
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Malar. , 12 March 2025
Sec. Vectors
Volume 3 - 2025 | https://doi.org/10.3389/fmala.2025.1540184
 Fadhila Kihwele1*
Fadhila Kihwele1* Olukayode G. Odufuwa1,2,3,4
Olukayode G. Odufuwa1,2,3,4 Joseph B. Muganga1
Joseph B. Muganga1 Emmanuel Mbuba1,2,3
Emmanuel Mbuba1,2,3 Rose Philipo1
Rose Philipo1 Jason Moore1,2,3
Jason Moore1,2,3 Ole Skovmand5
Ole Skovmand5 Rune Bosselmann6
Rune Bosselmann6 John Bradley4
John Bradley4 Sarah Moore1,2,3,7
Sarah Moore1,2,3,7 Zawadi Mageni Mboma1,7
Zawadi Mageni Mboma1,7Background: Unimproved housing is a risk factor for malaria. Therefore, netting incorporated with deltamethrin and piperonyl butoxide, supplied as a roll to screen opened eaves, windows, and holes in the walls of unimproved houses, could offer protection by killing and/or reducing the entry of mosquitoes into a house. This study assessed the community perceptions and the acceptability of insecticide-treated screens (ITS), previously described as insecticide-treated eave nets (ITENs) and insecticide-treated window screens (ITWS).
Methodology: A mixed-methods approach was implemented in three villages of the Chalinze District in Tanzania. This approach comprised in-depth interviews (IDIs) of the local carpenters who installed the ITS, focus group discussions (FGDs) with community members in both the ITS and control arms, and the administration of a structured questionnaire to members in the ITS arm. Data collection was conducted at 6 and 12 months post-installation. A thematic framework approach was used to identify and extract relevant themes from the qualitative data, including but not limited to community perceptions, acceptability, and adverse events, which were quantified using quantitative data. Furthermore, a separate structured questionnaire was administered during ITS installation to collect information on the time required for installation and the amount of netting used per house (214) in order to assess the cost implications of rolling out ITS in the community.
Results: The ITS were perceived to reduce the entry of mosquitoes, other insects, and crawling animals such as snakes and lizards into houses. This intervention was accepted in the community, whereby the majority (95%) of participants expressed willingness to purchase the netting if sold at an affordable price of 1,000–6,000 Tanzanian shillings (USD ≤2.50) per square meter. The average time for ITS installation was 1 h per house, using an average of 29.5 running meters of fabric netting from rolls with a width of 1.5 m. The average material cost of the ITS was USD 1.25 per kilogram in transport. In this study, the average installation cost per house was USD 6.6 using standard Tanzanian salary rates, half the annual cost of insecticide-treated nets (ITNs) for a five-person household.
Conclusion: Insecticide-treated screening for unimproved houses is a promising, adaptable, and acceptable tool to supplement the existing vector control tools. The community perceived the use of ITS as a feasible intervention. This study highlights the importance of intensive community engagement during the development of a novel intervention to promptly address concerns and improve its acceptability.
Tanzania reported a substantial increase of 1.3 million malaria cases in 2022 (World Health Organization, 2023a), and malaria remains a major public health concern in the country. Disease reduction has either stalled or, in certain regions of the country, reversed (Mohcdgec, 2020). This was attributed to several factors, including the increasing insecticide resistance in malaria vectors, the decreasing access and use of insecticide-treated nets (ITNs) due to rapid damage and loss, the intensive logistics needed to implement indoor residual spraying (IRS), and population growth (Russell et al., 2011; Sangoro et al., 2014; Ranson and Lissenden, 2016; Haji et al., 2020; Obi et al., 2020; Kihwele et al., 2023). It is also important to acknowledge that most of the malaria burden averted from the early 2000s to 2015 was attributed to the widespread use of vector control tools, particularly ITNs (Bhatt et al., 2015). Therefore, more cost-effective vector control tools that can supplement the existing ones are needed to increase impact and meet the national and global malaria burden reduction targets for 2030 (Who, 2016; Mohcdgec, 2020; Kihwele et al., 2023).
Most of the Afro-tropical Anopheles mosquitoes mainly bite humans indoors and at night (Finda et al., 2019), which makes the house a logical target for malaria control (Ngadjeu et al., 2020). Closing the gaps in house structures, including the eaves (gaps between the wall and the roof) and windows, is an effective method of reducing human–mosquito contact for malaria prevention (Fox et al., 2022). However, this is more feasible in houses with modern designs that minimize mosquito entry (Ogoma et al., 2010) than in unimproved houses (i.e., houses built with traditional or natural materials), which have open eaves and windows for indoor temperature cooling that mosquitoes can use for entry (Jatta et al., 2021). The malaria burden is highest in rural areas and is higher in unimproved houses (Tusting et al., 2017; Sarfo et al., 2023). It is therefore crucial to target unimproved houses in order to minimize the entry of mosquitoes. This could be achieved with insecticide-treated screens (ITS), previously described as insecticide-treated eave nets (ITENs) and insecticide-treated window screens (ITWS), for closing the gaps in houses such as that in eaves, windows, and wall holes (Odufuwa et al., 2022). ITS allow indoor airflow, act as a barrier against mosquito entry, and contain an insecticide to kill mosquitoes upon contact, protecting all household members equally and contributing to community protection. Intervention with ITS was developed by Vegro ApS (Copenhagen, Denmark). The ITS is a polyethylene net with a thickness of 0.152 mm, exceeding that of most standard ITNs. It incorporates 2 g/kg of the pyrethroid insecticide deltamethrin and 8 g/kg of the synergist piperonyl butoxide (PBO). PBO overcomes pyrethroid resistance in mosquitoes by inhibiting the enzymes that degrade pyrethroid insecticides, thereby improving the mosquito-killing efficacy of the ITS (Pryce et al., 2018; Gleave et al., 2021). The ITS have an ex-factory cost (Jiangsu, China) of 13 US cents per square meter or 8.7 US cents per running meter, with a cost of approximately 18 US cents per square meter, or 12 cents per running meter, delivered to the facility of the Bagamoyo branch of the Ifakara Health Institute (IHI) (2021 prices). For comparison, a PBO ITN procured by The Global Fund costs USD 2.32 for a size of 180 cm × 160 cm × 150 cm delivered to port (FCA),1 which is equal to approximately 17 cents per square meter. The ITS are supplied in rolls of 1.5 m width with hemmed edges and are packed in sacks, thereby reducing plastic use. As the ITS are installed on eaves and windows, areas where household members rarely contact, higher doses of the insecticide and mixtures of the active ingredient (AI) can be incorporated, which can be used for the management of the insecticide resistance of malaria vectors. A pilot study of a similar intervention, i.e., insecticide-treated eave curtains in Kenya, induced a remarkable reduction in the density of indoor mosquitoes (Odhiambo et al., 2016). The eave curtains were installed in semi-improved houses (i.e., constructed using both natural and processed materials) in Kenya. Therefore, in this study, the effect of ITS was evaluated in unimproved houses (mud houses).
