
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Trop. Dis., 10 July 2024
Sec. Neglected Tropical Diseases
Volume 5 - 2024 | https://doi.org/10.3389/fitd.2024.1419166
This article is part of the Research TopicFrontiers in Tropical Diseases Webinar SeriesView all 3 articles
The World Health Organization guidelines for verification of onchocerciasis elimination include demonstrating that the prevalence of exposure to the parasite in individuals born since transmission was interrupted needs to be less than 0.1%. The guidelines recommend using seropositivity to an Onchocerca volvulus specific antigen (Ov16) for this purpose. Ov16 seropositivity has most often been assessed using the Ov16 ELISA assay. Currently, the Ov16 ELISA assay includes internal positive and negative controls to monitor for proper assay performance but does not control for the quality of the dried blood spots (DBS) being tested. Previous studies have reported a high prevalence of antibodies recognizing Escherichia coli in children. Through the development of an ELISA assay to detect antibodies recognizing E. coli, a common commensal in humans, DBS may be prescreened for quality assurance prior to testing for Ov16. Results demonstrated antibodies to E. coli were detected in 100% of randomly selected serum samples collected from O. volvulus infected individuals residing in an onchocerciasis hyperendemic area. Furthermore, when DBS were improperly stored, the E. coli antibodies were found to decay over a period of one week, while remaining unchanged over the same period in properly stored samples. Similarly, E. coli antibodies were detected in 100% of a batch of field collected properly stored DBS, while being present only in 5% of a batch of improperly stored spots. This study demonstrates the value of E. coli ELISA for DBS quality control testing and validation of proper storage of collections of DBS for the Ov16 ELISA.
Onchocerciasis, or river blindness is one of the preventative chemotherapy neglected tropical diseases (PC-NTDs) targeted by the World Health Organization (WHO) and the international community for elimination (1). Onchocerciasis is caused by infection with the filarial parasite Onchocerca volvulus, which is transmitted by blackflies of the genus Simulium (2). The most recent data suggest that approximately 21 million Onchocerca volvulus infections persist, with the majority of cases found in Africa (3, 4). More importantly, many cases are stricken with lifelong health disparities which can include irreversible skin conditions and permanent blindness (5). Currently, the efforts to eliminate onchocerciasis have focused upon interrupting transmission of the parasite, primarily using a strategy of annual or semi-annual mass treatment of afflicted communities with Mectizan® (ivermectin), an anti-filarial drug that is donated by Merck “as much as needed for as long as needed” (6). Supplementing Mectizan® distribution with local community vector control has recently been shown to significantly reduce the time to elimination (7). Although the mass drug administration (MDA) program has reduced the disability-adjusted life years (DALYs) due to onchocerciasis since ivermectin treatments began in the 1990s, it is still estimated to cause over 1.5 million DALYs, with the largest DALY losses occurring in Nigeria, the Democratic Republic of Congo, South Sudan, and Cameroon (5). Given the expected decline in onchocerciasis with continued MDA programs in place across Africa, quality assurance in testing the population to ensure accurate results is integral to evaluating elimination status.
WHO has published guidelines for verification of the elimination of onchocerciasis (8). In brief, the elimination process involves three stages. The first stage involves community directed mass treatment with ivermectin (CDTI). A successful treatment is defined as one in which all endemic communities are treated (100% geographic coverage) and a minimum of 70% of the total population receives the drug (70% therapeutic coverage). At least 13 rounds of successful coverage must be delivered in a given focus before interruption of transmission is suspected.
The WHO guidelines then recommend that the countries conduct stop MDA surveys to confirm transmission has been interrupted (8). The stop MDA surveys recommended by the WHO involve demonstrating a prevalence of exposure in children under the age of 10 of less than 0.1%, as well as demonstrating a prevalence of vector flies carrying the infective stage of the parasite of less than 0.05% (8). If a stop MDA survey is successful, MDA can be halted in this focus. The WHO then recommends carrying out a post treatment surveillance (PTS) 3-5 years after MDA has ceased to confirm that transmission has not been re-established. The methods and metrics used in the PTS are the same as those used in the post-MDA survey. Once all foci in a country have successfully passed their PTS surveys, the country may submit a dossier to WHO applying for verification of the elimination of onchocerciasis.
The WHO guidelines call for measuring the prevalence of exposure in children using antibody response to a 16 kDa antigen specific to O. volvulus (Ov16). This antigen was initially shown to be specific for O. volvulus exposure using sera collected from other nematode infections of humans, although homologues to Ov16 can be found in other nematode parasites (9). For example, a homologue with 98 – 100% by BLASTp exists in Onchcerca ochengi (a cattle parasite sympatric with O. volvulus in much of Africa). The next nearest identity matches outside of the genus Onchocerca are Dirofilaria immitis at 80.58% and Brugia pahangi at 68.46% (10). Measurement of the prevalence of Ov16 positivity is commonly done using an Enzyme-linked Immunosorbent Assay (ELISA) assay with Ov16 recombinant antigen immobilized on the plate probed with antibodies eluted from DBS collected from children in the endemic communities (11, 12). Generally, the Ov16 ELISA is carried out in a central laboratory, and the DBS collected in the endemic communities are shipped to a central laboratory. While the protocols for the Ov16 ELISA assay includes both positive and negative controls, to date there has been no way to control for the quality of the DBS. There are guidelines available for appropriate collection and handling of DBS to ensure viability of the samples required to achieve valid results by testing laboratories upon receipt (13–15). However, proper collection and storage can be challenging when collecting samples in remote regions (16). Improper preparation or storage of DBS (e.g. exposure to high temperature or high humidity) can result in a relatively rapid loss of antibody activity. Though proper storage requirements recommend including indicators within a sample batch, these such indicators are unavailable or not utilized by the field collection teams. Thus, there is a need for additional ways to assess the quality of the DBS before they are tested in the Ov16 ELISA.
