- Ecoanthropologie, Centre National de la Recherche Scientifique/Muséum National d’Histoire Naturelle, University Paris Diderot, Sorbonne Paris Cité, Musée de l’Homme, Paris, France
Investigating the drivers of tool use in animals has recently received great attention because of its implication in understanding animals’ cognition and the evolution of tool use in hominins. The necessity hypothesis posits tool use as a necessary response to food scarcity, but its role is an ongoing debate. The largest body of literature comparing animal tool use frequencies is with regard to primates, particularly comparisons between the Pan species. This supports the hypothesis that tool use is rarer in wild bonobos because of differential manipulation abilities of chimpanzees rather than different ecological needs. In this article, I aim to enrich the discussion concerning the necessity hypothesis and the ecological drivers of tool use in apes. The higher feeding flexibility of bonobos may be a key aspect to explaining the lower use of feeding tools than that observed in chimpanzees. The diet flexibility of bonobos is similar to that of the lowest level of tool users among the wild great apes: the gorilla. Gorillas can thus help to shed further light on this debate. When fruit is scarce, Western gorillas and bonobos rely more on widely available proteinaceous herbs than chimpanzees, who remain highly frugivorous. Chimpanzees may thus face a greater necessity to search for an alternative to obtain high-quality food: tool-assisted feeding. An indirect piece of evidence for this higher level of herbivory is that the prevalence of gut ciliates in bonobos is double that of chimpanzees. In each animal species, a different combination of necessity, opportunities, predisposition, and learning processes are likely to be at play in the emergence of flexible tool use in animals.
Introduction
Investigating the drivers of tool use in mammals and birds has recently received much attention because of its relation to brain size, cognition, and culture (Matsuzawa, 2001; Lefebvre et al., 2004; Amodio et al., 2018). Understanding animal variation in tool use frequency has great implications for understanding the evolutionary foundations of tool use in hominins, particularly when studying primates (e.g., Luncz et al., 2019; Haslam, 2022; Lonsdorf and Sanz, 2022; Masi et al., 2022).
Over the past few years, we have witnessed an increase in the use of a more ecological approach in primate cognition studies, including tool use. However, the exploration of ecological drivers is often difficult, and subtle explanations for behavioral variants may remain hidden (Masi, 2011). Furthermore, the impact of ecological factors on a given behavior (e.g., tool use) may be dismissed because of the lack of correlation with environmental indicators (e.g., food availability), raising the question of whether or not the right association of the multiplicity of ecological variables was investigated.
The existence of ecological drivers of tool use in animals is an ongoing debate. Particularly, the necessity hypothesis posits tool use as a response to food scarcity, which is driven by the necessity to exploit novel food items during times of low food availability (Fox et al., 1999; Gruber et al., 2012). In this article, I aim to provide new perspectives and to enrich the discussion on the possible ecological drivers for tool-using behavior in the African apes. I underline the differences in their feeding flexibility to refine the discussion of the necessity hypothesis as a possible explanation for the large differences in tool use among the great apes.
Tool use and predisposition differences: the Pan mystery
Only 13 tool-using behaviors have been documented in wild bonobos (Pan paniscus, possibly 14; see Samuni et al., 2022), whereas more than 40 have been described in chimpanzees (Pan troglodytes; e.g., Kano, 1982; Ingmanson, 1996; Hohmann and Fruth, 2003; Sanz and Morgan, 2007; Gruber and Clay, 2016; Kalan et al., 2020; Fotang et al., 2023). Today, this difference is generally attributed to the lack of reports of tool use in wild bonobos when foraging (e.g., Samuni et al., 2022). Only one observation of a leaf sponge used to drink water has been documented in the wild (Hohmann and Fruth, 2003) despite the many zoo observations of bonobos using tools to obtain food (e.g., Gold, 2002; Shumaker et al., 2011; Boose et al., 2013). So why, despite their customary use in captivity, do bonobos use tools at a very low frequency and with poorer variety than closely related chimpanzees?
Using both an ecological and a cognitive approach, one recent study directly compared several possible hypotheses in the two Pan species for explaining differences in tool use frequency (Furuichi et al., 2014). The differences in tool use repertoires could not be explained by ecological variables (e.g., climate, habitat use, and availability of fruit, social insects, and nuts) investigated in the habitat of two communities representing the two species: the necessity hypothesis was thus considered unlikely (Furuichi et al., 2014). Because the comparison did not support the other hypotheses, the authors concluded that differential propensities for tool use between Pan species (in the wild) may reflect a divergent behavioral predisposition.
Follow-up studies investigated hypotheses such as the intrinsic predisposition hypothesis, which suggested that wild chimpanzees were more inclined to manipulate objects than wild bonobos (Koops et al., 2015a; Koops et al., 2015b). Based on the availability of tools or insect prey, bonobos did not have fewer ecological opportunities to fish for termites, leaving the different intrinsic motivation for tool use as the only candidate for explaining the variation between Pan species (Koops et al., 2015a). However, given the similar tool use abilities observed in captivity for Pan species, a different intrinsic manipulative capacity between them seems implausible. Similarly, such higher tool use in captive bonobos is not explicable by the higher opportunity for social learning in captivity, i.e., proximity to conspecifics, predator absence, and food provision, as suggested for some animal species (Amodio et al., 2018). Indeed, wild bonobos are also highly sociable during feeding (Kano, 1980; Kuroda, 1984; Hohmann and Fruth, 1993; White, 1994; Hohmann and Fruth, 2002; Surbeck and Hohmann, 2008), and experience similar predation pressure to chimpanzees. Furthermore, bonobos, but not chimpanzees, possess a tendon similar to the human flexor pollicis longus, which is responsible for our exceptional tool manufacture/use capacities (Susman et al., 1999; Diogo, 2018).
Having more tool-using models, may young chimpanzees be simply more used to observing individuals grabbing tools/objects than young bonobos, rather than having an intrinsic predisposition? However, this seems unluckily the result of social learning, because the objects manipulated by immature chimpanzees did not include adult tools and the behavior was present already in < 1-year-old infants (Koops et al., 2015b). Therefore, it was suggested that bonobos lost their predisposition for tool use as a trade-off between tool use motivation and social attention (Koops et al., 2015b), in contrast to chimpanzees who focus more on objects (e.g., tools; Kano et al., 2015). If we compare the manipulation data available from different wild chimpanzee communities, manipulation frequencies between the low-tool-using communities (Lamon et al., 2018) and the high-tool-using communities (Koops et al., 2015a; Koops et al., 2015b) indeed seems comparable. However, the most intriguing question is rather, why bonobos could afford to lose such a predisposition? As nutrition is a main driving factor for animal survival, why were social aspects privileged over nutritional ones? One overlooked hypothesis could be that bonobos may meet their nutritional requirements more easily than chimpanzees, particularly when fruit is scarce (Kano and Mulavwa, 1984; but see Yamakoshi, 2004). On the other hand, by engaging in the alternative feeding strategy of tool-assisted feeding, chimpanzees offer youngsters more opportunity for social learning. This could, per se, explain the different object manipulation rates found in Pan species.
