- 1Department of Animal Biology and Conservation (ABC), Faculty of Science, University of Buea, Buea, ;Cameroon
- 2Department of AgroEcoHealth, International Institute of Tropical Agriculture (IITA), Cotonou, ;Benin
- 3Department of Microbiology and Immunology, Drexel University College of Medicine, Philadelphia, PA, ;United States
- 4Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Rockville, MD, ;United States
- 5Department of Biomedical Sciences, University of Bamenda, Bambili, ;Cameroon
Background: Refugees are vulnerable populations especially in malaria endemic areas where the disease claims many lives and constitutes an emerging challenge for humanitarian response. This study assessed how the influx and settlement of Central African Republic (CAR) refugees influences malaria burden in the Gado-Badzere refugee camp, Eastern Cameroon.
Methods: A cross-sectional malariometric survey was conducted between November 2022 and October 2023 in 324 households comprising 1,304 individuals aged 1 month and above. Malaria parasite burden was determined using rapid diagnostic tests (RDTs) and Giemsa-stained microscopy. Demographic characteristics, malaria risk factors, treatment-seeking behaviors and costs to cure malaria were assessed using semi-structured questionnaires.
Results: Of the 1,304 participants, 525 (40.3%) were malaria parasite positive with moderate geometric mean parasite density (GMPD) of 1100 parasites/μl of blood. Plasmodium falciparum was the main species (99.8%), with mixed P. vivax infections (0.2%). Insecticide treated net (ITN) ownership was 53.7%, but its utilization was significantly low (22.4%) (P < 0.001). Reason for no ITN ownership was net damaged (74.7%). Net insufficiency (77.8%) accounted for non-frequent ITN use. Mean expenditure to treat malaria in the hospital was higher (USD 13.64 ± 8.67) than auto-medication (USD 1.13 ± 1.18). Significantly, malaria parasite prevalence and risk were higher for 0-5 years age (43.7%, OR = 1; P = 0.02), residents of sector 8 (49.2%, OR = 2.53; P < 0.001) of the camp, non-frequent ITN users (41.7%, OR = 2.08; P < 0.001), people living around stagnant water (44.4%, OR = 1.55; P < 0.001) and during the rainy season (43.5%, OR = 1.31; P = 0.02). The GMPD/µl was significantly higher in the 0-5 years age group (1456, P < 0.0001), inhabitants of sector 9 (1626, P = 0.04) and participants living around stagnant water (2097, P = 0.01).
Conclusion: The malaria burden in CAR refugees may represent the reservoir for malaria transmission, especially with the circulation of P. vivax. The improper use of ITNs could be ameliorated through sensitization. Seasonal chemoprevention mainly during the rainy season and Indoor Residual Spraying (IRS) might be implemented for effective malaria control in refugee settings.
1 Background
Eradicating human malaria has been a major global and public health objective, particularly in tropical countries, with sub-Saharan Africa bearing the highest burden (1). Since the early 2000s, extensive malaria interventions have been implemented, including artemisinin-based combination therapies (ACTs), large-scale distribution of insecticide treated nets (ITNs) and indoor residual spraying (IRS). These measures have significantly reduced malaria incidence and prevalence in many regions, including Cameroon (2). However, despite these efforts, residual malaria transmission remains a challenge in many high-incidence areas (3–5). Transmission is influenced by both biotic and abiotic factors, such as vector species’ biting patterns and infectiousness, as well as human behaviors and activities (6–8).
One critical human activity impacting malaria transmission is armed conflict, which causes population displacements, destroys infrastructures, collapses healthcare systems, and increases malaria risk in refugee-hosting areas (9–12). The United Nations High Commissioner for Refugees (UNHCR) guidance note on malaria programmes in refugee operations highlights the poor living conditions of refugees, including fragile household economics, inadequate shelter, poor nutrition, environmental exposure, and limited healthcare access, which exacerbate malaria risks (13). Cameroon has a long history of providing refuge to asylum seekers and refugees in the central African region and the country continues to be impacted by three complex humanitarian crises: the Lake Chad basin conflict, the North-west and South-west crisis and the Central African Republic (CAR) crisis. With over 480,000 refugees and asylum seekers, including about 349,000 from CAR, Cameroon remains significantly impacted (14).
The civil conflicts in CAR, driven by rebel groups, have led to massive population migrations, especially to Cameroon’s East Region, a high malaria prevalence area (2) The UNHCR notes that displaced populations in malaria-endemic regions pose substantial health risks and present new challenges for humanitarian response (13). Initially, malaria control programmes in refugee camps included the distribution of ITNs as the only form of physical protection against malaria, alongside free malaria testing and treatment for all age groups till 2020. However, since 2021, malaria testing and treatment has been restricted to children from 0-5 years, pregnant women and persons with special needs. Pregnant women and children, who are relatively immune-naïve against malaria, account for most cases and deaths (13). Consequently, the exemption of other age groups or categories from the free-of-charge health care services for malaria may impede malaria elimination or control in such vulnerable populations, which could constitute a reservoir for malaria dissemination in the country. These declarations makes malaria among refugees a disquieting issue in Cameroon where the entire country is exposed to the risk of transmission (15). Unfortunately, data on how the influx and settlement of people influences malaria prevalence and risk factors in Cameroon’s refugees is extremely limited.
This study aims to investigate the factors that lead to persistent malaria parasite infections in Cameroon’s second largest refugee camp, despite the prevention, control and treatment efforts by UN aid agencies. Specifically, it seeks to address the population’s attitudes and practices towards malaria prevention tools, shelter and environmental conditions, and treatment seeking behaviors and costs during malaria episodes.
2 Methods
2.1 Study area
The study was conducted in the Gado-Badzere refugee camp which was created after several years of civil conflicts in Central African Republic. The conflicts have triggered the displacement of over 329,000 refugees into Cameroon’s Adamawa, East and North Regions (14). The camp is the second largest in the country in terms of size and population. As of December 2023, the camp counts 23,544 refugees occupying 8,818 households, divided into 12 sectors, extending over a surface area of 55 hectares (16). The UNHCR in partnership with other aid agencies are present on the field to provide various support to the refugees.
Geographically, the camp is situated in the Garoua-Boulai Sub-division, Lom and Djerem Division, East Region of Cameroon (Figure 1). It is about 35km away from CAR border and is located between latitude 5°45’15.9114’’N and longitude 14°26’0.6’’E (UNHCR, 2024). The region is characterized by a wet equatorial climate with a mean temperature of 24°C on average and found between 500 and 1000m above sea level. The climate comprises four seasons, two dry seasons (mid-November to mid-March and mid-June to mid-August) and two rainy seasons (mid-March to mid-June and mid-August to mid-November) with mean annual rainfall varying between 1500 to 4000 mm/year. The East Region belongs to a holoendemic stratum with high and perennial malaria transmission (2, 17).
Most refugees live in huts constructed with mud or tarpaulin walls and grass-thatched or tarpaulin roofs (Figure 2). Majority are Muslims (93%) and the main ethnic group is the Fulani (85.5%). Many are house wives, livestock producers, manual workers, traders and farmers (16). Gado-Badzere has an Integrated Health Centre for medical assistance to both the local population and refugees.

Figure 2. Typical hut types in the study area. (A) a rectangular hut with grass-thatch roofing and tarpaulin walls. (B) a rectangular hut with grass thatch roofing and mud walls. (C, D) a round and rectangular hut respectively with tarpaulin roofing and walls.
2.2 Study design and population
This was a cross-sectional study on CAR refugees of all ages and both sexes, living in the Gado-Badzere refugee camp, between November 2022 and October 2023. Prior to data collection, the chief of each sector was informed by a community relay (translator) about the study protocol and the potential benefits of participation to the community. After obtaining a written informed consent/assent from the parents/care givers, we proceeded with the collection of samples at the different sectors of the site.
2.3 Sample size
The sample size (N) was estimated using the Lorentz formula for single proportion; N = Z2pq/d2. Z= 1.96 is the standard normal deviate (for a 95% confidence interval, CI), p = 46.4% is the prevalence of malaria recorded among a community in the East Region of Cameroon (18), q = 1-p is the contrary event and d = is the allowable error (5%). The minimum sample size was therefore estimated at N = 382 persons.
