
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Trop. Dis., 05 January 2024
Sec. Vector Biology
Volume 4 - 2023 | https://doi.org/10.3389/fitd.2023.1327349
This article is part of the Research Topic2022 in Review: Vector BiologyView all 5 articles
Phlebotomine sand flies are medically important as vectors of the protozoan parasites that cause leishmaniasis and other bacterial and viral pathogens. Previous work demonstrated that both sexes of certain species of sandflies are attracted to 1-octen-3-ol (octenol). Since 1-octen-3-ol exists as two enantiomeric isomers ─ ((R)-(-)- (R-form) and (S)-(+)- (S-form), we tested the hypothesis that the two enantiomeric forms and racemic mixture (R/S) attracted different sand fly species. We carried out field trials in a leishmaniasis endemic foci in Baringo County, Kenya. In a randomized design, trap captures of sandflies in CDC light traps baited with the R-, S- and racemic (R/S) forms of 1-octen-3-ol in hexane varied with the form and dose of the compound. Interestingly, of the captured species, only Phlebotomus martini, the vector of the parasite causing visceral leishmaniasis, exhibited a dose-dependent response to octenol; captures of both sexes of the species being generally 1.7-fold higher with the R- than S-form. There was no significant effect of treatment on captures of Sergentomyia species (S. schwetzi, S. antennata, S, clydei). Our findings have implications for surveillance of sandfly populations as part of leishmaniasis epidemiologic investigation.
Volatile organic compounds (VOCs) serve various biological functions including roles as semiochemicals - signalling chemicals mediating intra- and inter- organismal interactions (1, 2). They are chemically diverse and mediate a wide range of behaviors in arthropods, including host seeking, reproduction, and oviposition, to name a few (3). Examples include chemicals mainly in the classes: aldehydes, carboxylic acids, terpenes, ketones, alcohols, phenols, and sulfides (2). Their correct identification is key to as environmentally- friendly tools for monitoring and controlling vector populations.
The ‘mushroom alcohol’ 1-octen-3-ol, has been widely studied as an attractant for diverse blood-feeding arthropods to locate their vertebrate hosts. First demonstrated in tsetse flies (4), its kairomonal effect has also been reported in other insects such as horseflies (5), biting midges (6), and blackflies (7, 8). In mosquitoes, the dengue vector Aedes aegypti (9) and the malaria vector Anopheles gambiae (10) are attracted to octenol in combination with carbon dioxide, whereas the vector of West Nile virus vector Culex quinquefasciatus (11) is repelled by the blend. These findings suggest potential species-specific responses to this compound.
Notably, 1-octen-3-ol exists as two optical isomers or enantiomers: (R)- (–)-1-octen-3-ol (R-form) and (S)-(+)-1-octen-3-ol (S-form). Behavioral evidence suggests that certain insects respond differently to the different enantiomers of 1-octen-3-ol, although most studies have examined the effects of the racemic form (R/S). For instance, in Y-tube olfactometer assays using different mosquito species, Cook et al. (12) found higher behavioral response to the R- than S-form which translated to increased flight and attraction in Ae. aegypti but only increased activation in Cx. quinquefasciatus. Electrophysiological studies demonstrated higher sensitivity of the R- over the S-form in Aedes and Culex mosquitoes (12–15). In contrast, female beetles of the species Cis boleti, a known coloniser of tree fungus, exhibited greater attraction to the S- than R-form (16). In field studies, increased captures of the mosquito species Anopheles crucians, a malaria vector (17) and Ochlerotatus infirmatus an arbovirus vector (18), were found in traps baited with R- than S-forms (19). Similar discriminatory effect between the enantiomeric isomers have been reported in tsetse flies (4, 20, 21), indicating that the different enantiomers function differently in different insects.
Phlebotomine sand flies are vectors of Leishmania parasites and cause leishmaniasis that afflict humans besides other bacterial and viral pathogens (22, 23). To date, only a handful of studies have assessed the behavioral response of 1-octen-3-ol (racemic mixture) on sandflies. For instance, the sandfly species Lutzomyia longipalpis (Lutz and Neiva), and Phlebotomus duboscqi Neveu-Lemaire have been tested in laboratory assays (24, 25) and Lutzomyia intermedia (Lutz and Neiva) in field conditions (26) for their responses to the racemic mixture. However, evaluation of the individual enantiomers on sandfly attraction in the field has little been explored. In this study, we first carried out an enzyme-catalyzed kinetic resolution of racemic 1-octen-3-ol to obtain the individual enantiomeric isomers, and then tested the hypothesis that the individual enantiomeric and racemic forms differentially attracted sandflies in conventional traps.
Chemical profiling and enantiomeric excess (ee) of the two enantiomers; (S)-1-octen-3-ol and (R)-1-octen-3-ol were analyzed on an Agilent 6890N gas chromatography instrument equipped with 2-columns: a) chiral column, β-cyclodextrin capillary column 30 m length, 0.25 mm inner diameter, and 0.25 µm film thickness (J&W Scientific, USA), and b) achiral column HP-1 MS low bleed capillary column (30 m× 0.25 mm i.d., 0.25 μm) (J&W, USA). For all the analyses, we injected 0.2-1 µL samples, equivalent to a maximum of 100 ng on-column mass, into a cooling-on-column inlet system. The initial oven temperature was set at 30°C for 0.5 min and then ramped up to 150°C at a rate of 5°C/min. Subsequently, we increased the temperature to reach the target value of 230°C at a rate of 10°C/min, maintaining this temperature for 33 min. We used high purity nitrogen gas as a carrier gas, maintained at a constant linear velocity of 49 cm/s throughout the analysis. The Flame Ionization Detector (FID) was set at a temperature of 230°C, and data were acquired at a rate of 20 Hz.
Identities of the resolved enantiomeric forms, namely, (S)-1-octen-3-ol 1 and (R)-1-octen-3-ol 3, were established using nuclear magnetic resonance (NMR) analyses. Each compound (5 mg) was dissolved in CDCl3 (Cambridge Isotope Laboratories, Tewksbury, MA) and then transferred into 2.5 mm × 100 mm MATCH NMR tubes (Norell, Landisville, NJ). The NMR analyses encompassed both 1H NMR and 13C NMR spectroscopy. All NMR spectra were acquired at 22°C, utilizing a Bruker Avance II 600 console, which operates at 600 MHz for 1H NMR and 151 MHz for 13C NMR.
