- 1Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, United States
- 2Department of Pharmacology, Vanderbilt University, Nashville, TN, United States
- 3U.S. Department of Veterans Affairs, Tennessee Valley Healthcare System, Nashville, TN, United States
Objective: Hypertension is frequent in patients with systemic lupus erythematosus (SLE) and is a major contributor to increased cardiovascular risk. Isolevuglandins (IsoLGs) are downstream products of oxidative stress that drive hypertension and SLE disease activity in animal models. Antibodies to IsoLGs (anti-IsoLGs) are present in human SLE and associated with disease activity, but it is not known if concentrations are higher compared to control subjects or if they are associated with blood pressure (BP).
Methods: We measured serum anti-IsoLG IgG antibody concentrations by sandwich ELISA in 23 patients with SLE and 30 controls who had participated in a cross-sectional 24-hour ambulatory BP study. We examined the association between anti-IsoLG IgG antibodies and BP measurements in patients with SLE and controls by Spearman Rho (rs) and linear regression analysis.
Results: Serum anti-IsoLG IgG antibody concentrations were higher in patients with SLE than controls (P = 0.007) and inversely associated with BP in SLE but not controls. In patients with SLE antibody concentrations were inversely associated with office (rs = −0.418) and diurnal systolic BP (rs = −0.421); the relationship was stronger among patients not taking anti-hypertensives (office: rs = −0.740, diurnal systolic BP: rs = −0.802) and every 20% increase in antibody concentration was associated with 10 mmHg decrease in 24-hour systolic BP (P = 0.004).
Conclusion: Serum anti-IsoLG IgG antibody concentrations are higher in patients with SLE than controls and are inversely associated with 24-hour BP measurements. Since IsoLGs promote hypertension, it is possible that in SLE, IsoLG antibodies could help clear these hypertension-inducing antigens.
Introduction
Systemic lupus erythematosus (SLE) is a chronic, systemic, autoimmune disease associated with a 2–3-fold increased risk of cardiovascular events (1). Hypertension is a major risk factor for cardiovascular disease in SLE, and hypertension and resistant hypertension are increased 2-fold in SLE (2, 3). Furthermore, SLE-related resistant hypertension is associated with an almost 3-fold increased mortality (3). One mechanism contributing to hypertension in SLE could involve isolevuglandins (IsoLGs). IsoLGs are highly-reactive gamma-ketoaldehydes formed as the result of lipid peroxidation of arachidonic acid in the setting of oxidative stress (when reactive oxygen species are produced in excess of antioxidants); they bind to proteins rapidly causing misfolding, crosslinking and damage (4).
IsoLG-modified proteins are immunogenic and proinflammatory (5–7) and promote both SLE and hypertension in animal models (7, 8). Because IsoLGs bind proteins so rapidly, it is not possible to measure free IsoLGs (4). Thus, IsoLGs can be measured on the proteins they bind to by mass spectrometry and by flow cytometry. When measured by flow cytometry they are measured as the percentage of cells with IsoLG-adducted proteins and are termed “cellular IsoLGs” (4, 7–9). Cellular IsoLGs are increased in patients with SLE and patients with hypertension (7, 8); in a murine model of SLE, scavenging IsoLGs before they induced protein modifications decreased measures of disease activity such as nephritis and also lowered blood pressure (7). IsoLGs can drive immune-mediated hypertension through activation of the innate and adaptive immune systems; for example, the accumulation of cellular IsoLGs in dendritic cells leads to increased cytokine expression and T-cell proliferation and activation (8). The activated T-cells increase expression of interferon-γ (IFN-γ), interleukin-17A (IL-17A) and tumor necrosis factor-α (TNF-α), which increases blood pressure due to enhanced salt and water reabsorption and vasoconstriction (8, 10, 11).
We previously found that in patients with SLE, even though office blood pressure (BP) and renal function were relatively normal, 24-hour BP measurements were considerably higher compared to control subjects (12). We also found that anti-IsoLG IgG antibodies were increased in B6.Sle123 and NZBWF1 murine models of lupus compared to wild type mice and were present and associated with disease activity in humans with SLE (7). However, it is not known if concentrations of anti-IsoLG IgG antibodies are higher in patients with SLE than in control subjects or if they are associated with BP. Thus, the purpose of this study was to determine if anti-IsoLG IgG antibodies are increased and associated with 24-hour BP in patients with SLE.
