- 1Facultad de Medicina Veterinaria y Zootecnia, Universidad Autonoma del Estado de Mexico, Toluca, Mexico
- 2Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX, United States
- 3Comisión México-Estados Unidos para la Prevención de la Fiebre Aftosa y otras Enfermedades Exóticas de los Animales (CPA), Secretaria de Agricultura y Desarrollo Rural, Mexico City, Mexico
- 4Instituto Politecnico Nacional, Centro de Biotecnologia Genomica, Reynosa, Mexico
Cache Valley virus (CVV) was isolated from a clinically presenting horse in the Mexican Gulf region. CVV is linked to neuroinvasive disease in humans and fetal demise and defects in sheep. This is the first association between CVV and disease in horses. Implications for public and veterinary health are discussed.
Introduction
Cache Valley virus (CVV) is an arbovirus first isolated in 1956 from Culiseta inornata mosquitoes and named after the Cache Valley region in Utah, USA (1). It is a member of the Bunyamwera serogroup in the genus Orthobunyavirus, family Peribunyaviridae (2, 3). CVV is endemic in North and Central America, having been detected in various Culicidae species (4–6), wild and domesticated animals (6), and humans (7). Infections in people are linked to the central nervous system and are associated with lethal aseptic meningitis (8). Ovine epizootics lead to spontaneous abortions and congenital defects of the musculoskeletal and central nervous systems (4, 6, 8, 9). Here, we report for the first time the isolation of CVV from a horse in Mexico including clinical observations and the documentation of its phylogenetic analysis and relationships.
Materials and methods
Sampling site and horses
Seven female horses (Equus ferus caballus) born in the highlands near Mexico City, Mexico, were included in this study. All mares were verified by plaque reduction neutralization tests (PRNTs) to be seronegative to Venezuelan equine encephalitis virus (VEEV). Blood samples were obtained with a 10-mL Becton Dickinson Vacutainer ® (NYSE). An iButton chip (EDS, Lawrenceburg, KY) was implanted in their left hip to facilitate temperature monitoring. After 30 days, on 3 August 2014, the mares were transported 633 km away to a test site in the suburban periphery of Minatitlan City, Veracruz, Mexico (Figure 1). Horses were allowed to roam freely in an open pasture for grazing and monitored daily for signs of disease, temperature, and blood counts over a 22-day period (Table 1).
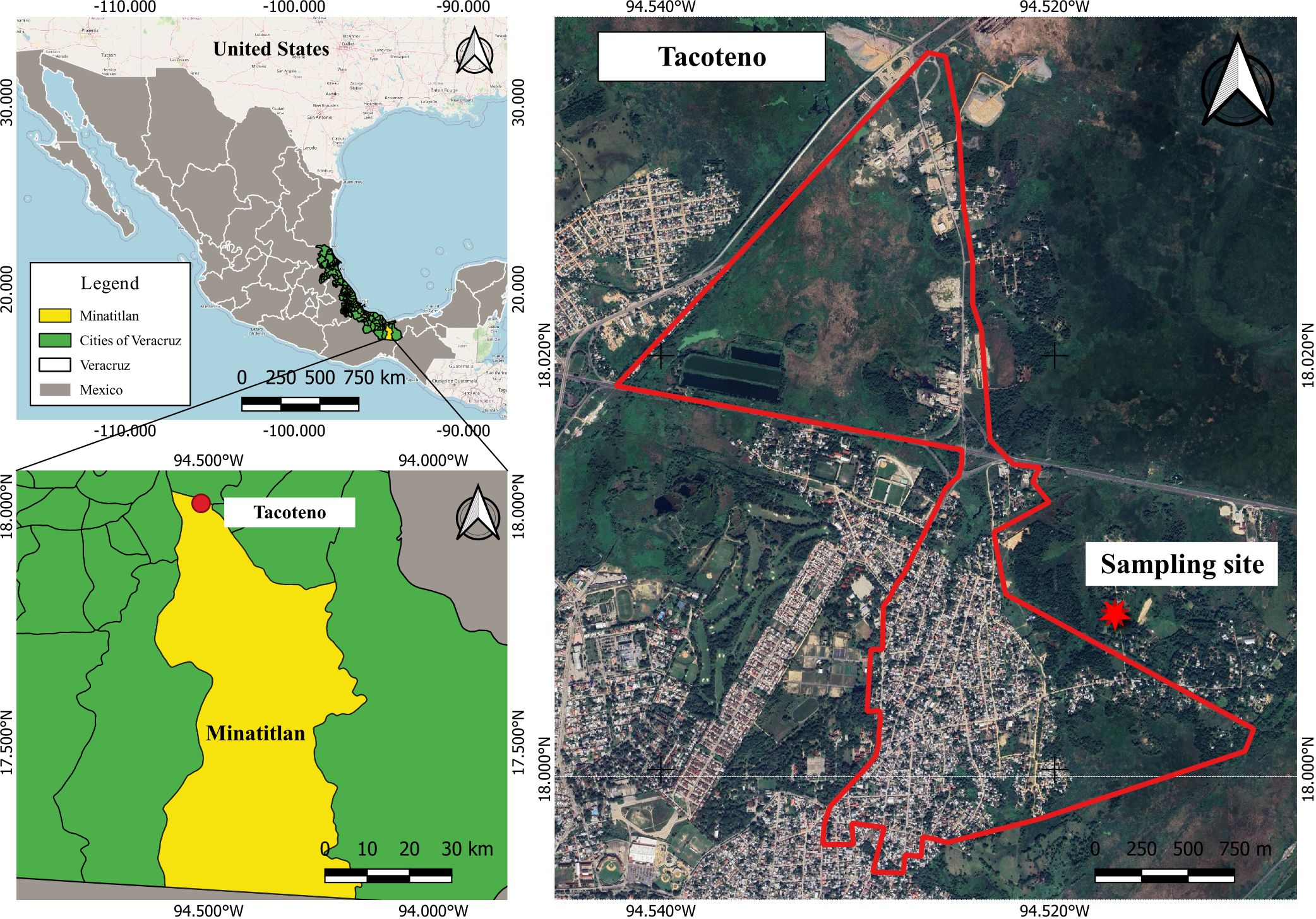
Figure 1. Geographical localization of Minatitlan City, state of Veracruz, Mexico. The map shows the location of the sampling site, near urban settlements and areas with wetlands, swamps, and grassland patches. The primary maps were generated using QGIS 3.16.6 (https://www.qgis.org). Free geographic data of administrative areas of Mexico were downloaded from the National Institute of Statistics and Geography, Mexico (INEGI, https://www.inegi.org.mx/app/mapas). Satellite images and street maps were obtained from Google Maps (https://www.google.com/maps).
