
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Trop. Dis., 20 August 2024
Sec. Major Tropical Diseases
Volume 5 - 2024 | https://doi.org/10.3389/fitd.2024.1412871
This article is part of the Research TopicNewer Initiatives in Detection, Drug Discovery and Therapeutic Management of Drug-Resistant TuberculosisView all 6 articles
 Camilla Genovese1,2
Camilla Genovese1,2 Marta Colaneri1,3
Marta Colaneri1,3 Simone Pagano1,2
Simone Pagano1,2 Marco Schiuma1
Marco Schiuma1 Lucia Galli1,2
Lucia Galli1,2 Federico Fama1,2
Federico Fama1,2 Spinello Antinori1,2
Spinello Antinori1,2 Andrea Gori1,2,3*
Andrea Gori1,2,3* Alessandro Torre1
Alessandro Torre1Introduction: In recent years, Europe has experienced a significant flux of migrants, often hailing from regions endemic for schistosomiasis and strongyloidiasis, diseases frequently overshadowed by tuberculosis (TB) in healthcare priorities. While TB remains a prevalent concern among this population, chronic schistosomiasis and strongyloidiasis are frequently neglected. Motivated by this observation, we aimed to investigate the prevalence of schistosomiasis and strongyloidiasis in patients attending our TB outpatient clinic.
Methods: We conducted a retrospective, observational study spanning from June 2020 to January 2024, focusing on patients attending the TB outpatient clinic of Luigi Sacco Hospital of Milan, Italy. Serology tests were performed in patients with a history suggestive of exposure to either Schistosoma spp. or Strongyloides stercoralis.
Results: Among the 228 patients included in the study, 84 (36.8%) individuals were born in Italy, one came from Spain, 80 (35.1%) from strongyloidiasis moderate or high endemic countries and 63 (27.6%) from areas endemic for schistosomiasis and strongyloidiasis. Of these patients, 160 (70.2%) were diagnosed with tuberculosis disease, while 68 (29.8%) had tuberculosis infection. The prevalence of schistosomiasis was 26.7%, while that of strongyloidiasis was 7.8%. Notably, 3 patients tested positive for both infections.
Conclusion: Our study highlights the often-underestimated prevalence of schistosomiasis and strongyloidiasis among migrants accessing healthcare for TB, underscoring the importance of increased awareness and targeted screening within this population.
Neglected tropical diseases (NTDs), including schistosomiasis and strongyloidiasis, have gained attention due to increased migration to Europe from endemic regions. Despite being considered rare in high-income countries, Schistosoma spp. infections affect nearly 780 million people worldwide, and Strongyloides stercoralis infections surpass 600 million people globally (1, 2). Other than the burden associated with the acute infections caused by these two microorganisms, chronic infections by Schistosoma spp. and Strongyloides stercoralis are associated with significant morbidity and mortality, highlighting the importance of gaining deeper knowledge about the prevalence of these conditions. Specifically, chronic schistosomiasis might be complicated by liver cirrhosis, kidney disease, bladder cancer, and infertility (3–5), while chronic strongyloidiasis can cause urticaria, diarrhea and asthma. Severe complications of strongyloidiasis usually affect immunocompromised individuals and include hyper- and disseminated- infections, which are life-threatening (6, 7).
Schistosomiasis is caused by 5 Schistosoma spp. species, Schistosoma haematobium, Schistosoma mansoni, Schistosoma. japonicum, Schistosoma intercalatum and Schistosoma mekongi, each endemic to different regions, predominantly in Africa, Asia (e.g., China, Cambodia, Laos, Philippines), and South America (e.g., Brazil, Venezuela) (8). Similarly, Strongyloides stercoralis is widespread in Africa, Asia, South America and in some Eastern European countries (9).
With increasing migration rates to Europe, the number of individuals at risk of these infections is rising. In fact, a recent systematic review by Asundi et al. reported a seroprevalence of 12.2% for strongyloidiasis and of 18.4% for schistosomiasis in migrants who reside in countries with low endemicities (10). Moreover, while these infections are often imported by tourists or migrants from endemic regions, in Italy, cases of strongyloidiasis have been reported in individuals born before 1952 (11), and autochthonous schistosomiasis cases have emerged in Corsica, France, in recent years (12). Epidemiological studies in Italy reveal in migrants varying prevalence rates of strongyloidiasis, ranging from 4.5% to 17%, and schistosomiasis ranging from 6% to 27% (11, 13–15). However, the actual burden may be underestimated, particularly among migrants, due to prioritization of other conditions such as tuberculosis (TB) or HIV.
