
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Trop. Dis., 17 January 2024
Sec. Neglected Tropical Diseases
Volume 4 - 2023 | https://doi.org/10.3389/fitd.2023.1303873
This article is part of the Research TopicWomen in Science - Emerging, Major & Neglected Tropical DiseasesView all 5 articles
Introduction: The most common soil-transmitted helminthic infection is caused by Ascaris lumbricoides (A. lumbricoides). Approximately 4 billion people are at risk of infection globally. The World Health Organisation recommends the administration of benzimidazole- containing deworming drugs (Albendazole and Mebendazole) to all susceptible populations. Due to this high drug pressure, these parasites may develop resistance to current benzimidazole drugs. The β-tubulin gene family is the target gene for benzimidazole deworming drugs. This systematic review aimed to highlight work that explored the genetic mutations in the β-tubulin gene family of A. lumbricoides that are associated with potential benzimidazole resistance.
Methods: An electronic search of several online databases was used to extract eligible articles using specific keywords related to the topic of interest.
Results: The majority of ascariasis infections occur in the subtropical and tropical regions of sub-Saharan Africa, the Americas and East Asia, although not enough studies were done to extensively cover this geographical range. In the β-tubulin gene family of A. lumbricoides the mutations at codons F200Y (TTC/Phenylalanine to TAC/Tyrosine), E198A (GAG, GAA/Glutamic acid to GCG, GCA/Alanine) and F167Y (TTC, TTT/Phenylalanine to TAC, TAT/Tyrosine) were associated with potential benzimidazole resistance.
Discussion: Resistant mutations were found in A. lumbricoides samples at codon F167Y from Haiti, Kenya and Panama. The first evidence of the mutation at codon F200Y was observed in Brazil. The codon E198A mutation was the least prevalent and most undetected.
Conclusion: There is a serious shortage of studies investigating the prevalence of β-tubulin gene family mutations in A. lumbricoides populations from endemic areas; this is a serious concern as resistance will negatively impact current mass drug administration programmes.
The World Health Organisation (WHO) has advocated that all countries move towards the elimination of neglected tropical diseases (NTDs), including soil-transmitted helminthiasis, which afflicts more than 1.5 billion people globally (24% of the global population) (1). The most prevalent soil-transmitted helminths (STHs) currently affecting the world’s population are Necator americanus, Strongyloides stercoralis, Trichuris trichiura, Ancylostoma duodenale, and Ascaris lumbricoides (2, 3). Ascariasis is the most common STH infection caused by A. lumbricoides, an intestinal roundworm, and infects an estimated 1.2 billion people worldwide (4) with a global approximation of 4 billion people at risk of infection (5). The heavy burden of A. lumbricoides infections causes more than 60,000 deaths annually (6) and an estimated 1.8 - 10.5 million disability-adjusted life years (DALYs) (7).
The majority of ascariasis infections occur in the subtropical and tropical areas of sub-Saharan Africa, the Americas and East Asia, particularly poverty-stricken areas with poor access to clean water and poor sanitary conditions (4). The prevalence of A. lumbricoides infections in sub-Saharan Africa is 13.6%, 15.6% in South America, and 18% in Southeast Asia and South Asia (8–10). Comparatively, with the introduction of modern waste management systems and sanitation in the United States (US) at the start of the 20th century, ascariasis prevalence has significantly decreased (11). However, cases of A. lumbricoides infections still occur as a result of emigration to the US and travel from the US to foreign countries where the prevalence of ascariasis infection is high (11). According to Hong et al. (12), additional factors that worsen the situation in developing countries include natural disasters, social instability, poor hygiene practices, inadequate healthcare facilities and systems, civil wars, and low-quality healthcare (12).
The current prevention strategy put forward by the WHO is the mass deworming drug administration to all susceptible populations, particularly school children, who experience the highest rates of parasite infections (13). In large-scale deworming programs, benzimidazole drugs such as Albendazole (a single dose of 400 mg taken orally) or Mebendazole (a single dose of 500 mg taken orally or two doses daily of 100 mg taken orally over three days) remain the current mainstays of treatment (7). These initiatives aim to reduce the morbidity caused by intestinal worms (7). In 2012, the London Declaration, led by the WHO Director General and the Bill Gates Foundation, committed funding and effort for the elimination of 10 NTDs, including STHs, by 2030 (14). The landmark feature of this declaration is that the world’s 13 leading pharmaceutical companies pledged to donate free anthelminthic drugs to all endemic countries. This exponentially accelerated the uptake of the mass drug administration (MDA) in many endemic countries. By 2021, 62 countries reported on the implementation of large-scale MDA treatment programmes for at least one of the targeted diseases. By that year, overall, 429 million were treated for soil-transmitted helminthiases (14). The only drugs used for the MDA in all the recipient endemic countries are the benzimidazoles (Mebendazole and Albendazole).
Continuous administration of the same drug treatment in single doses to large numbers of people provides opportunities for the evolution of potential drug resistance (15). Due to this high drug pressure among communities, A. lumbricoides may develop resistance to current benzimidazole drugs. The target for these benzimidazole drugs in A. lumbricoides is the β-tubulin proteins, where resistance is likely to develop, highlighting the need for surveillance systems to identify genetic mutations associated with benzimidazole resistance (16). However, there is a lack of knowledge on the mutations in β- tubulin gene family of A. lumbricoides that could potentially confer benzimidazole resistance and prevents the development of such tools (16).