The World Health Organization (WHO) recommends that the deployment of new vector control tools be accompanied by a well-designed community sensitization plan and the engagement of stakeholders at all levels to ensure that primary users fully understand the intervention, that their voices are heard, and that their concerns addressed promptly to increase acceptability and adherence (Sahan et al., 2017; Peto et al., 2018; Organização Mundial da S, 2019; Monroe et al., 2021). Several supplementary malaria control tools, such as the use of repellents and IRS implemented in different parts of Sub-Saharan Africa (SSA), struggle with insufficient community adherence, which also affects the impact of the intervention (Sangoro et al., 2014; Monroe et al., 2015; Maia et al., 2018; Opiyo and Paaijmans, 2020) largely due to a lack of understanding of the initiatives involved in upscaling or engaging end users early during the design, testing, and the implementation of these innovations for public health. Therefore, this study used mixed methods to explore the perceptions of the community and the acceptability of ITS before its availability in the market.
A mixed-methods approach was used in this study. To assess the practical aspects of ITS installation, a structured questionnaire was administered to accurately determine the installation time (in minutes) and the amount of ITS (in meters) required per house. Given the limited number of carpenters in each village (less than five), in-depth interviews (IDIs) were conducted to explore their individual perceptions, including any adverse effects encountered during the installation. Focus group discussions (FGDs) were conducted with community members to gather information on their acceptance, preference, and willingness to pay and the practices related to net care and repair. The findings from the FGDs were then quantified using data collected through a structured questionnaire administered to participants. All surveys were conducted at 6 and 12 months post-installation of the ITS. This study was conducted as part of a larger ITS trial (Odufuwa et al., 2022).
This study was conducted in three villages (Pongwe, Mazizi, and Kihangaiko) within Msata Council, Chalinze District, in coastal Tanzania. Msata Council has an estimated population of 24,000 residing in over 5,000 households (average size, 4.1 individuals) (The United Republic of Tanzania (URT) MoFaP et al., 2022). This region experiences approximately 28°C–29°C temperature and 1,200–2,100 mm of rainfall annually (Odufuwa et al., 2022), which contribute to the malaria transmission in this area. Msata Council, where the study was conducted, is characterized by subsistence farming as the primary occupation. Furthermore, residents largely live in unimproved houses with mud walls, open eaves, and unscreened windows, which increases their vulnerability to mosquito bites (Odufuwa et al., 2020).
Upon study approval from relevant authorities, a meeting with the leaders of the community was held to inform them of the study and the selection of villages for the study based on house design and perceived malaria prevalence. Following the selection of villages for the study, the community leaders announced the dates for sensitization meetings using public information systems and door-to-door visits, effectively reaching the whole community. The community sensitization meetings were conducted in all three villages through verbal presentations in Kiswahili. These presentations covered information about ITS, the benefits of using and caring for vector control tools, random selection of households for participation, and the importance of reporting any adverse events following ITS installation. At the end of each presentation, the community had the opportunity to ask questions, and appropriate responses were provided. A summary of the study, also written in Kiswahili, was distributed to the village leader and to all participants upon consent.
The health belief model (HBM), a commonly used approach to guide engagement or lack of health promotion behavior uptake and adherence (Jones et al., 2015), was used to guide and design the study tools to understand the community perceptions and the acceptability of ITS use for malaria control in Chalinze District.
Using the baseline information collected in May 2021, households were randomly allocated into two groups: the treatment group (ITS installation at baseline) and the control group (ITS installation at end line). The installation team was provided a list containing the following information: 1) the identification number of the household; 2) the geographic coordinates; 3) the hamlet name; and 4) the names (first, middle, and last) of the heads of the households of those randomly selected for ITS installation. The team consisted of three people: two carpenters and a field data collector, for ease and reliable data collection and for transport of the ITS and other equipment from one household to another (Figure 1A). After locating the assigned household using a global positioning system (GPS) device, written informed consent was obtained from the head of household or the available adult resident (>18 years old) before installation of the ITS (Figure 1B). A signed copy of the informed consent form was left in each household for reference and for contact with the project staff whenever necessary; a second one was taken for storage at IHI offices. The data collection forms (Supplementary File 1) were administered using the Open Data Kit (ODK) software installed on android tablet devices to record the duration of ITS installation per house. Two houses within participating villages were used for training and for development of the procedures used to install the ITS in all houses, which were applicable to all house types. The installation procedure commenced with the recording of the installation start time, followed by the carpenters rolling the ITS netting around the house until full coverage (Figure 1C). The data collector measured the width and the length of the rolled netting to record the total amount of installed netting. This was followed by attaching the ITS netting to the wooden beams of the roofs [houses with weak wooden beams were supported with additional plywood pieces measuring 2 cm × 2 cm (width × thickness), but of varying lengths based on the dimensions of each house] using a heavy-duty staple gun (Figure 1D). The second carpenter pulled the ITS netting inside the house for attachment against the wall or wood (Figure 1E). As the installation on the eaves was ongoing, the data collector measured the dimensions of the windows and then cut the ITS nets accordingly, with the addition of 30 cm × 10 cm (length × width) to account for the folding and flapping at the bottom (Figure 1F). Holes on the walls were also covered with appropriately measured ITS netting. After covering the eaves, the windows, and the holes on the walls with ITS, the end time was recorded. At the end of each installation day, the supervisors inspected the installation status of each house, and the houses in which the ITS were not properly installed were revisited on the same day or the next day to ensure proper installation of the ITS.

Figure 1. Installation of the insecticide-treated screens (ITS) in houses. (A) A roll of ITS. (B) Obtaining consent. (C) Rolling of the ITS around the house. (D) Stapling the ITS on the eaves. (E) Pulling the ITS indoor for eave screening. (F) Installing the ITS on the windows.
Qualitative studies using IDIs and FGDs were implemented. Among those who installed the ITS, a total of 14 carpenters were invited for the IDIs. For the FGDs, 30 households (15 per arm) from the list of households enrolled in the main study were randomly selected from each of the three villages (Odufuwa et al., 2022) using simple randomization of the baseline data for a respective village. Thereafter, purposive selection was employed to ensure equal number of participants from each gender per FGD, and households that refused the installation of ITS were represented. Thus, 12 participants (six men and six women for the FGD) from either the control or the treatment group were presented to the village leaders for invitation. Those who refused ITS installation were prioritized in order to capture detailed information on the reasons for ITS refusal. Two FGDs were implemented per village (a total of six FGDs per time period). A maximum of 12 participants for each FGD was included, which was facilitated by an experienced research scientist who ensured that everyone had the opportunity to contribute. Interviews were conducted at the premises of the public schools of the village to ensure neutrality. Participation in the interviews was voluntary. Participants were asked for their opinion on the ITS, any adverse events that had occurred since the installation, and whether they would be willing to pay for ITS, and at what cost.
The criteria for the selection of participants for both IDIs and FGDs were:
● Living in the study area for more than a year;
● Must be 18 years and older;
● Had ITS installed at the time of the interview (treatment) or not (control); and
● Carpenters who participated in the installation of the ITS.
In January 2022 (6 months post-installation), IDIs were conducted with 14 carpenters from the three villages (out of 20, as six had moved away from the study area for other work at the time of the interview). Efforts made to locate them by phone failed; hence, they were considered as lost to follow-up. However, this did not influence the sample size as the data reached saturation. In addition, six mixed-group FGDs and one survey using a structured questionnaire per time point (in January 2022, 6 months post-installation, and in July 2022, 12 months post-installation) were conducted. The carpenters and the users of ITS were asked about any adverse events during the FGDs and IDIs conducted in January 2022. All interviews were conducted in person, in Kiswahili, the national language that is most commonly used in the setting. During the ITS installation assessment, a structured questionnaire was administered to 214 consenting households. This survey, conducted during installation, recorded the installation time and the number of ITS distributed per house. The data collectors received training to begin timing immediately after consent was obtained and to measure the length and the width of the nets being installed, rather than the house structure, for consistency in the data collection.
