
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Trop. Dis. , 27 September 2024
Sec. Tropical Disease Epidemiology and Ecology
Volume 5 - 2024 | https://doi.org/10.3389/fitd.2024.1433013
Rickettsial infections present a substantial public health burden in India. Recent years have witnessed an increase in the incidence of Rickettsial infection-associated morbidity and mortality. These infections are primarily transmitted by vectors such as ticks, fleas, mites, and lice. This review aims to capture epidemiology, diagnosis and emerging disease trends of rickettsial infections, particularly Orientia tsutsugamushi (O. tsutsugamushi) in the Indian context. Diagnosis and treatment of Orientia infections remain challenging due to the lack of sensitive and specific diagnostic tools vis-a-vis clinical treatment in the absence of specific drugs targeting Rickettsial pathogens. Consequently, clinicians often rely on symptoms and epidemiological factors for diagnosis, highlighting the urgent need for improved diagnostics and therapeutic tools. A comprehensive understanding of the epidemiology of rickettsial diseases is essential for formulating effective preventive and control strategies. Identification of high-risk regions and populations by serological and genetic techniques may help the development of targeted interventions. Therefore, enhancing awareness among healthcare professionals and the public regarding epidemiology, clinical features, diagnosis and treatment interventions of rickettsiosis is crucial. This review summarizes the significance of comprehensive epidemiological investigations and diagnostic systems for understanding rickettsial infections in India.
Rickettsiosis is a group of arthropod-borne neglected tropical diseases caused by Rickettsia spp (1). transmitted by ticks, fleas, mites, and lice. These life-threatening diseases typically lead to multiorgan involvement and failure with high fatality rates. In India, the resurgence of rickettsial diseases can be attributed to increased exposure to vectors due to environmental changes. This is further enhanced by the unavailability of effective rickettsial vaccines (2). Scrub Typhus, caused by Orientia tsutsugamushi and transmitted by Leptotrombidium mites, is the most prevalent form of Rickettsiosis in India (3). Cases have been reported in several states of India, including Himachal Pradesh, Uttar Pradesh, Maharashtra, Tamilnadu, Delhi, Jammu and Kashmir, Rajasthan, Bihar, Assam, Kerala, Uttarakhand, Nagaland, Karnataka, and West Bengal. Scrub typhus causes small epidemics in densely populated hotspots, especially during monsoons and winter (4). Diagnosis of rickettsial infections, including scrub typhus, is challenging due to overlapping symptoms with other diseases like leptospirosis, dengue, and malaria (5).
Scrub typhus caused by Orientia tsutsugamushi is a dominating agent in Asian regions, with over one million cases and 150,000 deaths each year (6, 7). Severe disease in hospitalized patients may lead to multiorgan dysfunction and shock in approximately one-third of patients and can result in the death of about a quarter of cases despite therapy (8). Most rickettsioses cases are probably misdiagnosed due to a lack of precise and specific diagnostics. Clinicians largely rely on clinical symptoms or a characteristic “Eschar” at the site of the bite before initiating the treatment. The Weil-Felix test, though historically used, is not definitive for diagnosing scrub typhus. Currently, serological tests like the immunofluorescence assay (IFA) are considered the gold standard due to their higher accuracy and reliability. Improved and cost-effective diagnostic assays are urgently needed to enhance detection rates and facilitate early treatment. Frequent serological and molecular testing is needed to investigate and analyze the demographic characteristics of these pathogens. This review focuses on the epidemiology and diagnosis of rickettsial infections in India (Figure 1).
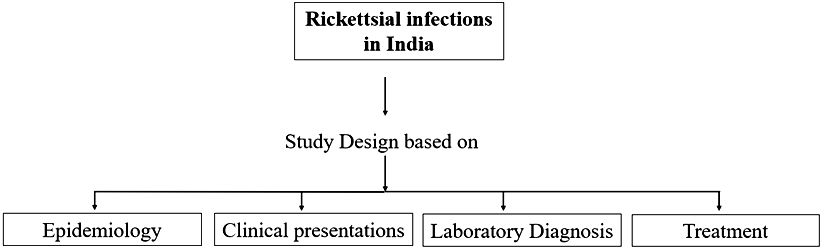
Figure 1. Schematic overview of the current state of knowledge of epidemiology, diagnosis and treatment of Rickettsia spp.
Rickettsial infections are prevalent across the world; however, their incidence and disease burden vary across countries due to vector and natural host constraints, climatic conditions, exposure rates, and access to diagnostics and healthcare systems. In Southeast Asia, rickettsial infections are the most common non-malarial diseases after dengue. The global epidemiology of scrub typhus indicates that it is endemic in many parts of Asia-Pacific; globalization and travel have led to the spread of the disease to non-endemic areas, complicating its epidemiological study (9). An elegant study by Lokida et al. (5), performed a comprehensive diagnosis of acute and convalescent blood from 975 hospitalized patients was tested using Rickettsia IgM and IgG by ELISA and IFA and Rickettsia-specific primers for Rickettsia rickettsii, Rickettsia typhi, and Orientia tsutsugamushi. The predominant rickettsial strains identified in Indonesia were R. typhi (30.8%), followed by R. rickettsii (5.8%) and O. tsutsugamushi (3.8%) vs 70-80% of O. tsutsugamushi in India. Hence, the strain differences between Indonesia and India may be responsible for higher severity, multi-organ failure and higher fatality in hospitalized patients observed in India (7). Scrub typhus, for example, has a seroprevalence of 9.3% to 27.9% in Asian regions, with over a million reported cases and a billion people are at risk worldwide (10). In Northern America, Rocky Mountain spotted fever (RMSF), caused by R. rickettsii, is the most severe and frequently reported rickettsial infection, with death rates ranging from 20-30% when untreated (11). Epidemic typhus, transmitted by human body lice, is prevalent in mountainous regions of Asia, South America, and Africa, with increased transmission rates during cold climates (12).