An optimized lipase-mediated synthetic route (LMER) using immobilized lipase B of Candida antarctica (Novozym 435) was selected for multi-gram synthesis and resolution of the two enantiomers following a high reported enantiomeric excess (ee) (27) (Figures 1, 2). Briefly, racemic 1-octen-3-ol (30 g, 233.97 mmol), (Sigma Aldrich Co. Ltd, Gillingham Dorset-UK) was dissolved in vinyl acetate 30 ml (28 g, 325.45 mmol), followed by addition of novozyme 435 (6g), stirred at room temperature for 60 hr. The reaction was stopped by filtering through glass wool to separate the enzyme, then the filtrate was concentrated in vacuo to yield 29.7 g of clear liquid. The filtrate was purified by column chromatography on silica gel (Et2O: pet ether 20%) to afford ‘crude’ (R)-1-octen-3-ol 1 (15 g) and (S) - 1-octen-3-yl acetate -2 (13 g ee 98.9%). 2 was deacetylated using KOH to yield (S)-1-octen-3-ol 3 (10 g) [α]D20 + 13.6° (MeOH). ‘Crude’ 1 was reintroduced into the enzyme pot, and the aforementioned process was repeated for 12 hr, to yield (R)-1-octen-3-ol 1 (11g) [α]D20 -15.5° (MeOH). 10 mg of compound 1 underwent acetylation, yielding compound 4 for subsequent enantiomeric excess analysis (ee > 99.9%). After testing three different substrate to enzyme ratios including 10:1, 10:2, and 10:4, the 10:2 ratio was found to be most suitable for the subsequent scale-up reaction.
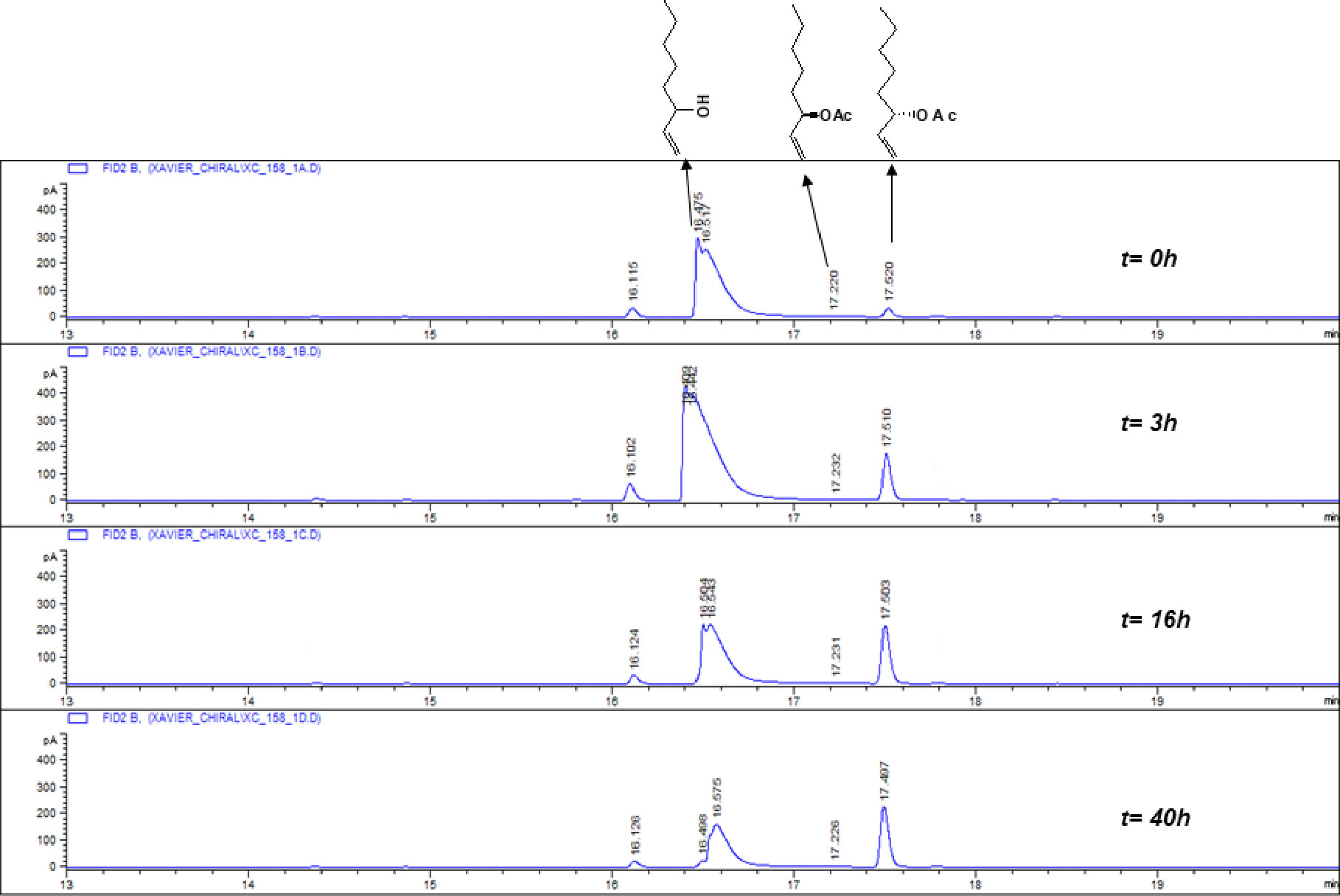
Figure 2 Representative GC-FID trace showing time lapse of a lipase-mediated resolution of racemic 1-octen-3-ol.
1 and 3: (R)-1-octen-3-ol and (S)-1-octen-3-ol. 1H NMR (CDCl3, 500 MHz): δ 1.36-1.42 (m,9H, CH2CH3), δ 1.46-1.99 (m,2H, CH2), δ 2.06(s,1H, OH), δ 4.06-4.10 (dt,1H, J = 6.7,13.0 Hz, C-OH), δ 5.08-5.10 (d,1H, J = 10.4 Hz, CHCH2), δ 5.19-5.23 (d,1H, J = 17.3 Hz, CHCH2), δ 5.83-5.88 (ddd,1H, J = 6.5,10.7,16.6Hz, CH).
1 and 3 (R)-1-octen-3-ol and (S)-1-octen-3-ol.13C NMR (CDCl3,126 MHz) δ: 14.0 (CH3), δ: 22.6,25.0,31.8,37.0 (CH2), δ: 73.3 (C-OH), δ: 144.5,141.4 (CHCH2).
Field evaluation was carried out in the surrounding community of Rabai (0.45866 N, 35.9889 E), in Marigat town located in Baringo County in the Kenyan Rift Valley. A map of the site is provided in Hassaballa et al. (28). The semi-arid ecology that characterizes the site is endemic for visceral and cutaneous leishmaniasis with presence of driving vectors including Phlebotomus martini and P. duboscqi (29, 30). Interspersed across the landscape is abundance of termite mounds, important habitat for sandflies (28, 29). The vegetation comprises mainly acacia and Prosopis juliflora trees, Cactus plants, Balanites spp, and Commiphora bushes. The area is sparsely inhabited, and people commonly indulge in subsistence agriculture and predominantly livestock keeping.