Results
Mosquito collection
We used Hamster Trinidad bait traps and collected the following mosquito species (N = 250): Culex (Melanoconion) taeniopus (10%), Culex (Melanoconion) iolambdis (10%), Culex nigripalpus (50%), Coquillettidia nigricans (10%), Culex quinquefasciatus (10%), Aedes taeniorhynchus (2%), Mansonia titillans (2%), Anopheles albimanus (2%), and unidentified species (4%).
Clinical observations
On 15 August 2014, one of seven mares presented the following symptoms: it developed profuse ocular discharge, jaundice, and pale mucous, accompanied by a mildly elevated heart rate and a high respiratory rate. The elevated respiratory rate persisted through 20 August 2014, measuring at least double the normal range on 3 of those 6 days and accompanied by a heart rate either at or slightly above the expected range throughout this period (Table 1). The mare displayed peristaltic movements with hyperactivity disorders on 19–20 August 2014, with symptoms worsening and the onset of diffuse diarrhea on the second day. By 22 August 2014, the mare was in prostration more than usual. The mare then recuperated, with no detectable illness on 26 August 2014 and no sequelae noted in a 2-month post-illness analysis. The mare had low hemoglobin and hematocrit measurements and elevated platelet counts throughout the study period, indicative of anemia and reactive thrombocytosis (Table 1).
Viral isolation and genome sequencing
Viral isolation from serum collected on 4–16 August 2014, as well as on 23 August 2014, was attempted in Vero cells. The mare’s primary serum obtained directly from the field was inoculated in the cells. From 9 to 12 August 2014, a cytopathic effect (CPE) was induced, and RNA was harvested from the cell culture supernatant and eluted in 50 µL of water using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions for viral species identification. This was followed by RNA extraction, and a cDNA library was generated using SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) with random hexamers according to the manufacturer’s instructions. This cDNA, along with cDNA generated from water and a stock of the TC-83 strain of VEEV to serve as negative and positive controls, respectively, was subjected to PCR with the VEEV 7894(+) and VEEV 395(−) primers as previously described (11). Upon discovering that VEEV was not detected from any of the cell culture supernatants obtained from CPE-positive isolation attempts from the mare in question, the RNA was subjected to next-generation sequencing using the Illumina NextSeq550 platform.
