
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Trop. Dis. , 11 June 2024
Sec. Neglected Tropical Diseases
Volume 5 - 2024 | https://doi.org/10.3389/fitd.2024.1354119
This article is part of the Research Topic Female Genital Schistosomiasis: Research Needed to Raise Awareness and Deliver Action View all 14 articles
 Sekeleghe A. Kayuni1,2,3*†
Sekeleghe A. Kayuni1,2,3*† Lucas J. Cunningham2†
Lucas J. Cunningham2† Dingase Kumwenda1,4†
Dingase Kumwenda1,4† Bright Mainga1,5
Bright Mainga1,5 David Lally1
David Lally1 Priscilla Chammudzi1
Priscilla Chammudzi1 Donales Kapira1
Donales Kapira1 Gladys Namacha1
Gladys Namacha1 Bessie Ntaba1
Bessie Ntaba1 Alice Chisale1
Alice Chisale1 Tereza Nchembe1
Tereza Nchembe1 Luis Kinley1,6
Luis Kinley1,6 Ephraim Chibwana1,6
Ephraim Chibwana1,6 Alexandra Juhasz2,7
Alexandra Juhasz2,7 Sam Jones2
Sam Jones2 John Archer2
John Archer2 Peter Makaula1
Peter Makaula1 John Chiphwanya8
John Chiphwanya8 E. James LaCourse2
E. James LaCourse2 Janelisa Musaya1,3‡
Janelisa Musaya1,3‡ J. Russell Stothard2‡
J. Russell Stothard2‡Female genital schistosomiasis (FGS) remains an often overlooked chronic complication of urogenital schistosomiasis in adolescent girls and women. Moreover, the role of zoonotic or hybrid schistosome infection(s) is poorly appreciated, but is increasingly becoming an emerging public health concern in sub-Saharan Africa. In Southern Malawi, during the “Hybridization in UroGenital Schistosomiasis (HUGS)” study visit, we describe the case of a 33-year-old woman with suspected FGS who partook in a detailed external assessment with internal cervical examination using a portable colposcope. She provided several biological samples for analysis with traditional and molecular parasitological methods—urine, cervicovaginal lavage (CVL), cervical swabs, and external mass and cervical biopsies—alongside provision of detailed demographic information after a thorough medical history questionnaire and an in-depth interview. These samples were screened for the presence of Schistosoma ova on microscopy and DNA genotyping using a novel real-time PCR assay in parallel to pre-published probe-based PCR assays capable of identifying and discriminating up to six named Schistosoma species. A further molecular screen of sexually transmitted infections (STIs) including Trichomonas vaginalis, Chlamydia spp., and human papilloma virus (HPV) was conducted on her genital swab and CVL. Overt FGS was diagnosed on clinical colposcopy alongside inspection of the cervical biopsy by microscopy, real-time PCR, and histopathology. The urine filtration, microscopy and real-time PCR of the CVL and swab were negative. This evidences the typical diagnostic challenge, and cases such as this will pose an unmet need in satisfactory patient management. In addition to Schistosoma haematobium, the presence of the zoonotic species Schistosoma mattheei and concurrent STIs raise questions as to the long-term effectiveness of the current control strategies of the National Control Programme to eliminate schistosomiasis as a public health problem. Improved availability of and regular accessibility to praziquantel treatment for women at risk such as this are urgently needed. Furthermore, targeted health education, increased community awareness, and dovetailing of synergistic activities and strategies with other health stakeholders such as those in sexual and reproductive health, as well as HIV/AIDS programs in the Ministry of Health, are needed here and in neighboring countries.
Although schistosomiasis has been studied since the early part of the 19th century and is the second most common parasitic disease affecting over 224 million people globally (1), the genital form of the disease remains overlooked and underappreciated. Genital schistosomiasis in both women and men is a consequence of a chronic infection caused by Schistosoma haematobium (2). These cases usually develop during the early stages of life and later become symptomatic as the disease progresses in later years. Ultimately, more definite organ-specific pathologies ensue thereafter.
Female genital schistosomiasis (FGS) is estimated to affect at least 56 million girls and women in sub-Saharan Africa (SSA) (3, 4). The first reported case originating from Egypt was presented by Professor Madden in 1899 (5). In certain schistosomiasis-endemic areas in SSA, where women are in contact with infested waters during their routine activities, FGS may be the most common gynecological morbidity (6).
In addition, FGS can cause a long list of ailments and complications typically manifesting as abdominal pain, offensive-smelling discharge, abnormal yellow discharge, dyspareunia, contact or post-coital bleeding, spot bleeding, burning sensations, itchiness in the genitals, decreased fertility, and spontaneous abortions, which arise from the inflammation in the cervical and vaginal mucosa caused by both viable and dead or dying schistosome eggs (2, 7–10). This inflammation and fibrotic sequelae give the cervix a pathognomonic sandy and a homogeneous grainy, patchy appearance. In addition to the clinical manifestations described above, abnormal neovascularization, vaginal bleeding, and rubbery papules have now been included in the World Health Organization (WHO) Pocket Atlas (11) (Supplementary Figure S1).