Qualitative data were analyzed following a thematic framework approach (Gale et al., 2013). All interviews were recorded using a tape recorder and later transcribed. Two research scientists spent time reading and familiarizing themselves with the transcripts before importing these to NVivo 12 Pro software for coding. All relevant themes and sub-themes were extracted, and the data were deductively analyzed. Patterns were used to develop categories, and selected quotations were translated for reporting (Given, 2012; Gale et al., 2013).
Using STATA 16 statistical software (College Station, TX, USA), we estimated the arithmetic mean and 95% confidence intervals for the hours spent on installation and the amount of ITS fabric (in meters) installed per house. Since the distribution was not normal, negative binomial regression models were employed to analyze both the hours spent for installation and the total amount of ITS fabric used per house. These models explored the potential influence of covariates: the wall type (mud vs. bricks), the number of holes in the walls (indicating the house condition), the number of windows (as a proxy for house size), villages (representing different housing settings), and technician performance. All covariates were adjusted for factor-fixed effects in all analyses. The likelihood-ratio test was used to examine the influence of each covariate on the models.
Between June and July 2021, a total of 248 households were visited for ITS installation. Of these, 31 (13%) households withdrew consent, 6 (2%) households were found to be damaged, and 2 (1%) households only had eaves without windows and therefore did not meet the eligibility criteria for installation. Analysis was performed on 214 houses, although two houses were used for training and were not considered for the analysis of installation time. The majority of the houses installed with ITS had the following structures: walls built with mud (86%, 185/214), mud floors (66%, 141/214), and iron sheet roofs (97%, 208/214). ITNs were present in most houses (79%, 168/214), largely delivered through the school net program or from antenatal clinics, with a few purchased from the market.
Post-installation of the ITS, the participants (55%, 105 out of 190) reported a perceived reduction in malaria infection within their households and a decrease in indoor mosquito density. They (87.9%) also observed a reduction in other insects indoors, such as cockroaches and flies. Another observation was a reduction of indoor crawling animals, including rodents, lizards, and snakes. The netting provided them a sense of general protection. However, almost all of the participants requested additional chemicals on the nets during the 12-month interviews as they (59%) perceived that the chemical efficacy had decreased over time. The community members also requested for a new design of the nets to cover doors as it was difficult to keep them shut in houses with children.
“Since screens have been installed in my house, no one suffered from malaria. We have tested twice, but the results were negative. But before having screens, we frequently had malaria cases. There are changes before and after the intervention. I am grateful for that.” (Participant from the Mazizi treatment group, 12 months post-installation)
“Nets have the best quality, but the efficiency of the chemicals starts decreasing 3 months after installation, and mosquitoes are not dying as it was when nets were installed. So to maintain efficiency, you should add chemicals after a certain period.” (Participant from the Kihangaiko treatment group, 6 months post-installation)
The ITS were well accepted in the community, particularly among the households in the control arm, in which no household refused the installation of ITS when it was their turn at the end of the study (12 months). As such, most of the participants showed interest in buying and self-installing the ITS, if sold at an affordable cost in the price range of 1,000–6,000 Tanzanian shillings (USD ≤2.50) per square meter. Low income was mentioned as a barrier to buying ITS. Similarly, quantitative findings indicated that the majority (>95%) of households reported that they would continue using ITS, recommend them to friends and family, and were willing to either purchase ITS or buy the materials for self-installation. On average, users were willing to pay 2,774 Tanzanian shillings for a 1-m × 1-m section of ITS fabric.
“You installed the intervention for free. We will buy the nets, but we must know the cost.” (Participant from the Pongwe treatment group, 6 months post-installation)
The team of carpenters, semi-skilled workers, and supervisors used an average of 1 h per house to install the ITS, and each team could, on average, install ITS in eight houses per day. However, the time required for installation varied depending on factors including the wall structure of the house, the number of holes on the walls, the number of windows, and the location of the house (Table 1). On average, each installation required approximately 29.5 m of ITS netting with a width of 1.5 m. The amount of netting used per house was associated with the number of holes on the walls and the total number of windows (Table 1).
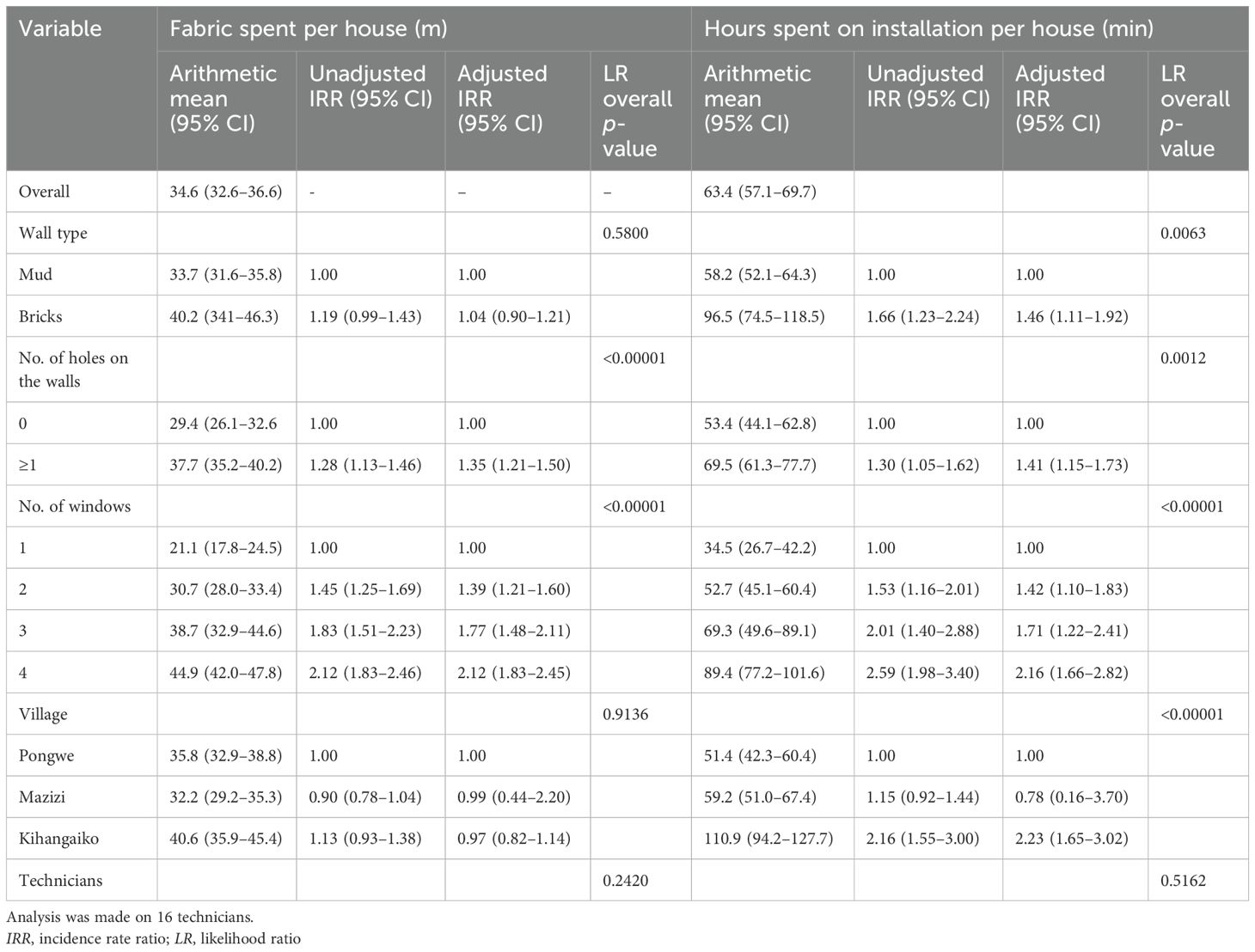
Table 1. Factors associated with the hours spent on installation and the insecticide-treated screen (ITS) fabric installed per house.