2.4 Assessment of refugee attitudes and practices regarding malaria transmission
A structured questionnaire was pretested and used for this survey. The questionnaire had four components: the first captured the socio-demographic characteristics of participants (gender, age, education level, marital status, occupation and religion); the second assessed the hut characteristics (good with closed eave spaces and poor with opened eave spaces) and environmental variables (seasonality, presence of vegetation and stagnant water around huts); the third examined the preventive measures of malaria (ITN ownership and use, sources of ITN acquisition, reason for non-ITN ownership, frequent ITN use and reasons for non-frequent ITN use); and the fourth addressed the treatment-seeking behaviors (hospital consultations, traditional pharmacopoeia and auto-medication) and costs during suspected cases of malaria.
The questionnaire was administered by a trained personnel alongside a translator to consenting household heads, their spouses or children from 18 years old in 324 randomly selected households in the camp. Before administration, the questionnaire was translated into “Fulfulde” or “Sango”, which are the common languages spoken by CAR citizens across the camp.
2.5 Sample collection and malaria diagnosis
Following consent/ascent, malaria diagnosis was performed on all individuals present in each household ensuing interview. Blood samples were obtained from individuals without a travel history and who had not received malaria treatment 2 weeks prior to the examination.
Approximately 3-4 mL of venous blood sample were collected from volunteer participants using sterile disposable 5ml syringes. Rapid diagnostic test (RDT) was performed on the field using a differential diagnosis between Plasmodium vivax (P. v) and P. falciparum (P. f) (Bioline™ Malaria Ag P.f/P.v test kit) by Abbott Diagnostics, Korea Inc. (LOT-number: 05DDG022A and REF number: 05FK80) according to the manufacturer’s instructions. Individuals positive for malaria were treated the same day with artemisinin-based combination therapy according to the WHO recommendations.
Thick blood smears were performed on the spot by dispensing about 6 µl of blood on clean grease-free slides. It was transported in a slide box to the laboratory of the Gado-Badzere Health Centre to identify malaria parasites and estimate parasite density on samples. The films were Giemsa stained and examined in the laboratory following standard procedures (19). All slides examined by a malaria research scientist were independently examined by two microscopists of the Gado-Badzere Integrated Health Centre. Parasite density was determined based on the number of parasites per 200 leukocytes count on a thick film, assuming a total white blood cell count of 8,000 cells/mL of whole blood. Specifically, parasite density was scored against 200 leukocytes after counting ≥ 100 parasites; and scored against 500 leukocytes after counting ≤ 99 parasites (20); otherwise, slides were declared negative if no parasites were detected after a count of 500 leukocytes. Malaria parasitemia was categorized as low (< 1000 parasites/μl blood), moderate (1000–4999 parasites/μl blood) and high (5000–99,999 parasites/μl blood) (21).
2.6 Data analysis
Data were coded and entered into spread sheets using Microsoft Excel 2016. After data cleaning, analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 20 (IBM-SPSS, Inc, Chicago, IL, USA) and MedCalc version 15.8 software. Data was summarized into means and standard deviations (SD), and percentages were used in the evaluation of the descriptive statistics. Independent Chi-square (χ2) and Fisher’s exact tests were used to compare proportions between categorical variables (gender, age groups, and sectors). Malaria parasite counts were log transformed before analysis. The geometric mean parasite density (GMPD) was used to compare the intensity of infection and differences were compared using Mann–Whitney U test and Kruskal-Wallis test where appropriate. Univariate and multivariate logistic regression models were used to determine the potential risk factors of malaria prevalence. The statistical level of significance was set at P <0.05.
3 Results
3.1 Socio-demographic characteristics, hut construction materials and environmental indices
Among the 324 households surveyed, female participants were more represented than males (78.7% vs. 21.3%). Participants’ ages ranged from 18 to 78 years, with a median age of 35.21 ± 12.18 years. Household sizes varied from 1 to 14 individuals per hut (median = 5.52 ± 2.09). The majority of participants were Muslims (91%), housewives (54.6%) with no formal education (71.9%). The main income-generating activities were small-scale business (15.1%) and farming (13.6%) as seen in Table 1.
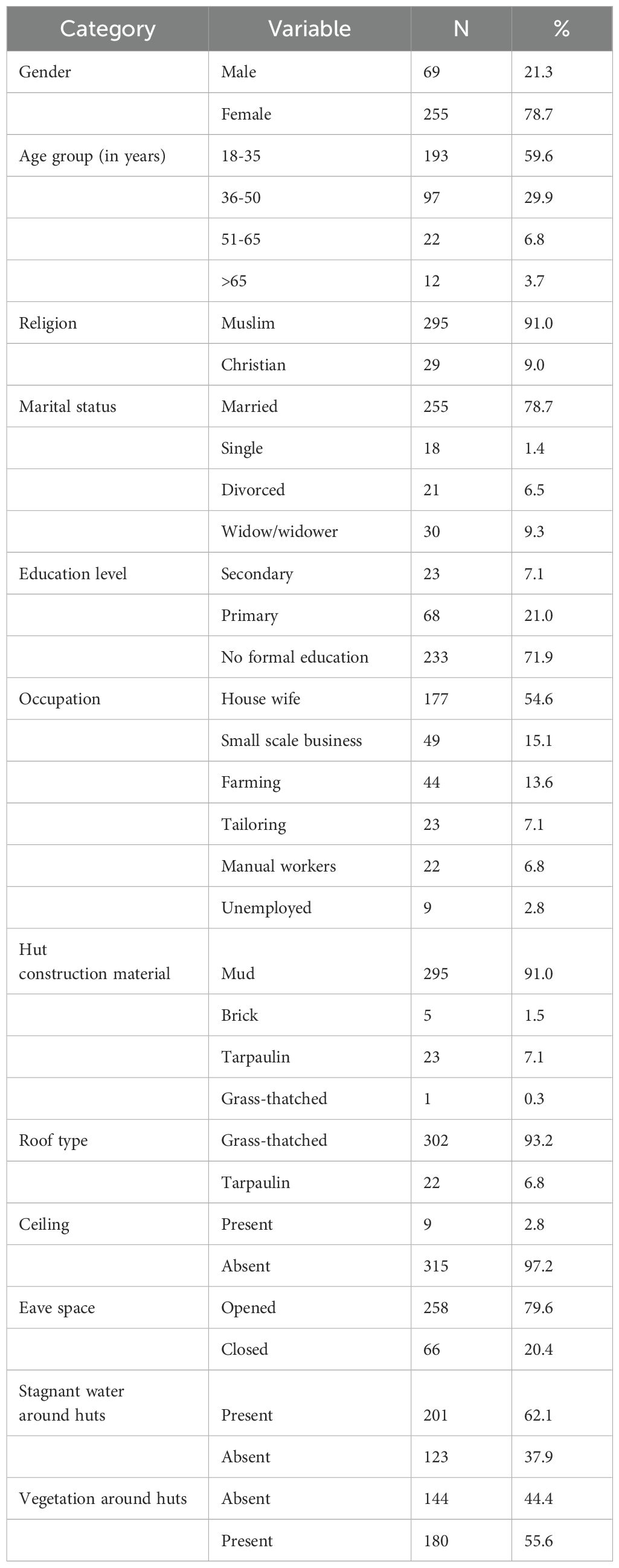
Table 1. Socio-demographic characteristics, physical characteristics of huts and environmental variables.
Most huts had mud walls (91%) and grass-thatched roofs (93.2%). No corrugated iron sheet roofing was observed in the camp. Ceilings were absent in most huts (97.2%) and eave spaces were mostly opened (79.6%). Many huts were surrounded by bushes (55.6%) and stagnant water (62.1%) (Table 1).
