
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Trop. Dis. , 10 March 2025
Sec. Neglected Tropical Diseases
Volume 6 - 2025 | https://doi.org/10.3389/fitd.2025.1532049
This article is part of the Research Topic Preventing and Controlling Tropical Infectious Diseases: Lessons from the Global South View all 5 articles
Background: Cutaneous leishmaniasis (CL) is a neglected skin disease that affects millions of people worldwide. Its visible symptoms and impact on physical appearance can lead to social rejection, stigma, and discrimination for patients with cutaneous leishmaniasis. It is a public health and social problem in East Africa. However, there was no conclusive evidence on the pooled prevalence of cutaneous leishmaniasis in East Africa. Thus, we conducted a systematic review and meta-analysis to examine the current evidence on the burden of cutaneous leishmaniasis in East Africa.
Objective: The main purpose of this study was to estimate the pooled prevalence of cutaneous leishmaniasis and its risk factors in East Africa.
Methods: This systematic review and meta-analysis were conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Stata software version 17. A forest plot and a random effects model were used to estimate the pooled prevalence of CL with a 95% confidence interval (CI) and heterogeneity of articles, respectively.
Results: A total of 221,365 study participants were included in the review. Based on the results, the forest plot was explained by I2 = 99.99% at p < 0.05. The prevalence of cutaneous leishmaniasis in primary studies ranged from 0.73% to 79.10%. The pooled prevalence of CL in East Africa was found to be 22.57% with a 95% CI (14.36, 30.78). The effect size showed statistically significant subgroup effects for study design, study setting, sample size, clinical forms of CL, year of publication, and study period at p < 0.05 according to the subgroup analyses.
Conclusion and recommendations: The pooled prevalence of cutaneous leishmaniasis in East Africa was high. This underscores the urgent need for targeted public health initiatives to mitigate the impact of this disease on affected populations. Collaborative efforts between governments, health organizations, and communities are crucial for the effective management and control of CL in the East Africa region.
Systematic review registration: https://www.crd.york.ac.uk, identifier CRD42024576017.
Leishmaniasis is considered a neglected disease by the World Health Organization (WHO) (1, 2). Leishmaniasis is caused by obligate intracellular protozoa of the genus Leishmania, belonging to the family Trypanosomatidae (3). Some of the Leishmania species known to cause disease in humans are L. donovani, L. infantum, L. major, L. tropica, and L. aethiopica. There are three main forms of leishmaniasis in the world, namely visceral (the most severe form because it is almost always fatal without treatment), cutaneous (the most common, usually causing skin ulcers), and mucocutaneous (affecting the mouth, nose, and throat) (4–6).
Cutaneous leishmaniasis (CL) is a global public health problem and a social challenge in many developing countries (7). CL is also prevalent in tropical and subtropical areas. East Africa, the Americas, Central Asia, the Middle East, and the Mediterranean basin account for approximately 95% of the global burden of disease (7, 8). The main victims of the disease are poor subjects from Africa, Asia, and Latin America, and it is associated with malnutrition, population displacement, poor housing, a weak immune system, and a lack of resources (9, 10). CL is highly endemic in Algeria, a country in North Africa, whereas its epidemiological prevalence is low in West African countries (11). In Algeria, Libya, Morocco, and Tunisia, the incidence is more than 1,000 new cases every year (12, 13). East Africa accounts for 80% of the cutaneous leishmaniasis cases reported worldwide (11, 14). According to a 2022 WHO report, out of the 200 countries and territories reporting to the WHO, 99 countries and territories are endemic for cutaneous leishmaniasis (5, 12).
The East African region remains one of the most impacted regions by cutaneous leishmaniasis globally. This significantly influences the health, well-being, and livelihoods of the affected communities and also hinders progress toward the control and elimination of this disease (8, 15). In the region, cutaneous leishmaniasis has numerous foci in Ethiopia, Sudan, Uganda, Kenya, Somalia, and Eritrea (16, 17). Since 2015, approximately 93% of the population of Sudan has been considered to be at risk of infection (18). The clinical form of the disease is estimated to be endemic by the WHO; however, there is a lack of accurate data describing the true extent of pooled CL cases in studies conducted in the region (8).
This systematic review and meta-analysis (SRMA) has consolidated the findings from various studies in different countries and settings to provide a holistic view of CL prevalence in the region. This review will aggregate data on the prevalence of cutaneous leishmaniasis in East Africa. The result of this systematic review and meta-analysis can inform public health policies and control strategies, ultimately reducing the burden of CL in the affected region. This may also highlight areas where research is lacking, directing future studies toward significant knowledge gaps or under-researched populations. This systematic review and meta-analysis will synthesize existing research, providing a comprehensive overview of the current knowledge of CL, including its epidemiology, clinical features, and treatment options in East Africa. Conducting a systematic review and meta-analysis of cutaneous leishmaniasis in East Africa is also crucial for empowering individuals, enhancing community health initiatives, and strengthening healthcare systems. By consolidating evidence and insights, this article will enable informed decision-making and targeted interventions that can significantly improve health outcomes for those affected by the disease in the region.
Investigating prevalence and incidence rates in different regions can also inform health policies and resource allocation. This review follows a rigorous methodology, which increases the reliability of the conclusions drawn. Overall, this systematic review and meta-analysis are essential for advancing the understanding and management of cutaneous leishmaniasis, ultimately contributing to improved patient outcomes and public health initiatives. The systematic review and meta-analysis were conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of 2020. Additionally, despite studies on CL prevalence in each nation, the pooled prevalence data are not known in the region. Thus, to the best of our knowledge, there has been no systematic review, and meta-analysis has been conducted on the pooled prevalence of CL using meta-regression in the East Africa region. As a result, the main objective of this review was to determine the pooled prevalence and conduct meta-regression and subgroup analyses of CL in the East Africa region.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 checklist was used to develop the protocol for this review. The review was registered in PROSPERO with the registration identification number CRD42024576017.
This is a systematic literature review and meta-analysis of primary studies on the prevalence and risk factors of cutaneous leishmaniasis in East Africa. To map the identified studies in this systematic review and meta-analysis, the PRISMA checklist was used.
Primary observational studies (cross-sectional and cohort) conducted to reveal the prevalence of cutaneous leishmaniasis in East Africa were included. Furthermore, studies that had been conducted at the hospital level and community level were included. All articles published only in English, with no limitation on the study period up to October 2024, were included. Study participants were patients with suspected cutaneous leishmaniasis in the East Africa region. The outcomes of interest were the pooled prevalence or incidence of cutaneous leishmaniasis in East Africa in 2024.
Duplicate studies, abstracts, editorial reports, reviews, commentaries, studies posted at preprint, conferences, reviewed papers, books, diaries, commentaries, and letters about cutaneous leishmaniasis were not included in this review. Studies written in languages other than English were excluded from this study. Despite contacting the corresponding author(s), articles with the absence of full text and difficulty in extracting data, studies that only reported qualitative findings and studies with methodological limitations such as incorrect outcome ascertainment criteria were also excluded.
Primarily, databases were searched for the same systematic review to avoid duplication. A literature search strategy was implemented to find published primary studies on the prevalence and determinants of cutaneous leishmaniasis in East Africa.
