- 1Rheumatology Research Group, Centre for Inflammatory Diseases, School of Clinical Sciences, Monash University, Clayton, VIC, Australia
- 2Rheumatology Department, Monash Health Hospital, Clayton, VIC, Australia
- 3Division of Rheumatology, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University Hospital, Chiang Mai, Thailand
- 4Division of Allergy, Immunology and Rheumatology, Taichung Veterans General Hospital, Taichung, Taiwan
- 5Rheumatology Division, National University Hospital, Singapore, Singapore
- 6Department of Medicine, Woodlands Health, Singapore, Singapore
- 7Division of Rheumatology, Department of Internal Medicine, Faculty of Medicine, Padjadjaran University/Hasan Sadikin General Hospital, Bandung, Indonesia
- 8Division of Rheumatology & Clinical Immunology, Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Pok Fu Lam, Hong Kong SAR, China
- 9Department of Rheumatology, Allergy and Immunology, Chang Gung Memorial Hospital, Taipei, Taiwan
- 10Department of Rheumatology, Allergy and Immunology, Chang Gung Memorial Hospital, Keelung, China
- 11Section of Rheumatology, University of Santo Tomas Hospital, Manila, Philippines
- 12Department of Rheumatology and Immunology, People’s Hospital Peking University Health Science Center, Beijing, China
- 13Department of Medicine, University of Malaya Medical Centre, Kuala Lumpur, Malaysia
- 14Institute of Rheumatology, Tokyo Women’s Medical University, Tokyo, Japan
- 15Department of Rheumatology, Sanno Hospital, Tokyo, Japan
- 16Rheumatology and Immunology Department, Peking University First Hospital, Beijing, China
- 17Department of Rheumatology, St Vincent’s Hospital, Melbourne, VIC, Australia
- 18Department of Nephrology, Teaching Hospital Kandy, Kandy, Sri Lanka
- 19Department of Rheumatology, Allergy & Immunology, Tan Tock Seng Hospital, Tan Tock Seng, Singapore
- 20Department of Internal Medicine, Keio University, Tokyo, Japan
- 21Department of Rheumatology and Applied Immunology, Saitama Medical University, Saitama, Japan
- 22Department of Rheumatology, Hanyang University Institute for Rheumatology Research and Hanyang Institute of Bioscience and Biotechnology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Republic of Korea
- 23Rheumatology Department, Liverpool Hospital, Liverpool, NSW, Australia
- 24Department of Medicine, University of Sydney, Sydney, NSW, Australia
- 25Department of Rheumatology, Flinders Medical Centre, Bedford Park, SA, Australia
- 26Department of Rheumatology, North Shore Hospital, Waitemata Health New Zealand, Auckland, New Zealand
- 27Asia Arthritis and Rheumatology Centre, Singapore, Singapore
- 28Department of Rheumatology, Greenlane Clinical Centre, Auckland, New Zealand
- 29Department of Rheumatology, Middlemore Hospital, Auckland, New Zealand
- 30The First Department of Internal Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan
- 31Department of Paediatrics, University of the Philippines, Manila, Philippines
- 32School of Public Health, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
Introduction: The guidelines for management of patients with systemic lupus erythematosus (SLE) recommend the use of anti-malarial (AM) drugs [commonly hydroxychloroquine (HCQ)] in all patients, unless contraindicated. We evaluated the prevalence of AM use in patients with SLE in countries across the Asia Pacific region.
Methods: We used data from the Asia Pacific Lupus Collaboration (APLC) cohort, collected prospectively from SLE patients meeting ACR or/and SLICC criteria, between 2013 and 2020. Demographic factors were collected at enrolment; disease activity indicators (SLEDAI-2K, PGA, SFI) and medication (glucocorticoids (GC), immunosuppressants (IS) and AM) details were captured at enrolment and at routine visits, and organ damage was assessed at enrolment and at annual visits using SLICC/ACR Damage Index. We examined medication use in relation to clinical and serological activity, defined based on SLEDAI-2K.
Results: We analyzed 4,086 patients and 41,653 visits of data; 3,222 (79%) patients used AM at least once during observation (AM-ever users), but this proportion varied significantly between countries (31%–95%). Overall, the total number of visits with AM use was 27,474 (66%). AM-never users were older and had lower disease activity at study enrolment when compared with AM-ever users. AM-ever users had lower GC and IS exposure; experienced fewer severe flares, and less organ damage.
Discussion: AM use was suboptimal and varied significantly across countries, highlighting disparities between current practice and SLE management guidelines. This study further reiterates that patients who used AM during the study period had lower exposure to GC and IS and experienced fewer severe flares and organ damage.
Introduction
Anti-malarial (AM) therapy, especially with hydroxychloroquine (HCQ), plays a significant role in the management of systemic lupus erythematosus (SLE). AM have a multifaceted mechanism of action including inhibition of Toll-like receptors (TLRs); inhibition of cytokine production, disruption of antigen presentation and autoantibody production, lysosomal alkalization and alteration of signaling pathways (1–4). These actions contribute to the drug's efficacy in active disease control including reduction in flares, steroid-sparing effects, prevention of organ damage, and mitigation of thrombotic effects of anti-phospholipid antibodies (2, 4). Antithrombotic effects of HCQ are particularly beneficial for patients with SLE who have an increased risk of thrombosis, and HCQ associated lipid profile improvement can contribute to overall cardiovascular health. Regular use of HCQ has also shown to be associated with improved survival in patients with SLE (5). Due to these numerous benefits, treatment with HCQ is recommended for all patients with SLE without contraindications in all major SLE management guidelines. The recently published Asia-Pacific League of Associations for Rheumatology (APLAR) consensus statements on the management of SLE recommends the routine use of HCQ for all Asian people with SLE unless contraindicated (6), with a maintenance dose of HCQ not to exceed 5 mg/kg per day of actual bodyweight. Similarly, the European League Against Rheumatism (EULAR) also recommends HCQ in all SLE patients at a target dose of 5 mg/kg/day, considering the individual's risk for flares and retinal toxicity (7). Overall, AM, HCQ in particular, is considered as a first-line therapy for SLE due to its efficacy in reducing disease activity and preventing flares. The risk of HCQ retinopathy, a potential adverse effect, is low and can be mitigated through regular monitoring (8).