Discussion
Reconsideration of the necessity hypothesis for Pan
In this framework, do bonobos really need to engage in energetic and cognitively demanding tool use to forage? If the necessity hypothesis is not a valid one, then the two Pan species should have similar feeding choices given the similar environmental conditions (i.e., food availability).
The previous comparative study investigating ecological correlates for differences in Pan tool repertoires excluded the necessity hypothesis because fruit availability revealed similar patterns for both species (Furuichi et al., 2014). However, this phenological comparison may be considered with caution as, first, the monthly proportion of trees with ripe fruit provides only a rough estimate of fruit availability in comparison to the estimation of crop abundance (e.g., score 0–4; Chapman et al., 1994). Second, because bonobos and chimpanzees respond differently to fruit scarcity, with bonobos relying less on fallback fruit (see below; Wrangham, 1986; Wrangham et al., 1996; Yamakoshi, 2004), considering the overall forest fruit productivity as opposed to only the species consumed by the apes may lead to an overestimation of fruit availability for bonobos. Finally, the comparison of food spatial distribution included trees of smaller sizes for bonobos (tree diameter: > 5 cm vs. > 10 cm; Furuichi et al., 2014) possibly leads, again, to an overestimation for bonobos. Considering this, and that the results of other previous studies, indicates differences in feeding choices between the two Pan species (Wrangham, 1986; Wrangham et al., 1996; Yamakoshi, 2004; Hohmann et al., 2010), a deeper re-examination of these differences in the context of tool use may be worthwhile for reconsidering the necessity hypothesis, for which bonobos may have a lower necessity than chimpanzees for tool-assisted feeding.
Both bonobos and chimpanzees are frugivorous and inhabit tropical forests, which are characterized by large seasonal variations in fruit availability (Brockman and van Schaik, 2005). Chimpanzees remain mainly frugivorous throughout the year by continuing to search for fruit when fruit is scarce, concurrently increasing the consumption of leaves and herbs as a secondary fallback strategy (Table 1; Wrangham et al., 1991; Tutin and Fernandez, 1993; Malenky and Wrangham, 1994; Kuroda et al., 1996; Wrangham et al., 1998; Basabose, 2002; Morgan and Sanz, 2006; N'guessan et al., 2009; Koops et al., 2013; Sanz and Morgan, 2013). During the low fruit season, chimpanzees may be more stimulated to search for highly nutritious (tool-needing) food (e.g., honey, ants, termites) to meet their daily nutritional and energetic needs.
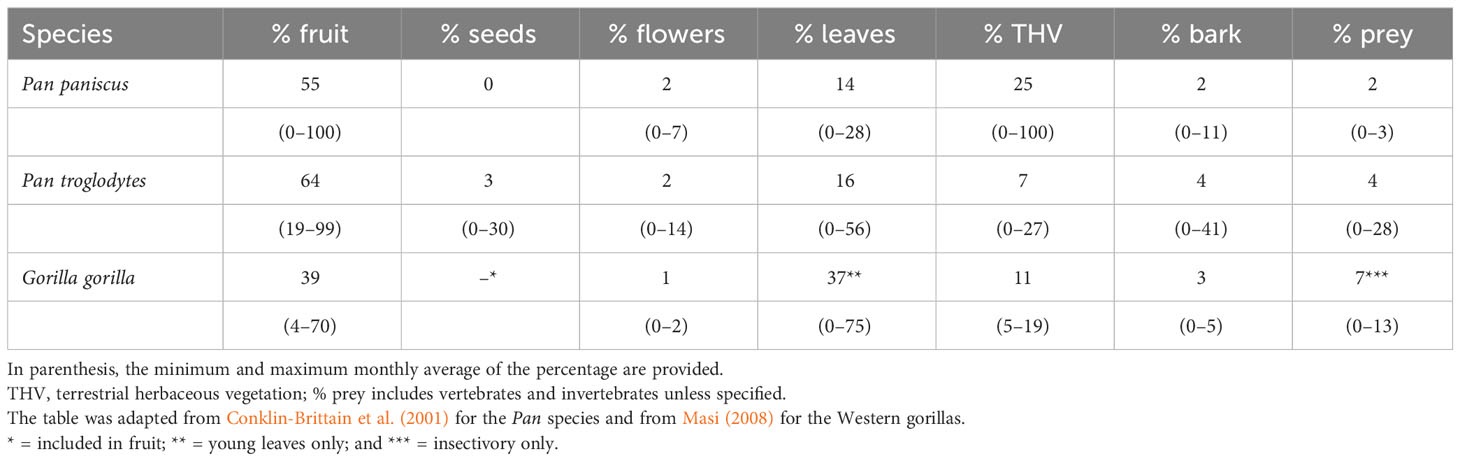
Table 1 Diet comparison among the two Pan species and Western gorillas in terms of (annual average) percentage of feeding time spent on each food type.
Bonobos feed more on high-quality herbs (terrestrial herbaceous vegetation, THV) than chimpanzees do (Malenky and Wrangham, 1994; Wrangham et al., 1996; Yamakoshi, 2004). To feed on herbs (Terada et al., 2015), bonobos visit inundated swamps more often than chimpanzees do (Hashimoto et al., 1998; Morgan et al., 2006; Mulavwa et al., 2010). Because bonobos rely more on vegetative food, they are said to occupy the Western gorillas’ (Gorilla gorilla) niche in a forest where gorillas are absent (Table 1; Wrangham et al., 1996; Yamakoshi, 2004; see below for more details on the Western gorillas’ diet). THV is not only more widely available than fruit, but it also provides high concentrations of nutrients such as proteins, non-structural carbohydrates, and lipids (e.g., Conklin-Brittain et al., 1998; Chapman and Chapman, 2002; Rode et al., 2003; Rothman et al., 2006; Masi et al., 2015). When fruit is scarce, bonobos feed more on herbs and reach nutritional intakes similar to that of chimpanzees (Kano and Mulavwa, 1984; Wrangham et al., 1991; Kuroda et al., 1996; Wrangham et al., 1996; Yamakoshi, 2004; Hohmann et al., 2010). Therefore, each year bonobos show greater flexibility than chimpanzees by consuming more herbaceous plants (Wrangham, 1986; for debate see Yamakoshi, 2004). This is also likely why bonobos have larger party sizes than chimpanzees (Furuichi et al., 2014).
In summary, as they remain mainly frugivorous year-round, during low-fruit periods chimpanzees may have different incentives than bonobos do, thus in turn use different strategies. The different rate of tool use may be the result of different feeding necessities, or a different response to a similar nutritional problem. In this view, chimpanzees are likely to be more constrained by their nutritional needs than bonobos, which may also explain why bonobos use fewer tools, specifically in feeding contexts (Furuichi et al., 2014; compare Sanz and Morgan, 2007 and Hohmann and Fruth, 2003). In addition, given the lower rate of feeding tolerance and food sharing in chimpanzees than in bonobos (Hare et al., 2007; Hare and Kwetuenda, 2010; Wobber et al., 2010; Lucchesi et al., 2021), they may be more likely to rely on alternative feeding strategies to decrease feeding competition.