Trials were conducted in February 2015 (experiment 1) and December 2018 (experiment 2) to coincide with peak sandfly activities in the dry season (28, 29). Each experiment comprised ten treatments, with the compounds formulated in hexane (31). These were racemic octenol R/S, R- and S- forms, all at three doses (0.1, 1 and 10 mg/ml), and a control trap baited with hexane only. The considered doses are within tested ranges of semiochemicals against other disease vectors in the field (31, 32). The treatment dose was used to bait a CDC light trap (Model 512, John W Hock, Gainesville, FL, USA) attached close to the fan (Supplementary Figure 1), and it released (1 ml solution) by diffusion from 1.5 ml polyethylene tubes with a pin hole in the center of the cap as described previously (31). CDC light trap is the conventional tool for surveillance of Phlebotomine sand flies and often without a bait (26, 28, 33). Generally, sandflies are considered poor flyers but move via jumping flights (34), although their flight ranges could vary by species. Thus, evaluation followed a randomised experiment targeting termite mounds which is a suitable sandfly microhabitat (28, 29), where treatments were individually positioned, with an inter-trap distance ~40 m. Traps were moved daily targeting new sets of termite mounds. Lures were renewed daily, as well. Because sandflies are nocturnal, traps were set at 18:00 h and retrieved at 06:00 h the following day.
Captured sandflies were immobilised using triethylamine, sorted, and then preserved in liquid nitrogen in the field. Thereafter, they were transported to the laboratory at the icipe Duduville Campus, Nairobi. Here, the samples were stored at −80°C until further processing. For identification, the head and genitalia from each sandfly specimen were excised and mounted on a slide and cover slip using Berlese’s medium. Species were determined by observing the dried slides (at least after a day) via microscopic examination of the cibarial armatures (Phlebotomus or Sergentomyia), male genitalia or female spermathecae and pharynx using published morphological keys of Kirk and Lewis (35) and Abonnenc and Minter (36).
(R)- (–)-1-octen-3-ol and (S)- (+)-1-octen-3-ol were enzymatically and kinetically resolved, and purity determined as 99.5% each and spectroscopically characterized. The racemic 1-octen-3-ol (98%, 50:50 R:S) was obtained from Sigma Aldrich (Louis, MO 63103, USA).
Sandflies were counted daily in each treatment and analysed using binomial regression following generalised linear models using the MASS package in R v 4.2.1. Data was analysed separately for the trial periods. The total abundance and that for species and sex encountered in adequate numbers were analysed separately and compared between the treatments. Treatment was considered the explanatory variable. Mean catches and standard errors for selected species were plotted. All results were considered significant at p ≤ 0.05.
In GC-FID analysis on an achiral column, one peak was detected for the racemic mixture of 1-octen-3-ol. However, when the analysis was carried out on a chiral column, two peaks resolved, with compound 1 eluting at 16.58 min and compound 3 at 17.41 min (Figure 2). In our enzyme-catalyzed kinetic resolution of racemic 1-octen-3-ol, monitored by gas chromatography-flame ionization detector (GC-FID) on the chiral column with 4 hr intervals over a total of 80 hr, maximum resolution of the two enantiomeric isomers occurred at 60 hr to give a reaction mixture of 45% acetate and 55% alcohol plus, vinyl acetate, and lipase (Figure 2).
As expected, the 1H NMR and 13C NMR spectra were identical to those of 1-octen-3-ol (37). Notably, the singlet at δ 2.06 ppm indicates the presence of the hydroxyl group (-OH). The multiplets at δ 1.36-1.99 ppm are consistent with methylene (CH2) groups, while the doublets and triplets at δ 4.06-5.88 ppm indicate the presence of olefinic protons. In the 13C NMR spectrum, peaks at δ 14.0 ppm correspond to methyl (CH3) group, while peaks at δ 22.6-37.0 ppm represent methylene (CH2) carbons. The signal at δ 73.3 ppm is associated with the carbon atom attached to the hydroxyl group (C-OH). Additionally, the peaks at δ 144.5 and 141.4 ppm correspond to the olefinic carbons (CH=CH2).
Experiment 1, carried out in 2015 resulted in captures of 1812 sandflies (female, f=1253, male, m=559) in four replicate trials. A minor proportion of the females captured were blood-fed (4.6%). The sandfly fauna was dominated by Sergentomyia species (94.9%, 1720/1812), notably, S. schwetzi (n=1149, 63.4%), followed by S. antennata (244, 13.5%) and S. clydei (n=126, 7.0%). Other sparsely captured Sergentomyia species included S. africana africana, S. adleri, S. squamipleuris and S.ingrami (Table 1). Only two Phlebotomus species were encountered including Phlebotomus martini (n=185,10.2%) and a sparse representation of P. duboscqi (n=2, 0.1%).
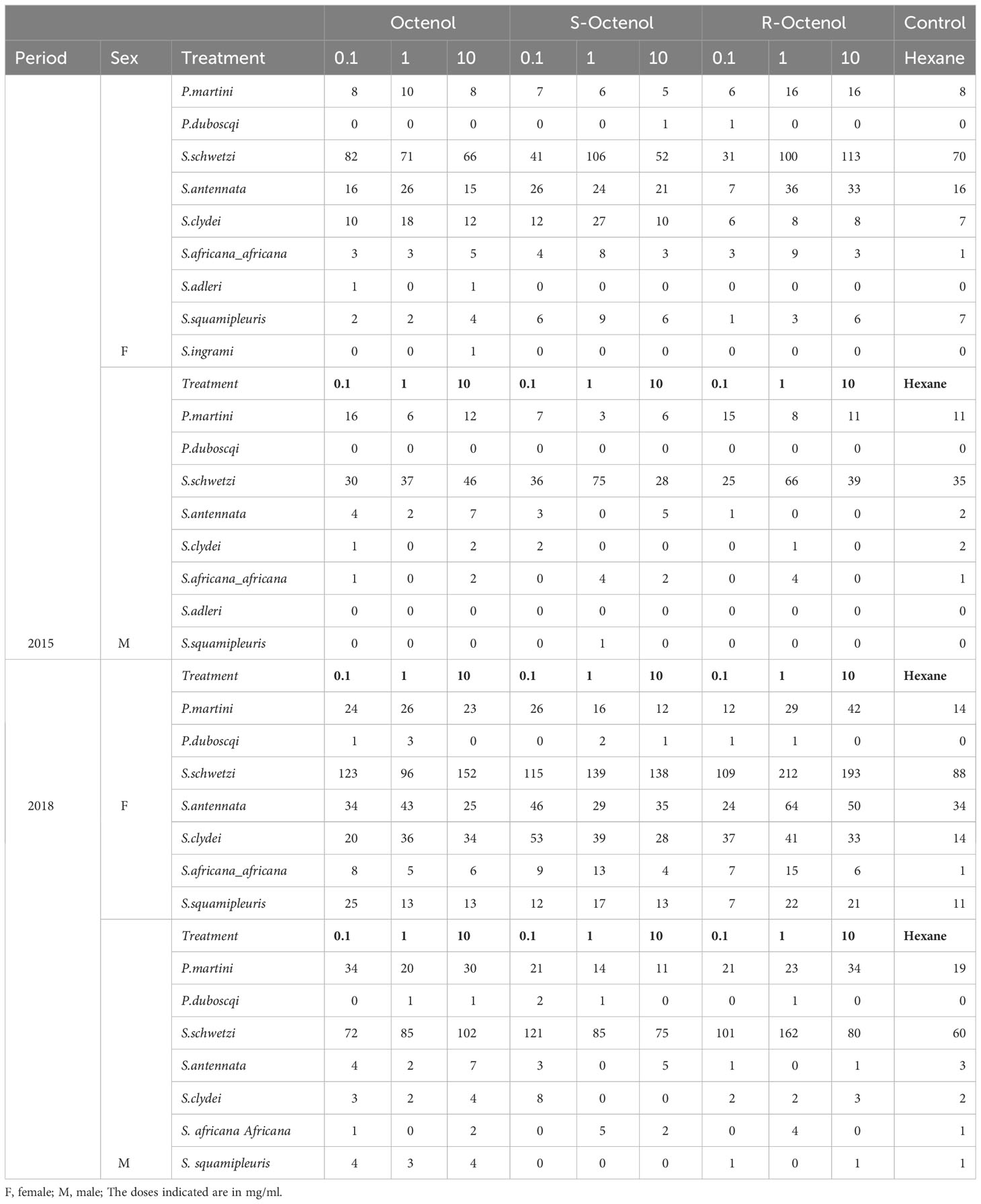
Table 1 Sandfly composition in field evaluations conducted in 2015 and 2018 in Rabai, Marigat sub-county, Kenya.