FGS has often been undiagnosed or misdiagnosed as a type of sexually transmitted infection (STI) owing to similarities in the symptoms and the technical diagnostic challenges in rural and resource-limited endemic settings (12). Moreover, the relationship between zoonotic and hybrid infections in the context of FGS has not been fully investigated despite an earlier report of the cattle-infecting species, Schistosoma mattheei, finding its characteristic ova in human cervical smears (13). Furthermore, zoonotic and hybrid schistosomes are increasingly becoming recognized as an emerging public health concern in SSA (14).
As part of a larger community-based study entitled “Hybridization in UroGenital Schistosomiasis (HUGS)” in the districts of Nsanje and Mangochi in Southern Malawi, we provide a detailed clinical case report of an adult woman diagnosed with a mixed-species FGS infection in Nsanje District. The local prevalence of urine egg-patent infection in women aged 18 years and over in Nsanje District was 42.0% (n = 193) at baseline in June 2022. A year later, in June 2023, a total of 29 women were invited to participate in an FGS sub-study. Of these, 15 (51.7%) had FGS lesions on colposcopy and 9 (31.0%) had egg-patent infection in their cervicovaginal lavage (CVL). In total, seven women had both FGS lesions on colposcopy and schistosome eggs in CVL, with a total of 19 women (65.5%) having FGS detected using real-time PCR analysis of the cervical swab and/or CVL.
The participant (hereinafter referred to as XX) is a 33-year-old woman born and raised in a rural community in Nsanje District, southern Malawi (Figure 1A). XX self-reported her water contact behavior, which includes daily use of community borehole water for her routine household chores; however, she frequently crosses the Shire River into Mozambique, where she has a small holding, a piece of common land for cultivating maize, rice, and potatoes (Figure 1B).
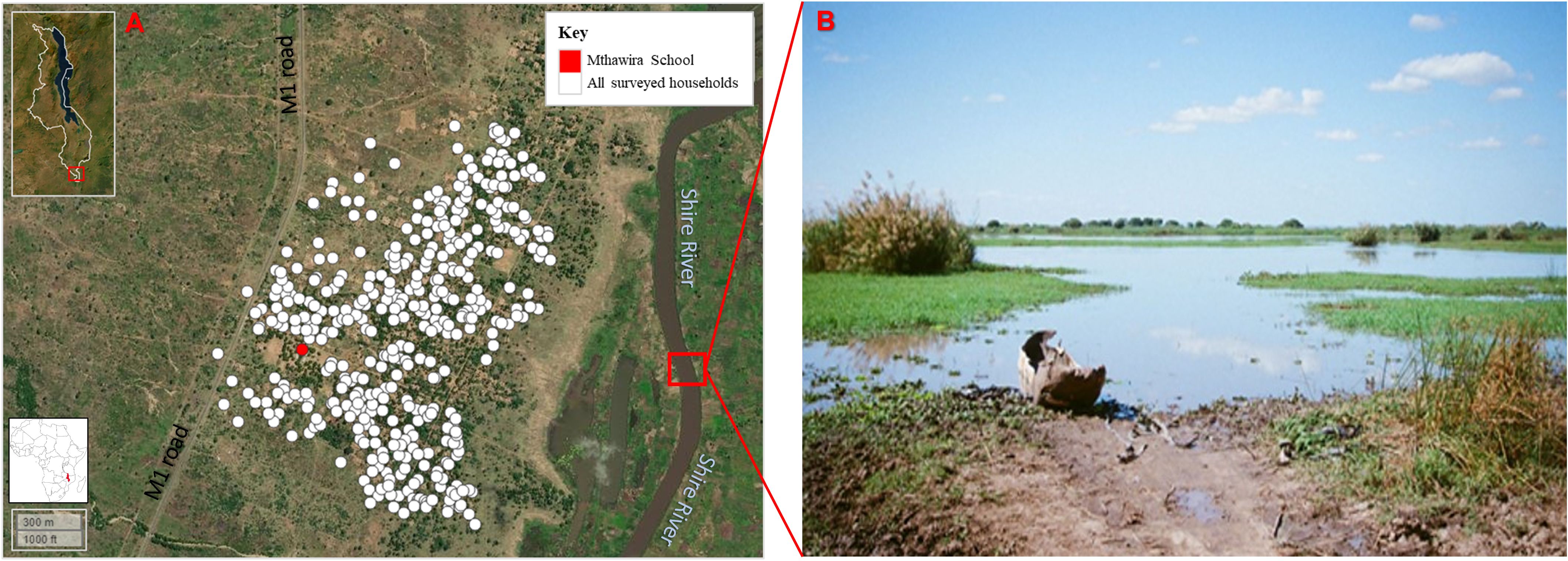
Figure 1 Map of Nsanje District in Southern Malawi, Africa (inset). (A) Community around Mthawira School, where XX lives. (B) Part of the Shire River where XX passes on the way to her garden. Intermediate snail hosts for schistosomiasis have been found in the river and in the flooded areas fringing this water body. Figure was generated using the “mapview” package version 2.10.0 of R Studio version 2021.09.0, build 351 (Posit, Boston, MA, USA). (Photo image courtesy of Dr Angus M. O’Ferrall).