Thus, this systematic review aims to gather information from published research literature globally regarding the prevalence of single nucleotide polymorphisms (SNPs) in the β-tubulin gene family of A. lumbricoides namely codons F167Y (TTC, TTT/Phenylalanine to TAC, TAT/Tyrosine), F200Y (TTC/Phenylalanine to TAC/Tyrosine) and E198A (GAG, GAA/Glutamic acid to GCG, GCA/Alanine) that may confer resistance to benzimidazole, methods used for SNP detection and also discuss the effect of Mebendazole and Albendazole drug resistance and treatment efficacy in endemic populations.
This systematic review collected relevant data from previous literature regarding SNPs found in the β-tubulin gene family of A. lumbricoides at codons F167Y, F200Y and E198A that could potentially confer benzimidazole resistance to current treatment regimens of Albendazole and Mebendazole. A narrative approach was followed to review relevant and available data on this topic.
ScienceDirect, Google Scholar, MEDLINE, PubMed and Institute for Scientific Information (ISI) Web of Knowledge databases were searched using the following keywords: ‘Ascaris lumbricoides’, ‘A. lumbricoides’, ‘β-tubulin gene mutations’, ‘benzimidazole resistance’, ‘F200Y’, ‘E198A’, ‘F167Y’ and ‘single nucleotide polymorphisms’. Individual keywords and a combination of the keywords were used to search for relevant literature. The relevant data was analyzed and reported following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (17) guidelines. This study included all English language-published literature globally from January 1990 to December 2022.
Appropriate literature was first selected by their titles, abstracts and full-text according to the exclusion and inclusion criteria by the main author (T.R.) and eligibility of the literature to be included in this study was approved and checked for discrepancies and duplicates by the two co-authors (P.N. and Z.L.M.K.). The quality of the relevant data extracted from the literature and presented in this study was separated into four categories: high quality, moderate quality, low quality and very low quality (18). This was assessed using the grading of recommendations, assessment, development and evaluations (GRADE) (19).
● Literature reporting on SNPs in the β-tubulin gene family of A. lumbricoides and the prevalence of these polymorphisms at codons F200Y, E198A and F167Y.
● Literature reporting on benzimidazole resistance in the β-tubulin gene family of A. lumbricoides due to SNPs at codons F200Y, E198A and F167Y.
● Literature published in all countries and in English from January 1990 to December 2022.
● Case-controlled studies, cross-sectional studies and cohort-appropriate studies.
● Published data in five databases.
● Literature published prior to January 1990.
● Articles not published in English.
● Reviews, Comments and editorials.
● Literature that is not relevant to A. lumbricoides and β-tubulin gene SNPs at codons F200Y, E198A and F167Y.
A total of 282 articles were found using the search criteria in the above-mentioned databases. Of the 282 articles, 192 were retained after the removal of duplicates. Further evaluation of the articles based on the eligibility criteria narrowed down the article selection to 12 articles. Finally, 8 of the 12 articles were selected based on A. lumbricoides SNPs (F200Y, E198A and F167Y) β-tubulin gene family data and benzimidazole resistance. A systematic flow diagram for the selection of articles chosen for this systematic review based on initial identification, screening, eligibility and final selection is represented in Figure 1.
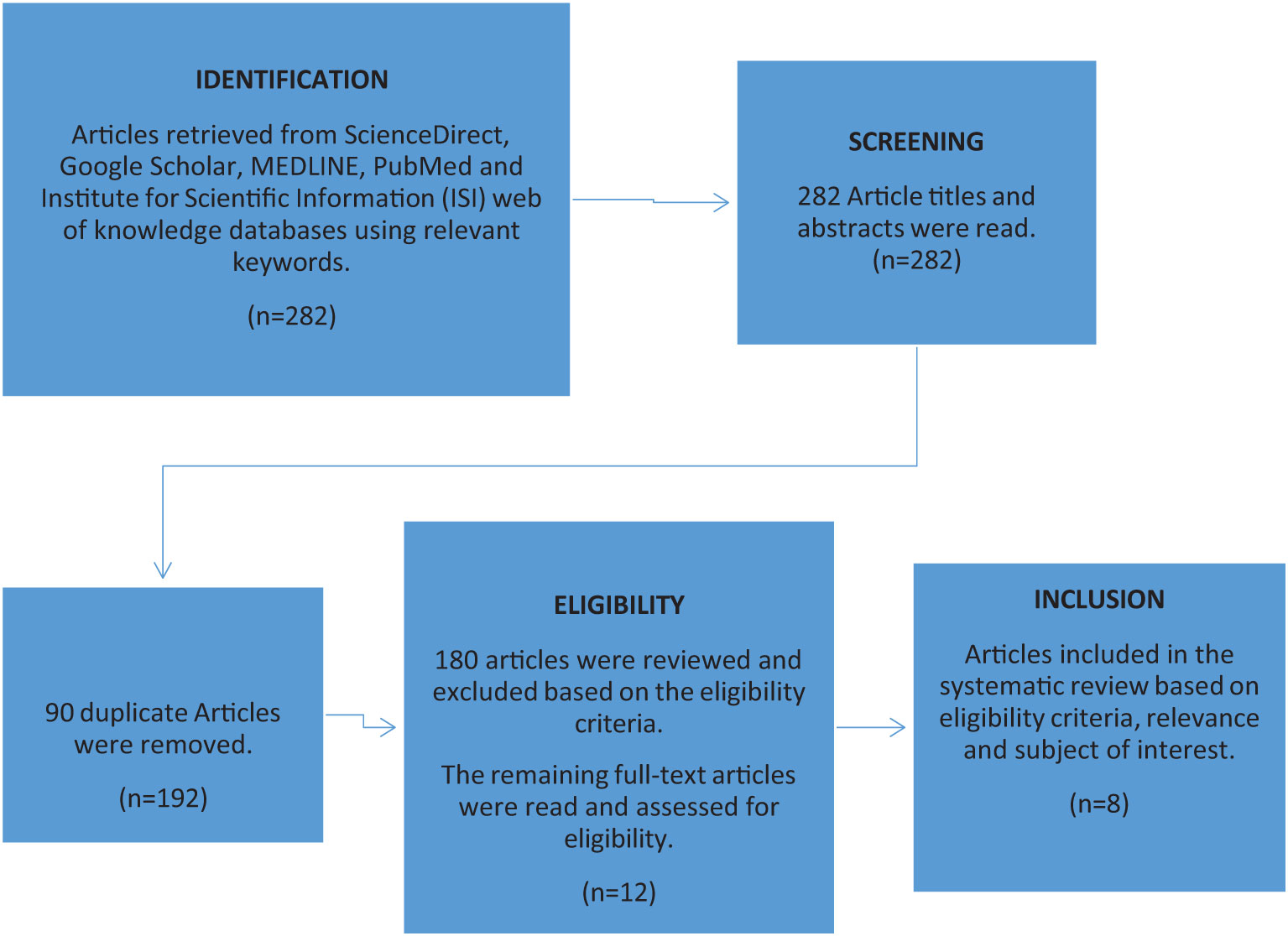
Figure 1 Systematic flow diagram representing the article selection process used to include relevant data from the initial identification, screening, eligibility and final selection of articles based on A. lumbricoides SNPs (F200Y, E198A and F167Y) β-tubulin gene family data and Benzimidazole resistance.
