- 1Centre National de la Recherche Scientifique/Institut National des Sciences de l'univers, Institut de Recherche pour le Développement, Mediterranean Institute of Oceanography, Aix Marseille Université, Université de Toulon, UM 110, Nouméa, New Caledonia
- 2Department of Biological Oceanography, Leibniz Institute for Baltic Sea Research, Rostock, Germany
Dinitrogen (N2) fixation (the reduction of atmospheric N2 to ammonium by specialized prokaryotic microbes), represents an important input of fixed nitrogen and contributes significantly to primary productivity in the oceans. Marine N2 fixation was discovered in the North Atlantic Ocean (NA) in the 1960s. Ever since, the NA has been subject to numerous studies that have looked into the diversity and abundance of N2-fixing microbes (diazotrophs), the spatial and temporal variability of N2 fixation rates, and the range of physical and chemical variables that control them. The NA provides 10–25% of the globally fixed N2, ranking as the third basin with the largest N2 fixation inputs in the world's oceans. This basin suffers a chronic depletion in phosphorus availability, more aeolian dust deposition than any other basin in the world's oceans, and significant nutrient inputs from important rivers like the Amazon and the Congo. These characteristics make it unique in comparison with other oceanic basins. After five decades of intensive research, here we present a comprehensive review of our current understanding of diazotrophic activity in the NA from both a geochemical and biological perspective. We discuss the advantages and disadvantages of current methods, future perspectives, and questions which remain to be answered.
Introduction
Dinitrogen (N2)fixation–the reduction of atmospheric nitrogen to ammonia- is an energy intensive process performed by prokaryotes from a variety of habitats, including rice fields, lacustrine waters, and the ocean (Karl et al., 2002). In the latter, N2 fixation provides an input of fixed nitrogen that may become the primary source of this nutrient when other inputs like coastal runoff, atmospheric deposition, or subsurface nutrient upwelling and diffusion are minor or insufficient to meet the photosynthetic phytoplankton demand. This can be the case in some of the remote and chronically stratified oligotrophic subtropical gyres, which make up approximately half of the world's ocean surfaces. In that sense, N2 fixation plays a key role in sustaining primary production on a global basis, powering the biological carbon pump and eventually conferring the oceans the ability to sequester atmospheric carbon dioxide (CO2). The availability of fixed nitrogen ultimately depends on the difference between “gains” and “losses” through N2 fixation vs. denitrification and anammox (Falkowski, 1997; Gruber and Galloway, 2008), and so does the magnitude and distribution of diazotrophic activity in the oceans (Weber and Deutsch, 2014). As a result, the complexity of the many reactions involved between the different nitrogen chemical forms and the diversity of the microorganisms responsible for them have encouraged a wide body of research in the last decades (Zehr and Kudela, 2011). A better understanding of the nitrogen cycle is needed in order to predict the behavior of our oceans in the light of climate change and other anthropogenic perturbations.
Probably due to the proximity to the wealthier surrounding countries of North America and Europe, the North Atlantic Ocean (NA) has been subject to the vast majority of the N2 fixation related studies conducted so far. Marine nitrogen fixation was first observed in the NA in the early 1900s. After Brandt (1899) introduced the concept of limiting nutrients in controlling phytoplankton production in the ocean, the German scientists Joseph Keutner and Wilhelm Benecke firstly reported that diazotrophic bacteria common in terrestrial environments like Clostridium and Azobacter were also present in the sea, and hence their role in providing these nutrients could be important (Mills, 2012). Modern marine N2 fixation research in the NA was pioneered by the research of Richard Dugdale, John Goering, and colleagues in the tropical and subtropical Northwest Atlantic (NWA) (Dugdale et al., 1961, 1964; Goering et al., 1966). These authors found considerable 15N atom % excess values (up to 0.9) after incubating isolated Trichodesmium colonies in the presence of 15N2. Intrigued by the recurrent observation of Trichodesmium in offshore nutrient-poor waters of the NWA, Goering et al. (1966) conducted further research in an area affected by the Amazon River plume. In subsequent years, the drawdown of dissolved inorganic carbon (DIC) at BATS in the absence of sufficient nitrate was tentatively attributed to N2 fixation, possibly in combination with atmospheric nitrogen deposition and deviations from Redfield stoichiometry (Michaels et al., 1994). Overcoming the temporal and spatial scale constraints of in situ 15N2 uptake measurements, the calculation of the N* parameter–a quasi-conservative tracer which indicates the excess of nitrate as compared to phosphate considering the Redfield ratio of nitrate:phosphate ratio (N:P) = 16:1 (Michaels et al., 1996; Gruber and Sarmiento, 1997)–allowed basin-scale estimations of nitrate concentrations in excess of that predicted by Redfield stoichiometry. Michaels et al. (1996) calculated a nitrogen excess generation rate of 51.8–89.6 Tg N y−1 in the top 1000 m of the Sargasso Sea, followed by Gruber and Sarmiento (1997) who estimated a fixed nitrogen excess rate of 28 Tg N y−1 for the tropical and subtropical NA. The estimated nitrogen excess was attributed to N2 fixation, predominantly by Trichodesmium (Gruber and Sarmiento, 1997). Trichodesmium is strongly limited by the availability of iron, which is a cofactor of its nitrogenase enzyme and makes an important part of its biomass (Berman-Frank et al., 2001). Early on, the importance of the input of Saharan dust with relatively high iron content to the NA was related to N2 fixation activity by Trichodesmium and the subsequent formation of a nitrogen excess signal (Duce et al., 1991; Michaels et al., 1996; Gruber and Sarmiento, 1997 the role of iron in shaping diazotrophic activity and diversity is further discussed in Section Responses to Atmospheric Dust Inputs below). At that time, the continued observation of dense surface blooms of Trichodesmium in the NWA and in the Caribbean Sea (e.g., Carpenter and Price, 1977), together with the associated high N2 fixation rates observed (Carpenter and Romans, 1991), pointed toward a prominent role for this conspicuous cyanobacterium in N2 fixation activity in the NA. Research was not strictly limited to Trichodesmium, as other diazotrophs could be studied with the microscopy-based techniques of the time, notably diatom-diazotroph associations (DDAs). These DDAs are commonly formed by the cyanobacteria Richelia intracellularis and Calothrix rhizosoleniae which form symbiotic associations with several diatom genera like Rhizosolenia, Hemiaulus, and Chaetoceros (e.g., Carpenter et al., 1999).
During the late 1990s and 2000s, the especial interest in Trichodesmium as a key species made way for a great deal of research initiatives focused on the independent contribution of this cyanobacterium to basin or global-scale N2 fixation (e.g., estimated at 22.4 Tg N y−1 for the NA by Capone et al., 2005). These added to a variety of studies on its physiology, autoecology, and distribution (recently reviewed by Bergman et al., 2012). Later studies found that Trichodesmium is mostly restricted to the tropical and subtropical NWA waters due to their growth temperature optimum (24–30°C; Breitbarth et al., 2007), and their preference for low dissolved inorganic nitrogen and calm stratified waters (Bergman et al., 2012).
The revolutionary application of polymerase chain reaction (PCR) techniques to amplify the nifH gene (Zehr et al., 1998)–the gene encoding the iron subunit of the nitrogenase enzyme- revolutionized the way diazotrophy was scrutinized in the ocean. Molecular studies unveiled the ubiquity of unicellular diazotrophic cyanobacteria (UCYN) which can equal or even exceed N2 fixation rates by Trichodesmium (Falcón et al., 2004; Montoya et al., 2004), as well as inhabit colder and more nutrient rich areas of the NA. Studies targeting the nifH gene in the tropical and subtropical NA have found a predominance of Trichodesmium, followed by UCYN-A, with the latter present in deeper and colder waters than the former (Langlois et al., 2005, 2008; Goebel et al., 2010). The distribution of these two phylotypes seems to follow a “shifting” pattern, so that Trichodesmium may dominate at the surface while UCYN-A dominates in deeper levels of the photic zone (Langlois et al., 2005). Additionally, current evidence points toward the dominance of Trichodesmium in the NWA while UCYN-A dominates in the NEA (Agawin et al., 2014). Other UCYN like Crocosphaera (UCYN-B) and UCYN-C phylotypes have also been recurrently detected in the NA, however in much lower abundances (e.g., Langlois et al., 2008).
Five decades have passed since the work of Dugdale et al. (1964) in the Sargasso Sea. Numerous studies on the ecological, biogeochemical and molecular aspects of diazotrophy have arisen since then, and work in the NA now represents the greatest number of N2 fixation and diazotrophic diversity studies of the world's oceans (Luo et al., 2012). Despite this, other facets of diazotrophy are not well-constrained yet. The discovery of new organisms with surprising metabolisms like the unicellular diazotrophic cyanobacterium UCYN-A (Candidatus Atelocyanobacterium thalassa; Zehr et al., 2008; Thompson et al., 2012), the measurement of significant N2 fixation rates in unexpected habitats (e.g., Bonnet et al., 2013), and the possibility that global N2 fixation rates have been systematically underestimated in the past (Großkopf et al., 2012) has left room for plenty of new questions still to be answered, including: how much do heterotrophic diazotrophs and UCYN contribute to total N2 fixation?, how much does N2 fixation contribute to new production and what is its role in the biological carbon pump?, can the methodological underestimation of available N2 fixation rates explain the current differences between denitrification and nitrogen fixation at a global scale?, to what extent published N2 fixation rates are affected by 15N2 gas contamination?, and, how will diazotrophs respond to climate change? The advent of new molecular biology methods, high resolution mass spectrometry techniques and single-cell techniques, together with more powerful modeling approaches should provide new insights and answers to these questions.
It is the purpose of this review to synthesize the knowledge gained on the distribution, magnitude and controls of diazotrophic activity and diversity in the pelagic NA through the years, providing an outlook toward better constraints of basin-scale N2 fixation rates and identification of the various diazotroph species including previously unexplored sites with diverging environmental conditions. Benthic -and coral- associated N2 fixation studies are out of the scope of this review. The reader is referred to comprehensive reviews on those topics (see Capone et al., 2008).
Physical and Chemical Controls of Diazotrophic Activity, Diversity, and Distribution
Diazotrophic activity and diversity is known to be regulated by a variety factors, broadly divided into physical (light, temperature, salinity, water column stability, turbulence, mesoscale variability, density fronts), and chemical factors (N:P ratios and iron availability, among other trace metals) (Luo et al., 2014). Both groups of controlling factors are inextricably connected as physical structures induce changes in nutrient availability patterns (e.g., diffusion through fronts, upwelling in the core of eddies, etc.). The NA spans a suite of regional environments where the physical and/or chemical constraints can be substantially different. For example, we can compare the turbulent, cold and nutrient rich waters of the Northwest African upwelling to the stratified and oligotrophic Sargasso Sea, the steep gradients of the Gulf Stream to the Amazon River plume, and the highest desert dust aeolian inputs in the world (Jickells, 2005). In this section we explore how these different factors shape the distribution and activity of diazotrophs in the NA.
Impact of Physical Factors on Diazotrophy
Hydrographic structures such as filaments, eddies or fronts can have profound effects on biological activity. Besides the changes in nutrient availability that these structures may cause, the spatial distribution of microorganisms can also change in response to physical forcing phenomena. For example, phytoplankton activity may be reduced when cells are transported to dimmer waters in an anticyclonic eddy, or cells can accumulate along a density front between riverine and open ocean waters.
In the case of diazotrophs, Trichodesmium is an obvious candidate to experience physical-driven changes in its spatial distribution given the buoyancy conferred by intracellular gas vesicles (Villareal and Carpenter, 2003). Local accumulations of Trichodesmium have been observed in anticyclonic eddies across the NA (Davis and McGillicuddy, 2006), with an increasing abundance toward the West. In the NEA however, Trichodesmium is too scarce and usually present as free trichomes instead of colonies (Moore et al., 2009; Fernández et al., 2010; Benavides et al., 2011). This low abundance probably prevents any structuring effects from mesoscale activity (Agawin et al., 2013). However, relative peaks of Trichodesmium abundance have been observed in typical NEA hydrographic structures such as the Azores Front or the upwelling filament off Cape Ghir (in the Northwest Africa coastal upwelling; Benavides et al., 2011).
In terms of N2 fixation activity, diazotrophs may respond to changes in nutrient availability caused by hydrographic phenomena. To date, research on this aspect of marine diazotrophy has been sparse, especially in the Atlantic as relative to the Pacific. The few studies available do not reveal a clear trend between hydrographic features and N2 fixation activity trends. For example, Agawin et al. (2013) could not find a relationship between anticyclonic/cyclonic eddies and N2 fixation rates have been observed in the eddy field downwind of the Canary Islands (Agawin et al., 2013). Ultimately, the study of how physical forcing influences N2 fixation requires research strategies capable of resolving meso- and submesoscale features.
Another important factor controlling the activity of nitrogen fixers is light. Light provides energy for N2 fixation and determines the circadian rhythm of autotrophic diazotrophs. In heterocystous diazotrophs, carbon and N2 fixation can take place concurrently, however non-heterocystous diazotrophs are challenged to protect nitrogenase from photosynthesis-derived oxygen. Trichodesmium possesses a photosynthetic apparatus adapted to a high-light regimes and uses a combined approach of high respiration rates and intracellular spatial segregation which allows it to fix both carbon and N2 during daylight hours (Capone, 1997; Berman-Frank et al., 2007), while UCYN (Zehr et al., 2008) species must perform carbon fixation during daylight, while using the obtained carbohydrates to sustain N2 fixation at night. As such, light controls the physiology of photosynthetic diazotrophs, while heterotrophic bacterial and archaeal diazotrophs may not be affected and have been shown to express the nifH gene independently of light availability (Church et al., 2005). However, the potential circadian rhythms of these diazotrophs is not yet well-understood.
On a greater scale, light levels structure how diazotrophs are distributed in the water column. While Trichodesmium usually prefers the top sunlit 75 m, most UCYN tend to appear at deeper depths and down to ~150 m (e.g., Langlois et al., 2008). The lower availability of light at these depths presumably results in a less efficient photosynthesis and hence less evolution of oxygen, thus making it easier for UCYN species to protect their nitrogenase (Karl et al., 2002). Alternatively, UCYN-A presents a more complex situation as this group is not capable of carrying out the oxygen-evolving steps of photosynthesis and because it is thought to be an extracellular symbiont of an eukaryotic algae that does require light for photosynthesis (Zehr et al., 2008; Thompson et al., 2012). While UCYN-A or Crocosphaera can appear at deeper levels of the water column than Trichodesmium, some studies have shown that their abundance still tends to be greater at the surface (Luo et al., 2012). Finally, the statistical analysis of global N2 fixation data and diazotroph abundance data indicates that solar radiation is the most influencing factor determining diazotrophic activity, and explains why the highest N2 fixation rates and abundances of diazotrophs have been reported for the tropical and subtropical bands of the oceans, which also receive the greatest amounts of light worldwide (Luo et al., 2014). However, it must be borne in mind that different diazotroph phylotypes have different responses to daily fluctuations in irradiance (e.g., Church et al., 2005). Finally, the presence and activity of heterotrophic diazotrophs in aphotic waters (e.g., Hewson et al., 2007; Bonnet et al., 2013; Rahav et al., 2013) challenges this strict control and defines physiological differences among diazotrophic species.