Upon examination in June 2023, she complained of lower abdominal pain (LAP), pain on micturition, itchiness of the vulvar, warty mass (Supplementary Figure S2), and vaginal pain with bleeding after coitus with her current husband. She was also concerned about failure to conceive following a miscarriage in 2020. She has two biological children, 14 and 11 years old, from her first husband (who had died more than 2 years prior). She is HIV-1 reactive and has been on antiretroviral treatment since 2008.
XX was requested to provide a urine sample for analysis to determine her schistosomiasis status. The urine sample was screened using point-of-care circulating cathodic antigen (POC-CCA) test, filtration of the 10 ml urine, and microscopy to visualize the Schistosoma spp. eggs (15). She was also requested to undergo an ultrasound scan of the abdomen and pelvis, as well as pelvic examination using a clean disinfected speculum for portable colposcopy using MobileODT EVA COLPO device (FemTech, Tel Aviv, Israel). For further investigations, CVL with 10 ml normal saline and a cervical swab were collected and stored in 100% ethanol for downstream molecular analysis using a novel real-time PCR assay described by Cunningham et al. (16) in parallel to pre-published probe-based real-time PCR assays targeting generic and species-specific markers (17, 18).
The CVL and swab were also analyzed for STIs using the EasyScreen™ STI Kit (SydPath, Sydney, Australia), which simultaneously detects the following 12 common STIs: Chlamydia trachomatis, Neisseria gonorrhoeae, Lymphogranuloma venereum (LGV), Mycoplasma genitalium, Trichomonas vaginalis, Ureaplasma spp., Candida spp., Mycoplasma hominis, Streptococcus agalactiae (group B Streptococcus), Treponema pallidum, and herpes simplex virus 1 (HSV1) and 2 (HSV2). In addition, real-time PCR testing for human papilloma virus (HPV) using the QIAscreen HPV PCR Test (QIAGEN Ltd., Manchester, UK), which is capable of detecting serotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66–68, was conducted on her CVL. Finally, visual inspection with acetic acid (VIA) was used to screen for cervical cancer (Supplementary Figure S3). Where suspicious lesion was observed in the cervicovaginal region and the external genitalia (vulvar mass), we conducted punch biopsy(ies) for histopathological examination and diagnosis. One vulvar biopsy and two cervical biopsy samples were collected from the participant.
Colposcopy revealed positive sandy patches, abnormal vessels, and rubbery patches on the cervix of the participant (Figure 2). On ultrasound scan, the only notable finding was a right ovarian cyst measuring 4.6 cm × 4.1 cm.
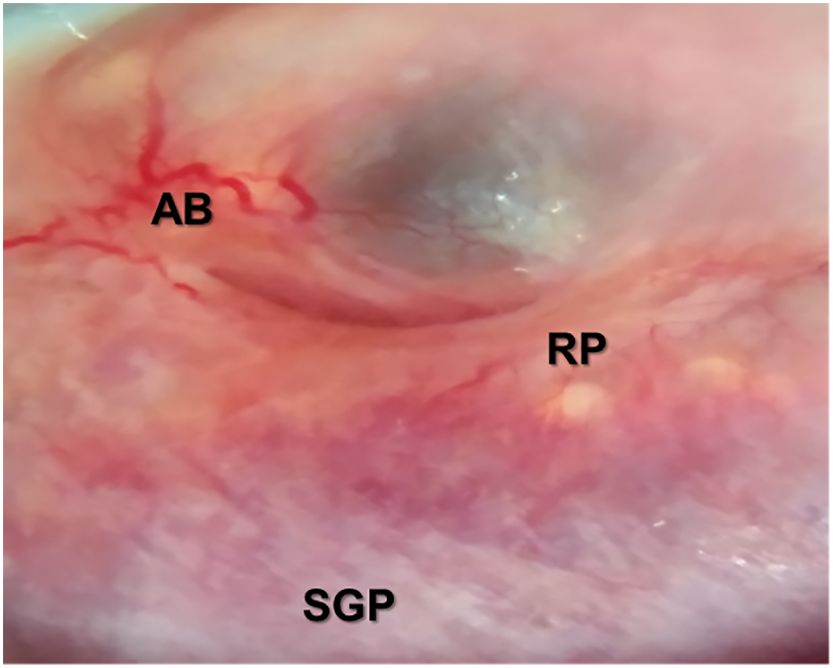
Figure 2 Image from the portable MobileODT EVA COLPO colposcopy showing sandy grainy patches (SGP), abnormal vessels and bleeding (AB), and rubbery papules (RP) as described in the WHO Pocket Atlas for female genital schistosomiasis (FGS) in Supplementary Figure S1. Magnification, ×20. (Image courtesy of Dr. Dingase Kumwenda).
The laboratory investigations showed that her urine POC-CCA, filtration, and microscopy were all negative (see Table 1). Microscopy and molecular DNA analysis using real-time PCR of the CVL and swab were also negative, while microscopy of the crushed cervical biopsy collected revealed numerous ova/shells characteristic of S. haematobium alongside a distinctive but minority of ova characteristic of S. mattheei (Figure 3A). Real-time PCR of the biopsy revealed a strong S. haematobium [cycle threshold (Ct) = 17] and weak S. mattheei (Ct = 33) DNA amplification signals.