The Italian Society of Tropical Medicine and Global Health (SIMET) has issued recommendations for managing these infections in non-endemic countries, advocating for screening in individuals born or residing in endemic areas. Specifically, screening of schistosomiasis and strongyloidiasis should be performed in all individuals who were born or have resided for a cumulative period of at least one year in areas endemic for either one of these two conditions (16, 17).
However, implementing screening programs is challenging, especially among hard-to-reach populations like asylum seekers and refugees (18, 19). Screening and prevention activities can therefore be performed in specific contexts: at the arrival in the hosting country or in reception centers and shelters. In these settings, though, resources are often limited and reserved to assess conditions which are considered more urgent, both from a clinical and a public health point of view. Since TB remains a prevalent condition among migrants, requiring months of treatment and several follow up appointments, TB outpatient clinics offer a unique opportunity for schistosomiasis and strongyloidiasis screening.
Motivated by these challenges, our study aimed to assess the prevalence of schistosomiasis and strongyloidiasis in our TB outpatient clinic.
We conducted a retrospective, monocentric, observational study including patients who attended the TB outpatient clinic of Luigi Sacco Hospital of Milan, Italy, from June 2020 to January 2024.
The primary objective of the study was to evaluate the prevalence of strongyloidiasis and schistosomiasis among patients attending the TB outpatient clinic who underwent serological testing for Schistosoma spp. and Strongyloides stercoralis.
The secondary objectives of the study were to delineate the demographic and clinical characteristics of patients who tested positive for the strongyloidiasis and schistosomiasis serological tests, and to discern variances between patients who tested positive vs negative on the strongyloidiasis and schistosomiasis serological examinations.
All patients aged more than 18 years evaluated at the TB outpatient clinic at Luigi Sacco Hospital were consecutively included in the study. Patients attending the TB outpatient clinic typically have either a TB disease (TBD, also known as active TB disease) or TB infection (TBI, also known as latent TB infection) (20). They are routinely referred to the clinic when diagnosed with either of the two abovementioned conditions in other settings (e.g., from the Infectious Diseases Inpatient Department or other hospitals which do not have outpatient department dedicated to TB patients).
During their initial evaluation, patients underwent routine microbiological and biochemical screening (e.g. full blood count, liver and renal function tests, C-reactive protein, erythrocyte sedimentation rate, serologies for HIV, hepatitis B and C and syphilis), accounting for around 3 mL of plasma and 8.5 mL of serum. Individuals at risk of past exposure to Schistosoma spp. or Strongyloides stercoralis underwent NovaTec Immunodiagnostica GmBh® enzyme-linked immunosorbent assay (ELISA) for the qualitative detection of serum IgG antibodies against Schistosoma mansoni and Strongyloides stercoralis (16, 17, 21).
Stool and urine microscopy parasitological tests were subsequently performed in those testing positive at serology. Specifically, urine collection was performed for every patient who tested positive for Schistosoma specific IgG and came from countries at risk for Schistosoma haematobium infection. Patients were given a plastic jar to collect their 24h-urine sample, which was then sent to the Microbiology laboratory of Luigi Sacco Hospital. Urine samples were centrifugated before realizing a microscopy exam (at 40x and 100x) for the detection of Schistosoma haematobium eggs. Instead, patients who tested positive for schistosomiasis or strongyloidiasis were asked to collect three Para-Pak® PLUS Ecofix stool samples produced in different days. Stool samples were also sent to Microbiology laboratory for microscopic examination (at 40x and 100x). Additionally, three other stool samples produced in different days were collected from patients who tested positive for strongyloidiasis and sent within 24 hours to the microbiology laboratory for specific stool culture.
Treatment with praziquantel or ivermectin was proposed to all patients with a probable schistosomiasis or strongyloidiasis (defined as a positive serology and a positive history of stay in an endemic country 3 months prior presentation) (16, 17, 21). Praziquantel, which required in-hospital administration, was given with food at the dosage of 40 mg/kg in a single dose for patients coming from countries endemic for Schistosoma mansoni, Schistosoma haematobium and Schistosoma intercalatum, while patients coming from countries endemic for Schistosoma japonicum and Schistosoma mekongi were administered 60 mg/kg divided in two subsequent doses (16). Ivermectin was given at the dosage of 200 mgc/kg in two consecutive days (22).
Individuals at risk for schistosomiasis were defined as individuals born or who have resided for at least one cumulative year in schistosomiasis-endemic countries. Individuals at risk for strongyloidiasis were defined as individuals born or who have resided for at least one cumulative year in countries with moderate to high endemicity for Strongyloides stercoralis, as well as individuals born or who have resided in Italy before 1952.