The 2018 trial (Experiment 2) captured 3802 sandflies (f=2545, m=1257) in 9 replicate experiments. Among the females captures were 79 blood-fed (3.1%). The sandfly composition mirrored the pattern observed for the 2015 captures. Sergentomyia sandflies were most abundant (93.9% of total captures) and highly represented by the species S. schwetzi (60.7%), S. antennata (10.8%), S. clydei (9.5%). The sand fly species P. martini (11.9%) occurred in higher numbers relative to P. duboscqi as the only Phlebotomus species (Table 1).
Regardless of dose, total captures in racemic R/S, S- and R-form were 1621, 1646 and 1939, respectively, with control traps recording the lowest (n=408) for the combined trapping periods (2015 and 2018) (Table 1).
Analysis between the treatments for total sandfly captures and for individual species (fairly represented) by sex was performed separately for each period. Despite a dose-response variation, total sandfly captures did not differ between the treatments both in 2015 (χ211,30 = 45.4, p=0.78) and 2018 (χ211,80 = 106.03, p=0.84). However, there were species-specific differences (Table 1).
Captures of female P. martini in 2015 were relatively highest for traps baited with the highest doses of the R-form (1 and 10 mg/ml) and lowest at all doses of the S-form. However, analysis of the 2015 data showed no significant variation in female P. martini captures between any of the treatments relative to the control trap that had only hexane (χ211,30 = 44.32, p=0.23). We found that traps baited with the R-form (0.1 mg/ml) and R/S (0.1 mg/ml) captured the highest number of male P. martini. These captures differed significantly from captures with the S-form (1mg/ml), which recorded the lowest captures. The mean captures are plotted in Figures 3A, B.
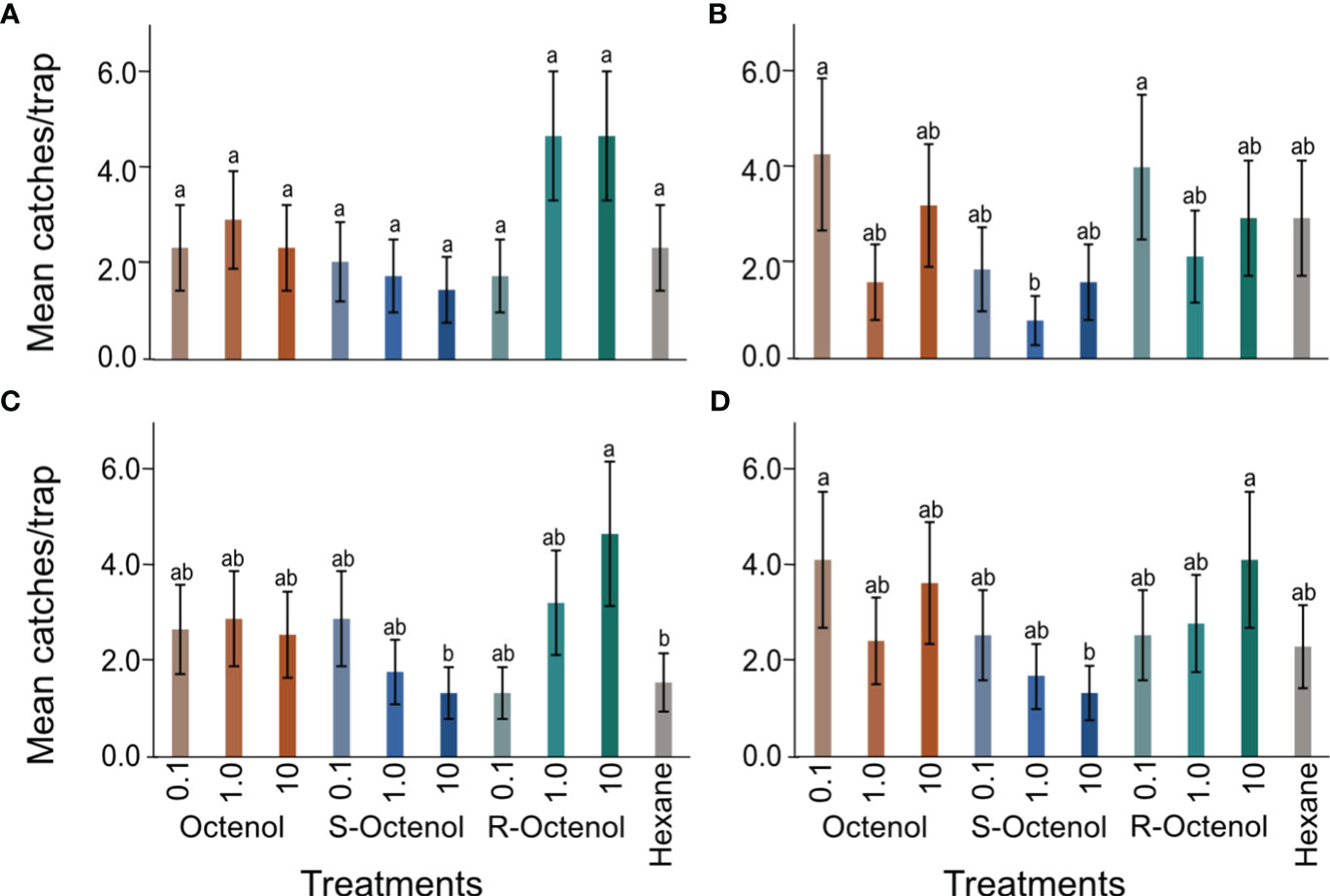
Figure 3 Mean ( ± se) captures of P. martini in the different treatments. (A) female and (B) male during 2015 (4 replicate trials); (C) female and (D) male in 2018 (n=9 replicate experiments). The dose for each form of octenol indicated is in mg/ml. se, standard error; Bars followed by different letters are significantly different from each other at p ≤ 0.05.