Escherichia coli is a common component of the human intestinal microbiome. It is present in over 90% of people’s intestines (17). Most known E. coli strains are non-pathogenic and some of the first exposures occur within the a month after birth initiating one of the earliest adaptive immune responses in life through production of IgG antibodies (17). In addition, E. coli is a commensal of many domestic animals (18) and can be isolated from many surfaces in the environment, especially in areas with limited infrastructure for water, sanitation and hygiene (18). Given its ubiquitous nature, a large proportion of individuals living in low resource settings produce antibodies to pathogenic strains of E. coli (19–21). We hypothesized that the near ubiquitous presence of E. coli in the human microbiome might mean that a large percentage of individuals might produce antibodies to this bacterium and that these antibodies might be detected using a crude extract prepared from a non-pathogenic strain of E. coli. In the initial experiments described below, antibodies against non-pathogenic E. coli were found in the all of the serum samples collected from O. volvulus infected individuals. This fact was exploited for development of the assay to demonstrate the presence of active antibodies in DBS, using an ELISA assay to detect antibodies recognizing E. coli.
To determine how frequently antibodies to E. coli were found in an onchocerciasis endemic population, 40 samples were randomly chosen from a serum bank of samples collected from individuals with microscopically confirmed infections with O. volvulus. The serum samples were diluted 1/20 in Phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4), containing 0.05% Tween 20 and 5% w/v bovine serum albumin (PBST-BSA) and tested for reactivity in the E. coli ELISA protocol described.
Synthetic blood spots were made using the following blood washing protocol using a total of 1 ml of human whole blood stored in 3.2% sodium citrate (single individual, gender unknown, obtained from BioIVT, Westbury, NY) combined with either banked serum from a known O. volvulus positive sample or an O. volvulus negative sample after erythrocyte washing. The whole blood was centrifuged at 500 x g for 5 minutes at room temperature. The supernatant was carefully removed from the pelleted erythrocytes and the pellet gently resuspended in 1ml sterile isotonic saline (154 mM NaCl). The solution was centrifuged again at 500 x g at room temperature, and resuspended again in 1ml isotonic saline to wash the erythrocytes. This wash process was repeated twice. After the final wash, the erythrocytes were resuspended in 1 ml of isotonic saline and divided into 5 aliquots of 200 µl each. The aliquots were centrifuged again at 500 x g at room temperature, the supernatant of isotonic saline was removed, and aliquots were made with the addition of 100 μl of O. volvulus positive serum or by addition of 100 µl of negative serum. The aliquots of 100 µl of washed erythrocytes were resuspended in 100 µl of either the positive or negative test sera and negative agglutination confirmed prior to creating DBS for downstream testing.
Blood spots were prepared by punching out 6 mm circles of Whatman #1 paper using a standard single hole paper punch. The circles were placed in plastic weigh boats and each circle was spotted with 10 µl of the washed erythrocyte/serum solutions containing either O. volvulus positive serum or negative serum as described above. The circles were allowed to completely dry at room temperature and the DBS were immediately stored in individual microcentrifuge tubes at -20°C.
Dried blood spots were prepared by saturating 6 mm circles of Whatman #1 paper with 10 µl of whole blood. To test the effects of humidity and temperature on antibody activity in DBS, half of the DBS were completely dried and stored at -20°C as described above. The other half of the DBS were not allowed to completely dry and were placed in an incubator at 37°C under 100% relative humidity. Paired duplicate DBS were removed from the DBS stored under each condition at days 1, 3, 5 and 7 after preparation, eluted and assayed for the presence of antibodies to E. coli using the ELISA protocol described below.
The E. coli ELISA was further tested using field collected DBS intended for testing in the Ov16 ELISA to monitor the progress of elimination following WHO guidelines. The DBS were packaged at the point of collection in each community in sealed plastic bags with a desiccant and sent to central laboratories for analysis. Upon receipt by the laboratory, each batch of DBS was inspected as part of the pre-testing process. One batch was noted to have color change in the desiccant (Bag 5) while another batch was received where the desiccant appeared normal (Bag 2). These were tested for the presence of antibodies recognizing E. coli using the E. coli ELISA described below.
A frozen stock of Escherichia coli BL21 (a non-pathogenic strain) was used to inoculate a plate containing Luria-Bertani (LB) agar, and the plate incubated overnight at 37°C. In the morning, 50 ml of LB broth was inoculated with a single colony from the plate and the culture allowed to grow overnight in a bacteriological shaker at 37°C and 300 revolutions per minute (RPM). The following morning, the 50 ml overnight culture was added to 1 l LB broth and the culture incubated in a bacteriological shaker at 37°C and 300 RPM until the optical density at 600 nm reached 1.0. The culture was then centrifuged at 17,000 x g for 10 minutes at 4°C. The cell pellet was resuspended in 10 ml lysis buffer (125 mM Tris(hydroxymethyl)aminomethane [Tris] pH 7.4, 150 mM sodium chlorids [NaCl], 1 mM Ethylenediaminetetraacetic Acid [EDTA], 1 mM dithiothreitol [DTT], 0.2 mg/ml lysozyme, 0.5% Triton x-100) and one tablet of Pierce Protease Inhibitor mini (Fisher Scientific) was dissolved in the solution. The solution was incubated for 30 minutes on ice to allow the cells to lyse. The lysate was then subjected to three cycles of sonication on ice for 1 minute each at a 50% duty cycle to lower its viscosity. The lysate was then subjected to centrifugation at 43,000 x g for 30 min. at 4°C to pellet the cellular debris. The supernatant was then passed through a 0.45 µm filter to sterilize it. The protein concentration of the filtrate was determined using the Bio Rad protein Assay (Bio Rad), adjusted to 2 mg/ml with lysis buffer and divided into 500 µl aliquots. The aliquots were stored at -80°C.
The E. coli ELISA was designed to adhere as closely as possible to the Ov16 ELISA protocol currently used by many of the countries in Africa and Latin America to monitor the progress of the efforts to interrupt transmission of O. volvulus. Thus, the E. coli ELISA utilizes most reagents and supplies in common with the Ov16 ELISA. A detailed protocol for the Ov16 ELISA has been published previously (22) and readers are directed to consult with this publication for additional details regarding the ELISA protocol in general. To carry out the E. coli ELISA, duplicate 6 mm circular punches of blood collected on Whatman #1 paper were placed into individual wells of a 96 well microtiter plate. A total of 200 μl of PBST-BSA was added to each well containing a DBS, and the plate was placed at 4°C overnight. In parallel, 100 μl of antigen coating solution (2 µg/ml E. coli antigen or 2 µg/ml BSA in 0.1 M NaHCO3 (pH 9.6) was added to wells of an Immulon 2 HB plate (Fisher Scientific). Columns 1-6 were coated with E. coli antigen, while Columns 7-12 were coated with BSA. The plate was covered and incubated overnight at 4°C. The following morning, the coated plate was washed 4 times with PBST, and 100 µl of PBST-BSA was added to each well. The plate was incubated at 4°C for one hour following the guidelines for use in the Ov16 ELISA approved by WHO, and the PBST-BSA removed. A total of 50 µl of the eluate from the DBS was then added to each well. Columns 1 and 7 each received the same samples, so that replicates were prepared, with each sample being placed in one well coated with E. coli and one coated with BSA. The plate was incubated at room temperature for 2 hours and washed four times with PBST. A total of 50 µl of goat anti-human IgG conjugated to alkaline phosphatase (Jackson ImmunoResearch) diluted 1/5000 in PBST was added to each well. The plate was sealed and incubated for one hour at room temperature. The plate was washed four times with PBST, and 50 μl of p-nitrophenyl phosphate (PNPP) substrate solution (Fisher Scientific, prepared according to the manufacturer’s instructions) was added to each well. The plate was allowed to develop until the optical density at 405 nm approached 0.1 in the wells coated with BSA. The reaction was stopped by the addition of 25 µl of 3 M NaOH to each well, and a final reading was taken and recorded.