3.2 Net ownership and use by respondents
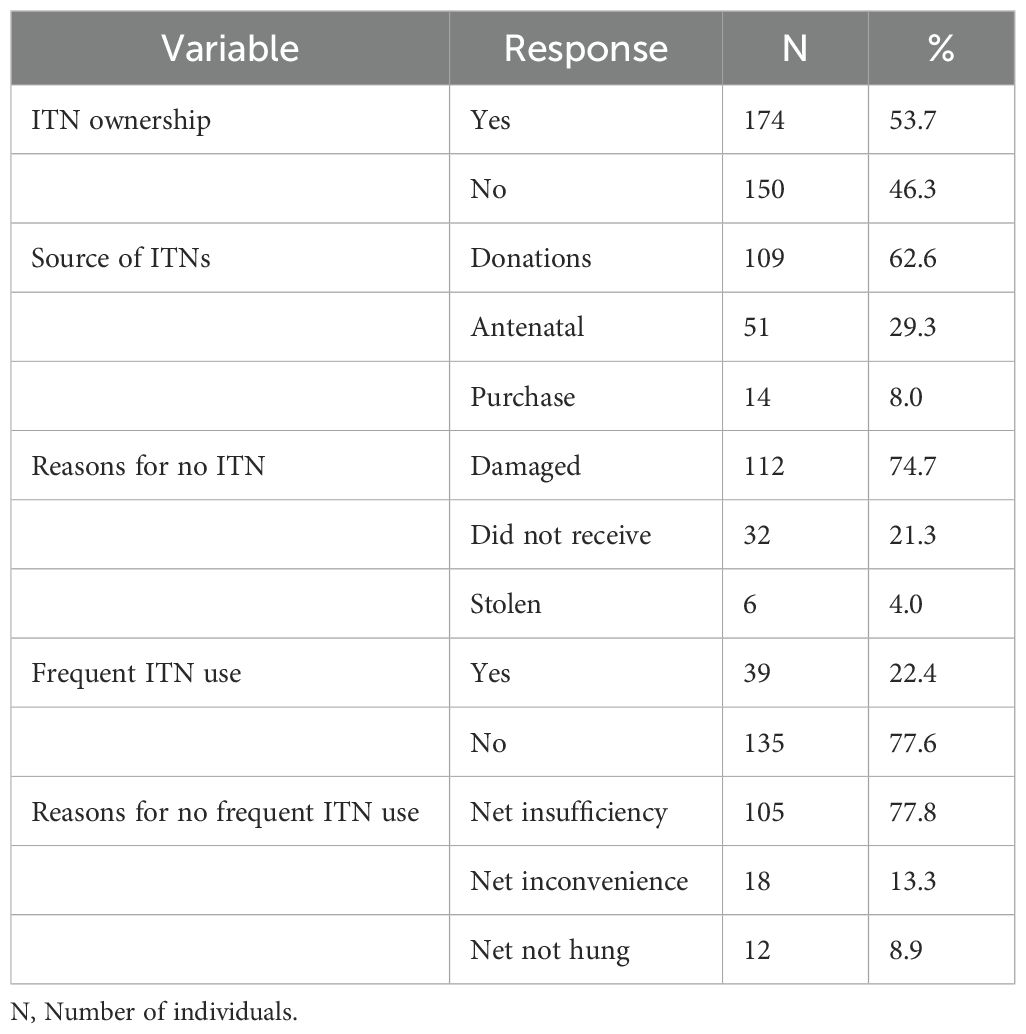
ITN ownership (defined as the proportion or percentage of households owning at least one bed net) was 53.7% with an average of 1.36 ± 0.61 nets per household (range = 1-3 nets). Most nets were acquired through donations from the government/United Nation aid agencies (62.6%) and antenatal visits (29.3%). Participants who did not own bed nets (46.3%) mainly reported that theirs were already damaged or torn (74.7%). Frequent ITN usage, calculated as the percentage of people sleeping under the bed nets every day was low (22.4%) and was mostly attributed to net insufficiency (77.8%) as seen in Table 2.
3.3 Malaria treatment-seeking behaviors and expenditures
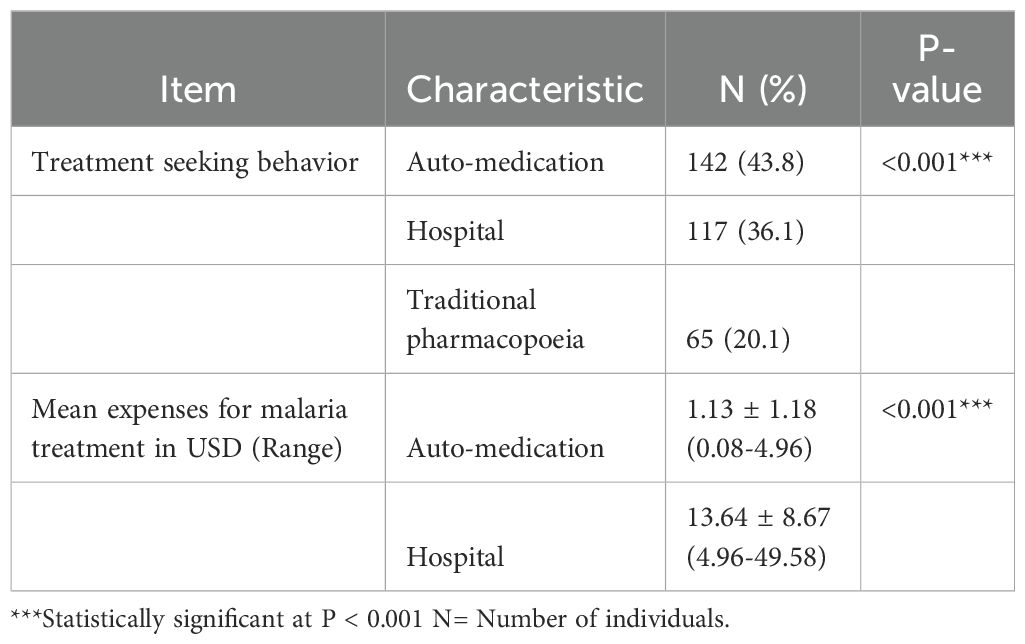
During suspected malaria cases, respondents opted for auto-medication, hospital treatment, or traditional pharmacopoeia. Auto-medication was significantly (P < 0.001) the most common (43.8%). Expenditures were significantly (P < 0.001) higher for hospital treatments (USD 13.64 ± 8.67) compared to auto-medication (USD 1.13 ± 1.18). Hospital consultation costs ranged from USD 4.96 to 49.58, and auto-medication costs ranged from USD 0.08 to 4.96 (Table 3).
3.4 Malaria prevalence and parasitemia by RDT and microscopy
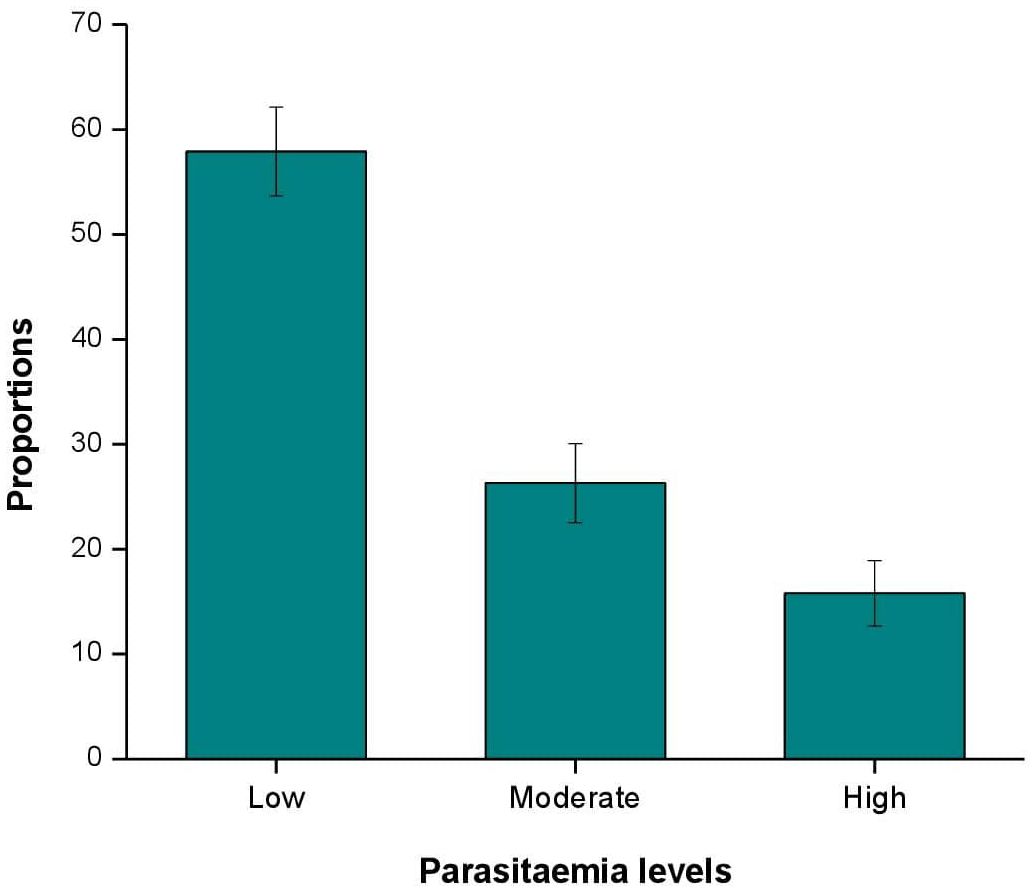
Of the 1,304 participants, 523 (40.1%) tested positive for malaria by RDT. Microscopy identified two additional cases, making the total 525 positives (40.3%). P. falciparum was the dominant species (99.8%), with a small proportion of mixed infections (0.2%) with P. vivax. Parasitemia levels varied significantly (P < 0.001). The prevalence of low parasitemia was 57.9% (95% CI: 53.7%-62.1%), followed by moderate parasitemia 26.3% (95% CI: 22.5%-30.1%) and lastly high parasitemia 15.8% (95% CI: 12.7%-18.9%) (Figure 3).