Contribution to the debate of the lowest level tool user: the gorillas
Further support for the necessity hypothesis explaining the different tool use rate in Pan may be found in the lowest-level tool user among the great apes: the gorilla. However, although only a small amount of tool use was observed in wild gorillas (Breuer et al., 2005; Wittiger and Sunderland-Groves, 2007; Grueter et al., 2013; Kinani and Zimmerman, 2014; Masi et al., 2022), captive gorillas’ use of tools is similar to orangutans, bonobos, and even chimpanzees (e.g., Parker et al., 1999; Shumaker et al., 2011). The low tool use has been considered as being linked to the absence of food requiring extractive foraging in a wild diet, rather than to low cognitive and manipulative capacities (McGrew, 1989; van Schaik et al., 1999). However, Western gorillas do rely on extractive foraging to ingest insects or spiny foods (Masi et al., 2012a; Masi et al., 2015; Masi et al., 2022; Auger et al., 2023). Another hypothesis for explaining this low rate of tool use in gorillas is their lower social tolerance and complexity than chimpanzees (Masi et al., 2009, Masi et al., 2012a; Goodall, 1986; Watts, 2003). In orangutans more sociable populations show higher variety of tool use, by providing more opportunities for social learning (Schuppli et al., 2017). The higher sociability and terrestriality of chimpanzees seem also responsible for the greater variety of tool use in comparison to orangutans (Meulman et al., 2012). As gorillas are the most terrestrial apes, sociability and terrestriality together cannot explain the observed differences in ape tool use. Therefore, are there ecological reasons why wild gorillas should not use tools?
If the necessity hypothesis explains the low level of tool use in bonobos and gorillas, they should have similar feeding choices. Western gorillas are seasonal frugivores with highly flexible diet (Remis, 1997; Doran-Sheehy et al., 2009; Masi et al., 2015). They change from being highly (70% of feeding time) frugivorous when fruit is available, to highly herbivorous when fruit is scarce (Doran-Sheehy et al., 2009; Masi et al., 2009; Masi et al., 2015; Masi and Breuer, 2018). In contrast to chimpanzees who consume THV as fallback food (Tutin et al., 1991; Wrangham et al., 1991; Kuroda et al., 1996; Wrangham et al., 1998; Koops et al., 2013), Western gorillas consistently consume highly proteinaceous herbs year-round and rely mainly on them and young leaves when fruit is scarce, like bonobos (Table 1; Remis, 1997; Doran-Sheehy et al., 2009; Masi et al., 2015). The presence of symbiotic ciliates in the gorilla gut and their enlarged hindgut (due to its large size) enables them to extract energy from fiber fermentation (Chivers and Hladik, 1980; Lambert, 1998). In the altitude forests, where fruit is scarce, mountain gorillas (Gorilla beringei beringei) rely almost entirely on herbaceous plants, thus experiencing even more annual stability in terms of food provision (Watts, 1984; Ganas et al., 2004). Under the necessity hypothesis, such higher stability should not stimulate a high rate of tool use, as is the case in the well-studied mountain gorillas.
However, all this raises the question of how bonobos can metabolize energy from fiber vegetation (THV, leaves) if they do not possess the same fermenting abilities of gorillas (i.e., a shorter hindgut tract)? Comparing studies using the same coproscopic method (Masi et al., 2012b; Narat et al., 2015), surprisingly, bonobos are shown to have a much higher density of gut symbiotic ciliates than chimpanzees and Western gorillas do (Figure 1; although study site differences cannot be excluded). Doubling ciliate density likely increases bonobo fermenting abilities (Landsoud-Soukate et al., 1995) in comparison to chimpanzees. Because Pan species share the same gut morphophysiology, this provides further indirect evidence of a more herbivorous diet in bonobos. These ciliates are highly present in both chimpanzees’ (80%) and bonobos’ (94%) feces thus witnessing their biological need for help in fermenting fibers, when compared to gorillas (31%; Masi et al., 2012b; Narat et al., 2015) that do so primarily with the enlarged hindgut.
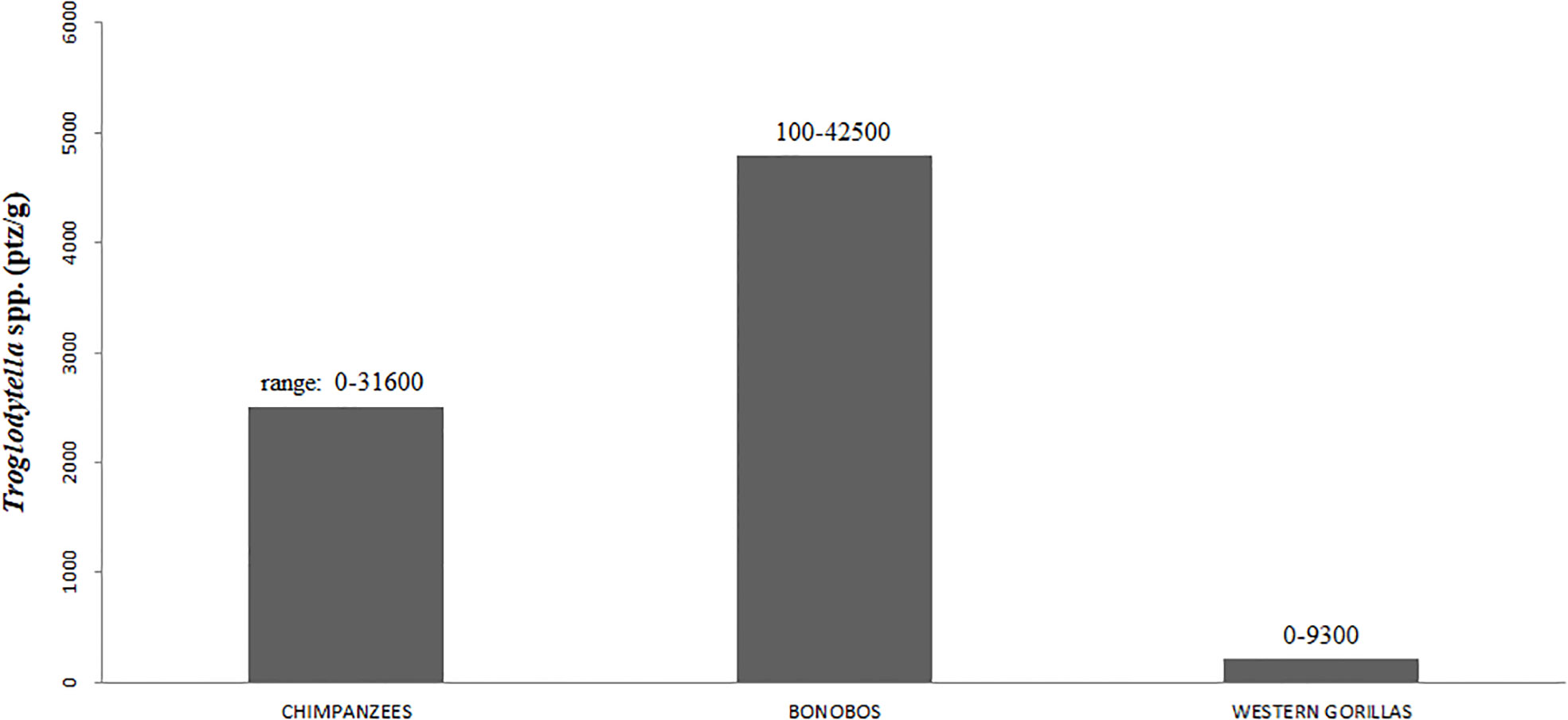
Figure 1 Mean corrected abundance of Troglodytella spp. (ptz/g) in chimpanzees (Pan troglodytes), bonobos (Pan paniscus), and Western gorillas (Gorilla gorilla). Ptz is the abbreviation for protozoa. The data were compiled from Masi et al. (2012b; chimpanzees and Western gorillas) and Narat et al. (2015; bonobos) using the same methods. Up on the bar is displayed the range of the corrected ciliate abundance.