Methods
Study population
We performed this study using stored serum samples and BP readings from a previous cross-sectional study of ambulatory 24-hour blood pressure in patients with SLE and control subjects, as previously published (12). To enter the study, patients with SLE needed to meet the American College of Rheumatology revised SLE classification criteria (13) and be 18 years of age or older. Control subjects could not have SLE or any other autoimmune disease; however, control subjects could have other medical problems. All subjects needed to be able to provide informed consent, operate the 24-hour blood pressure device and could not have atrial fibrillation, lymphedema, current use of anticoagulants, or conditions that could be worsened by frequent inflation of a cuff for blood pressure measurement. Additionally, for the present study, subjects had to have a sufficient volume of serum available for serum IsoLG IgG antibody measurement. Subjects were recruited from the Vanderbilt outpatient rheumatology clinic, patient referral, and through advertisement. All subjects provided written informed consent. The study was approved by the Vanderbilt University Medical Center Institutional Review Board (IRB# 110365).
Clinical measures
Information on demographics and medical history were collected by interview and review of medical records and recorded in a standardized manner. SLE disease activity was assessed by SLE disease activity index 2,000 (SLEDAI) (14), and disease damage was assessed by the Systemic Lupus International Collaborating Clinics (SLICC) score (15). Patients were deemed as having hypertension if they carried the clinical diagnosis or if they had a blood pressure >140/90 mmHg at their study visit (16). Patient reported function was assessed by the modified health assessment questionnaire (17). Patient reported pain, fatigue and global health scores were collected by 1–100 mm visual analogue scale. Erythrocyte sedimentation rate, creatinine and estimated glomerular filtration rate were obtained from the medical record from measurements obtained for routine clinical care. High-sensitivity C-reactive protein (hs-CRP) and complement C3 and C4 were measured separately by the hospital clinical laboratory from serum collected at the time of the study visit.
24-hour BP measurement
Twenty four-hour blood pressure was measured using the Card(x)plore blood pressure monitor (Meditech, Budapest, Hungary), as previously described (12). Blood pressure was measured at 15–30-minute intervals during the day (6 a.m.–10 p.m.) and 30-minute intervals at night (10 p.m.–6 a.m.). This study included subjects with 50% or more of expected blood pressure measurements for both day (i.e., ≥32 readings) and night (i.e., ≥8 readings). Diurnal and nocturnal blood pressure was defined by the patient's reported sleep schedule.
Serum-IsoLG IgG antibody detection by ELISA
Anti-IsoLG IgG antibodies were measured by ELISA using methods based on a prior study (7). Protein from isolated human peripheral blood mononuclear cells (PBMCs) and neutrophils was extracted using non-denaturating lysis buffer (20 mM Tris pH 8.0, 137 mM NaCl, 1.0% NP-20, 2 mM EDTA). IsoLG-protein adduction was performed by incubating 100 μg of protein with 100 μM IsoLG [prepared as previously described (18)] overnight at 4°C. Immunolon 2HB plates were coated with D11, a single chain fragment variable (scfv) recombinant custom antibody specific for IsoLGs (7, 19–21), at concentration 50 μg/mL in coating buffer (1.5 g Na2CO3, 2.93 g NAHCO3 to 1l dH2O, pH 9.6) overnight at 4°C. Plates were then washed 3 times with wash buffer (PBS, 0.05% Tween-20) and blocked with blocking buffer (PBS, 3% BSA, 3 mM EDTA) for 1 h at 37°C. IsoLG-adducted protein at concentration 50 μg/mL in binding buffer (PBS, 2% BSA, 3 mM EDTA, 0.05% Tween-20) was added to the wells and incubated for 1 h at 37°C. Plates were then washed 4 times. Subject serum (1:1,000 dilution in binding buffer) was added to wells and incubated for 1 h at 37°C. Binding buffer with no serum added was used as a negative control. Plates were then washed 4 times. Protein-G-HRP conjugate (1:1,000 dilution in secondary antibody diluent: PBS, 1% BSA, 0.05% Tween-20) was added to wells and incubated for 1 h at 37°C. Plates were washed 3 times, and TMB substrate solution (ThermoFisher) was then added to wells with further incubation in the dark for 30 min at room temperature. After the reaction was stopped using stop solution (ThermoFisher), absorbance was read at 450 nm on the GloMax® Discover microplate reader (Promega, United States). Subject serum samples and no-serum controls were run in 4 technical replicates. The same SLE serum sample was used on all plates to normalize for plate-to-plate variability. The intraassay coefficient of variation (CV) was 9.1% and interassay CV was 22% prior to normalization for plate-to-plate variability.