The endemic countries for strongyloidiasis and the high-risk countries for schistosomiasis were defined based on national and international recommendations (16, 17, 21).
Demographic and clinical data, including comorbidities and coinfections, were collected. Investigated comorbidities included liver diseases (e.g., cirrhosis and steatohepatitis). rheumatologic diseases (e.g., rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, sarcoidosis, psoriatic arthritis), diabetes mellitus (type 1 and type 2), cancer (both hematological and solid organ cancers), endocrine diseases, lung diseases (e.g., chronic obstructive pulmonary disease, asthma, bronchiectasis, interstitial lung diseases), coronary heart disease, chronic kidney disease (stage III to V), neurologic diseases (e.g., history of hemorrhagic or ischemic stroke, demyelinating diseases, peripheral neuropathies). Treatment with steroids was defined as patients receiving ≥ 15 mg of prednisone (or equivalent) for at least one month. Tobacco use was defined as ongoing tobacco use. Intravenous drug use was defined as ongoing intravenous drug use. Alcohol use was defined as at least 14 drinks per week, or at least 7 drinks per week for women and >65 years old. Eosinophilia was defined as eosinophils count greater than 500 cells/mm3.
Categorical data are presented as absolute frequencies and proportions. Continuous variables are presented as mean and standard deviation. Prevalence of positive serologies was calculated as the ratio between patients with a positive serology for either Schistosoma spp. or Strongyloides stercoralis over the total patient who were tested and is presented with a 95% confidence interval (CI). Concerning the secondary objectives, categorical variables were compared with Fisher exact tests, while continuous with Mann Whitney tests. Statistical significance threshold was set at 0.05. Analyses were performed in R-Studio software (v. 4.2.3).
The study was notified on March 20th 2024, to the Ethics Committee Lombardia 1 with number CET-133-2024, in accordance with local regulations. The study was conducted in accordance with local legislation and national requirements. All participants signed informed written consent for clinical and biological data collection as part of the Infectious Diseases Outpatient Department Registry of Luigi Sacco Hospital, which was approved by Ethics Committee Lombardia 1.
A total of 228 patients attended the TB outpatient clinic from June 2020 to January 2024.
The overall characteristics of patients attending the TB outpatient clinic are reported in Table 1. The median age of the patients attending the TB outpatient clinic was 46 years [IQR 35, 61]. One hundred-sixty individuals (70.2%) were diagnosed with TBD, while 68 (29.8%) had TBI.
While 84 (36.8%) individuals were born in Italy, 144 patients originated from foreign countries. Indeed, 80 (35.1%) patients originated from strongyloidiasis moderate- or high-endemic countries, and 63 (27.6%) patients were born in areas endemic for schistosomiasis and strongyloidiasis. Only one patient came from a foreign country which is not considered endemic for schistosomiasis and strongyloidiasis (Spain). The median length of stay in Italy for patients coming from foreign countries was 144 months [IQR 48, 240].
Strongyloidiasis screening was suggested for 170 patients attending the clinic. Of these, 153 (90%) were tested for strongyloidiasis serology. Of the 63 patients originating countries also endemic for schistosomiasis, 56 (88.8%) were tested for schistosomiasis. In the group of patients tested for strongyloidiasis serology, 22 were Italian and either had a history of stay in an endemic country or were born before 1952.
Among 56 patients tested for schistosomiasis serology, 15 patients were positive resulting in a prevalence of 26.7% [95% CI 16.9-39.9]. The most represented countries were China with 5 positive patients (33.3%) and Egypt with 2 positive patients (13.3%) (Figure 1). The median age was 43 years [IQR 32.5, 46] and notably, only 40% were males. The median length of stay in the original country was 324 months [IQR 239, 348]. Patients who resulted positive to schistosomiasis serology had a longer length of stay in Italy compared with those who tested negative to schistosomiasis serology, however not in a significant way (168 months [IQR 78, 234] vs 132 months [IQR 60, 240], respectively). Table 2 shows the characteristics of patients who were tested for schistosomiasis serology. No significant differences were observed between patients who tested positive with respect to those with a negative serology.