Lower frugivorous diet and necessity as driver for tool use in primates
In summary, during periods of fruit scarcity, chimpanzees may experience greater nutritional constraints than bonobos and Western gorillas, who generally rely more on a high-quality diet by feeding on THV and young leaves (e.g., Wrangham et al., 1991; Kuroda et al., 1996; Wrangham et al., 1996; White, 1998; Doran-Sheehy et al., 2009; Hohmann et al., 2010; Masi et al., 2015). A more herbivorous diet may thus elicit a lower tendency of tool use. Studies on different chimpanzee communities and other primates corroborate this.
In some chimpanzee communities, tool use is negatively correlated with fruit abundance and more leafy diets, leading to lower levels of tool use (Yamakoshi, 1998; Gruber et al., 2016). In savannah chimpanzees, tool use seems a necessary response to lower overall food abundance in comparison to forest habitats (Hernandez-Aguilar et al., 2007; Bogart and Pruetz, 2011). As chimpanzees use tools more after periods of fruit scarcity, necessity is seen as a major factor in the emergence of tool use (Gruber et al., 2012, Gruber et al., 2016; but see Sanz and Morgan, 2013). The largest-scale study on 144 chimpanzee communities showed that environmental variability, such as larger seasonality in rainfall and presence of savannah woodland habitats, fosters within-species behavioral diversification, including tool use (Kalan et al., 2020).
The necessity hypothesis seems plausible also when looking at tool-using monkeys. In capuchin monkeys, only groups inhabiting harsh (dryer) environments and experiencing extreme seasonal variation in intakes, use tools at a high frequency (Moura and Lee, 2004; Canale et al., 2009; Lee and Moura, 2015). When compared to the wet season, capuchins show higher tool use rates during the dry season (although no support for the necessity hypothesis was found; Spagnoletti et al., 2012; Falótico and Ottoni, 2023). Necessity has thus been suggested to foster tool use in primates (Lee and Moura, 2015). Interestingly, the other monkeys that possess a highly diverse repertoire of feeding tool techniques are the macaques (e.g., Carpenter, 1887; Gumert et al., 2009; Gumert and Malaivijitnond, 2012; Haslam et al., 2016; Falótico et al., 2017; Luncz et al., 2017; Pal et al., 2018). Macaques show advanced ecological flexibility by having adapted to various landscapes, tolerating wide climate fluctuations, often harsh ones (Fa, 1989). To cope with this, unsurprisingly, they show different alternative strategies, such as highly flexible diets and tool use (e.g., using stones to access seafood, Malaivijitnond et al., 2007).
Multifactorial influence on the emergence of tool using behavior
Suggesting that different tool use rates may reflect different diet flexibility highlights that the necessity hypothesis deserves further consideration. This view does not exclude the possible contribution of other factors to explain such differences.
In mammals, ecology is thought to be important in shaping the emergence of behavioral flexibility and a feeding-related culture, although the precise impact remains poorly understood (e.g., dolphins: Patterson and Mann, 2011; great apes: Gruber and Clay, 2016; Gruber, 2016; Grund et al., 2019; Kalan et al., 2020). Indeed, the necessity hypothesis is difficult to test as it depends on the correlations between tool use rates and multiple variables that may differ according to species habitat and sociality, and past and present environment.
Current directions investigating tool use in different animals underlines the importance of the species’ psychological predispositions in flexible tool use (Amodio et al., 2018). An example could be the wadging behavior and leaf sponging in chimpanzees, the latter being the absorption of water with leaves after depriving them of their juice (e.g., Goodall, 1964; McGrew, 1992; Sugiyama, 1995). Although Western gorillas ingest (digest) the whole fig fruit or Aframomum piths, chimpanzees make “wadges” out of them by extracting the juice and discarding indigestible fibers (personal observation). The gut’s inability to process fibers predisposes chimpanzees to extract juices from plants (wadging), like when they use leaf fibers to adsorb water (latent solution, Tennie et al., 2009).
In animals, environment, sociality, and cognition have been identified as main factors influencing the emergence of tool use, invention, and the transmission and retention of culture (Koops et al., 2014). It is likely that the specific behavioral propensity of a species also has its place here. A combination of ecological necessity and opportunities, propensity and learning processes may interplay in different ways in the emergence and maintenance of tool use in wild animals.
Finally, stimulating the discussion at the ecological level for interpreting behavioral differences in tool use will hopefully encourage future work in investigating in greater detail the interacting roles of environment (diet) with the cognitive abilities and behavioral predisposition of the study species.
Author contributions
SM: conceptualization; investigation; writing—original draft; and writing—review and editing.
Funding
Funding was received from the Muséum Nationale d’Histoire Naturelle. The author declares that financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
I thank Claudio Tennie, Victor Narat, Thomas Breuer, Laura Muniz, and Antonio Sanna for useful comments. I thank the relevant institutions that supported and granted me my long-term work: WWF Central African Republic (CAR), Dzanga-Ndoki National Park, Ministries of Education and Water and Forests of the CAR, University of Rome La Sapienza, Max Planck Institute, Muséum national d’Histoire naturelle (MNHN), Cleveland Metro Park Zoo, Action Transversal du Muséum, Federative Projects of the Department of “Homme et Environnement” of MNHN, LabEx BCDiv, ANR SAFEAPE, and CNRS. Lastly, I thank very much Steven Emilio Churchill for his review work.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author SM declares that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer-review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amodio P., Jelbert S., Clayton N. S. (2018). The interplay between psychological predispositions and skill learning in the evolution of tool use. Curr. Opin. Behav. Sci. 20, 130–137. doi: 10.1016/j.cobeha.2018.01.002
Auger C., Cipolletta C., Todd A., Fuh T., Sotto-Mayor A., Pouydebat E., et al. (2023). Feeling a bit peckish: seasonal and opportunistic insectivory for wild gorillas. Am. J. Biol. Anthropol. 182, 210–223. doi: 10.1002/ajpa.24811
Basabose A. K. (2002). Diet composition of chimpanzees inhabiting the Montane forest of Kahuzi, Democratic Republic of Congo. Am. J. Primatol 58 (1), 1–21. doi: 10.1002/ajp.10049
Bogart S. L., Pruetz J. D. (2011). Insectivory of savanna chimpanzees (Pan troglodytes verus) at Fongoli, Senegal. Am. J. Phys. Anthropol 145, 11–20. doi: 10.1002/ajpa.21452
Boose K. J., White F. J., Meinelt A. (2013). Sex differences in tool use acquisition in bonobos (Pan paniscus). Am. J. Primatol 75, 917–926. doi: 10.1002/ajp.22155
Breuer T., Ndoundou-Hockemba M., Fishlock V. (2005). First observation of tool use in wild gorillas. PloS Biol. 3, e380. doi: 10.1371/journal.pbio.0030380
Brockman D. K., van Schaik C. P. (2005). Seasonality in primates: studies of living and extinct human and non-human primates (Cambridge: Cambridge University Press).