Nutrient and Trace Metal Controls
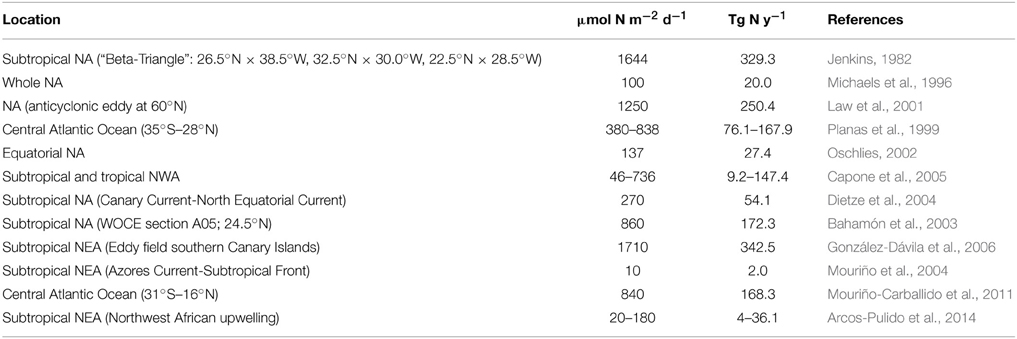
Fertilization of the photic layer of the oceans depends principally on upwelling, Ekman pumping, mixing, fronts, convection (in polar and temperate regions mainly) and vertical diffusion of deeper waters rich in remineralized nutrients, the remineralization efficiency on those deeper waters, and finally on how the aforementioned physical processes are constrained by the formation and transport of mode waters (Palter et al., 2005). Other external sources include atmospheric deposition, and internal sources include in situ regeneration processes like photic layer nitrification or dissolved organic matter (DOM) breakdown into bioavailable molecules through remineralization and photobleaching processes (e.g., Hedges, 2002; Yool et al., 2007). The NA is largely composed of a subtropical gyre where nitrate and phosphate concentrations are low (Figures 1A,B). This gyre is flanked by more nutrient-rich areas such as the Northwest African upwelling system, the Equatorial upwelling region, the Amazon River plume, the Gulf Stream, and the subpolar gyre. These nutrient-rich flanks fertilize the oligotrophic central gyre via advection and epipycnal (along isopycnals) mixing (Pelegrí et al., 2006). The NA subtropical gyre differs from others in that it receives the greatest quantity of desert dust worldwide, which in principle alleviates iron limitation in diazotrophs (Jickells, 2005). The extent to which nutrients and trace metals control N2 fixation vary depending on the diazotroph phylotype, their physiology and nutrient requirements (reviewed in Sohm et al., 2011c). These are trends that at present are only fairly clear for Trichodesmium (Bergman et al., 2012). In this section we explore how the distribution of nutrients and the input of aeolian trace metals controls N2 fixation and shapes the distribution of different diazotrophic phylotypes in the NA.
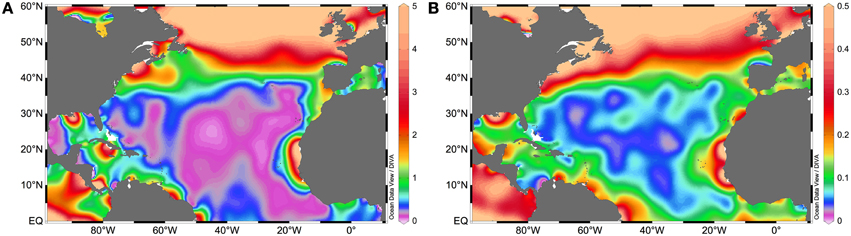
Figure 1. NA surface (A) nitrate and (B) phosphate concentrations (in μM). Data retrieved from the World Ocean Atlas 2013 Garcia et al., 2013.
Inorganic and Organic Nutrients Driving the Distribution of Diazotrophic Activity
N2 fixation provides a source of nitrogen that is unaccompanied by a concomitant input of phosphorus, eventually resulting in increased N:P ratios (with respect to the canonical Redfield ratio of N:P = 16:1; Redfield et al., 1963). This imbalance creates a fixed nitrogen excess signal in the NA (Redfield et al., 1963; Gruber and Sarmiento, 1997), which has been attributed to enhanced N2 fixation activity driven by the high aeolian iron supply (Michaels et al., 1996, 2001; Gruber and Sarmiento, 1997). As aforementioned, iron plays a key role in controlling diazotrophic activity and diversity in the ocean (see also Section Responses to Atmospheric Dust Inputs). This N:P imbalance results in a severe phosphorus deficiency in the NA basin, a feature which is remarkably different from the other basins of the world's oceans (Wu, 2000; Ammerman et al., 2003). Conversely, high concentrations of dissolved inorganic nitrogen forms (mainly nitrate and ammonium) are usually considered to inhibit diazotrophic activity, given that N2 fixation is energetically expensive relative to the assimilation of more readily available dissolved inorganic nitrogen forms (Holl and Montoya, 2005).
The comparison of nitrate and phosphate concentrations (Figures 1A,B), with the distribution of N:P ratios (Figure 2A), and that of integrated N2 fixation rates (Figure 2B) reveals a greater diazotrophic activity in relatively phosphate rich waters (0.1–0.3 μM; Figure 1B) where nitrate is low (<0.5 μM; Figure 1A), leading to low N:P ratios. Indeed, the relationship between phosphate supply or phosphate concentrations and N2 fixation rates has been documented for different locations of the NA (e.g., Fernández et al., 2012; Benavides et al., 2013d), although this does not hold true in all cases (Turk et al., 2011), because the response of diazotrophs to phosphate limitation may vary geographically and with each diazotroph phylotype (Turk-Kubo, 2012). In sites of nitrate and concomitant phosphate loading such as the Amazon River plume in the NWA or the Equatorial upwelling on the NEA, N2 fixation is thought to be fueled by excess phosphate remaining after the consumption of nitrate by non-N2 fixing phytoplankton (Subramaniam et al., 2008, 2013). As such, diazotroph abundances peak where nitrate is exhausted but there remains enough phosphate to fuel N2 fixation, such as in the mesohaline waters (salinities 30–35) of the Amazon River plume (Subramaniam et al., 2008). Integrated N2 fixation rates associated with the plume and East of the Caribbean Sea easily reach values >1000 μmol N m−2 d−1 (Figure 2B). Within the plume, DDAs dominate (mainly Richelia-Rhizosolenia symbioses which benefit from excess silica also provided by the river), closely followed by Trichodesmium. UCYN phylotypes are found where the plume meets the saltier waters of the open ocean (Foster et al., 2007). Similarly, in the NEA where the Congo River plume meets the Equatorial upwelling -note that the mouth of the Congo is in the southern hemisphere, but its plume affects the NA- and phosphate concentrations are relatively high (0.18 μM; Subramaniam et al., 2013), the abundances of DDAs and Trichodesmium are greatest within the plume and at the edge of the upwelling, while UCYN-A abounds within the area influenced by the upwelling (Foster et al., 2009). In general, high abundances of Trichodesmium are observed down to 75 m depth (Luo et al., 2012), and their geographical extent ranges from the waters adjacent to the Amazon plume to the eastern NA coast along the Equator.
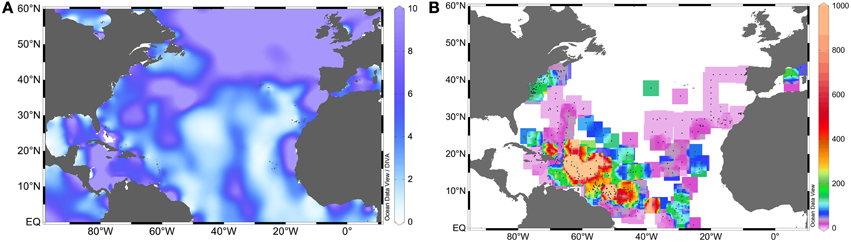
Figure 2. Surface NA (A) N:P ratios (as nitrate divided by phosphate concentrations retrieved from the World Ocean Atlas 2013, Garcia et al., 2013), and (B) integrated N2 fixation rates (in μmol N m−2 d−1) (data retrieved from the Luo et al. online database, Luo et al., 2012).
Away from relatively phosphate-rich waters, active diazotroph assemblages in the inner oligotrophic subtropical gyre are likely sustained by phosphate transferred vertically (diapycnal mixing), transported along isopycnals (epipycnal mixing), or advected from the surrounding gyre flanks (Pelegrí et al., 2006), such as excess phosphate from the Gulf Stream (Palter et al., 2011). Alternatively, dissolved organic phosphorus transported to the interior of the gyre from the productive NEA coastal and Equatorial upwelling sites (Mather et al., 2008; Reynolds et al., 2014) may play an important role in supporting N2 fixation (Mahaffey et al., 2014). The ability of the NEA coastal upwelling sites to partly sustain autotrophic and heterotrophic production within the subtropical gyre has been widely recognized. For example, the Canary Current upwelling exports ~60% of production as dissolved organic matter (DOM) (Álvarez-Salgado et al., 2007), while dissolved organic nitrogen and dissolved organic phosphorus are estimated to support 40–70% of export production within the gyre (Torres-Valdés et al., 2009; Reynolds et al., 2014). The role of DOM in sustaining N2 fixation is much less known. In the tropical and subtropical NA phosphate concentrations are too low to sustain Trichodesmium growth using this compound as the solely source of phosphorus (Sohm and Capone, 2006). Indeed, molecular biology approaches have demonstrated that Trichodesmium is capable of taking up dissolved organic phosphorus in the form of phosphomonoesters via alkaline phosphatase activity (Dyhrman et al., 2002), or as phosphonates via a carbon-phosphorus bond (C-P) lyase pathway (Dyhrman et al., 2006). The only other marine diazotroph that has been studied in terms of their dissolved organic phosphorus acquisition capability is Crocosphaera watsonii, which does not possess the genes to utilize phosphonates, but it does have the genes to hydrolyze phosphomonoesters, as well as a suite of genes for inorganic phosphorus transport (high-affinity and low-affinity phosphate transport genes) (Dyhrman et al., 2006). Despite these differences in dissolved organic phosphorus uptake capability, whether the differential distribution of Trichodesmium vs. Crocosphaera responds to phosphomonoesters and phosphonates distributions in the NA is unknown, a fact further worsened by the dearth of knowledge regarding the composition of the dissolved organic phosphorus pool in the ocean (Karl and Björkman, 2002; Kononova and Nesmeyanova, 2002).
Within the NA subtropical gyre, UCYN-A and Trichodesmium are the most abundant phylotypes (Figure 3), although they appear to be spatially segregated (Montoya et al., 2007; Agawin et al., 2014; Ratten et al., 2014). While UCYN occupy a wide latitudinal and longitudinal range (Figures 3C,D), Trichodesmium is constrained to <30°N in the NWA, with the exception of the data reported by Mulholland et al. on the US East Coast (Mulholland et al., 2012, Figure 3A). Indeed, most of the N2 fixation in the NWA is supported by Trichodesmium, and a greater contribution by UCYN phylotypes is found in the NEA (Montoya et al., 2007). UCYN-C is less abundant than the other UCYN and appears on a narrower latitudinal range (<20°N), mostly over the NWA (Figure 3F). Nevertheless, it must be borne in mind that matching N2 fixation activity to the presence on diazotrophs based solely on nifH abundance as determined by qPCR may lead to misleading results, and nifH expression can be high in low abundance phylotypes (Turk-Kubo, 2012).
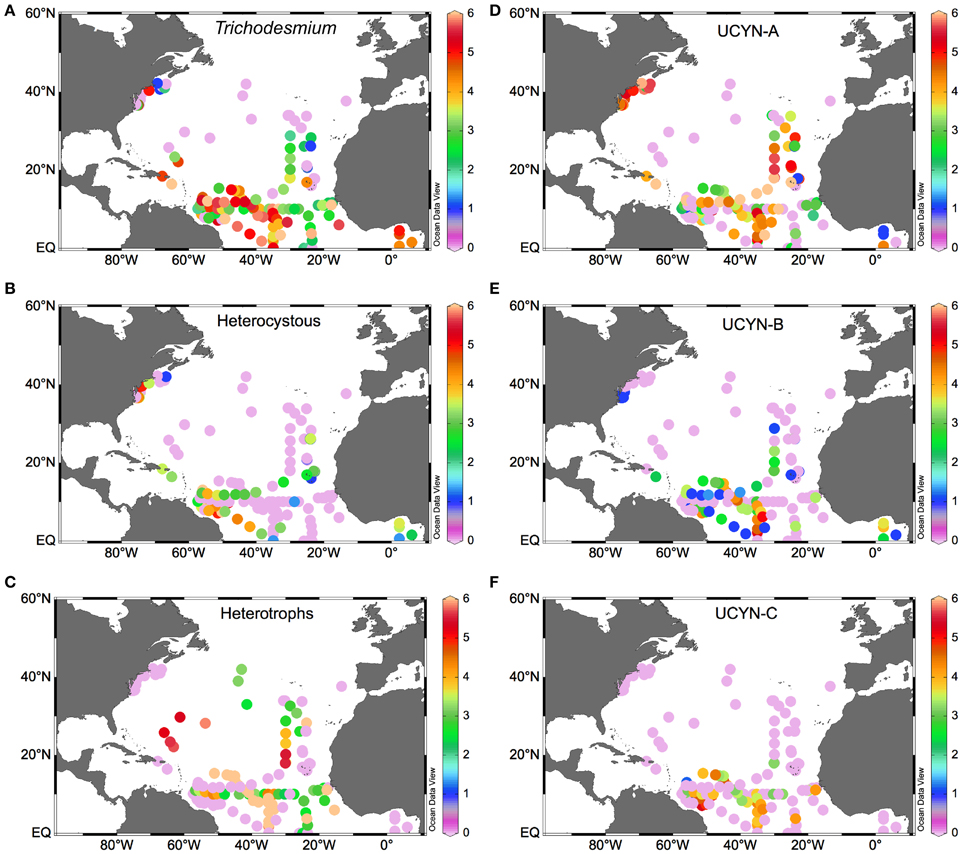
Figure 3. Surface distribution of diazotroph phylotypes in the NA (as log10 nifH copies L−1). (A) Trichodesmium, (B) symbiont heterocystous diazotrophs -sum of Richelia and Calothrix associated species-, (C) heterotrophic diazotrophs, (D) UCYN-A, (E) UCYN-B, (F) UCYN-C.
This spatial segregation may not be straightforward given that the low nitrate-high phosphate (low N:P ratio) conditions responsible for them occur in both the Amazon River plume and in the upwelling-affected areas of the NEA. However, upwelling-affected waters are colder, which likely explains the lower abundance of diazotrophic organisms in the NEA as compared to the NWA (Figure 3), and the lower N2 fixation rates (generally <1 nmol N L−1 d−1; Moore et al., 2009; Fernández et al., 2010; Benavides et al., 2011). The low N2 fixation rates of the NEA do not likely represent an important source of new production in this area (Benavides et al., 2013b). Thus, it appears that the fixed nitrogen requirements of autotrophic planktonic communities in the NEA are met by a combination of atmospheric fixed nitrogen deposition (as this area is located downwind of European industrialized countries), in situ remineralization of advected DOM and, to a minor extent, in situ photic nitrate regeneration (nitrification) and N2 fixation. However, the presence of N2 fixation activity and/or diazotrophic microorganisms in the cold and nutrient rich waters of the NA such as upwelling sites (Voss et al., 2004; Foster et al., 2009; Benavides et al., 2011; Subramaniam et al., 2013; Agawin et al., 2014) or mesopelagic waters (Hewson et al., 2007) has been observed, though their ecological role in these environments is unclear and warrants further study. High dissolved inorganic nitrogen environments have been usually regarded as inhibitory for diazotrophic activity (Holl and Montoya, 2005). However, this effect is not clear. Recent culture and field studies indicate that diazotrophy can continue upon exposure to dissolved inorganic nitrogen (nitrate and ammonium) as long as phosphate is available (reviewed in Knapp, 2012). Furthermore, active N2 fixation in dissolved inorganic nitrogen-rich environments suggests that restricting diazotrophy studies to the upper ocean layer of the tropics and subtropics can result in large underestimations of N2 fixation rates (Raimbault and Garcia, 2008; Benavides et al., 2011; Sohm et al., 2011b; Bonnet et al., 2013). Foster et al. (2009) found UCYN-A over the Equatorial upwelling region in the Gulf of Guinea. Although these authors did not find a high level of nifH expression in UCYN-A, in a later study Subramaniam et al. (2013) reported N2 fixation rates between 15 and 424 μmol N m−2 d−1 for the same site, with a ~50% contribution of the <10 μm fraction presumably including UCYN-A. Similarly, in the upwelling sites of Cape Silleiro (Northwest Iberian peninsula) and Cape Ghir (Northwest Africa), the <10 μm fraction contributed 60–90% of total N2 fixation, although the rates were much lower (Benavides et al., 2011). Finally, this group was found in high abundances along the East Coast of the US where it occurred at high dissolved inorganic nitrogen concentrations and comparatively low temperatures (Mulholland et al., 2012). Relatively high abundances of DDAs have been also reported off the eastern US coast, which could be due to actual in situ growth or as a result of transport from Amazon River plume affected waters along the Gulf Stream (Ratten et al., 2014).
