
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Malar., 28 January 2025
Sec. Case Management
Volume 3 - 2025 | https://doi.org/10.3389/fmala.2025.1519846
This article is part of the Research TopicAdvancing Malaria Eradication: Innovations, Implementations, and InsightsView all articles
Background: Malaria and schistosomiasis are among the most important parasitic diseases with overlapping geographical distribution in Ethiopia. The objective of this study, therefore, was to assess parasitological and clinical correlates and determinants of co-infection among malaria patients.
Methods: A health facility-based comparative cross-sectional study was conducted among malaria patients attending public health facilities in Damot Woyide district, Wolaita zone, South Ethiopia regional state, from December 2020 to June 2021. A total of 246 (123 Plasmodium-only and 123 Plasmodium–Schistosoma mansoni co-infected) study participants were sampled consecutively. A pre-tested structured questionnaire was used to collect data on demographic and risk factors. Detection and quantification of Schistosoma mansoni ova and malaria parasites were done with the Kato–Katz technique and blood film, respectively. Determination of hematological and biochemical parameters was done by the aLCose®HemoGo test system and the Cobas C 311 chemistry analyzer, respectively.
Results: The prevalence of Plasmodium–Schistosoma mansoni co-infection was 18.2%. Plasmodium–Schistosoma mansoni co-infected with a heavy intensity of Schistosoma mansoni had the highest parasitemia and was also significantly associated with malaria attack. In addition, anemia was associated with the presence of Plasmodium–Schistosoma mansoni co-infection. Schistosoma mansoni co-infection among malaria patients significantly increased mean Plasmodium density, serum glutamate oxaloacetate transaminase, serum glutamate pyruvate transaminase, alkaline phosphatase, direct bilirubin, and total protein. Hemoglobin and hematocrit were significantly lower among co-infected individuals. On multivariate analysis, being male (AOR=3.03, CI: 1.56-5.91,p=0.001), being in the 6-15 years age group (AOR = 5.85, CI: 2.45–13.93,p=0.00), the presence of stream (AOR = 4.1, CI: 1.65–10.15,p=0.002), having a water body distance of less than 1,000 m (AOR=2.33, CI: 1.23–4.41,p=0.009), and presence of irrigation practice (AOR= 3.24, CI: 1.69–6.18,p=0.00) were found to be significant risk factors of co-infection.
Conclusion: Malaria attack and level of parasitemia were associated with the intensity of concurrent Schistosoma mansoni infection. Co-infection results in the change of Plasmodium density, hematological, and biochemical parameters in malaria patients.
Malaria and Schistosomiasis are among the most important parasitic diseases with significant economic and public health consequences, particularly in Africa. Both parasites exert significant health, social, and financial burdens on infected individuals, households, and the health care system of the country in general (WHO, 2022; WHO, 2023). The two parasites have been associated with poverty, climate change, and several other related factors, which may result in their co-distribution and a high co-infection rate in humans (Brooker et al., 2007; Degarege et al., 2016).
Malaria causes an estimated 241 million cases and 627,000 deaths worldwide in 2020. The African region continues to carry the highest burden of malaria, accounting for about 95% of all malaria cases and 96% of all malaria deaths worldwide (WHO, 2023). In Ethiopia, malaria continues to be a major public health problem with over 68 percent which is approximately 74 million of the country’s total population living in areas at risk of malaria (Girum et al., 2019). In 2016, Ethiopia had an estimated three million new cases of malaria, resulting in 4,782 deaths and 365,900 Disability Adjusted Life Years (DALYs) in the same year (Girum et al., 2019). According to a malaria trend analysis conducted in the southern Ethiopian region’s Wolaita zone, Damot Woyide district is a high-transmission area where the prevalence of malaria is continuously rising (Legesse et al., 2015).
Schistosomiasis, on the other hand, remains a major cause of morbidity and mortality in several tropical and subtropical countries, with an estimated 240 million people infected worldwide and more than 700 million people living in endemic areas, mostly (90%) in Africa (W.H.O., 2018; WHO, 2022). In Ethiopia, intestinal schistosomiasis due to Schistosoma mansoni also remains the most widespread and predominant public health problem despite implementing several rounds of preventive chemotherapy. Every year, more than 35 million are at risk of infection, resulting in a pooled prevalence of 18% (Hussen et al., 2021; Ponpetch et al., 2021). Suitable environmental factors and human activities such as washing clothes, fetching water, swimming in rivers, bathing in open freshwater bodies, and engaging in irrigation farming are among the significant risk factors associated with schistosomiasis (Calasans et al., 2018; Bekana et al., 2021).
Several epidemiological studies show malaria and schistosomiasis co-endemic in many geographical areas. Due to this co-endemicity, multifactorial determinants like social-demographic (Abay et al., 2013; Getie et al., 2015), environmental (Booth et al., 2004; Chimbari et al., 2004), and behavioral factors (Juma et al., 2018) contribute to co-infection with schistosomiasis and malaria. Not only this, the different studies conducted in many countries reported different co-infection rates (Mazigo et al., 2010; Degarege et al., 2012; Mulu et al., 2013; Getie et al., 2015; Kinung’hi et al., 2017). Malaria and schistosomiasis co-infection, in turn, can affect parasitological (Getie et al., 2015; Dufera et al., 2016), hematological (Getie et al., 2015; Kinung’hi et al., 2017; Hailu et al., 2018; Abebe et al., 2024a), and biochemical parameters (Getie et al., 2015; Abebe et al., 2024b) among malaria patients thereby affecting the malaria clinical outcome
However, the current state of knowledge on the impact of co-infection on transmission dynamics and disease is contentious and inconclusive. Several studies found conflicting results regarding the effect of co-infection on malaria clinical outcomes. Some studies reported that co-infection increases malaria parasitemia (Degarege et al., 2012; Dufera et al., 2016).In contrast, others reported an association of co-infection with lower parasitemia (Sangweme et al., 2010; Getie et al., 2015), and still, others reported no correlation (Mazigo et al., 2010; Mulu et al., 2013). The disparity in findings was also observed concerning the hemoglobin level. Several studies have shown that co-infection reduces hemoglobin level (Degarege et al., 2012; Getie et al., 2015; Kinung’hi et al., 2017), while others found no effect of co-infection on hemoglobin level (Sangweme et al., 2010). Regarding pathological outcomes, some reported that co-infection with cerebral malaria was protective (Waknine-Grinberg et al., 2010), while others reported exacerbated hepatosplenomegaly (Wilson et al., 2011).
In Ethiopia, a previous related study was conducted to assess the prevalence and clinical correlates of Schistosoma mansoni co-infection among malaria-infected patients in Northwest Ethiopia, but, essential liver enzymes were not investigated in this study. In addition, renal involvement was not assessed, and potential risk factors were not considered in the analysis (Getie et al., 2015). The clinical and parasitological effects of Plasmodium–Schistosoma mansoni co-infection and factors favoring co-occurrence remain poorly understood. Therefore, this study aimed to assess parasitological and clinical correlates and determinants of co-infection among malaria-infected patients.
The study was conducted in four health centers in Damot Woyide district, Wolaita Zone, south Ethiopia regional state. The district is located 90 km and 384 km south of Hawassa and Addis Ababa, respectively. The administrative capital of the district is Bedesa. According to the Central Statistical Agency (CSA, 2015), the population of the district was 115,558 (57,670 males and 57,888 females) and it has twenty-three rural kebeles(the smallest administrative unit in Ethiopia) and two urban kebeles. There are four health centers (Bedessa, Girara, Sure Koyo, and Sake Health Center) and twenty-five health posts in the district. The majority of residents support their livelihoods life through subsistence farming (SNNPR, 2002). The area is known to be endemic to both malaria and schistosomiasis (Alemayehu and Tomass, 2015; Legesse et al., 2015) (Figure 1). Malaria transmission occurs in the district during two seasons: September to December, following heavy rain, and April to May, following light rain (health facility report). Previous research in the area found a high prevalence and intensity of Schistosoma mansoni infection, but a moderate prevalence of other soil-transmitted helminths (STHs) infections (81.9% vs. 32%) (Alemayehu and Tomass, 2015), and the co-endemicity of the two parasites in the districts make the area suitable for studying the effect of Schistosoma mansoni on Plasmodium infection. In addition to this, the area has no potable water access. Major coping strategies for the challenges of the area’s water supply are synchronizing different water sources and conserving water sources (Ermias et al., 2016). That is why native inhabitants of the community heavily rely on the “Bisare” stream for their water usage, as there is no sufficient safe water supply in the area (Ermias et al., 2016). The local community also uses the “Bisarestream” for irrigation.
