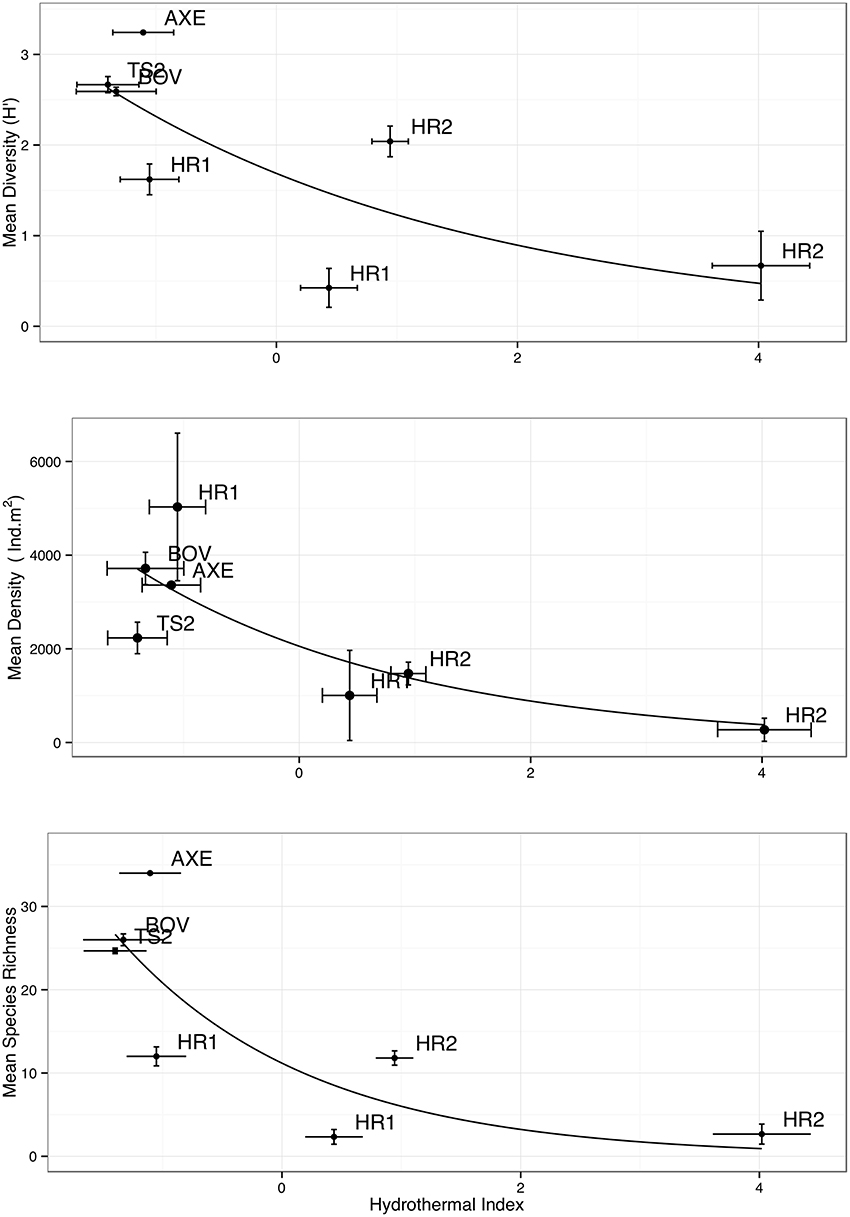
Figure 4. Comparison of “hydrothermal index” with mean diversity, abundance (m2), and species richness (using data from partitioned cores). Higher HI indicates greater levels of hydrothermal activity. Error bars denote ± 1 S.E.
Discussion
Controls on Faunal Diversity
Diversity was lower at hydrothermally active sites and declined exponentially toward the greatest levels of HI (Figure 4; Table 4), owing to the increased environmental toxicity creating unfavorable conditions for the majority of local fauna and the general absence of vent-endemic species. Whilst diversity was influenced by environmental distance (p < 0.05, Table 4), the relationship observed differed from the Bernardino et al. (2012) model in that it was maximal under background conditions and decreased toward greater hydrothermal influence, rather than maximal diversity occurring in areas of intermediate hydrothermal activity (Figure 4). The conceptual model of Bernardino et al. (2012) does not encompass the high biodiversity typical of deep-sea macrofauna, resulting in a disagreement with our observations (Figure 5). Therefore we accept hypothesis 2 and suggest that the proposed increase and subsequent decrease of diversity (Figure 5) with increasing hydrothermal influence (Bernardino et al., 2012) does not capture the high background biodiversity common in the deep-sea (Rex and Etter, 2010; Chown, 2012; Figure 5).
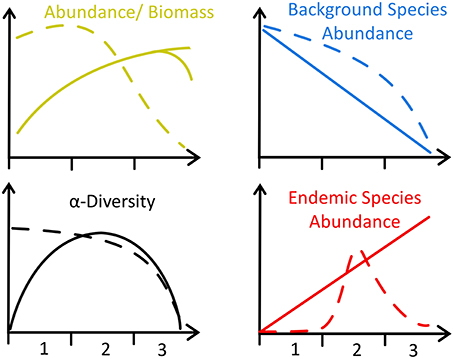
Figure 5. Schematic of four faunal trends in diffuse hydrothermal vents, adapted from Bernardino et al. (2012) (solid lines) to include Southern Ocean data (dashed lines). Axes labels refer to three distinct regions of the Bransfield Strait identified by this study with reference to previous work (Sahling et al., 2005; Aquilina et al., 2013): 1 = No hydrothermal activity (i.e., Control, Three Sisters and the Axe), 2 = Moderate hydrothermal activity (i.e., Hook Ridge 1, optimal S. contortum habitat), and 3 = Higher hydrothermal activity, unsuitable for most fauna.
Species accumulation curves from the Bransfield Strait, elsewhere in the Southern Ocean and Pacific SHV (Grassle and Petrecca, 1994; Flach and Heip, 1996; Levin et al., 2009; Neal et al., 2011) show that species richness was higher in background/ inactive sediments than at active vents (Figure 6), which is consistent with lower diversity at active vents (Figure 6). The Bransfield Strait SHV was generally more species rich than vents from the Manus Basin and comparable to NE Pacific vents at Middle Valley (Figure 6; Levin et al., 2009). The SHV at Middle Valley (2406–2411 m) is notably deeper than either the Bransfield vents (1054–1320 m) or the Manus Basin (1430–1634 m), suggesting that depth does not exert a consistent effect upon species richness, or abundance (Levin et al., 2009). Around the high temperature vents at the East Pacific Rise however, macrofaunal species richness peaked at intermediate levels of environmental stress (Gollner et al., 2015). This suggests that relationships between environmental gradients and macrofaunal communities may differ between different types of hydrothermal vent. At high temperature vents, meiofaunal taxa showed a similar pattern in species richness to that present here (Gollner et al., 2015), illustrating differences between taxa and environmental settings. Species richness was also generally lower in the Bransfield strait when compared with the West Antarctic shelf seas (Figure 6; Neal et al., 2011) although it should be noted that these samples were from sites ~500 m shallower and measure only polychaete species richness.