Samples were negative for VEEV by RT-PCR. The viral isolate derived from the sample from 11 August 2014, designated strain Mx14Eq03, was subjected to next-generation sequencing (NGS) using Illumina NextSeq. De-novo sequence assembly performed with ABySS version 2.03 (12) yielded a single viral species: CVV (GenBank accession OP137174-137176).
The L, M (Gc), M (Gn), and S segments of CVV Mx14Eq03 were aligned with all available CVV sequences as representative strains of closely related viruses (Table 2) using the MUSCLE algorithm in MegAlign Pro v17.2.1 (DNASTAR, Madison, WI). Alignments were trimmed to 528, 1,803, 525, and 754 nucleotide regions of the L, M (Gc), M (Gn), and S segments, respectively. The trees were generated from 62 L segments, 65 M (Gn) segments, 113 M (Gc) segments, and 52 S segments. The segments were analyzed separately because bunyaviruses are known to have undergone both recombination and reassortment. Phylogenetic analysis was performed using the phangorn package in R v4.1.3, and the GTR+G+I model of nucleotide substitution was employed (13). A maximum likelihood tree was generated using a rooted UPGMA tree for initialization with 1,000 iterations for bootstrapping analysis. The Mx14Eq03 strain was found by all four analyses to be a lineage 2 member of the CVV species (Figure 2).
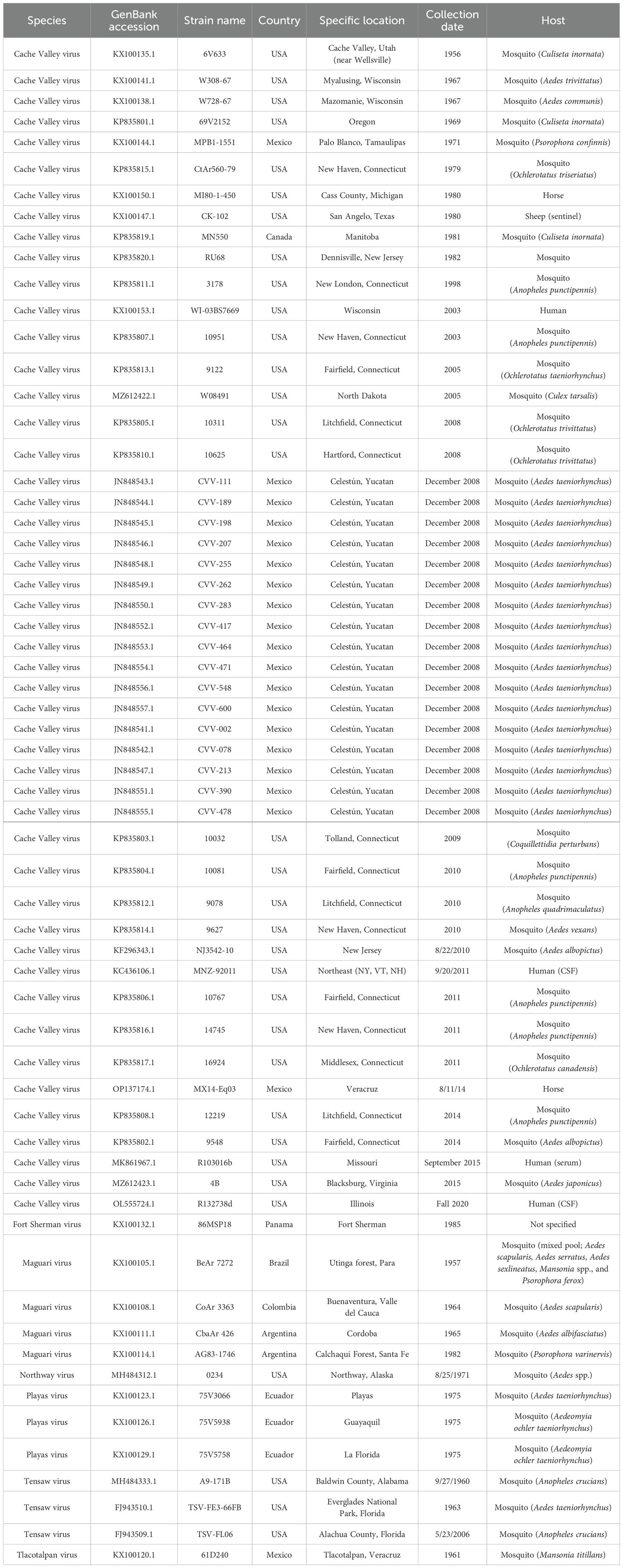
Table 2. Historical CVV strains isolated and closely related strains used in the phylogenetic analysis.