“Installing nets in mud houses is complicated, hence spending many hours like 3 h, but 1 h and a half is enough for brick houses.” (Carpenter from Mazizi, 6 months post-installation)
Carpenters believed that the installed ITS netting could last for many years as it was made of a stronger material than that readily available in the market. There is also the belief among the household participants that the ITS should be long-lasting based on its perceived good and strong quality. However, some of the participants reported holes in the ITS caused by rodents and the pins being detached due to strong winds. In future implementations, pins or nails should be used as a reinforcement during installation in order to enhance the strength and prevent detachment.
“Although the materials of these screens are more durable, the challenge is that rats are gnawing through the nets, creating holes that allow mosquitoes to enter the house.” (Participant from the Mazizi treatment group, 6 months post-installation)
Most of the participants across the study area, regardless of gender, prefer the color of the ITS to be dark gray. They noted that dust was not visible on the ITS netting shortly after installation. However, they noted that dust accumulation was noticeable after some time (>3 months), as dust is common in the area. Very few proposed having a variety of colors, such as white or blue, to provide options for customers to go with the color painted on their brick-walled houses. The majority of people were bothered by the dirty screens, as they do not know how to clean them. They recommended that producers and scientists consider this barrier for improvement and provide instructions for maintenance. The results from the questionnaire indicated that 99% of respondents expressed their approval of ITS, with 65.8% showing a preference for a gray netting color, while 34.2% did not like it. In addition, 87.7% recommended blue as an alternative color, with the other suggested colors including white, brown, pink, and green.
“My house is painted in blue color, but the screens have gray color. When installed, it will affect the beauty, and I don’t have options to choose another color. Please have different colors.” (Participant from the Mazizi control arm, 12 months post-installation)
“Most of the time, we the people don’t have choices of colors on health protection measures, so I can use any color, even white no problem.” (Participant from the Kihangaiko treatment arm, 6 months post-installation)
Of the 12 carpenters, two stated they experienced sneezing hours after work due to the close and extended contact with the chemicals on the ITS. The sneezing stopped within a couple of hours, without medical support. On the other hand, none of the community members faced any health outcomes following ITS installation in their houses. The carpenters showed interest in continuing to work with the project in the future.
“I sneezed for a couple of hours, but it didn’t last for a day because of the smell, after a certain period, I got used to it.” (Carpenter from Mazizi)
A few participants reported facing challenges in cleaning the ITS when they became dirty after several months of use (>3 months), as instructions on how to care for the ITS were not discussed during sensitization. A few repaired the ITS netting at their own expense, including the purchase of repair equipment, e.g., nails or paying for local carpenters for the service, while others waited for the project team to repair them. The participants, mostly from the control arm who received screens at the end of the project, indicated readiness to self-repair.
“Screens are dirty, but I am scared to clean. I have the fear that water can wash away insecticides.” (Participant from the Kihangaiko treatment arm, 6 months post-installation)
The ITS installation period collided with the coronavirus disease (COVID-19) pandemic. Therefore, some people refused the installation as they feared that free installation of the ITS would infect them with the virus. Moreover, some community members believed that the insecticide in the ITS might cause male sterilization. Thus, the qualitative research team visited households to address these fears and concerns and provided adequate information on ITS. In addition, they engaged influential individuals (e.g., community health workers and village leaders) to reinforce the messages shared by the sensitization team, resulting in improved community knowledge and acceptance of the intervention, particularly in the control group. Thus, at the end of the trial, when no health problems were observed among the neighbors with ITS, these members requested for ITS installation in their homes.
“I think the problem is the low level of education and misinformation. They think nets have chemicals which transmit COVID-19.” (Mazizi treatment arm, 6 months post-installation)
House screening has been suggested for malaria control (World Health Organization, 2023b) and has been proven to be an effective supplementary vector control intervention for the reduction of malaria burden and other diseases transmitted by mosquitoes in SSA (Ogoma et al., 2010; Lindsay et al., 2021; Abong'o et al., 2024; Saili et al., 2024). Moreover, combining treated screens with the use of ITNs has been linked to a reduction of human and mosquito contact indoors (Saili et al., 2024). While it is currently not scaled up, it is important to assess the acceptance and perception of the community as part of a randomized control trial of ITS in eastern Tanzania (Odufuwa et al., 2022).
Although ITS use was well accepted in the community, refusals during installation at the beginning of the study were observed. This was largely due to rumors associated with the outbreak of COVID-19 and the fear that ITS could cause male sterilization, which has also been recorded to affect the uptake of other health interventions (Schots et al., 2022; Chang et al., 2023; Woods et al., 2023). In our setting, the use of local remedies for the prevention of COVID-19 was widely echoed and supported by the community, which indirectly led to mistrust in these ITS, being manufactured outside of the country. These refusals were also influenced by the request for human blood for malaria testing using quantitative polymerase chain reaction (qPCR) (Schindler et al., 2019), as the primary outcome that determines the efficacy of ITS (the main study) (Odufuwa et al., 2022). The procedure required blood to be transferred to the laboratory for testing. This led some participants to believe that their blood might be misused for human immunodeficiency virus (HIV) and COVID-19 testing. They also feared testing positive for COVID-19 and being forced to accept vaccines, the safety and efficacy of which they were concerned about (Msuya et al., 2023). Another factor that influenced the refusals was the free installation of ITS. They indicated that “nothing can be offered for free in this world.” They were therefore concerned that their blood might be combined and sold to compensate for the cost of the ITS. All of these concerns were addressed during the first FGDs, which improved community awareness of the study objectives and the relationship between the project staff and the community members. Thereby, some members who refused the intervention at the beginning requested study inclusion after observing that the anticipated negative events were not experienced by their neighbors. Generally, people tended to question the need, the expected efficacy, and the possible side effects and may have also perceived hidden intentions behind the free intervention, particularly if it is a new intervention for public health, such as the ITS. It is therefore essential to fully engage potential end users before and during the development of an intervention in order to promptly address concerns and to improve the acceptability of a novel intervention; otherwise, the goals may not be fully attained (Sangoro et al., 2014).