Canale G. R., Guidorizzi C. E., Kierulff M. C. M., Gatto C. A. F. (2009). First record of tool use by wild populations of the yellow breasted capuchin monkey (Cebus xanthosternos) and new records for the bearded capuchin (Cebus libidinosus). Am. J. Primatol 71, 1–7. doi: 10.1002/ajp.20648
Chapman C. A., Chapman L. J. (2002). Foraging challenges of red colobus monkeys: influence of nutrients and secondary compounds. Comp. Biochem. Physiol. Part A 133, 861–875. doi: 10.1016/S1095-6433(02)00209-X
Chapman C. A., White F. J., Wrangham R. W. (1994). “Party size in chimpanzees and bonobos: a reevaluation of theory based on two similarly forested sites,” in Chimpanzee cultures. Eds. McGrew W. C., de Waal F. B. M., Heltne P. G. (Cambridge, Massachusetts, London: Harvard University Press), 41–57.
Chivers D. J., Hladik C. M. (1980). Morphology of the gastrointestinal tract in primates: comparisons with other mammals in relation to diet. J. Morphol 166, 337–386. doi: 10.1002/jmor.1051660306
Conklin-Brittain N. L., Knott C. D., Wrangham R. W. (2001). The feeding ecology of apes. apes: Challenges 21st century 445–465, 167–174.
Conklin-Brittain N. L., Wrangham R. W., Hunt D. H. (1998). Dietary response of chimpanzee and cercopithecines to seasonal variation in fruit abundance II Macronutrients. Int. J. Primat 19, 917–998. doi: 10.1023/A:1020370119096
Diogo R. (2018). First detailed anatomical study of bonobos reveals intra-specific variations and exposes just-so stories of human evolution, bipedalism, and tool use. Front. Ecol. Evol. 6. doi: 10.3389/fevo201800053
Doran-Sheehy D., Mongo P., Lodwick J., Conklin-Brittain N. L. (2009). Male and female western gorilla diet: preferred foods, use of fallback resources, and implications for ape versus old world monkey strategies. Am. J. Phy Ant 140, 727–738. doi: 10.1002/ajpa.21118
Fa J. (1989). The genus Macaca: a review of taxonomy and evolution. Mammal Rev. 19 (2), 45–81. doi: 10.1111/j1365-29071989tb00401x
Falótico T., Ottoni E. B. (2023). Greater tool use diversity is associated with increased terrestriality in wild capuchin monkeys. Am. J. Biol. Anthropol. 152, 105740. doi: 10.1002/ajpa.24740
Falótico T., Spagnoletti N., Haslam M., Luncz L. V., Malaivijitnond S., Gumert M. (2017). Analysis of sea almond (Terminalia catappa) cracking sites used by wild Burmese long-tailed macaques (Macaca fascicularis aurea). Am. J. Primatol 79, e22629. doi: 10.1002/ajp22629
Fotang C., Dutton P., Bröring U., Roos C., Willie J., Angwafo T. E., et al. (2023). Tool use by Nigeria-Cameroon chimpanzees for driver ant predation in Kom-Wum Forest Reserve, North-West Region Cameroon. Folia Primatol. 94 (1), 73–85. doi: 10.1163/14219980-bja10006
Fox E. A., Sitompul A. F., van Schaik C. P. (1999). “Intelligent tool use in wild Sumatran orangutans,” in The mentalities of gorillas and orangutans. Eds. Parker S. T., Mitchell R. W., Miles H. L. (Cambridge: Cambridge University Press), 99–116.
Furuichi T., Sanz C., Koops K., Sakamaki T., Ryu H., Tokuyama N., et al. (2014). Why do wild bonobos not use tools like chimpanzees do? Behaviour 152, 425–460. doi: 10.1163/1568539X-00003226
Ganas J., Robbins M. M., Nkurunungi J. B., Kaplin B. A., McNeilage A. (2004). Dietary variability of mountain gorillas in Bwindi Impenetrable National Park, Uganda. Int. J. Primatol 25, 1043–1072. doi: 10.1023/B:IJOP.0000043351.20129.44
Gold K. C. (2002). Ladder use and clubbing by a bonobo (Pan paniscus) in Apenheul Primate Park. Zoo Biol. 21, 607–611. doi: 10.1002/zoo.10064
Goodall J. (1964). Tool-using and aimed throwing in a community of free-living chimpanzees. Nature 201, 1264–1266. doi: 10.1038/2011264a0
Goodall J. (1986). The chimpanzees of Gombe: patterns of behaviour (Harvard: Cambridge, Massachusetts Belknap Press of Harvard University Press).
Gruber T. (2016). Great apes do not learn novel tool use easily: conservatism, functional fixedness, or cultural influence? Int. J. Primat 37 (2), 296–316. doi: 10.1007/s10764-016-9902-4
Gruber T., Clay Z. (2016). A comparison between bonobos and chimpanzees: a review and update. Evol. Anthrop 25, 239–252. doi: 10.1002/evan.21501
Gruber T., Potts K. B., Krupenye C., Byrne M.-R., Mackworth-Young C., McGrew W. C., et al. (2012). The influence of ecology on chimpanzee (Pan troglodytes) cultural behavior: a case study of five Ugandan chimpanzee communities. J. Comp. Psychol. 126, 446–457. doi: 10.1037/a0028702
Gruber T., Zuberbühler K., Neumann C. (2016). Travel fosters tool use in wild chimpanzees. eLIFE 5, e16371. doi: 10.7554/eLife16371
Grueter C. C., Robbins M. M., Ndagijimanab F., Tara S. (2013). Possible tool use in a mountain gorilla. Behav. Processes 100, 160–162. doi: 10.1016/j.beproc.2013.09.006
Grund C., Neumann C., Zuberbühler K., Gruber T. (2019). Necessity creates opportunities for chimpanzee tool use. Behav. Ecol. 30 (4), 1–9. doi: 10.1093/beheco/arz062
Gumert M. D., Kluck M., Malaivijitnond S. (2009). The physical characteristics and usage patterns of stone axe and pounding hammers used by long-tailed macaques in the Andaman Sea region of Thailand. Am. J. Primatol 71, 594–608. doi: 10.1002/ajp20694
Gumert M. D., Malaivijitnond S. (2012). Marine prey processed with stone tools by Burmese long-tailed macaques (Macaca fascicularis aurea) in intertidal habitats. Am. J. Phys. Anthropol 149, 447–457. doi: 10.1002/ajpa22143
Hare B., Kwetuenda S. (2010). Bonobos voluntarily share their own food with others. Curr. Biol. 20 (5), 230–231. doi: 10.1016/j.cub.2009.12.038
Hare B., Melis A. P., Woods V., Hastings S., Wrangham R. W. (2007). Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr. Biol. 17, 619–623. doi: 10.1016/j.cub.2007.02.040
Hashimoto C., Tashiro Y., Kimura D., Enomoto T., Ingmanson J., Idani G., et al. (1998). Habitat use and ranging of wild bonobos (Pan paniscus) at Wamba. Int. J. Primatol 19, 1045–1060. doi: 10.1023/A:1020378320913
Haslam M. (2022). Insights from orangutans into the evolution of tool use. Nature, 427–428. doi: 10.1038/d41586-022-00872-3
Haslam M., Pascual-Garrido A., Malaivijitnonds S., Gumert M. (2016). Stone tool transport by wild Burmese long-tailed macaques (Macaca fascicularis aurea). J. Archaeol Sci. Rep. 7, 408–413. doi: 10.1016/jjasrep201605040
Hernandez-Aguilar R. A., Moore J., Pickering T. R. (2007). Savanna chimpanzees use tools to harvest the underground storage organs of plants. Proc. Natl. Acad. Sci. U.S.A. 104, 19210–19213. doi: 10.1073/pnas.0707929104
Hohmann G., Fruth B. (2002). Dynamics in social organization of bonobos (Pan paniscus). In Behavioural diversity in chimpanzees and bonobos Boesch C., Hohmann G., Marchant L. F., editors. (New York: Cambridge University Press), p 138–150.