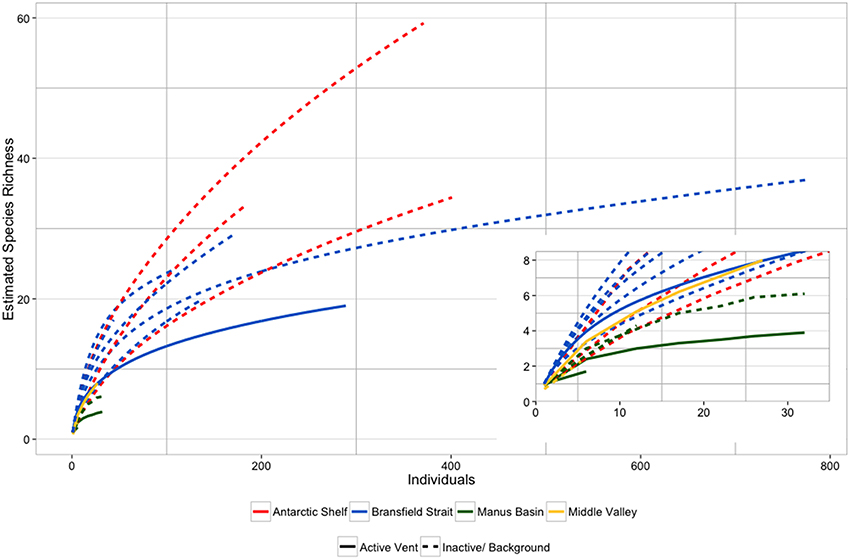
Figure 6. Comparative individual-based species accumulation curves between Bransfield Strait sites (this study) and literature values for other diffuse hydrothermal vents (Grassle et al., 1985; Levin et al., 2009) and typical bathyal sediments of the West Antarctic Peninsula (Neal et al., 2011). Data from the West Antarctic Peninsula are for polychaete species richness only.
Controls on Faunal Density
Macrofaunal density declined toward areas of highest hydrothermal advection (Figures 4, 6). Thus our data did not support our hypothesis that hydrothermal vents support increased faunal density in the Bransfield Strait (hypothesis 1). Mean density was higher at non-hydrothermally active sites in the Bransfield Strait than at Hook Ridge (Figure 3), driven mainly by the low faunal density at Hook Ridge 2, the site with the highest fluid advection rates (Aquilina et al., 2013). Environmental stress at Hook Ridge 2 may explain the low faunal abundance (Sahling et al., 2005), presumably reflecting the increased stress associated with elevated temperature or environmental toxicity (Figure 4). Bacterial mats were present at Hook Ridge 2 (Aquilina et al., 2013) but no complete specimens of vent endemic species were found.
It is likely that the relationship in density between SHV and local background areas depends upon toxicity at the vents (Levin et al., 2013), the amount of in-situ primary productivity and the availability of other sources of OM. In previous studies of comparable systems, macrofaunal density was not consistently enriched by the presence of hydrothermal flux, and has been observed to be both higher and lower than nearby inactive or background sediments (Grassle et al., 1985; Levin et al., 2009; Figure 7). Temperate or Polar latitude deep-sea SHV (a.k.a. “Hot muds”) with relatively high surface primary productivity (SPP) (Middle Valley or Bransfield Strait) tend to have lower densities than in nearby background sediments (Grassle et al., 1985; Levin et al., 2009), but where SPP is lower (e.g., Manus basin), density may be higher at SHV (Grassle et al., 1985; Levin et al., 2009; Figure 7). Export production and surface primary productivity is high in the Bransfield Strait (Wefer and Fischer, 1991; Kim et al., 2005), thus any extra chemosynthetic OM production may not be as significant to local fauna compared with deeper vents, or vents in less productive seas (Tarasov et al., 2005; Levin et al., 2009).
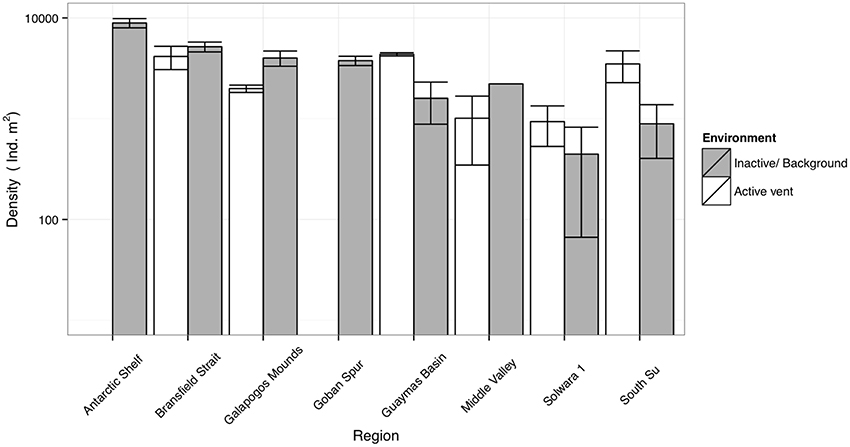
Figure 7. Comparative macrofaunal densities between Bransfield Strait sites (this study) and literature values for other diffuse hydrothermal vents (Grassle et al., 1985; Levin et al., 2009) and typical bathyal sediments of the West Antarctic Peninsula (polychaetes only; Neal et al., 2011) and Goban spur, NE Atlantic (Flach and Heip, 1996). Error bars denote ± 1 S.E.
Whilst these data do not fit Bernardino et al. (2012)'s model (Figure 5), we suggest that trends in density are much more dependent upon regional factors [e.g., export production or temperature sensitivity of local benthos (Clarke et al., 2009)]. The scope for regional variation means that for other systems, where SPP is lower (e.g., Manus Basin) or in-situ production higher (e.g., Middle Valley Clam beds), the existing model applies (Levin et al., 2009; Bernardino et al., 2012). Local OM production rates are also clearly important and areas of dense populations of vent-endemic megafauna (e.g., Clam beds at Middle Valley) can also enrich local macrofaunal density (Levin et al., 2009) but such habitats were not observed in the Bransfield Strait. At the basin scale, our observations do not agree with the model of increasing macrofaunal abundance toward vent (Figure 5), but at small scales (i.e., between Hook Ridge 1 replicates) there is a potential increase in faunal abundance and biomass associated with moderate levels of hydrothermal activity.
Deployment 25, from Hook Ridge 1, had both the highest vent-endemic and total faunal density. This suggests that chemosynthetic trophic support may have been enhancing local density and biomass, particularly for scavengers like crustaceans. However, the effect was clearly quite spatially limited as other sites at Hook Ridge had a much lower faunal abundance, suggesting that in situ OM production was patchily distributed at a scale of 10 s of meters (the estimated likely seabed separation of replicate deployments). This is consistent with sediment-hosted vents in the Arctic Ocean, which found limited macrofaunal utilization of chemosynthetically-derived OM, even within sediment-hosted vent fields (Sweetman et al., 2013). This kind of fine scale structuring of communities is consistent with other SHV (Bernardino et al., 2012). Elsewhere at Hook Ridge (particularly Hook Ridge 2), abundance per deployment was lower or similar to non-vent sites (Table 3), leading to the increase in abundance observed (Figure 4).
