- 1Department of Maternal, Infantile and Urological Sciences, Sapienza University of Rome, Rome, Italy
- 2Department of Obstetrics, Gynecology and Pediatrics, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
- 3Unit of Clinical Psychology, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
- 4Department of Anesthesiology and Intensive Care Medicine, Catholic University of the Sacred Heart, Rome, Italy
- 5Department of Internal Clinical Sciences, Anesthesiology and Cardiovascular Sciences, Sapienza University of Rome, Rome, Italy
Introduction: This retrospective cohort study aimed to observe the postnatal health of infants born to mothers with systemic autoimmune rheumatic diseases treated with hydroxychloroquine (HCQ) during pregnancy.
Methods: A total of 312 pregnancies of patients who suffered from different systemic autoimmune rheumatic diseases were considered. Pregnancy data were collected; a telephone follow-up questionnaire was successfully completed in 182 infants to detect the long-term pediatric outcome. The women who took hydroxychloroquine during pregnancy were defined as “HCQ group” and were compared to women who did not take hydroxychloroquine, “non-HCQ group”.
Results: A higher prevalence of women with multiple maternal diseases was detected in the HCQ group, in comparison to that of non-HCQ group (p = 0.0015). Despite HCQ group consisting of more complicated maternal conditions, the obstetrical and neonatal outcomes were similar between the two groups. Regarding postnatal health, 40% of infants in HCQ group revealed no pathologies versus 25% of the children in non-HCQ group (p = 0.0368).
Discussion: The protective role of HCQ on infants should be further evaluated in prospective multicenter long-term studies.
Introduction
Hydroxychloroquine (HCQ) is an old antimalarial drug, that has reached wide employment for treating systemic autoimmune rheumatic diseases. HCQ blocks the interaction of memory B-cells but not naive B-cells. A major mechanism of HCQ is preventing complement system activation. Moreover, HCQ inhibits various endolysosomal functions, including autophagy, endosomal Toll-like receptor activation, and calcium signaling. These effects impact several immune system processes, collectively reducing the production and release of pro-inflammatory cytokines (1).
HCQ, in APS, shows pleiotropic immunomodulant and protective effects through multiple pathways; HCQ can reduce the binding of the antiphospholipid antibodies (aPL) to the syncytiotrophoblast, restoring the expression of annexin A5 which has an anticoagulant effect (2) and antagonising aPL by the inhibition of trophoblast migration, invasion, and differentiation, even if partially (3). A reduction of antibody production was also demonstrated by a study showing a gradual decrease in the levels of circulating aPL in antiphospholipid syndrome (APS) during pregnancy (4, 5).
Key mechanisms include endothelial cell activation, where aPL bind to endothelial cells and trigger a pro-thrombotic state. This state is reinforced by the activation of monocytes, platelets, and neutrophils, often involving complement activation (6). Cellular activation in APS involves the binding of aPLs to specific receptors within lipid rafts, specialized microdomains in the cell membrane that trigger signal transduction (7). Gender differences have also been linked to APS outcomes, with research indicating a correlation between gender-specific immune responses and clinical manifestations in APS patients (8).
Moreover, its safety in pregnancy has been addressed in numerous studies (9–23). Studies by Parke et al., who examined SLE patients who received HCQ, revealed no association between its use and congenital abnormalities (11). In addition, they highlighted the beneficial effect of HCQ during pregnancy in alleviating skin lesions (9, 11). The 2020 American College of Rheumatology (ACR) recommends HCQ administration to all pregnant women with SLE (Systemic Erythematosus Lupus) if possible (15). Furthermore, several studies have shown that maternal use of HCQ is associated with decreased disease activity and lupus flares and reduced cardiac and cutaneous manifestations of neonatal lupus (13, 14). Other studies have presented the benefits of HCQ in lowering the risk of adverse pregnancy outcomes in patients with SLE (16–20). In recent years, larger literature surveys have also concluded that antimalarial HCQ has to be preferred over chloroquine (21). HCQ is also preferred to Azathioprine (AZA) to control disease activity during pregnancy in patients with SLE because of the association between AZA and the increased risk of childhood infections (22). HCQ is safe during breastfeeding and is present in the breast milk of women undergoing treatment since the level ingested by breastfed infants is less than 0.2 mg/kg/day, which is a small fraction of the adult therapeutic dose of 5 mg/kg/day, as the American Academy of Ophthalmology recommends (23), it unlikely causes toxic effects (24).
Given the increasingly widespread use of HCQ during pregnancy, our study aimed to observe the pregnancy outcome and the postnatal health in infants born to mothers with systemic autoimmune rheumatic diseases, treated or not with HCQ.
Materials and methods
This retrospective observational cohort study was conducted at IRCSS-Policlinico “A. Gemelli” in Rome, from 2000 to 2020.