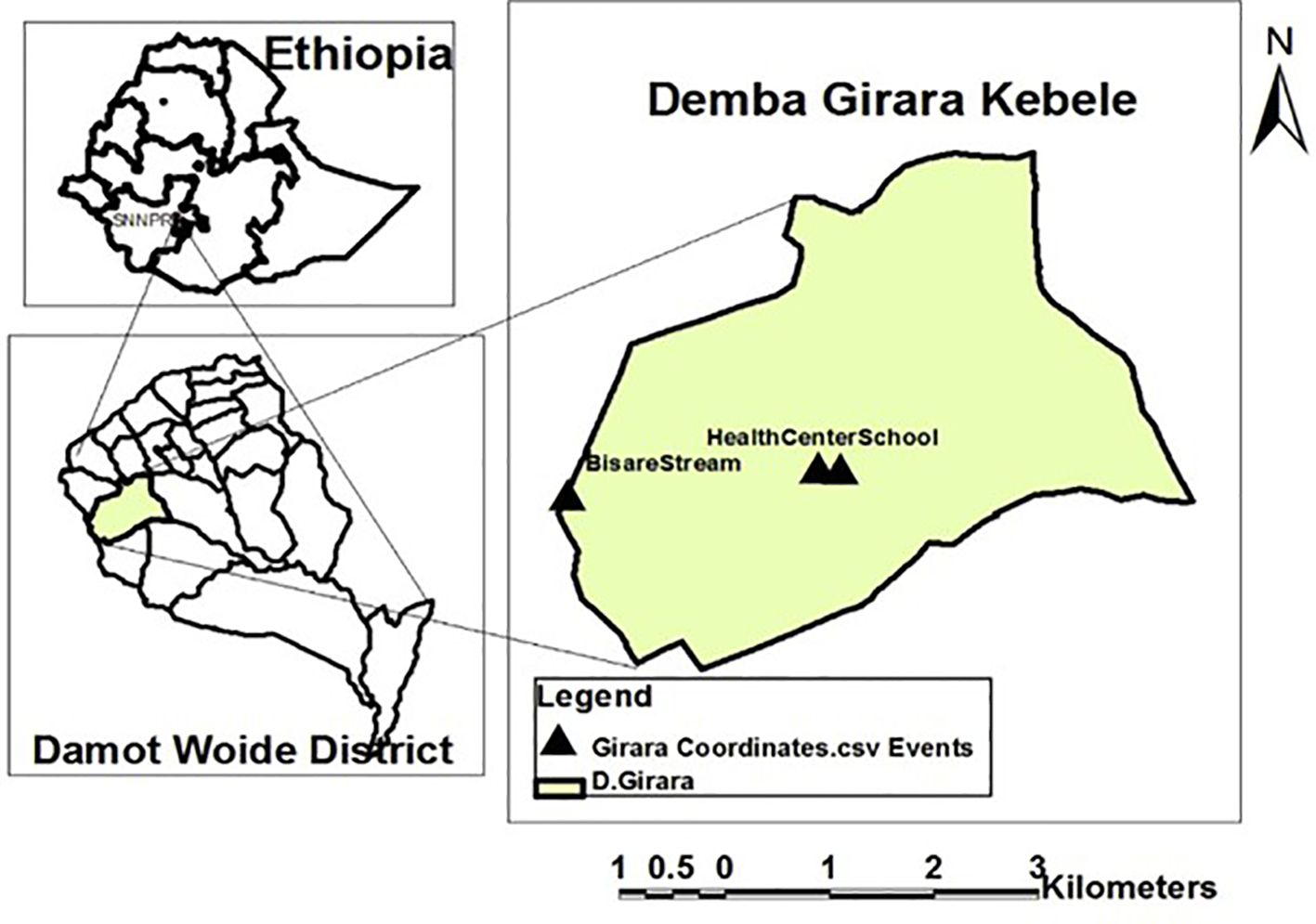
Figure 1. Study area map (Alemayehu and Tomass, 2015).
A health facility-based comparative cross-sectional study was conducted from December 2020 to June 2021 among malaria-suspected febrile patients. All microscopically confirmed Plasmodium-only infected and Plasmodium–Schistosoma mansoni co-infected patients attending public health facilities who met the inclusion criteria were included in the study (Figure 2).
The inclusion criteria were age of >5 years and being diagnosed with a confirmed malaria case. Exclusion criteria were with a history of antimalarial/anthelmintic medication use within 3 weeks before enrollment, patients who were co-morbid with confirmed chronic diseases, pregnant women attending an antenatal outpatient department, being on antiretroviral therapy, and concurrent infection with intestinal helminths other than Schistosoma mansoni.
The double population means formula was used for sample size determination.
112 participants for each group and a total of 224 (112*2)for both groups, then a 10% non-response rate added 224 *10/100 = 22.4, the total sample size was 224 + 22 = 246. Where β =0.1 (power 90%) and α = 5%[confidence interval (CI) 95%)] for two side t-tests. Where, N - Sample size in each of the groups, µ1 - Mean malaria parasitemia in Plasmodium-only-infected, µ2 - Mean malaria parasitemia in Plasmodium–Schistosoma mansoni co-infected group, µ1 - µ2- Difference in mean malaria parasitemia between the two groups, S12 -Variance of Plasmodium-only-infected, and S22 -Variance of Plasmodium–Schistosoma mansoni co-infected group, taken from a study done in Northwest Ethiopia (Getie et al., 2015). A consecutive sampling technique was employed to recruit all patients diagnosed with microscopically confirmed malaria from the four health facilities until the sample size was reached.
A pre-tested structured questionnaire was used to collect data on demographic and risk factors. Axillary body temperature was measured during a physical examination at the outpatient department of the public health facilities in the district. Other necessary clinical history was taken from the patients’ cards. In this study, a malaria attack was defined as P. falciparum-positive patients with a body temperature greater than 38°C and a malaria parasite density greater than or equal to 5000/µl, which is a case definition used elsewhere (Sokhna et al., 2004). The questionnaire was prepared in English and translated into both “Wolaita doona” and Amharic languages.
From each public health center, about five grams of fresh stool specimens were collected in screw-capped plastic, clean, and dry containers labeled with unique identification. A cold chain box was used to transport stool samples from each site of collection to the Girara Health Center for further processing and examination. Detection and quantification of Schistosoma mansoni ova were performed using a Kato-Katz thick smear utilizing a standardized template, measuring 41.7 mg of stool. The Kato-Katz thick smear was examined for Schistosoma mansoni ova after 24 hours of smear preparation to increase the sensitivity by improving the clarity of the ova during an examination. The number of eggs per gram of stool (infection intensity) was calculated by multiplying the number of eggs per Kato-Katz slide by 24, and infection intensities of Schistosoma mansoni were classified as follows: 1–99 infection intensity as light, 100–399 infection intensity as medium, and > 400 infection intensity as heavy (WHO, 2002).
A total of 5-mL venous blood was collected using a sterile disposable 5-mL syringe after cleaning the vein puncture site with an alcohol pad by trained laboratory personnel. A 2-mL blood sample was transferred into an anti-coagulated ethylene diamine tetraacetic acid (EDTA) tube for thick- and thin-blood film preparation and to measure hemoglobin and hematocrit levels. The remaining 3 mL of the blood were transferred to a serum separator tube for biochemical analysis.
Blood films were prepared at each public health facility from the 2 mL of anti-coagulated venous blood by placing two small drops of blood separately on a clean and pre-labeled microscopic glass slide. Prepared blood films were stored in a slide box and transported to the Girara health center for further processing and examination. Both thick and thin blood films were made on a single slide, and then the slide was allowed to dry, stained with 10% Giemsa stain for 10 minutes, and examined under an Olympus microscope with a 100X oil immersion objective lens. The thick blood smear was used to calculate the parasite density by counting the number of asexual parasites in a set number of white blood cells (WBCs) (typically 200) with a hand tally counter. The number of asexual parasites per µl of blood was calculated by dividing the number of asexual parasites by the number of WBCs counted and then multiplying by an assumed WBC density in 1µl of blood (typically 8000 per µl) (WHO, 2010). Finally, the level of parasitemia was graded as low (1-999/μl), moderate (1000-9999/μl), and High (> 10,000/μl), a method used elsewhere (Nega et al., 2015).
The remaining blood sample from the 2 mL of anti-coagulated venous blood was transported within 8 h of blood collection to Girara Health Center by using a cold chain system. The determination of hemoglobin and hematocrit levels was done by the aLCose® HemoGo blood hemoglobin test system (jjplus corporation, New Taipei City, Taiwan) following standard operating procedure.