Published articles were retrieved from major databases such as Public Medical Database (PubMed), Excerpta Medica Database (EMBASE), Scientific, Technical, and Other Publications in Scopus (SCOPUS), Health InterNetwork Access to Research Initiative (HINARI), Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Global Health using a standard search strategy. In addition, gray literature available through the Google search engine and Google Scholar were included. Furthermore, authors were contacted by e-mail for published articles without free access to download and review the full-length paper. Moreover, a manual search for articles to include in the review was conducted. If specific data points were missing, the researchers directly contacted the study authors to request the necessary information.
The search strings or terms used in Medline and PubMed used the following keywords: cutaneous leishmaniasis, human leishmaniasis, burden, prevalence, incidence, factors associated, East Africa, comorbidity, low-income countries, and Horn of Africa countries. The search terms were used to extract relevant articles in a combined form adapted to the requirements of the specific database. In the advanced search databases, the search strategy was built based on the terms mentioned above using Medical Subject Headings (MeSH) and “All fields” by linking terms with “AND” and “OR” Boolean operators as appropriate.
Between 30 August 2024 and 24 October 2024, a thorough and systematic literature search was conducted using electronic databases such as PubMed, EMBASE, Scopus, HINARI, CINAHL and Global Health, Advanced Google Search, and Google Scholar. The following keywords and MeSH terms were used in the search: [(prevalence OR burden OR frequency OR epidemiology OR magnitude OR incidence OR ‘‘Period Prevalence’’ OR ‘‘Point Prevalence’’ OR Incidence* OR ‘‘Attack Rate’’ OR ‘‘cumulative incidence’’ OR ‘‘Incidence Proportion’’ OR ‘‘Incidence Rate’’ OR ‘‘Person-time Rate’’ OR ‘‘Secondary Attack Rate’’ OR Burden) AND (‘‘associated factor’’ OR ‘‘risk factor’’ OR determinants OR factors OR factor OR associate factor OR associated factors OR predictors OR predictor) AND (“Leishmaniasis” OR “Oriental sore” OR “Cutaneous Leishmaniasis” OR “Diffuse Cutaneous Leishmaniasis” OR “human leishmaniasis” OR “Old World Cutaneous Leishmaniasis” OR “Mucocutaneous Leishmaniasis” OR “L. aethiopica” OR “Leishmania aethiopica” AND “low- and middle- income countries” OR “low- and middle- income country” OR “low and middle income” OR “low- and middle- income” OR “lower middle income” OR LMICs OR LMIC OR “LMICs” OR “lower middle income” OR “lower middle income countries” OR “lower middle income country” OR “lower middle income economies” OR “lower middle income setting” OR “lower middle income settings” OR ‘‘Developing Nations’’ OR ‘‘Developing Countries’’ OR “East Africa” OR “Horn of Africa” OR “Eastern Africa” OR “East of Africa” OR “Burundi” OR “Comoros” OR “Djibouti” OR “Eritrea” OR “Ethiopia” OR “Kenya” OR “Madagascar” OR “Malawi” OR “Mauritius” OR “Réunion” OR “Rwanda” OR “Seychelles” OR “Mozambique” Or “Sudan” OR “South Sudan” Or “Somalia” OR “Somaliland” OR “Tanzania” OR “Uganda” OR “Congo” OR “Mayotte” OR “Reunion”)].
Two independent reviewers screened articles using the title and abstract. The papers eligible for full-text review were reviewed by two independent reviewers for inclusion in the systematic review and meta-analysis. In all scenarios, a third reviewer was sought when two independent reviewers failed to agree on the inclusion of an article(or articles) during screening or full-text review. First, articles retrieved from databases and electronic search engines were exported to Mendeley so that duplicates were easily identified and removed. Second, the remaining papers were evaluated in the context of the topic, the study participants, the language, and the study area. Third, unrelated topics, studies conducted outside of East Africa, and articles in languages other than English were rejected. Finally, the remaining studies’ abstracts and full texts were reviewed entirely in order to identify the final included articles.
After carefully reviewing outcome measurements, data extraction was conducted. The outcome of interest for this review was the prevalence of cutaneous leishmaniasis in East Africa in 2024. The definition of variables used in the primary studies was considered. When there were differences in the definition of outcome and independent variables, data were collected and used for subgroup analysis for possible heterogeneity.
Three independent reviewers performed the quality assessment. The agreement between the reviewers was judged using Cohen’s kappa (K) coefficient statistics. To calculate “K,” a two-by-two contingency table was constructed with “High” and “Low” categories of quality assessment provided independently by the two reviewers based on a set of criteria.
The quality of each published paper was assessed using the standardized Joana Brig’s Institute (JBI) critical appraisal tool for cross-sectional studies. For analytical cross-sectional studies, an instrument with eight question items and eleven questions for cohort studies was employed.
All questions were of the “Yes” and “No” type, and scores of 1 and 2 were given for “Yes” and “No” responses, respectively. Only studies with a low risk were included in the systematic review and meta-analysis of the prevalence. For any disagreements between the assessors, the sources of the discrepancy were investigated by a thorough revision. Despite the detailed review, the reviewers’ average scores were calculated in cases of persistent disagreement. Similarly, each factor with each outcome variable was critically appraised for determinants. We used a similar cut-off point for prevalence studies. Moreover, the quality results of the primary studies were placed in a separate column in the data extraction format.
Once eligible studies were identified, with the full text of these potentially eligible studies was retrieved and independently assessed for eligibility by three independent reviewers. Any disagreement between them regarding the eligibility of particular studies was resolved through discussion. Finally, the relevant data were extracted using a prepared template in a Microsoft Excel spreadsheet. The summary table contains a list of study characteristic items such as the name of the first author(s), study year, country, study design, sample size, setting, clinical forms of CL, prevalence of CL in suspected CL patients, response rate, year of publication, and method of data collection.
For suspected CL patients, the logarithm of the proportion and the standard error of the logarithm of the prevalence were computed. Similarly, for determinants, the odds ratio, the logarithm of the odds ratio, and the standard error of the logarithm of the odds ratio were calculated. For any difficulties encountered during data extraction, the corresponding author(or authors) was/were contacted by any means of communication.
● Prevalence or incidence of CL in suspected CL patients in East Africa.
● Prevalence or incidence of CL risk factors in suspected CL patients in East Africa.
A Microsoft Excel spreadsheet was used to extract the data. The extracted data were exported to Stata software version 17 for further analysis. Tables, figures, and forest plots were used to describe and summarize the main investigations. All of the studies that were part of the review were conducted in various East African countries, and there was variation in the effect sizes. As a result, the random effects model was best suited to handle studies with such variability. Thus, a random effects model with a 95% confidence interval was used to pool the prevalence of CL among suspected CL patients in East Africa. Odds ratios with 95% confidence intervals were used to quantify the measure of association for factors affecting the prevalence of CL among suspected patients in East Africa. The existence of heterogeneity among studies was assessed using the forest plot and I2 heterogeneity test, adopting a 50% standard as recommended by the Cochrane guidelines. The I2 values of 25%, 50%, and 75% were interpreted as the presence of low, medium, and high heterogeneity, respectively. An I2 heterogeneity test of ≥50% and a p-value of < 0.05 denoted the presence of heterogeneity. To identify the influential studies that resulted in variation, sensitivity analysis was conducted using the “metaninf” command. For extreme outlier study(s), the extracted data were checked for any error that may have happened during the process of extraction. Finally, the article(s) were excluded from the analysis if the data were error-free. Similarly, subgroup analyses were employed using country, setting, sample size, and study year as grouping variables and sources of variation.