Hohmann G., Potts K., N'Guessan A., Fowler A., Mundry R., Ganzhorn J. U., et al. (2010). Plant foods consumed by Pan: exploring the variation of nutritional ecology across Africa. Am. J. Phys. Anthropol 141, 476–485. doi: 10.1002/ajpa.21168
Hohmann G., Fruth B. (1993). Field observations on meat sharing among bonobos (Pan paniscus). Folia Primatol 60, 225–229. doi: 10.1159/000156695
Hohmann G., Fruth B. (2003). Culture in bonobos? Between-species and within-species variation in behavior. Curr. Anthropol 44, 563–571. doi: 10.1086/377649
Ingmanson E. J. (1996). “Tool-using behaviors in wild Pan paniscus: social and ecological considerations,” in Reaching into thought: the minds of the great apes. Eds. Russon A. E., Bard K. A., Parker S. T. (Cambridge: Cambridge University Press), 190–210.
Kalan A. K., Kulik L., Arandjelovic M., Boesch C., Haas F., Dieguez P., et al. (2020). Environmental variability supports chimpanzee behavioural diversity. Nat. Commun. 11 (1), 4451. doi: 10.1038/s41467-020-18176-3
Kano T. (1980). Social behavior of wild pygmy chimpanzees (Pan paniscus) of Wamba, a preliminary report. J. Hum. Evol. 9, 243–260. doi: 10.1016/0047-2484(80)90053-6
Kano T. (1982). The use of leafy twigs for rain cover by the pygmy chimpanzees of Wamba. Primates 23, 453–457. doi: 10.1007/BF02381327
Kano F., Hirata S., Call J. (2015). Social attention in the two species of pan: Bonobos make more eye contact than chimpanzees. PloS One 10, 1–14. doi: 10.1371/journal.pone.0129684
Kano T., Mulavwa M. (1984). “Feeding ecology of the pygmy chimpanzees (Pan paniscus) of Wamba,” in The pygmy chimpanzee. Ed. Susman R. L. (New York: Plenum Press), 233–274.
Kinani J. F., Zimmerman D. (2014). Tool use for food acquisition in a wild mountain gorilla. Am. J. Primat 3), 353–357. doi: 10.1002/ajp22351
Koops K., Furuichi T., Hashimoto C. (2015a). Chimpanzees and bonobos differ in intrinsic motivation for tool use. Sci. Rep. 5, 11356. doi: 10.1038/srep11356
Koops K., Furuichi T., Hashimoto C., van Schaik C. P. (2015b). Sex differences in object manipulation in wild immature chimpanzees (Pan troglodytes schweinfurthii) and bonobos (Pan paniscus): Preparation for tool use? PloS One 10 (10), e0139909. doi: 10.1371/journalpone0139909
Koops K., McGrew W. C., Matsuzawa T. (2013). Ecology of culture: do environmental factors influence foraging tool use in wild chimpanzees, Pan troglodytes verus? Anim. Behav. 85, 175–185. doi: 10.1016/j.anbehav.2012.10.022
Koops K., Visalberghi E., van Schaik C. P. (2014). The ecology of primate material culture. Biol. Lett. 10, 20140508. doi: 10.1098/rsbl.2014.0508
Kuroda S. (1984). “Interaction over food among pygmy chimpanzees,” in The pygmy chimpanzee: evolutionary biology and behavior. Ed. Susman R. L. (New York: Plenum Press), 301–324.
Kuroda S., Nishihara T., Suzuki S., Oko R. A. (1996). “Sympatric chimpanzees and gorillas in the Ndoki Forest, Congo,” in Great ape societies,. Eds. McGrew W. C., Marchant L. F., Nishida T. (Cambridge: Cambridge Univ Press), 71–81.
Lambert J. E. (1998). Primate digestion: interactions among anatomy, physiology, and feeding ecology. Evol. Anthropol 7, 8–20. doi: 10.1002/(SICI)1520-6505(1998)7:1<8::AID-EVAN3>3.0.CO;2-C
Lamon N., Neumann C., Zuberbühler K. (2018). Development of object manipulation in wild chimpanzees. Anim. Behav. 135, 121–130. doi: 10.1016/j.anbehav.2017.11.003
Landsoud-Soukate J., Tutin C. E. G., Fernandez M. (1995). Intestinal parasites of sympatric gorillas and chimpanzees in the Lope Reserve, Gabon. Ann. Tropic Med. Parasitol. 89 (1), 73–79. doi: 10.1080/00034983.1995.11812931
Lee P. C., Moura A. C. de A. (2015). “Necessity, unpredictability and opportunity: an exploration of ecological and social drivers of behavioral innovation,” in Kaufman; kaufman (Org) animal creativity and innovation, 1ed (Cambridge, Massachusetts: Academic Press/Elsevier), 317–329.