The 3 mL of venous blood in the pre-labeled non-anti-coagulated serum separator tube were allowed to clot at the bench top for 30 min. Following that, blood was centrifuged at 3,000 rpm for 5 min and serum was separated. The test tubes with the patient’s identification were stored for 5 days until testing at 2°C–80°C. During transportation, the test tube rack is placed into the vaccine carrier cool box. Each test tube is individually protected by absorbent materials (cotton) to reduce shock or prevent breakage. Dry ice packs are placed at the bottom of the box and along the sides, specimens are placed in the center, and then extra ice packs are placed on top to keep the temperature at 2-8°C.
Serum was analyzed by a Cobas C 311 chemistry analyzer (Hitachi high-technologies corporation, Germany) for serum glutamate pyruvate transaminase (SGPT), serum glutamate oxaloacetate transaminase (SGOT), alkaline phosphates (ALP), bilirubin total (BIL-T), bilirubin direct (BIL-D), albumin (alb), total protein (TP), blood glucose, creatinine, and urea at Wachemo University Negist Eleni Mohamed Memorial comprehensive specialized hospital.
Before actual data collection, one day’s training was given to data collectors about data collection methods and how to handle ethical issues. A pre-test was conducted on 5% of the study sample size of patients not included in the study. Thus, the flow, clarity, and completeness of questions and the time needed to fill them were checked. Lastly, amendments were made to some of the unclear questions after discussion with the data collectors based on the results of the pre-test. To ensure that all necessary data were properly compiled, the supervisor (Head of the Health Center) and the principal investigator conducted regular supervision. Each day, during data collection, questionnaires were checked for completeness and consistency by the principal investigator. A necessary correction was made on incomplete questionnaires, otherwise discarded. The quality of the result from the biochemical analysis was assured by performing daily internal quality control and daily preventive maintenance. The functionality of the Giemsa stain was checked by preparing a smear from a known positive and negative sample from patients already diagnosed with malaria at Beddesa Health Center. Giemsa’s working solution was discarded after 24 hours, and a fresh working solution was prepared after use. A blood film was considered negative after examination of 1000 WBCs or 200 fields containing at least 10 WBCs per field revealing no asexual parasites. At least 10% of the randomly chosen positive and negative slides at Wachemo University Negist Eleni Mohamed Memorial Comprehensive Specialized Hospital were rechecked by a skilled lab technician who was blind to the previous result.
All raw data was coded and entered into SPSS version 25. The data was checked for missed values and outliers and was cleaned timely. Descriptive statistics were used to explore the data and to describe the demography of the participants. A Chi-square test was used to assess the associations of fever, Plasmodium infection, and anemia with the presence of Plasmodium–Schistosoma mansoni co-infection. An independent t-test was used to assess the mean difference in hematological (Hemoglobin and Hematocrit), biochemical parameters, and malaria parasite intensity (parasites/µL) among Plasmodium-only and Plasmodium–Schistosoma mansoni co-infected study participants. Analysis of variance (ANOVA) was used to compare the mean of Schistosoma mansoni infection intensity, parasitemia, hemoglobin, hematocrit, and biochemical parameters among subgroups. The multiple comparisons of mean Plasmodium density were made using Hochberg’s GT2 -post hoc test for Schistosoma mansoni infection intensity categories. The binary outcome of interest, the presence or absence of co-infection, was measured. Binary logistic regression and multivariate analysis were used to determine the statistical association between dependent and independent variables. Those variables associated with the dependent variable in the bivariate regression model (p-value< 0.25) were entered into multivariate analysis to control for possible confounders. The preliminary assumptions such as multi-collinearity and model fitness were checked. The odds ratio (OR) with a 95% CI was used to determine the strength of association between the presence of Plasmodium–Schistosoma mansoni co-infection and variables like sex, age, place of residence, presence of water body, farm working activities, length of stay, type of water body, proximity to a water body, and presence of irrigation practice. Statistical significance was defined at 95% confidence and a p-value less than 0.05.
Ethical clearance was obtained from the Ethical Review Board (IRB) of the Jimma University Institute of Health Science: Ref No: IHRPGD/947/20, Date: November,11,2020 After ethical clearance was received, permission to conduct the research was obtained from the Wolaita Zone Health Bureau and district health office. All participants were informed about the purpose, benefit, procedure, and potential risk of the study and their participation was voluntary. For children younger than eighty years, assent was obtained from parents or guardians on behalf of their children. Confidentiality of the information was maintained at each level of the study. The name of the participant was omitted from the questionnaire; instead, a number code was used to ensure confidentiality. All participants enrolled in the study received a free-of-charge diagnostic test on blood and stool. Those found to be positive for either or both of the infections including participants excluded in the study due to polyparasitism received the standard dose of treatment according to the National protocol by Health officers or Nurses at Health Centers in the district. Patients who were diagnosed with P. falciparum and P. vivax malaria were treated with artemether-lumefantrine (AL) and chloroquine, respectively. Individuals who were found positive for S. mansoni, Hymenolepis nana (H. nana), and Taenia spp were treated with Praziquantel while 400mg of albendazole was used to treat those who were positive for soil-transmitted helminths (STHs).
A total of 2,989 suspected malaria patients visited the public health facilities within Damot Woyide district, Wolaita Zone, from December 2020 to June 2021. Out of these, 675 (22.6%) were found positive for Plasmodium parasites, with a proportion of 69% (466/675) and 29.6% (200/675) for P. falciparum and P. vivax species, respectively. Out of the 675 malaria-positive participants, 123 (18.2%) were found to be co-infected with Schistosoma mansoni. For our study, 246 malaria-positive study participants (123 Plasmodium-only-infected and 123 Plasmodium–Schistosoma mansoni co-infected) who fulfilled the inclusion criteria were included in the study. Out of these, the majority were males (58.1%), resided in rural areas (74.8%), and in the age group between 6–15 years (35%) (Table 1). The median [interquartile range (IQR)] age of the study participants was 28 (SNNPR, 2002; WHO, 2002; Sokhna et al., 2004; Mazigo et al., 2010; Sangweme et al., 2010; Waknine-Grinberg et al., 2010; WHO, 2010; Wilson et al., 2011; Matangila et al., 2014; Alemayehu and Tomass, 2015; Nega et al., 2015; Dufera et al., 2016; Ermias et al., 2016; Alemayehu et al., 2017; Kinung’hi et al., 2017; Hailu et al., 2018; Tilla et al., 2019; Abebe et al., 2024a; Abebe et al., 2024b) years for malaria-only infected, and 17 (Juma et al., 2018; SNNPR, 2002; Booth et al., 2004; Chimbari et al., 2004; Mazigo et al., 2010; Sangweme et al., 2010; Waknine-Grinberg et al., 2010; Wilson et al., 2011; Degarege et al., 2012; Mulu et al., 2013; Alemayehu and Tomass, 2015; Getie et al., 2015; Dufera et al., 2016; Kinung’hi et al., 2017; Hailu et al., 2018; Abebe et al., 2024a; Abebe et al., 2024b) years for those Plasmodium–Schistosoma mansoni co-infected.