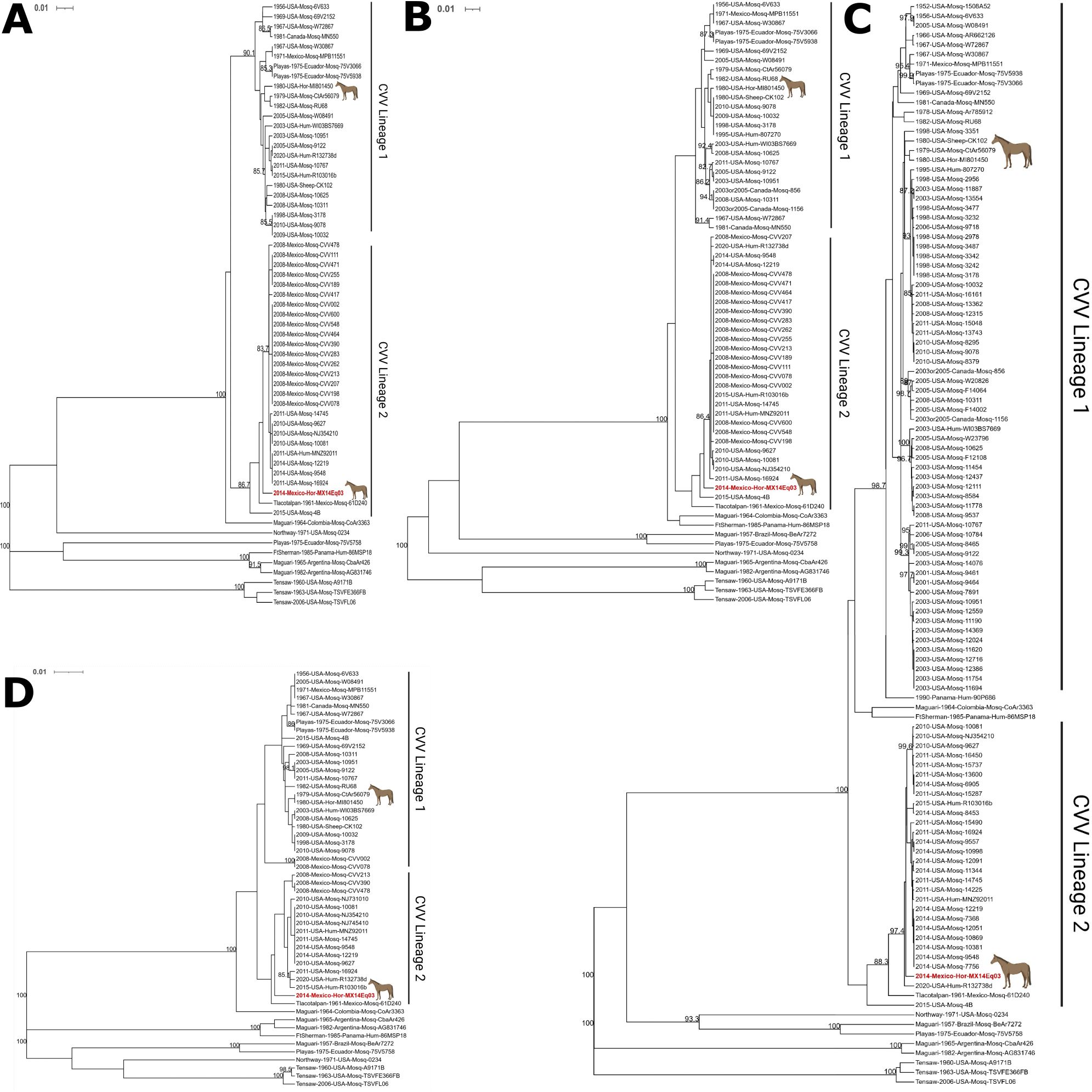
Figure 2. Maximum likelihood analysis of the (A) L segments, (B) Gn gene in the M segments, (C) Gc gene in the M segments, and (D) S segments of Cache Valley and closely related viruses. The Mx14Eq03 isolate is indicated in red. Equine isolates are indicated with a horse icon. Scale bars and branch lengths refer to the rate of nucleotide substitutions. Bootstrapping values greater than or equal to 80 are indicated at the corresponding nodes. Phylogenetic trees were visualized with iTOL v6.6 and annotated in BioRender.com.