Levels of organic carbon in surface sediment measured from Bransfield Strait sites (Yoon et al., 1994; Aquilina et al., 2014; Table 2) showed that Hook Ridge (0.25–1.35 wt% organic C) was comparable, to the control site (0.75–1.35 wt% organic C) and the Three Sisters (1.40 wt% organic C, Table 2), which suggests that chemosynthetic activity was not augmenting available organic matter. It is possible that the relatively high density of scavengers at Hook Ridge 1 increased turnover rates of available organic matter (Rowe et al., 1990), potentially masking local OM production. However, this should have resulted in increased macrofaunal biomass at Hook Ridge, which was not observed in either annelids or peracarids (Data Sheet S2). It is also possible that additional organic carbon produced at Hook Ridge 1 is largely retained in Sclerolinum tissue rather than being released into the sediment. It might be expected that the local OM production at SHV could enrich biomass, through increased food availability (Bernardino et al., 2012). However, in the Bransfield Strait, it seems that environmental conditions at active vents deter background fauna or that local production of chemosynthetically derived organic matter is insufficient to encourage interaction between endemic and background species (or both). Bottom water temperature in the Bransfield Strait is around −1.5°C (Clarke et al., 2009), compared with estimates of temperature at Hook Ridge [~24–48°C (Dählmann et al., 2001; Petersen et al., 2011)] This may indicate that temperature variation at Southern Ocean SHVs is more significant than in other areas of the deep-sea. Another factor that may structure Bransfield vent communities is the relative isolation of the Southern ocean caused by the Antarctic Circumpolar Current (ACC; Clarke et al., 2009; Neal et al., 2011). The ACC may have resulted in an impoverished community of vent-obligate or vent-tolerant fauna, relative to other SHVs where high temperature vents are in close proximity (e.g., Guaymas or Middle Valley).
Siboglinid Autecology
Although Hook Ridge 2 had the greatest hydrothermal advection rates (Aquilina et al., 2013), available sulfide was probably too low to support populations of S. contortum (Sahling et al., 2005) and was much lower than at Hook Ridge 1 [0–6 and 0–160 μmol L−1 respectively, (Aquilina et al., 2013)]. Sulfide concentrations at both sites were low for the typical range of sulfide concentrations expected at sediment-hosted vents [1.5–8 mM (Bernardino et al., 2012)]. Patterns in S. contortum density is consistent with Sahling et al. (2005) who concluded that S. contortum favored areas of sulfide concentration of around 100–150 μmol L−1, relatively low for SHV, and that in areas of higher hydrothermal influence, higher temperatures or the formation of siliceous surface crusts (Dählmann et al., 2001; Klinkhammer et al., 2001) made conditions unsuitable. There were no complete specimens (i.e., specimens with heads) of S. contortum or any other vent endemic species at Hook Ridge 2, but there were densities of S. contortum fragments similar to those at Hook Ridge 1 (0–1401 ind. m2). Video imagery also revealed patches of bacterial mat across Hook Ridge suggesting that there was at least patchy chemosynthetic activity across a wide area (Aquilina et al., 2013; Georgieva et al., 2015). It is probable that the differences in density of S. contortum between the two sites is either driven by sulfide availability (Aquilina et al., 2013), or temperature (Sahling et al., 2005), or both.
Ecological and geochemical (Aquilina et al., 2013) observations from across the Bransfield Strait support dividing the basin into three sub-regions, previously identified around Hook Ridge by Sahling et al. (2005) based on differences in S. contortum density, hydrothermal advection and sulfide availability, in support of hypothesis 3 (Figure 5): (1) background areas of no hydrothermal activity (e.g., Control); (2) areas of moderate hydrothermal activity supporting populations of S. contortum (Hook Ridge 1); and (3) areas of higher hydrothermal activity with no vent endemic fauna (Hook Ridge 2). Siliceous crusts were observed in areas of higher hydrothermal advection at Hook Ridge (Sahling et al., 2005; Aquilina et al., 2013) corresponding to, or possibly resulting in, areas of absence of S. contortum (Sahling et al., 2005, This Study). However, sulfide availability was quite variable at areas of siliceous crust, ranging between ~120 and 330 μmol L−1 (Sahling et al., 2005) and 0–6 μmol L−1 (Aquilina et al., 2013). Sulfide may either have been limiting (Aquilina et al., 2013) or prohibitively toxic (Sahling et al., 2005) in areas of higher hydrothermal activity and is likely crucial in structuring S. contortum populations. The apparently limited habitat niche favored by S. contortum, and the lack of any other vent endemic species (e.g., vesicomyid clams) conflicts with the Bernardino et al. model suggestion (Bernardino et al., 2012) that the proportion of vent endemic species should increase toward venting sites (Figure 5). This likely reflects a combination between the physiological constraints of S. contortum and the absence of a wider diversity of vent endemic species, illustrating the oceanographic and physiological restrictions that underpin communities of Southern Ocean benthos (Clarke et al., 2009; Chown, 2012).
Based on previous estimates of S. contortum density at Hook Ridge of up to 800 ind. m2 (Sahling et al., 2005), it was suggested that S. contortum tubes act as conduits, significantly increasing supply of dissolved iron and manganese to overlying waters by allowing them to bypass oxic sediment and thus avoid precipitation (Aquilina et al., 2014). S. contortum density in this study (up to 4520 ind. m2) was much higher than previous estimates (Sahling et al., 2005), demonstrating that population size varies widely in time and or space. Our observations suggest that the capacity of S. contortum to support pore fluid to bottom water exchange of dissolved metals may be more than originally estimated (Aquilina et al., 2014). Empty tubes may also facilitate transfer as suggested (Aquilina et al., 2014), and these were certainly far more numerous than complete specimens (up to 19 226 frag. m2). However, empty tubes commonly contained tissue fragments or sediment or were much shorter than complete specimens, potentially reducing their capacity to support fluid exchange. It is also unclear how long these fragments last or how quickly they are produced.
The presence of Siboglinum sp. at the non-Hook Ridge sites may suggest that these areas could have hosted some chemosynthetic activity (Juniper et al., 1992; Dando et al., 1994; Thornhill et al., 2008). Siboglinum fjordicum shows a similar endosymbiont composition to that of Oligobrachia mashikoi, a putative methanotroph (Thornhill et al., 2008; Cordes et al., 2010). However, Siboglinum spp. are also known from non-chemosynthetic sediments, potentially subsisting on dissolved organic matter (George, 1976; Southward et al., 1979; Shields and Blanco-Perez, 2013) and so are not necessarily indicative of active chemosynthesis. Methane was detected in sediment cores from the control site [deeper than 10cm b.s.f. (Aquilina et al., 2013)], which had the greatest abundance of Siboglinum and was not hydrothermally influenced. It is therefore suggested that Siboglinum sp. at the control site were utilizing thermogenic hydrocarbons, such as those observed elsewhere in Bransfield Strait sediments (Whiticar and Suess, 1990). It is likely that the production rate of these hydrocarbons is increased by the heat flux associated with hydrothermal activity (Whiticar and Suess, 1990), illustrating a potential indirect dependence upon hydrothermalism amongst some fauna. However, methane was not detected at the Three Sisters, which also hosted a small Siboglinum sp. population, suggesting that other OM sources are also possible.