In summary, N:P ratios seem to shape the distribution of diazotrophs and their associated N2 fixation rates, while the phosphorus requirements of diazotrophs could be met by direct uptake of dissolved organic phosphorus or hydrolysis of these compounds into inorganic phosphorus (however note that UCYN-A does not possess the genes needed to use dissolved organic phosphorus; Tripp et al., 2010). Trichodesmium needs low N:P ratios, UCYN can stand higher dissolved inorganic nitrogen concentrations, DDAs need low N:P accompanied by sufficient silica, and heterotrophic diazotrophs seem to be more flexible and better able to exploit the DOM accumulated in the inner subtropical gyre.
When dividing the NA into its western (80–40°W) and eastern (40–0°W) sides, and considering three latitudinal bands (Tropic 0–23.5°N, Subtropic 23.5–40°N, and Temperate >40°N), the distribution of nifH copies per liter of different diazotroph phylotypes show interesting patterns (based on the database of Luo et al., 2012; Table 1–note that the number of data points is strikingly different between latitude/longitude ranges-). The dominance of Trichodesmium in the tropical and subtropical NWA becomes evident, with one order of magnitude higher abundances than its eastern counterpart. Heterocystous diazotrophs are also clearly dominant in the NWA due to the influence of the Amazon River plume. The distribution of heterotrophic diazotrophs is more disparate, with two orders of magnitude higher abundance in the tropical NEA than in the NWA, but three orders of magnitude higher abundance in the subtropical NWA than in the NEA (Table 1). It is perhaps remarkable that heterotrophic diazotrophs in temperate waters are only one order of magnitude lower than in subtropical waters of the NWA. Nevertheless, the abundance of heterotrophic diazotrophs needs to be interpreted with great caution as their diversity is less explored than that of UCYN and contamination problems with PCR reagents are well known (Zehr et al., 2003). UCYN-A is also more abundant in the NWA as compared to the NEA. Their average abundances decrease by one order of magnitude by latitudinal band in the NWA, and surpass the average abundances of the NEA by more than two orders of magnitude. UCYN-B seem to be more homogenously distributed, with similar abundances in the tropical and subtropical NEA and NWA, and practically absent at temperate latitudes. This seems also to be the case of UCYN-C, which is however constrained to tropical latitudes. It should be noted that the NWA has been more extensively researched in terms of diazotrophic activity and diversity than the NEA, and therefore these averages could be biased by undersampling. This is clearly reflected by the generally higher standard deviation values of the NEA average nifH abundances, as well as by the number of data included for these analyses (NWA = 136 data points, NEA = 30 data points).
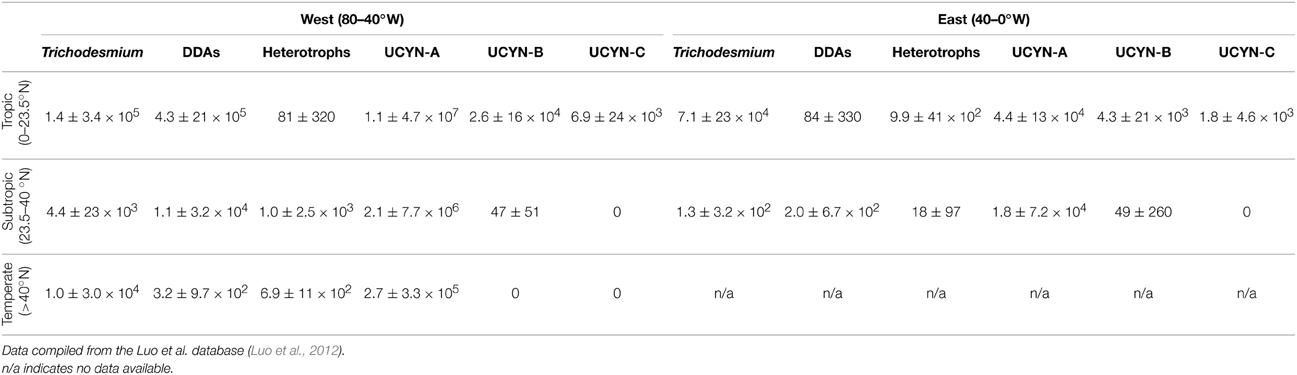
Table 1. Average ± standard deviation nifH copies L−1 of different diazotroph phylotypes as determined by qPCR.
Responses to Atmospheric Dust Inputs
The nitrogenase enzyme consists of an iron subunit (dinitrogenase reductase), and an iron-molybdenum subunit (dinitrogenase) (Postgate, 1982), which makes iron an essential metal for diazotrophs. Due to the low solubility of iron in the oxidized and slightly basic contemporary ocean (Liu and Millero, 2002), planktonic microorganisms have developed specialized iron scavenging mechanisms to provide them with the iron needed for the functioning of many photosynthetic and respiratory redox enzymes. For example, conspicuous pelagic diazotrophs like Trichodesmium and Crocosphaera express the IdiA protein in response to iron stress (Webb et al., 2001). Pioneering studies showed that the addition of iron enhanced growth and N2 fixation rates in cultured and field populations of Trichodesmium (Rueter, 1988; Paerl et al., 1994). Later, the cellular iron quotas of Trichodesmium were reported to be very high (Berman-Frank et al., 2001), with iron requirements during diazotrophic growth found to be as much as five-fold greater than when grown on ammonium (Kustka et al., 2003). When grown under moderate iron deficiency, cultured Crocosphaera responds by reducing its cellular size to overcome the limitation of growth and N2 fixation, while severe iron deficiency leads to disruption of the diazotrophic activity (Jacq et al., 2014). The release of extracellular polysaccharides has been hypothesized as an iron binding strategy in Crocosphaera (Sohm et al., 2011a), and in unidentified pico- and nano-sized diazotrophs of the NEA (Benavides et al., 2013c). UCYN-A increases nifH expression upon the addition of Fe or Saharan dust (Turk-Kubo, 2012; Krupke et al., 2014), suggesting that its N2 fixation activity may be partially controlled by dust deposition events, the chemical processes by which dust-derived Fe is dissolved into seawater, as well as any Fe-binding strategies of these microbes may have (Benavides et al., 2013c).
The biochemical mechanisms by which diazotrophs obtain iron in situ have yet to be fully characterized, but several experiments have confirmed that in situ N2 fixation activity responds to iron inputs. For example, Mills et al. (2004) reported a two- to three-fold increase of N2 fixation rates upon iron and phosphorus enrichment of seawater samples collected in the tropical NA. Langlois et al. (2012) observed an equivalent enhancement of N2 fixation rates in response to Saharan dust additions, which was also accompanied by an increase in the abundance of nifH phylotypes. In accordance with these artificially fertilized bioassays, natural peak inputs of Saharan dust to the NEA have been observed to increase N2 fixation rates and UCYN abundance by ~90–~60%, respectively (Benavides et al., 2013c). Dust inputs may also affect diazotrophic communities in areas distant from the NEA. For example, sporadic peaks in Trichodesmium abundance are often observed off Florida as a response to dust deposition (Lenes et al., 2001). This phenomenon produces enough dissolved organic nitrogen to promote toxic phytoplankton blooms (Lenes and Heil, 2010). These results confirm that N2 fixation reacts to dust and/or iron additions in time scales of hours to days.
The NA receives more desert dust than any other basin in the world, providing an estimated iron deposition of 6 Tg Fe y−1 (Prospero et al., 1996). Despite this great supply, the potential bioavailability of the iron deposited with dust is not well-understood (Mahowald et al., 2009). Significant correlations between dissolved iron concentrations, dust deposition rates (or dust deposition proxies like aerosol optical depth at 550 nm) and N2 fixation rates or diazotroph phylotypes abundance have been recurrently reported in the NA (e.g., Moore et al., 2009; Fernández et al., 2010; Benavides et al., 2013d; Ratten et al., 2014). In addition, it is now clear that the shifts in the geographical extent of coverage of the intertropical convergence zone (ITCZ) controls iron inputs and hence N2 fixation activity in the tropical and subtropical Atlantic (Schlosser et al., 2013). This “iron-rich cloud” acts as a frontier between the phosphorus-rich/iron-poor South Atlantic, and the phosphorus-poor/iron-rich NA (Capone, 2014).
Despite the evident control of iron on N2 fixation in the NA, it is unclear why the North Pacific has comparable N2 fixation rates given the negligible aeolian iron inputs to this basin (Mahowald et al., 2009). The predominance of different diazotroph phylotypes in the North Pacific and the North Atlantic has been hypothesized as an explanation for these differences (Deutsch et al., 2007), although the nifH sequences of different phylotypes isolated from the NA seem to be highly similar to those from the North Pacific (Langlois et al., 2005). As Luo et al. suggested (2014), other explanations include the saturation of N2 fixation by present iron supply, the adaptation of diazotrophs to low iron environments, and/or the biological unavailability of the iron deposited to the ocean. These authors also reported a poor relationship between N2 fixation and dust deposition from a global database (Luo et al., 2012), suggesting that iron is not the ultimate controlling factor of marine diazotrophy on a global basis (Luo et al., 2014). This implies that while empirical evidence suggests dust explains short-term diazotrophic responses, it cannot explain N2 fixation rates on a basin-scale or over geological time-scales in the NA (Straub et al., 2013).
N2 Fixation Contribution to New Production and Nitrogen Excess in the North Atlantic
Diazotrophic N2 fixation has been classically regarded as an important contribution to new production in chronically stratified oligotrophic areas of the oceans where the upwelling of deep nitrate rich waters is inhibited (Karl et al., 2002). Several studies in the NA have compared the upward diffusive flux of nitrate to 15N-labeled nitrate uptake rates. In most cases, nitrate uptake is greater than nitrate diffusion (e.g., Planas et al., 1999 see Table 2), a difference attributed to external fixed nitrogen inputs via atmospheric deposition, in situ remineralization of advected DOM, photic zone nitrification and/or N2 fixation (Capone et al., 2005; Landolfi et al., 2008; Mouriño-Carballido et al., 2011; Benavides et al., 2013b). The comparison of nitrate diffusive fluxes to in situ N2 fixation rates has resulted in a wide range of results (Tables 2, 3).
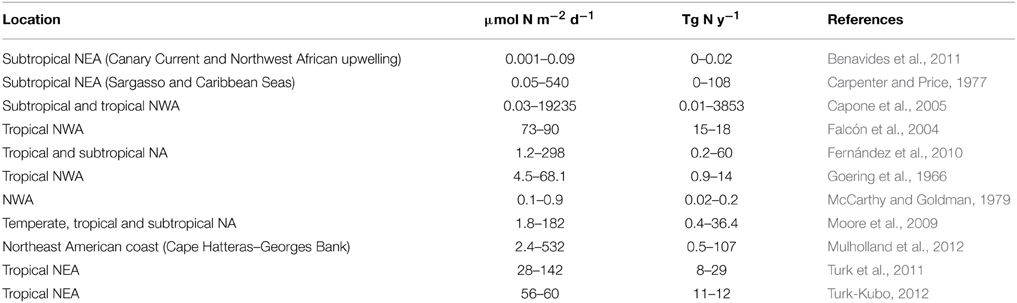
Table 3. List of integrated N2 fixation rates from the NA, compiled in Luo et al. Luo et al., 2012.
Capone et al. (2005) calculated that N2 fixation rates ranged from 50 to 180% of the parallel measured nitrate diapycnal fluxes in the tropical NWA, suggesting the major influence of diazotrophy in sustaining new production over this area. In the central subtropical and equatorial NA (~30 °W), Mouriño-Carballido et al. (2011) reported a contribution of N2 fixation to total new nitrogen inputs to the mixed layer of 22% (12°S to 16°N) and 2% (16 to 9°N). More recently, Painter et al. (2013) reported a contribution of 62% in an area north of that studied by Mouriño-Carballido et al. (26.5°N–29.5°W) 2011.
Differences between these estimates may arise from a variety of factors, including the geographical variability of hydrographic factors. The transport of nitrate into the surface layer occurs in two different ways, by advection, upwelling, or turbulent diffusion. Upwelling is caused by a vertically upward velocity (which is driven in general by the curl of the wind stress; Yoshida, 1967), or horizontal deformation fields in velocity (e.g., divergence or stretching deformation; Dippner et al., 2007). Turbulent diffusion depends on the gradient of the nitrate concentration and the characteristics of the turbulence field such as the vertical shear of the velocity field and the stability of the water column given by the vertical density gradient. The intensity of the turbulence and the depth of mixed layer are driven by the wind speed (Mellor and Yamada, 1982). Thus, nitrate uptake by phytoplankton is not directly coupled to N2 fixation, but the enhanced stability of the water column in the NWA as compared to the NEA translates into a lessened nitrate diffusion, which eventually makes the contribution of N2 fixation to total fixed nitrogen inputs greater than that derived from nitrate diffusion (Capone et al., 2005; Montoya et al., 2007).
In addition, the inherent seasonal variability of the NA, and the intermittent nature of mesoscale and submesoscale features may result in a highly variable contribution of N2 fixation to new production (Mouriño-Carballido et al., 2011; Painter et al., 2013). Finally, other factors such as the application of theoretical vs. empirical estimated turbulent diffusion coefficients (Kz) (Mouriño-Carballido et al., 2011; Arcos-Pulido et al., 2014), biases derived from measuring N2 fixation on sporadic Trichodesmium blooms (which might be not be representative of the long-term situation; Capone et al., 2005), 15N2 method underestimations (Mohr et al., 2010), or overestimations (Dabundo et al., 2014 see discussion below), and substantial nitrate regeneration in the photic zone (Yool et al., 2007), may also play an important role.
The N2 fixation rates reported in these studies are strikingly different. Capone et al. (2005) reported Trichodesmium-associated integrated N2 fixation rates averaging 239 μmol N m−2 d−1 (but up to 19235 μmol N m−2 d−1, see Table 3), while Mouriño-Carballido et al. (2011) and Painter et al. (2013) reported bulk seawater N2 fixation rates ranging from 1.3 to 154.5 μmol N m−2 d−1 and 15.4 to 95.6 μmol N m−2 d−1, respectively. Thus, it is clear that the contribution of N2 fixation to new production is greater in Trichodesmium-dominated waters of the NWA (Montoya et al., 2007), whereas minimal in the UCYN-dominated waters of the NEA (Benavides et al., 2013b; Arcos-Pulido et al., 2014).
Although N2 fixation has traditionally been regarded as an important source of new production, little is known about the fate of this fixed N2 or to what extent it fuels the biological carbon pump (Mulholland, 2007). The efficiency of fixed N2 transfer between trophic compartments and the mechanisms by which it is governed remain unclear (Mulholland, 2007), and whether the process is more efficient in Trichodesmium vs. UCYN or heterotrophic diazotrophs is yet to be determined. In principle, the transfer of fixed N2 should be highly efficient because the product of this reaction is ammonium, which is readily available to photoautotrophic microbes. Trichodesmium also releases large amounts of fixed N2 as labile dissolved organic nitrogen (Capone et al., 1994; Sipler et al., 2013), and the role of UCYN in providing these organic compounds could also be important (Benavides et al., 2013d). Modern high-resolution mass spectrometry techniques such as nano-scale secondary ion mass spectrometry (nanoSIMS) have recently been used to yield new insights into the efficiency of this transfer and the partitioning of fixed N2 between planktonic groups in different trophic levels. Symbiotic diazotrophs represent clear examples of fixed N2 transfer, like UCYN-A, which has been found to provide fixed N2 to its prymnesiophyte host from whom it receives carbon compounds (Thompson et al., 2012). This transfer has also been demonstrated in DDAs (Foster et al., 2011). However, the fate of N2 fixed by other non-symbiotic diazotrophs is unknown.