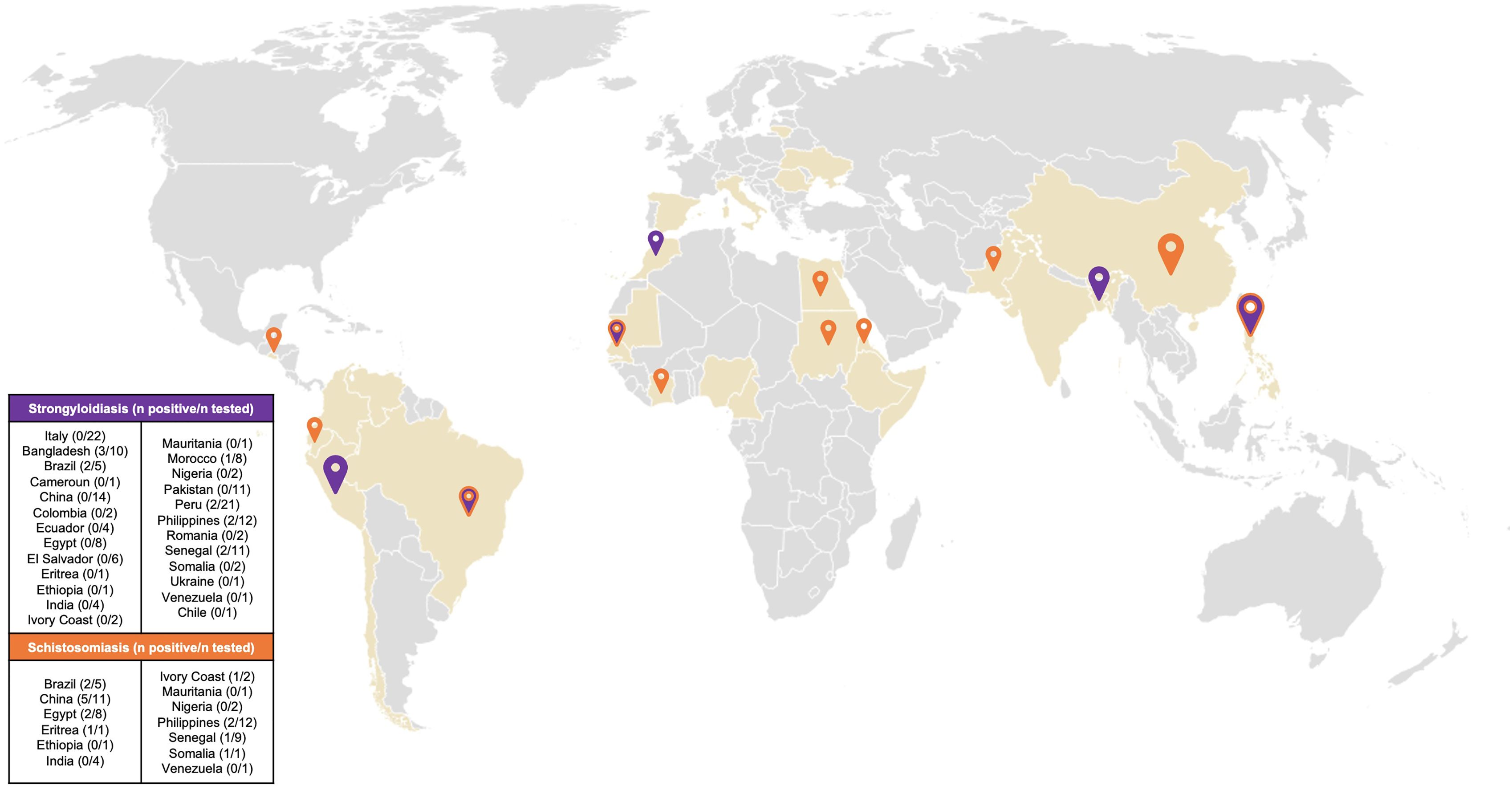
Figure 1. Map of country of origin of patients resulted positive to screening. Coloured countries are the countries of origin of all the patients attending the TB outpatient clinic. Orange pin: Country of origin of patients who resulted positive to Schistosoma spp screening. Purple pin: Country of origin of patients who resulted positive to Strongyloides stercoralis screening. Purple/orange pin: Country of origin of patients who resulted positive to Schistosoma spp. and/or Strongyloides stercoralis screening. Size of pins is proportional to the number of patients who tested positive to screening.