Despite this, the rates of AM use among cohorts enrolled in SLE clinical trials were as low as 66%–74% (9, 10), suggesting that adherence to these guidelines is sub-optimal. The prevalence of AM use in SLE is likely to vary globally, but it is generally considered high especially in the United States of America and Europe (11–13); however, local use can vary depending on healthcare infrastructure, access to medications, and adherence to treatment guidelines within each country (14). In Asia, the prevalence of AM use is reported to be more variable (15, 16) and limited to few countries. In this study, we addressed this knowledge gap by examining the prevalence of AM use across centers in 13 Asia-Pacific countries participating in a large, prospective observational study.
Patients and methods
Patients
We used patients enrolled in the Asia Pacific Lupus Collaboration (APLC) patient cohort (NCT03138941), prospectively followed between 2013 and 2020, to conduct this study (17). Participants were recruited from centers in Australia, China, Hong Kong, Indonesia, Japan, Korea, Malaysia, New Zealand, Philippines, Singapore, Sri Lanka, Taiwan, and Thailand and received standard of care treatment. All APLC patients were consenting adults who met either the 1997 American College of Rheumatology (ACR) Modified Classification Criteria for SLE (18) or the Systemic Lupus International Collaborating Clinics (SLICC) 2012 Classification Criteria (19). Each APLC site has obtained local ethics approval for patient recruitment, and to contribute to the centralized APLC dataset. Individual centers obtain valid informed consent in accordance with local authority regarding ethical conduct of human research. Monash University Human Research Ethics Committee has approved to store the central dataset in Monash University's secure servers and to perform analyses using collective data.
Variables
Patient demographics were captured at enrolment, while data on medications, disease activity, and pathology results were captured at enrolment as well as each 3–6 monthly routine visit. Medications were captured based on use at the time of each visit:
• Glucocorticoids (GC): prednisolone (PNL) or equivalent
• Anti-malarial drugs (AM): hydroxychloroquine (HCQ), chloroquine (CQ)
• Immunosuppressants (IS): methotrexate (MTX), azathioprine (AZA), mycophenolate mofetil (MMF), mycophenolic acid (MPA), leflunomide (LEF), ciclosporin (CyA), tacrolimus (TAC); cyclophosphamide (CYC)
• Biologics (in preceding 6 months): rituximab (RTX), belimumab (BEL)
Disease activity was measured using the SLE Disease Activity Index [SLEDAI]-2000 (2K) (20, 21), and Physician Global Assessment of activity (PGA) on a scale of 0 (no activity) to 3 (maximum activity) (22). Disease flares were captured using the SELENA flare index (SFI) (23, 24). Serological and clinical disease activity were defined based on the respective SLEDAI-2K domains. In addition, irreversible organ damage was captured using the SLICC-ACR Damage Index (SDI) (25), measured at recruitment and at annual visits.
Statistical analysis
Statistical analysis was performed using Stata version 18 (StataCorp, College Station, Texas, USA). Patient characteristics, including medication details were described using summary statistics for the whole study group as well as stratified by ever- vs. never- use of AM (ever: used at least once during the study observation period). Medication details were further explored at a visit level. Continuous variables were expressed as median [inter-quartile range (IQR)] and compared using Wilcoxon rank sum tests, while categorical variables were described as frequency (%) and compared using Chi-squared tests. Prevalence of AM use was estimated for each country at both the enrolment (first) visit and last visit of the study period.
Results
Patient characteristics at enrolment
Data from 4,086 patients and 41,653 visits with complete SLEDAI-2K descriptors, who were followed-up for a median [IQR] of 2.5 [1.0, 5.1] years, were analysed. Patient characteristics of the study cohort are shown in Table 1. In brief, 92% of study participants were female and 89% were of Asian ethnicity. The median [IQR] age at study enrolment and diagnosis was 39 years [30, 50] and 29 years [21, 39], respectively. Approximately 8% of patients had a family history of SLE; ∼5% were smokers at enrolment, and ∼53% had tertiary level education. According to the gross domestic product based on purchasing power parity per capita [GDP(PPP)] in 2020, Australia, Singapore, and Taiwan were grouped as countries with GDP(PPP) ≥international dollars (Int$) 50,000; Japan, Korea, Malaysia and New Zealand were grouped in “<Int$50,000 & ≥Int$20,000” category; and China, Indonesia, Philippines, Sri Lanka and Thailand were grouped in GDP(PPP) <Int$20,000 category. Approximately 35% of the study cohort was recruited from countries with GDP (PPP) <Int$20,000 (Table 1). A breakdown of patient characteristics by country is presented in Supplementary Table S1.
Approximately 79% of patients were on GC with a median [IQR] daily dose of 5 [2.1, 10] mg; 73% on AM, and nearly 60% were on IS at enrolment visit. Only about 6% (177/2,965) of the AM users were receiving CQ, while the remainder were taking HCQ (Table 1). CQ was predominantly used in Indonesia, followed by Thailand. A small number of patients in China, Philippines, and Singapore also received CQ in <10 visits.
