- 1Department of Ecology, Evolution, and Behavior, University of Minnesota, St. Paul, MN, United States
- 2Department of Neuroscience, University of Minnesota, Minneapolis, MN, United States
Living in a group setting is essential for the health and wellbeing of social carnivores; however, the managed formation of captive groups of territorial animals can be challenging because of the risk of aggression, injury, and even death. Numerous laboratory and clinical studies have implicated oxytocin in the formation of social bonds. Previously, we have shown that oxytocin (OT) administration reduced social vigilance in African lions. Here, we describe a series of case reports in which OT administration was used to facilitate social bonding in both familiar and unfamiliar conspecific pairs and groups of African lions and tigers, and we provide qualitative descriptions of the study animals’ behaviors before and after treatment. We hypothesized that the repeated intranasal administration of oxytocin over a period of 6–8 weeks would facilitate (1) the bonding process during introductions of unfamiliar individuals and (2) reconciliation between paired individuals that had developed ongoing negative social relationships. Following OT treatment, positive social interactions were either observed for the first time or were restored in all seven study groups.
1 Introduction
As human populations increase, available habitat for threatened and endangered species drastically decreases, becoming fragmented and often rendering natural group formation and dispersal impossible (Miller et al., 2020; Magliolo et al., 2022), thus necessitating human intervention to manage keystone species, such as large carnivores. The need for carnivore management is particularly acute in South Africa, where most wildlife reserves are fenced in order to mitigate human–wildlife conflict (Packer et al., 2013), and where there are roughly the same number of animals in captivity as in the wild (Thorn et al., 2012). In either situation, conservationists attempt to manage animal populations in ways that promote their natural behavior and ensure their positive welfare (Crooks et al., 2011; Gutierrez et al., 2021).
Sociality is an important component of behavioral management. Carnivores show a wide spectrum of social systems (Kleiman and Eisenberg, 1973; MacDonald, 1983; Clutton-Brock, 2021). In the more social carnivores, such as the African lion (Panthera leo) and the African wild dog (Lycaon pictus), social interactions are important for promoting health and wellbeing (Gutierrez et al., 2021), and social instability increases mortality (Fanshawe and Fitzgibbon, 1993; Carbone et al., 2005; Hunter et al., 2007). Even tigers (Panthera tigris), a naturally solitary species, show an increase in naturalistic behaviors and a decreased prevalence of stereotypic behaviors in captivity when housed with a companion (De Rouck et al., 2005; Vaz et al., 2017). Thus, whether relocating wild animals between fenced reserves to simulate natural dispersal, rescuing mistreated captive animals and bringing them to sanctuaries, or establishing new groups in a zoo setting, facilitating and maintaining cohesive bonds among group members is an essential aspect of proper wildlife management (van Dyk and Slotow, 2003; Hunter et al., 2007; Gusset et al., 2008a; Gusset et al., 2008b; Marneweck et al., 2019). Carnivores are highly territorial and attempting the introduction of unfamiliar individuals can pose substantial risks, including injury and death (Somers and Gusset, 2009; Marneweck et al., 2019; Robinson, 2019; Sblendorio, 2021; Salinas, 2022), even with the use of sedatives such as tranquilizers or selective serotonin reuptake inhibitors (SSRIs). Unfortunately, however, techniques and outcomes, especially negative outcomes, of alternative methodologies are seldom reported in the literature (Marneweck et al., 2019), leading to a positive publication bias (Boast et al., 2018). Documenting the successes and failures of introduction techniques is clearly essential to improving the welfare of managed populations (Thorn et al., 2012; Gutierrez et al., 2021).
Oxytocin (OT), a peptide that can act as both a neurotransmitter and a hormone, modulates complex social behavior and cognition across vertebrate taxa (Donaldson and Young, 2008; Anacker and Beery, 2013). It can be administered intranasally to enhance the development of social relationships by increasing the positive interpretation of, and response to, social cues from unfamiliar conspecifics (Romero et al., 2014; Oliva et al., 2015). Oxytocin may also facilitate positive social interactions by reducing associated anxiety and fear (Gimpl and Fahrenholz, 2001; Anagnostou et al., 2012; Sabihi et al., 2014; Zoicas et al., 2014) and increasing trust (Boccia et al., 2007), enhancing future involvement (Alvares et al., 2010), and promoting long-term bonds (Insel, 2010). Furthermore, OT mediates (Lukas and Neumann, 2013) and enhances social memory (Lin and Hsu, 2018), cementing positive experiences and maintaining prosocial behavioral effects beyond the treatment period (Oliva et al., 2015). Thus, oxytocin is uniquely suited to aid introductions by promoting anti-aggressive, pro-affiliative behaviors toward conspecifics (Calcagnoli et al., 2015; Oliva et al., 2015) while suppressing vigilance toward potential social threats (Ebitz et al., 2013). Importantly, lasting effects on affiliative behaviors can follow a single treatment of OT (Ebitz et al., 2013; Rault et al., 2013; Calcagnoli et al., 2015; Oliva et al., 2015); this property makes its use potentially highly advantageous for wildlife managers.
We previously showed that intranasal oxytocin in lions increases prosocial behaviors toward pride members and decreases vigilance toward unfamiliar conspecifics (Burkhart et al., 2022). Both pairs, as well as groups, of familiar lions were found to stay in closer proximity to one another during the OT trials, and they displayed an increase in tolerance toward neighbors while in possession of a non-food novel item. Importantly, adverse territorial reactions to unfamiliar “intruders” were also decreased following OT administration. A decrease in vigilance toward unfamiliar conspecifics, coupled with an increase in prosocial behavior, could therefore play a significant role in the success of introductions between unfamiliar lions or other territorial carnivores.
In this article we address whether or not intranasal administration of oxytocin can be used to facilitate social bonding as part of the captive management of large carnivores. We describe seven case studies in which OT was given to pairs or groups of felids in an attempt to enhance novel social bonds (introductions) or to promote more positive social interactions in established groups/pairings (reconciliations). For each case, we provide brief descriptive overviews of the study animals’ behaviors and living conditions prior to treatment and the number of treatments administered until the onset of affiliative behaviors and the absence of negative interactions, along with the observed changes throughout and following the treatment period. In all cases, we witnessed increased affiliative behaviors; in all but one case, the pairs/groups maintained positive relationships, and were able to coexist without intervention for over 1 year following OT administration, suggesting the promise of this method for long-term bonding.