Publication bias was calculated using the “metafunnel” command and Egger’s regression test. Accordingly, the funnel plot asymmetry and/or statistical significance in Egger’s regression test (p-value < 0.05) suggested publication bias. Therefore, using the “metatrim” command, a non-parametric trim and fill analysis method was conducted.
Using the Laired random effects model, the pooled proportion of CL among suspected CL patients was reported. The association between the determinants and the pooled proportion of CL among suspected CL patients was estimated based on the effect size. Furthermore, all statistical interpretations of the results were reported based on a 95% CI.
Ethical approval was not a concern since this was a systematic review and meta-analysis. The study results were published in a reputable peer-reviewed journal and presented at scientific research conferences.
The JBI quality appraisal criteria were applied to both cross-sectional and cohort study designs. All studies presented clear research questions and collected data to address questions, and all of the studies had representative samples and used appropriate statistical analysis. The studies included in this systematic review and meta-analysis had no considerable risk (had low risk). Therefore, all the studies were included in the review. The search strategy resulted in 532 records with PubMed=351, EMBASE=40, Global health=42, CINAHL=35, Scopus=49, and Google Scholar=15. Of these, 197 duplicate records were excluded, and after papers were screened using their titles and abstracts, 186 were excluded. Therefore, 149 articles were assessed for their eligibility. Of these, 114 articles were excluded as 31 were abstracts without full text, 37 studies had participants that were not the same as those in the review, nine studies had study outcomes that did not match with the review questions, 12 studies focused on general leishmaniasis, eight studies were repeat publications, seven studies were in a language other than English, and 10 studies had different study designs. Finally, 35 studies were included in the review, as shown in Figure 1.
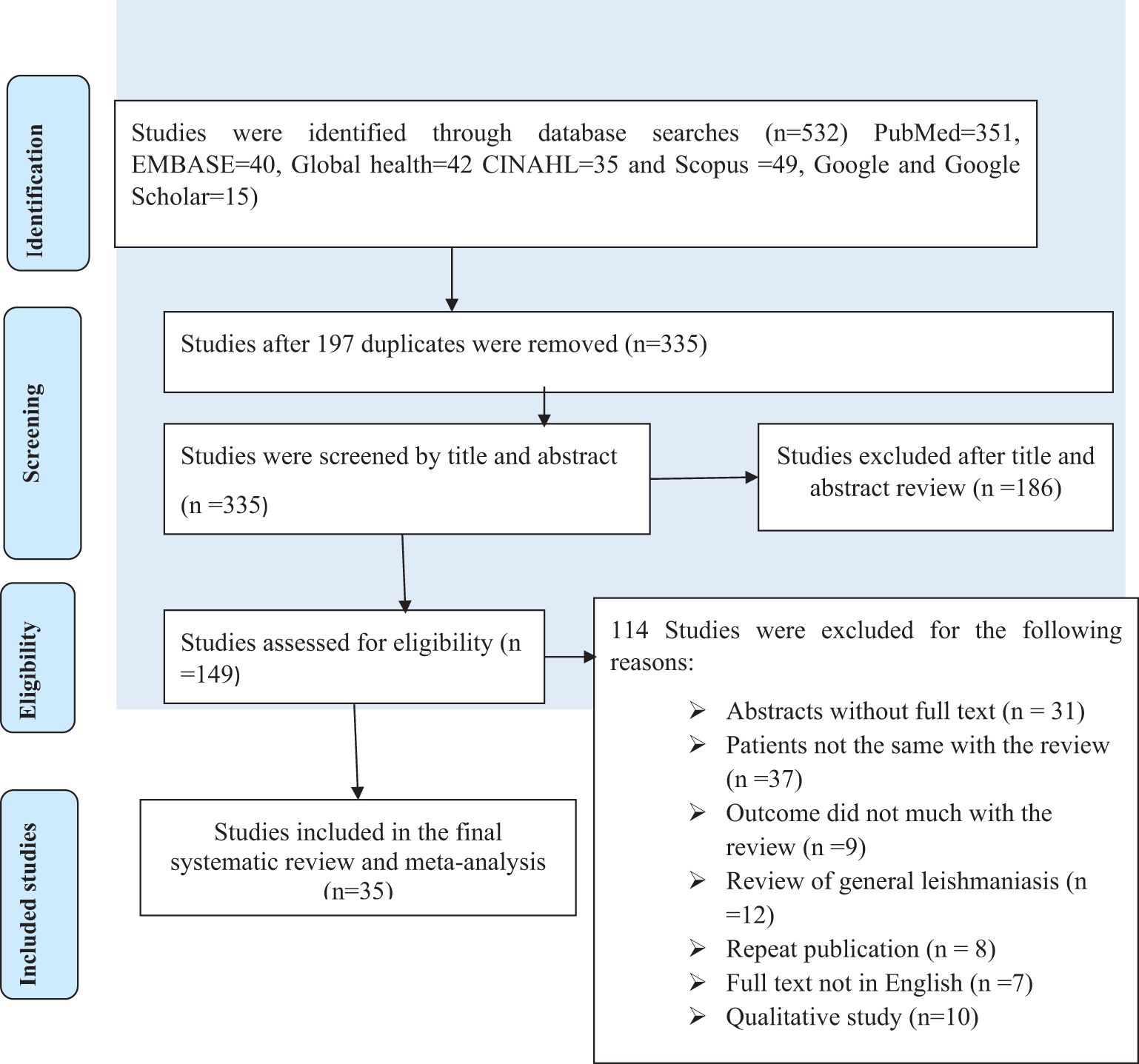
Figure 1. Flow chart of study selection for the systematic review and meta-analysis of the prevalence of cutaneous leishmaniasis in East Africa.
In total, 17 (48.6%) of the primary studies we found were published between 2016 and 2024; four (11.4%) of the primary studies included in the review were published between 2011 and 2015. A cross-sectional design was used in 29 (82.9%) of the studies included in the review, while 6 (17.1%) of the studies utilized a cohort study design.
The majority of the studies (85.7%) were from Ethiopia, while three (8.6%) were from Kenya.
A total of 221,365 study participants were included in the studies. The sample size in each primary study ranged from 94 to 71,325 in Ethiopia, as shown in Table 1.