Hydrothermal Activity
The hydrothermal index echoed geochemical variability (Aquilina et al., 2013, 2014) and treated environmental variability as coherent, rather than a series of isolated parameters (Sahling et al., 2005) permitting quantitative assessment of faunal responses (Dauwe and Middelburg, 1998; Brown et al., 2010). Ordination techniques were used to select model parameters but the spatial resolution of the environmental data was limited and did not facilitate the use of more established ordination analysis (e.g., BIO-ENV Clarke and Ainsworth, 1993). However, given the ship-time cost and pressure for large-scale environmental surveys of the deep sea to establish reference baselines to monitor anthropogenic and climate related changes in community composition, applied analytical techniques need to be developed where concurrent environmental sampling is poor. We propose that this approach, which can be adapted to any marine or aquatic habitat, can provide and support insights into establishing the influence of environmental gradients on faunal distribution. Here, we use it to test the conceptual models of Bernardino et al. (2012) and identify previously unknown trends in abundance and diversity that may be subject to regional as well as environmental factors.
One parameter missing from HI is temperature, for which data were unavailable. In the Southern Ocean, where benthos are relatively sensitive to temperature fluctuations (Barnes and Peck, 2008; Clarke et al., 2009), it is likely that the relatively hot conditions presented by SHVs represented a significant deterrent to background fauna. However, in Guaymas basin macrofaunal assemblages, concentrations of sulfide and methane were found to be drivers of compositional differences and temperature was not a significant factor (Portail et al., 2015). In the absence of temperature data, chlorinity profiles were used. Chloride depletion has been previously used as a proxy for the relative amounts of hydrothermal fluid present (Aquilina et al., 2013) and was considered proportional to temperature (Ginsburg et al., 1999). There are estimates of seafloor temperature at Hook Ridge from previous studies, of 25–48.5°C (Bohrmann et al., 1998; Dählmann et al., 2001) but downcore temperature profiles have not been measured. Although chlorinity is believed an acceptable proxy (Ginsburg et al., 1999; Aquilina et al., 2013), future multi-parameter indices for hydrothermal activity in SHV should consider incorporating temperature if possible.
Controls on Faunal Distribution and Community Structure
Environmental distance, represented by the changing influence of hydrothermal activity (Aquilina et al., 2013), clearly influenced assemblage structure. Diversity, abundance, and species richness all declined (GLM: p < 0.05; < 0.001; < 0.01, respectively) toward the area of highest hydrothermal flux (Figure 4; Table 4). This demonstrates a clear response of faunal assemblages to environmental stress and habitat partitioning, being more similar in regions of similar environmental conditions (Bernardino et al., 2012), rather than simple structuring in response to dispersal ability.
Since non-hydrothermally active sites did not exhibit strong variation in the selected geochemical parameters between upper and lower partitions, faunal variation downcore at non-vent sites was not relevant to the amount of hydrothermal flux. Therefore, to separate the influence of hydrothermalism from expected downcore variability, only data for upper partitions were used from non-vent sites (Table 3). It should be noted however that expected gradients, particularly in abundance, between upper and lower partitions will have contributed some of the model fit in a manner consistent with non-hydrothermally influenced sites.
The relatively rapid changes at HI levels of < 0 (indicated by the increased gradient, Figure 4) suggests a fairly narrow transition zone between background or vent-tolerant assemblages and those that favor hydrothermal conditions (Figure 4), with relatively little overlap in dominant taxa (Figure 2). Zonation in faunal distribution at hydrothermal vents is common, often associated with competition, environmental distance and availability of biogenic habitat (Levin et al., 2009, 2012; Bernardino et al., 2012; Marsh et al., 2012) and is analogous to more general disturbance gradients (Pearson and Rosenberg, 1978).
Proportions of different functional groups varied significantly between sites suggesting that niche variation, rather than geographical separation, was the dominant driver in the compositional differences observed (Nekola and White, 1999; McClain et al., 2012). For example, the motile deposit feeder Aricidea antarctica (Annelida, Paraonidae) occurred at all sites and was highly abundant at the control site but only minimally abundant elsewhere. Similarly, sessile deposit feeding terebellids (Polycirrus sp.) occurred in much higher relative density at Hook Ridge 2 (15% of abundance) than elsewhere in the Strait (0–5%). Polycirrus medusa (Annelida, Terebellidae, Grube, 1850) is known from a sediment-hosted vent in the Arctic (Sweetman et al., 2013), and “Ampharetid beds” are not uncommon in vent or seep environments (Bernardino et al., 2012), consistent with the relatively high density and biomass of terebellids at Hook Ridge 2. Patterns in terebellomorph polychaete at SHVs may represent similar mechanisms driving assemblage structure and habitat selection such as competition or tolerance to toxic environments. The majority (81%) of peracarids were found at Hook Ridge, demonstrating a clear preference to hydrothermally active sites, in comparison to annelids, for which only 19% of individuals counted were found at Hook Ridge sites. Hydrothermally-influenced sites were more suitable for scavengers and sessile deposit feeders, whilst sites without hydrothermal influence were more favored by motile deposit feeders.
The fact that many of the more numerous taxa were found at all sites demonstrates that their distribution was not limited by dispersal ability. Communities in the Bransfield Strait had a much higher proportion of species conserved across the basin than in other bathyal settings. For example, macroinvertebrates from the Monterey Canyon (McClain et al., 2011) experienced a reduction in shared species of 50% over ~7.5 km, compared to around 100 km in this study (classic Sørenson distance, presence-absence data). The relatively high conservation of species throughout the basin coupled with the changes in community structure observed between sites, further suggests that functional diversity, rather than dispersal ability, was very important in structuring local assemblages.
Overall, Pacific and Southern Ocean sedimented vents share a number of higher taxa (e.g., percarids isopods and tanaids) and polychaetes (terebellomorph and nereidid) (Grassle et al., 1985; Levin et al., 2009), but their relative abundances are quite variable. Pacific vent communities are distinct between eastern and western sites (Bernardino et al., 2012), in part reflecting geographic isolation. Eastern Pacific vents had a comparatively low abundance of peracarid crustaceans (relative to western Pacific and Bransfield Strait SHV) and tended to be more dominated by polychaete species characteristic of reducing environments (e.g., dorvilleids; Grassle et al., 1985; Grassle and Petrecca, 1994; Levin et al., 2009). Vent endemic fauna at sediment-hosted vents at the Chile Triple Junction (CTJ) (Thurber et al., unpublished data, cited in Levin et al., 2012) were also only represented by siboglinid polychaetes, as with the Bransfield Strait vents and it is likely, given their proximity, that the Bransfield vents are most similar to vents at the CTJ. Macrofauna from shallower sites around the South Shetland Islands were also numerically dominated by polychaetes, as with non-Hook Ridge areas of the Bransfield Strait, and structured in part by habitat (substratum) availability (Arnaud et al., 1998). These observations are semi-quantitative however, and include a wider range of size classes than analyzed here so were not directly comparable.