2 Methods
2.1 Subjects
All trials were performed in sanctuaries and reserves in South Africa between March 2021 and March 2022. Procedures were approved by the Institutional Animal Care and Use Committee of the University of Minnesota. Subjects (n = 16) were healthy adult felids [six females (F), and 10 males (M)]. No studies were performed on females who were lactating, pregnant, or in estrus. The introduction trials involved eight African lions (P. leo), whereas reconciliations involved six lions and two tigers (P. tigris). Detailed accounts of each individual’s life history and medical condition were obtained in advance, including origins, demographics, previous housing experience, groupings, known social encounters, time since arrival at the facility, and any other pertinent information. The animals’ behavioral compatibility, age, and health were taken into consideration by Jessica C. Burkhart and sanctuary/veterinary staff prior to OT treatment. The captive animals in this study were either spayed or on contraceptives; they were not paired for breeding purposes.
2.2 Trial types
Introductions are defined as the process of socializing two unfamiliar conspecifics placed in adjoining habitats that eventually leads to cohabitation. Reconciliations are defined as the process of re-bonding a pair or group of currently cohabiting animals that have recently begun to show signs of repeated aggression toward each other.
2.3 Trial protocol
Treatment schedules and protocols are similar for both introduction and reconciliation trials. Prior to beginning OT treatment, subjects were habituated to the researcher’s presence while their baseline measurements were observed. Where possible, pre- and post-treatment housing and social contact remained unchanged (details given below for each case), and we obtained qualitative descriptions of pre-treatment social behaviors from sanctuary staff.
Oxytocin was administered intranasally (Figure 1) by coaxing animals to a fence with a food reward (provided by sanctuary staff according to feeding guidelines for each animal). The food reward was held at the fence with a utensil, and OT was administered using an atomizer into the animal’s nostril (Figure 1). During a given trial, OT was given consecutively to all animals in a pair or group in order to facilitate bonding. Treatment was administered at approximately the same time each day. Non-food enrichment (e.g., boxes, spices, ungulate dung, etc.) was also provided to promote a positive milieu (Ford and Young, 2021), with each animal being given their own “play” item. Oxytocin administration generally occurred > 60 min after feeding (to allow the normal agitation from feeding time to subside). To ensure a safe social environment, unnecessary disruptions and distractions (i.e., construction, cleaning, viewers, volunteers, staff, etc.) were not permitted to be present during the trial or for at least 6 h thereafter.

Figure 1 Images of oxytocin (OT) being administered intranasally to a lion (left) and a tiger (right). Images courtesy of Lionsrock.
The doses were 10 international units (IUs) of OT for lions and 15 IUs for tigers (based on initial findings, see below). These doses are less than the required amount for inducing muscle contraction (Plumb’s Veterinary Drug Handbook). Intranasally administered OT bypasses the blood–brain barrier, peaking at 45 min and lasting ≈ 4 h. (Weisman et al., 2012; Lee et al., 2020).
Each trial was monitored and video recorded for analysis for approximately 90 min following administration. The subjects were also monitored on the days between OT treatments.
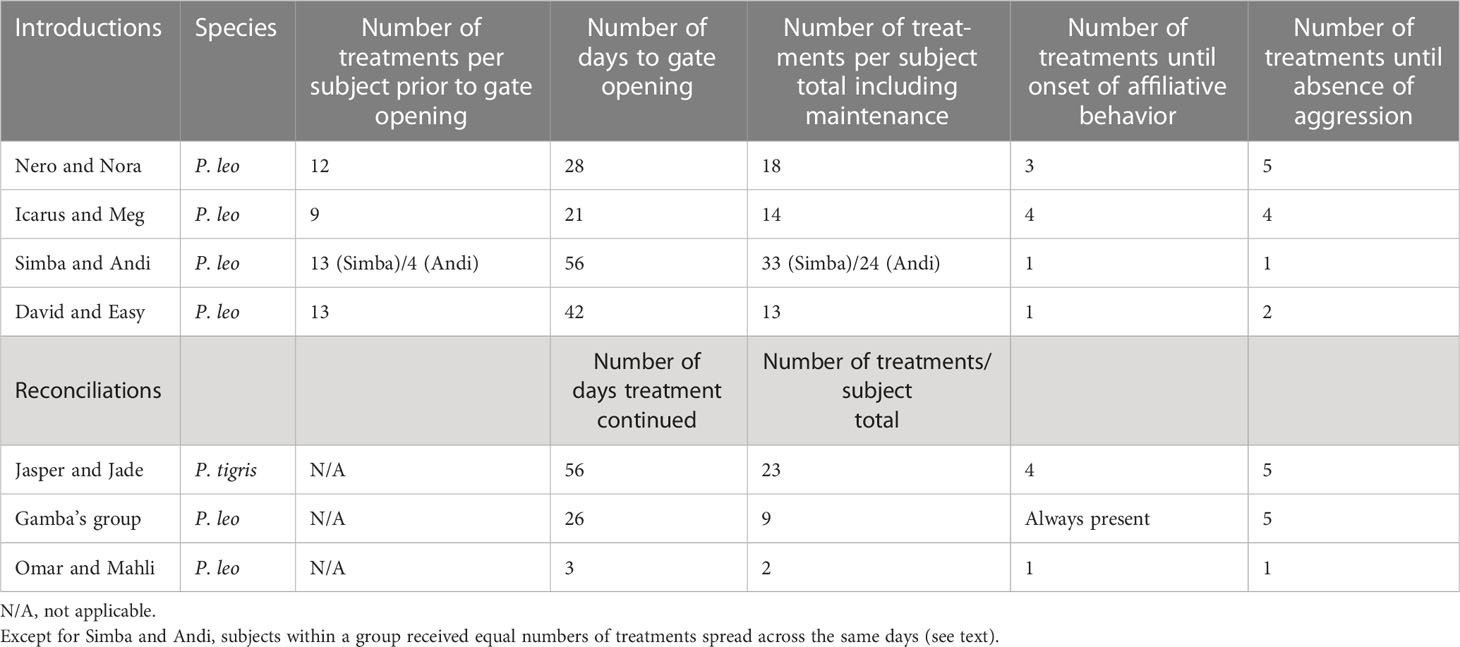
Although the specific protocols followed for each case are detailed below and in Table 1, the general framework is first described here. Oxytocin was initially given 3 or 4 times per week in ≈ 48-hour intervals. It was administered at this rate for a further 2–4 weeks, or until signs of fear and aggression had subsided for at least 1 week and prosocial behaviors were observed. Treatment frequency then decreased to twice per week for an additional 2–4 weeks.