Statistics
In preliminary studies the mean ± standard deviation anti-IsoLG antibody concentration was 0.893 ± 0.122 absorbance units in patients with SLE (7). Thus, a sample size of at least 22 patients and 22 controls would be needed to demonstrate a 1-standard deviation difference in anti-IsoLG antibodies between SLE and control subjects with 90% power. A secondary outcome was to determine the relationship between anti-IsoLG antibody concentration and 24-hour systolic BP. A sample size of 13 patients would provide 80% power to detect a correlation ≥0.7 or ≤−0.7. Data were compared by chi square (categorical) or Mann-Whitney U (continuous) tests. Correlation was conducted by Spearman Rho (rs) and linear regression analysis with anti-IsoLG IgG antibody concentrations were log-transformed to normalize residuals. Power was calculated using the PS Power and Sample Size program v3.1.6 (22). Data were analyzed and figures were created using IBM SPSS Statistics v27.
Results
Subject demographics
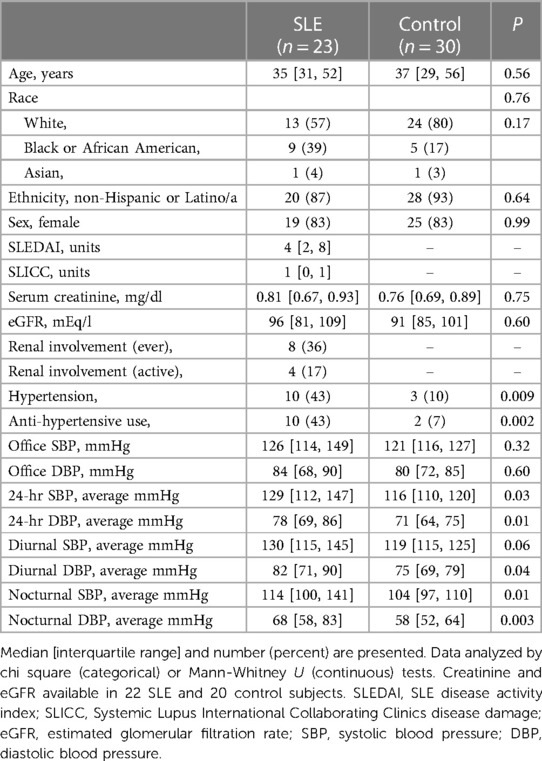
The SLE (n = 23) and control (n = 30) groups were similar in race, age (median age of 35 years and 37 years, respectively) and sex (83% female for SLE and control subjects) (Table 1). Disease activity in SLE patients was low to moderate (median SLEDAI = 4). A total of 8 (36%) of SLE patients had a history of renal involvement, however, serum creatinine and estimated GFR were similar in SLE and control groups (Table 1). Ten (43%) SLE and 3 (10%) control subjects had hypertension and 10 (43%) SLE patients and 2 (7%) control subjects were taking anti-hypertensive drugs. None of the SLE or control subjects had resistant hypertension, meaning uncontrolled office blood pressure on three or more anti-hypertensive agents including a diuretic. Most 24-hour BP measurements were significantly elevated in SLE vs. control subjects (Table 1), as previously published (12).
Anti-IsoLG antibodies in SLE vs. control subjects
Serum anti-IsoLG IgG antibody concentrations were higher in patients with SLE (median [interquartile range]: 1.30 units [0.93, 1.70 units]) than in control subjects (0.91 units [0.73, 1.28 units], P = 0.007; Figure 1).