Women included in the study suffered from different systemic autoimmune rheumatic diseases: Systemic Erythematosus Lupus (SLE), Antiphospholipid Syndrome APS, Rheumatoid Arthritis (RA), Primary Sjogren's Syndrome (pSjS), Systemic Sclerosis (SSc), Undifferentiated Connective Tissue Disease (UCTD), Antiphospholipid Antibody positivity with non-criteria manifestations (aPL). Pregnancy and delivery data were collected from delivery room records; a telephone follow-up questionnaire was subsequently performed to describe the long-term pediatric outcome.
A total of 312 pregnancies were considered, 309 singleton and 4 twin pregnancies, which were excluded from statistical analysis in the birth weight and birth weight percentile indicators. Among all pregnancies, 16 spontaneous abortion and 3 intrauterine deaths occurred, and 2 cases were lost to follow-up. Complete data for follow-up were obtained in 182 cases. Patients were divided into two groups: women who took HCQ (400 mg/day) during pregnancy (n = 135 pregnancies) (HCQ group) and women (n = 177 pregnancies) who did not take HCQ during pregnancy (non-HCQ group).
The distribution of the different Rheumatic Diseases among enrolled patients is presented in Figures 1a,b according to the group.
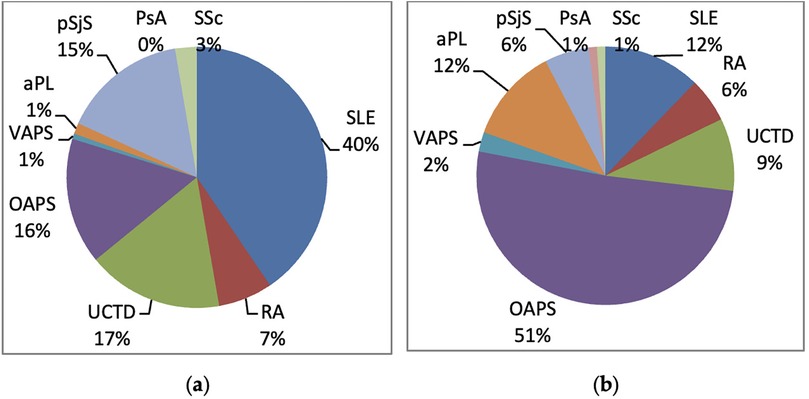
Figure 1. Distribution of autoimmune diseases in the two groups: (a) HCQ group; (b) non-HCQ group. aPL, antiphospholipid antibody positivity (aPL); VAPS, vascular antiphospholipid syndrome; OAPS, obstetric antiphospholipid syndrome; PsA, psoryatic arthritis; pSjS, primary Sjogren's syndrome; RA, rheumatoid arthritis; SLE, systemic erythematosus lupus; SSc, systemic sclerosis; UCTD, undifferentiated connective tissue disease.
All women in the study underwent clinical evaluation during pregnancy, and anamnestic, laboratory, and therapy data were collected retrospectively.
The anamnestic data included: patients' age at delivery, HCQ therapy taken during pregnancy and pre-conception, and any associated therapies [low-dose Aspirin, Low Molecular Weight Heparin (LWMH), Corticosteroids].
Delivery data were collected through the delivery room records at Policlinico “A. Gemelli” and, if patients had delivered elsewhere, by telephone questionnaire. Data included: mode of delivery (vaginal delivery or cesarean section); gestational age at the time of delivery; neonatal weight and weight percentile related to gestational age calculated according to Italian population curves (25); Apgar score at 1 and 5 min and admission to the neonatal intensive care unit.
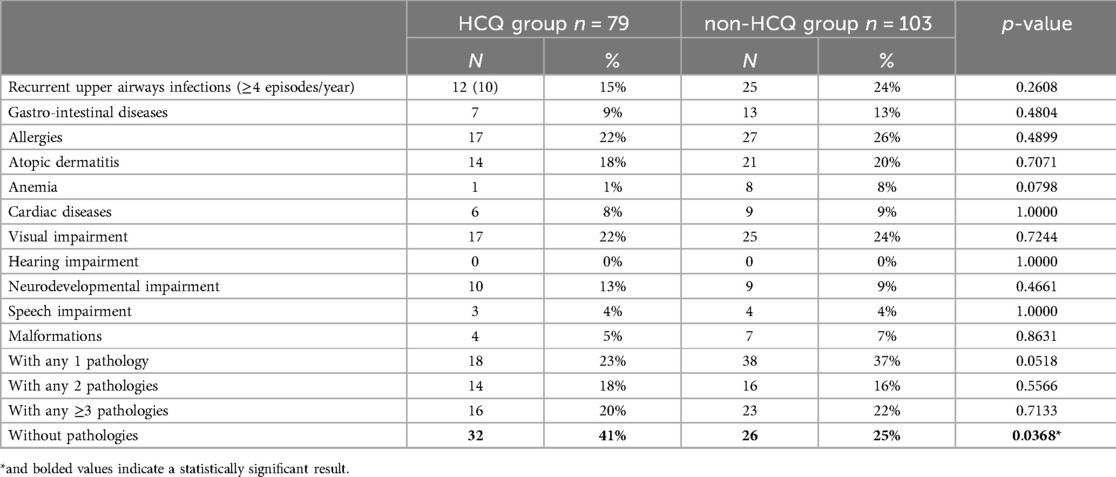
Follow-up data included children's age (in months); weight and height with their percentiles calculated according to CDC (26) growth curves; exclusive maternal breastfeeding and its duration; regularity of vaccinations; and presence of diseases such as recurrent upper respiratory tract infections (≥4 episodes/year), gastrointestinal diseases, allergic diseases, atopic dermatitis, anaemia, cardiac diseases, visual impairment, hearing impairment, neurobehavioral developmental disorders, particularly language, and presence of congenital malformations.