In 2018, the R-form at the dose of 10 mg/ml captured the highest number of female P. martini. This number was significantly different from that found for the S-form at the same dose, which with the control recorded the lowest captures (z=2.168; p=0.03). Likewise, as recorded in 2015, the lowest captures were recorded in traps baited with the S-form at the dose of 10 mg/ml. With regards to males during this period, significantly higher numbers were captured in traps baited with the R-form at the dose of 10 mg/ml and racemic R/S (0.1 mg/ml) compared to the S-form at 10 mg/ml which recorded the lowest captures. However, captures with the R-form (10 mg/ml) did not differ from captures recorded in the other treatments and control. The mean captures are plotted in Figures 3C, D.
After controlling for dose, total P. martini captures were 217, 134 and 233 in traps baited with R/S, S- and R-form, respectively. Control traps had the lowest captures (n=52). The data was for the combined trapping periods 2015 and 2018 (Table 1).
The most abundant species captured, S. schwetzi, was compared between treatments in both trapping periods. In 2015, there was no effect of treatments on captures of female S. schwetzi (χ211,30 = 45.88, p=0.71) and male (χ211,30 = 47.41, p=0.70). In 2018, the highest captures of S. schwetzi were obtained with the R-form (1 and 10 mg/ml) for females and at dose of 1mg/ml for males. However, no statistical significance was evident between the treatments and relative to captures recorded for females in the control (χ211,80 = 105.68, p=0.88) and males (χ211,80 = 104.98, p=0.81). The mean captures plotted for this species are indicated in Figures 4A–D.
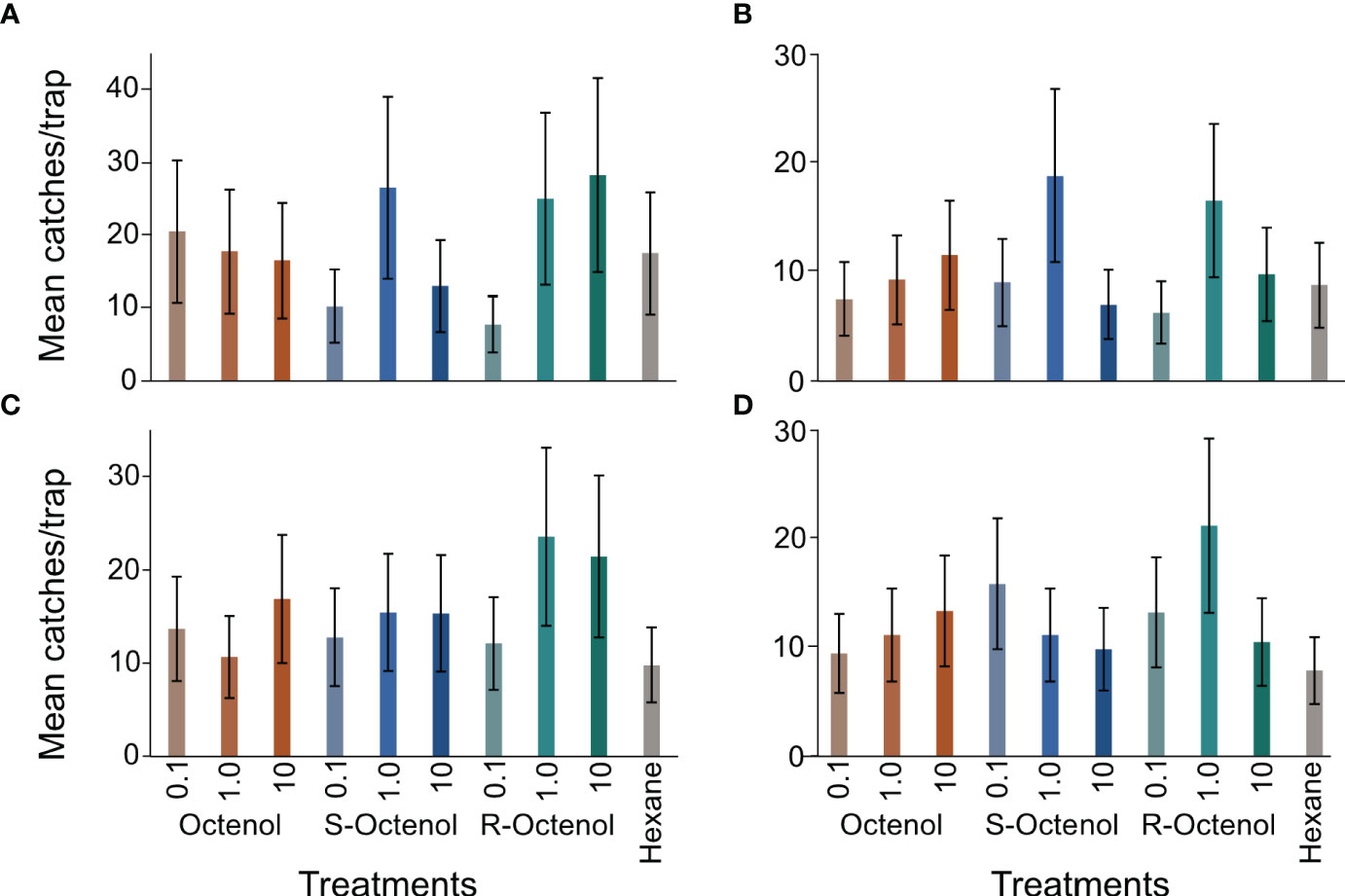
Figure 4 Mean ( ± se) captures of S. schwetzi in the different treatments. (A) female and (B) male during 2015 trials (4 replicate experiments); (C) female and (D) male in 2018 (n=9 replicate experiments). se, standard error; The dose for each form of octenol indicated is in mg/ml. There was no significant difference in collections of different sexes of this species among the treatments including the control at p ≤ 0.05.
Females of the two additional Sergentonyia species (S. antennata and S. clydei) encountered in relatively good numbers in 2018 were compared. Likewise, no significant difference was found between the treatments and control (S. antennata: (χ211,80 = 104.81, p=0.4956); S. clydei: (χ211,80 = 96.71, p=0.81). Nonetheless, S. antennata was most abundant in traps with the R-form (doses 1mg/ml and 10 mg/ml) and S. clydei with the S-form (0.1 mg/ml). The mean captures are represented graphically in Figures 5A, B.