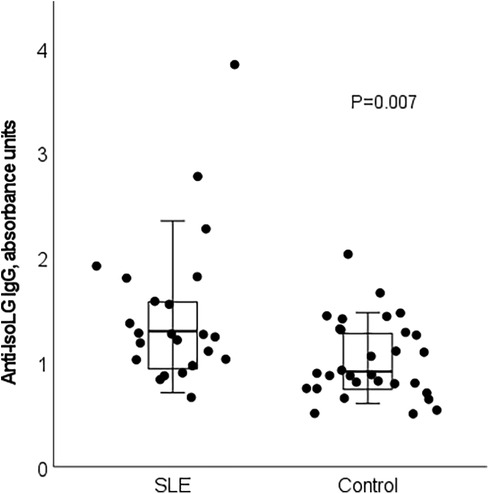
Figure 1. Serum anti-isoLG IgG antibodies are increased in patients with SLE compared to control subjects (P = 0.007). Box plot demonstrates median and interquartile range. Whiskers represent range excluding outliers >1.5× above or below the interquartile range.
Anti-IsoLG antibodies and relationship to blood pressure in SLE and controls
There was no consistent directionality of association or significant relationship between anti-IsoLG IgG antibodies and any BP measurement among control subjects (Supplementary Table 1). In contrast, among patients with SLE, the directionality of association was consistently inverse across all BP measurements (Table 2). The associations were significant for anti-IsoLG IgG antibody concentrations having an inverse association with office (rs = −0.418) and diurnal systolic BP (rs = −0.421) (Table 2) in patients with SLE. Anti-IsoLG IgG antibody concentrations tended to be higher in SLE patients who did not have hypertension (1.31 units [0.96 m 1.82 units]) vs. those who had hypertension (1.13 units [0.80, 1.56 units]), but this was not statistically significant (P = 0.42).
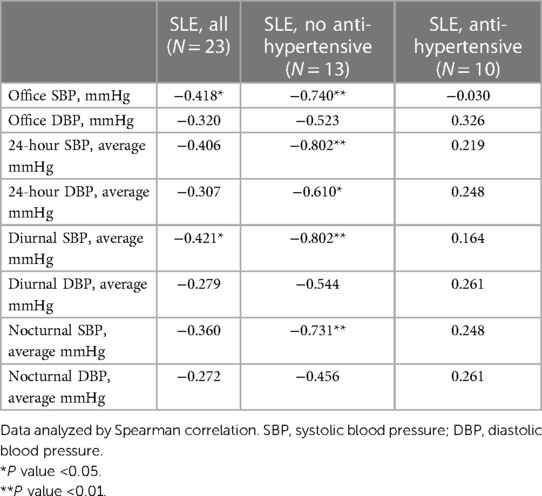
Table 2. Relationship between anti-isoLG IgG concentrations and blood pressure measurements in patients with SLE.
The relationship between anti-IsoLG IgG antibodies and BP was examined separately in those patients with SLE who were not taking anti-hypertensive drugs due to the effect of these drugs on BP. The demographic characteristics of this patient subset were similar to those of the entire group (Supplementary Table 2). Among SLE patients not taking anti-hypertensive drugs, anti-IsoLG IgG antibody concentrations were strongly and significantly inversely associated with office and 24-hour BP measurements including office systolic BP (SBP) (rs = −0.740), 24-hour SBP (rs = −0.802), 24-hour diastolic BP (DBP) (rs = −0.610), diurnal SBP (rs = −0.802), and nocturnal SBP (rs = −0.456) (Table 2). For example, every 20% increase in anti-IsoLG IgG concentration was associated with a 10 mmHg decrease in 24-hour systolic BP, P = 0.004 (Figure 2). This remained significant after adjustment for age (P adj = 0.03). In patients with SLE receiving antihypertensive drugs the association between ant-IsoLG antibodies and BP was not significant (Table 2).
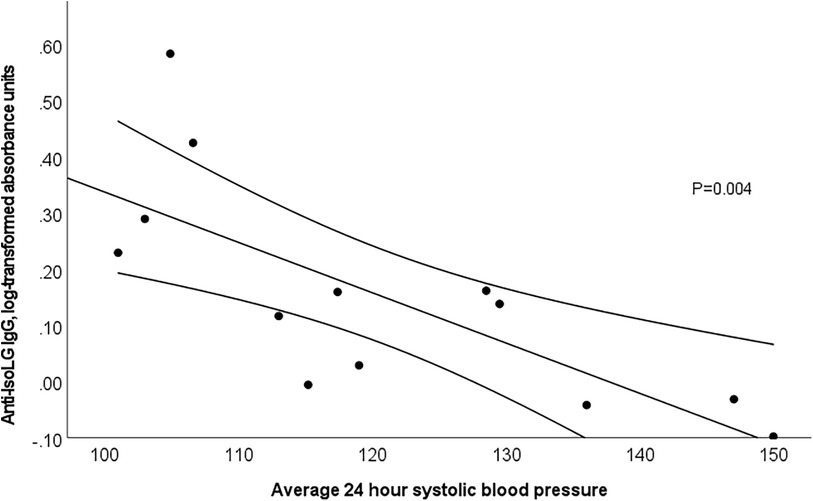
Figure 2. 24-hour systolic blood pressure and anti-isoLG IgG antibodies (log-transformed) are significantly inversely associated among SLE patients not taking an anti-hypertensive agent (P = 0.004). Linear regression with 95% confidence intervals.