The genus Rickettsia is divided into three major subgroups: Spotted Fever group (SFG), Typhus group (TG), and Scrub Typhus group (STG) (Table 1). The SFG comprises more than 30 different species, including R. rickettsii, the causative agent of Rocky Mountain Spotted Fever (RMSF) (13). Other SFG species include R. conorii, responsible for Mediterranean Spotted Fever (14), R. africae, which causes African Tick Bite Fever, and R. australis, the causative agent of Queensland Tick Typhus (15, 16). Examples of TG rickettsiae are R. typhi (also known as murine typhus) and R. prowazekii (also known as epidemic typhus) (17). Various species of Rickettsia have been identified worldwide between 1995 and 2024. This rapid discovery can be attributed to improved diagnostic methods as per global surveillance and with the help of advancements in molecular biology techniques. For instance, Rickettsia sibirica subsp. mongolitimonae, the causative agent of lymphangitis-associated rickettsiosis (LAR). It was first isolated in China from Hyalomma asiaticum ticks collected in Mongolia in 1991. Since then, China has reported the presence of eleven spotted fever group Rickettsia species, such as R. tarasevichiae, R. heilongjiangensis, R. sibirica strain BJ-90, and R. raoultii. Rickettsia conorii subsp. caspia, the agent of Astrakhan fever, is endemic to the Astrakhan region and has been described in Rhipicephalus sanguineus ticks in France. Israeli spotted fever (ISF) is caused by Rickettsia conorii subsp. israelensis and was first reported in Haifa Bay, Israel, in 1946. Mediterranean spotted fever (MSF), caused by Rickettsia conorii subsp. conorii is the most commonly reported rickettsiosis in various parts of Europe. Numerous other species, including R. sibirica, R. raoultii, and R. slovaca, have been implicated in human infections. In South-Eastern Mexico, Typhus Group Rickettsia (R. rickettsii and R. parkeri) infections have been reported in domesticated dogs. Recently, rickettsial infections caused by R. rhipicephali from ticks of wildlife fauna have been reported in parts of Costa Rica in Central America. Scrub typhus was recently reported in Dubai for the first time, and interestingly, the reported cases were located more than 500 kilometers away from the presumed endemic area, suggesting that the actual extent of the endemic region might be more significant than previously thought (18). In summary, rickettsial infections have a global presence, and the disease burden varies with distribution and coexistence of rickettsial species and vectors in the geographical region.
Scrub typhus is predominantly found in the endemic area known as the tsutsugamushi triangle in Southeast Asia and poses a significant public health impact on agricultural workers in the rural tropical region. Scrub forests, the secondary grassy vegetation resulting from forest clearance, were initially believed to be the ecological habitat of this typhus. However, it has been reported from diverse environments such as sandy beaches, semiarid and highland deserts (19), metropolitan centers, and even rice paddies.
In India, scrub typhus was first identified in 1943 near the India-Burma border, and the use of antibiotics and widespread pesticide application significantly reduced subsequent outbreaks during World War II (20). Rickettsial infections, including scrub typhus, have been reported in high-burden regions of India, including Uttar Pradesh, Bihar, Jharkhand, Madhya Pradesh, Andhra Pradesh, Maharashtra, Chhattisgarh, Orissa, Karnataka, and West Bengal (Figure 2). The earliest documented instances of scrub typhus in India provide descriptions of typhus fever group rickettsioses within the Indian population (21–23). Some reports suggest the emergence or reemergence of scrub typhus in several states of India (24–26). Several researchers have undertaken significant investigations on scrub typhus in Tamil Nadu (7, 8, 27, 28). Despite extensive studies on clinical and laboratory aspects, the burden of these infections and high mortality in severe cases, research on the molecular epidemiology of rickettsial strains has been limited.
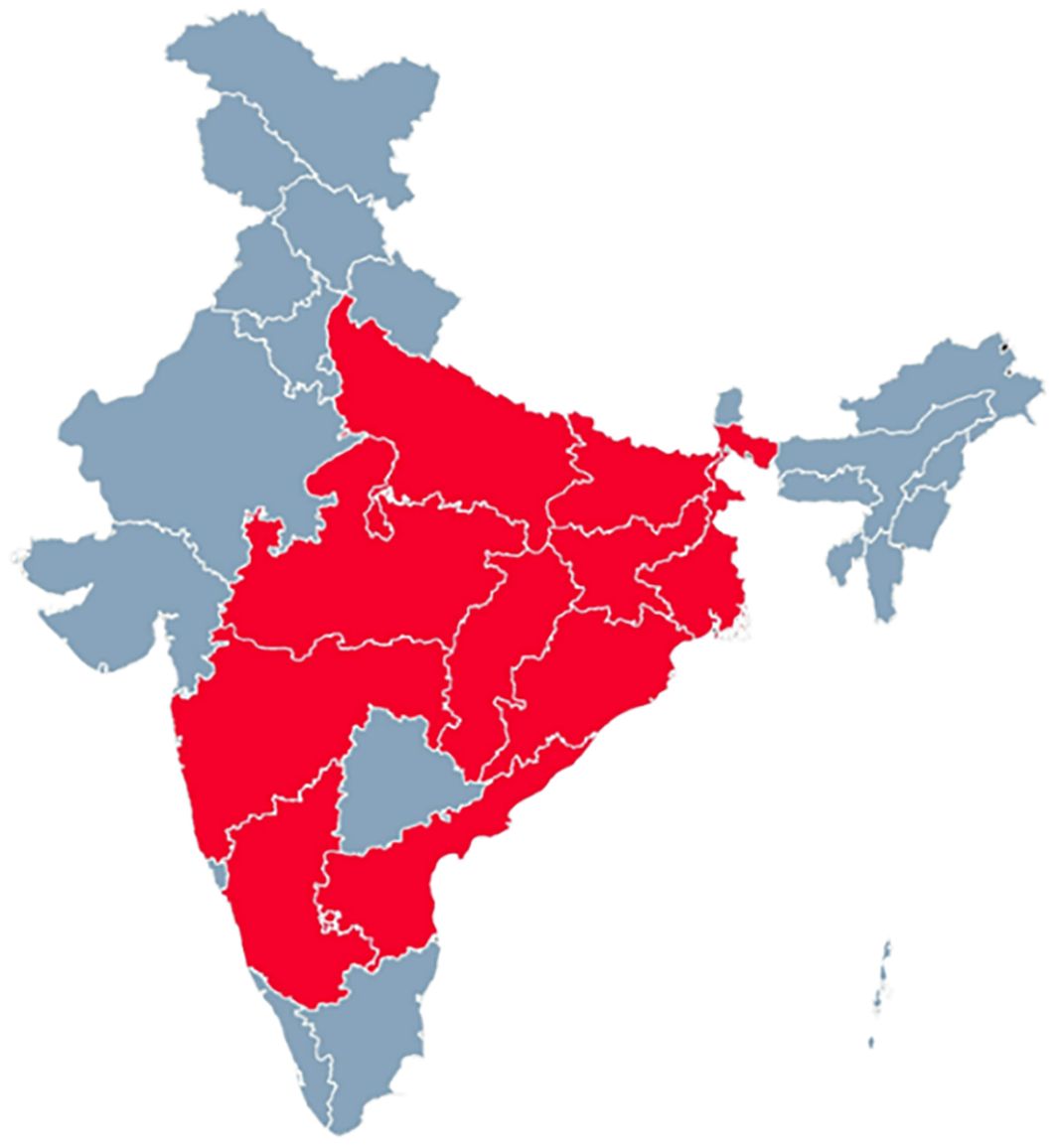
Figure 2. The geographical regions of India with a substantial prevalence of scrub typhus (high burden) are depicted in red, and the states exhibiting a low prevalence (low burden) are depicted in grey.
In India, beyond scrub typhus, which is nearly 70-80% of the reported rickettsial cases (29), several other rickettsial diseases are reported, including Indian Tick Typhus (Rickettsia conorii), Murine Typhus (Rickettsia typhi), Epidemic typhus (Rickettsia prowazekki), Q Fever (Coxiella burnetii), Trench fever (Bartonella quintana) and Rickettsialpox (Rickettsia akari) (30). Despite limited literature and underdiagnosis due to constrained awareness and diagnostic facilities, they pose significant health risks. Consequently, there is no literature on the distribution and prevalence of the rickettsial species in India (31). Orientia tsutsugamushi is the main causative agent of rickettsial infections in India; hence, subsequent sections of the review will focus exclusively on the epidemiology, diagnosis and treatment of Scrub typhus caused by O. tsutsugamushi.