3.5 Socio-demographic factors, malaria parasite and risk
Participants examined for malaria parasite were 1 month to 78 years old with a mean (SD) of 14.86 (15.43) years. The malaria rate per household (defined as the number of households with at least one infected individual) was 77.8% (252/324). The overall prevalence of malaria parasite in the study population was 40.3% (525/1,304) with a geometric mean parasite density (GMPD) of 1100 parasites/µl of blood.
Malaria parasite prevalence and density were comparable between males (42.5%, 1086 parasites/µl of blood) and females (38.6%, 1111 parasites/µl of blood). A significant difference was observed with age. Lower malaria parasite prevalence and risk was found in the 16-35 years (33.3%, OR = 0.64; P = 0.02) and >35 years (23.5%, OR = 0.39; P < 0.001) age groups, compared with the 0-5 years (47.3%) and 6-15 years age groups (48.5%). Of statistical significance (P < 0.001), the 0-5 years age group had the highest GMPD/µl of blood (1456) compared to their elder 6-15 years (971), 16-35 years (716) and >35 (904) counterparts. Although, not statistically significant (OR = 0.88, P = 0.10), participants living in huts with a family size of more than 5 members had lower GMPD/μl blood (1005) than households with less than 5 members (1227).
Several sectors of the camp were significantly associated with higher malaria parasite prevalence and risk namely; sector 8 (49.2%, OR = 2.53; P < 0.001), sector 5 (49.1%, OR = 2.52; P < 0.001), sector 3 (47.4%, OR = 2.35; P < 0.001), sector 4 (43.9%, OR = 2.04; P = 0.01) and sector 6 (41.2%, OR = 1.83; P = 0.03). Similarly, participants of some sectors were significantly (P = 0.04) associated with higher GMPD/µl of blood [sector 9 (1626), sector 6 (1511), sector 4 (1254), sector 5 (1110) and sector 3 (1099)] than others [sector 1 (1081), sector 11 (1074), sector 7 (994), sector 12 (977), sector 8 (932), sector 2 (900) and sector 10 (894)], as seen in Table 4.
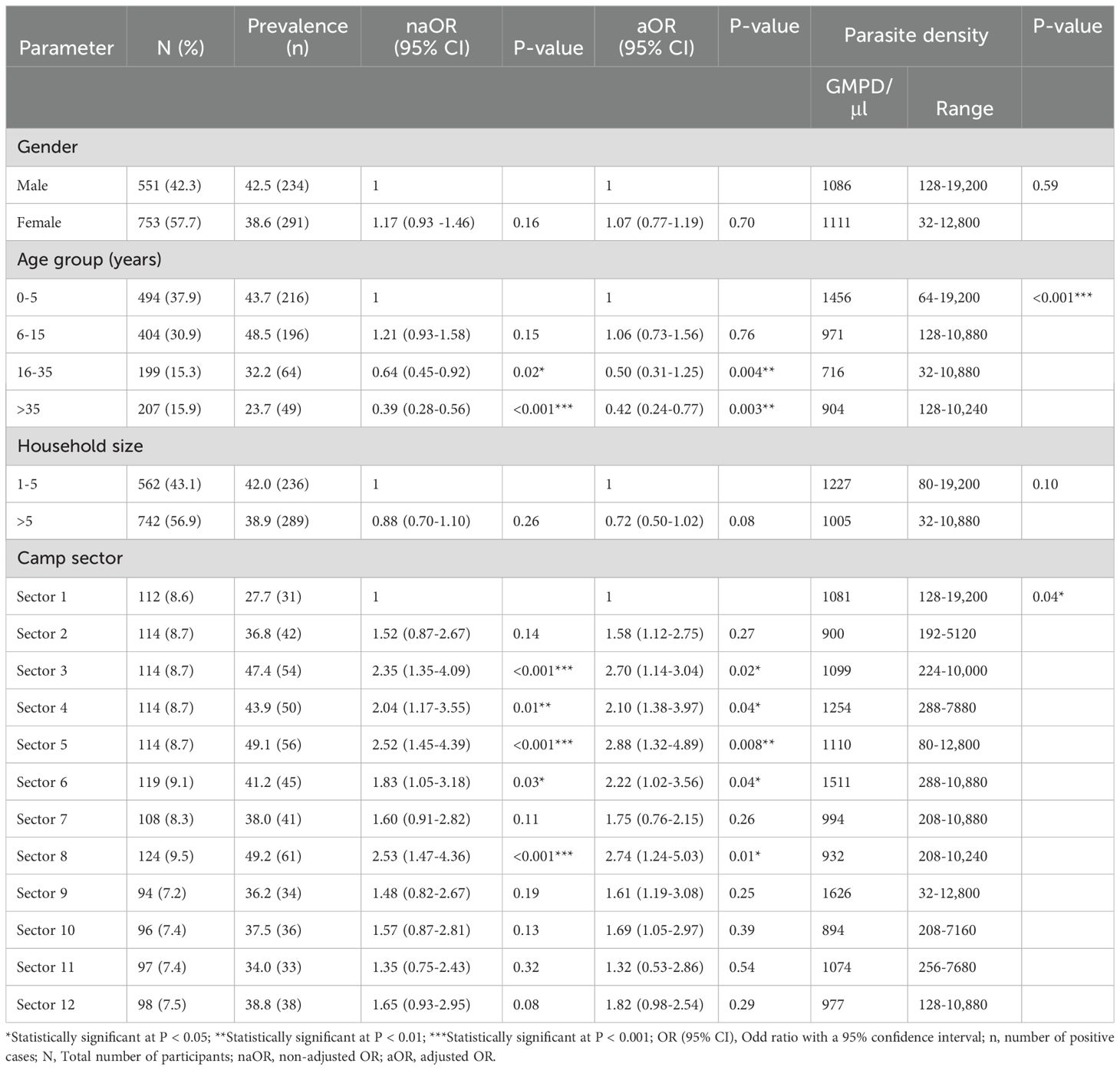
Table 4. Malaria prevalence and parasite density with respect to socio-demographic characteristics of CAR refugees.
3.6 ITN ownership, treatment options, hut constructions, environmental factors and malaria indices
Malaria parasite prevalence among non-frequent ITN users was significantly higher (41.7%) with 2.08 times odds of being infected by Plasmodium than ITN users (25.5%, OR = 2.08; P < 0.001). However, there was no significant difference between their GMPD/µl of blood. Participants who practiced traditional pharmacopoeia had higher malaria parasite prevalence (43.2%) and density (1399 parasites/µl) although not significant when compared with those who preferred hospital consultation or auto-medication (Table 5).