Conclusions
In this first community-ecology study of a sediment-hosted hydrothermal vent in the Southern Ocean we have shown that sediment-hosted hydrothermalism strongly influences infaunal communities in the deep Bransfield Strait. We assessed variation in macrofaunal communities along environmental distance gradients. Populations of vent endemic fauna, represented by a single species, had limited distribution but changes in macrofaunal community structure were still clearly evident between active vent and background sediments. The distribution of S. contortum, relative to sediment geochemistry, was consistent with previous observations (Sahling et al., 2005), supporting our hypothesis. Diversity, species richness, and density were all lower at hydrothermally active sites than in background sediments, confirming hypothesis two but rejecting hypothesis one we have identified several ways in which faunal trends for Bransfield Strait SHVs differ from generalized trends derived from other SHVs (Bernardino et al., 2012). Potential factors driving these differences include: physiological stress (Levin et al., 2013); other sources of OM (Wefer and Fischer, 1991; Palmer and Totterdell, 2001; Kim et al., 2005) undermining the significance of locally-produced OM, and sensitivity to environmental conditions of Southern Ocean benthos (Barnes and Peck, 2008).
Author Contributions
The authors have made the following declarations about their contributions. Conceived, designed and executed sampling programme: AG, CW, WR, and CS. Data collection and taxonomy: JB, AG, and CL. Performed analyses: JB and LB, Designed Hydrothermal Index: JB, LB, and CW. Literature review: JB. Wrote the paper: JB, CW. Prepared figures: JB. Commented on the paper: JB, CW, LB, CL, CS, WR, and AG.
Funding
JB was funded by a (NERC) Ph.D. Studentship (NE/L501542/1). This work was also funded by the NERC ChEsSo consortium (Chemosynthetically-driven Ecosystems South of the Polar Front, NERC Grant NE/DOI249X/I).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the Master and Crew of RRS James Cook cruise 055 for technical support and the Cruise Principal Scientific Officer Professor Paul Tyler. We gratefully acknowledge taxonomic contributions from P. Graham Oliver and Christer Erséus and geochemical data and insight provided by Laura Hepburn, Alfred Aquilina and Rachel Mills.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2016.00032
Data Sheet S1. Macrofaunal abundance by taxa (± S.E.) and site.
Data Sheet S2. Macrofaunal biomass (dry weight) of select taxa, compared between sites.
Data Sheet S3. Geochemical data used to construct the hydrothermal index.
References
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x
Aquilina, A., Connelly, D. P., Copley, J. T., Green, D. R., Hawkes, J. A., Hepburn, L., et al. (2013). Geochemical and visual indicators of hydrothermal fluid flow through a sediment-hosted volcanic ridge in the Central Bransfield Basin (Antarctica). PLoS ONE 8:e54686. doi: 10.1371/journal.pone.0054686
Aquilina, A., Homoky, W. B., Hawkes, J. A., Lyons, T. W., and Mills, R. A. (2014). Hydrothermal sediments are a source of water column Fe and Mn in the Bransfield Strait, Antarctica. Geochim. Cosmochim. Acta 137, 64–80. doi: 10.1016/j.gca.2014.04.003
Arnaud, P. M., López, C. M., Olaso, I., Ramil, F., Ramos-Esplá, A. A., and Ramos, A. (1998). Semi-quantitative study of macrobenthic fauna in the region of the South Shetland Islands and the Antarctica Peninsula. Polar Biol. 19, 160–166. doi: 10.1007/s003000050229
Barnard, J. L., and Karaman, G. S. (1991). The families and genera of marine gammaridean Amphipoda (except marine gammaroids). Part 2. Rec. Aust. Mus. Suppl. 13, 419–866. doi: 10.3853/j.0812-7387.13.1991.367
Barnes, D. K. A., and Peck, L. S. (2008). Vulnerability of Antarctic shelf biodiversity to predicted regional warming. Clim. Res. 37, 149–163. doi: 10.3354/cr00760
Beesley, P. L., Ross, G. J. B., and Glasby, C. (2000). Polychaetes & Allies: the Southern Synthesis. Fauna of Australia Vol. 4A Polychaeta, Myzostomida, Pogonophora, Echiura, Spincula. Melbourne, VIC: CSIRO.
Bemis, K., Lowell, R., and Farough, A. (2012). Diffuse flow on and around hydrothermal vents at mid-ocean Ridges. Oceanography 25, 182–191. doi: 10.5670/oceanog.2012.16
Bernardino, A. F., Levin, L. A., Thurber, A. R., and Smith, C. R. (2012). Comparative composition, diversity and trophic ecology of sediment Macrofauna at Vents, seeps and organic falls. PLoS ONE 7:e33515. doi: 10.1371/journal.pone.0033515
Bohrmann, G., Chin, C., Petersen, S., Sahling, H., Schwarz-Schampera, U., Greinert, J., et al. (1998). Hydrothermal activity at Hook Ridge in the Central Bransfield Basin, Antarctica. Geo Mar. Lett. 18, 277–284. doi: 10.1007/s003670050080
Brown, L. E., Milner, A. M., and Hannah, D. M. (2010). Predicting river ecosystem response to glacial meltwater dynamics: a case study of quantitative water sourcing and glaciality index approaches. Aquat. Sci. 72, 325–334. doi: 10.1007/s00027-010-0138-7
Chao, A., Chazdon, R. L., Colwell, R. K., and Shen, T.-J. (2005). A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 8, 148–159. doi: 10.1111/j.1461-0248.2004.00707.x
Chown, S. L. (2012). Antarctic Marine biodiversity and deep-sea Hydrothermal Vents. PLoS Biol. 10:e1001232. doi: 10.1371/journal.pbio.1001232
Clarke, A., and Crame, J. A. (1992). The Southern Ocean Benthic Fauna and climate change: a historical perspective. Philos. Trans. R. Soc. B Biol. Sci. 338, 299–309. doi: 10.1098/rstb.1992.0150
Clarke, A., and Crame, J. A. (2010). Evolutionary dynamics at high latitudes: speciation and extinction in polar marine faunas. Philos. Trans. R. Soc. B Biol. Sci. 365, 3655–3666. doi: 10.1098/rstb.2010.0270
Clarke, A., Griffiths, H. J., Barnes, D. K. A., Meredith, M. P., and Grant, S. M. (2009). Spatial variation in seabed temperatures in the Southern Ocean: implications for benthic ecology and biogeography. J. Geophys. Res. 114:G03003. doi: 10.1029/2008JG000886
Clarke, K. R., and Ainsworth, M. (1993). A method of linking multivariate community structure to environmental variables. Mar. Ecol. Prog. Ser. 92, 205–219. doi: 10.3354/meps092205