Concerning strongyloidiasis, of the 153 screened patients, 12 resulted positive for serology, resulting in a prevalence of 7.8% [95% CI 4.5-13.2]. Of those testing positive, the majority came from Bangladesh (25%) followed by Philippines (16.7%) and Peru (16.7%) (Figure 1). Of note, none of the screened Italian patients resulted positive to serology. The median age of patients who tested positive was 41.5 [IQR 31,48], half of them were men. The median length of stay in the country of origin was comparable between the patients who resulted positive (324 months [IQR 254.5, 420]) and the patients who resulted negative (324 months [IQR 264, 405]) to Strongyloides stercoralis serology. The median time spent in Italy was longer, but not significantly, in patients with a positive serology compared with those with a negative serology (168 months [IQR 67.5, 231] vs 144 months [IQR 60, 240], respectively). Similarly to schistosomiasis, no significant differences were observed between the patients who tested positive compared to those who tested negative to Strongyloides stercoralis serology, as reported by Table 3.
Only 3 patients tested positive for both schistosomiasis and strongyloidiasis serologies, two of them came from Brazil and one from Sudan.
A stool microscopy parasitological test was performed for 8 of the 15 patients who tested positive for schistosomiasis serology and for all the patients who tested positive for strongyloidiasis serology. Urinary microscopy examination was performed in 8 patients who tested positive for schistosomiasis. Only one patient from Bangladesh had a positive stool microscopy examination for Strongyloides stercoralis and one patient originating from Sudan had a positive urinary parasitological test for Schistosoma haematobium.
Blood tests showed eosinophilia in 11 (7.2%) of the screened patients. Of these, 3 tested positive for Schistosoma spp. serology and 3 tested positive for Strongyloides stercoralis serology. None of these patients tested positive for both infections. When asked for the presence of specific symptoms of schistosomiasis or strongyloidiasis, none of the patients who tested positive to either Schistosoma spp. or Strongyloides stercoralis serology showed any manifestation of disease at multiple physical examinations.
Ivermectin was administered to all patients who tested positive on serological screening for strongyloidiasis. Meanwhile, praziquantel was administered to 5 (33.3%) of the patients who tested positive for schistosomiasis, and none reported previous praziquantel treatment. No side effects were reported. The primary reason for withholding praziquantel from most patients was the known drug interaction between rifampin and praziquantel. Consequently, praziquantel was only offered to patients who completed their antitubercular treatment.
Our study aimed to assess the prevalence of two NTDs, schistosomiasis and strongyloidiasis, among patients attending the TB clinic at Luigi Sacco Hospital in Milan, Northern Italy. Additionally, we aimed to characterize the demographics and clinical features of patients testing positive for these infections, and to compare the characteristics of patients with positive and negative test results. TB remains a primary healthcare concern for marginalized populations, including asylum seekers, undocumented migrants, and refugees, often originating from regions endemic for schistosomiasis and strongyloidiasis. On one hand, TB is closely associated to conditions of immunosuppression and malnutrition (23), commonly observed in underserved populations (24). On the other hand, TB disease itself has been linked to the development of immunosuppression (25). The necessity for TBI screening in patients undergoing immunosuppressive therapies is well recognized across various healthcare settings. Given the potential for chronic strongyloidiasis to progress to disseminated infections under immunosuppressed conditions, it is prudent to consider screening for this condition in patients attending TB outpatient clinics, whether for TBD or TBI.
Schistosomiasis has not been linked to immunosuppression. However, chronic infection may remain asymptomatic for years while establishing unreversible lesions which can lead to liver cirrhosis, bladder cancer or infertility. Delay in the schistosomiasis diagnosis has often been described and was associated both with difficulties in accessing healthcare and poor awareness of physicians in non-endemic countries (26, 27).
In our cohort, the prevalence of schistosomiasis was 26.7% [95% CI 16.9-39.9], slightly higher compared to the prevalence in migrants of 18.4% proposed by Asundi et al., in their recent systematic review (10). This could be explained by the small sample size of our study. In Italy, studies have reported significant variations in the prevalence of these diseases among patients arriving from endemic regions, with rates ranging from 6% to 27.6% (13, 15). Notably, while the majority of imported schistosomiasis cases worldwide originate from Sub-Saharan Africa (28), our cohort exhibited a different distribution, with only 26% of positive cases originating from this region, primarily from Sudan, Senegal, and Eritrea. This unexpected finding could be attributed to comparable patient numbers across various countries of origin, as indicated by Italian migration reports (29). Interestingly, the median length of stay in Italy for patients testing positive for schistosomiasis in our study was higher (median: 168 months; IQR: 78-234) compared to a previously reported median of 110 months for asymptomatic patients in a multicenter study (27). This discrepancy may reflect increased awareness and screening efforts targeting recently arrived migrants in recent years. Furthermore, the median age of our cohort was notably higher (43 years) compared to the referenced study (27 years) (27), highlighting the importance of attention to individuals who have resided in Italy for a longer period and may have had limited access to healthcare services for an extended duration.