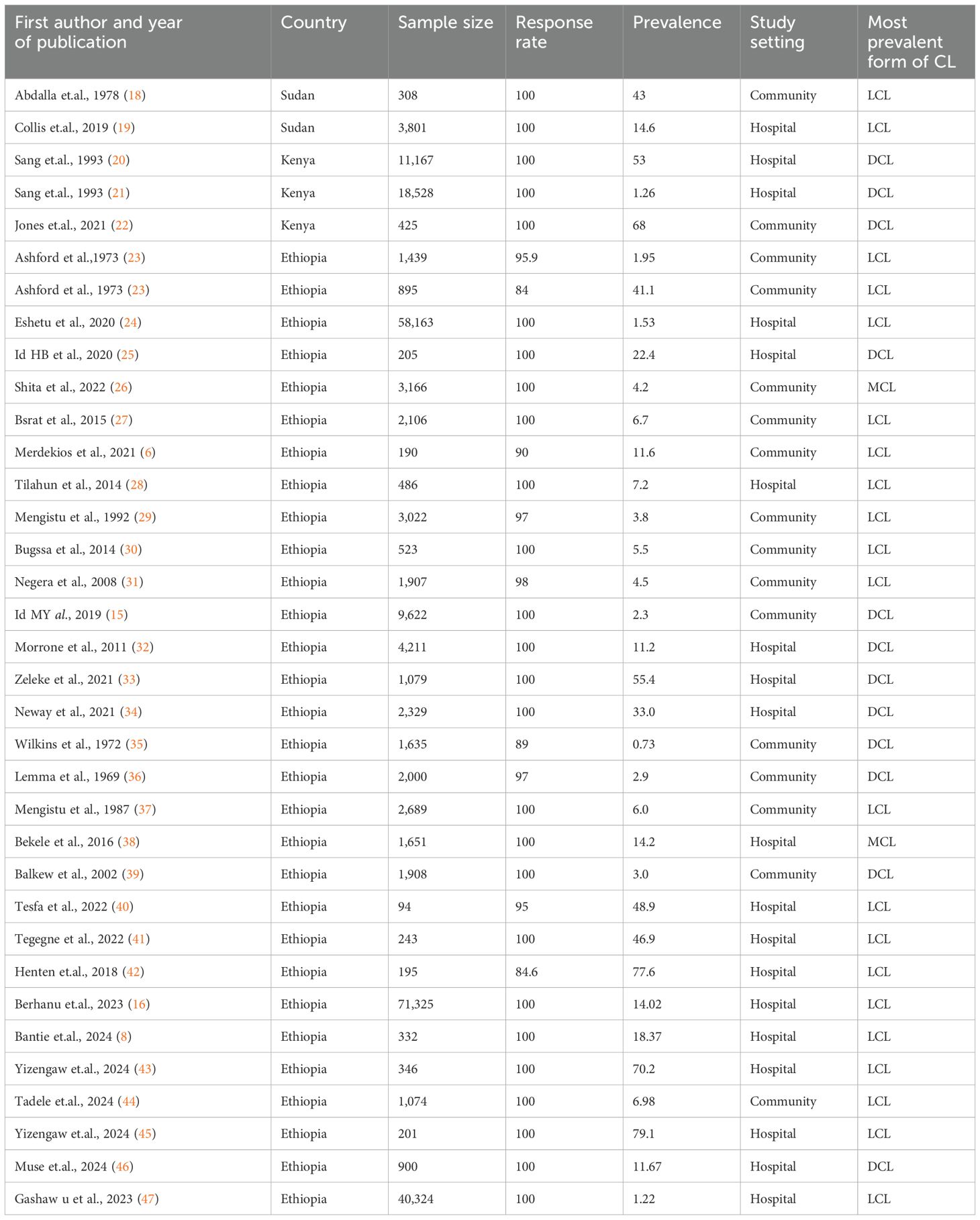
Table 1. Descriptive summary of the primary studies included in the systematic review and meta-analysis of the burden of cutaneous leishmaniasis among suspected cutaneous leishmaniasis patients in East Africa.
In a meta-analysis, a summary effect is typically estimated using two different types of models. These models, which include fixed and random effects, each have their own set of presumptions. According to a random effects model, which also assumes that the true effect size varies from investigation to investigation, the articles included in the analysis constitute a random sampling of effect sizes that could have been observed in each paper. Our estimation of these effects is the summary effect (variability in effect sizes is due to systematic error). The I² statistic quantifies the percentage of total variation across studies that is due to heterogeneity rather than chance. It ranges from 0% (no heterogeneity) to 100% (high heterogeneity). As can be seen from the forest plot, there was high heterogeneity among the included studies, which could be explained by I2 = 99.99% at p < 0.05. Therefore, a random effects model must be chosen to handle this variability. Thus, a random effects model was used to summarize the prevalence of CL in East Africa.
The prevalence of cutaneous leishmaniasis in the primary studies ranged from 0.73% to 79.10%. The pooled prevalence of CL in East Africa was found to be 22.57% with a 95% CI (14.36, 30.78), as shown in Figure 2.
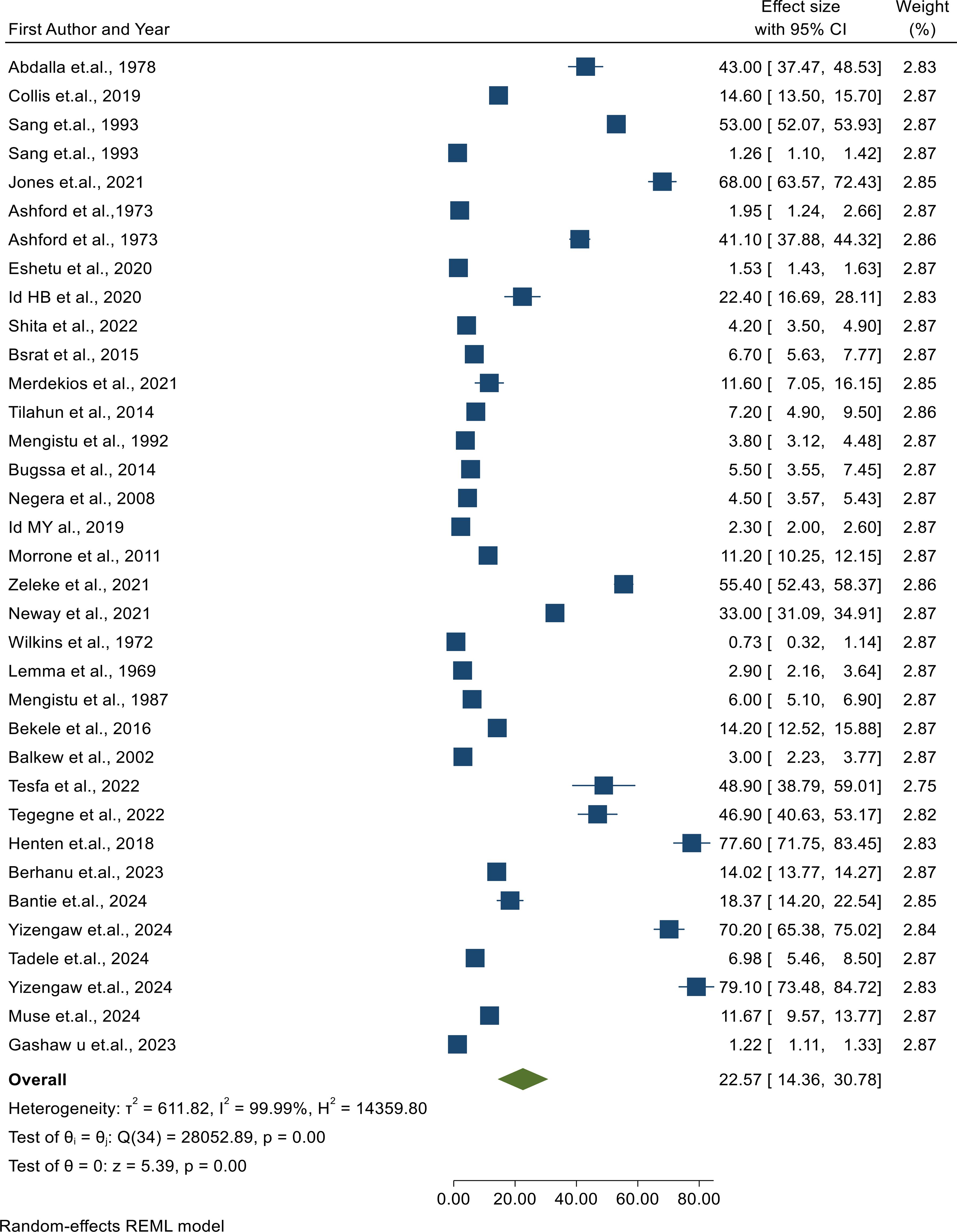
Figure 2. Forest plot showing the pooled prevalence of cutaneous leishmaniasis among patients suspected of having cutaneous leishmaniasis.