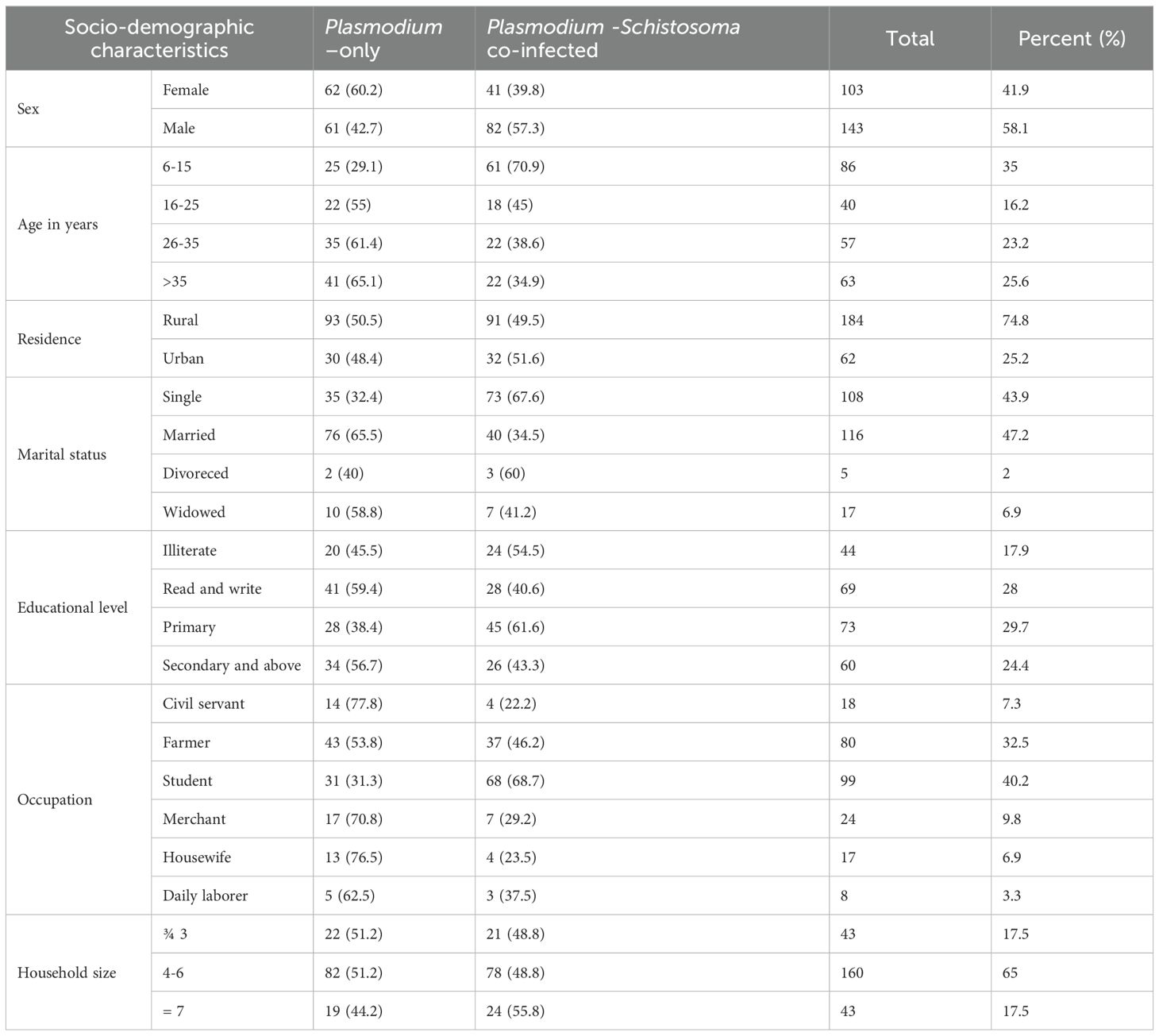
Table 1. Socio-demographic characteristics of study participants, Wolaita zone, south Ethiopia regional state, 2021.
Out of the total 246 malaria-positive participants included in the study, 71.1% (175/246) and 25.6% (63/246) were positive for P. falciparum and P. vivax respectively. The remaining 3.3% (8/246) had mixed infections. Of the 246 malaria-positive study participants, most Plasmodium infections were among males (58.1%), and within the age group of 6–15 years(35%). Similarly, most of the Plasmodium–Schistosoma mansoni co-infections were observed among male(66.7% (82/123) participants and in the age group between 6–15 years(49.6% (61/123)).
Approximately 72.5% (74/102) of P. falciparum and 27.2% (28/102) of P. vivax-infected participants were anemic, with a mean hemoglobin concentration of 10.2 ± 1.71, and 10.65 ± 1.17 respectively. The majority of anemic study participants were males [64.1% (66/103)] and aged between 6–15 years [37.9% (39/103)]. Moreover, the 6–15 years age group has the lowest mean Hemoglobin level compared to older age groups (F = 2.7, p = 0.046). Severe parasitemia (>10,000/μl) was found only in 4% (7/175) of the study participants with P. falciparum infection among Plasmodium–Schistosoma mansoni co-infected. Severe anemia (≤7g/dl) and severe hypoglycemia (≤ 40mg/dl) were found in 2.8% (7/246) and 8.9% (22/246) of the study participants, respectively. Mean malaria parasite density tends to decrease with increasing participant age groups. However, this effect was statistically not significant (F = 1.465, p = 0.225).
Infection intensity of Schistosoma mansoni among co-infected female patients was slightly higher than the male with mean Schistosoma mansoni infection intensity of 325.5 ± 167.5 and 301.8 ± 179.9, respectively (p=0.48). Similarly, the intensity of Schistosoma mansoni showed an age-related pattern with the highest (F= 3.706, p=0.014) mean being observed among individuals of the 16-25 years age group (mean Schistosoma mansoni infection intensity =355.17 ± 166.3) followed by the age group 6-15 (mean Schistosoma mansoni infection intensity =342.69 ± 160.5) compared to older age groups (Figures 3, 4).
After adjusting for possible confounders like sex, age, place of residence, presence of water body, farm working activities, length of stay, type of water body, proximity to a water body, and presence of irrigation practice, males are three times more likely to be co-infected than females (AOR = 3.03, CI: 1.56-5.91,p=0.001). Individuals within the 6-15 age range are six times more likely to be co-infected compared with those above 35 years (AOR = 5.85, CI: 2.45-13.93,p=0.00). Furthermore, people who live near a stream are four times more likely to have co-infection than people who live near other types of water bodies (AOR = 4.1, CI: 1.65-10.15,p=0.002). In addition, individuals living within a 1000m stretch of a water body are two times chance to get co-infection than those living above 1000m stretches (AOR = 2.33, CI: 1.23-4.41,p=0.009). Concerning irrigation practice, individuals practicing irrigation are three times more likely to get co-infection than those who do not practice irrigation (AOR = 3.24, CI: 1.69–6.18,p=0.00). The Hosmer-Lemeshow (HL) goodness-of-fit test (GOF) shows that the multivariate logistic regression model of co-infection fits well (HL χ2 = 5.421, df = 8, p = 0.71Parasitological correlates of Plasmodium–Schistosoma mansoni co-infection
The mean parasitemia among Plasmodium–Schistosoma mansoni co-infected individuals increased significantly as the level of Schistosoma mansoni infection intensity increased. There was a statistically significant mean difference in Plasmodium parasite density among light, moderate, and heavy Schistosoma mansoni co-infected individuals (F = 14.68, p< 0.001). In Hochberg’s GT2 post hoc analysis, this means the difference lies only among light and heavily co-infected malaria patients (1898.5 vs. 4260.85 parasite/μl, p<0.001) (Table 2).
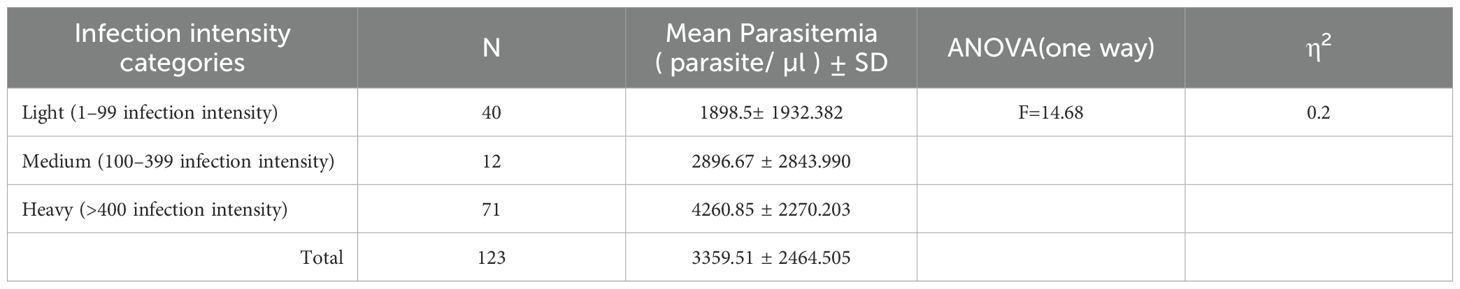
Table 2. One-way ANOVA analysis of malaria parasitemia specific to the intensity of Schistosoma mansoni among Plasmodium–Schistosoma mansoni co-infected patients, Wolaita zone, south Ethiopia regional state, 2021.
In this study, the prevalence of fever presentation (axillary body temperature = 38°C) was higher among Plasmodium-only infected than Plasmodium–Schistosoma mansoni co-infected patients. However, the observed difference in prevalence was suggestive rather than conclusive (χ2 = 3.739, p = 0.053). In addition, a higher proportion of anemia was observed among Plasmodium–Schistosoma mansoni co-infected patients [66% (68/103)] than Plasmodium-only infected patients [34%(35/103)] (χ2 = 18.18, p =0.00). There were no significant differences in mean Hemoglobin concentration between patients with light, moderate, and heavily co-infected malaria (F = 2.34, p = 0.101) (Supplementary Table 2).