Scrub typhus is documented from the tropics to the Himalayas and is associated with farming operations like paddy agriculture, oil palm and rubber plantations, and forestry. Chiggers, the larval stages of Leptotrombidium mites, are the primary vectors of the disease. Chigger activity is influenced by relative humidity and temperature stability in the tropics. A bimodal seasonal variation is observed in countries like China, Taiwan, and northern Japan, with summer and winter as the prime seasons. The endemic area of scrub typhus is dispersed across temperate zones due to significant seasonal variations in climate. L. deliense is the primary vector species in Southeast Asia and southern China (32). In Japan and Korea, L. akamushi, L. pallidum, and L. scutellare play major roles as vectors (33). L. chiangraiensis, a newly described vector species, appears to be predominant in Thailand’s paddy fields (34). Other Leptotrombidium species associated with disease transmission include L. fletcheri and L. Arenicola (35). Recent studies show that rodents and Leptotrombidium chiggers can adapt to new habitats, including semi-urban areas, and play a vital role in the ecology of scrub typhus.
Chiggers have one annual generation, which is most prevalent during late summer and early autumn. The mite’s life cycle ranges from two to three months in warmer climates and eight months in frigid temperatures. Chiggers commonly infest rodent ears owing to their proximity to blood vessels. Once infected, mice will remain infected and serve as carrier for life (36). Chiggers, rodents and secondary vegetation form a zoonotic triad crucial for the survival of O. tsutsugamushi in the wild (36). The vector’s life cycle includes four stages- eggs, larva, nymph, and adult. Only the larval stage is infectious as it feeds on mammals for necessary body secretions. Larvae are tiny, translucent orange in color and require a microscope for identification. Larvae have three pairs of legs and last one to two weeks before descending to the ground to transform into nymphs. Nymphs are brick red, last one to three weeks, and have four pairs of legs, with the first pair being the largest. Adults and nymphs are free-living and consume small invertebrates, eggs, and organic matter. Adult females deposit hundreds of eggs in the soil leading to severe host infestations, and typically live for about six months (37).
Chiggers serve as the primary vectors and reservoirs. Apart from chiggers, other ground-dwelling tiny animals and rodents in their natural environments also serve as reservoirs. Rats (Rattus rattus) (38), striped field mice (Apodemus agarius) (39), and house mice (Mus musculus) (40) are some of the animals that are most commonly associated with the disease. A clean and hygienic environment, free of rodents, is believed to reduce the risk of being exposed to chiggers.
Karp, Kato, and Gilliam were identified as the prototypical strains of O. tsutsugamushi in 1969 (41). The list of strains has since been expanded to include newer serotypes such as Kuroki, Shimokoshi, Kawasaki, and Boryong, among others. Strain typing can be done using immuno-fluorescent testing with strain-specific sera or monoclonal antibodies, although it was not useful in detecting the recent serotypes. Another method used in the identification of strains is by molecular techniques that use the 56-kDa Type-specific antigen (TSA), which is a recognized outer membrane protein in Orientia-infected individuals (42). Amplification of TSA followed by Restriction Fragment Length Polymorphism (RFLP) is typically used by identifying the hypervariable regions that are crucial for strain-level molecular typing (42, 43).
Sequencing has become the standard method for genotyping, comparing deduced sequences to existing genotype data and conducting phylogenetic analysis. Over 20 genotypes have been identified in O. tsutsugamushi due to genetic variation and specific antigens, including sta150, -58, -56, -49, -47, and -20, cloned and produced in Escherichia coli, are important targets for molecular diagnosis and evolutionary analysis (44).
Scrub typhus has an incubation period of 6 to 21 days. The exact mechanism of how Orientia bacteria move from the chigger bite site to internal organs is not well understood and is thought to involve the circulatory or lymphatic system. In a study by Shirai and colleagues (45), human volunteers developed fever, eschar, and regional lymphadenopathy after being bitten by infected chiggers. Chiggers transmit the infection through their bites, and bacteremia can be detected before the onset of fever. O. tsutsugamushi targets endothelial cells, macrophages, dendritic cells, polymorphonuclear leukocytes, and lymphocytes in various organs. Direct blood-borne spread of the disease has also been suggested. Host immune response plays a role in the self-limiting nature of scrub typhus, and chloramphenicol medication reduces recurrence rates. Infection does not provide long-term immunity, and reinfections can occur. Protective immunity from a heterologous serotype lasts one to three months, while a homologous serotype protects up to one to three years. The severity of the disease varies with different strains of O. tsutsugamushi.
The major challenge in the clinical diagnosis of scrub typhus is the presentation of non-specific febrile symptoms that overlap with several other tropical infectious diseases. Symptoms can range from fever, headache, myalgia, with lesser incidence of rash, eschar, regional lymphadenopathy, and neurological involvement. A few cases of conjunctival suffusion, cough, and gastrointestinal disturbances, along with rare complications like myocarditis and encephalitis, have also been reported. Owing to such a wide range of common and overlapping clinical manifestations, laboratory testing for confirmation becomes paramount. Delayed diagnosis or treatment can lead to complications like bronchopneumonia, thrombocytopenia, toxic hepatitis, and more severe conditions such as ARDS, MODS, septic shock, and multiorgan failure. Chigger bites are often painless, and the development of a single eschar at the bite site is common. If it happens, eschar formation is confirmatory but may not be present in all cases. Occasionally, eschars can resemble lesions caused by other diseases or non-infectious factors. Currently, clinicians rely on clinical suspicion and laboratory testing for diagnosis, with immunofluorescence assay (IFA) being the gold standard serological test. Prompt and accurate diagnosis is crucial for reducing fever and preventing more severe disease with multi-organ involvement. Different approaches for the diagnosis are described below.
In 1916, Weil and Felix discovered that typhus sera caused heterophile antibody agglutination in strains of Proteus vulgaris. Later, the test was extended to include scrub typhus. The Weil-Felix test uses non-specific antigens derived from non-motile strains of Proteus vulgaris (OX-19, OX-2) and Proteus mirabilis (OX-K) to detect Rickettsial infections (46). However, it cannot differentiate between different types of typhus or Rocky Mountain Spotted Fever. The appearance and rapid rise of Proteus agglutinins in the blood provide some evidence of Rickettsial infection, but the antibodies decline after a few months. Positive results can be seen in other infections like urinary tract infections, relapsing fever, febrile illnesses, and leptospirosis. Weil-Felix titers are not consistently elevated in scrub typhus, and subsequent infections do not increase OX-K agglutinins as in the first infection. Although the Weil-Felix test is considered the gold standard for diagnosis, it has limited value in early diagnosis and lacks specificity and sensitivity.