Whole blood used in the preparation of synthetic DBS was obtained under protocol 105951MODCR000003 approved by the Institutional Review Board for Human Subjects Research at the University of South Florida (USF). Serum samples from individuals with patent O. volvulus infection were obtained from a serum bank collected during the clinical trials to test the efficacy of ivermectin against O. volvulus infection conducted in Liberia in the 1980s (23). These samples were originally collected under a protocol approved by the Institutional Review Board for Human Subjects Research at Case Western Reserve University and University Hospitals, Cleveland, Ohio, USA. When the samples were utilized in this study, they were devoid of any individual identifiers. As a result, the USF Institutional Review Board for Human Subject Research concluded that “as these specimens were not collected specifically for this study and no one on our study team had access to the subject identifiers linked to the specimens, they are not considered human subjects research and do not meet the 45 Code of Federal Regulations Part 46 (45 CFR 46) definitions of human subject research”.
Dried blood spots were collected by programs supported by The Carter Center, which supports onchocerciasis elimination programs in Latin America and several countries in Africa. These samples were collected as part of normal disease surveillance activities and were not part of a research protocol. Furthermore, the samples were devoid of any individual identifiers. As above, since these samples were not collected specifically for this study and no one on our study team had access to the subject identifiers linked to the specimens, they were not considered human subjects research and do not meet the 45 CFR 46 definitions of human subject research.
The primary goal of this research was to develop a way to determine if active antibodies were present in DBS collected to monitor O. volvulus transmission in Africa. In particular, it was of interest to verify that antibodies to E. coli were present in populations from areas endemic for onchocerciasis. All of the random samples tested exhibited a level of reactivity to the E. coli antigen that was significantly greater than was seen in the wells without E. coli antigen (Figure 1; mean OD E. coli antigen 1.80, median OD E. coli antigen 1.78, 95% CI 1.67-1.94, versus mean OD blank 0.075, median OD blank 0.075, 95% CI 0.073-0.077, p= <0.0001, t test). This suggested that all of the samples tested contained antibodies that recognized E. coli.
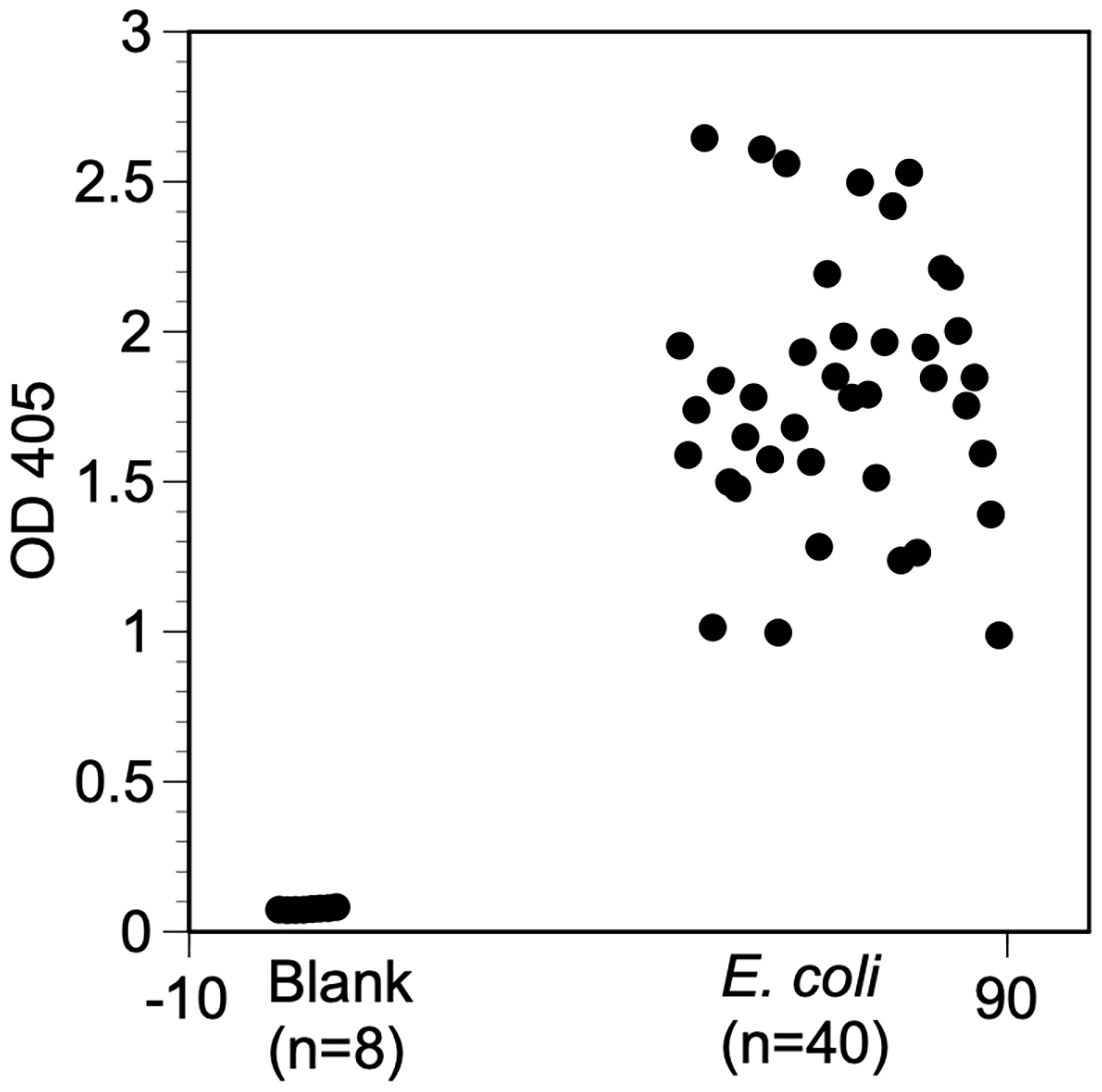
Figure 1 Reactivity of banked serum samples against E. coli antigen. Wells were coated with E. coli antigen prepared as described in Materials and methods or left uncoated. The E. coli ELISA protocol was then carried out and the OD at 405nm determined after the reactions were stopped. Blank = wells not coated with antigen and not exposed to sera. E. coli = wells coated with E. coli antigen and exposed to individual serum samples. Values represent the mean of duplicate wells for each sample.