In this study, malaria attacks were observed among 7.7% (19/246) of the study participants. In addition, a higher proportion of malaria attacks was identified among males 73.7% (14/19) than females 26.3% (5/19). After adjusting for sex and age, patients with a heavy egg load of Schistosoma mansoni were ten times more likely to experience malaria attacks than those who were free from Schistosoma mansoni infection (AOR = 9.834, CI: 2.487-38.828,p=0.001) (Table 3). Seventy-nine percent [79% (15/19)] of the study participants with malaria attacks had Schistosoma mansoni co-infection (Table 3).
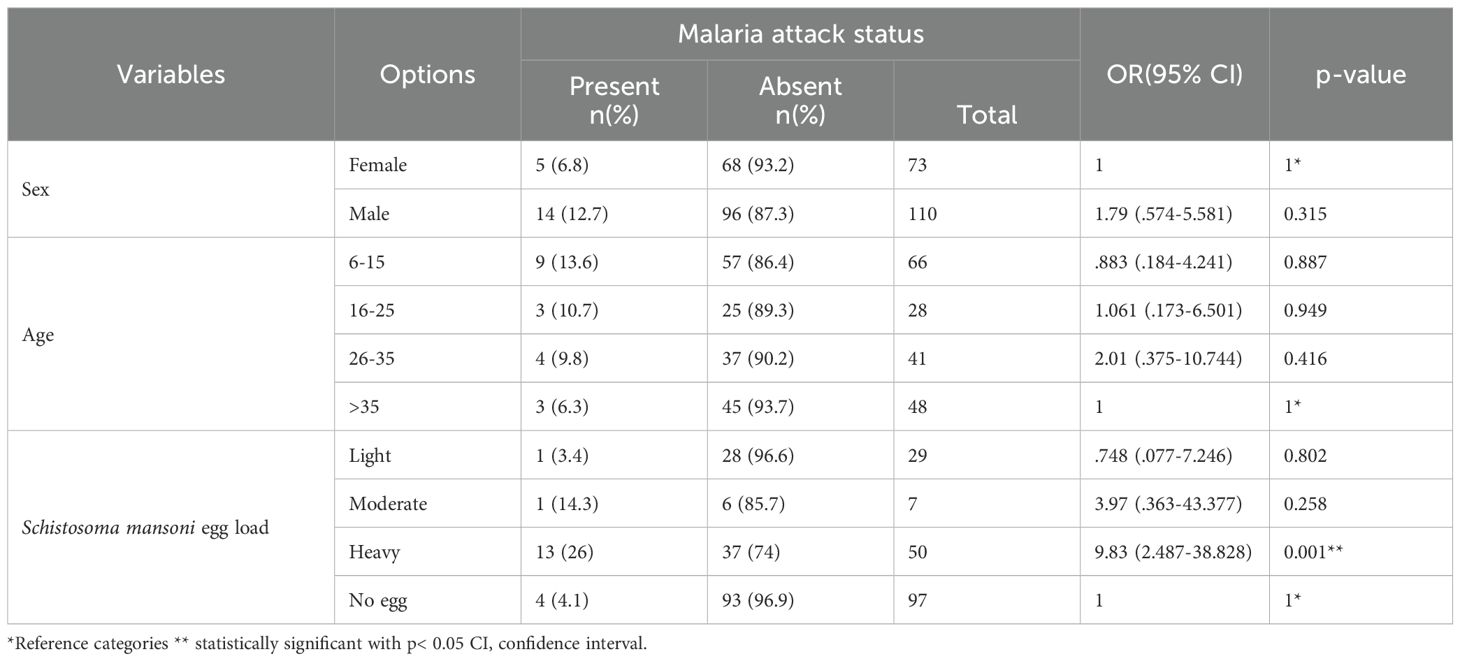
Table 3. Logistic regression model for malaria attack among study participants diagnosed with P. falciparum, adjusting for sex, age, and the intensity of Schistosoma mansoni,n=183, Wolaita zone, south Ethiopia regional state, 2021.
Between those with Plasmodium-only infection and those with Plasmodium–Schistosoma mansoni co-infection, there was a mean difference in clinical parameters. Co-infected individuals showed a statistically significant increase in mean Plasmodium density, SGPT, SGOT, ALP, BIL-D, and TP. However, the level of Hemoglobin and hematocrit was significantly lower among Plasmodium–Schistosoma mansoni co-infected individuals. In contrast, other parameters like urea, creatinine, and glucose were not statistically significant (Figures 5–7).
When comparing P. falciparum-Schistosoma mansoni co-infection to Plasmodium-only, and P. vivax-Schistosoma mansoni co-infection, there is a statistically significant increase in the mean Plasmodium density, SGPT, SGOT, and BIL-D among P. falciparum-Schistosoma mansoni co-infected. However, the hemoglobin and hematocrit level is significantly lower among P. falciparum-Schistosoma mansoni co-infected compared to Plasmodium –only, and P.vivax-Schistosoma mansoni co-infected. In contrast, the serum level of ALP was significantly higher among P. vivax-Schistosoma mansoni co-infected patients. In addition, the serum TP level was significantly higher among Plasmodium-only infected study participants (Table 4).
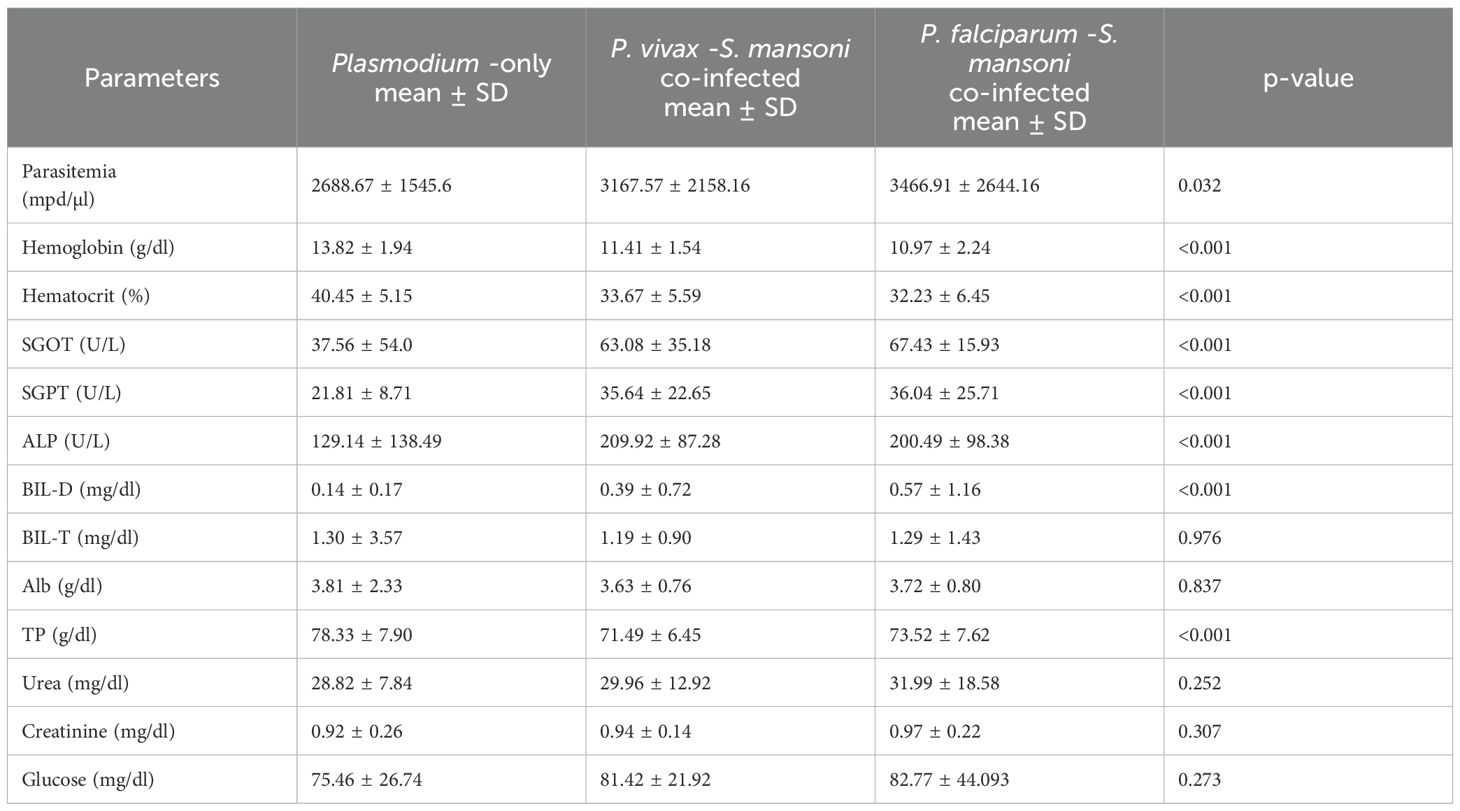
Table 4. One-way ANOVA analysis for clinical parameters, the mean difference between Plasmodium-only-infection and co-infection type among malaria patients, Wolaita zone, south Ethiopia regional state, 2021.