The prevalence of CL was observed to be highly influenced by different risk factors in many of the primary publications included in this review. The authors hypothesized that study design, study setting, sample size, year of publication, and study period may be the sources of the high heterogeneity among the studies included in the review, which was confirmed in the forest plot. In order to determine the most likely possible reason for heterogeneity, subgroup analyses were carried out on the effect sizes due to study design, data type, study setting, sample size, forms of CL, year of publication, and study period. Transmission dynamics and access to healthcare may differ between urban and rural settings, affecting the incidence and treatment outcomes of CL. Larger sample sizes generally provide more reliable estimates and reduce the influence of random variation. Analyzing the impact of sample size can help assess the robustness of findings. Over time, factors such as changes in treatment protocols, disease awareness, and vector control measures may influence the epidemiology and outcomes of CL. The effect size showed a statistically significant subgroup effect for study design, study setting, sample size, forms of CL, year of publication, and study period at p < 0.05 according to the subgroup outcome.
Regarding the study period, studies conducted after 2019 had significantly a slightly higher pooled level of CL prevalence [29.97% with a 95% CI (16.49% to 43.45%)] than articles conducted before 2019 [17.66% with a 95% CI (7.62% to 27.71%)] at p < 0.05. However, there was a considerable level of unexplained heterogeneity among the articles in each of these groups (I2; 2019+ = 100.00%; I2: < 2019 = 99.98%), as shown in Figure 3.
In comparison to the study setting, studies conducted at the hospital level had a significantly higher pooled level of CL prevalence [29.54% with a 95% CI (16.92% to 42.17%)] than articles conducted at the community level [16.71% with a 95% CI (6.37% to 27.05%)] at p < 0.05. However, there was a considerable level of unexplained heterogeneity across the articles in each of these groups (I2; hospital = 100.0%; I2: community = 99.97%), as shown in Figure 4.
In relation to sample size, studies conducted with a sample size of less than 1,000 had a significantly higher pooled level of CL prevalence [39.31% with a 95% CI (25.18% to 53.44%)] than articles conducted with a sample size greater than 1,000 [11.52% with a 95% CI (4.70% to 18.35%)] at p < 0.05. However, there was a considerable level of unexplained heterogeneity among the articles in each of these groups (I2; <1000 = 99.52%; I2: >1000 = 99.52%), as shown in Figure 5.
There are three forms of CL identified in this SRMA. Studies conducted in a majority of LCL cases had a significantly higher pooled level of LCL prevalence [24.18% with a 95% CI (12.88% to 35.48%)] than articles conducted in MCL [9.17% with a 95% CI (-0.63% to 18.97%)] at p < 0.05. However, there was a considerable level of unexplained heterogeneity among the articles in each of these groups (I2; with LCL = 99.99%; I2 with MCL = 99.14%), as shown in Figure 6.
A funnel plot was checked graphically to determine whether it was symmetrical or not, with the horizontal axis showing the effect estimates from individual studies and the vertical axis reflecting the standard error of the effect estimate. Articles with large effect sizes were dispersed at the top of the funnel plot in the diagram, whereas articles with small effect sizes were located at the bottom. The outcome of the plot resembled an inverted funnel with symmetry, showing that there was no publication bias, as shown in Figure 7. Finally, Egger’s test for small study effects was also done to identify publication bias statistically and was unable to show evidence of the existence of publication bias in this review (p =0.15).
The leave-one-out meta-analysis approach was used to assess the pooled prevalence of CL for the influence of each individual article on the overall pooled effect estimate. For each article, the displayed effect size corresponded to the overall effect size computed from a meta-analysis excluding that article. The leave-one-out forest plot also displayed a vertical line at the overall effect size based on the complete set of articles (with no omission) to help detect influential articles. We were unable to show evidence of the existence of excessive influence statistically due to the point estimate of all studies being below the confidence interval of the “combined” analysis, as shown in Figure 8.
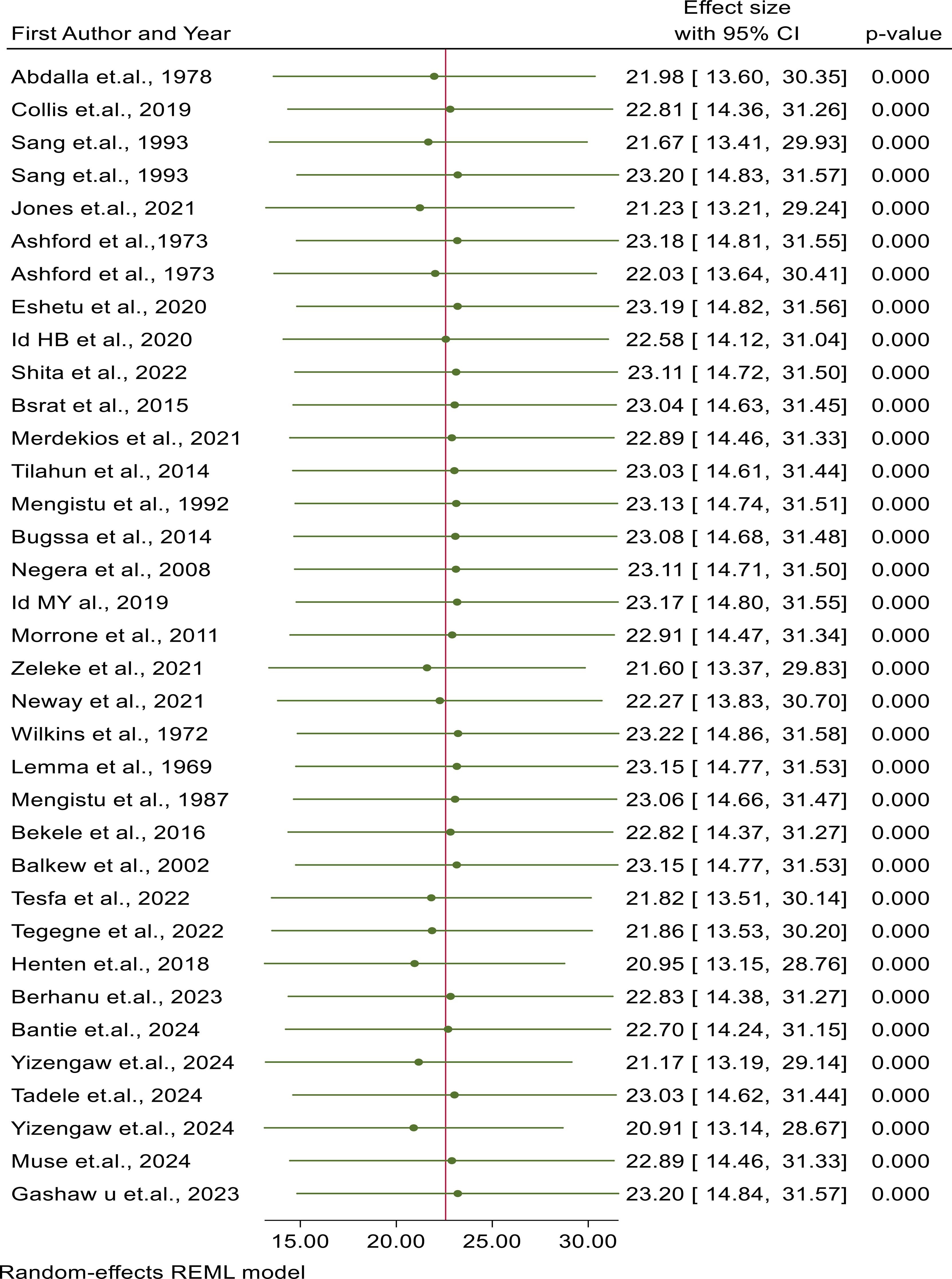
Figure 8. Sensitivity analysis of articles included in the review to check for the existence of heterogeneity.