Various clinical samples, such as buffy coats, defibrinated whole blood, triturated clots, plasma, necropsy tissue, skin biopsy, and arthropod samples, can be used to isolate Orientia tsutsugamushi. Giemsa-stained impression smears of the spleen or peritoneum surface can also reveal the organism. Mice are commonly inoculated with patients’ whole blood to monitor disease and mortality. Animal inoculation is preferred in cases where contamination is present in postmortem tissues. Cell culture techniques using cell lines like HeLa, BHK, VERO, L929, and primary monocytes are commonly used for isolating Orientia from clinical specimens (47, 48). Isolation success rates of Rickettsial pathogens from clinical specimens are typically around 20-40%, even under controlled cell culture conditions (49). Rickettsiae prefer lower temperatures than 37°C (50). Temperatures of 34-35°C seem optimum, and this also slows mammalian cell growth and helps establish long-lasting in vitro cultures (3). However, O. tsutsugamushi cultures form a clear cytopathic effect (CPE) on day 7-14 that becomes macroscopically visible on longer incubation of 3-4 weeks (51). CPE results in a distinct halo, accompanied by cell detachment and increased bacterial loads, as demonstrated by RT-PCR with lower CT values. O. tsutsugamushi in cell cultures can be demonstrated by Giemsa staining (52) or pink bodies by Gimenez staining (53). Biosafety level 3 facilities are required for cultivation, and the process can take up to 4-6 weeks to yield positive results like CPE by microscopy or CT values of 25-30 by RT-PCR.
The indirect fluorescent antibody assay (IFA) is the gold standard for detecting antibodies in scrub typhus. IFA uses epifluorescence to visualize antibodies bound to scrub typhus antigens (54). Significant antibody titers and IgM detection occur in primary infection in the first week, while IgG antibodies develop later. Reinfection can be identified by IgG antibodies on day 6. Although antigenic variation exists, it can detect IgG and IgM antibodies using antigens from Karp, Kato, and Gilliam serotypes (41). Detectable antibodies can persist for decades but may decrease over time due to strain heterogeneity and reinfection rates. IFA sensitivity and specificity vary with different antibody titers. The Infectious Disease Surveillance Centre (IDSC) in Japan suggests a method for identifying scrub typhus based on local strains and PCR testing (55). Detecting specific IgM antibodies to Rickettsia species using IFA provides evidence of recent Rickettsial infection; however, prozone phenomenon and rheumatoid factor interference must be considered. Micro-IFA allows testing with small volumes of serum and antigens, reducing processing time and resources. However, in endemic areas, diagnosing acute infection using serological testing should use paired acute and convalescent specimens to observe seroconversion or an increase in antibody titer.
Molecular techniques were utilized for diagnosing scrub typhus, where antigens such as 110-, 58-, 56-, and 47 kDa specific to scrub typhus are commonly used molecular targets. Notably, proteins with 47- and 56-kDa in the cell wall of O. tsutsugamushi show promise as antigenic targets for diagnosing Orientia spp (51, 56). PCR methods, including conventional (57), nested (N-PCR) (58), and real-time PCR (RT-PCR) (59–61), have improved scrub typhus diagnosis. Loop-Mediated Isothermal Amplification (LAMP) allows DNA identification at the point of treatment but has limitations compared to PCR (47). Molecular techniques can detect diseases earlier than serological tests. Target DNA segments such as 16S rRNA, htrA, gltA, ompA, ompB, and geneD have been used for PCR amplification (62, 63). Rickettsial species were isolated successfully using PCR from human blood, showing its sensitivity and early detection compared to serological techniques (64). Molecular techniques are also used to isolate rickettsial species from tick and flea samples. Nested PCR is found to be more sensitive and useful for sequencing and identifying new rickettsial species. Nested PCR was evaluated for detecting Rickettsia spp. in serum samples (65). RFLP can differentiate TG and SFG rickettsial strains with a detection limit of 10 rickettsial copies per assay (51).
Real-time PCR has replaced conventional and nested PCR extensively in the current molecular era due to its advantages, such as reduced contamination, improved sensitivity and accuracy, ability to perform multiplex PCR with different targets, rapid turnaround time, and high throughput analyses for epidemiologic investigations. Initially used for detecting R. prowazekii, real-time PCR has since been employed for various genus-, group-, and species-specific assays. For example, the qPCR assay was evaluated targeting the citrate synthase gene (gltA) of Rickettsia from the spotted fever and typhus groups, which detected a single target copy number per assay (66). This assay is useful in detecting low levels of Rickettsia in human samples. Similarly, SYBR Green-based qPCR assay was utilized to detect the rOmpA gene, with a sensitivity of five copies per reaction from infected tissues (51).
A multiplex qPCR assay was developed targeting the 47 kDa, gltA, and ompB genes to identify the scrub typhus, typhus, and spotted fever groups of rickettsiae, respectively (47). The detection limits for this assay were 24 copies/μl, 5 copies/μl, and 1 copy/μl, respectively. This assay was performed in 54 samples, and compared with the cell culture-based method, it has provided the most accurate results. Additionally, a real-time multiplex PCR assay was developed with increased sensitivity and specificity, detecting 2 gene copies in blood for three targets: scrub typhus (56 kDa gene), typhus group (17-kDa gene), and spotted fever group rickettsiae (ompA gene) (67). Multiplex assays have been widely used with increased sensitivity, although they may fail to detect certain targets in samples such as whole blood, where levels are relatively low compared to tissue samples (e.g., skin and eschar samples) with higher antigenemia.
The choice of primers used in PCR assays helps confirm the genus level, including SFGR and typhus group, as indicated by gltA, 17 kDa, and ompB targets. Amplification of the ompA gene provides conclusive evidence of the spotted fever group. The sensitivity of single-stage PCR assays using gltA alone was 33%, but sensitivity improved to 83% with sequential PCR. The highest sensitivity of 100% has been achieved using three single sequential PCR assays targeting ompA, ompB, and gltA (68). Multi-locus sequence typing (MLST) was performed using five genes (rrs, gltA, ompB, ompA, and sca4), which has helped differentiate rickettsial species and classify new species but for intra-species variation (69). Whole genome sequencing has been completed for 50 rickettsial genomes, enabling the development of a unique diagnostic tool specific to subsets of rickettsial species (70).