For introductions, animals were kept in adjacent areas and rotated between a mutual space (as enclosure space allowed) to become familiar with each other’s scent. As OT treatment frequency was decreased, rotations continued, and prosocial behaviors were monitored as intervals between treatments increased. During reconciliation treatments, animals were brought into smaller feeding camps, while positive enrichment items were given. Once prosocial behavior was maintained across greater intervals between treatments, we proceeded to the final stage of the introduction: opening the gate. To prepare for this, the gate was slowly lifted 6–8 inches on multiple occasions to signal that the barrier would be removed. On the final opening of the gate, we made certain that both animals were attentive to the situation and that each had enough space to flee if necessary. The gate was then lifted slowly while the animals were attentive. Once the gate was opened, animals were left in the same space together and monitored continuously for at least 24 h post opening.
We administered OT on the day that the gate was opened, and in 24-hour intervals for up to 3 consecutive days thereafter. In some cases, this was followed by a 1-week period in which treatments were given at 48-hour intervals; this frequency was then decreased to twice weekly as needed until prosocial behavior remained consistent. Subsequently, periodic maintenance doses were sometimes given upon observation of antisocial behaviors (e.g., avoidance, aggression). After the gate was opened, animals were monitored frequently for a minimum of 30 days, and behavioral observations gradually shifted from being undertaken by the researcher to the sanctuary staff.
During reconciliations, the frequency of OT administration was adjusted according to observed prosocial and/or aggressive behaviors and continued until signs of abnormal avoidance or aggression subsided, and prosocial behavior was consistently observed.
2.4 Outcomes
In both introductions and reconciliations, success was defined as the ability of the animals to cohabitate without additional pharmaceutical intervention. This decision ultimately belonged to the animals’ caretaking staff, and was made on the basis of behavioral measures, including the presence or absence of affiliative behaviors (proximity and prosocial behaviors), signs of stress (e.g., pacing, nervousness, avoidance, abnormal eating habits, etc.), aggression (e.g., vocalizations and physical acts of aggression), and tolerance (e.g., body posture and response to approach). During introductions, these behavioral measures were used to determine whether or not the gate could be left open (see Table 1), and the animals could be comfortably housed together following the ≈ 8-week introduction process. In the case of reconciliations, these behavioral measures were used to determine whether or not the animals could continue to cohabitate. Success was defined as the animals discontinuing antagonistic or stress-related behaviors and instead maintaining positive prosocial behaviors toward their enclosure mates.
3 Results
3.1 Introduction case studies
3.1.1 Nero and Nora (lions)
Nero (M, 9 years old) and Nora (F, 13 years old) were housed in a double-roomed feeding house leading into two small adjoining feeding camps, which were connected to a large, communal enclosure. Both lions had previously lived with other conspecifics, and Nora had been removed from one previous introduction attempt owing to persistent aggression. At the time of treatment, Nero was extremely aggressive toward both Nora and humans, and Nora showed no affiliative interest; thus, after spending 2 months in adjoining feeding camps, they had not been successfully paired. Importantly, the housing conditions and degree of social exposure were the same for this pair for the 2 months prior to OT treatment, during the treatment protocol, and also post-treatment, making the pre- and post-treatment periods essentially identical.
The OT treatments were initially given when Nero and Nora were physically separated by a gated fence. In total, 12 treatments were administered over a 4-week period before the gate was opened. The lions’ aggression both toward humans and each other declined with OT treatment, and their affiliative behavior increased. Before treatment, affiliative behavior was absent, and aggression was common at the fence line. The animals began to present affiliative behavior following the third treatment (lions were observed lying together and rubbing heads at the fence), and aggression ceased by the fifth treatment.
By the 12th treatment (day 28), it was considered safe for the animals to cohabitate, and the gate was opened. Initially, Nero entered onto Nora’s side of the feeding camps. Nora displayed reactive aggression when he neared her, at which point he withdrew, displaying no aggression. One week later, they were given access to the larger enclosure, and a series of maintenance doses were given (eight doses spread over 8 weeks) to decrease avoidance behavior. From that point onwards, the two animals have been allowed to cohabitate without further pharmaceutical management for > 1 year at the time of writing. The two lions have not had to be unpaired, nor have they required any additional OT treatments, having routinely spent the majority of their time in close proximity to each other and remaining continuously together without separation.
3.1.2 Icarus and Meg (lions)
Icarus (M, 16 years old) and Meg (F, 18 years old) were housed in a shared enclosure divided by a fence during introductions. Both lions had previously been paired with other, now deceased, conspecifics. At the time of the study, Icarus and Meg were not displaying aggression toward humans or other neighboring animals. However, when brought to the shared enclosure, they frequently displayed aggression toward each other through the common gate. Oxytocin treatment began 2 weeks after they were placed in the shared enclosure. This was the shortest pre-treatment social exposure time of all the cases described.
Nine treatments were administered over a 3-week period before the gate was opened. Prior to treatment, aggressive interactions occurred at the fence line and affiliative vocalizations between the two were absent. By the fourth treatment, aggression had ceased, and affiliative interactions (positive vocalizations) were present. When the gate was opened, Icarus walked into close proximity (i.e., < one body length) of Meg without eliciting aggression. Over the next 6-hour period, the two remained in close proximity, with only two instances of mild defensive aggression. Four maintenance treatments were administered over the following 30 days. The gates were subsequently left open at all times, meaning that it was deemed to be safe for the two lions to cohabitate without further intervention. They have remained amicably together for > 1 year after the final treatment.
3.1.3 Simba and Andi (lions)
Simba (M, 9 years old) and Andi (F, 10 years old) were housed in an enclosure that contained both shared and separate spaces. Neither Simba nor Andi had had any prior contact with other lions. Prior to OT treatment, they occupied neighboring enclosures for ≈ 2 years. At the time of treatment, Simba was unresponsive to both humans and other lions (including Andi), whereas Andi acted defensively toward Simba and displayed curiosity toward neighboring lions, and excessive affiliative behavior toward select humans and extreme skittishness and fear toward others. Simba and Andi were moved to a neutral habitat where they shared a common house 2 months prior to treatment, and there was no improvement in their social interactions with each other.