Regarding disease activity at enrolment, the median SLEDAI-2K score and PGA were 4 [2, 6] and 0.5 [0.1, 1.0], respectively. Approximately 62% of patients had serological activity; about half of these (1,260/2,544) had clinical activity in addition to serological activity. An additional 14% of the study cohort (n = 575) had clinical activity only. Clinical activity was most frequently observed in the renal domain, followed by cutaneous, musculoskeletal and hematological systems (Table 1). About 12% of patients experienced flares and ∼38% had irreversible organ damage. 3% (n = 116) had scores in the SDI domain of retinal change/optic atrophy at enrolment; SDI scoring does not separate these two pathologies and does not specifically capture AM-related retinal toxicity.
Clinical characteristics during the study observation period
A total of 3,222 (79%) of patients used AM, predominantly HCQ [n = 3,053 (74%)], at least once during the study observation period (AM-ever). Likewise, 85% and 70% had used GC and IS, respectively, at least once.
At the visit level, study participants were taking AM in 66% of visits [n = 27,474; HCQ in 62% of visits (n = 25,692)]; PNL in 78% of visits (n = 32,632), and IS in 60% of visits (n = 25,098) (Figure 1). HCQ users were found to have used this medication in almost every visit (median [IQR]%-time of HCQ use under observation period was = 100% [100%, 100%]) On average, patients received 300 mg of HCQ per day (median [IQR] dose = 300 [200, 400] mg/day). Few patients received HCQ dose >400 mg/day in <0.2% of visits (n = 48). Furthermore, AM use was least frequent in visits with clinical activity only (55%); in these visits, patients’ median [IQR] HCQ dose was 200 [200, 400] mg/day. During visits with serological activity with or without clinical activity, patients received an average daily dose of 300 mg [IQR: 200, 400] HCQ. The APLC registry did not capture body weight data; therefore, we were unable to determine the AM dose as mg/kg.
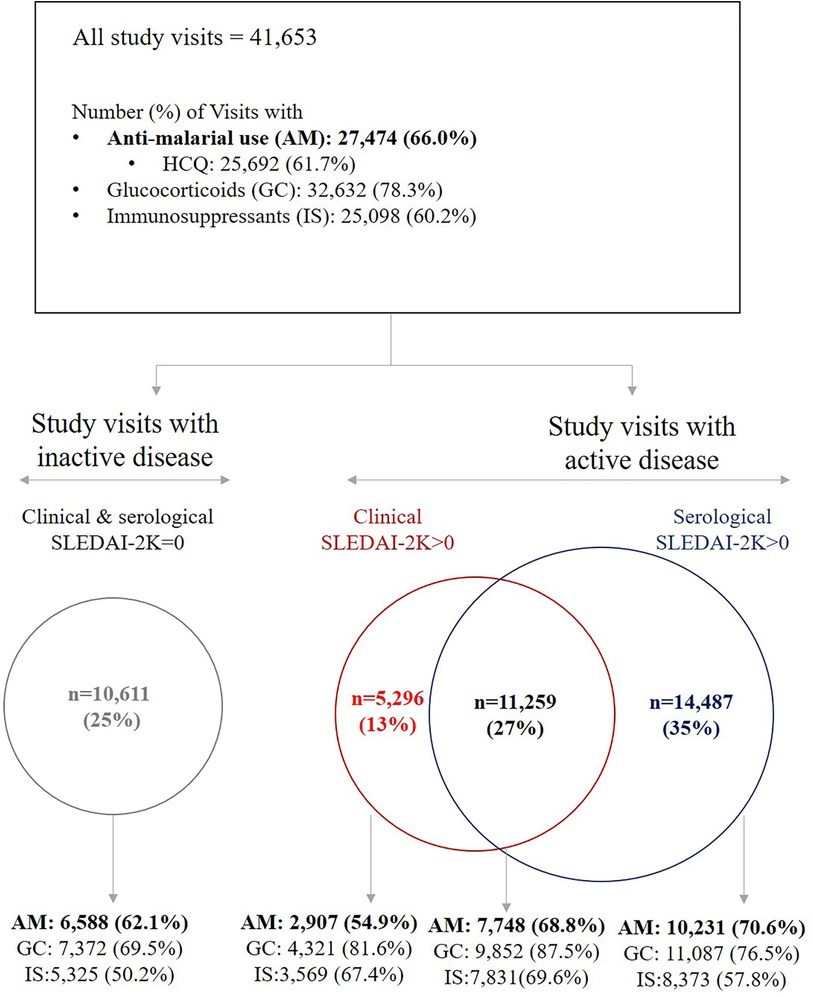
Figure 1. Medication use across study visits, stratified by clinical and serological disease activity, defined based on SLE disease activity Index (SLEDAI-2K). AM: anti-malarials, GC, glucocorticoids; HCQ, hydroxychloroquine; IS, immunosuppressants.
In terms of disease activity, the median [IQR] time-adjusted mean (TAM) SLEDAI-2K during the study observation period (also referred to as AMS) (26) was 3.0 [1.5, 4.7] while the median [IQR] TAM PGA was 0.4 [0.2, 0.7]. Patients experienced serological activity only in 35% of visits (n = 14,487); clinical activity only in 13% of visits (n = 5,296), and both clinical and serological activity in 27% of visits (n = 11,259). Patients had neither clinical nor serological activity (i.e., SLEDAI-2K = 0) in 25% of study visits (n = 10,611) (Figure 1). Approximately 48% had organ damage present at the end of study period. Fifty patients (1.4%) experienced new damage in the SDI retinal change/optic atrophy domain (Table 1).
Comparison of patient characteristics between AM-ever vs. AM-never patients
We compared patient characteristics of AM-ever patients to those who never used AM during study period [AM-never; n = 864 (21%)] (Tables 1). AM-never patients were older at SLE diagnosis and at study enrolment, and correspondingly had longer disease duration. Proportions of females and Asian patients were significantly higher in the AM-never group whereas the proportion of patients with tertiary education was significantly lower. Significantly higher proportion of patients in the AM-never group was from countries with GDP (PPP) <20,000 and received more GC and IS at enrolment. Although at enrolment the median SLEDAI-2K in the AM-never group was significantly lower than the AM-ever group (2[0, 6] vs. 4 [2, 6], p = 0.011), AM-ever patients were more likely to be experiencing a mild-to-moderate flare at enrolment.