Discussion and conclusion
In general, some areas of the south of Mexico have been characterized previously as endemic for VEEV for several orthobunyaviruses including Tlacotalpan virus (TLAV), Patois virus (PATV), and Barrita virus (BITV) (14) and for flaviviruses (11, 15, 16). Thus, the detection of CVV at the test site in the Coatzacoalcos river basin was expected since the virus has been known to circulate widely in the Yucatan Peninsula and also has been suspected to be spread in the lowland region of Veracruz (5, 15). Tlacotalpan virus, a virus considered to be a variant of CVV, was isolated from the Tlacotalpan Veracruz village, a town located 208 km north of our study site, in the 1960s (15).
Reinforcing such observations is the phylogenetic association as demonstrated in our phylogenetic tree and in segment L as shown in Figure 2. Although horses have been found to be seropositive for CVV in the Yucatan Peninsula and elsewhere (5), this is the first viral isolate obtained from this host outside of a single strain obtained from Michigan in 1980 (17).
The period of viremia of the mare showed an elevated heart rate and temperature. Visible signs of illness, including ocular discharge, jaundice, and pale mucus, began 3 days after the viremia and escalated to include additional gastrointestinal symptoms before resolving after 11 days with no apparent sequelae. We also noticed three distinct clinical signs, namely, depression, high fever, and respiratory distress, observed in other arboviral diseases likewise affecting equines. The disease linked to Culicoides mosquito bites is African horse sickness (AHS), known to be endemic in Africa and showing a similar pattern in our Minatitlan mare with the exception that AHS is highly lethal (18). During the study period, the mare seroconverted to VEEV. Low levels of neutralizing antibody against VEEV were first detected on the final day of CVV viremia, 12 August 2014, and the antibody titer steadily increased before reaching the upper limit of the assay on 21 August 2014. Results from the other six mares confirmed the presence of VEEV circulation at the test site during the study period. Sera collected from five of those six mares between 8 August 2014 and 15 August 2014 caused CPE in Vero cells and were subsequently identified as VEEV by RT-PCR and sequencing. Those five mares also seroconverted to VEEV, reaching neutralizing titers of 640 or greater between 13 August 2014 and 21 August 2014. Thus, although VEEV was not isolated from the mare that was infected with CVV, there is persuasive evidence that it was infected with VEEV during the study period. As the previous equine isolate was from a clinically normal animal (17), further study is needed to elucidate any causative relationship between CVV and adverse clinical outcomes in horses.
As expected, the phylogenetic analysis identified the Mx14Eq03 strain isolated in this study as a lineage 2 isolate of CVV. Lineage 1 strains of CVV are generally older and are dominated by strains from the USA and Canada (4, 6). CVV lineage 2, on the other hand, has emerged more recently and was identified in Mexico before its detection in the USA, suggesting possible introduction from Mexico or South America into the USA (4).
From a veterinary standpoint, the importance of our detection of CVV in the Mexican State of Veracruz is highlighted by its association with the sheep industry. The state of Veracruz, after the states of Hidalgo and Mexico, respectively, was recognized in 2021 as the third most important ovine production region of the Mexican Republic, with 730,015 head of sheep (19) that represent an important income source for many farmers. Infections of pregnant ewes can result in severe fetal malformations like arthrogryposis with hydranencephaly and death, with major agroeconomic losses (9). Active CVV circulation in the region also has implications for human health. It is likely that in tropical regions such as Veracruz, both veterinary and human cases are greatly underreported due to the simultaneous circulation of other arboviruses such as VEEV. Accurate, inexpensive, and rapid diagnostics at low containment are needed to improve differential diagnostics. Differential diagnostics should be a medically important component, especially in suspected arboviral diseases in equine herds including humans since the southern Mexican region is affected by other endemic neuroinvasive arboviral diseases such as the equine encephalitis (3, 11) and West Nile virus (16).