Anti-IsoLG IgG antibodies and relationship to SLE disease activity and features
The relationship between serum anti-IsoLG IgG antibody concentrations and clinical disease features was also assessed. Age was inversely associated with anti-IsoLG IgG (rs = −0.463, Supplementary Table 3) in SLE but not in control participants (rs = −0.012).
We previously found that anti-IsoLG IgG antibody concentrations were associated with disease activity assessed by SLEDAI in patients with SLE (7), however, in the current study this correlation was not significant (rs = 0.156). Antibody levels were associated with higher disease damage based on the SLICC score (rs = 0.338), and lower complement C3 (rs = −0.203) and C4 (rs = −0.320) concentrations but these were not statistically significant (Supplementary Table 3). Patients with active arthritis had significantly lower anti-IsoLG IgG antibody concentrations (median [IQR]: 0.93 units [0.81, 1.33]) vs. those without active arthritis ([1.44 units [1.07, 1.95]), but there was no significant difference based on whether the patient ever had arthritis or based on other clinical manifestations (Supplementary Table 4).
Discussion
The major findings of this study were that serum anti-IsoLG IgG antibody concentrations are higher in patients with SLE than in control subjects and that higher anti-IsoLG IgG antibody concentrations were associated with lower blood pressure in patients with SLE. This relationship was demonstrated most clearly among patients with SLE without the confounding effects of anti-hypertensive medications.
IsoLGs are the result of excess oxidative stress which occurs when reactive oxygen species are generated in excess of antioxidants; this causes lipid peroxidation of polyunsaturated fatty acids, yielding highly reactive dicarbonyls such as IsoLGs. IsoLGs bind covalently to lysine residues on proteins nearly instantaneously causing conformation changes and protein crosslinking (4). Because IsoLG adduction of proteins changes their conformation, it can result in antibody formation. Additionally, the IsoLG-adducted proteins increase endoplasmic reticulum stress, activate the receptor for advanced glycation end products, increase proinflammatory cytokine expression, and are antigens presented on MHC to activate T cells (5, 6, 23, 24).
Murine studies also demonstrate that IsoLGs drive immune-mediated hypertension. Dendritic cells present IsoLGs-adducted cellular proteins to T cells leading to cellular proliferation and activation to produce IFN-γ, IL-17A and TNF-α, which increase blood pressure due to effects salt and water reabsorption and vasoconstriction (8, 10, 11, 25–32). However, dendritic cell presentation of proteins with other oxidative stress modifications (e.g., malondialdehyde-adducted proteins) did not cause the T cell activation and proliferation (8). The adoptive transfer of dendritic cells treated with tert-butyl hydroperoxide to induce IsoLGs, significantly increased blood pressure in mice treated with low dose angiotensin II (8). Moreover, scavenging IsoLGs in two murine models of hypertension (angiotensin II-induced hypertension and deoxycorticosterone acetate plus NaCl models), and two murine models of SLE (B6.Sle123 and NZBWF1 models) significantly decreased blood pressure, renal injury (7, 8).
In addition to the impact on hypertension in murine lupus, we previously found that IsoLGs play a major role in lupus in mechanistic murine studies. In the B6.Sle123 and NZBWF1 models of murine lupus we found elevated cellular IsoLGs in splenic monocytes and dendritic cells compared to wildtype mice (7). We found that scavenging IsoLGs significantly decreased anti-double stranded DNA titers, splenic cellular expansion, nephritis, and blood pressure (7). There are several mechanisms that may explain why scavenging IsoLGs reduced murine lupus disease activity: (1) a reduction in proinflammatory cytokines (6), (2) a reduction in NETosis (9), (3) reduced T cell activation due to reduced dendritic cell presentation of IsoLG-adducted proteins to T cells, and (4) enhanced binding of transcription factor PU.1 to DNA leading to increased complement component C1q (7).