Community members showed interest and readiness to buy the ITS netting if available in the market, provided the price per meter was affordable [1,000–6,000 Tanzanian shillings (USD <2.50)]. However, this price exceeded the average price of the new ITNs made for the control of pyrethroid-resistant mosquitoes (Shepard et al., 2020).
It is quite encouraging that people are willing to pay for a product that costs 10 times more than a conventional ITN (USD 1–4). This could be attributed to the perceived benefits of ITS in the reduction of malaria, providing protection from mosquitoes and other pests, as well as the perceived long-lasting protection from ITS. However, willingness did not equate to an actual purchase as some of the respondents mentioned the influence of income on their willingness to purchase. Unfortunately, the majority of the villagers were within the low socioeconomic status; hence, self-purchasing the ITS may be too costly. It was also observed that three of the houses that withdrew from the study purchased similar products to be installed on their windows, indicating that wealthy households will purchase ITS if available, as seen elsewhere (Tusting et al., 2015; Tusting et al., 2017).
On average, nearly 30 running meters of ITS were used per house, which is equal to a consumption of 30 m × 1.5 m = 45 m2 of ITS netting. The carpenter team spent more than 1 h for installation in mud houses, consuming more time and resources to complete. In this study, the average installation cost per house was USD 26.9 when using the study site (the IHI) rates for the implementation teams, which included transport to the site, per diem, security, and other overheads, as well as equipment and wood. This was eight times the cost (combined net material and installation time) spent in a similar study in Kenya, in which eave nets were attached but no extra timber was added (Odhiambo et al., 2016).
Planning for a programmatic implementation of ITS, and using the same consumption rates, the budget would be as follows (see Table 2: material costs; Table 3: financial costs; Table 4: wages in Tanzania; and Table 5: implementation costs of the ITS, ITN, Eave tubes and IRS):
Fabric use and cost included a 10% margin to allow for wastage.
The operational costs were based on current wages in Tanzania for the types of workers involved, e.g., the salary of a carpenter was calculated on an annual salary of 8.8 million Tanzanian shillings. The per diem, security, and oversight rates were based on project costing. The productivity rate of eight installations per day breaks down to an operational cost per household of USD 6.30.
The rates for locally sourced materials, including the timber for frames, the nails, the staple guns, and the personal protective equipment (PPE), were taken from the project budget and came to just over USD 2.50 at current exchange rates. The ITS material, including trucking to the site of installation, came to approximately USD 8.80, giving a total material cost of USD 11.35 and a total installation cost per household of USD 17.60.
Even with using the IHI rates, the cost of ITS installation was still four times lower than that of a similar tool designed to kill mosquitoes upon installation in the eaves: the eave tubes (Tusting et al., 2015). The cost may be further significantly reduced if scaled up, as seen for ITNs.
The cost, expressed in USD per year per household, was very much dependent on how many years the intervention will last (Odhiambo et al., 2016). The cost per household year of protection with a 2-year dual-AI ITN, assuming three nets per household, was USD 6. IRS costs an average of USD 5.3 per person, hence USD 27 per household (Sternberg et al., 2021). Therefore, if cost-effectiveness is considered in terms of cost per household year of protection, ITS would match that of ITN if it lasts 4 years and that of IRS if it lasts 3 years. Durability of 3–4 years is technically feasible. In addition, cost-effectiveness is greater for households with more members. The condition and the type of houses in the study area had a significant impact on the time the carpenters spent installing the netting. The majority of the houses were mud houses (86%), and many of the houses were also not in good condition as the installation period was shortly after the end of the long rainy season, when much of the mud on the walls was damaged. The Kenyan site used heavy-duty staple machines throughout the installation, which was indicated as the major reason for the significant reduction in the installation time (Odhiambo et al., 2016). However, in our setting, the carpenters used staple machines, as well as hammers and nails, due to the delay in restocking the machines upon damage; therefore, it was necessary to account for 10% damage before procuring staple machines. Some of the houses in our setting had hardwood roof beams, which required the use of a hammer and nails. Another factor that significantly increased the amount of ITS netting used per house was the need to cover additional gaps in walls, with 26% of the houses having 2.0 m2 (0–20.5) gaps. Larger holes were found mainly in houses with a veranda and those sharing the same house roof with a veranda (Supplementary File 2). The installation time could have been reduced if several widths of the ITS fabric were supplied, e.g., 1.5-m and 2-m widths, in order to more easily cover wide eaves or large holes and openings. Other holes were found at the top of the doors and windows. Although these holes contributed to the amount of fabric used per house, it also suggests that ITS is an adaptable tool for this setting given that it could be used to cover not only eaves and windows but also all other forms of gaps in the household structure that mosquitoes could use for entry. In a nutshell, the cost of installing ITS could have been further reduced by promoting widespread adoption. If ITS were considered an essential component of every household, increased demand would likely lead to individual purchases and self-installation by residents. As the intervention gains popularity within the community, local carpenters would gain more experience, leading to increased efficiency and, potentially, a reduced installation time.
The members considered the nets as effective based on the reduction of the indoor entry of mosquitoes, insects, and reptiles such as snakes and lizards, which was partly associated with the physical barrier provided by the netting (Brake et al., 2022). The physical barrier also provided an additional benefit by reducing dust entry, which was also reported in the study conducted in Kenya (Abong'o et al., 2024). However, dust capture made the netting dirty, which detracted from the beautification of the house that people enjoyed when the netting was new. Educating residents on efficient ways of cleaning the netting such that the netting integrity and the insecticide effect are not reduced, for instance using a soft brush, would likely ensure that this drawback will not discourage its usage.
At 3 months after ITS installation, the FGD data collected at 6 months indicated a higher number of indoor mosquitoes observed to have gained entry through the door. Eaves were the main entry point of mosquitoes (Spitzen et al., 2016); therefore, their closure with the ITS, including the windows and wall holes, meant that entry through the door may have increased, as observed in another study in Tanzania where eaves were screened (Abong'o et al., 2022). Keeping doors closed is essential for the control of malaria vectors (Saili et al., 2024); however, many of the houses in our setting did not have well-fitted doors, and the few with well-fitted doors often had these doors left open during the day, particularly in houses with children, which was shown to reduce the impact of full house screening in a trial conducted in Gambia (Kirby et al., 2009). Curtains for doors have also been explored in an earlier study of ITS in Kenya (Odhiambo et al., 2016); however, misuse was observed as people would tilt the curtains to one side of the door, providing a space for mosquito entry. Promotional behavioral change campaigns toward correct closure of the insecticidal curtain could be conducted to address the problem. Furthermore, an intervention for a door should be self- and well-fitted without holes around the frame.
The observed increase in indoor mosquitoes after several months was partly due to the loss of the insecticide incorporated (Odufuwa, personal communication). As the insecticide wears out, the impact of the chemistries on the mosquitoes that have gained access into the house through the door may have been reduced. The loss of the insecticide in the nets could be attributed to evaporation (Kayedi et al., 2008) as the nets were neither washed nor cleaned. The ITS should be manufactured using a technology that allows a slow release of the incorporated insecticide for a long-lasting insecticidal effect over several years. The nets used in the Kenyan study contained deltamethrin only, and deltamethrin has a very low release rate of less than 1% per wash, measured as the wash-off rate. This net was also evaluated in a durability study and was shown to still be highly insecticidal after 3 years (Marius Allossogbe et al., 2017). The PBO net in the present study had higher release rates of deltamethrin (2.2%) and PBO (3.3%), which might explain the bigger loss due to evaporation as only the released insecticide is available for evaporation.