From a geochemical perspective, the recognition of biological N2 fixation as a significant input of fixed nitrogen to the NA has also been sustained by the recurrent observation of an excess fixed nitrogen signal over the intermediate waters of this basin (Michaels et al., 1996; Gruber and Sarmiento, 1997). The role of this excess in counterbalancing fixed nitrogen losses in oxygen minimum zones (OMZs) has been repeatedly discussed in the literature (e.g., Codispoti, 2007), and has even prompted improvements of the 15N2 fixation method that can potentially reconcile geochemical and biological estimates of N2 fixation in the ocean (Großkopf et al., 2012). Low phosphate availability can be driven by either diazotrophic phosphate-drawdown boosted by high iron availability in mid to low latitudes (Wu, 2000; Mather et al., 2008; Sohm and Capone, 2010) or by low non-Redfieldian (N:P <16) fast-growing phytoplankton nutrient uptake ratios at higher latitudes (Mills and Arrigo, 2010). Both N2 fixation and non-Redfieldian nutrient uptake patterns leave a fixed nitrogen excess signal (depicted by the N* parameter) which is maximal in mode waters (isopycnal surface σθ = 26.5 or 18°C water, and σθ = 27.1 or Subpolar Mode Water (SPMW) (e.g., Gruber and Sarmiento, 1997; Hansell et al., 2004), as shown in Figure 4. This excess nitrogen signal has been translated into basin-wide N2 fixation rates ranging from ~5 to 90 Tg N y−1 (Table 4) for the NA tropical and subtropical waters.
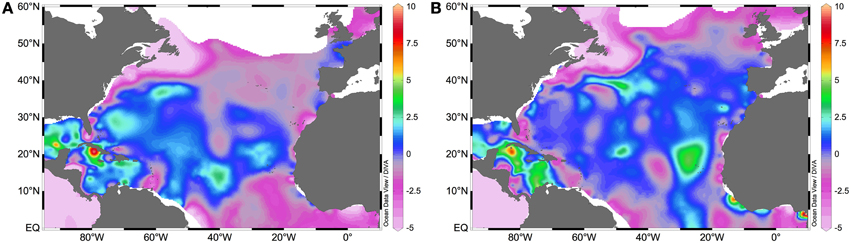
Figure 4. N* values (μM) at (A) σθ = 26.5 or 18°C water, and at (B) σθ = 27.1 or Subpolar Mode Water. N* was calculated as N* = [NO−3] − 16 × [PO3−4], where [NO−3] is the concentration of nitrate and [PO3−4] is the concentration of phosphate, both in μM. Nitrate and phosphate concentration data were retrieved from the World Ocean Atlas 2013 (Garcia et al., 2013).
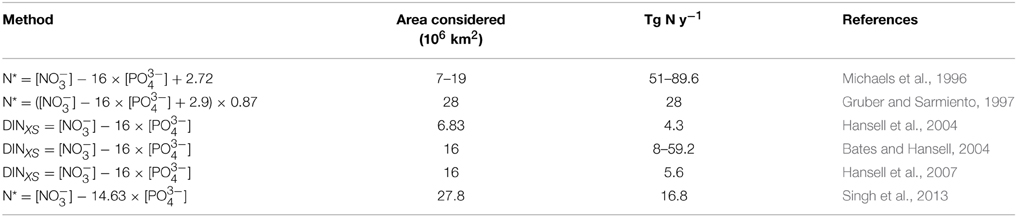
Table 4. Estimates of N2 fixation using a geochemical approach (N*) for different areas of the North Atlantic Ocean.
The rates estimated by Michaels et al. (1996) are four- to seven-fold higher than the most recent direct estimate rate (12.8 Tg N y−1; see the Supplementary Information of Großkopf et al., 2012), while the estimates of Hansell et al. (2004, 2007) are more conservative. However, it must be noted that these estimations rely on the extrapolation of nitrate excess formation rates over different areas of the NA (Table 4). In a recent paper, Singh et al. (2013) revisited the magnitude and distribution of N* in the NA taking into account other factors causing positive N* values. They calculated a basin-wide nitrate excess rate formation of 16.8 Tg N y−1, which is reasonably close to the average direct N2 fixation rate calculated by Großkopf et al. (2012). N2 fixation rates derived from N* estimates are potentially overestimated by non-diazotrophic positive N* signatures such as atmospheric deposition and of high N:P ratio compounds, and lateral advection of high N:P DOM (Landolfi et al., 2008; Letscher et al., 2013; Singh et al., 2013). Also, the high N:P uptake quotas typical of slow-growing picocyanobacteria which dominate subtropical gyres (i.e. Prochlorococcus) may reduce fixed nitrogen availability promoting diazotrophy which eventually produces a nitrogen excess signal in the thermocline of the NA subtropical gyre via (Mills and Arrigo, 2010). As Singh et al. (2013) stated, the N* parameter has to be interpreted with caution, because positive N* values are caused by a variety of processes other than N2 fixation. Predominantly, the temporal and spatial scales taken into account when deriving N2 fixation rates from N* and from direct 15N2 incubations are strikingly different. According to the timescales of thermocline ventilation, present N* observations likely reflect the N2 fixation activity that occurred a decade before (Gruber and Sarmiento, 1997). Moreover, positive N* signals travel downstream from the formation site along isopycnal surfaces (Hansell et al., 2004). As a result, there is a considerable spatial and temporal lag between the process and its signature. The N* signature is undoubtedly affected by atmospheric nitrogen deposition (which usually has high N:P; Duce et al., 1991), and by non-Redfieldian fluxes and remineralization of organic matter (Zamora et al., 2010; Singh et al., 2013). In turn, N2 fixation rates derived from 15N2 incubations assess the uptake of N2 in a closed volume of water (usually ranging from hundreds of mL to <5 L), along a limited incubation time (hours to days), in a specific geographical location and water column depth. In summary, while N* offers an easy and large-scale alternative to evaluate N2 fixation, it must be interpreted with great care, taking into account all other possible sources of positive N*, which might be difficult to constrain.
In general, the rates obtained with geochemical approaches exceed those obtained with biological approaches two to seven-fold (Tables 3, 4), although both types of estimates are highly variable and arguably have considerable uncertainties. This could either mean that geochemical-approach methods overestimate N2 fixation rates, or alternatively biological-approach methods underestimate (Mohr et al., 2010; Großkopf et al., 2012) or overestimate them (Dabundo et al., 2014). While the underestimation of N2 fixation rates by incomplete dissolution of the 15N2 bubble during short duration incubations generally translates into two to six-fold increases of the rates (Mohr et al., 2010; Großkopf et al., 2012; Wilson et al., 2012; Benavides et al., 2013d), the use of contaminated 15N2 gas stocks with other nitrogen compounds besides N2 (notably ammonium) may result in N2 fixation rates of 0.01 to 530 nmol N L−1 d−1 (as estimated by Dabundo et al., 2014). The upper limit of this broad range is of serious concern, as these rates surpass N2 fixation rates reported in many marine systems. However, the degree of contamination depends on the brand of 15N2 used (Dabundo et al., 2014), and thus not all published studies can be considered to be equally overestimated. Since current geochemical methods are not able to discern N2 fixation from other processes resulting in nitrogen excess signals, these methods are prone to result in overestimations of N2 fixation rates. Improvements in the large scale estimation of N* as well as in the empirical measurement of 15N2 fixation rates should bring denitrification and N2 fixation rates closer in the near future, eventually elucidating a contemporary balanced oceanic nitrogen cycle.
Crossing Boundaries: Heterotrophic and Aphotic N2 Fixation
Oceanic N2 fixation was once attributed solely to the filamentous cyanobacterium Trichodesmium (e.g., Capone, 1997). As discussed earlier, the advent of molecular techniques identified the previously unrecognized prevalence of UCYN (Zehr et al., 1998). These methodological approaches have also enabled the detection of non-cyanobacterial diazotrophs (bacteria and archaea), although their role in global marine N2 fixation has been largely overlooked (Farnelid and Riemann, 2008). Heterotrophic diazotroph nifH sequences group into four major clusters, namely Cluster I (including molybdenum nitrogenases from cyanobacteria, α-, β-, and γ-proteobacteria, and vanadium nitrogenases from γ-proteobacteria), Cluster II (composed of archaeal nitrogenases and bacterial iron nitrogenases, also including methanogens), Cluster III (comprising anaerobic bacteria and sulfate-reducing δ-proteobacteria), and Cluster IV (which includes non-functional archaeal nifH homologs). Present estimates indicate that heterotrophic nifH sequences account for >80% of the total number of nifH clones obtained from marine samples in a range of studies (Farnelid and Riemann, 2008). This high proportion highlights the need to understand the quantitative role of heterotrophic N2 fixation, although the usual nested PCR approach taken to obtain these clones (Zehr et al., 1998; Zehr and Turner, 2001) is not quantitative and hence the numerical majority of heterotrophic nifH derived from these methods is arguable (Riemann et al., 2010; Farnelid et al., 2011; Turk-Kubo et al., 2014) However, inconsistencies between the presence and the expression of their nifH genes has lead to skeptical points of view concerning their importance and contribution to total N2 fixation (Turk-Kubo et al., 2014).
The ecological role and the energy sources that maintain heterotrophic N2 fixation are uncertain. The differences between day and night heterotrophic nifH expression are not significant (Church et al., 2005; Moisander et al., 2014), suggesting the activity of these organisms could be independent of light. This opens the possibility of active N2 fixation below the euphotic zone, challenging the paradigm that N2 fixation is constrained to sunlit oligotrophic epipelagic waters. On the contrary, the predominance of non-cyanobacterial nitrogenase gene amplicons from surface oceanic waters collected around the world (Farnelid et al., 2011), suggests that heterotrophic diazotrophs may also obtain their energy through photoheterotrophic processes (e. g., Riemann et al., 2010; Moisander et al., 2014). This possibility is also supported by the significant relationship between solar light radiation and euphotic zone N2 fixation rates obtained worldwide (Luo et al., 2014), as previously hypothesized for the North Pacific by Karl et al. (2008). In any case, photic and aphotic heterotrophic diazotrophs probably belong to different phylotypes and obtain energy through different metabolic strategies.
Heterotrophic metabolisms imply that a source of bioavailable organic matter is needed for growth. At depth, fixed nitrogen concentrations are high and the lability of organic matter is low (Hedges, 2002), which inevitably leads to questions about the ecological benefit of aphotic heterotrophic N2 fixation. However, aphotic N2 fixation could well-occur in association with particles. Organic materials leaking from particles as a result of bacterial extracellular enzyme activity may be an important source of sustenance for heterotrophic N2 fixation (Riemann et al., 2010). In addition, particles may provide unique oxygen-deficient loci where the nitrogenase enzyme would be protected from destruction (Riemann et al., 2010). The pioneering work of Hans Paerl on particle-attached N2 fixation (Paerl, 1985; Paerl and Prufert, 1987) has, unfortunately, not been continued in modern microbial oceanography research, although some authors are currently working on the nitrogen metabolism of diazotrophic filamentous cyanobacteria aggregates (Klawonn et al., 2015). Heterotrophic N2 fixation activity has been confirmed in the coastal OMZ off Peru (Bonnet et al., 2013; Loescher et al., 2014), and in the oxygen-deficient waters of the Baltic Sea chemocline (Farnelid et al., 2013). However, matching rates to diazotroph phylotypes has been difficult (e.g., Turk-Kubo et al., 2014). Moreover, the mechanisms by which these organisms obtain energy are unknown. It has been hypothesized that in such environments, heterotrophic N2 fixation could be sustained by organic matter oxidation processes like denitrification or sulfate reduction, through the use of electron acceptors such as nitrate, nitrite or sulfate (Bonnet et al., 2013; Loescher et al., 2014).
In comparison with other oceanic basins, the NA OMZ is less pronounced than that of the Southeast Pacific (Stramma et al., 2008a). In principle, this would indicate a minor role for heterotrophic N2 fixation in the NA OMZ as compared to the Southeast Pacific, unless these heterotrophs rely on oxygen-poor microzones provided by particle-attached modes of living. In the NA, Baltar et al. (2009) observed an equatorward increase in bacterial abundance and activity in the mesopelagic and bathypelagic zones, related to a higher abundance of suspended particles. The potential role of these microhabitats in sustaining pelagic heterotrophic N2 fixation in the North Atlantic is an unsolved mystery. In the Sargasso Sea, Hewson et al. (2007) detected heterotrophic nifH genes in mesopelagic to abyssopelagic waters. Although these authors did not provide parallel N2 fixation rates, the presence of these phylotypes suggests that nitrogenase activity should be significant there. To our knowledge, there are no other aphotic diazotrophy studies in the NA. In this basin, the dependence of aphotic prokaryotes on particulate organic matter (Baltar et al., 2009) and on nutrient availability controlled by water mass mixing (Reinthaler et al., 2013) suggests that heterotrophic aphotic N2 fixation may be affected by these factors too. In the future, new investigations coupling organic matter characterization, nifH quantification and N2 fixation rate measurement in aphotic waters should help reveal the real importance of aphotic diazotrophy. Considering N2 fixation rates in aphotic waters could potentially increase global estimates enough to reconcile present differences between N2 fixation and denitrification (Bonnet et al., 2013), which has important consequences in the ability of our oceans to maintain uptake of CO2 (Codispoti, 2007).
What is there Left to be Known?
Ever since the pioneering work of Dugdale et al. (1961), the North Atlantic has been subject to a great amount of diazotrophy-related studies, with a clear NWA bias in their geographical distribution. To date, we have amassed considerable good knowledge of the distribution and magnitude of N2 fixation rates, the abundance and distribution of common qPCR target groups (most commonly UCYN-A, -B, -C, Trichodesmium and Het-1), and the importance of phosphorus and aeolian dust-related iron in controlling diazotrophic activity and diversity in this basin. After five decades of N2 fixation research in the North Atlantic, there are still open questions to be answered, which are also open questions for N2 fixation research in other basins:
1. How do different groups contribute to N2 fixation rates?: Many authors have used size-fractionation to ascribe rates to different groups of diazotrophs (e.g., Moore et al., 2009; Benavides et al., 2011). Such manipulations are prone to alter the behavior of the cells and to enhance dissolved organics release, among a variety of other problems (Mulholland, 2007; Benavides et al., 2013d). The comparison of in situ N2 fixation rates with parallel nifH copy abundance has been used extensively to indicate which groups are responsible for the measured activity (most studies are compiled in Luo et al., 2012), but the presence of nifH genes does not guarantee a measurable N2 fixation activity (Turk-Kubo, 2012). The tropical and subtropical NA has been long thought to be dominated by Trichodesmium, but despite they abound in the western NA, on a general basis UCYN phylotypes are more abundant (especially UCYN-A; Luo et al., 2012), and nifH expression by UCYN can be more important than by Trichodesmium locally, suggesting that in fact UCYN may be more representative of bulk N2 fixation rates than Trichodesmium, and changing classic paradigms of Trichodesmium dominance in the NA (Turk-Kubo, 2012). Still, the quantification of nifH transcripts does not indicate how to divide the measured N2 fixation activity among the active phylotypes found (Church et al., 2005). The identity-activity matching problem is worsened by the lack of specificity of currently published qPCR primer-probe sets, a problem which is being solved in part for some phylotypes like UCYN-A (Thompson et al., 2014). The development of single-cell approaches like nanoSIMS combined with halogen in situ hybridization (HISH-SIMS) has allowed the quantification of specific N2 fixation rates (Thompson et al., 2012; Krupke et al., 2013, 2014) although the HISH procedure has been shown to dilute the 15N signal, resulting in inaccurate rates (Musat et al., 2014). Other procedures like the combined use of catalyzed reporter deposition fluorescence in situ hybridization (CARD-FISH) with flow cytometry sorting holds great promise, but also comes with difficulties (McInnes et al., 2014). In summary, the combination of new methodologies and the development of single-cell approaches is significantly increasing our capability to quantify compound fluxes within and between planktonic cells, but there is much work to be done before straight-forward methodologies are developed and all aforementioned drawbacks are overcome. All in all, the days of compound “black box” studies are soon to be over.