Plasmodium–Schistosoma mansoni co-infection is a major public health concern in developing countries where their geographical distribution overlaps (Juma et al., 2018; Chimbari et al., 2004; Mazigo et al., 2010; Matangila et al., 2014; Kinung’hi et al., 2017). Previous studies conducted in Damot Woyide district, Wolaita zone, south Ethiopia regional state reported the presence of malaria and Schistosoma mansoni transmission in the district (Alemayehu and Tomass, 2015; Alemayehu et al., 2017; Tilla et al., 2019). The current study revealed that Damot Woyide district, Wolaita zone, is one of such epidemiological settings in Ethiopia where malaria and schistosomiasis transmission overlap with a co-infection rate of 18.2%.
The presence of streams(AOR = 4.1, CI: 1.65-10.15,p=0.002), small irrigation-based agricultural activities in the area (AOR = 3.24, CI: 1.69-6.18, p=0.00), as well as other social demographic characteristics such as age (AOR = 5.85, CI: 2.45-13.93, p=0.00) and sex (AOR = 3.03, CI: 1.56-5.91, p=0.001), were factors that showed significant associations with Plasmodium–Schistosoma mansoni co-infection among communities living in the study areas (Alemayehu and Tomass, 2015; Legesse et al., 2015; Alemayehu et al., 2017).In this study, living close to the water body was strongly associated with Plasmodium–Schistosoma mansoni co-infection. This is explained by a slight geographical variation in the risk of contracting schistosomiasis and malaria (Booth et al., 2004).
Moreover, the risk of co-infection was higher among individuals living around streams than in other types of water bodies. The possible explanation was that the previous study in the area identified the Bisare Stream and Adacha River as sources of Schistosoma mansoni infection (Alemayehu et al., 2017). Even though residents use both sources of water for irrigation, during a snail survey, most adult and cercaria-shedding snail intermediate hosts were identified from the stream compared to the river. Also, the physical characteristics, the slow velocity of the water body, vegetation density, and the presence of substrate like aquatic vegetation, mud, or rocks, of the stream are more conducive to Biomphalaria pfeifferi snail’s existence compared to the river, This might increase the co-infection rate (Alemayehu et al., 2017).
Another risk factor that was identified as an important determinant for Plasmodium -Schistosoma mansoni co-infection was the presence of irrigation practice. This result was in agreement with earlier studies conducted in Zimbabwe and Kenya (Juma et al., 2018; Chimbari et al., 2004). The presence of water bodies with suitable ecological conditions (Mereta et al., 2019), and irrigation practices create an ideal habitat for mosquitoes found in fields and irrigation ditches with stagnant water to lay their eggs and breed. Because more mosquitoes carrying the malaria parasite can flourish in these environments, there is an increased risk of malaria transmission. Similar to this, irrigation systems can produce stagnant or slow-moving water, which is preferred by the snails that serve as intermediate hosts for the schistosomiasis infection.
In the present study, co-infection status is strongly associated with gender. Males have been shown to have a higher co-infection rate compared to females. The observed difference between females and males can be linked with a higher frequency of contact with cercariae-contaminated water bodies by engaging in farming activities and outdoor night activities of males like irrigation, livestock care, and wild animal control that are active at night to minimize damage to their crops. This finding is supported by previous studies conducted in Ethiopia (Abay et al., 2013; Dufera et al., 2016; Kinung’hi et al., 2017). In contrast to this finding, a study from Kenya found no association between sex and co-infection; this is probably because of the few participants included in the study (Juma et al., 2018).
Additionally, in the present study, the risk of co-infection is higher among school-age children compared to adults this can be linked to the behavior and lifestyle of school-age children like bathing, swimming, rare cattle along water bodies, and lower-aged males also share tasks with their father by engaging outdoor night activities. Since mosquitoes are most active at dusk and dawn, malaria is frequently transmitted at these times. Participating in nighttime outdoor activities raises the possibility of coming into contact with mosquitoes that transmit malaria, particularly in regions where the disease is prevalent. Previous studies conducted in Western Ethiopia in 2020 (Dufera et al., 2016), Northwestern Ethiopia in 2015 (Getie et al., 2015), and Northeastern Ethiopia (Abay et al., 2013)also reported the association of age with co-infection.
Even though the current study could not identify gender and age relationships with Schistosoma mansoni infection, there is a difference in mean infection intensity between gender and age groups. Higher mean Schistosoma mansoni infection intensity was observed in the lower age group than in the older age group. It is expected that school-age children are the most affected due to high levels of unsafe water contact behavior such as swimming, bathing, and rare cattle near the grassy area along with water bodies. This finding is supported by other studies (Alemayehu and Tomass, 2015; Dufera et al., 2016; Alemayehu et al., 2017). Considering gender, female participants had slightly higher mean Schistosoma mansoni infection intensity than males, which is in contrast to previous studies (Alemayehu and Tomass, 2015; Alemayehu et al., 2017), but in agreement with the other study conducted in western Ethiopia (Abay et al., 2013). The observed gender variation could be attributed to the difference in the nature of the study participants, socioeconomic status, and immune status of the patients.
The co-infection rate of 18.2% reported in the current study is comparable to the rate reported in the study conducted in Kemise town, northeast Ethiopia (18.4%) (Abay et al., 2013), but lower than a study conducted at Dore Bafeno Health Center, southern Ethiopia (38.5%) (Degarege et al., 2012), and Northwest Ethiopia (19.5%) (Getie et al., 2015), the shores of Lake Victoria, northwestern Tanzania (22.6%) (Mazigo et al., 2010), southern Ethiopia (22.6%) (Mulu et al., 2013), and the Mara region, Northwestern Tanzania(81.9%) (Kinung’hi et al., 2017). However, it was higher than the finding of another study conducted in Zambia’s Zambezi District (2.7%) (Rutagwera and Tylleskär, 2012). The differences in the prevalence of co-infections among these studies could be attributed to the environmental and micro-climatic conditions that influence the two parasites. Some of these factors include types of water bodies, water contact practices, poverty, other socioeconomic factors, lack of effective preventative measures, limited access to health care, study design, and time of survey (Booth et al., 2004; Brooker et al., 2007; Ojo et al., 2019).
In the present study, the prevalence of anemia [(Hemoglobin levels ≤ 12 mg/dl)] (41.9%) among malaria patients was comparable with an earlier study done in Northwest Ethiopia (41.4%) (Hailu et al., 2018), and Congo (41.6%) (Matangila et al., 2014), but higher than a study done in Kenya (23.3%) (Valice et al., 2018). This finding was also lower than reports from studies conducted in Ethiopia (Getie et al., 2015; Tuasha et al., 2019). The observed variation in the prevalence of anemia could be explained by changing patterns of parasitic infection, nutritional status, the density of parasites, and differences in age distribution. Similar to the result of the current study, previous studies from Congo and Northwest Ethiopia reported a higher prevalence of anemia among co-infected[66% (68/103)] compared to Plasmodium–only (Matangila et al., 2014; Getie et al., 2015). In agreement with an earlier study (Kinung’hi et al., 2017), the current study did not find a significant statistical difference in the prevalence of anemia between sexes or age groups.
In this study, the rate of malaria attack [7.7%(19/246)] was significantly higher in patients heavily co-infected compared to those who were free from Schistosoma mansoni infection. This finding corroborates those of other studies that found that the heavy egg burden of Schistosoma mansoni increases the risk of developing severe malaria (Sokhna et al., 2004; Mulu et al., 2013; Getie et al., 2015). An increased risk of severe malaria in co-infected patients may result from the heavy egg burden of Schistosoma mansoni, which can affect liver function, exacerbate anemia, induce immunosuppression, alter cytokine profiles, and regulate the immunological response.