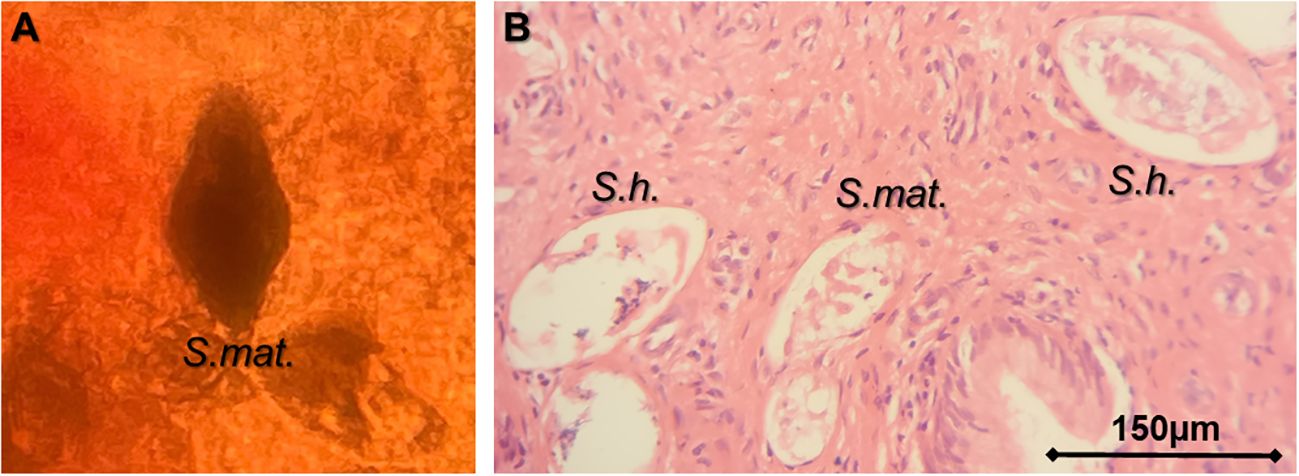
Figure 3 Images of the cervical tissue biopsy. (A) Microscopy of a crushed cervical biopsy. (B) Histopathological section showing eggs of Schistosoma haematobium (S.h.) and Schistosoma mattheei (S.mat.). (Photo image A courtesy of Professor J.R. Stothard and image B of Dr Sekeleghe Kayuni).
Histopathological examination of the cervical biopsy also showed S. haematobium and S. mattheei ova (Figure 3B). Biopsy of the external mass revealed fibrotic tissues with a single deformed ovum-like object therein. The STIs on the CVL and swab using the EasyScreen™ STI Kit were determined to be T. vaginalis, Ureaplasma spp., and M. hominis. Screening for HPV 16, 18, and other serotypes associated with cervical cancer all resulted negative.
Upon on-site review, XX was provided with a standard dose of praziquantel (PZQ) at 40 mg/kg body weight at three different intervals: immediately after examination, 1 week later, and then 1 month later during mass drug administration (MDA) exercise. All of the symptoms originally reported, except for the post-coital bleeding and infertility issue, completely resolved after the third PZQ dose. However, the vulvar mass had not regressed fully.
As described earlier and noted elsewhere in SSA, FGS is underdiagnosed in schistosomiasis-endemic areas and is commonly misdiagnosed as a STI in southern Malawi, where XX lives (19). A recent study completed in the same district and in the neighboring Chikwawa District revealed an extensive burden of FGS detected via clinical colposcopy alone, as was used in this case study, noting a prevalence of 21.5% compared with 6.8% upon egg-patent urine microscopy as a proxy for FGS diagnosis (20). Of note is that, in that study, a 15-year-old girl who often had abnormal vaginal bleeding and overt cervical abrasion observed, was positive for FGS, which was diagnosed after colposcopy and histopathological examination of the cervical biopsy with numerous ova present (21).
To our knowledge, this is the first case report in a schistosomiasis-endemic area describing the application of several diagnostic techniques to detect or incriminate FGS where most of the definite standard tests (e.g., urine filtration, CVL microscopy, and real-time PCR) each produced a negative result. Urine microscopy has been commonly used as a diagnostic proxy for FGS in most endemic areas with limited capacity (7, 22). However, it is known that FGS can present in the cervicovaginal fluid (23), with absence of schistosome eggs in the urine, as seen in this case (24).
In addition, portable colposcopy was able to describe grainy sandy patches, abnormal bleeding, and rubbery papules, descriptive of FGS in our participant. Portable colposcopy is becoming a valuable tool for the investigation and diagnosis of lesions in the female genital tract, including FGS (11, 25, 26). Recent advances have resulted in the development of low-cost devices coupled with capacity building of medical personnel. Collectively, this has contributed to the improved diagnosis and better management of genital infections such as FGS (27–29). However, there are limiting factors to consider in future scale-up, such as their costs and the training needs of local medical personnel.