Similar to the murine models, there are consistent alterations in cellular IsoLGs in the peripheral blood of patients with hypertension and with lupus compared those without. For example, twelve patients with hypertension had about a 3-fold increase in peripheral blood monocyte cellular IsoLGs measured by flow cytometry compared to 8 normotensive subjects and these were modestly associated with systolic blood pressure (8). Moreover, peripheral blood monocytes from 11 patients with SLE had significantly higher cellular IsoLGs compared to 10 control subjects (7).
Based on studies suggesting that IsoLGs are increased in and mechanistically crucial for the development of hypertension and SLE, we postulated that anti-IsoLG antibodies might provide insights regarding the pathogenesis of hypertension in patients with SLE. Our finding that higher anti-IsoLG IgG antibody concentrations were associated with lower blood pressure measurements was unexpected given the mechanistic association between IsoLGs and blood pressure. However, although IsoLGs can drive hypertension through immunologic responses, antibodies to them could have a protective effect for blood pressure in SLE patients. Mechanistically, these antibodies may clear the IsoLG-adducted proteins or otherwise prevent their presentation to T cells, which is an underlying mechanism of immune-mediated hypertension (8). If this is the case, such an antibody would have therapeutic potential and this idea will be examined in future studies. Before such future studies, the results of this study should be interpreted with caution since we demonstrated correlation rather than causation. Additionally, we observed a more striking relationship between the anti-IsoLG antibodies and systolic blood pressures; it is likely because diastolic blood pressure is lower, and correlations may be more difficult to observe.
At this time, it is not known what IsoLG-adducted proteins the anti-IsoLG IgG antibodies recognize. Just as there are a variety of antinuclear antibodies, the anti-IsoLG IgG antibodies measured in SLE patients may have different specificities compared to control subjects or other disease states. This may contribute to differences in the relationship between the anti-IsoLG IgG antibody concentrations and blood pressure among patients with SLE and control subjects.
In our prior study we found that serum anti-IsoLG IgG antibody concentrations were correlated with SLEDAI in 29 patients with SLE (7). In the current study, while anti-IsoLG IgG were positively associated with SLEDAI, the findings were modest and not statistically significant, likely because the current study was smaller (n = 23) and had a narrower range of disease activity (SLEDAI range 0–12 vs. 2–18) and heterogeneity of disease.
This study has strengths and limitations. The sample size was relatively small; however, 24-hour BP was carefully measured in the patients which permitted these detailed BP analyses. SLE disease activity was relatively mild as discussed above. Also, we did not have archived cells to measure cellular IsoLGs in the same patients contributing to the anti-IsoLG antibody data. Such studies will be done in the future to determine the relationship between the cellular IsoLGs and anti-IsoLG antibodies, particularly in the context of SLE-associated hypertension.
Data availability statement
The datasets presented in this article are not readily available because deidentified data will be made available to interested researchers with suitable approved data use agreement. Requests to access the datasets should be directed to Michelle Ormseth,bWljaGVsbGUub3Jtc2V0aEB2dW1jLm9yZw==.