During the study observation period the TAM SLEDAI-2K (AMS) was similar between the two groups but AM-never patients had higher exposure to GCs and IS medications. AM-never patients were also found to experience more severe flares and had higher mortality (Table 1). Although the AM-never group had a higher rate of organ damage present at enrolment, no statistically significant difference in organ damage accrual over time was observed between the two groups (Table 1).
Patient characteristics of HCQ users with ≥6 months of sustained use
Of the 3,053 HCQ-ever users, approximately 82% (n = 2,509) of patients had used HCQ for a sustained period of 6 months or more (≥6 m HCQ-users). Patients with <6 months of sustained HCQ use were younger and had significantly shorter study durations (median [IQR]: 0.2 [0, 0.7] years) (Supplementary Table S2). This indicates that they were recently enrolled and, therefore, had not been in the cohort for an extended period. Nearly 50% of these patients with <6 months of sustained HCQ were from countries with low GDP (PPP). While they experienced fewer disease flares and had less organ damage during the study period, their disease activity indicators such as TAM-SLEDAI (AMS) and TAM-PGA were similar to ≥6 m HCQ-users (Supplementary Table S2).
Prevalence of AM use by country
We further explored AM (-ever) use across the APLC participating countries (Figure 2; Supplementary Table S3). The prevalence of AM use significantly varied among countries. While countries such as Singapore, New Zealand, Philippines, Australia, China and South Korea had high prevalence of AM use (>85% of patients), other countries including Japan, Thailand and Indonesia had AM use in <50% of patients (Figure 2). Additionally, prevalence of GC used varied between 55% and 95%, and similarly, IS use ranged from 41% to 84% across countries (Figure 2a); notably the three countries with lowest AM use had highest use of GC, while countries with the lowest GC use all had >80% AM use. The prevalence of AM use significantly varied according to GDP(PPP) categories (Table 1). In terms of AM doses, while the median daily dose of HCQ in the overall cohort was 300 mg, it varied between 200 and 400 mg across countries, with a tendency toward lower doses in the majority of countries (Supplementary Table S3). Of note, HCQ was only approved for use in Japan in 2015, Thailand in 2004 and Indonesia in 2017, whereas approval in most other countries in the region predated the commencement of the APLC cohort.
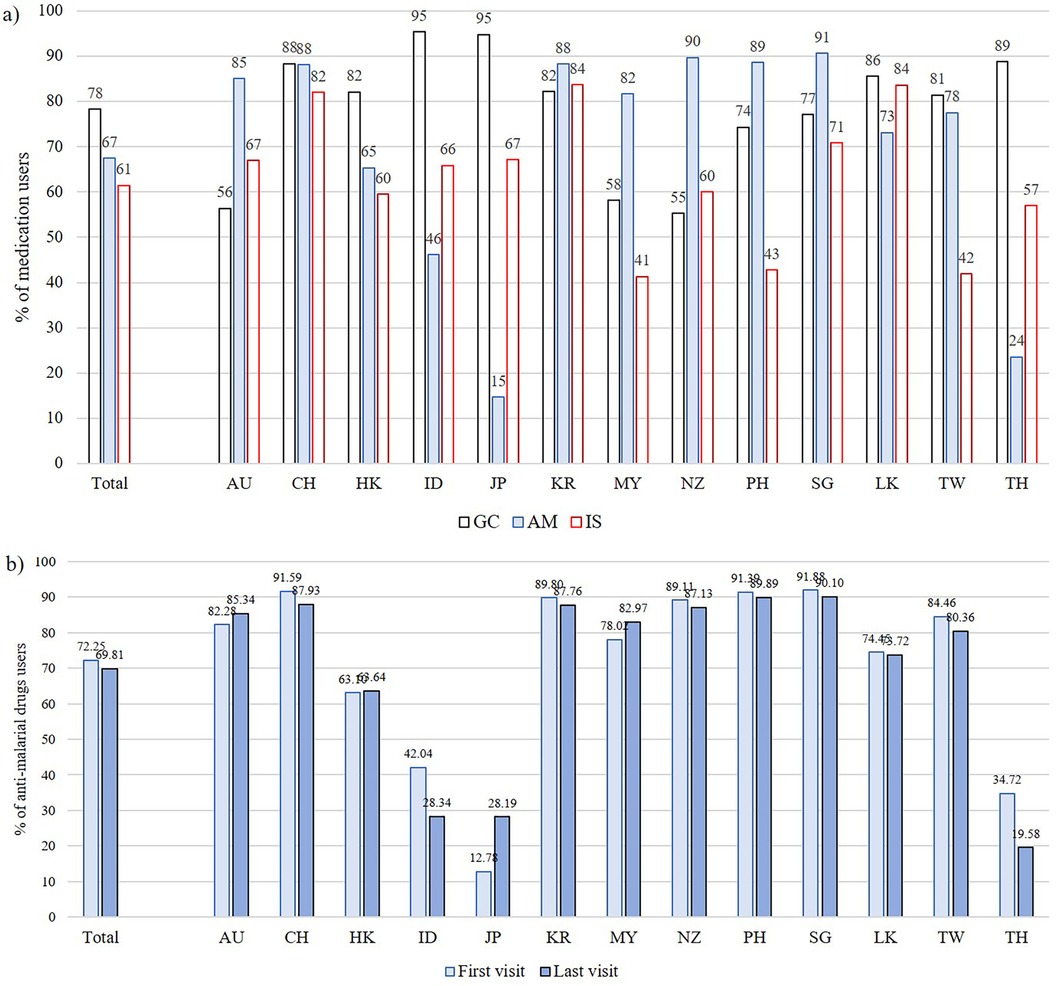
Figure 2. (a) Proportions of glucocorticoid (GC), anti-malarials (AM) and immunosuppressants (IS) use on a per-patient basis (-ever users), stratified by country; (b) proportions of AM use at first and last visit, stratified by country. AU, Australia; CH, China; HK, Hong Kong; ID, Indonesia; JP, Japan; KR, South Korea; MY, Malaysia; NZ, New Zealand; PH, the Philippines; SG, Singapore; LK, Sri Lanka; TW, Taiwan; TH, Thailand.