Among the women in HCQ group, n = 79 cases completed the telephone questionnaire, while in non-HCQ group n = 103 cases completed the questionnaire; the remaining cases were lost due to: non-traceability by phone, language barrier, and refusal. In the following flow chart, the distribution of the excluded cases is shown.
Statistical analysis
The appropriate statistical tests, parametric and nonparametric (Student's or Mann-Whitney's t-test, al X2 or Fisher's test), were used to analyse the comparison between variables among the two groups of patients. Multivariate logistic regression was performed to confirm the association between postnatal health (presence/absence of disease) and the mother's use of HCQ during pregnancy. The analysis has been corrected with confounding factors such as birth weight, birth weight percentile, gestational week at delivery, and the mother's age at delivery (Supplementary Table S1).
Results with p < 0.050 were considered statistically significant.
Results
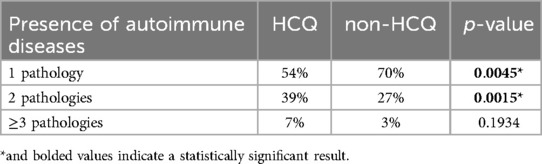
Women in the non-HCQ group were affected by a single autoimmune disease with a higher prevalence than women in the HCQ group (70% vs. 54%, p-value of 0.0045) (Figures 2a,b; Table 1). Accordingly, women in the HCQ group had a higher prevalence of multiple associated autoimmune diseases (39% vs. 27% in the non-HCQ group, with a p-value of 0.0015 (Figures 2a,b; Table 1).
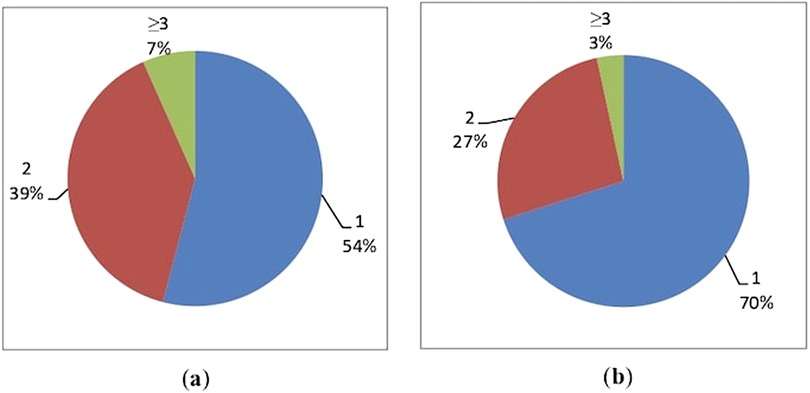
Figure 2. Distribution of associated autoimmune diseases in the two groups: (a) HCQ group; (b) non-HCQ group.
Other drugs taken during pregnancy
The intake of additional therapies besides HCQ in these high-risk pregnancies has been investigated, as shown in Figure 3.
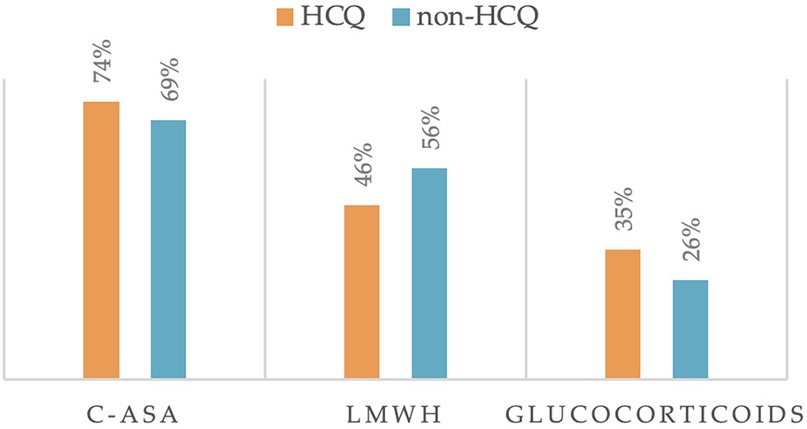
Figure 3. Other treatments taken during pregnancy (C-ASA, low dose aspirin; LMWH, low molecular weight heparin).