Then it was determined if the E. coli ELISA could be used to assay sera eluted from DBS. To accomplish this, 20 of the sera from the serum bank were used to prepare synthetic DBS, as described in Materials and Methods. These were then eluted and tested in the E. coli ELISA. All of the samples produced OD values that were significantly greater than background levels (Figure 2; mean OD E. coli antigen 0.692; median OD E. coli antigen 0.680, 95% CI 0.615-0.769 versus mean OD BSA 0.137; median OD BSA 0.131, 95% CI 0.123-0.147, p<0.0001 t test). Overall, the mean E. coli/BSA ratio of the samples tested was 5.15. This demonstrated that the E. coli ELISA could be used to check for active antibodies in eluted DBS using the elution process developed for the Ov16 ELISA.
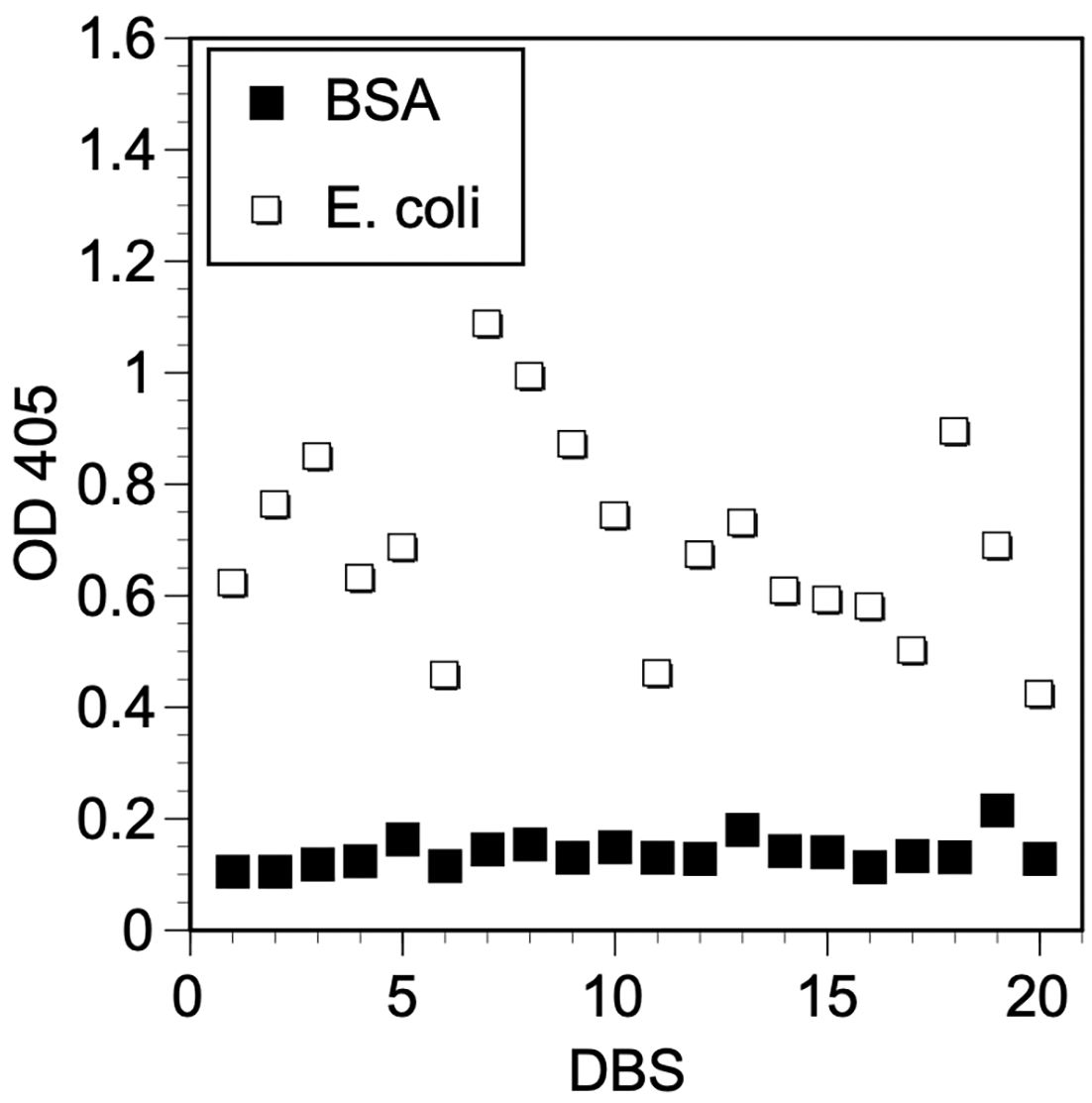
Figure 2 Reactivity of eluted synthetic DBS to E. coli antigen. Wells were coated with E. coli antigen prepared as described in Materials and Methods or BSA and developed with different eluted synthetic DBS prepared as described in Materials and Methods. Paired symbols represent the OD values obtained from each DBS when incubated with E. coli antigen or BSA coated wells. Values represent the mean of duplicate wells.