Antibiotics that target specific Rickettsia spp. remain the available therapeutic option (71). The choice of antibiotics depends on several factors, including severity of the infection, local resistance patterns, and individual patient considerations. Antibiotics like doxycycline, chloramphenicol, azithromycin, and fluoroquinolones are commonly used for the treatment of rickettsial infections (7). Doxycycline, a broad-spectrum antibiotic, is the preferred first-line drug due to its high efficacy against various rickettsial infections and can be administered intravenously or orally. It is important to note that tetracyclines, including doxycycline, have no reported cases of permanent dental staining possibly induced by doxycycline in young children. However, it has been recommended that the use of doxycycline be avoided in children aged less than 8 years (72). In cases where doxycycline cannot be used, such as in pregnant women, children, or individuals with contraindications to tetracyclines, chloramphenicol is an alternative drug of choice (7). However, chloramphenicol should be used cautiously during pregnancy due to possible teratogenic implications (73). For murine typhus infections caused by R. typhi, azithromycin is commonly administered orally or intravenously. In rare situations where other antibiotics are unsuitable or unavailable, fluoroquinolones like ciprofloxacin or levofloxacin are preferred (74). The differences in clinical severity of scrub typhus strains may be influenced by factors such as strain virulence, associated co-morbidities and immune status of the patients. The duration of treatment depends on the specific rickettsial infection, the severity of the illness, organ involvement and the patient’s clinical response. In severe cases, treatment may extend from 7 to 14 days or even longer (75). It is crucial for patients to complete the entire course of antibiotics as prescribed by the clinician, even if symptoms improve before the treatment is finished, to negate antibiotic resistance. Though the current drug arsenal can manage the treatment of O. tsutsugamushi infections, drug resistance is inevitable. O. tsutsugamushi infections are highly fatal and treatment delays due to lack of rapid and accurate diagnosis can drive serious patients to multiorgan failure and shock. Unfortunately, there are no apparently dedicated drug discovery programs for Rickettsial infections. Recently, Varghese and colleagues (7) conducted a multicentric, double-blind, randomized controlled clinical trial (INTREST) on 794 Scrub typhus patients in India. They compared the 7-day intravenous treatment of Doxycycline and Azithromycin alone and the combination of the two drugs. Combination IV therapy with doxycycline and azithromycin was found to be a better therapeutic option for the treatment of severe scrub typhus than monotherapy with either drug alone.
Scrub typhus caused by O. tsutsugamushi is an emerging neglected tropical zoonosis prevalent in various states of India with significant fatality rates and socio-economic impact. Clinical diagnosis of Orientia infections is challenging and must be addressed to facilitate early treatment. The overlap of common disease symptoms and clinical presentation with other endemic diseases makes clinical diagnosis difficult. In addition, lack of awareness about Rickettsial infections in the community and healthcare settings, limited access to skilled professionals and advanced diagnostic tools lead to delayed diagnosis and missed opportunities for early intervention. Though the disease is widespread across several states of India, there is a paucity of accurate epidemiological and clinical databases that would help to design strategies for timely diagnosis and therapeutic interventions. Serological approaches, while commonly used, have limitations such as cross-reactivity that can result in false positive or false negative results. More specific, accurate and standardized protocol-based rapid diagnostics for O. tsutsugamushi are urgently required to establish clinical diagnosis and initiate early treatment.
Advancements in molecular biology can help identify specific molecular targets and development of Orientia-specific diagnostics. Rapid diagnostic tests, particularly point-of-care assays, could enable quick and accurate detection, especially in resource-limited settings where access to sophisticated laboratory facilities is difficult. Public health interventions like focus on epidemiology, endemicity, vector control, disease monitoring, and emergence in certain endemic pockets are critical. Last but not least, dedicated programs on discovering new or repurposed drugs and new combination regimens for O. tsutsugamushi and other rickettsial pathogens, including controlled clinical trials, are urgently required to control this emerging disease.
VR: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SN: Conceptualization, Writing – review & editing. RS: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Guccione C, Colomba C, Iaria C, Cascio A. Rickettsiales in the WHO European Region: an update from a One Health perspective. Parasit Vectors. (2023) 16. doi: 10.1186/s13071-022-05646-4
2. Walker DH, Mendell NL. A scrub typhus vaccine presents a challenging unmet need. NPJ Vaccines. (2023) 8. doi: 10.1038/s41541-023-00605-1
3. Elliott I, Pearson I, Dahal P, Thomas NV, Roberts T, Newton PN. Scrub typhus ecology: A systematic review of Orientia in vectors and hosts. Parasit Vectors. (2019) 12. doi: 10.1186/s13071-019-3751-x
4. Kore VB, Mahajan SM. Recent threat of scrub typhus in India: A narrative review. Cureus. (2022). doi: 10.7759/cureus.30092
5. Lokida D, Hadi U, Lau CY, Kosasih H, Liang CJ, Rusli M, et al. Underdiagnoses of Rickettsia in patients hospitalized with acute fever in Indonesia: Observational study results. BMC Infect Dis. (2020) 20. doi: 10.1186/s12879-020-05057-9
6. Bonell A, Lubell Y, Newton PN, Crump JA, Paris DH. Estimating the burden of scrub typhus: A systematic review. PLoS Negl Trop Dis. (2017) 11. doi: 10.1371/journal.pntd.0005838
7. Varghese GM, Dayanand D, Gunasekaran K, Kundu D, Wyawahare M, Sharma N, et al. Intravenous doxycycline, azithromycin, or both for severe scrub typhus. New Engl J Med. (2023) 388. doi: 10.1056/nejmoa2208449
8. Varghese GM, Janardhanan J, Trowbridge P, Peter JV, Prakash JAJ, Sathyendra S, et al. Scrub typhus in South India: Clinical and laboratory manifestations, genetic variability, and outcome. Int J Infect Dis. (2013) 17. doi: 10.1016/j.ijid.2013.05.017
9. Xu G, Walker DH, Jupiter D, Melby PC, Arcari CM. A review of the global epidemiology of scrub typhus. PloS Negl Trop Dis. (2017) 11. doi: 10.1371/journal.pntd.0006062
10. Zaman K. Scrub typhus, a salient threat: Needs attention. PLoS Negl Trop Dis. (2023) 17. doi: 10.1371/journal.pntd.0011427
11. Hosahalli Vasanna S, Lim PPC, Khan TS, Dalal J. Secondary hemophagocytic lymphohistiocytosis associated with Rocky Mountain spotted fever in a toddler: A case report. EJHaem. (2022) 3. doi: 10.1002/jha2.405
12. Angelakis E, Bechah Y, Raoult D. The history of epidemic typhus. Microbiol Spectr. (2016) 4. doi: 10.1128/microbiolspec.poh-0010-2015
13. Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. (2007) 7. doi: 10.1016/S1473-3099(07)70261-X
14. Rovery C, Brouqui P, Raoult D. Questions on Mediterranean spotted fever a century after its discovery. Emerg Infect Dis. (2008) 14. doi: 10.3201/eid1409.071133
15. Stewart A, Armstrong M, Graves S, Hajkowicz K. Clinical Manifestations and Outcomes of Rickettsia australis Infection: A 15-year retrospective study of hospitalized patients. Trop Med Infect Dis. (2017) 2. doi: 10.3390/tropicalmed2020019
16. Stewart AGA, Smith S, Binotto E, McBride WJH, Hanson J. The epidemiology and clinical features of rickettsial diseases in North Queensland, Australia: Implications for patient identification and management. PLoS Negl Trop Dis. (2019) 13. doi: 10.1371/journal.pntd.0007583
17. Blanton LS. The rickettsioses: A practical update. Infect Dis Clin North Am. (2019) 33. doi: 10.1016/j.idc.2018.10.010
18. Izzard L, Fuller A, Blacksell SD, Paris DH, Richards AL, Aukkanit N, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. (2010) 48. doi: 10.1128/JCM.01526-10
19. Luce-Fedrow A, Lehman ML, Kelly DJ, Mullins K, Maina AN, Stewart RL, et al. A Review of Scrub Typhus (Orientia tsutsugamushi and related organisms): Then, now, and tomorrow. Trop Med Infect Dis. (2018) 3. doi: 10.3390/tropicalmed3010008
20. Kelly DJ, Richards AL, Temenak J, Strickman D, Dasch GA. The past and present threat of rickettsial diseases to military medicine and international public health. Clin Infect Dis. (2002) 34. doi: 10.1086/339908