To examine changes in AM use over time, we compared AM use at the last visit with the enrolment (first) visit (Figure 2b). Proportions of AM use slightly dropped or remained similar in many countries, except for Japan where the proportion of users changed from 12.7% at first visit to 28.2% at last visit, probably reflecting the relatively recent approval of HCQ. The reduction in AM users in Thailand and Indonesia was notable than the other countries; in Indonesia, AM use reduced from 42% to 28.3%, while in Thailand, it reduced from 34.7% to 19.6%. (Figure 2; Supplementary Table S3). Table 2 summarizes the number of patients with retinal changes or optic atrophy, as reported in SDI, at first and last assessments by country. Data in this table are limited to patients with ≥2 SDI surveys. Thailand and Indonesia, notably the countries with the greatest reductions in AM use as well as the highest use of CQ, had the highest proportions of patients with retinal changes/optic atrophy at the end of the study observation period (17.7% and 8.6%, respectively).

Table 2. Percentages of patients with positive scores in the retinal change or optic atrophy SDI domain at first and last visits by country.
Discussion
Despite recommendations for the use of AM therapy in the management of SLE in all patients with SLE unless contraindicated, we observed the use of these drugs in the Asia Pacific region is suboptimal, with significant variation across the 13 countries participating in the Asia Pacific Lupus Collaboration patient cohort. About one-fifth of this study cohort never received AM as part of their standard of care during the observation period. Patients who never received AM were older; had lower disease activity at enrolment, and were predominantly from less affluent countries. While the use of AM, especially HCQ, could be limited for some patients due to risk of retinal toxicity, <5% of the overall study cohort had any indication of possible retinal damage recorded using the SDI, although there were some differences across countries. Thailand and Indonesia had comparatively higher propotions of patients with retinal damage, lower proportions with AM use, and higher rates of CQ use compared to HCQ in other countries. The prevalence of AM use in Japan was also low, consistent with other observational studies from Japan (15) and likely related to the late approval of HCQ in Japan (2015) and historical factors related to CQ withdrawal in 1974 (27).
The reasons for sub-optimal use of AM therapy can be multi-factorial. As mentioned above, concerns about potential retinal toxicity associated with HCQ could have been a reason for reluctance in prescribing HCQ. In addition, patients might have discontinued or refused to start these drugs due to fear of vision issues, with patient preference being listed as a prevalent reason for HCQ discontinuation in a recent multicenter study (28). Other health concerns such as gastrointestinal disturbances, skin rashes and muscle weakness could contribute to non-adherence to HCQ as well. Lower prevalence of AM drug use in some lower GDP (PPP) countries highlight that use of these drugs, especially HCQ, could be limited due to cost, access to expert prescribers or healthcare infrastructure.
Current guidelines recommend HCQ dosing to be based on body weight, not exceeding 5 mg/kg/day (6, 7). A dose greater than this can lead to more than double the risk of retinal toxicity in patients with SLE (29). Very few patients received HCQ dose greater than 400 mg/day. This is in contrast to the observations reported from Europe where higher than recommended dosages of HCQ were prescribed to more than one-third of patients with lupus (12). However, the use of HCQ at less than the recommended dose can increase the risk of lupus flares (30). In this study, we observed that a significantly higher proportion of patients who never received AM experienced severe flares when compared with patients who received AM, and non-use of AM was also associated with higher GC and IS exposure and a higher prevalance of organ damage at study completion. While this is a descriptive study, we believe that these observations indirectly support the reported association of HCQ use with reduced flares and improved outcomes.
AM therapy is intended for long-term use to maintain disease control, even when symptoms are in remission. There is new evidence that HCQ should be given at a maintenace dose even when patients attain remission, as both reduction and discontinuation, compared to maintenance dose, can significantly increase the risk of flares (31). It was encouraging to observe that among HCQ-ever users, this medication was prescribed in almost every visit given the reported benefits of HCQ use in Asian patients with SLE such as protection against organ damage (32) and mortality (33).
There are some limitations to this study. Prescription of a medication does not guarantee patient adherence to taking the medication, and patient adherence to medications was not captured. There have been suggestions to monitor HCQ blood levels routinely in the management of SLE, as this has been shown to reduce the risk of active lupus and flares (34). It is important to note that only few centers from each country contributed data to the cohort, and data on per-country AM use therefore may not accurately represent the standard of care in the respective countries. While the study observation period spanned 2013 to 2020, not all centers joined at the same time. The impact of COVID-19 on HCQ use documented in this study is difficult to assess. Data pooling for this analysis was performed around the time the pandemic was emerging. During the COVID-19 pandemic, HCQ was widely discussed as a treatment for COVID-19, leading to shortages and affecting its availability for SLE patients (35). It is possible that the publicity around HCQ for COVID-19 may have led to confusion and conflicting information, affecting perceptions of its use in SLE. Strengths of the study include its size and the prospective data collection.