Concerning the prevalence of strongyloidiasis, our result was lower compared with the prevalence in migrants proposed by Asundi (10) and Buonfrate et al. (11) but was consistent with the one obtained by another multicentric study conducted in Italy (13). Interestingly, while Buonfrate et al. found a 1-8% seroprevalence of strongyloidiasis in Italians born before 1952, we identified none in our population (11). Nevertheless, despite this difference, screening is warranted in this kind of patients based on different prevalence studies (30, 31) and considering the risk of developing complicated forms of strongyloidiasis following immunosuppression. Interestingly, a significant proportion of patients born in Italy before 1952 sought treatment at our clinic for TBI, often as part of pre-immunosuppressive therapy screening protocols (e.g., prior to corticosteroids or immunomodulating agents). In our population, almost 10% of patients who were screened for strongyloidiasis had a positive history for rheumatologic conditions, while 11% were undergoing a steroid treatment. This underscores the importance of extending strongyloidiasis screening beyond Infectious Diseases wards to other clinical settings, such as Rheumatology and Hematology departments.
Three patients tested positive for both parasitic infections. While we recognize the possibility of false positive results in either test, in the absence of positive parasitological tests, the decision to treat these patients was based on adherence to current recommendations (16, 17, 21). Backing this approach of testing and treating patients, a recent study by Roure et al. proposed a significant disparity in healthcare costs between adopting a “test-and-treat” strategy compared to a “watchful wait” approach, particularly among long-term migrants (32).
Our study carries some limitations. Firstly, we were unable to obtain additional data regarding the progression of symptoms associated with these conditions. Although thorough physical examinations were conducted during each visit, it is possible that certain nonspecific symptoms may have been underreported due to language or cultural barriers. Secondly, we did not assess the response to antiparasitic treatment. Unfortunately, follow-up of these patients after completion of TB treatment can be challenging, as patients travel frequently or have unstable employment situations that are not conducive to regular outpatient clinic visits. Lastly, our sample size is limited. However, it’s important to note that we are examining two conditions that are not endemic in Italy, within a specific population. Studies at regional or national level are needed to comprehensively assess the prevalence of these overlooked conditions.
In conclusion, our study underlines the frequently underestimated prevalence of schistosomiasis and strongyloidiasis among patients seeking healthcare for tuberculosis. These findings emphasize the critical need for increased awareness and targeted screening efforts across various healthcare settings. While specialized departments such as Infectious Diseases outpatient clinics play a pivotal role, extending screening protocols to include other departments like Hematology, Rheumatology, as well as primary healthcare settings, is essential to effectively address these often-overlooked conditions within this vulnerable population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Comitato EticoTerritoriale LOMBARDIA 1. The studies were conducted inaccordance with the local legislation and institutional requirements.Written informed consent for participation was not required from theparticipants or the participants’ legal guardians/next of kin inaccordance with the national legislation and institutional requirements.
CG: Conceptualization, Writing – original draft, Writing – review & editing, Investigation. MC: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. SP: Conceptualization, Writing – original draft, Writing – review & editing. MS: Investigation, Writing – review & editing, Conceptualization. LG: Investigation, Writing – review & editing. FF: Writing – review & editing, Investigation. SA: Supervision, Validation, Writing – review & editing. AG: Writing – review & editing, Supervision, Validation. AT: Conceptualization, Investigation, Writing – review & editing, Supervision, Validation.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Schistosomiasis (Bilharzia) (n.). Available online at: https://www.who.int/health-topics/schistosomiasis#tab=tab_1 (Accessed February 18, 2024).
2. Buonfrate D, Bisanzio D, Giorli G, Odermatt P, Fürst T, Greenaway C, et al. The global prevalence of strongyloides stercoralis infection. Pathogens. (2020) 9:1–9. doi: 10.3390/PATHOGENS9060468
3. Tamarozzi F, Fittipaldo VA, Orth HM, Richter J, Buonfrate D, Riccardi N, et al. Diagnosis and clinical management of hepatosplenic schistosomiasis: A scoping review of the literature. PloS Negl Trop Dis. (2021) 15:e0009191. doi: 10.1371/JOURNAL.PNTD.0009191
4. Hotez PJ, Engels D, Gyapong M, Ducker C, Malecela MN. Female genital schistosomiasis. N Engl J Med. (2019) 381:271–80. doi: 10.1056/NEJMP1914709
5. Mostafa MH, Sheweita SA, O’Connor PJ. Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev. (1999) 12:97–111. doi: 10.1128/CMR.12.1.97
6. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. (2017) 144:263. doi: 10.1017/S0031182016000834
7. Tamarozzi F, Martello E, Giorli G, Fittipaldo A, Staffolani S, Montresor A, et al. Morbidity associated with chronic strongyloides stercoralis infection: A systematic review and meta-analysis. Am J Trop Med Hyg. (2019) 100:1305. doi: 10.4269/AJTMH.18-0895
8. CDC - DPDx - schistosomiasis infection(n.). Available online at: https://www.cdc.gov/dpdx/schistosomiasis/index.html (Accessed February 18, 2024).