21. Tattersall RN. Tsutsugamushi fever on the India-Burma border. Lancet. (1945) 246. doi: 10.1016/S0140-6736(45)91944-1
22. Kalra SL, Rao KN. Typhus fevers in Kashmir State. Part II. Murine typhus. Indian J Med Res. (1951) 39.
24. Madi D, Achappa B, Chakrapani M, Pavan M, Narayanan S, Yadlapati S, et al. Scrub typhus, a reemerging zoonosis – An Indian case series. Asian J Med Sci. (2014) 5. doi: 10.3126/ajms.v5i3.9213
25. Sethi S, Prasad A, Biswal M, Hallur VK, Mewara A, Gupta N, et al. Outbreak of scrub typhus in North India: A re-emerging epidemic. Trop Doct. (2014) 44. doi: 10.1177/0049475514523761
26. Sachdeva R, Sachdeva S. Scrub typhus: An underdiagnosed re-emerging zoonotic disease. Trop J Med Res. (2014) 17. doi: 10.4103/1119-0388.140445
27. Varghese GM, Janardhanan J, Mahajan SK, Tariang D, Trowbridge P, Prakash JAJ, et al. Molecular epidemiology and genetic diversity of Orientia tsutsugamushi from patients with scrub typhus in 3 regions of India. Emerg Infect Dis. (2015) 21. doi: 10.3201/eid2101.140580
28. Devasagayam E, Dayanand D, Kundu D, Kamath MS, Kirubakaran R, Varghese GM. The burden of scrub typhus in India: A systematic review. PLoS Negl Trop Dis. (2021) 15. doi: 10.1371/journal.pntd.0009619
29. Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. (2015) 9. doi: 10.1371/journal.pntd.0003971
30. Bhure M, Chavan K, Shinde S, A. Khan W, Chaudhari S. Rickettsial diseases of recent significance in India. Curr Topics Zoonoses. (2024). doi: 10.5772/intechopen.114139
31. Krishnamoorthi S, Goel S, Kaur J, Bisht K, Biswal M. A review of rickettsial diseases other than scrub typhus in India. Trop Med Infect Dis. (2023) 8. doi: 10.3390/tropicalmed8050280
32. Lv Y, Guo XG, Jin DC. Research progress on Leptotrombidium deliense. Korean J Parasitol. (2018) 56. doi: 10.3347/kjp.2018.56.4.313
33. Xiang R, Ren TG, Guo XG. Research history and progress of six vector chigger species of scrub typhus in China. Syst Appl Acarol. (2022) 27. doi: 10.11158/saa.27.9.11
34. Lerdthusnee K, Khuntirat B, Leepitakrat W, Tanskul P, Monkanna T, Khlaimanee N, et al. Scrub typhus: Vector competence of Leptotrombidium chiangraiensis chiggers and transmission efficacy and isolation of Orientia tsutsugamushi. Ann New York Acad Sci. (2003) 990. doi: 10.1111/j.1749-6632.2003.tb07333.x
35. Hase T, Roberts LW, Hildebrandt PK, Cavanaugh DC. Stylostome formation by Leptotrombidium mites (Acari: Trombiculidae). J Parasitol. (1978) 64. doi: 10.2307/3279967
36. Kuo CC, Huang JL, Shu PY, Lee PL, Kelt DA, Wang HC. Cascading effect of economic globalization on human risks of scrub typhus and tick-borne rickettsial diseases. Ecol Appl. (2012) 22. doi: 10.1890/12-0031.1
37. Bowles DE, Swaby JA. Field Guide to Venomous and Medically Important Invertebrates Affecting Military Operations : Identification, Biology, Symptoms, Treatment. Brooks City-Base, TX: USAF Institute for Operational Health (2006).
38. Tee TS, Kamalanathan M, Suan KA, Chun SS, Ming HT, Yasin RM, et al. Seroepidemiologic survey of Orientia tsutsugamushi, Rickettsia typhi, and TT118 spotted fever group rickettsiae in rubber estate workers in Malaysia. Am J Trop Med Hygiene. (1999) 61. doi: 10.4269/ajtmh.1999.61.73
39. Lee SH, Lee YS, Lee IY, Lim JW, Shin HK, Yu JR, et al. Monthly occurrence of vectors and reservoir rodents of scrub typhus in an endemic area of Jeollanam-do, Korea. Korean J Parasitol. (2012) 50. doi: 10.3347/kjp.2012.50.4.327
40. Zhang Y, Zhang Z, Zhao Y, Cheng S, Ren H. Identifying health effects of exposure to trichloroacetamide using transcriptomics and metabonomics in mice (Mus musculus). Environ Sci Technol. (2013) 47. doi: 10.1021/es3048976
41. Shishido A, Hikita M, Sato T, Kohno S. Particulate and soluble antigens of Rickettsia tsutsugamushi in the complement fixation test. J Immunol. (1969) 103. doi: 10.4049/jimmunol.103.3.480
42. Ohashi N, Nashimoto H, Ikeda H, Tamura A. Diversity of immunodominant 56-kDa type-specific antigen (TSA) of Rickettsia tsutsugamushi. Sequence and comparative analyses of the genes encoding TSA homologues from four antigenic variants. J Biol Chem. (1992) 267. doi: 10.1016/s0021-9258(18)42337-x
43. Kelly DJ, Fuerst PA, Ching WM, Richards AL. Scrub typhus: The geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. (2009) 48. doi: 10.1086/596576
44. Furuya Y, Yamamoto S, Otu M, Yoshida Y, Ohashi N, Murata M, et al. Use of monoclonal antibodies against Rickettsia tsutsugamushi Kawasaki for serodiagnosis by enzyme-linked immunosorbent assay. J Clin Microbiol. (1991) 29. doi: 10.1128/jcm.29.2.340-345.1991
45. Shirai A, Campbell RW, Gan E, Chan TC, Huxsoll DL. Serological analysis of Rickettsia tsutsugamushi isolates from North Queensland. Aust J Exp Biol Med Sci. (1982) 60. doi: 10.1038/icb.1982.22
46. Cruickshank R. The weil-felix reaction in typhus fever. J Hygiene. (1927) 27. doi: 10.1017/S0022172400031818
47. Paris DH, Blacksell SD, Newton PN, Day NPJ. Simple, rapid and sensitive detection of Orientia tsutsugamushi by loop-isothermal DNA amplification. Trans R Soc Trop Med Hyg. (2008) 102. doi: 10.1016/j.trstmh.2008.04.040
48. Seong SY, Kim HR, Huh MS, Park SG, Kang JS, Han TH, et al. Induction of neutralizing antibody in mice by immunization with recombinant 56 kDa protein of Orientia tsutsugamushi. Vaccine. (1997) 15. doi: 10.1016/S0264-410X(97)00112-6