Information regarding studies that were included, their relevant study population and A. lumbricoides sample type used for genetic analysis is described in Table 1. Table 2 presents the main results of the articles that were reviewed, assessed and included in this systematic review.
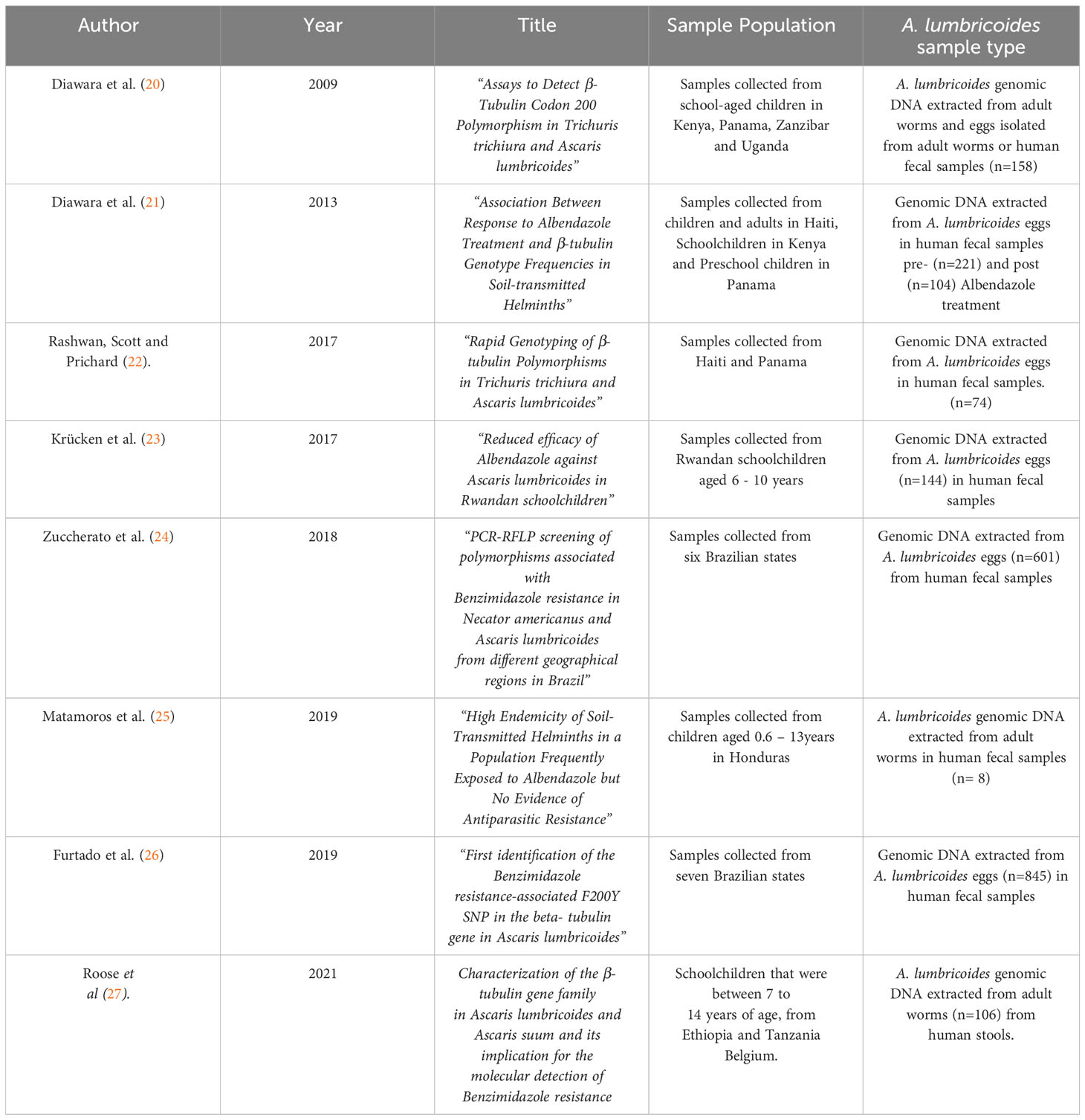
Table 1 Summary of articles included in this systematic review investigating the prevalence of the F200Y, E198A and F167Y SNPs in the β-tubulin gene family of A. lumbricoides, the target sample population of each study and their respective sample of choice from which genomic DNA was extracted.