A meta-regression with a significant amount of unexplained heterogeneity across articles was included in the review. This meta-analysis is very important to determine whether there are differences in study characteristics (methodological diversity) that could account for heterogeneity. This meta-analysis works only for reviews that use a random effects model. Hence, this review had a high heterogeneity between studies, so the study-level variables, such as sample size, study design, setting, study period, and country, were regressed to explain the existence of the heterogeneity. However, the results of the regression analysis showed that there was no statistically significant association between heterogeneity in the prevalence of cutaneous leishmaniasis due to country (P = 0.063), study period (P = 0.835), form of CL (P = 0.621), and setting (P = 0.860). Thus, country, study period, and setting were not identified as the causes of heterogeneity. Only sample size (P = 0.001, CI: -1.179 42.595) was identified as a possible cause of heterogeneity, as shown in Table 2.
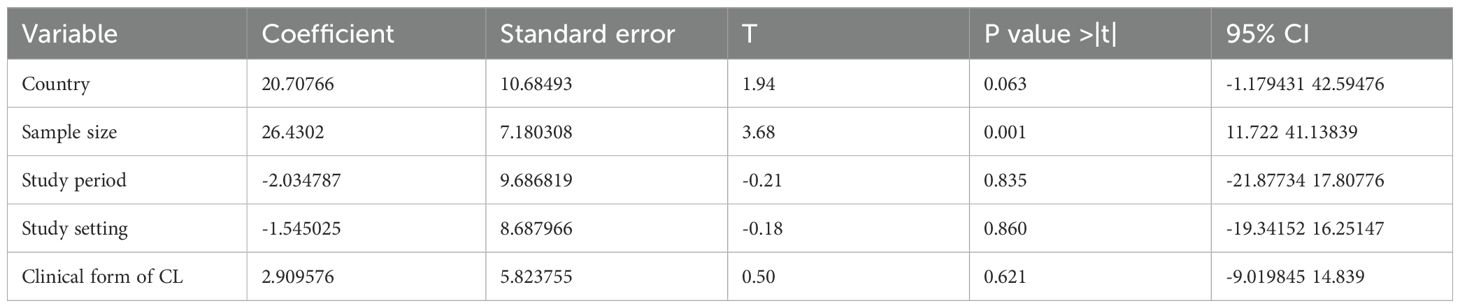
Table 2. Meta-regression for the included studies to identify the source of heterogeneity for the prevalence of cutaneous leishmaniasis in East Africa.
This systematic review and meta-analysis aimed to determine the pooled prevalence and associated determinants of CL in East Africa. Consequently, our intent for this study was to summarize the totality of the evidence regarding CL in East Africa after examining conflicting results from several individual research studies. The number of CL cases has significantly increased in East Africa in recent years, especially in Ethiopia, representing a major public health problem. It revealed that the presence of CL has been established in different regions of East Africa.
A total of 35 studies were included in the final analysis, providing an overall pooled prevalence of CL disease in East Africa of 22.57% (95% CI: 14.36% to 30.78%). Although the prevalence of CL varies from country to country and from study to study, in this review, the combined prevalence of CL was found to be high. It is higher than the findings from Burkina Faso (2.0%), Mali (0.6%), Nigeria (0.1%), Cameroon (0.8%), and Senegal (8.7%) (25, 27, 40, 48). This may be due to the region’s climate, particularly in rural and semi-urban areas, which provides favorable conditions for the sand fly vectors that transmit the disease. Other possible reasons may be that patients do not seek care due to the perceived self-healing nature of CL, poor access to health facilities as the majority of affected people live in remote rural areas, a lack of control tools, poverty, lack of access to healthcare, harmful practices related to CL, and insufficient public health infrastructure. This study found a higher pooled prevalence than in West Africa and North Africa. This may be due to East African countries having varying levels of healthcare access, public health infrastructure, and vector control measures compared to West Africa. In some East African countries, poverty and limited healthcare resources can also exacerbate the spread of the disease.
Regarding the study period, studies conducted after 2019 had a significantly slightly higher pooled level of CL prevalence [29.97% with a 95% CI (16.49% to 43.45%)] than articles conducted before 2019 [17.66% with a 95% CI (7.62% to 27.71%)]. There was high variability in the prevalence during the study period (25, 27, 40, 49). This may be due to disruptions caused by the COVID-19 pandemic, such as reduced healthcare access, shifted resources, and decreased surveillance, which may have led to underreporting or increased transmission due to reduced public health measures. Furthermore, migration and displacement due to conflict or economic opportunity may have led to higher exposure in endemic areas, facilitating the spread of the disease.
This may be due to a group of individuals over time, allowing researchers to observe the development of CL in real-time in cohort studies and snapshot studies that assess a population at a single point in time in cross-sectional studies. Other possible conditions may be due to missing cases that occurred before the study period or failure to capture individuals who are at risk but have not yet been infected in the case of cross-sectional studies.
Regarding the study setting, studies conducted at the hospital level had a significantly higher pooled level of CL prevalence [29.54% with a 95% CI (16.92% to 42.17%)] than articles conducted at the community level [16.71% with a 95% CI (6.37% to 27.05%)]. This finding is in line with other studies conducted in Ethiopia (8, 40, 42, 50). A possible reason may be due to hospitals primarily treating patients who exhibit symptoms severe enough to seek medical help, leading to a higher concentration of diagnosed cases. Furthermore, hospitals have the necessary facilities and expertise to diagnose CL effectively, increasing the likelihood of case identification. Finally, this may also be due to the fact that studies conducted at the hospital level recruited suspected patients presenting with signs and symptoms of disease and confirmed the type of disease they developed, compared to those conducted at the community level.
Regarding the sample size, studies conducted with a sample size of less than 1,000 had a significantly higher pooled level of CL prevalence [39.31% with a 95% CI (25.18% to 53.44%)] than articles conducted with a sample size greater than 1,000 [11.52% with a 95% CI (4.70% to 18.35%)]. There was a high variation in prevalence due to sample size from study to study (6, 31, 36). This may be due to larger studies including a more diverse and broader population, diluting the prevalence if many participants are not at risk and the higher prevalence of cutaneous leishmaniasis in studies with smaller sample sizes is often due to targeted sampling, increased detection sensitivity, and contextual factors that influence case identification.
Sample size could be a source of heterogeneity in this systematic review and meta-analysis for cutaneous leishmaniasis with a P-value of 0.015 (CI: 5.267154% to 44.20912%). One possible reason could be that larger studies generally have greater statistical power, which means that they are more capable of detecting true effects. Another explanation is that smaller studies may produce less reliable estimates, contributing to differences in effect sizes between studies when combined in a meta-analysis.