Clarke, K. R., Somerfield, P. J., and Chapman, M. G. (2006). On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray–Curtis coefficient for denuded assemblages. J. Exp. Mar. Biol. Ecol. 330, 55–80. doi: 10.1016/j.jembe.2005.12.017
Clarke, K. R., Somerfield, P. J., and Gorley, R. N. (2008). Testing of null hypotheses in exploratory community analyses: similarity profiles and biota-environment linkage. J. Exp. Mar. Biol. Ecol. 366, 56–69. doi: 10.1016/j.jembe.2008.07.009
Colwell, R. K., Chao, A., Gotelli, N. J., Lin, S. Y., Mao, C. X., Chazdon, R. L., et al. (2012). Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J. Plant Ecol. 5, 3–21. doi: 10.1093/jpe/rtr044
Colwell, R. K. (2013). EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 9. Available online at: http://purl.oclc.org/estimates
Connell, J. H. (1978). Diversity in tropical rain forests and coral reefs. Science 199, 1302–1310. doi: 10.1126/science.199.4335.1302
Cordes, E. E., Becker, E. L., Hourdez, S., and Fisher, C. R. (2010). Influence of foundation species, depth, and location on diversity and community composition at Gulf of Mexico lower-slope cold seeps. Deep Sea Res. Part II Top. Stud. Oceanogr. 57, 1870–1881. doi: 10.1016/j.dsr2.2010.05.010
Dählmann, A., Wallman, K., Sahling, H., Sarthou, G., Bohrmann, G., Petersen, S., et al. (2001). Hot vents in an ice-cold ocean: indications for phase separation at the southernmost area of hydrothermal activity, Bransfield Strait, Antarctica. Earth Planet. Sci. Lett. 193, 381–394. doi: 10.1016/S0012-821X(01)00535-0
Dando, P. R., Bussmann, I., Niven, S. J., O'Hara, S. C. M., Schmaljohann, R., and Taylor, L. J. (1994). A methane seep area in the Skagerrak, the habitat of the pogonophore Siboglinum poseidoni and the bivalve mollusc Thyasira sarsi. Mar. Ecol. Prog. Ser. 107, 157–167. doi: 10.3354/meps107157
Dauwe, B., and Middelburg, J. J. (1998). Amino acids and hexsoamines as indicators of organic matter degradation state in North Sea sediments. Limnol. Oceanogr. 43, 782–798. doi: 10.4319/lo.1998.43.5.0782
Dobson, A. J., and Barnett, A. (2008). An Introduction to Generalized Linear Models. Boca Raton, FL: Chapman & Hall.
Fauchald, K., and Jumars, P. A. (1979). The diet of worms: a study of polychaete feeding guilds. Oceanogr. Marine Biol. 17, 193–284.
Fauchald, K. (1977). The Polychaete Worms: Definitions and Keys to the Orders, Families and Genera. Natural History Museum of Los Angeles County.
Flach, E., and Heip, C. (1996). Vertical distribution of macrozoobenthos within the sediment on the continental slope of the Goban Spur area (NE Atlantic). Mar. Ecol. Prog. Ser. 141, 55–66. doi: 10.3354/meps141055
Fox, J., Weisberg, S., Adler, D., Bates, D., Baud-Bovy, G., Ellison, S. Core Team, et al. (2013). Companion to Applied Regression. Available online at: https://cran.r-project.org/
Gage, J. D., and Bett, B. J. (2005). “Deep-sea benthic sampling,” in Methods for the Study of the Marine Benthos, eds N. A. Holmes and A. D. McIntyre (Oxford: Blackwell Scientific Publications). doi: 10.1002/9781118542392
George, J. D. (1976). “Ecology of the Pogonophore, Siboglinum fiordicum Webb, in a shallow-water fjord community,” in Biology of Benthic Organisms: 11th European Symposium on Marine Biology, eds B. F. Keegan, P. J. S. Boaden, and P. O. Ceidigh (Galway: Elsevier).
Georgieva, M., Wiklund, H., Bell, J. B., Eilersten, M. H., Mills, R. A., Little, C. T. S., et al. (2015). A chemosynthetic weed: the tubeworm Sclerolinum contortum is a biploar, cosmopolitan species. BMC Evol. Biol. 15:280. doi: 10.1186/s12862-015-0559-y
Ginsburg, G. D., Milkov, A. V., Soloviev, V. A., Egorov, A. V., Cherkashev, G. A., Vogt, P. R., et al. (1999). Gas hydrate accumulation at the Håkon Mosby Mud Volcano. Geo Mar. Lett. 19, 57–67. doi: 10.1007/s003670050093
Gollner, S., Govenar, B., Fisher, C. R., and Bright, M. (2015). Size matters at deep-sea hydrothermal vents: different diversity and habitat fidelity patterns of meio- and macrofauna. Mar. Ecol. Prog. Ser. 520, 57–66. doi: 10.3354/meps11078
Gotelli, N. J., and Colwell, R. K. (2001). Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 4, 379–391. doi: 10.1046/j.1461-0248.2001.00230.x
Grassle, J. F., Brown-Leger, L. S., Morse-Porteous, L., Petrecca, R. F., and Williams, I. (1985). Deep-Sea Fauna of Sediments in the Vicinity of Hydrothermal Vents. Bull. Biol. Soc. Wash. 6, 443–452.
Grassle, J. F., and Petrecca, R. F. (1994). “Soft-sediment hydrothermal vent communities of Escanaba Trough,” in Geological, Hydrothermal, and Biological Studies at Escanaba Trough, Gorda Ridge, Offshore Northern California, eds R. A. Zierenberg and C. A. Reiss (Denver, CO: US Geological Survey), 327–335.
Hartmann-Schröder, G., and Rosenfeldt, P. (1988). Die Polychaeten der Polarstern-Reise ANT III/2 in die Antarktis 1984. Teil 1: Euphrosinidae bis Chaetopteridae, Vol. 85. Hamburg: Mitteilungen aus dem Hamburgischen zoologischen Museum und Institut.
Jumars, P. A., Dorgan, K. M., and Lindsay, S. M. (2015). Diet of worms emended: an update of polychaete feeding guilds. Ann. Rev. Mar. Sci. 7, 497–520. doi: 10.1146/annurev-marine-010814-020007
Juniper, S. K., Jonasson, I. R., Tunnicliffe, V., and Southward, A. J. (1992). Influence of tube building polychaete on hydrothermal chimney mineralization. Geology 20, 895–898.
Keuning, R., Schander, C., Kongsrud, J. A., and Willassen, E. (2011). Ecology of twelve species of Thyasiridae (Mollusca: Bivalvia). Mar. Pollut. Bull. 62, 786–791. doi: 10.1016/j.marpolbul.2011.01.004
Kharlamenko, V. I., Zhukova, N. V., Khotimchenko, S. V., Svetashev, V. I., and Kamenev, G. M. (1995). Fatty-acids as markers of food sources in a shallow-water hydrothermal ecosystem (Kraternaya Bight, Yankich island, Kurile Islands). Mar. Ecol. Prog. Ser. 120, 231–241. doi: 10.3354/meps120231
Kilgallen, N. H. (2007). Taxonomy of the Lysianassoidea of the Northeast Alantic and Mediterranean: An Interactive Identification Key and Studies on Problematic Groups. Ph.D. Thesis, Galway-Mayo Institute of Technology, Galway.