9. CDC - DPDx - strongyloidiasis(n.). Available online at: https://www.cdc.gov/dpdx/strongyloidiasis/index.html (Accessed February 18, 2024).
10. Asundi A, Beliavsky A, Liu XJ, Akaberi A, Schwarzer G, Bisoffi Z, et al. Prevalence of strongyloidiasis and schistosomiasis among migrants: a systematic review and meta-analysis. Lancet Glob Health. (2019) 7:e236–48. doi: 10.1016/S2214-109X(18)30490-X
11. Buonfrate D, Baldissera M, Abrescia F, Bassetti M, Caramaschi G, Giobbia M, et al. Epidemiology of Strongyloides stercoralis in northern Italy: results of a multicentre case–control study, February 2013 to July 2014. Eurosurveillance. (2016) 21:30310. doi: 10.2807/1560-7917.ES.2016.21.31.30310
12. Rothe C, Zimmer T, Schunk M, Wallrauch C, Helfrich K, Gültekin F, et al. Developing endemicity of schistosomiasis, corsica, France. Emerg Infect Dis. (2021) 27:319–21. doi: 10.3201/EID2701.204391
13. Martelli G, Di Girolamo C, Zammarchi L, Angheben A, Morandi M, Tais S, et al. Seroprevalence of five neglected parasitic diseases among immigrants accessing five infectious and tropical diseases units in Italy: a cross-sectional study. Clin Microbiol Infect. (2017) 23:335.e1–5. doi: 10.1016/J.CMI.2017.02.024
14. McCarthy AE, Weld LH, Barnett ED, So H, Coyle C, Greenaway C, et al. Spectrum of illness in international migrants seen at geoSentinel clinics in 1997–2009, part 2: migrants resettled internationally and evaluated for specific health concerns. Clin Infect Dis. (2013) 56:925–33. doi: 10.1093/CID/CIS1016
15. The hidden epidemic of schistosomiasis in recent African immigrants and asylum seekers to Italy on JSTOR(n.). Available online at: https://www.jstor.org/stable/44849551 (Accessed February 18, 2024).
16. Comelli A, Genovese C, Gobbi F, Brindicci G, Capone S, Corpolongo A, et al. Schistosomiasis in non-endemic areas: Italian consensus recommendations for screening, diagnosis and management by the Italian Society of Tropical Medicine and Global Health (SIMET), endorsed by the Committee for the Study of Parasitology of the Italian Association of Clinical Microbiologists (CoSP-AMCLI), the Italian Society of Parasitology (SoIPa), the Italian Society of Gastroenterology and Digestive Endoscopy (SIGE), the Italian Society of Gynaecology and Obstetrics (SIGO), the Italian Socie. Infection. (2023) 51:5 2023. doi: 10.1007/S15010-023-02050-7
17. Società Italiana di Medicina Tropicale e Salute Globale (SIMET). Strongiloidosi: Raccomandazioni per la gestione clinica in Italia. Società Italiana di Medicina Tropicale e Salute Globale (SIMET (2023) TIPOGRAFIA DE VITTORIA SRL.
18. Ingraham NE, Purcell LN, Karam BS, Dudley RA, Usher MG, Warlick CA, et al. Racial and Ethnic Disparities in Hospital Admissions from COVID-19: Determining the Impact of Neighborhood Deprivation and Primary Language. J Gen Intern Med. (2021) 36:3462-70. doi: 10.1007/s11606-021-06790-w
19. Lebano A, Hamed S, Bradby H, Gil-Salmerón A, Durá-Ferrandis E, Garcés-Ferrer J, et al. Migrants’ and refugees’ health status and healthcare in Europe: A scoping literature review. BMC Public Health. (2020) 20:1–22. doi: 10.1186/S12889-020-08749-8/TABLES/1
20. Migliori GB, Ong CWM, Petrone L, D’Ambrosio L, Centis R, Goletti D. The definition of tuberculosis infection based on the spectrum of tuberculosis disease. Breathe (Sheff). (2021) 17:210079. doi: 10.1183/20734735.0079-2021
21. European Centre for Disease Prevention and Control. Public health guidance on screening and vaccination for infectious diseases in newly arrived migrants within the EU/EEA. Stockholm: ECDC (2018). Available at: https://www.ecdc.europa.eu/en/publications-data/public-health-guidance-screening-and-vaccination-infectious-diseases-newly.