We found that Low dose Aspirin (100 mg, C-ASA) was taken by 74% of women in the HCQ group and 69% of women in the non-HCQ group (p = 0.3364). In addition, glucocorticoids were taken in 35% and 26% of cases, respectively (p = 0.1247). Regarding the intake of Low molecular weight heparin (LMWH), its use was very similar in the two groups (p = 0.0857). All differences were not statistically significant, meaning that the other drug intake did not influence the comparison between the two groups.
Despite the high number of patients with SLE, in our study population, we didn't report a high percentage of women who used Azathioprine in pregnancy. We reported 3 patients in the HCQ group and 3 patients in the non-HCQ group, but two of them were lost at follow-up due to non-traceability by phone. For this reason, we did not add it to the figure of additional therapies in Figure 3.
HCQ trend over time
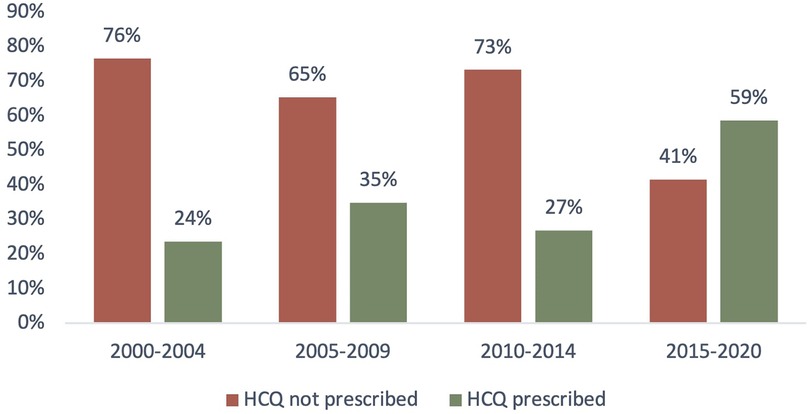
In our study population, the trend of HCQ intake over time was investigated, and we noticed an increasing use of this drug in the last two decades.
As shown in Figure 4, the prescription of HCQ over time has increased from 24% (years 2000–2004) to 59% in the last 5 years.
Obstetrical outcomes
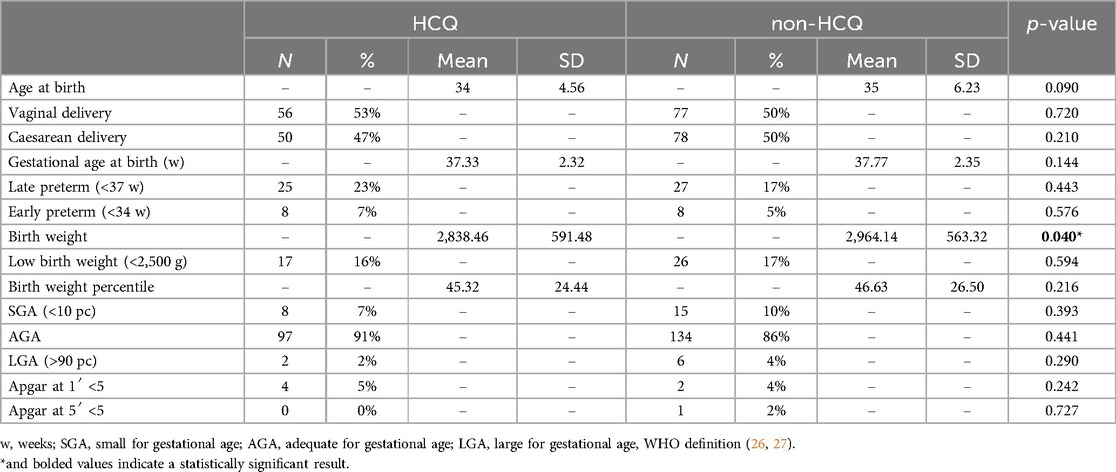
Obstetric outcomes of the two groups are shown in Table 2.
In HCQ group the mean maternal age at delivery was 34 years (SD 4.56), 106 live births, 10 spontaneous abortions, and 2 intrauterine deaths were recorded; the number of vaginal deliveries was 56, compared with a lower number (50) of Caesarean sections; the gestational age at delivery was 37.33 ± 2.32 weeks (mean ± SD). The number of infants born <37 weeks' gestation who were classified as “late preterm” was 23%, while the “early preterm” infants born <34 weeks were 7%.
In non-HCQ group, the mean maternal age was 35 years (SD 6.23); 155 live births, 6 spontaneous abortions and 1 intrauterine death were recorded; the number of vaginal deliveries was 77 and of Caesarean sections 78, thus accounting for 50% of cases; the mean week at delivery was 37.77 ± 2.35 (mean ± SD). The “late preterm” rate was 17%, while the “early preterm” rate was 5%.