Carpenters perceived the fabric of the ITS net to be long-lasting based on their comparison to the other nettings they have previously worked with. The participants had similar expectations. The nets used in this study were made of polyethylene, similar to that used for a commercial bed net product (Tsara Boost), but with a thicker filament to better resist fixing with staples and nails. As the current core vector control tool, ITNs are typically lost within a few months of distribution due to being gifted away or within 2 years due to damage (Koenker et al., 2014). These are avoidable in the case of ITS due to the installation; therefore, the intended areas of protection will remain protected throughout the year. The participants noted holes on the ITS, which could be attributable to strong winds, rodents, and cats. As these factors are unavoidable, the edges of the netting attached to the surface should be made stronger.
The majority of the people did not provide any form of care, such as repairing net holes and removing the dust off the net. This is largely because they were not informed of any care or maintenance practices. However, many still wanted to wash the nets, most especially the netting installed on the window. Washing of the netting will require uninstalling the nets, which could cause tearing of the fabric if not carefully removed. Although removal of the netting for washing was not observed, ITS users should be able to care for the netting without causing damage. A possible solution is the use of a soft brush to remove dust when necessary, while the netting remains installed. Another solution is by installing them as two overlapping screens, i.e., only fixed at the top and on one side and removable from the frame or the wall.
Carpenters and some inhabitants complained about sneezing. This is known as a side effect of cyano-pyrethroids (Hołyńska-Iwan and Szewczyk-Golec, 2020). The perceived effect only lasted for a few hours. This was perceived among carpenters as they refused to use the face masks provided due to inconvenience. In any case, none of the carpenters withdrew from the work due to any perceived side effects. Future studies should mandate the use of proper construction safety gear, including masks for carpenters, regardless of perceived discomfort.
This year-long study was too brief to evaluate the long-term effects and durability of the intervention. As household members do not interact with the ITS on a daily basis, it will take time for the ITS to degrade. Implementation of the intervention over multiple years would provide a more accurate assessment of its physical durability. In addition, expanding the study to other geographical regions and cultural settings is recommended to better understand how different communities respond to ITS use.
The findings of our study are likely generalizable to settings with similar socioeconomic characteristics and perceived levels of mosquito burden and malaria risk. However, in communities where eaves are not typically used for indoor cooling, the acceptance of ITS might be limited, as its primary application would be to cover windows, wall holes, and other entry points of mosquitoes. It is important to note that the ITS are particularly relevant for unimproved housing, which is common in rural areas.
The ITS were well accepted in the community based on their perceived contribution to the control of mosquitoes and malaria infection in the study area. However, innovations to control mosquito entry through the door are needed to ensure full house screening. Concurrently, in promoting behavioral change, efforts should prioritize the importance of keeping doors closed, particularly in households with children. This study underscores the significance of having continuous and tailored community sensitization for the acceptance and care of new vector control tools. It also revealed peoples’ willingness to pay for the installation and repair of ITS. Therefore, ITS scale-up could be donor-funded or community-driven given that the purchase of untreated screens was observed during the trial. Trials with longer duration are needed to evaluate the cost-effectiveness per household year of ITS.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Informed consent to participate was administered to the head of household or any adult resident (18 years and above). Ethical clearances to conduct the study were sought from the Ifakara Health Institute Institutional Review Board (IHI-IRB), National Institute for Medical Research (NIMR), Tanzania and LSHTM Observational / Interventions Research Ethics Committee referenced IHI/IRB/No: 19-2020, NIMR/HQ/R.8c/Vol.I/885 and 21639 – 1, respectively. Permission to publish has also been sought from NIMR, Tanzania. Approval for publication was sought from NIMR, referenced No. BD.242/437/01C/40.
FK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. OO: Data curation, Methodology, Writing – original draft, Writing – review & editing, Conceptualization, Formal Analysis, Investigation. JBM: Data curation, Writing – review & editing. EM: Writing – review & editing, Conceptualization. RP: Data curation, Project administration, Writing – review & editing. JM: Data curation, Supervision, Writing – review & editing, Conceptualization, Methodology. OS: Conceptualization, Writing – review & editing. RB: Conceptualization, Writing – review & editing. JB: Conceptualization, Supervision, Writing – review & editing, Investigation. SM: Conceptualization, Supervision, Writing – review & editing, Investigation. ZM: Conceptualization, Supervision, Writing – review & editing, Investigation.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study grant referenced MR/T0036771 & EPIDZR44 was funded by the Medical Research Council, United Kingdom (MRC-UK) through LSHTM, London.
Special appreciation goes to the communities of Pongwe, Mazizi, and Kihangaiko for their cooperation. We also acknowledge the contribution and the tenacity of the Vector Control Product Testing Unit (VCPTU) staff, field workers, and local carpenters during the work. A worthy appreciation goes to the head teacher of Pongwe - Mr. Joel Alfred Kajonga, Mazizi - Mr. George A. Maula, and Madesa - Ms. Dikla Bony Hemana, and the village leaders for their support and being accommodating throughout the study. A special thanks also goes to Abdu Nuha for assisting with the sourcing of equipment during the installation of ITS. Acknowledgment also goes to the staff of MCC47, Vegro Aps, and Biolytrics Vietnam Co., Ltd for manufacturing and chemical retention testing of ITS.
OO, EM, JBM, JM, and SM test vector control tools for numerous manufacturers of vector control tools including Moon Netting and Vegro Aps, RB works for Vergo Aps, and OS consults on the design of vector control tools for private and public companies.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmala.2025.1540184/full#supplementary-material
Supplementary file 1 | Data collection form.
Supplementary file 2 | Size of the Veranda.
Supplementary file 3 | Quantitative data on ITS installed and time spentper house.
AI, Active ingredient; COVID, Coronavirus; FGDs, Focus group discussions; ICF, Informed consent form; IDIs, In-depth interviews; ITNs, Insecticide-treated nets; ITS, Insecticide-treated screening; ODK, Open Data Kit; WHO, World Health Organization.
Abong'o B., Agumba S., Moshi V., Simwero J., Otima J., Ochomo E. (2024). Insecticide treated eaves screens provide additional marginal protection compared to untreated eave screens under semi-field conditions in western Kenya. Malariaworld J. 15, 1. doi: 10.5281/zenodo.10567425
Abong'o B., Gimnig J. E., Omoke D., Ochomo E., Walker E. D. (2022). Screening eaves of houses reduces indoor mosquito density in rural, western Kenya. Malar J. 21, 377. doi: 10.1186/s12936-022-04397-y
Bhatt S., Weiss D. J., Cameron E., Bisanzio D., Mappin B., Dalrymple U., et al. (2015). The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211. doi: 10.1038/nature15535
Brake S., Gomez-Maldonado D., Hummel M., Zohdy S., Peresin M. S. (2022). Understanding the current state-of-the-art of long-lasting insecticide nets and potential for sustainable alternatives. Curr. Res. Parasitol. Vector Borne Dis. 2, 100101. doi: 10.1016/j.crpvbd.2022.100101
Chang W., Cohen J., Wang D. Q., Abdulla S., Mahende M. K., Gavana T., et al. (2023). : Impact of 1,7-malaria reactive community-based testing and response (1,7-mRCTR) approach on malaria prevalence in Tanzania. Infect. Dis. Poverty 12, 116. doi: 10.1186/s40249-023-01166-0
Finda M. F., Moshi I. R., Monroe A., Limwagu A. J., Nyoni A. P., Swai J. K., et al. (2019). Linking human behaviours and malaria vector biting risk in South-Eastern Tanzania. PloS One 14, 1–23. doi: 10.1371/journal.pone.0217414
Fox T., Furnival-Adams J., Chaplin M., Napier M., Olanga E. A. (2022). House modifications for preventing malaria. Cochrane Database Syst. Rev. 10, CD013398. doi: 10.1002/14651858.cd013398.pub4
Gale N. K., Heath G., Cameron E., Rashid S., Redwood S. (2013). Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med. Res. Method. 13, 1–1. doi: 10.1186/1471-2288-13-117
Given L. (2012). “Thematic coding and analysis,” in The SAGE encyclopedia of qualitative research methods. (Thousand Oaks, California, USA: SAGE Publications).