Lefebvre L., Reader S. M., Sol D. (2004). Brains, innovations and evolution in birds and primates. Brain Behav. Evol. 63, 233–246. doi: 10.1159/000076784
Lonsdorf E. V., Sanz C. M. (2022). Behavioral and cognitive perspectives on the evolution of tool use from wild chimpanzees. Curr. Opin. Behav. Sci. 46, 101144. doi: 10.1016/j.cobeha.2022.101144
Lucchesi S., Cheng L., Deschner T., Mundry R., Wessling E. G., Surbeck M. (2021). Better together? How intergroup associations affect energy balance and feeding behavior in wild bonobos. Behav. Ecol. Sociobiol. 75, 1–17. doi: 10.1007/s00265-020-02943-9
Luncz L. V., Gill M., Proffitt T., Svensson M. S., Kulik L., Malaivijitnond S. (2019). Group-specific archaeological signatures of stone tool use in wild macaques. Elife 8, e46961. doi: 10.7554/eLife.46961.021
Luncz L. V., Svensson M. S., Haslam M., Malaivijitnond S., Proffitt T., Gumert M. (2017). Technological response of wild macaques (Macaca fascicularis) to anthropogenic change. Int. J. Primatol 38, 872–880. doi: 10.1007/s10764-017-9985-6
Malaivijitnond S., Lekprayoon C., Tandavanittj N., Panha S., Cheewatham C., Hamada Y. (2007). Stone-tool usage by Thai long-tailed macaques (Macaca fascicularis). Am. J. Primatol 69 (2), 227–233. doi: 10.1002/ajp20342
Malenky R. K., Wrangham R. W. (1994). A quantitative comparison of terrestrial herbaceous food consumption by Pan paniscus in the Lomako Forest, Zaire and Pan troglodytes in the Kibale Forest, Uganda. Am. J. Primatol 32, 1–12. doi: 10.1002/ajp.1350320102
Masi S. (2011). Differences in gorilla nettle-feeding between captivity and the wild: local traditions, species typical behaviours or merely the result of nutritional deficiencies? Anim. Cognit. 14, 921–925. doi: 10.1007/s10071-011-0457-7
Masi S., Breuer T. (2018). Dialium seed coprophagy in wild western gorillas: Multiple nutritional benefits and toxicity reduction hypotheses. Am. J. Primat 80 (4), e22752. doi: 10.1002/ajp.22752
Masi S., Chauffour S., Bain O., Todd A., Guillot J., Krief S. (2012b). Seasonal effects on great ape health: a case study of wild chimpanzees and western gorillas. PloS One 7 (12), e49805. doi: 10.1371/journal.pone.0049805
Masi S., Cipolletta C., Robbins M. M. (2009). Activity patterns of western lowland gorillas (Gorilla gorilla gorilla) at Bai-Hokou, Central African Republic. Am. J. Prim 71, 91–100. doi: 10.1002/ajp.20629
Masi S. (2008). Seasonal influence on feeding strategies, activity and energy budget of Western lowland gorillas (G. g. gorilla) in Bai Hokou, Central African Republic [PhD].
Masi S., Gustafsson E., Jalme M. S., Narat V., Todd A., Bomsel M. C., et al. (2012a). Unusual feeding behaviour in great apes, a window to understand origins of self-medication in humans: role of sociality and physiology on learning process. Physiol. Behav. 105 (2), 337–349. doi: 10.1016/j.physbeh.2011.08.012
Masi S., Mundry R., Cipolletta C., Ortmann S., Boitani L., Robbins M. M. (2015). Influence of seasonality on major nutrient intake in western gorillas but not on energy intake. PloS One 10 (7), e0129254. doi: 10.1371/journalpone0129254
Masi S., Pouydebat E., San-Galli A., Meulman E., Breuer T., Reeves J., et al. (2022). Free hand hitting of stone-like objects in wild gorillas. Sci. Rep. 12 (1), 1–10. doi: 10.1038/s41598-022-15542-7
Matsuzawa T. (2001). “Primate foundations of human intelligence: a view of tool use in nonhuman primates and fossil hominids,” in Primate origins of human cognition and behavior. Ed. Matsuzawa T. (Tokyo: Springer), 3–25.
McGrew W. C. (1989). “Why is ape tool use so confusing?,” in Comparative socioecology: The behavioural ecology of humans and other mammals. Eds. Standen V., Foley R. A. (Oxford: Blackwell Scientific Publications), 457–472.
Meulman E. J., Sanz C. M., Visalberghi E., van Schaik C. P. (2012). The role of terrestriality in promoting primate technology. Evolutionary Anthropology: Issues, News, and Reviews 21 (2), 58–68.
Morgan D., Sanz C. (2006). “Chimpanzee feeding ecology and comparisons with sympatric gorillas in the Goualougo Triangle, Republic of Congo,” in Primate feeding ecology in apes and other primates: ecological, physiological, and behavioural aspects. Eds. Hohmann G., Robbins M., Boesch C. (Cambridge: Cambridge University Press), 97–122.
Morgan D., Sanz C., Onononga J. R., Strindberg S. (2006). Ape abundance and habitat use in the Goualougo Triangle, Republic of Congo. Int. J. Primatol 27, 147–179. doi: 10.1007/s10764-005-9013-0
Moura A. C., Lee P. C. (2004). Capuchin stone tool use in Caatinga dry forest. Science 306, 1909. doi: 10.1126/science.1102558
Mulavwa M., Yangozene K., Yamba‐Yamba M., Motema‐Salo B., Mwanza N. N., Furuichi T., et al. (2010). Nest groups of wild bonobos at Wamba: selection of vegetation and tree species and relationships between nest group size and party size. Am. J. Primatol 72, 575–586. doi: 10.1002/ajp.20810
N'guessan A. K., Ortmann S., Boesch C. (2009). Daily energy balance and protein gain among Pan troglodytes verus in the Tai National Park, Cote d'Ivoire. Int. J. Primatol 30 (3), 481–496. doi: 10.1007/s10764-009-9354-1
Narat V., Guillot J., Pennec F., Lafosse S., Grüner A.C., Simmen B., et al. (2015). Intestinal helminths of wild bonobos in forest-savanna mosaic: Risk assessment of cross-species transmission with local people in the Democratic Republic of the Congo. EcoHealth 12 (4), 621–633. doi: 10.1007/s10393-015-1058-8
Pal A., Kumara H. N., Mishra P. S., Velankar A. D., Singh M. (2018). Extractive foraging and tool-aided behaviors in the wild Nicobar long-tailed macaque (Macaca fascicularis umbrosus). Primates 59 (2), 173–183. doi: 10.1007/s10329-017-0635-6
Parker S. T., Kerr M., Markowitz H., Gould J. (1999). “A survey of tool use in zoo gorillas,” in The mentalities of gorillas and orang-utans. Eds. Parker S. T., Mitchell R. W., Miles H. L. (Cambridge: Cambridge University Press), 188–193.