The experiments described above demonstrated that antibodies to E. coli could be detected in a large majority of individuals tested, both in sera and in synthetic DBS. However, it was important to show that the antibodies against E. coli could be used to distinguish properly prepared and stored DBS from those that had suffered degradation due to improper preparation or storage. To accomplish this, DBS were prepared by spotting whole blood on to Whatman #1 paper. Individual circles were prepared from the spotted blood using a 6 mm punch. Half of the spots were allowed to dry completely and stored dry at -20°C, representing ideal collection and storage procedures. The other half were not permitted to dry completely and transferred to a 37°C incubator under 100% humidity, representing improper collection and storage. The paired blood spots were assayed at days 1, 3, 5 and 7 post collection. On days 1 and 3, both properly prepared and improperly prepared DBS gave similar levels of reactivity in the ELISA, as measured by the ratio of reactivity in E. coli antigen coated wells versus paired BSA coated wells (Figure 3). However, by day 5, the activity in the improperly stored DBS began to decline. This decline continued through day 7 (Figure 3). In contrast the activity in the properly stored DBS did not change significantly throughout the course of the experiment (Figure 3).
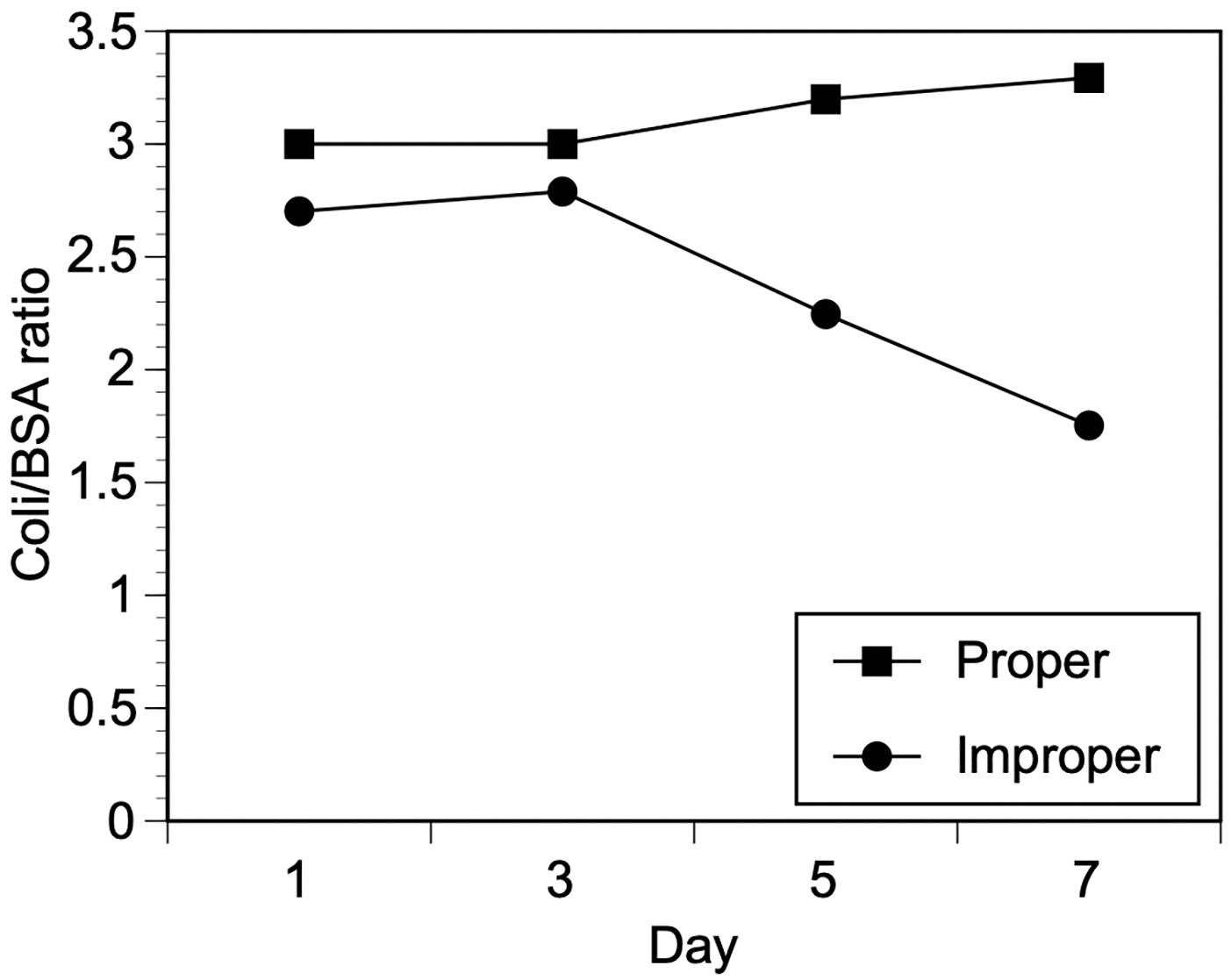
Figure 3 Effect of proper or improper storage conditions on reactivity of eluted DBS in the E. coli ELISA. DBS were prepared as described in Materials and Methods and subjected to proper or improper storage as described in Materials and Methods. The DBS were eluted and assayed using the E. coli ELISA. Values represent the mean of duplicate wells from two separate DBS for each storage condition and time point.
The Carter Center supports a number of onchocerciasis elimination programs worldwide, which utilize the Ov16 ELISA on DBS as one assay to monitor the progress of elimination and to make stop MDA decisions, following WHO guidelines. The results from random sampling provided from one batch of DBS where the desiccant appeared normal (Bag 2) and one where the desiccant appeared compromised (Bag 5) suggesting that there were active antibodies in the DBS from Bag 2 (the bag where the desiccant appeared dry), but the samples from Bag 5 (the bag where the desiccant appeared to have begun to change color) had suffered degradation (Figure 4). In the samples from Bag 2, 40/40 (100%) of the samples produced E. coli/BSA ratios that were above 2.5. In contrast, just 2/38 (5%) of the samples from Bag 5 produced E. coli/BSA ratios that were above 2.5. This difference was found to be statistically significant (X2 (1, N = 78) = 65.8237, p < 0.00001.