All the obstetrical parameters investigated were very similar in the two groups.
Multivariate logistic regression confirmed the association between lower maternal age and the use of HCQ (Supplementary Table S1).
Neonatal outcomes
Regarding neonatal outcomes, in the HCQ group we observed a mean birth weight of 2,838.46 ± 591.48 g (mean ± SD); the rate of low birth weight (<2,500 g) was 16%. In comparison, the mean birth weight in the non-HCQ group was 2,964.14 g ± 563.22 (mean ± SD); the rate of low birth weight (<2,500 g) was 17%. Birth weight resulted significantly different between the two groups (Table 2) even if multivariate logistic regression did not confirm this association (Supplementary Table S1).
The percentage of infants with percentile <10th, classified as SGA (Small for Gestational Age, WHO Definition) (26, 27) was very similar in the two groups (7% vs. 10%); similar results were found for infants >90th percentile, classified as LGA (Large for Gestational Age, WHO definition) (27, 28), (2% vs. 4%) (Table 2).
The Apgar index below 5 at 1′ and at 5′ was not different in the two groups.
Pediatric outcomes
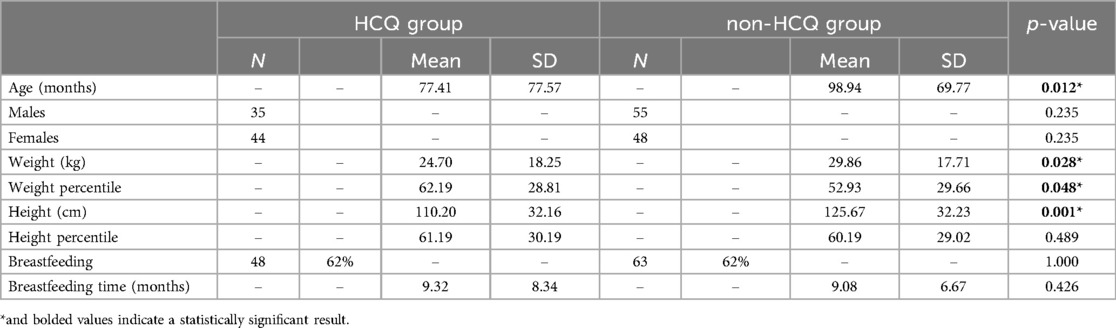
Pediatric outcomes are shown in Tables 3, 4.
Telephone follow-up questionnaires on children showed the following results: in HCQ group, data were collected from n = 79 children, 35 boys and 44 girls, while the non-HCQ group included n = 103 children, 55 boys and 48 girls. Mean age at follow-up resulted different between the two groups, being significantly higher in the non-HCQ group. We attributed this difference to the fact that HCQ use increased over time (as described in Figure 4), and the older children of the non-HCQ group came from those pregnancies where HCQ was not common.
Regarding the children's health status, in the HCQ group 15% of the cases suffered from recurrent upper airway diseases, 9% from gastro-intestinal diseases, 22% suffered from allergic diseases and 18% had atopic dermatitis; 1% were anaemic, 8% had cardiological problems, including 3 cases of Patent Foramen Ovale (PFO), 1 case of Congenital Heart Block (CHB), 2 cases of patency of Botallo's duct; 22% had visual impairment (4 cases of myopia, 1 of astigmatism, 4 of hypermetropia, and 8 cases of mixed disorders) while none of them had hearing deficits; 13% had neurodevelopmental disorders, 4% delayed or impaired speech development, and 5% congenital defects.
In the non-HCQ group, 24% of cases presented recurrent respiratory infections, 13% gastrointestinal diseases and 26% allergies; 20% had atopic dermatitis, 8% anaemia and 9% cardiological problems (7 cases of POF and 2 cases of electrical conduction abnormalities); 24% had visual impairment (9 cases of myopia, 6 cases of astigmatism, 3 cases of hypermetropia and 7 cases of mixed disorders) and none with hearing, while 9% had neurobehavioral developmental disorders and in particular 4% speech deficits; finally, 7% of them had congenital defects. In comparison, children in the non-HCQ group showed a slightly higher prevalence of all these disorders, but the difference did not reach statistical significance.
When the children were divided into three groups (distinguishing those with no pathologies from those with 1, 2, or ≥ 3 pathologies), 40% of the children in the HCQ group did not suffer from any pathology vs. 25% of the children in non-HCQ group. This difference was statistically significant with a p-value of 0.0368 (Table 4). Multivariate logistic regression confirmed the association between the mother's use of HCQ and the absence of disease in the children when correcting with other factors, possibly affecting the health of the baby, such as birth weight and birth weight percentile, week of delivery, modality of delivery, age of the mother at delivery (p = 0.008).
Discussion
We analysed retrospectively a large sample of pregnant women complicated by the presence of systemic autoimmune rheumatic diseases.