In summary, this study provides evidence that the use of AM drugs in SLE management in the Asia Pacific region is highly variable among countries, and is sub-optimal. This is concerning given the parallel findings of reduced rates of severe flare and of GC and IS use in patients using AM, and prior evidence on protective effects of HCQ against organ damage and mortality in SLE using Asian patient cohorts (32, 33). Our findings underscore the need to identify and implement strategies such as patient and physician education, patient adherence and improving healthcare access, in order to achieve optimal use of HCQ in SLE.
Data availability statement
The data underlying this article cannot be publicly shared due to the strict protocols and procedures outlined in the Asia Pacific Lupus Collaboration (APLC) Data Access Policy to protect patients’ privacy and to maintain data security and ethical principles. Access to APLC pooled data is subject to the specific guidelines outlined in the APLC Data Access Policy (available on request). The APLC welcomes requests for aggregate (summary) data or to perform analyses of new research questions, and such requests can be submitted to the APLC Steering Committee via the APLC Project Manager via the APLC website (www.asiapacificlupus.com).
Ethics statement
Each APLC site has obtained local ethics approval for patient recruitment, and to contribute de-identifiable data to the centralized APLC dataset. Individual centres obtain valid informed consent in accordance with local authority regarding ethical conduct of human research. Monash University Human Research Ethics Committee has approved to store the central dataset in Monash University's secure servers and to perform analyses using collective data. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RK-R: Writing – original draft, Conceptualization, Data curation, Formal Analysis, Writing – review & editing. AH: Data curation, Writing – review & editing. WL: Data curation, Writing – review & editing. Y-HC: Data curation, Writing – review & editing. JC: Data curation, Writing – review & editing. Al: Data curation, Writing – review & editing. LH: Data curation, Writing – review & editing. SC: Data curation, Writing – review & editing. SL: Data curation, Writing – review & editing. Y-JJW: Data curation, Writing – review & editing. SN: Data curation, Writing – review & editing. LZ: Data curation, Writing – review & editing. ZL: Data curation, Writing – review & editing. HY: Data curation, Writing – review & editing. SS: Data curation, Writing – review & editing. YK: Data curation, Writing – review & editing. MH: Data curation, Writing – review & editing. YH: Data curation, Writing – review & editing. ZZ: Data curation, Writing – review & editing. BB: Data curation, Writing – review & editing. MC: Data curation, Writing – review & editing. JK: Data curation, Writing – review & editing. TT: Data curation, Writing – review & editing. SO: Data curation, Writing – review & editing. S-CB: Data curation, Writing – review & editing. SO’N: Data curation, Writing – review & editing. FG: Data curation, Writing – review & editing. KN: Data curation, Writing – review & editing. AL: Writing – review & editing, Data curation. NT: Writing – review & editing, Data curation. SK: Writing – review & editing, Data curation. NO: Writing – review & editing, Data curation. MT: Writing – review & editing, Data curation. CT: Writing – review & editing, Data curation. YT: Writing – review & editing, Data curation. CL: Writing – review & editing, Data curation. VG: Writing – review & editing, Data curation. MN: Writing – review & editing, Data curation. EM: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The APLC received funding from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, EMD Serono, GSK, Janssen, and UCB Biopharma in support of its research activities such as data collection. S-CB is supported in part by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1A6A1A03038899).
Acknowledgments
The authors extend their gratitude to the patients enrolled in the Asia Pacific Lupus Collaboration cohort and to all the research support staff involved in data collection and entry.
Conflict of interest
RK-R has received grants from GSK and Novartis. AH has received a research grant from AstraZeneca, consulting fees from EUSA Pharma (UK), GSK and UCB Australia, and speaker fees/honoraria from AbbVie, Eli Lilly, Janssen, Limbic, Moose Republic and Novartis. ZL has received consulting fees and royalties from Abbott, AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, GSK, Janssen, MSD, Novartis, Pfizer, Roche and UCB Pharma. SN has received consulting fees from AstraZeneca, Biogen, Boehringer Ingelheim and Idorsia, and payment for lectures from AstraZeneca, Boehringer Ingelheim, GSK and Roche. MH has received institutional research grants from GSK KK and Novartis, and honoraria for lectures from AstraZeneca KK and Astellas Pharma. YK has received payment/honoraria from Asahi Kasei Pharma, AstraZeneca KK., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd, GSK KK., Janssen Pharmaceutical KK., Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc. and Sanofi KK ZZ has received payment/honoraria from