2. Where does the fixed N2 go?: N2 fixation is considered an input of “new nitrogen” (sensu Dugdale and Goering, 1967), as it comes from outside the system and not from in situ recycled production. As a new nitrogen source, diazotrophy-derived fixed nitrogen theoretically fuels export production (Capone et al., 2005). In practice however, current research has not resolved whether fixed N2 is preferentially transferred to higher trophic levels, recycled via the microbial loop, or if it fuels export production and the biological pump (Mulholland, 2007), nor have any of these fluxes been accurately quantified, largely due to the lack of appropriate methods. DDAs seem to clearly contribute to carbon sequestration and supply fixed nitrogen for remineralization at depth, as it has been seen in the Amazon River plume and at station ALOHA (Subramaniam et al., 2008; Karl et al., 2012), which is logical given the sinking character of these symbioses. The role of slow sinking diazotrophs like UCYN or floating colonies like those of Trichodesmium is relatively unknown. Over the past two decades several authors have quantified the release of recently fixed N2 in the form of ammonium and/or dissolved organic nitrogen, mainly from Trichodesmium (Glibert and Bronk, 1994; Mulholland et al., 2004), but also from smaller diazotrophs (Benavides et al., 2013a,d). The metabolism behind this behavior is unknown, but this activity is recurrently found in different marine systems and culture studies like those referenced above. The lability of dissolved organic nitrogen and its potential to fuel autotrophic production is not well-understood due to constraints in compositional analysis (Bronk et al., 2007). Recent advances in DOM characterization are currently starting to answer some of these questions. For example, Sipler et al. (2013) have shown that Trichodesmium produces labile dissolved organic nitrogen compounds capable of triggering toxic microalgae blooms. Integrative approaches including stable isotope tracing experiments in combination with state-of-the-art DOM characterization techniques hold great promise for new discoveries.
The other path of fixed nitrogen transfer is direct consumption of diazotrophs by higher trophic levels. Cyanobacteria had been traditionally considered being less palatable than other phytoplankton due to their low nutritional value and the toxin production in the cells (Sellner, 1997). There are two pathways that transport fixed nitrogen to upper trophic levels. One is the direct ingestion of cyanobacteria (e.g., for Trichodesmium filaments by a harpacticoid copepod Macrosetella gracilis; O'Neil and Roman, 1994), or the uptake of released fixed nitrogen (as described above) by heterotrophic bacteria, entering the microbial food web. Subsequently, microzooplankton sustaining their growth on fixed nitrogen can be consumed by mesozooplankton (Sellner et al., 1994; Montoya et al., 2002; Wannicke et al., 2013). Up the chain, this transport of fixed nitrogen can even stimulate fish production (Rolff et al., 2007). Montoya et al. (2002) estimated that the contribution of diazotrophy to the transfer of bulk surface particulate nitrogen can be as high as 30–40% in a bloom of Trichodesmium. However, when the abundance of cyanobacterial cells is low, the transfer may be also low and hence their contribution to mesozooplankton nutrition negligible (Wannicke et al., 2010). A mesocosm study revealed a strong dependency of grazing processes on the development of heterocysts (Chan et al., 2006). Successful growth could be interrupted by a high grazing activity of zooplankton, which may reduce the filament length and heterocyst frequency in cyanobacteria, as it occurs with Anabaena (Chan et al., 2006). In summary, cyanobacteria play a significant but mainly indirect role for the nitrogen transfer to higher trophic levels. The role of large colony forming species vs. the role of unicellular species cannot be currently assessed.
3. Are heterotrophic diazotrophs important?: Recently, there has been increased interest on diazotroph phylotypes other than cyanobacteria. Some authors have shown that non-cyanobacterial diazotrophs are widespread in the oceans and potentially very diverse (Riemann et al., 2010; Farnelid et al., 2011), as well as present in previously unrecognized sites like the dark pelagic realm (Hewson et al., 2007; Bonnet et al., 2013; Rahav et al., 2013). It has yet to be determined how much these phylotypes contribute to measured N2 fixation rates, given current difficulties in matching activity to identity, as discussed above. The ubiquity of heterotrophic diazotrophs in the world's oceans and their predominance in certain systems like the South Pacific Gyre (Halm et al., 2011) suggests that, although their specific activity might be lower than that of other diazotrophs like Trichodesmium, their N2 fixation rates are far from negligible and deserve further study (Falcón et al., 2004). There is an urgent need to develop methodologies that allow us to discern autotrophic from heterotrophic N2 fixation, as well as to further investigate the diversity of this group, which spreads among the four recognized nifH clusters (Riemann et al., 2010). Being heterotrophic, the N2 fixation activity of this group of diazotrophs may significantly differ from that of autotrophic cyanobacterial diazotrophs, posing a major challenge for microbial oceanographers and opening a variety of new questions which require novel and interdisciplinary approaches.
4. How will diazotrophy react to climate change?: Current CO2 partial pressure (pCO2) levels are expected to double by the end of this century (IPCC, 2007). The main consequences of this increase which will affect planktonic organisms are (a) seawater acidification, (b) increase of seawater temperature, (c) water column stratification, (d) expansion of OMZs, and (e) desertification of terrestrial systems and associated increase of desert dust inputs to the ocean (Boyd and Doney, 2002; Jickells, 2005; Stramma et al., 2008b). While seawater acidification can either enhance photosynthetic activity in some species, or be harmful for others (Malakoff, 2012), seawater warming and stratification will likely enhance the proliferation of diazotrophic cyanobacteria such as Trichodesmium (Lomas et al., 2012), although their response to increased pCO2 levels may depend on a variety of environmental factors (Shi et al., 2012). Indeed, culture experiments have revealed that the length of Trichodesmium filaments and their N2 fixation rates increase when exposed to increasing levels of CO2 (Levitan et al., 2007). However, experiments including several different diazotrophic strains suggest that the response to increased pCO2 is strain-specific and depends on the CO2 uptake kinetics of each diazotroph type (Hutchins et al., 2013). Similarly, field studies have found divergent responses to increasing pCO2 levels, indicating that different phylotypes are unequally affected by acidification, which results in a diluted effect on bulk N2 fixation field measurements (e.g., Gradoville et al., 2014). Heterotrophic N2 fixation in hypoxic zones (Hamersley et al., 2011), suggests that the predicted expansion of OMZs (Stramma et al., 2008b) will likely enhance global N2 fixation rates too, either by in situ heterotrophic N2 fixation, or by the enhancement of diazotrophy in other oceanic areas in order to balance increased denitrification occurring in the OMZs (Deutsch et al., 2007). N2 fixation by Trichodesmium and other diazotrophs would be further enhanced by an increased availability of iron through desert dust deposition in the oceans (Jickells, 2005). Michaels et al. (2001) proposed a theoretical climate-based N2 fixation feedback cycle. In this conceptual model, desertification causes a global increase of N2 fixation rates as a result of iron limitation alleviation through increased desert dust deposition, which in turn lowers atmospheric CO2 levels and decreases global temperatures. Global cooling may in turn decrease desert dust inputs diminishing N2 fixation and raising CO2 levels, which would again raise global temperatures. However, increased inputs of iron to the ocean will likely affect N2 fixation activity as a function of in situ phosphorus availability, suggesting that diazotrophic activity in the phosphorus-deficient NA may not be positively affected by increased dust inputs in the future (Garcia et al., 2014). In conclusion, diazotrophic responses to atmospheric CO2 levels will likely have an important role in carbon and nitrogen cycling and subsequent productivity in the future oceans, although their impact will depend on a variety of factors which are currently poorly constrained.
Summary and Concluding Remarks
The role of the NA in comparison with other basins: In Table 5 we compile the most recent basin-wide N2 fixation estimates for the main oceanic basins. Different basin-wide averaging methods yield different results, which are also affected by the latitudinal -and hence areal- range considered. All methods indicate that the NA ranks as the third most important oceanic basin in contributing to global N2 fixation (Table 5). Depending on the method used (geometric and arithmetic mean areal rates by Luo et al., 2012, or station-weighted mean areal rates by Großkopf et al., 2012), the percentage contribution of NA N2 fixation to the global sum ranges from 2.74 to 23.36%. Considering that the global arithmetic mean areal N2 fixation rate of Luo et al. (2012), and the global weighted mean of Großkopf et al. (2012) are closer to current geochemical global estimates of N2 fixation (100–200 Tg N y−1 Deutsch et al., 2007; Gruber and Galloway, 2008), we consider these estimates more appropriate and thus conclude that the NA most probably provides 10–25% of global fixed N2. The estimates presented here are somewhat uncertain given the current undersampling in other basins as compared to the NA (e.g., the South Atlantic, South Pacific, and especially the Indian Ocean), as well as the potential importance of diazotrophic activity at higher latitudes (>50°N or 50°S), and in the aphotic zone (>200 m).
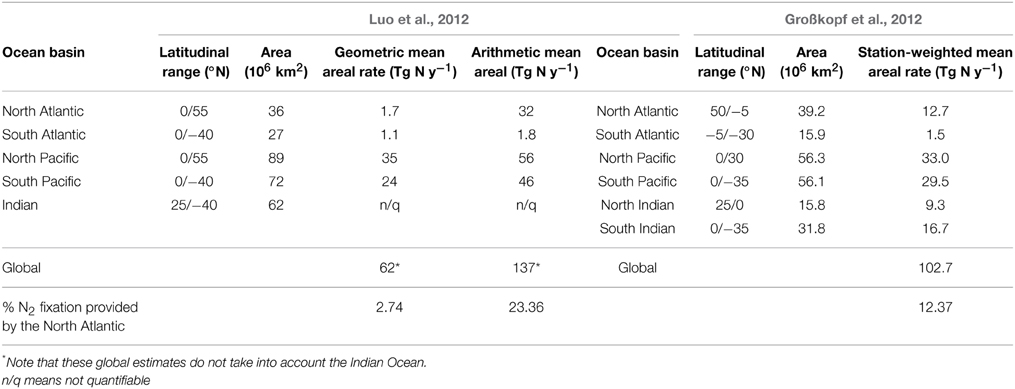
Table 5. Basin-wide N2 fixation rates compiled from the literature (Modified from Großkopf et al., 2012; Luo et al., 2012).
The biogeochemical and physical features of the NA influence the distribution and activity of diazotrophs. In the NWA, the low N:P ratio conditions and stratified water column appears to select for Trichodesmium. Although the NEA receives more iron-rich dust inputs than the NWA, the colder waters and more turbulent conditions of the NEA (influence of Northwest African upwelling) seems to constrain the proliferation of Trichodesmium. These colder and dissolved inorganic nitrogen-rich waters are preferentially inhabited by UCYN phylotypes. UCYN-A is the most widespread of the three, while UCYN-B and UCYN-C are constrained to tropical latitudes, with the latter more associated with coastal regions. The Amazon and Congo River plumes are characterized by high dissolved inorganic nitrogen and phosphate inputs, but the rapid consumption of dissolved inorganic nitrogen by non-diazotrophic phytoplankton leaves a low N:P signal which, combined with high silica inputs, sustains the proliferation of DDAs and UCYN (the latter closer to saltier open-ocean waters). Heterotrophic diazotrophs seem to be widespread and more flexible, capable of adapting to contrasting conditions and presumably able to exploit in situ DOM.
The wide distribution of small diazotrophs (UCYN and heterotrophs) predicts that their contribution to total basin-scale fixed nitrogen inputs is important. However, linking rates to species has been hampered by several difficulties (Zehr et al., 2007; Turk-Kubo et al., 2014) and so far UCYN-associated N2 fixation rates have been mostly calculated from size-fractionation studies (usually discerning between >10 and <10 μm fractions (e.g., Benavides et al., 2011), although new single-cell approaches are currently able to give specific N2 fixation rates for some phylotypes (Foster et al., 2011; Krupke et al., 2013). Still, N2 fixation rates in UCYN-dominated areas of the NEA are lower than those measured in the NWA, suggesting that the N2 fixation capacity of UCYN is lower than that of Trichodesmium and DDAs. Thus, it seems clear that the contribution of N2 fixation to new production is greater in Trichodesmium-dominated waters of the NWA (Montoya et al., 2007), but minimal in the UCYN-dominated waters of the NEA. The contribution of N2 fixation to new production is thus greater in Trichodesmium-dominated waters of the NWA. The low contribution of diazotrophic N2 fixation to new production in the NEA suggests that the fixed nitrogen requirements of autotrophic planktonic communities in the subtropical NEA and inner subtropical gyre are met by a combination of atmospheric fixed nitrogen deposition (as this area is located downwind of European industrialized countries), in situ remineralization of advected DOM and, to a minor extent, in situ photic nitrate regeneration (nitrification) and N2 fixation.
N2 fixation rates obtained with geochemical approaches exceed those obtained with biological approaches two to seven-fold. The differences between both groups of methods stem from nitrogen excess signals derived from processes other than N2 fixation (such as non-Redfieldian uptake ratios and atmospheric deposition of nitrogenous compounds), but also from methodological underestimations (and overestimations) of the 15N2 method. Future methodological developments should allow overcoming current drawbacks in the estimation of N2 fixation rates (Mohr et al., 2010; Dabundo et al., 2014). Nevertheless, our ability to reconcile present imbalances between global denitrification and N2 fixation rates will eventually depend on the accuracy of denitrification estimates and the quantification of other sources of fixed nitrogen to the ocean.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
MB was supported by a postdoctoral fellowship from the People Programme (Marie Skłodowska-Curie Actions) of the European Union's Seventh Framework Programme (FP7/2007–2013) under REA grant agreement number 625185. The authors would like to thank J. Dippner for his invaluable comments on the physical forcing section.