Kim, D., Kim, D.-Y., Park, J.-S., and Kim, Y.-J. (2005). Interannual variation of particle fluxes in the eastern Bransfield Strait, Antarctica: a response to the sea ice distribution. Deep Sea Res. Part I Oceanogr. Res. Pap. 52, 2140–2155. doi: 10.1016/j.dsr.2005.06.008
Klinkhammer, G. P., Chin, C. S., Keller, R. A., Dahlmann, A., Sahling, H., Sarthou, G., et al. (2001). Discovery of new hydrothermal vent sites in Bransfield Strait, Antarctica. Earth Planet. Sci. Lett. 193, 395–407. doi: 10.1016/S0012-821X(01)00536-2
Larson, B. I., Houghton, J. L., Lowell, R. P., Farough, A., and Meile, C. D. (2015). Subsurface conditions in hydrothermal vents inferred from diffuse flow composition, and models of reaction and transport. Earth Planet. Sci. Lett. 424, 245–255. doi: 10.1016/j.epsl.2015.05.033
Levin, L. A., Mendoza, G. F., Konotchick, T., and Lee, R. (2009). Macrobenthos community structure and trophic relationships within active and inactive Pacific hydrothermal sediments. Deep Sea Res. Part II Top. Stud. Oceanogr. 56, 1632–1648. doi: 10.1016/j.dsr2.2009.05.010
Levin, L. A., Orphan, V. J., Rouse, G. W., Rathburn, A. E., Ussler, W. III, Cook, G. S., et al. (2012). A hydrothermal seep on the Costa Rica margin: middle ground in a continuum of reducing ecosystems. Proc. R. Soc. B Biol. Sci. 279, 2580–2588. doi: 10.1098/rspb.2012.0205
Levin, L. A., Ziebis, W., Mendoza, G. F., Bertics, V. J., Washington, T., Gonzalez, J., et al. (2013). Ecological release and niche partitioning under stress: lessons from dorvilleid polychaetes in sulfidic sediments at methane seeps. Deep Sea Res. Part II Top. Stud. Oceanogr. 92, 214–233. doi: 10.1016/j.dsr2.2013.02.006
Marsh, L., Copley, J. T., Huvenne, V. A. I., Linse, K., Reid, W. D. K., Rogers, A. D., et al. (2012). Microdistribution of Faunal Assemblages at deep-sea hydrothermal vents in the Southern Ocean. PLoS ONE 7:e48348. doi: 10.1371/journal.pone.0048348
McClain, C. R., Nekola, J. C., Kuhnz, L., and Barry, J. P. (2011). Local-scale faunal turnover on the deep Pacific seafloor. Mar. Ecol. Prog. Ser. 422, 193–200. doi: 10.3354/meps08924
McClain, C. R., and Schlacher, T. A. (2015). On some hypotheses of diversity of animal life at great depths on the sea floor. Mar. Ecol. 36, 849–872. doi: 10.1111/maec.12288
McClain, C. R., Stegen, J. C., and Hurlbert, A. H. (2012). Dispersal, environmental niches and oceanic-scale turnover in deep-sea bivalves. Philos. Trans. R. Soc. B Biol. Sci. 279, 1993–2002. doi: 10.1098/rspb.2011.2166
Mincks, S. L., Smith, C. R., Jeffreys, R. M., and Sumida, P. Y. G. (2008). Trophic structure on the West Antarctic Peninsula shelf: detritivory and benthic inertia revealed by δ13C and δ15N analysis. Deep Sea Res. Part II Top. Stud. Oceanogr. 55, 2502–2514. doi: 10.1016/j.dsr2.2008.06.009
Neal, L., Mincks Hardy, S. L., Smith, C. R., and Glover, A. G. (2011). Polychaete species diversity on the West Antarctic Peninsula deep continental shelf. Mar. Ecol. Prog. Ser. 428, 119–134. doi: 10.3354/meps09012
Nekola, J. C., and White, P. S. (1999). The distance decay of similarity in biogeography and ecology. J. Biogeogr. 26, 867–878. doi: 10.1046/j.1365-2699.1999.00305.x
Netto, S. A., Gallucci, F., and Fonseca, G. (2009). Deep-sea meiofauna response to synthetic-based drilling mud discharge off SE Brazil. Deep Sea Res. Part II Top. Stud. Oceanogr. 56, 41–49. doi: 10.1016/j.dsr2.2008.08.018
Oksanen, J., Blanchet, G. G., Kindt, R., Legendre, P., Minchin, P. R., O'Hara, R. B., et al. (2013). Package ‘vegan’. Available online at: https://cran.r-project.org/
Palmer, J. R., and Totterdell, I. J. (2001). Production and export in a global ocean ecosystem model. Deep Sea Res. Part I Oceanogr. Res. Pap. 48, 1169–1198. doi: 10.1016/S0967-0637(00)00080-7
Pearson, T. H., and Rosenberg, R. (1978). Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. 16, 229–311.
Petersen, J. M., Zielinski, F. U., Pape, T., Seifert, R., Moraru, C., Amann, R., et al. (2011). Hydrogen is an energy source for hydrothermal vent symbioses. Nature 476, 176–180. doi: 10.1038/nature10325
Petersen, S., Herzig, P. M., Schwarz-Schampera, U., Hannington, M. D., and Jonasson, I. R. (2004). Hydrothermal precipitates associated with bimodal volcanism in the Central Bransfield Strait, Antarctica. Mineralium Deposita 39, 358–379. doi: 10.1007/s00126-004-0414-3
Poore, G. C. B. (2001). Isopoda Valvifera: diagnoses and relationships of the families. J. Crustac. Biol. 21, 205–230. doi: 10.1163/20021975-99990118
Portail, M., Olu, K., Escobar-Briones, E., Caprais, J. C., Menot, L., Waeles, M., et al. (2015). Comparative study of vent and seep macrofaunal communities in the Guaymas Basin. Biogeosciences 12, 5455–5479. doi: 10.5194/bg-12-5455-2015
R Core Team (2013). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
Reed, A. J., Morris, J. P., Linse, K., and Thatje, S. (2013). Plasticity in shell morphology and growth among deep-sea protobranch bivalves of the genus Yoldiella (Yoldiidae) from contrasting Southern Ocean regions. Deep Sea Res. Part I Oceanogr. Res. Pap. 81, 14–24. doi: 10.1016/j.dsr.2013.07.006
Rex, M. A., and Etter, R. J. (2010). Deep-Sea Biodiversity: Pattern and Scale. Cambridge, MA: Harvard University Press.