All in all, leading among the bunyavirus is the orthobunyavirus genus with more than 170 named viruses, many of them pathogens to humans and distributed worldwide. Among them, CVV is an example considered to be a reemerging zoonosis (3) and can cause serious diseases in humans and domestic animals. CVV disease and its putative impact remain an uncharted scenario in south Mexico worthy of renewed public and veterinary health attention by regional health institutions of the Mexican Republic.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The animal study was approved by the IRB protocol from the Facultad de Medicina, Universidad Autonoma del Estado de Mexico (UAEMex) 011/2015. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
GO: Investigation, Methodology, Writing – original draft, Writing – review & editing. JP: Investigation, Methodology, Software, Validation, Writing – review & editing. VF: Investigation, Methodology, Writing – review & editing. AG: Investigation, Methodology, Writing – review & editing. MS: Data curation, Methodology, Writing – review & editing. GR: Data curation, Methodology, Writing – review & editing. SW: Investigation, Methodology, Software, Validation, Writing – review & editing. KP: Investigation, Methodology, Software, Validation, Writing – review & editing. SW: Funding acquisition, Methodology, Resources, Validation, Writing – review & editing. JE: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was provided by SIP-IPN grants 20221576, 20230712, 20231063, and 20242499 to JE-F and by a WRCEVA grant (NIH R24 AI120942) to SW.
Acknowledgments
We are especially thankful in memory of the late Dr. Roberto Navarro-Lopez from CPA-SADER for supporting all aspects of the field efforts, experimental design, and development of the study. We are thankful for the field support provided by the Mexican Agriculture Ministry (CPA-SADER). We are thankful for the support in map design and data processing to Lihua Wei and Ricardo Palacios-Santana from IPN in Reynosa, Tamaulipas, Mexico. We would like to thank all the members of the World Reference Center for Emerging Viruses and Arboviruses, University of Texas Medical Branch, Galveston, Texas.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2024.1456666/full#supplementary-material
References
1. Holden P, Hess AD. Cache Valley virus, a previously undescribed mosquito-borne agent. Science. (1959) 130:1187–8. doi: 10.1126/science.130.3383.1187
2. International Committee on Taxonomy of Viruses. Taxonomy browser(2024). Available online at: https://ictv.global/taxonomy (Accessed August 13, 2024).
3. Gill CM, Beckham JD, Piquet AL, Tyler KL, Pastula DM. Five emerging neuroinvasive arboviral diseases: cache valley, eastern equine encephalitis, Jamestown Canyon, Powassan, and Usutu. Semin Neurol. (2019) 39:419–27. doi: 10.1055/s-0039-1687839
4. Armstrong PM, Andreadis TG, Anderson JF. Emergence of a new lineage of Cache Valley virus (Bunyaviridae: Orthobunyavirus) in the Northeastern United States. Am J Trop Med Hyg. (2015) 93:11–7. doi: 10.4269/ajtmh.15-0132
5. Farfan-Ale JA, Loroño-Pino MA, Garcia-Rejon JE, Soto V, Lin M, Staley M, et al. Detection of flaviviruses and orthobunyaviruses in mosquitoes in the Yucatan Peninsula of Mexico in 2008. Vector Borne Zoonotic Dis. (2010) 10:777–83. doi: 10.1089/vbz.2009.0196
6. Waddell L, Pachal N, Mascarenhas M, Greig J, Harding S, Young I, et al. Cache Valley virus: A scoping review of the global evidence. Zoonoses Public Health. (2019) 66:739–58. doi: 10.1111/zph.12621
7. Elliott RM, Schmaljohn CS. Bunyaviridae. In: Knipe DM, Howley PM, editors. Fields Virology, 6th Edition. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, PA (2013). p. 1245–8.