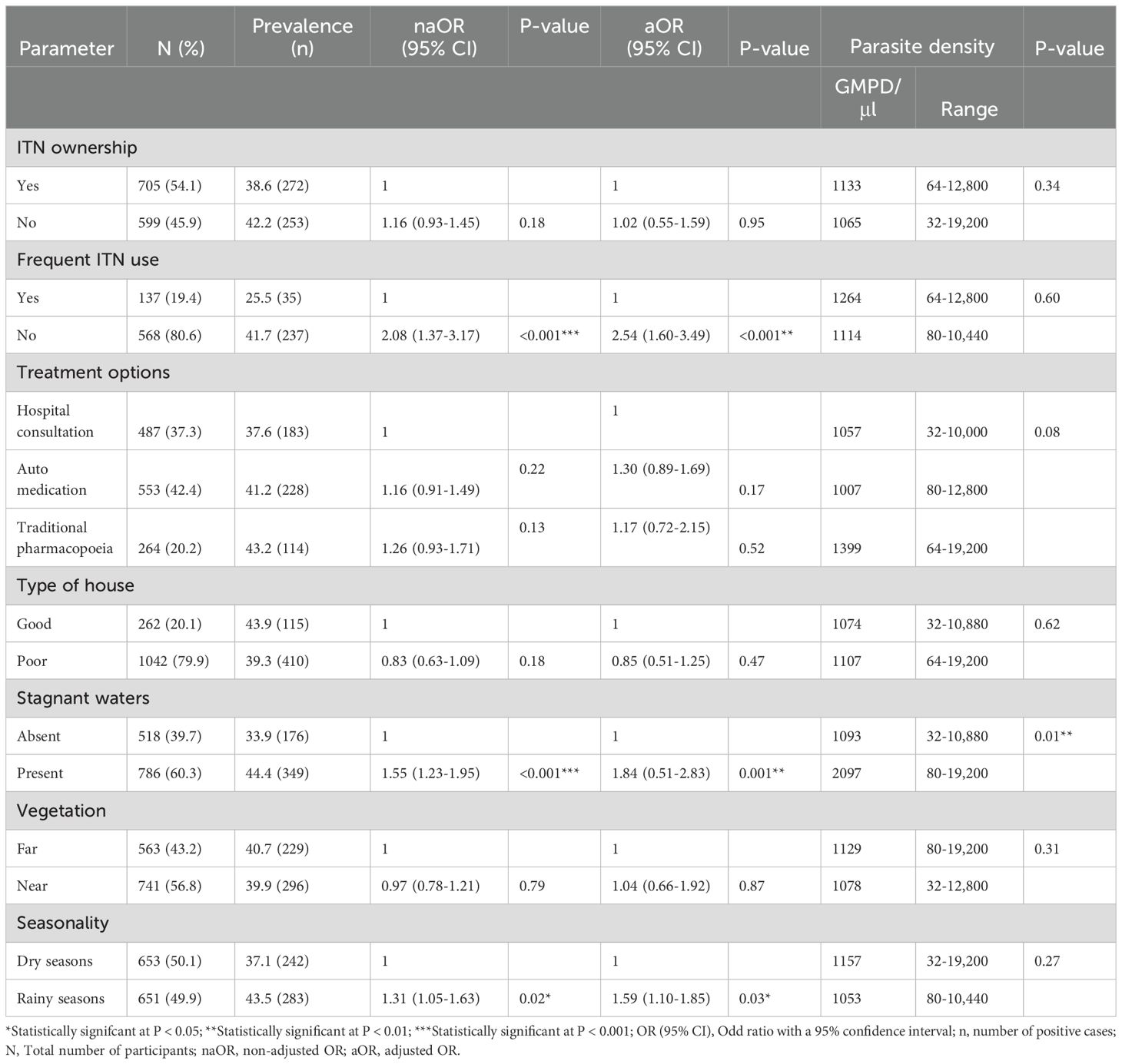
Table 5. ITN ownership and use, treatment options, hut construction, environmental factors and malaria indices.
Living in poorly constructed huts was not significantly associated with high malaria risk (OR = 0.83, P = 0.18). According to the environmental features, the presence of stagnant water around habitations (44.4%, OR = 1.55; P < 0.001) and the rainy seasons (43.5, OR = 1.31; P = 0.02) were significantly associated with higher malaria risk and prevalence. The nearness of huts to vegetation was not a risk factor for malaria parasite infection (OR = 0.97, P = 0.79). Participants living around stagnant waters had a significantly higher GMPD/µl of blood (2097, P = 0.01) compared to those who lived far (1093). However, the GMPD/µl of people dwelling near vegetation (1078) or far (1129) from it did not show a significant association with the participant’s GMPD/µl. With regards to seasonality, participant’s GMPD/µl of blood were comparable between the rainy seasons (1053) and the dry seasons (1157).
The results from both the univariate and multivariate binary logistic regression analyses showed that age groups, camp sectors, ITN usage, mosquito breeding sites around huts, and seasonality were each significantly associated with malaria parasite burden. The consistency of findings across both models highlights the robustness of these associations, suggesting that the identified factors are independently linked to malaria burden, even when adjusted for potential confounders. This alignment between the two analytical approaches strengthens the validity of the observed relationships and indicates that each factor plays a significant and independent role in influencing malaria risk within this population.
4 Discussion
The collaborative efforts of the local government, non-governmental organizations (NGOs) and aid agencies, are crucial in providing supplies, services and malaria prevention measures such as insecticide-treated bed nets (ITNs) and prophylaxis to refugees. This study highlights how socio-demographic and environmental factors, alongside the use of mosquito prevention methods, influence malaria infection rates among CAR refugees in the Gado-Badzere refugee camp.
ITNs were the primary preventive measure used by the camp’s residents. Despite the universal distribution of bed nets which occurred 6 months prior to the beginning of this study, individual coverage was only 53.7%. This rate is comparable to an Internally Displaced Person (IDP) camp in Uganda (57%) and the China-Myanmar border (76.4%) following mass ITN distribution (22, 23). However, this result contradicts what has been seen in an IDP camp in the Eastern Democratic Republic of Congo (DRC) where despite a high free distribution of bed nets, only 29% of IDP households owned a bed net (11). Up to 74.7% of people declared that their nets were already damaged or torn. This is probably due to the quality of the nets and the frequent washing due to the mud floors which soils the nets (11). This is in accordance with several authors who deplored a poor utilization of this tool by household members (7, 24). The non-ITN ownership could also be because people were absent during the distribution or their nets were stolen when dried outside as reported by (23). Interestingly, in another study majority of displaced persons reported selling their nets or exchanging them to meet immediate needs such as food (11). ITN ownership does not equate to frequent use. Bed net utilization was low, with net insufficiency cited as a significant barrier. Overcrowded households often lead to people sleeping on the floor without net coverage, increasing exposure to malaria transmission. ITN shortage in households were also reported by other authors (7, 11, 25). The frequent disuse of bed nets was due to net inconvenience (unpleasant chemical smell, needs frequent washing) (11). Some people deliberately refused to hang the net because it is cumbersome (confers more heat, difficulties in net installation) within their small huts and the lack of beds or matresses to fit in the nets have been well documented by some authors (7, 11). Disuse of nets due to inconvenience and the use of nets for other purposes, like fencing domestic animals and farms were common. This indicates a need for further sensitization on the importance of ITNs. These poor practices toward ITN use were also reported by several authors in different epidemiological settings (7, 25–27). In view of these findings, it is obvious that refugees are reluctant to use the bed nets. In such cases, additional or alternative control methods, such as Indoor Residual Spraying (IRS), should be evaluated and considered as complementary strategies.
Most refugees practiced self-medication during malaria episodes. Initially, aid agencies provided free healthcare services for malaria to all refugees, but later on only some age groups and categories were targeted. This might have favored auto-medication practices coupled with the high cost of hospital treatment compared to auto-medication observed in this study.
The overall malaria parasite prevalence in CAR refugees was 40.3% by microscopy and 40.1% by RDT. This confirms the concurrence of RDT in malaria diagnosis in endemic settings as reported by (28). This prevalence is higher compared to that published by National Malaria Control Programme (2) in the general population of the East region (34.2%), implying that these refugees may serve as the reservoir for malaria dissemination thereby increasing the risk of transmission to the host or nearby communities. The main species was P. falciparum, with mixed infections of P. falciparum and P. vivax in children aged 12, 9 and 6 years old. Similarly, other authors found 3 cases of mixed P. falciparum and P. vivax infections conducted by nested polymerase Chain reaction (PCR) on symptomatic refugees in a war-torn area in Pakistan where P. vivax was instead the common species (29). In that study, half of the vivax malaria patients were children and they attributed this situation to their poor yet-to-develop immunity against the parasites. Although P. vivax was uncommon in sub-Saharan Africa, several studies have documented its gradual emergence in many sub-Saharan African countries, including Cameroon. It is now obvious that P. vivax is gaining ground in Cameroon and has been reported in previous studies (30–34). There is a need to address P. vivax species in Cameroon’s refugee camps due to its dormant stages (hypnozoites) which are resistant to antimalarial drugs and are responsible for malaria relapses several months to years after the first infection (35). Moreover, its interaction with other Plasmodium species can regulate malaria severity (34). The high malaria prevalence can be attributed to the refugees’ residence in a high transmission region, where malaria thrives year-round (2, 17). Low and moderate parasitemia levels suggest that many participants were healthy carriers of the Plasmodium parasite, representing a significant reservoir for transmission if left untreated. Besides, refugee sites often promote mosquito breeding due to their marginal locations (13). The prevalence obtained in this study is comparable to that obtained by Gangueu et al. (18) who had a higher prevalence of 46.4% in asymptomatic pygmy people living in Cameroon’s East region. Indeed, the region has a favorable climate for the proliferation of the main malaria vectors Anopheles gambiae s.l in the country (36–39). Several studies also documented high malaria morbidity in displaced camps in Uganda, Sudan, Pakistan and the DRC (11, 23, 29, 40, 41).