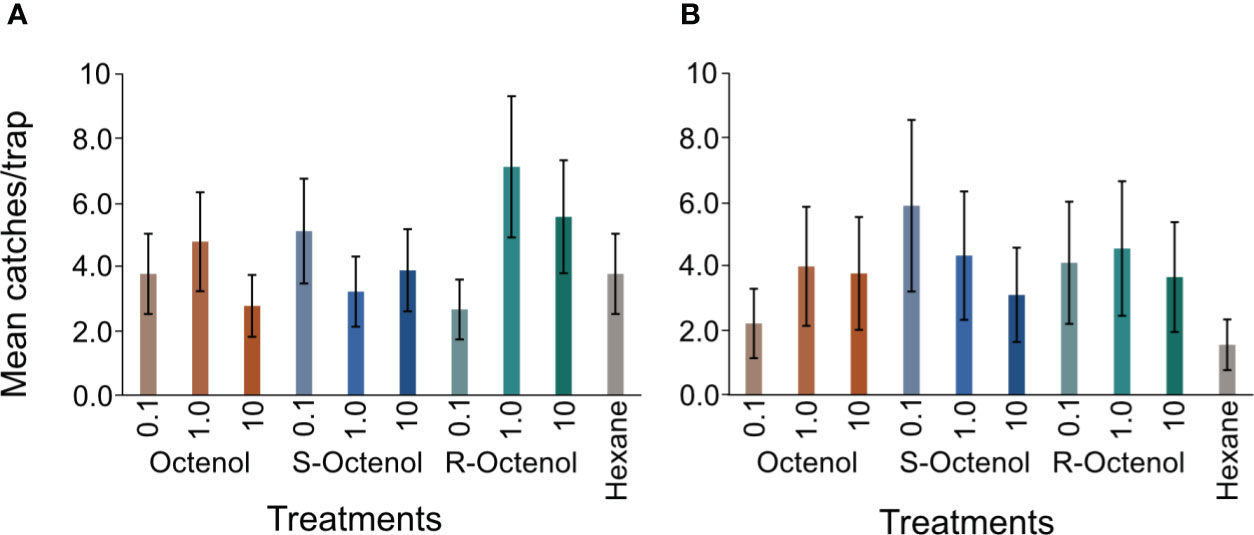
Figure 5 Mean ( ± se) captures of (A) S. antennata and (B) S. clydei in the different treatments in 2018 (n=9 replicate experiments). se, standard error; The dose for each form of octenol indicated is in mg/ml. There was no significant difference in collections of different sexes of this species among the treatments including the control at p ≤ 0.05.
This study evaluated whether the enantiomeric isomers of 1-octen-3-ol (R- and S-forms) and the racemic mixture exhibited differential responses in sandflies with regards to trap captures. Enzymatic resolution of the enantiomeric mixtures gave highly pure individual components (ee, 99.5%), whose structures were determined using spectroscopic methods. This suggests presence of minimal contaminants in the final products which were field evaluated. Thus, interference on sandfly responses by possible reaction contaminants may be ruled out from the field captures.
The trap composition comprised species dominated by those in the genus Sergentomyia. Phlebotomus species were represented by P. martini and P. duboscqi, vectors of causative Leishmania parasites of VL and CL, respectively (30, 38). The captures appear to mirror the sandfly fauna in the geographic area as reported previously (28, 30). Higher captures were recorded in the R- than S-forms for P. martini but not Sergentomyia species. These results suggest species-specific effect in behavioral response activity between the enantiomers. Variation in behavioral impact of octenol to different sandfly species, for instance, Lutzomyia intermedia and L. longipalpis (26, 39) and mosquito vectors (e.g. An. crucians, Oc. infirmatus (19) have been described previously. Our findings add to the few available data on the kairomonal effect of octenol on phlebotomine sandflies (26) and for the first time, the differential behavioral effect of its enantiomers in field settings.
The performance although dose-dependent was somewhat similar between male and female P. martini. Whereas the lowest captures of both sexes were associated with the S-form at the dose of 10mg/ml, highest captures were observed at specific doses with the R-form or the racemic mixture (Experiment 2). Invariably, these results suggest differential response of this sandfly species, and both sexes, to the two enantiomers. Octenol is represented in the volatile emissions of plants (40) and habitats like termite mounds (28), to which male and female sandflies exploit (41–43). Octenol is present also in the volatile emissions of human skin (44). Thus, the response of both sexes to octenol is not unexpected, indicating that it is part of the odour bouquet that drives variation in attraction to these substrates. Nonetheless, the relative proportion of the isomers in different natural substrates is unknown, although literature suggests a dominance of R- over S-form (4). Electrophysiological studies are needed to corroborate the differential sensitivity of this sandfly species to the different isomers.
In experiment 1 (2015) of the present study, overall higher captures of female P. martini at higher doses were recorded using the R-form (1 mg/ml, 10mg/ml). Analysis of the data indicated that males showed an apparent preference for the R-form at a lower dose instead (0.1 mg/ml). Males feed on plants entirely and could adapt to probably low levels released by plants compared to vertebrates. There was no variation in female captures of this species across treatments including the control. Lack of statistical significance could have been masked by the low numbers in the trapping efforts or number of replicates during this period. Additional studies to investigate release rates, seasonal and geographic effects of the enantiomeric isomers and racemic mixture on sand fly responses would be required.
Despite the overall abundance of Sergentomyia sandflies, among the species caught, S. schwetzi showed no variation between the treatments and relative to the control. This pattern contrasts to that found for P. martini, indicating divergence in behavioral activity elicited to octenol among sandfly species. Understanding the basis including sensitivity threshold via electrophysiological studies could shed light on species adaptation to resources mediated by volatile organic compounds such as octenol.
In conclusion, this study investigated the effects of enantiomers and racemic mixture of 1-octen-3-ol on trap catches of sandflies with CDC light traps under field conditions. Of the species encountered only P. martini exhibited a dose-dependent response to octenol; Captures of both sexes of the species were generally higher in the R- than S-form (at specific doses) indicative of clear differences in behavioral activity of the isomers in this sandfly species. Biologically, the (R)-1-octen-3-ol could be a more important kairomone that can be incorporated into traps for P. martini. Despite overall increased collections, no dose of the R-form performed better statistically, than the racemic mixture for this and the other sandfly species caught.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The manuscript presents research on animals that do not require ethical approval for their study.
DT: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Project administration. JJ: Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. XC: Conceptualization, Data curation, Formal Analysis, Funding administration, Investigation, Methodology, Validation, Writing – review & editing. LC: Data curation, Investigation, Validation, Writing – review & editing. IH: Investigation, Methodology, Writing – review & editing. BT: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study received funding from an icipe seed grant (SANVEC) awarded to DT and BT. Additional financial support was received through a Rothamsted International Fellowship awarded to XC in collaboration with Anthony Hooper, Rothamsted Research Institute, UK. We gratefully acknowledge the financial support for this research by the following organizations and agencies: the Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); the Australian Centre for International Agricultural Research (ACIAR); the Norwegian Agency for Development Cooperation (Norad); the Federal Democratic Republic of Ethiopia; and the Government of the Republic of Kenya. The views expressed herein do not necessarily reflect the official opinion of the donors.