Ethics statement
The studies involving humans were approved by Vanderbilt University Medical Center IRB. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AP: Data curation, Investigation, Writing – original draft, Writing – review & editing. AO: Writing – review & editing, Data curation, Investigation. SS: Data curation, Methodology, Writing – review & editing. QW: Data curation, Writing – review & editing, Methodology. OP: Data curation, Writing – review & editing. SD: Methodology, Writing – review & editing, Resources. JK: Data curation, Writing – review & editing, Methodology. DP: Methodology, Writing – review & editing, Funding acquisition. CS: Writing – review & editing, Conceptualization, Funding acquisition, Resources, Supervision. MO: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
This study was funded by NIH NIAMS R21 AR080372, NHLBI R01 HL 140145 and P01 HL116263, and NCATS 5UL1TR002243-03.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/flupu.2024.1377164/full#supplementary-material
References
1. Hak AE, Karlson EW, Feskanich D, Stampfer MJ, Costenbader KH. Systemic lupus erythematosus and the risk of cardiovascular disease: results from the nurses’ health study. Arthritis Rheum. (2009) 61:1396–402. doi: 10.1002/art.24537
2. Asanuma Y, Oeser A, Shintani AK, Turner E, Olsen N, Fazio S, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. (2003) 349:2407–15. doi: 10.1056/NEJMoa035611
3. Gandelman JS, Khan OA, Shuey MM, Neal JE, McNeer E, Dickson A, et al. Increased incidence of resistant hypertension in patients with systemic lupus erythematosus: a retrospective cohort study. Arthritis Care Res (Hoboken). (2020) 72:534–43. doi: 10.1002/acr.23880
4. Brame CJ, Salomon RG, Morrow JD, Roberts LJ 2nd. Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J Biol Chem. (1999) 274:13139–46. doi: 10.1074/jbc.274.19.13139
5. Guo L, Chen Z, Cox BE, Amarnath V, Epand RF, Epand RM, et al. Phosphatidylethanolamines modified by gamma-ketoaldehyde (gammaKA) induce endoplasmic reticulum stress and endothelial activation. J Biol Chem. (2011) 286:18170–80. doi: 10.1074/jbc.M110.213470
6. Tao H, Huang J, Yancey PG, Yermalitsky V, Blakemore JL, Zhang Y, et al. Scavenging of reactive dicarbonyls with 2-hydroxybenzylamine reduces atherosclerosis in hypercholesterolemic ldlr(-/-) mice. Nat Commun. (2020) 11:4084. doi: 10.1038/s41467-020-17915-w
7. Patrick DM, de la Visitacion N, Krishnan J, Chen W, Ormseth MJ, Stein CM, et al. Isolevuglandins disrupt PU.1-mediated C1q expression and promote autoimmunity and hypertension in systemic lupus erythematosus. JCI Insight. (2022) 7(13):e136678. doi: 10.1172/jci.insight.136678
8. Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. (2014) 124:4642–56. doi: 10.1172/JCI74084
9. Krishnan J, Visitacion N, Hennen EM, Amarnath V, Harrison DG, Patrick DM. IsoLGs (isolevuglandins) drive neutrophil migration in hypertension and are essential for the formation of neutrophil extracellular traps. Hypertension. (2022) 79(8):1644–55. doi: 10.1161/HYPERTENSIONAHA.122.19305
10. Murray EC, Nosalski R, MacRitchie N, Tomaszewski M, Maffia P, Harrison DG, et al. Therapeutic targeting of inflammation in hypertension: from novel mechanisms to translational perspective. Cardiovasc Res. (2021) 117:2589–609. doi: 10.1093/cvr/cvab330
11. Lu X, Crowley SD. The immune system in hypertension: a lost shaker of salt 2021 Lewis K. Dahl memorial lecture. Hypertension. (2022) 79:1339–47. doi: 10.1161/HYPERTENSIONAHA.122.18554
12. Carranza-Leon DA, Oeser A, Wu Q, Stein CM, Ormseth MJ, Chung CP. Ambulatory blood pressure in patients with systemic lupus erythematosus: association with markers of immune activation. Lupus. (2020) 29:1683–90. doi: 10.1177/0961203320951274
13. Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. (1997) 40:1725. doi: 10.1002/art.1780400928
14. Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. (2002) 29:288–91. 11838846
15. Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the systemic lupus international collaborating clinics/American college of rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. (1996) 39:363–9. doi: 10.1002/art.1780390303
16. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 international society of hypertension global hypertension practice guidelines. J Hypertens. (2020) 38:982–1004. doi: 10.1097/HJH.0000000000002453