The current study demonstrated a significant mean difference in Plasmodium parasite density, hemoglobin, hematocrit, SGPT, SGOT, ALP, BIL-D, and TP among Plasmodium -only and Plasmodium–Schistosoma mansoni co-infected. In contrast, a previous study only reported a significant mean difference in the mean hemoglobin level; the difference between other biochemical parameters was not statistically significant (Getie et al., 2015). This difference in findings can be attributed to the laboratory method employed, unproportional study participants allocated among strata, study design, sample size, and study setting in the previous study (Getie et al., 2015). Similar to earlier reports on the association of co-infection with lower hemoglobin levels (Getie et al., 2015; Kinung’hi et al., 2017), the present study showed that co-infection significantly lowers hemoglobin levels. The observed significantly higher mean in biochemical parameters among the co-infected group can be explained by the synergistic effect on liver pathology, leading to increased inflammation, impaired liver function, fibrosis, and raising the likelihood of serious liver consequences in co-infected people (Dufera et al., 2017).
In this study, a higher prevalence of severe parasitemia (>10,000/μL) was observed among P. falciparum-infected patients. This can be explained by the fact that P. falciparum infects both young and old red blood cells, and rapid replication increases the level of parasitemia during P. falciparum malaria infection. In contrast to an earlier study (Getie et al., 2015), a higher frequency of fever was observed among Plasmodium-only than in the co-infected counterpart. This may be due to anti-inflammatory markers being unregulated due to the dominance of heavy Schistosoma mansoni infection among Plasmodium–Schistosoma mansoni co-infected individuals. In Plasmodium-only infected Th1 pro-inflammatory mediators such as Tumor Necrosis Factor- (TNF-), which is pyrogenic, resulting in an aggravated fever presentation (Karunaweera et al., 1992).
In the current study, a higher mean malaria parasitemia was observed among Plasmodium–Schistosoma mansoni co-infected participants than Plasmodium-only infected participants. This finding concurs with results from western Ethiopia and southern Ethiopia, where co-infected malaria patients were more likely to have high parasitemia than Plasmodium-only infected patients (Degarege et al., 2012; Dufera et al., 2016). However, this contradicts a result from Zimbabwe and northwest Ethiopia, where a higher mean malaria parasite density was observed among Plasmodium-only infected individuals (Sangweme et al., 2010; Getie et al., 2015). This difference can be due to variation in Schistosoma mansoni infection intensity, which would affect Plasmodium density.
In this study, higher mean malaria parasitemia was observed among heavy Schistosoma mansoni co-infected patients than light-intense co-infected ones. In concordance with this finding, a study in northwest Ethiopia shows that co-infected patients with a moderate-heavy intensity of Schistosoma mansoni had significantly higher mean malaria parasitemia than co-infected patients with light-intense Schistosoma mansoni infection (Getie et al., 2015). Similarly, a study from western Ethiopia indicates that co-infection due to Plasmodium–Schistosoma mansoni reciprocally leads to an increase in parasite density (Dufera et al., 2016). Therefore, Schistosoma mansoni co-infection could affect malaria parasite density, depending on the intensity of Schistosoma mansoni infection.
In this study, the nutritional assessment and immunological profile of study participants were not done to see the effect of nutritional status and host immune response. These considerations may have contributed to strengthening the outcome of the present study.
Our findings suggest that co-infection is associated with altered Plasmodium parasite density, hematological, and biochemical parameters in malaria patients. A strong association between the intensity of the concomitant Schistosoma mansoni infection and Plasmodium parasite density was observed, which might be a load-related factor for the development of severe malaria with the course of infection. Moreover, malaria attacks were associated with the intensity of concurrent Schistosoma mansoni infections. Being male, being in the age group of 6–15, the presence of a stream, a water body distance within 1000m, and the presence of irrigation practice are risk factors of co-infection. The severity of Schistosoma mansoni infection is affected by age.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethical Review Board (IRB) of the Jimma University Institute of Health Science: Ref No: IHRPGD/947/20, Date: November,11,2020. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
KM: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. TB: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing. AZ: Conceptualization, Data curation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We sincerely thank Jimma University, the graduate school, for providing permission to conduct the study. We also thank Jimma University’s Faculty of Health Science and School of Medical Laboratory Sciences for enabling the study to be carried out. We do give special credit to Dr. Ayano Shanko, chief executive director, and Dr. Adane Desta, chief clinical director, Wachemo University Negist Eleni Mohamed Memorial Comprehensive Specialized Hospital, for their encouragement and unlimited support. They gave access to a hospital laboratory to conduct a biochemical analysis of the sample. We would like to thank the Damot Woyide district health professionals for their cooperation during the study. We want to provide my enormous thanks to my study participants for their willingness.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmala.2025.1519846/full#supplementary-material
Abay S. M., Tilahun M., Fikrie N., Habtewold A. (2013). Plasmodium falciparum and Schistosoma mansoni coinfection and the side benefit of artemether-lumefantrine in malaria patients. J. Infection Developing Countries. 7, 468–474. doi: 10.3855/jidc.2658
Abebe W., Asmare Z., Barasa S., Woldesenbet D., Lemma W., Derso A. (2024a). Assessment of the selected hematological profiles among malaria and Schistosoma mansoni co-infected patients, Northwest Ethiopia. J. parasitic diseases: Off. Organ Indian Soc. Parasitology. 48, 308–319. doi: 10.1007/s12639-024-01669-z
Abebe W., Asmare Z., Wondmagegn A., Awoke M., Adgo A., Derso A., et al. (2024b). Status of selected biochemical and coagulation profiles and platelet count in malaria and malaria-Schistosoma mansoni co-infection among patients attending at Dembiya selected Health Institutions, Northwest Ethiopia. Sci. Rep. 14, 6135. doi: 10.1038/s41598-024-56529-w
Alemayehu B., Tomass Z. (2015). Schistosoma mansoni infection prevalence and associated risk factors among schoolchildren in Demba Girara, Damot Woide District of Wolaita Zone, Southern Ethiopia. Asian Pacific J. Trop. Med. 8, 457–463. doi: 10.1016/j.apjtm.2015.05.009
Alemayehu B., Tomass Z., Wadilo F., Leja D., Liang S., Erko B. (2017). Epidemiology of intestinal helminthiasis among school children with emphasis on Schistosoma mansoni infection in Wolaita zone, Southern Ethiopia. BMC Public Health 17, 1–10. doi: 10.1186/s12889-017-4499-x
Bekana T., Berhe N., Eguale T., Aemero M., Medhin G., Tulu B., et al. (2021). Prevalence and factors associated with intestinal schistosomiasis and human fascioliasis among school children in Amhara Regional State, Ethiopia. Trop. Med. Health 49, 1–11. doi: 10.1186/s41182-021-00326-y
Booth M., Vennervald B. J., Kenty L., Butterworth A. E., Kariuki H. C., Kadzo H., et al. (2004). Micro-geographical variation in exposure to Schistosoma mansoni and malaria, and exacerbation of splenomegaly in Kenyan school-aged children. BMC Infect. diseases. 4, 1–11. doi: 10.1186/1471-2334-4-13
Brooker S., Akhwale W., Pullan R., Estambale B., Clarke S. E., Snow R. W., et al. (2007). Epidemiology of Plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia and prospects for combining control. Am. J. Trop. Med. hygiene. 77, 88. doi: 10.4269/ajtmh.2007.77.88
Calasans T. A. S., Souza G. T. R., Melo C. M., Madi R. R., Jeraldo V. (2018). Socioenvironmental factors associated with Schistosoma mansoni infection and intermediate hosts in an urban area of northeastern Brazil. PloS One 13, e0195519. doi: 10.1371/journal.pone.0195519
Chimbari M., Chirebvu E., Ndlela B. (2004). Malaria and schistosomiasis risks associated with surface and sprinkler irrigation systems in Zimbabwe. Acta tropica. 89, 205–213. doi: 10.1016/j.actatropica.2003.09.015
Degarege A., Degarege D., Veledar E., Erko B., Nacher M., Beck-Sague C. M., et al. (2016). Plasmodium falciparum infection status among children with Schistosoma in sub-Saharan Africa: a systematic review and meta-analysis. PloS Negl. Trop. diseases. 10, e0005193. doi: 10.1371/journal.pntd.0005193
Degarege A., Legesse M., Medhin G., Animut A., Erko B. (2012). Malaria and related outcomes in patients with intestinal helminths: a cross-sectional study. BMC Infect. diseases. 12, 1–9. doi: 10.1186/1471-2334-12-291
Dufera M., Berhe N., Petros B., Erko B. (2017). Periportal fibrosis in Schistosoma mansoni Mono and Malaria-Schistosoma Co-infected Individuals in Finchaa Sugar Estate, Western Ethiopia. Med. Health Sci. Res. J. 1, 01–06.doi: 10.20372/mhsr.v1i1.7
Dufera M., Petros B., Erko B., Berhe N. (2016). Reciprocal increase of malaria parasitaemia, S. mansoni egg count and anemia in malaria-Schistosomiasis mansoni co-infected individuals in Fincha Sugar Estate, western Ethiopia. J. Science Technol. Arts Res. 5, 10–21. doi: 10.4314/star.v4i4.00
Ermias M., Brook A., Kannan N. (2016). Assessment of urban water supply and sanitation: the case of Bedesa Town, Damot Woyde woreda of Wolaita Zone, Southern Ethiopia. J. Biology Agric. Healthcare. 6, 2224–3208.