References
Agawin, N., Benavides, M., Busquets, A., Ferriol, P., Stal, L. J., and Arístegui, J. (2014). Dominance of unicellular cyanobacteria in the diazotrophic community in the Atlantic Ocean. Limnol. Oceanogr. 59, 623–637. doi: 10.4319/lo.2014.59.2.0623
Agawin, N. S., Tovar-Sánchez, A., de Zarruk, K. K., Duarte, C. M., and Agustí, S. (2013). Variability in the abundance of Trichodesmium and nitrogen fixation activities in the subtropical NE Atlantic. J. Plankton Res. 35, 1126–1140. doi: 10.1093/plankt/fbt059
Álvarez-Salgado, X. A., Arístegui, J., Barton, E. D., and Hansell, D. A. (2007). Contribution of upwelling filaments to offshore carbon export in the subtropical Northeast Atlantic Ocean. Limnol. Oceanogr. 52, 1287–1292. doi: 10.4319/lo.2007.52.3.1287
Ammerman, J. W., Hood, R. R., Case, D. A., and Cotner, J. B. (2003). Phosphorus deficiency in the Atlantic: an emerging paradigm in oceanography. Eos Trans. Am. 84, 165–170. doi: 10.1029/2003EO180001
Arcos-Pulido, M., Rodríguez-Santana, A., Emelianov, M., Paka, V., Arístegui, J., Benavides, M., et al. (2014). Diapycnal nutrient fluxes on the northern boundary of Cape Ghir upwelling region. Deep Sea Res. Part I 84, 100–109. doi: 10.1016/j.dsr.2013.10.010
Bahamón, N., Velásquez, Z., and Cruzado, A. (2003). Chlorophyll and nitrogen flux in the tropical North Atlantic Ocean. Deep Sea Res. Part I 50, 1189–1203. doi: 10.1016/S0967-0637(03)00145-6
Baltar, F., Arístegui, J., Gasol, J. M., Sintes, E., and Herndl, G. J. (2009). Evidence of prokaryotic metabolism on suspended particulate organic matter in the dark waters of the subtropical North Atlantic. Limnol. Oceanogr. 54, 182–193. doi: 10.4319/lo.2009.54.1.0182
Bates, N. R., and Hansell, D. A. (2004). Temporal variability of excess nitrate in the subtropical mode water of the North Atlantic Ocean. Mar. Chem. 84, 225–241. doi: 10.1016/j.marchem.2003.08.003
Benavides, M., Agawin, N., Arístegui, J., Ferriol, P., and Stal, L. J. (2011). Nitrogen fixation by Trichodesmium and small diazotrophs in the subtropical northeast Atlantic. Aquat. Microb. Ecol. 65, 43–53. doi: 10.3354/ame01534
Benavides, M., Agawin, N., Arístegui, J., Peene, J., and Stal, L. J. (2013a). Dissolved organic nitrogen and carbon release by a marine unicellular diazotrophic cyanobacterium. Aquat. Microb. Ecol. 69, 69–80. doi: 10.3354/ame01621
Benavides, M., Arístegui, J., Agawin, N. S. R., Álvarez-Salgado, X. A., Álvarez, M., and Troupin, C. (2013b). Low contribution of N2 fixation to new production and excess nitrogen in the subtropical northeast Atlantic margin. Deep Sea Res. Part I 81, 36–48. doi: 10.1016/j.dsr.2013.07.004
Benavides, M., Arístegui, J., Agawin, N. S. R., López Cancio, J., and Hernández-León, S. (2013c). Enhancement of nitrogen fixation rates by unicellular diazotrophs vs. Trichodesmium after a dust deposition event in the Canary Islands. Limnol. Oceanogr. 58, 267–275. doi: 10.4319/lo.2013.58.1.0267
Benavides, M., Bronk, D. A., Agawin, N. S. R., Pérez-Hernández, M. D., Hernández-Guerra, A., and Arístegui, J. (2013d). Longitudinal variability of size-fractionated N2 fixation and DON release rates along 24.5°N in the subtropical North Atlantic. J Geophys. Res. Oceans 118, 3406–3415. doi: 10.1002/jgrc.20253
Bergman, B., Sandh, G., Lin, S., Larsson, J., and Carpenter, E. J. (2012). Trichodesmium - a widespread marine cyanobacterium with unusual nitrogen fixation properties. FEMS Microbiol. Rev. 37, 286–302. doi: 10.1111/j.1574-6976.2012.00352.x
Berman-Frank, I., Cullen, J. T., Shaked, Y., Sherrell, R. M., and Falkowski, P. G. (2001). Iron availability, cellular iron quotas, and nitrogen fixation in Trichodesmium. Limnol. Oceanogr. 46, 1249–1260. doi: 10.4319/lo.2001.46.6.1249
Berman-Frank, I., Quigg, A., Finkel, Z. V., Irwin, A. J., and Haramaty, L. (2007). Nitrogen-fixation strategies and Fe requirements in cyanobacteria. Limnol. Oceanogr. 52, 2260–2269. doi: 10.4319/lo.2007.52.5.2260
Bonnet, S., Dekaezemacker, J., Turk-Kubo, K. A., Moutin, T., Hamersley, R. M., Grosso, O., et al. (2013). Aphotic N2 Fixation in the Eastern Tropical South Pacific Ocean. PLoS ONE 8:e81265. doi: 10.1371/journal.pone.0081265.s001
Boyd, P. W., and Doney, S. C. (2002). Modelling regional responses by marine pelagic ecosystems to global climate change. Geophys. Res. Lett. 29, 53–1–53–4. doi: 10.1029/2001GL014130
Breitbarth, E., Oschlies, A., and LaRoche, J. (2007). Physiological constraints on the global distribution of Trichodesmium - effect of temperature on diazotrophy. Biogeosciences 4, 53–61. doi: 10.5194/bg-4-53-2007
Bronk, D. A., See, J. H., Bradley, P., and Killberg, L. (2007). DON as a source of bioavailable nitrogen for phytoplankton. Biogeosciences 4, 283–296. doi: 10.5194/bg-4-283-2007
Capone, D. G. (1997). Trichodesmium, a globally significant marine Cyanobacterium. Science 276, 1221–1229. doi: 10.1126/science.276.5316.1221
Capone, D. G. (2014). An iron curtain in the Atlantic Ocean forms a biogeochemical divide. Proc. Natl. Acad. Sci. U.S.A. 111, 1231–1232. doi: 10.1073/pnas.1322568111
Capone, D. G., Bronk, D. A., Mulholland, M. R., and Carpenter, E. J. (2008). Nitrogen in the Marine Environment. San Diego, CA: Academic Press.
Capone, D. G., Burns, J. A., Montoya, J. P., Subramaniam, A., Mahaffey, C., Gunderson, T., et al. (2005). Nitrogen fixation by Trichodesmium spp.: an important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Global Biogeochem. Cycles 19:GB2024. doi: 10.1029/2004GB002331
Capone, D. G., Ferrier, M. D., and Carpenter, E. J. (1994). Amino acid cycling in colonies of the planktonic marine cyanobacterium Trichodesmium thiebautii. Appl. Environ. Microbiol. 60, 3989–3995.
Carpenter, E. J., and Price, C. C. (1977). Nitrogen fixation, distribution, and production of Oscillatoria (Trichodesmium) spp. in the western Sargasso and Caribbean Seas. Limnol. Oceanogr. 22, 60–72. doi: 10.4319/lo.1977.22.1.0060
Carpenter, E. J., and Romans, K. (1991). Major role of the cyanobacterium Trichodesmium in nutrient cycling in the North Atlantic Ocean. Science 254:1356. doi: 10.1126/science.254.5036.1356
Carpenter, E. J., Montoya, J. P., Burns, J., Mulholland, M. R., Subramaniam, A., and Capone, D. G. (1999). Extensive bloom of a N2-fixing diatom/cyanobacterial association in the tropical Atlantic Ocean. Mar. Ecol. Prog. Ser. 185, 273–283. doi: 10.3354/meps185273
Chan, F., Marino, R. L., Howarth, R. W., and Pace, M. L. (2006). Ecological constraints on planktonic nitrogen fixation in saline estuaries. II. Grazing controls on cyanobacterial population dynamics. Mar. Ecol. Prog. Ser. 309, 41–53. doi: 10.3354/meps309041
Church, M. J., Short, C. M., Jenkins, B. D., Karl, D. M., and Zehr, J. P. (2005). Temporal Patterns of Nitrogenase Gene (nifH) Expression in the Oligotrophic North Pacific Ocean. Appl. Environ. Microbiol. 71, 5362–5370. doi: 10.1128/AEM.71.9.5362-5370.2005
Codispoti, L. A. (2007). An oceanic fixed nitrogen sink exceeding 400 Tg N a−1 vs the concept of homeostasis in the fixed-nitrogen inventory. Biogeosciences 4, 233–253. doi: 10.5194/bg-4-233-2007
Dabundo, R., Lehmann, M. F., Treibergs, L., Tobias, C. R., Altabet, M. A., Moisander, P. H., et al. (2014). The contamination of commercial 15N2 Gas Stocks with 15N-labeled nitrate and ammonium and consequences for nitrogen fixation measurements. PLoS ONE 9:e110335. doi: 10.1371/journal.pone.0110335
Davis, C. S., and McGillicuddy, D. J. Jr. (2006). Transatlantic abundance of the N2-fixing colonial cyanobacterium trichodesmium. Science 312, 1517–1520. doi: 10.1126/science.1123570
Deutsch, C., Sarmiento, J. L., Sigman, D. M., Gruber, N., and Dunne, J. P. (2007). Spatial coupling of nitrogen inputs and losses in the ocean. Nature 445, 163–167. doi: 10.1038/nature05392
Dietze, H., Oschlies, A., and Khler, P. (2004). Internal-wave-induced and double-diffusive nutrient fluxes to the nutrient-consuming surface layer in the oligotrophic subtropical North Atlantic. Ocean Dyn. 54, 1–7. doi: 10.1007/s10236-003-0060-9
Dippner, J. W., Nguyen, K. V., Hein, H., Ohde, T., and Loick, N. (2007). Monsoon-induced upwelling off the Vietnamese coast. Ocean Dynam. 57, 46–62. doi: 10.1007/s10236-006-0091-0
Duce, R. A., Liss, P. S., Merrill, J. T., Atlas, E. L., Buat-Menard, P., Hicks, B. B., et al. (1991). The atmospheric input of trace species to the world ocean. Global Biogeochem. Cycles 5, 193–259. doi: 10.1029/91GB01778
Dugdale, R. C., and Goering, J. J. (1967). Uptake of new and regenerated forms of nitrogen in primary productivity. Limnol. Oceanogr. 12, 196–206. doi: 10.4319/lo.1967.12.2.0196
Dugdale, R. C., Goering, J. J., and Ryther, J. H. (1964). High nitrogen fixation rates in the Sargasso Sea and the Arabian Sea. Limnol. Oceanogr. 9, 507–510. doi: 10.4319/lo.1964.9.4.0507
Dugdale, R. C., Menzel, D. W., and Ryther, J. H. (1961). Nitrogen fixation in the Sargasso Sea. Deep Sea Res. 7, 298–300. doi: 10.1016/0146-6313(61)90051-X
Dyhrman, S. T., Chappell, P. D., Haley, S. T., Moffett, J. W., Orchard, E. D., Waterbury, J. B., et al. (2006). Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 439, 68–71. doi: 10.1038/nature04203
Dyhrman, S. T., Webb, E. A., Anderson, D. M., Moffett, J. W., and Waterbury, J. B. (2002). Cell-specific detection of phosphorus stress in Trichodesmium from the Western North Atlantic. Limnol. Oceanogr. 47, 1832–1836. doi: 10.4319/lo.2002.47.6.1832
Falcón, L. I., Carpenter, E. J., Cipriano, F., Bergman, B., and Capone, D. G. (2004). N2 fixation by unicellular bacterioplankton from the Atlantic and Pacific Oceans: phylogeny and in situ rates. Appl. Environ. Microbiol. 70, 765–770. doi: 10.1128/AEM.70.2.765-770.2004
Falkowski, P. G. (1997). Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature 387, 272–275. doi: 10.1038/387272a0
Farnelid, H., and Riemann, L. (2008). “Heterotrophic N2-fixing bacteria: overlooked in the marine nitrogen cycle?,” in Nitrogen Fixation Research Progress, ed G. N. Couto (New York, NY: Nova Science Publishers, Inc.), 409–423.
Farnelid, H., Andersson, A. F., Bertilsson, S., Al-Soud, W. A., Hansen, L. H., Sørensen, S., et al. (2011). Nitrogenase gene amplicons from global marine surface waters are dominated by genes of non-cyanobacteria. PLoS ONE 6:e19223. doi: 10.1371/journal.pone.0019223
Farnelid, H., Bentzon-Tilia, M., Andersson, A. F., Bertilsson, S., Jost, G., Labrenz, M., et al. (2013). Active nitrogen-fixing heterotrophic bacteria at and below the chemocline of the central Baltic Sea. ISME J. 7, 1413–1423. doi: 10.1038/ismej.2013.26
Fernández, A., Grana, R., Mouriño-Carballido, B., Bode, A., Varela, M., Domínguez-Yanes, J. F., et al. (2012). Community N2 fixation and Trichodesmium spp. abundance along longitudinal gradients in the eastern subtropical North Atlantic. ICES J. Mar. Sci. 70, 223–231. doi: 10.1093/icesjms/fss142
Fernández, A., Mouriño-Carballido, B., Bode, A., Varela, M., and Marañón, E. (2010). Latitudinal distribution of Trichodesmium spp. and N2 fixation in the Atlantic Ocean. Biogeosciences 7, 3167–3176. doi: 10.5194/bg-7-3167-2010
Foster, R. A., Kuypers, M. M. M., Vagner, T., Paerl, R. W., Musat, N., and Zehr, J. P. (2011). Nitrogen fixation and transfer in open ocean diatom–cyanobacterial symbioses. ISME J. 5, 1484–1493. doi: 10.1038/ismej.2011.26
Foster, R. A., Subramaniam, A., and Zehr, J. P. (2009). Distribution and activity of diazotrophs in the Eastern Equatorial Atlantic. Environ. Microbiol. 11, 741–750. doi: 10.1111/j.1462-2920.2008.01796.x
Foster, R. A., Subramaniam, A., Mahaffey, C., Carpenter, E. J., Capone, D. G., and Zehr, J. P. (2007). Influence of the Amazon River plume on distributions of free-living and symbiotic cyanobacteria in the western tropical north Atlantic Ocean. Limnol. Oceanogr. 52, 517–532. doi: 10.4319/lo.2007.52.2.0517
Garcia, H. E., Locarnini, R. A., Boyer, T. P., Antonov, J. I., Baranova, O. K., Zweng, M. M., et al. (2013). “Dissolved inorganic nutrients (phosphate, nitrate, silicate),” in World Ocean Atlas 2013, Vol. 4, eds S. Levitus and A. Mishonov (NOAA Atlas NESDIS 76), 25.
Garcia, N. S., Fu, F., Sedwick, P. N., and Hutchins, D. A. (2014). Iron deficiency increases growth and nitrogen-fixation rates of phosphorus-deficient marine cyanobacteria. ISME J. 9, 1–8. doi: 10.1038/ismej.2014.104
Glibert, P. M., and Bronk, D. A. (1994). Release of dissolved organic nitrogen by marine diazotrophic cyanobacteria, Trichodesmium spp. Appl. Environ. Microbiol. 60, 3996–4000.
Goebel, N. L., Turk, K. A., Achilles, K. M., Paerl, R., Hewson, I., Morrison, A. E., et al. (2010). Abundance and distribution of major groups of diazotrophic cyanobacteria and their potential contribution to N2 fixation in the tropical Atlantic Ocean. Environ. Microbiol. 12, 3272–3289. doi: 10.1111/j.1462-2920.2010.02303.x
Goering, J. J., Dugdale, R. C., and Menzel, D. W. (1966). Estimates of in situ rates of nitrogen uptake by Trichodesmium sp. in the tropical Atlantic Ocean. Limnol. Oceanogr. 11, 614–620. doi: 10.4319/lo.1966.11.4.0614
González-Dávila, M., Santana-Casiano, J. M., and de Armas, D. (2006). The influence of island generated eddies on the carbon dioxide system, south of the Canary Islands. Mar. Chem. 99, 177–190. doi: 10.1016/j.marchem.2005.11.004
Gradoville, M. R., White, A. E., Böttjer, D., and Church, M. J. (2014). Diversity trumps acidification: Lack of evidence for carbon dioxide enhancement of Trichodesmium community nitrogen or carbon fixation at Station ALOHA. Limnol. Oceanogr. 59, 645–659. doi: 10.4319/lo.2014.59.3.0645
Großkopf, T., Mohr, W., Baustian, T., Schunck, H., Gill, D., Kuypers, M. M. M., et al. (2012). Doubling of marine dinitrogen-fixation rates based on direct measurements. Nature 488, 361–364. doi: 10.1038/nature11338
Gruber, N., and Galloway, J. N. (2008). An Earth-system perspective of the global nitrogen cycle. Nature 451, 293–296. doi: 10.1038/nature06592
Gruber, N., and Sarmiento, J. L. (1997). Global patterns of marine nitrogen fixation and denitrification. Global Biogeochem. Cycles 11, 235–266. doi: 10.1029/97GB00077
Halm, H., Lam, P., Ferdelman, T. G., Lavik, G., Dittmar, T., LaRoche, J., et al. (2011). Heterotrophic organisms dominate nitrogen fixation in the South Pacific Gyre. ISME J. 6, 1238–1249. doi: 10.1038/ismej.2011.182
Hamersley, M. R., Turk, K. A., Leinweber, A., Gruber, N., Zehr, J. P., Gunderson, T., et al. (2011). Nitrogen fixation within the water column associated with two hypoxic basins in the Southern California Bight. Aquat. Microb. Ecol. 63, 193–205. doi: 10.3354/ame01494
Hansell, D. A., Bates, N. R., and Olson, D. B. (2004). Excess nitrate and nitrogen fixation in the North Atlantic Ocean. Mar. Chem. 84, 243–265. doi: 10.1016/j.marchem.2003.08.004
Hansell, D. A., Olson, D. B., Dentener, F., and Zamora, L. M. (2007). Assessment of excess nitrate development in the subtropical North Atlantic. Mar. Chem. 106, 562–579. doi: 10.1016/j.marchem.2007.06.005
Hedges, J. I. (2002). “Why dissolved organics matter,” in Biogeochemistry of Dissolved Organic Matter, eds D. A. Hansell and C. A. Carlson (San Diego, CA: Academic Press), 1–33.