22. US Centers for Disease Control and Prevention. Clinical Care of Strongyloides. Available online at: https://www.cdc.gov/strongyloides/hcp/clinical-care/index.html (Accessed 02/07/2024).
23. Gupta K, Gupta R, Atreja A, Verma M, Vishvkarma S. Tuberculosis and nutrition. Lung India. (2009) 26:9–16. doi: 10.4103/0970-2113.45198
24. Ankomah A, Byaruhanga J, Woolley E, Boamah S, Akombi-Inyang B. Double burden of malnutrition among migrants and refugees in developed countries: A mixed-methods systematic review. PloS One. (2022) 17:e0273382. doi: 10.1371/JOURNAL.PONE.0273382
25. Roberts T, Beyers N, Aguirre A, Walzl G. Immunosuppression during Active Tuberculosis Is Characterized by Decreased Interferon-γ Production and CD25 Expression with Elevated Forkhead Box P3, Transforming Growth Factor-β, and Interleukin-4 mRNA Levels. J Infect Dis. (2007) 195:870–8. doi: 10.1086/511277
26. Riccardi N, Nosenzo F, Peraldo F, Sarocchi F, Taramasso L, Traverso P, et al. Increasing prevalence of genitourinary schistosomiasis in Europe in the Migrant Era: Neglected no more? PloS Negl Trop Dis. (2017) 11:e0005237. doi: 10.1371/JOURNAL.PNTD.0005237
27. Comelli A, Riccardi N, Canetti D, Spinicci M, Cenderello G, Magro P, et al. Delay in schistosomiasis diagnosis and treatment: a multicenter cohort study in Italy. J Travel Med. (2020) 27:taz075. doi: 10.1093/jtm/taz075
28. Lingscheid T, Kurth F, Clerinx J, Marocco S, Trevino B, Schunk M, et al. Schistosomiasis in european travelers and migrants: analysis of 14 years tropNet surveillance data. Am J Trop Med Hyg. (2017) 97:567–74. doi: 10.4269/AJTMH.17-0034
29. IstatData (n.). Available online at: https://esploradati.istat.it/databrowser/#/it/dw (Accessed April 1, 2024).
30. Pirisi M, Salvador E, Bisoffi Z, Gobbo M, Smirne C, Gigli C, et al. Unsuspected strongyloidiasis in hospitalised elderly patients with and without eosinophilia. Clin Microbiol Infect. (2006) 12:787–92. doi: 10.1111/J.1469-0691.2006.01500.X
31. Abrescia FF, Falda A, Caramaschi G, Scalzini A, Gobbi F, Angheben A, et al. Reemergence of strongyloidiasis, northern Italy. Emerg Infect Dis. (2009) 15:1531–3. doi: 10.3201/EID1509.090191
32. Roure S, López F, Oliva I, Pérez-Quílez O, March O, Chamorro A, et al. Schistosomiasis screening in non-endemic countries from a cost perspective: Knowledge gaps and research priorities. The case of African long-term residents in a Metropolitan Area, Spain. PloS Negl Trop Dis. (2023) 17:e0011221. doi: 10.1371/JOURNAL.PNTD.0011221
Keywords: neglected tropical diseases, tuberculosis, helminthiases, schistosomiasis, strongyloidiasis, prevalence
Citation: Genovese C, Colaneri M, Pagano S, Schiuma M, Galli L, Fama F, Antinori S, Gori A and Torre A (2024) Assessing the prevalence of schistosomiasis and strongyloidiasis in a tuberculosis clinic: the TB-TROPIcare study. Front. Trop. Dis 5:1412871. doi: 10.3389/fitd.2024.1412871
Received: 05 April 2024; Accepted: 01 August 2024;
Published: 20 August 2024.
Edited by:
Radha Gopalaswamy, National Institute of Research in Tuberculosis (ICMR), IndiaReviewed by:
Selvakumar Subbian, The State University of New Jersey, United StatesCopyright © 2024 Genovese, Colaneri, Pagano, Schiuma, Galli, Fama, Antinori, Gori and Torre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Gori, YW5kcmVhLmdvcmlAdW5pbWkuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.