Furthermore, although molecular techniques have advanced the diagnostic capability and high detection rate, real-time PCR did not detect sufficient schistosome genetic material in the CVL despite the colposcopy revealing classical lesions of FGS, i.e., grainy sandy patches, abnormal bleeding, and rubbery papules. These lesions have also been observed to increase the susceptibility to HIV by two- to fourfold, as well as HPV and possibly cervical cancer (2, 4, 30–32), hence the need for the improved availability of and accessibility to PZQ treatment for all diagnosed and suspected cases in endemic areas. For our case, she was HIV-1 reactive and on antiretroviral therapy, with HPV and VIA both negative. It was disappointing that the external vulvar mass, which is likely an external “bilharzoma” did not regress as well as expected given the known beneficial impact of treatment on external FGS (33, 34).
Several STIs were concurrently detected, namely, T. vaginalis, Ureaplasma spp., and M. hominis. These have been known to cause symptoms similar to those of FGS, such as LAP, dysuria, dyspareunia, vaginal itchiness, and infertility (7, 35). Although routine screening of asymptomatic patients or testing of symptomatic individuals for M. hominis, Ureaplasma urealyticum, and Ureaplasma parvum are not routinely recommended using multiplex PCR assays (36), such co-infections can result in the underdiagnosis, misdiagnosis, and mismanagement of FGS. Moreover, if left untreated, these increase the morbidity and the further reduction in well-being and quality of life. Sadly, these necessary diagnostic services to better diagnose FGS are not available in local public health settings, clearly indicating the dearth of quality diagnostics in infection and disease surveillance in Malawi.
After repeated PZQ treatments, which is above the routine standard of care, it was pertinent to note that her symptoms gradually resolved after receiving this increased level of PZQ administration. This correlates with prior reports of the ability of the drug to improve the well-being of patients beyond directly curing the disease (2, 37, 38). Repeated PZQ doses and consideration of higher doses of treatment up to 60 mg/kg in divided intervals can contribute to the more rapid reversal of these key morbidities (39).
Furthermore, our case is also unique in the detection of a zoonotic schistosome infection, S. mattheei, alongside the human schistosome S. haematobium in the cervical tissue biopsy collected, which has previously been reported in southern Malawi (40). Schistosoma mattheei has been known to commonly infect livestock such as cattle, sheep, and goats, with a few cases reported in humans with no different symptomatology from common human infection (41). Those with zoonotic S. mattheei infections do not appear to experience severe gastrointestinal symptoms, although they do live in close proximity to others who generally use the same water sources; therefore, their exposure history needs further consideration (Supplementary Figure S4). The communal water bodies can harbor competent intermediate snail hosts for both the human and zoonotic species of schistosomes, thereby transmitting the infection to humans and adjacent animals. This will certainly give rise to multispecies infections, which favor schistosome hybridization, which has been detected in the study area during this period (14). However, our case did not have unequivocal evidence of a hybrid detected, rather a mixed-species infection. The presence of zoonotic infection poses a challenge to the current disease control strategies of the Malawi Ministry of Health and the National Schistosomiasis Control Programme, which are focused on annual MDA with PZQ to individuals living only in areas with high and moderate burdens.
In a broader context, the discovery of mixed and hybrid infections in the region poses a need to understand their virulence and the severity of the pathologies caused by the hybrids as efforts toward eliminating schistosomiasis as a public health concern by 2030 are being advocated (42, 43). Further work is ongoing to describe this morbidity aspect. The unavailability of a histopathological and molecular diagnostic capacity in the district was a limitation for the quick availability of the test results for the participant.
In conclusion, FGS is a prevalent chronic manifestation of schistosomiasis, which is commonly caused not only by S. haematobium but also other schistosome species including S. mattheei, a zoonotic species detected in our case. Co-infection with other STIs was confirmed, highlighting the complexity of the diagnosis and treatment of FGS, where portable colposcopy and real-time PCR technologies may not be available. We advocate for the use of multiple diagnostic techniques in addition to a thorough history, water contact behavior, and pelvic examination in order to improve FGS diagnosis. Moreover, health education, community awareness, improved availability of and accessibility to PZQ treatment, and synergistic strategies with other health programs in the Ministry of Health, such as sexual and reproductive health, as well as HIV/AIDS, would greatly assist in the understanding, management, and holistic control interventions for FGS, STIs, and other infections in the country and region.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by College of Medicine Research Ethics Committee (COMREC) of Kamuzu University of Health Sciences (KUHeS) in Malawi (approval no. P.08/21/3381) and Liverpool School of Tropical Medicine Research Ethics Committee (LSTM-REC; approval no. 22–028). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. DKu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. BM: Writing – review & editing. DL: Investigation, Methodology, Resources, Writing – review & editing. PC: Investigation, Methodology, Resources, Writing – review & editing. DKa: Investigation, Methodology, Resources, Writing – review & editing. GN: Investigation, Methodology, Resources, Writing – review & editing. BN: Project administration, Resources, Writing – review & editing. AC: Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – review & editing. TN: Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – review & editing. LK: Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – review & editing. EC: Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – review & editing. AJ: Investigation, Methodology, Resources, Writing – review & editing. SJ: Investigation, Methodology, Project administration, Resources, Writing – review & editing. JA: Formal analysis, Investigation, Methodology, Project administration, Resources, Writing – review & editing. PM: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. JC: Investigation, Methodology, Resources, Supervision, Writing – review & editing. EL: Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. JM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. JS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is funded by Wellcome Trust and National Institute for Health Research (NIHR) through a Joint Investigator Award, grant number WT 220818/Z/20/Z.