One of the strengths of this review was the inclusion of studies from a varied range of databases, which served to increase the number of papers from the East African region. The scientific plausibility of the study was maintained by strictly following the standardized reporting checklists of PRISMA, which is another strength of this review. By analyzing data from multiple studies, meta-analyses yield robust evidence that can inform public health policies, interventions, and resource allocation tailored to the specific needs of East African populations.
This systematic review and meta-analysis provide an overview of the pooled prevalence of cutaneous leishmaniasis in East African countries. However, the findings of this study have certain limitations. Among the limitations, subgroup analysis for studies between all countries was difficult to carry out due to statistical constraints and a limited number of studies. This makes our review subject to a high degree of heterogeneity between articles. However, the random effects model was used to obtain pooled results that minimize this heterogeneity between articles.
The pooled prevalence of cutaneous leishmaniasis in East Africa was high. The pooled prevalence of CL in East Africa highlights a significant public health challenge that varies between countries. This pooled prevalence underscores the urgent need for targeted public health initiatives to mitigate the impact of this disease on affected populations. Collaborative efforts between governments, health organizations, and communities are crucial for the effective management and control of CL in the region.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
MHA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. SM: Conceptualization, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. MAA: Conceptualization, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We are immensely grateful to the School of Public Health, College of Medicine and Health Sciences, Bahir Dar University for providing the chance to conduct this systematic review and meta-analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Veeri RB, Gupta AK, Pal B, Siddiqui NA, Priya D, Das P. Assessment of quality of life using WHOQOL-BREF in patients with visceral leishmaniasis. Health Qual Life Outcomes. (2019) 17(1):1–7.
2. Tidman R, Kanankege KST, Bangert M, Abela-Ridder B. Global prevalence of 4 neglected foodborne trematodes targeted for control by WHO: A scoping review to highlight the gaps. PloS Negl Trop Dis. (2023) 224:1–24. doi: 10.1371/journal.pntd.0011073
3. Leta S, Dao THT, Mesele F, Alemayehu G. Visceral leishmaniasis in Ethiopia: an evolving disease. PloS Negl Trop Dis. (2014) 8:1–7. doi: 10.1371/journal.pntd.0003131
4. Teshome DF, Alemu S, Ayele TA, Atnafu A, Gelaye KA. Based intervention on hypertension management in Northwest Ethiopia, 2021: study protocol for a cluster randomised controlled trial. (2022), 1–10. doi: 10.1136/bmjopen-2021-051178
5. Report AC. Progress of mucocutaneous leishmaniasis to drug nonresponsive diffuse cutaneous leishmaniasis. (2020), 551–5.
6. Merdekios B, Pareyn M, Tadesse D, Getu S, Admassu B, Girma N, et al. Detection of cutaneous leishmaniasis foci in South Ethiopia. (2021) 105:156–8. doi: 10.4269/ajtmh.20-0708
7. Khazaei S, Hafshejani AM, Saatchi M. Epidemiological aspects of cutaneous leishmaniasis in Iran. (2015) 10:0–4. doi: 10.5812/archcid.28511
8. Bantie B, Kassaw G, Demelash AT, Abate MW, Nigat AB, Amare AT, et al. Magnitude and associated factors of cutaneous leishmaniasis among patients visiting Nefas Mewcha primary hospital, Northern Ethiopia, 2022: An institution- based Cross- sectional study. (2024), 1–9. doi: 10.1136/bmjopen-2023-075549
9. Piyasiri SB, Dewasurendra R. Diagnostic tools for cutaneous leishmaniasis caused by Leishmania donovani: A narrative review. (2023). doi: 10.3390/diagnostics13182989
10. Debash H, Ebrahim H, Bisetegn H. Epidemiological and clinical characteristics of cutaneous leishmaniasis among patients attending at Tefera Hailu Memorial Hospital, Sekota, Northeast Ethiopia: A five-year trend analysis (2016 – 2020). (2022). doi: 10.1177/20503121221129720
11. Khan W, Khan I, Ullah H, Zain SNM, Panhwar WA, Mehmood SA, et al. Cutaneous leishmaniasis – Awareness, knowledge and practices among general population in rural and urban areas in Malakand region, Pakistan. (2022) 82:1–6. doi: 10.1590/1519-6984.238665
12. Alzahrani MJ, Elfaki N, Abdalla YHA, Alkhadher MA, Ali MHM, Ahmed WAM. Cutaneous leishmaniasis: associated risk factors and prevention in Hubuna, Najran, Saudi Arabia. Int J Gen Med. (2023) 16:723–31. doi: 10.2147/IJGM.S401618
13. Ghatee MA, Taylor WR, Karamian M. The geographical distribution of cutaneous leishmaniasis causative agents in Iran and its neighboring countries, A review. (2020) 8:. doi: 10.3389/fpubh.2020.00011
14. Shirzadi MR, Javanbakht M, Jesri N. Spatial distribution of cutaneous leishmaniasis cases referred to health centers of three Khorasan provinces in Iran using ge- ographical information system. (2019) 48:1885–92.
15. Yohannes M, Abebe Z, Boelee E. Prevalence and environmental determinants of cutaneous leishmaniasis in rural communities in Tigray, northern Ethiopia. (2019) :. doi: 10.1371/journal.pntd.0007722
16. Berhanu A, Dugassa S, Maru M, Animut A. Heliyon Cutaneous leishmaniasis in Kutaber District, Ethiopia: Prevalence, sand fly fauna and community knowledge, attitude and practices. Heliyon. (2023) 9:e18286. doi: 10.1016/j.heliyon.2023.e18286
17. Karunaweera ND, Senanayake S, Ginige S, Silva H, Manamperi N, Samaranayake N, et al. Spatiotemporal distribution of cutaneous leishmaniasis in Sri Lanka and future case burden estimates. (2021) 30:1–16. doi: 10.1371/journal.pntd.0009346
18. Abdalla RE, Sherif H. Epidemic of cutaneous leishmaniasis in Northern Sudan Epidemic of cutaneous leishmaniasis 1n Northern Sudan. Ann Trop Med Parasitol. (2017) 4983.
19. Collis S, El-sa S, Atia AA, Bhattacharyya T, Hammad A, Den Boer M, et al. Epidemiological and molecular investigation of resurgent cutaneous leishmaniasis in Sudan. Int J Infect Dis. (2019) 88:14–20. doi: 10.1016/j.ijid.2019.08.018
20. Sang DK, Okelo GBA, Ndegwa CW, Division RWA, Diseases B. New foci of cutaneous leishmaniasis in central Kenya and the Rift Valley. (1993), 629–32. doi: 10.1016/0035-9203(93)90265-R
21. Sang DK, Okelo GBA, Chance ML, Okelo GBA, Cutaneous MLC. Cutaneous leishmaniasis due to Leishmania aethiopica, on Mount Elgon, Kenya Cutaneous leishmaniasis due to Leishmania aethiopica, on Mount. Ann Trop Med Parasitol. (2017) 4983.
22. Jones CM, Welburn SC. Leishmaniasis beyond East Africa. Front Vet Sci. (2021) 8:1–10. doi: 10.3389/fvets.2021.618766
23. Ashford R, Bray MA, Hutchinson MP. The epidemiology of cutaneous leishmaniasis in Ethiopia. Trans R Soc Trop Med Hyg. (1973) 67:568–601. doi: 10.1016/0035-9203(73)90088-6
24. Eshetu B, Mamo H. Cutaneous leishmaniasis in north-central Ethiopia: trend, clinical forms, geographic distribution, and determinants. (2020).