17. Pincus T, Summey JA, Soraci SA Jr., Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford health assessment questionnaire. Arthritis Rheum. (1983) 26:1346–53. doi: 10.1002/art.1780261107
18. May-Zhang LS, Yermalitsky V, Huang J, Pleasent T, Borja MS, Oda MN, et al. Modification by isolevuglandins, highly reactive gamma-ketoaldehydes, deleteriously alters high-density lipoprotein structure and function. J Biol Chem. (2018) 293:9176–87. doi: 10.1074/jbc.RA117.001099
19. Warden C, Simmons AJ, Pasic L, Pitzer A, Davies SS, Layer JH, et al. Direct detection of isolevuglandins in tissues using a D11 scFv-alkaline phosphatase fusion protein and immunofluorescence. J Vis Exp. (2021) 173. doi: 10.3791/62603
20. Yan HP, Roberts LJ, Davies SS, Pohlmann P, Parl FF, Estes S, et al. Isolevuglandins as a gauge of lipid peroxidation in human tumors. Free Radic Biol Med. (2017) 106:62–8. doi: 10.1016/j.freeradbiomed.2017.02.020
21. Davies SS, Talati M, Wang X, Mernaugh RL, Amarnath V, Fessel J, et al. Localization of isoketal adducts in vivo using a single-chain antibody. Free Radic Biol Med. (2004) 36:1163–74. doi: 10.1016/j.freeradbiomed.2004.02.014
22. Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. (1990) 11:116–28. doi: 10.1016/0197-2456(90)90005-M
23. Guo L, Chen Z, Amarnath V, Yancey PG, Van Lenten BJ, Savage JR, et al. Isolevuglandin-type lipid aldehydes induce the inflammatory response of macrophages by modifying phosphatidylethanolamines and activating the receptor for advanced glycation endproducts. Antioxid Redox Signal. (2015) 22:1633–45. doi: 10.1089/ars.2014.6078
24. Dixon KB, Davies SS, Kirabo A. Dendritic cells and isolevuglandins in immunity, inflammation, and hypertension. Am J Physiol Heart Circ Physiol. (2017) 312:H368–74. doi: 10.1152/ajpheart.00603.2016
25. Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, et al. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-gamma-/- and interleukin-17A-/- mice. Hypertension. (2015) 65:569–76. doi: 10.1161/HYPERTENSIONAHA.114.04975
26. Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L, et al. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension. (2016) 68:167–74. doi: 10.1161/HYPERTENSIONAHA.116.07493
27. Shahid M, Francis J, Majid DS. Tumor necrosis factor-alpha induces renal vasoconstriction as well as natriuresis in mice. Am J Physiol Renal Physiol. (2008) 295:F1836–44. doi: 10.1152/ajprenal.90297.2008
28. Kroetsch JT, Levy AS, Zhang H, Aschar-Sobbi R, Lidington D, Offermanns S, et al. Constitutive smooth muscle tumour necrosis factor regulates microvascular myogenic responsiveness and systemic blood pressure. Nat Commun. (2017) 8:14805. doi: 10.1038/ncomms14805
29. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. (2007) 204:2449–60. doi: 10.1084/jem.20070657
30. Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. (2010) 55:500–7. doi: 10.1161/HYPERTENSIONAHA.109.145094
31. Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. (2008) 51:1345–51. doi: 10.1161/HYPERTENSIONAHA.107.102152
32. Venegas-Pont M, Manigrasso MB, Grifoni SC, LaMarca BB, Maric C, Racusen LC, et al. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension. (2010) 56:643–9. doi: 10.1161/HYPERTENSIONAHA.110.157685
Keywords: SLE, isolevuglandins, hypertension, blood pressure, cardiovascular, inflammation, oxidative stress
Citation: Phothisane A, Oeser AM, Shaik S, Wu Q, Posey O, Davies SS, Krishnan J, Patrick DM, Stein CM and Ormseth MJ (2024) Serum isolevuglandin IgG antibody concentrations are increased in patients with systemic lupus erythematosus and associated with lower 24-hour blood pressure. Front. Lupus 2:1377164. doi: 10.3389/flupu.2024.1377164
Received: 26 January 2024; Accepted: 13 March 2024;
Published: 26 March 2024.
Edited by:
Ola Grimsholm, Medical University of Vienna, AustriaReviewed by:
Rahul Kakalij, University of Nebraska Medical Center, United StatesWenhai Shao, University of Cincinnati, United States
© 2024 Phothisane, Oeser, Shaik, Wu, Posey, Davies, Krishnan, Patrick, Stein and Ormseth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michelle J. Ormseth bWljaGVsbGUub3Jtc2V0aEB2dW1jLm9yZw==
 Anastasiia Phothisane1
Anastasiia Phothisane1 Shahensha Shaik
Shahensha Shaik Jaya Krishnan
Jaya Krishnan David M. Patrick
David M. Patrick Michelle J. Ormseth
Michelle J. Ormseth