Hewson, I., Moisander, P. H., Achilles, K. M., Carlson, C. A., Jenkins, B. D., Mondragon, E. A., et al. (2007). Characteristics of diazotrophs in surface to abyssopelagic waters of the Sargasso Sea. Aquat. Microb. Ecol. 46, 15–30. doi: 10.3354/ame046015
Holl, C. M., and Montoya, J. P. (2005). Interactions between nitrate uptake and nitrogen fixation in continuous cultures of the marine diazotroph Trichodesmium (Cyanobacteria). J. Phycol. 41, 1178–1183. doi: 10.1111/j.1529-8817.2005.00146.x
Hutchins, D. A., Fu, F.-X., Webb, E. A., Walworth, N., and Tagliabue, A. (2013). Taxon-specific response of marine nitrogen fixers to elevated carbon dioxide concentrations. Nature 6, 790–795. doi: 10.1038/ngeo1858
IPCC. (2007). Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
Jacq, V., Ridame, C., L'Helguen, S., Kaczmar, F., and Saliot, A. (2014). Response of the Unicellular Diazotrophic Cyanobacterium Crocosphaera watsonii to Iron Limitation. PLoS ONE 9:e86749. doi: 10.1371/journal.pone.0086749
Jenkins, W. J. (1982). Oxygen utilization rates in North Atlantic subtropical gyre and primary production in oligotrophic systems. Nature 300, 246–248. doi: 10.1038/300246a0
Jickells, T. D. (2005). Global iron connections between desert dust, Ocean biogeochemistry, and climate. Science 308, 67–71. doi: 10.1126/science.1105959
Karl, D. M., Bidigare, R. R., Church, M. J., Dore, J. E., Letelier, R. M., Mahaffey, C., et al. (2008). “The Nitrogen cycle in the North Pacific Trades Biome,” in Nitrogen in the Marine Environment, eds D. G. Capone, D. A. Bronk, M. R. Mulholl, and E. J. Carpenter (New York, NY: Academic Press), 705–769.
Karl, D. M., Church, M. J., Dore, J. E., Letelier, R. M., and Mahaffey, C. (2012). Predictable and efficient carbon sequestration in the North Pacific Ocean supported by symbiotic nitrogen fixation. Proc. Natl. Acad. Sci. U.S.A. 109, 1842–1849. doi: 10.1073/pnas.1120312109
Karl, D. M., Michaels, A. F., Bergman, B., Capone, D., Carpenter, E. J., Letelier, R. M., et al. (2002). Dinitrogen fixation in the world's oceans. Biogeochemistry 57/58, 47–98. doi: 10.1023/A:1015798105851
Karl, D., and Björkman, K. M. (2002). “Dynamics of dissolved organic phosphorus,” in Biogeochemistry of Marine Dissolved Organic Matter, eds D. A. Hansell, C. A. Carlson (Boston, MA: Elsevier Science), 249–366.
Klawonn, I., Bonaglia, S., Brüchert, V., and Ploug, H. (2015). Aerobic and anaerobic nitrogen transformation processes in N2-fixing cyanobacterial aggregates. ISME J. 9, 1456–1466. doi: 10.1038/ismej.2014.232
Knapp, A. N. (2012). The sensitivity of marine N2 fixation to dissolved inorganic nitrogen. Front Microbiol. 3:374. doi: 10.3389/fmicb.2012.00374
Kononova, S. V., and Nesmeyanova, M. A. (2002). Phosphonates and their degradation by microorganisms. Biochemistry 67, 184–195.
Krupke, A., Mohr, W., LaRoche, J., Fuchs, B. M., Amann, R. I., and Kuypers, M. M. (2014). The effect of nutrients on carbon and nitrogen fixation by the UCYN-A–haptophyte symbiosis. ISME J. doi: 10.1038/ismej.2014.253. [Epub ahead of print].
Krupke, A., Musat, N., LaRoche, J., Mohr, W., Fuchs, B. M., Amann, R. I., et al. (2013). In situ identification and N2 and C fixation rates of uncultivated cyanobacteria populations. Syst. Appl. Microbiol. 36, 259–271. doi: 10.1016/j.syapm.2013.02.002
Kustka, A. B., Sañudo-Wilhelmy, S. A., Carpenter, E. J., Capone, D., Burns, J., and Sunda, W. G. (2003). Iron requirements for dinitrogen-and ammonium-supported growth in cultures of Trichodesmium (IMS 101): Comparison with nitrogen fixation rates and iron: carbon ratios of field populations. Limnol. Oceanogr. 48, 1869–1884. doi: 10.4319/lo.2003.48.5.1869
Landolfi, A., Oschlies, A., and Sanders, R. (2008). Organic nutrients and excess nitrogen in the North Atlantic subtropical gyre. Biogeosciences 5, 1199–1213. doi: 10.5194/bg-5-1199-2008
Langlois, R. J., Hummer, D., and LaRoche, J. (2008). Abundances and Distributions of the dominant nifH Phylotypes in the Northern Atlantic Ocean. Appl. Environ. Microbiol. 74, 1922–1931. doi: 10.1128/AEM.01720-07
Langlois, R. J., LaRoche, J., and Raab, P. A. (2005). Diazotrophic diversity and distribution in the tropical and subtropical Atlantic Ocean. Appl. Environ. Microbiol. 71, 7910–7919. doi: 10.1128/AEM.71.12.7910-7919.2005
Langlois, R. J., Mills, M. M., Ridame, C., Croot, P., and LaRoche, J. (2012). Diazotrophic bacteria respond to Saharan dust additions. Mar. Ecol. Prog. Ser. 470, 1–14. doi: 10.3354/meps10109
Law, C. S., Martin, A. P., Liddicoat, M. I., and Watson, A. J. (2001). SF6 tracer study of an anticyclonic eddy in the North Atlantic: patch evolution, vertical mixing and nutrient supply to the mixed layer. Deep Sea Res. Part II 48, 705–724. doi: 10.1016/S0967-0645(00)00112-0
Lenes, J. M., and Heil, C. A. (2010). A historical analysis of the potential nutrient supply from the N2 fixing marine cyanobacterium Trichodesmium spp. to Karenia brevis blooms in the eastern Gulf of Mexico. J. Plankton Res. 32, 1421–1431. doi: 10.1093/plankt/fbq061
Lenes, J. M., Darrow, B. P., Cattrall, C., Heil, C. A., Callahan, M., Vargo, G. A., et al. (2001). Iron fertilization and the Trichodesmium response on the West Florida shelf. Limnol. Oceanogr. 46, 1261–1277. doi: 10.4319/lo.2001.46.6.1261
Letscher, R. T., Hansell, D. A., Carlson, C. A., Lumpkin, R., and Knapp, A. N. (2013). Dissolved organic nitrogen in the global surface ocean: distribution and fate. Global Biogeochem. Cycles 27, 141–153. doi: 10.1029/2012GB004449
Levitan, O., Rosenberg, E., Setlik, I., Setlikova, E., Grigel, J., Klepetar, J., et al. (2007). Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Glob. Change Biol. 13, 531–538. doi: 10.1111/j.1365-2486.2006.01314.x
Liu, X., and Millero, F. J. (2002). The solubility of iron in seawater. Mar. Chem. 77, 43–54. doi: 10.1016/S0304-4203(01)00074-3
Loescher, C. R., Großkopf, T., Desai, F. D., Gill, D., Schunck, H., Croot, P. L., et al. (2014). Facets of diazotrophy in the oxygen minimum zone waters off Peru. ISME J. 8, 2180–2192. doi: 10.1038/ismej.2014.71
Lomas, M. W., Hopkinson, B. M., Losh, J. L., Ryan, D. E., Shi, D. L., Xu, Y., et al. (2012). Effect of ocean acidification on cyanobacteria in the subtropical North Atlantic. Aquat. Microb. Ecol. 66, 211–222. doi: 10.3354/ame01576
Luo, Y. W., Doney, S. C., Anderson, L. A., Benavides, M., Berman-Frank, I., Bode, A., et al. (2012). Database of diazotrophs in global ocean: abundance, biomass and nitrogen fixation rates. Earth Syst. Sci. Data 4, 47–73. doi: 10.5194/essd-4-47-2012
Luo, Y. W., Lima, I. D., Karl, D. M., Deutsch, C. A., and Doney, S. C. (2014). Data-based assessment of environmental controls on global marine nitrogen fixation. Biogeosciences 11, 691–708. doi: 10.5194/bg-11-691-2014
Mahaffey, C., Reynolds, S., and Davis, C. E. (2014). Alkaline phosphatase activity in the subtropical ocean: insights from nutrient, dust and trace metal addition experiments. Front. Mar. Sci. 1:73. doi: 10.3389/fmars.2014.00073
Mahowald, N. M., Engelstaedter, S., Luo, C., Sealy, A., Artaxo, P., Benitez-Nelson, C., et al. (2009). Atmospheric iron deposition: global distribution, variability, and human perturbations. Annu. Rev. Mar. Sci. 1, 245–278. doi: 10.1146/annurev.marine.010908.163727
Malakoff, D. (2012). Researchers struggle to assess responses to ocean acidification. Science 338, 27–28. doi: 10.1126/science.338.6103.27
Mather, R. L., Reynolds, S. E., Wolff, G. A., Williams, R. G., Torres-Valdés, S., Woodward, E. M. S., et al. (2008). Phosphorus cycling in the North and South Atlantic Ocean subtropical gyres. Nature 1, 439–443. doi: 10.1038/ngeo232
McCarthy, J. J., and Goldman, J. C. (1979). Nitrogenous nutrition of marine phytoplankton in nutrient-depleted waters. Science 203:670. doi: 10.1126/science.203.4381.670
McInnes, A. S., Shepard, A. K., Raes, E. J., Waite, A. M., and Quigg, A. (2014). Simultaneous quantification of active carbon and nitrogen fixing communities and estimation of rates using fluorescence in situ hybridization and flow cytometry. Appl. Environ. Microbiol. 80, 6750–6759. doi: 10.1128/AEM.01962-14
Mellor, G., and Yamada, T. (1982). Development of a turbulence closure model for geophysical fluid problems. Rev. Geophys. 20, 851–875. doi: 10.1029/RG020i004p00851
Michaels, A. F., Bates, N. R., Buesseler, K. O., Carlson, C. A., and Knap, A. H. (1994). Carbon-cycle imbalances in the Sargasso Sea. Nature 372, 537–540. doi: 10.1038/372537a0
Michaels, A. F., Karl, D. M., and Capone, D. G. (2001). Element stoichiometry, new production and nitrogen fixation. Oceanography 14, 68–77. doi: 10.5670/oceanog.2001.08
Michaels, A. F., Olson, D., Sarmiento, J. L., Ammerman, J. W., Fanning, K., Jahnke, R., et al. (1996). Inputs, losses and transformations of nitrogen and phosphorus in the pelagic North Atlantic Ocean. Biogeochemistry 35, 181–226. doi: 10.1007/BF02179827
Mills, E. L. (2012). Biological Oceanography: An Early History 1870-1960. Toronto, ON: University of Toronto Press.
Mills, M. M., and Arrigo, K. R. (2010). Magnitude of oceanic nitrogen fixation influenced by the nutrient uptake ratio of phytoplankton. Nature 3, 412–416. doi: 10.1038/ngeo856
Mills, M. M., Ridame, C., Davey, M., La Roche, J., and Geider, R. J. (2004). Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature 429, 292–294. doi: 10.1038/nature02550
Mohr, W., Großkopf, T., Wallace, D. W. R., and LaRoche, J. (2010). Methodological Underestimation of Oceanic Nitrogen Fixation Rates. PLoS ONE 5:e12583. doi: 10.1371/journal.pone.0012583.t001
Moisander, P. H., Serros, T., Paerl, R. W., Beinart, R. A., and Zehr, J. P. (2014). Gammaproteobacterial diazotrophs and nifH gene expression in surface waters of the South Pacific Ocean. ISME J. 10, 1962–1973. doi: 10.1038/ismej.2014.49
Montoya, J. P., Carpenter, E. J., and Capone, D. G. (2002). Nitrogen fixation and nitrogen isotope abundances in zooplankton of the oligotrophic North Atlantic. Limnol. Oceanogr. 47, 1617–1628. doi: 10.4319/lo.2002.47.6.1617
Montoya, J. P., Holl, C. M., Zehr, J. P., Hansen, A., Villareal, T. A., and Capone, D. G. (2004). High rates of N2 fixation by unicellular diazotrophs in the oligotrophic Pacific Ocean. Nature 430, 1027–1032. doi: 10.1038/nature02824
Montoya, J. P., Voss, M., and Capone, D. G. (2007). Spatial variation in N2-fixation rate and diazotroph activity in the Tropical Atlantic. Biogeosciences 4, 369–376. doi: 10.5194/bg-4-369-2007
Moore, C. M., Mills, M. M., Achterberg, E. P., Geider, R. J., LaRoche, J., Lucas, M. I., et al. (2009). Large-scale distribution of Atlantic nitrogen fixation controlled by iron availability. Nat. Geosci. 2, 867–871. doi: 10.1038/ngeo667
Mouriño, B., Fernández, E., and Alves, M. (2004). Thermohaline structure, ageostrophic vertical velocity fields and phytoplankton distribution and production in the northeast Atlantic subtropical front. J. Geophys. Res. 109. doi: 10.1029/2003JC001990
Mouriño-Carballido, B., Graña, R., Fernández, A., Bode, A., Varela, M., Domínguez, J. F., et al. (2011). Importance of N2 fixation vs. nitrate eddy diffusion along a latitudinal transect in the Atlantic Ocean. Limnol. Oceanogr. 56, 999–1007. doi: 10.4319/lo.2011.56.3.0999
Mulholland, M. R. (2007). The fate of nitrogen fixed by diazotrophs in the ocean. Biogeosciences 4, 37–51. doi: 10.5194/bg-4-37-2007
Mulholland, M. R., Bernhardt, P. W., Blanco-Garcia, J. L., Mannino, A., Hyde, K., Mondragon, E., et al. (2012). Rates of dinitrogen fixation and the abundance of diazotrophs in North American coastal waters between Cape Hatteras and Georges Bank. Limnol. Oceanogr. 57, 1067–1083. doi: 10.4319/lo.2012.57.4.1067a
Mulholland, M. R., Bronk, D. A., and Capone, D. G. (2004). Dinitrogen fixation and release of ammonium and dissolved organic nitrogen by Trichodesmium IMS101. Aquat. Microb. Ecol. 37, 85–94. doi: 10.3354/ame037085
Musat, N., Stryhanyuk, H., Bombach, P., Adrian, L., Audinot, J.-N., and Richnow, H. H. (2014). The effect of FISH and CARD-FISH on the isotopic composition of 13C- and 15N-labeled Pseudomonas putida cells measured by nanoSIMS. Syst. Appl. Microbiol. 37, 267–276. doi: 10.1016/j.syapm.2014.02.002
O'Neil, J. M., and Roman, M. R. (1994). Ingestion of the cyanobacterium Trichodesmium spp. by pelagic harpacticoid copepods Macrosetella, Miracia and Oculosetella. Hydrobiologia 292, 235–240.
Oschlies, A. (2002). Nutrient supply to the surface waters of the North Atlantic: a model study. J. Geophys. Res. 107, 14–1–14–13. doi: 10.1029/2000JC000275
Paerl, H. W. (1985). Microzone formation: its role in the enhancement of aquatic N2 fixation. Limnol. Oceanogr. 30, 1246–1252. doi: 10.4319/lo.1985.30.6.1246
Paerl, H. W., and Prufert, L. E. (1987). Oxygen-poor microzones as potential sites of microbial N2 fixation in nitrogen-depleted aerobic marine waters. Appl. Environ. Microbiol. 53, 1078–1087.
Paerl, H. W., Prufert-Bebout, L. E., and Guo, C. (1994). Iron-stimulated N2 fixation and growth in natural and cultured populations of the planktonic marine cyanobacteria Trichodesmium spp. Appl. Environ. Microbiol. 60, 1044–1047.