25. Bisetegn H, Zeleke AJ, Gadisa E, Shumie G. Clinical, parasitological and molecular profiles of Cutaneous Leishmaniasis and its associated factors among clinically suspected patients attending Borumeda Hospital, North-. (2020), 1–13. doi: 10.1371/journal.pntd.0008507
26. Shita EY, Nibret E, Munshea A, Gashaw B. Burden and risk factors of cutaneous leishmaniasis in Ethiopia: a systematic review and meta-analysis. Int J Dermatol. (2022) 61:1336–45. doi: 10.1111/ijd.v61.11
27. Bsrat A, Berhe N, Balkew M, Yohannes M, Teklu T, Gadisa E. Epidemiological study of cutaneous leishmaniasis in Saesie Tsaeda-emba district, eastern Tigray, northern Ethiopia. (2015), 1–9. doi: 10.1186/s13071-015-0758-9
28. Tilahun F, Alemu W, Mulatu G. Magnitude and associated factors of cutaneous leishmaniasis; in Mekelle city, Ayder referral hospital. (2014) 3:189–99. doi: 10.11648/j.cmr.20140306.16
29. Mengistu G, Laskay T, Gemetchu T, Humber D, Ersamo M, Eva D, et al. Genene Mengistu”’, Tamas Laskay’, Teferi Gemetchu3, David Humber4, Mulugetta Ersamo’, David Eva&, Hailu Teferedegn’, Marie Anne Phelouzat7 and Dominique Frommel’**. (1992) :.
30. Bugssa G, Hailu A, Demtsu B. The current status of cutaneous leishmaniasis and the pattern of lesions in Ochollo primary school students, Ochollo, Southwestern Ethiopia. (2014) 3:111–6. doi: 10.11648/j.sjcm.20140306.13
31. Negera E, Gadisa E, Yamuah L, Engers H, Hussein J, Kuru T, et al. Outbreak of cutaneous leishmaniasis in Silti woreda, Ethiopia: risk factor assessment and causative agent identification. (2008). doi: 10.1016/j.trstmh.2008.03.021
32. Morrone A, Pitidis A, Chiara M, Dassoni F, Latini O, Ab G, et al. Transactions of the Royal Society of Tropical Medicine and Hygiene Epidemiological and geographical aspects of leishmaniasis in Tigray, northern Ethiopia: a retrospective analysis of medical records. Trans R Soc Trop Med Hyg. (2011) 105:273–80. doi: 10.1016/j.trstmh.2011.02.003
33. Zeleke AJ, Derso A, Yeshanew A, Mohammed R, Fikre H. Research article A ten-year trend of cutaneous leishmaniasis at university of Gondar hospital, Northwest Ethiopia: 2009-2018. (2021) 2021:. doi: 10.1155/2021/8860056
34. Neway S, Yeshitela B, Mebrat B, Gizealew A. Prevalence of cutaneous leishmaniasis in alert center, retrospective analysis, Addis Ababa. J Trop Dis Public Health. (2021) 3:110–21. doi: 10.52675/jhesp.949565
36. Lemma A, Foster WA, Gemetchu T, Preston PM, Bryceson A. Studies on leishmaniasis in Ethiopia. Ann Trop Med Parasitol. (2017) 4983.
37. Mengistu G, Humber DP, Ersumo M, Gizealew A. High prevalence of elephantiasis and cutaneous leishmaniasis in Ocholo, south- West Ethiopia. Ethiop Med J. (1987) 25:203–7.
38. Bekele S, Bekele Y, Mulatu F, Lemma T, Tilahun H, Gadisa E, et al. Recent trends of cutaneous leishmaniasis in alert hospital, Addis Ababa. Ethiop Med J. (2014) 1:1–11.
39. Balkew M, Gebre-Michael T, Berhe N, Ali A, Hailu A. Leishmaniasis in the middle course of the Ethiopian Rift Valley: II. Entomological observations. Ethiop Med J. (2002) 40:271–82.
40. Tesfa D, Manaye N, De Vries HJC, Van Griensven J, Enbiale W. Clinical pattern and treatment outcome of Cutaneous Leishmaniasis in two hospitals in Bahir Dar, Ethiopia (2017-2021). (2022). doi: 10.3855/jidc.15979
41. Tegegne B, Yimer M, Ejigu K, Alemu G. Eight-year trend analysis of cutaneous leishmaniasis cases in West Amhara region referred to Amhara public health institute Northwest, Ethiopia: A retrospective study. (2022) 2022:. doi: 10.1155/2022/6562092
42. Van Henten S, Adriaensen W, Fikre H, Akuffo H, Diro E, Hailu A, et al. EClinicalMedicine cutaneous leishmaniasis due to Leishmania aethiopica. EClinicalMedicine. (2018) 6:69–81. doi: 10.1016/j.eclinm.2018.12.009
43. Yizengaw E, Gashaw B, Yimer M, Takele Y, Nibret E, Yismaw G, et al. Demographic characteristics and clinical features of patients presenting with different forms of cutaneous leishmaniasis, in Lay. (2024), 1–17. doi: 10.1101/2024.02.15.24302809
44. Tadele G, Samuel A, Fite MB, Tasew G, Abera A. Prevalence and knowledge of cutaneous leishmaniasis in Aleku Area of Sayo District, Western Ethiopia: community- based cross-sectional study. J Med Public Health. (2024) .
45. Yizengaw E, Nibret E. Effects of cutaneous leishmaniasis on patients’ quality of life. BMC Infect Dis. (2024) 24:4–8. doi: 10.1186/s12879-024-09518-3
46. Muse AI, Ibrahim MO, Ibrahim MA. Clinical pattern and treatment outcome of cutaneous leishmaniasis patients in Somali region, eastern Ethiopia. Ski Heal Dis. (2024) . doi: 10.1002/ski2.416
47. Gashaw B, Yizengaw E, Yismaw G, Tebeje S, Tilahun F, Nibret E. Original Article Cutaneous Leishmaniasis: It ‘ s burden and challenge for patients and the health care system at Boru Meda Hospital., 1–7.
48. Oliveira F, Faye O, Dicko A, Coulibaly A, Sissoko IM, Sibiry S, et al. Prevalence of cutaneous leishmaniasis in districts of high and low endemicity in Mali. (2016), 1–12.
49. Diadie S, Manga IA, Sarr M, Balde E, Diop B, Mendy P. Cutaneous leishmaniasis in Senegal: When the practitioner is disarmed. (2024) 15:26–8.
Keywords: cutaneous leishmaniasis, heterogeneity, subgroup, prevalence and factors, meta-analysis
Citation: Ahmed MH, Mengiste SA and Asemahagn MA (2025) Prevalence of and risk factors for cutaneous leishmaniasis in East Africa: a systematic review and meta-analysis. Front. Trop. Dis. 6:1532049. doi: 10.3389/fitd.2025.1532049
Received: 21 November 2024; Accepted: 05 February 2025;
Published: 10 March 2025.
Edited by:
Rahul Shivahare, The Ohio State University, United StatesReviewed by:
Lucas Sousa Magalhães, Federal University of Alagoas, BrazilCopyright © 2025 Ahmed, Mengiste and Asemahagn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammedjud Hassen Ahmed, anVkaGFzc2VuNDIzQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.