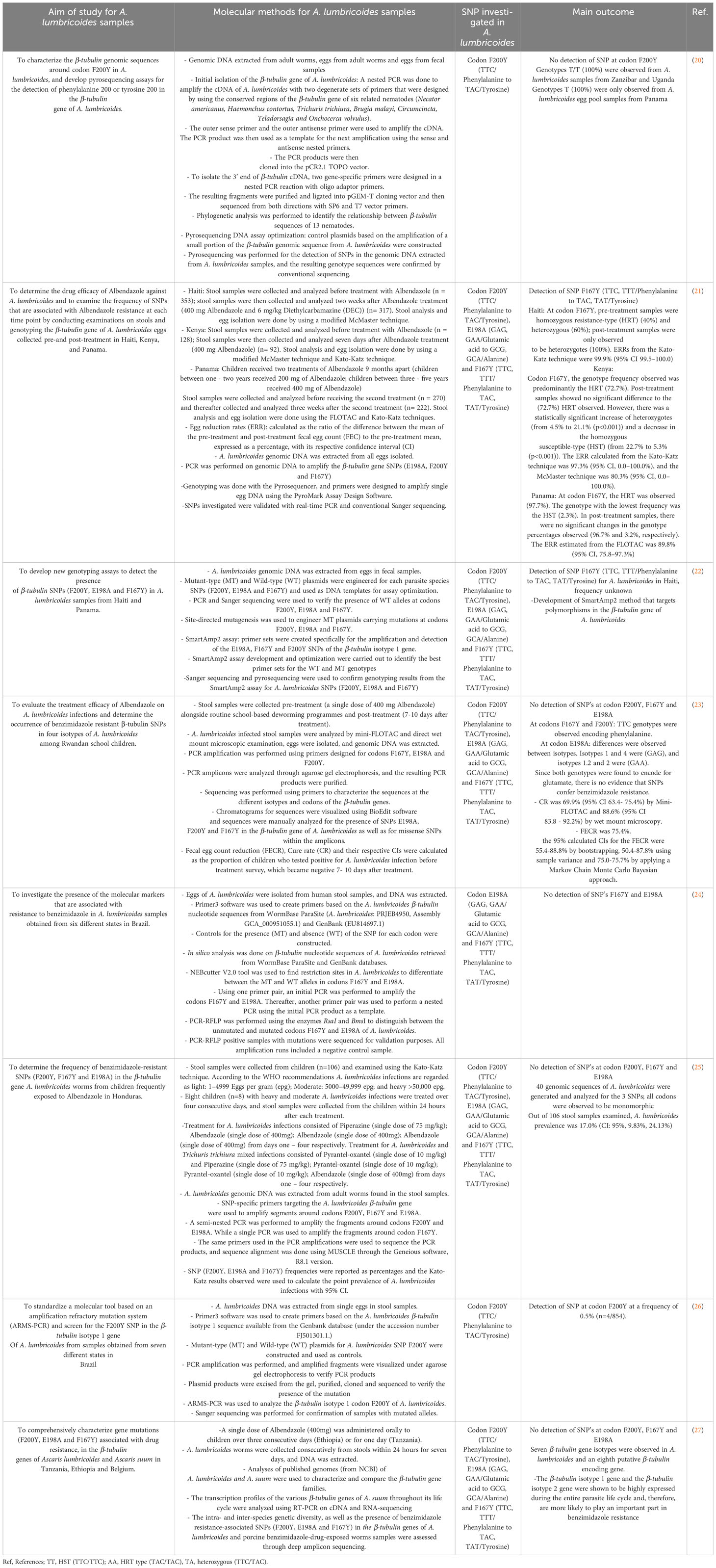
Table 2 Summary of articles included, identifying the prevalence of F200Y, E198A and F167Y polymorphisms in the β-tubulin gene family of A. lumbricoides.
This is the first systematic review that presents data on specific SNPs in codons F200Y, E198A, and F167Y in the β-tubulin gene family of A. lumbroicoides globally. Benzimidazole resistance in the in the β-tubulin gene family of helminths has been associated with amino acid substitutions from phenylalanine to tyrosine (TTC/Phenylalanine to TAC/Tyrosine) at codon 200 (F200Y SNP) (28). A similar SNP at codon 167, which also causes an amino acid substitution of phenylalanine to tyrosine (TTC, TTT/Phenylalanine to TAC, TAT/Tyrosine) (F167Y SNP) was also observed (29). Although rare, occasionally, a SNP at codon 198 can cause an amino acid substitution of glutamic acid to alanine (GAG, GAA/Glutamic acid to GCG, GCA/Alanine) (E198A SNP), which is also associated with benzimidazole resistance (26, 30).
Furtado et al. (26) screened the β-tubulin isotype 1 gene for SNPs at codon F200Y in A. lumbricoides samples obtained from seven states in Brazil; they observed a mutation at codon F200Y at 0.5% frequency (n=4/854) (26). Despite the low frequency observed, this is the first observation of an SNP detected at codon F200Y in A. lumbricoides, and the presence of this SNP is indicative of the potential of these parasite populations to develop resistance to current treatments and possibly at higher levels in the near future (26). This study did not indicate whether the sample population investigated had been previously exposed to benzimidazole treatment.