HCQ has been gradually implemented in our Center over the years, due to improved knowledge about the safety and benefits of its use in pregnancy and lactation (10, 18, 20). As expected, therefore, in our results we confirm that the births throughout the most recent years occurred in pregnancies with wider use of HCQ.
In addition, we observed that women with multiple comorbidities had a higher prevalence of HCQ use compared to women suffering from a single autoimmune disease.
Regarding pregnancy outcome, we found a high rate of live births, with a low occurrence of fetal losses. In part, this can be explained by the fact that women with early-stage miscarriages did not come under our observation and, therefore, do not appear in this case history; another possibility is that the management of systemic autoimmune diseases in pregnancy has been improved over the years. Most obstetric outcome indicators were very similar in the two groups, such as the week of delivery, the birth weight percentile, the rate of low birth weight, and the number of preterm births.
In our opinion, the similar pregnancy outcome in the two groups supports the beneficial effect of HCQ during gestation. In fact, literature data suggest that patients with multiple medical conditions have less favourable pregnancy outcomes (29). On this topic, a recent review by D'Ippolito et al. offers a thorough exploration of APS in pregnancy, delving into both well-established and novel mechanisms by which APS leads to obstetric complications. At the core of APS pathogenicity are aPL, which disrupt normal placental function by targeting trophoblast cells. This leads to placental thrombosis, inflammation, and impaired angiogenesis, causing adverse outcomes like recurrent miscarriages, fetal growth restriction, and preeclampsia (30). Moreover, further mechanisms are emerging for the identification of additional specificities and laboratory tests in those patients defined as seronegative (31).
Considering postnatal health, data on infants' follow-up was obtained through a telephone questionnaire. At follow-up, the children suffered from a substantial number of medical conditions: allergic diseases, atopic dermatitis, and visual impairment were the most represented. Our main finding was emphasized in the follow-up analysis, showing that infants in the HCQ group were disease-free at a significantly higher rate than those in the non-HCQ group. This observation suggests a potential role for HCQ in improving pregnancy outcomes and promoting infant health. Notably, this finding aligns with existing literature, which highlights HCQ benefits in improving live birth rates, reducing preterm births, and lowering the risk of neonatal complications such as cutaneous lupus and congenital heart block (32). These effects are due to the ability of HCQ to reduce inflammation, inhibit cytokine release, and modulate the immune environment in ways that may benefit fetal development and neonatal outcomes (32). The absence of an increased incidence of childhood infections in the HCQ group is particularly intriguing. This addresses an important safety concern regarding prenatal drug exposure and supports HCQ profile as a safe therapeutic option during pregnancy. By contributing to the growing body of evidence on HCQ exposure in pregnancy, our findings provide reassurance to clinicians and patients about its use in managing conditions such as SLE, Sjogren Syndrome and APS in pregnant women.
The present study has some limitations, as the absence of a control group from a general obstetric population limits our ability to identify the underlying causes of the high rate of observed medical conditions in infants. Moreover, incomplete data on NICU admissions prevented its inclusion in the statistical analysis, despite its potential value as a key indicator of neonatal health for future exploration. Additionally, the monocentric and retrospective design, along with the substantial number of cases lost to follow-up, further constrain the study's scope. Future research with a broader, multicentric and prospective approach is essential to generate more robust, long-term data. Moreover, further studies are needed to elucidate the mechanisms by which HCQ might influence fetal immune development and protect against disease manifestations in childhood.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical Committee of Fondazione Policlinico Universitario A. Gemelli, Rome. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
VM: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. FR: Data curation, Software, Validation, Writing – original draft. MD: Investigation, Visualization, Writing – review & editing. SB: Investigation, Writing – original draft. AS: Visualization, Writing – review & editing. AL: Resources, Supervision, Validation, Writing – review & editing. EG: Formal analysis, Writing – review & editing. SD: Conceptualization, Resources, Writing – review & editing. CG: Resources, Software, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GR declared a shared affiliation with the authors VM and CG to the handling editor at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/flupu.2025.1480867/full#supplementary-material
Abbreviations
HCQ, hydroxychloroquine; aPL, antiphospholipid antibodies; SLE, systemic erythematosus lupus; APS, antiphospholipid syndrome; RA, rheumatoid arthritis; pSjS, primary Sjogren's syndrome; SSc, systemic sclerosis; UCTD, undifferentiated connective tissue disease; AZA, azathioprine; VAPS, vascular antiphospholipid antibody syndrome; OAPS, obstetric antiphospholipid syndrome; PsA, psoriatic arthritis; LMWH, low molecular weight heparin; CDC, Centers for Disease Control and Prevention; cASA, low dose aspirin; SGA, small for gestational age; AGA, adequate gestational age; LGA, large for gestational age; W, weeks of gestation; PFO, patent foramen ovale; CHB, congenital heart block.