Robertson, B. P., Gardner, J. P. A., and Savage, C. (2015). Macrobenthic–mud relations strengthen the foundation for benthic index development: a case study from shallow, temperate New Zealand estuaries. Ecol. Indic. 58, 161–174. doi: 10.1016/j.ecolind.2015.05.039
Rogers, A. D., Tyler, P. A., Connelly, D. P., Copley, J. T., James, R., Larter, R. D., et al. (2012). The discovery of new Deep-Sea Hydrothermal Vent communities in the Southern Ocean and implications for biogeography. PLoS Biol. 10:e1001234. doi: 10.1371/journal.pbio.1001234
Roterman, C. N., Copley, J. T., Linse, K. T., Tyler, P. A., and Rogers, A. D. (2013). The biogeography of the yeti crabs (Kiwaidae) with notes on the phylogeny of the Chirostyloidea (Decapoda: Anomura). Proc. R. Soc. B Biol. Sci. 280:20130718. doi: 10.1098/rspb.2013.0718
Rowe, G. T., Sibuet, M., Deming, J., Tietjen, T., and Khripounoff, A. (1990). Organic carbon turnover time in deep-sea benthos. Prog. Oceanogr. 24, 141–160. doi: 10.1016/0079-6611(90)90026-X
Sahling, H., Wallman, K., Dählmann, A., Schmaljohann, R., and Petersen, S. (2005). The physicochemical habitat of Sclerolinum sp. at Hook Ridge hydrothermal vent, Bransfield Strait, Antarctica. Limnol. Oceanogr. 50, 598–606. doi: 10.4319/lo.2005.50.2.0598
Shields, M. A., and Blanco-Perez, R. (2013). Polychaete abundance, niomass and diversity patterns at the Mid-Atlantic Ridge, North Atlantic Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 98B, 315–325. doi: 10.1016/j.dsr2.2013.04.010
Smirnov, R. V. (2000). “Sclerolinum contortum,” in World Polychaeta Database. eds G. Read and K. Fauchald. Available online at: http://www.marinespecies.org/polychaeta/aphia.php?p=taxdetails&id=129130)
Somoza, L., Martıìnez-Frıìas, J., Smellie, J. L., Rey, J., and Maestro, A. (2004). Evidence for hydrothermal venting and sediment volcanism discharged after recent short-lived volcanic eruptions at Deception Island, Bransfield Strait, Antarctica. Mar. Geol. 203, 119–140. doi: 10.1016/S0025-3227(03)00285-8
Southward, A. J., Southward, E. C., Brattegard, T., and Bakke, T. (1979). Further Experiments on the value of Dissolved Organic Matter as Food for Siboglinum fjiordicum (Pogonophora). J. Mar. Biol. Assoc. U.K. 59, 133–148. doi: 10.1017/S0025315400046233
Sweetman, A. K., Levin, L. A., Rapp, H. T., and Schander, C. (2013). Faunal trophic structure at hydrothermal vents on the southern Mohn's Ridge, Arctic Ocean. Mar. Ecol. Prog. Ser. 473, 115. doi: 10.3354/meps10050
Tarasov, V. G., Gebruk, A. V., Mironov, A. N., and Moskalev, L. I. (2005). Deep-sea and shallow-water hydrothermal vent communities: two different phenomena? Chem. Geol. 224, 5–39. doi: 10.1016/j.chemgeo.2005.07.021
Thornhill, D. J., Wiley, A. A., Campbell, A. L., Bartol, F. F., Teske, A., and Halanych, K. M. (2008). Endosymbionts of Siboglinum fjordicum and the phylogeny of bacterial endosymbionts in siboglinidae (Annelida). Biol. Bull. 214, 135–144. doi: 10.2307/25066670
Tyler, P. A., Connelly, D. P., Copley, J. T., Linse, K., Mills, R. A., Pearce, D. A., et al. (2011). RRS James Cook Cruise JC55: Chemosynthetic Ecosystems of the Southern Ocean. Southampton: BODC Cruise Report.
Urich, T., Lanzén, A., Stokke, R., Pedersen, R. B., Bayer, C., Thorseth, I. H., et al. (2014). Microbial community structure and functioning in marine sediments associated with diffuse hydrothermal venting assessed by integrated meta-omics. Environ. Microbiol. 16, 2699–2710. doi: 10.1111/1462-2920.12283
Van Dover, C. L. (2010). Mining seafloor massive sulphides and biodiversity: what is at risk? ICES J. Mar. Sci. 68, 341–348. doi: 10.1093/icesjms/fsq086
Wefer, G., and Fischer, G. (1991). Annual primary production and export flux in the Southern Ocean from sediment trap data. Mar. Chem. 35, 597–613. doi: 10.1016/S0304-4203(09)90045-7
Whitaker, D., and Christmann, M. (2013). Package ‘clustsig’. Available online at: https://cran.r-project.org/
Whiticar, M. J., and Suess, E. (1990). Hydrothermal hydrocarbon gases in the sediments of the King George Basin, Bransfield Strait, Antarctica. Appl. Geochem. 5, 135–147. doi: 10.1016/0883-2927(90)90044-6
Yoon, H. I., Han, M. W., Park, B. K., Oh, J. K., and Chang, S. K. (1994). Depositional environment of near-surface sediments, King George Basin, Bransfield Strait, Antarctica. Geo Mar. Lett. 14, 1–9. doi: 10.1007/BF01204465
Zeppilli, D., Mea, M., Corinaldesi, C., and Danovaro, R. (2011). Mud volcanoes in the Mediterranean Sea are hot spots of exclusive meiobenthic species. Prog. Oceanogr. 91, 260–272. doi: 10.1016/j.pocean.2011.01.001
Zeppilli, D., Vanreusel, A., Pradillon, F., Fuchs, S., Mandon, P., James, T., et al. (2015). Rapid colonisation by nematodes on organic and inorganic substrata deployed at the deep-sea Lucky Strike hydrothermal vent field (Mid-Atlantic Ridge). Mar. Biodiv. 45, 489–504. doi: 10.1007/s12526-015-0348-2
Keywords: chemosynthetic, Southern Ocean, environmental distance, deep-sea, ecology, sedimented
Citation: Bell JB, Woulds C, Brown LE, Sweeting CJ, Reid WDK, Little CTS and Glover AG (2016) Macrofaunal Ecology of Sedimented Hydrothermal Vents in the Bransfield Strait, Antarctica. Front. Mar. Sci. 3:32. doi: 10.3389/fmars.2016.00032
Received: 29 September 2015; Accepted: 03 March 2016;
Published: 21 March 2016.
Edited by:
Ricardo Serrão Santos, University of the Azores, PortugalReviewed by:
Daniela Zeppilli, Institut Français de Recherche pour l'Exploitation de la Mer, FranceAmerico Montiel, Universidad de Magallanes, Chile
Copyright © 2016 Bell, Woulds, Brown, Sweeting, Reid, Little and Glover. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James B. Bell, Z3lqYmJAbGVlZHMuYWMudWs=
 James B. Bell
James B. Bell Clare Woulds1
Clare Woulds1 Christopher J. Sweeting
Christopher J. Sweeting William D. K. Reid
William D. K. Reid Adrian G. Glover
Adrian G. Glover