Parasite prevalence and density were age-dependent with the 16-30 and >30 years old being less infected, probably due to their high protective immunity against malaria compared to the younger age groups (0-5 years) who are still immune naïve or inefficiently using the bed net as reported elsewhere (12, 22, 36, 41, 42). This finding reaffirms that malaria remains a major cause of morbidity and mortality among refugee children (13, 43) and this situation calls for an immediate humanitarian response. In order to provide a sustainable solution to improve the well-being of this vulnerable age group, aid agencies have been providing free malaria diagnosis and treatment services in the camp. However, to strengthen protection and reduce malaria incidence in children under 5, the addition of seasonal malaria chemoprevention (SMC) especially in peak transmission seasons could be suggested as a valuable complement to existing interventions. Gender did not have a significant association with Plasmodium infection and risk, though males were more infected than females. This might be because men are mostly involved in outdoor activities more than females (29, 41, 44).
Malaria parasite infection rates varied by camp sector, with the most densely populated sectors showing higher prevalence and risk. Crowded living conditions increase exposure to mosquito bites, promoting malaria transmission as also indicated by certain authors (45–47). In addition, some authors reported that mosquitoes only bite people when they overlap in space and in time (48, 49).
The level of infection between frequent ITNs users and non-frequent users was assessed; non-frequent ITN users had a higher malaria prevalence and were 2 times more exposed to Plasmodium infection than frequent ITNs users. This increased malaria risk in non-frequent users of bed net was also reported by several studies in sub-Saharan Africa (36, 50–53). Modifications to bed net design, such as promoting round ITNs over rectangular ones, may make refugees more user-friendly in their cramped shelters. Additionally, continuous sensitization campaigns are essential to promote ITN use among displaced populations.
Initially, malaria consultations, diagnoses, and treatments were free for refugees of all age groups, which encouraged many to seek medical help during suspected malaria episodes. However, this free healthcare was later restricted to children aged 0-5 years, pregnant women, and individuals with special needs by aid agencies. In this study it was found that the parasite burden was highest in refugees who practiced traditional pharmacopoeia followed by auto-medication. This restriction might have led the exempted groups to resort to self-medication and traditional pharmacopoeia due to financial constraints and the significantly high costs of hospital treatments for malaria observed in this study. These treatment practices associated to high parasite burden could be explained in terms of delayed, poor or incomplete treatment.
As a result, the efficacy, dose and toxicity of the plant species used during traditional pharmacopeia remains questionable. Also, during auto-medication one maybe administered incorrect doses since drugs are at times sold depending on the amount of money the patient has. Self-medication practice with antimalarial and/or traditional drugs has also been reported by Foko et al. (34) in asymptomatic infection cases in different malaria epidemiological facets in Cameroon. Self-medication practices may lead to frequent malaria relapses, drug related side effects and even cause the emergence and spread of ACT resistance in P. falciparum parasites especially when used as first line treatments (34, 54).
In complex emergencies, adequate shelters are crucial to avoid malaria, yet the hut characteristics have shown not to be a risk factor for malaria in CAR refugees. This contradicts other studies that reported housing design significantly influence malaria transmission (51, 55–58). Among the environmental factors, the presence of stagnant water around huts and the rainy season were important malaria risk factors. This may suggests that during the rainy seasons, mosquito breeding sites (shallow pools of water and puddles) multiply and enable the easy and rapid proliferation of the different malaria vectors (59, 60).
The investigation of the malaria parasite burden and the variability of risk factors among CAR refugees are the strengths of this study. Despite the insights gained, it is essential to acknowledge the study’s inherent limitations. The diagnostic confirmation of Plasmodium species relied solely on RDTs and microscopy rather than PCR, which is known for its higher sensitivity and specificity in identifying malaria species. While RDTs and microscopy are widely used due to their practicality and cost-effectiveness in field settings, their lower sensitivity may have missed very low parasitemia levels, potentially leading to false negatives or missed cases. Furthermore, RDTs can yield false positives due to cross-reactivity with other infections, contributing to an underestimation of the true prevalence of malaria parasites in the study population. That notwithstanding, the low occurrence of P. vivax in this population calls for further investigation into the burden of sub-microscopic infections of P. vivax.
5 Conclusion
Findings of this study revealed that, malaria parasite burden was high among CAR refugees. The infrequent use of ITNs, age groups difference, mosquito breeding sites around huts, seasonality and living in particular sectors of the camp led to greater exposure to infection, high parasite prevalence and load. These provides vital information on the risk factors of Plasmodium infection to governments and NGOs operating in complex humanitarian crises zones. There is need for greater sensitization on the importance of the frequent use of ITNs in the camp during malaria-related communication. Complementary interventions such as the management of mosquito breeding habitats, IRS and seasonal malaria chemoprevention, especially during the rainy seasons should be considered when updating control interventions. The high cost to cure malaria in the hospital and the preference to self-medication in refugee settings could be averted by provision of free malaria diagnosis and treatment to all age groups and categories. In spite of this high falciparum malaria prevalence, the management of non-falciparum species is worth addressing in refugee camps.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of the Faculty of Health Sciences, University of Buea, Cameroon. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
ZW: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. FD: Conceptualization, Writing – review & editing. RT: Conceptualization, Formal analysis, Writing – review & editing. ML-D: Conceptualization, Data curation, Formal analysis, Resources, Writing – review & editing. CD: Writing – review & editing. RB: Writing – review & editing. EL: Resources, Writing – review & editing. HK: Resources, Writing – review & editing. IS: Conceptualization, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are grateful to the East Regional Delegation of Public Health and also to the United Nations High Commissioner for Refugee’s head office of Meiganga for their approval to conduct this study. We would like to thank the aid agency Africa Humanitarian Action (AHA) and the clinical staff of the Gado-Badzere Integrated Health Centre for their unconditional support during this study. Finally, we wish to appreciate all the community relays and CAR refugees for their participation during data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO. World malaria report 2023. Geneva: World Health Organization (2023) p. 1–356 p. Available at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023.
2. PNLP. Plan Stratégique National de Lutte contre le Paludisme au Cameroun 2019-2023. Yaounde, Cameroon (2019). p.103. Available at: https://pnlp.cm/ (accessed January 28, 2024).
3. Jumbam DT, Stevenson JC, Matoba J, Grieco JP, Ahern LN, Hamainza B, et al. Knowledge, attitudes and practices assessment of malaria interventions in rural Zambia. BMC Public Health. (2020) 20:1–15. doi: 10.1186/s12889-020-8235-6
4. Bamou R, Kopya E, Djamouko-Djonkam L, Awono-Ambene P, Tchuinkam T, Njiokou F, et al. Assessment of the Anophelinae blood seeking bionomic and pyrethroids resistance of local malaria vectors in the forest region of Southern Cameroon. JEZS. (2020) 8:1054–62.