References
1. Nirk EL, Reggiori F, Mauthe M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol Med. (2020) 12(8):e12476. doi: 10.15252/emmm.202012476
2. Mekinian A, Vicaut E, Cohen J, Bornes M, Kayem G, Fain O. Évaluation du bénéfice de l’utilisation d’hydroxychloroquine pour l’obtention d’une grossesse à terme non compliquée en cas de syndrome des antiphospholipides primaire: étude de phase II multicentrique randomisée en double insu versus placebo, HYDROSAPL. Gynecol Obstet Fertil Senol. (2018) 46:598–604. doi: 10.1016/j.gofs.2018.06.008
3. Albert CR, Schlesinger WJ, Viall CA, Mulla MJ, Brosens JJ, Chamley LW, et al. Effect of hydroxychloroquine on antiphospholipid antibody-induced changes in first trimester trophoblast function. Am J Reprod Immunol. (2014) 71:154–64. doi: 10.1111/aji.12184
4. De Carolis S, Botta A, Salvi S, di Pasquo E, Del Sordo G, Garufi C, et al. Is there any role for the hydroxychloroquine (HCQ) in refractory obstetrical antiphospholipid syndrome (APS) treatment? Autoimmun Rev. (2015) 14:760–2. doi: 10.1016/j.autrev.2015.04.010
5. Kravvariti E, Koutsogianni A, Samoli E, Sfikakis PP, Tektonidou MG. The effect of hydroxychloroquine on thrombosis prevention and antiphospholipid antibody levels in primary antiphospholipid syndrome: a pilot open label randomized prospective study. Autoimmun Rev. (2020) 19(4):102491. doi: 10.1016/j.autrev.2020.102491
6. Raschi E, Borghi MO, Tedesco F, Meroni PL. Antiphospholipid syndrome pathogenesis in 2023: an update of new mechanisms or just a reconsideration of the old ones? Rheumatology (Oxford). (2024) 63(SI):SI4–13. doi: 10.1093/rheumatology/kead603
7. Capozzi A, Manganelli V, Riitano G, Caissutti D, Longo A, Garofalo T, et al. Advances in the pathophysiology of thrombosis in antiphospholipid syndrome: molecular mechanisms and signaling through lipid rafts. J Clin Med. (2023) 12(3):891. doi: 10.3390/jcm12030891
8. Truglia S, Capozzi A, Mancuso S, Manganelli V, Rapino L, Riitano G, et al. Relationship between gender differences and clinical outcome in patients with the antiphospholipid syndrome. Front Immunol. (2022) 13:932181. doi: 10.3389/fimmu.2022.932181
9. Flint J, Panchal S, Hurrell A, van de Venne M, Gayed M, Schreiber K, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids: table 1. Rheumatology. (2016) 55:1693–7. doi: 10.1093/rheumatology/kev404
10. Ramsey-Goldman R. Treatment of inflammatory rheumatic disorders in pregnancy: what are the safest treatment options? Drug Saf. (1998) 19:389–410. doi: 10.2165/00002018-199819050-00006
11. Parke AL. Antimalarial drugs, pregnancy and lactation. Lupus. (1993) 2 Suppl 1:S21–3. doi: 10.1177/0961203393002001061
12. Andersson NW, Skov L, Andersen JT. Fetal safety of chloroquine and hydroxychloroquine use during pregnancy: a nationwide cohort study. Rheumatology (Oxford). (2021) 60(5):2317–26. doi: 10.1093/rheumatology/keaa592
13. Koh JH, Ko HS, Kwok SK, Ju JH, Park SH. Hydroxychloroquine and pregnancy on lupus flares in Korean patients with systemic lupus erythematosus. Lupus. (2015) 24:210–7. doi: 10.1177/0961203314555352
14. Izmirly PM, Costedoat-Chalumeau N, Pisoni CN, Khamashta MA, Kim MY, Saxena A, et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/ro-antibody- associated cardiac manifestations of neonatal lupus. Circulation. (2012) 126:76–82. doi: 10.1161/CIRCULATIONAHA.111.089268
15. Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, et al. American College of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol. (2020) 72:529–56. doi: 10.1002/art.41191
16. Hu Z, Gao R, Huang W, Wang H, Qin L. Effect of hydroxychloroquine on lupus activity, preeclampsia and intrauterine growth restriction in pregnant women with systemic lupus erythematosus and/or antiphospholipid syndrome: a systematic review and meta-analysis. J Clin Med. (2023) 12(2):485. doi: 10.3390/jcm12020485
17. Kroese SJ, de Hair MJH, Limper M, Lely AT, van Laar JM, Derksen RHWM, et al. Hydroxychloroquine use in lupus patients during pregnancy is associated with longer pregnancy duration in preterm births. J Immunol Res. (2017) 2017:2810202. doi: 10.1155/2017/2810202