Gleave K., Lissenden N., Chaplin M., Choi L., Ranson H. (2021). Piperonyl butoxide (PBO) combined with pyrethroids in insecticidetreated nets to prevent malaria in Africa (Review). Cochrane Database Systematic Rev. 5 (5), CD012776. doi: 10.1002/14651858.CD012776.pub3
Haji K. A., Khatib B. O., Obi E., Dimoso K., Koenker H., Babalola S., et al. (2020). Monitoring the durability of the long-lasting insecticidal nets Olyset((R)) and PermaNet((R)) 2.0 in similar use environments in Zanzibar. Malar J. 19, 187. doi: 10.1186/s12936-020-03258-w
Hołyńska-Iwan I., Szewczyk-Golec K. (2020). Pyrethroids: how they affect human and animal health. Medicina (Kaunas) 56, 582. doi: 10.3390/medicina56110582
Jatta E., Carrasco-Tenezaca M., Jawara M., Bradley J., Ceesay S., D'Alessandro U., et al. (2021). Impact of increased ventilation on indoor temperature and malaria mosquito density: an experimental study in The Gambia. J. R Soc. Interface 18, 20201030. doi: 10.1098/rsif.2020.1030
Jones C. L., Jensen J. D., Scherr C. L., Brown N. R., Christy K., Weaver J. (2015). The Health Belief Model as an explanatory framework in communication research: exploring parallel, serial, and moderated mediation. Health Commun. 30, 566–576. doi: 10.1080/10410236.2013.873363
Kayedi M. H., Lines J. D., Haghdoost A. A., Vatandoost M. H., Rassi Y., Khamisabady K. (2008). Evaluation of the effects of repeated hand washing, sunlight, smoke and dirt on the persistence of deltamethrin on insecticide-treated nets. Trans. R. Soc. Trop. Med. Hygiene 102, 811–816. doi: 10.1016/j.trstmh.2008.05.025
Kihwele F., Gavana T., Makungu C., Msuya H. M., Mlacha Y. P., Govella N. J., et al. (2023). Exploring activities and behaviours potentially increases school-age children’s vulnerability to malaria infections in south-eastern Tanzania. Malaria J. 22, 293. doi: 10.1186/s12936-023-04703-2
Kirby M. J., Ameh D., Bottomley C., Green C., Jawara M., Milligan P. J., et al. (2009). Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in The Gambia: a randomised controlled trial. Lancet 374, 998–1009. doi: 10.1016/S0140-6736(09)60871-0
Koenker H., Kilian A., Zegers de Beyl C., Onyefunafoa E. O., Selby R. A., Abeku T., et al. (2014). What happens to lost nets: a multi-country analysis of reasons for LLIN attrition using 14 household surveys in four countries. Malaria J. 13, 464. doi: 10.1186/1475-2875-13-464
Lindsay S. W., Davies M., Alabaster G., Altamirano H., Jatta E., Jawara M., et al. (2021). Recommendations for building out mosquito-transmitted diseases in sub-Saharan Africa: the DELIVER mnemonic. Philos. Trans. R Soc. Lond B Biol. Sci. 376, 20190814. doi: 10.1098/rstb.2019.0814
Maia M. F., Kliner M., Richardson M., Lengeler C., Moore S. J. (2018). Mosquito repellents for malaria prevention. Cochrane Database Syst. Rev. 2, CD011595. doi: 10.1002/14651858.CD011595.pub2
Marius Allossogbe V. G., Agossa F. R., Sahossi J. Z.-., Akinro B., Houtoukpe A., Anagonou R., et al. (2017). Comparative efficacy of five types of long-lasting insecticide-treated nets (PermaNet 3.0®, PermaNet 2.0®, Olyset Plus®, Olyset Net®, and LifeNet®) in a semi-natural environment against resistant Anopheles Gambiae sensu lato and Mansonia africana in Cove, Benin. Int. J. Mosq. Res. 4, 07–13. doi: 10.1603/ME13136
Mohcdgec (2020). National malaria strategic plan 2021–2025. Development 7, 274. 923,575.200-229.208.
Monroe A., ASamoah O., Lam Y., Koenker H., Psychas P., Lynch M., et al. (2015). Outdoor-sleeping and other night-time activities in northern Ghana: Implications for residual transmission and malaria prevention. Malaria J. 14, 35. doi: 10.1186/s12936-015-0543-4
Monroe A., Moore S., Olapeju B., Merritt A. P., Okumu F. (2021). Unlocking the human factor to increase effectiveness and sustainability of malaria vector control. Malar J. 20, 404. doi: 10.1186/s12936-021-03943-4
Msuya S. E., Manongi R. N., Jonas N., Mtei M., Amour C., Mgongo M. B., et al. (2023). COVID-19 vaccine uptake and associated factors in sub-saharan africa: evidence from a community-based survey in Tanzania. Vaccines (Basel). 11, 465. doi: 10.3390/vaccines11020465
Ngadjeu C. S., Doumbe-Belisse P., Talipouo A., Djamouko-Djonkam L., Awono-Ambene P., Kekeunou S., et al. (2020). Influence of house characteristics on mosquito distribution and malaria transmission in the city of Yaounde, Cameroon. Malar J. 19, 53. doi: 10.1186/s12936-020-3133-z
Obi E., Okoh F., Blaufuss S., Olapeju B., Akilah J., Okoko O. O., et al. (2020). Monitoring the physical and insecticidal durability of the long-lasting insecticidal net DawaPlus((R)) 2.0 in three States in Nigeria. Malar J. 19, 124. doi: 10.1186/s12936-020-03194-9
Odhiambo M. T. O., Vulule J. M., Afrane Y. A., Ombok M., Bosselmann R., Skovmand O. (2016). Supplementary effect and durability of prototype insecticide-treated eave curtains on indoor resting mosquitoes in Kadibo division, Western Kenya. MalariaWorld J. 7, 11. doi: 10.5281/zenodo.10818166
Odufuwa O. G., Moore S. J., Mboma Z. M., Mbuba E., Muganga J. B., Moore J., et al. (2022). Insecticide-treated eave nets and window screens for malaria control in Chalinze district, Tanzania: a study protocol for a household randomised control trial. Trials 23, 578. doi: 10.1186/s13063-022-06408-4
Odufuwa O. G., Ross A., Mlacha Y. P., Juma O., Mmbaga S., Msellemu D., et al. (2020). Household factors associated with access to insecticide-treated nets and house modification in Bagamoyo and Ulanga districts, Tanzania. Malar J. 19, 220. doi: 10.1186/s12936-020-03303-8
Ogoma S. B., Lweitoijera D. W., Ngonyani H., Furer B., Russell T. L., Mukabana W. R., et al. (2010). Screening mosquito house entry points as a potential method for integrated control of endophagic filariasis, arbovirus and malaria vectors. PloS Negl. Trop. Dis. 4, e773. doi: 10.1371/journal.pntd.0000773
Opiyo M. A., Paaijmans K. P. (2020). 'We spray and walk away': wall modifications decrease the impact of indoor residual spray campaigns through reductions in post-spray coverage. Malar J. 19, 30. doi: 10.1186/s12936-020-3102-6
Organização Mundial da S (2019). Guidelines for malaria vector control. (Geneva, Switzerland: World Health Organization (WHO)).