5. Mieguim Ngninpogni D, Ndo C, Ntonga Akono P, Nguemo A, Nguepi A, Metitsi DR, et al. Insights into factors sustaining persistence of high malaria transmission in forested areas of sub-Saharan Africa: the case of Mvoua, South Cameroon. Parasit Vectors. (2021) 14:1–10. doi: 10.1186/s13071-020-04525-0
6. Finda MF, Moshi IR, Monroe A, Limwagu AJ, Nyoni AP, Swai JK, et al. Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PloS One. (2019) 14:e0217414. doi: 10.1371/jisiournal.pone.0217414
7. Kala Chouakeu NA, Ngingahi LG, Bamou R, Talipouo A, Ngadjeu CS, Mayi MPA, et al. Knowledge, attitude, and practices (KAP) of human populations towards malaria control in four ecoepidemiological settings in Cameroon. J Trop Med. (2021) 2021:11. doi: 10.1155/2021/9925135
8. Bamou R, Sonhafouo-Chiana N, Mavridis K, Tchuinkam T, Wondji CS, Vontas J, et al. Status of Insecticide Resistance and Its Mechanisms in Anopheles Gambiae and Anopheles coluzzii Populations from Forest Settings in South Cameroon. Genes (Basel). (2019) 10:741. doi: 10.3390/genes10100741
9. Marou V, Vardavas CI, Aslanoglou K, Nikitara K, Plyta Z, Leonardi-Bee J, et al. The impact of conflict on infectious disease: a systematic literature review. Confl Health. (2024) 18:27. doi: 10.1186/s13031-023-00568-z
10. Charchuk R, Paul MKJ, Claude KM, Houston S, Hawkes MT. Burden of malaria is higher among children in an internal displacement camp compared to a neighbouring village in the Democratic Republic of the Congo. Malar J. (2016) 15:431. doi: 10.1186/s12936-016-1479-z
11. Brooks HM, Jean Paul MK, Claude KM, Mocanu V, Hawkes MT. Use and disuse of malaria bed nets in an internally displaced persons camp in the Democratic Republic of the Congo: a mixed-methods study. PloS One. (2017) 12:e0185290. doi: 10.1371/journal.pone.0185290
12. Hauser M, Kabuya J-BB, Mantus M, Kamavu LK, Sichivula JL, Matende WM, et al. Malaria in refugee children resettled to a holoendemic area of sub-saharan Africa. Clin Infect Dis. (2023) 76:e1104–13. doi: 10.1093/cid/ciac417
13. UNHCR. Guidance note on malaria programmes in refugee operations (2022). Available online at: www.unhcr.org. (Accessed January 28, 2024)
14. OCHA. United Nations Office for the Coordination of Humanitarian Affairs. Operational data portal (2023). Available online at: http://communityindustrygroup.org.au/wp-content/uploads/Working-with-Interpreters.pdf. (Accessed February 02, 2024)
15. Minsante. XIième Journée mondiale de lutte contre le paludisme “’prêt à vaincre le paludisme’” Nous sommes la génération qui peut éliminer le paludisme. Yaounde, Cameroon: Doss Press Minsante (2018) p. 1–20.
16. UNHCR. Profil du site de Gado (2024). Available online at: www.https://reliefweb.int/report/Cameroon/cameroun-profil-du-site-de-gado-decembre-2023. (Accessed February 13, 2024)
17. Antonio-Nkondjio C, Ndo C, Njiokou F, Bigoga JD, Awono-Ambene P, Etang J, et al. Review of malaria situation in Cameroon: Technical viewpoint on challenges and prospects for disease elimination. Parasites Vectors. (2019) 12:1–23. doi: 10.1186/s13071-019-3753-8
18. Diane Clotilde GD, Jeannette Y, Nadia NAC, Rosine NV, Joel ATR, Mbida M. Parasitaemia in asymptomatic Bantu and Baka Pygmy people piving in the East Region, Cameroon. Int J Trop Dis. (2020) 3. doi: 10.23937/2643-461x/1710039
19. Cheesbrough M. District Laboratory Practice in Tropical Countries. Cambridge, United Kingdom: Cambridge University Press (2009). 434 p.
20. WHO. Malaria Parasite Counting (2016). Available online at: https://apps.who.int/iris/handle/10665/274382. (Accessed October 10, 2022)
21. Kimbi HK, Nkesa SB, Ndamukong-Nyanga JL, Sumbele IUN, Atashili J, Atanga MBS. Knowledge and perceptions towards malaria prevention among vulnerable groups in the Buea Health District, Cameroon. BMC Public Health. (2014) 14:883. doi: 10.1186/1471-2458-14-883
22. Zhou G, Lo E, Zhong D, Wang X, Wang Y, Malla S, et al. Impact of interventions on malaria in internally displaced persons along the China–Myanmar border: 2011–2014. Malar J. (2016) 15:471. doi: 10.1186/s12936-016-1512-2
23. Spencer S, Grant AD, Piola P, Tukpo K, Okia M, Garcia M, et al. Malaria in camps for internally-displaced persons in Uganda: evaluation of an insecticide-treated bednet distribution programme. Trans R Soc Trop Med Hyg. (2004) 98:719–27. doi: 10.1016/j.trstmh.2004.01.012
24. Ordinioha B. The use and misuse of mass distributed free insecticide-treated bed nets in a semi-urban community in Rivers State, Nigeria. Ann Afr Med. (2012) 11:163. doi: 10.4103/1596-3519.96879
25. Talipouo A, Ngadjeu CS, Doumbe-Belisse P, Djamouko-Djonkam L, Sonhafouo-Chiana N, Kopya E, et al. Malaria prevention in the city of Yaoundé: Knowledge and practices of urban dwellers. Malar J. (2019) 18:1–13. doi: 10.1186/s12936-019-2799-6
26. Yandaï FH, Moundine K, Djoumbe E, Boulotigam K, Moukenet A, Kodindo ID, et al. Perception de risques du paludisme et utilisation des moustiquaires au TChad. Int J Biol Chem Sci. (2017) 11:228–36. doi: 10.4314/ijbcs.v11i1.18
27. Enama MLO, Ntonga PA, Mbida AM, Takap NN, Mbiada B, Hondt OEN, et al. Le paludisme: connaissances, attitudes et pratiques des chefs de ménage de la region de l’ouest-Cameroun. J Appl Biosci. (2020) 147:15117–24. doi: 10.35759/JABs.147.5
28. Teh RN, Sumbele IUN, Asoba Nkeudem G, Meduke DN, Ojong ST, Kimbi HK. Concurrence of CareStart™ Malaria HRP2 RDT with microscopy in population screening for Plasmodium falciparum infection in the Mount Cameroon area: predictors for RDT positivity. Trop Med Health. (2019) 47:1–11. doi: 10.1186/s41182-019-0145-x
29. Karim AM, Hussain I, Malik SK, Lee JH, Cho IH, Kim YB, et al. Epidemiology and clinical burden of malaria in the war-torn area, orakzai agency in Pakistan. PloS Negl Trop Dis. (2016) 10:e0004399. doi: 10.1371/journal.pntd.0004399
30. Mbenda HGN, Gouado I, Das A. An additional observation of Plasmodium vivax malaria infection in Duffy-negative individuals from Cameroon. J Infect Dev Ctries. (2016) 10:682–6. doi: 10.3855/jidc.7554
31. Fru-Cho J, Bumah VV, Safeukui I, Nkuo-Akenji T, Titanji VPK, Haldar K. Molecular typing reveals substantial Plasmodium vivax infection in asymptomatic adults in a rural area of Cameroon. Malar J. (2014) 13:1–11. doi: 10.1186/1475-2875-13-170
32. Russo G, Faggioni G, Paganotti GM, Djeunang Dongho GB, Pomponi A, De Santis R, et al. Molecular evidence of Plasmodium vivax infection in Duffy negative symptomatic individuals from Dschang, West Cameroon. Malar J. (2017) 16:1–9. doi: 10.1186/s12936-017-1722-2
33. Ngassa Mbenda HG, Das A. Molecular evidence of Plasmodium vivax mono and mixed malaria parasite infections in Duffy-negative native Cameroonians. PloS One. (2014) 9:e103262. doi: 10.1371/journal.pone.0103262
34. Kojom Foko LP, Hawadak J, Kouemo Motse FD, Eboumbou Moukoko CE, Kamgain Mawabo L, Pande V, et al. Non-falciparum species and submicroscopic infections in three epidemiological malaria facets in Cameroon. BMC Infect Dis. (2022) 22:900. doi: 10.1186/s12879-022-07901-6
35. Lin JT, Saunders DL, Meshnick SR. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol. (2014) 30:183–90. 10.1016/j.pt.2014.02.004