Roose et al. (27) observed at least seven different β-tubulin genes in A. lumbricoides samples from Ethiopia, Tanzania and Belgium, as well as an eighth putative β-tubulin encoding gene (27). The β-tubulin isotype 1 gene and the β-tubulin isotype 2 gene were shown to be highly expressed during the entire parasite life cycle and, therefore, are more likely to play an important part in benzimidazole resistance (27). This β-tubulin phylogeny is fairly complex; hence, it is challenging to establish a common nomenclature across species for the β-tubulin genes and makes the comparative analysis for the role of these genes in benzimidazole resistance difficult (27). Presently, in human A. lumbricoides species, there is a lack of knowledge about the genes that are associated with potential benzimidazole resistance in the β-tubulin gene family.
Four β-tubulin gene isotypes of A. lumbricoides were investigated by Krücken and colleagues (23) in Rwandan school children. They reported a reduced efficacy to benzimidazole treatment. Their observed cure rate was 69.9% (95% CI 63.4- 75.4%) by Mini-FLOTAC test and 88.6% (95% CI 83.8 - 92.2%) by wet mount microscopy and the 95% calculated confidence intervals for the fecal egg count reductions (FECRs) were less than 95% indicating a reduced efficacy towards treatment. These results observed cause for the suspicion of benzimidazole resistance; however, no SNPs at codons F167Y, E198A and F200Y were observed in the four investigated β-tubulin gene isotypes (23).
Rashwan and colleagues (22) developed a SmartAmp2 method that targeted polymorphisms in the β-tubulin isotype 1 gene of A. lumbricoides. They detected the SNP TTC>TAC in codon F167Y in Haiti; however, no information was given with regard to whether infected populations were previously exposed to benzimidazole (22). Consequently, for A. lumbricoides, the existing data is not yet well-defined for the use of these SNPs as a marker for benzimidazole resistance (31). In addition to this, due to the poor understanding of the roles of the different isotopes in the β-tubulin gene family that contribute to benzimidazole resistance in the Ascaris species, it is challenging to interpret and determine whether the absence of the possible resistance-conferring SNPs is relevant in a particular β-tubulin gene isotype without prior knowledge about other possibly relevant β-tubulin loci (27).
The characterization of the genes and potential mutations in the β-tubulin family of A. lumbricoides species will provide a framework for future research to determine the possible role of the β-tubulin gene SNPs in conferring benzimidazole resistance and its prevalence in a more methodical manner than previously possible (12). Diwara et al. (21) observed an SNP at codon F167Y at high frequencies from Kenya, Panama and Haiti populations that received benzimidazole treatment (21). In pre-treatment samples from Haiti, the homozygous resistance-type (HRT) (TAC/TAC at a 40% frequency) and heterozygous type (TTC/TAC at a 60% frequency) were observed. All post-treatment samples were found to be heterozygotes (TTC/TAC at a 100% frequency), and the ERR from the Kato-Katz technique was 99.9% (95% CI 99.5–100.0)21. In Kenya, the genotype frequency of codon F167Y observed was predominantly the HRT (TAC/TAC at a frequency of 72.7%) in pre-treatment samples. Post-treatment samples showed no significant difference to the HRT (TAC/TAC at a frequency of 72.7%) observed. However, there was a significant increase of heterozygotes (TTC/TAC, from 4.5% to 21.1% (p<0.001)) and a decrease of the homozygous susceptible-type (HST) (TTC/TTC, from 22.7% to 5.3% (p<0.001). The ERR calculated from the Kato-Katz was 97.3% (95% CI, 0.0–100.0%) and McMaster technique was 80.3% (95% CI, 0.0–100.0%)21. Pre-treatment samples from Panama predominantly had the HRT (TAC/TAC at a frequency of 97.7%), and the genotype with the lowest frequency was the HST (TTC/TTC at a frequency of 2.3%). In post-treatment samples, there were no significant changes in the genotype percentages observed, with a frequency of 96.7% and 3.2%, respectively. The ERR estimated from the FLOTAC was 89.8% (95% CI, 75.8–97.3%) (21).
Therefore, the HRT allele (TAC) at codon F167Y was at a high frequency pre – and post- treatment and remained in the population after the infection was cleared in Kenya and Panama. However, in samples from Haiti, the HRT genotype TAC/TAC frequency significantly increased after treatment (21). According to the WHO standardized thresholds for Albendazole, the drug is highly effective if the ERRs are between 92% - 100%; the estimated ERRs in this study showed the treatments to be successful (21). This may suggest that the SNP at codon F167Y may not impact the efficacy of the drug, although more testing needs to be done to confirm this (21).
Matamoros et al. (25) investigated the prevalence of SNPs in the β-tubulin gene family of 40 genomic sequences from A. lumbricoides samples that had received prior treatment with Albendazole in Honduras, where STH infections are highly endemic, no mutations at codons F200Y, F167Y and E198A were detected, and all sequences were observed to be monomorphic. They calculated the prevalence of A. lumbricoides infections in their sample population to be 17.0% (CI: 95%, 9.83%, 24.13%) (25). Similarly, Zuccherato et al. (24) collected A. lumbricoides samples from six Brazilian states to determine the presence of SNPs at codons E198A and F167Y in the β-tubulin gene family of A. lumbricoides; they were also unsuccessful in detecting the presence of any mutations. The authors did not state if the target population was previously exposed to benzimidazole treatment (24). Diwara A. et al. (20) developed a pyrosequencing assay to detect for an SNP at codon F200Y in the β-tubulin gene family of A. lumbricoides samples from Zanzibar, Uganda and Panama. The authors did not mention whether the sample population received prior treatment with benzimidazole. No mutations were observed, all genotypes found in Uganda and Zanzibar were T/T at a frequency of 100%, and genotypes observed from Panama samples were only T at a 100% frequency (20). Although these studies were unsuccessful in detecting SNPs in A. lumbricoides samples, the molecular methods developed for SNP detection will prove to be valuable tools for future research.