The study team acknowledge the generous support rendered by the Director of Health and Social Services, District Medical Officer, District Environmental Health Officer, District Laboratory Manager and Management and staff of Nsanje District Health Office; the In-charge and Nurse-Midwife of Tengani Health Centre; the Headteacher and teachers of Mthawira Primary School; and all community members including the case participant, TG, who participated in the HUGS Human longitudinal cohort study. Also grateful to the local community health workers: Abdul Salimu, Bossman Kutama, Christopher Thonje, Cynthia Issah, Grace Nyakamera, Harrison Makawa, Rester Fellow; and all traditional leaders around Mthawira school for their enthusiasm and support. We are particularly grateful to Dr Sarah Rollason and Dr Angus M. O'Ferrall for the valuable support and expertise in the study, Prof John Ellis and Dr Damien Stark for their assistance in facilitating the SydPath screen for STIs.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2024.1354119/full#supplementary-material
Supplementary Figure 1 | Images of the Classical presentation of Female Genital Schistosomiasis (FGS) lesions as described in the WHO Pocket Atlas for FGS.
Supplementary Figure 2 | Vulvar warty mass, pointed with black arrow in a circle, observed during examination of XX. (Photo image courtesy of Professor J.R. Stothard).
Supplementary Figure 3 | Flowchart of the Ultrasonography, Male and Female Genital Schistosomiasis sub-studies.
Supplementary Figure 4 | Daily activities at a typical water contact focus where this infested water source brings together women (and children) and livestock to interact with in a high-endemic area for urogenital schistosomiasis in Southern Malawi. (Photo image courtesy of Professor J.R. Stothard).
1. McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. Schistosomiasis. Nat Rev Dis Primers. (2018) 4:13. doi: 10.1038/s41572-018-0013-8
2. Bustinduy AL, Randriansolo B, Sturt AS, Kayuni SA, Leutscher PD, Webster BL, et al. An update on female and male genital schistosomiasis and a call to integrate efforts to escalate diagnosis, treatment and awareness in endemic and non-endemic settings: the time is now. Adv Parasitol. (2022) 115:1–44. doi: 10.1016/bs.apar.2021.12.003
3. Freer JB, Bourke CD, Durhuus GH, Kjetland EF, Prendergast AJ. Schistosomiasis in the first 1000 days. Lancet Infect Dis. (2018) 18:e193–203. doi: 10.1016/S1473-3099(17)30490-5
4. Sturt AS, Webb EL, Francis SC, Hayes RJ, Bustinduy AL. Beyond the barrier: Female Genital Schistosomiasis as a potential risk factor for HIV-1 acquisition. Acta Tropica. (2020) 209:105524. doi: 10.1016/j.actatropica.2020.105524
5. Madden F. A case of bilharzia of the vagina. Lancet. (1899) 153:1716. doi: 10.1016/S0140-6736(01)51385-9
6. Kjetland EF, Leutscher PDC, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol. (2012) 28:58–65. doi: 10.1016/j.pt.2011.10.008
7. Christinet V, Lazdins-Helds JK, Stothard JR, Reinhard-Rupp J. Female genital schistosomiasis (FGS): from case reports to a call for concerted action against this neglected gynaecological disease. Int J Parasitol. (2016) 46:395–404. doi: 10.1016/j.ijpara.2016.02.006
8. Jourdan PM, Roald B, Poggensee G, Gundersen SG, Kjetland EF. Increased vascularity in cervicovaginal mucosa with Schistosoma haematobium infection. PLoS Negl Trop Dis. (2011) 5:e1170. doi: 10.1371/journal.pntd.0001170
9. Feldmeier H, Poggensee G, Krantz I, Helling-Giese G. Female genital schistosomiasis. New challenges from a gender perspective. Trop Geographical Med. (1995) 47:S2–15.
10. Hegertun IEA, Sulheim Gundersen KM, Kleppa E, Zulu SG, Gundersen SG, Taylor M, et al. Schistosoma haematobium as a common cause of genital morbidity in girls: a cross-sectional study of children in South Africa. PLoS Negl Trop Dis. (2013) 7:e2104. doi: 10.1371/journal.pntd.0002104
11. WHO. Female genital schistosomiasis: a pocket atlas for clinical health-care professionals. Geneva, Switzerland: World Health Organization (2015). Report No.: 9241509295.