18. Seo MR, Chae J, Kim YM, Cha HS, Choi SJ, Oh S, et al. Hydroxychlroquine treatment during pregnancy in lupus patients is associated with lower risk of preeclampsia. Lupus. (2019) 28:722–30. doi: 10.1177/0961203319843343
19. Ye S, Zhao X, Liu Y, Ma Y, Wang Y, Zhao J. The use of hydroxychloroquine in pregnancy and its effect on perinatal outcomes in a population with autoimmune abnormalities. Clin Rheumatol. (2023) 42(4):1137–50. doi: 10.1007/s10067-022-06462-y
20. Hooper A, Bacal V, Bedaiwy MA. Does adding hydroxychloroquine to empiric treatment improve the live birth rate in refractory obstetrical antiphospholipid syndrome? A systematic review. Am J Reprod Immunol. (2023) 90(3):e13761. doi: 10.1111/aji.13761
21. Chambers CD, Johnson DL, Xu R, Luo Y, Felix R, Fine M, et al. Birth outcomes in women who have taken hydroxycholoroquine during pregnancy: a prospective cohort study. Arthritis Rheumatol. (2022) 74(4):711–24. doi: 10.1002/art.42015
22. Reynolds JA, Gayed M, Khamashta MA, Leone F, Toescu V, Bruce IN, et al. Outcomes of children born to mothers with systemic lupus erythematosus exposed to hydroxychloroquine or azathioprine. Rheumatology (Oxford). (2023) 62(3):1124–35. doi: 10.1093/rheumatology/keac372
23. Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF, American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. (2016) 123(6):1386–94. doi: 10.1016/j.ophtha.2016.01.058
24. Costedoat-Chalumeau N, Amoura Z, Huong DLT, Lechat P, Piette J-C. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases. Review of the literature. Autoimmun Rev. (2005) 4:111–5. doi: 10.1016/j.autrev.2004.11.009
25. Bertino E, Spada E, Occhi L, Coscia A, Giuliani F, Gagliardi L, et al. Neonatal anthropometric charts: the Italian neonatal study compared with other European studies. J Pediatr Gastroenterol Nutr. (2010) 51:353–61. doi: 10.1097/MPG.0b013e3181da213e
26. Chou JH, Roumiantsev S, Singh R. Peditools electronic growth chart calculators: applications in clinical care, research, and quality improvement. J Med Internet Res. (2020) 22:e16204. doi: 10.2196/16204
27. de Onis M, Habicht JP. Anthropometric reference data for international use: recommendations from a world health organization expert committee. Am J Clin Nutr. (1996) 64(4):650–8. doi: 10.1093/ajcn/64.4.650
28. WHO Expert Committee. Physical status: the use and interpretation of anthropometry. Report of a WHO expert committee. World Health Organ Tech Rep Ser. (1995) 854:1–452.8594834
29. Marziale A, Bettacchioli E, Picart G, Nafai S, Galinat H, Meroni PL, et al. Antiphospholipid autoantibody detection is important in all patients with systemic autoimmune diseases. J Autoimmun. (2020) 115:102524. doi: 10.1016/j.jaut.2020.102524
30. D'Ippolito S, Barbaro G, Paciullo C, Tersigni C, Scambia G, Di Simone N. Antiphospholipid syndrome in pregnancy: new and old pathogenetic mechanisms. Int J Mol Sci. (2023) 24(4):3195. doi: 10.3390/ijms24043195
31. Capozzi A, Riitano G, Mancuso S, Recalchi S, Manganelli V, Garofalo T, et al. Anti-vimentin/cardiolipin IgA in the anti-phospholipid syndrome: a new tool for “seronegative” diagnosis. Clin Exp Immunol. (2021) 205(3):326–32. doi: 10.1111/cei.13633
Keywords: hydroxychloroquine, postnatal health, pregnancy, autoimmunity, autoimmune diseases
Citation: Matys V, Rizzo F, De Carolis MP, Barresi S, Serio AM, Lanzone A, Garufi E, De Carolis S and Garufi C (2025) Postnatal health of infants born to mothers with autoimmune diseases when treated with hydroxychloroquine. Front. Lupus 3:1480867. doi: 10.3389/flupu.2025.1480867
Received: 14 August 2024; Accepted: 9 January 2025;
Published: 28 January 2025.
Edited by:
Laura Andreoli, University of Brescia, ItalyReviewed by:
Guilherme Ramires De Jesús, Rio de Janeiro State University, BrazilGloria Riitano, Sapienza University of Rome, Italy
Samar Al-Emadi, Hamad Medical Corporation, Qatar
Copyright: © 2025 Matys, Rizzo, De Carolis, Barresi, Serio, Lanzone, Garufi, De Carolis and Garufi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara De Carolis, c2FyYS5kZWNhcm9saXNAdW5pY2F0dC5pdA==
 Viviana Matys
Viviana Matys Francesca Rizzo
Francesca Rizzo Maria Pia De Carolis2
Maria Pia De Carolis2 Antonio Lanzone
Antonio Lanzone Sara De Carolis
Sara De Carolis Cristina Garufi
Cristina Garufi