36. Bamou R, Mbakop LR, Kopya E, Ndo C, Awono-Ambene P, Tchuinkam T, et al. Changes in malaria vector bionomics and transmission patterns in the equatorial forest region of Cameroon between 2000 and 2017. Parasit Vectors. (2018) 11:464. doi: 10.1186/s13071-018-3049-4
37. Tchuinkam T, Simard F, Lélé-Defo E, Téné-Fossog B, Tateng-Ngouateu A, Antonio-Nkondjio C, et al. Bionomics of Anopheline species and malaria transmission dynamics along an altitudinal transect in Western Cameroon. BMC Infect Dis. (2010) 10:119. doi: 10.1186/1471-2334-10-119
38. Chouakeu NAK, Tchuinkam T, Bamou R, Bindamu MM, Talipouo A, Kopya E, et al. Malaria transmission pattern across the Sahelian, humid savanna, highland and forest eco-epidemiological settings in Cameroon. Malar J. (2023) 22:116. doi: 10.1186/s12936-023-04544-z
39. Amvongo-Adjia N, Wirsiy EL, Riveron JM, Chounna Ndongmo WP, Enyong PA, Njiokou F, et al. Bionomics and vectorial role of anophelines in wetlands along the volcanic chain of Cameroon. Parasit Vectors. (2018) 11:1–12. doi: 10.1186/s13071-018-3041-z
40. Suliman MMA, Hamad BM, Albasheer MMA, Elhadi M, Amin Mustafa M, Elobied M, et al. Molecular evidence of high proportion of Plasmodium vivax malaria infection in White Nile area in Sudan. J Parasitol Res. (2016) 2016:2892371. doi: 10.1155/2016/2892371
41. Eshag HA, Elnzer E, Nahied E, Talib M, Mussa A, Muhajir AEMA, et al. Molecular epidemiology of malaria parasite amongst patients in a displaced people’s camp in Sudan. Trop Med Health. (2020) 48:3. doi: 10.1186/s41182-020-0192-3
42. Teh RN, Sumbele IUN, Meduke DN, Nkeudem GA, Ojong ST, Teh EA, et al. Insecticide-treated net ownership, utilization and knowledge of malaria in children residing in Batoke–Limbe, Mount Cameroon area: effect on malariometric and haematological indices. Malar J. (2021) 20:1–13. doi: 10.1186/s12936-021-03860-6
43. Hershey CL, Doocy S, Anderson J, Haskew C, Spiegel P, Moss WJ. Incidence and risk factors for malaria, pneumonia and diarrhea in children under 5 in UNHCR refugee camps: a retrospective study. Confl Health. (2011) 5:1–11. doi: 10.1186/1752-1505-5-24
44. Zubairi ABS, Nizami S, Raza A, Mehraj V, Rasheed AF, Ghanchi NK, et al. Severe plasmodium vivax malaria in Pakistan. Emerg Infect Dis. (2013) 19:1851–4. doi: 10.3201/eid1911.130495
45. Connolly MA, Gayer M, Ryan MJ, Salama P, Spiegel P, Heymann DL. Communicable diseases in complex emergencies: impact and challenges. Lancet. (2004) 364:1974–83. doi: 10.1016/S0140-6736(04)17481-3
46. Roberts B, Odong VN, Browne J, Ocaka KF, Geissler W, Sondorp E. An exploration of social determinants of health amongst internally displaced persons in northern Uganda. Confl Health. (2009) 3:10. doi: 10.1186/1752-1505-3-10
47. Wilder-Smith A. Tsunami in South Asia: What is the risk of post-disaster infectious disease outbreaks? Ann Acad Med Singapore. (2005) 34:625–31.
48. Makungu C, Stephen S, Kumburu S, Govella NJ, Dongus S, Hildon ZJ-L, et al. Informing new or improved vector control tools for reducing the malaria burden in Tanzania: a qualitative exploration of perceptions of mosquitoes and methods for their control among the residents of Dar es Salaam. Malar J. (2017) 16:410. doi: 10.1186/s12936-017-2056-9
49. Moshi IR, Ngowo H, Dillip A, Msellemu D, Madumla EP, Okumu FO, et al. Community perceptions on outdoor malaria transmission in Kilombero Valley, Southern Tanzania. Malar J. (2017) 16:274. doi: 10.1186/s12936-017-1924-7
50. Mugwagwa N, Mberikunashe J, Gombe NT, Tshimanga M, Bangure D, Mungati M. Factors associated with malaria infection in Honde valley, Mutasa district, Zimbabwe, 2014: a case control study. BMC Res Notes. (2015) 8:829. doi: 10.1186/s13104-015-1831-3
51. Essendi WM, Vardo-Zalik AM, Lo E, Machani MG, Zhou G, Githeko AK, et al. Epidemiological risk factors for clinical malaria infection in the highlands of Western Kenya. Malar J. (2019) 18:211. doi: 10.1186/s12936-019-2845-4
52. Bamou R, Nematchoua-Weyou Z, Lontsi-Demano M, Ningahi LG, Tchoumbou MA, Defo-Talom BA, et al. Performance assessment of a widely used rapid diagnostic test CareStart™ compared to microscopy for the detection of Plasmodium in asymptomatic patients in the Western region of Cameroon. Heliyon. (2021) 7:e06271. doi: 10.1016/j.heliyon.2021.e06271
53. Sumbele IUN, Teh RN, Nkeudem GA, Sandie SM, Moyeh MN, Shey RA, et al. Asymptomatic and sub-microscopic Plasmodium falciparum infection in children in the Mount Cameroon area: a cross-sectional study on altitudinal influence, haematological parameters and risk factors. Malar J. (2021) 20:1–14. doi: 10.1186/s12936-021-03916-7
54. Arya A, Foko LPK, Chaudhry S, Sharma A, Singh V. Artemisinin-based combination therapy (ACT) and drug resistance molecular markers: A systematic review of clinical studies from two malaria endemic regions–India and sub-Saharan Africa. Int J Parasitol Drugs Drug Resist. (2021) 15:43–56. doi: 10.1016/j.ijpddr.2020.11.006
55. Kaindoa EW, Finda M, Kiplagat J, Mkandawile G, Nyoni A, Coetzee M, et al. Housing gaps, mosquitoes and public viewpoints: a mixed methods assessment of relationships between house characteristics, malaria vector biting risk and community perspectives in rural Tanzania. Malar J. (2018) 17:98. doi: 10.1186/s12936-018-2450-y
56. Wanzirah H, Tusting LS, Arinaitwe E, Katureebe A, Maxwell K, Rek J, et al. Mind the gap: house structure and the risk of malaria in Uganda. PloS One. (2015) 10:e0117396. doi: 10.1371/journal.pone.0117396
57. Njie M, Dilger E, Lindsay SW, Kirby MJ. Importance of eaves to house entry by anopheline, but not culicine, mosquitoes. J Med Entomol. (2014) 46:505–10. doi: 10.1603/033.046.0314
58. Ngadjeu CS, Doumbe-Belisse P, Talipouo A, Djamouko-Djonkam L, Awono-Ambene P, Kekeunou S, et al. Influence of house characteristics on mosquito distribution and malaria transmission in the city of Yaoundé, Cameroon. Malar J. (2020) 19:53. doi: 10.1186/s12936-020-3133-z
59. Norris DE. Mosquito-borne diseases as a consequence of land use change. Ecohealth. (2004) 1:19–24. doi: 10.1007/s10393-004-0008-7
Keywords: malaria, risk factors, ITN, CAR refugees, Gado-Badzere, Cameroon
Citation: Nematchoua Weyou Z, Djemna Djieyep F, Ning Teh R, Lontsi-Demano M, Dieng CC, Bamou R, Lo E, Kuokuo Kimbi H and Ule Ngole Sumbele I (2024) Malaria parasite burden and heterogeneity of risk factors among Central African Republic refugees: a cross-sectional study in the Gado-Badzere refugee camp in Eastern Cameroon. Front. Trop. Dis 5:1508750. doi: 10.3389/fitd.2024.1508750
Received: 09 October 2024; Accepted: 13 November 2024;
Published: 03 December 2024.
Edited by:
Allassane Foungoye Ouattara, University of Nangui Abrogoua, Côte d'IvoireReviewed by:
Aldemir Branco de Oliveira Filho, Federal University of Pará, BrazilWilliam Hawley, Consultant to Ministry of Health of Indonesia, Indonesia
Copyright © 2024 Nematchoua Weyou, Djemna Djieyep, Ning Teh, Lontsi-Demano, Dieng, Bamou, Lo, Kuokuo Kimbi and Ule Ngole Sumbele. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zidedine Nematchoua Weyou, emlkZWRpbmVuZW1hdGNob3VhQGdtYWlsLmNvbQ==
 Zidedine Nematchoua Weyou
Zidedine Nematchoua Weyou Felicite Djemna Djieyep1
Felicite Djemna Djieyep1 Rene Ning Teh
Rene Ning Teh Eugenia Lo
Eugenia Lo Helen Kuokuo Kimbi
Helen Kuokuo Kimbi