12. Shukla JD, Kleppa E, Holmen S, Ndhlovu PD, Mtshali A, Sebitloane M, et al. The association between female genital schistosomiasis and other infections of the lower genital tract in adolescent girls and young women: A cross-sectional study in South Africa. J Lower Genital Tract Dis. (2023) 27:291–6. doi: 10.1097/LGT.0000000000000756
13. Berry A. First recorded finding of ova of the cattle parasite Schistosoma mattheei in human cervical smears. Trans R Soc Trop Med Hygiene. (1974) 68:263–4. doi: 10.1016/0035-9203(74)90033-9
14. Stothard JR, Kayuni SA, Al-Harbi MH, Musaya J, Webster BL. Future schistosome hybridizations: Will all Schistosoma haematobium hybrids please stand-up! PLoS Negl Trop Dis. (2020) 14:e0008201. doi: 10.1371/journal.pntd.0008201
15. Cheesbrough M. District laboratory practice in tropical countries. IJMS. (2018) 1:65–8. doi: 10.32441/ijms.v1i1
16. Cunningham L, Kayuni SA, Juhasz A, Makaula P, Lally D, Namacha G, et al. A rapid DNA screening method using high resolution melt analysis to detect Schistosoma haematobium and Schistosoma mattheei hybrids alongside other introgressing schistosomes. Front Trop Dis. (2024) 14. under review.
17. Obeng B, Aryeetey Y, De Dood C, Amoah A, Larbi I, Deelder A, et al. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann Trop Med Parasitol. (2008) 102:625–33. doi: 10.1179/136485908X337490
18. Alzaylaee H, Collins RA, Rinaldi G, Shechonge A, Ngatunga B, Morgan ER, et al. Schistosoma species detection by environmental DNA assays in African freshwaters. PLoS Negl Trop Dis. (2020) 14:e0008129. doi: 10.1371/journal.pntd.0008129
19. Swai B, Poggensee G, Mtweve S, Krantz I. Female genital schistosomiasis as an evidence of a neglected cause for reproductive ill-health: a retrospective histopathological study from Tanzania. BMC Infect Dis. (2006) 6:1–8. doi: 10.1186/1471-2334-6-134
20. Lamberti O, Kayuni S, Kumwenda D, Ngwira B, Singh V, Moktali V, et al. Female genital schistosomiasis burden and risk factors in two endemic areas in Malawi nested in the Morbidity Operational Research for Bilharziasis Implementation Decisions (MORBID) study. PLoS Negl Trop Dis. (2024) 18(5):e0012102. doi: 10.1371/journal.pntd.0012102
21. Kumwenda D. Schistosomes as a threat to human reproduction. Hurd H, editor. London, U.K: BugBitten: Springer Nature, BioMed Central Ltd (2022). Available at: https://blogs.biomedcentral.com/bugbitten/2022/07/08/schistosomes-as-a-threat-to-human-reproduction/.
22. Makia CM, Fesuh NB, Amabo EN, Gamba VA, Oluwole AS, Stothard R. Urogenital schistosomiasis (UGS) and female genital schistosomiasis (FGS) in Cameroon: an observational assessment of key reproductive health determinants of girls and women in the Matta Health Area. BMJ Open. (2023) 13:e063392. doi: 10.1136/bmjopen-2022-063392
23. Swart P, van der Merwe J. Wet-smear diagnosis of genital schistosomiasis. South Afr Med J. (1987) 72:631–2. doi: 10.10520/AJA20785135_8458
24. Poggensee G, Kiwelu I, Saria M, Richter J, Krantz I, Feldmeier H. Schistosomiasis of the lower reproductive tract without egg excretion in urine. Am J Trop Med hygiene. (1998) 59:782–3. doi: 10.4269/ajtmh.1998.59.782
25. Sow D, Ndiour CN, Thiam O, Ndiaye M, Diagne PN, Doucouré S, et al. Cytobrush and cotton swab as sampling tools for molecular diagnosis of female genital schistosomiasis in the uterine cervix. Curr Res Parasitol Vector Borne Dis. (2023) 4:100143. doi: 10.1016/j.crpvbd.2023.100143
26. Norseth HM, Ndhlovu PD, Kleppa E, Randrianasolo BS, Jourdan PM, Roald B, et al. The colposcopic atlas of schistosomiasis in the lower female genital tract based on studies in Malawi, Zimbabwe, Madagascar and South Africa. PLoS Negl Trop Dis. (2014) 8:e3229. doi: 10.1371/journal.pntd.0003229
27. Comelli A, Genovese C, Gobbi F, Brindicci G, Capone S, Corpolongo A, et al. Schistosomiasis in non-endemic areas: Italian consensus recommendations for screening, diagnosis and management by the Italian Society of Tropical Medicine and Global Health (SIMET), endorsed by the Committee for the Study of Parasitology of the Italian Association of Clinical Microbiologists (CoSP-AMCLI), the Italian Society of Parasitology (SoIPa), the Italian Society of Gastroenterology and Digestive Endoscopy (SIGE), the Italian Society of Gynaecology and Obstetrics (SIGO), the Italian Society of Colposcopy and Cervico-Vaginal Pathology (SICPCV), the Italian Society of General Medicine and Primary Care (SIMG), the Italian Society of Infectious and Tropical Diseases (SIMIT), the Italian Society of Pediatrics (SIP), the Italian Society of Paediatric Infectious Diseases (SITIP), the Italian Society of Urology (SIU). Infection. (2023) 51:1249–71. doi: 10.1007/s15010-023-02050-7