Benzimidazole drugs bind to β-tubulin proteins and disrupt the microtubule function (23). Thus, mutations in the β-tubulin gene family can alter the amino acid sequence of the β-tubulin protein, making the β-tubulin protein less sensitive to benzimidazoles (32). Which in turn makes the parasite resistant to the drug. An in silico study by Jones et al. (33) showed that in the β-tubulin gene family of the Ascaris genus, E198 is a crucial amino acid for benzimidazole binding of β-tubulins and, the E198A and F200Y SNPs both confer resistance by disrupting this key anchor point however, they did not observe any effect of the F167Y mutation (33).
The β-tubulin gene families of many helminths, such as Trichinella spiralis, filarial nematodes and H. contortus, have been used to perform in silico docking studies (32, 34).These studies noted the protein conformational changes that take place when resistance mutations are present and their effects on drug interactions (32–34). Presently, little work has been done to apply these methods to A. lumbricoides, and few studies have done research into the changes that may possibly occur between the individual β-tubulin isotypes within a genus or species by detecting the frequency of the F200Y, F167Y and E198A SNPs (20–27). Additionally, in human T. trichiura correlation of these SNPs in the β-tubulin gene with poor response to benzimidazole treatment has been reported, Rashwan and colleagues (22) observed that the frequency of SNPs (E198A and F167Y) increased significantly in individuals who responded poorly to Albendazole treatment as opposed to individuals who responded well (20, 22).
Given the current MDA programmes implemented around the globe (13), and taking into account the evidence of the existence of these SNPs in the β-tubulin gene family of A. lumbricoides presented by the studies analyzed in this systematic review (21, 22, 26) as well as evidence of these same SNPs conferring benzimidazole resistance in the β-tubulin genes of other veterinary nematodes (20, 22, 30), the possibility of benzimidazole resistance developing in A. lumbricoides cannot be ruled out. Future studies should focus on screening for these SNPs at codons F167Y, E198A and F200Y in the β-tubulin gene family of A. lumbricoides where infection rates are high and MDA programmes are ongoing. Future research also needs to be done on the treatment efficacy of Albendazole and Mebendazole in relation to these specific SNPs (F167Y, E198A and F200Y) in the β-tubulin gene family of A. lumbricoides. The data generated from such studies will be crucial in gaining a deeper understanding of the status of benzimidazole resistance in A. lumbricoides and could possibly incorporate approaches for prevention, management and treatment, thereby decreasing the economic and health burden due to benzimidazole anthelmintic resistance around the globe.
This narrative systematic review comprehensively analyzed published literature and included all nationalities, races, genders and geographical locations that complied with the search strategy and criteria used. However, there were a few limitations to this review. The literature included was limited to articles published in English; articles in other languages were excluded. Due to the lack of research on this topic, the literature included in this review does not have a broad geographical range or target population. The reported studies were only done in Africa: Kenya, Zanzibar, Uganda, Tanzania, Ethiopia and Rwanda; the Americas: Panama, Haiti, Brazil and Honduras. A. lumbricoides infection is also highly endemic in East Asia; however, no studies regarding this topic were found to be done in this population. Future studies that target highly endemic A. lumbricoides populations and larger sample populations may provide more insightful findings. Only a few articles included in this review were able to detect SNPs in A. lumbricoides, and some of the articles included in this review were not able to clearly define the SNP genotype frequencies and A. lumbricoides prevalence. Furthermore, literature analyzing the genomic DNA from pooled egg samples and adult A. lumbricoides worms did not differentiate the differences found between genotypes from eggs and worm samples. An analysis of whether SNPs are more easily detected from egg samples or worm samples would be beneficial for future studies. Only 50% (n=4/8) of the studies included had stated whether the populations investigated received prior exposure to benzimidazole treatment, and none of the articles included in this systematic review had indicated if the target populations had received multiple exposures to benzimidazole treatment. This information is vital as single exposure to treatment may not necessarily exert significant pressure on the development of benzimidazole resistance-associated SNPs. Populations undergoing MDA programmes that have been exposed to benzimidazole treatment multiple times are ideal for researching the selection pressure placed on A. lumbricoides samples by treatment methods while also considering possible naturally occurring SNPs.
In conclusion, the reported results on the occurrence of SNPs (F200Y, E198A and F167Y) and their potential role in conferring benzimidazole resistance in the β-tubulin gene family of A. lumbricoides are still limited compared to the number of countries that have accepted the donated benzimidazole drugs for the mass treatment of children in endemic countries. More extensive research needs to be aimed at characterizing and detecting SNPs in codon 200 (F200Y: TTC/Phenylalanine to TAC/Tyrosine), codon 198 (E198A: GAG, GAA/Glutamic acid to GCG, GCA/Alanine) and codon 167 (F167Y: TTC, TTT/Phenylalanine to TAC, TAT/Tyrosine) in the β-tubulin gene family of A. lumbricoides. This research should be widely applied in endemic and peri-urban areas where A. lumbricoides infection is the most predominant. This would be of value in determining if there are genetic variations of A. lumbricoides circulating within specific populations and if A. lumbricoides is developing resistance to the current benzimidazole treatment methods. Although the majority of ascariasis infections occur in the subtropical and tropical areas of sub-Saharan Africa, the Americas and East Asia, not enough studies were done to extensively cover this geographical range. More research should be done in areas that are highly endemic for A. lumbricoides infections. Resistant mutations were found in A. lumbricoides samples at codon F167Y (TTC, TTT/Phenylalanine to TAC, TAT/Tyrosine) from Haiti, Kenya and Panama at high frequencies. The first evidence of the resistant SNP at codon F200Y (TTC/Phenylalanine to TAC/Tyrosine) was also observed in Brazil at a low frequency. This could negatively impact current MDA programmes and could potentially propagate further studies on alternative treatment regimes. Identifying and characterizing these SNPs and their potential role in benzimidazole resistance will also be a key link for future research in treatment methods for resistant genotypes of the β-tubulin gene family of A. lumbricoides, such as developing treatments that target different genes of the parasite or developing alternative treatment methods.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
TR: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. PN: Formal Analysis, Project administration, Supervision, Validation, Writing – review & editing. ZM: Formal Analysis, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Research reported in this publication was supported by the South African Medical Research Council (SAMRC) (ZLMK MSC grant number: HDID5149/KR/202) through its Division of Research Capacity Development under the Research Capacity Development Initiative from funding received from the South African National Treasury.