Getie S., Wondimeneh Y., Getnet G., Workineh M., Worku L., Kassu A., et al. (2015). Prevalence and clinical correlates of Schistosoma mansoni co-infection among malaria infected patients, Northwest Ethiopia. BMC Res. Notes 8, 1–6. doi: 10.1186/s13104-015-1468-2
Girum T., Shumbej T., Shewangizaw M. (2019). Burden of malaria in Ethiopia, 2000-2016: findings from the Global Health Estimates 2016. Trop. Diseases Travel Med. Vaccines 5, 1–7. doi: 10.1186/s40794-019-0090-z
Hailu T., Yimer M., Mulu W., Abera B. (2018). Synergetic effects of Plasmodium, hookworm, and Schistosoma mansoni infections on hemoglobin level among febrile school age children in Jawe Worda, Northwest Ethiopia. J. Parasitol. Res. 2018, 9573413. doi: 10.1155/2018/9573413
Hussen S., Assegu D., Tadesse B. T., Shimelis T. (2021). Prevalence of Schistosoma mansoni infection in Ethiopia: a systematic review and meta-analysis. Trop. Diseases Travel Med. Vaccines 7, 1–12. doi: 10.1186/s40794-020-00127-x
Juma D. L., Shauri H., Abdullahi O., Mwangi T. (2018). Concomitant co-infections of malaria and schistosomiasis among school going children in the mining and agricultural concession areas of kwale county, Kenya. International Journal of Innovative Research and Advanced Studies (IJIRAS) 5 (10), 132–137.
Karunaweera N. D., Grau G. E., Gamage P., Carter R., Mendis K. N. (1992). Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivax malaria. Proc. Natl. Acad. Sci. 89, 3200–3203. doi: 10.1073/pnas.89.8.3200
Kinung’hi S. M., Mazigo H. D., Dunne D. W., Kepha S., Kaatano G., Kishamawe C., et al. (2017). Coinfection of intestinal schistosomiasis and malaria and association with haemoglobin levels and nutritional status in school children in Mara region, Northwestern Tanzania: a cross-sectional exploratory study. BMC Res. notes. 10, 1–11. doi: 10.1186/s13104-017-2904-2
Legesse D., Haji Y., Abreha S. (2015). Trend analysis of malaria occurrence in Wolaita Zone, Southern Ethiopia: retrospective cross-sectional study. Malaria Res. Treat. 2015, 123682. doi: 10.1155/2015/123682
Matangila J. R., Doua J. Y., Linsuke S., Madinga J., Inocêncio da Luz R., Van Geertruyden J.-P., et al. (2014). Malaria, schistosomiasis and soil transmitted helminth burden and their correlation with anemia in children attending primary schools in Kinshasa, Democratic Republic of Congo. PloS One 9, e110789. doi: 10.1371/journal.pone.0110789
Mazigo H. D., Waihenya R., Lwambo N. J., Mnyone L. L., Mahande A. M., Seni J., et al. (2010). Co-infections with Plasmodium falciparum, Schistosoma mansoni and intestinal helminths among schoolchildren in endemic areas of northwestern Tanzania. Parasites vectors. 3, 1–7. doi: 10.1186/1756-3305-3-44
Mereta S. T., Bedewi J., Yewhalaw D., Mandefro B., Abdie Y., Tegegne D., et al. (2019). Environmental determinants of distribution of freshwater snails and trematode infection in the Omo Gibe River Basin, southwest Ethiopia. Infect. Dis. poverty. 8, 1–10. doi: 10.1186/s40249-019-0604-y
Mulu A., Legesse M., Erko B., Belyhun Y., Nugussie D., Shimelis T., et al. (2013). Epidemiological and clinical correlates of malaria-helminth co-infections in Southern Ethiopia. Malaria J. 12, 1–7. doi: 10.1186/1475-2875-12-227
Nega D., Dana D., Tefera T., Eshetu T. (2015). Prevalence and predictors of asymptomatic malaria parasitemia among pregnant women in the rural surroundings of Arbaminch Town, South Ethiopia. PloS One 10, e0123630. doi: 10.1371/journal.pone.0123630
Ojo O., Adebayo A., Awobode H., Nguewa P., Anumudu C. (2019). Schistosoma haematobium and Plasmodium falciparum co-infection in Nigeria 2001–2018: a systematic review and meta-analysis. Sci. African. 6, e00186. doi: 10.1016/j.sciaf.2019.e00186
Ponpetch K., Erko B., Bekana T., Richards L., Liang S. (2021). Biogeographical characteristics of Schistosoma mansoni endemic areas in Ethiopia: a systematic review and meta analysis. Infect. Dis. Poverty. 10, 83. doi: 10.1186/s40249-021-00864-x
Rutagwera D. G., Tylleskär T. (2012). Co-infection with malaria, hookworm and schistosomiasis among school children in Zambezi: a school-based rapid survey. Med. J. Zambia. 39, 18–23.
Sangweme D. T., Midzi N., Zinyowera-Mutapuri S., Mduluza T., Diener-West M., Kumar N. (2010). Impact of schistosome infection on Plasmodium falciparum Malariometric indices and immune correlates in school age children in Burma Valley, Zimbabwe. PloS Negl. Trop. diseases. 4, e882. doi: 10.1371/journal.pntd.0000882
SNNPR (2002). Health profile of wolaita zone (Ethiopia: South Nation Nationality and Peaples Region Ethiopia).
Sokhna C., Le Hesran J.-Y., Mbaye P. A., Akiana J., Camara P., Diop M., et al. (2004). Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal. Malaria J. 3, 1–5. doi: 10.1186/1475-2875-3-43
Tilla T., Sorsa S., Asnake S. (2019). Prevalence of malaria and the associated risk factors among pregnant women attending antenatal care in health institutions of Damot Woyide district, Southern Ethiopia. J. Parasitol. Vector Biol. 11, 10–18. doi: 10.5897/JPVB2018.0350
Tuasha N., Hailemeskel E., Erko B., Petros B. (2019). Comorbidity of intestinal helminthiases among malaria outpatients of Wondo Genet health centers, southern Ethiopia: implications for integrated control. BMC Infect. diseases. 19, 1–8. doi: 10.1186/s12879-019-4290-y
Valice E. M., Wiegand R. E., Mwinzi P. N., Karanja D. M., Williamson J. M., Ochola E., et al. (2018). Relative contribution of schistosomiasis and malaria to anemia in western Kenya. Am. J. Trop. Med. Hygiene. 99, 713. doi: 10.4269/ajtmh.18-0069
Waknine-Grinberg J. H., Gold D., Ohayon A., Flescher E., Heyfets A., Doenhoff M. J., et al. (2010). Schistosoma mansoni infection reduces the incidence of murine cerebral malaria. Malaria J. 9, 1–11. doi: 10.1186/1475-2875-9-5
WHO (2002). Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee (Geneva: World Health Organization).
W.H.O. (2018). Schistosomiasis, and soil transmitted helminthiases: numbers of people treated in 2017. Wkly Epidemiol. Rec. 93, 681–692.
WHO (2022). Guideline on control and elimination of human schistosomiasis (Geneva: World Health Organization).
Keywords: malaria, Schistosoma mansoni, co-infection, Wolaita zone, Ethiopia
Citation: Mohamed K, Belay T and Zeynudin A (2025) Parasitological and clinical correlates of Plasmodium–Schistosoma mansoni co-infection and its determinants among Plasmodium-infected patients in the Wolaita Zone, South Ethiopia regional state. Front. Malar. 3:1519846. doi: 10.3389/fmala.2025.1519846
Received: 30 October 2024; Accepted: 06 January 2025;
Published: 28 January 2025.
Edited by:
Lau Yee Ling, University of Malaya, MalaysiaReviewed by:
Sanie Sesay, Sanofi, FranceCopyright © 2025 Mohamed, Belay and Zeynudin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kemal Mohamed, YWRvdGUxMjBAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.