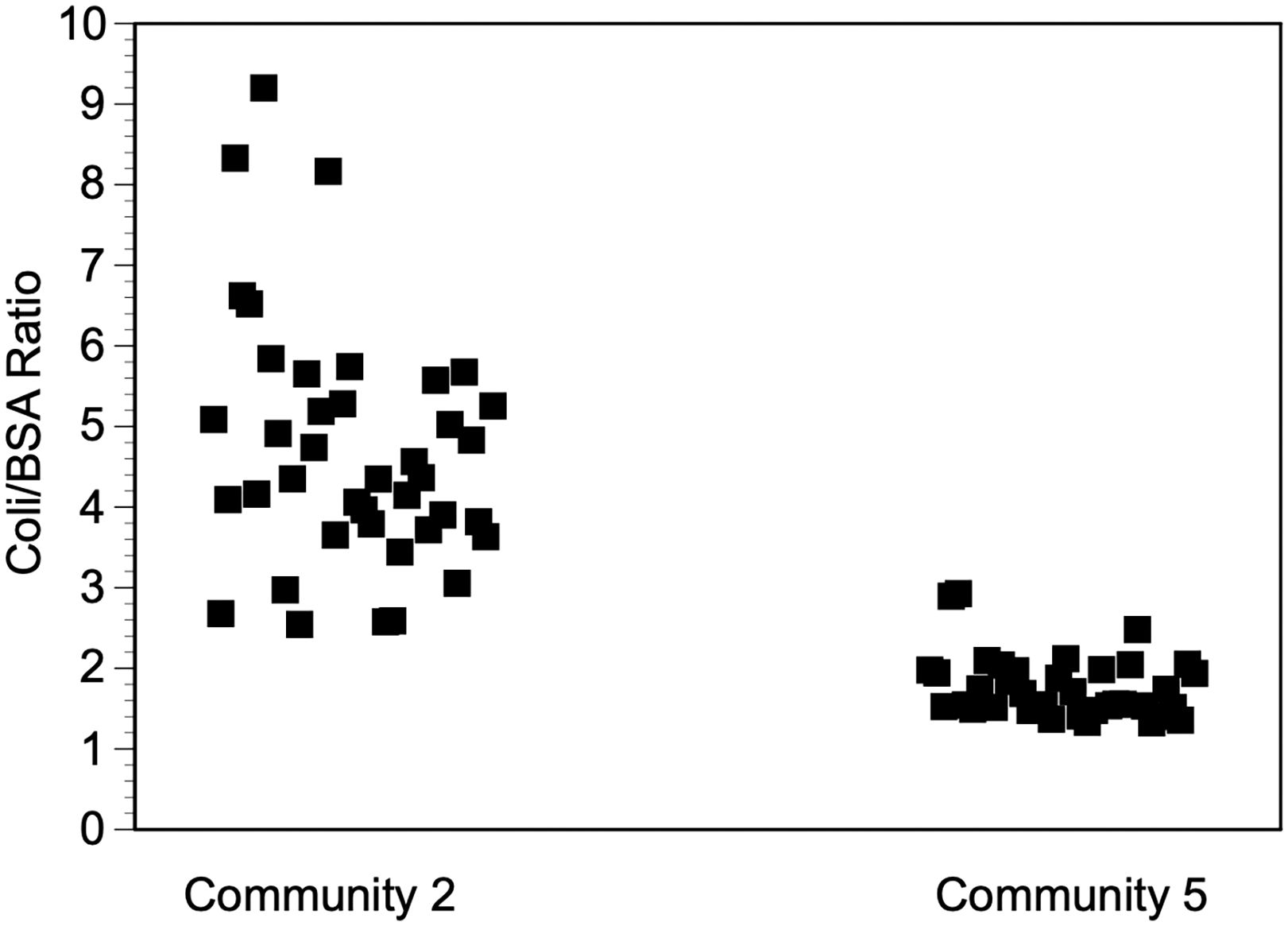
Figure 4 E. coli antibody levels in two field collections. DBS were collected as described in Materials and Methods and eluted and tested with the E. coli ELISA 2-4 weeks post collection. Each point represents the mean of two duplicate wells.
The E. coli antigen ELISA described herein has many characteristics that make it useful as a way to test the quality of DBS prior to their use in the Ov16 ELISA. As previously mentioned, E. coli is ubiquitous in nature and most strains are non-pathogenic with many individuals being exposed and eliciting antibody production at an early age (17). This and other research utilizes the diversity of BL21 E. coli in testing platforms due to its ability to create target antigens that could be detected within normal mammalian microbiomes with various E. coli antibodies noted in human serum (24, 25). The antigen preparation used in the E. coli ELISA is quite easy to produce, requiring no specialized equipment. The E. coli ELISA protocol itself closely follows the protocol developed for the Ov16 ELISA that is widely used to assess the prevalence of exposure to O. volvulus, and thus will be easily implemented by any laboratory that performs the Ov16 ELISA assay. The data presented above also suggest that is in agreement with previous studies (19–21) that the prevalence of antibodies to E. coli in the target population for the Ov16 ELISA is very high, likely approaching 100%. Finally, the results demonstrate that antibody reactivity in the DBS, using E. coli as a proxy for overall antibody reactivity in each DBS can be lost in improperly stored DBS, suggestive that measuring anti-E.coli antibodies is a useful way to monitor antibody integrity in DBS.
There are some practical considerations that will need to be refined as the E. coli ELISA is implemented by the laboratories that test samples from onchocerciasis endemic countries. First, in most of the experiments described above, each DBS was individually tested against E. coli antigen and against wells coated with BSA as a paired negative control. However, the samples tested gave OD readings in the BSA coated wells that were consistent, and did not differ significantly from the OD readings obtained from blank wells which contained no DBS eluates. It is therefore likely that it is not necessary to test every DBS against both E. coli antigen and BSA. It may be sufficient to only test the eluted DBS against E. coli and reference the results against blank wells included on every plate. Doing so would allow roughly twice as many DBS to be tested per plate.
It is possible to analyze the results of the E. coli ELISA either by using the raw OD values or by calculating the ratio of the OD obtained against the E. coli antigen and that obtained from a BSA coated well (or perhaps against the blank wells included on the plate). It is recommended to use the ratio, as the raw OD values may have some variation, depending upon how long the plate is allowed to develop, among other factors. Utilizing a ratio of the OD of a given sample to a negative well as a way to normalize results between plates, as there may be some plate-to-plate variation.
In the experiments reported above, the E. coli/BSA (or negative well) ratios for optimally preserved DBS samples varied from 2.5 to 9.2. In contrast, the ratio dropped below 2.5 in the improperly stored DBS on day 5 of the stability test, and just 2/38 DBS in the samples collected from Bag 5 from the batch which did not pass pre-testing visual inspection, were above 2.5. Taken together, this suggests that a cutoff of a ratio of 2.5 may represent a good starting point for accepting or rejecting DBS for the Ov16 ELISA. More testing of the E. coli ELISA by endemic country laboratories will be very useful in helping establish this cutoff, as the cutoff may be further fined tuned as more batches of samples are processed.