Painter, S. C., Patey, M. D., Forryan, A., and Torres-Valdés, S. (2013). Evaluating the balance between vertical diffusive nitrate supply and nitrogen fixation with reference to nitrate uptake in the eastern subtropical North Atlantic Ocean. J. Geophys. Res. Oceans 118, 5732–5749. doi: 10.1002/jgrc.20416
Palter, J. B., Lozier, M. S., and Barber, R. T. (2005). The effect of advection on the nutrient reservoir in the North Atlantic subtropical gyre. Nature 437, 687–692. doi: 10.1038/nature03969
Palter, J. B., Lozier, M. S., Sarmiento, J. L., and Williams, R. G. (2011). The supply of excess phosphate across the Gulf Stream and the maintenance of subtropical nitrogen fixation. Global Biogeochem. Cycles 25:GB4007. doi: 10.1029/2010GB003955
Pelegrí, J. L., Marrero-Díaz, A., and Ratsimandresy, A. W. (2006). Nutrient irrigation of the North Atlantic. Prog. Oceanogr. 70, 366–406. doi: 10.1016/j.pocean.2006.03.018
Planas, D., Agustí, S., Duarte, C. M., Granata, T. C., and Merino, M. (1999). Nitrate uptake and diffusive nitrate supply in the Central Atlantic. Limnol. Oceanogr. 44, 116–126. doi: 10.4319/lo.1999.44.1.0116
Postgate, J. R. (1982). The Fundamentals of Nitrogen Fixation. Cambridge: Cambridge University Press.
Prospero, J. M., Barrett, K., Church, T., Dentener, F., Duce, R. A., Galloway, J. N., et al. (1996). Atmospheric deposition of nutrients to the North Atlantic Basin. Biogeochemistry 35, 27–73. doi: 10.1007/BF02179824
Rahav, E., Bar-Zeev, E., Ohayon, S., Elifantz, H., Belkin, N., Herut, B., et al. (2013). Dinitrogen fixation in aphotic oxygenated marine environments. Front. Microbiol. 4:227. doi: 10.3389/fmicb.2013.00227
Raimbault, P., and Garcia, N. (2008). Evidence for efficient regenerated production and dinitrogen fixation in nitrogen-deficient waters of the South Pacific Ocean: impact on new and export production estimates. Biogeosciences 5, 323–338. doi: 10.5194/bg-5-323-2008
Ratten, J.-M., LaRoche, J., Desai, D., Shelley, R. U., Landing, W. M., Boyle, E., et al. (2014). Sources of iron and phosphate affect the distribution of diazotrophs in the North Atlantic. Deep Sea Res. Part II. 116, 332–341. doi: 10.1016/j.dsr2.2014.11.012
Redfield, A. C., Ketchum, B. H., Richards, F. A., Hill, M. N., Goldberg, E. D., Iselin, C. O., et al. (1963). “The influence of organisms on the composition of sea water,” in The Sea (London: Interscience), 26–77.
Reinthaler, T., Álvarez-Salgado, X. A., Álvarez, M., van Aken, H. M., and Herndl, G. J. (2013). Impact of water mass mixing on the biogeochemistry and microbiology of the Northeast Atlantic Deep Water. Global Biogeochem. Cycles 27, 1151–1162. doi: 10.1002/2013GB004634
Reynolds, S., Mahaffey, C., and Roussenov, V. (2014). Evidence for production and lateral transport of dissolved organic phosphorus in the eastern subtropical North Atlantic. Global Biogeochem. Cycles 28, 805–824. doi: 10.1002/2013GB004801
Riemann, L., Farnelid, H., and Steward, G. F. (2010). Nitrogenase genes in non-cyanobacterial plankton: prevalence, diversity and regulation in marine waters. Aquat. Microb. Ecol. 61, 235–247. doi: 10.3354/ame01431
Rolff, C., Almesjö, L., and Elmgren, R. (2007). Nitrogen fixation and abundance of the diazotrophic cyanobacterium Aphanizomenon sp. in the Baltic Proper. Mar. Ecol. Prog. Ser. 332, 107–118. doi: 10.3354/meps332107
Rueter, J. G. (1988). Iron stimulation of photosynthesis and nitrogen fixation in Anabaena 7120 and Trichodesmium (Cynophiceae). J. Phycol. 24, 249–254. doi: 10.1111/j.1529-8817.1988.tb04240.x
Schlosser, C., Klar, J. K., and Wake, B. D. (2013). Seasonal ITCZ migration dynamically controls the location of the (sub)tropical Atlantic biogeochemical divide. Proc. Natl. Acad. Sci. U.S.A. 111, 1438–1442. doi: 10.1073/pnas.1318670111
Sellner, K. G. (1997). Physiology, ecology, and toxic properties of marine cyanobacteria blooms. Limnol. Oceanogr. 42, 1089–1104. doi: 10.4319/lo.1997.42.5_part_2.1089
Sellner, K. G., Olson, M. M., and Kononen, K. (1994). Copepod grazing in a summer cyanobacteria bloom in the Gulf of Finland. Dev. Hydrobiol. 102, 249–254. doi: 10.1007/BF00229948
Shi, D., Kranz, S. A., and Kim, J. M. (2012). Ocean acidification slows nitrogen fixation and growth in the dominant diazotroph Trichodesmium under low-iron conditions. Proc. Natl. Acad. Sci. U.S.A. 109, E3094–E3100. doi: 10.1073/pnas.1216012109
Singh, A., Lomas, M. W., and Bates, N. R. (2013). Revisiting N2 fixation in the North Atlantic Ocean: significance of deviations from the Redfield Ratio, atmospheric deposition and climate variability. Deep Sea Res. Part II 93, 148–158. doi: 10.1016/j.dsr2.2013.04.008
Sipler, R. E., Bronk, D. A., Seitzinger, S. P., Lauck, R. J., McGuinness, L. R., Kirkpatrick, G. J., et al. (2013). Trichodesmium-derived dissolved organic matter is a source of nitrogen capable of supporting the growth of toxic red tide Karenia brevis. Mar. Ecol. Prog. Ser. 483, 31–45. doi: 10.3354/meps10258
Sohm, J. A., and Capone, D. G. (2006). Phosphorus dynamics of the tropical and subtropical north Atlantic: Trichodesmium spp. versus bulk plankton. Mar. Ecol. Prog. Ser. 317, 21–28. doi: 10.3354/meps317021
Sohm, J. A., and Capone, D. G. (2010). Zonal differences in phosphorus pools, turnover and deficiency across the tropical North Atlantic Ocean. Global Biogeochem. Cycles 24:GB2008. doi: 10.1029/2008GB003414
Sohm, J. A., Edwards, B. R., Wilson, B. G., and Webb, E. A. (2011a). Constitutive extracellular polysaccharide (EPS) production by specific isolates of Crocosphaera watsonii. Front Microbiol. 2:229. doi: 10.3389/fmicb.2011.00229
Sohm, J. A., Hilton, J. A., Noble, A. E., Zehr, J. P., Saito, M. A., and Webb, E. A. (2011b). Nitrogen fixation in the South Atlantic Gyre and the Benguela upwelling system. Geophys. Res. Lett. 38:L16608. doi: 10.1029/2011GL048315
Sohm, J. A., Webb, E. A., and Capone, D. G. (2011c). Emerging patterns of marine nitrogen fixation. Nat. Rev. Microbiol. 9, 499–508. doi: 10.1038/nrmicro2594
Stramma, L., Brandt, P., Schafstall, J., Schott, F., Fischer, J., and Körtzinger, A. (2008a). Oxygen minimum zone in the North Atlantic south and east of the Cape Verde Islands. J. Geophys. Res. 113:C04014. doi: 10.1029/2007JC004369
Stramma, L., Johnson, G. C., Sprintall, J., and Mohrholz, V. (2008b). Expanding oxygen-minimum zones in the Tropical Oceans. Science 320, 655–658. doi: 10.1126/science.1153847
Straub, M., Sigman, D. M., Ren, H., Martínez-García, A., Meckler, A. N., Hain, M. P., et al. (2013). Changes in North Atlantic nitrogen fixation controlled by ocean circulation. Nature 501, 200–203. doi: 10.1038/nature12397
Subramaniam, A., Mahaffey, C., Johns, W., and Mahowald, N. (2013). Equatorial upwelling enhances nitrogen fixation in the Atlantic Ocean. Geophys. Res. Lett. 40, 1766–1771. doi: 10.1002/grl.50250
Subramaniam, A., Yager, P. L., Carpenter, E. J., Mahaffey, C., Björkman, K., Cooley, S., et al. (2008). Amazon River enhances diazotrophy and carbon sequestration in the tropical North Atlantic Ocean. Proc. Natl. Acad. Sci. U.S.A. 105, 10460–10465. doi: 10.1073/pnas.0710279105
Thompson, A. W., Foster, R. A., Krupke, A., Carter, B. J., Musat, N., Vaulot, D., et al. (2012). Unicellular cyanobacterium symbiotic with a single-celled Eukaryotic Alga. Science 337, 1546–1550. doi: 10.1126/science.1222700
Thompson, A., Carter, B. J., Turk-Kubo, K., Malfatti, F., Azam, F., and Zehr, J. P. (2014). Genetic diversity of the unicellular nitrogen-fixing cyanobacteria UCYN-A and its prymnesiophyte host. Environ. Microbiol. 16, 3238–3249. doi: 10.1111/1462-2920.12490
Torres-Valdés, S., Roussenov, V. M., Sanders, R., Reynolds, S., Pan, X., Mather, R., et al. (2009). Distribution of dissolved organic nutrients and their effect on export production over the Atlantic Ocean. Global Biogeochem. Cycles 23:GB4019. doi: 10.1029/2008GB003389
Tripp, H. J., Bench, S. R., Turk, K. A., Foster, R. A., Desany, B. A., Niazi, F., et al. (2010). Metabolic streamlining in an open-ocean nitrogen-fixing cyanobacterium. Nature 464, 90–94. doi: 10.1038/nature08786
Turk, A. K., Rees, A. P., Zehr, J. P., Pereira, N., Swift, P., Shelley, R., et al. (2011). Nitrogen fixation and nitrogenase (nifH) expression in tropical waters of the eastern North Atlantic. ISME J. 5, 1201–1212. doi: 10.1038/ismej.2010.205
Turk-Kubo, K. A. (2012). Nitrogenase (nifH) gene expression in diazotrophic cyanobacteria in the Tropical North Atlantic in response to nutrient amendments. Front. Microbiol. 3:386. doi: 10.3389/fmicb.2012.00386
Turk-Kubo, K. A., Karamchandani, M., Capone, D. G., and Zehr, J. P. (2014). The paradox of marine heterotrophic nitrogen fixation: abundances of heterotrophic diazotrophs do not account for nitrogen fixation rates in the Eastern Tropical South Pacific. Environ. Microbiol. 16, 3095–3114. doi: 10.1111/1462-2920.12346
Villareal, T. A., and Carpenter, E. J. (2003). Buoyancy regulation and the potential for vertical migration in the oceanic Cyanobacterium Trichodesmium. Microb. Ecol. 45, 1–10. doi: 10.1007/s00248-002-1012-5
Voss, M., Croot, P. L., Lochte, K., Mills, M. M., and Peeken, I. (2004). Patterns of nitrogen fixation along 10°N in the tropical Atlantic. Geophys. Res. Lett. 31:L23SL09. doi: 10.1029/2004GL020127
Wannicke, N., Korth, F., Liskow, I., and Voss, M. (2013). Incorporation of diazotrophic fixed N2 by mesozooplankton: case studies in the southern Baltic Sea. J Mar. Sys. 117-118, 1–13. doi: 10.1016/j.jmarsys.2013.03.005
Wannicke, N., Liskow, I., and Voss, M. (2010). Impact of diazotrophy on N stable isotope signatures of nitrate and particulate organic nitrogen: case studies in the north-eastern tropical Atlantic Ocean. Isotopes Environ. Health 46, 337–354. doi: 10.1080/10256016.2010.505687
Webb, E. A., Moffett, J. W., and Waterbury, J. B. (2001). Iron Stress in Open-Ocean Cyanobacteria (Synechococcus, Trichodesmium, and Crocosphaera spp.): identification of the IdiA Protein. Appl. Environ. Microbiol. 67, 5444–5452. doi: 10.1128/AEM.67.12.5444-5452.2001
Weber, T., and Deutsch, C. (2014). Local versus basin-scale limitation of marine nitrogen fixation. Proc. Natl. Acad. Sci. U.S.A. 111, 8741–8746. doi: 10.1073/pnas.1317193111
Wilson, S. T., Böttjer, D., Church, M. J., and Karl, D. M. (2012). Comparative assessment of nitrogen fixation methodologies, conducted in the Oligotrophic North Pacific Ocean. Appl. Environ. Microbiol. 78, 6516–6523. doi: 10.1128/AEM.01146-12
Wu, J. (2000). Phosphate depletion in the Western North Atlantic Ocean. Science 289, 759–762. doi: 10.1126/science.289.5480.759
Yool, A., Martin, A. P., Fernández, C., and Clark, D. R. (2007). The significance of nitrification for oceanic new production. Nature 447, 999–1002. doi: 10.1038/nature05885
Yoshida, K. (1967). Circulation in the eastern tropical ocean with special references to upwelling and undercurrents. Jpn. J. Geophys. 4, 1–75.
Zamora, L., Landolfi, A., Oschlies, A., Hansell, D., Dietze, H., and Dentener, F. (2010). Atmospheric deposition of nutrients and excess N formation in the North Atlantic. Biogeosciences 7, 777–793. doi: 10.5194/bg-7-777-2010
Zehr, J. P., Bench, S. R., Carter, B. J., Hewson, I., Niazi, F., Shi, T., et al. (2008). Globally distributed uncultivated oceanic N2-fixing cyanobacteria lack oxygenic photosystem II. Science 322, 1110–1112. doi: 10.1126/science.1165340
Zehr, J. P., Crumbliss, L. L., Church, M. J., Omoregie, E. O., and Jenkins, B. D. (2003). Nitrogenase genes in PCR and RT-PCR reagents: implications for studies of diversity of functional genes. Biotechniques 35, 996–1013.
Zehr, J. P., and Kudela, R. M. (2011). Nitrogen cycle of the open Ocean: from genes to ecosystems. Annu. Rev. Mar. Sci. 3, 197–225. doi: 10.1146/annurev-marine-120709-142819
Zehr, J. P., Mellon, M. T., and Zani, S. (1998). New nitrogen-fixing microorganisms detected in oligotrophic oceans by amplification of nitrogenase (nifH) genes. Appl. Environ. Microbiol. 64, 3444–3450.
Zehr, J. P., Montoya, J. P., Jenkins, B. D., Hewson, I., Mondragon, E., Short, C. M., et al. (2007). Experiments linking nitrogenase gene expression to nitrogen fixation in the North Pacific subtropical gyre. Limnol. Oceanogr. 52, 169–183. doi: 10.4319/lo.2007.52.1.0169
Keywords: Nitrogen, nifH, tropical, upwelling, Amazon River plume, phosphorus, N*
Citation: Benavides M and Voss M (2015) Five decades of N2 fixation research in the North Atlantic Ocean. Front. Mar. Sci. 2:40. doi: 10.3389/fmars.2015.00040
Received: 22 January 2015; Accepted: 27 May 2015;
Published: 11 June 2015.
Edited by:
Claire Mahaffey, University of Liverpool, UKReviewed by:
Mark Moore, University of Southampton, UKAjit Subramaniam, Lamont-Doherty Earth Observatory/Columbia University, USA
Copyright © 2015 Benavides and Voss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mar Benavides, Centre National de la Recherche Scientifique/Institut National des Sciences de l'univers, Institut de Recherche pour le Développement, Mediterranean Institute of Oceanography, Aix Marseille Université, Université de Toulon, 101 Promenade Roger Laroque, BP A5, 98848 Nouméa cedex, New Caledonia,bWFyLmJlbmF2aWRlc0BpcmQuZnI=
 Mar Benavides
Mar Benavides Maren Voss
Maren Voss