49. Rodkvamtook W, Ruang-areerate T, Gaywee J, Richards AL, Jeamwattanalert P, Bodhidatta D, et al. Isolation and characterization of Orientia tsutsugamushi from rodents captured following a scrub typhus outbreak at a military training base, Bothong district, Chonburi province, central Thailand. Am J Trop Med Hygiene. (2011) 84. doi: 10.4269/ajtmh.2011.09-0768
50. Policastro PF, Munderloh UG, Fischer ER, Hackstadt T. Rickettsia rickettsii growth and temperature-inducible protein expression in embryonic tick cell lines. J Med Microbiol. (1997) 46. doi: 10.1099/00222615-46-10-839
51. Eremeeva ME, Dasch GA, Silverman DJ. Evaluation of a PCR assay for quantitation of Rickettsia rickettsii and closely related spotted fever group rickettsiae. J Clin Microbiol. (2003) 41. doi: 10.1128/JCM.41.12.5466-5472.2003
52. Schaechter M, Bozeman FM, Smadel JE. Study on the growth of rickettsiae. II. Morphologic observations of living rickettsiae in tissue culture cells. Virology. (1957) 3. doi: 10.1016/0042-6822(57)90030-2
53. Giménez DF. Staining rickettsiae in yolk-sac cultures. Biotechnic Histochem. (1964) 39. doi: 10.3109/10520296409061219
54. Robinson DM, Brown G, Gan E, Huxsoll DL. Adaptation of a microimmunofluorescence test to the study of human Rickettsia tsutsugamushi antibody. Am J Trop Med Hygiene. (1976) 25. doi: 10.4269/ajtmh.1976.25.900
55. Ogawa M, Hagiwara T, Kishimoto T, Shiga S, Yoshida Y, Furuya Y, et al. Scrub typhus in Japan: Epidemiology and clinical features of cases reported in 1998. Am J Trop Med Hygiene. (2002) 67. doi: 10.4269/ajtmh.2002.67.162
56. Huber E. Loop-mediated isothermal amplification assay targeting the 47-kda gene of Orientia tsutsugamushi: A rapid and sensitive alternative to real-time PCR. J Med Microbiol Diagn. (2012) 01. doi: 10.4172/2161-0703.1000112
57. Furuya Y, Yoshida Y, Katayama T, Kawamori F, Yamamoto S, Ohashi N, et al. Specific amplification of Rickettsia tsutsugamushi DNA from clinical specimens by polymerase chain reaction. J Clin Microbiol. (1991) 29. doi: 10.1128/jcm.29.11.2628-2630.1991
58. Furuya Y, Yoshida Y, Katayama T, Yamamoto S, Kawamura A. Serotype-specific amplification of Rickettsia tsutsugamushi DNA by nested polymerase chain reaction. J Clin Microbiol. (1993) 31. doi: 10.1128/jcm.31.6.1637-1640.1993
59. Jiang J, Paris DH, Blacksell SD, Aukkanit N, Newton PN, Phetsouvanh R, et al. Diversity of the 47-kD HtrA nucleic acid and translated amino acid sequences from 17 recent human isolates of Orientia. Vector-Borne Zoonotic Dis. (2013) 13. doi: 10.1089/vbz.2012.1112
60. Bakshi D, Singhal P, Mahajan SK, Subramaniam P, Tuteja U, Batra HV. Development of a real-time PCR assay for the diagnosis of scrub typhus cases in India and evidence of the prevalence of new genotype of O. tsutsugamushi. Acta Trop. (2007) 104. doi: 10.1016/j.actatropica.2007.07.013
61. Paris DH, Blacksell SD, Stenos J, Graves SR, Unsworth NB, Phetsouvanh R, et al. Real-time multiplex PCR assay for detection and differentiation of Rickettsiae and Orientiae. Trans R Soc Trop Med Hyg. (2008) 102. doi: 10.1016/j.trstmh.2007.11.001
62. Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. (2000) 28. doi: 10.1093/nar/28.12.e63
63. Blacksell SD, Paris DH, Chierakul W, Wuthiekanun V, Teeratakul A, Kantipong P, et al. Prospective evaluation of commercial antibody-based rapid tests in combination with a loop-mediated isothermal amplification PCR assay for detection of Orientia tsutsugamushi during the acute phase of scrub typhus infection. Clin Vaccine Immunol. (2012) 19. doi: 10.1128/CVI.05478-11
64. Tzianabos T, Anderson BE, McDade JE. Detection of Rickettsia rickettsii DNA in clinical specimens by using polymerase chain reaction technology. J Clin Microbiol. (1989) 27. doi: 10.1128/jcm.27.12.2866-2868.1989
65. Kim DM, Na RY, Tae YY, Ji HL, Jong TY, Shim SK, et al. Usefulness of nested PCR for the diagnosis of scrub typhus in clinical practice: A prospective study. Am J Trop Med Hygiene. (2006) 75. doi: 10.4269/ajtmh.2006.75.542
66. Stenos J, Graves SR, Unsworth NB. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group rickettsiae. Am J Trop Med Hygiene. (2005) 73. doi: 10.4269/ajtmh.2005.73.1083
67. Tantibhedhyangkul W, Wongsawat E, Silpasakorn S, Waywa D, Saenyasiri N, Suesuay J, et al. Use of multiplex real-time PCR to diagnose scrub typhus. J Clin Microbiol. (2017) 55. doi: 10.1128/JCM.02181-16
68. Santibáñez S, Portillo A, Santibáñez P, Palomar AM, Oteo JA. Usefulness of rickettsial PCR assays for the molecular diagnosis of human rickettsioses. Enferm Infecc Microbiol Clin. (2013) 31. doi: 10.1016/j.eimc.2012.08.001
69. Jiang J, Blair PJ, Felices V, Moron C, Cespedes M, Anaya E, et al. Phylogenetic analysis of a novel molecular isolate of spotted fever group Rickettsiae from northern Peru: Candidatus Rickettsia andeanae. Ann N Y Acad Sci. (2005) 1063. doi: 10.1196/annals.1355.054
70. He M, Zhang L, Hu H, Liu X, Zhang C, Xin Y, et al. Complete genome sequencing and comparative genomic analyses of a new spotted-fever Rickettsia heilongjiangensis strain B8. Emerg Microbes Infect. (2023) 12. doi: 10.1080/22221751.2022.2153085
71. Rolain JM, Maurin M, Vestris G, Raoult D. In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob Agents Chemother. (1998) 42. doi: 10.1128/aac.42.7.1537
72. Pöyhönen H, Nurmi M, Peltola V, Alaluusua S, Ruuskanen O, Lähdesmäki T. Dental staining after doxycycline use in children. J Antimicrobial Chemotherapy. (2017) 72. doi: 10.1093/jac/dkx245