8. Campbell GL, Mataczynski JD, Reisdorf ES, Powell JW, Martin DA, Lambert AJ, et al. Second human case of Cache Valley virus disease. Emerg Infect Dis. (2006) 12:854–6. doi: 10.3201/eid1205.051625
9. Calisher CH, Sever JL. Are North American Bunyamwera serogroup viruses etiologic agents of human congenital defects of the central nervous system? Emerg Infect Dis. (1995) 1:147–51. doi: 10.3201/eid0104.950409
10. Robinson NE. Current therapy in equine medicine 5. 5th ed. Philadelphia, Pa: W. B. Saunders (2003).
11. Adams AP, Navarro-Lopez R, Ramirez-Aguilar FJ, Lopez-Gonzalez I, Leal G, Flores-Mayorga JM, et al. Venezuelan equine encephalitis virus activity in the Gulf Coast region of Mexico, 2003-2010. PLoS Negl Trop Dis. (2012) 6:e1875. doi: 10.1371/journal.pntd.0001875
12. Simpson JT, Wong K, Jackman SD, Schein JE, Jones SJ, Birol I. ABySS: a parallel assembler for short read sequence data. Genome Res. (2009) 19:1117–23. doi: 10.1101/gr.089532.108
13. Schliep KP. phangorn: phylogenetic analysis in R. Bioinformatics. (2011) 27:592–3. doi: 10.1093/bioinformatics/btq706
14. Fernández D, Marciel de Souza W, Silvas JA, Deardorff ER, Widen SG, Estrada-Franco JG, et al. Barrita virus, a novel virus of the patois serogroup (Genus orthobunyavirus; family peribunyaviridae). Am J Trop Med Hyg. (2020) 103:190–2. doi: 10.4269/ajtmh.19-0906
15. Scherer WF, Campillo-Sainz C, Dickerman RW, Diaz-Najera A, Madalengoitia J. Isolation of Tlacotalpan virus, a new Bunyamwera-group virus from Mexican mosquitoes. Am J Trop Med Hyg. (1967) 16:79–91. doi: 10.4269/ajtmh.1967.16.79
16. Estrada-Franco JG, Navarro-Lopez R, Beasley DW, Coffey L, Carrara AS, Travassos da Rosa A, et al. West Nile virus in Mexico: evidence of widespread circulation since July 2002. Emerg Infect Dis. (2003) 9:1604–7. doi: 10.3201/eid0912.030564
17. McLean RG, Calisher CH, Parham GL. Isolation of Cache Valley virus and detection of antibody for selected arboviruses in Michigan horses in 1980. Am J Vet Res. (1987) 48:1039–41.
18. Mellor PS, Hamblin C. African horse sickness. Vet Res. (2004) 35:445–66. doi: 10.1051/vetres:2004021
19. Secretaria de Agricultura y Desarrollo Rural (SADER). Servicio de Informacion Agroalimentaria y Pesquera (SIAP). Ovino Poblacion ganadera 2012-2021 Cabezas (2021). Available at: https://www.gob.mx/cms/uploads/attachment/file/744954/Inventario_2021_ovino.pdf.
Keywords: orthobunyavirus, arbovirus, Cache Valley virus, encephalitis, horse
Citation: Ortega-Soriano G, Plante JA, Fabela-Becerril VA, Gonzalez-Perez AL, Solis-Hernandez M, Rafael G, Widen SG, Plante KS, Weaver SC and Estrada-Franco JG (2024) Cache Valley virus isolation from a horse in Veracruz State, Mexico. Front. Trop. Dis 5:1456666. doi: 10.3389/fitd.2024.1456666
Received: 28 June 2024; Accepted: 26 August 2024;
Published: 25 November 2024.
Edited by:
Richard Salvato, State Center for Health Surveillance, BrazilReviewed by:
Miguel A. Saldaña, University of Texas Medical Branch at Galveston, United StatesRaíssa Nunes, Federal University of Rio Grande do Sul, Brazil
Ludmila Baethgen, State Center for Health Surveillance, Brazil
Copyright © 2024 Ortega-Soriano, Plante, Fabela-Becerril, Gonzalez-Perez, Solis-Hernandez, Rafael, Widen, Plante, Weaver and Estrada-Franco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jose Guillermo Estrada-Franco, amVzdHJhZGFmQGlwbi5teA==
†These authors have contributed equally to this work
 Gamaliel Ortega-Soriano
Gamaliel Ortega-Soriano Jessica A. Plante2†
Jessica A. Plante2† Steven G. Widen
Steven G. Widen Kenneth S. Plante
Kenneth S. Plante Scott C. Weaver
Scott C. Weaver Jose Guillermo Estrada-Franco
Jose Guillermo Estrada-Franco