In collecting DBS to monitor O. volvulus exposure, field teams are usually instructed to dry the samples thoroughly and place them together in a sealed plastic bag with a desiccant. The bags are then placed in a cooler over ice packs to transport them to the laboratory for analysis. Thus, when problems arise, they usually affect an entire collection from a given community and are not confined to a single sample. Thus, it may only be necessary to test a randomly selected collection of DBS contained in each bag of samples in order to determine if the samples have active antibodies. Given the high prevalence of E. coli antibodies in the human population, the random sample tested need not be very large. In such situations, lot quality assurance (LQA) methods may be useful in determining how many randomly selected samples should be analyzed to determine if a lot (or bag of DBS in this case) can be accepted for further analysis. In determining sample size for an LQA analysis, one needs to consider multiple variables. First, the size of the lot (in this case the number of DBS in a given collection bag) needs to be determined. One then needs to choose the level of α and β error that are acceptable. In this case, the α error is the probability of accepting a bad lot, while the β error is the probability of rejecting a good lot. Finally, one needs to decide upon the upper and lower thresholds for the LQA analysis, in this case being the upper and lower bounds of proportion of truly positive samples in the lot. The sample size and number of positives that need to be obtained in the sample to pass the lot can then be determined using publicly available online calculators (e.g. (26)). In the case of the Ov16 ELISA, DBS are collected from children from individual communities in a transmission zone, and the lot size is usually between 50 and 200 DBS per community. Examples of the sample sizes needed for conducting an LQA on different lot sizes are given in Table 1. In this simulation, maximum lot sizes were entered as shown in the table, setting the upper threshold at 0.95 (95% positive) and the lower threshold at 0.7 (70% positive). The α and β error were both set at 0.05. As Table 1 shows, the number of samples needed to conduct an LQA is always less than half of the total number of samples in a lot, and the proportion of samples that needs to be tested declines as the number of samples in the lot increases.
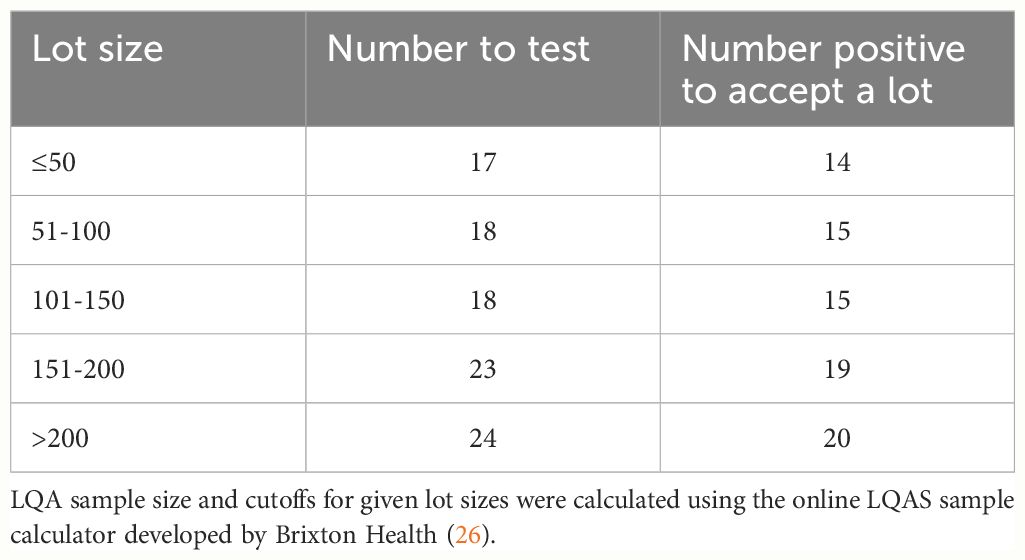
Table 1 Application of Lot Quality Assurance (LQA) to assay quality of DBS collections using the E. coli ELISA.
The Ov16 ELISA protocol contains multiple positive and negative internal controls that allow one to confirm that the assay conducted on every individual plate has been technically successful (22). However, these internal controls cannot measure the quality of the DBS that are being used in the assay. The E. coli ELISA assay described above uses reagents and supplies that are nearly identical to those used in the Ov16 ELISA and thus it offers a way to confirm the quality of the DBS prior to using them in the Ov16 ELISA, where it is expected that the vast majority of samples will test negative. It is very important that laboratories using the Ov16 ELISA in support of onchocerciasis elimination incorporate the E. coli ELISA as a way to confirm the quality of the DBS sent to them prior to using them in the Ov16 ELISA assay.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by University of South Florida Institutional Review Board for Human Subjects Research. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of a previous study, for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
HH: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. KM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. TU: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by contracts from the Task Force for Global Health and The Carter Center.
We are grateful to The Carter Center for providing some of the samples used in this study. We thank Dr. Vitaliano A. Cama for technical advice and critical reading of drafts of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hotez P. Enlarging the “Audacious Goal”: Elimination of the world’s high prevalence neglected tropical diseases. Vaccine. (2011) 29(Supplement 4):D104–10. doi: 10.1016/j.vaccine.2011.06.024
2. Greene BM, Warren KS, Mahmoud AAF. Onchocerciasis. In: Tropical and geographical medicine, 2nd ed. New York: McGraw-Hill Professional (1990) 429–38.