AbbVie, AstraZeneca KK., Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, GSK, Novartis, Pfizer, Roche, Sanofi, Janssen, UCB Pharma, and has participated in advisory boards for Beigene Ltd. FG was a Director on the Board of the Australian Rheumatology Association at the time of the study. MN has received an Investigator Grant from the National Health and Medical Research Council of Australia (NHMRC GNT1176538), research grants from Boehringer Ingelheim and Janssen, consulting fees from AstraZeneca and GSK; honoraria for presentations from AstraZeneca, Boehringer Ingelheim and GSK, and support for conference attendance from Boehringer Ingelheim. EM has received consulting fees from AbbVie, AstraZeneca, Biogen, Bristol Myers Squibb, Genentech, Gilead, Janssen, Novartis, and EMD Serono.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/flupu.2024.1461739/full#supplementary-material
References
1. Peng-Cheng L, Meng-Na L, Jian-Bin L, Shu-Jiao Y, Wu R. Advancements on the impact of hydroxychloroquine in systemic lupus erythematosus. Heliyon. (2024) 10(9):e30393. doi: 10.1016/j.heliyon.2024.e30393
2. Ponticelli C, Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE). Expert Opin Drug Saf. (2017) 16(3):411–9. doi: 10.1080/14740338.2017.1269168
3. Schrezenmeier E, Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. (2020) 16(3):155–66. doi: 10.1038/s41584-020-0372-x
4. Dima A, Jurcut C, Chasset F, Felten R, Arnaud L. Hydroxychloroquine in systemic lupus erythematosus: overview of current knowledge. Ther Adv Musculoskelet Dis. (2022) 14:1759720X211073001. doi: 10.1177/1759720X211073001
5. Alarcon GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alen J, Bastian HM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis. (2007) 66(9):1168–72. doi: 10.1136/ard.2006.068676
6. Mok CC, Hamijoyo L, Kasitanon N, Chen DY, Chen S, Yamaoka K, et al. The Asia-Pacific league of associations for rheumatology consensus statements on the management of systemic lupus erythematosus. Lancet Rheumatol. (2021) 3(7):E517–E31. doi: 10.1016/S2665-9913(21)00009-6
7. Fanouriakis A, Kostopoulou M, Andersen J, Aringer M, Arnaud L, Bae SC, et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis. (2023) 83:15–29. doi: 10.1136/ard-2023-224762
8. Weinlander E, Ringeisen AL, Mititelu M. Retinopathy in the era of routine hydroxychloroquine monitoring. J Rheumatol. (2016) 43(6):1254. doi: 10.3899/jrheum.151436
9. Morand EF, Vital EM, Petri M, van Vollenhoven R, Wallace DJ, Mosca M, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-I). Lancet. (2023) 401:1001–10. doi: 10.1016/S0140-6736(22)02607-1
10. Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. (2020) 382(3):211–21. doi: 10.1056/NEJMoa1912196
11. Winebrake J, Khalili L, Weiner J, Gartshteyn Y, Park L, Askanase AD. Rheumatologists’ perspective on hydroxychloroquine guidelines. Lupus Sci Med. (2020) 7(1):e000427. doi: 10.1136/lupus-2020-000427
12. Osmani Z, Schrama TJ, Zacouris-Verweij W, Andersen J, Frankel S, Bultink IEM, et al. Hydroxychloroquine treatment in European patients with lupus erythematosus: dosing, retinopathy screening and adherence. Lupus Sci Med. (2021) 8(1):e000478. doi: 10.1136/lupus-2021-000478
13. Wallace DJ, Tse K, Hanrahan L, Davies R, Petri MA. Hydroxychloroquine usage in US patients, their experiences of tolerability and adherence, and implications for treatment: survey results from 3127 patients with SLE conducted by the lupus foundation of America. Lupus Sci Med. (2019) 6(1):e000317. doi: 10.1136/lupus-2019-000317
14. Bethel M, Yang FM, Li S, Nahman NS, Oliver AM, Machua W, et al. Hydroxychloroquine in patients with systemic lupus erythematosus with end-stage renal disease. J Investig Med. (2016) 64(4):908–10. doi: 10.1136/jim-2016-000065
15. Hidekawa C, Yoshimi R, Saigusa Y, Tamura J, Kojitani N, Suzuki N, et al. Protective effect of hydroxychloroquine on infections in patients with systemic lupus erythematosus: an observational study using the LUNA registry. Front Immunol. (2023) 14:1227403. doi: 10.3389/fimmu.2023.1227403
16. Lee JE, Nam DR, Sung YK, Kim YJ, Jung SY. Nationwide patterns of hydroxychloroquine dosing and monitoring of retinal toxicity in patients with systemic lupus erythematosus. Sci Rep. (2023) 13(1):7270. doi: 10.1038/s41598-023-34022-0
17. Kandane-Rathnayake R, Golder V, Louthrenoo W, Luo SF, Jan Wu YJ, Li Z, et al. Development of the Asia Pacific lupus collaboration cohort. Int J Rheum Dis. (2019) 22(3):425–33. doi: 10.1111/1756-185X.13431
18. Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. (1997) 40(9):1725. doi: 10.1002/art.1780400928
19. Urowitz MB, Gladman DD, Ibanez D, Fortin PR, Bae SC, Gordon C, et al. Evolution of disease burden over five years in a multicenter inception systemic lupus erythematosus cohort. Arthritis Care Res (Hoboken). (2012) 64(1):132–7. doi: 10.1002/acr.20648
20. Touma Z, Urowitz MB, Ibanez D, Gladman DD. SLEDAI-2K 10 days versus SLEDAI-2K 30 days in a longitudinal evaluation. Lupus. (2011) 20(1):67–70. doi: 10.1177/0961203310385163
21. Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. (2002) 29(2):288–91.11838846
22. Chessa E, Piga M, Floris A, Devilliers H, Cauli A, Arnaud L. Use of physician global assessment in systemic lupus erythematosus: a systematic review of its psychometric properties. Rheumatology (Oxford). (2020) 59(12):3622–32. doi: 10.1093/rheumatology/keaa383