We thank the chief and community members for their support during field work at the study site. We are grateful to Dr. Anthony Hooper, Rothamsted Research Institute, UK, for his invaluable support throughout the multi-gram synthesis and resolution of racemic 1-octen-3-ol.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2023.1327349/full#supplementary-material
Supplementary Figure 1 | Trap design comprising the lure solution released from a 1.5 ml polyethylene tube with a pin hole attached close to the fan of a CDC trap.
1. Nyasembe VO, Torto B. Volatile phytochemicals as mosquito semiochemicals. Phytochem Lett (2014) 8:196–201. doi: 10.1016/j.phytol.2013.10.003
2. Tchouassi DP, Wanjiku C, Torto B. Chapter 33: Host-derived attractants for surveillance and control of mosquitoes. In: Sensory ecology of disease vectors. Wageningen Academic Publishers, The Netherlands (2022). p. 851–77. doi: 10.3920/978-90-8686-932-9_33
3. Torto B, Tchouassi DP. Chemical ecology and management of dengue vectors. Annu Rev Entomol (2024). doi: 10.1146/annurev-ento-020123-015755
4. Hall DR, Beevor PS, Cork A, Nesbitt BF, Vale GA. 1-Octen-3-ol. A potent olfactory stimulant and attractant for tsetse isolated from cattle odours. Int J Trop Insect Sci (1984) 5(5):335–9. doi: 10.1017/S1742758400008626
5. French FE, Kline DL. l-octen-3-ol, an effective attractant for tabanidae (Diptera). J Med Entomology. (1989) 26(5):459–61. doi: 10.1093/jmedent/26.5.459
6. Kline DL, Hagan DV, Wood JR. Culicoides responses to 1-octen-3-ol and carbon dioxide in salt marshes near Sea Island, Georgia, U.S.A. Med Vet Entomol (1994) 8(1):25–30. doi: 10.1111/j.1365-2915.1994.tb00379.x
7. Cheke R, Garms. R. Trials of attractants to enhance biconical trap catches of Simulium yahense and S. sanctipauli s.l. Trop Med Parasitol (1987) 38:62–3. Available at: https://api.semanticscholar.org/CorpusID:36041281
8. Atwood DW, Meisch MV. Evaluation of 1-octen-3-ol and carbon dioxide as black fly (Diptera: Simuliidae) attractants in Arkansas. J Am Mosq Control Assoc (1993) 9(2):143–6.
9. Bohbot J, Durand N, Vinyard B, Dickens J. Functional development of the octenol response in Aedes aEgypti. Front Physiol (2013) 4:39. doi: 10.3389/fphys.2013.00039
10. Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles Gambiae. Curr Biol (2007) 17(18):1533–44. doi: 10.1016/j.cub.2007.07.062
11. Xu P, Zhu F, Buss GK, Leal WS. 1-Octen-3-ol – the attractant that repels. F1000Research (2015) 4:156. doi: 10.12688/f1000research.6646.1
12. Cook JI, Majeed S, Ignell R, Pickett JA, Birkett MA, Logan JG. Enantiomeric selectivity in behavioural and electrophysiological responses of Aedes aEgypti and Culex quinquefasciatus mosquitoes. Bull Entomol Res (2011) 101(5):541–50. doi: 10.1017/S0007485311000162
13. Syed Z, Leal WS. Maxillary palps are broad spectrum odorant detectors in Culex quinquefasciatus. Chem Senses (2007) 32(8):727–38. doi: 10.1093/chemse/bjm040
14. Bohbot JD, Dickens JC. Characterization of an Enantioselective Odorant Receptor in the yellow fever mosquito Aedes aEgypti. PloS One (2009) 4(9):e7032. doi: 10.1371/journal.pone.0007032
15. Grant AJ, Dickens JC. Functional Characterization of the Octenol Receptor Neuron on the Maxillary Palps of the yellow fever mosquito, Aedes aEgypti. PloS One (2011) 6(6):e21785. doi: 10.1371/journal.pone.0021785
16. Thakeow P, Angeli S, Weißbecker B, Schütz S. Antennal and behavioral responses of Cis boleti to fungal odor of Trametes gibbosa. Chem Senses (2008) 33(4):379–87. doi: 10.1093/chemse/bjn005
17. Escobar D, Ascencio K, Ortiz A, Palma A, Fontecha G. Distribution and phylogenetic diversity of Anopheles species in malaria endemic areas of Honduras in an elimination setting. Parasit Vectors. (2020) 13(1):333. doi: 10.1186/s13071-020-04203-1
18. Wilke ABB, Vasquez C, Medina J, Carvajal A, Petrie W, Beier JC. Community composition and year-round abundance of vector species of mosquitoes make Miami-Dade County, Florida a receptive gateway for arbovirus entry to the United States. Sci Rep (2019) 9:8732. doi: 10.1038/s41598-019-45337-2
19. Kline DL, Allan SA, Bernier UR, Welch CH. Evaluation of the enantiomers of 1-octen-3-ol and 1-octyn-3-ol as attractants for mosquitoes associated with a freshwater swamp in Florida, U.S.A. Med Vet Entomol (2007) 21(4):323–31. doi: 10.1111/j.1365-2915.2007.00697.x
20. Bursell E. Effects of host odour on the behaviour of tsetse. Int J Trop Insect Sci (1984) 5(5):345–9. doi: 10.1017/S174275840000864X
21. Vale GA, Hall DR. The use of 1-octen-3-ol, acetone and carbon dioxide to improve baits for tsetse flies, Glossina spp. (Diptera: Glossinidae). Bull Entomol Res (1985) 75(2):219–32. doi: 10.1017/S0007485300014309
22. Tchouassi DP, Marklewitz M, Chepkorir E, Zirkel F, Agha SB, Tigoi CC, et al. Sand fly–associated Phlebovirus with evidence of neutralizing antibodies in humans, Kenya. Emerg Infect Dis (2019) 25(4):681–90. doi: 10.3201/eid2504.180750
23. Torto B, Hassaballa IB, Tchouassi DP. Chapter 8: Chemical ecology of sand fly plant-feeding behaviour. In: Sensory ecology of disease vectors. Wageningen Academic Publishers, The Netherlands (2022). p. 235–52. doi: 10.3920/978-90-8686-932-9_8
24. Magalhães-Junior JT, Barrouin-Melo SM, Corrêa AG, da Rocha Silva FB, MaChado VE, Govone JS, et al. A laboratory evaluation of alcohols as attractants for the sandfly Lutzomyia longipalpis (Diptera : Psychodidae). Parasit Vectors. (2014) 7(1):60. doi: 10.1186/1756-3305-7-60
25. Hassaballa IB, Matoke-Muhia D, Masiga DK, Sole CL, Torto B, Tchouassi DP. Behavioural responses of Phlebotomus duboscqi to plant-derived volatile organic compounds. Med Vet Entomol (2021) 35(4):625–32. doi: 10.1111/mve.12541
26. Andrade AJ, Andrade MR, Dias ES, Pinto MC, Eiras ÁE. Are light traps baited with kairomones effective in the capture of Lutzomyia longipalpis and Lutzomyia intermedia? An evaluation of synthetic human odor as an attractant for phlebotomine sand flies (Diptera: Psychodidae: Phlebotominae). Mem Inst Oswaldo Cruz. (2008) 103:337–43. doi: 10.1590/S0074-02762008000400004
27. Reinart-Okugbeni R, Ausmees K, Kriis K, Werner F, Rinken A, Kanger T. Chemoenzymatic synthesis and evaluation of 3-azabicyclo[3.2.0]heptane derivatives as dopaminergic ligands. Eur J Med Chem (2012) 55:255–61. doi: 10.1016/j.ejmech.2012.07.025
28. Hassaballa IB, Torto B, Sole CL, Tchouassi DP. Exploring the influence of different habitats and their volatile chemistry in modulating sand fly population structure in a leishmaniasis endemic foci, Kenya. PloS Negl Trop Dis (2021) 15(2):e0009062. doi: 10.1371/journal.pntd.0009062
29. Ngumbi PM, Lrungu LW, Robert LI, Gordon DM, Githure JL. Abundances and nocturnal activities of phlebotomine sandflies (Diptera: Psychodidae) in termite hills and animal burrows in Baringo District, Kenya. Afr J Health Sci (1998) 5(1):28–34.