3. Mshana MI, Silvestri V, Mushi V, Bonaventura WM, Tarimo D, Ngasala B, et al. Burden and factors associated with onchocerciasis transmission among school-aged children after more than 20 years of community directed treatment with Ivermectin in Ulanga district, Tanzania: a school-based cross-sectional study. PloS Glo Pub Health. (2023) 3:e0001919. doi: 10.1371/journal.pgph.0001919
4. Colebunders R, Kaiser C, Basáñez MG, Olliaro P, Lakwo T, Siewe Fodjo JN. Reducing onchocerciasis-associated morbidity in onchocerciasis-endemic foci with high ongoing transmission: a focus on the children. Int J Inf Dis. (2022) 116:302–5. doi: 10.1016/j.ijid.2022.01.042
5. Vinkeles Melchers NVS, Stolk WA, van Loon W, Pedrique B, Bakker R, Murdoch ME, et al. The burden of skin disease and eye disease due to onchocerciasis in countries formerly under the African Programme for Onchocerciasis Control mandate for 1990, 2020, and 2030. PloS Negl Trop Dis. (2021) 15:e0009604. doi: 10.1371/journal.pntd.0009604
6. Walsh J. Merck donates drug for river blindness. Science. (1987) 238:610. doi: 10.1126/science.238.4827.610.b
7. Jacob B, Michael E, Unnasch TR. Community-directed vector control to accelerate onchocerciasis elimination. Pathogens. (2024) 13:268. doi: 10.3390/pathogens13030268
8. World Health Organization. Onchocerciasis. In: Guidelines for stopping mass drug administration and verifying elimination of human onchocerciasis. Geneva, Switzerland: WHO Press, World Health Organization (2016).
9. Lobos E, Weiss N, Karam M, Taylor HR, Ottesen EA, Nutman TB. An immunogenic Onchocerca volvulus antigen: A specific and early marker of infection. Science. (1991) 251:1603–5. doi: 10.1126/science.2011741
10. National Institutes of Health. BLASTp proteins: national library of medicine (2024). Available online at: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins&PROGRAM=blastp&PAGE_TYPE=BlastSearch&BLAST_SPEC.
11. Richards FO, Katabarwa M, Bekele F, Tadesse Z, Mohammed A, Sauerbrey M, et al. Operational performance of the Onchocerca volvulus ‘OEPA’ Ov16 ELISA serological assay in mapping, stop mass drug administration, and post treatment surveillance surveys. Am J Trop Med Hyg. (2018) 99:749–52. doi: 10.4269/ajtmh.18-0341
12. Golden A, Faulx D, Kalnoky M, Stevens E, Yokobe L, Peck R, et al. Analysis of age-dependent trends in Ov16 IgG4 seroprevalence to onchocerciasis. Parasitol Vectors. (2016) 9:338. doi: 10.1186/s13071-016-1623-1
13. Centers For Disease Control and Prevention Maternal Child HIV Branch and International Lab Branch. HEI toolkit: DBS job aids for laboratory - receiving and storing dried blood spots (2024). Available online at: https://www.childrenandaids.org/HEI_Toolkit/DBS_Lab_JobAid.
14. Grüner N, Stambouli O, Ross RS. Dried blood spots–preparing and processing for use in immunoassays and in molecular techniques. J Vis Exp. (2015) 1397):52619. doi: 10.3791/52619
15. Malsagova K, Kopylov A, Stepanov A, Butkova T, Izotov A, Kaysheva A. Dried blood spot in laboratory: directions and prospects. Diagnostics (Basel). (2020) 10:248. doi: 10.3390/diagnostics10040248
16. Lim MD. Dried blood spots for global health diagnostics and surveillance: opportunities and challenges. Am J Trop Med Hyg. (2018) 99:256–65. doi: 10.4269/ajtmh.17-0889
17. Martinson JNV, Walk ST. Escherichia coli residency in the gut of healthy human adults. EcoSal Plus. (2020) 9. doi: 10.1128/ecosalplus.esp-0003-2020
18. Denamur E, Clermont O, Bonacorsi S, Gordon D. The population genetics of pathogenic Escherichia coli. Nat Rev Microbiol. (2021) 19:37–54. doi: 10.1038/s41579-020-0416-x
19. Lammie PJ, Moss DM, Brook Goodhew E, Hamlin K, Krolewiecki A, West SK, et al. Development of a new platform for neglected tropical disease surveillance. Int J Parasitol. (2012) 42:797–800. doi: 10.1016/j.ijpara.2012.07.002
20. Echeverria P, Burke DS, Blacklow NR, Cukor G, Charoenkul C, Yanggratoke S. Age-specific prevalence of antibody to rotavirus, Escherichia coli heat-labile enterotoxin, Norwalk virus, and hepatitis A virus in a rural community in Thailand. J Clin Microbiol. (1983) 17:923–5. doi: 10.1128/jcm.17.5.923-925.1983
21. Brüssow H, Sidoti J, Link H, Hoang YK, Barclay D, Dirren H, et al. Age-specific prevalence of antibody to enterotoxigenic Escherichia coli in Ecuadorian and German children. J Infect Dis. (1990) 162:974–7. doi: 10.1093/infdis/162.4.974
22. Oguttu D, Byamukama E, Katholi CR, Habomugisha P, Nahabwe C, Ngabirano M, et al. Serosurveillance to monitor onchocerciasis elimination: the Ugandan experience. Am J Trop Med Hyg. (2014) 90:339–45. doi: 10.4269/ajtmh.13-0546
23. Gallin MY, Edmonds K, Ellner JJ, Erttmann KD, White AT, Newland HS, et al. Cell-mediated immune responses in human infection with onchocerca volvulus. J Immunol. (1988) 140:1999–2007. doi: 10.4049/jimmunol.140.6.1999
24. Bong J-H, Kim J, Lee G-Y, Park J-H, Kim T-H, Kang M-J, et al. Fluorescence immunoassay of E. coli using anti-lipopolysaccharide antibodies isolated from human serum. Biosens Bioelectron. (2019) 126:518–28. doi: 10.1016/j.bios.2018.10.036
25. Andreishcheva EN, Vann WF. Escherichia coli BL21(DE3) chromosome contains a group II capsular gene cluster. Gene. (2006) 384:113–9. doi: 10.1016/j.gene.2006.07.020
26. Brixton Health. LQAS sampling plan calculator (Hypergeometric model) (2023). Available online at: http://www.brixtonhealth.com/hyperLQAS.html.
Keywords: onchocerciasis, river blindness, serology, elimination, Ov16
Citation: Hassan HK, Miley KM and Unnasch TR (2024) Verification of the reactivity of immunoglobulins in dried blood spots collected for onchocerciasis sero-surveillance by an Escherichia coli ELISA. Front. Trop. Dis 5:1419166. doi: 10.3389/fitd.2024.1419166
Received: 17 April 2024; Accepted: 26 June 2024;
Published: 10 July 2024.
Edited by:
Mark Taylor, Liverpool School of Tropical Medicine, United KingdomReviewed by:
Robert Adamu Shey, University of Buea, CameroonCopyright © 2024 Hassan, Miley and Unnasch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas R. Unnasch, dHVubmFzY2hAdXNmLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.