Oxytocin treatments were initially given to Simba and Andi while they were separated inside the neutral common house. Five treatments were administered to Andi, and 13 to Simba over an 8-week period before the gate was opened. For the first treatment, they were each only administered 3 IU of oxytocin because Andi became fearful of the atomizer. Despite the low dose in the first trial, the two animals were observed lying next to each other at the gate and playing (this had not been observed prior to treatment). By Andi’s second treatment (Simba’s ninth) affiliative head rubs were observed at the gate. Remarkably, although more than 2 years of prior exposure had not been effective, after only 8 weeks of oxytocin treatment, sanctuary staff decided that it would be safe to pair the two animals.
Upon opening the gate, Andi reacted with mild, defensive, fear-based aggression; Simba did not respond. All visits between the two were closely supervised for the first 8 weeks. Some antagonistic behaviors directed from Andi to Simba were observed, so several maintenance doses were given (15 treatments over an 8-week period). Following these maintenance doses, no further aggression was observed. Since then, the two have been safely cohabitating (for > 1 year at the time of writing). It is worth noting that this is the first time that either animal had ever been in physical contact with another lion.
3.1.4 David and Easy (lions)
David (M, 10 years old) and Easy (F, 16 years old), were housed in a double-roomed feeding house opening to a single feeding camp and a large, adjoining enclosure. Both lions had previously been paired with other, now deceased, animals. A prior attempt to introduce Easy (along with her late sister) to David > 2 years prior had been unsuccessful. For 1 year prior to treatment, staff rotated David and Easy in the common environment. Both animals displayed aggression toward humans, but only mildly toward each other, although David was seen to charge Easy at the fence, while Easy frequently paced and displayed agitation toward David. Treatments took place under the same housing and social exposure conditions as they had experienced for at least 1 year prior.
Thirteen oxytocin treatments were administered over a 6-week period before the gates were opened. On the day of the first treatment, the animals were brought into the double-roomed feeding house. Prior to treatment, aggression from David toward Easy was present, as well as agitation from Easy. Both behaviors were absent by the second treatment.
Upon opening the gate, David approached Easy, but received little initial response. Later that day, they were observed playing, and slept in contact that night. They were kept in the feeding area together for 3 days, and on the day that the gate was opened to the main habitat, a maintenance treatment of oxytocin was given. No further maintenance doses were necessary. The gates have since remained continuously open, with the pair successfully cohabitating without intervention (for > 1 year at the time of writing).
3.2 Reconciliation case studies
3.2.1 Jasper and Jade (tigers)
Jasper (M, 9 years old) and Jade (F, 9 years old) are tiger siblings that have been housed together since birth. At the time of treatment, they were housed in two adjacent feeding areas within a common one-hectare enclosure. Previously, the pair had reportedly maintained a strong social bond, but an incident that occurred 5 months prior to treatment, in which Jasper was aggressive toward a neighboring animal, led to defensive aggression by Jade, after which the two animals could not move into close proximity without displaying aggression (although they were not physically separated by a gate). The housing and social conditions during treatment were constant for 5 months prior to treatment; again, this allowed for direct comparisons between the animals’ pre- and post-treatment social behaviors to be made.
For treatment 1, only 10 IUs of oxytocin were given, but defensive aggression and stress-based pacing continued, and this trial was terminated early. For treatment 2 (day 3), the dosage was increased to 15 IUs, as we suspected that tigers (being a solitary species) might be less sensitive to the impacts of OT. On this day, animal caretakers noted a decrease in stress behaviors, and they allowed the animals to remain in the feeding area together for the full length of the trial (90 min). Prior to treatment, positive affiliative behaviors and vocalizations were absent between the two tigers, and fear-based aggression from Jade toward Jasper was consistently present. By the fourth treatment, positive vocalizations (chuffing) became present. By the fifth treatment, positive physical interaction was observed (head rubbing), and negative interaction had disappeared.
In total, 23 treatments were administered over an 8-week period. At this point, the pair was considered to have successfully reconciled, and treatment was concluded. The two animals remained in successful cohabitation from that time forward (> 1 year post treatment).
3.2.2 Gamba’s group (lions)
This group consists of four male lions, all 14 years of age, from two separate litters. They have been housed together since they were cubs. They share a three-hectare enclosure with one feeding area and a house with four rooms. Previously, this group had socialized well. However, ≈ 2 months prior to treatment, they began fighting, even leaving each other with deep wounds. The dominant male, Gamba, had become weak as a result of spondylosis. In addition, two females sharing a common fence line had begun exhibiting interest toward the four males. These two issues likely caused the increased aggression. Sanctuary staff were prepared to separate the group members if oxytocin treatment failed. Treatment took place in the same housing conditions as the group had been in for the previous 10+ years.
As the neighboring females presented a challenge to reconciliation, they were locked away from Gamba’s group during the 90-minute trial period following treatment. By the fifth OT treatment (day 13), no further altercations had occurred (even between treatment days when the females were present), and no further injuries or marks were found. We then progressed to allowing the males to visit the fence adjoining the females during OT trials to test the males’ reactive aggression toward each other in the females’ presence. We arranged for the males to enter the common area in different sequences so as to control for any influence of dominance relationships. The males did not display aggression toward each other or the females during any of these encounters. In summary, while consistent fighting and aggression had led to moderate injury prior to the first treatment, fighting ceased and positive interaction persisted even in the presence of females by the fifth treatment.
Moving forward, no further aggression between the males was observed, except for one minor altercation during feeding 6 weeks later, after which one follow-up maintenance dose of OT was administered. A total of nine treatments were given over a 4-week period, at which point aggression had subsided and affiliative behavior had been restored. One year later, no further injuries had been observed, and the males were successfully cohabitating without further intervention.
3.2.3 Omar and Mahli (lions)
Omar (M, 11 years old) and Mahli (F, 14 years old) had been introduced 4 months prior to reconciliation (independent of this study). Omar had previously been privately owned, but his social history is unknown. Mahli had previously been housed for 10 years with her two sisters but was separated from them because of fighting. Omar and Mahli were placed in a common neutral habitat together 1 year prior, but the gates were opened only after several months. According to staff, the integration initially appeared successful; however, after 4 months together, the keepers began to notice scratch marks on Omar’s back and hindquarters. Because this seemed to happen at night, keepers locked them apart in the evening. The OT was given (in the same setting) in an attempt to restore the pairs’ relationship.