73. Chung CY, Kwok AKH, Chung KL. Use of opthalmic medications during pregnancy. Hong Kong Med J. (2004) 10.
74. Gikas A, Doukakis S, Pediaditis J, Kastanakis S, Manios A, Tselentis Y. Comparison of the effectiveness of five different antibiotic regimens on infection with Rickettsia typhi: Therapeutic data from 87 cases. Am J Trop Med Hygiene. (2004) 70. doi: 10.4269/ajtmh.2004.70.576
75. Kim YS, Yun HJ, Shim SK, Koo SH, Kim SY, Kim S. A comparative trial of a single dose of azithromycin versus doxycycline for the treatment of mild scrub typhus. Clin Infect Dis. (2004) 39. doi: 10.1086/425008
76. Chaloemthanetphong A, Ahantarig A, Apanaskevich DA, Hirunkanokpun S, Baimai V, Trinachartvanit W. A novel Rickettsia, Candidatus Rickettsia takensis, and the first record of Candidatus Rickettsia laoensis in Dermacentor from Northwestern Thailand. Sci Rep. (2023) 13. doi: 10.1038/s41598-023-37206-w
77. Chao LL, Robinson M, Liang YF, Shih CM. First detection and molecular identification of Rickettsia massiliae, a human pathogen, in Rhipicephalus sanguineus ticks collected from Southern Taiwan. PloS Negl Trop Dis. (2022) 16. doi: 10.1371/journal.pntd.0010917
78. Latifian M, Khalili M, Farrokhnia M, Mostafavi E, Esmaeili S. Rickettsia conorii subsp. Israelensis infection: a case report from southeast Iran. BMC Infect Dis. (2022) 22. doi: 10.1186/s12879-022-07291-9
79. Sánchez-Montes S, Blum-Domínguez S, Lozano-Sardaneta YN, Zazueta-Islas HM, Solís-Cortés M, Ovando-Márquez O, et al. Molecular detection of Rickettsia sp. cf. Rickettsia monacensis in Ixodes sp. cf. Ixodes affinis collected from white-tailed deer in Campeche, Mexico. Parasitol Res. (2021) 120. doi: 10.1007/s00436-021-07128-5
80. Madeddu G, Mancini F, Caddeo A, Ciervo A, Babudieri S, Maida I, et al. Rickettsia monacensis as cause of Mediterranean spotted fever-like Illness, Italy. Emerg Infect Dis. (2012) 18. doi: 10.3201/eid1804.111583
81. Jado I, Oteo JA, Aldámiz M, Gil H, Escudero R, Ibarra V, et al. Rickettsia monacensis and human disease, Spain. Emerg Infect Dis. (2007) 13. doi: 10.3201/eid1309.060186
82. de Sousa R, dos Santos ML, Cruz C, Almeida V, Garrote AR, Ramirez F, et al. Rare case of Rickettsiosis caused by Rickettsia monacensis, Portugal, 2021. Emerg Infect Dis. (2022) 28. doi: 10.3201/eid2805.211836
83. Silva-Ramos CR, Hidalgo M, Faccini-Martínez ÁA. Clinical, epidemiological, and laboratory features of Rickettsia parkeri rickettsiosis: A systematic review. Ticks Tick Borne Dis. (2021) 12. doi: 10.1016/j.ttbdis.2021.101734
84. Szakacs TA, Wood H, Russell CB, Nelder MP, Patel SN. An apparent, locally acquired case of rickettsialpox (Rickettsia akari) in Ontario, Canada. J Assoc Med Microbiol Infect Dis Canada. (2020) 5. doi: 10.3138/jammi-2019-0028
85. Shtrek SV, Rudakov NV, Abramova NV, Samoylenko IE, Berezkina GVJ, Zelikman SY, et al. Evaluation of the effectiveness of the serological methods for the identification of antibodies in patients with tissue ricketsiosis on the territories of a different risk of Rickettsia sibirica infection. Klinichescheskaya Laboratornaya Diagnostika. (2018) 63. doi: 10.18821/0869-2084-2018-63-12-777-782
86. Noguchi M, Oshita S, Yamazoe N, Miyazaki M, Takemura YC. Important clinical features of Japanese spotted fever. Am J Trop Med Hygiene. (2018) 99. doi: 10.4269/ajtmh.17-0576
87. Álvarez-Hernández G, Roldán JFG, Milan NSH, Lash RR, Behravesh CB, Paddock CD. Rocky Mountain spotted fever in Mexico: past, present, and future. Lancet Infect Dis. (2017) 17. doi: 10.1016/S1473-3099(17)30173-1
88. Tosoni A, Mirijello A, Ciervo A, Mancini F, Rezza G, Damiano F, et al. Human Rickettsia aeschlimannii infection: First case with acute hepatitis and review of the literature. Eur Rev Med Pharmacol Sci. (2016) 20.
89. Brown LD, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. (2016) 3. doi: 10.1007/s40475-016-0070-6
90. Parola P, Rovery C, Rolain JM, Brouqui P, Davoust B, Raoult D. Rickettsia slovaca and R. raoultii in Tick-borne Rickettsioses. Emerg Infect Dis. (2009) 15. doi: 10.3201/eid1507.081449
91. Unsworth NB, Stenos J, Graves SR, Faa AG, Cox GE, Dyer JR, et al. Flinders Island spotted fever rickettsioses caused by the “marmionii” strain of Rickettsia honei, eastern Australia. Emerg Infect Dis. (2007) 13. doi: 10.3201/eid1304.060087
92. Kelly PJ. Rickettsia africae in the West Indies. Emerg Infect Dis. (2006) 12. doi: 10.3201/eid1202.050903
93. Fournier PE, Grunnenberger F, Jaulhac B, Gastinger G, Raoult D. Evidence of Rickettsia helvetica infection in humans, eastern France. Emerg Infect Dis. (2000) 6. doi: 10.3201/eid0604.000412
94. Driskell LO, Tucker AM, Woodard A, Wood RR, Wood DO. Fluorescence-activated cell sorting of Rickettsia prowazekii-infected host cells based on bacterial burden and early detection of fluorescent rickettsial transformants. PLoS One. (2016) 11. doi: 10.1371/journal.pone.0152365
Keywords: diagnosis, epidemiology, serology, PCR and RT-PCR, Rickettsia
Citation: Ravishankar V, Narayanan S and Shandil RK (2024) Rickettsial infections: prevalence and diagnosis of scrub typhus in India. Front. Trop. Dis 5:1433013. doi: 10.3389/fitd.2024.1433013
Received: 15 May 2024; Accepted: 30 August 2024;
Published: 27 September 2024.
Edited by:
Benjamin Cull, University of Minnesota Twin Cities, United StatesReviewed by:
Dewi Lokida, Indonesia Research Partnership on Infectious Disease (INA-RESPOND), IndonesiaCopyright © 2024 Ravishankar, Narayanan and Shandil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Radha Krishan Shandil, cmsuc2hhbmRpbEBmbmRyLmlu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.