30. Anjili CO, Ngumbi PM, Kaburi JC, Irungu LW. The phlebotomine sandfly fauna (Diptera: Psychodidae) of Kenya. J Vector Borne Dis (2011) 48(4):183–9.
31. Tchouassi DP, Sang R, Sole CL, Bastos ADS, Teal PEA, Borgemeister C, et al. Common Host-derived chemicals increase catches of disease-transmitting mosquitoes and can Improve early warning systems for Rift Valley fever virus. PloS Negl Trop Dis (2013) 7(1):e2007. doi: 10.1371/journal.pntd.0002007
32. Omondi WP, Owino EA, Odongo D, Mwangangi JM, Torto B, Tchouassi DP. Differential response to plant- and human-derived odorants in field surveillance of the dengue vector, Aedes aEgypti. Acta Trop (2019) 200:105163. doi: 10.1016/j.actatropica.2019.105163
33. Owino BO, Matoke-Muhia D, Alraey Y, Mwangi JM, Ingonga JM, Ngumbi PM, et al. Association of Phlebotomus guggisbergi with Leishmania major and Leishmania tropica in a complex transmission setting for cutaneous leishmaniasis in Gilgil, Nakuru County, Kenya. PloS Negl Trop Dis (2019) 13(10):e0007712. doi: 10.1371/journal.pntd.0007712
34. Killick-Kendrick R. Phlebotomine vectors of the leishmaniases: a review. Med Vet Entomol (1990) 4(1):1–24. doi: 10.1111/j.1365-2915.1990.tb00255.x
35. Kirk K, Lewis DJ. The phlebotominae of the Ethiopian Eegion.1. Trans R Entomol Soc London (1951) 102(8):383–510. doi: 10.1111/j.1365-2311.1951.tb00759.x
36. Abonnenc E, Minter DM. Bilingual keys for the identification of the sandflies of the Ethiopian region. Cahier ORSTOM (Entomologie medicale). (1965) 5:1–63.
37. Mosandl A, Heusinger G, Gessner M. Analytical and sensory differentiation of 1-octen-3-ol enantiomers. J Agric Food Chem (1986) 34(1):119–22. doi: 10.1021/jf00067a033
38. Anderson JM, Samake S, Jaramillo-Gutierrez G, Sissoko I, Coulibaly CA, Traoré B, et al. Seasonality and prevalence of Leishmania major infection in Phlebotomus duboscqi Neveu-Lemaire from two neighboring villages in Central Mali. PloS Negl Trop Dis (2011) 5(5):e1139. doi: 10.1371/journal.pntd.0001139
39. Beavers GM, Hanafi HA, Dykstra EA. Evaluation of 1-octen-3-ol and carbon dioxide as attractants for Phlebotomus papatasi (Diptera: Psychodidae) in southern Egypt. J Am Mosq Control Assoc (2004) 20(2):130–3.
40. Bendera M, Ekesi S, Ndung’u M, Srinivasan R, Torto B. A major host plant volatile, 1-octen-3-ol, contributes to mating in the legume pod borer, Maruca vitrata (Fabricius) (Lepidoptera: Crambidae). Naturwissenschaften (2015) 102(9–10):47. doi: 10.1007/s00114-015-1297-0
41. Junnila A, Müller GC, Schlein Y. Attraction of Phlebotomus papatasi to common fruit in the field. J Vector Ecol (2011) 36 Suppl 1:S206–211. doi: 10.1111/j.1948-7134.2011.00132.x
42. Lima LHG de M, Mesquita MR, Skrip L, de Souza Freitas MT, Silva VC, Kirstein OD, et al. DNA barcode for the identification of the sand fly Lutzomyia longipalpis plant feeding preferences in a tropical urban environment. Sci Rep (2016) 6:29742. doi: 10.1038/srep29742
43. Hassaballa IB, Sole CL, Cheseto X, Torto B, Tchouassi DP. Afrotropical sand fly-host plant relationships in a leishmaniasis endemic area, Kenya. PloS Negl Trop Dis (2021) 15(2):e0009041. doi: 10.1371/journal.pntd.0009041
44. Bernier UR, Kline DL, Barnard DR, Schreck CE, Yost RA. Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti). Anal Chem (2000) 72(4):747–56. doi: 10.102doi1/ac990963k
Keywords: volatile organic compounds, (R)-(-)-1-octen-3-ol, (S)-(+)-1-octen-3-ol, field evaluation, Phlebotomus martini, visceral leishmaniasis
Citation: Tchouassi DP, Jacob JW, Cheseto X, Chepkemoi LS, Hassaballa IB and Torto B (2024) Enzyme-catalyzed kinetic resolution of racemic 1-octen-3-ol and field evaluation of its enantiomeric isomers as attractants of sandflies. Front. Trop. Dis 4:1327349. doi: 10.3389/fitd.2023.1327349
Received: 24 October 2023; Accepted: 11 December 2023;
Published: 05 January 2024.
Edited by:
Paul O. Mireji, Kenya Agricultural and Livestock Research Organization, KenyaReviewed by:
Benson Wachira, Pwani University, KenyaCopyright © 2024 Tchouassi, Jacob, Cheseto, Chepkemoi, Hassaballa and Torto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David P. Tchouassi, ZHRjaG91YXNzaUBpY2lwZS5vcmc=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.