Peto T. J., Tripura R., Davoeung C., Nguon C., Nou S., Heng C., et al. (2018). Reflections on a community engagement strategy for mass antimalarial drug administration in Cambodia. Am. J. Trop. Med. Hygiene 98, 100–104. doi: 10.4269/ajtmh.17-0428
Pryce J., Richardson M., Lengeler C. (2018). Insecticide-treated nets for preventing malaria. Cochrane Database Syst. Rev. 11, CD000363. doi: 10.1002/14651858.CD000363.pub3
Ranson H., Lissenden N. (2016). Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 32, 187–196. doi: 10.1016/j.pt.2015.11.010
Russell T. L., Govella N. J., Azizi S., Drakeley C. J., Kachur S. P., Killeen G. F. (2011). Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malaria J. 10, 1–10. doi: 10.1186/1475-2875-10-80
Sahan K., Pell C., Smithuis F., Phyo A. K., Maung S. M., Indrasuta C., et al. (2017). Community engagement and the social context of targeted malaria treatment: a qualitative study in Kayin (Karen) State, Myanmar. Malaria J. 16, 1–10. doi: 10.1186/s12936-017-1718-y
Saili K., de Jager C., Masaninga F., Sangoro O. P., Nkya T. E., Likulunga L. E., et al. (2024). House screening reduces exposure to indoor host-seeking and biting malaria vectors: evidence from rural south-east Zambia. Trop. Med. Infect. Dis. 9, 20. doi: 10.3390/tropicalmed9010020
Sangoro O., Kelly A. H., Mtali S., Moore S. J. (2014). Feasibility of repellent use in a context of increasing outdoor transmission: A qualitative study in rural Tanzania. Malaria J. 13, 347. doi: 10.1186/1475-2875-13-347
Sarfo J. O., Amoadu M., Kordorwu P. Y., Adams A. K., Gyan T. B., Osman A. G., et al. (2023). Malaria amongst children under five in sub-Saharan Africa: a scoping review of prevalence, risk factors and preventive interventions. Eur. J. Med. Res. 28, 80. doi: 10.1186/s40001-023-01046-1
Schindler T., Deal A. C., Fink M., Guirou E., Moser K. A., Mwakasungula S. M., et al. (2019). A multiplex qPCR approach for detection of pfhrp2 and pfhrp3 gene deletions in multiple strain infections of Plasmodium falciparum. Sci. Rep. 9, 13107. doi: 10.1038/s41598-019-49389-2
Schots M. A. S., Coleman H. L. S., Lutwama G. W., Straetemans M., Jacobs E. (2022). The impact of the COVID-19 pandemic on healthcare access and utilisation in South Sudan: a cross-sectional mixed methods study. BMC Health Serv. Res. 22, 1559. doi: 10.1186/s12913-022-08929-9
Shepard D. S., Odumah J. U., Awolola S. T. (2020). Cost-effectiveness of PBO versus conventional long-lasting insecticidal bed nets in preventing symptomatic malaria in Nigeria: results of a pragmatic randomized trial. Am. J. Trop. Med. Hyg 104, 979–986. doi: 10.4269/ajtmh.20-0956
Spitzen J., Koelewijn T., Mukabana W. R., Takken W. (2016). Visualization of house-entry behaviour of malaria mosquitoes. Malar J. 15, 233. doi: 10.1186/s12936-016-1293-7
Sternberg E. D., Cook J., Alou L. P. A., Assi S. B., Koffi A. A., Doudou D. T., et al. (2021). Impact and cost-effectiveness of a lethal house lure against malaria transmission in central Côte d'Ivoire: a two-arm, cluster-randomised controlled trial. Lancet 397, 805–815. doi: 10.1016/S0140-6736(21)00250-6
The United Republic of Tanzania (URT) MoFaP, Tanzania National Bureau of Statistics and President’s Office - Finance and Planning, Office of the Chief Government Statistician, Zanzibar (2022). The 2022 population and housing census: administrative units population distribution report (Tanzania: the Ministry of Finance and Planning (MoFP) of the United Republic of Tanzania).
Tusting L. S., Bottomley C., Gibson H., Kleinschmidt I., Tatem A. J., Lindsay S. W., et al. (2017). Housing improvements and malaria risk in sub-saharan africa: A multi-country analysis of survey data. PloS Med. 14, e1002234. doi: 10.1371/journal.pmed.1002234
Tusting L. S., Ippolito M. M., Willey B. A., Kleinschmidt I., Dorsey G., Gosling R. D., et al. (2015). The evidence for improving housing to reduce malaria: a systematic review and meta-analysis. Malar J. 14, 209. doi: 10.1186/s12936-015-0724-1
Who (2016). Global technical strategy for malaria 2016-2030 (Geneva, Switzerland: World Health Organization), 1–35.
Woods J., Elmore S. N., Glenn L., Maues J., James D., Roberson M. L. (2023). A qualitative study of the impact of the COVID-19 pandemic on metastatic breast cancer care. J. Patient Exp. 10, 23743735231167973. doi: 10.1177/23743735231167973
World Health Organization (2023a). World malaria report. (Geneva, Switzerland: World Health Organization (WHO)).
Keywords: malaria, insecticide-treated screening, unimproved housing, eave-nets, windows, perception, acceptability, Tanzania
Citation: Kihwele F, Odufuwa OG, Muganga JB, Mbuba E, Philipo R, Moore J, Skovmand O, Bosselmann R, Bradley J, Moore S and Mboma ZM (2025) Community perceptions and acceptability of insecticide-treated screens for mosquito proofing of unimproved houses in Chalinze district, Tanzania: a mixed-methods study. Front. Malar. 3:1540184. doi: 10.3389/fmala.2025.1540184
Received: 05 December 2024; Accepted: 13 February 2025;
Published: 12 March 2025.
Edited by:
Andre Lin Ouedraogo, Bill and Melinda Gates Foundation, United StatesReviewed by:
Merveille Koissi Savi, Harvard University, United StatesCopyright © 2025 Kihwele, Odufuwa, Muganga, Mbuba, Philipo, Moore, Skovmand, Bosselmann, Bradley, Moore and Mboma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fadhila Kihwele, ZmtpaHdlbGVAaWhpLm9yLnR6
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.