In total, Omar and Mahli were given only two treatments of oxytocin; which were administered 2 days apart. During treatments, the two were brought together in a common feeding area and given enrichment to stimulate interaction. On both occasions, the animals displayed only positive interactions, playing and lying together. The gate was left open from the night of the second treatment onward, and no altercations took place over the following few months. However, Mahli resumed displaying antagonistic behaviors toward Omar 4 months after the second OT treatment.
4 Discussion
Our seven cases of oxytocin-facilitated socializations indicate the potential power of this intervention as a behavioral management tool. In all cases, subjects had positive interactions post-oxytocin treatment, and, in six cases, these effects continued long term. Subjects were successfully integrated and formed stable social bonds. This is a small-scale study in which each case was unique, and pairings were preplanned and adapted accordingly. In the only case that did not achieve lasting success (Omar and Mahli), the treatments did alleviate negative interactions for 4 months before antagonistic behavior resumed. Furthermore, this pair received the fewest treatments (only two doses), so it is possible that a more persistent dose schedule would have produced a more lasting impact.
Because of the well-known negative publication bias, it is difficult to measure the relative efficacy of different social integration management strategies (Boast et al., 2018; Marneweck et al., 2019). Nevertheless, pharmaceutical protocols, including SSRIs and tranquilizing agents (Hunter et al., 2007; Gaultier et al., 2005), have previously been used to aid in lion introductions, although with mixed success (based on veterinarian and sanctuary staff reports: P. Caldwell, personal communication, 2022; H. Pirker, personal communication, 2022). In addition, certain proteins, such as appeasing pheromones, are known to enhance positive social behavior (Moncho-Bogani et al., 2002; Beny and Kimchi, 2014; Liberles, 2014). However, unlike OT, the chemical composition of appeasing pheromones varies across species (Riddell et al., 2021), which may decrease their efficacy for any given species. One likely possibility (given their shared behavioral effects) is that pheromone administration, when successful, leads to oxytocin release, making OT the more proximate and relevant agent. Indeed, there are substantial structural opportunities for pheromone and oxytocin systems to interact in the brain (Wacker and Ludwig, 2012). Importantly, none of these aforementioned pharmaceutical agents is in widespread usage under a standardized protocol. Thus, there is essentially no “best practice” for pharmaceutically aided carnivore introduction.
Although our results are promising, there are significant limitations to consider should OT treatment become a tool for behavioral management. Our study lacked a randomized control structure. Ideally, we would have compared our strategy with a standardized “best practice”; however, as stated above, no such protocol exists. Alternatively, we could have attempted introductions without the aid of oxytocin. However, we could not justify a potentially fatal outcome in untreated animals. In the future, we plan to more carefully quantify specific behaviors in a control condition in standardized settings where animals can interact with each other in adjoining spaces with the gate still closed.
Nevertheless, we are able to retrospectively assess treatment impacts from behavioral changes reported before vs. after the OT treatment. For example, Nero and Nora displayed intense aggression for 2 months, which subsided within 2 weeks of treatment; Simba had shown no social interest in Andi for nearly 2 years in an adjoining enclosure yet began to show affiliative interest nearly immediately following the OT treatment; and Jade and Jasper had shown no increase in affiliative behavior or decrease in aggression over 5 months, but began showing affiliative interest by their second week of OT treatment. The housing conditions and social settings of the groups were the same during treatment as they had been before treatment (with pre-treatment time frames ranging from 2 weeks to over a decade). This allows us to reasonably rule out the possibility that affiliation would have increased in the same manner, in the same time frame, without oxytocin intervention.
Behavioral management with oxytocin should be performed with great caution. First, it is unlikely that an intramuscular, intravenous, or subcutaneous injection of oxytocin would induce behavioral change, as injected oxytocin may not reach the brain effectively (Mens et al., 1983; Ermisch et al., 1985; Landgraf, 1985; Leng and Ludwig, 2016; Lee et al., 2020). Second, there is substantial prior evidence indicating that the behavioral effects of OT depend on species, sex, rank, and even context (Insel and Shapiro, 1992; De Dreu, 2012; Dumais et al., 2013; DeWall et al., 2014; De Dreu and Kret, 2016; Ma et al., 2018; Lee et al., 2019). Because of this, oxytocin administration has the potential to cause behavioral problems rather than ameliorate them (De Dreu, 2012; De Dreu & Kret, 2016; Pedersen, 2017). For example, although OT can decrease social fear and anxiety and increase social interest (Labuschagne et al., 2010), it may not always override certain instincts or eradicate years of learned behavior. Indeed, we previously found that OT administration did not alter prosocial behaviors in the presence of a highly desired food item (Burkhart et al., 2022). Furthermore, recent work by Berendzen and colleagues (2022) has emphasized that the OT system may not be the only set of neural circuitry that is capable of governing affiliative behavior, which could potentially point to future adjunctive therapies targeting additional systems.
Importantly, social relationships are dynamic, and can be affected by multiple factors, including the animals’ housing, neighbors, staff interactions etc., all of which should be considered before commencing and/or continuing introduction or reconciliation treatments. Interindividual variation also plays a major role (von Rueden et al., 2015; Myers and Young, 2018), and certain combinations of individuals may prove to be incompatible (Hutchins and Kreger, 2006; Charles et al., 2022), depending on age, personality, medical condition, and previous life experiences. Our case studies suggest that the long-term effectiveness of oxytocin treatments may rely on the underlying compatibility of these individuals, emphasizing the necessity of collecting background and behavioral data on each animal prior to treatment. Finally, although we only included one case of tigers (a highly solitary species in the wild), the success shown here in captivity highlights the promise of OT treatment across a broad range of species.
In addition, oxytocin treatment may require the combined use of behavioral enrichment. Providing necessary social stimulation while under the influence is likely the most effective way to employ oxytocin's beneficial social impacts (Ford and Young, 2021). The cases reported in this study all involved extensive behavioral management and monitoring. Therefore, animal caretaking staff must not only be skilled in understanding animal behavior, but also be able to take the time to supplement OT treatments with enrichment and to monitor the animals through the entire process.