Patterson E. M., Mann J. (2011). The ecological conditions that favor tool use and innovation in wild bottlenose dolphins (Tursiops sp.). PloS One 6 (8). doi: 10.1371/annotation/2555a3f6-117f-42e2-b89c-c568bd6618c9
Remis M. J. (1997). Western lowland gorillas (Gorilla gorilla gorilla) as seasonal frugivores: use of variable resources. Am. J. Primatol 43 (2), 87–109. doi: 10.1002/(SICI)1098-2345(1997)43:2<87::AID-AJP1>3.0.CO;2-T
Rode K. D., Chapman C. A., Chapman L. J., McDowell L. R. (2003). Mineral resource availability and consumption by colobus in Kibale National Park, Uganda. Int. J. Primatol 24, 541–573. doi: 10.1023/A:1023788330155
Rothman J. M., Dierenfeld E. S., Molina D. O., Shaw A. V., Hintz H. F., Pell A. N. (2006). Nutritional chemistry of foods eaten by gorillas in Bwindi Impenetrable National Park, Uganda. Am. J. Primatol 68 (7), 675–691. doi: 10.1002/ajp.20243
Samuni L., Lemieux D., Lamb A., Galdino D., Surbeck M. (2022). Tool use behavior in three wild bonobo communities at Kokolopori. Am. J. Primatol. 84 (1), e23342. doi: 10.1002/ajp.23342
Sanz C., Morgan D. (2007). Chimpanzee tool technology in the Goualougo Triangle, Republic of Congo. J. Hum. Evol. 52, 420–433. doi: 10.1016/j.jhevol.2006.11.001
Sanz C. M., Morgan D. B. (2013). Ecological and social correlates of chimpanzee tool use. Phil Trans. Roy Soc. B 368, 20120416. doi: 10.1098/rstb.2012.0416
Schuppli C., Forss S., Meulman E., Atmoko S. U., van Noordwijk M., van Schaik C. (2017). The effects of sociability on exploratory tendency and innovation repertoires in wild Sumatran and Bornean orangutans. Sci. Rep. 7 (1), 15464. doi: 10.1038/s41598-017-15640-x
Shumaker R. W., Walkup K. R., Beck B. B. (2011). Animal tool behavior: the use and manufacture of tools by animals (Baltimore, MD: Johns Hopkins University Press).
Spagnoletti N., Visalberghi E., Ottoni E., Izar P., Fragaszy D. (2012). Stone tool use in wild bearded capuchin monkeys, Cebus libidinosus: is it a strategy to overcome food scarcity? Anim. Behav. 83, 1285–1294. doi: 10.1016/j.anbehav.2012.03.002
Sugiyama Y. (1995). Drinking tools of wild chimpanzees at bossou. Am. J. Primatol 37, 263–269. doi: 10.1002/ajp.1350370308
Surbeck M., Hohmann G. (2008). Primate hunting by bonobos at Lui Kotale, Salonga National Park. Curr. Biol. 18, R906–R907. doi: 10.1016/j.cub.2008.08.040
Susman R. L., Nyati L., Jassal M. S. (1999). Observations on the pollical palmar interosseus muscle (of Henle). Anat Rec 254, 159–165. doi: 10.1002/(SICI)1097-0185(19990201)254:2<159::AID-AR1>3.0.CO;2-H
Tennie C., Call J., Tomasello M. (2009). Ratcheting up the ratchet: on the evolution of cumulative culture. Phil Trans. R Soc. B 364, 2405–2415. doi: 10.1098/rstb20090052
Terada S., Nackoney J., Sakamaki T., Mulavwa M. N., Yumoto T., Furuichi T. (2015). Habitat use of bonobos (Pan paniscus) at Wamba: Selection of vegetation types for ranging, feeding, and night sleeping. Am. J. Primatol 77, 701–713. doi: 10.1002/ajp.22392
Tutin C. E. G., Fernandez M. (1993). Composition of the diet of chimpanzees and composition with that sympatric lowland gorillas in the Lopé Reserve, Gabon. Am. J. Primatol 30, 195–211. doi: 10.1002/ajp.1350300305
Tutin C. E. G., Fernandez M., Rogers M. E., Williamson E. A., McGrew W. B. (1991). Foraging profiles of sympatric lowland gorillas and chimpanzees in the Lopé Reserve, Gabon. Phil Trans. R Soc. Lond B 334 (1270), 179–186. doi: 10.1098/rstb.1991.0107
van Schaik C. P., Deaner R. O., Merrill M. Y. (1999). The conditions for tool use in primates: Implications for the evolution of material culture. J. Hum. Evol. 36, 719–741. doi: 10.1006/jhev.1999.0304
Watts D. P. (1984). Composition and variability of mountain gorilla diets in the Central Virungas. Am. J. Primatol 7, 323–356. doi: 10.1002/ajp.1350070403
Watts D. P. (2003). “Gorilla social relationships: a comparative overview,” in Gorilla biology: a multidisciplinary perspective. Eds. Taylor A. B., Goldsmith M. L. (New York: Cambridge University Press).
White F. (1994). “Food sharing in wild pygmy chimpanzees (Pan paniscus),” in Current primatology, Vol II: Social development, learning and behavior. Eds. Roeder J. J., Thierry B., Anderson J. R., Herrenschmidt N. (Strasbourg: Université Louis Pasteur), 1–10.
White F. J. (1998). Seasonality and socioecology: the importance of variation in fruit abundance to bonobo sociality. Int. J. Primatol 19 (6), 1013–1027. doi: 10.1023/A:1020374220004
Wittiger L., Sunderland-Groves J. L. (2007). Tool use during display behavior in wild Cross River gorillas. Am. J. Primatol 69, 1307–1311. doi: 10.1002/ajp.20436
Wobber V., Wrangham R. W., Hare B. (2010). Bonobos exhibit delayed development of social behavior and cognition relative to chimpanzees. Curr. Biol. 20, 226–230. doi: 10.1016/jcub200911070
Wrangham R. W. (1986). “Ecology and social relationships of two species of chimpanzee,” in Ecological aspects of social evolution: birds and mammals. Eds. Rubenstein D. I., Wrangham R. W. (Princeton, NJ: Princeton University Press), 352–378.
Wrangham R. W., Chapman C. A., Clark-Arcadi A. P., Isabirye-Basuta G. (1996). “Social ecology of Kanyawara chimpanzees: implications for understanding the costs of great ape groups,” in Great ape societies. Eds. McGrew W. C., Marchant L. F., Nishida T. (Cambridge: Cambridge Univ Press), 45–57.
Wrangham R. W., Conklin N. L., Chapman C. A., Hunt K. D. (1991). The significance of fibrous foods for Kibale Forest chimpanzees. Philosoph Transact R. Societ London B 334, 171–178. doi: 10.1098/rstb.1991.0106
Wrangham R. W., Conklin-Brittain N. L., Hunt K. D. (1998). Dietary response of chimpanzees and cercopithecines to seasonal variation in fruit abundance. I. Antifeedants. Int. J. Primatol 19 (6), 49–970. doi: 10.1023/A:1020318102257
Yamakoshi G. (1998). Dietary responses to fruit scarcity of wild chimpanzees at Bossou, Guinea: possible implications for ecological importance of tool use. Am. J. Phys. Anthropol 106 (3), 283–295. doi: 10.1002/(SICI)1096-8644(199807)106:3<283::AID-AJPA2>3.0.CO;2-O
Keywords: great apes, tool use, diet flexibility, behavioral predisposition, necessity hypothesis
Citation: Masi S (2023) Tool use, or not tool use, that is the question: is the necessity hypothesis really inconsequential for the African great apes? Front. Mamm. Sci. 2:1281030. doi: 10.3389/fmamm.2023.1281030
Received: 21 August 2023; Accepted: 20 September 2023;
Published: 09 October 2023.
Edited by:
Iain James Gordon, Australian National University, AustraliaReviewed by:
Steven Emilio Churchill, Duke University, United StatesCopyright © 2023 Masi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shelly Masi, bWFzaUBtbmhuLmZy
 Shelly Masi
Shelly Masi