28. Søfteland S, Sebitloane MH, Taylor M, Roald BB, Holmen S, Galappaththi-Arachchige HN, et al. A systematic review of handheld tools in lieu of colposcopy for cervical neoplasia and female genital schistosomiasis. Int J Gynaecol Obstet. (2021) 153:190–9. doi: 10.1002/ijgo.13538
29. Sturt A, Bristowe H, Webb E, Hansingo I, Phiri C, Mudenda M, et al. Visual diagnosis of female genital schistosomiasis in Zambian women from hand-held colposcopy: agreement of expert image review and association with clinical symptoms. Wellcome Open Res. (2023) 8:14. doi: 10.12688/wellcomeopenres
30. Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N, Gwanzura L, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. (2006) 20(4):593–600. doi: 10.1097/01.aids.0000210614.45212.0a
31. Patel P, Rose CE, Kjetland EF, Downs JA, Mbabazi PS, Sabin K, et al. Association of schistosomiasis and HIV infections: a systematic review and meta-analysis. Int J Infect Dis. (2020) 102:544–53. doi: 10.1016/j.ijid.2020.10.088
32. Hotez PJ, Engels D, Gyapong M, Ducker C, Malecela MN. Female genital schistosomiasis. N Engl J Med. (2019) 381:2493–5. doi: 10.1056/NEJMp1914709
33. Savioli L, Gabrielli A, Neve H. Vulvar Schistosoma haematobium lesion treated with praziquantel. Trop doctor. (1990) 20:45–6. doi: 10.1177/004947559002000119
34. Attili VR, Hira SK, Dube M. Schistosomal genital granulomas: a report of 10 cases. Sex Trans Dis. (1983) 59:269–72. doi: 10.1136/sti.59.4.269
35. Leutscher PDC, Ramarokoto C-E, Hoffmann S, Jensen JS, Ramaniraka V, Randrianasolo B, et al. Coexistence of urogenital schistosomiasis and sexually transmitted infection in women and men living in an area where Schistosoma haematobium is endemic. Clin Infect Dis. (2008) 47:775–82. doi: 10.1086/591127
36. Horner P, Donders G, Cusini M, Gomberg M, Jensen JS, Unemo M. Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women? - a position statement from the European STI Guidelines Editorial Board. J Eur Acad Dermatol Venereol. (2018) 32:1845–51. doi: 10.1111/jdv.15146
37. Wiegand RE, Fleming FM, de Vlas SJ, Odiere MR, Kinung'hi S, King CH, et al. Defining elimination as a public health problem for schistosomiasis control programmes: beyond prevalence of heavy-intensity infections. Lancet Global Health. (2022) 10:e1355–e9. doi: 10.1016/S2214-109X(22)00287-X
38. Zwang J, Olliaro PL. Clinical efficacy and tolerability of praziquantel for intestinal and urinary schistosomiasis—a meta-analysis of comparative and non-comparative clinical trials. PLoS Negl Trop Dis. (2014) 8:e3286. doi: 10.1371/journal.pntd.0003286
39. Payne L, Chiodini P, Bustinduy A. An update on schistosomiasis. Clin Microbiol Newsletter. (2023) 45:125–32. doi: 10.1016/j.clinmicnews.2023.08.001
40. Webster BL, Alharbi MH, Kayuni S, Makaula P, Halstead F, Christiansen R, et al. Schistosome interactions within the Schistosoma haematobium group, Malawi. Emerg Infect Dis. (2019) 25:1245–7. doi: 10.3201/eid2506.190020
41. Díaz AV, Walker M, Webster JP. Reaching the World Health Organization elimination targets for schistosomiasis: the importance of a One Health perspective. Philos Trans R Soc B. (2023) 378:20220274. doi: 10.1098/rstb.2022.0274
42. WHO. Ending the neglect to attain the sustainable development goals: One health: approach for action against neglected tropical diseases 2021–2030. Geneva, Switzerland: World Health Organization (2022).
Keywords: genital schistosomiasis, FGS, STIs, zoonotic, hybrid, real-time PCR, S. mattheei
Citation: Kayuni SA, Cunningham LJ, Kumwenda D, Mainga B, Lally D, Chammudzi P, Kapira D, Namacha G, Ntaba B, Chisale A, Nchembe T, Kinley L, Chibwana E, Juhasz A, Jones S, Archer J, Makaula P, Chiphwanya J, LaCourse EJ, Musaya J and Stothard JR (2024) Challenges in the diagnosis and control of female genital schistosomiasis in sub-Saharan Africa: an exemplar case report associated with mixed and putative hybrid schistosome infection in Nsanje District, Southern Malawi. Front. Trop. Dis 5:1354119. doi: 10.3389/fitd.2024.1354119
Received: 11 December 2023; Accepted: 29 April 2024;
Published: 11 June 2024.
Edited by:
Stephen Cose, University of London, United KingdomReviewed by:
Valentina Marchese, Bernhard Nocht Institute for Tropical Medicine (BNITM), GermanyCopyright © 2024 Kayuni, Cunningham, Kumwenda, Mainga, Lally, Chammudzi, Kapira, Namacha, Ntaba, Chisale, Nchembe, Kinley, Chibwana, Juhasz, Jones, Archer, Makaula, Chiphwanya, LaCourse, Musaya and Stothard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sekeleghe A. Kayuni, c2VrZWtheXVuaUBsaXZlLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.