Group dynamics add an additional level of complexity that affects social integrations (Boast et al., 2018; Marneweck et al., 2019). Increased oxytocin levels are correlated with in-group camaraderie (Port et al., 2017), and this should therefore be considered when placing a previously bonded pair with an unfamiliar individual. Rather than increasing interest between the unfamiliar individuals, oxytocin could potentially increase the bond between the familiar pair, leading to enhanced competition toward the newly introduced “outsider.” Nevertheless, our previous work has shown that a single treatment of OT led to decreased vigilance by groupmates toward outsiders (Burkhart et al., 2022). The case studies presented here indicated that oxytocin facilitated social bonds in both pairs and groups of big cats; in each case, we observed increased positive behavior and social interest between individuals. Although the administration of oxytocin alone may not always be sufficient to create lasting bonds, when used carefully in combination with behavioral management strategies, it may be an important catalyst of the process of alleviating aggression and mitigating fears between individuals, while promoting positive, stable social bonds. Importantly, without successful intervention, animals that cannot be paired or reconciled will remain in solitary conditions for a captive lifespan of over 20 years. This will likely lead to these animals presenting persistent stress-related behaviors, experiencing severe health conditions, and occupying more space within overcrowded captive facilities. More work will be necessary to investigate the most effective treatment schedules in different scenarios; however, it appears that oxytocin may be a useful tool in wildlife management.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of the University of Minnesota. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
JB developed and implemented all physical aspects of study under advisement from CP and SH. JB, SH, and CP were all involved in conception and design of the study and the writing and editing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Funding was provided by the Grad Program in EEB, University of Minnesota, and from the UMN AHC seed Award to SH. We would also like to thank Mary Kemen and Debbi Oswald for their generous donations.
Acknowledgments
We thank The Lions Foundation, the Kevin Richardson Wildlife Sanctuary, Lionsrock and Four Paws International, and Animal Defenders International for access and assistance with data collection. We thank Dr. Peter Caldwell for recommendations and advisement.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alvares G. A., Hickie I. B., Guastella A. J. (2010). Acute effects of intranasal oxytocin on subjective and behavioral responses to social rejection. Exp. Clin. Psychopharmacol. 18 (4), 316–321. doi: 10.1037/a0019719
Anacker A. M., Beery A. K. (2013). Life in groups: the roles of oxytocin in mammalian sociality. Front. Behav. Neurosci. 7, 185. doi: 10.3389/fnbeh.2013.00185
Anagnostou E., Soorya L., Chaplin W., Bartz J., Halpern D., Wasserman S., et al. (2012). Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Mol. Autism 3 (1), 16. doi: 10.1186/2040-2392-3-16
Beny Y., Kimchi T. (2014). Innate and learned aspects of pheromone-mediated social behaviours. Anim. Behav. 97, 301–311. doi: 10.1016/j.anbehav.2014.09.014
Boast L. K., Chelysheva E. V., van der Merwe V., Schmidt-Küntzel A., Walker E. H., Cilliers D., et al. (2018). “Cheetah translocation and reintroduction programs: past, present, and future,” in Cheetahs: biology and conservation: biodiversity of the world: conservation from genes to landscapes (Academic Press Cambridge Mass), 275–289. doi: 10.1016/B978-0-12-804088-1.00020-4
Boccia M. L., Goursaud A. P. S., Bachevalier J., Anderson K. D., Pedersen C. A. (2007). Peripherally administered non-peptide oxytocin antagonist, L368,899®, accumulates in limbic brain areas: a new pharmacological tool for the study of social motivation in non-human primates. Hormones Behav. 52 (3), 344–351. doi: 10.1016/j.yhbeh.2007.05.009
Burkhart J. C., Gupta S., Borrego N., Heilbronner S. R., Packer C. (2022). Oxytocin promotes social proximity and decreases vigilance in groups of African lions. iScience 25 (4), 104049. doi: 10.1016/j.isci.2022.104049
Calcagnoli F., Kreutzmann J. C., de Boer S. F., Althaus M., Koolhaas J. M. (2015). Acute and repeated intranasal oxytocin administration exerts anti-aggressive and pro-affiliative effects in male rats. Psychoneuroendocrinology 51, 112–121. doi: 10.1016/j.psyneuen.2014.09.019
Carbone C., Frame L., Frame G., Malcolm J., Fanshawe J., FitzGibbon C., et al. (2005). Feeding success of African wild dogs (Lycaon pictus) in the Serengeti: the effects of group size and kleptoparasitism. J. Zool 266 (2), 153–161. doi: 10.1017/S0952836905006710
Charles A., Henaut Y., Saint Jalme M., Mulot B., Lecu A., Delfour F. (2022). Studying antillean manatees’ (Trichechus manatus manatus) temperament in zoological parks: exploration of boldness, sociality and reactivity to humans. Appl. Anim. Behav. Sci. 246, C7–105512. doi: 10.1016/j.applanim.2021.105512
Clutton-Brock T. (2021). Social evolution in mammals. Science 373 (6561), eabc9699. doi: 10.1126/science.abc9699
Crooks K. R., Burdett C. L., Theobald D. M., Rondinini C., Boitani L. (2011). Global patterns of fragmentation and connectivity of mammalian carnivore habitat. Philos. Trans. R Soc. Lond B Biol. Sci. 366 (1578), 2642–2651. doi: 10.1098/rstb.2011.0120
De Dreu C. K. W. (2012). Oxytocin modulates cooperation within and competition between groups: an integrative review and research agenda. Hormones Behav. 61 (3), 419–428. doi: 10.1016/j.yhbeh.2011.12.009
De Dreu C. K. W., Kret M. E. (2016). Oxytocin conditions intergroup relations through upregulated in-group empathy, cooperation, conformity, and defense. Biol. Psychiatry 79 (3), 165–173. doi: 10.1016/j.biopsych.2015.03.020
De Rouck M., Kitchener A. C., Law G., Nelissen M. (2005). A comparative study of the influence of social housing conditions on the behaviour of captive tigers (Panthera tigris). Anim. Welfare 14 (3), 229–238. doi: 10.1017/S0962728600029390
DeWall C. N., Gillath O., Pressman S. D., Black L. L., Bartz J. A., Moskovitz J., et al. (2014). When the love hormone leads to violence: oxytocin increases intimate partner violence inclinations among high trait aggressive people. Soc. psychol. Pers. Sci. 5 (6), 691–697. doi: 10.1177/1948550613516876
Donaldson Z. R., Young L. J. (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322 (5903), 900–904. doi: 10.1126/science.1158668
Dumais K. M., Bredewold R., Mayer T. E., Veenema A. H. (2013). Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Hormones Behav. 64 (4), 693–701. doi: 10.1016/j.yhbeh.2013.08.012
Ebitz R. B., Watson K. K., Platt M. L. (2013). Oxytocin blunts social vigilance in the rhesus macaque. Proc. Natl. Acad. Sci. United States America 110 (28), 11630–11635. doi: 10.1073/pnas.1305230110
Ermisch A., Rühle H. J., Landgraf R., Hess J. (1985). Blood-brain barrier and peptides. J. Cereb Blood Flow Metab. 5 (3), 350–357. doi: 10.1038/jcbfm.1985.49
Fanshawe J. H., Fitzgibbon C. D. (1993). Factors influencing the hunting success of an African wild dog pack. Anim. Behav. 45 (3), 479–490. doi: 10.1006/anbe.1993.1059
Ford C. L., Young L. J. (2021). Translational opportunities for circuit-based social neuroscience: advancing 21st century psychiatry. Curr. Opin. Neurobiol. 68, 1–8. doi: 10.1016/j.conb.2020.11.007
Gaultier E., Falewee C., Bougrat L., Pageat P. (2005). The introduction of a female tiger (Panthera tigris) in a pre-established group of two neutered males: A case study. pp. 1–5. Purdue University Press: West Lafayette, IN, USA.