We would like to acknowledge the South African Medical Research Council (SAMRC) for funding.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The content and findings reported/illustrated are the sole deduction, view, and responsibility of the researchers and do not reflect the official position and sentiments of the SAMRC.
1. World health Organization. Soil-transmitted helminth infections (2020). Available at: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (Accessed 2022 Oct 28).
2. Naidoo P, Ghazi T, Chuturgoon AA, Naidoo RN, Ramsuran V, Mpaka-Mbatha MN, et al. SARS-CoV-2 and helminth co-infections, and environmental pollution exposure: An epidemiological and immunological perspective. Environ Int (2021) 156:1–14. doi: 10.1016/j.envint.2021.106695
3. Casulli A. New global targets for NTDs in the WHO roadmap 2021-2030. PloS Negl Trop Dis (2021) . 15(5):e0009373. doi: 10.1371/journal.pntd.0009373
4. Centers For Disease Control. Parasites – Ascariasis (2020). Available at: https://www.cdc.gov/parasites/ascariasis/index.html#:~:text=An%20estimated%20807%20million%E2%80%931.2,burden%20of%20parasitic%20disease%20worldwide (Accessed 2022 April 20).
5. Degarege A, Animut A, Medhin G, Legesse M, Erko B. The association between multiple intestinal helminth infections and blood group, anaemia and nutritional status in human populations from Dore Bafeno, southern Ethiopia. J Helminthol (2014) 88(2):152–9. doi: 10.1017/S0022149X12000855
6. Shah J, Shahidullah A. Ascaris lumbricoides: A Startling Discovery during Screening Colonoscopy. Case Rep Gastroenterol (2018) 12(2):224–9. doi: 10.1159/000489486
7. Brooker S. Estimating the global distribution and disease burden of intestinal nematode infections: Adding up the numbers – A review. Int J Parasitol (2010) . 40(10):1137–44. doi: 10.1016/j.ijpara.2010.04.004
8. Sartorius B, Cano J, Simpson H, Tusting L, Marczak L, Miller-Petrie M, et al. Prevalence and intensity of soil-transmitted helminth infections of children in sub-Saharan Africa, 2000-18: a geospatial analysis. Lancet Global Health (2021) . 9(1):52–60. doi: 10.1016/S2214-109X(20)30398-3
9. Chammartin F, Scholte R, Guimarães L, Tanner M, Utzinger J, Vounatsou P. Soil- transmitted helminth infection in South America: a systematic review and geostatistical meta-analysis. Lancet Infect Dis (2013) . 13:507–18. doi: 10.1016/S1473-3099(13)70071-9
10. Silver ZA, Kaliappan SP, Samuel P, Venugopal S, Kang G, Sarkar R, et al. Geographical distribution of soil transmitted helminths and the effects of community type in South Asia and South East Asia - A systematic review. PloS Negl Trop Dis (2018) . 12(1):e0006153. doi: 10.1371/journal.pntd.0006153
11. Starr MC, Montgomery SP. Soil-transmitted Helminthiasis in the United States: a systematic review-1940- 2010. Am J Trop Med Hyg (2011) . 85(4):680–4. doi: 10.4269/ajtmh.2011.11-0214
12. Hong S-T, Chai J-Y, Choi M-H, Huh S, Rim H-J, Lee S-H. A successful experience of soil-transmitted helminth control in the Republic of Korea. Korean J Parasitol (2006) . 44(3):177–85. doi: 10.3347/kjp.2006.44.3.177
13. Chai J-Y, Jung B-K, Hong S-J. Albendazole and Mebendazole as anti-parasitic and anti-cancer agents: An update. Korean J Parasitol (2021) . 59(3):189–225. doi: 10.3347/kjp.2021.59.3.189
14. The London declaration on ntds (2020). Available at: https://globalhealthprogress.org/collaboration/the-london-declaration-on-ntds-2/ (Accessed 2023 Sept 1).
15. Laxminarayan R, Bhutta Z, Duse A, Jenkins P, O’Brien T, Okeke IN, et al. Drug resistance. In: Jamison DT, Breman JG, Measham AR, editors. Disease Control Priorities in Developing Countries, 2nd. Washington (DC: The International Bank for Reconstruction and Development/The World Bank. Co-published by Oxford University Press, New York (2006). p. 1031–47.