23. Isenberg DA, Allen E, Farewell V, D'Cruz D, Alarcon GS, Aranow C, et al. An assessment of disease flare in patients with systemic lupus erythematosus: a comparison of BILAG 2004 and the flare version of SELENA. Ann Rheum Dis. (2011) 70(1):54–9. doi: 10.1136/ard.2010.132068
24. Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. (2005) 142(12 Pt 1):953–62. doi: 10.7326/0003-4819-142-12_Part_1-200506210-00004
25. Stoll T, Seifert B, Isenberg DA. SLICC/ACR damage index is valid, and renal and pulmonary organ scores are predictors of severe outcome in patients with systemic lupus erythematosus. Br J Rheumatol. (1996) 35(3):248–54. doi: 10.1093/rheumatology/35.3.248
26. Ibanez D, Urowitz MB, Gladman DD. Summarizing disease features over time: I. Adjusted mean SLEDAI derivation and application to an index of disease activity in lupus. J Rheumatol. (2003) 30(9):1977–82.12966601
27. Manabe A, Sada RM, Miyake H, Akebo H, Tsugihashi Y, Hatta K. An observational study to identify causative factors for not using hydroxychloroquine in systemic lupus erythematosus. Sci Rep. (2024) 14(1):7750. doi: 10.1038/s41598-024-58463-3
28. Fernandez-Ruiz R, Bornkamp N, Kim MY, Askanase A, Zezon A, Tseng C-E, et al. Discontinuation of hydroxychloroquine in older patients with systemic lupus erythematosus: a multicenter retrospective study. Arthritis Res Ther. (2020) 22(1):191. doi: 10.1186/s13075-020-02282-0
29. Almeida-Brasil CC, Hanly JG, Urowitz M, Clarke AE, Ruiz-Irastorza G, Gordon C, et al. Retinal toxicity in a multinational inception cohort of patients with systemic lupus on hydroxychloroquine. Lupus Sci Med. (2022) 9(1):e000789. doi: 10.1136/lupus-2022-000789
30. Jorge AM, Mancini C, Zhou B, Ho G, Zhang Y, Costenbader K, et al. Hydroxychloroquine dose per ophthalmology guidelines and the risk of systemic lupus erythematosus flares. JAMA. (2022) 328(14):1458–60. doi: 10.1001/jama.2022.13591
31. Almeida-Brasil CC, Hanly JG, Urowitz M, Clarke AE, Ruiz-Irastorza G, Gordon C, et al. Flares after hydroxychloroquine reduction or discontinuation: results from the systemic lupus international collaborating clinics (SLICC) inception cohort. Ann Rheum Dis. (2022) 81(3):370–8. doi: 10.1136/annrheumdis-2021-221295
32. Ramnarain A, Liam C, Milea D, Morand E, Kent J, Kandane-Rathnayake R. Predictors of organ damage in systemic lupus erythematosus in the Asia Pacific region: a systematic review. Arthritis Care Res (Hoboken). (2024) 76:720–32. doi: 10.1002/acr.25291
33. Cai T, Zhao J, Yang Y, Jiang Y, Zhang JA. Hydroxychloroquine use reduces mortality risk in systemic lupus erythematosus: a systematic review and meta-analysis of cohort studies. Lupus. (2022) 31(14):1714–25. doi: 10.1177/09612033221129774
34. Garg S, Chewning B, Hutson P, Astor BC, Bartels CM. Reference range of hydroxychloroquine blood levels that can reduce odds of active lupus and prevent flares. Arthritis Care Res (Hoboken). (2024) 76(2):241–50. doi: 10.1002/acr.25228
Keywords: anti-malarial therapy, lupus, SLE, Asia-Pacific region, observational cohort study
Citation: Kandane-Rathnayake R, Hoi A, Louthrenoo W, Chen Y-H, Cho J, Lateef A, Hamijoyo L, Chan S, Luo SF, Jan Wu Y-J, Navarra S, Zamora L, Li Z, Yao H, Sockalingam S, Katsumata Y, Harigai M, Hao Y, Zhang Z, Basnayake B, Chan M, Kikuchi J, Takeuchi T, Oon S, Bae S-C, O’Neill S, Goldblatt F, Ng KPL, Law A, Tugnet N, Kumar S, Ohkubo N, Tee ML, Tee C, Tanaka Y, Lau CS, Golder V, Nikpour M and Morand EF (2024) Sub-optimal use of anti-malarial therapy for SLE in the Asia Pacific region: observations from the Asia Pacific lupus cohort. Front. Lupus 2:1461739. doi: 10.3389/flupu.2024.1461739
Received: 9 July 2024; Accepted: 31 October 2024;
Published: 22 November 2024.
Edited by:
Ola Grimsholm, Medical University of Vienna, AustriaReviewed by:
Maddalena Larosa, University Hospital of Padua, ItalyGiuseppe Barilaro, Hospital Clinic of Barcelona, Spain
Copyright: © 2024 Kandane-Rathnayake, Hoi, Louthrenoo, Chen, Cho, Lateef, Hamijoyo, Chan, Luo, Jan Wu, Navarra, Zamora, Li, Yao, Sockalingam, Katsumata, Harigai, Hao, Zhang, Basnayake, Chan, Kikuchi, Takeuchi, Oon, Bae, O'Neill, Goldblatt, Ng, Law, Tugnet, Kumar, Ohkubo, Tee, Tee, Tanaka, Lau, Golder, Nikpour and Morand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rangi Kandane-Rathnayake, cmFuZ2kua2FuZGFuZS1yYXRobmF5YWtlQG1vbmFzaC5lZHU=
 Rangi Kandane-Rathnayake
Rangi Kandane-Rathnayake Alberta Hoi
Alberta Hoi Worawit Louthrenoo
Worawit Louthrenoo Yi-Hsing Chen4
Yi-Hsing Chen4 Jiacai Cho
Jiacai Cho Aisha Lateef
Aisha Lateef Laniyati Hamijoyo
Laniyati Hamijoyo Yeong-Jian Jan Wu
Yeong-Jian Jan Wu Sandra Navarra
Sandra Navarra Zhanguo Li
Zhanguo Li Haihong Yao
Haihong Yao Yasuhiro Katsumata
Yasuhiro Katsumata Masayoshi Harigai
Masayoshi Harigai Zhuoli Zhang
Zhuoli Zhang BMDB Basnayake
BMDB Basnayake Jun Kikuchi
Jun Kikuchi Sean O’Neill
Sean O’Neill Kristine (Pek Ling) Ng
Kristine (Pek Ling) Ng Nicola Tugnet
Nicola Tugnet Sunil Kumar
Sunil Kumar Naoaki Ohkubo
Naoaki Ohkubo Michael L. Tee
Michael L. Tee Cherica Tee
Cherica Tee Yoshiya Tanaka
Yoshiya Tanaka Chak S. Lau
Chak S. Lau Eric F. Morand
Eric F. Morand