Gimpl G., Fahrenholz F. (2001). The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 81 (2), 629–683. doi: 10.1152/physrev.2001.81.2.629
Gusset M., Maddock A. H., Gunther G. J., Szykman M., Slotow R., Walters M., et al. (2008a). Conflicting human interests over the re-introduction of endangered wild dogs in south Africa. Biodiver Conserv. 17 (1), 83–101. doi: 10.1007/s10531-007-9232-0
Gusset M., Ryan S. J., Hofmeyr M., Van Dyk G., Davies-Mostert H. T., Graf J. A., et al. (2008b). Efforts going to the dogs? evaluating attempts to re-introduce endangered wild dogs in south Africa. J. Appl. Ecol. 45 (1), 100–108. doi: 10.1111/j.1365-2664.2007.01357.x
Gutierrez S., Canington S. L., Eller A. R., Herrelko E. S., Sholts S. B. (2021). The intertwined history of non-human primate health and human medicine at the smithsonian's national zoo and conservation biology institute. Notes Records, 77 (1), rsnr20210009. doi: 10.1098/rsnr.2021.0009
Hunter L. T. B., Pretorius K., Carlisle L. C., Rickelton M., Walker C., Slotow R., et al. (2007). Restoring lions panthera leo to northern KwaZulu-natal, south Africa: short-term biological and technical success but equivocal long-term conservation. Oryx 41 (2), 196–204. doi: 10.1017/S003060530700172X
Hutchins M., Kreger M. D. (2006). Rhinoceros behaviour: implications for captive management and conservation. Int. Zoo Yearbook 40 (1), 150–173. doi: 10.1111/j.1748-1090.2006.00150.x
Insel T. R. (2010). The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 65 (6), 768–779. doi: 10.1016/j.neuron.2010.03.005
Insel T. R., Shapiro L. E. (1992). Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl. Acad. Sci. United States America 89 (13), 5981–5985. doi: 10.1073/pnas.89.13.5981
Kleiman D. G., Eisenberg J. F. (1973). Comparisons of canid and felid social systems from an evolutionary perspective. Anim. Behav. 21 (4), 637–659. doi: 10.1016/S0003-3472(73)80088-0
Labuschagne I., Phan K. L., Wood A., Angstadt M., Chua P., Heinrichs M., et al. (2010). Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology 35 (12), 2403–2413. doi: 10.1038/npp.2010.123
Landgraf R. (1985). Plasma oxytocin concentrations in man after different routes of administration of synthetic oxytocin. Exp. Clin. Endocrinol. 85 (2), 245–248. doi: 10.1055/s-0029-1210444
Lee W., Hiura L. C., Yang E., Broekman K. A., Ophir A. G., Curley J. P. (2019). Social status in mouse social hierarchies is associated with variation in oxytocin and vasopressin 1a receptor densities. Hormones Behav. 114. doi: 10.1016/j.yhbeh.2019.06.015
Lee M. R., Shnitko T. A., Blue S. W., Kaucher A. V., Winchell A. J., Erikson D. W., et al. (2020). Labeled oxytocin administered via the intranasal route reaches the brain in rhesus macaques. Nat. Commun. 11 (1). doi: 10.1038/s41467-020-15942-1
Leng G., Ludwig M. (2016). Intranasal oxytocin: myths and delusions. Biol. Psychiatry 79 (3), 243–250. doi: 10.1016/j.biopsych.2015.05.003
Liberles S. D. (2014). Mammalian pheromones. Annu. Rev. Physiol. 76, 151–175. doi: 10.1146/annurev-physiol-021113-170334
Lin Y. T., Hsu K. S. (2018). Oxytocin receptor signaling in the hippocampus: role in regulating neuronal excitability, network oscillatory activity, synaptic plasticity and social memory. Prog. Neurobiol. 171, 1–14. doi: 10.1016/j.pneurobio.2018.10.003
Lukas M., Neumann I. D. (2013). Oxytocin and vasopressin in rodent behaviors related to social dysfunctions in autism spectrum disorders. Behav. Brain Res. 251, 85–94. doi: 10.1016/j.bbr.2012.08.011
Ma X., Zhao W., Luo R., Zhou F., Geng Y., Xu L., et al. (2018). Sex- and context-dependent effects of oxytocin on social sharing. Neuroimage 183, 62–72. doi: 10.1016/j.neuroimage.2018.08.004
MacDonald D. W. (1983). The ecology of carnivore social behaviour. Nature 301 (5899), 379–384. doi: 10.1038/301379a0
Magliolo M., Naude V., van der Merwe V., Prost S., Orozco-terWengel P., Burger P., et al. (2022). Simulated genetic efficacy of metapopulation management and conservation value of captive reintroductions in a rapidly declining felid [Article|Early access]. Anim. Conserv. 26 (2), 250-263. doi: 10.1111/acv.12821
Marneweck C. J., Marchal A. F. J., Marneweck D. G., Beverley G., Davies-Mostert H. T., Parker D. M. (2019). A novel technique for artificial pack formation in African wild dogs using odour familiarity. Afr. J. Wildlife Res. 49 (1), 116–120. doi: 10.3957/056.049.0116
Mens W. B., Witter A., van Wimersma Greidanus T. B. (1983). Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 262 (1), 143–149. doi: 10.1016/0006-8993(83)90478-x
Miller S., Druce D., Dalton D., Harper C., Kotze A., Packer C., et al. (2020). Genetic rescue of an isolated African lion population [Article]. Conserv. Genet. 21 (1), 41–53. doi: 10.1007/s10592-019-01231-y
Moncho-Bogani J., Lanuza E., Hernández A., Novejarque A., Martínez-García F. (2002). Attractive properties of sexual pheromones in mice: innate or learned? Physiol. Behav. 77 (1), 167–176. doi: 10.1016/s0031-9384(02)00842-9