16. Redman E, Whitelaw F, Tait A, Burgess C, Bartley Y, Skuce PJ, et al. The emergence of resistance to the benzimidazole anthlemintics in parasitic nematodes of livestock is characterized by multiple independent hard and soft selective sweeps. PloS Negl Trop Dis (2015) . 9(2):e0003494. doi: 10.1371/journal.pntd.0003494
17. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) . 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
18. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3: Rating the quality of evidence. J Clin Epidemiol (2011) . 64(4):401–6. doi: 10.1016/j.jclinepi.2010.07.015
19. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1: Introduction GRADE evidence profiles and summary of findings tables. J Clin Epidemiol (2011) . 64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026
20. Diawara A, Drake LJ, Suswillo RR, Kihara J, Bundy DA, Scott ME, et al. Assays to detect β-tubulin codon 200 polymorphism in Trichuris trichiura and Ascaris lumbricoides. PloS Negl Trop Dis (2009) 3(3). doi: 10.1371/journal.pntd.0000397
21. Diawara A, Halpenny CM, Churcher TS, Mwandawiro C, Kihara J, Kaplan RM, et al. Association between response to albendazole treatment and β-tubulin genotype frequencies in soil-transmitted helminths. PloS Negl Trop Dis (2013) . 7(5):2247. doi: 10.1371/journal.pntd.0002247
22. Rashwan N, Scott M, Prichard R. Rapid Genotyping of beta-tubulin Polymorphisms in Trichuris trichiura and Ascaris lumbricoides. PloS Negl Trop Dis (2017) . 11(1):5205. doi: 10.1371/journal.pntd.0005205
23. Krücken J, Fraundorfer K, Mugisha JC, Ramünke S, Sifft KC, Geus D, et al. Reduced efficacy of Albendazole against Ascaris lumbricoides in Rwandan schoolchildren. Int J Parasitol: Drugs Drug Resistance (2017) 7(3):262–71. doi: 10.1016/j.ijpddr.2017.06.001
24. Zuccherato LW, Furtado LF, da Medeiros C, da Pinheiro C, Rabelo ÉM. PCR-RFLP screening of polymorphisms associated with benzimidazole resistance in Necator americanus and Ascaris lumbricoides from different geographical regions in Brazil. PloS Negl Trop Dis (2018) . 12(9):e0006766. doi: 10.1371/journal.pntd.0006766
25. Matamoros G, Rueda MM, Rodríguez C, Gabrie JA, Canales M, Fontecha G, et al. High endemicity of soil-transmitted helminths in a population frequently exposed to Albendazole but no evidence of antiparasitic resistance. Trop Med Infect Dis (2019) . 4(2):73. doi: 10.3390/tropicalmed4020073
26. Furtado LFV, Medeiros CDS, Zuccherato LW, Alves WP, de Oliveira VNGM, da Silva VJ, et al. First identification of the benzimidazole resistance-associated F200Y SNP in the beta-tubulin gene in Ascaris lumbricoides. PloS One (2019) 14(10):e0224108. doi: 10.1371/journal.pone.0224108
27. Roose S, Avramenko RW, Pollo SMJ, Wasmuth JD, Ame S, Ayana M, et al. Characterization of the β-tubulin gene family in Ascaris lumbricoides and Ascaris suum and its implication for the molecular detection of benzimidazole resistance. PloS Negl Trop Dis (2021) . 15(9):e0009777. doi: 10.1371/journal.pntd.0009777
28. Kwa MSG, Veenstra JG, Roos MH. Benzimidazole resistance in Haemonchus contortus is correlated with a conserved mutation at amino acid 200 in beta-tubulin isotype 1. Mol Biochem Parasitol (1994) . 63:299–303. doi: 10.1016/0166-6851(94)90066-3
29. Silvestre A, Cabaret J. Mutation in position 167 of isotype 1 beta-tubulin gene of trichostrongylid nematodes: Role in benzimidazole resistance. Mol Biochem Parasitol (2002) . 120(2):297–300. doi: 10.1016/s0166-6851(01)00455-8
30. Pitaksakulrat O, Chaiyasaeng M, Artchayasawat A, Eamudomkarn C, Thongsahuan S, Boonmars T. The first molecular identification of benzimidazole resistance in Haemonchus contortus from goats in Thailand. Vet World (2021) . 14(3):764–8. doi: 10.14202/vetworld.2021.764-768
31. Gandasegui J, Martínez-Valladares M, Grau-Pujol B, Krolewiecki AJ, Balaña-Fouce R, Gelaye W, et al. Role of DNA-detection–based tools for monitoring the soil-transmitted helminth treatment response in drug-efficacy trials. PloS Negl Trop Dis (2020) . 14(2):e0007931. doi: 10.1371/journal.pntd.0007931
32. Samant L, Halder S, Dhorajiwala T. Molecular docking studies of filarial β-tubulin protein models with antifilarial phytochemicals. Biomed Biotechnol Res J (BBRJ) (2019) . 3(3):162. doi: 10.4103/bbrj.bbrj_100_19
33. Jones BP, van Vliet AHM, LaCourse EJ, Beston M. Identification of key interactions of benzimidazole resistance-associated amino acid mutations in Ascaris β-tubulins by molecular docking simulations. Sci Rep (2022) 12:13725. doi: 10.1038/s41598-022-16765-4
Keywords: Ascaris lumbricoides, β-tubulin gene polymorphisms, benzimidazole drug resistance, Mebendazole and Albendazole, treatment efficacy
Citation: Ramkhelawan T, Naidoo P and Mkhize-Kwitshana ZL (2024) Single nucleotide polymorphisms in the β-tubulin gene family of Ascaris lumbricoides and their potential role in benzimidazole resistance: a systematic review. Front. Trop. Dis 4:1303873. doi: 10.3389/fitd.2023.1303873
Received: 28 September 2023; Accepted: 11 December 2023;
Published: 17 January 2024.
Edited by:
Stephanie Salyer, Centers for Disease Control and Prevention (CDC), United StatesCopyright © 2024 Ramkhelawan, Naidoo and Mkhize-Kwitshana. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teniel Ramkhelawan, dGVuei5yYW1raGVsYXdhbkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.