Myers P. J., Young J. K. (2018). Consistent individual behavior: evidence of personality in black bears. J. Ethol 36 (2), 117–124. doi: 10.1007/s10164-018-0541-4
Oliva J. L., Rault J. L., Appleton B., Lill A. (2015). Oxytocin enhances the appropriate use of human social cues by the domestic dog (Canis familiaris) in an object choice task. Anim. Cognit. 18 (3), 767–775. doi: 10.1007/s10071-015-0843-7
Packer C., Loveridge A., Canney S., Caro T., Garnett S. T., Pfeifer M., et al. (2013). Conserving large carnivores: dollars and fence. Ecol. Lett. 16 (5), 635–641. doi: 10.1111/ele.12091
Pedersen C. A. (2017). Oxytocin, tolerance, and the dark side of addiction. Int. Rev. Neurobiol. 136, 239–274. doi: 10.1016/bs.irn.2017.08.003
Port M., Schülke O., Ostner J. (2017). From individual to group territoriality: competitive environments promote the evolution of sociality. Am. Nat. 189 (3), E46–E57. doi: 10.1086/690218
Rault J. L., Carter C. S., Garner J. P., Marchant-Forde J. N., Richert B. T., Lay D. C. (2013). Repeated intranasal oxytocin administration in early life dysregulates the HPA axis and alters social behavior. Physiol. Behav. 112-113, 40–48. doi: 10.1016/j.physbeh.2013.02.007
Riddell P., Paris M. C. J., Joonè C. J., Pageat P., Paris D. B. B. P. (2021). Appeasing pheromones for the management of stress and aggression during conservation of wild canids: could the solution be right under our nose? Anim. (Basel) 11 (6). doi: 10.3390/ani11061574
Robinson M. (2019). Tiger kills potential mate on first date (London:CNN World). Available at: https://edition.cnn.com/2019/02/09/uk/tiger-dead-london-zoo-sumatran-scli-gbr-intl/index.html.
Romero T., Nagasawa M., Mogi K., Hasegawa T., Kikusui T. (2014). Oxytocin promotes social bonding in dogs. Proc. Natl. Acad. Sci. United States America 111 (25), 9085–9090. doi: 10.1073/pnas.1322868111
Sabihi S., Dong S. M., Durosko N. E., Leuner B. (2014). Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Front. Behav. Neurosci. 8. doi: 10.3389/fnbeh.2014.00258
Salinas R. (2022). Alabama Lioness killed by former San Antonio zoo lion during introduction (San Antonio, US:KSAT). Available at: https://www.ksat.com/news/local/2022/07/21/alabama-lioness-killed-by-former-san-antonio-zoo-lion-during-introduction/.
Sblendorio P. (2021). Endangered tiger killed in breeding introduction at zoo (Washington State, USA: Daily News). Available at: https://www.nydailynews.com/news/national/ny-endangered-tiger-dead-breeding-washington-zoo-20211006-3c7hr6fmyzf3npxqbsqsiko42m-story.html.
Somers M. J., Gusset M. (2009). “The role of social behaviour in carnivore reintroductions,” in Reintroduction of top-order predators (Wiley), 270–281. doi: 10.1002/9781444312034.ch12
Thorn M., Green M., Dalerum F., Bateman P. W., Scott D. M. (2012). What drives human–carnivore conflict in the north West province of south Africa? Biol. Conserv. 150 (1), 23–32. doi: 10.1016/j.biocon.2012.02.017
van Dyk G., Slotow R. (2003). The effects of fences and lions on the ecology of African wild dogs reintroduced to pilanesberg national park, south Africa. Afr. Zool 38 (1), 79–94. doi: 10.1080/15627020.2003.11657196
Vaz J., Narayan E. J., Dileep Kumar R., Thenmozhi K., Thiyagesan K., Baskaran N. (2017). Prevalence and determinants of stereotypic behaviours and physiological stress among tigers and leopards in Indian zoos. PloS One 12 (4), e0174711. doi: 10.1371/journal.pone.0174711
von Rueden C., Gavrilets S., Glowacki L. (2015). Solving the puzzle of collective action through inter-individual differences. Philos. Trans. R Soc. Lond B Biol. Sci. 370 (1683), 20150002. doi: 10.1098/rstb.2015.0002
Wacker D. W., Ludwig M. (2012). Vasopressin, oxytocin, and social odor recognition. Hormones Behav. 61 (3), 259–265. doi: 10.1016/j.yhbeh.2011.08.014
Weisman O., Zagoory-Sharon O., Feldman R. (2012). Intranasal oxytocin administration is reflected in human saliva. Psychoneuroendocrinology 37 (9), 1582–1586. doi: 10.1016/j.psyneuen.2012.02.014
Keywords: lion, tiger, conservation, reconciliation, oxytocin, wildlife management, introduction, socialization
Citation: Burkhart JC, Heilbronner SR and Packer C (2023) Oxytocin administration is a potential tool for behavioral management in felids. Front. Mamm. Sci. 2:1148214. doi: 10.3389/fmamm.2023.1148214
Received: 19 January 2023; Accepted: 12 May 2023;
Published: 15 June 2023.
Edited by:
Christian Timothy Chimimba, University of Pretoria, South AfricaReviewed by:
Kris Turlejski, Cardinal Stefan Wyszyński University, PolandLori K. Sheeran, Washington University, United States
Copyright © 2023 Burkhart, Heilbronner and Packer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica C. Burkhart, YnVya2gxMzVAdW1uLmVkdQ==
†These authors have contributed equally to this work
 Jessica C. Burkhart
Jessica C. Burkhart Sarah R. Heilbronner
Sarah R. Heilbronner Craig Packer
Craig Packer